User login
An overlooked cause of catatonia
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
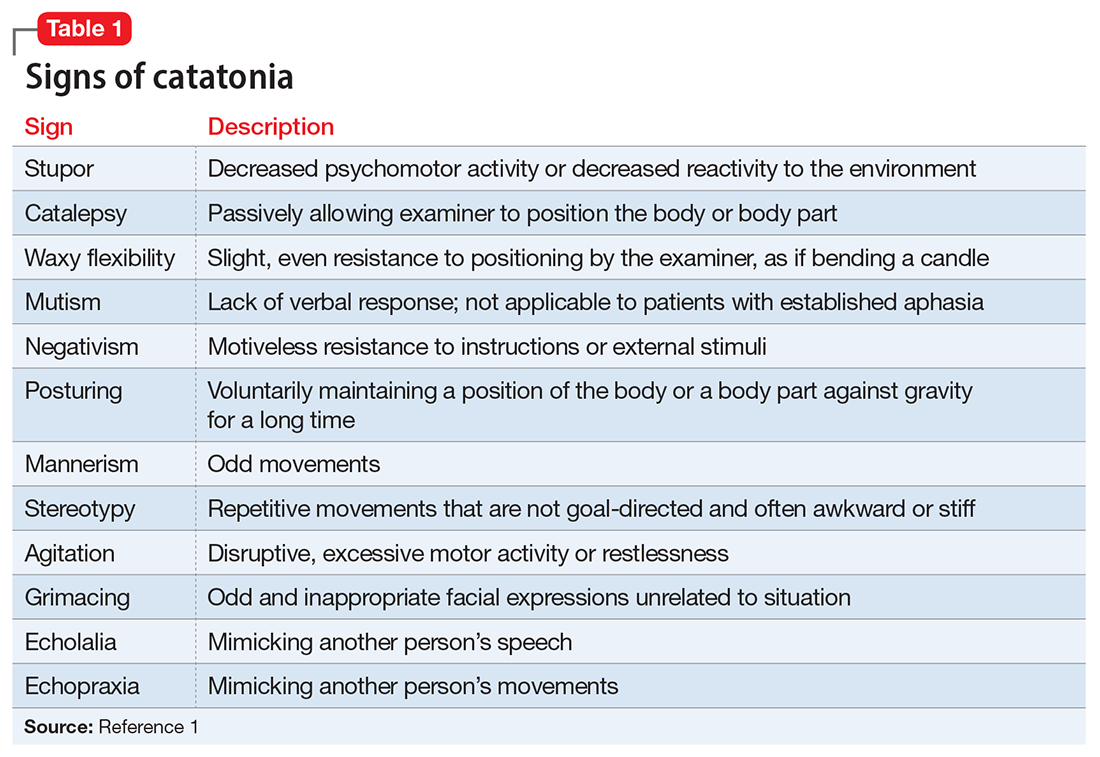
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.
EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
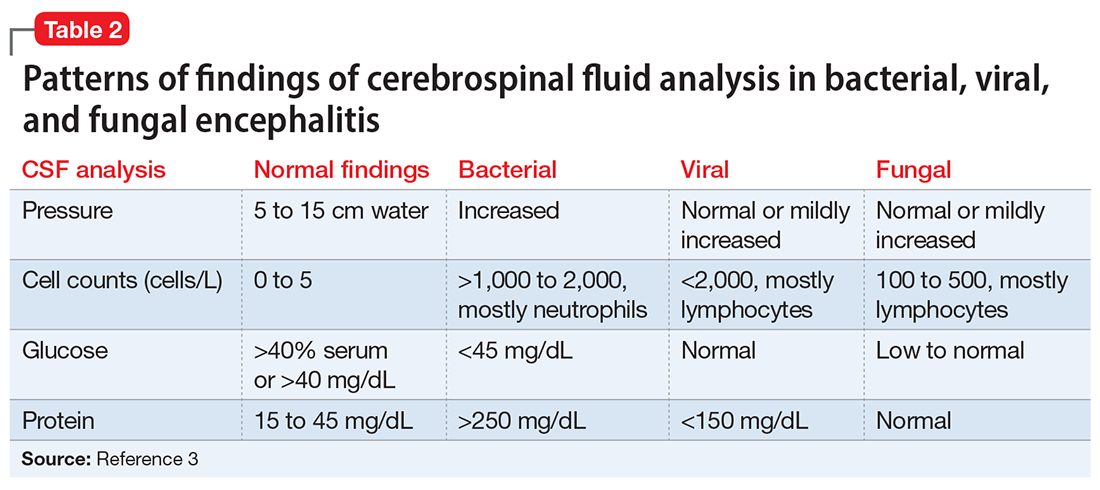
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).
[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.
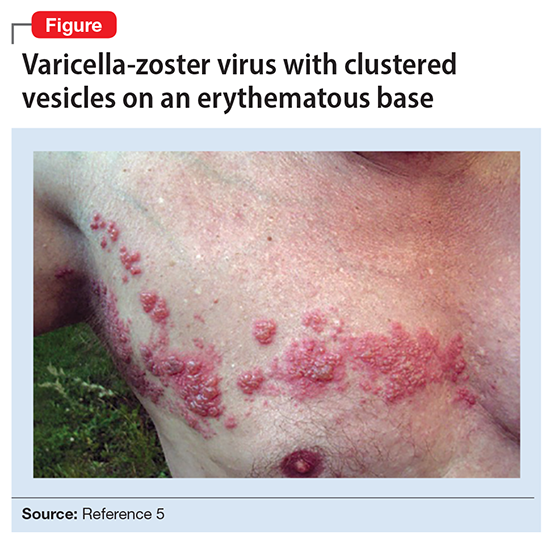
Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
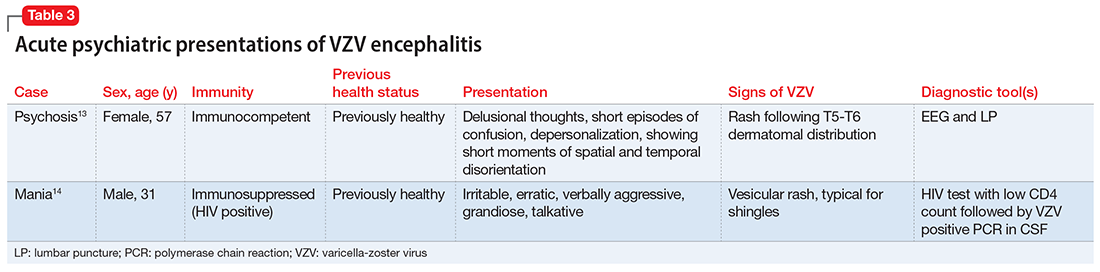
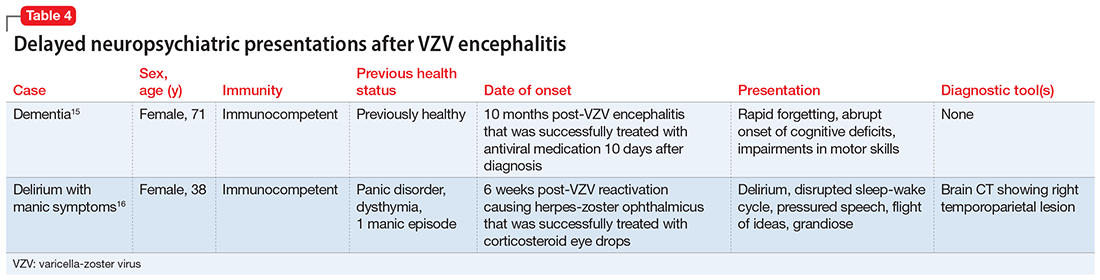
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16
To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-
Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.

EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).

[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.

Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16

To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-

Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.

EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).

[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.

Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16

To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-

Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
Hold or not to hold: Navigating involuntary commitment
CASE Depressed and suicidal
Police arrive at the home of Mr. H, age 50, after his wife calls 911. She reports he has depression and she saw him in bed brandishing a firearm as if he wanted to hurt himself. Upon arrival, the officers enter the house and find Mr. H in bed without a firearm. Mr. H says little to the officers about the alleged events, but acknowledges he has depression and is willing to go the hospital for further evaluation. Neither his wife nor the officers locate a firearm in the home.
EVALUATION Emergency detention
In the emergency department (ED), Mr. H’s laboratory results and physical examination findings are normal. He acknowledges feeling depressed over the past 2 weeks. Though he cannot identify any precipitants, he says he has experienced anhedonia, lack of appetite, decreased energy, and changes in his sleep patterns. When asked about the day’s events concerning the firearm, Mr. H becomes guarded and does not give a clear answer regarding having thoughts of suicide.
The evaluating psychiatrist obtains collateral from Mr. H’s wife and reviews his medical records. There are no active prescriptions on file and the psychiatrist notices that last year there was a suicide attempt involving a firearm. Following that episode, Mr. H was hospitalized, treated with sertraline 50 mg/d, and discharged with a diagnosis of major depressive disorder. There was no legal or substance abuse history.
In the ED, the psychiatrist conducts a psychiatric evaluation, including a suicide risk assessment, and determines Mr. H is at imminent risk of ending his life. Mr. H’s psychiatrist explains there are 2 treatment options: to be admitted to the hospital or to be discharged. The psychiatrist recommends hospital admission to Mr. H for his safety and stabilization. Mr. H says he prefers to return home.
Because the psychiatrist believes Mr. H is at imminent risk of ending his life and there is no less restrictive setting for treatment, he implements an emergency detention. In Ohio, this allows Mr. H to be held in the hospital for no more than 3 court days in accordance with state law. Before Mr. H’s emergency detention periods ends, the psychiatrist will need to decide whether Mr. H can be safely discharged. If the psychiatrist determines that Mr. H still needs treatment, the court will be petitioned for a civil commitment hearing.
[polldaddy:11189291]
The author’s observations
In some cases, courts allow information a psychiatrist does not directly obtain from a patient to be admitted as testimony in a civil commitment hearing. However, some jurisdictions consider sources of information not obtained directly from the patient as hearsay and thus inadmissible.1 Though each source listed may provide credible information that could be presented at a hearing, the psychiatrist should discuss with the patient the information obtained from these sources to ensure it is admissable.2 A discussion with Mr. H about the factors that led to his hospital arrival will avoid the psychiatrist’s reliance on what another person has heard or seen when providing testimony. Even when a psychiatrist is not faced with an issue of admissibility, caution must be taken with third-party reports.3
TREATMENT Civil commitment hearing
Before the emergency detention period expires, Mr. H’s psychiatrist determines that he remains at imminent risk of self-harm. To continue hospitalization, the psychiatrist files a petition for civil commitment and testifies at the commitment hearing. He reports that Mr. H suffers from a substantial mood disorder that grossly impairs his judgment and behavior. The psychiatrist also testifies that the least restrictive environment for treatment continues to be inpatient hospitalization, because Mr. H is still at imminent risk of harming himself.
Continue to: Following the psychiatrist's...
Following the psychiatrist’s testimony, the magistrate finds that Mr. H is a mentally ill person subject to hospitalization given his mood disorder that grossly impairs his judgment and behavior. The magistrate orders that Mr. H be civilly committed to the hospital.
[polldaddy:11189293]
The author’s observations
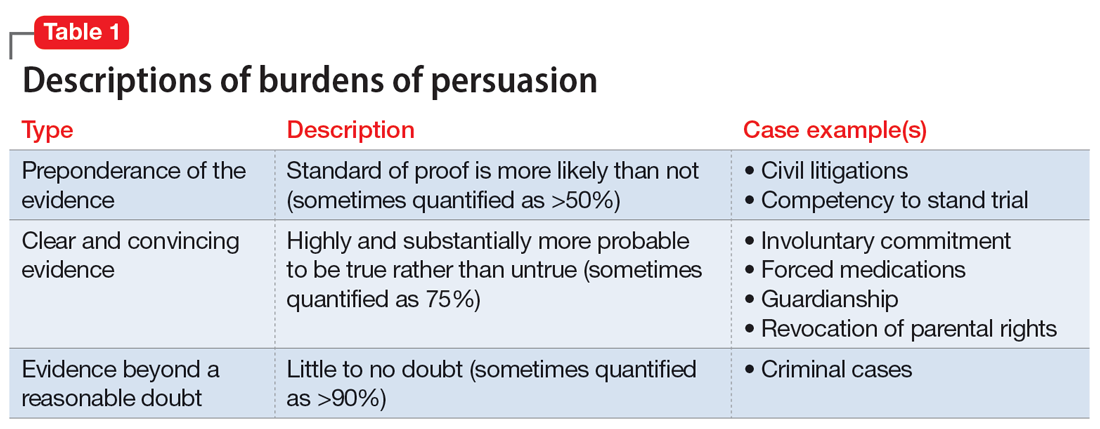
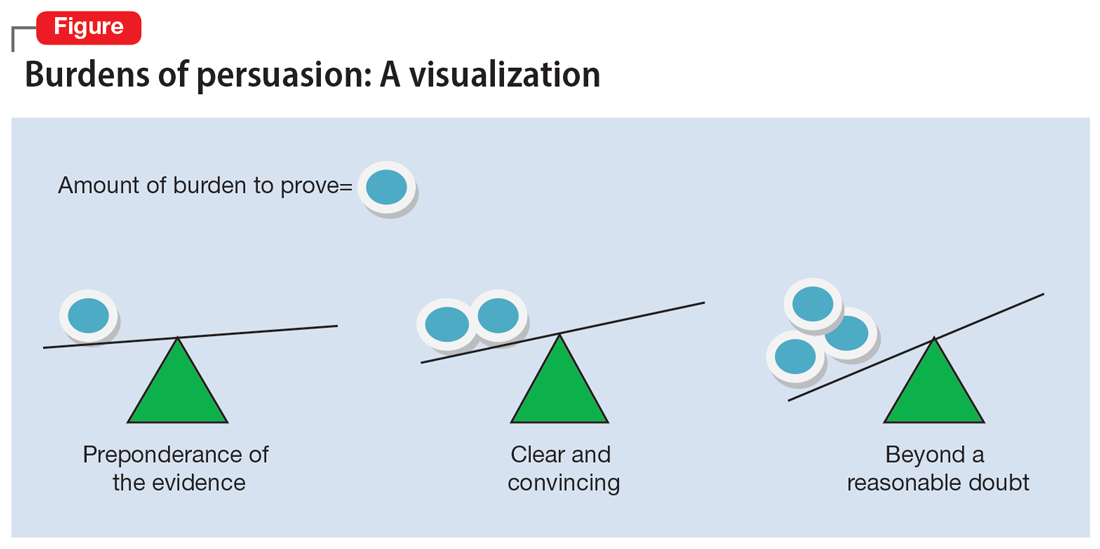
The psychiatrist’s testimony mirrors the language regarding civil commitment in the Ohio Revised Code.4 Other elements considered for mental illness in Ohio are a substantial disorder of memory, thought, orientation, or perception that grossly impairs one’s capacity to recognize reality or meet the demands of life.4 The definition of what constitutes a mental disorder varies by state, but the burden of persuasion—the standard by which the court must be convinced—is generally uniform.5 In the 1979 case Addington v Texas, the United States Supreme Court concluded that in a civil commitment hearing, the minimum standard of proof for involuntary commitment must be clear and convincing evidence.6 Neither medical certainty nor substantial probability are burdens of persuasions.6 Instead, these terms may be presented in a forensic report when an examiner outlines their opinion. Table 1 and the Figure provide more detail on burdens of persuasion.
TREATMENT Civil commitment and patient rights
At a regularly scheduled treatment team meeting, the team informs Mr. H that he has been civilly committed for further treatment. Mr. H becomes upset and tells the team the decision is a complete violation of his rights. After a long rant, Mr. H walks out of the room, saying, “I did not even know when this hearing was.” A member of the treatment team becomes concerned that Mr. H may not have been notified of the hearing.
[polldaddy:11189294]
The author’s observations
It is not clear if Mr. H had been notified of his civil commitment hearing. If Mr. H had not been notified, his rights would have been compromised. Lessard v Schmidt (1972) outlined that individuals involved in a civil commitment hearing should be afforded the same rights as those involved in criminal proceedings.7 Mr. H should have been notified of his hearing and afforded the opportunity to have the assignment of counsel, the right to appear, the right to testify, the right to present witnesses and other evidence, and the right to confront witnesses.
Without notification of the hearing, the only right that would have remained intact for Mr. H would have been the assignment of counsel in his absence and without his knowledge. If Mr. H had been notified of the hearing and did not want to attend, yet still desired legal counsel, he could have waived his presence voluntarily after discussing this option with his attorney.8,9
Continue to: OUTCOME Stabilization and discharge
OUTCOME Stabilization and discharge
During his 10-day stay, Mr. H is treated with sertraline 50 mg/d and engages in individual and group therapy. He shows noticeable improvement in his depressive symptoms and reports having no thoughts of suicide or self-harm. The treatment team determines it is appropriate to discharge him home (the firearm was never found) and involves his wife in safety planning and follow-up care. On the day of his discharge, Mr. H reflects on his treatment and civil commitment. He says, “I did not know a judge could order me to be hospitalized.”
[polldaddy:11189297]
The author’s observations
The physician’s decision to pursue civil commitment is best described by the legal doctrines of police powers and parens patriae. Other relevant ethical principles are described in Table 2.10

Though ethical principles may play a role in civil commitment, parens patriae and police powers is the answer with respect to the State.11Parens patriae is Latin for the “parent of the country” and grants the State the power to protect those residents who are most vulnerable. Police power is the authority of the State to enact and enforce rules that limit the rights of individuals for the greater good of ensuring health, safety, and welfare of all citizens.
Bottom Line
Psychiatrists are entrusted with recognizing when a patient, due to mental illness, is a danger to themselves or others and in need of treatment. After an emergency detention period, if the patient remains a danger to themselves or others and does not want to voluntarily receive treatment, a court hearing is required. As an expert witness, the treating psychiatrist should know the factors of law in their jurisdiction that determine civil commitment.
Related Resources
- Extreme Risk Protection Orders. Johns Hopkins Bloomberg School of Public Health. https://www.jhsph.edu/research/ centers-and-institutes/johns-hopkins-center-for-gun-violenceprevention-and-policy/research/extreme-risk-protectionorders/
- Gutheil TG. The Psychiatrist as Expert Witness. 2nd ed. American Psychiatric Association Publishing; 2009.
- Landmark Cases 2014. American Academy of Psychiatry and the Law. https://www.aapl.org/landmark-cases
Drug Brand Names
Sertraline • Zoloft
1. Pinals DA, Mossman D. Evaluation for Civil Commitment. Oxford University Press; 2012.
2. Thatcher BT, Mossman D. Testifying for civil commitment: help unwilling patients get the treatment they need. Current Psychiatry. 2009;8(11):51-56.
3. Marett CP, Mossman D. What is your liability for involuntary commitment based on faulty information? Current Psychiatry. 2017;16(3):21-25,33.
4. Ohio Rev Code § 5122.01 (2018).
5. The Burden of Proof. University of Minnesota. Accessed January 23, 2022. https://open.lib.umn.edu/criminallaw/chapter/2-4-the-burden-of-proof/
6. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2018.
7. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Suicide Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
8. Cook J. Good lawyering and bad role models: the role of respondent’s counsel in a civil commitment hearing. Georgetown Journal of Legal Ethics. 2000;14(1):179-195.
9. Ferris CE. The search for due process in civil commitment hearings: how procedural realities have altered substantive standards. Vanderbilt Law Rev. 2008;61(3):959-981.
10. Substance Abuse and Mental Health Services Administration. Civil Commitment and the Mental Health Care Continuum: Historical Trends and Principles for Law and Practice. 2019. Accessed January 23, 2022. https://www.samhsa.gov/resource/ebp/civil-commitment-mental-health-care-continuum-historical-trends-principles-law
11. Melton GB, Petrila J, Poythress NG, et al. Psychological Evaluations for the Courts: A Handbook for Mental Health Profession. 4th ed. Guilford Press; 2018.
CASE Depressed and suicidal
Police arrive at the home of Mr. H, age 50, after his wife calls 911. She reports he has depression and she saw him in bed brandishing a firearm as if he wanted to hurt himself. Upon arrival, the officers enter the house and find Mr. H in bed without a firearm. Mr. H says little to the officers about the alleged events, but acknowledges he has depression and is willing to go the hospital for further evaluation. Neither his wife nor the officers locate a firearm in the home.
EVALUATION Emergency detention
In the emergency department (ED), Mr. H’s laboratory results and physical examination findings are normal. He acknowledges feeling depressed over the past 2 weeks. Though he cannot identify any precipitants, he says he has experienced anhedonia, lack of appetite, decreased energy, and changes in his sleep patterns. When asked about the day’s events concerning the firearm, Mr. H becomes guarded and does not give a clear answer regarding having thoughts of suicide.
The evaluating psychiatrist obtains collateral from Mr. H’s wife and reviews his medical records. There are no active prescriptions on file and the psychiatrist notices that last year there was a suicide attempt involving a firearm. Following that episode, Mr. H was hospitalized, treated with sertraline 50 mg/d, and discharged with a diagnosis of major depressive disorder. There was no legal or substance abuse history.
In the ED, the psychiatrist conducts a psychiatric evaluation, including a suicide risk assessment, and determines Mr. H is at imminent risk of ending his life. Mr. H’s psychiatrist explains there are 2 treatment options: to be admitted to the hospital or to be discharged. The psychiatrist recommends hospital admission to Mr. H for his safety and stabilization. Mr. H says he prefers to return home.
Because the psychiatrist believes Mr. H is at imminent risk of ending his life and there is no less restrictive setting for treatment, he implements an emergency detention. In Ohio, this allows Mr. H to be held in the hospital for no more than 3 court days in accordance with state law. Before Mr. H’s emergency detention periods ends, the psychiatrist will need to decide whether Mr. H can be safely discharged. If the psychiatrist determines that Mr. H still needs treatment, the court will be petitioned for a civil commitment hearing.
[polldaddy:11189291]
The author’s observations
In some cases, courts allow information a psychiatrist does not directly obtain from a patient to be admitted as testimony in a civil commitment hearing. However, some jurisdictions consider sources of information not obtained directly from the patient as hearsay and thus inadmissible.1 Though each source listed may provide credible information that could be presented at a hearing, the psychiatrist should discuss with the patient the information obtained from these sources to ensure it is admissable.2 A discussion with Mr. H about the factors that led to his hospital arrival will avoid the psychiatrist’s reliance on what another person has heard or seen when providing testimony. Even when a psychiatrist is not faced with an issue of admissibility, caution must be taken with third-party reports.3
TREATMENT Civil commitment hearing
Before the emergency detention period expires, Mr. H’s psychiatrist determines that he remains at imminent risk of self-harm. To continue hospitalization, the psychiatrist files a petition for civil commitment and testifies at the commitment hearing. He reports that Mr. H suffers from a substantial mood disorder that grossly impairs his judgment and behavior. The psychiatrist also testifies that the least restrictive environment for treatment continues to be inpatient hospitalization, because Mr. H is still at imminent risk of harming himself.
Continue to: Following the psychiatrist's...
Following the psychiatrist’s testimony, the magistrate finds that Mr. H is a mentally ill person subject to hospitalization given his mood disorder that grossly impairs his judgment and behavior. The magistrate orders that Mr. H be civilly committed to the hospital.
[polldaddy:11189293]
The author’s observations
The psychiatrist’s testimony mirrors the language regarding civil commitment in the Ohio Revised Code.4 Other elements considered for mental illness in Ohio are a substantial disorder of memory, thought, orientation, or perception that grossly impairs one’s capacity to recognize reality or meet the demands of life.4 The definition of what constitutes a mental disorder varies by state, but the burden of persuasion—the standard by which the court must be convinced—is generally uniform.5 In the 1979 case Addington v Texas, the United States Supreme Court concluded that in a civil commitment hearing, the minimum standard of proof for involuntary commitment must be clear and convincing evidence.6 Neither medical certainty nor substantial probability are burdens of persuasions.6 Instead, these terms may be presented in a forensic report when an examiner outlines their opinion. Table 1 and the Figure provide more detail on burdens of persuasion.

TREATMENT Civil commitment and patient rights
At a regularly scheduled treatment team meeting, the team informs Mr. H that he has been civilly committed for further treatment. Mr. H becomes upset and tells the team the decision is a complete violation of his rights. After a long rant, Mr. H walks out of the room, saying, “I did not even know when this hearing was.” A member of the treatment team becomes concerned that Mr. H may not have been notified of the hearing.

[polldaddy:11189294]
The author’s observations
It is not clear if Mr. H had been notified of his civil commitment hearing. If Mr. H had not been notified, his rights would have been compromised. Lessard v Schmidt (1972) outlined that individuals involved in a civil commitment hearing should be afforded the same rights as those involved in criminal proceedings.7 Mr. H should have been notified of his hearing and afforded the opportunity to have the assignment of counsel, the right to appear, the right to testify, the right to present witnesses and other evidence, and the right to confront witnesses.
Without notification of the hearing, the only right that would have remained intact for Mr. H would have been the assignment of counsel in his absence and without his knowledge. If Mr. H had been notified of the hearing and did not want to attend, yet still desired legal counsel, he could have waived his presence voluntarily after discussing this option with his attorney.8,9
Continue to: OUTCOME Stabilization and discharge
OUTCOME Stabilization and discharge
During his 10-day stay, Mr. H is treated with sertraline 50 mg/d and engages in individual and group therapy. He shows noticeable improvement in his depressive symptoms and reports having no thoughts of suicide or self-harm. The treatment team determines it is appropriate to discharge him home (the firearm was never found) and involves his wife in safety planning and follow-up care. On the day of his discharge, Mr. H reflects on his treatment and civil commitment. He says, “I did not know a judge could order me to be hospitalized.”
[polldaddy:11189297]
The author’s observations
The physician’s decision to pursue civil commitment is best described by the legal doctrines of police powers and parens patriae. Other relevant ethical principles are described in Table 2.10

Though ethical principles may play a role in civil commitment, parens patriae and police powers is the answer with respect to the State.11Parens patriae is Latin for the “parent of the country” and grants the State the power to protect those residents who are most vulnerable. Police power is the authority of the State to enact and enforce rules that limit the rights of individuals for the greater good of ensuring health, safety, and welfare of all citizens.
Bottom Line
Psychiatrists are entrusted with recognizing when a patient, due to mental illness, is a danger to themselves or others and in need of treatment. After an emergency detention period, if the patient remains a danger to themselves or others and does not want to voluntarily receive treatment, a court hearing is required. As an expert witness, the treating psychiatrist should know the factors of law in their jurisdiction that determine civil commitment.
Related Resources
- Extreme Risk Protection Orders. Johns Hopkins Bloomberg School of Public Health. https://www.jhsph.edu/research/ centers-and-institutes/johns-hopkins-center-for-gun-violenceprevention-and-policy/research/extreme-risk-protectionorders/
- Gutheil TG. The Psychiatrist as Expert Witness. 2nd ed. American Psychiatric Association Publishing; 2009.
- Landmark Cases 2014. American Academy of Psychiatry and the Law. https://www.aapl.org/landmark-cases
Drug Brand Names
Sertraline • Zoloft
CASE Depressed and suicidal
Police arrive at the home of Mr. H, age 50, after his wife calls 911. She reports he has depression and she saw him in bed brandishing a firearm as if he wanted to hurt himself. Upon arrival, the officers enter the house and find Mr. H in bed without a firearm. Mr. H says little to the officers about the alleged events, but acknowledges he has depression and is willing to go the hospital for further evaluation. Neither his wife nor the officers locate a firearm in the home.
EVALUATION Emergency detention
In the emergency department (ED), Mr. H’s laboratory results and physical examination findings are normal. He acknowledges feeling depressed over the past 2 weeks. Though he cannot identify any precipitants, he says he has experienced anhedonia, lack of appetite, decreased energy, and changes in his sleep patterns. When asked about the day’s events concerning the firearm, Mr. H becomes guarded and does not give a clear answer regarding having thoughts of suicide.
The evaluating psychiatrist obtains collateral from Mr. H’s wife and reviews his medical records. There are no active prescriptions on file and the psychiatrist notices that last year there was a suicide attempt involving a firearm. Following that episode, Mr. H was hospitalized, treated with sertraline 50 mg/d, and discharged with a diagnosis of major depressive disorder. There was no legal or substance abuse history.
In the ED, the psychiatrist conducts a psychiatric evaluation, including a suicide risk assessment, and determines Mr. H is at imminent risk of ending his life. Mr. H’s psychiatrist explains there are 2 treatment options: to be admitted to the hospital or to be discharged. The psychiatrist recommends hospital admission to Mr. H for his safety and stabilization. Mr. H says he prefers to return home.
Because the psychiatrist believes Mr. H is at imminent risk of ending his life and there is no less restrictive setting for treatment, he implements an emergency detention. In Ohio, this allows Mr. H to be held in the hospital for no more than 3 court days in accordance with state law. Before Mr. H’s emergency detention periods ends, the psychiatrist will need to decide whether Mr. H can be safely discharged. If the psychiatrist determines that Mr. H still needs treatment, the court will be petitioned for a civil commitment hearing.
[polldaddy:11189291]
The author’s observations
In some cases, courts allow information a psychiatrist does not directly obtain from a patient to be admitted as testimony in a civil commitment hearing. However, some jurisdictions consider sources of information not obtained directly from the patient as hearsay and thus inadmissible.1 Though each source listed may provide credible information that could be presented at a hearing, the psychiatrist should discuss with the patient the information obtained from these sources to ensure it is admissable.2 A discussion with Mr. H about the factors that led to his hospital arrival will avoid the psychiatrist’s reliance on what another person has heard or seen when providing testimony. Even when a psychiatrist is not faced with an issue of admissibility, caution must be taken with third-party reports.3
TREATMENT Civil commitment hearing
Before the emergency detention period expires, Mr. H’s psychiatrist determines that he remains at imminent risk of self-harm. To continue hospitalization, the psychiatrist files a petition for civil commitment and testifies at the commitment hearing. He reports that Mr. H suffers from a substantial mood disorder that grossly impairs his judgment and behavior. The psychiatrist also testifies that the least restrictive environment for treatment continues to be inpatient hospitalization, because Mr. H is still at imminent risk of harming himself.
Continue to: Following the psychiatrist's...
Following the psychiatrist’s testimony, the magistrate finds that Mr. H is a mentally ill person subject to hospitalization given his mood disorder that grossly impairs his judgment and behavior. The magistrate orders that Mr. H be civilly committed to the hospital.
[polldaddy:11189293]
The author’s observations
The psychiatrist’s testimony mirrors the language regarding civil commitment in the Ohio Revised Code.4 Other elements considered for mental illness in Ohio are a substantial disorder of memory, thought, orientation, or perception that grossly impairs one’s capacity to recognize reality or meet the demands of life.4 The definition of what constitutes a mental disorder varies by state, but the burden of persuasion—the standard by which the court must be convinced—is generally uniform.5 In the 1979 case Addington v Texas, the United States Supreme Court concluded that in a civil commitment hearing, the minimum standard of proof for involuntary commitment must be clear and convincing evidence.6 Neither medical certainty nor substantial probability are burdens of persuasions.6 Instead, these terms may be presented in a forensic report when an examiner outlines their opinion. Table 1 and the Figure provide more detail on burdens of persuasion.

TREATMENT Civil commitment and patient rights
At a regularly scheduled treatment team meeting, the team informs Mr. H that he has been civilly committed for further treatment. Mr. H becomes upset and tells the team the decision is a complete violation of his rights. After a long rant, Mr. H walks out of the room, saying, “I did not even know when this hearing was.” A member of the treatment team becomes concerned that Mr. H may not have been notified of the hearing.

[polldaddy:11189294]
The author’s observations
It is not clear if Mr. H had been notified of his civil commitment hearing. If Mr. H had not been notified, his rights would have been compromised. Lessard v Schmidt (1972) outlined that individuals involved in a civil commitment hearing should be afforded the same rights as those involved in criminal proceedings.7 Mr. H should have been notified of his hearing and afforded the opportunity to have the assignment of counsel, the right to appear, the right to testify, the right to present witnesses and other evidence, and the right to confront witnesses.
Without notification of the hearing, the only right that would have remained intact for Mr. H would have been the assignment of counsel in his absence and without his knowledge. If Mr. H had been notified of the hearing and did not want to attend, yet still desired legal counsel, he could have waived his presence voluntarily after discussing this option with his attorney.8,9
Continue to: OUTCOME Stabilization and discharge
OUTCOME Stabilization and discharge
During his 10-day stay, Mr. H is treated with sertraline 50 mg/d and engages in individual and group therapy. He shows noticeable improvement in his depressive symptoms and reports having no thoughts of suicide or self-harm. The treatment team determines it is appropriate to discharge him home (the firearm was never found) and involves his wife in safety planning and follow-up care. On the day of his discharge, Mr. H reflects on his treatment and civil commitment. He says, “I did not know a judge could order me to be hospitalized.”
[polldaddy:11189297]
The author’s observations
The physician’s decision to pursue civil commitment is best described by the legal doctrines of police powers and parens patriae. Other relevant ethical principles are described in Table 2.10

Though ethical principles may play a role in civil commitment, parens patriae and police powers is the answer with respect to the State.11Parens patriae is Latin for the “parent of the country” and grants the State the power to protect those residents who are most vulnerable. Police power is the authority of the State to enact and enforce rules that limit the rights of individuals for the greater good of ensuring health, safety, and welfare of all citizens.
Bottom Line
Psychiatrists are entrusted with recognizing when a patient, due to mental illness, is a danger to themselves or others and in need of treatment. After an emergency detention period, if the patient remains a danger to themselves or others and does not want to voluntarily receive treatment, a court hearing is required. As an expert witness, the treating psychiatrist should know the factors of law in their jurisdiction that determine civil commitment.
Related Resources
- Extreme Risk Protection Orders. Johns Hopkins Bloomberg School of Public Health. https://www.jhsph.edu/research/ centers-and-institutes/johns-hopkins-center-for-gun-violenceprevention-and-policy/research/extreme-risk-protectionorders/
- Gutheil TG. The Psychiatrist as Expert Witness. 2nd ed. American Psychiatric Association Publishing; 2009.
- Landmark Cases 2014. American Academy of Psychiatry and the Law. https://www.aapl.org/landmark-cases
Drug Brand Names
Sertraline • Zoloft
1. Pinals DA, Mossman D. Evaluation for Civil Commitment. Oxford University Press; 2012.
2. Thatcher BT, Mossman D. Testifying for civil commitment: help unwilling patients get the treatment they need. Current Psychiatry. 2009;8(11):51-56.
3. Marett CP, Mossman D. What is your liability for involuntary commitment based on faulty information? Current Psychiatry. 2017;16(3):21-25,33.
4. Ohio Rev Code § 5122.01 (2018).
5. The Burden of Proof. University of Minnesota. Accessed January 23, 2022. https://open.lib.umn.edu/criminallaw/chapter/2-4-the-burden-of-proof/
6. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2018.
7. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Suicide Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
8. Cook J. Good lawyering and bad role models: the role of respondent’s counsel in a civil commitment hearing. Georgetown Journal of Legal Ethics. 2000;14(1):179-195.
9. Ferris CE. The search for due process in civil commitment hearings: how procedural realities have altered substantive standards. Vanderbilt Law Rev. 2008;61(3):959-981.
10. Substance Abuse and Mental Health Services Administration. Civil Commitment and the Mental Health Care Continuum: Historical Trends and Principles for Law and Practice. 2019. Accessed January 23, 2022. https://www.samhsa.gov/resource/ebp/civil-commitment-mental-health-care-continuum-historical-trends-principles-law
11. Melton GB, Petrila J, Poythress NG, et al. Psychological Evaluations for the Courts: A Handbook for Mental Health Profession. 4th ed. Guilford Press; 2018.
1. Pinals DA, Mossman D. Evaluation for Civil Commitment. Oxford University Press; 2012.
2. Thatcher BT, Mossman D. Testifying for civil commitment: help unwilling patients get the treatment they need. Current Psychiatry. 2009;8(11):51-56.
3. Marett CP, Mossman D. What is your liability for involuntary commitment based on faulty information? Current Psychiatry. 2017;16(3):21-25,33.
4. Ohio Rev Code § 5122.01 (2018).
5. The Burden of Proof. University of Minnesota. Accessed January 23, 2022. https://open.lib.umn.edu/criminallaw/chapter/2-4-the-burden-of-proof/
6. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2018.
7. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Suicide Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
8. Cook J. Good lawyering and bad role models: the role of respondent’s counsel in a civil commitment hearing. Georgetown Journal of Legal Ethics. 2000;14(1):179-195.
9. Ferris CE. The search for due process in civil commitment hearings: how procedural realities have altered substantive standards. Vanderbilt Law Rev. 2008;61(3):959-981.
10. Substance Abuse and Mental Health Services Administration. Civil Commitment and the Mental Health Care Continuum: Historical Trends and Principles for Law and Practice. 2019. Accessed January 23, 2022. https://www.samhsa.gov/resource/ebp/civil-commitment-mental-health-care-continuum-historical-trends-principles-law
11. Melton GB, Petrila J, Poythress NG, et al. Psychological Evaluations for the Courts: A Handbook for Mental Health Profession. 4th ed. Guilford Press; 2018.
Impaired cognition in a patient with schizophrenia and HIV
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.
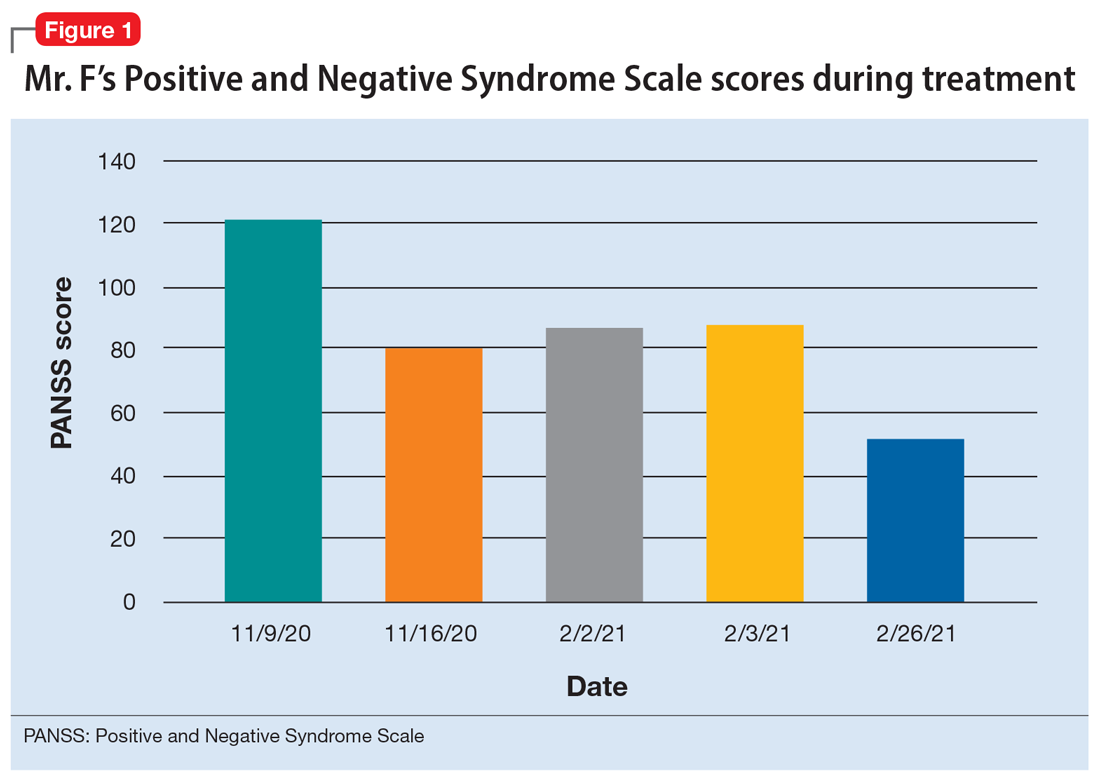
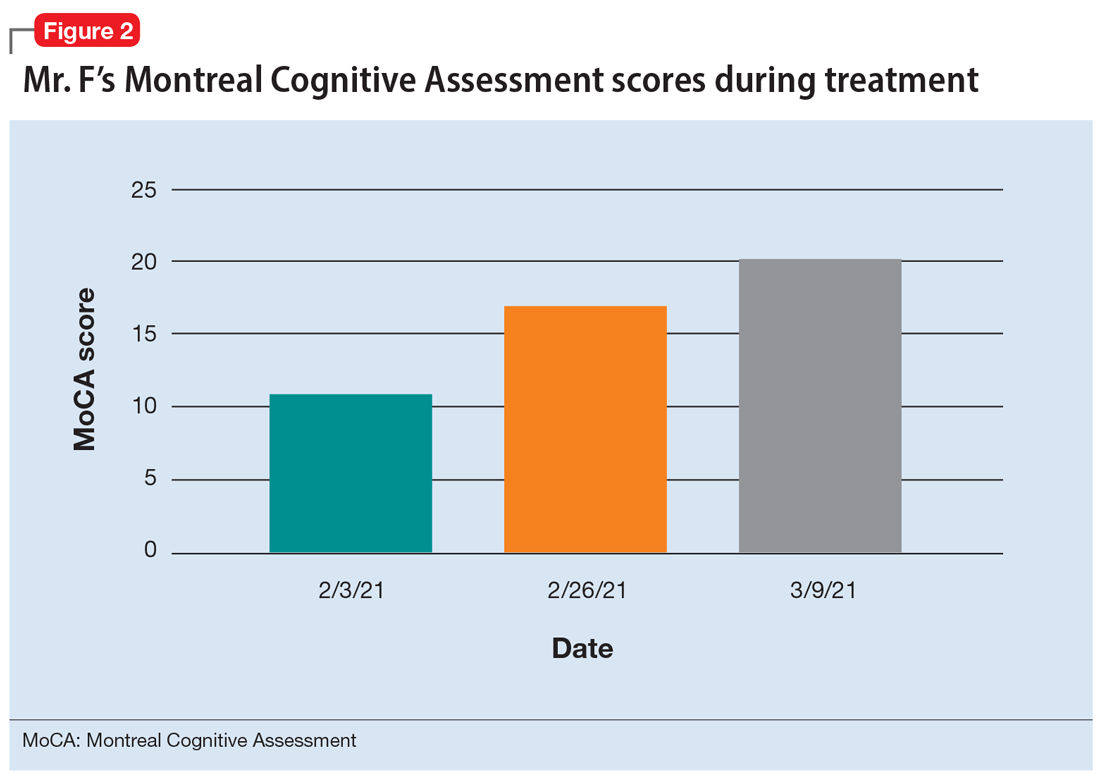
During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.
The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.

During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.

The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.

During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.

The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
Should clozapine be discontinued in a patient receiving chemotherapy?
CASE Schizophrenia, leukemia, and chemotherapy
Mr. A, age 30, has schizophrenia but has been stable on clozapine 600 mg/d. He presents to the emergency department with generalized pain that started in his right scapula, arm, elbow, and back. Laboratory tests and a diagnostic examination reveal severe leukocytosis, thrombocytopenia, and anemia, and clinicians diagnose Mr. A with B-cell acute lymphocytic leukemia (B-ALL). Upon admission, Mr. A is neutropenic with an absolute neutrophil count (ANC) of 1,420 µL (reference range 2,500 to 6,000 µL). The hematology team recommends chemotherapy. The treating clinicians also consult the psychiatry team for recommendations on how to best manage Mr. A’s schizophrenia during chemotherapy, including whether clozapine should be discontinued.
HISTORY Stable on clozapine for >10 years
Mr. A was diagnosed with schizophrenia at age 15 after developing paranoia and auditory hallucinations of people talking to him and to each other. He had been hospitalized multiple times for worsened auditory hallucinations and paranoia that led to significant agitation and violence. Previous treatment with multiple antipsychotics, including haloperidol, quetiapine, aripiprazole, olanzapine, risperidone, and ziprasidone, was not successful. Mr. A began clozapine >10 years ago, and his symptoms have been stable since, without any further psychiatric hospitalizations. Mr. A takes clozapine 600 mg/d and divalproex sodium 1,500 mg/d, which he tolerates well and without significant adverse effects. Though he continues to have intermittent auditory hallucinations, they are mild and manageable. Mr. A lives with his mother, who reports he occasionally talks to himself but when he does not take clozapine, the auditory hallucinations worsen and cause him to become paranoid and aggressive. His ANC is monitored monthly and had been normal for several years until he was diagnosed with B-ALL.
[polldaddy:11125941]
The authors’ observations
The decision to continue clozapine during chemotherapy is challenging and should weigh the risk of agranulocytosis against that of psychiatric destabilization. Because clozapine and chemotherapy are both associated with agranulocytosis, there is concern that concurrent treatment could increase this risk in an additive or synergistic manner. To the best of our knowledge, there are currently no controlled studies investigating the interactions between clozapine and chemotherapeutic agents. Evidence on the hematopoietic consequences of concurrent clozapine and chemotherapy treatment has been limited to case reports because the topic does not lend itself well to randomized controlled trials.
A recent systematic review found no adverse outcomes among the 27 published cases in which clozapine was continued during myelosuppressive chemotherapy.1 The most notable finding was an association between clozapine discontinuation and psychiatric decompensation, which was reported in 12 of 13 cases in which clozapine was prophylactically discontinued to minimize the risk of agranulocytosis.
Patient-specific factors must also be considered, such as the likelihood that psychotic symptoms will recur or worsen if clozapine is discontinued, as well as the extent to which symptom recurrence would interfere with cancer treatment. Clinicians should evaluate the feasibility of switching to another antipsychotic by obtaining a thorough history of the patient’s previous antipsychotics, doses, treatment duration, and response. However, many patients are treated with clozapine because their psychotic symptoms did not improve with other treatments. The character and severity of the patient’s psychotic symptoms when untreated or prior to clozapine treatment can provide a clearer understanding of how a recurrence of symptoms may interfere with cancer treatment. To formulate an accurate assessment of risks and benefits, it is necessary to consider both available evidence and patient-specific factors. The significant agitation and paranoia that Mr. A experienced when not taking clozapine was likely to disrupt chemotherapy. Thus, the adverse consequences of discontinuing clozapine were both severe and likely.
TREATMENT Continuing clozapine
After an extensive discussion of risks, benefits, and alternative treatments with the hematology and psychiatry teams, Mr. A and his family decide to continue clozapine with increased ANC monitoring during chemotherapy. Concurrent treatment was pursued with close collaboration among the patient, the patient’s family, and the hematology and pharmacy teams, and in careful consideration of the clozapine risk evaluation and mitigation strategy. Mr. A’s ANC was monitored daily during chemotherapy treatments and weekly in the intervals between treatments.
As expected, chemotherapy resulted in bone marrow suppression and pancytopenia. Mr. A’s ANC steadily decreased during the next 10 days until it reached 0 µL. This was consistent with the predicted ANC nadir between Day 10 and Day 14, after which recovery was expected. However, Mr. A’s ANC remained at 0 µL on Day 15.
[polldaddy:11125947]
Continue to: The authors' observations
The authors’ observations
Temporary decreases in ANC are expected during chemotherapy, and the timing of onset and recovery is often well characterized. Prior to Day 15, the observed progressive marrow suppression was solely due to chemotherapy. However, because Mr. A’s ANC remained 0 µL longer than anticipated, reevaluation of clozapine’s effects was warranted.
Timing, clinical course, and comprehensive hematologic monitoring can provide important clues as to whether clozapine may be responsible for prolonged neutropenia. Though a prolonged ANC of 0 µL raised concern for clozapine-induced agranulocytosis (CIAG), comprehensive monitoring of hematologic cell lines was reassuring because CIAG selectively targets granulocytic cells (neutrophils).2 In contrast, chemotherapy can affect other cell lineages, including lymphocytes, red blood cells, and platelets, which causes pancytopenia.3 For Mr. A, though the clinical presentation of pancytopenia was significant and concerning, it was inconsistent with CIAG.
Additionally, the patient’s baseline risk of CIAG should be considered. After 18 weeks of clozapine treatment, the risk of CIAG decreases to a level similar to that associated with other antipsychotics.4,5 Therefore, CIAG would be unlikely in a patient treated with clozapine for more than 1 year and who did not have a history of neutropenia, as was the case with Mr. A.
While bone marrow biopsy can help differentiate between the causes of agranulocytosis,6 it is highly invasive and may not be necessary if laboratory evidence is sufficient. However, if a treatment team is strongly considering discontinuing clozapine and there are no suitable alternatives, a biopsy may provide additional clarification.
TREATMENT CAR T-cell therapy and cancer remission
Clozapine is continued with daily monitoring. On Day 19, Mr. A’s ANC increases, reaching 2,600 µL by discharge on Day 40. Mr. A remains psychiatrically stable throughout his hospitalization and does not experience any complications associated with neutropenia, despite its prolonged duration.
Continue to: Unfortunately, multiple cycles of...
Unfortunately, multiple cycles of chemotherapy fail to induce remission. Mr. A is referred for CD19/CD22 chimeric antigen receptor (CAR) T-cell therapy, which helps achieve remission. Allogeneic hematopoietic stem cell transplant (HSCT) is recommended to maximize the likelihood of sustained remission.7 As with chemotherapy, Mr. A and his family agree with the multidisciplinary treatment recommendation to continue clozapine during both CAR T-cell therapy and HSCT, because the risks associated with psychiatric decompensation were greater than a potential increased risk of agranulocytosis. Clozapine treatment is continued throughout both therapies without issue.
Four months after HSCT, Mr. A is admitted for neutropenic fever and left face cellulitis. Upon admission, his ANC is 30 µL and subsequently decreases to 0 µL. In addition to neutropenia, Mr. A is also anemic and thrombocytopenic. He undergoes a bone marrow biopsy.
[polldaddy:11125950]
The authors’ observations
While no published cases have examined the bone marrow of patients experiencing CIAG, 2 retrospective studies have characterized 2 classes of bone marrow findings associated with drug-induced agranulocytosis resulting from nonchemotherapeutic agents (Table).8,9 Type I marrow appears hypercellular with adequate neutrophil precursors but an arrested neutrophil maturation, with few or no mature forms of neutrophils beyond myelocytes.8,9 Type II demonstrates a severe reduction or complete absence of granulocytic precursors with normal or increased erythropoiesis and megakaryocytes.8,9 These findings have been used to accurately differentiate between chemotherapy and nonchemotherapy drug-induced agranulocytosis,6 resulting in successful identification and discontinuation of the responsible agent.
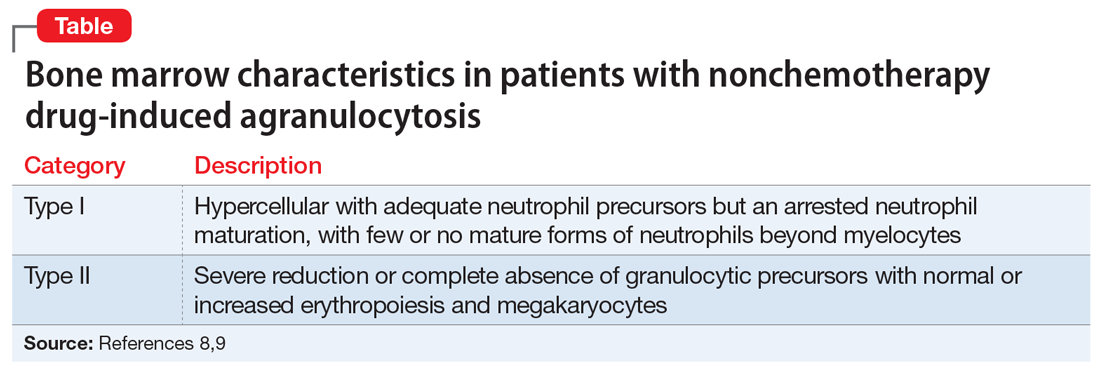
Mr. A’s bone marrow biopsy showed severe pancytopenia with profound neutropenia and normocytic anemia, without evidence of residual leukemia, inconsistent with Type I or Type II. Findings were suggestive of a myelodysplastic syndrome, consistent with secondary graft failure. Symptoms resolved after treatment with antibiotics, granulocyte colony-stimulating factor, epoetin alfa, and thrombopoietin. Mr. A’s ANC remained 0 µL for 22 days before returning to normal (>1,500 µL) by Day 29. He had no secondary complications resulting from neutropenia. As the clinical evidence suggested, Mr. A’s neutropenia was unlikely to be due to clozapine. Clozapine was continued throughout his cancer treatment, and he remained psychiatrically stable.
Clozapine, cancer treatments, and agranulocytosis
This case demonstrates that clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even with the development of agranulocytosis and prolonged neutropenia. Evidence to guide psychiatric clinicians to evaluate the likelihood that agranulocytosis is clozapine-induced is limited.
Continue to: We offer an algorithm...
We offer an algorithm to assist clinicians faced with this challenging clinical dilemma (Figure). Based on our experience and limited current evidence, we recommend continuing clozapine during cancer treatment unless there is clear evidence to suggest otherwise. Presently, no evidence in published literature suggests worsened outcomes in patients treated concurrently with clozapine and cancer therapies.
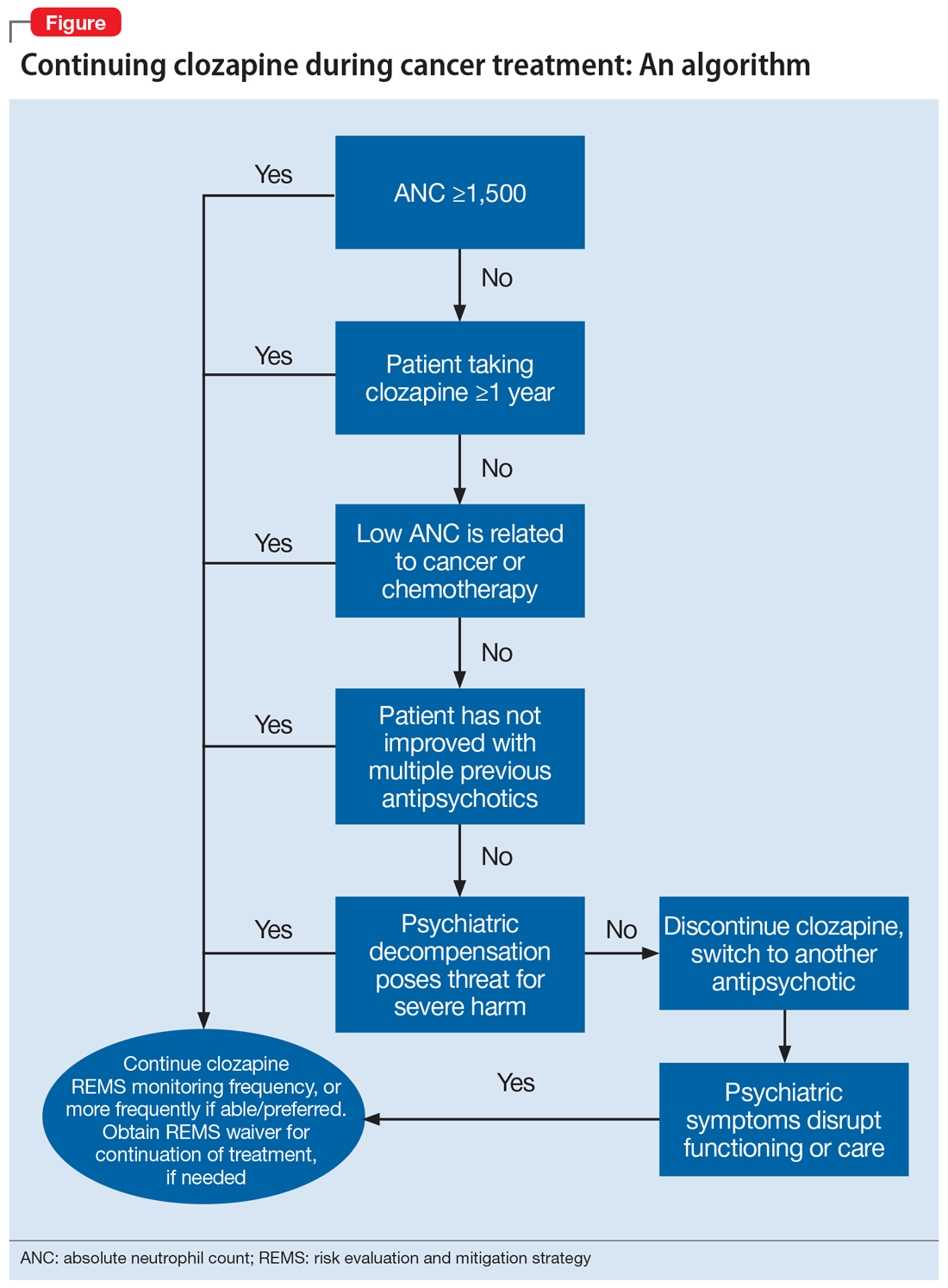
OUTCOME Cancer-free and psychiatrically stable
Mr. A continues clozapine therapy throughout all phases of treatment, without interruption. No adverse effects are determined to be secondary to clozapine. He remains psychiatrically stable throughout treatment, and able to participate and engage in his oncologic therapy. Mr. A is now more than 1 year in remission with no recurrence of graft failure, and his psychiatric symptoms continue to be well controlled with clozapine.
Bottom Line
Clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even in patients who develop agranulocytosis and prolonged neutropenia. Based on our experience and limited evidence, we offer an algorithm to assist clinicians faced with this challenging clinical dilemma.
Related Resources
- Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286. doi:10.1111/ejh.13285
- Daniel JS, Gross T. Managing clozapine-induced neutropenia and agranulocytosis. Current Psychiatry. 2016;15(12):51-53.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Divalproex sodium • Depakote
Epoetin alfa • Epogen
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
1. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286.
2. Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014:27(5):447-452.
3. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606-3618.
4. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
5. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483-488.
6. Azadeh N, Kelemen K, Fonseca R. Amitriptyline-induced agranulocytosis with bone marrow confirmation. Clin Lymphoma Myeloma Leuk. 2014;14(5):e183-e185.
7. Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9(13):1115-1125.
8. Apinantriyo B, Lekhakula A, Rujirojindakul P. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011;16(1):50-53.
9. Yang J, Zhong J, Xiao XH, et al. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr J. 2013;60(2):185-189.
CASE Schizophrenia, leukemia, and chemotherapy
Mr. A, age 30, has schizophrenia but has been stable on clozapine 600 mg/d. He presents to the emergency department with generalized pain that started in his right scapula, arm, elbow, and back. Laboratory tests and a diagnostic examination reveal severe leukocytosis, thrombocytopenia, and anemia, and clinicians diagnose Mr. A with B-cell acute lymphocytic leukemia (B-ALL). Upon admission, Mr. A is neutropenic with an absolute neutrophil count (ANC) of 1,420 µL (reference range 2,500 to 6,000 µL). The hematology team recommends chemotherapy. The treating clinicians also consult the psychiatry team for recommendations on how to best manage Mr. A’s schizophrenia during chemotherapy, including whether clozapine should be discontinued.
HISTORY Stable on clozapine for >10 years
Mr. A was diagnosed with schizophrenia at age 15 after developing paranoia and auditory hallucinations of people talking to him and to each other. He had been hospitalized multiple times for worsened auditory hallucinations and paranoia that led to significant agitation and violence. Previous treatment with multiple antipsychotics, including haloperidol, quetiapine, aripiprazole, olanzapine, risperidone, and ziprasidone, was not successful. Mr. A began clozapine >10 years ago, and his symptoms have been stable since, without any further psychiatric hospitalizations. Mr. A takes clozapine 600 mg/d and divalproex sodium 1,500 mg/d, which he tolerates well and without significant adverse effects. Though he continues to have intermittent auditory hallucinations, they are mild and manageable. Mr. A lives with his mother, who reports he occasionally talks to himself but when he does not take clozapine, the auditory hallucinations worsen and cause him to become paranoid and aggressive. His ANC is monitored monthly and had been normal for several years until he was diagnosed with B-ALL.
[polldaddy:11125941]
The authors’ observations
The decision to continue clozapine during chemotherapy is challenging and should weigh the risk of agranulocytosis against that of psychiatric destabilization. Because clozapine and chemotherapy are both associated with agranulocytosis, there is concern that concurrent treatment could increase this risk in an additive or synergistic manner. To the best of our knowledge, there are currently no controlled studies investigating the interactions between clozapine and chemotherapeutic agents. Evidence on the hematopoietic consequences of concurrent clozapine and chemotherapy treatment has been limited to case reports because the topic does not lend itself well to randomized controlled trials.
A recent systematic review found no adverse outcomes among the 27 published cases in which clozapine was continued during myelosuppressive chemotherapy.1 The most notable finding was an association between clozapine discontinuation and psychiatric decompensation, which was reported in 12 of 13 cases in which clozapine was prophylactically discontinued to minimize the risk of agranulocytosis.
Patient-specific factors must also be considered, such as the likelihood that psychotic symptoms will recur or worsen if clozapine is discontinued, as well as the extent to which symptom recurrence would interfere with cancer treatment. Clinicians should evaluate the feasibility of switching to another antipsychotic by obtaining a thorough history of the patient’s previous antipsychotics, doses, treatment duration, and response. However, many patients are treated with clozapine because their psychotic symptoms did not improve with other treatments. The character and severity of the patient’s psychotic symptoms when untreated or prior to clozapine treatment can provide a clearer understanding of how a recurrence of symptoms may interfere with cancer treatment. To formulate an accurate assessment of risks and benefits, it is necessary to consider both available evidence and patient-specific factors. The significant agitation and paranoia that Mr. A experienced when not taking clozapine was likely to disrupt chemotherapy. Thus, the adverse consequences of discontinuing clozapine were both severe and likely.
TREATMENT Continuing clozapine
After an extensive discussion of risks, benefits, and alternative treatments with the hematology and psychiatry teams, Mr. A and his family decide to continue clozapine with increased ANC monitoring during chemotherapy. Concurrent treatment was pursued with close collaboration among the patient, the patient’s family, and the hematology and pharmacy teams, and in careful consideration of the clozapine risk evaluation and mitigation strategy. Mr. A’s ANC was monitored daily during chemotherapy treatments and weekly in the intervals between treatments.
As expected, chemotherapy resulted in bone marrow suppression and pancytopenia. Mr. A’s ANC steadily decreased during the next 10 days until it reached 0 µL. This was consistent with the predicted ANC nadir between Day 10 and Day 14, after which recovery was expected. However, Mr. A’s ANC remained at 0 µL on Day 15.
[polldaddy:11125947]
Continue to: The authors' observations
The authors’ observations
Temporary decreases in ANC are expected during chemotherapy, and the timing of onset and recovery is often well characterized. Prior to Day 15, the observed progressive marrow suppression was solely due to chemotherapy. However, because Mr. A’s ANC remained 0 µL longer than anticipated, reevaluation of clozapine’s effects was warranted.
Timing, clinical course, and comprehensive hematologic monitoring can provide important clues as to whether clozapine may be responsible for prolonged neutropenia. Though a prolonged ANC of 0 µL raised concern for clozapine-induced agranulocytosis (CIAG), comprehensive monitoring of hematologic cell lines was reassuring because CIAG selectively targets granulocytic cells (neutrophils).2 In contrast, chemotherapy can affect other cell lineages, including lymphocytes, red blood cells, and platelets, which causes pancytopenia.3 For Mr. A, though the clinical presentation of pancytopenia was significant and concerning, it was inconsistent with CIAG.
Additionally, the patient’s baseline risk of CIAG should be considered. After 18 weeks of clozapine treatment, the risk of CIAG decreases to a level similar to that associated with other antipsychotics.4,5 Therefore, CIAG would be unlikely in a patient treated with clozapine for more than 1 year and who did not have a history of neutropenia, as was the case with Mr. A.
While bone marrow biopsy can help differentiate between the causes of agranulocytosis,6 it is highly invasive and may not be necessary if laboratory evidence is sufficient. However, if a treatment team is strongly considering discontinuing clozapine and there are no suitable alternatives, a biopsy may provide additional clarification.
TREATMENT CAR T-cell therapy and cancer remission
Clozapine is continued with daily monitoring. On Day 19, Mr. A’s ANC increases, reaching 2,600 µL by discharge on Day 40. Mr. A remains psychiatrically stable throughout his hospitalization and does not experience any complications associated with neutropenia, despite its prolonged duration.
Continue to: Unfortunately, multiple cycles of...
Unfortunately, multiple cycles of chemotherapy fail to induce remission. Mr. A is referred for CD19/CD22 chimeric antigen receptor (CAR) T-cell therapy, which helps achieve remission. Allogeneic hematopoietic stem cell transplant (HSCT) is recommended to maximize the likelihood of sustained remission.7 As with chemotherapy, Mr. A and his family agree with the multidisciplinary treatment recommendation to continue clozapine during both CAR T-cell therapy and HSCT, because the risks associated with psychiatric decompensation were greater than a potential increased risk of agranulocytosis. Clozapine treatment is continued throughout both therapies without issue.
Four months after HSCT, Mr. A is admitted for neutropenic fever and left face cellulitis. Upon admission, his ANC is 30 µL and subsequently decreases to 0 µL. In addition to neutropenia, Mr. A is also anemic and thrombocytopenic. He undergoes a bone marrow biopsy.
[polldaddy:11125950]
The authors’ observations
While no published cases have examined the bone marrow of patients experiencing CIAG, 2 retrospective studies have characterized 2 classes of bone marrow findings associated with drug-induced agranulocytosis resulting from nonchemotherapeutic agents (Table).8,9 Type I marrow appears hypercellular with adequate neutrophil precursors but an arrested neutrophil maturation, with few or no mature forms of neutrophils beyond myelocytes.8,9 Type II demonstrates a severe reduction or complete absence of granulocytic precursors with normal or increased erythropoiesis and megakaryocytes.8,9 These findings have been used to accurately differentiate between chemotherapy and nonchemotherapy drug-induced agranulocytosis,6 resulting in successful identification and discontinuation of the responsible agent.

Mr. A’s bone marrow biopsy showed severe pancytopenia with profound neutropenia and normocytic anemia, without evidence of residual leukemia, inconsistent with Type I or Type II. Findings were suggestive of a myelodysplastic syndrome, consistent with secondary graft failure. Symptoms resolved after treatment with antibiotics, granulocyte colony-stimulating factor, epoetin alfa, and thrombopoietin. Mr. A’s ANC remained 0 µL for 22 days before returning to normal (>1,500 µL) by Day 29. He had no secondary complications resulting from neutropenia. As the clinical evidence suggested, Mr. A’s neutropenia was unlikely to be due to clozapine. Clozapine was continued throughout his cancer treatment, and he remained psychiatrically stable.
Clozapine, cancer treatments, and agranulocytosis
This case demonstrates that clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even with the development of agranulocytosis and prolonged neutropenia. Evidence to guide psychiatric clinicians to evaluate the likelihood that agranulocytosis is clozapine-induced is limited.
Continue to: We offer an algorithm...
We offer an algorithm to assist clinicians faced with this challenging clinical dilemma (Figure). Based on our experience and limited current evidence, we recommend continuing clozapine during cancer treatment unless there is clear evidence to suggest otherwise. Presently, no evidence in published literature suggests worsened outcomes in patients treated concurrently with clozapine and cancer therapies.

OUTCOME Cancer-free and psychiatrically stable
Mr. A continues clozapine therapy throughout all phases of treatment, without interruption. No adverse effects are determined to be secondary to clozapine. He remains psychiatrically stable throughout treatment, and able to participate and engage in his oncologic therapy. Mr. A is now more than 1 year in remission with no recurrence of graft failure, and his psychiatric symptoms continue to be well controlled with clozapine.
Bottom Line
Clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even in patients who develop agranulocytosis and prolonged neutropenia. Based on our experience and limited evidence, we offer an algorithm to assist clinicians faced with this challenging clinical dilemma.
Related Resources
- Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286. doi:10.1111/ejh.13285
- Daniel JS, Gross T. Managing clozapine-induced neutropenia and agranulocytosis. Current Psychiatry. 2016;15(12):51-53.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Divalproex sodium • Depakote
Epoetin alfa • Epogen
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
CASE Schizophrenia, leukemia, and chemotherapy
Mr. A, age 30, has schizophrenia but has been stable on clozapine 600 mg/d. He presents to the emergency department with generalized pain that started in his right scapula, arm, elbow, and back. Laboratory tests and a diagnostic examination reveal severe leukocytosis, thrombocytopenia, and anemia, and clinicians diagnose Mr. A with B-cell acute lymphocytic leukemia (B-ALL). Upon admission, Mr. A is neutropenic with an absolute neutrophil count (ANC) of 1,420 µL (reference range 2,500 to 6,000 µL). The hematology team recommends chemotherapy. The treating clinicians also consult the psychiatry team for recommendations on how to best manage Mr. A’s schizophrenia during chemotherapy, including whether clozapine should be discontinued.
HISTORY Stable on clozapine for >10 years
Mr. A was diagnosed with schizophrenia at age 15 after developing paranoia and auditory hallucinations of people talking to him and to each other. He had been hospitalized multiple times for worsened auditory hallucinations and paranoia that led to significant agitation and violence. Previous treatment with multiple antipsychotics, including haloperidol, quetiapine, aripiprazole, olanzapine, risperidone, and ziprasidone, was not successful. Mr. A began clozapine >10 years ago, and his symptoms have been stable since, without any further psychiatric hospitalizations. Mr. A takes clozapine 600 mg/d and divalproex sodium 1,500 mg/d, which he tolerates well and without significant adverse effects. Though he continues to have intermittent auditory hallucinations, they are mild and manageable. Mr. A lives with his mother, who reports he occasionally talks to himself but when he does not take clozapine, the auditory hallucinations worsen and cause him to become paranoid and aggressive. His ANC is monitored monthly and had been normal for several years until he was diagnosed with B-ALL.
[polldaddy:11125941]
The authors’ observations
The decision to continue clozapine during chemotherapy is challenging and should weigh the risk of agranulocytosis against that of psychiatric destabilization. Because clozapine and chemotherapy are both associated with agranulocytosis, there is concern that concurrent treatment could increase this risk in an additive or synergistic manner. To the best of our knowledge, there are currently no controlled studies investigating the interactions between clozapine and chemotherapeutic agents. Evidence on the hematopoietic consequences of concurrent clozapine and chemotherapy treatment has been limited to case reports because the topic does not lend itself well to randomized controlled trials.
A recent systematic review found no adverse outcomes among the 27 published cases in which clozapine was continued during myelosuppressive chemotherapy.1 The most notable finding was an association between clozapine discontinuation and psychiatric decompensation, which was reported in 12 of 13 cases in which clozapine was prophylactically discontinued to minimize the risk of agranulocytosis.
Patient-specific factors must also be considered, such as the likelihood that psychotic symptoms will recur or worsen if clozapine is discontinued, as well as the extent to which symptom recurrence would interfere with cancer treatment. Clinicians should evaluate the feasibility of switching to another antipsychotic by obtaining a thorough history of the patient’s previous antipsychotics, doses, treatment duration, and response. However, many patients are treated with clozapine because their psychotic symptoms did not improve with other treatments. The character and severity of the patient’s psychotic symptoms when untreated or prior to clozapine treatment can provide a clearer understanding of how a recurrence of symptoms may interfere with cancer treatment. To formulate an accurate assessment of risks and benefits, it is necessary to consider both available evidence and patient-specific factors. The significant agitation and paranoia that Mr. A experienced when not taking clozapine was likely to disrupt chemotherapy. Thus, the adverse consequences of discontinuing clozapine were both severe and likely.
TREATMENT Continuing clozapine
After an extensive discussion of risks, benefits, and alternative treatments with the hematology and psychiatry teams, Mr. A and his family decide to continue clozapine with increased ANC monitoring during chemotherapy. Concurrent treatment was pursued with close collaboration among the patient, the patient’s family, and the hematology and pharmacy teams, and in careful consideration of the clozapine risk evaluation and mitigation strategy. Mr. A’s ANC was monitored daily during chemotherapy treatments and weekly in the intervals between treatments.
As expected, chemotherapy resulted in bone marrow suppression and pancytopenia. Mr. A’s ANC steadily decreased during the next 10 days until it reached 0 µL. This was consistent with the predicted ANC nadir between Day 10 and Day 14, after which recovery was expected. However, Mr. A’s ANC remained at 0 µL on Day 15.
[polldaddy:11125947]
Continue to: The authors' observations
The authors’ observations
Temporary decreases in ANC are expected during chemotherapy, and the timing of onset and recovery is often well characterized. Prior to Day 15, the observed progressive marrow suppression was solely due to chemotherapy. However, because Mr. A’s ANC remained 0 µL longer than anticipated, reevaluation of clozapine’s effects was warranted.
Timing, clinical course, and comprehensive hematologic monitoring can provide important clues as to whether clozapine may be responsible for prolonged neutropenia. Though a prolonged ANC of 0 µL raised concern for clozapine-induced agranulocytosis (CIAG), comprehensive monitoring of hematologic cell lines was reassuring because CIAG selectively targets granulocytic cells (neutrophils).2 In contrast, chemotherapy can affect other cell lineages, including lymphocytes, red blood cells, and platelets, which causes pancytopenia.3 For Mr. A, though the clinical presentation of pancytopenia was significant and concerning, it was inconsistent with CIAG.
Additionally, the patient’s baseline risk of CIAG should be considered. After 18 weeks of clozapine treatment, the risk of CIAG decreases to a level similar to that associated with other antipsychotics.4,5 Therefore, CIAG would be unlikely in a patient treated with clozapine for more than 1 year and who did not have a history of neutropenia, as was the case with Mr. A.
While bone marrow biopsy can help differentiate between the causes of agranulocytosis,6 it is highly invasive and may not be necessary if laboratory evidence is sufficient. However, if a treatment team is strongly considering discontinuing clozapine and there are no suitable alternatives, a biopsy may provide additional clarification.
TREATMENT CAR T-cell therapy and cancer remission
Clozapine is continued with daily monitoring. On Day 19, Mr. A’s ANC increases, reaching 2,600 µL by discharge on Day 40. Mr. A remains psychiatrically stable throughout his hospitalization and does not experience any complications associated with neutropenia, despite its prolonged duration.
Continue to: Unfortunately, multiple cycles of...
Unfortunately, multiple cycles of chemotherapy fail to induce remission. Mr. A is referred for CD19/CD22 chimeric antigen receptor (CAR) T-cell therapy, which helps achieve remission. Allogeneic hematopoietic stem cell transplant (HSCT) is recommended to maximize the likelihood of sustained remission.7 As with chemotherapy, Mr. A and his family agree with the multidisciplinary treatment recommendation to continue clozapine during both CAR T-cell therapy and HSCT, because the risks associated with psychiatric decompensation were greater than a potential increased risk of agranulocytosis. Clozapine treatment is continued throughout both therapies without issue.
Four months after HSCT, Mr. A is admitted for neutropenic fever and left face cellulitis. Upon admission, his ANC is 30 µL and subsequently decreases to 0 µL. In addition to neutropenia, Mr. A is also anemic and thrombocytopenic. He undergoes a bone marrow biopsy.
[polldaddy:11125950]
The authors’ observations
While no published cases have examined the bone marrow of patients experiencing CIAG, 2 retrospective studies have characterized 2 classes of bone marrow findings associated with drug-induced agranulocytosis resulting from nonchemotherapeutic agents (Table).8,9 Type I marrow appears hypercellular with adequate neutrophil precursors but an arrested neutrophil maturation, with few or no mature forms of neutrophils beyond myelocytes.8,9 Type II demonstrates a severe reduction or complete absence of granulocytic precursors with normal or increased erythropoiesis and megakaryocytes.8,9 These findings have been used to accurately differentiate between chemotherapy and nonchemotherapy drug-induced agranulocytosis,6 resulting in successful identification and discontinuation of the responsible agent.

Mr. A’s bone marrow biopsy showed severe pancytopenia with profound neutropenia and normocytic anemia, without evidence of residual leukemia, inconsistent with Type I or Type II. Findings were suggestive of a myelodysplastic syndrome, consistent with secondary graft failure. Symptoms resolved after treatment with antibiotics, granulocyte colony-stimulating factor, epoetin alfa, and thrombopoietin. Mr. A’s ANC remained 0 µL for 22 days before returning to normal (>1,500 µL) by Day 29. He had no secondary complications resulting from neutropenia. As the clinical evidence suggested, Mr. A’s neutropenia was unlikely to be due to clozapine. Clozapine was continued throughout his cancer treatment, and he remained psychiatrically stable.
Clozapine, cancer treatments, and agranulocytosis
This case demonstrates that clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even with the development of agranulocytosis and prolonged neutropenia. Evidence to guide psychiatric clinicians to evaluate the likelihood that agranulocytosis is clozapine-induced is limited.
Continue to: We offer an algorithm...
We offer an algorithm to assist clinicians faced with this challenging clinical dilemma (Figure). Based on our experience and limited current evidence, we recommend continuing clozapine during cancer treatment unless there is clear evidence to suggest otherwise. Presently, no evidence in published literature suggests worsened outcomes in patients treated concurrently with clozapine and cancer therapies.

OUTCOME Cancer-free and psychiatrically stable
Mr. A continues clozapine therapy throughout all phases of treatment, without interruption. No adverse effects are determined to be secondary to clozapine. He remains psychiatrically stable throughout treatment, and able to participate and engage in his oncologic therapy. Mr. A is now more than 1 year in remission with no recurrence of graft failure, and his psychiatric symptoms continue to be well controlled with clozapine.
Bottom Line
Clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even in patients who develop agranulocytosis and prolonged neutropenia. Based on our experience and limited evidence, we offer an algorithm to assist clinicians faced with this challenging clinical dilemma.
Related Resources
- Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286. doi:10.1111/ejh.13285
- Daniel JS, Gross T. Managing clozapine-induced neutropenia and agranulocytosis. Current Psychiatry. 2016;15(12):51-53.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Divalproex sodium • Depakote
Epoetin alfa • Epogen
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
1. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286.
2. Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014:27(5):447-452.
3. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606-3618.
4. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
5. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483-488.
6. Azadeh N, Kelemen K, Fonseca R. Amitriptyline-induced agranulocytosis with bone marrow confirmation. Clin Lymphoma Myeloma Leuk. 2014;14(5):e183-e185.
7. Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9(13):1115-1125.
8. Apinantriyo B, Lekhakula A, Rujirojindakul P. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011;16(1):50-53.
9. Yang J, Zhong J, Xiao XH, et al. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr J. 2013;60(2):185-189.
1. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286.
2. Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014:27(5):447-452.
3. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606-3618.
4. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
5. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483-488.
6. Azadeh N, Kelemen K, Fonseca R. Amitriptyline-induced agranulocytosis with bone marrow confirmation. Clin Lymphoma Myeloma Leuk. 2014;14(5):e183-e185.
7. Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9(13):1115-1125.
8. Apinantriyo B, Lekhakula A, Rujirojindakul P. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011;16(1):50-53.
9. Yang J, Zhong J, Xiao XH, et al. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr J. 2013;60(2):185-189.
The woman who kept passing out
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
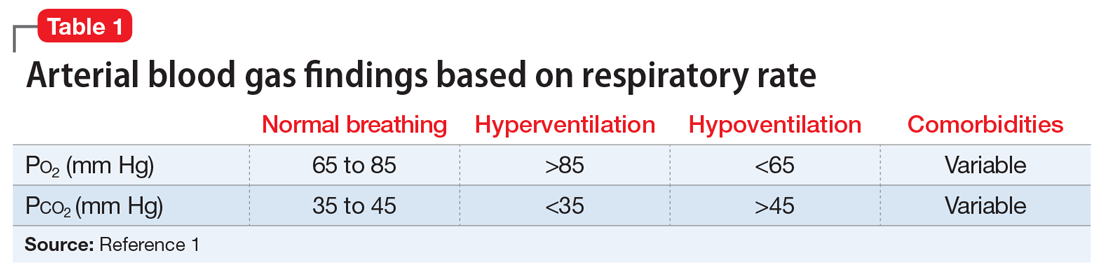
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
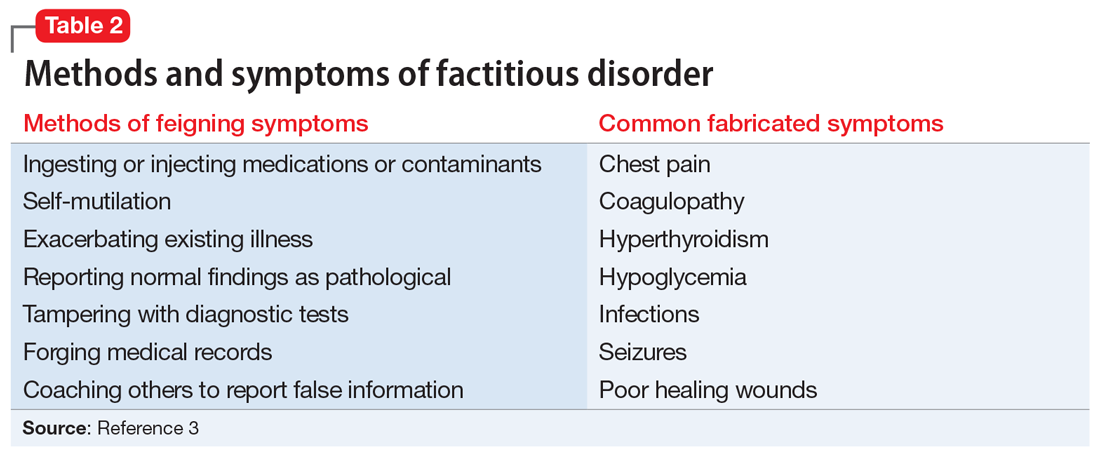
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
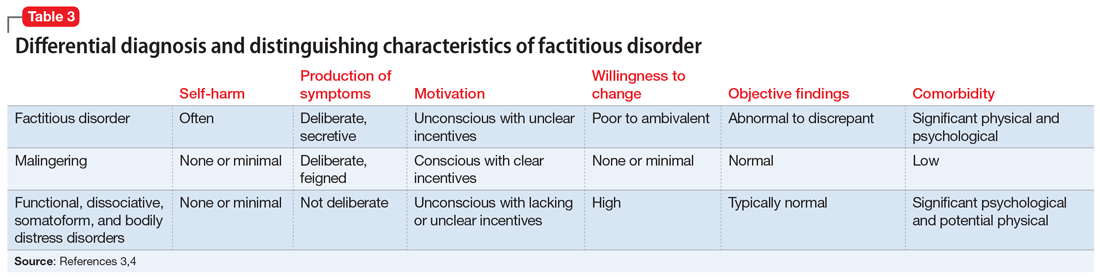
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.

HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).

Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3

Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).

Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.

HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).

Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3

Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).

Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.