User login
A 10-year-old boy with ‘voices in my head’: Is it a psychotic disorder?
CASE Auditory hallucinations?
M, age 10, has had multiple visits to the pediatric emergency department (PED) with the chief concern of excessive urinary frequency. At each visit, the medical workup has been negative and he was discharged home. After a few months, M’s parents bring their son back to the PED because he reports hearing “voices in my head” and “feeling tense and scared.” When these feelings become too overwhelming, M stops eating and experiences substantial fear and anxiety that require his mother’s repeated reassurances. M’s mother reports that 2 weeks before his most recent PED visit, he became increasingly anxious and disturbed, and said he was afraid most of the time, and worried about the safety of his family for no apparent reason.
The psychiatrist evaluates M in the PED and diagnoses him with unspecified schizophrenia spectrum and other psychotic disorder based on his persistent report of auditory and tactile hallucinations, including hearing a voice of a man telling him he was going to choke on his food and feeling someone touch his arm to soothe him during his anxious moments. M does not meet criteria for acute inpatient hospitalization, and is discharged home with referral to follow-up at our child and adolescent psychiatry outpatient clinic.
On subsequent evaluation in our clinic, M reports most of the same about his experience hearing “voices in my head” that repeatedly suggest “I might choke on my food and end up seriously ill in the hospital.” He started to hear the “voices” after he witnessed his sister choke while eating a few days earlier. He also mentions that the “voices” tell him “you have to use the restroom.” As a result, he uses the restroom several times before leaving for home and is frequently late for school. His parents accommodate his behavior—his mother allows him to use the bathroom multiple times, and his father overlooks the behavior as part of school anxiety.
At school, his teacher reports a concern for attention-deficit/hyperactivity disorder (ADHD) based on M’s continuous inattentiveness in class and dropping grades. He asks for bathroom breaks up to 15 times a day, which disrupts his class work.
These behaviors have led to a gradual 1-year decline in his overall functioning, including difficulty at school for requesting too many bathroom breaks; having to repeat the 3rd grade; and incurring multiple hospital visits for evaluation of his various complaints. M has become socially isolated and withdrawn from friends and family.
M’s developmental history is normal and his family history is negative for any psychiatric disorder. Medical history and physical examination are unremarkable. CT scan of his head is unremarkable, and all hematologic and biochemistry laboratory test values are within normal range.
[polldaddy:9971376]
Continue to: The authors' observations
The authors’ observations
Several factors may contribute to an increased chance of misdiagnosis of a psychiatric illness
On closer sequential evaluations with M and his family, we determined that the “voices” he was hearing were actually intrusive thoughts, and not hallucinations. M clarified this by saying that first he feels a “pressure”-like sensation in his head, followed by repeated intrusive thoughts of voiding his bladder that compel him to go to the restroom to try to urinate. He feels temporary relief after complying with the urge, even when he passes only a small amount of urine or just washes his hands. After a brief period of relief, this process repeats itself. Further, he was able to clarify his experience while eating food, where he first felt a “pressure”-like sensation in his head, followed by intrusive thoughts of choking that result in him not eating.
This led us to a more appropriate diagnosis of OCD (Table 11). The incidence of OCD has 2 peaks, with different gender distributions. The first peak occurs in childhood, with symptoms mostly arising between 7 and 12 years of age and affecting boys more often than girls. The second peak occurs in early adulthood, at a mean age of 21 years, with a slight female majority.2 However, OCD is often under recognized and undertreated, perhaps due to its extensive heterogeneity; symptom presentations and comorbidity patterns can vary noticeably between individual patients as well as age groups.
OCD is characterized by the presence of obsessions or compulsions that wax and wane in severity, are time-consuming (at least 1 hour per day), and cause subjective distress or interfere with life of the patient or the family. Adults with OCD recognize at some level that the obsessions and/or compulsions are excessive and unreasonable, although children are not required to have this insight to meet criteria for the diagnosis.1 Rating scales, such as the Children’s Yale-Brown Obsessive-Compulsive Scale, Dimensional Yale-Brown Obsessive-Compulsive Scale, and Family Accommodation Scale, are useful to obtain detailed information regarding OCD symptoms, tics, and other factors relevant to the diagnosis.
Continue to: M's symptomatology...
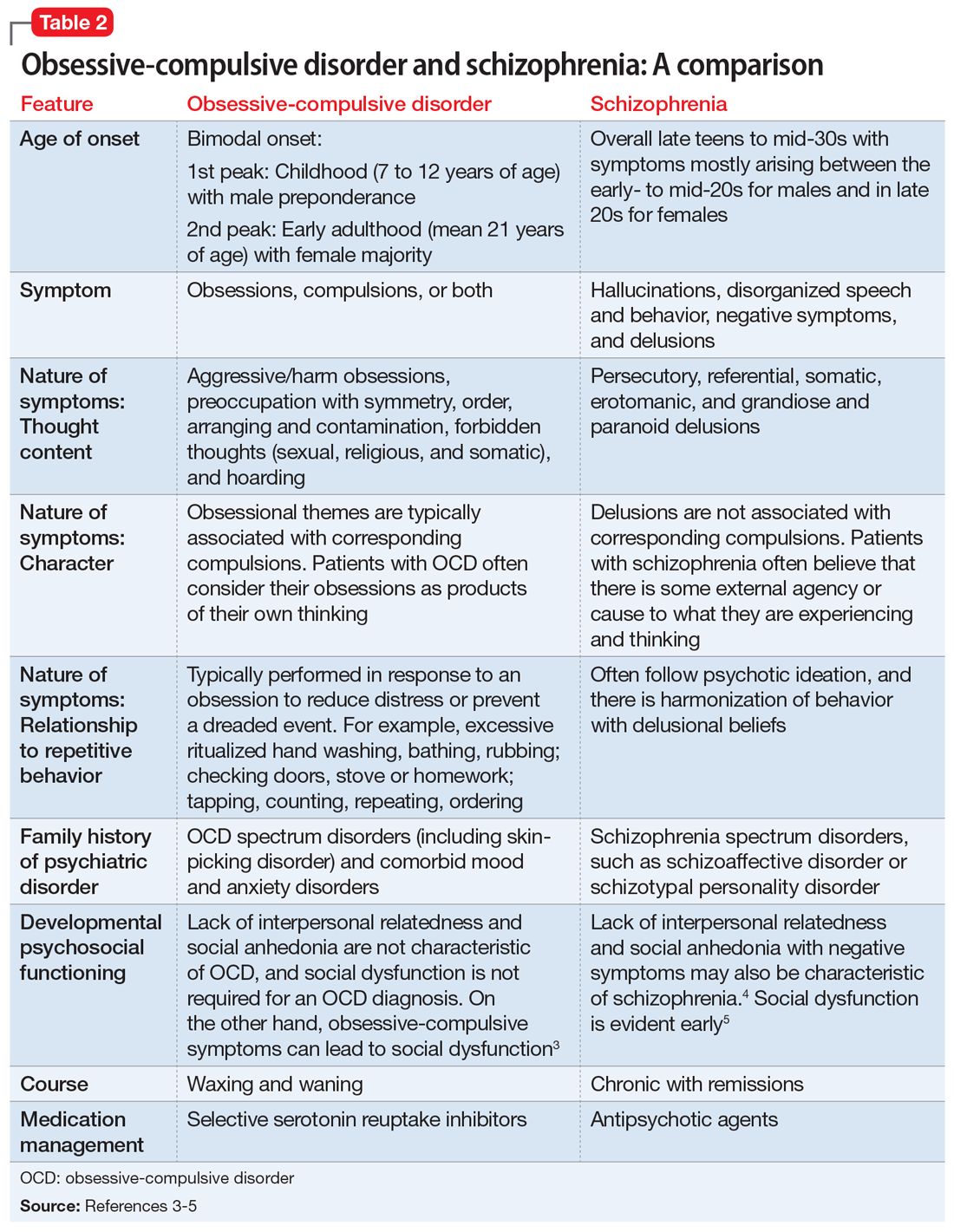
M’s symptomatology did not appear to be psychotic. He was screened for positive or negative symptoms of psychosis, which he and his family clearly denied. Moreover, M’s compulsions (going to the restroom) were typically performed in response to his obsessions (urge to void his bladder) to reduce his distress, which is different from schizophrenia, in which repetitive behaviors are performed in response to psychotic ideation, and not obsessions (Table 23-5).
M’s inattentiveness in the classroom was found to be related to his obsessions and compulsions, and not part of a symptom cluster characterizing ADHD. Teachers often interpret inattention and poor classroom performance as ADHD, but having detailed conversations with teachers often is helpful in understanding the nature of a child’s symptomology and making the appropriate diagnosis.
Establishing the correct clinical diagnosis is critical because it is the starting point in treatment. First-line medication for one condition may exacerbate the symptoms of others. For example, in addition to having a large adverse-effect burden, antipsychotics can induce de novo obsessive–compulsive symptoms (OCS) or exacerbate preexisting OCS, and selective serotonin reuptake inhibitors (SSRIs) may exacerbate psychosis in schizo-obsessive patients with a history of impulsivity and aggressiveness.6 Similarly, stimulant medications for ADHD may exacerbate OCS and may even induce them on their own.7,8
[polldaddy:9971377]
Continue to: The authors' observations
The authors’ observations
Studies have reported an average of 2.5 years from the onset of OCD symptoms to diagnosis in the United States.9 A key reason for this delay, which is more frequently encountered in pediatric patients, is secrecy. Children often feel embarrassed about their symptoms and conceal them until the interference with their functioning becomes extremely disabling. In some cases, symptoms may closely resemble normal childhood routines. In fact, some repetitive behaviors may be normal in some developmental stages, and OCD could be conceptualized as a pathological condition with continuity of normal behaviors during different developmental periods.10
Also, symptoms may go unnoticed for quite some time as unsuspecting and well-intentioned parents and family members become overly involved in the child’s rituals (eg, allowing for increasing frequent prolonged bathroom breaks or frequent change of clothing, etc.). This well-established phenomenon, termed accommodation, is defined as participation of family members in a child’s OCD–related rituals.11 Especially when symptoms are mild or the child is functioning well, accommodation can make it difficult for parents to realize the presence or nature of a problem, as they might tend to minimize their child’s symptoms as representing a unique personality trait or a special “quirk.” Parents generally will seek treatment when their child’s symptoms become more impairing and begin to interfere with social functioning, school performance, or family functioning.
The clinical picture is further complicated by comorbidity. Approximately 60% to 80% of children and adolescents with OCD have ≥1 comorbid psychiatric disorders. Some of the most common include tic disorders, ADHD, anxiety disorders, and mood or eating disorders.9
[polldaddy:9971379]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
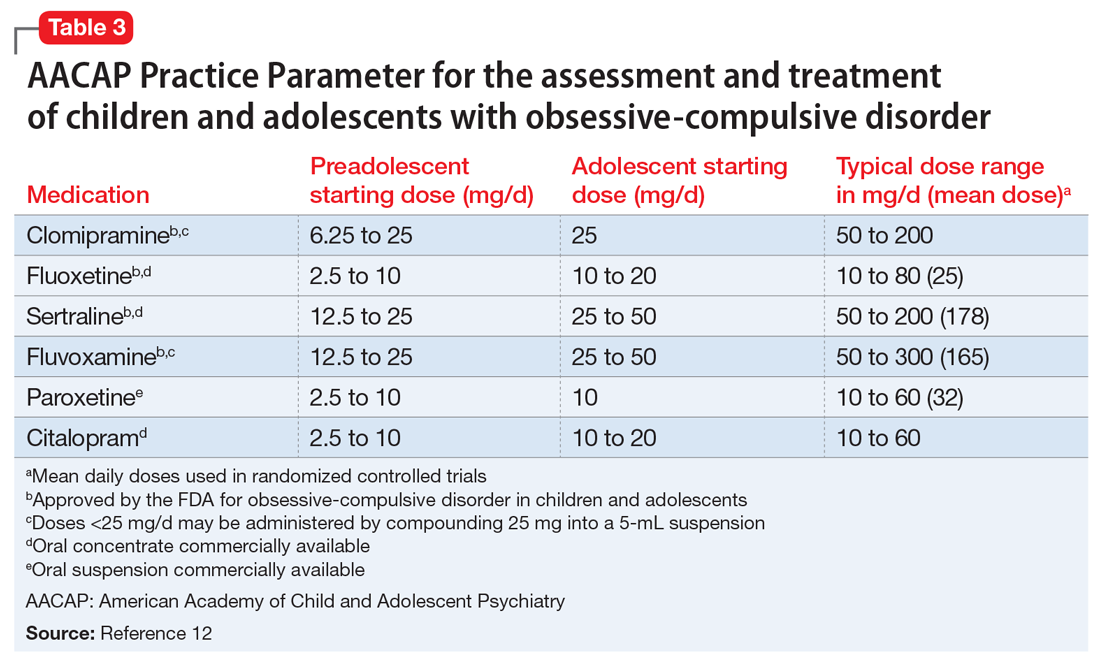
In keeping with American Academy of Child and Adolescent Psychiatry guidelines on treating OCD (Table 312), we start M on fluoxetine 10 mg/d. He also begins CBT. Fluoxetine is slowly titrated to 40 mg/d while M engages in learning and utilizing CBT techniques to manage his OCD.
The authors’ observations
The combination of CBT and medication has been suggested as the treatment of choice for moderate and severe OCD.12 The Pediatric OCD Treatment Study, a 5-year, 3-site outcome study designed to compare placebo, sertraline, CBT, and combined CBT and sertraline, concluded that the combined treatment (CBT plus sertraline) was more effective than CBT alone or sertraline alone.13 The effect sizes for the combined treatment, CBT alone, and sertraline alone were 1.4, 0.97, and 0.67, respectively. Remission rates for SSRIs alone are <33%.13,14
SSRIs are the first-line medication for OCD in children, adolescents, and adults (Table 312). Well-designed clinical trials have demonstrated the efficacy and safety of the SSRIs fluoxetine, sertraline, and fluvoxamine (alone or combined with CBT) in children and adolescents with OCD.13 Other SSRIs, such as citalopram, paroxetine, and escitalopram, also have demonstrated efficacy in children and adolescents with OCD, even though the FDA has not yet approved their use in pediatric patients.12 Despite a positive trial of paroxetine in pediatric OCD,12 there have been concerns related to its higher rates of treatment-emergent suicidality,15 lower likelihood of treatment response,16 and its particularly short half-life in pediatric patients.17
Clomipramine is a tricyclic antidepressant with serotonergic properties that is used alone or to boost the effect of an SSRI when there is a partial response. It should be introduced at a low dose in pediatric patients (before age 12) and closely monitored for anticholinergic and cardiac adverse effects. A systemic review and meta-analysis of early treatment responses of SSRIs and clomipramine in pediatric OCD indicated that the greatest benefits occurred early in treatment.18 Clomipramine was associated with a greater measured benefit compared with placebo than SSRIs; there was no evidence of a relationship between SSRI dosing and treatment effect, although data were limited. Adults and children with OCD demonstrated a similar degree and time course of response to SSRIs in OCD.18
Treatment should start with a low dose to reduce the risk of adverse effects with an adequate trial for 10 to 16 weeks at adequate doses. Most experts suggest that treatment should continue for at least 12 months after symptom resolution or stabilization, followed by a very gradual cessation.19
Continue to: OUTCOME Improvement in functioning
OUTCOME Improvement in functioning
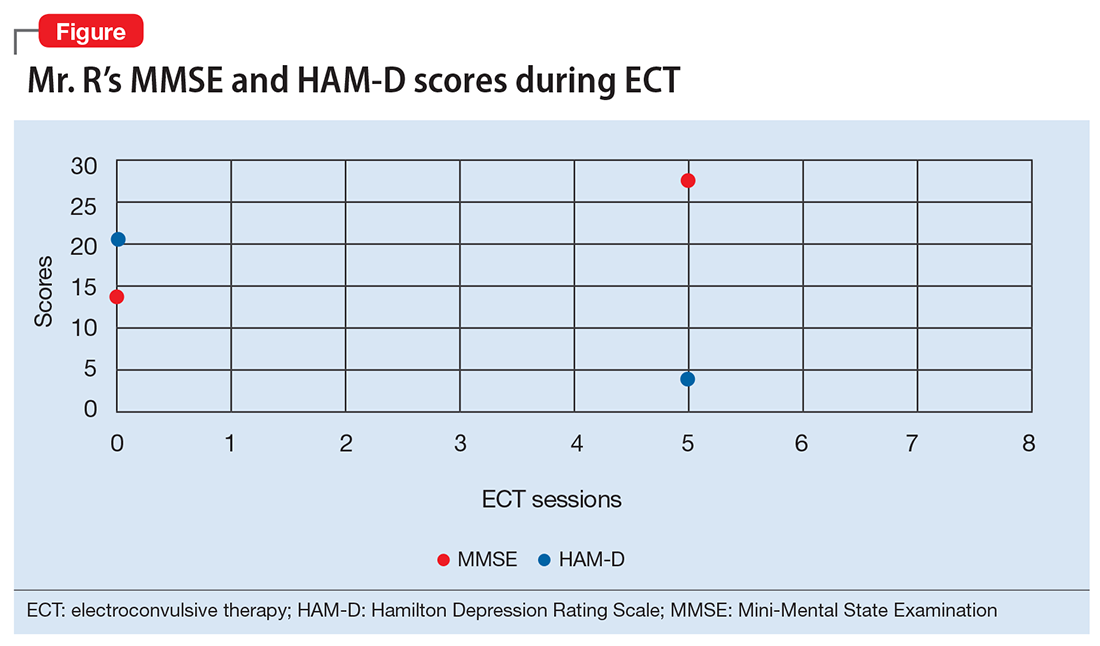
After 12 months of combined CBT and fluoxetine, M’s global assessment of functioning (GAF) scale score improves from 35 to 80, indicating major improvement in overall functional level.
Acknowledgement
The authors thank Uzoma Osuchukwu, MD, ex-fellow, Department of Child and Adolescent Psychiatry, Columbia University College of Physicians and Surgeons, Harlem Hospital Center, New York, New York, for his assistance with this article.
Bottom Line
Obsessive-compulsive disorder may masquerade as a schizophrenia spectrum disorder, particularly in younger patients. Accurate differentiation is crucial because antipsychotics can induce de novo obsessive-compulsive symptoms (OCS) or exacerbate preexisting OCS, and selective serotonin reuptake inhibitors may exacerbate psychosis in schizo-obsessive patients with a history of impulsivity and aggressiveness.
Related Resource
- Raveendranathan D, Shiva L, Sharma E, et al. Obsessive compulsive disorder masquerading as psychosis. Indian J Psychol Med. 2012;34(2):179-180.
Drug Brand Names
Citalopram • Celexa
Clomipramine • Anafranil
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Paroxetine • Paxil
Sertraline • Zoloft
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Geller D, Biederman J, Jones J, et al. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? A review of the pediatric literature. J Am Acad Child Adolesc Psychiatry.1998;37(4):420-427.
3. Huppert JD, Simpson HB, Nissenson KJ, et al. Quality of life and functional impairment in obsessive-compulsive disorder: A comparison of patients with and without comorbidity, patients in remission, and healthy controls. Depress Anxiety. 2009;26(1):39-45.
4. Sobel W, Wolski R, Cancro R, et al. Interpersonal relatedness and paranoid schizophrenia. Am J Psychiatry.1996;153(8):1084-1087.
5. Meares A. The diagnosis of prepsychotic schizophrenia. Lancet. 1959;1(7063):55-58.
6. Poyurovsky M, Weizman A, Weizman R. Obsessive-compulsive disorder in schizophrenia: Clinical characteristics and treatment. CNS Drugs. 2004;18(14):989-1010.
7. Kouris S. Methylphenidate-induced obsessive-compulsiveness. J Am Acad Child Adolesc Psychiatry. 1998;37(2):135.
8. Woolley JB, Heyman I. Dexamphetamine for obsessive-compulsive disorder. Am J Psychiatry. 2003;160(1):183.
9. Geller DA. Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatr Clin N Am. 2006;29(2):352-370.
10. Evans DW, Milanak ME, Medeiros B, et al. Magical beliefs and rituals in young children. Child Psychiatry Hum Dev. 2002;33(1):43-58.
11. Amir N, Freshman M, Foa E. Family distress and involvement in relatives of obsessive-compulsive disorder patients. J Anxiety Disord. 2000;14(3):209-217.
12. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):98-113.
13. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: The Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
14. Franklin ME, Sapyta J, Freeman JB, et al. Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: The Pediatric OCD Treatment Study II (POTS II) randomized controlled trial. JAMA. 2011;306(11):1224-1232.
15. Wagner KD, Asarnow JR, Vitiello B, et al. Out of the black box: treatment of resistant depression in adolescents and the antidepressant controversy. J Child Adolesc Psychopharmacol. 2012;22(1):5-10.
16. Sakolsky DJ, Perel JM, Emslie GJ, et al. Antidepressant exposure as a predictor of clinical outcomes in the treatment of resistant depression in adolescents (TORDIA) study. J Clin Psychopharmacol. 2011;31(1):92-97.
17. Findling RL. How (not) to dose antidepressants and antipsychotics for children. Current Psychiatry. 2007;6(6):79-83.
18. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016 Oct;55(10):851-859.e2.
19. Mancuso E, Faro A, Joshi G, et al. Treatment of pediatric obsessive-compulsive disorder: a review. J Child Adolesc Psychopharmacol. 2010;20(4):299-308.
CASE Auditory hallucinations?
M, age 10, has had multiple visits to the pediatric emergency department (PED) with the chief concern of excessive urinary frequency. At each visit, the medical workup has been negative and he was discharged home. After a few months, M’s parents bring their son back to the PED because he reports hearing “voices in my head” and “feeling tense and scared.” When these feelings become too overwhelming, M stops eating and experiences substantial fear and anxiety that require his mother’s repeated reassurances. M’s mother reports that 2 weeks before his most recent PED visit, he became increasingly anxious and disturbed, and said he was afraid most of the time, and worried about the safety of his family for no apparent reason.
The psychiatrist evaluates M in the PED and diagnoses him with unspecified schizophrenia spectrum and other psychotic disorder based on his persistent report of auditory and tactile hallucinations, including hearing a voice of a man telling him he was going to choke on his food and feeling someone touch his arm to soothe him during his anxious moments. M does not meet criteria for acute inpatient hospitalization, and is discharged home with referral to follow-up at our child and adolescent psychiatry outpatient clinic.
On subsequent evaluation in our clinic, M reports most of the same about his experience hearing “voices in my head” that repeatedly suggest “I might choke on my food and end up seriously ill in the hospital.” He started to hear the “voices” after he witnessed his sister choke while eating a few days earlier. He also mentions that the “voices” tell him “you have to use the restroom.” As a result, he uses the restroom several times before leaving for home and is frequently late for school. His parents accommodate his behavior—his mother allows him to use the bathroom multiple times, and his father overlooks the behavior as part of school anxiety.
At school, his teacher reports a concern for attention-deficit/hyperactivity disorder (ADHD) based on M’s continuous inattentiveness in class and dropping grades. He asks for bathroom breaks up to 15 times a day, which disrupts his class work.
These behaviors have led to a gradual 1-year decline in his overall functioning, including difficulty at school for requesting too many bathroom breaks; having to repeat the 3rd grade; and incurring multiple hospital visits for evaluation of his various complaints. M has become socially isolated and withdrawn from friends and family.
M’s developmental history is normal and his family history is negative for any psychiatric disorder. Medical history and physical examination are unremarkable. CT scan of his head is unremarkable, and all hematologic and biochemistry laboratory test values are within normal range.
[polldaddy:9971376]
Continue to: The authors' observations
The authors’ observations
Several factors may contribute to an increased chance of misdiagnosis of a psychiatric illness
On closer sequential evaluations with M and his family, we determined that the “voices” he was hearing were actually intrusive thoughts, and not hallucinations. M clarified this by saying that first he feels a “pressure”-like sensation in his head, followed by repeated intrusive thoughts of voiding his bladder that compel him to go to the restroom to try to urinate. He feels temporary relief after complying with the urge, even when he passes only a small amount of urine or just washes his hands. After a brief period of relief, this process repeats itself. Further, he was able to clarify his experience while eating food, where he first felt a “pressure”-like sensation in his head, followed by intrusive thoughts of choking that result in him not eating.
This led us to a more appropriate diagnosis of OCD (Table 11). The incidence of OCD has 2 peaks, with different gender distributions. The first peak occurs in childhood, with symptoms mostly arising between 7 and 12 years of age and affecting boys more often than girls. The second peak occurs in early adulthood, at a mean age of 21 years, with a slight female majority.2 However, OCD is often under recognized and undertreated, perhaps due to its extensive heterogeneity; symptom presentations and comorbidity patterns can vary noticeably between individual patients as well as age groups.
OCD is characterized by the presence of obsessions or compulsions that wax and wane in severity, are time-consuming (at least 1 hour per day), and cause subjective distress or interfere with life of the patient or the family. Adults with OCD recognize at some level that the obsessions and/or compulsions are excessive and unreasonable, although children are not required to have this insight to meet criteria for the diagnosis.1 Rating scales, such as the Children’s Yale-Brown Obsessive-Compulsive Scale, Dimensional Yale-Brown Obsessive-Compulsive Scale, and Family Accommodation Scale, are useful to obtain detailed information regarding OCD symptoms, tics, and other factors relevant to the diagnosis.
Continue to: M's symptomatology...
M’s symptomatology did not appear to be psychotic. He was screened for positive or negative symptoms of psychosis, which he and his family clearly denied. Moreover, M’s compulsions (going to the restroom) were typically performed in response to his obsessions (urge to void his bladder) to reduce his distress, which is different from schizophrenia, in which repetitive behaviors are performed in response to psychotic ideation, and not obsessions (Table 23-5).
M’s inattentiveness in the classroom was found to be related to his obsessions and compulsions, and not part of a symptom cluster characterizing ADHD. Teachers often interpret inattention and poor classroom performance as ADHD, but having detailed conversations with teachers often is helpful in understanding the nature of a child’s symptomology and making the appropriate diagnosis.
Establishing the correct clinical diagnosis is critical because it is the starting point in treatment. First-line medication for one condition may exacerbate the symptoms of others. For example, in addition to having a large adverse-effect burden, antipsychotics can induce de novo obsessive–compulsive symptoms (OCS) or exacerbate preexisting OCS, and selective serotonin reuptake inhibitors (SSRIs) may exacerbate psychosis in schizo-obsessive patients with a history of impulsivity and aggressiveness.6 Similarly, stimulant medications for ADHD may exacerbate OCS and may even induce them on their own.7,8
[polldaddy:9971377]
Continue to: The authors' observations
The authors’ observations
Studies have reported an average of 2.5 years from the onset of OCD symptoms to diagnosis in the United States.9 A key reason for this delay, which is more frequently encountered in pediatric patients, is secrecy. Children often feel embarrassed about their symptoms and conceal them until the interference with their functioning becomes extremely disabling. In some cases, symptoms may closely resemble normal childhood routines. In fact, some repetitive behaviors may be normal in some developmental stages, and OCD could be conceptualized as a pathological condition with continuity of normal behaviors during different developmental periods.10
Also, symptoms may go unnoticed for quite some time as unsuspecting and well-intentioned parents and family members become overly involved in the child’s rituals (eg, allowing for increasing frequent prolonged bathroom breaks or frequent change of clothing, etc.). This well-established phenomenon, termed accommodation, is defined as participation of family members in a child’s OCD–related rituals.11 Especially when symptoms are mild or the child is functioning well, accommodation can make it difficult for parents to realize the presence or nature of a problem, as they might tend to minimize their child’s symptoms as representing a unique personality trait or a special “quirk.” Parents generally will seek treatment when their child’s symptoms become more impairing and begin to interfere with social functioning, school performance, or family functioning.
The clinical picture is further complicated by comorbidity. Approximately 60% to 80% of children and adolescents with OCD have ≥1 comorbid psychiatric disorders. Some of the most common include tic disorders, ADHD, anxiety disorders, and mood or eating disorders.9
[polldaddy:9971379]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
In keeping with American Academy of Child and Adolescent Psychiatry guidelines on treating OCD (Table 312), we start M on fluoxetine 10 mg/d. He also begins CBT. Fluoxetine is slowly titrated to 40 mg/d while M engages in learning and utilizing CBT techniques to manage his OCD.
The authors’ observations
The combination of CBT and medication has been suggested as the treatment of choice for moderate and severe OCD.12 The Pediatric OCD Treatment Study, a 5-year, 3-site outcome study designed to compare placebo, sertraline, CBT, and combined CBT and sertraline, concluded that the combined treatment (CBT plus sertraline) was more effective than CBT alone or sertraline alone.13 The effect sizes for the combined treatment, CBT alone, and sertraline alone were 1.4, 0.97, and 0.67, respectively. Remission rates for SSRIs alone are <33%.13,14
SSRIs are the first-line medication for OCD in children, adolescents, and adults (Table 312). Well-designed clinical trials have demonstrated the efficacy and safety of the SSRIs fluoxetine, sertraline, and fluvoxamine (alone or combined with CBT) in children and adolescents with OCD.13 Other SSRIs, such as citalopram, paroxetine, and escitalopram, also have demonstrated efficacy in children and adolescents with OCD, even though the FDA has not yet approved their use in pediatric patients.12 Despite a positive trial of paroxetine in pediatric OCD,12 there have been concerns related to its higher rates of treatment-emergent suicidality,15 lower likelihood of treatment response,16 and its particularly short half-life in pediatric patients.17
Clomipramine is a tricyclic antidepressant with serotonergic properties that is used alone or to boost the effect of an SSRI when there is a partial response. It should be introduced at a low dose in pediatric patients (before age 12) and closely monitored for anticholinergic and cardiac adverse effects. A systemic review and meta-analysis of early treatment responses of SSRIs and clomipramine in pediatric OCD indicated that the greatest benefits occurred early in treatment.18 Clomipramine was associated with a greater measured benefit compared with placebo than SSRIs; there was no evidence of a relationship between SSRI dosing and treatment effect, although data were limited. Adults and children with OCD demonstrated a similar degree and time course of response to SSRIs in OCD.18
Treatment should start with a low dose to reduce the risk of adverse effects with an adequate trial for 10 to 16 weeks at adequate doses. Most experts suggest that treatment should continue for at least 12 months after symptom resolution or stabilization, followed by a very gradual cessation.19
Continue to: OUTCOME Improvement in functioning
OUTCOME Improvement in functioning
After 12 months of combined CBT and fluoxetine, M’s global assessment of functioning (GAF) scale score improves from 35 to 80, indicating major improvement in overall functional level.
Acknowledgement
The authors thank Uzoma Osuchukwu, MD, ex-fellow, Department of Child and Adolescent Psychiatry, Columbia University College of Physicians and Surgeons, Harlem Hospital Center, New York, New York, for his assistance with this article.
Bottom Line
Obsessive-compulsive disorder may masquerade as a schizophrenia spectrum disorder, particularly in younger patients. Accurate differentiation is crucial because antipsychotics can induce de novo obsessive-compulsive symptoms (OCS) or exacerbate preexisting OCS, and selective serotonin reuptake inhibitors may exacerbate psychosis in schizo-obsessive patients with a history of impulsivity and aggressiveness.
Related Resource
- Raveendranathan D, Shiva L, Sharma E, et al. Obsessive compulsive disorder masquerading as psychosis. Indian J Psychol Med. 2012;34(2):179-180.
Drug Brand Names
Citalopram • Celexa
Clomipramine • Anafranil
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Paroxetine • Paxil
Sertraline • Zoloft
CASE Auditory hallucinations?
M, age 10, has had multiple visits to the pediatric emergency department (PED) with the chief concern of excessive urinary frequency. At each visit, the medical workup has been negative and he was discharged home. After a few months, M’s parents bring their son back to the PED because he reports hearing “voices in my head” and “feeling tense and scared.” When these feelings become too overwhelming, M stops eating and experiences substantial fear and anxiety that require his mother’s repeated reassurances. M’s mother reports that 2 weeks before his most recent PED visit, he became increasingly anxious and disturbed, and said he was afraid most of the time, and worried about the safety of his family for no apparent reason.
The psychiatrist evaluates M in the PED and diagnoses him with unspecified schizophrenia spectrum and other psychotic disorder based on his persistent report of auditory and tactile hallucinations, including hearing a voice of a man telling him he was going to choke on his food and feeling someone touch his arm to soothe him during his anxious moments. M does not meet criteria for acute inpatient hospitalization, and is discharged home with referral to follow-up at our child and adolescent psychiatry outpatient clinic.
On subsequent evaluation in our clinic, M reports most of the same about his experience hearing “voices in my head” that repeatedly suggest “I might choke on my food and end up seriously ill in the hospital.” He started to hear the “voices” after he witnessed his sister choke while eating a few days earlier. He also mentions that the “voices” tell him “you have to use the restroom.” As a result, he uses the restroom several times before leaving for home and is frequently late for school. His parents accommodate his behavior—his mother allows him to use the bathroom multiple times, and his father overlooks the behavior as part of school anxiety.
At school, his teacher reports a concern for attention-deficit/hyperactivity disorder (ADHD) based on M’s continuous inattentiveness in class and dropping grades. He asks for bathroom breaks up to 15 times a day, which disrupts his class work.
These behaviors have led to a gradual 1-year decline in his overall functioning, including difficulty at school for requesting too many bathroom breaks; having to repeat the 3rd grade; and incurring multiple hospital visits for evaluation of his various complaints. M has become socially isolated and withdrawn from friends and family.
M’s developmental history is normal and his family history is negative for any psychiatric disorder. Medical history and physical examination are unremarkable. CT scan of his head is unremarkable, and all hematologic and biochemistry laboratory test values are within normal range.
[polldaddy:9971376]
Continue to: The authors' observations
The authors’ observations
Several factors may contribute to an increased chance of misdiagnosis of a psychiatric illness
On closer sequential evaluations with M and his family, we determined that the “voices” he was hearing were actually intrusive thoughts, and not hallucinations. M clarified this by saying that first he feels a “pressure”-like sensation in his head, followed by repeated intrusive thoughts of voiding his bladder that compel him to go to the restroom to try to urinate. He feels temporary relief after complying with the urge, even when he passes only a small amount of urine or just washes his hands. After a brief period of relief, this process repeats itself. Further, he was able to clarify his experience while eating food, where he first felt a “pressure”-like sensation in his head, followed by intrusive thoughts of choking that result in him not eating.
This led us to a more appropriate diagnosis of OCD (Table 11). The incidence of OCD has 2 peaks, with different gender distributions. The first peak occurs in childhood, with symptoms mostly arising between 7 and 12 years of age and affecting boys more often than girls. The second peak occurs in early adulthood, at a mean age of 21 years, with a slight female majority.2 However, OCD is often under recognized and undertreated, perhaps due to its extensive heterogeneity; symptom presentations and comorbidity patterns can vary noticeably between individual patients as well as age groups.
OCD is characterized by the presence of obsessions or compulsions that wax and wane in severity, are time-consuming (at least 1 hour per day), and cause subjective distress or interfere with life of the patient or the family. Adults with OCD recognize at some level that the obsessions and/or compulsions are excessive and unreasonable, although children are not required to have this insight to meet criteria for the diagnosis.1 Rating scales, such as the Children’s Yale-Brown Obsessive-Compulsive Scale, Dimensional Yale-Brown Obsessive-Compulsive Scale, and Family Accommodation Scale, are useful to obtain detailed information regarding OCD symptoms, tics, and other factors relevant to the diagnosis.
Continue to: M's symptomatology...
M’s symptomatology did not appear to be psychotic. He was screened for positive or negative symptoms of psychosis, which he and his family clearly denied. Moreover, M’s compulsions (going to the restroom) were typically performed in response to his obsessions (urge to void his bladder) to reduce his distress, which is different from schizophrenia, in which repetitive behaviors are performed in response to psychotic ideation, and not obsessions (Table 23-5).
M’s inattentiveness in the classroom was found to be related to his obsessions and compulsions, and not part of a symptom cluster characterizing ADHD. Teachers often interpret inattention and poor classroom performance as ADHD, but having detailed conversations with teachers often is helpful in understanding the nature of a child’s symptomology and making the appropriate diagnosis.
Establishing the correct clinical diagnosis is critical because it is the starting point in treatment. First-line medication for one condition may exacerbate the symptoms of others. For example, in addition to having a large adverse-effect burden, antipsychotics can induce de novo obsessive–compulsive symptoms (OCS) or exacerbate preexisting OCS, and selective serotonin reuptake inhibitors (SSRIs) may exacerbate psychosis in schizo-obsessive patients with a history of impulsivity and aggressiveness.6 Similarly, stimulant medications for ADHD may exacerbate OCS and may even induce them on their own.7,8
[polldaddy:9971377]
Continue to: The authors' observations
The authors’ observations
Studies have reported an average of 2.5 years from the onset of OCD symptoms to diagnosis in the United States.9 A key reason for this delay, which is more frequently encountered in pediatric patients, is secrecy. Children often feel embarrassed about their symptoms and conceal them until the interference with their functioning becomes extremely disabling. In some cases, symptoms may closely resemble normal childhood routines. In fact, some repetitive behaviors may be normal in some developmental stages, and OCD could be conceptualized as a pathological condition with continuity of normal behaviors during different developmental periods.10
Also, symptoms may go unnoticed for quite some time as unsuspecting and well-intentioned parents and family members become overly involved in the child’s rituals (eg, allowing for increasing frequent prolonged bathroom breaks or frequent change of clothing, etc.). This well-established phenomenon, termed accommodation, is defined as participation of family members in a child’s OCD–related rituals.11 Especially when symptoms are mild or the child is functioning well, accommodation can make it difficult for parents to realize the presence or nature of a problem, as they might tend to minimize their child’s symptoms as representing a unique personality trait or a special “quirk.” Parents generally will seek treatment when their child’s symptoms become more impairing and begin to interfere with social functioning, school performance, or family functioning.
The clinical picture is further complicated by comorbidity. Approximately 60% to 80% of children and adolescents with OCD have ≥1 comorbid psychiatric disorders. Some of the most common include tic disorders, ADHD, anxiety disorders, and mood or eating disorders.9
[polldaddy:9971379]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
In keeping with American Academy of Child and Adolescent Psychiatry guidelines on treating OCD (Table 312), we start M on fluoxetine 10 mg/d. He also begins CBT. Fluoxetine is slowly titrated to 40 mg/d while M engages in learning and utilizing CBT techniques to manage his OCD.
The authors’ observations
The combination of CBT and medication has been suggested as the treatment of choice for moderate and severe OCD.12 The Pediatric OCD Treatment Study, a 5-year, 3-site outcome study designed to compare placebo, sertraline, CBT, and combined CBT and sertraline, concluded that the combined treatment (CBT plus sertraline) was more effective than CBT alone or sertraline alone.13 The effect sizes for the combined treatment, CBT alone, and sertraline alone were 1.4, 0.97, and 0.67, respectively. Remission rates for SSRIs alone are <33%.13,14
SSRIs are the first-line medication for OCD in children, adolescents, and adults (Table 312). Well-designed clinical trials have demonstrated the efficacy and safety of the SSRIs fluoxetine, sertraline, and fluvoxamine (alone or combined with CBT) in children and adolescents with OCD.13 Other SSRIs, such as citalopram, paroxetine, and escitalopram, also have demonstrated efficacy in children and adolescents with OCD, even though the FDA has not yet approved their use in pediatric patients.12 Despite a positive trial of paroxetine in pediatric OCD,12 there have been concerns related to its higher rates of treatment-emergent suicidality,15 lower likelihood of treatment response,16 and its particularly short half-life in pediatric patients.17
Clomipramine is a tricyclic antidepressant with serotonergic properties that is used alone or to boost the effect of an SSRI when there is a partial response. It should be introduced at a low dose in pediatric patients (before age 12) and closely monitored for anticholinergic and cardiac adverse effects. A systemic review and meta-analysis of early treatment responses of SSRIs and clomipramine in pediatric OCD indicated that the greatest benefits occurred early in treatment.18 Clomipramine was associated with a greater measured benefit compared with placebo than SSRIs; there was no evidence of a relationship between SSRI dosing and treatment effect, although data were limited. Adults and children with OCD demonstrated a similar degree and time course of response to SSRIs in OCD.18
Treatment should start with a low dose to reduce the risk of adverse effects with an adequate trial for 10 to 16 weeks at adequate doses. Most experts suggest that treatment should continue for at least 12 months after symptom resolution or stabilization, followed by a very gradual cessation.19
Continue to: OUTCOME Improvement in functioning
OUTCOME Improvement in functioning
After 12 months of combined CBT and fluoxetine, M’s global assessment of functioning (GAF) scale score improves from 35 to 80, indicating major improvement in overall functional level.
Acknowledgement
The authors thank Uzoma Osuchukwu, MD, ex-fellow, Department of Child and Adolescent Psychiatry, Columbia University College of Physicians and Surgeons, Harlem Hospital Center, New York, New York, for his assistance with this article.
Bottom Line
Obsessive-compulsive disorder may masquerade as a schizophrenia spectrum disorder, particularly in younger patients. Accurate differentiation is crucial because antipsychotics can induce de novo obsessive-compulsive symptoms (OCS) or exacerbate preexisting OCS, and selective serotonin reuptake inhibitors may exacerbate psychosis in schizo-obsessive patients with a history of impulsivity and aggressiveness.
Related Resource
- Raveendranathan D, Shiva L, Sharma E, et al. Obsessive compulsive disorder masquerading as psychosis. Indian J Psychol Med. 2012;34(2):179-180.
Drug Brand Names
Citalopram • Celexa
Clomipramine • Anafranil
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Paroxetine • Paxil
Sertraline • Zoloft
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Geller D, Biederman J, Jones J, et al. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? A review of the pediatric literature. J Am Acad Child Adolesc Psychiatry.1998;37(4):420-427.
3. Huppert JD, Simpson HB, Nissenson KJ, et al. Quality of life and functional impairment in obsessive-compulsive disorder: A comparison of patients with and without comorbidity, patients in remission, and healthy controls. Depress Anxiety. 2009;26(1):39-45.
4. Sobel W, Wolski R, Cancro R, et al. Interpersonal relatedness and paranoid schizophrenia. Am J Psychiatry.1996;153(8):1084-1087.
5. Meares A. The diagnosis of prepsychotic schizophrenia. Lancet. 1959;1(7063):55-58.
6. Poyurovsky M, Weizman A, Weizman R. Obsessive-compulsive disorder in schizophrenia: Clinical characteristics and treatment. CNS Drugs. 2004;18(14):989-1010.
7. Kouris S. Methylphenidate-induced obsessive-compulsiveness. J Am Acad Child Adolesc Psychiatry. 1998;37(2):135.
8. Woolley JB, Heyman I. Dexamphetamine for obsessive-compulsive disorder. Am J Psychiatry. 2003;160(1):183.
9. Geller DA. Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatr Clin N Am. 2006;29(2):352-370.
10. Evans DW, Milanak ME, Medeiros B, et al. Magical beliefs and rituals in young children. Child Psychiatry Hum Dev. 2002;33(1):43-58.
11. Amir N, Freshman M, Foa E. Family distress and involvement in relatives of obsessive-compulsive disorder patients. J Anxiety Disord. 2000;14(3):209-217.
12. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):98-113.
13. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: The Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
14. Franklin ME, Sapyta J, Freeman JB, et al. Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: The Pediatric OCD Treatment Study II (POTS II) randomized controlled trial. JAMA. 2011;306(11):1224-1232.
15. Wagner KD, Asarnow JR, Vitiello B, et al. Out of the black box: treatment of resistant depression in adolescents and the antidepressant controversy. J Child Adolesc Psychopharmacol. 2012;22(1):5-10.
16. Sakolsky DJ, Perel JM, Emslie GJ, et al. Antidepressant exposure as a predictor of clinical outcomes in the treatment of resistant depression in adolescents (TORDIA) study. J Clin Psychopharmacol. 2011;31(1):92-97.
17. Findling RL. How (not) to dose antidepressants and antipsychotics for children. Current Psychiatry. 2007;6(6):79-83.
18. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016 Oct;55(10):851-859.e2.
19. Mancuso E, Faro A, Joshi G, et al. Treatment of pediatric obsessive-compulsive disorder: a review. J Child Adolesc Psychopharmacol. 2010;20(4):299-308.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Geller D, Biederman J, Jones J, et al. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? A review of the pediatric literature. J Am Acad Child Adolesc Psychiatry.1998;37(4):420-427.
3. Huppert JD, Simpson HB, Nissenson KJ, et al. Quality of life and functional impairment in obsessive-compulsive disorder: A comparison of patients with and without comorbidity, patients in remission, and healthy controls. Depress Anxiety. 2009;26(1):39-45.
4. Sobel W, Wolski R, Cancro R, et al. Interpersonal relatedness and paranoid schizophrenia. Am J Psychiatry.1996;153(8):1084-1087.
5. Meares A. The diagnosis of prepsychotic schizophrenia. Lancet. 1959;1(7063):55-58.
6. Poyurovsky M, Weizman A, Weizman R. Obsessive-compulsive disorder in schizophrenia: Clinical characteristics and treatment. CNS Drugs. 2004;18(14):989-1010.
7. Kouris S. Methylphenidate-induced obsessive-compulsiveness. J Am Acad Child Adolesc Psychiatry. 1998;37(2):135.
8. Woolley JB, Heyman I. Dexamphetamine for obsessive-compulsive disorder. Am J Psychiatry. 2003;160(1):183.
9. Geller DA. Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatr Clin N Am. 2006;29(2):352-370.
10. Evans DW, Milanak ME, Medeiros B, et al. Magical beliefs and rituals in young children. Child Psychiatry Hum Dev. 2002;33(1):43-58.
11. Amir N, Freshman M, Foa E. Family distress and involvement in relatives of obsessive-compulsive disorder patients. J Anxiety Disord. 2000;14(3):209-217.
12. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):98-113.
13. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: The Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
14. Franklin ME, Sapyta J, Freeman JB, et al. Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: The Pediatric OCD Treatment Study II (POTS II) randomized controlled trial. JAMA. 2011;306(11):1224-1232.
15. Wagner KD, Asarnow JR, Vitiello B, et al. Out of the black box: treatment of resistant depression in adolescents and the antidepressant controversy. J Child Adolesc Psychopharmacol. 2012;22(1):5-10.
16. Sakolsky DJ, Perel JM, Emslie GJ, et al. Antidepressant exposure as a predictor of clinical outcomes in the treatment of resistant depression in adolescents (TORDIA) study. J Clin Psychopharmacol. 2011;31(1):92-97.
17. Findling RL. How (not) to dose antidepressants and antipsychotics for children. Current Psychiatry. 2007;6(6):79-83.
18. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016 Oct;55(10):851-859.e2.
19. Mancuso E, Faro A, Joshi G, et al. Treatment of pediatric obsessive-compulsive disorder: a review. J Child Adolesc Psychopharmacol. 2010;20(4):299-308.
Decompensation in a 51-year-old woman with schizophrenia
CASE Psychotic and reclusive
Ms. A, age 51, has schizophrenia and has been doing well living at a supervised residential facility. She was stable on haloperidol, 10 mg twice a day, for years but recently became agitated, threatening her roommate and yelling during the night. Ms. A begins to refuse to take her haloperidol. She also refuses to attend several outpatient appointments. As a result, Ms. A is admitted to the psychiatric unit on an involuntary basis.
In the hospital, Ms. A rarely comes out of her room. When she does come out, she usually sits in a chair, talking to herself and occasionally yelling or crying in apparent distress. Ms. A refuses to engage with her treatment team and lies mute in her bed when they attempt to interview her. Her records indicate that previous medication trials have included
Over the next week, Ms. A begins to interact more appropriately with nursing sta
[polldaddy:9945425]
The authors’ observations
As a class, antipsychotics lead to symptom reduction in approximately 70% of patients.1 However, the degree of response can vary markedly between individuals; although some patients may experience almost complete resolution of symptoms, others are still markedly impaired, as in Ms. A’s case.
A substantial amount of literature suggests that although the practice is common, use of >1 antipsychotic does not significantly increase efficacy but increases risk of adverse effects, such as type 2 diabetes mellitus, metabolic syndrome, cognitive impairment, and extrapyramidal symptoms.2-4 One exception is augmentation of clozapine with a second antipsychotic, which in certain cases appears to offer greater efficacy than clozapine alone.1 Practice guidelines and evidence generally do not support the use of multiple antipsychotics, but 20% of patients take >1 antipsychotic.5,6 Although antipsychotic polypharmacy may be appropriate for some patients, current literature suggests it is being done more often than recommended.
Clozapine is considered the most efficacious option for treatment-resistant schizophrenia.7 Because of Ms. A’s history of recurrent hospitalizations, her extensive list of trialed medications, and her ongoing symptoms despite a sufficient trial of haloperidol, the treatment team gives serious consideration to switching Ms. A to clozapine. However, Ms. A is not able to tolerate blood draws without significant support from nursing staff, and it is likely she would be unable to tolerate the frequent blood monitoring required of patients receiving clozapine.
Because many of Ms. A’s symptoms were negative or depressive, including hypersomnia, psychomotor retardation, sadness with frequent crying spells, and reduced interest in activities, adding an antidepressant to Ms. A’s medication regimen was considered. A recent systematic review and meta-analysis showed that adding an antidepressant to an antipsychotic in patients with schizophrenia had small but beneficial effects on depressive and negative symptoms and a low risk of adverse effects.8 However, Ms. A declined this option.
TREATMENT Adding long-acting haloperidol
Ms. A had previously achieved therapeutic blood levels9 with oral haloperidol. Data suggest that compared with the oral form, long-acting injectable antipsychotics can both improve compliance and decrease rehospitalization rates.10-12 Because Ms. A previously had done well with haloperidol decanoate, 200 mg every 2 weeks, achieving a blood level of 16.2 ng/mL, and because she had a partial response to oral haloperidol, we add haloperidol decanoate, 100 mg every 2 weeks, to her regimen, with the intention of transitioning her to all-depot dosing. In addition, the treatment team tries to engage Ms. A in a discussion of potential psychological contributions to her current presentation. They note that Ms. A has her basic needs met on the unit and reports feeling safe there; thus, a fear of discharge may be contributing to her lack of engagement with the team. However, because of her limited communication, it is challenging to investigate this hypothesis or explore other possible psychological issues.
Despite increasing the dosing of haloperidol, Ms. A shows minimal improvement. She continues to stonewall her treatment team, and is unwilling or unable to engage in meaningful conversation. A review of her chart suggests that this hospital course is different from previous ones in which her average stay was a few weeks, and she generally was able to converse with the treatment team, participate in discussions about her care, and make decisions about her desire for discharge.
The team considers if additional factors could be impacting Ms. A’s current presentation. They raise the possibility that she could be going through menopause, and hormonal fluctuations may be contributing to her symptoms. Despite being on the unit for nearly 2 months, Ms. A has not required the use of sanitary products. She also reports to nursing staff that at times she feels flushed and sweaty, but she is afebrile and does not have other signs or symptoms of infection.
[polldaddy:9945428]
The authors’ observations
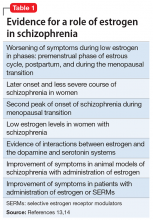
Evidence suggests that estrogen levels can influence the development and severity of symptoms of schizophrenia (Table 113,14). Rates of schizophrenia are lower in women, and women typically have a later onset of illness with less severe symptoms.13 Women also have a second peak incidence of schizophrenia between ages 45 and 50, corresponding with the hormonal changes associated with menopause and the associated drop in estrogen.14 Symptoms also fluctuate with hormonal cycles—women experience worsening symptoms during the premenstrual phase of the menstrual cycle, when estrogen levels are low, and an improvement of symptoms during high-estrogen phases of the cycle.14 Overall, low levels of estrogen also have been observed in women with schizophrenia relative to controls, although this may be partially attributable to treatment with antipsychotics.14
Estrogen affects various regions of the brain implicated in schizophrenia and likely imparts its behavioral effects through several different mechanisms. Estrogen can act on cells to directly impact intracellular signaling and to alter gene expression.15 Although most often thought of as being related to reproductive functions, estrogen receptors can be found in many cortical and subcortical regions of the brain, such as the hippocampus, substantia nigra, and prefrontal cortex. Estrogen receptor expression levels in certain brain regions have been found to be altered in individuals with schizophrenia.15 Estrogen also enhances neurogenesis and neuroplasticity, playing a role in learning and memory.16 Particularly relevant, estrogen has been shown to directly impact both the dopaminergic and serotonergic systems.15,17 In animal models, estrogen has been shown to decrease the behavioral effects induced by dopamine agonists and decrease symptoms of schizophrenia.18 The underlying molecular mechanisms by which estrogen has these effects are uncertain.
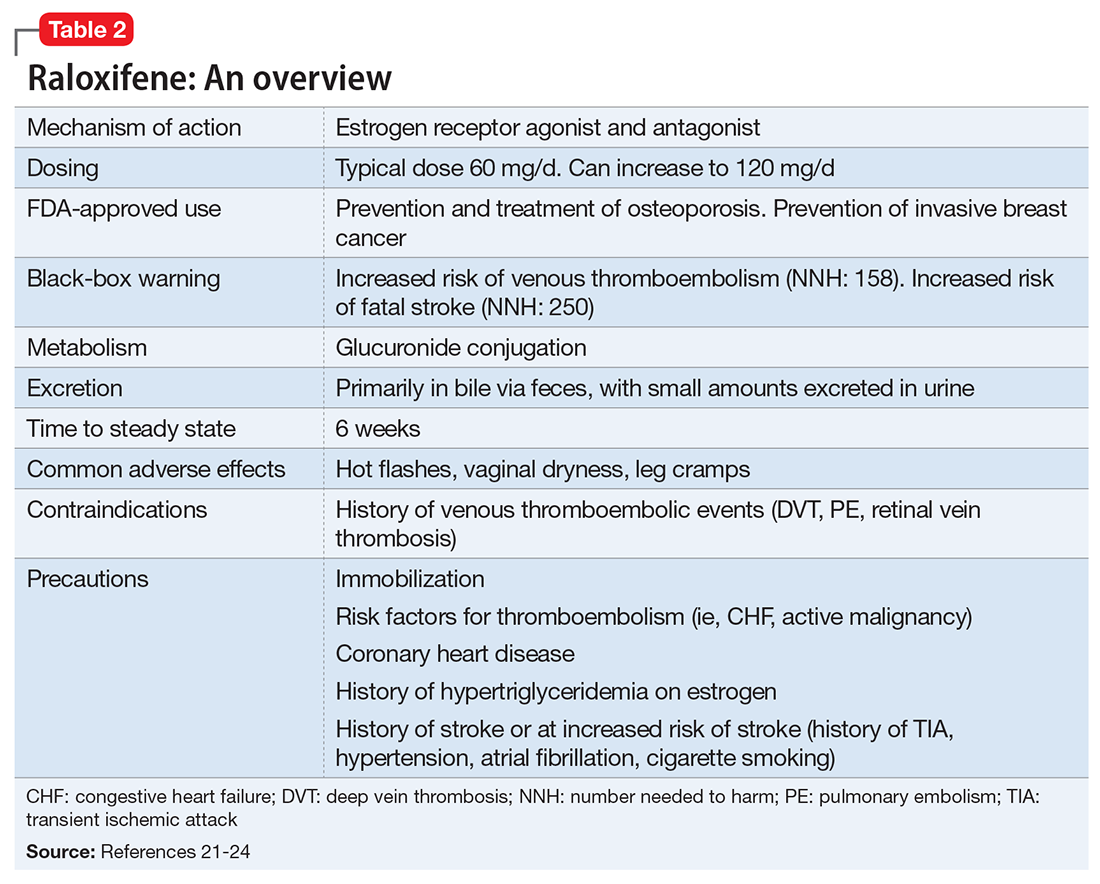
Given estrogen’s potentially protective effects, clinical trials have explored the role of estrogen as an adjuvant to antipsychotics for treating schizophrenia. Studies have shown that estrogen can improve psychotic symptoms in patients with schizophrenia.19,20 However, because estrogen administration can increase the risk of breast and uterine cancer, researchers are instead investigating selective estrogen receptor modulators (SERMs).14 These medications have mixed agonist and antagonist effects, with different effects on different tissues. Raloxifene is a SERM that acts as an estrogen agonist in some tissues, but an antagonist in uterine and breast tissue, which may minimize potential deleterious adverse effects (Table 221-24). Repeated randomized controlled trials have found promising results for use of raloxifene as an adjunctive treatment in peri- and postmenopausal women with schizophrenia, including those refractory to antipsychotic treatment.13,25-27
TREATMENT Address symptoms
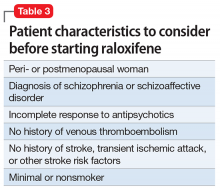
The treatment team takes steps to address Ms. A’s perimenopausal symptoms. For mild to moderate hot flashes, primary interventions are nonpharmacologic.28 Because Ms. A primarily reports her hot flashes at night, she is given lightweight pajamas and moved to the coolest room on the unit. Both bring some relief, and her hot flashes appear to be less distressing. The treatment team decides to consult Endocrinology to further investigate the feasibility of starting raloxifene (Table 3) because of their experience using this medication to manage osteoporosis.
[polldaddy:9945429]
The authors’ observations
Raloxifene is FDA-approved for treating osteoporosis and preventing invasive breast cancer.29 Because it is an estrogen antagonist in both breast and uterine tissues, raloxifene does not increase the risk of uterine or breast cancer. Large studies have shown rates of cardiovascular events are similar for raloxifene and placebo, and some studies have found that raloxifene treatment is associated with improvement in cardiovascular risk factors, including lower blood pressure, lower low-density lipoprotein cholesterol, and increased high-density lipoprotein cholesterol.29 Raloxifene does, however, increase risk of venous thromboembolism, including deep vein thrombosis and pulmonary embolism, and fatal stroke.29,30 Overall, the risk remains relatively low, with an absolute risk increase of fatal stroke of 0.7 per 1,000 woman-years (number needed to harm [NNH]: 250) and an absolute risk increase of venous thromboembolic events of 1.88 per 1,000 women-years (NNH: 158).31 However, raloxifene may not be appropriate for patients with independent risk factors for these events. Despite this, a large meta-analysis found a 10% decrease in mortality for patients taking raloxifene compared with those receiving placebo.32 Raloxifene also can cause hot flashes, muscle cramps, and flu-like symptoms.29
Diagnosis of menopause and perimenopause is largely clinical, with hormone testing generally recommended for women age <45 in whom the diagnosis may be unclear.28 Thus, Ms. A’s vasomotor symptoms and absence of a menstrual cycle for at least 2 months were diagnostic of perimenopause; a 12-month cessation in menstrual cycles is required for a diagnosis of menopause.28
OUTCOME Improvement with raloxifene
Because Ms. A is at relatively low risk for a thromboembolism or stroke, the benefit of raloxifene is thought to outweigh the risk, and she is started on raloxifene, 60 mg/d. Over the next 2 weeks, Ms. A becomes increasingly interactive, and is seen sitting at a table talking with other patients on multiple occasions. She spends time looking at fashion magazines, and engages in conversation about fashion with staff and other patients. She participates in group therapy for the first time during this hospital stay and begins to talk about discharge. She occasionally smiles and waves at her treatment team and participates more in the daily interview, although these interactions remain limited and on her terms. She maintains this improvement and is transferred to a psychiatric facility in her home county for ongoing care and discharge planning.
2. Citrome L, Jaffe A, Levine J, et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006-1013.
3. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1-3):91-100.
4. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527-542.
5. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28.
6. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378.
7. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
8. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9);876-886.
9. Ulrich S, Neuhof S, Braun V, et al. Therapeutic window of serum haloperidol concentration in acute schizophrenia and schizoaffective disorder. Pharmacopsychiatry. 1998;31(5):163-169.
10. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655.
11. MacEwan JP, Kamat SA, Duffy RA, et al. Hospital readmission rates among patients with schizophrenia treated with long-acting injectables or oral antipsychotics. Psychiatr Serv. 2016;67(11):1183-1188.
12. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768.
13. Usall J, Huerta-Ramos E, Iniesta R, et al; RALOPSYCAT Group. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72(11):1552-1557.
14. Seeman MV. Treating schizophrenia at the time of menopause. Maturitas. 2012;72(2):117-120.
15. Gogos A, Sbisa AM, Sun J, et al. A role for estrogen in schizophrenia: clinical and preclinical findings. Int J Endocrinol. 2015;2015:615356. doi: 10.1155/2015/615356.
16. Khan MM. Neurocognitive, neuroprotective, and cardiometabolic effects of raloxifene: potential for improving therapeutic outcomes in schizophrenia. CNS Drugs. 2016;30(7):589-601.
17. Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37.
18. Häfner H, Behrens S, De Vry J, et al. An animal model for the effects of estradiol on dopamine-mediated behavior: implications for sex differences in schizophrenia. Psychiatry Res. 1991;38(2):125-134.
19. Akhondzadeh S, Nejatisafa AA, Amini H, et al. Adjunctive estrogen treatment in women with chronic schizophrenia: a double-blind, randomized, and placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):1007-1012.
20. Kulkarni J, de Castella A, Fitzgerald PB, et al. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry. 2008;65(8):955-960.
21. Ellis AJ, Hendrick VM, Williams R, Komm BS. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921-934.
22. Morello KC, Wurz GT, DeGregorio MW. Pharmacokinetics of selective estrogen receptor modulators. Clin pharmacokinet. 2003;42(4):361-372.
23. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014;17(4):490-495.
24. Raloxifene Hydrochloride. Micromedex 2.0. Truven Health Analytics. www.micromedexsolutions.com. Accessed July 24, 2016.
25. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
26. Huerta-Ramos E, Iniesta R, Ochoa S, et al. Effects of raloxifene on cognition in postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 2014;24(2):223-231.
27. Kianimehr G, Fatehi F, Hashempoor S, et al. Raloxifene adjunctive therapy for postmenopausal women suffering from chronic schizophrenia: a randomized double-blind and placebo controlled trial. Daru. 2014;22:55.
28. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975-4011.
29. Ellis AJ, Hendrick VM, Williams R, et al. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921-934.
30. Adomaityte J, Farooq M, Qayyum R. Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis. Thromb Haemost. 2008;99(2):338-342.
31. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014;17(4):490-495.
32. Grady D, Cauley JA, Stock JL, et al. Effect of raloxifene on all-cause mortality. Am J Med. 2010;123(5):469.e1-461.e7.
CASE Psychotic and reclusive
Ms. A, age 51, has schizophrenia and has been doing well living at a supervised residential facility. She was stable on haloperidol, 10 mg twice a day, for years but recently became agitated, threatening her roommate and yelling during the night. Ms. A begins to refuse to take her haloperidol. She also refuses to attend several outpatient appointments. As a result, Ms. A is admitted to the psychiatric unit on an involuntary basis.
In the hospital, Ms. A rarely comes out of her room. When she does come out, she usually sits in a chair, talking to herself and occasionally yelling or crying in apparent distress. Ms. A refuses to engage with her treatment team and lies mute in her bed when they attempt to interview her. Her records indicate that previous medication trials have included
Over the next week, Ms. A begins to interact more appropriately with nursing sta
[polldaddy:9945425]
The authors’ observations
As a class, antipsychotics lead to symptom reduction in approximately 70% of patients.1 However, the degree of response can vary markedly between individuals; although some patients may experience almost complete resolution of symptoms, others are still markedly impaired, as in Ms. A’s case.
A substantial amount of literature suggests that although the practice is common, use of >1 antipsychotic does not significantly increase efficacy but increases risk of adverse effects, such as type 2 diabetes mellitus, metabolic syndrome, cognitive impairment, and extrapyramidal symptoms.2-4 One exception is augmentation of clozapine with a second antipsychotic, which in certain cases appears to offer greater efficacy than clozapine alone.1 Practice guidelines and evidence generally do not support the use of multiple antipsychotics, but 20% of patients take >1 antipsychotic.5,6 Although antipsychotic polypharmacy may be appropriate for some patients, current literature suggests it is being done more often than recommended.
Clozapine is considered the most efficacious option for treatment-resistant schizophrenia.7 Because of Ms. A’s history of recurrent hospitalizations, her extensive list of trialed medications, and her ongoing symptoms despite a sufficient trial of haloperidol, the treatment team gives serious consideration to switching Ms. A to clozapine. However, Ms. A is not able to tolerate blood draws without significant support from nursing staff, and it is likely she would be unable to tolerate the frequent blood monitoring required of patients receiving clozapine.
Because many of Ms. A’s symptoms were negative or depressive, including hypersomnia, psychomotor retardation, sadness with frequent crying spells, and reduced interest in activities, adding an antidepressant to Ms. A’s medication regimen was considered. A recent systematic review and meta-analysis showed that adding an antidepressant to an antipsychotic in patients with schizophrenia had small but beneficial effects on depressive and negative symptoms and a low risk of adverse effects.8 However, Ms. A declined this option.
TREATMENT Adding long-acting haloperidol
Ms. A had previously achieved therapeutic blood levels9 with oral haloperidol. Data suggest that compared with the oral form, long-acting injectable antipsychotics can both improve compliance and decrease rehospitalization rates.10-12 Because Ms. A previously had done well with haloperidol decanoate, 200 mg every 2 weeks, achieving a blood level of 16.2 ng/mL, and because she had a partial response to oral haloperidol, we add haloperidol decanoate, 100 mg every 2 weeks, to her regimen, with the intention of transitioning her to all-depot dosing. In addition, the treatment team tries to engage Ms. A in a discussion of potential psychological contributions to her current presentation. They note that Ms. A has her basic needs met on the unit and reports feeling safe there; thus, a fear of discharge may be contributing to her lack of engagement with the team. However, because of her limited communication, it is challenging to investigate this hypothesis or explore other possible psychological issues.
Despite increasing the dosing of haloperidol, Ms. A shows minimal improvement. She continues to stonewall her treatment team, and is unwilling or unable to engage in meaningful conversation. A review of her chart suggests that this hospital course is different from previous ones in which her average stay was a few weeks, and she generally was able to converse with the treatment team, participate in discussions about her care, and make decisions about her desire for discharge.
The team considers if additional factors could be impacting Ms. A’s current presentation. They raise the possibility that she could be going through menopause, and hormonal fluctuations may be contributing to her symptoms. Despite being on the unit for nearly 2 months, Ms. A has not required the use of sanitary products. She also reports to nursing staff that at times she feels flushed and sweaty, but she is afebrile and does not have other signs or symptoms of infection.
[polldaddy:9945428]
The authors’ observations
Evidence suggests that estrogen levels can influence the development and severity of symptoms of schizophrenia (Table 113,14). Rates of schizophrenia are lower in women, and women typically have a later onset of illness with less severe symptoms.13 Women also have a second peak incidence of schizophrenia between ages 45 and 50, corresponding with the hormonal changes associated with menopause and the associated drop in estrogen.14 Symptoms also fluctuate with hormonal cycles—women experience worsening symptoms during the premenstrual phase of the menstrual cycle, when estrogen levels are low, and an improvement of symptoms during high-estrogen phases of the cycle.14 Overall, low levels of estrogen also have been observed in women with schizophrenia relative to controls, although this may be partially attributable to treatment with antipsychotics.14
Estrogen affects various regions of the brain implicated in schizophrenia and likely imparts its behavioral effects through several different mechanisms. Estrogen can act on cells to directly impact intracellular signaling and to alter gene expression.15 Although most often thought of as being related to reproductive functions, estrogen receptors can be found in many cortical and subcortical regions of the brain, such as the hippocampus, substantia nigra, and prefrontal cortex. Estrogen receptor expression levels in certain brain regions have been found to be altered in individuals with schizophrenia.15 Estrogen also enhances neurogenesis and neuroplasticity, playing a role in learning and memory.16 Particularly relevant, estrogen has been shown to directly impact both the dopaminergic and serotonergic systems.15,17 In animal models, estrogen has been shown to decrease the behavioral effects induced by dopamine agonists and decrease symptoms of schizophrenia.18 The underlying molecular mechanisms by which estrogen has these effects are uncertain.
Given estrogen’s potentially protective effects, clinical trials have explored the role of estrogen as an adjuvant to antipsychotics for treating schizophrenia. Studies have shown that estrogen can improve psychotic symptoms in patients with schizophrenia.19,20 However, because estrogen administration can increase the risk of breast and uterine cancer, researchers are instead investigating selective estrogen receptor modulators (SERMs).14 These medications have mixed agonist and antagonist effects, with different effects on different tissues. Raloxifene is a SERM that acts as an estrogen agonist in some tissues, but an antagonist in uterine and breast tissue, which may minimize potential deleterious adverse effects (Table 221-24). Repeated randomized controlled trials have found promising results for use of raloxifene as an adjunctive treatment in peri- and postmenopausal women with schizophrenia, including those refractory to antipsychotic treatment.13,25-27
TREATMENT Address symptoms
The treatment team takes steps to address Ms. A’s perimenopausal symptoms. For mild to moderate hot flashes, primary interventions are nonpharmacologic.28 Because Ms. A primarily reports her hot flashes at night, she is given lightweight pajamas and moved to the coolest room on the unit. Both bring some relief, and her hot flashes appear to be less distressing. The treatment team decides to consult Endocrinology to further investigate the feasibility of starting raloxifene (Table 3) because of their experience using this medication to manage osteoporosis.
[polldaddy:9945429]
The authors’ observations
Raloxifene is FDA-approved for treating osteoporosis and preventing invasive breast cancer.29 Because it is an estrogen antagonist in both breast and uterine tissues, raloxifene does not increase the risk of uterine or breast cancer. Large studies have shown rates of cardiovascular events are similar for raloxifene and placebo, and some studies have found that raloxifene treatment is associated with improvement in cardiovascular risk factors, including lower blood pressure, lower low-density lipoprotein cholesterol, and increased high-density lipoprotein cholesterol.29 Raloxifene does, however, increase risk of venous thromboembolism, including deep vein thrombosis and pulmonary embolism, and fatal stroke.29,30 Overall, the risk remains relatively low, with an absolute risk increase of fatal stroke of 0.7 per 1,000 woman-years (number needed to harm [NNH]: 250) and an absolute risk increase of venous thromboembolic events of 1.88 per 1,000 women-years (NNH: 158).31 However, raloxifene may not be appropriate for patients with independent risk factors for these events. Despite this, a large meta-analysis found a 10% decrease in mortality for patients taking raloxifene compared with those receiving placebo.32 Raloxifene also can cause hot flashes, muscle cramps, and flu-like symptoms.29
Diagnosis of menopause and perimenopause is largely clinical, with hormone testing generally recommended for women age <45 in whom the diagnosis may be unclear.28 Thus, Ms. A’s vasomotor symptoms and absence of a menstrual cycle for at least 2 months were diagnostic of perimenopause; a 12-month cessation in menstrual cycles is required for a diagnosis of menopause.28
OUTCOME Improvement with raloxifene
Because Ms. A is at relatively low risk for a thromboembolism or stroke, the benefit of raloxifene is thought to outweigh the risk, and she is started on raloxifene, 60 mg/d. Over the next 2 weeks, Ms. A becomes increasingly interactive, and is seen sitting at a table talking with other patients on multiple occasions. She spends time looking at fashion magazines, and engages in conversation about fashion with staff and other patients. She participates in group therapy for the first time during this hospital stay and begins to talk about discharge. She occasionally smiles and waves at her treatment team and participates more in the daily interview, although these interactions remain limited and on her terms. She maintains this improvement and is transferred to a psychiatric facility in her home county for ongoing care and discharge planning.
CASE Psychotic and reclusive
Ms. A, age 51, has schizophrenia and has been doing well living at a supervised residential facility. She was stable on haloperidol, 10 mg twice a day, for years but recently became agitated, threatening her roommate and yelling during the night. Ms. A begins to refuse to take her haloperidol. She also refuses to attend several outpatient appointments. As a result, Ms. A is admitted to the psychiatric unit on an involuntary basis.
In the hospital, Ms. A rarely comes out of her room. When she does come out, she usually sits in a chair, talking to herself and occasionally yelling or crying in apparent distress. Ms. A refuses to engage with her treatment team and lies mute in her bed when they attempt to interview her. Her records indicate that previous medication trials have included
Over the next week, Ms. A begins to interact more appropriately with nursing sta
[polldaddy:9945425]
The authors’ observations
As a class, antipsychotics lead to symptom reduction in approximately 70% of patients.1 However, the degree of response can vary markedly between individuals; although some patients may experience almost complete resolution of symptoms, others are still markedly impaired, as in Ms. A’s case.
A substantial amount of literature suggests that although the practice is common, use of >1 antipsychotic does not significantly increase efficacy but increases risk of adverse effects, such as type 2 diabetes mellitus, metabolic syndrome, cognitive impairment, and extrapyramidal symptoms.2-4 One exception is augmentation of clozapine with a second antipsychotic, which in certain cases appears to offer greater efficacy than clozapine alone.1 Practice guidelines and evidence generally do not support the use of multiple antipsychotics, but 20% of patients take >1 antipsychotic.5,6 Although antipsychotic polypharmacy may be appropriate for some patients, current literature suggests it is being done more often than recommended.
Clozapine is considered the most efficacious option for treatment-resistant schizophrenia.7 Because of Ms. A’s history of recurrent hospitalizations, her extensive list of trialed medications, and her ongoing symptoms despite a sufficient trial of haloperidol, the treatment team gives serious consideration to switching Ms. A to clozapine. However, Ms. A is not able to tolerate blood draws without significant support from nursing staff, and it is likely she would be unable to tolerate the frequent blood monitoring required of patients receiving clozapine.
Because many of Ms. A’s symptoms were negative or depressive, including hypersomnia, psychomotor retardation, sadness with frequent crying spells, and reduced interest in activities, adding an antidepressant to Ms. A’s medication regimen was considered. A recent systematic review and meta-analysis showed that adding an antidepressant to an antipsychotic in patients with schizophrenia had small but beneficial effects on depressive and negative symptoms and a low risk of adverse effects.8 However, Ms. A declined this option.
TREATMENT Adding long-acting haloperidol
Ms. A had previously achieved therapeutic blood levels9 with oral haloperidol. Data suggest that compared with the oral form, long-acting injectable antipsychotics can both improve compliance and decrease rehospitalization rates.10-12 Because Ms. A previously had done well with haloperidol decanoate, 200 mg every 2 weeks, achieving a blood level of 16.2 ng/mL, and because she had a partial response to oral haloperidol, we add haloperidol decanoate, 100 mg every 2 weeks, to her regimen, with the intention of transitioning her to all-depot dosing. In addition, the treatment team tries to engage Ms. A in a discussion of potential psychological contributions to her current presentation. They note that Ms. A has her basic needs met on the unit and reports feeling safe there; thus, a fear of discharge may be contributing to her lack of engagement with the team. However, because of her limited communication, it is challenging to investigate this hypothesis or explore other possible psychological issues.
Despite increasing the dosing of haloperidol, Ms. A shows minimal improvement. She continues to stonewall her treatment team, and is unwilling or unable to engage in meaningful conversation. A review of her chart suggests that this hospital course is different from previous ones in which her average stay was a few weeks, and she generally was able to converse with the treatment team, participate in discussions about her care, and make decisions about her desire for discharge.
The team considers if additional factors could be impacting Ms. A’s current presentation. They raise the possibility that she could be going through menopause, and hormonal fluctuations may be contributing to her symptoms. Despite being on the unit for nearly 2 months, Ms. A has not required the use of sanitary products. She also reports to nursing staff that at times she feels flushed and sweaty, but she is afebrile and does not have other signs or symptoms of infection.
[polldaddy:9945428]
The authors’ observations
Evidence suggests that estrogen levels can influence the development and severity of symptoms of schizophrenia (Table 113,14). Rates of schizophrenia are lower in women, and women typically have a later onset of illness with less severe symptoms.13 Women also have a second peak incidence of schizophrenia between ages 45 and 50, corresponding with the hormonal changes associated with menopause and the associated drop in estrogen.14 Symptoms also fluctuate with hormonal cycles—women experience worsening symptoms during the premenstrual phase of the menstrual cycle, when estrogen levels are low, and an improvement of symptoms during high-estrogen phases of the cycle.14 Overall, low levels of estrogen also have been observed in women with schizophrenia relative to controls, although this may be partially attributable to treatment with antipsychotics.14
Estrogen affects various regions of the brain implicated in schizophrenia and likely imparts its behavioral effects through several different mechanisms. Estrogen can act on cells to directly impact intracellular signaling and to alter gene expression.15 Although most often thought of as being related to reproductive functions, estrogen receptors can be found in many cortical and subcortical regions of the brain, such as the hippocampus, substantia nigra, and prefrontal cortex. Estrogen receptor expression levels in certain brain regions have been found to be altered in individuals with schizophrenia.15 Estrogen also enhances neurogenesis and neuroplasticity, playing a role in learning and memory.16 Particularly relevant, estrogen has been shown to directly impact both the dopaminergic and serotonergic systems.15,17 In animal models, estrogen has been shown to decrease the behavioral effects induced by dopamine agonists and decrease symptoms of schizophrenia.18 The underlying molecular mechanisms by which estrogen has these effects are uncertain.
Given estrogen’s potentially protective effects, clinical trials have explored the role of estrogen as an adjuvant to antipsychotics for treating schizophrenia. Studies have shown that estrogen can improve psychotic symptoms in patients with schizophrenia.19,20 However, because estrogen administration can increase the risk of breast and uterine cancer, researchers are instead investigating selective estrogen receptor modulators (SERMs).14 These medications have mixed agonist and antagonist effects, with different effects on different tissues. Raloxifene is a SERM that acts as an estrogen agonist in some tissues, but an antagonist in uterine and breast tissue, which may minimize potential deleterious adverse effects (Table 221-24). Repeated randomized controlled trials have found promising results for use of raloxifene as an adjunctive treatment in peri- and postmenopausal women with schizophrenia, including those refractory to antipsychotic treatment.13,25-27
TREATMENT Address symptoms
The treatment team takes steps to address Ms. A’s perimenopausal symptoms. For mild to moderate hot flashes, primary interventions are nonpharmacologic.28 Because Ms. A primarily reports her hot flashes at night, she is given lightweight pajamas and moved to the coolest room on the unit. Both bring some relief, and her hot flashes appear to be less distressing. The treatment team decides to consult Endocrinology to further investigate the feasibility of starting raloxifene (Table 3) because of their experience using this medication to manage osteoporosis.
[polldaddy:9945429]
The authors’ observations
Raloxifene is FDA-approved for treating osteoporosis and preventing invasive breast cancer.29 Because it is an estrogen antagonist in both breast and uterine tissues, raloxifene does not increase the risk of uterine or breast cancer. Large studies have shown rates of cardiovascular events are similar for raloxifene and placebo, and some studies have found that raloxifene treatment is associated with improvement in cardiovascular risk factors, including lower blood pressure, lower low-density lipoprotein cholesterol, and increased high-density lipoprotein cholesterol.29 Raloxifene does, however, increase risk of venous thromboembolism, including deep vein thrombosis and pulmonary embolism, and fatal stroke.29,30 Overall, the risk remains relatively low, with an absolute risk increase of fatal stroke of 0.7 per 1,000 woman-years (number needed to harm [NNH]: 250) and an absolute risk increase of venous thromboembolic events of 1.88 per 1,000 women-years (NNH: 158).31 However, raloxifene may not be appropriate for patients with independent risk factors for these events. Despite this, a large meta-analysis found a 10% decrease in mortality for patients taking raloxifene compared with those receiving placebo.32 Raloxifene also can cause hot flashes, muscle cramps, and flu-like symptoms.29
Diagnosis of menopause and perimenopause is largely clinical, with hormone testing generally recommended for women age <45 in whom the diagnosis may be unclear.28 Thus, Ms. A’s vasomotor symptoms and absence of a menstrual cycle for at least 2 months were diagnostic of perimenopause; a 12-month cessation in menstrual cycles is required for a diagnosis of menopause.28
OUTCOME Improvement with raloxifene
Because Ms. A is at relatively low risk for a thromboembolism or stroke, the benefit of raloxifene is thought to outweigh the risk, and she is started on raloxifene, 60 mg/d. Over the next 2 weeks, Ms. A becomes increasingly interactive, and is seen sitting at a table talking with other patients on multiple occasions. She spends time looking at fashion magazines, and engages in conversation about fashion with staff and other patients. She participates in group therapy for the first time during this hospital stay and begins to talk about discharge. She occasionally smiles and waves at her treatment team and participates more in the daily interview, although these interactions remain limited and on her terms. She maintains this improvement and is transferred to a psychiatric facility in her home county for ongoing care and discharge planning.
2. Citrome L, Jaffe A, Levine J, et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006-1013.
3. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1-3):91-100.
4. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527-542.
5. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28.
6. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378.
7. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
8. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9);876-886.
9. Ulrich S, Neuhof S, Braun V, et al. Therapeutic window of serum haloperidol concentration in acute schizophrenia and schizoaffective disorder. Pharmacopsychiatry. 1998;31(5):163-169.
10. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655.
11. MacEwan JP, Kamat SA, Duffy RA, et al. Hospital readmission rates among patients with schizophrenia treated with long-acting injectables or oral antipsychotics. Psychiatr Serv. 2016;67(11):1183-1188.
12. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768.
13. Usall J, Huerta-Ramos E, Iniesta R, et al; RALOPSYCAT Group. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72(11):1552-1557.
14. Seeman MV. Treating schizophrenia at the time of menopause. Maturitas. 2012;72(2):117-120.
15. Gogos A, Sbisa AM, Sun J, et al. A role for estrogen in schizophrenia: clinical and preclinical findings. Int J Endocrinol. 2015;2015:615356. doi: 10.1155/2015/615356.
16. Khan MM. Neurocognitive, neuroprotective, and cardiometabolic effects of raloxifene: potential for improving therapeutic outcomes in schizophrenia. CNS Drugs. 2016;30(7):589-601.
17. Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37.
18. Häfner H, Behrens S, De Vry J, et al. An animal model for the effects of estradiol on dopamine-mediated behavior: implications for sex differences in schizophrenia. Psychiatry Res. 1991;38(2):125-134.
19. Akhondzadeh S, Nejatisafa AA, Amini H, et al. Adjunctive estrogen treatment in women with chronic schizophrenia: a double-blind, randomized, and placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):1007-1012.
20. Kulkarni J, de Castella A, Fitzgerald PB, et al. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry. 2008;65(8):955-960.
21. Ellis AJ, Hendrick VM, Williams R, Komm BS. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921-934.
22. Morello KC, Wurz GT, DeGregorio MW. Pharmacokinetics of selective estrogen receptor modulators. Clin pharmacokinet. 2003;42(4):361-372.
23. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014;17(4):490-495.
24. Raloxifene Hydrochloride. Micromedex 2.0. Truven Health Analytics. www.micromedexsolutions.com. Accessed July 24, 2016.
25. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
26. Huerta-Ramos E, Iniesta R, Ochoa S, et al. Effects of raloxifene on cognition in postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 2014;24(2):223-231.
27. Kianimehr G, Fatehi F, Hashempoor S, et al. Raloxifene adjunctive therapy for postmenopausal women suffering from chronic schizophrenia: a randomized double-blind and placebo controlled trial. Daru. 2014;22:55.
28. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975-4011.
29. Ellis AJ, Hendrick VM, Williams R, et al. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921-934.
30. Adomaityte J, Farooq M, Qayyum R. Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis. Thromb Haemost. 2008;99(2):338-342.
31. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014;17(4):490-495.
32. Grady D, Cauley JA, Stock JL, et al. Effect of raloxifene on all-cause mortality. Am J Med. 2010;123(5):469.e1-461.e7.
2. Citrome L, Jaffe A, Levine J, et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006-1013.
3. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1-3):91-100.
4. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527-542.
5. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28.
6. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378.
7. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
8. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9);876-886.
9. Ulrich S, Neuhof S, Braun V, et al. Therapeutic window of serum haloperidol concentration in acute schizophrenia and schizoaffective disorder. Pharmacopsychiatry. 1998;31(5):163-169.
10. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655.
11. MacEwan JP, Kamat SA, Duffy RA, et al. Hospital readmission rates among patients with schizophrenia treated with long-acting injectables or oral antipsychotics. Psychiatr Serv. 2016;67(11):1183-1188.
12. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768.
13. Usall J, Huerta-Ramos E, Iniesta R, et al; RALOPSYCAT Group. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72(11):1552-1557.
14. Seeman MV. Treating schizophrenia at the time of menopause. Maturitas. 2012;72(2):117-120.
15. Gogos A, Sbisa AM, Sun J, et al. A role for estrogen in schizophrenia: clinical and preclinical findings. Int J Endocrinol. 2015;2015:615356. doi: 10.1155/2015/615356.
16. Khan MM. Neurocognitive, neuroprotective, and cardiometabolic effects of raloxifene: potential for improving therapeutic outcomes in schizophrenia. CNS Drugs. 2016;30(7):589-601.
17. Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37.
18. Häfner H, Behrens S, De Vry J, et al. An animal model for the effects of estradiol on dopamine-mediated behavior: implications for sex differences in schizophrenia. Psychiatry Res. 1991;38(2):125-134.
19. Akhondzadeh S, Nejatisafa AA, Amini H, et al. Adjunctive estrogen treatment in women with chronic schizophrenia: a double-blind, randomized, and placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):1007-1012.
20. Kulkarni J, de Castella A, Fitzgerald PB, et al. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry. 2008;65(8):955-960.
21. Ellis AJ, Hendrick VM, Williams R, Komm BS. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921-934.
22. Morello KC, Wurz GT, DeGregorio MW. Pharmacokinetics of selective estrogen receptor modulators. Clin pharmacokinet. 2003;42(4):361-372.
23. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014;17(4):490-495.
24. Raloxifene Hydrochloride. Micromedex 2.0. Truven Health Analytics. www.micromedexsolutions.com. Accessed July 24, 2016.
25. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
26. Huerta-Ramos E, Iniesta R, Ochoa S, et al. Effects of raloxifene on cognition in postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 2014;24(2):223-231.
27. Kianimehr G, Fatehi F, Hashempoor S, et al. Raloxifene adjunctive therapy for postmenopausal women suffering from chronic schizophrenia: a randomized double-blind and placebo controlled trial. Daru. 2014;22:55.
28. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975-4011.
29. Ellis AJ, Hendrick VM, Williams R, et al. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921-934.
30. Adomaityte J, Farooq M, Qayyum R. Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis. Thromb Haemost. 2008;99(2):338-342.
31. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014;17(4):490-495.
32. Grady D, Cauley JA, Stock JL, et al. Effect of raloxifene on all-cause mortality. Am J Med. 2010;123(5):469.e1-461.e7.
Rapid weight loss, irritability, and nausea after restarting ADHD treatment
CASE Medication management
Mr. L, age 58, presents to the outpatient psychiatric clinic seeking treatment for attention-deficit/hyperactivity disorder (ADHD), which was first diagnosed 11 years ago. Since discontinuing his ADHD medication, lisdexamfetamine 60 mg/d, 8 months ago, he has not been completing tasks and has been distracted in his job as a limousine driver. Mr. L says that when he was taking the medication, “I could focus and prioritize.” He reports that he has trouble retaining information and is easily distracted. He says he generally is organized with appointments and keeping track of things but is messy, forgetful, tardy, and impatient. Procrastination is an ongoing problem. He denies misplacing things or being impulsive. Mr. L reports that as a child he was frequently reprimanded for talking in class. He states, “I get in trouble even now for talking too much.”
Mr. L is cooperative and polite, maintains good eye contact, and is alert. No psychomotor abnormalities are noted. His speech is spontaneous and coherent, with normal rate, rhythm, and volume. He reports that his mood is “all right,” and denies suicidal or homicidal ideation. His insight is full, judgment is intact, and thought is linear and logical. Mr. L sleeps 5 hours at night and takes a nap during the day, but his energy varies.
His psychiatric history is negative for suicide attempts or hospitalizations. Mr. L denies a history of major depressive episodes, manic symptoms, hallucinations, or delusions. Anxiety history is negative for excessive worrying, obsessions and compulsions, and panic attacks. Mr. L has no family history of mental illness or substance abuse, and he denies any personal history of drug use. He stopped using tobacco 14 years ago. Mr. L says he drinks 3 caffeinated drinks a day and 2 glasses of wine once a week. Previous medications included
A review of systems is negative. Vital signs are unremarkable. A recent electrocardiogram (EKG) showed normal sinus rhythm. Thyroid-stimulating hormone, comprehensive metabolic panel (CMP), lipids, iron, vitamin B12, folate, complete blood count (CBC), hemoglobin A1c, and urine analysis are normal, except for mildly elevated low-density lipoprotein. Testing for hepatitis C is negative.
The previous diagnosis of ADHD is confirmed, and Mr. L is started on
[polldaddy:9928295]
The author’s observations
Anxiety, irritability, agitation, and palpitations can all be symptoms of stimulant medications.1,2 There are numerous other iatrogenic causes, including steroid-based asthma treatments, thyroid medications, antidepressants in bipolar patients, and caffeine-based migraine treatments. Mr. L’s theory that his 15-lb weight loss was the result of his methylphenidate ER dose being too high was a reasonable one. Often, medication doses need to be adjusted with weight changes. His decrease in energy during the day could be explained by the methylphenidate ER controlling his hyperactive symptoms, which include high energy. At night, when the medication wears off, his hyperactivity symptoms could be returning, which would account for the increase in energy when he gets home from work. Although longer-acting stimulants tend to have a more benign adverse effects profile, they can cause insomnia if they are still in the patient’s system at bedtime. Shorter-acting stimulants wear off quickly but can be advantageous for patients who want to target concentration during certain times of day, such as for school and homework.
TREATMENT A surprising cause
The next month, Mr. L presents to the emergency room complaining of jitteriness, headache, and tingling in his fingers, and is evaluated for suspected carbon monoxide (CO) poisoning. Three months earlier, he had noted the odor of exhaust fumes in the limousine he drives 7 days a week. He took it to the mechanic twice for evaluation, but no cause was found. Despite his concerns, he continued to drive the car until an older client, in frail health, suddenly became short of breath and developed chest pain shortly after entering his vehicle, on a day when the odor was particularly bad. Before that, a family of passengers had complained of headaches upon entering his vehicle. The third time he brought his car to be checked, the mechanic identified an exhaust system leak.
[polldaddy:9928298]
The author’s observations
Work-up for suspected CO poisoning includes ABG, COHb level, CBC, basic metabolic panel, EKG, cardiac enzymes, and chest radiography, as well as other laboratory tests as deemed appropriate. Treatment includes oxygen by mask for low-level poisoning.
High levels of poisoning may require hyperbaric oxygen, which should be considered for patients who are unconscious or have an abnormal score on the Carbon Monoxide Neuropsychological Screening Battery, COHb of >40%, signs of cardiac ischemia or arrhythmia, history of ischemic heart disease with COHb level >20%, recurrent symptoms for up to 3 weeks, or symptoms that have not resolved with normobaric oxygen after 4 to 6 hours.9 Any pregnant woman with CO poisoning should receive hyperbaric therapy.10
OUTCOME Lasting improvement
Mr. L presents for follow-up in the psychiatric clinic 3 weeks after his emergency room visit. After his limousine was repaired, his symptoms resolved. He no longer experiences fatigue during the day with higher energy at night, palpitations, jitteriness, headache, or tingling. His concentration has improved, so he opts to stick with the 18-mg dose of methylphenidate ER rather than increase it to the initial dose. He places a CO detector in his vehicle, which proves to be a good decision when it gives him a warning that the exhaust leak had not been properly repaired.
[polldaddy:9928299]
The author’s observations
Although the correct cause of Mr. L’s symptoms was found incidentally, this case is an important reminder to always consider medical causes in the differential diagnosis. We are taught in medical school to look first for horses (more likely causes), not zebras (less likely causes), but sometimes zebras do occur. Be mindful that medical causes should be considered not only for symptoms of primary illnesses, but also for symptoms thought to be caused by adverse effects of medications. The differential diagnosis for Mr. L’s symptoms (palpitations, agitation, anxiety, irritability, weight loss, fatigue, nausea, and headache) included metabolic and endocrine abnormalities (thyroid disease, pheochromocytoma, hypoglycemia); psychiatric conditions (panic, bipolar disorder, depression); substance abuse (caffeine, cocaine, amphetamines); immune disorders; cardiac disorders; malignancy; toxic exposure; infectious sources; and nutritional deficiencies. CO poisoning can cause many of these symptoms (Table 2).1,2,8
Intentional CO poisoning should be considered in an obtunded or unconscious patient with depression. Patients may consider CO poisoning a more peaceful way to complete suicide than shooting, cutting, or hanging. As for unintentional poisoning, clinical suspicion can be increased by time of year, occupation, locale, and smoking status. Winter months increase risk because of the high use of heating devices, cars warming up in the garage, closed fireplace flues, and vehicle tailpipes blocked by snow. As in Mr. L’s case, occupation also may increase suspicion; drivers, mechanics, tollbooth operators, parking attendants, miners, and firefighters are all at increased risk for CO poisoning. Regarding locale, polluted urban environments as well as cold climates requiring heating sources cause higher risks for CO exposure. Rarely, excessive smoking can result in CO poisoning. The author once had a patient with schizophrenia who was admitted to the hospital with delirium. It was determined that he had CO poisoning from his 5-pack-a-day smoking habit.
Psychiatric patients often have the frustrating experience of their physical symptoms being attributed to psychiatric causes, which results in major medical issues being overlooked. We psychiatrists can fall into the same trap of overlooking medical illnesses, as indicated in this case, where Mr. L’s CO poisoning initially was attributed to adverse effects of his psychiatric medication.
1. Drugs.com. Amphetamine side effects. https://www.drugs.com/sfx/amphetamine-side-effects.html. Accessed December 7, 2017.
2. Golmirzaei J, Mahboobi H, Yazdanparast M, et al. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590-594.
3. Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol. 2015;131(3):191-203.
4. Carter D. Carbon monoxide: the forgotten killer. http://scot.nhs.uk/sehd/cmo/CMO(1998)19.pdf. Published September 7, 1998. Accessed January 10, 2018.
5. Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187-1195.
6. AA1Car. Troubleshoot odors & smells inside your car. http://www.aa1car.com/library/troubleshoot_odors.htm. Accessed December 7, 2017.
7. Rodkey FL, O’Neal JD, Collison HA, et al. Relative affinity of hemoglobin S and hemoglobin A for carbon monoxide and oxygen. Clin Chem. 1974;20(1):83-84.
8. Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med. 1987;146(1):52-56.
9. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603-1608.
10. Guzman JA. Carbon monoxide poisoning. Critical Care Clin. 2012;28(4):537-548.
CASE Medication management
Mr. L, age 58, presents to the outpatient psychiatric clinic seeking treatment for attention-deficit/hyperactivity disorder (ADHD), which was first diagnosed 11 years ago. Since discontinuing his ADHD medication, lisdexamfetamine 60 mg/d, 8 months ago, he has not been completing tasks and has been distracted in his job as a limousine driver. Mr. L says that when he was taking the medication, “I could focus and prioritize.” He reports that he has trouble retaining information and is easily distracted. He says he generally is organized with appointments and keeping track of things but is messy, forgetful, tardy, and impatient. Procrastination is an ongoing problem. He denies misplacing things or being impulsive. Mr. L reports that as a child he was frequently reprimanded for talking in class. He states, “I get in trouble even now for talking too much.”
Mr. L is cooperative and polite, maintains good eye contact, and is alert. No psychomotor abnormalities are noted. His speech is spontaneous and coherent, with normal rate, rhythm, and volume. He reports that his mood is “all right,” and denies suicidal or homicidal ideation. His insight is full, judgment is intact, and thought is linear and logical. Mr. L sleeps 5 hours at night and takes a nap during the day, but his energy varies.
His psychiatric history is negative for suicide attempts or hospitalizations. Mr. L denies a history of major depressive episodes, manic symptoms, hallucinations, or delusions. Anxiety history is negative for excessive worrying, obsessions and compulsions, and panic attacks. Mr. L has no family history of mental illness or substance abuse, and he denies any personal history of drug use. He stopped using tobacco 14 years ago. Mr. L says he drinks 3 caffeinated drinks a day and 2 glasses of wine once a week. Previous medications included
A review of systems is negative. Vital signs are unremarkable. A recent electrocardiogram (EKG) showed normal sinus rhythm. Thyroid-stimulating hormone, comprehensive metabolic panel (CMP), lipids, iron, vitamin B12, folate, complete blood count (CBC), hemoglobin A1c, and urine analysis are normal, except for mildly elevated low-density lipoprotein. Testing for hepatitis C is negative.
The previous diagnosis of ADHD is confirmed, and Mr. L is started on
[polldaddy:9928295]
The author’s observations
Anxiety, irritability, agitation, and palpitations can all be symptoms of stimulant medications.1,2 There are numerous other iatrogenic causes, including steroid-based asthma treatments, thyroid medications, antidepressants in bipolar patients, and caffeine-based migraine treatments. Mr. L’s theory that his 15-lb weight loss was the result of his methylphenidate ER dose being too high was a reasonable one. Often, medication doses need to be adjusted with weight changes. His decrease in energy during the day could be explained by the methylphenidate ER controlling his hyperactive symptoms, which include high energy. At night, when the medication wears off, his hyperactivity symptoms could be returning, which would account for the increase in energy when he gets home from work. Although longer-acting stimulants tend to have a more benign adverse effects profile, they can cause insomnia if they are still in the patient’s system at bedtime. Shorter-acting stimulants wear off quickly but can be advantageous for patients who want to target concentration during certain times of day, such as for school and homework.
TREATMENT A surprising cause
The next month, Mr. L presents to the emergency room complaining of jitteriness, headache, and tingling in his fingers, and is evaluated for suspected carbon monoxide (CO) poisoning. Three months earlier, he had noted the odor of exhaust fumes in the limousine he drives 7 days a week. He took it to the mechanic twice for evaluation, but no cause was found. Despite his concerns, he continued to drive the car until an older client, in frail health, suddenly became short of breath and developed chest pain shortly after entering his vehicle, on a day when the odor was particularly bad. Before that, a family of passengers had complained of headaches upon entering his vehicle. The third time he brought his car to be checked, the mechanic identified an exhaust system leak.
[polldaddy:9928298]
The author’s observations
Work-up for suspected CO poisoning includes ABG, COHb level, CBC, basic metabolic panel, EKG, cardiac enzymes, and chest radiography, as well as other laboratory tests as deemed appropriate. Treatment includes oxygen by mask for low-level poisoning.
High levels of poisoning may require hyperbaric oxygen, which should be considered for patients who are unconscious or have an abnormal score on the Carbon Monoxide Neuropsychological Screening Battery, COHb of >40%, signs of cardiac ischemia or arrhythmia, history of ischemic heart disease with COHb level >20%, recurrent symptoms for up to 3 weeks, or symptoms that have not resolved with normobaric oxygen after 4 to 6 hours.9 Any pregnant woman with CO poisoning should receive hyperbaric therapy.10
OUTCOME Lasting improvement
Mr. L presents for follow-up in the psychiatric clinic 3 weeks after his emergency room visit. After his limousine was repaired, his symptoms resolved. He no longer experiences fatigue during the day with higher energy at night, palpitations, jitteriness, headache, or tingling. His concentration has improved, so he opts to stick with the 18-mg dose of methylphenidate ER rather than increase it to the initial dose. He places a CO detector in his vehicle, which proves to be a good decision when it gives him a warning that the exhaust leak had not been properly repaired.
[polldaddy:9928299]
The author’s observations
Although the correct cause of Mr. L’s symptoms was found incidentally, this case is an important reminder to always consider medical causes in the differential diagnosis. We are taught in medical school to look first for horses (more likely causes), not zebras (less likely causes), but sometimes zebras do occur. Be mindful that medical causes should be considered not only for symptoms of primary illnesses, but also for symptoms thought to be caused by adverse effects of medications. The differential diagnosis for Mr. L’s symptoms (palpitations, agitation, anxiety, irritability, weight loss, fatigue, nausea, and headache) included metabolic and endocrine abnormalities (thyroid disease, pheochromocytoma, hypoglycemia); psychiatric conditions (panic, bipolar disorder, depression); substance abuse (caffeine, cocaine, amphetamines); immune disorders; cardiac disorders; malignancy; toxic exposure; infectious sources; and nutritional deficiencies. CO poisoning can cause many of these symptoms (Table 2).1,2,8
Intentional CO poisoning should be considered in an obtunded or unconscious patient with depression. Patients may consider CO poisoning a more peaceful way to complete suicide than shooting, cutting, or hanging. As for unintentional poisoning, clinical suspicion can be increased by time of year, occupation, locale, and smoking status. Winter months increase risk because of the high use of heating devices, cars warming up in the garage, closed fireplace flues, and vehicle tailpipes blocked by snow. As in Mr. L’s case, occupation also may increase suspicion; drivers, mechanics, tollbooth operators, parking attendants, miners, and firefighters are all at increased risk for CO poisoning. Regarding locale, polluted urban environments as well as cold climates requiring heating sources cause higher risks for CO exposure. Rarely, excessive smoking can result in CO poisoning. The author once had a patient with schizophrenia who was admitted to the hospital with delirium. It was determined that he had CO poisoning from his 5-pack-a-day smoking habit.
Psychiatric patients often have the frustrating experience of their physical symptoms being attributed to psychiatric causes, which results in major medical issues being overlooked. We psychiatrists can fall into the same trap of overlooking medical illnesses, as indicated in this case, where Mr. L’s CO poisoning initially was attributed to adverse effects of his psychiatric medication.
CASE Medication management
Mr. L, age 58, presents to the outpatient psychiatric clinic seeking treatment for attention-deficit/hyperactivity disorder (ADHD), which was first diagnosed 11 years ago. Since discontinuing his ADHD medication, lisdexamfetamine 60 mg/d, 8 months ago, he has not been completing tasks and has been distracted in his job as a limousine driver. Mr. L says that when he was taking the medication, “I could focus and prioritize.” He reports that he has trouble retaining information and is easily distracted. He says he generally is organized with appointments and keeping track of things but is messy, forgetful, tardy, and impatient. Procrastination is an ongoing problem. He denies misplacing things or being impulsive. Mr. L reports that as a child he was frequently reprimanded for talking in class. He states, “I get in trouble even now for talking too much.”
Mr. L is cooperative and polite, maintains good eye contact, and is alert. No psychomotor abnormalities are noted. His speech is spontaneous and coherent, with normal rate, rhythm, and volume. He reports that his mood is “all right,” and denies suicidal or homicidal ideation. His insight is full, judgment is intact, and thought is linear and logical. Mr. L sleeps 5 hours at night and takes a nap during the day, but his energy varies.
His psychiatric history is negative for suicide attempts or hospitalizations. Mr. L denies a history of major depressive episodes, manic symptoms, hallucinations, or delusions. Anxiety history is negative for excessive worrying, obsessions and compulsions, and panic attacks. Mr. L has no family history of mental illness or substance abuse, and he denies any personal history of drug use. He stopped using tobacco 14 years ago. Mr. L says he drinks 3 caffeinated drinks a day and 2 glasses of wine once a week. Previous medications included
A review of systems is negative. Vital signs are unremarkable. A recent electrocardiogram (EKG) showed normal sinus rhythm. Thyroid-stimulating hormone, comprehensive metabolic panel (CMP), lipids, iron, vitamin B12, folate, complete blood count (CBC), hemoglobin A1c, and urine analysis are normal, except for mildly elevated low-density lipoprotein. Testing for hepatitis C is negative.
The previous diagnosis of ADHD is confirmed, and Mr. L is started on
[polldaddy:9928295]
The author’s observations
Anxiety, irritability, agitation, and palpitations can all be symptoms of stimulant medications.1,2 There are numerous other iatrogenic causes, including steroid-based asthma treatments, thyroid medications, antidepressants in bipolar patients, and caffeine-based migraine treatments. Mr. L’s theory that his 15-lb weight loss was the result of his methylphenidate ER dose being too high was a reasonable one. Often, medication doses need to be adjusted with weight changes. His decrease in energy during the day could be explained by the methylphenidate ER controlling his hyperactive symptoms, which include high energy. At night, when the medication wears off, his hyperactivity symptoms could be returning, which would account for the increase in energy when he gets home from work. Although longer-acting stimulants tend to have a more benign adverse effects profile, they can cause insomnia if they are still in the patient’s system at bedtime. Shorter-acting stimulants wear off quickly but can be advantageous for patients who want to target concentration during certain times of day, such as for school and homework.
TREATMENT A surprising cause
The next month, Mr. L presents to the emergency room complaining of jitteriness, headache, and tingling in his fingers, and is evaluated for suspected carbon monoxide (CO) poisoning. Three months earlier, he had noted the odor of exhaust fumes in the limousine he drives 7 days a week. He took it to the mechanic twice for evaluation, but no cause was found. Despite his concerns, he continued to drive the car until an older client, in frail health, suddenly became short of breath and developed chest pain shortly after entering his vehicle, on a day when the odor was particularly bad. Before that, a family of passengers had complained of headaches upon entering his vehicle. The third time he brought his car to be checked, the mechanic identified an exhaust system leak.
[polldaddy:9928298]
The author’s observations
Work-up for suspected CO poisoning includes ABG, COHb level, CBC, basic metabolic panel, EKG, cardiac enzymes, and chest radiography, as well as other laboratory tests as deemed appropriate. Treatment includes oxygen by mask for low-level poisoning.
High levels of poisoning may require hyperbaric oxygen, which should be considered for patients who are unconscious or have an abnormal score on the Carbon Monoxide Neuropsychological Screening Battery, COHb of >40%, signs of cardiac ischemia or arrhythmia, history of ischemic heart disease with COHb level >20%, recurrent symptoms for up to 3 weeks, or symptoms that have not resolved with normobaric oxygen after 4 to 6 hours.9 Any pregnant woman with CO poisoning should receive hyperbaric therapy.10
OUTCOME Lasting improvement
Mr. L presents for follow-up in the psychiatric clinic 3 weeks after his emergency room visit. After his limousine was repaired, his symptoms resolved. He no longer experiences fatigue during the day with higher energy at night, palpitations, jitteriness, headache, or tingling. His concentration has improved, so he opts to stick with the 18-mg dose of methylphenidate ER rather than increase it to the initial dose. He places a CO detector in his vehicle, which proves to be a good decision when it gives him a warning that the exhaust leak had not been properly repaired.
[polldaddy:9928299]
The author’s observations
Although the correct cause of Mr. L’s symptoms was found incidentally, this case is an important reminder to always consider medical causes in the differential diagnosis. We are taught in medical school to look first for horses (more likely causes), not zebras (less likely causes), but sometimes zebras do occur. Be mindful that medical causes should be considered not only for symptoms of primary illnesses, but also for symptoms thought to be caused by adverse effects of medications. The differential diagnosis for Mr. L’s symptoms (palpitations, agitation, anxiety, irritability, weight loss, fatigue, nausea, and headache) included metabolic and endocrine abnormalities (thyroid disease, pheochromocytoma, hypoglycemia); psychiatric conditions (panic, bipolar disorder, depression); substance abuse (caffeine, cocaine, amphetamines); immune disorders; cardiac disorders; malignancy; toxic exposure; infectious sources; and nutritional deficiencies. CO poisoning can cause many of these symptoms (Table 2).1,2,8
Intentional CO poisoning should be considered in an obtunded or unconscious patient with depression. Patients may consider CO poisoning a more peaceful way to complete suicide than shooting, cutting, or hanging. As for unintentional poisoning, clinical suspicion can be increased by time of year, occupation, locale, and smoking status. Winter months increase risk because of the high use of heating devices, cars warming up in the garage, closed fireplace flues, and vehicle tailpipes blocked by snow. As in Mr. L’s case, occupation also may increase suspicion; drivers, mechanics, tollbooth operators, parking attendants, miners, and firefighters are all at increased risk for CO poisoning. Regarding locale, polluted urban environments as well as cold climates requiring heating sources cause higher risks for CO exposure. Rarely, excessive smoking can result in CO poisoning. The author once had a patient with schizophrenia who was admitted to the hospital with delirium. It was determined that he had CO poisoning from his 5-pack-a-day smoking habit.
Psychiatric patients often have the frustrating experience of their physical symptoms being attributed to psychiatric causes, which results in major medical issues being overlooked. We psychiatrists can fall into the same trap of overlooking medical illnesses, as indicated in this case, where Mr. L’s CO poisoning initially was attributed to adverse effects of his psychiatric medication.
1. Drugs.com. Amphetamine side effects. https://www.drugs.com/sfx/amphetamine-side-effects.html. Accessed December 7, 2017.
2. Golmirzaei J, Mahboobi H, Yazdanparast M, et al. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590-594.
3. Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol. 2015;131(3):191-203.
4. Carter D. Carbon monoxide: the forgotten killer. http://scot.nhs.uk/sehd/cmo/CMO(1998)19.pdf. Published September 7, 1998. Accessed January 10, 2018.
5. Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187-1195.
6. AA1Car. Troubleshoot odors & smells inside your car. http://www.aa1car.com/library/troubleshoot_odors.htm. Accessed December 7, 2017.
7. Rodkey FL, O’Neal JD, Collison HA, et al. Relative affinity of hemoglobin S and hemoglobin A for carbon monoxide and oxygen. Clin Chem. 1974;20(1):83-84.
8. Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med. 1987;146(1):52-56.
9. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603-1608.
10. Guzman JA. Carbon monoxide poisoning. Critical Care Clin. 2012;28(4):537-548.
1. Drugs.com. Amphetamine side effects. https://www.drugs.com/sfx/amphetamine-side-effects.html. Accessed December 7, 2017.
2. Golmirzaei J, Mahboobi H, Yazdanparast M, et al. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590-594.
3. Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol. 2015;131(3):191-203.
4. Carter D. Carbon monoxide: the forgotten killer. http://scot.nhs.uk/sehd/cmo/CMO(1998)19.pdf. Published September 7, 1998. Accessed January 10, 2018.
5. Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187-1195.
6. AA1Car. Troubleshoot odors & smells inside your car. http://www.aa1car.com/library/troubleshoot_odors.htm. Accessed December 7, 2017.
7. Rodkey FL, O’Neal JD, Collison HA, et al. Relative affinity of hemoglobin S and hemoglobin A for carbon monoxide and oxygen. Clin Chem. 1974;20(1):83-84.
8. Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med. 1987;146(1):52-56.
9. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603-1608.
10. Guzman JA. Carbon monoxide poisoning. Critical Care Clin. 2012;28(4):537-548.
A 95-year-old man with treatment-resistant depression
CASE Depressed, avoidant
Mr. R, age 95, has a history of recurrent major depressive disorder. He presents to the emergency department with depressive symptoms that began 6 weeks ago. His symptoms include depressed mood, hopelessness, anhedonia, anxiety, and insomnia. Co-occurring anorexia nervosa has resulted in a 20-lb weight loss. He denies suicidal ideation.
A mental status examination reveals profound psychomotor agitation, dysphoric mood, tearfulness, and mood-congruent delusions. Mr. R’s Mini-Mental State Examination (MMSE) score is 14/30; his Hamilton Depression Rating Scale (HAM-D) score is 21, indicating severe depression (19 to 22). However, the examiner feels that these scores may not reflect an accurate assessment because Mr. R gave flippant responses and did not cooperate during the interview. Physical examination is unremarkable. Previous medication trials included buspirone, escitalopram, and risperidone; none of these medications successfully alleviated his depressive symptoms.
On admission, Mr. R is given oral mirtazapine, 15 mg/d, and quetiapine, 25 mg/d, to target depressive mood, insomnia, and weight loss. Urgent intervention is indicated because his depressive symptoms are profoundly causing failure to thrive and are compromising his physical health. Mr. R’s deterioration concerns the physician team. Because of a history of failed pharmacotherapy trials, the team reassesses Mr. R’s treatment options.
[polldaddy:9903171]
The authors’ observations
The physician team recommends that Mr. R undergo ECT to obtain rapid relief from his depressive symptoms. After discussion of the potential risks and benefits, Mr. R agrees to this treatment. Quetiapine is discontinued prior to initiating ECT to avoid unnecessary medications; mirtazapine is continued.
Mr. R’s lack of response to previous antidepressants and significant deterioration were concerning. The physicians wanted to avoid higher-dose medications because of the risk of falls or somnolence. Their clinical experience and the literature supporting ECT for patients of Mr. R’s age lead them to select ECT as the most appropriate therapeutic option.
ECT has no absolute contraindications.1 The rate of ECT use in the United States has fluctuated over time because of factors unrelated to the efficacy and availability of ECT or alternative treatments.2 This form of intervention is also somewhat stigmatized.
Some psychiatrists are reluctant to prescribe ECT for geriatric patients because of concerns of potential neurocognitive or medical complications and risks during anesthesia. However, in the United States, older patients with depression are more likely to be treated with ECT than their younger counterparts.3 ECT usually induces greater immediate efficacy than antidepressants.4
Evidence supports using ECT in older patients
Multiple studies have found that ECT is a rapid, safe, and efficacious intervention for treating older persons with depression. Patients age >60 who receive ECT plus pharmacotherapy have lower HAM-D scores than those receiving pharmacotherapy alone.5 Overall, the rates of remission for depression range from 50% to 70%; yet geriatric patients who receive only ECT have response rates around 90%.6 Older age, presence of psychotic symptoms, and shorter duration of illness can predict a rapidly positive ECT response.7
When treated with ECT, older patients, including those age >85, have fewer subsequent episodes of depression compared with those who receive pharmacotherapy alone.1 Older individuals with physical illness or cognitive impairment respond to and tolerate ECT much like younger patients.6 Older patients receiving ECT may experience less cognitive decline than younger ones.7 Those in their ninth decade of life with treatment-resistant depression, psychotic features, and post-stroke depression often respond robustly with improvement following ECT.8
Remission rates also depend on the technique of administration. Interactions between electrode placement and stimulus parameter dosage affect efficacy and adverse effects.9 Right-sided, unilateral ECT induces less cognitive dysfunction compared with bilateral electrode placement,9 but bilateral ECT is more clinically effective.10 However, the efficacy of right-sided ECT is more dose-sensitive, and some data suggest that suboptimal response is due to insufficient stimulus dosages.11 One double-blind randomized controlled trial documented that when using a high-dose stimulus parameter, unilateral ECT is as effective as bilateral ECT.12 When there is a suboptimal response to unilateral ECT, bilateral ECT might be beneficial.12,13 For preventing relapse in older patients, increasing the interval between ECT treatments is more effective than stopping ECT abruptly.13
[polldaddy:9903172]
Indications of ECT
ECT is indicated for patients with severe depression, mania, and other conditions (Table).14 The most common indication for ECT in older persons is a history of treatment-resistant depression, with melancholia, psychosis, or suicidal ideations.1-6,12 There are also age-related and clinical factors to consider with ECT. This treatment provides a safe, rapid remission for patients age >65, even after adjusting for somatic conditions, duration of illness, medication resistance, or case severity.15 Compared with younger patients, older adults may not tolerate antidepressants as well because of age-related pharmacokinetic alterations, including increased sensitivity to anticholinergic and/or hypotensive effects.1
Factors that favor ECT include a previous good response to it; patient preference; and an indication for rapid intervention, such as suicidality, catatonia, dehydration, malnutrition, or a suboptimal result from pharmacotherapy.3 Mortality among individuals age >85 who receive ECT reportedly is lower than that among their counterparts who receive alternative treatments.16 ECT has been administered safely and effectively in patients with comorbid medical illnesses such as stroke, cerebral aneurysm, cardiovascular disease with ischemia or arrhythmia, dementia, and osteoporosis.17
Neurocognitive effects
Reports on the effects of ECT on neurocognitive functioning have varied. In some studies, performance improved or did not change in severely depressed older patients who received ECT.18,19 In older people who receive ECT, MMSE scores often return to baseline by the end of treatment.20 There often is only mild transient cognitive impairment in patients with late-life depression who receive ECT. Areas of concern include attention span, orientation, and speed of mental processing.20 Physicians should conduct cognitive tests before, during, and after ECT sessions to monitor their patient’s mental status.20
Cognitive stability can be maintained by administering ECT twice a week; applying right-sided, unilateral electrode placement; and using short, ultra-brief stimulus pulse width parameters.21 Cognitive impairment induced by ECT is not associated with age in geriatric patients with depression.22 Older adults who experienced longer postictal reorientation time periods have better outcomes than others who reach orientation faster; their intellectual impairment returned to baseline.20 Falling is another complication associated with ECT. A longitudinal cohort study found the incident of falls among patients receiving ECT was 13%.22 Risk factors for falls during a course of ECT include the number of treatments and the presence of coexisting Parkinson’s disease.23
OUTCOME Improvement
Mr. R receives 8 sessions of right-sided, unilateral ECT with an individualized dosage titration method. Treatments are completed with a stimulus intensity at 6 times seizure threshold, with an ultra-brief pulse width at 0.3 milliseconds. Mr. R’s mood and affect begin to improve after 3 ECT sessions. His MMSE score increases to 28/30 (Figure). His clinical improvement is progressively sustained; he develops an increasingly jovial attitude and experiences less anxiety. Mr. R’s confidence, appetite, and sleep also improve. There are no complications with treatment, and Mr. R has no complaints. After 8 ECT sessions, Mr. R has no affective symptoms and does not experience any cognitive impairment.
The authors’ observations
Depression among older people is a growing public health concern. It is a leading cause of disability, and often leads to nursing home placement.24 ECT is a safe, effective treatment for late-life depression, but is underutilized in patients age >75 because of concerns for cognitive impairment.6 However, there is evidence that response rates to ECT are higher in patients ages 45 to 85, compared with young individuals ages 18 to 45.25 ECT is a viable intervention for older depressed patients, particularly for those who do not tolerate or fail to respond to pharmacotherapy. Many of these patients are at risk for drug-induced toxicities or interactions or suicide.1
1. Kerner N, Prudic J. Current electroconvulsive therapy practice and research in the geriatric population. Neuropsychiatry (London). 2014;4(1):33-54.
2. Dombrovski AY, Mulsant BH. The evidence for electroconvulsive therapy (ECT) in the treatment of severe late-life depression. ECT: the preferred treatment for severe depression in late life. Int Psychogeriatr. 2007;19(1):10-14,27-35; discussion 24-26.
3. Olfson M, Marcus S, Sackeim HA, et al. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155(1):22-29.
4. Salzman C, Wong E, Wright BC. Drug and ECT treatment of depression in the elderly, 1996-2001: a literature review. Biol Psychiatry. 2002;52(3):265-284.
5. Kellner CH, Husain MM, Knapp RG, et al; CORE/PRIDE Work Group. A novel strategy for continuation ect in geriatric depression: phase 2 of the PRIDE study. A
6. Tew JD Jr, Mulsant BH, Haskett RF, et al. Acute efficacy of ECT in the treatment of major depression in the old-old. Am J Psychiatry. 1999;156(12):1865-1870.
7. Dombrovski AY, Mulsant BH, Haskett RF, et al. Predictors of remission after electroconvulsive therapy in unipolar major depression. J Clin Psychiatry. 2005;66(8):1043-1049.
8. Charles K. UpToDate. Unipolar major depression in adults: indications for efficacy of electroconvulsive therapy (ECT). https://www.uptodate.com/contents/unipolar-major-depression-in-adults-indications-for-and-efficacy-of-electroconvulsive-therapy-ect. Updated May 16, 2017. Accessed November 26, 2017.
9. Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425-434.
10. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
11. Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939-1945.
12. Stoppe A, Louzã M, Rosa M, et al. Fixed high dose electroconvulsive therapy in elderly with depression: a double-blind, randomized comparison of efficacy and tolerability between unilateral and bilateral electrode placement. J ECT. 2006;22(2):92-99.
13. Geduldig ET, Kellner CH. Electroconvulsive therapy in the elderly: new findings in geriatric depression. Curr Psychiatry Rep. 2016;18(4):40.
14. Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am J Psychiatry. 2000;157(suppl 4):1-45.
15. Rhebergen D, Huisman A, Bouckaert F, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. 2015;23(3):274-282.
16. Philibert RA, Richards L, Lynch CF, et al. Effect of ECT on mortality and clinical outcome in geriatric unipolar depression. J Clin Psychiatry. 1995;56(9):390-394.
17. Tomac TA, Rummans TA, Pileggi TS, et al. Safety and efficacy of electroconvulsive therapy in patients over age 85. Am J Geriatr Psychiatry. 1997;5(2):126-130.
18. Verwijk E, Comijs HC, Kok RM, et al. Short and long-term neurocognitive functioning after electroconvulsive therapy in depressed elderly: a prospective naturalistic study. Int Psychogeriatr. 2014;26(2):315-324.
19. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late life depression. Can J Psychiatry. 2002;47(8):734-741.
20. Bjolseth TM, Engedal K, Benth JS, et al. Speed of recovery from disorientation may predict the treatment outcome of electroconvulsive therapy (ECT) in elderly patients with major depression. J Affect Disord. 2016;190:178-186.
21. Sackeim HA, Prudic J, Nobler MS, et al. Ultra-brief pulse ECT and the affective and cognitive consequences of ECT. J ECT. 2001;17(1):77.
22. Bjolseth TM, Engedal K, Benth JS, et al. Baseline cognitive function does not predict the treatment outcome of electroconvulsive therapy (ECT) in late-life depression. J Affect Disord. 2015;185:67-75.
23. de Carle AJ, Kohn R. Electroconvulsive therapy and falls in the elderly. J ECT. 2000;16(3):252-257.
24. Hoover DR, Siegel M, Lucas J, et al. Depression in the first year of stay for elderly long-term nursing home residents in the USA. Int Psychogeriatr. 2010;22:1161.
25. O’Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. Report. Am J Geriatr Psychiatry. 2001; 9:382.
CASE Depressed, avoidant
Mr. R, age 95, has a history of recurrent major depressive disorder. He presents to the emergency department with depressive symptoms that began 6 weeks ago. His symptoms include depressed mood, hopelessness, anhedonia, anxiety, and insomnia. Co-occurring anorexia nervosa has resulted in a 20-lb weight loss. He denies suicidal ideation.
A mental status examination reveals profound psychomotor agitation, dysphoric mood, tearfulness, and mood-congruent delusions. Mr. R’s Mini-Mental State Examination (MMSE) score is 14/30; his Hamilton Depression Rating Scale (HAM-D) score is 21, indicating severe depression (19 to 22). However, the examiner feels that these scores may not reflect an accurate assessment because Mr. R gave flippant responses and did not cooperate during the interview. Physical examination is unremarkable. Previous medication trials included buspirone, escitalopram, and risperidone; none of these medications successfully alleviated his depressive symptoms.
On admission, Mr. R is given oral mirtazapine, 15 mg/d, and quetiapine, 25 mg/d, to target depressive mood, insomnia, and weight loss. Urgent intervention is indicated because his depressive symptoms are profoundly causing failure to thrive and are compromising his physical health. Mr. R’s deterioration concerns the physician team. Because of a history of failed pharmacotherapy trials, the team reassesses Mr. R’s treatment options.
[polldaddy:9903171]
The authors’ observations
The physician team recommends that Mr. R undergo ECT to obtain rapid relief from his depressive symptoms. After discussion of the potential risks and benefits, Mr. R agrees to this treatment. Quetiapine is discontinued prior to initiating ECT to avoid unnecessary medications; mirtazapine is continued.
Mr. R’s lack of response to previous antidepressants and significant deterioration were concerning. The physicians wanted to avoid higher-dose medications because of the risk of falls or somnolence. Their clinical experience and the literature supporting ECT for patients of Mr. R’s age lead them to select ECT as the most appropriate therapeutic option.
ECT has no absolute contraindications.1 The rate of ECT use in the United States has fluctuated over time because of factors unrelated to the efficacy and availability of ECT or alternative treatments.2 This form of intervention is also somewhat stigmatized.
Some psychiatrists are reluctant to prescribe ECT for geriatric patients because of concerns of potential neurocognitive or medical complications and risks during anesthesia. However, in the United States, older patients with depression are more likely to be treated with ECT than their younger counterparts.3 ECT usually induces greater immediate efficacy than antidepressants.4
Evidence supports using ECT in older patients
Multiple studies have found that ECT is a rapid, safe, and efficacious intervention for treating older persons with depression. Patients age >60 who receive ECT plus pharmacotherapy have lower HAM-D scores than those receiving pharmacotherapy alone.5 Overall, the rates of remission for depression range from 50% to 70%; yet geriatric patients who receive only ECT have response rates around 90%.6 Older age, presence of psychotic symptoms, and shorter duration of illness can predict a rapidly positive ECT response.7
When treated with ECT, older patients, including those age >85, have fewer subsequent episodes of depression compared with those who receive pharmacotherapy alone.1 Older individuals with physical illness or cognitive impairment respond to and tolerate ECT much like younger patients.6 Older patients receiving ECT may experience less cognitive decline than younger ones.7 Those in their ninth decade of life with treatment-resistant depression, psychotic features, and post-stroke depression often respond robustly with improvement following ECT.8
Remission rates also depend on the technique of administration. Interactions between electrode placement and stimulus parameter dosage affect efficacy and adverse effects.9 Right-sided, unilateral ECT induces less cognitive dysfunction compared with bilateral electrode placement,9 but bilateral ECT is more clinically effective.10 However, the efficacy of right-sided ECT is more dose-sensitive, and some data suggest that suboptimal response is due to insufficient stimulus dosages.11 One double-blind randomized controlled trial documented that when using a high-dose stimulus parameter, unilateral ECT is as effective as bilateral ECT.12 When there is a suboptimal response to unilateral ECT, bilateral ECT might be beneficial.12,13 For preventing relapse in older patients, increasing the interval between ECT treatments is more effective than stopping ECT abruptly.13
[polldaddy:9903172]
Indications of ECT
ECT is indicated for patients with severe depression, mania, and other conditions (Table).14 The most common indication for ECT in older persons is a history of treatment-resistant depression, with melancholia, psychosis, or suicidal ideations.1-6,12 There are also age-related and clinical factors to consider with ECT. This treatment provides a safe, rapid remission for patients age >65, even after adjusting for somatic conditions, duration of illness, medication resistance, or case severity.15 Compared with younger patients, older adults may not tolerate antidepressants as well because of age-related pharmacokinetic alterations, including increased sensitivity to anticholinergic and/or hypotensive effects.1
Factors that favor ECT include a previous good response to it; patient preference; and an indication for rapid intervention, such as suicidality, catatonia, dehydration, malnutrition, or a suboptimal result from pharmacotherapy.3 Mortality among individuals age >85 who receive ECT reportedly is lower than that among their counterparts who receive alternative treatments.16 ECT has been administered safely and effectively in patients with comorbid medical illnesses such as stroke, cerebral aneurysm, cardiovascular disease with ischemia or arrhythmia, dementia, and osteoporosis.17
Neurocognitive effects
Reports on the effects of ECT on neurocognitive functioning have varied. In some studies, performance improved or did not change in severely depressed older patients who received ECT.18,19 In older people who receive ECT, MMSE scores often return to baseline by the end of treatment.20 There often is only mild transient cognitive impairment in patients with late-life depression who receive ECT. Areas of concern include attention span, orientation, and speed of mental processing.20 Physicians should conduct cognitive tests before, during, and after ECT sessions to monitor their patient’s mental status.20
Cognitive stability can be maintained by administering ECT twice a week; applying right-sided, unilateral electrode placement; and using short, ultra-brief stimulus pulse width parameters.21 Cognitive impairment induced by ECT is not associated with age in geriatric patients with depression.22 Older adults who experienced longer postictal reorientation time periods have better outcomes than others who reach orientation faster; their intellectual impairment returned to baseline.20 Falling is another complication associated with ECT. A longitudinal cohort study found the incident of falls among patients receiving ECT was 13%.22 Risk factors for falls during a course of ECT include the number of treatments and the presence of coexisting Parkinson’s disease.23
OUTCOME Improvement
Mr. R receives 8 sessions of right-sided, unilateral ECT with an individualized dosage titration method. Treatments are completed with a stimulus intensity at 6 times seizure threshold, with an ultra-brief pulse width at 0.3 milliseconds. Mr. R’s mood and affect begin to improve after 3 ECT sessions. His MMSE score increases to 28/30 (Figure). His clinical improvement is progressively sustained; he develops an increasingly jovial attitude and experiences less anxiety. Mr. R’s confidence, appetite, and sleep also improve. There are no complications with treatment, and Mr. R has no complaints. After 8 ECT sessions, Mr. R has no affective symptoms and does not experience any cognitive impairment.
The authors’ observations
Depression among older people is a growing public health concern. It is a leading cause of disability, and often leads to nursing home placement.24 ECT is a safe, effective treatment for late-life depression, but is underutilized in patients age >75 because of concerns for cognitive impairment.6 However, there is evidence that response rates to ECT are higher in patients ages 45 to 85, compared with young individuals ages 18 to 45.25 ECT is a viable intervention for older depressed patients, particularly for those who do not tolerate or fail to respond to pharmacotherapy. Many of these patients are at risk for drug-induced toxicities or interactions or suicide.1
CASE Depressed, avoidant
Mr. R, age 95, has a history of recurrent major depressive disorder. He presents to the emergency department with depressive symptoms that began 6 weeks ago. His symptoms include depressed mood, hopelessness, anhedonia, anxiety, and insomnia. Co-occurring anorexia nervosa has resulted in a 20-lb weight loss. He denies suicidal ideation.
A mental status examination reveals profound psychomotor agitation, dysphoric mood, tearfulness, and mood-congruent delusions. Mr. R’s Mini-Mental State Examination (MMSE) score is 14/30; his Hamilton Depression Rating Scale (HAM-D) score is 21, indicating severe depression (19 to 22). However, the examiner feels that these scores may not reflect an accurate assessment because Mr. R gave flippant responses and did not cooperate during the interview. Physical examination is unremarkable. Previous medication trials included buspirone, escitalopram, and risperidone; none of these medications successfully alleviated his depressive symptoms.
On admission, Mr. R is given oral mirtazapine, 15 mg/d, and quetiapine, 25 mg/d, to target depressive mood, insomnia, and weight loss. Urgent intervention is indicated because his depressive symptoms are profoundly causing failure to thrive and are compromising his physical health. Mr. R’s deterioration concerns the physician team. Because of a history of failed pharmacotherapy trials, the team reassesses Mr. R’s treatment options.
[polldaddy:9903171]
The authors’ observations
The physician team recommends that Mr. R undergo ECT to obtain rapid relief from his depressive symptoms. After discussion of the potential risks and benefits, Mr. R agrees to this treatment. Quetiapine is discontinued prior to initiating ECT to avoid unnecessary medications; mirtazapine is continued.
Mr. R’s lack of response to previous antidepressants and significant deterioration were concerning. The physicians wanted to avoid higher-dose medications because of the risk of falls or somnolence. Their clinical experience and the literature supporting ECT for patients of Mr. R’s age lead them to select ECT as the most appropriate therapeutic option.
ECT has no absolute contraindications.1 The rate of ECT use in the United States has fluctuated over time because of factors unrelated to the efficacy and availability of ECT or alternative treatments.2 This form of intervention is also somewhat stigmatized.
Some psychiatrists are reluctant to prescribe ECT for geriatric patients because of concerns of potential neurocognitive or medical complications and risks during anesthesia. However, in the United States, older patients with depression are more likely to be treated with ECT than their younger counterparts.3 ECT usually induces greater immediate efficacy than antidepressants.4
Evidence supports using ECT in older patients
Multiple studies have found that ECT is a rapid, safe, and efficacious intervention for treating older persons with depression. Patients age >60 who receive ECT plus pharmacotherapy have lower HAM-D scores than those receiving pharmacotherapy alone.5 Overall, the rates of remission for depression range from 50% to 70%; yet geriatric patients who receive only ECT have response rates around 90%.6 Older age, presence of psychotic symptoms, and shorter duration of illness can predict a rapidly positive ECT response.7
When treated with ECT, older patients, including those age >85, have fewer subsequent episodes of depression compared with those who receive pharmacotherapy alone.1 Older individuals with physical illness or cognitive impairment respond to and tolerate ECT much like younger patients.6 Older patients receiving ECT may experience less cognitive decline than younger ones.7 Those in their ninth decade of life with treatment-resistant depression, psychotic features, and post-stroke depression often respond robustly with improvement following ECT.8
Remission rates also depend on the technique of administration. Interactions between electrode placement and stimulus parameter dosage affect efficacy and adverse effects.9 Right-sided, unilateral ECT induces less cognitive dysfunction compared with bilateral electrode placement,9 but bilateral ECT is more clinically effective.10 However, the efficacy of right-sided ECT is more dose-sensitive, and some data suggest that suboptimal response is due to insufficient stimulus dosages.11 One double-blind randomized controlled trial documented that when using a high-dose stimulus parameter, unilateral ECT is as effective as bilateral ECT.12 When there is a suboptimal response to unilateral ECT, bilateral ECT might be beneficial.12,13 For preventing relapse in older patients, increasing the interval between ECT treatments is more effective than stopping ECT abruptly.13
[polldaddy:9903172]
Indications of ECT
ECT is indicated for patients with severe depression, mania, and other conditions (Table).14 The most common indication for ECT in older persons is a history of treatment-resistant depression, with melancholia, psychosis, or suicidal ideations.1-6,12 There are also age-related and clinical factors to consider with ECT. This treatment provides a safe, rapid remission for patients age >65, even after adjusting for somatic conditions, duration of illness, medication resistance, or case severity.15 Compared with younger patients, older adults may not tolerate antidepressants as well because of age-related pharmacokinetic alterations, including increased sensitivity to anticholinergic and/or hypotensive effects.1
Factors that favor ECT include a previous good response to it; patient preference; and an indication for rapid intervention, such as suicidality, catatonia, dehydration, malnutrition, or a suboptimal result from pharmacotherapy.3 Mortality among individuals age >85 who receive ECT reportedly is lower than that among their counterparts who receive alternative treatments.16 ECT has been administered safely and effectively in patients with comorbid medical illnesses such as stroke, cerebral aneurysm, cardiovascular disease with ischemia or arrhythmia, dementia, and osteoporosis.17
Neurocognitive effects
Reports on the effects of ECT on neurocognitive functioning have varied. In some studies, performance improved or did not change in severely depressed older patients who received ECT.18,19 In older people who receive ECT, MMSE scores often return to baseline by the end of treatment.20 There often is only mild transient cognitive impairment in patients with late-life depression who receive ECT. Areas of concern include attention span, orientation, and speed of mental processing.20 Physicians should conduct cognitive tests before, during, and after ECT sessions to monitor their patient’s mental status.20
Cognitive stability can be maintained by administering ECT twice a week; applying right-sided, unilateral electrode placement; and using short, ultra-brief stimulus pulse width parameters.21 Cognitive impairment induced by ECT is not associated with age in geriatric patients with depression.22 Older adults who experienced longer postictal reorientation time periods have better outcomes than others who reach orientation faster; their intellectual impairment returned to baseline.20 Falling is another complication associated with ECT. A longitudinal cohort study found the incident of falls among patients receiving ECT was 13%.22 Risk factors for falls during a course of ECT include the number of treatments and the presence of coexisting Parkinson’s disease.23
OUTCOME Improvement
Mr. R receives 8 sessions of right-sided, unilateral ECT with an individualized dosage titration method. Treatments are completed with a stimulus intensity at 6 times seizure threshold, with an ultra-brief pulse width at 0.3 milliseconds. Mr. R’s mood and affect begin to improve after 3 ECT sessions. His MMSE score increases to 28/30 (Figure). His clinical improvement is progressively sustained; he develops an increasingly jovial attitude and experiences less anxiety. Mr. R’s confidence, appetite, and sleep also improve. There are no complications with treatment, and Mr. R has no complaints. After 8 ECT sessions, Mr. R has no affective symptoms and does not experience any cognitive impairment.
The authors’ observations
Depression among older people is a growing public health concern. It is a leading cause of disability, and often leads to nursing home placement.24 ECT is a safe, effective treatment for late-life depression, but is underutilized in patients age >75 because of concerns for cognitive impairment.6 However, there is evidence that response rates to ECT are higher in patients ages 45 to 85, compared with young individuals ages 18 to 45.25 ECT is a viable intervention for older depressed patients, particularly for those who do not tolerate or fail to respond to pharmacotherapy. Many of these patients are at risk for drug-induced toxicities or interactions or suicide.1
1. Kerner N, Prudic J. Current electroconvulsive therapy practice and research in the geriatric population. Neuropsychiatry (London). 2014;4(1):33-54.
2. Dombrovski AY, Mulsant BH. The evidence for electroconvulsive therapy (ECT) in the treatment of severe late-life depression. ECT: the preferred treatment for severe depression in late life. Int Psychogeriatr. 2007;19(1):10-14,27-35; discussion 24-26.
3. Olfson M, Marcus S, Sackeim HA, et al. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155(1):22-29.
4. Salzman C, Wong E, Wright BC. Drug and ECT treatment of depression in the elderly, 1996-2001: a literature review. Biol Psychiatry. 2002;52(3):265-284.
5. Kellner CH, Husain MM, Knapp RG, et al; CORE/PRIDE Work Group. A novel strategy for continuation ect in geriatric depression: phase 2 of the PRIDE study. A
6. Tew JD Jr, Mulsant BH, Haskett RF, et al. Acute efficacy of ECT in the treatment of major depression in the old-old. Am J Psychiatry. 1999;156(12):1865-1870.
7. Dombrovski AY, Mulsant BH, Haskett RF, et al. Predictors of remission after electroconvulsive therapy in unipolar major depression. J Clin Psychiatry. 2005;66(8):1043-1049.
8. Charles K. UpToDate. Unipolar major depression in adults: indications for efficacy of electroconvulsive therapy (ECT). https://www.uptodate.com/contents/unipolar-major-depression-in-adults-indications-for-and-efficacy-of-electroconvulsive-therapy-ect. Updated May 16, 2017. Accessed November 26, 2017.
9. Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425-434.
10. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
11. Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939-1945.
12. Stoppe A, Louzã M, Rosa M, et al. Fixed high dose electroconvulsive therapy in elderly with depression: a double-blind, randomized comparison of efficacy and tolerability between unilateral and bilateral electrode placement. J ECT. 2006;22(2):92-99.
13. Geduldig ET, Kellner CH. Electroconvulsive therapy in the elderly: new findings in geriatric depression. Curr Psychiatry Rep. 2016;18(4):40.
14. Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am J Psychiatry. 2000;157(suppl 4):1-45.
15. Rhebergen D, Huisman A, Bouckaert F, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. 2015;23(3):274-282.
16. Philibert RA, Richards L, Lynch CF, et al. Effect of ECT on mortality and clinical outcome in geriatric unipolar depression. J Clin Psychiatry. 1995;56(9):390-394.
17. Tomac TA, Rummans TA, Pileggi TS, et al. Safety and efficacy of electroconvulsive therapy in patients over age 85. Am J Geriatr Psychiatry. 1997;5(2):126-130.
18. Verwijk E, Comijs HC, Kok RM, et al. Short and long-term neurocognitive functioning after electroconvulsive therapy in depressed elderly: a prospective naturalistic study. Int Psychogeriatr. 2014;26(2):315-324.
19. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late life depression. Can J Psychiatry. 2002;47(8):734-741.
20. Bjolseth TM, Engedal K, Benth JS, et al. Speed of recovery from disorientation may predict the treatment outcome of electroconvulsive therapy (ECT) in elderly patients with major depression. J Affect Disord. 2016;190:178-186.
21. Sackeim HA, Prudic J, Nobler MS, et al. Ultra-brief pulse ECT and the affective and cognitive consequences of ECT. J ECT. 2001;17(1):77.
22. Bjolseth TM, Engedal K, Benth JS, et al. Baseline cognitive function does not predict the treatment outcome of electroconvulsive therapy (ECT) in late-life depression. J Affect Disord. 2015;185:67-75.
23. de Carle AJ, Kohn R. Electroconvulsive therapy and falls in the elderly. J ECT. 2000;16(3):252-257.
24. Hoover DR, Siegel M, Lucas J, et al. Depression in the first year of stay for elderly long-term nursing home residents in the USA. Int Psychogeriatr. 2010;22:1161.
25. O’Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. Report. Am J Geriatr Psychiatry. 2001; 9:382.
1. Kerner N, Prudic J. Current electroconvulsive therapy practice and research in the geriatric population. Neuropsychiatry (London). 2014;4(1):33-54.
2. Dombrovski AY, Mulsant BH. The evidence for electroconvulsive therapy (ECT) in the treatment of severe late-life depression. ECT: the preferred treatment for severe depression in late life. Int Psychogeriatr. 2007;19(1):10-14,27-35; discussion 24-26.
3. Olfson M, Marcus S, Sackeim HA, et al. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155(1):22-29.
4. Salzman C, Wong E, Wright BC. Drug and ECT treatment of depression in the elderly, 1996-2001: a literature review. Biol Psychiatry. 2002;52(3):265-284.
5. Kellner CH, Husain MM, Knapp RG, et al; CORE/PRIDE Work Group. A novel strategy for continuation ect in geriatric depression: phase 2 of the PRIDE study. A
6. Tew JD Jr, Mulsant BH, Haskett RF, et al. Acute efficacy of ECT in the treatment of major depression in the old-old. Am J Psychiatry. 1999;156(12):1865-1870.
7. Dombrovski AY, Mulsant BH, Haskett RF, et al. Predictors of remission after electroconvulsive therapy in unipolar major depression. J Clin Psychiatry. 2005;66(8):1043-1049.
8. Charles K. UpToDate. Unipolar major depression in adults: indications for efficacy of electroconvulsive therapy (ECT). https://www.uptodate.com/contents/unipolar-major-depression-in-adults-indications-for-and-efficacy-of-electroconvulsive-therapy-ect. Updated May 16, 2017. Accessed November 26, 2017.
9. Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425-434.
10. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
11. Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939-1945.
12. Stoppe A, Louzã M, Rosa M, et al. Fixed high dose electroconvulsive therapy in elderly with depression: a double-blind, randomized comparison of efficacy and tolerability between unilateral and bilateral electrode placement. J ECT. 2006;22(2):92-99.
13. Geduldig ET, Kellner CH. Electroconvulsive therapy in the elderly: new findings in geriatric depression. Curr Psychiatry Rep. 2016;18(4):40.
14. Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am J Psychiatry. 2000;157(suppl 4):1-45.
15. Rhebergen D, Huisman A, Bouckaert F, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. 2015;23(3):274-282.
16. Philibert RA, Richards L, Lynch CF, et al. Effect of ECT on mortality and clinical outcome in geriatric unipolar depression. J Clin Psychiatry. 1995;56(9):390-394.
17. Tomac TA, Rummans TA, Pileggi TS, et al. Safety and efficacy of electroconvulsive therapy in patients over age 85. Am J Geriatr Psychiatry. 1997;5(2):126-130.
18. Verwijk E, Comijs HC, Kok RM, et al. Short and long-term neurocognitive functioning after electroconvulsive therapy in depressed elderly: a prospective naturalistic study. Int Psychogeriatr. 2014;26(2):315-324.
19. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late life depression. Can J Psychiatry. 2002;47(8):734-741.
20. Bjolseth TM, Engedal K, Benth JS, et al. Speed of recovery from disorientation may predict the treatment outcome of electroconvulsive therapy (ECT) in elderly patients with major depression. J Affect Disord. 2016;190:178-186.
21. Sackeim HA, Prudic J, Nobler MS, et al. Ultra-brief pulse ECT and the affective and cognitive consequences of ECT. J ECT. 2001;17(1):77.
22. Bjolseth TM, Engedal K, Benth JS, et al. Baseline cognitive function does not predict the treatment outcome of electroconvulsive therapy (ECT) in late-life depression. J Affect Disord. 2015;185:67-75.
23. de Carle AJ, Kohn R. Electroconvulsive therapy and falls in the elderly. J ECT. 2000;16(3):252-257.
24. Hoover DR, Siegel M, Lucas J, et al. Depression in the first year of stay for elderly long-term nursing home residents in the USA. Int Psychogeriatr. 2010;22:1161.
25. O’Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. Report. Am J Geriatr Psychiatry. 2001; 9:382.
Self-mutilation after recent-onset psychosis
CASE Bleeding, bewildered
Mr. K, age 23, a South Asian male, is discovered in the bathroom bleeding profusely. Mr. K’s parents inform emergency medical services (EMS) personnel that Mr. K is “not in his right mind” and speculate that he is depressed. EMS personnel find Mr. K sitting in a pool of blood in the bathtub, holding a cloth over his pubic area and complaining of significant pain. They estimate that Mr. K has lost approximately 1 L of blood. Cursory evaluation reveals that his penis is severed; no other injuries or lacerations are notable. Mr. K states, “I did not want it anymore.” A kitchen knife that he used to self-amputate is found nearby. He is awake, alert, and able to follow simple directives.
In the emergency room, Mr. K is in mild-to-moderate distress. He has no history of medical illness, but his parents report that he previously required psychiatric treatment. Mr. K is not able to elaborate. He reluctantly discloses an intermittent history of Cannabis use. Physical examination reveals tachycardia (heart rate: 115 to 120 beats per minute), and despite blood loss, systolic hypertension (blood pressure: 142/70 to 167/70 mm Hg). His pulse oximetry is 97% to 99%; he is afebrile. Laboratory tests are notable for anemia (hemoglobin, 7.2 g/dL [reference range, 14.0 to 17.5 g/dL]; hematocrit, 21.2% [reference range, 41% to 50%]) and serum toxicology screen is positive for benzodiazepines, which had been administered en route to allay his distress.
Mr. K continues to hold pressure on his pubic area. When pressure is released, active arterial spurting of bright red blood is notable. Genital examination reveals a cleanly amputated phallus. Emergent surgical intervention is required to stop the hemorrhage and reattach the penis. Initially, Mr. K is opposed to reattachment, but after a brief discussion with his parents, he consents to surgery. Urology and plastic surgery consultations are elicited to perform the microvascular portion of the procedure.
[polldaddy:9881368]
The authors’ observations
Self-injurious behaviors occur in approximately 1% to 4% of adults in the United States, with chronic and severe self-injury occurring among approximately 1% of the U.S. population.1,2 Intentional GSM is a relatively rare catastrophic event that is often, but not solely, associated with severe mental illness. Because many cases go unreported, the prevalence of GSM is difficult to estimate.3,4 Although GSM has been described in both men and women, the literature has predominantly focused on GSM among men.5 Genital self-injury has been described in several (ie, ethnic/racial and religious) contexts and has been legally sanctioned.6-8
Psychiatric disorders associated with, and precipitating factors underlying, GSM have long remained elusive.8 GSM has been described in case reports and small case series in both psychiatric and urologic literature. These reports provide incomplete descriptions of the diagnostic conditions and psychosocial factors underlying male GSM.
A recent systematic review of 173 cases of men who engaged in GSM published in the past 115 years (since the first case of GSM was published in the psychiatric literature9) revealed that having some form of psychopathology elevates the probability of GSM10,11; rarely the individual did not have a psychiatric condition.11-17 Nearly one-half of the men had psychosis; most had a schizophrenia spectrum disorder diagnosis. Other psychiatric conditions associated with GSM include personality disorders, substance use disorder, and gender dysphoria. GSM is rarely associated with anxiety or mood disorders.
GSM is a heterogeneous form of self-injury that ranges from superficial genital lacerations, amputation, or castration to combinations of these injuries. Compared with individuals with other psychiatric disorders, a significantly greater proportion of individuals with schizophrenia spectrum disorders engage in self-amputation (auto-penectomy). By contrast, persons with gender dysphoria tend to engage in self-castration at significantly higher rates than those with other psychiatric conditions.11 Despite these trends, clinicians should not infer a specific psychiatric diagnosis based on the severity or type of self-inflicted injury.
HISTORY Command hallucinations
Postoperatively, Mr. K is managed in the trauma intensive care unit. During psychiatric consultation, Mr. K demonstrates a blunted affect. His speech is low in volume but clear and coherent. His thoughts are generally linear for specific lines of inquiry (eg, about perceived level of pain) but otherwise are impoverished. Mr. K often digresses into repetitively mumbled prayers. He appears distracted, as if responding to internal stimuli. Although he acknowledges the GSM, he does not discuss the factors underlying his decision to proceed with auto-penectomy. Over successive evaluations, he reluctantly discloses that he had been experiencing disparaging auditory hallucinations that told him that his penis “was too small” and commanded him to “cut it off.”
Psychiatric history reveals that Mr. K required psychiatric hospitalization 7 months earlier due to new-onset auditory hallucinations, paranoia, and thought disorganization, in the context of daily Cannabis use. At the time, the differential diagnosis included new-onset schizophrenia and substance-induced psychosis. His symptoms improved quickly with risperidone, 2 mg/d, and he was discharged in a stable condition with referrals for outpatient care. Mr. K admits he had stopped taking risperidone several weeks before the GSM because he was convinced that he had been cured. At that time, Mr. K had told his parents he was no longer required to take medication or engage in outpatient psychiatric treatment, and they did not question this. Mr. K struggled to sustain part-time employment (in a family business), having taken a leave of absence from graduate school after his first hospitalization. He continued to use Cannabis regularly but denies being intoxicated at the time of the GSM. Throughout his surgical hospitalization, Mr. K’s thoughts remain disorganized. He denies that the GSM was a suicide attempt or having current suicidal thoughts, intent, or plans. He also denies having religious preoccupations, over-valued religious beliefs, or delusions.
Mr. K identifies as heterosexual, and denies experiencing distress related to sexual orientation or gender identity or guilt related to sexual impulses or actions. He also denies having a history of trauma or victimization and does not report any symptoms of posttraumatic stress disorder or body dysmorphic disorder.
The authors’ observations
Little is known about how many individuals who engage in GSM eventually complete suicide. Although suicidal ideation and intent have been infrequently associated with GSM, suicide has been most notably reported among patients with schizophrenia spectrum disorders and psychotic mood disorders.11,18,23-26 For these individuals, suicidal ideation co-occurred with delusions, hallucinations, and pathological guilt preoccupations. Significant self-inflicted injury can be harbinger of distress that could lead to suicide if not optimally treated. Other psychosocial stressors, such as disruptions in interpersonal functioning arising from changes in or loss of social support or perceived rejection, may contribute to a patient’s level of distress, complicating underlying psychiatric disturbances and increasing vulnerability toward GSM.11,27
Substance use also increases vulnerability toward GSM.11,18,24,28 As is the case with patients who engage in various non-GSM self-injurious behaviors,29,30 substance use or intoxication likely contribute to disinhibition or a dissociative state, which enables individuals to engage in self-injury.30
A lack of access to treatment is a rare precipitant for GSM, except among individuals with gender dysphoria. Studies have found that many patients with gender dysphoria who performed self-castration did so in a premeditated manner with low suicidal intent, and the behavior often was related to a lack of or refusal for gender confirmation surgery.31-34
In the hospital setting, surgical/urological interventions need to be directed at the potentially life-threatening sequelae of self-injury. Although complications vary, depending on the type of injury incurred, urgent measures are needed to manage blood loss because hemorrhage can be fatal.23,35,36 Other consequences that can arise include urinary fistulae, urethral strictures, mummification of the glans penis, and development of sensory abnormalities after repair of the injured tissues or reattachment.8 More superficial injuries may require only hemostasis and simple suturing, whereas extensive injuries, such as complete amputation, can be addressed through microvascular techniques.
The psychiatrist’s role. The psychiatrist should act as an advocate for the GSM patient to create an environment conducive to healing. A patient who is experiencing hallucinations or delusions may feel overwhelmed by medical and familial attention. Pharmacologic treatment for prevailing mental illness, such as psychosis, should be initiated in the inpatient setting. An estimated 20% to 25% of those who self-inflict genital injury may repeatedly mutilate their genitals.19,28 Patients unduly influenced by command hallucinations, delusional thought processes, mood disturbances, or suicidal ideation may attempt to complete the injury, or reinjure themselves after surgical/urological intervention, which may require safety measures, such as 1:1 observation, restraints, or physical barriers, to prevent reinjury.37
Self-injury elicits strong, emotional responses from health care professionals, including fascination, apprehension, and hopelessness. Psychiatrists who care for such patients should monitor members of the patient’s treatment team for psychological reactions. In addition, the patient’s behavior while hospitalized may stir feelings of retaliation, anger, fear, and frustration.11,24,37 Collaborative relationships with medical and surgical specialties can help staff manage emotional reactions and avoid the inadvertent expression of those feelings in their interactions with the patient; these reactions might otherwise undermine treatment.24,34 Family education can help mitigate any guilt family members may harbor for not preventing the injury.37
Although efforts to understand the intended goal(s) and precipitants of the self-injury are likely to be worthwhile, the overwhelming distress associated with GSM and its emergent treatment may preclude intensive exploration.
TREATMENT Restarting medication
While on the surgical unit, Mr. K is restarted on risperidone, 2 mg/d. He appears to tolerate the medication without adverse effects. However, because Mr. K continues to experience auditory hallucinations, and the treatment team remains concerned that he might again experience commands to harm himself, he is transferred to an acute psychiatric inpatient setting.
Urology follow-up reveals necrosis/mummification of the replanted penis and an open scrotal wound. After discussing options with the patient and family, the urologist transfers Mr. K back to the surgical unit for wound closure and removal of the replanted penis. A urethrostomy is performed to allow for bladder emptying.
[polldaddy:9881371]
The authors’ observations
Because most published case reports of GSM among men have focused on acute treatment, there is a dearth of literature available on the long-term course of GSM to inform treatment strategies. Because recovery is a non-static process and a patient’s reactions to his injury will likely evolve over time, a multifaceted approach invoking psychiatric and psychotherapeutic interventions is necessary to help patients after initial injury and surgical management37,40-43 (Table 211,20,27,41).
OUTCOME Return to school, work
Mr. K is discharged with close follow-up at a specialized clinic for new-onset psychosis. Post-discharge treatment consists of education about the course of schizophrenia and the need for medication adherence to prevent relapse. Mr. K also is educated on the relationship between Cannabis use and psychosis, and he abstains from illicit substance use. Family involvement is encouraged to help with medication compliance and monitoring for symptom reemergence.
Therapy focuses on exploring the antecedents of the auto-penectomy, Mr. K’s body image issue concerns, and his feelings related to eventual prosthesis implantation. He insists that he cannot recall any precipitating factors for his self-injury other than the command hallucinations. He does not report sexual guilt, although he had been sexually active with his girlfriend in the months prior to his GSM, which goes against his family’s religious beliefs. He reports significant regret and shame for the self-mutilation, and blames himself for not informing family members about his hallucinations. Therapy involves addressing his attribution of blame using cognitive techniques and focuses on measures that can be taken to prevent further self-harm. Efforts are directed at exploring whether cultural and religious traditions impacted the therapeutic alliance, medication adherence, self-esteem and body image, sexuality, and future goals. Over the course of 1 year, he resumes his graduate studies and part-time work, and explores prosthetic placement for cosmetic purposes.
The authors’ observations
Research suggests that major self-mutilation among patients with psychotic illness is likely to occur during the first episode or early in the course of illness and/or with suboptimal treatment.44,45 Mr. K was enlisted in an intensive outpatient treatment program involving biweekly psychotherapy sessions and psychiatric follow-up. Initial sessions focused on education regarding the importance of medication adherence and exploration of signs and symptoms that might suggest reemergence of a psychotic decompensation. The psychiatrist monitored Mr. K closely to ensure he was able to tolerate his medications to mitigate the possibility that adverse effects would undermine adherence. Mr. K’s reactions to having a psychiatric illness also were explored because of concerns that such self-appraisals might trigger shame, embarrassment, denial, and other responses that might undermine treatment adherence. His family members were apprised of treatment goals and enlisted to foster adherence with medication and follow-up appointments.
Mr. K’s Cannabis use was addressed because ongoing use likely had a negative impact on his schizophrenia (ie, a greater propensity toward relapse and rehospitalization and a poorer therapeutic response to antipsychotic medication).46,47 He was strongly encouraged to avoid Cannabis and other illicit substances.
Psychiatrists can help in examining the meaning behind the injury while helping the patient to adapt to the sequelae and cultivate skills to meet functional demands.41 Once Mr. K’s psychotic symptoms were in remission, treatment began to address the antecedents of the GSM, as well as the resultant physical consequences. It was reasonable to explore how Mr. K now viewed his actions, as well as the consequences that his actions produced in terms of his physical appearance, sexual functioning, capacity for sexual intimacy, and reproductive potential. It was also important to recognize how such highly intimate and deeply personal self-schema are framed and organized against his cultural and religious background.27,33
Body image concerns and expectations for future urologic intervention also should be explored. Although Mr. K was not averse to such exploration, he did not spontaneously address such topics in great depth. The discussion was unforced and effectively left open as an issue that could be explored in future sessions.
1. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68(4):609-620.
2. Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501-1508.
3. Krasucki C, Kemp R, David A. A case study of female genital self-mutilation in schizophrenia. Br J Med Psychol. 1995;68(pt 2):179-186.
4. Lennon S. Genital self-mutilation in acute mania. Med J Aust. 1963;50(1):79-81.
5. Schweitzer I. Genital self-amputation and the Klingsor syndrome. Aust N Z J Psychiatry. 1990;24(4):566-569.
6. Anumonye A. Self-inflicted amputation of the penis in two Nigerian males. Niger Med J. 1973;3(1):51-52.
7. Bowman KM, Crook GH. Emotional changes following castration. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:81-96.
8. Eke N. Genital self-mutilation: there is no method in this madness. BJU Int. 2000;85(3):295-298.
9. Stroch D. Self-castration. JAMA. 1901;36(4):270.
10. Veeder TA, Leo RJ. Male genital self-mutilation: a comprehensive review of psychiatric disorders. Poster presented at: Academy of Psychosomatic Medicine Meeting, Austin, Texas, November 10, 2016.
11. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry. 2017;44:43-50.
12. Battle AO. The psychological appraisal of a patient who had performed self-castration. British Journal of Projective Psychology & Personality Study. 1973;18(2):5-17.
13. Bhatia MS, Arora S. Penile self-mutilation. Br J Psychiatry. 2001;178(1):86-87.
14. Gleeson MJ, Connolly J, Grainger R. Self-castration as treatment for alopecia. Br J Urol. 1993;71(5):614-615.
15. Hendershot E, Stutson AC, Adair TW. A case of extreme sexual self-mutilation. J Forensic Sci. 2010;55(1):245-247.
16. Hermann M, Thorstenson A. A rare case of male‐to‐eunuch gender dysphoria. Sex Med. 2015;3(4):331-333.
17. Nerli RB, Ravish IR, Amarkhed SS, et al. Genital self-mutilation in nonpsychotic heterosexual males: case report of two cases. Indian J Psychiatry. 2008;50(4):285-287.
18. Blacker KH, Wong N. Four cases of autocastration. Arch Gen Psychiatry. 1963;8:169-176.
19. Catalano G, Catalano MC, Carroll KM. Repetitive male genital self-mutilation: a case report and discussion of possible risk factors. J Sex Marital Ther. 2002;28(1):27-37.
20. Martin T, Gattaz WF. Psychiatric aspects of male genital self-mutilation. Psychopathology. 1991;24(3):170-178.
21. Money J. The Skoptic syndrome: castration and genital self-mutilation as an example of sexual body-image pathology. J Psychol Human Sex. 1988;1(1):113-128.
22. Nakaya M. On background factors of male genital self-mutilation. Psychopathology. 1996;29(4):242-248.
23. Borenstein A, Yaffe B, Seidman DS, et al. Successful microvascular replantation of an amputated penis. Isr J Med Sci. 1991;27(7):395-398.
24. Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36(4):441-446.
25. Mendez R, Kiely WF, Morrow JW. Self-emasculation. J Urol. 1972;107(6):981-985.
26. Siddique RA, Deshpande S. A case of genital self-mutilation in a patient with psychosis. German J Psychiatry. 2007;10(1):25-28.
27. Qureshi NA. Male genital self-mutilation with special emphasis on the sociocultural meanings. Neurosciences (Riyadh). 2009;14(2):178-181.
28. Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55(7):427-431.
29. Gahr M, Plener PL, Kölle MA, et al. Self-mutilation induced by psychotropic substances: a systematic review. Psychiatry Res. 2012;200(2-3):977-983.
30. Evren C, Sar V, Evren B, et al. Self-mutilation among male patients with alcohol dependency: the role of dissociation. Compr Psychiatry. 2008;49(5):489-495.
31. Brown GR. Autocastration and autopenectomy as surgical self-treatment in incarcerated persons with gender identity disorder. Int J Transgend. 2010;12(1):31-39.
32. Master VA, McAninch JW, Santucci RA. Genital self-mutilation and the Internet. J Urol. 2000;164(5):1656.
33. Premand NE, Eytan A. A case of non-psychotic autocastration: the importance of cultural factors. Psychiatry. 2005;68(2):174-178.
34. Simopoulos EF, Trinidad AC. Two cases of male genital self-mutilation: an examination of liaison dynamics. Psychosomatics. 2012;53(2):178-180.
35. Darewicz B, Galek L, Darewicz J, et al. Successful microsurgical replantation of an amputated penis. Int Urol Nephrol. 2001;33(2):385-386.
36. Raheem OA, Mirheydar HS, Patel ND, et al. Surgical management of traumatic penile amputation: a case report and review of the world literature. Sex Med. 2015;3(1):49-53.
37. Young LD, Feinsilver DL. Male genital self-mutilation: combined surgical and psychiatric care. Psychosomatics. 1986;27(7):513-517.
38. Walsh B. Clinical assessment of self-injury: a practical guide. J Clin Psychol. 2007;63(11):1057-1066.
39. Nafisi N, Stanley B. Developing and maintaining the therapeutic alliance with self-injuring patients. J Clin Psychol. 2007;63(11):1069-1079.
40. Fisch RZ. Genital self-mutilation in males: psychodynamic anatomy of a psychosis. Am J Psychother. 1987;41(3):453-458.
41. King PR. Cognitive-behavioral intervention in a case of self-mutilation. Clin Case Stud. 2014;13(2):181-189.
42. Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. J Ment Health Couns. 2006;28(2):166-185.
43. Walsh BW. Treating self-injury: a practical guide. New York, NY: The Guilford Press; 2006.
44. Large M, Babidge N, Andrews D, et al. Major self-mutilation in the first episode of psychosis. Schizophr Bull. 2009;35(5):1012-1021.
45. Large MM, Nielssen OB, Babidge N. Untreated psychosis is the main cause of major self-mutilation. Isr J Psychiatry Relat Sci. 2011;48(1):65.
46. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21.
47. Bowers MB Jr, Mazure CM, Nelson JC, et al. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16(1):81-85.
CASE Bleeding, bewildered
Mr. K, age 23, a South Asian male, is discovered in the bathroom bleeding profusely. Mr. K’s parents inform emergency medical services (EMS) personnel that Mr. K is “not in his right mind” and speculate that he is depressed. EMS personnel find Mr. K sitting in a pool of blood in the bathtub, holding a cloth over his pubic area and complaining of significant pain. They estimate that Mr. K has lost approximately 1 L of blood. Cursory evaluation reveals that his penis is severed; no other injuries or lacerations are notable. Mr. K states, “I did not want it anymore.” A kitchen knife that he used to self-amputate is found nearby. He is awake, alert, and able to follow simple directives.
In the emergency room, Mr. K is in mild-to-moderate distress. He has no history of medical illness, but his parents report that he previously required psychiatric treatment. Mr. K is not able to elaborate. He reluctantly discloses an intermittent history of Cannabis use. Physical examination reveals tachycardia (heart rate: 115 to 120 beats per minute), and despite blood loss, systolic hypertension (blood pressure: 142/70 to 167/70 mm Hg). His pulse oximetry is 97% to 99%; he is afebrile. Laboratory tests are notable for anemia (hemoglobin, 7.2 g/dL [reference range, 14.0 to 17.5 g/dL]; hematocrit, 21.2% [reference range, 41% to 50%]) and serum toxicology screen is positive for benzodiazepines, which had been administered en route to allay his distress.
Mr. K continues to hold pressure on his pubic area. When pressure is released, active arterial spurting of bright red blood is notable. Genital examination reveals a cleanly amputated phallus. Emergent surgical intervention is required to stop the hemorrhage and reattach the penis. Initially, Mr. K is opposed to reattachment, but after a brief discussion with his parents, he consents to surgery. Urology and plastic surgery consultations are elicited to perform the microvascular portion of the procedure.
[polldaddy:9881368]
The authors’ observations
Self-injurious behaviors occur in approximately 1% to 4% of adults in the United States, with chronic and severe self-injury occurring among approximately 1% of the U.S. population.1,2 Intentional GSM is a relatively rare catastrophic event that is often, but not solely, associated with severe mental illness. Because many cases go unreported, the prevalence of GSM is difficult to estimate.3,4 Although GSM has been described in both men and women, the literature has predominantly focused on GSM among men.5 Genital self-injury has been described in several (ie, ethnic/racial and religious) contexts and has been legally sanctioned.6-8
Psychiatric disorders associated with, and precipitating factors underlying, GSM have long remained elusive.8 GSM has been described in case reports and small case series in both psychiatric and urologic literature. These reports provide incomplete descriptions of the diagnostic conditions and psychosocial factors underlying male GSM.
A recent systematic review of 173 cases of men who engaged in GSM published in the past 115 years (since the first case of GSM was published in the psychiatric literature9) revealed that having some form of psychopathology elevates the probability of GSM10,11; rarely the individual did not have a psychiatric condition.11-17 Nearly one-half of the men had psychosis; most had a schizophrenia spectrum disorder diagnosis. Other psychiatric conditions associated with GSM include personality disorders, substance use disorder, and gender dysphoria. GSM is rarely associated with anxiety or mood disorders.
GSM is a heterogeneous form of self-injury that ranges from superficial genital lacerations, amputation, or castration to combinations of these injuries. Compared with individuals with other psychiatric disorders, a significantly greater proportion of individuals with schizophrenia spectrum disorders engage in self-amputation (auto-penectomy). By contrast, persons with gender dysphoria tend to engage in self-castration at significantly higher rates than those with other psychiatric conditions.11 Despite these trends, clinicians should not infer a specific psychiatric diagnosis based on the severity or type of self-inflicted injury.
HISTORY Command hallucinations
Postoperatively, Mr. K is managed in the trauma intensive care unit. During psychiatric consultation, Mr. K demonstrates a blunted affect. His speech is low in volume but clear and coherent. His thoughts are generally linear for specific lines of inquiry (eg, about perceived level of pain) but otherwise are impoverished. Mr. K often digresses into repetitively mumbled prayers. He appears distracted, as if responding to internal stimuli. Although he acknowledges the GSM, he does not discuss the factors underlying his decision to proceed with auto-penectomy. Over successive evaluations, he reluctantly discloses that he had been experiencing disparaging auditory hallucinations that told him that his penis “was too small” and commanded him to “cut it off.”
Psychiatric history reveals that Mr. K required psychiatric hospitalization 7 months earlier due to new-onset auditory hallucinations, paranoia, and thought disorganization, in the context of daily Cannabis use. At the time, the differential diagnosis included new-onset schizophrenia and substance-induced psychosis. His symptoms improved quickly with risperidone, 2 mg/d, and he was discharged in a stable condition with referrals for outpatient care. Mr. K admits he had stopped taking risperidone several weeks before the GSM because he was convinced that he had been cured. At that time, Mr. K had told his parents he was no longer required to take medication or engage in outpatient psychiatric treatment, and they did not question this. Mr. K struggled to sustain part-time employment (in a family business), having taken a leave of absence from graduate school after his first hospitalization. He continued to use Cannabis regularly but denies being intoxicated at the time of the GSM. Throughout his surgical hospitalization, Mr. K’s thoughts remain disorganized. He denies that the GSM was a suicide attempt or having current suicidal thoughts, intent, or plans. He also denies having religious preoccupations, over-valued religious beliefs, or delusions.
Mr. K identifies as heterosexual, and denies experiencing distress related to sexual orientation or gender identity or guilt related to sexual impulses or actions. He also denies having a history of trauma or victimization and does not report any symptoms of posttraumatic stress disorder or body dysmorphic disorder.
The authors’ observations
Little is known about how many individuals who engage in GSM eventually complete suicide. Although suicidal ideation and intent have been infrequently associated with GSM, suicide has been most notably reported among patients with schizophrenia spectrum disorders and psychotic mood disorders.11,18,23-26 For these individuals, suicidal ideation co-occurred with delusions, hallucinations, and pathological guilt preoccupations. Significant self-inflicted injury can be harbinger of distress that could lead to suicide if not optimally treated. Other psychosocial stressors, such as disruptions in interpersonal functioning arising from changes in or loss of social support or perceived rejection, may contribute to a patient’s level of distress, complicating underlying psychiatric disturbances and increasing vulnerability toward GSM.11,27
Substance use also increases vulnerability toward GSM.11,18,24,28 As is the case with patients who engage in various non-GSM self-injurious behaviors,29,30 substance use or intoxication likely contribute to disinhibition or a dissociative state, which enables individuals to engage in self-injury.30
A lack of access to treatment is a rare precipitant for GSM, except among individuals with gender dysphoria. Studies have found that many patients with gender dysphoria who performed self-castration did so in a premeditated manner with low suicidal intent, and the behavior often was related to a lack of or refusal for gender confirmation surgery.31-34
In the hospital setting, surgical/urological interventions need to be directed at the potentially life-threatening sequelae of self-injury. Although complications vary, depending on the type of injury incurred, urgent measures are needed to manage blood loss because hemorrhage can be fatal.23,35,36 Other consequences that can arise include urinary fistulae, urethral strictures, mummification of the glans penis, and development of sensory abnormalities after repair of the injured tissues or reattachment.8 More superficial injuries may require only hemostasis and simple suturing, whereas extensive injuries, such as complete amputation, can be addressed through microvascular techniques.
The psychiatrist’s role. The psychiatrist should act as an advocate for the GSM patient to create an environment conducive to healing. A patient who is experiencing hallucinations or delusions may feel overwhelmed by medical and familial attention. Pharmacologic treatment for prevailing mental illness, such as psychosis, should be initiated in the inpatient setting. An estimated 20% to 25% of those who self-inflict genital injury may repeatedly mutilate their genitals.19,28 Patients unduly influenced by command hallucinations, delusional thought processes, mood disturbances, or suicidal ideation may attempt to complete the injury, or reinjure themselves after surgical/urological intervention, which may require safety measures, such as 1:1 observation, restraints, or physical barriers, to prevent reinjury.37
Self-injury elicits strong, emotional responses from health care professionals, including fascination, apprehension, and hopelessness. Psychiatrists who care for such patients should monitor members of the patient’s treatment team for psychological reactions. In addition, the patient’s behavior while hospitalized may stir feelings of retaliation, anger, fear, and frustration.11,24,37 Collaborative relationships with medical and surgical specialties can help staff manage emotional reactions and avoid the inadvertent expression of those feelings in their interactions with the patient; these reactions might otherwise undermine treatment.24,34 Family education can help mitigate any guilt family members may harbor for not preventing the injury.37
Although efforts to understand the intended goal(s) and precipitants of the self-injury are likely to be worthwhile, the overwhelming distress associated with GSM and its emergent treatment may preclude intensive exploration.
TREATMENT Restarting medication
While on the surgical unit, Mr. K is restarted on risperidone, 2 mg/d. He appears to tolerate the medication without adverse effects. However, because Mr. K continues to experience auditory hallucinations, and the treatment team remains concerned that he might again experience commands to harm himself, he is transferred to an acute psychiatric inpatient setting.
Urology follow-up reveals necrosis/mummification of the replanted penis and an open scrotal wound. After discussing options with the patient and family, the urologist transfers Mr. K back to the surgical unit for wound closure and removal of the replanted penis. A urethrostomy is performed to allow for bladder emptying.
[polldaddy:9881371]
The authors’ observations
Because most published case reports of GSM among men have focused on acute treatment, there is a dearth of literature available on the long-term course of GSM to inform treatment strategies. Because recovery is a non-static process and a patient’s reactions to his injury will likely evolve over time, a multifaceted approach invoking psychiatric and psychotherapeutic interventions is necessary to help patients after initial injury and surgical management37,40-43 (Table 211,20,27,41).
OUTCOME Return to school, work
Mr. K is discharged with close follow-up at a specialized clinic for new-onset psychosis. Post-discharge treatment consists of education about the course of schizophrenia and the need for medication adherence to prevent relapse. Mr. K also is educated on the relationship between Cannabis use and psychosis, and he abstains from illicit substance use. Family involvement is encouraged to help with medication compliance and monitoring for symptom reemergence.
Therapy focuses on exploring the antecedents of the auto-penectomy, Mr. K’s body image issue concerns, and his feelings related to eventual prosthesis implantation. He insists that he cannot recall any precipitating factors for his self-injury other than the command hallucinations. He does not report sexual guilt, although he had been sexually active with his girlfriend in the months prior to his GSM, which goes against his family’s religious beliefs. He reports significant regret and shame for the self-mutilation, and blames himself for not informing family members about his hallucinations. Therapy involves addressing his attribution of blame using cognitive techniques and focuses on measures that can be taken to prevent further self-harm. Efforts are directed at exploring whether cultural and religious traditions impacted the therapeutic alliance, medication adherence, self-esteem and body image, sexuality, and future goals. Over the course of 1 year, he resumes his graduate studies and part-time work, and explores prosthetic placement for cosmetic purposes.
The authors’ observations
Research suggests that major self-mutilation among patients with psychotic illness is likely to occur during the first episode or early in the course of illness and/or with suboptimal treatment.44,45 Mr. K was enlisted in an intensive outpatient treatment program involving biweekly psychotherapy sessions and psychiatric follow-up. Initial sessions focused on education regarding the importance of medication adherence and exploration of signs and symptoms that might suggest reemergence of a psychotic decompensation. The psychiatrist monitored Mr. K closely to ensure he was able to tolerate his medications to mitigate the possibility that adverse effects would undermine adherence. Mr. K’s reactions to having a psychiatric illness also were explored because of concerns that such self-appraisals might trigger shame, embarrassment, denial, and other responses that might undermine treatment adherence. His family members were apprised of treatment goals and enlisted to foster adherence with medication and follow-up appointments.
Mr. K’s Cannabis use was addressed because ongoing use likely had a negative impact on his schizophrenia (ie, a greater propensity toward relapse and rehospitalization and a poorer therapeutic response to antipsychotic medication).46,47 He was strongly encouraged to avoid Cannabis and other illicit substances.
Psychiatrists can help in examining the meaning behind the injury while helping the patient to adapt to the sequelae and cultivate skills to meet functional demands.41 Once Mr. K’s psychotic symptoms were in remission, treatment began to address the antecedents of the GSM, as well as the resultant physical consequences. It was reasonable to explore how Mr. K now viewed his actions, as well as the consequences that his actions produced in terms of his physical appearance, sexual functioning, capacity for sexual intimacy, and reproductive potential. It was also important to recognize how such highly intimate and deeply personal self-schema are framed and organized against his cultural and religious background.27,33
Body image concerns and expectations for future urologic intervention also should be explored. Although Mr. K was not averse to such exploration, he did not spontaneously address such topics in great depth. The discussion was unforced and effectively left open as an issue that could be explored in future sessions.
CASE Bleeding, bewildered
Mr. K, age 23, a South Asian male, is discovered in the bathroom bleeding profusely. Mr. K’s parents inform emergency medical services (EMS) personnel that Mr. K is “not in his right mind” and speculate that he is depressed. EMS personnel find Mr. K sitting in a pool of blood in the bathtub, holding a cloth over his pubic area and complaining of significant pain. They estimate that Mr. K has lost approximately 1 L of blood. Cursory evaluation reveals that his penis is severed; no other injuries or lacerations are notable. Mr. K states, “I did not want it anymore.” A kitchen knife that he used to self-amputate is found nearby. He is awake, alert, and able to follow simple directives.
In the emergency room, Mr. K is in mild-to-moderate distress. He has no history of medical illness, but his parents report that he previously required psychiatric treatment. Mr. K is not able to elaborate. He reluctantly discloses an intermittent history of Cannabis use. Physical examination reveals tachycardia (heart rate: 115 to 120 beats per minute), and despite blood loss, systolic hypertension (blood pressure: 142/70 to 167/70 mm Hg). His pulse oximetry is 97% to 99%; he is afebrile. Laboratory tests are notable for anemia (hemoglobin, 7.2 g/dL [reference range, 14.0 to 17.5 g/dL]; hematocrit, 21.2% [reference range, 41% to 50%]) and serum toxicology screen is positive for benzodiazepines, which had been administered en route to allay his distress.
Mr. K continues to hold pressure on his pubic area. When pressure is released, active arterial spurting of bright red blood is notable. Genital examination reveals a cleanly amputated phallus. Emergent surgical intervention is required to stop the hemorrhage and reattach the penis. Initially, Mr. K is opposed to reattachment, but after a brief discussion with his parents, he consents to surgery. Urology and plastic surgery consultations are elicited to perform the microvascular portion of the procedure.
[polldaddy:9881368]
The authors’ observations
Self-injurious behaviors occur in approximately 1% to 4% of adults in the United States, with chronic and severe self-injury occurring among approximately 1% of the U.S. population.1,2 Intentional GSM is a relatively rare catastrophic event that is often, but not solely, associated with severe mental illness. Because many cases go unreported, the prevalence of GSM is difficult to estimate.3,4 Although GSM has been described in both men and women, the literature has predominantly focused on GSM among men.5 Genital self-injury has been described in several (ie, ethnic/racial and religious) contexts and has been legally sanctioned.6-8
Psychiatric disorders associated with, and precipitating factors underlying, GSM have long remained elusive.8 GSM has been described in case reports and small case series in both psychiatric and urologic literature. These reports provide incomplete descriptions of the diagnostic conditions and psychosocial factors underlying male GSM.
A recent systematic review of 173 cases of men who engaged in GSM published in the past 115 years (since the first case of GSM was published in the psychiatric literature9) revealed that having some form of psychopathology elevates the probability of GSM10,11; rarely the individual did not have a psychiatric condition.11-17 Nearly one-half of the men had psychosis; most had a schizophrenia spectrum disorder diagnosis. Other psychiatric conditions associated with GSM include personality disorders, substance use disorder, and gender dysphoria. GSM is rarely associated with anxiety or mood disorders.
GSM is a heterogeneous form of self-injury that ranges from superficial genital lacerations, amputation, or castration to combinations of these injuries. Compared with individuals with other psychiatric disorders, a significantly greater proportion of individuals with schizophrenia spectrum disorders engage in self-amputation (auto-penectomy). By contrast, persons with gender dysphoria tend to engage in self-castration at significantly higher rates than those with other psychiatric conditions.11 Despite these trends, clinicians should not infer a specific psychiatric diagnosis based on the severity or type of self-inflicted injury.
HISTORY Command hallucinations
Postoperatively, Mr. K is managed in the trauma intensive care unit. During psychiatric consultation, Mr. K demonstrates a blunted affect. His speech is low in volume but clear and coherent. His thoughts are generally linear for specific lines of inquiry (eg, about perceived level of pain) but otherwise are impoverished. Mr. K often digresses into repetitively mumbled prayers. He appears distracted, as if responding to internal stimuli. Although he acknowledges the GSM, he does not discuss the factors underlying his decision to proceed with auto-penectomy. Over successive evaluations, he reluctantly discloses that he had been experiencing disparaging auditory hallucinations that told him that his penis “was too small” and commanded him to “cut it off.”
Psychiatric history reveals that Mr. K required psychiatric hospitalization 7 months earlier due to new-onset auditory hallucinations, paranoia, and thought disorganization, in the context of daily Cannabis use. At the time, the differential diagnosis included new-onset schizophrenia and substance-induced psychosis. His symptoms improved quickly with risperidone, 2 mg/d, and he was discharged in a stable condition with referrals for outpatient care. Mr. K admits he had stopped taking risperidone several weeks before the GSM because he was convinced that he had been cured. At that time, Mr. K had told his parents he was no longer required to take medication or engage in outpatient psychiatric treatment, and they did not question this. Mr. K struggled to sustain part-time employment (in a family business), having taken a leave of absence from graduate school after his first hospitalization. He continued to use Cannabis regularly but denies being intoxicated at the time of the GSM. Throughout his surgical hospitalization, Mr. K’s thoughts remain disorganized. He denies that the GSM was a suicide attempt or having current suicidal thoughts, intent, or plans. He also denies having religious preoccupations, over-valued religious beliefs, or delusions.
Mr. K identifies as heterosexual, and denies experiencing distress related to sexual orientation or gender identity or guilt related to sexual impulses or actions. He also denies having a history of trauma or victimization and does not report any symptoms of posttraumatic stress disorder or body dysmorphic disorder.
The authors’ observations
Little is known about how many individuals who engage in GSM eventually complete suicide. Although suicidal ideation and intent have been infrequently associated with GSM, suicide has been most notably reported among patients with schizophrenia spectrum disorders and psychotic mood disorders.11,18,23-26 For these individuals, suicidal ideation co-occurred with delusions, hallucinations, and pathological guilt preoccupations. Significant self-inflicted injury can be harbinger of distress that could lead to suicide if not optimally treated. Other psychosocial stressors, such as disruptions in interpersonal functioning arising from changes in or loss of social support or perceived rejection, may contribute to a patient’s level of distress, complicating underlying psychiatric disturbances and increasing vulnerability toward GSM.11,27
Substance use also increases vulnerability toward GSM.11,18,24,28 As is the case with patients who engage in various non-GSM self-injurious behaviors,29,30 substance use or intoxication likely contribute to disinhibition or a dissociative state, which enables individuals to engage in self-injury.30
A lack of access to treatment is a rare precipitant for GSM, except among individuals with gender dysphoria. Studies have found that many patients with gender dysphoria who performed self-castration did so in a premeditated manner with low suicidal intent, and the behavior often was related to a lack of or refusal for gender confirmation surgery.31-34
In the hospital setting, surgical/urological interventions need to be directed at the potentially life-threatening sequelae of self-injury. Although complications vary, depending on the type of injury incurred, urgent measures are needed to manage blood loss because hemorrhage can be fatal.23,35,36 Other consequences that can arise include urinary fistulae, urethral strictures, mummification of the glans penis, and development of sensory abnormalities after repair of the injured tissues or reattachment.8 More superficial injuries may require only hemostasis and simple suturing, whereas extensive injuries, such as complete amputation, can be addressed through microvascular techniques.
The psychiatrist’s role. The psychiatrist should act as an advocate for the GSM patient to create an environment conducive to healing. A patient who is experiencing hallucinations or delusions may feel overwhelmed by medical and familial attention. Pharmacologic treatment for prevailing mental illness, such as psychosis, should be initiated in the inpatient setting. An estimated 20% to 25% of those who self-inflict genital injury may repeatedly mutilate their genitals.19,28 Patients unduly influenced by command hallucinations, delusional thought processes, mood disturbances, or suicidal ideation may attempt to complete the injury, or reinjure themselves after surgical/urological intervention, which may require safety measures, such as 1:1 observation, restraints, or physical barriers, to prevent reinjury.37
Self-injury elicits strong, emotional responses from health care professionals, including fascination, apprehension, and hopelessness. Psychiatrists who care for such patients should monitor members of the patient’s treatment team for psychological reactions. In addition, the patient’s behavior while hospitalized may stir feelings of retaliation, anger, fear, and frustration.11,24,37 Collaborative relationships with medical and surgical specialties can help staff manage emotional reactions and avoid the inadvertent expression of those feelings in their interactions with the patient; these reactions might otherwise undermine treatment.24,34 Family education can help mitigate any guilt family members may harbor for not preventing the injury.37
Although efforts to understand the intended goal(s) and precipitants of the self-injury are likely to be worthwhile, the overwhelming distress associated with GSM and its emergent treatment may preclude intensive exploration.
TREATMENT Restarting medication
While on the surgical unit, Mr. K is restarted on risperidone, 2 mg/d. He appears to tolerate the medication without adverse effects. However, because Mr. K continues to experience auditory hallucinations, and the treatment team remains concerned that he might again experience commands to harm himself, he is transferred to an acute psychiatric inpatient setting.
Urology follow-up reveals necrosis/mummification of the replanted penis and an open scrotal wound. After discussing options with the patient and family, the urologist transfers Mr. K back to the surgical unit for wound closure and removal of the replanted penis. A urethrostomy is performed to allow for bladder emptying.
[polldaddy:9881371]
The authors’ observations
Because most published case reports of GSM among men have focused on acute treatment, there is a dearth of literature available on the long-term course of GSM to inform treatment strategies. Because recovery is a non-static process and a patient’s reactions to his injury will likely evolve over time, a multifaceted approach invoking psychiatric and psychotherapeutic interventions is necessary to help patients after initial injury and surgical management37,40-43 (Table 211,20,27,41).
OUTCOME Return to school, work
Mr. K is discharged with close follow-up at a specialized clinic for new-onset psychosis. Post-discharge treatment consists of education about the course of schizophrenia and the need for medication adherence to prevent relapse. Mr. K also is educated on the relationship between Cannabis use and psychosis, and he abstains from illicit substance use. Family involvement is encouraged to help with medication compliance and monitoring for symptom reemergence.
Therapy focuses on exploring the antecedents of the auto-penectomy, Mr. K’s body image issue concerns, and his feelings related to eventual prosthesis implantation. He insists that he cannot recall any precipitating factors for his self-injury other than the command hallucinations. He does not report sexual guilt, although he had been sexually active with his girlfriend in the months prior to his GSM, which goes against his family’s religious beliefs. He reports significant regret and shame for the self-mutilation, and blames himself for not informing family members about his hallucinations. Therapy involves addressing his attribution of blame using cognitive techniques and focuses on measures that can be taken to prevent further self-harm. Efforts are directed at exploring whether cultural and religious traditions impacted the therapeutic alliance, medication adherence, self-esteem and body image, sexuality, and future goals. Over the course of 1 year, he resumes his graduate studies and part-time work, and explores prosthetic placement for cosmetic purposes.
The authors’ observations
Research suggests that major self-mutilation among patients with psychotic illness is likely to occur during the first episode or early in the course of illness and/or with suboptimal treatment.44,45 Mr. K was enlisted in an intensive outpatient treatment program involving biweekly psychotherapy sessions and psychiatric follow-up. Initial sessions focused on education regarding the importance of medication adherence and exploration of signs and symptoms that might suggest reemergence of a psychotic decompensation. The psychiatrist monitored Mr. K closely to ensure he was able to tolerate his medications to mitigate the possibility that adverse effects would undermine adherence. Mr. K’s reactions to having a psychiatric illness also were explored because of concerns that such self-appraisals might trigger shame, embarrassment, denial, and other responses that might undermine treatment adherence. His family members were apprised of treatment goals and enlisted to foster adherence with medication and follow-up appointments.
Mr. K’s Cannabis use was addressed because ongoing use likely had a negative impact on his schizophrenia (ie, a greater propensity toward relapse and rehospitalization and a poorer therapeutic response to antipsychotic medication).46,47 He was strongly encouraged to avoid Cannabis and other illicit substances.
Psychiatrists can help in examining the meaning behind the injury while helping the patient to adapt to the sequelae and cultivate skills to meet functional demands.41 Once Mr. K’s psychotic symptoms were in remission, treatment began to address the antecedents of the GSM, as well as the resultant physical consequences. It was reasonable to explore how Mr. K now viewed his actions, as well as the consequences that his actions produced in terms of his physical appearance, sexual functioning, capacity for sexual intimacy, and reproductive potential. It was also important to recognize how such highly intimate and deeply personal self-schema are framed and organized against his cultural and religious background.27,33
Body image concerns and expectations for future urologic intervention also should be explored. Although Mr. K was not averse to such exploration, he did not spontaneously address such topics in great depth. The discussion was unforced and effectively left open as an issue that could be explored in future sessions.
1. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68(4):609-620.
2. Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501-1508.
3. Krasucki C, Kemp R, David A. A case study of female genital self-mutilation in schizophrenia. Br J Med Psychol. 1995;68(pt 2):179-186.
4. Lennon S. Genital self-mutilation in acute mania. Med J Aust. 1963;50(1):79-81.
5. Schweitzer I. Genital self-amputation and the Klingsor syndrome. Aust N Z J Psychiatry. 1990;24(4):566-569.
6. Anumonye A. Self-inflicted amputation of the penis in two Nigerian males. Niger Med J. 1973;3(1):51-52.
7. Bowman KM, Crook GH. Emotional changes following castration. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:81-96.
8. Eke N. Genital self-mutilation: there is no method in this madness. BJU Int. 2000;85(3):295-298.
9. Stroch D. Self-castration. JAMA. 1901;36(4):270.
10. Veeder TA, Leo RJ. Male genital self-mutilation: a comprehensive review of psychiatric disorders. Poster presented at: Academy of Psychosomatic Medicine Meeting, Austin, Texas, November 10, 2016.
11. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry. 2017;44:43-50.
12. Battle AO. The psychological appraisal of a patient who had performed self-castration. British Journal of Projective Psychology & Personality Study. 1973;18(2):5-17.
13. Bhatia MS, Arora S. Penile self-mutilation. Br J Psychiatry. 2001;178(1):86-87.
14. Gleeson MJ, Connolly J, Grainger R. Self-castration as treatment for alopecia. Br J Urol. 1993;71(5):614-615.
15. Hendershot E, Stutson AC, Adair TW. A case of extreme sexual self-mutilation. J Forensic Sci. 2010;55(1):245-247.
16. Hermann M, Thorstenson A. A rare case of male‐to‐eunuch gender dysphoria. Sex Med. 2015;3(4):331-333.
17. Nerli RB, Ravish IR, Amarkhed SS, et al. Genital self-mutilation in nonpsychotic heterosexual males: case report of two cases. Indian J Psychiatry. 2008;50(4):285-287.
18. Blacker KH, Wong N. Four cases of autocastration. Arch Gen Psychiatry. 1963;8:169-176.
19. Catalano G, Catalano MC, Carroll KM. Repetitive male genital self-mutilation: a case report and discussion of possible risk factors. J Sex Marital Ther. 2002;28(1):27-37.
20. Martin T, Gattaz WF. Psychiatric aspects of male genital self-mutilation. Psychopathology. 1991;24(3):170-178.
21. Money J. The Skoptic syndrome: castration and genital self-mutilation as an example of sexual body-image pathology. J Psychol Human Sex. 1988;1(1):113-128.
22. Nakaya M. On background factors of male genital self-mutilation. Psychopathology. 1996;29(4):242-248.
23. Borenstein A, Yaffe B, Seidman DS, et al. Successful microvascular replantation of an amputated penis. Isr J Med Sci. 1991;27(7):395-398.
24. Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36(4):441-446.
25. Mendez R, Kiely WF, Morrow JW. Self-emasculation. J Urol. 1972;107(6):981-985.
26. Siddique RA, Deshpande S. A case of genital self-mutilation in a patient with psychosis. German J Psychiatry. 2007;10(1):25-28.
27. Qureshi NA. Male genital self-mutilation with special emphasis on the sociocultural meanings. Neurosciences (Riyadh). 2009;14(2):178-181.
28. Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55(7):427-431.
29. Gahr M, Plener PL, Kölle MA, et al. Self-mutilation induced by psychotropic substances: a systematic review. Psychiatry Res. 2012;200(2-3):977-983.
30. Evren C, Sar V, Evren B, et al. Self-mutilation among male patients with alcohol dependency: the role of dissociation. Compr Psychiatry. 2008;49(5):489-495.
31. Brown GR. Autocastration and autopenectomy as surgical self-treatment in incarcerated persons with gender identity disorder. Int J Transgend. 2010;12(1):31-39.
32. Master VA, McAninch JW, Santucci RA. Genital self-mutilation and the Internet. J Urol. 2000;164(5):1656.
33. Premand NE, Eytan A. A case of non-psychotic autocastration: the importance of cultural factors. Psychiatry. 2005;68(2):174-178.
34. Simopoulos EF, Trinidad AC. Two cases of male genital self-mutilation: an examination of liaison dynamics. Psychosomatics. 2012;53(2):178-180.
35. Darewicz B, Galek L, Darewicz J, et al. Successful microsurgical replantation of an amputated penis. Int Urol Nephrol. 2001;33(2):385-386.
36. Raheem OA, Mirheydar HS, Patel ND, et al. Surgical management of traumatic penile amputation: a case report and review of the world literature. Sex Med. 2015;3(1):49-53.
37. Young LD, Feinsilver DL. Male genital self-mutilation: combined surgical and psychiatric care. Psychosomatics. 1986;27(7):513-517.
38. Walsh B. Clinical assessment of self-injury: a practical guide. J Clin Psychol. 2007;63(11):1057-1066.
39. Nafisi N, Stanley B. Developing and maintaining the therapeutic alliance with self-injuring patients. J Clin Psychol. 2007;63(11):1069-1079.
40. Fisch RZ. Genital self-mutilation in males: psychodynamic anatomy of a psychosis. Am J Psychother. 1987;41(3):453-458.
41. King PR. Cognitive-behavioral intervention in a case of self-mutilation. Clin Case Stud. 2014;13(2):181-189.
42. Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. J Ment Health Couns. 2006;28(2):166-185.
43. Walsh BW. Treating self-injury: a practical guide. New York, NY: The Guilford Press; 2006.
44. Large M, Babidge N, Andrews D, et al. Major self-mutilation in the first episode of psychosis. Schizophr Bull. 2009;35(5):1012-1021.
45. Large MM, Nielssen OB, Babidge N. Untreated psychosis is the main cause of major self-mutilation. Isr J Psychiatry Relat Sci. 2011;48(1):65.
46. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21.
47. Bowers MB Jr, Mazure CM, Nelson JC, et al. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16(1):81-85.
1. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68(4):609-620.
2. Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501-1508.
3. Krasucki C, Kemp R, David A. A case study of female genital self-mutilation in schizophrenia. Br J Med Psychol. 1995;68(pt 2):179-186.
4. Lennon S. Genital self-mutilation in acute mania. Med J Aust. 1963;50(1):79-81.
5. Schweitzer I. Genital self-amputation and the Klingsor syndrome. Aust N Z J Psychiatry. 1990;24(4):566-569.
6. Anumonye A. Self-inflicted amputation of the penis in two Nigerian males. Niger Med J. 1973;3(1):51-52.
7. Bowman KM, Crook GH. Emotional changes following castration. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:81-96.
8. Eke N. Genital self-mutilation: there is no method in this madness. BJU Int. 2000;85(3):295-298.
9. Stroch D. Self-castration. JAMA. 1901;36(4):270.
10. Veeder TA, Leo RJ. Male genital self-mutilation: a comprehensive review of psychiatric disorders. Poster presented at: Academy of Psychosomatic Medicine Meeting, Austin, Texas, November 10, 2016.
11. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry. 2017;44:43-50.
12. Battle AO. The psychological appraisal of a patient who had performed self-castration. British Journal of Projective Psychology & Personality Study. 1973;18(2):5-17.
13. Bhatia MS, Arora S. Penile self-mutilation. Br J Psychiatry. 2001;178(1):86-87.
14. Gleeson MJ, Connolly J, Grainger R. Self-castration as treatment for alopecia. Br J Urol. 1993;71(5):614-615.
15. Hendershot E, Stutson AC, Adair TW. A case of extreme sexual self-mutilation. J Forensic Sci. 2010;55(1):245-247.
16. Hermann M, Thorstenson A. A rare case of male‐to‐eunuch gender dysphoria. Sex Med. 2015;3(4):331-333.
17. Nerli RB, Ravish IR, Amarkhed SS, et al. Genital self-mutilation in nonpsychotic heterosexual males: case report of two cases. Indian J Psychiatry. 2008;50(4):285-287.
18. Blacker KH, Wong N. Four cases of autocastration. Arch Gen Psychiatry. 1963;8:169-176.
19. Catalano G, Catalano MC, Carroll KM. Repetitive male genital self-mutilation: a case report and discussion of possible risk factors. J Sex Marital Ther. 2002;28(1):27-37.
20. Martin T, Gattaz WF. Psychiatric aspects of male genital self-mutilation. Psychopathology. 1991;24(3):170-178.
21. Money J. The Skoptic syndrome: castration and genital self-mutilation as an example of sexual body-image pathology. J Psychol Human Sex. 1988;1(1):113-128.
22. Nakaya M. On background factors of male genital self-mutilation. Psychopathology. 1996;29(4):242-248.
23. Borenstein A, Yaffe B, Seidman DS, et al. Successful microvascular replantation of an amputated penis. Isr J Med Sci. 1991;27(7):395-398.
24. Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36(4):441-446.
25. Mendez R, Kiely WF, Morrow JW. Self-emasculation. J Urol. 1972;107(6):981-985.
26. Siddique RA, Deshpande S. A case of genital self-mutilation in a patient with psychosis. German J Psychiatry. 2007;10(1):25-28.
27. Qureshi NA. Male genital self-mutilation with special emphasis on the sociocultural meanings. Neurosciences (Riyadh). 2009;14(2):178-181.
28. Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55(7):427-431.
29. Gahr M, Plener PL, Kölle MA, et al. Self-mutilation induced by psychotropic substances: a systematic review. Psychiatry Res. 2012;200(2-3):977-983.
30. Evren C, Sar V, Evren B, et al. Self-mutilation among male patients with alcohol dependency: the role of dissociation. Compr Psychiatry. 2008;49(5):489-495.
31. Brown GR. Autocastration and autopenectomy as surgical self-treatment in incarcerated persons with gender identity disorder. Int J Transgend. 2010;12(1):31-39.
32. Master VA, McAninch JW, Santucci RA. Genital self-mutilation and the Internet. J Urol. 2000;164(5):1656.
33. Premand NE, Eytan A. A case of non-psychotic autocastration: the importance of cultural factors. Psychiatry. 2005;68(2):174-178.
34. Simopoulos EF, Trinidad AC. Two cases of male genital self-mutilation: an examination of liaison dynamics. Psychosomatics. 2012;53(2):178-180.
35. Darewicz B, Galek L, Darewicz J, et al. Successful microsurgical replantation of an amputated penis. Int Urol Nephrol. 2001;33(2):385-386.
36. Raheem OA, Mirheydar HS, Patel ND, et al. Surgical management of traumatic penile amputation: a case report and review of the world literature. Sex Med. 2015;3(1):49-53.
37. Young LD, Feinsilver DL. Male genital self-mutilation: combined surgical and psychiatric care. Psychosomatics. 1986;27(7):513-517.
38. Walsh B. Clinical assessment of self-injury: a practical guide. J Clin Psychol. 2007;63(11):1057-1066.
39. Nafisi N, Stanley B. Developing and maintaining the therapeutic alliance with self-injuring patients. J Clin Psychol. 2007;63(11):1069-1079.
40. Fisch RZ. Genital self-mutilation in males: psychodynamic anatomy of a psychosis. Am J Psychother. 1987;41(3):453-458.
41. King PR. Cognitive-behavioral intervention in a case of self-mutilation. Clin Case Stud. 2014;13(2):181-189.
42. Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. J Ment Health Couns. 2006;28(2):166-185.
43. Walsh BW. Treating self-injury: a practical guide. New York, NY: The Guilford Press; 2006.
44. Large M, Babidge N, Andrews D, et al. Major self-mutilation in the first episode of psychosis. Schizophr Bull. 2009;35(5):1012-1021.
45. Large MM, Nielssen OB, Babidge N. Untreated psychosis is the main cause of major self-mutilation. Isr J Psychiatry Relat Sci. 2011;48(1):65.
46. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21.
47. Bowers MB Jr, Mazure CM, Nelson JC, et al. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16(1):81-85.