User login
Hemorrhagic Crescent Sign in Pseudocellulitis
To the Editor:
Cellulitis is the most common reason for skin-related hospital admissions.1 Despite its frequency, it is suspected that many cases of cellulitis are misdiagnosed as other etiologies presenting with similar symptoms such as a ruptured Baker cyst. These cysts are located behind the knee and can present with calf pain, peripheral edema, and erythema when ruptured. Symptoms of a ruptured Baker cyst can be indistinguishable from cellulitis as well as deep vein thrombosis (DVT), both manifesting with lower extremity pain, swelling, and erythema, making diagnosis challenging.2 The hemorrhagic crescent sign—a crescent of ecchymosis distal to the medial malleolus and on the foot that results from synovial injury or rupture—can be a useful diagnostic tool to differentiate between the causes of acute swelling and pain of the leg.2 When observed, the hemorrhagic crescent sign supports testing for synovial pathology at the knee.
A 63-year-old man presented to an outside hospital for evaluation of a fever (temperature, 101 °F [38.3 °C]), as well as pain, edema, and erythema of the right lower leg of 2 days’ duration. He had a history of leg cellulitis, gout, diabetes mellitus, lymphedema, and peripheral neuropathy. On admission, he was found to have elevated C-reactive protein (45 mg/L [reference range, <8 mg/L]) and mild leukocytosis (13,500 cells/μL [reference range, 4500–11,000 cells/μL]). A venous duplex scan did not demonstrate signs of thrombosis. Antibiotic therapy was started for suspected cellulitis including levofloxacin, piperacillin-tazobactam, and linezolid. Despite broad-spectrum antibiotic coverage, the patient continued to be febrile and experienced progressive erythema and swelling of the right lower leg, at which point he was transferred to our institution. A new antibiotic regimen of vancomycin, cefepime, and clindamycin was started and showed no improvement, after which dermatology was consulted.
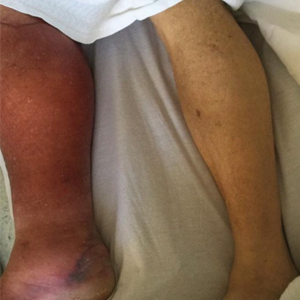
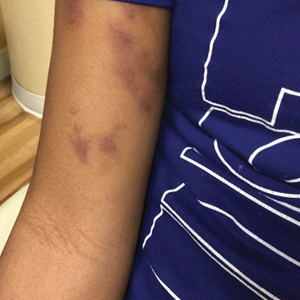
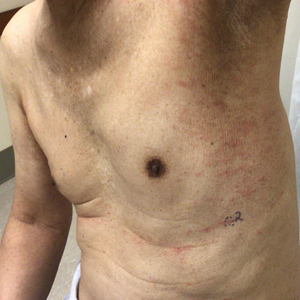
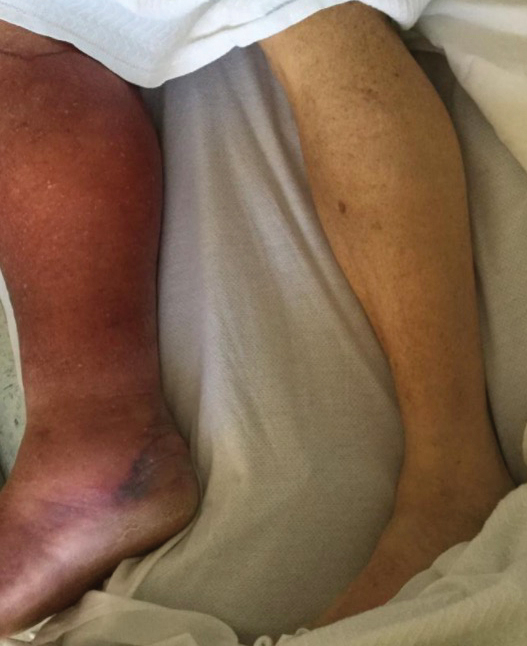
Physical examination revealed unilateral edema and calor of the right lower leg with a demarcated erythematous rash extending to the level of the knee. Furthermore, a hemorrhagic crescent sign was present below the right medial malleolus (Figure). The popliteal fossa was supple, though the patient was adamant that he had a Baker cyst. Punch biopsies demonstrated epidermal spongiosis and extensive edema with perivascular inflammation. No organisms were found by stain and culture. Ultrasound records confirmed a Baker cyst present at least 4 months prior; however, a repeat ultrasound showed resolution. A diagnosis of pseudocellulitis secondary to Baker cyst rupture was made, and corticosteroids were started, resulting in marked reduction in erythema and edema of the lower leg and hospital discharge.
This case highlights the importance of early involvement of dermatology when cellulitis is suspected. A study of 635 patients in the United Kingdom referred to dermatology for lower limb cellulitis found that 210 (33%) patients did not have cellulitis and only 18 (3%) required hospital admission.3 Dermatology consultations have been shown to benefit patients with inflammatory skin disease by decreasing length of stay and reducing readmissions.4
Our patient had several risk factors for cellulitis, including obesity, lymphedema, and chronic kidney disease, in addition to having fevers and unilateral involvement. However, failure of symptoms to improve with broad-spectrum antibiotics made a diagnosis of cellulitis less likely. In this case, a severe immune response to the ruptured Baker cyst mimicked the presentation of cellulitis.
Ruptured Baker cysts have been reported to cause acute leg swelling, mimicking the symptoms of cellulitis or DVT.2,5 The presence of a hemorrhagic crescent sign can be a useful diagnostic tool, as in our patient, because it has been reported as an indication of synovial injury or rupture, supporting the exclusion of cellulitis or DVT when it is observed.6 Prior reports have observed ecchymosis on the foot in as little as 1 day after the onset of calf swelling and at the lateral malleolus 3 days after the onset of calf swelling.5
Following suspicion of a ruptured Baker cyst causing pseudocellulitis, an ultrasound can be used to confirm the diagnosis. Ultrasonography shows a large hypoechoic space behind the calf muscles, which is pathognomonic of a ruptured Baker cyst.7
In conclusion, when a hemorrhagic crescent sign is observed, one should be suspicious for a ruptured Baker cyst or other synovial pathology as an etiology of pseudocellulitis. Early recognition of the hemorrhagic crescent sign can help rule out cellulitis and DVT, thereby reducing the amount of intravenous antibiotic prescribed, decreasing the length of hospital stay, and reducing readmission.
- Feldman SR, Fleischer AB, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730. doi:10.1001/archinte.158.7.726
- Von Schroeder HP, Ameli FM, Piazza D, et al. Ruptured Baker’s cyst causes ecchymosis of the foot. J Bone Joint Surg Br. 1993;75:316-317.
- Levell NJ, Wingfield CG, Garioch JJ. Severe lower limb cellulitis is best diagnosed by dermatologists and managed with shared care between primary and secondary care. Br J Dermatol. 2011;164:1326-1328.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;53:523-528.
- Dunlop D, Parker PJ, Keating JF. Ruptured Baker’s cyst causing posterior compartment syndrome. Injury. 1997;28:561-562.
- Kraag G, Thevathasan EM, Gordon DA, et al. The hemorrhagic crescent sign of acute synovial rupture. Ann Intern Med. 1976;85:477-478.
- Sato O, Kondoh K, Iyori K, et al. Midcalf ultrasonography for the diagnosis of ruptured Baker’s cysts. Surg Today. 2001;31:410-413. doi:10.1007/s005950170131
To the Editor:
Cellulitis is the most common reason for skin-related hospital admissions.1 Despite its frequency, it is suspected that many cases of cellulitis are misdiagnosed as other etiologies presenting with similar symptoms such as a ruptured Baker cyst. These cysts are located behind the knee and can present with calf pain, peripheral edema, and erythema when ruptured. Symptoms of a ruptured Baker cyst can be indistinguishable from cellulitis as well as deep vein thrombosis (DVT), both manifesting with lower extremity pain, swelling, and erythema, making diagnosis challenging.2 The hemorrhagic crescent sign—a crescent of ecchymosis distal to the medial malleolus and on the foot that results from synovial injury or rupture—can be a useful diagnostic tool to differentiate between the causes of acute swelling and pain of the leg.2 When observed, the hemorrhagic crescent sign supports testing for synovial pathology at the knee.
A 63-year-old man presented to an outside hospital for evaluation of a fever (temperature, 101 °F [38.3 °C]), as well as pain, edema, and erythema of the right lower leg of 2 days’ duration. He had a history of leg cellulitis, gout, diabetes mellitus, lymphedema, and peripheral neuropathy. On admission, he was found to have elevated C-reactive protein (45 mg/L [reference range, <8 mg/L]) and mild leukocytosis (13,500 cells/μL [reference range, 4500–11,000 cells/μL]). A venous duplex scan did not demonstrate signs of thrombosis. Antibiotic therapy was started for suspected cellulitis including levofloxacin, piperacillin-tazobactam, and linezolid. Despite broad-spectrum antibiotic coverage, the patient continued to be febrile and experienced progressive erythema and swelling of the right lower leg, at which point he was transferred to our institution. A new antibiotic regimen of vancomycin, cefepime, and clindamycin was started and showed no improvement, after which dermatology was consulted.
Physical examination revealed unilateral edema and calor of the right lower leg with a demarcated erythematous rash extending to the level of the knee. Furthermore, a hemorrhagic crescent sign was present below the right medial malleolus (Figure). The popliteal fossa was supple, though the patient was adamant that he had a Baker cyst. Punch biopsies demonstrated epidermal spongiosis and extensive edema with perivascular inflammation. No organisms were found by stain and culture. Ultrasound records confirmed a Baker cyst present at least 4 months prior; however, a repeat ultrasound showed resolution. A diagnosis of pseudocellulitis secondary to Baker cyst rupture was made, and corticosteroids were started, resulting in marked reduction in erythema and edema of the lower leg and hospital discharge.

This case highlights the importance of early involvement of dermatology when cellulitis is suspected. A study of 635 patients in the United Kingdom referred to dermatology for lower limb cellulitis found that 210 (33%) patients did not have cellulitis and only 18 (3%) required hospital admission.3 Dermatology consultations have been shown to benefit patients with inflammatory skin disease by decreasing length of stay and reducing readmissions.4
Our patient had several risk factors for cellulitis, including obesity, lymphedema, and chronic kidney disease, in addition to having fevers and unilateral involvement. However, failure of symptoms to improve with broad-spectrum antibiotics made a diagnosis of cellulitis less likely. In this case, a severe immune response to the ruptured Baker cyst mimicked the presentation of cellulitis.
Ruptured Baker cysts have been reported to cause acute leg swelling, mimicking the symptoms of cellulitis or DVT.2,5 The presence of a hemorrhagic crescent sign can be a useful diagnostic tool, as in our patient, because it has been reported as an indication of synovial injury or rupture, supporting the exclusion of cellulitis or DVT when it is observed.6 Prior reports have observed ecchymosis on the foot in as little as 1 day after the onset of calf swelling and at the lateral malleolus 3 days after the onset of calf swelling.5
Following suspicion of a ruptured Baker cyst causing pseudocellulitis, an ultrasound can be used to confirm the diagnosis. Ultrasonography shows a large hypoechoic space behind the calf muscles, which is pathognomonic of a ruptured Baker cyst.7
In conclusion, when a hemorrhagic crescent sign is observed, one should be suspicious for a ruptured Baker cyst or other synovial pathology as an etiology of pseudocellulitis. Early recognition of the hemorrhagic crescent sign can help rule out cellulitis and DVT, thereby reducing the amount of intravenous antibiotic prescribed, decreasing the length of hospital stay, and reducing readmission.
To the Editor:
Cellulitis is the most common reason for skin-related hospital admissions.1 Despite its frequency, it is suspected that many cases of cellulitis are misdiagnosed as other etiologies presenting with similar symptoms such as a ruptured Baker cyst. These cysts are located behind the knee and can present with calf pain, peripheral edema, and erythema when ruptured. Symptoms of a ruptured Baker cyst can be indistinguishable from cellulitis as well as deep vein thrombosis (DVT), both manifesting with lower extremity pain, swelling, and erythema, making diagnosis challenging.2 The hemorrhagic crescent sign—a crescent of ecchymosis distal to the medial malleolus and on the foot that results from synovial injury or rupture—can be a useful diagnostic tool to differentiate between the causes of acute swelling and pain of the leg.2 When observed, the hemorrhagic crescent sign supports testing for synovial pathology at the knee.
A 63-year-old man presented to an outside hospital for evaluation of a fever (temperature, 101 °F [38.3 °C]), as well as pain, edema, and erythema of the right lower leg of 2 days’ duration. He had a history of leg cellulitis, gout, diabetes mellitus, lymphedema, and peripheral neuropathy. On admission, he was found to have elevated C-reactive protein (45 mg/L [reference range, <8 mg/L]) and mild leukocytosis (13,500 cells/μL [reference range, 4500–11,000 cells/μL]). A venous duplex scan did not demonstrate signs of thrombosis. Antibiotic therapy was started for suspected cellulitis including levofloxacin, piperacillin-tazobactam, and linezolid. Despite broad-spectrum antibiotic coverage, the patient continued to be febrile and experienced progressive erythema and swelling of the right lower leg, at which point he was transferred to our institution. A new antibiotic regimen of vancomycin, cefepime, and clindamycin was started and showed no improvement, after which dermatology was consulted.
Physical examination revealed unilateral edema and calor of the right lower leg with a demarcated erythematous rash extending to the level of the knee. Furthermore, a hemorrhagic crescent sign was present below the right medial malleolus (Figure). The popliteal fossa was supple, though the patient was adamant that he had a Baker cyst. Punch biopsies demonstrated epidermal spongiosis and extensive edema with perivascular inflammation. No organisms were found by stain and culture. Ultrasound records confirmed a Baker cyst present at least 4 months prior; however, a repeat ultrasound showed resolution. A diagnosis of pseudocellulitis secondary to Baker cyst rupture was made, and corticosteroids were started, resulting in marked reduction in erythema and edema of the lower leg and hospital discharge.

This case highlights the importance of early involvement of dermatology when cellulitis is suspected. A study of 635 patients in the United Kingdom referred to dermatology for lower limb cellulitis found that 210 (33%) patients did not have cellulitis and only 18 (3%) required hospital admission.3 Dermatology consultations have been shown to benefit patients with inflammatory skin disease by decreasing length of stay and reducing readmissions.4
Our patient had several risk factors for cellulitis, including obesity, lymphedema, and chronic kidney disease, in addition to having fevers and unilateral involvement. However, failure of symptoms to improve with broad-spectrum antibiotics made a diagnosis of cellulitis less likely. In this case, a severe immune response to the ruptured Baker cyst mimicked the presentation of cellulitis.
Ruptured Baker cysts have been reported to cause acute leg swelling, mimicking the symptoms of cellulitis or DVT.2,5 The presence of a hemorrhagic crescent sign can be a useful diagnostic tool, as in our patient, because it has been reported as an indication of synovial injury or rupture, supporting the exclusion of cellulitis or DVT when it is observed.6 Prior reports have observed ecchymosis on the foot in as little as 1 day after the onset of calf swelling and at the lateral malleolus 3 days after the onset of calf swelling.5
Following suspicion of a ruptured Baker cyst causing pseudocellulitis, an ultrasound can be used to confirm the diagnosis. Ultrasonography shows a large hypoechoic space behind the calf muscles, which is pathognomonic of a ruptured Baker cyst.7
In conclusion, when a hemorrhagic crescent sign is observed, one should be suspicious for a ruptured Baker cyst or other synovial pathology as an etiology of pseudocellulitis. Early recognition of the hemorrhagic crescent sign can help rule out cellulitis and DVT, thereby reducing the amount of intravenous antibiotic prescribed, decreasing the length of hospital stay, and reducing readmission.
- Feldman SR, Fleischer AB, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730. doi:10.1001/archinte.158.7.726
- Von Schroeder HP, Ameli FM, Piazza D, et al. Ruptured Baker’s cyst causes ecchymosis of the foot. J Bone Joint Surg Br. 1993;75:316-317.
- Levell NJ, Wingfield CG, Garioch JJ. Severe lower limb cellulitis is best diagnosed by dermatologists and managed with shared care between primary and secondary care. Br J Dermatol. 2011;164:1326-1328.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;53:523-528.
- Dunlop D, Parker PJ, Keating JF. Ruptured Baker’s cyst causing posterior compartment syndrome. Injury. 1997;28:561-562.
- Kraag G, Thevathasan EM, Gordon DA, et al. The hemorrhagic crescent sign of acute synovial rupture. Ann Intern Med. 1976;85:477-478.
- Sato O, Kondoh K, Iyori K, et al. Midcalf ultrasonography for the diagnosis of ruptured Baker’s cysts. Surg Today. 2001;31:410-413. doi:10.1007/s005950170131
- Feldman SR, Fleischer AB, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730. doi:10.1001/archinte.158.7.726
- Von Schroeder HP, Ameli FM, Piazza D, et al. Ruptured Baker’s cyst causes ecchymosis of the foot. J Bone Joint Surg Br. 1993;75:316-317.
- Levell NJ, Wingfield CG, Garioch JJ. Severe lower limb cellulitis is best diagnosed by dermatologists and managed with shared care between primary and secondary care. Br J Dermatol. 2011;164:1326-1328.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;53:523-528.
- Dunlop D, Parker PJ, Keating JF. Ruptured Baker’s cyst causing posterior compartment syndrome. Injury. 1997;28:561-562.
- Kraag G, Thevathasan EM, Gordon DA, et al. The hemorrhagic crescent sign of acute synovial rupture. Ann Intern Med. 1976;85:477-478.
- Sato O, Kondoh K, Iyori K, et al. Midcalf ultrasonography for the diagnosis of ruptured Baker’s cysts. Surg Today. 2001;31:410-413. doi:10.1007/s005950170131
Practice Points
- Pseudocellulitis is common in patients presenting with cellulitislike symptoms.
- A hemorrhagic crescent at the medial malleolus should lead to the suspicion on bursa or joint pathology as a cause of pseudocellulitis.
Herpes Zoster and Varicella Encephalitis Following the Recombinant Zoster Vaccine
To the Editor:
Reported adverse effects following the recombinant zoster vaccine (RZV) include pyrexia, myalgia, and fatigue.1 We report the case of a patient who developed herpes zoster and subsequent varicella encephalitis within 8 days of receiving the second dose of the RZV.
A 75-year-old man presented to the emergency department with burning pain and pruritus involving the left hip and calf 2 days after receiving the second dose of the RZV. He had a history of chronic lymphocytic leukemia (CLL) and was being clinically monitored. He received the first dose of the RZV without complication 3 months prior. In the emergency department, he was diagnosed with “nerve pain,” given acetaminophen, and discharged home; however, he continued to have worsening pain 8 days later followed by a vesicular eruption that wrapped around the left leg and was concentrated on the inner thigh/groin area in a dermatomal distribution. His primary care physician diagnosed him with herpes zoster and prescribed valacyclovir 1000 mg every 8 hours for 7 days. Two days later, the patient developed weakness and confusion and returned to the emergency department. Upon admission, computed tomography and magnetic resonance imaging/magnetic resonance angiography of the brain was normal. A lumbar puncture confirmed varicella encephalitis via a polymerase chain reaction assay. He was treated with intravenous acyclovir and discharged to a rehabilitation facility. His course was further complicated by a subarachnoid hemorrhage and normal pressure hydrocephalus. He did not require a shunt but continues to have memory impairment, weakness, and cognitive impairment. He is steadily improving with rehabilitative services.
The RZV is an inactivated vaccine composed of the varicella-zoster virus (VZV) glycoprotein E antigen and an adjuvant, AS01B, that boosts both innate and adaptive immunity.2 It was approved by the US Food and Drug Administration in 2017 for prevention of herpes zoster in adults aged 50 years or older. It requires 2 separate injections administered 2 to 6 months apart. Its efficacy for the prevention of cutaneous herpes zoster and postherpetic neuralgia is 97% and 80% to 91%, respectively. It was developed to improve on the existing zoster vaccine live, which contains a live attenuated virus, with efficacy ranging from 38% to 70%.3
The Centers for Disease Control and Prevention initially recommended the RZV for immunocompetent individuals or those taking low-dose immunosuppressant medications as well those who have recovered from an immunocompromising illness. In immunocompetent patients, reported adverse effects include injection site pain and redness, headache, myalgia, fatigue, shivering, fever, and gastrointestinal tract symptoms; however, when the vaccine first came out, many of the studies excluded patients with CLL.4 Our patient’s herpes zoster and varicella encephalitis occurred following administration of the second dose of the RZV. Herpes zoster occurs from declining VZV-specific cell-mediated immunity. Given that the vaccine contains inactive virus, it is unlikely that our patient’s infection was the direct result of dissemination of the virus contained within the vaccine. The RZV specifically generates T-cell responses to the glycoprotein E subunit of VZV, which is thought to be responsible for the high levels of VZV-specific memory T cells with the RZV compared to the zoster vaccine live.5 However, this response does not occur until after the second dose of RZV. Although our patient already had 1 dose of RZV, it was unlikely that he had a substantial number of glycoprotein E and VZV-specific memory T cells to combat virus reactivation. Additionally, his CLL, though mild, may have resulted in an aberrant T-cell response in the presence of already low VZV-specific lymphocytes, allowing for reactivation and dissemination of the virus. Since then, there has been more of an emphasis on looking at the immunogenicity elicited by the vaccine in patients with CLL—both those who are treatment naive and those treated with Bruton tyrosine kinase inhibitors. Both groups of patients have demonstrated reduced immunogenicity in response to RZV, leaving the opportunity for viral reactivation in this patient population.6,7
The safety of the RZV has now been demonstrated in patients with CLL.7 However, even after RZV vaccination, patients with CLL are still at risk for herpes zoster reactivation and may have an aberrant response due to immune cell dysregulation. Our case demonstrates the need to increase monitoring of CLL patients for signs of viral reactivation and shift our focus to providing antiviral therapy quickly after symptom occurrence.
- Centers for Disease Control and Prevention. Shingles: about the vaccine. Updated January 24, 2022. Accessed February 7, 2024. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/about-vaccine.html
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108. doi:10.15585/mmwr.mm6703a5external icon
- Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95:169-183. doi:10.1016/j.mayocp.2019.02.024
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis [published correction appears in Lancet Infect Dis. 2020;20:E1]. Lancet Infect Dis. 2019;19:988-1000. doi:10.1016/S1473-3099(19)30163-X
- Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128:4429-4440.
- Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6:1732-1740. doi:10.1182/bloodadvances.2021006574
- Pleyer C, Cohen J, Soto S, et al. Response to the Shingrix varicella zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I). Blood. 2019;134(suppl 1):3053. doi:10.1182/blood-2019-121675
To the Editor:
Reported adverse effects following the recombinant zoster vaccine (RZV) include pyrexia, myalgia, and fatigue.1 We report the case of a patient who developed herpes zoster and subsequent varicella encephalitis within 8 days of receiving the second dose of the RZV.
A 75-year-old man presented to the emergency department with burning pain and pruritus involving the left hip and calf 2 days after receiving the second dose of the RZV. He had a history of chronic lymphocytic leukemia (CLL) and was being clinically monitored. He received the first dose of the RZV without complication 3 months prior. In the emergency department, he was diagnosed with “nerve pain,” given acetaminophen, and discharged home; however, he continued to have worsening pain 8 days later followed by a vesicular eruption that wrapped around the left leg and was concentrated on the inner thigh/groin area in a dermatomal distribution. His primary care physician diagnosed him with herpes zoster and prescribed valacyclovir 1000 mg every 8 hours for 7 days. Two days later, the patient developed weakness and confusion and returned to the emergency department. Upon admission, computed tomography and magnetic resonance imaging/magnetic resonance angiography of the brain was normal. A lumbar puncture confirmed varicella encephalitis via a polymerase chain reaction assay. He was treated with intravenous acyclovir and discharged to a rehabilitation facility. His course was further complicated by a subarachnoid hemorrhage and normal pressure hydrocephalus. He did not require a shunt but continues to have memory impairment, weakness, and cognitive impairment. He is steadily improving with rehabilitative services.
The RZV is an inactivated vaccine composed of the varicella-zoster virus (VZV) glycoprotein E antigen and an adjuvant, AS01B, that boosts both innate and adaptive immunity.2 It was approved by the US Food and Drug Administration in 2017 for prevention of herpes zoster in adults aged 50 years or older. It requires 2 separate injections administered 2 to 6 months apart. Its efficacy for the prevention of cutaneous herpes zoster and postherpetic neuralgia is 97% and 80% to 91%, respectively. It was developed to improve on the existing zoster vaccine live, which contains a live attenuated virus, with efficacy ranging from 38% to 70%.3
The Centers for Disease Control and Prevention initially recommended the RZV for immunocompetent individuals or those taking low-dose immunosuppressant medications as well those who have recovered from an immunocompromising illness. In immunocompetent patients, reported adverse effects include injection site pain and redness, headache, myalgia, fatigue, shivering, fever, and gastrointestinal tract symptoms; however, when the vaccine first came out, many of the studies excluded patients with CLL.4 Our patient’s herpes zoster and varicella encephalitis occurred following administration of the second dose of the RZV. Herpes zoster occurs from declining VZV-specific cell-mediated immunity. Given that the vaccine contains inactive virus, it is unlikely that our patient’s infection was the direct result of dissemination of the virus contained within the vaccine. The RZV specifically generates T-cell responses to the glycoprotein E subunit of VZV, which is thought to be responsible for the high levels of VZV-specific memory T cells with the RZV compared to the zoster vaccine live.5 However, this response does not occur until after the second dose of RZV. Although our patient already had 1 dose of RZV, it was unlikely that he had a substantial number of glycoprotein E and VZV-specific memory T cells to combat virus reactivation. Additionally, his CLL, though mild, may have resulted in an aberrant T-cell response in the presence of already low VZV-specific lymphocytes, allowing for reactivation and dissemination of the virus. Since then, there has been more of an emphasis on looking at the immunogenicity elicited by the vaccine in patients with CLL—both those who are treatment naive and those treated with Bruton tyrosine kinase inhibitors. Both groups of patients have demonstrated reduced immunogenicity in response to RZV, leaving the opportunity for viral reactivation in this patient population.6,7
The safety of the RZV has now been demonstrated in patients with CLL.7 However, even after RZV vaccination, patients with CLL are still at risk for herpes zoster reactivation and may have an aberrant response due to immune cell dysregulation. Our case demonstrates the need to increase monitoring of CLL patients for signs of viral reactivation and shift our focus to providing antiviral therapy quickly after symptom occurrence.
To the Editor:
Reported adverse effects following the recombinant zoster vaccine (RZV) include pyrexia, myalgia, and fatigue.1 We report the case of a patient who developed herpes zoster and subsequent varicella encephalitis within 8 days of receiving the second dose of the RZV.
A 75-year-old man presented to the emergency department with burning pain and pruritus involving the left hip and calf 2 days after receiving the second dose of the RZV. He had a history of chronic lymphocytic leukemia (CLL) and was being clinically monitored. He received the first dose of the RZV without complication 3 months prior. In the emergency department, he was diagnosed with “nerve pain,” given acetaminophen, and discharged home; however, he continued to have worsening pain 8 days later followed by a vesicular eruption that wrapped around the left leg and was concentrated on the inner thigh/groin area in a dermatomal distribution. His primary care physician diagnosed him with herpes zoster and prescribed valacyclovir 1000 mg every 8 hours for 7 days. Two days later, the patient developed weakness and confusion and returned to the emergency department. Upon admission, computed tomography and magnetic resonance imaging/magnetic resonance angiography of the brain was normal. A lumbar puncture confirmed varicella encephalitis via a polymerase chain reaction assay. He was treated with intravenous acyclovir and discharged to a rehabilitation facility. His course was further complicated by a subarachnoid hemorrhage and normal pressure hydrocephalus. He did not require a shunt but continues to have memory impairment, weakness, and cognitive impairment. He is steadily improving with rehabilitative services.
The RZV is an inactivated vaccine composed of the varicella-zoster virus (VZV) glycoprotein E antigen and an adjuvant, AS01B, that boosts both innate and adaptive immunity.2 It was approved by the US Food and Drug Administration in 2017 for prevention of herpes zoster in adults aged 50 years or older. It requires 2 separate injections administered 2 to 6 months apart. Its efficacy for the prevention of cutaneous herpes zoster and postherpetic neuralgia is 97% and 80% to 91%, respectively. It was developed to improve on the existing zoster vaccine live, which contains a live attenuated virus, with efficacy ranging from 38% to 70%.3
The Centers for Disease Control and Prevention initially recommended the RZV for immunocompetent individuals or those taking low-dose immunosuppressant medications as well those who have recovered from an immunocompromising illness. In immunocompetent patients, reported adverse effects include injection site pain and redness, headache, myalgia, fatigue, shivering, fever, and gastrointestinal tract symptoms; however, when the vaccine first came out, many of the studies excluded patients with CLL.4 Our patient’s herpes zoster and varicella encephalitis occurred following administration of the second dose of the RZV. Herpes zoster occurs from declining VZV-specific cell-mediated immunity. Given that the vaccine contains inactive virus, it is unlikely that our patient’s infection was the direct result of dissemination of the virus contained within the vaccine. The RZV specifically generates T-cell responses to the glycoprotein E subunit of VZV, which is thought to be responsible for the high levels of VZV-specific memory T cells with the RZV compared to the zoster vaccine live.5 However, this response does not occur until after the second dose of RZV. Although our patient already had 1 dose of RZV, it was unlikely that he had a substantial number of glycoprotein E and VZV-specific memory T cells to combat virus reactivation. Additionally, his CLL, though mild, may have resulted in an aberrant T-cell response in the presence of already low VZV-specific lymphocytes, allowing for reactivation and dissemination of the virus. Since then, there has been more of an emphasis on looking at the immunogenicity elicited by the vaccine in patients with CLL—both those who are treatment naive and those treated with Bruton tyrosine kinase inhibitors. Both groups of patients have demonstrated reduced immunogenicity in response to RZV, leaving the opportunity for viral reactivation in this patient population.6,7
The safety of the RZV has now been demonstrated in patients with CLL.7 However, even after RZV vaccination, patients with CLL are still at risk for herpes zoster reactivation and may have an aberrant response due to immune cell dysregulation. Our case demonstrates the need to increase monitoring of CLL patients for signs of viral reactivation and shift our focus to providing antiviral therapy quickly after symptom occurrence.
- Centers for Disease Control and Prevention. Shingles: about the vaccine. Updated January 24, 2022. Accessed February 7, 2024. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/about-vaccine.html
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108. doi:10.15585/mmwr.mm6703a5external icon
- Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95:169-183. doi:10.1016/j.mayocp.2019.02.024
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis [published correction appears in Lancet Infect Dis. 2020;20:E1]. Lancet Infect Dis. 2019;19:988-1000. doi:10.1016/S1473-3099(19)30163-X
- Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128:4429-4440.
- Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6:1732-1740. doi:10.1182/bloodadvances.2021006574
- Pleyer C, Cohen J, Soto S, et al. Response to the Shingrix varicella zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I). Blood. 2019;134(suppl 1):3053. doi:10.1182/blood-2019-121675
- Centers for Disease Control and Prevention. Shingles: about the vaccine. Updated January 24, 2022. Accessed February 7, 2024. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/about-vaccine.html
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108. doi:10.15585/mmwr.mm6703a5external icon
- Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95:169-183. doi:10.1016/j.mayocp.2019.02.024
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis [published correction appears in Lancet Infect Dis. 2020;20:E1]. Lancet Infect Dis. 2019;19:988-1000. doi:10.1016/S1473-3099(19)30163-X
- Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128:4429-4440.
- Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6:1732-1740. doi:10.1182/bloodadvances.2021006574
- Pleyer C, Cohen J, Soto S, et al. Response to the Shingrix varicella zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I). Blood. 2019;134(suppl 1):3053. doi:10.1182/blood-2019-121675
Practice Points
- Patients with chronic lymphocytic leukemia (CLL) are at risk for herpes zoster reactivation even with vaccination due to a decreased immune response. These patients may have an aberrant response due to immune cell dysregulation.
- It is important to increase monitoring of CLL patients for signs of viral reactivation and shift the focus to providing antiviral therapy quickly if herpes zoster symptoms occur.
Rapidly Progressive Necrotizing Myositis Mimicking Pyoderma Gangrenosum
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
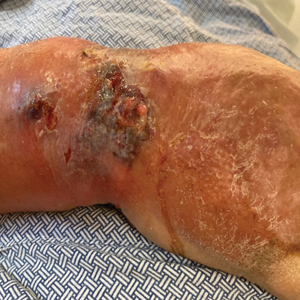
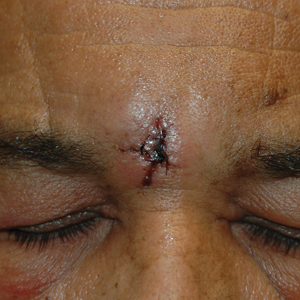
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.
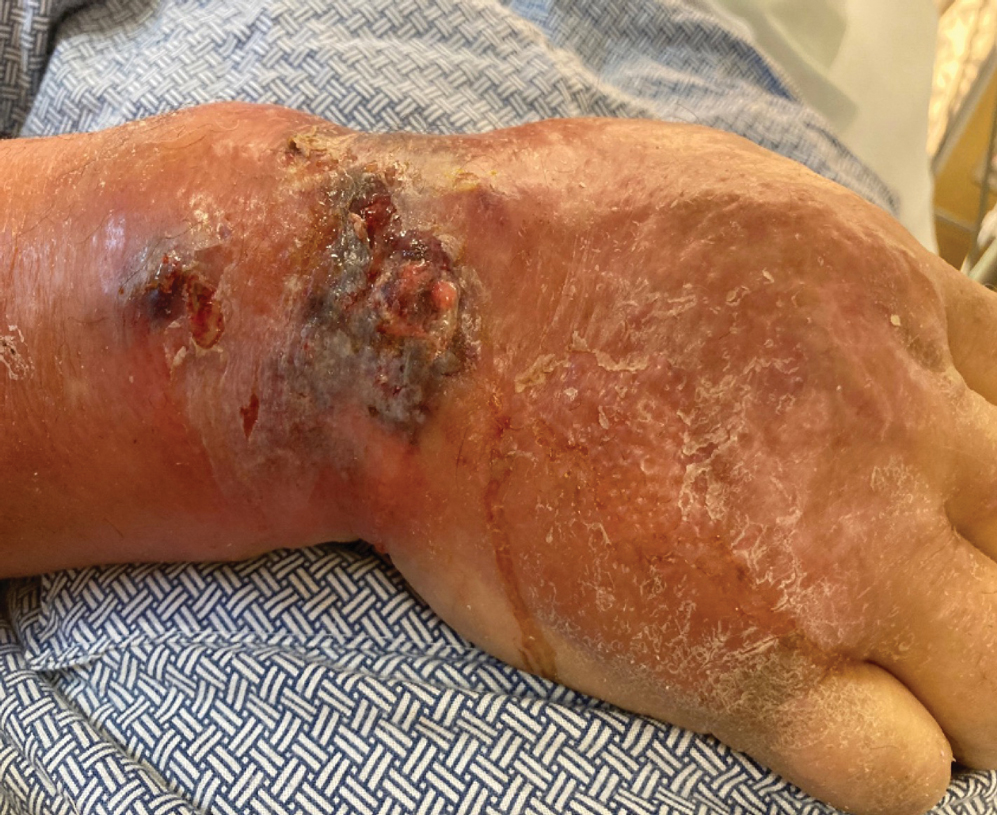
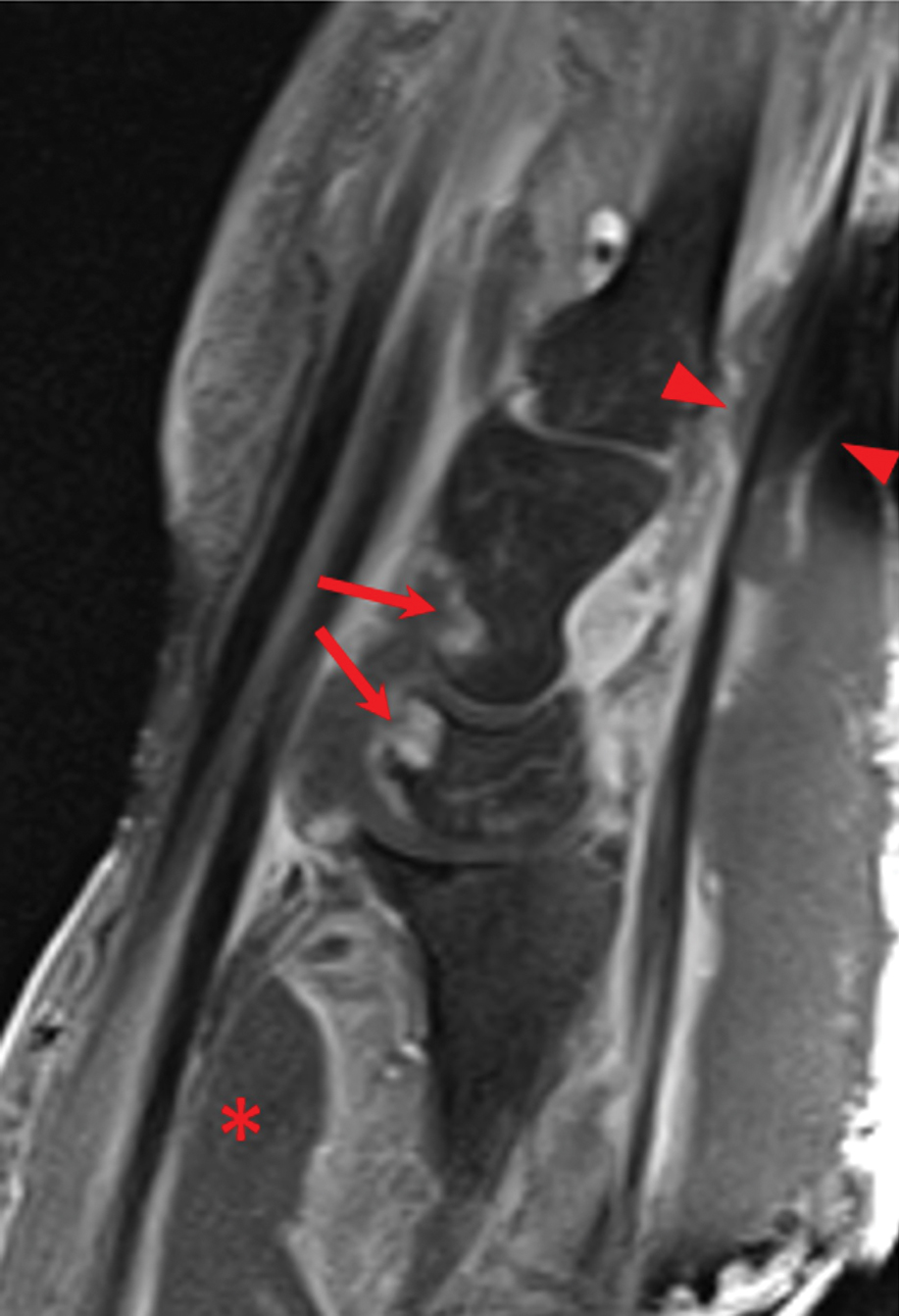
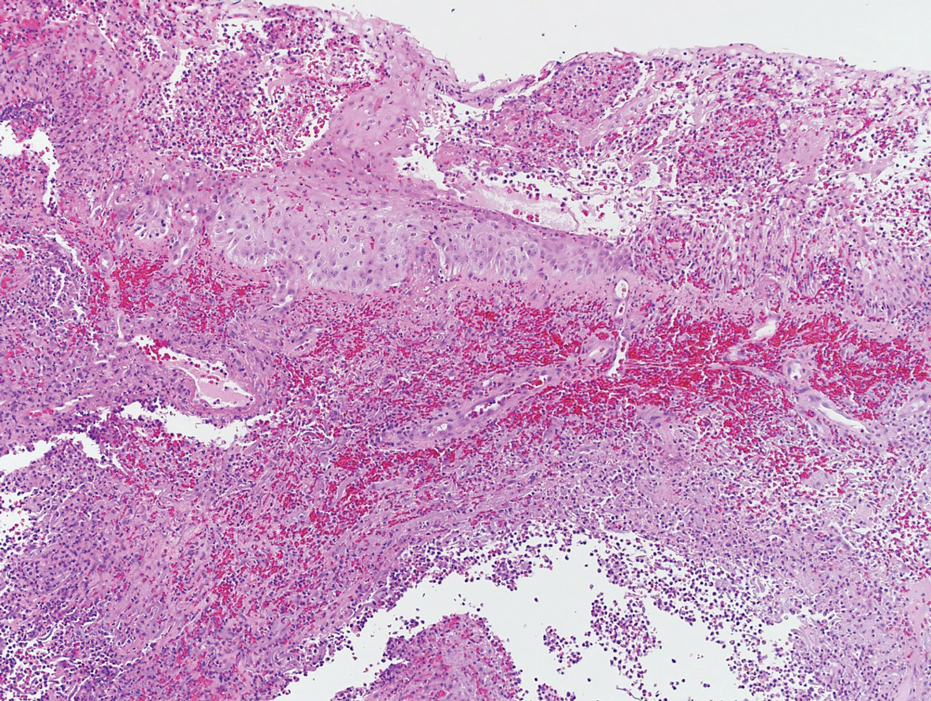
Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).
Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.
Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.

Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).

Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.

Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.

Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).

Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.

Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
Practice Points
- The accurate diagnosis of necrotizing myositis (NM) requires a multimodal approach with complete clinical, histological, and radiographic correlation.
- Necrotizing myositis can manifest as violaceous erythematous plaques, bullae, blisters, or vesicles with petechiae, marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane.
- The differential diagnosis of NM includes pyoderma gangrenosum.
Nonepidemic Kaposi Sarcoma: A Case of a Rare Epidemiologic Subtype
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

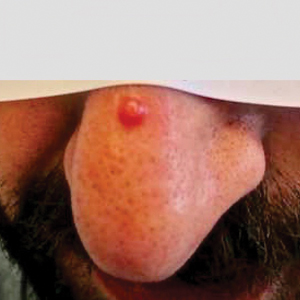
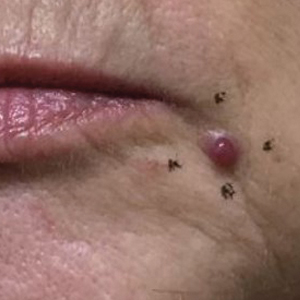
Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
Practice Points
- Nonepidemic Kaposi sarcoma (KS) is a recently described fifth subtype of the disease that typically occurs in younger men who are HIV-negative without detectable cellular or humoral immune deficiency.
- The cutaneous manifestations of nonepidemic KS are similar to those of classic KS, except that disease extent is limited and the prognosis is favorable in nonepidemic KS.
- Dermatologists should consider KS when a patient presents with clinically representative findings, even in the absence of typical risk factors such as immunosuppression.
Methotrexate-Induced Mucositis in a Patient With Angioimmunoblastic T-cell Lymphoma
To the Editor:
Angioimmunoblastic T-cell lymphoma (AITL) is an uncommon peripheral T-cell lymphoma that accounts for 1% to 2% of all forms of non-Hodgkin lymphoma and usually affects middle-aged individuals.1 It primarily appears on the skin and mimics an inflammatory dermatosis, leading to diagnostic and therapeutic delays.2 No gold-standard treatment has been identified for AITL; the prognosis often remains poor, with a 5-year progression-free survival rate of approximately 25%.3 Because of the rarity of AITL and the unmet need of a standard-of-care treatment regimen, relapsing and remitting disease is common and continues to challenge clinicians.
Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed at a higher dosage (>500 mg/m2) to manage cancers, including refractory AITL.4 In blocking dihydrofolate reductase, MTX reduces the folate pool, with the possible adverse effect of bone marrow suppression. Another important toxic effect is acute kidney injury, which may be due to an overdose of MTX or a patient’s predisposition to chronic kidney failure.4
A 50-year-old man was admitted to our inpatient clinic for evaluation of acute oral and genital mucositis. He had a 5-year history of AITL. He was previously treated by hematology with 3 lines of chemotherapy for multiple supradiaphragmatic and subdiaphragmatic localizations of lymphoma, without success. Six days prior to the current presentation, the hematologist started high-dose (3.5 g/m2) intravenous MTX therapy. Five days later, the patient developed transfusion-resistant pancytopenia and fever (maximum body temperature, 102.7°F [39.3°C]).
Physical examination at the current presentation revealed massive necrosis of the lower lip (Figure, A) and partial necrosis of the upper lip. Severe purulent balanoposthitis, causing penile edema and phimosis, complicated the clinical condition. Analysis of a specimen from a cutaneous swab of the penis showed infection with Pseudomonas aeruginosa and Enterococcus faecalis. Considering the clinical presentation and time of onset of signs and symptoms, a diagnosis of acute MTX-induced mucositis was made.
Rescue therapy was started immediately, including high-dose intravenous leucovorin (120 mg 4 times daily), oral sulfamethoxazole-trimethoprim (800 mg/160 mg 3 times daily for 3 days per week), and oral levofloxacin (500 mg/d). After 4 days of treatment, the patient was afebrile. Mucositis of the lips had almost resolved (Figure, B), and balanoposthitis also improved after this rescue therapy. Methotrexate was not resumed because rituximab had been started.
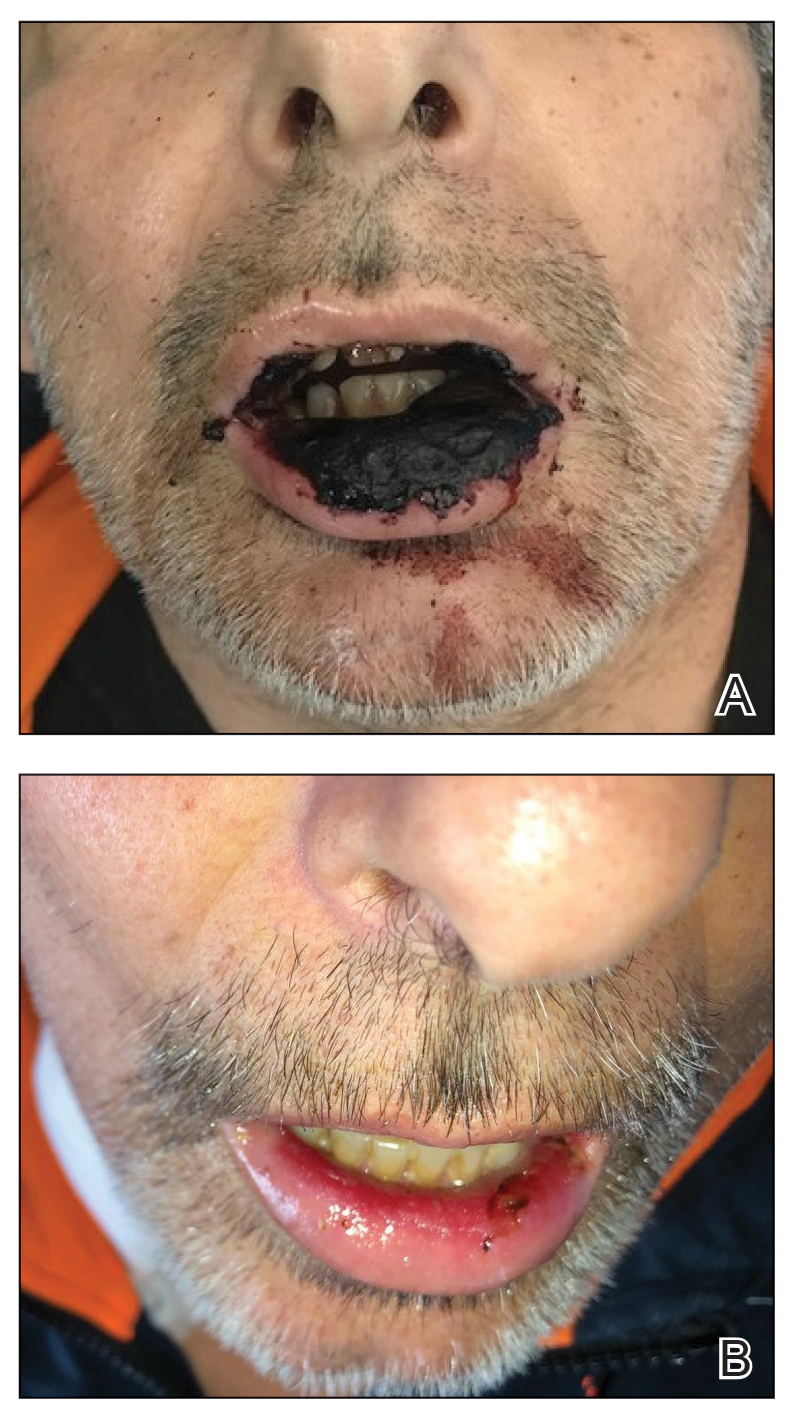
Methotrexate-induced mucositis is a rare severe skin manifestation of MTX toxicity. Prolonged renal toxicity from MTX can predispose a patient to massive myelosuppression, multiorgan failure, and mucositis.5 Pancytopenia manifests during the first 10 days of treatment. Because accumulation of MTX is higher in mucosal epithelial cells than in bone marrow stem cells, mucositis usually occurs during the first 7 days of administration, prior to onset of pancytopenia.
Skin involvement usually manifests as oral and genital mucositis due to direct toxicity against epithelial cells, with a pattern of severe keratinocyte necrosis on histopathology, known as MTX-induced epidermal necrosis.6 The principal condition in the differential diagnosis is Stevens-Johnson syndrome—including its severe form, toxic epidermal necrolysis—characterized by widespread blistering and more extensive skin detachment caused by an immune-mediated cytotoxic T-cell drug-specific reaction.7
To prevent MTX toxicity, liver and renal function should be assessed and a complete blood cell count should be performed before starting therapy. These tests should be repeated during treatment to monitor for MTX toxicity.
Leucovorin (folinic acid) counteracts MTX-induced epidermal necrosis by neutralizing the effect of MTX, including antitumoral effectiveness of the drug.8 For that reason, leucovorin cannot be started prophylactically.
The main challenges that we encountered in our patient's case were the rarity of reports of AITL in the literature and failure of 3 different lines of chemotherapy previously, which meant that MTX could not possibly be suspended because the drug represented the last therapeutic option. Our case confirms that timely clinical diagnosis and a rapid combined approach consisting of discontinuation of MTX and initiation of leucovorin rescue therapy represents an effective strategy to prevent further toxicity and to alleviate mucositis, even in patients with this rare subset of lymphoma.
- Swarup S, Kopel J, Thein, KZ, et al. Sequential complications of hypercalcemia, necrotizing granulomatous vasculitis, and aplastic anemia occurring in one patient with angioimmunoblastic T cell lymphoma. Am J Med Sci. 2021;361:375-382. doi:10.1016/j.amjms.2020.09.003
- Wang L, Lee HY, Koh HY, et al. Cutaneous presentation of angioimmunoblastic T-cell lymphoma: a harbinger of poor prognosis? Skinmed. 2016;14:469-471.
- Kameoka Y, Takahashi N, Itou S, et al. Analysis of clinical characteristics and prognostic factors for angioimmunoblastic T-cell lymphoma. Int J Hematol. 2015;101:536-542. doi:10.1007/s12185-015-1763-7
- Howard SC, McCormick J, Pui C-H, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471-1482. doi:10.1634/theoncologist.2015-0164
- Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949-959. doi:10.1200/JCO.2013.53.0808
- Yélamos O, Català A, Vilarrasa E, et al. Acute severe methotrexate toxicity in patients with psoriasis: a case series and discussion. Dermatology. 2014;229:306-309. doi:10.1159/000366501
- Delyon J, Ortonne N, Benayoun E, et al. Low-dose methotrexate-induced skin toxicity: keratinocyte dystrophy as a histologic marker.J Am Acad Dermatol. 2015;73:484-490. doi:10.1016/j.jaad.2015.06.015
- Chen T-J, Chung W-H, Chen C-B, et al. Methotrexate-induced epidermal necrosis: a case series of 24 patients. J Am Acad Dermatol. 2017;77:247-255.e2. doi:10.1016/j.jaad.2017.02.021
To the Editor:
Angioimmunoblastic T-cell lymphoma (AITL) is an uncommon peripheral T-cell lymphoma that accounts for 1% to 2% of all forms of non-Hodgkin lymphoma and usually affects middle-aged individuals.1 It primarily appears on the skin and mimics an inflammatory dermatosis, leading to diagnostic and therapeutic delays.2 No gold-standard treatment has been identified for AITL; the prognosis often remains poor, with a 5-year progression-free survival rate of approximately 25%.3 Because of the rarity of AITL and the unmet need of a standard-of-care treatment regimen, relapsing and remitting disease is common and continues to challenge clinicians.
Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed at a higher dosage (>500 mg/m2) to manage cancers, including refractory AITL.4 In blocking dihydrofolate reductase, MTX reduces the folate pool, with the possible adverse effect of bone marrow suppression. Another important toxic effect is acute kidney injury, which may be due to an overdose of MTX or a patient’s predisposition to chronic kidney failure.4
A 50-year-old man was admitted to our inpatient clinic for evaluation of acute oral and genital mucositis. He had a 5-year history of AITL. He was previously treated by hematology with 3 lines of chemotherapy for multiple supradiaphragmatic and subdiaphragmatic localizations of lymphoma, without success. Six days prior to the current presentation, the hematologist started high-dose (3.5 g/m2) intravenous MTX therapy. Five days later, the patient developed transfusion-resistant pancytopenia and fever (maximum body temperature, 102.7°F [39.3°C]).
Physical examination at the current presentation revealed massive necrosis of the lower lip (Figure, A) and partial necrosis of the upper lip. Severe purulent balanoposthitis, causing penile edema and phimosis, complicated the clinical condition. Analysis of a specimen from a cutaneous swab of the penis showed infection with Pseudomonas aeruginosa and Enterococcus faecalis. Considering the clinical presentation and time of onset of signs and symptoms, a diagnosis of acute MTX-induced mucositis was made.
Rescue therapy was started immediately, including high-dose intravenous leucovorin (120 mg 4 times daily), oral sulfamethoxazole-trimethoprim (800 mg/160 mg 3 times daily for 3 days per week), and oral levofloxacin (500 mg/d). After 4 days of treatment, the patient was afebrile. Mucositis of the lips had almost resolved (Figure, B), and balanoposthitis also improved after this rescue therapy. Methotrexate was not resumed because rituximab had been started.

Methotrexate-induced mucositis is a rare severe skin manifestation of MTX toxicity. Prolonged renal toxicity from MTX can predispose a patient to massive myelosuppression, multiorgan failure, and mucositis.5 Pancytopenia manifests during the first 10 days of treatment. Because accumulation of MTX is higher in mucosal epithelial cells than in bone marrow stem cells, mucositis usually occurs during the first 7 days of administration, prior to onset of pancytopenia.
Skin involvement usually manifests as oral and genital mucositis due to direct toxicity against epithelial cells, with a pattern of severe keratinocyte necrosis on histopathology, known as MTX-induced epidermal necrosis.6 The principal condition in the differential diagnosis is Stevens-Johnson syndrome—including its severe form, toxic epidermal necrolysis—characterized by widespread blistering and more extensive skin detachment caused by an immune-mediated cytotoxic T-cell drug-specific reaction.7
To prevent MTX toxicity, liver and renal function should be assessed and a complete blood cell count should be performed before starting therapy. These tests should be repeated during treatment to monitor for MTX toxicity.
Leucovorin (folinic acid) counteracts MTX-induced epidermal necrosis by neutralizing the effect of MTX, including antitumoral effectiveness of the drug.8 For that reason, leucovorin cannot be started prophylactically.
The main challenges that we encountered in our patient's case were the rarity of reports of AITL in the literature and failure of 3 different lines of chemotherapy previously, which meant that MTX could not possibly be suspended because the drug represented the last therapeutic option. Our case confirms that timely clinical diagnosis and a rapid combined approach consisting of discontinuation of MTX and initiation of leucovorin rescue therapy represents an effective strategy to prevent further toxicity and to alleviate mucositis, even in patients with this rare subset of lymphoma.
To the Editor:
Angioimmunoblastic T-cell lymphoma (AITL) is an uncommon peripheral T-cell lymphoma that accounts for 1% to 2% of all forms of non-Hodgkin lymphoma and usually affects middle-aged individuals.1 It primarily appears on the skin and mimics an inflammatory dermatosis, leading to diagnostic and therapeutic delays.2 No gold-standard treatment has been identified for AITL; the prognosis often remains poor, with a 5-year progression-free survival rate of approximately 25%.3 Because of the rarity of AITL and the unmet need of a standard-of-care treatment regimen, relapsing and remitting disease is common and continues to challenge clinicians.
Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed at a higher dosage (>500 mg/m2) to manage cancers, including refractory AITL.4 In blocking dihydrofolate reductase, MTX reduces the folate pool, with the possible adverse effect of bone marrow suppression. Another important toxic effect is acute kidney injury, which may be due to an overdose of MTX or a patient’s predisposition to chronic kidney failure.4
A 50-year-old man was admitted to our inpatient clinic for evaluation of acute oral and genital mucositis. He had a 5-year history of AITL. He was previously treated by hematology with 3 lines of chemotherapy for multiple supradiaphragmatic and subdiaphragmatic localizations of lymphoma, without success. Six days prior to the current presentation, the hematologist started high-dose (3.5 g/m2) intravenous MTX therapy. Five days later, the patient developed transfusion-resistant pancytopenia and fever (maximum body temperature, 102.7°F [39.3°C]).
Physical examination at the current presentation revealed massive necrosis of the lower lip (Figure, A) and partial necrosis of the upper lip. Severe purulent balanoposthitis, causing penile edema and phimosis, complicated the clinical condition. Analysis of a specimen from a cutaneous swab of the penis showed infection with Pseudomonas aeruginosa and Enterococcus faecalis. Considering the clinical presentation and time of onset of signs and symptoms, a diagnosis of acute MTX-induced mucositis was made.
Rescue therapy was started immediately, including high-dose intravenous leucovorin (120 mg 4 times daily), oral sulfamethoxazole-trimethoprim (800 mg/160 mg 3 times daily for 3 days per week), and oral levofloxacin (500 mg/d). After 4 days of treatment, the patient was afebrile. Mucositis of the lips had almost resolved (Figure, B), and balanoposthitis also improved after this rescue therapy. Methotrexate was not resumed because rituximab had been started.

Methotrexate-induced mucositis is a rare severe skin manifestation of MTX toxicity. Prolonged renal toxicity from MTX can predispose a patient to massive myelosuppression, multiorgan failure, and mucositis.5 Pancytopenia manifests during the first 10 days of treatment. Because accumulation of MTX is higher in mucosal epithelial cells than in bone marrow stem cells, mucositis usually occurs during the first 7 days of administration, prior to onset of pancytopenia.
Skin involvement usually manifests as oral and genital mucositis due to direct toxicity against epithelial cells, with a pattern of severe keratinocyte necrosis on histopathology, known as MTX-induced epidermal necrosis.6 The principal condition in the differential diagnosis is Stevens-Johnson syndrome—including its severe form, toxic epidermal necrolysis—characterized by widespread blistering and more extensive skin detachment caused by an immune-mediated cytotoxic T-cell drug-specific reaction.7
To prevent MTX toxicity, liver and renal function should be assessed and a complete blood cell count should be performed before starting therapy. These tests should be repeated during treatment to monitor for MTX toxicity.
Leucovorin (folinic acid) counteracts MTX-induced epidermal necrosis by neutralizing the effect of MTX, including antitumoral effectiveness of the drug.8 For that reason, leucovorin cannot be started prophylactically.
The main challenges that we encountered in our patient's case were the rarity of reports of AITL in the literature and failure of 3 different lines of chemotherapy previously, which meant that MTX could not possibly be suspended because the drug represented the last therapeutic option. Our case confirms that timely clinical diagnosis and a rapid combined approach consisting of discontinuation of MTX and initiation of leucovorin rescue therapy represents an effective strategy to prevent further toxicity and to alleviate mucositis, even in patients with this rare subset of lymphoma.
- Swarup S, Kopel J, Thein, KZ, et al. Sequential complications of hypercalcemia, necrotizing granulomatous vasculitis, and aplastic anemia occurring in one patient with angioimmunoblastic T cell lymphoma. Am J Med Sci. 2021;361:375-382. doi:10.1016/j.amjms.2020.09.003
- Wang L, Lee HY, Koh HY, et al. Cutaneous presentation of angioimmunoblastic T-cell lymphoma: a harbinger of poor prognosis? Skinmed. 2016;14:469-471.
- Kameoka Y, Takahashi N, Itou S, et al. Analysis of clinical characteristics and prognostic factors for angioimmunoblastic T-cell lymphoma. Int J Hematol. 2015;101:536-542. doi:10.1007/s12185-015-1763-7
- Howard SC, McCormick J, Pui C-H, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471-1482. doi:10.1634/theoncologist.2015-0164
- Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949-959. doi:10.1200/JCO.2013.53.0808
- Yélamos O, Català A, Vilarrasa E, et al. Acute severe methotrexate toxicity in patients with psoriasis: a case series and discussion. Dermatology. 2014;229:306-309. doi:10.1159/000366501
- Delyon J, Ortonne N, Benayoun E, et al. Low-dose methotrexate-induced skin toxicity: keratinocyte dystrophy as a histologic marker.J Am Acad Dermatol. 2015;73:484-490. doi:10.1016/j.jaad.2015.06.015
- Chen T-J, Chung W-H, Chen C-B, et al. Methotrexate-induced epidermal necrosis: a case series of 24 patients. J Am Acad Dermatol. 2017;77:247-255.e2. doi:10.1016/j.jaad.2017.02.021
- Swarup S, Kopel J, Thein, KZ, et al. Sequential complications of hypercalcemia, necrotizing granulomatous vasculitis, and aplastic anemia occurring in one patient with angioimmunoblastic T cell lymphoma. Am J Med Sci. 2021;361:375-382. doi:10.1016/j.amjms.2020.09.003
- Wang L, Lee HY, Koh HY, et al. Cutaneous presentation of angioimmunoblastic T-cell lymphoma: a harbinger of poor prognosis? Skinmed. 2016;14:469-471.
- Kameoka Y, Takahashi N, Itou S, et al. Analysis of clinical characteristics and prognostic factors for angioimmunoblastic T-cell lymphoma. Int J Hematol. 2015;101:536-542. doi:10.1007/s12185-015-1763-7
- Howard SC, McCormick J, Pui C-H, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471-1482. doi:10.1634/theoncologist.2015-0164
- Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949-959. doi:10.1200/JCO.2013.53.0808
- Yélamos O, Català A, Vilarrasa E, et al. Acute severe methotrexate toxicity in patients with psoriasis: a case series and discussion. Dermatology. 2014;229:306-309. doi:10.1159/000366501
- Delyon J, Ortonne N, Benayoun E, et al. Low-dose methotrexate-induced skin toxicity: keratinocyte dystrophy as a histologic marker.J Am Acad Dermatol. 2015;73:484-490. doi:10.1016/j.jaad.2015.06.015
- Chen T-J, Chung W-H, Chen C-B, et al. Methotrexate-induced epidermal necrosis: a case series of 24 patients. J Am Acad Dermatol. 2017;77:247-255.e2. doi:10.1016/j.jaad.2017.02.021
PRACTICE POINTS
- Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed to manage cancers such as refractory angioimmunoblastic T-cell lymphoma.
- Dermatologists should be aware of the potential mucocutaneous adverse effects of high-dosage MTX.
- To prevent MTX toxicity, liver and renal function should be assessed and a complete blood cell count should be performed before starting therapy.
Psychogenic Purpura
To the Editor:
A 14-year-old Black adolescent girl presented with episodic, painful, edematous plaques that occurred symmetrically on the arms and legs of 5 years’ duration. The plaques evolved into hyperpigmented patches within 24 to 48 hours before eventually resolving. Fatigue, headache, arthralgias of the arms and legs, chest pain, abdominal pain, nausea, and vomiting variably accompanied these episodes.
Prior to visiting our clinic, the patient had been seen by numerous specialists. A review of her medical records revealed an initial diagnosis of Henoch-Schönlein purpura (HSP), then urticarial vasculitis. She had been treated with antihistamines, topical and systemic steroids, hydroxychloroquine, mycophenolate mofetil, dapsone, azathioprine, and gabapentin. All treatments were ineffectual. She underwent extensive diagnostic testing and imaging, which were normal or noncontributory, including type I allergy testing; multiple exhaustive batteries of hematologic testing; and computed tomography/magnetic resonance imaging/magnetic resonance angiography of the brain, chest, abdomen, and pelvic region. Biopsies from symptomatic segments of the gastrointestinal tract were normal.
Chronic treatment with systemic steroids over 9 months resulted in gastritis and an episode of hematemesis requiring emergent hospitalization. A lengthy multidisciplinary evaluation was conducted at the patient’s local community hospital; the team concluded that she had an urticarial-type rash with accompanying symptoms that did not have an autoimmune, rheumatologic, or inflammatory basis.
The patient’s medical history was remarkable for recent-onset panic attacks. Her family medical history was noncontributory. Physical examination revealed multiple violaceous hyperpigmented patches diffusely located on the proximal upper arms (Figure 1). There were no additional findings on physical examination.
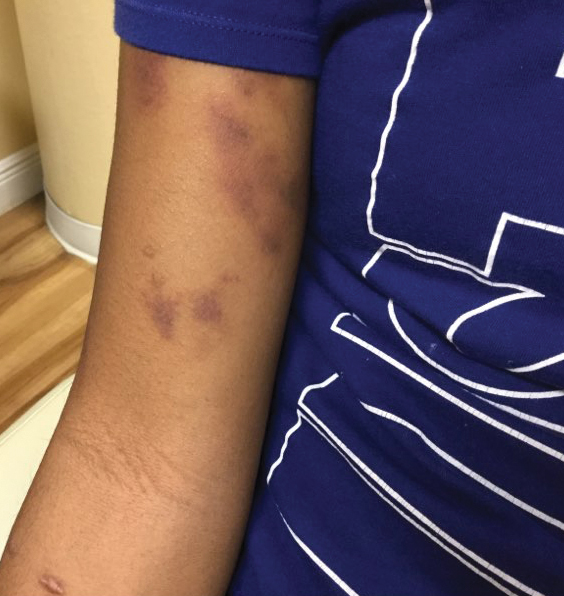
Punch biopsies were performed on lesional areas of the arm. Histopathology indicated a mild superficial perivascular dermal mixed infiltrate and extravasated erythrocytes (Figure 2). Direct immunofluorescence (DIF) testing was negative for vasculitis. Immunohistochemical stains for CD117 and tryptase demonstrated a slight increase in the number of dermal mast cells; however, the increase was not sufficient to diagnose cutaneous mastocytosis, which was in the differential. We proposed a diagnosis of psychogenic purpura (PP)(also known as Gardner-Diamond syndrome). She was treated with gabapentin, a selective serotonin reuptake inhibitor, and cognitive therapy. Unfortunately, after starting therapy the patient was lost to follow-up.
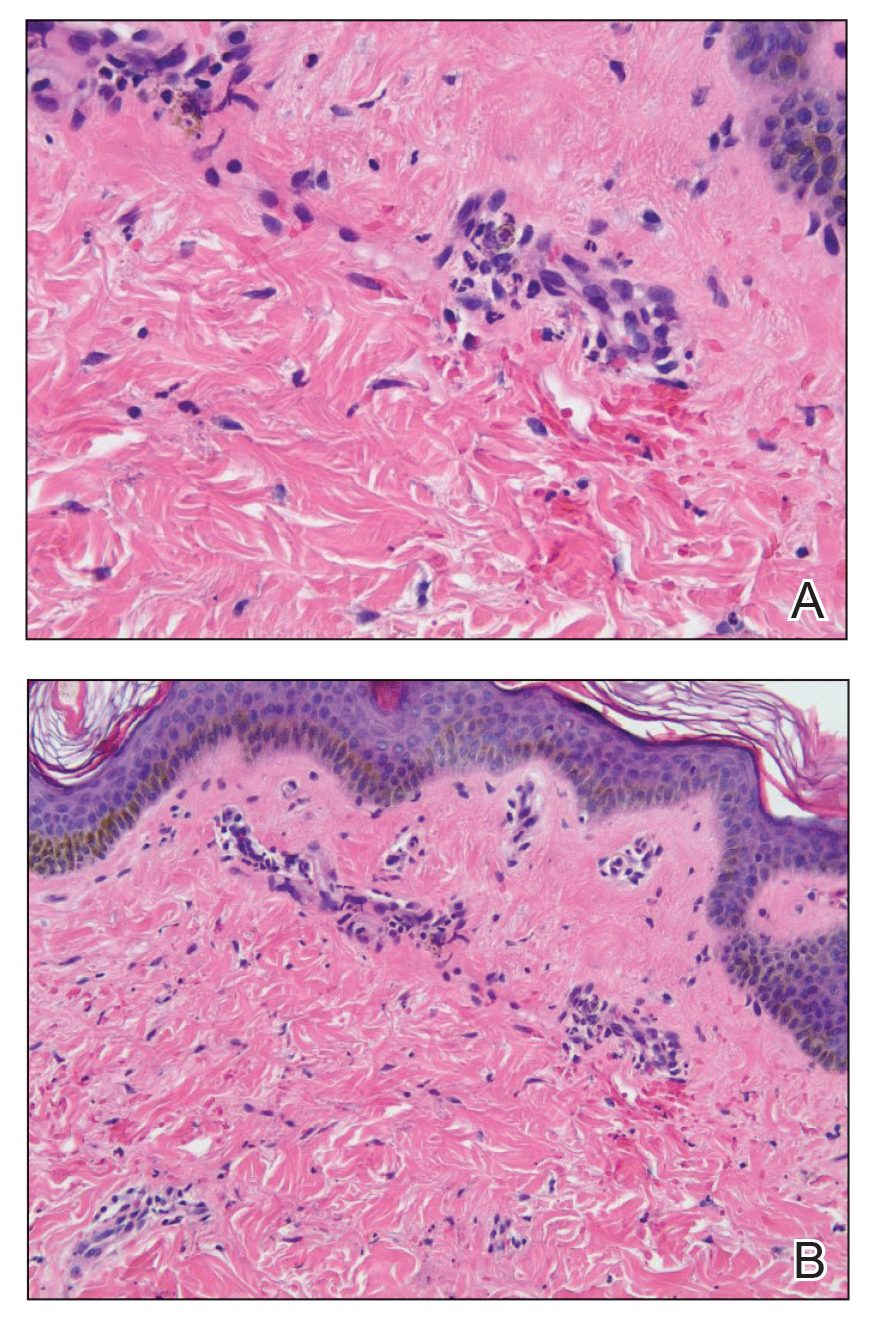
Psychogenic purpura is a rare vasculopathy of unknown etiology that may be a special form of factitious disorder.1,2 In one study, PP occurred predominantly in females aged 15 to 66 years, with a median onset age of 33 years.3 A prodrome of localized itching, burning, and/or pain precedes the development of edematous plaques. The plaques evolve into painful ecchymoses within 1 to 2 days and resolve in 10 days or fewer without treatment. Lesions most commonly occur on the extremities but may occur anywhere on the body. The most common associated finding is an underlying depressive disorder. Episodes may be accompanied by headache, dizziness, fatigue, fever, arthralgia, nausea, vomiting, abdominal pain, menstrual irregularities, myalgia, and urologic conditions.
In 1955, Gardner and Diamond4 described the first cases of PP in 4 female patients at Peter Bent Brigham Hospital in Boston, Massachusetts. The investigators were able to replicate the painful ecchymoses with intradermal injection of the patient’s own erythrocytes into the skin. They proposed that the underlying pathogenesis involved autosensitization to erythrocyte stroma.4 Since then, others have suggested that the pathogenesis may include autosensitization to erythrocyte phosphatidylserine, tonus dysregulation of venous capillaries, abnormal endothelial fibrin synthesis, and capillary wall instability.5-7
Histopathology typically reveals superficial and deep perivascular inflammation with extravasated erythrocytes. Direct immunofluorescence is negative for vasculitis.8 Diagnostics and laboratory findings for underlying systemic illness are negative or noncontributory. Cutaneous injection of 1 mL of the patient’s own washed erythrocytes may result in the formation of the characteristic painful plaques within 24 hours; however, this test is limited by lack of standardization and low sensitivity.3
Psychogenic purpura may share clinical features with cutaneous small vessel vasculitis, such as HSP or urticarial vasculitis. Some of the findings that our patient was experiencing, including purpura, arthralgia, and abdominal pain, are associated with HSP. However, HSP typically is self-limiting and classically features palpable purpura distributed across the lower extremities and buttocks. Histopathology demonstrates the classic findings of leukocytoclastic vasculitis; DIF typically is positive for perivascular IgA and C3 deposition. Increased serum IgA may be present.9 Urticarial vasculitis appears as erythematous indurated wheals that favor a proximal extremity and truncal distribution. They characteristically last longer than 24 hours, are frequently associated with nonprodromal pain or burning, and resolve with hyperpigmentation. Arthralgia and gastrointestinal, renal, pulmonary, cardiac, and neurologic symptoms may be present, especially in patients with low complement levels.10 Skin biopsy demonstrates leukocytoclasia that must be accompanied by vessel wall necrosis. Fibrinoid deposition, erythrocyte extravasation, or perivascular inflammation may be present. In 70% of cases revealing perivascular immunoglobulin, C3, and fibrinogen deposition, DIF is positive. Serum C1q autoantibody may be associated with the hypocomplementemic form.10
The classic histopathologic findings in leukocytoclastic vasculitis include transmural neutrophilic infiltration of the walls of small vessels, fibrinoid necrosis of vessel walls, leukocytoclasia, extravasated erythrocytes, and signs of endothelial cell damage.9 A prior punch biopsy in this patient demonstrated rare neutrophilic nuclear debris within the vessel walls without fibrin deposition. Although the presence of nuclear debris and extravasated erythrocytes could be compatible with a manifestation of urticarial vasculitis, the lack of direct evidence of vessel wall necrosis combined with subsequent biopsies unequivocally ruled out cutaneous small vessel vasculitis in our patient.
Psychogenic purpura has been reported to occur frequently in the background of psycho-emotional distress. In 1989, Ratnoff11 noted that many of the patients he was treating at the University Hospitals of Cleveland, Ohio, had a depressive syndrome. A review of patients treated at the Mayo Clinic in Rochester, Minnesota, illustrated concomitant psychiatric illnesses in 41 of 76 (54%) patients treated for PP, most commonly depressive, personality, and anxiety disorders.3
There is no consensus on therapy for PP. Treatment is based on providing symptomatic relief and relieving underlying psychiatric distress. Block et al12 found the use of selective serotonin reuptake inhibitors, tricyclic antidepressants, and psychotherapy to be successful in improving symptoms and reducing lesions at follow-up visits.
- Piette WW. Purpura: mechanisms and differential diagnosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:376-389.
- Harth W, Taube KM, Gieler U. Factitious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
- Sridharan M, Ali U, Hook CC, et al. The Mayo Clinic experience with psychogenic purpura (Gardner-Diamond syndrome). Am J Med Sci. 2019;357:411‐420.
- Gardner FH, Diamond LK. Autoerythrocyte sensitization; a form of purpura producing painful bruising following autosensitization to red blood cells in certain women. Blood. 1955;10:675-690.
- Groch GS, Finch SC, Rogoway W, et al. Studies in the pathogenesis of autoerythrocyte sensitization syndrome. Blood. 1966;28:19-33.
- Strunecká A, Krpejsová L, Palecek J, et al. Transbilayer redistribution of phosphatidylserine in erythrocytes of a patient with autoerythrocyte sensitization syndrome (psychogenic purpura). Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:829-841.
- Merlen JF. Ecchymotic patches of the fingers and Gardner-Diamond vascular purpura. Phlebologie. 1987;40:473-487.
- Ivanov OL, Lvov AN, Michenko AV, et al. Autoerythrocyte sensitization syndrome (Gardner-Diamond syndrome): review of the literature. J Eur Acad Dermatol Venereol. 2009;23:499-504.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Hamad A, Jithpratuck W, Krishnaswamy G. Urticarial vasculitis and associated disorders. Ann Allergy Asthma Immunol. 2017;118:394-398.
- Ratnoff OD. Psychogenic purpura (autoerythrocyte sensitization): an unsolved dilemma. Am J Med. 1989;87:16N-21N.
- Block ME, Sitenga JL, Lehrer M, et al. Gardner‐Diamond syndrome: a systematic review of treatment options for a rare psychodermatological disorder. Int J Dermatol. 2019;58:782-787.
To the Editor:
A 14-year-old Black adolescent girl presented with episodic, painful, edematous plaques that occurred symmetrically on the arms and legs of 5 years’ duration. The plaques evolved into hyperpigmented patches within 24 to 48 hours before eventually resolving. Fatigue, headache, arthralgias of the arms and legs, chest pain, abdominal pain, nausea, and vomiting variably accompanied these episodes.
Prior to visiting our clinic, the patient had been seen by numerous specialists. A review of her medical records revealed an initial diagnosis of Henoch-Schönlein purpura (HSP), then urticarial vasculitis. She had been treated with antihistamines, topical and systemic steroids, hydroxychloroquine, mycophenolate mofetil, dapsone, azathioprine, and gabapentin. All treatments were ineffectual. She underwent extensive diagnostic testing and imaging, which were normal or noncontributory, including type I allergy testing; multiple exhaustive batteries of hematologic testing; and computed tomography/magnetic resonance imaging/magnetic resonance angiography of the brain, chest, abdomen, and pelvic region. Biopsies from symptomatic segments of the gastrointestinal tract were normal.
Chronic treatment with systemic steroids over 9 months resulted in gastritis and an episode of hematemesis requiring emergent hospitalization. A lengthy multidisciplinary evaluation was conducted at the patient’s local community hospital; the team concluded that she had an urticarial-type rash with accompanying symptoms that did not have an autoimmune, rheumatologic, or inflammatory basis.
The patient’s medical history was remarkable for recent-onset panic attacks. Her family medical history was noncontributory. Physical examination revealed multiple violaceous hyperpigmented patches diffusely located on the proximal upper arms (Figure 1). There were no additional findings on physical examination.

Punch biopsies were performed on lesional areas of the arm. Histopathology indicated a mild superficial perivascular dermal mixed infiltrate and extravasated erythrocytes (Figure 2). Direct immunofluorescence (DIF) testing was negative for vasculitis. Immunohistochemical stains for CD117 and tryptase demonstrated a slight increase in the number of dermal mast cells; however, the increase was not sufficient to diagnose cutaneous mastocytosis, which was in the differential. We proposed a diagnosis of psychogenic purpura (PP)(also known as Gardner-Diamond syndrome). She was treated with gabapentin, a selective serotonin reuptake inhibitor, and cognitive therapy. Unfortunately, after starting therapy the patient was lost to follow-up.

Psychogenic purpura is a rare vasculopathy of unknown etiology that may be a special form of factitious disorder.1,2 In one study, PP occurred predominantly in females aged 15 to 66 years, with a median onset age of 33 years.3 A prodrome of localized itching, burning, and/or pain precedes the development of edematous plaques. The plaques evolve into painful ecchymoses within 1 to 2 days and resolve in 10 days or fewer without treatment. Lesions most commonly occur on the extremities but may occur anywhere on the body. The most common associated finding is an underlying depressive disorder. Episodes may be accompanied by headache, dizziness, fatigue, fever, arthralgia, nausea, vomiting, abdominal pain, menstrual irregularities, myalgia, and urologic conditions.
In 1955, Gardner and Diamond4 described the first cases of PP in 4 female patients at Peter Bent Brigham Hospital in Boston, Massachusetts. The investigators were able to replicate the painful ecchymoses with intradermal injection of the patient’s own erythrocytes into the skin. They proposed that the underlying pathogenesis involved autosensitization to erythrocyte stroma.4 Since then, others have suggested that the pathogenesis may include autosensitization to erythrocyte phosphatidylserine, tonus dysregulation of venous capillaries, abnormal endothelial fibrin synthesis, and capillary wall instability.5-7
Histopathology typically reveals superficial and deep perivascular inflammation with extravasated erythrocytes. Direct immunofluorescence is negative for vasculitis.8 Diagnostics and laboratory findings for underlying systemic illness are negative or noncontributory. Cutaneous injection of 1 mL of the patient’s own washed erythrocytes may result in the formation of the characteristic painful plaques within 24 hours; however, this test is limited by lack of standardization and low sensitivity.3
Psychogenic purpura may share clinical features with cutaneous small vessel vasculitis, such as HSP or urticarial vasculitis. Some of the findings that our patient was experiencing, including purpura, arthralgia, and abdominal pain, are associated with HSP. However, HSP typically is self-limiting and classically features palpable purpura distributed across the lower extremities and buttocks. Histopathology demonstrates the classic findings of leukocytoclastic vasculitis; DIF typically is positive for perivascular IgA and C3 deposition. Increased serum IgA may be present.9 Urticarial vasculitis appears as erythematous indurated wheals that favor a proximal extremity and truncal distribution. They characteristically last longer than 24 hours, are frequently associated with nonprodromal pain or burning, and resolve with hyperpigmentation. Arthralgia and gastrointestinal, renal, pulmonary, cardiac, and neurologic symptoms may be present, especially in patients with low complement levels.10 Skin biopsy demonstrates leukocytoclasia that must be accompanied by vessel wall necrosis. Fibrinoid deposition, erythrocyte extravasation, or perivascular inflammation may be present. In 70% of cases revealing perivascular immunoglobulin, C3, and fibrinogen deposition, DIF is positive. Serum C1q autoantibody may be associated with the hypocomplementemic form.10
The classic histopathologic findings in leukocytoclastic vasculitis include transmural neutrophilic infiltration of the walls of small vessels, fibrinoid necrosis of vessel walls, leukocytoclasia, extravasated erythrocytes, and signs of endothelial cell damage.9 A prior punch biopsy in this patient demonstrated rare neutrophilic nuclear debris within the vessel walls without fibrin deposition. Although the presence of nuclear debris and extravasated erythrocytes could be compatible with a manifestation of urticarial vasculitis, the lack of direct evidence of vessel wall necrosis combined with subsequent biopsies unequivocally ruled out cutaneous small vessel vasculitis in our patient.
Psychogenic purpura has been reported to occur frequently in the background of psycho-emotional distress. In 1989, Ratnoff11 noted that many of the patients he was treating at the University Hospitals of Cleveland, Ohio, had a depressive syndrome. A review of patients treated at the Mayo Clinic in Rochester, Minnesota, illustrated concomitant psychiatric illnesses in 41 of 76 (54%) patients treated for PP, most commonly depressive, personality, and anxiety disorders.3
There is no consensus on therapy for PP. Treatment is based on providing symptomatic relief and relieving underlying psychiatric distress. Block et al12 found the use of selective serotonin reuptake inhibitors, tricyclic antidepressants, and psychotherapy to be successful in improving symptoms and reducing lesions at follow-up visits.
To the Editor:
A 14-year-old Black adolescent girl presented with episodic, painful, edematous plaques that occurred symmetrically on the arms and legs of 5 years’ duration. The plaques evolved into hyperpigmented patches within 24 to 48 hours before eventually resolving. Fatigue, headache, arthralgias of the arms and legs, chest pain, abdominal pain, nausea, and vomiting variably accompanied these episodes.
Prior to visiting our clinic, the patient had been seen by numerous specialists. A review of her medical records revealed an initial diagnosis of Henoch-Schönlein purpura (HSP), then urticarial vasculitis. She had been treated with antihistamines, topical and systemic steroids, hydroxychloroquine, mycophenolate mofetil, dapsone, azathioprine, and gabapentin. All treatments were ineffectual. She underwent extensive diagnostic testing and imaging, which were normal or noncontributory, including type I allergy testing; multiple exhaustive batteries of hematologic testing; and computed tomography/magnetic resonance imaging/magnetic resonance angiography of the brain, chest, abdomen, and pelvic region. Biopsies from symptomatic segments of the gastrointestinal tract were normal.
Chronic treatment with systemic steroids over 9 months resulted in gastritis and an episode of hematemesis requiring emergent hospitalization. A lengthy multidisciplinary evaluation was conducted at the patient’s local community hospital; the team concluded that she had an urticarial-type rash with accompanying symptoms that did not have an autoimmune, rheumatologic, or inflammatory basis.
The patient’s medical history was remarkable for recent-onset panic attacks. Her family medical history was noncontributory. Physical examination revealed multiple violaceous hyperpigmented patches diffusely located on the proximal upper arms (Figure 1). There were no additional findings on physical examination.

Punch biopsies were performed on lesional areas of the arm. Histopathology indicated a mild superficial perivascular dermal mixed infiltrate and extravasated erythrocytes (Figure 2). Direct immunofluorescence (DIF) testing was negative for vasculitis. Immunohistochemical stains for CD117 and tryptase demonstrated a slight increase in the number of dermal mast cells; however, the increase was not sufficient to diagnose cutaneous mastocytosis, which was in the differential. We proposed a diagnosis of psychogenic purpura (PP)(also known as Gardner-Diamond syndrome). She was treated with gabapentin, a selective serotonin reuptake inhibitor, and cognitive therapy. Unfortunately, after starting therapy the patient was lost to follow-up.

Psychogenic purpura is a rare vasculopathy of unknown etiology that may be a special form of factitious disorder.1,2 In one study, PP occurred predominantly in females aged 15 to 66 years, with a median onset age of 33 years.3 A prodrome of localized itching, burning, and/or pain precedes the development of edematous plaques. The plaques evolve into painful ecchymoses within 1 to 2 days and resolve in 10 days or fewer without treatment. Lesions most commonly occur on the extremities but may occur anywhere on the body. The most common associated finding is an underlying depressive disorder. Episodes may be accompanied by headache, dizziness, fatigue, fever, arthralgia, nausea, vomiting, abdominal pain, menstrual irregularities, myalgia, and urologic conditions.
In 1955, Gardner and Diamond4 described the first cases of PP in 4 female patients at Peter Bent Brigham Hospital in Boston, Massachusetts. The investigators were able to replicate the painful ecchymoses with intradermal injection of the patient’s own erythrocytes into the skin. They proposed that the underlying pathogenesis involved autosensitization to erythrocyte stroma.4 Since then, others have suggested that the pathogenesis may include autosensitization to erythrocyte phosphatidylserine, tonus dysregulation of venous capillaries, abnormal endothelial fibrin synthesis, and capillary wall instability.5-7
Histopathology typically reveals superficial and deep perivascular inflammation with extravasated erythrocytes. Direct immunofluorescence is negative for vasculitis.8 Diagnostics and laboratory findings for underlying systemic illness are negative or noncontributory. Cutaneous injection of 1 mL of the patient’s own washed erythrocytes may result in the formation of the characteristic painful plaques within 24 hours; however, this test is limited by lack of standardization and low sensitivity.3
Psychogenic purpura may share clinical features with cutaneous small vessel vasculitis, such as HSP or urticarial vasculitis. Some of the findings that our patient was experiencing, including purpura, arthralgia, and abdominal pain, are associated with HSP. However, HSP typically is self-limiting and classically features palpable purpura distributed across the lower extremities and buttocks. Histopathology demonstrates the classic findings of leukocytoclastic vasculitis; DIF typically is positive for perivascular IgA and C3 deposition. Increased serum IgA may be present.9 Urticarial vasculitis appears as erythematous indurated wheals that favor a proximal extremity and truncal distribution. They characteristically last longer than 24 hours, are frequently associated with nonprodromal pain or burning, and resolve with hyperpigmentation. Arthralgia and gastrointestinal, renal, pulmonary, cardiac, and neurologic symptoms may be present, especially in patients with low complement levels.10 Skin biopsy demonstrates leukocytoclasia that must be accompanied by vessel wall necrosis. Fibrinoid deposition, erythrocyte extravasation, or perivascular inflammation may be present. In 70% of cases revealing perivascular immunoglobulin, C3, and fibrinogen deposition, DIF is positive. Serum C1q autoantibody may be associated with the hypocomplementemic form.10
The classic histopathologic findings in leukocytoclastic vasculitis include transmural neutrophilic infiltration of the walls of small vessels, fibrinoid necrosis of vessel walls, leukocytoclasia, extravasated erythrocytes, and signs of endothelial cell damage.9 A prior punch biopsy in this patient demonstrated rare neutrophilic nuclear debris within the vessel walls without fibrin deposition. Although the presence of nuclear debris and extravasated erythrocytes could be compatible with a manifestation of urticarial vasculitis, the lack of direct evidence of vessel wall necrosis combined with subsequent biopsies unequivocally ruled out cutaneous small vessel vasculitis in our patient.
Psychogenic purpura has been reported to occur frequently in the background of psycho-emotional distress. In 1989, Ratnoff11 noted that many of the patients he was treating at the University Hospitals of Cleveland, Ohio, had a depressive syndrome. A review of patients treated at the Mayo Clinic in Rochester, Minnesota, illustrated concomitant psychiatric illnesses in 41 of 76 (54%) patients treated for PP, most commonly depressive, personality, and anxiety disorders.3
There is no consensus on therapy for PP. Treatment is based on providing symptomatic relief and relieving underlying psychiatric distress. Block et al12 found the use of selective serotonin reuptake inhibitors, tricyclic antidepressants, and psychotherapy to be successful in improving symptoms and reducing lesions at follow-up visits.
- Piette WW. Purpura: mechanisms and differential diagnosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:376-389.
- Harth W, Taube KM, Gieler U. Factitious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
- Sridharan M, Ali U, Hook CC, et al. The Mayo Clinic experience with psychogenic purpura (Gardner-Diamond syndrome). Am J Med Sci. 2019;357:411‐420.
- Gardner FH, Diamond LK. Autoerythrocyte sensitization; a form of purpura producing painful bruising following autosensitization to red blood cells in certain women. Blood. 1955;10:675-690.
- Groch GS, Finch SC, Rogoway W, et al. Studies in the pathogenesis of autoerythrocyte sensitization syndrome. Blood. 1966;28:19-33.
- Strunecká A, Krpejsová L, Palecek J, et al. Transbilayer redistribution of phosphatidylserine in erythrocytes of a patient with autoerythrocyte sensitization syndrome (psychogenic purpura). Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:829-841.
- Merlen JF. Ecchymotic patches of the fingers and Gardner-Diamond vascular purpura. Phlebologie. 1987;40:473-487.
- Ivanov OL, Lvov AN, Michenko AV, et al. Autoerythrocyte sensitization syndrome (Gardner-Diamond syndrome): review of the literature. J Eur Acad Dermatol Venereol. 2009;23:499-504.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Hamad A, Jithpratuck W, Krishnaswamy G. Urticarial vasculitis and associated disorders. Ann Allergy Asthma Immunol. 2017;118:394-398.
- Ratnoff OD. Psychogenic purpura (autoerythrocyte sensitization): an unsolved dilemma. Am J Med. 1989;87:16N-21N.
- Block ME, Sitenga JL, Lehrer M, et al. Gardner‐Diamond syndrome: a systematic review of treatment options for a rare psychodermatological disorder. Int J Dermatol. 2019;58:782-787.
- Piette WW. Purpura: mechanisms and differential diagnosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:376-389.
- Harth W, Taube KM, Gieler U. Factitious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
- Sridharan M, Ali U, Hook CC, et al. The Mayo Clinic experience with psychogenic purpura (Gardner-Diamond syndrome). Am J Med Sci. 2019;357:411‐420.
- Gardner FH, Diamond LK. Autoerythrocyte sensitization; a form of purpura producing painful bruising following autosensitization to red blood cells in certain women. Blood. 1955;10:675-690.
- Groch GS, Finch SC, Rogoway W, et al. Studies in the pathogenesis of autoerythrocyte sensitization syndrome. Blood. 1966;28:19-33.
- Strunecká A, Krpejsová L, Palecek J, et al. Transbilayer redistribution of phosphatidylserine in erythrocytes of a patient with autoerythrocyte sensitization syndrome (psychogenic purpura). Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:829-841.
- Merlen JF. Ecchymotic patches of the fingers and Gardner-Diamond vascular purpura. Phlebologie. 1987;40:473-487.
- Ivanov OL, Lvov AN, Michenko AV, et al. Autoerythrocyte sensitization syndrome (Gardner-Diamond syndrome): review of the literature. J Eur Acad Dermatol Venereol. 2009;23:499-504.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Hamad A, Jithpratuck W, Krishnaswamy G. Urticarial vasculitis and associated disorders. Ann Allergy Asthma Immunol. 2017;118:394-398.
- Ratnoff OD. Psychogenic purpura (autoerythrocyte sensitization): an unsolved dilemma. Am J Med. 1989;87:16N-21N.
- Block ME, Sitenga JL, Lehrer M, et al. Gardner‐Diamond syndrome: a systematic review of treatment options for a rare psychodermatological disorder. Int J Dermatol. 2019;58:782-787.
PRACTICE POINTS
- Psychogenic purpura is a rare vasculopathy characterized by painful recurrent episodes of purpura. It is a diagnosis of exclusion that may manifest with signs similar to cutaneous small vessel vasculitis.
- Awareness of this condition could help prevent unnecessary diagnostics, medications, and adverse events.
Superficial Vascular Anomaly of the Glabella Mimicking a Cutaneous Cyst
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.
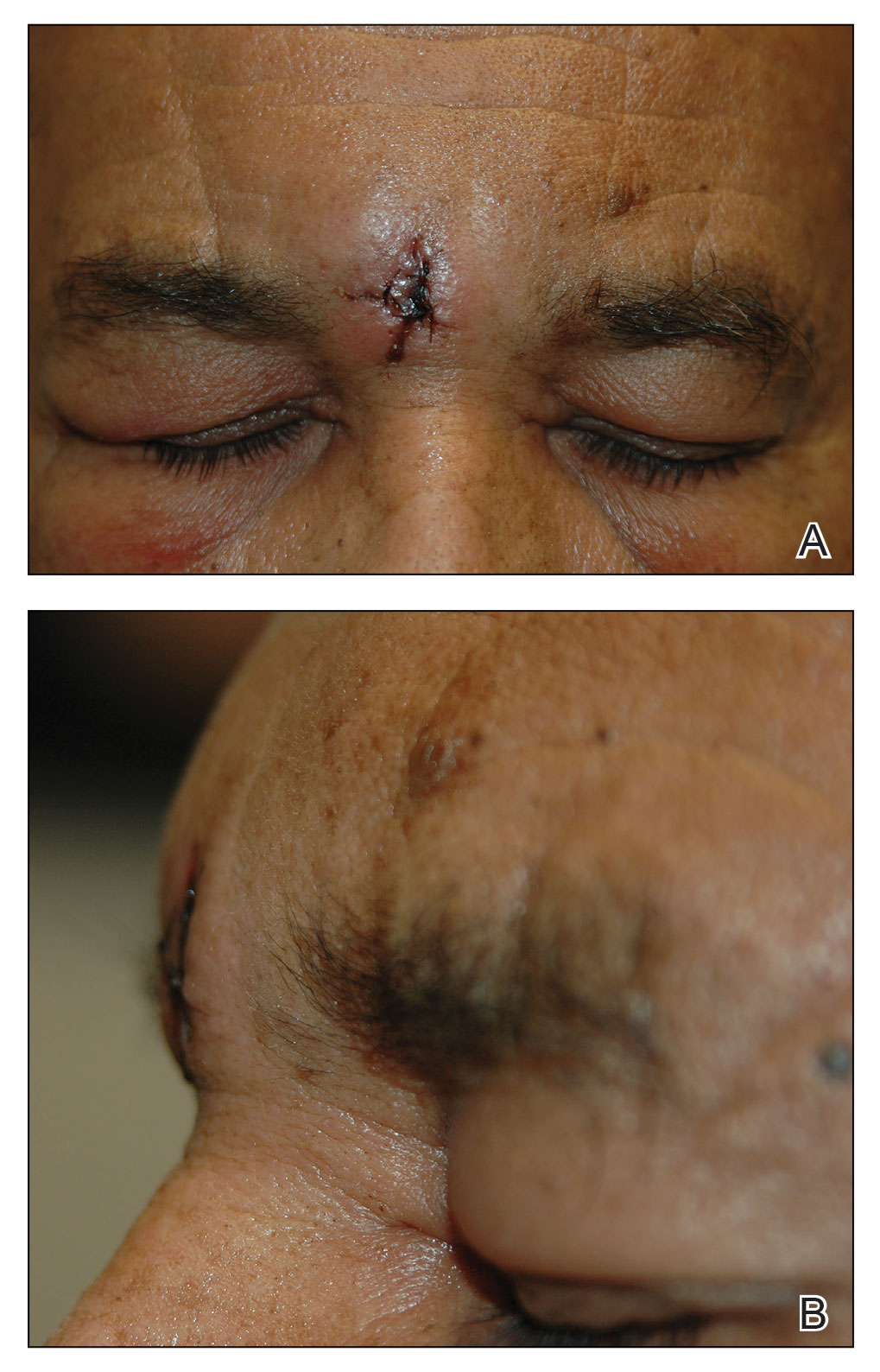
A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.

A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.

A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
Practice Points
- Vascular anomalies should be included in the differential diagnosis of soft cutaneous nodules, as management differs from cysts or lipomas.
- Preoperative evaluation for a cutaneous cyst excision on the head and neck should include ruling out findings of a vascular lesion through history, physical examination, and consideration of color Doppler ultrasonography in unclear cases.
- Surgical technique should involve sequential superficial incisions, descending at 1 to 2 mm per cut, until the suspected capsule is identified to minimize the risk for inadvertent injury to a cyst mimicker such as a vascular anomaly.
Multiple New-Onset Pyogenic Granulomas During Treatment With Paclitaxel and Ramucirumab
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).
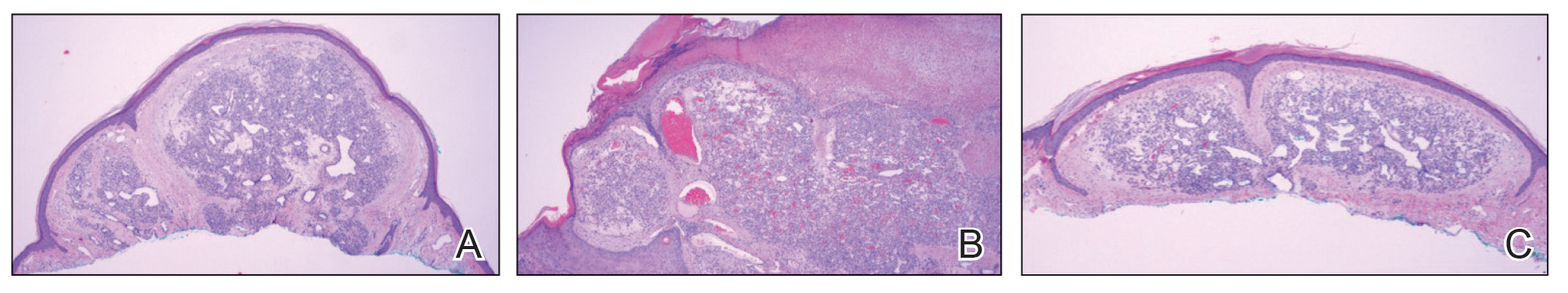
The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).

The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).

The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
Practice Points
- Pyogenic granulomas (PGs) are benign vascular tumors that clinically are characterized as small, eruptive, friable papules.
- Ramucirumab is a monoclonal antibody against vascular endothelial growth factor receptor 2.
- Some patients experience paradoxical formation of vascular tumors such as PGs when treated with combination therapy with ramucirumab and a taxane such as paclitaxel.
Cemiplimab-Associated Eruption of Generalized Eruptive Keratoacanthoma of Grzybowski
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
Practice Points
- Immunotherapy, including immune checkpoint inhibitors such as programmed cell-death protein 1 (PD-1) inhibitors, is associated with an array of immune-related adverse events that often manifest in a delayed and prolonged manner. They most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.
- Dermatologic adverse effects associated with PD-1 inhibitors include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.
- Eruptions of keratoacanthoma rarely have been reported following treatment with PD-1 inhibitors such as cemiplimab, nivolumab, and pembrolizumab.
Thalidomide Analogue Drug Eruption Along the Lines of Blaschko
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.
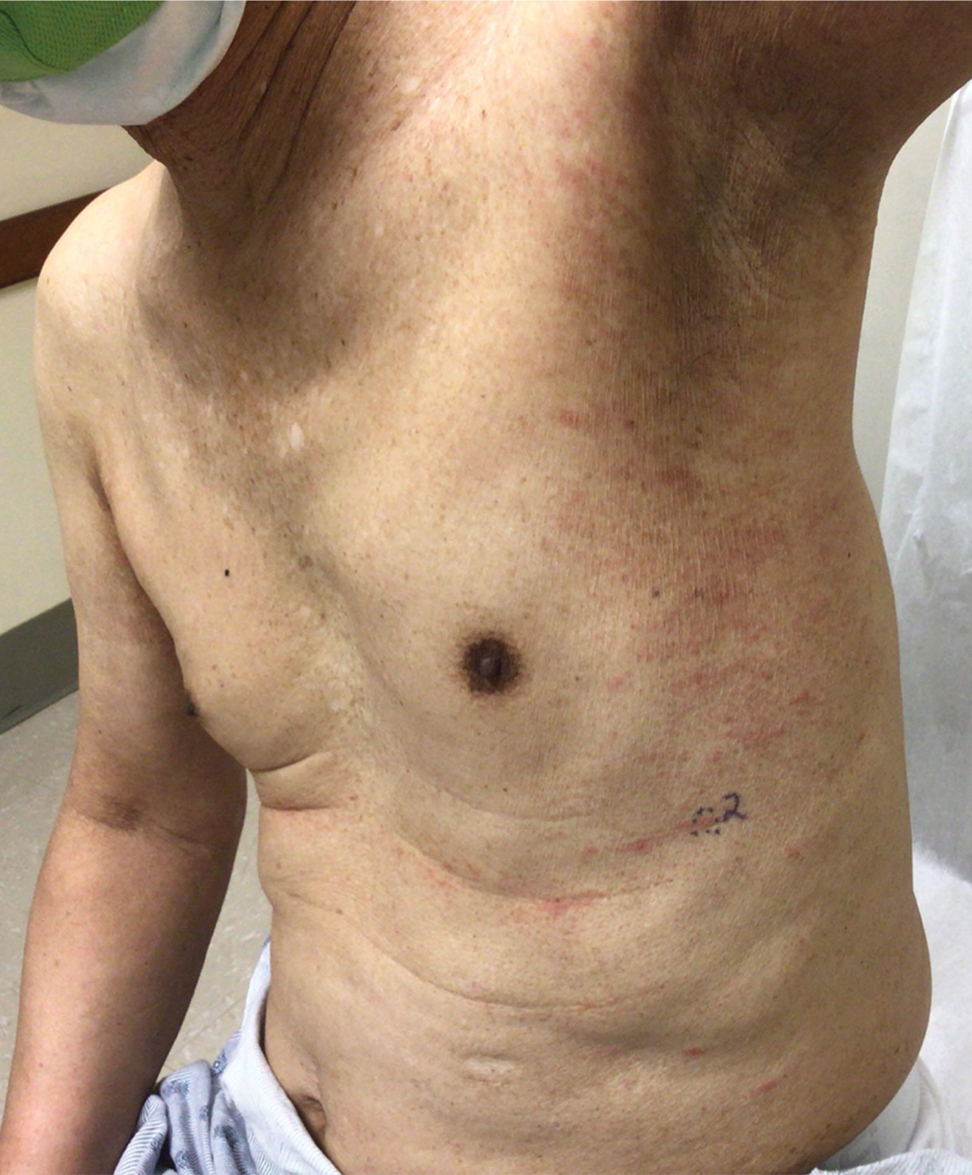
After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.
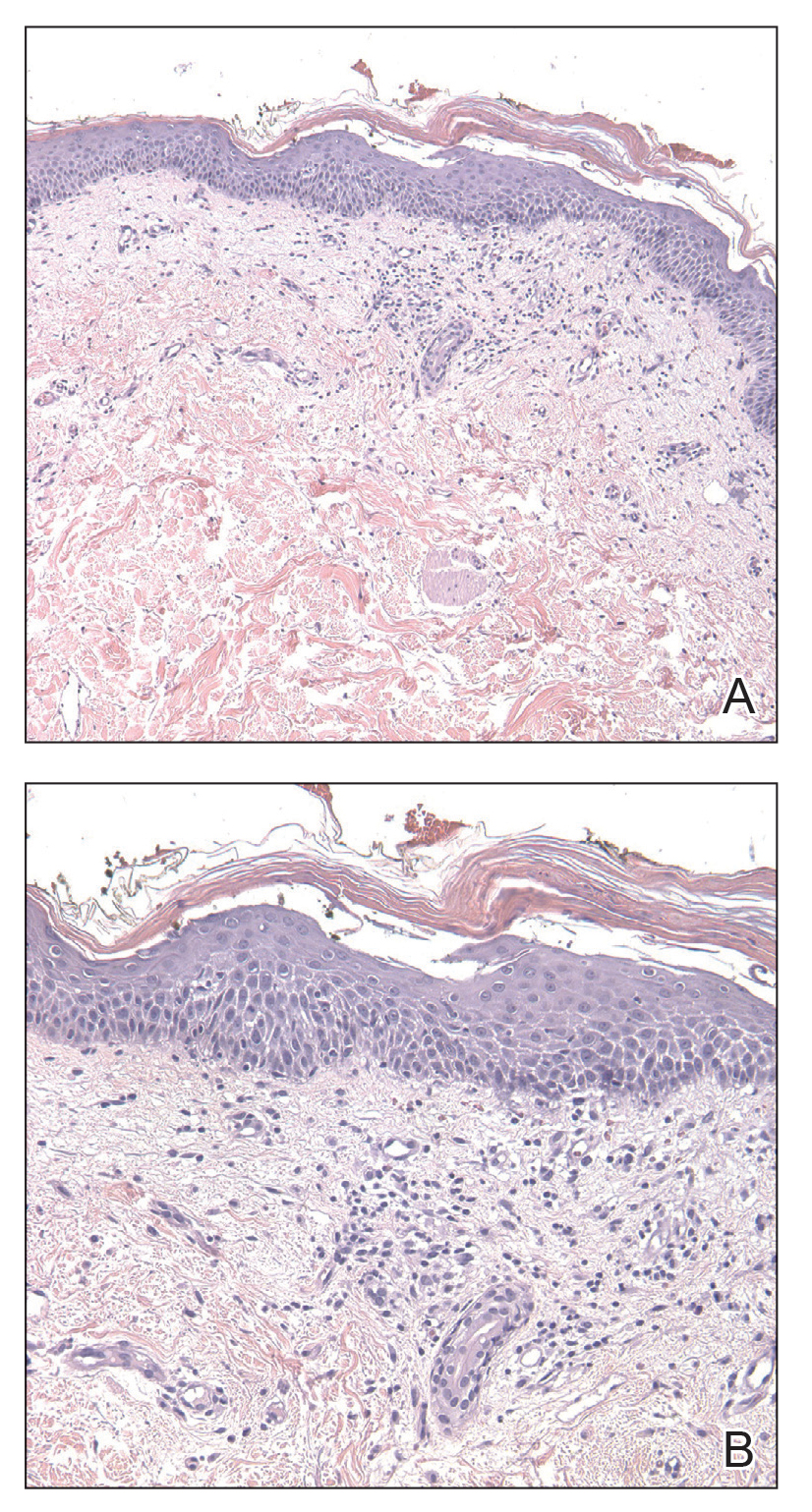
At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).
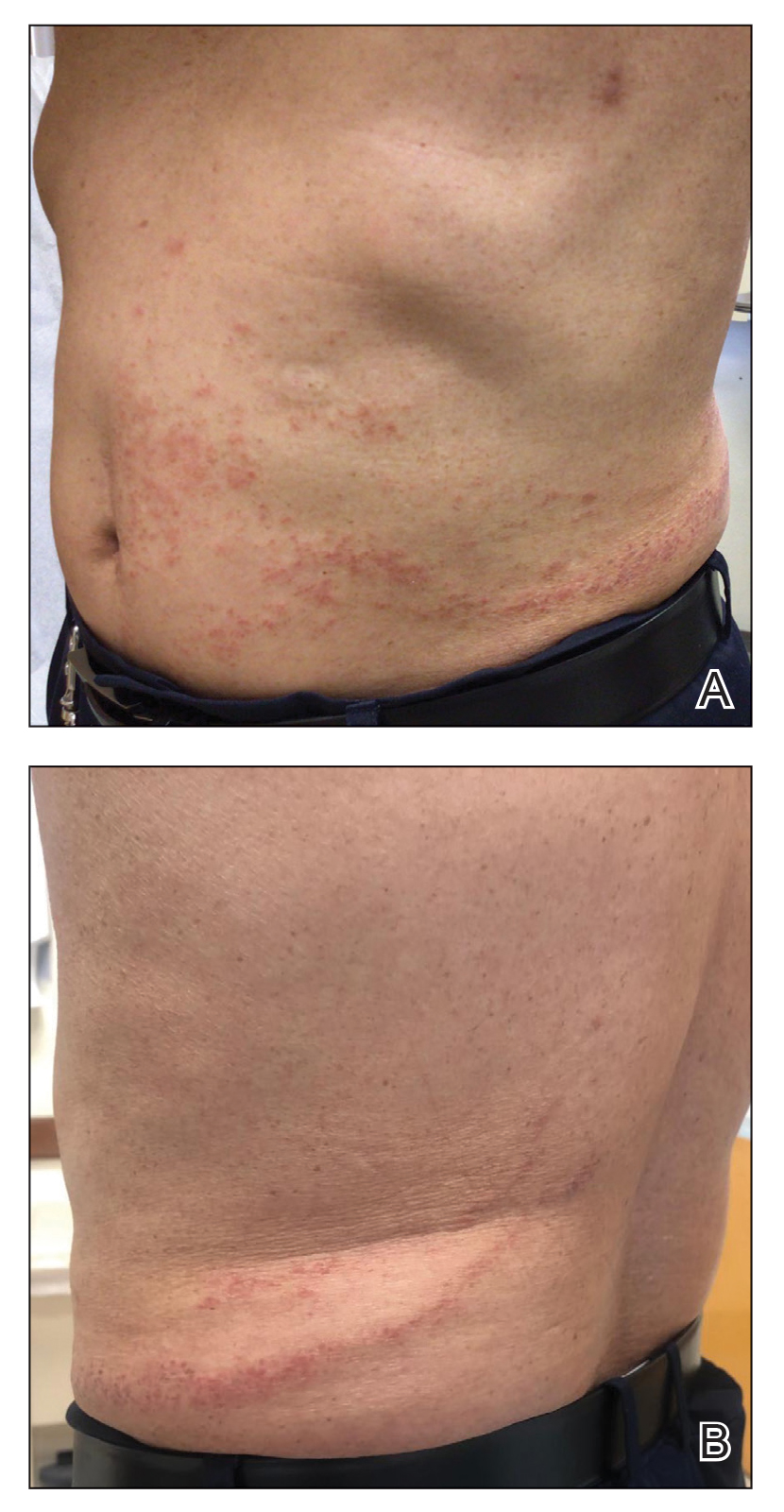
We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.

After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.

At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).

We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.

After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.

At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).

We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
Practice Points
- Dermatologists should be aware of the variety of cutaneous adverse events that can arise from the use of immunotherapeutic agents for hematologic malignancies.
- Some cutaneous reactions to immunotherapeutic medications, such as pityriasiform eruption and blaschkitis, generally are benign and may not necessitate halting an important therapy.