User login
Management of Late Pulmonary Complications After Hematopoietic Stem Cell Transplantation
Hematopoietic stem cell transplantation (HSCT) is increasingly being used to treat hematologic malignancies as well as nonmalignant diseases and solid tumors. Over the past 2 decades overall survival following transplant and transplant-related mortality have improved.1 With this increased survival, there is a need to focus on late complications after transplantation. Pulmonary complications are a common but sometimes underrecognized cause of late morbidity and mortality in HSCT patients. This article, the second of 2 articles on post-HSCT pulmonary complications, reviews late-onset complications, with a focus on the evaluation and treatment of bronchiolitis obliterans syndrome (BOS), one of the most common and serious late pulmonary complications in HSCT patients. The first article reviewed the management of early-onset pulmonary complications and included a basic overview of stem cell transplantation, discussion of factors associated with pulmonary complications, and a review of methods for assessing pretransplant risk for pulmonary complications in patients undergoing HSCT.2
Case Presentation
A 40-year-old white woman with a history of acute myeloid leukemia status post peripheral blood stem cell transplant presents with dyspnea on exertion, which she states started about 1 month ago and now is limiting her with even 1 flight of stairs. She also complains of mild dry cough and a 4- to 5-lb weight loss over the past 1 to 2 months. She has an occasional runny nose, but denies gastroesophageal reflux, fevers, chills, or night sweats. She has a history of matched related sibling donor transplant with busulfan and cyclophosphamide conditioning 1 year prior to presentation. She has had significant graft-versus-host disease (GVHD), affecting the liver, gastrointestinal tract, skin, and eyes.
On physical examination, heart rate is 110 beats/min, respiratory rate is 16 breaths/min, blood pressure is 92/58 mm Hg, and the patient is afebrile. Eye exam reveals scleral injection, mouth shows dry mucous membranes with a few white plaques, and the skin has chronic changes with a rash over both arms. Cardiac exam reveals tachycardia but regular rhythm and there are no murmurs, rubs, or gallops. Lungs are clear bilaterally and abdomen shows no organomegaly.
Laboratory exam shows a white blood cell count of 7800 cells/μL, hemoglobin level of 12.4 g/dL, and platelet count of 186 × 103/μL. Liver enzymes are mildly elevated. Chest radiograph shows clear lung fields bilaterally.
- What is the differential in this patient with dyspnea 1 year after transplantation?
Late pulmonary complications are generally accepted as those occurring more than 100 days post transplant. This period of time is characterized by chronic GVHD and impaired cellular and humoral immunity. Results of longitudinal studies of infections in adult HSCT patients suggest that special attention should be paid to allogeneic HSCT recipients for post-engraftment infectious pulmonary complications.3 Encapsulated bacteria such as Haemophilus influenzae and Streptococcus pneumoniae are the most frequent bacterial organisms causing late infectious pulmonary complications. Nontuberculous mycobacteria and Nocardia should also be considered. Depending upon geographic location, social and occupational risk factors, and prevalence, tuberculosis should also enter the differential.
There are many noninfectious late-onset pulmonary complications after HSCT. Unfortunately, the literature has divided pulmonary complications after HSCT using a range of criteria and classifications based upon timing, predominant pulmonary function test (PFT) findings, and etiology. These include early versus late, obstructive versus restrictive, and infectious versus noninfectious, which makes a comprehensive literature review of late pulmonary complications difficult. The most common noninfectious late-onset complications are bronchiolitis obliterans, cryptogenic organizing pneumonia (previously referred to as bronchiolitis obliterans organizing pneumonia, or BOOP), and interstitial pneumonia. Other rarely reported complications include eosinophilic pneumonia, pulmonary alveolar proteinosis, air leak syndrome, and pulmonary hypertension.
Case Continued
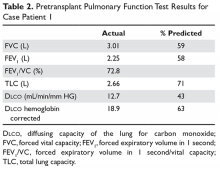
Because the patient does not have symptoms of infection, PFTs are obtained. Pretransplant PFTs and current PFTs are shown in Table 1.
- What is the diagnosis in this case?
Bronchiolitis Obliterans
BOS is one of the most common and most serious late-onset pulmonary diseases after allogeneic transplantation. It is considered the pulmonary form of chronic GVHD. BOS was first described in 1982 in patients with chronic GVHD after bone marrow transplantation.4 Many differing definitions of bronchiolitis obliterans have been described in the literature. A recent review of the topic cites 10 different published sets of criteria for the diagnosis of bronchiolitis obliterans.5 Traditionally, bronchiolitis obliterans was thought to occur in 2% to 8% of patients undergoing allogeneic HSCT, but these findings were from older studies that used a diagnosis based on very specific pathology findings. When more liberal diagnostic criteria are used, the incidence may be as high as 26% of allogeneic HSCT patients.6
Bronchiolitis obliterans is a progressive lung disease characterized by narrowing of the terminal airways and obliteration of the terminal bronchi. Pathology may show constrictive bronchiolitis but can also show lymphocytic bronchiolitis, which may be associated with a better outcome.7 As noted, bronchiolitis obliterans has traditionally been considered a pathologic diagnosis. Current diagnostic criteria have evolved based upon the difficulty in obtaining this diagnosis through transbronchial biopsy given the patchy nature of the disease.8 The gold standard of open lung biopsy is seldom pursued in the post-HSCT population as the procedure continues to carry a worrisome risk-benefit profile.
The 2005 National Institutes of Health (NIH) consensus development project on criteria for clinical trials in chronic GVHD developed a clinical strategy for diagnosing BOS using the following criteria: absence of active infection, decreased forced expiratory volume in 1 second (FEV1) < 75%, FEV1/forced vital capacity (FVC) ratio of < 70%, and evidence of air trapping on high-resolution computed tomography (HRCT) or PFTs (residual volume > 120%). These diagnostic criteria were applied to a small series of patients with clinically identified bronchiolitis obliterans or biopsy-proven bronchiolitis obliterans. Only 18% of these patients met the requirements for the NIH consensus definition.5 A 2011 study that applied the NIH criteria found an overall prevalence of 5.5% among all transplant recipients but a prevalence of 14% in patients with GVHD.9 In 2014, the NIH consensus development group updated their recommendations. The new criteria for diagnosis of BOS require the presence of airflow obstruction (FEV1/FVC < 70% or 5th percentile of predicted), FEV1 < 75% predicted with a ≥ 10% decline in fewer than 2 years, absence of infection, and presence of air trapping (by expiratory computed tomography [CT] scan or PFT with residual volume >120% predicted) (Table 2).
Some issues must be considered when determining airflow obstruction. The 2005 NIH working group recommends using Crapo as the reference set,11 but the National Health and Nutrition Examination Survey (NHANES) III reference values are the preferred reference set at this time12 and should be used in the United States. A recent article showed that the NHANES values were superior to older reference sets (however, they did not use Crapo as the comparison), although this study used the lower limit of normal as compared with the fixed 70% ratio.13 The 2014 NIH consensus group does not recommend a specific reference set and recognizes an FEV1/FVC ratio of 70% or less than the lower limit of normal as the cutoff value for airflow obstruction.10
Another issue in PFT interpretation is the finding of a decrease in FEV1 and FVC and normal total lung capacity, which is termed a nonspecific pattern. This pattern has been reported to occur in 9% of all PFTs and usually is associated with obstructive lung disease or obesity.14 A 2013 study described the nonspecific pattern as a BOS subgroup occurring in up to 31% of bronchiolitis obliterans patients.15
- What are the radiographic findings of BOS?
Chest radiograph is often normal in BOS. As discussed, air trapping can be documented using HRCT, according to the NIH clinical definition of bronchiolitis obliterans.16 A study that explored findings and trends seen on HRCT in HSCT patients with BOS found that the syndrome in these patients is characterized by central airway dilatation.17 Expiratory airway trapping on HRCT is the main finding, and this is best demonstrated on HRCT during inspiratory and expiratory phases.18 Other findings are bronchial wall thickening, parenchymal hypoattenuation, bronchiectasis, and centrilobular nodules.19
Galbán and colleagues developed a new technique called parametric response mapping that uses CT scanners to quantify normal parenchyma, functional small airway disease, emphysema, and parenchymal disease as relative lung volumes.20 This technique can detect airflow obstruction and small airway disease and was found to be a good method for detecting BOS after HSCT. In their study of parametric response mapping, the authors found that functional small airway disease affecting 28% or more of the total lung was highly indicative of bronchiolitis obliterans.20
- What therapies are used to treat BOS?
Traditionally, BOS has been treated with systemic immunosuppression. The recommended treatment had been systemic steroids at approximately 1 mg/ kg. However, it is increasingly recognized that BOS responds poorly to systemic steroids, and systemic steroids may actually be harmful and associated with increased mortality.15,21 The chronic GVHD recommendations from 2005 recommend ancillary therapy with inhaled corticosteroids and pulmonary rehabilitation.11 The updated 2011 German consensus statement lays out a clear management strategy for mild and moderate-severe disease with monitoring recommendations.22 The 2014 NIH chronic GVHD working group recommends fluticasone, azithromycin, and montelukast (ie, the FAM protocol) for treating BOS.23 FAM therapy in BOS may help lower the systemic steroid dose.24,25 Montelukast is not considered a treatment mainstay for BOS after lung transplant, but there is a study showing possible benefit in chronic GVHD.26 An evaluation of the natural history of a cohort of BOS patients treated with FAM therapy showed a rapid decline of FEV1 in the 6 months prior to diagnosis and treatment of BOS and subsequent stabilization following diagnosis and treatment.27 The benefit of high-dose inhaled corticosteroids or the combination of inhaled corticosteroids and long-acting beta-agonists has been demonstrated in small studies, which showed that these agents stabilized FEV1 and avoided the untoward side effects of systemic corticosteroids.28–30
Macrolide antibiotics have been explored as a treatment for BOS post HSCT because pilot studies suggested that azithromycin improved or stabilized FEV1 in patients with BOS after lung transplant or HSCT.31–33 Other studies of azithromycin have not shown benefit in the HSCT population after 3 months of therapy.34 A recent meta-analysis could neither support or refute the benefit of azithromycin for BOS after HSCT.35 In the lung transplant population, a study showed that patients who were started on azithromycin after transplant and continued on it 3 times a week had improved FEV1; these patients also had a reduced rate of BOS and improved overall and BOS-free survival 2 years after transplant.36 However, these benefits of azithromycin have not been observed in patients after HSCT. In fact, the ALLOZITHRO trial was stopped early because prophylactic azithromycin started at the time of the conditioning regimen with HSCT was associated with increased hematologic disease relapse, a decrease in airflow-decline-free survival, and reduced 2-year survival.30
Azithromycin is believed to exert an effect by its anti-inflammatory properties and perhaps by decreasing lung neutrophilia (it may be most beneficial in the subset of patients with high neutrophilia on bronchoalveolar lavage [BAL]).30 Adverse effects of chronic azithromycin include QT prolongation, cardiac arrhythmia, hearing loss, and antibiotic-resistant organism colonization.37,38
Other therapies include pulmonary rehabilitation, which may improve health-related quality of life and 6-minute walk distance,39 extracorporeal photopheresis,40 immunosuppression with calcineurin inhibitors or mycophenolate mofetil,21,41 and lung transplantation.42–44 A study with imatinib for the treatment of lung disease in steroid-refractory GVHD has shown promising results, but further validation with larger clinical trials is required.45
Case Continued
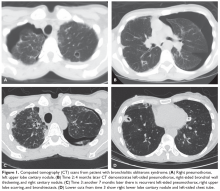
The patient is diagnosed with BOS and is treated for several months with prednisone 40 mg/day weaned over 3 months. She is started on inhaled corticosteroids, a proton pump inhibitor, and azithromycin 3 times per week, but she has a progressive decline in FEV1. She starts pulmonary rehabilitation but continues to functionally decline. Over the next year she develops bilateral pneumothoraces and bilateral cavitary nodules (Figure 1).
- What is causing this decline and the radiographic abnormalities?
Spontaneous air leak syndrome has been described in a little more than 1% of patients undergoing HSCT and has included pneumothorax and mediastinal and subcutaneous emphysema.46 It appears that air leak syndrome is more likely to occur in patients with chronic GVHD.47 The association between chronic GVHD and air leak syndrome could explain this patient’s recurrent pneumothoraces. The recurrent cavitary nodules are suspicious for infectious etiologies such as nontuberculous mycobacteria, tuberculosis, and fungal infections.
Case Continued
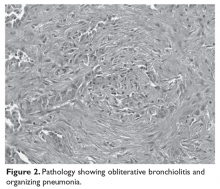
During an episode of pneumothorax, the patient undergoes chest tube placement, pleurodesis, and lung biopsy. Pathology reveals bronchiolitis obliterans as well as organizing pneumonia (Figure 2). No organisms are seen on acid-fast bacilli or GMS stains.
- What are the other late-onset noninfectious pulmonary complications?
Definitions of other late noninfectious pulmonary complications following HSCT are shown in Table 3.
Interstitial pneumonias may represent COP or may be idiopathic pneumonia syndrome with a later onset or a nonspecific interstitial pneumonia. This syndrome is poorly defined, with a number of differing definitions of the syndrome published in the literature.50–55
A rare pulmonary complication after HSCT is pulmonary veno-occlusive disease (PVOD). Pulmonary hypertension has been reported after HSCT,56 but PVOD is a subset of pulmonary hypertension. It is associated with pleural effusions and volume overload on chest radiography.57,58 It may present early or late after transplant and is poorly understood.
Besides obstructive and restrictive PFT abnormalities, changes in small airway function59 after transplant and loss in diffusing capacity of the lungs for carbon monoxide (D
Case Conclusion
The patient’s lung function continues to worsen, but no infectious etiologies are discovered. Ultimately, she dies of respiratory failure caused by progressive bronchiolitis obliterans.
Conclusion
Late pulmonary complications occur frequently in patients who have undergone HSCT. These complications can be classified as infectious versus noninfectious etiologies. Late-onset complications are more common in allogeneic transplantations because they are associated with chronic GVHD. These complications can be manifestations of pulmonary GHVD or can be infectious complications associated with prolonged immunosuppression. Appropriate monitoring for the development of BOS is essential. Early and aggressive treatment of respiratory infections and diagnostic bronchoscopy with BAL can help elucidate most infectious causes. Still, diagnostic challenges remain and multiple causes of respiratory deterioration can be present concurrently in the post-HSCT patient. Steroid therapy remains the mainstay treatment for most noninfectious pulmonary complications and should be strongly considered once infection is effectively ruled out.
1. Remberger M, Ackefors M, Berglund S, et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant 2011;17:1688–97.
2. Wood KL, Esguerra VG. Management of late pulmonary complications after hematopoietic stem cell transplantation. Hosp Phys Hematology-Oncology Board Review Manual 2018;13(1):36–48.
3. Ninin E, Milpied N, Moreau P, et al. Longitudinal study of bacterial, viral, and fungal infections in adult recipients of bone marrow transplants. Clin Infect Dis 2001;33:41–7.
4. Roca J, Granena A, Rodriguez-Roisin R, et al. Fatal airway disease in an adult with chronic graft-versus-host disease. Thorax 1982;37:77–8.
5. Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA 2009;302:306–14.
6. Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003;168:208–14.
7. Holbro A, Lehmann T, Girsberger S, et al. Lung histology predicts outcome of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013;19:973–80.
8. Chamberlain D, Maurer J, Chaparro C, Idolor L. Evaluation of transbronchial lung biopsy specimens in the diagnosis of bronchiolitis obliterans after lung transplantation. J Heart Lung Transplant 1994;13:963–71.
9. Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011;17:1072–8.
10. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21:389–401.
11. Couriel D, Carpenter PA, Cutler C, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant 2006;12:375–96.
12. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–68.
13. Williams KM, Hnatiuk O, Mitchell SA, et al. NHANES III equations enhance early detection and mortality prediction of bronchiolitis obliterans syndrome after hematopoietic SCT. Bone Marrow Transplant 2014;49:561–6.
14. Hyatt RE, Cowl CT, Bjoraker JA, Scanlon PD. Conditions associated with an abnormal nonspecific pattern of pulmonary function tests. Chest 2009;135:419–24.
15. Bergeron A, Godet C, Chevret S, et al. Bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: phenotypes and prognosis. Bone Marrow Transplant 2013;48:819–24.
16. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11:945–56.
17. Gazourian L, Coronata AM, Rogers AJ, et al. Airway dilation in bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. Respir Med 2013;107:276–83.
18. Gunn ML, Godwin JD, Kanne JP, et al. High-resolution CT findings of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. J Thorac Imaging 2008;23:244–50.
19. Sargent MA, Cairns RA, Murdoch MJ, et al. Obstructive lung disease in children after allogeneic bone marrow transplantation: evaluation with high-resolution CT. AJR Am J Roentgenol 1995;164:693–6.
20. Galban CJ, Boes JL, Bule M, et al. Parametric response mapping as an indicator of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014;20:1592–8.
21. Meyer KC, Raghu G, Verleden GM, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 2014;44:1479–1503.
22. Hildebrandt GC, Fazekas T, Lawitschka A, et al. Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD. Bone Marrow Transplant 2011;46:1283–95.
23. Carpenter PA, Kitko CL, Elad S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant 2015;21:1167–87.
24. Norman BC, Jacobsohn DA, Williams KM, et al. Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: a case series of eight patients. Bone Marrow Transplant 2011;46:1369–73.
25. Williams KM, Cheng GS, Pusic I, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016;22:710–6.
26. Or R, Gesundheit B, Resnick I, et al. Sparing effect by montelukast treatment for chronic graft versus host disease: a pilot study. Transplantation 2007;83:577–81.
27. Cheng GS, Storer B, Chien JW, et al. Lung function trajectory in bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplant. Ann Am Thorac Soc 2016;13:1932–9.
28. Bergeron A, Belle A, Chevret S, et al. Combined inhaled steroids and bronchodilatators in obstructive airway disease after allogeneic stem cell transplantation. Bone Marrow Transplant 2007;39:547–53.
29. Bashoura L, Gupta S, Jain A, et al. Inhaled corticosteroids stabilize constrictive bronchiolitis after hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;41:63–7.
30. Bergeron A, Chevret S, Granata A, et al. Effect of azithromycin on airflow decline-free survival after allogeneic hematopoietic stem cell transplant: the ALLOZITHRO randomized clinical trial. JAMA 2017;318:557–66.
31. Gerhardt SG, McDyer JF, Girgis RE, et al. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med 2003;168:121–5.
32. Khalid M, Al Saghir A, Saleemi S, et al. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur Respir J 2005;25:490–3.
33. Maimon N, Lipton JH, Chan CK, Marras TK. Macrolides in the treatment of bronchiolitis obliterans in allograft recipients. Bone Marrow Transplant 2009;44:69–73.
34. Lam DC, Lam B, Wong MK, et al. Effects of azithromycin in bronchiolitis obliterans syndrome after hematopoietic SCT--a randomized double-blinded placebo-controlled study. Bone Marrow Transplant 2011;46:1551–6.
35. Yadav H, Peters SG, Keogh KA, et al. Azithromycin for the treatment of obliterative bronchiolitis after hematopoietic stem cell transplantation: a systematic review and meta-analysis. Biol Blood Marrow Transplant 2016;22:2264–9.
36. Vos R, Vanaudenaerde BM, Verleden SE, et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur Respir J 2011;37:164–72.
37. Svanstrom H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013;368:1704–12.
38. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689–98.
39. Tran J, Norder EE, Diaz PT, et al. Pulmonary rehabilitation for bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012;18:1250–4.
40. Lucid CE, Savani BN, Engelhardt BG, et al. Extracorporeal photopheresis in patients with refractory bronchiolitis obliterans developing after allo-SCT. Bone Marrow Transplant 2011;46:426–9.
41. Hostettler KE, Halter JP, Gerull S, et al. Calcineurin inhibitors in bronchiolitis obliterans syndrome following stem cell transplantation. Eur Respir J 2014;43:221–32.
42. Holm AM, Riise GC, Brinch L, et al. Lung transplantation for bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation: unresolved questions. Transplantation 2013;96:e21–22.
43. Cheng GS, Edelman JD, Madtes DK, et al. Outcomes of lung transplantation after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014;20:1169–75.
44. Okumura H, Ohtake S, Ontachi Y, et al. Living-donor lobar lung transplantation for broncho-bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation: does bronchiolitis obliterans recur in transplanted lungs? Int J Hematol 2007;86:369–73.
45. Olivieri A, Cimminiello M, Corradini P, et al. Long-term outcome and prospective validation of NIH response criteria in 39 patients receiving imatinib for steroid-refractory chronic GVHD. Blood 2013;122:4111–8.
46. Rahmanian S, Wood KL. Bronchiolitis obliterans and the risk of pneumothorax after transbronchial biopsy. Respiratory Medicine CME 2010;3:87–9.
47. Sakai R, Kanamori H, Nakaseko C, et al. Air-leak syndrome following allo-SCT in adult patients: report from the Kanto Study Group for Cell Therapy in Japan. Bone Marrow Transplant 2011;46:379–84.
48. Visscher DW, Myers JL. Histologic spectrum of idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006;3:322–9.
49. Cordier JF. Cryptogenic organising pneumonia. Eur Respir J 2006;28:422–46.
50. Nishio N, Yagasaki H, Takahashi Y, et al. Late-onset non-infectious pulmonary complications following allogeneic hematopoietic stem cell transplantation in children. Bone Marrow Transplant 2009;44:303–8.
51. Ueda K, Watadani T, Maeda E, et al. Outcome and treatment of late-onset noninfectious pulmonary complications after allogeneic haematopoietic SCT. Bone Marrow Transplant 2010;45:1719–27.
52. Schlemmer F, Chevret S, Lorillon G, et al. Late-onset noninfectious interstitial lung disease after allogeneic hematopoietic stem cell transplantation. Respir Med 2014;108:1525–33.
53. Palmas A, Tefferi A, Myers JL, et al. Late-onset noninfectious pulmonary complications after allogeneic bone marrow transplantation. Br J Haematol 1998;100:680–7.
54. Sakaida E, Nakaseko C, Harima A, et al. Late-onset noninfectious pulmonary complications after allogeneic stem cell transplantation are significantly associated with chronic graft-versus-host disease and with the graft-versus-leukemia effect. Blood 2003;102:4236–42.
55. Solh M, Arat M, Cao Q, et al. Late-onset noninfectious pulmonary complications in adult allogeneic hematopoietic cell transplant recipients. Transplantation 2011;91:798–803.
56. Dandoy CE, Hirsch R, Chima R, et al. Pulmonary hypertension after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013;19:1546–56.
57. Bunte MC, Patnaik MM, Pritzker MR, Burns LJ. Pulmonary veno-occlusive disease following hematopoietic stem cell transplantation: a rare model of endothelial dysfunction. Bone Marrow Transplant 2008;41:677–86.
58. Troussard X, Bernaudin JF, Cordonnier C, et al. Pulmonary veno-occlusive disease after bone marrow transplantation. Thorax 1984;39:956–7.
59. Lahzami S, Schoeffel RE, Pechey V, et al. Small airways function declines after allogeneic haematopoietic stem cell transplantation. Eur Respir J 2011;38:1180–8.
60. Jain NA, Pophali PA, Klotz JK, et al. Repair of impaired pulmonary function is possible in very-long-term allogeneic stem cell transplantation survivors. Biol Blood Marrow Transplant 2014;20:209–13.
61. Barisione G, Bacigalupo A, Crimi E, et al. Changes in lung volumes and airway responsiveness following haematopoietic stem cell transplantation. Eur Respir J 2008;32:1576–82.
62. Kovalszki A, Schumaker GL, Klein A, et al. Reduced respiratory and skeletal muscle strength in survivors of sibling or unrelated donor hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;41:965–9.
63. Mathiesen S, Uhlving HH, Buchvald F, et al. Aerobic exercise capacity at long-term follow-up after paediatric allogeneic haematopoietic SCT. Bone Marrow Transplant 2014;49:1393–9.
Hematopoietic stem cell transplantation (HSCT) is increasingly being used to treat hematologic malignancies as well as nonmalignant diseases and solid tumors. Over the past 2 decades overall survival following transplant and transplant-related mortality have improved.1 With this increased survival, there is a need to focus on late complications after transplantation. Pulmonary complications are a common but sometimes underrecognized cause of late morbidity and mortality in HSCT patients. This article, the second of 2 articles on post-HSCT pulmonary complications, reviews late-onset complications, with a focus on the evaluation and treatment of bronchiolitis obliterans syndrome (BOS), one of the most common and serious late pulmonary complications in HSCT patients. The first article reviewed the management of early-onset pulmonary complications and included a basic overview of stem cell transplantation, discussion of factors associated with pulmonary complications, and a review of methods for assessing pretransplant risk for pulmonary complications in patients undergoing HSCT.2
Case Presentation
A 40-year-old white woman with a history of acute myeloid leukemia status post peripheral blood stem cell transplant presents with dyspnea on exertion, which she states started about 1 month ago and now is limiting her with even 1 flight of stairs. She also complains of mild dry cough and a 4- to 5-lb weight loss over the past 1 to 2 months. She has an occasional runny nose, but denies gastroesophageal reflux, fevers, chills, or night sweats. She has a history of matched related sibling donor transplant with busulfan and cyclophosphamide conditioning 1 year prior to presentation. She has had significant graft-versus-host disease (GVHD), affecting the liver, gastrointestinal tract, skin, and eyes.
On physical examination, heart rate is 110 beats/min, respiratory rate is 16 breaths/min, blood pressure is 92/58 mm Hg, and the patient is afebrile. Eye exam reveals scleral injection, mouth shows dry mucous membranes with a few white plaques, and the skin has chronic changes with a rash over both arms. Cardiac exam reveals tachycardia but regular rhythm and there are no murmurs, rubs, or gallops. Lungs are clear bilaterally and abdomen shows no organomegaly.
Laboratory exam shows a white blood cell count of 7800 cells/μL, hemoglobin level of 12.4 g/dL, and platelet count of 186 × 103/μL. Liver enzymes are mildly elevated. Chest radiograph shows clear lung fields bilaterally.
- What is the differential in this patient with dyspnea 1 year after transplantation?
Late pulmonary complications are generally accepted as those occurring more than 100 days post transplant. This period of time is characterized by chronic GVHD and impaired cellular and humoral immunity. Results of longitudinal studies of infections in adult HSCT patients suggest that special attention should be paid to allogeneic HSCT recipients for post-engraftment infectious pulmonary complications.3 Encapsulated bacteria such as Haemophilus influenzae and Streptococcus pneumoniae are the most frequent bacterial organisms causing late infectious pulmonary complications. Nontuberculous mycobacteria and Nocardia should also be considered. Depending upon geographic location, social and occupational risk factors, and prevalence, tuberculosis should also enter the differential.
There are many noninfectious late-onset pulmonary complications after HSCT. Unfortunately, the literature has divided pulmonary complications after HSCT using a range of criteria and classifications based upon timing, predominant pulmonary function test (PFT) findings, and etiology. These include early versus late, obstructive versus restrictive, and infectious versus noninfectious, which makes a comprehensive literature review of late pulmonary complications difficult. The most common noninfectious late-onset complications are bronchiolitis obliterans, cryptogenic organizing pneumonia (previously referred to as bronchiolitis obliterans organizing pneumonia, or BOOP), and interstitial pneumonia. Other rarely reported complications include eosinophilic pneumonia, pulmonary alveolar proteinosis, air leak syndrome, and pulmonary hypertension.
Case Continued
Because the patient does not have symptoms of infection, PFTs are obtained. Pretransplant PFTs and current PFTs are shown in Table 1.
- What is the diagnosis in this case?
Bronchiolitis Obliterans
BOS is one of the most common and most serious late-onset pulmonary diseases after allogeneic transplantation. It is considered the pulmonary form of chronic GVHD. BOS was first described in 1982 in patients with chronic GVHD after bone marrow transplantation.4 Many differing definitions of bronchiolitis obliterans have been described in the literature. A recent review of the topic cites 10 different published sets of criteria for the diagnosis of bronchiolitis obliterans.5 Traditionally, bronchiolitis obliterans was thought to occur in 2% to 8% of patients undergoing allogeneic HSCT, but these findings were from older studies that used a diagnosis based on very specific pathology findings. When more liberal diagnostic criteria are used, the incidence may be as high as 26% of allogeneic HSCT patients.6
Bronchiolitis obliterans is a progressive lung disease characterized by narrowing of the terminal airways and obliteration of the terminal bronchi. Pathology may show constrictive bronchiolitis but can also show lymphocytic bronchiolitis, which may be associated with a better outcome.7 As noted, bronchiolitis obliterans has traditionally been considered a pathologic diagnosis. Current diagnostic criteria have evolved based upon the difficulty in obtaining this diagnosis through transbronchial biopsy given the patchy nature of the disease.8 The gold standard of open lung biopsy is seldom pursued in the post-HSCT population as the procedure continues to carry a worrisome risk-benefit profile.
The 2005 National Institutes of Health (NIH) consensus development project on criteria for clinical trials in chronic GVHD developed a clinical strategy for diagnosing BOS using the following criteria: absence of active infection, decreased forced expiratory volume in 1 second (FEV1) < 75%, FEV1/forced vital capacity (FVC) ratio of < 70%, and evidence of air trapping on high-resolution computed tomography (HRCT) or PFTs (residual volume > 120%). These diagnostic criteria were applied to a small series of patients with clinically identified bronchiolitis obliterans or biopsy-proven bronchiolitis obliterans. Only 18% of these patients met the requirements for the NIH consensus definition.5 A 2011 study that applied the NIH criteria found an overall prevalence of 5.5% among all transplant recipients but a prevalence of 14% in patients with GVHD.9 In 2014, the NIH consensus development group updated their recommendations. The new criteria for diagnosis of BOS require the presence of airflow obstruction (FEV1/FVC < 70% or 5th percentile of predicted), FEV1 < 75% predicted with a ≥ 10% decline in fewer than 2 years, absence of infection, and presence of air trapping (by expiratory computed tomography [CT] scan or PFT with residual volume >120% predicted) (Table 2).
Some issues must be considered when determining airflow obstruction. The 2005 NIH working group recommends using Crapo as the reference set,11 but the National Health and Nutrition Examination Survey (NHANES) III reference values are the preferred reference set at this time12 and should be used in the United States. A recent article showed that the NHANES values were superior to older reference sets (however, they did not use Crapo as the comparison), although this study used the lower limit of normal as compared with the fixed 70% ratio.13 The 2014 NIH consensus group does not recommend a specific reference set and recognizes an FEV1/FVC ratio of 70% or less than the lower limit of normal as the cutoff value for airflow obstruction.10
Another issue in PFT interpretation is the finding of a decrease in FEV1 and FVC and normal total lung capacity, which is termed a nonspecific pattern. This pattern has been reported to occur in 9% of all PFTs and usually is associated with obstructive lung disease or obesity.14 A 2013 study described the nonspecific pattern as a BOS subgroup occurring in up to 31% of bronchiolitis obliterans patients.15
- What are the radiographic findings of BOS?
Chest radiograph is often normal in BOS. As discussed, air trapping can be documented using HRCT, according to the NIH clinical definition of bronchiolitis obliterans.16 A study that explored findings and trends seen on HRCT in HSCT patients with BOS found that the syndrome in these patients is characterized by central airway dilatation.17 Expiratory airway trapping on HRCT is the main finding, and this is best demonstrated on HRCT during inspiratory and expiratory phases.18 Other findings are bronchial wall thickening, parenchymal hypoattenuation, bronchiectasis, and centrilobular nodules.19
Galbán and colleagues developed a new technique called parametric response mapping that uses CT scanners to quantify normal parenchyma, functional small airway disease, emphysema, and parenchymal disease as relative lung volumes.20 This technique can detect airflow obstruction and small airway disease and was found to be a good method for detecting BOS after HSCT. In their study of parametric response mapping, the authors found that functional small airway disease affecting 28% or more of the total lung was highly indicative of bronchiolitis obliterans.20
- What therapies are used to treat BOS?
Traditionally, BOS has been treated with systemic immunosuppression. The recommended treatment had been systemic steroids at approximately 1 mg/ kg. However, it is increasingly recognized that BOS responds poorly to systemic steroids, and systemic steroids may actually be harmful and associated with increased mortality.15,21 The chronic GVHD recommendations from 2005 recommend ancillary therapy with inhaled corticosteroids and pulmonary rehabilitation.11 The updated 2011 German consensus statement lays out a clear management strategy for mild and moderate-severe disease with monitoring recommendations.22 The 2014 NIH chronic GVHD working group recommends fluticasone, azithromycin, and montelukast (ie, the FAM protocol) for treating BOS.23 FAM therapy in BOS may help lower the systemic steroid dose.24,25 Montelukast is not considered a treatment mainstay for BOS after lung transplant, but there is a study showing possible benefit in chronic GVHD.26 An evaluation of the natural history of a cohort of BOS patients treated with FAM therapy showed a rapid decline of FEV1 in the 6 months prior to diagnosis and treatment of BOS and subsequent stabilization following diagnosis and treatment.27 The benefit of high-dose inhaled corticosteroids or the combination of inhaled corticosteroids and long-acting beta-agonists has been demonstrated in small studies, which showed that these agents stabilized FEV1 and avoided the untoward side effects of systemic corticosteroids.28–30
Macrolide antibiotics have been explored as a treatment for BOS post HSCT because pilot studies suggested that azithromycin improved or stabilized FEV1 in patients with BOS after lung transplant or HSCT.31–33 Other studies of azithromycin have not shown benefit in the HSCT population after 3 months of therapy.34 A recent meta-analysis could neither support or refute the benefit of azithromycin for BOS after HSCT.35 In the lung transplant population, a study showed that patients who were started on azithromycin after transplant and continued on it 3 times a week had improved FEV1; these patients also had a reduced rate of BOS and improved overall and BOS-free survival 2 years after transplant.36 However, these benefits of azithromycin have not been observed in patients after HSCT. In fact, the ALLOZITHRO trial was stopped early because prophylactic azithromycin started at the time of the conditioning regimen with HSCT was associated with increased hematologic disease relapse, a decrease in airflow-decline-free survival, and reduced 2-year survival.30
Azithromycin is believed to exert an effect by its anti-inflammatory properties and perhaps by decreasing lung neutrophilia (it may be most beneficial in the subset of patients with high neutrophilia on bronchoalveolar lavage [BAL]).30 Adverse effects of chronic azithromycin include QT prolongation, cardiac arrhythmia, hearing loss, and antibiotic-resistant organism colonization.37,38
Other therapies include pulmonary rehabilitation, which may improve health-related quality of life and 6-minute walk distance,39 extracorporeal photopheresis,40 immunosuppression with calcineurin inhibitors or mycophenolate mofetil,21,41 and lung transplantation.42–44 A study with imatinib for the treatment of lung disease in steroid-refractory GVHD has shown promising results, but further validation with larger clinical trials is required.45
Case Continued
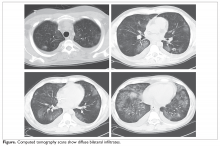
The patient is diagnosed with BOS and is treated for several months with prednisone 40 mg/day weaned over 3 months. She is started on inhaled corticosteroids, a proton pump inhibitor, and azithromycin 3 times per week, but she has a progressive decline in FEV1. She starts pulmonary rehabilitation but continues to functionally decline. Over the next year she develops bilateral pneumothoraces and bilateral cavitary nodules (Figure 1).
- What is causing this decline and the radiographic abnormalities?
Spontaneous air leak syndrome has been described in a little more than 1% of patients undergoing HSCT and has included pneumothorax and mediastinal and subcutaneous emphysema.46 It appears that air leak syndrome is more likely to occur in patients with chronic GVHD.47 The association between chronic GVHD and air leak syndrome could explain this patient’s recurrent pneumothoraces. The recurrent cavitary nodules are suspicious for infectious etiologies such as nontuberculous mycobacteria, tuberculosis, and fungal infections.
Case Continued
During an episode of pneumothorax, the patient undergoes chest tube placement, pleurodesis, and lung biopsy. Pathology reveals bronchiolitis obliterans as well as organizing pneumonia (Figure 2). No organisms are seen on acid-fast bacilli or GMS stains.
- What are the other late-onset noninfectious pulmonary complications?
Definitions of other late noninfectious pulmonary complications following HSCT are shown in Table 3.
Interstitial pneumonias may represent COP or may be idiopathic pneumonia syndrome with a later onset or a nonspecific interstitial pneumonia. This syndrome is poorly defined, with a number of differing definitions of the syndrome published in the literature.50–55
A rare pulmonary complication after HSCT is pulmonary veno-occlusive disease (PVOD). Pulmonary hypertension has been reported after HSCT,56 but PVOD is a subset of pulmonary hypertension. It is associated with pleural effusions and volume overload on chest radiography.57,58 It may present early or late after transplant and is poorly understood.
Besides obstructive and restrictive PFT abnormalities, changes in small airway function59 after transplant and loss in diffusing capacity of the lungs for carbon monoxide (D
Case Conclusion
The patient’s lung function continues to worsen, but no infectious etiologies are discovered. Ultimately, she dies of respiratory failure caused by progressive bronchiolitis obliterans.
Conclusion
Late pulmonary complications occur frequently in patients who have undergone HSCT. These complications can be classified as infectious versus noninfectious etiologies. Late-onset complications are more common in allogeneic transplantations because they are associated with chronic GVHD. These complications can be manifestations of pulmonary GHVD or can be infectious complications associated with prolonged immunosuppression. Appropriate monitoring for the development of BOS is essential. Early and aggressive treatment of respiratory infections and diagnostic bronchoscopy with BAL can help elucidate most infectious causes. Still, diagnostic challenges remain and multiple causes of respiratory deterioration can be present concurrently in the post-HSCT patient. Steroid therapy remains the mainstay treatment for most noninfectious pulmonary complications and should be strongly considered once infection is effectively ruled out.
Hematopoietic stem cell transplantation (HSCT) is increasingly being used to treat hematologic malignancies as well as nonmalignant diseases and solid tumors. Over the past 2 decades overall survival following transplant and transplant-related mortality have improved.1 With this increased survival, there is a need to focus on late complications after transplantation. Pulmonary complications are a common but sometimes underrecognized cause of late morbidity and mortality in HSCT patients. This article, the second of 2 articles on post-HSCT pulmonary complications, reviews late-onset complications, with a focus on the evaluation and treatment of bronchiolitis obliterans syndrome (BOS), one of the most common and serious late pulmonary complications in HSCT patients. The first article reviewed the management of early-onset pulmonary complications and included a basic overview of stem cell transplantation, discussion of factors associated with pulmonary complications, and a review of methods for assessing pretransplant risk for pulmonary complications in patients undergoing HSCT.2
Case Presentation
A 40-year-old white woman with a history of acute myeloid leukemia status post peripheral blood stem cell transplant presents with dyspnea on exertion, which she states started about 1 month ago and now is limiting her with even 1 flight of stairs. She also complains of mild dry cough and a 4- to 5-lb weight loss over the past 1 to 2 months. She has an occasional runny nose, but denies gastroesophageal reflux, fevers, chills, or night sweats. She has a history of matched related sibling donor transplant with busulfan and cyclophosphamide conditioning 1 year prior to presentation. She has had significant graft-versus-host disease (GVHD), affecting the liver, gastrointestinal tract, skin, and eyes.
On physical examination, heart rate is 110 beats/min, respiratory rate is 16 breaths/min, blood pressure is 92/58 mm Hg, and the patient is afebrile. Eye exam reveals scleral injection, mouth shows dry mucous membranes with a few white plaques, and the skin has chronic changes with a rash over both arms. Cardiac exam reveals tachycardia but regular rhythm and there are no murmurs, rubs, or gallops. Lungs are clear bilaterally and abdomen shows no organomegaly.
Laboratory exam shows a white blood cell count of 7800 cells/μL, hemoglobin level of 12.4 g/dL, and platelet count of 186 × 103/μL. Liver enzymes are mildly elevated. Chest radiograph shows clear lung fields bilaterally.
- What is the differential in this patient with dyspnea 1 year after transplantation?
Late pulmonary complications are generally accepted as those occurring more than 100 days post transplant. This period of time is characterized by chronic GVHD and impaired cellular and humoral immunity. Results of longitudinal studies of infections in adult HSCT patients suggest that special attention should be paid to allogeneic HSCT recipients for post-engraftment infectious pulmonary complications.3 Encapsulated bacteria such as Haemophilus influenzae and Streptococcus pneumoniae are the most frequent bacterial organisms causing late infectious pulmonary complications. Nontuberculous mycobacteria and Nocardia should also be considered. Depending upon geographic location, social and occupational risk factors, and prevalence, tuberculosis should also enter the differential.
There are many noninfectious late-onset pulmonary complications after HSCT. Unfortunately, the literature has divided pulmonary complications after HSCT using a range of criteria and classifications based upon timing, predominant pulmonary function test (PFT) findings, and etiology. These include early versus late, obstructive versus restrictive, and infectious versus noninfectious, which makes a comprehensive literature review of late pulmonary complications difficult. The most common noninfectious late-onset complications are bronchiolitis obliterans, cryptogenic organizing pneumonia (previously referred to as bronchiolitis obliterans organizing pneumonia, or BOOP), and interstitial pneumonia. Other rarely reported complications include eosinophilic pneumonia, pulmonary alveolar proteinosis, air leak syndrome, and pulmonary hypertension.
Case Continued
Because the patient does not have symptoms of infection, PFTs are obtained. Pretransplant PFTs and current PFTs are shown in Table 1.
- What is the diagnosis in this case?
Bronchiolitis Obliterans
BOS is one of the most common and most serious late-onset pulmonary diseases after allogeneic transplantation. It is considered the pulmonary form of chronic GVHD. BOS was first described in 1982 in patients with chronic GVHD after bone marrow transplantation.4 Many differing definitions of bronchiolitis obliterans have been described in the literature. A recent review of the topic cites 10 different published sets of criteria for the diagnosis of bronchiolitis obliterans.5 Traditionally, bronchiolitis obliterans was thought to occur in 2% to 8% of patients undergoing allogeneic HSCT, but these findings were from older studies that used a diagnosis based on very specific pathology findings. When more liberal diagnostic criteria are used, the incidence may be as high as 26% of allogeneic HSCT patients.6
Bronchiolitis obliterans is a progressive lung disease characterized by narrowing of the terminal airways and obliteration of the terminal bronchi. Pathology may show constrictive bronchiolitis but can also show lymphocytic bronchiolitis, which may be associated with a better outcome.7 As noted, bronchiolitis obliterans has traditionally been considered a pathologic diagnosis. Current diagnostic criteria have evolved based upon the difficulty in obtaining this diagnosis through transbronchial biopsy given the patchy nature of the disease.8 The gold standard of open lung biopsy is seldom pursued in the post-HSCT population as the procedure continues to carry a worrisome risk-benefit profile.
The 2005 National Institutes of Health (NIH) consensus development project on criteria for clinical trials in chronic GVHD developed a clinical strategy for diagnosing BOS using the following criteria: absence of active infection, decreased forced expiratory volume in 1 second (FEV1) < 75%, FEV1/forced vital capacity (FVC) ratio of < 70%, and evidence of air trapping on high-resolution computed tomography (HRCT) or PFTs (residual volume > 120%). These diagnostic criteria were applied to a small series of patients with clinically identified bronchiolitis obliterans or biopsy-proven bronchiolitis obliterans. Only 18% of these patients met the requirements for the NIH consensus definition.5 A 2011 study that applied the NIH criteria found an overall prevalence of 5.5% among all transplant recipients but a prevalence of 14% in patients with GVHD.9 In 2014, the NIH consensus development group updated their recommendations. The new criteria for diagnosis of BOS require the presence of airflow obstruction (FEV1/FVC < 70% or 5th percentile of predicted), FEV1 < 75% predicted with a ≥ 10% decline in fewer than 2 years, absence of infection, and presence of air trapping (by expiratory computed tomography [CT] scan or PFT with residual volume >120% predicted) (Table 2).
Some issues must be considered when determining airflow obstruction. The 2005 NIH working group recommends using Crapo as the reference set,11 but the National Health and Nutrition Examination Survey (NHANES) III reference values are the preferred reference set at this time12 and should be used in the United States. A recent article showed that the NHANES values were superior to older reference sets (however, they did not use Crapo as the comparison), although this study used the lower limit of normal as compared with the fixed 70% ratio.13 The 2014 NIH consensus group does not recommend a specific reference set and recognizes an FEV1/FVC ratio of 70% or less than the lower limit of normal as the cutoff value for airflow obstruction.10
Another issue in PFT interpretation is the finding of a decrease in FEV1 and FVC and normal total lung capacity, which is termed a nonspecific pattern. This pattern has been reported to occur in 9% of all PFTs and usually is associated with obstructive lung disease or obesity.14 A 2013 study described the nonspecific pattern as a BOS subgroup occurring in up to 31% of bronchiolitis obliterans patients.15
- What are the radiographic findings of BOS?
Chest radiograph is often normal in BOS. As discussed, air trapping can be documented using HRCT, according to the NIH clinical definition of bronchiolitis obliterans.16 A study that explored findings and trends seen on HRCT in HSCT patients with BOS found that the syndrome in these patients is characterized by central airway dilatation.17 Expiratory airway trapping on HRCT is the main finding, and this is best demonstrated on HRCT during inspiratory and expiratory phases.18 Other findings are bronchial wall thickening, parenchymal hypoattenuation, bronchiectasis, and centrilobular nodules.19
Galbán and colleagues developed a new technique called parametric response mapping that uses CT scanners to quantify normal parenchyma, functional small airway disease, emphysema, and parenchymal disease as relative lung volumes.20 This technique can detect airflow obstruction and small airway disease and was found to be a good method for detecting BOS after HSCT. In their study of parametric response mapping, the authors found that functional small airway disease affecting 28% or more of the total lung was highly indicative of bronchiolitis obliterans.20
- What therapies are used to treat BOS?
Traditionally, BOS has been treated with systemic immunosuppression. The recommended treatment had been systemic steroids at approximately 1 mg/ kg. However, it is increasingly recognized that BOS responds poorly to systemic steroids, and systemic steroids may actually be harmful and associated with increased mortality.15,21 The chronic GVHD recommendations from 2005 recommend ancillary therapy with inhaled corticosteroids and pulmonary rehabilitation.11 The updated 2011 German consensus statement lays out a clear management strategy for mild and moderate-severe disease with monitoring recommendations.22 The 2014 NIH chronic GVHD working group recommends fluticasone, azithromycin, and montelukast (ie, the FAM protocol) for treating BOS.23 FAM therapy in BOS may help lower the systemic steroid dose.24,25 Montelukast is not considered a treatment mainstay for BOS after lung transplant, but there is a study showing possible benefit in chronic GVHD.26 An evaluation of the natural history of a cohort of BOS patients treated with FAM therapy showed a rapid decline of FEV1 in the 6 months prior to diagnosis and treatment of BOS and subsequent stabilization following diagnosis and treatment.27 The benefit of high-dose inhaled corticosteroids or the combination of inhaled corticosteroids and long-acting beta-agonists has been demonstrated in small studies, which showed that these agents stabilized FEV1 and avoided the untoward side effects of systemic corticosteroids.28–30
Macrolide antibiotics have been explored as a treatment for BOS post HSCT because pilot studies suggested that azithromycin improved or stabilized FEV1 in patients with BOS after lung transplant or HSCT.31–33 Other studies of azithromycin have not shown benefit in the HSCT population after 3 months of therapy.34 A recent meta-analysis could neither support or refute the benefit of azithromycin for BOS after HSCT.35 In the lung transplant population, a study showed that patients who were started on azithromycin after transplant and continued on it 3 times a week had improved FEV1; these patients also had a reduced rate of BOS and improved overall and BOS-free survival 2 years after transplant.36 However, these benefits of azithromycin have not been observed in patients after HSCT. In fact, the ALLOZITHRO trial was stopped early because prophylactic azithromycin started at the time of the conditioning regimen with HSCT was associated with increased hematologic disease relapse, a decrease in airflow-decline-free survival, and reduced 2-year survival.30
Azithromycin is believed to exert an effect by its anti-inflammatory properties and perhaps by decreasing lung neutrophilia (it may be most beneficial in the subset of patients with high neutrophilia on bronchoalveolar lavage [BAL]).30 Adverse effects of chronic azithromycin include QT prolongation, cardiac arrhythmia, hearing loss, and antibiotic-resistant organism colonization.37,38
Other therapies include pulmonary rehabilitation, which may improve health-related quality of life and 6-minute walk distance,39 extracorporeal photopheresis,40 immunosuppression with calcineurin inhibitors or mycophenolate mofetil,21,41 and lung transplantation.42–44 A study with imatinib for the treatment of lung disease in steroid-refractory GVHD has shown promising results, but further validation with larger clinical trials is required.45
Case Continued
The patient is diagnosed with BOS and is treated for several months with prednisone 40 mg/day weaned over 3 months. She is started on inhaled corticosteroids, a proton pump inhibitor, and azithromycin 3 times per week, but she has a progressive decline in FEV1. She starts pulmonary rehabilitation but continues to functionally decline. Over the next year she develops bilateral pneumothoraces and bilateral cavitary nodules (Figure 1).
- What is causing this decline and the radiographic abnormalities?
Spontaneous air leak syndrome has been described in a little more than 1% of patients undergoing HSCT and has included pneumothorax and mediastinal and subcutaneous emphysema.46 It appears that air leak syndrome is more likely to occur in patients with chronic GVHD.47 The association between chronic GVHD and air leak syndrome could explain this patient’s recurrent pneumothoraces. The recurrent cavitary nodules are suspicious for infectious etiologies such as nontuberculous mycobacteria, tuberculosis, and fungal infections.
Case Continued
During an episode of pneumothorax, the patient undergoes chest tube placement, pleurodesis, and lung biopsy. Pathology reveals bronchiolitis obliterans as well as organizing pneumonia (Figure 2). No organisms are seen on acid-fast bacilli or GMS stains.
- What are the other late-onset noninfectious pulmonary complications?
Definitions of other late noninfectious pulmonary complications following HSCT are shown in Table 3.
Interstitial pneumonias may represent COP or may be idiopathic pneumonia syndrome with a later onset or a nonspecific interstitial pneumonia. This syndrome is poorly defined, with a number of differing definitions of the syndrome published in the literature.50–55
A rare pulmonary complication after HSCT is pulmonary veno-occlusive disease (PVOD). Pulmonary hypertension has been reported after HSCT,56 but PVOD is a subset of pulmonary hypertension. It is associated with pleural effusions and volume overload on chest radiography.57,58 It may present early or late after transplant and is poorly understood.
Besides obstructive and restrictive PFT abnormalities, changes in small airway function59 after transplant and loss in diffusing capacity of the lungs for carbon monoxide (D
Case Conclusion
The patient’s lung function continues to worsen, but no infectious etiologies are discovered. Ultimately, she dies of respiratory failure caused by progressive bronchiolitis obliterans.
Conclusion
Late pulmonary complications occur frequently in patients who have undergone HSCT. These complications can be classified as infectious versus noninfectious etiologies. Late-onset complications are more common in allogeneic transplantations because they are associated with chronic GVHD. These complications can be manifestations of pulmonary GHVD or can be infectious complications associated with prolonged immunosuppression. Appropriate monitoring for the development of BOS is essential. Early and aggressive treatment of respiratory infections and diagnostic bronchoscopy with BAL can help elucidate most infectious causes. Still, diagnostic challenges remain and multiple causes of respiratory deterioration can be present concurrently in the post-HSCT patient. Steroid therapy remains the mainstay treatment for most noninfectious pulmonary complications and should be strongly considered once infection is effectively ruled out.
1. Remberger M, Ackefors M, Berglund S, et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant 2011;17:1688–97.
2. Wood KL, Esguerra VG. Management of late pulmonary complications after hematopoietic stem cell transplantation. Hosp Phys Hematology-Oncology Board Review Manual 2018;13(1):36–48.
3. Ninin E, Milpied N, Moreau P, et al. Longitudinal study of bacterial, viral, and fungal infections in adult recipients of bone marrow transplants. Clin Infect Dis 2001;33:41–7.
4. Roca J, Granena A, Rodriguez-Roisin R, et al. Fatal airway disease in an adult with chronic graft-versus-host disease. Thorax 1982;37:77–8.
5. Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA 2009;302:306–14.
6. Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003;168:208–14.
7. Holbro A, Lehmann T, Girsberger S, et al. Lung histology predicts outcome of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013;19:973–80.
8. Chamberlain D, Maurer J, Chaparro C, Idolor L. Evaluation of transbronchial lung biopsy specimens in the diagnosis of bronchiolitis obliterans after lung transplantation. J Heart Lung Transplant 1994;13:963–71.
9. Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011;17:1072–8.
10. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21:389–401.
11. Couriel D, Carpenter PA, Cutler C, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant 2006;12:375–96.
12. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–68.
13. Williams KM, Hnatiuk O, Mitchell SA, et al. NHANES III equations enhance early detection and mortality prediction of bronchiolitis obliterans syndrome after hematopoietic SCT. Bone Marrow Transplant 2014;49:561–6.
14. Hyatt RE, Cowl CT, Bjoraker JA, Scanlon PD. Conditions associated with an abnormal nonspecific pattern of pulmonary function tests. Chest 2009;135:419–24.
15. Bergeron A, Godet C, Chevret S, et al. Bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: phenotypes and prognosis. Bone Marrow Transplant 2013;48:819–24.
16. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11:945–56.
17. Gazourian L, Coronata AM, Rogers AJ, et al. Airway dilation in bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. Respir Med 2013;107:276–83.
18. Gunn ML, Godwin JD, Kanne JP, et al. High-resolution CT findings of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. J Thorac Imaging 2008;23:244–50.
19. Sargent MA, Cairns RA, Murdoch MJ, et al. Obstructive lung disease in children after allogeneic bone marrow transplantation: evaluation with high-resolution CT. AJR Am J Roentgenol 1995;164:693–6.
20. Galban CJ, Boes JL, Bule M, et al. Parametric response mapping as an indicator of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014;20:1592–8.
21. Meyer KC, Raghu G, Verleden GM, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 2014;44:1479–1503.
22. Hildebrandt GC, Fazekas T, Lawitschka A, et al. Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD. Bone Marrow Transplant 2011;46:1283–95.
23. Carpenter PA, Kitko CL, Elad S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant 2015;21:1167–87.
24. Norman BC, Jacobsohn DA, Williams KM, et al. Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: a case series of eight patients. Bone Marrow Transplant 2011;46:1369–73.
25. Williams KM, Cheng GS, Pusic I, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016;22:710–6.
26. Or R, Gesundheit B, Resnick I, et al. Sparing effect by montelukast treatment for chronic graft versus host disease: a pilot study. Transplantation 2007;83:577–81.
27. Cheng GS, Storer B, Chien JW, et al. Lung function trajectory in bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplant. Ann Am Thorac Soc 2016;13:1932–9.
28. Bergeron A, Belle A, Chevret S, et al. Combined inhaled steroids and bronchodilatators in obstructive airway disease after allogeneic stem cell transplantation. Bone Marrow Transplant 2007;39:547–53.
29. Bashoura L, Gupta S, Jain A, et al. Inhaled corticosteroids stabilize constrictive bronchiolitis after hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;41:63–7.
30. Bergeron A, Chevret S, Granata A, et al. Effect of azithromycin on airflow decline-free survival after allogeneic hematopoietic stem cell transplant: the ALLOZITHRO randomized clinical trial. JAMA 2017;318:557–66.
31. Gerhardt SG, McDyer JF, Girgis RE, et al. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med 2003;168:121–5.
32. Khalid M, Al Saghir A, Saleemi S, et al. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur Respir J 2005;25:490–3.
33. Maimon N, Lipton JH, Chan CK, Marras TK. Macrolides in the treatment of bronchiolitis obliterans in allograft recipients. Bone Marrow Transplant 2009;44:69–73.
34. Lam DC, Lam B, Wong MK, et al. Effects of azithromycin in bronchiolitis obliterans syndrome after hematopoietic SCT--a randomized double-blinded placebo-controlled study. Bone Marrow Transplant 2011;46:1551–6.
35. Yadav H, Peters SG, Keogh KA, et al. Azithromycin for the treatment of obliterative bronchiolitis after hematopoietic stem cell transplantation: a systematic review and meta-analysis. Biol Blood Marrow Transplant 2016;22:2264–9.
36. Vos R, Vanaudenaerde BM, Verleden SE, et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur Respir J 2011;37:164–72.
37. Svanstrom H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013;368:1704–12.
38. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689–98.
39. Tran J, Norder EE, Diaz PT, et al. Pulmonary rehabilitation for bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012;18:1250–4.
40. Lucid CE, Savani BN, Engelhardt BG, et al. Extracorporeal photopheresis in patients with refractory bronchiolitis obliterans developing after allo-SCT. Bone Marrow Transplant 2011;46:426–9.
41. Hostettler KE, Halter JP, Gerull S, et al. Calcineurin inhibitors in bronchiolitis obliterans syndrome following stem cell transplantation. Eur Respir J 2014;43:221–32.
42. Holm AM, Riise GC, Brinch L, et al. Lung transplantation for bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation: unresolved questions. Transplantation 2013;96:e21–22.
43. Cheng GS, Edelman JD, Madtes DK, et al. Outcomes of lung transplantation after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014;20:1169–75.
44. Okumura H, Ohtake S, Ontachi Y, et al. Living-donor lobar lung transplantation for broncho-bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation: does bronchiolitis obliterans recur in transplanted lungs? Int J Hematol 2007;86:369–73.
45. Olivieri A, Cimminiello M, Corradini P, et al. Long-term outcome and prospective validation of NIH response criteria in 39 patients receiving imatinib for steroid-refractory chronic GVHD. Blood 2013;122:4111–8.
46. Rahmanian S, Wood KL. Bronchiolitis obliterans and the risk of pneumothorax after transbronchial biopsy. Respiratory Medicine CME 2010;3:87–9.
47. Sakai R, Kanamori H, Nakaseko C, et al. Air-leak syndrome following allo-SCT in adult patients: report from the Kanto Study Group for Cell Therapy in Japan. Bone Marrow Transplant 2011;46:379–84.
48. Visscher DW, Myers JL. Histologic spectrum of idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006;3:322–9.
49. Cordier JF. Cryptogenic organising pneumonia. Eur Respir J 2006;28:422–46.
50. Nishio N, Yagasaki H, Takahashi Y, et al. Late-onset non-infectious pulmonary complications following allogeneic hematopoietic stem cell transplantation in children. Bone Marrow Transplant 2009;44:303–8.
51. Ueda K, Watadani T, Maeda E, et al. Outcome and treatment of late-onset noninfectious pulmonary complications after allogeneic haematopoietic SCT. Bone Marrow Transplant 2010;45:1719–27.
52. Schlemmer F, Chevret S, Lorillon G, et al. Late-onset noninfectious interstitial lung disease after allogeneic hematopoietic stem cell transplantation. Respir Med 2014;108:1525–33.
53. Palmas A, Tefferi A, Myers JL, et al. Late-onset noninfectious pulmonary complications after allogeneic bone marrow transplantation. Br J Haematol 1998;100:680–7.
54. Sakaida E, Nakaseko C, Harima A, et al. Late-onset noninfectious pulmonary complications after allogeneic stem cell transplantation are significantly associated with chronic graft-versus-host disease and with the graft-versus-leukemia effect. Blood 2003;102:4236–42.
55. Solh M, Arat M, Cao Q, et al. Late-onset noninfectious pulmonary complications in adult allogeneic hematopoietic cell transplant recipients. Transplantation 2011;91:798–803.
56. Dandoy CE, Hirsch R, Chima R, et al. Pulmonary hypertension after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013;19:1546–56.
57. Bunte MC, Patnaik MM, Pritzker MR, Burns LJ. Pulmonary veno-occlusive disease following hematopoietic stem cell transplantation: a rare model of endothelial dysfunction. Bone Marrow Transplant 2008;41:677–86.
58. Troussard X, Bernaudin JF, Cordonnier C, et al. Pulmonary veno-occlusive disease after bone marrow transplantation. Thorax 1984;39:956–7.
59. Lahzami S, Schoeffel RE, Pechey V, et al. Small airways function declines after allogeneic haematopoietic stem cell transplantation. Eur Respir J 2011;38:1180–8.
60. Jain NA, Pophali PA, Klotz JK, et al. Repair of impaired pulmonary function is possible in very-long-term allogeneic stem cell transplantation survivors. Biol Blood Marrow Transplant 2014;20:209–13.
61. Barisione G, Bacigalupo A, Crimi E, et al. Changes in lung volumes and airway responsiveness following haematopoietic stem cell transplantation. Eur Respir J 2008;32:1576–82.
62. Kovalszki A, Schumaker GL, Klein A, et al. Reduced respiratory and skeletal muscle strength in survivors of sibling or unrelated donor hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;41:965–9.
63. Mathiesen S, Uhlving HH, Buchvald F, et al. Aerobic exercise capacity at long-term follow-up after paediatric allogeneic haematopoietic SCT. Bone Marrow Transplant 2014;49:1393–9.
1. Remberger M, Ackefors M, Berglund S, et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant 2011;17:1688–97.
2. Wood KL, Esguerra VG. Management of late pulmonary complications after hematopoietic stem cell transplantation. Hosp Phys Hematology-Oncology Board Review Manual 2018;13(1):36–48.
3. Ninin E, Milpied N, Moreau P, et al. Longitudinal study of bacterial, viral, and fungal infections in adult recipients of bone marrow transplants. Clin Infect Dis 2001;33:41–7.
4. Roca J, Granena A, Rodriguez-Roisin R, et al. Fatal airway disease in an adult with chronic graft-versus-host disease. Thorax 1982;37:77–8.
5. Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA 2009;302:306–14.
6. Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003;168:208–14.
7. Holbro A, Lehmann T, Girsberger S, et al. Lung histology predicts outcome of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013;19:973–80.
8. Chamberlain D, Maurer J, Chaparro C, Idolor L. Evaluation of transbronchial lung biopsy specimens in the diagnosis of bronchiolitis obliterans after lung transplantation. J Heart Lung Transplant 1994;13:963–71.
9. Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011;17:1072–8.
10. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21:389–401.
11. Couriel D, Carpenter PA, Cutler C, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant 2006;12:375–96.
12. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–68.
13. Williams KM, Hnatiuk O, Mitchell SA, et al. NHANES III equations enhance early detection and mortality prediction of bronchiolitis obliterans syndrome after hematopoietic SCT. Bone Marrow Transplant 2014;49:561–6.
14. Hyatt RE, Cowl CT, Bjoraker JA, Scanlon PD. Conditions associated with an abnormal nonspecific pattern of pulmonary function tests. Chest 2009;135:419–24.
15. Bergeron A, Godet C, Chevret S, et al. Bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: phenotypes and prognosis. Bone Marrow Transplant 2013;48:819–24.
16. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11:945–56.
17. Gazourian L, Coronata AM, Rogers AJ, et al. Airway dilation in bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. Respir Med 2013;107:276–83.
18. Gunn ML, Godwin JD, Kanne JP, et al. High-resolution CT findings of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. J Thorac Imaging 2008;23:244–50.
19. Sargent MA, Cairns RA, Murdoch MJ, et al. Obstructive lung disease in children after allogeneic bone marrow transplantation: evaluation with high-resolution CT. AJR Am J Roentgenol 1995;164:693–6.
20. Galban CJ, Boes JL, Bule M, et al. Parametric response mapping as an indicator of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014;20:1592–8.
21. Meyer KC, Raghu G, Verleden GM, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 2014;44:1479–1503.
22. Hildebrandt GC, Fazekas T, Lawitschka A, et al. Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD. Bone Marrow Transplant 2011;46:1283–95.
23. Carpenter PA, Kitko CL, Elad S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant 2015;21:1167–87.
24. Norman BC, Jacobsohn DA, Williams KM, et al. Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: a case series of eight patients. Bone Marrow Transplant 2011;46:1369–73.
25. Williams KM, Cheng GS, Pusic I, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016;22:710–6.
26. Or R, Gesundheit B, Resnick I, et al. Sparing effect by montelukast treatment for chronic graft versus host disease: a pilot study. Transplantation 2007;83:577–81.
27. Cheng GS, Storer B, Chien JW, et al. Lung function trajectory in bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplant. Ann Am Thorac Soc 2016;13:1932–9.
28. Bergeron A, Belle A, Chevret S, et al. Combined inhaled steroids and bronchodilatators in obstructive airway disease after allogeneic stem cell transplantation. Bone Marrow Transplant 2007;39:547–53.
29. Bashoura L, Gupta S, Jain A, et al. Inhaled corticosteroids stabilize constrictive bronchiolitis after hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;41:63–7.
30. Bergeron A, Chevret S, Granata A, et al. Effect of azithromycin on airflow decline-free survival after allogeneic hematopoietic stem cell transplant: the ALLOZITHRO randomized clinical trial. JAMA 2017;318:557–66.
31. Gerhardt SG, McDyer JF, Girgis RE, et al. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med 2003;168:121–5.
32. Khalid M, Al Saghir A, Saleemi S, et al. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur Respir J 2005;25:490–3.
33. Maimon N, Lipton JH, Chan CK, Marras TK. Macrolides in the treatment of bronchiolitis obliterans in allograft recipients. Bone Marrow Transplant 2009;44:69–73.
34. Lam DC, Lam B, Wong MK, et al. Effects of azithromycin in bronchiolitis obliterans syndrome after hematopoietic SCT--a randomized double-blinded placebo-controlled study. Bone Marrow Transplant 2011;46:1551–6.
35. Yadav H, Peters SG, Keogh KA, et al. Azithromycin for the treatment of obliterative bronchiolitis after hematopoietic stem cell transplantation: a systematic review and meta-analysis. Biol Blood Marrow Transplant 2016;22:2264–9.
36. Vos R, Vanaudenaerde BM, Verleden SE, et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur Respir J 2011;37:164–72.
37. Svanstrom H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013;368:1704–12.
38. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689–98.
39. Tran J, Norder EE, Diaz PT, et al. Pulmonary rehabilitation for bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012;18:1250–4.
40. Lucid CE, Savani BN, Engelhardt BG, et al. Extracorporeal photopheresis in patients with refractory bronchiolitis obliterans developing after allo-SCT. Bone Marrow Transplant 2011;46:426–9.
41. Hostettler KE, Halter JP, Gerull S, et al. Calcineurin inhibitors in bronchiolitis obliterans syndrome following stem cell transplantation. Eur Respir J 2014;43:221–32.
42. Holm AM, Riise GC, Brinch L, et al. Lung transplantation for bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation: unresolved questions. Transplantation 2013;96:e21–22.
43. Cheng GS, Edelman JD, Madtes DK, et al. Outcomes of lung transplantation after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014;20:1169–75.
44. Okumura H, Ohtake S, Ontachi Y, et al. Living-donor lobar lung transplantation for broncho-bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation: does bronchiolitis obliterans recur in transplanted lungs? Int J Hematol 2007;86:369–73.
45. Olivieri A, Cimminiello M, Corradini P, et al. Long-term outcome and prospective validation of NIH response criteria in 39 patients receiving imatinib for steroid-refractory chronic GVHD. Blood 2013;122:4111–8.
46. Rahmanian S, Wood KL. Bronchiolitis obliterans and the risk of pneumothorax after transbronchial biopsy. Respiratory Medicine CME 2010;3:87–9.
47. Sakai R, Kanamori H, Nakaseko C, et al. Air-leak syndrome following allo-SCT in adult patients: report from the Kanto Study Group for Cell Therapy in Japan. Bone Marrow Transplant 2011;46:379–84.
48. Visscher DW, Myers JL. Histologic spectrum of idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006;3:322–9.
49. Cordier JF. Cryptogenic organising pneumonia. Eur Respir J 2006;28:422–46.
50. Nishio N, Yagasaki H, Takahashi Y, et al. Late-onset non-infectious pulmonary complications following allogeneic hematopoietic stem cell transplantation in children. Bone Marrow Transplant 2009;44:303–8.
51. Ueda K, Watadani T, Maeda E, et al. Outcome and treatment of late-onset noninfectious pulmonary complications after allogeneic haematopoietic SCT. Bone Marrow Transplant 2010;45:1719–27.
52. Schlemmer F, Chevret S, Lorillon G, et al. Late-onset noninfectious interstitial lung disease after allogeneic hematopoietic stem cell transplantation. Respir Med 2014;108:1525–33.
53. Palmas A, Tefferi A, Myers JL, et al. Late-onset noninfectious pulmonary complications after allogeneic bone marrow transplantation. Br J Haematol 1998;100:680–7.
54. Sakaida E, Nakaseko C, Harima A, et al. Late-onset noninfectious pulmonary complications after allogeneic stem cell transplantation are significantly associated with chronic graft-versus-host disease and with the graft-versus-leukemia effect. Blood 2003;102:4236–42.
55. Solh M, Arat M, Cao Q, et al. Late-onset noninfectious pulmonary complications in adult allogeneic hematopoietic cell transplant recipients. Transplantation 2011;91:798–803.
56. Dandoy CE, Hirsch R, Chima R, et al. Pulmonary hypertension after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013;19:1546–56.
57. Bunte MC, Patnaik MM, Pritzker MR, Burns LJ. Pulmonary veno-occlusive disease following hematopoietic stem cell transplantation: a rare model of endothelial dysfunction. Bone Marrow Transplant 2008;41:677–86.
58. Troussard X, Bernaudin JF, Cordonnier C, et al. Pulmonary veno-occlusive disease after bone marrow transplantation. Thorax 1984;39:956–7.
59. Lahzami S, Schoeffel RE, Pechey V, et al. Small airways function declines after allogeneic haematopoietic stem cell transplantation. Eur Respir J 2011;38:1180–8.
60. Jain NA, Pophali PA, Klotz JK, et al. Repair of impaired pulmonary function is possible in very-long-term allogeneic stem cell transplantation survivors. Biol Blood Marrow Transplant 2014;20:209–13.
61. Barisione G, Bacigalupo A, Crimi E, et al. Changes in lung volumes and airway responsiveness following haematopoietic stem cell transplantation. Eur Respir J 2008;32:1576–82.
62. Kovalszki A, Schumaker GL, Klein A, et al. Reduced respiratory and skeletal muscle strength in survivors of sibling or unrelated donor hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;41:965–9.
63. Mathiesen S, Uhlving HH, Buchvald F, et al. Aerobic exercise capacity at long-term follow-up after paediatric allogeneic haematopoietic SCT. Bone Marrow Transplant 2014;49:1393–9.
Management of Early Pulmonary Complications After Hematopoietic Stem Cell Transplantation
Hematopoietic stem cell transplantation (HSCT) is widely used in the economically developed world to treat a variety of hematologic malignancies as well as nonmalignant diseases and solid tumors. An estimated 17,900 HSCTs were performed in 2011, and survival rates continue to increase.1 Pulmonary complications post HSCT are common, with rates ranging from 40% to 60%, and are associated with increased morbidity and mortality.2
Clinical diagnosis of pulmonary complications in the HSCT population has been aided by a previously well-defined chronology of the most common diseases.3 Historically, early pulmonary complications were defined as pulmonary complications occurring within 100 days of HSCT (corresponding to the acute graft-versus-host disease [GVHD] period). Late pulmonary complications are those that occur thereafter. This timeline, however, is now more variable given the increasing indications for HSCT, the use of reduced-intensity conditioning strategies, and varied individual immune reconstitution. This article discusses the management of early post-HSCT pulmonary complications; late post-HSCT pulmonary complications will be discussed in a separate follow-up article.
Transplant Basics
The development of pulmonary complications is affected by many factors associated with the transplant. Autologous transplantation involves the collection of a patient’s own stem cells, appropriate storage and processing, and re-implantation after induction therapy. During induction therapy, the patient undergoes high-dose chemotherapy or radiation therapy that ablates the bone marrow. The stem cells are then transfused back into the patient to repopulate the bone marrow. Allogeneic transplants involve the collection of stem cells from a donor. Donors are matched as closely as possible to the recipient’s histocompatibility antigen (HLA) haplotypes to prevent graft failure and rejection. The donor can be related or unrelated to the recipient. If there is not a possibility of a related match (from a sibling), then a national search is undertaken to look for a match through the National Marrow Donor Program. There are fewer transplant reactions and occurrences of GVHD if the major HLAs of the donor and recipient match. Table 1 reviews basic definitions pertaining to HSCT.
How the cells for transplantation are obtained is also an important factor in the rate of complications. There are 3 main sources: peripheral blood, bone marrow, and umbilical cord. Peripheral stem cell harvesting involves exposing the donor to granulocyte-colony stimulating factor (gCSF), which increases peripheral circulation of stem cells. These cells are then collected and infused into the recipient after the recipient has completed an induction regimen involving chemotherapy and/or radiation, depending on the protocol. This procedure is called peripheral blood stem cell transplant (PBSCT). Stem cells can also be directly harvested from bone marrow cells, which are collected from repeated aspiration of bone marrow from the posterior iliac crest.4 This technique is most common in children, whereas in adults peripheral blood stem cells are the most common source. Overall mortality does not differ based on the source of the stem cells. It is postulated that GVHD may be more common in patients undergoing PBSCT, but the graft failure rate may be lower.5
The third option is umbilical cord blood (UCB) as the source of stem cells. This involves the collection of umbilical cord blood that is prepared and frozen after birth. It has a smaller volume of cells, and although fewer cells are needed when using UCB, 2 separate donors may be required for a single adult recipient. The engraftment of the stem cells is slower and infections in the post-transplant period are more common. Prior reports indicate GVHD rates may be lower.4 While the use of UCB is not common in adults, the incidence has doubled over the past decade, increasing from 3% to 6%.
The conditioning regimen can influence pulmonary complications. Traditionally, an ablative transplant involves high-dose chemotherapy or radiation to eradicate the recipient’s bone marrow. This regimen can lead to many complications, especially in the immediate post-transplant period. In the past 10 years, there has been increasing interest in non-myeloablative, or reduced-intensity, conditioning transplants.6 These “mini transplants” involve smaller doses of chemotherapy or radiation, which do not totally eradicate the bone marrow; after the transplant a degree of chimerism develops where the donor and recipient stem cells coexist. The medications in the preparative regimen also should be considered because they can affect pulmonary complications after transplant. Certain chemotherapeutic agents such as carmustine, bleomycin, and many others can lead to acute and chronic presentations of pulmonary diseases such as hypersensitivity pneumonitis, pulmonary fibrosis, acute respiratory distress syndrome, and abnormal pulmonary function testing.
After the HSCT, GVHD can develop in more than 50% of allogeneic recipients.3 The incidence of GVHD has been reported to be increasing over the past 12 years.It is divided into acute GVHD (which traditionally happens in the first 100 days after transplant) and chronic GVHD (after day 100). This calendar-day–based system has been augmented based on a 2006 National Institutes of Health working group report emphasizing the importance of organ-specific features of chronic GVHD in the clinical presentation of GVHD.7 Histologic changes in chronic organ GVHD tend to include more fibrotic features, whereas in acute GVHD more inflammatory changes are seen. The NIH working group report also stressed the importance of obtaining a biopsy specimen for histopathologic review and interdisciplinary collaboration to arrive at a consensus diagnosis, and noted the limitations of using histologic changes as the sole determinant of a “gold standard” diagnosis.7 GVHD can directly predispose patients to pulmonary GVHD and indirectly predispose them to infectious complications because the mainstay of therapy for GVHD is increased immunosuppression.
Pretransplant Evaluation
Case Patient 1
A 56-year-old man is diagnosed with acute myeloid leukemia (AML) after presenting with signs and symptoms consistent with pancytopenia. He has a past medical history of chronic sinus congestion, arthritis, depression, chronic pain, and carpal tunnel surgery. He is employed as an oilfield worker and has a 40-pack-year smoking history, but he recently cut back to half a pack per day. He is being evaluated for allogeneic transplant with his brother as the donor and the planned conditioning regimen is total body irradiation (TBI), thiotepa, cyclophosphamide, and antithymocyte globulin with T-cell depletion. Routine pretransplant pulmonary function testing (PFT) reveals a restrictive pattern and he is sent for pretransplant pulmonary evaluation.
Physical exam reveals a chronically ill appearing man. He is afebrile, the respiratory rate is 16 breaths/min, blood pressure is 145/88 mm Hg, heart rate is 92 beats/min, and oxygen saturation is 95%. He is in no distress. Auscultation of the chest reveals slightly diminished breath sounds bilaterally but is clear and without wheezes, rhonchi, or rales. Heart exam shows regular rate and rhythm without murmurs, rubs, or gallops. Extremities reveal no edema or rashes. Otherwise, the remainder of the exam is normal. The patient’s PFT results are shown in Table 2.
- What aspects of this patient’s history put him at risk for pulmonary complications after transplantation?
Risk Factors for Pulmonary Complications
Predicting who is at risk for pulmonary complications is difficult. Complications are generally divided into infectious and noninfectious categories. Regardless of category, allogeneic HSCT recipients are at increased risk compared with autologous recipients, but even in autologous transplants, more than 25% of patients will develop pulmonary complications in the first year.8 Prior to transplant, patients undergo full PFT. Early on, many studies attempted to show relationships between various factors and post-transplant pulmonary complications. Factors that were implicated were forced expiratory volume in 1 second (FEV1), diffusing capacity of the lung for carbon monoxide (D
Another sometimes overlooked risk before transplantation is restrictive lung disease. One study showed a twofold increase in respiratory failure and mortality if there was pretransplant restriction based on TLC < 80%.16
An interesting study by one group in pretransplant evaluation found decreased muscle strength by maximal inspiratory muscle strength (PImax), maximal expiratory muscle strength (PEmax), dominant hand grip strength, and 6-minute walk test (6MWT) distance prior to allogeneic transplant, but did not find a relationship between these variables and mortality.17 While this study had a small sample size, these findings likely deserve continued investigation.18
- What methods are used to calculate risk for complications?
Risk Scoring Systems
Several pretransplantation risk scores have been developed. In a study that looked at more than 2500 allogeneic transplants, Parimon et al showed that risk of mortality and respiratory failure could be estimated prior to transplant using a scoring system—the Lung Function Score (LFS)—that combines the FEV1 and D
The Pretransplantation Assessment of Mortality score, initially developed in 2006, predicts mortality within the first 2 years after HSCT based on 8 clinical factors: disease risk, age at transplant, donor type, conditioning regimen, and markers of organ function (percentage of predicted FEV1, percentage of predicted D
- What other preoperative testing or interventions should be considered in this patient?
Since there is a high risk of infectious complications after transplant, the question of whether pretransplantation patients should undergo screening imaging may arise. There is no evidence that routine chest computed tomography (CT) reduces the risk of infectious complications after transplantation.26 An area that may be insufficiently addressed in the pretransplantation evaluation is smoking cessation counseling.27 Studies have shown an elevated risk of mortality in smokers.28-30 Others have found a higher incidence of respiratory failure but not an increased mortality.31 Overall, with the good rates of smoking cessation that can be accomplished, smokers should be counseled to quit before transplantation.
In summary, patients should undergo full PFTs prior to transplantation to help stratify risk for pulmonary complications and mortality and to establish a clinical baseline. The LFS (using FEV1 and D
Case Patient 1 Conclusion
The patient undergoes transplantation due to his lack of other treatment options. Evaluation prior to transplant, however, shows that he is at high risk for pulmonary complications. He has a LFS of 7 prior to transplant (using the D
Early Infectious Pulmonary Complications
Case Patient 2
A 27-year-old man with a medical history significant for AML and allogeneic HSCT presents with cough productive of a small amount of clear to white sputum, dyspnea on exertion, and fevers for 1 week. He also has mild nausea and a decrease in appetite. He underwent HSCT 2.5 months prior to admission, which was a matched unrelated bone marrow transplant with TBI and cyclophosphamide conditioning. His past medical history is significant only for exercise-induced asthma for which he takes a rescue inhaler infrequently prior to transplantation. His pretransplant PFTs showed normal spirometry with an FEV1 of 106% of predicted and D
Physical exam is notable for fever of 101.0°F, heart rate 80 beats/min, respiratory rate 16 breaths/ min, and blood pressure 142/78 mm Hg; an admission oxygen saturation is 93% on room air. Lungs show bibasilar crackles and the remainder of the exam is normal. Laboratory testing shows a white blood cell count of 2400 cells/μL, hemoglobin 7.6 g/dL, and platelet count 66 × 103/μL. Creatinine is 1.0 mg/dL. Chest radiograph shows ill-defined bilateral lower-lobe infiltrates. CT scans are shown in the Figure.
- For which infectious complications is this patient most at risk?
Pneumonia
A prospective trial in the HSCT population reported a pneumonia incidence rate of 68%, and pneumonia is more common in allogeneic HSCT with prolonged immunosuppressive therapy.32 Development of pneumonia within 100 days of transplant directly correlates with nonrelapsed mortality.33 Early detection is key, and bronchoscopy within the first 5 days of symptoms has been shown to change therapy in approximately 40% of cases but has not been shown to affect mortality.34 The clinical presentation of pneumonia in the HSCT population can be variable because of the presence of neutropenia and profound immunosuppression. Traditionally accepted diagnostic criteria of fevers, sputum production, and new infiltrates should be used with caution, and an appropriately high index of suspicion should be maintained. Progression to respiratory failure, regardless of causative organism of infection, portends a poor prognosis, with mortality rates estimated at 70% to 90%.35,36 Several transplant-specific factors may affect early infections. For instance, UCB transplants have been found to have a higher incidence of invasive aspergillosis and cytomegalovirus (CMV) infections but without higher mortality attributed to the infections.37
Bacterial Pneumonia
Bacterial pneumonia accounts for 20% to 50% of pneumonia cases in HSCT recipients.38 Gram-negative organisms, specifically Pseudomonas aeruginosa and Escherichia coli, were reported to be the most common pathologic bacteria in recent prospective trials, whereas previous retrospective trials showed that common community-acquired organisms were the most common cause of pneumonia in HSCT recipients.32,39 This underscores the importance of being aware of the clinical prevalence of microorganisms and local antibiograms, along with associated institutional susceptibility profiles. Initiation of immediate empiric broad-spectrum antibiotics is essential when bacterial pneumonia is suspected.
Viral Pneumonia
The prevalence of viral pneumonia in stem cell transplant recipients is estimated at 28%,32 with most cases being caused by community viral pathogens such as rhinovirus, respiratory syncytial virus (RSV), influenza A and B, and parainfluenza.39 The prevention, prophylaxis, and early treatment of viral pneumonias, specifically CMV infection, have decreased the mortality associated with early pneumonia after HSCT. Co-infection with bacterial organisms must be considered and has been associated with increased mortality in the intensive care unit setting.40
Supportive treatment with rhinovirus infection is sufficient as the disease is usually self-limited in immunocompromised patients. In contrast, infection with RSV in the lower respiratory tract is associated with increased mortality in prior reports, and recent studies suggest that further exploration of prophylaxis strategies is warranted.41 Treatment with ribavirin remains the backbone of therapy, but drug toxicity continues to limit its use. The addition of immunomodulators such as RSV immune globulin or palivizumab to ribavirin remains controversial, but a retrospective review suggests that early treatment may prevent progression to lower respiratory tract infection and lead to improved mortality.42 Infection with influenza A/B must be considered during influenza season. Treatment with oseltamivir may shorten the duration of disease when influenza A/B or parainfluenza are detected. Reactivation of latent herpes simplex virus during the pre-engraftment phase should also be considered. Treatment is similar to that in nonimmunocompromised hosts. When CMV pneumonia is suspected, careful history regarding compliance with prophylactic antivirals and CMV status of both the recipient and donor are key. A presumptive diagnosis can be made with the presence of appropriate clinical scenario, supportive radiographic images showing areas of ground-glass opacification or consolidation, and positive CMV polymerase chain reaction (PCR) assay. Visualization of inclusion bodies on lung biopsy tissue remains the gold standard for diagnosis. Treatment consists of CMV immunoglobulin and ganciclovir.
Fungal Pneumonia
Early fungal pneumonias have been associated with increased mortality in the HSCT population.43 Clinical suspicion should remain high and compliance with antifungal prophylaxis should be questioned thoroughly. Invasive aspergillosis (IA) remains the most common fungal infection. A bimodal distribution of onset of infection peaking on day 16 and again on day 96 has been described in the literature.44 Patients often present with classic pneumonia symptoms, but these may be accompanied by hemoptysis. Proven IA diagnosis requires visualization of fungal forms from biopsy or needle aspiration or a positive culture obtained in a sterile fashion.45 Most clinical data comes from experience with probable and possible diagnosis of IA. Bronchoalveolar lavage with testing with Aspergillus galactomannan assay has been shown to be clinically useful in establishing the clinical diagnosis in the HSCT population.46 Classic air-crescent findings on chest CT are helpful in establishing a possible diagnosis, but retrospective analysis reveals CT findings such as focal infiltrates and pulmonary nodular patterns are more common.47 First-line treatment with voriconazole has been shown to decrease short-term mortality attributable to IA but has not had an effect on long-term, all-cause mortality.48 Surgical resection is reserved for patients with refractory disease or patients presenting with massive hemoptysis.
Mucormycosis is an emerging disease with ever increasing prevalence in the HSCT population, reflecting the improved prophylaxis and treatment of IA. Initial clinical presentation is similar to IA, most commonly affecting the lung, although craniofacial involvement is classic for mucormycosis, especially in HSCT patients with diabetes.49Mucor infections can present with massive hemoptysis due to tissue invasion and disregard for tissue and fascial planes. Diagnosis of mucormycosis is associated with as much as a six-fold increase in risk for death. Diagnosis requires identification of the organism by examination or culture and biopsy is often necessary.50,51 Amphotericin B remains first-line therapy as mucormycosis is resistant to azole antifungals, with higher doses recommended for cerebral involvement.52
Candida pulmonary infections during the early HSCT period are becoming increasingly rare due to widespread use of fluconazole prophylaxis and early treatment of mucosal involvement during neutropenia. Endemic fungal infections such as blastomycosis, coccidioidomycosis, and histoplasmosis should be considered in patients inhabiting specific geographic areas or with recent travel to these areas.
- What test should be performed to evaluate for infectious causes of pneumonia?
Role of Flexible Fiberoptic Bronchoscopy
The utility of flexible fiberoptic bronchoscopy (FOB) in immune-compromised patients for the evaluation of pulmonary infiltrates is a frequently debated topic. Current studies suggest a diagnosis can be made in approximately 80% of cases in the immune-compromised population.32,53 Noninvasive testing such as urine and serum antigens, sputum cultures, Aspergillus galactomannan assays, viral nasal swabs, and PCR studies often lead to a diagnosis in appropriate clinical scenarios. Conservative management would dictate the use of noninvasive testing whenever possible, and randomized controlled trials have shown noninvasive testing to be noninferior to FOB in preventing need for mechanical ventilation, with no difference in overall mortality.54 FOB has been shown to be most useful in establishing a diagnosis when an infectious etiology is suspected.55 In multivariate analysis, a delay in the identification of the etiology of pulmonary infiltrate was associated with increased mortality.56 Additionally, early FOB was found to be superior to late FOB in revealing a diagnosis. 32,57 Despite its ability to detect the cause of pulmonary disease, direct antibiotic therapy, and possibly change therapy, FOB with diagnostic maneuvers has not been shown to affect mortality.58 In a large case series, FOB with bronchoalveolar lavage (BAL) revealed a diagnosis in approximately 30% to 50% of cases. The addition of transbronchial biopsy did not improve diagnostic utility.58 More recent studies have confirmed that the addition of transbronchial biopsy does not add to diagnostic yield and is associated with increased adverse events.59 The appropriate use of advanced techniques such as endobronchial ultrasound–guided transbronchial needle aspirations, endobronchial biopsy, and CT-guided navigational bronchoscopy has not been established and should be considered on a case-by-case basis. In summary, routine early BAL is the diagnostic test of choice, especially when infectious pulmonary complications are suspected.
Contraindications for FOB in this population mirror those in the general population. These include acute severe hypoxemic respiratory failure, myocardial ischemia or acute coronary syndrome within 2 weeks of procedure, severe thrombocytopenia, and inability to provide or obtain informed consent from patient or health care power of attorney. Coagulopathy and thrombocytopenia are common comorbid conditions in the HSCT population. A platelet count of < 20 × 103/µL has generally been used as a cut-off for routine FOB with BAL.60 Risks of the procedures should be discussed clearly with the patient, but simple FOB for airway evaluation and BAL is generally well tolerated even under these conditions.
Early Nonifectious Pulmonary Complications
Case Patient 2 Continued
Bronchoscopy with BAL performed the day after admission is unremarkable and stains and cultures are negative for viral, bacterial, and fungal organisms. The patient is initially started on broad-spectrum antibiotics, but his oxygenation continues to worsen to the point that he is placed on noninvasive positive pressure ventilation. He is started empirically on amphotericin B and eventually is intubated. VATS lung biopsy is ultimately performed and pathology is consistent with diffuse alveolar damage.
- Based on these biopsy findings, what is the diagnosis?
Based on the pathology consistent with diffuse alveolar damage, a diagnosis of idiopathic pneumonia syndrome (IPS) is made.
- What noninfectious pulmonary complications occur in the early post-transplant period?
The overall incidence of noninfectious pulmonary complications after HSCT is generally estimated at 20% to 30%.32 Acute pulmonary edema is a common very early noninfectious pulmonary complication and clinically the most straightforward to treat. Three distinct clinical syndromes—peri-engraftment respiratory distress syndrome (PERDS), diffuse alveolar hemorrhage (DAH), and IPS—comprise the remainder of the pertinent early noninfectious complications. Clinical presentation differs based upon the disease entity. Recent studies have evaluated the role of angiotensin-converting enzyme polymorphisms as a predictive marker for risk of developing early noninfectious pulmonary complications.61
Peri-Engraftment Respiratory Distress Syndrome
PERDS is a clinical syndrome comprising the cardinal features of erythematous rash and fever along with noncardiogenic pulmonary infiltrates and hypoxemia that occur in the peri-engraftment period, defined as recovery of absolute neutrophil count to > 500/μL on 2 consecutive days.62 PERDS occurs in the autologous HSCT population and may be a clinical correlate to early GVHD in the allogeneic HSCT population. It is hypothesized that the pathophysiology underlying PERDS is an autoimmune-related capillary leak caused by pro-inflammatory cytokine release.63 Treatment remains anecdotal and currently consists of supportive care and high-dose corticosteroids. Some have favored limiting the use of gCSF given its role in stimulating rapid white blood cell recovery.33 Prognosis is favorable, but progression to fulminant respiratory failure requiring mechanical ventilation portends a poor prognosis.
Diffuse Alveolar Hemorrhage
DAH is clinical syndrome consisting of diffuse alveolar infiltrates on pulmonary imaging combined with progressively bloodier return per aliquot during BAL in 3 different subsegments or more than 20% hemosiderin-laden macrophages on BAL fluid evaluation. Classically, DAH is defined in the absence of pulmonary infection or cardiac dysfunction. The pathophysiology is thought to be related to inflammation of pulmonary vasculature within the alveolar walls leading to alveolitis. Although no prospective trials exist, early use of high-dose corticosteroid therapy is thought to improve outcomes;64,65 a recent study, however, showed low-dose steroids may be associated with the lowest mortality.66 Mortality is directly linked to the presence of superimposed infection, need for mechanical ventilation, late onset, and development of multiorgan failure.67
Idiopathic Pneumonia Syndrome
IPS is a complex clinical syndrome whose pathology is felt to stem from a variety of possible lung insults such as direct myeloablative drug toxicity, occult pulmonary infection, or cytokine-driven inflammation. The ATS published an article further subcategorizing IPS as different clinical entities based upon whether the primary insult involves the vascular endothelium, interstitial tissue, and airway tissue, truly idiopathic, or unclassified.68 In clinical practice, IPS is defined as widespread alveolar injury in the absence of evidence of renal failure, heart failure, and excessive fluid resuscitation. In addition, negative testing for a variety of bacterial, viral, and fungal causes is also necessary.69 Clinical syndromes included within the IPS definition are ARDS, acute interstitial pneumonia, DAH, cryptogenic organizing pneumonia, and BOS.70 Risk factors for developing IPS include TBI, older age of recipient, acute GVHD, and underlying diagnosis of AML or myelodysplastic syndrome.12 In addition, it has been shown that risk for developing IPS is lower in patients undergoing allogeneic HSCT who receive non-myeloablative conditioning regimens.71 The pathologic finding in IPS is diffuse alveolar damage. A 2006 study in which investigators reviewed BAL samples from patients with IPS found that 3% of the patients had PCR evidence of human metapneumovirus infection, and a study in 2015 found PCR evidence of infection in 53% of BAL samples from patients diagnosed with IPS.72,73 This fuels the debate on whether IPS is truly an infection-driven process where the source of infection, pulmonary or otherwise, simply escapes detection. Various surfactant proteins, which play a role in decreasing surface tension within the alveolar interface and function as mediators within the innate immunity of the lung, have been studied in regard to development of IPS. Small retrospective studies have shown a trend toward lower pre-transplant serum protein surfactant D and the development of IPS.74
The diagnosis of IPS does not require pathologic diagnosis in most circumstances. The correct clinical findings in association with a negative infectious workup lead to a presumptive diagnosis of IPS. The extent of the infectious workup that must be completed to adequately rule out infection is often a difficult clinical question. Recent recommendations include BAL fluid evaluation for routine bacterial cultures, appropriate viral culture, and consideration of PCR testing to evaluate for Mycoplasma, Chlamydia, and Aspergillus antigens.75 Transbronchial biopsy continues to appear in recommendations, but is not routinely performed and should be completed as the patient’s clinical status permits.8,68 Table 3 reviews basic features of early noninfectious pulmonary complications.
Treatment of IPS centers around moderate to high doses of corticosteroids. Based on IPS experimental modes, tumor necrosis factor (TNF)-α has been implicated as an important mediator. Unfortunately, several studies evaluating etanercept have produced conflicting results, and this agent’s clinical effects on morbidity and mortality remain in question.76
- What treatment should be offered to the patient with diffuse alveolar damage on biopsy?
Treatment consists of supportive care and empiric broad-spectrum antibiotics with consideration of high-dose corticosteroids. Based upon early studies in murine models implicating TNF, pilot studies were performed evaluating etanercept as a possible safe and effective addition to high-dose systemic corticosteroids.77 Although these results were promising, data from a truncated randomized control clinical trial failed to show improvement in patient response in the adult population.76 More recent data from the same author suggests that pediatric populations with IPS are, however, responsive to etanercept and high-dose corticosteroid therapy.78 When IPS develops as a late complication, treatment with high-dose corticosteroids (2 mg/kg/day) and etanercept (0.4 mg/kg twice weekly) has been shown to improve 2-year survival.79
Case Patient 2 Conclusion
The patient is started on steroids and makes a speedy recovery. He is successfully extubated 5 days later.
Conclusion
Careful pretransplant evaluation, including a full set of pulmonary function tests, can help predict a patient’s risk for pulmonary complications after transplant, allowing risk factor modification strategies to be implemented prior to transplant, including smoking cessation. It also helps identify patients at high risk for complications who will require closer monitoring after transplantation. Early posttransplant complications include infectious and noninfectious entities. Bacterial, viral, and fungal pneumonias are in the differential of infectious pneumonia, and bronchoscopy can be helpful in establishing a diagnosis. A common, important noninfectious cause of early pulmonary complications is IPS, which is treated with steroids and sometimes anti-TNF therapy.
1. Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010;303:1617–24.
2. Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2004;170:22–48.
4. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006;354:1813–26.
5. Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012;367:1487–96.
6. Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009;15:367–9.
7. Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant 2006;12:31–47.
8. Afessa B, Abdulai RM, Kremers WK, et al. Risk factors and outcome of pulmonary complications after autologous hematopoietic stem cell transplant. Chest 2012;141:442–50.
9. Bolwell BJ. Are predictive factors clinically useful in bone marrow transplantation? Bone Marrow Transplant 2003;32:853–61.
10. Carlson K, Backlund L, Smedmyr B, et al. Pulmonary function and complications subsequent to autologous bone marrow transplantation. Bone Marrow Transplant 1994;14:805–11.
11. Clark JG, Schwartz DA, Flournoy N, et al. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med 1987;107:648–56.
12. Crawford SW, Fisher L. Predictive value of pulmonary function tests before marrow transplantation. Chest 1992; 101:1257–64.
13. Ghalie R, Szidon JP, Thompson L, et al. Evaluation of pulmonary complications after bone marrow transplantation: the role of pretransplant pulmonary function tests. Bone Marrow Transplant 1992;10:359–65.
14. Ho VT, Weller E, Lee SJ, et al. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001;7:223–9.
15. Horak DA, Schmidt GM, Zaia JA, et al. Pretransplant pulmonary function predicts cytomegalovirus-associated interstitial pneumonia following bone marrow transplantation. Chest 1992;102:1484–90.
16. Ramirez-Sarmiento A, Orozco-Levi M, Walter EC, et al. Influence of pretransplantation restrictive lung disease on allogeneic hematopoietic cell transplantation outcomes. Biol Blood Marrow Transplant 2010;16:199–206.
17. White AC, Terrin N, Miller KB, Ryan HF. Impaired respiratory and skeletal muscle strength in patients prior to hematopoietic stem-cell transplantation. Chest 2005;128145–52.
18. Afessa B. Pretransplant pulmonary evaluation of the blood and marrow transplant recipient. Chest 2005;128:8–10.
19. Parimon T, Madtes DK, Au DH, et al. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med 2005;172:384–90.
20. Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 2006;12:252–66.
21. Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med 2006;144:407–14.
22. Au BK, Gooley TA, Armand P, et al. Reevaluation of the pretransplant assessment of mortality score after allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant 2015;21:848–54.
23. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–9.
24. Chien JW, Sullivan KM. Carbon monoxide diffusion capacity: how low can you go for hematopoietic cell transplantation eligibility? Biol Blood Marrow Transplant 2009;15: 447–53.
25. Coffey DG, Pollyea DA, Myint H, et al. Adjusting DLCO for Hb and its effects on the Hematopoietic Cell Transplantation-specific Comorbidity Index. Bone Marrow Transplant 2013;48:1253–6.
26. Kasow KA, Krueger J, Srivastava DK, et al. Clinical utility of computed tomography screening of chest, abdomen, and sinuses before hematopoietic stem cell transplantation: the St. Jude experience. Biol Blood Marrow Transplant 2009;15:490–5.
27. Hamadani M, Craig M, Awan FT, Devine SM. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45: 1259–68.
28. Savani BN, Montero A, Wu C, et al. Prediction and prevention of transplant-related mortality from pulmonary causes after total body irradiation and allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:223–30.
29. Ehlers SL, Gastineau DA, Patten CA, et al. The impact of smoking on outcomes among patients undergoing hematopoietic SCT for the treatment of acute leukemia. Bone Marrow Transplant 2011;46:285–90.
30. Marks DI, Ballen K, Logan BR, et al. The effect of smoking on allogeneic transplant outcomes. Biol Blood Marrow Transplant 2009;15:1277–87.
31. Tran BT, Halperin A, Chien JW. Cigarette smoking and outcomes after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011;17:1004–11.
32. Lucena CM, Torres A, Rovira M, et al. Pulmonary complications in hematopoietic SCT: a prospective study. Bone Marrow Transplant 2014;49:1293–9.
33. Chi AK, Soubani AO, White AC, Miller KB. An update on pulmonary complications of hematopoietic stem cell transplantation. Chest 2013;144:1913–22.
34. Dunagan DP, Baker AM, Hurd DD, Haponik EF. Bronchoscopic evaluation of pulmonary infiltrates following bone marrow transplantation. Chest 1997;111:135–41.
35. Naeem N, Reed MD, Creger RJ, et al. Transfer of the hematopoietic stem cell transplant patient to the intensive care unit: does it really matter? Bone Marrow Transplant 2006;37:119–33.
36. Afessa B, Tefferi A, Hoagland HC, et al. Outcome of recipients of bone marrow transplants who require intensive care unit support. Mayo Clin Proc 1992;67:117–22.
37. Parody R, Martino R, de la Camara R, et al. Fungal and viral infections after allogeneic hematopoietic transplantation from unrelated donors in adults: improving outcomes over time. Bone Marrow Transplant 2015;50:274–81.
38. Orasch C, Weisser M, Mertz D, et al. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:521–6.
39. Aguilar-Guisado M, Jimenez-Jambrina M, Espigado I, et al. Pneumonia in allogeneic stem cell transplantation recipients: a multicenter prospective study. Clin Transplant 2011;25:E629–38.
40. Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS One 2009;4:e8540.
41. Hynicka LM, Ensor CR. Prophylaxis and treatment of respiratory syncytial virus in adult immunocompromised patients. Ann Pharmacother 2012;46:558–66.
42. Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 2011;2755–63.
43. Marr KA, Bowden RA. Fungal infections in patients undergoing blood and marrow transplantation. Transpl Infect Dis 1999;1:237–46.
44. Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis 1997;175:1459–66.
45. Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002;34:7–14.
46. Fisher CE, Stevens AM, Leisenring W, et al. Independent contribution of bronchoalveolar lavage and serum galactomannan in the diagnosis of invasive pulmonary aspergillosis. Transpl Infect Dis 2014;16:505–10.
47. Kojima R, Tateishi U, Kami M, et al. Chest computed tomography of late invasive aspergillosis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:506–11.
48. Salmeron G, Porcher R, Bergeron A, et al. Persistent poor long-term prognosis of allogeneic hematopoietic stem cell transplant recipients surviving invasive aspergillosis. Haematologica 2012;97:1357–63.
49. McNulty JS. Rhinocerebral mucormycosis: predisposing factors. Laryngoscope 1982;92(10 Pt 1):1140.
50. Walsh TJ, Gamaletsou MN, McGinnis MR, et al. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 2012;54 Suppl 1:S55–60.
51. Klingspor L, Saaedi B, Ljungman P, Szakos A. Epidemiology and outcomes of patients with invasive mould infections: a retrospective observational study from a single centre (2005-2009). Mycoses 2015;58:470–7.
52. Danion F, Aguilar C, Catherinot E, et al. Mucormycosis: new developments in a persistently devastating infection. Semin Respir Crit Care Med 2015;36:692–70.
53. Rano A, Agusti C, Jimenez P, et al. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax 2001;56:379–87.
54. Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med 2008;36:100–7.
55. Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest 2004;125:712–22.
56. Rano A, Agusti C, Benito N, et al. Prognostic factors of non-HIV immunocompromised patients with pulmonary infiltrates. Chest 2002;122:253–61.
57. Shannon VR, Andersson BS, Lei X, et al. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:647–55.
58. Patel NR, Lee PS, Kim JH, et al. The influence of diagnostic bronchoscopy on clinical outcomes comparing adult autologous and allogeneic bone marrow transplant patients. Chest 2005;127:1388–96.
59. Chellapandian D, Lehrnbecher T, Phillips B, et al. Bronchoalveolar lavage and lung biopsy in patients with cancer and hematopoietic stem-cell transplantation recipients: a systematic review and meta-analysis. J Clin Oncol 2015;33:501–9.
60. Carr IM, Koegelenberg CF, von Groote-Bidlingmaier F, et al. Blood loss during flexible bronchoscopy: a prospective observational study. Respiration 2012;84:312–8.
61. Miyamoto M, Onizuka M, Machida S, et al. ACE deletion polymorphism is associated with a high risk of non-infectious pulmonary complications after stem cell transplantation. Int J Hematol 2014;99:175–83.
62. Capizzi SA, Kumar S, Huneke NE, et al. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:1299–303.
63. Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:893–8.
64. Wanko SO, Broadwater G, Folz RJ, Chao NJ. Diffuse alveolar hemorrhage: retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol Blood Marrow Transplant 2006;12:949–53.
65. Metcalf JP, Rennard SI, Reed EC, et al. Corticosteroids as adjunctive therapy for diffuse alveolar hemorrhage associated with bone marrow transplantation. University of Nebraska Medical Center Bone Marrow Transplant Group. Am J Med 1994;96:327–34.
66. Rathi NK, Tanner AR, Dinh A, et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transplant 2015;50:420–6.
67. Afessa B, Tefferi A, Litzow MR, Peters SG. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med 2002;166:1364–8.
68. Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med 2011;183:1262–79.
69. Clark JG, Hansen JA, Hertz MI, Pet al. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Resp Dis 1993;147:1601–6.
70. Vande Vusse LK, Madtes DK. Early onset noninfectious pulmonary syndromes after hematopoietic cell transplantation. Clin Chest Med 2017;38:233–48.
71. Fukuda T, Hackman RC, Guthrie KA, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003;102:2777–85.
72. Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med 2006;144:344–9.
73. Seo S, Renaud C, Kuypers JM, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015;125:3789–97.
74. Nakane T, Nakamae H, Kamoi H, et al. Prognostic value of serum surfactant protein D level prior to transplant for the development of bronchiolitis obliterans syndrome and idiopathic pneumonia syndrome following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;42:43–9.
75. Gilbert CR, Lerner A, Baram M, Awsare BK. Utility of flexible bronchoscopy in the evaluation of pulmonary infiltrates in the hematopoietic stem cell transplant population—a single center fourteen year experience. Arch Bronconeumol 2013;49:189–95.
76. Yanik GA, Horowitz MM, Weisdorf DJ, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: blood and marrow transplant clinical trials network protocol. Biol Blood Marrow Transplant 2014;20:858–64.
77. Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood 2008;111:2470–5.
78. Yanik GA, Grupp SA, Pulsipher MA, et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children’s Oncology Group Study (ASCT0521). Biol Blood Marrow Transplant 2015;21:67–73.
79. Thompson J, Yin Z, D’Souza A, et al. Etanercept and corticosteroid therapy for the treatment of late-onset idiopathic pneumonia syndrome. Biol Blood Marrow Transplant J 2017; 23:1955–60.
Hematopoietic stem cell transplantation (HSCT) is widely used in the economically developed world to treat a variety of hematologic malignancies as well as nonmalignant diseases and solid tumors. An estimated 17,900 HSCTs were performed in 2011, and survival rates continue to increase.1 Pulmonary complications post HSCT are common, with rates ranging from 40% to 60%, and are associated with increased morbidity and mortality.2
Clinical diagnosis of pulmonary complications in the HSCT population has been aided by a previously well-defined chronology of the most common diseases.3 Historically, early pulmonary complications were defined as pulmonary complications occurring within 100 days of HSCT (corresponding to the acute graft-versus-host disease [GVHD] period). Late pulmonary complications are those that occur thereafter. This timeline, however, is now more variable given the increasing indications for HSCT, the use of reduced-intensity conditioning strategies, and varied individual immune reconstitution. This article discusses the management of early post-HSCT pulmonary complications; late post-HSCT pulmonary complications will be discussed in a separate follow-up article.
Transplant Basics
The development of pulmonary complications is affected by many factors associated with the transplant. Autologous transplantation involves the collection of a patient’s own stem cells, appropriate storage and processing, and re-implantation after induction therapy. During induction therapy, the patient undergoes high-dose chemotherapy or radiation therapy that ablates the bone marrow. The stem cells are then transfused back into the patient to repopulate the bone marrow. Allogeneic transplants involve the collection of stem cells from a donor. Donors are matched as closely as possible to the recipient’s histocompatibility antigen (HLA) haplotypes to prevent graft failure and rejection. The donor can be related or unrelated to the recipient. If there is not a possibility of a related match (from a sibling), then a national search is undertaken to look for a match through the National Marrow Donor Program. There are fewer transplant reactions and occurrences of GVHD if the major HLAs of the donor and recipient match. Table 1 reviews basic definitions pertaining to HSCT.
How the cells for transplantation are obtained is also an important factor in the rate of complications. There are 3 main sources: peripheral blood, bone marrow, and umbilical cord. Peripheral stem cell harvesting involves exposing the donor to granulocyte-colony stimulating factor (gCSF), which increases peripheral circulation of stem cells. These cells are then collected and infused into the recipient after the recipient has completed an induction regimen involving chemotherapy and/or radiation, depending on the protocol. This procedure is called peripheral blood stem cell transplant (PBSCT). Stem cells can also be directly harvested from bone marrow cells, which are collected from repeated aspiration of bone marrow from the posterior iliac crest.4 This technique is most common in children, whereas in adults peripheral blood stem cells are the most common source. Overall mortality does not differ based on the source of the stem cells. It is postulated that GVHD may be more common in patients undergoing PBSCT, but the graft failure rate may be lower.5
The third option is umbilical cord blood (UCB) as the source of stem cells. This involves the collection of umbilical cord blood that is prepared and frozen after birth. It has a smaller volume of cells, and although fewer cells are needed when using UCB, 2 separate donors may be required for a single adult recipient. The engraftment of the stem cells is slower and infections in the post-transplant period are more common. Prior reports indicate GVHD rates may be lower.4 While the use of UCB is not common in adults, the incidence has doubled over the past decade, increasing from 3% to 6%.
The conditioning regimen can influence pulmonary complications. Traditionally, an ablative transplant involves high-dose chemotherapy or radiation to eradicate the recipient’s bone marrow. This regimen can lead to many complications, especially in the immediate post-transplant period. In the past 10 years, there has been increasing interest in non-myeloablative, or reduced-intensity, conditioning transplants.6 These “mini transplants” involve smaller doses of chemotherapy or radiation, which do not totally eradicate the bone marrow; after the transplant a degree of chimerism develops where the donor and recipient stem cells coexist. The medications in the preparative regimen also should be considered because they can affect pulmonary complications after transplant. Certain chemotherapeutic agents such as carmustine, bleomycin, and many others can lead to acute and chronic presentations of pulmonary diseases such as hypersensitivity pneumonitis, pulmonary fibrosis, acute respiratory distress syndrome, and abnormal pulmonary function testing.
After the HSCT, GVHD can develop in more than 50% of allogeneic recipients.3 The incidence of GVHD has been reported to be increasing over the past 12 years.It is divided into acute GVHD (which traditionally happens in the first 100 days after transplant) and chronic GVHD (after day 100). This calendar-day–based system has been augmented based on a 2006 National Institutes of Health working group report emphasizing the importance of organ-specific features of chronic GVHD in the clinical presentation of GVHD.7 Histologic changes in chronic organ GVHD tend to include more fibrotic features, whereas in acute GVHD more inflammatory changes are seen. The NIH working group report also stressed the importance of obtaining a biopsy specimen for histopathologic review and interdisciplinary collaboration to arrive at a consensus diagnosis, and noted the limitations of using histologic changes as the sole determinant of a “gold standard” diagnosis.7 GVHD can directly predispose patients to pulmonary GVHD and indirectly predispose them to infectious complications because the mainstay of therapy for GVHD is increased immunosuppression.
Pretransplant Evaluation
Case Patient 1
A 56-year-old man is diagnosed with acute myeloid leukemia (AML) after presenting with signs and symptoms consistent with pancytopenia. He has a past medical history of chronic sinus congestion, arthritis, depression, chronic pain, and carpal tunnel surgery. He is employed as an oilfield worker and has a 40-pack-year smoking history, but he recently cut back to half a pack per day. He is being evaluated for allogeneic transplant with his brother as the donor and the planned conditioning regimen is total body irradiation (TBI), thiotepa, cyclophosphamide, and antithymocyte globulin with T-cell depletion. Routine pretransplant pulmonary function testing (PFT) reveals a restrictive pattern and he is sent for pretransplant pulmonary evaluation.
Physical exam reveals a chronically ill appearing man. He is afebrile, the respiratory rate is 16 breaths/min, blood pressure is 145/88 mm Hg, heart rate is 92 beats/min, and oxygen saturation is 95%. He is in no distress. Auscultation of the chest reveals slightly diminished breath sounds bilaterally but is clear and without wheezes, rhonchi, or rales. Heart exam shows regular rate and rhythm without murmurs, rubs, or gallops. Extremities reveal no edema or rashes. Otherwise, the remainder of the exam is normal. The patient’s PFT results are shown in Table 2.
- What aspects of this patient’s history put him at risk for pulmonary complications after transplantation?
Risk Factors for Pulmonary Complications
Predicting who is at risk for pulmonary complications is difficult. Complications are generally divided into infectious and noninfectious categories. Regardless of category, allogeneic HSCT recipients are at increased risk compared with autologous recipients, but even in autologous transplants, more than 25% of patients will develop pulmonary complications in the first year.8 Prior to transplant, patients undergo full PFT. Early on, many studies attempted to show relationships between various factors and post-transplant pulmonary complications. Factors that were implicated were forced expiratory volume in 1 second (FEV1), diffusing capacity of the lung for carbon monoxide (D
Another sometimes overlooked risk before transplantation is restrictive lung disease. One study showed a twofold increase in respiratory failure and mortality if there was pretransplant restriction based on TLC < 80%.16
An interesting study by one group in pretransplant evaluation found decreased muscle strength by maximal inspiratory muscle strength (PImax), maximal expiratory muscle strength (PEmax), dominant hand grip strength, and 6-minute walk test (6MWT) distance prior to allogeneic transplant, but did not find a relationship between these variables and mortality.17 While this study had a small sample size, these findings likely deserve continued investigation.18
- What methods are used to calculate risk for complications?
Risk Scoring Systems
Several pretransplantation risk scores have been developed. In a study that looked at more than 2500 allogeneic transplants, Parimon et al showed that risk of mortality and respiratory failure could be estimated prior to transplant using a scoring system—the Lung Function Score (LFS)—that combines the FEV1 and D
The Pretransplantation Assessment of Mortality score, initially developed in 2006, predicts mortality within the first 2 years after HSCT based on 8 clinical factors: disease risk, age at transplant, donor type, conditioning regimen, and markers of organ function (percentage of predicted FEV1, percentage of predicted D
- What other preoperative testing or interventions should be considered in this patient?
Since there is a high risk of infectious complications after transplant, the question of whether pretransplantation patients should undergo screening imaging may arise. There is no evidence that routine chest computed tomography (CT) reduces the risk of infectious complications after transplantation.26 An area that may be insufficiently addressed in the pretransplantation evaluation is smoking cessation counseling.27 Studies have shown an elevated risk of mortality in smokers.28-30 Others have found a higher incidence of respiratory failure but not an increased mortality.31 Overall, with the good rates of smoking cessation that can be accomplished, smokers should be counseled to quit before transplantation.
In summary, patients should undergo full PFTs prior to transplantation to help stratify risk for pulmonary complications and mortality and to establish a clinical baseline. The LFS (using FEV1 and D
Case Patient 1 Conclusion
The patient undergoes transplantation due to his lack of other treatment options. Evaluation prior to transplant, however, shows that he is at high risk for pulmonary complications. He has a LFS of 7 prior to transplant (using the D
Early Infectious Pulmonary Complications
Case Patient 2
A 27-year-old man with a medical history significant for AML and allogeneic HSCT presents with cough productive of a small amount of clear to white sputum, dyspnea on exertion, and fevers for 1 week. He also has mild nausea and a decrease in appetite. He underwent HSCT 2.5 months prior to admission, which was a matched unrelated bone marrow transplant with TBI and cyclophosphamide conditioning. His past medical history is significant only for exercise-induced asthma for which he takes a rescue inhaler infrequently prior to transplantation. His pretransplant PFTs showed normal spirometry with an FEV1 of 106% of predicted and D
Physical exam is notable for fever of 101.0°F, heart rate 80 beats/min, respiratory rate 16 breaths/ min, and blood pressure 142/78 mm Hg; an admission oxygen saturation is 93% on room air. Lungs show bibasilar crackles and the remainder of the exam is normal. Laboratory testing shows a white blood cell count of 2400 cells/μL, hemoglobin 7.6 g/dL, and platelet count 66 × 103/μL. Creatinine is 1.0 mg/dL. Chest radiograph shows ill-defined bilateral lower-lobe infiltrates. CT scans are shown in the Figure.
- For which infectious complications is this patient most at risk?
Pneumonia
A prospective trial in the HSCT population reported a pneumonia incidence rate of 68%, and pneumonia is more common in allogeneic HSCT with prolonged immunosuppressive therapy.32 Development of pneumonia within 100 days of transplant directly correlates with nonrelapsed mortality.33 Early detection is key, and bronchoscopy within the first 5 days of symptoms has been shown to change therapy in approximately 40% of cases but has not been shown to affect mortality.34 The clinical presentation of pneumonia in the HSCT population can be variable because of the presence of neutropenia and profound immunosuppression. Traditionally accepted diagnostic criteria of fevers, sputum production, and new infiltrates should be used with caution, and an appropriately high index of suspicion should be maintained. Progression to respiratory failure, regardless of causative organism of infection, portends a poor prognosis, with mortality rates estimated at 70% to 90%.35,36 Several transplant-specific factors may affect early infections. For instance, UCB transplants have been found to have a higher incidence of invasive aspergillosis and cytomegalovirus (CMV) infections but without higher mortality attributed to the infections.37
Bacterial Pneumonia
Bacterial pneumonia accounts for 20% to 50% of pneumonia cases in HSCT recipients.38 Gram-negative organisms, specifically Pseudomonas aeruginosa and Escherichia coli, were reported to be the most common pathologic bacteria in recent prospective trials, whereas previous retrospective trials showed that common community-acquired organisms were the most common cause of pneumonia in HSCT recipients.32,39 This underscores the importance of being aware of the clinical prevalence of microorganisms and local antibiograms, along with associated institutional susceptibility profiles. Initiation of immediate empiric broad-spectrum antibiotics is essential when bacterial pneumonia is suspected.
Viral Pneumonia
The prevalence of viral pneumonia in stem cell transplant recipients is estimated at 28%,32 with most cases being caused by community viral pathogens such as rhinovirus, respiratory syncytial virus (RSV), influenza A and B, and parainfluenza.39 The prevention, prophylaxis, and early treatment of viral pneumonias, specifically CMV infection, have decreased the mortality associated with early pneumonia after HSCT. Co-infection with bacterial organisms must be considered and has been associated with increased mortality in the intensive care unit setting.40
Supportive treatment with rhinovirus infection is sufficient as the disease is usually self-limited in immunocompromised patients. In contrast, infection with RSV in the lower respiratory tract is associated with increased mortality in prior reports, and recent studies suggest that further exploration of prophylaxis strategies is warranted.41 Treatment with ribavirin remains the backbone of therapy, but drug toxicity continues to limit its use. The addition of immunomodulators such as RSV immune globulin or palivizumab to ribavirin remains controversial, but a retrospective review suggests that early treatment may prevent progression to lower respiratory tract infection and lead to improved mortality.42 Infection with influenza A/B must be considered during influenza season. Treatment with oseltamivir may shorten the duration of disease when influenza A/B or parainfluenza are detected. Reactivation of latent herpes simplex virus during the pre-engraftment phase should also be considered. Treatment is similar to that in nonimmunocompromised hosts. When CMV pneumonia is suspected, careful history regarding compliance with prophylactic antivirals and CMV status of both the recipient and donor are key. A presumptive diagnosis can be made with the presence of appropriate clinical scenario, supportive radiographic images showing areas of ground-glass opacification or consolidation, and positive CMV polymerase chain reaction (PCR) assay. Visualization of inclusion bodies on lung biopsy tissue remains the gold standard for diagnosis. Treatment consists of CMV immunoglobulin and ganciclovir.
Fungal Pneumonia
Early fungal pneumonias have been associated with increased mortality in the HSCT population.43 Clinical suspicion should remain high and compliance with antifungal prophylaxis should be questioned thoroughly. Invasive aspergillosis (IA) remains the most common fungal infection. A bimodal distribution of onset of infection peaking on day 16 and again on day 96 has been described in the literature.44 Patients often present with classic pneumonia symptoms, but these may be accompanied by hemoptysis. Proven IA diagnosis requires visualization of fungal forms from biopsy or needle aspiration or a positive culture obtained in a sterile fashion.45 Most clinical data comes from experience with probable and possible diagnosis of IA. Bronchoalveolar lavage with testing with Aspergillus galactomannan assay has been shown to be clinically useful in establishing the clinical diagnosis in the HSCT population.46 Classic air-crescent findings on chest CT are helpful in establishing a possible diagnosis, but retrospective analysis reveals CT findings such as focal infiltrates and pulmonary nodular patterns are more common.47 First-line treatment with voriconazole has been shown to decrease short-term mortality attributable to IA but has not had an effect on long-term, all-cause mortality.48 Surgical resection is reserved for patients with refractory disease or patients presenting with massive hemoptysis.
Mucormycosis is an emerging disease with ever increasing prevalence in the HSCT population, reflecting the improved prophylaxis and treatment of IA. Initial clinical presentation is similar to IA, most commonly affecting the lung, although craniofacial involvement is classic for mucormycosis, especially in HSCT patients with diabetes.49Mucor infections can present with massive hemoptysis due to tissue invasion and disregard for tissue and fascial planes. Diagnosis of mucormycosis is associated with as much as a six-fold increase in risk for death. Diagnosis requires identification of the organism by examination or culture and biopsy is often necessary.50,51 Amphotericin B remains first-line therapy as mucormycosis is resistant to azole antifungals, with higher doses recommended for cerebral involvement.52
Candida pulmonary infections during the early HSCT period are becoming increasingly rare due to widespread use of fluconazole prophylaxis and early treatment of mucosal involvement during neutropenia. Endemic fungal infections such as blastomycosis, coccidioidomycosis, and histoplasmosis should be considered in patients inhabiting specific geographic areas or with recent travel to these areas.
- What test should be performed to evaluate for infectious causes of pneumonia?
Role of Flexible Fiberoptic Bronchoscopy
The utility of flexible fiberoptic bronchoscopy (FOB) in immune-compromised patients for the evaluation of pulmonary infiltrates is a frequently debated topic. Current studies suggest a diagnosis can be made in approximately 80% of cases in the immune-compromised population.32,53 Noninvasive testing such as urine and serum antigens, sputum cultures, Aspergillus galactomannan assays, viral nasal swabs, and PCR studies often lead to a diagnosis in appropriate clinical scenarios. Conservative management would dictate the use of noninvasive testing whenever possible, and randomized controlled trials have shown noninvasive testing to be noninferior to FOB in preventing need for mechanical ventilation, with no difference in overall mortality.54 FOB has been shown to be most useful in establishing a diagnosis when an infectious etiology is suspected.55 In multivariate analysis, a delay in the identification of the etiology of pulmonary infiltrate was associated with increased mortality.56 Additionally, early FOB was found to be superior to late FOB in revealing a diagnosis. 32,57 Despite its ability to detect the cause of pulmonary disease, direct antibiotic therapy, and possibly change therapy, FOB with diagnostic maneuvers has not been shown to affect mortality.58 In a large case series, FOB with bronchoalveolar lavage (BAL) revealed a diagnosis in approximately 30% to 50% of cases. The addition of transbronchial biopsy did not improve diagnostic utility.58 More recent studies have confirmed that the addition of transbronchial biopsy does not add to diagnostic yield and is associated with increased adverse events.59 The appropriate use of advanced techniques such as endobronchial ultrasound–guided transbronchial needle aspirations, endobronchial biopsy, and CT-guided navigational bronchoscopy has not been established and should be considered on a case-by-case basis. In summary, routine early BAL is the diagnostic test of choice, especially when infectious pulmonary complications are suspected.
Contraindications for FOB in this population mirror those in the general population. These include acute severe hypoxemic respiratory failure, myocardial ischemia or acute coronary syndrome within 2 weeks of procedure, severe thrombocytopenia, and inability to provide or obtain informed consent from patient or health care power of attorney. Coagulopathy and thrombocytopenia are common comorbid conditions in the HSCT population. A platelet count of < 20 × 103/µL has generally been used as a cut-off for routine FOB with BAL.60 Risks of the procedures should be discussed clearly with the patient, but simple FOB for airway evaluation and BAL is generally well tolerated even under these conditions.
Early Nonifectious Pulmonary Complications
Case Patient 2 Continued
Bronchoscopy with BAL performed the day after admission is unremarkable and stains and cultures are negative for viral, bacterial, and fungal organisms. The patient is initially started on broad-spectrum antibiotics, but his oxygenation continues to worsen to the point that he is placed on noninvasive positive pressure ventilation. He is started empirically on amphotericin B and eventually is intubated. VATS lung biopsy is ultimately performed and pathology is consistent with diffuse alveolar damage.
- Based on these biopsy findings, what is the diagnosis?
Based on the pathology consistent with diffuse alveolar damage, a diagnosis of idiopathic pneumonia syndrome (IPS) is made.
- What noninfectious pulmonary complications occur in the early post-transplant period?
The overall incidence of noninfectious pulmonary complications after HSCT is generally estimated at 20% to 30%.32 Acute pulmonary edema is a common very early noninfectious pulmonary complication and clinically the most straightforward to treat. Three distinct clinical syndromes—peri-engraftment respiratory distress syndrome (PERDS), diffuse alveolar hemorrhage (DAH), and IPS—comprise the remainder of the pertinent early noninfectious complications. Clinical presentation differs based upon the disease entity. Recent studies have evaluated the role of angiotensin-converting enzyme polymorphisms as a predictive marker for risk of developing early noninfectious pulmonary complications.61
Peri-Engraftment Respiratory Distress Syndrome
PERDS is a clinical syndrome comprising the cardinal features of erythematous rash and fever along with noncardiogenic pulmonary infiltrates and hypoxemia that occur in the peri-engraftment period, defined as recovery of absolute neutrophil count to > 500/μL on 2 consecutive days.62 PERDS occurs in the autologous HSCT population and may be a clinical correlate to early GVHD in the allogeneic HSCT population. It is hypothesized that the pathophysiology underlying PERDS is an autoimmune-related capillary leak caused by pro-inflammatory cytokine release.63 Treatment remains anecdotal and currently consists of supportive care and high-dose corticosteroids. Some have favored limiting the use of gCSF given its role in stimulating rapid white blood cell recovery.33 Prognosis is favorable, but progression to fulminant respiratory failure requiring mechanical ventilation portends a poor prognosis.
Diffuse Alveolar Hemorrhage
DAH is clinical syndrome consisting of diffuse alveolar infiltrates on pulmonary imaging combined with progressively bloodier return per aliquot during BAL in 3 different subsegments or more than 20% hemosiderin-laden macrophages on BAL fluid evaluation. Classically, DAH is defined in the absence of pulmonary infection or cardiac dysfunction. The pathophysiology is thought to be related to inflammation of pulmonary vasculature within the alveolar walls leading to alveolitis. Although no prospective trials exist, early use of high-dose corticosteroid therapy is thought to improve outcomes;64,65 a recent study, however, showed low-dose steroids may be associated with the lowest mortality.66 Mortality is directly linked to the presence of superimposed infection, need for mechanical ventilation, late onset, and development of multiorgan failure.67
Idiopathic Pneumonia Syndrome
IPS is a complex clinical syndrome whose pathology is felt to stem from a variety of possible lung insults such as direct myeloablative drug toxicity, occult pulmonary infection, or cytokine-driven inflammation. The ATS published an article further subcategorizing IPS as different clinical entities based upon whether the primary insult involves the vascular endothelium, interstitial tissue, and airway tissue, truly idiopathic, or unclassified.68 In clinical practice, IPS is defined as widespread alveolar injury in the absence of evidence of renal failure, heart failure, and excessive fluid resuscitation. In addition, negative testing for a variety of bacterial, viral, and fungal causes is also necessary.69 Clinical syndromes included within the IPS definition are ARDS, acute interstitial pneumonia, DAH, cryptogenic organizing pneumonia, and BOS.70 Risk factors for developing IPS include TBI, older age of recipient, acute GVHD, and underlying diagnosis of AML or myelodysplastic syndrome.12 In addition, it has been shown that risk for developing IPS is lower in patients undergoing allogeneic HSCT who receive non-myeloablative conditioning regimens.71 The pathologic finding in IPS is diffuse alveolar damage. A 2006 study in which investigators reviewed BAL samples from patients with IPS found that 3% of the patients had PCR evidence of human metapneumovirus infection, and a study in 2015 found PCR evidence of infection in 53% of BAL samples from patients diagnosed with IPS.72,73 This fuels the debate on whether IPS is truly an infection-driven process where the source of infection, pulmonary or otherwise, simply escapes detection. Various surfactant proteins, which play a role in decreasing surface tension within the alveolar interface and function as mediators within the innate immunity of the lung, have been studied in regard to development of IPS. Small retrospective studies have shown a trend toward lower pre-transplant serum protein surfactant D and the development of IPS.74
The diagnosis of IPS does not require pathologic diagnosis in most circumstances. The correct clinical findings in association with a negative infectious workup lead to a presumptive diagnosis of IPS. The extent of the infectious workup that must be completed to adequately rule out infection is often a difficult clinical question. Recent recommendations include BAL fluid evaluation for routine bacterial cultures, appropriate viral culture, and consideration of PCR testing to evaluate for Mycoplasma, Chlamydia, and Aspergillus antigens.75 Transbronchial biopsy continues to appear in recommendations, but is not routinely performed and should be completed as the patient’s clinical status permits.8,68 Table 3 reviews basic features of early noninfectious pulmonary complications.
Treatment of IPS centers around moderate to high doses of corticosteroids. Based on IPS experimental modes, tumor necrosis factor (TNF)-α has been implicated as an important mediator. Unfortunately, several studies evaluating etanercept have produced conflicting results, and this agent’s clinical effects on morbidity and mortality remain in question.76
- What treatment should be offered to the patient with diffuse alveolar damage on biopsy?
Treatment consists of supportive care and empiric broad-spectrum antibiotics with consideration of high-dose corticosteroids. Based upon early studies in murine models implicating TNF, pilot studies were performed evaluating etanercept as a possible safe and effective addition to high-dose systemic corticosteroids.77 Although these results were promising, data from a truncated randomized control clinical trial failed to show improvement in patient response in the adult population.76 More recent data from the same author suggests that pediatric populations with IPS are, however, responsive to etanercept and high-dose corticosteroid therapy.78 When IPS develops as a late complication, treatment with high-dose corticosteroids (2 mg/kg/day) and etanercept (0.4 mg/kg twice weekly) has been shown to improve 2-year survival.79
Case Patient 2 Conclusion
The patient is started on steroids and makes a speedy recovery. He is successfully extubated 5 days later.
Conclusion
Careful pretransplant evaluation, including a full set of pulmonary function tests, can help predict a patient’s risk for pulmonary complications after transplant, allowing risk factor modification strategies to be implemented prior to transplant, including smoking cessation. It also helps identify patients at high risk for complications who will require closer monitoring after transplantation. Early posttransplant complications include infectious and noninfectious entities. Bacterial, viral, and fungal pneumonias are in the differential of infectious pneumonia, and bronchoscopy can be helpful in establishing a diagnosis. A common, important noninfectious cause of early pulmonary complications is IPS, which is treated with steroids and sometimes anti-TNF therapy.
Hematopoietic stem cell transplantation (HSCT) is widely used in the economically developed world to treat a variety of hematologic malignancies as well as nonmalignant diseases and solid tumors. An estimated 17,900 HSCTs were performed in 2011, and survival rates continue to increase.1 Pulmonary complications post HSCT are common, with rates ranging from 40% to 60%, and are associated with increased morbidity and mortality.2
Clinical diagnosis of pulmonary complications in the HSCT population has been aided by a previously well-defined chronology of the most common diseases.3 Historically, early pulmonary complications were defined as pulmonary complications occurring within 100 days of HSCT (corresponding to the acute graft-versus-host disease [GVHD] period). Late pulmonary complications are those that occur thereafter. This timeline, however, is now more variable given the increasing indications for HSCT, the use of reduced-intensity conditioning strategies, and varied individual immune reconstitution. This article discusses the management of early post-HSCT pulmonary complications; late post-HSCT pulmonary complications will be discussed in a separate follow-up article.
Transplant Basics
The development of pulmonary complications is affected by many factors associated with the transplant. Autologous transplantation involves the collection of a patient’s own stem cells, appropriate storage and processing, and re-implantation after induction therapy. During induction therapy, the patient undergoes high-dose chemotherapy or radiation therapy that ablates the bone marrow. The stem cells are then transfused back into the patient to repopulate the bone marrow. Allogeneic transplants involve the collection of stem cells from a donor. Donors are matched as closely as possible to the recipient’s histocompatibility antigen (HLA) haplotypes to prevent graft failure and rejection. The donor can be related or unrelated to the recipient. If there is not a possibility of a related match (from a sibling), then a national search is undertaken to look for a match through the National Marrow Donor Program. There are fewer transplant reactions and occurrences of GVHD if the major HLAs of the donor and recipient match. Table 1 reviews basic definitions pertaining to HSCT.
How the cells for transplantation are obtained is also an important factor in the rate of complications. There are 3 main sources: peripheral blood, bone marrow, and umbilical cord. Peripheral stem cell harvesting involves exposing the donor to granulocyte-colony stimulating factor (gCSF), which increases peripheral circulation of stem cells. These cells are then collected and infused into the recipient after the recipient has completed an induction regimen involving chemotherapy and/or radiation, depending on the protocol. This procedure is called peripheral blood stem cell transplant (PBSCT). Stem cells can also be directly harvested from bone marrow cells, which are collected from repeated aspiration of bone marrow from the posterior iliac crest.4 This technique is most common in children, whereas in adults peripheral blood stem cells are the most common source. Overall mortality does not differ based on the source of the stem cells. It is postulated that GVHD may be more common in patients undergoing PBSCT, but the graft failure rate may be lower.5
The third option is umbilical cord blood (UCB) as the source of stem cells. This involves the collection of umbilical cord blood that is prepared and frozen after birth. It has a smaller volume of cells, and although fewer cells are needed when using UCB, 2 separate donors may be required for a single adult recipient. The engraftment of the stem cells is slower and infections in the post-transplant period are more common. Prior reports indicate GVHD rates may be lower.4 While the use of UCB is not common in adults, the incidence has doubled over the past decade, increasing from 3% to 6%.
The conditioning regimen can influence pulmonary complications. Traditionally, an ablative transplant involves high-dose chemotherapy or radiation to eradicate the recipient’s bone marrow. This regimen can lead to many complications, especially in the immediate post-transplant period. In the past 10 years, there has been increasing interest in non-myeloablative, or reduced-intensity, conditioning transplants.6 These “mini transplants” involve smaller doses of chemotherapy or radiation, which do not totally eradicate the bone marrow; after the transplant a degree of chimerism develops where the donor and recipient stem cells coexist. The medications in the preparative regimen also should be considered because they can affect pulmonary complications after transplant. Certain chemotherapeutic agents such as carmustine, bleomycin, and many others can lead to acute and chronic presentations of pulmonary diseases such as hypersensitivity pneumonitis, pulmonary fibrosis, acute respiratory distress syndrome, and abnormal pulmonary function testing.
After the HSCT, GVHD can develop in more than 50% of allogeneic recipients.3 The incidence of GVHD has been reported to be increasing over the past 12 years.It is divided into acute GVHD (which traditionally happens in the first 100 days after transplant) and chronic GVHD (after day 100). This calendar-day–based system has been augmented based on a 2006 National Institutes of Health working group report emphasizing the importance of organ-specific features of chronic GVHD in the clinical presentation of GVHD.7 Histologic changes in chronic organ GVHD tend to include more fibrotic features, whereas in acute GVHD more inflammatory changes are seen. The NIH working group report also stressed the importance of obtaining a biopsy specimen for histopathologic review and interdisciplinary collaboration to arrive at a consensus diagnosis, and noted the limitations of using histologic changes as the sole determinant of a “gold standard” diagnosis.7 GVHD can directly predispose patients to pulmonary GVHD and indirectly predispose them to infectious complications because the mainstay of therapy for GVHD is increased immunosuppression.
Pretransplant Evaluation
Case Patient 1
A 56-year-old man is diagnosed with acute myeloid leukemia (AML) after presenting with signs and symptoms consistent with pancytopenia. He has a past medical history of chronic sinus congestion, arthritis, depression, chronic pain, and carpal tunnel surgery. He is employed as an oilfield worker and has a 40-pack-year smoking history, but he recently cut back to half a pack per day. He is being evaluated for allogeneic transplant with his brother as the donor and the planned conditioning regimen is total body irradiation (TBI), thiotepa, cyclophosphamide, and antithymocyte globulin with T-cell depletion. Routine pretransplant pulmonary function testing (PFT) reveals a restrictive pattern and he is sent for pretransplant pulmonary evaluation.
Physical exam reveals a chronically ill appearing man. He is afebrile, the respiratory rate is 16 breaths/min, blood pressure is 145/88 mm Hg, heart rate is 92 beats/min, and oxygen saturation is 95%. He is in no distress. Auscultation of the chest reveals slightly diminished breath sounds bilaterally but is clear and without wheezes, rhonchi, or rales. Heart exam shows regular rate and rhythm without murmurs, rubs, or gallops. Extremities reveal no edema or rashes. Otherwise, the remainder of the exam is normal. The patient’s PFT results are shown in Table 2.
- What aspects of this patient’s history put him at risk for pulmonary complications after transplantation?
Risk Factors for Pulmonary Complications
Predicting who is at risk for pulmonary complications is difficult. Complications are generally divided into infectious and noninfectious categories. Regardless of category, allogeneic HSCT recipients are at increased risk compared with autologous recipients, but even in autologous transplants, more than 25% of patients will develop pulmonary complications in the first year.8 Prior to transplant, patients undergo full PFT. Early on, many studies attempted to show relationships between various factors and post-transplant pulmonary complications. Factors that were implicated were forced expiratory volume in 1 second (FEV1), diffusing capacity of the lung for carbon monoxide (D
Another sometimes overlooked risk before transplantation is restrictive lung disease. One study showed a twofold increase in respiratory failure and mortality if there was pretransplant restriction based on TLC < 80%.16
An interesting study by one group in pretransplant evaluation found decreased muscle strength by maximal inspiratory muscle strength (PImax), maximal expiratory muscle strength (PEmax), dominant hand grip strength, and 6-minute walk test (6MWT) distance prior to allogeneic transplant, but did not find a relationship between these variables and mortality.17 While this study had a small sample size, these findings likely deserve continued investigation.18
- What methods are used to calculate risk for complications?
Risk Scoring Systems
Several pretransplantation risk scores have been developed. In a study that looked at more than 2500 allogeneic transplants, Parimon et al showed that risk of mortality and respiratory failure could be estimated prior to transplant using a scoring system—the Lung Function Score (LFS)—that combines the FEV1 and D
The Pretransplantation Assessment of Mortality score, initially developed in 2006, predicts mortality within the first 2 years after HSCT based on 8 clinical factors: disease risk, age at transplant, donor type, conditioning regimen, and markers of organ function (percentage of predicted FEV1, percentage of predicted D
- What other preoperative testing or interventions should be considered in this patient?
Since there is a high risk of infectious complications after transplant, the question of whether pretransplantation patients should undergo screening imaging may arise. There is no evidence that routine chest computed tomography (CT) reduces the risk of infectious complications after transplantation.26 An area that may be insufficiently addressed in the pretransplantation evaluation is smoking cessation counseling.27 Studies have shown an elevated risk of mortality in smokers.28-30 Others have found a higher incidence of respiratory failure but not an increased mortality.31 Overall, with the good rates of smoking cessation that can be accomplished, smokers should be counseled to quit before transplantation.
In summary, patients should undergo full PFTs prior to transplantation to help stratify risk for pulmonary complications and mortality and to establish a clinical baseline. The LFS (using FEV1 and D
Case Patient 1 Conclusion
The patient undergoes transplantation due to his lack of other treatment options. Evaluation prior to transplant, however, shows that he is at high risk for pulmonary complications. He has a LFS of 7 prior to transplant (using the D
Early Infectious Pulmonary Complications
Case Patient 2
A 27-year-old man with a medical history significant for AML and allogeneic HSCT presents with cough productive of a small amount of clear to white sputum, dyspnea on exertion, and fevers for 1 week. He also has mild nausea and a decrease in appetite. He underwent HSCT 2.5 months prior to admission, which was a matched unrelated bone marrow transplant with TBI and cyclophosphamide conditioning. His past medical history is significant only for exercise-induced asthma for which he takes a rescue inhaler infrequently prior to transplantation. His pretransplant PFTs showed normal spirometry with an FEV1 of 106% of predicted and D
Physical exam is notable for fever of 101.0°F, heart rate 80 beats/min, respiratory rate 16 breaths/ min, and blood pressure 142/78 mm Hg; an admission oxygen saturation is 93% on room air. Lungs show bibasilar crackles and the remainder of the exam is normal. Laboratory testing shows a white blood cell count of 2400 cells/μL, hemoglobin 7.6 g/dL, and platelet count 66 × 103/μL. Creatinine is 1.0 mg/dL. Chest radiograph shows ill-defined bilateral lower-lobe infiltrates. CT scans are shown in the Figure.
- For which infectious complications is this patient most at risk?
Pneumonia
A prospective trial in the HSCT population reported a pneumonia incidence rate of 68%, and pneumonia is more common in allogeneic HSCT with prolonged immunosuppressive therapy.32 Development of pneumonia within 100 days of transplant directly correlates with nonrelapsed mortality.33 Early detection is key, and bronchoscopy within the first 5 days of symptoms has been shown to change therapy in approximately 40% of cases but has not been shown to affect mortality.34 The clinical presentation of pneumonia in the HSCT population can be variable because of the presence of neutropenia and profound immunosuppression. Traditionally accepted diagnostic criteria of fevers, sputum production, and new infiltrates should be used with caution, and an appropriately high index of suspicion should be maintained. Progression to respiratory failure, regardless of causative organism of infection, portends a poor prognosis, with mortality rates estimated at 70% to 90%.35,36 Several transplant-specific factors may affect early infections. For instance, UCB transplants have been found to have a higher incidence of invasive aspergillosis and cytomegalovirus (CMV) infections but without higher mortality attributed to the infections.37
Bacterial Pneumonia
Bacterial pneumonia accounts for 20% to 50% of pneumonia cases in HSCT recipients.38 Gram-negative organisms, specifically Pseudomonas aeruginosa and Escherichia coli, were reported to be the most common pathologic bacteria in recent prospective trials, whereas previous retrospective trials showed that common community-acquired organisms were the most common cause of pneumonia in HSCT recipients.32,39 This underscores the importance of being aware of the clinical prevalence of microorganisms and local antibiograms, along with associated institutional susceptibility profiles. Initiation of immediate empiric broad-spectrum antibiotics is essential when bacterial pneumonia is suspected.
Viral Pneumonia
The prevalence of viral pneumonia in stem cell transplant recipients is estimated at 28%,32 with most cases being caused by community viral pathogens such as rhinovirus, respiratory syncytial virus (RSV), influenza A and B, and parainfluenza.39 The prevention, prophylaxis, and early treatment of viral pneumonias, specifically CMV infection, have decreased the mortality associated with early pneumonia after HSCT. Co-infection with bacterial organisms must be considered and has been associated with increased mortality in the intensive care unit setting.40
Supportive treatment with rhinovirus infection is sufficient as the disease is usually self-limited in immunocompromised patients. In contrast, infection with RSV in the lower respiratory tract is associated with increased mortality in prior reports, and recent studies suggest that further exploration of prophylaxis strategies is warranted.41 Treatment with ribavirin remains the backbone of therapy, but drug toxicity continues to limit its use. The addition of immunomodulators such as RSV immune globulin or palivizumab to ribavirin remains controversial, but a retrospective review suggests that early treatment may prevent progression to lower respiratory tract infection and lead to improved mortality.42 Infection with influenza A/B must be considered during influenza season. Treatment with oseltamivir may shorten the duration of disease when influenza A/B or parainfluenza are detected. Reactivation of latent herpes simplex virus during the pre-engraftment phase should also be considered. Treatment is similar to that in nonimmunocompromised hosts. When CMV pneumonia is suspected, careful history regarding compliance with prophylactic antivirals and CMV status of both the recipient and donor are key. A presumptive diagnosis can be made with the presence of appropriate clinical scenario, supportive radiographic images showing areas of ground-glass opacification or consolidation, and positive CMV polymerase chain reaction (PCR) assay. Visualization of inclusion bodies on lung biopsy tissue remains the gold standard for diagnosis. Treatment consists of CMV immunoglobulin and ganciclovir.
Fungal Pneumonia
Early fungal pneumonias have been associated with increased mortality in the HSCT population.43 Clinical suspicion should remain high and compliance with antifungal prophylaxis should be questioned thoroughly. Invasive aspergillosis (IA) remains the most common fungal infection. A bimodal distribution of onset of infection peaking on day 16 and again on day 96 has been described in the literature.44 Patients often present with classic pneumonia symptoms, but these may be accompanied by hemoptysis. Proven IA diagnosis requires visualization of fungal forms from biopsy or needle aspiration or a positive culture obtained in a sterile fashion.45 Most clinical data comes from experience with probable and possible diagnosis of IA. Bronchoalveolar lavage with testing with Aspergillus galactomannan assay has been shown to be clinically useful in establishing the clinical diagnosis in the HSCT population.46 Classic air-crescent findings on chest CT are helpful in establishing a possible diagnosis, but retrospective analysis reveals CT findings such as focal infiltrates and pulmonary nodular patterns are more common.47 First-line treatment with voriconazole has been shown to decrease short-term mortality attributable to IA but has not had an effect on long-term, all-cause mortality.48 Surgical resection is reserved for patients with refractory disease or patients presenting with massive hemoptysis.
Mucormycosis is an emerging disease with ever increasing prevalence in the HSCT population, reflecting the improved prophylaxis and treatment of IA. Initial clinical presentation is similar to IA, most commonly affecting the lung, although craniofacial involvement is classic for mucormycosis, especially in HSCT patients with diabetes.49Mucor infections can present with massive hemoptysis due to tissue invasion and disregard for tissue and fascial planes. Diagnosis of mucormycosis is associated with as much as a six-fold increase in risk for death. Diagnosis requires identification of the organism by examination or culture and biopsy is often necessary.50,51 Amphotericin B remains first-line therapy as mucormycosis is resistant to azole antifungals, with higher doses recommended for cerebral involvement.52
Candida pulmonary infections during the early HSCT period are becoming increasingly rare due to widespread use of fluconazole prophylaxis and early treatment of mucosal involvement during neutropenia. Endemic fungal infections such as blastomycosis, coccidioidomycosis, and histoplasmosis should be considered in patients inhabiting specific geographic areas or with recent travel to these areas.
- What test should be performed to evaluate for infectious causes of pneumonia?
Role of Flexible Fiberoptic Bronchoscopy
The utility of flexible fiberoptic bronchoscopy (FOB) in immune-compromised patients for the evaluation of pulmonary infiltrates is a frequently debated topic. Current studies suggest a diagnosis can be made in approximately 80% of cases in the immune-compromised population.32,53 Noninvasive testing such as urine and serum antigens, sputum cultures, Aspergillus galactomannan assays, viral nasal swabs, and PCR studies often lead to a diagnosis in appropriate clinical scenarios. Conservative management would dictate the use of noninvasive testing whenever possible, and randomized controlled trials have shown noninvasive testing to be noninferior to FOB in preventing need for mechanical ventilation, with no difference in overall mortality.54 FOB has been shown to be most useful in establishing a diagnosis when an infectious etiology is suspected.55 In multivariate analysis, a delay in the identification of the etiology of pulmonary infiltrate was associated with increased mortality.56 Additionally, early FOB was found to be superior to late FOB in revealing a diagnosis. 32,57 Despite its ability to detect the cause of pulmonary disease, direct antibiotic therapy, and possibly change therapy, FOB with diagnostic maneuvers has not been shown to affect mortality.58 In a large case series, FOB with bronchoalveolar lavage (BAL) revealed a diagnosis in approximately 30% to 50% of cases. The addition of transbronchial biopsy did not improve diagnostic utility.58 More recent studies have confirmed that the addition of transbronchial biopsy does not add to diagnostic yield and is associated with increased adverse events.59 The appropriate use of advanced techniques such as endobronchial ultrasound–guided transbronchial needle aspirations, endobronchial biopsy, and CT-guided navigational bronchoscopy has not been established and should be considered on a case-by-case basis. In summary, routine early BAL is the diagnostic test of choice, especially when infectious pulmonary complications are suspected.
Contraindications for FOB in this population mirror those in the general population. These include acute severe hypoxemic respiratory failure, myocardial ischemia or acute coronary syndrome within 2 weeks of procedure, severe thrombocytopenia, and inability to provide or obtain informed consent from patient or health care power of attorney. Coagulopathy and thrombocytopenia are common comorbid conditions in the HSCT population. A platelet count of < 20 × 103/µL has generally been used as a cut-off for routine FOB with BAL.60 Risks of the procedures should be discussed clearly with the patient, but simple FOB for airway evaluation and BAL is generally well tolerated even under these conditions.
Early Nonifectious Pulmonary Complications
Case Patient 2 Continued
Bronchoscopy with BAL performed the day after admission is unremarkable and stains and cultures are negative for viral, bacterial, and fungal organisms. The patient is initially started on broad-spectrum antibiotics, but his oxygenation continues to worsen to the point that he is placed on noninvasive positive pressure ventilation. He is started empirically on amphotericin B and eventually is intubated. VATS lung biopsy is ultimately performed and pathology is consistent with diffuse alveolar damage.
- Based on these biopsy findings, what is the diagnosis?
Based on the pathology consistent with diffuse alveolar damage, a diagnosis of idiopathic pneumonia syndrome (IPS) is made.
- What noninfectious pulmonary complications occur in the early post-transplant period?
The overall incidence of noninfectious pulmonary complications after HSCT is generally estimated at 20% to 30%.32 Acute pulmonary edema is a common very early noninfectious pulmonary complication and clinically the most straightforward to treat. Three distinct clinical syndromes—peri-engraftment respiratory distress syndrome (PERDS), diffuse alveolar hemorrhage (DAH), and IPS—comprise the remainder of the pertinent early noninfectious complications. Clinical presentation differs based upon the disease entity. Recent studies have evaluated the role of angiotensin-converting enzyme polymorphisms as a predictive marker for risk of developing early noninfectious pulmonary complications.61
Peri-Engraftment Respiratory Distress Syndrome
PERDS is a clinical syndrome comprising the cardinal features of erythematous rash and fever along with noncardiogenic pulmonary infiltrates and hypoxemia that occur in the peri-engraftment period, defined as recovery of absolute neutrophil count to > 500/μL on 2 consecutive days.62 PERDS occurs in the autologous HSCT population and may be a clinical correlate to early GVHD in the allogeneic HSCT population. It is hypothesized that the pathophysiology underlying PERDS is an autoimmune-related capillary leak caused by pro-inflammatory cytokine release.63 Treatment remains anecdotal and currently consists of supportive care and high-dose corticosteroids. Some have favored limiting the use of gCSF given its role in stimulating rapid white blood cell recovery.33 Prognosis is favorable, but progression to fulminant respiratory failure requiring mechanical ventilation portends a poor prognosis.
Diffuse Alveolar Hemorrhage
DAH is clinical syndrome consisting of diffuse alveolar infiltrates on pulmonary imaging combined with progressively bloodier return per aliquot during BAL in 3 different subsegments or more than 20% hemosiderin-laden macrophages on BAL fluid evaluation. Classically, DAH is defined in the absence of pulmonary infection or cardiac dysfunction. The pathophysiology is thought to be related to inflammation of pulmonary vasculature within the alveolar walls leading to alveolitis. Although no prospective trials exist, early use of high-dose corticosteroid therapy is thought to improve outcomes;64,65 a recent study, however, showed low-dose steroids may be associated with the lowest mortality.66 Mortality is directly linked to the presence of superimposed infection, need for mechanical ventilation, late onset, and development of multiorgan failure.67
Idiopathic Pneumonia Syndrome
IPS is a complex clinical syndrome whose pathology is felt to stem from a variety of possible lung insults such as direct myeloablative drug toxicity, occult pulmonary infection, or cytokine-driven inflammation. The ATS published an article further subcategorizing IPS as different clinical entities based upon whether the primary insult involves the vascular endothelium, interstitial tissue, and airway tissue, truly idiopathic, or unclassified.68 In clinical practice, IPS is defined as widespread alveolar injury in the absence of evidence of renal failure, heart failure, and excessive fluid resuscitation. In addition, negative testing for a variety of bacterial, viral, and fungal causes is also necessary.69 Clinical syndromes included within the IPS definition are ARDS, acute interstitial pneumonia, DAH, cryptogenic organizing pneumonia, and BOS.70 Risk factors for developing IPS include TBI, older age of recipient, acute GVHD, and underlying diagnosis of AML or myelodysplastic syndrome.12 In addition, it has been shown that risk for developing IPS is lower in patients undergoing allogeneic HSCT who receive non-myeloablative conditioning regimens.71 The pathologic finding in IPS is diffuse alveolar damage. A 2006 study in which investigators reviewed BAL samples from patients with IPS found that 3% of the patients had PCR evidence of human metapneumovirus infection, and a study in 2015 found PCR evidence of infection in 53% of BAL samples from patients diagnosed with IPS.72,73 This fuels the debate on whether IPS is truly an infection-driven process where the source of infection, pulmonary or otherwise, simply escapes detection. Various surfactant proteins, which play a role in decreasing surface tension within the alveolar interface and function as mediators within the innate immunity of the lung, have been studied in regard to development of IPS. Small retrospective studies have shown a trend toward lower pre-transplant serum protein surfactant D and the development of IPS.74
The diagnosis of IPS does not require pathologic diagnosis in most circumstances. The correct clinical findings in association with a negative infectious workup lead to a presumptive diagnosis of IPS. The extent of the infectious workup that must be completed to adequately rule out infection is often a difficult clinical question. Recent recommendations include BAL fluid evaluation for routine bacterial cultures, appropriate viral culture, and consideration of PCR testing to evaluate for Mycoplasma, Chlamydia, and Aspergillus antigens.75 Transbronchial biopsy continues to appear in recommendations, but is not routinely performed and should be completed as the patient’s clinical status permits.8,68 Table 3 reviews basic features of early noninfectious pulmonary complications.
Treatment of IPS centers around moderate to high doses of corticosteroids. Based on IPS experimental modes, tumor necrosis factor (TNF)-α has been implicated as an important mediator. Unfortunately, several studies evaluating etanercept have produced conflicting results, and this agent’s clinical effects on morbidity and mortality remain in question.76
- What treatment should be offered to the patient with diffuse alveolar damage on biopsy?
Treatment consists of supportive care and empiric broad-spectrum antibiotics with consideration of high-dose corticosteroids. Based upon early studies in murine models implicating TNF, pilot studies were performed evaluating etanercept as a possible safe and effective addition to high-dose systemic corticosteroids.77 Although these results were promising, data from a truncated randomized control clinical trial failed to show improvement in patient response in the adult population.76 More recent data from the same author suggests that pediatric populations with IPS are, however, responsive to etanercept and high-dose corticosteroid therapy.78 When IPS develops as a late complication, treatment with high-dose corticosteroids (2 mg/kg/day) and etanercept (0.4 mg/kg twice weekly) has been shown to improve 2-year survival.79
Case Patient 2 Conclusion
The patient is started on steroids and makes a speedy recovery. He is successfully extubated 5 days later.
Conclusion
Careful pretransplant evaluation, including a full set of pulmonary function tests, can help predict a patient’s risk for pulmonary complications after transplant, allowing risk factor modification strategies to be implemented prior to transplant, including smoking cessation. It also helps identify patients at high risk for complications who will require closer monitoring after transplantation. Early posttransplant complications include infectious and noninfectious entities. Bacterial, viral, and fungal pneumonias are in the differential of infectious pneumonia, and bronchoscopy can be helpful in establishing a diagnosis. A common, important noninfectious cause of early pulmonary complications is IPS, which is treated with steroids and sometimes anti-TNF therapy.
1. Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010;303:1617–24.
2. Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2004;170:22–48.
4. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006;354:1813–26.
5. Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012;367:1487–96.
6. Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009;15:367–9.
7. Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant 2006;12:31–47.
8. Afessa B, Abdulai RM, Kremers WK, et al. Risk factors and outcome of pulmonary complications after autologous hematopoietic stem cell transplant. Chest 2012;141:442–50.
9. Bolwell BJ. Are predictive factors clinically useful in bone marrow transplantation? Bone Marrow Transplant 2003;32:853–61.
10. Carlson K, Backlund L, Smedmyr B, et al. Pulmonary function and complications subsequent to autologous bone marrow transplantation. Bone Marrow Transplant 1994;14:805–11.
11. Clark JG, Schwartz DA, Flournoy N, et al. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med 1987;107:648–56.
12. Crawford SW, Fisher L. Predictive value of pulmonary function tests before marrow transplantation. Chest 1992; 101:1257–64.
13. Ghalie R, Szidon JP, Thompson L, et al. Evaluation of pulmonary complications after bone marrow transplantation: the role of pretransplant pulmonary function tests. Bone Marrow Transplant 1992;10:359–65.
14. Ho VT, Weller E, Lee SJ, et al. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001;7:223–9.
15. Horak DA, Schmidt GM, Zaia JA, et al. Pretransplant pulmonary function predicts cytomegalovirus-associated interstitial pneumonia following bone marrow transplantation. Chest 1992;102:1484–90.
16. Ramirez-Sarmiento A, Orozco-Levi M, Walter EC, et al. Influence of pretransplantation restrictive lung disease on allogeneic hematopoietic cell transplantation outcomes. Biol Blood Marrow Transplant 2010;16:199–206.
17. White AC, Terrin N, Miller KB, Ryan HF. Impaired respiratory and skeletal muscle strength in patients prior to hematopoietic stem-cell transplantation. Chest 2005;128145–52.
18. Afessa B. Pretransplant pulmonary evaluation of the blood and marrow transplant recipient. Chest 2005;128:8–10.
19. Parimon T, Madtes DK, Au DH, et al. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med 2005;172:384–90.
20. Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 2006;12:252–66.
21. Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med 2006;144:407–14.
22. Au BK, Gooley TA, Armand P, et al. Reevaluation of the pretransplant assessment of mortality score after allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant 2015;21:848–54.
23. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–9.
24. Chien JW, Sullivan KM. Carbon monoxide diffusion capacity: how low can you go for hematopoietic cell transplantation eligibility? Biol Blood Marrow Transplant 2009;15: 447–53.
25. Coffey DG, Pollyea DA, Myint H, et al. Adjusting DLCO for Hb and its effects on the Hematopoietic Cell Transplantation-specific Comorbidity Index. Bone Marrow Transplant 2013;48:1253–6.
26. Kasow KA, Krueger J, Srivastava DK, et al. Clinical utility of computed tomography screening of chest, abdomen, and sinuses before hematopoietic stem cell transplantation: the St. Jude experience. Biol Blood Marrow Transplant 2009;15:490–5.
27. Hamadani M, Craig M, Awan FT, Devine SM. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45: 1259–68.
28. Savani BN, Montero A, Wu C, et al. Prediction and prevention of transplant-related mortality from pulmonary causes after total body irradiation and allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:223–30.
29. Ehlers SL, Gastineau DA, Patten CA, et al. The impact of smoking on outcomes among patients undergoing hematopoietic SCT for the treatment of acute leukemia. Bone Marrow Transplant 2011;46:285–90.
30. Marks DI, Ballen K, Logan BR, et al. The effect of smoking on allogeneic transplant outcomes. Biol Blood Marrow Transplant 2009;15:1277–87.
31. Tran BT, Halperin A, Chien JW. Cigarette smoking and outcomes after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011;17:1004–11.
32. Lucena CM, Torres A, Rovira M, et al. Pulmonary complications in hematopoietic SCT: a prospective study. Bone Marrow Transplant 2014;49:1293–9.
33. Chi AK, Soubani AO, White AC, Miller KB. An update on pulmonary complications of hematopoietic stem cell transplantation. Chest 2013;144:1913–22.
34. Dunagan DP, Baker AM, Hurd DD, Haponik EF. Bronchoscopic evaluation of pulmonary infiltrates following bone marrow transplantation. Chest 1997;111:135–41.
35. Naeem N, Reed MD, Creger RJ, et al. Transfer of the hematopoietic stem cell transplant patient to the intensive care unit: does it really matter? Bone Marrow Transplant 2006;37:119–33.
36. Afessa B, Tefferi A, Hoagland HC, et al. Outcome of recipients of bone marrow transplants who require intensive care unit support. Mayo Clin Proc 1992;67:117–22.
37. Parody R, Martino R, de la Camara R, et al. Fungal and viral infections after allogeneic hematopoietic transplantation from unrelated donors in adults: improving outcomes over time. Bone Marrow Transplant 2015;50:274–81.
38. Orasch C, Weisser M, Mertz D, et al. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:521–6.
39. Aguilar-Guisado M, Jimenez-Jambrina M, Espigado I, et al. Pneumonia in allogeneic stem cell transplantation recipients: a multicenter prospective study. Clin Transplant 2011;25:E629–38.
40. Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS One 2009;4:e8540.
41. Hynicka LM, Ensor CR. Prophylaxis and treatment of respiratory syncytial virus in adult immunocompromised patients. Ann Pharmacother 2012;46:558–66.
42. Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 2011;2755–63.
43. Marr KA, Bowden RA. Fungal infections in patients undergoing blood and marrow transplantation. Transpl Infect Dis 1999;1:237–46.
44. Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis 1997;175:1459–66.
45. Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002;34:7–14.
46. Fisher CE, Stevens AM, Leisenring W, et al. Independent contribution of bronchoalveolar lavage and serum galactomannan in the diagnosis of invasive pulmonary aspergillosis. Transpl Infect Dis 2014;16:505–10.
47. Kojima R, Tateishi U, Kami M, et al. Chest computed tomography of late invasive aspergillosis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:506–11.
48. Salmeron G, Porcher R, Bergeron A, et al. Persistent poor long-term prognosis of allogeneic hematopoietic stem cell transplant recipients surviving invasive aspergillosis. Haematologica 2012;97:1357–63.
49. McNulty JS. Rhinocerebral mucormycosis: predisposing factors. Laryngoscope 1982;92(10 Pt 1):1140.
50. Walsh TJ, Gamaletsou MN, McGinnis MR, et al. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 2012;54 Suppl 1:S55–60.
51. Klingspor L, Saaedi B, Ljungman P, Szakos A. Epidemiology and outcomes of patients with invasive mould infections: a retrospective observational study from a single centre (2005-2009). Mycoses 2015;58:470–7.
52. Danion F, Aguilar C, Catherinot E, et al. Mucormycosis: new developments in a persistently devastating infection. Semin Respir Crit Care Med 2015;36:692–70.
53. Rano A, Agusti C, Jimenez P, et al. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax 2001;56:379–87.
54. Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med 2008;36:100–7.
55. Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest 2004;125:712–22.
56. Rano A, Agusti C, Benito N, et al. Prognostic factors of non-HIV immunocompromised patients with pulmonary infiltrates. Chest 2002;122:253–61.
57. Shannon VR, Andersson BS, Lei X, et al. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:647–55.
58. Patel NR, Lee PS, Kim JH, et al. The influence of diagnostic bronchoscopy on clinical outcomes comparing adult autologous and allogeneic bone marrow transplant patients. Chest 2005;127:1388–96.
59. Chellapandian D, Lehrnbecher T, Phillips B, et al. Bronchoalveolar lavage and lung biopsy in patients with cancer and hematopoietic stem-cell transplantation recipients: a systematic review and meta-analysis. J Clin Oncol 2015;33:501–9.
60. Carr IM, Koegelenberg CF, von Groote-Bidlingmaier F, et al. Blood loss during flexible bronchoscopy: a prospective observational study. Respiration 2012;84:312–8.
61. Miyamoto M, Onizuka M, Machida S, et al. ACE deletion polymorphism is associated with a high risk of non-infectious pulmonary complications after stem cell transplantation. Int J Hematol 2014;99:175–83.
62. Capizzi SA, Kumar S, Huneke NE, et al. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:1299–303.
63. Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:893–8.
64. Wanko SO, Broadwater G, Folz RJ, Chao NJ. Diffuse alveolar hemorrhage: retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol Blood Marrow Transplant 2006;12:949–53.
65. Metcalf JP, Rennard SI, Reed EC, et al. Corticosteroids as adjunctive therapy for diffuse alveolar hemorrhage associated with bone marrow transplantation. University of Nebraska Medical Center Bone Marrow Transplant Group. Am J Med 1994;96:327–34.
66. Rathi NK, Tanner AR, Dinh A, et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transplant 2015;50:420–6.
67. Afessa B, Tefferi A, Litzow MR, Peters SG. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med 2002;166:1364–8.
68. Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med 2011;183:1262–79.
69. Clark JG, Hansen JA, Hertz MI, Pet al. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Resp Dis 1993;147:1601–6.
70. Vande Vusse LK, Madtes DK. Early onset noninfectious pulmonary syndromes after hematopoietic cell transplantation. Clin Chest Med 2017;38:233–48.
71. Fukuda T, Hackman RC, Guthrie KA, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003;102:2777–85.
72. Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med 2006;144:344–9.
73. Seo S, Renaud C, Kuypers JM, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015;125:3789–97.
74. Nakane T, Nakamae H, Kamoi H, et al. Prognostic value of serum surfactant protein D level prior to transplant for the development of bronchiolitis obliterans syndrome and idiopathic pneumonia syndrome following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;42:43–9.
75. Gilbert CR, Lerner A, Baram M, Awsare BK. Utility of flexible bronchoscopy in the evaluation of pulmonary infiltrates in the hematopoietic stem cell transplant population—a single center fourteen year experience. Arch Bronconeumol 2013;49:189–95.
76. Yanik GA, Horowitz MM, Weisdorf DJ, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: blood and marrow transplant clinical trials network protocol. Biol Blood Marrow Transplant 2014;20:858–64.
77. Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood 2008;111:2470–5.
78. Yanik GA, Grupp SA, Pulsipher MA, et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children’s Oncology Group Study (ASCT0521). Biol Blood Marrow Transplant 2015;21:67–73.
79. Thompson J, Yin Z, D’Souza A, et al. Etanercept and corticosteroid therapy for the treatment of late-onset idiopathic pneumonia syndrome. Biol Blood Marrow Transplant J 2017; 23:1955–60.
1. Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010;303:1617–24.
2. Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2004;170:22–48.
4. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006;354:1813–26.
5. Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012;367:1487–96.
6. Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009;15:367–9.
7. Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant 2006;12:31–47.
8. Afessa B, Abdulai RM, Kremers WK, et al. Risk factors and outcome of pulmonary complications after autologous hematopoietic stem cell transplant. Chest 2012;141:442–50.
9. Bolwell BJ. Are predictive factors clinically useful in bone marrow transplantation? Bone Marrow Transplant 2003;32:853–61.
10. Carlson K, Backlund L, Smedmyr B, et al. Pulmonary function and complications subsequent to autologous bone marrow transplantation. Bone Marrow Transplant 1994;14:805–11.
11. Clark JG, Schwartz DA, Flournoy N, et al. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med 1987;107:648–56.
12. Crawford SW, Fisher L. Predictive value of pulmonary function tests before marrow transplantation. Chest 1992; 101:1257–64.
13. Ghalie R, Szidon JP, Thompson L, et al. Evaluation of pulmonary complications after bone marrow transplantation: the role of pretransplant pulmonary function tests. Bone Marrow Transplant 1992;10:359–65.
14. Ho VT, Weller E, Lee SJ, et al. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001;7:223–9.
15. Horak DA, Schmidt GM, Zaia JA, et al. Pretransplant pulmonary function predicts cytomegalovirus-associated interstitial pneumonia following bone marrow transplantation. Chest 1992;102:1484–90.
16. Ramirez-Sarmiento A, Orozco-Levi M, Walter EC, et al. Influence of pretransplantation restrictive lung disease on allogeneic hematopoietic cell transplantation outcomes. Biol Blood Marrow Transplant 2010;16:199–206.
17. White AC, Terrin N, Miller KB, Ryan HF. Impaired respiratory and skeletal muscle strength in patients prior to hematopoietic stem-cell transplantation. Chest 2005;128145–52.
18. Afessa B. Pretransplant pulmonary evaluation of the blood and marrow transplant recipient. Chest 2005;128:8–10.
19. Parimon T, Madtes DK, Au DH, et al. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med 2005;172:384–90.
20. Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 2006;12:252–66.
21. Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med 2006;144:407–14.
22. Au BK, Gooley TA, Armand P, et al. Reevaluation of the pretransplant assessment of mortality score after allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant 2015;21:848–54.
23. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–9.
24. Chien JW, Sullivan KM. Carbon monoxide diffusion capacity: how low can you go for hematopoietic cell transplantation eligibility? Biol Blood Marrow Transplant 2009;15: 447–53.
25. Coffey DG, Pollyea DA, Myint H, et al. Adjusting DLCO for Hb and its effects on the Hematopoietic Cell Transplantation-specific Comorbidity Index. Bone Marrow Transplant 2013;48:1253–6.
26. Kasow KA, Krueger J, Srivastava DK, et al. Clinical utility of computed tomography screening of chest, abdomen, and sinuses before hematopoietic stem cell transplantation: the St. Jude experience. Biol Blood Marrow Transplant 2009;15:490–5.
27. Hamadani M, Craig M, Awan FT, Devine SM. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45: 1259–68.
28. Savani BN, Montero A, Wu C, et al. Prediction and prevention of transplant-related mortality from pulmonary causes after total body irradiation and allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:223–30.
29. Ehlers SL, Gastineau DA, Patten CA, et al. The impact of smoking on outcomes among patients undergoing hematopoietic SCT for the treatment of acute leukemia. Bone Marrow Transplant 2011;46:285–90.
30. Marks DI, Ballen K, Logan BR, et al. The effect of smoking on allogeneic transplant outcomes. Biol Blood Marrow Transplant 2009;15:1277–87.
31. Tran BT, Halperin A, Chien JW. Cigarette smoking and outcomes after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011;17:1004–11.
32. Lucena CM, Torres A, Rovira M, et al. Pulmonary complications in hematopoietic SCT: a prospective study. Bone Marrow Transplant 2014;49:1293–9.
33. Chi AK, Soubani AO, White AC, Miller KB. An update on pulmonary complications of hematopoietic stem cell transplantation. Chest 2013;144:1913–22.
34. Dunagan DP, Baker AM, Hurd DD, Haponik EF. Bronchoscopic evaluation of pulmonary infiltrates following bone marrow transplantation. Chest 1997;111:135–41.
35. Naeem N, Reed MD, Creger RJ, et al. Transfer of the hematopoietic stem cell transplant patient to the intensive care unit: does it really matter? Bone Marrow Transplant 2006;37:119–33.
36. Afessa B, Tefferi A, Hoagland HC, et al. Outcome of recipients of bone marrow transplants who require intensive care unit support. Mayo Clin Proc 1992;67:117–22.
37. Parody R, Martino R, de la Camara R, et al. Fungal and viral infections after allogeneic hematopoietic transplantation from unrelated donors in adults: improving outcomes over time. Bone Marrow Transplant 2015;50:274–81.
38. Orasch C, Weisser M, Mertz D, et al. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:521–6.
39. Aguilar-Guisado M, Jimenez-Jambrina M, Espigado I, et al. Pneumonia in allogeneic stem cell transplantation recipients: a multicenter prospective study. Clin Transplant 2011;25:E629–38.
40. Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS One 2009;4:e8540.
41. Hynicka LM, Ensor CR. Prophylaxis and treatment of respiratory syncytial virus in adult immunocompromised patients. Ann Pharmacother 2012;46:558–66.
42. Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 2011;2755–63.
43. Marr KA, Bowden RA. Fungal infections in patients undergoing blood and marrow transplantation. Transpl Infect Dis 1999;1:237–46.
44. Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis 1997;175:1459–66.
45. Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002;34:7–14.
46. Fisher CE, Stevens AM, Leisenring W, et al. Independent contribution of bronchoalveolar lavage and serum galactomannan in the diagnosis of invasive pulmonary aspergillosis. Transpl Infect Dis 2014;16:505–10.
47. Kojima R, Tateishi U, Kami M, et al. Chest computed tomography of late invasive aspergillosis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:506–11.
48. Salmeron G, Porcher R, Bergeron A, et al. Persistent poor long-term prognosis of allogeneic hematopoietic stem cell transplant recipients surviving invasive aspergillosis. Haematologica 2012;97:1357–63.
49. McNulty JS. Rhinocerebral mucormycosis: predisposing factors. Laryngoscope 1982;92(10 Pt 1):1140.
50. Walsh TJ, Gamaletsou MN, McGinnis MR, et al. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 2012;54 Suppl 1:S55–60.
51. Klingspor L, Saaedi B, Ljungman P, Szakos A. Epidemiology and outcomes of patients with invasive mould infections: a retrospective observational study from a single centre (2005-2009). Mycoses 2015;58:470–7.
52. Danion F, Aguilar C, Catherinot E, et al. Mucormycosis: new developments in a persistently devastating infection. Semin Respir Crit Care Med 2015;36:692–70.
53. Rano A, Agusti C, Jimenez P, et al. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax 2001;56:379–87.
54. Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med 2008;36:100–7.
55. Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest 2004;125:712–22.
56. Rano A, Agusti C, Benito N, et al. Prognostic factors of non-HIV immunocompromised patients with pulmonary infiltrates. Chest 2002;122:253–61.
57. Shannon VR, Andersson BS, Lei X, et al. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:647–55.
58. Patel NR, Lee PS, Kim JH, et al. The influence of diagnostic bronchoscopy on clinical outcomes comparing adult autologous and allogeneic bone marrow transplant patients. Chest 2005;127:1388–96.
59. Chellapandian D, Lehrnbecher T, Phillips B, et al. Bronchoalveolar lavage and lung biopsy in patients with cancer and hematopoietic stem-cell transplantation recipients: a systematic review and meta-analysis. J Clin Oncol 2015;33:501–9.
60. Carr IM, Koegelenberg CF, von Groote-Bidlingmaier F, et al. Blood loss during flexible bronchoscopy: a prospective observational study. Respiration 2012;84:312–8.
61. Miyamoto M, Onizuka M, Machida S, et al. ACE deletion polymorphism is associated with a high risk of non-infectious pulmonary complications after stem cell transplantation. Int J Hematol 2014;99:175–83.
62. Capizzi SA, Kumar S, Huneke NE, et al. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:1299–303.
63. Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:893–8.
64. Wanko SO, Broadwater G, Folz RJ, Chao NJ. Diffuse alveolar hemorrhage: retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol Blood Marrow Transplant 2006;12:949–53.
65. Metcalf JP, Rennard SI, Reed EC, et al. Corticosteroids as adjunctive therapy for diffuse alveolar hemorrhage associated with bone marrow transplantation. University of Nebraska Medical Center Bone Marrow Transplant Group. Am J Med 1994;96:327–34.
66. Rathi NK, Tanner AR, Dinh A, et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transplant 2015;50:420–6.
67. Afessa B, Tefferi A, Litzow MR, Peters SG. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med 2002;166:1364–8.
68. Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med 2011;183:1262–79.
69. Clark JG, Hansen JA, Hertz MI, Pet al. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Resp Dis 1993;147:1601–6.
70. Vande Vusse LK, Madtes DK. Early onset noninfectious pulmonary syndromes after hematopoietic cell transplantation. Clin Chest Med 2017;38:233–48.
71. Fukuda T, Hackman RC, Guthrie KA, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003;102:2777–85.
72. Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med 2006;144:344–9.
73. Seo S, Renaud C, Kuypers JM, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015;125:3789–97.
74. Nakane T, Nakamae H, Kamoi H, et al. Prognostic value of serum surfactant protein D level prior to transplant for the development of bronchiolitis obliterans syndrome and idiopathic pneumonia syndrome following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;42:43–9.
75. Gilbert CR, Lerner A, Baram M, Awsare BK. Utility of flexible bronchoscopy in the evaluation of pulmonary infiltrates in the hematopoietic stem cell transplant population—a single center fourteen year experience. Arch Bronconeumol 2013;49:189–95.
76. Yanik GA, Horowitz MM, Weisdorf DJ, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: blood and marrow transplant clinical trials network protocol. Biol Blood Marrow Transplant 2014;20:858–64.
77. Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood 2008;111:2470–5.
78. Yanik GA, Grupp SA, Pulsipher MA, et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children’s Oncology Group Study (ASCT0521). Biol Blood Marrow Transplant 2015;21:67–73.
79. Thompson J, Yin Z, D’Souza A, et al. Etanercept and corticosteroid therapy for the treatment of late-onset idiopathic pneumonia syndrome. Biol Blood Marrow Transplant J 2017; 23:1955–60.
Narcotic analgesia for breastfeeding women
Editor’s Note: This is the final installment of a six-part series that reviews key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte. This month’s edition of the Board Corner focuses on narcotic analgesia for breastfeeding women.
The American College of Obstetrics and Gynecologists “Practice Advisories” are released when there is an important clinical issue that needs immediate attention from ob.gyn. clinicians. On April 27, 2017, ACOG joined with the Society for Maternal-Fetal Medicine and the Academy of Breastfeeding Medicine to issue a new practice advisory with recommendations on opiate analgesia in breastfeeding women.1 These Practice Advisories contain material that may be tested on a board exam. We recommend that you read this Practice Advisory and review it carefully.
A. Morphine IV
B. Butorphanol
C. Acetaminophen with codeine
D. Hydromorphone
E. Morphine (given orally)
The correct answer is C.
Acetaminophen with codeine (aka Tylenol #3 or Tylenol #4) is the least appropriate first-choice narcotic in a breastfeeding postpartum patient after Cesarean delivery as it is the only narcotic among the choices that is metabolized by the enzyme CYP2D6. Morphine, butorphanol, and hydromorphone are all metabolized by CYP450. If a narcotic is indicated, it is better to choose one that is not metabolized by CYP2D6 because of potential side effects in both mothers and infants who may be “CYP2D6 ultrametabolizers” or “CYP2D6 poor metabolizers.”
Key points
The key points to remember are:
1. Do not use codeine or tramadol as a first-line narcotic choice in breastfeeding mothers, if possible, because of variable side effects in mothers and infants.
2. The preferred narcotics to use when breastfeeding are butorphanol, morphine, or hydromorphone.
3. If codeine or tramadol is used in breastfeeding women, the clinician should speak with the patient and family about the possible side effects and the recent labeling changes required by the Food and Drug Administration.
Literature summary
In April, the Food and Drug Administration issued a safety alert and said it is requiring labeling changes for prescription medications containing codeine and tramadol. Specifically, the agency warned against use of codeine and tramadol when breastfeeding. This change is the result of the fact that certain people metabolize codeine and tramadol differently. There are some people – considered CYP2D6 “ultrarapid metabolizers” – who can have levels of the drug in their breast milk that can cause excessive sleepiness and depressed breathing in infants. There is one report of an infant death resulting from codeine use. The frequency of these “rapid metabolizers” is about 4%-5% in the United States.
In addition to the effects on breastfed infants, there are effects on the mother as well. This is because 6% of patients in the United States are “poor metabolizers” who have insufficient pain relief, as well as greater side effects.
Hydrocodone and oxycodone are not addressed in the recent labeling changes. However, “ultrarapid metabolizers” do show more pain relief and pupil restriction.
When comparing codeine with nonsteroidal anti-inflammatory medications (NSAIDs) in abdominal surgery, nine randomized trials failed to show that codeine provided superior pain relief.
Hydromorphone, butorphanol, and morphine are not metabolized by CYP2D6 so the problems faced by “ultrarapid metabolizers” or “poor metabolizers” is not an issue.
Ob.gyns. should utilize regional anesthesia, NSAIDs, and acetaminophen (without codeine) to help decrease the risks of anesthesia while still ensuring adequate pain relief. Ob.gyns. should closely monitor their patients on narcotics for any side effects in both mothers and infants, especially central nervous system depression.
Dr. Kairis is an assistant professor in the department of gynecology and obstetrics at Loma Linda (Calif.) University Health and is the director of the Women’s Sexual Medicine Program there. She is on the editorial committee of the Ob/Gyn Board Master. Dr. Siddighi is editor in chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
1. Practice Advisory on Codeine and Tramadol for Breastfeeding Women. April 27, 2017. American College of Obstetricians and Gynecologists.
Editor’s Note: This is the final installment of a six-part series that reviews key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte. This month’s edition of the Board Corner focuses on narcotic analgesia for breastfeeding women.
The American College of Obstetrics and Gynecologists “Practice Advisories” are released when there is an important clinical issue that needs immediate attention from ob.gyn. clinicians. On April 27, 2017, ACOG joined with the Society for Maternal-Fetal Medicine and the Academy of Breastfeeding Medicine to issue a new practice advisory with recommendations on opiate analgesia in breastfeeding women.1 These Practice Advisories contain material that may be tested on a board exam. We recommend that you read this Practice Advisory and review it carefully.
A. Morphine IV
B. Butorphanol
C. Acetaminophen with codeine
D. Hydromorphone
E. Morphine (given orally)
The correct answer is C.
Acetaminophen with codeine (aka Tylenol #3 or Tylenol #4) is the least appropriate first-choice narcotic in a breastfeeding postpartum patient after Cesarean delivery as it is the only narcotic among the choices that is metabolized by the enzyme CYP2D6. Morphine, butorphanol, and hydromorphone are all metabolized by CYP450. If a narcotic is indicated, it is better to choose one that is not metabolized by CYP2D6 because of potential side effects in both mothers and infants who may be “CYP2D6 ultrametabolizers” or “CYP2D6 poor metabolizers.”
Key points
The key points to remember are:
1. Do not use codeine or tramadol as a first-line narcotic choice in breastfeeding mothers, if possible, because of variable side effects in mothers and infants.
2. The preferred narcotics to use when breastfeeding are butorphanol, morphine, or hydromorphone.
3. If codeine or tramadol is used in breastfeeding women, the clinician should speak with the patient and family about the possible side effects and the recent labeling changes required by the Food and Drug Administration.
Literature summary
In April, the Food and Drug Administration issued a safety alert and said it is requiring labeling changes for prescription medications containing codeine and tramadol. Specifically, the agency warned against use of codeine and tramadol when breastfeeding. This change is the result of the fact that certain people metabolize codeine and tramadol differently. There are some people – considered CYP2D6 “ultrarapid metabolizers” – who can have levels of the drug in their breast milk that can cause excessive sleepiness and depressed breathing in infants. There is one report of an infant death resulting from codeine use. The frequency of these “rapid metabolizers” is about 4%-5% in the United States.
In addition to the effects on breastfed infants, there are effects on the mother as well. This is because 6% of patients in the United States are “poor metabolizers” who have insufficient pain relief, as well as greater side effects.
Hydrocodone and oxycodone are not addressed in the recent labeling changes. However, “ultrarapid metabolizers” do show more pain relief and pupil restriction.
When comparing codeine with nonsteroidal anti-inflammatory medications (NSAIDs) in abdominal surgery, nine randomized trials failed to show that codeine provided superior pain relief.
Hydromorphone, butorphanol, and morphine are not metabolized by CYP2D6 so the problems faced by “ultrarapid metabolizers” or “poor metabolizers” is not an issue.
Ob.gyns. should utilize regional anesthesia, NSAIDs, and acetaminophen (without codeine) to help decrease the risks of anesthesia while still ensuring adequate pain relief. Ob.gyns. should closely monitor their patients on narcotics for any side effects in both mothers and infants, especially central nervous system depression.
Dr. Kairis is an assistant professor in the department of gynecology and obstetrics at Loma Linda (Calif.) University Health and is the director of the Women’s Sexual Medicine Program there. She is on the editorial committee of the Ob/Gyn Board Master. Dr. Siddighi is editor in chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
1. Practice Advisory on Codeine and Tramadol for Breastfeeding Women. April 27, 2017. American College of Obstetricians and Gynecologists.
Editor’s Note: This is the final installment of a six-part series that reviews key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte. This month’s edition of the Board Corner focuses on narcotic analgesia for breastfeeding women.
The American College of Obstetrics and Gynecologists “Practice Advisories” are released when there is an important clinical issue that needs immediate attention from ob.gyn. clinicians. On April 27, 2017, ACOG joined with the Society for Maternal-Fetal Medicine and the Academy of Breastfeeding Medicine to issue a new practice advisory with recommendations on opiate analgesia in breastfeeding women.1 These Practice Advisories contain material that may be tested on a board exam. We recommend that you read this Practice Advisory and review it carefully.
A. Morphine IV
B. Butorphanol
C. Acetaminophen with codeine
D. Hydromorphone
E. Morphine (given orally)
The correct answer is C.
Acetaminophen with codeine (aka Tylenol #3 or Tylenol #4) is the least appropriate first-choice narcotic in a breastfeeding postpartum patient after Cesarean delivery as it is the only narcotic among the choices that is metabolized by the enzyme CYP2D6. Morphine, butorphanol, and hydromorphone are all metabolized by CYP450. If a narcotic is indicated, it is better to choose one that is not metabolized by CYP2D6 because of potential side effects in both mothers and infants who may be “CYP2D6 ultrametabolizers” or “CYP2D6 poor metabolizers.”
Key points
The key points to remember are:
1. Do not use codeine or tramadol as a first-line narcotic choice in breastfeeding mothers, if possible, because of variable side effects in mothers and infants.
2. The preferred narcotics to use when breastfeeding are butorphanol, morphine, or hydromorphone.
3. If codeine or tramadol is used in breastfeeding women, the clinician should speak with the patient and family about the possible side effects and the recent labeling changes required by the Food and Drug Administration.
Literature summary
In April, the Food and Drug Administration issued a safety alert and said it is requiring labeling changes for prescription medications containing codeine and tramadol. Specifically, the agency warned against use of codeine and tramadol when breastfeeding. This change is the result of the fact that certain people metabolize codeine and tramadol differently. There are some people – considered CYP2D6 “ultrarapid metabolizers” – who can have levels of the drug in their breast milk that can cause excessive sleepiness and depressed breathing in infants. There is one report of an infant death resulting from codeine use. The frequency of these “rapid metabolizers” is about 4%-5% in the United States.
In addition to the effects on breastfed infants, there are effects on the mother as well. This is because 6% of patients in the United States are “poor metabolizers” who have insufficient pain relief, as well as greater side effects.
Hydrocodone and oxycodone are not addressed in the recent labeling changes. However, “ultrarapid metabolizers” do show more pain relief and pupil restriction.
When comparing codeine with nonsteroidal anti-inflammatory medications (NSAIDs) in abdominal surgery, nine randomized trials failed to show that codeine provided superior pain relief.
Hydromorphone, butorphanol, and morphine are not metabolized by CYP2D6 so the problems faced by “ultrarapid metabolizers” or “poor metabolizers” is not an issue.
Ob.gyns. should utilize regional anesthesia, NSAIDs, and acetaminophen (without codeine) to help decrease the risks of anesthesia while still ensuring adequate pain relief. Ob.gyns. should closely monitor their patients on narcotics for any side effects in both mothers and infants, especially central nervous system depression.
Dr. Kairis is an assistant professor in the department of gynecology and obstetrics at Loma Linda (Calif.) University Health and is the director of the Women’s Sexual Medicine Program there. She is on the editorial committee of the Ob/Gyn Board Master. Dr. Siddighi is editor in chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
1. Practice Advisory on Codeine and Tramadol for Breastfeeding Women. April 27, 2017. American College of Obstetricians and Gynecologists.
Pelvic organ prolapse: Effective treatments
Editor’s Note: This is the fifth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte. This month’s edition of the Board Corner focuses on pelvic organ prolapse.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material that is often tested on board exams. Earlier this year, ACOG released a revised Practice Bulletin (#176) updating its advice on the diagnosis and management of pelvic organ prolapse (POP).1 It is a well-written document summarizing most of the landmark articles published in the field of female pelvic medicine and reconstructive surgery. We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: Which of the following procedures is the most effective for a sexually-active patient with advanced prolapse?
A. Sacrospinous ligament suspension (SSLS)
B. Uterosacral ligament suspension (USLS)
C. Sacrocolpopexy (SCP)
D. Colpocleisis
E. Hysteropexy
A randomized trial comparing SSLS and USLS found the two apical procedures with native tissue repair are equally effective with comparable functional and adverse outcomes (answers A and B are incorrect). However, randomized trials comparing SCP to SSLS show that SCP with synthetic mesh has the lowest recurrence rate for prolapse. Colpocleisis is done for patients who are not sexually active (answer D is incorrect). Hysteropexy is performed for patients who desire preservation of the uterus. There is less available evidence on safety and efficacy, compared with hysterectomy at the time of prolapse repair (answer E is incorrect)
Key points
The key points to remember are:
1. SCP is the most effective prolapse repair technique.
2. USLS and SSLS fixation are equally effective when compared with one another.
3. Colpocleisis is a highly successful procedure for POP in patients who are not sexually active.
Literature summary
The lifetime risk for undergoing surgery for POP or stress incontinence is 20%. POP is the descent of one or more aspects of the vagina or uterus, which allows nearby organs to herniate into the vagina. POP should only be treated if it is symptomatic and bothersome for the patient. The pessary is an alternative to surgical treatment of prolapse.
Proven risk factors for POP are increased parity, vaginal delivery, age, obesity, chronic constipation, and certain congenital anomalies. A history should be taken to elucidate symptoms of prolapse, such as bulge, pressure, sexual dysfunction, lower urinary tract dysfunction, or defecatory dysfunction. It is also important to find out how much the POP is affecting her quality of life. A physical exam is best performed with a split speculum, with bladder empty, while the patient performs a Valsalva maneuver. We recommend using the POP-Q system to grade the severity of prolapse. The tone of the pelvic floor muscle should also be evaluated (absent, weak, normal, or strong) during pelvic exam.
The minimum testing necessary for a patient with POP is urinalysis and a postvoid residual. A stress test with a full bladder should also be done with and without reduction of the prolapse. If you’re considering surgery and the patient has advanced prolapse and/or other complicating factors – such as obstructive symptoms or significant neurologic disorder – you should consider performing urodynamic testing as well.
Native tissue, suture-based reconstructive repairs of the vagina include apical procedures, such as SSLS and USLS, in addition to anterior colporrhaphy and posterior repair. At 2-year follow-up, SSLS and USLS along with anterior colporrhaphy and posterior repair are equally effective for treatment of prolapse with comparable functional and adverse outcomes. SCP is more effective than SSLS but the abdominal procedure (not laparoscopic) may be associated with more complications. Currently, there are no published randomized trials comparing minimally-invasive SCP to USLS, but one is underway (clinicaltrials.gov).
Other procedures for POP include obliterative procedures such as colpocleisis, which is highly effective for patients who do not desire future vaginal intercourse and also has low morbidity. Preservation of the uterus by hysteropexy procedures (either transvaginal or transabdominal) are also options for women desiring to preserve their uterus, but these procedures have little safety and efficacy data. Regardless of the procedure performed, routine intraoperative cystoscopy should be done to assure ureteral patency and to rule out injury to the lower urinary tract.
Some type of prophylactic anti-incontinence procedure – retropubic or Burch – may be done at the time of vaginal prolapse repair or abdominal prolapse repair, respectively, in order to reduce the chance of postoperative stress urinary incontinence in a patient without symptoms of stress incontinence. The exception to this is in a patient who has an elevated postvoid residual or someone with a prior anti-incontinence procedure without symptoms of stress urinary incontinence.
Practice tips
Finally, here are some precautions and words of advice about the following POP procedures:
- Neither synthetic nor biologic grafts should be used to augment posterior repairs as these do not improve outcomes.
- Transvaginal repair of rectocele is superior to the transanal repair techniques.
- Synthetic mesh augmentation of the anterior vaginal wall may improve anatomic outcomes, but this comes at a cost (more reoperations and higher rate of complications). Thus, surgeons performing these procedures should have specialized training and the patient should have a unique indication and must undergo proper consent as recommended by ACOG.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Editor’s Note: This is the fifth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte. This month’s edition of the Board Corner focuses on pelvic organ prolapse.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material that is often tested on board exams. Earlier this year, ACOG released a revised Practice Bulletin (#176) updating its advice on the diagnosis and management of pelvic organ prolapse (POP).1 It is a well-written document summarizing most of the landmark articles published in the field of female pelvic medicine and reconstructive surgery. We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: Which of the following procedures is the most effective for a sexually-active patient with advanced prolapse?
A. Sacrospinous ligament suspension (SSLS)
B. Uterosacral ligament suspension (USLS)
C. Sacrocolpopexy (SCP)
D. Colpocleisis
E. Hysteropexy
A randomized trial comparing SSLS and USLS found the two apical procedures with native tissue repair are equally effective with comparable functional and adverse outcomes (answers A and B are incorrect). However, randomized trials comparing SCP to SSLS show that SCP with synthetic mesh has the lowest recurrence rate for prolapse. Colpocleisis is done for patients who are not sexually active (answer D is incorrect). Hysteropexy is performed for patients who desire preservation of the uterus. There is less available evidence on safety and efficacy, compared with hysterectomy at the time of prolapse repair (answer E is incorrect)
Key points
The key points to remember are:
1. SCP is the most effective prolapse repair technique.
2. USLS and SSLS fixation are equally effective when compared with one another.
3. Colpocleisis is a highly successful procedure for POP in patients who are not sexually active.
Literature summary
The lifetime risk for undergoing surgery for POP or stress incontinence is 20%. POP is the descent of one or more aspects of the vagina or uterus, which allows nearby organs to herniate into the vagina. POP should only be treated if it is symptomatic and bothersome for the patient. The pessary is an alternative to surgical treatment of prolapse.
Proven risk factors for POP are increased parity, vaginal delivery, age, obesity, chronic constipation, and certain congenital anomalies. A history should be taken to elucidate symptoms of prolapse, such as bulge, pressure, sexual dysfunction, lower urinary tract dysfunction, or defecatory dysfunction. It is also important to find out how much the POP is affecting her quality of life. A physical exam is best performed with a split speculum, with bladder empty, while the patient performs a Valsalva maneuver. We recommend using the POP-Q system to grade the severity of prolapse. The tone of the pelvic floor muscle should also be evaluated (absent, weak, normal, or strong) during pelvic exam.
The minimum testing necessary for a patient with POP is urinalysis and a postvoid residual. A stress test with a full bladder should also be done with and without reduction of the prolapse. If you’re considering surgery and the patient has advanced prolapse and/or other complicating factors – such as obstructive symptoms or significant neurologic disorder – you should consider performing urodynamic testing as well.
Native tissue, suture-based reconstructive repairs of the vagina include apical procedures, such as SSLS and USLS, in addition to anterior colporrhaphy and posterior repair. At 2-year follow-up, SSLS and USLS along with anterior colporrhaphy and posterior repair are equally effective for treatment of prolapse with comparable functional and adverse outcomes. SCP is more effective than SSLS but the abdominal procedure (not laparoscopic) may be associated with more complications. Currently, there are no published randomized trials comparing minimally-invasive SCP to USLS, but one is underway (clinicaltrials.gov).
Other procedures for POP include obliterative procedures such as colpocleisis, which is highly effective for patients who do not desire future vaginal intercourse and also has low morbidity. Preservation of the uterus by hysteropexy procedures (either transvaginal or transabdominal) are also options for women desiring to preserve their uterus, but these procedures have little safety and efficacy data. Regardless of the procedure performed, routine intraoperative cystoscopy should be done to assure ureteral patency and to rule out injury to the lower urinary tract.
Some type of prophylactic anti-incontinence procedure – retropubic or Burch – may be done at the time of vaginal prolapse repair or abdominal prolapse repair, respectively, in order to reduce the chance of postoperative stress urinary incontinence in a patient without symptoms of stress incontinence. The exception to this is in a patient who has an elevated postvoid residual or someone with a prior anti-incontinence procedure without symptoms of stress urinary incontinence.
Practice tips
Finally, here are some precautions and words of advice about the following POP procedures:
- Neither synthetic nor biologic grafts should be used to augment posterior repairs as these do not improve outcomes.
- Transvaginal repair of rectocele is superior to the transanal repair techniques.
- Synthetic mesh augmentation of the anterior vaginal wall may improve anatomic outcomes, but this comes at a cost (more reoperations and higher rate of complications). Thus, surgeons performing these procedures should have specialized training and the patient should have a unique indication and must undergo proper consent as recommended by ACOG.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Editor’s Note: This is the fifth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte. This month’s edition of the Board Corner focuses on pelvic organ prolapse.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material that is often tested on board exams. Earlier this year, ACOG released a revised Practice Bulletin (#176) updating its advice on the diagnosis and management of pelvic organ prolapse (POP).1 It is a well-written document summarizing most of the landmark articles published in the field of female pelvic medicine and reconstructive surgery. We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: Which of the following procedures is the most effective for a sexually-active patient with advanced prolapse?
A. Sacrospinous ligament suspension (SSLS)
B. Uterosacral ligament suspension (USLS)
C. Sacrocolpopexy (SCP)
D. Colpocleisis
E. Hysteropexy
A randomized trial comparing SSLS and USLS found the two apical procedures with native tissue repair are equally effective with comparable functional and adverse outcomes (answers A and B are incorrect). However, randomized trials comparing SCP to SSLS show that SCP with synthetic mesh has the lowest recurrence rate for prolapse. Colpocleisis is done for patients who are not sexually active (answer D is incorrect). Hysteropexy is performed for patients who desire preservation of the uterus. There is less available evidence on safety and efficacy, compared with hysterectomy at the time of prolapse repair (answer E is incorrect)
Key points
The key points to remember are:
1. SCP is the most effective prolapse repair technique.
2. USLS and SSLS fixation are equally effective when compared with one another.
3. Colpocleisis is a highly successful procedure for POP in patients who are not sexually active.
Literature summary
The lifetime risk for undergoing surgery for POP or stress incontinence is 20%. POP is the descent of one or more aspects of the vagina or uterus, which allows nearby organs to herniate into the vagina. POP should only be treated if it is symptomatic and bothersome for the patient. The pessary is an alternative to surgical treatment of prolapse.
Proven risk factors for POP are increased parity, vaginal delivery, age, obesity, chronic constipation, and certain congenital anomalies. A history should be taken to elucidate symptoms of prolapse, such as bulge, pressure, sexual dysfunction, lower urinary tract dysfunction, or defecatory dysfunction. It is also important to find out how much the POP is affecting her quality of life. A physical exam is best performed with a split speculum, with bladder empty, while the patient performs a Valsalva maneuver. We recommend using the POP-Q system to grade the severity of prolapse. The tone of the pelvic floor muscle should also be evaluated (absent, weak, normal, or strong) during pelvic exam.
The minimum testing necessary for a patient with POP is urinalysis and a postvoid residual. A stress test with a full bladder should also be done with and without reduction of the prolapse. If you’re considering surgery and the patient has advanced prolapse and/or other complicating factors – such as obstructive symptoms or significant neurologic disorder – you should consider performing urodynamic testing as well.
Native tissue, suture-based reconstructive repairs of the vagina include apical procedures, such as SSLS and USLS, in addition to anterior colporrhaphy and posterior repair. At 2-year follow-up, SSLS and USLS along with anterior colporrhaphy and posterior repair are equally effective for treatment of prolapse with comparable functional and adverse outcomes. SCP is more effective than SSLS but the abdominal procedure (not laparoscopic) may be associated with more complications. Currently, there are no published randomized trials comparing minimally-invasive SCP to USLS, but one is underway (clinicaltrials.gov).
Other procedures for POP include obliterative procedures such as colpocleisis, which is highly effective for patients who do not desire future vaginal intercourse and also has low morbidity. Preservation of the uterus by hysteropexy procedures (either transvaginal or transabdominal) are also options for women desiring to preserve their uterus, but these procedures have little safety and efficacy data. Regardless of the procedure performed, routine intraoperative cystoscopy should be done to assure ureteral patency and to rule out injury to the lower urinary tract.
Some type of prophylactic anti-incontinence procedure – retropubic or Burch – may be done at the time of vaginal prolapse repair or abdominal prolapse repair, respectively, in order to reduce the chance of postoperative stress urinary incontinence in a patient without symptoms of stress incontinence. The exception to this is in a patient who has an elevated postvoid residual or someone with a prior anti-incontinence procedure without symptoms of stress urinary incontinence.
Practice tips
Finally, here are some precautions and words of advice about the following POP procedures:
- Neither synthetic nor biologic grafts should be used to augment posterior repairs as these do not improve outcomes.
- Transvaginal repair of rectocele is superior to the transanal repair techniques.
- Synthetic mesh augmentation of the anterior vaginal wall may improve anatomic outcomes, but this comes at a cost (more reoperations and higher rate of complications). Thus, surgeons performing these procedures should have specialized training and the patient should have a unique indication and must undergo proper consent as recommended by ACOG.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
When to consider external cephalic version
Editor’s Note: This is the fourth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material often tested on board exams. In February 2016, ACOG issued a revised Practice Bulletin (#161) on external cephalic version outlining clinical considerations and recommendations and providing an algorithm for patient management.1 We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: According to the Practice Bulletin, which of the following is TRUE about external cephalic version (ECV)?
A. Success rate is lower in women with a previous cesarean delivery.
B. Placental location affects the success rate.
C. External cephalic version should be stopped after 15 minutes.
D. Women at 37 weeks’ gestation are preferred candidates.
E. Tocolysis decreases success rate.
The correct answer is D.
Women at 37 weeks’ gestation are the preferred candidates for an ECV because spontaneous version would likely have already occurred by this time and the risk of spontaneous reversion is lower. Answers A-C and E are incorrect statements.
Key points
Women at 37 weeks’ gestation are the preferred candidates for an ECV.
The overall pooled success rate for ECV is 58% with a 6% pooled complication rate.
The use of parenteral tocolysis has been associated with increased success rates of ECV, though there are not enough data to make a recommendation regarding use of regional anesthesia with the procedure.
Literature summary
ECV is a procedure designed to turn a fetus into vertex presentation by applying external pressure to a woman’s abdomen. Women at 37 weeks’ gestation are the preferred candidates. At this gestational age, spontaneous version is most likely to have occurred, and there is decreased risk of spontaneous reversion after the ECV. All patients who are near term and found to have a fetus in a nonvertex presentation should be offered an ECV as long as there are no contraindications to the procedure. ECV is not appropriate for women who have a contraindication to a vaginal delivery.
There are limited studies of ECV in women who undergo the procedure in early labor and in those who have had a previous uterine surgery. ECV success rates are not affected by a previous cesarean delivery, though the risks of uterine rupture are not clear. The procedure should be attempted only in settings where cesarean delivery services are immediately available.
The success rates of ECV have been reported to be anywhere from 16% to 100%, with an overall pooled success of 58% with a 6% pooled complication rate. Some studies have documented higher success rates with higher parity and a transverse or oblique fetal lie. However, placental location, maternal weight, and amniotic fluid volume have not been consistently found to be predictive of ECV success. The use of parenteral tocolysis has been associated with increased success rates of ECV, though there are not enough data to make a recommendation regarding use of regional anesthesia with the procedure.
ECV should be stopped in the face of a prolonged or significant fetal bradycardia or if the patient is experiencing intolerable levels of discomfort. However, there are no guidelines to recommend the total time limit of the procedure. After the ECV, there should be fetal heart rate monitoring for at least 30 minutes and anti-D immune globulin should be administered to those women who are Rh-negative if delivery is not anticipated in the next 72 hours.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Editor’s Note: This is the fourth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material often tested on board exams. In February 2016, ACOG issued a revised Practice Bulletin (#161) on external cephalic version outlining clinical considerations and recommendations and providing an algorithm for patient management.1 We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: According to the Practice Bulletin, which of the following is TRUE about external cephalic version (ECV)?
A. Success rate is lower in women with a previous cesarean delivery.
B. Placental location affects the success rate.
C. External cephalic version should be stopped after 15 minutes.
D. Women at 37 weeks’ gestation are preferred candidates.
E. Tocolysis decreases success rate.
The correct answer is D.
Women at 37 weeks’ gestation are the preferred candidates for an ECV because spontaneous version would likely have already occurred by this time and the risk of spontaneous reversion is lower. Answers A-C and E are incorrect statements.
Key points
Women at 37 weeks’ gestation are the preferred candidates for an ECV.
The overall pooled success rate for ECV is 58% with a 6% pooled complication rate.
The use of parenteral tocolysis has been associated with increased success rates of ECV, though there are not enough data to make a recommendation regarding use of regional anesthesia with the procedure.
Literature summary
ECV is a procedure designed to turn a fetus into vertex presentation by applying external pressure to a woman’s abdomen. Women at 37 weeks’ gestation are the preferred candidates. At this gestational age, spontaneous version is most likely to have occurred, and there is decreased risk of spontaneous reversion after the ECV. All patients who are near term and found to have a fetus in a nonvertex presentation should be offered an ECV as long as there are no contraindications to the procedure. ECV is not appropriate for women who have a contraindication to a vaginal delivery.
There are limited studies of ECV in women who undergo the procedure in early labor and in those who have had a previous uterine surgery. ECV success rates are not affected by a previous cesarean delivery, though the risks of uterine rupture are not clear. The procedure should be attempted only in settings where cesarean delivery services are immediately available.
The success rates of ECV have been reported to be anywhere from 16% to 100%, with an overall pooled success of 58% with a 6% pooled complication rate. Some studies have documented higher success rates with higher parity and a transverse or oblique fetal lie. However, placental location, maternal weight, and amniotic fluid volume have not been consistently found to be predictive of ECV success. The use of parenteral tocolysis has been associated with increased success rates of ECV, though there are not enough data to make a recommendation regarding use of regional anesthesia with the procedure.
ECV should be stopped in the face of a prolonged or significant fetal bradycardia or if the patient is experiencing intolerable levels of discomfort. However, there are no guidelines to recommend the total time limit of the procedure. After the ECV, there should be fetal heart rate monitoring for at least 30 minutes and anti-D immune globulin should be administered to those women who are Rh-negative if delivery is not anticipated in the next 72 hours.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Editor’s Note: This is the fourth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material often tested on board exams. In February 2016, ACOG issued a revised Practice Bulletin (#161) on external cephalic version outlining clinical considerations and recommendations and providing an algorithm for patient management.1 We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: According to the Practice Bulletin, which of the following is TRUE about external cephalic version (ECV)?
A. Success rate is lower in women with a previous cesarean delivery.
B. Placental location affects the success rate.
C. External cephalic version should be stopped after 15 minutes.
D. Women at 37 weeks’ gestation are preferred candidates.
E. Tocolysis decreases success rate.
The correct answer is D.
Women at 37 weeks’ gestation are the preferred candidates for an ECV because spontaneous version would likely have already occurred by this time and the risk of spontaneous reversion is lower. Answers A-C and E are incorrect statements.
Key points
Women at 37 weeks’ gestation are the preferred candidates for an ECV.
The overall pooled success rate for ECV is 58% with a 6% pooled complication rate.
The use of parenteral tocolysis has been associated with increased success rates of ECV, though there are not enough data to make a recommendation regarding use of regional anesthesia with the procedure.
Literature summary
ECV is a procedure designed to turn a fetus into vertex presentation by applying external pressure to a woman’s abdomen. Women at 37 weeks’ gestation are the preferred candidates. At this gestational age, spontaneous version is most likely to have occurred, and there is decreased risk of spontaneous reversion after the ECV. All patients who are near term and found to have a fetus in a nonvertex presentation should be offered an ECV as long as there are no contraindications to the procedure. ECV is not appropriate for women who have a contraindication to a vaginal delivery.
There are limited studies of ECV in women who undergo the procedure in early labor and in those who have had a previous uterine surgery. ECV success rates are not affected by a previous cesarean delivery, though the risks of uterine rupture are not clear. The procedure should be attempted only in settings where cesarean delivery services are immediately available.
The success rates of ECV have been reported to be anywhere from 16% to 100%, with an overall pooled success of 58% with a 6% pooled complication rate. Some studies have documented higher success rates with higher parity and a transverse or oblique fetal lie. However, placental location, maternal weight, and amniotic fluid volume have not been consistently found to be predictive of ECV success. The use of parenteral tocolysis has been associated with increased success rates of ECV, though there are not enough data to make a recommendation regarding use of regional anesthesia with the procedure.
ECV should be stopped in the face of a prolonged or significant fetal bradycardia or if the patient is experiencing intolerable levels of discomfort. However, there are no guidelines to recommend the total time limit of the procedure. After the ECV, there should be fetal heart rate monitoring for at least 30 minutes and anti-D immune globulin should be administered to those women who are Rh-negative if delivery is not anticipated in the next 72 hours.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Treating gonococcal infections
Editor’s Note: This is the third installment of a six-part monthly series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips.
Management of sexually transmitted infections will always be tested on any ob.gyn. examination. Since the American College of Obstetricians and Gynecologists issued two new committee opinions in 2015 focusing on the dual treatment for gonococcal infections and expedited partner therapy in the management of gonorrhea and chlamydial infection, it is wise to review this topic.1,2
A. Ciprofloxacin
B. Ciprofloxacin plus azithromycin
C. Cefixime plus azithromycin
D. Doxycycline plus azithromycin
E. Gentamicin plus azithromycin
The correct answer is E.
Dual therapy with gentamicin (240 mg single-intramuscular dose) and azithromycin (2 grams single-oral dose) is the treatment of choice for pregnant patients with severe penicillin or cephalosporin allergy. Gentamicin is safe during pregnancy. An alternative regimen is a single dose of azithromycin, if the patient is allergic to gentamicin and cephalosporin. For this patient, the physician needs to perform a test-of-cure 1 week after treatment.
Answer A is incorrect because neither doxycycline nor quinolones are recommended during pregnancy.
Answer B and C are incorrect because the patient is allergic to cephalosporin (ceftriaxone and cefixime).
Answer D in incorrect because neither doxycycline nor quinolones are recommended during pregnancy.
Key points
The key points to remember are:
- Ceftriaxone and azithromycin must be started on the same day.
- In patients with HIV, the treatment is same: Ceftriaxone 250-mg single-intramuscular dose plus azithromycin 1-g single-oral dose.
- The Centers for Disease Control and Prevention and state health departments will periodically update the most current information on gonococcal susceptibility.
- Despite nucleic acid amplification test (NAAT) being negative for Chlamydia trachomatis, dual treatment with a cephalosporin plus either azithromycin or doxycycline is recommended by ACOG to prevent resistance to cephalosporins.
- ACOG does not recommend test-of-cure after treatment of uncomplicated gonorrhea.
- ACOG does not recommend test-of-cure after treatment of pregnant patients.
- ACOG recommends test-of-cure for pharyngeal gonorrhea only if the patient received an alternative regimen treatment after 14 days.
- ACOG accepts both culture or NAAT as a test-of-cure.
- Cefixime is not a first-line regimen treatment for gonorrhea and dual therapy with ceftriaxone and azithromycin is the only recommended first-line regimen.
Literature summary
The guidelines from the ACOG Committee on Gynecologic Practice outline recommended antibiotic treatment regimens, considerations when treating special populations, and advice for expedited therapy for sexual partners.
- The first-line regimen is ceftriaxone 250-mg, single-intramuscular dose plus azithromycin 1-g, single-oral dose.
- The second-line regimen takes into account allergies and shortages. In the case of a severe penicillin allergy, use gemifloxacin 320-mg, single-oral dose plus azithromycin 2-g, single-oral dose. Another option is gentamicin, 240-mg, single-intramuscular dose plus azithromycin 2-g, single-oral dose. When ceftriaxone is not available, ACOG recommends cefixime, 400-mg, single-oral dose plus azithromycin 1-g, single-oral dose.
- In pregnancy, ACOG recommends the same treatment, with no need for test-of-cure if treated properly. For severe penicillin or cephalosporin allergy in pregnancy, use gentamicin 240-mg, single-intramuscular dose plus azithromycin 2-g, single-oral dose. For allergy to gentamicin and cephalosporins, use azithromycin 2-g single-oral dose and send a test-of-cure in 1 week. Do not use doxycycline or quinolones during pregnancy.
- In HIV infection, use the general recommended treatment regimen and there is no need for a test-of-cure if treated properly.
- For sex partners, ACOG recommends evaluation and treatment for partners in the last 60 days. They should abstain from sex for 7 days after treatment. Expedited partner therapy with cefixime and azithromycin is recommended, but review the legal the status of expedited partner therapy in your state before prescribing.
References
1. Obstet Gynecol. 2015 Nov;126(5):e95-9.
2. Obstet Gynecol. 2015 Jun;125(6):1526-8.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Editor’s Note: This is the third installment of a six-part monthly series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips.
Management of sexually transmitted infections will always be tested on any ob.gyn. examination. Since the American College of Obstetricians and Gynecologists issued two new committee opinions in 2015 focusing on the dual treatment for gonococcal infections and expedited partner therapy in the management of gonorrhea and chlamydial infection, it is wise to review this topic.1,2
A. Ciprofloxacin
B. Ciprofloxacin plus azithromycin
C. Cefixime plus azithromycin
D. Doxycycline plus azithromycin
E. Gentamicin plus azithromycin
The correct answer is E.
Dual therapy with gentamicin (240 mg single-intramuscular dose) and azithromycin (2 grams single-oral dose) is the treatment of choice for pregnant patients with severe penicillin or cephalosporin allergy. Gentamicin is safe during pregnancy. An alternative regimen is a single dose of azithromycin, if the patient is allergic to gentamicin and cephalosporin. For this patient, the physician needs to perform a test-of-cure 1 week after treatment.
Answer A is incorrect because neither doxycycline nor quinolones are recommended during pregnancy.
Answer B and C are incorrect because the patient is allergic to cephalosporin (ceftriaxone and cefixime).
Answer D in incorrect because neither doxycycline nor quinolones are recommended during pregnancy.
Key points
The key points to remember are:
- Ceftriaxone and azithromycin must be started on the same day.
- In patients with HIV, the treatment is same: Ceftriaxone 250-mg single-intramuscular dose plus azithromycin 1-g single-oral dose.
- The Centers for Disease Control and Prevention and state health departments will periodically update the most current information on gonococcal susceptibility.
- Despite nucleic acid amplification test (NAAT) being negative for Chlamydia trachomatis, dual treatment with a cephalosporin plus either azithromycin or doxycycline is recommended by ACOG to prevent resistance to cephalosporins.
- ACOG does not recommend test-of-cure after treatment of uncomplicated gonorrhea.
- ACOG does not recommend test-of-cure after treatment of pregnant patients.
- ACOG recommends test-of-cure for pharyngeal gonorrhea only if the patient received an alternative regimen treatment after 14 days.
- ACOG accepts both culture or NAAT as a test-of-cure.
- Cefixime is not a first-line regimen treatment for gonorrhea and dual therapy with ceftriaxone and azithromycin is the only recommended first-line regimen.
Literature summary
The guidelines from the ACOG Committee on Gynecologic Practice outline recommended antibiotic treatment regimens, considerations when treating special populations, and advice for expedited therapy for sexual partners.
- The first-line regimen is ceftriaxone 250-mg, single-intramuscular dose plus azithromycin 1-g, single-oral dose.
- The second-line regimen takes into account allergies and shortages. In the case of a severe penicillin allergy, use gemifloxacin 320-mg, single-oral dose plus azithromycin 2-g, single-oral dose. Another option is gentamicin, 240-mg, single-intramuscular dose plus azithromycin 2-g, single-oral dose. When ceftriaxone is not available, ACOG recommends cefixime, 400-mg, single-oral dose plus azithromycin 1-g, single-oral dose.
- In pregnancy, ACOG recommends the same treatment, with no need for test-of-cure if treated properly. For severe penicillin or cephalosporin allergy in pregnancy, use gentamicin 240-mg, single-intramuscular dose plus azithromycin 2-g, single-oral dose. For allergy to gentamicin and cephalosporins, use azithromycin 2-g single-oral dose and send a test-of-cure in 1 week. Do not use doxycycline or quinolones during pregnancy.
- In HIV infection, use the general recommended treatment regimen and there is no need for a test-of-cure if treated properly.
- For sex partners, ACOG recommends evaluation and treatment for partners in the last 60 days. They should abstain from sex for 7 days after treatment. Expedited partner therapy with cefixime and azithromycin is recommended, but review the legal the status of expedited partner therapy in your state before prescribing.
References
1. Obstet Gynecol. 2015 Nov;126(5):e95-9.
2. Obstet Gynecol. 2015 Jun;125(6):1526-8.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Editor’s Note: This is the third installment of a six-part monthly series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips.
Management of sexually transmitted infections will always be tested on any ob.gyn. examination. Since the American College of Obstetricians and Gynecologists issued two new committee opinions in 2015 focusing on the dual treatment for gonococcal infections and expedited partner therapy in the management of gonorrhea and chlamydial infection, it is wise to review this topic.1,2
A. Ciprofloxacin
B. Ciprofloxacin plus azithromycin
C. Cefixime plus azithromycin
D. Doxycycline plus azithromycin
E. Gentamicin plus azithromycin
The correct answer is E.
Dual therapy with gentamicin (240 mg single-intramuscular dose) and azithromycin (2 grams single-oral dose) is the treatment of choice for pregnant patients with severe penicillin or cephalosporin allergy. Gentamicin is safe during pregnancy. An alternative regimen is a single dose of azithromycin, if the patient is allergic to gentamicin and cephalosporin. For this patient, the physician needs to perform a test-of-cure 1 week after treatment.
Answer A is incorrect because neither doxycycline nor quinolones are recommended during pregnancy.
Answer B and C are incorrect because the patient is allergic to cephalosporin (ceftriaxone and cefixime).
Answer D in incorrect because neither doxycycline nor quinolones are recommended during pregnancy.
Key points
The key points to remember are:
- Ceftriaxone and azithromycin must be started on the same day.
- In patients with HIV, the treatment is same: Ceftriaxone 250-mg single-intramuscular dose plus azithromycin 1-g single-oral dose.
- The Centers for Disease Control and Prevention and state health departments will periodically update the most current information on gonococcal susceptibility.
- Despite nucleic acid amplification test (NAAT) being negative for Chlamydia trachomatis, dual treatment with a cephalosporin plus either azithromycin or doxycycline is recommended by ACOG to prevent resistance to cephalosporins.
- ACOG does not recommend test-of-cure after treatment of uncomplicated gonorrhea.
- ACOG does not recommend test-of-cure after treatment of pregnant patients.
- ACOG recommends test-of-cure for pharyngeal gonorrhea only if the patient received an alternative regimen treatment after 14 days.
- ACOG accepts both culture or NAAT as a test-of-cure.
- Cefixime is not a first-line regimen treatment for gonorrhea and dual therapy with ceftriaxone and azithromycin is the only recommended first-line regimen.
Literature summary
The guidelines from the ACOG Committee on Gynecologic Practice outline recommended antibiotic treatment regimens, considerations when treating special populations, and advice for expedited therapy for sexual partners.
- The first-line regimen is ceftriaxone 250-mg, single-intramuscular dose plus azithromycin 1-g, single-oral dose.
- The second-line regimen takes into account allergies and shortages. In the case of a severe penicillin allergy, use gemifloxacin 320-mg, single-oral dose plus azithromycin 2-g, single-oral dose. Another option is gentamicin, 240-mg, single-intramuscular dose plus azithromycin 2-g, single-oral dose. When ceftriaxone is not available, ACOG recommends cefixime, 400-mg, single-oral dose plus azithromycin 1-g, single-oral dose.
- In pregnancy, ACOG recommends the same treatment, with no need for test-of-cure if treated properly. For severe penicillin or cephalosporin allergy in pregnancy, use gentamicin 240-mg, single-intramuscular dose plus azithromycin 2-g, single-oral dose. For allergy to gentamicin and cephalosporins, use azithromycin 2-g single-oral dose and send a test-of-cure in 1 week. Do not use doxycycline or quinolones during pregnancy.
- In HIV infection, use the general recommended treatment regimen and there is no need for a test-of-cure if treated properly.
- For sex partners, ACOG recommends evaluation and treatment for partners in the last 60 days. They should abstain from sex for 7 days after treatment. Expedited partner therapy with cefixime and azithromycin is recommended, but review the legal the status of expedited partner therapy in your state before prescribing.
References
1. Obstet Gynecol. 2015 Nov;126(5):e95-9.
2. Obstet Gynecol. 2015 Jun;125(6):1526-8.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Zika virus in pregnancy
Editor’s Note: This is the second installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips. This month’s edition of the Board Corner focuses on Zika virus.
In 2016, The American Board of Obstetricians and Gynecologists assigned four maintenance of certification (MOC) articles dealing with Zika virus:
Zika Virus and Pregnancy: What Obstetric Health Care Providers Need to Know1
Update: Interim Guidance for Health Care Providers Caring for Women of Reproductive Age with Possible Zika Virus Exposure – United States, 20163
Interim Guidelines for Pregnant Women During a Zika Virus Outbreak – United States, 20164
The diagnosis and management of Zika virus in pregnancy is a hot topic that should be reviewed before any Obstetrics and Gynecology board exam. This month’s Board Corner will review the key elements for ob.gyns.
Let’s begin with a possible medical board question: Which of the following is LEAST LIKELY to be associated with congenital Zika virus infection?
A. Omphalocele
B. Microcephaly
C. Ventriculomegaly
D. Cataract
E. Intracranial calcifications
The correct answer is A.
Omphalocele has not been observed to be associated with Zika virus infection. Answers B-E are incorrect because reported fetal and neonatal abnormalities associated with Zika virus include microcephaly, ventriculomegaly, intracranial calcifications, brain atrophy, and cataracts.
Key points
The key points to remember are:
Transmission of Zika virus to humans most commonly occurs from the bite of an infected mosquito. Other modes of transmission have been reported, such as sexual transmission, maternal-fetal transmission, and transmission through blood transfusion.
Infection with Zika virus and maternal-fetal transmission have been documented in all trimesters of pregnancy. Reported fetal and neonatal sequelae include microcephaly, ventriculomegaly, intracranial calcifications, brain atrophy, and cataracts.
Immunoglobulin M (IgM) can also be detected as early as 4 days after the onset of illness. For those women who have laboratory-confirmed Zika virus infection during pregnancy, amniocentesis should be offered after 15 weeks gestation.
Literature summary
Zika virus is a mosquito-borne virus that is most commonly transmitted by the Aedes aegypti mosquito. This species of mosquito is also known to transmit dengue, yellow fever, and chikungunya viruses. Initial data suggest that the incubation period for Zika virus is a few days up to 2 weeks. Acute onset of fever, maculopapular rash, conjunctivitis, and arthralgia are the most common symptoms of Zika infection. Symptoms generally only last up to 7 days and are typically mild. Only about 20% of people infected with the virus become ill. There is currently no specific antiviral treatment available for Zika virus disease.
Testing for Zika virus can include detection of viral RNA, Zika antigen, or antibodies. Reverse transcription–polymerase chain reaction (RT-PCR) can be performed on serum, amniotic fluid, and tissue (such as placenta). It is recommended that RT-PCR testing of serum be performed within 7 days of the onset of symptoms. Immunoglobulin M (IgM) can also be detected as early as 4 days after the onset of illness.
Though fetal microcephaly can be detected ultrasonographically at 18-20 weeks gestation, the diagnosis can be challenging because there is no standard definition of fetal microcephaly. Because other intracranial abnormalities have been detected in association with congenital Zika infection, a complete ultrasound examination should be performed to assess the fetal neuroanatomy.
For those women who have laboratory-confirmed Zika virus infection during pregnancy, amniocentesis should be offered after 15 weeks gestation. RT-PCR can be used to test for the presence of Zika virus RNA in the amniotic fluid. Patients should be referred to a maternal-fetal medicine specialist for serial ultrasounds to assess fetal anatomy and monitor fetal growth every 3-4 weeks.
References
1. Obstet Gynecol. 2016 Apr;127(4):642-8.
2. Morb Mortal Wkly Rep. 2016;65:311-4.
3. Morb Mortal Wkly Rep. 2016;65:315-22.
4. Morb Mortal Wkly Rep. 2016;65:30-3.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Editor’s Note: This is the second installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips. This month’s edition of the Board Corner focuses on Zika virus.
In 2016, The American Board of Obstetricians and Gynecologists assigned four maintenance of certification (MOC) articles dealing with Zika virus:
Zika Virus and Pregnancy: What Obstetric Health Care Providers Need to Know1
Update: Interim Guidance for Health Care Providers Caring for Women of Reproductive Age with Possible Zika Virus Exposure – United States, 20163
Interim Guidelines for Pregnant Women During a Zika Virus Outbreak – United States, 20164
The diagnosis and management of Zika virus in pregnancy is a hot topic that should be reviewed before any Obstetrics and Gynecology board exam. This month’s Board Corner will review the key elements for ob.gyns.
Let’s begin with a possible medical board question: Which of the following is LEAST LIKELY to be associated with congenital Zika virus infection?
A. Omphalocele
B. Microcephaly
C. Ventriculomegaly
D. Cataract
E. Intracranial calcifications
The correct answer is A.
Omphalocele has not been observed to be associated with Zika virus infection. Answers B-E are incorrect because reported fetal and neonatal abnormalities associated with Zika virus include microcephaly, ventriculomegaly, intracranial calcifications, brain atrophy, and cataracts.
Key points
The key points to remember are:
Transmission of Zika virus to humans most commonly occurs from the bite of an infected mosquito. Other modes of transmission have been reported, such as sexual transmission, maternal-fetal transmission, and transmission through blood transfusion.
Infection with Zika virus and maternal-fetal transmission have been documented in all trimesters of pregnancy. Reported fetal and neonatal sequelae include microcephaly, ventriculomegaly, intracranial calcifications, brain atrophy, and cataracts.
Immunoglobulin M (IgM) can also be detected as early as 4 days after the onset of illness. For those women who have laboratory-confirmed Zika virus infection during pregnancy, amniocentesis should be offered after 15 weeks gestation.
Literature summary
Zika virus is a mosquito-borne virus that is most commonly transmitted by the Aedes aegypti mosquito. This species of mosquito is also known to transmit dengue, yellow fever, and chikungunya viruses. Initial data suggest that the incubation period for Zika virus is a few days up to 2 weeks. Acute onset of fever, maculopapular rash, conjunctivitis, and arthralgia are the most common symptoms of Zika infection. Symptoms generally only last up to 7 days and are typically mild. Only about 20% of people infected with the virus become ill. There is currently no specific antiviral treatment available for Zika virus disease.
Testing for Zika virus can include detection of viral RNA, Zika antigen, or antibodies. Reverse transcription–polymerase chain reaction (RT-PCR) can be performed on serum, amniotic fluid, and tissue (such as placenta). It is recommended that RT-PCR testing of serum be performed within 7 days of the onset of symptoms. Immunoglobulin M (IgM) can also be detected as early as 4 days after the onset of illness.
Though fetal microcephaly can be detected ultrasonographically at 18-20 weeks gestation, the diagnosis can be challenging because there is no standard definition of fetal microcephaly. Because other intracranial abnormalities have been detected in association with congenital Zika infection, a complete ultrasound examination should be performed to assess the fetal neuroanatomy.
For those women who have laboratory-confirmed Zika virus infection during pregnancy, amniocentesis should be offered after 15 weeks gestation. RT-PCR can be used to test for the presence of Zika virus RNA in the amniotic fluid. Patients should be referred to a maternal-fetal medicine specialist for serial ultrasounds to assess fetal anatomy and monitor fetal growth every 3-4 weeks.
References
1. Obstet Gynecol. 2016 Apr;127(4):642-8.
2. Morb Mortal Wkly Rep. 2016;65:311-4.
3. Morb Mortal Wkly Rep. 2016;65:315-22.
4. Morb Mortal Wkly Rep. 2016;65:30-3.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Editor’s Note: This is the second installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips. This month’s edition of the Board Corner focuses on Zika virus.
In 2016, The American Board of Obstetricians and Gynecologists assigned four maintenance of certification (MOC) articles dealing with Zika virus:
Zika Virus and Pregnancy: What Obstetric Health Care Providers Need to Know1
Update: Interim Guidance for Health Care Providers Caring for Women of Reproductive Age with Possible Zika Virus Exposure – United States, 20163
Interim Guidelines for Pregnant Women During a Zika Virus Outbreak – United States, 20164
The diagnosis and management of Zika virus in pregnancy is a hot topic that should be reviewed before any Obstetrics and Gynecology board exam. This month’s Board Corner will review the key elements for ob.gyns.
Let’s begin with a possible medical board question: Which of the following is LEAST LIKELY to be associated with congenital Zika virus infection?
A. Omphalocele
B. Microcephaly
C. Ventriculomegaly
D. Cataract
E. Intracranial calcifications
The correct answer is A.
Omphalocele has not been observed to be associated with Zika virus infection. Answers B-E are incorrect because reported fetal and neonatal abnormalities associated with Zika virus include microcephaly, ventriculomegaly, intracranial calcifications, brain atrophy, and cataracts.
Key points
The key points to remember are:
Transmission of Zika virus to humans most commonly occurs from the bite of an infected mosquito. Other modes of transmission have been reported, such as sexual transmission, maternal-fetal transmission, and transmission through blood transfusion.
Infection with Zika virus and maternal-fetal transmission have been documented in all trimesters of pregnancy. Reported fetal and neonatal sequelae include microcephaly, ventriculomegaly, intracranial calcifications, brain atrophy, and cataracts.
Immunoglobulin M (IgM) can also be detected as early as 4 days after the onset of illness. For those women who have laboratory-confirmed Zika virus infection during pregnancy, amniocentesis should be offered after 15 weeks gestation.
Literature summary
Zika virus is a mosquito-borne virus that is most commonly transmitted by the Aedes aegypti mosquito. This species of mosquito is also known to transmit dengue, yellow fever, and chikungunya viruses. Initial data suggest that the incubation period for Zika virus is a few days up to 2 weeks. Acute onset of fever, maculopapular rash, conjunctivitis, and arthralgia are the most common symptoms of Zika infection. Symptoms generally only last up to 7 days and are typically mild. Only about 20% of people infected with the virus become ill. There is currently no specific antiviral treatment available for Zika virus disease.
Testing for Zika virus can include detection of viral RNA, Zika antigen, or antibodies. Reverse transcription–polymerase chain reaction (RT-PCR) can be performed on serum, amniotic fluid, and tissue (such as placenta). It is recommended that RT-PCR testing of serum be performed within 7 days of the onset of symptoms. Immunoglobulin M (IgM) can also be detected as early as 4 days after the onset of illness.
Though fetal microcephaly can be detected ultrasonographically at 18-20 weeks gestation, the diagnosis can be challenging because there is no standard definition of fetal microcephaly. Because other intracranial abnormalities have been detected in association with congenital Zika infection, a complete ultrasound examination should be performed to assess the fetal neuroanatomy.
For those women who have laboratory-confirmed Zika virus infection during pregnancy, amniocentesis should be offered after 15 weeks gestation. RT-PCR can be used to test for the presence of Zika virus RNA in the amniotic fluid. Patients should be referred to a maternal-fetal medicine specialist for serial ultrasounds to assess fetal anatomy and monitor fetal growth every 3-4 weeks.
References
1. Obstet Gynecol. 2016 Apr;127(4):642-8.
2. Morb Mortal Wkly Rep. 2016;65:311-4.
3. Morb Mortal Wkly Rep. 2016;65:315-22.
4. Morb Mortal Wkly Rep. 2016;65:30-3.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Urinary incontinence
Editor’s Note: This is the first installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover key topics across the specialty, as well as general test-taking and study tips. This month’s edition of the Board Exam Corner focuses on urinary incontinence.
The literature
The American College of Obstetricians and Gynecologists and the American Urogynecologic Society recently published an updated Practice Bulletin regarding the diagnosis and management of urinary incontinence. Practice Bulletin No. 155 was also a maintenance of certification (MOC) assignment for 2016. The contents of this article are critical as urinary incontinence is a prevalent and treatable condition, and it will likely be part of this year’s board examination. This month’s Board Corner will review the key elements of the updated guidelines.
Let’s begin with a possible medical board question: An 80-year old patient has urinary leakage as a result of severe arthritis and poor vision, and is unable to reach the bathroom in time. What is the diagnosis?
A. Mixed urinary incontinence
B. Extraurethral incontinence
C. Functional incontinence
D. Postural incontinence
E. Ectopic ureter
The answer is C.
Functional incontinence occurs because of cognitive or mobility impairments in the presence of an intact lower urinary tract. The patient in the vignette has “restricted mobility” and a physical impairment that leads to urinary incontinence.
A is incorrect because mixed incontinence is an involuntary loss of urine associated with urgency and with physical exertion.
B and E are incorrect because extraurethral incontinence is urine leakage through channels other than the urethral meatus (e.g., vesicovaginal fistula or ectopic ureter).
D is incorrect because postural incontinence is the involuntary loss of urine associated with change in body positions (e.g., from sitting to standing).
The key points to remember are:
• Urinary incontinence is a prevalent condition that is treatable despite what many women believe.
• There are several types of incontinence but the main types are stress, urgency, and mixed incontinence.
• Basic office evaluation includes urinalysis and postvoid residual, but not urodynamics or cystoscopy.
• Treatment of stress incontinence includes weight loss, fluid management, pelvic floor muscle exercises (with or without assistance from a physical therapist), and suburethral sling or bulking agent injections.
• Treatment of urgency urinary incontinence includes bladder training, fluid management, decreasing caffeine, antimuscarinic and beta-3 agonists, and third-line treatments (botulinum toxin A injection, sacral neuromodulation, or tibial nerve stimulation).
• The mnemonic DIAPPERS is useful in remembering all of the reversible, nongenitourinary (i.e., functional) causes of incontinence. 
Test-taking tip of the month
If you finish the test early, take the time to go back to any question for which you made an educated guess. Reread the question carefully. Don’t change your answer unless there is a very good reason for doing so because your first impression is usually the right one. Don’t change an answer just because you have a gut feeling. However, there are good reasons to change your answers when you go back. For instance, consider changing your answer if rereading the question helped you to better understand what is being asked, if you reconsidered and conceptualized a better answer, if you remembered a new piece of information, or if you learned something from a later question that will help you better answer the earlier question.
Reference
Obstet Gynecol. 2015 Nov;126(5): e66-81.
Dr. Sam Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Editor’s Note: This is the first installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover key topics across the specialty, as well as general test-taking and study tips. This month’s edition of the Board Exam Corner focuses on urinary incontinence.
The literature
The American College of Obstetricians and Gynecologists and the American Urogynecologic Society recently published an updated Practice Bulletin regarding the diagnosis and management of urinary incontinence. Practice Bulletin No. 155 was also a maintenance of certification (MOC) assignment for 2016. The contents of this article are critical as urinary incontinence is a prevalent and treatable condition, and it will likely be part of this year’s board examination. This month’s Board Corner will review the key elements of the updated guidelines.
Let’s begin with a possible medical board question: An 80-year old patient has urinary leakage as a result of severe arthritis and poor vision, and is unable to reach the bathroom in time. What is the diagnosis?
A. Mixed urinary incontinence
B. Extraurethral incontinence
C. Functional incontinence
D. Postural incontinence
E. Ectopic ureter
The answer is C.
Functional incontinence occurs because of cognitive or mobility impairments in the presence of an intact lower urinary tract. The patient in the vignette has “restricted mobility” and a physical impairment that leads to urinary incontinence.
A is incorrect because mixed incontinence is an involuntary loss of urine associated with urgency and with physical exertion.
B and E are incorrect because extraurethral incontinence is urine leakage through channels other than the urethral meatus (e.g., vesicovaginal fistula or ectopic ureter).
D is incorrect because postural incontinence is the involuntary loss of urine associated with change in body positions (e.g., from sitting to standing).
The key points to remember are:
• Urinary incontinence is a prevalent condition that is treatable despite what many women believe.
• There are several types of incontinence but the main types are stress, urgency, and mixed incontinence.
• Basic office evaluation includes urinalysis and postvoid residual, but not urodynamics or cystoscopy.
• Treatment of stress incontinence includes weight loss, fluid management, pelvic floor muscle exercises (with or without assistance from a physical therapist), and suburethral sling or bulking agent injections.
• Treatment of urgency urinary incontinence includes bladder training, fluid management, decreasing caffeine, antimuscarinic and beta-3 agonists, and third-line treatments (botulinum toxin A injection, sacral neuromodulation, or tibial nerve stimulation).
• The mnemonic DIAPPERS is useful in remembering all of the reversible, nongenitourinary (i.e., functional) causes of incontinence. 
Test-taking tip of the month
If you finish the test early, take the time to go back to any question for which you made an educated guess. Reread the question carefully. Don’t change your answer unless there is a very good reason for doing so because your first impression is usually the right one. Don’t change an answer just because you have a gut feeling. However, there are good reasons to change your answers when you go back. For instance, consider changing your answer if rereading the question helped you to better understand what is being asked, if you reconsidered and conceptualized a better answer, if you remembered a new piece of information, or if you learned something from a later question that will help you better answer the earlier question.
Reference
Obstet Gynecol. 2015 Nov;126(5): e66-81.
Dr. Sam Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Editor’s Note: This is the first installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover key topics across the specialty, as well as general test-taking and study tips. This month’s edition of the Board Exam Corner focuses on urinary incontinence.
The literature
The American College of Obstetricians and Gynecologists and the American Urogynecologic Society recently published an updated Practice Bulletin regarding the diagnosis and management of urinary incontinence. Practice Bulletin No. 155 was also a maintenance of certification (MOC) assignment for 2016. The contents of this article are critical as urinary incontinence is a prevalent and treatable condition, and it will likely be part of this year’s board examination. This month’s Board Corner will review the key elements of the updated guidelines.
Let’s begin with a possible medical board question: An 80-year old patient has urinary leakage as a result of severe arthritis and poor vision, and is unable to reach the bathroom in time. What is the diagnosis?
A. Mixed urinary incontinence
B. Extraurethral incontinence
C. Functional incontinence
D. Postural incontinence
E. Ectopic ureter
The answer is C.
Functional incontinence occurs because of cognitive or mobility impairments in the presence of an intact lower urinary tract. The patient in the vignette has “restricted mobility” and a physical impairment that leads to urinary incontinence.
A is incorrect because mixed incontinence is an involuntary loss of urine associated with urgency and with physical exertion.
B and E are incorrect because extraurethral incontinence is urine leakage through channels other than the urethral meatus (e.g., vesicovaginal fistula or ectopic ureter).
D is incorrect because postural incontinence is the involuntary loss of urine associated with change in body positions (e.g., from sitting to standing).
The key points to remember are:
• Urinary incontinence is a prevalent condition that is treatable despite what many women believe.
• There are several types of incontinence but the main types are stress, urgency, and mixed incontinence.
• Basic office evaluation includes urinalysis and postvoid residual, but not urodynamics or cystoscopy.
• Treatment of stress incontinence includes weight loss, fluid management, pelvic floor muscle exercises (with or without assistance from a physical therapist), and suburethral sling or bulking agent injections.
• Treatment of urgency urinary incontinence includes bladder training, fluid management, decreasing caffeine, antimuscarinic and beta-3 agonists, and third-line treatments (botulinum toxin A injection, sacral neuromodulation, or tibial nerve stimulation).
• The mnemonic DIAPPERS is useful in remembering all of the reversible, nongenitourinary (i.e., functional) causes of incontinence. 
Test-taking tip of the month
If you finish the test early, take the time to go back to any question for which you made an educated guess. Reread the question carefully. Don’t change your answer unless there is a very good reason for doing so because your first impression is usually the right one. Don’t change an answer just because you have a gut feeling. However, there are good reasons to change your answers when you go back. For instance, consider changing your answer if rereading the question helped you to better understand what is being asked, if you reconsidered and conceptualized a better answer, if you remembered a new piece of information, or if you learned something from a later question that will help you better answer the earlier question.
Reference
Obstet Gynecol. 2015 Nov;126(5): e66-81.
Dr. Sam Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.