User login
Asthma: Newer Tx options mean more targeted therapy
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include
The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
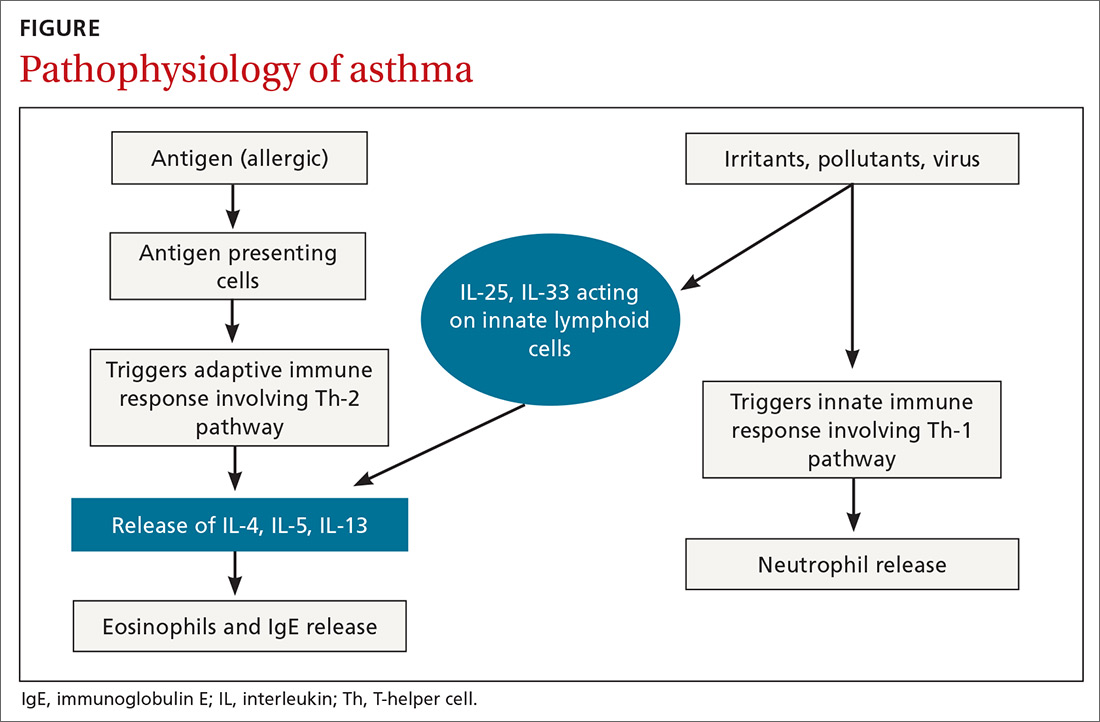
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4
In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
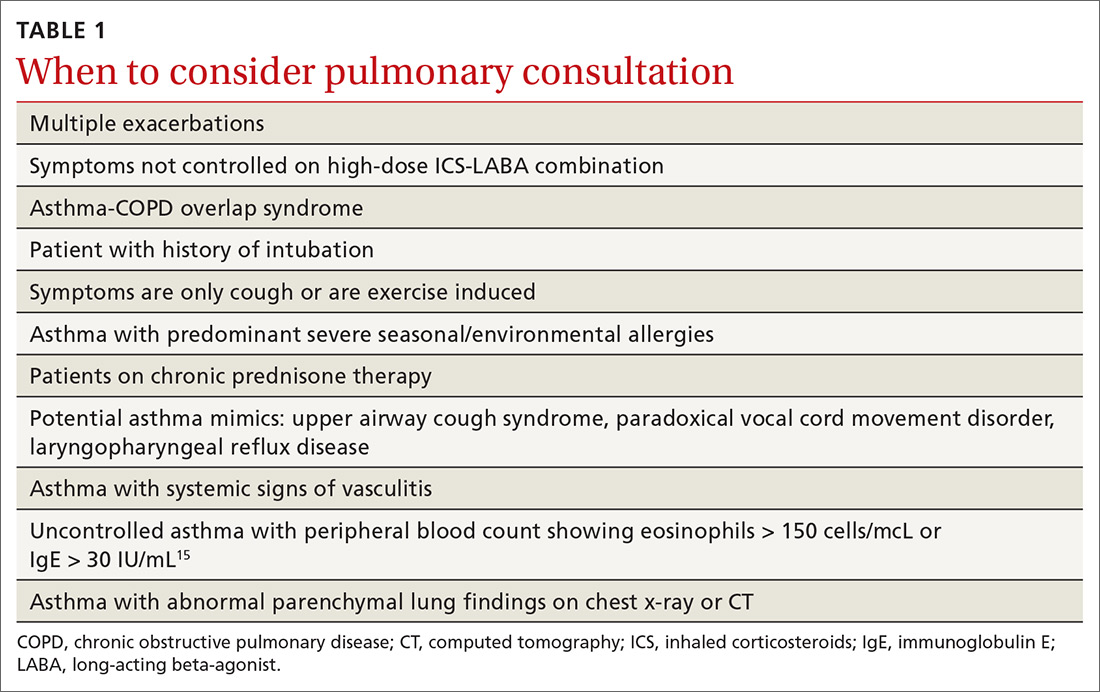
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
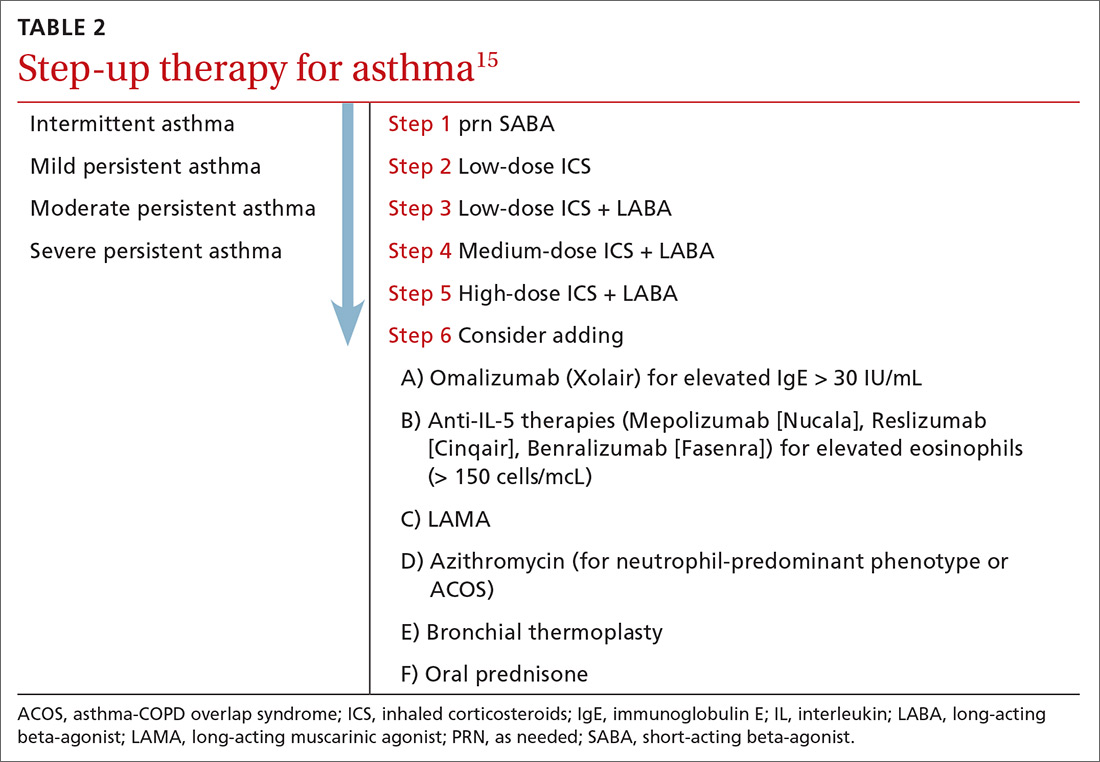
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.
Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
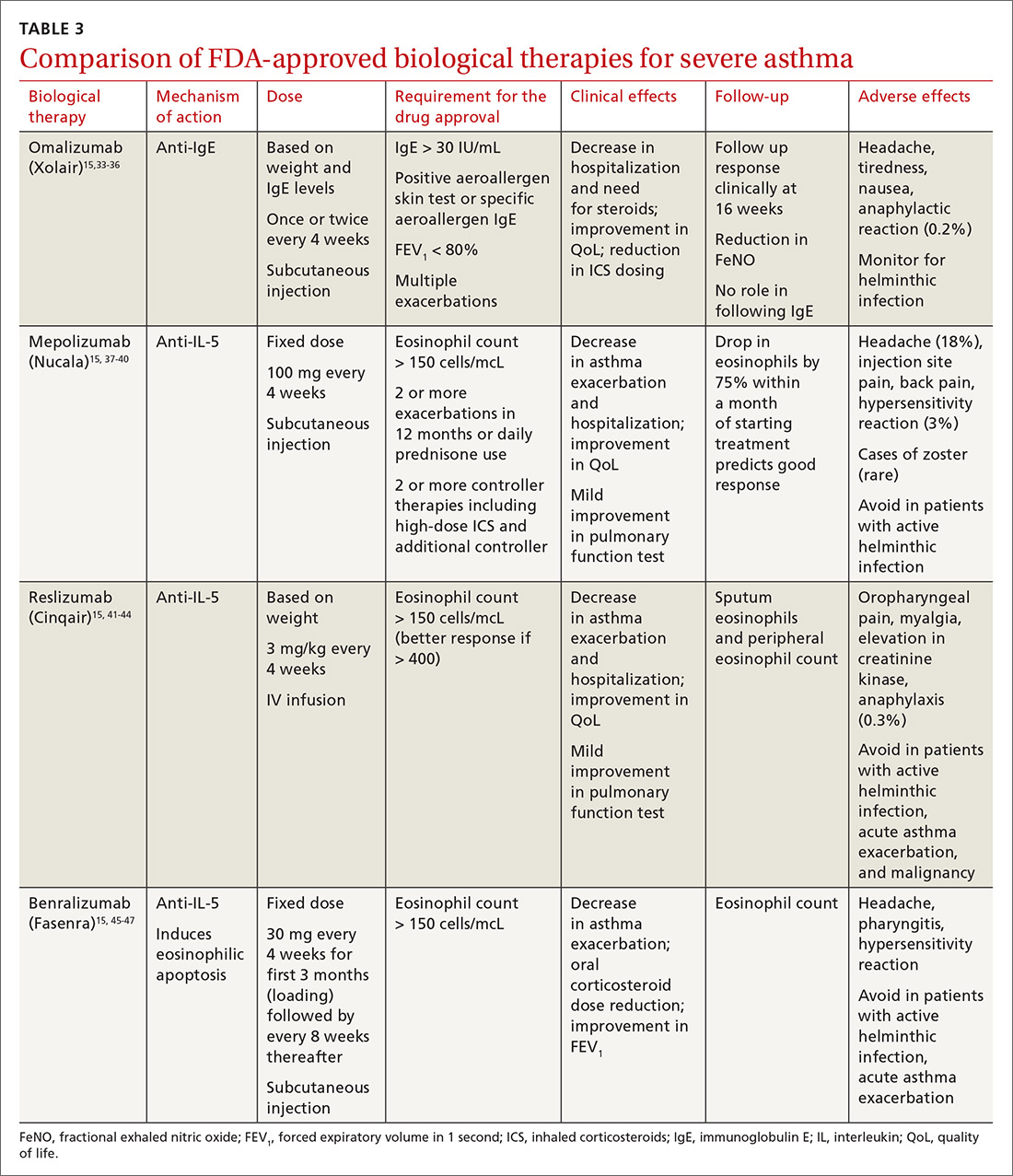
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.
Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; mrali@northwell.edu
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include
The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4
In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.
Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.
Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; mrali@northwell.edu
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include
The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4
In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.
Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.
Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; mrali@northwell.edu
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
PRACTICE RECOMMENDATIONS
› Consider inhaled corticosteroids (ICS) as your first choice for a long-term control agent to treat asthma; add a long-acting beta agonist (LABA) when needed. A
› Use long-acting muscarinic antagonists (LAMA) as add-on therapy for patients whose asthma is uncontrolled despite the use of low-dose ICS-LABA, or as an alternative to high-dose ICS-LABA. A
› Consider biological therapies for patients with asthma exacerbations that require steroids at least twice a year. B
› Use azithromycin as an add-on therapy to ICS-LABA for a select group of patients with uncontrolled persistent asthma (neutrophilic phenotype). C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Tips for self-care during the COVID-19 crisis
I think it’s fair to say, none of us have seen anything like this before. Yet here we are, and we must lead. We are many weeks into the COVID-19 crisis. We moved our offices home and tried not to miss a beat. Our patients need us more than ever – and in different ways.
Lest we become like the shoemaker’s daughter who has no shoes, let’s make sure we take care of ourselves. The shock waves from this pandemic are going to be massive and long lasting. I am already witnessing massive psychological growth on the part of my patients, and I hope, myself and my family. We must be strong as individuals and as a group of professionals.
Now more than ever, we need to set boundaries. So many are suffering. We must take stock of our own lives. Many of us are extremely fortunate. We have homes, families, and plenty of food. We are doctors performing essential services, and we can do so without risking our lives.
The priority is to make sure you are safe, and keeping your family and loved ones safe. As physicians, we have learned to distance ourselves from illness, but the coronavirus has affected us in disproportionate numbers.
To be physically and mentally strong, we must get enough sleep. This is exhausting for some and energizing for others. It is definitely a marathon not a sprint, so pace yourself. Eat well. This is no time for empty calories, and that goes for alcohol as well.
Create new routines. Exercise at the same time each day or perhaps twice a day. Try to be productive during certain hours, and relax at other times. Eat at similar times each day. We must strive to quickly create a “new normal” as we spend our days at home.
Find safe alternatives to your usual workout routine. Use YouTube and Instagram to help you find ways to stay fit in your own home. Ask friends for tips and consider sharing workout time with them via Zoom or FaceTime. New options are coming on line daily.
Make sure you are getting enough information to stay safe, and follow the advice of experts. Then turn off the news. I offer the same advice for financial worries. Try not to stress too much about finances right now. Most of us are feeling the pain of lost income and lost savings. Many of us have spouses or partners who suddenly found themselves out of work. Most likely, we will have ample ability to recover financially as we move forward and find ourselves with more work than ever.
Meditate. This may be advice you have been telling your patients for years but never found the time to try yourself. You can begin very simply with an app called Headspace or Calm. Google “5-minute meditation” on YouTube or find a meditation of any length you desire. If not now, when?
Reach out to one another. We can all use a caring word, or some humor or advice about how to move our practices online.
You may find your concentration is decreased, so be realistic in your expectations of yourself. I am finding shorter sessions more often are providing more comfort to some patients. Other patients are digging deeper than ever emotionally, and the work is becoming more rewarding.
Make sure you take a break to engage in positive activities. Read a book. Listen to soft music. Dim the lights. Watch the sunset, or be in nature if you can do so safely. Watch a TedTalk. Brush up on a foreign language. Take a deep breath. Journal. Puzzles, games, cooking, magazines, and humor all provide much needed respite from the stress. If you are lucky enough to be with family, try to take advantage of this unique time.
Try to avoid or minimize conflict with others. We need one another now more than ever. If you lose your cool, forgive yourself and make amends.
Even in these most challenging times, we must focus on what we are grateful for. Express gratitude to those around you as it will lift their mood as well. I know I am extremely grateful to be able to continue meaningful work when so many are unable to do so.
The next waves of this virus will be hitting our specialty directly so be strong and be prepared. It is an honor to serve, and we must rise to the occasion.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018), and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
I think it’s fair to say, none of us have seen anything like this before. Yet here we are, and we must lead. We are many weeks into the COVID-19 crisis. We moved our offices home and tried not to miss a beat. Our patients need us more than ever – and in different ways.
Lest we become like the shoemaker’s daughter who has no shoes, let’s make sure we take care of ourselves. The shock waves from this pandemic are going to be massive and long lasting. I am already witnessing massive psychological growth on the part of my patients, and I hope, myself and my family. We must be strong as individuals and as a group of professionals.
Now more than ever, we need to set boundaries. So many are suffering. We must take stock of our own lives. Many of us are extremely fortunate. We have homes, families, and plenty of food. We are doctors performing essential services, and we can do so without risking our lives.
The priority is to make sure you are safe, and keeping your family and loved ones safe. As physicians, we have learned to distance ourselves from illness, but the coronavirus has affected us in disproportionate numbers.
To be physically and mentally strong, we must get enough sleep. This is exhausting for some and energizing for others. It is definitely a marathon not a sprint, so pace yourself. Eat well. This is no time for empty calories, and that goes for alcohol as well.
Create new routines. Exercise at the same time each day or perhaps twice a day. Try to be productive during certain hours, and relax at other times. Eat at similar times each day. We must strive to quickly create a “new normal” as we spend our days at home.
Find safe alternatives to your usual workout routine. Use YouTube and Instagram to help you find ways to stay fit in your own home. Ask friends for tips and consider sharing workout time with them via Zoom or FaceTime. New options are coming on line daily.
Make sure you are getting enough information to stay safe, and follow the advice of experts. Then turn off the news. I offer the same advice for financial worries. Try not to stress too much about finances right now. Most of us are feeling the pain of lost income and lost savings. Many of us have spouses or partners who suddenly found themselves out of work. Most likely, we will have ample ability to recover financially as we move forward and find ourselves with more work than ever.
Meditate. This may be advice you have been telling your patients for years but never found the time to try yourself. You can begin very simply with an app called Headspace or Calm. Google “5-minute meditation” on YouTube or find a meditation of any length you desire. If not now, when?
Reach out to one another. We can all use a caring word, or some humor or advice about how to move our practices online.
You may find your concentration is decreased, so be realistic in your expectations of yourself. I am finding shorter sessions more often are providing more comfort to some patients. Other patients are digging deeper than ever emotionally, and the work is becoming more rewarding.
Make sure you take a break to engage in positive activities. Read a book. Listen to soft music. Dim the lights. Watch the sunset, or be in nature if you can do so safely. Watch a TedTalk. Brush up on a foreign language. Take a deep breath. Journal. Puzzles, games, cooking, magazines, and humor all provide much needed respite from the stress. If you are lucky enough to be with family, try to take advantage of this unique time.
Try to avoid or minimize conflict with others. We need one another now more than ever. If you lose your cool, forgive yourself and make amends.
Even in these most challenging times, we must focus on what we are grateful for. Express gratitude to those around you as it will lift their mood as well. I know I am extremely grateful to be able to continue meaningful work when so many are unable to do so.
The next waves of this virus will be hitting our specialty directly so be strong and be prepared. It is an honor to serve, and we must rise to the occasion.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018), and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
I think it’s fair to say, none of us have seen anything like this before. Yet here we are, and we must lead. We are many weeks into the COVID-19 crisis. We moved our offices home and tried not to miss a beat. Our patients need us more than ever – and in different ways.
Lest we become like the shoemaker’s daughter who has no shoes, let’s make sure we take care of ourselves. The shock waves from this pandemic are going to be massive and long lasting. I am already witnessing massive psychological growth on the part of my patients, and I hope, myself and my family. We must be strong as individuals and as a group of professionals.
Now more than ever, we need to set boundaries. So many are suffering. We must take stock of our own lives. Many of us are extremely fortunate. We have homes, families, and plenty of food. We are doctors performing essential services, and we can do so without risking our lives.
The priority is to make sure you are safe, and keeping your family and loved ones safe. As physicians, we have learned to distance ourselves from illness, but the coronavirus has affected us in disproportionate numbers.
To be physically and mentally strong, we must get enough sleep. This is exhausting for some and energizing for others. It is definitely a marathon not a sprint, so pace yourself. Eat well. This is no time for empty calories, and that goes for alcohol as well.
Create new routines. Exercise at the same time each day or perhaps twice a day. Try to be productive during certain hours, and relax at other times. Eat at similar times each day. We must strive to quickly create a “new normal” as we spend our days at home.
Find safe alternatives to your usual workout routine. Use YouTube and Instagram to help you find ways to stay fit in your own home. Ask friends for tips and consider sharing workout time with them via Zoom or FaceTime. New options are coming on line daily.
Make sure you are getting enough information to stay safe, and follow the advice of experts. Then turn off the news. I offer the same advice for financial worries. Try not to stress too much about finances right now. Most of us are feeling the pain of lost income and lost savings. Many of us have spouses or partners who suddenly found themselves out of work. Most likely, we will have ample ability to recover financially as we move forward and find ourselves with more work than ever.
Meditate. This may be advice you have been telling your patients for years but never found the time to try yourself. You can begin very simply with an app called Headspace or Calm. Google “5-minute meditation” on YouTube or find a meditation of any length you desire. If not now, when?
Reach out to one another. We can all use a caring word, or some humor or advice about how to move our practices online.
You may find your concentration is decreased, so be realistic in your expectations of yourself. I am finding shorter sessions more often are providing more comfort to some patients. Other patients are digging deeper than ever emotionally, and the work is becoming more rewarding.
Make sure you take a break to engage in positive activities. Read a book. Listen to soft music. Dim the lights. Watch the sunset, or be in nature if you can do so safely. Watch a TedTalk. Brush up on a foreign language. Take a deep breath. Journal. Puzzles, games, cooking, magazines, and humor all provide much needed respite from the stress. If you are lucky enough to be with family, try to take advantage of this unique time.
Try to avoid or minimize conflict with others. We need one another now more than ever. If you lose your cool, forgive yourself and make amends.
Even in these most challenging times, we must focus on what we are grateful for. Express gratitude to those around you as it will lift their mood as well. I know I am extremely grateful to be able to continue meaningful work when so many are unable to do so.
The next waves of this virus will be hitting our specialty directly so be strong and be prepared. It is an honor to serve, and we must rise to the occasion.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018), and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
Practicing solo and feeling grateful – despite COVID-19
I know that the world has gone upside down. It’s a nightmare, and people are filled with fear, and death is everywhere. In my little bubble of a world, however, I’ve been doing well.
I can’t lose my job, because I am my job. I’m a solo practitioner and have been for more than a decade. The restrictions to stay at home have not affected me, because I have a home office. Besides, I’m an introvert and see myself as a bit of a recluse, so the social distancing hasn’t been stressful. Conducting appointments by phone rather than face to face hasn’t undermined my work, since I can do everything that I do in my office over the phone. But I do it now in sweats and at my desk in my bedroom more often than not. I am prepared for a decrease in income as people lose their jobs, but that hasn’t happened yet. There are still people out there who are very motivated to come off their medications holistically. No rest for the wicked, as the saying goes.
On an emotional level, I feel calm because I’m not attached to material things, though I like them when they’re here. My children and friends have remained healthy, so I am grateful for that. I feel grounded in my belief that life goes on one way or another, and I trust in God to direct me wherever I need to go. Socially, I’ve been forced to be less lazy and cook more at home. As a result: less salt, MSG, and greasy food. I’ve spent a lot less on restaurants this past month and am eating less since I have to eat whatever I cook.
Can a person be more pandemic proof? I was joking with a friend about how pandemic-friendly my lifestyle is: spiritually, mentally, emotionally, physically, and socially. Oh, did I forget to mention the year supply of supplements in my office closet? They were for my patients, but those whole food green and red powders may come in handy, just in case.
So, that is how things are going for me. Please don’t hate me for not freaking out. When I read the news, I feel very sad for people who are suffering. I get angry at the politicians who can’t get their egos out of the way. But, I look at the sunshine outside my window, and I feel grateful that, at least in my case, I am not adding to the burden of suffering in the world. Not yet, anyway. I will keep trying to do the little bit that I do to help others for as long as I can.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
I know that the world has gone upside down. It’s a nightmare, and people are filled with fear, and death is everywhere. In my little bubble of a world, however, I’ve been doing well.
I can’t lose my job, because I am my job. I’m a solo practitioner and have been for more than a decade. The restrictions to stay at home have not affected me, because I have a home office. Besides, I’m an introvert and see myself as a bit of a recluse, so the social distancing hasn’t been stressful. Conducting appointments by phone rather than face to face hasn’t undermined my work, since I can do everything that I do in my office over the phone. But I do it now in sweats and at my desk in my bedroom more often than not. I am prepared for a decrease in income as people lose their jobs, but that hasn’t happened yet. There are still people out there who are very motivated to come off their medications holistically. No rest for the wicked, as the saying goes.
On an emotional level, I feel calm because I’m not attached to material things, though I like them when they’re here. My children and friends have remained healthy, so I am grateful for that. I feel grounded in my belief that life goes on one way or another, and I trust in God to direct me wherever I need to go. Socially, I’ve been forced to be less lazy and cook more at home. As a result: less salt, MSG, and greasy food. I’ve spent a lot less on restaurants this past month and am eating less since I have to eat whatever I cook.
Can a person be more pandemic proof? I was joking with a friend about how pandemic-friendly my lifestyle is: spiritually, mentally, emotionally, physically, and socially. Oh, did I forget to mention the year supply of supplements in my office closet? They were for my patients, but those whole food green and red powders may come in handy, just in case.
So, that is how things are going for me. Please don’t hate me for not freaking out. When I read the news, I feel very sad for people who are suffering. I get angry at the politicians who can’t get their egos out of the way. But, I look at the sunshine outside my window, and I feel grateful that, at least in my case, I am not adding to the burden of suffering in the world. Not yet, anyway. I will keep trying to do the little bit that I do to help others for as long as I can.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
I know that the world has gone upside down. It’s a nightmare, and people are filled with fear, and death is everywhere. In my little bubble of a world, however, I’ve been doing well.
I can’t lose my job, because I am my job. I’m a solo practitioner and have been for more than a decade. The restrictions to stay at home have not affected me, because I have a home office. Besides, I’m an introvert and see myself as a bit of a recluse, so the social distancing hasn’t been stressful. Conducting appointments by phone rather than face to face hasn’t undermined my work, since I can do everything that I do in my office over the phone. But I do it now in sweats and at my desk in my bedroom more often than not. I am prepared for a decrease in income as people lose their jobs, but that hasn’t happened yet. There are still people out there who are very motivated to come off their medications holistically. No rest for the wicked, as the saying goes.
On an emotional level, I feel calm because I’m not attached to material things, though I like them when they’re here. My children and friends have remained healthy, so I am grateful for that. I feel grounded in my belief that life goes on one way or another, and I trust in God to direct me wherever I need to go. Socially, I’ve been forced to be less lazy and cook more at home. As a result: less salt, MSG, and greasy food. I’ve spent a lot less on restaurants this past month and am eating less since I have to eat whatever I cook.
Can a person be more pandemic proof? I was joking with a friend about how pandemic-friendly my lifestyle is: spiritually, mentally, emotionally, physically, and socially. Oh, did I forget to mention the year supply of supplements in my office closet? They were for my patients, but those whole food green and red powders may come in handy, just in case.
So, that is how things are going for me. Please don’t hate me for not freaking out. When I read the news, I feel very sad for people who are suffering. I get angry at the politicians who can’t get their egos out of the way. But, I look at the sunshine outside my window, and I feel grateful that, at least in my case, I am not adding to the burden of suffering in the world. Not yet, anyway. I will keep trying to do the little bit that I do to help others for as long as I can.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
Is protocol-driven COVID-19 respiratory therapy doing more harm than good?
Physicians in the COVID-19 trenches are beginning to question whether standard respiratory therapy protocols for acute respiratory distress syndrome (ARDS) are the best approach for treating patients with COVID-19 pneumonia.
At issue is the standard use of ventilators for a virus whose presentation has not followed the standard for ARDS, but is looking more like high-altitude pulmonary edema (HAPE) in some patients.
In a letter to the editor published in the American Journal of Respiratory and Critical Care Medicine on March 30, and in an editorial accepted for publication in Intensive Care Medicine, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues make the case that protocol-driven ventilator use for patients with COVID-19 could be doing more harm than good.
Dr. Gattinoni noted that COVID-19 patients in ICUs in northern Italy had an atypical ARDS presentation with severe hypoxemia and well-preserved lung gas volume. He and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation–practicing patience to “buy time with minimum additional damage.”
Similar observations were made by Cameron Kyle-Sidell, MD, a critical care physician working in New York City, who has been speaking out about this issue on Twitter and who shared his own experiences in this video interview with WebMD chief medical officer John Whyte, MD.
The bottom line, as Dr. Kyle-Sidell and Dr. Gattinoni agree, is that protocol-driven ventilator use may be causing lung injury in COVID-19 patients.
Consider disease phenotype
In the editorial, Dr. Gattinoni and colleagues explained further that ventilator settings should be based on physiological findings – with different respiratory treatment based on disease phenotype rather than using standard protocols.
‘“This, of course, is a conceptual model, but based on the observations we have this far, I don’t know of any model which is better,” he said in an interview.
Anecdotal evidence has increasingly demonstrated that this proposed physiological approach is associated with much lower mortality rates among COVID-19 patients, he said.
While not willing to name the hospitals at this time, he said that one center in Europe has had a 0% mortality rate among COVID-19 patients in the ICU when using this approach, compared with a 60% mortality rate at a nearby hospital using a protocol-driven approach.
In his editorial, Dr. Gattinoni disputed the recently published recommendation from the Surviving Sepsis Campaign that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU.”
“Yet, COVID-19 pneumonia, despite falling in most of the circumstances under the Berlin definition of ARDS, is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance,” Dr. Gattinoni and colleagues wrote, noting that this was true for more than half of the 150 patients he and his colleagues had assessed, and that several other colleagues in northern Italy reported similar findings. “This remarkable combination is almost never seen in severe ARDS.”
Dr. Gattinoni and colleagues hypothesized that COVID-19 patterns at patient presentation depend on interaction between three sets of factors: 1) disease severity, host response, physiological reserve and comorbidities; 2) ventilatory responsiveness of the patient to hypoxemia; and 3) time elapsed between disease onset and hospitalization.
They identified two primary phenotypes based on the interaction of these factors: Type L, characterized by low elastance, low ventilator perfusion ratio, low lung weight, and low recruitability; and Type H, characterized by high elastance, high right-to-left shunt, high lung weight, and high recruitability.
“Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different,” Dr. Gattinoni said.
Patients may transition between phenotypes as their disease evolves. “If you start with the wrong protocol, at the end they become similar,” he said.
Rather, it is important to identify the phenotype at presentation to understand the pathophysiology and treat accordingly, he advised.
The phenotypes are best identified by CT scan, but signs implicit in each of the phenotypes, including respiratory system elastance and recruitability, can be used as surrogates if CT is unavailable, he noted.
“This is a kind of disease in which you don’t have to follow the protocol – you have to follow the physiology,” he said. “Unfortunately, many, many doctors around the world cannot think outside the protocol.”
In his interview with Dr. Whyte, Dr. Kyle-Sidell stressed that doctors must begin to consider other approaches. “We are desperate now, in the sense that everything we are doing does not seem to be working,” Dr. Kyle-Sidell said, noting that the first step toward improving outcomes is admitting that “this is something new.”
“I think it all starts from there, and I think we have the kind of scientific technology and the human capital in this country to solve this or at least have a very good shot at it,” he said.
Proposed treatment model
Dr. Gattinoni and his colleagues offered a proposed treatment model based on their conceptualization:
- Reverse hypoxemia through an increase in FiO2 to a level at which the Type L patient responds well, particularly for Type L patients who are not experiencing dyspnea.
- In Type L patients with dyspnea, try noninvasive options such as high-flow nasal cannula, continuous positive airway pressure, or noninvasive ventilation, and be sure to measure inspiratory esophageal pressure using esophageal manometry or surrogate measures. In intubated patients, determine P0.1 and P occlusion. High PEEP may decrease pleural pressure swings “and stop the vicious cycle that exacerbates lung injury,” but may be associated with high failure rates and delayed intubation.
- Intubate as soon as possible for esophageal pressure swings that increase from 5-10 cm H2O to above 15 cm H2O, which marks a transition from Type L to Type H phenotype and represents the level at which lung injury risk increases.
- For intubated and deeply sedated Type L patients who are hypercapnic, ventilate with volumes greater than 6 mL/kg up to 8-9 mL/kg as this high compliance results in tolerable strain without risk of ventilator-associated lung injury. Prone positioning should be used only as a rescue maneuver. Reduce PEEP to 8-10 cm H2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. Early intubation may avert the transition to Type H phenotype.
- Treat Type H phenotype like severe ARDS, including with higher PEEP if compatible with hemodynamics, and with prone positioning and extracorporeal support.
Dr. Gattinoni reported having no financial disclosures.
sworcester@mdedge.com
Physicians in the COVID-19 trenches are beginning to question whether standard respiratory therapy protocols for acute respiratory distress syndrome (ARDS) are the best approach for treating patients with COVID-19 pneumonia.
At issue is the standard use of ventilators for a virus whose presentation has not followed the standard for ARDS, but is looking more like high-altitude pulmonary edema (HAPE) in some patients.
In a letter to the editor published in the American Journal of Respiratory and Critical Care Medicine on March 30, and in an editorial accepted for publication in Intensive Care Medicine, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues make the case that protocol-driven ventilator use for patients with COVID-19 could be doing more harm than good.
Dr. Gattinoni noted that COVID-19 patients in ICUs in northern Italy had an atypical ARDS presentation with severe hypoxemia and well-preserved lung gas volume. He and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation–practicing patience to “buy time with minimum additional damage.”
Similar observations were made by Cameron Kyle-Sidell, MD, a critical care physician working in New York City, who has been speaking out about this issue on Twitter and who shared his own experiences in this video interview with WebMD chief medical officer John Whyte, MD.
The bottom line, as Dr. Kyle-Sidell and Dr. Gattinoni agree, is that protocol-driven ventilator use may be causing lung injury in COVID-19 patients.
Consider disease phenotype
In the editorial, Dr. Gattinoni and colleagues explained further that ventilator settings should be based on physiological findings – with different respiratory treatment based on disease phenotype rather than using standard protocols.
‘“This, of course, is a conceptual model, but based on the observations we have this far, I don’t know of any model which is better,” he said in an interview.
Anecdotal evidence has increasingly demonstrated that this proposed physiological approach is associated with much lower mortality rates among COVID-19 patients, he said.
While not willing to name the hospitals at this time, he said that one center in Europe has had a 0% mortality rate among COVID-19 patients in the ICU when using this approach, compared with a 60% mortality rate at a nearby hospital using a protocol-driven approach.
In his editorial, Dr. Gattinoni disputed the recently published recommendation from the Surviving Sepsis Campaign that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU.”
“Yet, COVID-19 pneumonia, despite falling in most of the circumstances under the Berlin definition of ARDS, is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance,” Dr. Gattinoni and colleagues wrote, noting that this was true for more than half of the 150 patients he and his colleagues had assessed, and that several other colleagues in northern Italy reported similar findings. “This remarkable combination is almost never seen in severe ARDS.”
Dr. Gattinoni and colleagues hypothesized that COVID-19 patterns at patient presentation depend on interaction between three sets of factors: 1) disease severity, host response, physiological reserve and comorbidities; 2) ventilatory responsiveness of the patient to hypoxemia; and 3) time elapsed between disease onset and hospitalization.
They identified two primary phenotypes based on the interaction of these factors: Type L, characterized by low elastance, low ventilator perfusion ratio, low lung weight, and low recruitability; and Type H, characterized by high elastance, high right-to-left shunt, high lung weight, and high recruitability.
“Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different,” Dr. Gattinoni said.
Patients may transition between phenotypes as their disease evolves. “If you start with the wrong protocol, at the end they become similar,” he said.
Rather, it is important to identify the phenotype at presentation to understand the pathophysiology and treat accordingly, he advised.
The phenotypes are best identified by CT scan, but signs implicit in each of the phenotypes, including respiratory system elastance and recruitability, can be used as surrogates if CT is unavailable, he noted.
“This is a kind of disease in which you don’t have to follow the protocol – you have to follow the physiology,” he said. “Unfortunately, many, many doctors around the world cannot think outside the protocol.”
In his interview with Dr. Whyte, Dr. Kyle-Sidell stressed that doctors must begin to consider other approaches. “We are desperate now, in the sense that everything we are doing does not seem to be working,” Dr. Kyle-Sidell said, noting that the first step toward improving outcomes is admitting that “this is something new.”
“I think it all starts from there, and I think we have the kind of scientific technology and the human capital in this country to solve this or at least have a very good shot at it,” he said.
Proposed treatment model
Dr. Gattinoni and his colleagues offered a proposed treatment model based on their conceptualization:
- Reverse hypoxemia through an increase in FiO2 to a level at which the Type L patient responds well, particularly for Type L patients who are not experiencing dyspnea.
- In Type L patients with dyspnea, try noninvasive options such as high-flow nasal cannula, continuous positive airway pressure, or noninvasive ventilation, and be sure to measure inspiratory esophageal pressure using esophageal manometry or surrogate measures. In intubated patients, determine P0.1 and P occlusion. High PEEP may decrease pleural pressure swings “and stop the vicious cycle that exacerbates lung injury,” but may be associated with high failure rates and delayed intubation.
- Intubate as soon as possible for esophageal pressure swings that increase from 5-10 cm H2O to above 15 cm H2O, which marks a transition from Type L to Type H phenotype and represents the level at which lung injury risk increases.
- For intubated and deeply sedated Type L patients who are hypercapnic, ventilate with volumes greater than 6 mL/kg up to 8-9 mL/kg as this high compliance results in tolerable strain without risk of ventilator-associated lung injury. Prone positioning should be used only as a rescue maneuver. Reduce PEEP to 8-10 cm H2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. Early intubation may avert the transition to Type H phenotype.
- Treat Type H phenotype like severe ARDS, including with higher PEEP if compatible with hemodynamics, and with prone positioning and extracorporeal support.
Dr. Gattinoni reported having no financial disclosures.
sworcester@mdedge.com
Physicians in the COVID-19 trenches are beginning to question whether standard respiratory therapy protocols for acute respiratory distress syndrome (ARDS) are the best approach for treating patients with COVID-19 pneumonia.
At issue is the standard use of ventilators for a virus whose presentation has not followed the standard for ARDS, but is looking more like high-altitude pulmonary edema (HAPE) in some patients.
In a letter to the editor published in the American Journal of Respiratory and Critical Care Medicine on March 30, and in an editorial accepted for publication in Intensive Care Medicine, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues make the case that protocol-driven ventilator use for patients with COVID-19 could be doing more harm than good.
Dr. Gattinoni noted that COVID-19 patients in ICUs in northern Italy had an atypical ARDS presentation with severe hypoxemia and well-preserved lung gas volume. He and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation–practicing patience to “buy time with minimum additional damage.”
Similar observations were made by Cameron Kyle-Sidell, MD, a critical care physician working in New York City, who has been speaking out about this issue on Twitter and who shared his own experiences in this video interview with WebMD chief medical officer John Whyte, MD.
The bottom line, as Dr. Kyle-Sidell and Dr. Gattinoni agree, is that protocol-driven ventilator use may be causing lung injury in COVID-19 patients.
Consider disease phenotype
In the editorial, Dr. Gattinoni and colleagues explained further that ventilator settings should be based on physiological findings – with different respiratory treatment based on disease phenotype rather than using standard protocols.
‘“This, of course, is a conceptual model, but based on the observations we have this far, I don’t know of any model which is better,” he said in an interview.
Anecdotal evidence has increasingly demonstrated that this proposed physiological approach is associated with much lower mortality rates among COVID-19 patients, he said.
While not willing to name the hospitals at this time, he said that one center in Europe has had a 0% mortality rate among COVID-19 patients in the ICU when using this approach, compared with a 60% mortality rate at a nearby hospital using a protocol-driven approach.
In his editorial, Dr. Gattinoni disputed the recently published recommendation from the Surviving Sepsis Campaign that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU.”
“Yet, COVID-19 pneumonia, despite falling in most of the circumstances under the Berlin definition of ARDS, is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance,” Dr. Gattinoni and colleagues wrote, noting that this was true for more than half of the 150 patients he and his colleagues had assessed, and that several other colleagues in northern Italy reported similar findings. “This remarkable combination is almost never seen in severe ARDS.”
Dr. Gattinoni and colleagues hypothesized that COVID-19 patterns at patient presentation depend on interaction between three sets of factors: 1) disease severity, host response, physiological reserve and comorbidities; 2) ventilatory responsiveness of the patient to hypoxemia; and 3) time elapsed between disease onset and hospitalization.
They identified two primary phenotypes based on the interaction of these factors: Type L, characterized by low elastance, low ventilator perfusion ratio, low lung weight, and low recruitability; and Type H, characterized by high elastance, high right-to-left shunt, high lung weight, and high recruitability.
“Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different,” Dr. Gattinoni said.
Patients may transition between phenotypes as their disease evolves. “If you start with the wrong protocol, at the end they become similar,” he said.
Rather, it is important to identify the phenotype at presentation to understand the pathophysiology and treat accordingly, he advised.
The phenotypes are best identified by CT scan, but signs implicit in each of the phenotypes, including respiratory system elastance and recruitability, can be used as surrogates if CT is unavailable, he noted.
“This is a kind of disease in which you don’t have to follow the protocol – you have to follow the physiology,” he said. “Unfortunately, many, many doctors around the world cannot think outside the protocol.”
In his interview with Dr. Whyte, Dr. Kyle-Sidell stressed that doctors must begin to consider other approaches. “We are desperate now, in the sense that everything we are doing does not seem to be working,” Dr. Kyle-Sidell said, noting that the first step toward improving outcomes is admitting that “this is something new.”
“I think it all starts from there, and I think we have the kind of scientific technology and the human capital in this country to solve this or at least have a very good shot at it,” he said.
Proposed treatment model
Dr. Gattinoni and his colleagues offered a proposed treatment model based on their conceptualization:
- Reverse hypoxemia through an increase in FiO2 to a level at which the Type L patient responds well, particularly for Type L patients who are not experiencing dyspnea.
- In Type L patients with dyspnea, try noninvasive options such as high-flow nasal cannula, continuous positive airway pressure, or noninvasive ventilation, and be sure to measure inspiratory esophageal pressure using esophageal manometry or surrogate measures. In intubated patients, determine P0.1 and P occlusion. High PEEP may decrease pleural pressure swings “and stop the vicious cycle that exacerbates lung injury,” but may be associated with high failure rates and delayed intubation.
- Intubate as soon as possible for esophageal pressure swings that increase from 5-10 cm H2O to above 15 cm H2O, which marks a transition from Type L to Type H phenotype and represents the level at which lung injury risk increases.
- For intubated and deeply sedated Type L patients who are hypercapnic, ventilate with volumes greater than 6 mL/kg up to 8-9 mL/kg as this high compliance results in tolerable strain without risk of ventilator-associated lung injury. Prone positioning should be used only as a rescue maneuver. Reduce PEEP to 8-10 cm H2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. Early intubation may avert the transition to Type H phenotype.
- Treat Type H phenotype like severe ARDS, including with higher PEEP if compatible with hemodynamics, and with prone positioning and extracorporeal support.
Dr. Gattinoni reported having no financial disclosures.
sworcester@mdedge.com
COVID-19 less severe in children, yet questions for pediatricians remain
COVID-19 is less severe in children, compared with adults, early data suggest. “Yet many questions remain, especially regarding the effects on children with special health care needs,” according to a viewpoint recently published in JAMA Pediatrics.
The COVID-19 pandemic also raises questions about clinic visits for healthy children in communities with widespread transmission and about the unintended effects of school closures and other measures aimed at slowing the spread of the disease, wrote Sonja A. Rasmussen, MD, and Lindsay A. Thompson, MD, both of the University of Florida, Gainesville.
In communities with widespread outbreaks, telephone triage and expanded use of telehealth may be needed to limit nonurgent clinic visits, they suggested.
“Community mitigation interventions, such as school closures, cancellation of mass gatherings, and closure of public places are appropriate” in places with widespread transmission, Dr. Rasmussen and Dr. Thompson wrote. “If these measures are required, pediatricians need to advocate to alleviate unintended consequences or inadvertent expansion of health disparities on children, such as by finding ways to maintain nutrition for those who depend on school lunches and provide online mental health services for stress management for families whose routines might be severely interrupted for an extended period of time.”
Continued preventive care for infants and vaccinations for younger children may be warranted, they wrote.
Clinical course
Overall, children have experienced lower-than-expected rates of COVID-19 disease, and deaths in this population appear to be rare, Dr. Rasmussen and Dr. Thompson wrote.
Common symptoms of COVID-19 in adults include fever, cough, myalgia, shortness of breath, headache, and diarrhea, and children have similar manifestations. In adults, older age and underlying illness increase the risk of severe disease. There has not been convincing evidence of intrauterine transmission of COVID-19, and whether breastfeeding can transmit the virus is unknown, they noted.
An analysis of more than 72,000 cases from China found that 1.2% were in patients aged 10-19 years, and 0.9% were in patients younger than 10 years. One death occurred in the adolescent age range. A separate analysis of 2,143 confirmed and suspected pediatric cases in China indicated that infants were at higher risk of severe disease (11%), compared with older children – 4% for those aged 11-15 years, and 3% in those 16 years and older.
There is less data available about the clinical course of COVID-19 in children in the United States, the authors noted. But among more than 4,000 patients with COVID-19 in the United States through March 16, no ICU admissions or deaths were reported for patients aged younger than 19 years (MMWR Morb Mortal Wkly Rep. 2020 Mar 26;69[12]:343-6).
Still, researchers have suggested that children with underlying illness may be at greater risk of COVID-19. In a study of 20 children with COVID-19 in China, 7 of the patients had a history of congenital or acquired disease, potentially indicating that they were more susceptible to the virus (Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718). Chest CT consolidations with surrounding halo sign was evident in half of the patients, and procalcitonin elevation was seen in 80% of the children; these were signs common in children, but not in adults with COVID-19.
“About 10% of children in the U.S. have asthma; many children live with other pulmonary, cardiac, neuromuscular, or genetic diseases that affect their ability to handle respiratory disease, and other children are immunosuppressed because of illness or its treatment,” Dr. Rasmussen and Dr. Thompson wrote. “It is possible that these children will experience COVID-19 differently than counterparts of the same ages who are healthy.”
The authors reported that they had no financial disclosures.
SOURCE: Rasmussen SA, Thompson LA. JAMA Pediatr. 2020 Apr 3. doi: 10.1001/jamapediatrics.2020.1224.
COVID-19 is less severe in children, compared with adults, early data suggest. “Yet many questions remain, especially regarding the effects on children with special health care needs,” according to a viewpoint recently published in JAMA Pediatrics.
The COVID-19 pandemic also raises questions about clinic visits for healthy children in communities with widespread transmission and about the unintended effects of school closures and other measures aimed at slowing the spread of the disease, wrote Sonja A. Rasmussen, MD, and Lindsay A. Thompson, MD, both of the University of Florida, Gainesville.
In communities with widespread outbreaks, telephone triage and expanded use of telehealth may be needed to limit nonurgent clinic visits, they suggested.
“Community mitigation interventions, such as school closures, cancellation of mass gatherings, and closure of public places are appropriate” in places with widespread transmission, Dr. Rasmussen and Dr. Thompson wrote. “If these measures are required, pediatricians need to advocate to alleviate unintended consequences or inadvertent expansion of health disparities on children, such as by finding ways to maintain nutrition for those who depend on school lunches and provide online mental health services for stress management for families whose routines might be severely interrupted for an extended period of time.”
Continued preventive care for infants and vaccinations for younger children may be warranted, they wrote.
Clinical course
Overall, children have experienced lower-than-expected rates of COVID-19 disease, and deaths in this population appear to be rare, Dr. Rasmussen and Dr. Thompson wrote.
Common symptoms of COVID-19 in adults include fever, cough, myalgia, shortness of breath, headache, and diarrhea, and children have similar manifestations. In adults, older age and underlying illness increase the risk of severe disease. There has not been convincing evidence of intrauterine transmission of COVID-19, and whether breastfeeding can transmit the virus is unknown, they noted.
An analysis of more than 72,000 cases from China found that 1.2% were in patients aged 10-19 years, and 0.9% were in patients younger than 10 years. One death occurred in the adolescent age range. A separate analysis of 2,143 confirmed and suspected pediatric cases in China indicated that infants were at higher risk of severe disease (11%), compared with older children – 4% for those aged 11-15 years, and 3% in those 16 years and older.
There is less data available about the clinical course of COVID-19 in children in the United States, the authors noted. But among more than 4,000 patients with COVID-19 in the United States through March 16, no ICU admissions or deaths were reported for patients aged younger than 19 years (MMWR Morb Mortal Wkly Rep. 2020 Mar 26;69[12]:343-6).
Still, researchers have suggested that children with underlying illness may be at greater risk of COVID-19. In a study of 20 children with COVID-19 in China, 7 of the patients had a history of congenital or acquired disease, potentially indicating that they were more susceptible to the virus (Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718). Chest CT consolidations with surrounding halo sign was evident in half of the patients, and procalcitonin elevation was seen in 80% of the children; these were signs common in children, but not in adults with COVID-19.
“About 10% of children in the U.S. have asthma; many children live with other pulmonary, cardiac, neuromuscular, or genetic diseases that affect their ability to handle respiratory disease, and other children are immunosuppressed because of illness or its treatment,” Dr. Rasmussen and Dr. Thompson wrote. “It is possible that these children will experience COVID-19 differently than counterparts of the same ages who are healthy.”
The authors reported that they had no financial disclosures.
SOURCE: Rasmussen SA, Thompson LA. JAMA Pediatr. 2020 Apr 3. doi: 10.1001/jamapediatrics.2020.1224.
COVID-19 is less severe in children, compared with adults, early data suggest. “Yet many questions remain, especially regarding the effects on children with special health care needs,” according to a viewpoint recently published in JAMA Pediatrics.
The COVID-19 pandemic also raises questions about clinic visits for healthy children in communities with widespread transmission and about the unintended effects of school closures and other measures aimed at slowing the spread of the disease, wrote Sonja A. Rasmussen, MD, and Lindsay A. Thompson, MD, both of the University of Florida, Gainesville.
In communities with widespread outbreaks, telephone triage and expanded use of telehealth may be needed to limit nonurgent clinic visits, they suggested.
“Community mitigation interventions, such as school closures, cancellation of mass gatherings, and closure of public places are appropriate” in places with widespread transmission, Dr. Rasmussen and Dr. Thompson wrote. “If these measures are required, pediatricians need to advocate to alleviate unintended consequences or inadvertent expansion of health disparities on children, such as by finding ways to maintain nutrition for those who depend on school lunches and provide online mental health services for stress management for families whose routines might be severely interrupted for an extended period of time.”
Continued preventive care for infants and vaccinations for younger children may be warranted, they wrote.
Clinical course
Overall, children have experienced lower-than-expected rates of COVID-19 disease, and deaths in this population appear to be rare, Dr. Rasmussen and Dr. Thompson wrote.
Common symptoms of COVID-19 in adults include fever, cough, myalgia, shortness of breath, headache, and diarrhea, and children have similar manifestations. In adults, older age and underlying illness increase the risk of severe disease. There has not been convincing evidence of intrauterine transmission of COVID-19, and whether breastfeeding can transmit the virus is unknown, they noted.
An analysis of more than 72,000 cases from China found that 1.2% were in patients aged 10-19 years, and 0.9% were in patients younger than 10 years. One death occurred in the adolescent age range. A separate analysis of 2,143 confirmed and suspected pediatric cases in China indicated that infants were at higher risk of severe disease (11%), compared with older children – 4% for those aged 11-15 years, and 3% in those 16 years and older.
There is less data available about the clinical course of COVID-19 in children in the United States, the authors noted. But among more than 4,000 patients with COVID-19 in the United States through March 16, no ICU admissions or deaths were reported for patients aged younger than 19 years (MMWR Morb Mortal Wkly Rep. 2020 Mar 26;69[12]:343-6).
Still, researchers have suggested that children with underlying illness may be at greater risk of COVID-19. In a study of 20 children with COVID-19 in China, 7 of the patients had a history of congenital or acquired disease, potentially indicating that they were more susceptible to the virus (Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718). Chest CT consolidations with surrounding halo sign was evident in half of the patients, and procalcitonin elevation was seen in 80% of the children; these were signs common in children, but not in adults with COVID-19.
“About 10% of children in the U.S. have asthma; many children live with other pulmonary, cardiac, neuromuscular, or genetic diseases that affect their ability to handle respiratory disease, and other children are immunosuppressed because of illness or its treatment,” Dr. Rasmussen and Dr. Thompson wrote. “It is possible that these children will experience COVID-19 differently than counterparts of the same ages who are healthy.”
The authors reported that they had no financial disclosures.
SOURCE: Rasmussen SA, Thompson LA. JAMA Pediatr. 2020 Apr 3. doi: 10.1001/jamapediatrics.2020.1224.
FROM JAMA PEDIATRICS
FDA grants emergency authorization for first rapid antibody test for COVID-19
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
Surge in firearm sales tied to COVID-19 fears, uncertainty presents risks
Use gentle assumptions and focus on home access to elicit positive answers.
In the wake of the 2012 shooting at Sandy Hook Elementary, in Newtown, Conn., after 20 children and seven adults were murdered, American gun sales surged on fears of new restrictions.
In the ensuing months, 20 more children and 40 more adults died from unintentional shootings believed to be tied to that surge in gun purchases.1 More recently, American gun sales surged in response to the COVID-19 pandemic with heated legal battles brewing over whether gun sales are essential.2,3 The results of this surge in sales are yet to fully manifest, but I would like to discuss several risks.
The public health risks of firearm access are well established: Nearly every measure of harm, from suicide to negligent injury and death to homicide to shootings of police, increase along with access to firearms.4 That firearms in the home are associated with greater likelihoods of suicide, negligent injury and death, and intrafamilial homicide has been recognized for decades as has the substantially heightened risk in the immediate period after a firearm is brought into the home.5,6 Defensive gun use is rare despite this being the nominal reason for firearm ownership among many.7 Even prior to recent events, there had been concerns of increased unsafe carrying and handling of firearms.8 It seems reasonable to expect such trends not to be diminished by recent events.
Added to this are several stressors, which one can reasonably expect to be associated with increased risks for unsafe use. There are new, broad social stressors from fear and uncertainty about COVID-19. Unemployment rates have skyrocketed, clinical care has been disrupted, and basic necessities have become scant. Children are home from school, unable to play with friends and unable to access mental health services as easily as before; risks of negligent and suicidal injuries and death may ensue. Couples and families are isolated in homes together for longer periods and with fewer avenues for relief; previously peaceful homes may see conflicts increase and homes with abuse have now trapped victims with their assailants. Social isolation is difficult for any person and may be even more traumatic for people with underlying vulnerabilities, including mental illness. The risks of being isolated in a home – struggling with worsening symptoms – with ready access to a firearm are self-evident.
- Consider reassessing for firearm access. Patients may be in new homes, or there may be new firearms in their homes. Use gentle assumptions and focus on home access over personal access to elicit the most true, positive answers, for example: “I understand there have been a lot of changes recently; how many guns are in the home now?”
- Reinforce safer storage practices. Simple measures, such as storing ammunition separately and using trigger locks or safes, can make a substantial difference in injury risks.
- Do not forget aging clients; suicide risk increases with age, and there may be substantial risks among the geriatric population for suicide and murder-suicide. If using telepsychiatry, realize that the abuser might be in the home or within earshot of any clinical encounter, and this might put the client at heightened risk, during and after telesessions.
- Highlight access to local and national resources, including the Disaster Distress Hotline (800-985-5990) and the National Suicide Prevention Lifeline (800-273-TALK). Promote both numbers, and note that some people may be more comfortable reaching out for help for “distress” than for “suicide.”
References
1. Levine PB and McKnight R. Science. 2017 Dec 8;358(6368):1324-8.
2. Levin D. “Coronavirus and firearms: Are gun shops essential businesses?” The New York Times. 2020 Mar 25.
3. Robertson L. “Neither hurricanes nor 9/11 caused as big a surge in gun sales as coronavirus.” Miami Herald. 2020 Mar 25.
4. Moyer MW. Scientific American. 2017 Oct;317(4):54-63.
5. Kellermann AL et al. J Trauma. 1998 Aug;45(2):263-7.
6. Wintemute GJ et al. New Engl J Med. 1999 Nov 18;341(21):1583-9.
7. Firearm Justifiable Homicides and Non-Fatal Self-Defense Gun Use: An Analysis of Federal Bureau of Investigation and National Crime Victimization Survey Data. Washington: Violence Policy Center; 2019 Jul.
8. Towers S et al. bioRxiv. 2019 Apr 18;613687.
Dr. Rozel is the medical director of resolve Crisis Services at UPMC Western Psychiatric Hospital and president of the American Association for Emergency Psychiatry. He also is associate professor of psychiatry and an adjunct professor of law at the University of Pittsburgh. He has no conflicts of interest but has worked for a gun dealer to teach sales staff how to recognize people in crisis (rather than sell a gun).
Use gentle assumptions and focus on home access to elicit positive answers.
Use gentle assumptions and focus on home access to elicit positive answers.
In the wake of the 2012 shooting at Sandy Hook Elementary, in Newtown, Conn., after 20 children and seven adults were murdered, American gun sales surged on fears of new restrictions.
In the ensuing months, 20 more children and 40 more adults died from unintentional shootings believed to be tied to that surge in gun purchases.1 More recently, American gun sales surged in response to the COVID-19 pandemic with heated legal battles brewing over whether gun sales are essential.2,3 The results of this surge in sales are yet to fully manifest, but I would like to discuss several risks.
The public health risks of firearm access are well established: Nearly every measure of harm, from suicide to negligent injury and death to homicide to shootings of police, increase along with access to firearms.4 That firearms in the home are associated with greater likelihoods of suicide, negligent injury and death, and intrafamilial homicide has been recognized for decades as has the substantially heightened risk in the immediate period after a firearm is brought into the home.5,6 Defensive gun use is rare despite this being the nominal reason for firearm ownership among many.7 Even prior to recent events, there had been concerns of increased unsafe carrying and handling of firearms.8 It seems reasonable to expect such trends not to be diminished by recent events.
Added to this are several stressors, which one can reasonably expect to be associated with increased risks for unsafe use. There are new, broad social stressors from fear and uncertainty about COVID-19. Unemployment rates have skyrocketed, clinical care has been disrupted, and basic necessities have become scant. Children are home from school, unable to play with friends and unable to access mental health services as easily as before; risks of negligent and suicidal injuries and death may ensue. Couples and families are isolated in homes together for longer periods and with fewer avenues for relief; previously peaceful homes may see conflicts increase and homes with abuse have now trapped victims with their assailants. Social isolation is difficult for any person and may be even more traumatic for people with underlying vulnerabilities, including mental illness. The risks of being isolated in a home – struggling with worsening symptoms – with ready access to a firearm are self-evident.
- Consider reassessing for firearm access. Patients may be in new homes, or there may be new firearms in their homes. Use gentle assumptions and focus on home access over personal access to elicit the most true, positive answers, for example: “I understand there have been a lot of changes recently; how many guns are in the home now?”
- Reinforce safer storage practices. Simple measures, such as storing ammunition separately and using trigger locks or safes, can make a substantial difference in injury risks.
- Do not forget aging clients; suicide risk increases with age, and there may be substantial risks among the geriatric population for suicide and murder-suicide. If using telepsychiatry, realize that the abuser might be in the home or within earshot of any clinical encounter, and this might put the client at heightened risk, during and after telesessions.
- Highlight access to local and national resources, including the Disaster Distress Hotline (800-985-5990) and the National Suicide Prevention Lifeline (800-273-TALK). Promote both numbers, and note that some people may be more comfortable reaching out for help for “distress” than for “suicide.”
References
1. Levine PB and McKnight R. Science. 2017 Dec 8;358(6368):1324-8.
2. Levin D. “Coronavirus and firearms: Are gun shops essential businesses?” The New York Times. 2020 Mar 25.
3. Robertson L. “Neither hurricanes nor 9/11 caused as big a surge in gun sales as coronavirus.” Miami Herald. 2020 Mar 25.
4. Moyer MW. Scientific American. 2017 Oct;317(4):54-63.
5. Kellermann AL et al. J Trauma. 1998 Aug;45(2):263-7.
6. Wintemute GJ et al. New Engl J Med. 1999 Nov 18;341(21):1583-9.
7. Firearm Justifiable Homicides and Non-Fatal Self-Defense Gun Use: An Analysis of Federal Bureau of Investigation and National Crime Victimization Survey Data. Washington: Violence Policy Center; 2019 Jul.
8. Towers S et al. bioRxiv. 2019 Apr 18;613687.
Dr. Rozel is the medical director of resolve Crisis Services at UPMC Western Psychiatric Hospital and president of the American Association for Emergency Psychiatry. He also is associate professor of psychiatry and an adjunct professor of law at the University of Pittsburgh. He has no conflicts of interest but has worked for a gun dealer to teach sales staff how to recognize people in crisis (rather than sell a gun).
In the wake of the 2012 shooting at Sandy Hook Elementary, in Newtown, Conn., after 20 children and seven adults were murdered, American gun sales surged on fears of new restrictions.
In the ensuing months, 20 more children and 40 more adults died from unintentional shootings believed to be tied to that surge in gun purchases.1 More recently, American gun sales surged in response to the COVID-19 pandemic with heated legal battles brewing over whether gun sales are essential.2,3 The results of this surge in sales are yet to fully manifest, but I would like to discuss several risks.
The public health risks of firearm access are well established: Nearly every measure of harm, from suicide to negligent injury and death to homicide to shootings of police, increase along with access to firearms.4 That firearms in the home are associated with greater likelihoods of suicide, negligent injury and death, and intrafamilial homicide has been recognized for decades as has the substantially heightened risk in the immediate period after a firearm is brought into the home.5,6 Defensive gun use is rare despite this being the nominal reason for firearm ownership among many.7 Even prior to recent events, there had been concerns of increased unsafe carrying and handling of firearms.8 It seems reasonable to expect such trends not to be diminished by recent events.
Added to this are several stressors, which one can reasonably expect to be associated with increased risks for unsafe use. There are new, broad social stressors from fear and uncertainty about COVID-19. Unemployment rates have skyrocketed, clinical care has been disrupted, and basic necessities have become scant. Children are home from school, unable to play with friends and unable to access mental health services as easily as before; risks of negligent and suicidal injuries and death may ensue. Couples and families are isolated in homes together for longer periods and with fewer avenues for relief; previously peaceful homes may see conflicts increase and homes with abuse have now trapped victims with their assailants. Social isolation is difficult for any person and may be even more traumatic for people with underlying vulnerabilities, including mental illness. The risks of being isolated in a home – struggling with worsening symptoms – with ready access to a firearm are self-evident.
- Consider reassessing for firearm access. Patients may be in new homes, or there may be new firearms in their homes. Use gentle assumptions and focus on home access over personal access to elicit the most true, positive answers, for example: “I understand there have been a lot of changes recently; how many guns are in the home now?”
- Reinforce safer storage practices. Simple measures, such as storing ammunition separately and using trigger locks or safes, can make a substantial difference in injury risks.
- Do not forget aging clients; suicide risk increases with age, and there may be substantial risks among the geriatric population for suicide and murder-suicide. If using telepsychiatry, realize that the abuser might be in the home or within earshot of any clinical encounter, and this might put the client at heightened risk, during and after telesessions.
- Highlight access to local and national resources, including the Disaster Distress Hotline (800-985-5990) and the National Suicide Prevention Lifeline (800-273-TALK). Promote both numbers, and note that some people may be more comfortable reaching out for help for “distress” than for “suicide.”
References
1. Levine PB and McKnight R. Science. 2017 Dec 8;358(6368):1324-8.
2. Levin D. “Coronavirus and firearms: Are gun shops essential businesses?” The New York Times. 2020 Mar 25.
3. Robertson L. “Neither hurricanes nor 9/11 caused as big a surge in gun sales as coronavirus.” Miami Herald. 2020 Mar 25.
4. Moyer MW. Scientific American. 2017 Oct;317(4):54-63.
5. Kellermann AL et al. J Trauma. 1998 Aug;45(2):263-7.
6. Wintemute GJ et al. New Engl J Med. 1999 Nov 18;341(21):1583-9.
7. Firearm Justifiable Homicides and Non-Fatal Self-Defense Gun Use: An Analysis of Federal Bureau of Investigation and National Crime Victimization Survey Data. Washington: Violence Policy Center; 2019 Jul.
8. Towers S et al. bioRxiv. 2019 Apr 18;613687.
Dr. Rozel is the medical director of resolve Crisis Services at UPMC Western Psychiatric Hospital and president of the American Association for Emergency Psychiatry. He also is associate professor of psychiatry and an adjunct professor of law at the University of Pittsburgh. He has no conflicts of interest but has worked for a gun dealer to teach sales staff how to recognize people in crisis (rather than sell a gun).
‘We will get through this’: Advice for lessening your pandemic anxiety
The COVID-19 pandemic is an experience that is unprecedented in our lifetime. It is having a pervasive effect due to how mysterious, potentially dangerous, and sustained it is. We don’t know how bad it’s going to get or how long it’s going to last. We have natural disasters like hurricanes and earthquakes, but they are limited in time and scope. But this global pandemic is something we can’t put our arms around just yet, breeding uncertainty, worry, and fear. This is where mental health professionals need to come in.
The populations being affected by this pandemic can be placed into different groups on the basis of their mental health consequences and needs. First you have, for lack of a better term, “the worried well.” These are people with no preexisting mental disorder who are naturally worried by this and are trying to take appropriate actions to protect themselves and prepare. For such individuals, the equivalent of mental health first-aid should be useful (we’ll come back to that in a moment). Given the proper guidance and sources of information, most such people should be able to manage the anxiety, worry, and dysphoria associated with this critical pandemic.
Then there are those who have preexisting mental conditions related to mood, anxiety, stress, or obsessive tendencies. They are probably going to have an increase in their symptoms, and as such, a corresponding need for adjusting treatment. This may require an increase in their existing medications or the addition of an ad hoc medication, or perhaps more frequent contact with their doctor or therapist.
Because travel and direct visitation is discouraged at the moment, virtual methods of communication should be used to speak with these patients. Such methods have long existed but haven’t been adopted in large numbers; this may be the impetus to finally make it happen. Using the telephone, FaceTime, Skype, WebEx, Zoom, and other means of videoconferencing should be feasible. As billing procedures are being adapted for this moment, there’s no reason why individuals shouldn’t be able to contact their mental health provider.
Substance abuse is also a condition vulnerable to the stress effects of this pandemic. This will prompt or tempt those to use substances that they’ve abused or turned to in the past as a way of self-medicating and assuaging their anxiety and worry.
It’s possible that the pandemic could find its way into delusions or exacerbate symptoms, but somewhat paradoxically, people with serious mental illnesses often respond more calmly to crises than do individuals without them. As a result, the number of these patients requiring emergency room admission for possible exacerbation of symptoms is probably not going to be that much greater than normal.
How to Cope With an Unprecedented Situation
For the worried well and for the clinicians who have understandable fears about exposure, there are several things you can try to manage your anxiety. There are concentric circles of concern that you have to maintain. Think of it like the instructions on an airplane when, if there’s a drop in cabin pressure, you’re asked to apply your own oxygen mask first before placing one on your child. In the same way, you must first think about protecting yourself by limiting your exposure and monitoring your own physical state for any symptoms. But then you must be concerned about your family, your friends, and also society. This is a situation where the impulse and the ethos of worrying about your fellow persons—being your brother’s keeper—is imperative.
The epidemic has been successfully managed in some countries, like Singapore and China, which, once they got on top of it, were able to limit contagion in a very dramatic way. But these are authoritarian governments. The United States doesn’t work that way, which is what makes appealing to the principle of caring for others so crucial. You can protect yourself, but if other people aren’t also protected, it may not matter. You have to worry not just about yourself but about everyone else.
When it comes to stress management, I recommend not catastrophizing or watching the news media 24/7. Distract yourself with other work or recreational activities. Reach out and communicate—virtually, of course—with friends, family, and healthcare providers as needed. Staying in touch acts not just as a diversion but also as an outlet for assuaging your feelings, your sense of being in this alone, feeling isolated.
There are also cognitive reframing mechanisms you can employ. Consider that although this is bad, some countries have already gone through it. And we’ll get through it too. You’ll understandably ask yourself what it would mean if you were to be exposed. In most cases you can say, “I’m going to have the flu and symptoms that are not going to be pleasant, but I’ve had the flu or serious sickness before.”
Remember that there are already antiretroviral treatments being tested in clinical trials and showing efficacy. It’s good to know that before this pandemic ends, some of these treatments will probably be clinically applied, mostly to those who are severely affected and in intensive care.
Diagnose yourself. Monitor your state. Determine whether the stress is really having an impact on you. Is it affecting your sleep, appetite, concentration, mood? And if you do have a preexisting psychiatric condition, don’t feel afraid to reach out to your mental health provider. Understand that you’re going to be anxious, which may aggravate your symptoms and require an adjustment in your treatment. That’s okay. It’s to be expected and your provider should be available to help you.
Controlling this outbreak via the same epidemiologic infectious disease prevention guidance that works in authoritarian societies is not going to be applicable here because of the liberties that we experience in American society. What will determine our success is the belief that we’re in this together, that we’re going to help each other. We should be proud of that, as it shows how Americans and people around the world stand up in situations like this.
Let’s also note that even though everybody is affected and undergoing previously unimaginable levels of anticipated stress and dislocation, it’s the healthcare providers who are really on the frontlines. They’re under tremendous pressure to continue to perform heroically, at great risk to themselves. They deserve a real debt of gratitude.
We will get through this, but as we do, it will not end until we’ve undergone an extreme test of our character. I certainly hope and trust that we will be up to it.
Dr. Jeffrey A. Lieberman is chairman of the Department of Psychiatry at Columbia University. He is a former president of the American Psychiatric Association.
Disclosure: Jeffrey A. Lieberman, MD, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Clintara; Intracellular Therapies. Received research grant from Alkermes; Biomarin; EnVivo/Forum; Genentech; Novartis/Novation; Sunovion. Patent: Repligen.
This article first appeared on Medscape.com.
The COVID-19 pandemic is an experience that is unprecedented in our lifetime. It is having a pervasive effect due to how mysterious, potentially dangerous, and sustained it is. We don’t know how bad it’s going to get or how long it’s going to last. We have natural disasters like hurricanes and earthquakes, but they are limited in time and scope. But this global pandemic is something we can’t put our arms around just yet, breeding uncertainty, worry, and fear. This is where mental health professionals need to come in.
The populations being affected by this pandemic can be placed into different groups on the basis of their mental health consequences and needs. First you have, for lack of a better term, “the worried well.” These are people with no preexisting mental disorder who are naturally worried by this and are trying to take appropriate actions to protect themselves and prepare. For such individuals, the equivalent of mental health first-aid should be useful (we’ll come back to that in a moment). Given the proper guidance and sources of information, most such people should be able to manage the anxiety, worry, and dysphoria associated with this critical pandemic.
Then there are those who have preexisting mental conditions related to mood, anxiety, stress, or obsessive tendencies. They are probably going to have an increase in their symptoms, and as such, a corresponding need for adjusting treatment. This may require an increase in their existing medications or the addition of an ad hoc medication, or perhaps more frequent contact with their doctor or therapist.
Because travel and direct visitation is discouraged at the moment, virtual methods of communication should be used to speak with these patients. Such methods have long existed but haven’t been adopted in large numbers; this may be the impetus to finally make it happen. Using the telephone, FaceTime, Skype, WebEx, Zoom, and other means of videoconferencing should be feasible. As billing procedures are being adapted for this moment, there’s no reason why individuals shouldn’t be able to contact their mental health provider.
Substance abuse is also a condition vulnerable to the stress effects of this pandemic. This will prompt or tempt those to use substances that they’ve abused or turned to in the past as a way of self-medicating and assuaging their anxiety and worry.
It’s possible that the pandemic could find its way into delusions or exacerbate symptoms, but somewhat paradoxically, people with serious mental illnesses often respond more calmly to crises than do individuals without them. As a result, the number of these patients requiring emergency room admission for possible exacerbation of symptoms is probably not going to be that much greater than normal.
How to Cope With an Unprecedented Situation
For the worried well and for the clinicians who have understandable fears about exposure, there are several things you can try to manage your anxiety. There are concentric circles of concern that you have to maintain. Think of it like the instructions on an airplane when, if there’s a drop in cabin pressure, you’re asked to apply your own oxygen mask first before placing one on your child. In the same way, you must first think about protecting yourself by limiting your exposure and monitoring your own physical state for any symptoms. But then you must be concerned about your family, your friends, and also society. This is a situation where the impulse and the ethos of worrying about your fellow persons—being your brother’s keeper—is imperative.
The epidemic has been successfully managed in some countries, like Singapore and China, which, once they got on top of it, were able to limit contagion in a very dramatic way. But these are authoritarian governments. The United States doesn’t work that way, which is what makes appealing to the principle of caring for others so crucial. You can protect yourself, but if other people aren’t also protected, it may not matter. You have to worry not just about yourself but about everyone else.
When it comes to stress management, I recommend not catastrophizing or watching the news media 24/7. Distract yourself with other work or recreational activities. Reach out and communicate—virtually, of course—with friends, family, and healthcare providers as needed. Staying in touch acts not just as a diversion but also as an outlet for assuaging your feelings, your sense of being in this alone, feeling isolated.
There are also cognitive reframing mechanisms you can employ. Consider that although this is bad, some countries have already gone through it. And we’ll get through it too. You’ll understandably ask yourself what it would mean if you were to be exposed. In most cases you can say, “I’m going to have the flu and symptoms that are not going to be pleasant, but I’ve had the flu or serious sickness before.”
Remember that there are already antiretroviral treatments being tested in clinical trials and showing efficacy. It’s good to know that before this pandemic ends, some of these treatments will probably be clinically applied, mostly to those who are severely affected and in intensive care.
Diagnose yourself. Monitor your state. Determine whether the stress is really having an impact on you. Is it affecting your sleep, appetite, concentration, mood? And if you do have a preexisting psychiatric condition, don’t feel afraid to reach out to your mental health provider. Understand that you’re going to be anxious, which may aggravate your symptoms and require an adjustment in your treatment. That’s okay. It’s to be expected and your provider should be available to help you.
Controlling this outbreak via the same epidemiologic infectious disease prevention guidance that works in authoritarian societies is not going to be applicable here because of the liberties that we experience in American society. What will determine our success is the belief that we’re in this together, that we’re going to help each other. We should be proud of that, as it shows how Americans and people around the world stand up in situations like this.
Let’s also note that even though everybody is affected and undergoing previously unimaginable levels of anticipated stress and dislocation, it’s the healthcare providers who are really on the frontlines. They’re under tremendous pressure to continue to perform heroically, at great risk to themselves. They deserve a real debt of gratitude.
We will get through this, but as we do, it will not end until we’ve undergone an extreme test of our character. I certainly hope and trust that we will be up to it.
Dr. Jeffrey A. Lieberman is chairman of the Department of Psychiatry at Columbia University. He is a former president of the American Psychiatric Association.
Disclosure: Jeffrey A. Lieberman, MD, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Clintara; Intracellular Therapies. Received research grant from Alkermes; Biomarin; EnVivo/Forum; Genentech; Novartis/Novation; Sunovion. Patent: Repligen.
This article first appeared on Medscape.com.
The COVID-19 pandemic is an experience that is unprecedented in our lifetime. It is having a pervasive effect due to how mysterious, potentially dangerous, and sustained it is. We don’t know how bad it’s going to get or how long it’s going to last. We have natural disasters like hurricanes and earthquakes, but they are limited in time and scope. But this global pandemic is something we can’t put our arms around just yet, breeding uncertainty, worry, and fear. This is where mental health professionals need to come in.
The populations being affected by this pandemic can be placed into different groups on the basis of their mental health consequences and needs. First you have, for lack of a better term, “the worried well.” These are people with no preexisting mental disorder who are naturally worried by this and are trying to take appropriate actions to protect themselves and prepare. For such individuals, the equivalent of mental health first-aid should be useful (we’ll come back to that in a moment). Given the proper guidance and sources of information, most such people should be able to manage the anxiety, worry, and dysphoria associated with this critical pandemic.
Then there are those who have preexisting mental conditions related to mood, anxiety, stress, or obsessive tendencies. They are probably going to have an increase in their symptoms, and as such, a corresponding need for adjusting treatment. This may require an increase in their existing medications or the addition of an ad hoc medication, or perhaps more frequent contact with their doctor or therapist.
Because travel and direct visitation is discouraged at the moment, virtual methods of communication should be used to speak with these patients. Such methods have long existed but haven’t been adopted in large numbers; this may be the impetus to finally make it happen. Using the telephone, FaceTime, Skype, WebEx, Zoom, and other means of videoconferencing should be feasible. As billing procedures are being adapted for this moment, there’s no reason why individuals shouldn’t be able to contact their mental health provider.
Substance abuse is also a condition vulnerable to the stress effects of this pandemic. This will prompt or tempt those to use substances that they’ve abused or turned to in the past as a way of self-medicating and assuaging their anxiety and worry.
It’s possible that the pandemic could find its way into delusions or exacerbate symptoms, but somewhat paradoxically, people with serious mental illnesses often respond more calmly to crises than do individuals without them. As a result, the number of these patients requiring emergency room admission for possible exacerbation of symptoms is probably not going to be that much greater than normal.
How to Cope With an Unprecedented Situation
For the worried well and for the clinicians who have understandable fears about exposure, there are several things you can try to manage your anxiety. There are concentric circles of concern that you have to maintain. Think of it like the instructions on an airplane when, if there’s a drop in cabin pressure, you’re asked to apply your own oxygen mask first before placing one on your child. In the same way, you must first think about protecting yourself by limiting your exposure and monitoring your own physical state for any symptoms. But then you must be concerned about your family, your friends, and also society. This is a situation where the impulse and the ethos of worrying about your fellow persons—being your brother’s keeper—is imperative.
The epidemic has been successfully managed in some countries, like Singapore and China, which, once they got on top of it, were able to limit contagion in a very dramatic way. But these are authoritarian governments. The United States doesn’t work that way, which is what makes appealing to the principle of caring for others so crucial. You can protect yourself, but if other people aren’t also protected, it may not matter. You have to worry not just about yourself but about everyone else.
When it comes to stress management, I recommend not catastrophizing or watching the news media 24/7. Distract yourself with other work or recreational activities. Reach out and communicate—virtually, of course—with friends, family, and healthcare providers as needed. Staying in touch acts not just as a diversion but also as an outlet for assuaging your feelings, your sense of being in this alone, feeling isolated.
There are also cognitive reframing mechanisms you can employ. Consider that although this is bad, some countries have already gone through it. And we’ll get through it too. You’ll understandably ask yourself what it would mean if you were to be exposed. In most cases you can say, “I’m going to have the flu and symptoms that are not going to be pleasant, but I’ve had the flu or serious sickness before.”
Remember that there are already antiretroviral treatments being tested in clinical trials and showing efficacy. It’s good to know that before this pandemic ends, some of these treatments will probably be clinically applied, mostly to those who are severely affected and in intensive care.
Diagnose yourself. Monitor your state. Determine whether the stress is really having an impact on you. Is it affecting your sleep, appetite, concentration, mood? And if you do have a preexisting psychiatric condition, don’t feel afraid to reach out to your mental health provider. Understand that you’re going to be anxious, which may aggravate your symptoms and require an adjustment in your treatment. That’s okay. It’s to be expected and your provider should be available to help you.
Controlling this outbreak via the same epidemiologic infectious disease prevention guidance that works in authoritarian societies is not going to be applicable here because of the liberties that we experience in American society. What will determine our success is the belief that we’re in this together, that we’re going to help each other. We should be proud of that, as it shows how Americans and people around the world stand up in situations like this.
Let’s also note that even though everybody is affected and undergoing previously unimaginable levels of anticipated stress and dislocation, it’s the healthcare providers who are really on the frontlines. They’re under tremendous pressure to continue to perform heroically, at great risk to themselves. They deserve a real debt of gratitude.
We will get through this, but as we do, it will not end until we’ve undergone an extreme test of our character. I certainly hope and trust that we will be up to it.
Dr. Jeffrey A. Lieberman is chairman of the Department of Psychiatry at Columbia University. He is a former president of the American Psychiatric Association.
Disclosure: Jeffrey A. Lieberman, MD, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Clintara; Intracellular Therapies. Received research grant from Alkermes; Biomarin; EnVivo/Forum; Genentech; Novartis/Novation; Sunovion. Patent: Repligen.
This article first appeared on Medscape.com.
Sunshine on my shoulders
On March 26, 2020, it’s hard to write or think of anything beyond the COVID-19 pandemic. Those of you who are on the front lines of the battle may find it strange that I am just a bit envious. Having stepped back from clinical medicine nearly a decade ago, it is frustrating to feel that there is little I can do to help other than offering to venture into the grocery store to shop for friends and neighbors who feel more vulnerable than I do.
Here in Maine, we are blessed by geographic isolation that for the moment seems to have damped the surge from the metropolitan centers to our south. But, the virus is here and, as the state with the oldest population, we are beginning to be affected.
For nearly a century, we could count on the outhouses here in Maine would be stocked with outdated Sears Roebucks catalogs when toilet paper was in short supply. Many outhouses remain but Sears Roebucks and its catalogs have disappeared from the landscape. I take a little comfort in the learning that I’m not the only human on the planet who can envision the horror of a week or even a day without toilet paper.
So I am left to sit on the sidelines and watch how my fellow Mainers are coping with the anxiety, depression, and loneliness that come with the forced social isolation. It is pretty clear that walking outside has become the coping strategy of choice. On a usual March day the walkers comprise a skimpy mix of dog walkers and wannabe arctic explorers testing the weather-defying capabilities of their high-tech outerwear. But, to say the least, this is not a usual March and the number of walkers has surged bolstered by gym rats forced off their sweat-drenched ellipticals and treadmills.
This increase in outdoor activity is clearly perceptible even on an overcast day, but it is far less than one would expect given the magnitude of the disruption to everyone’s routines. But, when the sun comes out! The doors fly open and onto the sidewalks and quiet rural roads spill scores of people I haven’t seen for months and in some cases decades. One can almost hear John Denver singing “sunshine on my shoulders makes me happy.” Everyone is smiling and waving to each other. It feels as though the community has, at least for a few hours, been able to throw off the burden of angst that the pandemic laid on us.
There has been a good bit of research about seasonal affective disorder, and I suspect that almost everyone has heard about the value of sunshine for depression. But it is unfortunate that the psychological benefits of just being outdoors – even on an overcast day – has gone pretty much unpublicized. As part of their marketing strategy, a local company that specializes in recreational clothing and gear is encouraging its customers to become “outsiders.” It may be that the pandemic will make more people realize the psychological benefits of being active outside. As physicians we should continue to encourage our patients to be more active and remind them that they don’t need to wait for a sunny day to do so.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
On March 26, 2020, it’s hard to write or think of anything beyond the COVID-19 pandemic. Those of you who are on the front lines of the battle may find it strange that I am just a bit envious. Having stepped back from clinical medicine nearly a decade ago, it is frustrating to feel that there is little I can do to help other than offering to venture into the grocery store to shop for friends and neighbors who feel more vulnerable than I do.
Here in Maine, we are blessed by geographic isolation that for the moment seems to have damped the surge from the metropolitan centers to our south. But, the virus is here and, as the state with the oldest population, we are beginning to be affected.
For nearly a century, we could count on the outhouses here in Maine would be stocked with outdated Sears Roebucks catalogs when toilet paper was in short supply. Many outhouses remain but Sears Roebucks and its catalogs have disappeared from the landscape. I take a little comfort in the learning that I’m not the only human on the planet who can envision the horror of a week or even a day without toilet paper.
So I am left to sit on the sidelines and watch how my fellow Mainers are coping with the anxiety, depression, and loneliness that come with the forced social isolation. It is pretty clear that walking outside has become the coping strategy of choice. On a usual March day the walkers comprise a skimpy mix of dog walkers and wannabe arctic explorers testing the weather-defying capabilities of their high-tech outerwear. But, to say the least, this is not a usual March and the number of walkers has surged bolstered by gym rats forced off their sweat-drenched ellipticals and treadmills.
This increase in outdoor activity is clearly perceptible even on an overcast day, but it is far less than one would expect given the magnitude of the disruption to everyone’s routines. But, when the sun comes out! The doors fly open and onto the sidewalks and quiet rural roads spill scores of people I haven’t seen for months and in some cases decades. One can almost hear John Denver singing “sunshine on my shoulders makes me happy.” Everyone is smiling and waving to each other. It feels as though the community has, at least for a few hours, been able to throw off the burden of angst that the pandemic laid on us.
There has been a good bit of research about seasonal affective disorder, and I suspect that almost everyone has heard about the value of sunshine for depression. But it is unfortunate that the psychological benefits of just being outdoors – even on an overcast day – has gone pretty much unpublicized. As part of their marketing strategy, a local company that specializes in recreational clothing and gear is encouraging its customers to become “outsiders.” It may be that the pandemic will make more people realize the psychological benefits of being active outside. As physicians we should continue to encourage our patients to be more active and remind them that they don’t need to wait for a sunny day to do so.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
On March 26, 2020, it’s hard to write or think of anything beyond the COVID-19 pandemic. Those of you who are on the front lines of the battle may find it strange that I am just a bit envious. Having stepped back from clinical medicine nearly a decade ago, it is frustrating to feel that there is little I can do to help other than offering to venture into the grocery store to shop for friends and neighbors who feel more vulnerable than I do.
Here in Maine, we are blessed by geographic isolation that for the moment seems to have damped the surge from the metropolitan centers to our south. But, the virus is here and, as the state with the oldest population, we are beginning to be affected.
For nearly a century, we could count on the outhouses here in Maine would be stocked with outdated Sears Roebucks catalogs when toilet paper was in short supply. Many outhouses remain but Sears Roebucks and its catalogs have disappeared from the landscape. I take a little comfort in the learning that I’m not the only human on the planet who can envision the horror of a week or even a day without toilet paper.
So I am left to sit on the sidelines and watch how my fellow Mainers are coping with the anxiety, depression, and loneliness that come with the forced social isolation. It is pretty clear that walking outside has become the coping strategy of choice. On a usual March day the walkers comprise a skimpy mix of dog walkers and wannabe arctic explorers testing the weather-defying capabilities of their high-tech outerwear. But, to say the least, this is not a usual March and the number of walkers has surged bolstered by gym rats forced off their sweat-drenched ellipticals and treadmills.
This increase in outdoor activity is clearly perceptible even on an overcast day, but it is far less than one would expect given the magnitude of the disruption to everyone’s routines. But, when the sun comes out! The doors fly open and onto the sidewalks and quiet rural roads spill scores of people I haven’t seen for months and in some cases decades. One can almost hear John Denver singing “sunshine on my shoulders makes me happy.” Everyone is smiling and waving to each other. It feels as though the community has, at least for a few hours, been able to throw off the burden of angst that the pandemic laid on us.
There has been a good bit of research about seasonal affective disorder, and I suspect that almost everyone has heard about the value of sunshine for depression. But it is unfortunate that the psychological benefits of just being outdoors – even on an overcast day – has gone pretty much unpublicized. As part of their marketing strategy, a local company that specializes in recreational clothing and gear is encouraging its customers to become “outsiders.” It may be that the pandemic will make more people realize the psychological benefits of being active outside. As physicians we should continue to encourage our patients to be more active and remind them that they don’t need to wait for a sunny day to do so.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
FDA okays emergency use of convalescent plasma for seriously ill COVID-19 patients
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to CBER_eIND_Covid-19@FDA.HHS.gov.
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to CBER_eIND_Covid-19@FDA.HHS.gov.
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to CBER_eIND_Covid-19@FDA.HHS.gov.
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”