User login
Chronically interrupted: The importance of communication with patient and family during the COVID-19 pandemic
Case narrative
A 35-year-old woman has worsening alcoholic cirrhosis and repeated admissions for ascites, hepato-renal syndrome, and alcoholic hepatitis. Upon recognition of her grave prognosis, we proceeded with a shared-management approach involving medicine, gastroenterology, social work, chaplaincy, and palliative care. When the team spoke with the patient’s health care proxy (HCP), family, and friends for collateral information and involvement in goals of care conversation, we realized that none were aware of her months-long decline and poor prognosis for recovery to hospital discharge.
Although several factors contributed to the disconnect between the patient and her support system, the obstacles were greatly exacerbated by profound changes in hospital protocol because of the COVID-19 pandemic. Physicians feel underprepared and challenged by prognostication and discussion of end of life during normal times; we believe COVID-19 has limited this essential physician role and led to tragic delays in effective communication and end of life planning.
Closing the loop
For patients with complex medical issues or those reaching end of life, effective communication within the health care system is critical. While inpatient teams often drive the plan, they care for their patients during a snapshot in time; contrarily, primary care providers and specialists often have established longitudinal relationships with their patients. Ergo, clinicians should communicate directly, and ideally with both patients and families, to achieve patient-centered and goal-concordant care.
For medically complex patients, PCPs tend to prefer verbal hand-offs. Timely and reliable communication between inpatient and outpatient providers has also been shown to prevent medical adverse events.1 Despite this, direct communication occurs infrequently.2 Given that hospitalists serve as primary inpatient providers for most general admissions, it is their responsibility to communicate with outpatient providers.
A multidisciplinary team redesigned the process by which PCPs were contacted following patient discharge. The transmission of information should ideally occur prior to discharge.3 Deficits in communication are extremely common and may negatively impact patient care, patient satisfaction, and patient safety.
Changes during the COVID-19 era
During the pandemic, patients have only one visitor per day, restricted visiting hours, and limited interactions with clinicians per implemented policies. Along with the increased burdens from personal protective equipment, remote hospital providers (social workers, case managers), and increased bureaucratic duties, COVID-19 has elucidated limitations in medical capacity and revealed the difficulties that clinicians face in communicating with patients and families, especially about serious illness.
Tasks include facilitating virtual goodbyes between dying patients and families, conducting family meetings via teleconference, and discussing patient care with specialists through virtual technologies.4 While these tasks are arguably more important during a global disaster, COVID-19 paradoxically restricts physical presence and severely hinders communication.5 Clinicians should continue to utilize core skills like building rapport, assessing patient/family perspectives and agenda, and using empathy.6 Patients tend to more frequently value functional outcomes while clinicians tend to default to treatment modalities.7 Additionally, goals of care and end of life discussions are associated with improved quality of life, fewer aggressive medical interventions near death, and even increased survival.
Given the limited resources and difficulties in communication during the pandemic, clinicians should place greater emphasis on values-based shared decision-making. Internet-based solutions are essential and widely used, and videoconferencing has been initiated at the institutional scale at many hospitals. Many clinicians with little experience are broadly implementing these technologies.7 Despite these technological innovations, issues still arise in how to communicate effectively in the hospital setting, and we must acknowledge that strategies require devices, Internet access, and technological literacy, highlighting disparities in access to quality health care.6 Conversations during the pandemic will require listening, empathy, responsive action, and the acknowledgment of the social determinants of health.7
Improving communication and transition of care
Multiple steps will be warranted to implement the safe transition process and improve communication. High-quality patient care encompasses careful review of medications, communication between inpatient and outpatient providers, and close follow-up at discharge. These steps serve to increase our reliance on patient compliance and the exchange of information about global progression of disease.
The quantitative and qualitative steps of transition of care should overcome disconnect between teams, specifically deficit areas regarding postdischarge communication, monitoring, and understanding of prognosis around the relevance to this era of COVID-19.
Dr. Haddad is a resident physician in the psychiatry residency program at Brigham and Women’s Hospital, Boston. Dr. Halporn is clinic director, Division of Adult Palliative Care, in the department of psychosocial oncology and palliative care, Dana-Farber Cancer Institute and Brigham and Women’s Hospital. Dr. Barkoudah is associate director of the Hospital Medicine Unit at Brigham and Women’s Hospital.
References
1. Goldman L et al. Passing the clinical baton: 6 principles to guide the hospitalist. Am J Med. 2001;111(9B):36S-39S. doi: 10.1016/s0002-9343(01)00968-8.
2. Kripalani S et al. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007 Feb 28;297(8):831-41. doi: 10.1001/jama.297.8.831.
3. Scotten M et al. Minding the gap: Interprofessional communication during inpatient and post discharge chasm care. Patient Educ Couns. 2015 Jul;98(7):895-900. doi: 10.1016/j.pec.2015.03.009.
4. Back A et al. Communication skills in the age of COVID-19. Ann Intern Med. 2020 Jun 2;172(11):759-60. doi: 10.7326/M20-1376.
5. Hart JL et al. Family-centered care during the COVID-19 era. J Pain Symptom Manage. 2020 Aug;60(2):e93-7. doi: 10.1016/j.jpainsymman.2020.04.017.
6. Rubinelli S et al. Implications of the current COVID-19 pandemic for communication in healthcare. Patient Educ Couns. 2020 Jun;103(6):1067-9. doi: 10.1016/j.pec.2020.04.021.
7. Simpson N et al. Don’t forget shared decision-making in the COVID-19 crisis. Intern Med J. 2020 Jun;50(6):761-3. doi: 10.1111/imj.14862.
Case narrative
A 35-year-old woman has worsening alcoholic cirrhosis and repeated admissions for ascites, hepato-renal syndrome, and alcoholic hepatitis. Upon recognition of her grave prognosis, we proceeded with a shared-management approach involving medicine, gastroenterology, social work, chaplaincy, and palliative care. When the team spoke with the patient’s health care proxy (HCP), family, and friends for collateral information and involvement in goals of care conversation, we realized that none were aware of her months-long decline and poor prognosis for recovery to hospital discharge.
Although several factors contributed to the disconnect between the patient and her support system, the obstacles were greatly exacerbated by profound changes in hospital protocol because of the COVID-19 pandemic. Physicians feel underprepared and challenged by prognostication and discussion of end of life during normal times; we believe COVID-19 has limited this essential physician role and led to tragic delays in effective communication and end of life planning.
Closing the loop
For patients with complex medical issues or those reaching end of life, effective communication within the health care system is critical. While inpatient teams often drive the plan, they care for their patients during a snapshot in time; contrarily, primary care providers and specialists often have established longitudinal relationships with their patients. Ergo, clinicians should communicate directly, and ideally with both patients and families, to achieve patient-centered and goal-concordant care.
For medically complex patients, PCPs tend to prefer verbal hand-offs. Timely and reliable communication between inpatient and outpatient providers has also been shown to prevent medical adverse events.1 Despite this, direct communication occurs infrequently.2 Given that hospitalists serve as primary inpatient providers for most general admissions, it is their responsibility to communicate with outpatient providers.
A multidisciplinary team redesigned the process by which PCPs were contacted following patient discharge. The transmission of information should ideally occur prior to discharge.3 Deficits in communication are extremely common and may negatively impact patient care, patient satisfaction, and patient safety.
Changes during the COVID-19 era
During the pandemic, patients have only one visitor per day, restricted visiting hours, and limited interactions with clinicians per implemented policies. Along with the increased burdens from personal protective equipment, remote hospital providers (social workers, case managers), and increased bureaucratic duties, COVID-19 has elucidated limitations in medical capacity and revealed the difficulties that clinicians face in communicating with patients and families, especially about serious illness.
Tasks include facilitating virtual goodbyes between dying patients and families, conducting family meetings via teleconference, and discussing patient care with specialists through virtual technologies.4 While these tasks are arguably more important during a global disaster, COVID-19 paradoxically restricts physical presence and severely hinders communication.5 Clinicians should continue to utilize core skills like building rapport, assessing patient/family perspectives and agenda, and using empathy.6 Patients tend to more frequently value functional outcomes while clinicians tend to default to treatment modalities.7 Additionally, goals of care and end of life discussions are associated with improved quality of life, fewer aggressive medical interventions near death, and even increased survival.
Given the limited resources and difficulties in communication during the pandemic, clinicians should place greater emphasis on values-based shared decision-making. Internet-based solutions are essential and widely used, and videoconferencing has been initiated at the institutional scale at many hospitals. Many clinicians with little experience are broadly implementing these technologies.7 Despite these technological innovations, issues still arise in how to communicate effectively in the hospital setting, and we must acknowledge that strategies require devices, Internet access, and technological literacy, highlighting disparities in access to quality health care.6 Conversations during the pandemic will require listening, empathy, responsive action, and the acknowledgment of the social determinants of health.7
Improving communication and transition of care
Multiple steps will be warranted to implement the safe transition process and improve communication. High-quality patient care encompasses careful review of medications, communication between inpatient and outpatient providers, and close follow-up at discharge. These steps serve to increase our reliance on patient compliance and the exchange of information about global progression of disease.
The quantitative and qualitative steps of transition of care should overcome disconnect between teams, specifically deficit areas regarding postdischarge communication, monitoring, and understanding of prognosis around the relevance to this era of COVID-19.
Dr. Haddad is a resident physician in the psychiatry residency program at Brigham and Women’s Hospital, Boston. Dr. Halporn is clinic director, Division of Adult Palliative Care, in the department of psychosocial oncology and palliative care, Dana-Farber Cancer Institute and Brigham and Women’s Hospital. Dr. Barkoudah is associate director of the Hospital Medicine Unit at Brigham and Women’s Hospital.
References
1. Goldman L et al. Passing the clinical baton: 6 principles to guide the hospitalist. Am J Med. 2001;111(9B):36S-39S. doi: 10.1016/s0002-9343(01)00968-8.
2. Kripalani S et al. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007 Feb 28;297(8):831-41. doi: 10.1001/jama.297.8.831.
3. Scotten M et al. Minding the gap: Interprofessional communication during inpatient and post discharge chasm care. Patient Educ Couns. 2015 Jul;98(7):895-900. doi: 10.1016/j.pec.2015.03.009.
4. Back A et al. Communication skills in the age of COVID-19. Ann Intern Med. 2020 Jun 2;172(11):759-60. doi: 10.7326/M20-1376.
5. Hart JL et al. Family-centered care during the COVID-19 era. J Pain Symptom Manage. 2020 Aug;60(2):e93-7. doi: 10.1016/j.jpainsymman.2020.04.017.
6. Rubinelli S et al. Implications of the current COVID-19 pandemic for communication in healthcare. Patient Educ Couns. 2020 Jun;103(6):1067-9. doi: 10.1016/j.pec.2020.04.021.
7. Simpson N et al. Don’t forget shared decision-making in the COVID-19 crisis. Intern Med J. 2020 Jun;50(6):761-3. doi: 10.1111/imj.14862.
Case narrative
A 35-year-old woman has worsening alcoholic cirrhosis and repeated admissions for ascites, hepato-renal syndrome, and alcoholic hepatitis. Upon recognition of her grave prognosis, we proceeded with a shared-management approach involving medicine, gastroenterology, social work, chaplaincy, and palliative care. When the team spoke with the patient’s health care proxy (HCP), family, and friends for collateral information and involvement in goals of care conversation, we realized that none were aware of her months-long decline and poor prognosis for recovery to hospital discharge.
Although several factors contributed to the disconnect between the patient and her support system, the obstacles were greatly exacerbated by profound changes in hospital protocol because of the COVID-19 pandemic. Physicians feel underprepared and challenged by prognostication and discussion of end of life during normal times; we believe COVID-19 has limited this essential physician role and led to tragic delays in effective communication and end of life planning.
Closing the loop
For patients with complex medical issues or those reaching end of life, effective communication within the health care system is critical. While inpatient teams often drive the plan, they care for their patients during a snapshot in time; contrarily, primary care providers and specialists often have established longitudinal relationships with their patients. Ergo, clinicians should communicate directly, and ideally with both patients and families, to achieve patient-centered and goal-concordant care.
For medically complex patients, PCPs tend to prefer verbal hand-offs. Timely and reliable communication between inpatient and outpatient providers has also been shown to prevent medical adverse events.1 Despite this, direct communication occurs infrequently.2 Given that hospitalists serve as primary inpatient providers for most general admissions, it is their responsibility to communicate with outpatient providers.
A multidisciplinary team redesigned the process by which PCPs were contacted following patient discharge. The transmission of information should ideally occur prior to discharge.3 Deficits in communication are extremely common and may negatively impact patient care, patient satisfaction, and patient safety.
Changes during the COVID-19 era
During the pandemic, patients have only one visitor per day, restricted visiting hours, and limited interactions with clinicians per implemented policies. Along with the increased burdens from personal protective equipment, remote hospital providers (social workers, case managers), and increased bureaucratic duties, COVID-19 has elucidated limitations in medical capacity and revealed the difficulties that clinicians face in communicating with patients and families, especially about serious illness.
Tasks include facilitating virtual goodbyes between dying patients and families, conducting family meetings via teleconference, and discussing patient care with specialists through virtual technologies.4 While these tasks are arguably more important during a global disaster, COVID-19 paradoxically restricts physical presence and severely hinders communication.5 Clinicians should continue to utilize core skills like building rapport, assessing patient/family perspectives and agenda, and using empathy.6 Patients tend to more frequently value functional outcomes while clinicians tend to default to treatment modalities.7 Additionally, goals of care and end of life discussions are associated with improved quality of life, fewer aggressive medical interventions near death, and even increased survival.
Given the limited resources and difficulties in communication during the pandemic, clinicians should place greater emphasis on values-based shared decision-making. Internet-based solutions are essential and widely used, and videoconferencing has been initiated at the institutional scale at many hospitals. Many clinicians with little experience are broadly implementing these technologies.7 Despite these technological innovations, issues still arise in how to communicate effectively in the hospital setting, and we must acknowledge that strategies require devices, Internet access, and technological literacy, highlighting disparities in access to quality health care.6 Conversations during the pandemic will require listening, empathy, responsive action, and the acknowledgment of the social determinants of health.7
Improving communication and transition of care
Multiple steps will be warranted to implement the safe transition process and improve communication. High-quality patient care encompasses careful review of medications, communication between inpatient and outpatient providers, and close follow-up at discharge. These steps serve to increase our reliance on patient compliance and the exchange of information about global progression of disease.
The quantitative and qualitative steps of transition of care should overcome disconnect between teams, specifically deficit areas regarding postdischarge communication, monitoring, and understanding of prognosis around the relevance to this era of COVID-19.
Dr. Haddad is a resident physician in the psychiatry residency program at Brigham and Women’s Hospital, Boston. Dr. Halporn is clinic director, Division of Adult Palliative Care, in the department of psychosocial oncology and palliative care, Dana-Farber Cancer Institute and Brigham and Women’s Hospital. Dr. Barkoudah is associate director of the Hospital Medicine Unit at Brigham and Women’s Hospital.
References
1. Goldman L et al. Passing the clinical baton: 6 principles to guide the hospitalist. Am J Med. 2001;111(9B):36S-39S. doi: 10.1016/s0002-9343(01)00968-8.
2. Kripalani S et al. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007 Feb 28;297(8):831-41. doi: 10.1001/jama.297.8.831.
3. Scotten M et al. Minding the gap: Interprofessional communication during inpatient and post discharge chasm care. Patient Educ Couns. 2015 Jul;98(7):895-900. doi: 10.1016/j.pec.2015.03.009.
4. Back A et al. Communication skills in the age of COVID-19. Ann Intern Med. 2020 Jun 2;172(11):759-60. doi: 10.7326/M20-1376.
5. Hart JL et al. Family-centered care during the COVID-19 era. J Pain Symptom Manage. 2020 Aug;60(2):e93-7. doi: 10.1016/j.jpainsymman.2020.04.017.
6. Rubinelli S et al. Implications of the current COVID-19 pandemic for communication in healthcare. Patient Educ Couns. 2020 Jun;103(6):1067-9. doi: 10.1016/j.pec.2020.04.021.
7. Simpson N et al. Don’t forget shared decision-making in the COVID-19 crisis. Intern Med J. 2020 Jun;50(6):761-3. doi: 10.1111/imj.14862.
AMA, hospital group sue federal government over surprise billing law
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
Apixaban a reasonable alternative to warfarin in patients with severe renal impairment
Background: Over 6 million Americans are prescribed anticoagulation; however, available anticoagulation options for patients with concomitant renal impairment are limited. Until recently, warfarin was the only recommended option because of a lack of data to support the use of alternative agents in such patients. This study evaluates the safety and effectiveness of apixaban, compared with warfarin, in patients with severe renal dysfunction.
Study design: Multicenter retrospective cohort study.
Setting: Seven hospitals in Michigan between January 2013 and December 2015 and including adult patients with CrCl less than 25 cc/min who were newly initiated on apixaban or warfarin.
Synopsis: Patients in the apixaban group (n=128) had a higher rate of heart failure, atrial fibrillation, stent placement, and hyperlipidemia, while the warfarin group (n=733) had a higher rate of prior venous thromboembolism. The primary outcome was time to first bleeding or thrombotic event. Apixaban was associated with a lower risk of thrombotic or bleeding events, compared with warfarin (HR, 0.47). Post-hoc analysis controlling for patient differences showed similar results. There was no statistical difference in the severity of events or overall mortality. Further subgroup analysis showed that 5 mg B.I.D. dosing was not associated with higher risk of bleeding than 2.5 mg B.I.D.
The main limitation is the retrospective observational design, which may have introduced confounding variables that were not accounted for in the analyses. The study also did not account for patient nonadherence to medication.
Bottom line: Apixaban is a reasonable alternative to warfarin in patients with severe renal impairment.
Citation: Hanni C et al. Outcomes associated with apixaban vs. warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11): 2366-71. doi: 10.1182/bloodadvances.2019000972.
Dr. Narayan is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Over 6 million Americans are prescribed anticoagulation; however, available anticoagulation options for patients with concomitant renal impairment are limited. Until recently, warfarin was the only recommended option because of a lack of data to support the use of alternative agents in such patients. This study evaluates the safety and effectiveness of apixaban, compared with warfarin, in patients with severe renal dysfunction.
Study design: Multicenter retrospective cohort study.
Setting: Seven hospitals in Michigan between January 2013 and December 2015 and including adult patients with CrCl less than 25 cc/min who were newly initiated on apixaban or warfarin.
Synopsis: Patients in the apixaban group (n=128) had a higher rate of heart failure, atrial fibrillation, stent placement, and hyperlipidemia, while the warfarin group (n=733) had a higher rate of prior venous thromboembolism. The primary outcome was time to first bleeding or thrombotic event. Apixaban was associated with a lower risk of thrombotic or bleeding events, compared with warfarin (HR, 0.47). Post-hoc analysis controlling for patient differences showed similar results. There was no statistical difference in the severity of events or overall mortality. Further subgroup analysis showed that 5 mg B.I.D. dosing was not associated with higher risk of bleeding than 2.5 mg B.I.D.
The main limitation is the retrospective observational design, which may have introduced confounding variables that were not accounted for in the analyses. The study also did not account for patient nonadherence to medication.
Bottom line: Apixaban is a reasonable alternative to warfarin in patients with severe renal impairment.
Citation: Hanni C et al. Outcomes associated with apixaban vs. warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11): 2366-71. doi: 10.1182/bloodadvances.2019000972.
Dr. Narayan is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Over 6 million Americans are prescribed anticoagulation; however, available anticoagulation options for patients with concomitant renal impairment are limited. Until recently, warfarin was the only recommended option because of a lack of data to support the use of alternative agents in such patients. This study evaluates the safety and effectiveness of apixaban, compared with warfarin, in patients with severe renal dysfunction.
Study design: Multicenter retrospective cohort study.
Setting: Seven hospitals in Michigan between January 2013 and December 2015 and including adult patients with CrCl less than 25 cc/min who were newly initiated on apixaban or warfarin.
Synopsis: Patients in the apixaban group (n=128) had a higher rate of heart failure, atrial fibrillation, stent placement, and hyperlipidemia, while the warfarin group (n=733) had a higher rate of prior venous thromboembolism. The primary outcome was time to first bleeding or thrombotic event. Apixaban was associated with a lower risk of thrombotic or bleeding events, compared with warfarin (HR, 0.47). Post-hoc analysis controlling for patient differences showed similar results. There was no statistical difference in the severity of events or overall mortality. Further subgroup analysis showed that 5 mg B.I.D. dosing was not associated with higher risk of bleeding than 2.5 mg B.I.D.
The main limitation is the retrospective observational design, which may have introduced confounding variables that were not accounted for in the analyses. The study also did not account for patient nonadherence to medication.
Bottom line: Apixaban is a reasonable alternative to warfarin in patients with severe renal impairment.
Citation: Hanni C et al. Outcomes associated with apixaban vs. warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11): 2366-71. doi: 10.1182/bloodadvances.2019000972.
Dr. Narayan is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
New data on rare myocarditis after COVID-19 vaccination
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
The top pediatric hospital medicine articles of 2020
The year 2020 was unlike any in recent history, particularly for those working in health care. With the onset of the SARs-CoV-2 pandemic, many physicians were met with increasing clinical demands, and hospitalists served an instrumental role in providing medical care as the world faced an unprecedented need for health care resources.
In addition, 2020 was a year in which many of us reflected on inequities both inside and outside of medicine. Many in health care witnessed the disproportionate burden that the SARs-CoV-2 pandemic placed on communities of color and inequities pertaining to vaccine distribution.
In spite of the challenges of 2020, the field of pediatric hospital medicine (PHM) has continued to grow and evolve, with an incredible amount of new literature published in 2020.
In this article, we identify the top 10 articles published in 2020, 5 of which are summarized below. These articles were presented at the Pediatric Update at SHM Converge 2021.
The top 5 articles
Association between parent comfort with English and adverse events among hospitalized children
Khan A et al. JAMA Pediatrics. December 2020.1
Background: Hospitalized children experience similar rates of medical errors compared to adult patients, but higher rates in areas that could cause harm.1 A major contributor to medical errors is communication failure, which language barriers frequently contribute to. Single-center data suggest that pediatric patients of families with limited comfort with English experience increased adverse events,2 but multicenter data are lacking.
Findings: This prospective cohort study observed adverse event rates among 2,148 patients from seven teaching hospitals from December 2014 to January 2017. Survey data revealed 147 of 1,666 (9%) parents of patient families expressed limited comfort in English, and Spanish was the predominant language in this group (71%). There were 217 adverse events reported, 142 (65%) of which were deemed preventable by study personnel. Nearly twice as many children of parents with limited comfort with English experienced an adverse event when compared to their English-speaking counterparts (26 of 147 [17.7%] vs. 146 of 1,519 [9.6%]; adjusted odds ratio, 2.1; 95% confidence interval, 1.2-3.7). Interpreter use was not measured.
Impact to practice: Children of parents with limited comfort with English are nearly twice as likely to experience adverse events when hospitalized. Hospitals should reflect on current practice and make efforts to improve their ability to identify and communicate with this vulnerable cohort.
Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children
Yeung F et al. Hospital Pediatrics. December 2020.3
Background: Peripheral intravenous catheter (PIV) insertion is performed on most hospitalized children. Unfortunately, PIVs frequently fail and need to be replaced. There is a widespread perception that infusing a crystalloid solution at a low rate through a PIV, a strategy known as “to keep vein open” (TKO) prolongs the patency of PIVs, however there is a lack of evidence to support this practice.4Findings: In this prospective, time-allocated study, 172 children were allocated to either a TKO strategy or a saline-lock strategy with a primary outcome of duration of PIV patency.3 Secondary outcomes included PIV–related complication rates and patient and caregiver satisfaction. The mean duration of PIV patency was 41.68 hours in the TKO group and 44.05 hours in the saline-lock group, which did not meet the prespecified definition of a clinically significant difference. There was no significant difference in prevalence of PIV-associated complications and patient satisfaction was similar between the two groups.
Impact to practice: Running fluid “to keep vein open” does not increase the duration of PIV patency compared to intermittent saline locks. Given that a TKO strategy limits a patient’s mobility, this low-value practice can be discontinued without increasing the risk of PIV failure.
Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol
Coon ER et al. Journal of Hospital Medicine. June 2020.5
Background: High Flow Nasal Cannula (HFNC) has been widely adopted for escalation of respiratory support in patients with bronchiolitis; however, its use is dictated by highly variant local protocols.6 Small-scale randomized control trials and systematic reviews show that early HFNC initiation in mild to moderate disease does not change patient outcomes.7Findings: In this retrospective cohort study of ward-based HFNC, the authors used the Pediatric Health Information System database to identify 12 hospitals that had adopted ward-based HFNC protocols. The study used an interrupted time series analysis to compare outcomes for patients ages 3-24 months hospitalized with bronchiolitis (n = 32,809) in the three seasons before and after protocol adoption. Ward-based HFNC adoption paradoxically increased ICU admission (absolute increase 3.1%, 95% confidence interval, 2.8-3.4%) and ICU length of stay (absolute difference 9.1 days/100 patients, 95% CI, 5.1-13.2). Total length of stay and rates of mechanical ventilation were similar between groups.5Impact to practice: Ward-based HFNC protocols are associated with increased ICU utilization. As bronchiolitis is the leading diagnosis in pediatrics, pediatric hospitals can lead ward-based quality efforts to decrease HFNC overutilization focused on decreased initiation or deimplementation.
Lower versus traditional treatment threshold for neonatal hypoglycemia
Van Kempen AAMW et al. New England Journal of Medicine. February 2020.8
Background: Hypoglycemia is the most common metabolic abnormality in newborns, and up to 30% of newborns are routinely monitored for hypoglycemia. There is no consensus regarding the appropriate threshold at which hypoglycemia should be treated in order to prevent neurologic injury. Prior studies of neonatal hypoglycemia have largely been observation and have yielded conflicting results.8Findings: In this multicenter, randomized, noninferiority trial, 689 infants born at 35 weeks gestational age or later with risk factors for hypoglycemia and a measured blood glucose of 36-46 mg/dL were randomized to either a lower glucose treatment threshold (36 mg/dL) or traditional glucose treatment threshold (47 mg/dL). The primary outcome was psychomotor development at 18 months, assessed via the Bayley Scale of Infant and Toddler Development, third edition. There was no significant difference in cognitive or motor scores at 18 months. The lower treatment threshold group had a higher frequency of severe hypoglycemia (< 36 mg/dL) and were more likely to have four or more episodes of hypoglycemia. The traditional treatment threshold group had more supplemental feeding and more IV glucose administration. Length of stay for the mother and baby did not differ between groups.8
Impact to practice: This prospective, randomized study suggests that reducing the treatment threshold for neonatal hypoglycemia did not affect neurodevelopmental at 18 months of age. In contrast, a recent meta-analysis by Shah et al. suggested that neonatal hypoglycemia was not associated with adverse neurodevelopmental outcomes in early childhood; however, differences in rates of neurodevelopmental impairment, low literacy, and low numeracy were detectable by age five.9
Factors associated with family experience in pediatric inpatient care
Feng JY et al. Pediatrics. March 2020 Mar.10
Background: Positive patient experience is associated with better health care outcomes and reduced health care use.11 Consequently, patient experience surveys have played a larger role in public reporting, financial risk sharing arrangements, and pay for performance programs. While adult studies have examined the importance of specific care dimensions for patient experience, data are lacking for inpatient pediatric populations.
Findings: A retrospective study collected Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys from 17,727 patients in 69 hospitals within the United States over a 14-month period.10 Of the 10 care dimensions analyzed, child comfort (aOR 1.50; 95% CI, 1.41-1.60) and nurse-parent communication (aOR 1.50; 95% CI, 1.42-1.58) were most strongly associated with a family’s willingness to recommend a hospital. Additional associated indices included preparing to leave the hospital (aOR 1.34; 95% CI, 1.27-1.41), doctor-parent communication (aOR 1.28; 95% CI, 1.21–1.35), and keeping parents informed (aOR 1.25; 95% CI, 1.18-1.33). Privacy and quietness, which are associated with positive patient experience in adult studies, were not significantly associated with willingness to recommend in this cohort.
Impact to practice: Hospitals seeking to improve patient experience will benefit most by focusing on improving patient comfort and nurse-parent communication. Factors that increase adult patient satisfaction may not be as important to the pediatric population and their families.
The other five articles that comprised the top 10 are listed below:
Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis
Coon ER et al. JAMA Pediatrics. September 2020.12
Clinical prediction rule for distinguishing bacterial from aseptic meningitis
Mintegi S et al. Pediatrics. September 2020.13
The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics: miniMAGIC Ullman AJ et al. Pediatrics. June 2020.14
A structured neonatal parenting elective: An approach for parenting leave during residency
Cree-Green M et al. Academic Pediatrics. Aug 2020.15
The KidzMed project: Teaching children to swallow tablet medication
Tse Y et al. Archives of Disease in Childhood. November 2020.16
Dr. Steed is an internal medicine and pediatrics hospitalist at Northwestern Memorial Hospital and Ann and Robert H. Lurie’s Children’s Hospital of Chicago. Dr. Fisher is a current fellow in hospice and palliative medicine and a clinical assistant professor at Michigan State University. Dr. Money is an assistant professor of pediatrics at the University of Utah and a fellowship-trained pediatric hospitalist at Utah Valley Hospital and Primary Children’s Hospital.
References
1. Khan A et al. Association between parent comfort with english and adverse events among hospitalized children. JAMA Pediatr. 2020 Dec 1;174(12):e203215. doi: 10.1001/jamapediatrics.2020.3215.
2. Wasserman M et al. Identifying and preventing medical errors in patients with limited English proficiency: Key findings and tools for the field. J Healthc Qual. May-Jun 2014;36(3):5-16. doi: 10.1111/jhq.12065.
3. Yeung F et al. Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children. Hosp Pediatr. 2020 Dec;10(12):1038-43. doi: 10.1542/hpeds.2020-0137.
4. Mok E et al. A randomized controlled trial for maintaining peripheral intravenous lock in children. Int J Nurs Pract. 2007 Feb;13(1):33-45. doi: 10.1111/j.1440-172X.2006.00607.x.
5. Coon ER et al. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020 Jun;15(6):325-30. doi: 10.12788/jhm.3417.
6. Kalburgi S and Halley T. High-flow nasal cannula use outside of the ICU setting. Pediatrics. 2020;146(5):e20194083. doi: 10.1542/peds.2019-4083.
7. Leyenaar JK and Ralston SL. Widespread adoption of low-value therapy: The case of bronchiolitis and high-flow oxygen. Pediatrics. 2020 Nov;146(5):e2020021188. doi: 10.1542/peds.2020-021188.
8. Van Kempen AAMW et al. Lower versus traditional treatment threshold for neonatal hypoglycemia. N Engl J Med. 2020 Feb 6;382(6):534-44. doi: 10.1056/NEJMoa1905593.
9. Shah R et al. Neonatal glycaemia and neurodevelopmental outcomes: A systematic review and meta-analysis. Neonatology. 2019;115(2):116-26. doi: 10.1159/000492859.
10. Feng JY et al. Factors associated with family experience in pediatric inpatient care. Pediatrics. 2020 Mar;145(3):e20191264. doi: 10.1542/peds.2019-1264.
11. Anhang Price R et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev. 2014 Oct;71(5):522-54. doi: 10.1177/1077558714541480.
12. Coon ER et al. Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis: The Bronchiolitis Follow-up Intervention Trial (BeneFIT) randomized clinical trial. JAMA Pediatr. 2020 Sep 1;174(9):e201937. doi: 10.1001/jamapediatrics.2020.1937.
13. Mintegi S et al. Clinical prediction rule for distinguishing bacterial from aseptic meningitis. Pediatrics. 2020 Sept;146(3): e20201126. doi: 10.1542/peds.2020-1126.
14. Ullman AJ et al. The Michigan Appropriateness Guide for Intravenous Catheters in pediatrics: miniMAGIC. Pediatrics. 2020 Jun;145(Suppl 3):S269-S284. doi: 10.1542/peds.2019-3474I.
15. Cree-Green M et al. A structured neonatal parenting elective: an approach for parenting leave during residency. Acad Pediatr. 2021 Jan-Feb;21(1):16-18. doi: 10.1016/j.acap.2020.02.008.
16. Tse Y et al. The KidzMed project: Teaching children to swallow tablet medication. Arch Dis Child. 2020 Nov;105(11):1105-7. doi: 10.1136/archdischild-2019-317512.
The year 2020 was unlike any in recent history, particularly for those working in health care. With the onset of the SARs-CoV-2 pandemic, many physicians were met with increasing clinical demands, and hospitalists served an instrumental role in providing medical care as the world faced an unprecedented need for health care resources.
In addition, 2020 was a year in which many of us reflected on inequities both inside and outside of medicine. Many in health care witnessed the disproportionate burden that the SARs-CoV-2 pandemic placed on communities of color and inequities pertaining to vaccine distribution.
In spite of the challenges of 2020, the field of pediatric hospital medicine (PHM) has continued to grow and evolve, with an incredible amount of new literature published in 2020.
In this article, we identify the top 10 articles published in 2020, 5 of which are summarized below. These articles were presented at the Pediatric Update at SHM Converge 2021.
The top 5 articles
Association between parent comfort with English and adverse events among hospitalized children
Khan A et al. JAMA Pediatrics. December 2020.1
Background: Hospitalized children experience similar rates of medical errors compared to adult patients, but higher rates in areas that could cause harm.1 A major contributor to medical errors is communication failure, which language barriers frequently contribute to. Single-center data suggest that pediatric patients of families with limited comfort with English experience increased adverse events,2 but multicenter data are lacking.
Findings: This prospective cohort study observed adverse event rates among 2,148 patients from seven teaching hospitals from December 2014 to January 2017. Survey data revealed 147 of 1,666 (9%) parents of patient families expressed limited comfort in English, and Spanish was the predominant language in this group (71%). There were 217 adverse events reported, 142 (65%) of which were deemed preventable by study personnel. Nearly twice as many children of parents with limited comfort with English experienced an adverse event when compared to their English-speaking counterparts (26 of 147 [17.7%] vs. 146 of 1,519 [9.6%]; adjusted odds ratio, 2.1; 95% confidence interval, 1.2-3.7). Interpreter use was not measured.
Impact to practice: Children of parents with limited comfort with English are nearly twice as likely to experience adverse events when hospitalized. Hospitals should reflect on current practice and make efforts to improve their ability to identify and communicate with this vulnerable cohort.
Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children
Yeung F et al. Hospital Pediatrics. December 2020.3
Background: Peripheral intravenous catheter (PIV) insertion is performed on most hospitalized children. Unfortunately, PIVs frequently fail and need to be replaced. There is a widespread perception that infusing a crystalloid solution at a low rate through a PIV, a strategy known as “to keep vein open” (TKO) prolongs the patency of PIVs, however there is a lack of evidence to support this practice.4Findings: In this prospective, time-allocated study, 172 children were allocated to either a TKO strategy or a saline-lock strategy with a primary outcome of duration of PIV patency.3 Secondary outcomes included PIV–related complication rates and patient and caregiver satisfaction. The mean duration of PIV patency was 41.68 hours in the TKO group and 44.05 hours in the saline-lock group, which did not meet the prespecified definition of a clinically significant difference. There was no significant difference in prevalence of PIV-associated complications and patient satisfaction was similar between the two groups.
Impact to practice: Running fluid “to keep vein open” does not increase the duration of PIV patency compared to intermittent saline locks. Given that a TKO strategy limits a patient’s mobility, this low-value practice can be discontinued without increasing the risk of PIV failure.
Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol
Coon ER et al. Journal of Hospital Medicine. June 2020.5
Background: High Flow Nasal Cannula (HFNC) has been widely adopted for escalation of respiratory support in patients with bronchiolitis; however, its use is dictated by highly variant local protocols.6 Small-scale randomized control trials and systematic reviews show that early HFNC initiation in mild to moderate disease does not change patient outcomes.7Findings: In this retrospective cohort study of ward-based HFNC, the authors used the Pediatric Health Information System database to identify 12 hospitals that had adopted ward-based HFNC protocols. The study used an interrupted time series analysis to compare outcomes for patients ages 3-24 months hospitalized with bronchiolitis (n = 32,809) in the three seasons before and after protocol adoption. Ward-based HFNC adoption paradoxically increased ICU admission (absolute increase 3.1%, 95% confidence interval, 2.8-3.4%) and ICU length of stay (absolute difference 9.1 days/100 patients, 95% CI, 5.1-13.2). Total length of stay and rates of mechanical ventilation were similar between groups.5Impact to practice: Ward-based HFNC protocols are associated with increased ICU utilization. As bronchiolitis is the leading diagnosis in pediatrics, pediatric hospitals can lead ward-based quality efforts to decrease HFNC overutilization focused on decreased initiation or deimplementation.
Lower versus traditional treatment threshold for neonatal hypoglycemia
Van Kempen AAMW et al. New England Journal of Medicine. February 2020.8
Background: Hypoglycemia is the most common metabolic abnormality in newborns, and up to 30% of newborns are routinely monitored for hypoglycemia. There is no consensus regarding the appropriate threshold at which hypoglycemia should be treated in order to prevent neurologic injury. Prior studies of neonatal hypoglycemia have largely been observation and have yielded conflicting results.8Findings: In this multicenter, randomized, noninferiority trial, 689 infants born at 35 weeks gestational age or later with risk factors for hypoglycemia and a measured blood glucose of 36-46 mg/dL were randomized to either a lower glucose treatment threshold (36 mg/dL) or traditional glucose treatment threshold (47 mg/dL). The primary outcome was psychomotor development at 18 months, assessed via the Bayley Scale of Infant and Toddler Development, third edition. There was no significant difference in cognitive or motor scores at 18 months. The lower treatment threshold group had a higher frequency of severe hypoglycemia (< 36 mg/dL) and were more likely to have four or more episodes of hypoglycemia. The traditional treatment threshold group had more supplemental feeding and more IV glucose administration. Length of stay for the mother and baby did not differ between groups.8
Impact to practice: This prospective, randomized study suggests that reducing the treatment threshold for neonatal hypoglycemia did not affect neurodevelopmental at 18 months of age. In contrast, a recent meta-analysis by Shah et al. suggested that neonatal hypoglycemia was not associated with adverse neurodevelopmental outcomes in early childhood; however, differences in rates of neurodevelopmental impairment, low literacy, and low numeracy were detectable by age five.9
Factors associated with family experience in pediatric inpatient care
Feng JY et al. Pediatrics. March 2020 Mar.10
Background: Positive patient experience is associated with better health care outcomes and reduced health care use.11 Consequently, patient experience surveys have played a larger role in public reporting, financial risk sharing arrangements, and pay for performance programs. While adult studies have examined the importance of specific care dimensions for patient experience, data are lacking for inpatient pediatric populations.
Findings: A retrospective study collected Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys from 17,727 patients in 69 hospitals within the United States over a 14-month period.10 Of the 10 care dimensions analyzed, child comfort (aOR 1.50; 95% CI, 1.41-1.60) and nurse-parent communication (aOR 1.50; 95% CI, 1.42-1.58) were most strongly associated with a family’s willingness to recommend a hospital. Additional associated indices included preparing to leave the hospital (aOR 1.34; 95% CI, 1.27-1.41), doctor-parent communication (aOR 1.28; 95% CI, 1.21–1.35), and keeping parents informed (aOR 1.25; 95% CI, 1.18-1.33). Privacy and quietness, which are associated with positive patient experience in adult studies, were not significantly associated with willingness to recommend in this cohort.
Impact to practice: Hospitals seeking to improve patient experience will benefit most by focusing on improving patient comfort and nurse-parent communication. Factors that increase adult patient satisfaction may not be as important to the pediatric population and their families.
The other five articles that comprised the top 10 are listed below:
Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis
Coon ER et al. JAMA Pediatrics. September 2020.12
Clinical prediction rule for distinguishing bacterial from aseptic meningitis
Mintegi S et al. Pediatrics. September 2020.13
The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics: miniMAGIC Ullman AJ et al. Pediatrics. June 2020.14
A structured neonatal parenting elective: An approach for parenting leave during residency
Cree-Green M et al. Academic Pediatrics. Aug 2020.15
The KidzMed project: Teaching children to swallow tablet medication
Tse Y et al. Archives of Disease in Childhood. November 2020.16
Dr. Steed is an internal medicine and pediatrics hospitalist at Northwestern Memorial Hospital and Ann and Robert H. Lurie’s Children’s Hospital of Chicago. Dr. Fisher is a current fellow in hospice and palliative medicine and a clinical assistant professor at Michigan State University. Dr. Money is an assistant professor of pediatrics at the University of Utah and a fellowship-trained pediatric hospitalist at Utah Valley Hospital and Primary Children’s Hospital.
References
1. Khan A et al. Association between parent comfort with english and adverse events among hospitalized children. JAMA Pediatr. 2020 Dec 1;174(12):e203215. doi: 10.1001/jamapediatrics.2020.3215.
2. Wasserman M et al. Identifying and preventing medical errors in patients with limited English proficiency: Key findings and tools for the field. J Healthc Qual. May-Jun 2014;36(3):5-16. doi: 10.1111/jhq.12065.
3. Yeung F et al. Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children. Hosp Pediatr. 2020 Dec;10(12):1038-43. doi: 10.1542/hpeds.2020-0137.
4. Mok E et al. A randomized controlled trial for maintaining peripheral intravenous lock in children. Int J Nurs Pract. 2007 Feb;13(1):33-45. doi: 10.1111/j.1440-172X.2006.00607.x.
5. Coon ER et al. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020 Jun;15(6):325-30. doi: 10.12788/jhm.3417.
6. Kalburgi S and Halley T. High-flow nasal cannula use outside of the ICU setting. Pediatrics. 2020;146(5):e20194083. doi: 10.1542/peds.2019-4083.
7. Leyenaar JK and Ralston SL. Widespread adoption of low-value therapy: The case of bronchiolitis and high-flow oxygen. Pediatrics. 2020 Nov;146(5):e2020021188. doi: 10.1542/peds.2020-021188.
8. Van Kempen AAMW et al. Lower versus traditional treatment threshold for neonatal hypoglycemia. N Engl J Med. 2020 Feb 6;382(6):534-44. doi: 10.1056/NEJMoa1905593.
9. Shah R et al. Neonatal glycaemia and neurodevelopmental outcomes: A systematic review and meta-analysis. Neonatology. 2019;115(2):116-26. doi: 10.1159/000492859.
10. Feng JY et al. Factors associated with family experience in pediatric inpatient care. Pediatrics. 2020 Mar;145(3):e20191264. doi: 10.1542/peds.2019-1264.
11. Anhang Price R et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev. 2014 Oct;71(5):522-54. doi: 10.1177/1077558714541480.
12. Coon ER et al. Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis: The Bronchiolitis Follow-up Intervention Trial (BeneFIT) randomized clinical trial. JAMA Pediatr. 2020 Sep 1;174(9):e201937. doi: 10.1001/jamapediatrics.2020.1937.
13. Mintegi S et al. Clinical prediction rule for distinguishing bacterial from aseptic meningitis. Pediatrics. 2020 Sept;146(3): e20201126. doi: 10.1542/peds.2020-1126.
14. Ullman AJ et al. The Michigan Appropriateness Guide for Intravenous Catheters in pediatrics: miniMAGIC. Pediatrics. 2020 Jun;145(Suppl 3):S269-S284. doi: 10.1542/peds.2019-3474I.
15. Cree-Green M et al. A structured neonatal parenting elective: an approach for parenting leave during residency. Acad Pediatr. 2021 Jan-Feb;21(1):16-18. doi: 10.1016/j.acap.2020.02.008.
16. Tse Y et al. The KidzMed project: Teaching children to swallow tablet medication. Arch Dis Child. 2020 Nov;105(11):1105-7. doi: 10.1136/archdischild-2019-317512.
The year 2020 was unlike any in recent history, particularly for those working in health care. With the onset of the SARs-CoV-2 pandemic, many physicians were met with increasing clinical demands, and hospitalists served an instrumental role in providing medical care as the world faced an unprecedented need for health care resources.
In addition, 2020 was a year in which many of us reflected on inequities both inside and outside of medicine. Many in health care witnessed the disproportionate burden that the SARs-CoV-2 pandemic placed on communities of color and inequities pertaining to vaccine distribution.
In spite of the challenges of 2020, the field of pediatric hospital medicine (PHM) has continued to grow and evolve, with an incredible amount of new literature published in 2020.
In this article, we identify the top 10 articles published in 2020, 5 of which are summarized below. These articles were presented at the Pediatric Update at SHM Converge 2021.
The top 5 articles
Association between parent comfort with English and adverse events among hospitalized children
Khan A et al. JAMA Pediatrics. December 2020.1
Background: Hospitalized children experience similar rates of medical errors compared to adult patients, but higher rates in areas that could cause harm.1 A major contributor to medical errors is communication failure, which language barriers frequently contribute to. Single-center data suggest that pediatric patients of families with limited comfort with English experience increased adverse events,2 but multicenter data are lacking.
Findings: This prospective cohort study observed adverse event rates among 2,148 patients from seven teaching hospitals from December 2014 to January 2017. Survey data revealed 147 of 1,666 (9%) parents of patient families expressed limited comfort in English, and Spanish was the predominant language in this group (71%). There were 217 adverse events reported, 142 (65%) of which were deemed preventable by study personnel. Nearly twice as many children of parents with limited comfort with English experienced an adverse event when compared to their English-speaking counterparts (26 of 147 [17.7%] vs. 146 of 1,519 [9.6%]; adjusted odds ratio, 2.1; 95% confidence interval, 1.2-3.7). Interpreter use was not measured.
Impact to practice: Children of parents with limited comfort with English are nearly twice as likely to experience adverse events when hospitalized. Hospitals should reflect on current practice and make efforts to improve their ability to identify and communicate with this vulnerable cohort.
Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children
Yeung F et al. Hospital Pediatrics. December 2020.3
Background: Peripheral intravenous catheter (PIV) insertion is performed on most hospitalized children. Unfortunately, PIVs frequently fail and need to be replaced. There is a widespread perception that infusing a crystalloid solution at a low rate through a PIV, a strategy known as “to keep vein open” (TKO) prolongs the patency of PIVs, however there is a lack of evidence to support this practice.4Findings: In this prospective, time-allocated study, 172 children were allocated to either a TKO strategy or a saline-lock strategy with a primary outcome of duration of PIV patency.3 Secondary outcomes included PIV–related complication rates and patient and caregiver satisfaction. The mean duration of PIV patency was 41.68 hours in the TKO group and 44.05 hours in the saline-lock group, which did not meet the prespecified definition of a clinically significant difference. There was no significant difference in prevalence of PIV-associated complications and patient satisfaction was similar between the two groups.
Impact to practice: Running fluid “to keep vein open” does not increase the duration of PIV patency compared to intermittent saline locks. Given that a TKO strategy limits a patient’s mobility, this low-value practice can be discontinued without increasing the risk of PIV failure.
Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol
Coon ER et al. Journal of Hospital Medicine. June 2020.5
Background: High Flow Nasal Cannula (HFNC) has been widely adopted for escalation of respiratory support in patients with bronchiolitis; however, its use is dictated by highly variant local protocols.6 Small-scale randomized control trials and systematic reviews show that early HFNC initiation in mild to moderate disease does not change patient outcomes.7Findings: In this retrospective cohort study of ward-based HFNC, the authors used the Pediatric Health Information System database to identify 12 hospitals that had adopted ward-based HFNC protocols. The study used an interrupted time series analysis to compare outcomes for patients ages 3-24 months hospitalized with bronchiolitis (n = 32,809) in the three seasons before and after protocol adoption. Ward-based HFNC adoption paradoxically increased ICU admission (absolute increase 3.1%, 95% confidence interval, 2.8-3.4%) and ICU length of stay (absolute difference 9.1 days/100 patients, 95% CI, 5.1-13.2). Total length of stay and rates of mechanical ventilation were similar between groups.5Impact to practice: Ward-based HFNC protocols are associated with increased ICU utilization. As bronchiolitis is the leading diagnosis in pediatrics, pediatric hospitals can lead ward-based quality efforts to decrease HFNC overutilization focused on decreased initiation or deimplementation.
Lower versus traditional treatment threshold for neonatal hypoglycemia
Van Kempen AAMW et al. New England Journal of Medicine. February 2020.8
Background: Hypoglycemia is the most common metabolic abnormality in newborns, and up to 30% of newborns are routinely monitored for hypoglycemia. There is no consensus regarding the appropriate threshold at which hypoglycemia should be treated in order to prevent neurologic injury. Prior studies of neonatal hypoglycemia have largely been observation and have yielded conflicting results.8Findings: In this multicenter, randomized, noninferiority trial, 689 infants born at 35 weeks gestational age or later with risk factors for hypoglycemia and a measured blood glucose of 36-46 mg/dL were randomized to either a lower glucose treatment threshold (36 mg/dL) or traditional glucose treatment threshold (47 mg/dL). The primary outcome was psychomotor development at 18 months, assessed via the Bayley Scale of Infant and Toddler Development, third edition. There was no significant difference in cognitive or motor scores at 18 months. The lower treatment threshold group had a higher frequency of severe hypoglycemia (< 36 mg/dL) and were more likely to have four or more episodes of hypoglycemia. The traditional treatment threshold group had more supplemental feeding and more IV glucose administration. Length of stay for the mother and baby did not differ between groups.8
Impact to practice: This prospective, randomized study suggests that reducing the treatment threshold for neonatal hypoglycemia did not affect neurodevelopmental at 18 months of age. In contrast, a recent meta-analysis by Shah et al. suggested that neonatal hypoglycemia was not associated with adverse neurodevelopmental outcomes in early childhood; however, differences in rates of neurodevelopmental impairment, low literacy, and low numeracy were detectable by age five.9
Factors associated with family experience in pediatric inpatient care
Feng JY et al. Pediatrics. March 2020 Mar.10
Background: Positive patient experience is associated with better health care outcomes and reduced health care use.11 Consequently, patient experience surveys have played a larger role in public reporting, financial risk sharing arrangements, and pay for performance programs. While adult studies have examined the importance of specific care dimensions for patient experience, data are lacking for inpatient pediatric populations.
Findings: A retrospective study collected Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys from 17,727 patients in 69 hospitals within the United States over a 14-month period.10 Of the 10 care dimensions analyzed, child comfort (aOR 1.50; 95% CI, 1.41-1.60) and nurse-parent communication (aOR 1.50; 95% CI, 1.42-1.58) were most strongly associated with a family’s willingness to recommend a hospital. Additional associated indices included preparing to leave the hospital (aOR 1.34; 95% CI, 1.27-1.41), doctor-parent communication (aOR 1.28; 95% CI, 1.21–1.35), and keeping parents informed (aOR 1.25; 95% CI, 1.18-1.33). Privacy and quietness, which are associated with positive patient experience in adult studies, were not significantly associated with willingness to recommend in this cohort.
Impact to practice: Hospitals seeking to improve patient experience will benefit most by focusing on improving patient comfort and nurse-parent communication. Factors that increase adult patient satisfaction may not be as important to the pediatric population and their families.
The other five articles that comprised the top 10 are listed below:
Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis
Coon ER et al. JAMA Pediatrics. September 2020.12
Clinical prediction rule for distinguishing bacterial from aseptic meningitis
Mintegi S et al. Pediatrics. September 2020.13
The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics: miniMAGIC Ullman AJ et al. Pediatrics. June 2020.14
A structured neonatal parenting elective: An approach for parenting leave during residency
Cree-Green M et al. Academic Pediatrics. Aug 2020.15
The KidzMed project: Teaching children to swallow tablet medication
Tse Y et al. Archives of Disease in Childhood. November 2020.16
Dr. Steed is an internal medicine and pediatrics hospitalist at Northwestern Memorial Hospital and Ann and Robert H. Lurie’s Children’s Hospital of Chicago. Dr. Fisher is a current fellow in hospice and palliative medicine and a clinical assistant professor at Michigan State University. Dr. Money is an assistant professor of pediatrics at the University of Utah and a fellowship-trained pediatric hospitalist at Utah Valley Hospital and Primary Children’s Hospital.
References
1. Khan A et al. Association between parent comfort with english and adverse events among hospitalized children. JAMA Pediatr. 2020 Dec 1;174(12):e203215. doi: 10.1001/jamapediatrics.2020.3215.
2. Wasserman M et al. Identifying and preventing medical errors in patients with limited English proficiency: Key findings and tools for the field. J Healthc Qual. May-Jun 2014;36(3):5-16. doi: 10.1111/jhq.12065.
3. Yeung F et al. Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children. Hosp Pediatr. 2020 Dec;10(12):1038-43. doi: 10.1542/hpeds.2020-0137.
4. Mok E et al. A randomized controlled trial for maintaining peripheral intravenous lock in children. Int J Nurs Pract. 2007 Feb;13(1):33-45. doi: 10.1111/j.1440-172X.2006.00607.x.
5. Coon ER et al. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020 Jun;15(6):325-30. doi: 10.12788/jhm.3417.
6. Kalburgi S and Halley T. High-flow nasal cannula use outside of the ICU setting. Pediatrics. 2020;146(5):e20194083. doi: 10.1542/peds.2019-4083.
7. Leyenaar JK and Ralston SL. Widespread adoption of low-value therapy: The case of bronchiolitis and high-flow oxygen. Pediatrics. 2020 Nov;146(5):e2020021188. doi: 10.1542/peds.2020-021188.
8. Van Kempen AAMW et al. Lower versus traditional treatment threshold for neonatal hypoglycemia. N Engl J Med. 2020 Feb 6;382(6):534-44. doi: 10.1056/NEJMoa1905593.
9. Shah R et al. Neonatal glycaemia and neurodevelopmental outcomes: A systematic review and meta-analysis. Neonatology. 2019;115(2):116-26. doi: 10.1159/000492859.
10. Feng JY et al. Factors associated with family experience in pediatric inpatient care. Pediatrics. 2020 Mar;145(3):e20191264. doi: 10.1542/peds.2019-1264.
11. Anhang Price R et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev. 2014 Oct;71(5):522-54. doi: 10.1177/1077558714541480.
12. Coon ER et al. Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis: The Bronchiolitis Follow-up Intervention Trial (BeneFIT) randomized clinical trial. JAMA Pediatr. 2020 Sep 1;174(9):e201937. doi: 10.1001/jamapediatrics.2020.1937.
13. Mintegi S et al. Clinical prediction rule for distinguishing bacterial from aseptic meningitis. Pediatrics. 2020 Sept;146(3): e20201126. doi: 10.1542/peds.2020-1126.
14. Ullman AJ et al. The Michigan Appropriateness Guide for Intravenous Catheters in pediatrics: miniMAGIC. Pediatrics. 2020 Jun;145(Suppl 3):S269-S284. doi: 10.1542/peds.2019-3474I.
15. Cree-Green M et al. A structured neonatal parenting elective: an approach for parenting leave during residency. Acad Pediatr. 2021 Jan-Feb;21(1):16-18. doi: 10.1016/j.acap.2020.02.008.
16. Tse Y et al. The KidzMed project: Teaching children to swallow tablet medication. Arch Dis Child. 2020 Nov;105(11):1105-7. doi: 10.1136/archdischild-2019-317512.
Timing of initiation of renal-replacement therapy in acute kidney injury
Background: Acute kidney injury (AKI) is a common complication that occurs in seriously ill patients admitted to the ICU, and many of these patients eventually require RRT. When complicated by major metabolic disorders, it is usually clear when therapy should be initiated. However, when these complications are absent, the most appropriate time to initiate RRT is unclear. There are potential advantages to performing early RRT in patients with severe AKI, such as restoring acid-base balance, preventing fluid accumulation, and preventing major electrolyte disturbances.
Study design: Multinational, randomized, controlled trial.
Setting: 168 hospitals in 15 countries.
Synopsis: Eligible patients were adults admitted to an ICU with severe AKI. Patients were randomly assigned to an accelerated strategy of RRT (initiated within 12 hours, 1,465 patients) or a standard strategy of RRT (held until conventional indications developed or AKI lasted more than 72 hours, 1,462 patients). RRT was performed in 1,418 (96.8%) in the accelerated group and 903 (61.8%) in the standard group. At 90 days, 643 deaths (43.9%) occurred in the accelerated group and 639 deaths (43.7%) occurred in the standard group (RR, 1.00; 95% CI, 0.93-1.09; P = .92). Among survivors at 90 days, 85 out of 814 accelerated patients (10.4%) and 49 of 815 standard patients (6.0%) continued to require RRT (RR, 1.75; 95% CI, 1.24-2.43), suggesting the possibility of increased dependence on long-term RRT if introduced early. Limitations include use of clinical equipoise to confirm full eligibility, introducing possible patient heterogeneity into the trial. In addition, broad discretion was given to clinicians on when to start RRT in the standard group resulting in variable initiation times.
Bottom line: In critically ill patients with severe AKI, earlier RRT did not result in lower mortality at 90 days compared with standard therapy and increased the risk of requiring RRT at 90 days.
Citation: Bagshaw SM et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383:240-51. doi: 10.1056/NEJMoa2000741.
Dr. Kim is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Acute kidney injury (AKI) is a common complication that occurs in seriously ill patients admitted to the ICU, and many of these patients eventually require RRT. When complicated by major metabolic disorders, it is usually clear when therapy should be initiated. However, when these complications are absent, the most appropriate time to initiate RRT is unclear. There are potential advantages to performing early RRT in patients with severe AKI, such as restoring acid-base balance, preventing fluid accumulation, and preventing major electrolyte disturbances.
Study design: Multinational, randomized, controlled trial.
Setting: 168 hospitals in 15 countries.
Synopsis: Eligible patients were adults admitted to an ICU with severe AKI. Patients were randomly assigned to an accelerated strategy of RRT (initiated within 12 hours, 1,465 patients) or a standard strategy of RRT (held until conventional indications developed or AKI lasted more than 72 hours, 1,462 patients). RRT was performed in 1,418 (96.8%) in the accelerated group and 903 (61.8%) in the standard group. At 90 days, 643 deaths (43.9%) occurred in the accelerated group and 639 deaths (43.7%) occurred in the standard group (RR, 1.00; 95% CI, 0.93-1.09; P = .92). Among survivors at 90 days, 85 out of 814 accelerated patients (10.4%) and 49 of 815 standard patients (6.0%) continued to require RRT (RR, 1.75; 95% CI, 1.24-2.43), suggesting the possibility of increased dependence on long-term RRT if introduced early. Limitations include use of clinical equipoise to confirm full eligibility, introducing possible patient heterogeneity into the trial. In addition, broad discretion was given to clinicians on when to start RRT in the standard group resulting in variable initiation times.
Bottom line: In critically ill patients with severe AKI, earlier RRT did not result in lower mortality at 90 days compared with standard therapy and increased the risk of requiring RRT at 90 days.
Citation: Bagshaw SM et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383:240-51. doi: 10.1056/NEJMoa2000741.
Dr. Kim is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Acute kidney injury (AKI) is a common complication that occurs in seriously ill patients admitted to the ICU, and many of these patients eventually require RRT. When complicated by major metabolic disorders, it is usually clear when therapy should be initiated. However, when these complications are absent, the most appropriate time to initiate RRT is unclear. There are potential advantages to performing early RRT in patients with severe AKI, such as restoring acid-base balance, preventing fluid accumulation, and preventing major electrolyte disturbances.
Study design: Multinational, randomized, controlled trial.
Setting: 168 hospitals in 15 countries.
Synopsis: Eligible patients were adults admitted to an ICU with severe AKI. Patients were randomly assigned to an accelerated strategy of RRT (initiated within 12 hours, 1,465 patients) or a standard strategy of RRT (held until conventional indications developed or AKI lasted more than 72 hours, 1,462 patients). RRT was performed in 1,418 (96.8%) in the accelerated group and 903 (61.8%) in the standard group. At 90 days, 643 deaths (43.9%) occurred in the accelerated group and 639 deaths (43.7%) occurred in the standard group (RR, 1.00; 95% CI, 0.93-1.09; P = .92). Among survivors at 90 days, 85 out of 814 accelerated patients (10.4%) and 49 of 815 standard patients (6.0%) continued to require RRT (RR, 1.75; 95% CI, 1.24-2.43), suggesting the possibility of increased dependence on long-term RRT if introduced early. Limitations include use of clinical equipoise to confirm full eligibility, introducing possible patient heterogeneity into the trial. In addition, broad discretion was given to clinicians on when to start RRT in the standard group resulting in variable initiation times.
Bottom line: In critically ill patients with severe AKI, earlier RRT did not result in lower mortality at 90 days compared with standard therapy and increased the risk of requiring RRT at 90 days.
Citation: Bagshaw SM et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383:240-51. doi: 10.1056/NEJMoa2000741.
Dr. Kim is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
The importance of self-compassion for hospitalists
A mindful way relate to ourselves
Physicians, clinicians, providers, healers, and now heroes, are some of the names we have been given throughout history. These titles bring together a universal concept in medicine that all human beings deserve compassion, understanding, and care. However, as health care providers we forget to show ourselves the same compassion we bestow upon others.
Self-compassion is a new way of relating to ourselves. As clinicians, we are trained investigators, delving deeper into what our patient is thinking and feeling. “Tell me more about that. How does that make you feel? That must have been (very painful/scary/frustrating).” These are a few statements we learned in patient interviewing to actively engage with patients, build rapport, solidify trust, validate their concerns, and ultimately obtain the information needed to diagnose and heal.
We know the importance of looking beyond the surface, as more often than not a deeper inspection reveals more to the story. We have uncovered cracks in the foundation, erosion of the roof, worn out siding, and a glimpse into the complexities that make up each individual. We look at our patients, loved ones, and the world with night-vision lenses to uncover what is deeper.
Clinicians are good at directing compassion toward others, but not as good at giving it to themselves.1 Many health care providers may see self-compassion as soft, weak, selfish, or unnecessary. However, mindful self-compassion is a positive practice that opens a pathway for healing, personal growth, and protection against the negative consequences of self-judgment, isolation, anxiety, burnout, and depression.
What is self-compassion?
Kristin Neff, PhD, an associate professor in educational psychology at the University of Texas at Austin, was the first to academically define self-compassion. Self-compassion brings together three core elements – kindness, humanity, and mindfulness.2 Self-compassion involves acting the same way toward yourself when you are having a difficult time as you would toward another person. Instead of mercilessly judging and criticizing yourself for self-perceived inadequacies or shortcomings, self-compassion allows you to ask yourself: “How can I give myself comfort and care in this moment?”
Mindfulness acknowledges a painful experience without resistance or judgment, while being present in the moment with things as they are. Self-compassion provides the emotional safety needed to mindfully open to our pain, disappointments, and defeats. Mindfulness and self-compassion both allow us to live with more acceptance toward ourselves and our lives. Mindfulness asks: “What am I experiencing right now?” Self-compassion asks: “What do I need right now?” When you feel compassion for yourself or another, you recognize that suffering, failure, and imperfection are all part of the shared human experience.
The physiology of self-compassion
When we practice self-compassion, we feel safe and cared for because there is a physiological pathway that explains this response. Self-compassion helps down-regulate the stress response (fight-flight-freeze). When we are triggered by a threat to our self-concept, we are likely to do one, two, or all of three things: we fight ourselves (self-criticism – often our first reaction when things go wrong), we flee from others (isolation), or we freeze (rumination).
Feeling threatened puts stress on the mind and body, and chronic stress leads to anxiety and depression, which hinders emotional and physical well-being. With self-criticism, we are both the attacker and the attacked. When we practice self-compassion, we are deactivating the threat-defense system and activating the care system, releasing oxytocin and endorphins, which reduce stress and increase feelings of safety and security.3
Why is self-compassion important to provider well-being?
Research has shown that individuals who are more self-compassionate tend to have greater happiness, life satisfaction, and motivation; better relationships and physical health; and less anxiety and depression. They also have the resilience needed to cope with stressful life events. The more we practice being kind and compassionate with ourselves, the more we’ll increase the habit of self-compassion, and extend compassion to our patients and loved ones in daily life.4
Why is self-compassion important? When we experience a setback at work or in life, we can become defensive, accuse others, or blame ourselves, especially when we are already under immense stress. These responses are not helpful, productive, or effective to the situation or our personal well-being. Although in the moment it may feel good to be reactive, it is a short-lived feeling that we trade for the longer-lasting effects of learning, resilience, and personal growth. Self-compassion teaches us to connect with our inner imperfections, and what makes us human, as to err is human.
To cultivate a habit of self-compassion itself, it is important to understand that self-compassion is a practice of goodwill, not good feelings. Self-compassion is aimed at the alleviation of suffering, but it does not erase any pain and suffering that does exist. The truth is, we can’t always control external forces – the events of 2020-2021 are a perfect example of this. As a result, we cannot utilize self-compassion as a practice to make our pain disappear or suppress strong emotions.
Instead, self-compassion helps us cultivate the resilience needed to mindfully acknowledge and accept a painful moment or experience, while reminding us to embrace ourselves with kindness and care in response. This builds our internal foundation with support, love, and self-care, while providing the optimal conditions for growth, resilience, and transformation
Self-compassion and the backdraft phenomenon
When you start the practice of self-compassion, you may experience backdraft, a phenomenon in which pain initially increases.5 Backdraft is similar to the stages of grief or when the flames of a burning house become larger when a door is opened and oxygen surges in. Practicing self-compassion may cause a tidal wave of emotions to come to the forefront, but it is likely that if this happens, it needs to happen.
Imagine yourself in a room with two versions of yourself. To the left is your best self that you present to the world, standing tall, organized, well kept, and without any noticeable imperfections. To the right, is the deepest part of your being, laying on the floor, filled with raw emotions – sadness, fear, anger, and love. This version of yourself is vulnerable, open, honest, and imperfect. When looking at each version of yourself, which one is the real you? The right? The left? Maybe it’s both?
Imagine what would happen if you walked over to the version of yourself on the right, sat down, and provided it comfort, and embraced yourself with love and kindness. What would happen if you gave that version of yourself a hug? Seeing your true self, with all the layers peeled away, at the very core of your being, vulnerable, and possibly broken, is a powerful and gut-wrenching experience. It may hurt at first, but once we embrace our own pain and suffering, that is where mindfulness and self-compassion intersect to begin the path to healing. It takes more strength and courage to be the version of ourselves on the right than the version on the left.
What is not self compassion?
Self-compassion is not self-pity, weakness, self-esteem, or selfishness. When individuals feel self-pity, they become immersed in their own problems and feel that they are the only ones in the world who are suffering. Self-compassion makes us more willing to accept, experience, and acknowledge difficult feelings with kindness. This paradoxically helps us process and let go of these feelings without long-term negative consequences, and with a better ability to recognize the suffering of others.
Self-compassion allows us to be our own inner ally and strengthens our ability to cope successfully when life gets hard. Self-compassion will not make you weak and vulnerable. It is a reliable source of inner strength that enhances resilience when faced with difficulties. Research shows self-compassionate people are better able to cope with tough situations like divorce, trauma, and crisis.
Self-compassion and self-esteem are important to well-being; however, they are not the same. Self-esteem refers to a judgment or evaluation of our sense of self-worth, perceived value, or how much we like ourselves. While self-compassion relates to the changing landscape of who we are with kindness and acceptance – especially in times when we feel useless, inadequate, or hopeless – self-esteem allows for greater self-clarity, independent of external circumstances, and acknowledges that all human beings deserve compassion and understanding, not because they possess certain traits or have a certain perceive valued, but because we share the human experience and the human condition of imperfection. Finally, self-compassion is not selfish, as practicing it helps people sustain the act of caring for others and decrease caregiver burnout.6,7
Strategies to practice self-compassion
There are many ways to practice self-compassion. Here are a few experiences created by Dr. Neff, a leader in the field.8
Experience 1: How would you treat a friend?
How do you think things might change if you responded to yourself in the same way you typically respond to a close friend when he or she is suffering? Why not try treating yourself like a good friend and see what happens.
Take out a sheet of paper and write down your answer to the following questions:
- First, think about times when a close friend feels really bad about him or herself or is really struggling in some way. How would you respond to your friend in this situation (especially when you’re at your best)? Write down what you typically do and say and note the tone in which you typically talk to your friends.
- Second, think about times when you feel bad about yourself or are struggling. How do you typically respond to yourself in these situations? Write down what you typically do and say, and note the tone in which you typically talk to your friends.
- Did you notice a difference? If so, ask yourself why. What factors or fears come into play that lead you to treat yourself and others so differently?
- Please write down how you think things might change if you responded to yourself in the same way you typically respond to a close friend when you’re suffering.
Experience 2: Take a self-compassion break
This practice can be used any time of day or night, with others or alone. It will help you remember to evoke the three aspects of self-compassion when you need it most.
Think of a situation in your life that is difficult, that is causing you stress. Call the situation to mind, and if you feel comfortable, allow yourself to experience these feelings and emotions, without judgment and without altering them to what you think they should be.
- Say to yourself one of the following: “This is a difficult moment,” “This is a moment of suffering,” “This is stress,” “This hurts,” or “Ouch.” Doing this step is “mindfulness”: A willingness to observe negative thoughts and emotions with openness and clarity, so that they are held in mindful awareness, without judgment.
- Find your equilibrium of observation with thoughts and feelings. Try not to suppress or deny them and try not to get caught up and swept away by them.
- Remind yourself of the shared human experience. Recognize that suffering and personal difficulty is something that we all go through rather than being something that happens to “me” alone. Remind yourself that “other people feel this way,” “I’m not alone,” and “we all have struggles in life.”
- Be kind to yourself and ask: “What do I need to hear right now to express kindness to myself?” Is there a phrase that speaks to you in your particular situation? For example: “May I give myself the compassion that I need; may I learn to accept myself as I am; may I forgive myself; may I be strong; may I be patient.” There is no wrong answer.
Exercise 3: Explore self-compassion through writing
Everybody has something about themselves that they don’t like; something that causes them to feel shame, to feel insecure, or not “good enough.” This exercise will help you write a letter to yourself about this issue from a place of acceptance and compassion. It can feel uncomfortable at first, but it gets easier with practice.
- Write about an issue you have that makes you feel inadequate or bad about yourself (physical appearance, work, or relationship issue) What emotions do you experience when you think about this aspect of yourself? Try to only feel your emotions exactly as they are – no more, no less – and then write about them.
- Write a letter as if you were talking to a dearly beloved friend who was struggling with the same concerns as you and has the same strengths and weaknesses as you. How would you convey deep compassion, especially for the pain you feel when they judge themselves so harshly? What would you write to your friend to remind them that they are only human, that all people have both strengths and weaknesses? As you write, try to infuse your letter with a strong sense of acceptance, kindness, caring, and desire for their health and happiness.
- After writing the letter, put it aside for a little while. Then come back and read it again, really letting the words sink in. Feel the compassion as it pours into you, soothing and comforting you. Love, connection, and acceptance are a part of your human right. To claim them you need only look within yourself.
Experience 4: Taking care of the caregiver
We work in the very stressful time of the COVID pandemic. As medical providers, we are caregivers to our patients and our families. Yet, we do not give ourselves time to rest, recover, and recharge. Remember, to care for others, you cannot pour from an empty cup.
- Give yourself permission to meet your own needs, recognizing that this will not only enhance your quality of life, it will also enhance your ability to be there for those that rely on you. Our time is limited but self-care can occur both at work and outside of work.
- When you are “off the clock,” be off the clock! Turn off notifications, don’t check email, and be present in your personal lives. If you are constantly answering patient calls or nursing questions until 10 p.m., that means your health care system is in need of an upgrade, as you need the appropriate coverage to give you time to care for yourself, just as well as you care for your patients.
- While at work you can practice self-care. Take 2 minutes to practice relaxation breathing. Take 1 minute to show yourself or another person gratitude. Take 5 minutes before you start writing your notes for the day to listen to relaxing music or a mindful podcast. Take 3 minutes to share three good things that happened in the day with your family or colleagues. Take 5-10 minutes to do chair yoga. Take a self-compassion break.
- Implement a 5-minute wellness break into your group’s daily function with some of the previous mentioned examples. This will allow you to care for and nurture yourself, while also caring for and nurturing others in an environment that cultivates your wellness goals.
As a hospitalist, I can attest that I did not show myself self-compassion nearly as often as I showed compassion to others. I am my own worst critic and my training taught me to suffer in silence, and not seek out others who are experiencing the same thing for fear of being perceived as weak, inadequate, or flawed.
This false notion that we need to always be tough, strong, and without emotion in order to be taken seriously, to advance, or be held in high regard is rubbish and only perpetuated by accepting it. In order to change the culture of medicine, we have to change the way we think and behave. I have practiced self-compassion exercises and it has enhanced my perspective to see that many of us are going through varying degrees of the same thing. It has shown me the positive effects on my inner being and my life. If you are ready to try something new that will benefit your psychological and emotional well-being, and help you through pain, suffering, struggles, and crisis, you have nothing to lose. Be the change, and show yourself self-compassion.
In summary, self-compassion is an attitude of warmth, curiosity, connection, and care. Learning to become more self-compassionate is a process of moving from striving to change our experience and ourselves toward embracing who we are already.9 The practice of self-compassion is giving ourselves what we need in the moment. Even if we are not ready, or it is too painful to fully accept or embrace, we can still plant the seeds that will, with time and patience, grow and bloom.
When we are mindful of our struggles, when we respond to ourselves with compassion, kindness, and give ourselves support in times of difficulty, we learn to embrace ourselves and our lives, our inner and outer imperfections, and provide ourselves with the strength needed to thrive in the most precarious and difficult situations. With self-compassion, we give the world the best of us, instead of what is left of us.
Dr. Williams is vice president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as vice president of the medical executive committee.
References
1. Sanchez-Reilly S et al. Caring for oneself to care for others: Physicians and their self-care. J Community Support Oncol. 2013;11(2):75-81. doi: 10.12788/j.suponc.0003.
2. Neff K. Self-Compassion: An Alternative Conceptualization of a Healthy Attitude Toward Oneself. Self Identity. 2010;2(2):85-101. doi: 10.1080/15298860309032.
3. Neff K et al. The forest and the trees: Examining the association of self-compassion and its positive and negative components with psychological functioning. Self Identity. 2018;17(6):627-45. doi: 10.1080/15298868.2018.1436587.
4. Zessin U et al. The relationship between self-compassion and well-being: A meta-analysis. Appl Psychol Health Well-Being. 2015;7(3):340-64. doi: 10.1111/aphw.12051.
5. Warren R et al. Self-criticism and self-compassion: Risk and resilience. Current Psychiatry. 2016 Dec;15(12):18-21,24-28,32.
6. Neff K. The Five Myths of Self-Compassion. Greater Good Magazine. 2015 Sep 30. https://greatergood.berkeley.edu/article/item/the_five_myths_of_self_compassion.
7. Neff KD and Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clin Psychol. 2013 Jan;69(1):28-44. doi: 10.1002/jclp.21923.
8. Neff K. Self-Compassion Guided Meditations and Exercises. https://self-compassion.org/category/exercises/#exercises.
9. Germer C and Neff KD. Mindful Self-Compassion (MSC), in “The handbook of mindfulness-based programs.” (London: Routledge, 2019, pp. 357-67).
A mindful way relate to ourselves
A mindful way relate to ourselves
Physicians, clinicians, providers, healers, and now heroes, are some of the names we have been given throughout history. These titles bring together a universal concept in medicine that all human beings deserve compassion, understanding, and care. However, as health care providers we forget to show ourselves the same compassion we bestow upon others.
Self-compassion is a new way of relating to ourselves. As clinicians, we are trained investigators, delving deeper into what our patient is thinking and feeling. “Tell me more about that. How does that make you feel? That must have been (very painful/scary/frustrating).” These are a few statements we learned in patient interviewing to actively engage with patients, build rapport, solidify trust, validate their concerns, and ultimately obtain the information needed to diagnose and heal.
We know the importance of looking beyond the surface, as more often than not a deeper inspection reveals more to the story. We have uncovered cracks in the foundation, erosion of the roof, worn out siding, and a glimpse into the complexities that make up each individual. We look at our patients, loved ones, and the world with night-vision lenses to uncover what is deeper.
Clinicians are good at directing compassion toward others, but not as good at giving it to themselves.1 Many health care providers may see self-compassion as soft, weak, selfish, or unnecessary. However, mindful self-compassion is a positive practice that opens a pathway for healing, personal growth, and protection against the negative consequences of self-judgment, isolation, anxiety, burnout, and depression.
What is self-compassion?
Kristin Neff, PhD, an associate professor in educational psychology at the University of Texas at Austin, was the first to academically define self-compassion. Self-compassion brings together three core elements – kindness, humanity, and mindfulness.2 Self-compassion involves acting the same way toward yourself when you are having a difficult time as you would toward another person. Instead of mercilessly judging and criticizing yourself for self-perceived inadequacies or shortcomings, self-compassion allows you to ask yourself: “How can I give myself comfort and care in this moment?”
Mindfulness acknowledges a painful experience without resistance or judgment, while being present in the moment with things as they are. Self-compassion provides the emotional safety needed to mindfully open to our pain, disappointments, and defeats. Mindfulness and self-compassion both allow us to live with more acceptance toward ourselves and our lives. Mindfulness asks: “What am I experiencing right now?” Self-compassion asks: “What do I need right now?” When you feel compassion for yourself or another, you recognize that suffering, failure, and imperfection are all part of the shared human experience.
The physiology of self-compassion
When we practice self-compassion, we feel safe and cared for because there is a physiological pathway that explains this response. Self-compassion helps down-regulate the stress response (fight-flight-freeze). When we are triggered by a threat to our self-concept, we are likely to do one, two, or all of three things: we fight ourselves (self-criticism – often our first reaction when things go wrong), we flee from others (isolation), or we freeze (rumination).
Feeling threatened puts stress on the mind and body, and chronic stress leads to anxiety and depression, which hinders emotional and physical well-being. With self-criticism, we are both the attacker and the attacked. When we practice self-compassion, we are deactivating the threat-defense system and activating the care system, releasing oxytocin and endorphins, which reduce stress and increase feelings of safety and security.3
Why is self-compassion important to provider well-being?
Research has shown that individuals who are more self-compassionate tend to have greater happiness, life satisfaction, and motivation; better relationships and physical health; and less anxiety and depression. They also have the resilience needed to cope with stressful life events. The more we practice being kind and compassionate with ourselves, the more we’ll increase the habit of self-compassion, and extend compassion to our patients and loved ones in daily life.4
Why is self-compassion important? When we experience a setback at work or in life, we can become defensive, accuse others, or blame ourselves, especially when we are already under immense stress. These responses are not helpful, productive, or effective to the situation or our personal well-being. Although in the moment it may feel good to be reactive, it is a short-lived feeling that we trade for the longer-lasting effects of learning, resilience, and personal growth. Self-compassion teaches us to connect with our inner imperfections, and what makes us human, as to err is human.
To cultivate a habit of self-compassion itself, it is important to understand that self-compassion is a practice of goodwill, not good feelings. Self-compassion is aimed at the alleviation of suffering, but it does not erase any pain and suffering that does exist. The truth is, we can’t always control external forces – the events of 2020-2021 are a perfect example of this. As a result, we cannot utilize self-compassion as a practice to make our pain disappear or suppress strong emotions.
Instead, self-compassion helps us cultivate the resilience needed to mindfully acknowledge and accept a painful moment or experience, while reminding us to embrace ourselves with kindness and care in response. This builds our internal foundation with support, love, and self-care, while providing the optimal conditions for growth, resilience, and transformation
Self-compassion and the backdraft phenomenon
When you start the practice of self-compassion, you may experience backdraft, a phenomenon in which pain initially increases.5 Backdraft is similar to the stages of grief or when the flames of a burning house become larger when a door is opened and oxygen surges in. Practicing self-compassion may cause a tidal wave of emotions to come to the forefront, but it is likely that if this happens, it needs to happen.
Imagine yourself in a room with two versions of yourself. To the left is your best self that you present to the world, standing tall, organized, well kept, and without any noticeable imperfections. To the right, is the deepest part of your being, laying on the floor, filled with raw emotions – sadness, fear, anger, and love. This version of yourself is vulnerable, open, honest, and imperfect. When looking at each version of yourself, which one is the real you? The right? The left? Maybe it’s both?
Imagine what would happen if you walked over to the version of yourself on the right, sat down, and provided it comfort, and embraced yourself with love and kindness. What would happen if you gave that version of yourself a hug? Seeing your true self, with all the layers peeled away, at the very core of your being, vulnerable, and possibly broken, is a powerful and gut-wrenching experience. It may hurt at first, but once we embrace our own pain and suffering, that is where mindfulness and self-compassion intersect to begin the path to healing. It takes more strength and courage to be the version of ourselves on the right than the version on the left.
What is not self compassion?
Self-compassion is not self-pity, weakness, self-esteem, or selfishness. When individuals feel self-pity, they become immersed in their own problems and feel that they are the only ones in the world who are suffering. Self-compassion makes us more willing to accept, experience, and acknowledge difficult feelings with kindness. This paradoxically helps us process and let go of these feelings without long-term negative consequences, and with a better ability to recognize the suffering of others.
Self-compassion allows us to be our own inner ally and strengthens our ability to cope successfully when life gets hard. Self-compassion will not make you weak and vulnerable. It is a reliable source of inner strength that enhances resilience when faced with difficulties. Research shows self-compassionate people are better able to cope with tough situations like divorce, trauma, and crisis.
Self-compassion and self-esteem are important to well-being; however, they are not the same. Self-esteem refers to a judgment or evaluation of our sense of self-worth, perceived value, or how much we like ourselves. While self-compassion relates to the changing landscape of who we are with kindness and acceptance – especially in times when we feel useless, inadequate, or hopeless – self-esteem allows for greater self-clarity, independent of external circumstances, and acknowledges that all human beings deserve compassion and understanding, not because they possess certain traits or have a certain perceive valued, but because we share the human experience and the human condition of imperfection. Finally, self-compassion is not selfish, as practicing it helps people sustain the act of caring for others and decrease caregiver burnout.6,7
Strategies to practice self-compassion
There are many ways to practice self-compassion. Here are a few experiences created by Dr. Neff, a leader in the field.8
Experience 1: How would you treat a friend?
How do you think things might change if you responded to yourself in the same way you typically respond to a close friend when he or she is suffering? Why not try treating yourself like a good friend and see what happens.
Take out a sheet of paper and write down your answer to the following questions:
- First, think about times when a close friend feels really bad about him or herself or is really struggling in some way. How would you respond to your friend in this situation (especially when you’re at your best)? Write down what you typically do and say and note the tone in which you typically talk to your friends.
- Second, think about times when you feel bad about yourself or are struggling. How do you typically respond to yourself in these situations? Write down what you typically do and say, and note the tone in which you typically talk to your friends.
- Did you notice a difference? If so, ask yourself why. What factors or fears come into play that lead you to treat yourself and others so differently?
- Please write down how you think things might change if you responded to yourself in the same way you typically respond to a close friend when you’re suffering.
Experience 2: Take a self-compassion break
This practice can be used any time of day or night, with others or alone. It will help you remember to evoke the three aspects of self-compassion when you need it most.
Think of a situation in your life that is difficult, that is causing you stress. Call the situation to mind, and if you feel comfortable, allow yourself to experience these feelings and emotions, without judgment and without altering them to what you think they should be.
- Say to yourself one of the following: “This is a difficult moment,” “This is a moment of suffering,” “This is stress,” “This hurts,” or “Ouch.” Doing this step is “mindfulness”: A willingness to observe negative thoughts and emotions with openness and clarity, so that they are held in mindful awareness, without judgment.
- Find your equilibrium of observation with thoughts and feelings. Try not to suppress or deny them and try not to get caught up and swept away by them.
- Remind yourself of the shared human experience. Recognize that suffering and personal difficulty is something that we all go through rather than being something that happens to “me” alone. Remind yourself that “other people feel this way,” “I’m not alone,” and “we all have struggles in life.”
- Be kind to yourself and ask: “What do I need to hear right now to express kindness to myself?” Is there a phrase that speaks to you in your particular situation? For example: “May I give myself the compassion that I need; may I learn to accept myself as I am; may I forgive myself; may I be strong; may I be patient.” There is no wrong answer.
Exercise 3: Explore self-compassion through writing
Everybody has something about themselves that they don’t like; something that causes them to feel shame, to feel insecure, or not “good enough.” This exercise will help you write a letter to yourself about this issue from a place of acceptance and compassion. It can feel uncomfortable at first, but it gets easier with practice.
- Write about an issue you have that makes you feel inadequate or bad about yourself (physical appearance, work, or relationship issue) What emotions do you experience when you think about this aspect of yourself? Try to only feel your emotions exactly as they are – no more, no less – and then write about them.
- Write a letter as if you were talking to a dearly beloved friend who was struggling with the same concerns as you and has the same strengths and weaknesses as you. How would you convey deep compassion, especially for the pain you feel when they judge themselves so harshly? What would you write to your friend to remind them that they are only human, that all people have both strengths and weaknesses? As you write, try to infuse your letter with a strong sense of acceptance, kindness, caring, and desire for their health and happiness.
- After writing the letter, put it aside for a little while. Then come back and read it again, really letting the words sink in. Feel the compassion as it pours into you, soothing and comforting you. Love, connection, and acceptance are a part of your human right. To claim them you need only look within yourself.
Experience 4: Taking care of the caregiver
We work in the very stressful time of the COVID pandemic. As medical providers, we are caregivers to our patients and our families. Yet, we do not give ourselves time to rest, recover, and recharge. Remember, to care for others, you cannot pour from an empty cup.
- Give yourself permission to meet your own needs, recognizing that this will not only enhance your quality of life, it will also enhance your ability to be there for those that rely on you. Our time is limited but self-care can occur both at work and outside of work.
- When you are “off the clock,” be off the clock! Turn off notifications, don’t check email, and be present in your personal lives. If you are constantly answering patient calls or nursing questions until 10 p.m., that means your health care system is in need of an upgrade, as you need the appropriate coverage to give you time to care for yourself, just as well as you care for your patients.
- While at work you can practice self-care. Take 2 minutes to practice relaxation breathing. Take 1 minute to show yourself or another person gratitude. Take 5 minutes before you start writing your notes for the day to listen to relaxing music or a mindful podcast. Take 3 minutes to share three good things that happened in the day with your family or colleagues. Take 5-10 minutes to do chair yoga. Take a self-compassion break.
- Implement a 5-minute wellness break into your group’s daily function with some of the previous mentioned examples. This will allow you to care for and nurture yourself, while also caring for and nurturing others in an environment that cultivates your wellness goals.
As a hospitalist, I can attest that I did not show myself self-compassion nearly as often as I showed compassion to others. I am my own worst critic and my training taught me to suffer in silence, and not seek out others who are experiencing the same thing for fear of being perceived as weak, inadequate, or flawed.
This false notion that we need to always be tough, strong, and without emotion in order to be taken seriously, to advance, or be held in high regard is rubbish and only perpetuated by accepting it. In order to change the culture of medicine, we have to change the way we think and behave. I have practiced self-compassion exercises and it has enhanced my perspective to see that many of us are going through varying degrees of the same thing. It has shown me the positive effects on my inner being and my life. If you are ready to try something new that will benefit your psychological and emotional well-being, and help you through pain, suffering, struggles, and crisis, you have nothing to lose. Be the change, and show yourself self-compassion.
In summary, self-compassion is an attitude of warmth, curiosity, connection, and care. Learning to become more self-compassionate is a process of moving from striving to change our experience and ourselves toward embracing who we are already.9 The practice of self-compassion is giving ourselves what we need in the moment. Even if we are not ready, or it is too painful to fully accept or embrace, we can still plant the seeds that will, with time and patience, grow and bloom.
When we are mindful of our struggles, when we respond to ourselves with compassion, kindness, and give ourselves support in times of difficulty, we learn to embrace ourselves and our lives, our inner and outer imperfections, and provide ourselves with the strength needed to thrive in the most precarious and difficult situations. With self-compassion, we give the world the best of us, instead of what is left of us.
Dr. Williams is vice president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as vice president of the medical executive committee.
References
1. Sanchez-Reilly S et al. Caring for oneself to care for others: Physicians and their self-care. J Community Support Oncol. 2013;11(2):75-81. doi: 10.12788/j.suponc.0003.
2. Neff K. Self-Compassion: An Alternative Conceptualization of a Healthy Attitude Toward Oneself. Self Identity. 2010;2(2):85-101. doi: 10.1080/15298860309032.
3. Neff K et al. The forest and the trees: Examining the association of self-compassion and its positive and negative components with psychological functioning. Self Identity. 2018;17(6):627-45. doi: 10.1080/15298868.2018.1436587.
4. Zessin U et al. The relationship between self-compassion and well-being: A meta-analysis. Appl Psychol Health Well-Being. 2015;7(3):340-64. doi: 10.1111/aphw.12051.
5. Warren R et al. Self-criticism and self-compassion: Risk and resilience. Current Psychiatry. 2016 Dec;15(12):18-21,24-28,32.
6. Neff K. The Five Myths of Self-Compassion. Greater Good Magazine. 2015 Sep 30. https://greatergood.berkeley.edu/article/item/the_five_myths_of_self_compassion.
7. Neff KD and Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clin Psychol. 2013 Jan;69(1):28-44. doi: 10.1002/jclp.21923.
8. Neff K. Self-Compassion Guided Meditations and Exercises. https://self-compassion.org/category/exercises/#exercises.
9. Germer C and Neff KD. Mindful Self-Compassion (MSC), in “The handbook of mindfulness-based programs.” (London: Routledge, 2019, pp. 357-67).
Physicians, clinicians, providers, healers, and now heroes, are some of the names we have been given throughout history. These titles bring together a universal concept in medicine that all human beings deserve compassion, understanding, and care. However, as health care providers we forget to show ourselves the same compassion we bestow upon others.
Self-compassion is a new way of relating to ourselves. As clinicians, we are trained investigators, delving deeper into what our patient is thinking and feeling. “Tell me more about that. How does that make you feel? That must have been (very painful/scary/frustrating).” These are a few statements we learned in patient interviewing to actively engage with patients, build rapport, solidify trust, validate their concerns, and ultimately obtain the information needed to diagnose and heal.
We know the importance of looking beyond the surface, as more often than not a deeper inspection reveals more to the story. We have uncovered cracks in the foundation, erosion of the roof, worn out siding, and a glimpse into the complexities that make up each individual. We look at our patients, loved ones, and the world with night-vision lenses to uncover what is deeper.
Clinicians are good at directing compassion toward others, but not as good at giving it to themselves.1 Many health care providers may see self-compassion as soft, weak, selfish, or unnecessary. However, mindful self-compassion is a positive practice that opens a pathway for healing, personal growth, and protection against the negative consequences of self-judgment, isolation, anxiety, burnout, and depression.
What is self-compassion?
Kristin Neff, PhD, an associate professor in educational psychology at the University of Texas at Austin, was the first to academically define self-compassion. Self-compassion brings together three core elements – kindness, humanity, and mindfulness.2 Self-compassion involves acting the same way toward yourself when you are having a difficult time as you would toward another person. Instead of mercilessly judging and criticizing yourself for self-perceived inadequacies or shortcomings, self-compassion allows you to ask yourself: “How can I give myself comfort and care in this moment?”
Mindfulness acknowledges a painful experience without resistance or judgment, while being present in the moment with things as they are. Self-compassion provides the emotional safety needed to mindfully open to our pain, disappointments, and defeats. Mindfulness and self-compassion both allow us to live with more acceptance toward ourselves and our lives. Mindfulness asks: “What am I experiencing right now?” Self-compassion asks: “What do I need right now?” When you feel compassion for yourself or another, you recognize that suffering, failure, and imperfection are all part of the shared human experience.
The physiology of self-compassion
When we practice self-compassion, we feel safe and cared for because there is a physiological pathway that explains this response. Self-compassion helps down-regulate the stress response (fight-flight-freeze). When we are triggered by a threat to our self-concept, we are likely to do one, two, or all of three things: we fight ourselves (self-criticism – often our first reaction when things go wrong), we flee from others (isolation), or we freeze (rumination).
Feeling threatened puts stress on the mind and body, and chronic stress leads to anxiety and depression, which hinders emotional and physical well-being. With self-criticism, we are both the attacker and the attacked. When we practice self-compassion, we are deactivating the threat-defense system and activating the care system, releasing oxytocin and endorphins, which reduce stress and increase feelings of safety and security.3
Why is self-compassion important to provider well-being?
Research has shown that individuals who are more self-compassionate tend to have greater happiness, life satisfaction, and motivation; better relationships and physical health; and less anxiety and depression. They also have the resilience needed to cope with stressful life events. The more we practice being kind and compassionate with ourselves, the more we’ll increase the habit of self-compassion, and extend compassion to our patients and loved ones in daily life.4
Why is self-compassion important? When we experience a setback at work or in life, we can become defensive, accuse others, or blame ourselves, especially when we are already under immense stress. These responses are not helpful, productive, or effective to the situation or our personal well-being. Although in the moment it may feel good to be reactive, it is a short-lived feeling that we trade for the longer-lasting effects of learning, resilience, and personal growth. Self-compassion teaches us to connect with our inner imperfections, and what makes us human, as to err is human.
To cultivate a habit of self-compassion itself, it is important to understand that self-compassion is a practice of goodwill, not good feelings. Self-compassion is aimed at the alleviation of suffering, but it does not erase any pain and suffering that does exist. The truth is, we can’t always control external forces – the events of 2020-2021 are a perfect example of this. As a result, we cannot utilize self-compassion as a practice to make our pain disappear or suppress strong emotions.
Instead, self-compassion helps us cultivate the resilience needed to mindfully acknowledge and accept a painful moment or experience, while reminding us to embrace ourselves with kindness and care in response. This builds our internal foundation with support, love, and self-care, while providing the optimal conditions for growth, resilience, and transformation
Self-compassion and the backdraft phenomenon
When you start the practice of self-compassion, you may experience backdraft, a phenomenon in which pain initially increases.5 Backdraft is similar to the stages of grief or when the flames of a burning house become larger when a door is opened and oxygen surges in. Practicing self-compassion may cause a tidal wave of emotions to come to the forefront, but it is likely that if this happens, it needs to happen.
Imagine yourself in a room with two versions of yourself. To the left is your best self that you present to the world, standing tall, organized, well kept, and without any noticeable imperfections. To the right, is the deepest part of your being, laying on the floor, filled with raw emotions – sadness, fear, anger, and love. This version of yourself is vulnerable, open, honest, and imperfect. When looking at each version of yourself, which one is the real you? The right? The left? Maybe it’s both?
Imagine what would happen if you walked over to the version of yourself on the right, sat down, and provided it comfort, and embraced yourself with love and kindness. What would happen if you gave that version of yourself a hug? Seeing your true self, with all the layers peeled away, at the very core of your being, vulnerable, and possibly broken, is a powerful and gut-wrenching experience. It may hurt at first, but once we embrace our own pain and suffering, that is where mindfulness and self-compassion intersect to begin the path to healing. It takes more strength and courage to be the version of ourselves on the right than the version on the left.
What is not self compassion?
Self-compassion is not self-pity, weakness, self-esteem, or selfishness. When individuals feel self-pity, they become immersed in their own problems and feel that they are the only ones in the world who are suffering. Self-compassion makes us more willing to accept, experience, and acknowledge difficult feelings with kindness. This paradoxically helps us process and let go of these feelings without long-term negative consequences, and with a better ability to recognize the suffering of others.
Self-compassion allows us to be our own inner ally and strengthens our ability to cope successfully when life gets hard. Self-compassion will not make you weak and vulnerable. It is a reliable source of inner strength that enhances resilience when faced with difficulties. Research shows self-compassionate people are better able to cope with tough situations like divorce, trauma, and crisis.
Self-compassion and self-esteem are important to well-being; however, they are not the same. Self-esteem refers to a judgment or evaluation of our sense of self-worth, perceived value, or how much we like ourselves. While self-compassion relates to the changing landscape of who we are with kindness and acceptance – especially in times when we feel useless, inadequate, or hopeless – self-esteem allows for greater self-clarity, independent of external circumstances, and acknowledges that all human beings deserve compassion and understanding, not because they possess certain traits or have a certain perceive valued, but because we share the human experience and the human condition of imperfection. Finally, self-compassion is not selfish, as practicing it helps people sustain the act of caring for others and decrease caregiver burnout.6,7
Strategies to practice self-compassion
There are many ways to practice self-compassion. Here are a few experiences created by Dr. Neff, a leader in the field.8
Experience 1: How would you treat a friend?
How do you think things might change if you responded to yourself in the same way you typically respond to a close friend when he or she is suffering? Why not try treating yourself like a good friend and see what happens.
Take out a sheet of paper and write down your answer to the following questions:
- First, think about times when a close friend feels really bad about him or herself or is really struggling in some way. How would you respond to your friend in this situation (especially when you’re at your best)? Write down what you typically do and say and note the tone in which you typically talk to your friends.
- Second, think about times when you feel bad about yourself or are struggling. How do you typically respond to yourself in these situations? Write down what you typically do and say, and note the tone in which you typically talk to your friends.
- Did you notice a difference? If so, ask yourself why. What factors or fears come into play that lead you to treat yourself and others so differently?
- Please write down how you think things might change if you responded to yourself in the same way you typically respond to a close friend when you’re suffering.
Experience 2: Take a self-compassion break
This practice can be used any time of day or night, with others or alone. It will help you remember to evoke the three aspects of self-compassion when you need it most.
Think of a situation in your life that is difficult, that is causing you stress. Call the situation to mind, and if you feel comfortable, allow yourself to experience these feelings and emotions, without judgment and without altering them to what you think they should be.
- Say to yourself one of the following: “This is a difficult moment,” “This is a moment of suffering,” “This is stress,” “This hurts,” or “Ouch.” Doing this step is “mindfulness”: A willingness to observe negative thoughts and emotions with openness and clarity, so that they are held in mindful awareness, without judgment.
- Find your equilibrium of observation with thoughts and feelings. Try not to suppress or deny them and try not to get caught up and swept away by them.
- Remind yourself of the shared human experience. Recognize that suffering and personal difficulty is something that we all go through rather than being something that happens to “me” alone. Remind yourself that “other people feel this way,” “I’m not alone,” and “we all have struggles in life.”
- Be kind to yourself and ask: “What do I need to hear right now to express kindness to myself?” Is there a phrase that speaks to you in your particular situation? For example: “May I give myself the compassion that I need; may I learn to accept myself as I am; may I forgive myself; may I be strong; may I be patient.” There is no wrong answer.
Exercise 3: Explore self-compassion through writing
Everybody has something about themselves that they don’t like; something that causes them to feel shame, to feel insecure, or not “good enough.” This exercise will help you write a letter to yourself about this issue from a place of acceptance and compassion. It can feel uncomfortable at first, but it gets easier with practice.
- Write about an issue you have that makes you feel inadequate or bad about yourself (physical appearance, work, or relationship issue) What emotions do you experience when you think about this aspect of yourself? Try to only feel your emotions exactly as they are – no more, no less – and then write about them.
- Write a letter as if you were talking to a dearly beloved friend who was struggling with the same concerns as you and has the same strengths and weaknesses as you. How would you convey deep compassion, especially for the pain you feel when they judge themselves so harshly? What would you write to your friend to remind them that they are only human, that all people have both strengths and weaknesses? As you write, try to infuse your letter with a strong sense of acceptance, kindness, caring, and desire for their health and happiness.
- After writing the letter, put it aside for a little while. Then come back and read it again, really letting the words sink in. Feel the compassion as it pours into you, soothing and comforting you. Love, connection, and acceptance are a part of your human right. To claim them you need only look within yourself.
Experience 4: Taking care of the caregiver
We work in the very stressful time of the COVID pandemic. As medical providers, we are caregivers to our patients and our families. Yet, we do not give ourselves time to rest, recover, and recharge. Remember, to care for others, you cannot pour from an empty cup.
- Give yourself permission to meet your own needs, recognizing that this will not only enhance your quality of life, it will also enhance your ability to be there for those that rely on you. Our time is limited but self-care can occur both at work and outside of work.
- When you are “off the clock,” be off the clock! Turn off notifications, don’t check email, and be present in your personal lives. If you are constantly answering patient calls or nursing questions until 10 p.m., that means your health care system is in need of an upgrade, as you need the appropriate coverage to give you time to care for yourself, just as well as you care for your patients.
- While at work you can practice self-care. Take 2 minutes to practice relaxation breathing. Take 1 minute to show yourself or another person gratitude. Take 5 minutes before you start writing your notes for the day to listen to relaxing music or a mindful podcast. Take 3 minutes to share three good things that happened in the day with your family or colleagues. Take 5-10 minutes to do chair yoga. Take a self-compassion break.
- Implement a 5-minute wellness break into your group’s daily function with some of the previous mentioned examples. This will allow you to care for and nurture yourself, while also caring for and nurturing others in an environment that cultivates your wellness goals.
As a hospitalist, I can attest that I did not show myself self-compassion nearly as often as I showed compassion to others. I am my own worst critic and my training taught me to suffer in silence, and not seek out others who are experiencing the same thing for fear of being perceived as weak, inadequate, or flawed.
This false notion that we need to always be tough, strong, and without emotion in order to be taken seriously, to advance, or be held in high regard is rubbish and only perpetuated by accepting it. In order to change the culture of medicine, we have to change the way we think and behave. I have practiced self-compassion exercises and it has enhanced my perspective to see that many of us are going through varying degrees of the same thing. It has shown me the positive effects on my inner being and my life. If you are ready to try something new that will benefit your psychological and emotional well-being, and help you through pain, suffering, struggles, and crisis, you have nothing to lose. Be the change, and show yourself self-compassion.
In summary, self-compassion is an attitude of warmth, curiosity, connection, and care. Learning to become more self-compassionate is a process of moving from striving to change our experience and ourselves toward embracing who we are already.9 The practice of self-compassion is giving ourselves what we need in the moment. Even if we are not ready, or it is too painful to fully accept or embrace, we can still plant the seeds that will, with time and patience, grow and bloom.
When we are mindful of our struggles, when we respond to ourselves with compassion, kindness, and give ourselves support in times of difficulty, we learn to embrace ourselves and our lives, our inner and outer imperfections, and provide ourselves with the strength needed to thrive in the most precarious and difficult situations. With self-compassion, we give the world the best of us, instead of what is left of us.
Dr. Williams is vice president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as vice president of the medical executive committee.
References
1. Sanchez-Reilly S et al. Caring for oneself to care for others: Physicians and their self-care. J Community Support Oncol. 2013;11(2):75-81. doi: 10.12788/j.suponc.0003.
2. Neff K. Self-Compassion: An Alternative Conceptualization of a Healthy Attitude Toward Oneself. Self Identity. 2010;2(2):85-101. doi: 10.1080/15298860309032.
3. Neff K et al. The forest and the trees: Examining the association of self-compassion and its positive and negative components with psychological functioning. Self Identity. 2018;17(6):627-45. doi: 10.1080/15298868.2018.1436587.
4. Zessin U et al. The relationship between self-compassion and well-being: A meta-analysis. Appl Psychol Health Well-Being. 2015;7(3):340-64. doi: 10.1111/aphw.12051.
5. Warren R et al. Self-criticism and self-compassion: Risk and resilience. Current Psychiatry. 2016 Dec;15(12):18-21,24-28,32.
6. Neff K. The Five Myths of Self-Compassion. Greater Good Magazine. 2015 Sep 30. https://greatergood.berkeley.edu/article/item/the_five_myths_of_self_compassion.
7. Neff KD and Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clin Psychol. 2013 Jan;69(1):28-44. doi: 10.1002/jclp.21923.
8. Neff K. Self-Compassion Guided Meditations and Exercises. https://self-compassion.org/category/exercises/#exercises.
9. Germer C and Neff KD. Mindful Self-Compassion (MSC), in “The handbook of mindfulness-based programs.” (London: Routledge, 2019, pp. 357-67).
Children and COVID-19: 7 million cases and still counting
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.
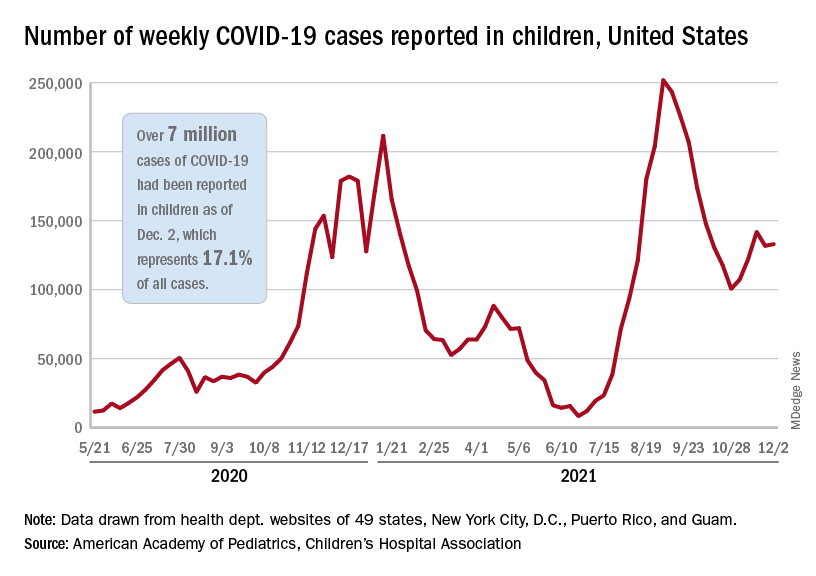
, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Compression therapy prevents recurrence of cellulitis
Background: Recurrent cellulitis is a common condition in patients with lower-extremity edema. Although some clinicians recommend compression garments as a preventative treatment, there are no data evaluating their efficacy for this purpose.
Study design: Participants were randomized to receive either education alone or education plus compression therapy. Neither the participants nor the assessors were blinded to the treatment arm.
Setting: Single-center study in Australia.
Synopsis: Participants with cellulitis who also had at least two previous episodes of cellulitis in the previous 2 years and had lower-extremity edema were enrolled. Of participants, 84 were randomized. Both groups received education regarding skin care, body weight, and exercise, while the compression therapy group also received compression garments and instructions for their use. The primary outcome was recurrent cellulitis. Patients in the control group were allowed to cross over after an episode of cellulitis. The trial was stopped early for efficacy. At the time the trial was halted, 17 of 43 (40%) participants in the control group had recurrent cellulitis, compared with only 6 of 41 (15%) in the intervention (hazard ratio, 0.23; 95% CI, 0.09-0.59; P = .002). Limitations include the lack of blinding, which could have introduced bias, although the diagnosis of recurrent cellulitis was made by clinicians external to the trial. This study supports the use of compression garments in preventing recurrent cellulitis in patients with lower-extremity edema.
Bottom line: Compression garments can be used to prevent recurrent cellulitis in patients with edema.
Citation: Webb E et al. Compression therapy to prevent recurrent cellulitis of the leg. N Engl J Med. 2020;383(7):630-9. doi:10.1056/NEJMoa1917197.
Dr. Herscher is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Recurrent cellulitis is a common condition in patients with lower-extremity edema. Although some clinicians recommend compression garments as a preventative treatment, there are no data evaluating their efficacy for this purpose.
Study design: Participants were randomized to receive either education alone or education plus compression therapy. Neither the participants nor the assessors were blinded to the treatment arm.
Setting: Single-center study in Australia.
Synopsis: Participants with cellulitis who also had at least two previous episodes of cellulitis in the previous 2 years and had lower-extremity edema were enrolled. Of participants, 84 were randomized. Both groups received education regarding skin care, body weight, and exercise, while the compression therapy group also received compression garments and instructions for their use. The primary outcome was recurrent cellulitis. Patients in the control group were allowed to cross over after an episode of cellulitis. The trial was stopped early for efficacy. At the time the trial was halted, 17 of 43 (40%) participants in the control group had recurrent cellulitis, compared with only 6 of 41 (15%) in the intervention (hazard ratio, 0.23; 95% CI, 0.09-0.59; P = .002). Limitations include the lack of blinding, which could have introduced bias, although the diagnosis of recurrent cellulitis was made by clinicians external to the trial. This study supports the use of compression garments in preventing recurrent cellulitis in patients with lower-extremity edema.
Bottom line: Compression garments can be used to prevent recurrent cellulitis in patients with edema.
Citation: Webb E et al. Compression therapy to prevent recurrent cellulitis of the leg. N Engl J Med. 2020;383(7):630-9. doi:10.1056/NEJMoa1917197.
Dr. Herscher is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Recurrent cellulitis is a common condition in patients with lower-extremity edema. Although some clinicians recommend compression garments as a preventative treatment, there are no data evaluating their efficacy for this purpose.
Study design: Participants were randomized to receive either education alone or education plus compression therapy. Neither the participants nor the assessors were blinded to the treatment arm.
Setting: Single-center study in Australia.
Synopsis: Participants with cellulitis who also had at least two previous episodes of cellulitis in the previous 2 years and had lower-extremity edema were enrolled. Of participants, 84 were randomized. Both groups received education regarding skin care, body weight, and exercise, while the compression therapy group also received compression garments and instructions for their use. The primary outcome was recurrent cellulitis. Patients in the control group were allowed to cross over after an episode of cellulitis. The trial was stopped early for efficacy. At the time the trial was halted, 17 of 43 (40%) participants in the control group had recurrent cellulitis, compared with only 6 of 41 (15%) in the intervention (hazard ratio, 0.23; 95% CI, 0.09-0.59; P = .002). Limitations include the lack of blinding, which could have introduced bias, although the diagnosis of recurrent cellulitis was made by clinicians external to the trial. This study supports the use of compression garments in preventing recurrent cellulitis in patients with lower-extremity edema.
Bottom line: Compression garments can be used to prevent recurrent cellulitis in patients with edema.
Citation: Webb E et al. Compression therapy to prevent recurrent cellulitis of the leg. N Engl J Med. 2020;383(7):630-9. doi:10.1056/NEJMoa1917197.
Dr. Herscher is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Apixaban outmatches rivaroxaban for VTE in study
Recurrent venous thromboembolism (VTE) – a composite of pulmonary embolism and deep vein thrombosis – was the primary effectiveness outcome in the retrospective analysis of new-user data from almost 40,000 patients, which was published in Annals of Internal Medicine. Safety was evaluated through a composite of intracranial and gastrointestinal bleeding.
After a median follow-up of 102 days in the apixaban group and 105 days in the rivaroxaban group, apixaban demonstrated superiority for both primary outcomes.
These real-world findings may guide selection of initial anticoagulant therapy, reported lead author Ghadeer K. Dawwas, PhD, MSc, MBA, of the University of Pennsylvania, Philadelphia, and colleagues.
“Randomized clinical trials comparing apixaban with rivaroxaban in patients with VTE are under way (for example, COBRRA (NCT03266783),” the investigators wrote. “Until the results from these trials become available (The estimated completion date for COBRRA is December 2023.), observational studies that use existing data can provide evidence on the effectiveness and safety of these alternatives to inform clinical practice.”
In the new research, apixaban was associated with a 23% lower rate of recurrent VTE (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87), including a 15% lower rate of deep vein thrombosis and a 41% lower rate of pulmonary embolism. Apixaban was associated with 40% fewer bleeding events (HR, 0.60; 95% CI, 0.53-0.69]), including a 40% lower rate of GI bleeding and a 46% lower rate of intracranial bleeding.
The study involved 37,236 patients with VTE, all of whom were diagnosed in at least one inpatient encounter and initiated direct oral anticoagulant (DOAC) therapy within 30 days, according to Optum’s deidentified Clinformatics Data Mart Database. Patients were evenly split into apixaban and rivaroxaban groups, with 18,618 individuals in each. Propensity score matching was used to minimize differences in baseline characteristics.
Apixaban was associated with an absolute reduction in recurrent VTE of 0.6% and 1.1% over 2 and 6 months, respectively, as well as reductions in bleeding of 1.1% and 1.5% over the same respective time periods.
The investigators noted that these findings were maintained in various sensitivity and subgroup analyses, including a model in which patients with VTE who had transient risk factors were compared with VTE patients exhibiting chronic risk factors.
“These findings suggest that apixaban has superior effectiveness and safety, compared with rivaroxaban and may provide guidance to clinicians and patients regarding selection of an anticoagulant for treatment of VTE,” Dr. Dawwas and colleagues concluded.
Study may have missed some nuance in possible outcomes, according to vascular surgeon
Thomas Wakefield, MD, a vascular surgeon and a professor of surgery at the University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, generally agreed with the investigators’ conclusion, although he noted that DOAC selection may also be influenced by other considerations.
“The results of this study suggest that, when choosing an agent for an individual patient, apixaban does appear to have an advantage over rivaroxaban related to recurrent VTE and bleeding,” Dr. Wakefield said in an interview. “One must keep in mind that these are not the only factors that are considered when choosing an agent and these are not the only two DOACs available. For example, rivaroxaban is given once per day while apixaban is given twice per day, and rivaroxaban has been shown to be successful in the treatment of other thrombotic disorders.”
Dr. Wakefield also pointed out that the study may have missed some nuance in possible outcomes.
“The current study looked at severe outcomes that resulted in inpatient hospitalization, so the generalization to strictly outpatient treatment and less severe outcomes cannot be inferred,” he said.
Damon E. Houghton, MD, of the department of medicine and a consultant in the department of vascular medicine and hematology at Mayo Clinic, Rochester, Minn., called the study a “very nice analysis,” highlighting the large sample size.
“The results are not a reason to abandon rivaroxaban altogether, but do suggest that, when otherwise appropriate for a patient, apixaban should be the first choice,” Dr. Houghton said in a written comment. “Hopefully this analysis will encourage more payers to create financial incentives that facilitate the use of apixaban in more patients.”
Randomized trial needed, says hematologist
Colleen Edwards, MD, of the departments of medicine, hematology, and medical oncology, at the Icahn School of Medicine at Mount Sinai, New York, had a more guarded view of the findings than Dr. Wakefield and Dr. Houghton.
“[The investigators] certainly seem to be doing a lot of statistical gymnastics in this paper,” Dr. Edwards said in an interview. “They used all kinds of surrogates in place of real data that you would get from a randomized trial.”
For example, Dr. Edwards noted the use of prescription refills as a surrogate for medication adherence, and emphasized that inpatient observational data may not reflect outpatient therapy.
“Inpatients are constantly missing their medicines all the time,” she said. “They’re holding it for procedures, they’re NPO, they’re off the floor, so they missed their medicine. So it’s just a very different patient population than the outpatient population, which is where venous thromboembolism is treated now, by and large.”
Although Dr. Edwards suggested that the findings might guide treatment selection “a little bit,” she noted that insurance constraints and costs play a greater role, and ultimately concluded that a randomized trial is needed to materially alter clinical decision-making.
“I think we really have to wait for randomized trial before we abandon our other choices,” she said.
The investigators disclosed relationships with Merck, Celgene, UCB, and others. Dr. Wakefield reported awaiting disclosures. Dr. Houghton and Dr. Edwards reported no relevant conflicts of interest.
Recurrent venous thromboembolism (VTE) – a composite of pulmonary embolism and deep vein thrombosis – was the primary effectiveness outcome in the retrospective analysis of new-user data from almost 40,000 patients, which was published in Annals of Internal Medicine. Safety was evaluated through a composite of intracranial and gastrointestinal bleeding.
After a median follow-up of 102 days in the apixaban group and 105 days in the rivaroxaban group, apixaban demonstrated superiority for both primary outcomes.
These real-world findings may guide selection of initial anticoagulant therapy, reported lead author Ghadeer K. Dawwas, PhD, MSc, MBA, of the University of Pennsylvania, Philadelphia, and colleagues.
“Randomized clinical trials comparing apixaban with rivaroxaban in patients with VTE are under way (for example, COBRRA (NCT03266783),” the investigators wrote. “Until the results from these trials become available (The estimated completion date for COBRRA is December 2023.), observational studies that use existing data can provide evidence on the effectiveness and safety of these alternatives to inform clinical practice.”
In the new research, apixaban was associated with a 23% lower rate of recurrent VTE (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87), including a 15% lower rate of deep vein thrombosis and a 41% lower rate of pulmonary embolism. Apixaban was associated with 40% fewer bleeding events (HR, 0.60; 95% CI, 0.53-0.69]), including a 40% lower rate of GI bleeding and a 46% lower rate of intracranial bleeding.
The study involved 37,236 patients with VTE, all of whom were diagnosed in at least one inpatient encounter and initiated direct oral anticoagulant (DOAC) therapy within 30 days, according to Optum’s deidentified Clinformatics Data Mart Database. Patients were evenly split into apixaban and rivaroxaban groups, with 18,618 individuals in each. Propensity score matching was used to minimize differences in baseline characteristics.
Apixaban was associated with an absolute reduction in recurrent VTE of 0.6% and 1.1% over 2 and 6 months, respectively, as well as reductions in bleeding of 1.1% and 1.5% over the same respective time periods.
The investigators noted that these findings were maintained in various sensitivity and subgroup analyses, including a model in which patients with VTE who had transient risk factors were compared with VTE patients exhibiting chronic risk factors.
“These findings suggest that apixaban has superior effectiveness and safety, compared with rivaroxaban and may provide guidance to clinicians and patients regarding selection of an anticoagulant for treatment of VTE,” Dr. Dawwas and colleagues concluded.
Study may have missed some nuance in possible outcomes, according to vascular surgeon
Thomas Wakefield, MD, a vascular surgeon and a professor of surgery at the University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, generally agreed with the investigators’ conclusion, although he noted that DOAC selection may also be influenced by other considerations.
“The results of this study suggest that, when choosing an agent for an individual patient, apixaban does appear to have an advantage over rivaroxaban related to recurrent VTE and bleeding,” Dr. Wakefield said in an interview. “One must keep in mind that these are not the only factors that are considered when choosing an agent and these are not the only two DOACs available. For example, rivaroxaban is given once per day while apixaban is given twice per day, and rivaroxaban has been shown to be successful in the treatment of other thrombotic disorders.”
Dr. Wakefield also pointed out that the study may have missed some nuance in possible outcomes.
“The current study looked at severe outcomes that resulted in inpatient hospitalization, so the generalization to strictly outpatient treatment and less severe outcomes cannot be inferred,” he said.
Damon E. Houghton, MD, of the department of medicine and a consultant in the department of vascular medicine and hematology at Mayo Clinic, Rochester, Minn., called the study a “very nice analysis,” highlighting the large sample size.
“The results are not a reason to abandon rivaroxaban altogether, but do suggest that, when otherwise appropriate for a patient, apixaban should be the first choice,” Dr. Houghton said in a written comment. “Hopefully this analysis will encourage more payers to create financial incentives that facilitate the use of apixaban in more patients.”
Randomized trial needed, says hematologist
Colleen Edwards, MD, of the departments of medicine, hematology, and medical oncology, at the Icahn School of Medicine at Mount Sinai, New York, had a more guarded view of the findings than Dr. Wakefield and Dr. Houghton.
“[The investigators] certainly seem to be doing a lot of statistical gymnastics in this paper,” Dr. Edwards said in an interview. “They used all kinds of surrogates in place of real data that you would get from a randomized trial.”
For example, Dr. Edwards noted the use of prescription refills as a surrogate for medication adherence, and emphasized that inpatient observational data may not reflect outpatient therapy.
“Inpatients are constantly missing their medicines all the time,” she said. “They’re holding it for procedures, they’re NPO, they’re off the floor, so they missed their medicine. So it’s just a very different patient population than the outpatient population, which is where venous thromboembolism is treated now, by and large.”
Although Dr. Edwards suggested that the findings might guide treatment selection “a little bit,” she noted that insurance constraints and costs play a greater role, and ultimately concluded that a randomized trial is needed to materially alter clinical decision-making.
“I think we really have to wait for randomized trial before we abandon our other choices,” she said.
The investigators disclosed relationships with Merck, Celgene, UCB, and others. Dr. Wakefield reported awaiting disclosures. Dr. Houghton and Dr. Edwards reported no relevant conflicts of interest.
Recurrent venous thromboembolism (VTE) – a composite of pulmonary embolism and deep vein thrombosis – was the primary effectiveness outcome in the retrospective analysis of new-user data from almost 40,000 patients, which was published in Annals of Internal Medicine. Safety was evaluated through a composite of intracranial and gastrointestinal bleeding.
After a median follow-up of 102 days in the apixaban group and 105 days in the rivaroxaban group, apixaban demonstrated superiority for both primary outcomes.
These real-world findings may guide selection of initial anticoagulant therapy, reported lead author Ghadeer K. Dawwas, PhD, MSc, MBA, of the University of Pennsylvania, Philadelphia, and colleagues.
“Randomized clinical trials comparing apixaban with rivaroxaban in patients with VTE are under way (for example, COBRRA (NCT03266783),” the investigators wrote. “Until the results from these trials become available (The estimated completion date for COBRRA is December 2023.), observational studies that use existing data can provide evidence on the effectiveness and safety of these alternatives to inform clinical practice.”
In the new research, apixaban was associated with a 23% lower rate of recurrent VTE (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87), including a 15% lower rate of deep vein thrombosis and a 41% lower rate of pulmonary embolism. Apixaban was associated with 40% fewer bleeding events (HR, 0.60; 95% CI, 0.53-0.69]), including a 40% lower rate of GI bleeding and a 46% lower rate of intracranial bleeding.
The study involved 37,236 patients with VTE, all of whom were diagnosed in at least one inpatient encounter and initiated direct oral anticoagulant (DOAC) therapy within 30 days, according to Optum’s deidentified Clinformatics Data Mart Database. Patients were evenly split into apixaban and rivaroxaban groups, with 18,618 individuals in each. Propensity score matching was used to minimize differences in baseline characteristics.
Apixaban was associated with an absolute reduction in recurrent VTE of 0.6% and 1.1% over 2 and 6 months, respectively, as well as reductions in bleeding of 1.1% and 1.5% over the same respective time periods.
The investigators noted that these findings were maintained in various sensitivity and subgroup analyses, including a model in which patients with VTE who had transient risk factors were compared with VTE patients exhibiting chronic risk factors.
“These findings suggest that apixaban has superior effectiveness and safety, compared with rivaroxaban and may provide guidance to clinicians and patients regarding selection of an anticoagulant for treatment of VTE,” Dr. Dawwas and colleagues concluded.
Study may have missed some nuance in possible outcomes, according to vascular surgeon
Thomas Wakefield, MD, a vascular surgeon and a professor of surgery at the University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, generally agreed with the investigators’ conclusion, although he noted that DOAC selection may also be influenced by other considerations.
“The results of this study suggest that, when choosing an agent for an individual patient, apixaban does appear to have an advantage over rivaroxaban related to recurrent VTE and bleeding,” Dr. Wakefield said in an interview. “One must keep in mind that these are not the only factors that are considered when choosing an agent and these are not the only two DOACs available. For example, rivaroxaban is given once per day while apixaban is given twice per day, and rivaroxaban has been shown to be successful in the treatment of other thrombotic disorders.”
Dr. Wakefield also pointed out that the study may have missed some nuance in possible outcomes.
“The current study looked at severe outcomes that resulted in inpatient hospitalization, so the generalization to strictly outpatient treatment and less severe outcomes cannot be inferred,” he said.
Damon E. Houghton, MD, of the department of medicine and a consultant in the department of vascular medicine and hematology at Mayo Clinic, Rochester, Minn., called the study a “very nice analysis,” highlighting the large sample size.
“The results are not a reason to abandon rivaroxaban altogether, but do suggest that, when otherwise appropriate for a patient, apixaban should be the first choice,” Dr. Houghton said in a written comment. “Hopefully this analysis will encourage more payers to create financial incentives that facilitate the use of apixaban in more patients.”
Randomized trial needed, says hematologist
Colleen Edwards, MD, of the departments of medicine, hematology, and medical oncology, at the Icahn School of Medicine at Mount Sinai, New York, had a more guarded view of the findings than Dr. Wakefield and Dr. Houghton.
“[The investigators] certainly seem to be doing a lot of statistical gymnastics in this paper,” Dr. Edwards said in an interview. “They used all kinds of surrogates in place of real data that you would get from a randomized trial.”
For example, Dr. Edwards noted the use of prescription refills as a surrogate for medication adherence, and emphasized that inpatient observational data may not reflect outpatient therapy.
“Inpatients are constantly missing their medicines all the time,” she said. “They’re holding it for procedures, they’re NPO, they’re off the floor, so they missed their medicine. So it’s just a very different patient population than the outpatient population, which is where venous thromboembolism is treated now, by and large.”
Although Dr. Edwards suggested that the findings might guide treatment selection “a little bit,” she noted that insurance constraints and costs play a greater role, and ultimately concluded that a randomized trial is needed to materially alter clinical decision-making.
“I think we really have to wait for randomized trial before we abandon our other choices,” she said.
The investigators disclosed relationships with Merck, Celgene, UCB, and others. Dr. Wakefield reported awaiting disclosures. Dr. Houghton and Dr. Edwards reported no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE




















