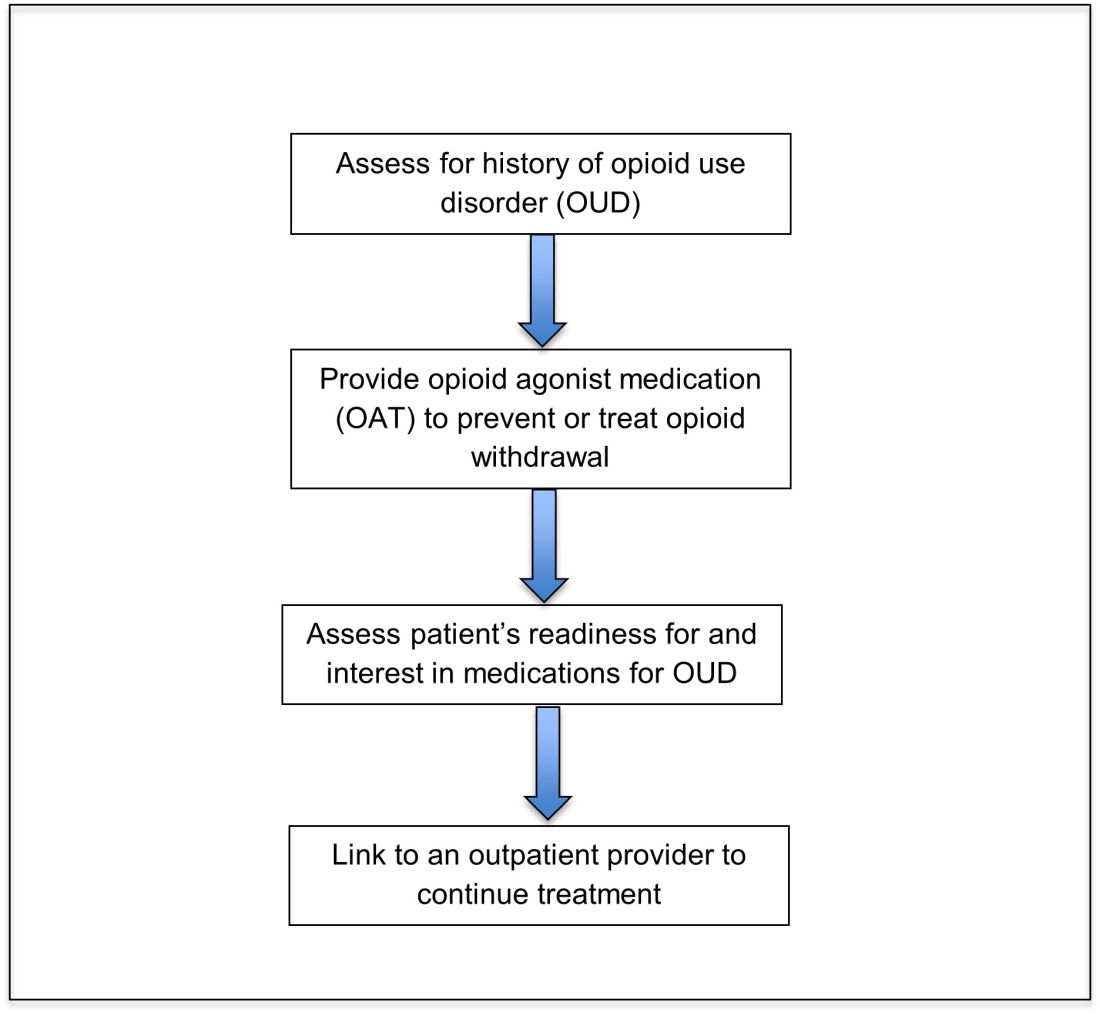
User login
Treatment of opioid use disorder in hospitalized patients
An opportunity for impact
Case
A 35-year-old woman with opioid use disorder (OUD) presents with fever, left arm redness, and swelling. She is admitted to the hospital for cellulitis treatment. On the day after admission she becomes agitated and develops nausea, diarrhea, and generalized pain. Opioid withdrawal is suspected. How should her opioid use be addressed while in the hospital?
Brief overview of the issue
Since 1999, there have been more than 800,000 deaths related to drug overdose in the United States, and in 2019 more than 70% of drug overdose deaths involved an opioid.1,2 Although effective treatments for OUD exist, less than 20% of those with OUD are engaged in treatment.3
In America, 4%-11% of hospitalized patients have OUD. Hospitalized patients with OUD often experience stigma surrounding their disease, and many inpatient clinicians lack knowledge regarding the care of patients with OUD. As a result, withdrawal symptoms may go untreated, which can erode trust in the medical system and contribute to patients’ leaving the hospital before their primary medical issue is fully addressed. Therefore, it is essential that inpatient clinicians be familiar with the management of this complex and vulnerable patient population. Initiating treatment for OUD in the hospital setting is feasible and effective, and can lead to increased engagement in OUD treatment even after the hospital stay.
Overview of the data
Assessing patients with suspected OUD
Assessment for OUD starts with an in-depth opioid use history including frequency, amount, and method of administration. Clinicians should gather information regarding use of other substances or nonprescribed medications, and take thorough psychiatric and social histories. A formal diagnosis of OUD can be made using the Fifth Edition Diagnostic and Statistical Manual for Mental Disorders (DSM-5) diagnostic criteria.
Recognizing and managing opioid withdrawal
OUD in hospitalized patients often becomes apparent when patients develop signs and symptoms of withdrawal. Decreasing physical discomfort related to withdrawal can allow inpatient clinicians to address the condition for which the patient was hospitalized, help to strengthen the patient-clinician relationship, and provide an opportunity to discuss long-term OUD treatment.
Signs and symptoms of opioid withdrawal include anxiety, restlessness, irritability, generalized pain, rhinorrhea, yawning, lacrimation, piloerection, anorexia, and nausea. Withdrawal can last days to weeks, depending on the half-life of the opioid that was used. Opioids with shorter half-lives, such as heroin or oxycodone, cause withdrawal with earlier onset and shorter duration than do opioids with longer half-lives, such as methadone. The degree of withdrawal can be quantified with validated tools, such as the Clinical Opiate Withdrawal Scale (COWS).
Treatment of opioid withdrawal should generally include the use of an opioid agonist such as methadone or buprenorphine. A 2017 Cochrane meta-analysis found methadone or buprenorphine to be more effective than clonidine in alleviating symptoms of withdrawal and in retaining patients in treatment.4 Clonidine, an alpha2-adrenergic agonist that binds to receptors in the locus coeruleus, does not alleviate opioid cravings, but may be used as an adjunctive treatment for associated autonomic withdrawal symptoms. Other adjunctive medications include analgesics, antiemetics, antidiarrheals, and antihistamines.
Opioid agonist treatment for opioid use disorder
Opioid agonist treatment (OAT) with methadone or buprenorphine is associated with decreased mortality, opioid use, and infectious complications, but remains underutilized.5 Hospitalized patients with OUD are frequently managed with a rapid opioid detoxification and then discharged without continued OUD treatment. Detoxification alone can lead to a relapse rate as high as 90%.6 Patients are at increased risk for overdose after withdrawal due to loss of tolerance. Inpatient clinicians can close this OUD treatment gap by familiarizing themselves with OAT and offering to initiate OAT for maintenance treatment in interested patients. In one study, patients started on buprenorphine while hospitalized were more likely to be engaged in treatment and less likely to report drug use at follow-up, compared to patients who were referred without starting the medication.7
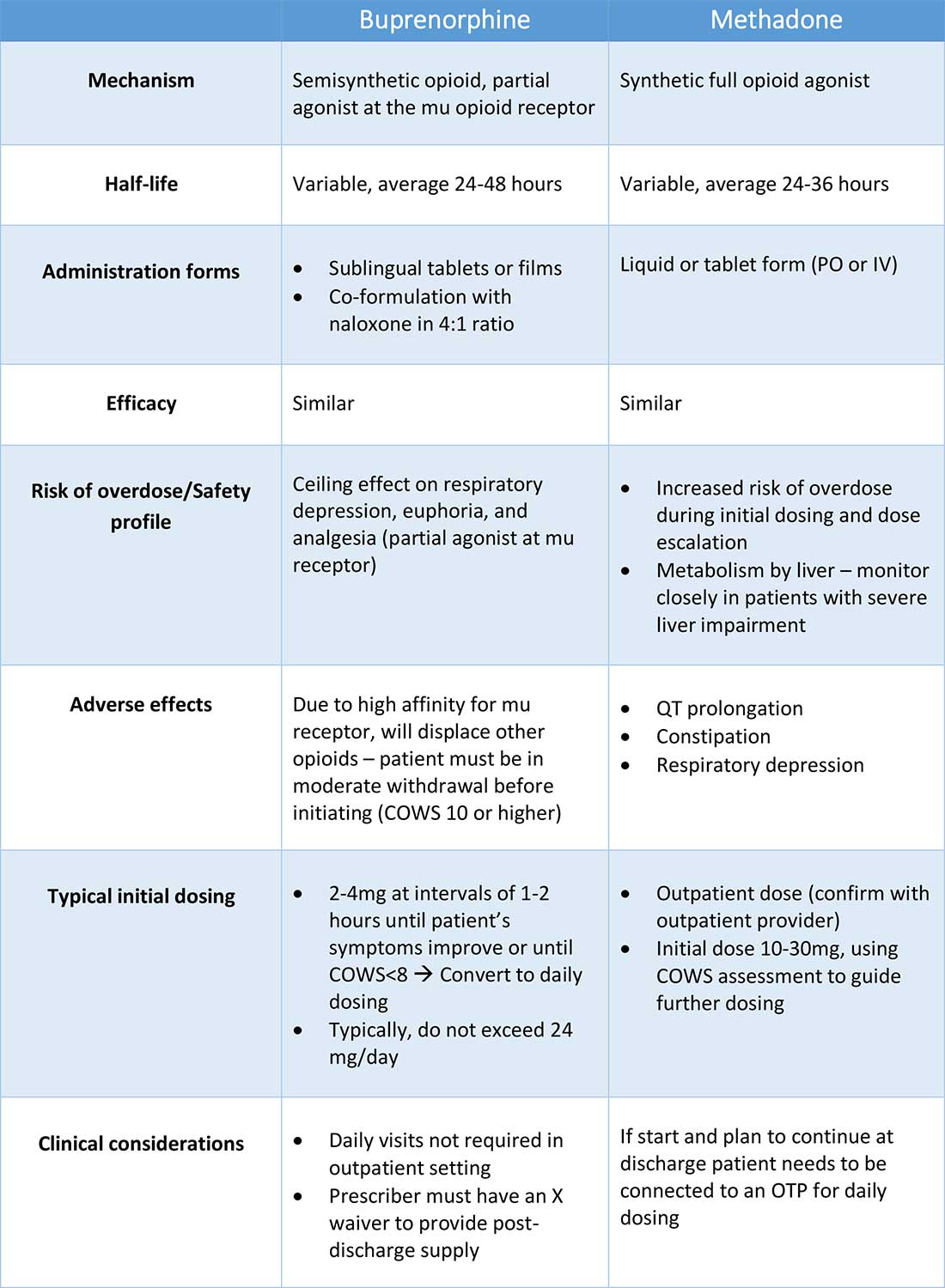
Buprenorphine
Buprenorphine is a partial agonist at the mu opioid receptor that can be ordered in the inpatient setting by any clinician. In the outpatient setting only DATA 2000 waivered clinicians can prescribe buprenorphine.8 Buprenorphine is most commonly coformulated with naloxone, an opioid antagonist, and is available in sublingual films or tablets. The naloxone component is not bioavailable when taken sublingually but becomes bioavailable if the drug is injected intravenously, leading to acute withdrawal.
Buprenorphine has a higher affinity for the mu opioid receptor than most opioids. If administered while other opioids are still present, it will displace the other opioid from the receptor but only partially stimulate the receptor, which can cause precipitated withdrawal. Buprenorphine initiation can start when the COWS score reflects moderate withdrawal. Many institutions use a threshold of 8-12 on the COWS scale. Typical dosing is 2-4 mg of buprenorphine at intervals of 1-2 hours as needed until the COWS score is less than 8, up to a maximum of 16 mg on day 1. The total dose from day 1 may be given as a daily dose beginning on day 2, up to a maximum total daily dose of 24 mg.
In recent years, a method of initiating buprenorphine called “micro-dosing” has gained traction. Very small doses of buprenorphine are given while a patient is receiving other opioids, thereby reducing the risk of precipitated withdrawal. This method can be helpful for patients who cannot tolerate withdrawal or who have recently taken long-acting opioids such as methadone. Such protocols should be utilized only at centers where consultation with an addiction specialist or experienced clinician is possible.
Despite evidence of buprenorphine’s efficacy, there are barriers to prescribing it. Physicians and advanced practitioners must be granted a waiver from the Drug Enforcement Administration to prescribe buprenorphine to outpatients. As of 2017, less than 10% of primary care physicians had obtained waivers.9 However, inpatient clinicians without a waiver can order buprenorphine and initiate treatment. Best practice is to do so with a specific plan for continuation at discharge. We encourage inpatient clinicians to obtain a waiver, so that a prescription can be given at discharge to bridge the patient to a first appointment with a community clinician who can continue treatment. As of April 27, 2021, providers treating fewer than 30 patients with OUD at one time may obtain a waiver without additional training.10
Methadone
Methadone is a full agonist at the mu opioid receptor. In the hospital setting, methadone can be ordered by any clinician to prevent and treat withdrawal. Commonly, doses of 10 mg can be given using the COWS score to guide the need for additional dosing. The patient can be reassessed every 1-2 hours to ensure that symptoms are improving, and that there is no sign of oversedation before giving additional methadone. For most patients, withdrawal can be managed with 20-40 mg of methadone daily.
In contrast to buprenorphine, methadone will not precipitate withdrawal and can be initiated even when patients are not yet showing withdrawal symptoms. Outpatient methadone treatment for OUD is federally regulated and can be delivered only in opioid treatment programs (OTPs).
Choosing methadone or buprenorphine in the inpatient setting
The choice between buprenorphine and methadone should take into consideration several factors, including patient preference, treatment history, and available outpatient treatment programs, which may vary widely by geographic region. Some patients benefit from the higher level of support and counseling available at OTPs. Methadone is available at all OTPs, and the availability of buprenorphine in this setting is increasing. Other patients may prefer the convenience and flexibility of buprenorphine treatment in an outpatient office setting.
Some patients have prior negative experiences with OAT. These can include prior precipitated withdrawal with buprenorphine induction, or negative experiences with the structure of OTPs. Clinicians are encouraged to provide counseling if patients have a history of precipitated withdrawal to assure them that this can be avoided with proper dosing. Clinicians should be familiar with available treatment options in their community and can refer to the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate OTPs and buprenorphine prescribers.
Polypharmacy and safety
If combined with benzodiazepines, alcohol, or other sedating agents, methadone or buprenorphine can increase risk of overdose. However, OUD treatment should not be withheld because of other substance use. Clinicians initiating treatment should counsel patients on the risk of concomitant substance use and provide overdose prevention education.
A brief note on naltrexone
Naltrexone, an opioid antagonist, is used more commonly in outpatient addiction treatment than in the inpatient setting, but inpatient clinicians should be aware of its use. It is available in oral and long-acting injectable formulations. Its utility in the inpatient setting may be limited as safe administration requires 7-10 days of opioid abstinence.
Discharge planning
Patients with OUD or who are started on OAT during a hospitalization should be linked to continued outpatient treatment. Before discharge it is best to ensure vaccinations for HAV, HBV, pneumococcus, and tetanus are up to date, and perform screening for HIV, hepatitis C, tuberculosis, and sexually transmitted infections if appropriate. All patients with OUD should be prescribed or provided with take-home naloxone for overdose reversal. Patients can also be referred to syringe service programs for additional harm reduction counseling and services.
Application of the data to our patient
For our patient, either methadone or buprenorphine could be used to treat her withdrawal. The COWS score should be used to assess withdrawal severity, and to guide appropriate timing of medication initiation. If she wishes to continue OAT after discharge, she should be linked to a clinician who can engage her in ongoing medical care. Prior to discharge she should also receive relevant vaccines and screening for infectious diseases as outlined above, as well as take-home naloxone (or a prescription).
Bottom line
Inpatient clinicians can play a pivotal role in patients’ lives by ensuring that patients with OUD receive OAT and are connected to outpatient care at discharge.
Dr. Linker is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai, New York. Ms. Hirt, Mr. Fine, and Mr. Villasanivis are medical students at the Icahn School of Medicine at Mount Sinai. Dr. Wang is assistant professor in the division of general internal medicine, Icahn School of Medicine at Mount Sinai. Dr. Herscher is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai.
References
1. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
2. Mattson CL et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-7. doi: 10.15585/mmwr.mm7006a4.
3. Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
4. Gowing L et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017 Feb;2017(2):CD002025. doi: 10.1002/14651858.CD002025.pub5.
5. Sordo L et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017 Apr 26;357:j1550. doi: 10.1136/bmj.j1550.
6. Smyth BP et al. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010 Jun;103(6):176-9. Available at www.drugsandalcohol.ie/13405.
7. Liebschutz JM. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1369-76. doi: 10.1001/jamainternmed.2014.2556.
8. Substance Abuse and Mental Health Services Administration. (Aug 20, 2020) Statutes, Regulations, and Guidelines.
9. McBain RK et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172(7):504-6. doi: 10.7326/M19-2403.
10. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. Apr 27, 2021.
11. Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Additional reading
Winetsky D. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018 Jan;13(1):62-4. doi: 10.12788/jhm.2861.
Donroe JH. Caring for patients with opioid use disorder in the hospital. Can Med Assoc J. 2016 Dec 6;188(17-18):1232-9. doi: 10.1503/cmaj.160290.
Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Key points
- Most patients with OUD are not engaged in evidence-based treatment. Clinicians have an opportunity to utilize the inpatient stay as a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
- Buprenorphine and methadone are effective opioid agonist medications used to treat OUD, and clinicians with the appropriate knowledge base can initiate either during the inpatient encounter, and link the patient to OUD treatment after the hospital stay.
Quiz
Caring for hospitalized patients with OUD
Most patients with OUD are not engaged in effective treatment. Hospitalization can be a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
1. Which is an effective and evidence-based medication for treating opioid withdrawal and OUD?
a) Naltrexone.
b) Buprenorphine.
c) Opioid detoxification.
d) Clonidine.
Explanation: Buprenorphine is effective for alleviating symptoms of withdrawal as well as for the long-term treatment of OUD. While naltrexone is also used to treat OUD, it is not useful for treating withdrawal. Clonidine can be a useful adjunctive medication for treating withdrawal but is not a long-term treatment for OUD. Nonpharmacologic detoxification is not an effective treatment for OUD and is associated with high relapse rates.
2. What scale can be used during a hospital stay to monitor patients with OUD at risk of opioid withdrawal, and to aid in buprenorphine initiation?
a) CIWA score.
b) PADUA score.
c) COWS score.
d) 4T score.
Explanation: COWS is the “clinical opiate withdrawal scale.” The COWS score should be calculated by a trained provider, and includes objective parameters (such as pulse) and subjective symptoms (such as GI upset, bone/joint aches.) It is recommended that agonist therapy be started when the COWS score is consistent with moderate withdrawal.
3. How can clinicians reliably find out if there are outpatient resources/clinics for patients with OUD in their area?
a) No way to find this out without personal knowledge.
b) Hospital providers and patients can visit www.samhsa.gov/find-help/national-helpline or call 1-800-662-HELP (4357) to find options for treatment for substance use disorders in their areas.
c) Dial “0” on any phone and ask.
d) Ask around at your hospital.
Explanation: The Substance Abuse and Mental Health Services Administration (SAMHSA) is an agency in the U.S. Department of Health and Human Services that is engaged in public health efforts to reduce the impact of substance abuse and mental illness on local communities. The agency’s website has helpful information about resources for substance use treatment.
4. Patients with OUD should be prescribed and given training about what medication that can be lifesaving when given during an opioid overdose?
a) Aspirin.
b) Naloxone.
c) Naltrexone.
d) Clonidine.
Explanation: Naloxone can be life-saving in the setting of an overdose. Best practice is to provide naloxone and training to patients with OUD.
5. When patients take buprenorphine soon after taking other opioids, there is concern for the development of which reaction:
a) Precipitated withdrawal.
b) Opioid overdose.
c) Allergic reaction.
d) Intoxication.
Explanation: Administering buprenorphine soon after taking other opioids can cause precipitated withdrawal, as buprenorphine binds with higher affinity to the mu receptor than many opioids. Precipitated withdrawal causes severe discomfort and can be dangerous for patients.
An opportunity for impact
An opportunity for impact
Case
A 35-year-old woman with opioid use disorder (OUD) presents with fever, left arm redness, and swelling. She is admitted to the hospital for cellulitis treatment. On the day after admission she becomes agitated and develops nausea, diarrhea, and generalized pain. Opioid withdrawal is suspected. How should her opioid use be addressed while in the hospital?
Brief overview of the issue
Since 1999, there have been more than 800,000 deaths related to drug overdose in the United States, and in 2019 more than 70% of drug overdose deaths involved an opioid.1,2 Although effective treatments for OUD exist, less than 20% of those with OUD are engaged in treatment.3
In America, 4%-11% of hospitalized patients have OUD. Hospitalized patients with OUD often experience stigma surrounding their disease, and many inpatient clinicians lack knowledge regarding the care of patients with OUD. As a result, withdrawal symptoms may go untreated, which can erode trust in the medical system and contribute to patients’ leaving the hospital before their primary medical issue is fully addressed. Therefore, it is essential that inpatient clinicians be familiar with the management of this complex and vulnerable patient population. Initiating treatment for OUD in the hospital setting is feasible and effective, and can lead to increased engagement in OUD treatment even after the hospital stay.
Overview of the data
Assessing patients with suspected OUD
Assessment for OUD starts with an in-depth opioid use history including frequency, amount, and method of administration. Clinicians should gather information regarding use of other substances or nonprescribed medications, and take thorough psychiatric and social histories. A formal diagnosis of OUD can be made using the Fifth Edition Diagnostic and Statistical Manual for Mental Disorders (DSM-5) diagnostic criteria.
Recognizing and managing opioid withdrawal
OUD in hospitalized patients often becomes apparent when patients develop signs and symptoms of withdrawal. Decreasing physical discomfort related to withdrawal can allow inpatient clinicians to address the condition for which the patient was hospitalized, help to strengthen the patient-clinician relationship, and provide an opportunity to discuss long-term OUD treatment.
Signs and symptoms of opioid withdrawal include anxiety, restlessness, irritability, generalized pain, rhinorrhea, yawning, lacrimation, piloerection, anorexia, and nausea. Withdrawal can last days to weeks, depending on the half-life of the opioid that was used. Opioids with shorter half-lives, such as heroin or oxycodone, cause withdrawal with earlier onset and shorter duration than do opioids with longer half-lives, such as methadone. The degree of withdrawal can be quantified with validated tools, such as the Clinical Opiate Withdrawal Scale (COWS).
Treatment of opioid withdrawal should generally include the use of an opioid agonist such as methadone or buprenorphine. A 2017 Cochrane meta-analysis found methadone or buprenorphine to be more effective than clonidine in alleviating symptoms of withdrawal and in retaining patients in treatment.4 Clonidine, an alpha2-adrenergic agonist that binds to receptors in the locus coeruleus, does not alleviate opioid cravings, but may be used as an adjunctive treatment for associated autonomic withdrawal symptoms. Other adjunctive medications include analgesics, antiemetics, antidiarrheals, and antihistamines.

Opioid agonist treatment for opioid use disorder
Opioid agonist treatment (OAT) with methadone or buprenorphine is associated with decreased mortality, opioid use, and infectious complications, but remains underutilized.5 Hospitalized patients with OUD are frequently managed with a rapid opioid detoxification and then discharged without continued OUD treatment. Detoxification alone can lead to a relapse rate as high as 90%.6 Patients are at increased risk for overdose after withdrawal due to loss of tolerance. Inpatient clinicians can close this OUD treatment gap by familiarizing themselves with OAT and offering to initiate OAT for maintenance treatment in interested patients. In one study, patients started on buprenorphine while hospitalized were more likely to be engaged in treatment and less likely to report drug use at follow-up, compared to patients who were referred without starting the medication.7
Buprenorphine
Buprenorphine is a partial agonist at the mu opioid receptor that can be ordered in the inpatient setting by any clinician. In the outpatient setting only DATA 2000 waivered clinicians can prescribe buprenorphine.8 Buprenorphine is most commonly coformulated with naloxone, an opioid antagonist, and is available in sublingual films or tablets. The naloxone component is not bioavailable when taken sublingually but becomes bioavailable if the drug is injected intravenously, leading to acute withdrawal.
Buprenorphine has a higher affinity for the mu opioid receptor than most opioids. If administered while other opioids are still present, it will displace the other opioid from the receptor but only partially stimulate the receptor, which can cause precipitated withdrawal. Buprenorphine initiation can start when the COWS score reflects moderate withdrawal. Many institutions use a threshold of 8-12 on the COWS scale. Typical dosing is 2-4 mg of buprenorphine at intervals of 1-2 hours as needed until the COWS score is less than 8, up to a maximum of 16 mg on day 1. The total dose from day 1 may be given as a daily dose beginning on day 2, up to a maximum total daily dose of 24 mg.
In recent years, a method of initiating buprenorphine called “micro-dosing” has gained traction. Very small doses of buprenorphine are given while a patient is receiving other opioids, thereby reducing the risk of precipitated withdrawal. This method can be helpful for patients who cannot tolerate withdrawal or who have recently taken long-acting opioids such as methadone. Such protocols should be utilized only at centers where consultation with an addiction specialist or experienced clinician is possible.
Despite evidence of buprenorphine’s efficacy, there are barriers to prescribing it. Physicians and advanced practitioners must be granted a waiver from the Drug Enforcement Administration to prescribe buprenorphine to outpatients. As of 2017, less than 10% of primary care physicians had obtained waivers.9 However, inpatient clinicians without a waiver can order buprenorphine and initiate treatment. Best practice is to do so with a specific plan for continuation at discharge. We encourage inpatient clinicians to obtain a waiver, so that a prescription can be given at discharge to bridge the patient to a first appointment with a community clinician who can continue treatment. As of April 27, 2021, providers treating fewer than 30 patients with OUD at one time may obtain a waiver without additional training.10
Methadone
Methadone is a full agonist at the mu opioid receptor. In the hospital setting, methadone can be ordered by any clinician to prevent and treat withdrawal. Commonly, doses of 10 mg can be given using the COWS score to guide the need for additional dosing. The patient can be reassessed every 1-2 hours to ensure that symptoms are improving, and that there is no sign of oversedation before giving additional methadone. For most patients, withdrawal can be managed with 20-40 mg of methadone daily.
In contrast to buprenorphine, methadone will not precipitate withdrawal and can be initiated even when patients are not yet showing withdrawal symptoms. Outpatient methadone treatment for OUD is federally regulated and can be delivered only in opioid treatment programs (OTPs).
Choosing methadone or buprenorphine in the inpatient setting
The choice between buprenorphine and methadone should take into consideration several factors, including patient preference, treatment history, and available outpatient treatment programs, which may vary widely by geographic region. Some patients benefit from the higher level of support and counseling available at OTPs. Methadone is available at all OTPs, and the availability of buprenorphine in this setting is increasing. Other patients may prefer the convenience and flexibility of buprenorphine treatment in an outpatient office setting.
Some patients have prior negative experiences with OAT. These can include prior precipitated withdrawal with buprenorphine induction, or negative experiences with the structure of OTPs. Clinicians are encouraged to provide counseling if patients have a history of precipitated withdrawal to assure them that this can be avoided with proper dosing. Clinicians should be familiar with available treatment options in their community and can refer to the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate OTPs and buprenorphine prescribers.

Polypharmacy and safety
If combined with benzodiazepines, alcohol, or other sedating agents, methadone or buprenorphine can increase risk of overdose. However, OUD treatment should not be withheld because of other substance use. Clinicians initiating treatment should counsel patients on the risk of concomitant substance use and provide overdose prevention education.
A brief note on naltrexone
Naltrexone, an opioid antagonist, is used more commonly in outpatient addiction treatment than in the inpatient setting, but inpatient clinicians should be aware of its use. It is available in oral and long-acting injectable formulations. Its utility in the inpatient setting may be limited as safe administration requires 7-10 days of opioid abstinence.
Discharge planning
Patients with OUD or who are started on OAT during a hospitalization should be linked to continued outpatient treatment. Before discharge it is best to ensure vaccinations for HAV, HBV, pneumococcus, and tetanus are up to date, and perform screening for HIV, hepatitis C, tuberculosis, and sexually transmitted infections if appropriate. All patients with OUD should be prescribed or provided with take-home naloxone for overdose reversal. Patients can also be referred to syringe service programs for additional harm reduction counseling and services.
Application of the data to our patient
For our patient, either methadone or buprenorphine could be used to treat her withdrawal. The COWS score should be used to assess withdrawal severity, and to guide appropriate timing of medication initiation. If she wishes to continue OAT after discharge, she should be linked to a clinician who can engage her in ongoing medical care. Prior to discharge she should also receive relevant vaccines and screening for infectious diseases as outlined above, as well as take-home naloxone (or a prescription).
Bottom line
Inpatient clinicians can play a pivotal role in patients’ lives by ensuring that patients with OUD receive OAT and are connected to outpatient care at discharge.
Dr. Linker is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai, New York. Ms. Hirt, Mr. Fine, and Mr. Villasanivis are medical students at the Icahn School of Medicine at Mount Sinai. Dr. Wang is assistant professor in the division of general internal medicine, Icahn School of Medicine at Mount Sinai. Dr. Herscher is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai.
References
1. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
2. Mattson CL et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-7. doi: 10.15585/mmwr.mm7006a4.
3. Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
4. Gowing L et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017 Feb;2017(2):CD002025. doi: 10.1002/14651858.CD002025.pub5.
5. Sordo L et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017 Apr 26;357:j1550. doi: 10.1136/bmj.j1550.
6. Smyth BP et al. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010 Jun;103(6):176-9. Available at www.drugsandalcohol.ie/13405.
7. Liebschutz JM. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1369-76. doi: 10.1001/jamainternmed.2014.2556.
8. Substance Abuse and Mental Health Services Administration. (Aug 20, 2020) Statutes, Regulations, and Guidelines.
9. McBain RK et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172(7):504-6. doi: 10.7326/M19-2403.
10. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. Apr 27, 2021.
11. Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Additional reading
Winetsky D. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018 Jan;13(1):62-4. doi: 10.12788/jhm.2861.
Donroe JH. Caring for patients with opioid use disorder in the hospital. Can Med Assoc J. 2016 Dec 6;188(17-18):1232-9. doi: 10.1503/cmaj.160290.
Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Key points
- Most patients with OUD are not engaged in evidence-based treatment. Clinicians have an opportunity to utilize the inpatient stay as a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
- Buprenorphine and methadone are effective opioid agonist medications used to treat OUD, and clinicians with the appropriate knowledge base can initiate either during the inpatient encounter, and link the patient to OUD treatment after the hospital stay.
Quiz
Caring for hospitalized patients with OUD
Most patients with OUD are not engaged in effective treatment. Hospitalization can be a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
1. Which is an effective and evidence-based medication for treating opioid withdrawal and OUD?
a) Naltrexone.
b) Buprenorphine.
c) Opioid detoxification.
d) Clonidine.
Explanation: Buprenorphine is effective for alleviating symptoms of withdrawal as well as for the long-term treatment of OUD. While naltrexone is also used to treat OUD, it is not useful for treating withdrawal. Clonidine can be a useful adjunctive medication for treating withdrawal but is not a long-term treatment for OUD. Nonpharmacologic detoxification is not an effective treatment for OUD and is associated with high relapse rates.
2. What scale can be used during a hospital stay to monitor patients with OUD at risk of opioid withdrawal, and to aid in buprenorphine initiation?
a) CIWA score.
b) PADUA score.
c) COWS score.
d) 4T score.
Explanation: COWS is the “clinical opiate withdrawal scale.” The COWS score should be calculated by a trained provider, and includes objective parameters (such as pulse) and subjective symptoms (such as GI upset, bone/joint aches.) It is recommended that agonist therapy be started when the COWS score is consistent with moderate withdrawal.
3. How can clinicians reliably find out if there are outpatient resources/clinics for patients with OUD in their area?
a) No way to find this out without personal knowledge.
b) Hospital providers and patients can visit www.samhsa.gov/find-help/national-helpline or call 1-800-662-HELP (4357) to find options for treatment for substance use disorders in their areas.
c) Dial “0” on any phone and ask.
d) Ask around at your hospital.
Explanation: The Substance Abuse and Mental Health Services Administration (SAMHSA) is an agency in the U.S. Department of Health and Human Services that is engaged in public health efforts to reduce the impact of substance abuse and mental illness on local communities. The agency’s website has helpful information about resources for substance use treatment.
4. Patients with OUD should be prescribed and given training about what medication that can be lifesaving when given during an opioid overdose?
a) Aspirin.
b) Naloxone.
c) Naltrexone.
d) Clonidine.
Explanation: Naloxone can be life-saving in the setting of an overdose. Best practice is to provide naloxone and training to patients with OUD.
5. When patients take buprenorphine soon after taking other opioids, there is concern for the development of which reaction:
a) Precipitated withdrawal.
b) Opioid overdose.
c) Allergic reaction.
d) Intoxication.
Explanation: Administering buprenorphine soon after taking other opioids can cause precipitated withdrawal, as buprenorphine binds with higher affinity to the mu receptor than many opioids. Precipitated withdrawal causes severe discomfort and can be dangerous for patients.
Case
A 35-year-old woman with opioid use disorder (OUD) presents with fever, left arm redness, and swelling. She is admitted to the hospital for cellulitis treatment. On the day after admission she becomes agitated and develops nausea, diarrhea, and generalized pain. Opioid withdrawal is suspected. How should her opioid use be addressed while in the hospital?
Brief overview of the issue
Since 1999, there have been more than 800,000 deaths related to drug overdose in the United States, and in 2019 more than 70% of drug overdose deaths involved an opioid.1,2 Although effective treatments for OUD exist, less than 20% of those with OUD are engaged in treatment.3
In America, 4%-11% of hospitalized patients have OUD. Hospitalized patients with OUD often experience stigma surrounding their disease, and many inpatient clinicians lack knowledge regarding the care of patients with OUD. As a result, withdrawal symptoms may go untreated, which can erode trust in the medical system and contribute to patients’ leaving the hospital before their primary medical issue is fully addressed. Therefore, it is essential that inpatient clinicians be familiar with the management of this complex and vulnerable patient population. Initiating treatment for OUD in the hospital setting is feasible and effective, and can lead to increased engagement in OUD treatment even after the hospital stay.
Overview of the data
Assessing patients with suspected OUD
Assessment for OUD starts with an in-depth opioid use history including frequency, amount, and method of administration. Clinicians should gather information regarding use of other substances or nonprescribed medications, and take thorough psychiatric and social histories. A formal diagnosis of OUD can be made using the Fifth Edition Diagnostic and Statistical Manual for Mental Disorders (DSM-5) diagnostic criteria.
Recognizing and managing opioid withdrawal
OUD in hospitalized patients often becomes apparent when patients develop signs and symptoms of withdrawal. Decreasing physical discomfort related to withdrawal can allow inpatient clinicians to address the condition for which the patient was hospitalized, help to strengthen the patient-clinician relationship, and provide an opportunity to discuss long-term OUD treatment.
Signs and symptoms of opioid withdrawal include anxiety, restlessness, irritability, generalized pain, rhinorrhea, yawning, lacrimation, piloerection, anorexia, and nausea. Withdrawal can last days to weeks, depending on the half-life of the opioid that was used. Opioids with shorter half-lives, such as heroin or oxycodone, cause withdrawal with earlier onset and shorter duration than do opioids with longer half-lives, such as methadone. The degree of withdrawal can be quantified with validated tools, such as the Clinical Opiate Withdrawal Scale (COWS).
Treatment of opioid withdrawal should generally include the use of an opioid agonist such as methadone or buprenorphine. A 2017 Cochrane meta-analysis found methadone or buprenorphine to be more effective than clonidine in alleviating symptoms of withdrawal and in retaining patients in treatment.4 Clonidine, an alpha2-adrenergic agonist that binds to receptors in the locus coeruleus, does not alleviate opioid cravings, but may be used as an adjunctive treatment for associated autonomic withdrawal symptoms. Other adjunctive medications include analgesics, antiemetics, antidiarrheals, and antihistamines.

Opioid agonist treatment for opioid use disorder
Opioid agonist treatment (OAT) with methadone or buprenorphine is associated with decreased mortality, opioid use, and infectious complications, but remains underutilized.5 Hospitalized patients with OUD are frequently managed with a rapid opioid detoxification and then discharged without continued OUD treatment. Detoxification alone can lead to a relapse rate as high as 90%.6 Patients are at increased risk for overdose after withdrawal due to loss of tolerance. Inpatient clinicians can close this OUD treatment gap by familiarizing themselves with OAT and offering to initiate OAT for maintenance treatment in interested patients. In one study, patients started on buprenorphine while hospitalized were more likely to be engaged in treatment and less likely to report drug use at follow-up, compared to patients who were referred without starting the medication.7
Buprenorphine
Buprenorphine is a partial agonist at the mu opioid receptor that can be ordered in the inpatient setting by any clinician. In the outpatient setting only DATA 2000 waivered clinicians can prescribe buprenorphine.8 Buprenorphine is most commonly coformulated with naloxone, an opioid antagonist, and is available in sublingual films or tablets. The naloxone component is not bioavailable when taken sublingually but becomes bioavailable if the drug is injected intravenously, leading to acute withdrawal.
Buprenorphine has a higher affinity for the mu opioid receptor than most opioids. If administered while other opioids are still present, it will displace the other opioid from the receptor but only partially stimulate the receptor, which can cause precipitated withdrawal. Buprenorphine initiation can start when the COWS score reflects moderate withdrawal. Many institutions use a threshold of 8-12 on the COWS scale. Typical dosing is 2-4 mg of buprenorphine at intervals of 1-2 hours as needed until the COWS score is less than 8, up to a maximum of 16 mg on day 1. The total dose from day 1 may be given as a daily dose beginning on day 2, up to a maximum total daily dose of 24 mg.
In recent years, a method of initiating buprenorphine called “micro-dosing” has gained traction. Very small doses of buprenorphine are given while a patient is receiving other opioids, thereby reducing the risk of precipitated withdrawal. This method can be helpful for patients who cannot tolerate withdrawal or who have recently taken long-acting opioids such as methadone. Such protocols should be utilized only at centers where consultation with an addiction specialist or experienced clinician is possible.
Despite evidence of buprenorphine’s efficacy, there are barriers to prescribing it. Physicians and advanced practitioners must be granted a waiver from the Drug Enforcement Administration to prescribe buprenorphine to outpatients. As of 2017, less than 10% of primary care physicians had obtained waivers.9 However, inpatient clinicians without a waiver can order buprenorphine and initiate treatment. Best practice is to do so with a specific plan for continuation at discharge. We encourage inpatient clinicians to obtain a waiver, so that a prescription can be given at discharge to bridge the patient to a first appointment with a community clinician who can continue treatment. As of April 27, 2021, providers treating fewer than 30 patients with OUD at one time may obtain a waiver without additional training.10
Methadone
Methadone is a full agonist at the mu opioid receptor. In the hospital setting, methadone can be ordered by any clinician to prevent and treat withdrawal. Commonly, doses of 10 mg can be given using the COWS score to guide the need for additional dosing. The patient can be reassessed every 1-2 hours to ensure that symptoms are improving, and that there is no sign of oversedation before giving additional methadone. For most patients, withdrawal can be managed with 20-40 mg of methadone daily.
In contrast to buprenorphine, methadone will not precipitate withdrawal and can be initiated even when patients are not yet showing withdrawal symptoms. Outpatient methadone treatment for OUD is federally regulated and can be delivered only in opioid treatment programs (OTPs).
Choosing methadone or buprenorphine in the inpatient setting
The choice between buprenorphine and methadone should take into consideration several factors, including patient preference, treatment history, and available outpatient treatment programs, which may vary widely by geographic region. Some patients benefit from the higher level of support and counseling available at OTPs. Methadone is available at all OTPs, and the availability of buprenorphine in this setting is increasing. Other patients may prefer the convenience and flexibility of buprenorphine treatment in an outpatient office setting.
Some patients have prior negative experiences with OAT. These can include prior precipitated withdrawal with buprenorphine induction, or negative experiences with the structure of OTPs. Clinicians are encouraged to provide counseling if patients have a history of precipitated withdrawal to assure them that this can be avoided with proper dosing. Clinicians should be familiar with available treatment options in their community and can refer to the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate OTPs and buprenorphine prescribers.

Polypharmacy and safety
If combined with benzodiazepines, alcohol, or other sedating agents, methadone or buprenorphine can increase risk of overdose. However, OUD treatment should not be withheld because of other substance use. Clinicians initiating treatment should counsel patients on the risk of concomitant substance use and provide overdose prevention education.
A brief note on naltrexone
Naltrexone, an opioid antagonist, is used more commonly in outpatient addiction treatment than in the inpatient setting, but inpatient clinicians should be aware of its use. It is available in oral and long-acting injectable formulations. Its utility in the inpatient setting may be limited as safe administration requires 7-10 days of opioid abstinence.
Discharge planning
Patients with OUD or who are started on OAT during a hospitalization should be linked to continued outpatient treatment. Before discharge it is best to ensure vaccinations for HAV, HBV, pneumococcus, and tetanus are up to date, and perform screening for HIV, hepatitis C, tuberculosis, and sexually transmitted infections if appropriate. All patients with OUD should be prescribed or provided with take-home naloxone for overdose reversal. Patients can also be referred to syringe service programs for additional harm reduction counseling and services.
Application of the data to our patient
For our patient, either methadone or buprenorphine could be used to treat her withdrawal. The COWS score should be used to assess withdrawal severity, and to guide appropriate timing of medication initiation. If she wishes to continue OAT after discharge, she should be linked to a clinician who can engage her in ongoing medical care. Prior to discharge she should also receive relevant vaccines and screening for infectious diseases as outlined above, as well as take-home naloxone (or a prescription).
Bottom line
Inpatient clinicians can play a pivotal role in patients’ lives by ensuring that patients with OUD receive OAT and are connected to outpatient care at discharge.
Dr. Linker is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai, New York. Ms. Hirt, Mr. Fine, and Mr. Villasanivis are medical students at the Icahn School of Medicine at Mount Sinai. Dr. Wang is assistant professor in the division of general internal medicine, Icahn School of Medicine at Mount Sinai. Dr. Herscher is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai.
References
1. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
2. Mattson CL et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-7. doi: 10.15585/mmwr.mm7006a4.
3. Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
4. Gowing L et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017 Feb;2017(2):CD002025. doi: 10.1002/14651858.CD002025.pub5.
5. Sordo L et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017 Apr 26;357:j1550. doi: 10.1136/bmj.j1550.
6. Smyth BP et al. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010 Jun;103(6):176-9. Available at www.drugsandalcohol.ie/13405.
7. Liebschutz JM. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1369-76. doi: 10.1001/jamainternmed.2014.2556.
8. Substance Abuse and Mental Health Services Administration. (Aug 20, 2020) Statutes, Regulations, and Guidelines.
9. McBain RK et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172(7):504-6. doi: 10.7326/M19-2403.
10. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. Apr 27, 2021.
11. Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Additional reading
Winetsky D. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018 Jan;13(1):62-4. doi: 10.12788/jhm.2861.
Donroe JH. Caring for patients with opioid use disorder in the hospital. Can Med Assoc J. 2016 Dec 6;188(17-18):1232-9. doi: 10.1503/cmaj.160290.
Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Key points
- Most patients with OUD are not engaged in evidence-based treatment. Clinicians have an opportunity to utilize the inpatient stay as a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
- Buprenorphine and methadone are effective opioid agonist medications used to treat OUD, and clinicians with the appropriate knowledge base can initiate either during the inpatient encounter, and link the patient to OUD treatment after the hospital stay.
Quiz
Caring for hospitalized patients with OUD
Most patients with OUD are not engaged in effective treatment. Hospitalization can be a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
1. Which is an effective and evidence-based medication for treating opioid withdrawal and OUD?
a) Naltrexone.
b) Buprenorphine.
c) Opioid detoxification.
d) Clonidine.
Explanation: Buprenorphine is effective for alleviating symptoms of withdrawal as well as for the long-term treatment of OUD. While naltrexone is also used to treat OUD, it is not useful for treating withdrawal. Clonidine can be a useful adjunctive medication for treating withdrawal but is not a long-term treatment for OUD. Nonpharmacologic detoxification is not an effective treatment for OUD and is associated with high relapse rates.
2. What scale can be used during a hospital stay to monitor patients with OUD at risk of opioid withdrawal, and to aid in buprenorphine initiation?
a) CIWA score.
b) PADUA score.
c) COWS score.
d) 4T score.
Explanation: COWS is the “clinical opiate withdrawal scale.” The COWS score should be calculated by a trained provider, and includes objective parameters (such as pulse) and subjective symptoms (such as GI upset, bone/joint aches.) It is recommended that agonist therapy be started when the COWS score is consistent with moderate withdrawal.
3. How can clinicians reliably find out if there are outpatient resources/clinics for patients with OUD in their area?
a) No way to find this out without personal knowledge.
b) Hospital providers and patients can visit www.samhsa.gov/find-help/national-helpline or call 1-800-662-HELP (4357) to find options for treatment for substance use disorders in their areas.
c) Dial “0” on any phone and ask.
d) Ask around at your hospital.
Explanation: The Substance Abuse and Mental Health Services Administration (SAMHSA) is an agency in the U.S. Department of Health and Human Services that is engaged in public health efforts to reduce the impact of substance abuse and mental illness on local communities. The agency’s website has helpful information about resources for substance use treatment.
4. Patients with OUD should be prescribed and given training about what medication that can be lifesaving when given during an opioid overdose?
a) Aspirin.
b) Naloxone.
c) Naltrexone.
d) Clonidine.
Explanation: Naloxone can be life-saving in the setting of an overdose. Best practice is to provide naloxone and training to patients with OUD.
5. When patients take buprenorphine soon after taking other opioids, there is concern for the development of which reaction:
a) Precipitated withdrawal.
b) Opioid overdose.
c) Allergic reaction.
d) Intoxication.
Explanation: Administering buprenorphine soon after taking other opioids can cause precipitated withdrawal, as buprenorphine binds with higher affinity to the mu receptor than many opioids. Precipitated withdrawal causes severe discomfort and can be dangerous for patients.
Compression therapy prevents recurrence of cellulitis
Background: Recurrent cellulitis is a common condition in patients with lower-extremity edema. Although some clinicians recommend compression garments as a preventative treatment, there are no data evaluating their efficacy for this purpose.
Study design: Participants were randomized to receive either education alone or education plus compression therapy. Neither the participants nor the assessors were blinded to the treatment arm.
Setting: Single-center study in Australia.
Synopsis: Participants with cellulitis who also had at least two previous episodes of cellulitis in the previous 2 years and had lower-extremity edema were enrolled. Of participants, 84 were randomized. Both groups received education regarding skin care, body weight, and exercise, while the compression therapy group also received compression garments and instructions for their use. The primary outcome was recurrent cellulitis. Patients in the control group were allowed to cross over after an episode of cellulitis. The trial was stopped early for efficacy. At the time the trial was halted, 17 of 43 (40%) participants in the control group had recurrent cellulitis, compared with only 6 of 41 (15%) in the intervention (hazard ratio, 0.23; 95% CI, 0.09-0.59; P = .002). Limitations include the lack of blinding, which could have introduced bias, although the diagnosis of recurrent cellulitis was made by clinicians external to the trial. This study supports the use of compression garments in preventing recurrent cellulitis in patients with lower-extremity edema.
Bottom line: Compression garments can be used to prevent recurrent cellulitis in patients with edema.
Citation: Webb E et al. Compression therapy to prevent recurrent cellulitis of the leg. N Engl J Med. 2020;383(7):630-9. doi:10.1056/NEJMoa1917197.
Dr. Herscher is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Recurrent cellulitis is a common condition in patients with lower-extremity edema. Although some clinicians recommend compression garments as a preventative treatment, there are no data evaluating their efficacy for this purpose.
Study design: Participants were randomized to receive either education alone or education plus compression therapy. Neither the participants nor the assessors were blinded to the treatment arm.
Setting: Single-center study in Australia.
Synopsis: Participants with cellulitis who also had at least two previous episodes of cellulitis in the previous 2 years and had lower-extremity edema were enrolled. Of participants, 84 were randomized. Both groups received education regarding skin care, body weight, and exercise, while the compression therapy group also received compression garments and instructions for their use. The primary outcome was recurrent cellulitis. Patients in the control group were allowed to cross over after an episode of cellulitis. The trial was stopped early for efficacy. At the time the trial was halted, 17 of 43 (40%) participants in the control group had recurrent cellulitis, compared with only 6 of 41 (15%) in the intervention (hazard ratio, 0.23; 95% CI, 0.09-0.59; P = .002). Limitations include the lack of blinding, which could have introduced bias, although the diagnosis of recurrent cellulitis was made by clinicians external to the trial. This study supports the use of compression garments in preventing recurrent cellulitis in patients with lower-extremity edema.
Bottom line: Compression garments can be used to prevent recurrent cellulitis in patients with edema.
Citation: Webb E et al. Compression therapy to prevent recurrent cellulitis of the leg. N Engl J Med. 2020;383(7):630-9. doi:10.1056/NEJMoa1917197.
Dr. Herscher is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Recurrent cellulitis is a common condition in patients with lower-extremity edema. Although some clinicians recommend compression garments as a preventative treatment, there are no data evaluating their efficacy for this purpose.
Study design: Participants were randomized to receive either education alone or education plus compression therapy. Neither the participants nor the assessors were blinded to the treatment arm.
Setting: Single-center study in Australia.
Synopsis: Participants with cellulitis who also had at least two previous episodes of cellulitis in the previous 2 years and had lower-extremity edema were enrolled. Of participants, 84 were randomized. Both groups received education regarding skin care, body weight, and exercise, while the compression therapy group also received compression garments and instructions for their use. The primary outcome was recurrent cellulitis. Patients in the control group were allowed to cross over after an episode of cellulitis. The trial was stopped early for efficacy. At the time the trial was halted, 17 of 43 (40%) participants in the control group had recurrent cellulitis, compared with only 6 of 41 (15%) in the intervention (hazard ratio, 0.23; 95% CI, 0.09-0.59; P = .002). Limitations include the lack of blinding, which could have introduced bias, although the diagnosis of recurrent cellulitis was made by clinicians external to the trial. This study supports the use of compression garments in preventing recurrent cellulitis in patients with lower-extremity edema.
Bottom line: Compression garments can be used to prevent recurrent cellulitis in patients with edema.
Citation: Webb E et al. Compression therapy to prevent recurrent cellulitis of the leg. N Engl J Med. 2020;383(7):630-9. doi:10.1056/NEJMoa1917197.
Dr. Herscher is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Postoperative Clostridium Difficile Infection Associated with Number of Antibiotics, Surgical Procedure Complexity
Clinical question: What are the factors that increase risk of Clostridium difficile infection (CDI) in postoperative patients?
Background: CDI has become an important infectious etiology for morbidity, lengthy and costly hospital admissions, and mortality. This study focused on the risks for postoperative patients to be infected with C. diff. Awareness of the risk factors for CDI allows for processes to be implemented that can decrease the rate of infection.
Study design: Retrospective, observational study.
Setting: Multiple Veterans Health Administration surgery programs.
Synopsis: The study investigated 468,386 surgical procedures in 134 surgical programs in 12 subspecialties over a four-year period. Overall, the postoperative CDI rate was 0.4% per year. Rates were higher in emergency or complex procedures, older patients, patients with longer preoperative hospital stays, and those who received three or more classes of antibiotics. CDI in postoperative patients was associated with five times higher risk of mortality, a 12 times higher risk of morbidity, and longer hospital stays (17.9 versus 3.6 days) compared with those without CDI. Further studies with a larger population size will confirm the findings of this study.
The study was conducted on middle-aged to elderly male veterans, and it can only be assumed that these results will translate to other populations. Nevertheless, CDI can lead to significant morbidity and mortality, and the study reinforces the importance of infection control and prevention to reduce CDI incidence and disease severity.
Bottom line: Postoperative CDI is significantly associated with the number of postoperative antibiotics, surgical procedure complexity, preoperative length of stay, and patient comorbidities.
Citation: Li X, Wilson M, Nylander W, Smith T, Lynn M, Gunnar W. Analysis of morbidity and mortality outcomes in postoperative Clostridium difficile infection in the Veterans Health Administration. JAMA Surg. 2015;25:1-9.
Clinical question: What are the factors that increase risk of Clostridium difficile infection (CDI) in postoperative patients?
Background: CDI has become an important infectious etiology for morbidity, lengthy and costly hospital admissions, and mortality. This study focused on the risks for postoperative patients to be infected with C. diff. Awareness of the risk factors for CDI allows for processes to be implemented that can decrease the rate of infection.
Study design: Retrospective, observational study.
Setting: Multiple Veterans Health Administration surgery programs.
Synopsis: The study investigated 468,386 surgical procedures in 134 surgical programs in 12 subspecialties over a four-year period. Overall, the postoperative CDI rate was 0.4% per year. Rates were higher in emergency or complex procedures, older patients, patients with longer preoperative hospital stays, and those who received three or more classes of antibiotics. CDI in postoperative patients was associated with five times higher risk of mortality, a 12 times higher risk of morbidity, and longer hospital stays (17.9 versus 3.6 days) compared with those without CDI. Further studies with a larger population size will confirm the findings of this study.
The study was conducted on middle-aged to elderly male veterans, and it can only be assumed that these results will translate to other populations. Nevertheless, CDI can lead to significant morbidity and mortality, and the study reinforces the importance of infection control and prevention to reduce CDI incidence and disease severity.
Bottom line: Postoperative CDI is significantly associated with the number of postoperative antibiotics, surgical procedure complexity, preoperative length of stay, and patient comorbidities.
Citation: Li X, Wilson M, Nylander W, Smith T, Lynn M, Gunnar W. Analysis of morbidity and mortality outcomes in postoperative Clostridium difficile infection in the Veterans Health Administration. JAMA Surg. 2015;25:1-9.
Clinical question: What are the factors that increase risk of Clostridium difficile infection (CDI) in postoperative patients?
Background: CDI has become an important infectious etiology for morbidity, lengthy and costly hospital admissions, and mortality. This study focused on the risks for postoperative patients to be infected with C. diff. Awareness of the risk factors for CDI allows for processes to be implemented that can decrease the rate of infection.
Study design: Retrospective, observational study.
Setting: Multiple Veterans Health Administration surgery programs.
Synopsis: The study investigated 468,386 surgical procedures in 134 surgical programs in 12 subspecialties over a four-year period. Overall, the postoperative CDI rate was 0.4% per year. Rates were higher in emergency or complex procedures, older patients, patients with longer preoperative hospital stays, and those who received three or more classes of antibiotics. CDI in postoperative patients was associated with five times higher risk of mortality, a 12 times higher risk of morbidity, and longer hospital stays (17.9 versus 3.6 days) compared with those without CDI. Further studies with a larger population size will confirm the findings of this study.
The study was conducted on middle-aged to elderly male veterans, and it can only be assumed that these results will translate to other populations. Nevertheless, CDI can lead to significant morbidity and mortality, and the study reinforces the importance of infection control and prevention to reduce CDI incidence and disease severity.
Bottom line: Postoperative CDI is significantly associated with the number of postoperative antibiotics, surgical procedure complexity, preoperative length of stay, and patient comorbidities.
Citation: Li X, Wilson M, Nylander W, Smith T, Lynn M, Gunnar W. Analysis of morbidity and mortality outcomes in postoperative Clostridium difficile infection in the Veterans Health Administration. JAMA Surg. 2015;25:1-9.
Most Postoperative Readmissions Due to Patient Factors
Clinical question: What is the etiology of 30-day readmissions in postoperative patients?
Background: As the focus of healthcare changes to a quality-focused model, readmissions impact physicians, reimbursements, and patients. Understanding the cause of readmissions becomes essential to preventing them. The etiology of 30-day readmissions in postoperative patients has not specifically been studied.
Study design: Retrospective analysis.
Setting: Academic tertiary-care center.
Synopsis: Using administrative claims data, an analysis of 22,559 patients who underwent a major surgical procedure between 2009 and 2013 was performed. A total of 56 surgeons within eight surgical subspecialties were analyzed, showing that variation in 30-day readmissions was largely due to patient-specific factors (82.8%) while only a minority were attributable to surgical subspecialty (14.5%) and individual surgeon levels (2.8%). Factors associated with readmission included race/ethnicity, comorbidities, postoperative complications, and extended length of stay.
Further studies within this area will need to be conducted focusing on one specific subspecialty and one surgeon to exclude confounding factors. Additional meta-analysis can then compare these individual studies. A larger population and multiple care centers will also further validate the findings. Understanding the cause of the readmissions in postoperative patients can prevent further readmissions, improve quality of care, and decrease healthcare costs. If patient factors are identified as a major cause for readmissions in postoperative patients, changes in preoperative management may need to be made.
Bottom line: Postoperative readmissions are more dependent on patient factors than surgeon- or surgical subspecialty-specific factors.
Citation: Gani F, Lucas DJ, Kim Y, Schneider EB, Pawlik TM. Understanding variation in 30-day surgical readmission in the era of accountable care: effect of the patient, surgeon, and surgical subspecialties. JAMA Surg. 2015;150(11):1042-1049.
Clinical question: What is the etiology of 30-day readmissions in postoperative patients?
Background: As the focus of healthcare changes to a quality-focused model, readmissions impact physicians, reimbursements, and patients. Understanding the cause of readmissions becomes essential to preventing them. The etiology of 30-day readmissions in postoperative patients has not specifically been studied.
Study design: Retrospective analysis.
Setting: Academic tertiary-care center.
Synopsis: Using administrative claims data, an analysis of 22,559 patients who underwent a major surgical procedure between 2009 and 2013 was performed. A total of 56 surgeons within eight surgical subspecialties were analyzed, showing that variation in 30-day readmissions was largely due to patient-specific factors (82.8%) while only a minority were attributable to surgical subspecialty (14.5%) and individual surgeon levels (2.8%). Factors associated with readmission included race/ethnicity, comorbidities, postoperative complications, and extended length of stay.
Further studies within this area will need to be conducted focusing on one specific subspecialty and one surgeon to exclude confounding factors. Additional meta-analysis can then compare these individual studies. A larger population and multiple care centers will also further validate the findings. Understanding the cause of the readmissions in postoperative patients can prevent further readmissions, improve quality of care, and decrease healthcare costs. If patient factors are identified as a major cause for readmissions in postoperative patients, changes in preoperative management may need to be made.
Bottom line: Postoperative readmissions are more dependent on patient factors than surgeon- or surgical subspecialty-specific factors.
Citation: Gani F, Lucas DJ, Kim Y, Schneider EB, Pawlik TM. Understanding variation in 30-day surgical readmission in the era of accountable care: effect of the patient, surgeon, and surgical subspecialties. JAMA Surg. 2015;150(11):1042-1049.
Clinical question: What is the etiology of 30-day readmissions in postoperative patients?
Background: As the focus of healthcare changes to a quality-focused model, readmissions impact physicians, reimbursements, and patients. Understanding the cause of readmissions becomes essential to preventing them. The etiology of 30-day readmissions in postoperative patients has not specifically been studied.
Study design: Retrospective analysis.
Setting: Academic tertiary-care center.
Synopsis: Using administrative claims data, an analysis of 22,559 patients who underwent a major surgical procedure between 2009 and 2013 was performed. A total of 56 surgeons within eight surgical subspecialties were analyzed, showing that variation in 30-day readmissions was largely due to patient-specific factors (82.8%) while only a minority were attributable to surgical subspecialty (14.5%) and individual surgeon levels (2.8%). Factors associated with readmission included race/ethnicity, comorbidities, postoperative complications, and extended length of stay.
Further studies within this area will need to be conducted focusing on one specific subspecialty and one surgeon to exclude confounding factors. Additional meta-analysis can then compare these individual studies. A larger population and multiple care centers will also further validate the findings. Understanding the cause of the readmissions in postoperative patients can prevent further readmissions, improve quality of care, and decrease healthcare costs. If patient factors are identified as a major cause for readmissions in postoperative patients, changes in preoperative management may need to be made.
Bottom line: Postoperative readmissions are more dependent on patient factors than surgeon- or surgical subspecialty-specific factors.
Citation: Gani F, Lucas DJ, Kim Y, Schneider EB, Pawlik TM. Understanding variation in 30-day surgical readmission in the era of accountable care: effect of the patient, surgeon, and surgical subspecialties. JAMA Surg. 2015;150(11):1042-1049.
Ischemic Hepatitis Associated with High Inpatient Mortality
Clinical question: What is the incidence and outcome of patients with ischemic hepatitis?
Background: Ischemic hepatitis, or shock liver, is often diagnosed in patients with massive increase in aminotransferase levels most often exceeding 1000 IU/L in the setting of hepatic hypoperfusion. The data on overall incidence and mortality of these patients are limited.
Study Design: Systematic review and meta-analysis.
Setting: Variable.
Synopsis: Using a combination of PubMed, Embase, and Web of Science, the study included 24 papers on incidence and outcomes of ischemic hepatitis published between 1965 and 2015 with a combined total of 1,782 cases. The incidence of ischemic hepatitis varied based on patient location with incidence of 2/1000 in all inpatient admissions and 2.5/100 in ICU admissions. The majority of patients suffered from cardiac comorbidities and decompensation during their admission. Inpatient mortality with ischemic hepatitis was 49%.
Interestingly, only 52.9% of patients had an episode of documented hypotension.
Hospitalists taking care of patients with massive rise in aminotransferases should consider ischemic hepatitis higher in their differential, even in the absence of documented hypotension.
There was significant variability in study design, sample size, and inclusion criteria among the studies, which reduces generalizability of this systematic review.
Bottom line: Ischemic hepatitis is associated with very high mortality and should be suspected in patients with high levels of alanine aminotransferase/aspartate aminotransferase even in the absence of documented hypotension.
Citation: Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321.
Short Take
Music Can Help Ease Pain and Anxiety after Surgery
A systematic review and meta-analysis showed that music reduces pain and anxiety and decreases the need for pain medication in postoperative patients regardless of type of music or at what interval of the operative period the music was initiated.
Citation: Hole J, Hirsch M, Ball E, Meads C. Music as an aid for postoperative recovery in adults: a systematic review and meta-analysis. Lancet. 2015;386(10004):1659-1671
Clinical question: What is the incidence and outcome of patients with ischemic hepatitis?
Background: Ischemic hepatitis, or shock liver, is often diagnosed in patients with massive increase in aminotransferase levels most often exceeding 1000 IU/L in the setting of hepatic hypoperfusion. The data on overall incidence and mortality of these patients are limited.
Study Design: Systematic review and meta-analysis.
Setting: Variable.
Synopsis: Using a combination of PubMed, Embase, and Web of Science, the study included 24 papers on incidence and outcomes of ischemic hepatitis published between 1965 and 2015 with a combined total of 1,782 cases. The incidence of ischemic hepatitis varied based on patient location with incidence of 2/1000 in all inpatient admissions and 2.5/100 in ICU admissions. The majority of patients suffered from cardiac comorbidities and decompensation during their admission. Inpatient mortality with ischemic hepatitis was 49%.
Interestingly, only 52.9% of patients had an episode of documented hypotension.
Hospitalists taking care of patients with massive rise in aminotransferases should consider ischemic hepatitis higher in their differential, even in the absence of documented hypotension.
There was significant variability in study design, sample size, and inclusion criteria among the studies, which reduces generalizability of this systematic review.
Bottom line: Ischemic hepatitis is associated with very high mortality and should be suspected in patients with high levels of alanine aminotransferase/aspartate aminotransferase even in the absence of documented hypotension.
Citation: Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321.
Short Take
Music Can Help Ease Pain and Anxiety after Surgery
A systematic review and meta-analysis showed that music reduces pain and anxiety and decreases the need for pain medication in postoperative patients regardless of type of music or at what interval of the operative period the music was initiated.
Citation: Hole J, Hirsch M, Ball E, Meads C. Music as an aid for postoperative recovery in adults: a systematic review and meta-analysis. Lancet. 2015;386(10004):1659-1671
Clinical question: What is the incidence and outcome of patients with ischemic hepatitis?
Background: Ischemic hepatitis, or shock liver, is often diagnosed in patients with massive increase in aminotransferase levels most often exceeding 1000 IU/L in the setting of hepatic hypoperfusion. The data on overall incidence and mortality of these patients are limited.
Study Design: Systematic review and meta-analysis.
Setting: Variable.
Synopsis: Using a combination of PubMed, Embase, and Web of Science, the study included 24 papers on incidence and outcomes of ischemic hepatitis published between 1965 and 2015 with a combined total of 1,782 cases. The incidence of ischemic hepatitis varied based on patient location with incidence of 2/1000 in all inpatient admissions and 2.5/100 in ICU admissions. The majority of patients suffered from cardiac comorbidities and decompensation during their admission. Inpatient mortality with ischemic hepatitis was 49%.
Interestingly, only 52.9% of patients had an episode of documented hypotension.
Hospitalists taking care of patients with massive rise in aminotransferases should consider ischemic hepatitis higher in their differential, even in the absence of documented hypotension.
There was significant variability in study design, sample size, and inclusion criteria among the studies, which reduces generalizability of this systematic review.
Bottom line: Ischemic hepatitis is associated with very high mortality and should be suspected in patients with high levels of alanine aminotransferase/aspartate aminotransferase even in the absence of documented hypotension.
Citation: Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321.
Short Take
Music Can Help Ease Pain and Anxiety after Surgery
A systematic review and meta-analysis showed that music reduces pain and anxiety and decreases the need for pain medication in postoperative patients regardless of type of music or at what interval of the operative period the music was initiated.
Citation: Hole J, Hirsch M, Ball E, Meads C. Music as an aid for postoperative recovery in adults: a systematic review and meta-analysis. Lancet. 2015;386(10004):1659-1671
AIMS65 Score Helps Predict Inpatient Mortality in Acute Upper Gastrointestinal Bleed
Clinical question: Does AIMS65 risk stratification score predict inpatient mortality in patients with acute upper gastrointestinal bleed (UGIB)?
Background: Acute UGIB is associated with significant morbidity and mortality, which makes it crucial to identify high-risk patients early. Several prognostic algorithms such as Glasgow-Blatchford (GBS) and pre-endoscopy (pre-RS) and post-endoscopy (post-RS) Rockall scores are available to triage such patients. The goal of this study was to validate AIMS65 score as a predictor of inpatient mortality in patients with acute UGIB compared to these other prognostic scores.
Study Design: Retrospective, cohort study.
Setting: Tertiary-care center in Australia, January 2010 to June 2013.
Synopsis: Using ICD-10 diagnosis codes, investigators identified 424 patients with UGIB requiring endoscopy. All patients were risk-stratified using AIMS65, GBS, pre-RS, and post-RS. The AIMS65 score was found to be superior in predicting inpatient mortality compared to GBS and pre-RS scores and statistically superior to all other scores in predicting need for ICU admission.
In addition to being a single-center, retrospective study, other limitations include the use of ICD-10 codes to identify patients. Further prospective studies are needed to further validate the AIMS65 in acute UGIB.
Bottom line: AIMS65 is a simple and useful tool in predicting inpatient mortality in patients with acute UGIB. However, its applicability in making clinical decisions remains unclear.
Citation: Robertson M, Majumdar A, Boyapati R, et al. Risk stratification in acute upper GI bleeding: comparison of the AIMS65 score with the Glasgow-Blatchford and Rockall scoring systems [published online ahead of print October 16, 2015]. Gastrointest Endosc. doi:10.1016/j.gie.2015.10.021.
Clinical question: Does AIMS65 risk stratification score predict inpatient mortality in patients with acute upper gastrointestinal bleed (UGIB)?
Background: Acute UGIB is associated with significant morbidity and mortality, which makes it crucial to identify high-risk patients early. Several prognostic algorithms such as Glasgow-Blatchford (GBS) and pre-endoscopy (pre-RS) and post-endoscopy (post-RS) Rockall scores are available to triage such patients. The goal of this study was to validate AIMS65 score as a predictor of inpatient mortality in patients with acute UGIB compared to these other prognostic scores.
Study Design: Retrospective, cohort study.
Setting: Tertiary-care center in Australia, January 2010 to June 2013.
Synopsis: Using ICD-10 diagnosis codes, investigators identified 424 patients with UGIB requiring endoscopy. All patients were risk-stratified using AIMS65, GBS, pre-RS, and post-RS. The AIMS65 score was found to be superior in predicting inpatient mortality compared to GBS and pre-RS scores and statistically superior to all other scores in predicting need for ICU admission.
In addition to being a single-center, retrospective study, other limitations include the use of ICD-10 codes to identify patients. Further prospective studies are needed to further validate the AIMS65 in acute UGIB.
Bottom line: AIMS65 is a simple and useful tool in predicting inpatient mortality in patients with acute UGIB. However, its applicability in making clinical decisions remains unclear.
Citation: Robertson M, Majumdar A, Boyapati R, et al. Risk stratification in acute upper GI bleeding: comparison of the AIMS65 score with the Glasgow-Blatchford and Rockall scoring systems [published online ahead of print October 16, 2015]. Gastrointest Endosc. doi:10.1016/j.gie.2015.10.021.
Clinical question: Does AIMS65 risk stratification score predict inpatient mortality in patients with acute upper gastrointestinal bleed (UGIB)?
Background: Acute UGIB is associated with significant morbidity and mortality, which makes it crucial to identify high-risk patients early. Several prognostic algorithms such as Glasgow-Blatchford (GBS) and pre-endoscopy (pre-RS) and post-endoscopy (post-RS) Rockall scores are available to triage such patients. The goal of this study was to validate AIMS65 score as a predictor of inpatient mortality in patients with acute UGIB compared to these other prognostic scores.
Study Design: Retrospective, cohort study.
Setting: Tertiary-care center in Australia, January 2010 to June 2013.
Synopsis: Using ICD-10 diagnosis codes, investigators identified 424 patients with UGIB requiring endoscopy. All patients were risk-stratified using AIMS65, GBS, pre-RS, and post-RS. The AIMS65 score was found to be superior in predicting inpatient mortality compared to GBS and pre-RS scores and statistically superior to all other scores in predicting need for ICU admission.
In addition to being a single-center, retrospective study, other limitations include the use of ICD-10 codes to identify patients. Further prospective studies are needed to further validate the AIMS65 in acute UGIB.
Bottom line: AIMS65 is a simple and useful tool in predicting inpatient mortality in patients with acute UGIB. However, its applicability in making clinical decisions remains unclear.
Citation: Robertson M, Majumdar A, Boyapati R, et al. Risk stratification in acute upper GI bleeding: comparison of the AIMS65 score with the Glasgow-Blatchford and Rockall scoring systems [published online ahead of print October 16, 2015]. Gastrointest Endosc. doi:10.1016/j.gie.2015.10.021.
No Mortality Benefit to Cardiac Catheterization in Patients with Stable Ischemic Heart Disease
Clinical question: Can cardiac catheterization prolong survival in patients with stable ischemic heart disease?
Background: Previous results from the COURAGE trial found no benefit of percutaneous intervention (PCI) as compared to medical therapy on a composite endpoint of death or nonfatal myocardial infarction or in total mortality at 4.6 years follow-up. The authors now report 15-year follow-up of the same patients.
Study design: Randomized, controlled trial.
Setting: The majority of the patients were from Veterans Affairs (VA) medical centers, although non-VA hospitals in the U.S. also were included.
Synopsis: Originally, 2,287 patients with stable ischemic heart disease and either an abnormal stress test or evidence of ischemia on ECG, as well at least 70% stenosis on angiography, were randomized to medical therapy or medical therapy plus PCI. Now, investigators have obtained extended follow-up information for 1,211 of the original patients (53%). They concluded that after 15 years of follow-up, there was no survival difference for the patients who initially received PCI in addition to medical management.
One limitation of the study was that it did not reflect important advances in both medical and interventional management of ischemic heart disease that have taken place since the study was conducted, which may affect patient mortality. It is also noteworthy that the investigators were unable to determine how many patients in the medical management group subsequently underwent revascularization after the study concluded and therefore may have crossed over between groups. Nevertheless, for now it appears that the major utility of PCI in stable ischemic heart disease is in symptomatic management.
Bottom Line: After 15 years of follow-up, there was still no mortality benefit to PCI as compared to optimal medical therapy for stable ischemic heart disease.
Citation: Sedlis SP, Hartigan PM, Teo KK, et al. Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med. 2015;373(20):1937-1946
Short Take
Cauti Infections Are Rarely Clinically Relevant and Associated with Low Complication Rate
A single-center retrospective study in the ICU setting shows that the definition of catheter-associated urinary tract infections (CAUTIs) is nonspecific and they’re mostly diagnosed when urine cultures are sent for workup of fever. Most of the time, there are alternative explanations for the fever.
Citation: Tedja R, Wentink J, O’Horo J, Thompson R, Sampathkumar P et al. Catheter-associated urinary tract infections in intensive care unit patients. Infect Control Hosp Epidemiol. 2015;36(11):1330-1334.
Clinical question: Can cardiac catheterization prolong survival in patients with stable ischemic heart disease?
Background: Previous results from the COURAGE trial found no benefit of percutaneous intervention (PCI) as compared to medical therapy on a composite endpoint of death or nonfatal myocardial infarction or in total mortality at 4.6 years follow-up. The authors now report 15-year follow-up of the same patients.
Study design: Randomized, controlled trial.
Setting: The majority of the patients were from Veterans Affairs (VA) medical centers, although non-VA hospitals in the U.S. also were included.
Synopsis: Originally, 2,287 patients with stable ischemic heart disease and either an abnormal stress test or evidence of ischemia on ECG, as well at least 70% stenosis on angiography, were randomized to medical therapy or medical therapy plus PCI. Now, investigators have obtained extended follow-up information for 1,211 of the original patients (53%). They concluded that after 15 years of follow-up, there was no survival difference for the patients who initially received PCI in addition to medical management.
One limitation of the study was that it did not reflect important advances in both medical and interventional management of ischemic heart disease that have taken place since the study was conducted, which may affect patient mortality. It is also noteworthy that the investigators were unable to determine how many patients in the medical management group subsequently underwent revascularization after the study concluded and therefore may have crossed over between groups. Nevertheless, for now it appears that the major utility of PCI in stable ischemic heart disease is in symptomatic management.
Bottom Line: After 15 years of follow-up, there was still no mortality benefit to PCI as compared to optimal medical therapy for stable ischemic heart disease.
Citation: Sedlis SP, Hartigan PM, Teo KK, et al. Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med. 2015;373(20):1937-1946
Short Take
Cauti Infections Are Rarely Clinically Relevant and Associated with Low Complication Rate
A single-center retrospective study in the ICU setting shows that the definition of catheter-associated urinary tract infections (CAUTIs) is nonspecific and they’re mostly diagnosed when urine cultures are sent for workup of fever. Most of the time, there are alternative explanations for the fever.
Citation: Tedja R, Wentink J, O’Horo J, Thompson R, Sampathkumar P et al. Catheter-associated urinary tract infections in intensive care unit patients. Infect Control Hosp Epidemiol. 2015;36(11):1330-1334.
Clinical question: Can cardiac catheterization prolong survival in patients with stable ischemic heart disease?
Background: Previous results from the COURAGE trial found no benefit of percutaneous intervention (PCI) as compared to medical therapy on a composite endpoint of death or nonfatal myocardial infarction or in total mortality at 4.6 years follow-up. The authors now report 15-year follow-up of the same patients.
Study design: Randomized, controlled trial.
Setting: The majority of the patients were from Veterans Affairs (VA) medical centers, although non-VA hospitals in the U.S. also were included.
Synopsis: Originally, 2,287 patients with stable ischemic heart disease and either an abnormal stress test or evidence of ischemia on ECG, as well at least 70% stenosis on angiography, were randomized to medical therapy or medical therapy plus PCI. Now, investigators have obtained extended follow-up information for 1,211 of the original patients (53%). They concluded that after 15 years of follow-up, there was no survival difference for the patients who initially received PCI in addition to medical management.
One limitation of the study was that it did not reflect important advances in both medical and interventional management of ischemic heart disease that have taken place since the study was conducted, which may affect patient mortality. It is also noteworthy that the investigators were unable to determine how many patients in the medical management group subsequently underwent revascularization after the study concluded and therefore may have crossed over between groups. Nevertheless, for now it appears that the major utility of PCI in stable ischemic heart disease is in symptomatic management.
Bottom Line: After 15 years of follow-up, there was still no mortality benefit to PCI as compared to optimal medical therapy for stable ischemic heart disease.
Citation: Sedlis SP, Hartigan PM, Teo KK, et al. Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med. 2015;373(20):1937-1946
Short Take
Cauti Infections Are Rarely Clinically Relevant and Associated with Low Complication Rate
A single-center retrospective study in the ICU setting shows that the definition of catheter-associated urinary tract infections (CAUTIs) is nonspecific and they’re mostly diagnosed when urine cultures are sent for workup of fever. Most of the time, there are alternative explanations for the fever.
Citation: Tedja R, Wentink J, O’Horo J, Thompson R, Sampathkumar P et al. Catheter-associated urinary tract infections in intensive care unit patients. Infect Control Hosp Epidemiol. 2015;36(11):1330-1334.
Increase in Broad-Spectrum Antibiotics Disproportionate to Rate of Resistant Organisms
Clinical question: Have healthcare-associated pneumonia (HCAP) guidelines improved treatment accuracy?
Background: Guidelines released in 2005 call for the use of broad-spectrum antibiotics for patients presenting with pneumonia who have had recent healthcare exposure. However, there is scant evidence to support the risk factors they identify, and the guidelines are likely to increase use of broad-spectrum antibiotics.
Study design: Observational, retrospective.
Setting: VA medical centers, 2006–2010.
Synopsis: In this study, VA medical center physicians evaluated 95,511 hospitalizations for pneumonia at 128 hospitals between 2006 and 2010, the years following the 2005 guidelines. Annual analyses were performed to assess antibiotics selection as well as evidence of resistant bacteria from blood and respiratory cultures. Researchers found that while the use of broad-spectrum antibiotics increased drastically during the study period (vancomycin from 16% to 31% and piperacillin-tazobactam from 16% to 27%, P<0.001 for both), the incidence of resistant organisms either decreased or remained stable.
Additionally, physicians were no better at matching broad-spectrum antibiotics to patients infected with resistant organisms at the end of the study period than they were at the start. They conclude that more research is urgently needed to identify patients at risk for resistant organisms in order to more appropriately prescribe broad-spectrum antibiotics.
This study did not evaluate patients’ clinical outcomes, so it is unclear whether they may have benefitted clinically from the implementation of the guidelines. For now, the optimal approach to empiric therapy for HCAP remains undefined.
Bottom line: Despite a marked increase in the use of broad-spectrum antibiotics for HCAP in the years following a change in treatment guidelines, doctors showed no improvement at matching these antibiotics to patients infected with resistant organisms.
Citation: Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403-1410.
Clinical question: Have healthcare-associated pneumonia (HCAP) guidelines improved treatment accuracy?
Background: Guidelines released in 2005 call for the use of broad-spectrum antibiotics for patients presenting with pneumonia who have had recent healthcare exposure. However, there is scant evidence to support the risk factors they identify, and the guidelines are likely to increase use of broad-spectrum antibiotics.
Study design: Observational, retrospective.
Setting: VA medical centers, 2006–2010.
Synopsis: In this study, VA medical center physicians evaluated 95,511 hospitalizations for pneumonia at 128 hospitals between 2006 and 2010, the years following the 2005 guidelines. Annual analyses were performed to assess antibiotics selection as well as evidence of resistant bacteria from blood and respiratory cultures. Researchers found that while the use of broad-spectrum antibiotics increased drastically during the study period (vancomycin from 16% to 31% and piperacillin-tazobactam from 16% to 27%, P<0.001 for both), the incidence of resistant organisms either decreased or remained stable.
Additionally, physicians were no better at matching broad-spectrum antibiotics to patients infected with resistant organisms at the end of the study period than they were at the start. They conclude that more research is urgently needed to identify patients at risk for resistant organisms in order to more appropriately prescribe broad-spectrum antibiotics.
This study did not evaluate patients’ clinical outcomes, so it is unclear whether they may have benefitted clinically from the implementation of the guidelines. For now, the optimal approach to empiric therapy for HCAP remains undefined.
Bottom line: Despite a marked increase in the use of broad-spectrum antibiotics for HCAP in the years following a change in treatment guidelines, doctors showed no improvement at matching these antibiotics to patients infected with resistant organisms.
Citation: Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403-1410.
Clinical question: Have healthcare-associated pneumonia (HCAP) guidelines improved treatment accuracy?
Background: Guidelines released in 2005 call for the use of broad-spectrum antibiotics for patients presenting with pneumonia who have had recent healthcare exposure. However, there is scant evidence to support the risk factors they identify, and the guidelines are likely to increase use of broad-spectrum antibiotics.
Study design: Observational, retrospective.
Setting: VA medical centers, 2006–2010.
Synopsis: In this study, VA medical center physicians evaluated 95,511 hospitalizations for pneumonia at 128 hospitals between 2006 and 2010, the years following the 2005 guidelines. Annual analyses were performed to assess antibiotics selection as well as evidence of resistant bacteria from blood and respiratory cultures. Researchers found that while the use of broad-spectrum antibiotics increased drastically during the study period (vancomycin from 16% to 31% and piperacillin-tazobactam from 16% to 27%, P<0.001 for both), the incidence of resistant organisms either decreased or remained stable.
Additionally, physicians were no better at matching broad-spectrum antibiotics to patients infected with resistant organisms at the end of the study period than they were at the start. They conclude that more research is urgently needed to identify patients at risk for resistant organisms in order to more appropriately prescribe broad-spectrum antibiotics.
This study did not evaluate patients’ clinical outcomes, so it is unclear whether they may have benefitted clinically from the implementation of the guidelines. For now, the optimal approach to empiric therapy for HCAP remains undefined.
Bottom line: Despite a marked increase in the use of broad-spectrum antibiotics for HCAP in the years following a change in treatment guidelines, doctors showed no improvement at matching these antibiotics to patients infected with resistant organisms.
Citation: Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403-1410.
Discontinuing Inhaled Corticosteroids in COPD Reduces Risk of Pneumonia
Clinical question: Is discontinuation of inhaled corticosteroids (ICSs) in patients with COPD associated with a decreased risk of pneumonia?
Background: ICSs are used in up to 85% of patients treated for COPD but may be associated with adverse systemic side effects including pneumonia. Trials looking at weaning patients off ICSs and replacing with long-acting bronchodilators have found few adverse outcomes; however, the benefits of discontinuation on adverse events, including pneumonia, have been unclear.
Study design: Case-control study.
Setting: Quebec health systems.
Synopsis: Using the Quebec health insurance databases, a study cohort of 103,386 patients with COPD on ICSs was created. Patients were followed for a mean of 4.9 years; 14,020 patients who were hospitalized for pneumonia or died from pneumonia outside the hospital were matched to control subjects. Discontinuation of ICSs was associated with a 37% decrease in serious pneumonia (relative risk [RR] 0.63; 95% CI, 0.60–0.66). The risk reduction occurred as early as one month after discontinuation of ICSs. Risk reduction was greater with fluticasone (RR 0.58; 95% CI, 0.54–0.61) than with budesonide (RR 0.87; 95% CI, 0.7–0.97).
Population size and follow-up may contribute to why risk reduction in pneumonia was seen in this study but not in other recent randomized trials on discontinuation of ICSs. A limitation of this study was its observational design; however, its results suggest that use of ICSs in COPD patients should be highly selective, as indiscriminate use can subject patients to elevated risk of hospitalization or death from pneumonia.
Bottom line: Discontinuation of ICSs in patients with COPD is associated with a decreased risk of contracting serious pneumonia. This reduction appears greatest with fluticasone.
Citation: Suissa S, Coulombe J, Ernst P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest. 2015;148(5):1177-1183.
Short Take
Increase in Rates of Prescription Drug Use and Polypharmacy Seen
The percentage of Americans who reported taking prescription medications increased substantially from 1999 to 2012 (51% to 59%), as did the percentage who reported taking at least five prescription medications.
Citation: Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1830.
Clinical question: Is discontinuation of inhaled corticosteroids (ICSs) in patients with COPD associated with a decreased risk of pneumonia?
Background: ICSs are used in up to 85% of patients treated for COPD but may be associated with adverse systemic side effects including pneumonia. Trials looking at weaning patients off ICSs and replacing with long-acting bronchodilators have found few adverse outcomes; however, the benefits of discontinuation on adverse events, including pneumonia, have been unclear.
Study design: Case-control study.
Setting: Quebec health systems.
Synopsis: Using the Quebec health insurance databases, a study cohort of 103,386 patients with COPD on ICSs was created. Patients were followed for a mean of 4.9 years; 14,020 patients who were hospitalized for pneumonia or died from pneumonia outside the hospital were matched to control subjects. Discontinuation of ICSs was associated with a 37% decrease in serious pneumonia (relative risk [RR] 0.63; 95% CI, 0.60–0.66). The risk reduction occurred as early as one month after discontinuation of ICSs. Risk reduction was greater with fluticasone (RR 0.58; 95% CI, 0.54–0.61) than with budesonide (RR 0.87; 95% CI, 0.7–0.97).
Population size and follow-up may contribute to why risk reduction in pneumonia was seen in this study but not in other recent randomized trials on discontinuation of ICSs. A limitation of this study was its observational design; however, its results suggest that use of ICSs in COPD patients should be highly selective, as indiscriminate use can subject patients to elevated risk of hospitalization or death from pneumonia.
Bottom line: Discontinuation of ICSs in patients with COPD is associated with a decreased risk of contracting serious pneumonia. This reduction appears greatest with fluticasone.
Citation: Suissa S, Coulombe J, Ernst P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest. 2015;148(5):1177-1183.
Short Take
Increase in Rates of Prescription Drug Use and Polypharmacy Seen
The percentage of Americans who reported taking prescription medications increased substantially from 1999 to 2012 (51% to 59%), as did the percentage who reported taking at least five prescription medications.
Citation: Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1830.
Clinical question: Is discontinuation of inhaled corticosteroids (ICSs) in patients with COPD associated with a decreased risk of pneumonia?
Background: ICSs are used in up to 85% of patients treated for COPD but may be associated with adverse systemic side effects including pneumonia. Trials looking at weaning patients off ICSs and replacing with long-acting bronchodilators have found few adverse outcomes; however, the benefits of discontinuation on adverse events, including pneumonia, have been unclear.
Study design: Case-control study.
Setting: Quebec health systems.
Synopsis: Using the Quebec health insurance databases, a study cohort of 103,386 patients with COPD on ICSs was created. Patients were followed for a mean of 4.9 years; 14,020 patients who were hospitalized for pneumonia or died from pneumonia outside the hospital were matched to control subjects. Discontinuation of ICSs was associated with a 37% decrease in serious pneumonia (relative risk [RR] 0.63; 95% CI, 0.60–0.66). The risk reduction occurred as early as one month after discontinuation of ICSs. Risk reduction was greater with fluticasone (RR 0.58; 95% CI, 0.54–0.61) than with budesonide (RR 0.87; 95% CI, 0.7–0.97).
Population size and follow-up may contribute to why risk reduction in pneumonia was seen in this study but not in other recent randomized trials on discontinuation of ICSs. A limitation of this study was its observational design; however, its results suggest that use of ICSs in COPD patients should be highly selective, as indiscriminate use can subject patients to elevated risk of hospitalization or death from pneumonia.
Bottom line: Discontinuation of ICSs in patients with COPD is associated with a decreased risk of contracting serious pneumonia. This reduction appears greatest with fluticasone.
Citation: Suissa S, Coulombe J, Ernst P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest. 2015;148(5):1177-1183.
Short Take
Increase in Rates of Prescription Drug Use and Polypharmacy Seen
The percentage of Americans who reported taking prescription medications increased substantially from 1999 to 2012 (51% to 59%), as did the percentage who reported taking at least five prescription medications.
Citation: Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1830.
MEDS Score for Sepsis Might Best Predict ED Mortality
Clinical question: Which illness severity score best predicts outcomes in emergency department (ED) patients presenting with infection?
Background: Several scoring models have been developed to predict illness severity and mortality in patients with infection. Some scores were developed specifically for patients with sepsis and others for patients in a general critical care setting. These different scoring models have not been specifically compared and validated in the ED setting in patients with infection of various severities.
Study design: Prospective, observational study.
Setting: Adult ED in a metropolitan tertiary, university-affiliated hospital.
Synopsis: Investigators prospectively identified 8,871 adult inpatients with infection from a single-center ED. Data to calculate five prediction models were collected. The models were:
- Mortality in Emergency Department Sepsis (MEDS) score;
- Acute Physiology and Chronic Health Evaluation II (APACHE II);
- Simplified Acute Physiology Score II (SAPS II);
- Sequential Organ Failure Assessment (SOFA); and
- Severe Sepsis Score (SSS).
Severity score performance was assessed for the overall cohort and for subgroups, including infection without systemic inflammatory response syndrome, sepsis, severe sepsis, and septic shock. The MEDS score best predicted mortality in the cohort, with an area under the receiver operating characteristics curve of 0.92. However, older scoring models such as the APACHE II and SAPS II still discriminated well, especially in patients who were admitted to the ICU. All scores tended to overestimate mortality.
Bottom line: The MEDS score may best predict illness severity in septic patients presenting to the ED, but other scoring models may be better-suited for specific patient populations.
Citation: Williams JM, Greenslade JH, Chu K, Brown AF, Lipman J. Severity scores in emergency department patients with presumed infection: a prospective validation study. Crit Care Med. 2016;44(3):539-547.
Clinical question: Which illness severity score best predicts outcomes in emergency department (ED) patients presenting with infection?
Background: Several scoring models have been developed to predict illness severity and mortality in patients with infection. Some scores were developed specifically for patients with sepsis and others for patients in a general critical care setting. These different scoring models have not been specifically compared and validated in the ED setting in patients with infection of various severities.
Study design: Prospective, observational study.
Setting: Adult ED in a metropolitan tertiary, university-affiliated hospital.
Synopsis: Investigators prospectively identified 8,871 adult inpatients with infection from a single-center ED. Data to calculate five prediction models were collected. The models were:
- Mortality in Emergency Department Sepsis (MEDS) score;
- Acute Physiology and Chronic Health Evaluation II (APACHE II);
- Simplified Acute Physiology Score II (SAPS II);
- Sequential Organ Failure Assessment (SOFA); and
- Severe Sepsis Score (SSS).
Severity score performance was assessed for the overall cohort and for subgroups, including infection without systemic inflammatory response syndrome, sepsis, severe sepsis, and septic shock. The MEDS score best predicted mortality in the cohort, with an area under the receiver operating characteristics curve of 0.92. However, older scoring models such as the APACHE II and SAPS II still discriminated well, especially in patients who were admitted to the ICU. All scores tended to overestimate mortality.
Bottom line: The MEDS score may best predict illness severity in septic patients presenting to the ED, but other scoring models may be better-suited for specific patient populations.
Citation: Williams JM, Greenslade JH, Chu K, Brown AF, Lipman J. Severity scores in emergency department patients with presumed infection: a prospective validation study. Crit Care Med. 2016;44(3):539-547.
Clinical question: Which illness severity score best predicts outcomes in emergency department (ED) patients presenting with infection?
Background: Several scoring models have been developed to predict illness severity and mortality in patients with infection. Some scores were developed specifically for patients with sepsis and others for patients in a general critical care setting. These different scoring models have not been specifically compared and validated in the ED setting in patients with infection of various severities.
Study design: Prospective, observational study.
Setting: Adult ED in a metropolitan tertiary, university-affiliated hospital.
Synopsis: Investigators prospectively identified 8,871 adult inpatients with infection from a single-center ED. Data to calculate five prediction models were collected. The models were:
- Mortality in Emergency Department Sepsis (MEDS) score;
- Acute Physiology and Chronic Health Evaluation II (APACHE II);
- Simplified Acute Physiology Score II (SAPS II);
- Sequential Organ Failure Assessment (SOFA); and
- Severe Sepsis Score (SSS).
Severity score performance was assessed for the overall cohort and for subgroups, including infection without systemic inflammatory response syndrome, sepsis, severe sepsis, and septic shock. The MEDS score best predicted mortality in the cohort, with an area under the receiver operating characteristics curve of 0.92. However, older scoring models such as the APACHE II and SAPS II still discriminated well, especially in patients who were admitted to the ICU. All scores tended to overestimate mortality.
Bottom line: The MEDS score may best predict illness severity in septic patients presenting to the ED, but other scoring models may be better-suited for specific patient populations.
Citation: Williams JM, Greenslade JH, Chu K, Brown AF, Lipman J. Severity scores in emergency department patients with presumed infection: a prospective validation study. Crit Care Med. 2016;44(3):539-547.





