User login
Symmetrical drug‐related intertriginous and flexural exanthema after coronary artery angiography
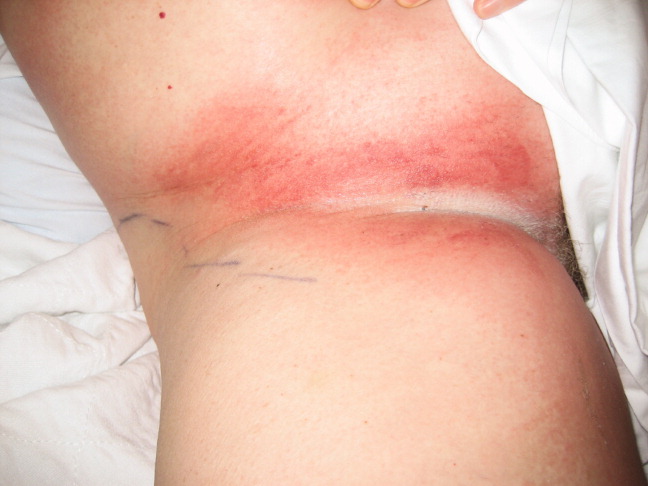
A 57‐year‐old woman developed a pruritic rash 6 hours after undergoing coronary angiography. On exam, symmetrical, eczematous plaques were noted in her bilateral groin (Figure 1), buttocks, axillae (Figure 2), and the intertriginous folds of her breasts. No palmar, plantar, or mucosal lesions were noted and laboratory tests were normal. This patient presents with symmetrical drug‐related intertriginous and flexural exanthema (SDRIFE) secondary to iodine‐based contrast dye. It is a type IV hypersensitivity reaction most often reported to nickel, mercury, and systemic antibiotics, although previous sensitization is often unknown. Also called baboon syndrome because its distribution mimics the pink bottom of a baboon, SDRIFE appears hours to days after exposure to the offending agent. The unusual distribution may be explained by high concentrations of the allergen in sweat. Resolution is typical with discontinuation of the offending drug, although antihistamines, topical steroids, and possibly oral steroids may be useful adjuncts.
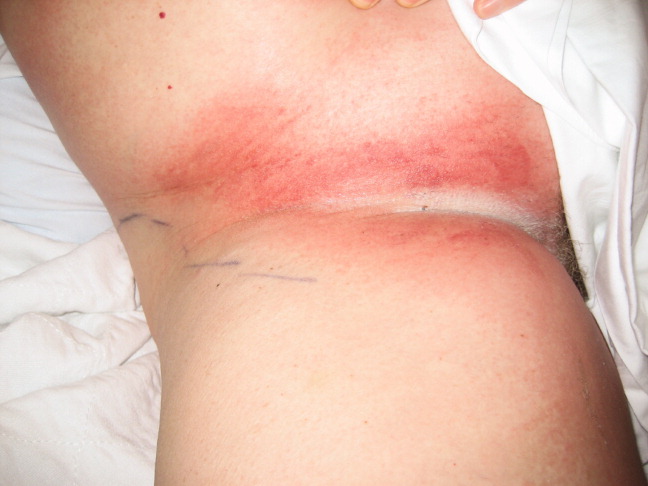
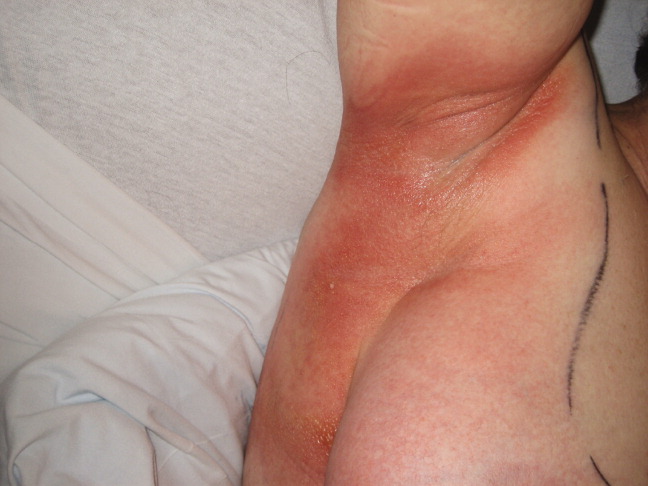
A 57‐year‐old woman developed a pruritic rash 6 hours after undergoing coronary angiography. On exam, symmetrical, eczematous plaques were noted in her bilateral groin (Figure 1), buttocks, axillae (Figure 2), and the intertriginous folds of her breasts. No palmar, plantar, or mucosal lesions were noted and laboratory tests were normal. This patient presents with symmetrical drug‐related intertriginous and flexural exanthema (SDRIFE) secondary to iodine‐based contrast dye. It is a type IV hypersensitivity reaction most often reported to nickel, mercury, and systemic antibiotics, although previous sensitization is often unknown. Also called baboon syndrome because its distribution mimics the pink bottom of a baboon, SDRIFE appears hours to days after exposure to the offending agent. The unusual distribution may be explained by high concentrations of the allergen in sweat. Resolution is typical with discontinuation of the offending drug, although antihistamines, topical steroids, and possibly oral steroids may be useful adjuncts.


A 57‐year‐old woman developed a pruritic rash 6 hours after undergoing coronary angiography. On exam, symmetrical, eczematous plaques were noted in her bilateral groin (Figure 1), buttocks, axillae (Figure 2), and the intertriginous folds of her breasts. No palmar, plantar, or mucosal lesions were noted and laboratory tests were normal. This patient presents with symmetrical drug‐related intertriginous and flexural exanthema (SDRIFE) secondary to iodine‐based contrast dye. It is a type IV hypersensitivity reaction most often reported to nickel, mercury, and systemic antibiotics, although previous sensitization is often unknown. Also called baboon syndrome because its distribution mimics the pink bottom of a baboon, SDRIFE appears hours to days after exposure to the offending agent. The unusual distribution may be explained by high concentrations of the allergen in sweat. Resolution is typical with discontinuation of the offending drug, although antihistamines, topical steroids, and possibly oral steroids may be useful adjuncts.


Out of Africa
I knew that he was going to die. I do not remember when it became evident to me, and I was not sure how to tell the family. I thought that I could arrange a family meeting and inform them of the sad reality in a calm, sympathetic manner. The patient had chronic lymphocytic leukemia, and his case was advanced. The only medication available to him was chlorambucil. As the days passed, I could not bring myself to call the family meeting because they had so much hope. Every day as we got results and I shared them, I would sandwich the bad news with some optimism to ease their pain. Well, his white blood cell count has come down, but his platelet count and red blood cell counts are very low, and this puts him in danger of bleeding. The medicine is bringing the white cell count down but has not yet brought the other cell counts up. What we can do is give him some blood. I tried not to allow despair to creep into my thoughts or my voice. I knew that the blood bank had no platelets or packed red blood cells. He was not eating or drinking, and we had placed a nasogastric tube through which his family fed him wheat or millet porridge (manufactured tube feeds are not widely available in Uganda). I tried not to think about the time that he had almost died a few weeks before.
I had been called to the bedside because the patient was in respiratory distress. The doctor on call was in his office when I arrived, and I wondered why he was not at the bedside. I took one look at the patient and had to step away for a moment to compose myself. I felt the tears threatening to come, but I had to stop them. This was not the time for emotions. I had to assess the patient and make some quick decisions. The doctor on call seemed to have given up. He was a young trainee in a system in which you treat when you can and, if the situation is hopeless, you move on to the next patient. There are no resources for perpetuating hope. This is so different from my practice in the United States, where if a patient wants everything done, we will do it. We are not taught when to give up hope, and futility does not figure into the allocation of resources. I looked at the patient struggling to breathe and felt that I had to do all that I could for him. I asked the doctor on call to place the patient on oxygen and hoped that the tanks were not empty. I was worried about a lot of things, such as pulmonary embolus, myocardial infarction, and pneumonia. Diagnosing any of these would not be easy (the hospital did not have a computed tomography scanner, and obtaining cardiac enzymes was not as simple as clicking a button on a computer). First things first: the chest X‐ray. I thanked God that we were in a private hospital, one of the best in the city of Kampala, so we were able to get a chest X‐ray right away. As we transported the patient (portable X‐rays are nonexistent), the resident told me that he had called the consultant (the equivalent of an attending physician in the United States), who happened to be out of town. The consultant instructed us to transfer the patient to Mulago Hospital (the largest tertiary center in Uganda with well over 1000 beds and some of the equipment that you might find in an American hospital). I wondered how an attending physician could be out of town and leave a resident in charge. The thought was disturbing, but I had no time to ponder it. I later learned that physicians are so poorly paid that many have their own private clinics. My patient got the X‐ray, and I reviewed it with the resident. Tuberculosis, he said. Tuberculosis was this resident's reality. Many patients who need chest X‐rays in Uganda have tuberculosis. As I reviewed the X‐ray, though, I was certain that this was congestive heart failure. However, in Uganda, congestive heart failure is rarely diagnosed in the hospital. Patients with an ejection fraction low enough to cause congestion generally die before they get to a hospital. I knew that some furosemide would work for this patient, but I could not get the resident to listen to me. He had orders from the consultant to transfer the patient immediately, and the ambulance was ready. I tried to convince the resident to administer furosemide before transferring the patient, but he feared administering a drug not approved by his superior. As the patient was loaded onto the ambulance, I reflected for a second on how different things would be if we were in the United States. We arrived at Mulago in record time, and I tried to get the intake doctors to understand what the problem was; however, they did not want to hear from the US doctor. I stared in frustration as they wasted valuable time. I wondered how long the patient would survive in respiratory distress with nothing being done. I called the patient's son and asked him to come to Mulago immediately. Miraculously, he had already been on his way. As I held the patient's hand, sure that he would die right then and there in a waiting area as nobody did anything, I saw the patient's son. I knew that he was a pharmacist, and I asked him to go to the pharmacy and buy furosemide and some syringes. In Uganda, one can buy any medication without a prescription. Luckily, the hospital pharmacy had the drug. We treated the patient, and in no time, his breathing had returned to normal.
I was jolted back to reality. He was dying, and I knew it. He had had many close calls. There was the time that he got the wrong blood during a blood transfusion. I informed the doctor on call as the blood was being administered that I thought the patient was getting a transfusion reaction because he had rigors. The physician on call suggested covering him in blankets, and I suggested stopping the infusion and administering steroids. The pack of blood showed that he was getting his blood type. The patient was typed and crossed again, and to our surprise, we got a different result. I went to the laboratory to perform a third, tie‐breaking cross match and was surprised to note that the reagents had passed their expiration date. However, I knew that these were small battles we were winning and that there was no winning the war.
I recognized that the challenges of practicing medicine in the developing world were many. I wondered how the patients of families with fewer resources survived. The answer was obvious: they didn't. I personally picked up blood when it was available from the blood bank and vividly remember walking from the blood bank at night to the private hospital with units of blood in each hand. Once we arrived at the hospital, I had to warm the blood to room temperature by holding it close to my own skin. Many tests that we perform routinely on a hospitalized patient in the United States are not available.
There was still the problem of breaking the news to the family. Despite everything that had been done and the many near misses that the patient had survived, he was still going to die. It turns out that the family was more intuitive than I thought. One day, the son came to me and asked how long his dad had. Not long, I said quietly. I thought about all that I could potentially do if I had the patient in the hospital at which I worked in the United States. Would it have made a difference? I do not know. It was impossible doctoring this patient, and I suspect doing it in a resource‐rich environment would not have made it any easier. You see this patient, perhaps the most important patient of my life, certainly a patient that I will never forget, was my father.
It had been 15 years since I had traveled to the United States for an education. I knew that my father was so incredibly proud of me. I think that he was the happiest I had ever seen him when he attended my graduation from medical school in Minnesota. I had been looking forward to this visit back home because it had been 3 years since I had last seen my family. I was somewhat concerned because my father had told me a week before I traveled that he was not feeling well. When I arrived, there seemed to be relief on my brother's face when he met me at the airport. We drove straight to the hospital, and along with the joy of seeing me, I could sense that my father was glad that I was home at this particular point in time. They had just received the diagnosis. He had leukemia, and they were glad that their doctor was home. They had particular faith in the daughter (sister) sent abroad for an education. Things would now be okay. Initially, I never got to choose the role of doctor that I played in the final chapter of my father's life. The decision was made for me out of my family's desperation to make sure that they had left no stone unturned to help my father, and I accepted it out of necessity. As my father became my father when I entered this world, I became his doctor when he was leaving it; there was never any question in my mind, as there never was in his. As it became clear that my father would not survive, I chose to continue the role of doctor. I have watched many patients die as a physician and have done my best to make sure that their passing is comfortable, peaceful, and dignified. The doctor could help this patient die, but the daughter could not watch her father go. When it was evident that he had only days to live and did not need this doctor or know his daughter, I flew back to the United States. Three days later my father died. I was not physically at his bedside, but my spirit was. I have no regrets. Although the head knows that he passed on, in my mind's eye, he is laughing and has a twinkle in his eye. I could not bear to see him without life. A piece of my heart is buried with him, and for this reason, I will never be out of Africa.
Acknowledgements
The author is indebted to J.B. Kisuule and seeks to honor his life of service. Thank you to Dr. Roy Ziegelstein for his help with this article.
I knew that he was going to die. I do not remember when it became evident to me, and I was not sure how to tell the family. I thought that I could arrange a family meeting and inform them of the sad reality in a calm, sympathetic manner. The patient had chronic lymphocytic leukemia, and his case was advanced. The only medication available to him was chlorambucil. As the days passed, I could not bring myself to call the family meeting because they had so much hope. Every day as we got results and I shared them, I would sandwich the bad news with some optimism to ease their pain. Well, his white blood cell count has come down, but his platelet count and red blood cell counts are very low, and this puts him in danger of bleeding. The medicine is bringing the white cell count down but has not yet brought the other cell counts up. What we can do is give him some blood. I tried not to allow despair to creep into my thoughts or my voice. I knew that the blood bank had no platelets or packed red blood cells. He was not eating or drinking, and we had placed a nasogastric tube through which his family fed him wheat or millet porridge (manufactured tube feeds are not widely available in Uganda). I tried not to think about the time that he had almost died a few weeks before.
I had been called to the bedside because the patient was in respiratory distress. The doctor on call was in his office when I arrived, and I wondered why he was not at the bedside. I took one look at the patient and had to step away for a moment to compose myself. I felt the tears threatening to come, but I had to stop them. This was not the time for emotions. I had to assess the patient and make some quick decisions. The doctor on call seemed to have given up. He was a young trainee in a system in which you treat when you can and, if the situation is hopeless, you move on to the next patient. There are no resources for perpetuating hope. This is so different from my practice in the United States, where if a patient wants everything done, we will do it. We are not taught when to give up hope, and futility does not figure into the allocation of resources. I looked at the patient struggling to breathe and felt that I had to do all that I could for him. I asked the doctor on call to place the patient on oxygen and hoped that the tanks were not empty. I was worried about a lot of things, such as pulmonary embolus, myocardial infarction, and pneumonia. Diagnosing any of these would not be easy (the hospital did not have a computed tomography scanner, and obtaining cardiac enzymes was not as simple as clicking a button on a computer). First things first: the chest X‐ray. I thanked God that we were in a private hospital, one of the best in the city of Kampala, so we were able to get a chest X‐ray right away. As we transported the patient (portable X‐rays are nonexistent), the resident told me that he had called the consultant (the equivalent of an attending physician in the United States), who happened to be out of town. The consultant instructed us to transfer the patient to Mulago Hospital (the largest tertiary center in Uganda with well over 1000 beds and some of the equipment that you might find in an American hospital). I wondered how an attending physician could be out of town and leave a resident in charge. The thought was disturbing, but I had no time to ponder it. I later learned that physicians are so poorly paid that many have their own private clinics. My patient got the X‐ray, and I reviewed it with the resident. Tuberculosis, he said. Tuberculosis was this resident's reality. Many patients who need chest X‐rays in Uganda have tuberculosis. As I reviewed the X‐ray, though, I was certain that this was congestive heart failure. However, in Uganda, congestive heart failure is rarely diagnosed in the hospital. Patients with an ejection fraction low enough to cause congestion generally die before they get to a hospital. I knew that some furosemide would work for this patient, but I could not get the resident to listen to me. He had orders from the consultant to transfer the patient immediately, and the ambulance was ready. I tried to convince the resident to administer furosemide before transferring the patient, but he feared administering a drug not approved by his superior. As the patient was loaded onto the ambulance, I reflected for a second on how different things would be if we were in the United States. We arrived at Mulago in record time, and I tried to get the intake doctors to understand what the problem was; however, they did not want to hear from the US doctor. I stared in frustration as they wasted valuable time. I wondered how long the patient would survive in respiratory distress with nothing being done. I called the patient's son and asked him to come to Mulago immediately. Miraculously, he had already been on his way. As I held the patient's hand, sure that he would die right then and there in a waiting area as nobody did anything, I saw the patient's son. I knew that he was a pharmacist, and I asked him to go to the pharmacy and buy furosemide and some syringes. In Uganda, one can buy any medication without a prescription. Luckily, the hospital pharmacy had the drug. We treated the patient, and in no time, his breathing had returned to normal.
I was jolted back to reality. He was dying, and I knew it. He had had many close calls. There was the time that he got the wrong blood during a blood transfusion. I informed the doctor on call as the blood was being administered that I thought the patient was getting a transfusion reaction because he had rigors. The physician on call suggested covering him in blankets, and I suggested stopping the infusion and administering steroids. The pack of blood showed that he was getting his blood type. The patient was typed and crossed again, and to our surprise, we got a different result. I went to the laboratory to perform a third, tie‐breaking cross match and was surprised to note that the reagents had passed their expiration date. However, I knew that these were small battles we were winning and that there was no winning the war.
I recognized that the challenges of practicing medicine in the developing world were many. I wondered how the patients of families with fewer resources survived. The answer was obvious: they didn't. I personally picked up blood when it was available from the blood bank and vividly remember walking from the blood bank at night to the private hospital with units of blood in each hand. Once we arrived at the hospital, I had to warm the blood to room temperature by holding it close to my own skin. Many tests that we perform routinely on a hospitalized patient in the United States are not available.
There was still the problem of breaking the news to the family. Despite everything that had been done and the many near misses that the patient had survived, he was still going to die. It turns out that the family was more intuitive than I thought. One day, the son came to me and asked how long his dad had. Not long, I said quietly. I thought about all that I could potentially do if I had the patient in the hospital at which I worked in the United States. Would it have made a difference? I do not know. It was impossible doctoring this patient, and I suspect doing it in a resource‐rich environment would not have made it any easier. You see this patient, perhaps the most important patient of my life, certainly a patient that I will never forget, was my father.
It had been 15 years since I had traveled to the United States for an education. I knew that my father was so incredibly proud of me. I think that he was the happiest I had ever seen him when he attended my graduation from medical school in Minnesota. I had been looking forward to this visit back home because it had been 3 years since I had last seen my family. I was somewhat concerned because my father had told me a week before I traveled that he was not feeling well. When I arrived, there seemed to be relief on my brother's face when he met me at the airport. We drove straight to the hospital, and along with the joy of seeing me, I could sense that my father was glad that I was home at this particular point in time. They had just received the diagnosis. He had leukemia, and they were glad that their doctor was home. They had particular faith in the daughter (sister) sent abroad for an education. Things would now be okay. Initially, I never got to choose the role of doctor that I played in the final chapter of my father's life. The decision was made for me out of my family's desperation to make sure that they had left no stone unturned to help my father, and I accepted it out of necessity. As my father became my father when I entered this world, I became his doctor when he was leaving it; there was never any question in my mind, as there never was in his. As it became clear that my father would not survive, I chose to continue the role of doctor. I have watched many patients die as a physician and have done my best to make sure that their passing is comfortable, peaceful, and dignified. The doctor could help this patient die, but the daughter could not watch her father go. When it was evident that he had only days to live and did not need this doctor or know his daughter, I flew back to the United States. Three days later my father died. I was not physically at his bedside, but my spirit was. I have no regrets. Although the head knows that he passed on, in my mind's eye, he is laughing and has a twinkle in his eye. I could not bear to see him without life. A piece of my heart is buried with him, and for this reason, I will never be out of Africa.
Acknowledgements
The author is indebted to J.B. Kisuule and seeks to honor his life of service. Thank you to Dr. Roy Ziegelstein for his help with this article.
I knew that he was going to die. I do not remember when it became evident to me, and I was not sure how to tell the family. I thought that I could arrange a family meeting and inform them of the sad reality in a calm, sympathetic manner. The patient had chronic lymphocytic leukemia, and his case was advanced. The only medication available to him was chlorambucil. As the days passed, I could not bring myself to call the family meeting because they had so much hope. Every day as we got results and I shared them, I would sandwich the bad news with some optimism to ease their pain. Well, his white blood cell count has come down, but his platelet count and red blood cell counts are very low, and this puts him in danger of bleeding. The medicine is bringing the white cell count down but has not yet brought the other cell counts up. What we can do is give him some blood. I tried not to allow despair to creep into my thoughts or my voice. I knew that the blood bank had no platelets or packed red blood cells. He was not eating or drinking, and we had placed a nasogastric tube through which his family fed him wheat or millet porridge (manufactured tube feeds are not widely available in Uganda). I tried not to think about the time that he had almost died a few weeks before.
I had been called to the bedside because the patient was in respiratory distress. The doctor on call was in his office when I arrived, and I wondered why he was not at the bedside. I took one look at the patient and had to step away for a moment to compose myself. I felt the tears threatening to come, but I had to stop them. This was not the time for emotions. I had to assess the patient and make some quick decisions. The doctor on call seemed to have given up. He was a young trainee in a system in which you treat when you can and, if the situation is hopeless, you move on to the next patient. There are no resources for perpetuating hope. This is so different from my practice in the United States, where if a patient wants everything done, we will do it. We are not taught when to give up hope, and futility does not figure into the allocation of resources. I looked at the patient struggling to breathe and felt that I had to do all that I could for him. I asked the doctor on call to place the patient on oxygen and hoped that the tanks were not empty. I was worried about a lot of things, such as pulmonary embolus, myocardial infarction, and pneumonia. Diagnosing any of these would not be easy (the hospital did not have a computed tomography scanner, and obtaining cardiac enzymes was not as simple as clicking a button on a computer). First things first: the chest X‐ray. I thanked God that we were in a private hospital, one of the best in the city of Kampala, so we were able to get a chest X‐ray right away. As we transported the patient (portable X‐rays are nonexistent), the resident told me that he had called the consultant (the equivalent of an attending physician in the United States), who happened to be out of town. The consultant instructed us to transfer the patient to Mulago Hospital (the largest tertiary center in Uganda with well over 1000 beds and some of the equipment that you might find in an American hospital). I wondered how an attending physician could be out of town and leave a resident in charge. The thought was disturbing, but I had no time to ponder it. I later learned that physicians are so poorly paid that many have their own private clinics. My patient got the X‐ray, and I reviewed it with the resident. Tuberculosis, he said. Tuberculosis was this resident's reality. Many patients who need chest X‐rays in Uganda have tuberculosis. As I reviewed the X‐ray, though, I was certain that this was congestive heart failure. However, in Uganda, congestive heart failure is rarely diagnosed in the hospital. Patients with an ejection fraction low enough to cause congestion generally die before they get to a hospital. I knew that some furosemide would work for this patient, but I could not get the resident to listen to me. He had orders from the consultant to transfer the patient immediately, and the ambulance was ready. I tried to convince the resident to administer furosemide before transferring the patient, but he feared administering a drug not approved by his superior. As the patient was loaded onto the ambulance, I reflected for a second on how different things would be if we were in the United States. We arrived at Mulago in record time, and I tried to get the intake doctors to understand what the problem was; however, they did not want to hear from the US doctor. I stared in frustration as they wasted valuable time. I wondered how long the patient would survive in respiratory distress with nothing being done. I called the patient's son and asked him to come to Mulago immediately. Miraculously, he had already been on his way. As I held the patient's hand, sure that he would die right then and there in a waiting area as nobody did anything, I saw the patient's son. I knew that he was a pharmacist, and I asked him to go to the pharmacy and buy furosemide and some syringes. In Uganda, one can buy any medication without a prescription. Luckily, the hospital pharmacy had the drug. We treated the patient, and in no time, his breathing had returned to normal.
I was jolted back to reality. He was dying, and I knew it. He had had many close calls. There was the time that he got the wrong blood during a blood transfusion. I informed the doctor on call as the blood was being administered that I thought the patient was getting a transfusion reaction because he had rigors. The physician on call suggested covering him in blankets, and I suggested stopping the infusion and administering steroids. The pack of blood showed that he was getting his blood type. The patient was typed and crossed again, and to our surprise, we got a different result. I went to the laboratory to perform a third, tie‐breaking cross match and was surprised to note that the reagents had passed their expiration date. However, I knew that these were small battles we were winning and that there was no winning the war.
I recognized that the challenges of practicing medicine in the developing world were many. I wondered how the patients of families with fewer resources survived. The answer was obvious: they didn't. I personally picked up blood when it was available from the blood bank and vividly remember walking from the blood bank at night to the private hospital with units of blood in each hand. Once we arrived at the hospital, I had to warm the blood to room temperature by holding it close to my own skin. Many tests that we perform routinely on a hospitalized patient in the United States are not available.
There was still the problem of breaking the news to the family. Despite everything that had been done and the many near misses that the patient had survived, he was still going to die. It turns out that the family was more intuitive than I thought. One day, the son came to me and asked how long his dad had. Not long, I said quietly. I thought about all that I could potentially do if I had the patient in the hospital at which I worked in the United States. Would it have made a difference? I do not know. It was impossible doctoring this patient, and I suspect doing it in a resource‐rich environment would not have made it any easier. You see this patient, perhaps the most important patient of my life, certainly a patient that I will never forget, was my father.
It had been 15 years since I had traveled to the United States for an education. I knew that my father was so incredibly proud of me. I think that he was the happiest I had ever seen him when he attended my graduation from medical school in Minnesota. I had been looking forward to this visit back home because it had been 3 years since I had last seen my family. I was somewhat concerned because my father had told me a week before I traveled that he was not feeling well. When I arrived, there seemed to be relief on my brother's face when he met me at the airport. We drove straight to the hospital, and along with the joy of seeing me, I could sense that my father was glad that I was home at this particular point in time. They had just received the diagnosis. He had leukemia, and they were glad that their doctor was home. They had particular faith in the daughter (sister) sent abroad for an education. Things would now be okay. Initially, I never got to choose the role of doctor that I played in the final chapter of my father's life. The decision was made for me out of my family's desperation to make sure that they had left no stone unturned to help my father, and I accepted it out of necessity. As my father became my father when I entered this world, I became his doctor when he was leaving it; there was never any question in my mind, as there never was in his. As it became clear that my father would not survive, I chose to continue the role of doctor. I have watched many patients die as a physician and have done my best to make sure that their passing is comfortable, peaceful, and dignified. The doctor could help this patient die, but the daughter could not watch her father go. When it was evident that he had only days to live and did not need this doctor or know his daughter, I flew back to the United States. Three days later my father died. I was not physically at his bedside, but my spirit was. I have no regrets. Although the head knows that he passed on, in my mind's eye, he is laughing and has a twinkle in his eye. I could not bear to see him without life. A piece of my heart is buried with him, and for this reason, I will never be out of Africa.
Acknowledgements
The author is indebted to J.B. Kisuule and seeks to honor his life of service. Thank you to Dr. Roy Ziegelstein for his help with this article.
Pediatric Hospitalists
There has been marked recent growth in the employment and utilization of both pediatric and adult hospitalists. Recent data demonstrate that approximately 25% of current pediatric hospitalist programs are less than 2 years old.1 Some have posited that this growth is due to increasing pressure from the public and payors to deliver cost‐effective and high‐quality care.2 However, little is known about the mechanisms by which those who deliver care in this framework are trained, nor the scope of clinical practice they provide.37 One study has shown that among those who direct pediatric hospitalist services there is a great degree of variability in the description of the roles, work patterns, and employment characteristics of hospitalists.1 That study provided only 1 perspective on the roles and career trajectories of those in the field. To better understand both the range and frequency of experiences, clinical and nonclinical roles, training, work expectations, and career plans, we conducted a national survey study of practicing pediatric hospitalists.
METHODS
Sample
We identified all 761 hospitals in the American Hospital Association (AHA)'s 2005 Annual Survey of Hospitals that reported to have both a hospitalist service (adult and/or pediatric) and pediatric beds. From these 761 hospitals, we selected a random sample of 213, stratified by:
Council of Teaching Hospital (COTH) designation
National Association of Children's Hospitals & Related Institutions (NACHRI) membership
Freestanding children's hospitals
Metropolitan Statistical Area (MSA) (urban versus rural location)
Hospital size (small: <250 total beds versus large: 250 total beds)
Some hospitals are included in more than 1 category. Thus, there is some overlap of hospitals in the analysis. Of these 213 hospitals, 97 were removed from the sample because they did not have at least 1 pediatric hospitalist. In a separate study, we surveyed hospitalist program directors at 112 of the remaining 116 hospitals from June through September 2006. These results have been published.1
Pediatric hospitalist program directors at these 112 participating hospitals were asked to provide the names of all practicing pediatric hospitalists in their respective programs. Ninety‐five of these program directors provided a list of hospitalists at their institutions, representing 85% of the hospitals in our previous study. A total of 530 practicing pediatric hospitalists were identified to us in this manner. Of these 530 hospitalists, 67% (N = 338) were from teaching hospitals, 71% (N = 374) were from children's hospitals, 43% (N = 230) were from freestanding children's hospitals, and 69% (N = 354) were from hospitals with 250 beds. These are not mutually exclusive categories.
Survey Instrument
We developed a structured questionnaire to be administered by mail. The survey contained 25 items and was designed to be completed in 10 minutes or less. The survey focused on exploring the characteristics of hospitalist clinical and nonclinical practice, service schedule, training, and career goals. The questionnaire was comprised of a mixture of fixed‐choice, Likert‐scale, and open‐ended questions.
Questionnaire Administration
In October 2006, the first mailing of questionnaires was sent via priority mail. The survey packet contained a personalized cover letter signed by the principal investigator (G.L.F.), the instrument, a business reply mail envelope, and a $5 bill as an incentive. Two additional mailings were sent to nonrespondents in November 2006 and January 2007.
Data Analysis
First, frequency distributions were calculated for all survey items. Next, comparisons were made between respondents indicating they held an academic appointment and those who did not. For the purposes of this analysis, academic pediatric hospitalists were defined as those respondents holding a full‐time or part‐time academic appointment. Nonacademic pediatric hospitalists were defined as respondents holding an adjunct or volunteer faculty position, or no academic appointment. Finally, chi‐square statistics were used to compare pediatric hospitalist responses by hospital demographics such as teaching status, children's hospital status, NACHRI freestanding hospital designation, and hospital bed size.
The study was approved by the University of Michigan Medical Institutional Review Board.
RESULTS
Response Rate
Of the initial 530 survey packets mailed, 18 were returned as undeliverable by the postal service and 431 physicians returned the survey. This yielded an overall response rate of 84%. Of the 431 respondents, 40 physicians were ineligible because they no longer provided inpatient care to children or did not consider themselves to be hospitalists. Thus, the final sample for analysis was 391.
Hospitalist Employment Characteristics
Demographics of Hospital Worksite
Of the 391 respondents, 61% (N = 237) were from teaching hospitals, 73% (N = 287) from children's hospitals, 47% (N = 182) from freestanding children's hospitals, and 66% (N = 258) from hospitals with more than 250 beds.
Physician Demographics
The mean age of respondents was 39 years and 59% were female. The majority were employed by a hospital or health system (56%), 20% were employed by a university, and 4% were employed by both. Eight percent reported employment by a general physician medical group, 7% were employed by a hospitalist‐only group, and 4% reported other sources of employment. Half of respondents (N = 196) reported holding a full‐time (40%) or part‐time (10%) academic appointment. Approximately half the respondents (N = 194) were considered nonacademic hospitalists.
More than half of respondents (54%; N = 211) had been practicing as hospitalists for at least 3 years. Reported time as a practicing hospitalist ranged from <1 year to 26 years, while the average length of time was 63 months (Table 1). These figures may be skewed because those hospitalists with higher turnover rates might have left their position during the period of time from when they were selected into the sample until the time of survey administration.
| Length of Time as Hospitalist | % (N) |
|---|---|
| |
| 12 months | 13 (51) |
| 13‐24 months | 18 (71) |
| 25‐36 months | 14 (56) |
| 37‐60 months | 17 (67) |
| >61 months | 37 (144) |
Clinical Practice
Most respondents reported that the pediatric inpatient unit (94%) and inpatient consultation service (51%) were a part of their regular clinical assignment (Table 2). A majority did not provide service in the normal newborn nursery (58%), subspecialty inpatient service (52%), pediatric intensive care unit (ICU) (70%), neonatal ICU (77%), transports (85%), outpatient clinics (66%), or as part of an emergency response team (53%).
| Part of Regular Clinical Assignment % (N) | Occasionally % (N) | Never % (N) | |
|---|---|---|---|
| |||
| Pediatric inpatient unit | 94 (368) | 3 (13) | 2 (9) |
| Inpatient consultation service | 51 (199) | 40 (155) | 9 (35) |
| Normal newborn nursery | 29 (110) | 13 (50) | 58 (223) |
| Emergency department | 25 (95) | 28 (108) | 47 (178) |
| Subspecialty inpatient service | 25 (92) | 23 (86) | 52 (196) |
| Emergency response team | 23 (87) | 24 (91) | 53 (201) |
| Outpatient/outreach clinics | 18 (68) | 16 (61) | 66 (253) |
| Pediatric ICU | 14 (54) | 16 (59) | 70 (268) |
| Neonatal ICU | 12 (44) | 11 (42) | 77 (294) |
| Transports | 9 (33) | 6 (23) | 85 (319) |
With regard to procedures, many (53%) respondents reported that they routinely perform or supervise lumbar punctures. Several services are never performed or never supervised by the majority of pediatric hospitalists, including infusion services (57%), peripherally inserted central catheter (PICC) placement (76%), central line placement (67%), and circumcision (85%).
Professional Roles and Parameters
Respondents reported that they participate in a variety of nonclinical activities. Ninety‐four percent of hospitalists were involved in education, and 45% reported having a leadership role in that area. The majority of respondents participated in quality improvement (QI) initiatives (84%) and practice guideline development (81%), with one‐quarter of hospitalists reporting a leadership role in each of these activities. Slightly more than half of respondents reported involvement in hospital administration (52%) and utilization review (55%) (Table 3).
| Participation | No Involvement % (N) | ||
|---|---|---|---|
| Participation of Any Type % (N) | Leadership Role % (N) | ||
| |||
| Education (students, house staff) | 94 (368) | 45 (177) | 6 (22) |
| Quality improvement initiatives | 84 (330) | 25 (99) | 16 (61) |
| Practice guideline development | 81 (313) | 26 (101) | 19 (74) |
| Utilization review | 55 (213) | 11 (41) | 45 (172) |
| Hospital administration | 52 (202) | 16 (60) | 48 (184) |
On average, hospitalists reported spending 61% of their time providing inpatient care (excluding clinical teaching) and 16% of their time providing clinical teaching or supervising residents. More than one‐third of respondents (38%) spent more than 75% of their time providing direct inpatient care. Research (3%), administrative duties (8%), and nonclinical teaching (3%) were reported to be a small part of hospitalist professional time.
Pediatric Hospitalist Service Schedule
The majority of respondents reported that their assigned clinical schedule was a combination of shift and call (61%).
When on service, over half of responding pediatric hospitalists (58%) reported that they spend 40 to 60 hours onsite per week. Less than one‐fifth of respondents (19%) reported that they provide <40 hours of onsite coverage when on service. Most (97%) provide some type of night coverage, including taking calls from home or providing onsite coverage.
Hospitalist Training and Continuing Education
Only 51 of the 391 respondents (13%) had received some type of fellowship training, mostly in general pediatrics or the pediatric subspecialties. Only 5 respondents had received fellowship training in hospital medicine.
Fifty‐eight percent of respondents reported that they had received no hospitalist‐specific training. One‐fifth reported that they received training through a workshop at a professional meeting, while fewer respondents had received hospitalist training though a continuing medical education (CME) course (16%) or a mentoring program (17%).
Respondents were asked to rate the adequacy of their respective training in preparing them for their work as hospitalists. The vast majority rated their training in general clinical skills (94%) and communication (85%) as fully adequate. However, respondents found their training for some of the nonclinical aspects of their positions to be deficient. Many respondents rated training for QI projects (38%) and hospital administrative duties (46%) as inadequate (Table 4).
| Fully Adequate % (N) | Somewhat Adequate % (N) | Not Adequate % (N) | NA % (N) | |
|---|---|---|---|---|
| ||||
| General clinical skills | 94 (367) | 5 (21) | 0 (0) | 0 (1) |
| Communication skills | 85 (330) | 14 (53) | 1 (5) | 0 (1) |
| Coordination of care | 73 (284) | 23 (89) | 4 (15) | 0 (1) |
| Clinical procedure experience | 67 (258) | 32 (123) | 1 (5) | 1 (2) |
| Teaching skills (resident and medical student teaching) | 64 (248) | 31 (120) | 3 (13) | 2 (8) |
| Attending newborn deliveries | 60 (233) | 18 (70) | 4 (14) | 19 (72) |
| Running resuscitation (codes) | 45 (173) | 46 (177) | 5 (21) | 5 (18) |
| Quality improvement projects | 14 (55) | 42 (162) | 38 (148) | 6 (22) |
| Hospital administrative duties | 10 (37) | 37 (144) | 46 (177) | 8 (31) |
Survey respondents were asked to indicate the extent to which they agreed or disagreed with 3 statements regarding hospitalist training. The majority of respondents believed that hospitalists need training in QI methods (70%). However, most pediatric hospitalists (73%) did not believe that additional training beyond residency should be required. Only one‐third (36%) of respondents agreed that current CME offerings are adequate for their needs as a pediatric hospitalist.
Career Goals and Expectations
Respondents were asked to select 1 or more reasons why they became pediatric hospitalists. The top factors influencing respondents' decision to become a hospitalist were reported to be a preference for the inpatient setting (73%), clinical variety (72%), enjoyment of teaching in the inpatient setting (58%), and a flexible schedule (52%) (Table 5).
| Factor | % (N) |
|---|---|
| |
| Prefer inpatient setting | 73 (284) |
| Clinical variety | 72 (281) |
| Enjoy teaching in inpatient setting | 58 (225) |
| Flexible schedule | 52 (202) |
| Defined hours | 41 (161) |
| Attractive career opportunities | 21 (80) |
| Salary | 18 (70) |
| Unsure of long‐term career direction | 13 (51) |
| Other | 7 (28) |
| Needed short‐term employment | 4 (15) |
| Only position available | 3 (10) |
The majority (85%) were satisfied with their position as a pediatric hospitalist, with 37% reporting that they were extremely satisfied. Over one‐half (61%) expected to remain a hospitalist for the duration of their career.
RESULTS BY ACADEMIC STATUS
Only significant differences between academic and nonacademic hospitalists are presented.
Clinical Practice by Academic Status
Nonacademic respondents were more likely than academic respondents to report regular service in the normal newborn nursery, pediatric ICU, neonatal ICU, transports, emergency department, and as part of an emergency response team. Academic respondents were more likely to report regular service in outpatient clinics. Nonacademic respondents were more likely than academic respondents to perform or supervise lumbar punctures, sedation services, PICC or central line insertions, and circumcisions (Table 6).
| Academic* (N = 196) | Nonacademic (N = 194) | P Value | |
|---|---|---|---|
| |||
| Regularly provides service | |||
| Normal newborn nursery | 16% | 42% | <0.0001 |
| Pediatric ICU | 9% | 20% | 0.0065 |
| Neonatal ICU | 4% | 20% | <0.0001 |
| Transports | 3% | 15% | <0.0001 |
| Emergency department | 16% | 34% | <0.0001 |
| Emergency response team | 17% | 29% | <0.0001 |
| Outpatient clinic | 23% | 13% | 0.0168 |
| Performs or supervises procedures | |||
| Lumbar puncture | 84% | 92% | 0.0152 |
| Sedation services | 50% | 64% | 0.0055 |
| PICC insertion | 8% | 18% | 0.0031 |
| Central line insertion | 11% | 23% | 0.0018 |
| Circumcision | 5% | 16% | 0.0002 |
| Holds leadership roles | |||
| Education (student or house staff) | 63% | 27% | <0.0001 |
| Hospital administration | 21% | 10% | <0.0001 |
| Quality improvement initiatives | 33% | 18% | 0.0005 |
Professional Roles and Parameters by Academic Status
Responding academic pediatric hospitalists were twice as likely as nonacademic respondents to have a leadership role in the education of students and house staff and to hold a leadership position in hospital administration. The academic respondents were also more likely to report a leadership role in QI initiatives (Table 6).
Clinical and Educational Activities by Academic Status
Academic pediatric hospitalist respondents reported spending on average 52% of their time providing inpatient care (excluding teaching), in contrast to the nonacademic hospitalist respondents who reported 71% of their time was spent providing inpatient care (P < 0.0001). Academic respondents also reported that 19% of their time was spent providing inpatient teaching or supervising residents, compared to 12% of nonacademic respondents (P < 0.0001). Responding academic pediatric hospitalists reported spending a greater proportion of time participating in nonclinical teaching activities (5% versus 2%; P < 0.0001), administrative duties (11% versus 5%; P < 0.0001), and research (4% versus 1%; P < 0.0001) compared to the nonacademic respondents.
Nonacademic respondents were more likely than academic respondents to report no hospitalist‐specific training (64% versus 54%; P = 0.0324).
RESULTS BY HOSPITAL CHARACTERISTICS
For each hospital characteristic, only significant differences between dichotomized groups are presented.
Children's Hospitals versus Other Hospitals
Clinical Practice
Pediatric hospitalist respondents practicing in NACHRI hospitals were more likely to report that they provide regular service for general pediatric inpatients (98% versus 86%; P < 0.0001) as well as subspecialty inpatients (27% versus 17%; P = 0.044). Non‐NACHRI pediatric hospitalist respondents were twice as likely to report the provision of regular service in the normal newborn nursery (49% versus 22%; P < 0.0001), the neonatal ICU (21% versus 8%, P = 0.002), and the emergency department (38% versus 20%; P < 0.0001).
Among respondents, pediatric hospitalists who were not working at a children's hospital were more likely to report that they sometimes or routinely performed lumbar punctures (93% versus 85%; P = 0.037), infusion services (36% versus 21%; P = 0.003), and were twice as likely to perform circumcision (16% versus 8%; P = 0.041) compared to those working at children's hospitals.
Professional Roles and Parameters
Respondents working in children's hospitals were twice as likely to hold a leadership position in utilization review (12% versus 6%; P = 0.012), though respondents from non‐NACHRI hospitals were more likely to at least participate in utilization review (58% versus 40%; P = 0.004).
Hospitalist Training
Respondents from non‐NACHRI hospitals were more likely to report that they had received no hospitalist‐specific training (68% versus 56%; P = 0.029). Those at NACHRI hospitals were twice as likely to have received hospitalist training through a mentoring program (20% versus 9%; P = 0.009).
Freestanding versus Nonfreestanding Children's Hospitals
Clinical Practice
Pediatric hospitalist respondents employed at institutions that are not freestanding children's hospitals were more likely to report that they provided regular service in the normal newborn nursery (42% versus 14%; P < 0.0001), pediatric ICU (22% versus 5%), emergency department (32% versus 17%; P < 0.0001), and outpatient clinics (23% versus 12%; P = 0.0068). They were also more likely to perform or supervise sedation services (63% versus 50%; P = 0.0116), infusion services (32% versus 17%; P = 0.0006), PICC insertions (19% versus 6%; P = 0.0002), central line insertions (23% versus 11%; P = 0.0024), and circumcisions (16% versus 3%; P < 0.0001).
Professional Roles and Parameters
Among respondents, pediatric hospitalists employed by nonfreestanding children's hospitals were more likely to report participation in utilization review (51% versus 38%; P = 0.02).
Hospital Size
Clinical Practice
Pediatric hospitalist respondents working at large hospitals were twice as likely to report that they regularly provided service in the pediatric ICU (18% versus 7%; P = 0.0072) and were more likely to regularly perform circumcisions (13% versus 5%; P = 0.0069). Respondents from small hospitals were more likely to provide regular service in the neonatal ICU (20% versus 7%; P = 0.0013).
COTH Status: Teaching versus Nonteaching Hospitals
Clinical Practice
Among survey respondents, pediatric hospitalists employed by COTH hospitals were more likely to provide regular service in the neonatal ICU, compared to their peers in nonteaching hospitals (15% versus 6%; P = 0.0109). Those employed by non‐COTH hospitals were more likely to provide service in subspecialty inpatient service (38% versus 16%; P < 0.0001), transports (14% versus 6%; P = 0.0227), inpatient consultation (61% versus 45%; P = 0.0086), and the emergency response team (29% versus 19%; P = 0.0021).
Professional Roles and Parameters
Respondents from COTH hospitals were more likely to have no involvement in utilization review, compared to their peers at non‐COTH hospitals (49% versus 37%; P = 0.0220).
DISCUSSION
This study provides the most comprehensive information available regarding the clinical and nonclinical roles, training, work expectations, and career plans of pediatric hospitalists. Among the most important of our findings is the distribution of the length of time that pediatric hospitalists had served in their roles. While over one‐third (37%) reported having been practicing as hospitalists for over 5 years, 45% of our respondents had been in practice for fewer than 3 years. This is consistent with both the perceptions of rapid growth of the field and with significant turnover of hospitalists.1, 8 It is important to note that our findings may actually overestimate the proportion of hospitalists with longer durations of employment as our sampling strategy would have been less likely to include those who left the field within the first 12 to 18 months of practice. Nevertheless, over half (61%) of our respondents expected to remain a hospitalist for the duration of their career and few reported choosing to become a hospitalist as a short‐term employment option. This finding has important implications for the future stability of the hospitalist workforce and the potential development of specific expertise among this cadre of clinicians.6
The demographic profile of pediatric hospitalists was also consistent with these findings. The mean age of 39 years for our respondents is indicative of a significant proportion of this group of physicians recently having completed their residency training. Further, the gender distribution approximates that of current pediatric residency graduates, thus indicating that that this is not a clinical choice for which there would be a skewed distribution as is the case in some pediatric subspecialties.9
Our findings were similar to the 2004 Ottolini et al.10 findings on the roles of pediatric hospitalists. Respondents in our study reported spending less time providing inpatient care (61% versus 75%), providing clinical teaching or supervising residents (16% versus 26%), performing administrative duties (8% versus 19%), and conducting research (3% versus 9%) compared with the respondents in the Ottolini et al.10 survey.
At this point in time, fewer than half of our respondents reported any hospitalist‐specific training, including workshops at professional meetings or CME coursework. As there are a paucity of fellowships offering postresidency training in pediatric hospital medicine, and most of the existing programs are newly established, few in practice have completed such programs.11 In addition, most respondents reported that current CME offerings do not meet their needs, and that they could have used additional QI training to prepare them for their role as pediatric hospitalists. However, almost three‐quarters of respondents (73%) do not believe any additional training beyond residency should be required. As such, it is unclear if a defined, unique body of knowledge specific to hospitalists is either needed or desired by those currently in the field.
Although there are a broad range of potential clinical roles within hospital medicine, and this clinical variety influenced most respondents' decisions to become hospitalists, the current scope of an individual hospitalist tends to become somewhat focused.12, 13 While we found almost all provided service on the pediatric inpatient unit, many fewer provided inpatient consultation and normal newborn care, or were involved in interhospital transport or as part of an emergency response team. There is also wide variation in the types of procedures performed or supervised by hospitalists at different institutions. More than half never perform or supervise infusion services, PICC or central line placement, or circumcision. The variation seen among hospitalists practicing in different hospital settings likely is a result, at least in part, of different needs in teaching hospitals for both service and for clinical experience of trainees. For example, our results demonstrate that pediatric hospitalists in nonteaching and non‐children's hospitals are more likely to have a broader scope of clinical care provision. Another potential issue is that some hospitalists may be employed by institutions which have no pediatric ICU, neonatal ICU, or other specialty unit. As such, these hospitalists would not have the opportunity to work in such settings.
Further, those without academic appointments are also more likely to have expanded clinical roles compared with their academic counterparts. This may be due to the fact that there is likely a greater number of subspecialty‐trained pediatric providers in academic centers and thus the need for hospitalists to cover specific services or perform specific procedures is lessened. There may also be a desire to prevent competition among care providers within the same institution. In contrast, hospitalists with academic appointments are more likely (though still uncommonly) to have taken leadership roles in hospital administration and QI initiatives. Thus, the nature of their efforts appears to expand into nonclinical delivery areas.
Clearly, hospitalists report they have assumed a significant role in the clinical teaching of trainees at all levels, with 94% of our respondents maintaining at least some involvement in education. On average, they spend 16% of their time in educational efforts. However, there are few data on the impact of their work in this area.5, 13 Studies in pediatrics to date have been limited to a few institutions,3, 5 and have not addressed the issue from the perspective of residency program directors or those who are in charge of inpatient curricula.
This study, like the majority of studies related to pediatric hospitalists, is hampered by the difficulty of identifying pediatric hospitalists. Rather than utilizing a hospital medicine membership list, which would be potentially biased by self‐selection, we attempted to obtain a more representative sample through utilization of the AHA database.
CONCLUSIONS
Findings from this study provide an additional perspective regarding pediatric hospitalists to add to our previous study of hospitalist program directors.1 However, the field is currently a moving target. Our data demonstrate that there is significant flux in the hospitalist workforce, uncertainty regarding turnover, and variation in the roles of these professionals in their clinical and nonclinical work environment. Moreover, additional studies of the educational impact of hospitalists on residency and medical student education are needed. Questions regarding the nature and degree of resident autonomy and experience conducting procedures in the hospitalist environment have been raised. These must be assessed through studies of residency program directors, their expectations of residents, and the curricula they have developed.
As with any new phenomenon, it will take time to understand the impact of hospitalists in a variety of domains. Additional research will be helpful in following the development of this field and the manner in which it will interface with existing medical practice and educational programs.
- ,,,; The Research Advisory Committee of the American Board of Pediatrics.Characteristics of the pediatric hospitalist workforce: its roles and work environment.Pediatrics.2007;120:33–39.
- .The evolution of the hospitalist model in the United States.Med Clin North Am.2002;86:687–706.
- ,.Hospitalists in children's hospitals: what we know now and what we need to know.J Pediatr.2006;148:296–299.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,,.Effect of a pediatric hospitalist system on housestaff education and experience.Arch Pediatr Adolesc Med.2002;156:877–883.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111:247–254.
- ,,,,,.Pediatric hospitalists in Canada and the United States: a survey of pediatric academic department chairs.Ambul Pediatr.2001;1:338–339.
- .Hospitalists in the United States: mission accomplished or work in progress?N Engl J Med.2004;350:1935–1936.
- ,.Pediatric workforce: a look at general pediatrics data from the American Board of Pediatrics.J Pediatr.2006;148:166–169.
- ,,,,PRIS survey: pediatric hospitalist roles and training needs [Abstr].Pediatr Res.2004;55:360A.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:1.e1–1.e7.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- ,,.Pediatric hospitalists fill varied roles in the care of newborns.Pediatr Ann.2003;32:802–810.
There has been marked recent growth in the employment and utilization of both pediatric and adult hospitalists. Recent data demonstrate that approximately 25% of current pediatric hospitalist programs are less than 2 years old.1 Some have posited that this growth is due to increasing pressure from the public and payors to deliver cost‐effective and high‐quality care.2 However, little is known about the mechanisms by which those who deliver care in this framework are trained, nor the scope of clinical practice they provide.37 One study has shown that among those who direct pediatric hospitalist services there is a great degree of variability in the description of the roles, work patterns, and employment characteristics of hospitalists.1 That study provided only 1 perspective on the roles and career trajectories of those in the field. To better understand both the range and frequency of experiences, clinical and nonclinical roles, training, work expectations, and career plans, we conducted a national survey study of practicing pediatric hospitalists.
METHODS
Sample
We identified all 761 hospitals in the American Hospital Association (AHA)'s 2005 Annual Survey of Hospitals that reported to have both a hospitalist service (adult and/or pediatric) and pediatric beds. From these 761 hospitals, we selected a random sample of 213, stratified by:
Council of Teaching Hospital (COTH) designation
National Association of Children's Hospitals & Related Institutions (NACHRI) membership
Freestanding children's hospitals
Metropolitan Statistical Area (MSA) (urban versus rural location)
Hospital size (small: <250 total beds versus large: 250 total beds)
Some hospitals are included in more than 1 category. Thus, there is some overlap of hospitals in the analysis. Of these 213 hospitals, 97 were removed from the sample because they did not have at least 1 pediatric hospitalist. In a separate study, we surveyed hospitalist program directors at 112 of the remaining 116 hospitals from June through September 2006. These results have been published.1
Pediatric hospitalist program directors at these 112 participating hospitals were asked to provide the names of all practicing pediatric hospitalists in their respective programs. Ninety‐five of these program directors provided a list of hospitalists at their institutions, representing 85% of the hospitals in our previous study. A total of 530 practicing pediatric hospitalists were identified to us in this manner. Of these 530 hospitalists, 67% (N = 338) were from teaching hospitals, 71% (N = 374) were from children's hospitals, 43% (N = 230) were from freestanding children's hospitals, and 69% (N = 354) were from hospitals with 250 beds. These are not mutually exclusive categories.
Survey Instrument
We developed a structured questionnaire to be administered by mail. The survey contained 25 items and was designed to be completed in 10 minutes or less. The survey focused on exploring the characteristics of hospitalist clinical and nonclinical practice, service schedule, training, and career goals. The questionnaire was comprised of a mixture of fixed‐choice, Likert‐scale, and open‐ended questions.
Questionnaire Administration
In October 2006, the first mailing of questionnaires was sent via priority mail. The survey packet contained a personalized cover letter signed by the principal investigator (G.L.F.), the instrument, a business reply mail envelope, and a $5 bill as an incentive. Two additional mailings were sent to nonrespondents in November 2006 and January 2007.
Data Analysis
First, frequency distributions were calculated for all survey items. Next, comparisons were made between respondents indicating they held an academic appointment and those who did not. For the purposes of this analysis, academic pediatric hospitalists were defined as those respondents holding a full‐time or part‐time academic appointment. Nonacademic pediatric hospitalists were defined as respondents holding an adjunct or volunteer faculty position, or no academic appointment. Finally, chi‐square statistics were used to compare pediatric hospitalist responses by hospital demographics such as teaching status, children's hospital status, NACHRI freestanding hospital designation, and hospital bed size.
The study was approved by the University of Michigan Medical Institutional Review Board.
RESULTS
Response Rate
Of the initial 530 survey packets mailed, 18 were returned as undeliverable by the postal service and 431 physicians returned the survey. This yielded an overall response rate of 84%. Of the 431 respondents, 40 physicians were ineligible because they no longer provided inpatient care to children or did not consider themselves to be hospitalists. Thus, the final sample for analysis was 391.
Hospitalist Employment Characteristics
Demographics of Hospital Worksite
Of the 391 respondents, 61% (N = 237) were from teaching hospitals, 73% (N = 287) from children's hospitals, 47% (N = 182) from freestanding children's hospitals, and 66% (N = 258) from hospitals with more than 250 beds.
Physician Demographics
The mean age of respondents was 39 years and 59% were female. The majority were employed by a hospital or health system (56%), 20% were employed by a university, and 4% were employed by both. Eight percent reported employment by a general physician medical group, 7% were employed by a hospitalist‐only group, and 4% reported other sources of employment. Half of respondents (N = 196) reported holding a full‐time (40%) or part‐time (10%) academic appointment. Approximately half the respondents (N = 194) were considered nonacademic hospitalists.
More than half of respondents (54%; N = 211) had been practicing as hospitalists for at least 3 years. Reported time as a practicing hospitalist ranged from <1 year to 26 years, while the average length of time was 63 months (Table 1). These figures may be skewed because those hospitalists with higher turnover rates might have left their position during the period of time from when they were selected into the sample until the time of survey administration.
| Length of Time as Hospitalist | % (N) |
|---|---|
| |
| 12 months | 13 (51) |
| 13‐24 months | 18 (71) |
| 25‐36 months | 14 (56) |
| 37‐60 months | 17 (67) |
| >61 months | 37 (144) |
Clinical Practice
Most respondents reported that the pediatric inpatient unit (94%) and inpatient consultation service (51%) were a part of their regular clinical assignment (Table 2). A majority did not provide service in the normal newborn nursery (58%), subspecialty inpatient service (52%), pediatric intensive care unit (ICU) (70%), neonatal ICU (77%), transports (85%), outpatient clinics (66%), or as part of an emergency response team (53%).
| Part of Regular Clinical Assignment % (N) | Occasionally % (N) | Never % (N) | |
|---|---|---|---|
| |||
| Pediatric inpatient unit | 94 (368) | 3 (13) | 2 (9) |
| Inpatient consultation service | 51 (199) | 40 (155) | 9 (35) |
| Normal newborn nursery | 29 (110) | 13 (50) | 58 (223) |
| Emergency department | 25 (95) | 28 (108) | 47 (178) |
| Subspecialty inpatient service | 25 (92) | 23 (86) | 52 (196) |
| Emergency response team | 23 (87) | 24 (91) | 53 (201) |
| Outpatient/outreach clinics | 18 (68) | 16 (61) | 66 (253) |
| Pediatric ICU | 14 (54) | 16 (59) | 70 (268) |
| Neonatal ICU | 12 (44) | 11 (42) | 77 (294) |
| Transports | 9 (33) | 6 (23) | 85 (319) |
With regard to procedures, many (53%) respondents reported that they routinely perform or supervise lumbar punctures. Several services are never performed or never supervised by the majority of pediatric hospitalists, including infusion services (57%), peripherally inserted central catheter (PICC) placement (76%), central line placement (67%), and circumcision (85%).
Professional Roles and Parameters
Respondents reported that they participate in a variety of nonclinical activities. Ninety‐four percent of hospitalists were involved in education, and 45% reported having a leadership role in that area. The majority of respondents participated in quality improvement (QI) initiatives (84%) and practice guideline development (81%), with one‐quarter of hospitalists reporting a leadership role in each of these activities. Slightly more than half of respondents reported involvement in hospital administration (52%) and utilization review (55%) (Table 3).
| Participation | No Involvement % (N) | ||
|---|---|---|---|
| Participation of Any Type % (N) | Leadership Role % (N) | ||
| |||
| Education (students, house staff) | 94 (368) | 45 (177) | 6 (22) |
| Quality improvement initiatives | 84 (330) | 25 (99) | 16 (61) |
| Practice guideline development | 81 (313) | 26 (101) | 19 (74) |
| Utilization review | 55 (213) | 11 (41) | 45 (172) |
| Hospital administration | 52 (202) | 16 (60) | 48 (184) |
On average, hospitalists reported spending 61% of their time providing inpatient care (excluding clinical teaching) and 16% of their time providing clinical teaching or supervising residents. More than one‐third of respondents (38%) spent more than 75% of their time providing direct inpatient care. Research (3%), administrative duties (8%), and nonclinical teaching (3%) were reported to be a small part of hospitalist professional time.
Pediatric Hospitalist Service Schedule
The majority of respondents reported that their assigned clinical schedule was a combination of shift and call (61%).
When on service, over half of responding pediatric hospitalists (58%) reported that they spend 40 to 60 hours onsite per week. Less than one‐fifth of respondents (19%) reported that they provide <40 hours of onsite coverage when on service. Most (97%) provide some type of night coverage, including taking calls from home or providing onsite coverage.
Hospitalist Training and Continuing Education
Only 51 of the 391 respondents (13%) had received some type of fellowship training, mostly in general pediatrics or the pediatric subspecialties. Only 5 respondents had received fellowship training in hospital medicine.
Fifty‐eight percent of respondents reported that they had received no hospitalist‐specific training. One‐fifth reported that they received training through a workshop at a professional meeting, while fewer respondents had received hospitalist training though a continuing medical education (CME) course (16%) or a mentoring program (17%).
Respondents were asked to rate the adequacy of their respective training in preparing them for their work as hospitalists. The vast majority rated their training in general clinical skills (94%) and communication (85%) as fully adequate. However, respondents found their training for some of the nonclinical aspects of their positions to be deficient. Many respondents rated training for QI projects (38%) and hospital administrative duties (46%) as inadequate (Table 4).
| Fully Adequate % (N) | Somewhat Adequate % (N) | Not Adequate % (N) | NA % (N) | |
|---|---|---|---|---|
| ||||
| General clinical skills | 94 (367) | 5 (21) | 0 (0) | 0 (1) |
| Communication skills | 85 (330) | 14 (53) | 1 (5) | 0 (1) |
| Coordination of care | 73 (284) | 23 (89) | 4 (15) | 0 (1) |
| Clinical procedure experience | 67 (258) | 32 (123) | 1 (5) | 1 (2) |
| Teaching skills (resident and medical student teaching) | 64 (248) | 31 (120) | 3 (13) | 2 (8) |
| Attending newborn deliveries | 60 (233) | 18 (70) | 4 (14) | 19 (72) |
| Running resuscitation (codes) | 45 (173) | 46 (177) | 5 (21) | 5 (18) |
| Quality improvement projects | 14 (55) | 42 (162) | 38 (148) | 6 (22) |
| Hospital administrative duties | 10 (37) | 37 (144) | 46 (177) | 8 (31) |
Survey respondents were asked to indicate the extent to which they agreed or disagreed with 3 statements regarding hospitalist training. The majority of respondents believed that hospitalists need training in QI methods (70%). However, most pediatric hospitalists (73%) did not believe that additional training beyond residency should be required. Only one‐third (36%) of respondents agreed that current CME offerings are adequate for their needs as a pediatric hospitalist.
Career Goals and Expectations
Respondents were asked to select 1 or more reasons why they became pediatric hospitalists. The top factors influencing respondents' decision to become a hospitalist were reported to be a preference for the inpatient setting (73%), clinical variety (72%), enjoyment of teaching in the inpatient setting (58%), and a flexible schedule (52%) (Table 5).
| Factor | % (N) |
|---|---|
| |
| Prefer inpatient setting | 73 (284) |
| Clinical variety | 72 (281) |
| Enjoy teaching in inpatient setting | 58 (225) |
| Flexible schedule | 52 (202) |
| Defined hours | 41 (161) |
| Attractive career opportunities | 21 (80) |
| Salary | 18 (70) |
| Unsure of long‐term career direction | 13 (51) |
| Other | 7 (28) |
| Needed short‐term employment | 4 (15) |
| Only position available | 3 (10) |
The majority (85%) were satisfied with their position as a pediatric hospitalist, with 37% reporting that they were extremely satisfied. Over one‐half (61%) expected to remain a hospitalist for the duration of their career.
RESULTS BY ACADEMIC STATUS
Only significant differences between academic and nonacademic hospitalists are presented.
Clinical Practice by Academic Status
Nonacademic respondents were more likely than academic respondents to report regular service in the normal newborn nursery, pediatric ICU, neonatal ICU, transports, emergency department, and as part of an emergency response team. Academic respondents were more likely to report regular service in outpatient clinics. Nonacademic respondents were more likely than academic respondents to perform or supervise lumbar punctures, sedation services, PICC or central line insertions, and circumcisions (Table 6).
| Academic* (N = 196) | Nonacademic (N = 194) | P Value | |
|---|---|---|---|
| |||
| Regularly provides service | |||
| Normal newborn nursery | 16% | 42% | <0.0001 |
| Pediatric ICU | 9% | 20% | 0.0065 |
| Neonatal ICU | 4% | 20% | <0.0001 |
| Transports | 3% | 15% | <0.0001 |
| Emergency department | 16% | 34% | <0.0001 |
| Emergency response team | 17% | 29% | <0.0001 |
| Outpatient clinic | 23% | 13% | 0.0168 |
| Performs or supervises procedures | |||
| Lumbar puncture | 84% | 92% | 0.0152 |
| Sedation services | 50% | 64% | 0.0055 |
| PICC insertion | 8% | 18% | 0.0031 |
| Central line insertion | 11% | 23% | 0.0018 |
| Circumcision | 5% | 16% | 0.0002 |
| Holds leadership roles | |||
| Education (student or house staff) | 63% | 27% | <0.0001 |
| Hospital administration | 21% | 10% | <0.0001 |
| Quality improvement initiatives | 33% | 18% | 0.0005 |
Professional Roles and Parameters by Academic Status
Responding academic pediatric hospitalists were twice as likely as nonacademic respondents to have a leadership role in the education of students and house staff and to hold a leadership position in hospital administration. The academic respondents were also more likely to report a leadership role in QI initiatives (Table 6).
Clinical and Educational Activities by Academic Status
Academic pediatric hospitalist respondents reported spending on average 52% of their time providing inpatient care (excluding teaching), in contrast to the nonacademic hospitalist respondents who reported 71% of their time was spent providing inpatient care (P < 0.0001). Academic respondents also reported that 19% of their time was spent providing inpatient teaching or supervising residents, compared to 12% of nonacademic respondents (P < 0.0001). Responding academic pediatric hospitalists reported spending a greater proportion of time participating in nonclinical teaching activities (5% versus 2%; P < 0.0001), administrative duties (11% versus 5%; P < 0.0001), and research (4% versus 1%; P < 0.0001) compared to the nonacademic respondents.
Nonacademic respondents were more likely than academic respondents to report no hospitalist‐specific training (64% versus 54%; P = 0.0324).
RESULTS BY HOSPITAL CHARACTERISTICS
For each hospital characteristic, only significant differences between dichotomized groups are presented.
Children's Hospitals versus Other Hospitals
Clinical Practice
Pediatric hospitalist respondents practicing in NACHRI hospitals were more likely to report that they provide regular service for general pediatric inpatients (98% versus 86%; P < 0.0001) as well as subspecialty inpatients (27% versus 17%; P = 0.044). Non‐NACHRI pediatric hospitalist respondents were twice as likely to report the provision of regular service in the normal newborn nursery (49% versus 22%; P < 0.0001), the neonatal ICU (21% versus 8%, P = 0.002), and the emergency department (38% versus 20%; P < 0.0001).
Among respondents, pediatric hospitalists who were not working at a children's hospital were more likely to report that they sometimes or routinely performed lumbar punctures (93% versus 85%; P = 0.037), infusion services (36% versus 21%; P = 0.003), and were twice as likely to perform circumcision (16% versus 8%; P = 0.041) compared to those working at children's hospitals.
Professional Roles and Parameters
Respondents working in children's hospitals were twice as likely to hold a leadership position in utilization review (12% versus 6%; P = 0.012), though respondents from non‐NACHRI hospitals were more likely to at least participate in utilization review (58% versus 40%; P = 0.004).
Hospitalist Training
Respondents from non‐NACHRI hospitals were more likely to report that they had received no hospitalist‐specific training (68% versus 56%; P = 0.029). Those at NACHRI hospitals were twice as likely to have received hospitalist training through a mentoring program (20% versus 9%; P = 0.009).
Freestanding versus Nonfreestanding Children's Hospitals
Clinical Practice
Pediatric hospitalist respondents employed at institutions that are not freestanding children's hospitals were more likely to report that they provided regular service in the normal newborn nursery (42% versus 14%; P < 0.0001), pediatric ICU (22% versus 5%), emergency department (32% versus 17%; P < 0.0001), and outpatient clinics (23% versus 12%; P = 0.0068). They were also more likely to perform or supervise sedation services (63% versus 50%; P = 0.0116), infusion services (32% versus 17%; P = 0.0006), PICC insertions (19% versus 6%; P = 0.0002), central line insertions (23% versus 11%; P = 0.0024), and circumcisions (16% versus 3%; P < 0.0001).
Professional Roles and Parameters
Among respondents, pediatric hospitalists employed by nonfreestanding children's hospitals were more likely to report participation in utilization review (51% versus 38%; P = 0.02).
Hospital Size
Clinical Practice
Pediatric hospitalist respondents working at large hospitals were twice as likely to report that they regularly provided service in the pediatric ICU (18% versus 7%; P = 0.0072) and were more likely to regularly perform circumcisions (13% versus 5%; P = 0.0069). Respondents from small hospitals were more likely to provide regular service in the neonatal ICU (20% versus 7%; P = 0.0013).
COTH Status: Teaching versus Nonteaching Hospitals
Clinical Practice
Among survey respondents, pediatric hospitalists employed by COTH hospitals were more likely to provide regular service in the neonatal ICU, compared to their peers in nonteaching hospitals (15% versus 6%; P = 0.0109). Those employed by non‐COTH hospitals were more likely to provide service in subspecialty inpatient service (38% versus 16%; P < 0.0001), transports (14% versus 6%; P = 0.0227), inpatient consultation (61% versus 45%; P = 0.0086), and the emergency response team (29% versus 19%; P = 0.0021).
Professional Roles and Parameters
Respondents from COTH hospitals were more likely to have no involvement in utilization review, compared to their peers at non‐COTH hospitals (49% versus 37%; P = 0.0220).
DISCUSSION
This study provides the most comprehensive information available regarding the clinical and nonclinical roles, training, work expectations, and career plans of pediatric hospitalists. Among the most important of our findings is the distribution of the length of time that pediatric hospitalists had served in their roles. While over one‐third (37%) reported having been practicing as hospitalists for over 5 years, 45% of our respondents had been in practice for fewer than 3 years. This is consistent with both the perceptions of rapid growth of the field and with significant turnover of hospitalists.1, 8 It is important to note that our findings may actually overestimate the proportion of hospitalists with longer durations of employment as our sampling strategy would have been less likely to include those who left the field within the first 12 to 18 months of practice. Nevertheless, over half (61%) of our respondents expected to remain a hospitalist for the duration of their career and few reported choosing to become a hospitalist as a short‐term employment option. This finding has important implications for the future stability of the hospitalist workforce and the potential development of specific expertise among this cadre of clinicians.6
The demographic profile of pediatric hospitalists was also consistent with these findings. The mean age of 39 years for our respondents is indicative of a significant proportion of this group of physicians recently having completed their residency training. Further, the gender distribution approximates that of current pediatric residency graduates, thus indicating that that this is not a clinical choice for which there would be a skewed distribution as is the case in some pediatric subspecialties.9
Our findings were similar to the 2004 Ottolini et al.10 findings on the roles of pediatric hospitalists. Respondents in our study reported spending less time providing inpatient care (61% versus 75%), providing clinical teaching or supervising residents (16% versus 26%), performing administrative duties (8% versus 19%), and conducting research (3% versus 9%) compared with the respondents in the Ottolini et al.10 survey.
At this point in time, fewer than half of our respondents reported any hospitalist‐specific training, including workshops at professional meetings or CME coursework. As there are a paucity of fellowships offering postresidency training in pediatric hospital medicine, and most of the existing programs are newly established, few in practice have completed such programs.11 In addition, most respondents reported that current CME offerings do not meet their needs, and that they could have used additional QI training to prepare them for their role as pediatric hospitalists. However, almost three‐quarters of respondents (73%) do not believe any additional training beyond residency should be required. As such, it is unclear if a defined, unique body of knowledge specific to hospitalists is either needed or desired by those currently in the field.
Although there are a broad range of potential clinical roles within hospital medicine, and this clinical variety influenced most respondents' decisions to become hospitalists, the current scope of an individual hospitalist tends to become somewhat focused.12, 13 While we found almost all provided service on the pediatric inpatient unit, many fewer provided inpatient consultation and normal newborn care, or were involved in interhospital transport or as part of an emergency response team. There is also wide variation in the types of procedures performed or supervised by hospitalists at different institutions. More than half never perform or supervise infusion services, PICC or central line placement, or circumcision. The variation seen among hospitalists practicing in different hospital settings likely is a result, at least in part, of different needs in teaching hospitals for both service and for clinical experience of trainees. For example, our results demonstrate that pediatric hospitalists in nonteaching and non‐children's hospitals are more likely to have a broader scope of clinical care provision. Another potential issue is that some hospitalists may be employed by institutions which have no pediatric ICU, neonatal ICU, or other specialty unit. As such, these hospitalists would not have the opportunity to work in such settings.
Further, those without academic appointments are also more likely to have expanded clinical roles compared with their academic counterparts. This may be due to the fact that there is likely a greater number of subspecialty‐trained pediatric providers in academic centers and thus the need for hospitalists to cover specific services or perform specific procedures is lessened. There may also be a desire to prevent competition among care providers within the same institution. In contrast, hospitalists with academic appointments are more likely (though still uncommonly) to have taken leadership roles in hospital administration and QI initiatives. Thus, the nature of their efforts appears to expand into nonclinical delivery areas.
Clearly, hospitalists report they have assumed a significant role in the clinical teaching of trainees at all levels, with 94% of our respondents maintaining at least some involvement in education. On average, they spend 16% of their time in educational efforts. However, there are few data on the impact of their work in this area.5, 13 Studies in pediatrics to date have been limited to a few institutions,3, 5 and have not addressed the issue from the perspective of residency program directors or those who are in charge of inpatient curricula.
This study, like the majority of studies related to pediatric hospitalists, is hampered by the difficulty of identifying pediatric hospitalists. Rather than utilizing a hospital medicine membership list, which would be potentially biased by self‐selection, we attempted to obtain a more representative sample through utilization of the AHA database.
CONCLUSIONS
Findings from this study provide an additional perspective regarding pediatric hospitalists to add to our previous study of hospitalist program directors.1 However, the field is currently a moving target. Our data demonstrate that there is significant flux in the hospitalist workforce, uncertainty regarding turnover, and variation in the roles of these professionals in their clinical and nonclinical work environment. Moreover, additional studies of the educational impact of hospitalists on residency and medical student education are needed. Questions regarding the nature and degree of resident autonomy and experience conducting procedures in the hospitalist environment have been raised. These must be assessed through studies of residency program directors, their expectations of residents, and the curricula they have developed.
As with any new phenomenon, it will take time to understand the impact of hospitalists in a variety of domains. Additional research will be helpful in following the development of this field and the manner in which it will interface with existing medical practice and educational programs.
There has been marked recent growth in the employment and utilization of both pediatric and adult hospitalists. Recent data demonstrate that approximately 25% of current pediatric hospitalist programs are less than 2 years old.1 Some have posited that this growth is due to increasing pressure from the public and payors to deliver cost‐effective and high‐quality care.2 However, little is known about the mechanisms by which those who deliver care in this framework are trained, nor the scope of clinical practice they provide.37 One study has shown that among those who direct pediatric hospitalist services there is a great degree of variability in the description of the roles, work patterns, and employment characteristics of hospitalists.1 That study provided only 1 perspective on the roles and career trajectories of those in the field. To better understand both the range and frequency of experiences, clinical and nonclinical roles, training, work expectations, and career plans, we conducted a national survey study of practicing pediatric hospitalists.
METHODS
Sample
We identified all 761 hospitals in the American Hospital Association (AHA)'s 2005 Annual Survey of Hospitals that reported to have both a hospitalist service (adult and/or pediatric) and pediatric beds. From these 761 hospitals, we selected a random sample of 213, stratified by:
Council of Teaching Hospital (COTH) designation
National Association of Children's Hospitals & Related Institutions (NACHRI) membership
Freestanding children's hospitals
Metropolitan Statistical Area (MSA) (urban versus rural location)
Hospital size (small: <250 total beds versus large: 250 total beds)
Some hospitals are included in more than 1 category. Thus, there is some overlap of hospitals in the analysis. Of these 213 hospitals, 97 were removed from the sample because they did not have at least 1 pediatric hospitalist. In a separate study, we surveyed hospitalist program directors at 112 of the remaining 116 hospitals from June through September 2006. These results have been published.1
Pediatric hospitalist program directors at these 112 participating hospitals were asked to provide the names of all practicing pediatric hospitalists in their respective programs. Ninety‐five of these program directors provided a list of hospitalists at their institutions, representing 85% of the hospitals in our previous study. A total of 530 practicing pediatric hospitalists were identified to us in this manner. Of these 530 hospitalists, 67% (N = 338) were from teaching hospitals, 71% (N = 374) were from children's hospitals, 43% (N = 230) were from freestanding children's hospitals, and 69% (N = 354) were from hospitals with 250 beds. These are not mutually exclusive categories.
Survey Instrument
We developed a structured questionnaire to be administered by mail. The survey contained 25 items and was designed to be completed in 10 minutes or less. The survey focused on exploring the characteristics of hospitalist clinical and nonclinical practice, service schedule, training, and career goals. The questionnaire was comprised of a mixture of fixed‐choice, Likert‐scale, and open‐ended questions.
Questionnaire Administration
In October 2006, the first mailing of questionnaires was sent via priority mail. The survey packet contained a personalized cover letter signed by the principal investigator (G.L.F.), the instrument, a business reply mail envelope, and a $5 bill as an incentive. Two additional mailings were sent to nonrespondents in November 2006 and January 2007.
Data Analysis
First, frequency distributions were calculated for all survey items. Next, comparisons were made between respondents indicating they held an academic appointment and those who did not. For the purposes of this analysis, academic pediatric hospitalists were defined as those respondents holding a full‐time or part‐time academic appointment. Nonacademic pediatric hospitalists were defined as respondents holding an adjunct or volunteer faculty position, or no academic appointment. Finally, chi‐square statistics were used to compare pediatric hospitalist responses by hospital demographics such as teaching status, children's hospital status, NACHRI freestanding hospital designation, and hospital bed size.
The study was approved by the University of Michigan Medical Institutional Review Board.
RESULTS
Response Rate
Of the initial 530 survey packets mailed, 18 were returned as undeliverable by the postal service and 431 physicians returned the survey. This yielded an overall response rate of 84%. Of the 431 respondents, 40 physicians were ineligible because they no longer provided inpatient care to children or did not consider themselves to be hospitalists. Thus, the final sample for analysis was 391.
Hospitalist Employment Characteristics
Demographics of Hospital Worksite
Of the 391 respondents, 61% (N = 237) were from teaching hospitals, 73% (N = 287) from children's hospitals, 47% (N = 182) from freestanding children's hospitals, and 66% (N = 258) from hospitals with more than 250 beds.
Physician Demographics
The mean age of respondents was 39 years and 59% were female. The majority were employed by a hospital or health system (56%), 20% were employed by a university, and 4% were employed by both. Eight percent reported employment by a general physician medical group, 7% were employed by a hospitalist‐only group, and 4% reported other sources of employment. Half of respondents (N = 196) reported holding a full‐time (40%) or part‐time (10%) academic appointment. Approximately half the respondents (N = 194) were considered nonacademic hospitalists.
More than half of respondents (54%; N = 211) had been practicing as hospitalists for at least 3 years. Reported time as a practicing hospitalist ranged from <1 year to 26 years, while the average length of time was 63 months (Table 1). These figures may be skewed because those hospitalists with higher turnover rates might have left their position during the period of time from when they were selected into the sample until the time of survey administration.
| Length of Time as Hospitalist | % (N) |
|---|---|
| |
| 12 months | 13 (51) |
| 13‐24 months | 18 (71) |
| 25‐36 months | 14 (56) |
| 37‐60 months | 17 (67) |
| >61 months | 37 (144) |
Clinical Practice
Most respondents reported that the pediatric inpatient unit (94%) and inpatient consultation service (51%) were a part of their regular clinical assignment (Table 2). A majority did not provide service in the normal newborn nursery (58%), subspecialty inpatient service (52%), pediatric intensive care unit (ICU) (70%), neonatal ICU (77%), transports (85%), outpatient clinics (66%), or as part of an emergency response team (53%).
| Part of Regular Clinical Assignment % (N) | Occasionally % (N) | Never % (N) | |
|---|---|---|---|
| |||
| Pediatric inpatient unit | 94 (368) | 3 (13) | 2 (9) |
| Inpatient consultation service | 51 (199) | 40 (155) | 9 (35) |
| Normal newborn nursery | 29 (110) | 13 (50) | 58 (223) |
| Emergency department | 25 (95) | 28 (108) | 47 (178) |
| Subspecialty inpatient service | 25 (92) | 23 (86) | 52 (196) |
| Emergency response team | 23 (87) | 24 (91) | 53 (201) |
| Outpatient/outreach clinics | 18 (68) | 16 (61) | 66 (253) |
| Pediatric ICU | 14 (54) | 16 (59) | 70 (268) |
| Neonatal ICU | 12 (44) | 11 (42) | 77 (294) |
| Transports | 9 (33) | 6 (23) | 85 (319) |
With regard to procedures, many (53%) respondents reported that they routinely perform or supervise lumbar punctures. Several services are never performed or never supervised by the majority of pediatric hospitalists, including infusion services (57%), peripherally inserted central catheter (PICC) placement (76%), central line placement (67%), and circumcision (85%).
Professional Roles and Parameters
Respondents reported that they participate in a variety of nonclinical activities. Ninety‐four percent of hospitalists were involved in education, and 45% reported having a leadership role in that area. The majority of respondents participated in quality improvement (QI) initiatives (84%) and practice guideline development (81%), with one‐quarter of hospitalists reporting a leadership role in each of these activities. Slightly more than half of respondents reported involvement in hospital administration (52%) and utilization review (55%) (Table 3).
| Participation | No Involvement % (N) | ||
|---|---|---|---|
| Participation of Any Type % (N) | Leadership Role % (N) | ||
| |||
| Education (students, house staff) | 94 (368) | 45 (177) | 6 (22) |
| Quality improvement initiatives | 84 (330) | 25 (99) | 16 (61) |
| Practice guideline development | 81 (313) | 26 (101) | 19 (74) |
| Utilization review | 55 (213) | 11 (41) | 45 (172) |
| Hospital administration | 52 (202) | 16 (60) | 48 (184) |
On average, hospitalists reported spending 61% of their time providing inpatient care (excluding clinical teaching) and 16% of their time providing clinical teaching or supervising residents. More than one‐third of respondents (38%) spent more than 75% of their time providing direct inpatient care. Research (3%), administrative duties (8%), and nonclinical teaching (3%) were reported to be a small part of hospitalist professional time.
Pediatric Hospitalist Service Schedule
The majority of respondents reported that their assigned clinical schedule was a combination of shift and call (61%).
When on service, over half of responding pediatric hospitalists (58%) reported that they spend 40 to 60 hours onsite per week. Less than one‐fifth of respondents (19%) reported that they provide <40 hours of onsite coverage when on service. Most (97%) provide some type of night coverage, including taking calls from home or providing onsite coverage.
Hospitalist Training and Continuing Education
Only 51 of the 391 respondents (13%) had received some type of fellowship training, mostly in general pediatrics or the pediatric subspecialties. Only 5 respondents had received fellowship training in hospital medicine.
Fifty‐eight percent of respondents reported that they had received no hospitalist‐specific training. One‐fifth reported that they received training through a workshop at a professional meeting, while fewer respondents had received hospitalist training though a continuing medical education (CME) course (16%) or a mentoring program (17%).
Respondents were asked to rate the adequacy of their respective training in preparing them for their work as hospitalists. The vast majority rated their training in general clinical skills (94%) and communication (85%) as fully adequate. However, respondents found their training for some of the nonclinical aspects of their positions to be deficient. Many respondents rated training for QI projects (38%) and hospital administrative duties (46%) as inadequate (Table 4).
| Fully Adequate % (N) | Somewhat Adequate % (N) | Not Adequate % (N) | NA % (N) | |
|---|---|---|---|---|
| ||||
| General clinical skills | 94 (367) | 5 (21) | 0 (0) | 0 (1) |
| Communication skills | 85 (330) | 14 (53) | 1 (5) | 0 (1) |
| Coordination of care | 73 (284) | 23 (89) | 4 (15) | 0 (1) |
| Clinical procedure experience | 67 (258) | 32 (123) | 1 (5) | 1 (2) |
| Teaching skills (resident and medical student teaching) | 64 (248) | 31 (120) | 3 (13) | 2 (8) |
| Attending newborn deliveries | 60 (233) | 18 (70) | 4 (14) | 19 (72) |
| Running resuscitation (codes) | 45 (173) | 46 (177) | 5 (21) | 5 (18) |
| Quality improvement projects | 14 (55) | 42 (162) | 38 (148) | 6 (22) |
| Hospital administrative duties | 10 (37) | 37 (144) | 46 (177) | 8 (31) |
Survey respondents were asked to indicate the extent to which they agreed or disagreed with 3 statements regarding hospitalist training. The majority of respondents believed that hospitalists need training in QI methods (70%). However, most pediatric hospitalists (73%) did not believe that additional training beyond residency should be required. Only one‐third (36%) of respondents agreed that current CME offerings are adequate for their needs as a pediatric hospitalist.
Career Goals and Expectations
Respondents were asked to select 1 or more reasons why they became pediatric hospitalists. The top factors influencing respondents' decision to become a hospitalist were reported to be a preference for the inpatient setting (73%), clinical variety (72%), enjoyment of teaching in the inpatient setting (58%), and a flexible schedule (52%) (Table 5).
| Factor | % (N) |
|---|---|
| |
| Prefer inpatient setting | 73 (284) |
| Clinical variety | 72 (281) |
| Enjoy teaching in inpatient setting | 58 (225) |
| Flexible schedule | 52 (202) |
| Defined hours | 41 (161) |
| Attractive career opportunities | 21 (80) |
| Salary | 18 (70) |
| Unsure of long‐term career direction | 13 (51) |
| Other | 7 (28) |
| Needed short‐term employment | 4 (15) |
| Only position available | 3 (10) |
The majority (85%) were satisfied with their position as a pediatric hospitalist, with 37% reporting that they were extremely satisfied. Over one‐half (61%) expected to remain a hospitalist for the duration of their career.
RESULTS BY ACADEMIC STATUS
Only significant differences between academic and nonacademic hospitalists are presented.
Clinical Practice by Academic Status
Nonacademic respondents were more likely than academic respondents to report regular service in the normal newborn nursery, pediatric ICU, neonatal ICU, transports, emergency department, and as part of an emergency response team. Academic respondents were more likely to report regular service in outpatient clinics. Nonacademic respondents were more likely than academic respondents to perform or supervise lumbar punctures, sedation services, PICC or central line insertions, and circumcisions (Table 6).
| Academic* (N = 196) | Nonacademic (N = 194) | P Value | |
|---|---|---|---|
| |||
| Regularly provides service | |||
| Normal newborn nursery | 16% | 42% | <0.0001 |
| Pediatric ICU | 9% | 20% | 0.0065 |
| Neonatal ICU | 4% | 20% | <0.0001 |
| Transports | 3% | 15% | <0.0001 |
| Emergency department | 16% | 34% | <0.0001 |
| Emergency response team | 17% | 29% | <0.0001 |
| Outpatient clinic | 23% | 13% | 0.0168 |
| Performs or supervises procedures | |||
| Lumbar puncture | 84% | 92% | 0.0152 |
| Sedation services | 50% | 64% | 0.0055 |
| PICC insertion | 8% | 18% | 0.0031 |
| Central line insertion | 11% | 23% | 0.0018 |
| Circumcision | 5% | 16% | 0.0002 |
| Holds leadership roles | |||
| Education (student or house staff) | 63% | 27% | <0.0001 |
| Hospital administration | 21% | 10% | <0.0001 |
| Quality improvement initiatives | 33% | 18% | 0.0005 |
Professional Roles and Parameters by Academic Status
Responding academic pediatric hospitalists were twice as likely as nonacademic respondents to have a leadership role in the education of students and house staff and to hold a leadership position in hospital administration. The academic respondents were also more likely to report a leadership role in QI initiatives (Table 6).
Clinical and Educational Activities by Academic Status
Academic pediatric hospitalist respondents reported spending on average 52% of their time providing inpatient care (excluding teaching), in contrast to the nonacademic hospitalist respondents who reported 71% of their time was spent providing inpatient care (P < 0.0001). Academic respondents also reported that 19% of their time was spent providing inpatient teaching or supervising residents, compared to 12% of nonacademic respondents (P < 0.0001). Responding academic pediatric hospitalists reported spending a greater proportion of time participating in nonclinical teaching activities (5% versus 2%; P < 0.0001), administrative duties (11% versus 5%; P < 0.0001), and research (4% versus 1%; P < 0.0001) compared to the nonacademic respondents.
Nonacademic respondents were more likely than academic respondents to report no hospitalist‐specific training (64% versus 54%; P = 0.0324).
RESULTS BY HOSPITAL CHARACTERISTICS
For each hospital characteristic, only significant differences between dichotomized groups are presented.
Children's Hospitals versus Other Hospitals
Clinical Practice
Pediatric hospitalist respondents practicing in NACHRI hospitals were more likely to report that they provide regular service for general pediatric inpatients (98% versus 86%; P < 0.0001) as well as subspecialty inpatients (27% versus 17%; P = 0.044). Non‐NACHRI pediatric hospitalist respondents were twice as likely to report the provision of regular service in the normal newborn nursery (49% versus 22%; P < 0.0001), the neonatal ICU (21% versus 8%, P = 0.002), and the emergency department (38% versus 20%; P < 0.0001).
Among respondents, pediatric hospitalists who were not working at a children's hospital were more likely to report that they sometimes or routinely performed lumbar punctures (93% versus 85%; P = 0.037), infusion services (36% versus 21%; P = 0.003), and were twice as likely to perform circumcision (16% versus 8%; P = 0.041) compared to those working at children's hospitals.
Professional Roles and Parameters
Respondents working in children's hospitals were twice as likely to hold a leadership position in utilization review (12% versus 6%; P = 0.012), though respondents from non‐NACHRI hospitals were more likely to at least participate in utilization review (58% versus 40%; P = 0.004).
Hospitalist Training
Respondents from non‐NACHRI hospitals were more likely to report that they had received no hospitalist‐specific training (68% versus 56%; P = 0.029). Those at NACHRI hospitals were twice as likely to have received hospitalist training through a mentoring program (20% versus 9%; P = 0.009).
Freestanding versus Nonfreestanding Children's Hospitals
Clinical Practice
Pediatric hospitalist respondents employed at institutions that are not freestanding children's hospitals were more likely to report that they provided regular service in the normal newborn nursery (42% versus 14%; P < 0.0001), pediatric ICU (22% versus 5%), emergency department (32% versus 17%; P < 0.0001), and outpatient clinics (23% versus 12%; P = 0.0068). They were also more likely to perform or supervise sedation services (63% versus 50%; P = 0.0116), infusion services (32% versus 17%; P = 0.0006), PICC insertions (19% versus 6%; P = 0.0002), central line insertions (23% versus 11%; P = 0.0024), and circumcisions (16% versus 3%; P < 0.0001).
Professional Roles and Parameters
Among respondents, pediatric hospitalists employed by nonfreestanding children's hospitals were more likely to report participation in utilization review (51% versus 38%; P = 0.02).
Hospital Size
Clinical Practice
Pediatric hospitalist respondents working at large hospitals were twice as likely to report that they regularly provided service in the pediatric ICU (18% versus 7%; P = 0.0072) and were more likely to regularly perform circumcisions (13% versus 5%; P = 0.0069). Respondents from small hospitals were more likely to provide regular service in the neonatal ICU (20% versus 7%; P = 0.0013).
COTH Status: Teaching versus Nonteaching Hospitals
Clinical Practice
Among survey respondents, pediatric hospitalists employed by COTH hospitals were more likely to provide regular service in the neonatal ICU, compared to their peers in nonteaching hospitals (15% versus 6%; P = 0.0109). Those employed by non‐COTH hospitals were more likely to provide service in subspecialty inpatient service (38% versus 16%; P < 0.0001), transports (14% versus 6%; P = 0.0227), inpatient consultation (61% versus 45%; P = 0.0086), and the emergency response team (29% versus 19%; P = 0.0021).
Professional Roles and Parameters
Respondents from COTH hospitals were more likely to have no involvement in utilization review, compared to their peers at non‐COTH hospitals (49% versus 37%; P = 0.0220).
DISCUSSION
This study provides the most comprehensive information available regarding the clinical and nonclinical roles, training, work expectations, and career plans of pediatric hospitalists. Among the most important of our findings is the distribution of the length of time that pediatric hospitalists had served in their roles. While over one‐third (37%) reported having been practicing as hospitalists for over 5 years, 45% of our respondents had been in practice for fewer than 3 years. This is consistent with both the perceptions of rapid growth of the field and with significant turnover of hospitalists.1, 8 It is important to note that our findings may actually overestimate the proportion of hospitalists with longer durations of employment as our sampling strategy would have been less likely to include those who left the field within the first 12 to 18 months of practice. Nevertheless, over half (61%) of our respondents expected to remain a hospitalist for the duration of their career and few reported choosing to become a hospitalist as a short‐term employment option. This finding has important implications for the future stability of the hospitalist workforce and the potential development of specific expertise among this cadre of clinicians.6
The demographic profile of pediatric hospitalists was also consistent with these findings. The mean age of 39 years for our respondents is indicative of a significant proportion of this group of physicians recently having completed their residency training. Further, the gender distribution approximates that of current pediatric residency graduates, thus indicating that that this is not a clinical choice for which there would be a skewed distribution as is the case in some pediatric subspecialties.9
Our findings were similar to the 2004 Ottolini et al.10 findings on the roles of pediatric hospitalists. Respondents in our study reported spending less time providing inpatient care (61% versus 75%), providing clinical teaching or supervising residents (16% versus 26%), performing administrative duties (8% versus 19%), and conducting research (3% versus 9%) compared with the respondents in the Ottolini et al.10 survey.
At this point in time, fewer than half of our respondents reported any hospitalist‐specific training, including workshops at professional meetings or CME coursework. As there are a paucity of fellowships offering postresidency training in pediatric hospital medicine, and most of the existing programs are newly established, few in practice have completed such programs.11 In addition, most respondents reported that current CME offerings do not meet their needs, and that they could have used additional QI training to prepare them for their role as pediatric hospitalists. However, almost three‐quarters of respondents (73%) do not believe any additional training beyond residency should be required. As such, it is unclear if a defined, unique body of knowledge specific to hospitalists is either needed or desired by those currently in the field.
Although there are a broad range of potential clinical roles within hospital medicine, and this clinical variety influenced most respondents' decisions to become hospitalists, the current scope of an individual hospitalist tends to become somewhat focused.12, 13 While we found almost all provided service on the pediatric inpatient unit, many fewer provided inpatient consultation and normal newborn care, or were involved in interhospital transport or as part of an emergency response team. There is also wide variation in the types of procedures performed or supervised by hospitalists at different institutions. More than half never perform or supervise infusion services, PICC or central line placement, or circumcision. The variation seen among hospitalists practicing in different hospital settings likely is a result, at least in part, of different needs in teaching hospitals for both service and for clinical experience of trainees. For example, our results demonstrate that pediatric hospitalists in nonteaching and non‐children's hospitals are more likely to have a broader scope of clinical care provision. Another potential issue is that some hospitalists may be employed by institutions which have no pediatric ICU, neonatal ICU, or other specialty unit. As such, these hospitalists would not have the opportunity to work in such settings.
Further, those without academic appointments are also more likely to have expanded clinical roles compared with their academic counterparts. This may be due to the fact that there is likely a greater number of subspecialty‐trained pediatric providers in academic centers and thus the need for hospitalists to cover specific services or perform specific procedures is lessened. There may also be a desire to prevent competition among care providers within the same institution. In contrast, hospitalists with academic appointments are more likely (though still uncommonly) to have taken leadership roles in hospital administration and QI initiatives. Thus, the nature of their efforts appears to expand into nonclinical delivery areas.
Clearly, hospitalists report they have assumed a significant role in the clinical teaching of trainees at all levels, with 94% of our respondents maintaining at least some involvement in education. On average, they spend 16% of their time in educational efforts. However, there are few data on the impact of their work in this area.5, 13 Studies in pediatrics to date have been limited to a few institutions,3, 5 and have not addressed the issue from the perspective of residency program directors or those who are in charge of inpatient curricula.
This study, like the majority of studies related to pediatric hospitalists, is hampered by the difficulty of identifying pediatric hospitalists. Rather than utilizing a hospital medicine membership list, which would be potentially biased by self‐selection, we attempted to obtain a more representative sample through utilization of the AHA database.
CONCLUSIONS
Findings from this study provide an additional perspective regarding pediatric hospitalists to add to our previous study of hospitalist program directors.1 However, the field is currently a moving target. Our data demonstrate that there is significant flux in the hospitalist workforce, uncertainty regarding turnover, and variation in the roles of these professionals in their clinical and nonclinical work environment. Moreover, additional studies of the educational impact of hospitalists on residency and medical student education are needed. Questions regarding the nature and degree of resident autonomy and experience conducting procedures in the hospitalist environment have been raised. These must be assessed through studies of residency program directors, their expectations of residents, and the curricula they have developed.
As with any new phenomenon, it will take time to understand the impact of hospitalists in a variety of domains. Additional research will be helpful in following the development of this field and the manner in which it will interface with existing medical practice and educational programs.
- ,,,; The Research Advisory Committee of the American Board of Pediatrics.Characteristics of the pediatric hospitalist workforce: its roles and work environment.Pediatrics.2007;120:33–39.
- .The evolution of the hospitalist model in the United States.Med Clin North Am.2002;86:687–706.
- ,.Hospitalists in children's hospitals: what we know now and what we need to know.J Pediatr.2006;148:296–299.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,,.Effect of a pediatric hospitalist system on housestaff education and experience.Arch Pediatr Adolesc Med.2002;156:877–883.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111:247–254.
- ,,,,,.Pediatric hospitalists in Canada and the United States: a survey of pediatric academic department chairs.Ambul Pediatr.2001;1:338–339.
- .Hospitalists in the United States: mission accomplished or work in progress?N Engl J Med.2004;350:1935–1936.
- ,.Pediatric workforce: a look at general pediatrics data from the American Board of Pediatrics.J Pediatr.2006;148:166–169.
- ,,,,PRIS survey: pediatric hospitalist roles and training needs [Abstr].Pediatr Res.2004;55:360A.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:1.e1–1.e7.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- ,,.Pediatric hospitalists fill varied roles in the care of newborns.Pediatr Ann.2003;32:802–810.
- ,,,; The Research Advisory Committee of the American Board of Pediatrics.Characteristics of the pediatric hospitalist workforce: its roles and work environment.Pediatrics.2007;120:33–39.
- .The evolution of the hospitalist model in the United States.Med Clin North Am.2002;86:687–706.
- ,.Hospitalists in children's hospitals: what we know now and what we need to know.J Pediatr.2006;148:296–299.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,,.Effect of a pediatric hospitalist system on housestaff education and experience.Arch Pediatr Adolesc Med.2002;156:877–883.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111:247–254.
- ,,,,,.Pediatric hospitalists in Canada and the United States: a survey of pediatric academic department chairs.Ambul Pediatr.2001;1:338–339.
- .Hospitalists in the United States: mission accomplished or work in progress?N Engl J Med.2004;350:1935–1936.
- ,.Pediatric workforce: a look at general pediatrics data from the American Board of Pediatrics.J Pediatr.2006;148:166–169.
- ,,,,PRIS survey: pediatric hospitalist roles and training needs [Abstr].Pediatr Res.2004;55:360A.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:1.e1–1.e7.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- ,,.Pediatric hospitalists fill varied roles in the care of newborns.Pediatr Ann.2003;32:802–810.
Copyright © 2009 Society of Hospital Medicine
Takotsubo Cardiomyopathy
Takotsubo cardiomyopathy, also known as transient left ventricular apical ballooning, stress cardiomyopathy, and broken heart syndrome, is a condition that mimics acute myocardial infarction. Patients typically present with chest pain, electrocardiographic changes consistent with acute ischemia or infarct, and elevated cardiac enzymes in the absence of significant coronary artery disease. Left ventriculography demonstrates a characteristic pattern of dysfunction: dyskinesis of the cardiac apex and hyperkinesis of the base. This resulting appearance of apical ballooning is reminiscent of the takotsubo, a Japanese octopus pot with a wide base and narrow top. The syndrome occurs almost exclusively in postmenopausal women and demonstrates a distinct temporal association with extreme emotional or physiological stress. The pathophysiology is poorly understood, but one theory suggests that the transient cardiomyopathy reflects myocardial stunning due to excessive sympathetic output.1 Treatment is supportive, and most patients rapidly recover normal systolic function.
A 57‐year‐old African American female with a past medical history significant only for chronic obstructive pulmonary disease presented with severe dyspnea that was progressive over several hours following the unexpected death of her son. She denied chest pain, palpitations, cough, or fever. On examination, she was afebrile with a blood pressure of 145/82 mm Hg, a pulse of 90 beats per minute, and a respiratory rate of 24 breaths per minute with oxygen saturations of 88% on room air. Lung examination revealed coarse breath sounds with a slightly prolonged expiration phase, but it was otherwise clear. Cardiac examination was unremarkable. Chest radiograph showed only emphysematous changes. Initial electrocardiogram and serial cardiac enzymes were negative. A computed tomography pulmonary angiogram showed no evidence of pulmonary embolism. The patient was admitted with the diagnosis of chronic obstructive pulmonary disease exacerbation and treated with supplemental oxygen, bronchodilators, and corticosteroids.
On the following day, the patient developed worsening dyspnea, hypoxia, and diffuse crackles on examination. Electrocardiogram at that time demonstrated ST‐segment elevations in leads V1 and V2 as well as T‐wave inversions in all precordial leads (Figure 1). The troponin‐I concentration was 1.92 ng/mL (<0.05 nL), and the brain natriuretic peptide concentration was 1425 pg/mL. The patient underwent urgent cardiac catheterization with no evidence of coronary artery obstruction. Left ventriculogram revealed a hyperdynamic base and akinetic apex extending into the mid‐heart (Figure 2). Left ventricular systolic function was severely reduced, with an estimated ejection fraction of 10% to 15%. The normal diastolic ventriculogram image is shown for comparison (Figure 3). These findings were felt to be consistent with takotsubo syndrome. The patient required inotropic support briefly but experienced full clinical recovery by the sixth hospital day.

DISCUSSION
Takotsubo cardiomyopathy was first described in Japan in 1991.2 Although the condition made a relatively recent debut in the United States, with the first case series published in 2003, subsequent reports have suggested that the condition is not rare.3 Recent analyses of Western populations estimate the prevalence to be approximately 2% among patients with acute coronary syndrome.1, 4, 5 Because women compose the majority of patients with takotsubo cardiomyopathy, the prevalence among women as a subset of patients with acute coronary syndrome is likely much higher. The syndrome has been described as a clinical entity in Japanese, European, Caucasian, and African American patients.2, 3 Interesting differences appear to exist among different ethnic groups. For example, evidence suggests that the condition is more likely to be precipitated by emotional stress in Caucasians, whereas physiological stress is a more frequent trigger in Asians.6
Although chest pain is described as a cardinal feature in takotsubo cardiomyopathy, existing data suggest that African American patients may lack this typical symptom. The first African American female reported with takotsubo syndrome presented with heart failure and hypotension in the absence of chest pain.1 Subsequently, Patel et al.7 reported 5 African American women with takotsubo syndrome. Three patients presented with dyspnea, and 2 presented with nausea: none of the patients experienced chest pain. Our case adds to this evidence by describing an African American woman with takotsubo syndrome whose presenting symptom was severe dyspnea without chest pain. Unlike the majority of reported cases, electrocardiographic and biomarker abnormalities were not present in our patient at admission. As with our patient, the diagnosis of takotsubo cardiomyopathy may initially be overlooked in African Americans because of the atypical presentation. As takotsubo syndrome becomes increasingly recognized in the United States, clinicians are encouraged to consider the diagnosis in African American women who present with severe dyspnea in the setting of extreme emotional or physiological stress. Further research on the pathophysiology of takotsubo cardiomyopathy is needed to explain why such differences in presenting symptoms may exist.
- ,,, et al.Neurohormonal features of myocardial stunning due to sudden emotional stress.N Engl J Med.2005;352:540–548.
- ,,, et al.Myocardial stunning due to simultaneous multivessel coronary spasm: a review of 5 cases.J Cardiol.1991;21:203–214.
- ,,, et al.A syndrome of transient left ventricular apical wall motion abnormality in the absence of coronary disease: a perspective from the United States.Cardiology.2003;100:61–66.
- ,,, et al.Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular ballooning syndrome.Am J Cardiol.2004;94:343–346.
- ,,, et al.Left ventricular apical ballooning: not an uncommon variant of acute myocardial infarction in women.Clin Cardiol.2006;29:9–12.
- ,.Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome.Heart Fail Rev.2005;10:311–316.
- ,,, et al.Takotsubo syndrome in African‐American women with atypical presentations: a single‐center experience.Clin Cardiol2007;30:14–18.
Takotsubo cardiomyopathy, also known as transient left ventricular apical ballooning, stress cardiomyopathy, and broken heart syndrome, is a condition that mimics acute myocardial infarction. Patients typically present with chest pain, electrocardiographic changes consistent with acute ischemia or infarct, and elevated cardiac enzymes in the absence of significant coronary artery disease. Left ventriculography demonstrates a characteristic pattern of dysfunction: dyskinesis of the cardiac apex and hyperkinesis of the base. This resulting appearance of apical ballooning is reminiscent of the takotsubo, a Japanese octopus pot with a wide base and narrow top. The syndrome occurs almost exclusively in postmenopausal women and demonstrates a distinct temporal association with extreme emotional or physiological stress. The pathophysiology is poorly understood, but one theory suggests that the transient cardiomyopathy reflects myocardial stunning due to excessive sympathetic output.1 Treatment is supportive, and most patients rapidly recover normal systolic function.
A 57‐year‐old African American female with a past medical history significant only for chronic obstructive pulmonary disease presented with severe dyspnea that was progressive over several hours following the unexpected death of her son. She denied chest pain, palpitations, cough, or fever. On examination, she was afebrile with a blood pressure of 145/82 mm Hg, a pulse of 90 beats per minute, and a respiratory rate of 24 breaths per minute with oxygen saturations of 88% on room air. Lung examination revealed coarse breath sounds with a slightly prolonged expiration phase, but it was otherwise clear. Cardiac examination was unremarkable. Chest radiograph showed only emphysematous changes. Initial electrocardiogram and serial cardiac enzymes were negative. A computed tomography pulmonary angiogram showed no evidence of pulmonary embolism. The patient was admitted with the diagnosis of chronic obstructive pulmonary disease exacerbation and treated with supplemental oxygen, bronchodilators, and corticosteroids.
On the following day, the patient developed worsening dyspnea, hypoxia, and diffuse crackles on examination. Electrocardiogram at that time demonstrated ST‐segment elevations in leads V1 and V2 as well as T‐wave inversions in all precordial leads (Figure 1). The troponin‐I concentration was 1.92 ng/mL (<0.05 nL), and the brain natriuretic peptide concentration was 1425 pg/mL. The patient underwent urgent cardiac catheterization with no evidence of coronary artery obstruction. Left ventriculogram revealed a hyperdynamic base and akinetic apex extending into the mid‐heart (Figure 2). Left ventricular systolic function was severely reduced, with an estimated ejection fraction of 10% to 15%. The normal diastolic ventriculogram image is shown for comparison (Figure 3). These findings were felt to be consistent with takotsubo syndrome. The patient required inotropic support briefly but experienced full clinical recovery by the sixth hospital day.



DISCUSSION
Takotsubo cardiomyopathy was first described in Japan in 1991.2 Although the condition made a relatively recent debut in the United States, with the first case series published in 2003, subsequent reports have suggested that the condition is not rare.3 Recent analyses of Western populations estimate the prevalence to be approximately 2% among patients with acute coronary syndrome.1, 4, 5 Because women compose the majority of patients with takotsubo cardiomyopathy, the prevalence among women as a subset of patients with acute coronary syndrome is likely much higher. The syndrome has been described as a clinical entity in Japanese, European, Caucasian, and African American patients.2, 3 Interesting differences appear to exist among different ethnic groups. For example, evidence suggests that the condition is more likely to be precipitated by emotional stress in Caucasians, whereas physiological stress is a more frequent trigger in Asians.6
Although chest pain is described as a cardinal feature in takotsubo cardiomyopathy, existing data suggest that African American patients may lack this typical symptom. The first African American female reported with takotsubo syndrome presented with heart failure and hypotension in the absence of chest pain.1 Subsequently, Patel et al.7 reported 5 African American women with takotsubo syndrome. Three patients presented with dyspnea, and 2 presented with nausea: none of the patients experienced chest pain. Our case adds to this evidence by describing an African American woman with takotsubo syndrome whose presenting symptom was severe dyspnea without chest pain. Unlike the majority of reported cases, electrocardiographic and biomarker abnormalities were not present in our patient at admission. As with our patient, the diagnosis of takotsubo cardiomyopathy may initially be overlooked in African Americans because of the atypical presentation. As takotsubo syndrome becomes increasingly recognized in the United States, clinicians are encouraged to consider the diagnosis in African American women who present with severe dyspnea in the setting of extreme emotional or physiological stress. Further research on the pathophysiology of takotsubo cardiomyopathy is needed to explain why such differences in presenting symptoms may exist.
Takotsubo cardiomyopathy, also known as transient left ventricular apical ballooning, stress cardiomyopathy, and broken heart syndrome, is a condition that mimics acute myocardial infarction. Patients typically present with chest pain, electrocardiographic changes consistent with acute ischemia or infarct, and elevated cardiac enzymes in the absence of significant coronary artery disease. Left ventriculography demonstrates a characteristic pattern of dysfunction: dyskinesis of the cardiac apex and hyperkinesis of the base. This resulting appearance of apical ballooning is reminiscent of the takotsubo, a Japanese octopus pot with a wide base and narrow top. The syndrome occurs almost exclusively in postmenopausal women and demonstrates a distinct temporal association with extreme emotional or physiological stress. The pathophysiology is poorly understood, but one theory suggests that the transient cardiomyopathy reflects myocardial stunning due to excessive sympathetic output.1 Treatment is supportive, and most patients rapidly recover normal systolic function.
A 57‐year‐old African American female with a past medical history significant only for chronic obstructive pulmonary disease presented with severe dyspnea that was progressive over several hours following the unexpected death of her son. She denied chest pain, palpitations, cough, or fever. On examination, she was afebrile with a blood pressure of 145/82 mm Hg, a pulse of 90 beats per minute, and a respiratory rate of 24 breaths per minute with oxygen saturations of 88% on room air. Lung examination revealed coarse breath sounds with a slightly prolonged expiration phase, but it was otherwise clear. Cardiac examination was unremarkable. Chest radiograph showed only emphysematous changes. Initial electrocardiogram and serial cardiac enzymes were negative. A computed tomography pulmonary angiogram showed no evidence of pulmonary embolism. The patient was admitted with the diagnosis of chronic obstructive pulmonary disease exacerbation and treated with supplemental oxygen, bronchodilators, and corticosteroids.
On the following day, the patient developed worsening dyspnea, hypoxia, and diffuse crackles on examination. Electrocardiogram at that time demonstrated ST‐segment elevations in leads V1 and V2 as well as T‐wave inversions in all precordial leads (Figure 1). The troponin‐I concentration was 1.92 ng/mL (<0.05 nL), and the brain natriuretic peptide concentration was 1425 pg/mL. The patient underwent urgent cardiac catheterization with no evidence of coronary artery obstruction. Left ventriculogram revealed a hyperdynamic base and akinetic apex extending into the mid‐heart (Figure 2). Left ventricular systolic function was severely reduced, with an estimated ejection fraction of 10% to 15%. The normal diastolic ventriculogram image is shown for comparison (Figure 3). These findings were felt to be consistent with takotsubo syndrome. The patient required inotropic support briefly but experienced full clinical recovery by the sixth hospital day.



DISCUSSION
Takotsubo cardiomyopathy was first described in Japan in 1991.2 Although the condition made a relatively recent debut in the United States, with the first case series published in 2003, subsequent reports have suggested that the condition is not rare.3 Recent analyses of Western populations estimate the prevalence to be approximately 2% among patients with acute coronary syndrome.1, 4, 5 Because women compose the majority of patients with takotsubo cardiomyopathy, the prevalence among women as a subset of patients with acute coronary syndrome is likely much higher. The syndrome has been described as a clinical entity in Japanese, European, Caucasian, and African American patients.2, 3 Interesting differences appear to exist among different ethnic groups. For example, evidence suggests that the condition is more likely to be precipitated by emotional stress in Caucasians, whereas physiological stress is a more frequent trigger in Asians.6
Although chest pain is described as a cardinal feature in takotsubo cardiomyopathy, existing data suggest that African American patients may lack this typical symptom. The first African American female reported with takotsubo syndrome presented with heart failure and hypotension in the absence of chest pain.1 Subsequently, Patel et al.7 reported 5 African American women with takotsubo syndrome. Three patients presented with dyspnea, and 2 presented with nausea: none of the patients experienced chest pain. Our case adds to this evidence by describing an African American woman with takotsubo syndrome whose presenting symptom was severe dyspnea without chest pain. Unlike the majority of reported cases, electrocardiographic and biomarker abnormalities were not present in our patient at admission. As with our patient, the diagnosis of takotsubo cardiomyopathy may initially be overlooked in African Americans because of the atypical presentation. As takotsubo syndrome becomes increasingly recognized in the United States, clinicians are encouraged to consider the diagnosis in African American women who present with severe dyspnea in the setting of extreme emotional or physiological stress. Further research on the pathophysiology of takotsubo cardiomyopathy is needed to explain why such differences in presenting symptoms may exist.
- ,,, et al.Neurohormonal features of myocardial stunning due to sudden emotional stress.N Engl J Med.2005;352:540–548.
- ,,, et al.Myocardial stunning due to simultaneous multivessel coronary spasm: a review of 5 cases.J Cardiol.1991;21:203–214.
- ,,, et al.A syndrome of transient left ventricular apical wall motion abnormality in the absence of coronary disease: a perspective from the United States.Cardiology.2003;100:61–66.
- ,,, et al.Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular ballooning syndrome.Am J Cardiol.2004;94:343–346.
- ,,, et al.Left ventricular apical ballooning: not an uncommon variant of acute myocardial infarction in women.Clin Cardiol.2006;29:9–12.
- ,.Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome.Heart Fail Rev.2005;10:311–316.
- ,,, et al.Takotsubo syndrome in African‐American women with atypical presentations: a single‐center experience.Clin Cardiol2007;30:14–18.
- ,,, et al.Neurohormonal features of myocardial stunning due to sudden emotional stress.N Engl J Med.2005;352:540–548.
- ,,, et al.Myocardial stunning due to simultaneous multivessel coronary spasm: a review of 5 cases.J Cardiol.1991;21:203–214.
- ,,, et al.A syndrome of transient left ventricular apical wall motion abnormality in the absence of coronary disease: a perspective from the United States.Cardiology.2003;100:61–66.
- ,,, et al.Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular ballooning syndrome.Am J Cardiol.2004;94:343–346.
- ,,, et al.Left ventricular apical ballooning: not an uncommon variant of acute myocardial infarction in women.Clin Cardiol.2006;29:9–12.
- ,.Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome.Heart Fail Rev.2005;10:311–316.
- ,,, et al.Takotsubo syndrome in African‐American women with atypical presentations: a single‐center experience.Clin Cardiol2007;30:14–18.
Legionella pneumonia and use of the Legionella urinary antigen test
A 33‐year‐old Caucasian woman presented to an outside hospital with a 10‐day history of fever, cough, and progressive dyspnea on exertion. Ten days prior to the onset of symptoms, she had traveled to Calgary, Alberta, Canada. Her niece and nephew had recently suffered upper respiratory symptoms. Additional review of systems was negative for joint pain, rash, diarrhea, or bloody stools. She had a history of ulcerative colitis, primary sclerosing cholangitis, and juvenile rheumatoid arthritis. Her outpatient medications included prednisone 10 mg daily, methotrexate 7.5 mg weekly, and ursodiol 200 mg 3 times daily. She was employed at a local hospital and her annual purified protein derivative (PPD) test had been negative. Computed tomography angiography demonstrated bilateral patchy consolidation. Vancomycin, levofloxacin, piperacillin/tazobactam, and fluconazole were initiated and she was transferred to our hospital for further evaluation.
On arrival, her vital signs were within normal limits. She was breathing comfortably but on auscultation had crackles at the right‐mid lung field. A complete blood cell count demonstrated a white blood cell count of 7000/L with left shift, hemoglobin 10.7 g/dL, and platelet count 156,000/L. Liver function tests showed albumin 2.6 g/dL, total bilirubin 9.0 mg/dL with conjugated fraction 6.6 mg/dL, alkaline phosphatase 586 U/L, aspartate aminotransferase 104 U/L, and alanine aminotransferase 72 U/L; these were all near her baseline. The basic metabolic panel was within normal limits. A chest X‐ray showed dense areas of consolidation in the lingula and left upper lobe. All antibiotics from the outside hospital were discontinued and empiric moxifloxacin was initiated.
On hospital day 1, she underwent bronchoscopy, which yielded cloudy fluid from the bronchoalveolar lavage (BAL). Initial BAL gram stain showed moderate white blood cells but no organisms; fungal smears and stains for acid fast bacilli were negative. Blood cultures and Legionella and Streptococcus urinary antigen tests were negative. The remainder of her hospital course was uneventful. Her shortness of breath improved and she remained afebrile. She was discharged home on a 10‐day course of moxifloxacin with close follow‐up. Six days after the BAL specimen was collected, the culture grew Legionella micdadei. Repeat chest film 2 weeks later demonstrated resolution of the original findings.
DISCUSSION
Legionella is responsible for 8000 to 18,000 hospitalizations for pneumonia annually.1 It is associated with community‐acquired, hospital‐acquired, and travel‐associated pneumonia. Twenty‐five Legionella species have been identified and 8 species are associated with pneumonia in humans.2 Community‐acquired and travel‐acquired Legionella pneumonia is most commonly caused by Legionella pneumophila; the second most common cause is L. micdadei.2, 3 It was initially identified in 1977 at the University of Pittsburgh in renal transplant patients with acute pneumonitis and is known as the Pittsburgh pneumonia agent. Similar cases were identified in a group of immunocompromised patients in Virginia, all of whom were receiving steroids and cytotoxic chemotherapy. It is unclear why L. micdadei predominates in this population, but is likely related to its decreased virulence compared to L. pneumophila. The definitive mode of transmission of L. micdadei is not known; it may be from contaminated water supplies but infections from inhalation of respiratory secretions have also been documented.2 While L. micdadei is not commonly seen in travel‐associated Legionella pneumonia, the patient's immunocompromised status secondary to the treatment of her underlying medical conditions made her particularly vulnerable. Given the temporal association with her trip, she was most likely exposed during her travels but her hospital employment should also be considered.
Legionella pneumonia is underdiagnosed because of difficulty distinguishing it from other types of pneumonia, failure to order diagnostic tests, and variable sensitivity of available diagnostic tests.4 Culture is considered the gold standard and is ideally performed from lower respiratory secretions, but variable sensitivity due to interlaboratory variation (range, 10%‐80%) limits its use.3, 4 Direct immunofluorescence assay (DFA) testing of respiratory secretions is available but also limited by poor sensitivity. Both culture and DFA have specificities approaching 100%. A newer test, the Legionella urinary antigen test, is an immunochromatographic assay. It is less technically difficult and results are available in less than 1 hour. The assay can detect the antigen in the urine starting 1 day after the onset of symptoms, and can remain positive for days or weeks following treatment.4
With the introduction and wide availability of the Legionella urinary antigen test, it is important to consider its limitations. While the test carries a high specificity, it detects only the soluble antigen of Legionella pneumophila serogroup 1. Thus, as in this case, the urinary test can be negative when infection is caused by other species such as L. micdadei. In the literature, the urine assay's sensitivity is variously reported at 45% to 100% with lower sensitivities in circumstances such as hospital‐acquired disease, where the association with other species is higher than in the community setting.3, 4 For instance, in nosocomial infections, the reported sensitivity is 45%.3 False‐positive results have also been seen in patients with serum sickness.4
The Legionella urinary antigen test has improved detection of Legionella pneumonia. Given its limitations, it is likely to be most accurate in community‐acquired and travel‐acquired cases.3 The Centers for Disease Control and Prevention recommend testing for Legionella in pneumonia patients requiring admission to the intensive care unit (ICU), immunocompromised patients, patients who traveled within 2 weeks of presentation, and those who have failed treatment with beta‐lactams or cephalosporins. A negative test does not rule out Legionella infection and additional testing with bronchoscopy may be indicated, especially in immunocompromised hosts.4
- Centers for Disease Control. Legionellosis Resource Site (Legionnaires' Disease and Pontiac Fever). Top 10 Things Every Clinician Needs to Know About Legionellosis. Available at http://www.cdc.gov/legionella/top10.htm. Accessed February2009.
- ,,.Disease due to the legionellaceae (other than Legionella pneumophila): historical, microbiological, clinical, and epidemiological review.Medicine.1989;68:116–132.
- ,,, et al.Clinical utility of urinary antigen detection for diagnosis of community‐acquired, travel‐associated, and nosocomial legionnaire's disease.J Clin Microbiol.2003;41(2):838–840.
- .Diagnosis of Legionella infection.Clin Infect Dis.2003;36:64–69.
A 33‐year‐old Caucasian woman presented to an outside hospital with a 10‐day history of fever, cough, and progressive dyspnea on exertion. Ten days prior to the onset of symptoms, she had traveled to Calgary, Alberta, Canada. Her niece and nephew had recently suffered upper respiratory symptoms. Additional review of systems was negative for joint pain, rash, diarrhea, or bloody stools. She had a history of ulcerative colitis, primary sclerosing cholangitis, and juvenile rheumatoid arthritis. Her outpatient medications included prednisone 10 mg daily, methotrexate 7.5 mg weekly, and ursodiol 200 mg 3 times daily. She was employed at a local hospital and her annual purified protein derivative (PPD) test had been negative. Computed tomography angiography demonstrated bilateral patchy consolidation. Vancomycin, levofloxacin, piperacillin/tazobactam, and fluconazole were initiated and she was transferred to our hospital for further evaluation.
On arrival, her vital signs were within normal limits. She was breathing comfortably but on auscultation had crackles at the right‐mid lung field. A complete blood cell count demonstrated a white blood cell count of 7000/L with left shift, hemoglobin 10.7 g/dL, and platelet count 156,000/L. Liver function tests showed albumin 2.6 g/dL, total bilirubin 9.0 mg/dL with conjugated fraction 6.6 mg/dL, alkaline phosphatase 586 U/L, aspartate aminotransferase 104 U/L, and alanine aminotransferase 72 U/L; these were all near her baseline. The basic metabolic panel was within normal limits. A chest X‐ray showed dense areas of consolidation in the lingula and left upper lobe. All antibiotics from the outside hospital were discontinued and empiric moxifloxacin was initiated.
On hospital day 1, she underwent bronchoscopy, which yielded cloudy fluid from the bronchoalveolar lavage (BAL). Initial BAL gram stain showed moderate white blood cells but no organisms; fungal smears and stains for acid fast bacilli were negative. Blood cultures and Legionella and Streptococcus urinary antigen tests were negative. The remainder of her hospital course was uneventful. Her shortness of breath improved and she remained afebrile. She was discharged home on a 10‐day course of moxifloxacin with close follow‐up. Six days after the BAL specimen was collected, the culture grew Legionella micdadei. Repeat chest film 2 weeks later demonstrated resolution of the original findings.
DISCUSSION
Legionella is responsible for 8000 to 18,000 hospitalizations for pneumonia annually.1 It is associated with community‐acquired, hospital‐acquired, and travel‐associated pneumonia. Twenty‐five Legionella species have been identified and 8 species are associated with pneumonia in humans.2 Community‐acquired and travel‐acquired Legionella pneumonia is most commonly caused by Legionella pneumophila; the second most common cause is L. micdadei.2, 3 It was initially identified in 1977 at the University of Pittsburgh in renal transplant patients with acute pneumonitis and is known as the Pittsburgh pneumonia agent. Similar cases were identified in a group of immunocompromised patients in Virginia, all of whom were receiving steroids and cytotoxic chemotherapy. It is unclear why L. micdadei predominates in this population, but is likely related to its decreased virulence compared to L. pneumophila. The definitive mode of transmission of L. micdadei is not known; it may be from contaminated water supplies but infections from inhalation of respiratory secretions have also been documented.2 While L. micdadei is not commonly seen in travel‐associated Legionella pneumonia, the patient's immunocompromised status secondary to the treatment of her underlying medical conditions made her particularly vulnerable. Given the temporal association with her trip, she was most likely exposed during her travels but her hospital employment should also be considered.
Legionella pneumonia is underdiagnosed because of difficulty distinguishing it from other types of pneumonia, failure to order diagnostic tests, and variable sensitivity of available diagnostic tests.4 Culture is considered the gold standard and is ideally performed from lower respiratory secretions, but variable sensitivity due to interlaboratory variation (range, 10%‐80%) limits its use.3, 4 Direct immunofluorescence assay (DFA) testing of respiratory secretions is available but also limited by poor sensitivity. Both culture and DFA have specificities approaching 100%. A newer test, the Legionella urinary antigen test, is an immunochromatographic assay. It is less technically difficult and results are available in less than 1 hour. The assay can detect the antigen in the urine starting 1 day after the onset of symptoms, and can remain positive for days or weeks following treatment.4
With the introduction and wide availability of the Legionella urinary antigen test, it is important to consider its limitations. While the test carries a high specificity, it detects only the soluble antigen of Legionella pneumophila serogroup 1. Thus, as in this case, the urinary test can be negative when infection is caused by other species such as L. micdadei. In the literature, the urine assay's sensitivity is variously reported at 45% to 100% with lower sensitivities in circumstances such as hospital‐acquired disease, where the association with other species is higher than in the community setting.3, 4 For instance, in nosocomial infections, the reported sensitivity is 45%.3 False‐positive results have also been seen in patients with serum sickness.4
The Legionella urinary antigen test has improved detection of Legionella pneumonia. Given its limitations, it is likely to be most accurate in community‐acquired and travel‐acquired cases.3 The Centers for Disease Control and Prevention recommend testing for Legionella in pneumonia patients requiring admission to the intensive care unit (ICU), immunocompromised patients, patients who traveled within 2 weeks of presentation, and those who have failed treatment with beta‐lactams or cephalosporins. A negative test does not rule out Legionella infection and additional testing with bronchoscopy may be indicated, especially in immunocompromised hosts.4
A 33‐year‐old Caucasian woman presented to an outside hospital with a 10‐day history of fever, cough, and progressive dyspnea on exertion. Ten days prior to the onset of symptoms, she had traveled to Calgary, Alberta, Canada. Her niece and nephew had recently suffered upper respiratory symptoms. Additional review of systems was negative for joint pain, rash, diarrhea, or bloody stools. She had a history of ulcerative colitis, primary sclerosing cholangitis, and juvenile rheumatoid arthritis. Her outpatient medications included prednisone 10 mg daily, methotrexate 7.5 mg weekly, and ursodiol 200 mg 3 times daily. She was employed at a local hospital and her annual purified protein derivative (PPD) test had been negative. Computed tomography angiography demonstrated bilateral patchy consolidation. Vancomycin, levofloxacin, piperacillin/tazobactam, and fluconazole were initiated and she was transferred to our hospital for further evaluation.
On arrival, her vital signs were within normal limits. She was breathing comfortably but on auscultation had crackles at the right‐mid lung field. A complete blood cell count demonstrated a white blood cell count of 7000/L with left shift, hemoglobin 10.7 g/dL, and platelet count 156,000/L. Liver function tests showed albumin 2.6 g/dL, total bilirubin 9.0 mg/dL with conjugated fraction 6.6 mg/dL, alkaline phosphatase 586 U/L, aspartate aminotransferase 104 U/L, and alanine aminotransferase 72 U/L; these were all near her baseline. The basic metabolic panel was within normal limits. A chest X‐ray showed dense areas of consolidation in the lingula and left upper lobe. All antibiotics from the outside hospital were discontinued and empiric moxifloxacin was initiated.
On hospital day 1, she underwent bronchoscopy, which yielded cloudy fluid from the bronchoalveolar lavage (BAL). Initial BAL gram stain showed moderate white blood cells but no organisms; fungal smears and stains for acid fast bacilli were negative. Blood cultures and Legionella and Streptococcus urinary antigen tests were negative. The remainder of her hospital course was uneventful. Her shortness of breath improved and she remained afebrile. She was discharged home on a 10‐day course of moxifloxacin with close follow‐up. Six days after the BAL specimen was collected, the culture grew Legionella micdadei. Repeat chest film 2 weeks later demonstrated resolution of the original findings.
DISCUSSION
Legionella is responsible for 8000 to 18,000 hospitalizations for pneumonia annually.1 It is associated with community‐acquired, hospital‐acquired, and travel‐associated pneumonia. Twenty‐five Legionella species have been identified and 8 species are associated with pneumonia in humans.2 Community‐acquired and travel‐acquired Legionella pneumonia is most commonly caused by Legionella pneumophila; the second most common cause is L. micdadei.2, 3 It was initially identified in 1977 at the University of Pittsburgh in renal transplant patients with acute pneumonitis and is known as the Pittsburgh pneumonia agent. Similar cases were identified in a group of immunocompromised patients in Virginia, all of whom were receiving steroids and cytotoxic chemotherapy. It is unclear why L. micdadei predominates in this population, but is likely related to its decreased virulence compared to L. pneumophila. The definitive mode of transmission of L. micdadei is not known; it may be from contaminated water supplies but infections from inhalation of respiratory secretions have also been documented.2 While L. micdadei is not commonly seen in travel‐associated Legionella pneumonia, the patient's immunocompromised status secondary to the treatment of her underlying medical conditions made her particularly vulnerable. Given the temporal association with her trip, she was most likely exposed during her travels but her hospital employment should also be considered.
Legionella pneumonia is underdiagnosed because of difficulty distinguishing it from other types of pneumonia, failure to order diagnostic tests, and variable sensitivity of available diagnostic tests.4 Culture is considered the gold standard and is ideally performed from lower respiratory secretions, but variable sensitivity due to interlaboratory variation (range, 10%‐80%) limits its use.3, 4 Direct immunofluorescence assay (DFA) testing of respiratory secretions is available but also limited by poor sensitivity. Both culture and DFA have specificities approaching 100%. A newer test, the Legionella urinary antigen test, is an immunochromatographic assay. It is less technically difficult and results are available in less than 1 hour. The assay can detect the antigen in the urine starting 1 day after the onset of symptoms, and can remain positive for days or weeks following treatment.4
With the introduction and wide availability of the Legionella urinary antigen test, it is important to consider its limitations. While the test carries a high specificity, it detects only the soluble antigen of Legionella pneumophila serogroup 1. Thus, as in this case, the urinary test can be negative when infection is caused by other species such as L. micdadei. In the literature, the urine assay's sensitivity is variously reported at 45% to 100% with lower sensitivities in circumstances such as hospital‐acquired disease, where the association with other species is higher than in the community setting.3, 4 For instance, in nosocomial infections, the reported sensitivity is 45%.3 False‐positive results have also been seen in patients with serum sickness.4
The Legionella urinary antigen test has improved detection of Legionella pneumonia. Given its limitations, it is likely to be most accurate in community‐acquired and travel‐acquired cases.3 The Centers for Disease Control and Prevention recommend testing for Legionella in pneumonia patients requiring admission to the intensive care unit (ICU), immunocompromised patients, patients who traveled within 2 weeks of presentation, and those who have failed treatment with beta‐lactams or cephalosporins. A negative test does not rule out Legionella infection and additional testing with bronchoscopy may be indicated, especially in immunocompromised hosts.4
- Centers for Disease Control. Legionellosis Resource Site (Legionnaires' Disease and Pontiac Fever). Top 10 Things Every Clinician Needs to Know About Legionellosis. Available at http://www.cdc.gov/legionella/top10.htm. Accessed February2009.
- ,,.Disease due to the legionellaceae (other than Legionella pneumophila): historical, microbiological, clinical, and epidemiological review.Medicine.1989;68:116–132.
- ,,, et al.Clinical utility of urinary antigen detection for diagnosis of community‐acquired, travel‐associated, and nosocomial legionnaire's disease.J Clin Microbiol.2003;41(2):838–840.
- .Diagnosis of Legionella infection.Clin Infect Dis.2003;36:64–69.
- Centers for Disease Control. Legionellosis Resource Site (Legionnaires' Disease and Pontiac Fever). Top 10 Things Every Clinician Needs to Know About Legionellosis. Available at http://www.cdc.gov/legionella/top10.htm. Accessed February2009.
- ,,.Disease due to the legionellaceae (other than Legionella pneumophila): historical, microbiological, clinical, and epidemiological review.Medicine.1989;68:116–132.
- ,,, et al.Clinical utility of urinary antigen detection for diagnosis of community‐acquired, travel‐associated, and nosocomial legionnaire's disease.J Clin Microbiol.2003;41(2):838–840.
- .Diagnosis of Legionella infection.Clin Infect Dis.2003;36:64–69.
Pediatric Hospital Medicine Fellowships
The field of pediatric hospital medicine is undergoing rapid growth. In 2002, there were approximately 600 pediatric hospitalists1 and in 2006 this number was estimated to be approximately 1000.2 A recent study found that approximately 25% of pediatric hospitalist practices are less than 2 years old.3 As such, there are many new physicians entering the field and most do so without specific training in hospital medicine prior to beginning their employment.4 There is also significant variability in the roles, work patterns, and scope of practice across institutions,3 and hospitalists are engaged in a wide variety of clinical, educational, and administrative functions.
A survey of pediatric department chairs in 2001 found that very few believed that any additional training beyond a pediatric residency was required to perform hospitalist medicine.5 However, since then the field has undergone significant growth. A more recent survey of practicing hospitalists found that 92% believed there was a need for additional training in a variety of domains.6 Specifically, respondents were most interested in achieving greater skill in performing critical care procedures and academic training. These hospitalists regarded pediatric hospitalist fellowships as the best way to gain the additional skills in teaching, research, and administration needed for their positions.
Nonetheless, for a variety of reasons, not the least of which is perhaps the paucity of hospitalist fellowship training programs, few hospitalists in practice today have completed a fellowship in hospital medicine. Over the past several years, a number of pediatric‐specific hospitalist fellowship programs have been initiated, yet little is known of their requirements or curricula. We conducted a study to explore the structure, components, and training goals of the pediatric hospitalist fellowship programs in North America.
MATERIALS AND METHODS
Sample
To examine the characteristics of pediatric hospitalist training in North America, we examined all 8 fellowships or training programs that were in existence in early 2007. The total sample included the following sites: Children's Hospital Boston, Children's Specialists of San Diego, Children's National Medical Center, Children's Healthcare of Atlanta, Texas Children's Hospital, All Children's Hospital, University of North Carolina, and The Hospital for Sick Children.
Survey Instrument
We constructed a 17‐item structured questionnaire to be administered by phone. The instrument was designed to be completed in approximately 10 minutes. Questionnaire items focused on documenting the goals, training, requirements, and clinical duties that characterize current pediatric hospitalist training programs. The questionnaire was comprised of a mixture of fixed‐choice and open‐ended questions. A draft of the instrument was shared with representatives of the Society of Hospital Medicine Pediatrics Committee for comment and suggestions.
Questionnaire Administration
The research team sent a prenotification letter to directors of the 8 pediatric hospitalist training programs to inform them of the research study. From February through June 2007, research staff contacted the directors of the programs, explained the purpose of the study, and obtained verbal consent.
Data Analysis
Responses were reviewed to compare and contrast the characteristics of the various programs. The study was approved by the University of Michigan Medical Institutional Review Board.
RESULTS
Response Rate
Of the 8 training programs, all completed the survey, representing a response rate of 100%. One institution offers 2 separate fellowship paths: academic and clinical.
Pediatric Hospitalist Fellowship and Training Program Overview
The first pediatric hospital medicine fellowship was initiated 15 years ago. However, the majority of pediatric hospitalist training programs in North America were established more recently, between 2003 and 2007.
Most pediatric hospitalist training programs offer 1 position per year. The duration of the training programs range from 1 to 3 years. Minimum clinical duties required by the programs vary from 4 to 8 months and the maximum amount of clinical time permitted ranges from 4 to 20 months. Most programs indicated that there is some flexibility in the clinical duties required or available to the fellows.
Six of the 8 programs offer an academic degree. Table 1 provides an overview of the programs, types of degrees offered, and funding sources for academic work. Subsequent tables provide blinded results to protect respondent confidentiality.
| Program | Year Established | Division | Number of Positions, 2007 | Duration of Program | Minimum Clinical Time | Maximum Clinical Time | Degree Possible? | Who Pays for Degree? |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Toronto‐Academic | 1992 | Pediatric medicine | 3 | 2 years | 4 months | 4 months | Yes: fellow's choice | Fellow |
| Children's Boston | 1998 | Emergency medicine | 1 | 2 years | 8 months | 12 months | Yes: MPH, MEd, MPP | Depart. funds; Externalfunds (creative) |
| Children's National | 2003 | Hospital medicine | 1‐2 | 2‐3 years | 6 months | 20 months | Yes: MPH | Faculty benefits |
| Children's Spec. San Diego | 2003 | Hospital medicine | 1 | 1‐2 years | 7 months | NA | Yes: MAS | Division |
| Toronto‐Clinical | 2004 | Pediatric medicine | 1 | 1 year | 8 months | 8 months | No | NA |
| Texas | 2005 | Emergency medicine | 1 | 2 years | 8 months | 8 months | Yes: MPH, MME | Varies |
| University of North Carolina | 2006 | General pediatrics and adolescent medicine | 1 | 1 year | 5 months | 6 months | No | NA |
| All Children's | 2007 | General pediatrics | 1 | 2 years | 8 months | 9 months | Yes: MPH, MS | External funding pending (federal grants) |
| Children's Atlanta | 2007 | Pediatric hospitalist section | 1 | 1 year | 6 months | 6 months | No | NA |
The number of fellowship or training program positions available each year has remained fairly consistent. However, to date, enrollment has not kept up with position availability (Table 2).
| Program | 2006‐2007 Positions Available | 2006‐2007 Fellows Enrolled | 2007‐2008 Positions Available |
|---|---|---|---|
| A | NA | NA | 1 |
| B | 2 | 1 | 2 |
| C | 1 | 1 | 1 |
| D | NA | NA | 1 |
| E | 1 | 0 | 2 |
| F | 1 | 0 | 1 |
| G | 2 | 0 | 3 |
| H | 1 | 2 | 1 |
| I | 1 | 1 | 0 |
Program Goals
Seven out of 8 programs reported the provision of advanced training in the clinical care of hospitalized patients, quality improvement (QI), and hospital administration to be central goals of their training program. Six respondents reported the provision of training in the education of medical students and residents to be a primary goal of their program, while 5 indicated training in health services research to be a primary goal.
Participation in General Hospital Activities
Trainees in all programs participate in clinical care, resident education, student education, research activities, and hospital committees. Seven out of 8 programs reported that fellows or trainees participate in patient safety activities and guideline development.
Formal Training
Half of the programs reported that they provide formal coursework in areas of education and hospital administration including quality improvement, resident teaching, and student teaching. Three of the 8 programs provide formal coursework in hospital economics.
Three of the 8 programs provide seminars in resident teaching, student teaching, hospital economics, and leading a healthcare team (Table 3).
| Programs | Resident Teaching | Student Teaching | Hospital Economics | Quality Improvement | Leading a Healthcare Team | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coursework | Seminars | Coursework | Seminars | Coursework | Seminars | Coursework | Seminars | Coursework | Seminars | |
| ||||||||||
| A | Yes | Yes | Yes | Yes | ||||||
| B | Yes | Yes | Yes | Yes | Yes | |||||
| C | Yes | Yes | Yes | Yes | Yes | |||||
| D | Yes | Yes | Yes | Yes | Yes | |||||
| E | Yes | Yes | Yes | Yes | ||||||
| F | Yes | |||||||||
| G | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| H | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| I | Yes | Yes | ||||||||
Seven of 8 pediatric hospitalist training programs provide formal coursework in epidemiology and research methodology. Six programs reported that they provide formal coursework in biostatistics and 5 in publications or grant writing. Four offer seminars in health economics, research methodology, and QI methodology (Table 4).
| Epidemiology | Biostatistics | Health Economics | Research Methodology | QI Methodology | Publications/Grant Writing | Translation Research | Educational Research | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | |
| ||||||||||||||||
| A | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| B | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||
| C | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||
| D | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||
| E | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||
| F | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||||
| G | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| H | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| I | ||||||||||||||||
Program Requirements
Seven pediatric hospitalist training programs require fellows to complete a research project. Six programs reported that they require fellows or trainees to complete a quality improvement project or participate on a hospital committee. Six of the programs require pediatric hospitalist fellows to attempt to present at a national meeting, and 4 programs require that fellows attempt to publish their research in a peer‐reviewed publication. Graduate degrees are required at 3 of the 8 pediatric hospitalist training programs (Table 5).
| QI Project | Research Project | Abstract/Presentation at National Meeting* | Peer‐Reviewed Publication* | Committee Participation at Hospital | Attending on General Ward Leading Resident Team | Specific Advanced Clinical Training | Graduate Degree Program | Other | |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| A | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| B | Yes | Yes | |||||||
| C | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| D | Yes | Yes | Yes | Yes | Yes | Yes | |||
| E | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| F | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| G | Yes | Yes | Yes | Yes | |||||
| H | |||||||||
| I | Yes | Yes | Yes | Journal club | |||||
Clinical Service Requirements
All programs indicated that they require the fellow or trainee to serve as an attending on the general pediatric ward. Five programs require the fellow or trainee to provide service at the fellow or PL‐3 level in the pediatric intensive care unit (PICU), anesthesia service, and transport team. Four programs reported that they require service in the emergency department, and 3 programs require service in the neonatal intensive care unit (NICU), newborn nursery, and general pediatric ward at the fellow or PL‐3 level. Only 2 programs require service in the pediatric subspecialty ward, and 1 program requires service in outpatient urgent care. No program requires primary care service (Table 6).
| PICU | NICU | Anesthesia | Primary Care (Outpatient) | Emergency Department | Urgent Care | Transport | General Pediatric Ward | Pediatric Subspecialty Ward | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Other Units | |
| |||||||||||||||||||
| A | Yes | Yes | Yes | Yes | Yes | Newborn nursery | |||||||||||||
| B | Yes | ||||||||||||||||||
| C | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Stepdown ICU | |||||||||||
| D | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||||||
| E | Yes | Yes | Yes | Yes | Yes | Child abuse, newborn nursery, subacute care rehabilitation facility | |||||||||||||
| F | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Variety of hospitals (county‐based) | |||||||||||
| G | Yes | Child abuse, consultation clinic, community‐based practice | |||||||||||||||||
| H | Yes | Child abuse, consultation clinic, community‐based practice | |||||||||||||||||
| I | Yes | Yes | Yes | Yes | Newborn nursery | ||||||||||||||
Pediatric Hospitalist Fellowship and Training Program Funding Sources
Five of the programs use department funds to finance the fellowship program. Four of the programs utilize the fellow or trainee's clinical work as a funding source. Two of the programs reported that the program is paid for through hospital funds.
Pediatric Hospitalist Fellow or Trainee Independence
Respondents indicated that fellows or trainees become increasingly independent over the course of the program. Fellows are supervised or mentored by hospitalists on staff. Half of the programs surveyed allow fellows or trainees to bill independently under certain circumstances (Table 7).
| Bill Independently? | Supervision? | |
|---|---|---|
| A | No: bill under a supervising attending | Supervised by hospitalist and given autonomy with supervision from hospitalist attending. |
| B | Yes | First couple of months during fellow's clinical period, more interaction with supervisors. Senior folks always available for consultation. |
| C | Yes: after 3 months | Clinical mentor (1 of 4 senior hospitalists) with whom they discuss patients on a more informal basis when on service. |
| D | Yes: on general wards, when functioning as attending | Fellows meet weekly with fellowship director. Hospitalist on call available for consult. |
| E | Fellows: no; faculty fellows: yes | Traditional fellowship role. Fellows complete several clinical electives with various levels of supervision. |
| F | Yes: after first 6 months | Fellows are supervised in their first year by hospitalist faculty. |
| G | No | Day to day in patient care, senior staff review as needed. Each fellow has 1 primary supervisor. When on service overnight, fellows call staff attending. |
| H | No | Day to day in patient care, senior staff review as needed. Each fellow has 1 primary supervisor. When on service overnight, fellows call staff attending. |
| I | Yes | Trainees are supervised by the director of the hospitalist program, the inpatient attending, and other hospitalists. |
DISCUSSION
There appear to be 2 distinct tracks for pediatric hospitalist training programs: clinical or academic specialization. However, this is not surprising, as most programs are relatively new and there are no standards or requirements for fellowship training from an external accrediting body. As such, the curriculum for these programs is likely driven by a combination of service requirements and local speculation on the needs of a future generation of pediatric hospitalists. Most programs also reported that they provide significant flexibility for each fellow based on their self‐perceived training needs and background.
Although there has been considerable emphasis on the potential educational role of hospitalists, formal coursework in teaching and education is not a part of the curriculum for half of the existing fellowship programs. Recent reports have demonstrated that hospitalists have received better teaching evaluations than traditional subspecialty attendings.7 However, this is in the absence of additional training in education and may reflect greater time that hospitalists might devote to their clinical trainees. The opportunity to further improve the educational training of hospitalists could be an important part of the fellowship experience.
Hospitalists have also been hypothesized to be in a prime position to either lead or have meaningful participation in quality improvement and cost‐saving efforts in the hospital setting. However, only half of programs provide formal coursework in QI and even fewer in areas of hospital economics.
Interestingly, most programs provide coursework in research methods, epidemiology, and grant writing. Requirements regarding clinical duties ranged from a minimum of 17% to a maximum of 67% of program time. It is unclear what the long‐term expectations in career achievement with regard to research will be for those physicians who spend the majority of their training time providing clinical care rather than in research. Previous authors have described the fallacy of expecting brief periods of coursework to prepare individuals for independent research careers.8 However, such coursework can certainly assist graduates of such programs to meaningfully participate in research projects and to put to valuable use their knowledge in both the educational and clinical aspects of their work. Though trainees enrolled in 1‐year programs will spend a larger proportion of their time providing clinical care based on program requirements, trainees in multiyear programs can choose to spend additional time performing clinical duties. Thus, 1 of the possible advantages of a 2‐year or 3‐year program may simply be the flexibility that the fellow has to tailor the program to his or her individual career goals.
Although previous studies have demonstrated that pediatric hospitalists may provide clinical service in a variety of hospital settings,2, 3, 911 most of the current fellowship programs do not provide extensive clinical experiences beyond the general pediatric ward. If hospitalists are to play a more comprehensive role in the care of the pediatric hospitalized patient, programs should consider expanding the scope of clinical training and exposure they provide.
The financial viability of hospitalist fellowship programs is also an important issue. If the additional training provided by these programs is felt to be of value to individual hospitals, it is likely that there will be an increase in the proportion of hospitals who wish to fund such training. A likely incentive for hospitals would be to position themselves to attract and retain hospitalists who possess a unique skill set for which they ascribe value for their patients and/or their bottom line.
Currently, in contrast to traditional, subspecialty‐based fellowships, half of the existing hospitalist fellowship programs allow hospitalist fellows to bill independently. This will have important implications both from an economic perspective, as well as relative to the perceptions of the degree of supervision provided by the respective training programs. This finding may also raise questions as to whether the need for additional clinical training after residency is really necessary to practice hospital medicine.
Whether the training and experience provided by these programs will be seen as a necessary precursor for careers in hospital medicine remains unknown. However, currently there appears to be a mismatch between what some hospitalists have identified as potential clinical educational needs6 with more than 50% desiring additional training in intensive care unit settings, and what is provided through the existing programs. In 2001, a survey of pediatric department chairs found that most did not believe additional formal training beyond residency was necessary to take on the role of a pediatric hospitalist.5 The value of pediatric hospitalist training programs may lie in their provision of or exposure to academic skill sets and the provision of administrative opportunities, in addition to targeted clinical training.
Potential Future Areas of Focus
The potential of a mismatch between education and practice or a training practice gap has been identified in internal medicine hospitalist training programs.12 To provide guidance to address this gap, Glasheen et al.13 assessed the spectrum and volume of specific diagnoses encountered in hospitals and the level of involvement of hospitalists in the care of these patients. They posit that training prioritized to the case mix expected to be encountered by hospitalists would be an appropriate concentration on which both tracked residency and fellowships could focus.
Of significant importance to many community physicians is the pattern of communication between hospitalists and the primary care physician of their patients. Recent reports have suggested this is a problem for many hospitalist programs.14 As such, it seems relevant that any hospitalist training program both develop a defined communication protocol and include instruction in physician‐to‐physician communication as a distinct part of their curriculum. Specifically, the importance of initial contact and timely discharge summaries should be addressed.
We did not explicitly ask respondents to discuss the scope of mentorship in their fellowship programs. However, based on respondents' descriptions of fellow or trainee supervision, we believe that the structure of mentorship programs likely varies across fellowships. Further study will be needed to determine the scope of mentorship in pediatric hospitalist training programs, and the impact of mentorship on training efficacy.
CONCLUSIONS
Pediatric hospitalist fellowship training programs are in the very early stages of their development. In time, greater structure across institutions will need to be put in place if they are to succeed in becoming a necessary prerequisite to the practice of hospital medicine. As the roles of hospitalists become more defined, the nature and extent of their advanced training needs will do so as well.
- ,.The emerging role of pediatric hospitalists.Clin Pediatr (Phila).2003;42(4):295–297.
- ,,, et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- ,,,, The Research Advisory Committee of the American Board of Pediatrics.Characteristics of the pediatric hospitalist workforce: its roles and work environment.Pediatrics.2007;120:33–39.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:1.e1–1.e7.
- ,,,,,.Pediatric hospitalists in Canada and the United States: a survey of pediatric academic department chairs.Ambul Pediatr.2001;1:338–339.
- ,,,.PRIS Survey: pediatric hospitalist roles and training needs [Abstract].Pediatr Res.2004;55:360A.
- ,.Third‐year medical students' evaluation of hospitalist and nonhospitalist faculty during the inpatient portion of their pediatrics clerkships.J Hosp Med.2007;2(1):17–22.
- .Challenges in the development of pediatric health services research.J Pediatr.2002;140:1–2.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31(3):847–852.
- New study highlights ingredients for reengineering success.Health Care Cost Reengineering Rep.1999;4(5):72–74,65.
- ,,.Pediatric hospitalists fill varied roles in the care of newborns.Pediatr Ann.2003;32(12):802–810.
- ,,,,.Closing the gap between internal medicine training and practice: recommendations from recent graduates.Am J Med.2005;118(6):680–685; discussion 685–687.
- ,,,,.The spectrum of community‐based hospitalist practice: a call to tailor internal medicine residency training.Arch Intern Med.2007;167(7):727–728.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
The field of pediatric hospital medicine is undergoing rapid growth. In 2002, there were approximately 600 pediatric hospitalists1 and in 2006 this number was estimated to be approximately 1000.2 A recent study found that approximately 25% of pediatric hospitalist practices are less than 2 years old.3 As such, there are many new physicians entering the field and most do so without specific training in hospital medicine prior to beginning their employment.4 There is also significant variability in the roles, work patterns, and scope of practice across institutions,3 and hospitalists are engaged in a wide variety of clinical, educational, and administrative functions.
A survey of pediatric department chairs in 2001 found that very few believed that any additional training beyond a pediatric residency was required to perform hospitalist medicine.5 However, since then the field has undergone significant growth. A more recent survey of practicing hospitalists found that 92% believed there was a need for additional training in a variety of domains.6 Specifically, respondents were most interested in achieving greater skill in performing critical care procedures and academic training. These hospitalists regarded pediatric hospitalist fellowships as the best way to gain the additional skills in teaching, research, and administration needed for their positions.
Nonetheless, for a variety of reasons, not the least of which is perhaps the paucity of hospitalist fellowship training programs, few hospitalists in practice today have completed a fellowship in hospital medicine. Over the past several years, a number of pediatric‐specific hospitalist fellowship programs have been initiated, yet little is known of their requirements or curricula. We conducted a study to explore the structure, components, and training goals of the pediatric hospitalist fellowship programs in North America.
MATERIALS AND METHODS
Sample
To examine the characteristics of pediatric hospitalist training in North America, we examined all 8 fellowships or training programs that were in existence in early 2007. The total sample included the following sites: Children's Hospital Boston, Children's Specialists of San Diego, Children's National Medical Center, Children's Healthcare of Atlanta, Texas Children's Hospital, All Children's Hospital, University of North Carolina, and The Hospital for Sick Children.
Survey Instrument
We constructed a 17‐item structured questionnaire to be administered by phone. The instrument was designed to be completed in approximately 10 minutes. Questionnaire items focused on documenting the goals, training, requirements, and clinical duties that characterize current pediatric hospitalist training programs. The questionnaire was comprised of a mixture of fixed‐choice and open‐ended questions. A draft of the instrument was shared with representatives of the Society of Hospital Medicine Pediatrics Committee for comment and suggestions.
Questionnaire Administration
The research team sent a prenotification letter to directors of the 8 pediatric hospitalist training programs to inform them of the research study. From February through June 2007, research staff contacted the directors of the programs, explained the purpose of the study, and obtained verbal consent.
Data Analysis
Responses were reviewed to compare and contrast the characteristics of the various programs. The study was approved by the University of Michigan Medical Institutional Review Board.
RESULTS
Response Rate
Of the 8 training programs, all completed the survey, representing a response rate of 100%. One institution offers 2 separate fellowship paths: academic and clinical.
Pediatric Hospitalist Fellowship and Training Program Overview
The first pediatric hospital medicine fellowship was initiated 15 years ago. However, the majority of pediatric hospitalist training programs in North America were established more recently, between 2003 and 2007.
Most pediatric hospitalist training programs offer 1 position per year. The duration of the training programs range from 1 to 3 years. Minimum clinical duties required by the programs vary from 4 to 8 months and the maximum amount of clinical time permitted ranges from 4 to 20 months. Most programs indicated that there is some flexibility in the clinical duties required or available to the fellows.
Six of the 8 programs offer an academic degree. Table 1 provides an overview of the programs, types of degrees offered, and funding sources for academic work. Subsequent tables provide blinded results to protect respondent confidentiality.
| Program | Year Established | Division | Number of Positions, 2007 | Duration of Program | Minimum Clinical Time | Maximum Clinical Time | Degree Possible? | Who Pays for Degree? |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Toronto‐Academic | 1992 | Pediatric medicine | 3 | 2 years | 4 months | 4 months | Yes: fellow's choice | Fellow |
| Children's Boston | 1998 | Emergency medicine | 1 | 2 years | 8 months | 12 months | Yes: MPH, MEd, MPP | Depart. funds; Externalfunds (creative) |
| Children's National | 2003 | Hospital medicine | 1‐2 | 2‐3 years | 6 months | 20 months | Yes: MPH | Faculty benefits |
| Children's Spec. San Diego | 2003 | Hospital medicine | 1 | 1‐2 years | 7 months | NA | Yes: MAS | Division |
| Toronto‐Clinical | 2004 | Pediatric medicine | 1 | 1 year | 8 months | 8 months | No | NA |
| Texas | 2005 | Emergency medicine | 1 | 2 years | 8 months | 8 months | Yes: MPH, MME | Varies |
| University of North Carolina | 2006 | General pediatrics and adolescent medicine | 1 | 1 year | 5 months | 6 months | No | NA |
| All Children's | 2007 | General pediatrics | 1 | 2 years | 8 months | 9 months | Yes: MPH, MS | External funding pending (federal grants) |
| Children's Atlanta | 2007 | Pediatric hospitalist section | 1 | 1 year | 6 months | 6 months | No | NA |
The number of fellowship or training program positions available each year has remained fairly consistent. However, to date, enrollment has not kept up with position availability (Table 2).
| Program | 2006‐2007 Positions Available | 2006‐2007 Fellows Enrolled | 2007‐2008 Positions Available |
|---|---|---|---|
| A | NA | NA | 1 |
| B | 2 | 1 | 2 |
| C | 1 | 1 | 1 |
| D | NA | NA | 1 |
| E | 1 | 0 | 2 |
| F | 1 | 0 | 1 |
| G | 2 | 0 | 3 |
| H | 1 | 2 | 1 |
| I | 1 | 1 | 0 |
Program Goals
Seven out of 8 programs reported the provision of advanced training in the clinical care of hospitalized patients, quality improvement (QI), and hospital administration to be central goals of their training program. Six respondents reported the provision of training in the education of medical students and residents to be a primary goal of their program, while 5 indicated training in health services research to be a primary goal.
Participation in General Hospital Activities
Trainees in all programs participate in clinical care, resident education, student education, research activities, and hospital committees. Seven out of 8 programs reported that fellows or trainees participate in patient safety activities and guideline development.
Formal Training
Half of the programs reported that they provide formal coursework in areas of education and hospital administration including quality improvement, resident teaching, and student teaching. Three of the 8 programs provide formal coursework in hospital economics.
Three of the 8 programs provide seminars in resident teaching, student teaching, hospital economics, and leading a healthcare team (Table 3).
| Programs | Resident Teaching | Student Teaching | Hospital Economics | Quality Improvement | Leading a Healthcare Team | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coursework | Seminars | Coursework | Seminars | Coursework | Seminars | Coursework | Seminars | Coursework | Seminars | |
| ||||||||||
| A | Yes | Yes | Yes | Yes | ||||||
| B | Yes | Yes | Yes | Yes | Yes | |||||
| C | Yes | Yes | Yes | Yes | Yes | |||||
| D | Yes | Yes | Yes | Yes | Yes | |||||
| E | Yes | Yes | Yes | Yes | ||||||
| F | Yes | |||||||||
| G | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| H | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| I | Yes | Yes | ||||||||
Seven of 8 pediatric hospitalist training programs provide formal coursework in epidemiology and research methodology. Six programs reported that they provide formal coursework in biostatistics and 5 in publications or grant writing. Four offer seminars in health economics, research methodology, and QI methodology (Table 4).
| Epidemiology | Biostatistics | Health Economics | Research Methodology | QI Methodology | Publications/Grant Writing | Translation Research | Educational Research | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | |
| ||||||||||||||||
| A | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| B | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||
| C | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||
| D | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||
| E | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||
| F | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||||
| G | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| H | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| I | ||||||||||||||||
Program Requirements
Seven pediatric hospitalist training programs require fellows to complete a research project. Six programs reported that they require fellows or trainees to complete a quality improvement project or participate on a hospital committee. Six of the programs require pediatric hospitalist fellows to attempt to present at a national meeting, and 4 programs require that fellows attempt to publish their research in a peer‐reviewed publication. Graduate degrees are required at 3 of the 8 pediatric hospitalist training programs (Table 5).
| QI Project | Research Project | Abstract/Presentation at National Meeting* | Peer‐Reviewed Publication* | Committee Participation at Hospital | Attending on General Ward Leading Resident Team | Specific Advanced Clinical Training | Graduate Degree Program | Other | |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| A | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| B | Yes | Yes | |||||||
| C | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| D | Yes | Yes | Yes | Yes | Yes | Yes | |||
| E | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| F | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| G | Yes | Yes | Yes | Yes | |||||
| H | |||||||||
| I | Yes | Yes | Yes | Journal club | |||||
Clinical Service Requirements
All programs indicated that they require the fellow or trainee to serve as an attending on the general pediatric ward. Five programs require the fellow or trainee to provide service at the fellow or PL‐3 level in the pediatric intensive care unit (PICU), anesthesia service, and transport team. Four programs reported that they require service in the emergency department, and 3 programs require service in the neonatal intensive care unit (NICU), newborn nursery, and general pediatric ward at the fellow or PL‐3 level. Only 2 programs require service in the pediatric subspecialty ward, and 1 program requires service in outpatient urgent care. No program requires primary care service (Table 6).
| PICU | NICU | Anesthesia | Primary Care (Outpatient) | Emergency Department | Urgent Care | Transport | General Pediatric Ward | Pediatric Subspecialty Ward | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Other Units | |
| |||||||||||||||||||
| A | Yes | Yes | Yes | Yes | Yes | Newborn nursery | |||||||||||||
| B | Yes | ||||||||||||||||||
| C | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Stepdown ICU | |||||||||||
| D | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||||||
| E | Yes | Yes | Yes | Yes | Yes | Child abuse, newborn nursery, subacute care rehabilitation facility | |||||||||||||
| F | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Variety of hospitals (county‐based) | |||||||||||
| G | Yes | Child abuse, consultation clinic, community‐based practice | |||||||||||||||||
| H | Yes | Child abuse, consultation clinic, community‐based practice | |||||||||||||||||
| I | Yes | Yes | Yes | Yes | Newborn nursery | ||||||||||||||
Pediatric Hospitalist Fellowship and Training Program Funding Sources
Five of the programs use department funds to finance the fellowship program. Four of the programs utilize the fellow or trainee's clinical work as a funding source. Two of the programs reported that the program is paid for through hospital funds.
Pediatric Hospitalist Fellow or Trainee Independence
Respondents indicated that fellows or trainees become increasingly independent over the course of the program. Fellows are supervised or mentored by hospitalists on staff. Half of the programs surveyed allow fellows or trainees to bill independently under certain circumstances (Table 7).
| Bill Independently? | Supervision? | |
|---|---|---|
| A | No: bill under a supervising attending | Supervised by hospitalist and given autonomy with supervision from hospitalist attending. |
| B | Yes | First couple of months during fellow's clinical period, more interaction with supervisors. Senior folks always available for consultation. |
| C | Yes: after 3 months | Clinical mentor (1 of 4 senior hospitalists) with whom they discuss patients on a more informal basis when on service. |
| D | Yes: on general wards, when functioning as attending | Fellows meet weekly with fellowship director. Hospitalist on call available for consult. |
| E | Fellows: no; faculty fellows: yes | Traditional fellowship role. Fellows complete several clinical electives with various levels of supervision. |
| F | Yes: after first 6 months | Fellows are supervised in their first year by hospitalist faculty. |
| G | No | Day to day in patient care, senior staff review as needed. Each fellow has 1 primary supervisor. When on service overnight, fellows call staff attending. |
| H | No | Day to day in patient care, senior staff review as needed. Each fellow has 1 primary supervisor. When on service overnight, fellows call staff attending. |
| I | Yes | Trainees are supervised by the director of the hospitalist program, the inpatient attending, and other hospitalists. |
DISCUSSION
There appear to be 2 distinct tracks for pediatric hospitalist training programs: clinical or academic specialization. However, this is not surprising, as most programs are relatively new and there are no standards or requirements for fellowship training from an external accrediting body. As such, the curriculum for these programs is likely driven by a combination of service requirements and local speculation on the needs of a future generation of pediatric hospitalists. Most programs also reported that they provide significant flexibility for each fellow based on their self‐perceived training needs and background.
Although there has been considerable emphasis on the potential educational role of hospitalists, formal coursework in teaching and education is not a part of the curriculum for half of the existing fellowship programs. Recent reports have demonstrated that hospitalists have received better teaching evaluations than traditional subspecialty attendings.7 However, this is in the absence of additional training in education and may reflect greater time that hospitalists might devote to their clinical trainees. The opportunity to further improve the educational training of hospitalists could be an important part of the fellowship experience.
Hospitalists have also been hypothesized to be in a prime position to either lead or have meaningful participation in quality improvement and cost‐saving efforts in the hospital setting. However, only half of programs provide formal coursework in QI and even fewer in areas of hospital economics.
Interestingly, most programs provide coursework in research methods, epidemiology, and grant writing. Requirements regarding clinical duties ranged from a minimum of 17% to a maximum of 67% of program time. It is unclear what the long‐term expectations in career achievement with regard to research will be for those physicians who spend the majority of their training time providing clinical care rather than in research. Previous authors have described the fallacy of expecting brief periods of coursework to prepare individuals for independent research careers.8 However, such coursework can certainly assist graduates of such programs to meaningfully participate in research projects and to put to valuable use their knowledge in both the educational and clinical aspects of their work. Though trainees enrolled in 1‐year programs will spend a larger proportion of their time providing clinical care based on program requirements, trainees in multiyear programs can choose to spend additional time performing clinical duties. Thus, 1 of the possible advantages of a 2‐year or 3‐year program may simply be the flexibility that the fellow has to tailor the program to his or her individual career goals.
Although previous studies have demonstrated that pediatric hospitalists may provide clinical service in a variety of hospital settings,2, 3, 911 most of the current fellowship programs do not provide extensive clinical experiences beyond the general pediatric ward. If hospitalists are to play a more comprehensive role in the care of the pediatric hospitalized patient, programs should consider expanding the scope of clinical training and exposure they provide.
The financial viability of hospitalist fellowship programs is also an important issue. If the additional training provided by these programs is felt to be of value to individual hospitals, it is likely that there will be an increase in the proportion of hospitals who wish to fund such training. A likely incentive for hospitals would be to position themselves to attract and retain hospitalists who possess a unique skill set for which they ascribe value for their patients and/or their bottom line.
Currently, in contrast to traditional, subspecialty‐based fellowships, half of the existing hospitalist fellowship programs allow hospitalist fellows to bill independently. This will have important implications both from an economic perspective, as well as relative to the perceptions of the degree of supervision provided by the respective training programs. This finding may also raise questions as to whether the need for additional clinical training after residency is really necessary to practice hospital medicine.
Whether the training and experience provided by these programs will be seen as a necessary precursor for careers in hospital medicine remains unknown. However, currently there appears to be a mismatch between what some hospitalists have identified as potential clinical educational needs6 with more than 50% desiring additional training in intensive care unit settings, and what is provided through the existing programs. In 2001, a survey of pediatric department chairs found that most did not believe additional formal training beyond residency was necessary to take on the role of a pediatric hospitalist.5 The value of pediatric hospitalist training programs may lie in their provision of or exposure to academic skill sets and the provision of administrative opportunities, in addition to targeted clinical training.
Potential Future Areas of Focus
The potential of a mismatch between education and practice or a training practice gap has been identified in internal medicine hospitalist training programs.12 To provide guidance to address this gap, Glasheen et al.13 assessed the spectrum and volume of specific diagnoses encountered in hospitals and the level of involvement of hospitalists in the care of these patients. They posit that training prioritized to the case mix expected to be encountered by hospitalists would be an appropriate concentration on which both tracked residency and fellowships could focus.
Of significant importance to many community physicians is the pattern of communication between hospitalists and the primary care physician of their patients. Recent reports have suggested this is a problem for many hospitalist programs.14 As such, it seems relevant that any hospitalist training program both develop a defined communication protocol and include instruction in physician‐to‐physician communication as a distinct part of their curriculum. Specifically, the importance of initial contact and timely discharge summaries should be addressed.
We did not explicitly ask respondents to discuss the scope of mentorship in their fellowship programs. However, based on respondents' descriptions of fellow or trainee supervision, we believe that the structure of mentorship programs likely varies across fellowships. Further study will be needed to determine the scope of mentorship in pediatric hospitalist training programs, and the impact of mentorship on training efficacy.
CONCLUSIONS
Pediatric hospitalist fellowship training programs are in the very early stages of their development. In time, greater structure across institutions will need to be put in place if they are to succeed in becoming a necessary prerequisite to the practice of hospital medicine. As the roles of hospitalists become more defined, the nature and extent of their advanced training needs will do so as well.
The field of pediatric hospital medicine is undergoing rapid growth. In 2002, there were approximately 600 pediatric hospitalists1 and in 2006 this number was estimated to be approximately 1000.2 A recent study found that approximately 25% of pediatric hospitalist practices are less than 2 years old.3 As such, there are many new physicians entering the field and most do so without specific training in hospital medicine prior to beginning their employment.4 There is also significant variability in the roles, work patterns, and scope of practice across institutions,3 and hospitalists are engaged in a wide variety of clinical, educational, and administrative functions.
A survey of pediatric department chairs in 2001 found that very few believed that any additional training beyond a pediatric residency was required to perform hospitalist medicine.5 However, since then the field has undergone significant growth. A more recent survey of practicing hospitalists found that 92% believed there was a need for additional training in a variety of domains.6 Specifically, respondents were most interested in achieving greater skill in performing critical care procedures and academic training. These hospitalists regarded pediatric hospitalist fellowships as the best way to gain the additional skills in teaching, research, and administration needed for their positions.
Nonetheless, for a variety of reasons, not the least of which is perhaps the paucity of hospitalist fellowship training programs, few hospitalists in practice today have completed a fellowship in hospital medicine. Over the past several years, a number of pediatric‐specific hospitalist fellowship programs have been initiated, yet little is known of their requirements or curricula. We conducted a study to explore the structure, components, and training goals of the pediatric hospitalist fellowship programs in North America.
MATERIALS AND METHODS
Sample
To examine the characteristics of pediatric hospitalist training in North America, we examined all 8 fellowships or training programs that were in existence in early 2007. The total sample included the following sites: Children's Hospital Boston, Children's Specialists of San Diego, Children's National Medical Center, Children's Healthcare of Atlanta, Texas Children's Hospital, All Children's Hospital, University of North Carolina, and The Hospital for Sick Children.
Survey Instrument
We constructed a 17‐item structured questionnaire to be administered by phone. The instrument was designed to be completed in approximately 10 minutes. Questionnaire items focused on documenting the goals, training, requirements, and clinical duties that characterize current pediatric hospitalist training programs. The questionnaire was comprised of a mixture of fixed‐choice and open‐ended questions. A draft of the instrument was shared with representatives of the Society of Hospital Medicine Pediatrics Committee for comment and suggestions.
Questionnaire Administration
The research team sent a prenotification letter to directors of the 8 pediatric hospitalist training programs to inform them of the research study. From February through June 2007, research staff contacted the directors of the programs, explained the purpose of the study, and obtained verbal consent.
Data Analysis
Responses were reviewed to compare and contrast the characteristics of the various programs. The study was approved by the University of Michigan Medical Institutional Review Board.
RESULTS
Response Rate
Of the 8 training programs, all completed the survey, representing a response rate of 100%. One institution offers 2 separate fellowship paths: academic and clinical.
Pediatric Hospitalist Fellowship and Training Program Overview
The first pediatric hospital medicine fellowship was initiated 15 years ago. However, the majority of pediatric hospitalist training programs in North America were established more recently, between 2003 and 2007.
Most pediatric hospitalist training programs offer 1 position per year. The duration of the training programs range from 1 to 3 years. Minimum clinical duties required by the programs vary from 4 to 8 months and the maximum amount of clinical time permitted ranges from 4 to 20 months. Most programs indicated that there is some flexibility in the clinical duties required or available to the fellows.
Six of the 8 programs offer an academic degree. Table 1 provides an overview of the programs, types of degrees offered, and funding sources for academic work. Subsequent tables provide blinded results to protect respondent confidentiality.
| Program | Year Established | Division | Number of Positions, 2007 | Duration of Program | Minimum Clinical Time | Maximum Clinical Time | Degree Possible? | Who Pays for Degree? |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Toronto‐Academic | 1992 | Pediatric medicine | 3 | 2 years | 4 months | 4 months | Yes: fellow's choice | Fellow |
| Children's Boston | 1998 | Emergency medicine | 1 | 2 years | 8 months | 12 months | Yes: MPH, MEd, MPP | Depart. funds; Externalfunds (creative) |
| Children's National | 2003 | Hospital medicine | 1‐2 | 2‐3 years | 6 months | 20 months | Yes: MPH | Faculty benefits |
| Children's Spec. San Diego | 2003 | Hospital medicine | 1 | 1‐2 years | 7 months | NA | Yes: MAS | Division |
| Toronto‐Clinical | 2004 | Pediatric medicine | 1 | 1 year | 8 months | 8 months | No | NA |
| Texas | 2005 | Emergency medicine | 1 | 2 years | 8 months | 8 months | Yes: MPH, MME | Varies |
| University of North Carolina | 2006 | General pediatrics and adolescent medicine | 1 | 1 year | 5 months | 6 months | No | NA |
| All Children's | 2007 | General pediatrics | 1 | 2 years | 8 months | 9 months | Yes: MPH, MS | External funding pending (federal grants) |
| Children's Atlanta | 2007 | Pediatric hospitalist section | 1 | 1 year | 6 months | 6 months | No | NA |
The number of fellowship or training program positions available each year has remained fairly consistent. However, to date, enrollment has not kept up with position availability (Table 2).
| Program | 2006‐2007 Positions Available | 2006‐2007 Fellows Enrolled | 2007‐2008 Positions Available |
|---|---|---|---|
| A | NA | NA | 1 |
| B | 2 | 1 | 2 |
| C | 1 | 1 | 1 |
| D | NA | NA | 1 |
| E | 1 | 0 | 2 |
| F | 1 | 0 | 1 |
| G | 2 | 0 | 3 |
| H | 1 | 2 | 1 |
| I | 1 | 1 | 0 |
Program Goals
Seven out of 8 programs reported the provision of advanced training in the clinical care of hospitalized patients, quality improvement (QI), and hospital administration to be central goals of their training program. Six respondents reported the provision of training in the education of medical students and residents to be a primary goal of their program, while 5 indicated training in health services research to be a primary goal.
Participation in General Hospital Activities
Trainees in all programs participate in clinical care, resident education, student education, research activities, and hospital committees. Seven out of 8 programs reported that fellows or trainees participate in patient safety activities and guideline development.
Formal Training
Half of the programs reported that they provide formal coursework in areas of education and hospital administration including quality improvement, resident teaching, and student teaching. Three of the 8 programs provide formal coursework in hospital economics.
Three of the 8 programs provide seminars in resident teaching, student teaching, hospital economics, and leading a healthcare team (Table 3).
| Programs | Resident Teaching | Student Teaching | Hospital Economics | Quality Improvement | Leading a Healthcare Team | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coursework | Seminars | Coursework | Seminars | Coursework | Seminars | Coursework | Seminars | Coursework | Seminars | |
| ||||||||||
| A | Yes | Yes | Yes | Yes | ||||||
| B | Yes | Yes | Yes | Yes | Yes | |||||
| C | Yes | Yes | Yes | Yes | Yes | |||||
| D | Yes | Yes | Yes | Yes | Yes | |||||
| E | Yes | Yes | Yes | Yes | ||||||
| F | Yes | |||||||||
| G | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| H | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| I | Yes | Yes | ||||||||
Seven of 8 pediatric hospitalist training programs provide formal coursework in epidemiology and research methodology. Six programs reported that they provide formal coursework in biostatistics and 5 in publications or grant writing. Four offer seminars in health economics, research methodology, and QI methodology (Table 4).
| Epidemiology | Biostatistics | Health Economics | Research Methodology | QI Methodology | Publications/Grant Writing | Translation Research | Educational Research | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | Course | Seminar | |
| ||||||||||||||||
| A | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| B | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||
| C | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||
| D | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||
| E | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||
| F | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||||
| G | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| H | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| I | ||||||||||||||||
Program Requirements
Seven pediatric hospitalist training programs require fellows to complete a research project. Six programs reported that they require fellows or trainees to complete a quality improvement project or participate on a hospital committee. Six of the programs require pediatric hospitalist fellows to attempt to present at a national meeting, and 4 programs require that fellows attempt to publish their research in a peer‐reviewed publication. Graduate degrees are required at 3 of the 8 pediatric hospitalist training programs (Table 5).
| QI Project | Research Project | Abstract/Presentation at National Meeting* | Peer‐Reviewed Publication* | Committee Participation at Hospital | Attending on General Ward Leading Resident Team | Specific Advanced Clinical Training | Graduate Degree Program | Other | |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| A | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| B | Yes | Yes | |||||||
| C | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| D | Yes | Yes | Yes | Yes | Yes | Yes | |||
| E | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| F | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| G | Yes | Yes | Yes | Yes | |||||
| H | |||||||||
| I | Yes | Yes | Yes | Journal club | |||||
Clinical Service Requirements
All programs indicated that they require the fellow or trainee to serve as an attending on the general pediatric ward. Five programs require the fellow or trainee to provide service at the fellow or PL‐3 level in the pediatric intensive care unit (PICU), anesthesia service, and transport team. Four programs reported that they require service in the emergency department, and 3 programs require service in the neonatal intensive care unit (NICU), newborn nursery, and general pediatric ward at the fellow or PL‐3 level. Only 2 programs require service in the pediatric subspecialty ward, and 1 program requires service in outpatient urgent care. No program requires primary care service (Table 6).
| PICU | NICU | Anesthesia | Primary Care (Outpatient) | Emergency Department | Urgent Care | Transport | General Pediatric Ward | Pediatric Subspecialty Ward | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Attd | Fellow | Other Units | |
| |||||||||||||||||||
| A | Yes | Yes | Yes | Yes | Yes | Newborn nursery | |||||||||||||
| B | Yes | ||||||||||||||||||
| C | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Stepdown ICU | |||||||||||
| D | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||||||
| E | Yes | Yes | Yes | Yes | Yes | Child abuse, newborn nursery, subacute care rehabilitation facility | |||||||||||||
| F | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Variety of hospitals (county‐based) | |||||||||||
| G | Yes | Child abuse, consultation clinic, community‐based practice | |||||||||||||||||
| H | Yes | Child abuse, consultation clinic, community‐based practice | |||||||||||||||||
| I | Yes | Yes | Yes | Yes | Newborn nursery | ||||||||||||||
Pediatric Hospitalist Fellowship and Training Program Funding Sources
Five of the programs use department funds to finance the fellowship program. Four of the programs utilize the fellow or trainee's clinical work as a funding source. Two of the programs reported that the program is paid for through hospital funds.
Pediatric Hospitalist Fellow or Trainee Independence
Respondents indicated that fellows or trainees become increasingly independent over the course of the program. Fellows are supervised or mentored by hospitalists on staff. Half of the programs surveyed allow fellows or trainees to bill independently under certain circumstances (Table 7).
| Bill Independently? | Supervision? | |
|---|---|---|
| A | No: bill under a supervising attending | Supervised by hospitalist and given autonomy with supervision from hospitalist attending. |
| B | Yes | First couple of months during fellow's clinical period, more interaction with supervisors. Senior folks always available for consultation. |
| C | Yes: after 3 months | Clinical mentor (1 of 4 senior hospitalists) with whom they discuss patients on a more informal basis when on service. |
| D | Yes: on general wards, when functioning as attending | Fellows meet weekly with fellowship director. Hospitalist on call available for consult. |
| E | Fellows: no; faculty fellows: yes | Traditional fellowship role. Fellows complete several clinical electives with various levels of supervision. |
| F | Yes: after first 6 months | Fellows are supervised in their first year by hospitalist faculty. |
| G | No | Day to day in patient care, senior staff review as needed. Each fellow has 1 primary supervisor. When on service overnight, fellows call staff attending. |
| H | No | Day to day in patient care, senior staff review as needed. Each fellow has 1 primary supervisor. When on service overnight, fellows call staff attending. |
| I | Yes | Trainees are supervised by the director of the hospitalist program, the inpatient attending, and other hospitalists. |
DISCUSSION
There appear to be 2 distinct tracks for pediatric hospitalist training programs: clinical or academic specialization. However, this is not surprising, as most programs are relatively new and there are no standards or requirements for fellowship training from an external accrediting body. As such, the curriculum for these programs is likely driven by a combination of service requirements and local speculation on the needs of a future generation of pediatric hospitalists. Most programs also reported that they provide significant flexibility for each fellow based on their self‐perceived training needs and background.
Although there has been considerable emphasis on the potential educational role of hospitalists, formal coursework in teaching and education is not a part of the curriculum for half of the existing fellowship programs. Recent reports have demonstrated that hospitalists have received better teaching evaluations than traditional subspecialty attendings.7 However, this is in the absence of additional training in education and may reflect greater time that hospitalists might devote to their clinical trainees. The opportunity to further improve the educational training of hospitalists could be an important part of the fellowship experience.
Hospitalists have also been hypothesized to be in a prime position to either lead or have meaningful participation in quality improvement and cost‐saving efforts in the hospital setting. However, only half of programs provide formal coursework in QI and even fewer in areas of hospital economics.
Interestingly, most programs provide coursework in research methods, epidemiology, and grant writing. Requirements regarding clinical duties ranged from a minimum of 17% to a maximum of 67% of program time. It is unclear what the long‐term expectations in career achievement with regard to research will be for those physicians who spend the majority of their training time providing clinical care rather than in research. Previous authors have described the fallacy of expecting brief periods of coursework to prepare individuals for independent research careers.8 However, such coursework can certainly assist graduates of such programs to meaningfully participate in research projects and to put to valuable use their knowledge in both the educational and clinical aspects of their work. Though trainees enrolled in 1‐year programs will spend a larger proportion of their time providing clinical care based on program requirements, trainees in multiyear programs can choose to spend additional time performing clinical duties. Thus, 1 of the possible advantages of a 2‐year or 3‐year program may simply be the flexibility that the fellow has to tailor the program to his or her individual career goals.
Although previous studies have demonstrated that pediatric hospitalists may provide clinical service in a variety of hospital settings,2, 3, 911 most of the current fellowship programs do not provide extensive clinical experiences beyond the general pediatric ward. If hospitalists are to play a more comprehensive role in the care of the pediatric hospitalized patient, programs should consider expanding the scope of clinical training and exposure they provide.
The financial viability of hospitalist fellowship programs is also an important issue. If the additional training provided by these programs is felt to be of value to individual hospitals, it is likely that there will be an increase in the proportion of hospitals who wish to fund such training. A likely incentive for hospitals would be to position themselves to attract and retain hospitalists who possess a unique skill set for which they ascribe value for their patients and/or their bottom line.
Currently, in contrast to traditional, subspecialty‐based fellowships, half of the existing hospitalist fellowship programs allow hospitalist fellows to bill independently. This will have important implications both from an economic perspective, as well as relative to the perceptions of the degree of supervision provided by the respective training programs. This finding may also raise questions as to whether the need for additional clinical training after residency is really necessary to practice hospital medicine.
Whether the training and experience provided by these programs will be seen as a necessary precursor for careers in hospital medicine remains unknown. However, currently there appears to be a mismatch between what some hospitalists have identified as potential clinical educational needs6 with more than 50% desiring additional training in intensive care unit settings, and what is provided through the existing programs. In 2001, a survey of pediatric department chairs found that most did not believe additional formal training beyond residency was necessary to take on the role of a pediatric hospitalist.5 The value of pediatric hospitalist training programs may lie in their provision of or exposure to academic skill sets and the provision of administrative opportunities, in addition to targeted clinical training.
Potential Future Areas of Focus
The potential of a mismatch between education and practice or a training practice gap has been identified in internal medicine hospitalist training programs.12 To provide guidance to address this gap, Glasheen et al.13 assessed the spectrum and volume of specific diagnoses encountered in hospitals and the level of involvement of hospitalists in the care of these patients. They posit that training prioritized to the case mix expected to be encountered by hospitalists would be an appropriate concentration on which both tracked residency and fellowships could focus.
Of significant importance to many community physicians is the pattern of communication between hospitalists and the primary care physician of their patients. Recent reports have suggested this is a problem for many hospitalist programs.14 As such, it seems relevant that any hospitalist training program both develop a defined communication protocol and include instruction in physician‐to‐physician communication as a distinct part of their curriculum. Specifically, the importance of initial contact and timely discharge summaries should be addressed.
We did not explicitly ask respondents to discuss the scope of mentorship in their fellowship programs. However, based on respondents' descriptions of fellow or trainee supervision, we believe that the structure of mentorship programs likely varies across fellowships. Further study will be needed to determine the scope of mentorship in pediatric hospitalist training programs, and the impact of mentorship on training efficacy.
CONCLUSIONS
Pediatric hospitalist fellowship training programs are in the very early stages of their development. In time, greater structure across institutions will need to be put in place if they are to succeed in becoming a necessary prerequisite to the practice of hospital medicine. As the roles of hospitalists become more defined, the nature and extent of their advanced training needs will do so as well.
- ,.The emerging role of pediatric hospitalists.Clin Pediatr (Phila).2003;42(4):295–297.
- ,,, et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- ,,,, The Research Advisory Committee of the American Board of Pediatrics.Characteristics of the pediatric hospitalist workforce: its roles and work environment.Pediatrics.2007;120:33–39.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:1.e1–1.e7.
- ,,,,,.Pediatric hospitalists in Canada and the United States: a survey of pediatric academic department chairs.Ambul Pediatr.2001;1:338–339.
- ,,,.PRIS Survey: pediatric hospitalist roles and training needs [Abstract].Pediatr Res.2004;55:360A.
- ,.Third‐year medical students' evaluation of hospitalist and nonhospitalist faculty during the inpatient portion of their pediatrics clerkships.J Hosp Med.2007;2(1):17–22.
- .Challenges in the development of pediatric health services research.J Pediatr.2002;140:1–2.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31(3):847–852.
- New study highlights ingredients for reengineering success.Health Care Cost Reengineering Rep.1999;4(5):72–74,65.
- ,,.Pediatric hospitalists fill varied roles in the care of newborns.Pediatr Ann.2003;32(12):802–810.
- ,,,,.Closing the gap between internal medicine training and practice: recommendations from recent graduates.Am J Med.2005;118(6):680–685; discussion 685–687.
- ,,,,.The spectrum of community‐based hospitalist practice: a call to tailor internal medicine residency training.Arch Intern Med.2007;167(7):727–728.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,.The emerging role of pediatric hospitalists.Clin Pediatr (Phila).2003;42(4):295–297.
- ,,, et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- ,,,, The Research Advisory Committee of the American Board of Pediatrics.Characteristics of the pediatric hospitalist workforce: its roles and work environment.Pediatrics.2007;120:33–39.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:1.e1–1.e7.
- ,,,,,.Pediatric hospitalists in Canada and the United States: a survey of pediatric academic department chairs.Ambul Pediatr.2001;1:338–339.
- ,,,.PRIS Survey: pediatric hospitalist roles and training needs [Abstract].Pediatr Res.2004;55:360A.
- ,.Third‐year medical students' evaluation of hospitalist and nonhospitalist faculty during the inpatient portion of their pediatrics clerkships.J Hosp Med.2007;2(1):17–22.
- .Challenges in the development of pediatric health services research.J Pediatr.2002;140:1–2.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31(3):847–852.
- New study highlights ingredients for reengineering success.Health Care Cost Reengineering Rep.1999;4(5):72–74,65.
- ,,.Pediatric hospitalists fill varied roles in the care of newborns.Pediatr Ann.2003;32(12):802–810.
- ,,,,.Closing the gap between internal medicine training and practice: recommendations from recent graduates.Am J Med.2005;118(6):680–685; discussion 685–687.
- ,,,,.The spectrum of community‐based hospitalist practice: a call to tailor internal medicine residency training.Arch Intern Med.2007;167(7):727–728.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
Copyright © 2009 Society of Hospital Medicine
Oseltamivir in Children with CA‐LCI
Influenza is a common cause of acute respiratory illness in children, resulting in hospitalization of both healthy and chronically ill children due to influenza‐related complications.1, 2 Currently, amantadine, rimantadine, oseltamivir, and zanamivir are approved for use in children to treat influenza. In early 2006, more than 90% of influenza isolates tested in the US were found to be resistant to the adamantanes, suggesting that these medications might be of limited benefit during future influenza seasons.3 To date, most isolates of influenza remain susceptible to neuraminidase inhibitors, zanamivir and oseltamivir. Zanamivir has not been used extensively in pediatrics because it is delivered by aerosolization, and is only approved by the US Food and Drug Administration (FDA) for children 7 years of age. Oseltamivir is administered orally and is FDA‐approved for use in children 1 year of age within 48 hours of onset of symptoms of influenza virus infection.
Studies performed in outpatient settings have shown that oseltamivir can lessen the severity and reduce the length of influenza illness by 36 hours when therapy is initiated within 2 days of the onset of symptoms.4 Treatment also reduced the frequency of new diagnoses of otitis media and decreased physician‐prescribed antibiotics.4
To date, there are limited data evaluating the use of oseltamivir in either adult or pediatric patients hospitalized with influenza. We sought to describe the use of antiviral medications among children hospitalized with community‐acquired laboratory‐confirmed influenza (CA‐LCI) and to evaluate the effect of a computer‐based electronic reminder to increase the rate of on‐label use of oseltamivir among hospitalized children.
PATIENTS AND METHODS
We performed a retrospective cohort study of patients 21 years of age who were hospitalized with CA‐LCI during 5 consecutive seasons from July 2000 through June 2005 (seasons 1‐5) at the Children's Hospital of Philadelphia (CHOP). CHOP is a 418‐bed tertiary care hospital with about 24,000 hospital admissions each year. Viral diagnostic studies are performed routinely on children hospitalized with acute respiratory symptoms of unknown etiology, which aids in assigning patients to cohorts. Patients who had laboratory confirmation of influenza performed at an outside institution were excluded from this analysis.
From June 2005 through May 2006 (season 6), an observational trial of an electronic clinical decision reminder was performed to assess a mechanism to increase the proportion of eligible children treated with oseltamivir. Patients were included in this analysis if they were 21 years of age and had a diagnostic specimen for influenza obtained less than 72 hours after admission. The CHOP Institutional Review Board approved this study with a waiver of informed consent.
Viral Diagnostic Testing
During the winter months from seasons 1‐5, nasopharyngeal aspirate specimens were initially tested using immunochromatographic membrane assays (IA) for respiratory syncytial virus (RSV) (NOW RSV; Binax, Inc., Scarborough, ME) and, if negative, for influenza virus types A and B (NOW Flu A, NOW Flu B; Binax). If negative, specimens were tested by direct fluorescent antibody (DFA) testing for multiple respiratory viruses, including influenza A and B. During the winter season, IA testing was performed multiple times each day, and DFA was performed once or twice daily with an 8 to 24 hour turnaround time after a specimen was obtained. For season 6, the testing algorithm was revised: a panel of real‐time polymerase chain reaction (PCR) assays were performed to detect nucleic acids from multiple respiratory viruses, including influenza virus types A and B, on specimens that tested negative for influenza and RSV by IA. PCR testing was performed multiple times each day, and specimen results were available within 24 hours of specimen submission. Comprehensive viral tube cultures were performed on specimens that were negative by IA and DFA (seasons 1‐5) or respiratory virus PCR panel (season 6).
Study Definitions
Patients were considered to have CA‐LCI if the first diagnostic specimen positive for influenza was obtained less than 72 hours after hospital admission. Prescriptions for oseltamivir that were consistent with the FDA recommendations were considered to be on‐label prescriptions. Prescriptions for oseltamivir given to patients who did not meet these FDA criteria were considered off‐label prescriptions.5 Patients were considered oseltamivir‐eligible if they were met the criteria for FDA approval for treatment with oseltamivir: at least 1 year of age with influenza symptoms of less than 48 hours duration. Patients who either by age and/or symptom duration were inconsistent with FDA labeling criteria for oseltamivir were deemed oseltamivir‐ineligible. This included those patients for whom influenza test results were received by the clinician more than 48 hours after symptom onset. Patients who were positive for influenza only by viral culture were considered oseltamivir‐ineligible since the time needed to culture influenza virus was >48 hours. Because of the abrupt onset of influenza symptoms, the duration of influenza symptoms was defined by chart review of the emergency room or admission note. A hierarchy of symptoms was used to define the initial onset of influenza‐related symptoms and include the following: (1) For all patients with a history of fever, onset of influenza was defined as the onset of fever as recorded in the first physician note. (2) For patients without a history of fever, the onset of respiratory symptoms was recorded as the onset of influenza. (3) For patients without a history of fever but in whom multiple respiratory symptoms were noted, the onset of symptoms was assigned as the beginning of the increased work of breathing.
Because influenza IA were performed at least 4 times a day during the influenza season, the date of result to clinician was determined to be the same date as specimen collection for patients who had a positive influenza IA. Patients were identified as having a positive influenza result to the clinician 1 day after specimen collection if the test was positive by DFA or PCR. A neurologic adverse event was defined as the occurrence of a seizure after initiation of oseltamivir therapy. A neuropsychiatric adverse event was defined as any significant new neuropsychiatric symptom (psychosis, encephalopathy) recorded after the initiation of oseltamivir therapy. We defined a dermatologic adverse event as the report of any skin findings recorded after the initiation of oseltamivir therapy.
Chronic medical conditions
Information from detailed chart review was used to identify children with Advisory Committee on Immunization Practices (ACIP) high‐risk medical conditions as previously described by our group (asthma, chronic pulmonary disease, cardiac disease, immunosuppression, hemoglobinopathies, chronic renal dysfunction, diabetes mellitus, inborn errors of metabolism, long‐term salicylate therapy, pregnancy, and neurological and neuromuscular disease [NNMD]).6
Electronic Reminder
During season 6, a computer‐based electronic reminder was designed. The reminder stated Consider OSELTAMIVIR if Age >1 year AND symptoms <48 hours. May shorten illness by 36 hours. Page ID approval for more info. The reminder was embedded within the influenza results for all positive determinations, so a clinician would see the reminder when viewing positive laboratory results (Meditech, Westwood, MA).
At the initiation of season 6, we determined prescription rates of oseltamivir in patients with CA‐LCI to measure the baseline rate of oseltamivir prescription. The electronic reminder was initiated during week 11 of influenza activity at our institution and continued through the end of the influenza season.
Data Collection
Two sources of antiviral prescription data were used. Inpatient prescription of antiviral medications was extracted from billing records and chart review; a 10% audit of the medication administration records showed that the billing records correctly identified oseltamivir prescription status in all cases reviewed. Patients with incomplete pharmacy data were removed from the analysis of prescription practices (n = 8). During all seasons studied, the infectious diseases pharmacist (T.A.M.) and an infectious diseases physician (T.E.Z.) reviewed requests for inpatient prescriptions for antiviral medications.
For season 6, daily review of infection control records was performed to conduct surveillance for children hospitalized with CA‐LCI. To determine symptom duration and use of antiviral medications, inpatient medical charts were reviewed at the time of initial identification and then daily thereafter.
Statistical Analysis
Dichotomous variables were created for prescription of oseltamivir, age 1 year and symptom duration of <48 hours at time of clinician receipt of influenza results. Descriptive analyses included calculating the frequencies for categorical variables. Categorical variables were compared using Fisher's exact test. The Cochrane‐Armitage test was employed to test for a trend in the prescription of oseltamivir by season. A 2‐tailed P value of <0.05 was considered significant for all statistical tests. All statistical calculations were performed using standard programs in SAS 9.1 (SAS Institute, Cary, NC), STATA 8.2 (Stata Corp., College Station, TX), and Excel (Microsoft, Redmond, WA).
Prior to the start of season 6, we determined that if the rate of oseltamivir prescription was 40% before initiation of the reminder, we would need 20 eligible patients to detect a difference of 40% or greater in subsequent prescription rates (with 80% power and an alpha of 0.05). Once this enrollment goal was met, an electronic reminder of the eligibility for oseltamivir was initiated.
RESULTS
Use of Antiviral Medications in Children Hospitalized with Influenza, 2000‐2005
From July 2000 to June 2005, 1,058 patients were admitted with laboratory confirmed influenza; 8 were excluded because confirmatory testing was done at an outside institution, 24 were repeat hospitalizations, 89 nosocomial cases, and 8 cases were in patients >21 years. Thus, 929 patients had CA‐LCI and were eligible for inclusion in this study. Most children were infected with influenza A and were 1 year of age (Table 1). During this study period, only 9.3% of study subjects were treated with antiviral medications, most of whom (91%) received oseltamivir. Eight patients received amantadine over all seasons studied.
| Characteristics | Patients Hospitalized with CA‐LCI (n = 929)* | Eligible to Receive Oseltamivir (n = 305)* |
|---|---|---|
| ||
| Age (years) | ||
| <1 | 342 (37) | 0 |
| 1 | 587 (63) | 305 (100) |
| Season | ||
| 2000‐2001 | 107 (11.5) | 32 (10) |
| 2001‐2002 | 252 (27) | 78 (26) |
| 2002‐2003 | 135 (14.5) | 31 (10) |
| 2003‐2004 | 243 (26) | 86 (28) |
| 2004‐2005 | 192 (21) | 78 (26) |
| Influenza type | ||
| A | 692 (75) | |
| B | 237 (25) | |
Overall, one‐third of patients (305/929; 33%) were eligible for treatment with oseltamivir. Among patients 1 year of age, approximately one‐half (305/587; 52%) were oseltamivir‐eligible. The additional 282 patients 1 year were ineligible because test results were returned to the clinician >48 hours after hospital admission. Only 49 (16.1%) of oseltamivir‐eligible patients were prescribed oseltamivir during hospitalization (Figure 1). The rate of prescription of oseltamivir increased over all seasons from 0% in 2000‐2001 to 20% in 2004‐2005. On‐label prescription rates increased from 0% in 2000‐2001 to 37.2% in 2004‐2005 (P < 0.0001; Figure 2).
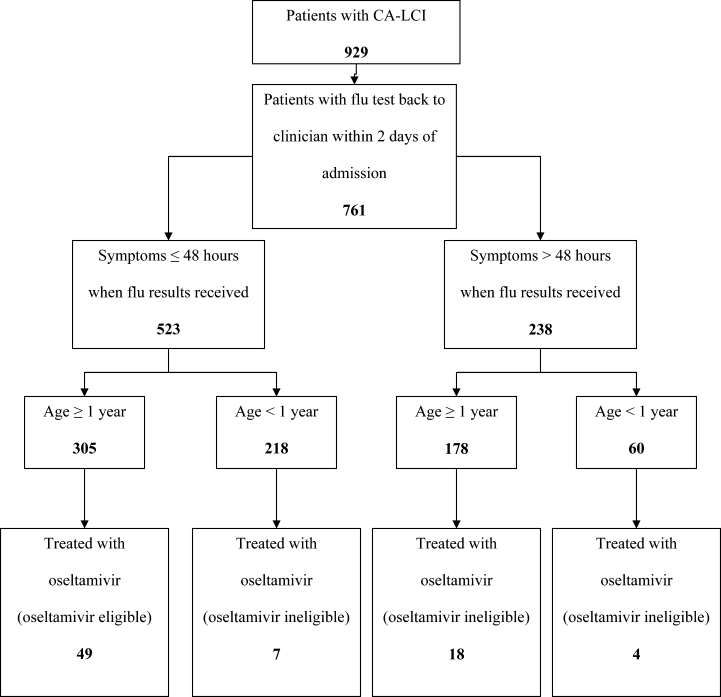
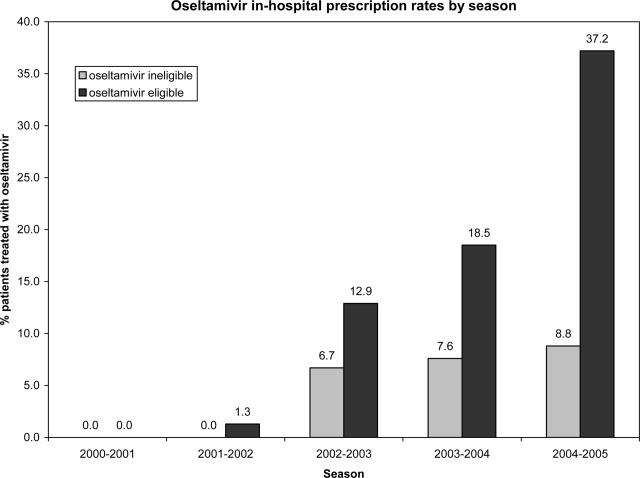
Off‐Label Oseltamivir Prescription
Oseltamivir was prescribed to 29 of the 624 patients who were determined to be oseltamivir‐ineligible. The rate of off‐label use increased over the seasons from 2000 to 2005 from 0% to 8.8% (P < 0.0001; Figure 1). Ineligible patients who received oseltamivir were 1 year of age (n = 11), had test results returned to the clinician 48 hours after hospital admission (n = 18), or both (n = 4). Most off‐label prescriptions occurred in patients who had chronic medical conditions (21/29; 72%), including cardiac disease (n = 9), asthma (n = 6), or prematurity (n = 5). Four of 11 patients 1 year of age who were treated with oseltamivir had influenza‐related respiratory failure. The oseltamivir dose for all patients 1 year of age was 2 mg/kg twice a day, all of whom survived to discharge.
Evaluation of a Computer‐Based Electronic Reminder Designed to Enhance the On‐Label Prescription of Oseltamivir
During season 6, an electronic reminder about the labeled use of oseltamivir was evaluated to determine its ability to increase the rate of prescription of oseltamivir among eligible children hospitalized with CA‐LCI. During season 6, most patients (226/311; 73%) were 1 year of age. A total of 84 patients were determined to be oseltamivir‐eligible (age 1 year and test results back to the clinician within 48 hours of symptom onset).
During the initial 10 weeks of local influenza activity, 20 oseltamivir‐eligible patients were admitted to our institution, and 8 received oseltamivir (40% prescription rate) (Table 2). In addition, 2 of 54 (3.7%) oseltamivir‐ineligible patients were also treated. The computer‐based electronic reminder was initiated in week 11 of the influenza season. After initiation of the reminder, 237 additional children with CA‐LCI were hospitalized, of whom 64 (27%) were determined to be oseltamivir‐eligible. The rate of on‐label prescription of oseltamivir was similar to that observed prior to initiation of the reminder: 16 of 64 patients eligible for antiviral therapy received oseltamivir (25% prescription rate) (Figure 3). An additional 8 patients were prescribed oseltamivir off‐label. The rate of oseltamivir prescription did not change significantly for either oseltamivir‐eligible (40‐25%) or oseltamivir‐ineligible (3.7‐4.6%) (Figure 4).
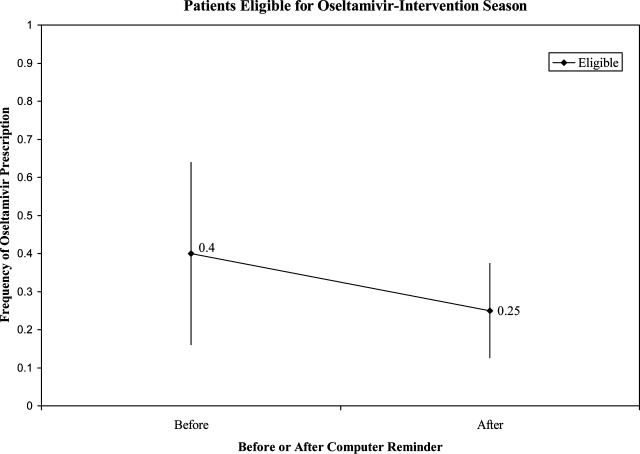
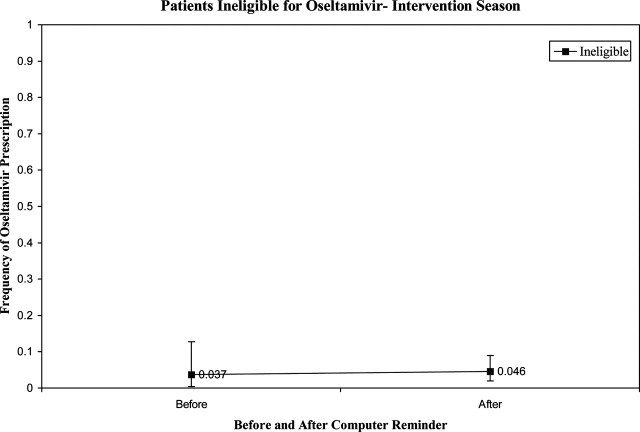
| Prompt Active? | Oseltamivir Use | Total | |
|---|---|---|---|
| Yes* | No | ||
| |||
| No | |||
| Eligible | 8 (40) | 12 | 20 |
| Ineligible | 2 (3.7) | 52 | 54 |
| Yes | |||
| Eligible | 16 (25) | 48 | 64 |
| Ineligible | 8 (4.6) | 165 | 173 |
| Total | 34 | 277 | 311 |
Dermatologic, Neurologic, and Neuropsychiatric Adverse Events
We reviewed the medical records of all patients treated with oseltamivir during the 6 study seasons to identify dermatologic, neurologic, and neuropsychiatric adverse outcomes that developed after the initiation of oseltamivir therapy. No new‐onset seizures, neuropsychiatric, or dermatologic reactions were identified among the children treated with oseltamivir.
DISCUSSION AND CONCLUSION
In this report, we describe the use of oseltamivir over 6 seasons in a cohort of children hospitalized with CA‐LCI at 1 tertiary care pediatric hospital and examine the impact of a mechanism designed to increase prescription among those eligible for oseltamivir. We found that only one‐third of patients hospitalized at our institution were eligible for oseltamivir treatment based on FDA‐approved indications. Of the eligible patients, few were prescribed oseltamivir during their hospitalization. During the sixth season, we employed a computer reminder system for oseltamivir prescription, which had no appreciable effect upon prescription rates. Despite the lack of effect of the electronic reminder system, we observed an increase of on‐label oseltamivir prescriptions over the entire study period. Finally, we identified 11 patients <1 year of age (3%) who were treated with oseltamivir. There were no adverse events identified in this group.
Although previous studies have addressed prescription rates of oseltamivir in children with influenza, few, if any, have looked at how these prescriptions correspond with FDA label criteria. In our cohort, only one‐third of hospitalized children were eligible for treatment with oseltamivir based upon their age and symptom duration at the time the results of rapid laboratory testing became available. Of those patients in our cohort eligible for oseltamivir, few were treated. The prescription of oseltamivir in seasons falls within the ranges found by Schrag et al.7 in their multistate review of pediatric influenza hospitalizations in 2003‐2004. They noted that use of antiviral medications varied by location of surveillance ranging from 3% in Connecticut to 34% in Colorado, indicating significant regional differences in prescription practices.7 Potential causes of low rates of appropriate use of oseltamivir include the observation that many physicians remain unaware of the potential severity of influenza infection in children.8 Additionally, physicians may differ on how to define the onset of influenza infection in children. A recent study published by Ohmit and Monto9 indicated that a fever and cough predicted 83% of children 5 to 12 years old who were determined to be influenza‐positive. Finally, many physicians who do not prescribe antiviral therapy may believe that their patients present too late for appropriate initiation of therapy.10
We identified 29 patients who received oseltamivir although they did not meet the FDA label criteria, of whom 72% had a chronic underlying condition. Moore et al.11 in their surveillance of influenza admissions in Canada found a similar trend. They described 26 of 29 (90%) hospitalized patients receiving antiinfluenza drugs had an underlying disease, and of those without a chronic condition, all had severe influenza‐related complications such as encephalopathy.11
Implementation of a computerized reminder to improve use of oseltamivir had no statistically significant effect on prescribing practice. Our sample size calculation was based on detecting a 40% difference in prescription rates, which limited our power to detect a smaller difference in prescription rates. A systematic review by Garg et al.12 identified barriers to the success of computer‐based decision support systems (CDS), which included failure of practitioners to use the system, poor integration of the system to the physician's workflow, and disagreement with what was recommended. Future enhancements to our inpatient electronic hospital record may allow for more targeted and robust CDS interventions.
We observed an increase in on‐label prescription rates of oseltamivir over the entire study period. We hypothesize that increased use of oseltamivir might be associated with growing concerns of pandemic influenza and attention to fatal influenza in children,13 as evidenced by the recent addition of influenza‐associated deaths in children to the list of nationally notifiable conditions in 2004.14
There has been considerable focus upon potential adverse events associated with treatment with oseltamivir in children. Reports have emerged, primarily from Japan, of neuropsychiatric and dermatologic adverse events of oseltamivir treatment.15 In the fall of 2006, the FDA added a precaution to the labeling of oseltamivir due to these neuropsychiatric events.16 In our treated cohort, no neurologic, neuropsychiatric, or dermatologic adverse events were identified. However, this finding is not surprising given the rarity of these adverse events and the limited number of children treated with oseltamivir in this study.
The strengths of this current study include a large cohort of laboratory‐confirmed influenza in hospitalized children over multiple influenza seasons. In addition, this is the first study of which we are aware that has assessed the number of children eligible for oseltamivir but not treated. The limitations of this study include misclassification bias related to the retrospective study design. Because of this design, onset of influenza symptoms was collected through chart review, and the time of receipt of influenza results from virology was based upon known laboratory turnover time, rather than actual knowledge of time of physician awareness of the result. To address this issue we used a conservative estimate of the time of receipt of influenza test results. In addition, the retrospective design prevented us from assessing the clinical decision‐making process, which led some patients to be treated with oseltamivir and others not. Our evaluation of the electronic reminder was designed to show a large change in prescription practices (ie, 40%), so it had insufficient power to detect a smaller impact. Finally, ascertainment bias may have limited our ability to identify adverse effects.
This study demonstrates that oseltamivir is prescribed infrequently among hospitalized children. Future studies are needed to determine whether appropriate use of oseltamivir improves outcomes among hospitalized children. Additional study of the safety and efficacy of oseltamivir in children aged <1 year is also needed given the large burden of disease in this age group.
Acknowledgements
We thank Michelle Precourt for her assistance with the computer‐based prompt. We also thank Drs. Anna Wheeler Rosenquist and Melissa Donovan for the original data collection for this project. This project was supported in part by the Centers for Disease Control and Prevention, grant H23/CCH32253‐02.
- ,,, et al.Influenza and the rates of hospitalization for respiratory disease among infants and young children.N Engl J Med.2000;342:232–239.
- ,,,,.The effect of influenza on hospitalizations, outpatient visits and courses of antibiotics in children.N Engl J Med.2000;342:225–231.
- Centers for Disease Control and Prevention (CDC). Diseases and Conditions. Seasonal Flu. CDC Health Alert: CDC recommends against the use of amantadine and rimantadine for the treatment or prophylaxis of influenza in the United States during the 2005–06 influenza season. January 14, 2006. Available at http://www.cdc.gov/flu/han011406.htm. Accessed November2008.
- ,,, et al.Oral oseltamivir treatment of influenza in children.Pediatr Infect Dis J.2001;20(2):127–133.
- ,,, et al.Off‐label drug use in hospitalized children.Arch Pediatr Adolesc Med.2007;161(3):282–290.
- ,,, et al.Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection.JAMA.2005;294:2188–2194.
- ,,, et al.Multistate surveillance for laboratory‐confirmed influenza‐associated hospitalizations in children 2003–2004.Pediatr Infect Dis J.2006;25(5):395–400.
- ,.Physician knowledge and perspectives regarding influenza and influenza vaccination.Hum Vaccin.2005;1(2):74–79.
- ,.Symptomatic predictors of influenza virus positivity in children during the influenza season.Clin Infect Dis.2006;43:564–568.
- ,,,,,.Effects of local variation, specialty, and beliefs on antiviral prescribing for influenza.Clin Infect Dis.2006;42:95–99.
- ,,, et al.Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004.Pediatrics.2006;118:e610–e619.
- ,,, et al.Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review.JAMA.2005;293:1223–1238.
- ,,, et al.Influenza‐associated deaths among children in the United States, 2003–2004.N Engl J Med.2005;353(24):2559–2567.
- Centers for Disease Control and Prevention (CDC). Diseases and Conditions. Seasonal Flu. Flu Activity 25(6):572.
- ,,.Post‐Marketing Adverse Event Reports. Review of Central Nervous System/Psychiatric Disorders Associated with the Use of Tamiflu, Drug: Oseltamivir Phosphate. Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Drug Evaluation and Research, Office of Surveillance and Epidemiology.2006. OSE PID #D060393 Oseltamivir—Neuropsychiatric Events.
Influenza is a common cause of acute respiratory illness in children, resulting in hospitalization of both healthy and chronically ill children due to influenza‐related complications.1, 2 Currently, amantadine, rimantadine, oseltamivir, and zanamivir are approved for use in children to treat influenza. In early 2006, more than 90% of influenza isolates tested in the US were found to be resistant to the adamantanes, suggesting that these medications might be of limited benefit during future influenza seasons.3 To date, most isolates of influenza remain susceptible to neuraminidase inhibitors, zanamivir and oseltamivir. Zanamivir has not been used extensively in pediatrics because it is delivered by aerosolization, and is only approved by the US Food and Drug Administration (FDA) for children 7 years of age. Oseltamivir is administered orally and is FDA‐approved for use in children 1 year of age within 48 hours of onset of symptoms of influenza virus infection.
Studies performed in outpatient settings have shown that oseltamivir can lessen the severity and reduce the length of influenza illness by 36 hours when therapy is initiated within 2 days of the onset of symptoms.4 Treatment also reduced the frequency of new diagnoses of otitis media and decreased physician‐prescribed antibiotics.4
To date, there are limited data evaluating the use of oseltamivir in either adult or pediatric patients hospitalized with influenza. We sought to describe the use of antiviral medications among children hospitalized with community‐acquired laboratory‐confirmed influenza (CA‐LCI) and to evaluate the effect of a computer‐based electronic reminder to increase the rate of on‐label use of oseltamivir among hospitalized children.
PATIENTS AND METHODS
We performed a retrospective cohort study of patients 21 years of age who were hospitalized with CA‐LCI during 5 consecutive seasons from July 2000 through June 2005 (seasons 1‐5) at the Children's Hospital of Philadelphia (CHOP). CHOP is a 418‐bed tertiary care hospital with about 24,000 hospital admissions each year. Viral diagnostic studies are performed routinely on children hospitalized with acute respiratory symptoms of unknown etiology, which aids in assigning patients to cohorts. Patients who had laboratory confirmation of influenza performed at an outside institution were excluded from this analysis.
From June 2005 through May 2006 (season 6), an observational trial of an electronic clinical decision reminder was performed to assess a mechanism to increase the proportion of eligible children treated with oseltamivir. Patients were included in this analysis if they were 21 years of age and had a diagnostic specimen for influenza obtained less than 72 hours after admission. The CHOP Institutional Review Board approved this study with a waiver of informed consent.
Viral Diagnostic Testing
During the winter months from seasons 1‐5, nasopharyngeal aspirate specimens were initially tested using immunochromatographic membrane assays (IA) for respiratory syncytial virus (RSV) (NOW RSV; Binax, Inc., Scarborough, ME) and, if negative, for influenza virus types A and B (NOW Flu A, NOW Flu B; Binax). If negative, specimens were tested by direct fluorescent antibody (DFA) testing for multiple respiratory viruses, including influenza A and B. During the winter season, IA testing was performed multiple times each day, and DFA was performed once or twice daily with an 8 to 24 hour turnaround time after a specimen was obtained. For season 6, the testing algorithm was revised: a panel of real‐time polymerase chain reaction (PCR) assays were performed to detect nucleic acids from multiple respiratory viruses, including influenza virus types A and B, on specimens that tested negative for influenza and RSV by IA. PCR testing was performed multiple times each day, and specimen results were available within 24 hours of specimen submission. Comprehensive viral tube cultures were performed on specimens that were negative by IA and DFA (seasons 1‐5) or respiratory virus PCR panel (season 6).
Study Definitions
Patients were considered to have CA‐LCI if the first diagnostic specimen positive for influenza was obtained less than 72 hours after hospital admission. Prescriptions for oseltamivir that were consistent with the FDA recommendations were considered to be on‐label prescriptions. Prescriptions for oseltamivir given to patients who did not meet these FDA criteria were considered off‐label prescriptions.5 Patients were considered oseltamivir‐eligible if they were met the criteria for FDA approval for treatment with oseltamivir: at least 1 year of age with influenza symptoms of less than 48 hours duration. Patients who either by age and/or symptom duration were inconsistent with FDA labeling criteria for oseltamivir were deemed oseltamivir‐ineligible. This included those patients for whom influenza test results were received by the clinician more than 48 hours after symptom onset. Patients who were positive for influenza only by viral culture were considered oseltamivir‐ineligible since the time needed to culture influenza virus was >48 hours. Because of the abrupt onset of influenza symptoms, the duration of influenza symptoms was defined by chart review of the emergency room or admission note. A hierarchy of symptoms was used to define the initial onset of influenza‐related symptoms and include the following: (1) For all patients with a history of fever, onset of influenza was defined as the onset of fever as recorded in the first physician note. (2) For patients without a history of fever, the onset of respiratory symptoms was recorded as the onset of influenza. (3) For patients without a history of fever but in whom multiple respiratory symptoms were noted, the onset of symptoms was assigned as the beginning of the increased work of breathing.
Because influenza IA were performed at least 4 times a day during the influenza season, the date of result to clinician was determined to be the same date as specimen collection for patients who had a positive influenza IA. Patients were identified as having a positive influenza result to the clinician 1 day after specimen collection if the test was positive by DFA or PCR. A neurologic adverse event was defined as the occurrence of a seizure after initiation of oseltamivir therapy. A neuropsychiatric adverse event was defined as any significant new neuropsychiatric symptom (psychosis, encephalopathy) recorded after the initiation of oseltamivir therapy. We defined a dermatologic adverse event as the report of any skin findings recorded after the initiation of oseltamivir therapy.
Chronic medical conditions
Information from detailed chart review was used to identify children with Advisory Committee on Immunization Practices (ACIP) high‐risk medical conditions as previously described by our group (asthma, chronic pulmonary disease, cardiac disease, immunosuppression, hemoglobinopathies, chronic renal dysfunction, diabetes mellitus, inborn errors of metabolism, long‐term salicylate therapy, pregnancy, and neurological and neuromuscular disease [NNMD]).6
Electronic Reminder
During season 6, a computer‐based electronic reminder was designed. The reminder stated Consider OSELTAMIVIR if Age >1 year AND symptoms <48 hours. May shorten illness by 36 hours. Page ID approval for more info. The reminder was embedded within the influenza results for all positive determinations, so a clinician would see the reminder when viewing positive laboratory results (Meditech, Westwood, MA).
At the initiation of season 6, we determined prescription rates of oseltamivir in patients with CA‐LCI to measure the baseline rate of oseltamivir prescription. The electronic reminder was initiated during week 11 of influenza activity at our institution and continued through the end of the influenza season.
Data Collection
Two sources of antiviral prescription data were used. Inpatient prescription of antiviral medications was extracted from billing records and chart review; a 10% audit of the medication administration records showed that the billing records correctly identified oseltamivir prescription status in all cases reviewed. Patients with incomplete pharmacy data were removed from the analysis of prescription practices (n = 8). During all seasons studied, the infectious diseases pharmacist (T.A.M.) and an infectious diseases physician (T.E.Z.) reviewed requests for inpatient prescriptions for antiviral medications.
For season 6, daily review of infection control records was performed to conduct surveillance for children hospitalized with CA‐LCI. To determine symptom duration and use of antiviral medications, inpatient medical charts were reviewed at the time of initial identification and then daily thereafter.
Statistical Analysis
Dichotomous variables were created for prescription of oseltamivir, age 1 year and symptom duration of <48 hours at time of clinician receipt of influenza results. Descriptive analyses included calculating the frequencies for categorical variables. Categorical variables were compared using Fisher's exact test. The Cochrane‐Armitage test was employed to test for a trend in the prescription of oseltamivir by season. A 2‐tailed P value of <0.05 was considered significant for all statistical tests. All statistical calculations were performed using standard programs in SAS 9.1 (SAS Institute, Cary, NC), STATA 8.2 (Stata Corp., College Station, TX), and Excel (Microsoft, Redmond, WA).
Prior to the start of season 6, we determined that if the rate of oseltamivir prescription was 40% before initiation of the reminder, we would need 20 eligible patients to detect a difference of 40% or greater in subsequent prescription rates (with 80% power and an alpha of 0.05). Once this enrollment goal was met, an electronic reminder of the eligibility for oseltamivir was initiated.
RESULTS
Use of Antiviral Medications in Children Hospitalized with Influenza, 2000‐2005
From July 2000 to June 2005, 1,058 patients were admitted with laboratory confirmed influenza; 8 were excluded because confirmatory testing was done at an outside institution, 24 were repeat hospitalizations, 89 nosocomial cases, and 8 cases were in patients >21 years. Thus, 929 patients had CA‐LCI and were eligible for inclusion in this study. Most children were infected with influenza A and were 1 year of age (Table 1). During this study period, only 9.3% of study subjects were treated with antiviral medications, most of whom (91%) received oseltamivir. Eight patients received amantadine over all seasons studied.
| Characteristics | Patients Hospitalized with CA‐LCI (n = 929)* | Eligible to Receive Oseltamivir (n = 305)* |
|---|---|---|
| ||
| Age (years) | ||
| <1 | 342 (37) | 0 |
| 1 | 587 (63) | 305 (100) |
| Season | ||
| 2000‐2001 | 107 (11.5) | 32 (10) |
| 2001‐2002 | 252 (27) | 78 (26) |
| 2002‐2003 | 135 (14.5) | 31 (10) |
| 2003‐2004 | 243 (26) | 86 (28) |
| 2004‐2005 | 192 (21) | 78 (26) |
| Influenza type | ||
| A | 692 (75) | |
| B | 237 (25) | |
Overall, one‐third of patients (305/929; 33%) were eligible for treatment with oseltamivir. Among patients 1 year of age, approximately one‐half (305/587; 52%) were oseltamivir‐eligible. The additional 282 patients 1 year were ineligible because test results were returned to the clinician >48 hours after hospital admission. Only 49 (16.1%) of oseltamivir‐eligible patients were prescribed oseltamivir during hospitalization (Figure 1). The rate of prescription of oseltamivir increased over all seasons from 0% in 2000‐2001 to 20% in 2004‐2005. On‐label prescription rates increased from 0% in 2000‐2001 to 37.2% in 2004‐2005 (P < 0.0001; Figure 2).


Off‐Label Oseltamivir Prescription
Oseltamivir was prescribed to 29 of the 624 patients who were determined to be oseltamivir‐ineligible. The rate of off‐label use increased over the seasons from 2000 to 2005 from 0% to 8.8% (P < 0.0001; Figure 1). Ineligible patients who received oseltamivir were 1 year of age (n = 11), had test results returned to the clinician 48 hours after hospital admission (n = 18), or both (n = 4). Most off‐label prescriptions occurred in patients who had chronic medical conditions (21/29; 72%), including cardiac disease (n = 9), asthma (n = 6), or prematurity (n = 5). Four of 11 patients 1 year of age who were treated with oseltamivir had influenza‐related respiratory failure. The oseltamivir dose for all patients 1 year of age was 2 mg/kg twice a day, all of whom survived to discharge.
Evaluation of a Computer‐Based Electronic Reminder Designed to Enhance the On‐Label Prescription of Oseltamivir
During season 6, an electronic reminder about the labeled use of oseltamivir was evaluated to determine its ability to increase the rate of prescription of oseltamivir among eligible children hospitalized with CA‐LCI. During season 6, most patients (226/311; 73%) were 1 year of age. A total of 84 patients were determined to be oseltamivir‐eligible (age 1 year and test results back to the clinician within 48 hours of symptom onset).
During the initial 10 weeks of local influenza activity, 20 oseltamivir‐eligible patients were admitted to our institution, and 8 received oseltamivir (40% prescription rate) (Table 2). In addition, 2 of 54 (3.7%) oseltamivir‐ineligible patients were also treated. The computer‐based electronic reminder was initiated in week 11 of the influenza season. After initiation of the reminder, 237 additional children with CA‐LCI were hospitalized, of whom 64 (27%) were determined to be oseltamivir‐eligible. The rate of on‐label prescription of oseltamivir was similar to that observed prior to initiation of the reminder: 16 of 64 patients eligible for antiviral therapy received oseltamivir (25% prescription rate) (Figure 3). An additional 8 patients were prescribed oseltamivir off‐label. The rate of oseltamivir prescription did not change significantly for either oseltamivir‐eligible (40‐25%) or oseltamivir‐ineligible (3.7‐4.6%) (Figure 4).


| Prompt Active? | Oseltamivir Use | Total | |
|---|---|---|---|
| Yes* | No | ||
| |||
| No | |||
| Eligible | 8 (40) | 12 | 20 |
| Ineligible | 2 (3.7) | 52 | 54 |
| Yes | |||
| Eligible | 16 (25) | 48 | 64 |
| Ineligible | 8 (4.6) | 165 | 173 |
| Total | 34 | 277 | 311 |
Dermatologic, Neurologic, and Neuropsychiatric Adverse Events
We reviewed the medical records of all patients treated with oseltamivir during the 6 study seasons to identify dermatologic, neurologic, and neuropsychiatric adverse outcomes that developed after the initiation of oseltamivir therapy. No new‐onset seizures, neuropsychiatric, or dermatologic reactions were identified among the children treated with oseltamivir.
DISCUSSION AND CONCLUSION
In this report, we describe the use of oseltamivir over 6 seasons in a cohort of children hospitalized with CA‐LCI at 1 tertiary care pediatric hospital and examine the impact of a mechanism designed to increase prescription among those eligible for oseltamivir. We found that only one‐third of patients hospitalized at our institution were eligible for oseltamivir treatment based on FDA‐approved indications. Of the eligible patients, few were prescribed oseltamivir during their hospitalization. During the sixth season, we employed a computer reminder system for oseltamivir prescription, which had no appreciable effect upon prescription rates. Despite the lack of effect of the electronic reminder system, we observed an increase of on‐label oseltamivir prescriptions over the entire study period. Finally, we identified 11 patients <1 year of age (3%) who were treated with oseltamivir. There were no adverse events identified in this group.
Although previous studies have addressed prescription rates of oseltamivir in children with influenza, few, if any, have looked at how these prescriptions correspond with FDA label criteria. In our cohort, only one‐third of hospitalized children were eligible for treatment with oseltamivir based upon their age and symptom duration at the time the results of rapid laboratory testing became available. Of those patients in our cohort eligible for oseltamivir, few were treated. The prescription of oseltamivir in seasons falls within the ranges found by Schrag et al.7 in their multistate review of pediatric influenza hospitalizations in 2003‐2004. They noted that use of antiviral medications varied by location of surveillance ranging from 3% in Connecticut to 34% in Colorado, indicating significant regional differences in prescription practices.7 Potential causes of low rates of appropriate use of oseltamivir include the observation that many physicians remain unaware of the potential severity of influenza infection in children.8 Additionally, physicians may differ on how to define the onset of influenza infection in children. A recent study published by Ohmit and Monto9 indicated that a fever and cough predicted 83% of children 5 to 12 years old who were determined to be influenza‐positive. Finally, many physicians who do not prescribe antiviral therapy may believe that their patients present too late for appropriate initiation of therapy.10
We identified 29 patients who received oseltamivir although they did not meet the FDA label criteria, of whom 72% had a chronic underlying condition. Moore et al.11 in their surveillance of influenza admissions in Canada found a similar trend. They described 26 of 29 (90%) hospitalized patients receiving antiinfluenza drugs had an underlying disease, and of those without a chronic condition, all had severe influenza‐related complications such as encephalopathy.11
Implementation of a computerized reminder to improve use of oseltamivir had no statistically significant effect on prescribing practice. Our sample size calculation was based on detecting a 40% difference in prescription rates, which limited our power to detect a smaller difference in prescription rates. A systematic review by Garg et al.12 identified barriers to the success of computer‐based decision support systems (CDS), which included failure of practitioners to use the system, poor integration of the system to the physician's workflow, and disagreement with what was recommended. Future enhancements to our inpatient electronic hospital record may allow for more targeted and robust CDS interventions.
We observed an increase in on‐label prescription rates of oseltamivir over the entire study period. We hypothesize that increased use of oseltamivir might be associated with growing concerns of pandemic influenza and attention to fatal influenza in children,13 as evidenced by the recent addition of influenza‐associated deaths in children to the list of nationally notifiable conditions in 2004.14
There has been considerable focus upon potential adverse events associated with treatment with oseltamivir in children. Reports have emerged, primarily from Japan, of neuropsychiatric and dermatologic adverse events of oseltamivir treatment.15 In the fall of 2006, the FDA added a precaution to the labeling of oseltamivir due to these neuropsychiatric events.16 In our treated cohort, no neurologic, neuropsychiatric, or dermatologic adverse events were identified. However, this finding is not surprising given the rarity of these adverse events and the limited number of children treated with oseltamivir in this study.
The strengths of this current study include a large cohort of laboratory‐confirmed influenza in hospitalized children over multiple influenza seasons. In addition, this is the first study of which we are aware that has assessed the number of children eligible for oseltamivir but not treated. The limitations of this study include misclassification bias related to the retrospective study design. Because of this design, onset of influenza symptoms was collected through chart review, and the time of receipt of influenza results from virology was based upon known laboratory turnover time, rather than actual knowledge of time of physician awareness of the result. To address this issue we used a conservative estimate of the time of receipt of influenza test results. In addition, the retrospective design prevented us from assessing the clinical decision‐making process, which led some patients to be treated with oseltamivir and others not. Our evaluation of the electronic reminder was designed to show a large change in prescription practices (ie, 40%), so it had insufficient power to detect a smaller impact. Finally, ascertainment bias may have limited our ability to identify adverse effects.
This study demonstrates that oseltamivir is prescribed infrequently among hospitalized children. Future studies are needed to determine whether appropriate use of oseltamivir improves outcomes among hospitalized children. Additional study of the safety and efficacy of oseltamivir in children aged <1 year is also needed given the large burden of disease in this age group.
Acknowledgements
We thank Michelle Precourt for her assistance with the computer‐based prompt. We also thank Drs. Anna Wheeler Rosenquist and Melissa Donovan for the original data collection for this project. This project was supported in part by the Centers for Disease Control and Prevention, grant H23/CCH32253‐02.
Influenza is a common cause of acute respiratory illness in children, resulting in hospitalization of both healthy and chronically ill children due to influenza‐related complications.1, 2 Currently, amantadine, rimantadine, oseltamivir, and zanamivir are approved for use in children to treat influenza. In early 2006, more than 90% of influenza isolates tested in the US were found to be resistant to the adamantanes, suggesting that these medications might be of limited benefit during future influenza seasons.3 To date, most isolates of influenza remain susceptible to neuraminidase inhibitors, zanamivir and oseltamivir. Zanamivir has not been used extensively in pediatrics because it is delivered by aerosolization, and is only approved by the US Food and Drug Administration (FDA) for children 7 years of age. Oseltamivir is administered orally and is FDA‐approved for use in children 1 year of age within 48 hours of onset of symptoms of influenza virus infection.
Studies performed in outpatient settings have shown that oseltamivir can lessen the severity and reduce the length of influenza illness by 36 hours when therapy is initiated within 2 days of the onset of symptoms.4 Treatment also reduced the frequency of new diagnoses of otitis media and decreased physician‐prescribed antibiotics.4
To date, there are limited data evaluating the use of oseltamivir in either adult or pediatric patients hospitalized with influenza. We sought to describe the use of antiviral medications among children hospitalized with community‐acquired laboratory‐confirmed influenza (CA‐LCI) and to evaluate the effect of a computer‐based electronic reminder to increase the rate of on‐label use of oseltamivir among hospitalized children.
PATIENTS AND METHODS
We performed a retrospective cohort study of patients 21 years of age who were hospitalized with CA‐LCI during 5 consecutive seasons from July 2000 through June 2005 (seasons 1‐5) at the Children's Hospital of Philadelphia (CHOP). CHOP is a 418‐bed tertiary care hospital with about 24,000 hospital admissions each year. Viral diagnostic studies are performed routinely on children hospitalized with acute respiratory symptoms of unknown etiology, which aids in assigning patients to cohorts. Patients who had laboratory confirmation of influenza performed at an outside institution were excluded from this analysis.
From June 2005 through May 2006 (season 6), an observational trial of an electronic clinical decision reminder was performed to assess a mechanism to increase the proportion of eligible children treated with oseltamivir. Patients were included in this analysis if they were 21 years of age and had a diagnostic specimen for influenza obtained less than 72 hours after admission. The CHOP Institutional Review Board approved this study with a waiver of informed consent.
Viral Diagnostic Testing
During the winter months from seasons 1‐5, nasopharyngeal aspirate specimens were initially tested using immunochromatographic membrane assays (IA) for respiratory syncytial virus (RSV) (NOW RSV; Binax, Inc., Scarborough, ME) and, if negative, for influenza virus types A and B (NOW Flu A, NOW Flu B; Binax). If negative, specimens were tested by direct fluorescent antibody (DFA) testing for multiple respiratory viruses, including influenza A and B. During the winter season, IA testing was performed multiple times each day, and DFA was performed once or twice daily with an 8 to 24 hour turnaround time after a specimen was obtained. For season 6, the testing algorithm was revised: a panel of real‐time polymerase chain reaction (PCR) assays were performed to detect nucleic acids from multiple respiratory viruses, including influenza virus types A and B, on specimens that tested negative for influenza and RSV by IA. PCR testing was performed multiple times each day, and specimen results were available within 24 hours of specimen submission. Comprehensive viral tube cultures were performed on specimens that were negative by IA and DFA (seasons 1‐5) or respiratory virus PCR panel (season 6).
Study Definitions
Patients were considered to have CA‐LCI if the first diagnostic specimen positive for influenza was obtained less than 72 hours after hospital admission. Prescriptions for oseltamivir that were consistent with the FDA recommendations were considered to be on‐label prescriptions. Prescriptions for oseltamivir given to patients who did not meet these FDA criteria were considered off‐label prescriptions.5 Patients were considered oseltamivir‐eligible if they were met the criteria for FDA approval for treatment with oseltamivir: at least 1 year of age with influenza symptoms of less than 48 hours duration. Patients who either by age and/or symptom duration were inconsistent with FDA labeling criteria for oseltamivir were deemed oseltamivir‐ineligible. This included those patients for whom influenza test results were received by the clinician more than 48 hours after symptom onset. Patients who were positive for influenza only by viral culture were considered oseltamivir‐ineligible since the time needed to culture influenza virus was >48 hours. Because of the abrupt onset of influenza symptoms, the duration of influenza symptoms was defined by chart review of the emergency room or admission note. A hierarchy of symptoms was used to define the initial onset of influenza‐related symptoms and include the following: (1) For all patients with a history of fever, onset of influenza was defined as the onset of fever as recorded in the first physician note. (2) For patients without a history of fever, the onset of respiratory symptoms was recorded as the onset of influenza. (3) For patients without a history of fever but in whom multiple respiratory symptoms were noted, the onset of symptoms was assigned as the beginning of the increased work of breathing.
Because influenza IA were performed at least 4 times a day during the influenza season, the date of result to clinician was determined to be the same date as specimen collection for patients who had a positive influenza IA. Patients were identified as having a positive influenza result to the clinician 1 day after specimen collection if the test was positive by DFA or PCR. A neurologic adverse event was defined as the occurrence of a seizure after initiation of oseltamivir therapy. A neuropsychiatric adverse event was defined as any significant new neuropsychiatric symptom (psychosis, encephalopathy) recorded after the initiation of oseltamivir therapy. We defined a dermatologic adverse event as the report of any skin findings recorded after the initiation of oseltamivir therapy.
Chronic medical conditions
Information from detailed chart review was used to identify children with Advisory Committee on Immunization Practices (ACIP) high‐risk medical conditions as previously described by our group (asthma, chronic pulmonary disease, cardiac disease, immunosuppression, hemoglobinopathies, chronic renal dysfunction, diabetes mellitus, inborn errors of metabolism, long‐term salicylate therapy, pregnancy, and neurological and neuromuscular disease [NNMD]).6
Electronic Reminder
During season 6, a computer‐based electronic reminder was designed. The reminder stated Consider OSELTAMIVIR if Age >1 year AND symptoms <48 hours. May shorten illness by 36 hours. Page ID approval for more info. The reminder was embedded within the influenza results for all positive determinations, so a clinician would see the reminder when viewing positive laboratory results (Meditech, Westwood, MA).
At the initiation of season 6, we determined prescription rates of oseltamivir in patients with CA‐LCI to measure the baseline rate of oseltamivir prescription. The electronic reminder was initiated during week 11 of influenza activity at our institution and continued through the end of the influenza season.
Data Collection
Two sources of antiviral prescription data were used. Inpatient prescription of antiviral medications was extracted from billing records and chart review; a 10% audit of the medication administration records showed that the billing records correctly identified oseltamivir prescription status in all cases reviewed. Patients with incomplete pharmacy data were removed from the analysis of prescription practices (n = 8). During all seasons studied, the infectious diseases pharmacist (T.A.M.) and an infectious diseases physician (T.E.Z.) reviewed requests for inpatient prescriptions for antiviral medications.
For season 6, daily review of infection control records was performed to conduct surveillance for children hospitalized with CA‐LCI. To determine symptom duration and use of antiviral medications, inpatient medical charts were reviewed at the time of initial identification and then daily thereafter.
Statistical Analysis
Dichotomous variables were created for prescription of oseltamivir, age 1 year and symptom duration of <48 hours at time of clinician receipt of influenza results. Descriptive analyses included calculating the frequencies for categorical variables. Categorical variables were compared using Fisher's exact test. The Cochrane‐Armitage test was employed to test for a trend in the prescription of oseltamivir by season. A 2‐tailed P value of <0.05 was considered significant for all statistical tests. All statistical calculations were performed using standard programs in SAS 9.1 (SAS Institute, Cary, NC), STATA 8.2 (Stata Corp., College Station, TX), and Excel (Microsoft, Redmond, WA).
Prior to the start of season 6, we determined that if the rate of oseltamivir prescription was 40% before initiation of the reminder, we would need 20 eligible patients to detect a difference of 40% or greater in subsequent prescription rates (with 80% power and an alpha of 0.05). Once this enrollment goal was met, an electronic reminder of the eligibility for oseltamivir was initiated.
RESULTS
Use of Antiviral Medications in Children Hospitalized with Influenza, 2000‐2005
From July 2000 to June 2005, 1,058 patients were admitted with laboratory confirmed influenza; 8 were excluded because confirmatory testing was done at an outside institution, 24 were repeat hospitalizations, 89 nosocomial cases, and 8 cases were in patients >21 years. Thus, 929 patients had CA‐LCI and were eligible for inclusion in this study. Most children were infected with influenza A and were 1 year of age (Table 1). During this study period, only 9.3% of study subjects were treated with antiviral medications, most of whom (91%) received oseltamivir. Eight patients received amantadine over all seasons studied.
| Characteristics | Patients Hospitalized with CA‐LCI (n = 929)* | Eligible to Receive Oseltamivir (n = 305)* |
|---|---|---|
| ||
| Age (years) | ||
| <1 | 342 (37) | 0 |
| 1 | 587 (63) | 305 (100) |
| Season | ||
| 2000‐2001 | 107 (11.5) | 32 (10) |
| 2001‐2002 | 252 (27) | 78 (26) |
| 2002‐2003 | 135 (14.5) | 31 (10) |
| 2003‐2004 | 243 (26) | 86 (28) |
| 2004‐2005 | 192 (21) | 78 (26) |
| Influenza type | ||
| A | 692 (75) | |
| B | 237 (25) | |
Overall, one‐third of patients (305/929; 33%) were eligible for treatment with oseltamivir. Among patients 1 year of age, approximately one‐half (305/587; 52%) were oseltamivir‐eligible. The additional 282 patients 1 year were ineligible because test results were returned to the clinician >48 hours after hospital admission. Only 49 (16.1%) of oseltamivir‐eligible patients were prescribed oseltamivir during hospitalization (Figure 1). The rate of prescription of oseltamivir increased over all seasons from 0% in 2000‐2001 to 20% in 2004‐2005. On‐label prescription rates increased from 0% in 2000‐2001 to 37.2% in 2004‐2005 (P < 0.0001; Figure 2).


Off‐Label Oseltamivir Prescription
Oseltamivir was prescribed to 29 of the 624 patients who were determined to be oseltamivir‐ineligible. The rate of off‐label use increased over the seasons from 2000 to 2005 from 0% to 8.8% (P < 0.0001; Figure 1). Ineligible patients who received oseltamivir were 1 year of age (n = 11), had test results returned to the clinician 48 hours after hospital admission (n = 18), or both (n = 4). Most off‐label prescriptions occurred in patients who had chronic medical conditions (21/29; 72%), including cardiac disease (n = 9), asthma (n = 6), or prematurity (n = 5). Four of 11 patients 1 year of age who were treated with oseltamivir had influenza‐related respiratory failure. The oseltamivir dose for all patients 1 year of age was 2 mg/kg twice a day, all of whom survived to discharge.
Evaluation of a Computer‐Based Electronic Reminder Designed to Enhance the On‐Label Prescription of Oseltamivir
During season 6, an electronic reminder about the labeled use of oseltamivir was evaluated to determine its ability to increase the rate of prescription of oseltamivir among eligible children hospitalized with CA‐LCI. During season 6, most patients (226/311; 73%) were 1 year of age. A total of 84 patients were determined to be oseltamivir‐eligible (age 1 year and test results back to the clinician within 48 hours of symptom onset).
During the initial 10 weeks of local influenza activity, 20 oseltamivir‐eligible patients were admitted to our institution, and 8 received oseltamivir (40% prescription rate) (Table 2). In addition, 2 of 54 (3.7%) oseltamivir‐ineligible patients were also treated. The computer‐based electronic reminder was initiated in week 11 of the influenza season. After initiation of the reminder, 237 additional children with CA‐LCI were hospitalized, of whom 64 (27%) were determined to be oseltamivir‐eligible. The rate of on‐label prescription of oseltamivir was similar to that observed prior to initiation of the reminder: 16 of 64 patients eligible for antiviral therapy received oseltamivir (25% prescription rate) (Figure 3). An additional 8 patients were prescribed oseltamivir off‐label. The rate of oseltamivir prescription did not change significantly for either oseltamivir‐eligible (40‐25%) or oseltamivir‐ineligible (3.7‐4.6%) (Figure 4).


| Prompt Active? | Oseltamivir Use | Total | |
|---|---|---|---|
| Yes* | No | ||
| |||
| No | |||
| Eligible | 8 (40) | 12 | 20 |
| Ineligible | 2 (3.7) | 52 | 54 |
| Yes | |||
| Eligible | 16 (25) | 48 | 64 |
| Ineligible | 8 (4.6) | 165 | 173 |
| Total | 34 | 277 | 311 |
Dermatologic, Neurologic, and Neuropsychiatric Adverse Events
We reviewed the medical records of all patients treated with oseltamivir during the 6 study seasons to identify dermatologic, neurologic, and neuropsychiatric adverse outcomes that developed after the initiation of oseltamivir therapy. No new‐onset seizures, neuropsychiatric, or dermatologic reactions were identified among the children treated with oseltamivir.
DISCUSSION AND CONCLUSION
In this report, we describe the use of oseltamivir over 6 seasons in a cohort of children hospitalized with CA‐LCI at 1 tertiary care pediatric hospital and examine the impact of a mechanism designed to increase prescription among those eligible for oseltamivir. We found that only one‐third of patients hospitalized at our institution were eligible for oseltamivir treatment based on FDA‐approved indications. Of the eligible patients, few were prescribed oseltamivir during their hospitalization. During the sixth season, we employed a computer reminder system for oseltamivir prescription, which had no appreciable effect upon prescription rates. Despite the lack of effect of the electronic reminder system, we observed an increase of on‐label oseltamivir prescriptions over the entire study period. Finally, we identified 11 patients <1 year of age (3%) who were treated with oseltamivir. There were no adverse events identified in this group.
Although previous studies have addressed prescription rates of oseltamivir in children with influenza, few, if any, have looked at how these prescriptions correspond with FDA label criteria. In our cohort, only one‐third of hospitalized children were eligible for treatment with oseltamivir based upon their age and symptom duration at the time the results of rapid laboratory testing became available. Of those patients in our cohort eligible for oseltamivir, few were treated. The prescription of oseltamivir in seasons falls within the ranges found by Schrag et al.7 in their multistate review of pediatric influenza hospitalizations in 2003‐2004. They noted that use of antiviral medications varied by location of surveillance ranging from 3% in Connecticut to 34% in Colorado, indicating significant regional differences in prescription practices.7 Potential causes of low rates of appropriate use of oseltamivir include the observation that many physicians remain unaware of the potential severity of influenza infection in children.8 Additionally, physicians may differ on how to define the onset of influenza infection in children. A recent study published by Ohmit and Monto9 indicated that a fever and cough predicted 83% of children 5 to 12 years old who were determined to be influenza‐positive. Finally, many physicians who do not prescribe antiviral therapy may believe that their patients present too late for appropriate initiation of therapy.10
We identified 29 patients who received oseltamivir although they did not meet the FDA label criteria, of whom 72% had a chronic underlying condition. Moore et al.11 in their surveillance of influenza admissions in Canada found a similar trend. They described 26 of 29 (90%) hospitalized patients receiving antiinfluenza drugs had an underlying disease, and of those without a chronic condition, all had severe influenza‐related complications such as encephalopathy.11
Implementation of a computerized reminder to improve use of oseltamivir had no statistically significant effect on prescribing practice. Our sample size calculation was based on detecting a 40% difference in prescription rates, which limited our power to detect a smaller difference in prescription rates. A systematic review by Garg et al.12 identified barriers to the success of computer‐based decision support systems (CDS), which included failure of practitioners to use the system, poor integration of the system to the physician's workflow, and disagreement with what was recommended. Future enhancements to our inpatient electronic hospital record may allow for more targeted and robust CDS interventions.
We observed an increase in on‐label prescription rates of oseltamivir over the entire study period. We hypothesize that increased use of oseltamivir might be associated with growing concerns of pandemic influenza and attention to fatal influenza in children,13 as evidenced by the recent addition of influenza‐associated deaths in children to the list of nationally notifiable conditions in 2004.14
There has been considerable focus upon potential adverse events associated with treatment with oseltamivir in children. Reports have emerged, primarily from Japan, of neuropsychiatric and dermatologic adverse events of oseltamivir treatment.15 In the fall of 2006, the FDA added a precaution to the labeling of oseltamivir due to these neuropsychiatric events.16 In our treated cohort, no neurologic, neuropsychiatric, or dermatologic adverse events were identified. However, this finding is not surprising given the rarity of these adverse events and the limited number of children treated with oseltamivir in this study.
The strengths of this current study include a large cohort of laboratory‐confirmed influenza in hospitalized children over multiple influenza seasons. In addition, this is the first study of which we are aware that has assessed the number of children eligible for oseltamivir but not treated. The limitations of this study include misclassification bias related to the retrospective study design. Because of this design, onset of influenza symptoms was collected through chart review, and the time of receipt of influenza results from virology was based upon known laboratory turnover time, rather than actual knowledge of time of physician awareness of the result. To address this issue we used a conservative estimate of the time of receipt of influenza test results. In addition, the retrospective design prevented us from assessing the clinical decision‐making process, which led some patients to be treated with oseltamivir and others not. Our evaluation of the electronic reminder was designed to show a large change in prescription practices (ie, 40%), so it had insufficient power to detect a smaller impact. Finally, ascertainment bias may have limited our ability to identify adverse effects.
This study demonstrates that oseltamivir is prescribed infrequently among hospitalized children. Future studies are needed to determine whether appropriate use of oseltamivir improves outcomes among hospitalized children. Additional study of the safety and efficacy of oseltamivir in children aged <1 year is also needed given the large burden of disease in this age group.
Acknowledgements
We thank Michelle Precourt for her assistance with the computer‐based prompt. We also thank Drs. Anna Wheeler Rosenquist and Melissa Donovan for the original data collection for this project. This project was supported in part by the Centers for Disease Control and Prevention, grant H23/CCH32253‐02.
- ,,, et al.Influenza and the rates of hospitalization for respiratory disease among infants and young children.N Engl J Med.2000;342:232–239.
- ,,,,.The effect of influenza on hospitalizations, outpatient visits and courses of antibiotics in children.N Engl J Med.2000;342:225–231.
- Centers for Disease Control and Prevention (CDC). Diseases and Conditions. Seasonal Flu. CDC Health Alert: CDC recommends against the use of amantadine and rimantadine for the treatment or prophylaxis of influenza in the United States during the 2005–06 influenza season. January 14, 2006. Available at http://www.cdc.gov/flu/han011406.htm. Accessed November2008.
- ,,, et al.Oral oseltamivir treatment of influenza in children.Pediatr Infect Dis J.2001;20(2):127–133.
- ,,, et al.Off‐label drug use in hospitalized children.Arch Pediatr Adolesc Med.2007;161(3):282–290.
- ,,, et al.Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection.JAMA.2005;294:2188–2194.
- ,,, et al.Multistate surveillance for laboratory‐confirmed influenza‐associated hospitalizations in children 2003–2004.Pediatr Infect Dis J.2006;25(5):395–400.
- ,.Physician knowledge and perspectives regarding influenza and influenza vaccination.Hum Vaccin.2005;1(2):74–79.
- ,.Symptomatic predictors of influenza virus positivity in children during the influenza season.Clin Infect Dis.2006;43:564–568.
- ,,,,,.Effects of local variation, specialty, and beliefs on antiviral prescribing for influenza.Clin Infect Dis.2006;42:95–99.
- ,,, et al.Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004.Pediatrics.2006;118:e610–e619.
- ,,, et al.Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review.JAMA.2005;293:1223–1238.
- ,,, et al.Influenza‐associated deaths among children in the United States, 2003–2004.N Engl J Med.2005;353(24):2559–2567.
- Centers for Disease Control and Prevention (CDC). Diseases and Conditions. Seasonal Flu. Flu Activity 25(6):572.
- ,,.Post‐Marketing Adverse Event Reports. Review of Central Nervous System/Psychiatric Disorders Associated with the Use of Tamiflu, Drug: Oseltamivir Phosphate. Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Drug Evaluation and Research, Office of Surveillance and Epidemiology.2006. OSE PID #D060393 Oseltamivir—Neuropsychiatric Events.
- ,,, et al.Influenza and the rates of hospitalization for respiratory disease among infants and young children.N Engl J Med.2000;342:232–239.
- ,,,,.The effect of influenza on hospitalizations, outpatient visits and courses of antibiotics in children.N Engl J Med.2000;342:225–231.
- Centers for Disease Control and Prevention (CDC). Diseases and Conditions. Seasonal Flu. CDC Health Alert: CDC recommends against the use of amantadine and rimantadine for the treatment or prophylaxis of influenza in the United States during the 2005–06 influenza season. January 14, 2006. Available at http://www.cdc.gov/flu/han011406.htm. Accessed November2008.
- ,,, et al.Oral oseltamivir treatment of influenza in children.Pediatr Infect Dis J.2001;20(2):127–133.
- ,,, et al.Off‐label drug use in hospitalized children.Arch Pediatr Adolesc Med.2007;161(3):282–290.
- ,,, et al.Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection.JAMA.2005;294:2188–2194.
- ,,, et al.Multistate surveillance for laboratory‐confirmed influenza‐associated hospitalizations in children 2003–2004.Pediatr Infect Dis J.2006;25(5):395–400.
- ,.Physician knowledge and perspectives regarding influenza and influenza vaccination.Hum Vaccin.2005;1(2):74–79.
- ,.Symptomatic predictors of influenza virus positivity in children during the influenza season.Clin Infect Dis.2006;43:564–568.
- ,,,,,.Effects of local variation, specialty, and beliefs on antiviral prescribing for influenza.Clin Infect Dis.2006;42:95–99.
- ,,, et al.Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004.Pediatrics.2006;118:e610–e619.
- ,,, et al.Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review.JAMA.2005;293:1223–1238.
- ,,, et al.Influenza‐associated deaths among children in the United States, 2003–2004.N Engl J Med.2005;353(24):2559–2567.
- Centers for Disease Control and Prevention (CDC). Diseases and Conditions. Seasonal Flu. Flu Activity 25(6):572.
- ,,.Post‐Marketing Adverse Event Reports. Review of Central Nervous System/Psychiatric Disorders Associated with the Use of Tamiflu, Drug: Oseltamivir Phosphate. Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Drug Evaluation and Research, Office of Surveillance and Epidemiology.2006. OSE PID #D060393 Oseltamivir—Neuropsychiatric Events.
Copyright © 2009 Society of Hospital Medicine
Positively False
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A51‐year‐old woman presented after 5 days of fever, rigors, anorexia, right‐sided abdominal pain, nausea, and dizziness. She had 2 loose stools the day before admission, without blood or mucus, but otherwise had recently been constipated. She denied cough, shortness of breath, chest pain, headache, sore throat, rash, arthritis, or dysuria.
In a 51‐year‐old woman with right‐sided abdominal pain and systemic symptoms, major concerns include biliary disease, liver abscess, or appendicitis. Right‐sided diverticulitis would be more unusual. Pyelonephritis infrequently presents with epigastric and lower quadrant pain, instead of flank pain. Basilar pneumonia may present with abdominal pain, but this is less likely in the absence of respiratory symptoms.
The patient used an albuterol inhaler for mild asthma and had experienced an episode of herpes zoster 7 years prior, but was otherwise well. Her surgical history was notable for a remote appendectomy. She was a native of the Dominican Republic who had lived in the United States for the past 20 years. She visited the Dominican Republic for 3 weeks every year, with her last visit occurring about 10 months before. She was a cleaning and maintenance worker. She had 2 adult children in good health, was divorced from her husband, and had not been sexually active for the past 8 years. The patient had no pets or other animal exposures. She did not smoke, drink alcohol, or use intravenous drugs.
The remote episode of shingles makes me a bit worried about chronic human immunodeficiency virus (HIV) infection. As a native of and traveler to the Dominican Republic, she is at risk for a variety of tropical pathogens. Hyperinfection syndrome from strongyloides can cause fever and bacteremia, but this is almost always associated with significant immunosuppression. Dengue fever has become very common in the Caribbean, but should occur within 2 weeks of travel. Her work in cleaning and maintenance might bring her into contact with rats and mice, putting her at risk for leptospirosis. This can present as a fairly nonspecific febrile syndrome, but this is unlikely without a major complaint of headache.
The patient appeared fatigued. Her temperature was 39.7C, her heart rate was 110 beats per minute, and her blood pressure 80/62 mm Hg. The oropharynx was normal. Mild cervical lymphadenopathy was present (less than 1 cm in diameter). The chest was clear and the cardiac examination unremarkable. Bowel sounds were present. Moderate right‐sided abdominal tenderness was noted, somewhat more marked in the right lower quadrant, without guarding or rebound. There was no hepatosplenomegaly. There was no rash. A bedside right upper quadrant ultrasound was negative for gallstones.
Her low blood pressure is concerning for bacterial sepsis. The negative right upper quadrant ultrasound makes cholecystitis or cholangitis less likely, but does not exclude diverticulitis or pelvic inflammatory disease. She lacks peritoneal signs, but they may be absent in these conditions. Another worrisome finding on her physical examination is cervical lymphadenopathy. In an older patient, this raises the specter of malignancy. In a younger patient, it could suggest a mononucleosis syndrome from Epstein‐Barr virus (EBV) or cytomegalovirus (CMV). In addition, HIV must be considered in any patient with unexplained lymphadenopathy.
She received intravenous levofloxacin and 1 L of intravenous normal saline, with a rise in her blood pressure to 100/59 mm Hg. Her white blood cell count was 5.0, with 71% polys and 9% bands. The hematocrit was 32%, with a normal mean corpuscular volume. The erythrocyte sedimentation rate (ESR) was 109 mm/hour. The platelet count, serum electrolytes, creatinine, aminotransferases, alkaline phosphatase, bilirubin, amylase, and lipase were normal. The serum level of lactate dehydrogenase (LDH) was 498 unit/L (normal range, 107231 units/L). Computed tomography (CT) scan of the abdomen showed multiple enlarged lymph nodes up to 1.2 cm in size along the gastrohepatic ligament and the mesentery, with mild associated fat stranding, consistent with mesenteric lymphadenitis.
Her blood pressure has responded to fluids; perhaps she was just volume‐depleted from not eating for several days. She has bandemia, which is consistent with acute bacterial infection, but might also signify a stress response. The low hematocrit and high ESR raise the possibility of anemia of chronic disease; perhaps her illness is more longstanding than her presentation suggests. Mesenteric lymphadenitis may be related to EBV and HIV, but in a patient originally from the Caribbean, it raises the possibility of gastrointestinal tuberculosis or histoplasmosis. Human T‐cell lymphotropic virus type 1 (HTLV‐1) is also endemic in the Caribbean, and may cause adult T‐cell leukemia/lymphoma (ATLL), which could explain her lymphadenopathy and elevated LDH. Mesenteric lymphadenitis is also characteristic of several bacterial infections, especially Yersinia, Salmonella, and Bartonella. The lack of diarrhea makes yersiniosis doubtful, and the absence of cat exposure makes bartonellosis unlikely. Salmonella infection is also associated with diarrhea, except for typhoid fever, in which patients have diarrhea, constipation, or normal stools.
A urine culture and 3 sets of blood cultures obtained prior to the initiation of antibiotics were negative. A blood smear showed no malaria or babesia parasites. The patient's fever continued for the first 2 days of her hospitalization, but subsequently abated. The patient had no loose stools during her hospitalization. Serologies for EBV, hepatitis A, CMV, and toxoplasmosis were indicative of remote infection. Serum rapid plasma reagin (RPR), Bartonella antibodies, antinuclear antibodies, hepatitis B surface antigen, and hepatitis C antibody were negative. Tuberculin skin testing and urinary histoplasma antigen were negative. By the fifth hospital day, she had been afebrile for over 48 hours, and her abdominal pain had improved, though it had not completely resolved. She was discharged to complete a 10‐day course of levofloxacin.
It is not clear to me whether she has just experienced a spontaneous remission in her illness, or whether her disease course has truly been modified with antibiotics. Could she have typhoid fever or another salmonellosis? The patient has not traveled abroad in several months. If she had typhoid fever, the source of infection would have to be imported food, or exposure to a family member who was a carrier. The negative tuberculin skin test makes tuberculosis less likely, but does not exclude it entirely. Similarly, while a negative histoplasma urinary antigen essentially rules out acute disseminated histoplasmosis, as would be seen in acquired immune deficiency syndrome (AIDS), it is less sensitive for more chronic forms of disseminated histoplasmosis, including gastrointestinal involvement.
One week later, the patient returned to the emergency department with recurrent abdominal pain, anorexia, fever, night sweats, and increased swelling of the lymph nodes in her neck. She had lost a total of 4.4 kg (10 lb) since the onset of her illness. Physical examination revealed moderate (up to 2 cm), slightly tender cervical and inguinal lymphadenopathy, and continued moderate right‐sided abdominal tenderness. Mesenteric, retroperitoneal, and inguinal lymphadenopathy was more prominent than on the prior CT scan (Figure 1).She was told by the emergency room physicians that the HIV test obtained during the prior hospitalization was positive. Further questioning elicited that her estranged husband had been promiscuous prior to their separation. She was transferred to a second hospital for further care. Blood cultures were sent for fungi and acid‐fast bacilli.
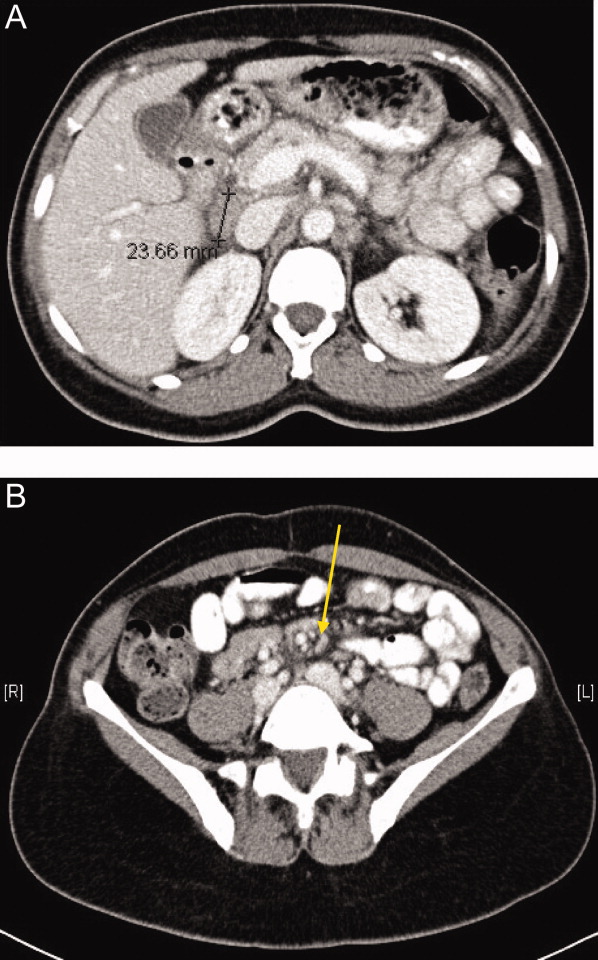
Everyone with fever of unknown origin (FUO) deserves an HIV test. Is this just lymphadenopathy from HIV, or is she suffering from an opportunistic infection? Mycobacterium avium infection is an attractive explanation for her fever, abdominal pain, and lymphadenopathy, but I would hold off on empiric treatment until the results of a CD4+ cell count were available. Could she have a secondary, HIV‐related lymphoma?
Full review of records from the outside hospital showed that the patient had a positive HIV enzyme immunoassay, with an indeterminate HIV Western blot. The patient's CD4 cell count was 313/cm3, with a CD4/CD8 ratio within the normal range. Her HIV enzyme immunoassay was repeated and found to be negative, and the HIV viral load was undetectable.
The HIV enzyme immunoassay is only a screening test, and must be confirmed with a positive HIV Western blot. (In most clinical laboratories, this is done automatically before the test is reported.) Indeterminate HIV Western blots are common in acute HIV infection, but outside of this setting, most patients with indeterminate HIV Western blots turn out not to have HIV infection. I am still very concerned about lymphoma, and would pursue a lymph node biopsy. Disseminated tuberculosis is still possible.
Biopsy of a right inguinal lymph node was performed on the third day of the second hospitalization. The patient was persistently febrile, developed swelling of the knees, ankles, and interphalangeal joints of the second and third digits of the left hand, and complained of pruritus. Anti‐double‐stranded DNA antibody, antineutrophilic cytoplasmic antibody, and Brucella antibody were negative. Antibody against cyclic citrullinated peptide (CCP) was weakly positive.
Now she has a more florid syndrome, with arthritic symptoms. Sarcoidosis is an attractive explanation for her fever, lymphadenopathy, and arthritis. Lupus could explain some features of her presentation, but the negative serology makes it unlikely. Antibody to cyclic citrullinated peptide is a newer diagnostic test for rheumatoid arthritis. Although anti‐CCP is more specific than rheumatoid factor for the diagnosis of rheumatoid arthritis, this result could still be a false positive, particularly given the low titer. As well, the patient has more impressive lymphadenopathy than is usual for rheumatoid arthritis. Reactive arthritis can follow enteric infection with Campylobacter, Salmonella, Yersinia, and Shigella, but lymphadenopathy is not a feature. Arthritis may be prominent in parvovirus B19, rubella, disseminated gonococcal infection, and Lyme disease, but mesenteric lymphadenitis and a prolonged, waxing and waning course would not be expected with any of these. I worry that the arthritis and pruritus are paraneoplastic manifestations of lymphoma.
The inguinal lymph node biopsy showed markedly distorted nodal architecture with an atypical proliferation of small‐sized to large‐sized lymphoid cells, with predominantly round nuclei, vesicular chromatin, small nucleoli, and variable amounts of clear to eosinophilic cytoplasm (Figure 2). Residual follicles and occasional apoptotic bodies and mitoses were seen. Large B‐cells were frequently present, which stained positive for EBV‐associated mRNA by in situ hybridization studies. Immunoperoxidase staining revealed that the atypical cell population was largely composed of CD4+ T‐cells. T‐cell receptor gene rearrangement studies demonstrated that the T‐cell population was monoclonal. These results were consistent with angioimmunoblastic T‐cell lymphoma (AITL). Positron emission tomography (PET) scanning was performed, showing diffuse fluorodeoxyglucose (FDG)‐avid lymphadenopathy involving cervical, axillary, mediastinal, retroperitoneal, mesenteric, and inguinal lymph nodes, up to 2 cm in diameter. The patient completed 6 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), as well as experimental treatment with denileukin diftitox. Her fever and arthritis subsided quickly, and she was clinically well and free of disease by CT scans and PET scans 1 year after diagnosis.
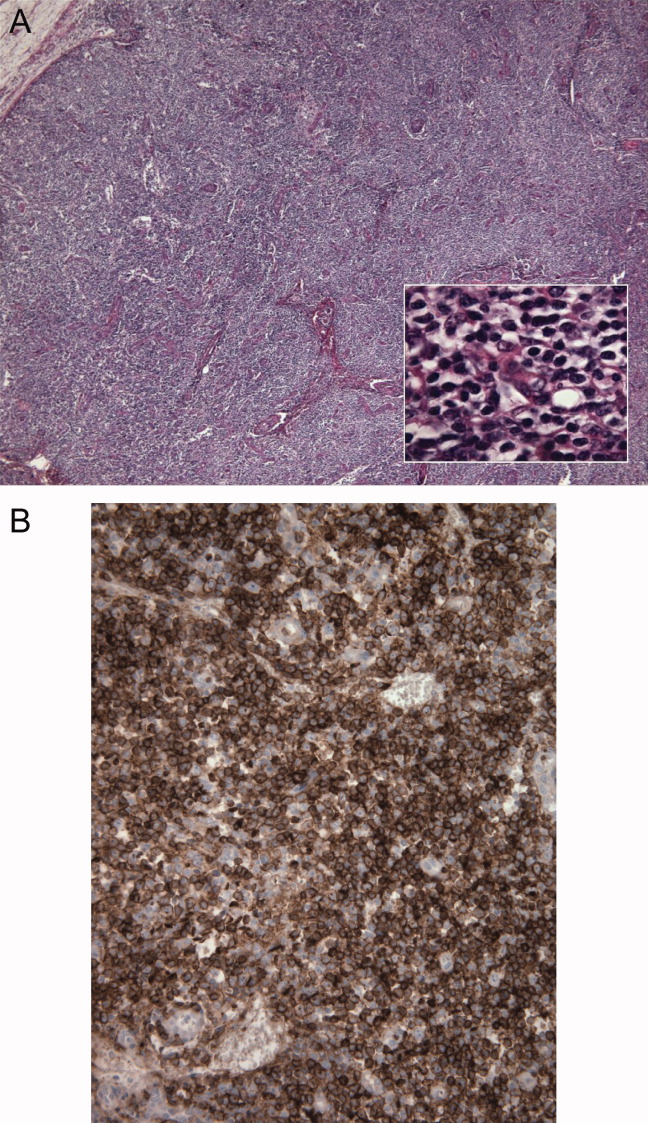
COMMENTARY
The differential diagnosis of FUO is one of the largest in medicine, encompassing a dizzying range of infectious, inflammatory, and neoplastic conditions. This has made the development of standardized diagnostic algorithms difficult.1 The workup of patients with FUO begins with a detailed history and physical examination, followed by a core set of microbiology cultures, imaging studies, and blood tests on all patients. Further testing is individualized, based on key clinical findings, also known as pivot points.2 Unfortunately, many of the diseases presenting as FUO have overlapping symptoms and signs, somewhat limiting the utility of pivot points. In a prospective study, 81% of these potentially diagnostic clues were misleading, although 19% of them contributed to the final diagnosis.3 Key clinical findings in patients with FUO may trigger a barrage of diagnostic tests, often leading to a large number of false‐positive results.4 Clinicians who investigate patients with FUO must remember that many clues are diagnostic dead ends, and should be wary of drawing positively‐false conclusions.
Fever pattern is usually not helpful in the diagnosis of FUO, with occasional exceptions, such as the tertian and quartan fevers in some forms of malaria. A minority of patients with Hodgkin's disease have Pel‐Ebstein fevers, in which 1 to 2 weeks of fever alternate with an afebrile period of similar or longer duration. More often, fever in lymphoma waxes and wanes unpredictably,5 a circumstance that may lead to the mistaken impression of response to antibiotics, as in this case.
Tissue biopsies are helpful in FUO when suggestive findings are present on physical examination or imaging studies. In patients with lymphadenopathy and FUO, lymph node biopsy is a high‐yield procedure, contributing to the final diagnosis 46% of the time. This is exceeded only by biopsies of skin lesions, which have a 63% diagnostic yield in FUO.3 In older patients with FUO, temporal artery biopsies have a significant diagnostic yield, in the range of 16% to 17%. Liver biopsies have a similar yield (14‐17%), but a higher risk of complications. Bone marrow biopsies have a low yield in most FUO patients.1
AITL makes up 1% of all non‐Hodgkin's lymphomas. AITL was once known as angioimmunoblastic lymphadenopathy, and was thought to be either a disorder of immune regulation or a premalignant lymphoid disease. However, molecular diagnostic techniques have established that monoclonal T‐cell populations and cytogenetic abnormalities are usually present at the time of diagnosis.6, 7 The prognosis in AITL is unfavorable. Disease is usually widespread at the time of diagnosis. Most patients achieve complete remission with anthracycline‐based chemotherapy, such as CHOP, but the duration of remission is often brief, and median survival after diagnosis is only 3 years.6 Novel treatments under investigation include denileukin diftitox,8 a fusion protein of interleukin‐2 (IL‐2) conjugated to diphtheria toxin, which leads to apoptosis of cells expressing the IL‐2 receptor; rituximab, which targets the reactive population of B‐cells in AITL, rather than the malignant clone of T‐cells; and antiangiogenic therapy, such as thalidomide.9
The diagnosis of AITL is usually elusive, and the average patient has symptoms for 4 months prior to diagnosis.6 This patient's clinical presentation, while certainly not specific, was typical of AITL. AITL usually presents as fever of unknown origin with generalized, nonbulky lymphadenopathy. Fever is present in 57% of patients with AITL, and up to 2% of FUO is caused by AITL.6, 10 Other features of this patient's illness, such as night sweats, weight loss, pruritus, and arthritis, are common in AITL.
T‐cell depletion and immune dysregulation are frequent in AITL, explaining why AITL shares a number of clinical features with HIV disease. These include a high incidence of drug rashes, immune thrombocytopenic purpura, polyclonal hypergammaglobulinemia, and autoantibodies.6, 7 As with HIV patients, death in AITL is often due to opportunistic infections or diffuse large B‐cell lymphomas.7 Over 95% of patients with AITL display a proliferation of EBV‐infected B cells, presumably from immune dysregulation, and the B‐cell lymphomas in these patients are usually EBV‐positive.11
Because AITL is a proinflammatory state, false‐positive antibody tests are fairly common. The occasional occurrence of positive HIV enzyme immunoassays, with indeterminate Western blot results, may be a particular source of diagnostic confusion.12, 13 The significance of indeterminate Western blots is often erroneously communicated to patients, as happened here. Indeterminate HIV Western blots may be seen in HIV seroconversion, HIV‐2 infection, or advanced HIV disease with loss of core antibody. They may also result from laboratory error, multiparity, syphilis, malaria, or cross‐reacting antibodies in autoimmune diseases. Indeterminate HIV Western blots in low‐risk patients usually do not represent true HIV infection.14
KEY POINTS FOR HOSPITALISTS
-
FUO is one of the most challenging diagnoses faced by hospitalists. Biopsies of new skin lesions and enlarged lymph nodes are particularly high‐yield diagnostic procedures in FUO. Fever pattern is generally not helpful in the diagnosis of FUO, with few exceptions, such as the tertian fevers of malaria.
-
Misleading and false‐positive tests results often occur in the course of FUO evaluation, due to the sheer number of tests ordered, and the higher likelihood of false‐positive serologic tests in the setting of inflammatory states.
-
AITL may be an elusive diagnosis in FUO with diverse clinical features, including weight loss, night sweats, rashes, arthritis, autoantibodies, immune dysregulation, and opportunistic infections. The prognosis traditionally has been guarded, but may be more hopeful in an era of emerging molecular therapies.
- ,,.A comprehensive evidence‐based approach to fever of unknown origin.Arch Intern Med.2003;163:545–551.
- ,.The art of diagnosis: solving the clinicopathological exercise.N Engl J Med.1982;306:1263–1268.
- ,,, et al.A prospective multicenter study on fever of unknown origin: the yield of a structured diagnostic protocol.Medicine (Baltimore).2007;86:26–38.
- ,,.Fever of unknown origin (FUO). II. Diagnostic procedures in a prospective multicenter study of 167 patients. The Netherlands FUO Study Group.Medicine (Baltimore).1997;76:401–414.
- ,.Neoplastic diseases. In:Murray HW, ed.FUO: Fever of Undetermined Origin.New York, NY:Futura Publishing;1983:39–48.
- ,,, et al.Angioimmunoblastic T‐cell lymphoma: clinical and laboratory features at diagnosis in 77 patients.Medicine (Baltimore).2007;86:282–292.
- ,,.Angioimmunoblastic T‐cell lymphoma.Br J Haematol.2003;121:681–691.
- ,,, et al.Phase II trial of denileukin diftitox for relapsed/refractory T‐cell non‐Hodgkin lymphoma.Br J Haematol.2007;136:439–447.
- ,.Angioimmunoblastic T‐cell lymphoma: still a dismal prognosis with current treatment approaches.Leuk Lymphoma.2007;48:645–646.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO Study Group.Medicine (Baltimore).1997;76:392–400.
- ,,, et al.Histologic evolution of angioimmunoblastic T‐cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression.Am J Surg Pathol.2007;31:1077–1088.
- ,,,.Angioimmunoblastic lymphadenopathy, immunoblastic lymphoma, and false‐positive seroconversion for human immunodeficiency virus.Ann Intern Med.1987;107:114.
- ,.Angioimmunoblastic T‐cell lymphoma associated with an antibody to human immunodeficiency virus protein.Int J Hematol.2003;78:160–162.
- ,,.Communicating indeterminate HIV Western blot test results to clients: an observational study of three community testing sites.AIDS Patient Care STDS.2006;20:620–627.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A51‐year‐old woman presented after 5 days of fever, rigors, anorexia, right‐sided abdominal pain, nausea, and dizziness. She had 2 loose stools the day before admission, without blood or mucus, but otherwise had recently been constipated. She denied cough, shortness of breath, chest pain, headache, sore throat, rash, arthritis, or dysuria.
In a 51‐year‐old woman with right‐sided abdominal pain and systemic symptoms, major concerns include biliary disease, liver abscess, or appendicitis. Right‐sided diverticulitis would be more unusual. Pyelonephritis infrequently presents with epigastric and lower quadrant pain, instead of flank pain. Basilar pneumonia may present with abdominal pain, but this is less likely in the absence of respiratory symptoms.
The patient used an albuterol inhaler for mild asthma and had experienced an episode of herpes zoster 7 years prior, but was otherwise well. Her surgical history was notable for a remote appendectomy. She was a native of the Dominican Republic who had lived in the United States for the past 20 years. She visited the Dominican Republic for 3 weeks every year, with her last visit occurring about 10 months before. She was a cleaning and maintenance worker. She had 2 adult children in good health, was divorced from her husband, and had not been sexually active for the past 8 years. The patient had no pets or other animal exposures. She did not smoke, drink alcohol, or use intravenous drugs.
The remote episode of shingles makes me a bit worried about chronic human immunodeficiency virus (HIV) infection. As a native of and traveler to the Dominican Republic, she is at risk for a variety of tropical pathogens. Hyperinfection syndrome from strongyloides can cause fever and bacteremia, but this is almost always associated with significant immunosuppression. Dengue fever has become very common in the Caribbean, but should occur within 2 weeks of travel. Her work in cleaning and maintenance might bring her into contact with rats and mice, putting her at risk for leptospirosis. This can present as a fairly nonspecific febrile syndrome, but this is unlikely without a major complaint of headache.
The patient appeared fatigued. Her temperature was 39.7C, her heart rate was 110 beats per minute, and her blood pressure 80/62 mm Hg. The oropharynx was normal. Mild cervical lymphadenopathy was present (less than 1 cm in diameter). The chest was clear and the cardiac examination unremarkable. Bowel sounds were present. Moderate right‐sided abdominal tenderness was noted, somewhat more marked in the right lower quadrant, without guarding or rebound. There was no hepatosplenomegaly. There was no rash. A bedside right upper quadrant ultrasound was negative for gallstones.
Her low blood pressure is concerning for bacterial sepsis. The negative right upper quadrant ultrasound makes cholecystitis or cholangitis less likely, but does not exclude diverticulitis or pelvic inflammatory disease. She lacks peritoneal signs, but they may be absent in these conditions. Another worrisome finding on her physical examination is cervical lymphadenopathy. In an older patient, this raises the specter of malignancy. In a younger patient, it could suggest a mononucleosis syndrome from Epstein‐Barr virus (EBV) or cytomegalovirus (CMV). In addition, HIV must be considered in any patient with unexplained lymphadenopathy.
She received intravenous levofloxacin and 1 L of intravenous normal saline, with a rise in her blood pressure to 100/59 mm Hg. Her white blood cell count was 5.0, with 71% polys and 9% bands. The hematocrit was 32%, with a normal mean corpuscular volume. The erythrocyte sedimentation rate (ESR) was 109 mm/hour. The platelet count, serum electrolytes, creatinine, aminotransferases, alkaline phosphatase, bilirubin, amylase, and lipase were normal. The serum level of lactate dehydrogenase (LDH) was 498 unit/L (normal range, 107231 units/L). Computed tomography (CT) scan of the abdomen showed multiple enlarged lymph nodes up to 1.2 cm in size along the gastrohepatic ligament and the mesentery, with mild associated fat stranding, consistent with mesenteric lymphadenitis.
Her blood pressure has responded to fluids; perhaps she was just volume‐depleted from not eating for several days. She has bandemia, which is consistent with acute bacterial infection, but might also signify a stress response. The low hematocrit and high ESR raise the possibility of anemia of chronic disease; perhaps her illness is more longstanding than her presentation suggests. Mesenteric lymphadenitis may be related to EBV and HIV, but in a patient originally from the Caribbean, it raises the possibility of gastrointestinal tuberculosis or histoplasmosis. Human T‐cell lymphotropic virus type 1 (HTLV‐1) is also endemic in the Caribbean, and may cause adult T‐cell leukemia/lymphoma (ATLL), which could explain her lymphadenopathy and elevated LDH. Mesenteric lymphadenitis is also characteristic of several bacterial infections, especially Yersinia, Salmonella, and Bartonella. The lack of diarrhea makes yersiniosis doubtful, and the absence of cat exposure makes bartonellosis unlikely. Salmonella infection is also associated with diarrhea, except for typhoid fever, in which patients have diarrhea, constipation, or normal stools.
A urine culture and 3 sets of blood cultures obtained prior to the initiation of antibiotics were negative. A blood smear showed no malaria or babesia parasites. The patient's fever continued for the first 2 days of her hospitalization, but subsequently abated. The patient had no loose stools during her hospitalization. Serologies for EBV, hepatitis A, CMV, and toxoplasmosis were indicative of remote infection. Serum rapid plasma reagin (RPR), Bartonella antibodies, antinuclear antibodies, hepatitis B surface antigen, and hepatitis C antibody were negative. Tuberculin skin testing and urinary histoplasma antigen were negative. By the fifth hospital day, she had been afebrile for over 48 hours, and her abdominal pain had improved, though it had not completely resolved. She was discharged to complete a 10‐day course of levofloxacin.
It is not clear to me whether she has just experienced a spontaneous remission in her illness, or whether her disease course has truly been modified with antibiotics. Could she have typhoid fever or another salmonellosis? The patient has not traveled abroad in several months. If she had typhoid fever, the source of infection would have to be imported food, or exposure to a family member who was a carrier. The negative tuberculin skin test makes tuberculosis less likely, but does not exclude it entirely. Similarly, while a negative histoplasma urinary antigen essentially rules out acute disseminated histoplasmosis, as would be seen in acquired immune deficiency syndrome (AIDS), it is less sensitive for more chronic forms of disseminated histoplasmosis, including gastrointestinal involvement.
One week later, the patient returned to the emergency department with recurrent abdominal pain, anorexia, fever, night sweats, and increased swelling of the lymph nodes in her neck. She had lost a total of 4.4 kg (10 lb) since the onset of her illness. Physical examination revealed moderate (up to 2 cm), slightly tender cervical and inguinal lymphadenopathy, and continued moderate right‐sided abdominal tenderness. Mesenteric, retroperitoneal, and inguinal lymphadenopathy was more prominent than on the prior CT scan (Figure 1).She was told by the emergency room physicians that the HIV test obtained during the prior hospitalization was positive. Further questioning elicited that her estranged husband had been promiscuous prior to their separation. She was transferred to a second hospital for further care. Blood cultures were sent for fungi and acid‐fast bacilli.

Everyone with fever of unknown origin (FUO) deserves an HIV test. Is this just lymphadenopathy from HIV, or is she suffering from an opportunistic infection? Mycobacterium avium infection is an attractive explanation for her fever, abdominal pain, and lymphadenopathy, but I would hold off on empiric treatment until the results of a CD4+ cell count were available. Could she have a secondary, HIV‐related lymphoma?
Full review of records from the outside hospital showed that the patient had a positive HIV enzyme immunoassay, with an indeterminate HIV Western blot. The patient's CD4 cell count was 313/cm3, with a CD4/CD8 ratio within the normal range. Her HIV enzyme immunoassay was repeated and found to be negative, and the HIV viral load was undetectable.
The HIV enzyme immunoassay is only a screening test, and must be confirmed with a positive HIV Western blot. (In most clinical laboratories, this is done automatically before the test is reported.) Indeterminate HIV Western blots are common in acute HIV infection, but outside of this setting, most patients with indeterminate HIV Western blots turn out not to have HIV infection. I am still very concerned about lymphoma, and would pursue a lymph node biopsy. Disseminated tuberculosis is still possible.
Biopsy of a right inguinal lymph node was performed on the third day of the second hospitalization. The patient was persistently febrile, developed swelling of the knees, ankles, and interphalangeal joints of the second and third digits of the left hand, and complained of pruritus. Anti‐double‐stranded DNA antibody, antineutrophilic cytoplasmic antibody, and Brucella antibody were negative. Antibody against cyclic citrullinated peptide (CCP) was weakly positive.
Now she has a more florid syndrome, with arthritic symptoms. Sarcoidosis is an attractive explanation for her fever, lymphadenopathy, and arthritis. Lupus could explain some features of her presentation, but the negative serology makes it unlikely. Antibody to cyclic citrullinated peptide is a newer diagnostic test for rheumatoid arthritis. Although anti‐CCP is more specific than rheumatoid factor for the diagnosis of rheumatoid arthritis, this result could still be a false positive, particularly given the low titer. As well, the patient has more impressive lymphadenopathy than is usual for rheumatoid arthritis. Reactive arthritis can follow enteric infection with Campylobacter, Salmonella, Yersinia, and Shigella, but lymphadenopathy is not a feature. Arthritis may be prominent in parvovirus B19, rubella, disseminated gonococcal infection, and Lyme disease, but mesenteric lymphadenitis and a prolonged, waxing and waning course would not be expected with any of these. I worry that the arthritis and pruritus are paraneoplastic manifestations of lymphoma.
The inguinal lymph node biopsy showed markedly distorted nodal architecture with an atypical proliferation of small‐sized to large‐sized lymphoid cells, with predominantly round nuclei, vesicular chromatin, small nucleoli, and variable amounts of clear to eosinophilic cytoplasm (Figure 2). Residual follicles and occasional apoptotic bodies and mitoses were seen. Large B‐cells were frequently present, which stained positive for EBV‐associated mRNA by in situ hybridization studies. Immunoperoxidase staining revealed that the atypical cell population was largely composed of CD4+ T‐cells. T‐cell receptor gene rearrangement studies demonstrated that the T‐cell population was monoclonal. These results were consistent with angioimmunoblastic T‐cell lymphoma (AITL). Positron emission tomography (PET) scanning was performed, showing diffuse fluorodeoxyglucose (FDG)‐avid lymphadenopathy involving cervical, axillary, mediastinal, retroperitoneal, mesenteric, and inguinal lymph nodes, up to 2 cm in diameter. The patient completed 6 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), as well as experimental treatment with denileukin diftitox. Her fever and arthritis subsided quickly, and she was clinically well and free of disease by CT scans and PET scans 1 year after diagnosis.

COMMENTARY
The differential diagnosis of FUO is one of the largest in medicine, encompassing a dizzying range of infectious, inflammatory, and neoplastic conditions. This has made the development of standardized diagnostic algorithms difficult.1 The workup of patients with FUO begins with a detailed history and physical examination, followed by a core set of microbiology cultures, imaging studies, and blood tests on all patients. Further testing is individualized, based on key clinical findings, also known as pivot points.2 Unfortunately, many of the diseases presenting as FUO have overlapping symptoms and signs, somewhat limiting the utility of pivot points. In a prospective study, 81% of these potentially diagnostic clues were misleading, although 19% of them contributed to the final diagnosis.3 Key clinical findings in patients with FUO may trigger a barrage of diagnostic tests, often leading to a large number of false‐positive results.4 Clinicians who investigate patients with FUO must remember that many clues are diagnostic dead ends, and should be wary of drawing positively‐false conclusions.
Fever pattern is usually not helpful in the diagnosis of FUO, with occasional exceptions, such as the tertian and quartan fevers in some forms of malaria. A minority of patients with Hodgkin's disease have Pel‐Ebstein fevers, in which 1 to 2 weeks of fever alternate with an afebrile period of similar or longer duration. More often, fever in lymphoma waxes and wanes unpredictably,5 a circumstance that may lead to the mistaken impression of response to antibiotics, as in this case.
Tissue biopsies are helpful in FUO when suggestive findings are present on physical examination or imaging studies. In patients with lymphadenopathy and FUO, lymph node biopsy is a high‐yield procedure, contributing to the final diagnosis 46% of the time. This is exceeded only by biopsies of skin lesions, which have a 63% diagnostic yield in FUO.3 In older patients with FUO, temporal artery biopsies have a significant diagnostic yield, in the range of 16% to 17%. Liver biopsies have a similar yield (14‐17%), but a higher risk of complications. Bone marrow biopsies have a low yield in most FUO patients.1
AITL makes up 1% of all non‐Hodgkin's lymphomas. AITL was once known as angioimmunoblastic lymphadenopathy, and was thought to be either a disorder of immune regulation or a premalignant lymphoid disease. However, molecular diagnostic techniques have established that monoclonal T‐cell populations and cytogenetic abnormalities are usually present at the time of diagnosis.6, 7 The prognosis in AITL is unfavorable. Disease is usually widespread at the time of diagnosis. Most patients achieve complete remission with anthracycline‐based chemotherapy, such as CHOP, but the duration of remission is often brief, and median survival after diagnosis is only 3 years.6 Novel treatments under investigation include denileukin diftitox,8 a fusion protein of interleukin‐2 (IL‐2) conjugated to diphtheria toxin, which leads to apoptosis of cells expressing the IL‐2 receptor; rituximab, which targets the reactive population of B‐cells in AITL, rather than the malignant clone of T‐cells; and antiangiogenic therapy, such as thalidomide.9
The diagnosis of AITL is usually elusive, and the average patient has symptoms for 4 months prior to diagnosis.6 This patient's clinical presentation, while certainly not specific, was typical of AITL. AITL usually presents as fever of unknown origin with generalized, nonbulky lymphadenopathy. Fever is present in 57% of patients with AITL, and up to 2% of FUO is caused by AITL.6, 10 Other features of this patient's illness, such as night sweats, weight loss, pruritus, and arthritis, are common in AITL.
T‐cell depletion and immune dysregulation are frequent in AITL, explaining why AITL shares a number of clinical features with HIV disease. These include a high incidence of drug rashes, immune thrombocytopenic purpura, polyclonal hypergammaglobulinemia, and autoantibodies.6, 7 As with HIV patients, death in AITL is often due to opportunistic infections or diffuse large B‐cell lymphomas.7 Over 95% of patients with AITL display a proliferation of EBV‐infected B cells, presumably from immune dysregulation, and the B‐cell lymphomas in these patients are usually EBV‐positive.11
Because AITL is a proinflammatory state, false‐positive antibody tests are fairly common. The occasional occurrence of positive HIV enzyme immunoassays, with indeterminate Western blot results, may be a particular source of diagnostic confusion.12, 13 The significance of indeterminate Western blots is often erroneously communicated to patients, as happened here. Indeterminate HIV Western blots may be seen in HIV seroconversion, HIV‐2 infection, or advanced HIV disease with loss of core antibody. They may also result from laboratory error, multiparity, syphilis, malaria, or cross‐reacting antibodies in autoimmune diseases. Indeterminate HIV Western blots in low‐risk patients usually do not represent true HIV infection.14
KEY POINTS FOR HOSPITALISTS
-
FUO is one of the most challenging diagnoses faced by hospitalists. Biopsies of new skin lesions and enlarged lymph nodes are particularly high‐yield diagnostic procedures in FUO. Fever pattern is generally not helpful in the diagnosis of FUO, with few exceptions, such as the tertian fevers of malaria.
-
Misleading and false‐positive tests results often occur in the course of FUO evaluation, due to the sheer number of tests ordered, and the higher likelihood of false‐positive serologic tests in the setting of inflammatory states.
-
AITL may be an elusive diagnosis in FUO with diverse clinical features, including weight loss, night sweats, rashes, arthritis, autoantibodies, immune dysregulation, and opportunistic infections. The prognosis traditionally has been guarded, but may be more hopeful in an era of emerging molecular therapies.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A51‐year‐old woman presented after 5 days of fever, rigors, anorexia, right‐sided abdominal pain, nausea, and dizziness. She had 2 loose stools the day before admission, without blood or mucus, but otherwise had recently been constipated. She denied cough, shortness of breath, chest pain, headache, sore throat, rash, arthritis, or dysuria.
In a 51‐year‐old woman with right‐sided abdominal pain and systemic symptoms, major concerns include biliary disease, liver abscess, or appendicitis. Right‐sided diverticulitis would be more unusual. Pyelonephritis infrequently presents with epigastric and lower quadrant pain, instead of flank pain. Basilar pneumonia may present with abdominal pain, but this is less likely in the absence of respiratory symptoms.
The patient used an albuterol inhaler for mild asthma and had experienced an episode of herpes zoster 7 years prior, but was otherwise well. Her surgical history was notable for a remote appendectomy. She was a native of the Dominican Republic who had lived in the United States for the past 20 years. She visited the Dominican Republic for 3 weeks every year, with her last visit occurring about 10 months before. She was a cleaning and maintenance worker. She had 2 adult children in good health, was divorced from her husband, and had not been sexually active for the past 8 years. The patient had no pets or other animal exposures. She did not smoke, drink alcohol, or use intravenous drugs.
The remote episode of shingles makes me a bit worried about chronic human immunodeficiency virus (HIV) infection. As a native of and traveler to the Dominican Republic, she is at risk for a variety of tropical pathogens. Hyperinfection syndrome from strongyloides can cause fever and bacteremia, but this is almost always associated with significant immunosuppression. Dengue fever has become very common in the Caribbean, but should occur within 2 weeks of travel. Her work in cleaning and maintenance might bring her into contact with rats and mice, putting her at risk for leptospirosis. This can present as a fairly nonspecific febrile syndrome, but this is unlikely without a major complaint of headache.
The patient appeared fatigued. Her temperature was 39.7C, her heart rate was 110 beats per minute, and her blood pressure 80/62 mm Hg. The oropharynx was normal. Mild cervical lymphadenopathy was present (less than 1 cm in diameter). The chest was clear and the cardiac examination unremarkable. Bowel sounds were present. Moderate right‐sided abdominal tenderness was noted, somewhat more marked in the right lower quadrant, without guarding or rebound. There was no hepatosplenomegaly. There was no rash. A bedside right upper quadrant ultrasound was negative for gallstones.
Her low blood pressure is concerning for bacterial sepsis. The negative right upper quadrant ultrasound makes cholecystitis or cholangitis less likely, but does not exclude diverticulitis or pelvic inflammatory disease. She lacks peritoneal signs, but they may be absent in these conditions. Another worrisome finding on her physical examination is cervical lymphadenopathy. In an older patient, this raises the specter of malignancy. In a younger patient, it could suggest a mononucleosis syndrome from Epstein‐Barr virus (EBV) or cytomegalovirus (CMV). In addition, HIV must be considered in any patient with unexplained lymphadenopathy.
She received intravenous levofloxacin and 1 L of intravenous normal saline, with a rise in her blood pressure to 100/59 mm Hg. Her white blood cell count was 5.0, with 71% polys and 9% bands. The hematocrit was 32%, with a normal mean corpuscular volume. The erythrocyte sedimentation rate (ESR) was 109 mm/hour. The platelet count, serum electrolytes, creatinine, aminotransferases, alkaline phosphatase, bilirubin, amylase, and lipase were normal. The serum level of lactate dehydrogenase (LDH) was 498 unit/L (normal range, 107231 units/L). Computed tomography (CT) scan of the abdomen showed multiple enlarged lymph nodes up to 1.2 cm in size along the gastrohepatic ligament and the mesentery, with mild associated fat stranding, consistent with mesenteric lymphadenitis.
Her blood pressure has responded to fluids; perhaps she was just volume‐depleted from not eating for several days. She has bandemia, which is consistent with acute bacterial infection, but might also signify a stress response. The low hematocrit and high ESR raise the possibility of anemia of chronic disease; perhaps her illness is more longstanding than her presentation suggests. Mesenteric lymphadenitis may be related to EBV and HIV, but in a patient originally from the Caribbean, it raises the possibility of gastrointestinal tuberculosis or histoplasmosis. Human T‐cell lymphotropic virus type 1 (HTLV‐1) is also endemic in the Caribbean, and may cause adult T‐cell leukemia/lymphoma (ATLL), which could explain her lymphadenopathy and elevated LDH. Mesenteric lymphadenitis is also characteristic of several bacterial infections, especially Yersinia, Salmonella, and Bartonella. The lack of diarrhea makes yersiniosis doubtful, and the absence of cat exposure makes bartonellosis unlikely. Salmonella infection is also associated with diarrhea, except for typhoid fever, in which patients have diarrhea, constipation, or normal stools.
A urine culture and 3 sets of blood cultures obtained prior to the initiation of antibiotics were negative. A blood smear showed no malaria or babesia parasites. The patient's fever continued for the first 2 days of her hospitalization, but subsequently abated. The patient had no loose stools during her hospitalization. Serologies for EBV, hepatitis A, CMV, and toxoplasmosis were indicative of remote infection. Serum rapid plasma reagin (RPR), Bartonella antibodies, antinuclear antibodies, hepatitis B surface antigen, and hepatitis C antibody were negative. Tuberculin skin testing and urinary histoplasma antigen were negative. By the fifth hospital day, she had been afebrile for over 48 hours, and her abdominal pain had improved, though it had not completely resolved. She was discharged to complete a 10‐day course of levofloxacin.
It is not clear to me whether she has just experienced a spontaneous remission in her illness, or whether her disease course has truly been modified with antibiotics. Could she have typhoid fever or another salmonellosis? The patient has not traveled abroad in several months. If she had typhoid fever, the source of infection would have to be imported food, or exposure to a family member who was a carrier. The negative tuberculin skin test makes tuberculosis less likely, but does not exclude it entirely. Similarly, while a negative histoplasma urinary antigen essentially rules out acute disseminated histoplasmosis, as would be seen in acquired immune deficiency syndrome (AIDS), it is less sensitive for more chronic forms of disseminated histoplasmosis, including gastrointestinal involvement.
One week later, the patient returned to the emergency department with recurrent abdominal pain, anorexia, fever, night sweats, and increased swelling of the lymph nodes in her neck. She had lost a total of 4.4 kg (10 lb) since the onset of her illness. Physical examination revealed moderate (up to 2 cm), slightly tender cervical and inguinal lymphadenopathy, and continued moderate right‐sided abdominal tenderness. Mesenteric, retroperitoneal, and inguinal lymphadenopathy was more prominent than on the prior CT scan (Figure 1).She was told by the emergency room physicians that the HIV test obtained during the prior hospitalization was positive. Further questioning elicited that her estranged husband had been promiscuous prior to their separation. She was transferred to a second hospital for further care. Blood cultures were sent for fungi and acid‐fast bacilli.

Everyone with fever of unknown origin (FUO) deserves an HIV test. Is this just lymphadenopathy from HIV, or is she suffering from an opportunistic infection? Mycobacterium avium infection is an attractive explanation for her fever, abdominal pain, and lymphadenopathy, but I would hold off on empiric treatment until the results of a CD4+ cell count were available. Could she have a secondary, HIV‐related lymphoma?
Full review of records from the outside hospital showed that the patient had a positive HIV enzyme immunoassay, with an indeterminate HIV Western blot. The patient's CD4 cell count was 313/cm3, with a CD4/CD8 ratio within the normal range. Her HIV enzyme immunoassay was repeated and found to be negative, and the HIV viral load was undetectable.
The HIV enzyme immunoassay is only a screening test, and must be confirmed with a positive HIV Western blot. (In most clinical laboratories, this is done automatically before the test is reported.) Indeterminate HIV Western blots are common in acute HIV infection, but outside of this setting, most patients with indeterminate HIV Western blots turn out not to have HIV infection. I am still very concerned about lymphoma, and would pursue a lymph node biopsy. Disseminated tuberculosis is still possible.
Biopsy of a right inguinal lymph node was performed on the third day of the second hospitalization. The patient was persistently febrile, developed swelling of the knees, ankles, and interphalangeal joints of the second and third digits of the left hand, and complained of pruritus. Anti‐double‐stranded DNA antibody, antineutrophilic cytoplasmic antibody, and Brucella antibody were negative. Antibody against cyclic citrullinated peptide (CCP) was weakly positive.
Now she has a more florid syndrome, with arthritic symptoms. Sarcoidosis is an attractive explanation for her fever, lymphadenopathy, and arthritis. Lupus could explain some features of her presentation, but the negative serology makes it unlikely. Antibody to cyclic citrullinated peptide is a newer diagnostic test for rheumatoid arthritis. Although anti‐CCP is more specific than rheumatoid factor for the diagnosis of rheumatoid arthritis, this result could still be a false positive, particularly given the low titer. As well, the patient has more impressive lymphadenopathy than is usual for rheumatoid arthritis. Reactive arthritis can follow enteric infection with Campylobacter, Salmonella, Yersinia, and Shigella, but lymphadenopathy is not a feature. Arthritis may be prominent in parvovirus B19, rubella, disseminated gonococcal infection, and Lyme disease, but mesenteric lymphadenitis and a prolonged, waxing and waning course would not be expected with any of these. I worry that the arthritis and pruritus are paraneoplastic manifestations of lymphoma.
The inguinal lymph node biopsy showed markedly distorted nodal architecture with an atypical proliferation of small‐sized to large‐sized lymphoid cells, with predominantly round nuclei, vesicular chromatin, small nucleoli, and variable amounts of clear to eosinophilic cytoplasm (Figure 2). Residual follicles and occasional apoptotic bodies and mitoses were seen. Large B‐cells were frequently present, which stained positive for EBV‐associated mRNA by in situ hybridization studies. Immunoperoxidase staining revealed that the atypical cell population was largely composed of CD4+ T‐cells. T‐cell receptor gene rearrangement studies demonstrated that the T‐cell population was monoclonal. These results were consistent with angioimmunoblastic T‐cell lymphoma (AITL). Positron emission tomography (PET) scanning was performed, showing diffuse fluorodeoxyglucose (FDG)‐avid lymphadenopathy involving cervical, axillary, mediastinal, retroperitoneal, mesenteric, and inguinal lymph nodes, up to 2 cm in diameter. The patient completed 6 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), as well as experimental treatment with denileukin diftitox. Her fever and arthritis subsided quickly, and she was clinically well and free of disease by CT scans and PET scans 1 year after diagnosis.

COMMENTARY
The differential diagnosis of FUO is one of the largest in medicine, encompassing a dizzying range of infectious, inflammatory, and neoplastic conditions. This has made the development of standardized diagnostic algorithms difficult.1 The workup of patients with FUO begins with a detailed history and physical examination, followed by a core set of microbiology cultures, imaging studies, and blood tests on all patients. Further testing is individualized, based on key clinical findings, also known as pivot points.2 Unfortunately, many of the diseases presenting as FUO have overlapping symptoms and signs, somewhat limiting the utility of pivot points. In a prospective study, 81% of these potentially diagnostic clues were misleading, although 19% of them contributed to the final diagnosis.3 Key clinical findings in patients with FUO may trigger a barrage of diagnostic tests, often leading to a large number of false‐positive results.4 Clinicians who investigate patients with FUO must remember that many clues are diagnostic dead ends, and should be wary of drawing positively‐false conclusions.
Fever pattern is usually not helpful in the diagnosis of FUO, with occasional exceptions, such as the tertian and quartan fevers in some forms of malaria. A minority of patients with Hodgkin's disease have Pel‐Ebstein fevers, in which 1 to 2 weeks of fever alternate with an afebrile period of similar or longer duration. More often, fever in lymphoma waxes and wanes unpredictably,5 a circumstance that may lead to the mistaken impression of response to antibiotics, as in this case.
Tissue biopsies are helpful in FUO when suggestive findings are present on physical examination or imaging studies. In patients with lymphadenopathy and FUO, lymph node biopsy is a high‐yield procedure, contributing to the final diagnosis 46% of the time. This is exceeded only by biopsies of skin lesions, which have a 63% diagnostic yield in FUO.3 In older patients with FUO, temporal artery biopsies have a significant diagnostic yield, in the range of 16% to 17%. Liver biopsies have a similar yield (14‐17%), but a higher risk of complications. Bone marrow biopsies have a low yield in most FUO patients.1
AITL makes up 1% of all non‐Hodgkin's lymphomas. AITL was once known as angioimmunoblastic lymphadenopathy, and was thought to be either a disorder of immune regulation or a premalignant lymphoid disease. However, molecular diagnostic techniques have established that monoclonal T‐cell populations and cytogenetic abnormalities are usually present at the time of diagnosis.6, 7 The prognosis in AITL is unfavorable. Disease is usually widespread at the time of diagnosis. Most patients achieve complete remission with anthracycline‐based chemotherapy, such as CHOP, but the duration of remission is often brief, and median survival after diagnosis is only 3 years.6 Novel treatments under investigation include denileukin diftitox,8 a fusion protein of interleukin‐2 (IL‐2) conjugated to diphtheria toxin, which leads to apoptosis of cells expressing the IL‐2 receptor; rituximab, which targets the reactive population of B‐cells in AITL, rather than the malignant clone of T‐cells; and antiangiogenic therapy, such as thalidomide.9
The diagnosis of AITL is usually elusive, and the average patient has symptoms for 4 months prior to diagnosis.6 This patient's clinical presentation, while certainly not specific, was typical of AITL. AITL usually presents as fever of unknown origin with generalized, nonbulky lymphadenopathy. Fever is present in 57% of patients with AITL, and up to 2% of FUO is caused by AITL.6, 10 Other features of this patient's illness, such as night sweats, weight loss, pruritus, and arthritis, are common in AITL.
T‐cell depletion and immune dysregulation are frequent in AITL, explaining why AITL shares a number of clinical features with HIV disease. These include a high incidence of drug rashes, immune thrombocytopenic purpura, polyclonal hypergammaglobulinemia, and autoantibodies.6, 7 As with HIV patients, death in AITL is often due to opportunistic infections or diffuse large B‐cell lymphomas.7 Over 95% of patients with AITL display a proliferation of EBV‐infected B cells, presumably from immune dysregulation, and the B‐cell lymphomas in these patients are usually EBV‐positive.11
Because AITL is a proinflammatory state, false‐positive antibody tests are fairly common. The occasional occurrence of positive HIV enzyme immunoassays, with indeterminate Western blot results, may be a particular source of diagnostic confusion.12, 13 The significance of indeterminate Western blots is often erroneously communicated to patients, as happened here. Indeterminate HIV Western blots may be seen in HIV seroconversion, HIV‐2 infection, or advanced HIV disease with loss of core antibody. They may also result from laboratory error, multiparity, syphilis, malaria, or cross‐reacting antibodies in autoimmune diseases. Indeterminate HIV Western blots in low‐risk patients usually do not represent true HIV infection.14
KEY POINTS FOR HOSPITALISTS
-
FUO is one of the most challenging diagnoses faced by hospitalists. Biopsies of new skin lesions and enlarged lymph nodes are particularly high‐yield diagnostic procedures in FUO. Fever pattern is generally not helpful in the diagnosis of FUO, with few exceptions, such as the tertian fevers of malaria.
-
Misleading and false‐positive tests results often occur in the course of FUO evaluation, due to the sheer number of tests ordered, and the higher likelihood of false‐positive serologic tests in the setting of inflammatory states.
-
AITL may be an elusive diagnosis in FUO with diverse clinical features, including weight loss, night sweats, rashes, arthritis, autoantibodies, immune dysregulation, and opportunistic infections. The prognosis traditionally has been guarded, but may be more hopeful in an era of emerging molecular therapies.
- ,,.A comprehensive evidence‐based approach to fever of unknown origin.Arch Intern Med.2003;163:545–551.
- ,.The art of diagnosis: solving the clinicopathological exercise.N Engl J Med.1982;306:1263–1268.
- ,,, et al.A prospective multicenter study on fever of unknown origin: the yield of a structured diagnostic protocol.Medicine (Baltimore).2007;86:26–38.
- ,,.Fever of unknown origin (FUO). II. Diagnostic procedures in a prospective multicenter study of 167 patients. The Netherlands FUO Study Group.Medicine (Baltimore).1997;76:401–414.
- ,.Neoplastic diseases. In:Murray HW, ed.FUO: Fever of Undetermined Origin.New York, NY:Futura Publishing;1983:39–48.
- ,,, et al.Angioimmunoblastic T‐cell lymphoma: clinical and laboratory features at diagnosis in 77 patients.Medicine (Baltimore).2007;86:282–292.
- ,,.Angioimmunoblastic T‐cell lymphoma.Br J Haematol.2003;121:681–691.
- ,,, et al.Phase II trial of denileukin diftitox for relapsed/refractory T‐cell non‐Hodgkin lymphoma.Br J Haematol.2007;136:439–447.
- ,.Angioimmunoblastic T‐cell lymphoma: still a dismal prognosis with current treatment approaches.Leuk Lymphoma.2007;48:645–646.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO Study Group.Medicine (Baltimore).1997;76:392–400.
- ,,, et al.Histologic evolution of angioimmunoblastic T‐cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression.Am J Surg Pathol.2007;31:1077–1088.
- ,,,.Angioimmunoblastic lymphadenopathy, immunoblastic lymphoma, and false‐positive seroconversion for human immunodeficiency virus.Ann Intern Med.1987;107:114.
- ,.Angioimmunoblastic T‐cell lymphoma associated with an antibody to human immunodeficiency virus protein.Int J Hematol.2003;78:160–162.
- ,,.Communicating indeterminate HIV Western blot test results to clients: an observational study of three community testing sites.AIDS Patient Care STDS.2006;20:620–627.
- ,,.A comprehensive evidence‐based approach to fever of unknown origin.Arch Intern Med.2003;163:545–551.
- ,.The art of diagnosis: solving the clinicopathological exercise.N Engl J Med.1982;306:1263–1268.
- ,,, et al.A prospective multicenter study on fever of unknown origin: the yield of a structured diagnostic protocol.Medicine (Baltimore).2007;86:26–38.
- ,,.Fever of unknown origin (FUO). II. Diagnostic procedures in a prospective multicenter study of 167 patients. The Netherlands FUO Study Group.Medicine (Baltimore).1997;76:401–414.
- ,.Neoplastic diseases. In:Murray HW, ed.FUO: Fever of Undetermined Origin.New York, NY:Futura Publishing;1983:39–48.
- ,,, et al.Angioimmunoblastic T‐cell lymphoma: clinical and laboratory features at diagnosis in 77 patients.Medicine (Baltimore).2007;86:282–292.
- ,,.Angioimmunoblastic T‐cell lymphoma.Br J Haematol.2003;121:681–691.
- ,,, et al.Phase II trial of denileukin diftitox for relapsed/refractory T‐cell non‐Hodgkin lymphoma.Br J Haematol.2007;136:439–447.
- ,.Angioimmunoblastic T‐cell lymphoma: still a dismal prognosis with current treatment approaches.Leuk Lymphoma.2007;48:645–646.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO Study Group.Medicine (Baltimore).1997;76:392–400.
- ,,, et al.Histologic evolution of angioimmunoblastic T‐cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression.Am J Surg Pathol.2007;31:1077–1088.
- ,,,.Angioimmunoblastic lymphadenopathy, immunoblastic lymphoma, and false‐positive seroconversion for human immunodeficiency virus.Ann Intern Med.1987;107:114.
- ,.Angioimmunoblastic T‐cell lymphoma associated with an antibody to human immunodeficiency virus protein.Int J Hematol.2003;78:160–162.
- ,,.Communicating indeterminate HIV Western blot test results to clients: an observational study of three community testing sites.AIDS Patient Care STDS.2006;20:620–627.
Research Roundup
Question: Does gentamicin use affect clinical outcomes and prognosis or just creatinine clearance?
Background: Impaired kidney function in patients with endocarditis predicts worse outcomes in both morbidity and mortality. Given that the aminoglycosides can be nephrotoxic, it has been debated whether physicians should abandon its use in these patients.
Study design: Prospective, observational, cohort study.
Setting: Two tertiary-care hospitals serving Copenhagen, Denmark, from 2002-2007.
Synopsis: The study identified 373 patients as having definite or probable infective endocarditis. ("Probable" meant patients underwent the same treatment for endocarditis as those with confirmed disease.) Gentamicin treatment decreased estimated creatinine clearance (CrCl) by 0.5% per day of treatment, with more significant decreases in CrCl associated with length of treatment and age. It did not increase the risk of in-hospital or post-discharge mortality, nor did it increase the need for dialysis. The mean duration of follow up was 562 days.
Bottom line: With appropriate monitoring, gentamicin is a reasonable treatment choice for patients with endocarditis when clinically indicated. Patient-centered outcomes are not negatively impacted by its use.
Citation: Buchholtz K, Larsen CT, Hassager C, Bruun NE. Severity of gentamicin’s nephrotoxic effect on patients with infective endocarditis: a prospective observational cohort study of 373 patients. Clin Infect Dis. 2009;48:65-71.
In the Literature: For the latest HM literature reviews, visit www.the-hospitalist.org and search "literature."
—Reviewed by Michael Kedansky, MD, Victor Weaver, MD, Michael Goldman, MD, Lisa Gushwa, MD, Paul Hicks, MD, Barbara Eckstein, MD, and Christine Kneisel, MD, Department of Family and Community Medicine, University of Arizona
Question: Does gentamicin use affect clinical outcomes and prognosis or just creatinine clearance?
Background: Impaired kidney function in patients with endocarditis predicts worse outcomes in both morbidity and mortality. Given that the aminoglycosides can be nephrotoxic, it has been debated whether physicians should abandon its use in these patients.
Study design: Prospective, observational, cohort study.
Setting: Two tertiary-care hospitals serving Copenhagen, Denmark, from 2002-2007.
Synopsis: The study identified 373 patients as having definite or probable infective endocarditis. ("Probable" meant patients underwent the same treatment for endocarditis as those with confirmed disease.) Gentamicin treatment decreased estimated creatinine clearance (CrCl) by 0.5% per day of treatment, with more significant decreases in CrCl associated with length of treatment and age. It did not increase the risk of in-hospital or post-discharge mortality, nor did it increase the need for dialysis. The mean duration of follow up was 562 days.
Bottom line: With appropriate monitoring, gentamicin is a reasonable treatment choice for patients with endocarditis when clinically indicated. Patient-centered outcomes are not negatively impacted by its use.
Citation: Buchholtz K, Larsen CT, Hassager C, Bruun NE. Severity of gentamicin’s nephrotoxic effect on patients with infective endocarditis: a prospective observational cohort study of 373 patients. Clin Infect Dis. 2009;48:65-71.
In the Literature: For the latest HM literature reviews, visit www.the-hospitalist.org and search "literature."
—Reviewed by Michael Kedansky, MD, Victor Weaver, MD, Michael Goldman, MD, Lisa Gushwa, MD, Paul Hicks, MD, Barbara Eckstein, MD, and Christine Kneisel, MD, Department of Family and Community Medicine, University of Arizona
Question: Does gentamicin use affect clinical outcomes and prognosis or just creatinine clearance?
Background: Impaired kidney function in patients with endocarditis predicts worse outcomes in both morbidity and mortality. Given that the aminoglycosides can be nephrotoxic, it has been debated whether physicians should abandon its use in these patients.
Study design: Prospective, observational, cohort study.
Setting: Two tertiary-care hospitals serving Copenhagen, Denmark, from 2002-2007.
Synopsis: The study identified 373 patients as having definite or probable infective endocarditis. ("Probable" meant patients underwent the same treatment for endocarditis as those with confirmed disease.) Gentamicin treatment decreased estimated creatinine clearance (CrCl) by 0.5% per day of treatment, with more significant decreases in CrCl associated with length of treatment and age. It did not increase the risk of in-hospital or post-discharge mortality, nor did it increase the need for dialysis. The mean duration of follow up was 562 days.
Bottom line: With appropriate monitoring, gentamicin is a reasonable treatment choice for patients with endocarditis when clinically indicated. Patient-centered outcomes are not negatively impacted by its use.
Citation: Buchholtz K, Larsen CT, Hassager C, Bruun NE. Severity of gentamicin’s nephrotoxic effect on patients with infective endocarditis: a prospective observational cohort study of 373 patients. Clin Infect Dis. 2009;48:65-71.
In the Literature: For the latest HM literature reviews, visit www.the-hospitalist.org and search "literature."
—Reviewed by Michael Kedansky, MD, Victor Weaver, MD, Michael Goldman, MD, Lisa Gushwa, MD, Paul Hicks, MD, Barbara Eckstein, MD, and Christine Kneisel, MD, Department of Family and Community Medicine, University of Arizona
Head of the Class
Brian Tyson, MD, was named medical director of the hospitalist program at St. Bernardine Medical Center in San Bernardino, Calif., about a year ago, but he still can't learn enough about business drivers, communication, and leadership skills. That's why he attended SHM's Leadership Academy last week in Honolulu.
"I needed some things to work on. ...I think we all need to," says Dr. Tyson, whose HM program is operated by Cogent Healthcare. "This is that opportunity. When people have done things and run things, you don't need to reinvent the wheel. It's easier sometimes to get the keys to success from someone who’s been there."
More than a hundred hospitalists apparently agreed, joining Dr. Tyson at the four-day session. Dr. Tyson found the insights into business particularly helpful, especially because fiscal matters are not a major focus of medical school. Analyzing his managerial personality and identifying his program's strengths and weaknesses were "eye opening," as was the chance to discuss staffing and budget issues with HM directors from different parts of the country.
The leadership program offers two tracks, and the first course must be completed before taking the advanced level. A first-time attendee, Dr. Tyson says he is looking forward to completing the second part of the academy.
"Medicine is changing so much, this helps us manage our programs," he says. "Having the tools to be able to do that is absolutely necessary."
Brian Tyson, MD, was named medical director of the hospitalist program at St. Bernardine Medical Center in San Bernardino, Calif., about a year ago, but he still can't learn enough about business drivers, communication, and leadership skills. That's why he attended SHM's Leadership Academy last week in Honolulu.
"I needed some things to work on. ...I think we all need to," says Dr. Tyson, whose HM program is operated by Cogent Healthcare. "This is that opportunity. When people have done things and run things, you don't need to reinvent the wheel. It's easier sometimes to get the keys to success from someone who’s been there."
More than a hundred hospitalists apparently agreed, joining Dr. Tyson at the four-day session. Dr. Tyson found the insights into business particularly helpful, especially because fiscal matters are not a major focus of medical school. Analyzing his managerial personality and identifying his program's strengths and weaknesses were "eye opening," as was the chance to discuss staffing and budget issues with HM directors from different parts of the country.
The leadership program offers two tracks, and the first course must be completed before taking the advanced level. A first-time attendee, Dr. Tyson says he is looking forward to completing the second part of the academy.
"Medicine is changing so much, this helps us manage our programs," he says. "Having the tools to be able to do that is absolutely necessary."
Brian Tyson, MD, was named medical director of the hospitalist program at St. Bernardine Medical Center in San Bernardino, Calif., about a year ago, but he still can't learn enough about business drivers, communication, and leadership skills. That's why he attended SHM's Leadership Academy last week in Honolulu.
"I needed some things to work on. ...I think we all need to," says Dr. Tyson, whose HM program is operated by Cogent Healthcare. "This is that opportunity. When people have done things and run things, you don't need to reinvent the wheel. It's easier sometimes to get the keys to success from someone who’s been there."
More than a hundred hospitalists apparently agreed, joining Dr. Tyson at the four-day session. Dr. Tyson found the insights into business particularly helpful, especially because fiscal matters are not a major focus of medical school. Analyzing his managerial personality and identifying his program's strengths and weaknesses were "eye opening," as was the chance to discuss staffing and budget issues with HM directors from different parts of the country.
The leadership program offers two tracks, and the first course must be completed before taking the advanced level. A first-time attendee, Dr. Tyson says he is looking forward to completing the second part of the academy.
"Medicine is changing so much, this helps us manage our programs," he says. "Having the tools to be able to do that is absolutely necessary."