User login
Age‐Specific CSF Protein Reference Values
Emergency department evaluation of a febrile neonate or young infant routinely includes lumbar puncture and cerebrospinal fluid (CSF) analysis to diagnose meningitis or encephalitis. In addition to CSF Gram stain and culture, clinicians generally request a laboratory report for the CSF cell count, glucose content and protein concentration. Interpretation of these ancillary tests requires knowledge of normal reference values. In adult medicine, the accepted reference value for CSF protein concentration at the level of the lumbar spine is 15 mg/dL to45 mg/dL.1 There is general consensus among reference texts and published original studies dating back to Widell2 in 1958 that adult CSF protein reference values are not valid in the pediatric population. A healthy neonate's CSF protein concentration is normally twice to 3 times that of an adult, and declines with age from birth to early childhood. The most rapid rate of decline is thought to occur in the first 6 months of life as the infant's blood‐CSF barrier matures.3 However, published studies47 differ in the reported rate, timing, and magnitude of this decline; on close review these studies have significant limitations which call into question the appropriateness of using these values in clinical practice. Perhaps in recognition of the limited evidence, textbooks of general pediatrics,810 hospital medicine,1113 emergency medicine,14, 15 infectious diseases,16, 17 neonatology,18 and neurology19, 20 frequently publish norms for pediatric CSF protein concentration without reference to any original research studies.
Because ethical considerations prohibit subjecting young infants to a potentially painfully procedure (ie, lumbar puncture) before they are able to assent, we sought to define a study population that approximates a group of healthy infants. Our objectives were to quantify age‐related declines in CSF protein concentration and to determine accurate, age‐specific reference values for CSF protein concentration in a population of neonates and young infants who presented for medical care with an indication for lumbar puncture and were subsequently found to have no condition associated with elevated or depressed CSF protein concentration.
Methods
Study Design and Setting
This cross‐sectional study was performed at The Children's Hospital of Philadelphia (Philadelphia, PA), an urban, tertiary‐care children's hospital. The Committees for the Protection of Human Subjects approved this study with a waiver of informed consent.
Study Participants
Infants 56 days of age or younger were eligible for inclusion if they had a lumbar puncture performed as part of their emergency department evaluation between January 1, 2005 and June 30, 2007. Children in this age range were selected as they routinely undergo lumbar puncture when presenting with fever at our institution.21, 22 Patients undergoing lumbar puncture in the emergency department were identified using 2 different data sources to ensure accurate identification of all eligible infants: (1) Emergency department computerized order entry records identified all infants with CSF testing (including CSF Gram stain, culture, cell count, glucose, or protein) performed during the study period, and (2) Clinical Virology Laboratory records identified all infants in whom CSF herpes simplex virus or enterovirus testing was performed. Medical records of infants identified by these 2 sources were reviewed to determine study eligibility.
Subjects with conditions known or suspected to cause abnormal CSF protein concentration were systematically excluded from the final analysis. Exclusion criteria included traumatic lumbar puncture (defined as CSF sample with >500 red blood cells per mm3), serious bacterial infection (including meningitis, urinary tract infection, bacteremia, pneumonia, osteomyelitis, or septic arthritis), congenital infection, CSF positive for enterovirus by polymerase chain reaction (PCR) testing, seizure prior to presentation, presence of a ventricular shunt device, elevated serum bilirubin, and absent CSF protein measurements or CSF red blood cell counts. The presence of lysed red blood cells in the CSF secondary to a traumatic lumbar puncture or subarachnoid hemorrhage alters the CSF protein.23 We also excluded subjects who had CSF assays done on samples drawn by accessing a ventricular shunt device, as there may be up to a 300% regional difference in CSF protein concentration between the cranial and caudal ends of the neuroaxis.1 Bilirubin in the CSF sample at a concentration of 5 mg/dL biases the CSF protein concentration measurement by an average of 13.7 mg/dL.24 Quantitative protein assay was performed on the institution's standard Vitros chemistry system; the protein assay is a modified biuret reaction.
Study Definitions
CSF pleocytosis was defined as a CSF white blood cell count (WBC) >22/mm3 (for infants age 28 days) or >15/mm3 (for infants 2956 days of age).25 Bacterial meningitis was defined as isolation of a bacterial pathogen from the CSF. Bacteremia was defined as isolation of a bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Bacterial pneumonia was defined as a new discrete infiltrate on chest radiograph as documented by an attending pediatric radiologist in conjunction with growth of a respiratory bacterial pathogen from blood culture. Urinary tract infection was defined as growth of a single known pathogen in culture as follows: (1) 1000 colony‐forming units/mL for cultures obtained by suprapubic aspiration, (2) 50,000 cfu/mL from a catheterized specimen, or (3) 10,000 cfu/mL from catheterized specimen in conjunction with a positive urinalysis.26 Positive urinalysis was defined as trace or greater leukocyte esterase by dip stick, or >9 WBC per high‐power filed on standard microscopic exam of centrifuged urine, or >10 WBC/mm3 by hemocytometer count of uncentrifuged urine.27, 28 We defined osteomyelitis as growth of pathogenic bacteria from blood, bone, or subperiosteal aspirate culture in a subject with fever and localized tenderness, edema or erythema at the site of bony infection, and compatible imaging; and septic arthritis as growth of pathogenic bacteria from synovial fluid or blood culture from a subject with purulent synovial fluid or positive Gram stain of synovial fluid.
A temperature 38.0C by any method qualified as fever. Prematurity was defined as a gestational age less than 37 weeks. Seizure included any clinical description of the event within 48 hours of presentation to the Emergency Department, or documented seizure activity on electroencephalogram. Enterovirus season was defined as June 1st to October 31st of each year.29
Data Collection and Statistical Analysis
Information collected included the following: demographics, vital signs, history of present illness, birth history, clinical findings, results of laboratory testing and imaging within 48 hours of presentation, antibiotics administered, and duration of visit to the Emergency Department or admission to the hospital.
Categorical data were described using frequencies and percents, and continuous variables were described using mean, median, interquartile range, and 90th and 95th percentile values. Linear regression was used to determine the association between age and CSF protein concentration. Because the CSF protein concentrations had a skewed distribution (P < 0.001, Shapiro‐Wilk test), our analyses were performed using logarithmically transformed CSF protein values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent change in CSF protein with increasing age. Two‐sample Wilcoxon rank‐sum tests were subsequently used to compare the distribution of CSF protein concentrations amongst four predefined age categories to facilitate implementation of our results into clinical practice: 014 days, 1528 days, 2942 days, and 4356 days. The analyses were repeated while excluding preterm infants, patients receiving antibiotics before lumbar puncture, and patients with CSF pleocytosis to determine the impact of these factors on CSF protein concentrations. Data were analyzed using STATA v10 (Stata Corporation, College Station, TX). Two‐tailed P values < 0.05 were considered statistically significant.
Results
During the study period, 1064 infants age 56 days of age or younger underwent lumbar puncture in the emergency department. Of these, 689 (65%) met sequential exclusion criteria as follows: traumatic lumbar puncture (n = 330); transported from an outside medical facility (n = 90); bacterial meningitis (n = 6); noncentral nervous system serious bacterial infections (n = 135); CSF positive for herpes simplex virus by PCR (n = 2); CSF positive for enterovirus by PCR (n = 45); congenital syphilis (n = 1); seizures (n = 28); abnormal central nervous system imaging (n = 2); and ventricular shunt device (n = 1). An additional 44 patients had lumbar puncture and CSF testing but the protein assay was never done or never reported and 5 patients did not have a CSF red blood cell count available. No cases were excluded for elevated serum bilirubin. Infants may have met multiple exclusion criteria. The remaining 375 (35%) subjects were included in the final analysis. The median patient age was 36 days (interquartile range: 2247 days); 139 (37%) were 28 days of age or younger. Overall, 205 (55%) were male, 211 (56%) were black, and 145 (39%) presented during enterovirus season. Most (43 of 57) preterm infants were born between 34 weeks to 37 weeks gestation. Antibiotics were administered before lumbar puncture to 42 (11%) infants and 312 (83%) infants had fever.
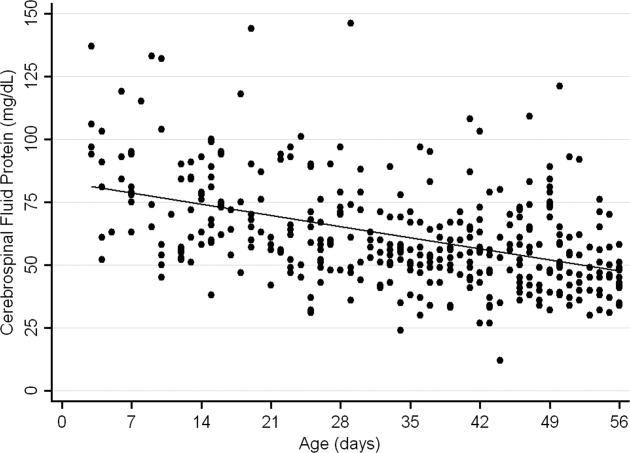
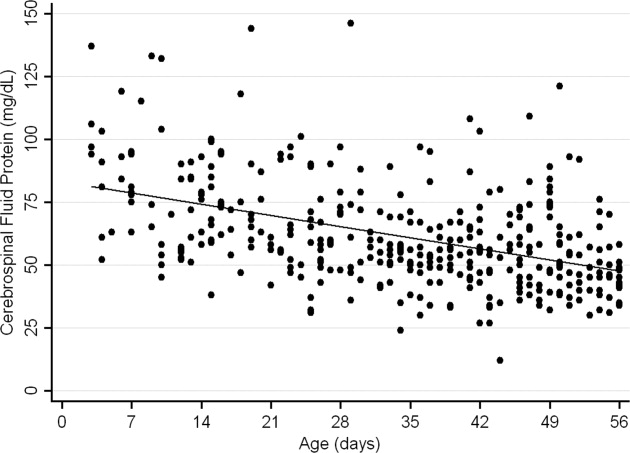
The median CSF protein value was 58 mg/dL (interquartile range: 4872 mg/dL). There was an age‐related declined in CSF protein concentration (Figure 1). In linear regression, the CSF protein concentration decreased 6.8% (95% confidence interval [CI], 5.48.1%; P < 0.001) for each 1‐week increase in age.0
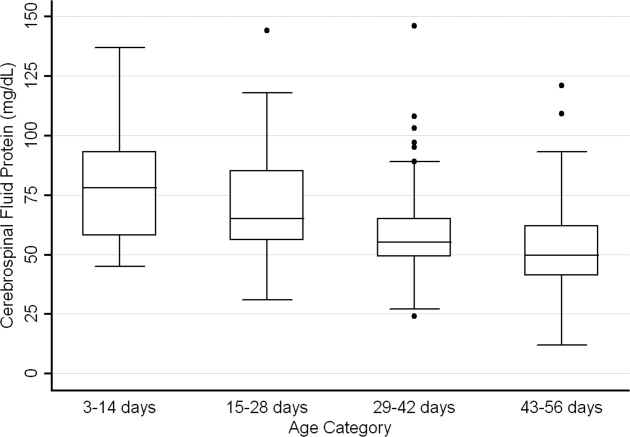
CSF protein concentrations were higher for infants 28 days of age than for infants 2956 days of age (P < 0.001, Wilcoxon rank‐sum test). The median CSF protein concentrations were 68 mg/dL (95th percentile value, 115 mg/dL) for infants 28 days of age and 54 mg/dL (95th percentile value, 89 mg/dL) for infants 2956 days. CSF protein concentrations by 2‐week age intervals are shown in Table 1. The 95th percentile CSF protein concentrations were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL (Table 1). CSF protein concentration decreased significantly across each age interval when compared with infants in the next highest age category (P < 0.02 for all pair‐wise comparisons, Wilcoxon rank‐sum test).
| Value | 014 days (n = 52) | 1528 days (n = 87) | 2942 days (n = 110) | 4356 days (n = 126) | All Infants (n = 375) |
|---|---|---|---|---|---|
| |||||
| Mean (SD) | 79 (23) | 69 (20) | 58 (17) | 53 (17) | 62 (21) |
| Median (IQR) | 78 (5893) | 65 (5685) | 55 (4965) | 50 (4162) | 58 (4872) |
| 90th percentile | 106 | 95 | 79 | 75 | 91 |
| 95th percentile | 132 | 100 | 89 | 83 | 99 |
| 95th percentile* | 132 | 101 | 89 | 82 | 97 |
| 95th percentile | 132 | 100 | 87 | 74 | 97 |
Age‐specific 95th percentile CSF protein values changed by <1% when infants receiving antibiotics before lumbar puncture were excluded (Table 1). Age‐specific CSF protein values changed minimally when preterm infants were excluded with the exception of infants 4356 days of age where the 95th percentile value was 9.7% lower than when all infants were included (Table 1); the 90th percentile values in this age group were more comparable at 75 mg/dL and 71 mg/dL, respectively, in the subgroups with and without preterm infants. Age‐specific 95th percentile CSF protein values changes by <1% when patients with CSF pleocytosis were excluded.
Discussion
We examined CSF protein values in neonates and young infants to establish reference values and to bring the literature up to date at a time when molecular tools are commonly used in clinical practice. We also quantified the age‐related decline in CSF protein concentrations over the first two months of life. Our findings provide age‐specific reference ranges for CSF protein concentrations in neonates and young infants. These findings are particularly important because a variety of infectious (eg, herpes simplex virus infection) and noninfectious (eg, subarachnoid or intraventricular hemorrhage) conditions may occur in the absence of appreciable elevations in the CSF WBC.
CSF protein concentrations depend on serum protein concentrations and on the permeability of the blood‐CSF barrier. Immaturity of the blood‐CSF barrier is thought to result in higher CSF protein concentrations for neonates and young infants compared with older children and adults. Though previous studies agree that CSF protein concentrations depend on age, the reported age‐specific values and rates of decline vary considerably.47, 3032 Additionally, these prior studies are limited by (1) small sample size, (2) variable inclusion and exclusion criteria, (3) variable laboratory techniques to quantify protein concentration in a CSF sample, and (4) presentation of mean, standard deviation, and range values rather than the 75th, 90th, or 95th percentile values necessary to define a clinically meaningful reference range.
The median and mean values found in this study were generally comparable to previously published values (Table 2). In addition, we have quantified the age‐related decline in CSF protein concentrations identified in previous studies. While our large sample size allowed us to define narrower reference intervals than most previous studies, direct comparison of values used to define reference ranges was hampered by lack of consistent reporting of data across studies. Ahmed et al.5 and Bonadio et al.4 reported only mean and standard deviation values. When data are skewed, as is the case for CSF protein values, the standard deviation will be grossly inflated, making extrapolation to percentile values unreliable. The 90th percentile value of 87 mg/dL reported by Wong et al.7 for infants 060 days of age was similar to the value of 91 mg/dL for infants 56 days of age and younger found in this study. Biou et al.6 reported the following 95th percentile values: ages 18 days, 108 mg/dL; ages 830 days, 90 mg/dL; and ages 12 months, 77 mg/dL. These values are lower than those reported in our study. The reason for such differences is not clear. The exclusion criteria were similar between the two studies though Biou et al.6 did not include preterm infants. When we excluded preterm infants from our analysis, no age‐specific result decreased by more than 5%, making the inclusion of this population an unlikely explanation for the differences between the two studies.
| Author | Year | Number of Infants | Age (days) | Median (mg/dL) | Mean SD (mg/dL) |
|---|---|---|---|---|---|
| |||||
| Bonadio et al.4 | 1992 | 35 | 030 | 84 45 | |
| 40 | 3060 | 59 25 | |||
| Ahmed et al.5 | 1996 | 17 | 07 | 81 31 | |
| 33 | 814 | 69 23 | |||
| 25 | 1521 | 60 23 | |||
| 33 | 2230 | 54 16 | |||
| Biou et al.6 | 2000 | 26 | 18 | 71 | |
| 76 | 830 | 59 | |||
| 155 | 3060 | 47 | |||
| Wong et al.7 | 2000 | 99 | 060 | 60 | 59 21 |
CSF protein concentration is a method‐dependent value; the results depend a great deal on what technique the laboratory uses. Two common methods used in the past few decades are Biuret Colorimetry and Turbidimetric; reported values are approximately 25% higher with the Biuret method compared with the Turbidimetric method.33 A CSF protein reference value is only clinically useful if the method used to define the norm is specified and equivalent to currently used methods. Similar to our study, Biou et al.6 and Wong et al.7 used the Biuret (Vitros) method. The method of protein measurement was not specified by other studies.4, 5
This study had several limitations that could cause us to overestimate the upper bound of the reference range. First, spectrum bias is possible in this observational study. Individual physicians determined whether lumbar puncture was warranted, a limitation that could potentially lead to the disproportionate inclusion of infants with conditions associated with higher CSF protein concentrations. We do not believe that this limitation would meaningfully affect our results because febrile infants 56 days of age or younger routinely undergo lumbar puncture at our institution, regardless of illness severity, and patients diagnosed with conditions known or suspected to increase CSF protein concentrations were excluded. Second, infants with aseptic meningitisa condition that can be associated with elevated CSF protein concentrationsmay have been misclassified as uninfected. Though we excluded patients with positive CSF enteroviral PCR tests, some infants were not tested and other viruses (eg, parechoviruses)34 not detected by the enterovirus PCR may also cause aseptic meningitis. Third, certain antibiotics including ampicillin and vancomycin are known to interfere with the CSF protein assay used in our laboratory.24 Forty‐two of the 375 subjects included in our final analysis received antibiotics prior to lumbar puncture. When receiving antibiotics prior to lumbar puncture were excluded from analysis, the CSF protein concentrations were within 1% of the overall study population, suggesting that antibiotic administration before lumbar puncture did not influence our results in any meaningful way. We would not expect any of these limitations to disproportionately affect patients in 1 particular age category.
In conclusion, the CSF protein concentration values reported here represent the largest series to‐date for this young age group. Our study quantifies the age‐related decline in CSF protein concentration from birth to 56 days of life. Our work designing this study, specifically the exclusion criteria, refines the approach to defining normal CSF protein values in children. As CSF protein values decline steadily with increasing age, the selection of reference values is a balance of accuracy and convenience. Age‐specific reference values by 2‐week increments would be most accurate. However, considering reference values by month of age, as is the convention for CSF WBCs, is far more practical. The 95th percentile values by age category in our study were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL. The 95th percentile values were 115 mg/dL for infants 28 days and 89 mg/dL for infants 2956 days. We feel that either approach is reasonable. These values can be used to accurately interpret the results of CSF studies in neonates and young infants.
- ,.Henry's Clinical Diagnosis and Management by Laboratory Methods.21st ed.Philadelphia, PA:W.B. Saunders, Inc.;2006.
- .On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo‐encephalitis.Acta Paediatr Suppl.1958;47(Suppl 115):1–102.
- ,.Development of the blood‐CSF barrier.Dev Med Child Neurol.1983;25(2):152–161.
- ,,,,.Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks.Pediatr Infect Dis J.1992;11(7):589–591.
- ,,, et al.Cerebrospinal fluid values in the term neonate.Pediatr Infect Dis J.1996;15(4):298–303.
- ,,,,,.Cerebrospinal fluid protein concentrations in children: age‐related values in patients without disorders of the central nervous system.Clin Chem.2000;46(3):399–403.
- ,,,,.Cerebrospinal fluid protein concentration in pediatric patients: defining clinically relevant reference values.Arch Pediatr Adolesc Med.2000;154(8):827–831.
- ,,.Nelson Textbook of Pediatrics.17th ed.Philadelphia, PA:Saunders;2004.
- ,,,.Oski's pediatrics : principles 2006.
- Robertson J, Shilkofski N, eds.Johns Hopkins: The Harriet Lane Handbook: A Manual for Pediatric House Officers.17 ed.Philadelphia, PA:Elsevier Mosby;2005.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia, PA:Lippincott Williams 2005.
- ,,,.Pediatric Hospital Medicine: Textbook of Inpatient Management.Philadelphia, PA:Lippincott Williams 2008.
- ,.Comprehensive pediatric hospital medicine.Philadelphia, PA:Mosby Elsevier;2007.
- ,,.Textbook of Pediatric Emergency Medicine.5th ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,.Pediatric Emergency Medicine.Philadelphia, PA:Saunders Elsevier;2008.
- ,,,.Textbook of Pediatric Infectious Diseases.5th ed.Philadelphia, PA:Saunders;2004.
- ,.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier Saunders;2006.
- ,.Avery's diseases of the newborn.7th ed.Philadelphia, PA:Saunders;1998.
- ,.Child Neurology.6th ed.Philadelphia, PA:Lippincott Williams 2000.
- ,.Pediatric Neurology: Principles and Practice.3rd ed.St. Louis, MO:Mosby;1999.
- ,.Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age.Arch Pediatr Adolesc Med.1999;153(5):508–511.
- ,,.The efficacy of routine outpatient management without antibiotics of fever in selected infants.Pediatrics.1999;103(3):627–631.
- ,,.Bacterial sepsis and meningitis. In: Remington JS, Klein JO, Wilson CB, Baker CJ, eds.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier, Inc.;2006:247–295.
- NCCLS.Interference testing in Clinical Chemistry, NCCLS Document EP7.Wayne, PA:NCCLS;1986.
- ,,,.Lack of cerebrospinal fluid pleocytosis in young infants with enterovirus infections of the central nervous system.Pediatr Emerg Care.2010;26(2):77–81.
- ,,, et al.Clinical and demographic factors associated with urinary tract infection in young febrile infants.Pediatrics.2005;116(3):644–648.
- ,,,,.Enhanced urinalysis as a screening test for urinary tract infection.Pediatrics.1993;91(6):1196–1199.
- ,,,.Screening for urinary tract infection in infants in the emergency department: which test is best?Pediatrics.1998;101(6):E1.
- ,,,,,.Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger.Pediatrics.2007;120(3):489–496.
- .The normal cerebro‐spinal fluid in children.Archf Dis Child.1928:96–108.
- .The cerebrospinal fluid in the healthy newborn infant.S Afr Med J.1968;42(35):933–935.
- ,,.Cerebrospinal fluid evaluation in neonates: comparison of high‐risk infants with and without meningitis.J Pediatr.1976;88(3):473–477.
- ,.Estimation of reference intervals for total protein in cerebrospinal fluid.Clin Chem.1989;35(8):1766–1770.
- ,,,,,.Severe neonatal parechovirus infection and similarity with enterovirus infection.Pediatr Infect Dis J.2008;27(3):241–245.
Emergency department evaluation of a febrile neonate or young infant routinely includes lumbar puncture and cerebrospinal fluid (CSF) analysis to diagnose meningitis or encephalitis. In addition to CSF Gram stain and culture, clinicians generally request a laboratory report for the CSF cell count, glucose content and protein concentration. Interpretation of these ancillary tests requires knowledge of normal reference values. In adult medicine, the accepted reference value for CSF protein concentration at the level of the lumbar spine is 15 mg/dL to45 mg/dL.1 There is general consensus among reference texts and published original studies dating back to Widell2 in 1958 that adult CSF protein reference values are not valid in the pediatric population. A healthy neonate's CSF protein concentration is normally twice to 3 times that of an adult, and declines with age from birth to early childhood. The most rapid rate of decline is thought to occur in the first 6 months of life as the infant's blood‐CSF barrier matures.3 However, published studies47 differ in the reported rate, timing, and magnitude of this decline; on close review these studies have significant limitations which call into question the appropriateness of using these values in clinical practice. Perhaps in recognition of the limited evidence, textbooks of general pediatrics,810 hospital medicine,1113 emergency medicine,14, 15 infectious diseases,16, 17 neonatology,18 and neurology19, 20 frequently publish norms for pediatric CSF protein concentration without reference to any original research studies.
Because ethical considerations prohibit subjecting young infants to a potentially painfully procedure (ie, lumbar puncture) before they are able to assent, we sought to define a study population that approximates a group of healthy infants. Our objectives were to quantify age‐related declines in CSF protein concentration and to determine accurate, age‐specific reference values for CSF protein concentration in a population of neonates and young infants who presented for medical care with an indication for lumbar puncture and were subsequently found to have no condition associated with elevated or depressed CSF protein concentration.
Methods
Study Design and Setting
This cross‐sectional study was performed at The Children's Hospital of Philadelphia (Philadelphia, PA), an urban, tertiary‐care children's hospital. The Committees for the Protection of Human Subjects approved this study with a waiver of informed consent.
Study Participants
Infants 56 days of age or younger were eligible for inclusion if they had a lumbar puncture performed as part of their emergency department evaluation between January 1, 2005 and June 30, 2007. Children in this age range were selected as they routinely undergo lumbar puncture when presenting with fever at our institution.21, 22 Patients undergoing lumbar puncture in the emergency department were identified using 2 different data sources to ensure accurate identification of all eligible infants: (1) Emergency department computerized order entry records identified all infants with CSF testing (including CSF Gram stain, culture, cell count, glucose, or protein) performed during the study period, and (2) Clinical Virology Laboratory records identified all infants in whom CSF herpes simplex virus or enterovirus testing was performed. Medical records of infants identified by these 2 sources were reviewed to determine study eligibility.
Subjects with conditions known or suspected to cause abnormal CSF protein concentration were systematically excluded from the final analysis. Exclusion criteria included traumatic lumbar puncture (defined as CSF sample with >500 red blood cells per mm3), serious bacterial infection (including meningitis, urinary tract infection, bacteremia, pneumonia, osteomyelitis, or septic arthritis), congenital infection, CSF positive for enterovirus by polymerase chain reaction (PCR) testing, seizure prior to presentation, presence of a ventricular shunt device, elevated serum bilirubin, and absent CSF protein measurements or CSF red blood cell counts. The presence of lysed red blood cells in the CSF secondary to a traumatic lumbar puncture or subarachnoid hemorrhage alters the CSF protein.23 We also excluded subjects who had CSF assays done on samples drawn by accessing a ventricular shunt device, as there may be up to a 300% regional difference in CSF protein concentration between the cranial and caudal ends of the neuroaxis.1 Bilirubin in the CSF sample at a concentration of 5 mg/dL biases the CSF protein concentration measurement by an average of 13.7 mg/dL.24 Quantitative protein assay was performed on the institution's standard Vitros chemistry system; the protein assay is a modified biuret reaction.
Study Definitions
CSF pleocytosis was defined as a CSF white blood cell count (WBC) >22/mm3 (for infants age 28 days) or >15/mm3 (for infants 2956 days of age).25 Bacterial meningitis was defined as isolation of a bacterial pathogen from the CSF. Bacteremia was defined as isolation of a bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Bacterial pneumonia was defined as a new discrete infiltrate on chest radiograph as documented by an attending pediatric radiologist in conjunction with growth of a respiratory bacterial pathogen from blood culture. Urinary tract infection was defined as growth of a single known pathogen in culture as follows: (1) 1000 colony‐forming units/mL for cultures obtained by suprapubic aspiration, (2) 50,000 cfu/mL from a catheterized specimen, or (3) 10,000 cfu/mL from catheterized specimen in conjunction with a positive urinalysis.26 Positive urinalysis was defined as trace or greater leukocyte esterase by dip stick, or >9 WBC per high‐power filed on standard microscopic exam of centrifuged urine, or >10 WBC/mm3 by hemocytometer count of uncentrifuged urine.27, 28 We defined osteomyelitis as growth of pathogenic bacteria from blood, bone, or subperiosteal aspirate culture in a subject with fever and localized tenderness, edema or erythema at the site of bony infection, and compatible imaging; and septic arthritis as growth of pathogenic bacteria from synovial fluid or blood culture from a subject with purulent synovial fluid or positive Gram stain of synovial fluid.
A temperature 38.0C by any method qualified as fever. Prematurity was defined as a gestational age less than 37 weeks. Seizure included any clinical description of the event within 48 hours of presentation to the Emergency Department, or documented seizure activity on electroencephalogram. Enterovirus season was defined as June 1st to October 31st of each year.29
Data Collection and Statistical Analysis
Information collected included the following: demographics, vital signs, history of present illness, birth history, clinical findings, results of laboratory testing and imaging within 48 hours of presentation, antibiotics administered, and duration of visit to the Emergency Department or admission to the hospital.
Categorical data were described using frequencies and percents, and continuous variables were described using mean, median, interquartile range, and 90th and 95th percentile values. Linear regression was used to determine the association between age and CSF protein concentration. Because the CSF protein concentrations had a skewed distribution (P < 0.001, Shapiro‐Wilk test), our analyses were performed using logarithmically transformed CSF protein values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent change in CSF protein with increasing age. Two‐sample Wilcoxon rank‐sum tests were subsequently used to compare the distribution of CSF protein concentrations amongst four predefined age categories to facilitate implementation of our results into clinical practice: 014 days, 1528 days, 2942 days, and 4356 days. The analyses were repeated while excluding preterm infants, patients receiving antibiotics before lumbar puncture, and patients with CSF pleocytosis to determine the impact of these factors on CSF protein concentrations. Data were analyzed using STATA v10 (Stata Corporation, College Station, TX). Two‐tailed P values < 0.05 were considered statistically significant.
Results
During the study period, 1064 infants age 56 days of age or younger underwent lumbar puncture in the emergency department. Of these, 689 (65%) met sequential exclusion criteria as follows: traumatic lumbar puncture (n = 330); transported from an outside medical facility (n = 90); bacterial meningitis (n = 6); noncentral nervous system serious bacterial infections (n = 135); CSF positive for herpes simplex virus by PCR (n = 2); CSF positive for enterovirus by PCR (n = 45); congenital syphilis (n = 1); seizures (n = 28); abnormal central nervous system imaging (n = 2); and ventricular shunt device (n = 1). An additional 44 patients had lumbar puncture and CSF testing but the protein assay was never done or never reported and 5 patients did not have a CSF red blood cell count available. No cases were excluded for elevated serum bilirubin. Infants may have met multiple exclusion criteria. The remaining 375 (35%) subjects were included in the final analysis. The median patient age was 36 days (interquartile range: 2247 days); 139 (37%) were 28 days of age or younger. Overall, 205 (55%) were male, 211 (56%) were black, and 145 (39%) presented during enterovirus season. Most (43 of 57) preterm infants were born between 34 weeks to 37 weeks gestation. Antibiotics were administered before lumbar puncture to 42 (11%) infants and 312 (83%) infants had fever.
The median CSF protein value was 58 mg/dL (interquartile range: 4872 mg/dL). There was an age‐related declined in CSF protein concentration (Figure 1). In linear regression, the CSF protein concentration decreased 6.8% (95% confidence interval [CI], 5.48.1%; P < 0.001) for each 1‐week increase in age.0


CSF protein concentrations were higher for infants 28 days of age than for infants 2956 days of age (P < 0.001, Wilcoxon rank‐sum test). The median CSF protein concentrations were 68 mg/dL (95th percentile value, 115 mg/dL) for infants 28 days of age and 54 mg/dL (95th percentile value, 89 mg/dL) for infants 2956 days. CSF protein concentrations by 2‐week age intervals are shown in Table 1. The 95th percentile CSF protein concentrations were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL (Table 1). CSF protein concentration decreased significantly across each age interval when compared with infants in the next highest age category (P < 0.02 for all pair‐wise comparisons, Wilcoxon rank‐sum test).
| Value | 014 days (n = 52) | 1528 days (n = 87) | 2942 days (n = 110) | 4356 days (n = 126) | All Infants (n = 375) |
|---|---|---|---|---|---|
| |||||
| Mean (SD) | 79 (23) | 69 (20) | 58 (17) | 53 (17) | 62 (21) |
| Median (IQR) | 78 (5893) | 65 (5685) | 55 (4965) | 50 (4162) | 58 (4872) |
| 90th percentile | 106 | 95 | 79 | 75 | 91 |
| 95th percentile | 132 | 100 | 89 | 83 | 99 |
| 95th percentile* | 132 | 101 | 89 | 82 | 97 |
| 95th percentile | 132 | 100 | 87 | 74 | 97 |
Age‐specific 95th percentile CSF protein values changed by <1% when infants receiving antibiotics before lumbar puncture were excluded (Table 1). Age‐specific CSF protein values changed minimally when preterm infants were excluded with the exception of infants 4356 days of age where the 95th percentile value was 9.7% lower than when all infants were included (Table 1); the 90th percentile values in this age group were more comparable at 75 mg/dL and 71 mg/dL, respectively, in the subgroups with and without preterm infants. Age‐specific 95th percentile CSF protein values changes by <1% when patients with CSF pleocytosis were excluded.
Discussion
We examined CSF protein values in neonates and young infants to establish reference values and to bring the literature up to date at a time when molecular tools are commonly used in clinical practice. We also quantified the age‐related decline in CSF protein concentrations over the first two months of life. Our findings provide age‐specific reference ranges for CSF protein concentrations in neonates and young infants. These findings are particularly important because a variety of infectious (eg, herpes simplex virus infection) and noninfectious (eg, subarachnoid or intraventricular hemorrhage) conditions may occur in the absence of appreciable elevations in the CSF WBC.
CSF protein concentrations depend on serum protein concentrations and on the permeability of the blood‐CSF barrier. Immaturity of the blood‐CSF barrier is thought to result in higher CSF protein concentrations for neonates and young infants compared with older children and adults. Though previous studies agree that CSF protein concentrations depend on age, the reported age‐specific values and rates of decline vary considerably.47, 3032 Additionally, these prior studies are limited by (1) small sample size, (2) variable inclusion and exclusion criteria, (3) variable laboratory techniques to quantify protein concentration in a CSF sample, and (4) presentation of mean, standard deviation, and range values rather than the 75th, 90th, or 95th percentile values necessary to define a clinically meaningful reference range.
The median and mean values found in this study were generally comparable to previously published values (Table 2). In addition, we have quantified the age‐related decline in CSF protein concentrations identified in previous studies. While our large sample size allowed us to define narrower reference intervals than most previous studies, direct comparison of values used to define reference ranges was hampered by lack of consistent reporting of data across studies. Ahmed et al.5 and Bonadio et al.4 reported only mean and standard deviation values. When data are skewed, as is the case for CSF protein values, the standard deviation will be grossly inflated, making extrapolation to percentile values unreliable. The 90th percentile value of 87 mg/dL reported by Wong et al.7 for infants 060 days of age was similar to the value of 91 mg/dL for infants 56 days of age and younger found in this study. Biou et al.6 reported the following 95th percentile values: ages 18 days, 108 mg/dL; ages 830 days, 90 mg/dL; and ages 12 months, 77 mg/dL. These values are lower than those reported in our study. The reason for such differences is not clear. The exclusion criteria were similar between the two studies though Biou et al.6 did not include preterm infants. When we excluded preterm infants from our analysis, no age‐specific result decreased by more than 5%, making the inclusion of this population an unlikely explanation for the differences between the two studies.
| Author | Year | Number of Infants | Age (days) | Median (mg/dL) | Mean SD (mg/dL) |
|---|---|---|---|---|---|
| |||||
| Bonadio et al.4 | 1992 | 35 | 030 | 84 45 | |
| 40 | 3060 | 59 25 | |||
| Ahmed et al.5 | 1996 | 17 | 07 | 81 31 | |
| 33 | 814 | 69 23 | |||
| 25 | 1521 | 60 23 | |||
| 33 | 2230 | 54 16 | |||
| Biou et al.6 | 2000 | 26 | 18 | 71 | |
| 76 | 830 | 59 | |||
| 155 | 3060 | 47 | |||
| Wong et al.7 | 2000 | 99 | 060 | 60 | 59 21 |
CSF protein concentration is a method‐dependent value; the results depend a great deal on what technique the laboratory uses. Two common methods used in the past few decades are Biuret Colorimetry and Turbidimetric; reported values are approximately 25% higher with the Biuret method compared with the Turbidimetric method.33 A CSF protein reference value is only clinically useful if the method used to define the norm is specified and equivalent to currently used methods. Similar to our study, Biou et al.6 and Wong et al.7 used the Biuret (Vitros) method. The method of protein measurement was not specified by other studies.4, 5
This study had several limitations that could cause us to overestimate the upper bound of the reference range. First, spectrum bias is possible in this observational study. Individual physicians determined whether lumbar puncture was warranted, a limitation that could potentially lead to the disproportionate inclusion of infants with conditions associated with higher CSF protein concentrations. We do not believe that this limitation would meaningfully affect our results because febrile infants 56 days of age or younger routinely undergo lumbar puncture at our institution, regardless of illness severity, and patients diagnosed with conditions known or suspected to increase CSF protein concentrations were excluded. Second, infants with aseptic meningitisa condition that can be associated with elevated CSF protein concentrationsmay have been misclassified as uninfected. Though we excluded patients with positive CSF enteroviral PCR tests, some infants were not tested and other viruses (eg, parechoviruses)34 not detected by the enterovirus PCR may also cause aseptic meningitis. Third, certain antibiotics including ampicillin and vancomycin are known to interfere with the CSF protein assay used in our laboratory.24 Forty‐two of the 375 subjects included in our final analysis received antibiotics prior to lumbar puncture. When receiving antibiotics prior to lumbar puncture were excluded from analysis, the CSF protein concentrations were within 1% of the overall study population, suggesting that antibiotic administration before lumbar puncture did not influence our results in any meaningful way. We would not expect any of these limitations to disproportionately affect patients in 1 particular age category.
In conclusion, the CSF protein concentration values reported here represent the largest series to‐date for this young age group. Our study quantifies the age‐related decline in CSF protein concentration from birth to 56 days of life. Our work designing this study, specifically the exclusion criteria, refines the approach to defining normal CSF protein values in children. As CSF protein values decline steadily with increasing age, the selection of reference values is a balance of accuracy and convenience. Age‐specific reference values by 2‐week increments would be most accurate. However, considering reference values by month of age, as is the convention for CSF WBCs, is far more practical. The 95th percentile values by age category in our study were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL. The 95th percentile values were 115 mg/dL for infants 28 days and 89 mg/dL for infants 2956 days. We feel that either approach is reasonable. These values can be used to accurately interpret the results of CSF studies in neonates and young infants.
Emergency department evaluation of a febrile neonate or young infant routinely includes lumbar puncture and cerebrospinal fluid (CSF) analysis to diagnose meningitis or encephalitis. In addition to CSF Gram stain and culture, clinicians generally request a laboratory report for the CSF cell count, glucose content and protein concentration. Interpretation of these ancillary tests requires knowledge of normal reference values. In adult medicine, the accepted reference value for CSF protein concentration at the level of the lumbar spine is 15 mg/dL to45 mg/dL.1 There is general consensus among reference texts and published original studies dating back to Widell2 in 1958 that adult CSF protein reference values are not valid in the pediatric population. A healthy neonate's CSF protein concentration is normally twice to 3 times that of an adult, and declines with age from birth to early childhood. The most rapid rate of decline is thought to occur in the first 6 months of life as the infant's blood‐CSF barrier matures.3 However, published studies47 differ in the reported rate, timing, and magnitude of this decline; on close review these studies have significant limitations which call into question the appropriateness of using these values in clinical practice. Perhaps in recognition of the limited evidence, textbooks of general pediatrics,810 hospital medicine,1113 emergency medicine,14, 15 infectious diseases,16, 17 neonatology,18 and neurology19, 20 frequently publish norms for pediatric CSF protein concentration without reference to any original research studies.
Because ethical considerations prohibit subjecting young infants to a potentially painfully procedure (ie, lumbar puncture) before they are able to assent, we sought to define a study population that approximates a group of healthy infants. Our objectives were to quantify age‐related declines in CSF protein concentration and to determine accurate, age‐specific reference values for CSF protein concentration in a population of neonates and young infants who presented for medical care with an indication for lumbar puncture and were subsequently found to have no condition associated with elevated or depressed CSF protein concentration.
Methods
Study Design and Setting
This cross‐sectional study was performed at The Children's Hospital of Philadelphia (Philadelphia, PA), an urban, tertiary‐care children's hospital. The Committees for the Protection of Human Subjects approved this study with a waiver of informed consent.
Study Participants
Infants 56 days of age or younger were eligible for inclusion if they had a lumbar puncture performed as part of their emergency department evaluation between January 1, 2005 and June 30, 2007. Children in this age range were selected as they routinely undergo lumbar puncture when presenting with fever at our institution.21, 22 Patients undergoing lumbar puncture in the emergency department were identified using 2 different data sources to ensure accurate identification of all eligible infants: (1) Emergency department computerized order entry records identified all infants with CSF testing (including CSF Gram stain, culture, cell count, glucose, or protein) performed during the study period, and (2) Clinical Virology Laboratory records identified all infants in whom CSF herpes simplex virus or enterovirus testing was performed. Medical records of infants identified by these 2 sources were reviewed to determine study eligibility.
Subjects with conditions known or suspected to cause abnormal CSF protein concentration were systematically excluded from the final analysis. Exclusion criteria included traumatic lumbar puncture (defined as CSF sample with >500 red blood cells per mm3), serious bacterial infection (including meningitis, urinary tract infection, bacteremia, pneumonia, osteomyelitis, or septic arthritis), congenital infection, CSF positive for enterovirus by polymerase chain reaction (PCR) testing, seizure prior to presentation, presence of a ventricular shunt device, elevated serum bilirubin, and absent CSF protein measurements or CSF red blood cell counts. The presence of lysed red blood cells in the CSF secondary to a traumatic lumbar puncture or subarachnoid hemorrhage alters the CSF protein.23 We also excluded subjects who had CSF assays done on samples drawn by accessing a ventricular shunt device, as there may be up to a 300% regional difference in CSF protein concentration between the cranial and caudal ends of the neuroaxis.1 Bilirubin in the CSF sample at a concentration of 5 mg/dL biases the CSF protein concentration measurement by an average of 13.7 mg/dL.24 Quantitative protein assay was performed on the institution's standard Vitros chemistry system; the protein assay is a modified biuret reaction.
Study Definitions
CSF pleocytosis was defined as a CSF white blood cell count (WBC) >22/mm3 (for infants age 28 days) or >15/mm3 (for infants 2956 days of age).25 Bacterial meningitis was defined as isolation of a bacterial pathogen from the CSF. Bacteremia was defined as isolation of a bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Bacterial pneumonia was defined as a new discrete infiltrate on chest radiograph as documented by an attending pediatric radiologist in conjunction with growth of a respiratory bacterial pathogen from blood culture. Urinary tract infection was defined as growth of a single known pathogen in culture as follows: (1) 1000 colony‐forming units/mL for cultures obtained by suprapubic aspiration, (2) 50,000 cfu/mL from a catheterized specimen, or (3) 10,000 cfu/mL from catheterized specimen in conjunction with a positive urinalysis.26 Positive urinalysis was defined as trace or greater leukocyte esterase by dip stick, or >9 WBC per high‐power filed on standard microscopic exam of centrifuged urine, or >10 WBC/mm3 by hemocytometer count of uncentrifuged urine.27, 28 We defined osteomyelitis as growth of pathogenic bacteria from blood, bone, or subperiosteal aspirate culture in a subject with fever and localized tenderness, edema or erythema at the site of bony infection, and compatible imaging; and septic arthritis as growth of pathogenic bacteria from synovial fluid or blood culture from a subject with purulent synovial fluid or positive Gram stain of synovial fluid.
A temperature 38.0C by any method qualified as fever. Prematurity was defined as a gestational age less than 37 weeks. Seizure included any clinical description of the event within 48 hours of presentation to the Emergency Department, or documented seizure activity on electroencephalogram. Enterovirus season was defined as June 1st to October 31st of each year.29
Data Collection and Statistical Analysis
Information collected included the following: demographics, vital signs, history of present illness, birth history, clinical findings, results of laboratory testing and imaging within 48 hours of presentation, antibiotics administered, and duration of visit to the Emergency Department or admission to the hospital.
Categorical data were described using frequencies and percents, and continuous variables were described using mean, median, interquartile range, and 90th and 95th percentile values. Linear regression was used to determine the association between age and CSF protein concentration. Because the CSF protein concentrations had a skewed distribution (P < 0.001, Shapiro‐Wilk test), our analyses were performed using logarithmically transformed CSF protein values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent change in CSF protein with increasing age. Two‐sample Wilcoxon rank‐sum tests were subsequently used to compare the distribution of CSF protein concentrations amongst four predefined age categories to facilitate implementation of our results into clinical practice: 014 days, 1528 days, 2942 days, and 4356 days. The analyses were repeated while excluding preterm infants, patients receiving antibiotics before lumbar puncture, and patients with CSF pleocytosis to determine the impact of these factors on CSF protein concentrations. Data were analyzed using STATA v10 (Stata Corporation, College Station, TX). Two‐tailed P values < 0.05 were considered statistically significant.
Results
During the study period, 1064 infants age 56 days of age or younger underwent lumbar puncture in the emergency department. Of these, 689 (65%) met sequential exclusion criteria as follows: traumatic lumbar puncture (n = 330); transported from an outside medical facility (n = 90); bacterial meningitis (n = 6); noncentral nervous system serious bacterial infections (n = 135); CSF positive for herpes simplex virus by PCR (n = 2); CSF positive for enterovirus by PCR (n = 45); congenital syphilis (n = 1); seizures (n = 28); abnormal central nervous system imaging (n = 2); and ventricular shunt device (n = 1). An additional 44 patients had lumbar puncture and CSF testing but the protein assay was never done or never reported and 5 patients did not have a CSF red blood cell count available. No cases were excluded for elevated serum bilirubin. Infants may have met multiple exclusion criteria. The remaining 375 (35%) subjects were included in the final analysis. The median patient age was 36 days (interquartile range: 2247 days); 139 (37%) were 28 days of age or younger. Overall, 205 (55%) were male, 211 (56%) were black, and 145 (39%) presented during enterovirus season. Most (43 of 57) preterm infants were born between 34 weeks to 37 weeks gestation. Antibiotics were administered before lumbar puncture to 42 (11%) infants and 312 (83%) infants had fever.
The median CSF protein value was 58 mg/dL (interquartile range: 4872 mg/dL). There was an age‐related declined in CSF protein concentration (Figure 1). In linear regression, the CSF protein concentration decreased 6.8% (95% confidence interval [CI], 5.48.1%; P < 0.001) for each 1‐week increase in age.0


CSF protein concentrations were higher for infants 28 days of age than for infants 2956 days of age (P < 0.001, Wilcoxon rank‐sum test). The median CSF protein concentrations were 68 mg/dL (95th percentile value, 115 mg/dL) for infants 28 days of age and 54 mg/dL (95th percentile value, 89 mg/dL) for infants 2956 days. CSF protein concentrations by 2‐week age intervals are shown in Table 1. The 95th percentile CSF protein concentrations were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL (Table 1). CSF protein concentration decreased significantly across each age interval when compared with infants in the next highest age category (P < 0.02 for all pair‐wise comparisons, Wilcoxon rank‐sum test).
| Value | 014 days (n = 52) | 1528 days (n = 87) | 2942 days (n = 110) | 4356 days (n = 126) | All Infants (n = 375) |
|---|---|---|---|---|---|
| |||||
| Mean (SD) | 79 (23) | 69 (20) | 58 (17) | 53 (17) | 62 (21) |
| Median (IQR) | 78 (5893) | 65 (5685) | 55 (4965) | 50 (4162) | 58 (4872) |
| 90th percentile | 106 | 95 | 79 | 75 | 91 |
| 95th percentile | 132 | 100 | 89 | 83 | 99 |
| 95th percentile* | 132 | 101 | 89 | 82 | 97 |
| 95th percentile | 132 | 100 | 87 | 74 | 97 |
Age‐specific 95th percentile CSF protein values changed by <1% when infants receiving antibiotics before lumbar puncture were excluded (Table 1). Age‐specific CSF protein values changed minimally when preterm infants were excluded with the exception of infants 4356 days of age where the 95th percentile value was 9.7% lower than when all infants were included (Table 1); the 90th percentile values in this age group were more comparable at 75 mg/dL and 71 mg/dL, respectively, in the subgroups with and without preterm infants. Age‐specific 95th percentile CSF protein values changes by <1% when patients with CSF pleocytosis were excluded.
Discussion
We examined CSF protein values in neonates and young infants to establish reference values and to bring the literature up to date at a time when molecular tools are commonly used in clinical practice. We also quantified the age‐related decline in CSF protein concentrations over the first two months of life. Our findings provide age‐specific reference ranges for CSF protein concentrations in neonates and young infants. These findings are particularly important because a variety of infectious (eg, herpes simplex virus infection) and noninfectious (eg, subarachnoid or intraventricular hemorrhage) conditions may occur in the absence of appreciable elevations in the CSF WBC.
CSF protein concentrations depend on serum protein concentrations and on the permeability of the blood‐CSF barrier. Immaturity of the blood‐CSF barrier is thought to result in higher CSF protein concentrations for neonates and young infants compared with older children and adults. Though previous studies agree that CSF protein concentrations depend on age, the reported age‐specific values and rates of decline vary considerably.47, 3032 Additionally, these prior studies are limited by (1) small sample size, (2) variable inclusion and exclusion criteria, (3) variable laboratory techniques to quantify protein concentration in a CSF sample, and (4) presentation of mean, standard deviation, and range values rather than the 75th, 90th, or 95th percentile values necessary to define a clinically meaningful reference range.
The median and mean values found in this study were generally comparable to previously published values (Table 2). In addition, we have quantified the age‐related decline in CSF protein concentrations identified in previous studies. While our large sample size allowed us to define narrower reference intervals than most previous studies, direct comparison of values used to define reference ranges was hampered by lack of consistent reporting of data across studies. Ahmed et al.5 and Bonadio et al.4 reported only mean and standard deviation values. When data are skewed, as is the case for CSF protein values, the standard deviation will be grossly inflated, making extrapolation to percentile values unreliable. The 90th percentile value of 87 mg/dL reported by Wong et al.7 for infants 060 days of age was similar to the value of 91 mg/dL for infants 56 days of age and younger found in this study. Biou et al.6 reported the following 95th percentile values: ages 18 days, 108 mg/dL; ages 830 days, 90 mg/dL; and ages 12 months, 77 mg/dL. These values are lower than those reported in our study. The reason for such differences is not clear. The exclusion criteria were similar between the two studies though Biou et al.6 did not include preterm infants. When we excluded preterm infants from our analysis, no age‐specific result decreased by more than 5%, making the inclusion of this population an unlikely explanation for the differences between the two studies.
| Author | Year | Number of Infants | Age (days) | Median (mg/dL) | Mean SD (mg/dL) |
|---|---|---|---|---|---|
| |||||
| Bonadio et al.4 | 1992 | 35 | 030 | 84 45 | |
| 40 | 3060 | 59 25 | |||
| Ahmed et al.5 | 1996 | 17 | 07 | 81 31 | |
| 33 | 814 | 69 23 | |||
| 25 | 1521 | 60 23 | |||
| 33 | 2230 | 54 16 | |||
| Biou et al.6 | 2000 | 26 | 18 | 71 | |
| 76 | 830 | 59 | |||
| 155 | 3060 | 47 | |||
| Wong et al.7 | 2000 | 99 | 060 | 60 | 59 21 |
CSF protein concentration is a method‐dependent value; the results depend a great deal on what technique the laboratory uses. Two common methods used in the past few decades are Biuret Colorimetry and Turbidimetric; reported values are approximately 25% higher with the Biuret method compared with the Turbidimetric method.33 A CSF protein reference value is only clinically useful if the method used to define the norm is specified and equivalent to currently used methods. Similar to our study, Biou et al.6 and Wong et al.7 used the Biuret (Vitros) method. The method of protein measurement was not specified by other studies.4, 5
This study had several limitations that could cause us to overestimate the upper bound of the reference range. First, spectrum bias is possible in this observational study. Individual physicians determined whether lumbar puncture was warranted, a limitation that could potentially lead to the disproportionate inclusion of infants with conditions associated with higher CSF protein concentrations. We do not believe that this limitation would meaningfully affect our results because febrile infants 56 days of age or younger routinely undergo lumbar puncture at our institution, regardless of illness severity, and patients diagnosed with conditions known or suspected to increase CSF protein concentrations were excluded. Second, infants with aseptic meningitisa condition that can be associated with elevated CSF protein concentrationsmay have been misclassified as uninfected. Though we excluded patients with positive CSF enteroviral PCR tests, some infants were not tested and other viruses (eg, parechoviruses)34 not detected by the enterovirus PCR may also cause aseptic meningitis. Third, certain antibiotics including ampicillin and vancomycin are known to interfere with the CSF protein assay used in our laboratory.24 Forty‐two of the 375 subjects included in our final analysis received antibiotics prior to lumbar puncture. When receiving antibiotics prior to lumbar puncture were excluded from analysis, the CSF protein concentrations were within 1% of the overall study population, suggesting that antibiotic administration before lumbar puncture did not influence our results in any meaningful way. We would not expect any of these limitations to disproportionately affect patients in 1 particular age category.
In conclusion, the CSF protein concentration values reported here represent the largest series to‐date for this young age group. Our study quantifies the age‐related decline in CSF protein concentration from birth to 56 days of life. Our work designing this study, specifically the exclusion criteria, refines the approach to defining normal CSF protein values in children. As CSF protein values decline steadily with increasing age, the selection of reference values is a balance of accuracy and convenience. Age‐specific reference values by 2‐week increments would be most accurate. However, considering reference values by month of age, as is the convention for CSF WBCs, is far more practical. The 95th percentile values by age category in our study were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL. The 95th percentile values were 115 mg/dL for infants 28 days and 89 mg/dL for infants 2956 days. We feel that either approach is reasonable. These values can be used to accurately interpret the results of CSF studies in neonates and young infants.
- ,.Henry's Clinical Diagnosis and Management by Laboratory Methods.21st ed.Philadelphia, PA:W.B. Saunders, Inc.;2006.
- .On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo‐encephalitis.Acta Paediatr Suppl.1958;47(Suppl 115):1–102.
- ,.Development of the blood‐CSF barrier.Dev Med Child Neurol.1983;25(2):152–161.
- ,,,,.Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks.Pediatr Infect Dis J.1992;11(7):589–591.
- ,,, et al.Cerebrospinal fluid values in the term neonate.Pediatr Infect Dis J.1996;15(4):298–303.
- ,,,,,.Cerebrospinal fluid protein concentrations in children: age‐related values in patients without disorders of the central nervous system.Clin Chem.2000;46(3):399–403.
- ,,,,.Cerebrospinal fluid protein concentration in pediatric patients: defining clinically relevant reference values.Arch Pediatr Adolesc Med.2000;154(8):827–831.
- ,,.Nelson Textbook of Pediatrics.17th ed.Philadelphia, PA:Saunders;2004.
- ,,,.Oski's pediatrics : principles 2006.
- Robertson J, Shilkofski N, eds.Johns Hopkins: The Harriet Lane Handbook: A Manual for Pediatric House Officers.17 ed.Philadelphia, PA:Elsevier Mosby;2005.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia, PA:Lippincott Williams 2005.
- ,,,.Pediatric Hospital Medicine: Textbook of Inpatient Management.Philadelphia, PA:Lippincott Williams 2008.
- ,.Comprehensive pediatric hospital medicine.Philadelphia, PA:Mosby Elsevier;2007.
- ,,.Textbook of Pediatric Emergency Medicine.5th ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,.Pediatric Emergency Medicine.Philadelphia, PA:Saunders Elsevier;2008.
- ,,,.Textbook of Pediatric Infectious Diseases.5th ed.Philadelphia, PA:Saunders;2004.
- ,.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier Saunders;2006.
- ,.Avery's diseases of the newborn.7th ed.Philadelphia, PA:Saunders;1998.
- ,.Child Neurology.6th ed.Philadelphia, PA:Lippincott Williams 2000.
- ,.Pediatric Neurology: Principles and Practice.3rd ed.St. Louis, MO:Mosby;1999.
- ,.Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age.Arch Pediatr Adolesc Med.1999;153(5):508–511.
- ,,.The efficacy of routine outpatient management without antibiotics of fever in selected infants.Pediatrics.1999;103(3):627–631.
- ,,.Bacterial sepsis and meningitis. In: Remington JS, Klein JO, Wilson CB, Baker CJ, eds.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier, Inc.;2006:247–295.
- NCCLS.Interference testing in Clinical Chemistry, NCCLS Document EP7.Wayne, PA:NCCLS;1986.
- ,,,.Lack of cerebrospinal fluid pleocytosis in young infants with enterovirus infections of the central nervous system.Pediatr Emerg Care.2010;26(2):77–81.
- ,,, et al.Clinical and demographic factors associated with urinary tract infection in young febrile infants.Pediatrics.2005;116(3):644–648.
- ,,,,.Enhanced urinalysis as a screening test for urinary tract infection.Pediatrics.1993;91(6):1196–1199.
- ,,,.Screening for urinary tract infection in infants in the emergency department: which test is best?Pediatrics.1998;101(6):E1.
- ,,,,,.Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger.Pediatrics.2007;120(3):489–496.
- .The normal cerebro‐spinal fluid in children.Archf Dis Child.1928:96–108.
- .The cerebrospinal fluid in the healthy newborn infant.S Afr Med J.1968;42(35):933–935.
- ,,.Cerebrospinal fluid evaluation in neonates: comparison of high‐risk infants with and without meningitis.J Pediatr.1976;88(3):473–477.
- ,.Estimation of reference intervals for total protein in cerebrospinal fluid.Clin Chem.1989;35(8):1766–1770.
- ,,,,,.Severe neonatal parechovirus infection and similarity with enterovirus infection.Pediatr Infect Dis J.2008;27(3):241–245.
- ,.Henry's Clinical Diagnosis and Management by Laboratory Methods.21st ed.Philadelphia, PA:W.B. Saunders, Inc.;2006.
- .On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo‐encephalitis.Acta Paediatr Suppl.1958;47(Suppl 115):1–102.
- ,.Development of the blood‐CSF barrier.Dev Med Child Neurol.1983;25(2):152–161.
- ,,,,.Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks.Pediatr Infect Dis J.1992;11(7):589–591.
- ,,, et al.Cerebrospinal fluid values in the term neonate.Pediatr Infect Dis J.1996;15(4):298–303.
- ,,,,,.Cerebrospinal fluid protein concentrations in children: age‐related values in patients without disorders of the central nervous system.Clin Chem.2000;46(3):399–403.
- ,,,,.Cerebrospinal fluid protein concentration in pediatric patients: defining clinically relevant reference values.Arch Pediatr Adolesc Med.2000;154(8):827–831.
- ,,.Nelson Textbook of Pediatrics.17th ed.Philadelphia, PA:Saunders;2004.
- ,,,.Oski's pediatrics : principles 2006.
- Robertson J, Shilkofski N, eds.Johns Hopkins: The Harriet Lane Handbook: A Manual for Pediatric House Officers.17 ed.Philadelphia, PA:Elsevier Mosby;2005.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia, PA:Lippincott Williams 2005.
- ,,,.Pediatric Hospital Medicine: Textbook of Inpatient Management.Philadelphia, PA:Lippincott Williams 2008.
- ,.Comprehensive pediatric hospital medicine.Philadelphia, PA:Mosby Elsevier;2007.
- ,,.Textbook of Pediatric Emergency Medicine.5th ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,.Pediatric Emergency Medicine.Philadelphia, PA:Saunders Elsevier;2008.
- ,,,.Textbook of Pediatric Infectious Diseases.5th ed.Philadelphia, PA:Saunders;2004.
- ,.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier Saunders;2006.
- ,.Avery's diseases of the newborn.7th ed.Philadelphia, PA:Saunders;1998.
- ,.Child Neurology.6th ed.Philadelphia, PA:Lippincott Williams 2000.
- ,.Pediatric Neurology: Principles and Practice.3rd ed.St. Louis, MO:Mosby;1999.
- ,.Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age.Arch Pediatr Adolesc Med.1999;153(5):508–511.
- ,,.The efficacy of routine outpatient management without antibiotics of fever in selected infants.Pediatrics.1999;103(3):627–631.
- ,,.Bacterial sepsis and meningitis. In: Remington JS, Klein JO, Wilson CB, Baker CJ, eds.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier, Inc.;2006:247–295.
- NCCLS.Interference testing in Clinical Chemistry, NCCLS Document EP7.Wayne, PA:NCCLS;1986.
- ,,,.Lack of cerebrospinal fluid pleocytosis in young infants with enterovirus infections of the central nervous system.Pediatr Emerg Care.2010;26(2):77–81.
- ,,, et al.Clinical and demographic factors associated with urinary tract infection in young febrile infants.Pediatrics.2005;116(3):644–648.
- ,,,,.Enhanced urinalysis as a screening test for urinary tract infection.Pediatrics.1993;91(6):1196–1199.
- ,,,.Screening for urinary tract infection in infants in the emergency department: which test is best?Pediatrics.1998;101(6):E1.
- ,,,,,.Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger.Pediatrics.2007;120(3):489–496.
- .The normal cerebro‐spinal fluid in children.Archf Dis Child.1928:96–108.
- .The cerebrospinal fluid in the healthy newborn infant.S Afr Med J.1968;42(35):933–935.
- ,,.Cerebrospinal fluid evaluation in neonates: comparison of high‐risk infants with and without meningitis.J Pediatr.1976;88(3):473–477.
- ,.Estimation of reference intervals for total protein in cerebrospinal fluid.Clin Chem.1989;35(8):1766–1770.
- ,,,,,.Severe neonatal parechovirus infection and similarity with enterovirus infection.Pediatr Infect Dis J.2008;27(3):241–245.
Copyright © 2010 Society of Hospital Medicine
Oseltamivir in Children with CA‐LCI
Influenza is a common cause of acute respiratory illness in children, resulting in hospitalization of both healthy and chronically ill children due to influenza‐related complications.1, 2 Currently, amantadine, rimantadine, oseltamivir, and zanamivir are approved for use in children to treat influenza. In early 2006, more than 90% of influenza isolates tested in the US were found to be resistant to the adamantanes, suggesting that these medications might be of limited benefit during future influenza seasons.3 To date, most isolates of influenza remain susceptible to neuraminidase inhibitors, zanamivir and oseltamivir. Zanamivir has not been used extensively in pediatrics because it is delivered by aerosolization, and is only approved by the US Food and Drug Administration (FDA) for children 7 years of age. Oseltamivir is administered orally and is FDA‐approved for use in children 1 year of age within 48 hours of onset of symptoms of influenza virus infection.
Studies performed in outpatient settings have shown that oseltamivir can lessen the severity and reduce the length of influenza illness by 36 hours when therapy is initiated within 2 days of the onset of symptoms.4 Treatment also reduced the frequency of new diagnoses of otitis media and decreased physician‐prescribed antibiotics.4
To date, there are limited data evaluating the use of oseltamivir in either adult or pediatric patients hospitalized with influenza. We sought to describe the use of antiviral medications among children hospitalized with community‐acquired laboratory‐confirmed influenza (CA‐LCI) and to evaluate the effect of a computer‐based electronic reminder to increase the rate of on‐label use of oseltamivir among hospitalized children.
PATIENTS AND METHODS
We performed a retrospective cohort study of patients 21 years of age who were hospitalized with CA‐LCI during 5 consecutive seasons from July 2000 through June 2005 (seasons 1‐5) at the Children's Hospital of Philadelphia (CHOP). CHOP is a 418‐bed tertiary care hospital with about 24,000 hospital admissions each year. Viral diagnostic studies are performed routinely on children hospitalized with acute respiratory symptoms of unknown etiology, which aids in assigning patients to cohorts. Patients who had laboratory confirmation of influenza performed at an outside institution were excluded from this analysis.
From June 2005 through May 2006 (season 6), an observational trial of an electronic clinical decision reminder was performed to assess a mechanism to increase the proportion of eligible children treated with oseltamivir. Patients were included in this analysis if they were 21 years of age and had a diagnostic specimen for influenza obtained less than 72 hours after admission. The CHOP Institutional Review Board approved this study with a waiver of informed consent.
Viral Diagnostic Testing
During the winter months from seasons 1‐5, nasopharyngeal aspirate specimens were initially tested using immunochromatographic membrane assays (IA) for respiratory syncytial virus (RSV) (NOW RSV; Binax, Inc., Scarborough, ME) and, if negative, for influenza virus types A and B (NOW Flu A, NOW Flu B; Binax). If negative, specimens were tested by direct fluorescent antibody (DFA) testing for multiple respiratory viruses, including influenza A and B. During the winter season, IA testing was performed multiple times each day, and DFA was performed once or twice daily with an 8 to 24 hour turnaround time after a specimen was obtained. For season 6, the testing algorithm was revised: a panel of real‐time polymerase chain reaction (PCR) assays were performed to detect nucleic acids from multiple respiratory viruses, including influenza virus types A and B, on specimens that tested negative for influenza and RSV by IA. PCR testing was performed multiple times each day, and specimen results were available within 24 hours of specimen submission. Comprehensive viral tube cultures were performed on specimens that were negative by IA and DFA (seasons 1‐5) or respiratory virus PCR panel (season 6).
Study Definitions
Patients were considered to have CA‐LCI if the first diagnostic specimen positive for influenza was obtained less than 72 hours after hospital admission. Prescriptions for oseltamivir that were consistent with the FDA recommendations were considered to be on‐label prescriptions. Prescriptions for oseltamivir given to patients who did not meet these FDA criteria were considered off‐label prescriptions.5 Patients were considered oseltamivir‐eligible if they were met the criteria for FDA approval for treatment with oseltamivir: at least 1 year of age with influenza symptoms of less than 48 hours duration. Patients who either by age and/or symptom duration were inconsistent with FDA labeling criteria for oseltamivir were deemed oseltamivir‐ineligible. This included those patients for whom influenza test results were received by the clinician more than 48 hours after symptom onset. Patients who were positive for influenza only by viral culture were considered oseltamivir‐ineligible since the time needed to culture influenza virus was >48 hours. Because of the abrupt onset of influenza symptoms, the duration of influenza symptoms was defined by chart review of the emergency room or admission note. A hierarchy of symptoms was used to define the initial onset of influenza‐related symptoms and include the following: (1) For all patients with a history of fever, onset of influenza was defined as the onset of fever as recorded in the first physician note. (2) For patients without a history of fever, the onset of respiratory symptoms was recorded as the onset of influenza. (3) For patients without a history of fever but in whom multiple respiratory symptoms were noted, the onset of symptoms was assigned as the beginning of the increased work of breathing.
Because influenza IA were performed at least 4 times a day during the influenza season, the date of result to clinician was determined to be the same date as specimen collection for patients who had a positive influenza IA. Patients were identified as having a positive influenza result to the clinician 1 day after specimen collection if the test was positive by DFA or PCR. A neurologic adverse event was defined as the occurrence of a seizure after initiation of oseltamivir therapy. A neuropsychiatric adverse event was defined as any significant new neuropsychiatric symptom (psychosis, encephalopathy) recorded after the initiation of oseltamivir therapy. We defined a dermatologic adverse event as the report of any skin findings recorded after the initiation of oseltamivir therapy.
Chronic medical conditions
Information from detailed chart review was used to identify children with Advisory Committee on Immunization Practices (ACIP) high‐risk medical conditions as previously described by our group (asthma, chronic pulmonary disease, cardiac disease, immunosuppression, hemoglobinopathies, chronic renal dysfunction, diabetes mellitus, inborn errors of metabolism, long‐term salicylate therapy, pregnancy, and neurological and neuromuscular disease [NNMD]).6
Electronic Reminder
During season 6, a computer‐based electronic reminder was designed. The reminder stated Consider OSELTAMIVIR if Age >1 year AND symptoms <48 hours. May shorten illness by 36 hours. Page ID approval for more info. The reminder was embedded within the influenza results for all positive determinations, so a clinician would see the reminder when viewing positive laboratory results (Meditech, Westwood, MA).
At the initiation of season 6, we determined prescription rates of oseltamivir in patients with CA‐LCI to measure the baseline rate of oseltamivir prescription. The electronic reminder was initiated during week 11 of influenza activity at our institution and continued through the end of the influenza season.
Data Collection
Two sources of antiviral prescription data were used. Inpatient prescription of antiviral medications was extracted from billing records and chart review; a 10% audit of the medication administration records showed that the billing records correctly identified oseltamivir prescription status in all cases reviewed. Patients with incomplete pharmacy data were removed from the analysis of prescription practices (n = 8). During all seasons studied, the infectious diseases pharmacist (T.A.M.) and an infectious diseases physician (T.E.Z.) reviewed requests for inpatient prescriptions for antiviral medications.
For season 6, daily review of infection control records was performed to conduct surveillance for children hospitalized with CA‐LCI. To determine symptom duration and use of antiviral medications, inpatient medical charts were reviewed at the time of initial identification and then daily thereafter.
Statistical Analysis
Dichotomous variables were created for prescription of oseltamivir, age 1 year and symptom duration of <48 hours at time of clinician receipt of influenza results. Descriptive analyses included calculating the frequencies for categorical variables. Categorical variables were compared using Fisher's exact test. The Cochrane‐Armitage test was employed to test for a trend in the prescription of oseltamivir by season. A 2‐tailed P value of <0.05 was considered significant for all statistical tests. All statistical calculations were performed using standard programs in SAS 9.1 (SAS Institute, Cary, NC), STATA 8.2 (Stata Corp., College Station, TX), and Excel (Microsoft, Redmond, WA).
Prior to the start of season 6, we determined that if the rate of oseltamivir prescription was 40% before initiation of the reminder, we would need 20 eligible patients to detect a difference of 40% or greater in subsequent prescription rates (with 80% power and an alpha of 0.05). Once this enrollment goal was met, an electronic reminder of the eligibility for oseltamivir was initiated.
RESULTS
Use of Antiviral Medications in Children Hospitalized with Influenza, 2000‐2005
From July 2000 to June 2005, 1,058 patients were admitted with laboratory confirmed influenza; 8 were excluded because confirmatory testing was done at an outside institution, 24 were repeat hospitalizations, 89 nosocomial cases, and 8 cases were in patients >21 years. Thus, 929 patients had CA‐LCI and were eligible for inclusion in this study. Most children were infected with influenza A and were 1 year of age (Table 1). During this study period, only 9.3% of study subjects were treated with antiviral medications, most of whom (91%) received oseltamivir. Eight patients received amantadine over all seasons studied.
| Characteristics | Patients Hospitalized with CA‐LCI (n = 929)* | Eligible to Receive Oseltamivir (n = 305)* |
|---|---|---|
| ||
| Age (years) | ||
| <1 | 342 (37) | 0 |
| 1 | 587 (63) | 305 (100) |
| Season | ||
| 2000‐2001 | 107 (11.5) | 32 (10) |
| 2001‐2002 | 252 (27) | 78 (26) |
| 2002‐2003 | 135 (14.5) | 31 (10) |
| 2003‐2004 | 243 (26) | 86 (28) |
| 2004‐2005 | 192 (21) | 78 (26) |
| Influenza type | ||
| A | 692 (75) | |
| B | 237 (25) | |
Overall, one‐third of patients (305/929; 33%) were eligible for treatment with oseltamivir. Among patients 1 year of age, approximately one‐half (305/587; 52%) were oseltamivir‐eligible. The additional 282 patients 1 year were ineligible because test results were returned to the clinician >48 hours after hospital admission. Only 49 (16.1%) of oseltamivir‐eligible patients were prescribed oseltamivir during hospitalization (Figure 1). The rate of prescription of oseltamivir increased over all seasons from 0% in 2000‐2001 to 20% in 2004‐2005. On‐label prescription rates increased from 0% in 2000‐2001 to 37.2% in 2004‐2005 (P < 0.0001; Figure 2).
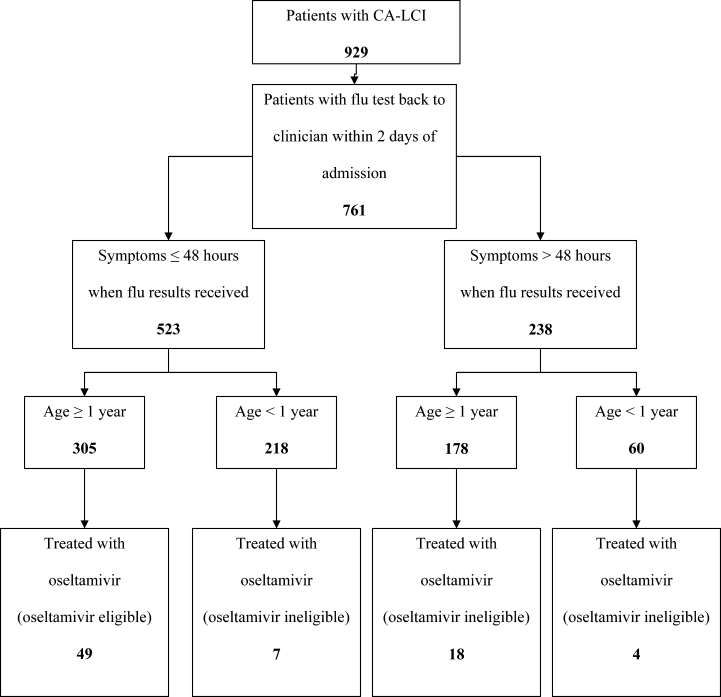
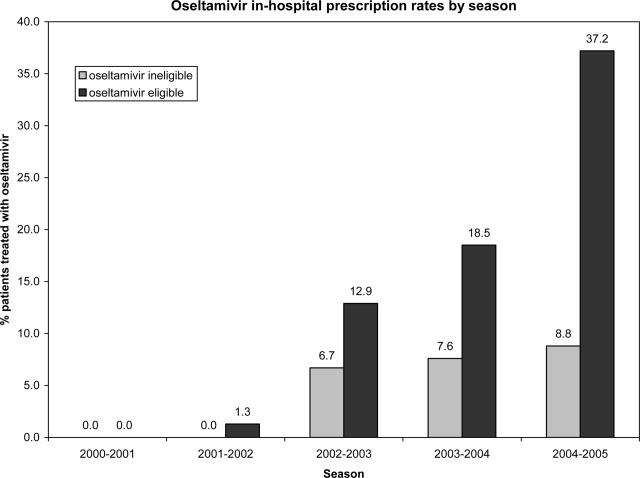
Off‐Label Oseltamivir Prescription
Oseltamivir was prescribed to 29 of the 624 patients who were determined to be oseltamivir‐ineligible. The rate of off‐label use increased over the seasons from 2000 to 2005 from 0% to 8.8% (P < 0.0001; Figure 1). Ineligible patients who received oseltamivir were 1 year of age (n = 11), had test results returned to the clinician 48 hours after hospital admission (n = 18), or both (n = 4). Most off‐label prescriptions occurred in patients who had chronic medical conditions (21/29; 72%), including cardiac disease (n = 9), asthma (n = 6), or prematurity (n = 5). Four of 11 patients 1 year of age who were treated with oseltamivir had influenza‐related respiratory failure. The oseltamivir dose for all patients 1 year of age was 2 mg/kg twice a day, all of whom survived to discharge.
Evaluation of a Computer‐Based Electronic Reminder Designed to Enhance the On‐Label Prescription of Oseltamivir
During season 6, an electronic reminder about the labeled use of oseltamivir was evaluated to determine its ability to increase the rate of prescription of oseltamivir among eligible children hospitalized with CA‐LCI. During season 6, most patients (226/311; 73%) were 1 year of age. A total of 84 patients were determined to be oseltamivir‐eligible (age 1 year and test results back to the clinician within 48 hours of symptom onset).
During the initial 10 weeks of local influenza activity, 20 oseltamivir‐eligible patients were admitted to our institution, and 8 received oseltamivir (40% prescription rate) (Table 2). In addition, 2 of 54 (3.7%) oseltamivir‐ineligible patients were also treated. The computer‐based electronic reminder was initiated in week 11 of the influenza season. After initiation of the reminder, 237 additional children with CA‐LCI were hospitalized, of whom 64 (27%) were determined to be oseltamivir‐eligible. The rate of on‐label prescription of oseltamivir was similar to that observed prior to initiation of the reminder: 16 of 64 patients eligible for antiviral therapy received oseltamivir (25% prescription rate) (Figure 3). An additional 8 patients were prescribed oseltamivir off‐label. The rate of oseltamivir prescription did not change significantly for either oseltamivir‐eligible (40‐25%) or oseltamivir‐ineligible (3.7‐4.6%) (Figure 4).
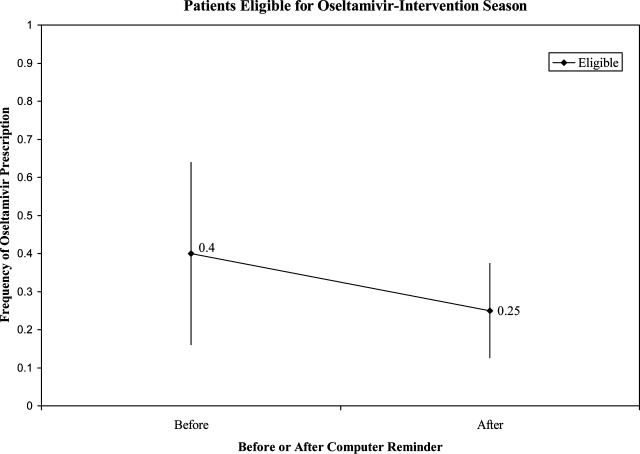
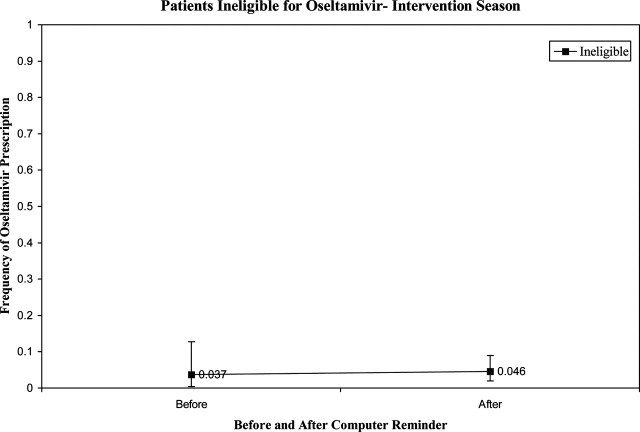
| Prompt Active? | Oseltamivir Use | Total | |
|---|---|---|---|
| Yes* | No | ||
| |||
| No | |||
| Eligible | 8 (40) | 12 | 20 |
| Ineligible | 2 (3.7) | 52 | 54 |
| Yes | |||
| Eligible | 16 (25) | 48 | 64 |
| Ineligible | 8 (4.6) | 165 | 173 |
| Total | 34 | 277 | 311 |
Dermatologic, Neurologic, and Neuropsychiatric Adverse Events
We reviewed the medical records of all patients treated with oseltamivir during the 6 study seasons to identify dermatologic, neurologic, and neuropsychiatric adverse outcomes that developed after the initiation of oseltamivir therapy. No new‐onset seizures, neuropsychiatric, or dermatologic reactions were identified among the children treated with oseltamivir.
DISCUSSION AND CONCLUSION
In this report, we describe the use of oseltamivir over 6 seasons in a cohort of children hospitalized with CA‐LCI at 1 tertiary care pediatric hospital and examine the impact of a mechanism designed to increase prescription among those eligible for oseltamivir. We found that only one‐third of patients hospitalized at our institution were eligible for oseltamivir treatment based on FDA‐approved indications. Of the eligible patients, few were prescribed oseltamivir during their hospitalization. During the sixth season, we employed a computer reminder system for oseltamivir prescription, which had no appreciable effect upon prescription rates. Despite the lack of effect of the electronic reminder system, we observed an increase of on‐label oseltamivir prescriptions over the entire study period. Finally, we identified 11 patients <1 year of age (3%) who were treated with oseltamivir. There were no adverse events identified in this group.
Although previous studies have addressed prescription rates of oseltamivir in children with influenza, few, if any, have looked at how these prescriptions correspond with FDA label criteria. In our cohort, only one‐third of hospitalized children were eligible for treatment with oseltamivir based upon their age and symptom duration at the time the results of rapid laboratory testing became available. Of those patients in our cohort eligible for oseltamivir, few were treated. The prescription of oseltamivir in seasons falls within the ranges found by Schrag et al.7 in their multistate review of pediatric influenza hospitalizations in 2003‐2004. They noted that use of antiviral medications varied by location of surveillance ranging from 3% in Connecticut to 34% in Colorado, indicating significant regional differences in prescription practices.7 Potential causes of low rates of appropriate use of oseltamivir include the observation that many physicians remain unaware of the potential severity of influenza infection in children.8 Additionally, physicians may differ on how to define the onset of influenza infection in children. A recent study published by Ohmit and Monto9 indicated that a fever and cough predicted 83% of children 5 to 12 years old who were determined to be influenza‐positive. Finally, many physicians who do not prescribe antiviral therapy may believe that their patients present too late for appropriate initiation of therapy.10
We identified 29 patients who received oseltamivir although they did not meet the FDA label criteria, of whom 72% had a chronic underlying condition. Moore et al.11 in their surveillance of influenza admissions in Canada found a similar trend. They described 26 of 29 (90%) hospitalized patients receiving antiinfluenza drugs had an underlying disease, and of those without a chronic condition, all had severe influenza‐related complications such as encephalopathy.11
Implementation of a computerized reminder to improve use of oseltamivir had no statistically significant effect on prescribing practice. Our sample size calculation was based on detecting a 40% difference in prescription rates, which limited our power to detect a smaller difference in prescription rates. A systematic review by Garg et al.12 identified barriers to the success of computer‐based decision support systems (CDS), which included failure of practitioners to use the system, poor integration of the system to the physician's workflow, and disagreement with what was recommended. Future enhancements to our inpatient electronic hospital record may allow for more targeted and robust CDS interventions.
We observed an increase in on‐label prescription rates of oseltamivir over the entire study period. We hypothesize that increased use of oseltamivir might be associated with growing concerns of pandemic influenza and attention to fatal influenza in children,13 as evidenced by the recent addition of influenza‐associated deaths in children to the list of nationally notifiable conditions in 2004.14
There has been considerable focus upon potential adverse events associated with treatment with oseltamivir in children. Reports have emerged, primarily from Japan, of neuropsychiatric and dermatologic adverse events of oseltamivir treatment.15 In the fall of 2006, the FDA added a precaution to the labeling of oseltamivir due to these neuropsychiatric events.16 In our treated cohort, no neurologic, neuropsychiatric, or dermatologic adverse events were identified. However, this finding is not surprising given the rarity of these adverse events and the limited number of children treated with oseltamivir in this study.
The strengths of this current study include a large cohort of laboratory‐confirmed influenza in hospitalized children over multiple influenza seasons. In addition, this is the first study of which we are aware that has assessed the number of children eligible for oseltamivir but not treated. The limitations of this study include misclassification bias related to the retrospective study design. Because of this design, onset of influenza symptoms was collected through chart review, and the time of receipt of influenza results from virology was based upon known laboratory turnover time, rather than actual knowledge of time of physician awareness of the result. To address this issue we used a conservative estimate of the time of receipt of influenza test results. In addition, the retrospective design prevented us from assessing the clinical decision‐making process, which led some patients to be treated with oseltamivir and others not. Our evaluation of the electronic reminder was designed to show a large change in prescription practices (ie, 40%), so it had insufficient power to detect a smaller impact. Finally, ascertainment bias may have limited our ability to identify adverse effects.
This study demonstrates that oseltamivir is prescribed infrequently among hospitalized children. Future studies are needed to determine whether appropriate use of oseltamivir improves outcomes among hospitalized children. Additional study of the safety and efficacy of oseltamivir in children aged <1 year is also needed given the large burden of disease in this age group.
Acknowledgements
We thank Michelle Precourt for her assistance with the computer‐based prompt. We also thank Drs. Anna Wheeler Rosenquist and Melissa Donovan for the original data collection for this project. This project was supported in part by the Centers for Disease Control and Prevention, grant H23/CCH32253‐02.
- ,,, et al.Influenza and the rates of hospitalization for respiratory disease among infants and young children.N Engl J Med.2000;342:232–239.
- ,,,,.The effect of influenza on hospitalizations, outpatient visits and courses of antibiotics in children.N Engl J Med.2000;342:225–231.
- Centers for Disease Control and Prevention (CDC). Diseases and Conditions. Seasonal Flu. CDC Health Alert: CDC recommends against the use of amantadine and rimantadine for the treatment or prophylaxis of influenza in the United States during the 2005–06 influenza season. January 14, 2006. Available at http://www.cdc.gov/flu/han011406.htm. Accessed November2008.
- ,,, et al.Oral oseltamivir treatment of influenza in children.Pediatr Infect Dis J.2001;20(2):127–133.
- ,,, et al.Off‐label drug use in hospitalized children.Arch Pediatr Adolesc Med.2007;161(3):282–290.
- ,,, et al.Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection.JAMA.2005;294:2188–2194.
- ,,, et al.Multistate surveillance for laboratory‐confirmed influenza‐associated hospitalizations in children 2003–2004.Pediatr Infect Dis J.2006;25(5):395–400.
- ,.Physician knowledge and perspectives regarding influenza and influenza vaccination.Hum Vaccin.2005;1(2):74–79.
- ,.Symptomatic predictors of influenza virus positivity in children during the influenza season.Clin Infect Dis.2006;43:564–568.
- ,,,,,.Effects of local variation, specialty, and beliefs on antiviral prescribing for influenza.Clin Infect Dis.2006;42:95–99.
- ,,, et al.Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004.Pediatrics.2006;118:e610–e619.
- ,,, et al.Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review.JAMA.2005;293:1223–1238.
- ,,, et al.Influenza‐associated deaths among children in the United States, 2003–2004.N Engl J Med.2005;353(24):2559–2567.
- Centers for Disease Control and Prevention (CDC). Diseases and Conditions. Seasonal Flu. Flu Activity 25(6):572.
- ,,.Post‐Marketing Adverse Event Reports. Review of Central Nervous System/Psychiatric Disorders Associated with the Use of Tamiflu, Drug: Oseltamivir Phosphate. Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Drug Evaluation and Research, Office of Surveillance and Epidemiology.2006. OSE PID #D060393 Oseltamivir—Neuropsychiatric Events.
Influenza is a common cause of acute respiratory illness in children, resulting in hospitalization of both healthy and chronically ill children due to influenza‐related complications.1, 2 Currently, amantadine, rimantadine, oseltamivir, and zanamivir are approved for use in children to treat influenza. In early 2006, more than 90% of influenza isolates tested in the US were found to be resistant to the adamantanes, suggesting that these medications might be of limited benefit during future influenza seasons.3 To date, most isolates of influenza remain susceptible to neuraminidase inhibitors, zanamivir and oseltamivir. Zanamivir has not been used extensively in pediatrics because it is delivered by aerosolization, and is only approved by the US Food and Drug Administration (FDA) for children 7 years of age. Oseltamivir is administered orally and is FDA‐approved for use in children 1 year of age within 48 hours of onset of symptoms of influenza virus infection.
Studies performed in outpatient settings have shown that oseltamivir can lessen the severity and reduce the length of influenza illness by 36 hours when therapy is initiated within 2 days of the onset of symptoms.4 Treatment also reduced the frequency of new diagnoses of otitis media and decreased physician‐prescribed antibiotics.4
To date, there are limited data evaluating the use of oseltamivir in either adult or pediatric patients hospitalized with influenza. We sought to describe the use of antiviral medications among children hospitalized with community‐acquired laboratory‐confirmed influenza (CA‐LCI) and to evaluate the effect of a computer‐based electronic reminder to increase the rate of on‐label use of oseltamivir among hospitalized children.
PATIENTS AND METHODS
We performed a retrospective cohort study of patients 21 years of age who were hospitalized with CA‐LCI during 5 consecutive seasons from July 2000 through June 2005 (seasons 1‐5) at the Children's Hospital of Philadelphia (CHOP). CHOP is a 418‐bed tertiary care hospital with about 24,000 hospital admissions each year. Viral diagnostic studies are performed routinely on children hospitalized with acute respiratory symptoms of unknown etiology, which aids in assigning patients to cohorts. Patients who had laboratory confirmation of influenza performed at an outside institution were excluded from this analysis.
From June 2005 through May 2006 (season 6), an observational trial of an electronic clinical decision reminder was performed to assess a mechanism to increase the proportion of eligible children treated with oseltamivir. Patients were included in this analysis if they were 21 years of age and had a diagnostic specimen for influenza obtained less than 72 hours after admission. The CHOP Institutional Review Board approved this study with a waiver of informed consent.
Viral Diagnostic Testing
During the winter months from seasons 1‐5, nasopharyngeal aspirate specimens were initially tested using immunochromatographic membrane assays (IA) for respiratory syncytial virus (RSV) (NOW RSV; Binax, Inc., Scarborough, ME) and, if negative, for influenza virus types A and B (NOW Flu A, NOW Flu B; Binax). If negative, specimens were tested by direct fluorescent antibody (DFA) testing for multiple respiratory viruses, including influenza A and B. During the winter season, IA testing was performed multiple times each day, and DFA was performed once or twice daily with an 8 to 24 hour turnaround time after a specimen was obtained. For season 6, the testing algorithm was revised: a panel of real‐time polymerase chain reaction (PCR) assays were performed to detect nucleic acids from multiple respiratory viruses, including influenza virus types A and B, on specimens that tested negative for influenza and RSV by IA. PCR testing was performed multiple times each day, and specimen results were available within 24 hours of specimen submission. Comprehensive viral tube cultures were performed on specimens that were negative by IA and DFA (seasons 1‐5) or respiratory virus PCR panel (season 6).
Study Definitions
Patients were considered to have CA‐LCI if the first diagnostic specimen positive for influenza was obtained less than 72 hours after hospital admission. Prescriptions for oseltamivir that were consistent with the FDA recommendations were considered to be on‐label prescriptions. Prescriptions for oseltamivir given to patients who did not meet these FDA criteria were considered off‐label prescriptions.5 Patients were considered oseltamivir‐eligible if they were met the criteria for FDA approval for treatment with oseltamivir: at least 1 year of age with influenza symptoms of less than 48 hours duration. Patients who either by age and/or symptom duration were inconsistent with FDA labeling criteria for oseltamivir were deemed oseltamivir‐ineligible. This included those patients for whom influenza test results were received by the clinician more than 48 hours after symptom onset. Patients who were positive for influenza only by viral culture were considered oseltamivir‐ineligible since the time needed to culture influenza virus was >48 hours. Because of the abrupt onset of influenza symptoms, the duration of influenza symptoms was defined by chart review of the emergency room or admission note. A hierarchy of symptoms was used to define the initial onset of influenza‐related symptoms and include the following: (1) For all patients with a history of fever, onset of influenza was defined as the onset of fever as recorded in the first physician note. (2) For patients without a history of fever, the onset of respiratory symptoms was recorded as the onset of influenza. (3) For patients without a history of fever but in whom multiple respiratory symptoms were noted, the onset of symptoms was assigned as the beginning of the increased work of breathing.
Because influenza IA were performed at least 4 times a day during the influenza season, the date of result to clinician was determined to be the same date as specimen collection for patients who had a positive influenza IA. Patients were identified as having a positive influenza result to the clinician 1 day after specimen collection if the test was positive by DFA or PCR. A neurologic adverse event was defined as the occurrence of a seizure after initiation of oseltamivir therapy. A neuropsychiatric adverse event was defined as any significant new neuropsychiatric symptom (psychosis, encephalopathy) recorded after the initiation of oseltamivir therapy. We defined a dermatologic adverse event as the report of any skin findings recorded after the initiation of oseltamivir therapy.
Chronic medical conditions
Information from detailed chart review was used to identify children with Advisory Committee on Immunization Practices (ACIP) high‐risk medical conditions as previously described by our group (asthma, chronic pulmonary disease, cardiac disease, immunosuppression, hemoglobinopathies, chronic renal dysfunction, diabetes mellitus, inborn errors of metabolism, long‐term salicylate therapy, pregnancy, and neurological and neuromuscular disease [NNMD]).6
Electronic Reminder
During season 6, a computer‐based electronic reminder was designed. The reminder stated Consider OSELTAMIVIR if Age >1 year AND symptoms <48 hours. May shorten illness by 36 hours. Page ID approval for more info. The reminder was embedded within the influenza results for all positive determinations, so a clinician would see the reminder when viewing positive laboratory results (Meditech, Westwood, MA).
At the initiation of season 6, we determined prescription rates of oseltamivir in patients with CA‐LCI to measure the baseline rate of oseltamivir prescription. The electronic reminder was initiated during week 11 of influenza activity at our institution and continued through the end of the influenza season.
Data Collection
Two sources of antiviral prescription data were used. Inpatient prescription of antiviral medications was extracted from billing records and chart review; a 10% audit of the medication administration records showed that the billing records correctly identified oseltamivir prescription status in all cases reviewed. Patients with incomplete pharmacy data were removed from the analysis of prescription practices (n = 8). During all seasons studied, the infectious diseases pharmacist (T.A.M.) and an infectious diseases physician (T.E.Z.) reviewed requests for inpatient prescriptions for antiviral medications.
For season 6, daily review of infection control records was performed to conduct surveillance for children hospitalized with CA‐LCI. To determine symptom duration and use of antiviral medications, inpatient medical charts were reviewed at the time of initial identification and then daily thereafter.
Statistical Analysis
Dichotomous variables were created for prescription of oseltamivir, age 1 year and symptom duration of <48 hours at time of clinician receipt of influenza results. Descriptive analyses included calculating the frequencies for categorical variables. Categorical variables were compared using Fisher's exact test. The Cochrane‐Armitage test was employed to test for a trend in the prescription of oseltamivir by season. A 2‐tailed P value of <0.05 was considered significant for all statistical tests. All statistical calculations were performed using standard programs in SAS 9.1 (SAS Institute, Cary, NC), STATA 8.2 (Stata Corp., College Station, TX), and Excel (Microsoft, Redmond, WA).
Prior to the start of season 6, we determined that if the rate of oseltamivir prescription was 40% before initiation of the reminder, we would need 20 eligible patients to detect a difference of 40% or greater in subsequent prescription rates (with 80% power and an alpha of 0.05). Once this enrollment goal was met, an electronic reminder of the eligibility for oseltamivir was initiated.
RESULTS
Use of Antiviral Medications in Children Hospitalized with Influenza, 2000‐2005
From July 2000 to June 2005, 1,058 patients were admitted with laboratory confirmed influenza; 8 were excluded because confirmatory testing was done at an outside institution, 24 were repeat hospitalizations, 89 nosocomial cases, and 8 cases were in patients >21 years. Thus, 929 patients had CA‐LCI and were eligible for inclusion in this study. Most children were infected with influenza A and were 1 year of age (Table 1). During this study period, only 9.3% of study subjects were treated with antiviral medications, most of whom (91%) received oseltamivir. Eight patients received amantadine over all seasons studied.
| Characteristics | Patients Hospitalized with CA‐LCI (n = 929)* | Eligible to Receive Oseltamivir (n = 305)* |
|---|---|---|
| ||
| Age (years) | ||
| <1 | 342 (37) | 0 |
| 1 | 587 (63) | 305 (100) |
| Season | ||
| 2000‐2001 | 107 (11.5) | 32 (10) |
| 2001‐2002 | 252 (27) | 78 (26) |
| 2002‐2003 | 135 (14.5) | 31 (10) |
| 2003‐2004 | 243 (26) | 86 (28) |
| 2004‐2005 | 192 (21) | 78 (26) |
| Influenza type | ||
| A | 692 (75) | |
| B | 237 (25) | |
Overall, one‐third of patients (305/929; 33%) were eligible for treatment with oseltamivir. Among patients 1 year of age, approximately one‐half (305/587; 52%) were oseltamivir‐eligible. The additional 282 patients 1 year were ineligible because test results were returned to the clinician >48 hours after hospital admission. Only 49 (16.1%) of oseltamivir‐eligible patients were prescribed oseltamivir during hospitalization (Figure 1). The rate of prescription of oseltamivir increased over all seasons from 0% in 2000‐2001 to 20% in 2004‐2005. On‐label prescription rates increased from 0% in 2000‐2001 to 37.2% in 2004‐2005 (P < 0.0001; Figure 2).


Off‐Label Oseltamivir Prescription
Oseltamivir was prescribed to 29 of the 624 patients who were determined to be oseltamivir‐ineligible. The rate of off‐label use increased over the seasons from 2000 to 2005 from 0% to 8.8% (P < 0.0001; Figure 1). Ineligible patients who received oseltamivir were 1 year of age (n = 11), had test results returned to the clinician 48 hours after hospital admission (n = 18), or both (n = 4). Most off‐label prescriptions occurred in patients who had chronic medical conditions (21/29; 72%), including cardiac disease (n = 9), asthma (n = 6), or prematurity (n = 5). Four of 11 patients 1 year of age who were treated with oseltamivir had influenza‐related respiratory failure. The oseltamivir dose for all patients 1 year of age was 2 mg/kg twice a day, all of whom survived to discharge.
Evaluation of a Computer‐Based Electronic Reminder Designed to Enhance the On‐Label Prescription of Oseltamivir
During season 6, an electronic reminder about the labeled use of oseltamivir was evaluated to determine its ability to increase the rate of prescription of oseltamivir among eligible children hospitalized with CA‐LCI. During season 6, most patients (226/311; 73%) were 1 year of age. A total of 84 patients were determined to be oseltamivir‐eligible (age 1 year and test results back to the clinician within 48 hours of symptom onset).
During the initial 10 weeks of local influenza activity, 20 oseltamivir‐eligible patients were admitted to our institution, and 8 received oseltamivir (40% prescription rate) (Table 2). In addition, 2 of 54 (3.7%) oseltamivir‐ineligible patients were also treated. The computer‐based electronic reminder was initiated in week 11 of the influenza season. After initiation of the reminder, 237 additional children with CA‐LCI were hospitalized, of whom 64 (27%) were determined to be oseltamivir‐eligible. The rate of on‐label prescription of oseltamivir was similar to that observed prior to initiation of the reminder: 16 of 64 patients eligible for antiviral therapy received oseltamivir (25% prescription rate) (Figure 3). An additional 8 patients were prescribed oseltamivir off‐label. The rate of oseltamivir prescription did not change significantly for either oseltamivir‐eligible (40‐25%) or oseltamivir‐ineligible (3.7‐4.6%) (Figure 4).


| Prompt Active? | Oseltamivir Use | Total | |
|---|---|---|---|
| Yes* | No | ||
| |||
| No | |||
| Eligible | 8 (40) | 12 | 20 |
| Ineligible | 2 (3.7) | 52 | 54 |
| Yes | |||
| Eligible | 16 (25) | 48 | 64 |
| Ineligible | 8 (4.6) | 165 | 173 |
| Total | 34 | 277 | 311 |
Dermatologic, Neurologic, and Neuropsychiatric Adverse Events
We reviewed the medical records of all patients treated with oseltamivir during the 6 study seasons to identify dermatologic, neurologic, and neuropsychiatric adverse outcomes that developed after the initiation of oseltamivir therapy. No new‐onset seizures, neuropsychiatric, or dermatologic reactions were identified among the children treated with oseltamivir.
DISCUSSION AND CONCLUSION
In this report, we describe the use of oseltamivir over 6 seasons in a cohort of children hospitalized with CA‐LCI at 1 tertiary care pediatric hospital and examine the impact of a mechanism designed to increase prescription among those eligible for oseltamivir. We found that only one‐third of patients hospitalized at our institution were eligible for oseltamivir treatment based on FDA‐approved indications. Of the eligible patients, few were prescribed oseltamivir during their hospitalization. During the sixth season, we employed a computer reminder system for oseltamivir prescription, which had no appreciable effect upon prescription rates. Despite the lack of effect of the electronic reminder system, we observed an increase of on‐label oseltamivir prescriptions over the entire study period. Finally, we identified 11 patients <1 year of age (3%) who were treated with oseltamivir. There were no adverse events identified in this group.
Although previous studies have addressed prescription rates of oseltamivir in children with influenza, few, if any, have looked at how these prescriptions correspond with FDA label criteria. In our cohort, only one‐third of hospitalized children were eligible for treatment with oseltamivir based upon their age and symptom duration at the time the results of rapid laboratory testing became available. Of those patients in our cohort eligible for oseltamivir, few were treated. The prescription of oseltamivir in seasons falls within the ranges found by Schrag et al.7 in their multistate review of pediatric influenza hospitalizations in 2003‐2004. They noted that use of antiviral medications varied by location of surveillance ranging from 3% in Connecticut to 34% in Colorado, indicating significant regional differences in prescription practices.7 Potential causes of low rates of appropriate use of oseltamivir include the observation that many physicians remain unaware of the potential severity of influenza infection in children.8 Additionally, physicians may differ on how to define the onset of influenza infection in children. A recent study published by Ohmit and Monto9 indicated that a fever and cough predicted 83% of children 5 to 12 years old who were determined to be influenza‐positive. Finally, many physicians who do not prescribe antiviral therapy may believe that their patients present too late for appropriate initiation of therapy.10
We identified 29 patients who received oseltamivir although they did not meet the FDA label criteria, of whom 72% had a chronic underlying condition. Moore et al.11 in their surveillance of influenza admissions in Canada found a similar trend. They described 26 of 29 (90%) hospitalized patients receiving antiinfluenza drugs had an underlying disease, and of those without a chronic condition, all had severe influenza‐related complications such as encephalopathy.11
Implementation of a computerized reminder to improve use of oseltamivir had no statistically significant effect on prescribing practice. Our sample size calculation was based on detecting a 40% difference in prescription rates, which limited our power to detect a smaller difference in prescription rates. A systematic review by Garg et al.12 identified barriers to the success of computer‐based decision support systems (CDS), which included failure of practitioners to use the system, poor integration of the system to the physician's workflow, and disagreement with what was recommended. Future enhancements to our inpatient electronic hospital record may allow for more targeted and robust CDS interventions.
We observed an increase in on‐label prescription rates of oseltamivir over the entire study period. We hypothesize that increased use of oseltamivir might be associated with growing concerns of pandemic influenza and attention to fatal influenza in children,13 as evidenced by the recent addition of influenza‐associated deaths in children to the list of nationally notifiable conditions in 2004.14
There has been considerable focus upon potential adverse events associated with treatment with oseltamivir in children. Reports have emerged, primarily from Japan, of neuropsychiatric and dermatologic adverse events of oseltamivir treatment.15 In the fall of 2006, the FDA added a precaution to the labeling of oseltamivir due to these neuropsychiatric events.16 In our treated cohort, no neurologic, neuropsychiatric, or dermatologic adverse events were identified. However, this finding is not surprising given the rarity of these adverse events and the limited number of children treated with oseltamivir in this study.
The strengths of this current study include a large cohort of laboratory‐confirmed influenza in hospitalized children over multiple influenza seasons. In addition, this is the first study of which we are aware that has assessed the number of children eligible for oseltamivir but not treated. The limitations of this study include misclassification bias related to the retrospective study design. Because of this design, onset of influenza symptoms was collected through chart review, and the time of receipt of influenza results from virology was based upon known laboratory turnover time, rather than actual knowledge of time of physician awareness of the result. To address this issue we used a conservative estimate of the time of receipt of influenza test results. In addition, the retrospective design prevented us from assessing the clinical decision‐making process, which led some patients to be treated with oseltamivir and others not. Our evaluation of the electronic reminder was designed to show a large change in prescription practices (ie, 40%), so it had insufficient power to detect a smaller impact. Finally, ascertainment bias may have limited our ability to identify adverse effects.
This study demonstrates that oseltamivir is prescribed infrequently among hospitalized children. Future studies are needed to determine whether appropriate use of oseltamivir improves outcomes among hospitalized children. Additional study of the safety and efficacy of oseltamivir in children aged <1 year is also needed given the large burden of disease in this age group.
Acknowledgements
We thank Michelle Precourt for her assistance with the computer‐based prompt. We also thank Drs. Anna Wheeler Rosenquist and Melissa Donovan for the original data collection for this project. This project was supported in part by the Centers for Disease Control and Prevention, grant H23/CCH32253‐02.
Influenza is a common cause of acute respiratory illness in children, resulting in hospitalization of both healthy and chronically ill children due to influenza‐related complications.1, 2 Currently, amantadine, rimantadine, oseltamivir, and zanamivir are approved for use in children to treat influenza. In early 2006, more than 90% of influenza isolates tested in the US were found to be resistant to the adamantanes, suggesting that these medications might be of limited benefit during future influenza seasons.3 To date, most isolates of influenza remain susceptible to neuraminidase inhibitors, zanamivir and oseltamivir. Zanamivir has not been used extensively in pediatrics because it is delivered by aerosolization, and is only approved by the US Food and Drug Administration (FDA) for children 7 years of age. Oseltamivir is administered orally and is FDA‐approved for use in children 1 year of age within 48 hours of onset of symptoms of influenza virus infection.
Studies performed in outpatient settings have shown that oseltamivir can lessen the severity and reduce the length of influenza illness by 36 hours when therapy is initiated within 2 days of the onset of symptoms.4 Treatment also reduced the frequency of new diagnoses of otitis media and decreased physician‐prescribed antibiotics.4
To date, there are limited data evaluating the use of oseltamivir in either adult or pediatric patients hospitalized with influenza. We sought to describe the use of antiviral medications among children hospitalized with community‐acquired laboratory‐confirmed influenza (CA‐LCI) and to evaluate the effect of a computer‐based electronic reminder to increase the rate of on‐label use of oseltamivir among hospitalized children.
PATIENTS AND METHODS
We performed a retrospective cohort study of patients 21 years of age who were hospitalized with CA‐LCI during 5 consecutive seasons from July 2000 through June 2005 (seasons 1‐5) at the Children's Hospital of Philadelphia (CHOP). CHOP is a 418‐bed tertiary care hospital with about 24,000 hospital admissions each year. Viral diagnostic studies are performed routinely on children hospitalized with acute respiratory symptoms of unknown etiology, which aids in assigning patients to cohorts. Patients who had laboratory confirmation of influenza performed at an outside institution were excluded from this analysis.
From June 2005 through May 2006 (season 6), an observational trial of an electronic clinical decision reminder was performed to assess a mechanism to increase the proportion of eligible children treated with oseltamivir. Patients were included in this analysis if they were 21 years of age and had a diagnostic specimen for influenza obtained less than 72 hours after admission. The CHOP Institutional Review Board approved this study with a waiver of informed consent.
Viral Diagnostic Testing
During the winter months from seasons 1‐5, nasopharyngeal aspirate specimens were initially tested using immunochromatographic membrane assays (IA) for respiratory syncytial virus (RSV) (NOW RSV; Binax, Inc., Scarborough, ME) and, if negative, for influenza virus types A and B (NOW Flu A, NOW Flu B; Binax). If negative, specimens were tested by direct fluorescent antibody (DFA) testing for multiple respiratory viruses, including influenza A and B. During the winter season, IA testing was performed multiple times each day, and DFA was performed once or twice daily with an 8 to 24 hour turnaround time after a specimen was obtained. For season 6, the testing algorithm was revised: a panel of real‐time polymerase chain reaction (PCR) assays were performed to detect nucleic acids from multiple respiratory viruses, including influenza virus types A and B, on specimens that tested negative for influenza and RSV by IA. PCR testing was performed multiple times each day, and specimen results were available within 24 hours of specimen submission. Comprehensive viral tube cultures were performed on specimens that were negative by IA and DFA (seasons 1‐5) or respiratory virus PCR panel (season 6).
Study Definitions
Patients were considered to have CA‐LCI if the first diagnostic specimen positive for influenza was obtained less than 72 hours after hospital admission. Prescriptions for oseltamivir that were consistent with the FDA recommendations were considered to be on‐label prescriptions. Prescriptions for oseltamivir given to patients who did not meet these FDA criteria were considered off‐label prescriptions.5 Patients were considered oseltamivir‐eligible if they were met the criteria for FDA approval for treatment with oseltamivir: at least 1 year of age with influenza symptoms of less than 48 hours duration. Patients who either by age and/or symptom duration were inconsistent with FDA labeling criteria for oseltamivir were deemed oseltamivir‐ineligible. This included those patients for whom influenza test results were received by the clinician more than 48 hours after symptom onset. Patients who were positive for influenza only by viral culture were considered oseltamivir‐ineligible since the time needed to culture influenza virus was >48 hours. Because of the abrupt onset of influenza symptoms, the duration of influenza symptoms was defined by chart review of the emergency room or admission note. A hierarchy of symptoms was used to define the initial onset of influenza‐related symptoms and include the following: (1) For all patients with a history of fever, onset of influenza was defined as the onset of fever as recorded in the first physician note. (2) For patients without a history of fever, the onset of respiratory symptoms was recorded as the onset of influenza. (3) For patients without a history of fever but in whom multiple respiratory symptoms were noted, the onset of symptoms was assigned as the beginning of the increased work of breathing.
Because influenza IA were performed at least 4 times a day during the influenza season, the date of result to clinician was determined to be the same date as specimen collection for patients who had a positive influenza IA. Patients were identified as having a positive influenza result to the clinician 1 day after specimen collection if the test was positive by DFA or PCR. A neurologic adverse event was defined as the occurrence of a seizure after initiation of oseltamivir therapy. A neuropsychiatric adverse event was defined as any significant new neuropsychiatric symptom (psychosis, encephalopathy) recorded after the initiation of oseltamivir therapy. We defined a dermatologic adverse event as the report of any skin findings recorded after the initiation of oseltamivir therapy.
Chronic medical conditions
Information from detailed chart review was used to identify children with Advisory Committee on Immunization Practices (ACIP) high‐risk medical conditions as previously described by our group (asthma, chronic pulmonary disease, cardiac disease, immunosuppression, hemoglobinopathies, chronic renal dysfunction, diabetes mellitus, inborn errors of metabolism, long‐term salicylate therapy, pregnancy, and neurological and neuromuscular disease [NNMD]).6
Electronic Reminder
During season 6, a computer‐based electronic reminder was designed. The reminder stated Consider OSELTAMIVIR if Age >1 year AND symptoms <48 hours. May shorten illness by 36 hours. Page ID approval for more info. The reminder was embedded within the influenza results for all positive determinations, so a clinician would see the reminder when viewing positive laboratory results (Meditech, Westwood, MA).
At the initiation of season 6, we determined prescription rates of oseltamivir in patients with CA‐LCI to measure the baseline rate of oseltamivir prescription. The electronic reminder was initiated during week 11 of influenza activity at our institution and continued through the end of the influenza season.
Data Collection
Two sources of antiviral prescription data were used. Inpatient prescription of antiviral medications was extracted from billing records and chart review; a 10% audit of the medication administration records showed that the billing records correctly identified oseltamivir prescription status in all cases reviewed. Patients with incomplete pharmacy data were removed from the analysis of prescription practices (n = 8). During all seasons studied, the infectious diseases pharmacist (T.A.M.) and an infectious diseases physician (T.E.Z.) reviewed requests for inpatient prescriptions for antiviral medications.
For season 6, daily review of infection control records was performed to conduct surveillance for children hospitalized with CA‐LCI. To determine symptom duration and use of antiviral medications, inpatient medical charts were reviewed at the time of initial identification and then daily thereafter.
Statistical Analysis
Dichotomous variables were created for prescription of oseltamivir, age 1 year and symptom duration of <48 hours at time of clinician receipt of influenza results. Descriptive analyses included calculating the frequencies for categorical variables. Categorical variables were compared using Fisher's exact test. The Cochrane‐Armitage test was employed to test for a trend in the prescription of oseltamivir by season. A 2‐tailed P value of <0.05 was considered significant for all statistical tests. All statistical calculations were performed using standard programs in SAS 9.1 (SAS Institute, Cary, NC), STATA 8.2 (Stata Corp., College Station, TX), and Excel (Microsoft, Redmond, WA).
Prior to the start of season 6, we determined that if the rate of oseltamivir prescription was 40% before initiation of the reminder, we would need 20 eligible patients to detect a difference of 40% or greater in subsequent prescription rates (with 80% power and an alpha of 0.05). Once this enrollment goal was met, an electronic reminder of the eligibility for oseltamivir was initiated.
RESULTS
Use of Antiviral Medications in Children Hospitalized with Influenza, 2000‐2005
From July 2000 to June 2005, 1,058 patients were admitted with laboratory confirmed influenza; 8 were excluded because confirmatory testing was done at an outside institution, 24 were repeat hospitalizations, 89 nosocomial cases, and 8 cases were in patients >21 years. Thus, 929 patients had CA‐LCI and were eligible for inclusion in this study. Most children were infected with influenza A and were 1 year of age (Table 1). During this study period, only 9.3% of study subjects were treated with antiviral medications, most of whom (91%) received oseltamivir. Eight patients received amantadine over all seasons studied.
| Characteristics | Patients Hospitalized with CA‐LCI (n = 929)* | Eligible to Receive Oseltamivir (n = 305)* |
|---|---|---|
| ||
| Age (years) | ||
| <1 | 342 (37) | 0 |
| 1 | 587 (63) | 305 (100) |
| Season | ||
| 2000‐2001 | 107 (11.5) | 32 (10) |
| 2001‐2002 | 252 (27) | 78 (26) |
| 2002‐2003 | 135 (14.5) | 31 (10) |
| 2003‐2004 | 243 (26) | 86 (28) |
| 2004‐2005 | 192 (21) | 78 (26) |
| Influenza type | ||
| A | 692 (75) | |
| B | 237 (25) | |
Overall, one‐third of patients (305/929; 33%) were eligible for treatment with oseltamivir. Among patients 1 year of age, approximately one‐half (305/587; 52%) were oseltamivir‐eligible. The additional 282 patients 1 year were ineligible because test results were returned to the clinician >48 hours after hospital admission. Only 49 (16.1%) of oseltamivir‐eligible patients were prescribed oseltamivir during hospitalization (Figure 1). The rate of prescription of oseltamivir increased over all seasons from 0% in 2000‐2001 to 20% in 2004‐2005. On‐label prescription rates increased from 0% in 2000‐2001 to 37.2% in 2004‐2005 (P < 0.0001; Figure 2).


Off‐Label Oseltamivir Prescription
Oseltamivir was prescribed to 29 of the 624 patients who were determined to be oseltamivir‐ineligible. The rate of off‐label use increased over the seasons from 2000 to 2005 from 0% to 8.8% (P < 0.0001; Figure 1). Ineligible patients who received oseltamivir were 1 year of age (n = 11), had test results returned to the clinician 48 hours after hospital admission (n = 18), or both (n = 4). Most off‐label prescriptions occurred in patients who had chronic medical conditions (21/29; 72%), including cardiac disease (n = 9), asthma (n = 6), or prematurity (n = 5). Four of 11 patients 1 year of age who were treated with oseltamivir had influenza‐related respiratory failure. The oseltamivir dose for all patients 1 year of age was 2 mg/kg twice a day, all of whom survived to discharge.
Evaluation of a Computer‐Based Electronic Reminder Designed to Enhance the On‐Label Prescription of Oseltamivir
During season 6, an electronic reminder about the labeled use of oseltamivir was evaluated to determine its ability to increase the rate of prescription of oseltamivir among eligible children hospitalized with CA‐LCI. During season 6, most patients (226/311; 73%) were 1 year of age. A total of 84 patients were determined to be oseltamivir‐eligible (age 1 year and test results back to the clinician within 48 hours of symptom onset).
During the initial 10 weeks of local influenza activity, 20 oseltamivir‐eligible patients were admitted to our institution, and 8 received oseltamivir (40% prescription rate) (Table 2). In addition, 2 of 54 (3.7%) oseltamivir‐ineligible patients were also treated. The computer‐based electronic reminder was initiated in week 11 of the influenza season. After initiation of the reminder, 237 additional children with CA‐LCI were hospitalized, of whom 64 (27%) were determined to be oseltamivir‐eligible. The rate of on‐label prescription of oseltamivir was similar to that observed prior to initiation of the reminder: 16 of 64 patients eligible for antiviral therapy received oseltamivir (25% prescription rate) (Figure 3). An additional 8 patients were prescribed oseltamivir off‐label. The rate of oseltamivir prescription did not change significantly for either oseltamivir‐eligible (40‐25%) or oseltamivir‐ineligible (3.7‐4.6%) (Figure 4).


| Prompt Active? | Oseltamivir Use | Total | |
|---|---|---|---|
| Yes* | No | ||
| |||
| No | |||
| Eligible | 8 (40) | 12 | 20 |
| Ineligible | 2 (3.7) | 52 | 54 |
| Yes | |||
| Eligible | 16 (25) | 48 | 64 |
| Ineligible | 8 (4.6) | 165 | 173 |
| Total | 34 | 277 | 311 |
Dermatologic, Neurologic, and Neuropsychiatric Adverse Events
We reviewed the medical records of all patients treated with oseltamivir during the 6 study seasons to identify dermatologic, neurologic, and neuropsychiatric adverse outcomes that developed after the initiation of oseltamivir therapy. No new‐onset seizures, neuropsychiatric, or dermatologic reactions were identified among the children treated with oseltamivir.
DISCUSSION AND CONCLUSION
In this report, we describe the use of oseltamivir over 6 seasons in a cohort of children hospitalized with CA‐LCI at 1 tertiary care pediatric hospital and examine the impact of a mechanism designed to increase prescription among those eligible for oseltamivir. We found that only one‐third of patients hospitalized at our institution were eligible for oseltamivir treatment based on FDA‐approved indications. Of the eligible patients, few were prescribed oseltamivir during their hospitalization. During the sixth season, we employed a computer reminder system for oseltamivir prescription, which had no appreciable effect upon prescription rates. Despite the lack of effect of the electronic reminder system, we observed an increase of on‐label oseltamivir prescriptions over the entire study period. Finally, we identified 11 patients <1 year of age (3%) who were treated with oseltamivir. There were no adverse events identified in this group.
Although previous studies have addressed prescription rates of oseltamivir in children with influenza, few, if any, have looked at how these prescriptions correspond with FDA label criteria. In our cohort, only one‐third of hospitalized children were eligible for treatment with oseltamivir based upon their age and symptom duration at the time the results of rapid laboratory testing became available. Of those patients in our cohort eligible for oseltamivir, few were treated. The prescription of oseltamivir in seasons falls within the ranges found by Schrag et al.7 in their multistate review of pediatric influenza hospitalizations in 2003‐2004. They noted that use of antiviral medications varied by location of surveillance ranging from 3% in Connecticut to 34% in Colorado, indicating significant regional differences in prescription practices.7 Potential causes of low rates of appropriate use of oseltamivir include the observation that many physicians remain unaware of the potential severity of influenza infection in children.8 Additionally, physicians may differ on how to define the onset of influenza infection in children. A recent study published by Ohmit and Monto9 indicated that a fever and cough predicted 83% of children 5 to 12 years old who were determined to be influenza‐positive. Finally, many physicians who do not prescribe antiviral therapy may believe that their patients present too late for appropriate initiation of therapy.10
We identified 29 patients who received oseltamivir although they did not meet the FDA label criteria, of whom 72% had a chronic underlying condition. Moore et al.11 in their surveillance of influenza admissions in Canada found a similar trend. They described 26 of 29 (90%) hospitalized patients receiving antiinfluenza drugs had an underlying disease, and of those without a chronic condition, all had severe influenza‐related complications such as encephalopathy.11
Implementation of a computerized reminder to improve use of oseltamivir had no statistically significant effect on prescribing practice. Our sample size calculation was based on detecting a 40% difference in prescription rates, which limited our power to detect a smaller difference in prescription rates. A systematic review by Garg et al.12 identified barriers to the success of computer‐based decision support systems (CDS), which included failure of practitioners to use the system, poor integration of the system to the physician's workflow, and disagreement with what was recommended. Future enhancements to our inpatient electronic hospital record may allow for more targeted and robust CDS interventions.
We observed an increase in on‐label prescription rates of oseltamivir over the entire study period. We hypothesize that increased use of oseltamivir might be associated with growing concerns of pandemic influenza and attention to fatal influenza in children,13 as evidenced by the recent addition of influenza‐associated deaths in children to the list of nationally notifiable conditions in 2004.14
There has been considerable focus upon potential adverse events associated with treatment with oseltamivir in children. Reports have emerged, primarily from Japan, of neuropsychiatric and dermatologic adverse events of oseltamivir treatment.15 In the fall of 2006, the FDA added a precaution to the labeling of oseltamivir due to these neuropsychiatric events.16 In our treated cohort, no neurologic, neuropsychiatric, or dermatologic adverse events were identified. However, this finding is not surprising given the rarity of these adverse events and the limited number of children treated with oseltamivir in this study.
The strengths of this current study include a large cohort of laboratory‐confirmed influenza in hospitalized children over multiple influenza seasons. In addition, this is the first study of which we are aware that has assessed the number of children eligible for oseltamivir but not treated. The limitations of this study include misclassification bias related to the retrospective study design. Because of this design, onset of influenza symptoms was collected through chart review, and the time of receipt of influenza results from virology was based upon known laboratory turnover time, rather than actual knowledge of time of physician awareness of the result. To address this issue we used a conservative estimate of the time of receipt of influenza test results. In addition, the retrospective design prevented us from assessing the clinical decision‐making process, which led some patients to be treated with oseltamivir and others not. Our evaluation of the electronic reminder was designed to show a large change in prescription practices (ie, 40%), so it had insufficient power to detect a smaller impact. Finally, ascertainment bias may have limited our ability to identify adverse effects.
This study demonstrates that oseltamivir is prescribed infrequently among hospitalized children. Future studies are needed to determine whether appropriate use of oseltamivir improves outcomes among hospitalized children. Additional study of the safety and efficacy of oseltamivir in children aged <1 year is also needed given the large burden of disease in this age group.
Acknowledgements
We thank Michelle Precourt for her assistance with the computer‐based prompt. We also thank Drs. Anna Wheeler Rosenquist and Melissa Donovan for the original data collection for this project. This project was supported in part by the Centers for Disease Control and Prevention, grant H23/CCH32253‐02.
- ,,, et al.Influenza and the rates of hospitalization for respiratory disease among infants and young children.N Engl J Med.2000;342:232–239.
- ,,,,.The effect of influenza on hospitalizations, outpatient visits and courses of antibiotics in children.N Engl J Med.2000;342:225–231.
- Centers for Disease Control and Prevention (CDC). Diseases and Conditions. Seasonal Flu. CDC Health Alert: CDC recommends against the use of amantadine and rimantadine for the treatment or prophylaxis of influenza in the United States during the 2005–06 influenza season. January 14, 2006. Available at http://www.cdc.gov/flu/han011406.htm. Accessed November2008.
- ,,, et al.Oral oseltamivir treatment of influenza in children.Pediatr Infect Dis J.2001;20(2):127–133.
- ,,, et al.Off‐label drug use in hospitalized children.Arch Pediatr Adolesc Med.2007;161(3):282–290.
- ,,, et al.Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection.JAMA.2005;294:2188–2194.
- ,,, et al.Multistate surveillance for laboratory‐confirmed influenza‐associated hospitalizations in children 2003–2004.Pediatr Infect Dis J.2006;25(5):395–400.
- ,.Physician knowledge and perspectives regarding influenza and influenza vaccination.Hum Vaccin.2005;1(2):74–79.
- ,.Symptomatic predictors of influenza virus positivity in children during the influenza season.Clin Infect Dis.2006;43:564–568.
- ,,,,,.Effects of local variation, specialty, and beliefs on antiviral prescribing for influenza.Clin Infect Dis.2006;42:95–99.
- ,,, et al.Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004.Pediatrics.2006;118:e610–e619.
- ,,, et al.Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review.JAMA.2005;293:1223–1238.
- ,,, et al.Influenza‐associated deaths among children in the United States, 2003–2004.N Engl J Med.2005;353(24):2559–2567.
- Centers for Disease Control and Prevention (CDC). Diseases and Conditions. Seasonal Flu. Flu Activity 25(6):572.
- ,,.Post‐Marketing Adverse Event Reports. Review of Central Nervous System/Psychiatric Disorders Associated with the Use of Tamiflu, Drug: Oseltamivir Phosphate. Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Drug Evaluation and Research, Office of Surveillance and Epidemiology.2006. OSE PID #D060393 Oseltamivir—Neuropsychiatric Events.
- ,,, et al.Influenza and the rates of hospitalization for respiratory disease among infants and young children.N Engl J Med.2000;342:232–239.
- ,,,,.The effect of influenza on hospitalizations, outpatient visits and courses of antibiotics in children.N Engl J Med.2000;342:225–231.
- Centers for Disease Control and Prevention (CDC). Diseases and Conditions. Seasonal Flu. CDC Health Alert: CDC recommends against the use of amantadine and rimantadine for the treatment or prophylaxis of influenza in the United States during the 2005–06 influenza season. January 14, 2006. Available at http://www.cdc.gov/flu/han011406.htm. Accessed November2008.
- ,,, et al.Oral oseltamivir treatment of influenza in children.Pediatr Infect Dis J.2001;20(2):127–133.
- ,,, et al.Off‐label drug use in hospitalized children.Arch Pediatr Adolesc Med.2007;161(3):282–290.
- ,,, et al.Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection.JAMA.2005;294:2188–2194.
- ,,, et al.Multistate surveillance for laboratory‐confirmed influenza‐associated hospitalizations in children 2003–2004.Pediatr Infect Dis J.2006;25(5):395–400.
- ,.Physician knowledge and perspectives regarding influenza and influenza vaccination.Hum Vaccin.2005;1(2):74–79.
- ,.Symptomatic predictors of influenza virus positivity in children during the influenza season.Clin Infect Dis.2006;43:564–568.
- ,,,,,.Effects of local variation, specialty, and beliefs on antiviral prescribing for influenza.Clin Infect Dis.2006;42:95–99.
- ,,, et al.Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004.Pediatrics.2006;118:e610–e619.
- ,,, et al.Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review.JAMA.2005;293:1223–1238.
- ,,, et al.Influenza‐associated deaths among children in the United States, 2003–2004.N Engl J Med.2005;353(24):2559–2567.
- Centers for Disease Control and Prevention (CDC). Diseases and Conditions. Seasonal Flu. Flu Activity 25(6):572.
- ,,.Post‐Marketing Adverse Event Reports. Review of Central Nervous System/Psychiatric Disorders Associated with the Use of Tamiflu, Drug: Oseltamivir Phosphate. Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Drug Evaluation and Research, Office of Surveillance and Epidemiology.2006. OSE PID #D060393 Oseltamivir—Neuropsychiatric Events.
Copyright © 2009 Society of Hospital Medicine