User login
Older RBCs may sometimes be better
New research suggests that, overall, the age of transfused red blood cells (RBCs) does not significantly impact outcomes in critically ill adults, but, in some cases, older RBCs may be the better choice.
The study showed no significant difference in 90-day mortality whether patients received RBCs stored for a mean of 11.8 days or 22.4 days.
Likewise, there were no significant differences in most other study endpoints.
However, febrile nonhemolytic transfusion reactions were more frequent in the short-term storage group.
And among the most severely ill patients, the transfusion of older RBCs was associated with fewer deaths at 90 days.
“Older blood appears to be like a good red wine—better with some age,” said study author D. James Cooper, MD, of Monash University and Alfred Hospital in Melbourne, Victoria, Australia.
“The findings of our trial confirm that the current duration of storage of red blood cells for transfusion is both safe and optimal.”
Dr Cooper and his colleagues reported their findings in NEJM.
The researchers conducted this trial from November 2012 through December 2016 at 59 centers in 5 countries—Australia, New Zealand, Ireland, Finland, and Saudi Arabia.
The study included nearly 5000 critically ill adults who were randomized to receive either the freshest available RBCs or the oldest available RBCs.
There were 2457 patients in the short-term storage group, where the mean RBC storage duration was 11.8 ± 5.3 days. And there were 2462 patients in the long-term storage group, where the mean RBC storage duration was 22.4 ± 7.5 days.
Baseline characteristics were largely similar between the 2 groups. However, patients were significantly older in the short-term storage group, with a mean age of 62.5 ± 16.8 years, compared to 61.4 ± 17.3 years in the long-term storage group (P=0.02).
The median time from randomization to first RBC transfusion was similar between the groups—1.6 hours in the short-term group and 1.5 hours in the long-term group. The mean number of RBC units was also similar—4.1 ± 6.0 and 4.0 ± 6.2, respectively. The use of other blood products was similar as well.
90-day mortality
The study’s primary endpoint was 90-day mortality, which was 24.8% (n=610) in the short-term storage group and 24.1% (n=594) in the long-term storage group. The unadjusted (u) odds ratio (OR) was 1.04 (P=0.57).
When the researchers adjusted for APACHE III risk of death, patient age, hemoglobin at randomization, blood group, and site, the adjusted (a) OR was 1.04 (P=0.59).
When the researchers looked at patient subgroups, they found a significant difference between the storage groups when it came to 90-day mortality according to APACHE III risk of death.
Patients with an APACHE III predicted risk of death at hospital discharge at a median of 21.5% or higher had a significantly higher rate of 90-day mortality if they received the freshest available RBCs rather than the oldest available RBCs—37.7% and 34.0%, respectively (uOR=1.18, P=0.05).
There were no significant differences in 90-day mortality in the other subgroups.
Secondary endpoints
There were no significant between-group differences in secondary endpoints, with the exception of febrile nonhemolytic transfusion reaction. The incidence of this outcome was 5.0% in the short-term storage group and 3.6% in the long-term group (uOR=1.42, P=0.01; aOR=1.45, P=0.01).
Other secondary endpoints included (data in the short-term and long-term groups, respectively):
- Death at day 28 (19.4% and 18.8%, uOR=1.04, P=0.61)
- Death at day 180 (28.5% and 28.1%, uOR=1.02, P=0.75)
- Persistent organ dysfunction or death at day 28 (23.3% and 22.3%, uOR=1.06, P=0.39)
- New bloodstream infection (1.4% and 1.6%, uOR=0.90, P=0.65)
- Duration of hospital stay (median 14.5 days and 14.7 days, P=0.42)
- Duration of stay in the intensive care unit (median 4.2 days for both, P=0.86)
- Invasive mechanical ventilation (58.6% and 59.3%, uOR=0.97, P=0.64)
- Renal-replacement therapy (13.9% and 14.6%, uOR=0.97, P=0.48).

New research suggests that, overall, the age of transfused red blood cells (RBCs) does not significantly impact outcomes in critically ill adults, but, in some cases, older RBCs may be the better choice.
The study showed no significant difference in 90-day mortality whether patients received RBCs stored for a mean of 11.8 days or 22.4 days.
Likewise, there were no significant differences in most other study endpoints.
However, febrile nonhemolytic transfusion reactions were more frequent in the short-term storage group.
And among the most severely ill patients, the transfusion of older RBCs was associated with fewer deaths at 90 days.
“Older blood appears to be like a good red wine—better with some age,” said study author D. James Cooper, MD, of Monash University and Alfred Hospital in Melbourne, Victoria, Australia.
“The findings of our trial confirm that the current duration of storage of red blood cells for transfusion is both safe and optimal.”
Dr Cooper and his colleagues reported their findings in NEJM.
The researchers conducted this trial from November 2012 through December 2016 at 59 centers in 5 countries—Australia, New Zealand, Ireland, Finland, and Saudi Arabia.
The study included nearly 5000 critically ill adults who were randomized to receive either the freshest available RBCs or the oldest available RBCs.
There were 2457 patients in the short-term storage group, where the mean RBC storage duration was 11.8 ± 5.3 days. And there were 2462 patients in the long-term storage group, where the mean RBC storage duration was 22.4 ± 7.5 days.
Baseline characteristics were largely similar between the 2 groups. However, patients were significantly older in the short-term storage group, with a mean age of 62.5 ± 16.8 years, compared to 61.4 ± 17.3 years in the long-term storage group (P=0.02).
The median time from randomization to first RBC transfusion was similar between the groups—1.6 hours in the short-term group and 1.5 hours in the long-term group. The mean number of RBC units was also similar—4.1 ± 6.0 and 4.0 ± 6.2, respectively. The use of other blood products was similar as well.
90-day mortality
The study’s primary endpoint was 90-day mortality, which was 24.8% (n=610) in the short-term storage group and 24.1% (n=594) in the long-term storage group. The unadjusted (u) odds ratio (OR) was 1.04 (P=0.57).
When the researchers adjusted for APACHE III risk of death, patient age, hemoglobin at randomization, blood group, and site, the adjusted (a) OR was 1.04 (P=0.59).
When the researchers looked at patient subgroups, they found a significant difference between the storage groups when it came to 90-day mortality according to APACHE III risk of death.
Patients with an APACHE III predicted risk of death at hospital discharge at a median of 21.5% or higher had a significantly higher rate of 90-day mortality if they received the freshest available RBCs rather than the oldest available RBCs—37.7% and 34.0%, respectively (uOR=1.18, P=0.05).
There were no significant differences in 90-day mortality in the other subgroups.
Secondary endpoints
There were no significant between-group differences in secondary endpoints, with the exception of febrile nonhemolytic transfusion reaction. The incidence of this outcome was 5.0% in the short-term storage group and 3.6% in the long-term group (uOR=1.42, P=0.01; aOR=1.45, P=0.01).
Other secondary endpoints included (data in the short-term and long-term groups, respectively):
- Death at day 28 (19.4% and 18.8%, uOR=1.04, P=0.61)
- Death at day 180 (28.5% and 28.1%, uOR=1.02, P=0.75)
- Persistent organ dysfunction or death at day 28 (23.3% and 22.3%, uOR=1.06, P=0.39)
- New bloodstream infection (1.4% and 1.6%, uOR=0.90, P=0.65)
- Duration of hospital stay (median 14.5 days and 14.7 days, P=0.42)
- Duration of stay in the intensive care unit (median 4.2 days for both, P=0.86)
- Invasive mechanical ventilation (58.6% and 59.3%, uOR=0.97, P=0.64)
- Renal-replacement therapy (13.9% and 14.6%, uOR=0.97, P=0.48).

New research suggests that, overall, the age of transfused red blood cells (RBCs) does not significantly impact outcomes in critically ill adults, but, in some cases, older RBCs may be the better choice.
The study showed no significant difference in 90-day mortality whether patients received RBCs stored for a mean of 11.8 days or 22.4 days.
Likewise, there were no significant differences in most other study endpoints.
However, febrile nonhemolytic transfusion reactions were more frequent in the short-term storage group.
And among the most severely ill patients, the transfusion of older RBCs was associated with fewer deaths at 90 days.
“Older blood appears to be like a good red wine—better with some age,” said study author D. James Cooper, MD, of Monash University and Alfred Hospital in Melbourne, Victoria, Australia.
“The findings of our trial confirm that the current duration of storage of red blood cells for transfusion is both safe and optimal.”
Dr Cooper and his colleagues reported their findings in NEJM.
The researchers conducted this trial from November 2012 through December 2016 at 59 centers in 5 countries—Australia, New Zealand, Ireland, Finland, and Saudi Arabia.
The study included nearly 5000 critically ill adults who were randomized to receive either the freshest available RBCs or the oldest available RBCs.
There were 2457 patients in the short-term storage group, where the mean RBC storage duration was 11.8 ± 5.3 days. And there were 2462 patients in the long-term storage group, where the mean RBC storage duration was 22.4 ± 7.5 days.
Baseline characteristics were largely similar between the 2 groups. However, patients were significantly older in the short-term storage group, with a mean age of 62.5 ± 16.8 years, compared to 61.4 ± 17.3 years in the long-term storage group (P=0.02).
The median time from randomization to first RBC transfusion was similar between the groups—1.6 hours in the short-term group and 1.5 hours in the long-term group. The mean number of RBC units was also similar—4.1 ± 6.0 and 4.0 ± 6.2, respectively. The use of other blood products was similar as well.
90-day mortality
The study’s primary endpoint was 90-day mortality, which was 24.8% (n=610) in the short-term storage group and 24.1% (n=594) in the long-term storage group. The unadjusted (u) odds ratio (OR) was 1.04 (P=0.57).
When the researchers adjusted for APACHE III risk of death, patient age, hemoglobin at randomization, blood group, and site, the adjusted (a) OR was 1.04 (P=0.59).
When the researchers looked at patient subgroups, they found a significant difference between the storage groups when it came to 90-day mortality according to APACHE III risk of death.
Patients with an APACHE III predicted risk of death at hospital discharge at a median of 21.5% or higher had a significantly higher rate of 90-day mortality if they received the freshest available RBCs rather than the oldest available RBCs—37.7% and 34.0%, respectively (uOR=1.18, P=0.05).
There were no significant differences in 90-day mortality in the other subgroups.
Secondary endpoints
There were no significant between-group differences in secondary endpoints, with the exception of febrile nonhemolytic transfusion reaction. The incidence of this outcome was 5.0% in the short-term storage group and 3.6% in the long-term group (uOR=1.42, P=0.01; aOR=1.45, P=0.01).
Other secondary endpoints included (data in the short-term and long-term groups, respectively):
- Death at day 28 (19.4% and 18.8%, uOR=1.04, P=0.61)
- Death at day 180 (28.5% and 28.1%, uOR=1.02, P=0.75)
- Persistent organ dysfunction or death at day 28 (23.3% and 22.3%, uOR=1.06, P=0.39)
- New bloodstream infection (1.4% and 1.6%, uOR=0.90, P=0.65)
- Duration of hospital stay (median 14.5 days and 14.7 days, P=0.42)
- Duration of stay in the intensive care unit (median 4.2 days for both, P=0.86)
- Invasive mechanical ventilation (58.6% and 59.3%, uOR=0.97, P=0.64)
- Renal-replacement therapy (13.9% and 14.6%, uOR=0.97, P=0.48).

Daratumumab combos approved to treat MM in Japan
The Ministry of Health, Labor and Welfare in Japan has approved the use of daratumumab (DARZALEX®) in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat adults with relapsed or refractory multiple myeloma (MM).
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
The drug is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab.
The approval of daratumumab is based on data from the phase 3 POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
The Ministry of Health, Labor and Welfare in Japan has approved the use of daratumumab (DARZALEX®) in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat adults with relapsed or refractory multiple myeloma (MM).
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
The drug is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab.
The approval of daratumumab is based on data from the phase 3 POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
The Ministry of Health, Labor and Welfare in Japan has approved the use of daratumumab (DARZALEX®) in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat adults with relapsed or refractory multiple myeloma (MM).
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
The drug is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab.
The approval of daratumumab is based on data from the phase 3 POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
FDA grants factor IX therapy orphan designation
The US Food and Drug Administration (FDA) has granted orphan drug designation to CB 2679d/ISU304, a clinical stage drug candidate for hemophilia B.
CB 2679d/ISU304 is a next-generation coagulation factor IX variant that may allow for subcutaneous prophylactic treatment of patients with hemophilia B.
The product is being developed by Catalyst Biosciences, Inc. and ISU Abxis.
The companies are currently conducting a phase 1/2 trial of CB 2679d/ISU304 in patients with severe hemophilia B.
Catalyst Biosciences and ISU Abxis plan to have interim, top-line results from this trial by the end of 2017 and complete results in early 2018.
CB 2679d/ISU304 also has orphan medicinal product designation from the European Commission.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to CB 2679d/ISU304, a clinical stage drug candidate for hemophilia B.
CB 2679d/ISU304 is a next-generation coagulation factor IX variant that may allow for subcutaneous prophylactic treatment of patients with hemophilia B.
The product is being developed by Catalyst Biosciences, Inc. and ISU Abxis.
The companies are currently conducting a phase 1/2 trial of CB 2679d/ISU304 in patients with severe hemophilia B.
Catalyst Biosciences and ISU Abxis plan to have interim, top-line results from this trial by the end of 2017 and complete results in early 2018.
CB 2679d/ISU304 also has orphan medicinal product designation from the European Commission.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to CB 2679d/ISU304, a clinical stage drug candidate for hemophilia B.
CB 2679d/ISU304 is a next-generation coagulation factor IX variant that may allow for subcutaneous prophylactic treatment of patients with hemophilia B.
The product is being developed by Catalyst Biosciences, Inc. and ISU Abxis.
The companies are currently conducting a phase 1/2 trial of CB 2679d/ISU304 in patients with severe hemophilia B.
Catalyst Biosciences and ISU Abxis plan to have interim, top-line results from this trial by the end of 2017 and complete results in early 2018.
CB 2679d/ISU304 also has orphan medicinal product designation from the European Commission.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
Bowel rest or early feeding for acute pancreatitis
Clinical question: When should you start enteral feedings in patients with acute pancreatitis?
Background: Oral intake stimulates pancreatic exocrine activity and therefore bowel rest has been one of the mainstays of acute pancreatitis treatment. However, some studies suggest that enteral nutrition may reduce the risk of infection by supporting the gut’s protective barrier limiting bacterial translocation and sepsis. Studies thus far comparing early versus delayed enteral nutrition in acute pancreatitis have been conflicting.
Setting: Europe, New Zealand, United States, and China.
Synopsis: Study authors attempted to compare the length of hospital stay, mortality, and readmission in hospitalized patients with acute pancreatitis who received early versus delayed feeding. The authors searched for randomized clinical trials that compared early feeding (less than 48 hours after hospitalization) versus delayed feeding (more than 48 hours after hospitalization).
The authors found and analyzed 11 randomized trials comprising 948 patients in which early and delayed feeding strategies were compared. Their review suggests that early feeding in patients with acute pancreatitis is not associated with increased adverse events and may reduce length of hospital stay. Their analysis was limited by markedly different feeding protocols that precluded performing a meta-analysis. Their analysis was also limited by including studies that had high risk or unclear risk of bias and by the small size of most trials limiting power to detect differences in outcome.
Bottom line: Optimal route and timing of nutrition in patients with acute pancreatitis remains unsettled.
Citation: Vaughn VM, Shuster D, Rogers MAM, et al. Early versus delayed feeding in patients with acute pancreatitis: a systematic review. Ann Intern Med. 2017;166(12):883-92.
Dr. Teixeira is a hospitalist at Ochsner Health System, New Orleans.
Clinical question: When should you start enteral feedings in patients with acute pancreatitis?
Background: Oral intake stimulates pancreatic exocrine activity and therefore bowel rest has been one of the mainstays of acute pancreatitis treatment. However, some studies suggest that enteral nutrition may reduce the risk of infection by supporting the gut’s protective barrier limiting bacterial translocation and sepsis. Studies thus far comparing early versus delayed enteral nutrition in acute pancreatitis have been conflicting.
Setting: Europe, New Zealand, United States, and China.
Synopsis: Study authors attempted to compare the length of hospital stay, mortality, and readmission in hospitalized patients with acute pancreatitis who received early versus delayed feeding. The authors searched for randomized clinical trials that compared early feeding (less than 48 hours after hospitalization) versus delayed feeding (more than 48 hours after hospitalization).
The authors found and analyzed 11 randomized trials comprising 948 patients in which early and delayed feeding strategies were compared. Their review suggests that early feeding in patients with acute pancreatitis is not associated with increased adverse events and may reduce length of hospital stay. Their analysis was limited by markedly different feeding protocols that precluded performing a meta-analysis. Their analysis was also limited by including studies that had high risk or unclear risk of bias and by the small size of most trials limiting power to detect differences in outcome.
Bottom line: Optimal route and timing of nutrition in patients with acute pancreatitis remains unsettled.
Citation: Vaughn VM, Shuster D, Rogers MAM, et al. Early versus delayed feeding in patients with acute pancreatitis: a systematic review. Ann Intern Med. 2017;166(12):883-92.
Dr. Teixeira is a hospitalist at Ochsner Health System, New Orleans.
Clinical question: When should you start enteral feedings in patients with acute pancreatitis?
Background: Oral intake stimulates pancreatic exocrine activity and therefore bowel rest has been one of the mainstays of acute pancreatitis treatment. However, some studies suggest that enteral nutrition may reduce the risk of infection by supporting the gut’s protective barrier limiting bacterial translocation and sepsis. Studies thus far comparing early versus delayed enteral nutrition in acute pancreatitis have been conflicting.
Setting: Europe, New Zealand, United States, and China.
Synopsis: Study authors attempted to compare the length of hospital stay, mortality, and readmission in hospitalized patients with acute pancreatitis who received early versus delayed feeding. The authors searched for randomized clinical trials that compared early feeding (less than 48 hours after hospitalization) versus delayed feeding (more than 48 hours after hospitalization).
The authors found and analyzed 11 randomized trials comprising 948 patients in which early and delayed feeding strategies were compared. Their review suggests that early feeding in patients with acute pancreatitis is not associated with increased adverse events and may reduce length of hospital stay. Their analysis was limited by markedly different feeding protocols that precluded performing a meta-analysis. Their analysis was also limited by including studies that had high risk or unclear risk of bias and by the small size of most trials limiting power to detect differences in outcome.
Bottom line: Optimal route and timing of nutrition in patients with acute pancreatitis remains unsettled.
Citation: Vaughn VM, Shuster D, Rogers MAM, et al. Early versus delayed feeding in patients with acute pancreatitis: a systematic review. Ann Intern Med. 2017;166(12):883-92.
Dr. Teixeira is a hospitalist at Ochsner Health System, New Orleans.
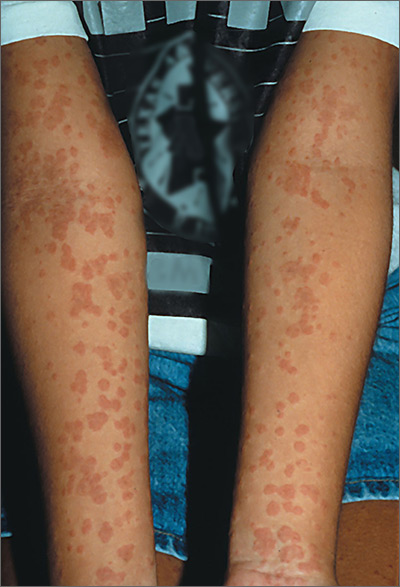
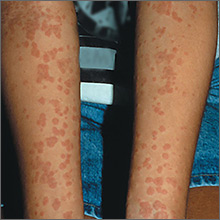
Nonpruritic rash on arms
Based on the results of the punch biopsy and the slight scale seen on the periphery of the lesions (a collarette scale pattern), the FP made a diagnosis of pityriasis rosea.
The distribution of the lesions in this case was not typical for pityriasis rosea; lesions are typically found on the trunk (not the arms) and may start with a herald patch. Given the distribution of the lesions in this case, the more precise diagnosis was inverse pityriasis rosea.
The physician explained to the patient and her mother that the rash would resolve spontaneously and was unlikely to leave any scarring. Six months later, the FP saw the mother for an unrelated issue and she said her daughter’s rash had gotten better within a month of her daughter’s visit, and there had been no scarring.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 896-900.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Based on the results of the punch biopsy and the slight scale seen on the periphery of the lesions (a collarette scale pattern), the FP made a diagnosis of pityriasis rosea.
The distribution of the lesions in this case was not typical for pityriasis rosea; lesions are typically found on the trunk (not the arms) and may start with a herald patch. Given the distribution of the lesions in this case, the more precise diagnosis was inverse pityriasis rosea.
The physician explained to the patient and her mother that the rash would resolve spontaneously and was unlikely to leave any scarring. Six months later, the FP saw the mother for an unrelated issue and she said her daughter’s rash had gotten better within a month of her daughter’s visit, and there had been no scarring.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 896-900.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Based on the results of the punch biopsy and the slight scale seen on the periphery of the lesions (a collarette scale pattern), the FP made a diagnosis of pityriasis rosea.
The distribution of the lesions in this case was not typical for pityriasis rosea; lesions are typically found on the trunk (not the arms) and may start with a herald patch. Given the distribution of the lesions in this case, the more precise diagnosis was inverse pityriasis rosea.
The physician explained to the patient and her mother that the rash would resolve spontaneously and was unlikely to leave any scarring. Six months later, the FP saw the mother for an unrelated issue and she said her daughter’s rash had gotten better within a month of her daughter’s visit, and there had been no scarring.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 896-900.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Delayed-release metformin proves promising for diabetic renal disease
LISBON – in a 16-week, dose-ranging, phase 2 trial performed in patients with type 2 diabetes mellitus and chronic kidney disease (CKD).
There was also a reduced incidence of gastrointestinal side effects with metformin DR versus immediate-release (IR) metformin (less than 16% at all doses tested vs. 28%), particularly with regard to nausea (1%-3% vs. 10%).
“Based on concerns about the potential for lactic acidosis, primarily in patients with fairly severe renal impairment, metformin use is contraindicated in patients with stage IV chronic kidney disease,” Dr. Frias observed. Furthermore its use is restricted in patients with stage IIIB CKD, with European guidelines recommending lower starting (500 mg) and maximum (1,000 mg) daily doses, and U.S. guidelines recommending continuing treatment with caution in those already on metformin and not initiating metformin in new patients with this stage of kidney disease.
Treating patients with type 2 diabetes mellitus (T2DM) and later stages of CKD is challenging, as there are issues with almost all of the available alternatives to metformin, he noted. For instance, insulin use and sulfonylureas carry the risk of hypoglycemia, which is higher in patients with stage IIIB/IV renal disease than without. The dipeptidyl peptidase-4 inhibitors are “modestly able to reduce A1c, but generally do much better in combination with metformin, which is often contraindicated in these patients,” Dr. Frias said. Sodium glucose cotransporter 2 inhibitors are “generally not effective” in this patient group, he said.
Metformin DR is being specifically developed to manage patients with T2DM patients and stage IIIB/IV CKD, Dr. Frias said. Its enteric coating helps it bypass the stomach and upper intestine and so ensures that the majority of metformin absorption occurs in the lower bowel to reduce systemic exposure while retaining its positive effects on glycemic mechanisms such as the secretion of glucagon-like peptide 1.
In the current phase 2 study, 571 patients with T2DM and stage I/II CKD were recruited. Patients with stage IIIB/IV were not included because of the restrictions on the use of metformin.
Patients were randomized to receive placebo or metformin DR (600 mg, 900 mg, 1,200 mg, and 1,500 mg twice daily) in a double-blind comparison, with a single-blind reference arm of metformin IR, (1,000 mg once daily for the first week then 1,000 mg twice daily) also included as part of the study design.
The change in hemoglobin A1c (HbA1c) from baseline levels to week 16 of treatment, the primary endpoint, was significantly (P less than .05) greater with metformin DR) than with placebo (–0.49%, –0.62%, and –0.06%, respectively). Changes in fasting plasma glucose (FPG) from week 4 to week 16 were also higher with metformin DR than with placebo, with the 1,200 metformin DR dose achieving a 25.1 mg/dL drop in FPG, “almost 80% of the fasting glucose–lowering capacity of the immediate release formulation.”
While the changes in HbA1c (-1.10%) and FPG (-32.6 m/dL) were greatest with metformin IR, the lower systemic exposure needs to be considered, Dr. Frias said. The plasma exposure with metformin DR was less than 37% that of metformin IR.
“If we normalize for systemic exposure, so for any given unit, if you will, of systemic exposure, you actually had [a 1.5-fold] improved hemoglobin A1c with the delayed-release formulation, and a twofold increase in the fasting glucose,” Dr. Frias reported. “So from a practical point of view, if you needed to reach those ‘safe’ plasma concentrations with an immediate-release formulation, you would have to lower [the dose of] that formulation, probably to a dose that would not be efficacious for a patient.”
As for safety, any adverse event (AE) occurred in 41.7% of placebo-treated patients, in 47.9% of metformin IR–treated patients, and in 55.3%, 48.4%, 39.6%, and 43.8%, of those taking metformin DR at the respective doses of 600 mg, 900 mg, 1,200 mg, and 1, 500 mg.
Serious AEs were recorded in 4.2% of placebo-treated patients, 1.1% of metformin IR-treated patients, and in 1.1%, 0%, 4.2%, and 1.0% those taking increasing doses of metformin DR.
There were fewer AEs related to study medication (12.8%, 13.7%, 14.6%, and 9.4%) and subsequently resulting in discontinuation (3.2%, 2.1%, 7.3%, 2.1%) with metformin DR than with metformin IR (25.5%, 8.5%). Of placebo-treated patients, 6.3% developed a treatment-related AE, and 6.3% discontinued the study as a result.
“The improved risk/benefit profile that’s seen [in this study] would lead you to think that this would be a formulation that would be effective, particularly in patients with CKD IIIB or IV,” Dr. Frias concluded, noting that further studies would need to look into this possibility further.
The study was funded by Elcelyx Therapeutics. Dr. Frias disclosed receiving research support from Abbvie, Eli Lilly, IONIS, Janssen, Johnson & Johnson, Merck, Mylan, Novartis, Pfizer, and vTv therapeutics. He has also received research support from and participated in scientific advisory boards for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk, and Theracos. A coauthor is an employee of Elcelyx Therapeutics and disclosed being a shareholder of the company.
LISBON – in a 16-week, dose-ranging, phase 2 trial performed in patients with type 2 diabetes mellitus and chronic kidney disease (CKD).
There was also a reduced incidence of gastrointestinal side effects with metformin DR versus immediate-release (IR) metformin (less than 16% at all doses tested vs. 28%), particularly with regard to nausea (1%-3% vs. 10%).
“Based on concerns about the potential for lactic acidosis, primarily in patients with fairly severe renal impairment, metformin use is contraindicated in patients with stage IV chronic kidney disease,” Dr. Frias observed. Furthermore its use is restricted in patients with stage IIIB CKD, with European guidelines recommending lower starting (500 mg) and maximum (1,000 mg) daily doses, and U.S. guidelines recommending continuing treatment with caution in those already on metformin and not initiating metformin in new patients with this stage of kidney disease.
Treating patients with type 2 diabetes mellitus (T2DM) and later stages of CKD is challenging, as there are issues with almost all of the available alternatives to metformin, he noted. For instance, insulin use and sulfonylureas carry the risk of hypoglycemia, which is higher in patients with stage IIIB/IV renal disease than without. The dipeptidyl peptidase-4 inhibitors are “modestly able to reduce A1c, but generally do much better in combination with metformin, which is often contraindicated in these patients,” Dr. Frias said. Sodium glucose cotransporter 2 inhibitors are “generally not effective” in this patient group, he said.
Metformin DR is being specifically developed to manage patients with T2DM patients and stage IIIB/IV CKD, Dr. Frias said. Its enteric coating helps it bypass the stomach and upper intestine and so ensures that the majority of metformin absorption occurs in the lower bowel to reduce systemic exposure while retaining its positive effects on glycemic mechanisms such as the secretion of glucagon-like peptide 1.
In the current phase 2 study, 571 patients with T2DM and stage I/II CKD were recruited. Patients with stage IIIB/IV were not included because of the restrictions on the use of metformin.
Patients were randomized to receive placebo or metformin DR (600 mg, 900 mg, 1,200 mg, and 1,500 mg twice daily) in a double-blind comparison, with a single-blind reference arm of metformin IR, (1,000 mg once daily for the first week then 1,000 mg twice daily) also included as part of the study design.
The change in hemoglobin A1c (HbA1c) from baseline levels to week 16 of treatment, the primary endpoint, was significantly (P less than .05) greater with metformin DR) than with placebo (–0.49%, –0.62%, and –0.06%, respectively). Changes in fasting plasma glucose (FPG) from week 4 to week 16 were also higher with metformin DR than with placebo, with the 1,200 metformin DR dose achieving a 25.1 mg/dL drop in FPG, “almost 80% of the fasting glucose–lowering capacity of the immediate release formulation.”
While the changes in HbA1c (-1.10%) and FPG (-32.6 m/dL) were greatest with metformin IR, the lower systemic exposure needs to be considered, Dr. Frias said. The plasma exposure with metformin DR was less than 37% that of metformin IR.
“If we normalize for systemic exposure, so for any given unit, if you will, of systemic exposure, you actually had [a 1.5-fold] improved hemoglobin A1c with the delayed-release formulation, and a twofold increase in the fasting glucose,” Dr. Frias reported. “So from a practical point of view, if you needed to reach those ‘safe’ plasma concentrations with an immediate-release formulation, you would have to lower [the dose of] that formulation, probably to a dose that would not be efficacious for a patient.”
As for safety, any adverse event (AE) occurred in 41.7% of placebo-treated patients, in 47.9% of metformin IR–treated patients, and in 55.3%, 48.4%, 39.6%, and 43.8%, of those taking metformin DR at the respective doses of 600 mg, 900 mg, 1,200 mg, and 1, 500 mg.
Serious AEs were recorded in 4.2% of placebo-treated patients, 1.1% of metformin IR-treated patients, and in 1.1%, 0%, 4.2%, and 1.0% those taking increasing doses of metformin DR.
There were fewer AEs related to study medication (12.8%, 13.7%, 14.6%, and 9.4%) and subsequently resulting in discontinuation (3.2%, 2.1%, 7.3%, 2.1%) with metformin DR than with metformin IR (25.5%, 8.5%). Of placebo-treated patients, 6.3% developed a treatment-related AE, and 6.3% discontinued the study as a result.
“The improved risk/benefit profile that’s seen [in this study] would lead you to think that this would be a formulation that would be effective, particularly in patients with CKD IIIB or IV,” Dr. Frias concluded, noting that further studies would need to look into this possibility further.
The study was funded by Elcelyx Therapeutics. Dr. Frias disclosed receiving research support from Abbvie, Eli Lilly, IONIS, Janssen, Johnson & Johnson, Merck, Mylan, Novartis, Pfizer, and vTv therapeutics. He has also received research support from and participated in scientific advisory boards for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk, and Theracos. A coauthor is an employee of Elcelyx Therapeutics and disclosed being a shareholder of the company.
LISBON – in a 16-week, dose-ranging, phase 2 trial performed in patients with type 2 diabetes mellitus and chronic kidney disease (CKD).
There was also a reduced incidence of gastrointestinal side effects with metformin DR versus immediate-release (IR) metformin (less than 16% at all doses tested vs. 28%), particularly with regard to nausea (1%-3% vs. 10%).
“Based on concerns about the potential for lactic acidosis, primarily in patients with fairly severe renal impairment, metformin use is contraindicated in patients with stage IV chronic kidney disease,” Dr. Frias observed. Furthermore its use is restricted in patients with stage IIIB CKD, with European guidelines recommending lower starting (500 mg) and maximum (1,000 mg) daily doses, and U.S. guidelines recommending continuing treatment with caution in those already on metformin and not initiating metformin in new patients with this stage of kidney disease.
Treating patients with type 2 diabetes mellitus (T2DM) and later stages of CKD is challenging, as there are issues with almost all of the available alternatives to metformin, he noted. For instance, insulin use and sulfonylureas carry the risk of hypoglycemia, which is higher in patients with stage IIIB/IV renal disease than without. The dipeptidyl peptidase-4 inhibitors are “modestly able to reduce A1c, but generally do much better in combination with metformin, which is often contraindicated in these patients,” Dr. Frias said. Sodium glucose cotransporter 2 inhibitors are “generally not effective” in this patient group, he said.
Metformin DR is being specifically developed to manage patients with T2DM patients and stage IIIB/IV CKD, Dr. Frias said. Its enteric coating helps it bypass the stomach and upper intestine and so ensures that the majority of metformin absorption occurs in the lower bowel to reduce systemic exposure while retaining its positive effects on glycemic mechanisms such as the secretion of glucagon-like peptide 1.
In the current phase 2 study, 571 patients with T2DM and stage I/II CKD were recruited. Patients with stage IIIB/IV were not included because of the restrictions on the use of metformin.
Patients were randomized to receive placebo or metformin DR (600 mg, 900 mg, 1,200 mg, and 1,500 mg twice daily) in a double-blind comparison, with a single-blind reference arm of metformin IR, (1,000 mg once daily for the first week then 1,000 mg twice daily) also included as part of the study design.
The change in hemoglobin A1c (HbA1c) from baseline levels to week 16 of treatment, the primary endpoint, was significantly (P less than .05) greater with metformin DR) than with placebo (–0.49%, –0.62%, and –0.06%, respectively). Changes in fasting plasma glucose (FPG) from week 4 to week 16 were also higher with metformin DR than with placebo, with the 1,200 metformin DR dose achieving a 25.1 mg/dL drop in FPG, “almost 80% of the fasting glucose–lowering capacity of the immediate release formulation.”
While the changes in HbA1c (-1.10%) and FPG (-32.6 m/dL) were greatest with metformin IR, the lower systemic exposure needs to be considered, Dr. Frias said. The plasma exposure with metformin DR was less than 37% that of metformin IR.
“If we normalize for systemic exposure, so for any given unit, if you will, of systemic exposure, you actually had [a 1.5-fold] improved hemoglobin A1c with the delayed-release formulation, and a twofold increase in the fasting glucose,” Dr. Frias reported. “So from a practical point of view, if you needed to reach those ‘safe’ plasma concentrations with an immediate-release formulation, you would have to lower [the dose of] that formulation, probably to a dose that would not be efficacious for a patient.”
As for safety, any adverse event (AE) occurred in 41.7% of placebo-treated patients, in 47.9% of metformin IR–treated patients, and in 55.3%, 48.4%, 39.6%, and 43.8%, of those taking metformin DR at the respective doses of 600 mg, 900 mg, 1,200 mg, and 1, 500 mg.
Serious AEs were recorded in 4.2% of placebo-treated patients, 1.1% of metformin IR-treated patients, and in 1.1%, 0%, 4.2%, and 1.0% those taking increasing doses of metformin DR.
There were fewer AEs related to study medication (12.8%, 13.7%, 14.6%, and 9.4%) and subsequently resulting in discontinuation (3.2%, 2.1%, 7.3%, 2.1%) with metformin DR than with metformin IR (25.5%, 8.5%). Of placebo-treated patients, 6.3% developed a treatment-related AE, and 6.3% discontinued the study as a result.
“The improved risk/benefit profile that’s seen [in this study] would lead you to think that this would be a formulation that would be effective, particularly in patients with CKD IIIB or IV,” Dr. Frias concluded, noting that further studies would need to look into this possibility further.
The study was funded by Elcelyx Therapeutics. Dr. Frias disclosed receiving research support from Abbvie, Eli Lilly, IONIS, Janssen, Johnson & Johnson, Merck, Mylan, Novartis, Pfizer, and vTv therapeutics. He has also received research support from and participated in scientific advisory boards for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk, and Theracos. A coauthor is an employee of Elcelyx Therapeutics and disclosed being a shareholder of the company.
AT EASD 2017
Key clinical point: A delayed-release formulation of metformin appears have a risk/benefit profile that would enable use in patients with type 2 diabetes and chronic kidney disease.
Major finding: Change in HbA1c at week 16 (primary endpoint) was –0.49%, –0.62%, and –0.06%, for metformin DR 1,200 mg, 1,500 mg, and placebo, respectively, P less than .05).
Data source: A 16-week, dose-ranging phase 2 trial involving 571 patients with T2DM and CKD.
Disclosures: The study was funded by Elcelyx Therapeutics. Dr. Frias disclosed receiving research support from Abbvie, Eli Lilly, IONIS, Janssen, Johnson & Johnson, Merck, Mylan, Novartis, Pfizer, and vTv therapeutics. He has also received research support from and participated in scientific advisory boards for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk, and Theracos. A coauthor is an employee of Elcelyx Therapeutics and disclosed being a shareholder of the company.
Rituximab maintenance halves MCL death risk after ASCT
, results of a phase 3 trial show.
After 50.2 months median follow-up, the overall survival rate for patients aged 65 or younger randomized to rituximab maintenance after four cycles of induction chemotherapy with rituximab, dexamethasone, cytarabine, and a platinum derivative (R-DHAP) followed by ASCT was 89%, compared with 80% for patients randomized to observation (P = .004), reported Steven Le Gouill, MD, PhD, of University Hospital Hotel-Dieu, in Nantes, Frances, and colleagues.
In an unadjusted regression analysis, the difference translated into a hazard ratio for death within 4 years of 0.50 (P = .004) favoring rituximab, they wrote in the Sept. 28, 2017 issue of The New England Journal of Medicine.
“[A]n induction regimen with four courses of R-DHAP followed by transplantation without total-body irradiation resulted in a high rate of complete response. A 3-year course of rituximab maintenance therapy administered every 2 months prolonged overall survival among young patients with mantle cell lymphoma,” the investigators wrote (N Engl J Med. 2017;377:1250-60).
Dr. Le Gouill and his colleagues hypothesized that relapses following treatment for MCL may be caused by residual malignant cells that chemotherapy and ASCT fail to eradicate, suggesting that maintenance therapy with rituximab could help to suppress residual disease, prolong the duration of responses, and extend both progression-free and overall survival.
They cited an earlier study by members of the European Mantle Cell Lymphoma Network showing that among patients aged 60 and older who had a response to eight cycles of chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), maintenance therapy with rituximab was associated with an 87% 4-year overall survival rate vs. 63% for patients maintained on interferon alfa (P = .005) (N Engl J Med. 2012;367:520-31).
For the current study, the researchers enrolled 299 patients, of whom 257 went on to ASCT, and 240 of whom were randomized and were included in an intention-to-treat (ITT) analysis.
Patients received induction with four cycles of R-DHAP. Those patients who had partial responses or tumor mass shrinkage of less than 75% on CT received a rescue induction with four cycles of R-CHOP.
Those patients with complete or partial responses could then go on to transplantation after a conditioning regimen of R-BEAM (rituximab, carmustine, etoposide, cytarabine, and melphalan).
Patients randomized after ASCT to rituximab received it every 2 months for 3 years in an intravenous infusion at a dose of 375 mg/m2.
After a median of 50.2 months from randomization, the rate of 4-year event-free survival (no disease progression, relapse, death, or severe infection), the primary endpoint, was 79% for patients maintained on rituximab vs. 61% for those on observation alone (P = .001).
The 4-year progression-free survival rate also favored rituximab at 83% vs. 64%, respectively (P less than .001), with respective overall survival rates of 89% and 80%.
The median event-free survival, progression-free survival, and overall survival was not reached in either study arm.
For the 59 patients who for various reasons did not undergo randomization, the median progression-free survival was 11.0 months, and the median overall survival was 30.6 months.
In all, 83 of the 120 patients randomized to rituximab completed the scheduled 3 years of therapy. Maintenance therapy was stopped for disease progression in 16 patients and because of neutropenia in 9. There were 13 deaths in the rituximab arm, including 3 deaths from second malignancies.
Of the 120 patients assigned to observation, 37 had disease progression during the study period, and 24 died, one from a second malignancy.
Four patients in each study arm had serious infections after ASCT, including one case each of spondylitis, pyelonephritis, septicemia, and varicella pneumonia in the rituximab group, and septicemia, cellulitis, meningitis, and severe pneumonia in the observation group.
Lymphoma was the cause of death in 8 patients assigned to rituximab, and in 16 assigned to observation.
The investigators noted that although some centers use total-body irradiation for conditioning prior to transplant, this modality is not available in all centers and is associated with both short- and long-term toxicities. The progression-free survival results seen in this trial, where only ablative drug regimens were used “suggest that total-body irradiation–based conditioning regimens may not be superior to chemotherapy alone when an effective regimen is used during induction,” they wrote.
The study was supported by Roche and Amgen. Dr. Le Gouill disclosed fees for consulting and honoraria from Roche, Janssen-Cilag, and Celgene. Multiple coauthors disclosed similar relationships with industry.
, results of a phase 3 trial show.
After 50.2 months median follow-up, the overall survival rate for patients aged 65 or younger randomized to rituximab maintenance after four cycles of induction chemotherapy with rituximab, dexamethasone, cytarabine, and a platinum derivative (R-DHAP) followed by ASCT was 89%, compared with 80% for patients randomized to observation (P = .004), reported Steven Le Gouill, MD, PhD, of University Hospital Hotel-Dieu, in Nantes, Frances, and colleagues.
In an unadjusted regression analysis, the difference translated into a hazard ratio for death within 4 years of 0.50 (P = .004) favoring rituximab, they wrote in the Sept. 28, 2017 issue of The New England Journal of Medicine.
“[A]n induction regimen with four courses of R-DHAP followed by transplantation without total-body irradiation resulted in a high rate of complete response. A 3-year course of rituximab maintenance therapy administered every 2 months prolonged overall survival among young patients with mantle cell lymphoma,” the investigators wrote (N Engl J Med. 2017;377:1250-60).
Dr. Le Gouill and his colleagues hypothesized that relapses following treatment for MCL may be caused by residual malignant cells that chemotherapy and ASCT fail to eradicate, suggesting that maintenance therapy with rituximab could help to suppress residual disease, prolong the duration of responses, and extend both progression-free and overall survival.
They cited an earlier study by members of the European Mantle Cell Lymphoma Network showing that among patients aged 60 and older who had a response to eight cycles of chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), maintenance therapy with rituximab was associated with an 87% 4-year overall survival rate vs. 63% for patients maintained on interferon alfa (P = .005) (N Engl J Med. 2012;367:520-31).
For the current study, the researchers enrolled 299 patients, of whom 257 went on to ASCT, and 240 of whom were randomized and were included in an intention-to-treat (ITT) analysis.
Patients received induction with four cycles of R-DHAP. Those patients who had partial responses or tumor mass shrinkage of less than 75% on CT received a rescue induction with four cycles of R-CHOP.
Those patients with complete or partial responses could then go on to transplantation after a conditioning regimen of R-BEAM (rituximab, carmustine, etoposide, cytarabine, and melphalan).
Patients randomized after ASCT to rituximab received it every 2 months for 3 years in an intravenous infusion at a dose of 375 mg/m2.
After a median of 50.2 months from randomization, the rate of 4-year event-free survival (no disease progression, relapse, death, or severe infection), the primary endpoint, was 79% for patients maintained on rituximab vs. 61% for those on observation alone (P = .001).
The 4-year progression-free survival rate also favored rituximab at 83% vs. 64%, respectively (P less than .001), with respective overall survival rates of 89% and 80%.
The median event-free survival, progression-free survival, and overall survival was not reached in either study arm.
For the 59 patients who for various reasons did not undergo randomization, the median progression-free survival was 11.0 months, and the median overall survival was 30.6 months.
In all, 83 of the 120 patients randomized to rituximab completed the scheduled 3 years of therapy. Maintenance therapy was stopped for disease progression in 16 patients and because of neutropenia in 9. There were 13 deaths in the rituximab arm, including 3 deaths from second malignancies.
Of the 120 patients assigned to observation, 37 had disease progression during the study period, and 24 died, one from a second malignancy.
Four patients in each study arm had serious infections after ASCT, including one case each of spondylitis, pyelonephritis, septicemia, and varicella pneumonia in the rituximab group, and septicemia, cellulitis, meningitis, and severe pneumonia in the observation group.
Lymphoma was the cause of death in 8 patients assigned to rituximab, and in 16 assigned to observation.
The investigators noted that although some centers use total-body irradiation for conditioning prior to transplant, this modality is not available in all centers and is associated with both short- and long-term toxicities. The progression-free survival results seen in this trial, where only ablative drug regimens were used “suggest that total-body irradiation–based conditioning regimens may not be superior to chemotherapy alone when an effective regimen is used during induction,” they wrote.
The study was supported by Roche and Amgen. Dr. Le Gouill disclosed fees for consulting and honoraria from Roche, Janssen-Cilag, and Celgene. Multiple coauthors disclosed similar relationships with industry.
, results of a phase 3 trial show.
After 50.2 months median follow-up, the overall survival rate for patients aged 65 or younger randomized to rituximab maintenance after four cycles of induction chemotherapy with rituximab, dexamethasone, cytarabine, and a platinum derivative (R-DHAP) followed by ASCT was 89%, compared with 80% for patients randomized to observation (P = .004), reported Steven Le Gouill, MD, PhD, of University Hospital Hotel-Dieu, in Nantes, Frances, and colleagues.
In an unadjusted regression analysis, the difference translated into a hazard ratio for death within 4 years of 0.50 (P = .004) favoring rituximab, they wrote in the Sept. 28, 2017 issue of The New England Journal of Medicine.
“[A]n induction regimen with four courses of R-DHAP followed by transplantation without total-body irradiation resulted in a high rate of complete response. A 3-year course of rituximab maintenance therapy administered every 2 months prolonged overall survival among young patients with mantle cell lymphoma,” the investigators wrote (N Engl J Med. 2017;377:1250-60).
Dr. Le Gouill and his colleagues hypothesized that relapses following treatment for MCL may be caused by residual malignant cells that chemotherapy and ASCT fail to eradicate, suggesting that maintenance therapy with rituximab could help to suppress residual disease, prolong the duration of responses, and extend both progression-free and overall survival.
They cited an earlier study by members of the European Mantle Cell Lymphoma Network showing that among patients aged 60 and older who had a response to eight cycles of chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), maintenance therapy with rituximab was associated with an 87% 4-year overall survival rate vs. 63% for patients maintained on interferon alfa (P = .005) (N Engl J Med. 2012;367:520-31).
For the current study, the researchers enrolled 299 patients, of whom 257 went on to ASCT, and 240 of whom were randomized and were included in an intention-to-treat (ITT) analysis.
Patients received induction with four cycles of R-DHAP. Those patients who had partial responses or tumor mass shrinkage of less than 75% on CT received a rescue induction with four cycles of R-CHOP.
Those patients with complete or partial responses could then go on to transplantation after a conditioning regimen of R-BEAM (rituximab, carmustine, etoposide, cytarabine, and melphalan).
Patients randomized after ASCT to rituximab received it every 2 months for 3 years in an intravenous infusion at a dose of 375 mg/m2.
After a median of 50.2 months from randomization, the rate of 4-year event-free survival (no disease progression, relapse, death, or severe infection), the primary endpoint, was 79% for patients maintained on rituximab vs. 61% for those on observation alone (P = .001).
The 4-year progression-free survival rate also favored rituximab at 83% vs. 64%, respectively (P less than .001), with respective overall survival rates of 89% and 80%.
The median event-free survival, progression-free survival, and overall survival was not reached in either study arm.
For the 59 patients who for various reasons did not undergo randomization, the median progression-free survival was 11.0 months, and the median overall survival was 30.6 months.
In all, 83 of the 120 patients randomized to rituximab completed the scheduled 3 years of therapy. Maintenance therapy was stopped for disease progression in 16 patients and because of neutropenia in 9. There were 13 deaths in the rituximab arm, including 3 deaths from second malignancies.
Of the 120 patients assigned to observation, 37 had disease progression during the study period, and 24 died, one from a second malignancy.
Four patients in each study arm had serious infections after ASCT, including one case each of spondylitis, pyelonephritis, septicemia, and varicella pneumonia in the rituximab group, and septicemia, cellulitis, meningitis, and severe pneumonia in the observation group.
Lymphoma was the cause of death in 8 patients assigned to rituximab, and in 16 assigned to observation.
The investigators noted that although some centers use total-body irradiation for conditioning prior to transplant, this modality is not available in all centers and is associated with both short- and long-term toxicities. The progression-free survival results seen in this trial, where only ablative drug regimens were used “suggest that total-body irradiation–based conditioning regimens may not be superior to chemotherapy alone when an effective regimen is used during induction,” they wrote.
The study was supported by Roche and Amgen. Dr. Le Gouill disclosed fees for consulting and honoraria from Roche, Janssen-Cilag, and Celgene. Multiple coauthors disclosed similar relationships with industry.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Following stem cell transplantation, rituximab maintenance cut in half the risk for death in patients with mantle cell lymphoma.
Major finding: Four-year overall survival was 89% with rituximab maintenance vs. 80% for observation alone.
Data source: Randomized phase 3 trial in 240 patients aged 65 and younger at diagnosis of mantle cell lymphoma.
Disclosures: The study was supported by Roche and Amgen. Dr. Le Gouill disclosed fees for consulting and honoraria from Roche, Janssen-Cilag, and Celgene. Multiple coauthors disclosed similar relationships with industry.
Tips for Living With Ataxia
Click here to download the PDF.
Click here to download the PDF.
Click here to download the PDF.
FDA approves first duodenoscope with disposable distal cap
, according to an FDA press release.
A disposable distal cap will improve the ability to clean and reprocess the duodenoscope. Without being thoroughly cleaned and disinfected, contaminated tissue can remain and potentially can be transmitted to other patients.
A previous version of the Pentax duodenoscope, the ED-3490TK, was subject to a January 2017 FDA Safety Alert, because of the potential for cracks and gaps to develop in the adhesive sealing the duodenoscope’s distal cap.
“Since the issue of duodenoscope-associated transmission of infections first received widespread attention in 2015, the AGA Center for GI Innovation and Technology has been working closely with regulators and endoscope manufacturers to identify and address problems in scope design and develop a path forward to ensure zero device-associated infections,” said V. Raman Muthusamy, MD, AGAF, FACG, FASGE, chair, AGA Center for GI Innovation and Technology. “We applaud Pentax for answering our call for innovation to improve patient safety for this common and life-saving GI procedure. We encourage all device manufacturers to continue on a path of innovation to better support gastroenterologists and, most importantly, the patients we serve.”
, according to an FDA press release.
A disposable distal cap will improve the ability to clean and reprocess the duodenoscope. Without being thoroughly cleaned and disinfected, contaminated tissue can remain and potentially can be transmitted to other patients.
A previous version of the Pentax duodenoscope, the ED-3490TK, was subject to a January 2017 FDA Safety Alert, because of the potential for cracks and gaps to develop in the adhesive sealing the duodenoscope’s distal cap.
“Since the issue of duodenoscope-associated transmission of infections first received widespread attention in 2015, the AGA Center for GI Innovation and Technology has been working closely with regulators and endoscope manufacturers to identify and address problems in scope design and develop a path forward to ensure zero device-associated infections,” said V. Raman Muthusamy, MD, AGAF, FACG, FASGE, chair, AGA Center for GI Innovation and Technology. “We applaud Pentax for answering our call for innovation to improve patient safety for this common and life-saving GI procedure. We encourage all device manufacturers to continue on a path of innovation to better support gastroenterologists and, most importantly, the patients we serve.”
, according to an FDA press release.
A disposable distal cap will improve the ability to clean and reprocess the duodenoscope. Without being thoroughly cleaned and disinfected, contaminated tissue can remain and potentially can be transmitted to other patients.
A previous version of the Pentax duodenoscope, the ED-3490TK, was subject to a January 2017 FDA Safety Alert, because of the potential for cracks and gaps to develop in the adhesive sealing the duodenoscope’s distal cap.
“Since the issue of duodenoscope-associated transmission of infections first received widespread attention in 2015, the AGA Center for GI Innovation and Technology has been working closely with regulators and endoscope manufacturers to identify and address problems in scope design and develop a path forward to ensure zero device-associated infections,” said V. Raman Muthusamy, MD, AGAF, FACG, FASGE, chair, AGA Center for GI Innovation and Technology. “We applaud Pentax for answering our call for innovation to improve patient safety for this common and life-saving GI procedure. We encourage all device manufacturers to continue on a path of innovation to better support gastroenterologists and, most importantly, the patients we serve.”
Caffeine offers no perks for Parkinson’s patients
Caffeine consumption has no significant impact on motor skills in patients with Parkinson’s disease, based on data from a double-blind, randomized, placebo-controlled trial of 121 adults. The findings were published online Sept. 27 in Neurology.
Data from previous studies have suggested a link between caffeine consumption and reduced risk of Parkinson’s disease, wrote Ronald B. Postuma, MD, of McGill University in Montreal, and his colleagues (Neurology. 2017 Sep 27. doi: 10.1212/WNL.0000000000004568). In addition, Dr. Postuma and his coauthors found a small impact of caffeine on motor skills in patients with existing Parkinson’s as a secondary outcome in a 2012 study on the role of caffeine on daytime sleepiness. Based on these findings, they designed a multicenter, randomized, controlled trial of 121 adults with Parkinson’s to assess the impact of caffeine. The average age of the patients was 62 years and the average disease duration was 4 years.
Motor skills worsened in the caffeine group by an average of 0.16 points on the Movement Disorder Society–sponsored Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III during patients’ “on” state and by 0.64 points in the placebo group; the difference was not significant.
The researchers found no differences between groups in the clinical global impression of change based on both patient and examiner assessment. In addition, no differences appeared in depression or anxiety, or in quality of life.
The study findings included long-term follow up data from 88 patients assessed at 12 months and 66 patients assessed at 18 months. The results were similar to the 6-month results, and the study was stopped, although the original design included a 4-year extension.
A total of 29 patients in the caffeine group and 31 patients in the placebo group reported adverse events, and 7 patients in the caffeine group and 5 in the placebo group discontinued the study because of side effects. A serious adverse event was reported by one patient in each group, but neither was deemed related to the interventions.
The findings contrast with the effects of caffeine in the 6-week study in 2012 that showed a significant, 3.2-point improvement in motor skills on the MDS-UPDRS part III with caffeine use, as well as reduced daytime sleepiness, the researchers said (Neurology. 2012;79:651-8). Interpretations of the different findings between the trials may be constrained by factors including differences in the study populations, speed of dose escalation, and trial duration and the possible short-term nature of caffeine’s impact, they noted. “Regardless, our core finding is that caffeine cannot be recommended as symptomatic therapy for parkinsonism.” However, “since caffeine is safe and generally well tolerated, it seems reasonable to empirically try intermittent moderate doses of caffeine for somnolence, and repeat if improvement is seen,” they added.
Dr. Postuma disclosed grant funding from the Canadian Institute of Health Research, the Webster Foundation, and Fonds de Recherche du Québec-Santé for this study.
The appeal of an inexpensive, well-tolerated intervention such as caffeine to improve motor symptoms in Parkinson’s patients gave Dr. Postuma and his associates a good reason to try to replicate the positive results they found in an earlier study of caffeine.
The investigators found no benefit from caffeine, although their study was designed to have more than four times as many participants as the pilot study, was adequately powered to detect the same level of improvement as before, and was planned to have an extended follow-up period to look for persistent effects. The trial ended early and enrolled approximately half of its intended total, but was well run and did not suffer from differential compliance or loss to follow-up.
Although the small number of participants resulted in a wide confidence interval and cannot exclude a small effect, this trial suggests that caffeine does not significantly improve Parkinson’s disease symptoms and that it should not be a priority for future Parkinson’s disease intervention studies.
Charles B. Hall, PhD, is affiliated with the department of epidemiology and population health at Albert Einstein College of Medicine, New York. He disclosed salary support from the National Institute of Occupational Safety and Health, National Institute of Aging, National Cancer Institute, and National Center for Advancing Translational Sciences. His comments are derived from an editorial that accompanied Dr. Postuma and colleagues’ study (Neurology. 2017 Sep 27. doi: 10.1212/WNL.0000000000004584).
The appeal of an inexpensive, well-tolerated intervention such as caffeine to improve motor symptoms in Parkinson’s patients gave Dr. Postuma and his associates a good reason to try to replicate the positive results they found in an earlier study of caffeine.
The investigators found no benefit from caffeine, although their study was designed to have more than four times as many participants as the pilot study, was adequately powered to detect the same level of improvement as before, and was planned to have an extended follow-up period to look for persistent effects. The trial ended early and enrolled approximately half of its intended total, but was well run and did not suffer from differential compliance or loss to follow-up.
Although the small number of participants resulted in a wide confidence interval and cannot exclude a small effect, this trial suggests that caffeine does not significantly improve Parkinson’s disease symptoms and that it should not be a priority for future Parkinson’s disease intervention studies.
Charles B. Hall, PhD, is affiliated with the department of epidemiology and population health at Albert Einstein College of Medicine, New York. He disclosed salary support from the National Institute of Occupational Safety and Health, National Institute of Aging, National Cancer Institute, and National Center for Advancing Translational Sciences. His comments are derived from an editorial that accompanied Dr. Postuma and colleagues’ study (Neurology. 2017 Sep 27. doi: 10.1212/WNL.0000000000004584).
The appeal of an inexpensive, well-tolerated intervention such as caffeine to improve motor symptoms in Parkinson’s patients gave Dr. Postuma and his associates a good reason to try to replicate the positive results they found in an earlier study of caffeine.
The investigators found no benefit from caffeine, although their study was designed to have more than four times as many participants as the pilot study, was adequately powered to detect the same level of improvement as before, and was planned to have an extended follow-up period to look for persistent effects. The trial ended early and enrolled approximately half of its intended total, but was well run and did not suffer from differential compliance or loss to follow-up.
Although the small number of participants resulted in a wide confidence interval and cannot exclude a small effect, this trial suggests that caffeine does not significantly improve Parkinson’s disease symptoms and that it should not be a priority for future Parkinson’s disease intervention studies.
Charles B. Hall, PhD, is affiliated with the department of epidemiology and population health at Albert Einstein College of Medicine, New York. He disclosed salary support from the National Institute of Occupational Safety and Health, National Institute of Aging, National Cancer Institute, and National Center for Advancing Translational Sciences. His comments are derived from an editorial that accompanied Dr. Postuma and colleagues’ study (Neurology. 2017 Sep 27. doi: 10.1212/WNL.0000000000004584).
Caffeine consumption has no significant impact on motor skills in patients with Parkinson’s disease, based on data from a double-blind, randomized, placebo-controlled trial of 121 adults. The findings were published online Sept. 27 in Neurology.
Data from previous studies have suggested a link between caffeine consumption and reduced risk of Parkinson’s disease, wrote Ronald B. Postuma, MD, of McGill University in Montreal, and his colleagues (Neurology. 2017 Sep 27. doi: 10.1212/WNL.0000000000004568). In addition, Dr. Postuma and his coauthors found a small impact of caffeine on motor skills in patients with existing Parkinson’s as a secondary outcome in a 2012 study on the role of caffeine on daytime sleepiness. Based on these findings, they designed a multicenter, randomized, controlled trial of 121 adults with Parkinson’s to assess the impact of caffeine. The average age of the patients was 62 years and the average disease duration was 4 years.
Motor skills worsened in the caffeine group by an average of 0.16 points on the Movement Disorder Society–sponsored Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III during patients’ “on” state and by 0.64 points in the placebo group; the difference was not significant.
The researchers found no differences between groups in the clinical global impression of change based on both patient and examiner assessment. In addition, no differences appeared in depression or anxiety, or in quality of life.
The study findings included long-term follow up data from 88 patients assessed at 12 months and 66 patients assessed at 18 months. The results were similar to the 6-month results, and the study was stopped, although the original design included a 4-year extension.
A total of 29 patients in the caffeine group and 31 patients in the placebo group reported adverse events, and 7 patients in the caffeine group and 5 in the placebo group discontinued the study because of side effects. A serious adverse event was reported by one patient in each group, but neither was deemed related to the interventions.
The findings contrast with the effects of caffeine in the 6-week study in 2012 that showed a significant, 3.2-point improvement in motor skills on the MDS-UPDRS part III with caffeine use, as well as reduced daytime sleepiness, the researchers said (Neurology. 2012;79:651-8). Interpretations of the different findings between the trials may be constrained by factors including differences in the study populations, speed of dose escalation, and trial duration and the possible short-term nature of caffeine’s impact, they noted. “Regardless, our core finding is that caffeine cannot be recommended as symptomatic therapy for parkinsonism.” However, “since caffeine is safe and generally well tolerated, it seems reasonable to empirically try intermittent moderate doses of caffeine for somnolence, and repeat if improvement is seen,” they added.
Dr. Postuma disclosed grant funding from the Canadian Institute of Health Research, the Webster Foundation, and Fonds de Recherche du Québec-Santé for this study.
Caffeine consumption has no significant impact on motor skills in patients with Parkinson’s disease, based on data from a double-blind, randomized, placebo-controlled trial of 121 adults. The findings were published online Sept. 27 in Neurology.
Data from previous studies have suggested a link between caffeine consumption and reduced risk of Parkinson’s disease, wrote Ronald B. Postuma, MD, of McGill University in Montreal, and his colleagues (Neurology. 2017 Sep 27. doi: 10.1212/WNL.0000000000004568). In addition, Dr. Postuma and his coauthors found a small impact of caffeine on motor skills in patients with existing Parkinson’s as a secondary outcome in a 2012 study on the role of caffeine on daytime sleepiness. Based on these findings, they designed a multicenter, randomized, controlled trial of 121 adults with Parkinson’s to assess the impact of caffeine. The average age of the patients was 62 years and the average disease duration was 4 years.
Motor skills worsened in the caffeine group by an average of 0.16 points on the Movement Disorder Society–sponsored Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III during patients’ “on” state and by 0.64 points in the placebo group; the difference was not significant.
The researchers found no differences between groups in the clinical global impression of change based on both patient and examiner assessment. In addition, no differences appeared in depression or anxiety, or in quality of life.
The study findings included long-term follow up data from 88 patients assessed at 12 months and 66 patients assessed at 18 months. The results were similar to the 6-month results, and the study was stopped, although the original design included a 4-year extension.
A total of 29 patients in the caffeine group and 31 patients in the placebo group reported adverse events, and 7 patients in the caffeine group and 5 in the placebo group discontinued the study because of side effects. A serious adverse event was reported by one patient in each group, but neither was deemed related to the interventions.
The findings contrast with the effects of caffeine in the 6-week study in 2012 that showed a significant, 3.2-point improvement in motor skills on the MDS-UPDRS part III with caffeine use, as well as reduced daytime sleepiness, the researchers said (Neurology. 2012;79:651-8). Interpretations of the different findings between the trials may be constrained by factors including differences in the study populations, speed of dose escalation, and trial duration and the possible short-term nature of caffeine’s impact, they noted. “Regardless, our core finding is that caffeine cannot be recommended as symptomatic therapy for parkinsonism.” However, “since caffeine is safe and generally well tolerated, it seems reasonable to empirically try intermittent moderate doses of caffeine for somnolence, and repeat if improvement is seen,” they added.
Dr. Postuma disclosed grant funding from the Canadian Institute of Health Research, the Webster Foundation, and Fonds de Recherche du Québec-Santé for this study.
FROM NEUROLOGY
Key clinical point:
Major finding: Motor skills declined by an average of 0.16 points in the caffeine group and 0.64 points in the placebo group; the difference was not significant.
Data source: A double-blind, multicenter, placebo-controlled trial of 121 adults with Parkinson’s.
Disclosures: Dr. Postuma disclosed grant funding from the Canadian Institute of Health Research, the Webster Foundation, and Fonds de Recherche du Québec-Santé for this study.