User login
Social media attention increases citation rates for rheumatology journal articles
Social media might be the way to go for authors hoping to bump up the number of times their articles are cited by other articles, according to a presentation at the annual European Congress of Rheumatology that showed how Altmetric scores influence citation rates in journals.
Altmetrics are nontraditional bibliometrics designed to calculate scholarly impact based on online attention. The Altmetric Attention Score and donut provide a collated record of online attention. The colorful badge, which accompanies an increasing number of journal papers online, offers readers a full record of all original shares and mentions of an individual piece of scholarly content across a range of platforms, including Twitter, Facebook, online news media, blogs, Google+, Mendeley, and others.
Dimensions badges, also found on journal sites, count citations from any kind of scientific or mainstream publication. Journal citations remain one of the most recognized proxies for impact in medical research.
Assessing data from over 2,000 articles published in the two official journals of the European League Against Rheumatism – Annals of the Rheumatic Diseases (ARD) and RMD Open – during January 2015–November 2019, Paul Studenic, MD, PhD (@Stiddyo), of the Medical University of Vienna, and coauthor Caroline Ospelt, MD, PhD (@CarolineOspelt), of University Hospital Zürich, found that Altmetric Attention Scores are higher for articles published more recently, with Twitter showing by far the highest activity among the score’s subcategories.
“The total number of Twitter mentions increased by 2.8 per year from 2015 to 2019, indicating that more recently published articles were more often picked up on Twitter,” Dr. Studenic said in an interview. He noted that only original tweets that link to an article are given a full count of 1, while retweets or reposts have less impact on the score.
There are exceptions to this finding that newer articles have higher scores than older articles, he noted. A rheumatology article with one of the highest Altmetric Attention Scores (407) is a piece on the effect of habitual knuckle cracking that published in ARD in 1990. “It has one of the most colorful donuts with 42 news outlets, plus blogs, tweets, Facebook pages, Wikipedia mentions, and Mendeley reads,” Dr. Studenic said. But the article’s citation count is only 20.
“I would not say that the Altmetric Score has anything to do with the quality of the study, it’s just a measure of online popularity. So, you might be a brilliant research team that published a perfect study, but it is not of that much interest to editors, or it might not be tweeted by the journal itself because it was not found to be that interesting. In this case you will get your citations through your scientific community,” he said.
“Particularly if you look now at Altmetric Scores for what is being published on COVID-19, the numbers do not represent at all any profoundness of scientific quality, but there is a lot of tweeting of these articles and a lot of attention,” he said.
Overall, the odds for reaching the top 25% of citations increased with the time since publication. The time since publication accounted for 10% of the variability in the probability of reaching the top 25% of citations, whereas the Altmetric Attention Score accounted for about 5% of the variability.
Besides time since publication and Altmetric Attention Score, the type of article also influences citation count, the researchers found. The Altmetric Attention Score was more likely to boost the citation rate for original research and editorials, but it did little for correspondences.
The influence of Altmetric Attention Score on citation count of editorials added 16% to the 12% of variability explained by publication time. “We never found an effect for correspondence articles,” Dr. Studenic added.
Online popularity is something more likely to favor younger researchers, given their greater engagement online, he said. But he stressed that social media savvy is not absolutely necessary. “If you aren’t on social media and you want to build up a network, you can still do it by other means and your manuscript will still be seen, but I would say in that case that the attention kind of runs behind your back, whereas if you’re active on social media, you can steer it more effectively.”
The study had no outside funding. Both authors are social media advisers to ARD and RMD Open, and Dr. Ospelt is an associate editor of RMD Open and an editorial board member of ARD. Dr. Studenic reported receiving research or grant support from AbbVie, and Dr. Ospelt reported receiving consultancy fees from Gilead Sciences.
SOURCE: Studenic P and Ospelt C. Ann Rheum Dis. 2020 Jun;79(suppl 1):208.
Social media might be the way to go for authors hoping to bump up the number of times their articles are cited by other articles, according to a presentation at the annual European Congress of Rheumatology that showed how Altmetric scores influence citation rates in journals.
Altmetrics are nontraditional bibliometrics designed to calculate scholarly impact based on online attention. The Altmetric Attention Score and donut provide a collated record of online attention. The colorful badge, which accompanies an increasing number of journal papers online, offers readers a full record of all original shares and mentions of an individual piece of scholarly content across a range of platforms, including Twitter, Facebook, online news media, blogs, Google+, Mendeley, and others.
Dimensions badges, also found on journal sites, count citations from any kind of scientific or mainstream publication. Journal citations remain one of the most recognized proxies for impact in medical research.
Assessing data from over 2,000 articles published in the two official journals of the European League Against Rheumatism – Annals of the Rheumatic Diseases (ARD) and RMD Open – during January 2015–November 2019, Paul Studenic, MD, PhD (@Stiddyo), of the Medical University of Vienna, and coauthor Caroline Ospelt, MD, PhD (@CarolineOspelt), of University Hospital Zürich, found that Altmetric Attention Scores are higher for articles published more recently, with Twitter showing by far the highest activity among the score’s subcategories.
“The total number of Twitter mentions increased by 2.8 per year from 2015 to 2019, indicating that more recently published articles were more often picked up on Twitter,” Dr. Studenic said in an interview. He noted that only original tweets that link to an article are given a full count of 1, while retweets or reposts have less impact on the score.
There are exceptions to this finding that newer articles have higher scores than older articles, he noted. A rheumatology article with one of the highest Altmetric Attention Scores (407) is a piece on the effect of habitual knuckle cracking that published in ARD in 1990. “It has one of the most colorful donuts with 42 news outlets, plus blogs, tweets, Facebook pages, Wikipedia mentions, and Mendeley reads,” Dr. Studenic said. But the article’s citation count is only 20.
“I would not say that the Altmetric Score has anything to do with the quality of the study, it’s just a measure of online popularity. So, you might be a brilliant research team that published a perfect study, but it is not of that much interest to editors, or it might not be tweeted by the journal itself because it was not found to be that interesting. In this case you will get your citations through your scientific community,” he said.
“Particularly if you look now at Altmetric Scores for what is being published on COVID-19, the numbers do not represent at all any profoundness of scientific quality, but there is a lot of tweeting of these articles and a lot of attention,” he said.
Overall, the odds for reaching the top 25% of citations increased with the time since publication. The time since publication accounted for 10% of the variability in the probability of reaching the top 25% of citations, whereas the Altmetric Attention Score accounted for about 5% of the variability.
Besides time since publication and Altmetric Attention Score, the type of article also influences citation count, the researchers found. The Altmetric Attention Score was more likely to boost the citation rate for original research and editorials, but it did little for correspondences.
The influence of Altmetric Attention Score on citation count of editorials added 16% to the 12% of variability explained by publication time. “We never found an effect for correspondence articles,” Dr. Studenic added.
Online popularity is something more likely to favor younger researchers, given their greater engagement online, he said. But he stressed that social media savvy is not absolutely necessary. “If you aren’t on social media and you want to build up a network, you can still do it by other means and your manuscript will still be seen, but I would say in that case that the attention kind of runs behind your back, whereas if you’re active on social media, you can steer it more effectively.”
The study had no outside funding. Both authors are social media advisers to ARD and RMD Open, and Dr. Ospelt is an associate editor of RMD Open and an editorial board member of ARD. Dr. Studenic reported receiving research or grant support from AbbVie, and Dr. Ospelt reported receiving consultancy fees from Gilead Sciences.
SOURCE: Studenic P and Ospelt C. Ann Rheum Dis. 2020 Jun;79(suppl 1):208.
Social media might be the way to go for authors hoping to bump up the number of times their articles are cited by other articles, according to a presentation at the annual European Congress of Rheumatology that showed how Altmetric scores influence citation rates in journals.
Altmetrics are nontraditional bibliometrics designed to calculate scholarly impact based on online attention. The Altmetric Attention Score and donut provide a collated record of online attention. The colorful badge, which accompanies an increasing number of journal papers online, offers readers a full record of all original shares and mentions of an individual piece of scholarly content across a range of platforms, including Twitter, Facebook, online news media, blogs, Google+, Mendeley, and others.
Dimensions badges, also found on journal sites, count citations from any kind of scientific or mainstream publication. Journal citations remain one of the most recognized proxies for impact in medical research.
Assessing data from over 2,000 articles published in the two official journals of the European League Against Rheumatism – Annals of the Rheumatic Diseases (ARD) and RMD Open – during January 2015–November 2019, Paul Studenic, MD, PhD (@Stiddyo), of the Medical University of Vienna, and coauthor Caroline Ospelt, MD, PhD (@CarolineOspelt), of University Hospital Zürich, found that Altmetric Attention Scores are higher for articles published more recently, with Twitter showing by far the highest activity among the score’s subcategories.
“The total number of Twitter mentions increased by 2.8 per year from 2015 to 2019, indicating that more recently published articles were more often picked up on Twitter,” Dr. Studenic said in an interview. He noted that only original tweets that link to an article are given a full count of 1, while retweets or reposts have less impact on the score.
There are exceptions to this finding that newer articles have higher scores than older articles, he noted. A rheumatology article with one of the highest Altmetric Attention Scores (407) is a piece on the effect of habitual knuckle cracking that published in ARD in 1990. “It has one of the most colorful donuts with 42 news outlets, plus blogs, tweets, Facebook pages, Wikipedia mentions, and Mendeley reads,” Dr. Studenic said. But the article’s citation count is only 20.
“I would not say that the Altmetric Score has anything to do with the quality of the study, it’s just a measure of online popularity. So, you might be a brilliant research team that published a perfect study, but it is not of that much interest to editors, or it might not be tweeted by the journal itself because it was not found to be that interesting. In this case you will get your citations through your scientific community,” he said.
“Particularly if you look now at Altmetric Scores for what is being published on COVID-19, the numbers do not represent at all any profoundness of scientific quality, but there is a lot of tweeting of these articles and a lot of attention,” he said.
Overall, the odds for reaching the top 25% of citations increased with the time since publication. The time since publication accounted for 10% of the variability in the probability of reaching the top 25% of citations, whereas the Altmetric Attention Score accounted for about 5% of the variability.
Besides time since publication and Altmetric Attention Score, the type of article also influences citation count, the researchers found. The Altmetric Attention Score was more likely to boost the citation rate for original research and editorials, but it did little for correspondences.
The influence of Altmetric Attention Score on citation count of editorials added 16% to the 12% of variability explained by publication time. “We never found an effect for correspondence articles,” Dr. Studenic added.
Online popularity is something more likely to favor younger researchers, given their greater engagement online, he said. But he stressed that social media savvy is not absolutely necessary. “If you aren’t on social media and you want to build up a network, you can still do it by other means and your manuscript will still be seen, but I would say in that case that the attention kind of runs behind your back, whereas if you’re active on social media, you can steer it more effectively.”
The study had no outside funding. Both authors are social media advisers to ARD and RMD Open, and Dr. Ospelt is an associate editor of RMD Open and an editorial board member of ARD. Dr. Studenic reported receiving research or grant support from AbbVie, and Dr. Ospelt reported receiving consultancy fees from Gilead Sciences.
SOURCE: Studenic P and Ospelt C. Ann Rheum Dis. 2020 Jun;79(suppl 1):208.
FROM THE EULAR 2020 E-CONGRESS
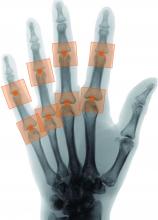
Automated RA image scoring could be coming
A novel program that aims to automate the Sharp-van der Heijde scoring of radiographs of patients with rheumatoid arthritis has shown good reliability in identifying regions of interest and matching human reader scoring for joint-space narrowing, according to a report given at the annual European Congress of Rheumatology, held online this year because of COVID-19.
First author and presenter Thomas Deimel, MD, and colleagues at the Medical University of Vienna said their program, called autoscoRA, may be a solution to the problem of readers having to make subjective calls on the severity of damage seen on radiographs.
Although the work continues to be validated, Dr. Deimel, a resident at the university, is confident in the system as is. “I think for joint space narrowing, we’re there at the point where this could be used and could be as good as a human reader in terms of reliability,” he said in an interview. To find out, the group plans to compare the variability between autoscoRA and a gold-standard human reader against the variability seen between human readers. If the two measures of variability are similar, it would provide a strong endorsement.
The effort is far from the first to develop an automatic scoring system for RA images, but no fully automated system has emerged as reliable, according to Dr. Deimel. He thinks one main issue for others has been lack of access to a sufficient data set to train systems. It can be difficult to find enough training images because many types of joint damage are comparatively uncommon. The problem is made even worse because images can be hard to interpret: The shapes that the system must decipher can be misleading, especially in positions of tendon insertion or ligament attachment that can resemble damage. Differing angles of view between various training images can also complicate matters.
The autoscoRA program is based on modifications of a form of convolutional neural network called the VGG16 architecture. The team used 2,207 images from 270 patients to train autoscoRA, 1,150 images from 133 patients for validation, and 1,834 images from 237 patients to test it.
The group had access to a high-quality data set of almost 6,000 hand radiographs from their institution, the result of foresight of principal investigator Daniel Aletaha, MD, and his predecessor Josef Smolen, MD. They “thought ahead and started collecting data and had all of it scored,” Dr. Deimel said. The work wasn’t all completed ahead of time, though. Dr. Deimel had to pull images from the hospital’s system sort through them manually.
The group also benefited from close proximity to computer scientists, including coauthor Georg Langs from the Medical University of Vienna’s computational imaging research lab. “We were lucky that we have a computer science department that is very much involved in medical imaging,” Dr. Deimel said.
The trained system successfully identified regions of interest in 96% of joints. It calculated the same score as the human reader in 80.5% of metacarpophalangeal joints and 72.3% of proximal interphalangeal joints. It deviated by more than 1 point from the gold-standard score in just 1.8% of metacarpophalangeal joints and 1.7% of proximal interphalangeal joints.
The researchers aim next to extend the program to bone erosions and also to images of the wrists and feet. They also hope to use scores from the program in clinical trials to measure a treatment’s effect, in registries of routine patient visits where thousands of such images along with clinical data could form the basis of informative observational studies, and in clinical practice, though likely with human oversight.
The study received no outside financial support. Dr. Deimel had no relevant financial disclosures. Mr. Langs reported being cofounder and shareholder of contextflow and receiving grants from Novartis, Siemens Healthineers, and NVIDIA. Dr. Aletaha reported financial relationships with many companies marketing drugs for rheumatoid arthritis.
SOURCE: Deimel T et al. Ann Rheum Dis. 2020 Jun;79(suppl 1):39-40.
A novel program that aims to automate the Sharp-van der Heijde scoring of radiographs of patients with rheumatoid arthritis has shown good reliability in identifying regions of interest and matching human reader scoring for joint-space narrowing, according to a report given at the annual European Congress of Rheumatology, held online this year because of COVID-19.
First author and presenter Thomas Deimel, MD, and colleagues at the Medical University of Vienna said their program, called autoscoRA, may be a solution to the problem of readers having to make subjective calls on the severity of damage seen on radiographs.
Although the work continues to be validated, Dr. Deimel, a resident at the university, is confident in the system as is. “I think for joint space narrowing, we’re there at the point where this could be used and could be as good as a human reader in terms of reliability,” he said in an interview. To find out, the group plans to compare the variability between autoscoRA and a gold-standard human reader against the variability seen between human readers. If the two measures of variability are similar, it would provide a strong endorsement.
The effort is far from the first to develop an automatic scoring system for RA images, but no fully automated system has emerged as reliable, according to Dr. Deimel. He thinks one main issue for others has been lack of access to a sufficient data set to train systems. It can be difficult to find enough training images because many types of joint damage are comparatively uncommon. The problem is made even worse because images can be hard to interpret: The shapes that the system must decipher can be misleading, especially in positions of tendon insertion or ligament attachment that can resemble damage. Differing angles of view between various training images can also complicate matters.
The autoscoRA program is based on modifications of a form of convolutional neural network called the VGG16 architecture. The team used 2,207 images from 270 patients to train autoscoRA, 1,150 images from 133 patients for validation, and 1,834 images from 237 patients to test it.
The group had access to a high-quality data set of almost 6,000 hand radiographs from their institution, the result of foresight of principal investigator Daniel Aletaha, MD, and his predecessor Josef Smolen, MD. They “thought ahead and started collecting data and had all of it scored,” Dr. Deimel said. The work wasn’t all completed ahead of time, though. Dr. Deimel had to pull images from the hospital’s system sort through them manually.
The group also benefited from close proximity to computer scientists, including coauthor Georg Langs from the Medical University of Vienna’s computational imaging research lab. “We were lucky that we have a computer science department that is very much involved in medical imaging,” Dr. Deimel said.
The trained system successfully identified regions of interest in 96% of joints. It calculated the same score as the human reader in 80.5% of metacarpophalangeal joints and 72.3% of proximal interphalangeal joints. It deviated by more than 1 point from the gold-standard score in just 1.8% of metacarpophalangeal joints and 1.7% of proximal interphalangeal joints.
The researchers aim next to extend the program to bone erosions and also to images of the wrists and feet. They also hope to use scores from the program in clinical trials to measure a treatment’s effect, in registries of routine patient visits where thousands of such images along with clinical data could form the basis of informative observational studies, and in clinical practice, though likely with human oversight.
The study received no outside financial support. Dr. Deimel had no relevant financial disclosures. Mr. Langs reported being cofounder and shareholder of contextflow and receiving grants from Novartis, Siemens Healthineers, and NVIDIA. Dr. Aletaha reported financial relationships with many companies marketing drugs for rheumatoid arthritis.
SOURCE: Deimel T et al. Ann Rheum Dis. 2020 Jun;79(suppl 1):39-40.
A novel program that aims to automate the Sharp-van der Heijde scoring of radiographs of patients with rheumatoid arthritis has shown good reliability in identifying regions of interest and matching human reader scoring for joint-space narrowing, according to a report given at the annual European Congress of Rheumatology, held online this year because of COVID-19.
First author and presenter Thomas Deimel, MD, and colleagues at the Medical University of Vienna said their program, called autoscoRA, may be a solution to the problem of readers having to make subjective calls on the severity of damage seen on radiographs.
Although the work continues to be validated, Dr. Deimel, a resident at the university, is confident in the system as is. “I think for joint space narrowing, we’re there at the point where this could be used and could be as good as a human reader in terms of reliability,” he said in an interview. To find out, the group plans to compare the variability between autoscoRA and a gold-standard human reader against the variability seen between human readers. If the two measures of variability are similar, it would provide a strong endorsement.
The effort is far from the first to develop an automatic scoring system for RA images, but no fully automated system has emerged as reliable, according to Dr. Deimel. He thinks one main issue for others has been lack of access to a sufficient data set to train systems. It can be difficult to find enough training images because many types of joint damage are comparatively uncommon. The problem is made even worse because images can be hard to interpret: The shapes that the system must decipher can be misleading, especially in positions of tendon insertion or ligament attachment that can resemble damage. Differing angles of view between various training images can also complicate matters.
The autoscoRA program is based on modifications of a form of convolutional neural network called the VGG16 architecture. The team used 2,207 images from 270 patients to train autoscoRA, 1,150 images from 133 patients for validation, and 1,834 images from 237 patients to test it.
The group had access to a high-quality data set of almost 6,000 hand radiographs from their institution, the result of foresight of principal investigator Daniel Aletaha, MD, and his predecessor Josef Smolen, MD. They “thought ahead and started collecting data and had all of it scored,” Dr. Deimel said. The work wasn’t all completed ahead of time, though. Dr. Deimel had to pull images from the hospital’s system sort through them manually.
The group also benefited from close proximity to computer scientists, including coauthor Georg Langs from the Medical University of Vienna’s computational imaging research lab. “We were lucky that we have a computer science department that is very much involved in medical imaging,” Dr. Deimel said.
The trained system successfully identified regions of interest in 96% of joints. It calculated the same score as the human reader in 80.5% of metacarpophalangeal joints and 72.3% of proximal interphalangeal joints. It deviated by more than 1 point from the gold-standard score in just 1.8% of metacarpophalangeal joints and 1.7% of proximal interphalangeal joints.
The researchers aim next to extend the program to bone erosions and also to images of the wrists and feet. They also hope to use scores from the program in clinical trials to measure a treatment’s effect, in registries of routine patient visits where thousands of such images along with clinical data could form the basis of informative observational studies, and in clinical practice, though likely with human oversight.
The study received no outside financial support. Dr. Deimel had no relevant financial disclosures. Mr. Langs reported being cofounder and shareholder of contextflow and receiving grants from Novartis, Siemens Healthineers, and NVIDIA. Dr. Aletaha reported financial relationships with many companies marketing drugs for rheumatoid arthritis.
SOURCE: Deimel T et al. Ann Rheum Dis. 2020 Jun;79(suppl 1):39-40.
FROM THE EULAR 2020 E-CONGRESS
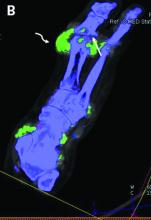
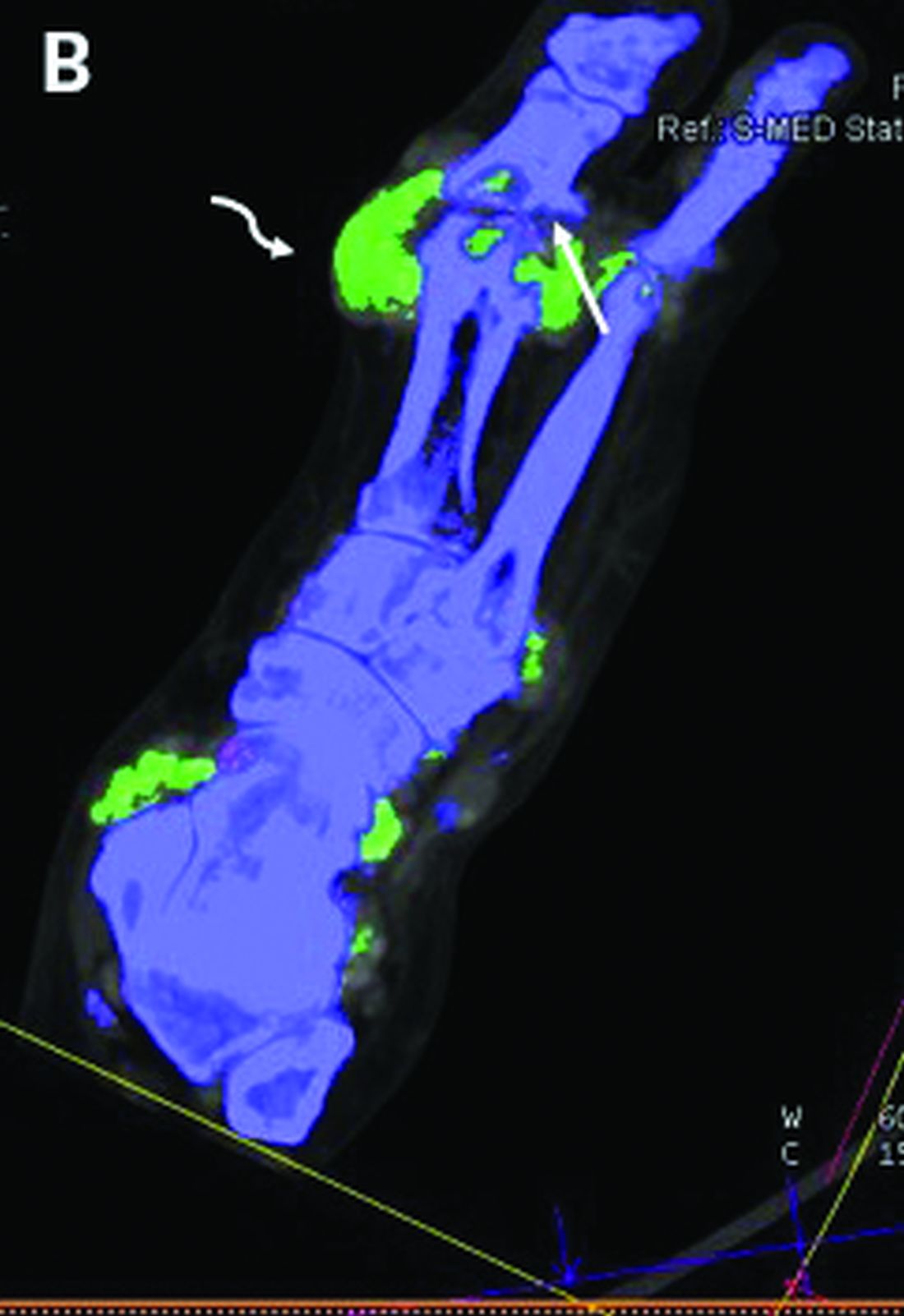
DECT has mixed performance in differentiating gout vs. CPPD
Dual-energy computed tomography (DECT) appears to have limited utility for differentiating between gout and calcium pyrophosphate deposition disease (CPPD), according to a German prospective cohort study. Findings were reported at the annual European Congress of Rheumatology, held online this year because of COVID-19.
“Differentiation of gout and pseudogout, or CPPD, is sometimes difficult,” said presenting investigator Valentin S. Schäfer, MD, associate professor of internal medicine and head of the department of rheumatology and clinical immunology at University Hospital Bonn (Germany).
“Arthrocentesis and subsequent polarization microscopy remains the gold standard,” he noted. “Novel diagnostic approaches, such as DECT, have recently been validated for gout, but limited data [are] available on the use of DECT in patients with CPPD.”
The investigators studied 30 patients: 22 with suspected gout and 8 with suspected CPPD. All underwent arthrocentesis with subsequent polarization microscopy for definitive diagnosis, plus clinical examination, ultrasound examination, conventional radiography, DECT, and assessment of 12 laboratory parameters.
For diagnosis of gout, DECT had a sensitivity and specificity of 59.1% and 100%, respectively, Dr. Schäfer reported, noting that this sensitivity falls considerably short of the 90% previously reported for gout.
Corresponding sensitivity and specificity were 90.9% and 75% for ultrasound, 58.8% and 100% for conventional radiography, and 81.8% and 87.5% for the rheumatologists’ suspected clinical diagnosis.
For diagnosis of CPPD, DECT had sensitivity of 37.5% and specificity of 81.8%. Corresponding values were 87.5% and 91% for ultrasound, 0% and 94.1% for conventional radiography, and 75.0% and 100% for suspected clinical diagnosis.
None of the 12 laboratory parameters studied – uric acid, C-reactive protein, organic phosphate, and leukocytes, among others – significantly differentiated between conditions.
“Both ultrasound and suspected clinical diagnosis had higher sensitivities than DECT for gout and CPPD,” Dr. Schäfer concluded. “Further studies with larger patient cohorts and perhaps modified scan protocols are needed in order to determine the diagnostic utility of DECT in CPPD.”
Findings in context
“Noninvasive, accurate methods for distinguishing between gout and CPPD will improve clinical care,” Sara K. Tedeschi, MD, MPH, predicted in an interview.
“Arthrocentesis is painful in an acutely inflamed joint, can be difficult to perform on small joints, and is underutilized in clinical practice,” she elaborated. And ultrasound is operator dependent and does not quantify crystal volume in and around the joint.
The question addressed by the study is therefore clinically relevant, according to Dr. Tedeschi, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, Boston.
However, among the patients with CPPD, the study did not report specific phenotypes (acute inflammatory arthritis, chronic inflammatory arthritis, and osteoarthritis with calcium pyrophosphate deposits), she noted. “It is difficult to draw conclusions about the sensitivity or specificity of DECT for CPPD without this information, especially among just 8 CPPD patients.”
In addition, among the patients with gout, the proportion having new-onset disease with flare duration less than 6 weeks and the proportion with tophi were unknown, both of which affected DECT sensitivity in the previous study that reported 90% sensitivity. “Based on the 95% confidence interval in the present study, it is possible that with a larger sample size, DECT sensitivity for gout would have been higher,” she pointed out. “We also do not know the DECT software settings, which impact DECT interpretation as positive or negative for the crystal of interest.”
Finally, “it would be relevant to know what joints were aspirated and imaged in each group,” Dr. Tedeschi said. “For example, if the first metatarsophalangeal (MTP) joint was aspirated and imaged for half of the gout patients but for none of the CPPD patients, that may affect the study interpretation.”
The study did not receive any specific funding. Dr. Schäfer disclosed a variety of financial relationships with multiple pharmaceutical companies. Dr. Tedeschi disclosed receiving grant support from the National Institutes of Health to study imaging modalities for CPPD, and being first author on a study comparing the sensitivity of DECT, ultrasound, and x-ray for acute CPP crystal arthritis.
SOURCE: Kravchenko D et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:196.
Dual-energy computed tomography (DECT) appears to have limited utility for differentiating between gout and calcium pyrophosphate deposition disease (CPPD), according to a German prospective cohort study. Findings were reported at the annual European Congress of Rheumatology, held online this year because of COVID-19.
“Differentiation of gout and pseudogout, or CPPD, is sometimes difficult,” said presenting investigator Valentin S. Schäfer, MD, associate professor of internal medicine and head of the department of rheumatology and clinical immunology at University Hospital Bonn (Germany).
“Arthrocentesis and subsequent polarization microscopy remains the gold standard,” he noted. “Novel diagnostic approaches, such as DECT, have recently been validated for gout, but limited data [are] available on the use of DECT in patients with CPPD.”
The investigators studied 30 patients: 22 with suspected gout and 8 with suspected CPPD. All underwent arthrocentesis with subsequent polarization microscopy for definitive diagnosis, plus clinical examination, ultrasound examination, conventional radiography, DECT, and assessment of 12 laboratory parameters.
For diagnosis of gout, DECT had a sensitivity and specificity of 59.1% and 100%, respectively, Dr. Schäfer reported, noting that this sensitivity falls considerably short of the 90% previously reported for gout.
Corresponding sensitivity and specificity were 90.9% and 75% for ultrasound, 58.8% and 100% for conventional radiography, and 81.8% and 87.5% for the rheumatologists’ suspected clinical diagnosis.
For diagnosis of CPPD, DECT had sensitivity of 37.5% and specificity of 81.8%. Corresponding values were 87.5% and 91% for ultrasound, 0% and 94.1% for conventional radiography, and 75.0% and 100% for suspected clinical diagnosis.
None of the 12 laboratory parameters studied – uric acid, C-reactive protein, organic phosphate, and leukocytes, among others – significantly differentiated between conditions.
“Both ultrasound and suspected clinical diagnosis had higher sensitivities than DECT for gout and CPPD,” Dr. Schäfer concluded. “Further studies with larger patient cohorts and perhaps modified scan protocols are needed in order to determine the diagnostic utility of DECT in CPPD.”
Findings in context
“Noninvasive, accurate methods for distinguishing between gout and CPPD will improve clinical care,” Sara K. Tedeschi, MD, MPH, predicted in an interview.
“Arthrocentesis is painful in an acutely inflamed joint, can be difficult to perform on small joints, and is underutilized in clinical practice,” she elaborated. And ultrasound is operator dependent and does not quantify crystal volume in and around the joint.
The question addressed by the study is therefore clinically relevant, according to Dr. Tedeschi, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, Boston.
However, among the patients with CPPD, the study did not report specific phenotypes (acute inflammatory arthritis, chronic inflammatory arthritis, and osteoarthritis with calcium pyrophosphate deposits), she noted. “It is difficult to draw conclusions about the sensitivity or specificity of DECT for CPPD without this information, especially among just 8 CPPD patients.”
In addition, among the patients with gout, the proportion having new-onset disease with flare duration less than 6 weeks and the proportion with tophi were unknown, both of which affected DECT sensitivity in the previous study that reported 90% sensitivity. “Based on the 95% confidence interval in the present study, it is possible that with a larger sample size, DECT sensitivity for gout would have been higher,” she pointed out. “We also do not know the DECT software settings, which impact DECT interpretation as positive or negative for the crystal of interest.”
Finally, “it would be relevant to know what joints were aspirated and imaged in each group,” Dr. Tedeschi said. “For example, if the first metatarsophalangeal (MTP) joint was aspirated and imaged for half of the gout patients but for none of the CPPD patients, that may affect the study interpretation.”
The study did not receive any specific funding. Dr. Schäfer disclosed a variety of financial relationships with multiple pharmaceutical companies. Dr. Tedeschi disclosed receiving grant support from the National Institutes of Health to study imaging modalities for CPPD, and being first author on a study comparing the sensitivity of DECT, ultrasound, and x-ray for acute CPP crystal arthritis.
SOURCE: Kravchenko D et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:196.
Dual-energy computed tomography (DECT) appears to have limited utility for differentiating between gout and calcium pyrophosphate deposition disease (CPPD), according to a German prospective cohort study. Findings were reported at the annual European Congress of Rheumatology, held online this year because of COVID-19.
“Differentiation of gout and pseudogout, or CPPD, is sometimes difficult,” said presenting investigator Valentin S. Schäfer, MD, associate professor of internal medicine and head of the department of rheumatology and clinical immunology at University Hospital Bonn (Germany).
“Arthrocentesis and subsequent polarization microscopy remains the gold standard,” he noted. “Novel diagnostic approaches, such as DECT, have recently been validated for gout, but limited data [are] available on the use of DECT in patients with CPPD.”
The investigators studied 30 patients: 22 with suspected gout and 8 with suspected CPPD. All underwent arthrocentesis with subsequent polarization microscopy for definitive diagnosis, plus clinical examination, ultrasound examination, conventional radiography, DECT, and assessment of 12 laboratory parameters.
For diagnosis of gout, DECT had a sensitivity and specificity of 59.1% and 100%, respectively, Dr. Schäfer reported, noting that this sensitivity falls considerably short of the 90% previously reported for gout.
Corresponding sensitivity and specificity were 90.9% and 75% for ultrasound, 58.8% and 100% for conventional radiography, and 81.8% and 87.5% for the rheumatologists’ suspected clinical diagnosis.
For diagnosis of CPPD, DECT had sensitivity of 37.5% and specificity of 81.8%. Corresponding values were 87.5% and 91% for ultrasound, 0% and 94.1% for conventional radiography, and 75.0% and 100% for suspected clinical diagnosis.
None of the 12 laboratory parameters studied – uric acid, C-reactive protein, organic phosphate, and leukocytes, among others – significantly differentiated between conditions.
“Both ultrasound and suspected clinical diagnosis had higher sensitivities than DECT for gout and CPPD,” Dr. Schäfer concluded. “Further studies with larger patient cohorts and perhaps modified scan protocols are needed in order to determine the diagnostic utility of DECT in CPPD.”
Findings in context
“Noninvasive, accurate methods for distinguishing between gout and CPPD will improve clinical care,” Sara K. Tedeschi, MD, MPH, predicted in an interview.
“Arthrocentesis is painful in an acutely inflamed joint, can be difficult to perform on small joints, and is underutilized in clinical practice,” she elaborated. And ultrasound is operator dependent and does not quantify crystal volume in and around the joint.
The question addressed by the study is therefore clinically relevant, according to Dr. Tedeschi, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, Boston.
However, among the patients with CPPD, the study did not report specific phenotypes (acute inflammatory arthritis, chronic inflammatory arthritis, and osteoarthritis with calcium pyrophosphate deposits), she noted. “It is difficult to draw conclusions about the sensitivity or specificity of DECT for CPPD without this information, especially among just 8 CPPD patients.”
In addition, among the patients with gout, the proportion having new-onset disease with flare duration less than 6 weeks and the proportion with tophi were unknown, both of which affected DECT sensitivity in the previous study that reported 90% sensitivity. “Based on the 95% confidence interval in the present study, it is possible that with a larger sample size, DECT sensitivity for gout would have been higher,” she pointed out. “We also do not know the DECT software settings, which impact DECT interpretation as positive or negative for the crystal of interest.”
Finally, “it would be relevant to know what joints were aspirated and imaged in each group,” Dr. Tedeschi said. “For example, if the first metatarsophalangeal (MTP) joint was aspirated and imaged for half of the gout patients but for none of the CPPD patients, that may affect the study interpretation.”
The study did not receive any specific funding. Dr. Schäfer disclosed a variety of financial relationships with multiple pharmaceutical companies. Dr. Tedeschi disclosed receiving grant support from the National Institutes of Health to study imaging modalities for CPPD, and being first author on a study comparing the sensitivity of DECT, ultrasound, and x-ray for acute CPP crystal arthritis.
SOURCE: Kravchenko D et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:196.
FROM THE EULAR 2020 E-CONGRESS
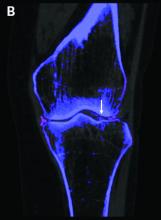
EULAR gives pointers on intra-articular injection best practices
New EULAR recommendations for the intra-articular (IA) treatment of arthropathies aim to facilitate uniformity and quality of care for this mainstay of rheumatologic practice, according to a report on the new guidance that was presented at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Until now there were no official recommendations on how best to use it in everyday practice. “This is the first time that there’s been a joint effort to develop evidence-based recommendations,” Jacqueline Usón, MD, PhD, associate professor medicine at Rey Juan Carlos University in Madrid, said in an interview. “Everything that we are saying is pretty logical, but it’s nice to see it put in recommendations based on evidence.”
IA therapy has been around for decades and is key for treating adults with a number of different conditions where synovitis, effusion, pain, or all three, are present, such as inflammatory arthritis and osteoarthritis, Dr. Usón observed during her presentation.
“Today, commonly used injectables are not only corticosteroids but also local anesthetics, hyaluronic acid, blood products, and maybe pharmaceuticals,” she said, adding that “there is a wide variation in the way intra-articular therapies are used and delivered to patients.” Health professionals also have very different views and habits depending on geographic locations and health care systems, she observed. Ironing out the variation was one of the main objectives of the recommendations.
As one of the two conveners of the EULAR task force behind the recommendations, Dr. Usón, herself a rheumatologist at University Hospital of Móstoles, pointed out that the task force brought together a range of specialties – rheumatologists, orthopedic surgeons, radiologists, nuclear medicine specialists, among others, as well as patients – to ensure that the best advice could be given.
The task force followed EULAR standard operating procedures for developing recommendations, with discussion groups, systematic literature reviews, and Delphi technique-based consensus all being employed. The literature search considered publications from 1946 up until 2019.
“We agreed on the need for more background information from health professionals and patients, so we developed two surveys: One for health professionals with 160 items, [for which] we obtained 186 responses from 26 countries; and the patient survey was made up of 44 items, translated into 10 different languages, and we obtained 200 responses,” she said.
The results of the systematic literature review and surveys were used to help form expert consensus, leading to 5 overarching principles and 11 recommendations that look at before, during, and after intra-articular therapy.
Five overarching principles
The first overarching principle recognizes the widespread use of IA therapies and that their use is specific to the disease that is being treated and “may not be interchangeable across indications,” Dr. Usón said. The second principle concerns improving patient-centered outcomes, which are “those that are relevant to the patient,” and include the benefits, harms, preferences, or implications for self-management.
“Contextual factors are important and contribute to the effect of IAT [intra-articular treatment],” she said, discussing the third principle. “These include effective communication, patient expectations, or settings [where the procedure takes place]. In addition, one should take into account that the route of delivery has in itself a placebo effect. We found that in different RCTs [randomized controlled trials], the pooled placebo effect of IA saline is moderate to large.”
The fourth principle looks at ensuring that patients and clinicians make an informed and shared decision, which is again highlighted by the first recommendation. The fifth, and last, overarching principle acknowledges that IA injections may be given by a range of health care professionals.
Advice for before, during, and after injection
Patients need to be “fully informed of the nature of the procedure, the injectable used, and potential effects – benefits and risks – [and] informed consent should be obtained and documented,” said Dr. Usón, outlining the first recommendation. “That seems common,” she said in the interview, “but when we did the survey, we realize that many patients didn’t [give consent], and the doctors didn’t even ask for it. This is why it’s a very general statement, and it’s our first recommendation. The agreement was 99%!”
The recommendations also look at the optimal settings for performing injections, such as providing a professional and private, well-lighted room, and having a resuscitation kit nearby in case patients faint. Accuracy is important, Dr. Usón said, and imaging, such as ultrasound, should be used where available to ensure accurate injection into the joint. This is an area where further research could be performed, she said, urging young rheumatologists and health professionals to consider this. “Intra-articular therapy is something that you learn and do, but you never really investigate in it,” she said.
One recommendation states that when intra-articular injections are being given to pregnant patients, the safety of injected compound must be considered, both for the mother and for the fetus. There is another recommendation on the need to perform IA injections under aseptic conditions, and another stating that patients should be offered local anesthetics, after explaining the pros and cons.
Special populations of patients are also considered, Dr. Usón said. For example, the guidance advises warning patients with diabetes of the risk of transient glycemia after IA glucocorticoids and the need to monitor their blood glucose levels carefully for a couple of days afterward.
As a rule, “IAT is not a contraindication to people with clotting or bleeding disorders, or taking antithrombotic medications,” she said, unless they are at a high risk of bleeding.
Importantly, the recommendations cover when IAT can be performed after joint replacement surgery (after at least 3 months), and the need to “avoid overuse of injected joints” while also avoiding complete immobilization for at least 24 hours afterward. The recommendations very generally cover re-injections, but not how long intervals between injections should be. When asked about interval duration after her presentation, Dr. Usón said that the usual advice is to give IA injections no more than 2-3 times a year, but it depends on the injectable.
“It wasn’t our intention to review the efficacy and the safety of the different injectables, nor to review the use of IAT in different types of joint diseases,” she said. “We do lack a lot of information, a lot of evidence in this, and I really would hope that new rheumatologists start looking into and start investigating in this topic,” she added.
Recommendations will increase awareness of good clinical practice
“IA injections are commonly administered in the rheumatology setting. This is because [IA injection] is often a useful treatment for acute flare of arthritis, particularly when it is limited to a few joints,” observed Ai Lyn Tan, MD, associate professor and honorary consultant rheumatologist at the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine.
IA injection “also relieves symptoms relatively quickly for patients; however, the response can be variable, and there are side effects associated with IA injections,” Dr. Tan added in an interview.
There is a lack of universally accepted recommendations, Dr. Tan observed, noting that while there might be some local guidelines on how to safely perform IA injections these were often not standardized and were subject to being continually updated to try to improve the experience for patients.
“It is therefore timely to learn about the new EULAR recommendations for IA injections. The advantage of this will be to increase awareness of good clinical practice for performing IA injections.”
Dr. Tan had no relevant conflicts of interest.
SOURCE: EULAR COVID-19 Recommendations. E-congress content available until Sept. 1, 2020.
New EULAR recommendations for the intra-articular (IA) treatment of arthropathies aim to facilitate uniformity and quality of care for this mainstay of rheumatologic practice, according to a report on the new guidance that was presented at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Until now there were no official recommendations on how best to use it in everyday practice. “This is the first time that there’s been a joint effort to develop evidence-based recommendations,” Jacqueline Usón, MD, PhD, associate professor medicine at Rey Juan Carlos University in Madrid, said in an interview. “Everything that we are saying is pretty logical, but it’s nice to see it put in recommendations based on evidence.”
IA therapy has been around for decades and is key for treating adults with a number of different conditions where synovitis, effusion, pain, or all three, are present, such as inflammatory arthritis and osteoarthritis, Dr. Usón observed during her presentation.
“Today, commonly used injectables are not only corticosteroids but also local anesthetics, hyaluronic acid, blood products, and maybe pharmaceuticals,” she said, adding that “there is a wide variation in the way intra-articular therapies are used and delivered to patients.” Health professionals also have very different views and habits depending on geographic locations and health care systems, she observed. Ironing out the variation was one of the main objectives of the recommendations.
As one of the two conveners of the EULAR task force behind the recommendations, Dr. Usón, herself a rheumatologist at University Hospital of Móstoles, pointed out that the task force brought together a range of specialties – rheumatologists, orthopedic surgeons, radiologists, nuclear medicine specialists, among others, as well as patients – to ensure that the best advice could be given.
The task force followed EULAR standard operating procedures for developing recommendations, with discussion groups, systematic literature reviews, and Delphi technique-based consensus all being employed. The literature search considered publications from 1946 up until 2019.
“We agreed on the need for more background information from health professionals and patients, so we developed two surveys: One for health professionals with 160 items, [for which] we obtained 186 responses from 26 countries; and the patient survey was made up of 44 items, translated into 10 different languages, and we obtained 200 responses,” she said.
The results of the systematic literature review and surveys were used to help form expert consensus, leading to 5 overarching principles and 11 recommendations that look at before, during, and after intra-articular therapy.
Five overarching principles
The first overarching principle recognizes the widespread use of IA therapies and that their use is specific to the disease that is being treated and “may not be interchangeable across indications,” Dr. Usón said. The second principle concerns improving patient-centered outcomes, which are “those that are relevant to the patient,” and include the benefits, harms, preferences, or implications for self-management.
“Contextual factors are important and contribute to the effect of IAT [intra-articular treatment],” she said, discussing the third principle. “These include effective communication, patient expectations, or settings [where the procedure takes place]. In addition, one should take into account that the route of delivery has in itself a placebo effect. We found that in different RCTs [randomized controlled trials], the pooled placebo effect of IA saline is moderate to large.”
The fourth principle looks at ensuring that patients and clinicians make an informed and shared decision, which is again highlighted by the first recommendation. The fifth, and last, overarching principle acknowledges that IA injections may be given by a range of health care professionals.
Advice for before, during, and after injection
Patients need to be “fully informed of the nature of the procedure, the injectable used, and potential effects – benefits and risks – [and] informed consent should be obtained and documented,” said Dr. Usón, outlining the first recommendation. “That seems common,” she said in the interview, “but when we did the survey, we realize that many patients didn’t [give consent], and the doctors didn’t even ask for it. This is why it’s a very general statement, and it’s our first recommendation. The agreement was 99%!”
The recommendations also look at the optimal settings for performing injections, such as providing a professional and private, well-lighted room, and having a resuscitation kit nearby in case patients faint. Accuracy is important, Dr. Usón said, and imaging, such as ultrasound, should be used where available to ensure accurate injection into the joint. This is an area where further research could be performed, she said, urging young rheumatologists and health professionals to consider this. “Intra-articular therapy is something that you learn and do, but you never really investigate in it,” she said.
One recommendation states that when intra-articular injections are being given to pregnant patients, the safety of injected compound must be considered, both for the mother and for the fetus. There is another recommendation on the need to perform IA injections under aseptic conditions, and another stating that patients should be offered local anesthetics, after explaining the pros and cons.
Special populations of patients are also considered, Dr. Usón said. For example, the guidance advises warning patients with diabetes of the risk of transient glycemia after IA glucocorticoids and the need to monitor their blood glucose levels carefully for a couple of days afterward.
As a rule, “IAT is not a contraindication to people with clotting or bleeding disorders, or taking antithrombotic medications,” she said, unless they are at a high risk of bleeding.
Importantly, the recommendations cover when IAT can be performed after joint replacement surgery (after at least 3 months), and the need to “avoid overuse of injected joints” while also avoiding complete immobilization for at least 24 hours afterward. The recommendations very generally cover re-injections, but not how long intervals between injections should be. When asked about interval duration after her presentation, Dr. Usón said that the usual advice is to give IA injections no more than 2-3 times a year, but it depends on the injectable.
“It wasn’t our intention to review the efficacy and the safety of the different injectables, nor to review the use of IAT in different types of joint diseases,” she said. “We do lack a lot of information, a lot of evidence in this, and I really would hope that new rheumatologists start looking into and start investigating in this topic,” she added.
Recommendations will increase awareness of good clinical practice
“IA injections are commonly administered in the rheumatology setting. This is because [IA injection] is often a useful treatment for acute flare of arthritis, particularly when it is limited to a few joints,” observed Ai Lyn Tan, MD, associate professor and honorary consultant rheumatologist at the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine.
IA injection “also relieves symptoms relatively quickly for patients; however, the response can be variable, and there are side effects associated with IA injections,” Dr. Tan added in an interview.
There is a lack of universally accepted recommendations, Dr. Tan observed, noting that while there might be some local guidelines on how to safely perform IA injections these were often not standardized and were subject to being continually updated to try to improve the experience for patients.
“It is therefore timely to learn about the new EULAR recommendations for IA injections. The advantage of this will be to increase awareness of good clinical practice for performing IA injections.”
Dr. Tan had no relevant conflicts of interest.
SOURCE: EULAR COVID-19 Recommendations. E-congress content available until Sept. 1, 2020.
New EULAR recommendations for the intra-articular (IA) treatment of arthropathies aim to facilitate uniformity and quality of care for this mainstay of rheumatologic practice, according to a report on the new guidance that was presented at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Until now there were no official recommendations on how best to use it in everyday practice. “This is the first time that there’s been a joint effort to develop evidence-based recommendations,” Jacqueline Usón, MD, PhD, associate professor medicine at Rey Juan Carlos University in Madrid, said in an interview. “Everything that we are saying is pretty logical, but it’s nice to see it put in recommendations based on evidence.”
IA therapy has been around for decades and is key for treating adults with a number of different conditions where synovitis, effusion, pain, or all three, are present, such as inflammatory arthritis and osteoarthritis, Dr. Usón observed during her presentation.
“Today, commonly used injectables are not only corticosteroids but also local anesthetics, hyaluronic acid, blood products, and maybe pharmaceuticals,” she said, adding that “there is a wide variation in the way intra-articular therapies are used and delivered to patients.” Health professionals also have very different views and habits depending on geographic locations and health care systems, she observed. Ironing out the variation was one of the main objectives of the recommendations.
As one of the two conveners of the EULAR task force behind the recommendations, Dr. Usón, herself a rheumatologist at University Hospital of Móstoles, pointed out that the task force brought together a range of specialties – rheumatologists, orthopedic surgeons, radiologists, nuclear medicine specialists, among others, as well as patients – to ensure that the best advice could be given.
The task force followed EULAR standard operating procedures for developing recommendations, with discussion groups, systematic literature reviews, and Delphi technique-based consensus all being employed. The literature search considered publications from 1946 up until 2019.
“We agreed on the need for more background information from health professionals and patients, so we developed two surveys: One for health professionals with 160 items, [for which] we obtained 186 responses from 26 countries; and the patient survey was made up of 44 items, translated into 10 different languages, and we obtained 200 responses,” she said.
The results of the systematic literature review and surveys were used to help form expert consensus, leading to 5 overarching principles and 11 recommendations that look at before, during, and after intra-articular therapy.
Five overarching principles
The first overarching principle recognizes the widespread use of IA therapies and that their use is specific to the disease that is being treated and “may not be interchangeable across indications,” Dr. Usón said. The second principle concerns improving patient-centered outcomes, which are “those that are relevant to the patient,” and include the benefits, harms, preferences, or implications for self-management.
“Contextual factors are important and contribute to the effect of IAT [intra-articular treatment],” she said, discussing the third principle. “These include effective communication, patient expectations, or settings [where the procedure takes place]. In addition, one should take into account that the route of delivery has in itself a placebo effect. We found that in different RCTs [randomized controlled trials], the pooled placebo effect of IA saline is moderate to large.”
The fourth principle looks at ensuring that patients and clinicians make an informed and shared decision, which is again highlighted by the first recommendation. The fifth, and last, overarching principle acknowledges that IA injections may be given by a range of health care professionals.
Advice for before, during, and after injection
Patients need to be “fully informed of the nature of the procedure, the injectable used, and potential effects – benefits and risks – [and] informed consent should be obtained and documented,” said Dr. Usón, outlining the first recommendation. “That seems common,” she said in the interview, “but when we did the survey, we realize that many patients didn’t [give consent], and the doctors didn’t even ask for it. This is why it’s a very general statement, and it’s our first recommendation. The agreement was 99%!”
The recommendations also look at the optimal settings for performing injections, such as providing a professional and private, well-lighted room, and having a resuscitation kit nearby in case patients faint. Accuracy is important, Dr. Usón said, and imaging, such as ultrasound, should be used where available to ensure accurate injection into the joint. This is an area where further research could be performed, she said, urging young rheumatologists and health professionals to consider this. “Intra-articular therapy is something that you learn and do, but you never really investigate in it,” she said.
One recommendation states that when intra-articular injections are being given to pregnant patients, the safety of injected compound must be considered, both for the mother and for the fetus. There is another recommendation on the need to perform IA injections under aseptic conditions, and another stating that patients should be offered local anesthetics, after explaining the pros and cons.
Special populations of patients are also considered, Dr. Usón said. For example, the guidance advises warning patients with diabetes of the risk of transient glycemia after IA glucocorticoids and the need to monitor their blood glucose levels carefully for a couple of days afterward.
As a rule, “IAT is not a contraindication to people with clotting or bleeding disorders, or taking antithrombotic medications,” she said, unless they are at a high risk of bleeding.
Importantly, the recommendations cover when IAT can be performed after joint replacement surgery (after at least 3 months), and the need to “avoid overuse of injected joints” while also avoiding complete immobilization for at least 24 hours afterward. The recommendations very generally cover re-injections, but not how long intervals between injections should be. When asked about interval duration after her presentation, Dr. Usón said that the usual advice is to give IA injections no more than 2-3 times a year, but it depends on the injectable.
“It wasn’t our intention to review the efficacy and the safety of the different injectables, nor to review the use of IAT in different types of joint diseases,” she said. “We do lack a lot of information, a lot of evidence in this, and I really would hope that new rheumatologists start looking into and start investigating in this topic,” she added.
Recommendations will increase awareness of good clinical practice
“IA injections are commonly administered in the rheumatology setting. This is because [IA injection] is often a useful treatment for acute flare of arthritis, particularly when it is limited to a few joints,” observed Ai Lyn Tan, MD, associate professor and honorary consultant rheumatologist at the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine.
IA injection “also relieves symptoms relatively quickly for patients; however, the response can be variable, and there are side effects associated with IA injections,” Dr. Tan added in an interview.
There is a lack of universally accepted recommendations, Dr. Tan observed, noting that while there might be some local guidelines on how to safely perform IA injections these were often not standardized and were subject to being continually updated to try to improve the experience for patients.
“It is therefore timely to learn about the new EULAR recommendations for IA injections. The advantage of this will be to increase awareness of good clinical practice for performing IA injections.”
Dr. Tan had no relevant conflicts of interest.
SOURCE: EULAR COVID-19 Recommendations. E-congress content available until Sept. 1, 2020.
FROM THE EULAR 2020 E-CONGRESS
Belimumab safely improved renal function in lupus nephritis patients
compared with control patients who only received standard therapy, in a randomized, multicenter trial with 446 evaluable patients, a finding that may help extend this treatment to a new group of lupus patients.
“The largest” treatment study of lupus nephritis reported to date showed that belimumab, approved by the Food and Drug Administration in 2011 for treating patients with systemic lupus erythematosus (SLE), administered at a standard dosage of 10 mg intravenously every 4 weeks, “significantly improved multiple lupus nephritis renal responses versus standard therapy alone while maintaining an acceptable safety profile,” Richard A. Furie, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
The study’s primary endpoint was a composite measure that Dr. Furie and associates called the Primary Endpoint Renal Response, which required patients to have achieved a urinary protein-to-creatinine ratio of 0.7 or less (compared with an enrollment level of 1.0 or greater), an estimated glomerular filtration rate (eGFR) of at least 60 mL/min/1.73 kg/m2 and no more than 20% below its preflare level, and continuation on the assigned treatment regimen. After 104 weeks on this treatment, which followed a 60-day induction phase that included treatment with a high-dose glucocorticoid, the percentages of patients who met the Primary Endpoint Renal Response criteria were 32% in the control arm who received standard treatment at the discretion of their treating clinicians plus placebo infusions and 43% in patients who received belimumab infusions in addition to their standard care. This calculated out to a 55% relative increase in this response with belimumab, a statistically significant result, reported Dr. Furie, professor of medicine at Hofstra University, Hempstead, N.Y., and chief of rheumatology at Northwell Health in Manhasset, N.Y.
Patients who received belimumab also had similar and statistically significant levels of improvement for several secondary endpoints, including one called Complete Renal Response, which required a protein-to-creatinine ratio of no greater than 0.5, an eGFR of at least 90 mL/min per 1.73 kg/m2 and no more than 10% below its preflare level, and maintaining the assigned treatment. The Complete Renal Response after 104 weeks was 20% among control patients and 30% among those maintained on belimumab, a 74% relative improvement that was statistically significant. The total percentage of patients with any renal-related event after 104 weeks was 28% among the control patients and 16% among those who received belimumab, a statistically significant difference.
“The fact that the primary and all key secondary endpoints were successfully attained is a major accomplishment in lupus nephritis as well as in any SLE study,” Dr. Furie said in an interview. The study’s 2-year design “provided insight into the durability of the response,” and the steady divergence of the endpoint events in the two study arms beginning after about 24 weeks into the randomized phase “provided data regarding the rapidity of onset of action.” Collectively, the endpoints “mimic our real-life treatment goals: reduce disease activity, prevent flares, preserve renal function, lower steroid treatment, and do it all safely,” he concluded.
Results confirm benefit to subset of patients
“Belimumab is a safe and effective treatment for a significant subset of patients with lupus. We already knew that. Now we have even more confirmation,” commented Joan T. Merrill, MD, a professor of medicine at the University of Oklahoma Health Sciences Center and a rheumatologist who specializes in SLE at the Oklahoma Medical Research Foundation, both in Oklahoma City. “There have already been at least four international trials demonstrating belimumab’s efficacy in general lupus. Some patients in these earlier trials had nephritis, so it should not be surprising to see similar results in a trial restricted to patients with active nephritis, given the drug’s mechanism of action. Belimumab has repeatedly shown early and sustained benefits above what background treatments achieve, and belimumab has also proven to be safe to add to standard-of-care treatments,” she said in an interview.
The BLISS-LN (Efficacy and Safety of Belimumab in Patients With Active Lupus Nephritis) study enrolled patients at any of 118 centers in 20 countries, including the United States. All patients enrolled in the trial were adults with biopsy-confirmed, clinically active lupus nephritis and a urinary protein-to-creatinine ratio of at least 1.0, and need for induction therapy. The 60-day induction run-in phase began with high-dose glucocorticoids plus either cyclophosphamide or mycophenolate mofetil (CellCept), followed by maintenance on low-dose glucocorticoids and either azathioprine or mycophenolate mofetil. Nearly three-quarters of patients received mycophenolate mofetil–based induction. Once treatment with either belimumab or placebo began in the study’s main phase, the glucocorticoid dosage had to drop with tapering to no more than 10 mg/day within 24 weeks or the patient was considered a treatment failure.
Thoughts on current and future use of belimumab
The current labeled indication for belimumab is for “treatment of patients aged 5 years and older with active, autoantibody-positive systemic lupus erythematosus who are receiving standard therapy,” an inclusive SLE population, but the label also adds this caveat: “Limitations of use: The efficacy of Benlysta has not been evaluated in patients with severe active lupus nephritis or severe active central nervous system lupus.” According to Dr. Furie’s report, GlaxoSmithKline, the company that markets belimumab, plans to seek a labeled indication for lupus nephritis for the drug during 2020.
“I doubt the drug is widely used as yet in clinical practice for lupus nephritis,” although it is being prescribed to selected SLE patients in current, routine practice, said Dr. Merrill, a coinvestigator on some belimumab studies. What also remains unknown is the efficacy of belimumab monotherapy. “We don’t know which subset of patients might benefit from belimumab alone,” she noted. Nor is it known whether belimumab treatment of patients with SLE but without lupus nephritis will forestall later development of lupus nephritis.
“With the introduction of the subcutaneous formulation a few years ago, there has been greater belimumab use” overall in patients with SLE, said Dr. Furie, and with a safety and efficacy record now established in five separate, reported studies in addition to the new BLISS-LN study: BLISS-52, BLISS-76, BLISS-SC, BLISS-NE ASIA, and PLUTO. “The pivotal studies [BLISS-52 and BLISS-76] were done in patients with SLE but without nephritis in need of aggressive induction therapy. About 15% of the trial cohorts had low-level renal involvement,” and post hoc analyses suggested that the benefit in those patients was similar to patients without renal involvement, which led to the BLISS-LN study. “In theory, no SLE patients with high-level nephritis should be on belimumab at this time,” based on its labeling, although some SLE patients with low-level renal disease may now receive the drug because they also have other affected organs, such as skin and joints, Dr. Furie said.
“These are encouraging results,” commented George K. Bertsias, MD, a rheumatologist and SLE specialist at the University of Crete in Heraklion, Greece. He particularly cited the “significant effect from add-on belimumab” on top of treatment with mycophenolate mofetil, an “established and effective treatment for lupus nephritis. The data provide additional evidence for the efficacy of belimumab in SLE, and also in lupus nephritis,” he said in an interview, and “having an official labeled indication for active nephritis will enhance use of the drug” in such patients. “Considering the favorable effects of the drug on SLE, especially preventing major flares, and on lupus nephritis it is possible that the drug will be particularly suitable for SLE patients who are at high risk for developing lupus nephritis, although such an effect remains to be determined.” Until now, belimumab has generally been prescribed to SLE patients who have disease manifestations in organs outside of the kidneys, he noted.
BLISS-LN was sponsored by GlaxoSmithKline. Dr. Furie is a consultant to and has received research funding from GlaxoSmithKline, and several of the study’s coauthors are employees of the company. Dr. Merrill has been a consultant to GlaxoSmithKline as well as to several other companies and has been a coinvestigator on belimumab studies. Dr. Bertsias has been a consultant to Novartis and has received research funding from GlaxoSmithKline.
SOURCE: Furie RA et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:103, Abstract OP0164.
compared with control patients who only received standard therapy, in a randomized, multicenter trial with 446 evaluable patients, a finding that may help extend this treatment to a new group of lupus patients.
“The largest” treatment study of lupus nephritis reported to date showed that belimumab, approved by the Food and Drug Administration in 2011 for treating patients with systemic lupus erythematosus (SLE), administered at a standard dosage of 10 mg intravenously every 4 weeks, “significantly improved multiple lupus nephritis renal responses versus standard therapy alone while maintaining an acceptable safety profile,” Richard A. Furie, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
The study’s primary endpoint was a composite measure that Dr. Furie and associates called the Primary Endpoint Renal Response, which required patients to have achieved a urinary protein-to-creatinine ratio of 0.7 or less (compared with an enrollment level of 1.0 or greater), an estimated glomerular filtration rate (eGFR) of at least 60 mL/min/1.73 kg/m2 and no more than 20% below its preflare level, and continuation on the assigned treatment regimen. After 104 weeks on this treatment, which followed a 60-day induction phase that included treatment with a high-dose glucocorticoid, the percentages of patients who met the Primary Endpoint Renal Response criteria were 32% in the control arm who received standard treatment at the discretion of their treating clinicians plus placebo infusions and 43% in patients who received belimumab infusions in addition to their standard care. This calculated out to a 55% relative increase in this response with belimumab, a statistically significant result, reported Dr. Furie, professor of medicine at Hofstra University, Hempstead, N.Y., and chief of rheumatology at Northwell Health in Manhasset, N.Y.
Patients who received belimumab also had similar and statistically significant levels of improvement for several secondary endpoints, including one called Complete Renal Response, which required a protein-to-creatinine ratio of no greater than 0.5, an eGFR of at least 90 mL/min per 1.73 kg/m2 and no more than 10% below its preflare level, and maintaining the assigned treatment. The Complete Renal Response after 104 weeks was 20% among control patients and 30% among those maintained on belimumab, a 74% relative improvement that was statistically significant. The total percentage of patients with any renal-related event after 104 weeks was 28% among the control patients and 16% among those who received belimumab, a statistically significant difference.
“The fact that the primary and all key secondary endpoints were successfully attained is a major accomplishment in lupus nephritis as well as in any SLE study,” Dr. Furie said in an interview. The study’s 2-year design “provided insight into the durability of the response,” and the steady divergence of the endpoint events in the two study arms beginning after about 24 weeks into the randomized phase “provided data regarding the rapidity of onset of action.” Collectively, the endpoints “mimic our real-life treatment goals: reduce disease activity, prevent flares, preserve renal function, lower steroid treatment, and do it all safely,” he concluded.
Results confirm benefit to subset of patients
“Belimumab is a safe and effective treatment for a significant subset of patients with lupus. We already knew that. Now we have even more confirmation,” commented Joan T. Merrill, MD, a professor of medicine at the University of Oklahoma Health Sciences Center and a rheumatologist who specializes in SLE at the Oklahoma Medical Research Foundation, both in Oklahoma City. “There have already been at least four international trials demonstrating belimumab’s efficacy in general lupus. Some patients in these earlier trials had nephritis, so it should not be surprising to see similar results in a trial restricted to patients with active nephritis, given the drug’s mechanism of action. Belimumab has repeatedly shown early and sustained benefits above what background treatments achieve, and belimumab has also proven to be safe to add to standard-of-care treatments,” she said in an interview.
The BLISS-LN (Efficacy and Safety of Belimumab in Patients With Active Lupus Nephritis) study enrolled patients at any of 118 centers in 20 countries, including the United States. All patients enrolled in the trial were adults with biopsy-confirmed, clinically active lupus nephritis and a urinary protein-to-creatinine ratio of at least 1.0, and need for induction therapy. The 60-day induction run-in phase began with high-dose glucocorticoids plus either cyclophosphamide or mycophenolate mofetil (CellCept), followed by maintenance on low-dose glucocorticoids and either azathioprine or mycophenolate mofetil. Nearly three-quarters of patients received mycophenolate mofetil–based induction. Once treatment with either belimumab or placebo began in the study’s main phase, the glucocorticoid dosage had to drop with tapering to no more than 10 mg/day within 24 weeks or the patient was considered a treatment failure.
Thoughts on current and future use of belimumab
The current labeled indication for belimumab is for “treatment of patients aged 5 years and older with active, autoantibody-positive systemic lupus erythematosus who are receiving standard therapy,” an inclusive SLE population, but the label also adds this caveat: “Limitations of use: The efficacy of Benlysta has not been evaluated in patients with severe active lupus nephritis or severe active central nervous system lupus.” According to Dr. Furie’s report, GlaxoSmithKline, the company that markets belimumab, plans to seek a labeled indication for lupus nephritis for the drug during 2020.
“I doubt the drug is widely used as yet in clinical practice for lupus nephritis,” although it is being prescribed to selected SLE patients in current, routine practice, said Dr. Merrill, a coinvestigator on some belimumab studies. What also remains unknown is the efficacy of belimumab monotherapy. “We don’t know which subset of patients might benefit from belimumab alone,” she noted. Nor is it known whether belimumab treatment of patients with SLE but without lupus nephritis will forestall later development of lupus nephritis.
“With the introduction of the subcutaneous formulation a few years ago, there has been greater belimumab use” overall in patients with SLE, said Dr. Furie, and with a safety and efficacy record now established in five separate, reported studies in addition to the new BLISS-LN study: BLISS-52, BLISS-76, BLISS-SC, BLISS-NE ASIA, and PLUTO. “The pivotal studies [BLISS-52 and BLISS-76] were done in patients with SLE but without nephritis in need of aggressive induction therapy. About 15% of the trial cohorts had low-level renal involvement,” and post hoc analyses suggested that the benefit in those patients was similar to patients without renal involvement, which led to the BLISS-LN study. “In theory, no SLE patients with high-level nephritis should be on belimumab at this time,” based on its labeling, although some SLE patients with low-level renal disease may now receive the drug because they also have other affected organs, such as skin and joints, Dr. Furie said.
“These are encouraging results,” commented George K. Bertsias, MD, a rheumatologist and SLE specialist at the University of Crete in Heraklion, Greece. He particularly cited the “significant effect from add-on belimumab” on top of treatment with mycophenolate mofetil, an “established and effective treatment for lupus nephritis. The data provide additional evidence for the efficacy of belimumab in SLE, and also in lupus nephritis,” he said in an interview, and “having an official labeled indication for active nephritis will enhance use of the drug” in such patients. “Considering the favorable effects of the drug on SLE, especially preventing major flares, and on lupus nephritis it is possible that the drug will be particularly suitable for SLE patients who are at high risk for developing lupus nephritis, although such an effect remains to be determined.” Until now, belimumab has generally been prescribed to SLE patients who have disease manifestations in organs outside of the kidneys, he noted.
BLISS-LN was sponsored by GlaxoSmithKline. Dr. Furie is a consultant to and has received research funding from GlaxoSmithKline, and several of the study’s coauthors are employees of the company. Dr. Merrill has been a consultant to GlaxoSmithKline as well as to several other companies and has been a coinvestigator on belimumab studies. Dr. Bertsias has been a consultant to Novartis and has received research funding from GlaxoSmithKline.
SOURCE: Furie RA et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:103, Abstract OP0164.
compared with control patients who only received standard therapy, in a randomized, multicenter trial with 446 evaluable patients, a finding that may help extend this treatment to a new group of lupus patients.
“The largest” treatment study of lupus nephritis reported to date showed that belimumab, approved by the Food and Drug Administration in 2011 for treating patients with systemic lupus erythematosus (SLE), administered at a standard dosage of 10 mg intravenously every 4 weeks, “significantly improved multiple lupus nephritis renal responses versus standard therapy alone while maintaining an acceptable safety profile,” Richard A. Furie, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
The study’s primary endpoint was a composite measure that Dr. Furie and associates called the Primary Endpoint Renal Response, which required patients to have achieved a urinary protein-to-creatinine ratio of 0.7 or less (compared with an enrollment level of 1.0 or greater), an estimated glomerular filtration rate (eGFR) of at least 60 mL/min/1.73 kg/m2 and no more than 20% below its preflare level, and continuation on the assigned treatment regimen. After 104 weeks on this treatment, which followed a 60-day induction phase that included treatment with a high-dose glucocorticoid, the percentages of patients who met the Primary Endpoint Renal Response criteria were 32% in the control arm who received standard treatment at the discretion of their treating clinicians plus placebo infusions and 43% in patients who received belimumab infusions in addition to their standard care. This calculated out to a 55% relative increase in this response with belimumab, a statistically significant result, reported Dr. Furie, professor of medicine at Hofstra University, Hempstead, N.Y., and chief of rheumatology at Northwell Health in Manhasset, N.Y.
Patients who received belimumab also had similar and statistically significant levels of improvement for several secondary endpoints, including one called Complete Renal Response, which required a protein-to-creatinine ratio of no greater than 0.5, an eGFR of at least 90 mL/min per 1.73 kg/m2 and no more than 10% below its preflare level, and maintaining the assigned treatment. The Complete Renal Response after 104 weeks was 20% among control patients and 30% among those maintained on belimumab, a 74% relative improvement that was statistically significant. The total percentage of patients with any renal-related event after 104 weeks was 28% among the control patients and 16% among those who received belimumab, a statistically significant difference.
“The fact that the primary and all key secondary endpoints were successfully attained is a major accomplishment in lupus nephritis as well as in any SLE study,” Dr. Furie said in an interview. The study’s 2-year design “provided insight into the durability of the response,” and the steady divergence of the endpoint events in the two study arms beginning after about 24 weeks into the randomized phase “provided data regarding the rapidity of onset of action.” Collectively, the endpoints “mimic our real-life treatment goals: reduce disease activity, prevent flares, preserve renal function, lower steroid treatment, and do it all safely,” he concluded.
Results confirm benefit to subset of patients
“Belimumab is a safe and effective treatment for a significant subset of patients with lupus. We already knew that. Now we have even more confirmation,” commented Joan T. Merrill, MD, a professor of medicine at the University of Oklahoma Health Sciences Center and a rheumatologist who specializes in SLE at the Oklahoma Medical Research Foundation, both in Oklahoma City. “There have already been at least four international trials demonstrating belimumab’s efficacy in general lupus. Some patients in these earlier trials had nephritis, so it should not be surprising to see similar results in a trial restricted to patients with active nephritis, given the drug’s mechanism of action. Belimumab has repeatedly shown early and sustained benefits above what background treatments achieve, and belimumab has also proven to be safe to add to standard-of-care treatments,” she said in an interview.
The BLISS-LN (Efficacy and Safety of Belimumab in Patients With Active Lupus Nephritis) study enrolled patients at any of 118 centers in 20 countries, including the United States. All patients enrolled in the trial were adults with biopsy-confirmed, clinically active lupus nephritis and a urinary protein-to-creatinine ratio of at least 1.0, and need for induction therapy. The 60-day induction run-in phase began with high-dose glucocorticoids plus either cyclophosphamide or mycophenolate mofetil (CellCept), followed by maintenance on low-dose glucocorticoids and either azathioprine or mycophenolate mofetil. Nearly three-quarters of patients received mycophenolate mofetil–based induction. Once treatment with either belimumab or placebo began in the study’s main phase, the glucocorticoid dosage had to drop with tapering to no more than 10 mg/day within 24 weeks or the patient was considered a treatment failure.
Thoughts on current and future use of belimumab
The current labeled indication for belimumab is for “treatment of patients aged 5 years and older with active, autoantibody-positive systemic lupus erythematosus who are receiving standard therapy,” an inclusive SLE population, but the label also adds this caveat: “Limitations of use: The efficacy of Benlysta has not been evaluated in patients with severe active lupus nephritis or severe active central nervous system lupus.” According to Dr. Furie’s report, GlaxoSmithKline, the company that markets belimumab, plans to seek a labeled indication for lupus nephritis for the drug during 2020.
“I doubt the drug is widely used as yet in clinical practice for lupus nephritis,” although it is being prescribed to selected SLE patients in current, routine practice, said Dr. Merrill, a coinvestigator on some belimumab studies. What also remains unknown is the efficacy of belimumab monotherapy. “We don’t know which subset of patients might benefit from belimumab alone,” she noted. Nor is it known whether belimumab treatment of patients with SLE but without lupus nephritis will forestall later development of lupus nephritis.
“With the introduction of the subcutaneous formulation a few years ago, there has been greater belimumab use” overall in patients with SLE, said Dr. Furie, and with a safety and efficacy record now established in five separate, reported studies in addition to the new BLISS-LN study: BLISS-52, BLISS-76, BLISS-SC, BLISS-NE ASIA, and PLUTO. “The pivotal studies [BLISS-52 and BLISS-76] were done in patients with SLE but without nephritis in need of aggressive induction therapy. About 15% of the trial cohorts had low-level renal involvement,” and post hoc analyses suggested that the benefit in those patients was similar to patients without renal involvement, which led to the BLISS-LN study. “In theory, no SLE patients with high-level nephritis should be on belimumab at this time,” based on its labeling, although some SLE patients with low-level renal disease may now receive the drug because they also have other affected organs, such as skin and joints, Dr. Furie said.
“These are encouraging results,” commented George K. Bertsias, MD, a rheumatologist and SLE specialist at the University of Crete in Heraklion, Greece. He particularly cited the “significant effect from add-on belimumab” on top of treatment with mycophenolate mofetil, an “established and effective treatment for lupus nephritis. The data provide additional evidence for the efficacy of belimumab in SLE, and also in lupus nephritis,” he said in an interview, and “having an official labeled indication for active nephritis will enhance use of the drug” in such patients. “Considering the favorable effects of the drug on SLE, especially preventing major flares, and on lupus nephritis it is possible that the drug will be particularly suitable for SLE patients who are at high risk for developing lupus nephritis, although such an effect remains to be determined.” Until now, belimumab has generally been prescribed to SLE patients who have disease manifestations in organs outside of the kidneys, he noted.
BLISS-LN was sponsored by GlaxoSmithKline. Dr. Furie is a consultant to and has received research funding from GlaxoSmithKline, and several of the study’s coauthors are employees of the company. Dr. Merrill has been a consultant to GlaxoSmithKline as well as to several other companies and has been a coinvestigator on belimumab studies. Dr. Bertsias has been a consultant to Novartis and has received research funding from GlaxoSmithKline.
SOURCE: Furie RA et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:103, Abstract OP0164.
FROM THE EULAR 2020 E-CONGRESS
New registry focuses on rheumatic immune-related AEs of cancer therapy
Its first findings were reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“We have limited knowledge on the interrelationships between malignant and rheumatic diseases on both the clinical and molecular level, and we have a large unmet need for management guidelines in the case of the coincidence of both disease entities,” noted lead author Karolina Benesova, MD, of the department of hematology, oncology, and rheumatology at University Hospital Heidelberg (Germany).
The TRheuMa registry – Therapy-Induced Rheumatic Symptoms in Patients with Malignancy – is one of three registries in a multicenter observational project exploring various contexts between malignant and rheumatic diseases. Over its first 22 months, the registry recruited 69 patients having rheumatic symptoms as a result of immune checkpoint inhibitor therapy or other cancer therapies.
Registry findings
The largest shares of patients had non–small cell lung cancer (38%) or melanoma (33%), Dr. Benesova reported. The immune checkpoint inhibitors most commonly received were pembrolizumab (Keytruda), nivolumab (Opdivo), and ipilimumab (Yervoy).
The immune-related adverse events usually presented with symptoms of de novo spondyloarthritis or psoriatic arthritis (42%), late-onset RA (17%), or polymyalgia rheumatica (14%). But 16% of the patients were experiencing a flare of a preexisting rheumatic and musculoskeletal disease.
Laboratory findings differed somewhat from those of classical rheumatic and musculoskeletal diseases, according to Dr. Benesova. Specific findings were rare; in particular, most patients did not have detectable autoantibodies. However, 76% had an elevated C-reactive protein level and 39% had an elevated soluble CD25 level. In addition, nearly all patients (96%) undergoing joint ultrasound had pathologic findings.
“Based on our experiences from interdisciplinary care together with our local oncologists, we have developed a therapeutic algorithm for rheumatic immune-related adverse events,” she reported, noting that the algorithm is consistent with recently published recommendations in this area.
The large majority of patients were adequately treated with prednisone at a dose greater than 10 mg (40%) or at a dose of 10 mg or less with or without an NSAID (40%), while some received NSAID monotherapy (14%).
“We have a growing proportion of patients on conventional or biological [disease-modifying antirheumatic drugs],” Dr. Benesova noted. “These are mostly patients with preexisting rheumatic and musculoskeletal disease or highly suspected de novo classical rheumatic and musculoskeletal disease under checkpoint inhibitor therapy.”
Patients with melanoma having a rheumatic immune-related adverse event had a better response to their therapy than historical counterparts who did not have such events: 39% of the former had a complete response, relative to merely 4% of the latter.
Only a small proportion of patients overall (9%) had to discontinue immune checkpoint inhibitor therapy because of their adverse event, and some of them may be eligible for rechallenge if their cancer progresses, Dr. Benesova noted.
“There is still a lot to be done,” she stated, such as better elucidating the nature of these adverse events [whether transient side effects or a triggering of chronic rheumatic and musculoskeletal diseases], the need for a defensive treatment strategy, and the advisability of closer monitoring of high-risk patients given immune checkpoint inhibitors. “We are aiming at solving these questions in the next few years,” she concluded.
Findings in context
“Registries are important to gain prospective data on patient outcomes,” Sabina Sandigursky, MD, an instructor in the division of rheumatology at the Laura and Isaac Perlmutter Cancer Center at New York University, commented in an interview. “One must be careful, while interpreting these data, especially since they are not randomized, controlled trials.”
Patterns may differ at other centers, too, she pointed out. “The German registry reported a predominance of spondyloarthritis-like disease; however, our patients have a predominance of small-joint involvement. It is unclear what accounts for this difference.”
Individual institutions in North America are similarly collecting data on this patient population, with efforts underway to compile those data to provide a larger picture, according to Dr. Sandigursky.
“Many of the syndromes that we consider to be rheumatic immune-related adverse events have been well described by groups from the U.S., Canada, Australia, and European Union,” she concluded. “From this registry, we can observe how patients are being treated in real time since this information is largely consensus based.”
The study did not receive any specific funding. Dr. Benesova disclosed grant/research support from AbbVie, Novartis, Rheumaliga Baden-Wurttemberg, and the University of Heidelberg, and consultancies, speaker fees, and/or travel reimbursements from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Medac, Merck Sharp & Dohme, Mundipharma, Novartis, Pfizer, Roche, and UCB. Some of her coauthors also disclosed financial relationships with industry. Dr. Sandigursky disclosed having no relevant conflicts of interest.
SOURCE: Benesova K et al. Ann Rheum Dis 2020;79[suppl 1]:168-9, Abstract OP0270.
Its first findings were reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“We have limited knowledge on the interrelationships between malignant and rheumatic diseases on both the clinical and molecular level, and we have a large unmet need for management guidelines in the case of the coincidence of both disease entities,” noted lead author Karolina Benesova, MD, of the department of hematology, oncology, and rheumatology at University Hospital Heidelberg (Germany).
The TRheuMa registry – Therapy-Induced Rheumatic Symptoms in Patients with Malignancy – is one of three registries in a multicenter observational project exploring various contexts between malignant and rheumatic diseases. Over its first 22 months, the registry recruited 69 patients having rheumatic symptoms as a result of immune checkpoint inhibitor therapy or other cancer therapies.
Registry findings
The largest shares of patients had non–small cell lung cancer (38%) or melanoma (33%), Dr. Benesova reported. The immune checkpoint inhibitors most commonly received were pembrolizumab (Keytruda), nivolumab (Opdivo), and ipilimumab (Yervoy).
The immune-related adverse events usually presented with symptoms of de novo spondyloarthritis or psoriatic arthritis (42%), late-onset RA (17%), or polymyalgia rheumatica (14%). But 16% of the patients were experiencing a flare of a preexisting rheumatic and musculoskeletal disease.
Laboratory findings differed somewhat from those of classical rheumatic and musculoskeletal diseases, according to Dr. Benesova. Specific findings were rare; in particular, most patients did not have detectable autoantibodies. However, 76% had an elevated C-reactive protein level and 39% had an elevated soluble CD25 level. In addition, nearly all patients (96%) undergoing joint ultrasound had pathologic findings.
“Based on our experiences from interdisciplinary care together with our local oncologists, we have developed a therapeutic algorithm for rheumatic immune-related adverse events,” she reported, noting that the algorithm is consistent with recently published recommendations in this area.
The large majority of patients were adequately treated with prednisone at a dose greater than 10 mg (40%) or at a dose of 10 mg or less with or without an NSAID (40%), while some received NSAID monotherapy (14%).
“We have a growing proportion of patients on conventional or biological [disease-modifying antirheumatic drugs],” Dr. Benesova noted. “These are mostly patients with preexisting rheumatic and musculoskeletal disease or highly suspected de novo classical rheumatic and musculoskeletal disease under checkpoint inhibitor therapy.”
Patients with melanoma having a rheumatic immune-related adverse event had a better response to their therapy than historical counterparts who did not have such events: 39% of the former had a complete response, relative to merely 4% of the latter.
Only a small proportion of patients overall (9%) had to discontinue immune checkpoint inhibitor therapy because of their adverse event, and some of them may be eligible for rechallenge if their cancer progresses, Dr. Benesova noted.
“There is still a lot to be done,” she stated, such as better elucidating the nature of these adverse events [whether transient side effects or a triggering of chronic rheumatic and musculoskeletal diseases], the need for a defensive treatment strategy, and the advisability of closer monitoring of high-risk patients given immune checkpoint inhibitors. “We are aiming at solving these questions in the next few years,” she concluded.
Findings in context
“Registries are important to gain prospective data on patient outcomes,” Sabina Sandigursky, MD, an instructor in the division of rheumatology at the Laura and Isaac Perlmutter Cancer Center at New York University, commented in an interview. “One must be careful, while interpreting these data, especially since they are not randomized, controlled trials.”
Patterns may differ at other centers, too, she pointed out. “The German registry reported a predominance of spondyloarthritis-like disease; however, our patients have a predominance of small-joint involvement. It is unclear what accounts for this difference.”
Individual institutions in North America are similarly collecting data on this patient population, with efforts underway to compile those data to provide a larger picture, according to Dr. Sandigursky.
“Many of the syndromes that we consider to be rheumatic immune-related adverse events have been well described by groups from the U.S., Canada, Australia, and European Union,” she concluded. “From this registry, we can observe how patients are being treated in real time since this information is largely consensus based.”
The study did not receive any specific funding. Dr. Benesova disclosed grant/research support from AbbVie, Novartis, Rheumaliga Baden-Wurttemberg, and the University of Heidelberg, and consultancies, speaker fees, and/or travel reimbursements from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Medac, Merck Sharp & Dohme, Mundipharma, Novartis, Pfizer, Roche, and UCB. Some of her coauthors also disclosed financial relationships with industry. Dr. Sandigursky disclosed having no relevant conflicts of interest.
SOURCE: Benesova K et al. Ann Rheum Dis 2020;79[suppl 1]:168-9, Abstract OP0270.
Its first findings were reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“We have limited knowledge on the interrelationships between malignant and rheumatic diseases on both the clinical and molecular level, and we have a large unmet need for management guidelines in the case of the coincidence of both disease entities,” noted lead author Karolina Benesova, MD, of the department of hematology, oncology, and rheumatology at University Hospital Heidelberg (Germany).
The TRheuMa registry – Therapy-Induced Rheumatic Symptoms in Patients with Malignancy – is one of three registries in a multicenter observational project exploring various contexts between malignant and rheumatic diseases. Over its first 22 months, the registry recruited 69 patients having rheumatic symptoms as a result of immune checkpoint inhibitor therapy or other cancer therapies.
Registry findings
The largest shares of patients had non–small cell lung cancer (38%) or melanoma (33%), Dr. Benesova reported. The immune checkpoint inhibitors most commonly received were pembrolizumab (Keytruda), nivolumab (Opdivo), and ipilimumab (Yervoy).
The immune-related adverse events usually presented with symptoms of de novo spondyloarthritis or psoriatic arthritis (42%), late-onset RA (17%), or polymyalgia rheumatica (14%). But 16% of the patients were experiencing a flare of a preexisting rheumatic and musculoskeletal disease.
Laboratory findings differed somewhat from those of classical rheumatic and musculoskeletal diseases, according to Dr. Benesova. Specific findings were rare; in particular, most patients did not have detectable autoantibodies. However, 76% had an elevated C-reactive protein level and 39% had an elevated soluble CD25 level. In addition, nearly all patients (96%) undergoing joint ultrasound had pathologic findings.
“Based on our experiences from interdisciplinary care together with our local oncologists, we have developed a therapeutic algorithm for rheumatic immune-related adverse events,” she reported, noting that the algorithm is consistent with recently published recommendations in this area.
The large majority of patients were adequately treated with prednisone at a dose greater than 10 mg (40%) or at a dose of 10 mg or less with or without an NSAID (40%), while some received NSAID monotherapy (14%).
“We have a growing proportion of patients on conventional or biological [disease-modifying antirheumatic drugs],” Dr. Benesova noted. “These are mostly patients with preexisting rheumatic and musculoskeletal disease or highly suspected de novo classical rheumatic and musculoskeletal disease under checkpoint inhibitor therapy.”
Patients with melanoma having a rheumatic immune-related adverse event had a better response to their therapy than historical counterparts who did not have such events: 39% of the former had a complete response, relative to merely 4% of the latter.
Only a small proportion of patients overall (9%) had to discontinue immune checkpoint inhibitor therapy because of their adverse event, and some of them may be eligible for rechallenge if their cancer progresses, Dr. Benesova noted.
“There is still a lot to be done,” she stated, such as better elucidating the nature of these adverse events [whether transient side effects or a triggering of chronic rheumatic and musculoskeletal diseases], the need for a defensive treatment strategy, and the advisability of closer monitoring of high-risk patients given immune checkpoint inhibitors. “We are aiming at solving these questions in the next few years,” she concluded.
Findings in context
“Registries are important to gain prospective data on patient outcomes,” Sabina Sandigursky, MD, an instructor in the division of rheumatology at the Laura and Isaac Perlmutter Cancer Center at New York University, commented in an interview. “One must be careful, while interpreting these data, especially since they are not randomized, controlled trials.”
Patterns may differ at other centers, too, she pointed out. “The German registry reported a predominance of spondyloarthritis-like disease; however, our patients have a predominance of small-joint involvement. It is unclear what accounts for this difference.”
Individual institutions in North America are similarly collecting data on this patient population, with efforts underway to compile those data to provide a larger picture, according to Dr. Sandigursky.
“Many of the syndromes that we consider to be rheumatic immune-related adverse events have been well described by groups from the U.S., Canada, Australia, and European Union,” she concluded. “From this registry, we can observe how patients are being treated in real time since this information is largely consensus based.”
The study did not receive any specific funding. Dr. Benesova disclosed grant/research support from AbbVie, Novartis, Rheumaliga Baden-Wurttemberg, and the University of Heidelberg, and consultancies, speaker fees, and/or travel reimbursements from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Medac, Merck Sharp & Dohme, Mundipharma, Novartis, Pfizer, Roche, and UCB. Some of her coauthors also disclosed financial relationships with industry. Dr. Sandigursky disclosed having no relevant conflicts of interest.
SOURCE: Benesova K et al. Ann Rheum Dis 2020;79[suppl 1]:168-9, Abstract OP0270.
FROM THE EULAR 2020 E-CONGRESS
Studies give new insight on starting, stopping etanercept in nonradiographic axSpA
The results from a pair of clinical trials should help to take the guesswork out of starting and stopping the tumor necrosis factor inhibitor etanercept (Enbrel) in patients with nonradiographic axial spondyloarthritis (nr-axSpA). The trials were reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Optimal use of etanercept in this disease is still being defined, according to the investigators. Its effects, if any, when given very early in the disease course is unclear, and guidance is conflicting when it comes to stopping the drug after inactive disease is achieved.
In the Dutch randomized controlled PrevAS trial of 80 patients with suspected very early nr-axSpA, initiating etanercept instead of placebo did not significantly improve the odds of achieving a 20% improvement in Assessment of Spondyloarthritis International Society (ASAS 20) response criteria at week 16.
And in the multinational, open-label, phase 4 RE-EMBARK trial, three-quarters of the 119 patients with nr-axSpA who achieved inactive disease on etanercept and stopped the drug experienced a flare within 40 weeks. However, the majority were able to regain disease inactivity after restarting the drug.
Findings in context
“We all have some patients like this [PrevAS population] where we strongly believe they have axial spondyloarthritis but do not fully qualify,” Nigil Haroon MD, PhD, said in an interview. “From a clinical decision-making process, we may diagnose these patients with axial spondyloarthritis, but due to restrictions in access to medications, we have difficulty accessing biologic medications for them. Hence, this study has practical implications.”
“It has already been shown in other, much larger studies that, even in patients who satisfy the criteria of axial spondyloarthritis, those who are MRI and CRP [C-reactive protein] negative are unlikely to respond, so the results are not surprising,” commented Dr. Haroon, who is codirector of the spondylitis program at the University Health Network and associate professor of medicine and rheumatology at the University of Toronto.
Although intended to be a population with suspected very early disease, several of the PrevAS patients would have met ASAS criteria for the disease at baseline, Dr. Haroon cautioned. In addition, the small sample size precluded subgroup analyses.
“The overall conclusion should be, this is a negative study, rather than state there was a trend to better improvement on etanercept. Although there are practical implications, as mentioned, I don’t think this study, with the numbers and the results presented, will change clinical practice,” he said.
The question of stopping biologics in nr-axSpA was previously addressed in the ABILITY-3 randomized trial of adalimumab (Humira), which found that flares were significantly more common with stopping versus continuing the drug and only about half of patients were able to get back in remission by restarting the drug, according to Dr. Haroon.
However, the RE-EMBARK and ABILITY-3 studies differed in both design and patient population, he noted. For example, the mean disease duration was only about 2 years in the former study, compared with 7 years in the latter.
The initial 59% rate of attaining inactive disease on etanercept in RE-EMBARK was “impressive,” Dr. Haroon said, “but as this was an open-label study, higher values are expected.”
“The message in both studies is that stopping biologics completely is not a good idea as the majority of patients, 70%-75%, will relapse within a short period,” he concluded. “However, it should be kept in mind that these [RE-EMBARK] patients received biologic only for a short 24-week period. This study does not answer the question of whether nonradiographic axial spondyloarthritis patients with sustained inactive disease can be taken off biologics abruptly without a taper over time.”
Details of the studies
In the PrevAS trial, Tamara Rusman, a PhD candidate in Rheumatology at the VU University Medical Center Amsterdam and coinvestigators studied patients meeting Calin criteria for inflammatory back pain who had high disease activity plus either HLA-B27 positivity with at least one feature of axial spondyloarthritis or HLA-B27 negativity with two features.
This population is of interest because “most studies have included only patients with nonradiographic axial spondyloarthritis with a positive MRI of the sacroiliac joints and/or an elevated C-reactive protein level,” she noted.
Results showed that, during 16 weeks of treatment, etanercept users had a nonsignficantly higher rate of achieving an ASAS 20 response with etanercept versus placebo users (17% vs. 11%; hazard ratio, 2.1; P = .2). The etanercept group also had a somewhat higher rate of response as defined by the Ankylosing Spondylitis Disease Activity Score CRP (ASDAS-CRP) criterion (25% vs. 13%; hazard ratio, 1.1; P = .8).
“Based on these data, early treatment in inflammatory back pain patients prone to develop axial spondyloarthritis seems not to be useful,” Ms. Rusman concluded. “However, monitoring of these patients should be continued since they remain a risk group for developing axial spondyloarthritis.”
In the RE-EMBARK trial, investigators led by Filip Van den Bosch, MD, PhD, Rheumatology Head-of-Clinic at Ghent (Belgium) University Hospital, started with a cohort of 208 patients with nr-axSpA who were given etanercept and background NSAIDs for 24 weeks.
“Current guidelines do not agree on whether a TNF-blocking agent or another biological DMARD should be tapered once a status of low disease activity or remission is achieved,” he noted.
Overall, 59% of the patients achieved inactive disease (defined as an ASDAS-CRP < 1.3) and discontinued etanercept.
During the next 40 weeks, 24% of these patients maintained inactive disease with only the background NSAID therapy. Among the 75% who experienced a flare, defined as an ASDAS with erythrocyte sedimentation rate (ASDAS-ESR) score of 2.1 or greater, the median time to flare was 16.1 weeks. Fully 62% of this group were able to regain disease inactivity within 12 weeks of restarting etanercept.
In a comparative analysis, relative to the RE-EMBARK patients discontinuing etanercept, similar patients who continued etanercept on the companion EMBARK trial had a longer time to flare (P < .0001) and an 85% lower risk of this outcome.
“There were no new safety signals identified, and as expected, the number of treatment-emergent adverse events dropped during the drug-free period and, interestingly, remained stable over retreatment,” Dr. Van den Bosch noted.
“Temporarily discontinuing etanercept may be an option for some patients with stable inactive nonradiographic axial spondyloarthritis,” he concluded.
The PrevAS trial was financially supported by Pfizer and ReumaNederland. Ms. Rusman declared no relevant conflicts of interest; four coauthors reported financial relationship(s) with Pfizer and other pharmaceutical companies. The RE-EMBARK trial was sponsored by Pfizer. Dr. Van den Bosch disclosed receiving grant/research support from AbbVie, Merck, and UCB, and consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Four coauthors reported financial ties to Pfizer and other pharmaceutical companies, and five coauthors were employees and shareholders of Pfizer.
SOURCES: Rusman T et al. Ann Rheum Dis. 2020;79[suppl 1]:72-3; and Van den Bosch F et al. Ann Rheum Dis. 2020;79[suppl 1]:70.
The results from a pair of clinical trials should help to take the guesswork out of starting and stopping the tumor necrosis factor inhibitor etanercept (Enbrel) in patients with nonradiographic axial spondyloarthritis (nr-axSpA). The trials were reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Optimal use of etanercept in this disease is still being defined, according to the investigators. Its effects, if any, when given very early in the disease course is unclear, and guidance is conflicting when it comes to stopping the drug after inactive disease is achieved.
In the Dutch randomized controlled PrevAS trial of 80 patients with suspected very early nr-axSpA, initiating etanercept instead of placebo did not significantly improve the odds of achieving a 20% improvement in Assessment of Spondyloarthritis International Society (ASAS 20) response criteria at week 16.
And in the multinational, open-label, phase 4 RE-EMBARK trial, three-quarters of the 119 patients with nr-axSpA who achieved inactive disease on etanercept and stopped the drug experienced a flare within 40 weeks. However, the majority were able to regain disease inactivity after restarting the drug.
Findings in context
“We all have some patients like this [PrevAS population] where we strongly believe they have axial spondyloarthritis but do not fully qualify,” Nigil Haroon MD, PhD, said in an interview. “From a clinical decision-making process, we may diagnose these patients with axial spondyloarthritis, but due to restrictions in access to medications, we have difficulty accessing biologic medications for them. Hence, this study has practical implications.”
“It has already been shown in other, much larger studies that, even in patients who satisfy the criteria of axial spondyloarthritis, those who are MRI and CRP [C-reactive protein] negative are unlikely to respond, so the results are not surprising,” commented Dr. Haroon, who is codirector of the spondylitis program at the University Health Network and associate professor of medicine and rheumatology at the University of Toronto.
Although intended to be a population with suspected very early disease, several of the PrevAS patients would have met ASAS criteria for the disease at baseline, Dr. Haroon cautioned. In addition, the small sample size precluded subgroup analyses.
“The overall conclusion should be, this is a negative study, rather than state there was a trend to better improvement on etanercept. Although there are practical implications, as mentioned, I don’t think this study, with the numbers and the results presented, will change clinical practice,” he said.
The question of stopping biologics in nr-axSpA was previously addressed in the ABILITY-3 randomized trial of adalimumab (Humira), which found that flares were significantly more common with stopping versus continuing the drug and only about half of patients were able to get back in remission by restarting the drug, according to Dr. Haroon.
However, the RE-EMBARK and ABILITY-3 studies differed in both design and patient population, he noted. For example, the mean disease duration was only about 2 years in the former study, compared with 7 years in the latter.
The initial 59% rate of attaining inactive disease on etanercept in RE-EMBARK was “impressive,” Dr. Haroon said, “but as this was an open-label study, higher values are expected.”
“The message in both studies is that stopping biologics completely is not a good idea as the majority of patients, 70%-75%, will relapse within a short period,” he concluded. “However, it should be kept in mind that these [RE-EMBARK] patients received biologic only for a short 24-week period. This study does not answer the question of whether nonradiographic axial spondyloarthritis patients with sustained inactive disease can be taken off biologics abruptly without a taper over time.”
Details of the studies
In the PrevAS trial, Tamara Rusman, a PhD candidate in Rheumatology at the VU University Medical Center Amsterdam and coinvestigators studied patients meeting Calin criteria for inflammatory back pain who had high disease activity plus either HLA-B27 positivity with at least one feature of axial spondyloarthritis or HLA-B27 negativity with two features.
This population is of interest because “most studies have included only patients with nonradiographic axial spondyloarthritis with a positive MRI of the sacroiliac joints and/or an elevated C-reactive protein level,” she noted.
Results showed that, during 16 weeks of treatment, etanercept users had a nonsignficantly higher rate of achieving an ASAS 20 response with etanercept versus placebo users (17% vs. 11%; hazard ratio, 2.1; P = .2). The etanercept group also had a somewhat higher rate of response as defined by the Ankylosing Spondylitis Disease Activity Score CRP (ASDAS-CRP) criterion (25% vs. 13%; hazard ratio, 1.1; P = .8).
“Based on these data, early treatment in inflammatory back pain patients prone to develop axial spondyloarthritis seems not to be useful,” Ms. Rusman concluded. “However, monitoring of these patients should be continued since they remain a risk group for developing axial spondyloarthritis.”
In the RE-EMBARK trial, investigators led by Filip Van den Bosch, MD, PhD, Rheumatology Head-of-Clinic at Ghent (Belgium) University Hospital, started with a cohort of 208 patients with nr-axSpA who were given etanercept and background NSAIDs for 24 weeks.
“Current guidelines do not agree on whether a TNF-blocking agent or another biological DMARD should be tapered once a status of low disease activity or remission is achieved,” he noted.
Overall, 59% of the patients achieved inactive disease (defined as an ASDAS-CRP < 1.3) and discontinued etanercept.
During the next 40 weeks, 24% of these patients maintained inactive disease with only the background NSAID therapy. Among the 75% who experienced a flare, defined as an ASDAS with erythrocyte sedimentation rate (ASDAS-ESR) score of 2.1 or greater, the median time to flare was 16.1 weeks. Fully 62% of this group were able to regain disease inactivity within 12 weeks of restarting etanercept.
In a comparative analysis, relative to the RE-EMBARK patients discontinuing etanercept, similar patients who continued etanercept on the companion EMBARK trial had a longer time to flare (P < .0001) and an 85% lower risk of this outcome.
“There were no new safety signals identified, and as expected, the number of treatment-emergent adverse events dropped during the drug-free period and, interestingly, remained stable over retreatment,” Dr. Van den Bosch noted.
“Temporarily discontinuing etanercept may be an option for some patients with stable inactive nonradiographic axial spondyloarthritis,” he concluded.
The PrevAS trial was financially supported by Pfizer and ReumaNederland. Ms. Rusman declared no relevant conflicts of interest; four coauthors reported financial relationship(s) with Pfizer and other pharmaceutical companies. The RE-EMBARK trial was sponsored by Pfizer. Dr. Van den Bosch disclosed receiving grant/research support from AbbVie, Merck, and UCB, and consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Four coauthors reported financial ties to Pfizer and other pharmaceutical companies, and five coauthors were employees and shareholders of Pfizer.
SOURCES: Rusman T et al. Ann Rheum Dis. 2020;79[suppl 1]:72-3; and Van den Bosch F et al. Ann Rheum Dis. 2020;79[suppl 1]:70.
The results from a pair of clinical trials should help to take the guesswork out of starting and stopping the tumor necrosis factor inhibitor etanercept (Enbrel) in patients with nonradiographic axial spondyloarthritis (nr-axSpA). The trials were reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Optimal use of etanercept in this disease is still being defined, according to the investigators. Its effects, if any, when given very early in the disease course is unclear, and guidance is conflicting when it comes to stopping the drug after inactive disease is achieved.
In the Dutch randomized controlled PrevAS trial of 80 patients with suspected very early nr-axSpA, initiating etanercept instead of placebo did not significantly improve the odds of achieving a 20% improvement in Assessment of Spondyloarthritis International Society (ASAS 20) response criteria at week 16.
And in the multinational, open-label, phase 4 RE-EMBARK trial, three-quarters of the 119 patients with nr-axSpA who achieved inactive disease on etanercept and stopped the drug experienced a flare within 40 weeks. However, the majority were able to regain disease inactivity after restarting the drug.
Findings in context
“We all have some patients like this [PrevAS population] where we strongly believe they have axial spondyloarthritis but do not fully qualify,” Nigil Haroon MD, PhD, said in an interview. “From a clinical decision-making process, we may diagnose these patients with axial spondyloarthritis, but due to restrictions in access to medications, we have difficulty accessing biologic medications for them. Hence, this study has practical implications.”
“It has already been shown in other, much larger studies that, even in patients who satisfy the criteria of axial spondyloarthritis, those who are MRI and CRP [C-reactive protein] negative are unlikely to respond, so the results are not surprising,” commented Dr. Haroon, who is codirector of the spondylitis program at the University Health Network and associate professor of medicine and rheumatology at the University of Toronto.
Although intended to be a population with suspected very early disease, several of the PrevAS patients would have met ASAS criteria for the disease at baseline, Dr. Haroon cautioned. In addition, the small sample size precluded subgroup analyses.
“The overall conclusion should be, this is a negative study, rather than state there was a trend to better improvement on etanercept. Although there are practical implications, as mentioned, I don’t think this study, with the numbers and the results presented, will change clinical practice,” he said.
The question of stopping biologics in nr-axSpA was previously addressed in the ABILITY-3 randomized trial of adalimumab (Humira), which found that flares were significantly more common with stopping versus continuing the drug and only about half of patients were able to get back in remission by restarting the drug, according to Dr. Haroon.
However, the RE-EMBARK and ABILITY-3 studies differed in both design and patient population, he noted. For example, the mean disease duration was only about 2 years in the former study, compared with 7 years in the latter.
The initial 59% rate of attaining inactive disease on etanercept in RE-EMBARK was “impressive,” Dr. Haroon said, “but as this was an open-label study, higher values are expected.”
“The message in both studies is that stopping biologics completely is not a good idea as the majority of patients, 70%-75%, will relapse within a short period,” he concluded. “However, it should be kept in mind that these [RE-EMBARK] patients received biologic only for a short 24-week period. This study does not answer the question of whether nonradiographic axial spondyloarthritis patients with sustained inactive disease can be taken off biologics abruptly without a taper over time.”
Details of the studies
In the PrevAS trial, Tamara Rusman, a PhD candidate in Rheumatology at the VU University Medical Center Amsterdam and coinvestigators studied patients meeting Calin criteria for inflammatory back pain who had high disease activity plus either HLA-B27 positivity with at least one feature of axial spondyloarthritis or HLA-B27 negativity with two features.
This population is of interest because “most studies have included only patients with nonradiographic axial spondyloarthritis with a positive MRI of the sacroiliac joints and/or an elevated C-reactive protein level,” she noted.
Results showed that, during 16 weeks of treatment, etanercept users had a nonsignficantly higher rate of achieving an ASAS 20 response with etanercept versus placebo users (17% vs. 11%; hazard ratio, 2.1; P = .2). The etanercept group also had a somewhat higher rate of response as defined by the Ankylosing Spondylitis Disease Activity Score CRP (ASDAS-CRP) criterion (25% vs. 13%; hazard ratio, 1.1; P = .8).
“Based on these data, early treatment in inflammatory back pain patients prone to develop axial spondyloarthritis seems not to be useful,” Ms. Rusman concluded. “However, monitoring of these patients should be continued since they remain a risk group for developing axial spondyloarthritis.”
In the RE-EMBARK trial, investigators led by Filip Van den Bosch, MD, PhD, Rheumatology Head-of-Clinic at Ghent (Belgium) University Hospital, started with a cohort of 208 patients with nr-axSpA who were given etanercept and background NSAIDs for 24 weeks.
“Current guidelines do not agree on whether a TNF-blocking agent or another biological DMARD should be tapered once a status of low disease activity or remission is achieved,” he noted.
Overall, 59% of the patients achieved inactive disease (defined as an ASDAS-CRP < 1.3) and discontinued etanercept.
During the next 40 weeks, 24% of these patients maintained inactive disease with only the background NSAID therapy. Among the 75% who experienced a flare, defined as an ASDAS with erythrocyte sedimentation rate (ASDAS-ESR) score of 2.1 or greater, the median time to flare was 16.1 weeks. Fully 62% of this group were able to regain disease inactivity within 12 weeks of restarting etanercept.
In a comparative analysis, relative to the RE-EMBARK patients discontinuing etanercept, similar patients who continued etanercept on the companion EMBARK trial had a longer time to flare (P < .0001) and an 85% lower risk of this outcome.
“There were no new safety signals identified, and as expected, the number of treatment-emergent adverse events dropped during the drug-free period and, interestingly, remained stable over retreatment,” Dr. Van den Bosch noted.
“Temporarily discontinuing etanercept may be an option for some patients with stable inactive nonradiographic axial spondyloarthritis,” he concluded.
The PrevAS trial was financially supported by Pfizer and ReumaNederland. Ms. Rusman declared no relevant conflicts of interest; four coauthors reported financial relationship(s) with Pfizer and other pharmaceutical companies. The RE-EMBARK trial was sponsored by Pfizer. Dr. Van den Bosch disclosed receiving grant/research support from AbbVie, Merck, and UCB, and consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Four coauthors reported financial ties to Pfizer and other pharmaceutical companies, and five coauthors were employees and shareholders of Pfizer.
SOURCES: Rusman T et al. Ann Rheum Dis. 2020;79[suppl 1]:72-3; and Van den Bosch F et al. Ann Rheum Dis. 2020;79[suppl 1]:70.
FROM THE EULAR 2020 E-CONGRESS
Key clinical point: In nonradiographic axial spondyloarthritis (nr-axSpA), etanercept does not have significant benefit by 16 weeks when started in very early disease, and the majority of patients who achieved inactive disease on the drug and then stopped it experienced a flare within 40 weeks.
Major finding: Patients with suspected very early disease who took etanercept did not have a significantly greater rate of achieving a 20% improvement in Assessment of Spondyloarthritis International Society (ASAS 20) response criteria at week 16 than did those taking placebo (17% vs. 11%; hazard ratio, 2.1; P = .2). In a separate trial, 75% of patients who achieved inactive disease with etanercept and then stopped the drug had a flare within 40 weeks, but 62% of this group were able to regain disease inactivity within 12 weeks of restarting etanercept.
Study details: A randomized, placebo-controlled PrevAS trial involved 80 patients with suspected very early nr-axSpA who started either etanercept or placebo, and the multicenter, open-label, phase 4 RE-EMBARK trial involved 119 patients achieving inactive nr-axSpA on etanercept.
Disclosures: The PrevAS trial was financially supported by Pfizer and ReumaNederland. Ms. Rusman declared no relevant conflicts of interest; four coauthors reported financial relationship(s) with Pfizer and other pharmaceutical companies. The RE-EMBARK trial was sponsored by Pfizer. Dr. Van den Bosch disclosed receiving grant/research support from AbbVie, Merck, and UCB and consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Four coauthors reported financial ties to Pfizer and other pharmaceutical companies, and five coauthors were employees and shareholders of Pfizer.
Sources: Rusman T et al. Ann Rheum Dis. 2020;79[suppl 1]:72-3; and Van den Bosch F et al. Ann Rheum Dis. 2020;79[suppl 1]:70.
Antinuclear antibody test interpretation guidance gets updated
New recommendations from the European League Against Rheumatism on interpreting the results of antinuclear antibody (ANA) testing advised taking the test methodology into account because of differences in performance.
ANA results vary not only by the test being used but also by the underlying disease they are being used to assess, warned Pier Luigi Meroni, MD, director of the Immunorheumatology Research Laboratory at the IRCCS Istituto Auxologico Italiano in Milan.
“Antinuclear antibody testing is a known diagnostic tool. But the recent advances in methodologies strongly suggests that we have to update our knowledge for a better interpretation of the results,” Dr. Meroni said in his presentation at the annual European Congress of Rheumatology, held online this year due to COVID-19.
There is “no doubt that ANA testing is useful,” he continued, adding that ANA is used as a primary screening tool in many rheumatic diseases, notably systemic lupus erythematosus (SLE), primary Sjögren’s syndrome, and systemic sclerosis. It’s also recently been suggested as an important entry criterion for the classification of SLE.
In fact, the 2019 SLE classification criteria – developed by EULAR in collaboration with the American College of Rheumatology – state that “testing by immunofluorescence on HEp-2 cells or a solid-phase ANA screening immunoassay with at least equivalent performance is highly recommended,” Dr. Meroni said.
The ideas underpinning that recommendation was that “ANA expression is invariable in SLE, and that ANA-negative lupus is quite rare,” he explained. Also, as SLE expression persists over time, ANA testing could be used for classification at any point in the disease course. These assumptions have been borne out in several studies, with very small percentages of patients (6% or less) having ANA-negative lupus, and more than 80% having a positive HEp-2 test over time, even with immunosuppressive treatment.
Which test methodology to use?
There are several methods that can be used to detect ANA, including the preferred HEp-2 indirect fluorescence assay (IFA), several solid-phase assays (SpA), and line- or dot-blot immunoassays. The issue is which assay should be used in which disease?
The performance of a particular assay can depend on the disease in which they are used. For instance, while the HEp-2 IFA and SpA are equivalent in SLE and in other connective tissue diseases, “this is not the case for other autoimmune diseases in which basically we don’t know exactly all the autoantigens,” Dr. Meroni explained. “Most of the autoantigens are undefined. They cannot be found in solid-phase kits, and we have to use the IFA for detecting all these autoantibodies.”
Importantly, neither the IFA nor the SpA is superior to the other. “We just say that one technique can detect relevant antibodies that are not detectable by the other one, and maybe the combination of the two techniques can be the right strategy to get the highest sensitivity,” Dr. Meroni said.
“Clinicians should be aware of the type of assay used for ANA detection,” he said, “because there are strong differences in the performance, for example between IFA and SpA, and such differences can have important clinical and relevant consequences.”
The test selected will depend on if the aim is to exclude or confirm a disease, and the optimal strategy will depend on pretest probability. For instance, IFA is more sensitive than SpA for SLE and scleroderma, whereas IFA is less sensitive than SpA for Sjögren’s. For SLE, it is suggested to use both the IFA and SpA. A combination of both tests is also considered optimal for scleroderma. SpA testing offers the best sensitivity for Sjögren’s.
“The story is a little bit more complicated for inflammatory myopathies in which we don’t have assays able to detect all the autoantibodies,” Dr. Meroni said. In that situation, several different techniques have to be used to check if the SpA results fit with the IFA pattern.
In 2019, the ACR released its own position statement on ANA testing, highlighting that it supported the use of the HEp-2 IFA assay as the preferred option for ANA testing and that labs should specify the methods being used to test for ANA when reporting their results. The ACR position statement also noted that “ordering health care professionals should select specific ANA subserologies based on a patient’s signs and symptoms and when there is a high pretest suspicion for a specific condition.”
Dr. Meroni disclosed serving as a consultant to Inova Diagnostics, Thermo Fisher Scientific, Pfizer, AbbVie, Merck Sharp & Dohme, and UCB.
New recommendations from the European League Against Rheumatism on interpreting the results of antinuclear antibody (ANA) testing advised taking the test methodology into account because of differences in performance.
ANA results vary not only by the test being used but also by the underlying disease they are being used to assess, warned Pier Luigi Meroni, MD, director of the Immunorheumatology Research Laboratory at the IRCCS Istituto Auxologico Italiano in Milan.
“Antinuclear antibody testing is a known diagnostic tool. But the recent advances in methodologies strongly suggests that we have to update our knowledge for a better interpretation of the results,” Dr. Meroni said in his presentation at the annual European Congress of Rheumatology, held online this year due to COVID-19.
There is “no doubt that ANA testing is useful,” he continued, adding that ANA is used as a primary screening tool in many rheumatic diseases, notably systemic lupus erythematosus (SLE), primary Sjögren’s syndrome, and systemic sclerosis. It’s also recently been suggested as an important entry criterion for the classification of SLE.
In fact, the 2019 SLE classification criteria – developed by EULAR in collaboration with the American College of Rheumatology – state that “testing by immunofluorescence on HEp-2 cells or a solid-phase ANA screening immunoassay with at least equivalent performance is highly recommended,” Dr. Meroni said.
The ideas underpinning that recommendation was that “ANA expression is invariable in SLE, and that ANA-negative lupus is quite rare,” he explained. Also, as SLE expression persists over time, ANA testing could be used for classification at any point in the disease course. These assumptions have been borne out in several studies, with very small percentages of patients (6% or less) having ANA-negative lupus, and more than 80% having a positive HEp-2 test over time, even with immunosuppressive treatment.
Which test methodology to use?
There are several methods that can be used to detect ANA, including the preferred HEp-2 indirect fluorescence assay (IFA), several solid-phase assays (SpA), and line- or dot-blot immunoassays. The issue is which assay should be used in which disease?
The performance of a particular assay can depend on the disease in which they are used. For instance, while the HEp-2 IFA and SpA are equivalent in SLE and in other connective tissue diseases, “this is not the case for other autoimmune diseases in which basically we don’t know exactly all the autoantigens,” Dr. Meroni explained. “Most of the autoantigens are undefined. They cannot be found in solid-phase kits, and we have to use the IFA for detecting all these autoantibodies.”
Importantly, neither the IFA nor the SpA is superior to the other. “We just say that one technique can detect relevant antibodies that are not detectable by the other one, and maybe the combination of the two techniques can be the right strategy to get the highest sensitivity,” Dr. Meroni said.
“Clinicians should be aware of the type of assay used for ANA detection,” he said, “because there are strong differences in the performance, for example between IFA and SpA, and such differences can have important clinical and relevant consequences.”
The test selected will depend on if the aim is to exclude or confirm a disease, and the optimal strategy will depend on pretest probability. For instance, IFA is more sensitive than SpA for SLE and scleroderma, whereas IFA is less sensitive than SpA for Sjögren’s. For SLE, it is suggested to use both the IFA and SpA. A combination of both tests is also considered optimal for scleroderma. SpA testing offers the best sensitivity for Sjögren’s.
“The story is a little bit more complicated for inflammatory myopathies in which we don’t have assays able to detect all the autoantibodies,” Dr. Meroni said. In that situation, several different techniques have to be used to check if the SpA results fit with the IFA pattern.
In 2019, the ACR released its own position statement on ANA testing, highlighting that it supported the use of the HEp-2 IFA assay as the preferred option for ANA testing and that labs should specify the methods being used to test for ANA when reporting their results. The ACR position statement also noted that “ordering health care professionals should select specific ANA subserologies based on a patient’s signs and symptoms and when there is a high pretest suspicion for a specific condition.”
Dr. Meroni disclosed serving as a consultant to Inova Diagnostics, Thermo Fisher Scientific, Pfizer, AbbVie, Merck Sharp & Dohme, and UCB.
New recommendations from the European League Against Rheumatism on interpreting the results of antinuclear antibody (ANA) testing advised taking the test methodology into account because of differences in performance.
ANA results vary not only by the test being used but also by the underlying disease they are being used to assess, warned Pier Luigi Meroni, MD, director of the Immunorheumatology Research Laboratory at the IRCCS Istituto Auxologico Italiano in Milan.
“Antinuclear antibody testing is a known diagnostic tool. But the recent advances in methodologies strongly suggests that we have to update our knowledge for a better interpretation of the results,” Dr. Meroni said in his presentation at the annual European Congress of Rheumatology, held online this year due to COVID-19.
There is “no doubt that ANA testing is useful,” he continued, adding that ANA is used as a primary screening tool in many rheumatic diseases, notably systemic lupus erythematosus (SLE), primary Sjögren’s syndrome, and systemic sclerosis. It’s also recently been suggested as an important entry criterion for the classification of SLE.
In fact, the 2019 SLE classification criteria – developed by EULAR in collaboration with the American College of Rheumatology – state that “testing by immunofluorescence on HEp-2 cells or a solid-phase ANA screening immunoassay with at least equivalent performance is highly recommended,” Dr. Meroni said.
The ideas underpinning that recommendation was that “ANA expression is invariable in SLE, and that ANA-negative lupus is quite rare,” he explained. Also, as SLE expression persists over time, ANA testing could be used for classification at any point in the disease course. These assumptions have been borne out in several studies, with very small percentages of patients (6% or less) having ANA-negative lupus, and more than 80% having a positive HEp-2 test over time, even with immunosuppressive treatment.
Which test methodology to use?
There are several methods that can be used to detect ANA, including the preferred HEp-2 indirect fluorescence assay (IFA), several solid-phase assays (SpA), and line- or dot-blot immunoassays. The issue is which assay should be used in which disease?
The performance of a particular assay can depend on the disease in which they are used. For instance, while the HEp-2 IFA and SpA are equivalent in SLE and in other connective tissue diseases, “this is not the case for other autoimmune diseases in which basically we don’t know exactly all the autoantigens,” Dr. Meroni explained. “Most of the autoantigens are undefined. They cannot be found in solid-phase kits, and we have to use the IFA for detecting all these autoantibodies.”
Importantly, neither the IFA nor the SpA is superior to the other. “We just say that one technique can detect relevant antibodies that are not detectable by the other one, and maybe the combination of the two techniques can be the right strategy to get the highest sensitivity,” Dr. Meroni said.
“Clinicians should be aware of the type of assay used for ANA detection,” he said, “because there are strong differences in the performance, for example between IFA and SpA, and such differences can have important clinical and relevant consequences.”
The test selected will depend on if the aim is to exclude or confirm a disease, and the optimal strategy will depend on pretest probability. For instance, IFA is more sensitive than SpA for SLE and scleroderma, whereas IFA is less sensitive than SpA for Sjögren’s. For SLE, it is suggested to use both the IFA and SpA. A combination of both tests is also considered optimal for scleroderma. SpA testing offers the best sensitivity for Sjögren’s.
“The story is a little bit more complicated for inflammatory myopathies in which we don’t have assays able to detect all the autoantibodies,” Dr. Meroni said. In that situation, several different techniques have to be used to check if the SpA results fit with the IFA pattern.
In 2019, the ACR released its own position statement on ANA testing, highlighting that it supported the use of the HEp-2 IFA assay as the preferred option for ANA testing and that labs should specify the methods being used to test for ANA when reporting their results. The ACR position statement also noted that “ordering health care professionals should select specific ANA subserologies based on a patient’s signs and symptoms and when there is a high pretest suspicion for a specific condition.”
Dr. Meroni disclosed serving as a consultant to Inova Diagnostics, Thermo Fisher Scientific, Pfizer, AbbVie, Merck Sharp & Dohme, and UCB.
FROM THE EULAR 2020 E-CONGRESS
Seropositivity in RA linked with doubled pneumonia incidence
from a single U.S. medical system.
“Patients with seropositive RA, particularly RF [rheumatoid factor]-positive RA, had increased risk for pneumonia throughout the RA disease course that was not explained by measured confounders, including smoking status, multimorbidity, medications, and [erythrocyte sedimentation rate] level,” Jeffrey A. Sparks, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“There has been much interest about the relationship between lung inflammation and the generation of RF and CCP [cyclic citrullinated protein] prior to the onset of RA. We hypothesized that patients with seropositive RA might have subclinical lung injury that could predispose them to pneumonia after clinical RA onset,” Dr. Sparks said in an interview. “Pneumonia is one of the most common serious infections in both patients with RA and the general population, and it causes serious morbidity and mortality.”
The doubled relative risk for pneumonia seen in the findings “translates into a clinically meaningful finding when considering the high rate and the many patients at risk since RA is relatively common,” said Dr. Sparks, a rheumatologist at Brigham and Women’s Hospital in Boston.
“Patients with RF-positive RA who present with symptoms concerning for pneumonia should be evaluated carefully for this and for other possible pulmonary manifestations of RA. Vaccination for pneumonia should be strongly considered for patients with RA who are on disease-modifying antirheumatic drugs, and we hope that our report encourages clinicians and patients” to undertake vaccination, he said.
His study used a database of more than 60,000 patients diagnosed with RA as of November 2013 in the records of a large Boston-area medical system that includes physicians affiliated with Brigham and Women’s Hospital and Massachusetts General Hospital. The researchers applied a validated algorithm for calculating a patient’s probability of having RA, and at the level of 97% probability they narrowed the cohort down to just under 10,000 patients. Additional winnowing because of missing data or a history of pneumonia yielded a study group of 4,110, which included 3,279 (80%) who were seropositive for either or both CCP and RF, and 831 (20%) who were seronegative. During a median follow-up of 7.8 years and total follow-up of more than 32,000 patient-years, the overall pneumonia incidence was 5.8%, with a 2.8% rate among the seronegatives and a 6.6% rate among seropositives. After adjustment for age, sex, glucocorticoid use, disease-modifying antirheumatic drug use, and several other possible confounders, the researchers calculated a 99% relative increased rate of pneumonia among all seropositive patients, compared with the seronegatives.
Further analysis looked at pneumonia incidence rates among patients positive only for CCP antibody, positive only for RF antibody, or both, compared with seronegative patients. This showed that CCP seropositivity had no statistically significant link with incident pneumonia, while RF seropositivity linked with a statistically significant, roughly twofold higher rate. Only 6% of all seropositive patients were positive only for CCP antibody, 59% were positive specifically for RF antibody, and 35% for both.
The data Dr. Sparks presented did not include information on pneumonia type, the timing of the pneumonia, compared with the onset of RA, disease activity, or smoking intensity.
“We anticipated that both RF positive and CCP positive would each be associated with pneumonia, so it was somewhat surprising that we only detected this for RF,” Dr. Sparks said. But he added that, because the number of patients with only CCP positivity was relatively so small, “it is still possible that CCP [antibody] could also increase pneumonia risk.”
The study had no commercial funding. Dr. Sparks had no disclosures.
SOURCE: Sparks JA et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:73, Abstract OP0111.
from a single U.S. medical system.
“Patients with seropositive RA, particularly RF [rheumatoid factor]-positive RA, had increased risk for pneumonia throughout the RA disease course that was not explained by measured confounders, including smoking status, multimorbidity, medications, and [erythrocyte sedimentation rate] level,” Jeffrey A. Sparks, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“There has been much interest about the relationship between lung inflammation and the generation of RF and CCP [cyclic citrullinated protein] prior to the onset of RA. We hypothesized that patients with seropositive RA might have subclinical lung injury that could predispose them to pneumonia after clinical RA onset,” Dr. Sparks said in an interview. “Pneumonia is one of the most common serious infections in both patients with RA and the general population, and it causes serious morbidity and mortality.”
The doubled relative risk for pneumonia seen in the findings “translates into a clinically meaningful finding when considering the high rate and the many patients at risk since RA is relatively common,” said Dr. Sparks, a rheumatologist at Brigham and Women’s Hospital in Boston.
“Patients with RF-positive RA who present with symptoms concerning for pneumonia should be evaluated carefully for this and for other possible pulmonary manifestations of RA. Vaccination for pneumonia should be strongly considered for patients with RA who are on disease-modifying antirheumatic drugs, and we hope that our report encourages clinicians and patients” to undertake vaccination, he said.
His study used a database of more than 60,000 patients diagnosed with RA as of November 2013 in the records of a large Boston-area medical system that includes physicians affiliated with Brigham and Women’s Hospital and Massachusetts General Hospital. The researchers applied a validated algorithm for calculating a patient’s probability of having RA, and at the level of 97% probability they narrowed the cohort down to just under 10,000 patients. Additional winnowing because of missing data or a history of pneumonia yielded a study group of 4,110, which included 3,279 (80%) who were seropositive for either or both CCP and RF, and 831 (20%) who were seronegative. During a median follow-up of 7.8 years and total follow-up of more than 32,000 patient-years, the overall pneumonia incidence was 5.8%, with a 2.8% rate among the seronegatives and a 6.6% rate among seropositives. After adjustment for age, sex, glucocorticoid use, disease-modifying antirheumatic drug use, and several other possible confounders, the researchers calculated a 99% relative increased rate of pneumonia among all seropositive patients, compared with the seronegatives.
Further analysis looked at pneumonia incidence rates among patients positive only for CCP antibody, positive only for RF antibody, or both, compared with seronegative patients. This showed that CCP seropositivity had no statistically significant link with incident pneumonia, while RF seropositivity linked with a statistically significant, roughly twofold higher rate. Only 6% of all seropositive patients were positive only for CCP antibody, 59% were positive specifically for RF antibody, and 35% for both.
The data Dr. Sparks presented did not include information on pneumonia type, the timing of the pneumonia, compared with the onset of RA, disease activity, or smoking intensity.
“We anticipated that both RF positive and CCP positive would each be associated with pneumonia, so it was somewhat surprising that we only detected this for RF,” Dr. Sparks said. But he added that, because the number of patients with only CCP positivity was relatively so small, “it is still possible that CCP [antibody] could also increase pneumonia risk.”
The study had no commercial funding. Dr. Sparks had no disclosures.
SOURCE: Sparks JA et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:73, Abstract OP0111.
from a single U.S. medical system.
“Patients with seropositive RA, particularly RF [rheumatoid factor]-positive RA, had increased risk for pneumonia throughout the RA disease course that was not explained by measured confounders, including smoking status, multimorbidity, medications, and [erythrocyte sedimentation rate] level,” Jeffrey A. Sparks, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“There has been much interest about the relationship between lung inflammation and the generation of RF and CCP [cyclic citrullinated protein] prior to the onset of RA. We hypothesized that patients with seropositive RA might have subclinical lung injury that could predispose them to pneumonia after clinical RA onset,” Dr. Sparks said in an interview. “Pneumonia is one of the most common serious infections in both patients with RA and the general population, and it causes serious morbidity and mortality.”
The doubled relative risk for pneumonia seen in the findings “translates into a clinically meaningful finding when considering the high rate and the many patients at risk since RA is relatively common,” said Dr. Sparks, a rheumatologist at Brigham and Women’s Hospital in Boston.
“Patients with RF-positive RA who present with symptoms concerning for pneumonia should be evaluated carefully for this and for other possible pulmonary manifestations of RA. Vaccination for pneumonia should be strongly considered for patients with RA who are on disease-modifying antirheumatic drugs, and we hope that our report encourages clinicians and patients” to undertake vaccination, he said.
His study used a database of more than 60,000 patients diagnosed with RA as of November 2013 in the records of a large Boston-area medical system that includes physicians affiliated with Brigham and Women’s Hospital and Massachusetts General Hospital. The researchers applied a validated algorithm for calculating a patient’s probability of having RA, and at the level of 97% probability they narrowed the cohort down to just under 10,000 patients. Additional winnowing because of missing data or a history of pneumonia yielded a study group of 4,110, which included 3,279 (80%) who were seropositive for either or both CCP and RF, and 831 (20%) who were seronegative. During a median follow-up of 7.8 years and total follow-up of more than 32,000 patient-years, the overall pneumonia incidence was 5.8%, with a 2.8% rate among the seronegatives and a 6.6% rate among seropositives. After adjustment for age, sex, glucocorticoid use, disease-modifying antirheumatic drug use, and several other possible confounders, the researchers calculated a 99% relative increased rate of pneumonia among all seropositive patients, compared with the seronegatives.
Further analysis looked at pneumonia incidence rates among patients positive only for CCP antibody, positive only for RF antibody, or both, compared with seronegative patients. This showed that CCP seropositivity had no statistically significant link with incident pneumonia, while RF seropositivity linked with a statistically significant, roughly twofold higher rate. Only 6% of all seropositive patients were positive only for CCP antibody, 59% were positive specifically for RF antibody, and 35% for both.
The data Dr. Sparks presented did not include information on pneumonia type, the timing of the pneumonia, compared with the onset of RA, disease activity, or smoking intensity.
“We anticipated that both RF positive and CCP positive would each be associated with pneumonia, so it was somewhat surprising that we only detected this for RF,” Dr. Sparks said. But he added that, because the number of patients with only CCP positivity was relatively so small, “it is still possible that CCP [antibody] could also increase pneumonia risk.”
The study had no commercial funding. Dr. Sparks had no disclosures.
SOURCE: Sparks JA et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:73, Abstract OP0111.
FROM THE EULAR 2020 E-CONGRESS
Entheseal lesions, bone density linked with incident PsA in psoriasis patients
Structural entheseal lesions and reduced bone mineral density detected using high-resolution CT imaging of a pair of knuckle joints in patients with psoriasis strongly linked with subsequent development of psoriatic arthritis (PsA) in a single-center study with 114 patients followed for an average of 2.3 years.
“These findings substantiate the concept of mechano-inflammation in the pathogenesis of psoriatic disease,” and suggest that interventions with high efficacy for controlling entheseal inflammation may be a “particularly valuable strategy in interfering with the onset of PsA in patients with psoriatic disease,” David Simon, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
The study, which is now published in Arthritis & Rheumatology, began with 377 patients with psoriasis who had been referred to the University Hospital in Erlangen, Germany, during 2011-2018, and who tested positive on the German Psoriasis Arthritis Diagnostic questionnaire. The researchers excluded patients with existing signs of PsA, any arthritis or enthesitis or other signs of inflammatory rheumatic disease, and they also excluded patients who had not undergoing a high-resolution peripheral quantitative CT (HR-pQCT) examination of the second and third metacarpal joints of the patient’s nondominant hand, which left 114 patients for their analysis. During a mean follow-up of 28 months, 24 patients (27%) developed PsA. The study patients were an average age of 45 years, and they had been diagnosed with psoriasis for an average of about 16 years.
Dr. Simon and associates used the baseline HR-pQCT scans to make two assessments of each patient: the presence of structural entheseal lesions (SEL) in the two metacarpal joints and the calculated volumetric bone mineral density (vBMD). Their analysis showed that the number and severity of SEL were increased among patients who later developed PsA. In a multivariable model that adjusted for age, sex, body mass index, duration of psoriasis, and arthralgia, patients with any SEL had a fivefold higher rate of developing PsA, compared with patients with no SEL, reported Dr. Simon, a rheumatologist at Erlangen University Hospital.
The analysis of vBMD also showed a strong link between bone density at the entheseal sites of the two studied joints and subsequent PsA development. For every standard deviation increase in vBMD at these sites the subsequent rate of PsA incidence fell by about 67% in an analysis that controlled for the same covariants as well as presence of SEL. The same relationship between higher vBMD and a lower risk for PsA held for both total vBMD measurement and for cortical vBMD, but only at the entheseal site. Levels of vBMD at the intra-articular site of the joints had no statistically significant relationship with subsequent PsA development.
The two metrics also appeared to identify additive risks. Nearly 90% of patients with at least one SEL who also had low vBMD at the entheseal site developed PsA during follow-up, compared with about a 50% rate among patients with at least one SEL but high vBMD.
The imaging method used to run these analyses, HR-pQCT, remains for the time being a “research technique” that “is not generalizable for routine practice,” but further development of this method or of a surrogate measure might make it feasible for future widespread practice, commented Iain McInnes, MD, PhD, president of the European League Against Rheumatism and professor of rheumatology and director of the Institute of Infection, Immunity, and Inflammation at the University of Glasgow.
“We’ve thought for many years that psoriasis and psoriatic arthritis are on a spectrum, and this work is consistent with the idea that some patients with psoriasis develop tissue involvement at entheses and joints,” Dr. McInnes said in an interview. The higher incidence of PsA seen in patients with adverse SEL and vBMD markers was in an “interesting range” that warrants further study. A next step is to run an intervention study in which patients with these adverse markers would receive an intervention randomized against placebo to see if it improved their outcomes, he suggested. Good candidate agents to study in psoriasis patients who have these adverse markers include drugs that inhibit the action of interleukin-17, drugs that target the p19 cytokine subunit of IL-23, and possibly Janus kinase inhibitor drugs.
Dr. Simon has been a consultant to AbbVie and Eli Lilly, a speaker on behalf of Eli Lilly, Janssen, and Novartis, and has received research funding from Eli Lilly and Novartis. Dr. McInnes has been a consultant to AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB, and he has received research funding from Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, and UCB.
SOURCE: Simon D et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:33-4, Abstract OP0051.
Structural entheseal lesions and reduced bone mineral density detected using high-resolution CT imaging of a pair of knuckle joints in patients with psoriasis strongly linked with subsequent development of psoriatic arthritis (PsA) in a single-center study with 114 patients followed for an average of 2.3 years.
“These findings substantiate the concept of mechano-inflammation in the pathogenesis of psoriatic disease,” and suggest that interventions with high efficacy for controlling entheseal inflammation may be a “particularly valuable strategy in interfering with the onset of PsA in patients with psoriatic disease,” David Simon, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
The study, which is now published in Arthritis & Rheumatology, began with 377 patients with psoriasis who had been referred to the University Hospital in Erlangen, Germany, during 2011-2018, and who tested positive on the German Psoriasis Arthritis Diagnostic questionnaire. The researchers excluded patients with existing signs of PsA, any arthritis or enthesitis or other signs of inflammatory rheumatic disease, and they also excluded patients who had not undergoing a high-resolution peripheral quantitative CT (HR-pQCT) examination of the second and third metacarpal joints of the patient’s nondominant hand, which left 114 patients for their analysis. During a mean follow-up of 28 months, 24 patients (27%) developed PsA. The study patients were an average age of 45 years, and they had been diagnosed with psoriasis for an average of about 16 years.
Dr. Simon and associates used the baseline HR-pQCT scans to make two assessments of each patient: the presence of structural entheseal lesions (SEL) in the two metacarpal joints and the calculated volumetric bone mineral density (vBMD). Their analysis showed that the number and severity of SEL were increased among patients who later developed PsA. In a multivariable model that adjusted for age, sex, body mass index, duration of psoriasis, and arthralgia, patients with any SEL had a fivefold higher rate of developing PsA, compared with patients with no SEL, reported Dr. Simon, a rheumatologist at Erlangen University Hospital.
The analysis of vBMD also showed a strong link between bone density at the entheseal sites of the two studied joints and subsequent PsA development. For every standard deviation increase in vBMD at these sites the subsequent rate of PsA incidence fell by about 67% in an analysis that controlled for the same covariants as well as presence of SEL. The same relationship between higher vBMD and a lower risk for PsA held for both total vBMD measurement and for cortical vBMD, but only at the entheseal site. Levels of vBMD at the intra-articular site of the joints had no statistically significant relationship with subsequent PsA development.
The two metrics also appeared to identify additive risks. Nearly 90% of patients with at least one SEL who also had low vBMD at the entheseal site developed PsA during follow-up, compared with about a 50% rate among patients with at least one SEL but high vBMD.
The imaging method used to run these analyses, HR-pQCT, remains for the time being a “research technique” that “is not generalizable for routine practice,” but further development of this method or of a surrogate measure might make it feasible for future widespread practice, commented Iain McInnes, MD, PhD, president of the European League Against Rheumatism and professor of rheumatology and director of the Institute of Infection, Immunity, and Inflammation at the University of Glasgow.
“We’ve thought for many years that psoriasis and psoriatic arthritis are on a spectrum, and this work is consistent with the idea that some patients with psoriasis develop tissue involvement at entheses and joints,” Dr. McInnes said in an interview. The higher incidence of PsA seen in patients with adverse SEL and vBMD markers was in an “interesting range” that warrants further study. A next step is to run an intervention study in which patients with these adverse markers would receive an intervention randomized against placebo to see if it improved their outcomes, he suggested. Good candidate agents to study in psoriasis patients who have these adverse markers include drugs that inhibit the action of interleukin-17, drugs that target the p19 cytokine subunit of IL-23, and possibly Janus kinase inhibitor drugs.
Dr. Simon has been a consultant to AbbVie and Eli Lilly, a speaker on behalf of Eli Lilly, Janssen, and Novartis, and has received research funding from Eli Lilly and Novartis. Dr. McInnes has been a consultant to AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB, and he has received research funding from Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, and UCB.
SOURCE: Simon D et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:33-4, Abstract OP0051.
Structural entheseal lesions and reduced bone mineral density detected using high-resolution CT imaging of a pair of knuckle joints in patients with psoriasis strongly linked with subsequent development of psoriatic arthritis (PsA) in a single-center study with 114 patients followed for an average of 2.3 years.
“These findings substantiate the concept of mechano-inflammation in the pathogenesis of psoriatic disease,” and suggest that interventions with high efficacy for controlling entheseal inflammation may be a “particularly valuable strategy in interfering with the onset of PsA in patients with psoriatic disease,” David Simon, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
The study, which is now published in Arthritis & Rheumatology, began with 377 patients with psoriasis who had been referred to the University Hospital in Erlangen, Germany, during 2011-2018, and who tested positive on the German Psoriasis Arthritis Diagnostic questionnaire. The researchers excluded patients with existing signs of PsA, any arthritis or enthesitis or other signs of inflammatory rheumatic disease, and they also excluded patients who had not undergoing a high-resolution peripheral quantitative CT (HR-pQCT) examination of the second and third metacarpal joints of the patient’s nondominant hand, which left 114 patients for their analysis. During a mean follow-up of 28 months, 24 patients (27%) developed PsA. The study patients were an average age of 45 years, and they had been diagnosed with psoriasis for an average of about 16 years.
Dr. Simon and associates used the baseline HR-pQCT scans to make two assessments of each patient: the presence of structural entheseal lesions (SEL) in the two metacarpal joints and the calculated volumetric bone mineral density (vBMD). Their analysis showed that the number and severity of SEL were increased among patients who later developed PsA. In a multivariable model that adjusted for age, sex, body mass index, duration of psoriasis, and arthralgia, patients with any SEL had a fivefold higher rate of developing PsA, compared with patients with no SEL, reported Dr. Simon, a rheumatologist at Erlangen University Hospital.
The analysis of vBMD also showed a strong link between bone density at the entheseal sites of the two studied joints and subsequent PsA development. For every standard deviation increase in vBMD at these sites the subsequent rate of PsA incidence fell by about 67% in an analysis that controlled for the same covariants as well as presence of SEL. The same relationship between higher vBMD and a lower risk for PsA held for both total vBMD measurement and for cortical vBMD, but only at the entheseal site. Levels of vBMD at the intra-articular site of the joints had no statistically significant relationship with subsequent PsA development.
The two metrics also appeared to identify additive risks. Nearly 90% of patients with at least one SEL who also had low vBMD at the entheseal site developed PsA during follow-up, compared with about a 50% rate among patients with at least one SEL but high vBMD.
The imaging method used to run these analyses, HR-pQCT, remains for the time being a “research technique” that “is not generalizable for routine practice,” but further development of this method or of a surrogate measure might make it feasible for future widespread practice, commented Iain McInnes, MD, PhD, president of the European League Against Rheumatism and professor of rheumatology and director of the Institute of Infection, Immunity, and Inflammation at the University of Glasgow.
“We’ve thought for many years that psoriasis and psoriatic arthritis are on a spectrum, and this work is consistent with the idea that some patients with psoriasis develop tissue involvement at entheses and joints,” Dr. McInnes said in an interview. The higher incidence of PsA seen in patients with adverse SEL and vBMD markers was in an “interesting range” that warrants further study. A next step is to run an intervention study in which patients with these adverse markers would receive an intervention randomized against placebo to see if it improved their outcomes, he suggested. Good candidate agents to study in psoriasis patients who have these adverse markers include drugs that inhibit the action of interleukin-17, drugs that target the p19 cytokine subunit of IL-23, and possibly Janus kinase inhibitor drugs.
Dr. Simon has been a consultant to AbbVie and Eli Lilly, a speaker on behalf of Eli Lilly, Janssen, and Novartis, and has received research funding from Eli Lilly and Novartis. Dr. McInnes has been a consultant to AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB, and he has received research funding from Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, and UCB.
SOURCE: Simon D et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:33-4, Abstract OP0051.
FROM THE EULAR 2020 E-CONGRESS