User login
Leg lymphedema after gynecologic lymphadenectomy exceeds expectations
NEW ORLEANS – Leg lymphedema occurred in 19%-40% of women with a gynecologic cancer who underwent surgery with lymphadenectomy in a prospective study of 821 U.S. patients.
The incidence of lymphedema of the lower extremity (LLE) during 2 years of follow-up was 18% among 672 endometrial cancer patients, 25% among 124 cervical cancer patients, and 40% among 24 vulvar cancer patients, Jay W. Carlson, DO, said at the annual meeting of the Society of Gynecologic Oncology.
Although the study followed patients for 2 years after surgery, 84% of the LLE events occurred within the first 6 months after surgery, and 95% within the first 12 months. The robust incidence rates documented in this study contrasted with a general perception that LLE is relatively uncommon, leading Dr. Carlson to note that the new data show “the incidence of LLE is under recognized.” The findings also bucked conventional wisdom by showing no link between the incidence of LLE and number of lymph nodes dissected or with use of radiation treatment, said Dr. Carlson, a gynecologic oncologist at Mercy Clinic Women’s Oncology in Springfield, Mo.
To better define the incidence of LLE after lymphadenectomy for gynecologic cancers, the Gynecologic Oncology Group organized the Lymphedema and Gynecologic Cancer (LEG) study, run at more than 70 U.S. centers during June 2012–November 2014. The study enrolled patients scheduled for surgery to treat endometrial, cervical, or vulvar cancer, and applied systematic leg measurement to patients just before and at several prespecified times following surgery through 2 years of follow-up.
The study began with a total of 1,054 patients, but the final analysis that Dr. Carlson presented excluded patients who did not actually undergo lymphadenectomy during their surgery, did not have leg volume data available both before and after their surgery, or had a comorbidity or change in body mass that could have caused the change in leg size. The researchers also required patients identified with LLE to have completed the Gynecologic Cancer Lymphedema Questionnaire (Gynecol Oncol. 2010 May;117[2]:317-23) and tallied a score of at least 4, and to have at least a 10% increase in leg volume at the time of diagnosis, compared with the presurgical volume.
The exclusions yielded a total of 672 patients with endometrial cancer, including 127 who developed LLE (19%); 124 patients with cervical cancer, including 31 who developed LLE (25%); and 25 patients with vulvar cancer, including 10 who developed LLE (40%), Dr. Carlson reported.
Analysis of the patients who developed LLE showed no significant association with type of surgery (open, robotic, or laparoscopic), and no significant associations with several patient-specific factors including age, race, cancer stage, surgical blood loss, or serum albumin, he said.
mzoler@MDedge.com
On Twitter @mitchelzoler
SOURCE: Carlson J et al. SGO 2018, Abstract 11.
NEW ORLEANS – Leg lymphedema occurred in 19%-40% of women with a gynecologic cancer who underwent surgery with lymphadenectomy in a prospective study of 821 U.S. patients.
The incidence of lymphedema of the lower extremity (LLE) during 2 years of follow-up was 18% among 672 endometrial cancer patients, 25% among 124 cervical cancer patients, and 40% among 24 vulvar cancer patients, Jay W. Carlson, DO, said at the annual meeting of the Society of Gynecologic Oncology.
Although the study followed patients for 2 years after surgery, 84% of the LLE events occurred within the first 6 months after surgery, and 95% within the first 12 months. The robust incidence rates documented in this study contrasted with a general perception that LLE is relatively uncommon, leading Dr. Carlson to note that the new data show “the incidence of LLE is under recognized.” The findings also bucked conventional wisdom by showing no link between the incidence of LLE and number of lymph nodes dissected or with use of radiation treatment, said Dr. Carlson, a gynecologic oncologist at Mercy Clinic Women’s Oncology in Springfield, Mo.
To better define the incidence of LLE after lymphadenectomy for gynecologic cancers, the Gynecologic Oncology Group organized the Lymphedema and Gynecologic Cancer (LEG) study, run at more than 70 U.S. centers during June 2012–November 2014. The study enrolled patients scheduled for surgery to treat endometrial, cervical, or vulvar cancer, and applied systematic leg measurement to patients just before and at several prespecified times following surgery through 2 years of follow-up.
The study began with a total of 1,054 patients, but the final analysis that Dr. Carlson presented excluded patients who did not actually undergo lymphadenectomy during their surgery, did not have leg volume data available both before and after their surgery, or had a comorbidity or change in body mass that could have caused the change in leg size. The researchers also required patients identified with LLE to have completed the Gynecologic Cancer Lymphedema Questionnaire (Gynecol Oncol. 2010 May;117[2]:317-23) and tallied a score of at least 4, and to have at least a 10% increase in leg volume at the time of diagnosis, compared with the presurgical volume.
The exclusions yielded a total of 672 patients with endometrial cancer, including 127 who developed LLE (19%); 124 patients with cervical cancer, including 31 who developed LLE (25%); and 25 patients with vulvar cancer, including 10 who developed LLE (40%), Dr. Carlson reported.
Analysis of the patients who developed LLE showed no significant association with type of surgery (open, robotic, or laparoscopic), and no significant associations with several patient-specific factors including age, race, cancer stage, surgical blood loss, or serum albumin, he said.
mzoler@MDedge.com
On Twitter @mitchelzoler
SOURCE: Carlson J et al. SGO 2018, Abstract 11.
NEW ORLEANS – Leg lymphedema occurred in 19%-40% of women with a gynecologic cancer who underwent surgery with lymphadenectomy in a prospective study of 821 U.S. patients.
The incidence of lymphedema of the lower extremity (LLE) during 2 years of follow-up was 18% among 672 endometrial cancer patients, 25% among 124 cervical cancer patients, and 40% among 24 vulvar cancer patients, Jay W. Carlson, DO, said at the annual meeting of the Society of Gynecologic Oncology.
Although the study followed patients for 2 years after surgery, 84% of the LLE events occurred within the first 6 months after surgery, and 95% within the first 12 months. The robust incidence rates documented in this study contrasted with a general perception that LLE is relatively uncommon, leading Dr. Carlson to note that the new data show “the incidence of LLE is under recognized.” The findings also bucked conventional wisdom by showing no link between the incidence of LLE and number of lymph nodes dissected or with use of radiation treatment, said Dr. Carlson, a gynecologic oncologist at Mercy Clinic Women’s Oncology in Springfield, Mo.
To better define the incidence of LLE after lymphadenectomy for gynecologic cancers, the Gynecologic Oncology Group organized the Lymphedema and Gynecologic Cancer (LEG) study, run at more than 70 U.S. centers during June 2012–November 2014. The study enrolled patients scheduled for surgery to treat endometrial, cervical, or vulvar cancer, and applied systematic leg measurement to patients just before and at several prespecified times following surgery through 2 years of follow-up.
The study began with a total of 1,054 patients, but the final analysis that Dr. Carlson presented excluded patients who did not actually undergo lymphadenectomy during their surgery, did not have leg volume data available both before and after their surgery, or had a comorbidity or change in body mass that could have caused the change in leg size. The researchers also required patients identified with LLE to have completed the Gynecologic Cancer Lymphedema Questionnaire (Gynecol Oncol. 2010 May;117[2]:317-23) and tallied a score of at least 4, and to have at least a 10% increase in leg volume at the time of diagnosis, compared with the presurgical volume.
The exclusions yielded a total of 672 patients with endometrial cancer, including 127 who developed LLE (19%); 124 patients with cervical cancer, including 31 who developed LLE (25%); and 25 patients with vulvar cancer, including 10 who developed LLE (40%), Dr. Carlson reported.
Analysis of the patients who developed LLE showed no significant association with type of surgery (open, robotic, or laparoscopic), and no significant associations with several patient-specific factors including age, race, cancer stage, surgical blood loss, or serum albumin, he said.
mzoler@MDedge.com
On Twitter @mitchelzoler
SOURCE: Carlson J et al. SGO 2018, Abstract 11.
REPORTING FROM SGO 2018
Key clinical point: Leg lymphedema is common following lymphadenectomy for a gynecologic cancer.
Major finding: Leg lymphedema incidence was 19%-40% during 2-year follow-up after lymphadenectomy during gynecologic cancer surgery.
Study details: LEG, a multicenter, U.S. prospective study with 821 gynecologic cancer patients in the final analysis.
Disclosures: LEG had no commercial funding. Dr. Carlson had no disclosures.
Source: Carlson J et al. SGO 2018, Abstract 11.
Disproportionately low U.S. research funding targets gynecologic cancers
NEW ORLEANS – The National Cancer Institute is woefully underfunding gynecologic cancer research, compared with several other cancer types, when the money the institute is spending annually is factored by the incidence and lethal impact of each cancer using U.S. data from 2007 to 2014.
That period featured “systematic and pervasive underfunding of gynecologic cancers in relation to other cancer sites,” Ryan J. Spencer, MD, said at the annual meeting of the Society of Gynecologic Oncology. The trends over the period he studied worsened with time and pose the risk that progress in gynecologic cancers – uterine, cervical, and ovarian – will “lag behind” other cancers’ progress in prevention, treatment, and improved survival, said Dr. Spencer, a gynecologic oncologist at the University of Wisconsin–Madison.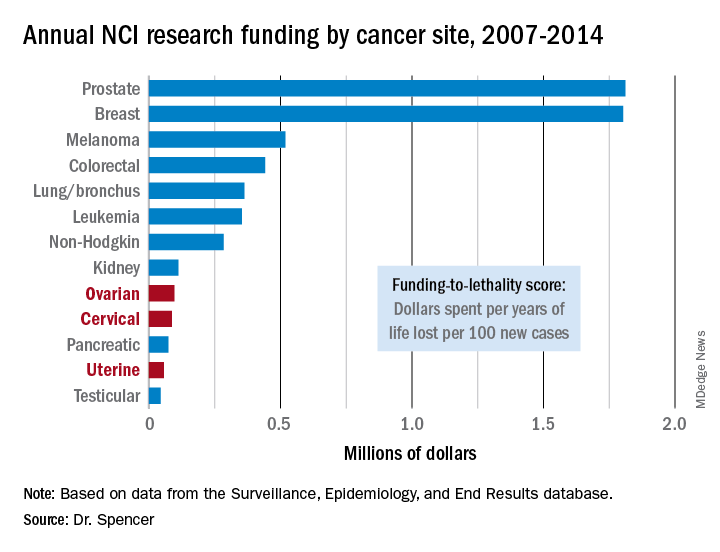
Additional time trend analyses showed that the annual funding-to-lethality score for each of the three gynecologic cancers declined during the period studied.
“We must do everything we can to reverse these trends,” Dr. Spencer concluded.
SOURCE: Spencer R et al. SGO 2018, Abstract 3.
The data reported by Dr. Spencer and his associates are very sobering. They present an elegant analysis that documents a lag and decline in funding for gynecologic cancers that factors in the lethality of various cancers. By several other measures as well, funding for research into gynecologic cancers has been slipping in recent years. During 2011-2016, we saw a 90% drop in enrollment into U.S. clinical trials for gynecologic cancers, and from a peak in 2012-2016 the total number of trials for gynecologic cancers fell by more than two-thirds.
Paola A. Gehrig, MD , is professor of ob.gyn. and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no disclosures. She made these comments as designated discussant for the report.
The data reported by Dr. Spencer and his associates are very sobering. They present an elegant analysis that documents a lag and decline in funding for gynecologic cancers that factors in the lethality of various cancers. By several other measures as well, funding for research into gynecologic cancers has been slipping in recent years. During 2011-2016, we saw a 90% drop in enrollment into U.S. clinical trials for gynecologic cancers, and from a peak in 2012-2016 the total number of trials for gynecologic cancers fell by more than two-thirds.
Paola A. Gehrig, MD , is professor of ob.gyn. and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no disclosures. She made these comments as designated discussant for the report.
The data reported by Dr. Spencer and his associates are very sobering. They present an elegant analysis that documents a lag and decline in funding for gynecologic cancers that factors in the lethality of various cancers. By several other measures as well, funding for research into gynecologic cancers has been slipping in recent years. During 2011-2016, we saw a 90% drop in enrollment into U.S. clinical trials for gynecologic cancers, and from a peak in 2012-2016 the total number of trials for gynecologic cancers fell by more than two-thirds.
Paola A. Gehrig, MD , is professor of ob.gyn. and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no disclosures. She made these comments as designated discussant for the report.
NEW ORLEANS – The National Cancer Institute is woefully underfunding gynecologic cancer research, compared with several other cancer types, when the money the institute is spending annually is factored by the incidence and lethal impact of each cancer using U.S. data from 2007 to 2014.
That period featured “systematic and pervasive underfunding of gynecologic cancers in relation to other cancer sites,” Ryan J. Spencer, MD, said at the annual meeting of the Society of Gynecologic Oncology. The trends over the period he studied worsened with time and pose the risk that progress in gynecologic cancers – uterine, cervical, and ovarian – will “lag behind” other cancers’ progress in prevention, treatment, and improved survival, said Dr. Spencer, a gynecologic oncologist at the University of Wisconsin–Madison.
Additional time trend analyses showed that the annual funding-to-lethality score for each of the three gynecologic cancers declined during the period studied.
“We must do everything we can to reverse these trends,” Dr. Spencer concluded.
SOURCE: Spencer R et al. SGO 2018, Abstract 3.
NEW ORLEANS – The National Cancer Institute is woefully underfunding gynecologic cancer research, compared with several other cancer types, when the money the institute is spending annually is factored by the incidence and lethal impact of each cancer using U.S. data from 2007 to 2014.
That period featured “systematic and pervasive underfunding of gynecologic cancers in relation to other cancer sites,” Ryan J. Spencer, MD, said at the annual meeting of the Society of Gynecologic Oncology. The trends over the period he studied worsened with time and pose the risk that progress in gynecologic cancers – uterine, cervical, and ovarian – will “lag behind” other cancers’ progress in prevention, treatment, and improved survival, said Dr. Spencer, a gynecologic oncologist at the University of Wisconsin–Madison.
Additional time trend analyses showed that the annual funding-to-lethality score for each of the three gynecologic cancers declined during the period studied.
“We must do everything we can to reverse these trends,” Dr. Spencer concluded.
SOURCE: Spencer R et al. SGO 2018, Abstract 3.
REPORTING FROM SGO 2018
Key clinical point: The National Cancer Institute underfunds gynecologic cancer research.
Major finding: Ovarian cancer research funding averaged $97,000 per year of life lost per 100 new cases, compared with $1.8 million for both breast and prostate cancer.
Study details: A review of U.S. data collected by the National Cancer Institute during 2007-2014.
Disclosures: Dr. Spencer had no disclosures.
Source: Spencer R et al. SGO 2018, Abstract 3.
VIDEO: Indocyanine green finds more sentinel lymph nodes
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Indocyanine green (ICG) worked better than isosulfan blue for mapping sentinel lymph nodes (SLNs) in a pivotal phase 3 trial with 176 patients who had stage I endometrial or cervical cancer.
Four injections of ICG resulted in detection of 96% of the identified SLNs in these patients, including bilateral SLNs in 78% of the patients. In contrast, four injections with isosulfan blue dye led to detection of 74% of all SLNs and identified bilateral SLNs in 31% of the patients, Michael M. Frumovitz, MD, said at the annual meeting of the Society for Gynecologic Oncology.
The FILM trial randomized 176 patients with stage I endometrial or cervical cancer at eight centers in the United States or Canada between December 2015 and May 2017. Patients first received one of the tagging agents and then the second, and then underwent mapping using white light to detect blue-tagged SLNs and near-infrared light to find green-tagged SLNs. The patients were aged 63 years on average, and 96% had endometrial cancer.
The researchers identified 279 sentinel lymph nodes that stained only green, nine SLNs that stained only blue, and 248 SLNs tagged with both dyes. They confirmed tumor cells within all nine of SLNs tagged with blue dye only, in 95% of those tagged with ICG only, and in 92% of the SLNs stained with both dyes. The isosulfan blue dye identified SLNs in two patients who did not have any SLNs detected by the ICG, whereas the ICG identified SLNs in 22 patients who did not have any SLNs detected using the blue dye. Sixteen patients had metastatic disease that had moved to 21 SLNs. The ICG system identified all 21 involved lymph nodes; the blue dye identified 13 of the 21 affected SLNs (62%).
Dr. Frumovitz and his associates designed FILM as primarily a test of noninferiority. The per-protocol analysis with 163 patients showed that ICG was noninferior to isosulfan blue (P less than .001). Once the results demonstrated noninferiority, the study protocol allowed the researchers to test for superiority in the full, intention-to-treat cohort of 176 patients. The results showed that ICG was significantly superior to isosulfan blue (P less than .001). In addition, ICG treatment produced no allergic or other adverse reactions, Dr. Frumovitz said.
Once ICG and the associated near-infrared detection camera receive FDA marketing approval, “I think this will become the standard within 5 years,” he predicted in an interview.
The results also showed that using both ICG and isosulfan blue was not better than using ICG alone. “If you’re using both dyes, you can drop the blue dye. At MD Anderson we’ve used only ICG for about the past year,” Dr. Frumovitz said.
FILM was sponsored by Novadaq/Stryker, the company developing the ICG PINPOINT imaging system. Dr. Frumovitz has been a consultant to Novadaq/Stryker and Genentech and has received research funding from Novadaq/Stryker and Navidea. Dr. Backes has been a consultant to Tesaro and has received research funding from Clovis, Eisai, and ImmunoGen. Dr. Buda had no disclosures.
SOURCE: Frumovitz MM. SGO 2018, Abstract 12. Backes FJ. SGO 2018, Abstract 13.
The results from the FILM trial are potentially practice changing. The findings presented by Michael M. Frumovitz, MD, and his associates showed that indocyanine green is superior to isosulfan blue dye for mapping sentinel lymph nodes in patients with stage I endometrial or cervical cancer. The results also showed that using both dyes was no better than using indocyanine green alone.
The report by Floor J. Backes, MD, addressed an important and still unresolved question in treating patients with stage I or II endometrial cancer: What is the significance of finding isolated tumor cells in sentinel lymph nodes in these patients? The retrospective findings she presented showed that the presence of isolated tumor cells had no apparent effect on recurrence-free survival, recurrence pattern, or patient response to various treatments. This suggested th at treatment decisions in these patients should depend on other high-risk uterine factors but not on whether some lymph nodes contained isolated tumor cells.
Brent Smith, MD , is a gynecologic oncologist at the Ohio State University, Columbus. He had no disclosures. Dr. Smith made these comments in a video interview.
The results from the FILM trial are potentially practice changing. The findings presented by Michael M. Frumovitz, MD, and his associates showed that indocyanine green is superior to isosulfan blue dye for mapping sentinel lymph nodes in patients with stage I endometrial or cervical cancer. The results also showed that using both dyes was no better than using indocyanine green alone.
The report by Floor J. Backes, MD, addressed an important and still unresolved question in treating patients with stage I or II endometrial cancer: What is the significance of finding isolated tumor cells in sentinel lymph nodes in these patients? The retrospective findings she presented showed that the presence of isolated tumor cells had no apparent effect on recurrence-free survival, recurrence pattern, or patient response to various treatments. This suggested th at treatment decisions in these patients should depend on other high-risk uterine factors but not on whether some lymph nodes contained isolated tumor cells.
Brent Smith, MD , is a gynecologic oncologist at the Ohio State University, Columbus. He had no disclosures. Dr. Smith made these comments in a video interview.
The results from the FILM trial are potentially practice changing. The findings presented by Michael M. Frumovitz, MD, and his associates showed that indocyanine green is superior to isosulfan blue dye for mapping sentinel lymph nodes in patients with stage I endometrial or cervical cancer. The results also showed that using both dyes was no better than using indocyanine green alone.
The report by Floor J. Backes, MD, addressed an important and still unresolved question in treating patients with stage I or II endometrial cancer: What is the significance of finding isolated tumor cells in sentinel lymph nodes in these patients? The retrospective findings she presented showed that the presence of isolated tumor cells had no apparent effect on recurrence-free survival, recurrence pattern, or patient response to various treatments. This suggested th at treatment decisions in these patients should depend on other high-risk uterine factors but not on whether some lymph nodes contained isolated tumor cells.
Brent Smith, MD , is a gynecologic oncologist at the Ohio State University, Columbus. He had no disclosures. Dr. Smith made these comments in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Indocyanine green (ICG) worked better than isosulfan blue for mapping sentinel lymph nodes (SLNs) in a pivotal phase 3 trial with 176 patients who had stage I endometrial or cervical cancer.
Four injections of ICG resulted in detection of 96% of the identified SLNs in these patients, including bilateral SLNs in 78% of the patients. In contrast, four injections with isosulfan blue dye led to detection of 74% of all SLNs and identified bilateral SLNs in 31% of the patients, Michael M. Frumovitz, MD, said at the annual meeting of the Society for Gynecologic Oncology.
The FILM trial randomized 176 patients with stage I endometrial or cervical cancer at eight centers in the United States or Canada between December 2015 and May 2017. Patients first received one of the tagging agents and then the second, and then underwent mapping using white light to detect blue-tagged SLNs and near-infrared light to find green-tagged SLNs. The patients were aged 63 years on average, and 96% had endometrial cancer.
The researchers identified 279 sentinel lymph nodes that stained only green, nine SLNs that stained only blue, and 248 SLNs tagged with both dyes. They confirmed tumor cells within all nine of SLNs tagged with blue dye only, in 95% of those tagged with ICG only, and in 92% of the SLNs stained with both dyes. The isosulfan blue dye identified SLNs in two patients who did not have any SLNs detected by the ICG, whereas the ICG identified SLNs in 22 patients who did not have any SLNs detected using the blue dye. Sixteen patients had metastatic disease that had moved to 21 SLNs. The ICG system identified all 21 involved lymph nodes; the blue dye identified 13 of the 21 affected SLNs (62%).
Dr. Frumovitz and his associates designed FILM as primarily a test of noninferiority. The per-protocol analysis with 163 patients showed that ICG was noninferior to isosulfan blue (P less than .001). Once the results demonstrated noninferiority, the study protocol allowed the researchers to test for superiority in the full, intention-to-treat cohort of 176 patients. The results showed that ICG was significantly superior to isosulfan blue (P less than .001). In addition, ICG treatment produced no allergic or other adverse reactions, Dr. Frumovitz said.
Once ICG and the associated near-infrared detection camera receive FDA marketing approval, “I think this will become the standard within 5 years,” he predicted in an interview.
The results also showed that using both ICG and isosulfan blue was not better than using ICG alone. “If you’re using both dyes, you can drop the blue dye. At MD Anderson we’ve used only ICG for about the past year,” Dr. Frumovitz said.
FILM was sponsored by Novadaq/Stryker, the company developing the ICG PINPOINT imaging system. Dr. Frumovitz has been a consultant to Novadaq/Stryker and Genentech and has received research funding from Novadaq/Stryker and Navidea. Dr. Backes has been a consultant to Tesaro and has received research funding from Clovis, Eisai, and ImmunoGen. Dr. Buda had no disclosures.
SOURCE: Frumovitz MM. SGO 2018, Abstract 12. Backes FJ. SGO 2018, Abstract 13.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Indocyanine green (ICG) worked better than isosulfan blue for mapping sentinel lymph nodes (SLNs) in a pivotal phase 3 trial with 176 patients who had stage I endometrial or cervical cancer.
Four injections of ICG resulted in detection of 96% of the identified SLNs in these patients, including bilateral SLNs in 78% of the patients. In contrast, four injections with isosulfan blue dye led to detection of 74% of all SLNs and identified bilateral SLNs in 31% of the patients, Michael M. Frumovitz, MD, said at the annual meeting of the Society for Gynecologic Oncology.
The FILM trial randomized 176 patients with stage I endometrial or cervical cancer at eight centers in the United States or Canada between December 2015 and May 2017. Patients first received one of the tagging agents and then the second, and then underwent mapping using white light to detect blue-tagged SLNs and near-infrared light to find green-tagged SLNs. The patients were aged 63 years on average, and 96% had endometrial cancer.
The researchers identified 279 sentinel lymph nodes that stained only green, nine SLNs that stained only blue, and 248 SLNs tagged with both dyes. They confirmed tumor cells within all nine of SLNs tagged with blue dye only, in 95% of those tagged with ICG only, and in 92% of the SLNs stained with both dyes. The isosulfan blue dye identified SLNs in two patients who did not have any SLNs detected by the ICG, whereas the ICG identified SLNs in 22 patients who did not have any SLNs detected using the blue dye. Sixteen patients had metastatic disease that had moved to 21 SLNs. The ICG system identified all 21 involved lymph nodes; the blue dye identified 13 of the 21 affected SLNs (62%).
Dr. Frumovitz and his associates designed FILM as primarily a test of noninferiority. The per-protocol analysis with 163 patients showed that ICG was noninferior to isosulfan blue (P less than .001). Once the results demonstrated noninferiority, the study protocol allowed the researchers to test for superiority in the full, intention-to-treat cohort of 176 patients. The results showed that ICG was significantly superior to isosulfan blue (P less than .001). In addition, ICG treatment produced no allergic or other adverse reactions, Dr. Frumovitz said.
Once ICG and the associated near-infrared detection camera receive FDA marketing approval, “I think this will become the standard within 5 years,” he predicted in an interview.
The results also showed that using both ICG and isosulfan blue was not better than using ICG alone. “If you’re using both dyes, you can drop the blue dye. At MD Anderson we’ve used only ICG for about the past year,” Dr. Frumovitz said.
FILM was sponsored by Novadaq/Stryker, the company developing the ICG PINPOINT imaging system. Dr. Frumovitz has been a consultant to Novadaq/Stryker and Genentech and has received research funding from Novadaq/Stryker and Navidea. Dr. Backes has been a consultant to Tesaro and has received research funding from Clovis, Eisai, and ImmunoGen. Dr. Buda had no disclosures.
SOURCE: Frumovitz MM. SGO 2018, Abstract 12. Backes FJ. SGO 2018, Abstract 13.
REPORTING FROM SGO 2018
Key clinical point: Indocyanine green surpassed isosulfan blue for sentinel lymph node mapping in a pivotal trial.
Major finding: Researchers mapped sentinel lymph nodes in 96% of patients with indocyanine green and in 74% with isosulfan blue.
Study details: FILM, a multicenter, randomized phase 3 trial with 176 patients.
Disclosures: FILM was sponsored by Novadaq/Stryker, the company developing the ICG PINPOINT imaging system. Dr. Frumovitz has been a consultant to Novadaq/Stryker and Genentech and has received research funding from Novadaq/Stryker and Navidea. Dr. Backes has been a consultant to Tesaro and has received research funding from Clovis, Eisai, and ImmunoGen. Dr. Buda had no disclosures.
Source: Frumovitz MM. SGO 2018, Abstract 12. Backes FJ. SGO 2018, Abstract 13.
Trastuzumab plus chemo shows efficacy for high HER2 endometrial cancer
NEW ORLEANS – Adding the anti-HER2 antibody trastuzumab to a standard chemotherapy regimen produced markedly improved responses among patients with an advanced stage or recurrent uterine serous carcinoma that also overexpressed the HER2/neu cell receptor protein in a controlled, multicenter, phase 2 study with 58 evaluable patients.
The 30 patients who received trastuzumab along with carboplatin plus paclitaxel had a median progression-free survival of 12.6 months compared with 8.0 months among the 28 control patients who received only carboplatin plus paclitaxel, a hazard reduction of 56% with trastuzumab that was statistically significant (P = .0052), Alessandro D. Santin, MD, said at the annual meeting of the Society of Gynecologic Oncology.
In the subgroup of 41 patients with advanced (not recurrent) uterine serous carcinoma (USC) the incremental difference in median progression-free survival among patients treated with trastuzumab was 8.6 months, based on a 17.9-month duration in the 20 patients who received trastuzumab and 9.3 months in 20 control patients on chemotherapy only (P = .013).
“We’ll push to have the trastuzumab, carboplatin, and paclitaxel regimen in the guidelines” for treating patients with USC that overexpresses HER2/neu, agreed Amanda N. Fader, MD, director of gynecologic oncology at Johns Hopkins Medicine in Baltimore and lead author on the study reported by Dr. Santin.
Shortly after Dr. Santin’s report at the meeting, the results appeared in an article published online March 27 in Journal of Clinical Oncology.
The phase 2 trial ran at 11 U.S. centers during 2011-2016, and randomized 61 patients with stage III or IV or recurrent USC and high HER2 protein expression measured by immunohistochemistry and amplification of the HER2 gene shown by fluorescence in situ hybridization. The researchers collected data from 58 treated patients. Among the 17 patients with recurrent disease, those who received trastuzumab had a median progression-free survival of 9.2 months compared with 6.0 months in the controls, a hazard reduction of 86% with trastuzumab that was statistically significant (P = .0029). The addition of trastuzumab to carboplatin and paclitaxel was well tolerated.
The greatest benefit from the trastuzumab plus chemotherapy regimen might occur when used as first-line treatment, Dr. Santin suggested. He expressed interest in running a second study to validate the current findings, and a new study that would combine a second antibody directed against HER2, pertuzumab (Perjeta) with trastuzumab, carboplatin, and paclitaxel.
A key factor in making the trastuzumab plus chemotherapy regimen more widely available to USC patients would be routinely screening these patients for high levels of HER2/neu expression immediately after USC is diagnosed. “At Yale and Johns Hopkins this testing is done on all USC patients. That’s not standard care everywhere, but it should be based on these data,” Dr. Santin said in an interview.
SOURCE: Fader A et al. Abstract 22. J Clin Oncol. 2018 Mar 27. doi: 10.1200/JCO.2017.76.5966.
NEW ORLEANS – Adding the anti-HER2 antibody trastuzumab to a standard chemotherapy regimen produced markedly improved responses among patients with an advanced stage or recurrent uterine serous carcinoma that also overexpressed the HER2/neu cell receptor protein in a controlled, multicenter, phase 2 study with 58 evaluable patients.
The 30 patients who received trastuzumab along with carboplatin plus paclitaxel had a median progression-free survival of 12.6 months compared with 8.0 months among the 28 control patients who received only carboplatin plus paclitaxel, a hazard reduction of 56% with trastuzumab that was statistically significant (P = .0052), Alessandro D. Santin, MD, said at the annual meeting of the Society of Gynecologic Oncology.
In the subgroup of 41 patients with advanced (not recurrent) uterine serous carcinoma (USC) the incremental difference in median progression-free survival among patients treated with trastuzumab was 8.6 months, based on a 17.9-month duration in the 20 patients who received trastuzumab and 9.3 months in 20 control patients on chemotherapy only (P = .013).
“We’ll push to have the trastuzumab, carboplatin, and paclitaxel regimen in the guidelines” for treating patients with USC that overexpresses HER2/neu, agreed Amanda N. Fader, MD, director of gynecologic oncology at Johns Hopkins Medicine in Baltimore and lead author on the study reported by Dr. Santin.
Shortly after Dr. Santin’s report at the meeting, the results appeared in an article published online March 27 in Journal of Clinical Oncology.
The phase 2 trial ran at 11 U.S. centers during 2011-2016, and randomized 61 patients with stage III or IV or recurrent USC and high HER2 protein expression measured by immunohistochemistry and amplification of the HER2 gene shown by fluorescence in situ hybridization. The researchers collected data from 58 treated patients. Among the 17 patients with recurrent disease, those who received trastuzumab had a median progression-free survival of 9.2 months compared with 6.0 months in the controls, a hazard reduction of 86% with trastuzumab that was statistically significant (P = .0029). The addition of trastuzumab to carboplatin and paclitaxel was well tolerated.
The greatest benefit from the trastuzumab plus chemotherapy regimen might occur when used as first-line treatment, Dr. Santin suggested. He expressed interest in running a second study to validate the current findings, and a new study that would combine a second antibody directed against HER2, pertuzumab (Perjeta) with trastuzumab, carboplatin, and paclitaxel.
A key factor in making the trastuzumab plus chemotherapy regimen more widely available to USC patients would be routinely screening these patients for high levels of HER2/neu expression immediately after USC is diagnosed. “At Yale and Johns Hopkins this testing is done on all USC patients. That’s not standard care everywhere, but it should be based on these data,” Dr. Santin said in an interview.
SOURCE: Fader A et al. Abstract 22. J Clin Oncol. 2018 Mar 27. doi: 10.1200/JCO.2017.76.5966.
NEW ORLEANS – Adding the anti-HER2 antibody trastuzumab to a standard chemotherapy regimen produced markedly improved responses among patients with an advanced stage or recurrent uterine serous carcinoma that also overexpressed the HER2/neu cell receptor protein in a controlled, multicenter, phase 2 study with 58 evaluable patients.
The 30 patients who received trastuzumab along with carboplatin plus paclitaxel had a median progression-free survival of 12.6 months compared with 8.0 months among the 28 control patients who received only carboplatin plus paclitaxel, a hazard reduction of 56% with trastuzumab that was statistically significant (P = .0052), Alessandro D. Santin, MD, said at the annual meeting of the Society of Gynecologic Oncology.
In the subgroup of 41 patients with advanced (not recurrent) uterine serous carcinoma (USC) the incremental difference in median progression-free survival among patients treated with trastuzumab was 8.6 months, based on a 17.9-month duration in the 20 patients who received trastuzumab and 9.3 months in 20 control patients on chemotherapy only (P = .013).
“We’ll push to have the trastuzumab, carboplatin, and paclitaxel regimen in the guidelines” for treating patients with USC that overexpresses HER2/neu, agreed Amanda N. Fader, MD, director of gynecologic oncology at Johns Hopkins Medicine in Baltimore and lead author on the study reported by Dr. Santin.
Shortly after Dr. Santin’s report at the meeting, the results appeared in an article published online March 27 in Journal of Clinical Oncology.
The phase 2 trial ran at 11 U.S. centers during 2011-2016, and randomized 61 patients with stage III or IV or recurrent USC and high HER2 protein expression measured by immunohistochemistry and amplification of the HER2 gene shown by fluorescence in situ hybridization. The researchers collected data from 58 treated patients. Among the 17 patients with recurrent disease, those who received trastuzumab had a median progression-free survival of 9.2 months compared with 6.0 months in the controls, a hazard reduction of 86% with trastuzumab that was statistically significant (P = .0029). The addition of trastuzumab to carboplatin and paclitaxel was well tolerated.
The greatest benefit from the trastuzumab plus chemotherapy regimen might occur when used as first-line treatment, Dr. Santin suggested. He expressed interest in running a second study to validate the current findings, and a new study that would combine a second antibody directed against HER2, pertuzumab (Perjeta) with trastuzumab, carboplatin, and paclitaxel.
A key factor in making the trastuzumab plus chemotherapy regimen more widely available to USC patients would be routinely screening these patients for high levels of HER2/neu expression immediately after USC is diagnosed. “At Yale and Johns Hopkins this testing is done on all USC patients. That’s not standard care everywhere, but it should be based on these data,” Dr. Santin said in an interview.
SOURCE: Fader A et al. Abstract 22. J Clin Oncol. 2018 Mar 27. doi: 10.1200/JCO.2017.76.5966.
REPORTING FROM SGO 2018
Key clinical point: Trastuzumab plus chemotherapy improved progression-free survival in a phase 2 study.
Major finding: Median progression-free survival was 12.6 months with trastuzumab and 8.0 months in controls.
Study details: A multicenter, phase 2 study that enrolled 58 evaluable patients.
Disclosures: Trastuzumab was provided at no charge by Genentech. Dr. Santin and Dr. Fader had no relevant disclosures.
Source: Fader A et al. Abstract 22. J Clin Oncol. 2018 Mar 27. doi: 10.1200/JCO.2017.76.5966)
views
The biological relevance of HER2 amplification has been clearly established in the results from this and other studies. But we must keep in kind that the results reported by Dr. Santin have applicability to a subset of a subset of patients. Specifically, about 10% of endometrial cancers are uterine serous carcinoma (USC) (although they account for roughly 40% of deaths among endometrial cancer patients), about half of USC patients show a high level of HER2/neu expression by immunohistochemistry, and about 60% of these high-expressing USC also have HER2 gene amplification. The study presented by Dr. Santin ran at 11 centers for 5.5 years and enrolled just 61 patients, fewer than the planned enrollment target of 100 patients.
Michael A. Bookman, MD , is director of gynecologic oncology therapeutics at the Permanente Medical Group in San Francisco. He has been an adviser to AstraZeneca, Bayer, Clovis, Merck, Pfizer, and Tesaro, and he has participated in trials funded by Abbvie, Genentech, Immunogen, Mateon, and Roche. He made these comments as designated discussant for the study.
Residual single-site ovarian cancer surpasses multisite outcomes
NEW ORLEANS – When complete resection of advanced-stage, epithelial ovarian cancer is not possible, surgical resection that leaves a small volume of residual tumor at a single site produces significantly better outcomes than leaving minimal residual cancer at multiple sites, according to a review of 510 patients at two U.S. centers.
“In the past, we separated these patients based on whether they had a complete resection, R0 disease, or had 1 cm or less of residual disease” regardless of the number of sites with this small amount of residual tumor. The third category was patients with more than 1 cm of residual tumor at one or more sites, explained Dr. Manning-Geist in an interview. “What we did was break down the patients with 1 cm or less of residual tumor into those with one site or multiple sites. This is the first reported study to use number of sites” as a clinical characteristic for analysis in this context.
The message from the findings is that, while the goal of debulking surgery in patients with advanced epithelial ovarian cancer is complete tumor resection, if that can’t be achieved, the next goal is to leave residual tumor at just a single site, she concluded. A question that remains is whether primary debulking surgery is preferable to neoadjuvant treatment followed by interval debulking surgery. In the results Dr. Manning-Geist presented, patients who underwent primary debulking had better outcomes than those with neoadjuvant therapy followed by interval debulking, but these two subgroups also had different clinical characteristics.
The study used data from 510 patients with stage IIIC or IV epithelial ovarian cancer treated at either Brigham and Women’s or Massachusetts General Hospital during 2010-2015. The study cohort included 240 patients who underwent primary debulking surgery and 270 who first received neoadjuvant chemotherapy and then underwent interval debulking surgery. The patients who received neoadjuvant therapy were, on average, older (65 years vs. 63 years), had a higher prevalence of stage IV disease (44% vs. 16%), and had a higher prevalence of tumors with serous histology (93% vs. 77%), compared with patients who underwent primary debulking.
Complete tumor resections occurred in 39% of the primary debulking patients and in 64% of those who received neoadjuvant therapy; residual disease of 1 cm or less at one site occurred in 17% and 13%, respectively; minimal residual disease at multiple sites remained in 28% and 17% respectively; and the remaining patients had residual disease of more than 1 cm in at least one site, 16% and 6% respectively.
For this analysis, Dr. Manning-Geist and her associates considered residual disease at any of seven possible sites: diaphragm, upper abdomen, bowel mesentary, bowel serosa, abdominal peritoneum, pelvis, and nodal. Even if multiple individual metastases remained within one of these sites after surgery, it was categorized as a single site of residual disease.
Among patients who underwent primary debulking surgery, progression-free survival persisted for a median of 23 months among patients with full resection, 19 months in patients with a single site with minimal residual disease, 13 months among those with multiple sites of residual disease, and 10 months in patients with more than 1 cm of residual tumor. Median overall survival in these four subgroups was not yet reached, 64 months, 50 months, and 49 months, respectively.
Among patients who received neoadjuvant chemotherapy and then underwent interval debulking surgery, median durations of progression-free survival were 14 months, 12 months, 10 months, and 6 months, respectively. Median overall survival rates were 58 months, 37 months, 26 months, and 33 months, respectively. Within each of these four analyses, the differences in both survival and progression-free survival across the four subgroups was statistically significant, with a P less than .001 for each analysis.
In multivariate analyses, among patients who underwent primary debulking surgery, the significant linkages with worsening progression-free and overall survival were age, cancer stage, and amount and site number of residual disease. Among patients who received neoadjuvant chemotherapy followed by interval debulking residual disease diameter and site number of residual tumor was the only significant determinant for both progression-free and overall survival, Dr. Manning-Geist reported.
Dr. Manning-Geist had no disclosures.
SOURCE: Manning-Geist B et al. SGO 2018, Abstract 43.
NEW ORLEANS – When complete resection of advanced-stage, epithelial ovarian cancer is not possible, surgical resection that leaves a small volume of residual tumor at a single site produces significantly better outcomes than leaving minimal residual cancer at multiple sites, according to a review of 510 patients at two U.S. centers.
“In the past, we separated these patients based on whether they had a complete resection, R0 disease, or had 1 cm or less of residual disease” regardless of the number of sites with this small amount of residual tumor. The third category was patients with more than 1 cm of residual tumor at one or more sites, explained Dr. Manning-Geist in an interview. “What we did was break down the patients with 1 cm or less of residual tumor into those with one site or multiple sites. This is the first reported study to use number of sites” as a clinical characteristic for analysis in this context.
The message from the findings is that, while the goal of debulking surgery in patients with advanced epithelial ovarian cancer is complete tumor resection, if that can’t be achieved, the next goal is to leave residual tumor at just a single site, she concluded. A question that remains is whether primary debulking surgery is preferable to neoadjuvant treatment followed by interval debulking surgery. In the results Dr. Manning-Geist presented, patients who underwent primary debulking had better outcomes than those with neoadjuvant therapy followed by interval debulking, but these two subgroups also had different clinical characteristics.
The study used data from 510 patients with stage IIIC or IV epithelial ovarian cancer treated at either Brigham and Women’s or Massachusetts General Hospital during 2010-2015. The study cohort included 240 patients who underwent primary debulking surgery and 270 who first received neoadjuvant chemotherapy and then underwent interval debulking surgery. The patients who received neoadjuvant therapy were, on average, older (65 years vs. 63 years), had a higher prevalence of stage IV disease (44% vs. 16%), and had a higher prevalence of tumors with serous histology (93% vs. 77%), compared with patients who underwent primary debulking.
Complete tumor resections occurred in 39% of the primary debulking patients and in 64% of those who received neoadjuvant therapy; residual disease of 1 cm or less at one site occurred in 17% and 13%, respectively; minimal residual disease at multiple sites remained in 28% and 17% respectively; and the remaining patients had residual disease of more than 1 cm in at least one site, 16% and 6% respectively.
For this analysis, Dr. Manning-Geist and her associates considered residual disease at any of seven possible sites: diaphragm, upper abdomen, bowel mesentary, bowel serosa, abdominal peritoneum, pelvis, and nodal. Even if multiple individual metastases remained within one of these sites after surgery, it was categorized as a single site of residual disease.
Among patients who underwent primary debulking surgery, progression-free survival persisted for a median of 23 months among patients with full resection, 19 months in patients with a single site with minimal residual disease, 13 months among those with multiple sites of residual disease, and 10 months in patients with more than 1 cm of residual tumor. Median overall survival in these four subgroups was not yet reached, 64 months, 50 months, and 49 months, respectively.
Among patients who received neoadjuvant chemotherapy and then underwent interval debulking surgery, median durations of progression-free survival were 14 months, 12 months, 10 months, and 6 months, respectively. Median overall survival rates were 58 months, 37 months, 26 months, and 33 months, respectively. Within each of these four analyses, the differences in both survival and progression-free survival across the four subgroups was statistically significant, with a P less than .001 for each analysis.
In multivariate analyses, among patients who underwent primary debulking surgery, the significant linkages with worsening progression-free and overall survival were age, cancer stage, and amount and site number of residual disease. Among patients who received neoadjuvant chemotherapy followed by interval debulking residual disease diameter and site number of residual tumor was the only significant determinant for both progression-free and overall survival, Dr. Manning-Geist reported.
Dr. Manning-Geist had no disclosures.
SOURCE: Manning-Geist B et al. SGO 2018, Abstract 43.
NEW ORLEANS – When complete resection of advanced-stage, epithelial ovarian cancer is not possible, surgical resection that leaves a small volume of residual tumor at a single site produces significantly better outcomes than leaving minimal residual cancer at multiple sites, according to a review of 510 patients at two U.S. centers.
“In the past, we separated these patients based on whether they had a complete resection, R0 disease, or had 1 cm or less of residual disease” regardless of the number of sites with this small amount of residual tumor. The third category was patients with more than 1 cm of residual tumor at one or more sites, explained Dr. Manning-Geist in an interview. “What we did was break down the patients with 1 cm or less of residual tumor into those with one site or multiple sites. This is the first reported study to use number of sites” as a clinical characteristic for analysis in this context.
The message from the findings is that, while the goal of debulking surgery in patients with advanced epithelial ovarian cancer is complete tumor resection, if that can’t be achieved, the next goal is to leave residual tumor at just a single site, she concluded. A question that remains is whether primary debulking surgery is preferable to neoadjuvant treatment followed by interval debulking surgery. In the results Dr. Manning-Geist presented, patients who underwent primary debulking had better outcomes than those with neoadjuvant therapy followed by interval debulking, but these two subgroups also had different clinical characteristics.
The study used data from 510 patients with stage IIIC or IV epithelial ovarian cancer treated at either Brigham and Women’s or Massachusetts General Hospital during 2010-2015. The study cohort included 240 patients who underwent primary debulking surgery and 270 who first received neoadjuvant chemotherapy and then underwent interval debulking surgery. The patients who received neoadjuvant therapy were, on average, older (65 years vs. 63 years), had a higher prevalence of stage IV disease (44% vs. 16%), and had a higher prevalence of tumors with serous histology (93% vs. 77%), compared with patients who underwent primary debulking.
Complete tumor resections occurred in 39% of the primary debulking patients and in 64% of those who received neoadjuvant therapy; residual disease of 1 cm or less at one site occurred in 17% and 13%, respectively; minimal residual disease at multiple sites remained in 28% and 17% respectively; and the remaining patients had residual disease of more than 1 cm in at least one site, 16% and 6% respectively.
For this analysis, Dr. Manning-Geist and her associates considered residual disease at any of seven possible sites: diaphragm, upper abdomen, bowel mesentary, bowel serosa, abdominal peritoneum, pelvis, and nodal. Even if multiple individual metastases remained within one of these sites after surgery, it was categorized as a single site of residual disease.
Among patients who underwent primary debulking surgery, progression-free survival persisted for a median of 23 months among patients with full resection, 19 months in patients with a single site with minimal residual disease, 13 months among those with multiple sites of residual disease, and 10 months in patients with more than 1 cm of residual tumor. Median overall survival in these four subgroups was not yet reached, 64 months, 50 months, and 49 months, respectively.
Among patients who received neoadjuvant chemotherapy and then underwent interval debulking surgery, median durations of progression-free survival were 14 months, 12 months, 10 months, and 6 months, respectively. Median overall survival rates were 58 months, 37 months, 26 months, and 33 months, respectively. Within each of these four analyses, the differences in both survival and progression-free survival across the four subgroups was statistically significant, with a P less than .001 for each analysis.
In multivariate analyses, among patients who underwent primary debulking surgery, the significant linkages with worsening progression-free and overall survival were age, cancer stage, and amount and site number of residual disease. Among patients who received neoadjuvant chemotherapy followed by interval debulking residual disease diameter and site number of residual tumor was the only significant determinant for both progression-free and overall survival, Dr. Manning-Geist reported.
Dr. Manning-Geist had no disclosures.
SOURCE: Manning-Geist B et al. SGO 2018, Abstract 43.
REPORTING FROM SGO 2018
Key clinical point:
Major finding: Median overall survival after primary debulking was 64 months with single-site residual disease and 50 months with multisite disease.
Study details: Retrospective review of 510 patients from two U.S. centers.
Disclosures: Dr. Manning-Geist had no disclosures.
Source: Manning-Geist BL et al. SGO 2018, Abstract 43.
VIDEO: Interventions target opioid overprescribing after gynecologic surgery
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – U.S. clinicians prescribe opioid tablets to postsurgical patients too often and at too high a pill count, according to results from two independent studies that examined prescribing patterns and opioid use in patients following gynecologic surgery.
In addition, “setting preoperative expectations about pain management led to increased compliance at discharge,” said Dr. Mark, a gynecologic oncologist at Roswell Park Comprehensive Cancer Center in Buffalo, N.Y.
Findings from the second study, of 122 women who underwent gynecologic surgery at Women and Infants Hospital in Providence, R.I., showed that 32% did not use any opioids for pain following hospital discharge, and that opioid use during hospitalization was a significant predictor of postdischarge opioid needs. This finding provided a way to devise a new prescribing guide for postsurgical patients based on their opioid use while hospitalized, said Erica Weston, MD, a gynecologic oncologist at Johns Hopkins University, Baltimore.
“No question, we are overprescribing,” Dr. Dowdy said, and described a program he and his colleagues at Mayo recently put in place that capped routine opioid pill prescriptions following various surgeries based on historic patient needs. For example, most laparotomy patients receive a prescription for 10 opioid doses on discharge. Based on the first 6 months of this program, it’s on track to cut the annual number of opioid tablets prescribed to postsurgical patients at Mayo by 35,000 for all gynecologic surgeries and by 1.5 million tablets for all Mayo surgical subspecialties, he said.
The study reported by Dr. Mark ran after the Roswell Park gynecologic oncology department implemented new guidelines for dispensing pain control medications following surgery. The guidelines called for comprehensive teaching for patients about pain expectations and pain management both before and after surgery and also established four dispensing categories:
- Patients undergoing minimally invasive or outpatient surgery and with no history of chronic pain and low opioid need while hospitalized received the default dispense of 600 mg ibuprofen every 6 hours as needed for 7 days and 500 mg acetaminophen every 6 hours as needed for 7 days.
- Patients who underwent this surgery but required 5 or more opioid tablets while hospitalized or those with a history of chronic pain and opioid use received the ibuprofen and acetaminophen regimen plus 12 opioid tablets, a 3-day supply with 1 tablet taken every 6 hours as needed.
- Patients who underwent laparotomy and had no chronic pain and opioid history and low opioid use while hospitalized received the ibuprofen and acetaminophen regimen plus 12 opioid tablets, a 3-day supply.
- Patients who underwent laparotomy and showed a higher opioid need based on their use during the 24 hours before discharge received the ibuprofen and acetaminophen regimen plus 24 opioid tablets for 3 days so they could take 2 tablets every 6 hours as needed.
Dr. Mark and his associates collected data from 337 patients managed with these guidelines during June 2017–January 2018 and compared them with 626 patients who underwent gynecologic surgery at Roswell Park during July 2016–June 2017. The data showed the average number of opioid tablets dispensed per patient for all discharges fell from 31.7 before the new guideline to 3.5, an 89% reduction. For the subgroup of patients who had undergone a laparotomy, the average pill number fell from 43.6 to 11.6, a 72% drop. Among patients treated with minimally invasive or outpatient surgery, average tablets dispensed fell from 28.1 before to 0.9 after, a 97% reduction. The reduction among opioid-naive patients was 90%, and it was 83% among patients who used opioids prior to their surgery.
Under the new program, patients requested an opioid refill 14% of the time after laparotomy and 8% of the time after minimally invasive surgery, rates that did not significantly differ from the prior era. Average postoperative pain scores were identical among patients treated under the new dispensing guidelines and those treated during the prior years, and 96% of patients said they were satisfied with the care they received during the new, restricted dispensing period, Dr. Mark reported.
The single-center experience reviewed by Dr. Weston tracked opioid use by 122 women who underwent a minimally invasive hysterectomy at Women and Infants both as inpatients and out to both 1-2 weeks and 4-6 weeks following discharge. The patients were an average age of 61 years, and included 16% who reported chronic pain and 5% with a history of chronic opioid use.
During the inpatient phase, median opioid use was three doses, with 25% of the patients using no opioids. During the first 1-2 weeks following discharge, median opioid use was nine tablets, with 37% of the patients using no opioids. By the 4- to 6-week follow-up (which collected data from 114 of the patients), median opioid use was a cumulative 11 tablets with 67% of the patients reporting no opioid use during the time between their first and second follow-up visit. During the total postdischarge period, 90% of the patients used 30 or fewer opioid tablets.
A multivariate analysis of the findings showed that opioid use while in hospital was the only significant predictor of opioid use after discharge. Age of 65 years or older showed a nonsignificant trend toward less postdischarge opioid use.
Based on these data Dr. Weston and her associates proposed a formula for estimating a patient’s opioid needs at discharge: Gynecologic surgery patients who needed no opioid medication as inpatients could receive 1-5 opioid tablets at discharge, patients who used opioids at or below the median level should receive 10-15 tablets at discharge, and those who used more than the median number of opioid tablets as inpatients should receive 25-30 tablets at discharge. For patients who undergo surgery as outpatients and have no record of pain medication needs, Dr. Weston recommended discharging them with a prescription for 25-30 tablets, possibly reducing this to 10-15 tablets for patients aged 65 years or older.
Dr. Mark, Dr. Weston, and Dr. Dowdy had no disclosures.
SOURCE: Mark J et al. SGO 2018, Abstract 7. Weston E et al. SGO 2018, Abstract 8.
The important and thought provoking reports by Dr. Mark and by Dr. Weston and their associates present innovative ways for clinicians to address the opioid crisis by prescribing fewer narcotics to postsurgical patients when they leave the hospital. Their work suggests that doing this can have little or no negative impact on patient satisfaction with their care. Their findings give us important documentation for prescribing fewer opioid tablets, while still giving patients adequate pain relief.

Their findings also provide clear documentation that, in current practice, without guidance like this opioids are often overprescribed, not out of negligence but because clinicians are simply not sure what a patient will need once they go home following gynecologic surgery. By reviewing the pain medication a patient needed in hospital we can better estimate what patients will need when they go home, and we can better avoid giving patients more opioid tablets than they will really need.
Brent Smith, MD , is a gynecologic oncologist at Ohio State University, Columbus. He had no disclosures. Dr. Smith made these comments in a video interview.
The important and thought provoking reports by Dr. Mark and by Dr. Weston and their associates present innovative ways for clinicians to address the opioid crisis by prescribing fewer narcotics to postsurgical patients when they leave the hospital. Their work suggests that doing this can have little or no negative impact on patient satisfaction with their care. Their findings give us important documentation for prescribing fewer opioid tablets, while still giving patients adequate pain relief.

Their findings also provide clear documentation that, in current practice, without guidance like this opioids are often overprescribed, not out of negligence but because clinicians are simply not sure what a patient will need once they go home following gynecologic surgery. By reviewing the pain medication a patient needed in hospital we can better estimate what patients will need when they go home, and we can better avoid giving patients more opioid tablets than they will really need.
Brent Smith, MD , is a gynecologic oncologist at Ohio State University, Columbus. He had no disclosures. Dr. Smith made these comments in a video interview.
The important and thought provoking reports by Dr. Mark and by Dr. Weston and their associates present innovative ways for clinicians to address the opioid crisis by prescribing fewer narcotics to postsurgical patients when they leave the hospital. Their work suggests that doing this can have little or no negative impact on patient satisfaction with their care. Their findings give us important documentation for prescribing fewer opioid tablets, while still giving patients adequate pain relief.

Their findings also provide clear documentation that, in current practice, without guidance like this opioids are often overprescribed, not out of negligence but because clinicians are simply not sure what a patient will need once they go home following gynecologic surgery. By reviewing the pain medication a patient needed in hospital we can better estimate what patients will need when they go home, and we can better avoid giving patients more opioid tablets than they will really need.
Brent Smith, MD , is a gynecologic oncologist at Ohio State University, Columbus. He had no disclosures. Dr. Smith made these comments in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – U.S. clinicians prescribe opioid tablets to postsurgical patients too often and at too high a pill count, according to results from two independent studies that examined prescribing patterns and opioid use in patients following gynecologic surgery.
In addition, “setting preoperative expectations about pain management led to increased compliance at discharge,” said Dr. Mark, a gynecologic oncologist at Roswell Park Comprehensive Cancer Center in Buffalo, N.Y.
Findings from the second study, of 122 women who underwent gynecologic surgery at Women and Infants Hospital in Providence, R.I., showed that 32% did not use any opioids for pain following hospital discharge, and that opioid use during hospitalization was a significant predictor of postdischarge opioid needs. This finding provided a way to devise a new prescribing guide for postsurgical patients based on their opioid use while hospitalized, said Erica Weston, MD, a gynecologic oncologist at Johns Hopkins University, Baltimore.
“No question, we are overprescribing,” Dr. Dowdy said, and described a program he and his colleagues at Mayo recently put in place that capped routine opioid pill prescriptions following various surgeries based on historic patient needs. For example, most laparotomy patients receive a prescription for 10 opioid doses on discharge. Based on the first 6 months of this program, it’s on track to cut the annual number of opioid tablets prescribed to postsurgical patients at Mayo by 35,000 for all gynecologic surgeries and by 1.5 million tablets for all Mayo surgical subspecialties, he said.
The study reported by Dr. Mark ran after the Roswell Park gynecologic oncology department implemented new guidelines for dispensing pain control medications following surgery. The guidelines called for comprehensive teaching for patients about pain expectations and pain management both before and after surgery and also established four dispensing categories:
- Patients undergoing minimally invasive or outpatient surgery and with no history of chronic pain and low opioid need while hospitalized received the default dispense of 600 mg ibuprofen every 6 hours as needed for 7 days and 500 mg acetaminophen every 6 hours as needed for 7 days.
- Patients who underwent this surgery but required 5 or more opioid tablets while hospitalized or those with a history of chronic pain and opioid use received the ibuprofen and acetaminophen regimen plus 12 opioid tablets, a 3-day supply with 1 tablet taken every 6 hours as needed.
- Patients who underwent laparotomy and had no chronic pain and opioid history and low opioid use while hospitalized received the ibuprofen and acetaminophen regimen plus 12 opioid tablets, a 3-day supply.
- Patients who underwent laparotomy and showed a higher opioid need based on their use during the 24 hours before discharge received the ibuprofen and acetaminophen regimen plus 24 opioid tablets for 3 days so they could take 2 tablets every 6 hours as needed.
Dr. Mark and his associates collected data from 337 patients managed with these guidelines during June 2017–January 2018 and compared them with 626 patients who underwent gynecologic surgery at Roswell Park during July 2016–June 2017. The data showed the average number of opioid tablets dispensed per patient for all discharges fell from 31.7 before the new guideline to 3.5, an 89% reduction. For the subgroup of patients who had undergone a laparotomy, the average pill number fell from 43.6 to 11.6, a 72% drop. Among patients treated with minimally invasive or outpatient surgery, average tablets dispensed fell from 28.1 before to 0.9 after, a 97% reduction. The reduction among opioid-naive patients was 90%, and it was 83% among patients who used opioids prior to their surgery.
Under the new program, patients requested an opioid refill 14% of the time after laparotomy and 8% of the time after minimally invasive surgery, rates that did not significantly differ from the prior era. Average postoperative pain scores were identical among patients treated under the new dispensing guidelines and those treated during the prior years, and 96% of patients said they were satisfied with the care they received during the new, restricted dispensing period, Dr. Mark reported.
The single-center experience reviewed by Dr. Weston tracked opioid use by 122 women who underwent a minimally invasive hysterectomy at Women and Infants both as inpatients and out to both 1-2 weeks and 4-6 weeks following discharge. The patients were an average age of 61 years, and included 16% who reported chronic pain and 5% with a history of chronic opioid use.
During the inpatient phase, median opioid use was three doses, with 25% of the patients using no opioids. During the first 1-2 weeks following discharge, median opioid use was nine tablets, with 37% of the patients using no opioids. By the 4- to 6-week follow-up (which collected data from 114 of the patients), median opioid use was a cumulative 11 tablets with 67% of the patients reporting no opioid use during the time between their first and second follow-up visit. During the total postdischarge period, 90% of the patients used 30 or fewer opioid tablets.
A multivariate analysis of the findings showed that opioid use while in hospital was the only significant predictor of opioid use after discharge. Age of 65 years or older showed a nonsignificant trend toward less postdischarge opioid use.
Based on these data Dr. Weston and her associates proposed a formula for estimating a patient’s opioid needs at discharge: Gynecologic surgery patients who needed no opioid medication as inpatients could receive 1-5 opioid tablets at discharge, patients who used opioids at or below the median level should receive 10-15 tablets at discharge, and those who used more than the median number of opioid tablets as inpatients should receive 25-30 tablets at discharge. For patients who undergo surgery as outpatients and have no record of pain medication needs, Dr. Weston recommended discharging them with a prescription for 25-30 tablets, possibly reducing this to 10-15 tablets for patients aged 65 years or older.
Dr. Mark, Dr. Weston, and Dr. Dowdy had no disclosures.
SOURCE: Mark J et al. SGO 2018, Abstract 7. Weston E et al. SGO 2018, Abstract 8.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – U.S. clinicians prescribe opioid tablets to postsurgical patients too often and at too high a pill count, according to results from two independent studies that examined prescribing patterns and opioid use in patients following gynecologic surgery.
In addition, “setting preoperative expectations about pain management led to increased compliance at discharge,” said Dr. Mark, a gynecologic oncologist at Roswell Park Comprehensive Cancer Center in Buffalo, N.Y.
Findings from the second study, of 122 women who underwent gynecologic surgery at Women and Infants Hospital in Providence, R.I., showed that 32% did not use any opioids for pain following hospital discharge, and that opioid use during hospitalization was a significant predictor of postdischarge opioid needs. This finding provided a way to devise a new prescribing guide for postsurgical patients based on their opioid use while hospitalized, said Erica Weston, MD, a gynecologic oncologist at Johns Hopkins University, Baltimore.
“No question, we are overprescribing,” Dr. Dowdy said, and described a program he and his colleagues at Mayo recently put in place that capped routine opioid pill prescriptions following various surgeries based on historic patient needs. For example, most laparotomy patients receive a prescription for 10 opioid doses on discharge. Based on the first 6 months of this program, it’s on track to cut the annual number of opioid tablets prescribed to postsurgical patients at Mayo by 35,000 for all gynecologic surgeries and by 1.5 million tablets for all Mayo surgical subspecialties, he said.
The study reported by Dr. Mark ran after the Roswell Park gynecologic oncology department implemented new guidelines for dispensing pain control medications following surgery. The guidelines called for comprehensive teaching for patients about pain expectations and pain management both before and after surgery and also established four dispensing categories:
- Patients undergoing minimally invasive or outpatient surgery and with no history of chronic pain and low opioid need while hospitalized received the default dispense of 600 mg ibuprofen every 6 hours as needed for 7 days and 500 mg acetaminophen every 6 hours as needed for 7 days.
- Patients who underwent this surgery but required 5 or more opioid tablets while hospitalized or those with a history of chronic pain and opioid use received the ibuprofen and acetaminophen regimen plus 12 opioid tablets, a 3-day supply with 1 tablet taken every 6 hours as needed.
- Patients who underwent laparotomy and had no chronic pain and opioid history and low opioid use while hospitalized received the ibuprofen and acetaminophen regimen plus 12 opioid tablets, a 3-day supply.
- Patients who underwent laparotomy and showed a higher opioid need based on their use during the 24 hours before discharge received the ibuprofen and acetaminophen regimen plus 24 opioid tablets for 3 days so they could take 2 tablets every 6 hours as needed.
Dr. Mark and his associates collected data from 337 patients managed with these guidelines during June 2017–January 2018 and compared them with 626 patients who underwent gynecologic surgery at Roswell Park during July 2016–June 2017. The data showed the average number of opioid tablets dispensed per patient for all discharges fell from 31.7 before the new guideline to 3.5, an 89% reduction. For the subgroup of patients who had undergone a laparotomy, the average pill number fell from 43.6 to 11.6, a 72% drop. Among patients treated with minimally invasive or outpatient surgery, average tablets dispensed fell from 28.1 before to 0.9 after, a 97% reduction. The reduction among opioid-naive patients was 90%, and it was 83% among patients who used opioids prior to their surgery.
Under the new program, patients requested an opioid refill 14% of the time after laparotomy and 8% of the time after minimally invasive surgery, rates that did not significantly differ from the prior era. Average postoperative pain scores were identical among patients treated under the new dispensing guidelines and those treated during the prior years, and 96% of patients said they were satisfied with the care they received during the new, restricted dispensing period, Dr. Mark reported.
The single-center experience reviewed by Dr. Weston tracked opioid use by 122 women who underwent a minimally invasive hysterectomy at Women and Infants both as inpatients and out to both 1-2 weeks and 4-6 weeks following discharge. The patients were an average age of 61 years, and included 16% who reported chronic pain and 5% with a history of chronic opioid use.
During the inpatient phase, median opioid use was three doses, with 25% of the patients using no opioids. During the first 1-2 weeks following discharge, median opioid use was nine tablets, with 37% of the patients using no opioids. By the 4- to 6-week follow-up (which collected data from 114 of the patients), median opioid use was a cumulative 11 tablets with 67% of the patients reporting no opioid use during the time between their first and second follow-up visit. During the total postdischarge period, 90% of the patients used 30 or fewer opioid tablets.
A multivariate analysis of the findings showed that opioid use while in hospital was the only significant predictor of opioid use after discharge. Age of 65 years or older showed a nonsignificant trend toward less postdischarge opioid use.
Based on these data Dr. Weston and her associates proposed a formula for estimating a patient’s opioid needs at discharge: Gynecologic surgery patients who needed no opioid medication as inpatients could receive 1-5 opioid tablets at discharge, patients who used opioids at or below the median level should receive 10-15 tablets at discharge, and those who used more than the median number of opioid tablets as inpatients should receive 25-30 tablets at discharge. For patients who undergo surgery as outpatients and have no record of pain medication needs, Dr. Weston recommended discharging them with a prescription for 25-30 tablets, possibly reducing this to 10-15 tablets for patients aged 65 years or older.
Dr. Mark, Dr. Weston, and Dr. Dowdy had no disclosures.
SOURCE: Mark J et al. SGO 2018, Abstract 7. Weston E et al. SGO 2018, Abstract 8.
REPORTING FROM SGO 2018
Key clinical point: Patients who underwent gynecologic surgery received adequate pain relief when receiving fewer opioid tablets.
Major finding: A protocol that restricted opioid dispensing successfully cut the discharge allotment of opioid tablets by 89%.
Study details: A single-center review of 337 patients, and a second single-center experience with 122 patients.
Disclosures: Dr. Mark, Dr. Weston, and Dr. Dowdy had no disclosures.
Source: Mark J et al. SGO 2018, Abstract 7. Weston E et al. SGO 2018, Abstract 8.
VIDEO: Cervical cancer laparotomy outperforms minimally invasive surgery
NEW ORLEANS – Use of minimally invasive radical hysterectomy to treat early-stage cervical cancer has grown over the past decade, and in current U.S. practice, roughly half of these cases are done with a minimally-invasive approach, with the rest done by conventional laparotomy. But the first data ever reported from a large, prospective trial that compared the efficacy of both methods for cervical cancer had the unexpected finding that disease-free survival following minimally invasive procedures significantly lagged behind radical hysterectomies done by open laparotomy, Pedro T. Ramirez, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Just after this report came results from a second study that used propensity score–adjusted observational data from the National Cancer Database and found significantly worse overall survival following minimally invasive radical hysterectomy for early-stage cervical cancer, compared with laparotomy, said J. Alejandro Rauh-Hain, MD, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston.
Both findings were “very surprising,” said Dr. Rauh-Hain in a video interview. “I was pretty sure we’d see no difference” in outcomes between minimally invasive radical hysterectomies and the same surgery either done by laparoscope or robotically assisted.
Prior prospective comparisons of minimally invasive and open surgical methods for other cancer types, including endometrial, gastric, and ovarian, showed no differences in cancer recurrences and survival, which led to widening use of minimally invasive surgery (MIS) for cervical cancer despite no direct evidence supporting equivalence, Dr. Rauh-Hain noted. “We adopted it with no data. It made sense that cervical cancer would be the same as endometrial cancer,” he explained.
The Laparoscopic Approach to Cervical Cancer (LACC) trial ran at 33 centers in 12 countries, including six U.S. centers. The study randomized women during 2008-2017 who had stage 1A1, 1A2, or 1B1 cervical cancer to either MIS or open surgery for a radical hysterectomy. Each participating center had to submit to a trial review committee full case records for 10 patients and unedited surgical videos of two patients who had previously undergone a minimally invasive radical hysterectomy at the center to document local prowess with MIS.
Dr. Ramirez and his colleagues designed LACC to prove the noninferiority of MIS and calculated an expected enrollment of 740 patients based on statistical expectations, but the study stopped early after enrolling 631 patients because of the adverse outcomes identified in the MIS patients, with a median follow-up of 2.5 years instead of the planned follow-up of 4.5 years. The study reached the 4.5-year follow-up in about 39% of patients. Of the 312 patients randomized to undergo laparotomy, 88% actually underwent the surgery; of the 319 patients randomized to MIS, 91% received this surgery, with 16% of the MIS procedures done using robotic assistance.
The study’s primary endpoint was disease-free survival at 4.5 years, which occurred in 86% of the MIS patients and in 96.5% of the laparotomy patients, a difference that failed to meet the study’s prespecified definition of noninferiority for MIS, reported Dr. Ramirez, a professor of gynecologic oncology and director of Minimally Invasive Surgery Research and Education at the MD Anderson Cancer Center. In addition, several secondary analyses of the data all showed starkly superior outcomes in the laparotomy subgroup.
Disease-free survival among all patients regardless of follow-up duration occurred in 98% of laparotomy patients and 92% of MIS patients, which translated into a 3.74 hazard ratio (P = .002) for disease recurrence or death among the MIS patients when compared with laparotomy patients. The all-cause mortality rates were 1% in the laparotomy patients and 6% among the MIS patients, a hazard ratio of 6.00 (P = .004). The risk of local or regional recurrences was more than fourfold higher in the MIS patients. A blinded, central panel adjudicated all recurrences identified during the study.
The LACC results “should be discussed with patients scheduled to undergo radical hysterectomy” for cervical cancer, Dr. Ramirez concluded.
The observational data from the National Cancer Database used in the analysis led by Dr. Rauh-Hain came from 2,221 patients hospitalized and treated with radical hysterectomy and pelvic lymph node dissection at a U.S. center during 2010-2012 for either stage 1A2 or 1B1 cervical cancer. Among these patients, 47.5% underwent MIS, with 79% of those procedures done with robotic assistance, while the other 52.5% underwent open laparotomy, Dr. Rauh-Hain reported. Additional analysis of data from this database by the researchers showed that, although the first report of MIS for radical hysterectomy appeared in 1992, the approach remained largely unused in U.S. practice until 2007, when use of MIS began to sharply rise. By 2010, about a third of radical hysterectomies for cervical cancer involved MIS, and usage increased still further during 2011 and 2012 to produce a nearly 48% rate during the 3-year study period.
The primary endpoint of Dr. Rauh-Hain’s analysis was overall survival following propensity-score matching of the MIS and laparotomy patients using 13 demographic and clinical criteria. The analysis showed 4-year mortality rates of 5.8% among the laparotomy patients and 8.4% among the MIS patients, which calculated to a relatively increased mortality hazard from MIS of 48% (P = .02).
Dr. Rauh-Hain also reported results from an interrupted time series analysis using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This analysis compared annual 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer and found that, after survival rates showed a gradual, steady rise during the years culminating in 2006, once MIS began being more widely used in 2007 survival rates began to drop, with a statistically significant annualized decline of 1% through 2010.
Based on the results from both studies, “at MD Anderson we discuss the results with patients,” with the consequence that the percentage of patients treated with laparotomy is now increasing, Dr. Rauh-Hain said. The results from both studies “are concerning,” he explained.
SOURCE: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
The findings from these studies appear valid and should be discussed with patients.
The findings raise a major question: Why has minimally invasive surgery (MIS) led to worse survival rates than laparotomy? Several possible explanations can be hypothesized: The uterine manipulator used in MIS led to local spread of cancer cells; MIS involves a learning curve and initial attempts at MIS did not remove enough of the tumor; and MIS led to increased exposure of the peritoneal cavity to the cancer. The findings also raise another question: Why has MIS for cervical cancer performed less well than MIS for cancers from other organs, such as endometrial and prostate?
The overall survival results from the LACC trial calculate out to 4.75 added deaths per year for every 1,000 patients treated with MIS, compared with laparoscopy. But the National Inpatient Sample data suggest that MIS cuts mortality by about two deaths per year per 1,000 patients, compared with laparotomy, and mortality data from a different analysis (Gynecol Oncol. 2012 Oct;127[1]:11-7) suggest that MIS might prevent six deaths annually for every 1,000 patients, compared with laparotomy. Overall, these three sets of findings suggest roughly comparable mortality outcomes from MIS and laparotomy, but with MIS having the bonus of fewer complications and less need for transfusions.
The cautions and concerns raised by the LACC trial and Dr. Rauh-Hain’s analysis of observational data cannot be easily dismissed. We need to figure out why the results from both studies show worse survival and recurrence rates with MIS, and we need to identify whether subgroups of patients exist who might clearly benefit from either the MIS or open-surgery approach.
Shitanshu Uppal, MD , is a gynecologic oncologist at the University of Michigan in Ann Arbor. He made these comments as designated discussant for the two studies. He had no disclosures.
The findings from these studies appear valid and should be discussed with patients.
The findings raise a major question: Why has minimally invasive surgery (MIS) led to worse survival rates than laparotomy? Several possible explanations can be hypothesized: The uterine manipulator used in MIS led to local spread of cancer cells; MIS involves a learning curve and initial attempts at MIS did not remove enough of the tumor; and MIS led to increased exposure of the peritoneal cavity to the cancer. The findings also raise another question: Why has MIS for cervical cancer performed less well than MIS for cancers from other organs, such as endometrial and prostate?
The overall survival results from the LACC trial calculate out to 4.75 added deaths per year for every 1,000 patients treated with MIS, compared with laparoscopy. But the National Inpatient Sample data suggest that MIS cuts mortality by about two deaths per year per 1,000 patients, compared with laparotomy, and mortality data from a different analysis (Gynecol Oncol. 2012 Oct;127[1]:11-7) suggest that MIS might prevent six deaths annually for every 1,000 patients, compared with laparotomy. Overall, these three sets of findings suggest roughly comparable mortality outcomes from MIS and laparotomy, but with MIS having the bonus of fewer complications and less need for transfusions.
The cautions and concerns raised by the LACC trial and Dr. Rauh-Hain’s analysis of observational data cannot be easily dismissed. We need to figure out why the results from both studies show worse survival and recurrence rates with MIS, and we need to identify whether subgroups of patients exist who might clearly benefit from either the MIS or open-surgery approach.
Shitanshu Uppal, MD , is a gynecologic oncologist at the University of Michigan in Ann Arbor. He made these comments as designated discussant for the two studies. He had no disclosures.
The findings from these studies appear valid and should be discussed with patients.
The findings raise a major question: Why has minimally invasive surgery (MIS) led to worse survival rates than laparotomy? Several possible explanations can be hypothesized: The uterine manipulator used in MIS led to local spread of cancer cells; MIS involves a learning curve and initial attempts at MIS did not remove enough of the tumor; and MIS led to increased exposure of the peritoneal cavity to the cancer. The findings also raise another question: Why has MIS for cervical cancer performed less well than MIS for cancers from other organs, such as endometrial and prostate?
The overall survival results from the LACC trial calculate out to 4.75 added deaths per year for every 1,000 patients treated with MIS, compared with laparoscopy. But the National Inpatient Sample data suggest that MIS cuts mortality by about two deaths per year per 1,000 patients, compared with laparotomy, and mortality data from a different analysis (Gynecol Oncol. 2012 Oct;127[1]:11-7) suggest that MIS might prevent six deaths annually for every 1,000 patients, compared with laparotomy. Overall, these three sets of findings suggest roughly comparable mortality outcomes from MIS and laparotomy, but with MIS having the bonus of fewer complications and less need for transfusions.
The cautions and concerns raised by the LACC trial and Dr. Rauh-Hain’s analysis of observational data cannot be easily dismissed. We need to figure out why the results from both studies show worse survival and recurrence rates with MIS, and we need to identify whether subgroups of patients exist who might clearly benefit from either the MIS or open-surgery approach.
Shitanshu Uppal, MD , is a gynecologic oncologist at the University of Michigan in Ann Arbor. He made these comments as designated discussant for the two studies. He had no disclosures.
NEW ORLEANS – Use of minimally invasive radical hysterectomy to treat early-stage cervical cancer has grown over the past decade, and in current U.S. practice, roughly half of these cases are done with a minimally-invasive approach, with the rest done by conventional laparotomy. But the first data ever reported from a large, prospective trial that compared the efficacy of both methods for cervical cancer had the unexpected finding that disease-free survival following minimally invasive procedures significantly lagged behind radical hysterectomies done by open laparotomy, Pedro T. Ramirez, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Just after this report came results from a second study that used propensity score–adjusted observational data from the National Cancer Database and found significantly worse overall survival following minimally invasive radical hysterectomy for early-stage cervical cancer, compared with laparotomy, said J. Alejandro Rauh-Hain, MD, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston.
Both findings were “very surprising,” said Dr. Rauh-Hain in a video interview. “I was pretty sure we’d see no difference” in outcomes between minimally invasive radical hysterectomies and the same surgery either done by laparoscope or robotically assisted.
Prior prospective comparisons of minimally invasive and open surgical methods for other cancer types, including endometrial, gastric, and ovarian, showed no differences in cancer recurrences and survival, which led to widening use of minimally invasive surgery (MIS) for cervical cancer despite no direct evidence supporting equivalence, Dr. Rauh-Hain noted. “We adopted it with no data. It made sense that cervical cancer would be the same as endometrial cancer,” he explained.
The Laparoscopic Approach to Cervical Cancer (LACC) trial ran at 33 centers in 12 countries, including six U.S. centers. The study randomized women during 2008-2017 who had stage 1A1, 1A2, or 1B1 cervical cancer to either MIS or open surgery for a radical hysterectomy. Each participating center had to submit to a trial review committee full case records for 10 patients and unedited surgical videos of two patients who had previously undergone a minimally invasive radical hysterectomy at the center to document local prowess with MIS.
Dr. Ramirez and his colleagues designed LACC to prove the noninferiority of MIS and calculated an expected enrollment of 740 patients based on statistical expectations, but the study stopped early after enrolling 631 patients because of the adverse outcomes identified in the MIS patients, with a median follow-up of 2.5 years instead of the planned follow-up of 4.5 years. The study reached the 4.5-year follow-up in about 39% of patients. Of the 312 patients randomized to undergo laparotomy, 88% actually underwent the surgery; of the 319 patients randomized to MIS, 91% received this surgery, with 16% of the MIS procedures done using robotic assistance.
The study’s primary endpoint was disease-free survival at 4.5 years, which occurred in 86% of the MIS patients and in 96.5% of the laparotomy patients, a difference that failed to meet the study’s prespecified definition of noninferiority for MIS, reported Dr. Ramirez, a professor of gynecologic oncology and director of Minimally Invasive Surgery Research and Education at the MD Anderson Cancer Center. In addition, several secondary analyses of the data all showed starkly superior outcomes in the laparotomy subgroup.
Disease-free survival among all patients regardless of follow-up duration occurred in 98% of laparotomy patients and 92% of MIS patients, which translated into a 3.74 hazard ratio (P = .002) for disease recurrence or death among the MIS patients when compared with laparotomy patients. The all-cause mortality rates were 1% in the laparotomy patients and 6% among the MIS patients, a hazard ratio of 6.00 (P = .004). The risk of local or regional recurrences was more than fourfold higher in the MIS patients. A blinded, central panel adjudicated all recurrences identified during the study.
The LACC results “should be discussed with patients scheduled to undergo radical hysterectomy” for cervical cancer, Dr. Ramirez concluded.
The observational data from the National Cancer Database used in the analysis led by Dr. Rauh-Hain came from 2,221 patients hospitalized and treated with radical hysterectomy and pelvic lymph node dissection at a U.S. center during 2010-2012 for either stage 1A2 or 1B1 cervical cancer. Among these patients, 47.5% underwent MIS, with 79% of those procedures done with robotic assistance, while the other 52.5% underwent open laparotomy, Dr. Rauh-Hain reported. Additional analysis of data from this database by the researchers showed that, although the first report of MIS for radical hysterectomy appeared in 1992, the approach remained largely unused in U.S. practice until 2007, when use of MIS began to sharply rise. By 2010, about a third of radical hysterectomies for cervical cancer involved MIS, and usage increased still further during 2011 and 2012 to produce a nearly 48% rate during the 3-year study period.
The primary endpoint of Dr. Rauh-Hain’s analysis was overall survival following propensity-score matching of the MIS and laparotomy patients using 13 demographic and clinical criteria. The analysis showed 4-year mortality rates of 5.8% among the laparotomy patients and 8.4% among the MIS patients, which calculated to a relatively increased mortality hazard from MIS of 48% (P = .02).
Dr. Rauh-Hain also reported results from an interrupted time series analysis using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This analysis compared annual 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer and found that, after survival rates showed a gradual, steady rise during the years culminating in 2006, once MIS began being more widely used in 2007 survival rates began to drop, with a statistically significant annualized decline of 1% through 2010.
Based on the results from both studies, “at MD Anderson we discuss the results with patients,” with the consequence that the percentage of patients treated with laparotomy is now increasing, Dr. Rauh-Hain said. The results from both studies “are concerning,” he explained.
SOURCE: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
NEW ORLEANS – Use of minimally invasive radical hysterectomy to treat early-stage cervical cancer has grown over the past decade, and in current U.S. practice, roughly half of these cases are done with a minimally-invasive approach, with the rest done by conventional laparotomy. But the first data ever reported from a large, prospective trial that compared the efficacy of both methods for cervical cancer had the unexpected finding that disease-free survival following minimally invasive procedures significantly lagged behind radical hysterectomies done by open laparotomy, Pedro T. Ramirez, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Just after this report came results from a second study that used propensity score–adjusted observational data from the National Cancer Database and found significantly worse overall survival following minimally invasive radical hysterectomy for early-stage cervical cancer, compared with laparotomy, said J. Alejandro Rauh-Hain, MD, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston.
Both findings were “very surprising,” said Dr. Rauh-Hain in a video interview. “I was pretty sure we’d see no difference” in outcomes between minimally invasive radical hysterectomies and the same surgery either done by laparoscope or robotically assisted.
Prior prospective comparisons of minimally invasive and open surgical methods for other cancer types, including endometrial, gastric, and ovarian, showed no differences in cancer recurrences and survival, which led to widening use of minimally invasive surgery (MIS) for cervical cancer despite no direct evidence supporting equivalence, Dr. Rauh-Hain noted. “We adopted it with no data. It made sense that cervical cancer would be the same as endometrial cancer,” he explained.
The Laparoscopic Approach to Cervical Cancer (LACC) trial ran at 33 centers in 12 countries, including six U.S. centers. The study randomized women during 2008-2017 who had stage 1A1, 1A2, or 1B1 cervical cancer to either MIS or open surgery for a radical hysterectomy. Each participating center had to submit to a trial review committee full case records for 10 patients and unedited surgical videos of two patients who had previously undergone a minimally invasive radical hysterectomy at the center to document local prowess with MIS.
Dr. Ramirez and his colleagues designed LACC to prove the noninferiority of MIS and calculated an expected enrollment of 740 patients based on statistical expectations, but the study stopped early after enrolling 631 patients because of the adverse outcomes identified in the MIS patients, with a median follow-up of 2.5 years instead of the planned follow-up of 4.5 years. The study reached the 4.5-year follow-up in about 39% of patients. Of the 312 patients randomized to undergo laparotomy, 88% actually underwent the surgery; of the 319 patients randomized to MIS, 91% received this surgery, with 16% of the MIS procedures done using robotic assistance.
The study’s primary endpoint was disease-free survival at 4.5 years, which occurred in 86% of the MIS patients and in 96.5% of the laparotomy patients, a difference that failed to meet the study’s prespecified definition of noninferiority for MIS, reported Dr. Ramirez, a professor of gynecologic oncology and director of Minimally Invasive Surgery Research and Education at the MD Anderson Cancer Center. In addition, several secondary analyses of the data all showed starkly superior outcomes in the laparotomy subgroup.
Disease-free survival among all patients regardless of follow-up duration occurred in 98% of laparotomy patients and 92% of MIS patients, which translated into a 3.74 hazard ratio (P = .002) for disease recurrence or death among the MIS patients when compared with laparotomy patients. The all-cause mortality rates were 1% in the laparotomy patients and 6% among the MIS patients, a hazard ratio of 6.00 (P = .004). The risk of local or regional recurrences was more than fourfold higher in the MIS patients. A blinded, central panel adjudicated all recurrences identified during the study.
The LACC results “should be discussed with patients scheduled to undergo radical hysterectomy” for cervical cancer, Dr. Ramirez concluded.
The observational data from the National Cancer Database used in the analysis led by Dr. Rauh-Hain came from 2,221 patients hospitalized and treated with radical hysterectomy and pelvic lymph node dissection at a U.S. center during 2010-2012 for either stage 1A2 or 1B1 cervical cancer. Among these patients, 47.5% underwent MIS, with 79% of those procedures done with robotic assistance, while the other 52.5% underwent open laparotomy, Dr. Rauh-Hain reported. Additional analysis of data from this database by the researchers showed that, although the first report of MIS for radical hysterectomy appeared in 1992, the approach remained largely unused in U.S. practice until 2007, when use of MIS began to sharply rise. By 2010, about a third of radical hysterectomies for cervical cancer involved MIS, and usage increased still further during 2011 and 2012 to produce a nearly 48% rate during the 3-year study period.
The primary endpoint of Dr. Rauh-Hain’s analysis was overall survival following propensity-score matching of the MIS and laparotomy patients using 13 demographic and clinical criteria. The analysis showed 4-year mortality rates of 5.8% among the laparotomy patients and 8.4% among the MIS patients, which calculated to a relatively increased mortality hazard from MIS of 48% (P = .02).
Dr. Rauh-Hain also reported results from an interrupted time series analysis using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This analysis compared annual 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer and found that, after survival rates showed a gradual, steady rise during the years culminating in 2006, once MIS began being more widely used in 2007 survival rates began to drop, with a statistically significant annualized decline of 1% through 2010.
Based on the results from both studies, “at MD Anderson we discuss the results with patients,” with the consequence that the percentage of patients treated with laparotomy is now increasing, Dr. Rauh-Hain said. The results from both studies “are concerning,” he explained.
SOURCE: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
REPORTING FROM SGO 2018
Key clinical point: Laparotomy produced better survival than did minimally invasive surgery for cervical cancer.
Major finding: Disease-free survival after 4.5 years was 96.5% with laparotomy and 86.0% with minimally invasive surgery.
Study details: LACC was a multicenter, randomized trial with 631 patients. The observational study included 2,221 patients from the National Cancer Database during 2010-2012.
Disclosures: Dr. Ramirez and Dr. Rauh-Hain had no disclosures.
Source: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
Combined PARP, checkpoint inhibitor regimens show ovarian cancer activity
NEW ORLEANS – Results from the first two trials to assess the efficacy of combined treatment with a PARP inhibitor plus an immune checkpoint inhibitor in patients with ovarian cancer showed promising results in both phase 2.
The second trial treated 34 patients with platinum-sensitive ovarian cancer with a “chemotherapy-sparing” combination of the PARP inhibitor olaparib (Lynparza) and the immune checkpoint inhibitor durvalumab (Imfinzi) and showed an objective response rate of 72% and a disease control rate of 81%, reported Yvette Drew, MD, of the Northern Institute for Cancer Research in Newcastle upon Tyne, England.
These responses in the two uncontrolled series appeared to exceed historical rates on monotherapy with a PARP inhibitor or immune checkpoint inhibitor, leading both Dr. Konstantinopoulos and Dr. Drew to suggest possible synergy between the two drug classes. The response rates “were higher than previously reported” with either drug alone in platinum-resistant or -refractory ovarian cancer patients, noted Dr. Konstantinopoulos, the director of translational research in the Gynecologic Oncology Program at the Dana-Farber Cancer Institute in Boston.
In both phase 2 studies, the drugs were generally well tolerated, with grade 3 or higher adverse effects roughly matching what’s been reported using monotherapy with each of the tested drugs. The most common was anemia, in 12%-19% of patients. The data on niraparib plus pembrolizumab that Dr. Konstantinopoulos reported also included nine patients in a phase 1 study that had compared the dosages used in the phase 2 study against a second regimen that used a higher niraparib dosage.
The TOPACIO (Niraparib in Combination With Pembrolizumab in Patients With Triple-Negative Breast Cancer or Ovarian Cancer) trial enrolled ovarian cancer patients who had been on a platinum regimen for at least 6 months and had received no more than five prior lines of treatment. The phase 1/2 study MEDIOLA (MEDI4736 in Combination With Olaparib in Patients With Advanced Solid Tumors) enrolled only patients with a BRCA mutation and at least two prior lines of treatment. The substantial differences in response and control rates between the two studies can be at least partially attributed to differences in their enrollment criteria and other study details, commented Amir A. Jazaeri, MD, director of the Gynecologic Cancer Immunotherapy Program at the University of Texas MD Anderson Cancer Center in Houston.
TOPACIO was funded by Tesaro and Merck. MEDIOLA was funded by AstraZeneca. Dr. Konstantinopoulos is an adviser to AstraZeneca, Merck, and Pfizer. Dr. Drew is an adviser to AstraZeneca and Clovis. Dr. Jazaeri is an adviser to Areva, has received travel support from AstraZeneca and Genentech as well as research funding from AstraZeneca, Bristol-Myers Squibb, Iovance, and Pfizer.
SOURCE: Konstantinopoulos PA and Drew Y. SGO 2018.
NEW ORLEANS – Results from the first two trials to assess the efficacy of combined treatment with a PARP inhibitor plus an immune checkpoint inhibitor in patients with ovarian cancer showed promising results in both phase 2.
The second trial treated 34 patients with platinum-sensitive ovarian cancer with a “chemotherapy-sparing” combination of the PARP inhibitor olaparib (Lynparza) and the immune checkpoint inhibitor durvalumab (Imfinzi) and showed an objective response rate of 72% and a disease control rate of 81%, reported Yvette Drew, MD, of the Northern Institute for Cancer Research in Newcastle upon Tyne, England.
These responses in the two uncontrolled series appeared to exceed historical rates on monotherapy with a PARP inhibitor or immune checkpoint inhibitor, leading both Dr. Konstantinopoulos and Dr. Drew to suggest possible synergy between the two drug classes. The response rates “were higher than previously reported” with either drug alone in platinum-resistant or -refractory ovarian cancer patients, noted Dr. Konstantinopoulos, the director of translational research in the Gynecologic Oncology Program at the Dana-Farber Cancer Institute in Boston.
In both phase 2 studies, the drugs were generally well tolerated, with grade 3 or higher adverse effects roughly matching what’s been reported using monotherapy with each of the tested drugs. The most common was anemia, in 12%-19% of patients. The data on niraparib plus pembrolizumab that Dr. Konstantinopoulos reported also included nine patients in a phase 1 study that had compared the dosages used in the phase 2 study against a second regimen that used a higher niraparib dosage.
The TOPACIO (Niraparib in Combination With Pembrolizumab in Patients With Triple-Negative Breast Cancer or Ovarian Cancer) trial enrolled ovarian cancer patients who had been on a platinum regimen for at least 6 months and had received no more than five prior lines of treatment. The phase 1/2 study MEDIOLA (MEDI4736 in Combination With Olaparib in Patients With Advanced Solid Tumors) enrolled only patients with a BRCA mutation and at least two prior lines of treatment. The substantial differences in response and control rates between the two studies can be at least partially attributed to differences in their enrollment criteria and other study details, commented Amir A. Jazaeri, MD, director of the Gynecologic Cancer Immunotherapy Program at the University of Texas MD Anderson Cancer Center in Houston.
TOPACIO was funded by Tesaro and Merck. MEDIOLA was funded by AstraZeneca. Dr. Konstantinopoulos is an adviser to AstraZeneca, Merck, and Pfizer. Dr. Drew is an adviser to AstraZeneca and Clovis. Dr. Jazaeri is an adviser to Areva, has received travel support from AstraZeneca and Genentech as well as research funding from AstraZeneca, Bristol-Myers Squibb, Iovance, and Pfizer.
SOURCE: Konstantinopoulos PA and Drew Y. SGO 2018.
NEW ORLEANS – Results from the first two trials to assess the efficacy of combined treatment with a PARP inhibitor plus an immune checkpoint inhibitor in patients with ovarian cancer showed promising results in both phase 2.
The second trial treated 34 patients with platinum-sensitive ovarian cancer with a “chemotherapy-sparing” combination of the PARP inhibitor olaparib (Lynparza) and the immune checkpoint inhibitor durvalumab (Imfinzi) and showed an objective response rate of 72% and a disease control rate of 81%, reported Yvette Drew, MD, of the Northern Institute for Cancer Research in Newcastle upon Tyne, England.
These responses in the two uncontrolled series appeared to exceed historical rates on monotherapy with a PARP inhibitor or immune checkpoint inhibitor, leading both Dr. Konstantinopoulos and Dr. Drew to suggest possible synergy between the two drug classes. The response rates “were higher than previously reported” with either drug alone in platinum-resistant or -refractory ovarian cancer patients, noted Dr. Konstantinopoulos, the director of translational research in the Gynecologic Oncology Program at the Dana-Farber Cancer Institute in Boston.
In both phase 2 studies, the drugs were generally well tolerated, with grade 3 or higher adverse effects roughly matching what’s been reported using monotherapy with each of the tested drugs. The most common was anemia, in 12%-19% of patients. The data on niraparib plus pembrolizumab that Dr. Konstantinopoulos reported also included nine patients in a phase 1 study that had compared the dosages used in the phase 2 study against a second regimen that used a higher niraparib dosage.
The TOPACIO (Niraparib in Combination With Pembrolizumab in Patients With Triple-Negative Breast Cancer or Ovarian Cancer) trial enrolled ovarian cancer patients who had been on a platinum regimen for at least 6 months and had received no more than five prior lines of treatment. The phase 1/2 study MEDIOLA (MEDI4736 in Combination With Olaparib in Patients With Advanced Solid Tumors) enrolled only patients with a BRCA mutation and at least two prior lines of treatment. The substantial differences in response and control rates between the two studies can be at least partially attributed to differences in their enrollment criteria and other study details, commented Amir A. Jazaeri, MD, director of the Gynecologic Cancer Immunotherapy Program at the University of Texas MD Anderson Cancer Center in Houston.
TOPACIO was funded by Tesaro and Merck. MEDIOLA was funded by AstraZeneca. Dr. Konstantinopoulos is an adviser to AstraZeneca, Merck, and Pfizer. Dr. Drew is an adviser to AstraZeneca and Clovis. Dr. Jazaeri is an adviser to Areva, has received travel support from AstraZeneca and Genentech as well as research funding from AstraZeneca, Bristol-Myers Squibb, Iovance, and Pfizer.
SOURCE: Konstantinopoulos PA and Drew Y. SGO 2018.
REPORTING FROM SGO 2018
Key clinical point:
Major finding: Disease control rate was 67% in the TOPACIO study and 81% in the MEDIOLA study.
Study details: TOPACIO was a multicenter phase 1/2 study with 62 total patients. MEDIOLA was a multicenter phase 2 study with 34 patients.
Disclosures: TOPACIO was funded by Tesaro and Merck. MEDIOLA was funded by AstraZeneca. Dr. Konstantinopoulos is an adviser to AstraZeneca, Merck, and Pfizer. Dr. Drew is an adviser to AstraZeneca and Clovis. Dr. Jazaeri is an adviser to Areva, has received travel support from AstraZeneca and Genentech as well as research funding from AstraZeneca, Bristol-Myers Squibb, Iovance, and Pfizer.
Source: Konstantinopoulos PA and Drew Y. SGO 2018.
VIDEO: Pelvic radiation surpasses brachytherapy/chemo for early endometrial cancer
NEW ORLEANS – Pelvic radiation was as effective for producing recurrence-free survival as vaginal cuff brachytherapy plus chemotherapy but with less acute toxicity and fewer local recurrences in women with high-risk stage I or stage II endometrial cancer in a multicenter, randomized trial with 601 patients.
These findings should result in wider use of pelvic radiation as the preferred treatment for these patients, Marcus E. Randall, MD, said at the annual meeting of the Society of Gynecologic Oncology. “It will change practice,” he predicted.
Dr. Randall and his colleagues from the Gynecologic Oncology Group (which recently became part of NRG Oncology) designed the trial, GOG-0249, to address recent interest in using vaginal cuff brachytherapy plus chemotherapy with carboplatin and paclitaxel as an alternative to the more standard approach of pelvic radiation for treating women with either high-risk stage I or stage II endometrial cancers. Clinicians had considered the brachytherapy plus chemotherapy approach a reasonable option by “extrapolating from studies with advanced” endometrial cancer, but with no direct evidence to support this alternative, Dr. Randall explained in a video interview.
To generate evidence, the researchers enrolled 601 patients at several participating U.S. centers and followed them for a median of 53 months (4.4 years), with 259 patients treated and followed in the pelvic radiation arm and 268 patients treated and followed in the brachytherapy plus chemotherapy arm. Clinicians administered the complete planned treatment regimen to 91% of patients assigned to pelvic radiation and to 87% of those assigned to brachytherapy plus chemotherapy. Three quarters of enrolled patients had high-risk stage I disease, and the entire study group averaged about 62 years old.
The trial’s primary endpoint was recurrence-free survival, which occurred in 78% of the pelvic radiation patients and 79% of brachytherapy plus chemotherapy patients after 5 years when analyzed on an intention-to-treat basis. The two subgroups also showed similar rates of overall survival during follow-up.
Although the two treatments produced essentially identical outcomes for the primary result, they showed two important differences on secondary outcomes, reported Dr. Randall, professor and chair of radiation medicine at the University of Kentucky in Lexington.
Acute adverse effects rated as grade 3 severity or higher occurred in 11% of the pelvic radiation patients and in 64% of the brachytherapy plus chemotherapy patients, although late toxicities occurred at similar rates (13% and 12%, respectively) in the two subgroups.
Local pelvic and para-aortic nodal recurrences occurred in 4% of the pelvic radiation patients and in 9% of the brachytherapy plus chemotherapy patients, a 53% relative risk reduction with pelvic radiation. The difference in the nodal recurrences was apparent within the first year of follow-up, and the difference in rates continued to steadily widen over time after that. However the rates of both vaginal and distant recurrences were very similar in the two treatment arms. Distant recurrences were the most common type of treatment failure, occurring in about 18% of patients in both subgroups during complete follow-up.
“Pelvic radiation therapy remains an appropriate and preferable treatment for high-risk, early stage endometrial carcinoma,” Dr. Randall concluded.
SOURCE: Randall ME et al. SGO 2018.
NEW ORLEANS – Pelvic radiation was as effective for producing recurrence-free survival as vaginal cuff brachytherapy plus chemotherapy but with less acute toxicity and fewer local recurrences in women with high-risk stage I or stage II endometrial cancer in a multicenter, randomized trial with 601 patients.
These findings should result in wider use of pelvic radiation as the preferred treatment for these patients, Marcus E. Randall, MD, said at the annual meeting of the Society of Gynecologic Oncology. “It will change practice,” he predicted.
Dr. Randall and his colleagues from the Gynecologic Oncology Group (which recently became part of NRG Oncology) designed the trial, GOG-0249, to address recent interest in using vaginal cuff brachytherapy plus chemotherapy with carboplatin and paclitaxel as an alternative to the more standard approach of pelvic radiation for treating women with either high-risk stage I or stage II endometrial cancers. Clinicians had considered the brachytherapy plus chemotherapy approach a reasonable option by “extrapolating from studies with advanced” endometrial cancer, but with no direct evidence to support this alternative, Dr. Randall explained in a video interview.
To generate evidence, the researchers enrolled 601 patients at several participating U.S. centers and followed them for a median of 53 months (4.4 years), with 259 patients treated and followed in the pelvic radiation arm and 268 patients treated and followed in the brachytherapy plus chemotherapy arm. Clinicians administered the complete planned treatment regimen to 91% of patients assigned to pelvic radiation and to 87% of those assigned to brachytherapy plus chemotherapy. Three quarters of enrolled patients had high-risk stage I disease, and the entire study group averaged about 62 years old.
The trial’s primary endpoint was recurrence-free survival, which occurred in 78% of the pelvic radiation patients and 79% of brachytherapy plus chemotherapy patients after 5 years when analyzed on an intention-to-treat basis. The two subgroups also showed similar rates of overall survival during follow-up.
Although the two treatments produced essentially identical outcomes for the primary result, they showed two important differences on secondary outcomes, reported Dr. Randall, professor and chair of radiation medicine at the University of Kentucky in Lexington.
Acute adverse effects rated as grade 3 severity or higher occurred in 11% of the pelvic radiation patients and in 64% of the brachytherapy plus chemotherapy patients, although late toxicities occurred at similar rates (13% and 12%, respectively) in the two subgroups.
Local pelvic and para-aortic nodal recurrences occurred in 4% of the pelvic radiation patients and in 9% of the brachytherapy plus chemotherapy patients, a 53% relative risk reduction with pelvic radiation. The difference in the nodal recurrences was apparent within the first year of follow-up, and the difference in rates continued to steadily widen over time after that. However the rates of both vaginal and distant recurrences were very similar in the two treatment arms. Distant recurrences were the most common type of treatment failure, occurring in about 18% of patients in both subgroups during complete follow-up.
“Pelvic radiation therapy remains an appropriate and preferable treatment for high-risk, early stage endometrial carcinoma,” Dr. Randall concluded.
SOURCE: Randall ME et al. SGO 2018.
NEW ORLEANS – Pelvic radiation was as effective for producing recurrence-free survival as vaginal cuff brachytherapy plus chemotherapy but with less acute toxicity and fewer local recurrences in women with high-risk stage I or stage II endometrial cancer in a multicenter, randomized trial with 601 patients.
These findings should result in wider use of pelvic radiation as the preferred treatment for these patients, Marcus E. Randall, MD, said at the annual meeting of the Society of Gynecologic Oncology. “It will change practice,” he predicted.
Dr. Randall and his colleagues from the Gynecologic Oncology Group (which recently became part of NRG Oncology) designed the trial, GOG-0249, to address recent interest in using vaginal cuff brachytherapy plus chemotherapy with carboplatin and paclitaxel as an alternative to the more standard approach of pelvic radiation for treating women with either high-risk stage I or stage II endometrial cancers. Clinicians had considered the brachytherapy plus chemotherapy approach a reasonable option by “extrapolating from studies with advanced” endometrial cancer, but with no direct evidence to support this alternative, Dr. Randall explained in a video interview.
To generate evidence, the researchers enrolled 601 patients at several participating U.S. centers and followed them for a median of 53 months (4.4 years), with 259 patients treated and followed in the pelvic radiation arm and 268 patients treated and followed in the brachytherapy plus chemotherapy arm. Clinicians administered the complete planned treatment regimen to 91% of patients assigned to pelvic radiation and to 87% of those assigned to brachytherapy plus chemotherapy. Three quarters of enrolled patients had high-risk stage I disease, and the entire study group averaged about 62 years old.
The trial’s primary endpoint was recurrence-free survival, which occurred in 78% of the pelvic radiation patients and 79% of brachytherapy plus chemotherapy patients after 5 years when analyzed on an intention-to-treat basis. The two subgroups also showed similar rates of overall survival during follow-up.
Although the two treatments produced essentially identical outcomes for the primary result, they showed two important differences on secondary outcomes, reported Dr. Randall, professor and chair of radiation medicine at the University of Kentucky in Lexington.
Acute adverse effects rated as grade 3 severity or higher occurred in 11% of the pelvic radiation patients and in 64% of the brachytherapy plus chemotherapy patients, although late toxicities occurred at similar rates (13% and 12%, respectively) in the two subgroups.
Local pelvic and para-aortic nodal recurrences occurred in 4% of the pelvic radiation patients and in 9% of the brachytherapy plus chemotherapy patients, a 53% relative risk reduction with pelvic radiation. The difference in the nodal recurrences was apparent within the first year of follow-up, and the difference in rates continued to steadily widen over time after that. However the rates of both vaginal and distant recurrences were very similar in the two treatment arms. Distant recurrences were the most common type of treatment failure, occurring in about 18% of patients in both subgroups during complete follow-up.
“Pelvic radiation therapy remains an appropriate and preferable treatment for high-risk, early stage endometrial carcinoma,” Dr. Randall concluded.
SOURCE: Randall ME et al. SGO 2018.
REPORTING FROM SGO 2018
Key clinical point:
Major finding: Acute, higher-grade toxicities occurred in 11% of pelvic radiation patients and 64% of brachytherapy/chemotherapy patients.
Study details: GOG-0249, a multicenter, randomized phase III trial with 601 patients.
Disclosures: GOG-0249 had no commercial funding. Dr. Randall had no disclosures.
Source: Randall ME et al. SGO 2018.
VIDEO: Everolimus/letrozole promising for recurrent endometrial cancer
NEW ORLEANS – Combined treatment for 28 days with the mammalian target of rapamycin inhibitor everolimus plus the aromatase inhibitor letrozole in 37 women with recurrent endometrial cancer produced an overall objective response rate of 24% and an average progression-free survival rate of 6.3 months in a randomized phase 2 study with a total of 74 patients. The treatment was also relatively well tolerated, with more serious adverse vents of anemia and hyperglycemia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
But the most attention-grabbing finding of this study was that, in the subset of 15 women who had received no prior chemotherapy, the objective response rate on this regimen was 53% and median progression-free survival was 21.6 months, Brian M. Slomovitz, MD, said at the annual meeting of the Society of Gynecologic Oncology.
This level of response in the chemotherapy-naive subgroup was “very high, and not what we expected,” Dr. Slomovitz, , professor of ob.gyn. and human genetics and director of gynecologic oncology at the University of Miami, said in a video interview. “This is something we need to further investigate to see if we can make this part of standard care.”
Although he conceded that the data from this study were too limited to warrant a regulatory indication, he suggested that it might be enough to gain the everolimus plus letrozole combination used in the study citation as a treatment option in clinical guidelines. The next step should be a phase 3 trial that compares the everolimus plus letrozole combination with the traditional chemotherapy regimen of carboplatin plus paclitaxel, Dr. Slomovitz added.
The Everolimus and Letrozole or Hormonal Therapy to Treat Endometrial Cancer phase 2 trial, initiated by the Gynecologic Oncology Group, ran at 26 U.S. centers, and randomized patients with stage III or IV recurrent, advanced, or persistent endometrial cancer who had either no or at most one prior course of chemotherapy. The study design also excluded patients who had previously been treated with an mammalian target of rapamycin inhibitor or hormonal therapy for their endometrial cancer. The control arm placed 37 patients on a standard hormonal therapy regimen of tamoxifen plus medroxyprogesterone, with 36 of patients in this subgroup evaluable.
The overall results in the two treatment arms were roughly similar, except for the striking benefit seen with everolimus(Afinitor) plus letrozole(Femara) in the chemotherapy-naive patents; 15 patients in each arm had not received any chemotherapy before entering the study. In this subgroup, among the patients who received conventional hormonal therapy, the objective response rate was 43% and progression-free survival was 6.6 months.
The two arms also differed by their pattern of grade 3 or 4 adverse events. Among the patients on everolimus plus letrozole, the most common were anemia (24%) and hyperglycemia (14%), both expected consequences of the regimen. The hormonal therapy arm led to an 8% incidence of thromboembolic events, which did not occur in the everolimus plus letrozole arm.
Another attraction of the everolimus and letrozole regimen is that it is oral and avoids the need for drug infusions, Dr. Slomovitz noted.
The investigator-initiated study received funding from Novartis, the company that markets everolimus and letrozole. Dr. Slomovitz has been an advisor to Advaxis, AstraZeneca, Clovis, Genmab, Jannsen, and Tesaro, and he has received research funding from Novartis.
SOURCE: Slomovitz BM et al. SGO 2018, abstract 1.
The results from this study are exciting, with fairly compelling response rates. The rate of progression-free survival with everolimus and letrozole treatment is very impressive, compared with traditional chemotherapy using carboplatin and paclitaxel. The median progression-free survival rate of 21.6 months seen among the chemotherapy naive patients who received everolimus and letrozole showed a 7-month edge over the 14-month median progression-free survival previously reported with chemotherapy. This difference is very provocative and could be practice changing, but it of course needs further evaluation.
Paola A. Gehrig, MD , is professor of ob.gyn. and director of gynecologic oncology at the University of North Carolina in Chapel Hill. She made these comments as designated discussant for the study. She had no disclosures.
The results from this study are exciting, with fairly compelling response rates. The rate of progression-free survival with everolimus and letrozole treatment is very impressive, compared with traditional chemotherapy using carboplatin and paclitaxel. The median progression-free survival rate of 21.6 months seen among the chemotherapy naive patients who received everolimus and letrozole showed a 7-month edge over the 14-month median progression-free survival previously reported with chemotherapy. This difference is very provocative and could be practice changing, but it of course needs further evaluation.
Paola A. Gehrig, MD , is professor of ob.gyn. and director of gynecologic oncology at the University of North Carolina in Chapel Hill. She made these comments as designated discussant for the study. She had no disclosures.
The results from this study are exciting, with fairly compelling response rates. The rate of progression-free survival with everolimus and letrozole treatment is very impressive, compared with traditional chemotherapy using carboplatin and paclitaxel. The median progression-free survival rate of 21.6 months seen among the chemotherapy naive patients who received everolimus and letrozole showed a 7-month edge over the 14-month median progression-free survival previously reported with chemotherapy. This difference is very provocative and could be practice changing, but it of course needs further evaluation.
Paola A. Gehrig, MD , is professor of ob.gyn. and director of gynecologic oncology at the University of North Carolina in Chapel Hill. She made these comments as designated discussant for the study. She had no disclosures.
NEW ORLEANS – Combined treatment for 28 days with the mammalian target of rapamycin inhibitor everolimus plus the aromatase inhibitor letrozole in 37 women with recurrent endometrial cancer produced an overall objective response rate of 24% and an average progression-free survival rate of 6.3 months in a randomized phase 2 study with a total of 74 patients. The treatment was also relatively well tolerated, with more serious adverse vents of anemia and hyperglycemia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
But the most attention-grabbing finding of this study was that, in the subset of 15 women who had received no prior chemotherapy, the objective response rate on this regimen was 53% and median progression-free survival was 21.6 months, Brian M. Slomovitz, MD, said at the annual meeting of the Society of Gynecologic Oncology.
This level of response in the chemotherapy-naive subgroup was “very high, and not what we expected,” Dr. Slomovitz, , professor of ob.gyn. and human genetics and director of gynecologic oncology at the University of Miami, said in a video interview. “This is something we need to further investigate to see if we can make this part of standard care.”
Although he conceded that the data from this study were too limited to warrant a regulatory indication, he suggested that it might be enough to gain the everolimus plus letrozole combination used in the study citation as a treatment option in clinical guidelines. The next step should be a phase 3 trial that compares the everolimus plus letrozole combination with the traditional chemotherapy regimen of carboplatin plus paclitaxel, Dr. Slomovitz added.
The Everolimus and Letrozole or Hormonal Therapy to Treat Endometrial Cancer phase 2 trial, initiated by the Gynecologic Oncology Group, ran at 26 U.S. centers, and randomized patients with stage III or IV recurrent, advanced, or persistent endometrial cancer who had either no or at most one prior course of chemotherapy. The study design also excluded patients who had previously been treated with an mammalian target of rapamycin inhibitor or hormonal therapy for their endometrial cancer. The control arm placed 37 patients on a standard hormonal therapy regimen of tamoxifen plus medroxyprogesterone, with 36 of patients in this subgroup evaluable.
The overall results in the two treatment arms were roughly similar, except for the striking benefit seen with everolimus(Afinitor) plus letrozole(Femara) in the chemotherapy-naive patents; 15 patients in each arm had not received any chemotherapy before entering the study. In this subgroup, among the patients who received conventional hormonal therapy, the objective response rate was 43% and progression-free survival was 6.6 months.
The two arms also differed by their pattern of grade 3 or 4 adverse events. Among the patients on everolimus plus letrozole, the most common were anemia (24%) and hyperglycemia (14%), both expected consequences of the regimen. The hormonal therapy arm led to an 8% incidence of thromboembolic events, which did not occur in the everolimus plus letrozole arm.
Another attraction of the everolimus and letrozole regimen is that it is oral and avoids the need for drug infusions, Dr. Slomovitz noted.
The investigator-initiated study received funding from Novartis, the company that markets everolimus and letrozole. Dr. Slomovitz has been an advisor to Advaxis, AstraZeneca, Clovis, Genmab, Jannsen, and Tesaro, and he has received research funding from Novartis.
SOURCE: Slomovitz BM et al. SGO 2018, abstract 1.
NEW ORLEANS – Combined treatment for 28 days with the mammalian target of rapamycin inhibitor everolimus plus the aromatase inhibitor letrozole in 37 women with recurrent endometrial cancer produced an overall objective response rate of 24% and an average progression-free survival rate of 6.3 months in a randomized phase 2 study with a total of 74 patients. The treatment was also relatively well tolerated, with more serious adverse vents of anemia and hyperglycemia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
But the most attention-grabbing finding of this study was that, in the subset of 15 women who had received no prior chemotherapy, the objective response rate on this regimen was 53% and median progression-free survival was 21.6 months, Brian M. Slomovitz, MD, said at the annual meeting of the Society of Gynecologic Oncology.
This level of response in the chemotherapy-naive subgroup was “very high, and not what we expected,” Dr. Slomovitz, , professor of ob.gyn. and human genetics and director of gynecologic oncology at the University of Miami, said in a video interview. “This is something we need to further investigate to see if we can make this part of standard care.”
Although he conceded that the data from this study were too limited to warrant a regulatory indication, he suggested that it might be enough to gain the everolimus plus letrozole combination used in the study citation as a treatment option in clinical guidelines. The next step should be a phase 3 trial that compares the everolimus plus letrozole combination with the traditional chemotherapy regimen of carboplatin plus paclitaxel, Dr. Slomovitz added.
The Everolimus and Letrozole or Hormonal Therapy to Treat Endometrial Cancer phase 2 trial, initiated by the Gynecologic Oncology Group, ran at 26 U.S. centers, and randomized patients with stage III or IV recurrent, advanced, or persistent endometrial cancer who had either no or at most one prior course of chemotherapy. The study design also excluded patients who had previously been treated with an mammalian target of rapamycin inhibitor or hormonal therapy for their endometrial cancer. The control arm placed 37 patients on a standard hormonal therapy regimen of tamoxifen plus medroxyprogesterone, with 36 of patients in this subgroup evaluable.
The overall results in the two treatment arms were roughly similar, except for the striking benefit seen with everolimus(Afinitor) plus letrozole(Femara) in the chemotherapy-naive patents; 15 patients in each arm had not received any chemotherapy before entering the study. In this subgroup, among the patients who received conventional hormonal therapy, the objective response rate was 43% and progression-free survival was 6.6 months.
The two arms also differed by their pattern of grade 3 or 4 adverse events. Among the patients on everolimus plus letrozole, the most common were anemia (24%) and hyperglycemia (14%), both expected consequences of the regimen. The hormonal therapy arm led to an 8% incidence of thromboembolic events, which did not occur in the everolimus plus letrozole arm.
Another attraction of the everolimus and letrozole regimen is that it is oral and avoids the need for drug infusions, Dr. Slomovitz noted.
The investigator-initiated study received funding from Novartis, the company that markets everolimus and letrozole. Dr. Slomovitz has been an advisor to Advaxis, AstraZeneca, Clovis, Genmab, Jannsen, and Tesaro, and he has received research funding from Novartis.
SOURCE: Slomovitz BM et al. SGO 2018, abstract 1.
REPORTING FROM SGO 2018
Key clinical point: Treatment of recurrent endometrial cancer with everolimus and letrozole shows promise.
Major finding: Among 15 chemotherapy-naive patients, objective responses occurred in 53% and median progression-free survival was 21.6 months.
Study details: A multicenter, phase 2 randomized study in 74 patients.
Disclosures: The investigator-initiated study received funding from Novartis, the company that markets everolimus(Afinitor) and letrozole(Femara). Dr. Slomovitz has been an advisor to Advaxis, AstraZeneca, Clovis, Genmab, Jannsen, and Tesaro, and he has received research funding from Novartis.
Source: Slomovitz BM et al. SGO 2018, abstract 1.
























