User login
European Society of Cardiology (ESC): Annual Congress
High-dose statins don’t prevent postop AF
BARCELONA – Intensive perioperative statin therapy in patients undergoing CABG surgery doesn’t protect against postop atrial fibrillation or myocardial injury, according to a large randomized clinical trial hailed as the "definitive" study addressing this issue.
"There are many reasons why these patients should be put on statin treatment, but the prevention of postop complications is not one of them," Dr. Barbara Casadei said in presenting the findings of the Statin Therapy in Cardiac Surgery (STICS) trial at the annual congress of the European Society of Cardiology.
The STICS results are at odds with conventional wisdom. ESC guidelines give a favorable class IIa, level of evidence B recommendation that "statins should be considered for prevention of new-onset atrial fibrillation after coronary artery bypass grafting, either isolated or in combination with valvular interventions."
"STICS was a very carefully conducted, large scale, robust study that I think has definitely closed the door on this issue," commented Dr. Keith A.A. Fox, professor of cardiology at the University of Edinburgh and chair of the scientific and clinical program committee at ESC Congress 2014.
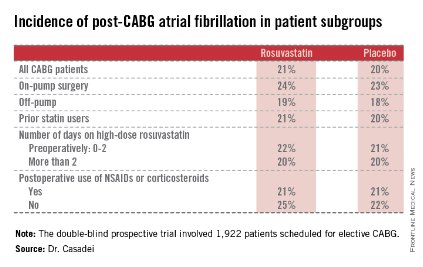
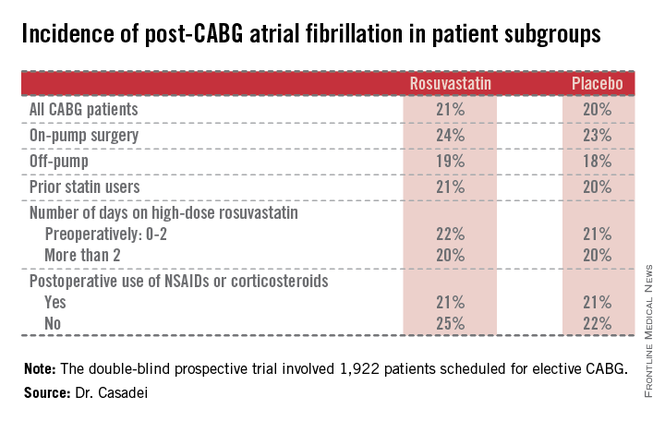
STICS was a double-blind prospective trial in which 1,922 patients scheduled for elective CABG were randomized to 20 mg per day of rosuvastatin (Crestor) or placebo starting up to 8 days prior to surgery and continued for 5 days postop. All participants were in sinus rhythm preoperatively, with no history of AF, said Dr. Casadei, professor of cardiovascular medicine at the University of Oxford, England.
The two coprimary endpoints in STICS were the incidence of new-onset AF during 5 days of postop Holter monitoring, and evidence of postop myocardial injury as demonstrated in serial troponin I assays.
Postop AF occurred in 21% of those given high-intensity therapy with rosuvastatin and 20% of placebo-treated controls. There was no subgroup where rosuvastatin was protective (see graphic).
Troponin I measurements obtained 6, 24, 48, and 120 hours postop showed areas under the curve that were superimposable in the two study groups, meaning perioperative high-dose statin therapy provided absolutely no protection against postop cardiac muscle injury.
Mean hospital length of stay and ICU time didn’t differ between the two groups, either.
The impetus for conducting STICS was recognition that the guidelines’ endorsement of perioperative high-dose statin therapy in conjunction with cardiac surgery was based upon a series of small randomized trials with serious limitations. Although the results of a meta-analysis of the 14 prior trials looked impressive at first glance – a 17% incidence of postop AF in statin-treated patients, compared with 30% in controls, for a near-halving of the risk of this important complication – these 14 studies totaled 1,300 patients, and there were many methodologic shortcomings.
The STICS researchers hypothesized that a large, well-designed trial – bigger than all previous studies combined – would shore up the previously shaky supporting evidence and perhaps provide grounds for statins to win a new indication from regulatory agencies. Post-CABG AF is associated with a doubled risk of stroke and mortality, and excess hospital costs of $8,000-$18,000 dollars per patient.
Discussant Dr. Paulus Kirchhof, a member of the task force that developed the current ESC guidelines (Europace 2010;12:1360-420), said those guidelines now clearly need to be revisited. Beyond that, he added, STICS provides important new contributions in understanding the pathophysiology of AF.
"We know that AF is caused by several vicious circles, and we believe that inflammation could influence those and cause AF. And we also thought that postop AF was the condition where inflammation plays the biggest role. Based upon the negative results with this anti-inflammatory intervention, I think we have to question this concept a bit," said Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham, England.
Dr. Casadei countered that she’s not ready to write off postop inflammation entirely as a major trigger of new-onset AF following CABG.
"The inflammation is there. We know from experimental work in animals that there is a strong association between inflammation and postop atrial fibrillation, but whether the association is causal, I think, is still debated. However, it may be that the anti-inflammatory effect of statins is not sufficiently strong to actually prevent this complication," she said.
Discussant Dr. Steven Nissen praised STICS as "an outstanding trial."
"I also think there’s a terribly important lesson here, which is the power of self-delusion in medicine. When we base our guidelines on small, poorly controlled trials, we are often making mistakes. This is one of countless examples where when someone finally does a careful, thoughtful trial, we find out that something that people believe just isn’t true. We can’t cut corners with evidence. We need good randomized trials," declared Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
The STICS trial was funded primarily by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. In addition, Dr. Casadei reported receiving an unrestricted grant from AstraZeneca in conjunction with the trial.

|
| Dr. Hiren Shah |
There are two key lessons from the results of the STICS trial. First, extrapolation of results from biochemical pathways and measured cellular markers does not always translate into meaningful clinical outcomes. Thus, it has long been known from several large trials that statin therapy effectively and rapidly lowers CRP levels both in hyper- and normocholesterolemic patients and that statins are effective in decreasing systemic inflammation. It has also been known that inflammation contributes to the development and maintenance of AF, so it was postulated that by improving endothelial nitric oxide availability, reducing inflammation, and decreasing oxidative stress, and through neurohormonal activation, statins would reduce the incidence of post-op AF. This link was so strong that clinical guidelines adopted limited data from small trials to make treatment recommendations.
This leads us to consider the second key lesson from this study. Trials with small sample size, even when combined across many other trials (1,300 patients were involved across 14 trials in this case), do not always yield reliable results, especially when they have significant limitations, notably not always being blind and having been performed in statin-naive patients only. The large, randomized, and well-designed STICS trial puts to rest an important issue, given the high prevalence of AF after cardiac surgery, which is associated with a longer length of stay, an increased risk of stroke, higher mortality, and greater costs, and should prompt us to consider further evaluation of different strategies to reduce this significant complication.
Dr. Hiren Shah is medical director of the medicine and cardiac telemetry hospitalist unit at Northwestern Memorial Hospital in Chicago and an adviser to Hospitalist News. He is the national chair of the Clinician Committee for ACP’s Initiative on Stroke Prevention and Atrial Fibrillation and is the lead physician for the Society of Hospital Medicine’s National Atrial Fibrillation Initiative.

|
| Dr. Hiren Shah |
There are two key lessons from the results of the STICS trial. First, extrapolation of results from biochemical pathways and measured cellular markers does not always translate into meaningful clinical outcomes. Thus, it has long been known from several large trials that statin therapy effectively and rapidly lowers CRP levels both in hyper- and normocholesterolemic patients and that statins are effective in decreasing systemic inflammation. It has also been known that inflammation contributes to the development and maintenance of AF, so it was postulated that by improving endothelial nitric oxide availability, reducing inflammation, and decreasing oxidative stress, and through neurohormonal activation, statins would reduce the incidence of post-op AF. This link was so strong that clinical guidelines adopted limited data from small trials to make treatment recommendations.
This leads us to consider the second key lesson from this study. Trials with small sample size, even when combined across many other trials (1,300 patients were involved across 14 trials in this case), do not always yield reliable results, especially when they have significant limitations, notably not always being blind and having been performed in statin-naive patients only. The large, randomized, and well-designed STICS trial puts to rest an important issue, given the high prevalence of AF after cardiac surgery, which is associated with a longer length of stay, an increased risk of stroke, higher mortality, and greater costs, and should prompt us to consider further evaluation of different strategies to reduce this significant complication.
Dr. Hiren Shah is medical director of the medicine and cardiac telemetry hospitalist unit at Northwestern Memorial Hospital in Chicago and an adviser to Hospitalist News. He is the national chair of the Clinician Committee for ACP’s Initiative on Stroke Prevention and Atrial Fibrillation and is the lead physician for the Society of Hospital Medicine’s National Atrial Fibrillation Initiative.

|
| Dr. Hiren Shah |
There are two key lessons from the results of the STICS trial. First, extrapolation of results from biochemical pathways and measured cellular markers does not always translate into meaningful clinical outcomes. Thus, it has long been known from several large trials that statin therapy effectively and rapidly lowers CRP levels both in hyper- and normocholesterolemic patients and that statins are effective in decreasing systemic inflammation. It has also been known that inflammation contributes to the development and maintenance of AF, so it was postulated that by improving endothelial nitric oxide availability, reducing inflammation, and decreasing oxidative stress, and through neurohormonal activation, statins would reduce the incidence of post-op AF. This link was so strong that clinical guidelines adopted limited data from small trials to make treatment recommendations.
This leads us to consider the second key lesson from this study. Trials with small sample size, even when combined across many other trials (1,300 patients were involved across 14 trials in this case), do not always yield reliable results, especially when they have significant limitations, notably not always being blind and having been performed in statin-naive patients only. The large, randomized, and well-designed STICS trial puts to rest an important issue, given the high prevalence of AF after cardiac surgery, which is associated with a longer length of stay, an increased risk of stroke, higher mortality, and greater costs, and should prompt us to consider further evaluation of different strategies to reduce this significant complication.
Dr. Hiren Shah is medical director of the medicine and cardiac telemetry hospitalist unit at Northwestern Memorial Hospital in Chicago and an adviser to Hospitalist News. He is the national chair of the Clinician Committee for ACP’s Initiative on Stroke Prevention and Atrial Fibrillation and is the lead physician for the Society of Hospital Medicine’s National Atrial Fibrillation Initiative.
BARCELONA – Intensive perioperative statin therapy in patients undergoing CABG surgery doesn’t protect against postop atrial fibrillation or myocardial injury, according to a large randomized clinical trial hailed as the "definitive" study addressing this issue.
"There are many reasons why these patients should be put on statin treatment, but the prevention of postop complications is not one of them," Dr. Barbara Casadei said in presenting the findings of the Statin Therapy in Cardiac Surgery (STICS) trial at the annual congress of the European Society of Cardiology.

The STICS results are at odds with conventional wisdom. ESC guidelines give a favorable class IIa, level of evidence B recommendation that "statins should be considered for prevention of new-onset atrial fibrillation after coronary artery bypass grafting, either isolated or in combination with valvular interventions."
"STICS was a very carefully conducted, large scale, robust study that I think has definitely closed the door on this issue," commented Dr. Keith A.A. Fox, professor of cardiology at the University of Edinburgh and chair of the scientific and clinical program committee at ESC Congress 2014.
STICS was a double-blind prospective trial in which 1,922 patients scheduled for elective CABG were randomized to 20 mg per day of rosuvastatin (Crestor) or placebo starting up to 8 days prior to surgery and continued for 5 days postop. All participants were in sinus rhythm preoperatively, with no history of AF, said Dr. Casadei, professor of cardiovascular medicine at the University of Oxford, England.
The two coprimary endpoints in STICS were the incidence of new-onset AF during 5 days of postop Holter monitoring, and evidence of postop myocardial injury as demonstrated in serial troponin I assays.
Postop AF occurred in 21% of those given high-intensity therapy with rosuvastatin and 20% of placebo-treated controls. There was no subgroup where rosuvastatin was protective (see graphic).
Troponin I measurements obtained 6, 24, 48, and 120 hours postop showed areas under the curve that were superimposable in the two study groups, meaning perioperative high-dose statin therapy provided absolutely no protection against postop cardiac muscle injury.
Mean hospital length of stay and ICU time didn’t differ between the two groups, either.
The impetus for conducting STICS was recognition that the guidelines’ endorsement of perioperative high-dose statin therapy in conjunction with cardiac surgery was based upon a series of small randomized trials with serious limitations. Although the results of a meta-analysis of the 14 prior trials looked impressive at first glance – a 17% incidence of postop AF in statin-treated patients, compared with 30% in controls, for a near-halving of the risk of this important complication – these 14 studies totaled 1,300 patients, and there were many methodologic shortcomings.
The STICS researchers hypothesized that a large, well-designed trial – bigger than all previous studies combined – would shore up the previously shaky supporting evidence and perhaps provide grounds for statins to win a new indication from regulatory agencies. Post-CABG AF is associated with a doubled risk of stroke and mortality, and excess hospital costs of $8,000-$18,000 dollars per patient.
Discussant Dr. Paulus Kirchhof, a member of the task force that developed the current ESC guidelines (Europace 2010;12:1360-420), said those guidelines now clearly need to be revisited. Beyond that, he added, STICS provides important new contributions in understanding the pathophysiology of AF.
"We know that AF is caused by several vicious circles, and we believe that inflammation could influence those and cause AF. And we also thought that postop AF was the condition where inflammation plays the biggest role. Based upon the negative results with this anti-inflammatory intervention, I think we have to question this concept a bit," said Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham, England.
Dr. Casadei countered that she’s not ready to write off postop inflammation entirely as a major trigger of new-onset AF following CABG.
"The inflammation is there. We know from experimental work in animals that there is a strong association between inflammation and postop atrial fibrillation, but whether the association is causal, I think, is still debated. However, it may be that the anti-inflammatory effect of statins is not sufficiently strong to actually prevent this complication," she said.
Discussant Dr. Steven Nissen praised STICS as "an outstanding trial."
"I also think there’s a terribly important lesson here, which is the power of self-delusion in medicine. When we base our guidelines on small, poorly controlled trials, we are often making mistakes. This is one of countless examples where when someone finally does a careful, thoughtful trial, we find out that something that people believe just isn’t true. We can’t cut corners with evidence. We need good randomized trials," declared Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
The STICS trial was funded primarily by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. In addition, Dr. Casadei reported receiving an unrestricted grant from AstraZeneca in conjunction with the trial.
BARCELONA – Intensive perioperative statin therapy in patients undergoing CABG surgery doesn’t protect against postop atrial fibrillation or myocardial injury, according to a large randomized clinical trial hailed as the "definitive" study addressing this issue.
"There are many reasons why these patients should be put on statin treatment, but the prevention of postop complications is not one of them," Dr. Barbara Casadei said in presenting the findings of the Statin Therapy in Cardiac Surgery (STICS) trial at the annual congress of the European Society of Cardiology.

The STICS results are at odds with conventional wisdom. ESC guidelines give a favorable class IIa, level of evidence B recommendation that "statins should be considered for prevention of new-onset atrial fibrillation after coronary artery bypass grafting, either isolated or in combination with valvular interventions."
"STICS was a very carefully conducted, large scale, robust study that I think has definitely closed the door on this issue," commented Dr. Keith A.A. Fox, professor of cardiology at the University of Edinburgh and chair of the scientific and clinical program committee at ESC Congress 2014.
STICS was a double-blind prospective trial in which 1,922 patients scheduled for elective CABG were randomized to 20 mg per day of rosuvastatin (Crestor) or placebo starting up to 8 days prior to surgery and continued for 5 days postop. All participants were in sinus rhythm preoperatively, with no history of AF, said Dr. Casadei, professor of cardiovascular medicine at the University of Oxford, England.
The two coprimary endpoints in STICS were the incidence of new-onset AF during 5 days of postop Holter monitoring, and evidence of postop myocardial injury as demonstrated in serial troponin I assays.
Postop AF occurred in 21% of those given high-intensity therapy with rosuvastatin and 20% of placebo-treated controls. There was no subgroup where rosuvastatin was protective (see graphic).
Troponin I measurements obtained 6, 24, 48, and 120 hours postop showed areas under the curve that were superimposable in the two study groups, meaning perioperative high-dose statin therapy provided absolutely no protection against postop cardiac muscle injury.
Mean hospital length of stay and ICU time didn’t differ between the two groups, either.
The impetus for conducting STICS was recognition that the guidelines’ endorsement of perioperative high-dose statin therapy in conjunction with cardiac surgery was based upon a series of small randomized trials with serious limitations. Although the results of a meta-analysis of the 14 prior trials looked impressive at first glance – a 17% incidence of postop AF in statin-treated patients, compared with 30% in controls, for a near-halving of the risk of this important complication – these 14 studies totaled 1,300 patients, and there were many methodologic shortcomings.
The STICS researchers hypothesized that a large, well-designed trial – bigger than all previous studies combined – would shore up the previously shaky supporting evidence and perhaps provide grounds for statins to win a new indication from regulatory agencies. Post-CABG AF is associated with a doubled risk of stroke and mortality, and excess hospital costs of $8,000-$18,000 dollars per patient.
Discussant Dr. Paulus Kirchhof, a member of the task force that developed the current ESC guidelines (Europace 2010;12:1360-420), said those guidelines now clearly need to be revisited. Beyond that, he added, STICS provides important new contributions in understanding the pathophysiology of AF.
"We know that AF is caused by several vicious circles, and we believe that inflammation could influence those and cause AF. And we also thought that postop AF was the condition where inflammation plays the biggest role. Based upon the negative results with this anti-inflammatory intervention, I think we have to question this concept a bit," said Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham, England.
Dr. Casadei countered that she’s not ready to write off postop inflammation entirely as a major trigger of new-onset AF following CABG.
"The inflammation is there. We know from experimental work in animals that there is a strong association between inflammation and postop atrial fibrillation, but whether the association is causal, I think, is still debated. However, it may be that the anti-inflammatory effect of statins is not sufficiently strong to actually prevent this complication," she said.
Discussant Dr. Steven Nissen praised STICS as "an outstanding trial."
"I also think there’s a terribly important lesson here, which is the power of self-delusion in medicine. When we base our guidelines on small, poorly controlled trials, we are often making mistakes. This is one of countless examples where when someone finally does a careful, thoughtful trial, we find out that something that people believe just isn’t true. We can’t cut corners with evidence. We need good randomized trials," declared Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
The STICS trial was funded primarily by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. In addition, Dr. Casadei reported receiving an unrestricted grant from AstraZeneca in conjunction with the trial.
AT THE ESC CONGRESS 2014
Key clinical point: Perioperative statin therapy in patients undergoing CABG failed to protect against new-onset postop atrial fibrillation.
Major finding: The incidence of postop atrial fibrillation within 5 days post-CABG was 21% in patients randomized to 20 mg/day of rosuvastatin and 20% in placebo-treated controls.
Data source: The multicenter STICS trial included 1,922 randomized patients scheduled for elective CABG.
Disclosures: STICS was funded by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. The presenter reported having received a research grant from AstraZeneca.
Stroke risk skyrocketed after intracranial hemorrhage in warfarin users
BARCELONA – Patients who have an intracranial hemorrhage while on warfarin for atrial fibrillation are at sharply increased risk for an ischemic stroke during the following year, a period when many of them are off warfarin, according to a Danish national study.
The absolute risk of a first ischemic stroke among survivors of an intracranial hemorrhage (ICH) while on warfarin was roughly 10% within the first month, 20% within 3 months, and 32% at 1 year. These rates exclude strokes that occurred within the first 7 days after an ICH, Dr. Peter B. Nielsen reported at the annual congress of the European Society of Cardiology.
The design of this real-world national registry study doesn’t permit definitive conclusions to be drawn regarding causality. It is telling, however, that the rate of warfarin use among Danish atrial fibrillation (AF) patients in the year after an ICH plunged by 72%; thus, only about one in four of patients who regularly filled warfarin prescriptions up until the time of their ICH did so afterward, according to Dr. Nielsen of Aalborg (Denmark) University.
He reported on 58,815 warfarin-treated Danes with AF with no prior ICH, ischemic stroke, transient ischemic attack, or systemic embolism at baseline who were followed for up to 13 years, during which 1,639 of them were diagnosed with ICH.
During 261,681 person-years of follow-up of the cohort that remained free of ICH, 6,843 patients were diagnosed with a first ischemic stroke, transient ischemic attack, or systemic embolism. Among the group who had an ICH, that rate was 3.67-fold greater.
Moreover, all-cause mortality occurred in 946 patients with an ICH during 2,404 person-years of follow-up, a rate 5.6-fold greater than in those who remained on warfarin and free of ICH.
When the analysis was restricted to only 2 years of follow-up, the event rate ratios became even more dramatic: AF patients with an ICH were at subsequent 5.4- and 11.8-fold greater risks of ischemic stroke and all-cause mortality, respectively, within the next 2 years, compared with those who were free of ICH.
The optimal timing of resumption of warfarin following an ICH is unclear. Various small studies have come up with recommendations ranging from 1 to 30 weeks, Dr. Nielsen noted.
"Caution may lead to undertreatment and increased risk of ischemic stroke and thrombotic events," he noted. "The question now in my mind is, Who, having survived an ICH, can benefit from a return to warfarin or a switch to a novel oral anticoagulant?"
Discussant Dr. Christopher B. Granger, commenting on the Danish study results, said, "I think this is very important information that there’s this extraordinary risk of ischemic stroke in the year after ICH. I don’t think this has been described before. It of course puts us in a tough bind because we don’t know the safety of returning to warfarin use. This raises the issue in my mind of whether there might be a role for a left atrial appendage occlusion device as an option in this population that’s presumably going to be at high risk of recurrent ICH upon resumption of oral anticoagulation," said Dr. Granger, professor of medicine and director of the cardiac care unit at Duke University Medical Center in Durham, N.C.
When it was pointed out that 3 months of oral anticoagulant therapy is required after placement of the Watchman, a left atrial appendage occlusion device, he responded, "Yes, at least the way it has been developed so far – but this might be an opportunity to test an alternative approach."
Dr. Nielsen and Dr. Granger reported having no relevant financial conflicts.
BARCELONA – Patients who have an intracranial hemorrhage while on warfarin for atrial fibrillation are at sharply increased risk for an ischemic stroke during the following year, a period when many of them are off warfarin, according to a Danish national study.
The absolute risk of a first ischemic stroke among survivors of an intracranial hemorrhage (ICH) while on warfarin was roughly 10% within the first month, 20% within 3 months, and 32% at 1 year. These rates exclude strokes that occurred within the first 7 days after an ICH, Dr. Peter B. Nielsen reported at the annual congress of the European Society of Cardiology.
The design of this real-world national registry study doesn’t permit definitive conclusions to be drawn regarding causality. It is telling, however, that the rate of warfarin use among Danish atrial fibrillation (AF) patients in the year after an ICH plunged by 72%; thus, only about one in four of patients who regularly filled warfarin prescriptions up until the time of their ICH did so afterward, according to Dr. Nielsen of Aalborg (Denmark) University.
He reported on 58,815 warfarin-treated Danes with AF with no prior ICH, ischemic stroke, transient ischemic attack, or systemic embolism at baseline who were followed for up to 13 years, during which 1,639 of them were diagnosed with ICH.
During 261,681 person-years of follow-up of the cohort that remained free of ICH, 6,843 patients were diagnosed with a first ischemic stroke, transient ischemic attack, or systemic embolism. Among the group who had an ICH, that rate was 3.67-fold greater.
Moreover, all-cause mortality occurred in 946 patients with an ICH during 2,404 person-years of follow-up, a rate 5.6-fold greater than in those who remained on warfarin and free of ICH.
When the analysis was restricted to only 2 years of follow-up, the event rate ratios became even more dramatic: AF patients with an ICH were at subsequent 5.4- and 11.8-fold greater risks of ischemic stroke and all-cause mortality, respectively, within the next 2 years, compared with those who were free of ICH.
The optimal timing of resumption of warfarin following an ICH is unclear. Various small studies have come up with recommendations ranging from 1 to 30 weeks, Dr. Nielsen noted.
"Caution may lead to undertreatment and increased risk of ischemic stroke and thrombotic events," he noted. "The question now in my mind is, Who, having survived an ICH, can benefit from a return to warfarin or a switch to a novel oral anticoagulant?"
Discussant Dr. Christopher B. Granger, commenting on the Danish study results, said, "I think this is very important information that there’s this extraordinary risk of ischemic stroke in the year after ICH. I don’t think this has been described before. It of course puts us in a tough bind because we don’t know the safety of returning to warfarin use. This raises the issue in my mind of whether there might be a role for a left atrial appendage occlusion device as an option in this population that’s presumably going to be at high risk of recurrent ICH upon resumption of oral anticoagulation," said Dr. Granger, professor of medicine and director of the cardiac care unit at Duke University Medical Center in Durham, N.C.
When it was pointed out that 3 months of oral anticoagulant therapy is required after placement of the Watchman, a left atrial appendage occlusion device, he responded, "Yes, at least the way it has been developed so far – but this might be an opportunity to test an alternative approach."
Dr. Nielsen and Dr. Granger reported having no relevant financial conflicts.
BARCELONA – Patients who have an intracranial hemorrhage while on warfarin for atrial fibrillation are at sharply increased risk for an ischemic stroke during the following year, a period when many of them are off warfarin, according to a Danish national study.
The absolute risk of a first ischemic stroke among survivors of an intracranial hemorrhage (ICH) while on warfarin was roughly 10% within the first month, 20% within 3 months, and 32% at 1 year. These rates exclude strokes that occurred within the first 7 days after an ICH, Dr. Peter B. Nielsen reported at the annual congress of the European Society of Cardiology.
The design of this real-world national registry study doesn’t permit definitive conclusions to be drawn regarding causality. It is telling, however, that the rate of warfarin use among Danish atrial fibrillation (AF) patients in the year after an ICH plunged by 72%; thus, only about one in four of patients who regularly filled warfarin prescriptions up until the time of their ICH did so afterward, according to Dr. Nielsen of Aalborg (Denmark) University.
He reported on 58,815 warfarin-treated Danes with AF with no prior ICH, ischemic stroke, transient ischemic attack, or systemic embolism at baseline who were followed for up to 13 years, during which 1,639 of them were diagnosed with ICH.
During 261,681 person-years of follow-up of the cohort that remained free of ICH, 6,843 patients were diagnosed with a first ischemic stroke, transient ischemic attack, or systemic embolism. Among the group who had an ICH, that rate was 3.67-fold greater.
Moreover, all-cause mortality occurred in 946 patients with an ICH during 2,404 person-years of follow-up, a rate 5.6-fold greater than in those who remained on warfarin and free of ICH.
When the analysis was restricted to only 2 years of follow-up, the event rate ratios became even more dramatic: AF patients with an ICH were at subsequent 5.4- and 11.8-fold greater risks of ischemic stroke and all-cause mortality, respectively, within the next 2 years, compared with those who were free of ICH.
The optimal timing of resumption of warfarin following an ICH is unclear. Various small studies have come up with recommendations ranging from 1 to 30 weeks, Dr. Nielsen noted.
"Caution may lead to undertreatment and increased risk of ischemic stroke and thrombotic events," he noted. "The question now in my mind is, Who, having survived an ICH, can benefit from a return to warfarin or a switch to a novel oral anticoagulant?"
Discussant Dr. Christopher B. Granger, commenting on the Danish study results, said, "I think this is very important information that there’s this extraordinary risk of ischemic stroke in the year after ICH. I don’t think this has been described before. It of course puts us in a tough bind because we don’t know the safety of returning to warfarin use. This raises the issue in my mind of whether there might be a role for a left atrial appendage occlusion device as an option in this population that’s presumably going to be at high risk of recurrent ICH upon resumption of oral anticoagulation," said Dr. Granger, professor of medicine and director of the cardiac care unit at Duke University Medical Center in Durham, N.C.
When it was pointed out that 3 months of oral anticoagulant therapy is required after placement of the Watchman, a left atrial appendage occlusion device, he responded, "Yes, at least the way it has been developed so far – but this might be an opportunity to test an alternative approach."
Dr. Nielsen and Dr. Granger reported having no relevant financial conflicts.
AT THE ESC CONGRESS 2014
Key clinical point: Taking patients with atrial fibrillation off warfarin, even temporarily, after an intracranial hemorrhage comes at a high cost in terms of the subsequent rate of ischemic stroke.
Major finding: The incidence of a first ischemic stroke was 32% during the first year after patients on warfarin for atrial fibrillation had an intracranial hemorrhage.
Data source: A Danish national registry study that included nearly 59,000 patients on warfarin for atrial fibrillation who were followed for up to 13 years.
Disclosures: The study was supported by institutional funds. The presenter reported having no relevant financial conflicts.
Pneumococcal Vaccine Protects Against Cardiac and Cerebrovascular Events
BARCELONA – Influenza vaccine has been shown to provide protection against cardiovascular events, but can the same be said for pneumococcal vaccine?
Yes, particularly in the elderly and in patients at high baseline cardiovascular risk, according to a meta-analysis presented by Dr. Dimitrios Terentes-Printzios at the annual congress of the European Society of Cardiology.
He analyzed 11 published studies comprising 332,267 subjects followed for a mean of 20 months. Because the studies focused on different populations and in some cases reached conflicting conclusions, he performed a series of subgroup analyses to gain a clearer picture.
One of these analyses found that the cardioprotective effects of pneumococcal vaccination wane over time. In studies with follow-up of less than 1 year, the relative risk of total cardiovascular events was 0.72, meaning that patients who received pneumococcal vaccine had a significant 28% relative risk reduction compared with those who did not. In studies with follow-up in excess of 1 year, however, there was no cardioprotective effect, according to Dr. Terentes-Printzios of Athens Medical School.
Significant protection against total cardiovascular events was seen in elderly vaccinated patients, with a 20% relative risk reduction, and in subjects at high baseline cardiovascular risk, who had an 8% risk reduction if they received pneumococcal vaccine.
Breaking down the specific endpoints, subjects who got pneumococcal vaccine had a statistically significant 8% reduction in the risk of cardiovascular mortality. However, vaccination provided no significant protective effect against acute MI or cerebrovascular events except in the elderly, where the relative risk reductions were 10% and 14%, respectively.
These cardio- and cerebrovascular protective benefits of the pneumococcal vaccine can be viewed as added value, given that the primary reason physicians prescribe the vaccine is its demonstrated ability to reduce the risk of invasive pneumococcal infection by up to 60%.
Dr. Terentes-Printzios reported having no financial conflicts.
BARCELONA – Influenza vaccine has been shown to provide protection against cardiovascular events, but can the same be said for pneumococcal vaccine?
Yes, particularly in the elderly and in patients at high baseline cardiovascular risk, according to a meta-analysis presented by Dr. Dimitrios Terentes-Printzios at the annual congress of the European Society of Cardiology.
He analyzed 11 published studies comprising 332,267 subjects followed for a mean of 20 months. Because the studies focused on different populations and in some cases reached conflicting conclusions, he performed a series of subgroup analyses to gain a clearer picture.
One of these analyses found that the cardioprotective effects of pneumococcal vaccination wane over time. In studies with follow-up of less than 1 year, the relative risk of total cardiovascular events was 0.72, meaning that patients who received pneumococcal vaccine had a significant 28% relative risk reduction compared with those who did not. In studies with follow-up in excess of 1 year, however, there was no cardioprotective effect, according to Dr. Terentes-Printzios of Athens Medical School.
Significant protection against total cardiovascular events was seen in elderly vaccinated patients, with a 20% relative risk reduction, and in subjects at high baseline cardiovascular risk, who had an 8% risk reduction if they received pneumococcal vaccine.
Breaking down the specific endpoints, subjects who got pneumococcal vaccine had a statistically significant 8% reduction in the risk of cardiovascular mortality. However, vaccination provided no significant protective effect against acute MI or cerebrovascular events except in the elderly, where the relative risk reductions were 10% and 14%, respectively.
These cardio- and cerebrovascular protective benefits of the pneumococcal vaccine can be viewed as added value, given that the primary reason physicians prescribe the vaccine is its demonstrated ability to reduce the risk of invasive pneumococcal infection by up to 60%.
Dr. Terentes-Printzios reported having no financial conflicts.
BARCELONA – Influenza vaccine has been shown to provide protection against cardiovascular events, but can the same be said for pneumococcal vaccine?
Yes, particularly in the elderly and in patients at high baseline cardiovascular risk, according to a meta-analysis presented by Dr. Dimitrios Terentes-Printzios at the annual congress of the European Society of Cardiology.
He analyzed 11 published studies comprising 332,267 subjects followed for a mean of 20 months. Because the studies focused on different populations and in some cases reached conflicting conclusions, he performed a series of subgroup analyses to gain a clearer picture.
One of these analyses found that the cardioprotective effects of pneumococcal vaccination wane over time. In studies with follow-up of less than 1 year, the relative risk of total cardiovascular events was 0.72, meaning that patients who received pneumococcal vaccine had a significant 28% relative risk reduction compared with those who did not. In studies with follow-up in excess of 1 year, however, there was no cardioprotective effect, according to Dr. Terentes-Printzios of Athens Medical School.
Significant protection against total cardiovascular events was seen in elderly vaccinated patients, with a 20% relative risk reduction, and in subjects at high baseline cardiovascular risk, who had an 8% risk reduction if they received pneumococcal vaccine.
Breaking down the specific endpoints, subjects who got pneumococcal vaccine had a statistically significant 8% reduction in the risk of cardiovascular mortality. However, vaccination provided no significant protective effect against acute MI or cerebrovascular events except in the elderly, where the relative risk reductions were 10% and 14%, respectively.
These cardio- and cerebrovascular protective benefits of the pneumococcal vaccine can be viewed as added value, given that the primary reason physicians prescribe the vaccine is its demonstrated ability to reduce the risk of invasive pneumococcal infection by up to 60%.
Dr. Terentes-Printzios reported having no financial conflicts.
AT THE ESC CONGRESS 2014
Pneumococcal vaccine protects against cardiac and cerebrovascular events
BARCELONA – Influenza vaccine has been shown to provide protection against cardiovascular events, but can the same be said for pneumococcal vaccine?
Yes, particularly in the elderly and in patients at high baseline cardiovascular risk, according to a meta-analysis presented by Dr. Dimitrios Terentes-Printzios at the annual congress of the European Society of Cardiology.
He analyzed 11 published studies comprising 332,267 subjects followed for a mean of 20 months. Because the studies focused on different populations and in some cases reached conflicting conclusions, he performed a series of subgroup analyses to gain a clearer picture.
One of these analyses found that the cardioprotective effects of pneumococcal vaccination wane over time. In studies with follow-up of less than 1 year, the relative risk of total cardiovascular events was 0.72, meaning that patients who received pneumococcal vaccine had a significant 28% relative risk reduction compared with those who did not. In studies with follow-up in excess of 1 year, however, there was no cardioprotective effect, according to Dr. Terentes-Printzios of Athens Medical School.
Significant protection against total cardiovascular events was seen in elderly vaccinated patients, with a 20% relative risk reduction, and in subjects at high baseline cardiovascular risk, who had an 8% risk reduction if they received pneumococcal vaccine.
Breaking down the specific endpoints, subjects who got pneumococcal vaccine had a statistically significant 8% reduction in the risk of cardiovascular mortality. However, vaccination provided no significant protective effect against acute MI or cerebrovascular events except in the elderly, where the relative risk reductions were 10% and 14%, respectively.
These cardio- and cerebrovascular protective benefits of the pneumococcal vaccine can be viewed as added value, given that the primary reason physicians prescribe the vaccine is its demonstrated ability to reduce the risk of invasive pneumococcal infection by up to 60%.
Dr. Terentes-Printzios reported having no financial conflicts.
Many patients with pneumonia are known to be at increased risk for cardiovascular events. The American Heart Association’s Get With The Guidelines – Resuscitation database found that in many stroke and cardiopulmonary arrests, pneumonia was a preexisting condition or was acquired during hospitalization.
Hence, it’s expected that preventing pneumonia with vaccination will also prevent subsequent death from cardiovascular reasons. Pneumonia vaccination will prevent not only cardiovascular events but death in general.
This study found more compelling reasons for us in the front line to vaccinate eligible candidates for flu and pneumonia, especially with fall already here.
Many patients with pneumonia are known to be at increased risk for cardiovascular events. The American Heart Association’s Get With The Guidelines – Resuscitation database found that in many stroke and cardiopulmonary arrests, pneumonia was a preexisting condition or was acquired during hospitalization.
Hence, it’s expected that preventing pneumonia with vaccination will also prevent subsequent death from cardiovascular reasons. Pneumonia vaccination will prevent not only cardiovascular events but death in general.
This study found more compelling reasons for us in the front line to vaccinate eligible candidates for flu and pneumonia, especially with fall already here.
Many patients with pneumonia are known to be at increased risk for cardiovascular events. The American Heart Association’s Get With The Guidelines – Resuscitation database found that in many stroke and cardiopulmonary arrests, pneumonia was a preexisting condition or was acquired during hospitalization.
Hence, it’s expected that preventing pneumonia with vaccination will also prevent subsequent death from cardiovascular reasons. Pneumonia vaccination will prevent not only cardiovascular events but death in general.
This study found more compelling reasons for us in the front line to vaccinate eligible candidates for flu and pneumonia, especially with fall already here.
BARCELONA – Influenza vaccine has been shown to provide protection against cardiovascular events, but can the same be said for pneumococcal vaccine?
Yes, particularly in the elderly and in patients at high baseline cardiovascular risk, according to a meta-analysis presented by Dr. Dimitrios Terentes-Printzios at the annual congress of the European Society of Cardiology.
He analyzed 11 published studies comprising 332,267 subjects followed for a mean of 20 months. Because the studies focused on different populations and in some cases reached conflicting conclusions, he performed a series of subgroup analyses to gain a clearer picture.
One of these analyses found that the cardioprotective effects of pneumococcal vaccination wane over time. In studies with follow-up of less than 1 year, the relative risk of total cardiovascular events was 0.72, meaning that patients who received pneumococcal vaccine had a significant 28% relative risk reduction compared with those who did not. In studies with follow-up in excess of 1 year, however, there was no cardioprotective effect, according to Dr. Terentes-Printzios of Athens Medical School.
Significant protection against total cardiovascular events was seen in elderly vaccinated patients, with a 20% relative risk reduction, and in subjects at high baseline cardiovascular risk, who had an 8% risk reduction if they received pneumococcal vaccine.
Breaking down the specific endpoints, subjects who got pneumococcal vaccine had a statistically significant 8% reduction in the risk of cardiovascular mortality. However, vaccination provided no significant protective effect against acute MI or cerebrovascular events except in the elderly, where the relative risk reductions were 10% and 14%, respectively.
These cardio- and cerebrovascular protective benefits of the pneumococcal vaccine can be viewed as added value, given that the primary reason physicians prescribe the vaccine is its demonstrated ability to reduce the risk of invasive pneumococcal infection by up to 60%.
Dr. Terentes-Printzios reported having no financial conflicts.
BARCELONA – Influenza vaccine has been shown to provide protection against cardiovascular events, but can the same be said for pneumococcal vaccine?
Yes, particularly in the elderly and in patients at high baseline cardiovascular risk, according to a meta-analysis presented by Dr. Dimitrios Terentes-Printzios at the annual congress of the European Society of Cardiology.
He analyzed 11 published studies comprising 332,267 subjects followed for a mean of 20 months. Because the studies focused on different populations and in some cases reached conflicting conclusions, he performed a series of subgroup analyses to gain a clearer picture.
One of these analyses found that the cardioprotective effects of pneumococcal vaccination wane over time. In studies with follow-up of less than 1 year, the relative risk of total cardiovascular events was 0.72, meaning that patients who received pneumococcal vaccine had a significant 28% relative risk reduction compared with those who did not. In studies with follow-up in excess of 1 year, however, there was no cardioprotective effect, according to Dr. Terentes-Printzios of Athens Medical School.
Significant protection against total cardiovascular events was seen in elderly vaccinated patients, with a 20% relative risk reduction, and in subjects at high baseline cardiovascular risk, who had an 8% risk reduction if they received pneumococcal vaccine.
Breaking down the specific endpoints, subjects who got pneumococcal vaccine had a statistically significant 8% reduction in the risk of cardiovascular mortality. However, vaccination provided no significant protective effect against acute MI or cerebrovascular events except in the elderly, where the relative risk reductions were 10% and 14%, respectively.
These cardio- and cerebrovascular protective benefits of the pneumococcal vaccine can be viewed as added value, given that the primary reason physicians prescribe the vaccine is its demonstrated ability to reduce the risk of invasive pneumococcal infection by up to 60%.
Dr. Terentes-Printzios reported having no financial conflicts.
AT THE ESC CONGRESS 2014
Key clinical point: Pneumococcal vaccine provides ancillary cardio- and cerebrovascular protective benefits, particularly in the elderly.
Major finding: Patients who got the pneumococcal vaccine had a statistically significant 8% reduction in the risk of cardiovascular mortality.
Data source: A meta-analysis of 11 studies comprising more than 332,000 patients.
Disclosures: The presenter reported having no conflicts of interest regarding this study.
Thrombectomy during primary PCI lacks 1-year benefits
BARCELONA – Routine thrombus aspiration in patients with acute ST-elevation myocardial infarction failed to produce a 1-year survival benefit over conventional care in a multicenter, randomized trial with more than 7,000 patients, negating a previously reported 1-year survival boost from thrombus aspiration in a single-center study with about 1,000 patients.
"Our recommendation is that routine thrombus aspiration not be done" in patients with ST-elevation myocardial infarction [STEMI] undergoing percutaneous coronary intervention [PCI], Dr. Bo Lagerqvist said at the annual congress of the European Society of Cardiology. A year ago, he and his associates reported no 30-day mortality benefit from routine thrombus aspiration during PCI in the same TASTE (Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia) study (N. Eng. J. Med. 2013;369:1587-97).
But Dr. Lagerqvist and other experts left the door open to thrombus aspiration for cause during PCI, a niche that likely will continue to fuel interest in a technique that has soared in popularity despite its unproven benefit.
"The abundance of devices [for performing thrombus aspiration] on the market contrasts with the paucity of data" showing benefit, commented Dr. Gilles Montalescot, professor of cardiology at University Hospital La Pitié Salpêtrière in Paris.
"I don’t expect a reduction in use of thrombus aspiration despite the results," commented Dr. Carlo Di Mario, an interventional cardiologist at Royal Brompton Hospital in London. "Cardiologists use thrombus aspiration to create a larger lumen" when an infarct-related artery is blocked with a large thrombus. "The alternative it to use a balloon, which can cause embolization and which just exchanges one device for another."
TASTE randomized 7,244 acute STEMI patients undergoing PCI at 29 centers in Sweden and 1 center each in Iceland and Denmark. They averaged about 66 years of age, and 78% of patients had either full occlusion or low coronary flow through the infarct-related artery at the time of hospitalization.
One year after intervention, the rates of all-cause death, repeat STEMI, and of stent thrombosis were each identical in the patients who received thrombus aspiration and those who did not, reported Dr Lagerqvist, a cardiologist at Uppsala (Sweden) University Hospital. In addition, the absence of a benefit was consistent across several subgroups included in the analysis, which meant the researchers found no type of acute STEMI patient who clearly benefited from thrombus aspiration. Concurrent with his report at the meeting the results also appeared online (N. Engl. J. Med. 2014 [doi: 10.1056/NEJMoa1405707]).
"We saw no danger from thrombus aspiration, and it is relatively inexpensive, so it is possible to use it, but we don’t know how to identify the patients who might benefit from it, even patients with a full coronary occlusion" from thrombus, he said during a press conference. Before the TASTE results were known, roughly 50% of Swedish primary PCI cases for acute STEMI also received thrombus aspiration; today, use is "very, very low," Dr. Lagerqvist said.
Dr. Lagerqvist and others acknowledged that even more definitive data on the role of thrombus aspiration during PCI for acute STEMI will come from the TOTAL (Trial of Routine Aspiration Thrombectomy With PCI vs. PCI Alone in Patients With STEMI Undergoing Primary PCI) study, which has randomized nearly 11,000 patients and is expected to report results next year. Dr. Sanjit Jolly, an interventional cardiologist at McMaster University in Hamilton, Ont., and lead investigator of TOTAL, made this written statement about the new TASTE report:
"Thrombectomy is a one-time intervention unlike an ongoing drug therapy (i.e., ticagrelor). Therefore, the hypothesis that late events may have been more responsive to thrombectomy than early events is likely unrealistic. While TASTE excludes a 50% reduction in mortality with thrombectomy, it was not powered for realistic 20%-25% reductions in outcomes. Therefore, clinicians should reserve judgment and await the results of the largest trial examining this question, the TOTAL trial. The TOTAL trial has randomized nearly 11,000 patients to thrombectomy vs. PCI alone in STEMI and uses a composite outcome with blinded adjudication of outcomes. The TOTAL trial is powered for a 20% relative risk reduction and is expected to be presented in early 2015."
TASTE received no commercial support. Dr. Lagerqvist and Dr. Di Marco had no disclosures. Dr. Montalescot has received honoraria and research support from several companies including Medtronic, which markets a thrombectomy catheter. Dr. Jolly has received research support from Medtronic.
On Twitter @mitchelzoler
Thrombus aspiration is easy to use, it can make percutaneous coronary interventions easier to perform, and is probably safe, but there is insufficient evidence to recommend its use. One-year results from a relatively small, single-center study had suggested that it produced beneficial effects. But the TASTE study is the largest test of thrombectomy to date, and we must accept its findings.
It is not good to have so many devices on the market for performing thrombus aspiration when no data show what they achieve. The results from the TOTAL trial next year should be definitive, but I think that TOTAL will bring an end to thrombus aspiration during primary PCI. Thrombectomy risks the danger of displaced thrombus. When a large thrombus totally occludes an artery, there is a need for thrombus aspiration, so I don’t think the technique will totally disappear. But routine use for all patients will die out. We also need to study the possible impact of thrombectomy on reducing the risk for heart failure following STEMI.
Dr. Gilles Montalescot is professor of cardiology at University Hospital La Pitié Salpêtrière in Paris. He has received honoraria and grant support from several drug and device companies, including Medtronic, which markets a thrombectomy device. He made these comments as designated discussant for the study and in an interview.
Thrombus aspiration is easy to use, it can make percutaneous coronary interventions easier to perform, and is probably safe, but there is insufficient evidence to recommend its use. One-year results from a relatively small, single-center study had suggested that it produced beneficial effects. But the TASTE study is the largest test of thrombectomy to date, and we must accept its findings.
It is not good to have so many devices on the market for performing thrombus aspiration when no data show what they achieve. The results from the TOTAL trial next year should be definitive, but I think that TOTAL will bring an end to thrombus aspiration during primary PCI. Thrombectomy risks the danger of displaced thrombus. When a large thrombus totally occludes an artery, there is a need for thrombus aspiration, so I don’t think the technique will totally disappear. But routine use for all patients will die out. We also need to study the possible impact of thrombectomy on reducing the risk for heart failure following STEMI.
Dr. Gilles Montalescot is professor of cardiology at University Hospital La Pitié Salpêtrière in Paris. He has received honoraria and grant support from several drug and device companies, including Medtronic, which markets a thrombectomy device. He made these comments as designated discussant for the study and in an interview.
Thrombus aspiration is easy to use, it can make percutaneous coronary interventions easier to perform, and is probably safe, but there is insufficient evidence to recommend its use. One-year results from a relatively small, single-center study had suggested that it produced beneficial effects. But the TASTE study is the largest test of thrombectomy to date, and we must accept its findings.
It is not good to have so many devices on the market for performing thrombus aspiration when no data show what they achieve. The results from the TOTAL trial next year should be definitive, but I think that TOTAL will bring an end to thrombus aspiration during primary PCI. Thrombectomy risks the danger of displaced thrombus. When a large thrombus totally occludes an artery, there is a need for thrombus aspiration, so I don’t think the technique will totally disappear. But routine use for all patients will die out. We also need to study the possible impact of thrombectomy on reducing the risk for heart failure following STEMI.
Dr. Gilles Montalescot is professor of cardiology at University Hospital La Pitié Salpêtrière in Paris. He has received honoraria and grant support from several drug and device companies, including Medtronic, which markets a thrombectomy device. He made these comments as designated discussant for the study and in an interview.
BARCELONA – Routine thrombus aspiration in patients with acute ST-elevation myocardial infarction failed to produce a 1-year survival benefit over conventional care in a multicenter, randomized trial with more than 7,000 patients, negating a previously reported 1-year survival boost from thrombus aspiration in a single-center study with about 1,000 patients.
"Our recommendation is that routine thrombus aspiration not be done" in patients with ST-elevation myocardial infarction [STEMI] undergoing percutaneous coronary intervention [PCI], Dr. Bo Lagerqvist said at the annual congress of the European Society of Cardiology. A year ago, he and his associates reported no 30-day mortality benefit from routine thrombus aspiration during PCI in the same TASTE (Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia) study (N. Eng. J. Med. 2013;369:1587-97).
But Dr. Lagerqvist and other experts left the door open to thrombus aspiration for cause during PCI, a niche that likely will continue to fuel interest in a technique that has soared in popularity despite its unproven benefit.
"The abundance of devices [for performing thrombus aspiration] on the market contrasts with the paucity of data" showing benefit, commented Dr. Gilles Montalescot, professor of cardiology at University Hospital La Pitié Salpêtrière in Paris.
"I don’t expect a reduction in use of thrombus aspiration despite the results," commented Dr. Carlo Di Mario, an interventional cardiologist at Royal Brompton Hospital in London. "Cardiologists use thrombus aspiration to create a larger lumen" when an infarct-related artery is blocked with a large thrombus. "The alternative it to use a balloon, which can cause embolization and which just exchanges one device for another."
TASTE randomized 7,244 acute STEMI patients undergoing PCI at 29 centers in Sweden and 1 center each in Iceland and Denmark. They averaged about 66 years of age, and 78% of patients had either full occlusion or low coronary flow through the infarct-related artery at the time of hospitalization.
One year after intervention, the rates of all-cause death, repeat STEMI, and of stent thrombosis were each identical in the patients who received thrombus aspiration and those who did not, reported Dr Lagerqvist, a cardiologist at Uppsala (Sweden) University Hospital. In addition, the absence of a benefit was consistent across several subgroups included in the analysis, which meant the researchers found no type of acute STEMI patient who clearly benefited from thrombus aspiration. Concurrent with his report at the meeting the results also appeared online (N. Engl. J. Med. 2014 [doi: 10.1056/NEJMoa1405707]).
"We saw no danger from thrombus aspiration, and it is relatively inexpensive, so it is possible to use it, but we don’t know how to identify the patients who might benefit from it, even patients with a full coronary occlusion" from thrombus, he said during a press conference. Before the TASTE results were known, roughly 50% of Swedish primary PCI cases for acute STEMI also received thrombus aspiration; today, use is "very, very low," Dr. Lagerqvist said.
Dr. Lagerqvist and others acknowledged that even more definitive data on the role of thrombus aspiration during PCI for acute STEMI will come from the TOTAL (Trial of Routine Aspiration Thrombectomy With PCI vs. PCI Alone in Patients With STEMI Undergoing Primary PCI) study, which has randomized nearly 11,000 patients and is expected to report results next year. Dr. Sanjit Jolly, an interventional cardiologist at McMaster University in Hamilton, Ont., and lead investigator of TOTAL, made this written statement about the new TASTE report:
"Thrombectomy is a one-time intervention unlike an ongoing drug therapy (i.e., ticagrelor). Therefore, the hypothesis that late events may have been more responsive to thrombectomy than early events is likely unrealistic. While TASTE excludes a 50% reduction in mortality with thrombectomy, it was not powered for realistic 20%-25% reductions in outcomes. Therefore, clinicians should reserve judgment and await the results of the largest trial examining this question, the TOTAL trial. The TOTAL trial has randomized nearly 11,000 patients to thrombectomy vs. PCI alone in STEMI and uses a composite outcome with blinded adjudication of outcomes. The TOTAL trial is powered for a 20% relative risk reduction and is expected to be presented in early 2015."
TASTE received no commercial support. Dr. Lagerqvist and Dr. Di Marco had no disclosures. Dr. Montalescot has received honoraria and research support from several companies including Medtronic, which markets a thrombectomy catheter. Dr. Jolly has received research support from Medtronic.
On Twitter @mitchelzoler
BARCELONA – Routine thrombus aspiration in patients with acute ST-elevation myocardial infarction failed to produce a 1-year survival benefit over conventional care in a multicenter, randomized trial with more than 7,000 patients, negating a previously reported 1-year survival boost from thrombus aspiration in a single-center study with about 1,000 patients.
"Our recommendation is that routine thrombus aspiration not be done" in patients with ST-elevation myocardial infarction [STEMI] undergoing percutaneous coronary intervention [PCI], Dr. Bo Lagerqvist said at the annual congress of the European Society of Cardiology. A year ago, he and his associates reported no 30-day mortality benefit from routine thrombus aspiration during PCI in the same TASTE (Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia) study (N. Eng. J. Med. 2013;369:1587-97).
But Dr. Lagerqvist and other experts left the door open to thrombus aspiration for cause during PCI, a niche that likely will continue to fuel interest in a technique that has soared in popularity despite its unproven benefit.
"The abundance of devices [for performing thrombus aspiration] on the market contrasts with the paucity of data" showing benefit, commented Dr. Gilles Montalescot, professor of cardiology at University Hospital La Pitié Salpêtrière in Paris.
"I don’t expect a reduction in use of thrombus aspiration despite the results," commented Dr. Carlo Di Mario, an interventional cardiologist at Royal Brompton Hospital in London. "Cardiologists use thrombus aspiration to create a larger lumen" when an infarct-related artery is blocked with a large thrombus. "The alternative it to use a balloon, which can cause embolization and which just exchanges one device for another."
TASTE randomized 7,244 acute STEMI patients undergoing PCI at 29 centers in Sweden and 1 center each in Iceland and Denmark. They averaged about 66 years of age, and 78% of patients had either full occlusion or low coronary flow through the infarct-related artery at the time of hospitalization.
One year after intervention, the rates of all-cause death, repeat STEMI, and of stent thrombosis were each identical in the patients who received thrombus aspiration and those who did not, reported Dr Lagerqvist, a cardiologist at Uppsala (Sweden) University Hospital. In addition, the absence of a benefit was consistent across several subgroups included in the analysis, which meant the researchers found no type of acute STEMI patient who clearly benefited from thrombus aspiration. Concurrent with his report at the meeting the results also appeared online (N. Engl. J. Med. 2014 [doi: 10.1056/NEJMoa1405707]).
"We saw no danger from thrombus aspiration, and it is relatively inexpensive, so it is possible to use it, but we don’t know how to identify the patients who might benefit from it, even patients with a full coronary occlusion" from thrombus, he said during a press conference. Before the TASTE results were known, roughly 50% of Swedish primary PCI cases for acute STEMI also received thrombus aspiration; today, use is "very, very low," Dr. Lagerqvist said.
Dr. Lagerqvist and others acknowledged that even more definitive data on the role of thrombus aspiration during PCI for acute STEMI will come from the TOTAL (Trial of Routine Aspiration Thrombectomy With PCI vs. PCI Alone in Patients With STEMI Undergoing Primary PCI) study, which has randomized nearly 11,000 patients and is expected to report results next year. Dr. Sanjit Jolly, an interventional cardiologist at McMaster University in Hamilton, Ont., and lead investigator of TOTAL, made this written statement about the new TASTE report:
"Thrombectomy is a one-time intervention unlike an ongoing drug therapy (i.e., ticagrelor). Therefore, the hypothesis that late events may have been more responsive to thrombectomy than early events is likely unrealistic. While TASTE excludes a 50% reduction in mortality with thrombectomy, it was not powered for realistic 20%-25% reductions in outcomes. Therefore, clinicians should reserve judgment and await the results of the largest trial examining this question, the TOTAL trial. The TOTAL trial has randomized nearly 11,000 patients to thrombectomy vs. PCI alone in STEMI and uses a composite outcome with blinded adjudication of outcomes. The TOTAL trial is powered for a 20% relative risk reduction and is expected to be presented in early 2015."
TASTE received no commercial support. Dr. Lagerqvist and Dr. Di Marco had no disclosures. Dr. Montalescot has received honoraria and research support from several companies including Medtronic, which markets a thrombectomy catheter. Dr. Jolly has received research support from Medtronic.
On Twitter @mitchelzoler
AT ESC 2014
Key clinical point: Thrombus aspiration during primary PCI in acute STEMI patients produced no survival benefit 1 year after treatment in a randomized trial with 7,000 patients.
Major finding: The 1-year all-cause death rate was 5.3% in patients who received thrombectomy and 5.6% in those who did not, a nonsignificant difference.
Data source: TASTE, a multicenter study with 7,244 acute STEMI patients who underwent primary PCI.
Disclosures: TASTE received no commercial support. Dr. Lagerqvist and Dr. Di Marco had no disclosures. Dr. Montalescot has received honoraria and research support from several companies including Medtronic, which markets a thrombectomy catheter. Dr. Jolly has received research support from Medtronic.
Modest hypertension control in diabetic patients boosts survival
BARCELONA – A modest amount of blood pressure control over an average of 4 years produced a significant, long-term survival benefit in patients with type 2 diabetes, based on a 10-year follow-up of more than 8,000 patients.
"It is critically important to maintain active blood-pressure lowering in both the short and long term in order to derive the greatest possible reductions in mortality and major cardiovascular events" in patients with type 2 diabetes, Dr. John Chalmers said at the annual congress of the European Society of Cardiology.
The results he reported showed that during 10 years of total follow-up, 4 years of concerted therapy with a dual antihypertensive-drug formulation on top of background therapy produced an average drop in blood pressure of 5.6/2.2 mm Hg, which was linked to a relative 9% decrease in mortality through the entire 10-year period.
Dr. Chalmers’ analysis used data from an average 6-year follow-up of patients after they completed a 4-year trial that had randomized them to treatment with either the combined formulation of the angiotensin-converting enzyme inhibitor perindopril and the diuretic indapamide, or to placebo. The 6-year assessment of these patients after they finished the closely regulated treatment phase while in the clinical trial is "the longest follow-up on the impact of blood pressure reduction in patients with diabetes," commented Dr. Lars Rydén, a cardiologist at the Karolinska Institute in Stockholm.
"The greatest part of the overall, cumulative benefit [over a 10-year period] was contributed by the carry forward of the in-trial benefits of active BP lowering," said Dr. Chalmers, senior director of the George Institute for Global Health in Sydney, Australia.
His study focused on data collected from patients enrolled in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation) trial. This study randomized 11,140 patients with type 2 diabetes who were at least 55 years old and had at least one additional cardiovascular disease risk factor, to treatment with the combined formulation on top of their preexisting medications or to placebo plus existing medications (Lancet 2007;370:829-40). Patients in the placebo group were unable to receive an angiotensin-converting enzyme inhibitor during the study. The study was sponsored by Servier, the company that markets a combined perindopril and indapamide formulation (Preterax).
At enrollment, ADVANCE patients averaged 66 years old, had an average 8-year history of type 2 diabetes, and had an average blood pressure of 145/81 mm Hg. Three-quarters were on an antihypertensive regimen of some kind at entry into the study.
During the study’s 4 years of active treatment, patients randomized to receive the perindopril and indapamide combination had significantly reduced blood pressure, compared with the control patients. After 4 years, this linked to a 9% relative drop in the combined incidence of major macrovascular and microvascular events (including cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction). Compared with the control group, this cut in events was statistically significant for the study’s primary endpoint. The antihypertensive intervention also was linked to a statistically significant, 14% relative drop in all-cause deaths.
To assess the longer-term impact of this 4-year intervention, Dr. Chalmers and his associates ran a post-trial observational study, ADVANCE-ON. Initially, 8,494 of the patients who completed ADVANCE (83% of surviving patients) agreed to participate in ADVANCE-ON; after another 6 years of follow-up, 5,131 patients remained under observation. During the extended 6 years of follow-up, patients from both the initial intervention arm and the initial control arm all maintained average blood pressures of roughly 137/75 mm Hg. The blood pressures of the original intervention and control patient arms "fully converged," Dr. Chalmers said.
During this 6-year period, patients received care from their personal physicians and could receive any antihypertensive regimen their physician prescribed. At their first follow-up medical examination after the end of the main ADVANCE study, about 40% of patients were on no antihypertensive drugs, 23% were on one drug, 21% were on two drugs, and the remainder were on higher numbers of antihypertensive drugs.
During the 6-year post-trial period, patients who had previously been in the active-treatment arm had a 6% relative reduction in all-cause mortality, compared with patients originally in the control arm, a difference that was not significant.
But when the researchers combined the 6-year post-trial follow-up with the in-trial results for a total median 10-year follow-up, they found an overall 9% relative cut in mortality in the intervention patients, compared with the controls, and a relative 12% drop in cardiovascular mortality.
The persistent, long-term benefits were "attenuated" compared with the shorter-term benefits seen during the active phase, but nonetheless they persisted and were consistent across all subgroups studied, Dr. Chalmers said.
The ADVANCE and ADVANCE-ON studies were partly funded by Servier. Dr. Chalmers has received research grants and honoraria from that company. Dr. Rydén has been an adviser to AstraZeneca, Roche, Bristol-Myers Squibb, and Sanofi, and received research grants from Roche.
On Twitter @mitchelzoler
The findings from ADVANCE-ON are important. Clinicians need clear evidence that controlling a patient’s blood pressure produces long-term benefits, and the results from ADVANCE-ON provide this. We need many more studies that track the long-term impact of blood pressure reduction. Currently, a large fraction of patients with diabetes are not maintained at the recommended blood pressure levels. This report presents the longest follow-up of any antihypertensive intervention in patients with diabetes.
The findings also show that a small blood pressure reduction can have an important and long-lasting effect. This highlights the value of blood pressure control, even when the reduction achieved in an individual patient seems small.

|
|
I believe that the effects linked with blood pressure reduction in ADVANCE-ON are likely generalizable to any antihypertensive regimen that produces a similar magnitude of effect. It is the blood pressure effect rather than the specific antihypertensive drugs used that is that critical, although drugs that affect the renin-angiotensin-aldosterone system are clearly the first choice for patients with diabetes and hypertension.
Dr. Lars Rydén is a cardiologist at the Karolinska Institute in Stockholm. He has been an adviser to AstraZeneca, Roche, Bristol-Myers Squibb, and Sanofi, and received research grants from Roche. He made these comments as designated discussant for the ADVANCE-ON report and in an interview.
The findings from ADVANCE-ON are important. Clinicians need clear evidence that controlling a patient’s blood pressure produces long-term benefits, and the results from ADVANCE-ON provide this. We need many more studies that track the long-term impact of blood pressure reduction. Currently, a large fraction of patients with diabetes are not maintained at the recommended blood pressure levels. This report presents the longest follow-up of any antihypertensive intervention in patients with diabetes.
The findings also show that a small blood pressure reduction can have an important and long-lasting effect. This highlights the value of blood pressure control, even when the reduction achieved in an individual patient seems small.

|
|
I believe that the effects linked with blood pressure reduction in ADVANCE-ON are likely generalizable to any antihypertensive regimen that produces a similar magnitude of effect. It is the blood pressure effect rather than the specific antihypertensive drugs used that is that critical, although drugs that affect the renin-angiotensin-aldosterone system are clearly the first choice for patients with diabetes and hypertension.
Dr. Lars Rydén is a cardiologist at the Karolinska Institute in Stockholm. He has been an adviser to AstraZeneca, Roche, Bristol-Myers Squibb, and Sanofi, and received research grants from Roche. He made these comments as designated discussant for the ADVANCE-ON report and in an interview.
The findings from ADVANCE-ON are important. Clinicians need clear evidence that controlling a patient’s blood pressure produces long-term benefits, and the results from ADVANCE-ON provide this. We need many more studies that track the long-term impact of blood pressure reduction. Currently, a large fraction of patients with diabetes are not maintained at the recommended blood pressure levels. This report presents the longest follow-up of any antihypertensive intervention in patients with diabetes.
The findings also show that a small blood pressure reduction can have an important and long-lasting effect. This highlights the value of blood pressure control, even when the reduction achieved in an individual patient seems small.

|
|
I believe that the effects linked with blood pressure reduction in ADVANCE-ON are likely generalizable to any antihypertensive regimen that produces a similar magnitude of effect. It is the blood pressure effect rather than the specific antihypertensive drugs used that is that critical, although drugs that affect the renin-angiotensin-aldosterone system are clearly the first choice for patients with diabetes and hypertension.
Dr. Lars Rydén is a cardiologist at the Karolinska Institute in Stockholm. He has been an adviser to AstraZeneca, Roche, Bristol-Myers Squibb, and Sanofi, and received research grants from Roche. He made these comments as designated discussant for the ADVANCE-ON report and in an interview.
BARCELONA – A modest amount of blood pressure control over an average of 4 years produced a significant, long-term survival benefit in patients with type 2 diabetes, based on a 10-year follow-up of more than 8,000 patients.
"It is critically important to maintain active blood-pressure lowering in both the short and long term in order to derive the greatest possible reductions in mortality and major cardiovascular events" in patients with type 2 diabetes, Dr. John Chalmers said at the annual congress of the European Society of Cardiology.
The results he reported showed that during 10 years of total follow-up, 4 years of concerted therapy with a dual antihypertensive-drug formulation on top of background therapy produced an average drop in blood pressure of 5.6/2.2 mm Hg, which was linked to a relative 9% decrease in mortality through the entire 10-year period.
Dr. Chalmers’ analysis used data from an average 6-year follow-up of patients after they completed a 4-year trial that had randomized them to treatment with either the combined formulation of the angiotensin-converting enzyme inhibitor perindopril and the diuretic indapamide, or to placebo. The 6-year assessment of these patients after they finished the closely regulated treatment phase while in the clinical trial is "the longest follow-up on the impact of blood pressure reduction in patients with diabetes," commented Dr. Lars Rydén, a cardiologist at the Karolinska Institute in Stockholm.
"The greatest part of the overall, cumulative benefit [over a 10-year period] was contributed by the carry forward of the in-trial benefits of active BP lowering," said Dr. Chalmers, senior director of the George Institute for Global Health in Sydney, Australia.
His study focused on data collected from patients enrolled in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation) trial. This study randomized 11,140 patients with type 2 diabetes who were at least 55 years old and had at least one additional cardiovascular disease risk factor, to treatment with the combined formulation on top of their preexisting medications or to placebo plus existing medications (Lancet 2007;370:829-40). Patients in the placebo group were unable to receive an angiotensin-converting enzyme inhibitor during the study. The study was sponsored by Servier, the company that markets a combined perindopril and indapamide formulation (Preterax).
At enrollment, ADVANCE patients averaged 66 years old, had an average 8-year history of type 2 diabetes, and had an average blood pressure of 145/81 mm Hg. Three-quarters were on an antihypertensive regimen of some kind at entry into the study.
During the study’s 4 years of active treatment, patients randomized to receive the perindopril and indapamide combination had significantly reduced blood pressure, compared with the control patients. After 4 years, this linked to a 9% relative drop in the combined incidence of major macrovascular and microvascular events (including cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction). Compared with the control group, this cut in events was statistically significant for the study’s primary endpoint. The antihypertensive intervention also was linked to a statistically significant, 14% relative drop in all-cause deaths.
To assess the longer-term impact of this 4-year intervention, Dr. Chalmers and his associates ran a post-trial observational study, ADVANCE-ON. Initially, 8,494 of the patients who completed ADVANCE (83% of surviving patients) agreed to participate in ADVANCE-ON; after another 6 years of follow-up, 5,131 patients remained under observation. During the extended 6 years of follow-up, patients from both the initial intervention arm and the initial control arm all maintained average blood pressures of roughly 137/75 mm Hg. The blood pressures of the original intervention and control patient arms "fully converged," Dr. Chalmers said.
During this 6-year period, patients received care from their personal physicians and could receive any antihypertensive regimen their physician prescribed. At their first follow-up medical examination after the end of the main ADVANCE study, about 40% of patients were on no antihypertensive drugs, 23% were on one drug, 21% were on two drugs, and the remainder were on higher numbers of antihypertensive drugs.
During the 6-year post-trial period, patients who had previously been in the active-treatment arm had a 6% relative reduction in all-cause mortality, compared with patients originally in the control arm, a difference that was not significant.
But when the researchers combined the 6-year post-trial follow-up with the in-trial results for a total median 10-year follow-up, they found an overall 9% relative cut in mortality in the intervention patients, compared with the controls, and a relative 12% drop in cardiovascular mortality.
The persistent, long-term benefits were "attenuated" compared with the shorter-term benefits seen during the active phase, but nonetheless they persisted and were consistent across all subgroups studied, Dr. Chalmers said.
The ADVANCE and ADVANCE-ON studies were partly funded by Servier. Dr. Chalmers has received research grants and honoraria from that company. Dr. Rydén has been an adviser to AstraZeneca, Roche, Bristol-Myers Squibb, and Sanofi, and received research grants from Roche.
On Twitter @mitchelzoler
BARCELONA – A modest amount of blood pressure control over an average of 4 years produced a significant, long-term survival benefit in patients with type 2 diabetes, based on a 10-year follow-up of more than 8,000 patients.
"It is critically important to maintain active blood-pressure lowering in both the short and long term in order to derive the greatest possible reductions in mortality and major cardiovascular events" in patients with type 2 diabetes, Dr. John Chalmers said at the annual congress of the European Society of Cardiology.
The results he reported showed that during 10 years of total follow-up, 4 years of concerted therapy with a dual antihypertensive-drug formulation on top of background therapy produced an average drop in blood pressure of 5.6/2.2 mm Hg, which was linked to a relative 9% decrease in mortality through the entire 10-year period.
Dr. Chalmers’ analysis used data from an average 6-year follow-up of patients after they completed a 4-year trial that had randomized them to treatment with either the combined formulation of the angiotensin-converting enzyme inhibitor perindopril and the diuretic indapamide, or to placebo. The 6-year assessment of these patients after they finished the closely regulated treatment phase while in the clinical trial is "the longest follow-up on the impact of blood pressure reduction in patients with diabetes," commented Dr. Lars Rydén, a cardiologist at the Karolinska Institute in Stockholm.
"The greatest part of the overall, cumulative benefit [over a 10-year period] was contributed by the carry forward of the in-trial benefits of active BP lowering," said Dr. Chalmers, senior director of the George Institute for Global Health in Sydney, Australia.
His study focused on data collected from patients enrolled in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation) trial. This study randomized 11,140 patients with type 2 diabetes who were at least 55 years old and had at least one additional cardiovascular disease risk factor, to treatment with the combined formulation on top of their preexisting medications or to placebo plus existing medications (Lancet 2007;370:829-40). Patients in the placebo group were unable to receive an angiotensin-converting enzyme inhibitor during the study. The study was sponsored by Servier, the company that markets a combined perindopril and indapamide formulation (Preterax).
At enrollment, ADVANCE patients averaged 66 years old, had an average 8-year history of type 2 diabetes, and had an average blood pressure of 145/81 mm Hg. Three-quarters were on an antihypertensive regimen of some kind at entry into the study.
During the study’s 4 years of active treatment, patients randomized to receive the perindopril and indapamide combination had significantly reduced blood pressure, compared with the control patients. After 4 years, this linked to a 9% relative drop in the combined incidence of major macrovascular and microvascular events (including cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction). Compared with the control group, this cut in events was statistically significant for the study’s primary endpoint. The antihypertensive intervention also was linked to a statistically significant, 14% relative drop in all-cause deaths.
To assess the longer-term impact of this 4-year intervention, Dr. Chalmers and his associates ran a post-trial observational study, ADVANCE-ON. Initially, 8,494 of the patients who completed ADVANCE (83% of surviving patients) agreed to participate in ADVANCE-ON; after another 6 years of follow-up, 5,131 patients remained under observation. During the extended 6 years of follow-up, patients from both the initial intervention arm and the initial control arm all maintained average blood pressures of roughly 137/75 mm Hg. The blood pressures of the original intervention and control patient arms "fully converged," Dr. Chalmers said.
During this 6-year period, patients received care from their personal physicians and could receive any antihypertensive regimen their physician prescribed. At their first follow-up medical examination after the end of the main ADVANCE study, about 40% of patients were on no antihypertensive drugs, 23% were on one drug, 21% were on two drugs, and the remainder were on higher numbers of antihypertensive drugs.
During the 6-year post-trial period, patients who had previously been in the active-treatment arm had a 6% relative reduction in all-cause mortality, compared with patients originally in the control arm, a difference that was not significant.
But when the researchers combined the 6-year post-trial follow-up with the in-trial results for a total median 10-year follow-up, they found an overall 9% relative cut in mortality in the intervention patients, compared with the controls, and a relative 12% drop in cardiovascular mortality.
The persistent, long-term benefits were "attenuated" compared with the shorter-term benefits seen during the active phase, but nonetheless they persisted and were consistent across all subgroups studied, Dr. Chalmers said.
The ADVANCE and ADVANCE-ON studies were partly funded by Servier. Dr. Chalmers has received research grants and honoraria from that company. Dr. Rydén has been an adviser to AstraZeneca, Roche, Bristol-Myers Squibb, and Sanofi, and received research grants from Roche.
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2014
Key clinical point: The survival benefit from 4 years of concerted antihypertensive treatment in patients with type 2 diabetes persisted for at least another 6 years.
Major finding: During a median 10-year follow-up, 4 years of active antihypertensive treatment cut mortality by 9%, compared with controls in diabetic patients.
Data source: ADVANCE-ON, a 10-year follow-up of 8,494 patients initially enrolled in ADVANCE, a 4-year, controlled, randomized trial that began with 11,140 patients with type 2 diabetes and at least one other cardiovascular risk factor.
Disclosures:. The ADVANCE and ADVANCE-ON studies were partly funded by Servier. Dr. Chalmers has received research grants and honoraria from that company. Dr. Rydén has been an adviser to AstraZeneca, Roche, Bristol-Myers Squibb, and Sanofi, and received research grants from Roche.
VIDEO: Rivaroxaban provides advantages for cardioversion in AF
BARCELONA – The first-ever prospective, randomized trial of a novel oral anticoagulant in patients with atrial fibrillation undergoing elective cardioversion showed oral rivaroxaban at 20 mg once daily to be an effective and safe alternative to standard-of-care warfarin. But the study, known as X-VeRT, also showed rivaroxaban offers something in addition: more expeditious cardioversion amenable to reliable scheduling.
Dr. Riccardo Cappato, who presented the X-VeRT results at the annual congress of the European Society of Cardiology, explains in this video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – The first-ever prospective, randomized trial of a novel oral anticoagulant in patients with atrial fibrillation undergoing elective cardioversion showed oral rivaroxaban at 20 mg once daily to be an effective and safe alternative to standard-of-care warfarin. But the study, known as X-VeRT, also showed rivaroxaban offers something in addition: more expeditious cardioversion amenable to reliable scheduling.
Dr. Riccardo Cappato, who presented the X-VeRT results at the annual congress of the European Society of Cardiology, explains in this video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – The first-ever prospective, randomized trial of a novel oral anticoagulant in patients with atrial fibrillation undergoing elective cardioversion showed oral rivaroxaban at 20 mg once daily to be an effective and safe alternative to standard-of-care warfarin. But the study, known as X-VeRT, also showed rivaroxaban offers something in addition: more expeditious cardioversion amenable to reliable scheduling.
Dr. Riccardo Cappato, who presented the X-VeRT results at the annual congress of the European Society of Cardiology, explains in this video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2014
Rivaroxaban shows advantages in AF cardioversion
BARCELONA – Rivaroxaban appears to be a safe and effective alternative to warfarin in patients with atrial fibrillation undergoing elective cardioversion, according to the findings of the first-ever prospective, randomized trial of a novel oral anticoagulant for this application.
Additionally, the X-VeRT trial showed that rivaroxaban (Xarelto) may offer an attractive, highly practical advantage over warfarin, the current guideline-recommended standard of care for cardioversion: Namely, rivaroxaban enabled patients to undergo the procedure more expeditiously, Dr. Riccardo Cappato noted in presenting the X-VeRT findings at the annual congress of the European Society of Cardiology.
Indeed, patients in the early-cardioversion arm of the trial safely underwent the procedure as early as 4 hours after taking their first 20-mg dose of rivaroxaban.
Moreover, patients in the delayed-conversion study arm, which required at least 3 consecutive weeks of effective oral anticoagulation preprocedurally, underwent cardioversion an average of 8 days earlier if randomized to rivaroxaban rather than to warfarin or another vitamin K antagonist. That’s because many warfarin-treated patients couldn’t maintain their international normalized ratio (INR) within the target range of 2.0-3.0 for 3 straight weeks, explained Dr. Cappato, professor of electrophysiology and chief of the arrhythmia and electrophysiology center at the University of Milan’s San Donato Polyclinic Hospital.
X-VeRT included 1,504 patients with nonvalvular atrial fibrillation (AF) at 141 centers in 16 countries. All were scheduled for elective cardioversion. They were randomized 2:1 to rivaroxaban 20 mg once daily or to warfarin at a dose adjusted to maintain an INR of 2.0-3.0.
Of those patients, 872 were assigned to an early-cardioversion strategy. Their cardioversion took place after 1-5 days on study medication, provided transesophageal echocardiography had ruled out a left atrial thrombus or they were already on chronic warfarin with their last three INRs in the target range. The other 632 patients were cardioverted using a delayed strategy in which they had to be on study medication for 3-8 weeks before the procedure.
The primary efficacy outcome in X-VeRT was the composite rate of stroke, TIA, peripheral embolism, MI, and cardiovascular death. The incidence was 0.51% in the rivaroxaban group and not statistically different at 1.02% in the warfarin group. The primary safety outcome – major bleeding – occurred in 0.6% of rivaroxaban-treated patients and 0.8% on warfarin.
The median time to cardioversion in the early-cardioversion strategy arm was similar regardless of which drug was used. Of note, however, in the delayed-strategy group, the median time to cardioversion was 22 days in patients on rivaroxaban, compared with 30 days with warfarin.
Of patients in the delayed-strategy group, 77% of those on rivaroxaban were cardioverted as scheduled, compared with 36% on warfarin. Only one patient in the rivaroxaban group was unable to undergo cardioversion before the 8-week cutoff because of inadequate anticoagulation as defined by less than 80% compliance in pill taking; in contrast, 95 warfarin-treated patients in the delayed-strategy group weren’t cardioverted because they missed the 8-week cutoff because of problematic INRs.
In an interview, ESC spokesman Dr. Jurrien Ten Berg predicted X-VeRT will be practice changing. He believes that on the basis of these study results, many physicians will view elective cardioversion as an excellent time to switch patients from warfarin, with all its inherent problems, to rivaroxaban, especially if they qualify for early cardioversion.
"I think this will absolutely change our policy. Here we’re talking about a once-daily pill that makes it possible to do a cardioversion early, and I think that’s a major advantage. If you delay the cardioversion, anything can happen. We know the vitamin K antagonists are unreliable, especially in the first weeks, when even if the INR is fine you don’t really know if the patient is well anticoagulated. For me, the totally of evidence, including the retrospective analyses of the large atrial fibrillation stroke prevention trials, is enough now to use a NOAC for several days and then do an early cardioversion," said Dr. Ten Berg, a cardiologist at St. Antonius Hospital, Nieuwegein, the Netherlands.
Discussant Dr. Christoph Bode called X-VeRT "a landmark trial" and "brilliant work."
"This should be included in the next update of the guidelines," said Dr. Bode, professor and chair of internal medicine and cardiology at the University of Freiburg, Germany.
However, Dr. Steven Nissen took a more skeptical, albeit clearly a minority, view.
"I think this study shows neither safety nor efficacy for this regimen. There were just a handful of events in both groups, too few to draw any conclusions. I don’t think the guidelines should be changed. I think the proper interpretation of the study is that it’s inconclusive," said Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
Dr. Cappato noted in response that he’d stated at the outset that X-VeRT, even at more than 1,500 patients, was underpowered statistically. Event rates in well-anticoagulated patients undergoing cardioversion are so low that a study to establish noninferiority for rivaroxaban would require 25,000-30,000 participants, which is not going to happen.
"Many physicians are already switching to NOACs [novel oral anticoagulants] for cardioversion despite the absence of good evidence. We thought bringing forward this solid, methodologically sound information from X-VeRT would provide more consistent support for those who are doing this or considering it," the cardiologist said.
Simultaneous with his presentation in Barcelona, the X-VeRT (Explore the Efficacy and Safety of Once-Daily Oral Rivaroxaban for the Prevention of Cardiovascular Events in Patients With Nonvalvular Atrial Fibrillation Scheduled for Cardioversion) study was published online (Eur. Heart J. 2014 [doi:10.1093/eurheart/ehu367]).
Dr. Cappato reported receiving investigator fees from and serving as a consultant to and on speakers bureaus for numerous pharmaceutical companies, including Bayer HealthCare, which sponsored X-VeRT. Dr. Bode has received honoraria from Bayer HealthCare. Dr. Ten Berg and Dr. Nissen reported having no financial conflicts.
BARCELONA – Rivaroxaban appears to be a safe and effective alternative to warfarin in patients with atrial fibrillation undergoing elective cardioversion, according to the findings of the first-ever prospective, randomized trial of a novel oral anticoagulant for this application.
Additionally, the X-VeRT trial showed that rivaroxaban (Xarelto) may offer an attractive, highly practical advantage over warfarin, the current guideline-recommended standard of care for cardioversion: Namely, rivaroxaban enabled patients to undergo the procedure more expeditiously, Dr. Riccardo Cappato noted in presenting the X-VeRT findings at the annual congress of the European Society of Cardiology.
Indeed, patients in the early-cardioversion arm of the trial safely underwent the procedure as early as 4 hours after taking their first 20-mg dose of rivaroxaban.
Moreover, patients in the delayed-conversion study arm, which required at least 3 consecutive weeks of effective oral anticoagulation preprocedurally, underwent cardioversion an average of 8 days earlier if randomized to rivaroxaban rather than to warfarin or another vitamin K antagonist. That’s because many warfarin-treated patients couldn’t maintain their international normalized ratio (INR) within the target range of 2.0-3.0 for 3 straight weeks, explained Dr. Cappato, professor of electrophysiology and chief of the arrhythmia and electrophysiology center at the University of Milan’s San Donato Polyclinic Hospital.
X-VeRT included 1,504 patients with nonvalvular atrial fibrillation (AF) at 141 centers in 16 countries. All were scheduled for elective cardioversion. They were randomized 2:1 to rivaroxaban 20 mg once daily or to warfarin at a dose adjusted to maintain an INR of 2.0-3.0.
Of those patients, 872 were assigned to an early-cardioversion strategy. Their cardioversion took place after 1-5 days on study medication, provided transesophageal echocardiography had ruled out a left atrial thrombus or they were already on chronic warfarin with their last three INRs in the target range. The other 632 patients were cardioverted using a delayed strategy in which they had to be on study medication for 3-8 weeks before the procedure.
The primary efficacy outcome in X-VeRT was the composite rate of stroke, TIA, peripheral embolism, MI, and cardiovascular death. The incidence was 0.51% in the rivaroxaban group and not statistically different at 1.02% in the warfarin group. The primary safety outcome – major bleeding – occurred in 0.6% of rivaroxaban-treated patients and 0.8% on warfarin.
The median time to cardioversion in the early-cardioversion strategy arm was similar regardless of which drug was used. Of note, however, in the delayed-strategy group, the median time to cardioversion was 22 days in patients on rivaroxaban, compared with 30 days with warfarin.
Of patients in the delayed-strategy group, 77% of those on rivaroxaban were cardioverted as scheduled, compared with 36% on warfarin. Only one patient in the rivaroxaban group was unable to undergo cardioversion before the 8-week cutoff because of inadequate anticoagulation as defined by less than 80% compliance in pill taking; in contrast, 95 warfarin-treated patients in the delayed-strategy group weren’t cardioverted because they missed the 8-week cutoff because of problematic INRs.
In an interview, ESC spokesman Dr. Jurrien Ten Berg predicted X-VeRT will be practice changing. He believes that on the basis of these study results, many physicians will view elective cardioversion as an excellent time to switch patients from warfarin, with all its inherent problems, to rivaroxaban, especially if they qualify for early cardioversion.
"I think this will absolutely change our policy. Here we’re talking about a once-daily pill that makes it possible to do a cardioversion early, and I think that’s a major advantage. If you delay the cardioversion, anything can happen. We know the vitamin K antagonists are unreliable, especially in the first weeks, when even if the INR is fine you don’t really know if the patient is well anticoagulated. For me, the totally of evidence, including the retrospective analyses of the large atrial fibrillation stroke prevention trials, is enough now to use a NOAC for several days and then do an early cardioversion," said Dr. Ten Berg, a cardiologist at St. Antonius Hospital, Nieuwegein, the Netherlands.
Discussant Dr. Christoph Bode called X-VeRT "a landmark trial" and "brilliant work."
"This should be included in the next update of the guidelines," said Dr. Bode, professor and chair of internal medicine and cardiology at the University of Freiburg, Germany.
However, Dr. Steven Nissen took a more skeptical, albeit clearly a minority, view.
"I think this study shows neither safety nor efficacy for this regimen. There were just a handful of events in both groups, too few to draw any conclusions. I don’t think the guidelines should be changed. I think the proper interpretation of the study is that it’s inconclusive," said Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
Dr. Cappato noted in response that he’d stated at the outset that X-VeRT, even at more than 1,500 patients, was underpowered statistically. Event rates in well-anticoagulated patients undergoing cardioversion are so low that a study to establish noninferiority for rivaroxaban would require 25,000-30,000 participants, which is not going to happen.
"Many physicians are already switching to NOACs [novel oral anticoagulants] for cardioversion despite the absence of good evidence. We thought bringing forward this solid, methodologically sound information from X-VeRT would provide more consistent support for those who are doing this or considering it," the cardiologist said.
Simultaneous with his presentation in Barcelona, the X-VeRT (Explore the Efficacy and Safety of Once-Daily Oral Rivaroxaban for the Prevention of Cardiovascular Events in Patients With Nonvalvular Atrial Fibrillation Scheduled for Cardioversion) study was published online (Eur. Heart J. 2014 [doi:10.1093/eurheart/ehu367]).
Dr. Cappato reported receiving investigator fees from and serving as a consultant to and on speakers bureaus for numerous pharmaceutical companies, including Bayer HealthCare, which sponsored X-VeRT. Dr. Bode has received honoraria from Bayer HealthCare. Dr. Ten Berg and Dr. Nissen reported having no financial conflicts.
BARCELONA – Rivaroxaban appears to be a safe and effective alternative to warfarin in patients with atrial fibrillation undergoing elective cardioversion, according to the findings of the first-ever prospective, randomized trial of a novel oral anticoagulant for this application.
Additionally, the X-VeRT trial showed that rivaroxaban (Xarelto) may offer an attractive, highly practical advantage over warfarin, the current guideline-recommended standard of care for cardioversion: Namely, rivaroxaban enabled patients to undergo the procedure more expeditiously, Dr. Riccardo Cappato noted in presenting the X-VeRT findings at the annual congress of the European Society of Cardiology.
Indeed, patients in the early-cardioversion arm of the trial safely underwent the procedure as early as 4 hours after taking their first 20-mg dose of rivaroxaban.
Moreover, patients in the delayed-conversion study arm, which required at least 3 consecutive weeks of effective oral anticoagulation preprocedurally, underwent cardioversion an average of 8 days earlier if randomized to rivaroxaban rather than to warfarin or another vitamin K antagonist. That’s because many warfarin-treated patients couldn’t maintain their international normalized ratio (INR) within the target range of 2.0-3.0 for 3 straight weeks, explained Dr. Cappato, professor of electrophysiology and chief of the arrhythmia and electrophysiology center at the University of Milan’s San Donato Polyclinic Hospital.
X-VeRT included 1,504 patients with nonvalvular atrial fibrillation (AF) at 141 centers in 16 countries. All were scheduled for elective cardioversion. They were randomized 2:1 to rivaroxaban 20 mg once daily or to warfarin at a dose adjusted to maintain an INR of 2.0-3.0.
Of those patients, 872 were assigned to an early-cardioversion strategy. Their cardioversion took place after 1-5 days on study medication, provided transesophageal echocardiography had ruled out a left atrial thrombus or they were already on chronic warfarin with their last three INRs in the target range. The other 632 patients were cardioverted using a delayed strategy in which they had to be on study medication for 3-8 weeks before the procedure.
The primary efficacy outcome in X-VeRT was the composite rate of stroke, TIA, peripheral embolism, MI, and cardiovascular death. The incidence was 0.51% in the rivaroxaban group and not statistically different at 1.02% in the warfarin group. The primary safety outcome – major bleeding – occurred in 0.6% of rivaroxaban-treated patients and 0.8% on warfarin.
The median time to cardioversion in the early-cardioversion strategy arm was similar regardless of which drug was used. Of note, however, in the delayed-strategy group, the median time to cardioversion was 22 days in patients on rivaroxaban, compared with 30 days with warfarin.
Of patients in the delayed-strategy group, 77% of those on rivaroxaban were cardioverted as scheduled, compared with 36% on warfarin. Only one patient in the rivaroxaban group was unable to undergo cardioversion before the 8-week cutoff because of inadequate anticoagulation as defined by less than 80% compliance in pill taking; in contrast, 95 warfarin-treated patients in the delayed-strategy group weren’t cardioverted because they missed the 8-week cutoff because of problematic INRs.
In an interview, ESC spokesman Dr. Jurrien Ten Berg predicted X-VeRT will be practice changing. He believes that on the basis of these study results, many physicians will view elective cardioversion as an excellent time to switch patients from warfarin, with all its inherent problems, to rivaroxaban, especially if they qualify for early cardioversion.
"I think this will absolutely change our policy. Here we’re talking about a once-daily pill that makes it possible to do a cardioversion early, and I think that’s a major advantage. If you delay the cardioversion, anything can happen. We know the vitamin K antagonists are unreliable, especially in the first weeks, when even if the INR is fine you don’t really know if the patient is well anticoagulated. For me, the totally of evidence, including the retrospective analyses of the large atrial fibrillation stroke prevention trials, is enough now to use a NOAC for several days and then do an early cardioversion," said Dr. Ten Berg, a cardiologist at St. Antonius Hospital, Nieuwegein, the Netherlands.
Discussant Dr. Christoph Bode called X-VeRT "a landmark trial" and "brilliant work."
"This should be included in the next update of the guidelines," said Dr. Bode, professor and chair of internal medicine and cardiology at the University of Freiburg, Germany.
However, Dr. Steven Nissen took a more skeptical, albeit clearly a minority, view.
"I think this study shows neither safety nor efficacy for this regimen. There were just a handful of events in both groups, too few to draw any conclusions. I don’t think the guidelines should be changed. I think the proper interpretation of the study is that it’s inconclusive," said Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
Dr. Cappato noted in response that he’d stated at the outset that X-VeRT, even at more than 1,500 patients, was underpowered statistically. Event rates in well-anticoagulated patients undergoing cardioversion are so low that a study to establish noninferiority for rivaroxaban would require 25,000-30,000 participants, which is not going to happen.
"Many physicians are already switching to NOACs [novel oral anticoagulants] for cardioversion despite the absence of good evidence. We thought bringing forward this solid, methodologically sound information from X-VeRT would provide more consistent support for those who are doing this or considering it," the cardiologist said.
Simultaneous with his presentation in Barcelona, the X-VeRT (Explore the Efficacy and Safety of Once-Daily Oral Rivaroxaban for the Prevention of Cardiovascular Events in Patients With Nonvalvular Atrial Fibrillation Scheduled for Cardioversion) study was published online (Eur. Heart J. 2014 [doi:10.1093/eurheart/ehu367]).
Dr. Cappato reported receiving investigator fees from and serving as a consultant to and on speakers bureaus for numerous pharmaceutical companies, including Bayer HealthCare, which sponsored X-VeRT. Dr. Bode has received honoraria from Bayer HealthCare. Dr. Ten Berg and Dr. Nissen reported having no financial conflicts.
AT THE ESC CONGRESS 2014
Key clinical point: Patients in atrial fibrillation were able to safely undergo cardioversion as early as 4 hours after taking their first 20-mg dose of oral rivaroxaban.
Major finding: Patients with AF assigned to rivaroxaban in conjunction with cardioversion had a 0.51% rate of major cardiovascular events, similar to the 1.02% rate in patients on warfarin or other vitamin K antagonists. These rates weren’t statistically different; nor were the major bleeding rates in the two groups.
Data source: X-VeRT was a randomized, prospective, open-label, phase IIIb clinical trial involving 1,504 patients at 141 centers in 16 countries.
Disclosures: The X-VeRT trial was sponsored by Bayer HealthCare. The presenter serves as a consultant to and on speakers bureaus for Bayer and other pharmaceutical companies.
VIDEO: Long-term impact of high cholesterol in younger adults underappreciated
BARCELONA – Dr. Ann Marie Navar-Boggan has a beef with current American and European cholesterol guidelines, which she believes seriously underestimate the long-term adverse impact of hypercholesterolemia in younger adults.
Here are the data Dr. Navar-Boggan of the Duke Clinical Research Institute, Durham, N.C., presented at the annual congress of the European Society of Cardiology to bolster her argument for more aggressive treatment of hypercholesterolemia in this population.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – Dr. Ann Marie Navar-Boggan has a beef with current American and European cholesterol guidelines, which she believes seriously underestimate the long-term adverse impact of hypercholesterolemia in younger adults.
Here are the data Dr. Navar-Boggan of the Duke Clinical Research Institute, Durham, N.C., presented at the annual congress of the European Society of Cardiology to bolster her argument for more aggressive treatment of hypercholesterolemia in this population.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – Dr. Ann Marie Navar-Boggan has a beef with current American and European cholesterol guidelines, which she believes seriously underestimate the long-term adverse impact of hypercholesterolemia in younger adults.
Here are the data Dr. Navar-Boggan of the Duke Clinical Research Institute, Durham, N.C., presented at the annual congress of the European Society of Cardiology to bolster her argument for more aggressive treatment of hypercholesterolemia in this population.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2014
VIDEO: Consider local anesthesia for transfemoral TAVR
BARCELONA – An analysis of FRANCE 2 registry showed that local anesthesia during the transfemoral aortic valve replacement procedure is a safe and effective option, compared with general anesthesia.
The study was performed based on data from 2010 and 2011, and at the annual congress of the European Society of Cardiology, Dr. Romain Chopard of the University Hospital of Besançon, Paris, and his coinvestigators reported that the practice is rather common – even routine in some centers – today.
But, performing TAVR under local anesthesia is not common practice in the United States, said Dr. Deepak L. Bhatt, executive director of interventional cardiovascular programs at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
In a video, Dr. Bhatt shares his thoughts on performing local anesthesia and why it hasn’t taken hold in the United States. He also shares his advice with physicians who perform the TAVR procedure.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
BARCELONA – An analysis of FRANCE 2 registry showed that local anesthesia during the transfemoral aortic valve replacement procedure is a safe and effective option, compared with general anesthesia.
The study was performed based on data from 2010 and 2011, and at the annual congress of the European Society of Cardiology, Dr. Romain Chopard of the University Hospital of Besançon, Paris, and his coinvestigators reported that the practice is rather common – even routine in some centers – today.
But, performing TAVR under local anesthesia is not common practice in the United States, said Dr. Deepak L. Bhatt, executive director of interventional cardiovascular programs at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
In a video, Dr. Bhatt shares his thoughts on performing local anesthesia and why it hasn’t taken hold in the United States. He also shares his advice with physicians who perform the TAVR procedure.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
BARCELONA – An analysis of FRANCE 2 registry showed that local anesthesia during the transfemoral aortic valve replacement procedure is a safe and effective option, compared with general anesthesia.
The study was performed based on data from 2010 and 2011, and at the annual congress of the European Society of Cardiology, Dr. Romain Chopard of the University Hospital of Besançon, Paris, and his coinvestigators reported that the practice is rather common – even routine in some centers – today.
But, performing TAVR under local anesthesia is not common practice in the United States, said Dr. Deepak L. Bhatt, executive director of interventional cardiovascular programs at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
In a video, Dr. Bhatt shares his thoughts on performing local anesthesia and why it hasn’t taken hold in the United States. He also shares his advice with physicians who perform the TAVR procedure.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
AT THE ESC CONGRESS 2014