User login
Strong gender difference for stroke in diabetes patients with restless legs syndrome
CHICAGO – Stroke risk in diabetic women with restless legs syndrome (RLS) is triple that of diabetic men with the sensorimotor disease, Zoe Heis reported at the annual meeting of the American College of Cardiology.
The mechanism underlying this marked gender discrepancy in risk requires further investigation, as does the highly practical question of whether improved diabetic control can reduce the stroke risk, said Ms. Heis of the Center for Integrative Research on Cardiovascular Aging at Aurora Health Care in Milwaukee.
She presented a retrospective cohort study of 385 patients diagnosed with RLS during 2011-2013 at a community sleep center using the International RLS Study Group criteria. Along with 770 propensity-matched controls, they were followed until mid-2015. At baseline, 40% of the RLS patients had diabetes and 70% had hypertension, as did 32% and 63% of controls, respectively.
Stroke occurred in 7.5% of the RLS group and 4.2% of matched controls. The presence of diabetes more than doubled the stroke risk in both groups.
The risk of stroke was 18.2% in diabetic women with RLS and 7% in diabetic men with RLS. In a multivariate analysis that controlled for potential confounding factors, this translated to a threefold increased stroke risk.
Diabetes was associated with a doubling of stroke risk in subjects without RLS, but the risk was similar in men and women, according to Ms. Heis.
In addition to diabetes and female gender, the other major predictor of increased stroke risk in patients with RLS was, not surprisingly, hypertension. It was associated with a 13-fold increased likelihood of stroke, she noted.
RLS was initially linked to increased risk of coronary heart disease in a report from the Nurses’ Health Study (Circulation. 2012 Oct 2;126[14]:1689-94). In 2015, another research group linked more severe RLS to an increased risk of stroke. Ms. Heis and her coinvestigators carried out the current study to test their hypothesis that, since diabetes is a condition that accelerates cardiovascular disease, the endocrine disorder would boost stroke risk more in subjects with RLS than in those without it.
Ms. Heis reported having no financial conflicts regarding her study, which was conducted free of commercial support.
CHICAGO – Stroke risk in diabetic women with restless legs syndrome (RLS) is triple that of diabetic men with the sensorimotor disease, Zoe Heis reported at the annual meeting of the American College of Cardiology.
The mechanism underlying this marked gender discrepancy in risk requires further investigation, as does the highly practical question of whether improved diabetic control can reduce the stroke risk, said Ms. Heis of the Center for Integrative Research on Cardiovascular Aging at Aurora Health Care in Milwaukee.
She presented a retrospective cohort study of 385 patients diagnosed with RLS during 2011-2013 at a community sleep center using the International RLS Study Group criteria. Along with 770 propensity-matched controls, they were followed until mid-2015. At baseline, 40% of the RLS patients had diabetes and 70% had hypertension, as did 32% and 63% of controls, respectively.
Stroke occurred in 7.5% of the RLS group and 4.2% of matched controls. The presence of diabetes more than doubled the stroke risk in both groups.
The risk of stroke was 18.2% in diabetic women with RLS and 7% in diabetic men with RLS. In a multivariate analysis that controlled for potential confounding factors, this translated to a threefold increased stroke risk.
Diabetes was associated with a doubling of stroke risk in subjects without RLS, but the risk was similar in men and women, according to Ms. Heis.
In addition to diabetes and female gender, the other major predictor of increased stroke risk in patients with RLS was, not surprisingly, hypertension. It was associated with a 13-fold increased likelihood of stroke, she noted.
RLS was initially linked to increased risk of coronary heart disease in a report from the Nurses’ Health Study (Circulation. 2012 Oct 2;126[14]:1689-94). In 2015, another research group linked more severe RLS to an increased risk of stroke. Ms. Heis and her coinvestigators carried out the current study to test their hypothesis that, since diabetes is a condition that accelerates cardiovascular disease, the endocrine disorder would boost stroke risk more in subjects with RLS than in those without it.
Ms. Heis reported having no financial conflicts regarding her study, which was conducted free of commercial support.
CHICAGO – Stroke risk in diabetic women with restless legs syndrome (RLS) is triple that of diabetic men with the sensorimotor disease, Zoe Heis reported at the annual meeting of the American College of Cardiology.
The mechanism underlying this marked gender discrepancy in risk requires further investigation, as does the highly practical question of whether improved diabetic control can reduce the stroke risk, said Ms. Heis of the Center for Integrative Research on Cardiovascular Aging at Aurora Health Care in Milwaukee.
She presented a retrospective cohort study of 385 patients diagnosed with RLS during 2011-2013 at a community sleep center using the International RLS Study Group criteria. Along with 770 propensity-matched controls, they were followed until mid-2015. At baseline, 40% of the RLS patients had diabetes and 70% had hypertension, as did 32% and 63% of controls, respectively.
Stroke occurred in 7.5% of the RLS group and 4.2% of matched controls. The presence of diabetes more than doubled the stroke risk in both groups.
The risk of stroke was 18.2% in diabetic women with RLS and 7% in diabetic men with RLS. In a multivariate analysis that controlled for potential confounding factors, this translated to a threefold increased stroke risk.
Diabetes was associated with a doubling of stroke risk in subjects without RLS, but the risk was similar in men and women, according to Ms. Heis.
In addition to diabetes and female gender, the other major predictor of increased stroke risk in patients with RLS was, not surprisingly, hypertension. It was associated with a 13-fold increased likelihood of stroke, she noted.
RLS was initially linked to increased risk of coronary heart disease in a report from the Nurses’ Health Study (Circulation. 2012 Oct 2;126[14]:1689-94). In 2015, another research group linked more severe RLS to an increased risk of stroke. Ms. Heis and her coinvestigators carried out the current study to test their hypothesis that, since diabetes is a condition that accelerates cardiovascular disease, the endocrine disorder would boost stroke risk more in subjects with RLS than in those without it.
Ms. Heis reported having no financial conflicts regarding her study, which was conducted free of commercial support.
AT ACC 16
Key clinical point: Diabetic women who have restless legs syndrome face a sharply elevated risk of stroke.
Major finding: Stroke occurred in 18.2% of diabetic women with restless legs syndrome, compared with 7% of men.
Data source: This retrospective cohort study included 385 patients with restless legs syndrome and 770 propensity-matched controls.
Disclosures: This study was conducted free of commercial support. The presenter reported having no financial conflicts.
Drilling down on end-of-life health care costs in heart failure
CHICAGO – Health care costs for heart failure patients spike dramatically in the last 6 months of life, with lack of adherence to guideline-directed outpatient medical therapy being the major modifiable factor driving the costs, Jason P. Swindle, Ph.D., reported at the annual meeting of the American College of Cardiology.
He presented a retrospective study of heart failure–related and total health care costs during the final 24 months of life for 48,026 Medicare Advantage managed care plan members with heart failure.
The researchers were interested in exploring possible racial/ethnic differences in costs, particularly in light of evidence that African Americans have a higher risk of heart failure and higher all-cause mortality. And while a first look at the data indicated racial differences in the size of end-of-life cost spikes, those differences lost their significance in multivariate analysis.
“Lack of guideline-directed outpatient heart failure therapy was a key. Also older age and the presence of coronary heart disease – those were really the big three items that were driving the spike in costs,” said Dr. Swindle of the Chicago office of Optum, a health care consulting group.
He was quick to add that, since the study was based upon administrative data, the lack of adherence to guideline-directed therapy may be unrelated to physician prescribing.
“We see the prescriptions that patients are actually filling. Patients may very well be seeing their cardiologist and being prescribed a medication, but they simply don’t fill the prescription,” he explained in an interview.
Over patients’ final 2 years of life, semiannual all-cause health care costs climbed from a baseline of roughly $10,000 during months 24-19 before death by about 4-fold during months 6-1 before death. Heart failure–related medical costs jumped 10-fold in Asians, 7.8-fold in Hispanics, 6.6-fold in African Americans, and 6.7-fold in whites. Most of the increases occurred in the final 6 months.
Zeroing in on the final 6 months of life, the mean cumulative total medical costs were $44,599, with heart failure–related medical costs accounting for $24,818 of that figure. Total medical costs averaged just under $5,000 during month 6 prior to death and rose roughly 3.5-fold over the remaining months.
This study was supported by Novartis Pharmaceuticals.
CHICAGO – Health care costs for heart failure patients spike dramatically in the last 6 months of life, with lack of adherence to guideline-directed outpatient medical therapy being the major modifiable factor driving the costs, Jason P. Swindle, Ph.D., reported at the annual meeting of the American College of Cardiology.
He presented a retrospective study of heart failure–related and total health care costs during the final 24 months of life for 48,026 Medicare Advantage managed care plan members with heart failure.
The researchers were interested in exploring possible racial/ethnic differences in costs, particularly in light of evidence that African Americans have a higher risk of heart failure and higher all-cause mortality. And while a first look at the data indicated racial differences in the size of end-of-life cost spikes, those differences lost their significance in multivariate analysis.
“Lack of guideline-directed outpatient heart failure therapy was a key. Also older age and the presence of coronary heart disease – those were really the big three items that were driving the spike in costs,” said Dr. Swindle of the Chicago office of Optum, a health care consulting group.
He was quick to add that, since the study was based upon administrative data, the lack of adherence to guideline-directed therapy may be unrelated to physician prescribing.
“We see the prescriptions that patients are actually filling. Patients may very well be seeing their cardiologist and being prescribed a medication, but they simply don’t fill the prescription,” he explained in an interview.
Over patients’ final 2 years of life, semiannual all-cause health care costs climbed from a baseline of roughly $10,000 during months 24-19 before death by about 4-fold during months 6-1 before death. Heart failure–related medical costs jumped 10-fold in Asians, 7.8-fold in Hispanics, 6.6-fold in African Americans, and 6.7-fold in whites. Most of the increases occurred in the final 6 months.
Zeroing in on the final 6 months of life, the mean cumulative total medical costs were $44,599, with heart failure–related medical costs accounting for $24,818 of that figure. Total medical costs averaged just under $5,000 during month 6 prior to death and rose roughly 3.5-fold over the remaining months.
This study was supported by Novartis Pharmaceuticals.
CHICAGO – Health care costs for heart failure patients spike dramatically in the last 6 months of life, with lack of adherence to guideline-directed outpatient medical therapy being the major modifiable factor driving the costs, Jason P. Swindle, Ph.D., reported at the annual meeting of the American College of Cardiology.
He presented a retrospective study of heart failure–related and total health care costs during the final 24 months of life for 48,026 Medicare Advantage managed care plan members with heart failure.
The researchers were interested in exploring possible racial/ethnic differences in costs, particularly in light of evidence that African Americans have a higher risk of heart failure and higher all-cause mortality. And while a first look at the data indicated racial differences in the size of end-of-life cost spikes, those differences lost their significance in multivariate analysis.
“Lack of guideline-directed outpatient heart failure therapy was a key. Also older age and the presence of coronary heart disease – those were really the big three items that were driving the spike in costs,” said Dr. Swindle of the Chicago office of Optum, a health care consulting group.
He was quick to add that, since the study was based upon administrative data, the lack of adherence to guideline-directed therapy may be unrelated to physician prescribing.
“We see the prescriptions that patients are actually filling. Patients may very well be seeing their cardiologist and being prescribed a medication, but they simply don’t fill the prescription,” he explained in an interview.
Over patients’ final 2 years of life, semiannual all-cause health care costs climbed from a baseline of roughly $10,000 during months 24-19 before death by about 4-fold during months 6-1 before death. Heart failure–related medical costs jumped 10-fold in Asians, 7.8-fold in Hispanics, 6.6-fold in African Americans, and 6.7-fold in whites. Most of the increases occurred in the final 6 months.
Zeroing in on the final 6 months of life, the mean cumulative total medical costs were $44,599, with heart failure–related medical costs accounting for $24,818 of that figure. Total medical costs averaged just under $5,000 during month 6 prior to death and rose roughly 3.5-fold over the remaining months.
This study was supported by Novartis Pharmaceuticals.
AT ACC 16
Key clinical point: Addressing lack of adherence to guideline-directed medical therapy could curb end-of-life health care costs in heart failure.
Major finding: Total monthly medical costs in heart failure patients during their final 6 months of life climbed roughly 3.5-fold.
Data source: This was a retrospective study of total and heart failure–related health care costs during the final 24 months of life for more than 48,000 patients with heart failure.
Disclosures: This study was supported by Novartis Pharmaceuticals. Dr. Swindle is an employee of Optum, which conducted the research.
Reassurance on cardiovascular safety of Breo Ellipta for COPD
CHICAGO – Using an inhaled long-acting beta agonist and corticosteroid to treat patients with moderate chronic obstructive pulmonary disease who have known cardiovascular disease had no impact on the cardiovascular event rate in the landmark SUMMIT trial, Dr. David E. Newby reported at the annual meeting of the American College of Cardiology.
SUMMIT (the Study to Understand Mortality and Morbidity) was a randomized, double-blind, placebo-controlled, 43-country, four-arm clinical trial in which 16,485 patients with moderate COPD were placed on once-daily inhaled fluticasone furoate/vilanterol 100/25 mcg by dry powder inhaler (Breo Ellipta), either drug alone, or placebo for an average of 2 years. Two-thirds of participants had overt cardiovascular disease; the rest were at high risk as defined by age greater than 60 years plus the presence of two or more cardiovascular risk factors.
This was the first large prospective clinical trial to investigate the impact of respiratory therapy on survival in patients with two commonly comorbid conditions. The incidence of major adverse cardiovascular events – a prespecified secondary endpoint – was of major interest because some other long-acting beta agonists have been linked to increased cardiovascular risk, explained Dr. Newby of the University of Edinburgh.
The primary endpoint in SUMMIT was all-cause mortality. The 12.2% relative risk reduction in the fluticasone furoate/vilanterol group, compared with placebo, didn’t achieve statistical significance. Neither did the 7.4% reduction in the secondary composite endpoint of cardiovascular death, MI, stroke, unstable angina, or TIA seen in the corticosteroid/LABA group, which is reassuring from a safety standpoint, he noted.
A positive result was seen for another secondary endpoint: the rate of lung function decline as measured by forced expiratory volume in 1 second. The rate of decline was 8 mL/year less with fluticasone furoate/vilanterol, compared with placebo.
The Breo Ellipta group also had significantly lower rates of moderate or severe COPD exacerbations, hospitalization for exacerbations, and improved quality of life as measured by the St. George’s Respiratory Questionnaire – COPD total score at 12 months.
The SUMMIT trial was sponsored by GlaxoSmithKline. The presenter is a consultant to GSK and eight other pharmaceutical companies.
CHICAGO – Using an inhaled long-acting beta agonist and corticosteroid to treat patients with moderate chronic obstructive pulmonary disease who have known cardiovascular disease had no impact on the cardiovascular event rate in the landmark SUMMIT trial, Dr. David E. Newby reported at the annual meeting of the American College of Cardiology.
SUMMIT (the Study to Understand Mortality and Morbidity) was a randomized, double-blind, placebo-controlled, 43-country, four-arm clinical trial in which 16,485 patients with moderate COPD were placed on once-daily inhaled fluticasone furoate/vilanterol 100/25 mcg by dry powder inhaler (Breo Ellipta), either drug alone, or placebo for an average of 2 years. Two-thirds of participants had overt cardiovascular disease; the rest were at high risk as defined by age greater than 60 years plus the presence of two or more cardiovascular risk factors.
This was the first large prospective clinical trial to investigate the impact of respiratory therapy on survival in patients with two commonly comorbid conditions. The incidence of major adverse cardiovascular events – a prespecified secondary endpoint – was of major interest because some other long-acting beta agonists have been linked to increased cardiovascular risk, explained Dr. Newby of the University of Edinburgh.
The primary endpoint in SUMMIT was all-cause mortality. The 12.2% relative risk reduction in the fluticasone furoate/vilanterol group, compared with placebo, didn’t achieve statistical significance. Neither did the 7.4% reduction in the secondary composite endpoint of cardiovascular death, MI, stroke, unstable angina, or TIA seen in the corticosteroid/LABA group, which is reassuring from a safety standpoint, he noted.
A positive result was seen for another secondary endpoint: the rate of lung function decline as measured by forced expiratory volume in 1 second. The rate of decline was 8 mL/year less with fluticasone furoate/vilanterol, compared with placebo.
The Breo Ellipta group also had significantly lower rates of moderate or severe COPD exacerbations, hospitalization for exacerbations, and improved quality of life as measured by the St. George’s Respiratory Questionnaire – COPD total score at 12 months.
The SUMMIT trial was sponsored by GlaxoSmithKline. The presenter is a consultant to GSK and eight other pharmaceutical companies.
CHICAGO – Using an inhaled long-acting beta agonist and corticosteroid to treat patients with moderate chronic obstructive pulmonary disease who have known cardiovascular disease had no impact on the cardiovascular event rate in the landmark SUMMIT trial, Dr. David E. Newby reported at the annual meeting of the American College of Cardiology.
SUMMIT (the Study to Understand Mortality and Morbidity) was a randomized, double-blind, placebo-controlled, 43-country, four-arm clinical trial in which 16,485 patients with moderate COPD were placed on once-daily inhaled fluticasone furoate/vilanterol 100/25 mcg by dry powder inhaler (Breo Ellipta), either drug alone, or placebo for an average of 2 years. Two-thirds of participants had overt cardiovascular disease; the rest were at high risk as defined by age greater than 60 years plus the presence of two or more cardiovascular risk factors.
This was the first large prospective clinical trial to investigate the impact of respiratory therapy on survival in patients with two commonly comorbid conditions. The incidence of major adverse cardiovascular events – a prespecified secondary endpoint – was of major interest because some other long-acting beta agonists have been linked to increased cardiovascular risk, explained Dr. Newby of the University of Edinburgh.
The primary endpoint in SUMMIT was all-cause mortality. The 12.2% relative risk reduction in the fluticasone furoate/vilanterol group, compared with placebo, didn’t achieve statistical significance. Neither did the 7.4% reduction in the secondary composite endpoint of cardiovascular death, MI, stroke, unstable angina, or TIA seen in the corticosteroid/LABA group, which is reassuring from a safety standpoint, he noted.
A positive result was seen for another secondary endpoint: the rate of lung function decline as measured by forced expiratory volume in 1 second. The rate of decline was 8 mL/year less with fluticasone furoate/vilanterol, compared with placebo.
The Breo Ellipta group also had significantly lower rates of moderate or severe COPD exacerbations, hospitalization for exacerbations, and improved quality of life as measured by the St. George’s Respiratory Questionnaire – COPD total score at 12 months.
The SUMMIT trial was sponsored by GlaxoSmithKline. The presenter is a consultant to GSK and eight other pharmaceutical companies.
AT ACC 16
Key clinical point: Once-daily inhaled fluticasone furoate/vilanterol 100/25 mcg to treat moderate COPD in patients with comorbid cardiovascular disease did not increase their risk of cardiovascular events.
Major finding: The major adverse cardiovascular event rate in patients on once-daily inhaled fluticasone furoate/vilanterol 100/25 mcg was 7.4% less than with placebo, a nonsignificant difference.
Data source: This was a randomized, double-blind, placebo-controlled, 2-year clinical trial including 16,485 patients with moderate COPD and overt cardiovascular disease or at high risk for it.
Disclosures: The SUMMIT trial was sponsored by GlaxoSmithKline. The presenter is a consultant to GSK and eight other pharmaceutical companies.
AF and Stroke May Be Temporally Related
CHICAGO – One-third of a large cohort of patients with an implantable cardiac device in place at the time of an ischemic stroke had one or more episodes of atrial fibrillation within the prior 30 days, Dr. Rhea C. Pimentel reported at the annual meeting of the American College of Cardiology.
The in-hospital mortality rate of these atrial fibrillation–related strokes was high: 11 of 42 (26%) such patients died during their stroke hospitalization, compared with 6 of 83 (7%) whose strokes were not temporally related to atrial fibrillation (AF), noted Dr. Pimentel, an electrophysiologist at the University of Kansas Medical Center in Kansas City.
Data from the Framingham Heart Study and other sources suggest that stroke in patients who have AF carries about double the mortality rate of strokes in patients without AF. Mortality associated with AF-related stroke in the Kansas study was probably so much higher because the hospital serves as a comprehensive stroke center, drawing patients from considerable distances across the Midwest, she said.
Dr. Pimentel reported on 125 patients who presented with an ischemic stroke when a cardiac monitoring device was in place. This is believed to be the largest such patient series ever reported. Their mean age was 73 years, and 41% were women. The mean CHADS2 score was 3.96, with a CHA2DS2-VASc score of 5.28. Of the patients, 62% had a pacemaker; the rest had an implantable cardioverter-defibrillator or cardiac resynchronization device. One-quarter of the group had a prior history of AF, and a fifth were on an oral anticoagulant – warfarin, in 70% of cases – at the time of their stroke.
The investigators defined a stroke-related AF episode as a total of at least 1 hour spent in AF during the 30 days preceding the stroke. Eighty percent of affected patients had paroxysmal AF. They typically fulfilled the 1-hour AF requirement via multiple short, self-terminated episodes rather than in an hour-long episode.
Being on an oral anticoagulant had no impact on in-hospital mortality, which was 14.2% in patients on warfarin or a newer anticoagulant and 14.3% in those who were not. Dr. Pimentel noted that she was presenting the results of the investigators’ initial look at the data. They are in the process of obtaining the patients’ international normalized ratio data, which “should be very enlightening,” she said.
She and her coinvestigators also plan to subdivide their 30-day study period into 5-day segments to learn just how soon after an AF episode the strokes occurred. Researchers at Stanford (Calif.) University have reported that the greatest stroke risk in patients with AF is in the first 5 days after an AF episode, and the Kansas group would like to confirm that observation.
In addition, because it remains an unresolved question whether any amount of AF is safe, Dr. Pimentel and her coworkers are considering reanalyzing their data using a cutoff of 6 minutes of AF rather than 1 hour during the 30 days prior to stroke.
The study was conducted free of commercial support. Dr. Pimentel reported having no relevant financial conflicts.
CHICAGO – One-third of a large cohort of patients with an implantable cardiac device in place at the time of an ischemic stroke had one or more episodes of atrial fibrillation within the prior 30 days, Dr. Rhea C. Pimentel reported at the annual meeting of the American College of Cardiology.
The in-hospital mortality rate of these atrial fibrillation–related strokes was high: 11 of 42 (26%) such patients died during their stroke hospitalization, compared with 6 of 83 (7%) whose strokes were not temporally related to atrial fibrillation (AF), noted Dr. Pimentel, an electrophysiologist at the University of Kansas Medical Center in Kansas City.
Data from the Framingham Heart Study and other sources suggest that stroke in patients who have AF carries about double the mortality rate of strokes in patients without AF. Mortality associated with AF-related stroke in the Kansas study was probably so much higher because the hospital serves as a comprehensive stroke center, drawing patients from considerable distances across the Midwest, she said.
Dr. Pimentel reported on 125 patients who presented with an ischemic stroke when a cardiac monitoring device was in place. This is believed to be the largest such patient series ever reported. Their mean age was 73 years, and 41% were women. The mean CHADS2 score was 3.96, with a CHA2DS2-VASc score of 5.28. Of the patients, 62% had a pacemaker; the rest had an implantable cardioverter-defibrillator or cardiac resynchronization device. One-quarter of the group had a prior history of AF, and a fifth were on an oral anticoagulant – warfarin, in 70% of cases – at the time of their stroke.
The investigators defined a stroke-related AF episode as a total of at least 1 hour spent in AF during the 30 days preceding the stroke. Eighty percent of affected patients had paroxysmal AF. They typically fulfilled the 1-hour AF requirement via multiple short, self-terminated episodes rather than in an hour-long episode.
Being on an oral anticoagulant had no impact on in-hospital mortality, which was 14.2% in patients on warfarin or a newer anticoagulant and 14.3% in those who were not. Dr. Pimentel noted that she was presenting the results of the investigators’ initial look at the data. They are in the process of obtaining the patients’ international normalized ratio data, which “should be very enlightening,” she said.
She and her coinvestigators also plan to subdivide their 30-day study period into 5-day segments to learn just how soon after an AF episode the strokes occurred. Researchers at Stanford (Calif.) University have reported that the greatest stroke risk in patients with AF is in the first 5 days after an AF episode, and the Kansas group would like to confirm that observation.
In addition, because it remains an unresolved question whether any amount of AF is safe, Dr. Pimentel and her coworkers are considering reanalyzing their data using a cutoff of 6 minutes of AF rather than 1 hour during the 30 days prior to stroke.
The study was conducted free of commercial support. Dr. Pimentel reported having no relevant financial conflicts.
CHICAGO – One-third of a large cohort of patients with an implantable cardiac device in place at the time of an ischemic stroke had one or more episodes of atrial fibrillation within the prior 30 days, Dr. Rhea C. Pimentel reported at the annual meeting of the American College of Cardiology.
The in-hospital mortality rate of these atrial fibrillation–related strokes was high: 11 of 42 (26%) such patients died during their stroke hospitalization, compared with 6 of 83 (7%) whose strokes were not temporally related to atrial fibrillation (AF), noted Dr. Pimentel, an electrophysiologist at the University of Kansas Medical Center in Kansas City.
Data from the Framingham Heart Study and other sources suggest that stroke in patients who have AF carries about double the mortality rate of strokes in patients without AF. Mortality associated with AF-related stroke in the Kansas study was probably so much higher because the hospital serves as a comprehensive stroke center, drawing patients from considerable distances across the Midwest, she said.
Dr. Pimentel reported on 125 patients who presented with an ischemic stroke when a cardiac monitoring device was in place. This is believed to be the largest such patient series ever reported. Their mean age was 73 years, and 41% were women. The mean CHADS2 score was 3.96, with a CHA2DS2-VASc score of 5.28. Of the patients, 62% had a pacemaker; the rest had an implantable cardioverter-defibrillator or cardiac resynchronization device. One-quarter of the group had a prior history of AF, and a fifth were on an oral anticoagulant – warfarin, in 70% of cases – at the time of their stroke.
The investigators defined a stroke-related AF episode as a total of at least 1 hour spent in AF during the 30 days preceding the stroke. Eighty percent of affected patients had paroxysmal AF. They typically fulfilled the 1-hour AF requirement via multiple short, self-terminated episodes rather than in an hour-long episode.
Being on an oral anticoagulant had no impact on in-hospital mortality, which was 14.2% in patients on warfarin or a newer anticoagulant and 14.3% in those who were not. Dr. Pimentel noted that she was presenting the results of the investigators’ initial look at the data. They are in the process of obtaining the patients’ international normalized ratio data, which “should be very enlightening,” she said.
She and her coinvestigators also plan to subdivide their 30-day study period into 5-day segments to learn just how soon after an AF episode the strokes occurred. Researchers at Stanford (Calif.) University have reported that the greatest stroke risk in patients with AF is in the first 5 days after an AF episode, and the Kansas group would like to confirm that observation.
In addition, because it remains an unresolved question whether any amount of AF is safe, Dr. Pimentel and her coworkers are considering reanalyzing their data using a cutoff of 6 minutes of AF rather than 1 hour during the 30 days prior to stroke.
The study was conducted free of commercial support. Dr. Pimentel reported having no relevant financial conflicts.
AT ACC 16
AF and stroke may be temporally related
CHICAGO – One-third of a large cohort of patients with an implantable cardiac device in place at the time of an ischemic stroke had one or more episodes of atrial fibrillation within the prior 30 days, Dr. Rhea C. Pimentel reported at the annual meeting of the American College of Cardiology.
The in-hospital mortality rate of these atrial fibrillation–related strokes was high: 11 of 42 (26%) such patients died during their stroke hospitalization, compared with 6 of 83 (7%) whose strokes were not temporally related to atrial fibrillation (AF), noted Dr. Pimentel, an electrophysiologist at the University of Kansas Medical Center in Kansas City.
Data from the Framingham Heart Study and other sources suggest that stroke in patients who have AF carries about double the mortality rate of strokes in patients without AF. Mortality associated with AF-related stroke in the Kansas study was probably so much higher because the hospital serves as a comprehensive stroke center, drawing patients from considerable distances across the Midwest, she said.
Dr. Pimentel reported on 125 patients who presented with an ischemic stroke when a cardiac monitoring device was in place. This is believed to be the largest such patient series ever reported. Their mean age was 73 years, and 41% were women. The mean CHADS2 score was 3.96, with a CHA2DS2-VASc score of 5.28. Of the patients, 62% had a pacemaker; the rest had an implantable cardioverter-defibrillator or cardiac resynchronization device. One-quarter of the group had a prior history of AF, and a fifth were on an oral anticoagulant – warfarin, in 70% of cases – at the time of their stroke.
The investigators defined a stroke-related AF episode as a total of at least 1 hour spent in AF during the 30 days preceding the stroke. Eighty percent of affected patients had paroxysmal AF. They typically fulfilled the 1-hour AF requirement via multiple short, self-terminated episodes rather than in an hour-long episode.
Being on an oral anticoagulant had no impact on in-hospital mortality, which was 14.2% in patients on warfarin or a newer anticoagulant and 14.3% in those who were not. Dr. Pimentel noted that she was presenting the results of the investigators’ initial look at the data. They are in the process of obtaining the patients’ international normalized ratio data, which “should be very enlightening,” she said.
She and her coinvestigators also plan to subdivide their 30-day study period into 5-day segments to learn just how soon after an AF episode the strokes occurred. Researchers at Stanford (Calif.) University have reported that the greatest stroke risk in patients with AF is in the first 5 days after an AF episode, and the Kansas group would like to confirm that observation.
In addition, because it remains an unresolved question whether any amount of AF is safe, Dr. Pimentel and her coworkers are considering reanalyzing their data using a cutoff of 6 minutes of AF rather than 1 hour during the 30 days prior to stroke.
The study was conducted free of commercial support. Dr. Pimentel reported having no relevant financial conflicts.
CHICAGO – One-third of a large cohort of patients with an implantable cardiac device in place at the time of an ischemic stroke had one or more episodes of atrial fibrillation within the prior 30 days, Dr. Rhea C. Pimentel reported at the annual meeting of the American College of Cardiology.
The in-hospital mortality rate of these atrial fibrillation–related strokes was high: 11 of 42 (26%) such patients died during their stroke hospitalization, compared with 6 of 83 (7%) whose strokes were not temporally related to atrial fibrillation (AF), noted Dr. Pimentel, an electrophysiologist at the University of Kansas Medical Center in Kansas City.
Data from the Framingham Heart Study and other sources suggest that stroke in patients who have AF carries about double the mortality rate of strokes in patients without AF. Mortality associated with AF-related stroke in the Kansas study was probably so much higher because the hospital serves as a comprehensive stroke center, drawing patients from considerable distances across the Midwest, she said.
Dr. Pimentel reported on 125 patients who presented with an ischemic stroke when a cardiac monitoring device was in place. This is believed to be the largest such patient series ever reported. Their mean age was 73 years, and 41% were women. The mean CHADS2 score was 3.96, with a CHA2DS2-VASc score of 5.28. Of the patients, 62% had a pacemaker; the rest had an implantable cardioverter-defibrillator or cardiac resynchronization device. One-quarter of the group had a prior history of AF, and a fifth were on an oral anticoagulant – warfarin, in 70% of cases – at the time of their stroke.
The investigators defined a stroke-related AF episode as a total of at least 1 hour spent in AF during the 30 days preceding the stroke. Eighty percent of affected patients had paroxysmal AF. They typically fulfilled the 1-hour AF requirement via multiple short, self-terminated episodes rather than in an hour-long episode.
Being on an oral anticoagulant had no impact on in-hospital mortality, which was 14.2% in patients on warfarin or a newer anticoagulant and 14.3% in those who were not. Dr. Pimentel noted that she was presenting the results of the investigators’ initial look at the data. They are in the process of obtaining the patients’ international normalized ratio data, which “should be very enlightening,” she said.
She and her coinvestigators also plan to subdivide their 30-day study period into 5-day segments to learn just how soon after an AF episode the strokes occurred. Researchers at Stanford (Calif.) University have reported that the greatest stroke risk in patients with AF is in the first 5 days after an AF episode, and the Kansas group would like to confirm that observation.
In addition, because it remains an unresolved question whether any amount of AF is safe, Dr. Pimentel and her coworkers are considering reanalyzing their data using a cutoff of 6 minutes of AF rather than 1 hour during the 30 days prior to stroke.
The study was conducted free of commercial support. Dr. Pimentel reported having no relevant financial conflicts.
CHICAGO – One-third of a large cohort of patients with an implantable cardiac device in place at the time of an ischemic stroke had one or more episodes of atrial fibrillation within the prior 30 days, Dr. Rhea C. Pimentel reported at the annual meeting of the American College of Cardiology.
The in-hospital mortality rate of these atrial fibrillation–related strokes was high: 11 of 42 (26%) such patients died during their stroke hospitalization, compared with 6 of 83 (7%) whose strokes were not temporally related to atrial fibrillation (AF), noted Dr. Pimentel, an electrophysiologist at the University of Kansas Medical Center in Kansas City.
Data from the Framingham Heart Study and other sources suggest that stroke in patients who have AF carries about double the mortality rate of strokes in patients without AF. Mortality associated with AF-related stroke in the Kansas study was probably so much higher because the hospital serves as a comprehensive stroke center, drawing patients from considerable distances across the Midwest, she said.
Dr. Pimentel reported on 125 patients who presented with an ischemic stroke when a cardiac monitoring device was in place. This is believed to be the largest such patient series ever reported. Their mean age was 73 years, and 41% were women. The mean CHADS2 score was 3.96, with a CHA2DS2-VASc score of 5.28. Of the patients, 62% had a pacemaker; the rest had an implantable cardioverter-defibrillator or cardiac resynchronization device. One-quarter of the group had a prior history of AF, and a fifth were on an oral anticoagulant – warfarin, in 70% of cases – at the time of their stroke.
The investigators defined a stroke-related AF episode as a total of at least 1 hour spent in AF during the 30 days preceding the stroke. Eighty percent of affected patients had paroxysmal AF. They typically fulfilled the 1-hour AF requirement via multiple short, self-terminated episodes rather than in an hour-long episode.
Being on an oral anticoagulant had no impact on in-hospital mortality, which was 14.2% in patients on warfarin or a newer anticoagulant and 14.3% in those who were not. Dr. Pimentel noted that she was presenting the results of the investigators’ initial look at the data. They are in the process of obtaining the patients’ international normalized ratio data, which “should be very enlightening,” she said.
She and her coinvestigators also plan to subdivide their 30-day study period into 5-day segments to learn just how soon after an AF episode the strokes occurred. Researchers at Stanford (Calif.) University have reported that the greatest stroke risk in patients with AF is in the first 5 days after an AF episode, and the Kansas group would like to confirm that observation.
In addition, because it remains an unresolved question whether any amount of AF is safe, Dr. Pimentel and her coworkers are considering reanalyzing their data using a cutoff of 6 minutes of AF rather than 1 hour during the 30 days prior to stroke.
The study was conducted free of commercial support. Dr. Pimentel reported having no relevant financial conflicts.
AT ACC 16
Key clinical point: One-third of patients with an ischemic stroke while they had an implantable cardiac device had atrial fibrillation in the 30 days prior.
Major finding: The in-hospital mortality rate was 26% in patients with AF-related stroke, compared with 7% in those without AF.
Data source: A single-center, retrospective study of 125 patients who had an ischemic stroke while wearing a pacemaker or other implanted cardiac device.
Disclosures: The study was conducted free of commercial support. The presenter reported having no financial conflicts of interest.
Acute Heart Failure Mortality Climbs With Severity of Peripheral Edema
CHICAGO – Breathlessness typically results in hospital admission for patients with heart failure, but peripheral edema is what prolongs their stay, according to Dr. John G.F. Cleland.
Moreover, it’s not only hospital length of stay that climbs with increasing severity of peripheral edema on admission. So does mortality, both during the index admission and long term, Dr. Cleland reported at the annual meeting of the American College of Cardiology.
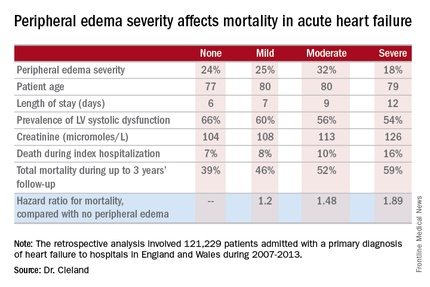
He presented a retrospective analysis of 121,229 patients admitted with a primary diagnosis of heart failure to more than 90% of the hospitals in England and Wales during 2007-2013.
“It turns out that the majority of patients we’re seeing admitted with heart failure at U.K. hospitals are not admitted because of severe breathlessness at rest; they’re admitted for increasing fluid retention. They have lots of peripheral edema, which is actually associated with bad outcome. The patients who are very breathless tend to have the better outcome because they have left heart failure. The ones who are full of peripheral edema have more renal dysfunction and anemia, and they’re more likely to have right heart failure,” said Dr. Cleland, professor of cardiology at Imperial College London.
“I’m not saying the breathless group isn’t a target, but this group with peripheral edema is a bigger target – and we’re not designing trials to address their problems,” he added.
Compared with patients with no peripheral edema on hospital admission, the risk of mortality during that hospitalization and up to 3 years of subsequent follow-up was increased 1.2-fold in those with mild peripheral edema, 1.48-fold with moderate peripheral edema, and 1.89-fold in those with severe peripheral edema.
“We’re designing all the big clinical trials in acute heart failure to capture what I regard as neither fish nor fowl. They’re recruiting patients 6-12 hours after admission for acute heart failure. But by that point they’ve pretty well responded to their intravenous diuretics, and we’re just catching the tail end of their breathlessness. They’re not an emergency. The problem is really their peripheral edema, and that’s a day 2/day 3 problem. The first 6 hours of care really isn’t relevant to this group,” according to the internationally renowned heart failure researcher.
Indeed, he said he’d like to see the term “acute heart failure” laid to rest.
“If you think of acute decompensated heart failure, you think of patients wearing an oxygen mask coming in by an ambulance with blue lights flashing, and it’s an emergency. We need to move the mind-set. We shouldn’t call it acute heart failure at all, we should start talking about hospitalized heart failure, of which some is acute but much of it is subacute in people who have been deteriorating over several weeks and have just gotten to the point where they’re not coping at home anymore. They call a taxi or a friend who takes them to the hospital. Then they take a wheelchair from the taxi to the ER, but they don’t really need an ER at all,” Dr. Cleland said.
Many of these patients could be redirected to a different sort of facility at great cost savings, he added.
“In the United Kingdom and I think in the States, we’re now talking about furosemide lounges where, rather than admit the patient, you can bring them up as a day case, give them intravenous therapy, then [have them] go home at night, perhaps coming back for 3 or 4 days if needed. People are also now looking at home infusion services, and there’s a nice device for giving subcutaneous doses of furosemide as well,” the cardiologist said.
Apart from diuretics, there really aren’t any effective medications at present for peripheral edema in heart failure. But there are novel investigational agents worthy of evaluation, according to Dr. Cleland, including drugs aimed at improving mitochondrial function, agents that inhibit channels that allow edema to gather, and iron therapy.
The study was supported by the British Society for Heart Failure and the National Institute for Cardiovascular Outcomes Research. Dr. Cleland reported having no relevant financial conflicts.
CHICAGO – Breathlessness typically results in hospital admission for patients with heart failure, but peripheral edema is what prolongs their stay, according to Dr. John G.F. Cleland.
Moreover, it’s not only hospital length of stay that climbs with increasing severity of peripheral edema on admission. So does mortality, both during the index admission and long term, Dr. Cleland reported at the annual meeting of the American College of Cardiology.
He presented a retrospective analysis of 121,229 patients admitted with a primary diagnosis of heart failure to more than 90% of the hospitals in England and Wales during 2007-2013.
“It turns out that the majority of patients we’re seeing admitted with heart failure at U.K. hospitals are not admitted because of severe breathlessness at rest; they’re admitted for increasing fluid retention. They have lots of peripheral edema, which is actually associated with bad outcome. The patients who are very breathless tend to have the better outcome because they have left heart failure. The ones who are full of peripheral edema have more renal dysfunction and anemia, and they’re more likely to have right heart failure,” said Dr. Cleland, professor of cardiology at Imperial College London.
“I’m not saying the breathless group isn’t a target, but this group with peripheral edema is a bigger target – and we’re not designing trials to address their problems,” he added.
Compared with patients with no peripheral edema on hospital admission, the risk of mortality during that hospitalization and up to 3 years of subsequent follow-up was increased 1.2-fold in those with mild peripheral edema, 1.48-fold with moderate peripheral edema, and 1.89-fold in those with severe peripheral edema.

“We’re designing all the big clinical trials in acute heart failure to capture what I regard as neither fish nor fowl. They’re recruiting patients 6-12 hours after admission for acute heart failure. But by that point they’ve pretty well responded to their intravenous diuretics, and we’re just catching the tail end of their breathlessness. They’re not an emergency. The problem is really their peripheral edema, and that’s a day 2/day 3 problem. The first 6 hours of care really isn’t relevant to this group,” according to the internationally renowned heart failure researcher.
Indeed, he said he’d like to see the term “acute heart failure” laid to rest.
“If you think of acute decompensated heart failure, you think of patients wearing an oxygen mask coming in by an ambulance with blue lights flashing, and it’s an emergency. We need to move the mind-set. We shouldn’t call it acute heart failure at all, we should start talking about hospitalized heart failure, of which some is acute but much of it is subacute in people who have been deteriorating over several weeks and have just gotten to the point where they’re not coping at home anymore. They call a taxi or a friend who takes them to the hospital. Then they take a wheelchair from the taxi to the ER, but they don’t really need an ER at all,” Dr. Cleland said.
Many of these patients could be redirected to a different sort of facility at great cost savings, he added.
“In the United Kingdom and I think in the States, we’re now talking about furosemide lounges where, rather than admit the patient, you can bring them up as a day case, give them intravenous therapy, then [have them] go home at night, perhaps coming back for 3 or 4 days if needed. People are also now looking at home infusion services, and there’s a nice device for giving subcutaneous doses of furosemide as well,” the cardiologist said.
Apart from diuretics, there really aren’t any effective medications at present for peripheral edema in heart failure. But there are novel investigational agents worthy of evaluation, according to Dr. Cleland, including drugs aimed at improving mitochondrial function, agents that inhibit channels that allow edema to gather, and iron therapy.
The study was supported by the British Society for Heart Failure and the National Institute for Cardiovascular Outcomes Research. Dr. Cleland reported having no relevant financial conflicts.
CHICAGO – Breathlessness typically results in hospital admission for patients with heart failure, but peripheral edema is what prolongs their stay, according to Dr. John G.F. Cleland.
Moreover, it’s not only hospital length of stay that climbs with increasing severity of peripheral edema on admission. So does mortality, both during the index admission and long term, Dr. Cleland reported at the annual meeting of the American College of Cardiology.
He presented a retrospective analysis of 121,229 patients admitted with a primary diagnosis of heart failure to more than 90% of the hospitals in England and Wales during 2007-2013.
“It turns out that the majority of patients we’re seeing admitted with heart failure at U.K. hospitals are not admitted because of severe breathlessness at rest; they’re admitted for increasing fluid retention. They have lots of peripheral edema, which is actually associated with bad outcome. The patients who are very breathless tend to have the better outcome because they have left heart failure. The ones who are full of peripheral edema have more renal dysfunction and anemia, and they’re more likely to have right heart failure,” said Dr. Cleland, professor of cardiology at Imperial College London.
“I’m not saying the breathless group isn’t a target, but this group with peripheral edema is a bigger target – and we’re not designing trials to address their problems,” he added.
Compared with patients with no peripheral edema on hospital admission, the risk of mortality during that hospitalization and up to 3 years of subsequent follow-up was increased 1.2-fold in those with mild peripheral edema, 1.48-fold with moderate peripheral edema, and 1.89-fold in those with severe peripheral edema.

“We’re designing all the big clinical trials in acute heart failure to capture what I regard as neither fish nor fowl. They’re recruiting patients 6-12 hours after admission for acute heart failure. But by that point they’ve pretty well responded to their intravenous diuretics, and we’re just catching the tail end of their breathlessness. They’re not an emergency. The problem is really their peripheral edema, and that’s a day 2/day 3 problem. The first 6 hours of care really isn’t relevant to this group,” according to the internationally renowned heart failure researcher.
Indeed, he said he’d like to see the term “acute heart failure” laid to rest.
“If you think of acute decompensated heart failure, you think of patients wearing an oxygen mask coming in by an ambulance with blue lights flashing, and it’s an emergency. We need to move the mind-set. We shouldn’t call it acute heart failure at all, we should start talking about hospitalized heart failure, of which some is acute but much of it is subacute in people who have been deteriorating over several weeks and have just gotten to the point where they’re not coping at home anymore. They call a taxi or a friend who takes them to the hospital. Then they take a wheelchair from the taxi to the ER, but they don’t really need an ER at all,” Dr. Cleland said.
Many of these patients could be redirected to a different sort of facility at great cost savings, he added.
“In the United Kingdom and I think in the States, we’re now talking about furosemide lounges where, rather than admit the patient, you can bring them up as a day case, give them intravenous therapy, then [have them] go home at night, perhaps coming back for 3 or 4 days if needed. People are also now looking at home infusion services, and there’s a nice device for giving subcutaneous doses of furosemide as well,” the cardiologist said.
Apart from diuretics, there really aren’t any effective medications at present for peripheral edema in heart failure. But there are novel investigational agents worthy of evaluation, according to Dr. Cleland, including drugs aimed at improving mitochondrial function, agents that inhibit channels that allow edema to gather, and iron therapy.
The study was supported by the British Society for Heart Failure and the National Institute for Cardiovascular Outcomes Research. Dr. Cleland reported having no relevant financial conflicts.
AT ACC 16
Acute heart failure mortality climbs with severity of peripheral edema
CHICAGO – Breathlessness typically results in hospital admission for patients with heart failure, but peripheral edema is what prolongs their stay, according to Dr. John G.F. Cleland.
Moreover, it’s not only hospital length of stay that climbs with increasing severity of peripheral edema on admission. So does mortality, both during the index admission and long term, Dr. Cleland reported at the annual meeting of the American College of Cardiology.
He presented a retrospective analysis of 121,229 patients admitted with a primary diagnosis of heart failure to more than 90% of the hospitals in England and Wales during 2007-2013.
“It turns out that the majority of patients we’re seeing admitted with heart failure at U.K. hospitals are not admitted because of severe breathlessness at rest; they’re admitted for increasing fluid retention. They have lots of peripheral edema, which is actually associated with bad outcome. The patients who are very breathless tend to have the better outcome because they have left heart failure. The ones who are full of peripheral edema have more renal dysfunction and anemia, and they’re more likely to have right heart failure,” said Dr. Cleland, professor of cardiology at Imperial College London.
“I’m not saying the breathless group isn’t a target, but this group with peripheral edema is a bigger target – and we’re not designing trials to address their problems,” he added.
Compared with patients with no peripheral edema on hospital admission, the risk of mortality during that hospitalization and up to 3 years of subsequent follow-up was increased 1.2-fold in those with mild peripheral edema, 1.48-fold with moderate peripheral edema, and 1.89-fold in those with severe peripheral edema.
“We’re designing all the big clinical trials in acute heart failure to capture what I regard as neither fish nor fowl. They’re recruiting patients 6-12 hours after admission for acute heart failure. But by that point they’ve pretty well responded to their intravenous diuretics, and we’re just catching the tail end of their breathlessness. They’re not an emergency. The problem is really their peripheral edema, and that’s a day 2/day 3 problem. The first 6 hours of care really isn’t relevant to this group,” according to the internationally renowned heart failure researcher.
Indeed, he said he’d like to see the term “acute heart failure” laid to rest.
“If you think of acute decompensated heart failure, you think of patients wearing an oxygen mask coming in by an ambulance with blue lights flashing, and it’s an emergency. We need to move the mind-set. We shouldn’t call it acute heart failure at all, we should start talking about hospitalized heart failure, of which some is acute but much of it is subacute in people who have been deteriorating over several weeks and have just gotten to the point where they’re not coping at home anymore. They call a taxi or a friend who takes them to the hospital. Then they take a wheelchair from the taxi to the ER, but they don’t really need an ER at all,” Dr. Cleland said.
Many of these patients could be redirected to a different sort of facility at great cost savings, he added.
“In the United Kingdom and I think in the States, we’re now talking about furosemide lounges where, rather than admit the patient, you can bring them up as a day case, give them intravenous therapy, then [have them] go home at night, perhaps coming back for 3 or 4 days if needed. People are also now looking at home infusion services, and there’s a nice device for giving subcutaneous doses of furosemide as well,” the cardiologist said.
Apart from diuretics, there really aren’t any effective medications at present for peripheral edema in heart failure. But there are novel investigational agents worthy of evaluation, according to Dr. Cleland, including drugs aimed at improving mitochondrial function, agents that inhibit channels that allow edema to gather, and iron therapy.
The study was supported by the British Society for Heart Failure and the National Institute for Cardiovascular Outcomes Research. Dr. Cleland reported having no relevant financial conflicts.
CHICAGO – Breathlessness typically results in hospital admission for patients with heart failure, but peripheral edema is what prolongs their stay, according to Dr. John G.F. Cleland.
Moreover, it’s not only hospital length of stay that climbs with increasing severity of peripheral edema on admission. So does mortality, both during the index admission and long term, Dr. Cleland reported at the annual meeting of the American College of Cardiology.
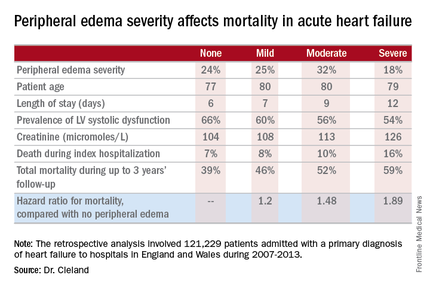
He presented a retrospective analysis of 121,229 patients admitted with a primary diagnosis of heart failure to more than 90% of the hospitals in England and Wales during 2007-2013.
“It turns out that the majority of patients we’re seeing admitted with heart failure at U.K. hospitals are not admitted because of severe breathlessness at rest; they’re admitted for increasing fluid retention. They have lots of peripheral edema, which is actually associated with bad outcome. The patients who are very breathless tend to have the better outcome because they have left heart failure. The ones who are full of peripheral edema have more renal dysfunction and anemia, and they’re more likely to have right heart failure,” said Dr. Cleland, professor of cardiology at Imperial College London.
“I’m not saying the breathless group isn’t a target, but this group with peripheral edema is a bigger target – and we’re not designing trials to address their problems,” he added.
Compared with patients with no peripheral edema on hospital admission, the risk of mortality during that hospitalization and up to 3 years of subsequent follow-up was increased 1.2-fold in those with mild peripheral edema, 1.48-fold with moderate peripheral edema, and 1.89-fold in those with severe peripheral edema.

“We’re designing all the big clinical trials in acute heart failure to capture what I regard as neither fish nor fowl. They’re recruiting patients 6-12 hours after admission for acute heart failure. But by that point they’ve pretty well responded to their intravenous diuretics, and we’re just catching the tail end of their breathlessness. They’re not an emergency. The problem is really their peripheral edema, and that’s a day 2/day 3 problem. The first 6 hours of care really isn’t relevant to this group,” according to the internationally renowned heart failure researcher.
Indeed, he said he’d like to see the term “acute heart failure” laid to rest.
“If you think of acute decompensated heart failure, you think of patients wearing an oxygen mask coming in by an ambulance with blue lights flashing, and it’s an emergency. We need to move the mind-set. We shouldn’t call it acute heart failure at all, we should start talking about hospitalized heart failure, of which some is acute but much of it is subacute in people who have been deteriorating over several weeks and have just gotten to the point where they’re not coping at home anymore. They call a taxi or a friend who takes them to the hospital. Then they take a wheelchair from the taxi to the ER, but they don’t really need an ER at all,” Dr. Cleland said.
Many of these patients could be redirected to a different sort of facility at great cost savings, he added.
“In the United Kingdom and I think in the States, we’re now talking about furosemide lounges where, rather than admit the patient, you can bring them up as a day case, give them intravenous therapy, then [have them] go home at night, perhaps coming back for 3 or 4 days if needed. People are also now looking at home infusion services, and there’s a nice device for giving subcutaneous doses of furosemide as well,” the cardiologist said.
Apart from diuretics, there really aren’t any effective medications at present for peripheral edema in heart failure. But there are novel investigational agents worthy of evaluation, according to Dr. Cleland, including drugs aimed at improving mitochondrial function, agents that inhibit channels that allow edema to gather, and iron therapy.
The study was supported by the British Society for Heart Failure and the National Institute for Cardiovascular Outcomes Research. Dr. Cleland reported having no relevant financial conflicts.
CHICAGO – Breathlessness typically results in hospital admission for patients with heart failure, but peripheral edema is what prolongs their stay, according to Dr. John G.F. Cleland.
Moreover, it’s not only hospital length of stay that climbs with increasing severity of peripheral edema on admission. So does mortality, both during the index admission and long term, Dr. Cleland reported at the annual meeting of the American College of Cardiology.
He presented a retrospective analysis of 121,229 patients admitted with a primary diagnosis of heart failure to more than 90% of the hospitals in England and Wales during 2007-2013.
“It turns out that the majority of patients we’re seeing admitted with heart failure at U.K. hospitals are not admitted because of severe breathlessness at rest; they’re admitted for increasing fluid retention. They have lots of peripheral edema, which is actually associated with bad outcome. The patients who are very breathless tend to have the better outcome because they have left heart failure. The ones who are full of peripheral edema have more renal dysfunction and anemia, and they’re more likely to have right heart failure,” said Dr. Cleland, professor of cardiology at Imperial College London.
“I’m not saying the breathless group isn’t a target, but this group with peripheral edema is a bigger target – and we’re not designing trials to address their problems,” he added.
Compared with patients with no peripheral edema on hospital admission, the risk of mortality during that hospitalization and up to 3 years of subsequent follow-up was increased 1.2-fold in those with mild peripheral edema, 1.48-fold with moderate peripheral edema, and 1.89-fold in those with severe peripheral edema.

“We’re designing all the big clinical trials in acute heart failure to capture what I regard as neither fish nor fowl. They’re recruiting patients 6-12 hours after admission for acute heart failure. But by that point they’ve pretty well responded to their intravenous diuretics, and we’re just catching the tail end of their breathlessness. They’re not an emergency. The problem is really their peripheral edema, and that’s a day 2/day 3 problem. The first 6 hours of care really isn’t relevant to this group,” according to the internationally renowned heart failure researcher.
Indeed, he said he’d like to see the term “acute heart failure” laid to rest.
“If you think of acute decompensated heart failure, you think of patients wearing an oxygen mask coming in by an ambulance with blue lights flashing, and it’s an emergency. We need to move the mind-set. We shouldn’t call it acute heart failure at all, we should start talking about hospitalized heart failure, of which some is acute but much of it is subacute in people who have been deteriorating over several weeks and have just gotten to the point where they’re not coping at home anymore. They call a taxi or a friend who takes them to the hospital. Then they take a wheelchair from the taxi to the ER, but they don’t really need an ER at all,” Dr. Cleland said.
Many of these patients could be redirected to a different sort of facility at great cost savings, he added.
“In the United Kingdom and I think in the States, we’re now talking about furosemide lounges where, rather than admit the patient, you can bring them up as a day case, give them intravenous therapy, then [have them] go home at night, perhaps coming back for 3 or 4 days if needed. People are also now looking at home infusion services, and there’s a nice device for giving subcutaneous doses of furosemide as well,” the cardiologist said.
Apart from diuretics, there really aren’t any effective medications at present for peripheral edema in heart failure. But there are novel investigational agents worthy of evaluation, according to Dr. Cleland, including drugs aimed at improving mitochondrial function, agents that inhibit channels that allow edema to gather, and iron therapy.
The study was supported by the British Society for Heart Failure and the National Institute for Cardiovascular Outcomes Research. Dr. Cleland reported having no relevant financial conflicts.
AT ACC 16
Key clinical point: Leg swelling warrants greater attention in patients hospitalized for acute heart failure.
Major finding: In-hospital mortality was more than twice as great in patients admitted for acute heart failure with severe peripheral edema, compared with no leg swelling.
Data source: A retrospective study of more than 121,000 patients hospitalized for acute heart failure in England and Wales.
Disclosures: The study was supported by the British Society for Heart Failure and the National Institute for Cardiovascular Outcomes Research. The presenter reported having no relevant financial conflicts.
Valve hemodynamic deterioration 2.5% at 1 year
CHICAGO – The incidence of valve hemodynamic deterioration in the first year after transcatheter aortic valve replacement is about 2.5%, but this event wasn’t clearly associated with adverse clinical outcomes out to 18 months of follow-up in an analysis of the large U.S. registry collaboratively maintained by the Society of Thoracic Surgeons and the American College of Cardiology.
“These findings, especially the patient and procedural predictors of valve hemodynamic deterioration we identified, may help to inform TAVR care, including patient selection, surveillance, and preventive strategies,” Dr. Sreekanth Vemulapalli reported at the annual meeting of the American College of Cardiology.
Recent reports have linked TAVR to subsequent development of leaflet abnormalities and valve thrombosis, with widely ranging estimates of incidence. Definitive answers as to the true rate of these adverse events and the underlying mechanisms will come from ongoing prospective studies using advanced imaging via four-dimensional CT or transesophageal echocardiography, but those studies will take years to complete, noted Dr. Vemulapalli of the Duke Clinical Research Institute in Durham, N.C.
In the meantime, he continued, the STS/ACC Transcatheter Valve Therapy Registry provides a unique opportunity to shed light on the incidence and consequences of valve hemodynamic deterioration (VHD) in real-world clinical practice. The registry includes all commercial TAVR procedures performed in the United States, with transthoracic echocardiograms obtained pre- and post-TAVR, at 30 days, and at 1 year after the procedure.
To examine the short- and longer-term rates of VHD, which Dr. Vemulapalli and his coinvestigators defined as an increase in the mean aortic valve gradient of 10 mm or more, the researchers assembled two separate patient cohorts. They comprised a short-term–risk group of 10,095 patients who underwent TAVR at 334 sites, with an incidence of VHD of 2.1% during the first 30 days after the procedure, and 3,175 patients at 254 sites, whose incidence of VHD from day 30 through 1 year post TAVR was 2.5%.
The combined rate of VHD and all-cause mortality during the first 30 days was 7.1%. For the long-term cohort, the combined endpoint rate from day 30 to 1 year was 23.5%.
Importantly, the occurrence of VHD was not associated with an excess of the composite endpoint of mortality, stroke, heart failure hospitalization, or aortic valve reintervention at 1 year in either the short- or long-term cohort. The same held true in an analysis covering the period of 12-18 months post TAVR, according to Dr. Vemulapalli.
In a multivariate analysis, the significant predictors of VHD in the short-term cohort were male sex; increased body mass index, with the risk rising stepwise with every additional 5 kg/m above normal weight; baseline severe chronic lung disease; a valve-in-valve procedure; a larger baseline aortic valve gradient; a TAVR valve size of 23 mm or less; and severe patient/prosthesis mismatch.
In the long-term cohort, the risk factors for VHD were hospital discharge on a factor Xa inhibitor and a larger predischarge aortic valve gradient.
Change in left ventricular ejection fraction over the course of the study bore no relation to VHD risk. Neither did which of the two commercially available TAVR valves a patient received.
This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. Vemulapalli reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.
CHICAGO – The incidence of valve hemodynamic deterioration in the first year after transcatheter aortic valve replacement is about 2.5%, but this event wasn’t clearly associated with adverse clinical outcomes out to 18 months of follow-up in an analysis of the large U.S. registry collaboratively maintained by the Society of Thoracic Surgeons and the American College of Cardiology.
“These findings, especially the patient and procedural predictors of valve hemodynamic deterioration we identified, may help to inform TAVR care, including patient selection, surveillance, and preventive strategies,” Dr. Sreekanth Vemulapalli reported at the annual meeting of the American College of Cardiology.
Recent reports have linked TAVR to subsequent development of leaflet abnormalities and valve thrombosis, with widely ranging estimates of incidence. Definitive answers as to the true rate of these adverse events and the underlying mechanisms will come from ongoing prospective studies using advanced imaging via four-dimensional CT or transesophageal echocardiography, but those studies will take years to complete, noted Dr. Vemulapalli of the Duke Clinical Research Institute in Durham, N.C.
In the meantime, he continued, the STS/ACC Transcatheter Valve Therapy Registry provides a unique opportunity to shed light on the incidence and consequences of valve hemodynamic deterioration (VHD) in real-world clinical practice. The registry includes all commercial TAVR procedures performed in the United States, with transthoracic echocardiograms obtained pre- and post-TAVR, at 30 days, and at 1 year after the procedure.
To examine the short- and longer-term rates of VHD, which Dr. Vemulapalli and his coinvestigators defined as an increase in the mean aortic valve gradient of 10 mm or more, the researchers assembled two separate patient cohorts. They comprised a short-term–risk group of 10,095 patients who underwent TAVR at 334 sites, with an incidence of VHD of 2.1% during the first 30 days after the procedure, and 3,175 patients at 254 sites, whose incidence of VHD from day 30 through 1 year post TAVR was 2.5%.
The combined rate of VHD and all-cause mortality during the first 30 days was 7.1%. For the long-term cohort, the combined endpoint rate from day 30 to 1 year was 23.5%.
Importantly, the occurrence of VHD was not associated with an excess of the composite endpoint of mortality, stroke, heart failure hospitalization, or aortic valve reintervention at 1 year in either the short- or long-term cohort. The same held true in an analysis covering the period of 12-18 months post TAVR, according to Dr. Vemulapalli.
In a multivariate analysis, the significant predictors of VHD in the short-term cohort were male sex; increased body mass index, with the risk rising stepwise with every additional 5 kg/m above normal weight; baseline severe chronic lung disease; a valve-in-valve procedure; a larger baseline aortic valve gradient; a TAVR valve size of 23 mm or less; and severe patient/prosthesis mismatch.
In the long-term cohort, the risk factors for VHD were hospital discharge on a factor Xa inhibitor and a larger predischarge aortic valve gradient.
Change in left ventricular ejection fraction over the course of the study bore no relation to VHD risk. Neither did which of the two commercially available TAVR valves a patient received.
This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. Vemulapalli reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.
CHICAGO – The incidence of valve hemodynamic deterioration in the first year after transcatheter aortic valve replacement is about 2.5%, but this event wasn’t clearly associated with adverse clinical outcomes out to 18 months of follow-up in an analysis of the large U.S. registry collaboratively maintained by the Society of Thoracic Surgeons and the American College of Cardiology.
“These findings, especially the patient and procedural predictors of valve hemodynamic deterioration we identified, may help to inform TAVR care, including patient selection, surveillance, and preventive strategies,” Dr. Sreekanth Vemulapalli reported at the annual meeting of the American College of Cardiology.
Recent reports have linked TAVR to subsequent development of leaflet abnormalities and valve thrombosis, with widely ranging estimates of incidence. Definitive answers as to the true rate of these adverse events and the underlying mechanisms will come from ongoing prospective studies using advanced imaging via four-dimensional CT or transesophageal echocardiography, but those studies will take years to complete, noted Dr. Vemulapalli of the Duke Clinical Research Institute in Durham, N.C.
In the meantime, he continued, the STS/ACC Transcatheter Valve Therapy Registry provides a unique opportunity to shed light on the incidence and consequences of valve hemodynamic deterioration (VHD) in real-world clinical practice. The registry includes all commercial TAVR procedures performed in the United States, with transthoracic echocardiograms obtained pre- and post-TAVR, at 30 days, and at 1 year after the procedure.
To examine the short- and longer-term rates of VHD, which Dr. Vemulapalli and his coinvestigators defined as an increase in the mean aortic valve gradient of 10 mm or more, the researchers assembled two separate patient cohorts. They comprised a short-term–risk group of 10,095 patients who underwent TAVR at 334 sites, with an incidence of VHD of 2.1% during the first 30 days after the procedure, and 3,175 patients at 254 sites, whose incidence of VHD from day 30 through 1 year post TAVR was 2.5%.
The combined rate of VHD and all-cause mortality during the first 30 days was 7.1%. For the long-term cohort, the combined endpoint rate from day 30 to 1 year was 23.5%.
Importantly, the occurrence of VHD was not associated with an excess of the composite endpoint of mortality, stroke, heart failure hospitalization, or aortic valve reintervention at 1 year in either the short- or long-term cohort. The same held true in an analysis covering the period of 12-18 months post TAVR, according to Dr. Vemulapalli.
In a multivariate analysis, the significant predictors of VHD in the short-term cohort were male sex; increased body mass index, with the risk rising stepwise with every additional 5 kg/m above normal weight; baseline severe chronic lung disease; a valve-in-valve procedure; a larger baseline aortic valve gradient; a TAVR valve size of 23 mm or less; and severe patient/prosthesis mismatch.
In the long-term cohort, the risk factors for VHD were hospital discharge on a factor Xa inhibitor and a larger predischarge aortic valve gradient.
Change in left ventricular ejection fraction over the course of the study bore no relation to VHD risk. Neither did which of the two commercially available TAVR valves a patient received.
This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. Vemulapalli reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.
AT ACC 16
Key clinical point: The incidence of valve hemodynamic deterioration after transaortic valve replacement is 2.5% from day 30 through 12 months post procedure.
Major finding: Patients who experienced valve hemodynamic deterioration had a rate of adverse clinical outcomes similar to those without valve deterioration.
Data source: This was a retrospective study of 18-month outcomes in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, which covers all commercial transcatheter valve replacements done in the United States.
Disclosures: This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. The presenter reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.
Kawasaki disease and infections aren’t mutually exclusive
CHICAGO – Kawasaki disease and concurrent bacterial or viral infection are by no means mutually exclusive, Dr. Cathie-Kim Le cautioned at the annual meeting of the American College of Cardiology.
“Recognizing that both can coexist will ensure timely IVIg [intravenous immunoglobulin] treatment and appropriate containment of coronary artery complications,” said Dr. Le of Sainte-Justine University Hospital in Montreal.
She presented a retrospective study of 128 patients, mean age 3.4 years, admitted to the pediatric academic tertiary care center with a discharge diagnosis of Kawasaki disease. During their hospitalization all of them underwent a work-up for bacterial and viral infectious diseases, which proved positive in 33% of cases. Roughly 40% of subjects had incomplete Kawasaki disease, meaning they lacked a sufficient number of signs of mucocutaneous inflammation to fulfill the epidemiologic case definition; however, their prevalence of concomitant infection was similar to that of the group with classic Kawasaki disease.
Among the most common types of infections in patients with Kawasaki disease were otitis media, which accounted for 17% of the infections; upper respiratory infections, 21%; and group A streptococcal pharyngitis and pneumonia, which accounted for 14% each.
There were no differences in clinical presentation or laboratory values between children with or without concomitant infection. Nor was myocardial profiling useful in differentiating patients with concomitant infection from those without: Ventricular shortening fraction scores and N-terminal probrain natriuretic peptide levels were similar in the two groups of Kawasaki disease patients.
Acute coronary dilatation occurred in 26% of Kawasaki disease patients with concomitant infection and similarly in 30% of those without infection. Coronary aneurysm, the most serious complication of Kawasaki disease, occurred in 14% of patients with infection and an identical proportion of the uninfected.
Almost all patients received IVIg. Resistance to the effects of IVIg occurred in 36% of patients with concomitant infection, a rate twice that seen in the group without infection. Coronary aneurysms and dilatations were significantly more common in IVIg-resistant patients, regardless of their infection status.
Dr. Le reported having no relevant financial conflicts.
CHICAGO – Kawasaki disease and concurrent bacterial or viral infection are by no means mutually exclusive, Dr. Cathie-Kim Le cautioned at the annual meeting of the American College of Cardiology.
“Recognizing that both can coexist will ensure timely IVIg [intravenous immunoglobulin] treatment and appropriate containment of coronary artery complications,” said Dr. Le of Sainte-Justine University Hospital in Montreal.
She presented a retrospective study of 128 patients, mean age 3.4 years, admitted to the pediatric academic tertiary care center with a discharge diagnosis of Kawasaki disease. During their hospitalization all of them underwent a work-up for bacterial and viral infectious diseases, which proved positive in 33% of cases. Roughly 40% of subjects had incomplete Kawasaki disease, meaning they lacked a sufficient number of signs of mucocutaneous inflammation to fulfill the epidemiologic case definition; however, their prevalence of concomitant infection was similar to that of the group with classic Kawasaki disease.
Among the most common types of infections in patients with Kawasaki disease were otitis media, which accounted for 17% of the infections; upper respiratory infections, 21%; and group A streptococcal pharyngitis and pneumonia, which accounted for 14% each.
There were no differences in clinical presentation or laboratory values between children with or without concomitant infection. Nor was myocardial profiling useful in differentiating patients with concomitant infection from those without: Ventricular shortening fraction scores and N-terminal probrain natriuretic peptide levels were similar in the two groups of Kawasaki disease patients.
Acute coronary dilatation occurred in 26% of Kawasaki disease patients with concomitant infection and similarly in 30% of those without infection. Coronary aneurysm, the most serious complication of Kawasaki disease, occurred in 14% of patients with infection and an identical proportion of the uninfected.
Almost all patients received IVIg. Resistance to the effects of IVIg occurred in 36% of patients with concomitant infection, a rate twice that seen in the group without infection. Coronary aneurysms and dilatations were significantly more common in IVIg-resistant patients, regardless of their infection status.
Dr. Le reported having no relevant financial conflicts.
CHICAGO – Kawasaki disease and concurrent bacterial or viral infection are by no means mutually exclusive, Dr. Cathie-Kim Le cautioned at the annual meeting of the American College of Cardiology.
“Recognizing that both can coexist will ensure timely IVIg [intravenous immunoglobulin] treatment and appropriate containment of coronary artery complications,” said Dr. Le of Sainte-Justine University Hospital in Montreal.
She presented a retrospective study of 128 patients, mean age 3.4 years, admitted to the pediatric academic tertiary care center with a discharge diagnosis of Kawasaki disease. During their hospitalization all of them underwent a work-up for bacterial and viral infectious diseases, which proved positive in 33% of cases. Roughly 40% of subjects had incomplete Kawasaki disease, meaning they lacked a sufficient number of signs of mucocutaneous inflammation to fulfill the epidemiologic case definition; however, their prevalence of concomitant infection was similar to that of the group with classic Kawasaki disease.
Among the most common types of infections in patients with Kawasaki disease were otitis media, which accounted for 17% of the infections; upper respiratory infections, 21%; and group A streptococcal pharyngitis and pneumonia, which accounted for 14% each.
There were no differences in clinical presentation or laboratory values between children with or without concomitant infection. Nor was myocardial profiling useful in differentiating patients with concomitant infection from those without: Ventricular shortening fraction scores and N-terminal probrain natriuretic peptide levels were similar in the two groups of Kawasaki disease patients.
Acute coronary dilatation occurred in 26% of Kawasaki disease patients with concomitant infection and similarly in 30% of those without infection. Coronary aneurysm, the most serious complication of Kawasaki disease, occurred in 14% of patients with infection and an identical proportion of the uninfected.
Almost all patients received IVIg. Resistance to the effects of IVIg occurred in 36% of patients with concomitant infection, a rate twice that seen in the group without infection. Coronary aneurysms and dilatations were significantly more common in IVIg-resistant patients, regardless of their infection status.
Dr. Le reported having no relevant financial conflicts.
AT ACC 16
Key clinical point: One-third of Kawasaki patients have a concomitant common infection that may delay timely diagnosis.
Major finding: Coronary aneurysm, the most serious complication of Kawasaki disease, occurred in 14% of patients with an infection and in 14% of the uninfected.
Data source: A retrospective study of 128 children with Kawasaki disease.
Disclosures: The study presenter reported having no relevant financial conflicts.
Primary arrhythmia syndromes: Common cause of pediatric sudden cardiac death
CHICAGO – Just over one-half of all sudden deaths in a large pediatric case series were due to a primary arrhythmia syndrome, Dr. Grazia Delle Donne reported at the annual meeting of the American College of Cardiology.
She presented an analysis of all patients under the age of 18 years who were referred to London’s Royal Brompton Hospital for post mortem examination following presumed sudden cardiac death during 1991-2013. Royal Brompton is a national referral center for sudden cardiac death.
The review was undertaken because sudden cardiac death in the pediatric population occurs infrequently. Little is known about the prevalence of the various causes, noted Dr. Delle Donne of Royal Brompton.
Of the 398 subjects, 266 (67%) were female. The median age at death was 14 years. Twenty-two percent of the fatalities occurred during or immediately after exercise. Thirty-nine percent occurred while at rest.
Thirty-one percent of subjects had a family history of sudden cardiac death, another 14% had a family history of cardiomyopathy, and in 5% of cases there was a significant family history of arrhythmia.
Five percent of the children were known to have congenital heart disease. Eighteen percent of the children had a history of syncope.
Investigators determined that a primary arrhythmia syndrome such as long QT or Brugada syndrome was the cause of sudden death in 54% of cases. Death was attributed to cardiomyopathy in 15% cases, congenital heart disease in 8%, myocarditis in 6%, and coronary anomalies in 5%, with miscellaneous causes accounting for the remainder.
Dr. Delle Donne reported having no financial conflicts of interest regarding her presentation.
CHICAGO – Just over one-half of all sudden deaths in a large pediatric case series were due to a primary arrhythmia syndrome, Dr. Grazia Delle Donne reported at the annual meeting of the American College of Cardiology.
She presented an analysis of all patients under the age of 18 years who were referred to London’s Royal Brompton Hospital for post mortem examination following presumed sudden cardiac death during 1991-2013. Royal Brompton is a national referral center for sudden cardiac death.
The review was undertaken because sudden cardiac death in the pediatric population occurs infrequently. Little is known about the prevalence of the various causes, noted Dr. Delle Donne of Royal Brompton.
Of the 398 subjects, 266 (67%) were female. The median age at death was 14 years. Twenty-two percent of the fatalities occurred during or immediately after exercise. Thirty-nine percent occurred while at rest.
Thirty-one percent of subjects had a family history of sudden cardiac death, another 14% had a family history of cardiomyopathy, and in 5% of cases there was a significant family history of arrhythmia.
Five percent of the children were known to have congenital heart disease. Eighteen percent of the children had a history of syncope.
Investigators determined that a primary arrhythmia syndrome such as long QT or Brugada syndrome was the cause of sudden death in 54% of cases. Death was attributed to cardiomyopathy in 15% cases, congenital heart disease in 8%, myocarditis in 6%, and coronary anomalies in 5%, with miscellaneous causes accounting for the remainder.
Dr. Delle Donne reported having no financial conflicts of interest regarding her presentation.
CHICAGO – Just over one-half of all sudden deaths in a large pediatric case series were due to a primary arrhythmia syndrome, Dr. Grazia Delle Donne reported at the annual meeting of the American College of Cardiology.
She presented an analysis of all patients under the age of 18 years who were referred to London’s Royal Brompton Hospital for post mortem examination following presumed sudden cardiac death during 1991-2013. Royal Brompton is a national referral center for sudden cardiac death.
The review was undertaken because sudden cardiac death in the pediatric population occurs infrequently. Little is known about the prevalence of the various causes, noted Dr. Delle Donne of Royal Brompton.
Of the 398 subjects, 266 (67%) were female. The median age at death was 14 years. Twenty-two percent of the fatalities occurred during or immediately after exercise. Thirty-nine percent occurred while at rest.
Thirty-one percent of subjects had a family history of sudden cardiac death, another 14% had a family history of cardiomyopathy, and in 5% of cases there was a significant family history of arrhythmia.
Five percent of the children were known to have congenital heart disease. Eighteen percent of the children had a history of syncope.
Investigators determined that a primary arrhythmia syndrome such as long QT or Brugada syndrome was the cause of sudden death in 54% of cases. Death was attributed to cardiomyopathy in 15% cases, congenital heart disease in 8%, myocarditis in 6%, and coronary anomalies in 5%, with miscellaneous causes accounting for the remainder.
Dr. Delle Donne reported having no financial conflicts of interest regarding her presentation.
AT ACC 16
Key clinical point: Primary arrhythmia syndromes accounted for most cases of sudden cardiac death in a large pediatric case series.
Major finding: Family history of sudden cardiac death was present in 31% of 398 cases.
Data source: A retrospective review of all 398 cases of sudden cardiac death in childhood referred for post mortem examination at a British center during 1991-2013.
Disclosures: Dr. Delle Donne reported having no financial conflicts of interest.













