User login
Study finds increase in dermatologic prescriptions for postsurgical antibiotics
WASHINGTON – , according to an analysis presented at the annual meeting of the American Academy of Dermatology.
John Barbieri, MD, a dermatologist and postdoctoral research fellow at the University of Pennsylvania, Philadelphia, who presented the results, and colleagues examined data about antibiotic use in dermatology between 2008 and 2016. Overall, antibiotic use decreased during this period, primarily for chronic conditions such as acne and rosacea. On the other hand, antibiotic use associated with surgical visits increased by approximately 70%.
Using data from 2008 to 2016 in the Optum Clinformatics DataMart deidentified commercial claims database, the researchers performed a repeated cross-sectional analysis of oral antibiotic prescriptions associated with encounters for surgical procedures performed by dermatologists. They found that oral antibiotic prescriptions increased from 2.9% to 4.4% of visits for benign excisions, from 4.2% to 6.3% of visits for malignant excisions, and from 9.9% to 13.8% of visits for Mohs procedures during this time period. Oral antibiotic prescribing was more common for procedures that entailed a flap or graft and among patients with diabetes, female patients, and younger patients.
The investigators observed greater than twofold variation in antibiotic-prescribing rates across geographic census divisions. “If higher-prescribing divisions were to develop antibiotic prescribing rates similar to [those of] lower-prescribing divisions, antibiotic use could be decreased by over 50%,” Dr. Barbieri said. Before prescribing antibiotic prophylaxis, dermatologists should consider which patients benefit most from it, he added.
The investigators also examined the duration of antibiotic courses. The median course duration of postoperative antibiotics was 7 days. Randomized, controlled trials that collectively included more than 600 patients have failed to demonstrate a benefit of long postoperative courses of antibiotics, compared with perioperative antibiotics alone, said Dr. Barbieri. “While it may be hard to have true perioperative antibiotics available in a dermatology surgical clinic, there likely are opportunities to reduce these postoperative courses to a day or 3 days from this mean of 7 days that we observed in the study.” Reducing these courses would decrease the risk of antibiotic-associated complications such as nausea, diarrhea, and skin rashes, he added.
SOURCE: AAD 2019, Abstract 11356.
WASHINGTON – , according to an analysis presented at the annual meeting of the American Academy of Dermatology.
John Barbieri, MD, a dermatologist and postdoctoral research fellow at the University of Pennsylvania, Philadelphia, who presented the results, and colleagues examined data about antibiotic use in dermatology between 2008 and 2016. Overall, antibiotic use decreased during this period, primarily for chronic conditions such as acne and rosacea. On the other hand, antibiotic use associated with surgical visits increased by approximately 70%.
Using data from 2008 to 2016 in the Optum Clinformatics DataMart deidentified commercial claims database, the researchers performed a repeated cross-sectional analysis of oral antibiotic prescriptions associated with encounters for surgical procedures performed by dermatologists. They found that oral antibiotic prescriptions increased from 2.9% to 4.4% of visits for benign excisions, from 4.2% to 6.3% of visits for malignant excisions, and from 9.9% to 13.8% of visits for Mohs procedures during this time period. Oral antibiotic prescribing was more common for procedures that entailed a flap or graft and among patients with diabetes, female patients, and younger patients.
The investigators observed greater than twofold variation in antibiotic-prescribing rates across geographic census divisions. “If higher-prescribing divisions were to develop antibiotic prescribing rates similar to [those of] lower-prescribing divisions, antibiotic use could be decreased by over 50%,” Dr. Barbieri said. Before prescribing antibiotic prophylaxis, dermatologists should consider which patients benefit most from it, he added.
The investigators also examined the duration of antibiotic courses. The median course duration of postoperative antibiotics was 7 days. Randomized, controlled trials that collectively included more than 600 patients have failed to demonstrate a benefit of long postoperative courses of antibiotics, compared with perioperative antibiotics alone, said Dr. Barbieri. “While it may be hard to have true perioperative antibiotics available in a dermatology surgical clinic, there likely are opportunities to reduce these postoperative courses to a day or 3 days from this mean of 7 days that we observed in the study.” Reducing these courses would decrease the risk of antibiotic-associated complications such as nausea, diarrhea, and skin rashes, he added.
SOURCE: AAD 2019, Abstract 11356.
WASHINGTON – , according to an analysis presented at the annual meeting of the American Academy of Dermatology.
John Barbieri, MD, a dermatologist and postdoctoral research fellow at the University of Pennsylvania, Philadelphia, who presented the results, and colleagues examined data about antibiotic use in dermatology between 2008 and 2016. Overall, antibiotic use decreased during this period, primarily for chronic conditions such as acne and rosacea. On the other hand, antibiotic use associated with surgical visits increased by approximately 70%.
Using data from 2008 to 2016 in the Optum Clinformatics DataMart deidentified commercial claims database, the researchers performed a repeated cross-sectional analysis of oral antibiotic prescriptions associated with encounters for surgical procedures performed by dermatologists. They found that oral antibiotic prescriptions increased from 2.9% to 4.4% of visits for benign excisions, from 4.2% to 6.3% of visits for malignant excisions, and from 9.9% to 13.8% of visits for Mohs procedures during this time period. Oral antibiotic prescribing was more common for procedures that entailed a flap or graft and among patients with diabetes, female patients, and younger patients.
The investigators observed greater than twofold variation in antibiotic-prescribing rates across geographic census divisions. “If higher-prescribing divisions were to develop antibiotic prescribing rates similar to [those of] lower-prescribing divisions, antibiotic use could be decreased by over 50%,” Dr. Barbieri said. Before prescribing antibiotic prophylaxis, dermatologists should consider which patients benefit most from it, he added.
The investigators also examined the duration of antibiotic courses. The median course duration of postoperative antibiotics was 7 days. Randomized, controlled trials that collectively included more than 600 patients have failed to demonstrate a benefit of long postoperative courses of antibiotics, compared with perioperative antibiotics alone, said Dr. Barbieri. “While it may be hard to have true perioperative antibiotics available in a dermatology surgical clinic, there likely are opportunities to reduce these postoperative courses to a day or 3 days from this mean of 7 days that we observed in the study.” Reducing these courses would decrease the risk of antibiotic-associated complications such as nausea, diarrhea, and skin rashes, he added.
SOURCE: AAD 2019, Abstract 11356.
REPORTING FROM AAD 2019
Misleading information, reimbursement among the barriers to teledermatology progress
WASHINGTON – Suephy C. Chen, MD, said at the annual meeting of the American Academy of Dermatology.
Even with disclaimers, there are people who want a “quick and easy answer,” and these apps can provide misleading information that “can lead them down a wrong diagnostic pathway,” said Dr. Chen, professor of dermatology and director of the teledermatology service at Emory University, Atlanta. Users not only include lower income or uninsured patients, but busy, high-powered executives.
Apps focused on photo storage are used to help patients track lesions for changes, with some apps dedicated to total body mole mapping. However, while these apps may empower patients to perform regular self skin checks, there is a question of whether they are HIPAA secure, Dr. Chen said. Another issue is that the many different app choices on the market may make it difficult for providers to keep up with which app a particular patient is using, she added. “If you have 10 different patients coming in with 10 different apps, it’s going to be really hard for you to learn all of those and be able to manipulate that easily, especially in the 15-minute slot.”
Smartphone and tablet apps that offer reminders to perform monthly skin checks or apply sunscreen when outdoors are plentiful. Dr. Chen noted that, while the efficacy of these apps are not known, they are similar to less high-tech technology like alarms or calendar reminders. “[They] are really kind of neat and fun. It’s kind of boring to just get a reminder, and you tune it out if you get a reminder on your calendars, so this may be a new way to help people,” she said.
Wearables also track users’ sun exposure, and range from a UV sensor on the thumb that measures sun exposure over a period of months to clip-on wearables and temporary tattoos that tell users when to apply or reapply sunscreen. Some devices allow entry of an individual’s Fitzpatrick skin type and can detect temperature and humidity, she noted.
Risk-calculating apps use images taken from smartphone cameras to determine the risk of melanoma, using algorithms that consider color and pattern recognition, but these apps are not as accurate as dermatologists, she said. In a study published in 2013, the app that sent images directly to a dermatologist was the most effective, compared with apps that relied on an automated algorithm to analyze the images (JAMA Dermatol. 2013 Apr;149[4]:422-6).
One of the conclusions the authors made was that feedback was slow for the one that required the image be sent to a dermatologist. “As opposed to just a minute and spitting out the result, it took 24 hours. My argument is 24 hours is still a lot faster than if you tried to call and get an appointment with a dermatologist,” Dr. Chen commented.
One step above teledermatology is teledermoscopy, or using a mobile, smartphone-attached device to send images to a dermatologist over a secure cloud service for review. “Most of us would agree that it would just take too long to do a live video with a patient,” Dr. Chen pointed out. “They may as well just come in anyway. It’ll take you 40 minutes to be able to take a look at that mole on the video, but to do it in a store-and-forward format can be quite efficient.”
However, she noted that one barrier to entry for teledermoscopy is defining the type of service, such as whether apps will offer provider-to-provider or patient-to-provider services. “That is fraught with its own details and issues, especially with photo quality.”
Another barrier, reimbursement from Centers for Medicare & Medicaid Services for teledermatology, is “the real sticking point,” Dr. Chen continued. Under a 2019 CMS Final Rule, telemedicine is only covered if the patient is already established within the practice, and reimbursement for Healthcare Common Procedure Coding System codes G2010 and G2012 relating to telemedicine ranges between $12 and $14.
Based on her back-of-the-envelope calculation, she added, “I would have to see 180 patients in a half-day session by this method in order to generate my salary, and that would just be impossible.”
Dr. Chen said that teledermatology is the “way of the future” and hopes the CMS Final Rule is reconsidered so the technology can be used to help solve some of the growing issues in the dermatology field. “There’s no way we can meet the demands of an increasingly aging population by an in-person brick and mortar sort of paradigm,” she said, noting that, even in an urban setting, it can be difficult to see a dermatologist.
Dr. Chen reports relationships with BioPharmX, Dermecular Therapeutics, Leo Pharma, Phoenix Tissue Repair, Trevi Therapeutics, and Unilever.
WASHINGTON – Suephy C. Chen, MD, said at the annual meeting of the American Academy of Dermatology.
Even with disclaimers, there are people who want a “quick and easy answer,” and these apps can provide misleading information that “can lead them down a wrong diagnostic pathway,” said Dr. Chen, professor of dermatology and director of the teledermatology service at Emory University, Atlanta. Users not only include lower income or uninsured patients, but busy, high-powered executives.
Apps focused on photo storage are used to help patients track lesions for changes, with some apps dedicated to total body mole mapping. However, while these apps may empower patients to perform regular self skin checks, there is a question of whether they are HIPAA secure, Dr. Chen said. Another issue is that the many different app choices on the market may make it difficult for providers to keep up with which app a particular patient is using, she added. “If you have 10 different patients coming in with 10 different apps, it’s going to be really hard for you to learn all of those and be able to manipulate that easily, especially in the 15-minute slot.”
Smartphone and tablet apps that offer reminders to perform monthly skin checks or apply sunscreen when outdoors are plentiful. Dr. Chen noted that, while the efficacy of these apps are not known, they are similar to less high-tech technology like alarms or calendar reminders. “[They] are really kind of neat and fun. It’s kind of boring to just get a reminder, and you tune it out if you get a reminder on your calendars, so this may be a new way to help people,” she said.
Wearables also track users’ sun exposure, and range from a UV sensor on the thumb that measures sun exposure over a period of months to clip-on wearables and temporary tattoos that tell users when to apply or reapply sunscreen. Some devices allow entry of an individual’s Fitzpatrick skin type and can detect temperature and humidity, she noted.
Risk-calculating apps use images taken from smartphone cameras to determine the risk of melanoma, using algorithms that consider color and pattern recognition, but these apps are not as accurate as dermatologists, she said. In a study published in 2013, the app that sent images directly to a dermatologist was the most effective, compared with apps that relied on an automated algorithm to analyze the images (JAMA Dermatol. 2013 Apr;149[4]:422-6).
One of the conclusions the authors made was that feedback was slow for the one that required the image be sent to a dermatologist. “As opposed to just a minute and spitting out the result, it took 24 hours. My argument is 24 hours is still a lot faster than if you tried to call and get an appointment with a dermatologist,” Dr. Chen commented.
One step above teledermatology is teledermoscopy, or using a mobile, smartphone-attached device to send images to a dermatologist over a secure cloud service for review. “Most of us would agree that it would just take too long to do a live video with a patient,” Dr. Chen pointed out. “They may as well just come in anyway. It’ll take you 40 minutes to be able to take a look at that mole on the video, but to do it in a store-and-forward format can be quite efficient.”
However, she noted that one barrier to entry for teledermoscopy is defining the type of service, such as whether apps will offer provider-to-provider or patient-to-provider services. “That is fraught with its own details and issues, especially with photo quality.”
Another barrier, reimbursement from Centers for Medicare & Medicaid Services for teledermatology, is “the real sticking point,” Dr. Chen continued. Under a 2019 CMS Final Rule, telemedicine is only covered if the patient is already established within the practice, and reimbursement for Healthcare Common Procedure Coding System codes G2010 and G2012 relating to telemedicine ranges between $12 and $14.
Based on her back-of-the-envelope calculation, she added, “I would have to see 180 patients in a half-day session by this method in order to generate my salary, and that would just be impossible.”
Dr. Chen said that teledermatology is the “way of the future” and hopes the CMS Final Rule is reconsidered so the technology can be used to help solve some of the growing issues in the dermatology field. “There’s no way we can meet the demands of an increasingly aging population by an in-person brick and mortar sort of paradigm,” she said, noting that, even in an urban setting, it can be difficult to see a dermatologist.
Dr. Chen reports relationships with BioPharmX, Dermecular Therapeutics, Leo Pharma, Phoenix Tissue Repair, Trevi Therapeutics, and Unilever.
WASHINGTON – Suephy C. Chen, MD, said at the annual meeting of the American Academy of Dermatology.
Even with disclaimers, there are people who want a “quick and easy answer,” and these apps can provide misleading information that “can lead them down a wrong diagnostic pathway,” said Dr. Chen, professor of dermatology and director of the teledermatology service at Emory University, Atlanta. Users not only include lower income or uninsured patients, but busy, high-powered executives.
Apps focused on photo storage are used to help patients track lesions for changes, with some apps dedicated to total body mole mapping. However, while these apps may empower patients to perform regular self skin checks, there is a question of whether they are HIPAA secure, Dr. Chen said. Another issue is that the many different app choices on the market may make it difficult for providers to keep up with which app a particular patient is using, she added. “If you have 10 different patients coming in with 10 different apps, it’s going to be really hard for you to learn all of those and be able to manipulate that easily, especially in the 15-minute slot.”
Smartphone and tablet apps that offer reminders to perform monthly skin checks or apply sunscreen when outdoors are plentiful. Dr. Chen noted that, while the efficacy of these apps are not known, they are similar to less high-tech technology like alarms or calendar reminders. “[They] are really kind of neat and fun. It’s kind of boring to just get a reminder, and you tune it out if you get a reminder on your calendars, so this may be a new way to help people,” she said.
Wearables also track users’ sun exposure, and range from a UV sensor on the thumb that measures sun exposure over a period of months to clip-on wearables and temporary tattoos that tell users when to apply or reapply sunscreen. Some devices allow entry of an individual’s Fitzpatrick skin type and can detect temperature and humidity, she noted.
Risk-calculating apps use images taken from smartphone cameras to determine the risk of melanoma, using algorithms that consider color and pattern recognition, but these apps are not as accurate as dermatologists, she said. In a study published in 2013, the app that sent images directly to a dermatologist was the most effective, compared with apps that relied on an automated algorithm to analyze the images (JAMA Dermatol. 2013 Apr;149[4]:422-6).
One of the conclusions the authors made was that feedback was slow for the one that required the image be sent to a dermatologist. “As opposed to just a minute and spitting out the result, it took 24 hours. My argument is 24 hours is still a lot faster than if you tried to call and get an appointment with a dermatologist,” Dr. Chen commented.
One step above teledermatology is teledermoscopy, or using a mobile, smartphone-attached device to send images to a dermatologist over a secure cloud service for review. “Most of us would agree that it would just take too long to do a live video with a patient,” Dr. Chen pointed out. “They may as well just come in anyway. It’ll take you 40 minutes to be able to take a look at that mole on the video, but to do it in a store-and-forward format can be quite efficient.”
However, she noted that one barrier to entry for teledermoscopy is defining the type of service, such as whether apps will offer provider-to-provider or patient-to-provider services. “That is fraught with its own details and issues, especially with photo quality.”
Another barrier, reimbursement from Centers for Medicare & Medicaid Services for teledermatology, is “the real sticking point,” Dr. Chen continued. Under a 2019 CMS Final Rule, telemedicine is only covered if the patient is already established within the practice, and reimbursement for Healthcare Common Procedure Coding System codes G2010 and G2012 relating to telemedicine ranges between $12 and $14.
Based on her back-of-the-envelope calculation, she added, “I would have to see 180 patients in a half-day session by this method in order to generate my salary, and that would just be impossible.”
Dr. Chen said that teledermatology is the “way of the future” and hopes the CMS Final Rule is reconsidered so the technology can be used to help solve some of the growing issues in the dermatology field. “There’s no way we can meet the demands of an increasingly aging population by an in-person brick and mortar sort of paradigm,” she said, noting that, even in an urban setting, it can be difficult to see a dermatologist.
Dr. Chen reports relationships with BioPharmX, Dermecular Therapeutics, Leo Pharma, Phoenix Tissue Repair, Trevi Therapeutics, and Unilever.
EXPERT ANALYSIS FROM AAD 2019
Ligelizumab maintains urticaria control for up to 1 year
WASHINGTON – in an open-label extension study, Diane Baker, MD, said at the annual meeting of the American Academy of Dermatology.
About 75% of the cohort experienced complete disease control at least once during the study. Novartis is developing ligelizumab (QGE031) as a treatment option for patients with spontaneous chronic urticaria (CSU) whose symptoms are inadequately controlled by H1-antihistamines. Like omalizumab (Xolair), which is approved in the United States and Europe for treatment of CSU, ligelizumab is a humanized anti-IgE monoclonal antibody. But the investigational agent binds to IgE with greater affinity than omalizumab, said Dr. Baker, a dermatologist who practices in Portland, Ore.
The extension study was a follow-up to a 12-week, phase 2, dose-finding trial of 382 CSU patients. In the study, which was not powered for efficacy endpoints, 51% of those who received 72 mg subcutaneously every 4 weeks had a Hives Severity Score of 0 by week 12, compared with 42% of those who received 240 mg every 4 weeks and 26% of those taking the omalizumab comparator. Additionally, 47% of those in the 72-mg group and 46% of the 240-mg group achieved a score of 0 on another indicator, the Urticaria Activity Score, which measures symptoms over 7 days (UAS7).
The extension study, which evaluated the 240-mg dose, showed the durability of that response, with 52% of those in the 240-mg group maintained a UAS7 of 0 at 1 year, according to Dr. Baker. By the end of the year, most patients (75.8%) had experienced at least one period of complete symptom control, and 84.0% experienced a UAS of 6 or lower at least once.
Adverse events were common in the cohort, with 84% experiencing at least one. But most (78%) were mild or moderate, and there was no clear side effect pattern, Dr. Baker said. Eight patients discontinued treatment because of an adverse event, and another eight dropped out because of lack of efficacy. Other reasons for discontinuation were pregnancy, protocol deviation, and physician or patient decision.
Novartis has launched two 1-year, phase 3 trials randomizing patients to 72 mg or 240 mg of ligelizumab or 300 mg of omalizumab every 4 weeks in a similar patient population, Dr. Baker said. PEARL 1 and PEARL 2, the largest pivotal trials to date in CSU, will enroll more than 2,000 patients, according to a company press release.
Dr. Baker is a clinical trials investigator for Novartis.
SOURCE: Baker D et al. AAD 2019, Session S034.
WASHINGTON – in an open-label extension study, Diane Baker, MD, said at the annual meeting of the American Academy of Dermatology.
About 75% of the cohort experienced complete disease control at least once during the study. Novartis is developing ligelizumab (QGE031) as a treatment option for patients with spontaneous chronic urticaria (CSU) whose symptoms are inadequately controlled by H1-antihistamines. Like omalizumab (Xolair), which is approved in the United States and Europe for treatment of CSU, ligelizumab is a humanized anti-IgE monoclonal antibody. But the investigational agent binds to IgE with greater affinity than omalizumab, said Dr. Baker, a dermatologist who practices in Portland, Ore.
The extension study was a follow-up to a 12-week, phase 2, dose-finding trial of 382 CSU patients. In the study, which was not powered for efficacy endpoints, 51% of those who received 72 mg subcutaneously every 4 weeks had a Hives Severity Score of 0 by week 12, compared with 42% of those who received 240 mg every 4 weeks and 26% of those taking the omalizumab comparator. Additionally, 47% of those in the 72-mg group and 46% of the 240-mg group achieved a score of 0 on another indicator, the Urticaria Activity Score, which measures symptoms over 7 days (UAS7).
The extension study, which evaluated the 240-mg dose, showed the durability of that response, with 52% of those in the 240-mg group maintained a UAS7 of 0 at 1 year, according to Dr. Baker. By the end of the year, most patients (75.8%) had experienced at least one period of complete symptom control, and 84.0% experienced a UAS of 6 or lower at least once.
Adverse events were common in the cohort, with 84% experiencing at least one. But most (78%) were mild or moderate, and there was no clear side effect pattern, Dr. Baker said. Eight patients discontinued treatment because of an adverse event, and another eight dropped out because of lack of efficacy. Other reasons for discontinuation were pregnancy, protocol deviation, and physician or patient decision.
Novartis has launched two 1-year, phase 3 trials randomizing patients to 72 mg or 240 mg of ligelizumab or 300 mg of omalizumab every 4 weeks in a similar patient population, Dr. Baker said. PEARL 1 and PEARL 2, the largest pivotal trials to date in CSU, will enroll more than 2,000 patients, according to a company press release.
Dr. Baker is a clinical trials investigator for Novartis.
SOURCE: Baker D et al. AAD 2019, Session S034.
WASHINGTON – in an open-label extension study, Diane Baker, MD, said at the annual meeting of the American Academy of Dermatology.
About 75% of the cohort experienced complete disease control at least once during the study. Novartis is developing ligelizumab (QGE031) as a treatment option for patients with spontaneous chronic urticaria (CSU) whose symptoms are inadequately controlled by H1-antihistamines. Like omalizumab (Xolair), which is approved in the United States and Europe for treatment of CSU, ligelizumab is a humanized anti-IgE monoclonal antibody. But the investigational agent binds to IgE with greater affinity than omalizumab, said Dr. Baker, a dermatologist who practices in Portland, Ore.
The extension study was a follow-up to a 12-week, phase 2, dose-finding trial of 382 CSU patients. In the study, which was not powered for efficacy endpoints, 51% of those who received 72 mg subcutaneously every 4 weeks had a Hives Severity Score of 0 by week 12, compared with 42% of those who received 240 mg every 4 weeks and 26% of those taking the omalizumab comparator. Additionally, 47% of those in the 72-mg group and 46% of the 240-mg group achieved a score of 0 on another indicator, the Urticaria Activity Score, which measures symptoms over 7 days (UAS7).
The extension study, which evaluated the 240-mg dose, showed the durability of that response, with 52% of those in the 240-mg group maintained a UAS7 of 0 at 1 year, according to Dr. Baker. By the end of the year, most patients (75.8%) had experienced at least one period of complete symptom control, and 84.0% experienced a UAS of 6 or lower at least once.
Adverse events were common in the cohort, with 84% experiencing at least one. But most (78%) were mild or moderate, and there was no clear side effect pattern, Dr. Baker said. Eight patients discontinued treatment because of an adverse event, and another eight dropped out because of lack of efficacy. Other reasons for discontinuation were pregnancy, protocol deviation, and physician or patient decision.
Novartis has launched two 1-year, phase 3 trials randomizing patients to 72 mg or 240 mg of ligelizumab or 300 mg of omalizumab every 4 weeks in a similar patient population, Dr. Baker said. PEARL 1 and PEARL 2, the largest pivotal trials to date in CSU, will enroll more than 2,000 patients, according to a company press release.
Dr. Baker is a clinical trials investigator for Novartis.
SOURCE: Baker D et al. AAD 2019, Session S034.
REPORTING FROM AAD 2019
Hormonal management strategies for hidradenitis suppurativa target androgens
WASHINGTON – Hidradenitis suppurativa (HS) management should be individualized in patients, with consideration of their comorbidities, and therapies should be layered and rotated to improve efficacy, Ginette Okoye, MD, said at the annual meeting of the American Academy of Dermatology.
, spironolactone, and oral contraceptives, said Dr. Okoye, professor and chair of dermatology at Howard University, Washington. A patient’s comorbidities can help tailor which treatments to use, so if a patient with HS also has androgenetic alopecia, finasteride can be considered, while spironolactone, with or without an OC, can be considered for a patient with acne – and metformin can be considered for a patient with diabetes or prediabetes, or polycystic ovary syndrome (PCOS), she commented.
The main goal behind hormonal and metabolic therapies in patients with HS is to decrease androgens. Metformin, the oral hypoglycemic drug, reduces ovarian androgen production, and increases insulin-receptor sensitivity, and is an option for patients with HS, and can also treat comorbid conditions these patients tend to have, such as obesity, insulin resistance, and PCOS, she noted. Metformin dosing is 1,500 to 2,000 mg a day, starting at 500 mg per day with an evening meal, titrating up 500 mg every 2-4 weeks based on how patients tolerate side effects such as diarrhea, nausea, vomiting, and flatulence. Lactic acidosis is a less common side effect, but the risk increases for patients with renal and hepatic impairment or excessive alcohol intake, and for those who are undergoing a radiological procedure with contrast or who are over 65 years of age. While metformin alone, in her experience, does not make a big difference, it can be helpful when combined with other treatments such as antibiotics and biologics, and in patients with these comorbidities, she said.
Pregnant women with HS can benefit from treatment with metformin, but dermatologists should consult with the patient’s obstetrician-gynecologist as the medication is classified as pregnancy category B. In addition, metformin should not be given to patients with a glomerular filtration rate (GFR) less than 45 mL/min, and long-term use is associated with low vitamin B12 levels, she said.
“I often layer this with the antibiotic therapy, so my patient may be on clindamycin, rifampin, and metformin,” said Dr. Okoye. “If they are, you can give them a much lower dose of metformin since rifampin increases the plasma concentration of metformin.”
Patients with HS may also respond well to finasteride at doses between 1 mg and 5 mg once daily, an off-label use for this medication. Finasteride, which targets type 2 5-alpha-reductase, reduces the levels of dihydrotestosterone within hair follicles, which can improve HS symptoms, she said. However, she discusses potential side effects of finasteride use with patients, which include reduced libido, abnormal ejaculation, breast tenderness, prostate cancer, and depression. She also referred to postmarketing data suggesting that finasteride can lead to post-finasteride syndrome, characterized by symptoms that include depression and anhedonia, even long after stopping treatment, she said.
“I still think that it’s worth a try,” Dr. Okoye commented. “Many of our HS patients already are dealing with depression because of their disease. ... In 3 months, we talk about their symptoms, [and] make sure that they’re feeling okay before continuing.”
While finasteride is not appropriate for women of childbearing potential (pregnancy category X), it can be an option for women with HS who are of childbearing age but are not at risk for becoming pregnant, Dr. Okoye added, which can be determined by discussing a patient’s family planning goals. For example, she said, “if you have a woman of childbearing age but she’s in a same-sex relationship and has no intention of having children, then maybe finasteride is an option for her.”
The mineralocorticoid- and aldosterone-receptor antagonist spironolactone, used off label for acne treatment, also has antiandrogenic properties and is an option for patients with HS “at the higher end of the dosing spectrum” with 100-200 mg daily. However, Dr. Okoye referred to a recently published single-center retrospective study that showed a low daily dose of 75 mg was effective for HS (J Am Acad Dermatol. 2019 Jan;80[1]:114-9).
While spironolactone increases the risk of hyperkalemia, in patients with no preexisting renal disease under 50 years of age, monitoring is not necessary because there is little to no risk of clinical hyperkalemia in these patients, she said. Combining spironolactone or finasteride with OCs may increase antiandrogenic activity, she noted.
The data on effectiveness of hormonal contraceptives are mixed with regard to treatment of HS, with some studies showing benefit or worsening of the disease with OC use. “I think one of the reasons the data is so ‘dirty’ is because OCs range widely in terms of their ingredients and in terms of how androgenic their progesterones are,” Dr. Okoye commented.
OCs increase the risk of venous thromboembolism (VTE), but Dr. Okoye noted the risk is less than a patient would experience during pregnancy. “When you talk to dermatologists, there are two camps: some dermatologists who are very comfortable prescribing OCs, and dermatologists who prefer not to, given the risk of VTEs,” she said. However, risk should also be applied to patient population and location, she noted.
“If you are in an area [where] you serve a patient population that has fewer options for access to care, and if you don’t prescribe the OCs, those patients have to wait several months before getting on therapy, said Dr. Okoye. “Maybe that’s a case where you might want to start the OC [with] one or two refills while they find an OB, but it’s really up to you and your risk aversion.”
Dietary factors may also contribute to HS, but more studies are needed to analyze how sugar and carbohydrates contribute to the condition. Instead of taking for granted that a patient will understand what reducing dietary carbohydrate and sugar intake means, Dr. Okoye said, “I like to get very specific; ask them what they’re drinking on a daily basis.”
With regard to weight loss, there is little to link significant weight loss and symptom improvement. However, weight loss could help with comorbid conditions in patients with HS, like metabolic syndrome, and subsequent skin reduction may reduce friction of intertriginous areas, she pointed out.
Dr. Okoye reports receiving grants and/or research funding from Eli Lilly.
WASHINGTON – Hidradenitis suppurativa (HS) management should be individualized in patients, with consideration of their comorbidities, and therapies should be layered and rotated to improve efficacy, Ginette Okoye, MD, said at the annual meeting of the American Academy of Dermatology.
, spironolactone, and oral contraceptives, said Dr. Okoye, professor and chair of dermatology at Howard University, Washington. A patient’s comorbidities can help tailor which treatments to use, so if a patient with HS also has androgenetic alopecia, finasteride can be considered, while spironolactone, with or without an OC, can be considered for a patient with acne – and metformin can be considered for a patient with diabetes or prediabetes, or polycystic ovary syndrome (PCOS), she commented.
The main goal behind hormonal and metabolic therapies in patients with HS is to decrease androgens. Metformin, the oral hypoglycemic drug, reduces ovarian androgen production, and increases insulin-receptor sensitivity, and is an option for patients with HS, and can also treat comorbid conditions these patients tend to have, such as obesity, insulin resistance, and PCOS, she noted. Metformin dosing is 1,500 to 2,000 mg a day, starting at 500 mg per day with an evening meal, titrating up 500 mg every 2-4 weeks based on how patients tolerate side effects such as diarrhea, nausea, vomiting, and flatulence. Lactic acidosis is a less common side effect, but the risk increases for patients with renal and hepatic impairment or excessive alcohol intake, and for those who are undergoing a radiological procedure with contrast or who are over 65 years of age. While metformin alone, in her experience, does not make a big difference, it can be helpful when combined with other treatments such as antibiotics and biologics, and in patients with these comorbidities, she said.
Pregnant women with HS can benefit from treatment with metformin, but dermatologists should consult with the patient’s obstetrician-gynecologist as the medication is classified as pregnancy category B. In addition, metformin should not be given to patients with a glomerular filtration rate (GFR) less than 45 mL/min, and long-term use is associated with low vitamin B12 levels, she said.
“I often layer this with the antibiotic therapy, so my patient may be on clindamycin, rifampin, and metformin,” said Dr. Okoye. “If they are, you can give them a much lower dose of metformin since rifampin increases the plasma concentration of metformin.”
Patients with HS may also respond well to finasteride at doses between 1 mg and 5 mg once daily, an off-label use for this medication. Finasteride, which targets type 2 5-alpha-reductase, reduces the levels of dihydrotestosterone within hair follicles, which can improve HS symptoms, she said. However, she discusses potential side effects of finasteride use with patients, which include reduced libido, abnormal ejaculation, breast tenderness, prostate cancer, and depression. She also referred to postmarketing data suggesting that finasteride can lead to post-finasteride syndrome, characterized by symptoms that include depression and anhedonia, even long after stopping treatment, she said.
“I still think that it’s worth a try,” Dr. Okoye commented. “Many of our HS patients already are dealing with depression because of their disease. ... In 3 months, we talk about their symptoms, [and] make sure that they’re feeling okay before continuing.”
While finasteride is not appropriate for women of childbearing potential (pregnancy category X), it can be an option for women with HS who are of childbearing age but are not at risk for becoming pregnant, Dr. Okoye added, which can be determined by discussing a patient’s family planning goals. For example, she said, “if you have a woman of childbearing age but she’s in a same-sex relationship and has no intention of having children, then maybe finasteride is an option for her.”
The mineralocorticoid- and aldosterone-receptor antagonist spironolactone, used off label for acne treatment, also has antiandrogenic properties and is an option for patients with HS “at the higher end of the dosing spectrum” with 100-200 mg daily. However, Dr. Okoye referred to a recently published single-center retrospective study that showed a low daily dose of 75 mg was effective for HS (J Am Acad Dermatol. 2019 Jan;80[1]:114-9).
While spironolactone increases the risk of hyperkalemia, in patients with no preexisting renal disease under 50 years of age, monitoring is not necessary because there is little to no risk of clinical hyperkalemia in these patients, she said. Combining spironolactone or finasteride with OCs may increase antiandrogenic activity, she noted.
The data on effectiveness of hormonal contraceptives are mixed with regard to treatment of HS, with some studies showing benefit or worsening of the disease with OC use. “I think one of the reasons the data is so ‘dirty’ is because OCs range widely in terms of their ingredients and in terms of how androgenic their progesterones are,” Dr. Okoye commented.
OCs increase the risk of venous thromboembolism (VTE), but Dr. Okoye noted the risk is less than a patient would experience during pregnancy. “When you talk to dermatologists, there are two camps: some dermatologists who are very comfortable prescribing OCs, and dermatologists who prefer not to, given the risk of VTEs,” she said. However, risk should also be applied to patient population and location, she noted.
“If you are in an area [where] you serve a patient population that has fewer options for access to care, and if you don’t prescribe the OCs, those patients have to wait several months before getting on therapy, said Dr. Okoye. “Maybe that’s a case where you might want to start the OC [with] one or two refills while they find an OB, but it’s really up to you and your risk aversion.”
Dietary factors may also contribute to HS, but more studies are needed to analyze how sugar and carbohydrates contribute to the condition. Instead of taking for granted that a patient will understand what reducing dietary carbohydrate and sugar intake means, Dr. Okoye said, “I like to get very specific; ask them what they’re drinking on a daily basis.”
With regard to weight loss, there is little to link significant weight loss and symptom improvement. However, weight loss could help with comorbid conditions in patients with HS, like metabolic syndrome, and subsequent skin reduction may reduce friction of intertriginous areas, she pointed out.
Dr. Okoye reports receiving grants and/or research funding from Eli Lilly.
WASHINGTON – Hidradenitis suppurativa (HS) management should be individualized in patients, with consideration of their comorbidities, and therapies should be layered and rotated to improve efficacy, Ginette Okoye, MD, said at the annual meeting of the American Academy of Dermatology.
, spironolactone, and oral contraceptives, said Dr. Okoye, professor and chair of dermatology at Howard University, Washington. A patient’s comorbidities can help tailor which treatments to use, so if a patient with HS also has androgenetic alopecia, finasteride can be considered, while spironolactone, with or without an OC, can be considered for a patient with acne – and metformin can be considered for a patient with diabetes or prediabetes, or polycystic ovary syndrome (PCOS), she commented.
The main goal behind hormonal and metabolic therapies in patients with HS is to decrease androgens. Metformin, the oral hypoglycemic drug, reduces ovarian androgen production, and increases insulin-receptor sensitivity, and is an option for patients with HS, and can also treat comorbid conditions these patients tend to have, such as obesity, insulin resistance, and PCOS, she noted. Metformin dosing is 1,500 to 2,000 mg a day, starting at 500 mg per day with an evening meal, titrating up 500 mg every 2-4 weeks based on how patients tolerate side effects such as diarrhea, nausea, vomiting, and flatulence. Lactic acidosis is a less common side effect, but the risk increases for patients with renal and hepatic impairment or excessive alcohol intake, and for those who are undergoing a radiological procedure with contrast or who are over 65 years of age. While metformin alone, in her experience, does not make a big difference, it can be helpful when combined with other treatments such as antibiotics and biologics, and in patients with these comorbidities, she said.
Pregnant women with HS can benefit from treatment with metformin, but dermatologists should consult with the patient’s obstetrician-gynecologist as the medication is classified as pregnancy category B. In addition, metformin should not be given to patients with a glomerular filtration rate (GFR) less than 45 mL/min, and long-term use is associated with low vitamin B12 levels, she said.
“I often layer this with the antibiotic therapy, so my patient may be on clindamycin, rifampin, and metformin,” said Dr. Okoye. “If they are, you can give them a much lower dose of metformin since rifampin increases the plasma concentration of metformin.”
Patients with HS may also respond well to finasteride at doses between 1 mg and 5 mg once daily, an off-label use for this medication. Finasteride, which targets type 2 5-alpha-reductase, reduces the levels of dihydrotestosterone within hair follicles, which can improve HS symptoms, she said. However, she discusses potential side effects of finasteride use with patients, which include reduced libido, abnormal ejaculation, breast tenderness, prostate cancer, and depression. She also referred to postmarketing data suggesting that finasteride can lead to post-finasteride syndrome, characterized by symptoms that include depression and anhedonia, even long after stopping treatment, she said.
“I still think that it’s worth a try,” Dr. Okoye commented. “Many of our HS patients already are dealing with depression because of their disease. ... In 3 months, we talk about their symptoms, [and] make sure that they’re feeling okay before continuing.”
While finasteride is not appropriate for women of childbearing potential (pregnancy category X), it can be an option for women with HS who are of childbearing age but are not at risk for becoming pregnant, Dr. Okoye added, which can be determined by discussing a patient’s family planning goals. For example, she said, “if you have a woman of childbearing age but she’s in a same-sex relationship and has no intention of having children, then maybe finasteride is an option for her.”
The mineralocorticoid- and aldosterone-receptor antagonist spironolactone, used off label for acne treatment, also has antiandrogenic properties and is an option for patients with HS “at the higher end of the dosing spectrum” with 100-200 mg daily. However, Dr. Okoye referred to a recently published single-center retrospective study that showed a low daily dose of 75 mg was effective for HS (J Am Acad Dermatol. 2019 Jan;80[1]:114-9).
While spironolactone increases the risk of hyperkalemia, in patients with no preexisting renal disease under 50 years of age, monitoring is not necessary because there is little to no risk of clinical hyperkalemia in these patients, she said. Combining spironolactone or finasteride with OCs may increase antiandrogenic activity, she noted.
The data on effectiveness of hormonal contraceptives are mixed with regard to treatment of HS, with some studies showing benefit or worsening of the disease with OC use. “I think one of the reasons the data is so ‘dirty’ is because OCs range widely in terms of their ingredients and in terms of how androgenic their progesterones are,” Dr. Okoye commented.
OCs increase the risk of venous thromboembolism (VTE), but Dr. Okoye noted the risk is less than a patient would experience during pregnancy. “When you talk to dermatologists, there are two camps: some dermatologists who are very comfortable prescribing OCs, and dermatologists who prefer not to, given the risk of VTEs,” she said. However, risk should also be applied to patient population and location, she noted.
“If you are in an area [where] you serve a patient population that has fewer options for access to care, and if you don’t prescribe the OCs, those patients have to wait several months before getting on therapy, said Dr. Okoye. “Maybe that’s a case where you might want to start the OC [with] one or two refills while they find an OB, but it’s really up to you and your risk aversion.”
Dietary factors may also contribute to HS, but more studies are needed to analyze how sugar and carbohydrates contribute to the condition. Instead of taking for granted that a patient will understand what reducing dietary carbohydrate and sugar intake means, Dr. Okoye said, “I like to get very specific; ask them what they’re drinking on a daily basis.”
With regard to weight loss, there is little to link significant weight loss and symptom improvement. However, weight loss could help with comorbid conditions in patients with HS, like metabolic syndrome, and subsequent skin reduction may reduce friction of intertriginous areas, she pointed out.
Dr. Okoye reports receiving grants and/or research funding from Eli Lilly.
EXPERT ANALYSIS FROM AAD 19
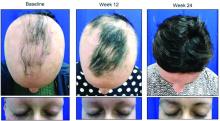
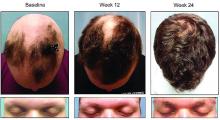
Deuterium-altered ruxolitinib may be an effective treatment for alopecia areata
WASHINGTON – Yet another inhibitor of the Janus kinase enzyme has debuted with positive phase 2 results for patients with even longstanding alopecia areata.
About half of those who took 8 mg of CPT-543, a chemically altered form of ruxolitinib, twice a day for 24 weeks, regrew hair – including eyebrows and eyelashes – by at least 50%. The dose-ranging study also found that a 4 mg twice-daily dose promoted the same growth in 21%, James V. Cassella, PhD, said during a late breaking clinical trials session at the annual meeting of the American Academy of Dermatology.
Adverse events were mild, including headache, reported in 26% of the 8 mg group. However, investigators “are keeping an eye” on infections and blood chemistry, and in light of the confirmed increased risk of herpes zoster and suspected increased risk of thromboembolic events with ??ruxolitinib, said Dr. Cassella, chief development officer of Concert Pharmaceuticals, which is developing the molecule.
Ruxolitinib, an inhibitor of both JAK1 and JAK2, is available under the name Jakafi and is approved for the treatment of myelofibrosis and polycythemia vera. The addition of deuterium slows its metabolism, increasing half-life and bioavailability without affecting receptor selectivity or potency, according to the company.
The company’s (35). The primary endpoint was the proportion of patients with at least a 50% relative reduction in scalp hair loss as measured by the Severity of Alopecia Tool (SALT) at 24 weeks, from baseline. Secondarily, the trial examined response by alopecia subtype (patchy or complete) and individual SALT changes compared with baseline.
Patients were generally in their mid-30s, and about 75% were female. The mean current alopecia episode was about 5 years. The mean SALT score at baseline was about 89, with 100 being complete hair loss.
By week 24, 47% of those taking 8 mg twice daily and 21% of those taking 4 mg twice daily experienced the primary endpoint of at least 50% SALT reduction from baseline. Almost 9% of those taking placebo also hit the target. These patients all had patchy alopecia and may have been coming out of an alopecia episode during the trial, Dr. Cassella said.
Week 12 was the inflection point for response division, with both active groups significantly outperforming the placebo group. By week 16, 30% of the 8 mg group and about 15% of the 4 mg group had already hit the primary endpoint. Response in the 4 mg group climbed slowly until the end of the trial, while in the 8 mg group, response ascended more quickly. Response was still trending upward when the study stopped.
“We think we have not hit the ceiling effect with this drug,” Dr. Cassella said. “There is some evidence that response would continue to increase after 24 weeks.”
Patchy alopecia and alopecia universalis appeared to respond best to treatment in both dosage groups. There was no response in either group for patients with alopecia ophiasis or totalis.
Headache was the most common adverse event, and appeared to be dose-dependent, occurring in 11% of placebo patients, 17% of the 4 mg group, and 26% of the 8 mg group. Six patients developed increased blood creatinine phosphokinase levels (one in the placebo group, three in the 4 mg group, and two in the 8 mg group). There were no thromboembolic events. Three patients in the placebo group and two in the 8 mg group discontinued the medication due to unspecified adverse events.
In early March, the company announced an open-label dose-finding study, which will randomize 60 patients with moderate-to-severe alopecia areata to either 8 mg twice daily or 16 mg once daily over a 24-week treatment period. Concert intends to conduct a food-effect trial to assess the relative bioavailability of oral doses of CTP-543 under fasted and fed conditions in 14 healthy volunteers in the first half of 2019.
SOURCE: Casella J. AAD 2019; S034, Abstract 11291.
WASHINGTON – Yet another inhibitor of the Janus kinase enzyme has debuted with positive phase 2 results for patients with even longstanding alopecia areata.
About half of those who took 8 mg of CPT-543, a chemically altered form of ruxolitinib, twice a day for 24 weeks, regrew hair – including eyebrows and eyelashes – by at least 50%. The dose-ranging study also found that a 4 mg twice-daily dose promoted the same growth in 21%, James V. Cassella, PhD, said during a late breaking clinical trials session at the annual meeting of the American Academy of Dermatology.
Adverse events were mild, including headache, reported in 26% of the 8 mg group. However, investigators “are keeping an eye” on infections and blood chemistry, and in light of the confirmed increased risk of herpes zoster and suspected increased risk of thromboembolic events with ??ruxolitinib, said Dr. Cassella, chief development officer of Concert Pharmaceuticals, which is developing the molecule.
Ruxolitinib, an inhibitor of both JAK1 and JAK2, is available under the name Jakafi and is approved for the treatment of myelofibrosis and polycythemia vera. The addition of deuterium slows its metabolism, increasing half-life and bioavailability without affecting receptor selectivity or potency, according to the company.
The company’s (35). The primary endpoint was the proportion of patients with at least a 50% relative reduction in scalp hair loss as measured by the Severity of Alopecia Tool (SALT) at 24 weeks, from baseline. Secondarily, the trial examined response by alopecia subtype (patchy or complete) and individual SALT changes compared with baseline.
Patients were generally in their mid-30s, and about 75% were female. The mean current alopecia episode was about 5 years. The mean SALT score at baseline was about 89, with 100 being complete hair loss.
By week 24, 47% of those taking 8 mg twice daily and 21% of those taking 4 mg twice daily experienced the primary endpoint of at least 50% SALT reduction from baseline. Almost 9% of those taking placebo also hit the target. These patients all had patchy alopecia and may have been coming out of an alopecia episode during the trial, Dr. Cassella said.
Week 12 was the inflection point for response division, with both active groups significantly outperforming the placebo group. By week 16, 30% of the 8 mg group and about 15% of the 4 mg group had already hit the primary endpoint. Response in the 4 mg group climbed slowly until the end of the trial, while in the 8 mg group, response ascended more quickly. Response was still trending upward when the study stopped.
“We think we have not hit the ceiling effect with this drug,” Dr. Cassella said. “There is some evidence that response would continue to increase after 24 weeks.”
Patchy alopecia and alopecia universalis appeared to respond best to treatment in both dosage groups. There was no response in either group for patients with alopecia ophiasis or totalis.
Headache was the most common adverse event, and appeared to be dose-dependent, occurring in 11% of placebo patients, 17% of the 4 mg group, and 26% of the 8 mg group. Six patients developed increased blood creatinine phosphokinase levels (one in the placebo group, three in the 4 mg group, and two in the 8 mg group). There were no thromboembolic events. Three patients in the placebo group and two in the 8 mg group discontinued the medication due to unspecified adverse events.
In early March, the company announced an open-label dose-finding study, which will randomize 60 patients with moderate-to-severe alopecia areata to either 8 mg twice daily or 16 mg once daily over a 24-week treatment period. Concert intends to conduct a food-effect trial to assess the relative bioavailability of oral doses of CTP-543 under fasted and fed conditions in 14 healthy volunteers in the first half of 2019.
SOURCE: Casella J. AAD 2019; S034, Abstract 11291.
WASHINGTON – Yet another inhibitor of the Janus kinase enzyme has debuted with positive phase 2 results for patients with even longstanding alopecia areata.
About half of those who took 8 mg of CPT-543, a chemically altered form of ruxolitinib, twice a day for 24 weeks, regrew hair – including eyebrows and eyelashes – by at least 50%. The dose-ranging study also found that a 4 mg twice-daily dose promoted the same growth in 21%, James V. Cassella, PhD, said during a late breaking clinical trials session at the annual meeting of the American Academy of Dermatology.
Adverse events were mild, including headache, reported in 26% of the 8 mg group. However, investigators “are keeping an eye” on infections and blood chemistry, and in light of the confirmed increased risk of herpes zoster and suspected increased risk of thromboembolic events with ??ruxolitinib, said Dr. Cassella, chief development officer of Concert Pharmaceuticals, which is developing the molecule.
Ruxolitinib, an inhibitor of both JAK1 and JAK2, is available under the name Jakafi and is approved for the treatment of myelofibrosis and polycythemia vera. The addition of deuterium slows its metabolism, increasing half-life and bioavailability without affecting receptor selectivity or potency, according to the company.
The company’s (35). The primary endpoint was the proportion of patients with at least a 50% relative reduction in scalp hair loss as measured by the Severity of Alopecia Tool (SALT) at 24 weeks, from baseline. Secondarily, the trial examined response by alopecia subtype (patchy or complete) and individual SALT changes compared with baseline.
Patients were generally in their mid-30s, and about 75% were female. The mean current alopecia episode was about 5 years. The mean SALT score at baseline was about 89, with 100 being complete hair loss.
By week 24, 47% of those taking 8 mg twice daily and 21% of those taking 4 mg twice daily experienced the primary endpoint of at least 50% SALT reduction from baseline. Almost 9% of those taking placebo also hit the target. These patients all had patchy alopecia and may have been coming out of an alopecia episode during the trial, Dr. Cassella said.
Week 12 was the inflection point for response division, with both active groups significantly outperforming the placebo group. By week 16, 30% of the 8 mg group and about 15% of the 4 mg group had already hit the primary endpoint. Response in the 4 mg group climbed slowly until the end of the trial, while in the 8 mg group, response ascended more quickly. Response was still trending upward when the study stopped.
“We think we have not hit the ceiling effect with this drug,” Dr. Cassella said. “There is some evidence that response would continue to increase after 24 weeks.”
Patchy alopecia and alopecia universalis appeared to respond best to treatment in both dosage groups. There was no response in either group for patients with alopecia ophiasis or totalis.
Headache was the most common adverse event, and appeared to be dose-dependent, occurring in 11% of placebo patients, 17% of the 4 mg group, and 26% of the 8 mg group. Six patients developed increased blood creatinine phosphokinase levels (one in the placebo group, three in the 4 mg group, and two in the 8 mg group). There were no thromboembolic events. Three patients in the placebo group and two in the 8 mg group discontinued the medication due to unspecified adverse events.
In early March, the company announced an open-label dose-finding study, which will randomize 60 patients with moderate-to-severe alopecia areata to either 8 mg twice daily or 16 mg once daily over a 24-week treatment period. Concert intends to conduct a food-effect trial to assess the relative bioavailability of oral doses of CTP-543 under fasted and fed conditions in 14 healthy volunteers in the first half of 2019.
SOURCE: Casella J. AAD 2019; S034, Abstract 11291.
REPORTING FROM AAD 19
The 39th ASLMS meeting is now underway
WASHINGTON – At the annual meeting of the American Academy of Dermatology, the taking place March 27-31, 2019, in Denver.

“ASLMS is always an amazing meeting, and it’s a unique meeting,” said past president Mathew Avram, MD, director of the Dermatology Laser & Cosmetic Center at Massachusetts General Hospital, Boston. “At its core, it’s a scientific meeting ... you can take things back to your practice that change the practice of medicine.”
Current ASLMS president Eric Bernstein, MD, of Main Line Center for Laser Surgery, Ardmore, Pa., pointed out that, in addition to doctors and other health care practitioners, other available and accessible attendees include the engineers who build the lasers. And this year, injectables are being incorporated into the program.
MDedge reporter Doug Brunk will be reporting from the meeting.
WASHINGTON – At the annual meeting of the American Academy of Dermatology, the taking place March 27-31, 2019, in Denver.

“ASLMS is always an amazing meeting, and it’s a unique meeting,” said past president Mathew Avram, MD, director of the Dermatology Laser & Cosmetic Center at Massachusetts General Hospital, Boston. “At its core, it’s a scientific meeting ... you can take things back to your practice that change the practice of medicine.”
Current ASLMS president Eric Bernstein, MD, of Main Line Center for Laser Surgery, Ardmore, Pa., pointed out that, in addition to doctors and other health care practitioners, other available and accessible attendees include the engineers who build the lasers. And this year, injectables are being incorporated into the program.
MDedge reporter Doug Brunk will be reporting from the meeting.
WASHINGTON – At the annual meeting of the American Academy of Dermatology, the taking place March 27-31, 2019, in Denver.

“ASLMS is always an amazing meeting, and it’s a unique meeting,” said past president Mathew Avram, MD, director of the Dermatology Laser & Cosmetic Center at Massachusetts General Hospital, Boston. “At its core, it’s a scientific meeting ... you can take things back to your practice that change the practice of medicine.”
Current ASLMS president Eric Bernstein, MD, of Main Line Center for Laser Surgery, Ardmore, Pa., pointed out that, in addition to doctors and other health care practitioners, other available and accessible attendees include the engineers who build the lasers. And this year, injectables are being incorporated into the program.
MDedge reporter Doug Brunk will be reporting from the meeting.
REPORTING FROM AAD 2019
Spending on combination products for acne has increased significantly
WASHINGTON – Expenditures for combination acne products increased from $82 million in 1996 to $487 million in 2016, according to an analysis presented at the annual meeting of the American Academy of Dermatology.
During a late-breaking research session, David Li, a fourth-year medical student at Tufts University, Boston, presented the results of a retrospective cost analysis study, conducted to identify trends in overall spending for combination acne products. Spending measures were adjusted for inflation to 2016 U.S. dollars.
he noted. Combination products are more expensive than the sum of their component parts, and prescribing generic formulations of individual acne treatments could potentially reduce costs, but possible advantages of combination products for acne include improved patient adherence and increased efficacy.
Mr. Li, a research fellow in the department of dermatology at Brigham and Women’s Hospital in Boston, and his colleagues drafted a comprehensive list of available combination products (eight brand-name products and two generics). Most combine benzoyl peroxide with another common acne medication. To analyze trends in medication use, they performed a retrospective cost analysis using Medical Expenditure Panel Survey (MEPS) data from 1996 to 2016, searching for these combination medications to gather the annual number of prescriptions, number of users, expenditures, and aggregate demographics for each product. The data were weighted to represent national estimates.
They also used data from the National Average Drug Acquisition Cost (NADAC) database, used by the Centers for Medicare & Medicaid Services as a pricing benchmark, and calculated the difference between the unit price of each combination product and the sum of the prices of its generic components. They multiplied this difference by the median number of units prescribed annually for the given combination.
The researchers found that most users of combination acne products were younger than 18 years (23%), female (55%), and white (83%), and the most commonly prescribed combination product changed over time. From 1996 to 2002, Benzamycin (benzoyl peroxide and erythromycin) was the most frequently prescribed combination. Several years later, its place was taken by BenzaClin (benzoyl peroxide and clindamycin) from 2003 to 2010, followed by Ziana (clindamycin and tretinoin) in 2011 and Epiduo (adapalene and benzoyl peroxide) from 2012 to 2016.
“Spending has increased steadily from a little bit over $82 million in 1996 to nearly half a billion dollars in 2016,” Mr. Li said. “That’s a rise of more than 500% in the last 20 years. Based on the median pricing and utilization data that we derived from the NADAC database, we determined that substitution with component generics can provide median annual savings of at least a quarter billion dollars each year.”
Although the data indicate a trend toward increased use of and spending on new, branded combination products, the literature includes “minimal data to suggest whether one combination acne product is better than the next one, or how it compares to its component medications when used in combination,” he said. He and his colleagues found no comparative data apart from a 2001 study that examined Benzamycin and BenzaClin, which suggested that there was no difference in efficacy or tolerability between the products.
The present study is limited by reporting bias and recall bias because it relies partly on MEPS, a survey, and the NADAC pricing database had information only for 2013-2016. The researchers consequently used the most recent prices to calculate potential savings.
“Until we have more meaningful data to suggest otherwise, we’re in a state of equipoise,” said Mr. Li.
The research was funded by the National Center for Advancing Translational Sciences, part of the National Institutes of Health.
SOURCE: Li D et al. AAD 2019, Abstract 11333.
WASHINGTON – Expenditures for combination acne products increased from $82 million in 1996 to $487 million in 2016, according to an analysis presented at the annual meeting of the American Academy of Dermatology.
During a late-breaking research session, David Li, a fourth-year medical student at Tufts University, Boston, presented the results of a retrospective cost analysis study, conducted to identify trends in overall spending for combination acne products. Spending measures were adjusted for inflation to 2016 U.S. dollars.
he noted. Combination products are more expensive than the sum of their component parts, and prescribing generic formulations of individual acne treatments could potentially reduce costs, but possible advantages of combination products for acne include improved patient adherence and increased efficacy.
Mr. Li, a research fellow in the department of dermatology at Brigham and Women’s Hospital in Boston, and his colleagues drafted a comprehensive list of available combination products (eight brand-name products and two generics). Most combine benzoyl peroxide with another common acne medication. To analyze trends in medication use, they performed a retrospective cost analysis using Medical Expenditure Panel Survey (MEPS) data from 1996 to 2016, searching for these combination medications to gather the annual number of prescriptions, number of users, expenditures, and aggregate demographics for each product. The data were weighted to represent national estimates.
They also used data from the National Average Drug Acquisition Cost (NADAC) database, used by the Centers for Medicare & Medicaid Services as a pricing benchmark, and calculated the difference between the unit price of each combination product and the sum of the prices of its generic components. They multiplied this difference by the median number of units prescribed annually for the given combination.
The researchers found that most users of combination acne products were younger than 18 years (23%), female (55%), and white (83%), and the most commonly prescribed combination product changed over time. From 1996 to 2002, Benzamycin (benzoyl peroxide and erythromycin) was the most frequently prescribed combination. Several years later, its place was taken by BenzaClin (benzoyl peroxide and clindamycin) from 2003 to 2010, followed by Ziana (clindamycin and tretinoin) in 2011 and Epiduo (adapalene and benzoyl peroxide) from 2012 to 2016.
“Spending has increased steadily from a little bit over $82 million in 1996 to nearly half a billion dollars in 2016,” Mr. Li said. “That’s a rise of more than 500% in the last 20 years. Based on the median pricing and utilization data that we derived from the NADAC database, we determined that substitution with component generics can provide median annual savings of at least a quarter billion dollars each year.”
Although the data indicate a trend toward increased use of and spending on new, branded combination products, the literature includes “minimal data to suggest whether one combination acne product is better than the next one, or how it compares to its component medications when used in combination,” he said. He and his colleagues found no comparative data apart from a 2001 study that examined Benzamycin and BenzaClin, which suggested that there was no difference in efficacy or tolerability between the products.
The present study is limited by reporting bias and recall bias because it relies partly on MEPS, a survey, and the NADAC pricing database had information only for 2013-2016. The researchers consequently used the most recent prices to calculate potential savings.
“Until we have more meaningful data to suggest otherwise, we’re in a state of equipoise,” said Mr. Li.
The research was funded by the National Center for Advancing Translational Sciences, part of the National Institutes of Health.
SOURCE: Li D et al. AAD 2019, Abstract 11333.
WASHINGTON – Expenditures for combination acne products increased from $82 million in 1996 to $487 million in 2016, according to an analysis presented at the annual meeting of the American Academy of Dermatology.
During a late-breaking research session, David Li, a fourth-year medical student at Tufts University, Boston, presented the results of a retrospective cost analysis study, conducted to identify trends in overall spending for combination acne products. Spending measures were adjusted for inflation to 2016 U.S. dollars.
he noted. Combination products are more expensive than the sum of their component parts, and prescribing generic formulations of individual acne treatments could potentially reduce costs, but possible advantages of combination products for acne include improved patient adherence and increased efficacy.
Mr. Li, a research fellow in the department of dermatology at Brigham and Women’s Hospital in Boston, and his colleagues drafted a comprehensive list of available combination products (eight brand-name products and two generics). Most combine benzoyl peroxide with another common acne medication. To analyze trends in medication use, they performed a retrospective cost analysis using Medical Expenditure Panel Survey (MEPS) data from 1996 to 2016, searching for these combination medications to gather the annual number of prescriptions, number of users, expenditures, and aggregate demographics for each product. The data were weighted to represent national estimates.
They also used data from the National Average Drug Acquisition Cost (NADAC) database, used by the Centers for Medicare & Medicaid Services as a pricing benchmark, and calculated the difference between the unit price of each combination product and the sum of the prices of its generic components. They multiplied this difference by the median number of units prescribed annually for the given combination.
The researchers found that most users of combination acne products were younger than 18 years (23%), female (55%), and white (83%), and the most commonly prescribed combination product changed over time. From 1996 to 2002, Benzamycin (benzoyl peroxide and erythromycin) was the most frequently prescribed combination. Several years later, its place was taken by BenzaClin (benzoyl peroxide and clindamycin) from 2003 to 2010, followed by Ziana (clindamycin and tretinoin) in 2011 and Epiduo (adapalene and benzoyl peroxide) from 2012 to 2016.
“Spending has increased steadily from a little bit over $82 million in 1996 to nearly half a billion dollars in 2016,” Mr. Li said. “That’s a rise of more than 500% in the last 20 years. Based on the median pricing and utilization data that we derived from the NADAC database, we determined that substitution with component generics can provide median annual savings of at least a quarter billion dollars each year.”
Although the data indicate a trend toward increased use of and spending on new, branded combination products, the literature includes “minimal data to suggest whether one combination acne product is better than the next one, or how it compares to its component medications when used in combination,” he said. He and his colleagues found no comparative data apart from a 2001 study that examined Benzamycin and BenzaClin, which suggested that there was no difference in efficacy or tolerability between the products.
The present study is limited by reporting bias and recall bias because it relies partly on MEPS, a survey, and the NADAC pricing database had information only for 2013-2016. The researchers consequently used the most recent prices to calculate potential savings.
“Until we have more meaningful data to suggest otherwise, we’re in a state of equipoise,” said Mr. Li.
The research was funded by the National Center for Advancing Translational Sciences, part of the National Institutes of Health.
SOURCE: Li D et al. AAD 2019, Abstract 11333.
REPORTING FROM AAD 2019
TNF inhibitor–induced psoriasis in IBD patients a consideration
WASHINGTON – (IBD), Sophia Delano, MD, said during a session on the cutaneous effects of IBD at the annual meeting of the American Academy of Dermatology.
This is a paradoxical reaction, which can happen “weeks to years after starting a TNF blocker,” with about 70% of cases occurring during the first year of therapy, said Dr. Delano, an attending physician in the dermatology program at Boston Children’s Hospital.
Those receiving infliximab are more likely to develop TNF inhibitor–induced psoriasis, compared with those on adalimumab or etanercept. TNF inhibitor–induced psoriasis may not track with gastrointestinal activity, and some patients whose gastrointestinal disease is responding to treatment can begin to develop psoriasis, she noted.
The clinical presentation of TNF inhibitor–induced psoriasis can also vary. In one study of 216 cases, 26.9% of patients had a mixed morphology, with the most common presentations including plaque psoriasis (44.8%) and palmoplantar pustular psoriasis (36.3%). Other presentations were psoriasiform dermatitis (19.9%), scalp involvement with alopecia (7.5%), and generalized pustular psoriasis (10.9%). Locations affected were the soles of the feet (45.8%), extremities (45.4%), palms (44.9%), scalp (36.1%), and trunk (32.4%), Dr. Delano said.
TNF inhibitor–induced psoriasis is likely a class effect, she said, noting that, in the same review, symptoms resolved in 47.7% of patients who discontinued TNF inhibitors, in 36.7% of patients who switched to another TNF inhibitor, and in 32.9% of patients who continued their original therapy (J Am Acad Dermatol. 2017 Feb;76[2]:334-41). In the study, Crohn’s disease and RA were the most common diseases, in 40.7% and 37.0% of the patients, respectively.
There have been case reports of TNF antagonist–induced lupus-like syndrome (TAILS), which is more common in patients with RA and ulcerative colitis. TAILS occurs more often in women than in men; can present similarly to systemic lupus erythematosus, subacute cutaneous lupus erythematosus, and chronic cutaneous lupus; and resolves by stopping TNF inhibitor treatment, Dr. Delano said.
Skin cancer risk, infections, and injection site reactions
Both adult and pediatric patients treated with TNF inhibitors for IBD may be at increased risk for lymphoma, visceral tumors, melanoma, and nonmelanoma skin cancers. Dr. Delano referred to a study published in 2014, which identified 972 reports of melanoma in the Food and Drug Administration’s Adverse Event Reporting System database associated with TNF inhibitor use; of these, 69 cases involved patients using more than one TNF inhibitor. Infliximab, golimumab, etanercept, and adalimumab were associated with a safety signal for melanoma, but not certolizumab (Br J Dermatol. 2014 May;170[5]:1170-2).
Dr. Delano observed that thiopurines such as azathioprine are also associated with an increased cancer risk, as noted in one retrospective study that found that the risk of nonmelanoma skin cancer was 2.1 times higher in a mostly white male cohort with ulcerative colitis during treatment with thiopurines, compared with patients not treated with thiopurines (Am J Gastroenterol. 2014 Nov;109[11]:1781-93). A greater duration of treatment (more than 6 months) and higher doses were associated with higher risks.
Adalimumab, golimumab, and certolizumab can also cause injection site reactions, typically within 1- 2 days of injection, said Dr. Delano. In these cases, symptoms of erythema, warmth, burning, or pruritus are worse at the beginning of treatment and can be relieved by rotating the injection site as well as providing cool compresses, topical steroids, antihistamines, and supportive care.
“If you have a patient with a worsening reaction, consider it may represent the type 1 IgE-related hypersensitivity requiring desensitization to continue that systemic,” she noted.
Cutaneous bacterial, fungal, and viral infections such as molluscum contagiosum, verruca vulgaris, herpes simplex, and varicella zoster can occur as a result of TNF inhibition as well, and can be difficult to clear because of immunosuppression, she added.
Dr. Delano reported no relevant conflicts of interest.
WASHINGTON – (IBD), Sophia Delano, MD, said during a session on the cutaneous effects of IBD at the annual meeting of the American Academy of Dermatology.
This is a paradoxical reaction, which can happen “weeks to years after starting a TNF blocker,” with about 70% of cases occurring during the first year of therapy, said Dr. Delano, an attending physician in the dermatology program at Boston Children’s Hospital.
Those receiving infliximab are more likely to develop TNF inhibitor–induced psoriasis, compared with those on adalimumab or etanercept. TNF inhibitor–induced psoriasis may not track with gastrointestinal activity, and some patients whose gastrointestinal disease is responding to treatment can begin to develop psoriasis, she noted.
The clinical presentation of TNF inhibitor–induced psoriasis can also vary. In one study of 216 cases, 26.9% of patients had a mixed morphology, with the most common presentations including plaque psoriasis (44.8%) and palmoplantar pustular psoriasis (36.3%). Other presentations were psoriasiform dermatitis (19.9%), scalp involvement with alopecia (7.5%), and generalized pustular psoriasis (10.9%). Locations affected were the soles of the feet (45.8%), extremities (45.4%), palms (44.9%), scalp (36.1%), and trunk (32.4%), Dr. Delano said.
TNF inhibitor–induced psoriasis is likely a class effect, she said, noting that, in the same review, symptoms resolved in 47.7% of patients who discontinued TNF inhibitors, in 36.7% of patients who switched to another TNF inhibitor, and in 32.9% of patients who continued their original therapy (J Am Acad Dermatol. 2017 Feb;76[2]:334-41). In the study, Crohn’s disease and RA were the most common diseases, in 40.7% and 37.0% of the patients, respectively.
There have been case reports of TNF antagonist–induced lupus-like syndrome (TAILS), which is more common in patients with RA and ulcerative colitis. TAILS occurs more often in women than in men; can present similarly to systemic lupus erythematosus, subacute cutaneous lupus erythematosus, and chronic cutaneous lupus; and resolves by stopping TNF inhibitor treatment, Dr. Delano said.
Skin cancer risk, infections, and injection site reactions
Both adult and pediatric patients treated with TNF inhibitors for IBD may be at increased risk for lymphoma, visceral tumors, melanoma, and nonmelanoma skin cancers. Dr. Delano referred to a study published in 2014, which identified 972 reports of melanoma in the Food and Drug Administration’s Adverse Event Reporting System database associated with TNF inhibitor use; of these, 69 cases involved patients using more than one TNF inhibitor. Infliximab, golimumab, etanercept, and adalimumab were associated with a safety signal for melanoma, but not certolizumab (Br J Dermatol. 2014 May;170[5]:1170-2).
Dr. Delano observed that thiopurines such as azathioprine are also associated with an increased cancer risk, as noted in one retrospective study that found that the risk of nonmelanoma skin cancer was 2.1 times higher in a mostly white male cohort with ulcerative colitis during treatment with thiopurines, compared with patients not treated with thiopurines (Am J Gastroenterol. 2014 Nov;109[11]:1781-93). A greater duration of treatment (more than 6 months) and higher doses were associated with higher risks.
Adalimumab, golimumab, and certolizumab can also cause injection site reactions, typically within 1- 2 days of injection, said Dr. Delano. In these cases, symptoms of erythema, warmth, burning, or pruritus are worse at the beginning of treatment and can be relieved by rotating the injection site as well as providing cool compresses, topical steroids, antihistamines, and supportive care.
“If you have a patient with a worsening reaction, consider it may represent the type 1 IgE-related hypersensitivity requiring desensitization to continue that systemic,” she noted.
Cutaneous bacterial, fungal, and viral infections such as molluscum contagiosum, verruca vulgaris, herpes simplex, and varicella zoster can occur as a result of TNF inhibition as well, and can be difficult to clear because of immunosuppression, she added.
Dr. Delano reported no relevant conflicts of interest.
WASHINGTON – (IBD), Sophia Delano, MD, said during a session on the cutaneous effects of IBD at the annual meeting of the American Academy of Dermatology.
This is a paradoxical reaction, which can happen “weeks to years after starting a TNF blocker,” with about 70% of cases occurring during the first year of therapy, said Dr. Delano, an attending physician in the dermatology program at Boston Children’s Hospital.
Those receiving infliximab are more likely to develop TNF inhibitor–induced psoriasis, compared with those on adalimumab or etanercept. TNF inhibitor–induced psoriasis may not track with gastrointestinal activity, and some patients whose gastrointestinal disease is responding to treatment can begin to develop psoriasis, she noted.
The clinical presentation of TNF inhibitor–induced psoriasis can also vary. In one study of 216 cases, 26.9% of patients had a mixed morphology, with the most common presentations including plaque psoriasis (44.8%) and palmoplantar pustular psoriasis (36.3%). Other presentations were psoriasiform dermatitis (19.9%), scalp involvement with alopecia (7.5%), and generalized pustular psoriasis (10.9%). Locations affected were the soles of the feet (45.8%), extremities (45.4%), palms (44.9%), scalp (36.1%), and trunk (32.4%), Dr. Delano said.
TNF inhibitor–induced psoriasis is likely a class effect, she said, noting that, in the same review, symptoms resolved in 47.7% of patients who discontinued TNF inhibitors, in 36.7% of patients who switched to another TNF inhibitor, and in 32.9% of patients who continued their original therapy (J Am Acad Dermatol. 2017 Feb;76[2]:334-41). In the study, Crohn’s disease and RA were the most common diseases, in 40.7% and 37.0% of the patients, respectively.
There have been case reports of TNF antagonist–induced lupus-like syndrome (TAILS), which is more common in patients with RA and ulcerative colitis. TAILS occurs more often in women than in men; can present similarly to systemic lupus erythematosus, subacute cutaneous lupus erythematosus, and chronic cutaneous lupus; and resolves by stopping TNF inhibitor treatment, Dr. Delano said.
Skin cancer risk, infections, and injection site reactions
Both adult and pediatric patients treated with TNF inhibitors for IBD may be at increased risk for lymphoma, visceral tumors, melanoma, and nonmelanoma skin cancers. Dr. Delano referred to a study published in 2014, which identified 972 reports of melanoma in the Food and Drug Administration’s Adverse Event Reporting System database associated with TNF inhibitor use; of these, 69 cases involved patients using more than one TNF inhibitor. Infliximab, golimumab, etanercept, and adalimumab were associated with a safety signal for melanoma, but not certolizumab (Br J Dermatol. 2014 May;170[5]:1170-2).
Dr. Delano observed that thiopurines such as azathioprine are also associated with an increased cancer risk, as noted in one retrospective study that found that the risk of nonmelanoma skin cancer was 2.1 times higher in a mostly white male cohort with ulcerative colitis during treatment with thiopurines, compared with patients not treated with thiopurines (Am J Gastroenterol. 2014 Nov;109[11]:1781-93). A greater duration of treatment (more than 6 months) and higher doses were associated with higher risks.
Adalimumab, golimumab, and certolizumab can also cause injection site reactions, typically within 1- 2 days of injection, said Dr. Delano. In these cases, symptoms of erythema, warmth, burning, or pruritus are worse at the beginning of treatment and can be relieved by rotating the injection site as well as providing cool compresses, topical steroids, antihistamines, and supportive care.
“If you have a patient with a worsening reaction, consider it may represent the type 1 IgE-related hypersensitivity requiring desensitization to continue that systemic,” she noted.
Cutaneous bacterial, fungal, and viral infections such as molluscum contagiosum, verruca vulgaris, herpes simplex, and varicella zoster can occur as a result of TNF inhibition as well, and can be difficult to clear because of immunosuppression, she added.
Dr. Delano reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM AAD 2019
Acne take home messages from the AAD annual meeting
WASHINGTON – In an interview at the close of the annual meeting of the American Academy of Dermatology, .
Both Dr. Harper, who practices in Birmingham, Ala., and is the immediate past president of the American Acne & Rosacea Society, and Dr. Keri, of the department of dermatology at the University of Miami and the Miami VA Healthcare System, spoke during several acne sessions. Among the topics they discussed during the interview were a relatively recent meta-analysis that provides reassuring information about depression and isotretinoin, how to start patients on spironolactone, and the use of antibiotics – and benzoyl peroxide.
They emphasized the importance of not withholding treatment for patients who need it and the psychosocial impact of acne. “Patients need to get to the treatment they need ... faster,” Dr. Harper said. “We want to treat sooner, and we want to prevent scarring,” Dr. Keri added.
Dr. Keri disclosed relationships with Hoffmann–La Roche, Ortho Dermatologics, and Pierre Fabre Dermatologie. Dr. Harper has no relevant financial disclosures.
WASHINGTON – In an interview at the close of the annual meeting of the American Academy of Dermatology, .
Both Dr. Harper, who practices in Birmingham, Ala., and is the immediate past president of the American Acne & Rosacea Society, and Dr. Keri, of the department of dermatology at the University of Miami and the Miami VA Healthcare System, spoke during several acne sessions. Among the topics they discussed during the interview were a relatively recent meta-analysis that provides reassuring information about depression and isotretinoin, how to start patients on spironolactone, and the use of antibiotics – and benzoyl peroxide.
They emphasized the importance of not withholding treatment for patients who need it and the psychosocial impact of acne. “Patients need to get to the treatment they need ... faster,” Dr. Harper said. “We want to treat sooner, and we want to prevent scarring,” Dr. Keri added.
Dr. Keri disclosed relationships with Hoffmann–La Roche, Ortho Dermatologics, and Pierre Fabre Dermatologie. Dr. Harper has no relevant financial disclosures.
WASHINGTON – In an interview at the close of the annual meeting of the American Academy of Dermatology, .
Both Dr. Harper, who practices in Birmingham, Ala., and is the immediate past president of the American Acne & Rosacea Society, and Dr. Keri, of the department of dermatology at the University of Miami and the Miami VA Healthcare System, spoke during several acne sessions. Among the topics they discussed during the interview were a relatively recent meta-analysis that provides reassuring information about depression and isotretinoin, how to start patients on spironolactone, and the use of antibiotics – and benzoyl peroxide.
They emphasized the importance of not withholding treatment for patients who need it and the psychosocial impact of acne. “Patients need to get to the treatment they need ... faster,” Dr. Harper said. “We want to treat sooner, and we want to prevent scarring,” Dr. Keri added.
Dr. Keri disclosed relationships with Hoffmann–La Roche, Ortho Dermatologics, and Pierre Fabre Dermatologie. Dr. Harper has no relevant financial disclosures.
Whether diet, vitamins, or supplements can benefit patients with vitiligo remains unclear
WASHINGTON – Many patients with vitiligo are interested in treating their condition with vitamins, supplements, or a modified diet, but research on whether these measures have an impact remains limited, Nada Elbuluk, MD, said at the annual meeting of the American Academy of Dermatology.
, “we need more well designed, controlled studies in the future to know where this belongs in our treatment armamentarium,” said Dr. Elbuluk of the department of dermatology at the University of Southern California, Los Angeles.
During a session at the AAD meeting, Dr. Elbuluk, who is also director of the pigmentary disorders clinic at USC, reviewed the evidence for the use of these adjunctive therapies in patients with vitiligo.
Vitamins
The pathogenesis of vitiligo includes the overproduction of reactive oxygen species and oxidative stress, factors that contribute to melanocyte damage and death. In addition, many patients with vitiligo are deficient in certain vitamins and minerals, the basis of the hypothesis that supplementation could be beneficial, according to Dr. Elbuluk.
Vitamin B12 and folic acid contribute to DNA repair, synthesis, and methylation, and researchers have hypothesized that these vitamins also play a role in melanin synthesis. In a review of the literature, Dr. Elbuluk and her colleagues found four studies that evaluated vitamin B12 and folic acid in vitiligo. In one study, a controlled trial in which patients took B12 and folic acid with and without phototherapy, the investigators observed no significant difference in repigmentation between groups. The other three studies were uncontrolled and thus provide an insufficient understanding of the effect of B12 and folic acid, said Dr. Elbuluk.
Vitamin D is involved in melanocyte and keratinocyte growth and differentiation, and inhibits T cell activation. Data indicate that low vitamin D levels are common in patients with vitiligo and comorbid autoimmune diseases. In one study, patients who received narrow-band UVB had an increase in vitamin D levels that could contribute to photo-induced melanogenesis, and an open-label study indicated that patients who took vitamin D daily (without phototherapy) for 6 months had an increase of repigmentation over time. “Topical vitamin D analogs have also been used in vitiligo treatment with varying success,” Dr. Elbuluk noted.
“I check vitamin D levels on my patients and make sure that they are within normal range. But I think the degree of supplementation and its role in vitiligo needs to be further elucidated,” she said. And because vitamin D is fat soluble, there is a risk of toxicity if a patient takes too much.
Vitamin C, vitamin E, and alpha-lipoic acid have antioxidant properties. In a double-blind, randomized, controlled trial, one group of patients took vitamins C and E and alpha-lipoic acid for 2 months before and during treatment with narrow-band UVB twice per week (Clin Exp Dermatol. 2007 Nov;32[6]:631-6). Another group underwent phototherapy without supplementation. A significantly greater proportion of patients who received the antioxidants obtained more than 75% repigmentation compared with those who did not. In another study, 73% of patients who received oral vitamin E and narrow band UVB phototherapy had marked to excellent repigmentation, compared with 55.6% of those who had phototherapy only (J Clin Pharmacol. 2009 Jul;49[7]:852-5).
The results of these studies support the idea that antioxidants can stabilize disease, reduce oxidative stress, and improve the effect of phototherapy, Dr. Elbuluk said.
Herbal supplements
Several research teams have examined Ginkgo biloba as a possible treatment for vitiligo. This plant is native to China and has antioxidant and anti-inflammatory properties; its most common side effect is gastrointestinal distress. Because it entails a risk of coagulopathy, it may not be appropriate for patients receiving anticoagulant treatment, Dr. Elbuluk pointed out. In a double-blinded, randomized, controlled trial comparing ginkgo biloba alone with placebo in patients with vitiligo, treatment was associated with cessation of active disease in most patients, and more than 40% of patients receiving ginkgo biloba had 75% repigmentation or more.
Polypodium leucotomos, a fern native to Central and South America, protects against UV radiation damage, modulates the immune system, and has anti-inflammatory and antioxidant effects. It has a good safety profile and is well tolerated at a dose of 240 mg/day, she said. It sometimes causes gastrointestinal discomfort or pruritus. Several randomized, controlled trials in patients with vitiligo showed that supplementation with polypodium leucotomos improves repigmentation, particularly in photo-exposed areas, she noted.
Khellin is an extract from the Mediterranean khella plant that is thought to stimulate melanocyte proliferation and melanogenesis. Several studies have examined khellin supplementation in combination with phototherapy. Khellin can be administered orally or topically and appears to be more beneficial than sunlight or phototherapy alone in stabilizing disease or inducing repigmentation. Oral khellin can cause many side effects, including nausea, transaminitis, and hypotension, so researchers have been more interested in using topical khellin as a liposomal vehicle to improve drug delivery, Dr. Elbuluk said.
Minerals
Some patients with vitiligo have deficiencies in zinc and copper. Zinc is an antioxidant that aids wound healing, protects against free radicals, supports melanogenesis, and possibly prevents melanocyte death, but can cause gastrointestinal irritation. Copper, too, is an antioxidant and coenzyme involved in melanogenesis. One study compared topical steroid treatment with and without oral zinc supplementation. Dual treatment was associated with greater repigmentation, but the difference was not statistically significant. No studies have examined copper supplementation, she said.
L-phenylalanine, diet, and green tea
Investigators have proposed that the amino acid L-phenylalanine, a precursor to tyrosine in the pathway of melanin synthesis, might interfere with antibody production against melanocytes. This supplement is administered orally by weight, typically in conjunction with phototherapy or sunlight. Various studies have observed positive outcomes of L-phenylalanine combined with phototherapy or sunlight. L-phenylalanine tends to be safe and has been administered to children with vitiligo.
Many patients with vitiligo “have already tried diets by the time they come to me,” said Dr. Elbuluk. No controlled studies have analyzed the role of diet in the prevention or treatment of vitiligo, but case reports describe gluten-free diets in this population, including one report of a patient with celiac disease whose vitiligo improved after adoption of such a diet. Another case report described a patient without celiac disease who had refractory acrofacial vitiligo, which improved after the adoption of a gluten-free diet. Evidence supports a gluten-free diet for patients with celiac disease, but does not support this challenging diet for people without celiac disease, she pointed out.
Green tea includes catechins, which have antioxidant and anti-inflammatory properties. Its main component is epigallocatechin gallate (EGCG), which is thought to modulate T cell mediated responses. In one animal study, administration of EGCG delayed the onset of vitiligo and decreased the area of depigmentation in a mouse model. Although these findings are promising, clinical trials are needed to determine whether EGCG is beneficial in humans with vitiligo, said Dr. Elbuluk.
The literature on diets and supplementation as treatments for vitiligo has several shortcomings, with studies that used heterogeneous methodologies, and many that used nonstandard outcome measures that have not been validated. Sample sizes often are small, and many trials are uncontrolled. “These limitations make it harder to make sense of the data and have take-home conclusions,” Dr. Elbuluk said.
She had no disclosures.
SOURCE: Elbuluk N. AAD 19, Session S002.
WASHINGTON – Many patients with vitiligo are interested in treating their condition with vitamins, supplements, or a modified diet, but research on whether these measures have an impact remains limited, Nada Elbuluk, MD, said at the annual meeting of the American Academy of Dermatology.
, “we need more well designed, controlled studies in the future to know where this belongs in our treatment armamentarium,” said Dr. Elbuluk of the department of dermatology at the University of Southern California, Los Angeles.
During a session at the AAD meeting, Dr. Elbuluk, who is also director of the pigmentary disorders clinic at USC, reviewed the evidence for the use of these adjunctive therapies in patients with vitiligo.
Vitamins
The pathogenesis of vitiligo includes the overproduction of reactive oxygen species and oxidative stress, factors that contribute to melanocyte damage and death. In addition, many patients with vitiligo are deficient in certain vitamins and minerals, the basis of the hypothesis that supplementation could be beneficial, according to Dr. Elbuluk.
Vitamin B12 and folic acid contribute to DNA repair, synthesis, and methylation, and researchers have hypothesized that these vitamins also play a role in melanin synthesis. In a review of the literature, Dr. Elbuluk and her colleagues found four studies that evaluated vitamin B12 and folic acid in vitiligo. In one study, a controlled trial in which patients took B12 and folic acid with and without phototherapy, the investigators observed no significant difference in repigmentation between groups. The other three studies were uncontrolled and thus provide an insufficient understanding of the effect of B12 and folic acid, said Dr. Elbuluk.
Vitamin D is involved in melanocyte and keratinocyte growth and differentiation, and inhibits T cell activation. Data indicate that low vitamin D levels are common in patients with vitiligo and comorbid autoimmune diseases. In one study, patients who received narrow-band UVB had an increase in vitamin D levels that could contribute to photo-induced melanogenesis, and an open-label study indicated that patients who took vitamin D daily (without phototherapy) for 6 months had an increase of repigmentation over time. “Topical vitamin D analogs have also been used in vitiligo treatment with varying success,” Dr. Elbuluk noted.
“I check vitamin D levels on my patients and make sure that they are within normal range. But I think the degree of supplementation and its role in vitiligo needs to be further elucidated,” she said. And because vitamin D is fat soluble, there is a risk of toxicity if a patient takes too much.
Vitamin C, vitamin E, and alpha-lipoic acid have antioxidant properties. In a double-blind, randomized, controlled trial, one group of patients took vitamins C and E and alpha-lipoic acid for 2 months before and during treatment with narrow-band UVB twice per week (Clin Exp Dermatol. 2007 Nov;32[6]:631-6). Another group underwent phototherapy without supplementation. A significantly greater proportion of patients who received the antioxidants obtained more than 75% repigmentation compared with those who did not. In another study, 73% of patients who received oral vitamin E and narrow band UVB phototherapy had marked to excellent repigmentation, compared with 55.6% of those who had phototherapy only (J Clin Pharmacol. 2009 Jul;49[7]:852-5).
The results of these studies support the idea that antioxidants can stabilize disease, reduce oxidative stress, and improve the effect of phototherapy, Dr. Elbuluk said.
Herbal supplements
Several research teams have examined Ginkgo biloba as a possible treatment for vitiligo. This plant is native to China and has antioxidant and anti-inflammatory properties; its most common side effect is gastrointestinal distress. Because it entails a risk of coagulopathy, it may not be appropriate for patients receiving anticoagulant treatment, Dr. Elbuluk pointed out. In a double-blinded, randomized, controlled trial comparing ginkgo biloba alone with placebo in patients with vitiligo, treatment was associated with cessation of active disease in most patients, and more than 40% of patients receiving ginkgo biloba had 75% repigmentation or more.
Polypodium leucotomos, a fern native to Central and South America, protects against UV radiation damage, modulates the immune system, and has anti-inflammatory and antioxidant effects. It has a good safety profile and is well tolerated at a dose of 240 mg/day, she said. It sometimes causes gastrointestinal discomfort or pruritus. Several randomized, controlled trials in patients with vitiligo showed that supplementation with polypodium leucotomos improves repigmentation, particularly in photo-exposed areas, she noted.
Khellin is an extract from the Mediterranean khella plant that is thought to stimulate melanocyte proliferation and melanogenesis. Several studies have examined khellin supplementation in combination with phototherapy. Khellin can be administered orally or topically and appears to be more beneficial than sunlight or phototherapy alone in stabilizing disease or inducing repigmentation. Oral khellin can cause many side effects, including nausea, transaminitis, and hypotension, so researchers have been more interested in using topical khellin as a liposomal vehicle to improve drug delivery, Dr. Elbuluk said.
Minerals
Some patients with vitiligo have deficiencies in zinc and copper. Zinc is an antioxidant that aids wound healing, protects against free radicals, supports melanogenesis, and possibly prevents melanocyte death, but can cause gastrointestinal irritation. Copper, too, is an antioxidant and coenzyme involved in melanogenesis. One study compared topical steroid treatment with and without oral zinc supplementation. Dual treatment was associated with greater repigmentation, but the difference was not statistically significant. No studies have examined copper supplementation, she said.
L-phenylalanine, diet, and green tea
Investigators have proposed that the amino acid L-phenylalanine, a precursor to tyrosine in the pathway of melanin synthesis, might interfere with antibody production against melanocytes. This supplement is administered orally by weight, typically in conjunction with phototherapy or sunlight. Various studies have observed positive outcomes of L-phenylalanine combined with phototherapy or sunlight. L-phenylalanine tends to be safe and has been administered to children with vitiligo.
Many patients with vitiligo “have already tried diets by the time they come to me,” said Dr. Elbuluk. No controlled studies have analyzed the role of diet in the prevention or treatment of vitiligo, but case reports describe gluten-free diets in this population, including one report of a patient with celiac disease whose vitiligo improved after adoption of such a diet. Another case report described a patient without celiac disease who had refractory acrofacial vitiligo, which improved after the adoption of a gluten-free diet. Evidence supports a gluten-free diet for patients with celiac disease, but does not support this challenging diet for people without celiac disease, she pointed out.
Green tea includes catechins, which have antioxidant and anti-inflammatory properties. Its main component is epigallocatechin gallate (EGCG), which is thought to modulate T cell mediated responses. In one animal study, administration of EGCG delayed the onset of vitiligo and decreased the area of depigmentation in a mouse model. Although these findings are promising, clinical trials are needed to determine whether EGCG is beneficial in humans with vitiligo, said Dr. Elbuluk.
The literature on diets and supplementation as treatments for vitiligo has several shortcomings, with studies that used heterogeneous methodologies, and many that used nonstandard outcome measures that have not been validated. Sample sizes often are small, and many trials are uncontrolled. “These limitations make it harder to make sense of the data and have take-home conclusions,” Dr. Elbuluk said.
She had no disclosures.
SOURCE: Elbuluk N. AAD 19, Session S002.
WASHINGTON – Many patients with vitiligo are interested in treating their condition with vitamins, supplements, or a modified diet, but research on whether these measures have an impact remains limited, Nada Elbuluk, MD, said at the annual meeting of the American Academy of Dermatology.
, “we need more well designed, controlled studies in the future to know where this belongs in our treatment armamentarium,” said Dr. Elbuluk of the department of dermatology at the University of Southern California, Los Angeles.
During a session at the AAD meeting, Dr. Elbuluk, who is also director of the pigmentary disorders clinic at USC, reviewed the evidence for the use of these adjunctive therapies in patients with vitiligo.
Vitamins
The pathogenesis of vitiligo includes the overproduction of reactive oxygen species and oxidative stress, factors that contribute to melanocyte damage and death. In addition, many patients with vitiligo are deficient in certain vitamins and minerals, the basis of the hypothesis that supplementation could be beneficial, according to Dr. Elbuluk.
Vitamin B12 and folic acid contribute to DNA repair, synthesis, and methylation, and researchers have hypothesized that these vitamins also play a role in melanin synthesis. In a review of the literature, Dr. Elbuluk and her colleagues found four studies that evaluated vitamin B12 and folic acid in vitiligo. In one study, a controlled trial in which patients took B12 and folic acid with and without phototherapy, the investigators observed no significant difference in repigmentation between groups. The other three studies were uncontrolled and thus provide an insufficient understanding of the effect of B12 and folic acid, said Dr. Elbuluk.
Vitamin D is involved in melanocyte and keratinocyte growth and differentiation, and inhibits T cell activation. Data indicate that low vitamin D levels are common in patients with vitiligo and comorbid autoimmune diseases. In one study, patients who received narrow-band UVB had an increase in vitamin D levels that could contribute to photo-induced melanogenesis, and an open-label study indicated that patients who took vitamin D daily (without phototherapy) for 6 months had an increase of repigmentation over time. “Topical vitamin D analogs have also been used in vitiligo treatment with varying success,” Dr. Elbuluk noted.
“I check vitamin D levels on my patients and make sure that they are within normal range. But I think the degree of supplementation and its role in vitiligo needs to be further elucidated,” she said. And because vitamin D is fat soluble, there is a risk of toxicity if a patient takes too much.
Vitamin C, vitamin E, and alpha-lipoic acid have antioxidant properties. In a double-blind, randomized, controlled trial, one group of patients took vitamins C and E and alpha-lipoic acid for 2 months before and during treatment with narrow-band UVB twice per week (Clin Exp Dermatol. 2007 Nov;32[6]:631-6). Another group underwent phototherapy without supplementation. A significantly greater proportion of patients who received the antioxidants obtained more than 75% repigmentation compared with those who did not. In another study, 73% of patients who received oral vitamin E and narrow band UVB phototherapy had marked to excellent repigmentation, compared with 55.6% of those who had phototherapy only (J Clin Pharmacol. 2009 Jul;49[7]:852-5).
The results of these studies support the idea that antioxidants can stabilize disease, reduce oxidative stress, and improve the effect of phototherapy, Dr. Elbuluk said.
Herbal supplements
Several research teams have examined Ginkgo biloba as a possible treatment for vitiligo. This plant is native to China and has antioxidant and anti-inflammatory properties; its most common side effect is gastrointestinal distress. Because it entails a risk of coagulopathy, it may not be appropriate for patients receiving anticoagulant treatment, Dr. Elbuluk pointed out. In a double-blinded, randomized, controlled trial comparing ginkgo biloba alone with placebo in patients with vitiligo, treatment was associated with cessation of active disease in most patients, and more than 40% of patients receiving ginkgo biloba had 75% repigmentation or more.
Polypodium leucotomos, a fern native to Central and South America, protects against UV radiation damage, modulates the immune system, and has anti-inflammatory and antioxidant effects. It has a good safety profile and is well tolerated at a dose of 240 mg/day, she said. It sometimes causes gastrointestinal discomfort or pruritus. Several randomized, controlled trials in patients with vitiligo showed that supplementation with polypodium leucotomos improves repigmentation, particularly in photo-exposed areas, she noted.
Khellin is an extract from the Mediterranean khella plant that is thought to stimulate melanocyte proliferation and melanogenesis. Several studies have examined khellin supplementation in combination with phototherapy. Khellin can be administered orally or topically and appears to be more beneficial than sunlight or phototherapy alone in stabilizing disease or inducing repigmentation. Oral khellin can cause many side effects, including nausea, transaminitis, and hypotension, so researchers have been more interested in using topical khellin as a liposomal vehicle to improve drug delivery, Dr. Elbuluk said.
Minerals
Some patients with vitiligo have deficiencies in zinc and copper. Zinc is an antioxidant that aids wound healing, protects against free radicals, supports melanogenesis, and possibly prevents melanocyte death, but can cause gastrointestinal irritation. Copper, too, is an antioxidant and coenzyme involved in melanogenesis. One study compared topical steroid treatment with and without oral zinc supplementation. Dual treatment was associated with greater repigmentation, but the difference was not statistically significant. No studies have examined copper supplementation, she said.
L-phenylalanine, diet, and green tea
Investigators have proposed that the amino acid L-phenylalanine, a precursor to tyrosine in the pathway of melanin synthesis, might interfere with antibody production against melanocytes. This supplement is administered orally by weight, typically in conjunction with phototherapy or sunlight. Various studies have observed positive outcomes of L-phenylalanine combined with phototherapy or sunlight. L-phenylalanine tends to be safe and has been administered to children with vitiligo.
Many patients with vitiligo “have already tried diets by the time they come to me,” said Dr. Elbuluk. No controlled studies have analyzed the role of diet in the prevention or treatment of vitiligo, but case reports describe gluten-free diets in this population, including one report of a patient with celiac disease whose vitiligo improved after adoption of such a diet. Another case report described a patient without celiac disease who had refractory acrofacial vitiligo, which improved after the adoption of a gluten-free diet. Evidence supports a gluten-free diet for patients with celiac disease, but does not support this challenging diet for people without celiac disease, she pointed out.
Green tea includes catechins, which have antioxidant and anti-inflammatory properties. Its main component is epigallocatechin gallate (EGCG), which is thought to modulate T cell mediated responses. In one animal study, administration of EGCG delayed the onset of vitiligo and decreased the area of depigmentation in a mouse model. Although these findings are promising, clinical trials are needed to determine whether EGCG is beneficial in humans with vitiligo, said Dr. Elbuluk.
The literature on diets and supplementation as treatments for vitiligo has several shortcomings, with studies that used heterogeneous methodologies, and many that used nonstandard outcome measures that have not been validated. Sample sizes often are small, and many trials are uncontrolled. “These limitations make it harder to make sense of the data and have take-home conclusions,” Dr. Elbuluk said.
She had no disclosures.
SOURCE: Elbuluk N. AAD 19, Session S002.
REPORTING FROM AAD 19