User login
-
CHP/CCUS: Low blood cancer risk for most patients
The reason is that patients will inevitably “go online and see that [the conditions are] associated with lots of bad things; it can really cause patients psychosocial harm if there is no one to explain what their risk is and also provide risk-specific management,” Dr. Weeks said at the annual meeting of the Society of Hematologic Oncology in Houston.
CHIP and CCUS are precursors of myeloid malignancies but for most patients, the risk of progression is less than 1%. CHIPS and CCUS are also associated with cardiovascular, rheumatologic, hepatic, and other diseases.
CHIP is defined by somatic mutations in myeloid malignancy driver genes with a variant allele fraction of 2% or more; CCUS is when those molecular features are accompanied by an unexplained and persistent anemia, thrombocytopenia, or neutropenia.
A small 2017 study suggested that about a third of patients with otherwise unexplained cytopenias have CCUS.
With the increasing use of next generation sequencing for tissue and liquid biopsies and other uses, the incidental diagnosis of both conditions is increasing.
Fortunately, Dr. Weeks’ group recently published a tool for predicting the risk of progression to myeloid malignancy.
Their “clonal hematopoiesis risk score” (CHRS) was developed and validated in over 400,000 healthy volunteers in the UK Biobank, with additional validation in cohorts from Dana Farber and the University of Pavia, Italy.
The CHRS incorporates eight high-risk genetic and clinical prognostic factors, including the type and number of genetic mutations in blood cells, factors related to red blood cell volume, and age over 65. It’s available online.
“You just input the patient’s information and it spits out if the patient is low, intermediate, or high risk for progression to any myeloid malignancy,” Dr. Weeks told her audience.
High-risk patients have about a 50% 10-year cumulative incidence of myeloid malignancy. The large majority of patients are low risk, however, and have a 10-year cumulative incidence of less than 1%. Patients in the middle have a 10-year risk of about 8%.
The low-risk group “is the population of people who probably don’t need to see a specialist,” and can be followed with an annual CBC with their primary care doctors plus further workup with any clinical change. Patients should also be evaluated for cardiovascular and other comorbidity risks.
“It’s the high-risk group we worry most about,” Dr. Weeks said. “We see them more often and repeat the next-generation sequencing” annually with a CBC at least every 6 months and a bone marrow biopsy with any clinical change.
“This is the population we would shuttle towards a clinical trial, as this is the population most likely to benefit,” she said.
The overarching goal of the several ongoing studies in CHIP/CCUS is to find a way to prevent progression to blood cancer. They range from prospective cohorts and single arm pilot studies to randomized clinical trials. One trial is evaluating canakinumab to prevent progression. “Intervention in clonal hematopoiesis might have the dual benefit of both preventing hematologic malignancy as well as reducing [the] inflammatory comorbidities,” Dr. Weeks said.
The reason is that patients will inevitably “go online and see that [the conditions are] associated with lots of bad things; it can really cause patients psychosocial harm if there is no one to explain what their risk is and also provide risk-specific management,” Dr. Weeks said at the annual meeting of the Society of Hematologic Oncology in Houston.
CHIP and CCUS are precursors of myeloid malignancies but for most patients, the risk of progression is less than 1%. CHIPS and CCUS are also associated with cardiovascular, rheumatologic, hepatic, and other diseases.
CHIP is defined by somatic mutations in myeloid malignancy driver genes with a variant allele fraction of 2% or more; CCUS is when those molecular features are accompanied by an unexplained and persistent anemia, thrombocytopenia, or neutropenia.
A small 2017 study suggested that about a third of patients with otherwise unexplained cytopenias have CCUS.
With the increasing use of next generation sequencing for tissue and liquid biopsies and other uses, the incidental diagnosis of both conditions is increasing.
Fortunately, Dr. Weeks’ group recently published a tool for predicting the risk of progression to myeloid malignancy.
Their “clonal hematopoiesis risk score” (CHRS) was developed and validated in over 400,000 healthy volunteers in the UK Biobank, with additional validation in cohorts from Dana Farber and the University of Pavia, Italy.
The CHRS incorporates eight high-risk genetic and clinical prognostic factors, including the type and number of genetic mutations in blood cells, factors related to red blood cell volume, and age over 65. It’s available online.
“You just input the patient’s information and it spits out if the patient is low, intermediate, or high risk for progression to any myeloid malignancy,” Dr. Weeks told her audience.
High-risk patients have about a 50% 10-year cumulative incidence of myeloid malignancy. The large majority of patients are low risk, however, and have a 10-year cumulative incidence of less than 1%. Patients in the middle have a 10-year risk of about 8%.
The low-risk group “is the population of people who probably don’t need to see a specialist,” and can be followed with an annual CBC with their primary care doctors plus further workup with any clinical change. Patients should also be evaluated for cardiovascular and other comorbidity risks.
“It’s the high-risk group we worry most about,” Dr. Weeks said. “We see them more often and repeat the next-generation sequencing” annually with a CBC at least every 6 months and a bone marrow biopsy with any clinical change.
“This is the population we would shuttle towards a clinical trial, as this is the population most likely to benefit,” she said.
The overarching goal of the several ongoing studies in CHIP/CCUS is to find a way to prevent progression to blood cancer. They range from prospective cohorts and single arm pilot studies to randomized clinical trials. One trial is evaluating canakinumab to prevent progression. “Intervention in clonal hematopoiesis might have the dual benefit of both preventing hematologic malignancy as well as reducing [the] inflammatory comorbidities,” Dr. Weeks said.
The reason is that patients will inevitably “go online and see that [the conditions are] associated with lots of bad things; it can really cause patients psychosocial harm if there is no one to explain what their risk is and also provide risk-specific management,” Dr. Weeks said at the annual meeting of the Society of Hematologic Oncology in Houston.
CHIP and CCUS are precursors of myeloid malignancies but for most patients, the risk of progression is less than 1%. CHIPS and CCUS are also associated with cardiovascular, rheumatologic, hepatic, and other diseases.
CHIP is defined by somatic mutations in myeloid malignancy driver genes with a variant allele fraction of 2% or more; CCUS is when those molecular features are accompanied by an unexplained and persistent anemia, thrombocytopenia, or neutropenia.
A small 2017 study suggested that about a third of patients with otherwise unexplained cytopenias have CCUS.
With the increasing use of next generation sequencing for tissue and liquid biopsies and other uses, the incidental diagnosis of both conditions is increasing.
Fortunately, Dr. Weeks’ group recently published a tool for predicting the risk of progression to myeloid malignancy.
Their “clonal hematopoiesis risk score” (CHRS) was developed and validated in over 400,000 healthy volunteers in the UK Biobank, with additional validation in cohorts from Dana Farber and the University of Pavia, Italy.
The CHRS incorporates eight high-risk genetic and clinical prognostic factors, including the type and number of genetic mutations in blood cells, factors related to red blood cell volume, and age over 65. It’s available online.
“You just input the patient’s information and it spits out if the patient is low, intermediate, or high risk for progression to any myeloid malignancy,” Dr. Weeks told her audience.
High-risk patients have about a 50% 10-year cumulative incidence of myeloid malignancy. The large majority of patients are low risk, however, and have a 10-year cumulative incidence of less than 1%. Patients in the middle have a 10-year risk of about 8%.
The low-risk group “is the population of people who probably don’t need to see a specialist,” and can be followed with an annual CBC with their primary care doctors plus further workup with any clinical change. Patients should also be evaluated for cardiovascular and other comorbidity risks.
“It’s the high-risk group we worry most about,” Dr. Weeks said. “We see them more often and repeat the next-generation sequencing” annually with a CBC at least every 6 months and a bone marrow biopsy with any clinical change.
“This is the population we would shuttle towards a clinical trial, as this is the population most likely to benefit,” she said.
The overarching goal of the several ongoing studies in CHIP/CCUS is to find a way to prevent progression to blood cancer. They range from prospective cohorts and single arm pilot studies to randomized clinical trials. One trial is evaluating canakinumab to prevent progression. “Intervention in clonal hematopoiesis might have the dual benefit of both preventing hematologic malignancy as well as reducing [the] inflammatory comorbidities,” Dr. Weeks said.
FROM SOHO 2023
Domestic violence in health care is real and underreported
To protect survivors’ identities, some names have been changed or shortened.
Natasha Abadilla, MD, met the man who would become her abuser while working abroad for a public health nonprofit. When he began emotionally and physically abusing her, she did everything she could to hide it.
“My coworkers knew nothing of the abuse. I became an expert in applying makeup to hide the bruises,” recalls Dr. Abadilla, now a second-year resident and pediatric neurologist at Lucile Packard Children’s Hospital at Stanford.
Dr. Abadilla says she strongly identifies as a hard worker and – to this day – hopes her work did not falter despite her partner’s constant drain on her. But the impact of the abuse continued to affect her for years. Like many survivors of domestic violence, she struggled with PTSD and depression.
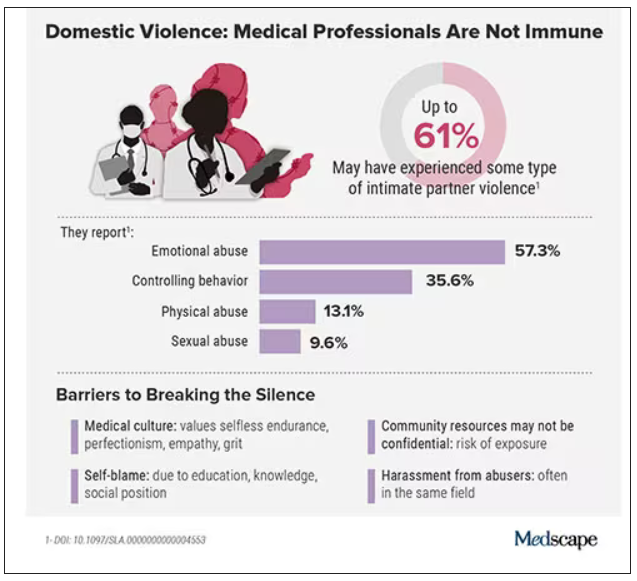
Health care workers are often the first point of contact for survivors of domestic violence. Experts and advocates continue to push for more training for clinicians to identify and respond to signs among their patients. Often missing from this conversation is the reality that those tasked with screening can also be victims of intimate partner violence themselves.
What’s more: The very strengths that medical professionals often pride themselves on – perfectionism, empathy, grit – can make it harder for them to identify abuse in their own relationships and push through humiliation and shame to seek help.
Dr. Abadilla is exceptional among survivors in the medical field. Rather than keep her experience quiet, she has shared it publicly.
Awareness, she believes, can save lives.
An understudied problem in an underserved group
The majority of research on health care workers in this area has focused on workplace violence, which 62% experience worldwide. But intimate partner violence remains understudied and underdiscussed. Some medical professionals are even saddled with a “double burden,” facing trauma at work and at home, note the authors of a 2022 meta-analysis published in the journal Trauma, Violence, & Abuse.
The problem has had dire consequences. In recent years, many health care workers have been killed by their abusers:
- In 2016, Casey M. Drawert, MD, a Texas-based critical care anesthesiologist, was fatally shot by her husband in a murder-suicide.
- In 2018, Tamara O’Neal, MD, an ER physician, and Dayna Less, a first-year pharmacy resident, were killed by Dr. O’Neal’s ex-fiancé at Mercy Hospital in Chicago.
- In 2019, Sarah Hawley, MD, a first-year University of Utah resident, was fatally shot by her boyfriend in a murder-suicide.
- In 2021, Moria Kinsey, a nurse practitioner in Tahlequah, Okla., was murdered by a physician.
- In July of 2023, Gwendolyn Lavonne Riddick, DO, an ob.gyn. in North Carolina, was fatally shot by the father of her 3-year-old son.
There are others.
In the wake of these tragedies, calls for health care workers to screen each other as well as patients have grown. But for an untold number of survivors, breaking the silence is still not possible due to concerns about their reputation, professional consequences, the threat of harassment from abusers who are often in the same field, a medical culture of selfless endurance, and a lack of appropriate resources.
While the vast majority have stayed silent, those who have spoken out say there’s a need for targeted interventions to educate medical professionals as well as more supportive policies throughout the health care system.
Are health care workers more at risk?
Although more studies are needed, research indicates health care workers experience domestic violence at rates comparable to those of other populations, whereas some data suggest rates may be higher.
In the United States, more than one in three women and one in four men experience some form of intimate partner violence in their lifetime. Similarly, a 2020 study found that 24% of 400 physicians responding to a survey reported a history of domestic violence, with 15% reporting verbal abuse, 8% reporting physical violence, 4% reporting sexual abuse, and 4% reporting stalking.
Meanwhile, in an anonymous survey completed by 882 practicing surgeons and trainees in the United States from late 2018 to early 2019, more than 60% reported experiencing some type of intimate partner violence, most commonly emotional abuse.
Recent studies in the United Kingdom, Australia, and elsewhere show that significant numbers of medical professionals are fighting this battle. A 2019 study of more than 2,000 nurses, midwives, and health care assistants in the United Kingdom found that nurses were three times more likely to experience domestic violence than the average person.
What would help solve this problem: More study of health care worker-survivors as a unique group with unique risk factors. In general, domestic violence is most prevalent among women and people in marginalized groups. But young adults, such as medical students and trainees, can face an increased risk due to economic strain. Major life changes, such as relocating for residency, can also drive up stress and fray social connections, further isolating victims.
Why it’s so much harder for medical professionals to reveal abuse
For medical professionals accustomed to being strong and forging on, identifying as a victim of abuse can seem like a personal contradiction. It can feel easier to separate their personal and professional lives rather than face a complex reality.
In a personal essay on KevinMD.com, medical student Chloe N. L. Lee describes this emotional turmoil. “As an aspiring psychiatrist, I questioned my character judgment (how did I end up with a misogynistic abuser?) and wondered if I ought to have known better. I worried that my colleagues would deem me unfit to care for patients. And I thought that this was not supposed to happen to women like me,” Ms. Lee writes.
Kimberly, a licensed therapist, experienced a similar pattern of self-blame when her partner began exhibiting violent behavior. “For a long time, I felt guilty because I said to myself, You’re a therapist. You’re supposed to know this,” she recalls. At the same time, she felt driven to help him and sought couples therapy as his violence escalated.
Whitney, a pharmacist, recognized the “hallmarks” of abuse in her relationship, but she coped by compartmentalizing. Whitney says she was vulnerable to her abuser as a young college student who struggled financially. As he showered her with gifts, she found herself waving away red flags like aggressiveness or overprotectiveness.
After Whitney graduated, her partner’s emotional manipulation escalated into frequent physical assaults. When he gave her a black eye, she could not bring herself to go into work. She quit her job without notice. Despite a spotless record, none of her coworkers ever reached out to investigate her sudden departure.
It would take 8 years for Whitney to acknowledge the abuse and seize a moment to escape. She fled with just her purse and started over in a new city, rebuilding her life in the midst of harassment and threats from her ex. She says she’s grateful to be alive.
An imperfect system doesn’t help
Health care workers rarely ask for support or disclose abuse at work. Some have cited stigma, a lack of confidentiality (especially when the abuser is also in health care), fears about colleagues’ judgment, and a culture that doesn’t prioritize self-care.
Sometimes policies get in the way: In a 2021 qualitative study of interviews with 21 female physician-survivors in the United Kingdom, many said that despite the intense stress of abuse and recovery, they were unable to take any time off.
Of 180 UK-based midwife-survivors interviewed in a 2018 study, only 60 sought support at work and 30 received it. Many said their supervisors pressured them to report the abuse and get back to work, called social services behind their back, or reported them to their professional regulator. “I was treated like the perpetrator,” one said. Barbara Hernandez, PhD, a researcher who studies physician-survivors and director of physician vitality at Loma Linda University in southern California, says workplace violence and mistreatment from patients or colleagues – and a poor institutional response – can make those in health care feel like they have to “shut up and put up,” priming them to also tolerate abuse at home.
When survivors do reach out, there can be a disconnect between the resources they need and those they’re offered, Dr. Hernandez adds. In a recent survey of 400 physicians she conducted, respondents typically said they would advise a physician-survivor to “get to a shelter quickly.” But when roles were reversed, they admitted going to a shelter was the least feasible option. Support groups can also be problematic in smaller communities where physicians might be recognized or see their own patients.
Complicating matters further, the violence often comes from within the medical community. This can lead to particularly malicious abuse tactics like sending false accusations to a victim’s regulatory college or board; prolonged court and custody battles to drain them of all resources and their ability to hold a job; or even sabotage, harassment, or violence at work. The sheen of the abuser’s public persona, on the other hand, can guard them from any accountability.
For example, one physician-survivor said her ex-partner, a psychiatrist, coerced her into believing she was mentally ill, claimed she was “psychotic” in order to take back their children after she left, and had numerous colleagues serve as character witnesses in court for him, “saying he couldn’t have done any of these things, how great he is, and what a wonderful father he is.”
Slow progress is still progress
After Sherilyn M. Gordon-Burroughs, MD, a Texas-based transplant surgeon, mother, and educator, was killed by her husband in a murder-suicide in 2017, her friends Barbara Lee Bass, MD, president of the American College of Surgeons, and Patricia L. Turner, MD, were spurred into action. Together, they founded the ACS Intimate Partner Violence Task Force. Their mission is to educate surgeons to identify the signs of intimate partner violence (IPV) in themselves and their colleagues and connect them with resources.
“There is a concerted effort to close that gap,” says D’Andrea K. Joseph, MD, cochair of the task force and chief of trauma and acute care surgery at NYU Langone in New York. In the future, Dr. Joseph predicts, “making this a part of the curriculum, that it’s standardized for residents and trainees, that there is a safe place for victims ... and that we can band together and really recognize and assist our colleagues who are in trouble.”
Resources created by the ACS IPV task force, such as the toolkit and curriculum, provide a model for other health care leaders. But there have been few similar initiatives aimed at increasing IPV intervention within the medical system.
What you can do in your workplace
In her essay, Ms. Lee explains that a major turning point came when a physician friend explicitly asked if she was experiencing abuse. He then gently confirmed she was, and asked without judgment how he could support her, an approach that mirrors advice from the National Domestic Violence Hotline.
“Having a physician validate that this was, indeed, an abusive situation helped enormously ... I believe it may have saved my life,” she writes.
That validation can be crucial, and Dr. Abadilla urges other physicians to regularly check in with colleagues, especially those who seem particularly positive with a go-getter attitude and yet may not seem themselves. That was how she presented when she was struggling the most.
Supporting systemic changes within your organization and beyond is also important. The authors of the 2022 meta-analysis stress the need for domestic violence training, legislative changes, paid leave, and union support.
Finding strength in recovery
Over a decade after escaping her abuser, Whitney says she’s only just begun to share her experience, but what she’s learned has made her a better pharmacist. She says she’s more attuned to subtle signs something could be off with patients and coworkers. When someone makes comments about feeling anxious or that they can’t do anything right, it’s important to ask why, she says.
Recently, Kimberly has opened up to her mentor and other therapists, many of whom have shared that they’re also survivors.
“The last thing I said to [my abuser] is you think you’ve won and you’re hurting me, but what you’ve done to me – I’m going to utilize this and I’m going to help other people,” Kimberly says. “This pain that I have will go away, and I’m going to save the lives of others.”
A version of this article first appeared on Medscape.com.
To protect survivors’ identities, some names have been changed or shortened.
Natasha Abadilla, MD, met the man who would become her abuser while working abroad for a public health nonprofit. When he began emotionally and physically abusing her, she did everything she could to hide it.
“My coworkers knew nothing of the abuse. I became an expert in applying makeup to hide the bruises,” recalls Dr. Abadilla, now a second-year resident and pediatric neurologist at Lucile Packard Children’s Hospital at Stanford.
Dr. Abadilla says she strongly identifies as a hard worker and – to this day – hopes her work did not falter despite her partner’s constant drain on her. But the impact of the abuse continued to affect her for years. Like many survivors of domestic violence, she struggled with PTSD and depression.
Health care workers are often the first point of contact for survivors of domestic violence. Experts and advocates continue to push for more training for clinicians to identify and respond to signs among their patients. Often missing from this conversation is the reality that those tasked with screening can also be victims of intimate partner violence themselves.
What’s more: The very strengths that medical professionals often pride themselves on – perfectionism, empathy, grit – can make it harder for them to identify abuse in their own relationships and push through humiliation and shame to seek help.
Dr. Abadilla is exceptional among survivors in the medical field. Rather than keep her experience quiet, she has shared it publicly.
Awareness, she believes, can save lives.
An understudied problem in an underserved group
The majority of research on health care workers in this area has focused on workplace violence, which 62% experience worldwide. But intimate partner violence remains understudied and underdiscussed. Some medical professionals are even saddled with a “double burden,” facing trauma at work and at home, note the authors of a 2022 meta-analysis published in the journal Trauma, Violence, & Abuse.
The problem has had dire consequences. In recent years, many health care workers have been killed by their abusers:
- In 2016, Casey M. Drawert, MD, a Texas-based critical care anesthesiologist, was fatally shot by her husband in a murder-suicide.
- In 2018, Tamara O’Neal, MD, an ER physician, and Dayna Less, a first-year pharmacy resident, were killed by Dr. O’Neal’s ex-fiancé at Mercy Hospital in Chicago.
- In 2019, Sarah Hawley, MD, a first-year University of Utah resident, was fatally shot by her boyfriend in a murder-suicide.
- In 2021, Moria Kinsey, a nurse practitioner in Tahlequah, Okla., was murdered by a physician.
- In July of 2023, Gwendolyn Lavonne Riddick, DO, an ob.gyn. in North Carolina, was fatally shot by the father of her 3-year-old son.
There are others.
In the wake of these tragedies, calls for health care workers to screen each other as well as patients have grown. But for an untold number of survivors, breaking the silence is still not possible due to concerns about their reputation, professional consequences, the threat of harassment from abusers who are often in the same field, a medical culture of selfless endurance, and a lack of appropriate resources.
While the vast majority have stayed silent, those who have spoken out say there’s a need for targeted interventions to educate medical professionals as well as more supportive policies throughout the health care system.
Are health care workers more at risk?
Although more studies are needed, research indicates health care workers experience domestic violence at rates comparable to those of other populations, whereas some data suggest rates may be higher.
In the United States, more than one in three women and one in four men experience some form of intimate partner violence in their lifetime. Similarly, a 2020 study found that 24% of 400 physicians responding to a survey reported a history of domestic violence, with 15% reporting verbal abuse, 8% reporting physical violence, 4% reporting sexual abuse, and 4% reporting stalking.
Meanwhile, in an anonymous survey completed by 882 practicing surgeons and trainees in the United States from late 2018 to early 2019, more than 60% reported experiencing some type of intimate partner violence, most commonly emotional abuse.
Recent studies in the United Kingdom, Australia, and elsewhere show that significant numbers of medical professionals are fighting this battle. A 2019 study of more than 2,000 nurses, midwives, and health care assistants in the United Kingdom found that nurses were three times more likely to experience domestic violence than the average person.
What would help solve this problem: More study of health care worker-survivors as a unique group with unique risk factors. In general, domestic violence is most prevalent among women and people in marginalized groups. But young adults, such as medical students and trainees, can face an increased risk due to economic strain. Major life changes, such as relocating for residency, can also drive up stress and fray social connections, further isolating victims.
Why it’s so much harder for medical professionals to reveal abuse
For medical professionals accustomed to being strong and forging on, identifying as a victim of abuse can seem like a personal contradiction. It can feel easier to separate their personal and professional lives rather than face a complex reality.
In a personal essay on KevinMD.com, medical student Chloe N. L. Lee describes this emotional turmoil. “As an aspiring psychiatrist, I questioned my character judgment (how did I end up with a misogynistic abuser?) and wondered if I ought to have known better. I worried that my colleagues would deem me unfit to care for patients. And I thought that this was not supposed to happen to women like me,” Ms. Lee writes.
Kimberly, a licensed therapist, experienced a similar pattern of self-blame when her partner began exhibiting violent behavior. “For a long time, I felt guilty because I said to myself, You’re a therapist. You’re supposed to know this,” she recalls. At the same time, she felt driven to help him and sought couples therapy as his violence escalated.
Whitney, a pharmacist, recognized the “hallmarks” of abuse in her relationship, but she coped by compartmentalizing. Whitney says she was vulnerable to her abuser as a young college student who struggled financially. As he showered her with gifts, she found herself waving away red flags like aggressiveness or overprotectiveness.
After Whitney graduated, her partner’s emotional manipulation escalated into frequent physical assaults. When he gave her a black eye, she could not bring herself to go into work. She quit her job without notice. Despite a spotless record, none of her coworkers ever reached out to investigate her sudden departure.
It would take 8 years for Whitney to acknowledge the abuse and seize a moment to escape. She fled with just her purse and started over in a new city, rebuilding her life in the midst of harassment and threats from her ex. She says she’s grateful to be alive.
An imperfect system doesn’t help
Health care workers rarely ask for support or disclose abuse at work. Some have cited stigma, a lack of confidentiality (especially when the abuser is also in health care), fears about colleagues’ judgment, and a culture that doesn’t prioritize self-care.
Sometimes policies get in the way: In a 2021 qualitative study of interviews with 21 female physician-survivors in the United Kingdom, many said that despite the intense stress of abuse and recovery, they were unable to take any time off.
Of 180 UK-based midwife-survivors interviewed in a 2018 study, only 60 sought support at work and 30 received it. Many said their supervisors pressured them to report the abuse and get back to work, called social services behind their back, or reported them to their professional regulator. “I was treated like the perpetrator,” one said. Barbara Hernandez, PhD, a researcher who studies physician-survivors and director of physician vitality at Loma Linda University in southern California, says workplace violence and mistreatment from patients or colleagues – and a poor institutional response – can make those in health care feel like they have to “shut up and put up,” priming them to also tolerate abuse at home.
When survivors do reach out, there can be a disconnect between the resources they need and those they’re offered, Dr. Hernandez adds. In a recent survey of 400 physicians she conducted, respondents typically said they would advise a physician-survivor to “get to a shelter quickly.” But when roles were reversed, they admitted going to a shelter was the least feasible option. Support groups can also be problematic in smaller communities where physicians might be recognized or see their own patients.
Complicating matters further, the violence often comes from within the medical community. This can lead to particularly malicious abuse tactics like sending false accusations to a victim’s regulatory college or board; prolonged court and custody battles to drain them of all resources and their ability to hold a job; or even sabotage, harassment, or violence at work. The sheen of the abuser’s public persona, on the other hand, can guard them from any accountability.
For example, one physician-survivor said her ex-partner, a psychiatrist, coerced her into believing she was mentally ill, claimed she was “psychotic” in order to take back their children after she left, and had numerous colleagues serve as character witnesses in court for him, “saying he couldn’t have done any of these things, how great he is, and what a wonderful father he is.”
Slow progress is still progress
After Sherilyn M. Gordon-Burroughs, MD, a Texas-based transplant surgeon, mother, and educator, was killed by her husband in a murder-suicide in 2017, her friends Barbara Lee Bass, MD, president of the American College of Surgeons, and Patricia L. Turner, MD, were spurred into action. Together, they founded the ACS Intimate Partner Violence Task Force. Their mission is to educate surgeons to identify the signs of intimate partner violence (IPV) in themselves and their colleagues and connect them with resources.
“There is a concerted effort to close that gap,” says D’Andrea K. Joseph, MD, cochair of the task force and chief of trauma and acute care surgery at NYU Langone in New York. In the future, Dr. Joseph predicts, “making this a part of the curriculum, that it’s standardized for residents and trainees, that there is a safe place for victims ... and that we can band together and really recognize and assist our colleagues who are in trouble.”
Resources created by the ACS IPV task force, such as the toolkit and curriculum, provide a model for other health care leaders. But there have been few similar initiatives aimed at increasing IPV intervention within the medical system.
What you can do in your workplace
In her essay, Ms. Lee explains that a major turning point came when a physician friend explicitly asked if she was experiencing abuse. He then gently confirmed she was, and asked without judgment how he could support her, an approach that mirrors advice from the National Domestic Violence Hotline.
“Having a physician validate that this was, indeed, an abusive situation helped enormously ... I believe it may have saved my life,” she writes.
That validation can be crucial, and Dr. Abadilla urges other physicians to regularly check in with colleagues, especially those who seem particularly positive with a go-getter attitude and yet may not seem themselves. That was how she presented when she was struggling the most.
Supporting systemic changes within your organization and beyond is also important. The authors of the 2022 meta-analysis stress the need for domestic violence training, legislative changes, paid leave, and union support.
Finding strength in recovery
Over a decade after escaping her abuser, Whitney says she’s only just begun to share her experience, but what she’s learned has made her a better pharmacist. She says she’s more attuned to subtle signs something could be off with patients and coworkers. When someone makes comments about feeling anxious or that they can’t do anything right, it’s important to ask why, she says.
Recently, Kimberly has opened up to her mentor and other therapists, many of whom have shared that they’re also survivors.
“The last thing I said to [my abuser] is you think you’ve won and you’re hurting me, but what you’ve done to me – I’m going to utilize this and I’m going to help other people,” Kimberly says. “This pain that I have will go away, and I’m going to save the lives of others.”
A version of this article first appeared on Medscape.com.
To protect survivors’ identities, some names have been changed or shortened.
Natasha Abadilla, MD, met the man who would become her abuser while working abroad for a public health nonprofit. When he began emotionally and physically abusing her, she did everything she could to hide it.
“My coworkers knew nothing of the abuse. I became an expert in applying makeup to hide the bruises,” recalls Dr. Abadilla, now a second-year resident and pediatric neurologist at Lucile Packard Children’s Hospital at Stanford.
Dr. Abadilla says she strongly identifies as a hard worker and – to this day – hopes her work did not falter despite her partner’s constant drain on her. But the impact of the abuse continued to affect her for years. Like many survivors of domestic violence, she struggled with PTSD and depression.
Health care workers are often the first point of contact for survivors of domestic violence. Experts and advocates continue to push for more training for clinicians to identify and respond to signs among their patients. Often missing from this conversation is the reality that those tasked with screening can also be victims of intimate partner violence themselves.
What’s more: The very strengths that medical professionals often pride themselves on – perfectionism, empathy, grit – can make it harder for them to identify abuse in their own relationships and push through humiliation and shame to seek help.
Dr. Abadilla is exceptional among survivors in the medical field. Rather than keep her experience quiet, she has shared it publicly.
Awareness, she believes, can save lives.
An understudied problem in an underserved group
The majority of research on health care workers in this area has focused on workplace violence, which 62% experience worldwide. But intimate partner violence remains understudied and underdiscussed. Some medical professionals are even saddled with a “double burden,” facing trauma at work and at home, note the authors of a 2022 meta-analysis published in the journal Trauma, Violence, & Abuse.
The problem has had dire consequences. In recent years, many health care workers have been killed by their abusers:
- In 2016, Casey M. Drawert, MD, a Texas-based critical care anesthesiologist, was fatally shot by her husband in a murder-suicide.
- In 2018, Tamara O’Neal, MD, an ER physician, and Dayna Less, a first-year pharmacy resident, were killed by Dr. O’Neal’s ex-fiancé at Mercy Hospital in Chicago.
- In 2019, Sarah Hawley, MD, a first-year University of Utah resident, was fatally shot by her boyfriend in a murder-suicide.
- In 2021, Moria Kinsey, a nurse practitioner in Tahlequah, Okla., was murdered by a physician.
- In July of 2023, Gwendolyn Lavonne Riddick, DO, an ob.gyn. in North Carolina, was fatally shot by the father of her 3-year-old son.
There are others.
In the wake of these tragedies, calls for health care workers to screen each other as well as patients have grown. But for an untold number of survivors, breaking the silence is still not possible due to concerns about their reputation, professional consequences, the threat of harassment from abusers who are often in the same field, a medical culture of selfless endurance, and a lack of appropriate resources.
While the vast majority have stayed silent, those who have spoken out say there’s a need for targeted interventions to educate medical professionals as well as more supportive policies throughout the health care system.
Are health care workers more at risk?
Although more studies are needed, research indicates health care workers experience domestic violence at rates comparable to those of other populations, whereas some data suggest rates may be higher.
In the United States, more than one in three women and one in four men experience some form of intimate partner violence in their lifetime. Similarly, a 2020 study found that 24% of 400 physicians responding to a survey reported a history of domestic violence, with 15% reporting verbal abuse, 8% reporting physical violence, 4% reporting sexual abuse, and 4% reporting stalking.
Meanwhile, in an anonymous survey completed by 882 practicing surgeons and trainees in the United States from late 2018 to early 2019, more than 60% reported experiencing some type of intimate partner violence, most commonly emotional abuse.
Recent studies in the United Kingdom, Australia, and elsewhere show that significant numbers of medical professionals are fighting this battle. A 2019 study of more than 2,000 nurses, midwives, and health care assistants in the United Kingdom found that nurses were three times more likely to experience domestic violence than the average person.
What would help solve this problem: More study of health care worker-survivors as a unique group with unique risk factors. In general, domestic violence is most prevalent among women and people in marginalized groups. But young adults, such as medical students and trainees, can face an increased risk due to economic strain. Major life changes, such as relocating for residency, can also drive up stress and fray social connections, further isolating victims.
Why it’s so much harder for medical professionals to reveal abuse
For medical professionals accustomed to being strong and forging on, identifying as a victim of abuse can seem like a personal contradiction. It can feel easier to separate their personal and professional lives rather than face a complex reality.
In a personal essay on KevinMD.com, medical student Chloe N. L. Lee describes this emotional turmoil. “As an aspiring psychiatrist, I questioned my character judgment (how did I end up with a misogynistic abuser?) and wondered if I ought to have known better. I worried that my colleagues would deem me unfit to care for patients. And I thought that this was not supposed to happen to women like me,” Ms. Lee writes.
Kimberly, a licensed therapist, experienced a similar pattern of self-blame when her partner began exhibiting violent behavior. “For a long time, I felt guilty because I said to myself, You’re a therapist. You’re supposed to know this,” she recalls. At the same time, she felt driven to help him and sought couples therapy as his violence escalated.
Whitney, a pharmacist, recognized the “hallmarks” of abuse in her relationship, but she coped by compartmentalizing. Whitney says she was vulnerable to her abuser as a young college student who struggled financially. As he showered her with gifts, she found herself waving away red flags like aggressiveness or overprotectiveness.
After Whitney graduated, her partner’s emotional manipulation escalated into frequent physical assaults. When he gave her a black eye, she could not bring herself to go into work. She quit her job without notice. Despite a spotless record, none of her coworkers ever reached out to investigate her sudden departure.
It would take 8 years for Whitney to acknowledge the abuse and seize a moment to escape. She fled with just her purse and started over in a new city, rebuilding her life in the midst of harassment and threats from her ex. She says she’s grateful to be alive.
An imperfect system doesn’t help
Health care workers rarely ask for support or disclose abuse at work. Some have cited stigma, a lack of confidentiality (especially when the abuser is also in health care), fears about colleagues’ judgment, and a culture that doesn’t prioritize self-care.
Sometimes policies get in the way: In a 2021 qualitative study of interviews with 21 female physician-survivors in the United Kingdom, many said that despite the intense stress of abuse and recovery, they were unable to take any time off.
Of 180 UK-based midwife-survivors interviewed in a 2018 study, only 60 sought support at work and 30 received it. Many said their supervisors pressured them to report the abuse and get back to work, called social services behind their back, or reported them to their professional regulator. “I was treated like the perpetrator,” one said. Barbara Hernandez, PhD, a researcher who studies physician-survivors and director of physician vitality at Loma Linda University in southern California, says workplace violence and mistreatment from patients or colleagues – and a poor institutional response – can make those in health care feel like they have to “shut up and put up,” priming them to also tolerate abuse at home.
When survivors do reach out, there can be a disconnect between the resources they need and those they’re offered, Dr. Hernandez adds. In a recent survey of 400 physicians she conducted, respondents typically said they would advise a physician-survivor to “get to a shelter quickly.” But when roles were reversed, they admitted going to a shelter was the least feasible option. Support groups can also be problematic in smaller communities where physicians might be recognized or see their own patients.
Complicating matters further, the violence often comes from within the medical community. This can lead to particularly malicious abuse tactics like sending false accusations to a victim’s regulatory college or board; prolonged court and custody battles to drain them of all resources and their ability to hold a job; or even sabotage, harassment, or violence at work. The sheen of the abuser’s public persona, on the other hand, can guard them from any accountability.
For example, one physician-survivor said her ex-partner, a psychiatrist, coerced her into believing she was mentally ill, claimed she was “psychotic” in order to take back their children after she left, and had numerous colleagues serve as character witnesses in court for him, “saying he couldn’t have done any of these things, how great he is, and what a wonderful father he is.”
Slow progress is still progress
After Sherilyn M. Gordon-Burroughs, MD, a Texas-based transplant surgeon, mother, and educator, was killed by her husband in a murder-suicide in 2017, her friends Barbara Lee Bass, MD, president of the American College of Surgeons, and Patricia L. Turner, MD, were spurred into action. Together, they founded the ACS Intimate Partner Violence Task Force. Their mission is to educate surgeons to identify the signs of intimate partner violence (IPV) in themselves and their colleagues and connect them with resources.
“There is a concerted effort to close that gap,” says D’Andrea K. Joseph, MD, cochair of the task force and chief of trauma and acute care surgery at NYU Langone in New York. In the future, Dr. Joseph predicts, “making this a part of the curriculum, that it’s standardized for residents and trainees, that there is a safe place for victims ... and that we can band together and really recognize and assist our colleagues who are in trouble.”
Resources created by the ACS IPV task force, such as the toolkit and curriculum, provide a model for other health care leaders. But there have been few similar initiatives aimed at increasing IPV intervention within the medical system.
What you can do in your workplace
In her essay, Ms. Lee explains that a major turning point came when a physician friend explicitly asked if she was experiencing abuse. He then gently confirmed she was, and asked without judgment how he could support her, an approach that mirrors advice from the National Domestic Violence Hotline.
“Having a physician validate that this was, indeed, an abusive situation helped enormously ... I believe it may have saved my life,” she writes.
That validation can be crucial, and Dr. Abadilla urges other physicians to regularly check in with colleagues, especially those who seem particularly positive with a go-getter attitude and yet may not seem themselves. That was how she presented when she was struggling the most.
Supporting systemic changes within your organization and beyond is also important. The authors of the 2022 meta-analysis stress the need for domestic violence training, legislative changes, paid leave, and union support.
Finding strength in recovery
Over a decade after escaping her abuser, Whitney says she’s only just begun to share her experience, but what she’s learned has made her a better pharmacist. She says she’s more attuned to subtle signs something could be off with patients and coworkers. When someone makes comments about feeling anxious or that they can’t do anything right, it’s important to ask why, she says.
Recently, Kimberly has opened up to her mentor and other therapists, many of whom have shared that they’re also survivors.
“The last thing I said to [my abuser] is you think you’ve won and you’re hurting me, but what you’ve done to me – I’m going to utilize this and I’m going to help other people,” Kimberly says. “This pain that I have will go away, and I’m going to save the lives of others.”
A version of this article first appeared on Medscape.com.
One in five doctors with long COVID can no longer work: Survey
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Resident creates AI alternative to U.S. News med school ranking
For decades, pre-med students depended on the annual medical school rankings by U.S. News and World Report to decide where to apply for physician education. But after several prominent med schools pulled out of the rankings, one resident began experimenting with artificial intelligence (AI) to create an alternative.
Brandon Turner MD, MSc, a radiation oncology resident at Massachusetts General Hospital in Boston, developed a free do-it-yourself tool using AI that allows prospective students to rank medical schools based on considerations that are most important to them. His research was published online in JAMA Network Open.
“One of the flaws with conventional ranking systems is that the metrics used in these tools are weighted based on the preferences and views of the people who developed these rankings, but those may not work for everyone,” Dr. Turner told this news organization.
He explained that there are different types of metrics used in the U.S. News ranking: one for research and the other for primary care. “The research rankings carry the most prestige and are the ones that most people know about,” he explained. These metrics take into account factors such as how many grant dollars the medical school receives and the average size of those grants per faculty member, Dr. Turner said.
Admission metrics are also included – for example, the median grade point average or MCAT scores of students who have been accepted. “These don’t tell you anything about the research output of the school, only about how selective the school is,” he said.
Primary care metrics might focus on how many graduates of a given school go into primary care, or how other schools rate the quality of primary care training at a given school – a process called peer assessment, Dr. Turner said.
But even though these might be helpful, students may be more interested in the cost of attendance, average debt, representation of minorities, and how many graduates pass their boards, he said. “U.S. News metrics don’t capture these things, but I included them in my algorithm.”
A U.S. News spokesperson said that the publication continues to help students and their families make decisions about their future education. The spokesperson cited U.S. News’ explanation of how it calculates its rankings. “A school’s overall Best Medical Schools rank should be one consideration and not the lone determinant in where a student applies and accepts,” the article states.
Dr. Turner agreed ranking systems are a good starting point when researching med schools, “but the values reflected in the ranking may not reflect an individual’s goals.”
Tyra-Lee Brett, a premed student at the University of South Florida, Tampa, believes an additional tool for students to evaluate medical schools is needed – and she could potentially see herself using Dr. Turner’s creation.
Still, Ms. Brett, a premed trustee of the American Medical Student Association, doesn’t regard any ranking tool as the “be all and end all.” Rather, she feels that the most effective tool would be based on students’ lived experiences. The AMSA is developing a scorecard in which students grade schools based on their opinions about such issues as housing, family planning, and environmental health, she said.
No prior judgments
To develop his algorithm, Dr. Turner used a branch of AI called “unsupervised learning.” It doesn’t make a prior judgment about what the data should look like, Dr. Turner explained.
“You’re just analyzing natural trends within the data.”
The algorithm tries to find and discover clusters or patterns within the data. “It’s like saying to the algorithm: ‘I want you to tell me what schools you think should be grouped together based on the data I feed you,’ which is the data that the user selects based on his or her personal preferences.”
U.S. News has been transparent about the metrics it uses, Dr. Turner notes. “When I started looking into how rankings are developed, I saw that there was transparency, and the reasoning for choosing the metrics used to develop the ranking was pretty sound,” he said.
“But I didn’t see any justification as to why they chose the particular metrics and weighted them in the way that they did.”
Dr. Turner extracted data from the 2023 U.S. News report, which ranked 109 allopathic medical schools, and applied several scenarios to the results to create his alternative ranking system.
In one scenario, he used the same research metrics used by U.S. News, such as a peer research assessment, median federal research activity per full-time faculty member, median GPA, median MCAT, acceptance rate, and faculty-student ratio.
In another scenario, he included four additional metrics: debt, in-state cost of attendance, USMLE Step 1 passing rate, and percentage of underrepresented students with minority race or ethnicity at the school.
For example, a user can rank the importance of the diversity of the class, amount of debt students expect to incur, and amount of research funding the medical school receives. After selecting those factors, the tool generates tiered results displayed in a circle, a shape chosen to avoid the appearance of the hierarchy associated with traditional rankings, Dr. Turner said.
“A prospective student might not care about acceptance rates and MCAT scores, and instead cares about diversity and debt,” Dr. Turner said. He looks forward to extending this approach to the ranking of colleges as well.
‘Imperfect measures’
“The model and interesting online tool that Dr. Turner created allows a premed [student] to generate custom rankings that are in line with their own priorities,” said Christopher Worsham, MD, MPH, a critical care physician in Mass General’s division of pulmonary and critical care medicine.
But Dr. Worsham, also a teaching associate at Harvard Medical School’s department of health care policy, expressed concern that factors figuring into the rankings by U.S. News and Dr. Turner’s alternative “are imperfect measures of medical school quality.”
For example, a student interested in research might favor federal research funding in their customized rankings with Dr. Turner’s model. “But higher research funding doesn’t necessarily translate into a better education for students, particularly when differentiating between two major research systems,” Dr. Worsham noted.
Dr. Worsham added that neither ranking system accurately predicts the quality of doctors graduating from the schools. Instead, he’d like to see ranking systems based on which schools’ graduates deliver the best patient outcomes, whether that’s through direct patient care, impactful research, or leadership within the health care system.
Michael Sauder, PhD, professor of sociology at the University of Iowa, Iowa City, said the model could offer a valuable alternative to the U.S. News ranking system. It might help users develop their own criteria for determining the ranking of medical schools, which is a big improvement over a “one-size-fits-all” approach, Dr. Sauder said.
And Hanna Stotland, an admission consultant based in Chicago, noted that most students rely on rankings because they “don’t have the luxury of advisers who know the ins and outs of different medical schools.” Given the role that rankings play, Ms. Stotland expects that every new ranking tool will have some influence on students.
This tool in particular “has the potential to be useful for students who have identified values they want their medical school to share.” For example, students who care about racial diversity “could use it to easily identify schools that are successful on that metric,” Ms. Stotland said.
Sujay Ratna, a 2nd-year med student at Icahn School of Medicine at Mount Sinai in New York, said he considered the U.S. News ranking his “go-to tool” when he was applying to med school.
But after reading Dr. Turner’s article, the AMSA membership vice president tried the algorithm. “I definitely would have used it had it existed when I was thinking of what schools to apply to and what [schools] to attend.”
The study had no specific funding. Dr. Turner, Dr. Worsham, Dr. Sauder, Ms. Stotland, Ms. Brett, and Mr. Ratna report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For decades, pre-med students depended on the annual medical school rankings by U.S. News and World Report to decide where to apply for physician education. But after several prominent med schools pulled out of the rankings, one resident began experimenting with artificial intelligence (AI) to create an alternative.
Brandon Turner MD, MSc, a radiation oncology resident at Massachusetts General Hospital in Boston, developed a free do-it-yourself tool using AI that allows prospective students to rank medical schools based on considerations that are most important to them. His research was published online in JAMA Network Open.
“One of the flaws with conventional ranking systems is that the metrics used in these tools are weighted based on the preferences and views of the people who developed these rankings, but those may not work for everyone,” Dr. Turner told this news organization.
He explained that there are different types of metrics used in the U.S. News ranking: one for research and the other for primary care. “The research rankings carry the most prestige and are the ones that most people know about,” he explained. These metrics take into account factors such as how many grant dollars the medical school receives and the average size of those grants per faculty member, Dr. Turner said.
Admission metrics are also included – for example, the median grade point average or MCAT scores of students who have been accepted. “These don’t tell you anything about the research output of the school, only about how selective the school is,” he said.
Primary care metrics might focus on how many graduates of a given school go into primary care, or how other schools rate the quality of primary care training at a given school – a process called peer assessment, Dr. Turner said.
But even though these might be helpful, students may be more interested in the cost of attendance, average debt, representation of minorities, and how many graduates pass their boards, he said. “U.S. News metrics don’t capture these things, but I included them in my algorithm.”
A U.S. News spokesperson said that the publication continues to help students and their families make decisions about their future education. The spokesperson cited U.S. News’ explanation of how it calculates its rankings. “A school’s overall Best Medical Schools rank should be one consideration and not the lone determinant in where a student applies and accepts,” the article states.
Dr. Turner agreed ranking systems are a good starting point when researching med schools, “but the values reflected in the ranking may not reflect an individual’s goals.”
Tyra-Lee Brett, a premed student at the University of South Florida, Tampa, believes an additional tool for students to evaluate medical schools is needed – and she could potentially see herself using Dr. Turner’s creation.
Still, Ms. Brett, a premed trustee of the American Medical Student Association, doesn’t regard any ranking tool as the “be all and end all.” Rather, she feels that the most effective tool would be based on students’ lived experiences. The AMSA is developing a scorecard in which students grade schools based on their opinions about such issues as housing, family planning, and environmental health, she said.
No prior judgments
To develop his algorithm, Dr. Turner used a branch of AI called “unsupervised learning.” It doesn’t make a prior judgment about what the data should look like, Dr. Turner explained.
“You’re just analyzing natural trends within the data.”
The algorithm tries to find and discover clusters or patterns within the data. “It’s like saying to the algorithm: ‘I want you to tell me what schools you think should be grouped together based on the data I feed you,’ which is the data that the user selects based on his or her personal preferences.”
U.S. News has been transparent about the metrics it uses, Dr. Turner notes. “When I started looking into how rankings are developed, I saw that there was transparency, and the reasoning for choosing the metrics used to develop the ranking was pretty sound,” he said.
“But I didn’t see any justification as to why they chose the particular metrics and weighted them in the way that they did.”
Dr. Turner extracted data from the 2023 U.S. News report, which ranked 109 allopathic medical schools, and applied several scenarios to the results to create his alternative ranking system.
In one scenario, he used the same research metrics used by U.S. News, such as a peer research assessment, median federal research activity per full-time faculty member, median GPA, median MCAT, acceptance rate, and faculty-student ratio.
In another scenario, he included four additional metrics: debt, in-state cost of attendance, USMLE Step 1 passing rate, and percentage of underrepresented students with minority race or ethnicity at the school.
For example, a user can rank the importance of the diversity of the class, amount of debt students expect to incur, and amount of research funding the medical school receives. After selecting those factors, the tool generates tiered results displayed in a circle, a shape chosen to avoid the appearance of the hierarchy associated with traditional rankings, Dr. Turner said.
“A prospective student might not care about acceptance rates and MCAT scores, and instead cares about diversity and debt,” Dr. Turner said. He looks forward to extending this approach to the ranking of colleges as well.
‘Imperfect measures’
“The model and interesting online tool that Dr. Turner created allows a premed [student] to generate custom rankings that are in line with their own priorities,” said Christopher Worsham, MD, MPH, a critical care physician in Mass General’s division of pulmonary and critical care medicine.
But Dr. Worsham, also a teaching associate at Harvard Medical School’s department of health care policy, expressed concern that factors figuring into the rankings by U.S. News and Dr. Turner’s alternative “are imperfect measures of medical school quality.”
For example, a student interested in research might favor federal research funding in their customized rankings with Dr. Turner’s model. “But higher research funding doesn’t necessarily translate into a better education for students, particularly when differentiating between two major research systems,” Dr. Worsham noted.
Dr. Worsham added that neither ranking system accurately predicts the quality of doctors graduating from the schools. Instead, he’d like to see ranking systems based on which schools’ graduates deliver the best patient outcomes, whether that’s through direct patient care, impactful research, or leadership within the health care system.
Michael Sauder, PhD, professor of sociology at the University of Iowa, Iowa City, said the model could offer a valuable alternative to the U.S. News ranking system. It might help users develop their own criteria for determining the ranking of medical schools, which is a big improvement over a “one-size-fits-all” approach, Dr. Sauder said.
And Hanna Stotland, an admission consultant based in Chicago, noted that most students rely on rankings because they “don’t have the luxury of advisers who know the ins and outs of different medical schools.” Given the role that rankings play, Ms. Stotland expects that every new ranking tool will have some influence on students.
This tool in particular “has the potential to be useful for students who have identified values they want their medical school to share.” For example, students who care about racial diversity “could use it to easily identify schools that are successful on that metric,” Ms. Stotland said.
Sujay Ratna, a 2nd-year med student at Icahn School of Medicine at Mount Sinai in New York, said he considered the U.S. News ranking his “go-to tool” when he was applying to med school.
But after reading Dr. Turner’s article, the AMSA membership vice president tried the algorithm. “I definitely would have used it had it existed when I was thinking of what schools to apply to and what [schools] to attend.”
The study had no specific funding. Dr. Turner, Dr. Worsham, Dr. Sauder, Ms. Stotland, Ms. Brett, and Mr. Ratna report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For decades, pre-med students depended on the annual medical school rankings by U.S. News and World Report to decide where to apply for physician education. But after several prominent med schools pulled out of the rankings, one resident began experimenting with artificial intelligence (AI) to create an alternative.
Brandon Turner MD, MSc, a radiation oncology resident at Massachusetts General Hospital in Boston, developed a free do-it-yourself tool using AI that allows prospective students to rank medical schools based on considerations that are most important to them. His research was published online in JAMA Network Open.
“One of the flaws with conventional ranking systems is that the metrics used in these tools are weighted based on the preferences and views of the people who developed these rankings, but those may not work for everyone,” Dr. Turner told this news organization.
He explained that there are different types of metrics used in the U.S. News ranking: one for research and the other for primary care. “The research rankings carry the most prestige and are the ones that most people know about,” he explained. These metrics take into account factors such as how many grant dollars the medical school receives and the average size of those grants per faculty member, Dr. Turner said.
Admission metrics are also included – for example, the median grade point average or MCAT scores of students who have been accepted. “These don’t tell you anything about the research output of the school, only about how selective the school is,” he said.
Primary care metrics might focus on how many graduates of a given school go into primary care, or how other schools rate the quality of primary care training at a given school – a process called peer assessment, Dr. Turner said.
But even though these might be helpful, students may be more interested in the cost of attendance, average debt, representation of minorities, and how many graduates pass their boards, he said. “U.S. News metrics don’t capture these things, but I included them in my algorithm.”
A U.S. News spokesperson said that the publication continues to help students and their families make decisions about their future education. The spokesperson cited U.S. News’ explanation of how it calculates its rankings. “A school’s overall Best Medical Schools rank should be one consideration and not the lone determinant in where a student applies and accepts,” the article states.
Dr. Turner agreed ranking systems are a good starting point when researching med schools, “but the values reflected in the ranking may not reflect an individual’s goals.”
Tyra-Lee Brett, a premed student at the University of South Florida, Tampa, believes an additional tool for students to evaluate medical schools is needed – and she could potentially see herself using Dr. Turner’s creation.
Still, Ms. Brett, a premed trustee of the American Medical Student Association, doesn’t regard any ranking tool as the “be all and end all.” Rather, she feels that the most effective tool would be based on students’ lived experiences. The AMSA is developing a scorecard in which students grade schools based on their opinions about such issues as housing, family planning, and environmental health, she said.
No prior judgments
To develop his algorithm, Dr. Turner used a branch of AI called “unsupervised learning.” It doesn’t make a prior judgment about what the data should look like, Dr. Turner explained.
“You’re just analyzing natural trends within the data.”
The algorithm tries to find and discover clusters or patterns within the data. “It’s like saying to the algorithm: ‘I want you to tell me what schools you think should be grouped together based on the data I feed you,’ which is the data that the user selects based on his or her personal preferences.”
U.S. News has been transparent about the metrics it uses, Dr. Turner notes. “When I started looking into how rankings are developed, I saw that there was transparency, and the reasoning for choosing the metrics used to develop the ranking was pretty sound,” he said.
“But I didn’t see any justification as to why they chose the particular metrics and weighted them in the way that they did.”
Dr. Turner extracted data from the 2023 U.S. News report, which ranked 109 allopathic medical schools, and applied several scenarios to the results to create his alternative ranking system.
In one scenario, he used the same research metrics used by U.S. News, such as a peer research assessment, median federal research activity per full-time faculty member, median GPA, median MCAT, acceptance rate, and faculty-student ratio.
In another scenario, he included four additional metrics: debt, in-state cost of attendance, USMLE Step 1 passing rate, and percentage of underrepresented students with minority race or ethnicity at the school.
For example, a user can rank the importance of the diversity of the class, amount of debt students expect to incur, and amount of research funding the medical school receives. After selecting those factors, the tool generates tiered results displayed in a circle, a shape chosen to avoid the appearance of the hierarchy associated with traditional rankings, Dr. Turner said.
“A prospective student might not care about acceptance rates and MCAT scores, and instead cares about diversity and debt,” Dr. Turner said. He looks forward to extending this approach to the ranking of colleges as well.
‘Imperfect measures’
“The model and interesting online tool that Dr. Turner created allows a premed [student] to generate custom rankings that are in line with their own priorities,” said Christopher Worsham, MD, MPH, a critical care physician in Mass General’s division of pulmonary and critical care medicine.
But Dr. Worsham, also a teaching associate at Harvard Medical School’s department of health care policy, expressed concern that factors figuring into the rankings by U.S. News and Dr. Turner’s alternative “are imperfect measures of medical school quality.”
For example, a student interested in research might favor federal research funding in their customized rankings with Dr. Turner’s model. “But higher research funding doesn’t necessarily translate into a better education for students, particularly when differentiating between two major research systems,” Dr. Worsham noted.
Dr. Worsham added that neither ranking system accurately predicts the quality of doctors graduating from the schools. Instead, he’d like to see ranking systems based on which schools’ graduates deliver the best patient outcomes, whether that’s through direct patient care, impactful research, or leadership within the health care system.
Michael Sauder, PhD, professor of sociology at the University of Iowa, Iowa City, said the model could offer a valuable alternative to the U.S. News ranking system. It might help users develop their own criteria for determining the ranking of medical schools, which is a big improvement over a “one-size-fits-all” approach, Dr. Sauder said.
And Hanna Stotland, an admission consultant based in Chicago, noted that most students rely on rankings because they “don’t have the luxury of advisers who know the ins and outs of different medical schools.” Given the role that rankings play, Ms. Stotland expects that every new ranking tool will have some influence on students.
This tool in particular “has the potential to be useful for students who have identified values they want their medical school to share.” For example, students who care about racial diversity “could use it to easily identify schools that are successful on that metric,” Ms. Stotland said.
Sujay Ratna, a 2nd-year med student at Icahn School of Medicine at Mount Sinai in New York, said he considered the U.S. News ranking his “go-to tool” when he was applying to med school.
But after reading Dr. Turner’s article, the AMSA membership vice president tried the algorithm. “I definitely would have used it had it existed when I was thinking of what schools to apply to and what [schools] to attend.”
The study had no specific funding. Dr. Turner, Dr. Worsham, Dr. Sauder, Ms. Stotland, Ms. Brett, and Mr. Ratna report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA to step up oversight of cosmetics, assess ‘forever chemicals’
They are also preparing to assess potential risks of so-called forever chemicals in these products.
The Food and Drug Administration last year gained new authority over cosmetics when Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by adding this bill to a December budget package.
“On average, consumers in the U.S. use six to 12 cosmetics products daily. But, until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced,” Namandjé Bumpus, PhD, FDA’s chief scientist, said in a press release.
In the statement, the FDA announced the release of a draft guidance document that is intended to help companies comply with the transparency requirements slated to kick in this December. The agency is accepting comments on this draft guidance through Sept. 7.
“Later this year, registration and listing of cosmetic product facilities and products will become a requirement, making information about cosmetic products, including the ingredients used in products and the facilities where they are produced, readily available to the agency,” Dr. Bumpus said.
The products, according to the FDA statement, include makeup, nail polishes, shaving creams, other grooming products, perfumes, face and body cleansers, hair products, moisturizers, and other skin care items.
MoCRA “represents a sea change in how FDA regulates the cosmetics industry,” attorneys Frederick R. Ball, Alyson Walker Lotman, and Kelly A. Bonner, wrote in an article for the Food and Drug Law Institute published in spring 2023.
The FDA has called the MoCRA law “the most significant expansion” of its authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic Act was passed in 1938.
The agency is in the process of expanding its staff to carry out newly authorized duties, including the tracking of adverse events. The FDA budget request for fiscal 2024, which begins Oct. 1, seeks $5 million for work needed to implement MoCRA.
PFAS, or ‘forever chemicals’
Some of the requested FDA funding is intended to prepare the agency to assess the use of per-and polyfluoroalkyl substances (PFAS) in cosmetics.
MoCRA sets a 3-year deadline for the FDA to issue an assessment of the use and potential risks of PFAS in cosmetics products. PFAS are sometimes added as ingredients in some cosmetic products, including lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara, according to the FDA. Sometimes the presence of PFAS in cosmetics is unintentional and is the result of impurities in raw materials or is due to the breakdown of ingredients, the FDA said.
The FDA’s website says that so far, the available research doesn’t allow for “definitive conclusions about the potential health risks of PFAS in cosmetics.”
The Centers for Disease Control and Prevention has stated that research has suggested potential links between high levels of certain PFAS, in general, with increased cholesterol levels, changes in liver enzyme levels, increased risk of hypertension or preeclampsia in pregnant women, and increased risk of kidney or testicular cancer.
PFAS compounds often are used to resist grease, oil, water, and heat in industrial settings. They are used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they can reduce friction, according to a National Academies of Sciences, Engineering, and Medicine report on PFAS that was issued last year.
PFAS are known as “forever chemicals” because they contain a carbon-fluorine bond, which does not break naturally. Even when PFAS are transformed in the body, they can assume other forms of PFAS that preserve the troublesome carbon-fluorine bond. With PFAS, the human body is confronted with a substance it doesn’t have the tools to process.
This is in contrast to proteins and carbohydrates, which are in a sense prepackaged for relatively easy disassembly in the human body. Many of these compounds have weak links that enzymes and stomach acid can take apart, such as sulfur-to-sulfur (disulfide) bonds. That’s why protein-based biotech drugs are injected instead of administered as pills. The ultimate goal of this digestion is for the body to gain energy from these compounds.
But with PFAS, the body faces the challenge of carbon-fluorine bonds that are very hard to break down, and there is no payoff for these efforts, Graham F. Peaslee, PhD, professor of physics at the University of Notre Dame (Indiana), told this news organization.
“Nothing will naturally eat it because when you break the bond, it’s like eating celery,” he said. “You use more calories to eat the celery than you gain back from it.”
Interest from a U.S. senator
Dr. Peaslee was one of the authors of a 2021 article about PFAS in cosmetics that appeared in the journal Environmental Science and Technology Letters.
In the article, Dr. Peaslee and colleagues reported on their screening of 231 cosmetic products purchased in the United States and Canada using particle-induced gamma-ray emission spectroscopy. They found cases of undisclosed PFAS in cosmetic products. Foundations, mascaras, and lip products were noted as being especially problematic.
Sen. Susan Collins (R-ME) cited Dr. Peaslee’s article in a 2021 floor speech as she argued for having the FDA ban the intentional addition of PFAS to cosmetics.
“The findings of this study are particularly alarming, as many of these products are subject to direct human exposure,” Sen. Collins said. “For example, lipstick is often inadvertently ingested, and mascara is sometimes absorbed through tear ducts.”
In addition, workers at cosmetics plants may be exposed to PFAS and discarded cosmetics that have these compounds, which could potentially contaminate drinking water, Sen. Collins said. In 2021, she introduced legislation seeking a ban on PFAS that are intentionally added to cosmetics. That legislation did not advance through the Senate.
But the Senate Appropriations Committee, on which Sen. Collins is the ranking Republican, wants the FDA to keep a ban on PFAS in mind.
The Senate Agriculture Appropriations subcommittee, which oversees the FDA’s budget, raised the issue of PFAS and cosmetics in a June report. The FDA should develop a plan outlining research needed to inform “regulatory decision making, including potential development of a proposed rule to ban intentionally added PFAS substances in cosmetics,” the subcommittee said.
A version of this article first appeared on Medscape.com.
They are also preparing to assess potential risks of so-called forever chemicals in these products.
The Food and Drug Administration last year gained new authority over cosmetics when Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by adding this bill to a December budget package.
“On average, consumers in the U.S. use six to 12 cosmetics products daily. But, until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced,” Namandjé Bumpus, PhD, FDA’s chief scientist, said in a press release.
In the statement, the FDA announced the release of a draft guidance document that is intended to help companies comply with the transparency requirements slated to kick in this December. The agency is accepting comments on this draft guidance through Sept. 7.
“Later this year, registration and listing of cosmetic product facilities and products will become a requirement, making information about cosmetic products, including the ingredients used in products and the facilities where they are produced, readily available to the agency,” Dr. Bumpus said.
The products, according to the FDA statement, include makeup, nail polishes, shaving creams, other grooming products, perfumes, face and body cleansers, hair products, moisturizers, and other skin care items.
MoCRA “represents a sea change in how FDA regulates the cosmetics industry,” attorneys Frederick R. Ball, Alyson Walker Lotman, and Kelly A. Bonner, wrote in an article for the Food and Drug Law Institute published in spring 2023.
The FDA has called the MoCRA law “the most significant expansion” of its authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic Act was passed in 1938.
The agency is in the process of expanding its staff to carry out newly authorized duties, including the tracking of adverse events. The FDA budget request for fiscal 2024, which begins Oct. 1, seeks $5 million for work needed to implement MoCRA.
PFAS, or ‘forever chemicals’
Some of the requested FDA funding is intended to prepare the agency to assess the use of per-and polyfluoroalkyl substances (PFAS) in cosmetics.
MoCRA sets a 3-year deadline for the FDA to issue an assessment of the use and potential risks of PFAS in cosmetics products. PFAS are sometimes added as ingredients in some cosmetic products, including lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara, according to the FDA. Sometimes the presence of PFAS in cosmetics is unintentional and is the result of impurities in raw materials or is due to the breakdown of ingredients, the FDA said.
The FDA’s website says that so far, the available research doesn’t allow for “definitive conclusions about the potential health risks of PFAS in cosmetics.”
The Centers for Disease Control and Prevention has stated that research has suggested potential links between high levels of certain PFAS, in general, with increased cholesterol levels, changes in liver enzyme levels, increased risk of hypertension or preeclampsia in pregnant women, and increased risk of kidney or testicular cancer.
PFAS compounds often are used to resist grease, oil, water, and heat in industrial settings. They are used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they can reduce friction, according to a National Academies of Sciences, Engineering, and Medicine report on PFAS that was issued last year.
PFAS are known as “forever chemicals” because they contain a carbon-fluorine bond, which does not break naturally. Even when PFAS are transformed in the body, they can assume other forms of PFAS that preserve the troublesome carbon-fluorine bond. With PFAS, the human body is confronted with a substance it doesn’t have the tools to process.
This is in contrast to proteins and carbohydrates, which are in a sense prepackaged for relatively easy disassembly in the human body. Many of these compounds have weak links that enzymes and stomach acid can take apart, such as sulfur-to-sulfur (disulfide) bonds. That’s why protein-based biotech drugs are injected instead of administered as pills. The ultimate goal of this digestion is for the body to gain energy from these compounds.
But with PFAS, the body faces the challenge of carbon-fluorine bonds that are very hard to break down, and there is no payoff for these efforts, Graham F. Peaslee, PhD, professor of physics at the University of Notre Dame (Indiana), told this news organization.
“Nothing will naturally eat it because when you break the bond, it’s like eating celery,” he said. “You use more calories to eat the celery than you gain back from it.”
Interest from a U.S. senator
Dr. Peaslee was one of the authors of a 2021 article about PFAS in cosmetics that appeared in the journal Environmental Science and Technology Letters.
In the article, Dr. Peaslee and colleagues reported on their screening of 231 cosmetic products purchased in the United States and Canada using particle-induced gamma-ray emission spectroscopy. They found cases of undisclosed PFAS in cosmetic products. Foundations, mascaras, and lip products were noted as being especially problematic.
Sen. Susan Collins (R-ME) cited Dr. Peaslee’s article in a 2021 floor speech as she argued for having the FDA ban the intentional addition of PFAS to cosmetics.
“The findings of this study are particularly alarming, as many of these products are subject to direct human exposure,” Sen. Collins said. “For example, lipstick is often inadvertently ingested, and mascara is sometimes absorbed through tear ducts.”
In addition, workers at cosmetics plants may be exposed to PFAS and discarded cosmetics that have these compounds, which could potentially contaminate drinking water, Sen. Collins said. In 2021, she introduced legislation seeking a ban on PFAS that are intentionally added to cosmetics. That legislation did not advance through the Senate.
But the Senate Appropriations Committee, on which Sen. Collins is the ranking Republican, wants the FDA to keep a ban on PFAS in mind.
The Senate Agriculture Appropriations subcommittee, which oversees the FDA’s budget, raised the issue of PFAS and cosmetics in a June report. The FDA should develop a plan outlining research needed to inform “regulatory decision making, including potential development of a proposed rule to ban intentionally added PFAS substances in cosmetics,” the subcommittee said.
A version of this article first appeared on Medscape.com.
They are also preparing to assess potential risks of so-called forever chemicals in these products.
The Food and Drug Administration last year gained new authority over cosmetics when Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by adding this bill to a December budget package.
“On average, consumers in the U.S. use six to 12 cosmetics products daily. But, until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced,” Namandjé Bumpus, PhD, FDA’s chief scientist, said in a press release.
In the statement, the FDA announced the release of a draft guidance document that is intended to help companies comply with the transparency requirements slated to kick in this December. The agency is accepting comments on this draft guidance through Sept. 7.
“Later this year, registration and listing of cosmetic product facilities and products will become a requirement, making information about cosmetic products, including the ingredients used in products and the facilities where they are produced, readily available to the agency,” Dr. Bumpus said.
The products, according to the FDA statement, include makeup, nail polishes, shaving creams, other grooming products, perfumes, face and body cleansers, hair products, moisturizers, and other skin care items.
MoCRA “represents a sea change in how FDA regulates the cosmetics industry,” attorneys Frederick R. Ball, Alyson Walker Lotman, and Kelly A. Bonner, wrote in an article for the Food and Drug Law Institute published in spring 2023.
The FDA has called the MoCRA law “the most significant expansion” of its authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic Act was passed in 1938.
The agency is in the process of expanding its staff to carry out newly authorized duties, including the tracking of adverse events. The FDA budget request for fiscal 2024, which begins Oct. 1, seeks $5 million for work needed to implement MoCRA.
PFAS, or ‘forever chemicals’
Some of the requested FDA funding is intended to prepare the agency to assess the use of per-and polyfluoroalkyl substances (PFAS) in cosmetics.
MoCRA sets a 3-year deadline for the FDA to issue an assessment of the use and potential risks of PFAS in cosmetics products. PFAS are sometimes added as ingredients in some cosmetic products, including lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara, according to the FDA. Sometimes the presence of PFAS in cosmetics is unintentional and is the result of impurities in raw materials or is due to the breakdown of ingredients, the FDA said.
The FDA’s website says that so far, the available research doesn’t allow for “definitive conclusions about the potential health risks of PFAS in cosmetics.”
The Centers for Disease Control and Prevention has stated that research has suggested potential links between high levels of certain PFAS, in general, with increased cholesterol levels, changes in liver enzyme levels, increased risk of hypertension or preeclampsia in pregnant women, and increased risk of kidney or testicular cancer.
PFAS compounds often are used to resist grease, oil, water, and heat in industrial settings. They are used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they can reduce friction, according to a National Academies of Sciences, Engineering, and Medicine report on PFAS that was issued last year.
PFAS are known as “forever chemicals” because they contain a carbon-fluorine bond, which does not break naturally. Even when PFAS are transformed in the body, they can assume other forms of PFAS that preserve the troublesome carbon-fluorine bond. With PFAS, the human body is confronted with a substance it doesn’t have the tools to process.
This is in contrast to proteins and carbohydrates, which are in a sense prepackaged for relatively easy disassembly in the human body. Many of these compounds have weak links that enzymes and stomach acid can take apart, such as sulfur-to-sulfur (disulfide) bonds. That’s why protein-based biotech drugs are injected instead of administered as pills. The ultimate goal of this digestion is for the body to gain energy from these compounds.
But with PFAS, the body faces the challenge of carbon-fluorine bonds that are very hard to break down, and there is no payoff for these efforts, Graham F. Peaslee, PhD, professor of physics at the University of Notre Dame (Indiana), told this news organization.
“Nothing will naturally eat it because when you break the bond, it’s like eating celery,” he said. “You use more calories to eat the celery than you gain back from it.”
Interest from a U.S. senator
Dr. Peaslee was one of the authors of a 2021 article about PFAS in cosmetics that appeared in the journal Environmental Science and Technology Letters.
In the article, Dr. Peaslee and colleagues reported on their screening of 231 cosmetic products purchased in the United States and Canada using particle-induced gamma-ray emission spectroscopy. They found cases of undisclosed PFAS in cosmetic products. Foundations, mascaras, and lip products were noted as being especially problematic.
Sen. Susan Collins (R-ME) cited Dr. Peaslee’s article in a 2021 floor speech as she argued for having the FDA ban the intentional addition of PFAS to cosmetics.
“The findings of this study are particularly alarming, as many of these products are subject to direct human exposure,” Sen. Collins said. “For example, lipstick is often inadvertently ingested, and mascara is sometimes absorbed through tear ducts.”
In addition, workers at cosmetics plants may be exposed to PFAS and discarded cosmetics that have these compounds, which could potentially contaminate drinking water, Sen. Collins said. In 2021, she introduced legislation seeking a ban on PFAS that are intentionally added to cosmetics. That legislation did not advance through the Senate.
But the Senate Appropriations Committee, on which Sen. Collins is the ranking Republican, wants the FDA to keep a ban on PFAS in mind.
The Senate Agriculture Appropriations subcommittee, which oversees the FDA’s budget, raised the issue of PFAS and cosmetics in a June report. The FDA should develop a plan outlining research needed to inform “regulatory decision making, including potential development of a proposed rule to ban intentionally added PFAS substances in cosmetics,” the subcommittee said.
A version of this article first appeared on Medscape.com.
Hemophilia: Concizumab lessens bleeding, could expand treatment options
Concizumab (Novo Nordisk), a subcutaneous monoclonal antibody administered once daily, shows significant reductions in annualized bleeding rates in patients with hemophilia A or B with inhibitors, potentially representing the first subcutaneous treatment option for patients with hemophilia B with inhibitors.
“These results demonstrate the potential of concizumab as an efficacious treatment option for people living with hemophilia A or B with inhibitors – the latter a population with severely limited treatment options,” first author Tadashi Matsushita, MD, PhD, of the department of transfusion medicine, Nagoya (Japan) University Hospital, said in an interview regarding the study, published in the New England Journal of Medicine.
The results are from the prospective, multicenter, phase 3 explorer7 trial, involving 133 patients, including 80 with hemophilia A and 53 had hemophilia B, all with inhibitors, a complication of hemophilia therapy in which antibodies ‘inhibit’ clot formation and complicate standard treatment.
The patients, aged 12 or older and all receiving on-demand treatment with bypassing agents, were randomized to receive no prophylaxis for at least 24 weeks (n = 19) or concizumab prophylaxis for at least 32 weeks (n = 33), with the remaining patients nonrandomly assigned to two groups receiving concizumab prophylaxis for at least 24 weeks (n = 81).
For the primary endpoint of the estimated mean annualized bleeding rate, the rate in the no-prophylaxis group was 11.8 episodes versus just 1.7 episodes in the concizumab prophylaxis 32-week group (rate ratio, 0.14; P < .001).
The overall median annualized bleeding rate for patients in all three groups receiving concizumab was zero episodes.
Annualized rates of treated spontaneous, joint, and target joint bleeding episodes were also lower in the concizumab groups versus the no-prophylaxis group, with annualized rate ratios that were similar to the annualized rate ratio for the primary endpoint.
While similar annualized bleeding rates were observed in hemophilia subtypes, the study wasn’t powered to show superiority according the hemophilia A or B, the authors noted.
Plasma concentrations of concizumab remained stable over the course of the study.
There were no significant differences in terms of key secondary endpoints of change in bodily pain and physical functioning scores from the start of treatment to week 24.
Pause for safety
Treatment in the study was paused for 6 months from March 2020 to August 2020 following nonfatal thromboembolic events occurring in three patients receiving concizumab, including one in the explorer7 trial and two in the concurrent explorer8 trial, evaluating the drug in patients with hemophilia without inhibitors.
The authors wrote that the three patients had all received concomitant treatment for bleeding and had thrombotic risk factors including obesity and other comorbidities.
The trial resumed following mitigation measures that included revising the dosing regimen to include a 1–mg/kg concizumab loading dose, followed by a subcutaneous once-daily dose of 0.2 mg/kg concizumab. No further thromboembolic events were reported after the pause. Otherwise, adverse events were mainly low grade, with serious events being rare.
Option for hemophilia B important
Of the two disease types, hemophilia A is much more common, with an estimated prevalence in the United States of 12 cases per 100,000 males versus hemophilia B, which has a rate of only 3.7 cases per 100,000, according to the Centers for Disease Control and Prevention. Women make up only about 1% of cases with moderate to severe hemophilia.
A standard treatment of hemophilia A or B is prophylaxis with factor replacement therapies allow for improved clotting and reduced bleeding. However, one caveat is the need for intravenous injections, as often as once daily in some cases.
The development of inhibitors in response to replacement therapy may further necessitate the need for treatment with bypassing agents for breakthrough bleeding.
As an alternative, non–factor replacement therapies can promote coagulation, notably the factor VIII mimetic emicizumab, given by subcutaneous injection, and approved by the Food and Drug Administration in 2018 for patients with and without inhibitors.
Emicizumab is recommended by the World Federation on Hemophilia over bypassing agents as prophylaxis for patients with hemophilia A and persistent inhibitors.
Importantly, however, there are no effective prophylactic treatments or easily administered subcutaneous therapies available for hemophilia B with inhibitors, underscoring the potential importance of concizumab, which targets the tissue factor pathway inhibitor protein, linked to coagulation.
“Concizumab has the potential to become the first subcutaneous and first-in-class treatment for hemophilia B with inhibitors,” Dr. Matsushita said. “There are other therapies investigated for hemophilia B with and without inhibitors still in clinical development,” he noted.
FDA resubmission planned
In May, Novo Nordisk announced that the FDA had rejected its application for concizumab, requesting more information on the drug’s manufacturing process and its system for the monitoring and dosing of patients to ensure proper drug administration. In a statement, Novo Nordisk reported its plans to move ahead.
“Novo Nordisk has begun addressing the FDA’s feedback and is working closely with the FDA as plans for resubmission continue,” the company reported. “We are confident in the potential of concizumab to address a significant unmet need, particularly for people with hemophilia B with inhibitors who currently have limited prophylactic options and are committed to bringing this important treatment to people with hemophilia with inhibitors living in the U.S.A.”
Meanwhile in Canada, concizumab was approved in March 2023 for the treatment of adolescent and adult patients with hemophilia B with inhibitors and who require routine prophylaxis to prevent or reduce the frequency of bleeding episodes.
The authors wrote that concizumab continues to be investigated across all hemophilia subtypes, including in the explorer10 study, which is evaluating the drug in children living with hemophilia A or B, with and without inhibitors.
The study was supported by Novo Nordisk. Dr. Matsushita reported speaking for and participating on a scientific advisory board of Novo Nordisk.
Concizumab (Novo Nordisk), a subcutaneous monoclonal antibody administered once daily, shows significant reductions in annualized bleeding rates in patients with hemophilia A or B with inhibitors, potentially representing the first subcutaneous treatment option for patients with hemophilia B with inhibitors.
“These results demonstrate the potential of concizumab as an efficacious treatment option for people living with hemophilia A or B with inhibitors – the latter a population with severely limited treatment options,” first author Tadashi Matsushita, MD, PhD, of the department of transfusion medicine, Nagoya (Japan) University Hospital, said in an interview regarding the study, published in the New England Journal of Medicine.
The results are from the prospective, multicenter, phase 3 explorer7 trial, involving 133 patients, including 80 with hemophilia A and 53 had hemophilia B, all with inhibitors, a complication of hemophilia therapy in which antibodies ‘inhibit’ clot formation and complicate standard treatment.
The patients, aged 12 or older and all receiving on-demand treatment with bypassing agents, were randomized to receive no prophylaxis for at least 24 weeks (n = 19) or concizumab prophylaxis for at least 32 weeks (n = 33), with the remaining patients nonrandomly assigned to two groups receiving concizumab prophylaxis for at least 24 weeks (n = 81).
For the primary endpoint of the estimated mean annualized bleeding rate, the rate in the no-prophylaxis group was 11.8 episodes versus just 1.7 episodes in the concizumab prophylaxis 32-week group (rate ratio, 0.14; P < .001).
The overall median annualized bleeding rate for patients in all three groups receiving concizumab was zero episodes.
Annualized rates of treated spontaneous, joint, and target joint bleeding episodes were also lower in the concizumab groups versus the no-prophylaxis group, with annualized rate ratios that were similar to the annualized rate ratio for the primary endpoint.
While similar annualized bleeding rates were observed in hemophilia subtypes, the study wasn’t powered to show superiority according the hemophilia A or B, the authors noted.
Plasma concentrations of concizumab remained stable over the course of the study.
There were no significant differences in terms of key secondary endpoints of change in bodily pain and physical functioning scores from the start of treatment to week 24.
Pause for safety
Treatment in the study was paused for 6 months from March 2020 to August 2020 following nonfatal thromboembolic events occurring in three patients receiving concizumab, including one in the explorer7 trial and two in the concurrent explorer8 trial, evaluating the drug in patients with hemophilia without inhibitors.
The authors wrote that the three patients had all received concomitant treatment for bleeding and had thrombotic risk factors including obesity and other comorbidities.
The trial resumed following mitigation measures that included revising the dosing regimen to include a 1–mg/kg concizumab loading dose, followed by a subcutaneous once-daily dose of 0.2 mg/kg concizumab. No further thromboembolic events were reported after the pause. Otherwise, adverse events were mainly low grade, with serious events being rare.
Option for hemophilia B important
Of the two disease types, hemophilia A is much more common, with an estimated prevalence in the United States of 12 cases per 100,000 males versus hemophilia B, which has a rate of only 3.7 cases per 100,000, according to the Centers for Disease Control and Prevention. Women make up only about 1% of cases with moderate to severe hemophilia.
A standard treatment of hemophilia A or B is prophylaxis with factor replacement therapies allow for improved clotting and reduced bleeding. However, one caveat is the need for intravenous injections, as often as once daily in some cases.
The development of inhibitors in response to replacement therapy may further necessitate the need for treatment with bypassing agents for breakthrough bleeding.
As an alternative, non–factor replacement therapies can promote coagulation, notably the factor VIII mimetic emicizumab, given by subcutaneous injection, and approved by the Food and Drug Administration in 2018 for patients with and without inhibitors.
Emicizumab is recommended by the World Federation on Hemophilia over bypassing agents as prophylaxis for patients with hemophilia A and persistent inhibitors.
Importantly, however, there are no effective prophylactic treatments or easily administered subcutaneous therapies available for hemophilia B with inhibitors, underscoring the potential importance of concizumab, which targets the tissue factor pathway inhibitor protein, linked to coagulation.
“Concizumab has the potential to become the first subcutaneous and first-in-class treatment for hemophilia B with inhibitors,” Dr. Matsushita said. “There are other therapies investigated for hemophilia B with and without inhibitors still in clinical development,” he noted.
FDA resubmission planned
In May, Novo Nordisk announced that the FDA had rejected its application for concizumab, requesting more information on the drug’s manufacturing process and its system for the monitoring and dosing of patients to ensure proper drug administration. In a statement, Novo Nordisk reported its plans to move ahead.
“Novo Nordisk has begun addressing the FDA’s feedback and is working closely with the FDA as plans for resubmission continue,” the company reported. “We are confident in the potential of concizumab to address a significant unmet need, particularly for people with hemophilia B with inhibitors who currently have limited prophylactic options and are committed to bringing this important treatment to people with hemophilia with inhibitors living in the U.S.A.”
Meanwhile in Canada, concizumab was approved in March 2023 for the treatment of adolescent and adult patients with hemophilia B with inhibitors and who require routine prophylaxis to prevent or reduce the frequency of bleeding episodes.
The authors wrote that concizumab continues to be investigated across all hemophilia subtypes, including in the explorer10 study, which is evaluating the drug in children living with hemophilia A or B, with and without inhibitors.
The study was supported by Novo Nordisk. Dr. Matsushita reported speaking for and participating on a scientific advisory board of Novo Nordisk.
Concizumab (Novo Nordisk), a subcutaneous monoclonal antibody administered once daily, shows significant reductions in annualized bleeding rates in patients with hemophilia A or B with inhibitors, potentially representing the first subcutaneous treatment option for patients with hemophilia B with inhibitors.
“These results demonstrate the potential of concizumab as an efficacious treatment option for people living with hemophilia A or B with inhibitors – the latter a population with severely limited treatment options,” first author Tadashi Matsushita, MD, PhD, of the department of transfusion medicine, Nagoya (Japan) University Hospital, said in an interview regarding the study, published in the New England Journal of Medicine.
The results are from the prospective, multicenter, phase 3 explorer7 trial, involving 133 patients, including 80 with hemophilia A and 53 had hemophilia B, all with inhibitors, a complication of hemophilia therapy in which antibodies ‘inhibit’ clot formation and complicate standard treatment.
The patients, aged 12 or older and all receiving on-demand treatment with bypassing agents, were randomized to receive no prophylaxis for at least 24 weeks (n = 19) or concizumab prophylaxis for at least 32 weeks (n = 33), with the remaining patients nonrandomly assigned to two groups receiving concizumab prophylaxis for at least 24 weeks (n = 81).
For the primary endpoint of the estimated mean annualized bleeding rate, the rate in the no-prophylaxis group was 11.8 episodes versus just 1.7 episodes in the concizumab prophylaxis 32-week group (rate ratio, 0.14; P < .001).
The overall median annualized bleeding rate for patients in all three groups receiving concizumab was zero episodes.
Annualized rates of treated spontaneous, joint, and target joint bleeding episodes were also lower in the concizumab groups versus the no-prophylaxis group, with annualized rate ratios that were similar to the annualized rate ratio for the primary endpoint.
While similar annualized bleeding rates were observed in hemophilia subtypes, the study wasn’t powered to show superiority according the hemophilia A or B, the authors noted.
Plasma concentrations of concizumab remained stable over the course of the study.
There were no significant differences in terms of key secondary endpoints of change in bodily pain and physical functioning scores from the start of treatment to week 24.
Pause for safety
Treatment in the study was paused for 6 months from March 2020 to August 2020 following nonfatal thromboembolic events occurring in three patients receiving concizumab, including one in the explorer7 trial and two in the concurrent explorer8 trial, evaluating the drug in patients with hemophilia without inhibitors.
The authors wrote that the three patients had all received concomitant treatment for bleeding and had thrombotic risk factors including obesity and other comorbidities.
The trial resumed following mitigation measures that included revising the dosing regimen to include a 1–mg/kg concizumab loading dose, followed by a subcutaneous once-daily dose of 0.2 mg/kg concizumab. No further thromboembolic events were reported after the pause. Otherwise, adverse events were mainly low grade, with serious events being rare.
Option for hemophilia B important
Of the two disease types, hemophilia A is much more common, with an estimated prevalence in the United States of 12 cases per 100,000 males versus hemophilia B, which has a rate of only 3.7 cases per 100,000, according to the Centers for Disease Control and Prevention. Women make up only about 1% of cases with moderate to severe hemophilia.
A standard treatment of hemophilia A or B is prophylaxis with factor replacement therapies allow for improved clotting and reduced bleeding. However, one caveat is the need for intravenous injections, as often as once daily in some cases.
The development of inhibitors in response to replacement therapy may further necessitate the need for treatment with bypassing agents for breakthrough bleeding.
As an alternative, non–factor replacement therapies can promote coagulation, notably the factor VIII mimetic emicizumab, given by subcutaneous injection, and approved by the Food and Drug Administration in 2018 for patients with and without inhibitors.
Emicizumab is recommended by the World Federation on Hemophilia over bypassing agents as prophylaxis for patients with hemophilia A and persistent inhibitors.
Importantly, however, there are no effective prophylactic treatments or easily administered subcutaneous therapies available for hemophilia B with inhibitors, underscoring the potential importance of concizumab, which targets the tissue factor pathway inhibitor protein, linked to coagulation.
“Concizumab has the potential to become the first subcutaneous and first-in-class treatment for hemophilia B with inhibitors,” Dr. Matsushita said. “There are other therapies investigated for hemophilia B with and without inhibitors still in clinical development,” he noted.
FDA resubmission planned
In May, Novo Nordisk announced that the FDA had rejected its application for concizumab, requesting more information on the drug’s manufacturing process and its system for the monitoring and dosing of patients to ensure proper drug administration. In a statement, Novo Nordisk reported its plans to move ahead.
“Novo Nordisk has begun addressing the FDA’s feedback and is working closely with the FDA as plans for resubmission continue,” the company reported. “We are confident in the potential of concizumab to address a significant unmet need, particularly for people with hemophilia B with inhibitors who currently have limited prophylactic options and are committed to bringing this important treatment to people with hemophilia with inhibitors living in the U.S.A.”
Meanwhile in Canada, concizumab was approved in March 2023 for the treatment of adolescent and adult patients with hemophilia B with inhibitors and who require routine prophylaxis to prevent or reduce the frequency of bleeding episodes.
The authors wrote that concizumab continues to be investigated across all hemophilia subtypes, including in the explorer10 study, which is evaluating the drug in children living with hemophilia A or B, with and without inhibitors.
The study was supported by Novo Nordisk. Dr. Matsushita reported speaking for and participating on a scientific advisory board of Novo Nordisk.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Medicare announces 10 drugs targeted for price cuts in 2026
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
Post-SCT, better survival in children with healthy gut diversity
“To the best of our knowledge, we present the first evidence of an association between pretransplantation lower gut microbiota diversity and poorer outcome in children undergoing allo-HSCT,” the authors report, in research published in the journal Blood. “Our findings underscore the importance of pre-transplant gut microbiota diversity and compositional structure in influencing allo-HSCT-related clinical outcomes in the pediatric setting.”
While allogeneic hematopoietic stem cell transplantation (allo-HSCT) can be potentially curative of hematologic malignancies, the stem cell transplantation process can wreak havoc on gut microbiota, because of factors including the conditioning regimen, antibiotic exposure, and dietary changes.
Specifically, the process can cause a substantial decrease in necessary alpha diversity and a potential expansion of possibly pathogenic bacteria.
While poor gut microbiota diversity has been linked to higher mortality in adult patients receiving allo-HSCT, research on the effects in pediatric patients is lacking.
“The gut microbiota of children differs from adults’ one, and this accounts for the need for specific pediatric studies on the gut microbiota-to–allo-HSCT relationship,” the authors write.
For the multicenter study, first author Riccardo Masetti, MD, PhD, of the department of pediatric oncology and hematology at the University of Bologna, Italy, and colleagues analyzed the gut microbiota diversity of 90 pediatric allo-HSCT recipients at four centers in Italy and one in Poland, stratifying the patients into groups of higher and lower diversity pretransplantation and again at the time of neutrophil engraftment.
Overall, gut microbiota diversity significantly declined from before allo-HSCT to afterward, at the time of neutrophil engraftment (P < .0001), with lower diversity observed in patients 3 years of age or younger.
With a median follow-up of 52 months, compared with the lower diversity group, those with higher diversity prior to transplantation had a significantly higher probability of overall survival (hazard ratio, 0.26; P = .011), after adjustment for age, graft source, donor type, intensity of conditioning regimen, center, and type of disease, with estimated overall survival at 52 months after allo-HSCT of 88.9% for the higher diversity group and 62.7% for the lower diversity group.
The cumulative incidence of grade II-IV acute GvHD was significantly lower for the higher diversity group versus lower diversity (20.0 versus 44.4, respectively; P = .017), as were the incidence rates of grade III-IV acute GvHD (2.2 versus 20.0; P = .007).
There were, however, no significant differences between the low and high diversity gut microbiota groups in relapse-free survival (P = .091).
The higher diversity group notably had higher relative abundances of potentially health-related bacterial families, including Ruminococcaceae and Oscillospiraceae, while the lower diversity group showed an overabundance of Enterococcaceae and Enterobacteriaceae.
Of note, the results differ from those observed in adults, among whom gut microbiota diversity before as well as after transplantation has been significantly associated with transplant outcomes, whereas with children, the association was limited to diversity prior to transplant.
In general, children have significantly lower diversity of gut microbiota than adults, with varying functional properties, and microbiota that is more easily modified by environmental factors, with larger changes occurring upon exposure to external stressors, the authors explain.
“Considering these different ecological properties compared to adults, we hypothesize that allo-HSCT–induced dysbiosis in the pediatric setting may imply loss of age-related gut microbiota signatures, including alpha diversity, with high interpatient variability,” they say.
Characteristics that were associated with higher or lower gut microbiota diversity prior to allo-HSCT included the treating center, suggesting that the geographical region may affect the diversity and the type of antibiotic exposure prior to the transplant.
Limitations included that “we didn’t assess other pretransplant characteristics such as the type of chemotherapy received, or the lifestyle, and this should be addressed in future studies on larger cohorts,” Dr. Masetti said in an interview.
While lengthy delays in screening of samples are barriers in the use of the gut microbiome as a tool in clinical practice, he noted that clinicians can take key measures to improve the microbiota.
“[Preventive measures] include the avoidance of unnecessary antibiotic treatment, which has a detrimental effect on the microbiota,” he said. “Moreover, some dietary changes may promote microbiota health.”
In addition, key measures can be taken during the allo-HSCT to preserve the microbiota, he added.
“In our center, we use enteral nutrition with a nasogastric tube rather than parenteral nutrition, which helps the microbiota to recover faster,” Dr. Masetti explained. “Moreover, other interventional measures such as fecal microbiota transplantation or the use of probiotics are under testing.”
“In particular, our data emphasize the importance of an overall healthy network, rather than the abundance of specific families or genera, in preventing complications and unfavorable outcomes.”
Commenting on the study, Robert Jenq, MD, an assistant professor in the departments of genomic medicine and stem cell transplantation and cellular therapy at the University of Texas M.D. Anderson Cancer Center, Houston, noted that with the growing evidence of the effects of poor gut microbiota diversity on clinical outcomes, multiple early-phase clinical trials are being conducted to test various strategies to prevent or treat gut injury.
“I’m not aware of any one approach that has shown enough promise to warrant being tested in multicenter studies yet, but it’s still a bit early,” Dr. Jenq said.“In the meantime, discontinuing or de-escalating antibiotics when medically safe, and encouraging patients to eat as much as they’re able to is a reasonable recommendation.”
Dr. Jenq added that, with most of the data on the issue being retrospective, a causative role has not been established, and “the finding of an association between the gut microbiota composition and survival, while interesting and provocative, does not provide evidence that intervening on the gut microbiota will lead to a clinical benefit.”
“I’m hopeful that randomized clinical trials will eventually demonstrate that we can protect or restore the gut microbiota, and this will lead to substantial clinical benefits, but this remains to be seen,” he said.
The authors had no disclosures to report. Dr. Jenq is an advisor for Seres Therapeutics, Prolacta Biosciences, and MaaT Pharma.
“To the best of our knowledge, we present the first evidence of an association between pretransplantation lower gut microbiota diversity and poorer outcome in children undergoing allo-HSCT,” the authors report, in research published in the journal Blood. “Our findings underscore the importance of pre-transplant gut microbiota diversity and compositional structure in influencing allo-HSCT-related clinical outcomes in the pediatric setting.”
While allogeneic hematopoietic stem cell transplantation (allo-HSCT) can be potentially curative of hematologic malignancies, the stem cell transplantation process can wreak havoc on gut microbiota, because of factors including the conditioning regimen, antibiotic exposure, and dietary changes.
Specifically, the process can cause a substantial decrease in necessary alpha diversity and a potential expansion of possibly pathogenic bacteria.
While poor gut microbiota diversity has been linked to higher mortality in adult patients receiving allo-HSCT, research on the effects in pediatric patients is lacking.
“The gut microbiota of children differs from adults’ one, and this accounts for the need for specific pediatric studies on the gut microbiota-to–allo-HSCT relationship,” the authors write.
For the multicenter study, first author Riccardo Masetti, MD, PhD, of the department of pediatric oncology and hematology at the University of Bologna, Italy, and colleagues analyzed the gut microbiota diversity of 90 pediatric allo-HSCT recipients at four centers in Italy and one in Poland, stratifying the patients into groups of higher and lower diversity pretransplantation and again at the time of neutrophil engraftment.
Overall, gut microbiota diversity significantly declined from before allo-HSCT to afterward, at the time of neutrophil engraftment (P < .0001), with lower diversity observed in patients 3 years of age or younger.
With a median follow-up of 52 months, compared with the lower diversity group, those with higher diversity prior to transplantation had a significantly higher probability of overall survival (hazard ratio, 0.26; P = .011), after adjustment for age, graft source, donor type, intensity of conditioning regimen, center, and type of disease, with estimated overall survival at 52 months after allo-HSCT of 88.9% for the higher diversity group and 62.7% for the lower diversity group.
The cumulative incidence of grade II-IV acute GvHD was significantly lower for the higher diversity group versus lower diversity (20.0 versus 44.4, respectively; P = .017), as were the incidence rates of grade III-IV acute GvHD (2.2 versus 20.0; P = .007).
There were, however, no significant differences between the low and high diversity gut microbiota groups in relapse-free survival (P = .091).
The higher diversity group notably had higher relative abundances of potentially health-related bacterial families, including Ruminococcaceae and Oscillospiraceae, while the lower diversity group showed an overabundance of Enterococcaceae and Enterobacteriaceae.
Of note, the results differ from those observed in adults, among whom gut microbiota diversity before as well as after transplantation has been significantly associated with transplant outcomes, whereas with children, the association was limited to diversity prior to transplant.
In general, children have significantly lower diversity of gut microbiota than adults, with varying functional properties, and microbiota that is more easily modified by environmental factors, with larger changes occurring upon exposure to external stressors, the authors explain.
“Considering these different ecological properties compared to adults, we hypothesize that allo-HSCT–induced dysbiosis in the pediatric setting may imply loss of age-related gut microbiota signatures, including alpha diversity, with high interpatient variability,” they say.
Characteristics that were associated with higher or lower gut microbiota diversity prior to allo-HSCT included the treating center, suggesting that the geographical region may affect the diversity and the type of antibiotic exposure prior to the transplant.
Limitations included that “we didn’t assess other pretransplant characteristics such as the type of chemotherapy received, or the lifestyle, and this should be addressed in future studies on larger cohorts,” Dr. Masetti said in an interview.
While lengthy delays in screening of samples are barriers in the use of the gut microbiome as a tool in clinical practice, he noted that clinicians can take key measures to improve the microbiota.
“[Preventive measures] include the avoidance of unnecessary antibiotic treatment, which has a detrimental effect on the microbiota,” he said. “Moreover, some dietary changes may promote microbiota health.”
In addition, key measures can be taken during the allo-HSCT to preserve the microbiota, he added.
“In our center, we use enteral nutrition with a nasogastric tube rather than parenteral nutrition, which helps the microbiota to recover faster,” Dr. Masetti explained. “Moreover, other interventional measures such as fecal microbiota transplantation or the use of probiotics are under testing.”
“In particular, our data emphasize the importance of an overall healthy network, rather than the abundance of specific families or genera, in preventing complications and unfavorable outcomes.”
Commenting on the study, Robert Jenq, MD, an assistant professor in the departments of genomic medicine and stem cell transplantation and cellular therapy at the University of Texas M.D. Anderson Cancer Center, Houston, noted that with the growing evidence of the effects of poor gut microbiota diversity on clinical outcomes, multiple early-phase clinical trials are being conducted to test various strategies to prevent or treat gut injury.
“I’m not aware of any one approach that has shown enough promise to warrant being tested in multicenter studies yet, but it’s still a bit early,” Dr. Jenq said.“In the meantime, discontinuing or de-escalating antibiotics when medically safe, and encouraging patients to eat as much as they’re able to is a reasonable recommendation.”
Dr. Jenq added that, with most of the data on the issue being retrospective, a causative role has not been established, and “the finding of an association between the gut microbiota composition and survival, while interesting and provocative, does not provide evidence that intervening on the gut microbiota will lead to a clinical benefit.”
“I’m hopeful that randomized clinical trials will eventually demonstrate that we can protect or restore the gut microbiota, and this will lead to substantial clinical benefits, but this remains to be seen,” he said.
The authors had no disclosures to report. Dr. Jenq is an advisor for Seres Therapeutics, Prolacta Biosciences, and MaaT Pharma.
“To the best of our knowledge, we present the first evidence of an association between pretransplantation lower gut microbiota diversity and poorer outcome in children undergoing allo-HSCT,” the authors report, in research published in the journal Blood. “Our findings underscore the importance of pre-transplant gut microbiota diversity and compositional structure in influencing allo-HSCT-related clinical outcomes in the pediatric setting.”
While allogeneic hematopoietic stem cell transplantation (allo-HSCT) can be potentially curative of hematologic malignancies, the stem cell transplantation process can wreak havoc on gut microbiota, because of factors including the conditioning regimen, antibiotic exposure, and dietary changes.
Specifically, the process can cause a substantial decrease in necessary alpha diversity and a potential expansion of possibly pathogenic bacteria.
While poor gut microbiota diversity has been linked to higher mortality in adult patients receiving allo-HSCT, research on the effects in pediatric patients is lacking.
“The gut microbiota of children differs from adults’ one, and this accounts for the need for specific pediatric studies on the gut microbiota-to–allo-HSCT relationship,” the authors write.
For the multicenter study, first author Riccardo Masetti, MD, PhD, of the department of pediatric oncology and hematology at the University of Bologna, Italy, and colleagues analyzed the gut microbiota diversity of 90 pediatric allo-HSCT recipients at four centers in Italy and one in Poland, stratifying the patients into groups of higher and lower diversity pretransplantation and again at the time of neutrophil engraftment.
Overall, gut microbiota diversity significantly declined from before allo-HSCT to afterward, at the time of neutrophil engraftment (P < .0001), with lower diversity observed in patients 3 years of age or younger.
With a median follow-up of 52 months, compared with the lower diversity group, those with higher diversity prior to transplantation had a significantly higher probability of overall survival (hazard ratio, 0.26; P = .011), after adjustment for age, graft source, donor type, intensity of conditioning regimen, center, and type of disease, with estimated overall survival at 52 months after allo-HSCT of 88.9% for the higher diversity group and 62.7% for the lower diversity group.
The cumulative incidence of grade II-IV acute GvHD was significantly lower for the higher diversity group versus lower diversity (20.0 versus 44.4, respectively; P = .017), as were the incidence rates of grade III-IV acute GvHD (2.2 versus 20.0; P = .007).
There were, however, no significant differences between the low and high diversity gut microbiota groups in relapse-free survival (P = .091).
The higher diversity group notably had higher relative abundances of potentially health-related bacterial families, including Ruminococcaceae and Oscillospiraceae, while the lower diversity group showed an overabundance of Enterococcaceae and Enterobacteriaceae.
Of note, the results differ from those observed in adults, among whom gut microbiota diversity before as well as after transplantation has been significantly associated with transplant outcomes, whereas with children, the association was limited to diversity prior to transplant.
In general, children have significantly lower diversity of gut microbiota than adults, with varying functional properties, and microbiota that is more easily modified by environmental factors, with larger changes occurring upon exposure to external stressors, the authors explain.
“Considering these different ecological properties compared to adults, we hypothesize that allo-HSCT–induced dysbiosis in the pediatric setting may imply loss of age-related gut microbiota signatures, including alpha diversity, with high interpatient variability,” they say.
Characteristics that were associated with higher or lower gut microbiota diversity prior to allo-HSCT included the treating center, suggesting that the geographical region may affect the diversity and the type of antibiotic exposure prior to the transplant.
Limitations included that “we didn’t assess other pretransplant characteristics such as the type of chemotherapy received, or the lifestyle, and this should be addressed in future studies on larger cohorts,” Dr. Masetti said in an interview.
While lengthy delays in screening of samples are barriers in the use of the gut microbiome as a tool in clinical practice, he noted that clinicians can take key measures to improve the microbiota.
“[Preventive measures] include the avoidance of unnecessary antibiotic treatment, which has a detrimental effect on the microbiota,” he said. “Moreover, some dietary changes may promote microbiota health.”
In addition, key measures can be taken during the allo-HSCT to preserve the microbiota, he added.
“In our center, we use enteral nutrition with a nasogastric tube rather than parenteral nutrition, which helps the microbiota to recover faster,” Dr. Masetti explained. “Moreover, other interventional measures such as fecal microbiota transplantation or the use of probiotics are under testing.”
“In particular, our data emphasize the importance of an overall healthy network, rather than the abundance of specific families or genera, in preventing complications and unfavorable outcomes.”
Commenting on the study, Robert Jenq, MD, an assistant professor in the departments of genomic medicine and stem cell transplantation and cellular therapy at the University of Texas M.D. Anderson Cancer Center, Houston, noted that with the growing evidence of the effects of poor gut microbiota diversity on clinical outcomes, multiple early-phase clinical trials are being conducted to test various strategies to prevent or treat gut injury.
“I’m not aware of any one approach that has shown enough promise to warrant being tested in multicenter studies yet, but it’s still a bit early,” Dr. Jenq said.“In the meantime, discontinuing or de-escalating antibiotics when medically safe, and encouraging patients to eat as much as they’re able to is a reasonable recommendation.”
Dr. Jenq added that, with most of the data on the issue being retrospective, a causative role has not been established, and “the finding of an association between the gut microbiota composition and survival, while interesting and provocative, does not provide evidence that intervening on the gut microbiota will lead to a clinical benefit.”
“I’m hopeful that randomized clinical trials will eventually demonstrate that we can protect or restore the gut microbiota, and this will lead to substantial clinical benefits, but this remains to be seen,” he said.
The authors had no disclosures to report. Dr. Jenq is an advisor for Seres Therapeutics, Prolacta Biosciences, and MaaT Pharma.
FROM BLOOD
Lymphoma specialist to lead MD Anderson’s cancer medicine division
“My research uncovered a series of physicians who served as ‘clinical champions’ and dramatically sped the process of drug development,” Dr. Flowers recalled in an interview. “This early career research inspired me to become the type of clinical champion that I uncovered.”
Over his career, hematologist-oncologist Dr. Flowers has developed lifesaving therapies for lymphoma, which has transformed into a highly treatable and even curable disease. He’s listed as a coauthor of hundreds of peer-reviewed cancer studies, reports, and medical society guidelines. And he’s revealed stark disparities in blood cancer care: His research shows that non-White patients suffer from worse outcomes, regardless of factors like income and insurance coverage.
The University of Texas MD Anderson Cancer Center, Houston, recently named physician-scientist Dr. Flowers as division head of cancer medicine, a position he’s held on an interim basis. As of Sept. 1, he will permanently oversee 300 faculty and more than 2,000 staff members.
A running start in Seattle
For Dr. Flowers, track and field is a sport that runs in the family. His grandfather was a top runner in both high school and college, and both Dr. Flowers and his brother ran competitively in Seattle, where they grew up. But Dr. Flowers chose a career in oncology, earning a medical degree at Stanford and master’s degrees at both Stanford and the University of Washington, Seattle.
The late Kenneth Melmon, MD, a groundbreaking pharmacologist, was a major influence. “He was one of the first people that I met when I began as an undergraduate at Stanford. We grew to be long-standing friends, and he demonstrated what outstanding mentorship looks like. In our research collaboration, we investigated the work of Dr. Gertrude Elion and Dr. George Hitchings involving the translation of pharmacological data from cellular and animal models to clinically useful drugs including 6-mercaptopurine, allopurinol, azathioprine, acyclovir, and zidovudine.”
The late Oliver Press, MD, a blood cancer specialist, inspired Dr. Flower’s interest in lymphoma. “I began work with him during an internship at the University of Washington. Ollie was a great inspiration and a key leader in the development of innovative therapies for lymphoma. He embodied the role of a clinical champion translating work in radioimmunotherapy to new therapeutics for patients with lymphomas. Working with him ultimately led me to pursue a career in hematology and oncology with a focus on the care for patients with lymphomas.”
Career blooms as lymphoma care advances
Dr. Flowers went on to Emory University, Atlanta, where he served as scientific director of the Research Informatics Shared Resource and a faculty member in the department of biomedical informatics. “I applied my training in informatics and my clinical expertise to support active grants from the Burroughs Wellcome Fund for Innovation in Regulatory Science and from the National Cancer Institute to develop informatics tools for pathology image analysis and prognostic modeling.”
For 13 years, he also served the Winship Cancer Institute as director of the Emory Healthcare lymphoma program (where his patients included Kansas City Chiefs football star Eric Berry), and for 4 years as scientific director of research informatics. Meanwhile, Dr. Flowers helped develop national practice guidelines for the American Society of Clinical Oncology, the American Cancer Society, and the American College of Radiology. He also chaired the ASCO guideline on management of febrile neutropenia.
In 2019, MD Anderson hired Dr. Flowers as chair of the department of lymphoma/myeloma. A year later, he was appointed division head ad interim for cancer medicine.
“Chris is a unique leader who expertly combines mentorship, sponsorship, and bidirectional open, honest communication,” said Sairah Ahmed, MD, associate professor of lymphoma at MD Anderson. “He doesn’t just empower his team to reach their goals. He also inspires those around him to turn vision into reality.”
As Dr. Flowers noted, many patients with lymphoma are now able to recover and live normal lives. He himself played a direct role himself in boosting lifespans.
“I have been fortunate to play a role in the development of several treatments that have led to advances in first-line therapy for patients with aggressive lymphomas. I partnered with others at MD Anderson, including Dr. Sattva Neelapu and Dr. Jason Westin, who have developed novel therapies like chimeric antigen receptor T-cell therapy for patients with relapse lymphomas,” he said. “Leaders in the field at MD Anderson like Dr. Michael Wang have developed new oral treatments for patients with rare lymphoma subtypes like mantle cell lymphoma. Other colleagues such as Dr. Nathan Fowler and Dr. Loretta Nastoupil have focused on the care for patients with indolent lymphomas and developed less-toxic therapies that are now in common use.”
Exposing the disparities in blood cancer care
Dr. Flowers, who’s African American, has also been a leader in health disparity research. In 2016, for example, he was coauthor of a study into non-Hodgkin’s lymphoma that revealed that Blacks in the United States have dramatically lower survival rates than Whites. The 10-year survival rate for Black women with chronic lymphocytic leukemia was just 47%, for example, compared with 66% for White females. “Although incidence rates of lymphoid neoplasms are generally higher among Whites, Black men tend to have poorer survival,” Dr. Flowers and colleagues wrote.
In a 2021 report for the ASCO Educational Book, Dr. Flowers and hematologist-oncologist Demetria Smith-Graziani, MD, now with Emory University, explored disparities across blood cancers and barriers to minority enrollment in clinical trials. “Some approaches that clinicians can apply to address these disparities include increasing systems-level awareness, improving access to care, and reducing biases in clinical setting,” the authors wrote.
Luis Malpica Castillo, MD, assistant professor of lymphoma at MD Anderson Cancer Center, lauded the work of Dr. Flowers in expanding opportunities for minority patients with the disease.
“During the past years, Dr. Flowers’ work has not only had a positive impact on the Texan community, but minority populations living with cancer in the United States and abroad,” he said. “Currently, we are implementing cancer care networks aimed to increase diversity in clinical trials by enrolling a larger number of Hispanic and African American patients, who otherwise may not have benefited from novel therapies. The ultimate goal is to provide high-quality care to all patients living with cancer.”
In addition to his research work, Dr. Flowers is an advocate for diversity within the hematology community. He’s a founding member and former chair of the American Society of Hematology’s Committee on Diversity, Equity and Inclusion (formerly the Committee on Promoting Diversity), and he helped develop the society’s Minority Recruitment Initiative.
What’s next for Dr. Flowers? For one, he plans to continue working as a mentor; he received the ASH Mentor Award in honor of his service in 2022. “I am strongly committed to increasing the number of tenure-track investigators trained in clinical and translational cancer research and to promote their career development.”
And he looks forward to helping develop MD Anderson’s recently announced $2.5 billion hospital in Austin. “This will extend the exceptional care that we provide as the No. 1 cancer center in the United States,” he said. “It will also create new opportunities for research and collaboration with experts at UT Austin.”
When he’s not in clinic, Dr. Flowers embraces his lifelong love of speeding through life on his own two feet. He’s even inspired his children to share his passion. “I run most days of the week,” he said. “Running provides a great opportunity to think and process new research ideas, work through leadership challenges, and sometimes just to relax and let go of the day.”
“My research uncovered a series of physicians who served as ‘clinical champions’ and dramatically sped the process of drug development,” Dr. Flowers recalled in an interview. “This early career research inspired me to become the type of clinical champion that I uncovered.”
Over his career, hematologist-oncologist Dr. Flowers has developed lifesaving therapies for lymphoma, which has transformed into a highly treatable and even curable disease. He’s listed as a coauthor of hundreds of peer-reviewed cancer studies, reports, and medical society guidelines. And he’s revealed stark disparities in blood cancer care: His research shows that non-White patients suffer from worse outcomes, regardless of factors like income and insurance coverage.
The University of Texas MD Anderson Cancer Center, Houston, recently named physician-scientist Dr. Flowers as division head of cancer medicine, a position he’s held on an interim basis. As of Sept. 1, he will permanently oversee 300 faculty and more than 2,000 staff members.
A running start in Seattle
For Dr. Flowers, track and field is a sport that runs in the family. His grandfather was a top runner in both high school and college, and both Dr. Flowers and his brother ran competitively in Seattle, where they grew up. But Dr. Flowers chose a career in oncology, earning a medical degree at Stanford and master’s degrees at both Stanford and the University of Washington, Seattle.
The late Kenneth Melmon, MD, a groundbreaking pharmacologist, was a major influence. “He was one of the first people that I met when I began as an undergraduate at Stanford. We grew to be long-standing friends, and he demonstrated what outstanding mentorship looks like. In our research collaboration, we investigated the work of Dr. Gertrude Elion and Dr. George Hitchings involving the translation of pharmacological data from cellular and animal models to clinically useful drugs including 6-mercaptopurine, allopurinol, azathioprine, acyclovir, and zidovudine.”
The late Oliver Press, MD, a blood cancer specialist, inspired Dr. Flower’s interest in lymphoma. “I began work with him during an internship at the University of Washington. Ollie was a great inspiration and a key leader in the development of innovative therapies for lymphoma. He embodied the role of a clinical champion translating work in radioimmunotherapy to new therapeutics for patients with lymphomas. Working with him ultimately led me to pursue a career in hematology and oncology with a focus on the care for patients with lymphomas.”
Career blooms as lymphoma care advances
Dr. Flowers went on to Emory University, Atlanta, where he served as scientific director of the Research Informatics Shared Resource and a faculty member in the department of biomedical informatics. “I applied my training in informatics and my clinical expertise to support active grants from the Burroughs Wellcome Fund for Innovation in Regulatory Science and from the National Cancer Institute to develop informatics tools for pathology image analysis and prognostic modeling.”
For 13 years, he also served the Winship Cancer Institute as director of the Emory Healthcare lymphoma program (where his patients included Kansas City Chiefs football star Eric Berry), and for 4 years as scientific director of research informatics. Meanwhile, Dr. Flowers helped develop national practice guidelines for the American Society of Clinical Oncology, the American Cancer Society, and the American College of Radiology. He also chaired the ASCO guideline on management of febrile neutropenia.
In 2019, MD Anderson hired Dr. Flowers as chair of the department of lymphoma/myeloma. A year later, he was appointed division head ad interim for cancer medicine.
“Chris is a unique leader who expertly combines mentorship, sponsorship, and bidirectional open, honest communication,” said Sairah Ahmed, MD, associate professor of lymphoma at MD Anderson. “He doesn’t just empower his team to reach their goals. He also inspires those around him to turn vision into reality.”
As Dr. Flowers noted, many patients with lymphoma are now able to recover and live normal lives. He himself played a direct role himself in boosting lifespans.
“I have been fortunate to play a role in the development of several treatments that have led to advances in first-line therapy for patients with aggressive lymphomas. I partnered with others at MD Anderson, including Dr. Sattva Neelapu and Dr. Jason Westin, who have developed novel therapies like chimeric antigen receptor T-cell therapy for patients with relapse lymphomas,” he said. “Leaders in the field at MD Anderson like Dr. Michael Wang have developed new oral treatments for patients with rare lymphoma subtypes like mantle cell lymphoma. Other colleagues such as Dr. Nathan Fowler and Dr. Loretta Nastoupil have focused on the care for patients with indolent lymphomas and developed less-toxic therapies that are now in common use.”
Exposing the disparities in blood cancer care
Dr. Flowers, who’s African American, has also been a leader in health disparity research. In 2016, for example, he was coauthor of a study into non-Hodgkin’s lymphoma that revealed that Blacks in the United States have dramatically lower survival rates than Whites. The 10-year survival rate for Black women with chronic lymphocytic leukemia was just 47%, for example, compared with 66% for White females. “Although incidence rates of lymphoid neoplasms are generally higher among Whites, Black men tend to have poorer survival,” Dr. Flowers and colleagues wrote.
In a 2021 report for the ASCO Educational Book, Dr. Flowers and hematologist-oncologist Demetria Smith-Graziani, MD, now with Emory University, explored disparities across blood cancers and barriers to minority enrollment in clinical trials. “Some approaches that clinicians can apply to address these disparities include increasing systems-level awareness, improving access to care, and reducing biases in clinical setting,” the authors wrote.
Luis Malpica Castillo, MD, assistant professor of lymphoma at MD Anderson Cancer Center, lauded the work of Dr. Flowers in expanding opportunities for minority patients with the disease.
“During the past years, Dr. Flowers’ work has not only had a positive impact on the Texan community, but minority populations living with cancer in the United States and abroad,” he said. “Currently, we are implementing cancer care networks aimed to increase diversity in clinical trials by enrolling a larger number of Hispanic and African American patients, who otherwise may not have benefited from novel therapies. The ultimate goal is to provide high-quality care to all patients living with cancer.”
In addition to his research work, Dr. Flowers is an advocate for diversity within the hematology community. He’s a founding member and former chair of the American Society of Hematology’s Committee on Diversity, Equity and Inclusion (formerly the Committee on Promoting Diversity), and he helped develop the society’s Minority Recruitment Initiative.
What’s next for Dr. Flowers? For one, he plans to continue working as a mentor; he received the ASH Mentor Award in honor of his service in 2022. “I am strongly committed to increasing the number of tenure-track investigators trained in clinical and translational cancer research and to promote their career development.”
And he looks forward to helping develop MD Anderson’s recently announced $2.5 billion hospital in Austin. “This will extend the exceptional care that we provide as the No. 1 cancer center in the United States,” he said. “It will also create new opportunities for research and collaboration with experts at UT Austin.”
When he’s not in clinic, Dr. Flowers embraces his lifelong love of speeding through life on his own two feet. He’s even inspired his children to share his passion. “I run most days of the week,” he said. “Running provides a great opportunity to think and process new research ideas, work through leadership challenges, and sometimes just to relax and let go of the day.”
“My research uncovered a series of physicians who served as ‘clinical champions’ and dramatically sped the process of drug development,” Dr. Flowers recalled in an interview. “This early career research inspired me to become the type of clinical champion that I uncovered.”
Over his career, hematologist-oncologist Dr. Flowers has developed lifesaving therapies for lymphoma, which has transformed into a highly treatable and even curable disease. He’s listed as a coauthor of hundreds of peer-reviewed cancer studies, reports, and medical society guidelines. And he’s revealed stark disparities in blood cancer care: His research shows that non-White patients suffer from worse outcomes, regardless of factors like income and insurance coverage.
The University of Texas MD Anderson Cancer Center, Houston, recently named physician-scientist Dr. Flowers as division head of cancer medicine, a position he’s held on an interim basis. As of Sept. 1, he will permanently oversee 300 faculty and more than 2,000 staff members.
A running start in Seattle
For Dr. Flowers, track and field is a sport that runs in the family. His grandfather was a top runner in both high school and college, and both Dr. Flowers and his brother ran competitively in Seattle, where they grew up. But Dr. Flowers chose a career in oncology, earning a medical degree at Stanford and master’s degrees at both Stanford and the University of Washington, Seattle.
The late Kenneth Melmon, MD, a groundbreaking pharmacologist, was a major influence. “He was one of the first people that I met when I began as an undergraduate at Stanford. We grew to be long-standing friends, and he demonstrated what outstanding mentorship looks like. In our research collaboration, we investigated the work of Dr. Gertrude Elion and Dr. George Hitchings involving the translation of pharmacological data from cellular and animal models to clinically useful drugs including 6-mercaptopurine, allopurinol, azathioprine, acyclovir, and zidovudine.”
The late Oliver Press, MD, a blood cancer specialist, inspired Dr. Flower’s interest in lymphoma. “I began work with him during an internship at the University of Washington. Ollie was a great inspiration and a key leader in the development of innovative therapies for lymphoma. He embodied the role of a clinical champion translating work in radioimmunotherapy to new therapeutics for patients with lymphomas. Working with him ultimately led me to pursue a career in hematology and oncology with a focus on the care for patients with lymphomas.”
Career blooms as lymphoma care advances
Dr. Flowers went on to Emory University, Atlanta, where he served as scientific director of the Research Informatics Shared Resource and a faculty member in the department of biomedical informatics. “I applied my training in informatics and my clinical expertise to support active grants from the Burroughs Wellcome Fund for Innovation in Regulatory Science and from the National Cancer Institute to develop informatics tools for pathology image analysis and prognostic modeling.”
For 13 years, he also served the Winship Cancer Institute as director of the Emory Healthcare lymphoma program (where his patients included Kansas City Chiefs football star Eric Berry), and for 4 years as scientific director of research informatics. Meanwhile, Dr. Flowers helped develop national practice guidelines for the American Society of Clinical Oncology, the American Cancer Society, and the American College of Radiology. He also chaired the ASCO guideline on management of febrile neutropenia.
In 2019, MD Anderson hired Dr. Flowers as chair of the department of lymphoma/myeloma. A year later, he was appointed division head ad interim for cancer medicine.
“Chris is a unique leader who expertly combines mentorship, sponsorship, and bidirectional open, honest communication,” said Sairah Ahmed, MD, associate professor of lymphoma at MD Anderson. “He doesn’t just empower his team to reach their goals. He also inspires those around him to turn vision into reality.”
As Dr. Flowers noted, many patients with lymphoma are now able to recover and live normal lives. He himself played a direct role himself in boosting lifespans.
“I have been fortunate to play a role in the development of several treatments that have led to advances in first-line therapy for patients with aggressive lymphomas. I partnered with others at MD Anderson, including Dr. Sattva Neelapu and Dr. Jason Westin, who have developed novel therapies like chimeric antigen receptor T-cell therapy for patients with relapse lymphomas,” he said. “Leaders in the field at MD Anderson like Dr. Michael Wang have developed new oral treatments for patients with rare lymphoma subtypes like mantle cell lymphoma. Other colleagues such as Dr. Nathan Fowler and Dr. Loretta Nastoupil have focused on the care for patients with indolent lymphomas and developed less-toxic therapies that are now in common use.”
Exposing the disparities in blood cancer care
Dr. Flowers, who’s African American, has also been a leader in health disparity research. In 2016, for example, he was coauthor of a study into non-Hodgkin’s lymphoma that revealed that Blacks in the United States have dramatically lower survival rates than Whites. The 10-year survival rate for Black women with chronic lymphocytic leukemia was just 47%, for example, compared with 66% for White females. “Although incidence rates of lymphoid neoplasms are generally higher among Whites, Black men tend to have poorer survival,” Dr. Flowers and colleagues wrote.
In a 2021 report for the ASCO Educational Book, Dr. Flowers and hematologist-oncologist Demetria Smith-Graziani, MD, now with Emory University, explored disparities across blood cancers and barriers to minority enrollment in clinical trials. “Some approaches that clinicians can apply to address these disparities include increasing systems-level awareness, improving access to care, and reducing biases in clinical setting,” the authors wrote.
Luis Malpica Castillo, MD, assistant professor of lymphoma at MD Anderson Cancer Center, lauded the work of Dr. Flowers in expanding opportunities for minority patients with the disease.
“During the past years, Dr. Flowers’ work has not only had a positive impact on the Texan community, but minority populations living with cancer in the United States and abroad,” he said. “Currently, we are implementing cancer care networks aimed to increase diversity in clinical trials by enrolling a larger number of Hispanic and African American patients, who otherwise may not have benefited from novel therapies. The ultimate goal is to provide high-quality care to all patients living with cancer.”
In addition to his research work, Dr. Flowers is an advocate for diversity within the hematology community. He’s a founding member and former chair of the American Society of Hematology’s Committee on Diversity, Equity and Inclusion (formerly the Committee on Promoting Diversity), and he helped develop the society’s Minority Recruitment Initiative.
What’s next for Dr. Flowers? For one, he plans to continue working as a mentor; he received the ASH Mentor Award in honor of his service in 2022. “I am strongly committed to increasing the number of tenure-track investigators trained in clinical and translational cancer research and to promote their career development.”
And he looks forward to helping develop MD Anderson’s recently announced $2.5 billion hospital in Austin. “This will extend the exceptional care that we provide as the No. 1 cancer center in the United States,” he said. “It will also create new opportunities for research and collaboration with experts at UT Austin.”
When he’s not in clinic, Dr. Flowers embraces his lifelong love of speeding through life on his own two feet. He’s even inspired his children to share his passion. “I run most days of the week,” he said. “Running provides a great opportunity to think and process new research ideas, work through leadership challenges, and sometimes just to relax and let go of the day.”
SCD: Survival disparities seen across insurance types
“To the best of our knowledge, this is the first United States nationwide investigation into lifetime survival for individuals with sickle cell disease covered by Medicare and Medicaid and disparities in survival by public insurance type, using comprehensive claims data collected from all 50 states,” the authors report in the study, published in Blood Advances.
“Our study underscores the persistent life expectancy shortfall for patients with sickle cell disease, the burden of premature mortality during adulthood, and survival disparities by insurance status,” they write.
SCD, which disproportionately affects people of African descent, significantly increases the risk of various acute and chronic life-threatening complications, including acute pain crisis, stroke, acute chest syndrome, chronic pain, symptomatic anemia, infections, and organ damage.
In recent years, advances ranging from newborn screening to prophylactic antibiotics have significantly improved the survival rates of children with SCD in the United States. However, premature mortality in adulthood has remained well above that of the general public.
A recent study of a U.S. birth cohort predicted life expectancy with SCD to be approximately 2 decades shorter than in those without the disease. However, those projections were based on a simulation model, and other life expectancy estimates have had sample sizes with state or regional limitations.
With no population-level individual data–based periodic life tables existing for individuals with SCD, the authors turned to nationwide data on Medicare and Medicaid beneficiaries to get a better idea of survival predictions.
For the study, they identified 94,616 individuals diagnosed with SCD who had not undergone transplant and were receiving common care, based on nationwide Medicare and Medicaid claim data from 2008 to 2016 on beneficiaries in all 50 states.
Of the patients, 74% were Black and 53% resided in the South at the index date. The patients had a mean entry age overall of 26.6 years across insurance types.
The results showed projected life expectancy at birth with SCD to be 52.6 years, with the life expectancy at birth for females significantly longer, compared with that of males (55.0 versus 49.3 years). For the general population, U.S. life expectancy estimates have been reported to be about 76 years.
Specifically, life expectancy in the cohort into adulthood was 35.4 years at 18 years of age; 24.1 years at 35 years of age; 19.6 years at 45 years old; 13.2 years at 65 years of age; and 5.4 years at 85 years old.
Likewise, survival probability rates were high during childhood, with survival probability at 18 years being 0.98, declining to 0.804 at age 30; 0.628 at age 45; 0.267 at age 65 and 0.70 at age 85.
Black individuals had a significantly shorter life expectancy at birth than non-Black individuals (52.2 vs. 55.1 years), with the survival expectancy for Black individuals significantly shorter than the 75-year life expectancy estimated by the 2016 U.S. Centers for Disease Control and Prevention life table for the Black population in general.
Of the patients, only 5% had Medicare old age and survivor’s insurance, and 4% had Medicare disability insurance benefits or end-stage renal disease (ESRD), while 48% had Medicaid, and 43% were dually eligible for Medicare and Medicaid. The racial and regional distributions were similar across insurance types.
Adults covered by Medicare had a life expectancy of 39.8 years at 18 years of age, an improvement over life expectancy for those with Medicare for disabilities or ESRD and those dually insured by Medicare and Medicaid.
And the shorter life expectancy with Medicare and Medicaid was also observed among beneficiaries aged 65 or older, compared with those enrolled in Medicare old age and survivor’s insurance.
“Evidently, the life expectancy gap persists among patients with sickle cell disease, even though they are protected by public insurance,” the authors report.
The shorter life expectancy among those with dual eligibility for Medicare and Medicaid is consistent with that of the general population, the authors add.
“It is well-recognized that the dual-eligible individuals are a vulnerable population with some of the most complex and expensive health care needs,” they write. “They are more likely to have fewer socioeconomic resources, more chronic conditions, and poorer survival outcomes than single eligible.”
First author Boshen Jiao, PhD, MPH, of the Harvard T.H. Chan School of Public Health, in Boston, noted that socioeconomic factors, on a broader level, have an important impact on SCD.
“Even the first and most established FDA-approved medication for sickle cell disease, hydroxyurea, suffers from underutilization,” he told MDedge. “This issue can be associated with disparities along racial lines, particularly concerning access to treatments.”
Such factors can also play a role in the lack of a well-coordinated transition program needed to prevent gaps in care between youth and adulthood, as well as a lack of access to specialized care needed to potentially initiate new therapies.
Ultimately, the decline in survival seen from childhood to adulthood in SCD “emphasizes the pivotal role of health care during the critical transition phase from childhood to adulthood for individuals with sickle cell disease,” Dr. Jiao said. “Moreover, the prospect of a curative therapy that can intervene in the early stages of life, prior to adulthood, stands as a desirable goal.”
The authors urge that “future studies should uncover factors influencing survival outcomes and explore policy options to address the unmet needs of the disabled or dually eligible population with SCD.” They had no disclosures to report.
A version of this article first appeared on Medscape.com.
“To the best of our knowledge, this is the first United States nationwide investigation into lifetime survival for individuals with sickle cell disease covered by Medicare and Medicaid and disparities in survival by public insurance type, using comprehensive claims data collected from all 50 states,” the authors report in the study, published in Blood Advances.
“Our study underscores the persistent life expectancy shortfall for patients with sickle cell disease, the burden of premature mortality during adulthood, and survival disparities by insurance status,” they write.
SCD, which disproportionately affects people of African descent, significantly increases the risk of various acute and chronic life-threatening complications, including acute pain crisis, stroke, acute chest syndrome, chronic pain, symptomatic anemia, infections, and organ damage.
In recent years, advances ranging from newborn screening to prophylactic antibiotics have significantly improved the survival rates of children with SCD in the United States. However, premature mortality in adulthood has remained well above that of the general public.
A recent study of a U.S. birth cohort predicted life expectancy with SCD to be approximately 2 decades shorter than in those without the disease. However, those projections were based on a simulation model, and other life expectancy estimates have had sample sizes with state or regional limitations.
With no population-level individual data–based periodic life tables existing for individuals with SCD, the authors turned to nationwide data on Medicare and Medicaid beneficiaries to get a better idea of survival predictions.
For the study, they identified 94,616 individuals diagnosed with SCD who had not undergone transplant and were receiving common care, based on nationwide Medicare and Medicaid claim data from 2008 to 2016 on beneficiaries in all 50 states.
Of the patients, 74% were Black and 53% resided in the South at the index date. The patients had a mean entry age overall of 26.6 years across insurance types.
The results showed projected life expectancy at birth with SCD to be 52.6 years, with the life expectancy at birth for females significantly longer, compared with that of males (55.0 versus 49.3 years). For the general population, U.S. life expectancy estimates have been reported to be about 76 years.
Specifically, life expectancy in the cohort into adulthood was 35.4 years at 18 years of age; 24.1 years at 35 years of age; 19.6 years at 45 years old; 13.2 years at 65 years of age; and 5.4 years at 85 years old.
Likewise, survival probability rates were high during childhood, with survival probability at 18 years being 0.98, declining to 0.804 at age 30; 0.628 at age 45; 0.267 at age 65 and 0.70 at age 85.
Black individuals had a significantly shorter life expectancy at birth than non-Black individuals (52.2 vs. 55.1 years), with the survival expectancy for Black individuals significantly shorter than the 75-year life expectancy estimated by the 2016 U.S. Centers for Disease Control and Prevention life table for the Black population in general.
Of the patients, only 5% had Medicare old age and survivor’s insurance, and 4% had Medicare disability insurance benefits or end-stage renal disease (ESRD), while 48% had Medicaid, and 43% were dually eligible for Medicare and Medicaid. The racial and regional distributions were similar across insurance types.
Adults covered by Medicare had a life expectancy of 39.8 years at 18 years of age, an improvement over life expectancy for those with Medicare for disabilities or ESRD and those dually insured by Medicare and Medicaid.
And the shorter life expectancy with Medicare and Medicaid was also observed among beneficiaries aged 65 or older, compared with those enrolled in Medicare old age and survivor’s insurance.
“Evidently, the life expectancy gap persists among patients with sickle cell disease, even though they are protected by public insurance,” the authors report.
The shorter life expectancy among those with dual eligibility for Medicare and Medicaid is consistent with that of the general population, the authors add.
“It is well-recognized that the dual-eligible individuals are a vulnerable population with some of the most complex and expensive health care needs,” they write. “They are more likely to have fewer socioeconomic resources, more chronic conditions, and poorer survival outcomes than single eligible.”
First author Boshen Jiao, PhD, MPH, of the Harvard T.H. Chan School of Public Health, in Boston, noted that socioeconomic factors, on a broader level, have an important impact on SCD.
“Even the first and most established FDA-approved medication for sickle cell disease, hydroxyurea, suffers from underutilization,” he told MDedge. “This issue can be associated with disparities along racial lines, particularly concerning access to treatments.”
Such factors can also play a role in the lack of a well-coordinated transition program needed to prevent gaps in care between youth and adulthood, as well as a lack of access to specialized care needed to potentially initiate new therapies.
Ultimately, the decline in survival seen from childhood to adulthood in SCD “emphasizes the pivotal role of health care during the critical transition phase from childhood to adulthood for individuals with sickle cell disease,” Dr. Jiao said. “Moreover, the prospect of a curative therapy that can intervene in the early stages of life, prior to adulthood, stands as a desirable goal.”
The authors urge that “future studies should uncover factors influencing survival outcomes and explore policy options to address the unmet needs of the disabled or dually eligible population with SCD.” They had no disclosures to report.
A version of this article first appeared on Medscape.com.
“To the best of our knowledge, this is the first United States nationwide investigation into lifetime survival for individuals with sickle cell disease covered by Medicare and Medicaid and disparities in survival by public insurance type, using comprehensive claims data collected from all 50 states,” the authors report in the study, published in Blood Advances.
“Our study underscores the persistent life expectancy shortfall for patients with sickle cell disease, the burden of premature mortality during adulthood, and survival disparities by insurance status,” they write.
SCD, which disproportionately affects people of African descent, significantly increases the risk of various acute and chronic life-threatening complications, including acute pain crisis, stroke, acute chest syndrome, chronic pain, symptomatic anemia, infections, and organ damage.
In recent years, advances ranging from newborn screening to prophylactic antibiotics have significantly improved the survival rates of children with SCD in the United States. However, premature mortality in adulthood has remained well above that of the general public.
A recent study of a U.S. birth cohort predicted life expectancy with SCD to be approximately 2 decades shorter than in those without the disease. However, those projections were based on a simulation model, and other life expectancy estimates have had sample sizes with state or regional limitations.
With no population-level individual data–based periodic life tables existing for individuals with SCD, the authors turned to nationwide data on Medicare and Medicaid beneficiaries to get a better idea of survival predictions.
For the study, they identified 94,616 individuals diagnosed with SCD who had not undergone transplant and were receiving common care, based on nationwide Medicare and Medicaid claim data from 2008 to 2016 on beneficiaries in all 50 states.
Of the patients, 74% were Black and 53% resided in the South at the index date. The patients had a mean entry age overall of 26.6 years across insurance types.
The results showed projected life expectancy at birth with SCD to be 52.6 years, with the life expectancy at birth for females significantly longer, compared with that of males (55.0 versus 49.3 years). For the general population, U.S. life expectancy estimates have been reported to be about 76 years.
Specifically, life expectancy in the cohort into adulthood was 35.4 years at 18 years of age; 24.1 years at 35 years of age; 19.6 years at 45 years old; 13.2 years at 65 years of age; and 5.4 years at 85 years old.
Likewise, survival probability rates were high during childhood, with survival probability at 18 years being 0.98, declining to 0.804 at age 30; 0.628 at age 45; 0.267 at age 65 and 0.70 at age 85.
Black individuals had a significantly shorter life expectancy at birth than non-Black individuals (52.2 vs. 55.1 years), with the survival expectancy for Black individuals significantly shorter than the 75-year life expectancy estimated by the 2016 U.S. Centers for Disease Control and Prevention life table for the Black population in general.
Of the patients, only 5% had Medicare old age and survivor’s insurance, and 4% had Medicare disability insurance benefits or end-stage renal disease (ESRD), while 48% had Medicaid, and 43% were dually eligible for Medicare and Medicaid. The racial and regional distributions were similar across insurance types.
Adults covered by Medicare had a life expectancy of 39.8 years at 18 years of age, an improvement over life expectancy for those with Medicare for disabilities or ESRD and those dually insured by Medicare and Medicaid.
And the shorter life expectancy with Medicare and Medicaid was also observed among beneficiaries aged 65 or older, compared with those enrolled in Medicare old age and survivor’s insurance.
“Evidently, the life expectancy gap persists among patients with sickle cell disease, even though they are protected by public insurance,” the authors report.
The shorter life expectancy among those with dual eligibility for Medicare and Medicaid is consistent with that of the general population, the authors add.
“It is well-recognized that the dual-eligible individuals are a vulnerable population with some of the most complex and expensive health care needs,” they write. “They are more likely to have fewer socioeconomic resources, more chronic conditions, and poorer survival outcomes than single eligible.”
First author Boshen Jiao, PhD, MPH, of the Harvard T.H. Chan School of Public Health, in Boston, noted that socioeconomic factors, on a broader level, have an important impact on SCD.
“Even the first and most established FDA-approved medication for sickle cell disease, hydroxyurea, suffers from underutilization,” he told MDedge. “This issue can be associated with disparities along racial lines, particularly concerning access to treatments.”
Such factors can also play a role in the lack of a well-coordinated transition program needed to prevent gaps in care between youth and adulthood, as well as a lack of access to specialized care needed to potentially initiate new therapies.
Ultimately, the decline in survival seen from childhood to adulthood in SCD “emphasizes the pivotal role of health care during the critical transition phase from childhood to adulthood for individuals with sickle cell disease,” Dr. Jiao said. “Moreover, the prospect of a curative therapy that can intervene in the early stages of life, prior to adulthood, stands as a desirable goal.”
The authors urge that “future studies should uncover factors influencing survival outcomes and explore policy options to address the unmet needs of the disabled or dually eligible population with SCD.” They had no disclosures to report.
A version of this article first appeared on Medscape.com.
FROM BLOOD ADVANCES