User login
Ixekizumab psoriasis outcomes, sliced and diced
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
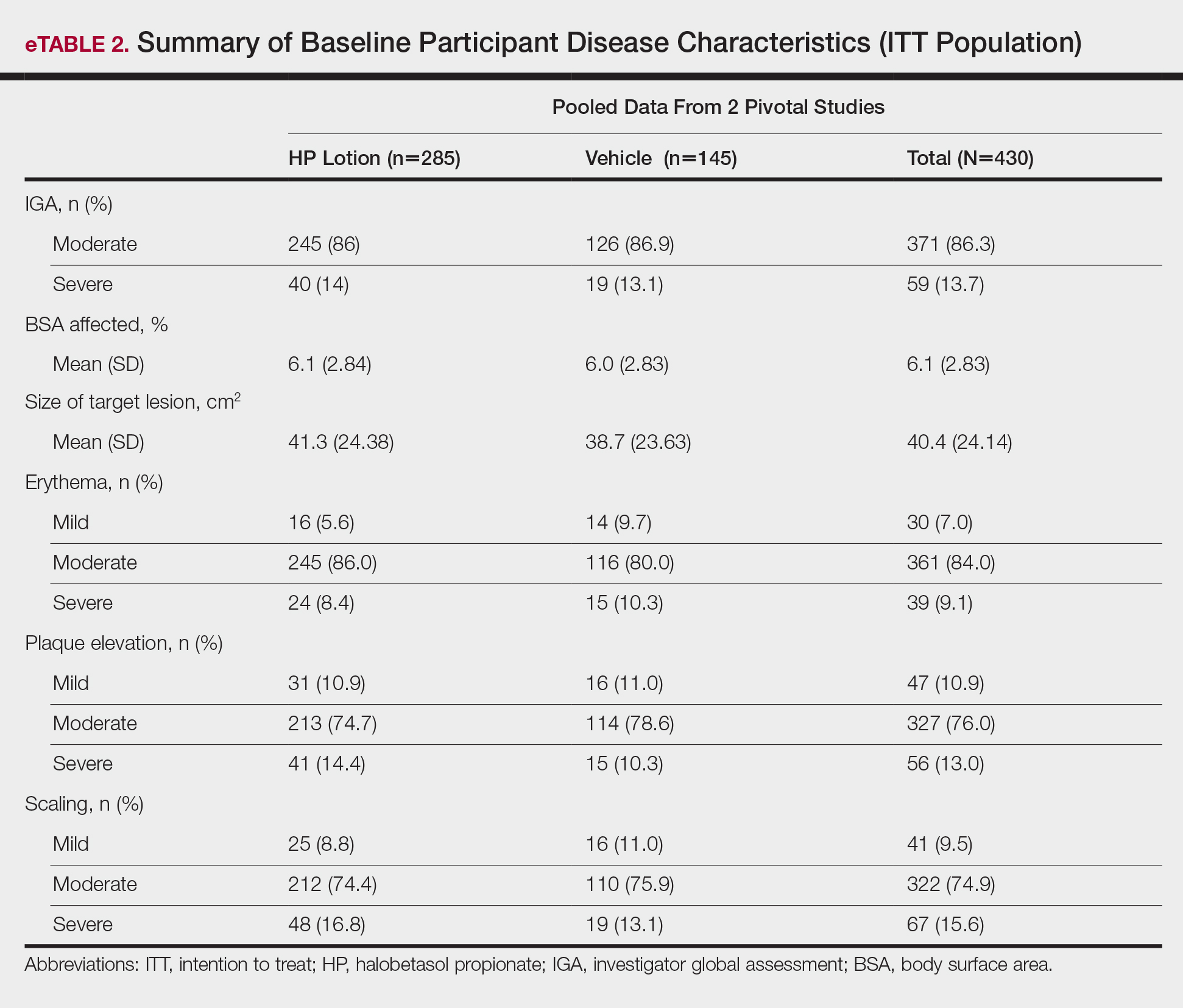
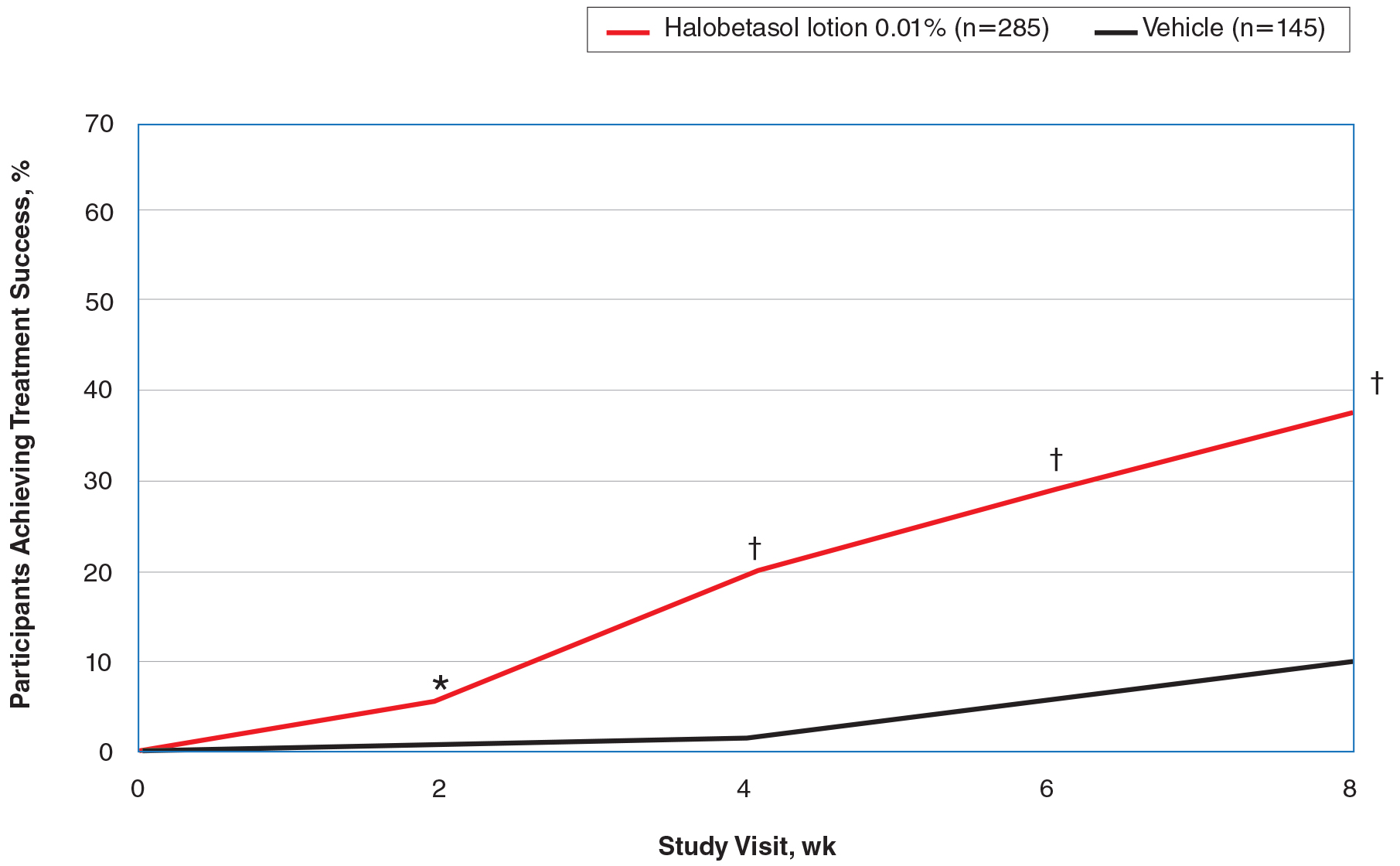
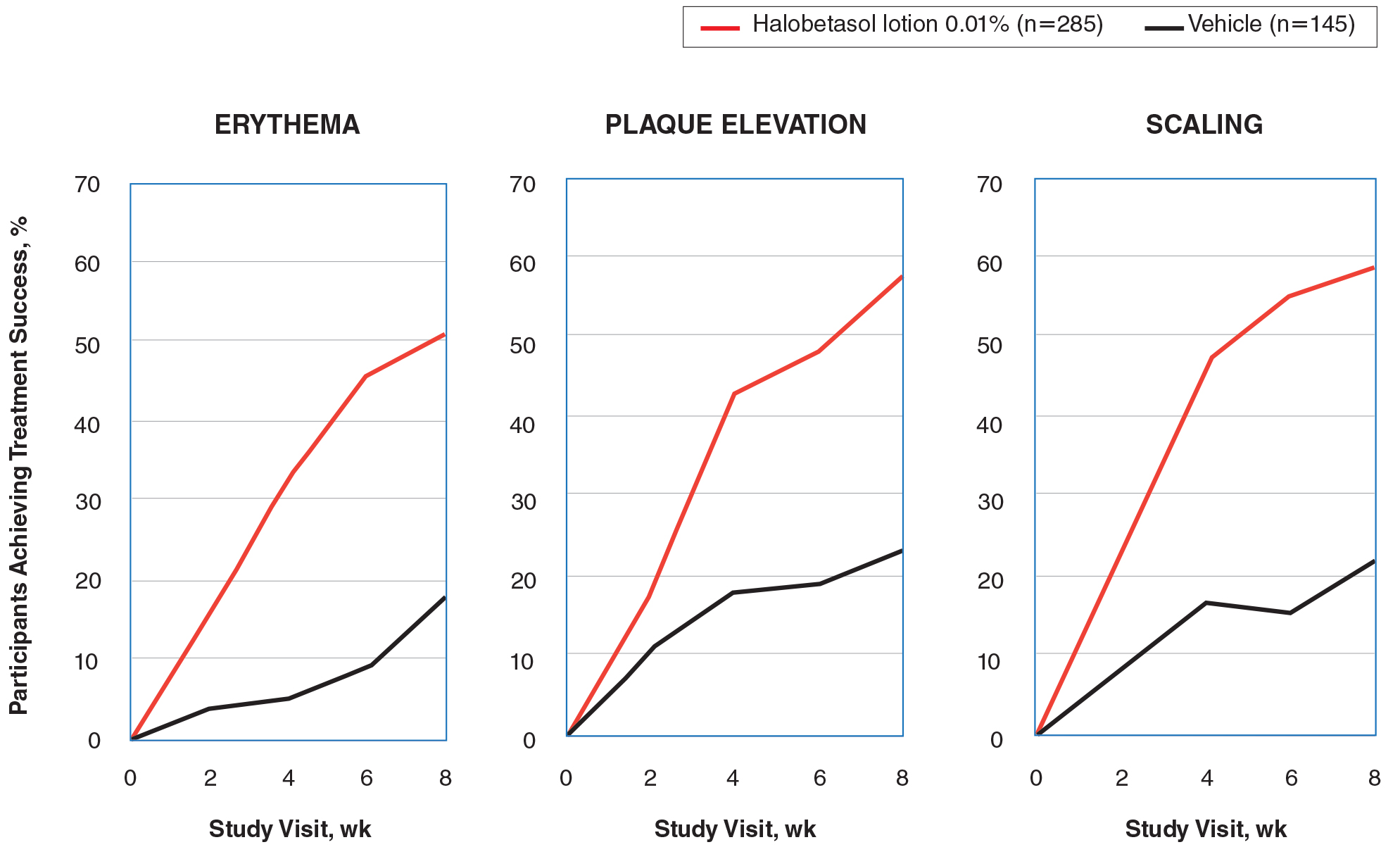
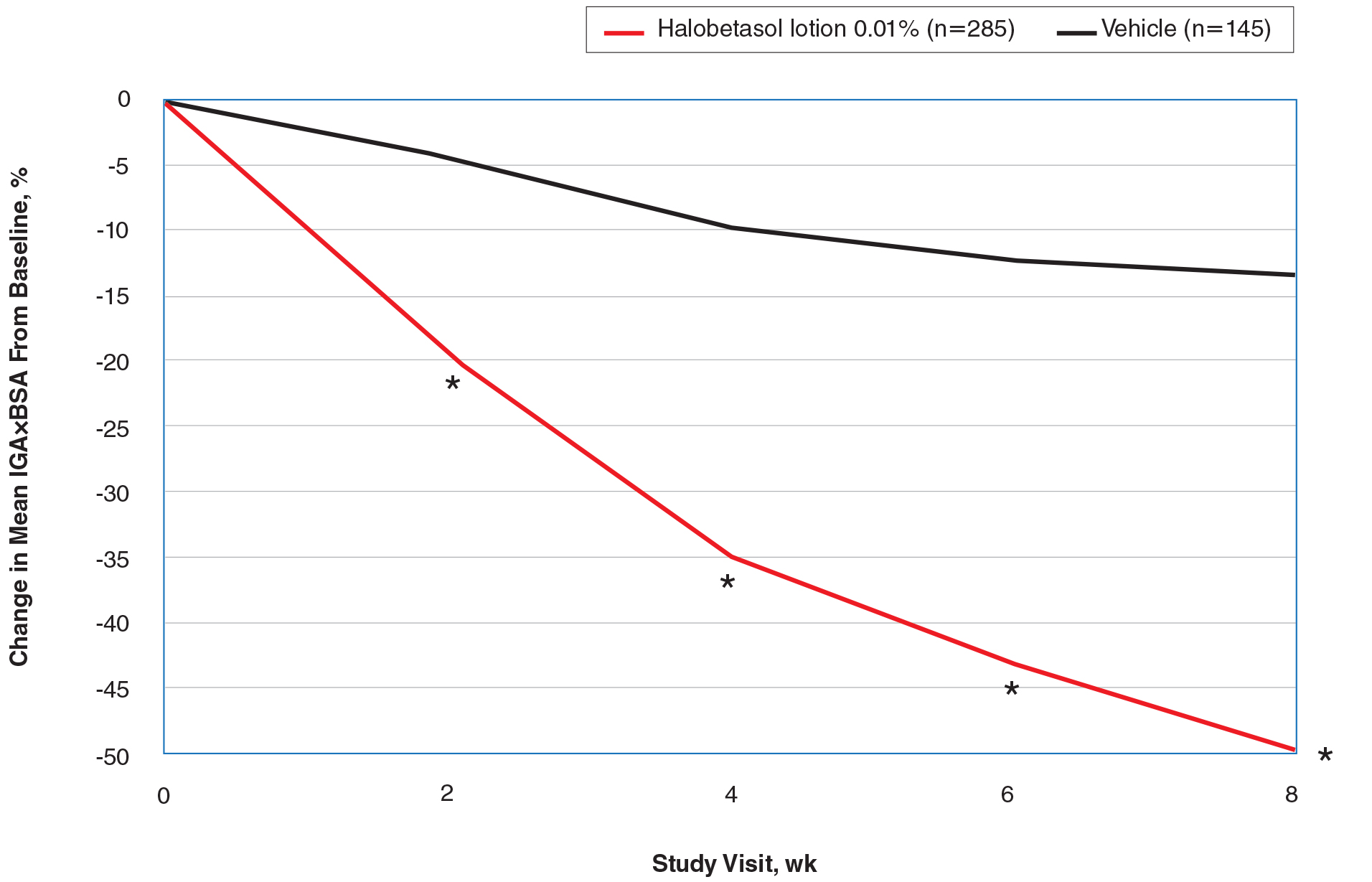
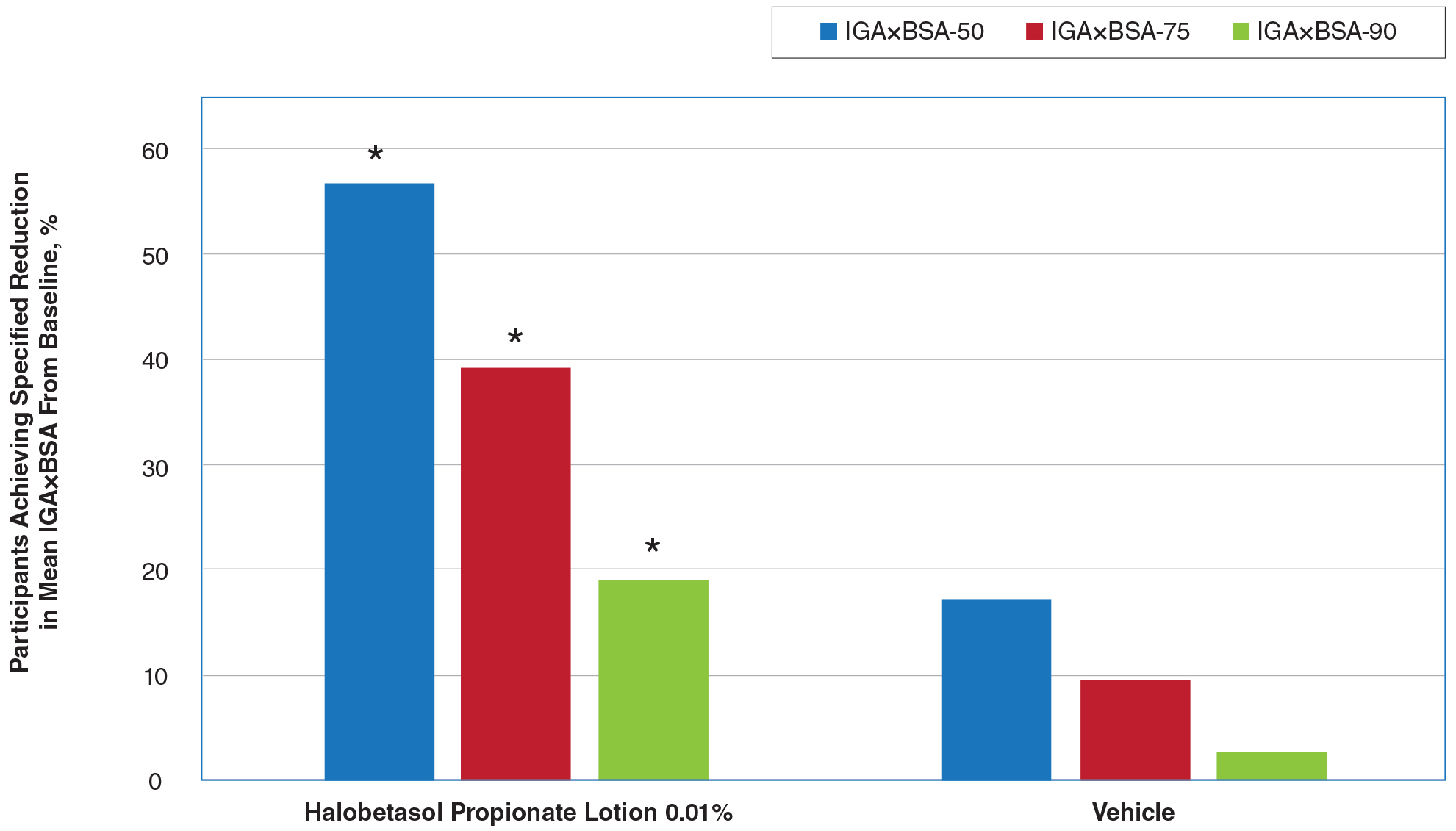
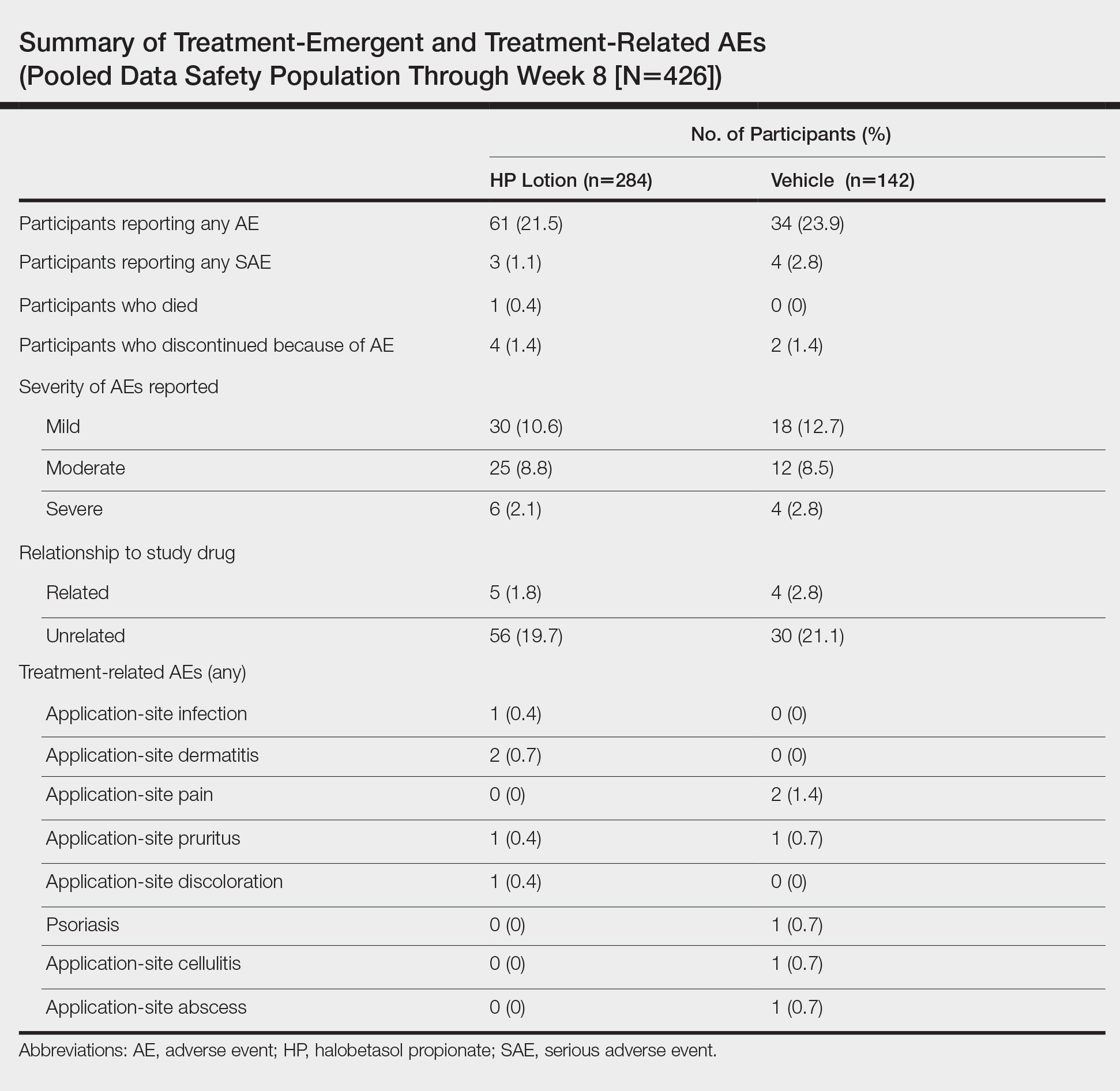
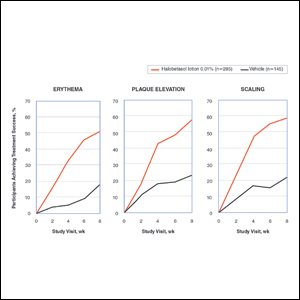
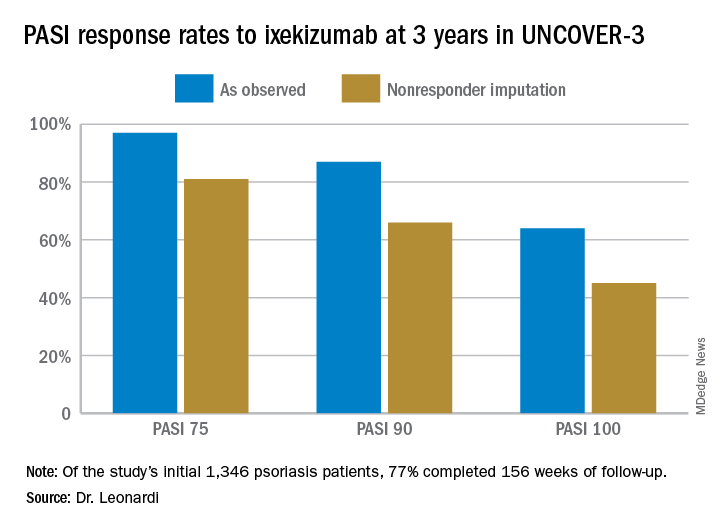
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.

However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.

However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
AAD, NPF release two joint guidelines on treatment, management of psoriasis
The .
These guidelines are the first of two papers to be published in the Journal of the American Academy of Dermatology (JAAD), with four more guidelines on psoriasis to be published later this year in JAAD on phototherapy, topical therapy, nonbiologic systemic medications, and treatment of pediatric patients.
The guideline on biologics updates the 2008 AAD guidelines on psoriasis. In an interview, Alan Menter, MD, cochair of the guidelines work group and lead author of the biologics paper, said the guidelines for biologics were needed because of major advances with the availability of new biologics over the last decade. For example, three tumor necrosis factor–alpha (TNF-alpha) inhibitors were available in 2008, but that number has increased to 10 biologics and now includes agents such as those targeting interleukin (IL)-12/IL-23, IL-17 and IL-23.
In addition, the new guidelines from AAD were developed to represent improvements in the management of patients with moderate to severe psoriasis as well as the relationship between psoriasis and related comorbidities.
“Major advances in new biologic drugs [are] now available to patients, plus [there have been] significant advances in our understanding of comorbid conditions,” such as cardiovascular comorbidities, said Dr. Menter, chairman of the division of dermatology, Baylor University Medical Center, and clinical professor of dermatology, University of Texas, both in Dallas.
The working group for each set of guidelines consisted of dermatologists, patient representatives, a cardiologist, and a rheumatologist. The biologic guidelines working group analyzed studies published between January 2008 and December 2018 and issued a series of recommendations based on published evidence for the effectiveness, adverse events, and switching for Food and Drug Administration–approved TNF-alpha inhibitors (etanercept, infliximab, adalimumab, certolizumab, and TNF-alpha biosimilars); IL-12/IL-23 inhibitors (ustekinumab); IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); and IL-23 inhibitors (guselkumab and tildrakizumab, and risankizumab, which is still under FDA review) for monotherapy or combination therapy in patients with moderate to severe psoriasis.
The biologic guidelines noted that, while FDA-approved biologics were deemed safe overall for patients with moderate to severe psoriasis, dermatologists should recognize the adverse effects of these therapies, monitor for infections, and counsel their patients against modifying or discontinuing therapy without first consulting a dermatologist. In general, the working group noted that failure with one biologic does not necessarily mean that a patient will experience failure with a different biologic, even among TNF-alpha and IL-12/IL-23 inhibitors. However, reduced efficacy for a patient receiving a specific TNF-alpha inhibitor may predict reduced efficacy when switching to a different TNF-alpha inhibitor, they said.
In the psoriasis comorbidity guideline, the working group examined the therapeutic interventions for psoriasis-related comorbidities such as psoriatic arthritis (PsA), cardiovascular disease, metabolic syndrome, and inflammatory bowel disease. They also provided recommendations on the effect of psoriasis on mental health, quality of life, and lifestyle choices such as smoking and alcohol use.
With respect to cardiovascular disease, the dermatologist should ensure that patients are aware of the association between risk factors for cardiovascular disease and psoriasis, and that they undergo screening for these risk factors, consider lifestyle changes to reduce risk of cardiovascular disease, and consult with cardiologists and primary care providers based on individual risk, the guideline states. The working group recommended that patients with psoriasis undergo screening for hypertension, diabetes, and hyperlipidemia based on national guidelines, with more frequent screening recommended for patients with psoriasis greater than 10% body surface area or who are eligible for systemic or phototherapy.
In both the biologic and the comorbidity guidelines, the working groups stressed the importance of patient education and the role of the dermatologist in educating patients so that shared decision-making can occur. They noted that education was related to improved quality of life for these patients.
“Both the comorbidities guidelines and the biologic guidelines will help educate the psoriasis population with input from dermatologists in clinical practices,” Dr. Menter said.
However, both working groups noted there are still significant gaps in research, such as the effects of treatment combinations for new biologics and the lack of biomarkers that would identify which biologics are best suited for individual psoriasis patients.
There is also little known about the complex relationship between psoriasis and its comorbidities, and how psoriasis treatment can potentially prevent future disease. To ensure treatment of psoriasis-related comorbidities, dermatologists should consider psoriasis as a systemic disease with multiple comorbidities and interact with primary care doctors, cardiologists, and other providers involved in the care of the patients, Dr. Menter said.
There were no specific funding sources reported for the guidelines. Several authors reported relationships with industry, including pharmaceutical companies with drugs and products involving psoriasis, during the development of the guidelines. If a potential conflict was noted, the working group member recused himself or herself from discussion and drafting of recommendations, according to the paper. Dr. Menter’s disclosure includes serving as a consultant, speaker, investigator, and adviser, and receiving honoraria, from multiple pharmaceutical companies.
SOURCE: Menter A et al. J Am Acad Dermatol. 2019 Feb 13. doi: 10.1016/j.jaad.2018.11.057. Elmets CA et al. J Am Acad Dermatol. 2019 Feb 13. doi: 10.1016/j.jaad.2018.11.058.
The .
These guidelines are the first of two papers to be published in the Journal of the American Academy of Dermatology (JAAD), with four more guidelines on psoriasis to be published later this year in JAAD on phototherapy, topical therapy, nonbiologic systemic medications, and treatment of pediatric patients.
The guideline on biologics updates the 2008 AAD guidelines on psoriasis. In an interview, Alan Menter, MD, cochair of the guidelines work group and lead author of the biologics paper, said the guidelines for biologics were needed because of major advances with the availability of new biologics over the last decade. For example, three tumor necrosis factor–alpha (TNF-alpha) inhibitors were available in 2008, but that number has increased to 10 biologics and now includes agents such as those targeting interleukin (IL)-12/IL-23, IL-17 and IL-23.
In addition, the new guidelines from AAD were developed to represent improvements in the management of patients with moderate to severe psoriasis as well as the relationship between psoriasis and related comorbidities.
“Major advances in new biologic drugs [are] now available to patients, plus [there have been] significant advances in our understanding of comorbid conditions,” such as cardiovascular comorbidities, said Dr. Menter, chairman of the division of dermatology, Baylor University Medical Center, and clinical professor of dermatology, University of Texas, both in Dallas.
The working group for each set of guidelines consisted of dermatologists, patient representatives, a cardiologist, and a rheumatologist. The biologic guidelines working group analyzed studies published between January 2008 and December 2018 and issued a series of recommendations based on published evidence for the effectiveness, adverse events, and switching for Food and Drug Administration–approved TNF-alpha inhibitors (etanercept, infliximab, adalimumab, certolizumab, and TNF-alpha biosimilars); IL-12/IL-23 inhibitors (ustekinumab); IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); and IL-23 inhibitors (guselkumab and tildrakizumab, and risankizumab, which is still under FDA review) for monotherapy or combination therapy in patients with moderate to severe psoriasis.
The biologic guidelines noted that, while FDA-approved biologics were deemed safe overall for patients with moderate to severe psoriasis, dermatologists should recognize the adverse effects of these therapies, monitor for infections, and counsel their patients against modifying or discontinuing therapy without first consulting a dermatologist. In general, the working group noted that failure with one biologic does not necessarily mean that a patient will experience failure with a different biologic, even among TNF-alpha and IL-12/IL-23 inhibitors. However, reduced efficacy for a patient receiving a specific TNF-alpha inhibitor may predict reduced efficacy when switching to a different TNF-alpha inhibitor, they said.
In the psoriasis comorbidity guideline, the working group examined the therapeutic interventions for psoriasis-related comorbidities such as psoriatic arthritis (PsA), cardiovascular disease, metabolic syndrome, and inflammatory bowel disease. They also provided recommendations on the effect of psoriasis on mental health, quality of life, and lifestyle choices such as smoking and alcohol use.
With respect to cardiovascular disease, the dermatologist should ensure that patients are aware of the association between risk factors for cardiovascular disease and psoriasis, and that they undergo screening for these risk factors, consider lifestyle changes to reduce risk of cardiovascular disease, and consult with cardiologists and primary care providers based on individual risk, the guideline states. The working group recommended that patients with psoriasis undergo screening for hypertension, diabetes, and hyperlipidemia based on national guidelines, with more frequent screening recommended for patients with psoriasis greater than 10% body surface area or who are eligible for systemic or phototherapy.
In both the biologic and the comorbidity guidelines, the working groups stressed the importance of patient education and the role of the dermatologist in educating patients so that shared decision-making can occur. They noted that education was related to improved quality of life for these patients.
“Both the comorbidities guidelines and the biologic guidelines will help educate the psoriasis population with input from dermatologists in clinical practices,” Dr. Menter said.
However, both working groups noted there are still significant gaps in research, such as the effects of treatment combinations for new biologics and the lack of biomarkers that would identify which biologics are best suited for individual psoriasis patients.
There is also little known about the complex relationship between psoriasis and its comorbidities, and how psoriasis treatment can potentially prevent future disease. To ensure treatment of psoriasis-related comorbidities, dermatologists should consider psoriasis as a systemic disease with multiple comorbidities and interact with primary care doctors, cardiologists, and other providers involved in the care of the patients, Dr. Menter said.
There were no specific funding sources reported for the guidelines. Several authors reported relationships with industry, including pharmaceutical companies with drugs and products involving psoriasis, during the development of the guidelines. If a potential conflict was noted, the working group member recused himself or herself from discussion and drafting of recommendations, according to the paper. Dr. Menter’s disclosure includes serving as a consultant, speaker, investigator, and adviser, and receiving honoraria, from multiple pharmaceutical companies.
SOURCE: Menter A et al. J Am Acad Dermatol. 2019 Feb 13. doi: 10.1016/j.jaad.2018.11.057. Elmets CA et al. J Am Acad Dermatol. 2019 Feb 13. doi: 10.1016/j.jaad.2018.11.058.
The .
These guidelines are the first of two papers to be published in the Journal of the American Academy of Dermatology (JAAD), with four more guidelines on psoriasis to be published later this year in JAAD on phototherapy, topical therapy, nonbiologic systemic medications, and treatment of pediatric patients.
The guideline on biologics updates the 2008 AAD guidelines on psoriasis. In an interview, Alan Menter, MD, cochair of the guidelines work group and lead author of the biologics paper, said the guidelines for biologics were needed because of major advances with the availability of new biologics over the last decade. For example, three tumor necrosis factor–alpha (TNF-alpha) inhibitors were available in 2008, but that number has increased to 10 biologics and now includes agents such as those targeting interleukin (IL)-12/IL-23, IL-17 and IL-23.
In addition, the new guidelines from AAD were developed to represent improvements in the management of patients with moderate to severe psoriasis as well as the relationship between psoriasis and related comorbidities.
“Major advances in new biologic drugs [are] now available to patients, plus [there have been] significant advances in our understanding of comorbid conditions,” such as cardiovascular comorbidities, said Dr. Menter, chairman of the division of dermatology, Baylor University Medical Center, and clinical professor of dermatology, University of Texas, both in Dallas.
The working group for each set of guidelines consisted of dermatologists, patient representatives, a cardiologist, and a rheumatologist. The biologic guidelines working group analyzed studies published between January 2008 and December 2018 and issued a series of recommendations based on published evidence for the effectiveness, adverse events, and switching for Food and Drug Administration–approved TNF-alpha inhibitors (etanercept, infliximab, adalimumab, certolizumab, and TNF-alpha biosimilars); IL-12/IL-23 inhibitors (ustekinumab); IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); and IL-23 inhibitors (guselkumab and tildrakizumab, and risankizumab, which is still under FDA review) for monotherapy or combination therapy in patients with moderate to severe psoriasis.
The biologic guidelines noted that, while FDA-approved biologics were deemed safe overall for patients with moderate to severe psoriasis, dermatologists should recognize the adverse effects of these therapies, monitor for infections, and counsel their patients against modifying or discontinuing therapy without first consulting a dermatologist. In general, the working group noted that failure with one biologic does not necessarily mean that a patient will experience failure with a different biologic, even among TNF-alpha and IL-12/IL-23 inhibitors. However, reduced efficacy for a patient receiving a specific TNF-alpha inhibitor may predict reduced efficacy when switching to a different TNF-alpha inhibitor, they said.
In the psoriasis comorbidity guideline, the working group examined the therapeutic interventions for psoriasis-related comorbidities such as psoriatic arthritis (PsA), cardiovascular disease, metabolic syndrome, and inflammatory bowel disease. They also provided recommendations on the effect of psoriasis on mental health, quality of life, and lifestyle choices such as smoking and alcohol use.
With respect to cardiovascular disease, the dermatologist should ensure that patients are aware of the association between risk factors for cardiovascular disease and psoriasis, and that they undergo screening for these risk factors, consider lifestyle changes to reduce risk of cardiovascular disease, and consult with cardiologists and primary care providers based on individual risk, the guideline states. The working group recommended that patients with psoriasis undergo screening for hypertension, diabetes, and hyperlipidemia based on national guidelines, with more frequent screening recommended for patients with psoriasis greater than 10% body surface area or who are eligible for systemic or phototherapy.
In both the biologic and the comorbidity guidelines, the working groups stressed the importance of patient education and the role of the dermatologist in educating patients so that shared decision-making can occur. They noted that education was related to improved quality of life for these patients.
“Both the comorbidities guidelines and the biologic guidelines will help educate the psoriasis population with input from dermatologists in clinical practices,” Dr. Menter said.
However, both working groups noted there are still significant gaps in research, such as the effects of treatment combinations for new biologics and the lack of biomarkers that would identify which biologics are best suited for individual psoriasis patients.
There is also little known about the complex relationship between psoriasis and its comorbidities, and how psoriasis treatment can potentially prevent future disease. To ensure treatment of psoriasis-related comorbidities, dermatologists should consider psoriasis as a systemic disease with multiple comorbidities and interact with primary care doctors, cardiologists, and other providers involved in the care of the patients, Dr. Menter said.
There were no specific funding sources reported for the guidelines. Several authors reported relationships with industry, including pharmaceutical companies with drugs and products involving psoriasis, during the development of the guidelines. If a potential conflict was noted, the working group member recused himself or herself from discussion and drafting of recommendations, according to the paper. Dr. Menter’s disclosure includes serving as a consultant, speaker, investigator, and adviser, and receiving honoraria, from multiple pharmaceutical companies.
SOURCE: Menter A et al. J Am Acad Dermatol. 2019 Feb 13. doi: 10.1016/j.jaad.2018.11.057. Elmets CA et al. J Am Acad Dermatol. 2019 Feb 13. doi: 10.1016/j.jaad.2018.11.058.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
New Topical Treatments for Psoriasis



TNF inhibitor prices rose despite increased drug class competition
according to a new analysis of Medicare claims data and wholesale acquisition costs published online in JAMA Internal Medicine.
A research team led by Alvaro San-Juan-Rodriguez, PharmD, of the University of Pittsburgh said their analysis illustrates “a market failure contributing to the rising costs of prescription drugs.”
Before 2009, etanercept (Enbrel), infliximab (Remicade), and adalimumab (Humira) were the only tumor necrosis factor (TNF) inhibitors approved by the Food and Drug Administration for treating rheumatoid arthritis; infliximab and adalimumab are also approved to treat inflammatory bowel disease. In 2009, subcutaneous golimumab (Simponi) and certolizumab pegol (Cimzia) entered the market, followed by intravenous golimumab (Simponi ARIA) in 2013.
The researchers used an interrupted time series analysis with a linear model that “regressed the annual cost of existing TNF inhibitors against a continuous variable for month, two indicator variables for each period after market entry of new drugs, and the interactions between them.”
Using estimates from this model, the researchers calculated the trends in costs that would have been expected if new anti-TNFs had not entered the market. They examined costs for TNF inhibitors typically reimbursed under Medicare Part D (Enbrel, Humira, Simponi, and Cimzia) and adjusted the data for increases in manufacturer rebates, but “owing to lack of data,” they could not “assess how purchasing prices for drugs typically reimbursed under Medicare Part B [Remicade and Simponi ARIA] changed over time.” All estimates for annual costs of treatment were based on dosing recommendations for a standard 80-kg patient with rheumatoid arthritis.
The annual treatment costs with existing TNF inhibitors increased after the three new agents entered the market. For example, when wholesale acquisition cost data was applied, annual treatment costs with existing TNF inhibitors increased by 144% from April 2009 to December 2016 after new drug entry (from $15.809 to $38,574). However, in the absence of new drugs’ entry, the researchers estimated that annual treatment costs would have increased by 34% (from $15,809 to $21,184).
Medicare annual treatment costs increased by 139% (from $14,901 to $35,613), compared with a 43% increase expected in the absence of new drugs’ entry (from $14,901 to $21,308). Medicare spending increased in parallel with increases in annual treatment costs, but out-of-pocket costs and manufacturer coverage gap discounts remained relatively constant over time.
The research team noted that if cost trends had not changed after the entry of new products, the costs of Enbrel, Remicade, and Humira in December 2016 would have been 40%-45% lower.
“These increases were born solely by Medicare, while patient out-of-pocket spending remained flat. In addition, these increases were not offset by manufacturer discounts in the Medicare Part D coverage gap. The rising costs of existing products may reflect manufacturers’ opportunism in response to payers’ increased willingness to pay for TNF inhibitors after market entry of new, more expensive agents,” the research team noted.
The study was funded in part by the Myers Family Foundation and one author reported funding from the National Heart, Lung, and Blood Institute.
SOURCE: San-Juan-Rodriguez A et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7656
according to a new analysis of Medicare claims data and wholesale acquisition costs published online in JAMA Internal Medicine.
A research team led by Alvaro San-Juan-Rodriguez, PharmD, of the University of Pittsburgh said their analysis illustrates “a market failure contributing to the rising costs of prescription drugs.”
Before 2009, etanercept (Enbrel), infliximab (Remicade), and adalimumab (Humira) were the only tumor necrosis factor (TNF) inhibitors approved by the Food and Drug Administration for treating rheumatoid arthritis; infliximab and adalimumab are also approved to treat inflammatory bowel disease. In 2009, subcutaneous golimumab (Simponi) and certolizumab pegol (Cimzia) entered the market, followed by intravenous golimumab (Simponi ARIA) in 2013.
The researchers used an interrupted time series analysis with a linear model that “regressed the annual cost of existing TNF inhibitors against a continuous variable for month, two indicator variables for each period after market entry of new drugs, and the interactions between them.”
Using estimates from this model, the researchers calculated the trends in costs that would have been expected if new anti-TNFs had not entered the market. They examined costs for TNF inhibitors typically reimbursed under Medicare Part D (Enbrel, Humira, Simponi, and Cimzia) and adjusted the data for increases in manufacturer rebates, but “owing to lack of data,” they could not “assess how purchasing prices for drugs typically reimbursed under Medicare Part B [Remicade and Simponi ARIA] changed over time.” All estimates for annual costs of treatment were based on dosing recommendations for a standard 80-kg patient with rheumatoid arthritis.
The annual treatment costs with existing TNF inhibitors increased after the three new agents entered the market. For example, when wholesale acquisition cost data was applied, annual treatment costs with existing TNF inhibitors increased by 144% from April 2009 to December 2016 after new drug entry (from $15.809 to $38,574). However, in the absence of new drugs’ entry, the researchers estimated that annual treatment costs would have increased by 34% (from $15,809 to $21,184).
Medicare annual treatment costs increased by 139% (from $14,901 to $35,613), compared with a 43% increase expected in the absence of new drugs’ entry (from $14,901 to $21,308). Medicare spending increased in parallel with increases in annual treatment costs, but out-of-pocket costs and manufacturer coverage gap discounts remained relatively constant over time.
The research team noted that if cost trends had not changed after the entry of new products, the costs of Enbrel, Remicade, and Humira in December 2016 would have been 40%-45% lower.
“These increases were born solely by Medicare, while patient out-of-pocket spending remained flat. In addition, these increases were not offset by manufacturer discounts in the Medicare Part D coverage gap. The rising costs of existing products may reflect manufacturers’ opportunism in response to payers’ increased willingness to pay for TNF inhibitors after market entry of new, more expensive agents,” the research team noted.
The study was funded in part by the Myers Family Foundation and one author reported funding from the National Heart, Lung, and Blood Institute.
SOURCE: San-Juan-Rodriguez A et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7656
according to a new analysis of Medicare claims data and wholesale acquisition costs published online in JAMA Internal Medicine.
A research team led by Alvaro San-Juan-Rodriguez, PharmD, of the University of Pittsburgh said their analysis illustrates “a market failure contributing to the rising costs of prescription drugs.”
Before 2009, etanercept (Enbrel), infliximab (Remicade), and adalimumab (Humira) were the only tumor necrosis factor (TNF) inhibitors approved by the Food and Drug Administration for treating rheumatoid arthritis; infliximab and adalimumab are also approved to treat inflammatory bowel disease. In 2009, subcutaneous golimumab (Simponi) and certolizumab pegol (Cimzia) entered the market, followed by intravenous golimumab (Simponi ARIA) in 2013.
The researchers used an interrupted time series analysis with a linear model that “regressed the annual cost of existing TNF inhibitors against a continuous variable for month, two indicator variables for each period after market entry of new drugs, and the interactions between them.”
Using estimates from this model, the researchers calculated the trends in costs that would have been expected if new anti-TNFs had not entered the market. They examined costs for TNF inhibitors typically reimbursed under Medicare Part D (Enbrel, Humira, Simponi, and Cimzia) and adjusted the data for increases in manufacturer rebates, but “owing to lack of data,” they could not “assess how purchasing prices for drugs typically reimbursed under Medicare Part B [Remicade and Simponi ARIA] changed over time.” All estimates for annual costs of treatment were based on dosing recommendations for a standard 80-kg patient with rheumatoid arthritis.
The annual treatment costs with existing TNF inhibitors increased after the three new agents entered the market. For example, when wholesale acquisition cost data was applied, annual treatment costs with existing TNF inhibitors increased by 144% from April 2009 to December 2016 after new drug entry (from $15.809 to $38,574). However, in the absence of new drugs’ entry, the researchers estimated that annual treatment costs would have increased by 34% (from $15,809 to $21,184).
Medicare annual treatment costs increased by 139% (from $14,901 to $35,613), compared with a 43% increase expected in the absence of new drugs’ entry (from $14,901 to $21,308). Medicare spending increased in parallel with increases in annual treatment costs, but out-of-pocket costs and manufacturer coverage gap discounts remained relatively constant over time.
The research team noted that if cost trends had not changed after the entry of new products, the costs of Enbrel, Remicade, and Humira in December 2016 would have been 40%-45% lower.
“These increases were born solely by Medicare, while patient out-of-pocket spending remained flat. In addition, these increases were not offset by manufacturer discounts in the Medicare Part D coverage gap. The rising costs of existing products may reflect manufacturers’ opportunism in response to payers’ increased willingness to pay for TNF inhibitors after market entry of new, more expensive agents,” the research team noted.
The study was funded in part by the Myers Family Foundation and one author reported funding from the National Heart, Lung, and Blood Institute.
SOURCE: San-Juan-Rodriguez A et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7656
FROM JAMA INTERNAL MEDICINE
What’s new with adalimumab? Plenty
WAIKOLOA, HAWAII – A flurry of recent impressive , identifies a simple biomarker predictive of the likelihood of a favorable PASI 75 response, and highlights a disconnect in psoriatic arthritis (PsA) patients between clinical response as reflected in disease activity and radiographic progression of joint disease, according to Kristina C. Duffin, MD.
Also, a new citrate-free version of adalimumab (Humira) is available. It requires a new prescription, and an additional prior authorization is mandated by some insurers. But this is a welcome innovation for patients bothered by significant burning and stinging with their injections of classic adalimumab, Dr. Duffin, cochair of the department of dermatology at the University of Utah, Salt Lake City, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
New long-term safety data
Adalimumab is a market leader in biologic therapy for psoriasis. But the long-term experience with biologics in dermatology is still relatively limited, so the recent publication of two large studies providing encouraging evidence of the long-term safety of adalimumab is noteworthy.
Craig L. Leonardi, MD, of Saint Louis University, St. Louis, Mo., was first author of an analysis of long-term safety data from 18 clinical trials in adults with moderate to severe plaque psoriasis. The key takeaway, in Dr. Duffin’s view, was that the rate of adverse events, including serious infections and malignancies other than nonmelanoma skin cancer, remained stable over time out to 240 weeks of follow-up in patients on continuous treatment, with no new safety signals emerging (Br J Dermatol. 2019 Jan;180[1]:76-85).
However, randomized clinical trials often paint an overly rosy safety picture because of their strict inclusion and exclusion criteria.
“We single out patients for clinical trials because they’re especially healthy. That doesn’t happen in real-world registries,” she noted.
That’s why a systematic review of adalimumab’s safety performance in 10 real-world registries of adalimumab-treated psoriasis patients is particularly informative. The registries included in the systematic review, led by Bruce E. Strober, MD, PhD, professor of dermatology at the University of Connecticut, Farmington, didn’t all measure the same outcomes. But the three registries that documented major adverse cardiovascular events showed rates of less than 0.1 to less than 1 per 100 patient-years. Rates of malignancies other than nonmelanoma skin cancer were consistently in the 0.3-0.6 events per 100 patient-years range, similar to what has been reported in studies of other systemic psoriasis therapies, biologic as well as nonbiologic (J Eur Acad Dermatol Venereol. 2018 Dec;32[12]:2126-33).
Overall infection rates reported in the real-world registries ranged from 7.7 to 14.7 events per 100 patient-years, which is actually considerably lower than in the clinical trials. Rates of serious infections ranged from less than one up to two events per 100 patient-years, with the most common ones being cellulitis and pneumonia, consistent with the randomized trial experience.
Predicting response to adalimumab
A prospective, multicenter, observational cohort study of 544 psoriasis patients on adalimumab monotherapy conducted by U.K. investigators concluded that a patient’s serum drug level is the single most important predictor of treatment response. A cut point of 3.2 mcg/mL, which is considered the minimal effective circulating drug level, was associated with a 65% probability of a 75% improvement in Psoriasis Area and Severity Index from baseline, or PASI 75 response. The higher the serum drug level, the greater the likelihood of a PASI 75 response, up to a serum level of 7 mcg/mL, which was associated with an 81% probability of achieving PASI 75. Beyond 7 mcg/mL, however, the relationship with treatment response plateaued. Importantly, drug levels measured early on – at 1-12 weeks into therapy – were predictive of response 6 months later. So were steady-state levels (J Invest Dermatol. 2019 Jan;139[1]:115-23).
This is clinically useful information, Dr. Duffin observed.
“I’m hoping we’re going to see more real-world use of checking drug levels,” she said.
Indeed, even though the approved dosing of adalimumab for psoriasis is 40 mg by subcutaneous injection every 2 weeks, the new American Academy of Dermatology/National Psoriasis Foundation joint guidelines for treatment of psoriasis with biologics declare that “a maintenance dose of adalimumab at 40 mg/week is recommended for better disease control in some patients” (J Am Acad Dermatol. 2019 Feb 7. doi: 10.1016/j.jaad.2018.11.057. [Epub ahead of print]).
The new guidelines provide support for dermatologists who decide weekly therapy is best for a given patient, and adalimumab drug levels could prove useful in identifying the patient subgroup likely to benefit.
Dr. Duffin is often consulted by other physicians as to whether they should check for neutralizing antibodies in patients who appear to be losing therapeutic efficacy on a given biologic. She’s not a fan of the practice.
“There are commercial assays out there, but it’s very hard to interpret them because we don’t really know if they’re truly measuring neutralizing antibodies. And the cost is not insignificant; it can be hundreds of dollars,” she noted.
She believes a straightforward measurement of the serum biologic level is a better strategy.
“It makes sense: This is an indirect way of determining if there’s been neutralization of the drug, rather than trying to check the antibody that’s doing it, which is fraught with problems,” Dr. Duffin said.
Radiographic progression and clinical PsA activity on adalimumab don’t always correlate
A post hoc analysis of the randomized, double-blind, placebo-controlled ADEPT trial in PsA patients demonstrated that inhibition of radiographic progression as measured by change in modified total Sharp score from baseline through 24 weeks of adalimumab therapy was greater than expected based upon control of clinical disease activity (Rheumatology [Oxford]. 2019 Jan 3. doi: 10.1093/rheumatology/key417. [Epub ahead of print]).
One implication of the disconnect between radiographic progression and clinical disease documented in this study is that a dermatologist shouldn’t be too quick to change from adalimumab to another biologic just because a patient with PsA reports continued but bearable joint pain. And the converse is also true.
“I think that we as dermatologists probably shouldn’t be reassured when a patient says, ‘My joints feel great!” That’s because you may not necessarily be able to predict lack of progression in Sharp score based upon clinical response,” Dr. Duffin cautioned. “I think you should still have a rheumatologist check in with the patient and do x-rays periodically. The rheumatologist I work with does that, usually about on a yearly basis.”
Another key finding in the ADEPT analysis was that concomitant methotrexate had no added effect in terms of preventing joint destruction. This underscores the prescience of the first-ever collaborative American College of Rheumatology/National Psoriasis Foundation guidelines for the treatment of PsA (Arthritis Care Res (Hoboken). 2019 Jan;71[1]:2-29).
The new guidelines recommend that, in a psoriasis patient with confirmed PsA, the first-line treatment is a tumor necrosis factor (TNF) inhibitor. Agents from this class are preferred over other biologics because they are backed by a larger body of data regarding inhibition of joint disease progression. If the patient fails on the first TNF inhibitor prescribed, second-line therapy is another TNF inhibitor. So is third-line therapy.
Adalimumab citrate free
Not only does this new iteration of adalimumab do away with citrate as a buffer because it can cause pain and burning, it also utilizes a thinner 29-gauge needle rather than the standard 27-gauge. And the needle cover isn’t made with natural rubber latex. Also, both the pen and prefilled syringe contain half the volume of liquid, compared with the classic version of the biologic, so it’s 40 mg of drug in 0.4 mL rather than in 0.8 mL.
The packaging of adalimumab citrate free is different. It comes in a blue box to distinguish the product from the classic version.
Dr. Duffin reported receiving research grants from and serving as a consultant to AbbVie, which markets adalimumab, as well as close to a dozen other pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – A flurry of recent impressive , identifies a simple biomarker predictive of the likelihood of a favorable PASI 75 response, and highlights a disconnect in psoriatic arthritis (PsA) patients between clinical response as reflected in disease activity and radiographic progression of joint disease, according to Kristina C. Duffin, MD.
Also, a new citrate-free version of adalimumab (Humira) is available. It requires a new prescription, and an additional prior authorization is mandated by some insurers. But this is a welcome innovation for patients bothered by significant burning and stinging with their injections of classic adalimumab, Dr. Duffin, cochair of the department of dermatology at the University of Utah, Salt Lake City, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
New long-term safety data
Adalimumab is a market leader in biologic therapy for psoriasis. But the long-term experience with biologics in dermatology is still relatively limited, so the recent publication of two large studies providing encouraging evidence of the long-term safety of adalimumab is noteworthy.
Craig L. Leonardi, MD, of Saint Louis University, St. Louis, Mo., was first author of an analysis of long-term safety data from 18 clinical trials in adults with moderate to severe plaque psoriasis. The key takeaway, in Dr. Duffin’s view, was that the rate of adverse events, including serious infections and malignancies other than nonmelanoma skin cancer, remained stable over time out to 240 weeks of follow-up in patients on continuous treatment, with no new safety signals emerging (Br J Dermatol. 2019 Jan;180[1]:76-85).
However, randomized clinical trials often paint an overly rosy safety picture because of their strict inclusion and exclusion criteria.
“We single out patients for clinical trials because they’re especially healthy. That doesn’t happen in real-world registries,” she noted.
That’s why a systematic review of adalimumab’s safety performance in 10 real-world registries of adalimumab-treated psoriasis patients is particularly informative. The registries included in the systematic review, led by Bruce E. Strober, MD, PhD, professor of dermatology at the University of Connecticut, Farmington, didn’t all measure the same outcomes. But the three registries that documented major adverse cardiovascular events showed rates of less than 0.1 to less than 1 per 100 patient-years. Rates of malignancies other than nonmelanoma skin cancer were consistently in the 0.3-0.6 events per 100 patient-years range, similar to what has been reported in studies of other systemic psoriasis therapies, biologic as well as nonbiologic (J Eur Acad Dermatol Venereol. 2018 Dec;32[12]:2126-33).
Overall infection rates reported in the real-world registries ranged from 7.7 to 14.7 events per 100 patient-years, which is actually considerably lower than in the clinical trials. Rates of serious infections ranged from less than one up to two events per 100 patient-years, with the most common ones being cellulitis and pneumonia, consistent with the randomized trial experience.
Predicting response to adalimumab
A prospective, multicenter, observational cohort study of 544 psoriasis patients on adalimumab monotherapy conducted by U.K. investigators concluded that a patient’s serum drug level is the single most important predictor of treatment response. A cut point of 3.2 mcg/mL, which is considered the minimal effective circulating drug level, was associated with a 65% probability of a 75% improvement in Psoriasis Area and Severity Index from baseline, or PASI 75 response. The higher the serum drug level, the greater the likelihood of a PASI 75 response, up to a serum level of 7 mcg/mL, which was associated with an 81% probability of achieving PASI 75. Beyond 7 mcg/mL, however, the relationship with treatment response plateaued. Importantly, drug levels measured early on – at 1-12 weeks into therapy – were predictive of response 6 months later. So were steady-state levels (J Invest Dermatol. 2019 Jan;139[1]:115-23).
This is clinically useful information, Dr. Duffin observed.
“I’m hoping we’re going to see more real-world use of checking drug levels,” she said.
Indeed, even though the approved dosing of adalimumab for psoriasis is 40 mg by subcutaneous injection every 2 weeks, the new American Academy of Dermatology/National Psoriasis Foundation joint guidelines for treatment of psoriasis with biologics declare that “a maintenance dose of adalimumab at 40 mg/week is recommended for better disease control in some patients” (J Am Acad Dermatol. 2019 Feb 7. doi: 10.1016/j.jaad.2018.11.057. [Epub ahead of print]).
The new guidelines provide support for dermatologists who decide weekly therapy is best for a given patient, and adalimumab drug levels could prove useful in identifying the patient subgroup likely to benefit.
Dr. Duffin is often consulted by other physicians as to whether they should check for neutralizing antibodies in patients who appear to be losing therapeutic efficacy on a given biologic. She’s not a fan of the practice.
“There are commercial assays out there, but it’s very hard to interpret them because we don’t really know if they’re truly measuring neutralizing antibodies. And the cost is not insignificant; it can be hundreds of dollars,” she noted.
She believes a straightforward measurement of the serum biologic level is a better strategy.
“It makes sense: This is an indirect way of determining if there’s been neutralization of the drug, rather than trying to check the antibody that’s doing it, which is fraught with problems,” Dr. Duffin said.
Radiographic progression and clinical PsA activity on adalimumab don’t always correlate
A post hoc analysis of the randomized, double-blind, placebo-controlled ADEPT trial in PsA patients demonstrated that inhibition of radiographic progression as measured by change in modified total Sharp score from baseline through 24 weeks of adalimumab therapy was greater than expected based upon control of clinical disease activity (Rheumatology [Oxford]. 2019 Jan 3. doi: 10.1093/rheumatology/key417. [Epub ahead of print]).
One implication of the disconnect between radiographic progression and clinical disease documented in this study is that a dermatologist shouldn’t be too quick to change from adalimumab to another biologic just because a patient with PsA reports continued but bearable joint pain. And the converse is also true.
“I think that we as dermatologists probably shouldn’t be reassured when a patient says, ‘My joints feel great!” That’s because you may not necessarily be able to predict lack of progression in Sharp score based upon clinical response,” Dr. Duffin cautioned. “I think you should still have a rheumatologist check in with the patient and do x-rays periodically. The rheumatologist I work with does that, usually about on a yearly basis.”
Another key finding in the ADEPT analysis was that concomitant methotrexate had no added effect in terms of preventing joint destruction. This underscores the prescience of the first-ever collaborative American College of Rheumatology/National Psoriasis Foundation guidelines for the treatment of PsA (Arthritis Care Res (Hoboken). 2019 Jan;71[1]:2-29).
The new guidelines recommend that, in a psoriasis patient with confirmed PsA, the first-line treatment is a tumor necrosis factor (TNF) inhibitor. Agents from this class are preferred over other biologics because they are backed by a larger body of data regarding inhibition of joint disease progression. If the patient fails on the first TNF inhibitor prescribed, second-line therapy is another TNF inhibitor. So is third-line therapy.
Adalimumab citrate free
Not only does this new iteration of adalimumab do away with citrate as a buffer because it can cause pain and burning, it also utilizes a thinner 29-gauge needle rather than the standard 27-gauge. And the needle cover isn’t made with natural rubber latex. Also, both the pen and prefilled syringe contain half the volume of liquid, compared with the classic version of the biologic, so it’s 40 mg of drug in 0.4 mL rather than in 0.8 mL.
The packaging of adalimumab citrate free is different. It comes in a blue box to distinguish the product from the classic version.
Dr. Duffin reported receiving research grants from and serving as a consultant to AbbVie, which markets adalimumab, as well as close to a dozen other pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – A flurry of recent impressive , identifies a simple biomarker predictive of the likelihood of a favorable PASI 75 response, and highlights a disconnect in psoriatic arthritis (PsA) patients between clinical response as reflected in disease activity and radiographic progression of joint disease, according to Kristina C. Duffin, MD.
Also, a new citrate-free version of adalimumab (Humira) is available. It requires a new prescription, and an additional prior authorization is mandated by some insurers. But this is a welcome innovation for patients bothered by significant burning and stinging with their injections of classic adalimumab, Dr. Duffin, cochair of the department of dermatology at the University of Utah, Salt Lake City, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
New long-term safety data
Adalimumab is a market leader in biologic therapy for psoriasis. But the long-term experience with biologics in dermatology is still relatively limited, so the recent publication of two large studies providing encouraging evidence of the long-term safety of adalimumab is noteworthy.
Craig L. Leonardi, MD, of Saint Louis University, St. Louis, Mo., was first author of an analysis of long-term safety data from 18 clinical trials in adults with moderate to severe plaque psoriasis. The key takeaway, in Dr. Duffin’s view, was that the rate of adverse events, including serious infections and malignancies other than nonmelanoma skin cancer, remained stable over time out to 240 weeks of follow-up in patients on continuous treatment, with no new safety signals emerging (Br J Dermatol. 2019 Jan;180[1]:76-85).
However, randomized clinical trials often paint an overly rosy safety picture because of their strict inclusion and exclusion criteria.
“We single out patients for clinical trials because they’re especially healthy. That doesn’t happen in real-world registries,” she noted.
That’s why a systematic review of adalimumab’s safety performance in 10 real-world registries of adalimumab-treated psoriasis patients is particularly informative. The registries included in the systematic review, led by Bruce E. Strober, MD, PhD, professor of dermatology at the University of Connecticut, Farmington, didn’t all measure the same outcomes. But the three registries that documented major adverse cardiovascular events showed rates of less than 0.1 to less than 1 per 100 patient-years. Rates of malignancies other than nonmelanoma skin cancer were consistently in the 0.3-0.6 events per 100 patient-years range, similar to what has been reported in studies of other systemic psoriasis therapies, biologic as well as nonbiologic (J Eur Acad Dermatol Venereol. 2018 Dec;32[12]:2126-33).
Overall infection rates reported in the real-world registries ranged from 7.7 to 14.7 events per 100 patient-years, which is actually considerably lower than in the clinical trials. Rates of serious infections ranged from less than one up to two events per 100 patient-years, with the most common ones being cellulitis and pneumonia, consistent with the randomized trial experience.
Predicting response to adalimumab
A prospective, multicenter, observational cohort study of 544 psoriasis patients on adalimumab monotherapy conducted by U.K. investigators concluded that a patient’s serum drug level is the single most important predictor of treatment response. A cut point of 3.2 mcg/mL, which is considered the minimal effective circulating drug level, was associated with a 65% probability of a 75% improvement in Psoriasis Area and Severity Index from baseline, or PASI 75 response. The higher the serum drug level, the greater the likelihood of a PASI 75 response, up to a serum level of 7 mcg/mL, which was associated with an 81% probability of achieving PASI 75. Beyond 7 mcg/mL, however, the relationship with treatment response plateaued. Importantly, drug levels measured early on – at 1-12 weeks into therapy – were predictive of response 6 months later. So were steady-state levels (J Invest Dermatol. 2019 Jan;139[1]:115-23).
This is clinically useful information, Dr. Duffin observed.
“I’m hoping we’re going to see more real-world use of checking drug levels,” she said.
Indeed, even though the approved dosing of adalimumab for psoriasis is 40 mg by subcutaneous injection every 2 weeks, the new American Academy of Dermatology/National Psoriasis Foundation joint guidelines for treatment of psoriasis with biologics declare that “a maintenance dose of adalimumab at 40 mg/week is recommended for better disease control in some patients” (J Am Acad Dermatol. 2019 Feb 7. doi: 10.1016/j.jaad.2018.11.057. [Epub ahead of print]).
The new guidelines provide support for dermatologists who decide weekly therapy is best for a given patient, and adalimumab drug levels could prove useful in identifying the patient subgroup likely to benefit.
Dr. Duffin is often consulted by other physicians as to whether they should check for neutralizing antibodies in patients who appear to be losing therapeutic efficacy on a given biologic. She’s not a fan of the practice.
“There are commercial assays out there, but it’s very hard to interpret them because we don’t really know if they’re truly measuring neutralizing antibodies. And the cost is not insignificant; it can be hundreds of dollars,” she noted.
She believes a straightforward measurement of the serum biologic level is a better strategy.
“It makes sense: This is an indirect way of determining if there’s been neutralization of the drug, rather than trying to check the antibody that’s doing it, which is fraught with problems,” Dr. Duffin said.
Radiographic progression and clinical PsA activity on adalimumab don’t always correlate
A post hoc analysis of the randomized, double-blind, placebo-controlled ADEPT trial in PsA patients demonstrated that inhibition of radiographic progression as measured by change in modified total Sharp score from baseline through 24 weeks of adalimumab therapy was greater than expected based upon control of clinical disease activity (Rheumatology [Oxford]. 2019 Jan 3. doi: 10.1093/rheumatology/key417. [Epub ahead of print]).
One implication of the disconnect between radiographic progression and clinical disease documented in this study is that a dermatologist shouldn’t be too quick to change from adalimumab to another biologic just because a patient with PsA reports continued but bearable joint pain. And the converse is also true.
“I think that we as dermatologists probably shouldn’t be reassured when a patient says, ‘My joints feel great!” That’s because you may not necessarily be able to predict lack of progression in Sharp score based upon clinical response,” Dr. Duffin cautioned. “I think you should still have a rheumatologist check in with the patient and do x-rays periodically. The rheumatologist I work with does that, usually about on a yearly basis.”
Another key finding in the ADEPT analysis was that concomitant methotrexate had no added effect in terms of preventing joint destruction. This underscores the prescience of the first-ever collaborative American College of Rheumatology/National Psoriasis Foundation guidelines for the treatment of PsA (Arthritis Care Res (Hoboken). 2019 Jan;71[1]:2-29).
The new guidelines recommend that, in a psoriasis patient with confirmed PsA, the first-line treatment is a tumor necrosis factor (TNF) inhibitor. Agents from this class are preferred over other biologics because they are backed by a larger body of data regarding inhibition of joint disease progression. If the patient fails on the first TNF inhibitor prescribed, second-line therapy is another TNF inhibitor. So is third-line therapy.
Adalimumab citrate free
Not only does this new iteration of adalimumab do away with citrate as a buffer because it can cause pain and burning, it also utilizes a thinner 29-gauge needle rather than the standard 27-gauge. And the needle cover isn’t made with natural rubber latex. Also, both the pen and prefilled syringe contain half the volume of liquid, compared with the classic version of the biologic, so it’s 40 mg of drug in 0.4 mL rather than in 0.8 mL.
The packaging of adalimumab citrate free is different. It comes in a blue box to distinguish the product from the classic version.
Dr. Duffin reported receiving research grants from and serving as a consultant to AbbVie, which markets adalimumab, as well as close to a dozen other pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Biologics curb coronary artery plaques in severe psoriasis
Treatment with biologic therapy significantly improves coronary plaque profiles in patients with severe psoriasis, based on data from 121 adult patients who completed a year of follow-up.
A previous study showed a reduced rate of nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death among individuals treated with biologic therapies, wrote Youssef A. Elnabawi, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and his colleagues.
Psoriasis “provides a reliable model to study inflammatory atherogenesis and the longitudinal impact of modulating specific cytokines on vascular behavior, while treating the primary skin disease with [Food and Drug Administration]–approved biologic therapies,” the researchers said.
In a study published in Cardiovascular Research, patients given biologics showed a 5% reduction in total coronary plaque burden after 1 year, as well as a 64% improvement in Psoriasis Area Severity Index scores. In addition, the decrease in noncalcified plaque burden in the biologics group was significantly greater, compared with the nonbiologics group (P =.03), and remained significant after controlling for standard cardiovascular risk factors.
When broken down by biologic, “we observed the greatest percent reduction of noncalcified plaque burden in patients on [anti-interleukin (IL)–17] therapy with a reduction in necrotic core suggesting a potential role for IL-17 in atherosclerotic pathways,” Dr. Elnabawi and his colleagues wrote.
(from 2.0 mg/dL to 1.4 mg/dL), but no change in the nonbiologics group.
The study population included patients naive to biologic or systemic psoriasis therapies who were assessed via clinical and laboratory data and coronary computed tomography angiography at baseline and after 1 year. A total of 89 participants with moderate to severe psoriasis received biologics, including adalimumab, etanercept, ustekinumab, secukinumab, and ixekizumab; 32 psoriasis patients received no biologics and served as a reference group. The average age of the patients was 50 years, and 58% were male. At baseline, patients had low cardiovascular risk based on Framingham scores, and moderate to severe skin disease.
The findings were limited by several factors, including the observational nature of the study, small study population, and the open-label use of biologics, as well as the use of coronary indices, rather than actual cardiovascular events, to assess cardiovascular disease risk, the researchers noted.
However, the results, combined with results from previous studies in animal models, “support further investigation of IL-17 blockade on coronary disease in humans,” they said.
The study was supported by the National Heart, Lung, and Blood Institute, with additional support from the National Institutes of Health Medical Research Scholars Program, the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors. Dr. Elnabawi had no financial conflicts to disclose.
SOURCE: Elnabawi YA et al. Cardiovasc Res. 2019. doi: 10.1093/cvr/cvz009.
Treatment with biologic therapy significantly improves coronary plaque profiles in patients with severe psoriasis, based on data from 121 adult patients who completed a year of follow-up.
A previous study showed a reduced rate of nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death among individuals treated with biologic therapies, wrote Youssef A. Elnabawi, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and his colleagues.
Psoriasis “provides a reliable model to study inflammatory atherogenesis and the longitudinal impact of modulating specific cytokines on vascular behavior, while treating the primary skin disease with [Food and Drug Administration]–approved biologic therapies,” the researchers said.
In a study published in Cardiovascular Research, patients given biologics showed a 5% reduction in total coronary plaque burden after 1 year, as well as a 64% improvement in Psoriasis Area Severity Index scores. In addition, the decrease in noncalcified plaque burden in the biologics group was significantly greater, compared with the nonbiologics group (P =.03), and remained significant after controlling for standard cardiovascular risk factors.
When broken down by biologic, “we observed the greatest percent reduction of noncalcified plaque burden in patients on [anti-interleukin (IL)–17] therapy with a reduction in necrotic core suggesting a potential role for IL-17 in atherosclerotic pathways,” Dr. Elnabawi and his colleagues wrote.
(from 2.0 mg/dL to 1.4 mg/dL), but no change in the nonbiologics group.
The study population included patients naive to biologic or systemic psoriasis therapies who were assessed via clinical and laboratory data and coronary computed tomography angiography at baseline and after 1 year. A total of 89 participants with moderate to severe psoriasis received biologics, including adalimumab, etanercept, ustekinumab, secukinumab, and ixekizumab; 32 psoriasis patients received no biologics and served as a reference group. The average age of the patients was 50 years, and 58% were male. At baseline, patients had low cardiovascular risk based on Framingham scores, and moderate to severe skin disease.
The findings were limited by several factors, including the observational nature of the study, small study population, and the open-label use of biologics, as well as the use of coronary indices, rather than actual cardiovascular events, to assess cardiovascular disease risk, the researchers noted.
However, the results, combined with results from previous studies in animal models, “support further investigation of IL-17 blockade on coronary disease in humans,” they said.
The study was supported by the National Heart, Lung, and Blood Institute, with additional support from the National Institutes of Health Medical Research Scholars Program, the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors. Dr. Elnabawi had no financial conflicts to disclose.
SOURCE: Elnabawi YA et al. Cardiovasc Res. 2019. doi: 10.1093/cvr/cvz009.
Treatment with biologic therapy significantly improves coronary plaque profiles in patients with severe psoriasis, based on data from 121 adult patients who completed a year of follow-up.
A previous study showed a reduced rate of nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death among individuals treated with biologic therapies, wrote Youssef A. Elnabawi, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and his colleagues.
Psoriasis “provides a reliable model to study inflammatory atherogenesis and the longitudinal impact of modulating specific cytokines on vascular behavior, while treating the primary skin disease with [Food and Drug Administration]–approved biologic therapies,” the researchers said.
In a study published in Cardiovascular Research, patients given biologics showed a 5% reduction in total coronary plaque burden after 1 year, as well as a 64% improvement in Psoriasis Area Severity Index scores. In addition, the decrease in noncalcified plaque burden in the biologics group was significantly greater, compared with the nonbiologics group (P =.03), and remained significant after controlling for standard cardiovascular risk factors.
When broken down by biologic, “we observed the greatest percent reduction of noncalcified plaque burden in patients on [anti-interleukin (IL)–17] therapy with a reduction in necrotic core suggesting a potential role for IL-17 in atherosclerotic pathways,” Dr. Elnabawi and his colleagues wrote.
(from 2.0 mg/dL to 1.4 mg/dL), but no change in the nonbiologics group.
The study population included patients naive to biologic or systemic psoriasis therapies who were assessed via clinical and laboratory data and coronary computed tomography angiography at baseline and after 1 year. A total of 89 participants with moderate to severe psoriasis received biologics, including adalimumab, etanercept, ustekinumab, secukinumab, and ixekizumab; 32 psoriasis patients received no biologics and served as a reference group. The average age of the patients was 50 years, and 58% were male. At baseline, patients had low cardiovascular risk based on Framingham scores, and moderate to severe skin disease.
The findings were limited by several factors, including the observational nature of the study, small study population, and the open-label use of biologics, as well as the use of coronary indices, rather than actual cardiovascular events, to assess cardiovascular disease risk, the researchers noted.
However, the results, combined with results from previous studies in animal models, “support further investigation of IL-17 blockade on coronary disease in humans,” they said.
The study was supported by the National Heart, Lung, and Blood Institute, with additional support from the National Institutes of Health Medical Research Scholars Program, the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors. Dr. Elnabawi had no financial conflicts to disclose.
SOURCE: Elnabawi YA et al. Cardiovasc Res. 2019. doi: 10.1093/cvr/cvz009.
FROM CARDIOVASCULAR RESEARCH
Key clinical point: Psoriasis patients treated with biologics also showed improvement in coronary artery profiles after 1 year, compared with patients not treated with biologics.
Major finding: Biologic therapy was associated with a 5% reduction in total coronary plaque burden from baseline.
Study details: The data come from 121 psoriasis patients in a prospective, observational study.
Disclosures: The study was supported by the National Heart, Lung, and Blood Institute, with additional support from the National Institutes of Health Medical Research Scholars Program, the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors. Dr. Elnabawi had no financial conflicts to disclose.
Source: Elnabawi YA et al. Cardiovasc Res. 2019. doi: 10.1093/cvr/cvz009.
Different disease features found with family history of psoriasis versus PsA
the results of a retrospective cohort study suggest.
A family history of psoriasis was associated with younger onset of psoriatic disease and the presence of enthesitis, while by contrast, a family history of psoriatic arthritis (PsA) was associated with lower risk of plaque psoriasis and higher risk of deformities, according to Dilek Solmaz, MD, of the University of Ottawa and her coauthors, who reported their findings in Arthritis Care & Research.
“The link between family history of psoriasis/psoriatic arthritis and pustular/plaque phenotypes may point to a different genetic background and pathogenic mechanisms in these subsets,” the investigators wrote.
Most, if not all, previous studies evaluating family history have grouped psoriasis and PsA together, according to Dr. Solmaz and her colleagues, rather than looking at the individual effects of psoriasis or PsA family history that may lead to unique disease phenotypes, as was done in the present study.
The investigators based their retrospective analysis on patients recruited in a longitudinal, multicenter database in Turkey and Canada. The mean age of patients in the study was 48 years; nearly 65% were female.
Out of 1,393 patients in the database, 444 had a family history of psoriasis or PsA. That included 335 patients with a psoriasis-only family history and 74 with a family history of PsA; another 35 patients weren’t sure about having a family history of PsA or psoriasis and were left out of the analysis.
Plaque psoriasis was more common in individuals with a family history of only psoriasis, while pustular psoriasis was more common in those with a PsA family history, the investigators reported.
In multivariate analyses, having a family member with psoriasis was a risk factor for younger age of psoriasis onset (odds ratio, 0.976; 95% confidence interval, 0.964-0.989; P less than .001) as well as a higher risk for enthesitis (OR, 1.931; 95% CI, 1.276-2.922; P = .002) when compared against patients without a family history of psoriasis.
Patients with a family history of PsA were more likely to have deformities (OR, 2.557; 95% CI, 1.250-5.234; P less than .010) and lower risk of plaque-type psoriasis (OR, 0.417; 95% CI, 0.213-0.816; P less than .011) than patients without a family history of PsA.
Disease onset was earlier among patients with a family history of psoriasis at a mean of 28.1 years versus 31.9 years for those with a family history of PsA (P less than .001).
Dr. Solmaz and her colleagues reported no conflicts of interest related to the research, which was supported in part by the Turkish Society for Rheumatology, the Scientific and Technological Research Council of Turkey, and Union Chimique Belge.
SOURCE: Solmaz D et al. Arthritis Care Res (Hoboken). 2019 Jan 25. doi: 10.1002/acr.23836.
the results of a retrospective cohort study suggest.
A family history of psoriasis was associated with younger onset of psoriatic disease and the presence of enthesitis, while by contrast, a family history of psoriatic arthritis (PsA) was associated with lower risk of plaque psoriasis and higher risk of deformities, according to Dilek Solmaz, MD, of the University of Ottawa and her coauthors, who reported their findings in Arthritis Care & Research.
“The link between family history of psoriasis/psoriatic arthritis and pustular/plaque phenotypes may point to a different genetic background and pathogenic mechanisms in these subsets,” the investigators wrote.
Most, if not all, previous studies evaluating family history have grouped psoriasis and PsA together, according to Dr. Solmaz and her colleagues, rather than looking at the individual effects of psoriasis or PsA family history that may lead to unique disease phenotypes, as was done in the present study.
The investigators based their retrospective analysis on patients recruited in a longitudinal, multicenter database in Turkey and Canada. The mean age of patients in the study was 48 years; nearly 65% were female.
Out of 1,393 patients in the database, 444 had a family history of psoriasis or PsA. That included 335 patients with a psoriasis-only family history and 74 with a family history of PsA; another 35 patients weren’t sure about having a family history of PsA or psoriasis and were left out of the analysis.
Plaque psoriasis was more common in individuals with a family history of only psoriasis, while pustular psoriasis was more common in those with a PsA family history, the investigators reported.
In multivariate analyses, having a family member with psoriasis was a risk factor for younger age of psoriasis onset (odds ratio, 0.976; 95% confidence interval, 0.964-0.989; P less than .001) as well as a higher risk for enthesitis (OR, 1.931; 95% CI, 1.276-2.922; P = .002) when compared against patients without a family history of psoriasis.
Patients with a family history of PsA were more likely to have deformities (OR, 2.557; 95% CI, 1.250-5.234; P less than .010) and lower risk of plaque-type psoriasis (OR, 0.417; 95% CI, 0.213-0.816; P less than .011) than patients without a family history of PsA.
Disease onset was earlier among patients with a family history of psoriasis at a mean of 28.1 years versus 31.9 years for those with a family history of PsA (P less than .001).
Dr. Solmaz and her colleagues reported no conflicts of interest related to the research, which was supported in part by the Turkish Society for Rheumatology, the Scientific and Technological Research Council of Turkey, and Union Chimique Belge.
SOURCE: Solmaz D et al. Arthritis Care Res (Hoboken). 2019 Jan 25. doi: 10.1002/acr.23836.
the results of a retrospective cohort study suggest.
A family history of psoriasis was associated with younger onset of psoriatic disease and the presence of enthesitis, while by contrast, a family history of psoriatic arthritis (PsA) was associated with lower risk of plaque psoriasis and higher risk of deformities, according to Dilek Solmaz, MD, of the University of Ottawa and her coauthors, who reported their findings in Arthritis Care & Research.
“The link between family history of psoriasis/psoriatic arthritis and pustular/plaque phenotypes may point to a different genetic background and pathogenic mechanisms in these subsets,” the investigators wrote.
Most, if not all, previous studies evaluating family history have grouped psoriasis and PsA together, according to Dr. Solmaz and her colleagues, rather than looking at the individual effects of psoriasis or PsA family history that may lead to unique disease phenotypes, as was done in the present study.
The investigators based their retrospective analysis on patients recruited in a longitudinal, multicenter database in Turkey and Canada. The mean age of patients in the study was 48 years; nearly 65% were female.
Out of 1,393 patients in the database, 444 had a family history of psoriasis or PsA. That included 335 patients with a psoriasis-only family history and 74 with a family history of PsA; another 35 patients weren’t sure about having a family history of PsA or psoriasis and were left out of the analysis.
Plaque psoriasis was more common in individuals with a family history of only psoriasis, while pustular psoriasis was more common in those with a PsA family history, the investigators reported.
In multivariate analyses, having a family member with psoriasis was a risk factor for younger age of psoriasis onset (odds ratio, 0.976; 95% confidence interval, 0.964-0.989; P less than .001) as well as a higher risk for enthesitis (OR, 1.931; 95% CI, 1.276-2.922; P = .002) when compared against patients without a family history of psoriasis.
Patients with a family history of PsA were more likely to have deformities (OR, 2.557; 95% CI, 1.250-5.234; P less than .010) and lower risk of plaque-type psoriasis (OR, 0.417; 95% CI, 0.213-0.816; P less than .011) than patients without a family history of PsA.
Disease onset was earlier among patients with a family history of psoriasis at a mean of 28.1 years versus 31.9 years for those with a family history of PsA (P less than .001).
Dr. Solmaz and her colleagues reported no conflicts of interest related to the research, which was supported in part by the Turkish Society for Rheumatology, the Scientific and Technological Research Council of Turkey, and Union Chimique Belge.
SOURCE: Solmaz D et al. Arthritis Care Res (Hoboken). 2019 Jan 25. doi: 10.1002/acr.23836.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Family histories of psoriasis and psoriatic arthritis were linked to different skin phenotypes, disease severity, and musculoskeletal features.
Major finding: Compared with no family history, psoriasis family history was a risk factor for enthesitis (odds ratio, 1.931) and younger age of onset (OR, 0.976) while psoriatic arthritis family history was linked to higher risk of deformities (OR, 2.557) and lower risk of plaque-type psoriasis (OR, 0.417).
Study details: A retrospective analysis including 1,393 Turkish or Canadian patients enrolled in a psoriatic arthritis database.
Disclosures: The study authors reported no conflicts of interest related to the research, which was supported in part by the Turkish Society for Rheumatology, the Scientific and Technological Research Council of Turkey, and Union Chimique Belge.
Source: Solmaz D et al. Arthritis Care Res (Hoboken). 2019 Jan 25. doi: 10.1002/acr.23836.
Clearance of Psoriasis After Ischemic Stroke
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
Practice Points
- Psoriasis is exacerbated in the presence of stress, and psoriatic lesions often have a symmetric distribution, which is evidence that the nervous system is involved in the pathophysiology of the condition.
- Various neuropeptides are involved in the pathophysiology of psoriasis, including substance P, nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide.
- Peripheral nerve damage results in decreased secretion of neuropeptides, which can lead to remission of psoriasis.
Safety and Efficacy of Halobetasol Propionate Lotion 0.01% in the Treatment of Moderate to Severe Plaque Psoriasis: A Pooled Analysis of 2 Phase 3 Studies
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.
A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).
Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).
Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).
Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).
BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).
IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).
By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).
Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.
Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.
A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).
Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).
Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).
Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).
BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).
IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).
By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).
Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.
Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.
A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).
Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).
Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).
Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).
BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).
IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).
By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).
Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.
Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
Psoriasis Treatment in Patients With Sickle Cell Disease
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Practice Points
• Tumor necrosis factor α contributes both to the vascular inflammatory state seen in sickle cell disease as well as the cycle of inflammation seen in the development of psoriasis.
• Tumor necrosis factor α inhibitors may be the drug of choice for patients with both psoriasis and sickle cell disease.