User login
What’s New in Topical Treatments for Psoriasis
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
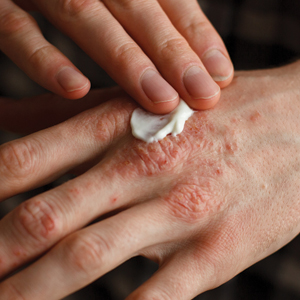
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
Guselkumab tops secukinumab over 48 weeks for plaque psoriasis
GRAND CAYMAN, CAYMAN ISLANDS – Guselkumab bested secukinumab in a 48-week-long study of plaque psoriasis, with 84.5% of patients on the interleukin (IL)-23 blocker hitting at least a 90% improvement in their Psoriasis Area Severity Index (PASI), compared with 70% of those taking secukinumab, which blocks IL-17.
The drug was numerically, but not significantly, better than secukinumab in the PASI 75 response at weeks 12 and 48 (84.6% for guselkumab at both time points vs. 80.2% for secukinumab at both time points). This finding on the primary secondary endpoint knocked the P values of the other five into “nominally significant” ranges. But the responses were still good enough for researchers to tag guselkumab as noninferior to its competitor, Jeffrey M. Sobell, MD, said at the meeting provided by Global Academy for Medical Education.
The difference also speaks to the difference in the drugs’ onset of action and its peak efficacy, said Dr. Sobell, of the department of dermatology at Tufts University, Boston.
“In both groups, the PASI 90 increased similarly in the first month,” to about 20%, he commented. “But at week 12 and after, it was consistently higher in guselkumab, peaking around week 28. Secukinumab peaked around weeks 16 to 20 and then slowly declined.”
Despite not being statistically significant, the other secondary efficacy endpoints were certainly enough to pique the audience’s attention. At week 48, guselkumab topped secukinumab in both PASI 100 (58.2% vs. 48.4%, respectively) and Investigator’s Global Assessment (IGA) scores of 0 (62.2% vs. 50.4%) and 0-1 (85% vs. 74.9%).
ECLIPSE randomized 1,048 patients with moderate to severe plaque psoriasis to 100-mg subcutaneous guselkumab at weeks 0, 4, and 12, followed by dosing every 8 weeks, or to 300-mg subcutaneous secukinumab administered by two subcutaneous injections of 150 mg at weeks 0, 1, 2, 3, and 4, followed by dosing every 4 weeks. The primary endpoint of the study was the proportion of patients achieving a PASI 90 response at week 48. Secondary endpoints were assessed at weeks 12 and 48, with safety monitoring through week 56.
The mean baseline Body Surface Area score was 24, and the mean PASI score was 20. Patients had already been treated with phototherapy (51.8%), nonbiologic systemic medications (53.7%), and biologics (29%). About 37% were naive to both nonbiologics and biologics.
Both drugs were well tolerated, with no unanticipated adverse events. Through week 44, the discontinuation rates were 5% for guselkumab and 9% for secukinumab. Adverse events were common in both arms (77.9% and 81.6%, respectively). Serious adverse events occurred in 6.2% and 7.2%, respectively. These included serious infections in six patients taking guselkumab and five taking secukinumab. Superficial Candida infections occurred in 2% of the guselkumab group and 5.7% of the secukinumab group; Tinea infections occurred in 1.7% and 4.5%, respectively.
The session was sponsored by Janssen, the manufacturer of guselkumab (Tremfya). Dr. Sobell is a consultant for Janssen and also disclosed relationships with AbbVie, Amgen, Celgene, Eli Lilly, Merck, Novartis, Regeneron, and Sun Pharma. Secukinumab is marketed as Cosentyx.
Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
GRAND CAYMAN, CAYMAN ISLANDS – Guselkumab bested secukinumab in a 48-week-long study of plaque psoriasis, with 84.5% of patients on the interleukin (IL)-23 blocker hitting at least a 90% improvement in their Psoriasis Area Severity Index (PASI), compared with 70% of those taking secukinumab, which blocks IL-17.
The drug was numerically, but not significantly, better than secukinumab in the PASI 75 response at weeks 12 and 48 (84.6% for guselkumab at both time points vs. 80.2% for secukinumab at both time points). This finding on the primary secondary endpoint knocked the P values of the other five into “nominally significant” ranges. But the responses were still good enough for researchers to tag guselkumab as noninferior to its competitor, Jeffrey M. Sobell, MD, said at the meeting provided by Global Academy for Medical Education.
The difference also speaks to the difference in the drugs’ onset of action and its peak efficacy, said Dr. Sobell, of the department of dermatology at Tufts University, Boston.
“In both groups, the PASI 90 increased similarly in the first month,” to about 20%, he commented. “But at week 12 and after, it was consistently higher in guselkumab, peaking around week 28. Secukinumab peaked around weeks 16 to 20 and then slowly declined.”
Despite not being statistically significant, the other secondary efficacy endpoints were certainly enough to pique the audience’s attention. At week 48, guselkumab topped secukinumab in both PASI 100 (58.2% vs. 48.4%, respectively) and Investigator’s Global Assessment (IGA) scores of 0 (62.2% vs. 50.4%) and 0-1 (85% vs. 74.9%).
ECLIPSE randomized 1,048 patients with moderate to severe plaque psoriasis to 100-mg subcutaneous guselkumab at weeks 0, 4, and 12, followed by dosing every 8 weeks, or to 300-mg subcutaneous secukinumab administered by two subcutaneous injections of 150 mg at weeks 0, 1, 2, 3, and 4, followed by dosing every 4 weeks. The primary endpoint of the study was the proportion of patients achieving a PASI 90 response at week 48. Secondary endpoints were assessed at weeks 12 and 48, with safety monitoring through week 56.
The mean baseline Body Surface Area score was 24, and the mean PASI score was 20. Patients had already been treated with phototherapy (51.8%), nonbiologic systemic medications (53.7%), and biologics (29%). About 37% were naive to both nonbiologics and biologics.
Both drugs were well tolerated, with no unanticipated adverse events. Through week 44, the discontinuation rates were 5% for guselkumab and 9% for secukinumab. Adverse events were common in both arms (77.9% and 81.6%, respectively). Serious adverse events occurred in 6.2% and 7.2%, respectively. These included serious infections in six patients taking guselkumab and five taking secukinumab. Superficial Candida infections occurred in 2% of the guselkumab group and 5.7% of the secukinumab group; Tinea infections occurred in 1.7% and 4.5%, respectively.
The session was sponsored by Janssen, the manufacturer of guselkumab (Tremfya). Dr. Sobell is a consultant for Janssen and also disclosed relationships with AbbVie, Amgen, Celgene, Eli Lilly, Merck, Novartis, Regeneron, and Sun Pharma. Secukinumab is marketed as Cosentyx.
Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
GRAND CAYMAN, CAYMAN ISLANDS – Guselkumab bested secukinumab in a 48-week-long study of plaque psoriasis, with 84.5% of patients on the interleukin (IL)-23 blocker hitting at least a 90% improvement in their Psoriasis Area Severity Index (PASI), compared with 70% of those taking secukinumab, which blocks IL-17.
The drug was numerically, but not significantly, better than secukinumab in the PASI 75 response at weeks 12 and 48 (84.6% for guselkumab at both time points vs. 80.2% for secukinumab at both time points). This finding on the primary secondary endpoint knocked the P values of the other five into “nominally significant” ranges. But the responses were still good enough for researchers to tag guselkumab as noninferior to its competitor, Jeffrey M. Sobell, MD, said at the meeting provided by Global Academy for Medical Education.
The difference also speaks to the difference in the drugs’ onset of action and its peak efficacy, said Dr. Sobell, of the department of dermatology at Tufts University, Boston.
“In both groups, the PASI 90 increased similarly in the first month,” to about 20%, he commented. “But at week 12 and after, it was consistently higher in guselkumab, peaking around week 28. Secukinumab peaked around weeks 16 to 20 and then slowly declined.”
Despite not being statistically significant, the other secondary efficacy endpoints were certainly enough to pique the audience’s attention. At week 48, guselkumab topped secukinumab in both PASI 100 (58.2% vs. 48.4%, respectively) and Investigator’s Global Assessment (IGA) scores of 0 (62.2% vs. 50.4%) and 0-1 (85% vs. 74.9%).
ECLIPSE randomized 1,048 patients with moderate to severe plaque psoriasis to 100-mg subcutaneous guselkumab at weeks 0, 4, and 12, followed by dosing every 8 weeks, or to 300-mg subcutaneous secukinumab administered by two subcutaneous injections of 150 mg at weeks 0, 1, 2, 3, and 4, followed by dosing every 4 weeks. The primary endpoint of the study was the proportion of patients achieving a PASI 90 response at week 48. Secondary endpoints were assessed at weeks 12 and 48, with safety monitoring through week 56.
The mean baseline Body Surface Area score was 24, and the mean PASI score was 20. Patients had already been treated with phototherapy (51.8%), nonbiologic systemic medications (53.7%), and biologics (29%). About 37% were naive to both nonbiologics and biologics.
Both drugs were well tolerated, with no unanticipated adverse events. Through week 44, the discontinuation rates were 5% for guselkumab and 9% for secukinumab. Adverse events were common in both arms (77.9% and 81.6%, respectively). Serious adverse events occurred in 6.2% and 7.2%, respectively. These included serious infections in six patients taking guselkumab and five taking secukinumab. Superficial Candida infections occurred in 2% of the guselkumab group and 5.7% of the secukinumab group; Tinea infections occurred in 1.7% and 4.5%, respectively.
The session was sponsored by Janssen, the manufacturer of guselkumab (Tremfya). Dr. Sobell is a consultant for Janssen and also disclosed relationships with AbbVie, Amgen, Celgene, Eli Lilly, Merck, Novartis, Regeneron, and Sun Pharma. Secukinumab is marketed as Cosentyx.
Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
REPORTING FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
Key clinical point: Guselkumab outperformed secukinumab for patients with moderate to severe plaque psoriasis.
Major finding: PASI 90 was achieved in 84.5% of patients on guselkumab and 70% on secukinumab.
Study details: The phase 3 study randomized 1,048 patients to guselkumab or secukinumab.
Disclosures: The session was sponsored by Janssen, the manufacturer of guselkumab (Tremfya). Dr. Sobell is a consultant for Janssen and also disclosed relationships with AbbVie, Amgen, Celgene, Eli Lilly, Merck, Novartis, Regeneron, and Sun Pharma.
Comorbidities may cut effectiveness of psoriasis biologics
PARIS – in response to biologic therapy, according to the results of the prospective observational PSO-BIO-REAL study.
The clinical importance of this finding lies in the fact that comorbidities are highly prevalent among patients with moderate to severe psoriasis. Indeed, fully 64% of the 846 participants in PSO-BIO-REAL had at least one major comorbid condition at baseline, Finn Ziegler said at the annual congress of the European Academy of Dermatology and Venereology.
“I think this reflects a picture that has been seen in other studies,” noted Mr. Ziegler, director of global patient access at Leo Pharma in Ballerup, Denmark.
The purpose of the 12-month PSO-BIO-REAL (PSOriasis treated with BIOlogics in REAL life) study was to assess the effectiveness of a variety of biologic agents in a real-world population typical of patients encountered in routine clinical practice, as opposed to more restrictive format of often-cited randomized trials, which generally feature a lengthy list of exclusions. One-third of participants were from the United States, with the rest drawn from four Western European countries. Their mean age was 47 years, with an 18.4-year history of psoriasis and a baseline Psoriasis Area and Severity Index (PASI) score of 14.3.
Sixty percent of participants were starting treatment with a biologic agent for the first time. The other 40% had prior biologic experience. At physician discretion, 61% of enrollees were put on a tumor necrosis factor inhibitor, either etanercept (Enbrel), adalimumab (Humira), or infliximab (Remicade); 30% initiated treatment with the interleukin-12/23 inhibitor ustekinumab (Stelara); and 9% received secukinumab (Cosentyx), an interleukin-17 inhibitor.
The five most common comorbid conditions present at baseline were hypertension, present in 33.5% of participants; psoriatic arthritis (PsA), present in 28.1%; hyperlipidemia, 20.9%; diabetes, 13.9%, and depression, present in 13.7% of the psoriasis patients.
Baseline comorbidities were significantly more common among the biologic-experienced patients. For example, their prevalence of hypertension was 42%, compared with 28% in the biologic-naive group. PsA was present in 35% of the biologic-experienced and 23% of the biologic-naive patients. Nineteen percent of biologic-experienced patients had diabetes at baseline, as did 11% of the biologic-naive group.
During the 12-month study, 3.7% of patients developed a new comorbidity, the most common being anxiety, hypertension, PsA, depression, and hyperlipidemia.
The primary outcome in the study was the complete clearance rate – a PASI 100 response – at 6 months. It ranged from a high of 31% in patients with no baseline comorbid conditions to a low of 16.5% in those with three or more. The results were similar at 12 months.
Conversely, an inadequate therapeutic response as defined by a PASI 50 or less at 6 months occurred in 15% of psoriasis patients with no baseline comorbidities, 27% with one, 35% with two comorbid conditions, and 28% with three or more.
The major caveat regarding this study is that the observed association between comorbid conditions and complete clearance rates doesn’t prove causality, Mr. Ziegler noted.
The PSO-BIO-REAL study was sponsored by Amgen, AstraZeneca, and Leo Pharma. Mr. Ziegler is a Leo executive.
SOURCE: Ziegler F. EADV Congress, Abstract FC04.01.
PARIS – in response to biologic therapy, according to the results of the prospective observational PSO-BIO-REAL study.
The clinical importance of this finding lies in the fact that comorbidities are highly prevalent among patients with moderate to severe psoriasis. Indeed, fully 64% of the 846 participants in PSO-BIO-REAL had at least one major comorbid condition at baseline, Finn Ziegler said at the annual congress of the European Academy of Dermatology and Venereology.
“I think this reflects a picture that has been seen in other studies,” noted Mr. Ziegler, director of global patient access at Leo Pharma in Ballerup, Denmark.
The purpose of the 12-month PSO-BIO-REAL (PSOriasis treated with BIOlogics in REAL life) study was to assess the effectiveness of a variety of biologic agents in a real-world population typical of patients encountered in routine clinical practice, as opposed to more restrictive format of often-cited randomized trials, which generally feature a lengthy list of exclusions. One-third of participants were from the United States, with the rest drawn from four Western European countries. Their mean age was 47 years, with an 18.4-year history of psoriasis and a baseline Psoriasis Area and Severity Index (PASI) score of 14.3.
Sixty percent of participants were starting treatment with a biologic agent for the first time. The other 40% had prior biologic experience. At physician discretion, 61% of enrollees were put on a tumor necrosis factor inhibitor, either etanercept (Enbrel), adalimumab (Humira), or infliximab (Remicade); 30% initiated treatment with the interleukin-12/23 inhibitor ustekinumab (Stelara); and 9% received secukinumab (Cosentyx), an interleukin-17 inhibitor.
The five most common comorbid conditions present at baseline were hypertension, present in 33.5% of participants; psoriatic arthritis (PsA), present in 28.1%; hyperlipidemia, 20.9%; diabetes, 13.9%, and depression, present in 13.7% of the psoriasis patients.
Baseline comorbidities were significantly more common among the biologic-experienced patients. For example, their prevalence of hypertension was 42%, compared with 28% in the biologic-naive group. PsA was present in 35% of the biologic-experienced and 23% of the biologic-naive patients. Nineteen percent of biologic-experienced patients had diabetes at baseline, as did 11% of the biologic-naive group.
During the 12-month study, 3.7% of patients developed a new comorbidity, the most common being anxiety, hypertension, PsA, depression, and hyperlipidemia.
The primary outcome in the study was the complete clearance rate – a PASI 100 response – at 6 months. It ranged from a high of 31% in patients with no baseline comorbid conditions to a low of 16.5% in those with three or more. The results were similar at 12 months.
Conversely, an inadequate therapeutic response as defined by a PASI 50 or less at 6 months occurred in 15% of psoriasis patients with no baseline comorbidities, 27% with one, 35% with two comorbid conditions, and 28% with three or more.
The major caveat regarding this study is that the observed association between comorbid conditions and complete clearance rates doesn’t prove causality, Mr. Ziegler noted.
The PSO-BIO-REAL study was sponsored by Amgen, AstraZeneca, and Leo Pharma. Mr. Ziegler is a Leo executive.
SOURCE: Ziegler F. EADV Congress, Abstract FC04.01.
PARIS – in response to biologic therapy, according to the results of the prospective observational PSO-BIO-REAL study.
The clinical importance of this finding lies in the fact that comorbidities are highly prevalent among patients with moderate to severe psoriasis. Indeed, fully 64% of the 846 participants in PSO-BIO-REAL had at least one major comorbid condition at baseline, Finn Ziegler said at the annual congress of the European Academy of Dermatology and Venereology.
“I think this reflects a picture that has been seen in other studies,” noted Mr. Ziegler, director of global patient access at Leo Pharma in Ballerup, Denmark.
The purpose of the 12-month PSO-BIO-REAL (PSOriasis treated with BIOlogics in REAL life) study was to assess the effectiveness of a variety of biologic agents in a real-world population typical of patients encountered in routine clinical practice, as opposed to more restrictive format of often-cited randomized trials, which generally feature a lengthy list of exclusions. One-third of participants were from the United States, with the rest drawn from four Western European countries. Their mean age was 47 years, with an 18.4-year history of psoriasis and a baseline Psoriasis Area and Severity Index (PASI) score of 14.3.
Sixty percent of participants were starting treatment with a biologic agent for the first time. The other 40% had prior biologic experience. At physician discretion, 61% of enrollees were put on a tumor necrosis factor inhibitor, either etanercept (Enbrel), adalimumab (Humira), or infliximab (Remicade); 30% initiated treatment with the interleukin-12/23 inhibitor ustekinumab (Stelara); and 9% received secukinumab (Cosentyx), an interleukin-17 inhibitor.
The five most common comorbid conditions present at baseline were hypertension, present in 33.5% of participants; psoriatic arthritis (PsA), present in 28.1%; hyperlipidemia, 20.9%; diabetes, 13.9%, and depression, present in 13.7% of the psoriasis patients.
Baseline comorbidities were significantly more common among the biologic-experienced patients. For example, their prevalence of hypertension was 42%, compared with 28% in the biologic-naive group. PsA was present in 35% of the biologic-experienced and 23% of the biologic-naive patients. Nineteen percent of biologic-experienced patients had diabetes at baseline, as did 11% of the biologic-naive group.
During the 12-month study, 3.7% of patients developed a new comorbidity, the most common being anxiety, hypertension, PsA, depression, and hyperlipidemia.
The primary outcome in the study was the complete clearance rate – a PASI 100 response – at 6 months. It ranged from a high of 31% in patients with no baseline comorbid conditions to a low of 16.5% in those with three or more. The results were similar at 12 months.
Conversely, an inadequate therapeutic response as defined by a PASI 50 or less at 6 months occurred in 15% of psoriasis patients with no baseline comorbidities, 27% with one, 35% with two comorbid conditions, and 28% with three or more.
The major caveat regarding this study is that the observed association between comorbid conditions and complete clearance rates doesn’t prove causality, Mr. Ziegler noted.
The PSO-BIO-REAL study was sponsored by Amgen, AstraZeneca, and Leo Pharma. Mr. Ziegler is a Leo executive.
SOURCE: Ziegler F. EADV Congress, Abstract FC04.01.
REPORTING FROM THE EADV CONGRESS
Key clinical point: As the number of baseline comorbid conditions increases, the complete clearance rate in response to biologic agents for psoriasis falls.
Major finding: The complete clearance rate after 6 months of biologic therapy ranged from a high of 31% in patients with no baseline comorbid conditions to a low of 16.5% in those with three or more.
Study details: This multinational, prospective, observational, 12-month study included 846 patients initiating biologic therapy for moderate to severe psoriasis.
Disclosures: The PSO-BIO-REAL study was sponsored by Amgen, AstraZeneca, and Leo Pharma and was presented by a Leo executive.
Source: Ziegler F. EADV Congress, Abstract FC04.01.
Brodalumab raced past ustekinumab to PASI 100
PARIS – The interleukin-17 receptor inhibitor
That’s according to a post hoc pooled analysis of the phase 3 randomized AMAGINE-1 and -3 trials that Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Other interleukin-17 inhibitors have also outperformed ustekinumab (Stelara) in head-to-head, randomized trials. What’s unique about this new secondary analysis of the AMAGINE trials is the demonstration that the complete clearance rate – that is, 100% improvement in Psoriasis Area and Severity Index (PASI) – with brodalumab (Siliq) was consistent, regardless of a psoriasis patient’s prior treatment history, according to Dr. Reich, professor of dermatology at Georg-August-University in Göttingen, Germany, and a partner at the Dermatologikum Hamburg.
“I don’t want to niche brodalumab as a rescue drug; but if you need a response in a patient who has failed a biologic, then obviously, this is a pretty good choice,” he said.
Typically, psoriasis patients who have previously failed to respond favorably to a biologic agent have a substantially lower complete clearance rate when placed on another biologic than do those who are biologic naive or haven’t been on a nonbiologic systemic therapy.
“I think it’s interesting that there is very little impact of previous treatment response with regard to this analysis when it comes to brodalumab,” the dermatologist observed. “It goes down a little bit, but if you compare it to ustekinumab, you see a very good robustness despite previous therapy.”
His presentation focused on the 339 AMAGINE-2 or AMAGINE-3 participants randomized to brodalumab at the approved dose of 210 mg by subcutaneous injection every 2 weeks, or to subcutaneous ustekinumab at the approved dose of 45 mg or 90 mg, depending upon body weight, on day 1, week 4, and then every 12 weeks in the 52-week trials.
It took 14 weeks for 50% of patients assigned to brodalumab to achieve a PASI 100 response, and 44 weeks to accomplish the same in the ustekinumab group. At 52 weeks, the PASI 100 response rate was 76% for brodalumab and 52% for ustekinumab.
This was a competing-risk analysis – a methodology relatively new to dermatology – in which the coprimary endpoint was inadequate response to treatment, as defined by a static Physician’s Global Assessment score of 3 or more or two consecutive sPGAs of at least 2 over a 4-week interval at any point from week 16 on. The inadequate response rate was 20% in the brodalumab group and 40% with ustekinumab.
Looking in the brodalumab group at PASI 100 response rates in relation to prior treatments, the complete clearance rate at week 52 was 76% in those with no prior systemic treatment at study entry, 78% in those with a history of nonbiologic systemic treatment, 75% in patients who hadn’t experienced treatment failure when previously on another biologic agent, and 70% in those with a baseline history of failure on a different biologic.
The corresponding PASI 100 rates in the ustekinumab group were strikingly lower, at 58%, 55%, 41%, and 30%.
Leo Pharma funded Dr. Reich’s post hoc analysis; Leo markets brodalumab in Europe. Dr. Reich reported receiving research funding from and serving as a consultant to that pharmaceutical company and numerous others involved in developing new drugs for psoriasis and atopic dermatitis.
SOURCE: Reich K. EADV Congress, Abstract #FC03.06.
PARIS – The interleukin-17 receptor inhibitor
That’s according to a post hoc pooled analysis of the phase 3 randomized AMAGINE-1 and -3 trials that Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Other interleukin-17 inhibitors have also outperformed ustekinumab (Stelara) in head-to-head, randomized trials. What’s unique about this new secondary analysis of the AMAGINE trials is the demonstration that the complete clearance rate – that is, 100% improvement in Psoriasis Area and Severity Index (PASI) – with brodalumab (Siliq) was consistent, regardless of a psoriasis patient’s prior treatment history, according to Dr. Reich, professor of dermatology at Georg-August-University in Göttingen, Germany, and a partner at the Dermatologikum Hamburg.
“I don’t want to niche brodalumab as a rescue drug; but if you need a response in a patient who has failed a biologic, then obviously, this is a pretty good choice,” he said.
Typically, psoriasis patients who have previously failed to respond favorably to a biologic agent have a substantially lower complete clearance rate when placed on another biologic than do those who are biologic naive or haven’t been on a nonbiologic systemic therapy.
“I think it’s interesting that there is very little impact of previous treatment response with regard to this analysis when it comes to brodalumab,” the dermatologist observed. “It goes down a little bit, but if you compare it to ustekinumab, you see a very good robustness despite previous therapy.”
His presentation focused on the 339 AMAGINE-2 or AMAGINE-3 participants randomized to brodalumab at the approved dose of 210 mg by subcutaneous injection every 2 weeks, or to subcutaneous ustekinumab at the approved dose of 45 mg or 90 mg, depending upon body weight, on day 1, week 4, and then every 12 weeks in the 52-week trials.
It took 14 weeks for 50% of patients assigned to brodalumab to achieve a PASI 100 response, and 44 weeks to accomplish the same in the ustekinumab group. At 52 weeks, the PASI 100 response rate was 76% for brodalumab and 52% for ustekinumab.
This was a competing-risk analysis – a methodology relatively new to dermatology – in which the coprimary endpoint was inadequate response to treatment, as defined by a static Physician’s Global Assessment score of 3 or more or two consecutive sPGAs of at least 2 over a 4-week interval at any point from week 16 on. The inadequate response rate was 20% in the brodalumab group and 40% with ustekinumab.
Looking in the brodalumab group at PASI 100 response rates in relation to prior treatments, the complete clearance rate at week 52 was 76% in those with no prior systemic treatment at study entry, 78% in those with a history of nonbiologic systemic treatment, 75% in patients who hadn’t experienced treatment failure when previously on another biologic agent, and 70% in those with a baseline history of failure on a different biologic.
The corresponding PASI 100 rates in the ustekinumab group were strikingly lower, at 58%, 55%, 41%, and 30%.
Leo Pharma funded Dr. Reich’s post hoc analysis; Leo markets brodalumab in Europe. Dr. Reich reported receiving research funding from and serving as a consultant to that pharmaceutical company and numerous others involved in developing new drugs for psoriasis and atopic dermatitis.
SOURCE: Reich K. EADV Congress, Abstract #FC03.06.
PARIS – The interleukin-17 receptor inhibitor
That’s according to a post hoc pooled analysis of the phase 3 randomized AMAGINE-1 and -3 trials that Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Other interleukin-17 inhibitors have also outperformed ustekinumab (Stelara) in head-to-head, randomized trials. What’s unique about this new secondary analysis of the AMAGINE trials is the demonstration that the complete clearance rate – that is, 100% improvement in Psoriasis Area and Severity Index (PASI) – with brodalumab (Siliq) was consistent, regardless of a psoriasis patient’s prior treatment history, according to Dr. Reich, professor of dermatology at Georg-August-University in Göttingen, Germany, and a partner at the Dermatologikum Hamburg.
“I don’t want to niche brodalumab as a rescue drug; but if you need a response in a patient who has failed a biologic, then obviously, this is a pretty good choice,” he said.
Typically, psoriasis patients who have previously failed to respond favorably to a biologic agent have a substantially lower complete clearance rate when placed on another biologic than do those who are biologic naive or haven’t been on a nonbiologic systemic therapy.
“I think it’s interesting that there is very little impact of previous treatment response with regard to this analysis when it comes to brodalumab,” the dermatologist observed. “It goes down a little bit, but if you compare it to ustekinumab, you see a very good robustness despite previous therapy.”
His presentation focused on the 339 AMAGINE-2 or AMAGINE-3 participants randomized to brodalumab at the approved dose of 210 mg by subcutaneous injection every 2 weeks, or to subcutaneous ustekinumab at the approved dose of 45 mg or 90 mg, depending upon body weight, on day 1, week 4, and then every 12 weeks in the 52-week trials.
It took 14 weeks for 50% of patients assigned to brodalumab to achieve a PASI 100 response, and 44 weeks to accomplish the same in the ustekinumab group. At 52 weeks, the PASI 100 response rate was 76% for brodalumab and 52% for ustekinumab.
This was a competing-risk analysis – a methodology relatively new to dermatology – in which the coprimary endpoint was inadequate response to treatment, as defined by a static Physician’s Global Assessment score of 3 or more or two consecutive sPGAs of at least 2 over a 4-week interval at any point from week 16 on. The inadequate response rate was 20% in the brodalumab group and 40% with ustekinumab.
Looking in the brodalumab group at PASI 100 response rates in relation to prior treatments, the complete clearance rate at week 52 was 76% in those with no prior systemic treatment at study entry, 78% in those with a history of nonbiologic systemic treatment, 75% in patients who hadn’t experienced treatment failure when previously on another biologic agent, and 70% in those with a baseline history of failure on a different biologic.
The corresponding PASI 100 rates in the ustekinumab group were strikingly lower, at 58%, 55%, 41%, and 30%.
Leo Pharma funded Dr. Reich’s post hoc analysis; Leo markets brodalumab in Europe. Dr. Reich reported receiving research funding from and serving as a consultant to that pharmaceutical company and numerous others involved in developing new drugs for psoriasis and atopic dermatitis.
SOURCE: Reich K. EADV Congress, Abstract #FC03.06.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Complete clearance rates in psoriasis patients on brodalumab were similar regardless of treatment history.
Major finding: Half of brodalumab-treated patients with moderate-to-severe psoriasis experienced complete clearance at 14 weeks; it took 44 weeks in patients assigned to ustekinumab.
Study details: This was a post hoc analysis of 52-week outcomes in more than 900 participants in the phase 3 AMAGINE-2 and AMAGINE-3 randomized head-to-head comparisons of brodalumab and ustekinumab.
Disclosures: Leo Pharma funded the post hoc analysis. The presenter reported receiving research funding from and serving as a consultant to that pharmaceutical company and numerous others involved in developing new drugs for psoriasis and atopic dermatitis.
Source: Reich K. EADV Congress, Abstract #FC03.06.
Chronic infections such as HCV, HIV, and TB cause unique problems for psoriasis patients
In a review of therapeutic issues for psoriasis patients who have such chronic infections as hepatitis, HIV, or latent tuberculosis infection (LTBI) or those who fall into the category of special populations (pregnant women or children), significant concerns were directly tied to the mode of action of the drugs involved.
In particular, “Most systemic agents for psoriasis are immunosuppressive, which poses a unique treatment challenge in patients with psoriasis with chronic infections because they are already immunosuppressed,” according to Shivani B. Kaushik, MD, a resident in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and her colleague Mark G. Lebwohl, MD, professor and system chair of the department.
For example, the reviewers detailed a report of hepatitis B virus (HBV) and hepatitis C virus (HCV) reactivation in patients with psoriasis who were taking biologics. Virus reactivation was noted in 2/175 patients who were positive for anti-HBc antibody, 3/97 patients with HCV infection, and 8/40 patients who were positive for HBsAg (the surface antigen of HBV). From this, they concluded that “biologics pose minimal risk for viral reactivation in patients with anti-HCV or anti-HBc antibodies, but they are of considerable risk in HBsAg-positive patients.” (J Amer Acad Derm. 2019 Jan;80:43-53).
Giving a specific example, Dr. Kaushik and her colleague pointed out that the safety of ustekinumab in patients with psoriasis with concurrent HCV and HBV infection was not clear. Viral reactivation and hepatocellular cancer were reported in one of four patients with HCV and in two of seven HBsAg-positive patients; and yet, another study showed that the successful use of ustekinumab for psoriasis had no impact on liver function or viral load in a patient with coexisting HCV.
Overall, “Patients should not be treated with immunosuppressive therapies during the acute stage. However, biologic treatment can be initiated in patients with chronic or resolved hepatitis under close monitoring and collaboration with a gastroenterologist,” the researchers stated.
In addition, they pointed out that methotrexate, another commonly prescribed drug for psoriasis, is absolutely contraindicated, although the use of cyclosporine remains controversial for those patients who are HCV-antibody positive.
“Most systemic agents used in psoriasis are immunosuppressive and require appropriate screening, monitoring, and prophylaxis when used in [psoriasis] patients with chronic infections, such as hepatitis, HIV, and LTBI,” the authors concluded.
The authors reported receiving funding from a number of pharmaceutical companies.
SOURCE: Kaushik BS et al. J Amer Acad Derm. 2019;80:43-53.
In a review of therapeutic issues for psoriasis patients who have such chronic infections as hepatitis, HIV, or latent tuberculosis infection (LTBI) or those who fall into the category of special populations (pregnant women or children), significant concerns were directly tied to the mode of action of the drugs involved.
In particular, “Most systemic agents for psoriasis are immunosuppressive, which poses a unique treatment challenge in patients with psoriasis with chronic infections because they are already immunosuppressed,” according to Shivani B. Kaushik, MD, a resident in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and her colleague Mark G. Lebwohl, MD, professor and system chair of the department.
For example, the reviewers detailed a report of hepatitis B virus (HBV) and hepatitis C virus (HCV) reactivation in patients with psoriasis who were taking biologics. Virus reactivation was noted in 2/175 patients who were positive for anti-HBc antibody, 3/97 patients with HCV infection, and 8/40 patients who were positive for HBsAg (the surface antigen of HBV). From this, they concluded that “biologics pose minimal risk for viral reactivation in patients with anti-HCV or anti-HBc antibodies, but they are of considerable risk in HBsAg-positive patients.” (J Amer Acad Derm. 2019 Jan;80:43-53).
Giving a specific example, Dr. Kaushik and her colleague pointed out that the safety of ustekinumab in patients with psoriasis with concurrent HCV and HBV infection was not clear. Viral reactivation and hepatocellular cancer were reported in one of four patients with HCV and in two of seven HBsAg-positive patients; and yet, another study showed that the successful use of ustekinumab for psoriasis had no impact on liver function or viral load in a patient with coexisting HCV.
Overall, “Patients should not be treated with immunosuppressive therapies during the acute stage. However, biologic treatment can be initiated in patients with chronic or resolved hepatitis under close monitoring and collaboration with a gastroenterologist,” the researchers stated.
In addition, they pointed out that methotrexate, another commonly prescribed drug for psoriasis, is absolutely contraindicated, although the use of cyclosporine remains controversial for those patients who are HCV-antibody positive.
“Most systemic agents used in psoriasis are immunosuppressive and require appropriate screening, monitoring, and prophylaxis when used in [psoriasis] patients with chronic infections, such as hepatitis, HIV, and LTBI,” the authors concluded.
The authors reported receiving funding from a number of pharmaceutical companies.
SOURCE: Kaushik BS et al. J Amer Acad Derm. 2019;80:43-53.
In a review of therapeutic issues for psoriasis patients who have such chronic infections as hepatitis, HIV, or latent tuberculosis infection (LTBI) or those who fall into the category of special populations (pregnant women or children), significant concerns were directly tied to the mode of action of the drugs involved.
In particular, “Most systemic agents for psoriasis are immunosuppressive, which poses a unique treatment challenge in patients with psoriasis with chronic infections because they are already immunosuppressed,” according to Shivani B. Kaushik, MD, a resident in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and her colleague Mark G. Lebwohl, MD, professor and system chair of the department.
For example, the reviewers detailed a report of hepatitis B virus (HBV) and hepatitis C virus (HCV) reactivation in patients with psoriasis who were taking biologics. Virus reactivation was noted in 2/175 patients who were positive for anti-HBc antibody, 3/97 patients with HCV infection, and 8/40 patients who were positive for HBsAg (the surface antigen of HBV). From this, they concluded that “biologics pose minimal risk for viral reactivation in patients with anti-HCV or anti-HBc antibodies, but they are of considerable risk in HBsAg-positive patients.” (J Amer Acad Derm. 2019 Jan;80:43-53).
Giving a specific example, Dr. Kaushik and her colleague pointed out that the safety of ustekinumab in patients with psoriasis with concurrent HCV and HBV infection was not clear. Viral reactivation and hepatocellular cancer were reported in one of four patients with HCV and in two of seven HBsAg-positive patients; and yet, another study showed that the successful use of ustekinumab for psoriasis had no impact on liver function or viral load in a patient with coexisting HCV.
Overall, “Patients should not be treated with immunosuppressive therapies during the acute stage. However, biologic treatment can be initiated in patients with chronic or resolved hepatitis under close monitoring and collaboration with a gastroenterologist,” the researchers stated.
In addition, they pointed out that methotrexate, another commonly prescribed drug for psoriasis, is absolutely contraindicated, although the use of cyclosporine remains controversial for those patients who are HCV-antibody positive.
“Most systemic agents used in psoriasis are immunosuppressive and require appropriate screening, monitoring, and prophylaxis when used in [psoriasis] patients with chronic infections, such as hepatitis, HIV, and LTBI,” the authors concluded.
The authors reported receiving funding from a number of pharmaceutical companies.
SOURCE: Kaushik BS et al. J Amer Acad Derm. 2019;80:43-53.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Longterm maintenance of PASI 75 responses observed with tildrakizumab
PARIS – Dermatologists are likely to do a double-take when they see the long-term efficacy and safety data for tildrakizumab (Ilumya), a high-affinity humanized monoclonal antibody targeting interleukin-23 p19, relative to the performance of older and more familiar biologic agents with other targets in psoriasis, Diamant Thaçi, MD, predicted at the annual congress of the European Academy of Dermatology and Venereology.
“The time to relapse [off tildrakizumab] is very different from what we are used to with other biologics; for example, the tumor necrosis factor inhibitors,” observed Dr. Thaçi, professor and chairman of the department of dermatology at the University of Lübeck (Germany).
He presented the 148-week, follow-up results of a pooled analysis of the open-label extension studies of reSURFACE 1 and reSURFACE 2, two pivotal phase 3 randomized double-blind international trials of 1,862 patients with moderate to severe chronic plaque psoriasis. The primary outcomes through week 12, which were instrumental in gaining marketing approval for tildrakizumab for treating psoriasis in 2018 from the Food and Drug Administration and the European Medicines Agency, have been published in the Lancet (2017 Jul 15;390[10091]:276-88).
Dr. Thaçi’s analysis of the 148-week outcomes was restricted to the patients who had at least a 75% improvement from baseline in Psoriasis Area and Severity Index scores (PASI 75) at week 28. Nearly 80% of patients on tildrakizumab reached that threshold at week 28 in reSURFACE 1, as did 73% in reSURFACE 2.
The question asked in the extension study was, How do responders to tildrakizumab at 28 weeks fare after nearly 3 years on the drug? And the answer was: very well. Maintenance of at least a PASI 75 response was observed at 148 weeks in 91% of patients on tildrakizumab at the approved 100-mg dose and 92% of those on the 200-mg dose. The FDA-approved regimen is 100 mg by subcutaneous injection at weeks 0 and 4, and then every 12 weeks after that.
An intriguing feature of reSURFACE 1 was that a subset of PASI 75 responders at week 28 got taken off tildrakizumab at that point and switched to double-blind placebo, then restarted on their earlier dose of tildrakizumab upon relapse, which was defined as loss of at least 50% of the achieved on-drug PASI improvement.
At week 64, fully 48 weeks after their last dose of tildrakizumab, the relapse rate was 54% in the group formerly on 100 mg of tildrakizumab and slightly better at 47% in those formerly on 200 mg. The median time to relapse was 226 days in the 100-mg group and 258 days in the higher-dose arm. Those are exceptionally long times to relapse, and it’s useful information to file away in the event a psoriasis patient needs to discontinue biologic therapy for a period of time, Dr. Thaçi observed.
At week 64 – again, off active treatment since week 16 – 63% of the tildrakizumab 100-mg group had lost their previous PASI 75 response, as had 52% who were formerly on tildrakizumab at 200 mg.
The long-term safety profile of tildrakizumab paralleled that of placebo. For example, the exposure-adjusted adverse event rates of serious infections and major adverse cardiovascular events were closely similar in the placebo, tildrakizumab 100 mg, and tildrakizumab 200 mg groups.
There were two notable between-group differences in adverse events of interest: injection site reactions occurred at a rate of 5.36 per 100 person-years with placebo, compared with 1.94 and 2.3 per 100 person-years with tildrakizumab at 100 and 200 mg, respectively; and the incidence of nonmelanoma skin cancer was 0.97 cases per 100 person-years in the placebo arm, versus 0.5 and 0.49 cases per 100 person-years in the two tildrakizumab arms.
Dr. Thaçi did not present PASI 90 response outcomes because, at the time the reSURFACE trials were planned, PASI 75 was considered state of the art. The PASI 90 data are still being crunched but will be available soon. The 4- and 5-year follow-up data from the long-term extension studies are also on their way.
The reSURFACE 1 and reSURFACE 2 trials and their extension studies were funded by Sun Pharma and Merck. Dr. Thaçi reported receiving research grants from and serving as a consultant and paid scientific advisor to those pharmaceutical companies and more than a dozen others.
PARIS – Dermatologists are likely to do a double-take when they see the long-term efficacy and safety data for tildrakizumab (Ilumya), a high-affinity humanized monoclonal antibody targeting interleukin-23 p19, relative to the performance of older and more familiar biologic agents with other targets in psoriasis, Diamant Thaçi, MD, predicted at the annual congress of the European Academy of Dermatology and Venereology.
“The time to relapse [off tildrakizumab] is very different from what we are used to with other biologics; for example, the tumor necrosis factor inhibitors,” observed Dr. Thaçi, professor and chairman of the department of dermatology at the University of Lübeck (Germany).
He presented the 148-week, follow-up results of a pooled analysis of the open-label extension studies of reSURFACE 1 and reSURFACE 2, two pivotal phase 3 randomized double-blind international trials of 1,862 patients with moderate to severe chronic plaque psoriasis. The primary outcomes through week 12, which were instrumental in gaining marketing approval for tildrakizumab for treating psoriasis in 2018 from the Food and Drug Administration and the European Medicines Agency, have been published in the Lancet (2017 Jul 15;390[10091]:276-88).
Dr. Thaçi’s analysis of the 148-week outcomes was restricted to the patients who had at least a 75% improvement from baseline in Psoriasis Area and Severity Index scores (PASI 75) at week 28. Nearly 80% of patients on tildrakizumab reached that threshold at week 28 in reSURFACE 1, as did 73% in reSURFACE 2.
The question asked in the extension study was, How do responders to tildrakizumab at 28 weeks fare after nearly 3 years on the drug? And the answer was: very well. Maintenance of at least a PASI 75 response was observed at 148 weeks in 91% of patients on tildrakizumab at the approved 100-mg dose and 92% of those on the 200-mg dose. The FDA-approved regimen is 100 mg by subcutaneous injection at weeks 0 and 4, and then every 12 weeks after that.
An intriguing feature of reSURFACE 1 was that a subset of PASI 75 responders at week 28 got taken off tildrakizumab at that point and switched to double-blind placebo, then restarted on their earlier dose of tildrakizumab upon relapse, which was defined as loss of at least 50% of the achieved on-drug PASI improvement.
At week 64, fully 48 weeks after their last dose of tildrakizumab, the relapse rate was 54% in the group formerly on 100 mg of tildrakizumab and slightly better at 47% in those formerly on 200 mg. The median time to relapse was 226 days in the 100-mg group and 258 days in the higher-dose arm. Those are exceptionally long times to relapse, and it’s useful information to file away in the event a psoriasis patient needs to discontinue biologic therapy for a period of time, Dr. Thaçi observed.
At week 64 – again, off active treatment since week 16 – 63% of the tildrakizumab 100-mg group had lost their previous PASI 75 response, as had 52% who were formerly on tildrakizumab at 200 mg.
The long-term safety profile of tildrakizumab paralleled that of placebo. For example, the exposure-adjusted adverse event rates of serious infections and major adverse cardiovascular events were closely similar in the placebo, tildrakizumab 100 mg, and tildrakizumab 200 mg groups.
There were two notable between-group differences in adverse events of interest: injection site reactions occurred at a rate of 5.36 per 100 person-years with placebo, compared with 1.94 and 2.3 per 100 person-years with tildrakizumab at 100 and 200 mg, respectively; and the incidence of nonmelanoma skin cancer was 0.97 cases per 100 person-years in the placebo arm, versus 0.5 and 0.49 cases per 100 person-years in the two tildrakizumab arms.
Dr. Thaçi did not present PASI 90 response outcomes because, at the time the reSURFACE trials were planned, PASI 75 was considered state of the art. The PASI 90 data are still being crunched but will be available soon. The 4- and 5-year follow-up data from the long-term extension studies are also on their way.
The reSURFACE 1 and reSURFACE 2 trials and their extension studies were funded by Sun Pharma and Merck. Dr. Thaçi reported receiving research grants from and serving as a consultant and paid scientific advisor to those pharmaceutical companies and more than a dozen others.
PARIS – Dermatologists are likely to do a double-take when they see the long-term efficacy and safety data for tildrakizumab (Ilumya), a high-affinity humanized monoclonal antibody targeting interleukin-23 p19, relative to the performance of older and more familiar biologic agents with other targets in psoriasis, Diamant Thaçi, MD, predicted at the annual congress of the European Academy of Dermatology and Venereology.
“The time to relapse [off tildrakizumab] is very different from what we are used to with other biologics; for example, the tumor necrosis factor inhibitors,” observed Dr. Thaçi, professor and chairman of the department of dermatology at the University of Lübeck (Germany).
He presented the 148-week, follow-up results of a pooled analysis of the open-label extension studies of reSURFACE 1 and reSURFACE 2, two pivotal phase 3 randomized double-blind international trials of 1,862 patients with moderate to severe chronic plaque psoriasis. The primary outcomes through week 12, which were instrumental in gaining marketing approval for tildrakizumab for treating psoriasis in 2018 from the Food and Drug Administration and the European Medicines Agency, have been published in the Lancet (2017 Jul 15;390[10091]:276-88).
Dr. Thaçi’s analysis of the 148-week outcomes was restricted to the patients who had at least a 75% improvement from baseline in Psoriasis Area and Severity Index scores (PASI 75) at week 28. Nearly 80% of patients on tildrakizumab reached that threshold at week 28 in reSURFACE 1, as did 73% in reSURFACE 2.
The question asked in the extension study was, How do responders to tildrakizumab at 28 weeks fare after nearly 3 years on the drug? And the answer was: very well. Maintenance of at least a PASI 75 response was observed at 148 weeks in 91% of patients on tildrakizumab at the approved 100-mg dose and 92% of those on the 200-mg dose. The FDA-approved regimen is 100 mg by subcutaneous injection at weeks 0 and 4, and then every 12 weeks after that.
An intriguing feature of reSURFACE 1 was that a subset of PASI 75 responders at week 28 got taken off tildrakizumab at that point and switched to double-blind placebo, then restarted on their earlier dose of tildrakizumab upon relapse, which was defined as loss of at least 50% of the achieved on-drug PASI improvement.
At week 64, fully 48 weeks after their last dose of tildrakizumab, the relapse rate was 54% in the group formerly on 100 mg of tildrakizumab and slightly better at 47% in those formerly on 200 mg. The median time to relapse was 226 days in the 100-mg group and 258 days in the higher-dose arm. Those are exceptionally long times to relapse, and it’s useful information to file away in the event a psoriasis patient needs to discontinue biologic therapy for a period of time, Dr. Thaçi observed.
At week 64 – again, off active treatment since week 16 – 63% of the tildrakizumab 100-mg group had lost their previous PASI 75 response, as had 52% who were formerly on tildrakizumab at 200 mg.
The long-term safety profile of tildrakizumab paralleled that of placebo. For example, the exposure-adjusted adverse event rates of serious infections and major adverse cardiovascular events were closely similar in the placebo, tildrakizumab 100 mg, and tildrakizumab 200 mg groups.
There were two notable between-group differences in adverse events of interest: injection site reactions occurred at a rate of 5.36 per 100 person-years with placebo, compared with 1.94 and 2.3 per 100 person-years with tildrakizumab at 100 and 200 mg, respectively; and the incidence of nonmelanoma skin cancer was 0.97 cases per 100 person-years in the placebo arm, versus 0.5 and 0.49 cases per 100 person-years in the two tildrakizumab arms.
Dr. Thaçi did not present PASI 90 response outcomes because, at the time the reSURFACE trials were planned, PASI 75 was considered state of the art. The PASI 90 data are still being crunched but will be available soon. The 4- and 5-year follow-up data from the long-term extension studies are also on their way.
The reSURFACE 1 and reSURFACE 2 trials and their extension studies were funded by Sun Pharma and Merck. Dr. Thaçi reported receiving research grants from and serving as a consultant and paid scientific advisor to those pharmaceutical companies and more than a dozen others.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Inhibition of interleukin-23 p19 via tildrakizumab pays major long-term dividends.
Major finding: Of patients with a PASI 75 response to tildrakizumab 100 mg at 6 months, 91% maintained that level of response through 148 weeks.
Study details: This was a long-term, prospective, open-label extension study of the phase 3 reSURFACE 1 and 2 trials of 1,862 psoriasis patients.
Disclosures: The reSURFACE 1 and reSURFACE 2 trials and their extension study were funded by Sun Pharma and Merck. The presenter reported receiving research grants from and serving as a consultant to those pharmaceutical companies and more than a dozen others.
CONDOR trial: Most psoriasis patients can be downshifted to reduced-dose biologics
PARIS – with long-term maintenance of disease control and no adverse consequences, Juul van den Reek, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.

She presented the results of the CONDOR trial, the first-ever formal, randomized, controlled trial of tightly regulated dose reduction of biologics, compared with usual care standard-dose therapy. “Our current advice is we think you can try to reduce the dose because there are a lot of patients who benefit from this,” declared Dr. van den Reek, a dermatologist at Radboud University, Nijmegen, the Netherlands.
The advantages of this strategy are twofold: lower expenditures for this costly collection of medications and less exposure to any long-term, drug-related health risks, she noted.
CONDOR was a Dutch six-center, 12-month, open-label, unblinded, noninferiority, randomized trial including 111 patients. Participants had to have stable low disease activity as defined by both Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI) scores of 5 or less for at least 6 months while on standard-dose etanercept (Enbrel), adalimumab (Humira), or ustekinumab (Stelara) prior to enrollment. In fact, the average baseline PASI score was less than 2, with a DLQI of 0.
Participants were randomized to usual care – the customary approved dose of biologic therapy – or a drop down to 67% of that dose, achieved through prolongation of the dosing interval. If the reduced-dose patients kept their PASI and DLQI scores at 5 or less for 3 months straight, they dropped further to 50% of their original dose. However, patients who exceeded those thresholds were immediately returned to their previously effective dose.
The primary endpoint in this noninferiority trial was the difference in mean PASI scores between the dose-reduction and usual-care groups at 12 months. The prespecified margin for noninferiority was a difference of 0.5 PASI points. And that’s where the results get dicey: The mean difference turned out to be 1.1 PASI points in favor of usual care, meaning that, according to the study ground rules, dose reduction was not statistically noninferior. In hindsight, however, that 0.5-point margin was ill-considered and too narrowly defined.
“Within the chosen margins, the dose-reduction strategy seemed inferior. But what is the clinical relevance of a mean difference of 1.1 PASI points, when the accepted minimal clinically important difference is 3.2 points?” Dr. van den Reek observed.
There was no significant between-group difference in DLQI scores at 12 months. Nor did the two study arms differ in terms of the prespecified secondary endpoint of persistent disease flares as defined by a PASI or DLQI greater than 5 for 3 consecutive months: five patients in the reduced-dose group and three in the usual-care arm experienced such flares. There were no serious adverse events or other safety signals related to the intervention.
At 12 months, 50% of patients in the dose-reduction group were well maintained on 50% of their original approved-dose biologic and another 17% were doing well on 67% of their former dose.
Session chair Dedee Murrell, MD, professor of dermatology at the University of New South Wales, Sydney, noted that neither patients nor dermatologists were blinded as to treatment status in CONDOR. She then asked the question on everybody’s minds: Was there any loss of treatment efficacy when patients in the dose-reduction arm needed to resume higher-dose therapy?
No, Dr. van den Reek replied. She added that planned future CONDOR analyses include a cost-effectiveness determination as well as measurement of serum drug levels and identification of antidrug antibodies, information that might prove helpful in identifying an enriched population of patients most likely to respond favorably to biologic dose reduction. In addition, CONDOR-X, a long-term extension study, is ongoing in order to learn how patients on reduced-dose biologics fare after the 12-month mark.
The CONDOR trial was funded by the Netherlands Organization for Health Research and Development; Dr. van den Reek reported having no financial conflicts of interest.
PARIS – with long-term maintenance of disease control and no adverse consequences, Juul van den Reek, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.

She presented the results of the CONDOR trial, the first-ever formal, randomized, controlled trial of tightly regulated dose reduction of biologics, compared with usual care standard-dose therapy. “Our current advice is we think you can try to reduce the dose because there are a lot of patients who benefit from this,” declared Dr. van den Reek, a dermatologist at Radboud University, Nijmegen, the Netherlands.
The advantages of this strategy are twofold: lower expenditures for this costly collection of medications and less exposure to any long-term, drug-related health risks, she noted.
CONDOR was a Dutch six-center, 12-month, open-label, unblinded, noninferiority, randomized trial including 111 patients. Participants had to have stable low disease activity as defined by both Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI) scores of 5 or less for at least 6 months while on standard-dose etanercept (Enbrel), adalimumab (Humira), or ustekinumab (Stelara) prior to enrollment. In fact, the average baseline PASI score was less than 2, with a DLQI of 0.
Participants were randomized to usual care – the customary approved dose of biologic therapy – or a drop down to 67% of that dose, achieved through prolongation of the dosing interval. If the reduced-dose patients kept their PASI and DLQI scores at 5 or less for 3 months straight, they dropped further to 50% of their original dose. However, patients who exceeded those thresholds were immediately returned to their previously effective dose.
The primary endpoint in this noninferiority trial was the difference in mean PASI scores between the dose-reduction and usual-care groups at 12 months. The prespecified margin for noninferiority was a difference of 0.5 PASI points. And that’s where the results get dicey: The mean difference turned out to be 1.1 PASI points in favor of usual care, meaning that, according to the study ground rules, dose reduction was not statistically noninferior. In hindsight, however, that 0.5-point margin was ill-considered and too narrowly defined.
“Within the chosen margins, the dose-reduction strategy seemed inferior. But what is the clinical relevance of a mean difference of 1.1 PASI points, when the accepted minimal clinically important difference is 3.2 points?” Dr. van den Reek observed.
There was no significant between-group difference in DLQI scores at 12 months. Nor did the two study arms differ in terms of the prespecified secondary endpoint of persistent disease flares as defined by a PASI or DLQI greater than 5 for 3 consecutive months: five patients in the reduced-dose group and three in the usual-care arm experienced such flares. There were no serious adverse events or other safety signals related to the intervention.
At 12 months, 50% of patients in the dose-reduction group were well maintained on 50% of their original approved-dose biologic and another 17% were doing well on 67% of their former dose.
Session chair Dedee Murrell, MD, professor of dermatology at the University of New South Wales, Sydney, noted that neither patients nor dermatologists were blinded as to treatment status in CONDOR. She then asked the question on everybody’s minds: Was there any loss of treatment efficacy when patients in the dose-reduction arm needed to resume higher-dose therapy?
No, Dr. van den Reek replied. She added that planned future CONDOR analyses include a cost-effectiveness determination as well as measurement of serum drug levels and identification of antidrug antibodies, information that might prove helpful in identifying an enriched population of patients most likely to respond favorably to biologic dose reduction. In addition, CONDOR-X, a long-term extension study, is ongoing in order to learn how patients on reduced-dose biologics fare after the 12-month mark.
The CONDOR trial was funded by the Netherlands Organization for Health Research and Development; Dr. van den Reek reported having no financial conflicts of interest.
PARIS – with long-term maintenance of disease control and no adverse consequences, Juul van den Reek, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.

She presented the results of the CONDOR trial, the first-ever formal, randomized, controlled trial of tightly regulated dose reduction of biologics, compared with usual care standard-dose therapy. “Our current advice is we think you can try to reduce the dose because there are a lot of patients who benefit from this,” declared Dr. van den Reek, a dermatologist at Radboud University, Nijmegen, the Netherlands.
The advantages of this strategy are twofold: lower expenditures for this costly collection of medications and less exposure to any long-term, drug-related health risks, she noted.
CONDOR was a Dutch six-center, 12-month, open-label, unblinded, noninferiority, randomized trial including 111 patients. Participants had to have stable low disease activity as defined by both Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI) scores of 5 or less for at least 6 months while on standard-dose etanercept (Enbrel), adalimumab (Humira), or ustekinumab (Stelara) prior to enrollment. In fact, the average baseline PASI score was less than 2, with a DLQI of 0.
Participants were randomized to usual care – the customary approved dose of biologic therapy – or a drop down to 67% of that dose, achieved through prolongation of the dosing interval. If the reduced-dose patients kept their PASI and DLQI scores at 5 or less for 3 months straight, they dropped further to 50% of their original dose. However, patients who exceeded those thresholds were immediately returned to their previously effective dose.
The primary endpoint in this noninferiority trial was the difference in mean PASI scores between the dose-reduction and usual-care groups at 12 months. The prespecified margin for noninferiority was a difference of 0.5 PASI points. And that’s where the results get dicey: The mean difference turned out to be 1.1 PASI points in favor of usual care, meaning that, according to the study ground rules, dose reduction was not statistically noninferior. In hindsight, however, that 0.5-point margin was ill-considered and too narrowly defined.
“Within the chosen margins, the dose-reduction strategy seemed inferior. But what is the clinical relevance of a mean difference of 1.1 PASI points, when the accepted minimal clinically important difference is 3.2 points?” Dr. van den Reek observed.
There was no significant between-group difference in DLQI scores at 12 months. Nor did the two study arms differ in terms of the prespecified secondary endpoint of persistent disease flares as defined by a PASI or DLQI greater than 5 for 3 consecutive months: five patients in the reduced-dose group and three in the usual-care arm experienced such flares. There were no serious adverse events or other safety signals related to the intervention.
At 12 months, 50% of patients in the dose-reduction group were well maintained on 50% of their original approved-dose biologic and another 17% were doing well on 67% of their former dose.
Session chair Dedee Murrell, MD, professor of dermatology at the University of New South Wales, Sydney, noted that neither patients nor dermatologists were blinded as to treatment status in CONDOR. She then asked the question on everybody’s minds: Was there any loss of treatment efficacy when patients in the dose-reduction arm needed to resume higher-dose therapy?
No, Dr. van den Reek replied. She added that planned future CONDOR analyses include a cost-effectiveness determination as well as measurement of serum drug levels and identification of antidrug antibodies, information that might prove helpful in identifying an enriched population of patients most likely to respond favorably to biologic dose reduction. In addition, CONDOR-X, a long-term extension study, is ongoing in order to learn how patients on reduced-dose biologics fare after the 12-month mark.
The CONDOR trial was funded by the Netherlands Organization for Health Research and Development; Dr. van den Reek reported having no financial conflicts of interest.
REPORTING FROM THE EADV CONGRESS
Key clinical point: An attempt at dose reduction is worthwhile in psoriasis patients well controlled on full-dose biologic therapy.
Major finding: Two-thirds of psoriasis patients maintained disease control after 12 months on reduced-dose biologic therapy.
Study details: This was a Dutch six-center, 12-month, open-label, unblinded, noninferiority, randomized trial of 111 psoriasis patients with stable low disease activity on standard-dose biologics at enrollment.
Disclosures: The CONDOR trial was funded by the Netherlands Organization for Health Research and Development; the presenter reported having no financial conflicts of interest.
Algorithm proposes approach for managing TNF inhibitor–induced psoriasis
Patients with tumor necrosis factor inhibitor–induced psoriasis could potentially be switched to a different drug class if they have moderate to severe skin eruption or mild skin eruption with an uncontrolled underlying disease such as inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis, according to a new treatment algorithm proposed by researchers from Brigham and Women’s Hospital and Harvard Medical School in Boston.
The researchers outlined the prevalence of tumor necrosis factor–alpha inhibitor (TNFi)-induced psoriasis in a literature review of inflammatory bowel disease (IBD), psoriasis, psoriatic arthritis (PsA), and rheumatoid arthritis (RA) and identified an estimated rate of between 2.3% and 5% in patients with RA and between 1.6% and 2.7% in patients with IBD. Although there have been reports of TNFi-induced psoriasis in patients with psoriasis and PsA, the prevalence is unclear, they wrote in the Journal of Psoriasis and Psoriatic Arthritis.
The authors then created an algorithm to manage and treat TNFi-induced psoriasiform skin eruptions with decisions to continue therapy and “treat through” symptoms, switch to a different anti-TNF therapy, or switch to a different drug class based on severity of symptoms, whether the underlying disease is well controlled, and how patients with those underlying diseases have fared with those specific therapies or agents.
“We’ve shifted gears over the past decade, and we’ve gone from having very few agents and trying to keep patients desperately on one or two agents because we didn’t want to have to give up on them for their other comorbid disease, whether it was Crohn’s, colitis, RA, or whatever it may be,” senior author Joseph Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston, said in an interview. “We’re now in an area where we can have an algorithm like this, and we have so many more mechanistic options to move to.”
Dr. Merola, who is board certified in dermatology and rheumatology, said the algorithm is meant to “open a dialogue” with other specialists in different areas and raise awareness of treatments in related but separate fields. For diseases not often seen by more than one specialty, with the exception of psoriasis and PsA, he said that “the idea is to start a dialogue and increase communication between specialists.”
Dr. Merola noted that while the algorithm in many respects is meant to guide a physician in a specialty in appropriate medication decisions, at the same time he hopes that “it opens a dialogue and communication with the other specialty who tends to oversee this particular disease state or class of medicine to really work together to try to find the right drug for the right person.”
For patients with a mild skin eruption and a controlled underlying disease, the algorithm recommends a “treat through” approach by continuing anti-TNF therapy and treating psoriasis symptoms with topical steroids, ultraviolet therapy, methotrexate, cyclosporine, or acitretin, and to consider dapsone in cases of pustular psoriasis. However, the researchers noted that “treat through” studies have reported complete symptom resolution in 26%-41% of patients.
For patients with recalcitrant or worsening TNFi-induced psoriasis or patients with mild skin eruptions with an uncontrolled underlying disease, the researchers proposed considering switching to a different anti-TNF therapy, although studies have shown complete resolution of symptoms in only 5%-37% of patients.
If patients worsen from there, or if they have moderate to-severe skin eruption with uncontrolled underlying disease, they could be considered for switching to a different drug class and treated based on their underlying disease, along with treatment for psoriasis symptoms. This approach has been shown to completely resolve lesions in up to 64% of cases, they said. IBD patients could benefit from ustekinumab, vedolizumab, 6-mercaptopurine, or azathioprine as an alternative to anti-TNF therapy. Those patients with psoriasis should be considered for guselkumab, while ustekinumab, ixekizumab, secukinumab, and apremilast are effective treatments for patients with psoriasis and PsA. Patients with RA could receive treatment with tocilizumab, rituximab, abatacept, and tofacitinib, the authors wrote.
Dr. Merola reported serving as a consultant and/or investigator for Merck Research Laboratories, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GlaxoSmithKline, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
SOURCE: Li SJ et al. J Psoriasis Psoriatic Arthritis. 2018 Nov 21. doi: 10.1177/2475530318810851.
Patients with tumor necrosis factor inhibitor–induced psoriasis could potentially be switched to a different drug class if they have moderate to severe skin eruption or mild skin eruption with an uncontrolled underlying disease such as inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis, according to a new treatment algorithm proposed by researchers from Brigham and Women’s Hospital and Harvard Medical School in Boston.
The researchers outlined the prevalence of tumor necrosis factor–alpha inhibitor (TNFi)-induced psoriasis in a literature review of inflammatory bowel disease (IBD), psoriasis, psoriatic arthritis (PsA), and rheumatoid arthritis (RA) and identified an estimated rate of between 2.3% and 5% in patients with RA and between 1.6% and 2.7% in patients with IBD. Although there have been reports of TNFi-induced psoriasis in patients with psoriasis and PsA, the prevalence is unclear, they wrote in the Journal of Psoriasis and Psoriatic Arthritis.
The authors then created an algorithm to manage and treat TNFi-induced psoriasiform skin eruptions with decisions to continue therapy and “treat through” symptoms, switch to a different anti-TNF therapy, or switch to a different drug class based on severity of symptoms, whether the underlying disease is well controlled, and how patients with those underlying diseases have fared with those specific therapies or agents.
“We’ve shifted gears over the past decade, and we’ve gone from having very few agents and trying to keep patients desperately on one or two agents because we didn’t want to have to give up on them for their other comorbid disease, whether it was Crohn’s, colitis, RA, or whatever it may be,” senior author Joseph Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston, said in an interview. “We’re now in an area where we can have an algorithm like this, and we have so many more mechanistic options to move to.”
Dr. Merola, who is board certified in dermatology and rheumatology, said the algorithm is meant to “open a dialogue” with other specialists in different areas and raise awareness of treatments in related but separate fields. For diseases not often seen by more than one specialty, with the exception of psoriasis and PsA, he said that “the idea is to start a dialogue and increase communication between specialists.”
Dr. Merola noted that while the algorithm in many respects is meant to guide a physician in a specialty in appropriate medication decisions, at the same time he hopes that “it opens a dialogue and communication with the other specialty who tends to oversee this particular disease state or class of medicine to really work together to try to find the right drug for the right person.”
For patients with a mild skin eruption and a controlled underlying disease, the algorithm recommends a “treat through” approach by continuing anti-TNF therapy and treating psoriasis symptoms with topical steroids, ultraviolet therapy, methotrexate, cyclosporine, or acitretin, and to consider dapsone in cases of pustular psoriasis. However, the researchers noted that “treat through” studies have reported complete symptom resolution in 26%-41% of patients.
For patients with recalcitrant or worsening TNFi-induced psoriasis or patients with mild skin eruptions with an uncontrolled underlying disease, the researchers proposed considering switching to a different anti-TNF therapy, although studies have shown complete resolution of symptoms in only 5%-37% of patients.
If patients worsen from there, or if they have moderate to-severe skin eruption with uncontrolled underlying disease, they could be considered for switching to a different drug class and treated based on their underlying disease, along with treatment for psoriasis symptoms. This approach has been shown to completely resolve lesions in up to 64% of cases, they said. IBD patients could benefit from ustekinumab, vedolizumab, 6-mercaptopurine, or azathioprine as an alternative to anti-TNF therapy. Those patients with psoriasis should be considered for guselkumab, while ustekinumab, ixekizumab, secukinumab, and apremilast are effective treatments for patients with psoriasis and PsA. Patients with RA could receive treatment with tocilizumab, rituximab, abatacept, and tofacitinib, the authors wrote.
Dr. Merola reported serving as a consultant and/or investigator for Merck Research Laboratories, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GlaxoSmithKline, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
SOURCE: Li SJ et al. J Psoriasis Psoriatic Arthritis. 2018 Nov 21. doi: 10.1177/2475530318810851.
Patients with tumor necrosis factor inhibitor–induced psoriasis could potentially be switched to a different drug class if they have moderate to severe skin eruption or mild skin eruption with an uncontrolled underlying disease such as inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis, according to a new treatment algorithm proposed by researchers from Brigham and Women’s Hospital and Harvard Medical School in Boston.
The researchers outlined the prevalence of tumor necrosis factor–alpha inhibitor (TNFi)-induced psoriasis in a literature review of inflammatory bowel disease (IBD), psoriasis, psoriatic arthritis (PsA), and rheumatoid arthritis (RA) and identified an estimated rate of between 2.3% and 5% in patients with RA and between 1.6% and 2.7% in patients with IBD. Although there have been reports of TNFi-induced psoriasis in patients with psoriasis and PsA, the prevalence is unclear, they wrote in the Journal of Psoriasis and Psoriatic Arthritis.
The authors then created an algorithm to manage and treat TNFi-induced psoriasiform skin eruptions with decisions to continue therapy and “treat through” symptoms, switch to a different anti-TNF therapy, or switch to a different drug class based on severity of symptoms, whether the underlying disease is well controlled, and how patients with those underlying diseases have fared with those specific therapies or agents.
“We’ve shifted gears over the past decade, and we’ve gone from having very few agents and trying to keep patients desperately on one or two agents because we didn’t want to have to give up on them for their other comorbid disease, whether it was Crohn’s, colitis, RA, or whatever it may be,” senior author Joseph Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston, said in an interview. “We’re now in an area where we can have an algorithm like this, and we have so many more mechanistic options to move to.”
Dr. Merola, who is board certified in dermatology and rheumatology, said the algorithm is meant to “open a dialogue” with other specialists in different areas and raise awareness of treatments in related but separate fields. For diseases not often seen by more than one specialty, with the exception of psoriasis and PsA, he said that “the idea is to start a dialogue and increase communication between specialists.”
Dr. Merola noted that while the algorithm in many respects is meant to guide a physician in a specialty in appropriate medication decisions, at the same time he hopes that “it opens a dialogue and communication with the other specialty who tends to oversee this particular disease state or class of medicine to really work together to try to find the right drug for the right person.”
For patients with a mild skin eruption and a controlled underlying disease, the algorithm recommends a “treat through” approach by continuing anti-TNF therapy and treating psoriasis symptoms with topical steroids, ultraviolet therapy, methotrexate, cyclosporine, or acitretin, and to consider dapsone in cases of pustular psoriasis. However, the researchers noted that “treat through” studies have reported complete symptom resolution in 26%-41% of patients.
For patients with recalcitrant or worsening TNFi-induced psoriasis or patients with mild skin eruptions with an uncontrolled underlying disease, the researchers proposed considering switching to a different anti-TNF therapy, although studies have shown complete resolution of symptoms in only 5%-37% of patients.
If patients worsen from there, or if they have moderate to-severe skin eruption with uncontrolled underlying disease, they could be considered for switching to a different drug class and treated based on their underlying disease, along with treatment for psoriasis symptoms. This approach has been shown to completely resolve lesions in up to 64% of cases, they said. IBD patients could benefit from ustekinumab, vedolizumab, 6-mercaptopurine, or azathioprine as an alternative to anti-TNF therapy. Those patients with psoriasis should be considered for guselkumab, while ustekinumab, ixekizumab, secukinumab, and apremilast are effective treatments for patients with psoriasis and PsA. Patients with RA could receive treatment with tocilizumab, rituximab, abatacept, and tofacitinib, the authors wrote.
Dr. Merola reported serving as a consultant and/or investigator for Merck Research Laboratories, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GlaxoSmithKline, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
SOURCE: Li SJ et al. J Psoriasis Psoriatic Arthritis. 2018 Nov 21. doi: 10.1177/2475530318810851.
FROM JOURNAL OF PSORIASIS AND PSORIATIC ARTHRITIS
Debunking Psoriasis Myths: Psoriasis Is More Than Skin Deep
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
Weight loss cuts risk of psoriatic arthritis
CHICAGO – Overweight and obese psoriasis patients have it within their power to reduce their risk of developing psoriatic arthritis through weight loss, according to a large British longitudinal study.
Of the three modifiable lifestyle factors evaluated in the study as potential risk factors for the development of psoriatic arthritis in psoriasis patients – body mass index, smoking, and alcohol intake – reduction in BMI over time was clearly the winning strategy, Neil McHugh, MD, said at the annual meeting of the American College of Rheumatology.
The message from this study of 90,189 incident cases of psoriasis identified in the U.K. Clinical Practice Research Datalink was unequivocal: “If you’re overweight and have psoriasis and you lose weight, you reduce your chance of developing a nasty form of arthritis,” said Dr. McHugh, professor of pharmacoepidemiology and a rheumatologist at the University of Bath, England.
“As psoriatic arthritis affects around 20% of people with psoriasis, weight reduction amongst those who are obese may have the potential to greatly reduce their risk of psoriatic arthritis in addition to providing additional health benefits,” he added.
Among the more than 90,000 patients diagnosed with psoriasis, 1,409 subsequently developed psoriatic arthritis, with an overall incidence rate of 2.72 cases per 1,000 person-years. Baseline BMI was strongly associated in stepwise fashion with subsequent psoriatic arthritis. Psoriasis patients with a baseline BMI of 25-29.9 kg/m2 were at an adjusted 1.76-fold increased risk of later developing psoriatic arthritis, compared with psoriasis patients having a BMI of less than 25. For those with a BMI of 30-34.9 kg/m2, the risk of subsequent psoriatic arthritis was increased 2.04-fold. And for those with a baseline BMI of 35 kg/m2 or more, the risk was increased 2.42-fold in analyses adjusted for age, sex, psoriasis duration and severity, history of trauma, and diabetes.
In contrast, the risk of developing psoriatic arthritis wasn’t significantly different between psoriasis patients who were nonsmokers, ex-smokers, or current smokers. And while there was a significantly increased risk of developing psoriatic arthritis in psoriasis patients who were current drinkers, compared with nondrinkers, the risk in ex-drinkers and heavy drinkers was similar to that in nondrinkers, a counterintuitive finding Dr. McHugh suspects was a distortion due to small numbers.
While the observed relationship between baseline BMI and subsequent risk of psoriatic arthritis was informative, it only tells part of the story, since body weight so often changes over time. Dr. McHugh and his coinvestigators had data on change in BMI over the course of 10 years of follow-up in 15,627 psoriasis patients free of psoriatic arthritis at the time their psoriasis was diagnosed. The researchers developed a BMI risk calculator that expressed the effect of change in BMI over time on the cumulative risk of developing psoriatic arthritis.
“We were able to show that if, for instance, you started with a BMI of 25 at baseline and ended up with a BMI of 30, your risk of psoriatic arthritis goes up by 13%, whereas if you start at 30 and come down to 25, your risk decreases by 13%. And the more weight you lose, the greater you reduce your risk of developing psoriatic arthritis,” the rheumatologist explained in an interview.
Indeed, with more extreme changes in BMI over the course of a decade following diagnosis of psoriasis – for example, dropping from a baseline BMI of 36 kg/m2 to 23 kg/m2 – the risk of developing psoriatic arthritis fell by close to 30%.
Dr. McHugh reported having no financial conflicts regarding this study, funded by the U.K. National Institute for Health Research.
SOURCE: Green A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2134.
CHICAGO – Overweight and obese psoriasis patients have it within their power to reduce their risk of developing psoriatic arthritis through weight loss, according to a large British longitudinal study.
Of the three modifiable lifestyle factors evaluated in the study as potential risk factors for the development of psoriatic arthritis in psoriasis patients – body mass index, smoking, and alcohol intake – reduction in BMI over time was clearly the winning strategy, Neil McHugh, MD, said at the annual meeting of the American College of Rheumatology.
The message from this study of 90,189 incident cases of psoriasis identified in the U.K. Clinical Practice Research Datalink was unequivocal: “If you’re overweight and have psoriasis and you lose weight, you reduce your chance of developing a nasty form of arthritis,” said Dr. McHugh, professor of pharmacoepidemiology and a rheumatologist at the University of Bath, England.
“As psoriatic arthritis affects around 20% of people with psoriasis, weight reduction amongst those who are obese may have the potential to greatly reduce their risk of psoriatic arthritis in addition to providing additional health benefits,” he added.
Among the more than 90,000 patients diagnosed with psoriasis, 1,409 subsequently developed psoriatic arthritis, with an overall incidence rate of 2.72 cases per 1,000 person-years. Baseline BMI was strongly associated in stepwise fashion with subsequent psoriatic arthritis. Psoriasis patients with a baseline BMI of 25-29.9 kg/m2 were at an adjusted 1.76-fold increased risk of later developing psoriatic arthritis, compared with psoriasis patients having a BMI of less than 25. For those with a BMI of 30-34.9 kg/m2, the risk of subsequent psoriatic arthritis was increased 2.04-fold. And for those with a baseline BMI of 35 kg/m2 or more, the risk was increased 2.42-fold in analyses adjusted for age, sex, psoriasis duration and severity, history of trauma, and diabetes.
In contrast, the risk of developing psoriatic arthritis wasn’t significantly different between psoriasis patients who were nonsmokers, ex-smokers, or current smokers. And while there was a significantly increased risk of developing psoriatic arthritis in psoriasis patients who were current drinkers, compared with nondrinkers, the risk in ex-drinkers and heavy drinkers was similar to that in nondrinkers, a counterintuitive finding Dr. McHugh suspects was a distortion due to small numbers.
While the observed relationship between baseline BMI and subsequent risk of psoriatic arthritis was informative, it only tells part of the story, since body weight so often changes over time. Dr. McHugh and his coinvestigators had data on change in BMI over the course of 10 years of follow-up in 15,627 psoriasis patients free of psoriatic arthritis at the time their psoriasis was diagnosed. The researchers developed a BMI risk calculator that expressed the effect of change in BMI over time on the cumulative risk of developing psoriatic arthritis.
“We were able to show that if, for instance, you started with a BMI of 25 at baseline and ended up with a BMI of 30, your risk of psoriatic arthritis goes up by 13%, whereas if you start at 30 and come down to 25, your risk decreases by 13%. And the more weight you lose, the greater you reduce your risk of developing psoriatic arthritis,” the rheumatologist explained in an interview.
Indeed, with more extreme changes in BMI over the course of a decade following diagnosis of psoriasis – for example, dropping from a baseline BMI of 36 kg/m2 to 23 kg/m2 – the risk of developing psoriatic arthritis fell by close to 30%.
Dr. McHugh reported having no financial conflicts regarding this study, funded by the U.K. National Institute for Health Research.
SOURCE: Green A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2134.
CHICAGO – Overweight and obese psoriasis patients have it within their power to reduce their risk of developing psoriatic arthritis through weight loss, according to a large British longitudinal study.
Of the three modifiable lifestyle factors evaluated in the study as potential risk factors for the development of psoriatic arthritis in psoriasis patients – body mass index, smoking, and alcohol intake – reduction in BMI over time was clearly the winning strategy, Neil McHugh, MD, said at the annual meeting of the American College of Rheumatology.
The message from this study of 90,189 incident cases of psoriasis identified in the U.K. Clinical Practice Research Datalink was unequivocal: “If you’re overweight and have psoriasis and you lose weight, you reduce your chance of developing a nasty form of arthritis,” said Dr. McHugh, professor of pharmacoepidemiology and a rheumatologist at the University of Bath, England.
“As psoriatic arthritis affects around 20% of people with psoriasis, weight reduction amongst those who are obese may have the potential to greatly reduce their risk of psoriatic arthritis in addition to providing additional health benefits,” he added.
Among the more than 90,000 patients diagnosed with psoriasis, 1,409 subsequently developed psoriatic arthritis, with an overall incidence rate of 2.72 cases per 1,000 person-years. Baseline BMI was strongly associated in stepwise fashion with subsequent psoriatic arthritis. Psoriasis patients with a baseline BMI of 25-29.9 kg/m2 were at an adjusted 1.76-fold increased risk of later developing psoriatic arthritis, compared with psoriasis patients having a BMI of less than 25. For those with a BMI of 30-34.9 kg/m2, the risk of subsequent psoriatic arthritis was increased 2.04-fold. And for those with a baseline BMI of 35 kg/m2 or more, the risk was increased 2.42-fold in analyses adjusted for age, sex, psoriasis duration and severity, history of trauma, and diabetes.
In contrast, the risk of developing psoriatic arthritis wasn’t significantly different between psoriasis patients who were nonsmokers, ex-smokers, or current smokers. And while there was a significantly increased risk of developing psoriatic arthritis in psoriasis patients who were current drinkers, compared with nondrinkers, the risk in ex-drinkers and heavy drinkers was similar to that in nondrinkers, a counterintuitive finding Dr. McHugh suspects was a distortion due to small numbers.
While the observed relationship between baseline BMI and subsequent risk of psoriatic arthritis was informative, it only tells part of the story, since body weight so often changes over time. Dr. McHugh and his coinvestigators had data on change in BMI over the course of 10 years of follow-up in 15,627 psoriasis patients free of psoriatic arthritis at the time their psoriasis was diagnosed. The researchers developed a BMI risk calculator that expressed the effect of change in BMI over time on the cumulative risk of developing psoriatic arthritis.
“We were able to show that if, for instance, you started with a BMI of 25 at baseline and ended up with a BMI of 30, your risk of psoriatic arthritis goes up by 13%, whereas if you start at 30 and come down to 25, your risk decreases by 13%. And the more weight you lose, the greater you reduce your risk of developing psoriatic arthritis,” the rheumatologist explained in an interview.
Indeed, with more extreme changes in BMI over the course of a decade following diagnosis of psoriasis – for example, dropping from a baseline BMI of 36 kg/m2 to 23 kg/m2 – the risk of developing psoriatic arthritis fell by close to 30%.
Dr. McHugh reported having no financial conflicts regarding this study, funded by the U.K. National Institute for Health Research.
SOURCE: Green A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2134.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: A psoriasis patient’s risk of developing psoriatic arthritis increases stepwise with greater body mass index, and the converse is true as well.
Study details: This study included more than 90,000 patients with a diagnosis of psoriasis in the U.K. Clinical Practice Research Datalink.
Disclosures: The presenter reported having no financial conflicts regarding this study, funded by the U.K. National Institute for Health Research.
Source: Green A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2134.