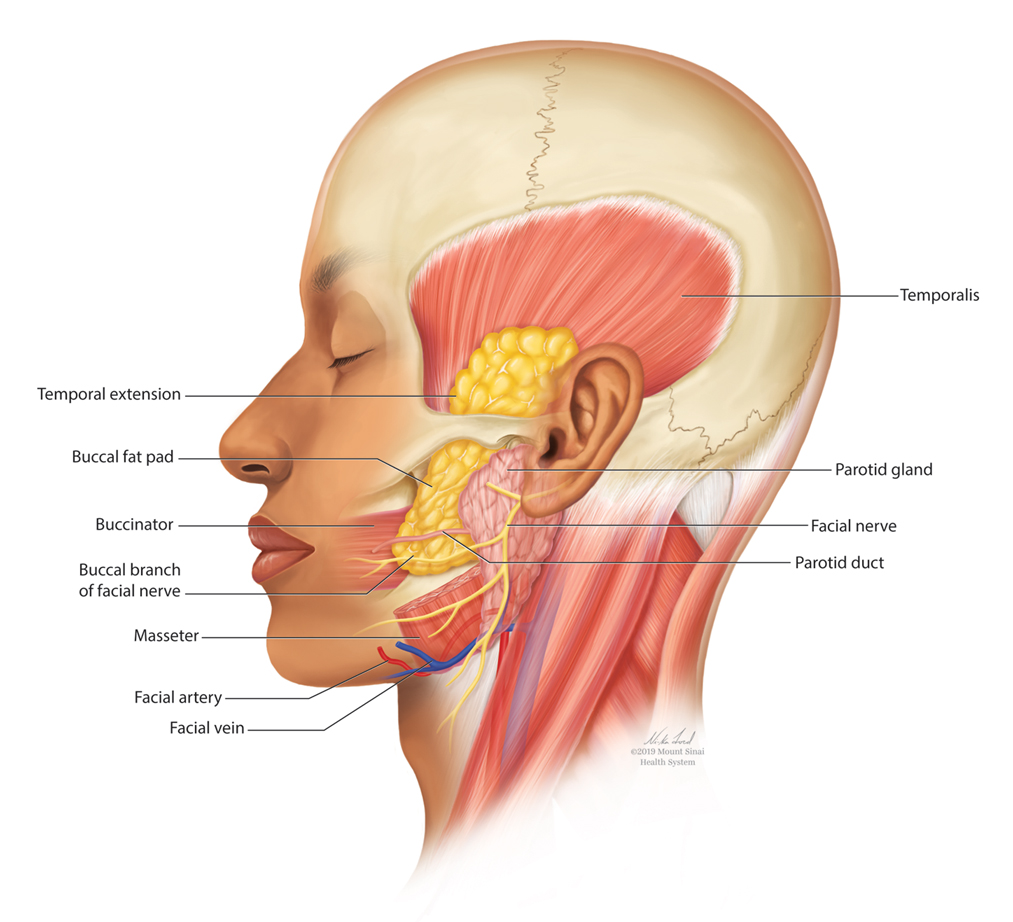
User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
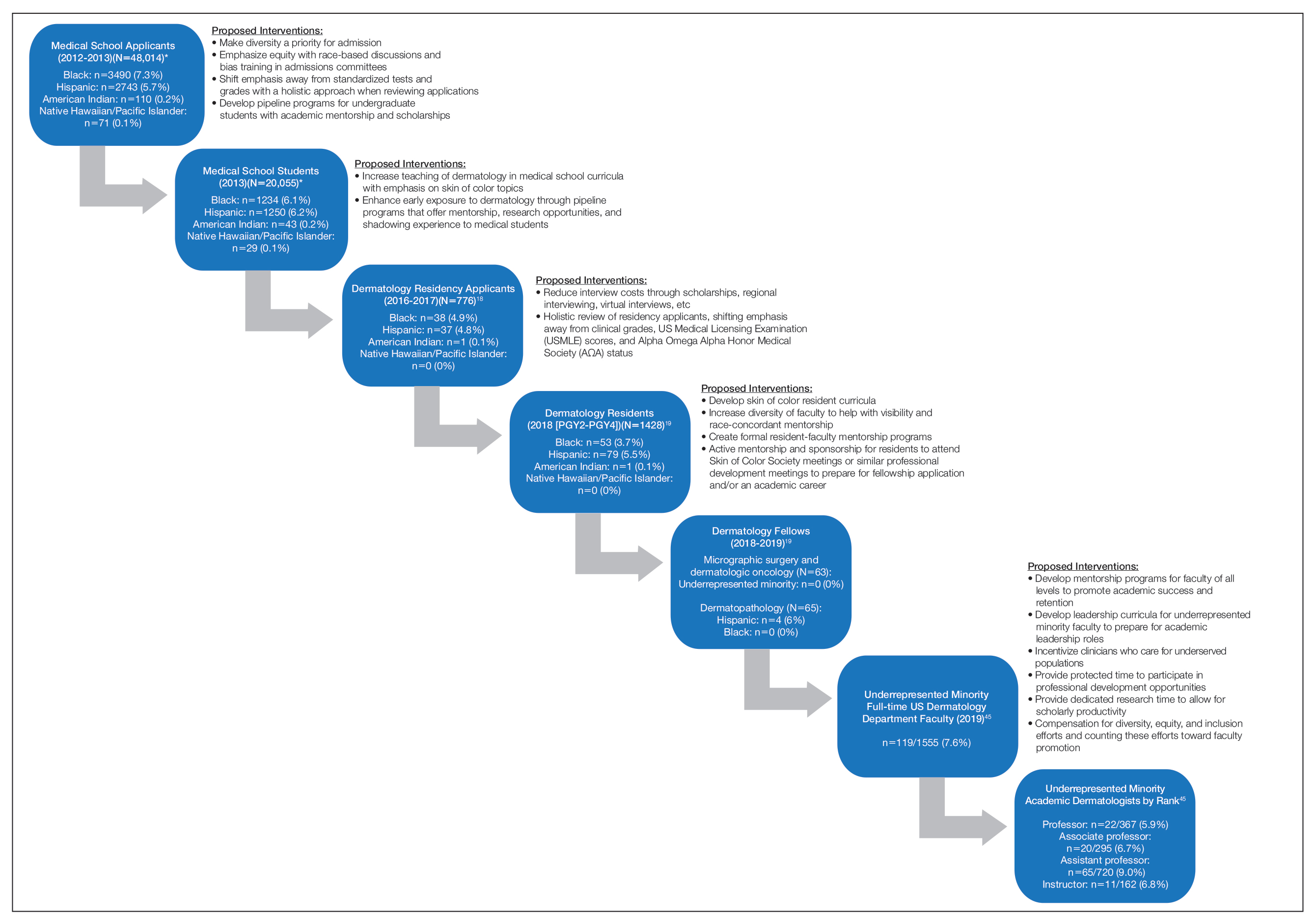
What’s in a White Coat? The Changing Trends in Physician Attire and What it Means for Dermatology
The White Coat Ceremony is an enduring memory from my medical school years. Amidst the tumult of memories of seemingly endless sleepless nights spent in libraries and cramming for clerkship examinations between surgical cases, I recall a sunny spring day in 2016 where I gathered with my classmates, family, and friends in the medical school campus courtyard. There were several short, mostly forgotten speeches after which proud fathers and mothers, partners, or siblings slipped the all-important white coat onto the shoulders of the physicians-to-be. At that moment, I felt the weight of tradition centuries in the making resting on my shoulders. Of course, the pomp of the ceremony might have felt a tad overblown had I known that the whole thing had fewer years under its belt than the movie Die Hard.
That’s right, the first White Coat Ceremony was held 5 years after the release of that Bruce Willis classic. Dr. Arnold Gold, a pediatric neurologist on faculty at Columbia University, conceived the ceremony in 1993, and it spread rapidly to medical schools—and later nursing schools—across the United States.1 Although the values highlighted by the White Coat Ceremony—humanism and compassion in medicine—are timeless, the ceremony itself is a more modern undertaking. What, then, of the white coat itself? Is it the timeless symbol of doctoring—of medicine—that we all presume it to be? Or is it a symbol of modern marketing, just a trend that caught on? And is it encountering its twilight—as trends often do—in the face of changing fashion and, more fundamentally, in changes to who our physicians are and to their roles in our society?
The Cleanliness of the White Coat
Until the end of the 19th century, physicians in the Western world most frequently dressed in black formal wear. The rationale behind this attire seems to have been twofold. First, society as a whole perceived the physician’s work as a serious and formal matter, and any medical encounter had to reflect the gravity of the occasion. Additionally, physicians’ visits often were a portent of impending demise, as physicians in the era prior to antibiotics and antisepsis frequently had little to offer their patients outside of—at best—anecdotal treatments and—at worst—sheer quackery.2 Black may have seemed a respectful choice for patients who likely faced dire outcomes regardless of the treatment afforded.3
With the turn of the century came a new understanding of the concepts of antisepsis and disease transmission. While Joseph Lister first published on the use of antisepsis in 1867, his practices did not become commonplace until the early 1900s.4 Around the same time came the Flexner report,5 the publication of William Osler’s Principles and Practice of Medicine,6 and the establishment of the modern medical residency, all of which contributed to the shift from the patient’s own bedside and to the hospital as the house of medicine, with cleanliness and antisepsis as part of its core principles.7 The white coat arose as a symbol of purity and freedom from disease. Throughout the 20th century and into the 21st, it has remained the predominant symbol of cleanliness and professionalism for the medical practitioner.
Patient Preference of Physician Attire
Although the white coat may serve as a professional symbol and is well respected medicine, it also plays an important role in the layperson’s perception of their health care providers.8 There is little denying that patients prefer their physicians, almost uniformly, to wear a white coat. A systematic review of physician attire that included 30 studies mainly from North America, Europe, and the United Kingdom found that patient preference for formal attire and white coats is near universal.9 Patients routinely rate physicians wearing a white coat as more intelligent and trustworthy and feel more confident in the care they will receive.10-13 They also freely admit that a physician’s appearance influences their satisfaction with their care.14 The recent adoption of the fleece, or softshell, jacket has not yet pervaded patients’ perceptions of what is considered appropriate physician attire. A 500-respondent survey found that patients were more likely to rate a model wearing a white coat as more professional and experienced compared to the same model wearing a fleece or softshell jacket or other formal attire sans white coat.15
Closer examination of the same data, however, reveals results reproduced with startling consistency across several studies, which suggest those of us adopting other attire need not dig those white coats out of the closet just yet. First, while many studies point to patient preference for white coats, this preference is uniformly strongest in older patients, beginning around age 40 years and becoming an entrenched preference in those older than 65 years.9,14,16-18 On the other hand, younger patient populations display little to no such preference, and some studies indicate that younger patients actually prefer scrubs over formal attire in specific settings such as surgical offices, procedural spaces, or the emergency department.12,14,19 This suggests that bias in favor of traditional physician garb may be more linked to age demographics and may continue to shift as the overall population ages. Additionally, although patients might profess a strong preference for physician attire in theory, it often does not translate into any impact on the patient’s perception of the physician following a clinic visit. The large systematic review on the topic noted that only 25% of studies that surveyed patients about a clinical visit following the encounter reported that physician attire influenced their satisfaction with that visit, suggesting that attire may be less likely to influence patients in the real-world context of receiving care.9 In fact, a prospective study of patient perception of medical staff and interactions found that staff style of dress not only had no bearing on the perception of staff or visit satisfaction but that patients often failed to even accurately recall physician attire when surveyed.20 Another survey study echoed these conclusions, finding that physician attire had no effect on the perception of a proposed treatment plan.21
What do we know about patient perception of physician attire in the dermatology setting specifically, where visits can be unique in their tendency to transition from medical to procedural in the span of a 15-minute encounter depending on the patient’s chief concern? A survey study of dermatology patients at the general, surgical, and wound care dermatology clinics of an academic medical center (Miami, Florida) found that professional attire with a white coat was strongly preferred across a litany of scenarios assessing many aspects of dermatologic care.21 Similarly, a study of patients visiting a single institution’s dermatology and pediatric dermatology clinics surveyed patients and parents regarding attire prior to an appointment and specifically asked if a white coat should be worn.13 Fifty-four percent of the adult patients (n=176) surveyed professed a preference for physicians in white coats, with a stronger preference for white coats reported by those 50 years and older (55%; n=113). Parents or guardians presenting to the pediatric dermatology clinic, on the other hand, favored less formal attire.13 A recent, real-world study performed at an outpatient dermatology clinic examined the influence of changing physician attire on a patient’s perceptions of care received during clinic encounters. They found no substantial difference in patient satisfaction scores before and following the adoption of a new clinic uniform that transitioned from formal attire to fitted scrubs.22
Racial and Gender Bias Affecting Attire Preference
With any study of preference, there is the underlying concern over respondent bias. Many of the studies discussed here have found secondarily that a patient’s implicit bias does not end at the clothes their physician is wearing. The survey study of dermatology patients from the academic medical center in Miami, Florida, found that patients preferred that Black physicians of either sex be garbed in professional attire at all times but generally were more accepting of White physicians in less formal attire.21 Adamson et al23 published a response to the study’s findings urging dermatologists to recognize that a physician’s race and gender influence patients’ perceptions in much the same way that physician attire seems to and encouraged the development of a more diverse dermatologic workforce to help combat this prejudice. The issue of bias is not limited to the specialty of dermatology; the recent survey study by Xun et al15 found that respondents consistently rated female models garbed in physician attire as less professional than male model counterparts. Additionally, female models wearing white coats were mistakenly identified as medical technicians, physician assistants, or nurses with substantially more frequency than males, despite being clothed in the traditional physician garb. Several other publications on the subject have uncovered implicit bias, though it is rarely, if ever, the principle focus of the study.10,24,25 As is unfortunately true in many professions, female physicians and physicians from ethnic minorities face barriers to being perceived as fully competent physicians.
Impact of the COVID-19 Pandemic
Finally, of course, there is the ever-present question of the effect of the pandemic. Although the exact role of the white coat as a fomite for infection—and especially for the spread of viral illness—remains controversial, the perception nonetheless has helped catalyze the movement to alternatives such as short-sleeved white coats, technical jackets, and more recently, fitted scrubs.26-29 As with much in this realm, facts seem less important than perceptions; Zahrina et al30 found that when patients were presented with information regarding the risk for microbial contamination associated with white coats, preference for physicians in professional garb plummeted from 72% to only 22%. To date no articles have examined patient perceptions of the white coat in the context of microbial transmission in the age of COVID-19, but future articles on this topic are likely and may serve to further the demise of the white coat.
Final Thoughts
From my vantage point, it seems the white coat will be claimed by the outgoing tide. During this most recent residency interview season, I do not recall a single medical student wearing a short white coat. The closest I came was a quick glimpse of a crumpled white jacket slung over an arm or stuffed in a shoulder bag. Rotating interns and residents from other services on rotation in our department present in softshell or fleece jackets. Fitted scrubs in the newest trendy colors speckle a previously all-white canvas. I, for one, have not donned my own white coat in at least a year, and perhaps it is all for the best. Physician attire is one small aspect of the practice of medicine and likely bears little, if any, relation to the wearer’s qualifications. Our focus should be on building rapport with our patients, providing high-quality care, reducing the risk for nosocomial infection, and developing a health care system that is fair and equitable for patients and health care workers alike, not on who is wearing what. Perhaps the introduction of new physician attire is a small part of the disruption we need to help address persistent gender and racial biases in our field and help shepherd our patients and colleagues to a worldview that is more open and accepting of physicians of diverse backgrounds.
- White Coat Ceremony. Gold Foundation website. Accessed December 26, 2021. https://www.gold-foundation.org/programs/white-coat-ceremony/
- Shryock RH. The Development of Modern Medicine. University of Pennsylvania Press; 2017.
- Hochberg MS. The doctor’s white coat—an historical perspective. Virtual Mentor. 2007;9:310-314.
- Lister J. On the antiseptic principle in the practice of surgery. Lancet. 1867;90:353-356.
- Flexner A. Medical Education in the United States and Canada: A Report to the Carnegie Foundation for the Advancement of Teaching. Carnegie Foundation for the Advancement of Teaching; 1910.
- Osler W. Principles and Practice of Medicine: Designed for the Use of Practitioners and Students of Medicine. D. Appleton & Company; 1892.
- Blumhagen DW. The doctor’s white coat: the image of the physician in modern America. Ann Intern Med. 1979;91:111-116.
- Verghese BG, Kashinath SK, Jadhav N, et al. Physician attire: physicians’ perspectives on attire in a community hospital setting among non-surgical specialties. J Community Hosp Intern Med Perspect. 2020;10:1-5.
- Petrilli CM, Mack M, Petrilli JJ, et al. Understanding the role of physician attire on patient perceptions: a systematic review of the literature—targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5:E006678.
- Rehman SU, Nietert PJ, Cope DW, et al. What to wear today? effect of doctor’s attire on the trust and confidence of patients. Am J Med. 2005;118:1279-1286.
- Jennings JD, Ciaravino SG, Ramsey FV, et al. Physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474:1908-1918.
- Gherardi G, Cameron J, West A, et al. Are we dressed to impress? a descriptive survey assessing patients preference of doctors’ attire in the hospital setting. Clin Med (Lond). 2009;9:519-524.
- Thomas MW, Burkhart CN, Lugo-Somolinos A, et al. Patients’ perceptions of physician attire in dermatology clinics. Arch Dermatol. 2011;147:505-506.
- Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8:E021239.
- Xun H, Chen J, Sun AH, et al. Public perceptions of physician attire and professionalism in the US. JAMA Network Open. 2021;4:E2117779.
- Kamata K, Kuriyama A, Chopra V, et al. Patient preferences for physician attire: a multicenter study in Japan [published online February 11, 2020]. J Hosp Med. 2020;15:204-210.
- Budny AM, Rogers LC, Mandracchia VJ, et al. The physician’s attire and its influence on patient confidence. J Am Podiatr Assoc. 2006;96:132-138.
- Lill MM, Wilkinson TJ. Judging a book by its cover: descriptive survey of patients’ preferences for doctors’ appearance and mode of address. Br Med J. 2005;331:1524-1527.
- Hossler EW, Shipp D, Palmer M, et al. Impact of provider attire on patient satisfaction in an outpatient dermatology clinic. Cutis. 2018;102:127-129.
- Boon D, Wardrope J. What should doctors wear in the accident and emergency department? patients’ perception. J Accid Emerg Med. 1994;11:175-177.
- Fox JD, Prado G, Baquerizo Nole KL, et al. Patient preference in dermatologist attire in the medical, surgical, and wound care settings. JAMA Dermatol. 2016;152:913-919.
- Bray JK, Porter C, Feldman SR. The effect of physician appearance on patient perceptions of treatment plans. Dermatol Online J. 2021;27. doi:10.5070/D327553611
- Adamson AS, Wright SW, Pandya AG. A missed opportunity to discuss racial and gender bias in dermatology. JAMA Dermatol. 2017;153:110-111.
- Hartmans C, Heremans S, Lagrain M, et al. The doctor’s new clothes: professional or fashionable? Primary Health Care. 2013;3:135.
- Kurihara H, Maeno T, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13:2.
- Treakle AM, Thom KA, Furuno JP, et al. Bacterial contamination of health care workers’ white coats. Am J Infect Control. 2009;37:101-105.
- Banu A, Anand M, Nagi N, et al. White coats as a vehicle for bacterial dissemination. J Clin Diagn Res. 2012;6:1381-1384.
- Haun N, Hooper-Lane C, Safdar N. Healthcare personnel attire and devices as fomites: a systematic review. Infect Control Hosp Epidemiol. 2016;37:1367-1373.
- Tse G, Withey S, Yeo JM, et al. Bare below the elbows: was the target the white coat? J Hosp Infect. 2015;91:299-301.
- Zahrina AZ, Haymond P, Rosanna P, et al. Does the attire of a primary care physician affect patients’ perceptions and their levels of trust in the doctor? Malays Fam Physician. 2018;13:3-11.
The White Coat Ceremony is an enduring memory from my medical school years. Amidst the tumult of memories of seemingly endless sleepless nights spent in libraries and cramming for clerkship examinations between surgical cases, I recall a sunny spring day in 2016 where I gathered with my classmates, family, and friends in the medical school campus courtyard. There were several short, mostly forgotten speeches after which proud fathers and mothers, partners, or siblings slipped the all-important white coat onto the shoulders of the physicians-to-be. At that moment, I felt the weight of tradition centuries in the making resting on my shoulders. Of course, the pomp of the ceremony might have felt a tad overblown had I known that the whole thing had fewer years under its belt than the movie Die Hard.
That’s right, the first White Coat Ceremony was held 5 years after the release of that Bruce Willis classic. Dr. Arnold Gold, a pediatric neurologist on faculty at Columbia University, conceived the ceremony in 1993, and it spread rapidly to medical schools—and later nursing schools—across the United States.1 Although the values highlighted by the White Coat Ceremony—humanism and compassion in medicine—are timeless, the ceremony itself is a more modern undertaking. What, then, of the white coat itself? Is it the timeless symbol of doctoring—of medicine—that we all presume it to be? Or is it a symbol of modern marketing, just a trend that caught on? And is it encountering its twilight—as trends often do—in the face of changing fashion and, more fundamentally, in changes to who our physicians are and to their roles in our society?
The Cleanliness of the White Coat
Until the end of the 19th century, physicians in the Western world most frequently dressed in black formal wear. The rationale behind this attire seems to have been twofold. First, society as a whole perceived the physician’s work as a serious and formal matter, and any medical encounter had to reflect the gravity of the occasion. Additionally, physicians’ visits often were a portent of impending demise, as physicians in the era prior to antibiotics and antisepsis frequently had little to offer their patients outside of—at best—anecdotal treatments and—at worst—sheer quackery.2 Black may have seemed a respectful choice for patients who likely faced dire outcomes regardless of the treatment afforded.3
With the turn of the century came a new understanding of the concepts of antisepsis and disease transmission. While Joseph Lister first published on the use of antisepsis in 1867, his practices did not become commonplace until the early 1900s.4 Around the same time came the Flexner report,5 the publication of William Osler’s Principles and Practice of Medicine,6 and the establishment of the modern medical residency, all of which contributed to the shift from the patient’s own bedside and to the hospital as the house of medicine, with cleanliness and antisepsis as part of its core principles.7 The white coat arose as a symbol of purity and freedom from disease. Throughout the 20th century and into the 21st, it has remained the predominant symbol of cleanliness and professionalism for the medical practitioner.
Patient Preference of Physician Attire
Although the white coat may serve as a professional symbol and is well respected medicine, it also plays an important role in the layperson’s perception of their health care providers.8 There is little denying that patients prefer their physicians, almost uniformly, to wear a white coat. A systematic review of physician attire that included 30 studies mainly from North America, Europe, and the United Kingdom found that patient preference for formal attire and white coats is near universal.9 Patients routinely rate physicians wearing a white coat as more intelligent and trustworthy and feel more confident in the care they will receive.10-13 They also freely admit that a physician’s appearance influences their satisfaction with their care.14 The recent adoption of the fleece, or softshell, jacket has not yet pervaded patients’ perceptions of what is considered appropriate physician attire. A 500-respondent survey found that patients were more likely to rate a model wearing a white coat as more professional and experienced compared to the same model wearing a fleece or softshell jacket or other formal attire sans white coat.15
Closer examination of the same data, however, reveals results reproduced with startling consistency across several studies, which suggest those of us adopting other attire need not dig those white coats out of the closet just yet. First, while many studies point to patient preference for white coats, this preference is uniformly strongest in older patients, beginning around age 40 years and becoming an entrenched preference in those older than 65 years.9,14,16-18 On the other hand, younger patient populations display little to no such preference, and some studies indicate that younger patients actually prefer scrubs over formal attire in specific settings such as surgical offices, procedural spaces, or the emergency department.12,14,19 This suggests that bias in favor of traditional physician garb may be more linked to age demographics and may continue to shift as the overall population ages. Additionally, although patients might profess a strong preference for physician attire in theory, it often does not translate into any impact on the patient’s perception of the physician following a clinic visit. The large systematic review on the topic noted that only 25% of studies that surveyed patients about a clinical visit following the encounter reported that physician attire influenced their satisfaction with that visit, suggesting that attire may be less likely to influence patients in the real-world context of receiving care.9 In fact, a prospective study of patient perception of medical staff and interactions found that staff style of dress not only had no bearing on the perception of staff or visit satisfaction but that patients often failed to even accurately recall physician attire when surveyed.20 Another survey study echoed these conclusions, finding that physician attire had no effect on the perception of a proposed treatment plan.21
What do we know about patient perception of physician attire in the dermatology setting specifically, where visits can be unique in their tendency to transition from medical to procedural in the span of a 15-minute encounter depending on the patient’s chief concern? A survey study of dermatology patients at the general, surgical, and wound care dermatology clinics of an academic medical center (Miami, Florida) found that professional attire with a white coat was strongly preferred across a litany of scenarios assessing many aspects of dermatologic care.21 Similarly, a study of patients visiting a single institution’s dermatology and pediatric dermatology clinics surveyed patients and parents regarding attire prior to an appointment and specifically asked if a white coat should be worn.13 Fifty-four percent of the adult patients (n=176) surveyed professed a preference for physicians in white coats, with a stronger preference for white coats reported by those 50 years and older (55%; n=113). Parents or guardians presenting to the pediatric dermatology clinic, on the other hand, favored less formal attire.13 A recent, real-world study performed at an outpatient dermatology clinic examined the influence of changing physician attire on a patient’s perceptions of care received during clinic encounters. They found no substantial difference in patient satisfaction scores before and following the adoption of a new clinic uniform that transitioned from formal attire to fitted scrubs.22
Racial and Gender Bias Affecting Attire Preference
With any study of preference, there is the underlying concern over respondent bias. Many of the studies discussed here have found secondarily that a patient’s implicit bias does not end at the clothes their physician is wearing. The survey study of dermatology patients from the academic medical center in Miami, Florida, found that patients preferred that Black physicians of either sex be garbed in professional attire at all times but generally were more accepting of White physicians in less formal attire.21 Adamson et al23 published a response to the study’s findings urging dermatologists to recognize that a physician’s race and gender influence patients’ perceptions in much the same way that physician attire seems to and encouraged the development of a more diverse dermatologic workforce to help combat this prejudice. The issue of bias is not limited to the specialty of dermatology; the recent survey study by Xun et al15 found that respondents consistently rated female models garbed in physician attire as less professional than male model counterparts. Additionally, female models wearing white coats were mistakenly identified as medical technicians, physician assistants, or nurses with substantially more frequency than males, despite being clothed in the traditional physician garb. Several other publications on the subject have uncovered implicit bias, though it is rarely, if ever, the principle focus of the study.10,24,25 As is unfortunately true in many professions, female physicians and physicians from ethnic minorities face barriers to being perceived as fully competent physicians.
Impact of the COVID-19 Pandemic
Finally, of course, there is the ever-present question of the effect of the pandemic. Although the exact role of the white coat as a fomite for infection—and especially for the spread of viral illness—remains controversial, the perception nonetheless has helped catalyze the movement to alternatives such as short-sleeved white coats, technical jackets, and more recently, fitted scrubs.26-29 As with much in this realm, facts seem less important than perceptions; Zahrina et al30 found that when patients were presented with information regarding the risk for microbial contamination associated with white coats, preference for physicians in professional garb plummeted from 72% to only 22%. To date no articles have examined patient perceptions of the white coat in the context of microbial transmission in the age of COVID-19, but future articles on this topic are likely and may serve to further the demise of the white coat.
Final Thoughts
From my vantage point, it seems the white coat will be claimed by the outgoing tide. During this most recent residency interview season, I do not recall a single medical student wearing a short white coat. The closest I came was a quick glimpse of a crumpled white jacket slung over an arm or stuffed in a shoulder bag. Rotating interns and residents from other services on rotation in our department present in softshell or fleece jackets. Fitted scrubs in the newest trendy colors speckle a previously all-white canvas. I, for one, have not donned my own white coat in at least a year, and perhaps it is all for the best. Physician attire is one small aspect of the practice of medicine and likely bears little, if any, relation to the wearer’s qualifications. Our focus should be on building rapport with our patients, providing high-quality care, reducing the risk for nosocomial infection, and developing a health care system that is fair and equitable for patients and health care workers alike, not on who is wearing what. Perhaps the introduction of new physician attire is a small part of the disruption we need to help address persistent gender and racial biases in our field and help shepherd our patients and colleagues to a worldview that is more open and accepting of physicians of diverse backgrounds.
The White Coat Ceremony is an enduring memory from my medical school years. Amidst the tumult of memories of seemingly endless sleepless nights spent in libraries and cramming for clerkship examinations between surgical cases, I recall a sunny spring day in 2016 where I gathered with my classmates, family, and friends in the medical school campus courtyard. There were several short, mostly forgotten speeches after which proud fathers and mothers, partners, or siblings slipped the all-important white coat onto the shoulders of the physicians-to-be. At that moment, I felt the weight of tradition centuries in the making resting on my shoulders. Of course, the pomp of the ceremony might have felt a tad overblown had I known that the whole thing had fewer years under its belt than the movie Die Hard.
That’s right, the first White Coat Ceremony was held 5 years after the release of that Bruce Willis classic. Dr. Arnold Gold, a pediatric neurologist on faculty at Columbia University, conceived the ceremony in 1993, and it spread rapidly to medical schools—and later nursing schools—across the United States.1 Although the values highlighted by the White Coat Ceremony—humanism and compassion in medicine—are timeless, the ceremony itself is a more modern undertaking. What, then, of the white coat itself? Is it the timeless symbol of doctoring—of medicine—that we all presume it to be? Or is it a symbol of modern marketing, just a trend that caught on? And is it encountering its twilight—as trends often do—in the face of changing fashion and, more fundamentally, in changes to who our physicians are and to their roles in our society?
The Cleanliness of the White Coat
Until the end of the 19th century, physicians in the Western world most frequently dressed in black formal wear. The rationale behind this attire seems to have been twofold. First, society as a whole perceived the physician’s work as a serious and formal matter, and any medical encounter had to reflect the gravity of the occasion. Additionally, physicians’ visits often were a portent of impending demise, as physicians in the era prior to antibiotics and antisepsis frequently had little to offer their patients outside of—at best—anecdotal treatments and—at worst—sheer quackery.2 Black may have seemed a respectful choice for patients who likely faced dire outcomes regardless of the treatment afforded.3
With the turn of the century came a new understanding of the concepts of antisepsis and disease transmission. While Joseph Lister first published on the use of antisepsis in 1867, his practices did not become commonplace until the early 1900s.4 Around the same time came the Flexner report,5 the publication of William Osler’s Principles and Practice of Medicine,6 and the establishment of the modern medical residency, all of which contributed to the shift from the patient’s own bedside and to the hospital as the house of medicine, with cleanliness and antisepsis as part of its core principles.7 The white coat arose as a symbol of purity and freedom from disease. Throughout the 20th century and into the 21st, it has remained the predominant symbol of cleanliness and professionalism for the medical practitioner.
Patient Preference of Physician Attire
Although the white coat may serve as a professional symbol and is well respected medicine, it also plays an important role in the layperson’s perception of their health care providers.8 There is little denying that patients prefer their physicians, almost uniformly, to wear a white coat. A systematic review of physician attire that included 30 studies mainly from North America, Europe, and the United Kingdom found that patient preference for formal attire and white coats is near universal.9 Patients routinely rate physicians wearing a white coat as more intelligent and trustworthy and feel more confident in the care they will receive.10-13 They also freely admit that a physician’s appearance influences their satisfaction with their care.14 The recent adoption of the fleece, or softshell, jacket has not yet pervaded patients’ perceptions of what is considered appropriate physician attire. A 500-respondent survey found that patients were more likely to rate a model wearing a white coat as more professional and experienced compared to the same model wearing a fleece or softshell jacket or other formal attire sans white coat.15
Closer examination of the same data, however, reveals results reproduced with startling consistency across several studies, which suggest those of us adopting other attire need not dig those white coats out of the closet just yet. First, while many studies point to patient preference for white coats, this preference is uniformly strongest in older patients, beginning around age 40 years and becoming an entrenched preference in those older than 65 years.9,14,16-18 On the other hand, younger patient populations display little to no such preference, and some studies indicate that younger patients actually prefer scrubs over formal attire in specific settings such as surgical offices, procedural spaces, or the emergency department.12,14,19 This suggests that bias in favor of traditional physician garb may be more linked to age demographics and may continue to shift as the overall population ages. Additionally, although patients might profess a strong preference for physician attire in theory, it often does not translate into any impact on the patient’s perception of the physician following a clinic visit. The large systematic review on the topic noted that only 25% of studies that surveyed patients about a clinical visit following the encounter reported that physician attire influenced their satisfaction with that visit, suggesting that attire may be less likely to influence patients in the real-world context of receiving care.9 In fact, a prospective study of patient perception of medical staff and interactions found that staff style of dress not only had no bearing on the perception of staff or visit satisfaction but that patients often failed to even accurately recall physician attire when surveyed.20 Another survey study echoed these conclusions, finding that physician attire had no effect on the perception of a proposed treatment plan.21
What do we know about patient perception of physician attire in the dermatology setting specifically, where visits can be unique in their tendency to transition from medical to procedural in the span of a 15-minute encounter depending on the patient’s chief concern? A survey study of dermatology patients at the general, surgical, and wound care dermatology clinics of an academic medical center (Miami, Florida) found that professional attire with a white coat was strongly preferred across a litany of scenarios assessing many aspects of dermatologic care.21 Similarly, a study of patients visiting a single institution’s dermatology and pediatric dermatology clinics surveyed patients and parents regarding attire prior to an appointment and specifically asked if a white coat should be worn.13 Fifty-four percent of the adult patients (n=176) surveyed professed a preference for physicians in white coats, with a stronger preference for white coats reported by those 50 years and older (55%; n=113). Parents or guardians presenting to the pediatric dermatology clinic, on the other hand, favored less formal attire.13 A recent, real-world study performed at an outpatient dermatology clinic examined the influence of changing physician attire on a patient’s perceptions of care received during clinic encounters. They found no substantial difference in patient satisfaction scores before and following the adoption of a new clinic uniform that transitioned from formal attire to fitted scrubs.22
Racial and Gender Bias Affecting Attire Preference
With any study of preference, there is the underlying concern over respondent bias. Many of the studies discussed here have found secondarily that a patient’s implicit bias does not end at the clothes their physician is wearing. The survey study of dermatology patients from the academic medical center in Miami, Florida, found that patients preferred that Black physicians of either sex be garbed in professional attire at all times but generally were more accepting of White physicians in less formal attire.21 Adamson et al23 published a response to the study’s findings urging dermatologists to recognize that a physician’s race and gender influence patients’ perceptions in much the same way that physician attire seems to and encouraged the development of a more diverse dermatologic workforce to help combat this prejudice. The issue of bias is not limited to the specialty of dermatology; the recent survey study by Xun et al15 found that respondents consistently rated female models garbed in physician attire as less professional than male model counterparts. Additionally, female models wearing white coats were mistakenly identified as medical technicians, physician assistants, or nurses with substantially more frequency than males, despite being clothed in the traditional physician garb. Several other publications on the subject have uncovered implicit bias, though it is rarely, if ever, the principle focus of the study.10,24,25 As is unfortunately true in many professions, female physicians and physicians from ethnic minorities face barriers to being perceived as fully competent physicians.
Impact of the COVID-19 Pandemic
Finally, of course, there is the ever-present question of the effect of the pandemic. Although the exact role of the white coat as a fomite for infection—and especially for the spread of viral illness—remains controversial, the perception nonetheless has helped catalyze the movement to alternatives such as short-sleeved white coats, technical jackets, and more recently, fitted scrubs.26-29 As with much in this realm, facts seem less important than perceptions; Zahrina et al30 found that when patients were presented with information regarding the risk for microbial contamination associated with white coats, preference for physicians in professional garb plummeted from 72% to only 22%. To date no articles have examined patient perceptions of the white coat in the context of microbial transmission in the age of COVID-19, but future articles on this topic are likely and may serve to further the demise of the white coat.
Final Thoughts
From my vantage point, it seems the white coat will be claimed by the outgoing tide. During this most recent residency interview season, I do not recall a single medical student wearing a short white coat. The closest I came was a quick glimpse of a crumpled white jacket slung over an arm or stuffed in a shoulder bag. Rotating interns and residents from other services on rotation in our department present in softshell or fleece jackets. Fitted scrubs in the newest trendy colors speckle a previously all-white canvas. I, for one, have not donned my own white coat in at least a year, and perhaps it is all for the best. Physician attire is one small aspect of the practice of medicine and likely bears little, if any, relation to the wearer’s qualifications. Our focus should be on building rapport with our patients, providing high-quality care, reducing the risk for nosocomial infection, and developing a health care system that is fair and equitable for patients and health care workers alike, not on who is wearing what. Perhaps the introduction of new physician attire is a small part of the disruption we need to help address persistent gender and racial biases in our field and help shepherd our patients and colleagues to a worldview that is more open and accepting of physicians of diverse backgrounds.
- White Coat Ceremony. Gold Foundation website. Accessed December 26, 2021. https://www.gold-foundation.org/programs/white-coat-ceremony/
- Shryock RH. The Development of Modern Medicine. University of Pennsylvania Press; 2017.
- Hochberg MS. The doctor’s white coat—an historical perspective. Virtual Mentor. 2007;9:310-314.
- Lister J. On the antiseptic principle in the practice of surgery. Lancet. 1867;90:353-356.
- Flexner A. Medical Education in the United States and Canada: A Report to the Carnegie Foundation for the Advancement of Teaching. Carnegie Foundation for the Advancement of Teaching; 1910.
- Osler W. Principles and Practice of Medicine: Designed for the Use of Practitioners and Students of Medicine. D. Appleton & Company; 1892.
- Blumhagen DW. The doctor’s white coat: the image of the physician in modern America. Ann Intern Med. 1979;91:111-116.
- Verghese BG, Kashinath SK, Jadhav N, et al. Physician attire: physicians’ perspectives on attire in a community hospital setting among non-surgical specialties. J Community Hosp Intern Med Perspect. 2020;10:1-5.
- Petrilli CM, Mack M, Petrilli JJ, et al. Understanding the role of physician attire on patient perceptions: a systematic review of the literature—targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5:E006678.
- Rehman SU, Nietert PJ, Cope DW, et al. What to wear today? effect of doctor’s attire on the trust and confidence of patients. Am J Med. 2005;118:1279-1286.
- Jennings JD, Ciaravino SG, Ramsey FV, et al. Physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474:1908-1918.
- Gherardi G, Cameron J, West A, et al. Are we dressed to impress? a descriptive survey assessing patients preference of doctors’ attire in the hospital setting. Clin Med (Lond). 2009;9:519-524.
- Thomas MW, Burkhart CN, Lugo-Somolinos A, et al. Patients’ perceptions of physician attire in dermatology clinics. Arch Dermatol. 2011;147:505-506.
- Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8:E021239.
- Xun H, Chen J, Sun AH, et al. Public perceptions of physician attire and professionalism in the US. JAMA Network Open. 2021;4:E2117779.
- Kamata K, Kuriyama A, Chopra V, et al. Patient preferences for physician attire: a multicenter study in Japan [published online February 11, 2020]. J Hosp Med. 2020;15:204-210.
- Budny AM, Rogers LC, Mandracchia VJ, et al. The physician’s attire and its influence on patient confidence. J Am Podiatr Assoc. 2006;96:132-138.
- Lill MM, Wilkinson TJ. Judging a book by its cover: descriptive survey of patients’ preferences for doctors’ appearance and mode of address. Br Med J. 2005;331:1524-1527.
- Hossler EW, Shipp D, Palmer M, et al. Impact of provider attire on patient satisfaction in an outpatient dermatology clinic. Cutis. 2018;102:127-129.
- Boon D, Wardrope J. What should doctors wear in the accident and emergency department? patients’ perception. J Accid Emerg Med. 1994;11:175-177.
- Fox JD, Prado G, Baquerizo Nole KL, et al. Patient preference in dermatologist attire in the medical, surgical, and wound care settings. JAMA Dermatol. 2016;152:913-919.
- Bray JK, Porter C, Feldman SR. The effect of physician appearance on patient perceptions of treatment plans. Dermatol Online J. 2021;27. doi:10.5070/D327553611
- Adamson AS, Wright SW, Pandya AG. A missed opportunity to discuss racial and gender bias in dermatology. JAMA Dermatol. 2017;153:110-111.
- Hartmans C, Heremans S, Lagrain M, et al. The doctor’s new clothes: professional or fashionable? Primary Health Care. 2013;3:135.
- Kurihara H, Maeno T, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13:2.
- Treakle AM, Thom KA, Furuno JP, et al. Bacterial contamination of health care workers’ white coats. Am J Infect Control. 2009;37:101-105.
- Banu A, Anand M, Nagi N, et al. White coats as a vehicle for bacterial dissemination. J Clin Diagn Res. 2012;6:1381-1384.
- Haun N, Hooper-Lane C, Safdar N. Healthcare personnel attire and devices as fomites: a systematic review. Infect Control Hosp Epidemiol. 2016;37:1367-1373.
- Tse G, Withey S, Yeo JM, et al. Bare below the elbows: was the target the white coat? J Hosp Infect. 2015;91:299-301.
- Zahrina AZ, Haymond P, Rosanna P, et al. Does the attire of a primary care physician affect patients’ perceptions and their levels of trust in the doctor? Malays Fam Physician. 2018;13:3-11.
- White Coat Ceremony. Gold Foundation website. Accessed December 26, 2021. https://www.gold-foundation.org/programs/white-coat-ceremony/
- Shryock RH. The Development of Modern Medicine. University of Pennsylvania Press; 2017.
- Hochberg MS. The doctor’s white coat—an historical perspective. Virtual Mentor. 2007;9:310-314.
- Lister J. On the antiseptic principle in the practice of surgery. Lancet. 1867;90:353-356.
- Flexner A. Medical Education in the United States and Canada: A Report to the Carnegie Foundation for the Advancement of Teaching. Carnegie Foundation for the Advancement of Teaching; 1910.
- Osler W. Principles and Practice of Medicine: Designed for the Use of Practitioners and Students of Medicine. D. Appleton & Company; 1892.
- Blumhagen DW. The doctor’s white coat: the image of the physician in modern America. Ann Intern Med. 1979;91:111-116.
- Verghese BG, Kashinath SK, Jadhav N, et al. Physician attire: physicians’ perspectives on attire in a community hospital setting among non-surgical specialties. J Community Hosp Intern Med Perspect. 2020;10:1-5.
- Petrilli CM, Mack M, Petrilli JJ, et al. Understanding the role of physician attire on patient perceptions: a systematic review of the literature—targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5:E006678.
- Rehman SU, Nietert PJ, Cope DW, et al. What to wear today? effect of doctor’s attire on the trust and confidence of patients. Am J Med. 2005;118:1279-1286.
- Jennings JD, Ciaravino SG, Ramsey FV, et al. Physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474:1908-1918.
- Gherardi G, Cameron J, West A, et al. Are we dressed to impress? a descriptive survey assessing patients preference of doctors’ attire in the hospital setting. Clin Med (Lond). 2009;9:519-524.
- Thomas MW, Burkhart CN, Lugo-Somolinos A, et al. Patients’ perceptions of physician attire in dermatology clinics. Arch Dermatol. 2011;147:505-506.
- Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8:E021239.
- Xun H, Chen J, Sun AH, et al. Public perceptions of physician attire and professionalism in the US. JAMA Network Open. 2021;4:E2117779.
- Kamata K, Kuriyama A, Chopra V, et al. Patient preferences for physician attire: a multicenter study in Japan [published online February 11, 2020]. J Hosp Med. 2020;15:204-210.
- Budny AM, Rogers LC, Mandracchia VJ, et al. The physician’s attire and its influence on patient confidence. J Am Podiatr Assoc. 2006;96:132-138.
- Lill MM, Wilkinson TJ. Judging a book by its cover: descriptive survey of patients’ preferences for doctors’ appearance and mode of address. Br Med J. 2005;331:1524-1527.
- Hossler EW, Shipp D, Palmer M, et al. Impact of provider attire on patient satisfaction in an outpatient dermatology clinic. Cutis. 2018;102:127-129.
- Boon D, Wardrope J. What should doctors wear in the accident and emergency department? patients’ perception. J Accid Emerg Med. 1994;11:175-177.
- Fox JD, Prado G, Baquerizo Nole KL, et al. Patient preference in dermatologist attire in the medical, surgical, and wound care settings. JAMA Dermatol. 2016;152:913-919.
- Bray JK, Porter C, Feldman SR. The effect of physician appearance on patient perceptions of treatment plans. Dermatol Online J. 2021;27. doi:10.5070/D327553611
- Adamson AS, Wright SW, Pandya AG. A missed opportunity to discuss racial and gender bias in dermatology. JAMA Dermatol. 2017;153:110-111.
- Hartmans C, Heremans S, Lagrain M, et al. The doctor’s new clothes: professional or fashionable? Primary Health Care. 2013;3:135.
- Kurihara H, Maeno T, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13:2.
- Treakle AM, Thom KA, Furuno JP, et al. Bacterial contamination of health care workers’ white coats. Am J Infect Control. 2009;37:101-105.
- Banu A, Anand M, Nagi N, et al. White coats as a vehicle for bacterial dissemination. J Clin Diagn Res. 2012;6:1381-1384.
- Haun N, Hooper-Lane C, Safdar N. Healthcare personnel attire and devices as fomites: a systematic review. Infect Control Hosp Epidemiol. 2016;37:1367-1373.
- Tse G, Withey S, Yeo JM, et al. Bare below the elbows: was the target the white coat? J Hosp Infect. 2015;91:299-301.
- Zahrina AZ, Haymond P, Rosanna P, et al. Does the attire of a primary care physician affect patients’ perceptions and their levels of trust in the doctor? Malays Fam Physician. 2018;13:3-11.
Resident Pearls
- Until the end of the 19th century, Western physicians most commonly wore black formal wear. The rise of the physician’s white coat occurred in conjunction with the shift to hospital medicine.
- Patient surveys repeatedly have demonstrated a preference for physicians to wear white coats; whether or not this has any bearing on patient satisfaction in real-world scenarios is less clear.
- The impact of the COVID-19 pandemic on trends in white coat wear has not yet been elucidated.
Blisters in a Comatose Elderly Woman
The Diagnosis: Coma Blisters
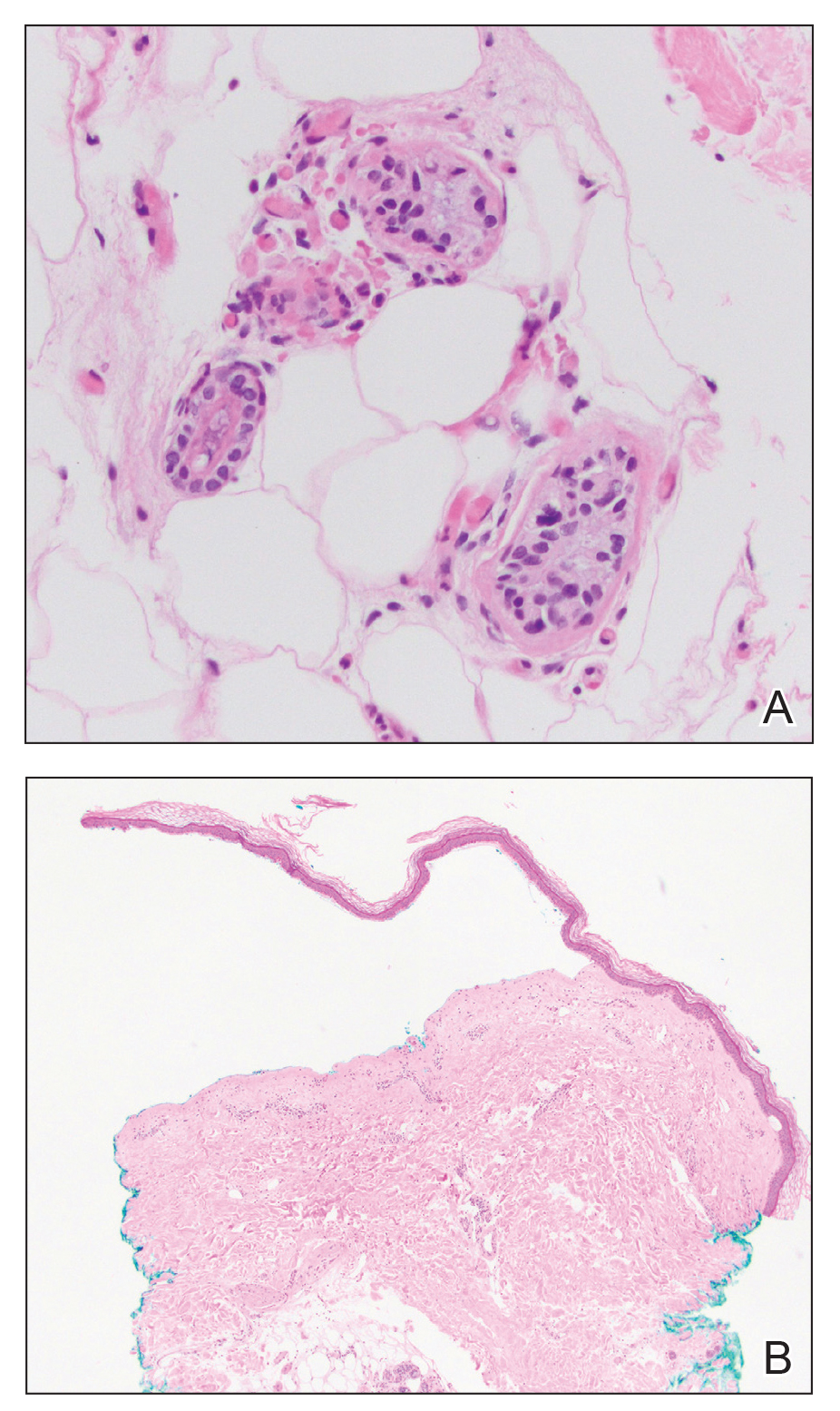
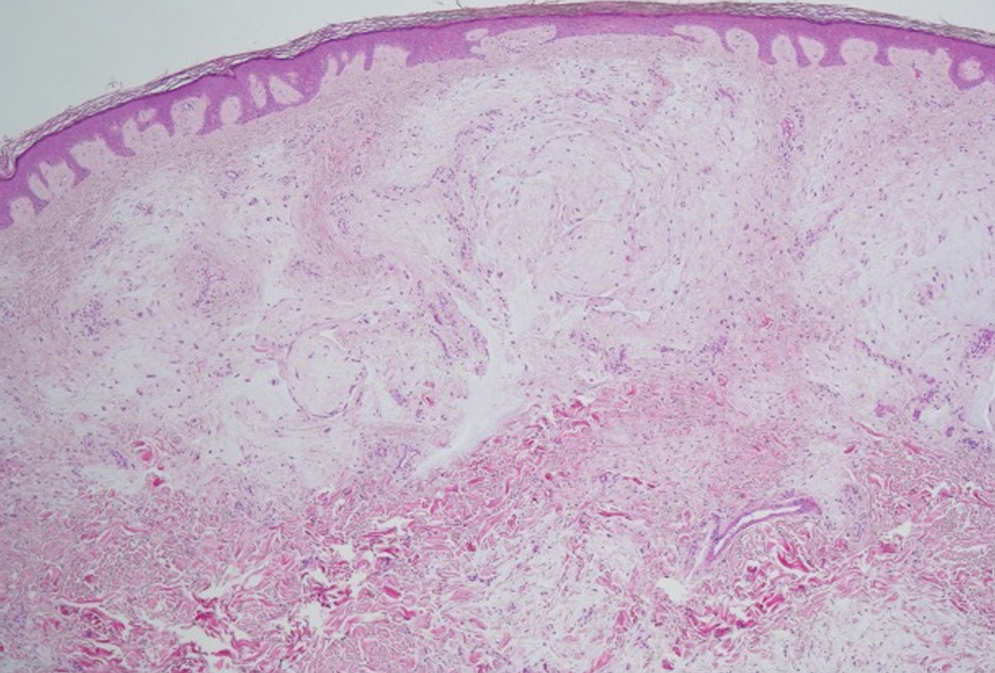
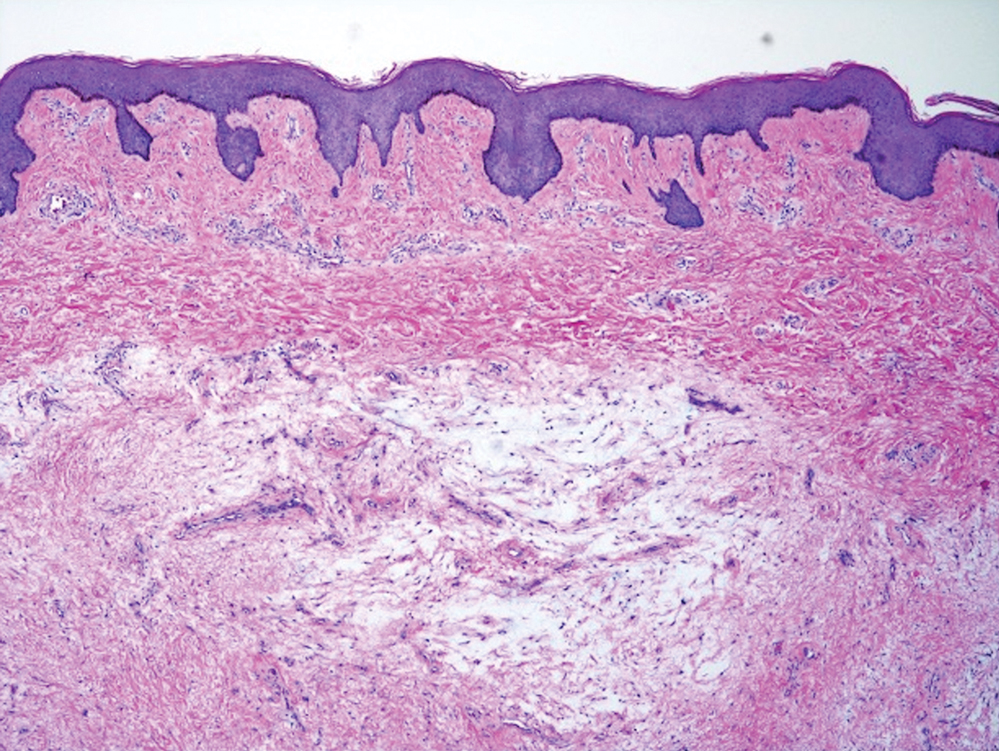
Histologic examination revealed pauci-inflammatory subepidermal blisters with swelling of eccrine cells, signaling impending gland necrosis (Figure). Direct immunofluorescence testing on perilesional skin was negative. These findings would be inconsistent for diagnoses of edema blisters (most commonly seen in patients with an acute exacerbation of chronic lower extremity edema), friction blisters (intraepidermal blisters seen on histopathology), and bullous pemphigoid (linear IgG and/or C3 staining along the basement membrane zone on direct immunofluorescence testing is characteristic). Although eccrine gland alterations have been seen in toxic epidermal necrolysis,1 the mucous membranes are involved in more than 90% of cases, making the diagnosis less likely. Furthermore, interface changes including prominent keratinocyte necrosis were not seen on histology.
Given the localized nature of the lesions in our patient and negative direct immunofluorescence studies, a diagnosis of coma blisters was made. Gentle wound care practices to the areas of denuded skin were implemented with complete resolution. The patient’s condition gradually improved, and she was extubated and discharged home.
Coma blisters are self-limited bullous lesions that have been reported in comatose patients as early as 1812 when Napoleon’s surgeon first noticed cutaneous blisters in comatose French soldiers being treated for carbon monoxide intoxication.2 Since then, barbiturate overdose has remained the most common association, but coma blisters have occurred in the absence of specific drug exposures. Clinically, erythematous or violaceous plaques typically appear within 24 hours of drug ingestion, and progression to large tense bullae usually occurs within 48 to 72 hours of unconsciousness.3 They characteristically occur in pressure-dependent areas, but reports have shown lesions in non–pressure-dependent areas, including the penis and mouth.1,4 Spontaneous resolution within 1 to 2 weeks is typical.5
The underlying pathogenesis remains controversial, as multiple mechanisms have been suggested, but clear causal evidence is lacking. The original proposition that direct effects of drug toxicity caused the cutaneous observations was later refuted after similar bullous lesions with eccrine gland necrosis were reported in comatose patients with neurologic conditions.6 It is largely accepted that pressure-induced local ischemia—proportional to the duration and amount of pressure—leads to tissue injury and is critical to the pathogenesis. During periods of ischemia, the most metabolically active tissues will undergo necrosis first; however, in eccrine glands, the earliest and most severe damage does not seem to occur in the most metabolically active cells.7 Additionally, this would not provide a viable explanation for coma blisters with eccrine gland necrosis developing in variable non–pressuredependent areas.
Moreover, drug- and non–drug-induced coma blisters can appear identically, but specific histopathologic differences have been reported. The most notable markers of non–drug-induced coma blisters are the absence of an inflammatory infiltrate in the epidermis and the presence of thrombosis in dermal vessels.8 Demonstration of necrotic changes in the secretory portion of the eccrine gland is considered the histopathologic hallmark for drug-induced coma blisters, but other findings can include subepidermal or intraepidermal bullae; perivascular infiltrates; and focal necrosis of the epidermis, dermis, subcutis, or epidermal appendages.6 Arteriolar wall necrosis and dermal inflammatory infiltrates also have been observed.7
Benzodiazepines have been widely prescribed and abused since their development, and overdose is much more common today than with barbiturates.9 Coma blisters rarely have been documented in the setting of isolated benzodiazepine overdose, and of the few cases, only one report implicated lorazepam as the causative agent.4,7 The characteristic finding of eccrine gland necrosis consistently was seen in our patient. This case not only emphasizes the need for greater awareness of the association between benzodiazepine overdose and coma blisters but also the importance of clinical context when considering diagnoses. It is essential to note that coma blisters themselves are nonspecific, and the diagnosis of drug-induced coma blisters warrants confirmatory toxicologic analysis.
- Ferreli C, Sulica VI, Aste N, et al. Drug-induced sweat gland necrosis in a non-comatose patient: a case presentation. J Eur Acad Dermatol Venereol. 2003;17:443-445.
- Larrey DJ. Memoires de Chirurgie Militaire et Campagnes. Smith and Buisson; 1812.
- Agarwal A, Bansal M, Conner K. Coma blisters with hypoxemic respiratory failure. Dermatol Online J. 2012;18:10.
- Varma AJ, Fisher BK, Sarin MK. Diazepam-induced coma with bullae and eccrine sweat gland necrosis. Arch Intern Med. 1977;137:1207-1210.
- Rocha J, Pereira T, Ventura F, et al. Coma blisters. Case Rep Dermatol. 2009;1:66-70.
- Arndt KA, Mihm MC, Parrish JA. Bullae: a cutaneous sign of a variety of neurologic diseases. J Invest Dermatol. 1973;60:312-320.
- Sánchez Yus E, Requena L, Simón P. Histopathology of cutaneous changes in drug-induced coma. Am J Dermatopathol. 1993;15:208-216.
- Kato N, Ueno H, Mimura M. Histopathology of cutaneous changes in non-drug-induced coma. Am J Dermatopathol. 1996;18:344-350.
- Kang M, Ghassemzadeh S. Benzodiazepine Toxicity. StatPearls Publishing; 2018.
The Diagnosis: Coma Blisters
Histologic examination revealed pauci-inflammatory subepidermal blisters with swelling of eccrine cells, signaling impending gland necrosis (Figure). Direct immunofluorescence testing on perilesional skin was negative. These findings would be inconsistent for diagnoses of edema blisters (most commonly seen in patients with an acute exacerbation of chronic lower extremity edema), friction blisters (intraepidermal blisters seen on histopathology), and bullous pemphigoid (linear IgG and/or C3 staining along the basement membrane zone on direct immunofluorescence testing is characteristic). Although eccrine gland alterations have been seen in toxic epidermal necrolysis,1 the mucous membranes are involved in more than 90% of cases, making the diagnosis less likely. Furthermore, interface changes including prominent keratinocyte necrosis were not seen on histology.
Given the localized nature of the lesions in our patient and negative direct immunofluorescence studies, a diagnosis of coma blisters was made. Gentle wound care practices to the areas of denuded skin were implemented with complete resolution. The patient’s condition gradually improved, and she was extubated and discharged home.
Coma blisters are self-limited bullous lesions that have been reported in comatose patients as early as 1812 when Napoleon’s surgeon first noticed cutaneous blisters in comatose French soldiers being treated for carbon monoxide intoxication.2 Since then, barbiturate overdose has remained the most common association, but coma blisters have occurred in the absence of specific drug exposures. Clinically, erythematous or violaceous plaques typically appear within 24 hours of drug ingestion, and progression to large tense bullae usually occurs within 48 to 72 hours of unconsciousness.3 They characteristically occur in pressure-dependent areas, but reports have shown lesions in non–pressure-dependent areas, including the penis and mouth.1,4 Spontaneous resolution within 1 to 2 weeks is typical.5
The underlying pathogenesis remains controversial, as multiple mechanisms have been suggested, but clear causal evidence is lacking. The original proposition that direct effects of drug toxicity caused the cutaneous observations was later refuted after similar bullous lesions with eccrine gland necrosis were reported in comatose patients with neurologic conditions.6 It is largely accepted that pressure-induced local ischemia—proportional to the duration and amount of pressure—leads to tissue injury and is critical to the pathogenesis. During periods of ischemia, the most metabolically active tissues will undergo necrosis first; however, in eccrine glands, the earliest and most severe damage does not seem to occur in the most metabolically active cells.7 Additionally, this would not provide a viable explanation for coma blisters with eccrine gland necrosis developing in variable non–pressuredependent areas.
Moreover, drug- and non–drug-induced coma blisters can appear identically, but specific histopathologic differences have been reported. The most notable markers of non–drug-induced coma blisters are the absence of an inflammatory infiltrate in the epidermis and the presence of thrombosis in dermal vessels.8 Demonstration of necrotic changes in the secretory portion of the eccrine gland is considered the histopathologic hallmark for drug-induced coma blisters, but other findings can include subepidermal or intraepidermal bullae; perivascular infiltrates; and focal necrosis of the epidermis, dermis, subcutis, or epidermal appendages.6 Arteriolar wall necrosis and dermal inflammatory infiltrates also have been observed.7
Benzodiazepines have been widely prescribed and abused since their development, and overdose is much more common today than with barbiturates.9 Coma blisters rarely have been documented in the setting of isolated benzodiazepine overdose, and of the few cases, only one report implicated lorazepam as the causative agent.4,7 The characteristic finding of eccrine gland necrosis consistently was seen in our patient. This case not only emphasizes the need for greater awareness of the association between benzodiazepine overdose and coma blisters but also the importance of clinical context when considering diagnoses. It is essential to note that coma blisters themselves are nonspecific, and the diagnosis of drug-induced coma blisters warrants confirmatory toxicologic analysis.
The Diagnosis: Coma Blisters
Histologic examination revealed pauci-inflammatory subepidermal blisters with swelling of eccrine cells, signaling impending gland necrosis (Figure). Direct immunofluorescence testing on perilesional skin was negative. These findings would be inconsistent for diagnoses of edema blisters (most commonly seen in patients with an acute exacerbation of chronic lower extremity edema), friction blisters (intraepidermal blisters seen on histopathology), and bullous pemphigoid (linear IgG and/or C3 staining along the basement membrane zone on direct immunofluorescence testing is characteristic). Although eccrine gland alterations have been seen in toxic epidermal necrolysis,1 the mucous membranes are involved in more than 90% of cases, making the diagnosis less likely. Furthermore, interface changes including prominent keratinocyte necrosis were not seen on histology.
Given the localized nature of the lesions in our patient and negative direct immunofluorescence studies, a diagnosis of coma blisters was made. Gentle wound care practices to the areas of denuded skin were implemented with complete resolution. The patient’s condition gradually improved, and she was extubated and discharged home.
Coma blisters are self-limited bullous lesions that have been reported in comatose patients as early as 1812 when Napoleon’s surgeon first noticed cutaneous blisters in comatose French soldiers being treated for carbon monoxide intoxication.2 Since then, barbiturate overdose has remained the most common association, but coma blisters have occurred in the absence of specific drug exposures. Clinically, erythematous or violaceous plaques typically appear within 24 hours of drug ingestion, and progression to large tense bullae usually occurs within 48 to 72 hours of unconsciousness.3 They characteristically occur in pressure-dependent areas, but reports have shown lesions in non–pressure-dependent areas, including the penis and mouth.1,4 Spontaneous resolution within 1 to 2 weeks is typical.5
The underlying pathogenesis remains controversial, as multiple mechanisms have been suggested, but clear causal evidence is lacking. The original proposition that direct effects of drug toxicity caused the cutaneous observations was later refuted after similar bullous lesions with eccrine gland necrosis were reported in comatose patients with neurologic conditions.6 It is largely accepted that pressure-induced local ischemia—proportional to the duration and amount of pressure—leads to tissue injury and is critical to the pathogenesis. During periods of ischemia, the most metabolically active tissues will undergo necrosis first; however, in eccrine glands, the earliest and most severe damage does not seem to occur in the most metabolically active cells.7 Additionally, this would not provide a viable explanation for coma blisters with eccrine gland necrosis developing in variable non–pressuredependent areas.
Moreover, drug- and non–drug-induced coma blisters can appear identically, but specific histopathologic differences have been reported. The most notable markers of non–drug-induced coma blisters are the absence of an inflammatory infiltrate in the epidermis and the presence of thrombosis in dermal vessels.8 Demonstration of necrotic changes in the secretory portion of the eccrine gland is considered the histopathologic hallmark for drug-induced coma blisters, but other findings can include subepidermal or intraepidermal bullae; perivascular infiltrates; and focal necrosis of the epidermis, dermis, subcutis, or epidermal appendages.6 Arteriolar wall necrosis and dermal inflammatory infiltrates also have been observed.7
Benzodiazepines have been widely prescribed and abused since their development, and overdose is much more common today than with barbiturates.9 Coma blisters rarely have been documented in the setting of isolated benzodiazepine overdose, and of the few cases, only one report implicated lorazepam as the causative agent.4,7 The characteristic finding of eccrine gland necrosis consistently was seen in our patient. This case not only emphasizes the need for greater awareness of the association between benzodiazepine overdose and coma blisters but also the importance of clinical context when considering diagnoses. It is essential to note that coma blisters themselves are nonspecific, and the diagnosis of drug-induced coma blisters warrants confirmatory toxicologic analysis.
- Ferreli C, Sulica VI, Aste N, et al. Drug-induced sweat gland necrosis in a non-comatose patient: a case presentation. J Eur Acad Dermatol Venereol. 2003;17:443-445.
- Larrey DJ. Memoires de Chirurgie Militaire et Campagnes. Smith and Buisson; 1812.
- Agarwal A, Bansal M, Conner K. Coma blisters with hypoxemic respiratory failure. Dermatol Online J. 2012;18:10.
- Varma AJ, Fisher BK, Sarin MK. Diazepam-induced coma with bullae and eccrine sweat gland necrosis. Arch Intern Med. 1977;137:1207-1210.
- Rocha J, Pereira T, Ventura F, et al. Coma blisters. Case Rep Dermatol. 2009;1:66-70.
- Arndt KA, Mihm MC, Parrish JA. Bullae: a cutaneous sign of a variety of neurologic diseases. J Invest Dermatol. 1973;60:312-320.
- Sánchez Yus E, Requena L, Simón P. Histopathology of cutaneous changes in drug-induced coma. Am J Dermatopathol. 1993;15:208-216.
- Kato N, Ueno H, Mimura M. Histopathology of cutaneous changes in non-drug-induced coma. Am J Dermatopathol. 1996;18:344-350.
- Kang M, Ghassemzadeh S. Benzodiazepine Toxicity. StatPearls Publishing; 2018.
- Ferreli C, Sulica VI, Aste N, et al. Drug-induced sweat gland necrosis in a non-comatose patient: a case presentation. J Eur Acad Dermatol Venereol. 2003;17:443-445.
- Larrey DJ. Memoires de Chirurgie Militaire et Campagnes. Smith and Buisson; 1812.
- Agarwal A, Bansal M, Conner K. Coma blisters with hypoxemic respiratory failure. Dermatol Online J. 2012;18:10.
- Varma AJ, Fisher BK, Sarin MK. Diazepam-induced coma with bullae and eccrine sweat gland necrosis. Arch Intern Med. 1977;137:1207-1210.
- Rocha J, Pereira T, Ventura F, et al. Coma blisters. Case Rep Dermatol. 2009;1:66-70.
- Arndt KA, Mihm MC, Parrish JA. Bullae: a cutaneous sign of a variety of neurologic diseases. J Invest Dermatol. 1973;60:312-320.
- Sánchez Yus E, Requena L, Simón P. Histopathology of cutaneous changes in drug-induced coma. Am J Dermatopathol. 1993;15:208-216.
- Kato N, Ueno H, Mimura M. Histopathology of cutaneous changes in non-drug-induced coma. Am J Dermatopathol. 1996;18:344-350.
- Kang M, Ghassemzadeh S. Benzodiazepine Toxicity. StatPearls Publishing; 2018.
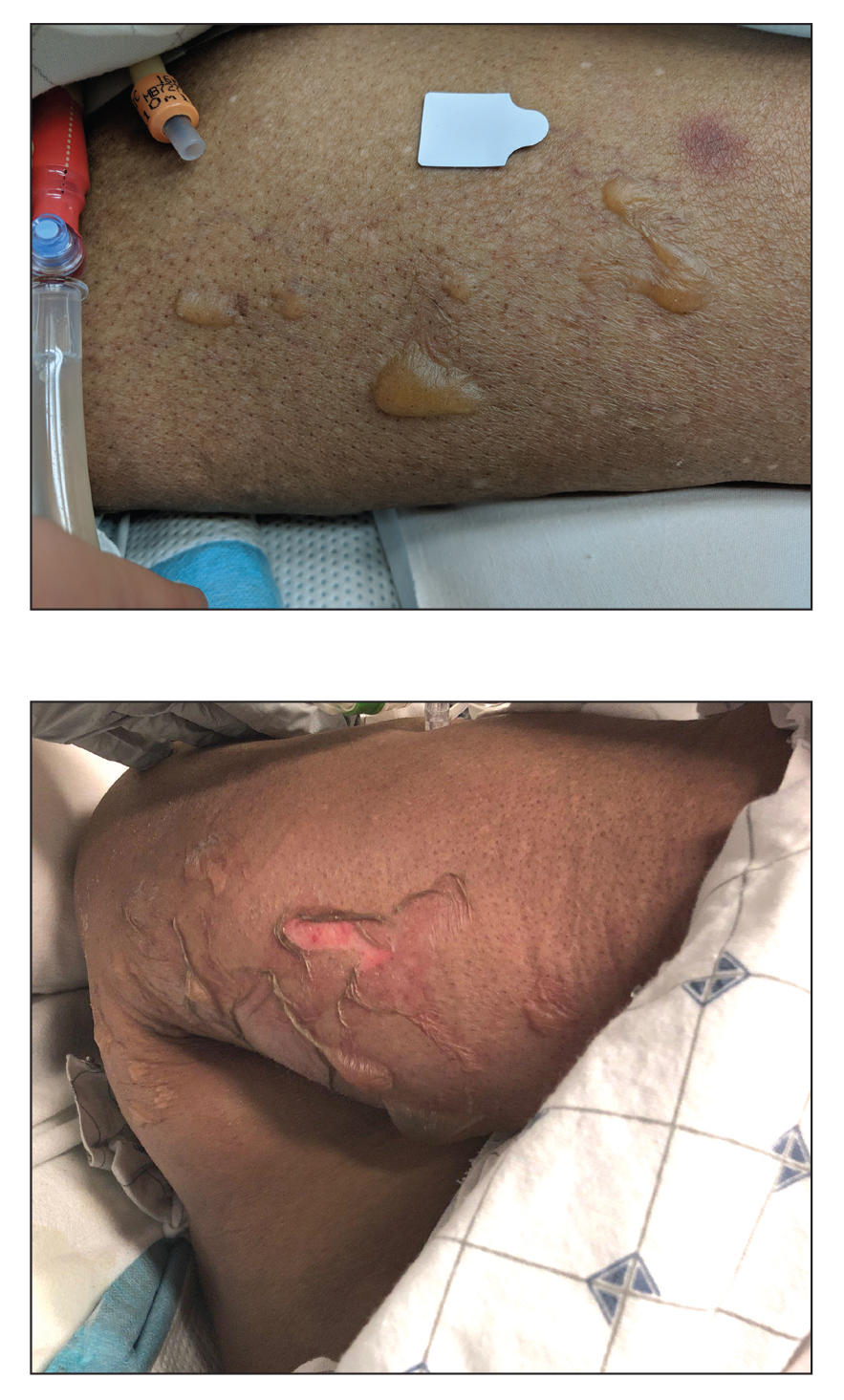
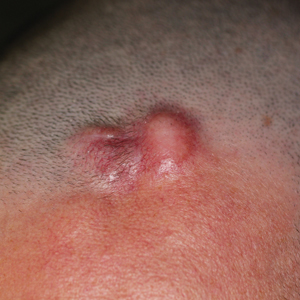
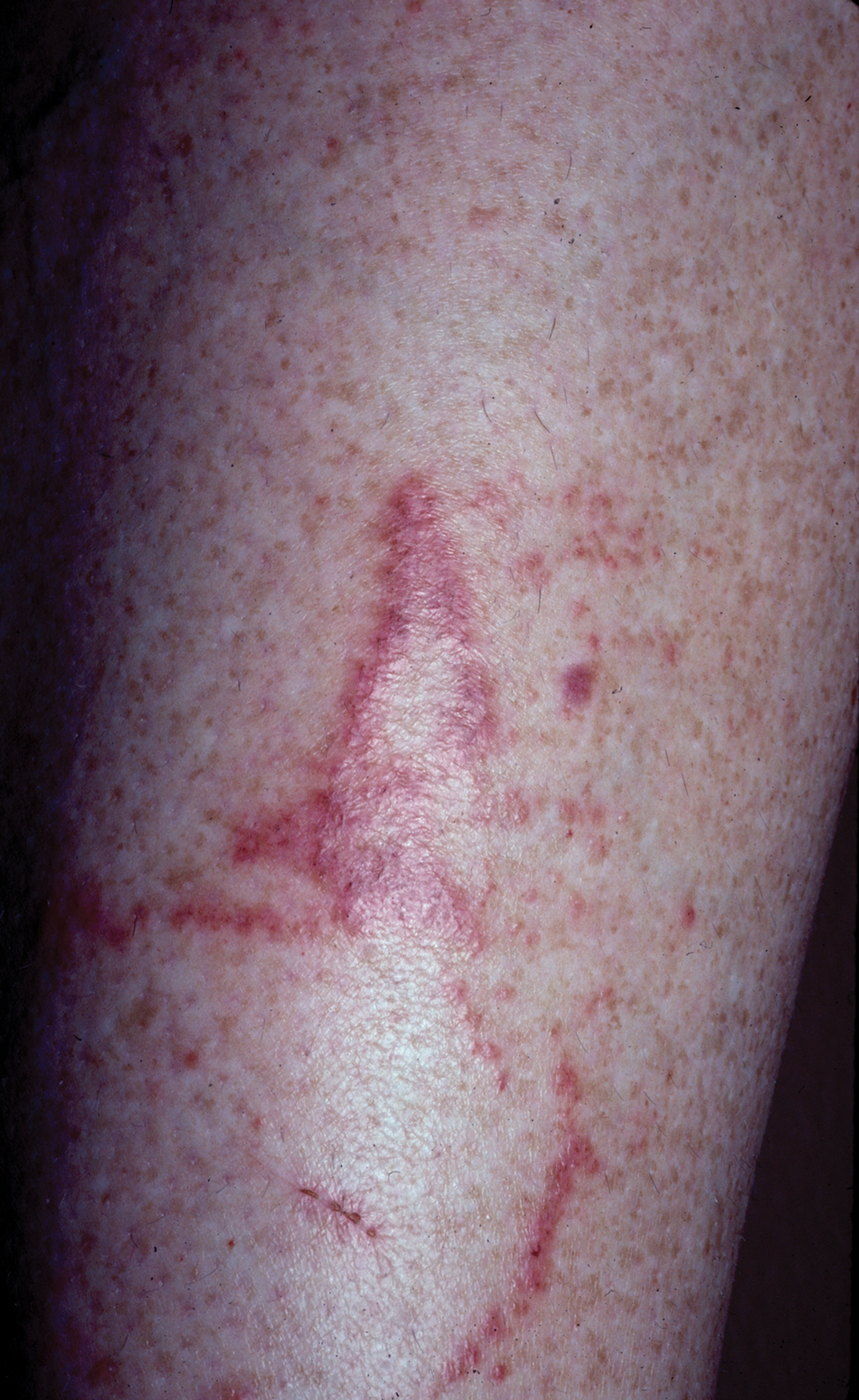
An 82-year-old woman presented to the emergency department after her daughter found her unconscious in the bathroom laying on her right side. Her medical history was notable for hypertension and asthma for which she was on losartan, furosemide, diltiazem, and albuterol. She recently had been prescribed lorazepam for insomnia and had started taking the medication 2 days prior. She underwent intubation and was noted to have flaccid, fluid-filled bullae on the right thigh (top) along with large areas of desquamation on the right lateral arm (bottom) with minimal surrounding erythema. There was no mucous membrane involvement. Urine toxicology was positive for benzodiazepines and negative for all other drugs, including barbiturates.
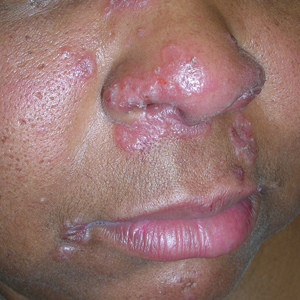
Enlarging Nodule on the Back
The Diagnosis: Cutaneous Myxoma
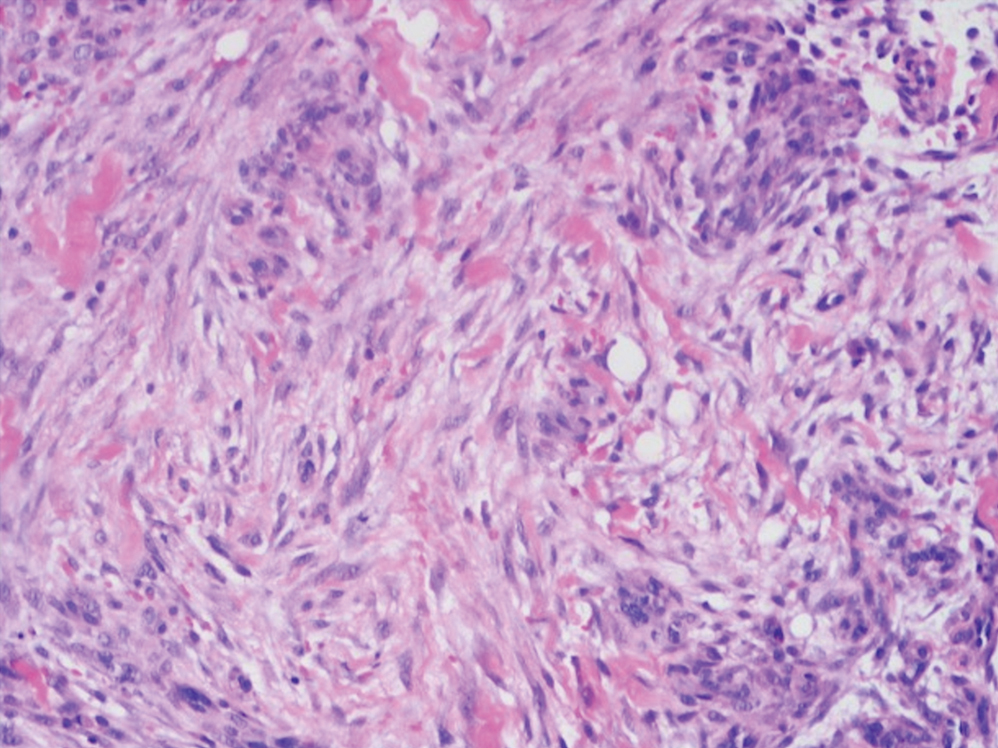
Microscopic analysis showed features of cutaneous myxoma (quiz images). The epidermis was essentially unremarkable. Stellate to spindle cells with bland nuclear chromatin were present in the dermis with abundant pools of myxoid stroma. Colloidal iron staining highlighted the markedly increased dermal mucin.
Cutaneous myxomas (also referred to as superficial angiomyxomas) are rare, well-demarcated tumors of the dermis and subcutis.1,2 They can present as solitary, fleshcolored nodules on the trunk, lower extremities, head, or neck, and they often measure between 1 and 5 cm.2,3 Histologically, cutaneous myxomas are hypocellular with some stellate fibroblasts, occasional epithelial structures, and an abundant myxoid stroma, with notable thinwalled small blood vessels.2,4 These lesions contain pools of mucin and are positive for mesenchymal mucin stains such as colloidal iron and Alcian blue.1 Moreover, perivascular neutrophils are a distinguishing characteristic of cutaneous myxomas.4
Multiple cutaneous myxomas should raise concern for Carney complex,1,5 a genodermatologic syndrome that arises due to a mutation in the protein kinase CAMP-dependent type I regulatory subunit alpha gene, PRKAR1A, on chromosome 2.1,5 Additional cutaneous manifestations include blue nevi, lentigines, and café-aulait macules.5 Carney complex also is known for endocrine overactivity and cardiac myxomas, which can cause serious embolic complications.1
Recommended management is complete excision with close follow-up, as these lesions may recur in up to one-third of cases. Although there is a potential for recurrence, metastases are uncommon.3 Even without recurrence in the presenting location, follow-up should include screening for manifestations of Carney complex.1,3
The clinical and histological differential for cutaneous myxoma may include nerve sheath myxoma or neurofibroma. A nerve sheath myxoma is a dermal tumor that manifests as a solitary, flesh-colored nodule, measuring less than 2 cm. These lesions commonly present on the head, neck, and upper body.6 Cutaneous myxomas can grow larger than 2 cm, but these two lesions have a great deal of overlap in their other features.3,6 Thus, histology can be used to distinguish them.
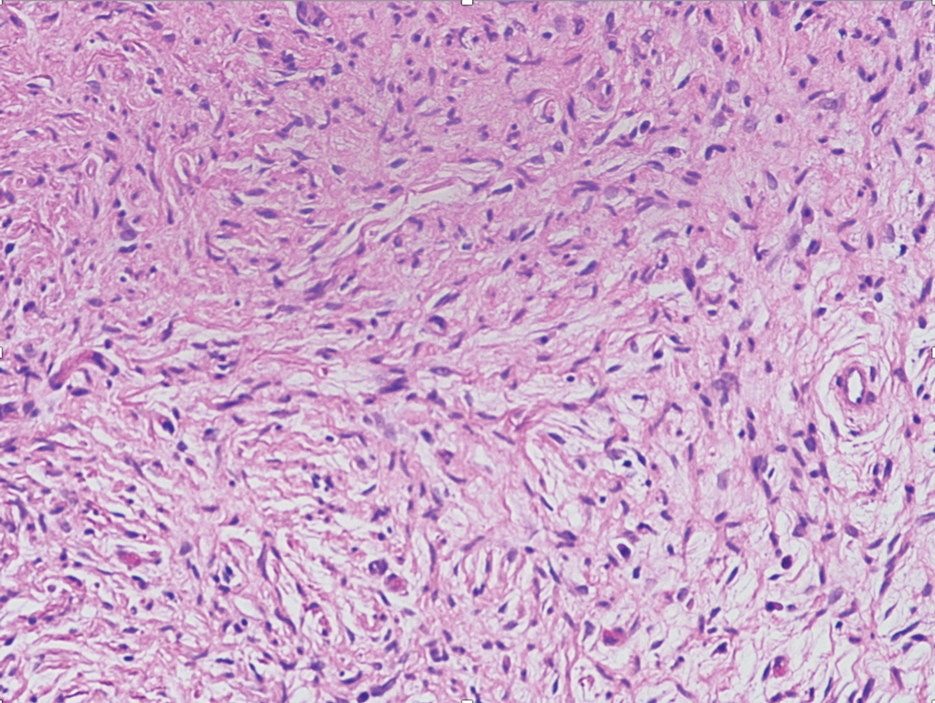
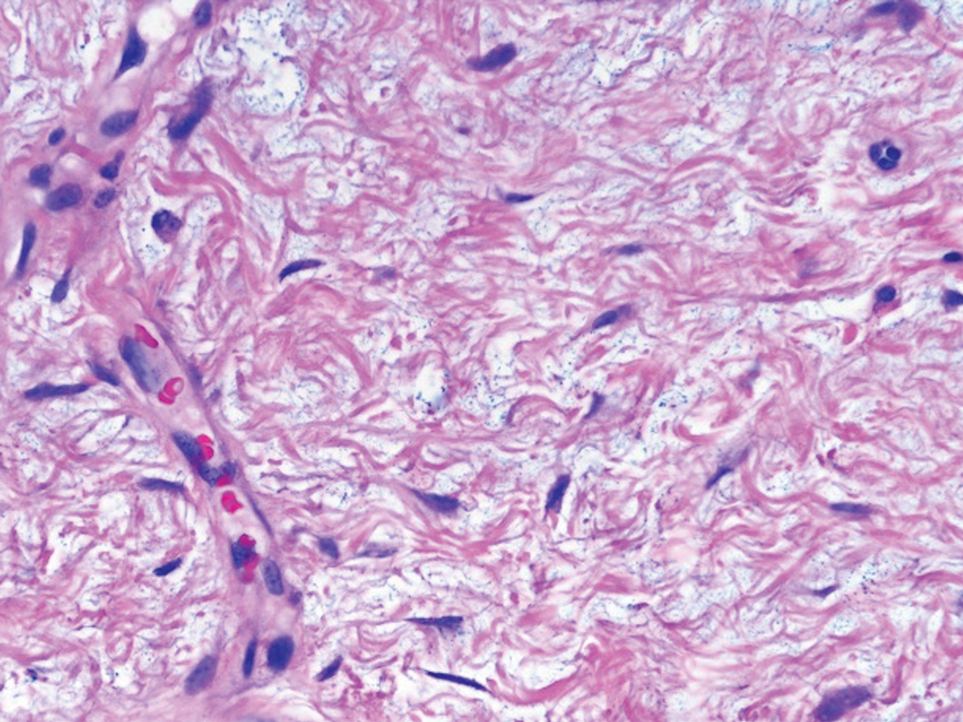
Nerve sheath myxomas are circumscribed nonencapsulated tumors of the dermis composed of multilobular aggregates of spindle to epithelioid cells in a mucinous matrix (Figure 1). Clefts often are present around the cell aggregates. Despite previously being termed myxoid neurothekeomas, nerve sheath myxomas are S-100 positive, whereas cellular neurothekeomas are S-100 negative and likely not of neural origin. Cutaneous myxomas, in contrast to nerve sheath myxomas, are S-100 negative. Nerve sheath myxomas are more cellular and lack the characteristic mucin pools compared with cutaneous myxomas.1,2,6 Neurofibromas frequently are flesh colored and pedunculated, as was the lesion in our patient, yet they are vastly different microscopically. The stroma of neurofibromas can vary, but cellularity typically is greater than a cutaneous myxoma and consists of increased numbers of bland spindle cells with wavy nuclei (Schwann cells) and fibrillar cytoplasm as well as mast cells and fibroblasts (Figure 2). Neurofibromas stain positively for S-100 and SOX-10 (Sry-related HMg-box 10).2,7 In addition to café-au-lait macules, axillary freckling, optic gliomas, and positive family history, neurofibromas are associated with neurofibromatosis type 1, which is linked to a defect in a tumor suppressor gene that codes for neurofibromin.7
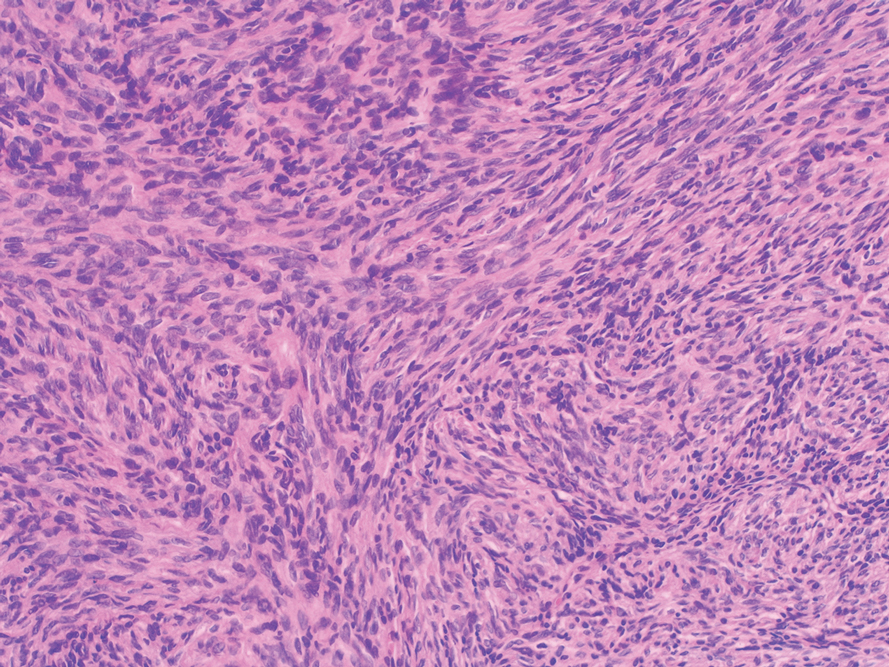
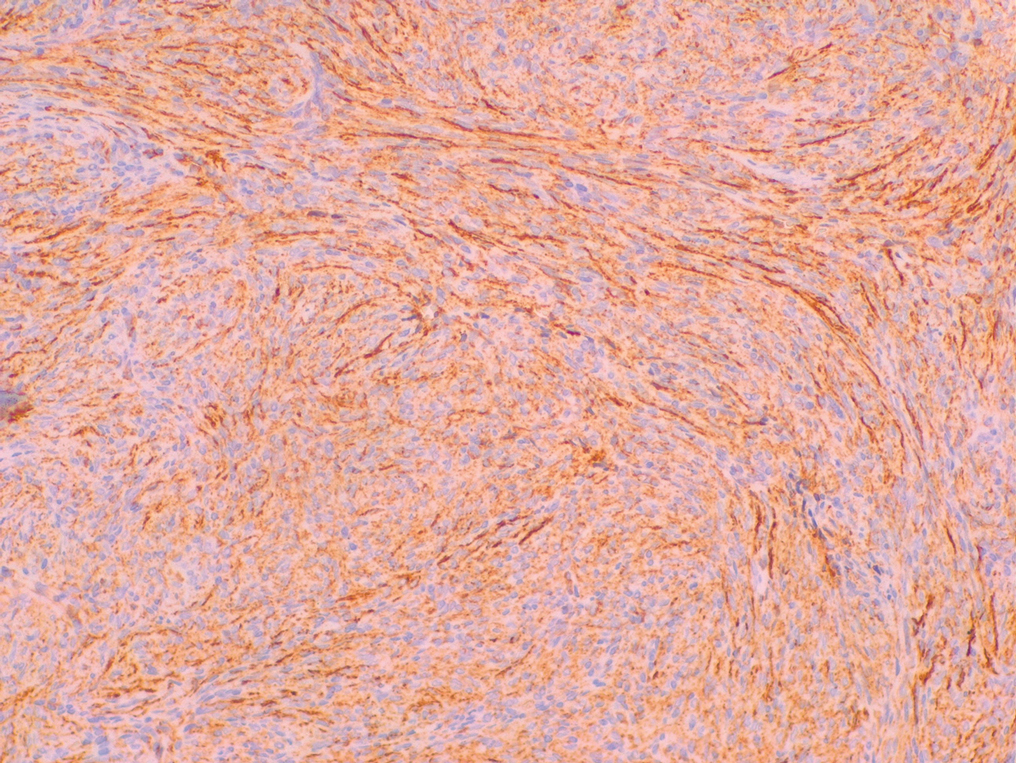
Nodular fasciitis is a self-limited myofibroblastic neoplasm that contains fusion genes, with the most common being myosin-9–ubiquitin specific peptidase 6, MYH9-USP6, which leads to overexpression of USP6. Nodular fasciitis presents as a solitary, rapidly enlarging nodule affecting the subcutaneous tissue, muscles, or fascia.8,9 It usually presents in the third or fourth decades of life.8 The arms are the most common location in adults, while the most commonly affected site in children is the head or neck. Histopathology reveals a characteristic tissue culture pattern with a proliferation of plump spindle and stellate fibroblasts as well as myofibroblasts (Figure 3). Early lesions have haphazard spindle cells with a proliferation of small blood vessels and extravasated erythrocytes. Despite increased mitotic figures, cellular atypia is rare. The fibroblasts and myofibroblasts react positively for vimentin and muscle-specific actin.8 This lesion is highly cellular comparatively and notably lacks the perivascular neutrophils and epithelial structures that would be expected in a cutaneous myxoma.4,8
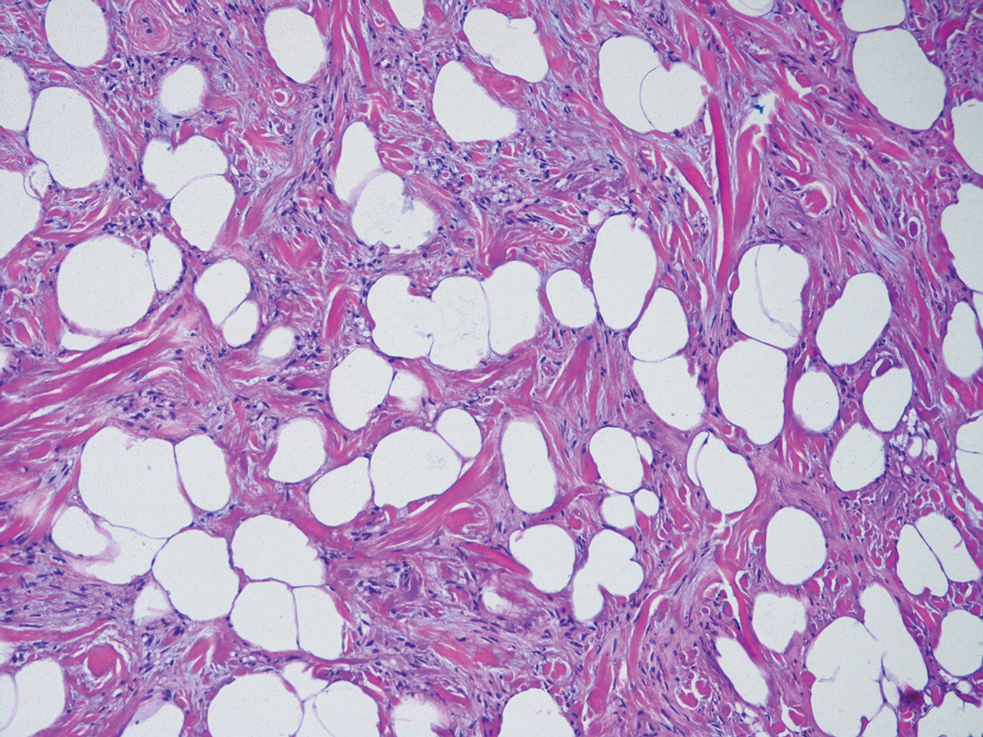
Spindle cell lipomas, solitary subcutaneous masses commonly presenting on the upper back in middle-aged men, also can mimic cutaneous myxomas.4 Histologically, these lesions may contain short bundles of spindle cells arranged in a school of fish–like pattern, mature adipocytes, or myxoid stroma and characteristic CD34 positivity (Figure 4). Spindle cell lipomas often will present with ropey collagen, which can easily distinguish them from cutaneous myxomas.4
- Lanjewar DN, Bhatia VO, Lanjewar SD, et al. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460-462.
- Choi HJ, Kim YJ, Yim JH, et al. Unusual presentation of solitary cutaneous myxoma. J Eur Acad Dermatol Venereol. 2007;21:403-404. doi:10.1111/j.1468-3083.2006.01881.x
- Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59:1-3. doi:10.4103/0019-5154.139893
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918.
- Sarfo A, Helm K, Flamm A. Cutaneous myxomas and a psammomatous melanotic schwannoma in a patient with Carney complex. J Cutan Pathol. 2019;46:93-96. doi:10.1111/cup.13385
- Gill P, Abi Daoud MS. Multiple cellular neurothekeomas in a middleaged woman including the lower extremity: a case report and review of the current literature. J Cutan Pathol. 2019;46:67-73. doi:10.1111/ cup.13366
- Ohgaki H, Kim Y, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr Opin Neurol. 2010;23:583-591. doi:10.1097/WCO.0b013e3283405b5f
- Luna A, Molinari L, Bollea Garlatti LA, et al. Nodular fasciitis, a forgotten entity. Int J Dermatol. 2019;58:190-193. doi:10.1111/ijd.14219
- Patel N, Chrisinger J, Demicco E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017; 30:1577-1588.
The Diagnosis: Cutaneous Myxoma
Microscopic analysis showed features of cutaneous myxoma (quiz images). The epidermis was essentially unremarkable. Stellate to spindle cells with bland nuclear chromatin were present in the dermis with abundant pools of myxoid stroma. Colloidal iron staining highlighted the markedly increased dermal mucin.
Cutaneous myxomas (also referred to as superficial angiomyxomas) are rare, well-demarcated tumors of the dermis and subcutis.1,2 They can present as solitary, fleshcolored nodules on the trunk, lower extremities, head, or neck, and they often measure between 1 and 5 cm.2,3 Histologically, cutaneous myxomas are hypocellular with some stellate fibroblasts, occasional epithelial structures, and an abundant myxoid stroma, with notable thinwalled small blood vessels.2,4 These lesions contain pools of mucin and are positive for mesenchymal mucin stains such as colloidal iron and Alcian blue.1 Moreover, perivascular neutrophils are a distinguishing characteristic of cutaneous myxomas.4
Multiple cutaneous myxomas should raise concern for Carney complex,1,5 a genodermatologic syndrome that arises due to a mutation in the protein kinase CAMP-dependent type I regulatory subunit alpha gene, PRKAR1A, on chromosome 2.1,5 Additional cutaneous manifestations include blue nevi, lentigines, and café-aulait macules.5 Carney complex also is known for endocrine overactivity and cardiac myxomas, which can cause serious embolic complications.1
Recommended management is complete excision with close follow-up, as these lesions may recur in up to one-third of cases. Although there is a potential for recurrence, metastases are uncommon.3 Even without recurrence in the presenting location, follow-up should include screening for manifestations of Carney complex.1,3
The clinical and histological differential for cutaneous myxoma may include nerve sheath myxoma or neurofibroma. A nerve sheath myxoma is a dermal tumor that manifests as a solitary, flesh-colored nodule, measuring less than 2 cm. These lesions commonly present on the head, neck, and upper body.6 Cutaneous myxomas can grow larger than 2 cm, but these two lesions have a great deal of overlap in their other features.3,6 Thus, histology can be used to distinguish them.
Nerve sheath myxomas are circumscribed nonencapsulated tumors of the dermis composed of multilobular aggregates of spindle to epithelioid cells in a mucinous matrix (Figure 1). Clefts often are present around the cell aggregates. Despite previously being termed myxoid neurothekeomas, nerve sheath myxomas are S-100 positive, whereas cellular neurothekeomas are S-100 negative and likely not of neural origin. Cutaneous myxomas, in contrast to nerve sheath myxomas, are S-100 negative. Nerve sheath myxomas are more cellular and lack the characteristic mucin pools compared with cutaneous myxomas.1,2,6 Neurofibromas frequently are flesh colored and pedunculated, as was the lesion in our patient, yet they are vastly different microscopically. The stroma of neurofibromas can vary, but cellularity typically is greater than a cutaneous myxoma and consists of increased numbers of bland spindle cells with wavy nuclei (Schwann cells) and fibrillar cytoplasm as well as mast cells and fibroblasts (Figure 2). Neurofibromas stain positively for S-100 and SOX-10 (Sry-related HMg-box 10).2,7 In addition to café-au-lait macules, axillary freckling, optic gliomas, and positive family history, neurofibromas are associated with neurofibromatosis type 1, which is linked to a defect in a tumor suppressor gene that codes for neurofibromin.7
Nodular fasciitis is a self-limited myofibroblastic neoplasm that contains fusion genes, with the most common being myosin-9–ubiquitin specific peptidase 6, MYH9-USP6, which leads to overexpression of USP6. Nodular fasciitis presents as a solitary, rapidly enlarging nodule affecting the subcutaneous tissue, muscles, or fascia.8,9 It usually presents in the third or fourth decades of life.8 The arms are the most common location in adults, while the most commonly affected site in children is the head or neck. Histopathology reveals a characteristic tissue culture pattern with a proliferation of plump spindle and stellate fibroblasts as well as myofibroblasts (Figure 3). Early lesions have haphazard spindle cells with a proliferation of small blood vessels and extravasated erythrocytes. Despite increased mitotic figures, cellular atypia is rare. The fibroblasts and myofibroblasts react positively for vimentin and muscle-specific actin.8 This lesion is highly cellular comparatively and notably lacks the perivascular neutrophils and epithelial structures that would be expected in a cutaneous myxoma.4,8
Spindle cell lipomas, solitary subcutaneous masses commonly presenting on the upper back in middle-aged men, also can mimic cutaneous myxomas.4 Histologically, these lesions may contain short bundles of spindle cells arranged in a school of fish–like pattern, mature adipocytes, or myxoid stroma and characteristic CD34 positivity (Figure 4). Spindle cell lipomas often will present with ropey collagen, which can easily distinguish them from cutaneous myxomas.4
The Diagnosis: Cutaneous Myxoma
Microscopic analysis showed features of cutaneous myxoma (quiz images). The epidermis was essentially unremarkable. Stellate to spindle cells with bland nuclear chromatin were present in the dermis with abundant pools of myxoid stroma. Colloidal iron staining highlighted the markedly increased dermal mucin.
Cutaneous myxomas (also referred to as superficial angiomyxomas) are rare, well-demarcated tumors of the dermis and subcutis.1,2 They can present as solitary, fleshcolored nodules on the trunk, lower extremities, head, or neck, and they often measure between 1 and 5 cm.2,3 Histologically, cutaneous myxomas are hypocellular with some stellate fibroblasts, occasional epithelial structures, and an abundant myxoid stroma, with notable thinwalled small blood vessels.2,4 These lesions contain pools of mucin and are positive for mesenchymal mucin stains such as colloidal iron and Alcian blue.1 Moreover, perivascular neutrophils are a distinguishing characteristic of cutaneous myxomas.4
Multiple cutaneous myxomas should raise concern for Carney complex,1,5 a genodermatologic syndrome that arises due to a mutation in the protein kinase CAMP-dependent type I regulatory subunit alpha gene, PRKAR1A, on chromosome 2.1,5 Additional cutaneous manifestations include blue nevi, lentigines, and café-aulait macules.5 Carney complex also is known for endocrine overactivity and cardiac myxomas, which can cause serious embolic complications.1
Recommended management is complete excision with close follow-up, as these lesions may recur in up to one-third of cases. Although there is a potential for recurrence, metastases are uncommon.3 Even without recurrence in the presenting location, follow-up should include screening for manifestations of Carney complex.1,3
The clinical and histological differential for cutaneous myxoma may include nerve sheath myxoma or neurofibroma. A nerve sheath myxoma is a dermal tumor that manifests as a solitary, flesh-colored nodule, measuring less than 2 cm. These lesions commonly present on the head, neck, and upper body.6 Cutaneous myxomas can grow larger than 2 cm, but these two lesions have a great deal of overlap in their other features.3,6 Thus, histology can be used to distinguish them.
Nerve sheath myxomas are circumscribed nonencapsulated tumors of the dermis composed of multilobular aggregates of spindle to epithelioid cells in a mucinous matrix (Figure 1). Clefts often are present around the cell aggregates. Despite previously being termed myxoid neurothekeomas, nerve sheath myxomas are S-100 positive, whereas cellular neurothekeomas are S-100 negative and likely not of neural origin. Cutaneous myxomas, in contrast to nerve sheath myxomas, are S-100 negative. Nerve sheath myxomas are more cellular and lack the characteristic mucin pools compared with cutaneous myxomas.1,2,6 Neurofibromas frequently are flesh colored and pedunculated, as was the lesion in our patient, yet they are vastly different microscopically. The stroma of neurofibromas can vary, but cellularity typically is greater than a cutaneous myxoma and consists of increased numbers of bland spindle cells with wavy nuclei (Schwann cells) and fibrillar cytoplasm as well as mast cells and fibroblasts (Figure 2). Neurofibromas stain positively for S-100 and SOX-10 (Sry-related HMg-box 10).2,7 In addition to café-au-lait macules, axillary freckling, optic gliomas, and positive family history, neurofibromas are associated with neurofibromatosis type 1, which is linked to a defect in a tumor suppressor gene that codes for neurofibromin.7
Nodular fasciitis is a self-limited myofibroblastic neoplasm that contains fusion genes, with the most common being myosin-9–ubiquitin specific peptidase 6, MYH9-USP6, which leads to overexpression of USP6. Nodular fasciitis presents as a solitary, rapidly enlarging nodule affecting the subcutaneous tissue, muscles, or fascia.8,9 It usually presents in the third or fourth decades of life.8 The arms are the most common location in adults, while the most commonly affected site in children is the head or neck. Histopathology reveals a characteristic tissue culture pattern with a proliferation of plump spindle and stellate fibroblasts as well as myofibroblasts (Figure 3). Early lesions have haphazard spindle cells with a proliferation of small blood vessels and extravasated erythrocytes. Despite increased mitotic figures, cellular atypia is rare. The fibroblasts and myofibroblasts react positively for vimentin and muscle-specific actin.8 This lesion is highly cellular comparatively and notably lacks the perivascular neutrophils and epithelial structures that would be expected in a cutaneous myxoma.4,8
Spindle cell lipomas, solitary subcutaneous masses commonly presenting on the upper back in middle-aged men, also can mimic cutaneous myxomas.4 Histologically, these lesions may contain short bundles of spindle cells arranged in a school of fish–like pattern, mature adipocytes, or myxoid stroma and characteristic CD34 positivity (Figure 4). Spindle cell lipomas often will present with ropey collagen, which can easily distinguish them from cutaneous myxomas.4
- Lanjewar DN, Bhatia VO, Lanjewar SD, et al. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460-462.
- Choi HJ, Kim YJ, Yim JH, et al. Unusual presentation of solitary cutaneous myxoma. J Eur Acad Dermatol Venereol. 2007;21:403-404. doi:10.1111/j.1468-3083.2006.01881.x
- Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59:1-3. doi:10.4103/0019-5154.139893
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918.
- Sarfo A, Helm K, Flamm A. Cutaneous myxomas and a psammomatous melanotic schwannoma in a patient with Carney complex. J Cutan Pathol. 2019;46:93-96. doi:10.1111/cup.13385
- Gill P, Abi Daoud MS. Multiple cellular neurothekeomas in a middleaged woman including the lower extremity: a case report and review of the current literature. J Cutan Pathol. 2019;46:67-73. doi:10.1111/ cup.13366
- Ohgaki H, Kim Y, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr Opin Neurol. 2010;23:583-591. doi:10.1097/WCO.0b013e3283405b5f
- Luna A, Molinari L, Bollea Garlatti LA, et al. Nodular fasciitis, a forgotten entity. Int J Dermatol. 2019;58:190-193. doi:10.1111/ijd.14219
- Patel N, Chrisinger J, Demicco E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017; 30:1577-1588.
- Lanjewar DN, Bhatia VO, Lanjewar SD, et al. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460-462.
- Choi HJ, Kim YJ, Yim JH, et al. Unusual presentation of solitary cutaneous myxoma. J Eur Acad Dermatol Venereol. 2007;21:403-404. doi:10.1111/j.1468-3083.2006.01881.x
- Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59:1-3. doi:10.4103/0019-5154.139893
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918.
- Sarfo A, Helm K, Flamm A. Cutaneous myxomas and a psammomatous melanotic schwannoma in a patient with Carney complex. J Cutan Pathol. 2019;46:93-96. doi:10.1111/cup.13385
- Gill P, Abi Daoud MS. Multiple cellular neurothekeomas in a middleaged woman including the lower extremity: a case report and review of the current literature. J Cutan Pathol. 2019;46:67-73. doi:10.1111/ cup.13366
- Ohgaki H, Kim Y, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr Opin Neurol. 2010;23:583-591. doi:10.1097/WCO.0b013e3283405b5f
- Luna A, Molinari L, Bollea Garlatti LA, et al. Nodular fasciitis, a forgotten entity. Int J Dermatol. 2019;58:190-193. doi:10.1111/ijd.14219
- Patel N, Chrisinger J, Demicco E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017; 30:1577-1588.
A 43-year-old man with an unremarkable medical history presented to our clinic with an enlarging painful nodule on the upper back that was present for years without bleeding or ulceration. He denied prior treatment or any similar lesions. Physical examination was notable for a 2×1.5-cm, pedunculated, flesh-colored nodule on the left upper back. A shave excision of the lesion was performed.
Erythematous Indurated Nodule on the Forehead
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.
Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1
A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.
Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1
A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.
Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1
A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
A 39-year-old man presented with an enlarging asymptomatic nodule on the forehead of more than 3 years’ duration. Physical examination revealed a 3.4×2.3-cm, indurated, firm, erythematous nodule on the frontotemporal scalp. The patient denied any history of trauma to the area.
Sarcoidosis
THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous. In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjusting for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
- Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
- Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66:121.e1-121.e14.
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/oxfordjournals.aje.a009096
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis if the features in 170 patients. Respir Med. 2003;97:978-982.
- Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
- Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
- Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
- Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
- James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
- Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
- Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
- Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous. In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjusting for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous. In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjusting for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
- Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
- Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66:121.e1-121.e14.
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/oxfordjournals.aje.a009096
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis if the features in 170 patients. Respir Med. 2003;97:978-982.
- Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
- Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
- Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
- Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
- James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
- Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
- Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
- Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
- Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
- Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66:121.e1-121.e14.
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/oxfordjournals.aje.a009096
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis if the features in 170 patients. Respir Med. 2003;97:978-982.
- Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
- Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
- Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
- Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
- James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
- Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
- Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
- Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
Contact Allergy to Topical Medicaments, Part 2: Steroids, Immunomodulators, and Anesthetics, Oh My!
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5
Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.
Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5
Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.
Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5
Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.
Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
Practice Points
- Allergic contact dermatitis (ACD) should be suspected in patients with persistent or worsening dermatitis after use of topical medications.
- Cross-reactions commonly occur between structurally similar compounds and occasionally between molecules from different drug classes.
- Some cases of topical medicament ACD remain elusive after patch testing, particularly drugs with potent immunomodulating effects.
Buccal Fat Pad Reduction With Intraoperative Fat Transfer to the Temple
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.
Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.
Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.
Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
Practice Points
- Buccal fat pad reduction is an increasingly popular procedure for facial shaping.
- Buccal fat pad reduction in addition to natural aging can result in volume depletion of the temporal fossae.
- Removed buccal fat can be transferred to the temples for increased volume.
Aquatic Antagonists: Jellyfish Stings
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8
The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10
Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6
Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8
The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10
Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6
Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8
The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10
Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6
Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
Practice Points
- Jellyfish stings occur an estimated 150 million times annually worldwide, with numbers expected to rise due to climate change.
- Most stings result in painful self-limited cutaneous symptoms that resolve spontaneously. Box jellyfish (Cubozoa) stings carry a greater risk for causing severe systemic reactions.
- Treatment of skin reactions includes removal of tentacles and hot water immersion. Vinegar dousing for at least 30 seconds is recommended for box jellyfish stings. Supportive care and monitoring for cardiovascular collapse are key. The role of antivenin is uncertain.
The Leaky Pipeline: A Narrative Review of Diversity in Dermatology
With a majority-minority population expected in the United States by 2044, improving diversity and cultural competency in the dermatology workforce is now more important than ever. A more diverse workforce increases the cultural competence of all providers, provides greater opportunities for mentorship and sponsorship of underrepresented minority (URM) trainees, establishes a more inclusive environment for learners, and enhances the knowledge and productivity of the workforce.1-3 Additionally, it is imperative to address clinical care disparities seen in minority patients in dermatology, including treatment of skin cancer, psoriasis, acne, atopic dermatitis, and other diseases.4-7
Despite the attention that has been devoted to improving diversity in medicine,8-10 dermatology remains one of the least diverse specialties, prompting additional calls to action within the field.11 Why does the lack of diversity still exist in dermatology, and what is the path to correcting this problem? In this article, we review the evidence of diversity barriers at different stages of medical education training that may impede academic advancement for minority learners pursuing careers in dermatology.
Undergraduate Medical Education
The term leaky pipeline refers to the progressive decline in the number of URMs along a given career path, including in dermatology. The Association of American Medical Colleges defines URMs as racial/ethnic populations that are “underrepresented in the medical profession relative to their numbers in the general population.”9 The first leak in the pipeline is that URMs are not applying to medical school. From 2002 and 2017, rates of both application and matriculation to medical school were lower by 30% to 70% in URM groups compared to White students, including Hispanic, Black, and American Indian/Alaska Native students.12,13 The decision not to apply to medical school was greater in URM undergraduate students irrespective of scholastic ability as measured by SAT scores.14
A striking statistic is that the number of Black men matriculating into medical school in 2014 was less than it was in 1978 despite the increase in the number of US medical schools and efforts to recruit more diverse student populations. The Association of American Medical Colleges identified potential reasons for this decline, including poor early education, lack of mentorship, negative perceptions of Black men due to racial stereotypes, and lack of financial and academic resources to support the application process.8,13,15-17 Implicit racial bias by admission committees also may play a role.
Medical School Matriculation and Applying to Dermatology Residency
There is greater representation of URM students in medical school than in dermatology residency, which means URM students are either not applying to dermatology programs or they are not matching into the specialty. In the Electronic Residency Application Service’s 2016-2017 application cycle (N=776), there were 76 (9.8%) URM dermatology residency applicants.18 In 2018, there was a notable decline in representation of Black students among residency applicants (4.9%) to matched residents (3.7%), and there were only 133 (9.3%) URM dermatology residents in total (PGY2-PGY4 classes).19 The lack of exposure to medical subspecialties and the recommendation by medical schools for URM medical students to pursue careers in primary care have been cited as reasons that these students may not apply to residency programs in specialty care.20,21 The presence of an Accreditation Council for Graduate Medical Education dermatology residency program, fellowships, and dermatology interest groups at their medical schools correlated with higher proportions of URM students applying to dermatology programs.20
Underrepresented minority students face critical challenges during medical school, including receiving lower grades in both standardized and school-designated assessments and clerkship grades.21,22 A 2019 National Board of Medical Examiners study found that Hispanic and Black test takers scored 12.1 and 16.6 points lower than White men, respectively, on the
A recent cross-sectional study showed that lack of equitable resources, lack of support, financial constrictions, and lack of group identity were 4 barriers to URM students matching into dermatology.26 Dermatology is a competitive specialty with the highest median Electronic Residency Application Service applications submitted per US applicant (n=90)27 and an approximate total cost per US applicant of $10,781.28,29 Disadvantaged URM applicants noted relying on loans while non-URM applicants cited family financial support as being beneficial.26 In addition, an increasing number of applicants take gap years for research, which pose additional costs for finances and resources. In contrast, mentorship and participation in pipeline/enrichment programs were factors associated with URM students matching into dermatology.26
Dermatology Residency and the Transition to Advanced Dermatology Fellowships
Similar to the transition from medical school into dermatology residency, URM dermatology residents are either not applying to fellowships or are not getting in. In the 2018-2019 academic year, there were no Black, Hispanic, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native Mohs micrographic surgery and dermatologic oncology fellows.19 Similarly, there were no Black, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native dermatopathology fellows. There were 4 (6%) Hispanic dermatopathology fellows.19
There also is marked underrepresentation of minority groups—and minimal growth over time—in the dermatology procedural subspecialty. Whereas the percentage of female Mohs surgeons increased considerably from 1985 to 2005 (12.7% to 40.9%, respectively), the percentage of URM Mohs surgeons remained steady from 4.2% to 4.6%, respectively, and remained at 4.5% in 2014.30
There are no available data on the race/ethnicity of fellowship applicants, as these demographic data for the application process have not been consistently or traditionally collected. The reasons why there are so few URM dermatology fellows is not known; whether this is due to a lack of mentorship or whether other factors lead to residents not applying for advanced training needs further study. Financial factors related to prolonged training, which include lower salaries and delayed loan repayment, may present barriers to applying to fellowships.
Lack of URM Academic Faculty in Dermatology
At the academic faculty level, URM representation continues to worsen. Lett et al31 found that there is declining racial and ethnic representation in clinical academic medicine relative to US census data for 16 US medical specialties, including dermatology, with growing underrepresentation of Black and Hispanic faculty at the associate professor and full professor levels and underrepresentation in all faculty ranks. From 1970 to 2018, URM faculty in dermatology only increased from 4.8% to 7.4%, respectively. Non-URM female and male faculty members increased by 13.8 and 10.8 faculty members per year, respectively, while URM female and male faculty members increased by 1.2 and 0.8 faculty members per year, respectively.32
Underrepresentation of minorities seen in dermatology faculty may result from clinical demands, minority taxation (defined as the extensive service requirements uniquely experienced by URM faculty to disproportionately serve as representatives on academic committees and to mentor URM students), and barriers to academic promotion, which are challenges uniquely encountered by URMs in academic dermatology.33 Increased clinical demand may result from the fact that URM physicians are more likely to care for underserved populations, those of lower socioeconomic status, non-English–speaking patients, those on Medicaid, and those who are uninsured, which may impact renumeration. Minority tax experienced by URM faculty includes mentoring URM medical students, providing cultural expertise to departments and institutions, and participating in community service projects and outreach programs. Specifically, many institutional committees require the participation of a URM member, resulting in URM faculty members experiencing higher committee service burden. Many, if not all, of these responsibilities often are not compensated through salary or academic promotion.
A Call to Action
There are several steps that can be taken to create a pathway to dermatology that is inclusive, flexible, and supportive of URMs.
• Increase early exposure to dermatology in medical school. Early exposure and mentorship opportunities are associated with higher rates of students pursuing specialty field careers.34 Increased early opportunities allow for URM students to consider and explore a career in dermatology; receive mentorship; and ensure that dermatology, including topics related to skin of color (SOC), is incorporated into their learning. The American Academy of Dermatology has contributed to these efforts by its presence at every national meeting of the Student National Medical Association and Latino Medical Student Association, as well as its involvement with Nth Dimensions, which offers various educational opportunities for URM medical students.
• Implement equitable grading and holistic review processes in medical school. Racial/ethnic differences in clinical grading and standardized test scores in medical school demonstrate why holistic review of dermatology residency applicants is needed and why other metrics such as USMLE scores and AΩA status should be de-emphasized or eliminated when evaluating candidates. To support equity, many medical schools have eliminated honors grading, and some schools have eliminated AΩA distinction.
• Increase diversity of dermatology residents and residency programs. Implicit bias training for a medical school admissions committee has been shown to increase diversity in medical school enrollment.35 Whether implicit bias training and other diversity training may benefit dermatology residency selection must be examined, including study of unintended consequences, such as reduced diversity, increased microaggressions toward minority colleagues, and the illusion of fairness.36-39 Increasing representation is not sufficient—creating inclusive residency training environments is a critical parallel aim. Prioritizing diversity in dermatology residency recruitment is imperative. Creating dermatology residency positions specifically for URM residents may be an important option, as done at the University of Pennsylvania (Philadelphia, Pennsylvania) and Duke University (Durham, North Carolina).
• Create effective programs for URM mentorship. Due to the competitive nature of dermatology residency, the need for mentors in dermatology is critically vital for URM medical students, especially those without a home dermatology program at their medical school. Further development of formal mentorship and pipeline programs is essential at both the local and national levels. Some national examples of these initiatives include diversity mentorship programs offered by the American Academy of Dermatology, Skin of Color Society, Women’s Dermatologic Society, and Student National Medical Association. Many institutional programs also offer invaluable opportunities, such as the summer research fellowship at the University of California, San Francisco (UCSF); visiting clerkship grants for URMs at the University of Pennsylvania (Philadelphia, Pennsylvania) and Johns Hopkins University (Baltimore, Maryland); and integrated programs, such as the Visiting Elective Scholarship Program at UCSF, which provides funding and faculty mentorship for URM students completing an away rotation at UCSF.
• Establish longitudinal skin-of-color curricula and increased opportunities for research. More robust SOC training may lead to an increasingly diverse workforce. It is important that medical student and dermatology resident and fellow education include training on SOC to ensure high-quality care to diverse patient populations, which also may enhance the knowledge of trainees, encourage clinical and research interest in this field, and reduce health care disparities. Increasing research opportunities and offering formalized longitudinal training in SOC as well as incorporating more diverse images in medical school education may foster greater interest in this field at a time when trainees are establishing their career interests. At present, there is considerable room for improvement. Nijhawan et al40 surveyed 63 dermatology chief residents and 41 program directors and found only 14.3% and 14.6%, respectively, reported having an expert who conducts clinic specializing in SOC. Only 52.4% and 65.9% reported having didactic sessions or lectures focused on SOC diseases, and 30.2% and 12.2% reported having a dedicated rotation for residents to gain experience in SOC.40 A more recent study showed that when faculty were asked to incorporate more SOC content into lectures, the most commonly identified barrier to implementation was a lack of SOC images.41 Additionally, there remains a paucity of published research on this topic, with SOC articles representing only 2.7% of the literature.42 These numbers demonstrate the continued need for a more inclusive and comprehensive curriculum in dermatology residency programs and more robust funding for SOC research.
• Recruit and support URM faculty. Increasing diversity in dermatology residency programs likely will increase the number of potential URMs pursuing additional fellowship training and academic dermatology with active career mentorship and support. In addition, promoting faculty retention by combatting the progressive loss of URMs at all faculty levels is paramount. Mentorship for URM physicians has been shown to play a key role in the decision to pursue academic medicine as well as academic productivity and job satisfaction.43,44 The visibility, cultural competency, clinical work, academic productivity, and mentorship efforts that URM faculty provide are essential to enhancing patient care, teaching diverse groups of learners, and recruiting more diverse trainees. Protected time to participate in professional development opportunities has been shown to improve recruitment and retention of URM faculty and offer additional opportunities for junior faculty to find mentors.35,36 Incentivizing clinical care of underserved populations also may augment financial stability for URM physicians who choose to care for these patients. Finally, diversity work and community service should be legitimized and count toward faculty promotion.
Conclusion
There are numerous factors that contribute to the leaky pipeline in dermatology (eFigure). Many challenges that are unique to the URM population disadvantage these students from entering medical school, applying to dermatology residency, matching into dermatology fellowships, pursuing and staying in faculty positions, and achieving faculty advancement into leadership positions. With each progressive step along this trajectory, there is less minority representation. All dermatologists, regardless of race/ethnicity, need to play an active role and must prioritize diversity, equity, and inclusion efforts at all levels of education and training for the betterment of the specialty.
- Dixon G, Kind T, Wright J, et al. Factors that influence the choice of academic pediatrics by underrepresented minorities. Pediatrics. 2019;144:E20182759. doi:10.1542/peds.2018-2759
- Yehia BR, Cronholm PF, Wilson N, et al. Mentorship and pursuit of academic medicine careers: a mixed methods study of residents from diverse backgrounds. BMC Med Educ. 2014:14:2-26. doi:10.1186/1472-6920-14-26
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145. doi:10.1001/jama.300.10.1135
- Hsu DY, Gordon K, Silverberg JI. The patient burden of psoriasis in the United States. J Am Acad Dermatol. 2016;75:33-41. doi:10.1016/j.jaad.2016.03.048
- Silverberg JI. Racial and ethnic disparities in atopic dermatitis. Curr Dermatol Rep. 2015;4:44-48.
- Buster KJ, Sevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Smedley BD, Stith AY, Colburn L, et al. The Right Thing To Do, The Smart Thing to Do: Enhancing Diversity in the Health Professions. National Academies Press; 2001.
- Association of American Medical Colleges. Minorities in medical education: fact and figures 2019. Accessed December 9, 2021. https://www.aamc.org/datareports/workforce/report/diversity-medicine-facts-and-figures-2019
- Liaison Committee on Medical Education (LCME) standards on diversity. University of South Florida Health website. Accessed December 9, 2021. https://health.usf.edu/~/media/Files/Medicine/MD%20Program/Diversity/LCMEStandardsonDiversity1.ashx?la=en
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500. doi:10.1001/jamadermatol.2017.0296
- Lett LA, Murdock HM, Orji W, et al. Trends in racial/ethnic representation among US medical students. JAMA Netw Open. 2019;2:e1910490. doi:10.1001/jamanetworkopen.2019.10490
- Association of American Medical Colleges. Altering the course: Black males in medicine. Published 2015. Accessed December 8, 2021. https://store.aamc.org/downloadable/download/sample/sample_id/84/
- Barr DA, Gonzalez ME, Wanat SF. The leaky pipeline: factors associated with early decline in interest in premedical studies among underrepresented minority undergraduate students. Acad Med. 2008;83:5:503-511. doi:10.1097/ACM.0b013e31816bda16
- Flores RL. The rising gap between rich and poor: a look at the persistence of educational disparities in the United States and why we should worry. Cogent Soc Sci. 2017;3:1323698.
- Jackson D. Why am I behind? an examination of low income and minority students’ preparedness for college. McNair Sch J. 2012;13:121-138.
- Rothstein R. The racial achievement gap, segregated schools, andsegregated neighborhoods: a constitutional insult. Race Soc Probl. 2015;7:21-30.
- Association of American Medical Colleges. Residency Applicants From US MD Granting Medical Schools to ACGME-Accredited Programs by Specialty and Race/Ethnicity. Association of American Medical Colleges; 2017.
- Brotherton SE, Etzel SL. Graduate medical education, 2018-2019. JAMA. 2019;322:996-1016. doi:10.1001/jama.2019.10155
- Barnes LA, Bae GH, Nambudiri V. Sex and racial/ethnic diversity of US medical students and their exposure to dermatology programs. JAMA Dermatol. 2019;155:490-491. doi:10.1001/jamadermatol.2018.5025
- Soliman YS, Rzepecki AK, Guzman AK. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Low D, Pollack SW, Liao Z, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance and United States medical licensing examination scores. Acad Med. 2019;94;364-370. doi:10.1097/ACM.0000000000002366
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the alpha omega honor society. JAMA Intern Med. 2017;177:659-665. doi:10.1001/jamainternmed.2016.9623
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey [published online March 17, 2014]. Dermatol Res Pract. doi:10.1155/2014/692760
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Results of the 2019 NRMP applicant survey by preferred specialty and applicant type. National Resident Matching Program website. Published July 2019. Accessed December 8, 2021. https://www.nrmp.org/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Polacco MA, Lally J, Walls A, et al. Digging into debt: the financial burden associated with the otolaryngology match. Otolaryngol Head Neck Surg. 2017;12:1091-1096. doi:10.1177/0194599816686538
- Feng H, Feng PW, Geronemus RG. Diversity in the US Mohs micrographic surgery workforce. Dermatol Surg. 2020:46:1451-1455. doi:10.1097/DSS.0000000000002080
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS ONE. 2018;13:e0207274. doi:10.1371/journal.pone.020727432. Xierali IM, Nivet MA, Pandya AG. US Dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970-2018. JAMA Dermatol. 2020;156:280-287. doi:10.1001/jamadermatol.2019.4297
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Intl J Womens Dermatol. 2020;6:57-60. doi:10.1016/j.ijwd.2019.09.009
- Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86:2335-2338. doi:10.2106/00004623-200410000-00031
- Capers Q, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369. doi:10.1097/ACM.0000000000001388
- Dobbin F, Kalev A. Why diversity programs fail. Harvard Business Rev. 2016;52-60. Accessed December 8, 2021. https://hbr.org/2016/07/why-diversity-programs-fail
- Kalev A, Dobbin F, Kelly E. Best practices or best guesses? assessing the efficacy of corporate affirmative action and diversity policies. Am Sociol Rev. 2006;71:589-617.
- Sanchez JI, Medkik N. The effects of diversity awareness training on differential treatment. Group Organ Manag. 2004;29:517-536.
- Kaiser CR, Major B, Jurcevic I, et al. Presumed fair: ironic effects of organizational diversity structures. J Pers Soc Psychol. 2013;104:504-519. doi:10.1037/a0030838
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-617.
- Jia JL, Gordon JS, Lester JC, et al. Integrating skin of color and sexual and gender minority content into dermatology residency curricula: a prospective program initiative [published online April 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.018
- Amuzie AU, Lia JL, Taylor SC, et al. Skin of color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Beech BM, Calles-Escandon J, Hairston KC, et al. Mentoring programs for underrepresented minority faculty in academic medical center: a systematic review of the literature. Acad Med. 2013;88:541-549. doi:10.1097/ACM.0b013e31828589e3
- Daley S, Wingard DL, Reznik V. Improving the retention of underrepresented minority faculty in academic medicine. J Natl Med Assoc. 2006;98:1435-1440. doi:10.1016/s0027-9684(15)31449-8
- Association of American Medical Colleges. US medical school faculty by sex, race/ethnicity, rank, and department, 2019. Published December 31, 2019. Accessed December 20, 2021. https://www.aamc.org/media/8476/download?attachment
With a majority-minority population expected in the United States by 2044, improving diversity and cultural competency in the dermatology workforce is now more important than ever. A more diverse workforce increases the cultural competence of all providers, provides greater opportunities for mentorship and sponsorship of underrepresented minority (URM) trainees, establishes a more inclusive environment for learners, and enhances the knowledge and productivity of the workforce.1-3 Additionally, it is imperative to address clinical care disparities seen in minority patients in dermatology, including treatment of skin cancer, psoriasis, acne, atopic dermatitis, and other diseases.4-7
Despite the attention that has been devoted to improving diversity in medicine,8-10 dermatology remains one of the least diverse specialties, prompting additional calls to action within the field.11 Why does the lack of diversity still exist in dermatology, and what is the path to correcting this problem? In this article, we review the evidence of diversity barriers at different stages of medical education training that may impede academic advancement for minority learners pursuing careers in dermatology.
Undergraduate Medical Education
The term leaky pipeline refers to the progressive decline in the number of URMs along a given career path, including in dermatology. The Association of American Medical Colleges defines URMs as racial/ethnic populations that are “underrepresented in the medical profession relative to their numbers in the general population.”9 The first leak in the pipeline is that URMs are not applying to medical school. From 2002 and 2017, rates of both application and matriculation to medical school were lower by 30% to 70% in URM groups compared to White students, including Hispanic, Black, and American Indian/Alaska Native students.12,13 The decision not to apply to medical school was greater in URM undergraduate students irrespective of scholastic ability as measured by SAT scores.14
A striking statistic is that the number of Black men matriculating into medical school in 2014 was less than it was in 1978 despite the increase in the number of US medical schools and efforts to recruit more diverse student populations. The Association of American Medical Colleges identified potential reasons for this decline, including poor early education, lack of mentorship, negative perceptions of Black men due to racial stereotypes, and lack of financial and academic resources to support the application process.8,13,15-17 Implicit racial bias by admission committees also may play a role.
Medical School Matriculation and Applying to Dermatology Residency
There is greater representation of URM students in medical school than in dermatology residency, which means URM students are either not applying to dermatology programs or they are not matching into the specialty. In the Electronic Residency Application Service’s 2016-2017 application cycle (N=776), there were 76 (9.8%) URM dermatology residency applicants.18 In 2018, there was a notable decline in representation of Black students among residency applicants (4.9%) to matched residents (3.7%), and there were only 133 (9.3%) URM dermatology residents in total (PGY2-PGY4 classes).19 The lack of exposure to medical subspecialties and the recommendation by medical schools for URM medical students to pursue careers in primary care have been cited as reasons that these students may not apply to residency programs in specialty care.20,21 The presence of an Accreditation Council for Graduate Medical Education dermatology residency program, fellowships, and dermatology interest groups at their medical schools correlated with higher proportions of URM students applying to dermatology programs.20
Underrepresented minority students face critical challenges during medical school, including receiving lower grades in both standardized and school-designated assessments and clerkship grades.21,22 A 2019 National Board of Medical Examiners study found that Hispanic and Black test takers scored 12.1 and 16.6 points lower than White men, respectively, on the
A recent cross-sectional study showed that lack of equitable resources, lack of support, financial constrictions, and lack of group identity were 4 barriers to URM students matching into dermatology.26 Dermatology is a competitive specialty with the highest median Electronic Residency Application Service applications submitted per US applicant (n=90)27 and an approximate total cost per US applicant of $10,781.28,29 Disadvantaged URM applicants noted relying on loans while non-URM applicants cited family financial support as being beneficial.26 In addition, an increasing number of applicants take gap years for research, which pose additional costs for finances and resources. In contrast, mentorship and participation in pipeline/enrichment programs were factors associated with URM students matching into dermatology.26
Dermatology Residency and the Transition to Advanced Dermatology Fellowships
Similar to the transition from medical school into dermatology residency, URM dermatology residents are either not applying to fellowships or are not getting in. In the 2018-2019 academic year, there were no Black, Hispanic, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native Mohs micrographic surgery and dermatologic oncology fellows.19 Similarly, there were no Black, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native dermatopathology fellows. There were 4 (6%) Hispanic dermatopathology fellows.19
There also is marked underrepresentation of minority groups—and minimal growth over time—in the dermatology procedural subspecialty. Whereas the percentage of female Mohs surgeons increased considerably from 1985 to 2005 (12.7% to 40.9%, respectively), the percentage of URM Mohs surgeons remained steady from 4.2% to 4.6%, respectively, and remained at 4.5% in 2014.30
There are no available data on the race/ethnicity of fellowship applicants, as these demographic data for the application process have not been consistently or traditionally collected. The reasons why there are so few URM dermatology fellows is not known; whether this is due to a lack of mentorship or whether other factors lead to residents not applying for advanced training needs further study. Financial factors related to prolonged training, which include lower salaries and delayed loan repayment, may present barriers to applying to fellowships.
Lack of URM Academic Faculty in Dermatology
At the academic faculty level, URM representation continues to worsen. Lett et al31 found that there is declining racial and ethnic representation in clinical academic medicine relative to US census data for 16 US medical specialties, including dermatology, with growing underrepresentation of Black and Hispanic faculty at the associate professor and full professor levels and underrepresentation in all faculty ranks. From 1970 to 2018, URM faculty in dermatology only increased from 4.8% to 7.4%, respectively. Non-URM female and male faculty members increased by 13.8 and 10.8 faculty members per year, respectively, while URM female and male faculty members increased by 1.2 and 0.8 faculty members per year, respectively.32
Underrepresentation of minorities seen in dermatology faculty may result from clinical demands, minority taxation (defined as the extensive service requirements uniquely experienced by URM faculty to disproportionately serve as representatives on academic committees and to mentor URM students), and barriers to academic promotion, which are challenges uniquely encountered by URMs in academic dermatology.33 Increased clinical demand may result from the fact that URM physicians are more likely to care for underserved populations, those of lower socioeconomic status, non-English–speaking patients, those on Medicaid, and those who are uninsured, which may impact renumeration. Minority tax experienced by URM faculty includes mentoring URM medical students, providing cultural expertise to departments and institutions, and participating in community service projects and outreach programs. Specifically, many institutional committees require the participation of a URM member, resulting in URM faculty members experiencing higher committee service burden. Many, if not all, of these responsibilities often are not compensated through salary or academic promotion.
A Call to Action
There are several steps that can be taken to create a pathway to dermatology that is inclusive, flexible, and supportive of URMs.
• Increase early exposure to dermatology in medical school. Early exposure and mentorship opportunities are associated with higher rates of students pursuing specialty field careers.34 Increased early opportunities allow for URM students to consider and explore a career in dermatology; receive mentorship; and ensure that dermatology, including topics related to skin of color (SOC), is incorporated into their learning. The American Academy of Dermatology has contributed to these efforts by its presence at every national meeting of the Student National Medical Association and Latino Medical Student Association, as well as its involvement with Nth Dimensions, which offers various educational opportunities for URM medical students.
• Implement equitable grading and holistic review processes in medical school. Racial/ethnic differences in clinical grading and standardized test scores in medical school demonstrate why holistic review of dermatology residency applicants is needed and why other metrics such as USMLE scores and AΩA status should be de-emphasized or eliminated when evaluating candidates. To support equity, many medical schools have eliminated honors grading, and some schools have eliminated AΩA distinction.
• Increase diversity of dermatology residents and residency programs. Implicit bias training for a medical school admissions committee has been shown to increase diversity in medical school enrollment.35 Whether implicit bias training and other diversity training may benefit dermatology residency selection must be examined, including study of unintended consequences, such as reduced diversity, increased microaggressions toward minority colleagues, and the illusion of fairness.36-39 Increasing representation is not sufficient—creating inclusive residency training environments is a critical parallel aim. Prioritizing diversity in dermatology residency recruitment is imperative. Creating dermatology residency positions specifically for URM residents may be an important option, as done at the University of Pennsylvania (Philadelphia, Pennsylvania) and Duke University (Durham, North Carolina).
• Create effective programs for URM mentorship. Due to the competitive nature of dermatology residency, the need for mentors in dermatology is critically vital for URM medical students, especially those without a home dermatology program at their medical school. Further development of formal mentorship and pipeline programs is essential at both the local and national levels. Some national examples of these initiatives include diversity mentorship programs offered by the American Academy of Dermatology, Skin of Color Society, Women’s Dermatologic Society, and Student National Medical Association. Many institutional programs also offer invaluable opportunities, such as the summer research fellowship at the University of California, San Francisco (UCSF); visiting clerkship grants for URMs at the University of Pennsylvania (Philadelphia, Pennsylvania) and Johns Hopkins University (Baltimore, Maryland); and integrated programs, such as the Visiting Elective Scholarship Program at UCSF, which provides funding and faculty mentorship for URM students completing an away rotation at UCSF.
• Establish longitudinal skin-of-color curricula and increased opportunities for research. More robust SOC training may lead to an increasingly diverse workforce. It is important that medical student and dermatology resident and fellow education include training on SOC to ensure high-quality care to diverse patient populations, which also may enhance the knowledge of trainees, encourage clinical and research interest in this field, and reduce health care disparities. Increasing research opportunities and offering formalized longitudinal training in SOC as well as incorporating more diverse images in medical school education may foster greater interest in this field at a time when trainees are establishing their career interests. At present, there is considerable room for improvement. Nijhawan et al40 surveyed 63 dermatology chief residents and 41 program directors and found only 14.3% and 14.6%, respectively, reported having an expert who conducts clinic specializing in SOC. Only 52.4% and 65.9% reported having didactic sessions or lectures focused on SOC diseases, and 30.2% and 12.2% reported having a dedicated rotation for residents to gain experience in SOC.40 A more recent study showed that when faculty were asked to incorporate more SOC content into lectures, the most commonly identified barrier to implementation was a lack of SOC images.41 Additionally, there remains a paucity of published research on this topic, with SOC articles representing only 2.7% of the literature.42 These numbers demonstrate the continued need for a more inclusive and comprehensive curriculum in dermatology residency programs and more robust funding for SOC research.
• Recruit and support URM faculty. Increasing diversity in dermatology residency programs likely will increase the number of potential URMs pursuing additional fellowship training and academic dermatology with active career mentorship and support. In addition, promoting faculty retention by combatting the progressive loss of URMs at all faculty levels is paramount. Mentorship for URM physicians has been shown to play a key role in the decision to pursue academic medicine as well as academic productivity and job satisfaction.43,44 The visibility, cultural competency, clinical work, academic productivity, and mentorship efforts that URM faculty provide are essential to enhancing patient care, teaching diverse groups of learners, and recruiting more diverse trainees. Protected time to participate in professional development opportunities has been shown to improve recruitment and retention of URM faculty and offer additional opportunities for junior faculty to find mentors.35,36 Incentivizing clinical care of underserved populations also may augment financial stability for URM physicians who choose to care for these patients. Finally, diversity work and community service should be legitimized and count toward faculty promotion.
Conclusion
There are numerous factors that contribute to the leaky pipeline in dermatology (eFigure). Many challenges that are unique to the URM population disadvantage these students from entering medical school, applying to dermatology residency, matching into dermatology fellowships, pursuing and staying in faculty positions, and achieving faculty advancement into leadership positions. With each progressive step along this trajectory, there is less minority representation. All dermatologists, regardless of race/ethnicity, need to play an active role and must prioritize diversity, equity, and inclusion efforts at all levels of education and training for the betterment of the specialty.
With a majority-minority population expected in the United States by 2044, improving diversity and cultural competency in the dermatology workforce is now more important than ever. A more diverse workforce increases the cultural competence of all providers, provides greater opportunities for mentorship and sponsorship of underrepresented minority (URM) trainees, establishes a more inclusive environment for learners, and enhances the knowledge and productivity of the workforce.1-3 Additionally, it is imperative to address clinical care disparities seen in minority patients in dermatology, including treatment of skin cancer, psoriasis, acne, atopic dermatitis, and other diseases.4-7
Despite the attention that has been devoted to improving diversity in medicine,8-10 dermatology remains one of the least diverse specialties, prompting additional calls to action within the field.11 Why does the lack of diversity still exist in dermatology, and what is the path to correcting this problem? In this article, we review the evidence of diversity barriers at different stages of medical education training that may impede academic advancement for minority learners pursuing careers in dermatology.
Undergraduate Medical Education
The term leaky pipeline refers to the progressive decline in the number of URMs along a given career path, including in dermatology. The Association of American Medical Colleges defines URMs as racial/ethnic populations that are “underrepresented in the medical profession relative to their numbers in the general population.”9 The first leak in the pipeline is that URMs are not applying to medical school. From 2002 and 2017, rates of both application and matriculation to medical school were lower by 30% to 70% in URM groups compared to White students, including Hispanic, Black, and American Indian/Alaska Native students.12,13 The decision not to apply to medical school was greater in URM undergraduate students irrespective of scholastic ability as measured by SAT scores.14
A striking statistic is that the number of Black men matriculating into medical school in 2014 was less than it was in 1978 despite the increase in the number of US medical schools and efforts to recruit more diverse student populations. The Association of American Medical Colleges identified potential reasons for this decline, including poor early education, lack of mentorship, negative perceptions of Black men due to racial stereotypes, and lack of financial and academic resources to support the application process.8,13,15-17 Implicit racial bias by admission committees also may play a role.
Medical School Matriculation and Applying to Dermatology Residency
There is greater representation of URM students in medical school than in dermatology residency, which means URM students are either not applying to dermatology programs or they are not matching into the specialty. In the Electronic Residency Application Service’s 2016-2017 application cycle (N=776), there were 76 (9.8%) URM dermatology residency applicants.18 In 2018, there was a notable decline in representation of Black students among residency applicants (4.9%) to matched residents (3.7%), and there were only 133 (9.3%) URM dermatology residents in total (PGY2-PGY4 classes).19 The lack of exposure to medical subspecialties and the recommendation by medical schools for URM medical students to pursue careers in primary care have been cited as reasons that these students may not apply to residency programs in specialty care.20,21 The presence of an Accreditation Council for Graduate Medical Education dermatology residency program, fellowships, and dermatology interest groups at their medical schools correlated with higher proportions of URM students applying to dermatology programs.20
Underrepresented minority students face critical challenges during medical school, including receiving lower grades in both standardized and school-designated assessments and clerkship grades.21,22 A 2019 National Board of Medical Examiners study found that Hispanic and Black test takers scored 12.1 and 16.6 points lower than White men, respectively, on the
A recent cross-sectional study showed that lack of equitable resources, lack of support, financial constrictions, and lack of group identity were 4 barriers to URM students matching into dermatology.26 Dermatology is a competitive specialty with the highest median Electronic Residency Application Service applications submitted per US applicant (n=90)27 and an approximate total cost per US applicant of $10,781.28,29 Disadvantaged URM applicants noted relying on loans while non-URM applicants cited family financial support as being beneficial.26 In addition, an increasing number of applicants take gap years for research, which pose additional costs for finances and resources. In contrast, mentorship and participation in pipeline/enrichment programs were factors associated with URM students matching into dermatology.26
Dermatology Residency and the Transition to Advanced Dermatology Fellowships
Similar to the transition from medical school into dermatology residency, URM dermatology residents are either not applying to fellowships or are not getting in. In the 2018-2019 academic year, there were no Black, Hispanic, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native Mohs micrographic surgery and dermatologic oncology fellows.19 Similarly, there were no Black, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native dermatopathology fellows. There were 4 (6%) Hispanic dermatopathology fellows.19
There also is marked underrepresentation of minority groups—and minimal growth over time—in the dermatology procedural subspecialty. Whereas the percentage of female Mohs surgeons increased considerably from 1985 to 2005 (12.7% to 40.9%, respectively), the percentage of URM Mohs surgeons remained steady from 4.2% to 4.6%, respectively, and remained at 4.5% in 2014.30
There are no available data on the race/ethnicity of fellowship applicants, as these demographic data for the application process have not been consistently or traditionally collected. The reasons why there are so few URM dermatology fellows is not known; whether this is due to a lack of mentorship or whether other factors lead to residents not applying for advanced training needs further study. Financial factors related to prolonged training, which include lower salaries and delayed loan repayment, may present barriers to applying to fellowships.
Lack of URM Academic Faculty in Dermatology
At the academic faculty level, URM representation continues to worsen. Lett et al31 found that there is declining racial and ethnic representation in clinical academic medicine relative to US census data for 16 US medical specialties, including dermatology, with growing underrepresentation of Black and Hispanic faculty at the associate professor and full professor levels and underrepresentation in all faculty ranks. From 1970 to 2018, URM faculty in dermatology only increased from 4.8% to 7.4%, respectively. Non-URM female and male faculty members increased by 13.8 and 10.8 faculty members per year, respectively, while URM female and male faculty members increased by 1.2 and 0.8 faculty members per year, respectively.32
Underrepresentation of minorities seen in dermatology faculty may result from clinical demands, minority taxation (defined as the extensive service requirements uniquely experienced by URM faculty to disproportionately serve as representatives on academic committees and to mentor URM students), and barriers to academic promotion, which are challenges uniquely encountered by URMs in academic dermatology.33 Increased clinical demand may result from the fact that URM physicians are more likely to care for underserved populations, those of lower socioeconomic status, non-English–speaking patients, those on Medicaid, and those who are uninsured, which may impact renumeration. Minority tax experienced by URM faculty includes mentoring URM medical students, providing cultural expertise to departments and institutions, and participating in community service projects and outreach programs. Specifically, many institutional committees require the participation of a URM member, resulting in URM faculty members experiencing higher committee service burden. Many, if not all, of these responsibilities often are not compensated through salary or academic promotion.
A Call to Action
There are several steps that can be taken to create a pathway to dermatology that is inclusive, flexible, and supportive of URMs.
• Increase early exposure to dermatology in medical school. Early exposure and mentorship opportunities are associated with higher rates of students pursuing specialty field careers.34 Increased early opportunities allow for URM students to consider and explore a career in dermatology; receive mentorship; and ensure that dermatology, including topics related to skin of color (SOC), is incorporated into their learning. The American Academy of Dermatology has contributed to these efforts by its presence at every national meeting of the Student National Medical Association and Latino Medical Student Association, as well as its involvement with Nth Dimensions, which offers various educational opportunities for URM medical students.
• Implement equitable grading and holistic review processes in medical school. Racial/ethnic differences in clinical grading and standardized test scores in medical school demonstrate why holistic review of dermatology residency applicants is needed and why other metrics such as USMLE scores and AΩA status should be de-emphasized or eliminated when evaluating candidates. To support equity, many medical schools have eliminated honors grading, and some schools have eliminated AΩA distinction.
• Increase diversity of dermatology residents and residency programs. Implicit bias training for a medical school admissions committee has been shown to increase diversity in medical school enrollment.35 Whether implicit bias training and other diversity training may benefit dermatology residency selection must be examined, including study of unintended consequences, such as reduced diversity, increased microaggressions toward minority colleagues, and the illusion of fairness.36-39 Increasing representation is not sufficient—creating inclusive residency training environments is a critical parallel aim. Prioritizing diversity in dermatology residency recruitment is imperative. Creating dermatology residency positions specifically for URM residents may be an important option, as done at the University of Pennsylvania (Philadelphia, Pennsylvania) and Duke University (Durham, North Carolina).
• Create effective programs for URM mentorship. Due to the competitive nature of dermatology residency, the need for mentors in dermatology is critically vital for URM medical students, especially those without a home dermatology program at their medical school. Further development of formal mentorship and pipeline programs is essential at both the local and national levels. Some national examples of these initiatives include diversity mentorship programs offered by the American Academy of Dermatology, Skin of Color Society, Women’s Dermatologic Society, and Student National Medical Association. Many institutional programs also offer invaluable opportunities, such as the summer research fellowship at the University of California, San Francisco (UCSF); visiting clerkship grants for URMs at the University of Pennsylvania (Philadelphia, Pennsylvania) and Johns Hopkins University (Baltimore, Maryland); and integrated programs, such as the Visiting Elective Scholarship Program at UCSF, which provides funding and faculty mentorship for URM students completing an away rotation at UCSF.
• Establish longitudinal skin-of-color curricula and increased opportunities for research. More robust SOC training may lead to an increasingly diverse workforce. It is important that medical student and dermatology resident and fellow education include training on SOC to ensure high-quality care to diverse patient populations, which also may enhance the knowledge of trainees, encourage clinical and research interest in this field, and reduce health care disparities. Increasing research opportunities and offering formalized longitudinal training in SOC as well as incorporating more diverse images in medical school education may foster greater interest in this field at a time when trainees are establishing their career interests. At present, there is considerable room for improvement. Nijhawan et al40 surveyed 63 dermatology chief residents and 41 program directors and found only 14.3% and 14.6%, respectively, reported having an expert who conducts clinic specializing in SOC. Only 52.4% and 65.9% reported having didactic sessions or lectures focused on SOC diseases, and 30.2% and 12.2% reported having a dedicated rotation for residents to gain experience in SOC.40 A more recent study showed that when faculty were asked to incorporate more SOC content into lectures, the most commonly identified barrier to implementation was a lack of SOC images.41 Additionally, there remains a paucity of published research on this topic, with SOC articles representing only 2.7% of the literature.42 These numbers demonstrate the continued need for a more inclusive and comprehensive curriculum in dermatology residency programs and more robust funding for SOC research.
• Recruit and support URM faculty. Increasing diversity in dermatology residency programs likely will increase the number of potential URMs pursuing additional fellowship training and academic dermatology with active career mentorship and support. In addition, promoting faculty retention by combatting the progressive loss of URMs at all faculty levels is paramount. Mentorship for URM physicians has been shown to play a key role in the decision to pursue academic medicine as well as academic productivity and job satisfaction.43,44 The visibility, cultural competency, clinical work, academic productivity, and mentorship efforts that URM faculty provide are essential to enhancing patient care, teaching diverse groups of learners, and recruiting more diverse trainees. Protected time to participate in professional development opportunities has been shown to improve recruitment and retention of URM faculty and offer additional opportunities for junior faculty to find mentors.35,36 Incentivizing clinical care of underserved populations also may augment financial stability for URM physicians who choose to care for these patients. Finally, diversity work and community service should be legitimized and count toward faculty promotion.
Conclusion
There are numerous factors that contribute to the leaky pipeline in dermatology (eFigure). Many challenges that are unique to the URM population disadvantage these students from entering medical school, applying to dermatology residency, matching into dermatology fellowships, pursuing and staying in faculty positions, and achieving faculty advancement into leadership positions. With each progressive step along this trajectory, there is less minority representation. All dermatologists, regardless of race/ethnicity, need to play an active role and must prioritize diversity, equity, and inclusion efforts at all levels of education and training for the betterment of the specialty.
- Dixon G, Kind T, Wright J, et al. Factors that influence the choice of academic pediatrics by underrepresented minorities. Pediatrics. 2019;144:E20182759. doi:10.1542/peds.2018-2759
- Yehia BR, Cronholm PF, Wilson N, et al. Mentorship and pursuit of academic medicine careers: a mixed methods study of residents from diverse backgrounds. BMC Med Educ. 2014:14:2-26. doi:10.1186/1472-6920-14-26
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145. doi:10.1001/jama.300.10.1135
- Hsu DY, Gordon K, Silverberg JI. The patient burden of psoriasis in the United States. J Am Acad Dermatol. 2016;75:33-41. doi:10.1016/j.jaad.2016.03.048
- Silverberg JI. Racial and ethnic disparities in atopic dermatitis. Curr Dermatol Rep. 2015;4:44-48.
- Buster KJ, Sevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Smedley BD, Stith AY, Colburn L, et al. The Right Thing To Do, The Smart Thing to Do: Enhancing Diversity in the Health Professions. National Academies Press; 2001.
- Association of American Medical Colleges. Minorities in medical education: fact and figures 2019. Accessed December 9, 2021. https://www.aamc.org/datareports/workforce/report/diversity-medicine-facts-and-figures-2019
- Liaison Committee on Medical Education (LCME) standards on diversity. University of South Florida Health website. Accessed December 9, 2021. https://health.usf.edu/~/media/Files/Medicine/MD%20Program/Diversity/LCMEStandardsonDiversity1.ashx?la=en
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500. doi:10.1001/jamadermatol.2017.0296
- Lett LA, Murdock HM, Orji W, et al. Trends in racial/ethnic representation among US medical students. JAMA Netw Open. 2019;2:e1910490. doi:10.1001/jamanetworkopen.2019.10490
- Association of American Medical Colleges. Altering the course: Black males in medicine. Published 2015. Accessed December 8, 2021. https://store.aamc.org/downloadable/download/sample/sample_id/84/
- Barr DA, Gonzalez ME, Wanat SF. The leaky pipeline: factors associated with early decline in interest in premedical studies among underrepresented minority undergraduate students. Acad Med. 2008;83:5:503-511. doi:10.1097/ACM.0b013e31816bda16
- Flores RL. The rising gap between rich and poor: a look at the persistence of educational disparities in the United States and why we should worry. Cogent Soc Sci. 2017;3:1323698.
- Jackson D. Why am I behind? an examination of low income and minority students’ preparedness for college. McNair Sch J. 2012;13:121-138.
- Rothstein R. The racial achievement gap, segregated schools, andsegregated neighborhoods: a constitutional insult. Race Soc Probl. 2015;7:21-30.
- Association of American Medical Colleges. Residency Applicants From US MD Granting Medical Schools to ACGME-Accredited Programs by Specialty and Race/Ethnicity. Association of American Medical Colleges; 2017.
- Brotherton SE, Etzel SL. Graduate medical education, 2018-2019. JAMA. 2019;322:996-1016. doi:10.1001/jama.2019.10155
- Barnes LA, Bae GH, Nambudiri V. Sex and racial/ethnic diversity of US medical students and their exposure to dermatology programs. JAMA Dermatol. 2019;155:490-491. doi:10.1001/jamadermatol.2018.5025
- Soliman YS, Rzepecki AK, Guzman AK. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Low D, Pollack SW, Liao Z, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance and United States medical licensing examination scores. Acad Med. 2019;94;364-370. doi:10.1097/ACM.0000000000002366
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the alpha omega honor society. JAMA Intern Med. 2017;177:659-665. doi:10.1001/jamainternmed.2016.9623
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey [published online March 17, 2014]. Dermatol Res Pract. doi:10.1155/2014/692760
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Results of the 2019 NRMP applicant survey by preferred specialty and applicant type. National Resident Matching Program website. Published July 2019. Accessed December 8, 2021. https://www.nrmp.org/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Polacco MA, Lally J, Walls A, et al. Digging into debt: the financial burden associated with the otolaryngology match. Otolaryngol Head Neck Surg. 2017;12:1091-1096. doi:10.1177/0194599816686538
- Feng H, Feng PW, Geronemus RG. Diversity in the US Mohs micrographic surgery workforce. Dermatol Surg. 2020:46:1451-1455. doi:10.1097/DSS.0000000000002080
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS ONE. 2018;13:e0207274. doi:10.1371/journal.pone.020727432. Xierali IM, Nivet MA, Pandya AG. US Dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970-2018. JAMA Dermatol. 2020;156:280-287. doi:10.1001/jamadermatol.2019.4297
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Intl J Womens Dermatol. 2020;6:57-60. doi:10.1016/j.ijwd.2019.09.009
- Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86:2335-2338. doi:10.2106/00004623-200410000-00031
- Capers Q, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369. doi:10.1097/ACM.0000000000001388
- Dobbin F, Kalev A. Why diversity programs fail. Harvard Business Rev. 2016;52-60. Accessed December 8, 2021. https://hbr.org/2016/07/why-diversity-programs-fail
- Kalev A, Dobbin F, Kelly E. Best practices or best guesses? assessing the efficacy of corporate affirmative action and diversity policies. Am Sociol Rev. 2006;71:589-617.
- Sanchez JI, Medkik N. The effects of diversity awareness training on differential treatment. Group Organ Manag. 2004;29:517-536.
- Kaiser CR, Major B, Jurcevic I, et al. Presumed fair: ironic effects of organizational diversity structures. J Pers Soc Psychol. 2013;104:504-519. doi:10.1037/a0030838
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-617.
- Jia JL, Gordon JS, Lester JC, et al. Integrating skin of color and sexual and gender minority content into dermatology residency curricula: a prospective program initiative [published online April 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.018
- Amuzie AU, Lia JL, Taylor SC, et al. Skin of color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Beech BM, Calles-Escandon J, Hairston KC, et al. Mentoring programs for underrepresented minority faculty in academic medical center: a systematic review of the literature. Acad Med. 2013;88:541-549. doi:10.1097/ACM.0b013e31828589e3
- Daley S, Wingard DL, Reznik V. Improving the retention of underrepresented minority faculty in academic medicine. J Natl Med Assoc. 2006;98:1435-1440. doi:10.1016/s0027-9684(15)31449-8
- Association of American Medical Colleges. US medical school faculty by sex, race/ethnicity, rank, and department, 2019. Published December 31, 2019. Accessed December 20, 2021. https://www.aamc.org/media/8476/download?attachment
- Dixon G, Kind T, Wright J, et al. Factors that influence the choice of academic pediatrics by underrepresented minorities. Pediatrics. 2019;144:E20182759. doi:10.1542/peds.2018-2759
- Yehia BR, Cronholm PF, Wilson N, et al. Mentorship and pursuit of academic medicine careers: a mixed methods study of residents from diverse backgrounds. BMC Med Educ. 2014:14:2-26. doi:10.1186/1472-6920-14-26
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145. doi:10.1001/jama.300.10.1135
- Hsu DY, Gordon K, Silverberg JI. The patient burden of psoriasis in the United States. J Am Acad Dermatol. 2016;75:33-41. doi:10.1016/j.jaad.2016.03.048
- Silverberg JI. Racial and ethnic disparities in atopic dermatitis. Curr Dermatol Rep. 2015;4:44-48.
- Buster KJ, Sevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Smedley BD, Stith AY, Colburn L, et al. The Right Thing To Do, The Smart Thing to Do: Enhancing Diversity in the Health Professions. National Academies Press; 2001.
- Association of American Medical Colleges. Minorities in medical education: fact and figures 2019. Accessed December 9, 2021. https://www.aamc.org/datareports/workforce/report/diversity-medicine-facts-and-figures-2019
- Liaison Committee on Medical Education (LCME) standards on diversity. University of South Florida Health website. Accessed December 9, 2021. https://health.usf.edu/~/media/Files/Medicine/MD%20Program/Diversity/LCMEStandardsonDiversity1.ashx?la=en
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500. doi:10.1001/jamadermatol.2017.0296
- Lett LA, Murdock HM, Orji W, et al. Trends in racial/ethnic representation among US medical students. JAMA Netw Open. 2019;2:e1910490. doi:10.1001/jamanetworkopen.2019.10490
- Association of American Medical Colleges. Altering the course: Black males in medicine. Published 2015. Accessed December 8, 2021. https://store.aamc.org/downloadable/download/sample/sample_id/84/
- Barr DA, Gonzalez ME, Wanat SF. The leaky pipeline: factors associated with early decline in interest in premedical studies among underrepresented minority undergraduate students. Acad Med. 2008;83:5:503-511. doi:10.1097/ACM.0b013e31816bda16
- Flores RL. The rising gap between rich and poor: a look at the persistence of educational disparities in the United States and why we should worry. Cogent Soc Sci. 2017;3:1323698.
- Jackson D. Why am I behind? an examination of low income and minority students’ preparedness for college. McNair Sch J. 2012;13:121-138.
- Rothstein R. The racial achievement gap, segregated schools, andsegregated neighborhoods: a constitutional insult. Race Soc Probl. 2015;7:21-30.
- Association of American Medical Colleges. Residency Applicants From US MD Granting Medical Schools to ACGME-Accredited Programs by Specialty and Race/Ethnicity. Association of American Medical Colleges; 2017.
- Brotherton SE, Etzel SL. Graduate medical education, 2018-2019. JAMA. 2019;322:996-1016. doi:10.1001/jama.2019.10155
- Barnes LA, Bae GH, Nambudiri V. Sex and racial/ethnic diversity of US medical students and their exposure to dermatology programs. JAMA Dermatol. 2019;155:490-491. doi:10.1001/jamadermatol.2018.5025
- Soliman YS, Rzepecki AK, Guzman AK. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Low D, Pollack SW, Liao Z, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance and United States medical licensing examination scores. Acad Med. 2019;94;364-370. doi:10.1097/ACM.0000000000002366
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the alpha omega honor society. JAMA Intern Med. 2017;177:659-665. doi:10.1001/jamainternmed.2016.9623
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey [published online March 17, 2014]. Dermatol Res Pract. doi:10.1155/2014/692760
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Results of the 2019 NRMP applicant survey by preferred specialty and applicant type. National Resident Matching Program website. Published July 2019. Accessed December 8, 2021. https://www.nrmp.org/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Polacco MA, Lally J, Walls A, et al. Digging into debt: the financial burden associated with the otolaryngology match. Otolaryngol Head Neck Surg. 2017;12:1091-1096. doi:10.1177/0194599816686538
- Feng H, Feng PW, Geronemus RG. Diversity in the US Mohs micrographic surgery workforce. Dermatol Surg. 2020:46:1451-1455. doi:10.1097/DSS.0000000000002080
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS ONE. 2018;13:e0207274. doi:10.1371/journal.pone.020727432. Xierali IM, Nivet MA, Pandya AG. US Dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970-2018. JAMA Dermatol. 2020;156:280-287. doi:10.1001/jamadermatol.2019.4297
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Intl J Womens Dermatol. 2020;6:57-60. doi:10.1016/j.ijwd.2019.09.009
- Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86:2335-2338. doi:10.2106/00004623-200410000-00031
- Capers Q, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369. doi:10.1097/ACM.0000000000001388
- Dobbin F, Kalev A. Why diversity programs fail. Harvard Business Rev. 2016;52-60. Accessed December 8, 2021. https://hbr.org/2016/07/why-diversity-programs-fail
- Kalev A, Dobbin F, Kelly E. Best practices or best guesses? assessing the efficacy of corporate affirmative action and diversity policies. Am Sociol Rev. 2006;71:589-617.
- Sanchez JI, Medkik N. The effects of diversity awareness training on differential treatment. Group Organ Manag. 2004;29:517-536.
- Kaiser CR, Major B, Jurcevic I, et al. Presumed fair: ironic effects of organizational diversity structures. J Pers Soc Psychol. 2013;104:504-519. doi:10.1037/a0030838
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-617.
- Jia JL, Gordon JS, Lester JC, et al. Integrating skin of color and sexual and gender minority content into dermatology residency curricula: a prospective program initiative [published online April 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.018
- Amuzie AU, Lia JL, Taylor SC, et al. Skin of color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Beech BM, Calles-Escandon J, Hairston KC, et al. Mentoring programs for underrepresented minority faculty in academic medical center: a systematic review of the literature. Acad Med. 2013;88:541-549. doi:10.1097/ACM.0b013e31828589e3
- Daley S, Wingard DL, Reznik V. Improving the retention of underrepresented minority faculty in academic medicine. J Natl Med Assoc. 2006;98:1435-1440. doi:10.1016/s0027-9684(15)31449-8
- Association of American Medical Colleges. US medical school faculty by sex, race/ethnicity, rank, and department, 2019. Published December 31, 2019. Accessed December 20, 2021. https://www.aamc.org/media/8476/download?attachment
Practice Points
- Dermatology remains the second least diverse specialty in medicine, which has important implications for the workforce and clinical excellence of the specialty.
- Barriers presenting at different stages of medical education and training result in the loss of underrepresented minority (URM) learners pursuing or advancing careers in dermatology.
- Understanding these barriers is the first step to creating and implementing important structural changes to the way we mentor, teach, and support URM students in the specialty.
Teledermatology During the COVID-19 Pandemic: Lessons Learned and Future Directions
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.