User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Risk for erectile dysfunction sixfold higher in men with COVID-19
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular risks elevated in transgender youth
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The pandemic is making periods unbearable for some women
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Hyperphagia, anxiety eased with carbetocin in patients with Prader-Willi syndrome
Children and adolescents with Prader-Willi syndrome (PWS) who received three daily, intranasal doses of carbetocin, an investigational, long-acting oxytocin analogue, had significant improvement in hyperphagia and anxiety during 8 weeks on treatment, compared with placebo in a multicenter, phase 3 trial with 119 patients.
The treatment also appeared safe during up to 56 additional weeks on active treatment, with no serious adverse effects nor “unexpected” events, and once completing the study about 95% of enrolled patients opted to remain on active treatment, Cheri L. Deal, MD, PhD, said at the annual meeting of the Endocrine Society.
Based on “the significant results for the placebo-controlled period, as well as for those finishing the 56-week extension, we may well have a new armament for helping these kids and their families deal with the unrelenting hunger of patients with PWS as well as some of the behavioral symptoms,” Dr. Deal, chief of endocrinology and diabetes at the Sainte-Justine Mother-Child University of Montreal Hospital, said in an interview. No treatment currently has labeling for addressing the hyperphagia or anxiety that is characteristic and often problematic for children and adolescents with PWS, an autosomal dominant genetic disease with an incidence of about 1 in 15,000 births and an estimated U.S. prevalence of about 9,000 cases, or about 1 case for every 37,000 people.
‘Gorgeous’ safety
“The results looked pretty positive, and we’re encouraged by what appears to be a good safety profile, so overall I think the PWS community is very excited by the results and is very interested in getting access to this drug,” commented Theresa V. Strong, PhD, director of research programs for the Foundation for Prader-Willi Research in Walnut, Calif., a group not involved with the study. Currently, “we have no effective treatments for these difficult behaviors” of hyperphagia and anxiety. Surveys and studies run by the foundation have documented that hyperphagia and anxiety “were the two most important symptoms that families would like to see treated,” Dr. Strong added in an interview.
PWS “is complex and affects almost every aspect of the lives of affected people and their families. Any treatment that can chip away at some of the problems these patients have can be a huge benefit to the patients and their families,” said Jennifer L. Miller, MD, a professor of pediatric endocrinology at the University of Florida, Gainesville, and a coinvestigator on the study.
But the finding that carbetocin appeared to address, at least in part, this unmet need while compiling a safety record that Dr. Miller called “gorgeous” and “remarkable,” also came with a few limitations.
Fewer patients than planned, and muddled outcomes
The CARE-PWS trial aimed to enroll 175 patients, but fell short once the COVID-19 pandemic hit. Plus the trial had two prespecified primary endpoints – improvements in a measure of hyperphagia, and in a measure of obsessive and compulsive behaviors – specifically in the 40 patients who received the higher of the two dosages studied, 9.6 mg t.i.d. intranasally. Neither endpoint showed significant improvement among the patients on this dosage, compared with the 40 patients who received placebo, although both outcomes trended in the right direction in the actively treated patients.
The study’s positive results came in a secondary treatment group, 39 patients who received 3.2 mg t.i.d., also intranasally. This subgroup had significant benefit, compared with placebo, for reducing hyperphagia symptoms as measured on the Hyperphagia Questionnaire for Clinical Trials (HQ-CT) Total Score. After the first 8 weeks on treatment, patients on the lower carbetocin dosage had an average reduction in their HQ-CT score of greater than 5 points, more than double the reduction seen among control patients who received placebo.
Those on the 3.2-mg t.i.d. dosage also showed significant improvements, compared with placebo, for anxiety, measured by the PWS Anxiety and Distress Questionnaire Total Score, as well as on measures of clinical global impression of severity, and of clinical global impression of change. Like the higher-dosage patients the lower-dosage subgroup did not show a significant difference compared with placebo for the other primary endpoint, change in obsessive and compulsive behaviors as measured by the Children’s Yale-Brown Obsessive-Compulsive Scale Total Score, although also like the higher dosage the effect from the lower dosage trended toward benefit.
A further limitation was that, at the time of her report, presented in abstract OR16-3 at the meeting, Dr. Deal could only present complete 64-week follow-up for 72 patients, although this reassuringly showed that, as time on the 9.6-mg t.i.d. dosage continued beyond 8 weeks, patients gradually improved their HQ-CT response so that by 64 weeks on treatment their hyperphagia score had improved as much as in the patients who received the lower dosage.
In short, documented benefits occurred on the lower dosage, especially for clinically meaningful symptoms like hyperphagia and anxiety, but the study’s overall results were not fully consistent by statistical criteria.
Benefiting an unmet need?
“While it is regrettable that we did not get to 175 patients because of COVID-19, the dataset is significant enough for me to feel that the FDA [Food and Drug Administration] needs to take a very serious look and consider approval,” Dr. Deal said in an interview. “Once safety is assured, which I think it is, I can only hope that regulatory officials understand their unmet needs of this rare disease community and will allow the drug to move to the next stage.”
“This is a very rare disease, and having families participate in trials is really challenging,” especially while the COVID-19 pandemic continues, Dr. Strong said. For the pediatric and adolescent patients targeted in this study “it will take a while for COVID to go away and for families to feel safe again being in a trial, so a real concern is that a need for more clinical trials is not terribly feasible now. Given that the safety profile looked good and one dose seemed to have good efficacy, as long as the long-term data continue to look good we’d love for the FDA to look at the existing data and see whether there is a path forward.”
Dr. Miller highlighted the limitations of what the CARE-PWS findings show.
“Given that it was only an 8-week trial of drug against placebo, and the fact that the primary outcomes weren’t met for the higher dose, my thought is that potentially we need to study more patients for a longer period at the 3.2-mg dose,” she said. She acknowledged that the metric used in the study to assess obsessive and compulsive behaviors is “very difficult” to apply to patients with PWS because of uncertainties in scoring obsessions in patients “who are not very good at telling you what they’re thinking.” Plus, “it’s absolutely not a problem that we did not see an effect on obsession and compulsions if the treatment potentially improves anxiety and hyperphagia, which are very common.” A treatment that reliably reduces these symptoms “would be amazing,” Dr. Miller added.
“PWS is very rare, so it’s very hard to do trials. Maybe the FDA will approve carbetocin because it was safe and gave a signal of efficacy at the lower dose. But my thought is that additional treatment trials are needed with only the lower dose and with longer duration,” she said.
CARE-PWS enrolled patients with nutritional phase 3 PWS who were aged 7-18 years at any of 24 sites in the United States, Canada, or Australia during 2018-2020. They averaged about 12 years of age, and 56% were girls.
The most common adverse effect from carbetocin was flushing, occurring in 14% of those on the lower dose and 21% on the higher dose, but not in any placebo patient. Other adverse effects more common on the lower dose than in the placebo group included headache in 16%, and diarrhea in 9%.
Carbetocin is not only long-lasting in circulation, it also has better affinity for oxytocin receptors than for vasopressin receptors, reducing the potential for causing hyponatremia. The idea to use it in patients with PWS followed prior studies with oxytocin, which had shown dopamine interactions that reduced anxiety and influenced food ingestion behavior. Brain autopsy studies had shown that patients with Prader-Willi syndrome have substantially fewer neurons than usual producing oxytocin. Treatment with intranasal carbetocin had shown efficacy for improving hyperphagia and behavior in a controlled phase 2 study with 37 patients.
Carbetocin is approved for use in reducing excessive bleeding after childbirth, particularly cesarean, in more than 20 countries outside the United States.
CARE-PWS was sponsored by Levo Therapeutics, the company developing carbetocin. Dr. Deal has been an adviser to Levo Therapeutics. Dr. Strong is an employee of the Foundation for Prader-Willi Research, which has received support from Levo Therapeutics as well as from other drug companies, but which receives most of its funding from individuals. Dr. Miller has received research funding from Levo Therapeutics and also from Harmony Biosciences, Rhythm Pharmaceuticals, and Soleno Therapeutics.
Children and adolescents with Prader-Willi syndrome (PWS) who received three daily, intranasal doses of carbetocin, an investigational, long-acting oxytocin analogue, had significant improvement in hyperphagia and anxiety during 8 weeks on treatment, compared with placebo in a multicenter, phase 3 trial with 119 patients.
The treatment also appeared safe during up to 56 additional weeks on active treatment, with no serious adverse effects nor “unexpected” events, and once completing the study about 95% of enrolled patients opted to remain on active treatment, Cheri L. Deal, MD, PhD, said at the annual meeting of the Endocrine Society.
Based on “the significant results for the placebo-controlled period, as well as for those finishing the 56-week extension, we may well have a new armament for helping these kids and their families deal with the unrelenting hunger of patients with PWS as well as some of the behavioral symptoms,” Dr. Deal, chief of endocrinology and diabetes at the Sainte-Justine Mother-Child University of Montreal Hospital, said in an interview. No treatment currently has labeling for addressing the hyperphagia or anxiety that is characteristic and often problematic for children and adolescents with PWS, an autosomal dominant genetic disease with an incidence of about 1 in 15,000 births and an estimated U.S. prevalence of about 9,000 cases, or about 1 case for every 37,000 people.
‘Gorgeous’ safety
“The results looked pretty positive, and we’re encouraged by what appears to be a good safety profile, so overall I think the PWS community is very excited by the results and is very interested in getting access to this drug,” commented Theresa V. Strong, PhD, director of research programs for the Foundation for Prader-Willi Research in Walnut, Calif., a group not involved with the study. Currently, “we have no effective treatments for these difficult behaviors” of hyperphagia and anxiety. Surveys and studies run by the foundation have documented that hyperphagia and anxiety “were the two most important symptoms that families would like to see treated,” Dr. Strong added in an interview.
PWS “is complex and affects almost every aspect of the lives of affected people and their families. Any treatment that can chip away at some of the problems these patients have can be a huge benefit to the patients and their families,” said Jennifer L. Miller, MD, a professor of pediatric endocrinology at the University of Florida, Gainesville, and a coinvestigator on the study.
But the finding that carbetocin appeared to address, at least in part, this unmet need while compiling a safety record that Dr. Miller called “gorgeous” and “remarkable,” also came with a few limitations.
Fewer patients than planned, and muddled outcomes
The CARE-PWS trial aimed to enroll 175 patients, but fell short once the COVID-19 pandemic hit. Plus the trial had two prespecified primary endpoints – improvements in a measure of hyperphagia, and in a measure of obsessive and compulsive behaviors – specifically in the 40 patients who received the higher of the two dosages studied, 9.6 mg t.i.d. intranasally. Neither endpoint showed significant improvement among the patients on this dosage, compared with the 40 patients who received placebo, although both outcomes trended in the right direction in the actively treated patients.
The study’s positive results came in a secondary treatment group, 39 patients who received 3.2 mg t.i.d., also intranasally. This subgroup had significant benefit, compared with placebo, for reducing hyperphagia symptoms as measured on the Hyperphagia Questionnaire for Clinical Trials (HQ-CT) Total Score. After the first 8 weeks on treatment, patients on the lower carbetocin dosage had an average reduction in their HQ-CT score of greater than 5 points, more than double the reduction seen among control patients who received placebo.
Those on the 3.2-mg t.i.d. dosage also showed significant improvements, compared with placebo, for anxiety, measured by the PWS Anxiety and Distress Questionnaire Total Score, as well as on measures of clinical global impression of severity, and of clinical global impression of change. Like the higher-dosage patients the lower-dosage subgroup did not show a significant difference compared with placebo for the other primary endpoint, change in obsessive and compulsive behaviors as measured by the Children’s Yale-Brown Obsessive-Compulsive Scale Total Score, although also like the higher dosage the effect from the lower dosage trended toward benefit.
A further limitation was that, at the time of her report, presented in abstract OR16-3 at the meeting, Dr. Deal could only present complete 64-week follow-up for 72 patients, although this reassuringly showed that, as time on the 9.6-mg t.i.d. dosage continued beyond 8 weeks, patients gradually improved their HQ-CT response so that by 64 weeks on treatment their hyperphagia score had improved as much as in the patients who received the lower dosage.
In short, documented benefits occurred on the lower dosage, especially for clinically meaningful symptoms like hyperphagia and anxiety, but the study’s overall results were not fully consistent by statistical criteria.
Benefiting an unmet need?
“While it is regrettable that we did not get to 175 patients because of COVID-19, the dataset is significant enough for me to feel that the FDA [Food and Drug Administration] needs to take a very serious look and consider approval,” Dr. Deal said in an interview. “Once safety is assured, which I think it is, I can only hope that regulatory officials understand their unmet needs of this rare disease community and will allow the drug to move to the next stage.”
“This is a very rare disease, and having families participate in trials is really challenging,” especially while the COVID-19 pandemic continues, Dr. Strong said. For the pediatric and adolescent patients targeted in this study “it will take a while for COVID to go away and for families to feel safe again being in a trial, so a real concern is that a need for more clinical trials is not terribly feasible now. Given that the safety profile looked good and one dose seemed to have good efficacy, as long as the long-term data continue to look good we’d love for the FDA to look at the existing data and see whether there is a path forward.”
Dr. Miller highlighted the limitations of what the CARE-PWS findings show.
“Given that it was only an 8-week trial of drug against placebo, and the fact that the primary outcomes weren’t met for the higher dose, my thought is that potentially we need to study more patients for a longer period at the 3.2-mg dose,” she said. She acknowledged that the metric used in the study to assess obsessive and compulsive behaviors is “very difficult” to apply to patients with PWS because of uncertainties in scoring obsessions in patients “who are not very good at telling you what they’re thinking.” Plus, “it’s absolutely not a problem that we did not see an effect on obsession and compulsions if the treatment potentially improves anxiety and hyperphagia, which are very common.” A treatment that reliably reduces these symptoms “would be amazing,” Dr. Miller added.
“PWS is very rare, so it’s very hard to do trials. Maybe the FDA will approve carbetocin because it was safe and gave a signal of efficacy at the lower dose. But my thought is that additional treatment trials are needed with only the lower dose and with longer duration,” she said.
CARE-PWS enrolled patients with nutritional phase 3 PWS who were aged 7-18 years at any of 24 sites in the United States, Canada, or Australia during 2018-2020. They averaged about 12 years of age, and 56% were girls.
The most common adverse effect from carbetocin was flushing, occurring in 14% of those on the lower dose and 21% on the higher dose, but not in any placebo patient. Other adverse effects more common on the lower dose than in the placebo group included headache in 16%, and diarrhea in 9%.
Carbetocin is not only long-lasting in circulation, it also has better affinity for oxytocin receptors than for vasopressin receptors, reducing the potential for causing hyponatremia. The idea to use it in patients with PWS followed prior studies with oxytocin, which had shown dopamine interactions that reduced anxiety and influenced food ingestion behavior. Brain autopsy studies had shown that patients with Prader-Willi syndrome have substantially fewer neurons than usual producing oxytocin. Treatment with intranasal carbetocin had shown efficacy for improving hyperphagia and behavior in a controlled phase 2 study with 37 patients.
Carbetocin is approved for use in reducing excessive bleeding after childbirth, particularly cesarean, in more than 20 countries outside the United States.
CARE-PWS was sponsored by Levo Therapeutics, the company developing carbetocin. Dr. Deal has been an adviser to Levo Therapeutics. Dr. Strong is an employee of the Foundation for Prader-Willi Research, which has received support from Levo Therapeutics as well as from other drug companies, but which receives most of its funding from individuals. Dr. Miller has received research funding from Levo Therapeutics and also from Harmony Biosciences, Rhythm Pharmaceuticals, and Soleno Therapeutics.
Children and adolescents with Prader-Willi syndrome (PWS) who received three daily, intranasal doses of carbetocin, an investigational, long-acting oxytocin analogue, had significant improvement in hyperphagia and anxiety during 8 weeks on treatment, compared with placebo in a multicenter, phase 3 trial with 119 patients.
The treatment also appeared safe during up to 56 additional weeks on active treatment, with no serious adverse effects nor “unexpected” events, and once completing the study about 95% of enrolled patients opted to remain on active treatment, Cheri L. Deal, MD, PhD, said at the annual meeting of the Endocrine Society.
Based on “the significant results for the placebo-controlled period, as well as for those finishing the 56-week extension, we may well have a new armament for helping these kids and their families deal with the unrelenting hunger of patients with PWS as well as some of the behavioral symptoms,” Dr. Deal, chief of endocrinology and diabetes at the Sainte-Justine Mother-Child University of Montreal Hospital, said in an interview. No treatment currently has labeling for addressing the hyperphagia or anxiety that is characteristic and often problematic for children and adolescents with PWS, an autosomal dominant genetic disease with an incidence of about 1 in 15,000 births and an estimated U.S. prevalence of about 9,000 cases, or about 1 case for every 37,000 people.
‘Gorgeous’ safety
“The results looked pretty positive, and we’re encouraged by what appears to be a good safety profile, so overall I think the PWS community is very excited by the results and is very interested in getting access to this drug,” commented Theresa V. Strong, PhD, director of research programs for the Foundation for Prader-Willi Research in Walnut, Calif., a group not involved with the study. Currently, “we have no effective treatments for these difficult behaviors” of hyperphagia and anxiety. Surveys and studies run by the foundation have documented that hyperphagia and anxiety “were the two most important symptoms that families would like to see treated,” Dr. Strong added in an interview.
PWS “is complex and affects almost every aspect of the lives of affected people and their families. Any treatment that can chip away at some of the problems these patients have can be a huge benefit to the patients and their families,” said Jennifer L. Miller, MD, a professor of pediatric endocrinology at the University of Florida, Gainesville, and a coinvestigator on the study.
But the finding that carbetocin appeared to address, at least in part, this unmet need while compiling a safety record that Dr. Miller called “gorgeous” and “remarkable,” also came with a few limitations.
Fewer patients than planned, and muddled outcomes
The CARE-PWS trial aimed to enroll 175 patients, but fell short once the COVID-19 pandemic hit. Plus the trial had two prespecified primary endpoints – improvements in a measure of hyperphagia, and in a measure of obsessive and compulsive behaviors – specifically in the 40 patients who received the higher of the two dosages studied, 9.6 mg t.i.d. intranasally. Neither endpoint showed significant improvement among the patients on this dosage, compared with the 40 patients who received placebo, although both outcomes trended in the right direction in the actively treated patients.
The study’s positive results came in a secondary treatment group, 39 patients who received 3.2 mg t.i.d., also intranasally. This subgroup had significant benefit, compared with placebo, for reducing hyperphagia symptoms as measured on the Hyperphagia Questionnaire for Clinical Trials (HQ-CT) Total Score. After the first 8 weeks on treatment, patients on the lower carbetocin dosage had an average reduction in their HQ-CT score of greater than 5 points, more than double the reduction seen among control patients who received placebo.
Those on the 3.2-mg t.i.d. dosage also showed significant improvements, compared with placebo, for anxiety, measured by the PWS Anxiety and Distress Questionnaire Total Score, as well as on measures of clinical global impression of severity, and of clinical global impression of change. Like the higher-dosage patients the lower-dosage subgroup did not show a significant difference compared with placebo for the other primary endpoint, change in obsessive and compulsive behaviors as measured by the Children’s Yale-Brown Obsessive-Compulsive Scale Total Score, although also like the higher dosage the effect from the lower dosage trended toward benefit.
A further limitation was that, at the time of her report, presented in abstract OR16-3 at the meeting, Dr. Deal could only present complete 64-week follow-up for 72 patients, although this reassuringly showed that, as time on the 9.6-mg t.i.d. dosage continued beyond 8 weeks, patients gradually improved their HQ-CT response so that by 64 weeks on treatment their hyperphagia score had improved as much as in the patients who received the lower dosage.
In short, documented benefits occurred on the lower dosage, especially for clinically meaningful symptoms like hyperphagia and anxiety, but the study’s overall results were not fully consistent by statistical criteria.
Benefiting an unmet need?
“While it is regrettable that we did not get to 175 patients because of COVID-19, the dataset is significant enough for me to feel that the FDA [Food and Drug Administration] needs to take a very serious look and consider approval,” Dr. Deal said in an interview. “Once safety is assured, which I think it is, I can only hope that regulatory officials understand their unmet needs of this rare disease community and will allow the drug to move to the next stage.”
“This is a very rare disease, and having families participate in trials is really challenging,” especially while the COVID-19 pandemic continues, Dr. Strong said. For the pediatric and adolescent patients targeted in this study “it will take a while for COVID to go away and for families to feel safe again being in a trial, so a real concern is that a need for more clinical trials is not terribly feasible now. Given that the safety profile looked good and one dose seemed to have good efficacy, as long as the long-term data continue to look good we’d love for the FDA to look at the existing data and see whether there is a path forward.”
Dr. Miller highlighted the limitations of what the CARE-PWS findings show.
“Given that it was only an 8-week trial of drug against placebo, and the fact that the primary outcomes weren’t met for the higher dose, my thought is that potentially we need to study more patients for a longer period at the 3.2-mg dose,” she said. She acknowledged that the metric used in the study to assess obsessive and compulsive behaviors is “very difficult” to apply to patients with PWS because of uncertainties in scoring obsessions in patients “who are not very good at telling you what they’re thinking.” Plus, “it’s absolutely not a problem that we did not see an effect on obsession and compulsions if the treatment potentially improves anxiety and hyperphagia, which are very common.” A treatment that reliably reduces these symptoms “would be amazing,” Dr. Miller added.
“PWS is very rare, so it’s very hard to do trials. Maybe the FDA will approve carbetocin because it was safe and gave a signal of efficacy at the lower dose. But my thought is that additional treatment trials are needed with only the lower dose and with longer duration,” she said.
CARE-PWS enrolled patients with nutritional phase 3 PWS who were aged 7-18 years at any of 24 sites in the United States, Canada, or Australia during 2018-2020. They averaged about 12 years of age, and 56% were girls.
The most common adverse effect from carbetocin was flushing, occurring in 14% of those on the lower dose and 21% on the higher dose, but not in any placebo patient. Other adverse effects more common on the lower dose than in the placebo group included headache in 16%, and diarrhea in 9%.
Carbetocin is not only long-lasting in circulation, it also has better affinity for oxytocin receptors than for vasopressin receptors, reducing the potential for causing hyponatremia. The idea to use it in patients with PWS followed prior studies with oxytocin, which had shown dopamine interactions that reduced anxiety and influenced food ingestion behavior. Brain autopsy studies had shown that patients with Prader-Willi syndrome have substantially fewer neurons than usual producing oxytocin. Treatment with intranasal carbetocin had shown efficacy for improving hyperphagia and behavior in a controlled phase 2 study with 37 patients.
Carbetocin is approved for use in reducing excessive bleeding after childbirth, particularly cesarean, in more than 20 countries outside the United States.
CARE-PWS was sponsored by Levo Therapeutics, the company developing carbetocin. Dr. Deal has been an adviser to Levo Therapeutics. Dr. Strong is an employee of the Foundation for Prader-Willi Research, which has received support from Levo Therapeutics as well as from other drug companies, but which receives most of its funding from individuals. Dr. Miller has received research funding from Levo Therapeutics and also from Harmony Biosciences, Rhythm Pharmaceuticals, and Soleno Therapeutics.
FROM ENDO 2021
Study suggests no added risk of blood clots in COVID-19 outpatients
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
Cardiovascular disease remains leading cause of type 2 diabetes mortality
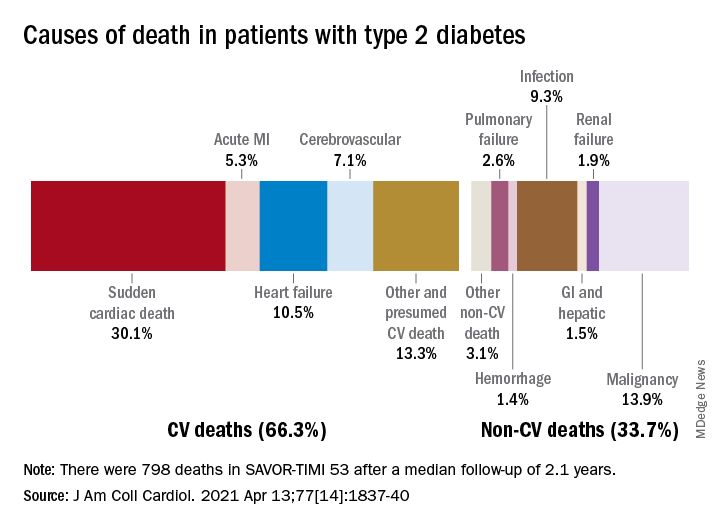
Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.

Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.

Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
In low-risk thyroid cancer, no advantage found for postsurgical iodine
After 3 years of follow-up in patients with low-risk differentiated thyroid cancer (DTC), there is no significant difference in the rate of tumor-related events in those treated with postsurgical radioactive iodine (RAI) relative to those who are not, according to a randomized phase 3 trial.
The controversy about whether postoperative RAI is beneficial or an overtreatment in low-risk DTC has persisted for years, but it can now be addressed with level one data, according to Sophie Leboulleux, MD, PhD, who heads the thyroid cancer division at Gustave Roussy Cancer Institute, Villejuif, France.
The trial, called ESTIMABL2, included 776 low-risk DTC patients managed at 35 participating treatment centers in France. Two to five months after surgery, patients were randomized to receive RAI or to follow-up without RAI if there were no suspicious findings, such as changes in lateral neck lymph nodes, on ultrasonography.
Three years after randomization in 729 evaluable patients, tumor-related effects occurred in 4.1% of those randomized to RAI and 4.9% of those randomized to follow-up without RAI.
“Thus, 95.1% of patients in the followed group and 95.9% in the RAI group had no event over 3 years of follow-up. The difference of 0.8% was noninferior by the prespecified definition,” Dr. Leboulleux reported at the annual meeting of the Endocrine Society.
The 0.8% difference in events is noninferior
The prespecified definition was a less than 5% difference in events at the end of 5 years. The 0.8% difference in this study was comfortably within the 95% confidence interval (–3.3%-1.8%).
On yearly evaluations under levothyroxine treatment, events were defined as an indication for treatment, whether surgery or RAI administration if there was abnormal RAI intake on the posttherapeutic whole-body scan or elevated thyroglobulin or thyroglobulin antibodies. In the latter case, this was an event even in the absence of abnormal neck ultrasonography.
“Elevated thyroglobulin levels on levothyroxine treatment were defined as greater than 2 ng/mL in the group that did not received RAI and greater than 1 ng/mL in the group that did,” Dr. Leboulleux reported.
Thyroglobulin antibodies were considered elevated with a greater than 50% increase over the upper limit of normal on two consecutive determinations 6 months apart, Dr. Leboulleux said. The same or similar definitions of an event have been used previously.
Of the study population, 83% were women and almost all (96%) had papillary DTC. The mean age was 52 years. Of the tumors, 81.1% were T1b, of which more than half were unevaluable for node status and about 40% were node negative. The remaining tumors were grade T1a, of which about two-thirds had unevaluable node status and the remainder were confirmed node negative.
Prior studies also question RAI benefit
Several retrospective studies have also failed to associated RAI with a significant protection against thyroid events in low-risk DTC. In the most recent guidelines, the American Thoracic Society recommended against routine use of RAI following surgery in low-risk DTC patients. However, these guidelines acknowledged this is a “weak recommendation” based on “low-quality evidence.”
Recurrence rates following surgery in low-risk DTC are not zero. According to a review article coauthored by Dr. Leboulleux, these may occur in up to 3% of patients. This measurable risk might explain why many published retrospective data suggest low-risk DTC patients are continuing to receive postsurgical RAI. In one, using data taken from the Surveillance, Epidemiology, and End Results (SEER) database, nearly 25% of 17,286 low-risk papillary thyroid cancers had received postsurgical RAI.
The ESTIMABL2 trial is one of two randomized studies launched over the last several years to address the controversy regarding postsurgical RAI in low-risk DTC with prospective randomized data. The ongoing IoN trial is the other. The researchers plan a much longer follow-up, with data not expected until 2031.
In fact, one criticism of the ESTIMABL2 is “the low event rate and relatively short follow-up,” reported Megan Haymart, MD, a professor in the division of metabolism, endocrinology, and diabetes at the University of Michigan, Ann Arbor.
“The strengths of this study include the involvement of 35 French centers, the use of a cohort that is representative of the distribution of low-risk thyroid cancer at large, and the use of biochemical markers during follow-up,” Dr. Haymart said in an interview.
Ultimately, despite the limited follow-up, “this is a clinically important study as it supports the recent paradigm shift towards less use of RAI for low-risk thyroid cancer,” she said.
Dr. Leboulleux and Dr. Haymart report no relevant conflicts of interest.
After 3 years of follow-up in patients with low-risk differentiated thyroid cancer (DTC), there is no significant difference in the rate of tumor-related events in those treated with postsurgical radioactive iodine (RAI) relative to those who are not, according to a randomized phase 3 trial.
The controversy about whether postoperative RAI is beneficial or an overtreatment in low-risk DTC has persisted for years, but it can now be addressed with level one data, according to Sophie Leboulleux, MD, PhD, who heads the thyroid cancer division at Gustave Roussy Cancer Institute, Villejuif, France.
The trial, called ESTIMABL2, included 776 low-risk DTC patients managed at 35 participating treatment centers in France. Two to five months after surgery, patients were randomized to receive RAI or to follow-up without RAI if there were no suspicious findings, such as changes in lateral neck lymph nodes, on ultrasonography.
Three years after randomization in 729 evaluable patients, tumor-related effects occurred in 4.1% of those randomized to RAI and 4.9% of those randomized to follow-up without RAI.
“Thus, 95.1% of patients in the followed group and 95.9% in the RAI group had no event over 3 years of follow-up. The difference of 0.8% was noninferior by the prespecified definition,” Dr. Leboulleux reported at the annual meeting of the Endocrine Society.
The 0.8% difference in events is noninferior
The prespecified definition was a less than 5% difference in events at the end of 5 years. The 0.8% difference in this study was comfortably within the 95% confidence interval (–3.3%-1.8%).
On yearly evaluations under levothyroxine treatment, events were defined as an indication for treatment, whether surgery or RAI administration if there was abnormal RAI intake on the posttherapeutic whole-body scan or elevated thyroglobulin or thyroglobulin antibodies. In the latter case, this was an event even in the absence of abnormal neck ultrasonography.
“Elevated thyroglobulin levels on levothyroxine treatment were defined as greater than 2 ng/mL in the group that did not received RAI and greater than 1 ng/mL in the group that did,” Dr. Leboulleux reported.
Thyroglobulin antibodies were considered elevated with a greater than 50% increase over the upper limit of normal on two consecutive determinations 6 months apart, Dr. Leboulleux said. The same or similar definitions of an event have been used previously.
Of the study population, 83% were women and almost all (96%) had papillary DTC. The mean age was 52 years. Of the tumors, 81.1% were T1b, of which more than half were unevaluable for node status and about 40% were node negative. The remaining tumors were grade T1a, of which about two-thirds had unevaluable node status and the remainder were confirmed node negative.
Prior studies also question RAI benefit
Several retrospective studies have also failed to associated RAI with a significant protection against thyroid events in low-risk DTC. In the most recent guidelines, the American Thoracic Society recommended against routine use of RAI following surgery in low-risk DTC patients. However, these guidelines acknowledged this is a “weak recommendation” based on “low-quality evidence.”
Recurrence rates following surgery in low-risk DTC are not zero. According to a review article coauthored by Dr. Leboulleux, these may occur in up to 3% of patients. This measurable risk might explain why many published retrospective data suggest low-risk DTC patients are continuing to receive postsurgical RAI. In one, using data taken from the Surveillance, Epidemiology, and End Results (SEER) database, nearly 25% of 17,286 low-risk papillary thyroid cancers had received postsurgical RAI.
The ESTIMABL2 trial is one of two randomized studies launched over the last several years to address the controversy regarding postsurgical RAI in low-risk DTC with prospective randomized data. The ongoing IoN trial is the other. The researchers plan a much longer follow-up, with data not expected until 2031.
In fact, one criticism of the ESTIMABL2 is “the low event rate and relatively short follow-up,” reported Megan Haymart, MD, a professor in the division of metabolism, endocrinology, and diabetes at the University of Michigan, Ann Arbor.
“The strengths of this study include the involvement of 35 French centers, the use of a cohort that is representative of the distribution of low-risk thyroid cancer at large, and the use of biochemical markers during follow-up,” Dr. Haymart said in an interview.
Ultimately, despite the limited follow-up, “this is a clinically important study as it supports the recent paradigm shift towards less use of RAI for low-risk thyroid cancer,” she said.
Dr. Leboulleux and Dr. Haymart report no relevant conflicts of interest.
After 3 years of follow-up in patients with low-risk differentiated thyroid cancer (DTC), there is no significant difference in the rate of tumor-related events in those treated with postsurgical radioactive iodine (RAI) relative to those who are not, according to a randomized phase 3 trial.
The controversy about whether postoperative RAI is beneficial or an overtreatment in low-risk DTC has persisted for years, but it can now be addressed with level one data, according to Sophie Leboulleux, MD, PhD, who heads the thyroid cancer division at Gustave Roussy Cancer Institute, Villejuif, France.
The trial, called ESTIMABL2, included 776 low-risk DTC patients managed at 35 participating treatment centers in France. Two to five months after surgery, patients were randomized to receive RAI or to follow-up without RAI if there were no suspicious findings, such as changes in lateral neck lymph nodes, on ultrasonography.
Three years after randomization in 729 evaluable patients, tumor-related effects occurred in 4.1% of those randomized to RAI and 4.9% of those randomized to follow-up without RAI.
“Thus, 95.1% of patients in the followed group and 95.9% in the RAI group had no event over 3 years of follow-up. The difference of 0.8% was noninferior by the prespecified definition,” Dr. Leboulleux reported at the annual meeting of the Endocrine Society.
The 0.8% difference in events is noninferior
The prespecified definition was a less than 5% difference in events at the end of 5 years. The 0.8% difference in this study was comfortably within the 95% confidence interval (–3.3%-1.8%).
On yearly evaluations under levothyroxine treatment, events were defined as an indication for treatment, whether surgery or RAI administration if there was abnormal RAI intake on the posttherapeutic whole-body scan or elevated thyroglobulin or thyroglobulin antibodies. In the latter case, this was an event even in the absence of abnormal neck ultrasonography.
“Elevated thyroglobulin levels on levothyroxine treatment were defined as greater than 2 ng/mL in the group that did not received RAI and greater than 1 ng/mL in the group that did,” Dr. Leboulleux reported.
Thyroglobulin antibodies were considered elevated with a greater than 50% increase over the upper limit of normal on two consecutive determinations 6 months apart, Dr. Leboulleux said. The same or similar definitions of an event have been used previously.
Of the study population, 83% were women and almost all (96%) had papillary DTC. The mean age was 52 years. Of the tumors, 81.1% were T1b, of which more than half were unevaluable for node status and about 40% were node negative. The remaining tumors were grade T1a, of which about two-thirds had unevaluable node status and the remainder were confirmed node negative.
Prior studies also question RAI benefit
Several retrospective studies have also failed to associated RAI with a significant protection against thyroid events in low-risk DTC. In the most recent guidelines, the American Thoracic Society recommended against routine use of RAI following surgery in low-risk DTC patients. However, these guidelines acknowledged this is a “weak recommendation” based on “low-quality evidence.”
Recurrence rates following surgery in low-risk DTC are not zero. According to a review article coauthored by Dr. Leboulleux, these may occur in up to 3% of patients. This measurable risk might explain why many published retrospective data suggest low-risk DTC patients are continuing to receive postsurgical RAI. In one, using data taken from the Surveillance, Epidemiology, and End Results (SEER) database, nearly 25% of 17,286 low-risk papillary thyroid cancers had received postsurgical RAI.
The ESTIMABL2 trial is one of two randomized studies launched over the last several years to address the controversy regarding postsurgical RAI in low-risk DTC with prospective randomized data. The ongoing IoN trial is the other. The researchers plan a much longer follow-up, with data not expected until 2031.
In fact, one criticism of the ESTIMABL2 is “the low event rate and relatively short follow-up,” reported Megan Haymart, MD, a professor in the division of metabolism, endocrinology, and diabetes at the University of Michigan, Ann Arbor.
“The strengths of this study include the involvement of 35 French centers, the use of a cohort that is representative of the distribution of low-risk thyroid cancer at large, and the use of biochemical markers during follow-up,” Dr. Haymart said in an interview.
Ultimately, despite the limited follow-up, “this is a clinically important study as it supports the recent paradigm shift towards less use of RAI for low-risk thyroid cancer,” she said.
Dr. Leboulleux and Dr. Haymart report no relevant conflicts of interest.
FROM ENDO 2021
Excess deaths jump 23% in U.S. in 2020, mostly because of COVID-19
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
Coffee could be the secret weapon against NAFLD
Treatment of obesity through exercise and diet is unquestionably the foundation of care for patients with nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH). But drinking at least several cups of coffee a day makes for additional powerful medicine, said Manal F. Abdelmalek, MD, MPH, at the Gastroenterology Updates, IBD, Liver Disease Conference.
“I do recommend at least two to three cups of coffee per day for my patients with NAFLD,” said Dr. Abdelmalek, professor of medicine and a gastroenterologist at Duke University, Durham, N.C.
Her thinking on this recommendation has been influenced by a meta-analysis of 16 studies including more than 3,000 coffee drinkers and 132,000 nonconsumers; the meta-analysis concluded that coffee drinkers were 39% less likely to develop cirrhosis. There was evidence of a dose-response effect: Consumers of two or more cups daily had a 47% reduction in the risk of cirrhosis, compared with the nondrinkers, while more modest consumption was associated with a 34% reduction. Moreover, the investigators found that coffee consumption was also associated with a 27% reduction in the likelihood of developing advanced hepatic fibrosis, compared with that of non–coffee drinkers.
“What’s even more provocative is the evidence that coffee decreases risk of hepatocellular carcinoma,” the gastroenterologist said.
She highlighted a U.K. meta-analysis of 18 cohort studies with 2.27 million participants and 2,905 cases, along with 8 case-control studies featuring a collective 1,825 cases and 4,652 controls. The investigators reported that drinking at least two cups of coffee per day was associated with a 35% reduction in the risk of hepatocellular carcinoma independent of a patient’s stage of liver disease or the presence or absence of high alcohol consumption, smoking, obesity, type 2 diabetes, or hepatitis B or C infection.
“This is very impressive data and certainly not something you should ignore,” according to Dr. Abdelmalek.
There is also “fairly strong” data that coffee reduces the risk of developing type 2 diabetes, she continued. The mechanism of these benefits is unclear.
“It’s not known if it’s caffeine or some other constituent of the bean; a phenol, for example. The story behind tea is not as compelling as for coffee, so it may be something beyond caffeine,” according to Dr. Abdelmalek.
Session moderator Norah A. Terrault, MD, MPH, noted that drinking at least two cups of coffee per day has also been associated with reduced risk of cirrhosis in patients with hepatitis B or hepatitis C infection. So she too is on board the coffee express.
“I’m also a big proponent of recommending coffee. We take so much away from the patients, it’s nice to give them back something, right?” said Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
Diet and exercise
Most of the major gastroenterology professional societies emphasize in their practice guidelines for NAFLD that diet and routine physical activity are mandatory. If sustained, these lifestyle modifications can improve NASH and hepatic fibrosis, as well as reduce the risk of portal hypertension and liver cancer. Dr. Abdelmalek counsels her patients to aim for at least 150 minutes per week of moderate or vigorous aerobic and/or resistance exercise. She doesn’t care about the exercise intensity or type, noting that what she considers to be “a beautifully done intervention trial” in 220 patients over the course of 12 months concluded that both moderate and vigorous exercise achieved a significant reduction in intrahepatic triglyceride content.
“Tailor exercise to what patients can do, what they enjoy, and what they can sustain,” she advised.
She identifies and addresses all modifiable risk factors for NAFLD, including hypertension, diabetes, abdominal obesity, smoking, excessive alcohol intake, obstructive sleep apnea, and an unhealthy diet high in fat, red meat, and fructose.
“The primary message I tell my patients interested in dieting is: I want you to find the right approach for you. There is no right or wrong answer. For some of my patients, it’s intermittent fasting and having their first meal at 2 or 3 o’clock in the afternoon. For others it’s a Weight Watchers approach, or a Mediterranean diet, or it’s high protein. The bottom line of my approach is a gravitation away from excess carbohydrates and fats, and beyond that if I can achieve weight loss through caloric restriction or intermittent fasting, I try to tailor that to my patients’ preferences. I do send them to nutritionists,” the gastroenterologist said.
A 7%-10% weight loss has been shown to result in resolution of NASH in 64%-90% of patients. However, only about 10% of patients who achieve clinically meaningful weight loss short term are able to maintain it at 1 year, so ongoing follow-up is essential.
At present there is no FDA-approved therapy for NAFLD/NASH. Beyond diet and exercise – and coffee – there is the option of antiobesity weight-loss drug therapy, which is about as effective as successful lifestyle modification, and bariatric surgery, which is dramatically effective. French surgeons recently reported in a prospective single-center study of 180 severely obese patients with NASH who underwent bariatric surgery that, at 5 years’ follow-up, 84% of them had resolution of NASH with no worsening of liver fibrosis. Indeed, 63% of patients with mild fibrosis at baseline experienced complete resolution of their fibrosis at follow-up, as did 46% of those with more severe baseline bridging fibrosis.
Dr. Abdelmalek reported having no financial conflicts of interest regarding her presentation.
Treatment of obesity through exercise and diet is unquestionably the foundation of care for patients with nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH). But drinking at least several cups of coffee a day makes for additional powerful medicine, said Manal F. Abdelmalek, MD, MPH, at the Gastroenterology Updates, IBD, Liver Disease Conference.
“I do recommend at least two to three cups of coffee per day for my patients with NAFLD,” said Dr. Abdelmalek, professor of medicine and a gastroenterologist at Duke University, Durham, N.C.
Her thinking on this recommendation has been influenced by a meta-analysis of 16 studies including more than 3,000 coffee drinkers and 132,000 nonconsumers; the meta-analysis concluded that coffee drinkers were 39% less likely to develop cirrhosis. There was evidence of a dose-response effect: Consumers of two or more cups daily had a 47% reduction in the risk of cirrhosis, compared with the nondrinkers, while more modest consumption was associated with a 34% reduction. Moreover, the investigators found that coffee consumption was also associated with a 27% reduction in the likelihood of developing advanced hepatic fibrosis, compared with that of non–coffee drinkers.
“What’s even more provocative is the evidence that coffee decreases risk of hepatocellular carcinoma,” the gastroenterologist said.
She highlighted a U.K. meta-analysis of 18 cohort studies with 2.27 million participants and 2,905 cases, along with 8 case-control studies featuring a collective 1,825 cases and 4,652 controls. The investigators reported that drinking at least two cups of coffee per day was associated with a 35% reduction in the risk of hepatocellular carcinoma independent of a patient’s stage of liver disease or the presence or absence of high alcohol consumption, smoking, obesity, type 2 diabetes, or hepatitis B or C infection.
“This is very impressive data and certainly not something you should ignore,” according to Dr. Abdelmalek.
There is also “fairly strong” data that coffee reduces the risk of developing type 2 diabetes, she continued. The mechanism of these benefits is unclear.
“It’s not known if it’s caffeine or some other constituent of the bean; a phenol, for example. The story behind tea is not as compelling as for coffee, so it may be something beyond caffeine,” according to Dr. Abdelmalek.
Session moderator Norah A. Terrault, MD, MPH, noted that drinking at least two cups of coffee per day has also been associated with reduced risk of cirrhosis in patients with hepatitis B or hepatitis C infection. So she too is on board the coffee express.
“I’m also a big proponent of recommending coffee. We take so much away from the patients, it’s nice to give them back something, right?” said Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
Diet and exercise
Most of the major gastroenterology professional societies emphasize in their practice guidelines for NAFLD that diet and routine physical activity are mandatory. If sustained, these lifestyle modifications can improve NASH and hepatic fibrosis, as well as reduce the risk of portal hypertension and liver cancer. Dr. Abdelmalek counsels her patients to aim for at least 150 minutes per week of moderate or vigorous aerobic and/or resistance exercise. She doesn’t care about the exercise intensity or type, noting that what she considers to be “a beautifully done intervention trial” in 220 patients over the course of 12 months concluded that both moderate and vigorous exercise achieved a significant reduction in intrahepatic triglyceride content.
“Tailor exercise to what patients can do, what they enjoy, and what they can sustain,” she advised.
She identifies and addresses all modifiable risk factors for NAFLD, including hypertension, diabetes, abdominal obesity, smoking, excessive alcohol intake, obstructive sleep apnea, and an unhealthy diet high in fat, red meat, and fructose.
“The primary message I tell my patients interested in dieting is: I want you to find the right approach for you. There is no right or wrong answer. For some of my patients, it’s intermittent fasting and having their first meal at 2 or 3 o’clock in the afternoon. For others it’s a Weight Watchers approach, or a Mediterranean diet, or it’s high protein. The bottom line of my approach is a gravitation away from excess carbohydrates and fats, and beyond that if I can achieve weight loss through caloric restriction or intermittent fasting, I try to tailor that to my patients’ preferences. I do send them to nutritionists,” the gastroenterologist said.
A 7%-10% weight loss has been shown to result in resolution of NASH in 64%-90% of patients. However, only about 10% of patients who achieve clinically meaningful weight loss short term are able to maintain it at 1 year, so ongoing follow-up is essential.
At present there is no FDA-approved therapy for NAFLD/NASH. Beyond diet and exercise – and coffee – there is the option of antiobesity weight-loss drug therapy, which is about as effective as successful lifestyle modification, and bariatric surgery, which is dramatically effective. French surgeons recently reported in a prospective single-center study of 180 severely obese patients with NASH who underwent bariatric surgery that, at 5 years’ follow-up, 84% of them had resolution of NASH with no worsening of liver fibrosis. Indeed, 63% of patients with mild fibrosis at baseline experienced complete resolution of their fibrosis at follow-up, as did 46% of those with more severe baseline bridging fibrosis.
Dr. Abdelmalek reported having no financial conflicts of interest regarding her presentation.
Treatment of obesity through exercise and diet is unquestionably the foundation of care for patients with nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH). But drinking at least several cups of coffee a day makes for additional powerful medicine, said Manal F. Abdelmalek, MD, MPH, at the Gastroenterology Updates, IBD, Liver Disease Conference.
“I do recommend at least two to three cups of coffee per day for my patients with NAFLD,” said Dr. Abdelmalek, professor of medicine and a gastroenterologist at Duke University, Durham, N.C.
Her thinking on this recommendation has been influenced by a meta-analysis of 16 studies including more than 3,000 coffee drinkers and 132,000 nonconsumers; the meta-analysis concluded that coffee drinkers were 39% less likely to develop cirrhosis. There was evidence of a dose-response effect: Consumers of two or more cups daily had a 47% reduction in the risk of cirrhosis, compared with the nondrinkers, while more modest consumption was associated with a 34% reduction. Moreover, the investigators found that coffee consumption was also associated with a 27% reduction in the likelihood of developing advanced hepatic fibrosis, compared with that of non–coffee drinkers.
“What’s even more provocative is the evidence that coffee decreases risk of hepatocellular carcinoma,” the gastroenterologist said.
She highlighted a U.K. meta-analysis of 18 cohort studies with 2.27 million participants and 2,905 cases, along with 8 case-control studies featuring a collective 1,825 cases and 4,652 controls. The investigators reported that drinking at least two cups of coffee per day was associated with a 35% reduction in the risk of hepatocellular carcinoma independent of a patient’s stage of liver disease or the presence or absence of high alcohol consumption, smoking, obesity, type 2 diabetes, or hepatitis B or C infection.
“This is very impressive data and certainly not something you should ignore,” according to Dr. Abdelmalek.
There is also “fairly strong” data that coffee reduces the risk of developing type 2 diabetes, she continued. The mechanism of these benefits is unclear.
“It’s not known if it’s caffeine or some other constituent of the bean; a phenol, for example. The story behind tea is not as compelling as for coffee, so it may be something beyond caffeine,” according to Dr. Abdelmalek.
Session moderator Norah A. Terrault, MD, MPH, noted that drinking at least two cups of coffee per day has also been associated with reduced risk of cirrhosis in patients with hepatitis B or hepatitis C infection. So she too is on board the coffee express.
“I’m also a big proponent of recommending coffee. We take so much away from the patients, it’s nice to give them back something, right?” said Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
Diet and exercise
Most of the major gastroenterology professional societies emphasize in their practice guidelines for NAFLD that diet and routine physical activity are mandatory. If sustained, these lifestyle modifications can improve NASH and hepatic fibrosis, as well as reduce the risk of portal hypertension and liver cancer. Dr. Abdelmalek counsels her patients to aim for at least 150 minutes per week of moderate or vigorous aerobic and/or resistance exercise. She doesn’t care about the exercise intensity or type, noting that what she considers to be “a beautifully done intervention trial” in 220 patients over the course of 12 months concluded that both moderate and vigorous exercise achieved a significant reduction in intrahepatic triglyceride content.
“Tailor exercise to what patients can do, what they enjoy, and what they can sustain,” she advised.
She identifies and addresses all modifiable risk factors for NAFLD, including hypertension, diabetes, abdominal obesity, smoking, excessive alcohol intake, obstructive sleep apnea, and an unhealthy diet high in fat, red meat, and fructose.
“The primary message I tell my patients interested in dieting is: I want you to find the right approach for you. There is no right or wrong answer. For some of my patients, it’s intermittent fasting and having their first meal at 2 or 3 o’clock in the afternoon. For others it’s a Weight Watchers approach, or a Mediterranean diet, or it’s high protein. The bottom line of my approach is a gravitation away from excess carbohydrates and fats, and beyond that if I can achieve weight loss through caloric restriction or intermittent fasting, I try to tailor that to my patients’ preferences. I do send them to nutritionists,” the gastroenterologist said.
A 7%-10% weight loss has been shown to result in resolution of NASH in 64%-90% of patients. However, only about 10% of patients who achieve clinically meaningful weight loss short term are able to maintain it at 1 year, so ongoing follow-up is essential.
At present there is no FDA-approved therapy for NAFLD/NASH. Beyond diet and exercise – and coffee – there is the option of antiobesity weight-loss drug therapy, which is about as effective as successful lifestyle modification, and bariatric surgery, which is dramatically effective. French surgeons recently reported in a prospective single-center study of 180 severely obese patients with NASH who underwent bariatric surgery that, at 5 years’ follow-up, 84% of them had resolution of NASH with no worsening of liver fibrosis. Indeed, 63% of patients with mild fibrosis at baseline experienced complete resolution of their fibrosis at follow-up, as did 46% of those with more severe baseline bridging fibrosis.
Dr. Abdelmalek reported having no financial conflicts of interest regarding her presentation.
FROM GUILD 2021
AstraZeneca COVID vaccine: Clotting disorder mechanism revealed?
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.