User login
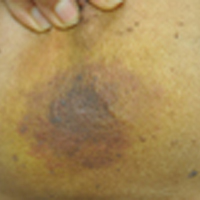
Enlarging Breast Lesion
The Diagnosis: Radiation-Associated Angiosarcoma
At the time of presentation, a 4-mm lesional punch biopsy was obtained (Figure), which revealed an epithelioid neoplasm within the dermis expressing CD31 and CD34, and staining negatively for S-100, CD45, and estrogen and progesterone receptors. The histologic and immunophenotypic findings were compatible with the diagnosis of angiosarcoma. Given the patient’s history of radiation for breast carcinoma several years ago, this tumor was consistent with radiation-associated angiosarcoma (RAAS).
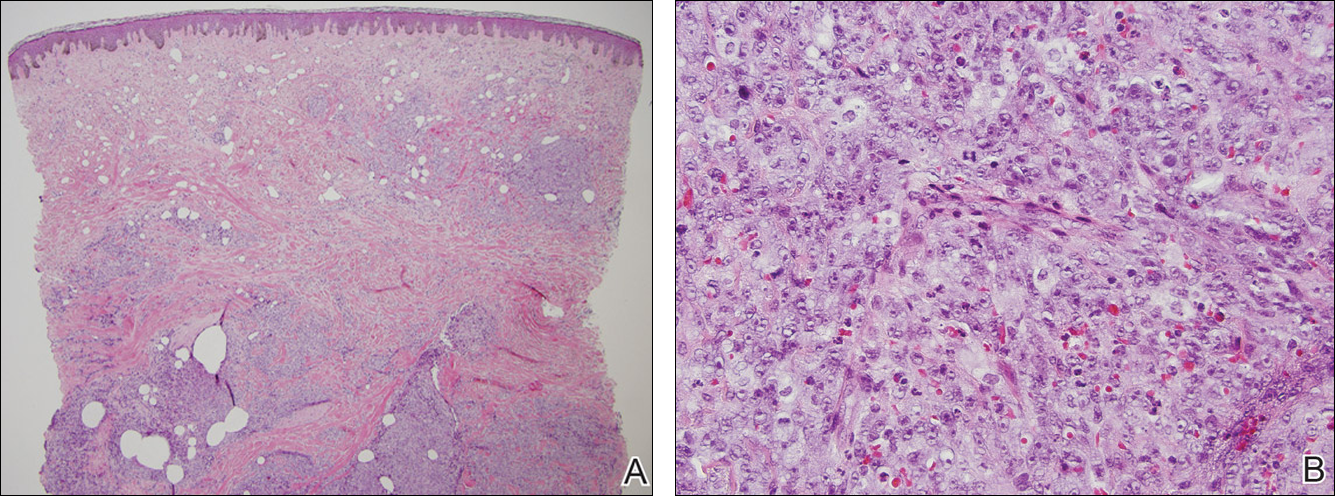
Development of secondary angiosarcoma has been linked to both prior radiation (RAAS) and chronic lymphedema (Stewart-Treves syndrome).1 Radiation-associated angiosarcoma is defined as a “pathologically confirmed breast or chest wall angiosarcoma arising within a previously irradiated field.”2 The incidence of RAAS is estimated to be 0.9 per 1000 individuals following radiation treatment of breast cancer over the subsequent 15 years and a mean time from radiation to development of 7 years.1 Incidence is expected to increase in the future due to improved likelihood of surviving early-stage breast carcinoma and the increased use of external beam radiation therapy for management of breast cancer.
Differentiating between primary and secondary angiosarcoma of the breast is important. Although primary breast angiosarcoma usually arises in women aged 30 to 40 years, RAAS tends to arise in older women (mean age, 68 years) and is seen only in those women with prior radiation.2 Additionally, high-level amplification of MYC, a known photo-oncogene, on chromosome 8 is a key genetic alteration of RAAS that helps to distinguish it from primary angiosarcoma, though this variance may be present in only half of RAAS cases.3 Immunohistochemical analysis of tumor cells for MYC expression correlates well with this amplification and also is helpful in distinguishing atypical vascular lesions from RAAS.4 Atypical vascular lesions, similar to RAAS, occur years after radiation exposure and may have a similar clinical presentation. Atypical vascular lesions do not progress to angiosarcoma in reported cases, but clinical and histologic overlap with RAAS make the diagnosis difficult.5 In these cases, analysis with fluorescence in situ hybridization or immunohistochemistry for the MYC amplification is important to differentiate these tumors.6
At the time of presentation, the majority of patients with RAAS of the breast have localized disease, often with a variable presentation. In all known cases, there have been skin changes present, emphasizing the importance of both patient and clinician vigilance on a regular basis in at-risk individuals. In one study, the most common presentation was breast ecchymosis, which was observed in 55% of patients.7 These lesions involve the dermis and are commonly mistaken for benign conditions such as infection or hemorrhage.2 In 2 other studies, RAAS most often manifested as a skin nodule or apparent tumor, closely followed by either a rash or bruiselike presentation.1,2
The overall recommendation for management of patients with ecchymotic skin lesions in previously irradiated regions is to obtain a biopsy specimen for tissue diagnosis. Although there is no standard of care for the management of RAAS, a multidisciplinary approach involving specialists from oncology, surgical oncology, and radiation oncology is recommended. Most often, radical surgery encompassing both the breast parenchyma and the at-risk radiated skin is performed. Extensive surgery has demonstrated the best survival benefits compared to mastectomy alone.7 Chemotherapeutics also may be used as adjuncts to surgery, which have been determined to decrease local recurrence rates but have no proven survival benefits.2 Adverse prognostic factors for survival are tumor size greater than 10 cm and development of local and/or distant metastases.2 Following the diagnosis of RAAS, our patient underwent radical mastectomy with adjuvant chemotherapy and remained disease free 6 months after surgery.
In summary, RAAS is a well-known, albeit relatively uncommon, consequence of radiation therapy. Dermatologists, oncologists, and primary care providers play an important role in recognizing this entity when evaluating patients with ecchymotic lesions as well as nodules or tumors within an irradiated field. Biopsy should be obtained promptly to prevent delay in diagnosis and to expedite referral to appropriate specialists for further evaluation and treatment.
- Seinen JM, Emelie S, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700-2706.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Ginter PS, Mosquera JM, MacDonald TY, et al. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol. 2014;45:709-716.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75-85.
- Fernandez AP, Sun Y, Tubbs RR, et al. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol. 2012;39:234-242.
- Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol. 2012;19:3801-3808.
The Diagnosis: Radiation-Associated Angiosarcoma
At the time of presentation, a 4-mm lesional punch biopsy was obtained (Figure), which revealed an epithelioid neoplasm within the dermis expressing CD31 and CD34, and staining negatively for S-100, CD45, and estrogen and progesterone receptors. The histologic and immunophenotypic findings were compatible with the diagnosis of angiosarcoma. Given the patient’s history of radiation for breast carcinoma several years ago, this tumor was consistent with radiation-associated angiosarcoma (RAAS).

Development of secondary angiosarcoma has been linked to both prior radiation (RAAS) and chronic lymphedema (Stewart-Treves syndrome).1 Radiation-associated angiosarcoma is defined as a “pathologically confirmed breast or chest wall angiosarcoma arising within a previously irradiated field.”2 The incidence of RAAS is estimated to be 0.9 per 1000 individuals following radiation treatment of breast cancer over the subsequent 15 years and a mean time from radiation to development of 7 years.1 Incidence is expected to increase in the future due to improved likelihood of surviving early-stage breast carcinoma and the increased use of external beam radiation therapy for management of breast cancer.
Differentiating between primary and secondary angiosarcoma of the breast is important. Although primary breast angiosarcoma usually arises in women aged 30 to 40 years, RAAS tends to arise in older women (mean age, 68 years) and is seen only in those women with prior radiation.2 Additionally, high-level amplification of MYC, a known photo-oncogene, on chromosome 8 is a key genetic alteration of RAAS that helps to distinguish it from primary angiosarcoma, though this variance may be present in only half of RAAS cases.3 Immunohistochemical analysis of tumor cells for MYC expression correlates well with this amplification and also is helpful in distinguishing atypical vascular lesions from RAAS.4 Atypical vascular lesions, similar to RAAS, occur years after radiation exposure and may have a similar clinical presentation. Atypical vascular lesions do not progress to angiosarcoma in reported cases, but clinical and histologic overlap with RAAS make the diagnosis difficult.5 In these cases, analysis with fluorescence in situ hybridization or immunohistochemistry for the MYC amplification is important to differentiate these tumors.6
At the time of presentation, the majority of patients with RAAS of the breast have localized disease, often with a variable presentation. In all known cases, there have been skin changes present, emphasizing the importance of both patient and clinician vigilance on a regular basis in at-risk individuals. In one study, the most common presentation was breast ecchymosis, which was observed in 55% of patients.7 These lesions involve the dermis and are commonly mistaken for benign conditions such as infection or hemorrhage.2 In 2 other studies, RAAS most often manifested as a skin nodule or apparent tumor, closely followed by either a rash or bruiselike presentation.1,2
The overall recommendation for management of patients with ecchymotic skin lesions in previously irradiated regions is to obtain a biopsy specimen for tissue diagnosis. Although there is no standard of care for the management of RAAS, a multidisciplinary approach involving specialists from oncology, surgical oncology, and radiation oncology is recommended. Most often, radical surgery encompassing both the breast parenchyma and the at-risk radiated skin is performed. Extensive surgery has demonstrated the best survival benefits compared to mastectomy alone.7 Chemotherapeutics also may be used as adjuncts to surgery, which have been determined to decrease local recurrence rates but have no proven survival benefits.2 Adverse prognostic factors for survival are tumor size greater than 10 cm and development of local and/or distant metastases.2 Following the diagnosis of RAAS, our patient underwent radical mastectomy with adjuvant chemotherapy and remained disease free 6 months after surgery.
In summary, RAAS is a well-known, albeit relatively uncommon, consequence of radiation therapy. Dermatologists, oncologists, and primary care providers play an important role in recognizing this entity when evaluating patients with ecchymotic lesions as well as nodules or tumors within an irradiated field. Biopsy should be obtained promptly to prevent delay in diagnosis and to expedite referral to appropriate specialists for further evaluation and treatment.
The Diagnosis: Radiation-Associated Angiosarcoma
At the time of presentation, a 4-mm lesional punch biopsy was obtained (Figure), which revealed an epithelioid neoplasm within the dermis expressing CD31 and CD34, and staining negatively for S-100, CD45, and estrogen and progesterone receptors. The histologic and immunophenotypic findings were compatible with the diagnosis of angiosarcoma. Given the patient’s history of radiation for breast carcinoma several years ago, this tumor was consistent with radiation-associated angiosarcoma (RAAS).

Development of secondary angiosarcoma has been linked to both prior radiation (RAAS) and chronic lymphedema (Stewart-Treves syndrome).1 Radiation-associated angiosarcoma is defined as a “pathologically confirmed breast or chest wall angiosarcoma arising within a previously irradiated field.”2 The incidence of RAAS is estimated to be 0.9 per 1000 individuals following radiation treatment of breast cancer over the subsequent 15 years and a mean time from radiation to development of 7 years.1 Incidence is expected to increase in the future due to improved likelihood of surviving early-stage breast carcinoma and the increased use of external beam radiation therapy for management of breast cancer.
Differentiating between primary and secondary angiosarcoma of the breast is important. Although primary breast angiosarcoma usually arises in women aged 30 to 40 years, RAAS tends to arise in older women (mean age, 68 years) and is seen only in those women with prior radiation.2 Additionally, high-level amplification of MYC, a known photo-oncogene, on chromosome 8 is a key genetic alteration of RAAS that helps to distinguish it from primary angiosarcoma, though this variance may be present in only half of RAAS cases.3 Immunohistochemical analysis of tumor cells for MYC expression correlates well with this amplification and also is helpful in distinguishing atypical vascular lesions from RAAS.4 Atypical vascular lesions, similar to RAAS, occur years after radiation exposure and may have a similar clinical presentation. Atypical vascular lesions do not progress to angiosarcoma in reported cases, but clinical and histologic overlap with RAAS make the diagnosis difficult.5 In these cases, analysis with fluorescence in situ hybridization or immunohistochemistry for the MYC amplification is important to differentiate these tumors.6
At the time of presentation, the majority of patients with RAAS of the breast have localized disease, often with a variable presentation. In all known cases, there have been skin changes present, emphasizing the importance of both patient and clinician vigilance on a regular basis in at-risk individuals. In one study, the most common presentation was breast ecchymosis, which was observed in 55% of patients.7 These lesions involve the dermis and are commonly mistaken for benign conditions such as infection or hemorrhage.2 In 2 other studies, RAAS most often manifested as a skin nodule or apparent tumor, closely followed by either a rash or bruiselike presentation.1,2
The overall recommendation for management of patients with ecchymotic skin lesions in previously irradiated regions is to obtain a biopsy specimen for tissue diagnosis. Although there is no standard of care for the management of RAAS, a multidisciplinary approach involving specialists from oncology, surgical oncology, and radiation oncology is recommended. Most often, radical surgery encompassing both the breast parenchyma and the at-risk radiated skin is performed. Extensive surgery has demonstrated the best survival benefits compared to mastectomy alone.7 Chemotherapeutics also may be used as adjuncts to surgery, which have been determined to decrease local recurrence rates but have no proven survival benefits.2 Adverse prognostic factors for survival are tumor size greater than 10 cm and development of local and/or distant metastases.2 Following the diagnosis of RAAS, our patient underwent radical mastectomy with adjuvant chemotherapy and remained disease free 6 months after surgery.
In summary, RAAS is a well-known, albeit relatively uncommon, consequence of radiation therapy. Dermatologists, oncologists, and primary care providers play an important role in recognizing this entity when evaluating patients with ecchymotic lesions as well as nodules or tumors within an irradiated field. Biopsy should be obtained promptly to prevent delay in diagnosis and to expedite referral to appropriate specialists for further evaluation and treatment.
- Seinen JM, Emelie S, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700-2706.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Ginter PS, Mosquera JM, MacDonald TY, et al. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol. 2014;45:709-716.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75-85.
- Fernandez AP, Sun Y, Tubbs RR, et al. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol. 2012;39:234-242.
- Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol. 2012;19:3801-3808.
- Seinen JM, Emelie S, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700-2706.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Ginter PS, Mosquera JM, MacDonald TY, et al. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol. 2014;45:709-716.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75-85.
- Fernandez AP, Sun Y, Tubbs RR, et al. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol. 2012;39:234-242.
- Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol. 2012;19:3801-3808.

A 75-year-old woman with a history of stage II invasive ductal carcinoma of the right breast presented to the dermatology clinic with an enlarging, indurated, ecchymotic plaque on the inferior aspect of the right breast of 2 months’ duration. The patient underwent a lumpectomy, radiation, and adjuvant chemotherapy 13 years prior to presentation. Review of systems was otherwise noncontributory.
Painful Nail with Longitudinal Erythronychia
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
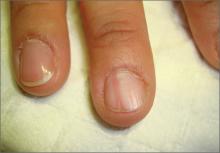
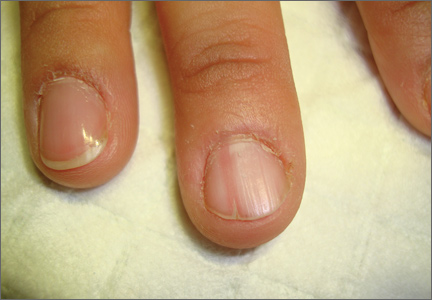
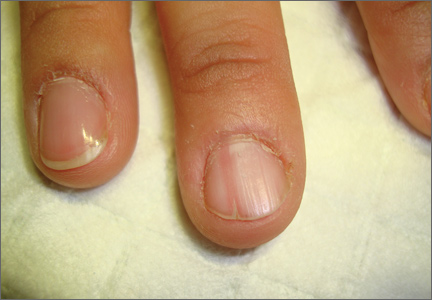
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
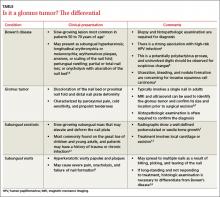
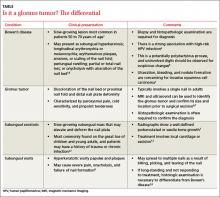
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
Patients report relief of symptoms following excision, although it may take several weeks for the pain to resolve completely.1 The rate of recurrence following excision is estimated at 10% to 20%.1 This may be due to incomplete excision or the development of a new lesion. Therefore, patients should be re-evaluated and considered for possible re-exploration if symptoms return or persist for more than 3 months after the excision.13
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; thomas.beachkofsky@us.af.mil
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
Patients report relief of symptoms following excision, although it may take several weeks for the pain to resolve completely.1 The rate of recurrence following excision is estimated at 10% to 20%.1 This may be due to incomplete excision or the development of a new lesion. Therefore, patients should be re-evaluated and considered for possible re-exploration if symptoms return or persist for more than 3 months after the excision.13
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; thomas.beachkofsky@us.af.mil
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
Patients report relief of symptoms following excision, although it may take several weeks for the pain to resolve completely.1 The rate of recurrence following excision is estimated at 10% to 20%.1 This may be due to incomplete excision or the development of a new lesion. Therefore, patients should be re-evaluated and considered for possible re-exploration if symptoms return or persist for more than 3 months after the excision.13
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; thomas.beachkofsky@us.af.mil
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
Painful nail with longitudinal erythronychia
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting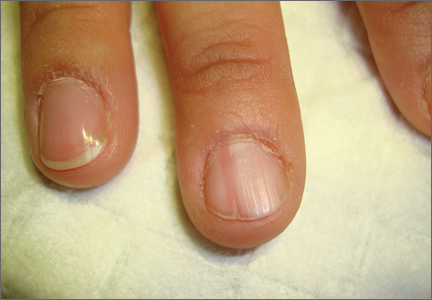
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; thomas.beachkofsky@us.af.mil
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; thomas.beachkofsky@us.af.mil
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; thomas.beachkofsky@us.af.mil
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
Pruritic rash on trunk
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 48-YEAR-OLD HISPANIC MAN came into our dermatology clinic with a 2-month history of a pruritic rash that was confined mainly to the trunk. Prior to this visit, he had tried topical corticosteroids and antifungals, but they had not helped.
His trunk showed erythematous macules and reticulate patches with interspersed thin urticarial plaques without scale (FIGURE). Given that the patient had no vesicles or lichenification (which one would expect with eczematous dermatitis) and that the topical steroids did not provide any relief, we performed a biopsy.
FIGURE
Erythematous macules and reticulate patches without scale
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Secondary syphilis
Our patient’s punch biopsy showed an unremarkable epidermis but a superficial perivascular infiltrate. On higher magnification, the infiltrate proved to be predominately plasma cells. After further investigation and interview, the patient revealed a history of unprotected sex with multiple women; his rapid plasma reagin (RPR) was elevated with a titer of 1:256. Specific treponemal antibody tests confirmed the diagnosis of syphilis. The patient’s human immunodeficiency virus (HIV) test was negative.
Syphilis, a systemic disease with varied dermatological findings, has been described as “the great imitator.” Although it is on the list of differential diagnoses for multiple conditions, it is rarely the culprit—especially given how uncommon it has become in 20th century medicine. With the worldwide HIV epidemic, safe sex programs effectively dropped the incidence of primary and secondary syphilis in the United States to the lowest in recorded history in the year 2001 at 2.17/100,000.1
More recently, however, this infection appears to be making a comeback. Beginning in 2002, its incidence started to rise, reaching 4.6/100,000 in 2009.1
Secondary syphilis usually appears 6 to 8 weeks after the appearance of the primary chancre. As the pathogen spreads into the bloodstream, a host of systemic symptoms may occur, including an influenza-like illness of body aches, fever, fatigue, and headache. While the exanthem of secondary syphilis is traditionally described as a nonpruritic, papular eruption involving the trunk, extremities, face, palms, and soles, a number of cutaneous manifestations are possible, including localized alopecia and syphilids.2 In addition, a number of atypical cases are described in the literature, although none has described an urticarial variant, as seen in our case.
The differential included urticaria and lupus erythematosus
The differential diagnosis for our patient included urticaria, telangiectasia macularis eruptiva perstans, subacute cutaneous lupus erythematosus, and mycosis fungoides. All of these conditions can be distinguished from secondary syphilis by serology and/or biopsy.
Urticaria is a common dermatologic problem with numerous etiologies. It presents as pruritic raised edematous erythematous wheels that blanch with pressure. Although it affects 15% to 25% of the general population at least once in their lives,3 it may progress to life-threatening anaphylaxis. Isolated acute urticaria usually responds to oral antihistamines.
Telangiectasia macularis eruptiva perstans is a form of cutaneous mastocytosis that appears as persistent macules that are red to brown and may exhibit telangiectasia.4 Systemic disease may be evaluated using serum tryptase levels. Patients without systemic disease are managed with oral antihistamines.
Subacute cutaneous lupus erythematosus (SCLE) often presents precipitously as erythematous maculopapular lesions that may coalesce into annular or papulosquamous plaques.5 It has a predilection for sun-exposed areas and is more common in women.5 Multiple drugs have been associated with SCLE, including phenytoin, calcium channel blockers, and thiazide diuretics.6 Treatment consists of discontinuing the offending drug (if one is identified), avoiding (or protecting against) sun exposure, and using topical corticosteroids, oral corticosteroids, and/or antimalarials.
Mycosis fungoides is a form of primary cutaneous T-cell lymphoma that more commonly affects males.7 It begins as erythematous pruritic patches that typically involve the sun-spared areas of the lower abdomen and proximal extremities; it progresses slowly.7 As lesions develop into plaques, they may appear psoriasiform. Treatment depends on the stage of the disease and ranges from topical corticosteroids to systemic radiation and chemotherapy.8
Serology greatly aids diagnosis
If syphilis is not treated during the primary stage, it may progress directly into latency or into the second stage of infection. Preventing progression into late findings hinges upon proper diagnostics. While the initial suspicion should begin with history and physical examination, serology is most frequently used to confirm the presence of Treponema pallidum.
It may take as long as 3 weeks after the appearance of the primary chancre for serology to become positive.9 During this interval, directly visualizing the pathogen via dark-field microscopy may be useful. Following this interval, nontreponemal serology such as the RPR and venereal disease research laboratory (VDRL) are frequently used as the initial serology. These rapid tests detect the antibody to cardiolipin and are relatively inexpensive.
Infection is confirmed with specific treponemal tests, including the fluorescent treponemal antibody absorption (FTA-abs), treponemal enzyme immunoassay, and treponemal particle agglutination tests. These tests are specific for T pallidum and confirm a positive RPR or VDRL. However, specific treponemal tests will not differentiate syphilis from nonvenereal treponematoses such as Bejel, Yaws, and Pinta.10
The common belief is that nontreponemal tests may become negative after successful treatment, and treponemal tests will remain positive indefinitely after successful treatment. However, a study found that 28% of patients treated during primary syphilis and 44% of patients treated during secondary syphilis had positive nontreponemal tests 3 years after treatment.11 In the same study, nearly a quarter of patients treated during primary syphilis no longer had positive FTA-abs 3 years after treatment.11
Penicillin remains the first-line treatment
Once the presence of T pallidum is confirmed, treatment depends on the stage of infection (TABLE). In nonallergic patients, benzathine penicillin G is the standard of care. It should be administered as a single intramuscular (IM) dose of 2.4 million units during primary, secondary, and early latent syphilis12 (strength of recommendation [SOR]: C). Late latent and tertiary syphilis require 3 to 4 weeks of penicillin therapy that is usually achieved with 3 weekly IM injections of 2.4 million units benzathine penicillin G12 (SOR: C). Owing largely to the selective permeability of the blood-brain barrier, neurosyphilis requires a larger dose of 3 million to 4 million units intravenous aqueous crystalline benzathine penicillin every 4 hours for 10 to 14 days12 (SOR: C).
Penicillin desensitization should be considered in penicillin-allergic patients, particularly in those who are pregnant or have HIV infection.12
Treatment success can be determined by a 4-fold decline in RPR/VDRL titer over a period of 3 to 6 months after treatment. During the first 24 hours after initial treatment, patients may develop an acute febrile illness known as the Jarisch-Herxheimer reaction. This is largely the result of massive lysis of the pathogen, spilling large quantities of inflammatory cytokines into the bloodstream.13
Table
Syphilis treatment by stage of infection12
| Stage | Time since exposure | Treatment |
|---|---|---|
| Primary | 10-90 days | Adults Children |
| Secondary | 4-10 weeks | Adults Children |
| Early latent | After primary or secondary stages, <1 year | Adults Children |
| Late latent | >1 year of no symptoms | Adults Children |
| Tertiary | Months to years | Adults See above |
| Neurosyphilis (at any stage) | Any time after infection | Aqueous crystalline penicillin G 18-24 million units/d, administered as 3-4 million units IV every 4 hours or continuous infusion, for 10-14 days Alternative |
| IM, intramuscular; IV, intravenous. | ||
Our patient’s symptoms resolved with penicillin
Given the nebulous history of exposure, we treated the patient as having late latent syphilis (rather than secondary syphilis) and administered 2.4 million units benzathine penicillin G IM weekly for 3 weeks. After this treatment course, the pruritic lesions resolved and the patient’s RPR titer dropped to 1:8 in 3 months.
Our case demonstrates a unique atypical presentation of secondary syphilis. To our knowledge, there is no mention of secondary syphilis mimicking urticaria in the literature. The pruritus that accompanied the lesions was also atypical; however, one study noted 42% of patients experience this symptom in secondary syphilis.14 Fortunately, serological studies confirmed the diagnosis and the patient’s symptoms resolved with standard therapy.
CORRESPONDENCE
Peter L. Mattei, MD, 641 Bainbridge Drive, Mullica Hill, NJ 08062; peterlmattei@gmail.com
1. Centers for Disease Control and Prevention. Summary of notifiable diseases: United States, 2009. MMWR Morb Mortal Wkly Rep. 2011;58:1-100.
2. Bolognia JL, Jorizzo JL, Rapini RP. eds. Dermatology (e-dition). 2nd ed. Mosby Elsevier; 2008. Available at: http://www.expertconsultbook.com/expertconsult/op/book.do?method=display&type=bookPage&decorator=none&eid=4-u1.0-B978-1-4160-2999-1..50002-3&isbn=978-1-4160-2999-1. Accessed March 29, 2010.
3. Fonacier LS, Dreskin SC, Leung DY. Allergic skin diseases. J Allergy Clin Immunol. 2010;125(2 suppl 2):S138-S149.
4. Nguyen NQ. Telangiectasia macularis eruptiva perstans. Dermatol Online J. 2004;10:1.-
5. Wechsler HL. Cutaneous disease in systemic lupus erythematosus. Clin Dermatol. 1985;3:79-87.
6. Rothfield N, Sontheimer RD, Bernstein M. Lupus erythematosus: systemic and cutaneous manifestations. Clin Dermatol. 2006;24:348-362.
7. Galper SL, Smith BD, Wilson LD. Diagnosis and management of mycosis fungoides. Oncology (Williston Park). 2010;24:491-501.
8. Lansigan F, Foss FM. Current and emerging treatment strategies for cutaneous T-cell lymphoma. Drugs. 2010;70:273-286.
9. Eccleston K, Collins L, Higgins SP. Primary syphilis. Int J STD AIDS. 2008;19:145-151.
10. Koff AB, Rosen T. Nonvenereal treponematoses: yaws, endemic syphilis, and pinta. J Am Acad Dermatol. 1993;29:519-535.
11. Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114:1005-1009.
12. Workowski KA, Berman SM. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
13. Mandell GL, Bennet JE, Dolin R. Principles and Practice of Infectious Diseases. 6th ed. New York, NY: Elsevier Health Sciences; 2005:2768–2784.
14. Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 48-YEAR-OLD HISPANIC MAN came into our dermatology clinic with a 2-month history of a pruritic rash that was confined mainly to the trunk. Prior to this visit, he had tried topical corticosteroids and antifungals, but they had not helped.
His trunk showed erythematous macules and reticulate patches with interspersed thin urticarial plaques without scale (FIGURE). Given that the patient had no vesicles or lichenification (which one would expect with eczematous dermatitis) and that the topical steroids did not provide any relief, we performed a biopsy.
FIGURE
Erythematous macules and reticulate patches without scale
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Secondary syphilis
Our patient’s punch biopsy showed an unremarkable epidermis but a superficial perivascular infiltrate. On higher magnification, the infiltrate proved to be predominately plasma cells. After further investigation and interview, the patient revealed a history of unprotected sex with multiple women; his rapid plasma reagin (RPR) was elevated with a titer of 1:256. Specific treponemal antibody tests confirmed the diagnosis of syphilis. The patient’s human immunodeficiency virus (HIV) test was negative.
Syphilis, a systemic disease with varied dermatological findings, has been described as “the great imitator.” Although it is on the list of differential diagnoses for multiple conditions, it is rarely the culprit—especially given how uncommon it has become in 20th century medicine. With the worldwide HIV epidemic, safe sex programs effectively dropped the incidence of primary and secondary syphilis in the United States to the lowest in recorded history in the year 2001 at 2.17/100,000.1
More recently, however, this infection appears to be making a comeback. Beginning in 2002, its incidence started to rise, reaching 4.6/100,000 in 2009.1
Secondary syphilis usually appears 6 to 8 weeks after the appearance of the primary chancre. As the pathogen spreads into the bloodstream, a host of systemic symptoms may occur, including an influenza-like illness of body aches, fever, fatigue, and headache. While the exanthem of secondary syphilis is traditionally described as a nonpruritic, papular eruption involving the trunk, extremities, face, palms, and soles, a number of cutaneous manifestations are possible, including localized alopecia and syphilids.2 In addition, a number of atypical cases are described in the literature, although none has described an urticarial variant, as seen in our case.
The differential included urticaria and lupus erythematosus
The differential diagnosis for our patient included urticaria, telangiectasia macularis eruptiva perstans, subacute cutaneous lupus erythematosus, and mycosis fungoides. All of these conditions can be distinguished from secondary syphilis by serology and/or biopsy.
Urticaria is a common dermatologic problem with numerous etiologies. It presents as pruritic raised edematous erythematous wheels that blanch with pressure. Although it affects 15% to 25% of the general population at least once in their lives,3 it may progress to life-threatening anaphylaxis. Isolated acute urticaria usually responds to oral antihistamines.
Telangiectasia macularis eruptiva perstans is a form of cutaneous mastocytosis that appears as persistent macules that are red to brown and may exhibit telangiectasia.4 Systemic disease may be evaluated using serum tryptase levels. Patients without systemic disease are managed with oral antihistamines.
Subacute cutaneous lupus erythematosus (SCLE) often presents precipitously as erythematous maculopapular lesions that may coalesce into annular or papulosquamous plaques.5 It has a predilection for sun-exposed areas and is more common in women.5 Multiple drugs have been associated with SCLE, including phenytoin, calcium channel blockers, and thiazide diuretics.6 Treatment consists of discontinuing the offending drug (if one is identified), avoiding (or protecting against) sun exposure, and using topical corticosteroids, oral corticosteroids, and/or antimalarials.
Mycosis fungoides is a form of primary cutaneous T-cell lymphoma that more commonly affects males.7 It begins as erythematous pruritic patches that typically involve the sun-spared areas of the lower abdomen and proximal extremities; it progresses slowly.7 As lesions develop into plaques, they may appear psoriasiform. Treatment depends on the stage of the disease and ranges from topical corticosteroids to systemic radiation and chemotherapy.8
Serology greatly aids diagnosis
If syphilis is not treated during the primary stage, it may progress directly into latency or into the second stage of infection. Preventing progression into late findings hinges upon proper diagnostics. While the initial suspicion should begin with history and physical examination, serology is most frequently used to confirm the presence of Treponema pallidum.
It may take as long as 3 weeks after the appearance of the primary chancre for serology to become positive.9 During this interval, directly visualizing the pathogen via dark-field microscopy may be useful. Following this interval, nontreponemal serology such as the RPR and venereal disease research laboratory (VDRL) are frequently used as the initial serology. These rapid tests detect the antibody to cardiolipin and are relatively inexpensive.
Infection is confirmed with specific treponemal tests, including the fluorescent treponemal antibody absorption (FTA-abs), treponemal enzyme immunoassay, and treponemal particle agglutination tests. These tests are specific for T pallidum and confirm a positive RPR or VDRL. However, specific treponemal tests will not differentiate syphilis from nonvenereal treponematoses such as Bejel, Yaws, and Pinta.10
The common belief is that nontreponemal tests may become negative after successful treatment, and treponemal tests will remain positive indefinitely after successful treatment. However, a study found that 28% of patients treated during primary syphilis and 44% of patients treated during secondary syphilis had positive nontreponemal tests 3 years after treatment.11 In the same study, nearly a quarter of patients treated during primary syphilis no longer had positive FTA-abs 3 years after treatment.11
Penicillin remains the first-line treatment
Once the presence of T pallidum is confirmed, treatment depends on the stage of infection (TABLE). In nonallergic patients, benzathine penicillin G is the standard of care. It should be administered as a single intramuscular (IM) dose of 2.4 million units during primary, secondary, and early latent syphilis12 (strength of recommendation [SOR]: C). Late latent and tertiary syphilis require 3 to 4 weeks of penicillin therapy that is usually achieved with 3 weekly IM injections of 2.4 million units benzathine penicillin G12 (SOR: C). Owing largely to the selective permeability of the blood-brain barrier, neurosyphilis requires a larger dose of 3 million to 4 million units intravenous aqueous crystalline benzathine penicillin every 4 hours for 10 to 14 days12 (SOR: C).
Penicillin desensitization should be considered in penicillin-allergic patients, particularly in those who are pregnant or have HIV infection.12
Treatment success can be determined by a 4-fold decline in RPR/VDRL titer over a period of 3 to 6 months after treatment. During the first 24 hours after initial treatment, patients may develop an acute febrile illness known as the Jarisch-Herxheimer reaction. This is largely the result of massive lysis of the pathogen, spilling large quantities of inflammatory cytokines into the bloodstream.13
Table
Syphilis treatment by stage of infection12
| Stage | Time since exposure | Treatment |
|---|---|---|
| Primary | 10-90 days | Adults Children |
| Secondary | 4-10 weeks | Adults Children |
| Early latent | After primary or secondary stages, <1 year | Adults Children |
| Late latent | >1 year of no symptoms | Adults Children |
| Tertiary | Months to years | Adults See above |
| Neurosyphilis (at any stage) | Any time after infection | Aqueous crystalline penicillin G 18-24 million units/d, administered as 3-4 million units IV every 4 hours or continuous infusion, for 10-14 days Alternative |
| IM, intramuscular; IV, intravenous. | ||
Our patient’s symptoms resolved with penicillin
Given the nebulous history of exposure, we treated the patient as having late latent syphilis (rather than secondary syphilis) and administered 2.4 million units benzathine penicillin G IM weekly for 3 weeks. After this treatment course, the pruritic lesions resolved and the patient’s RPR titer dropped to 1:8 in 3 months.
Our case demonstrates a unique atypical presentation of secondary syphilis. To our knowledge, there is no mention of secondary syphilis mimicking urticaria in the literature. The pruritus that accompanied the lesions was also atypical; however, one study noted 42% of patients experience this symptom in secondary syphilis.14 Fortunately, serological studies confirmed the diagnosis and the patient’s symptoms resolved with standard therapy.
CORRESPONDENCE
Peter L. Mattei, MD, 641 Bainbridge Drive, Mullica Hill, NJ 08062; peterlmattei@gmail.com
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 48-YEAR-OLD HISPANIC MAN came into our dermatology clinic with a 2-month history of a pruritic rash that was confined mainly to the trunk. Prior to this visit, he had tried topical corticosteroids and antifungals, but they had not helped.
His trunk showed erythematous macules and reticulate patches with interspersed thin urticarial plaques without scale (FIGURE). Given that the patient had no vesicles or lichenification (which one would expect with eczematous dermatitis) and that the topical steroids did not provide any relief, we performed a biopsy.
FIGURE
Erythematous macules and reticulate patches without scale
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Secondary syphilis
Our patient’s punch biopsy showed an unremarkable epidermis but a superficial perivascular infiltrate. On higher magnification, the infiltrate proved to be predominately plasma cells. After further investigation and interview, the patient revealed a history of unprotected sex with multiple women; his rapid plasma reagin (RPR) was elevated with a titer of 1:256. Specific treponemal antibody tests confirmed the diagnosis of syphilis. The patient’s human immunodeficiency virus (HIV) test was negative.
Syphilis, a systemic disease with varied dermatological findings, has been described as “the great imitator.” Although it is on the list of differential diagnoses for multiple conditions, it is rarely the culprit—especially given how uncommon it has become in 20th century medicine. With the worldwide HIV epidemic, safe sex programs effectively dropped the incidence of primary and secondary syphilis in the United States to the lowest in recorded history in the year 2001 at 2.17/100,000.1
More recently, however, this infection appears to be making a comeback. Beginning in 2002, its incidence started to rise, reaching 4.6/100,000 in 2009.1
Secondary syphilis usually appears 6 to 8 weeks after the appearance of the primary chancre. As the pathogen spreads into the bloodstream, a host of systemic symptoms may occur, including an influenza-like illness of body aches, fever, fatigue, and headache. While the exanthem of secondary syphilis is traditionally described as a nonpruritic, papular eruption involving the trunk, extremities, face, palms, and soles, a number of cutaneous manifestations are possible, including localized alopecia and syphilids.2 In addition, a number of atypical cases are described in the literature, although none has described an urticarial variant, as seen in our case.
The differential included urticaria and lupus erythematosus
The differential diagnosis for our patient included urticaria, telangiectasia macularis eruptiva perstans, subacute cutaneous lupus erythematosus, and mycosis fungoides. All of these conditions can be distinguished from secondary syphilis by serology and/or biopsy.
Urticaria is a common dermatologic problem with numerous etiologies. It presents as pruritic raised edematous erythematous wheels that blanch with pressure. Although it affects 15% to 25% of the general population at least once in their lives,3 it may progress to life-threatening anaphylaxis. Isolated acute urticaria usually responds to oral antihistamines.
Telangiectasia macularis eruptiva perstans is a form of cutaneous mastocytosis that appears as persistent macules that are red to brown and may exhibit telangiectasia.4 Systemic disease may be evaluated using serum tryptase levels. Patients without systemic disease are managed with oral antihistamines.
Subacute cutaneous lupus erythematosus (SCLE) often presents precipitously as erythematous maculopapular lesions that may coalesce into annular or papulosquamous plaques.5 It has a predilection for sun-exposed areas and is more common in women.5 Multiple drugs have been associated with SCLE, including phenytoin, calcium channel blockers, and thiazide diuretics.6 Treatment consists of discontinuing the offending drug (if one is identified), avoiding (or protecting against) sun exposure, and using topical corticosteroids, oral corticosteroids, and/or antimalarials.
Mycosis fungoides is a form of primary cutaneous T-cell lymphoma that more commonly affects males.7 It begins as erythematous pruritic patches that typically involve the sun-spared areas of the lower abdomen and proximal extremities; it progresses slowly.7 As lesions develop into plaques, they may appear psoriasiform. Treatment depends on the stage of the disease and ranges from topical corticosteroids to systemic radiation and chemotherapy.8
Serology greatly aids diagnosis
If syphilis is not treated during the primary stage, it may progress directly into latency or into the second stage of infection. Preventing progression into late findings hinges upon proper diagnostics. While the initial suspicion should begin with history and physical examination, serology is most frequently used to confirm the presence of Treponema pallidum.
It may take as long as 3 weeks after the appearance of the primary chancre for serology to become positive.9 During this interval, directly visualizing the pathogen via dark-field microscopy may be useful. Following this interval, nontreponemal serology such as the RPR and venereal disease research laboratory (VDRL) are frequently used as the initial serology. These rapid tests detect the antibody to cardiolipin and are relatively inexpensive.
Infection is confirmed with specific treponemal tests, including the fluorescent treponemal antibody absorption (FTA-abs), treponemal enzyme immunoassay, and treponemal particle agglutination tests. These tests are specific for T pallidum and confirm a positive RPR or VDRL. However, specific treponemal tests will not differentiate syphilis from nonvenereal treponematoses such as Bejel, Yaws, and Pinta.10
The common belief is that nontreponemal tests may become negative after successful treatment, and treponemal tests will remain positive indefinitely after successful treatment. However, a study found that 28% of patients treated during primary syphilis and 44% of patients treated during secondary syphilis had positive nontreponemal tests 3 years after treatment.11 In the same study, nearly a quarter of patients treated during primary syphilis no longer had positive FTA-abs 3 years after treatment.11
Penicillin remains the first-line treatment
Once the presence of T pallidum is confirmed, treatment depends on the stage of infection (TABLE). In nonallergic patients, benzathine penicillin G is the standard of care. It should be administered as a single intramuscular (IM) dose of 2.4 million units during primary, secondary, and early latent syphilis12 (strength of recommendation [SOR]: C). Late latent and tertiary syphilis require 3 to 4 weeks of penicillin therapy that is usually achieved with 3 weekly IM injections of 2.4 million units benzathine penicillin G12 (SOR: C). Owing largely to the selective permeability of the blood-brain barrier, neurosyphilis requires a larger dose of 3 million to 4 million units intravenous aqueous crystalline benzathine penicillin every 4 hours for 10 to 14 days12 (SOR: C).
Penicillin desensitization should be considered in penicillin-allergic patients, particularly in those who are pregnant or have HIV infection.12
Treatment success can be determined by a 4-fold decline in RPR/VDRL titer over a period of 3 to 6 months after treatment. During the first 24 hours after initial treatment, patients may develop an acute febrile illness known as the Jarisch-Herxheimer reaction. This is largely the result of massive lysis of the pathogen, spilling large quantities of inflammatory cytokines into the bloodstream.13
Table
Syphilis treatment by stage of infection12
| Stage | Time since exposure | Treatment |
|---|---|---|
| Primary | 10-90 days | Adults Children |
| Secondary | 4-10 weeks | Adults Children |
| Early latent | After primary or secondary stages, <1 year | Adults Children |
| Late latent | >1 year of no symptoms | Adults Children |
| Tertiary | Months to years | Adults See above |
| Neurosyphilis (at any stage) | Any time after infection | Aqueous crystalline penicillin G 18-24 million units/d, administered as 3-4 million units IV every 4 hours or continuous infusion, for 10-14 days Alternative |
| IM, intramuscular; IV, intravenous. | ||
Our patient’s symptoms resolved with penicillin
Given the nebulous history of exposure, we treated the patient as having late latent syphilis (rather than secondary syphilis) and administered 2.4 million units benzathine penicillin G IM weekly for 3 weeks. After this treatment course, the pruritic lesions resolved and the patient’s RPR titer dropped to 1:8 in 3 months.
Our case demonstrates a unique atypical presentation of secondary syphilis. To our knowledge, there is no mention of secondary syphilis mimicking urticaria in the literature. The pruritus that accompanied the lesions was also atypical; however, one study noted 42% of patients experience this symptom in secondary syphilis.14 Fortunately, serological studies confirmed the diagnosis and the patient’s symptoms resolved with standard therapy.
CORRESPONDENCE
Peter L. Mattei, MD, 641 Bainbridge Drive, Mullica Hill, NJ 08062; peterlmattei@gmail.com
1. Centers for Disease Control and Prevention. Summary of notifiable diseases: United States, 2009. MMWR Morb Mortal Wkly Rep. 2011;58:1-100.
2. Bolognia JL, Jorizzo JL, Rapini RP. eds. Dermatology (e-dition). 2nd ed. Mosby Elsevier; 2008. Available at: http://www.expertconsultbook.com/expertconsult/op/book.do?method=display&type=bookPage&decorator=none&eid=4-u1.0-B978-1-4160-2999-1..50002-3&isbn=978-1-4160-2999-1. Accessed March 29, 2010.
3. Fonacier LS, Dreskin SC, Leung DY. Allergic skin diseases. J Allergy Clin Immunol. 2010;125(2 suppl 2):S138-S149.
4. Nguyen NQ. Telangiectasia macularis eruptiva perstans. Dermatol Online J. 2004;10:1.-
5. Wechsler HL. Cutaneous disease in systemic lupus erythematosus. Clin Dermatol. 1985;3:79-87.
6. Rothfield N, Sontheimer RD, Bernstein M. Lupus erythematosus: systemic and cutaneous manifestations. Clin Dermatol. 2006;24:348-362.
7. Galper SL, Smith BD, Wilson LD. Diagnosis and management of mycosis fungoides. Oncology (Williston Park). 2010;24:491-501.
8. Lansigan F, Foss FM. Current and emerging treatment strategies for cutaneous T-cell lymphoma. Drugs. 2010;70:273-286.
9. Eccleston K, Collins L, Higgins SP. Primary syphilis. Int J STD AIDS. 2008;19:145-151.
10. Koff AB, Rosen T. Nonvenereal treponematoses: yaws, endemic syphilis, and pinta. J Am Acad Dermatol. 1993;29:519-535.
11. Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114:1005-1009.
12. Workowski KA, Berman SM. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
13. Mandell GL, Bennet JE, Dolin R. Principles and Practice of Infectious Diseases. 6th ed. New York, NY: Elsevier Health Sciences; 2005:2768–2784.
14. Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164.
1. Centers for Disease Control and Prevention. Summary of notifiable diseases: United States, 2009. MMWR Morb Mortal Wkly Rep. 2011;58:1-100.
2. Bolognia JL, Jorizzo JL, Rapini RP. eds. Dermatology (e-dition). 2nd ed. Mosby Elsevier; 2008. Available at: http://www.expertconsultbook.com/expertconsult/op/book.do?method=display&type=bookPage&decorator=none&eid=4-u1.0-B978-1-4160-2999-1..50002-3&isbn=978-1-4160-2999-1. Accessed March 29, 2010.
3. Fonacier LS, Dreskin SC, Leung DY. Allergic skin diseases. J Allergy Clin Immunol. 2010;125(2 suppl 2):S138-S149.
4. Nguyen NQ. Telangiectasia macularis eruptiva perstans. Dermatol Online J. 2004;10:1.-
5. Wechsler HL. Cutaneous disease in systemic lupus erythematosus. Clin Dermatol. 1985;3:79-87.
6. Rothfield N, Sontheimer RD, Bernstein M. Lupus erythematosus: systemic and cutaneous manifestations. Clin Dermatol. 2006;24:348-362.
7. Galper SL, Smith BD, Wilson LD. Diagnosis and management of mycosis fungoides. Oncology (Williston Park). 2010;24:491-501.
8. Lansigan F, Foss FM. Current and emerging treatment strategies for cutaneous T-cell lymphoma. Drugs. 2010;70:273-286.
9. Eccleston K, Collins L, Higgins SP. Primary syphilis. Int J STD AIDS. 2008;19:145-151.
10. Koff AB, Rosen T. Nonvenereal treponematoses: yaws, endemic syphilis, and pinta. J Am Acad Dermatol. 1993;29:519-535.
11. Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114:1005-1009.
12. Workowski KA, Berman SM. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
13. Mandell GL, Bennet JE, Dolin R. Principles and Practice of Infectious Diseases. 6th ed. New York, NY: Elsevier Health Sciences; 2005:2768–2784.
14. Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164.
Alopecia with perifollicular papules and pustules
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 23-Year-Old African American Man sought care at our medical center because he had been losing hair over the vertex of his scalp for the past several years. He indicated that his father had early-onset male patterned alopecia. As a result, he considered his hair loss “genetic.” However, he described waxing and waning flares of painful pustules associated with occasional spontaneous bleeding and discharge of purulent material that occurred in the same area as the hair loss.
Physical examination revealed multiple perifollicular papules and pustules on the vertex of his scalp with interspersed patches of alopecia (FIGURE 1). There were no lesions elsewhere on his body and his past medical history was otherwise unremarkable.
FIGURE 1
Alopecia with a painful twist
This 23-year-old patient said that he had spontaneous bleeding and discharge of purulent material in the area of his hair loss.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU MANAGE THIS PATIENT?
Diagnosis: Folliculitis decalvans
Folliculitis decalvans (FD) is a highly inflammatory form of scarring alopecia characterized by inflammatory perifollicular papules and pustules. The term scarring alopecia refers to the fact that the follicular epithelium has been replaced by connective tissue, ultimately resulting in permanent hair loss. This manifests clinically as patches of skin without terminal or vellus hairs, whereas a nonscarring alopecia would demonstrate preservation of the vellus hairs. Left untreated, advancing permanent hair loss ensues and may result in an end-stage pattern of tufted folliculitis or polytrichia, where interspersed dilated follicular openings house multiple hairs.
Affected areas commonly include the vertex and occipital scalp. Common symptoms include pain, itching, burning, and occasionally spontaneous bleeding or discharge of purulent material.1
FD generally occurs in young and middle-aged African Americans with a slight predominance in males. It accounts for 11% of all primary scarring alopecias.2,3 The etiology of this inflammatory process is not fully understood; however, scalp colonization with Staphylococcus aureus has been implicated as a contributing factor.4 Other reports suggest patients may have an altered host immune response and/or genetic predisposition for this condition.2,3
The differential includes various scarring, nonscarring alopecias
Since clinical findings of FD can range from relatively nonspecific mild disease at its onset to the end stage described above, a detailed patient history is needed. The following scarring and nonscarring alopecias should be considered in the diff erential diagnosis: dissecting cellulitis of the scalp, central centrifugal cicatricial alopecia (CCCA), acne keloidalis nuchae, erosive pustular dermatosis, lichen planopilaris (LPP), inflammatory tinea capitis, and secondary syphilis.
Dissecting cellulitis of the scalp is a distinctive, often debilitating disease commonly seen in young adult African American men. It is considered part of the follicular occlusion tetrad that also includes hidradenitis suppurativa, acne conglobata, and pilonidal cysts. It presents as a scarring alopecia with firm scalp nodules that rapidly develop into boggy, fluctuant, oval to linear sinuses that may eventually discharge purulent material.
In contrast to FD, dissecting scalp cellulitis lesions interconnect via sinus tract formation so that pressure on one fluctuant area may result in purulent discharge from perfo-rations several centimeters away.5 Although both dissecting cellulitis and FD are considered primary neutrophilic scarring alopecias, the presence of true sinus tract formation can be a distinguishing finding.
CCCA is the most common form of scarring alopecia among African Americans and is particularly seen among African American women.5 It generally presents on the scalp vertex like FD, but it is much less inflamma-tory and typically causes only mild pruritus or tenderness of the involved areas.
Although numerous theories have been suggested, the etiology is unknown. The pathogenesis is thought to be associated with premature desquamation of the inner root sheath, which can be demonstrated on biopsy. Also seen histologically is lymphocytic perifollicular inflammation and polytrichia.6
Acne keloidalis nuchae is also a scarring alopecia. It is seen most commonly in African American men and presents as keloid-like papules and plaques with occasional pustules characteristically on the occipital scalp and posterior neck. In contrast to FD, acne keloidalis nuchae papules coalesce and may form firm, hairless, protuberant keloid-like plaques that may be painful and cosmetically disfiguring. The cause of acne keloidalis nuchae is unknown.
Shaving or cutting tight curly hair too short and subsequently having the new hair curve back and penetrate the skin may be the precipitating event. Thus, a history of close shaving should make one suspect this diagnosis. Histologic analysis reveals a chronic, predominantly lymphocytic folliculitis with eventual follicular destruction.
Erosive pustular dermatosis is a rare disorder that primarily aff ects the elderly. It is characterized by a chronic amicrobial pustular dermatosis with extensive boggy, crusted, erosive plaques on the scalp resulting in scarring alopecia. Most cases have an onset after the age of 40. Therefore, age of onset may help diff erentiate between erosive pustular dermatosis and FD.
The cause of erosive pustular dermatosis is unknown. It is thought to be related to local trauma, such as chronic sun exposure, occurring months to years prior to the onset of lesions or as an autoimmune process.6 Histologic specimens show nonspecific changes including parakeratosis or hyperkeratotic scale with atrophy or erosion of the epidermis, while an inflammatory infiltrate with lymphocytes and plasma cells is found in the dermis.
LPP is seen more commonly in women than men, and Caucasians are more often aff ected than African Americans. It presents with erythema, perifollicular scale, and scattered patches of scarring alopecia. Half of involved cases develop concomitant clinical features of lichen planus. When present, these characteristics may help distinguish it from FD and other scarring alopecias.6
The etiology of LPP is unknown, but is thought to be similar to the presumed cause of lichen planus: a T-cell?mediated autoimmune response that damages basal keratinocytes.5 Histologic findings include a band-like mononuclear cell infiltrate obscuring the interface between follicular epithelium and dermis at the superficial part of the follicle with occasional interfollicular epidermal changes consistent with lichen planus.
Inflammatory tinea capitis is a common dermatophyte infection of the scalp that aff ects children and adults alike. Typically, it is easily distinguished from FD. However, severe cases may result in a highly inflammatory pustular eruption with alopecia—with or without a kerion—which can make diff erentiation difficult.
In contrast to FD, the alopecia associated with tinea capitis is usually nonscarring, although this depends on the extent and depth of infection. Also, tinea capitis may present with either discrete patches or involve the entire scalp, whereas FD is usually localized to the vertex or occiput (as noted earlier). Correct diagnosis can be accomplished by means of light microscopy and fungal culture.
Secondary syphilis is usually a sexually transmitted disease, but it can also be acquired perinatally. It often presents with a “moth-eaten” alopecia and should be considered when examining patients with patchy alopecia such as that seen in FD. These lesions manifest 3 to 10 weeks after the onset of primary syphilis. Early in its course, the condition is reversible, but if it becomes chronic, the condition will cause a scarring alopecia.
The presence of other stigmata, including a generalized pruritic papulosquamous eruption with involvement of the palms and soles, mucosal lesions ranging from superficial ulcers to large gray plaques, and condylomata lata, should help to diff erentiate syphilis from FD.
Serologic tests such as rapid plasma reagin and venereal disease research laboratory assays are often preferred for routine screening. If the index of suspicion is high, confirmatory testing with direct antibody as-says such as a microhemagglutination assay or fluorescent treponemal antibody absorption test is indicated.
Biopsy is needed for the diagnosis
Two scalp biopsies should be performed to make the diagnosis. Recommended guidelines for sampling the scalp include performance of 4-mm punch biopsies extending into the fat at 2 diff erent clinically active sites.7 One biopsy should be processed for standard horizontal sectioning, but the second biopsy should be bisected vertically, with half sent for histologic examination and the other half for tissue culture (fungal and bacterial). An additional subsequent biopsy for direct immunofluorescence may also be considered if the initial biopsies are nondiagnostic.
Bacterial and fungal cultures collected from an intact pustule on the scalp with a standard culture swab should also be undertaken with pustular disease. If scale is present, a potassium hydroxide examination can help establish the diagnosis of a fungal etiology.
Doxycycline, intralesional corticosteroids are the first line of Tx
Management of FD can be difficult, and long-term treatment is often necessary. You’ll need to explain to patients that their current hair loss is permanent and that the goal of treatment is to decrease inflammation and prevent further balding.
After initial bacterial cultures and sensitivities are obtained, primary treatment is aimed at eliminating S aureus colonization. Often, this requires oral antibiotic therapy, most commonly doxycycline 100 mg twice daily5(strength of recommendation [SOR]: C). Topical antibiotics, however, may be used in mild cases; options include 2% mupirocin, 1% clindamycin, 1.5% fusidic acid, or 2% erythromycin applied twice daily1(SOR: C). In recalcitrant cases, a common treatment regimen includes oral rifampin 300 mg and clindamycin 300 mg twice daily for 10 weeks4(SOR: C).
Adjunctive topical and intralesional corticosteroids may help reduce inflammation and provide symptomatic relief from itching, burning, and pain. Topical class I or II corticosteroids can be used twice daily, whereas intralesional triamcinolone acetonide (combined with topical and/or oral antibiotics) may be administered every 4 to 6 weeks, starting at a concentration of 10 mg/mL1(SOR: C). Oral corticosteroids should only be considered for highly active and rapidly progressive symptoms.
Dapsone may also be considered as a treatment option for FD due to its antimicrobial activity and anti-inflammatory action directed toward neutrophil metabolism. Relapse, however, is frequent after treatment withdrawal1(SOR: C).
Improvement, but anticipated chronicity
We prescribed oral doxycycline 100 mg twice daily for our patient, as well as clobetasol 0.05% topical solution, to be applied to the affected area in the morning and evening.
We told our patient that FD is a chronic relapsing disorder and that while we could not make the condition go away completely, we could control it. We advised the patient to follow up every 2 months for the next 6 months, then every 6 months to ensure there was no progression or need to change the treatment regimen.
The patient’s symptoms improved after the first 2 months. After weaning the patient off doxycycline over a 6-month period, we planned to transition the patient to topical clindamycin solution twice daily.
In some cases, the patient can be weaned off oral antibiotics once the condition is controlled, but for most patients, continuous systemic therapy is needed.
CORRESPONDENCE Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/ SG05D/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; wiscooj@gmail.com
1. Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244.
2. Douwes KE, Landthaler M, Szeimies RM. Simultaneous occur-rence of folliculitis decalvans capillitii in identical twins. Br J Dermatol. 2000;143:195-197.
3. Chandrawansa PH, Giam YC. Folliculitis decalvans-a retrospective study in a tertiary referred center, over five years. Singapore Med J. 2003;44:84-87.
4. Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical histological and therapeutic findings. Br J Dermatol. 1999;140:328-333.
5. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
6. Somani N, Bergfeld WF. Cicatricial alopecia: classification and histopathology. Dermatol Ther. 2008;21:221-237.
7. Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored workshop on cicatricial alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 23-Year-Old African American Man sought care at our medical center because he had been losing hair over the vertex of his scalp for the past several years. He indicated that his father had early-onset male patterned alopecia. As a result, he considered his hair loss “genetic.” However, he described waxing and waning flares of painful pustules associated with occasional spontaneous bleeding and discharge of purulent material that occurred in the same area as the hair loss.
Physical examination revealed multiple perifollicular papules and pustules on the vertex of his scalp with interspersed patches of alopecia (FIGURE 1). There were no lesions elsewhere on his body and his past medical history was otherwise unremarkable.
FIGURE 1
Alopecia with a painful twist
This 23-year-old patient said that he had spontaneous bleeding and discharge of purulent material in the area of his hair loss.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU MANAGE THIS PATIENT?
Diagnosis: Folliculitis decalvans
Folliculitis decalvans (FD) is a highly inflammatory form of scarring alopecia characterized by inflammatory perifollicular papules and pustules. The term scarring alopecia refers to the fact that the follicular epithelium has been replaced by connective tissue, ultimately resulting in permanent hair loss. This manifests clinically as patches of skin without terminal or vellus hairs, whereas a nonscarring alopecia would demonstrate preservation of the vellus hairs. Left untreated, advancing permanent hair loss ensues and may result in an end-stage pattern of tufted folliculitis or polytrichia, where interspersed dilated follicular openings house multiple hairs.
Affected areas commonly include the vertex and occipital scalp. Common symptoms include pain, itching, burning, and occasionally spontaneous bleeding or discharge of purulent material.1
FD generally occurs in young and middle-aged African Americans with a slight predominance in males. It accounts for 11% of all primary scarring alopecias.2,3 The etiology of this inflammatory process is not fully understood; however, scalp colonization with Staphylococcus aureus has been implicated as a contributing factor.4 Other reports suggest patients may have an altered host immune response and/or genetic predisposition for this condition.2,3
The differential includes various scarring, nonscarring alopecias
Since clinical findings of FD can range from relatively nonspecific mild disease at its onset to the end stage described above, a detailed patient history is needed. The following scarring and nonscarring alopecias should be considered in the diff erential diagnosis: dissecting cellulitis of the scalp, central centrifugal cicatricial alopecia (CCCA), acne keloidalis nuchae, erosive pustular dermatosis, lichen planopilaris (LPP), inflammatory tinea capitis, and secondary syphilis.
Dissecting cellulitis of the scalp is a distinctive, often debilitating disease commonly seen in young adult African American men. It is considered part of the follicular occlusion tetrad that also includes hidradenitis suppurativa, acne conglobata, and pilonidal cysts. It presents as a scarring alopecia with firm scalp nodules that rapidly develop into boggy, fluctuant, oval to linear sinuses that may eventually discharge purulent material.
In contrast to FD, dissecting scalp cellulitis lesions interconnect via sinus tract formation so that pressure on one fluctuant area may result in purulent discharge from perfo-rations several centimeters away.5 Although both dissecting cellulitis and FD are considered primary neutrophilic scarring alopecias, the presence of true sinus tract formation can be a distinguishing finding.
CCCA is the most common form of scarring alopecia among African Americans and is particularly seen among African American women.5 It generally presents on the scalp vertex like FD, but it is much less inflamma-tory and typically causes only mild pruritus or tenderness of the involved areas.
Although numerous theories have been suggested, the etiology is unknown. The pathogenesis is thought to be associated with premature desquamation of the inner root sheath, which can be demonstrated on biopsy. Also seen histologically is lymphocytic perifollicular inflammation and polytrichia.6
Acne keloidalis nuchae is also a scarring alopecia. It is seen most commonly in African American men and presents as keloid-like papules and plaques with occasional pustules characteristically on the occipital scalp and posterior neck. In contrast to FD, acne keloidalis nuchae papules coalesce and may form firm, hairless, protuberant keloid-like plaques that may be painful and cosmetically disfiguring. The cause of acne keloidalis nuchae is unknown.
Shaving or cutting tight curly hair too short and subsequently having the new hair curve back and penetrate the skin may be the precipitating event. Thus, a history of close shaving should make one suspect this diagnosis. Histologic analysis reveals a chronic, predominantly lymphocytic folliculitis with eventual follicular destruction.
Erosive pustular dermatosis is a rare disorder that primarily aff ects the elderly. It is characterized by a chronic amicrobial pustular dermatosis with extensive boggy, crusted, erosive plaques on the scalp resulting in scarring alopecia. Most cases have an onset after the age of 40. Therefore, age of onset may help diff erentiate between erosive pustular dermatosis and FD.
The cause of erosive pustular dermatosis is unknown. It is thought to be related to local trauma, such as chronic sun exposure, occurring months to years prior to the onset of lesions or as an autoimmune process.6 Histologic specimens show nonspecific changes including parakeratosis or hyperkeratotic scale with atrophy or erosion of the epidermis, while an inflammatory infiltrate with lymphocytes and plasma cells is found in the dermis.
LPP is seen more commonly in women than men, and Caucasians are more often aff ected than African Americans. It presents with erythema, perifollicular scale, and scattered patches of scarring alopecia. Half of involved cases develop concomitant clinical features of lichen planus. When present, these characteristics may help distinguish it from FD and other scarring alopecias.6
The etiology of LPP is unknown, but is thought to be similar to the presumed cause of lichen planus: a T-cell?mediated autoimmune response that damages basal keratinocytes.5 Histologic findings include a band-like mononuclear cell infiltrate obscuring the interface between follicular epithelium and dermis at the superficial part of the follicle with occasional interfollicular epidermal changes consistent with lichen planus.
Inflammatory tinea capitis is a common dermatophyte infection of the scalp that aff ects children and adults alike. Typically, it is easily distinguished from FD. However, severe cases may result in a highly inflammatory pustular eruption with alopecia—with or without a kerion—which can make diff erentiation difficult.
In contrast to FD, the alopecia associated with tinea capitis is usually nonscarring, although this depends on the extent and depth of infection. Also, tinea capitis may present with either discrete patches or involve the entire scalp, whereas FD is usually localized to the vertex or occiput (as noted earlier). Correct diagnosis can be accomplished by means of light microscopy and fungal culture.
Secondary syphilis is usually a sexually transmitted disease, but it can also be acquired perinatally. It often presents with a “moth-eaten” alopecia and should be considered when examining patients with patchy alopecia such as that seen in FD. These lesions manifest 3 to 10 weeks after the onset of primary syphilis. Early in its course, the condition is reversible, but if it becomes chronic, the condition will cause a scarring alopecia.
The presence of other stigmata, including a generalized pruritic papulosquamous eruption with involvement of the palms and soles, mucosal lesions ranging from superficial ulcers to large gray plaques, and condylomata lata, should help to diff erentiate syphilis from FD.
Serologic tests such as rapid plasma reagin and venereal disease research laboratory assays are often preferred for routine screening. If the index of suspicion is high, confirmatory testing with direct antibody as-says such as a microhemagglutination assay or fluorescent treponemal antibody absorption test is indicated.
Biopsy is needed for the diagnosis
Two scalp biopsies should be performed to make the diagnosis. Recommended guidelines for sampling the scalp include performance of 4-mm punch biopsies extending into the fat at 2 diff erent clinically active sites.7 One biopsy should be processed for standard horizontal sectioning, but the second biopsy should be bisected vertically, with half sent for histologic examination and the other half for tissue culture (fungal and bacterial). An additional subsequent biopsy for direct immunofluorescence may also be considered if the initial biopsies are nondiagnostic.
Bacterial and fungal cultures collected from an intact pustule on the scalp with a standard culture swab should also be undertaken with pustular disease. If scale is present, a potassium hydroxide examination can help establish the diagnosis of a fungal etiology.
Doxycycline, intralesional corticosteroids are the first line of Tx
Management of FD can be difficult, and long-term treatment is often necessary. You’ll need to explain to patients that their current hair loss is permanent and that the goal of treatment is to decrease inflammation and prevent further balding.
After initial bacterial cultures and sensitivities are obtained, primary treatment is aimed at eliminating S aureus colonization. Often, this requires oral antibiotic therapy, most commonly doxycycline 100 mg twice daily5(strength of recommendation [SOR]: C). Topical antibiotics, however, may be used in mild cases; options include 2% mupirocin, 1% clindamycin, 1.5% fusidic acid, or 2% erythromycin applied twice daily1(SOR: C). In recalcitrant cases, a common treatment regimen includes oral rifampin 300 mg and clindamycin 300 mg twice daily for 10 weeks4(SOR: C).
Adjunctive topical and intralesional corticosteroids may help reduce inflammation and provide symptomatic relief from itching, burning, and pain. Topical class I or II corticosteroids can be used twice daily, whereas intralesional triamcinolone acetonide (combined with topical and/or oral antibiotics) may be administered every 4 to 6 weeks, starting at a concentration of 10 mg/mL1(SOR: C). Oral corticosteroids should only be considered for highly active and rapidly progressive symptoms.
Dapsone may also be considered as a treatment option for FD due to its antimicrobial activity and anti-inflammatory action directed toward neutrophil metabolism. Relapse, however, is frequent after treatment withdrawal1(SOR: C).
Improvement, but anticipated chronicity
We prescribed oral doxycycline 100 mg twice daily for our patient, as well as clobetasol 0.05% topical solution, to be applied to the affected area in the morning and evening.
We told our patient that FD is a chronic relapsing disorder and that while we could not make the condition go away completely, we could control it. We advised the patient to follow up every 2 months for the next 6 months, then every 6 months to ensure there was no progression or need to change the treatment regimen.
The patient’s symptoms improved after the first 2 months. After weaning the patient off doxycycline over a 6-month period, we planned to transition the patient to topical clindamycin solution twice daily.
In some cases, the patient can be weaned off oral antibiotics once the condition is controlled, but for most patients, continuous systemic therapy is needed.
CORRESPONDENCE Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/ SG05D/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; wiscooj@gmail.com
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 23-Year-Old African American Man sought care at our medical center because he had been losing hair over the vertex of his scalp for the past several years. He indicated that his father had early-onset male patterned alopecia. As a result, he considered his hair loss “genetic.” However, he described waxing and waning flares of painful pustules associated with occasional spontaneous bleeding and discharge of purulent material that occurred in the same area as the hair loss.
Physical examination revealed multiple perifollicular papules and pustules on the vertex of his scalp with interspersed patches of alopecia (FIGURE 1). There were no lesions elsewhere on his body and his past medical history was otherwise unremarkable.
FIGURE 1
Alopecia with a painful twist
This 23-year-old patient said that he had spontaneous bleeding and discharge of purulent material in the area of his hair loss.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU MANAGE THIS PATIENT?
Diagnosis: Folliculitis decalvans
Folliculitis decalvans (FD) is a highly inflammatory form of scarring alopecia characterized by inflammatory perifollicular papules and pustules. The term scarring alopecia refers to the fact that the follicular epithelium has been replaced by connective tissue, ultimately resulting in permanent hair loss. This manifests clinically as patches of skin without terminal or vellus hairs, whereas a nonscarring alopecia would demonstrate preservation of the vellus hairs. Left untreated, advancing permanent hair loss ensues and may result in an end-stage pattern of tufted folliculitis or polytrichia, where interspersed dilated follicular openings house multiple hairs.
Affected areas commonly include the vertex and occipital scalp. Common symptoms include pain, itching, burning, and occasionally spontaneous bleeding or discharge of purulent material.1
FD generally occurs in young and middle-aged African Americans with a slight predominance in males. It accounts for 11% of all primary scarring alopecias.2,3 The etiology of this inflammatory process is not fully understood; however, scalp colonization with Staphylococcus aureus has been implicated as a contributing factor.4 Other reports suggest patients may have an altered host immune response and/or genetic predisposition for this condition.2,3
The differential includes various scarring, nonscarring alopecias
Since clinical findings of FD can range from relatively nonspecific mild disease at its onset to the end stage described above, a detailed patient history is needed. The following scarring and nonscarring alopecias should be considered in the diff erential diagnosis: dissecting cellulitis of the scalp, central centrifugal cicatricial alopecia (CCCA), acne keloidalis nuchae, erosive pustular dermatosis, lichen planopilaris (LPP), inflammatory tinea capitis, and secondary syphilis.
Dissecting cellulitis of the scalp is a distinctive, often debilitating disease commonly seen in young adult African American men. It is considered part of the follicular occlusion tetrad that also includes hidradenitis suppurativa, acne conglobata, and pilonidal cysts. It presents as a scarring alopecia with firm scalp nodules that rapidly develop into boggy, fluctuant, oval to linear sinuses that may eventually discharge purulent material.
In contrast to FD, dissecting scalp cellulitis lesions interconnect via sinus tract formation so that pressure on one fluctuant area may result in purulent discharge from perfo-rations several centimeters away.5 Although both dissecting cellulitis and FD are considered primary neutrophilic scarring alopecias, the presence of true sinus tract formation can be a distinguishing finding.
CCCA is the most common form of scarring alopecia among African Americans and is particularly seen among African American women.5 It generally presents on the scalp vertex like FD, but it is much less inflamma-tory and typically causes only mild pruritus or tenderness of the involved areas.
Although numerous theories have been suggested, the etiology is unknown. The pathogenesis is thought to be associated with premature desquamation of the inner root sheath, which can be demonstrated on biopsy. Also seen histologically is lymphocytic perifollicular inflammation and polytrichia.6
Acne keloidalis nuchae is also a scarring alopecia. It is seen most commonly in African American men and presents as keloid-like papules and plaques with occasional pustules characteristically on the occipital scalp and posterior neck. In contrast to FD, acne keloidalis nuchae papules coalesce and may form firm, hairless, protuberant keloid-like plaques that may be painful and cosmetically disfiguring. The cause of acne keloidalis nuchae is unknown.
Shaving or cutting tight curly hair too short and subsequently having the new hair curve back and penetrate the skin may be the precipitating event. Thus, a history of close shaving should make one suspect this diagnosis. Histologic analysis reveals a chronic, predominantly lymphocytic folliculitis with eventual follicular destruction.
Erosive pustular dermatosis is a rare disorder that primarily aff ects the elderly. It is characterized by a chronic amicrobial pustular dermatosis with extensive boggy, crusted, erosive plaques on the scalp resulting in scarring alopecia. Most cases have an onset after the age of 40. Therefore, age of onset may help diff erentiate between erosive pustular dermatosis and FD.
The cause of erosive pustular dermatosis is unknown. It is thought to be related to local trauma, such as chronic sun exposure, occurring months to years prior to the onset of lesions or as an autoimmune process.6 Histologic specimens show nonspecific changes including parakeratosis or hyperkeratotic scale with atrophy or erosion of the epidermis, while an inflammatory infiltrate with lymphocytes and plasma cells is found in the dermis.
LPP is seen more commonly in women than men, and Caucasians are more often aff ected than African Americans. It presents with erythema, perifollicular scale, and scattered patches of scarring alopecia. Half of involved cases develop concomitant clinical features of lichen planus. When present, these characteristics may help distinguish it from FD and other scarring alopecias.6
The etiology of LPP is unknown, but is thought to be similar to the presumed cause of lichen planus: a T-cell?mediated autoimmune response that damages basal keratinocytes.5 Histologic findings include a band-like mononuclear cell infiltrate obscuring the interface between follicular epithelium and dermis at the superficial part of the follicle with occasional interfollicular epidermal changes consistent with lichen planus.
Inflammatory tinea capitis is a common dermatophyte infection of the scalp that aff ects children and adults alike. Typically, it is easily distinguished from FD. However, severe cases may result in a highly inflammatory pustular eruption with alopecia—with or without a kerion—which can make diff erentiation difficult.
In contrast to FD, the alopecia associated with tinea capitis is usually nonscarring, although this depends on the extent and depth of infection. Also, tinea capitis may present with either discrete patches or involve the entire scalp, whereas FD is usually localized to the vertex or occiput (as noted earlier). Correct diagnosis can be accomplished by means of light microscopy and fungal culture.
Secondary syphilis is usually a sexually transmitted disease, but it can also be acquired perinatally. It often presents with a “moth-eaten” alopecia and should be considered when examining patients with patchy alopecia such as that seen in FD. These lesions manifest 3 to 10 weeks after the onset of primary syphilis. Early in its course, the condition is reversible, but if it becomes chronic, the condition will cause a scarring alopecia.
The presence of other stigmata, including a generalized pruritic papulosquamous eruption with involvement of the palms and soles, mucosal lesions ranging from superficial ulcers to large gray plaques, and condylomata lata, should help to diff erentiate syphilis from FD.
Serologic tests such as rapid plasma reagin and venereal disease research laboratory assays are often preferred for routine screening. If the index of suspicion is high, confirmatory testing with direct antibody as-says such as a microhemagglutination assay or fluorescent treponemal antibody absorption test is indicated.
Biopsy is needed for the diagnosis
Two scalp biopsies should be performed to make the diagnosis. Recommended guidelines for sampling the scalp include performance of 4-mm punch biopsies extending into the fat at 2 diff erent clinically active sites.7 One biopsy should be processed for standard horizontal sectioning, but the second biopsy should be bisected vertically, with half sent for histologic examination and the other half for tissue culture (fungal and bacterial). An additional subsequent biopsy for direct immunofluorescence may also be considered if the initial biopsies are nondiagnostic.
Bacterial and fungal cultures collected from an intact pustule on the scalp with a standard culture swab should also be undertaken with pustular disease. If scale is present, a potassium hydroxide examination can help establish the diagnosis of a fungal etiology.
Doxycycline, intralesional corticosteroids are the first line of Tx
Management of FD can be difficult, and long-term treatment is often necessary. You’ll need to explain to patients that their current hair loss is permanent and that the goal of treatment is to decrease inflammation and prevent further balding.
After initial bacterial cultures and sensitivities are obtained, primary treatment is aimed at eliminating S aureus colonization. Often, this requires oral antibiotic therapy, most commonly doxycycline 100 mg twice daily5(strength of recommendation [SOR]: C). Topical antibiotics, however, may be used in mild cases; options include 2% mupirocin, 1% clindamycin, 1.5% fusidic acid, or 2% erythromycin applied twice daily1(SOR: C). In recalcitrant cases, a common treatment regimen includes oral rifampin 300 mg and clindamycin 300 mg twice daily for 10 weeks4(SOR: C).
Adjunctive topical and intralesional corticosteroids may help reduce inflammation and provide symptomatic relief from itching, burning, and pain. Topical class I or II corticosteroids can be used twice daily, whereas intralesional triamcinolone acetonide (combined with topical and/or oral antibiotics) may be administered every 4 to 6 weeks, starting at a concentration of 10 mg/mL1(SOR: C). Oral corticosteroids should only be considered for highly active and rapidly progressive symptoms.
Dapsone may also be considered as a treatment option for FD due to its antimicrobial activity and anti-inflammatory action directed toward neutrophil metabolism. Relapse, however, is frequent after treatment withdrawal1(SOR: C).
Improvement, but anticipated chronicity
We prescribed oral doxycycline 100 mg twice daily for our patient, as well as clobetasol 0.05% topical solution, to be applied to the affected area in the morning and evening.
We told our patient that FD is a chronic relapsing disorder and that while we could not make the condition go away completely, we could control it. We advised the patient to follow up every 2 months for the next 6 months, then every 6 months to ensure there was no progression or need to change the treatment regimen.
The patient’s symptoms improved after the first 2 months. After weaning the patient off doxycycline over a 6-month period, we planned to transition the patient to topical clindamycin solution twice daily.
In some cases, the patient can be weaned off oral antibiotics once the condition is controlled, but for most patients, continuous systemic therapy is needed.
CORRESPONDENCE Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/ SG05D/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; wiscooj@gmail.com
1. Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244.
2. Douwes KE, Landthaler M, Szeimies RM. Simultaneous occur-rence of folliculitis decalvans capillitii in identical twins. Br J Dermatol. 2000;143:195-197.
3. Chandrawansa PH, Giam YC. Folliculitis decalvans-a retrospective study in a tertiary referred center, over five years. Singapore Med J. 2003;44:84-87.
4. Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical histological and therapeutic findings. Br J Dermatol. 1999;140:328-333.
5. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
6. Somani N, Bergfeld WF. Cicatricial alopecia: classification and histopathology. Dermatol Ther. 2008;21:221-237.
7. Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored workshop on cicatricial alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110.
1. Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244.
2. Douwes KE, Landthaler M, Szeimies RM. Simultaneous occur-rence of folliculitis decalvans capillitii in identical twins. Br J Dermatol. 2000;143:195-197.
3. Chandrawansa PH, Giam YC. Folliculitis decalvans-a retrospective study in a tertiary referred center, over five years. Singapore Med J. 2003;44:84-87.
4. Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical histological and therapeutic findings. Br J Dermatol. 1999;140:328-333.
5. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
6. Somani N, Bergfeld WF. Cicatricial alopecia: classification and histopathology. Dermatol Ther. 2008;21:221-237.
7. Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored workshop on cicatricial alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110.
Verrucous papule on thigh
A 21-year-old man came into our medical center to have a lesion on his thigh examined. He said the lesion developed a few months earlier at the site of minimal trauma. He noted that, over the previous few months, the lesion had progressively darkened and it bled sporadically. On examination, we noted a solitary 7.5-mm firm, blue-black verrucous papule over the right medial thigh (FIGURES 1A AND 1B). There were no other lesions.
The patient indicated that he had gotten sunburned many times in the past. He also said that he had an aunt who’d had a melanoma.
FIGURE 1
A lesion that bled sporadically
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Angiokeratoma
An angiokeratoma is a benign pink-red to blue-black variably sized papule or plaque that is typically 2 to 10 mm in diameter.1 Angiokeratomas are composed of a series of subepidermal dilated capillaries that have a characteristic hyperkeratotic surface and bleed easily.2 These lesions are rare, with a prevalence estimated to be 0.16% in the general population.3
The pathogenesis of angiokeratoma formation is unclear; however, multiple theories exist. The development of these lesions may be related to repeated trauma or friction at a particular site.4 Alternatively, increased venous blood pressure or primary degeneration of vascular elastic tissue could explain their development.5 While their cause is unclear, the initial event in the development of an angiokeratoma is believed to be the development of a vascular ectasia within the papillary dermis. The epidermal reaction appears to be a secondary phenomenon due to increased proliferative capacity on the surface of the vessels.5
The most common form—as seen in this case—is the solitary or sporadic angiokeratoma. It comprises 70% to 83% of all cases of angiokeratomas3 and usually develops on the lower extremities. Angiokeratomas typically arise during the first 2 decades of life,6 and are more common in men.3 Other types of angiokeratomas include angiokeratoma of Mibelli, angiokeratoma of Fordyce, angiokeratoma circumscriptum, and angiokeratoma corporis diffusum (Fabry’s disease).7,8
Angiokeratoma of Mibelli is characterized by pink to dark red papules or verrucoid nodules that occur most commonly in men7 and involve the bony prominences, such as the elbows.
Fordyce lesions involve the scrotum or vulva and are usually numerous and related to conditions with elevated venous pressure.
Angiokeratoma circumscriptum usually present as papules that commonly coalesce to form plaques.
Fabry’s disease, or angiokeratoma corporis diffusum, is an X-linked recessive disease related to a deficiency in alpha-galactosidase A. This leads to multiple, variably sized angiokeratomas occurring in childhood that are concentrated between the umbilicus and the knees. This disease invariably leads to involvement of other organs, which may result in renal failure, myocardial infarction, or cerebrovascular accidents.1,7
A mimicker of melanoma
An angiokeratoma is an uncommon, though important, mimicker of melanoma. (For more on other lesions that can be confused with melanoma, see “Nonmelanocytic melanoma mimickers”.)
Melanoma is the most aggressive and potentially life-threatening neoplasm in the differential diagnosis of an angiokeratoma. Risk factors for melanoma include increasing age, fair skin and hair color, tendency for freckling, number of moles (5 large or >50 small nevi doubles the risk of melanoma), a personal or first-degree family history of melanoma, and a history of intermittent sunburns.9-12
A number of nonmelanocytic lesions can be confused with melanoma. They include the following:
Actinic keratoses (AKs) are a type of keratinocytic neoplasm that typically develops on the sun-exposed skin of the elderly. An AK is typically 3 to 10 mm in size, pink to red in color, and has scaling secondary to local hyperkeratosis. If these lesions are left untreated, they can develop into squamous cell carcinomas (SCCs) at a rate of 0.24% annually.15,16 Thus, AKs are more often a concern for SCC than for melanoma. However, the pigmented variant of an AK can clinically and histologically raise concern for melanoma due to its pigmentation and microscopic evidence of melanin within keratinocytes and macrophages.15 If it is not possible to differentiate an AK from melanoma clinically or histologically, immunohistochemistry is often required to make the final diagnosis. For example, immunohistochemical staining with S-100 can be used to identify epidermal melanocytes and distinguish them from atypical keratinocytes.17
Basal cell carcinoma (BCC) is the most common skin cancer.18 While most BCCs are amelanocytic, 7% of BCCs are pigmented and present as irregularly pigmented nodules with irregular telangiectatic vessels on their surface. The center of a BCC may be depressed or ulcerated and may easily crust or bleed. Definitive diagnosis may be made histologically. A BCC typically consists of columns of basaloid cells with atypical nuclei, sparse cytoplasm, and peripheral cellular palisading.19 BCCs are easily differentiated from melanoma using immunohistochemistry, as they are negative for traditional melanocytic markers.17
Seborrheic keratoses (SKs) are among the most common skin lesions and represent a benign proliferation of immature keratinocytes. The appearance of an SK can vary from a smooth peppered appearance to a rough surface that may be irregularly pigmented, dry, and fissured. Given their range of presentation, it is common for SKs to be biopsied to evaluate for melanoma and occasionally BCC.20
Dermatofibromas (DFs) are common benign skin lesions that typically appear as pink-to brown-colored firm nodules that represent a localized response to skin injury and inflammation. DFs are typically 3 to 10 mm in diameter and are most commonly located on the anterior surface of the thigh. Histologic analysis of a DF reveals an acanthotic epidermis with a proliferation of spindle cells in the mid and lower dermis, with capillaries dispersed throughout. A common finding in DFs is the trapping of collagen within the spindle cell at the periphery of the lesion.21
How to diagnose angiokeratoma
The clinical presentation typically suffices in making the diagnosis of an angiokeratoma. If dermoscopy is performed, the characteristic findings include the presence of scale and purple lacunae13 (FIGURE 2). However, when there is suspicion of melanoma or the clinical diagnosis is in doubt, the entire lesion should be removed (with narrow margins) in order to obtain a definitive diagnosis. Histological findings consist of dilated subepidermal vessels associated with epidermal hyperkeratosis.3
FIGURE 2
A view from the dermatoscope
No need to treat, unless there are cosmetic concerns
If the diagnosis is straightforward and a biopsy is not needed, no treatment is necessary because simple angiokeratomas are benign entities. However, treatment may be considered for cosmetic purposes, or to prevent bothersome bleeding. Angiokeratomas may be removed via shave or standard excision, electrodessication and curettage, or destroyed with a laser. For Fabry’s disease, in which numerous angiokeratomas pose a cosmetic concern, laser therapy, including the use of an argon, copper, Nd:Yag, KTP 532-nm, or Candela V-beam laser, is preferred.14
In our patient’s case, we performed a 2-mm punch biopsy, which revealed that the lesion was an angiokeratoma. It was subsequently removed by shave biopsy with clear margins.
CORRESPONDENCE
Thomas M. Beachkofsky, MD, Capt, USAF, MC, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/SG05D/ Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; tbeachkofsky@yahoo.com
1. Karen JK, Hale EK, Ma L. Angiokeratoma corporis diffusum. Dermatol Online J. [Internet]. 2005;11:8. Available at: http://dermatology.cdlib.org/114/NYU/NYUtexts/0419054.html. Accessed September 24, 2010.
2. Schiller PI, Itin PH. Angiokeratomas: an update. Dermatology. 1996;193:275-282.
3. Zaballos P, Dauft C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318-325.
4. Kim JH, Nam TS, Kim SH. Solitary angiokeratoma developed in one area of lymphangioma circumscriptum. J Korean Med Sci. 1988;3:169-170.
5. Sion-Vardy N, Manor E, Puterman M, et al. Solitary angiokeratoma of the tongue. Med Oral Patol Oral Cir Bucal. 2008;13:12-14.
6. Vascular tumors and malformations In: Habif TP, Campbell JL, Dinulos JG, et al, eds. Skin Disease: Diagnosis and Treatment. New York, NY: Mosby; 2004:486–487.
7. Leis-Dosil VM, Alijo-Serrano F, Aviles-Izquierdo JA, et al. Angiokeratoma of the glans penis: clinical, histopathological and dermoscopic correlation. Dermatol Online J. [Internet]. 2007;13:19. Available from: http://dermatology.cdlib.org/132/case_presentations/angiokeratoma/dosil.html. Accessed September 24, 2010.
8. Erkek E, Basar MM, Bagci Y, et al. Fordyce angiokeratomas as clues to local venous hypertension. Arch Dermatol. 2005;141:1325-1326.
9. Rager EL, Bridgeford EP, Ollila DW. Cutaneous melanoma: update on prevention, screening, diagnosis, and treatment. Am Fam Physician. 2005;72:269-276.
10. Chudnovsky Y, Khavari PA, Adams AE. Melanoma genetics and the development of rational therapeutics. J Clin Invest. 2005;115:813-824.
11. Ortiz CA, Goodwin JS, Freeman JL. The effect of socioeconomic factors on incidence, stage at diagnosis, and survival of cutaneous melanoma. Med Sci Monit. 2005;11:163-172.
12. Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma. JAMA. 2004;292:2771-2776.
13. Johr RH, Soyer P, Argenziano G, et al. Dermoscopy: The Essentials. New York, NY: Mosby; 2007:130.
14. Enjolras O. Vascular malformations. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Philadelphia, Pa: Mosby; 2003: 1621–1622.
15. Peris K, Micantonio T, Piccolo D, et al. Dermoscopic features of actinic keratosis. J Dtsch Dermatol Ges. 2007;5:970-976.
16. McIntyre WJ, Downs MR, Bedwell SA. Treatment options for actinic keratosis. Am Fam Physician. 2007;76:667-671.
17. Kamil ZS, Tong LC, Habeeb AA, et al. Non-melanocytic mimics of melanoma: part 1: intraepidermal mimics. J Clin Pathol. 2009;62:120-127.
18. Wong CS, Strange RC, Lear JT. Basal cell carcinoma. BMJ. 2003;327:794-798.
19. Menzies SW. Dermoscopy of pigmented basal cell carcinoma. Clin Dermatol. 2002;20:268-269.
20. Braun RP, Rabinovitz H, Oliviero M, et al. Dermoscopic diagnosis of seborrheic keratosis. Clin Dermatol. 2002;20:270-272.
21. Agero AL, Taliercio S, Dusza SW, et al. Conventional and polarized dermoscopy features of dermatofibroma. Arch Dermatol. 2006;142:1431-1437.
A 21-year-old man came into our medical center to have a lesion on his thigh examined. He said the lesion developed a few months earlier at the site of minimal trauma. He noted that, over the previous few months, the lesion had progressively darkened and it bled sporadically. On examination, we noted a solitary 7.5-mm firm, blue-black verrucous papule over the right medial thigh (FIGURES 1A AND 1B). There were no other lesions.
The patient indicated that he had gotten sunburned many times in the past. He also said that he had an aunt who’d had a melanoma.
FIGURE 1
A lesion that bled sporadically
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Angiokeratoma
An angiokeratoma is a benign pink-red to blue-black variably sized papule or plaque that is typically 2 to 10 mm in diameter.1 Angiokeratomas are composed of a series of subepidermal dilated capillaries that have a characteristic hyperkeratotic surface and bleed easily.2 These lesions are rare, with a prevalence estimated to be 0.16% in the general population.3
The pathogenesis of angiokeratoma formation is unclear; however, multiple theories exist. The development of these lesions may be related to repeated trauma or friction at a particular site.4 Alternatively, increased venous blood pressure or primary degeneration of vascular elastic tissue could explain their development.5 While their cause is unclear, the initial event in the development of an angiokeratoma is believed to be the development of a vascular ectasia within the papillary dermis. The epidermal reaction appears to be a secondary phenomenon due to increased proliferative capacity on the surface of the vessels.5
The most common form—as seen in this case—is the solitary or sporadic angiokeratoma. It comprises 70% to 83% of all cases of angiokeratomas3 and usually develops on the lower extremities. Angiokeratomas typically arise during the first 2 decades of life,6 and are more common in men.3 Other types of angiokeratomas include angiokeratoma of Mibelli, angiokeratoma of Fordyce, angiokeratoma circumscriptum, and angiokeratoma corporis diffusum (Fabry’s disease).7,8
Angiokeratoma of Mibelli is characterized by pink to dark red papules or verrucoid nodules that occur most commonly in men7 and involve the bony prominences, such as the elbows.
Fordyce lesions involve the scrotum or vulva and are usually numerous and related to conditions with elevated venous pressure.
Angiokeratoma circumscriptum usually present as papules that commonly coalesce to form plaques.
Fabry’s disease, or angiokeratoma corporis diffusum, is an X-linked recessive disease related to a deficiency in alpha-galactosidase A. This leads to multiple, variably sized angiokeratomas occurring in childhood that are concentrated between the umbilicus and the knees. This disease invariably leads to involvement of other organs, which may result in renal failure, myocardial infarction, or cerebrovascular accidents.1,7
A mimicker of melanoma
An angiokeratoma is an uncommon, though important, mimicker of melanoma. (For more on other lesions that can be confused with melanoma, see “Nonmelanocytic melanoma mimickers”.)
Melanoma is the most aggressive and potentially life-threatening neoplasm in the differential diagnosis of an angiokeratoma. Risk factors for melanoma include increasing age, fair skin and hair color, tendency for freckling, number of moles (5 large or >50 small nevi doubles the risk of melanoma), a personal or first-degree family history of melanoma, and a history of intermittent sunburns.9-12
A number of nonmelanocytic lesions can be confused with melanoma. They include the following:
Actinic keratoses (AKs) are a type of keratinocytic neoplasm that typically develops on the sun-exposed skin of the elderly. An AK is typically 3 to 10 mm in size, pink to red in color, and has scaling secondary to local hyperkeratosis. If these lesions are left untreated, they can develop into squamous cell carcinomas (SCCs) at a rate of 0.24% annually.15,16 Thus, AKs are more often a concern for SCC than for melanoma. However, the pigmented variant of an AK can clinically and histologically raise concern for melanoma due to its pigmentation and microscopic evidence of melanin within keratinocytes and macrophages.15 If it is not possible to differentiate an AK from melanoma clinically or histologically, immunohistochemistry is often required to make the final diagnosis. For example, immunohistochemical staining with S-100 can be used to identify epidermal melanocytes and distinguish them from atypical keratinocytes.17
Basal cell carcinoma (BCC) is the most common skin cancer.18 While most BCCs are amelanocytic, 7% of BCCs are pigmented and present as irregularly pigmented nodules with irregular telangiectatic vessels on their surface. The center of a BCC may be depressed or ulcerated and may easily crust or bleed. Definitive diagnosis may be made histologically. A BCC typically consists of columns of basaloid cells with atypical nuclei, sparse cytoplasm, and peripheral cellular palisading.19 BCCs are easily differentiated from melanoma using immunohistochemistry, as they are negative for traditional melanocytic markers.17
Seborrheic keratoses (SKs) are among the most common skin lesions and represent a benign proliferation of immature keratinocytes. The appearance of an SK can vary from a smooth peppered appearance to a rough surface that may be irregularly pigmented, dry, and fissured. Given their range of presentation, it is common for SKs to be biopsied to evaluate for melanoma and occasionally BCC.20
Dermatofibromas (DFs) are common benign skin lesions that typically appear as pink-to brown-colored firm nodules that represent a localized response to skin injury and inflammation. DFs are typically 3 to 10 mm in diameter and are most commonly located on the anterior surface of the thigh. Histologic analysis of a DF reveals an acanthotic epidermis with a proliferation of spindle cells in the mid and lower dermis, with capillaries dispersed throughout. A common finding in DFs is the trapping of collagen within the spindle cell at the periphery of the lesion.21
How to diagnose angiokeratoma
The clinical presentation typically suffices in making the diagnosis of an angiokeratoma. If dermoscopy is performed, the characteristic findings include the presence of scale and purple lacunae13 (FIGURE 2). However, when there is suspicion of melanoma or the clinical diagnosis is in doubt, the entire lesion should be removed (with narrow margins) in order to obtain a definitive diagnosis. Histological findings consist of dilated subepidermal vessels associated with epidermal hyperkeratosis.3
FIGURE 2
A view from the dermatoscope
No need to treat, unless there are cosmetic concerns
If the diagnosis is straightforward and a biopsy is not needed, no treatment is necessary because simple angiokeratomas are benign entities. However, treatment may be considered for cosmetic purposes, or to prevent bothersome bleeding. Angiokeratomas may be removed via shave or standard excision, electrodessication and curettage, or destroyed with a laser. For Fabry’s disease, in which numerous angiokeratomas pose a cosmetic concern, laser therapy, including the use of an argon, copper, Nd:Yag, KTP 532-nm, or Candela V-beam laser, is preferred.14
In our patient’s case, we performed a 2-mm punch biopsy, which revealed that the lesion was an angiokeratoma. It was subsequently removed by shave biopsy with clear margins.
CORRESPONDENCE
Thomas M. Beachkofsky, MD, Capt, USAF, MC, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/SG05D/ Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; tbeachkofsky@yahoo.com
A 21-year-old man came into our medical center to have a lesion on his thigh examined. He said the lesion developed a few months earlier at the site of minimal trauma. He noted that, over the previous few months, the lesion had progressively darkened and it bled sporadically. On examination, we noted a solitary 7.5-mm firm, blue-black verrucous papule over the right medial thigh (FIGURES 1A AND 1B). There were no other lesions.
The patient indicated that he had gotten sunburned many times in the past. He also said that he had an aunt who’d had a melanoma.
FIGURE 1
A lesion that bled sporadically
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Angiokeratoma
An angiokeratoma is a benign pink-red to blue-black variably sized papule or plaque that is typically 2 to 10 mm in diameter.1 Angiokeratomas are composed of a series of subepidermal dilated capillaries that have a characteristic hyperkeratotic surface and bleed easily.2 These lesions are rare, with a prevalence estimated to be 0.16% in the general population.3
The pathogenesis of angiokeratoma formation is unclear; however, multiple theories exist. The development of these lesions may be related to repeated trauma or friction at a particular site.4 Alternatively, increased venous blood pressure or primary degeneration of vascular elastic tissue could explain their development.5 While their cause is unclear, the initial event in the development of an angiokeratoma is believed to be the development of a vascular ectasia within the papillary dermis. The epidermal reaction appears to be a secondary phenomenon due to increased proliferative capacity on the surface of the vessels.5
The most common form—as seen in this case—is the solitary or sporadic angiokeratoma. It comprises 70% to 83% of all cases of angiokeratomas3 and usually develops on the lower extremities. Angiokeratomas typically arise during the first 2 decades of life,6 and are more common in men.3 Other types of angiokeratomas include angiokeratoma of Mibelli, angiokeratoma of Fordyce, angiokeratoma circumscriptum, and angiokeratoma corporis diffusum (Fabry’s disease).7,8
Angiokeratoma of Mibelli is characterized by pink to dark red papules or verrucoid nodules that occur most commonly in men7 and involve the bony prominences, such as the elbows.
Fordyce lesions involve the scrotum or vulva and are usually numerous and related to conditions with elevated venous pressure.
Angiokeratoma circumscriptum usually present as papules that commonly coalesce to form plaques.
Fabry’s disease, or angiokeratoma corporis diffusum, is an X-linked recessive disease related to a deficiency in alpha-galactosidase A. This leads to multiple, variably sized angiokeratomas occurring in childhood that are concentrated between the umbilicus and the knees. This disease invariably leads to involvement of other organs, which may result in renal failure, myocardial infarction, or cerebrovascular accidents.1,7
A mimicker of melanoma
An angiokeratoma is an uncommon, though important, mimicker of melanoma. (For more on other lesions that can be confused with melanoma, see “Nonmelanocytic melanoma mimickers”.)
Melanoma is the most aggressive and potentially life-threatening neoplasm in the differential diagnosis of an angiokeratoma. Risk factors for melanoma include increasing age, fair skin and hair color, tendency for freckling, number of moles (5 large or >50 small nevi doubles the risk of melanoma), a personal or first-degree family history of melanoma, and a history of intermittent sunburns.9-12
A number of nonmelanocytic lesions can be confused with melanoma. They include the following:
Actinic keratoses (AKs) are a type of keratinocytic neoplasm that typically develops on the sun-exposed skin of the elderly. An AK is typically 3 to 10 mm in size, pink to red in color, and has scaling secondary to local hyperkeratosis. If these lesions are left untreated, they can develop into squamous cell carcinomas (SCCs) at a rate of 0.24% annually.15,16 Thus, AKs are more often a concern for SCC than for melanoma. However, the pigmented variant of an AK can clinically and histologically raise concern for melanoma due to its pigmentation and microscopic evidence of melanin within keratinocytes and macrophages.15 If it is not possible to differentiate an AK from melanoma clinically or histologically, immunohistochemistry is often required to make the final diagnosis. For example, immunohistochemical staining with S-100 can be used to identify epidermal melanocytes and distinguish them from atypical keratinocytes.17
Basal cell carcinoma (BCC) is the most common skin cancer.18 While most BCCs are amelanocytic, 7% of BCCs are pigmented and present as irregularly pigmented nodules with irregular telangiectatic vessels on their surface. The center of a BCC may be depressed or ulcerated and may easily crust or bleed. Definitive diagnosis may be made histologically. A BCC typically consists of columns of basaloid cells with atypical nuclei, sparse cytoplasm, and peripheral cellular palisading.19 BCCs are easily differentiated from melanoma using immunohistochemistry, as they are negative for traditional melanocytic markers.17
Seborrheic keratoses (SKs) are among the most common skin lesions and represent a benign proliferation of immature keratinocytes. The appearance of an SK can vary from a smooth peppered appearance to a rough surface that may be irregularly pigmented, dry, and fissured. Given their range of presentation, it is common for SKs to be biopsied to evaluate for melanoma and occasionally BCC.20
Dermatofibromas (DFs) are common benign skin lesions that typically appear as pink-to brown-colored firm nodules that represent a localized response to skin injury and inflammation. DFs are typically 3 to 10 mm in diameter and are most commonly located on the anterior surface of the thigh. Histologic analysis of a DF reveals an acanthotic epidermis with a proliferation of spindle cells in the mid and lower dermis, with capillaries dispersed throughout. A common finding in DFs is the trapping of collagen within the spindle cell at the periphery of the lesion.21
How to diagnose angiokeratoma
The clinical presentation typically suffices in making the diagnosis of an angiokeratoma. If dermoscopy is performed, the characteristic findings include the presence of scale and purple lacunae13 (FIGURE 2). However, when there is suspicion of melanoma or the clinical diagnosis is in doubt, the entire lesion should be removed (with narrow margins) in order to obtain a definitive diagnosis. Histological findings consist of dilated subepidermal vessels associated with epidermal hyperkeratosis.3
FIGURE 2
A view from the dermatoscope
No need to treat, unless there are cosmetic concerns
If the diagnosis is straightforward and a biopsy is not needed, no treatment is necessary because simple angiokeratomas are benign entities. However, treatment may be considered for cosmetic purposes, or to prevent bothersome bleeding. Angiokeratomas may be removed via shave or standard excision, electrodessication and curettage, or destroyed with a laser. For Fabry’s disease, in which numerous angiokeratomas pose a cosmetic concern, laser therapy, including the use of an argon, copper, Nd:Yag, KTP 532-nm, or Candela V-beam laser, is preferred.14
In our patient’s case, we performed a 2-mm punch biopsy, which revealed that the lesion was an angiokeratoma. It was subsequently removed by shave biopsy with clear margins.
CORRESPONDENCE
Thomas M. Beachkofsky, MD, Capt, USAF, MC, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/SG05D/ Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; tbeachkofsky@yahoo.com
1. Karen JK, Hale EK, Ma L. Angiokeratoma corporis diffusum. Dermatol Online J. [Internet]. 2005;11:8. Available at: http://dermatology.cdlib.org/114/NYU/NYUtexts/0419054.html. Accessed September 24, 2010.
2. Schiller PI, Itin PH. Angiokeratomas: an update. Dermatology. 1996;193:275-282.
3. Zaballos P, Dauft C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318-325.
4. Kim JH, Nam TS, Kim SH. Solitary angiokeratoma developed in one area of lymphangioma circumscriptum. J Korean Med Sci. 1988;3:169-170.
5. Sion-Vardy N, Manor E, Puterman M, et al. Solitary angiokeratoma of the tongue. Med Oral Patol Oral Cir Bucal. 2008;13:12-14.
6. Vascular tumors and malformations In: Habif TP, Campbell JL, Dinulos JG, et al, eds. Skin Disease: Diagnosis and Treatment. New York, NY: Mosby; 2004:486–487.
7. Leis-Dosil VM, Alijo-Serrano F, Aviles-Izquierdo JA, et al. Angiokeratoma of the glans penis: clinical, histopathological and dermoscopic correlation. Dermatol Online J. [Internet]. 2007;13:19. Available from: http://dermatology.cdlib.org/132/case_presentations/angiokeratoma/dosil.html. Accessed September 24, 2010.
8. Erkek E, Basar MM, Bagci Y, et al. Fordyce angiokeratomas as clues to local venous hypertension. Arch Dermatol. 2005;141:1325-1326.
9. Rager EL, Bridgeford EP, Ollila DW. Cutaneous melanoma: update on prevention, screening, diagnosis, and treatment. Am Fam Physician. 2005;72:269-276.
10. Chudnovsky Y, Khavari PA, Adams AE. Melanoma genetics and the development of rational therapeutics. J Clin Invest. 2005;115:813-824.
11. Ortiz CA, Goodwin JS, Freeman JL. The effect of socioeconomic factors on incidence, stage at diagnosis, and survival of cutaneous melanoma. Med Sci Monit. 2005;11:163-172.
12. Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma. JAMA. 2004;292:2771-2776.
13. Johr RH, Soyer P, Argenziano G, et al. Dermoscopy: The Essentials. New York, NY: Mosby; 2007:130.
14. Enjolras O. Vascular malformations. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Philadelphia, Pa: Mosby; 2003: 1621–1622.
15. Peris K, Micantonio T, Piccolo D, et al. Dermoscopic features of actinic keratosis. J Dtsch Dermatol Ges. 2007;5:970-976.
16. McIntyre WJ, Downs MR, Bedwell SA. Treatment options for actinic keratosis. Am Fam Physician. 2007;76:667-671.
17. Kamil ZS, Tong LC, Habeeb AA, et al. Non-melanocytic mimics of melanoma: part 1: intraepidermal mimics. J Clin Pathol. 2009;62:120-127.
18. Wong CS, Strange RC, Lear JT. Basal cell carcinoma. BMJ. 2003;327:794-798.
19. Menzies SW. Dermoscopy of pigmented basal cell carcinoma. Clin Dermatol. 2002;20:268-269.
20. Braun RP, Rabinovitz H, Oliviero M, et al. Dermoscopic diagnosis of seborrheic keratosis. Clin Dermatol. 2002;20:270-272.
21. Agero AL, Taliercio S, Dusza SW, et al. Conventional and polarized dermoscopy features of dermatofibroma. Arch Dermatol. 2006;142:1431-1437.
1. Karen JK, Hale EK, Ma L. Angiokeratoma corporis diffusum. Dermatol Online J. [Internet]. 2005;11:8. Available at: http://dermatology.cdlib.org/114/NYU/NYUtexts/0419054.html. Accessed September 24, 2010.
2. Schiller PI, Itin PH. Angiokeratomas: an update. Dermatology. 1996;193:275-282.
3. Zaballos P, Dauft C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318-325.
4. Kim JH, Nam TS, Kim SH. Solitary angiokeratoma developed in one area of lymphangioma circumscriptum. J Korean Med Sci. 1988;3:169-170.
5. Sion-Vardy N, Manor E, Puterman M, et al. Solitary angiokeratoma of the tongue. Med Oral Patol Oral Cir Bucal. 2008;13:12-14.
6. Vascular tumors and malformations In: Habif TP, Campbell JL, Dinulos JG, et al, eds. Skin Disease: Diagnosis and Treatment. New York, NY: Mosby; 2004:486–487.
7. Leis-Dosil VM, Alijo-Serrano F, Aviles-Izquierdo JA, et al. Angiokeratoma of the glans penis: clinical, histopathological and dermoscopic correlation. Dermatol Online J. [Internet]. 2007;13:19. Available from: http://dermatology.cdlib.org/132/case_presentations/angiokeratoma/dosil.html. Accessed September 24, 2010.
8. Erkek E, Basar MM, Bagci Y, et al. Fordyce angiokeratomas as clues to local venous hypertension. Arch Dermatol. 2005;141:1325-1326.
9. Rager EL, Bridgeford EP, Ollila DW. Cutaneous melanoma: update on prevention, screening, diagnosis, and treatment. Am Fam Physician. 2005;72:269-276.
10. Chudnovsky Y, Khavari PA, Adams AE. Melanoma genetics and the development of rational therapeutics. J Clin Invest. 2005;115:813-824.
11. Ortiz CA, Goodwin JS, Freeman JL. The effect of socioeconomic factors on incidence, stage at diagnosis, and survival of cutaneous melanoma. Med Sci Monit. 2005;11:163-172.
12. Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma. JAMA. 2004;292:2771-2776.
13. Johr RH, Soyer P, Argenziano G, et al. Dermoscopy: The Essentials. New York, NY: Mosby; 2007:130.
14. Enjolras O. Vascular malformations. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Philadelphia, Pa: Mosby; 2003: 1621–1622.
15. Peris K, Micantonio T, Piccolo D, et al. Dermoscopic features of actinic keratosis. J Dtsch Dermatol Ges. 2007;5:970-976.
16. McIntyre WJ, Downs MR, Bedwell SA. Treatment options for actinic keratosis. Am Fam Physician. 2007;76:667-671.
17. Kamil ZS, Tong LC, Habeeb AA, et al. Non-melanocytic mimics of melanoma: part 1: intraepidermal mimics. J Clin Pathol. 2009;62:120-127.
18. Wong CS, Strange RC, Lear JT. Basal cell carcinoma. BMJ. 2003;327:794-798.
19. Menzies SW. Dermoscopy of pigmented basal cell carcinoma. Clin Dermatol. 2002;20:268-269.
20. Braun RP, Rabinovitz H, Oliviero M, et al. Dermoscopic diagnosis of seborrheic keratosis. Clin Dermatol. 2002;20:270-272.
21. Agero AL, Taliercio S, Dusza SW, et al. Conventional and polarized dermoscopy features of dermatofibroma. Arch Dermatol. 2006;142:1431-1437.
Verrucous nodules on the ankle
A 56-year-old woman came into our medical center complaining of multiple pruritic, slowly growing scaly nodules over her right ankle (FIGURE 1A AND 1B). She indicated that the lesions started as small pink “bumps” at the staple sites of an open reduction and internal fixation surgery of her talus that she’d had 8 years ago.
There were no lesions elsewhere on her body and her past medical history was otherwise unremarkable.
FIGURE 1
Multiple pruritic, scaly nodules
What is your diagnosis?
How would you manage this condition?
Diagnosis: Hypertrophic lichen planus
Hypertrophic lichen planus (HLP), a variant of lichen planus (LP), is a lesion that is usually found on the distal extremities. HLP plaques evolve from initial characteristic LP lesions (purple, planar, pruritic, polygonal papules or plaque) to form reddish-brown to violaceous, hypertrophic, verrucous round-to-elongated plaques. Primary lesions may be spread by scratching or other trauma and often develop dark brown hyperpigmentation over several years. Like other variants of LP, HLP most commonly affects adults 30 to 60 years of age, with a slight female predominance.1
HLP may be idiopathic, drug induced, or associated with a systemic disease. Although many drugs have been linked to this lesion, the most commonly reported medications are gold salts, beta-blockers, antimalarials, thiazides, furosemide, and penicillamines. If your patient has HLP and is taking one of these medications, you should consider discontinuing the medication.1 As with other forms of LP, HLP has been associated with hepatitis C. Consider transaminases and a hepatitis panel for all patients with HLP. Other HLP-associated conditions include venous insufficiency, herpes simplex virus, and varicella-zoster virus.1
When the history confuses the diagnosis
When there are surrounding classic LP lesions, the diagnosis of HLP is fairly straightforward. However, when the patient has a history of surgery or trauma preceding the lesions and no surrounding classic LP lesions, the diagnosis may be less clear-cut. In such cases, the differential diagnosis includes lichen simplex chronicus, mycetoma, chromoblastomycosis, and squamous cell carcinoma.
Lichen simplex chronicus can be distinguished from HLP by reviewing the patient’s history. Patients who describe habitual rubbing or scratching of the area are likely to have lichen simplex chronicus. On exam, lichen simplex chronicus lesions are slightly erythematous, scaly, well-demarcated, and firm. There are rough plaques with exaggerated skin lines (lichenification) rather than the verrucous surface typically seen with HLP lesions. Wickham’s striae (seen in LP) are not seen with lichen simplex chronicus, and the lesions are localized only to easily reached areas.2
Mycetoma is a tumor-like lesion produced by a fungus (eumycetoma) or bacteria (actinomycetoma), typically encountered in arid areas rather than humid environments.3 These chronic, localized, nonpainful subcutaneous nodules develop on the foot and lower extremity after traumatic inoculation with the bacteria or fungus. Mycetomas persist for many years and classically present with a triad of tumefaction, draining sinus tracts, and “sulfur grains” that distinguish it from the dry, hyperkeratotic lesions of HLP. Diagnosis requires biopsy for histologic examination and both fungal and bacterial culture in order to choose the appropriate therapy.
Chromoblastomycosis is a deep fungal infection most commonly caused by the pigmented fungus Phialophora verrucosa found in tropical climates.4 The fungi enter the skin of the lower extremity after minor trauma, resulting in a gradually expanding verrucous nodule or plaque. The nodular variant is often pedunculated with classic pigmented cauliflower-like florets. While the nodular variant is localized, the plaque variant may spread laterally, possibly metastasizing through lymphatic channels with a concomitant bacterial infection. There is also a characteristic unpleasant odor with lymph stasis.
On potassium hydroxide (KOH) mounts or histologic examination, the thick-walled cells (muriform bodies) of chromoblastomycosis are diagnostic. Patients with chromoblastomycosis have seen response rates >60% with 10 to 24 months of daily itraconazole (200 mg) therapy.5
Squamous cell carcinoma (SCC) is the second most common skin cancer and affects more than 250,000 Americans each year. While associated with sun exposure, it has also been linked to ionizing radiation, arsenic, human papilloma virus, cigarette smoking, and chronic nonhealing wounds and scars such as Marjolin’s ulcer.1
Marjolin’s ulcer usually appears as a triad of nodule formation, induration, and ulceration at a scar site and thus may be confused with HLP. It is more common than sun-induced SCC in Asian and dark-skinned individuals.6 Marjolin’s ulcer will usually present in the fifth decade, years after the initial insult. Diagnosis is supported by the clinical appearance and history of a preceding scar at the site. Marjolin’s ulcer has a higher rate of recurrence and metastasis than other forms of SCC, and thus should be treated aggressively.7,8
A biopsy may be needed
A drop of immersion oil can confirm your HLP suspicions by revealing the white, lacy reticular network of Wickham’s striae.1 Other clinical clues to the diagnosis of LP or one of its variants include a white reticular, erythematous, or ulcerative appearance on the buccal mucosa in addition to a dorsal pterygium and/or diffuse pitting on the nails.
A deep shave or punch biopsy may be necessary, however, when the clinical diagnosis is unclear. Histological findings demonstrate focal hyperorthokeratosis, saw-toothed rete ridges, vacuolar change at the basal layer, and a band-like lymphocytic infiltrate.
Corticosteroids are the treatment of choice
There have been few large-scale prospective studies exploring the treatment of HLP. However, treatment for HLP is similar to that of LP and typically begins with topical class I or II glucocorticoids or intralesional injections of triamcinolone. Narrow-band ultraviolet-B (UVB) markedly reduces pruritus and flattens plaques, and is considered second-line treatment (strength of recommendation [SOR]: C).9-11 The retinoid acitretin may be effective for severe HLP at oral dosages of 30 mg/d for 8 weeks (SOR: A).12 Azathioprine and cyclosporine have also been used successfully, but risk of renal dysfunction, hypertension, and increased viral and fungal infections make these agents third-line therapies (SOR: C).13-15
A good outcome for our patient
Our patient applied clobetasol ointment 0.05% to the affected areas twice daily until the lesions went away (approximately 2 months later).
CORRESPONDENCE
Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/SGOMD/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; wiscoderm@yahoo.com
1. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
2. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia, Pa: Mosby; 2004:65.
3. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2301.
4. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2375.
5. Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31(3 Pt 2):S91-S102.
6. Chuang TY, Reizner GT, Elpern DJ, et al. Nonmelanoma skin cancer in Japanese ethnic Hawaiians in Kauai, Hawaii: an incidence report. J Am Acad Dermatol. 1995;33:422-426.
7. Treves N, Pack GT. The development of cancer in burn scars. An analysis and report of thirty-four cases. Surg Gynecol Obstet. 1930;51:749.-
8. Lifeso RM, Rooney RJ, el-Shaker M. Post-traumatic squamous-cell carcinoma. J Bone Joint Surg. 1990;72:12-18.
9. Gambichler T, Breuckmann F, Boms S, et al. Narrow-band UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
10. Saricaoglu H, Karadogan SK, Baskan EB. Narrowband UVB therapy in the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2003;19:265-267.
11. Taneja A, Taylor CR. Narrow-band UVB for lichen planus treatment. Int J Dermatol. 2002;41:282-283.
12. Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin: a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol 1991;24:434-437.
13. Kossard S, Artemi P. Acitretin for hypertrophic lichen planus-like reaction in a burn scar. Arch Dermatol. 2000;136:591-594.
14. Lear JT, English JS. Erosive and generalized lichen planus responsive to azathioprine. Clin Exp Dermatol. 1996;21:56-57.
15. Ho VC, Gupta AK, Ellis CN, et al. Treatment of severe lichen planus with cyclosporine. J Am Acad Dermatol. 1990;22:64-68.
A 56-year-old woman came into our medical center complaining of multiple pruritic, slowly growing scaly nodules over her right ankle (FIGURE 1A AND 1B). She indicated that the lesions started as small pink “bumps” at the staple sites of an open reduction and internal fixation surgery of her talus that she’d had 8 years ago.
There were no lesions elsewhere on her body and her past medical history was otherwise unremarkable.
FIGURE 1
Multiple pruritic, scaly nodules
What is your diagnosis?
How would you manage this condition?
Diagnosis: Hypertrophic lichen planus
Hypertrophic lichen planus (HLP), a variant of lichen planus (LP), is a lesion that is usually found on the distal extremities. HLP plaques evolve from initial characteristic LP lesions (purple, planar, pruritic, polygonal papules or plaque) to form reddish-brown to violaceous, hypertrophic, verrucous round-to-elongated plaques. Primary lesions may be spread by scratching or other trauma and often develop dark brown hyperpigmentation over several years. Like other variants of LP, HLP most commonly affects adults 30 to 60 years of age, with a slight female predominance.1
HLP may be idiopathic, drug induced, or associated with a systemic disease. Although many drugs have been linked to this lesion, the most commonly reported medications are gold salts, beta-blockers, antimalarials, thiazides, furosemide, and penicillamines. If your patient has HLP and is taking one of these medications, you should consider discontinuing the medication.1 As with other forms of LP, HLP has been associated with hepatitis C. Consider transaminases and a hepatitis panel for all patients with HLP. Other HLP-associated conditions include venous insufficiency, herpes simplex virus, and varicella-zoster virus.1
When the history confuses the diagnosis
When there are surrounding classic LP lesions, the diagnosis of HLP is fairly straightforward. However, when the patient has a history of surgery or trauma preceding the lesions and no surrounding classic LP lesions, the diagnosis may be less clear-cut. In such cases, the differential diagnosis includes lichen simplex chronicus, mycetoma, chromoblastomycosis, and squamous cell carcinoma.
Lichen simplex chronicus can be distinguished from HLP by reviewing the patient’s history. Patients who describe habitual rubbing or scratching of the area are likely to have lichen simplex chronicus. On exam, lichen simplex chronicus lesions are slightly erythematous, scaly, well-demarcated, and firm. There are rough plaques with exaggerated skin lines (lichenification) rather than the verrucous surface typically seen with HLP lesions. Wickham’s striae (seen in LP) are not seen with lichen simplex chronicus, and the lesions are localized only to easily reached areas.2
Mycetoma is a tumor-like lesion produced by a fungus (eumycetoma) or bacteria (actinomycetoma), typically encountered in arid areas rather than humid environments.3 These chronic, localized, nonpainful subcutaneous nodules develop on the foot and lower extremity after traumatic inoculation with the bacteria or fungus. Mycetomas persist for many years and classically present with a triad of tumefaction, draining sinus tracts, and “sulfur grains” that distinguish it from the dry, hyperkeratotic lesions of HLP. Diagnosis requires biopsy for histologic examination and both fungal and bacterial culture in order to choose the appropriate therapy.
Chromoblastomycosis is a deep fungal infection most commonly caused by the pigmented fungus Phialophora verrucosa found in tropical climates.4 The fungi enter the skin of the lower extremity after minor trauma, resulting in a gradually expanding verrucous nodule or plaque. The nodular variant is often pedunculated with classic pigmented cauliflower-like florets. While the nodular variant is localized, the plaque variant may spread laterally, possibly metastasizing through lymphatic channels with a concomitant bacterial infection. There is also a characteristic unpleasant odor with lymph stasis.
On potassium hydroxide (KOH) mounts or histologic examination, the thick-walled cells (muriform bodies) of chromoblastomycosis are diagnostic. Patients with chromoblastomycosis have seen response rates >60% with 10 to 24 months of daily itraconazole (200 mg) therapy.5
Squamous cell carcinoma (SCC) is the second most common skin cancer and affects more than 250,000 Americans each year. While associated with sun exposure, it has also been linked to ionizing radiation, arsenic, human papilloma virus, cigarette smoking, and chronic nonhealing wounds and scars such as Marjolin’s ulcer.1
Marjolin’s ulcer usually appears as a triad of nodule formation, induration, and ulceration at a scar site and thus may be confused with HLP. It is more common than sun-induced SCC in Asian and dark-skinned individuals.6 Marjolin’s ulcer will usually present in the fifth decade, years after the initial insult. Diagnosis is supported by the clinical appearance and history of a preceding scar at the site. Marjolin’s ulcer has a higher rate of recurrence and metastasis than other forms of SCC, and thus should be treated aggressively.7,8
A biopsy may be needed
A drop of immersion oil can confirm your HLP suspicions by revealing the white, lacy reticular network of Wickham’s striae.1 Other clinical clues to the diagnosis of LP or one of its variants include a white reticular, erythematous, or ulcerative appearance on the buccal mucosa in addition to a dorsal pterygium and/or diffuse pitting on the nails.
A deep shave or punch biopsy may be necessary, however, when the clinical diagnosis is unclear. Histological findings demonstrate focal hyperorthokeratosis, saw-toothed rete ridges, vacuolar change at the basal layer, and a band-like lymphocytic infiltrate.
Corticosteroids are the treatment of choice
There have been few large-scale prospective studies exploring the treatment of HLP. However, treatment for HLP is similar to that of LP and typically begins with topical class I or II glucocorticoids or intralesional injections of triamcinolone. Narrow-band ultraviolet-B (UVB) markedly reduces pruritus and flattens plaques, and is considered second-line treatment (strength of recommendation [SOR]: C).9-11 The retinoid acitretin may be effective for severe HLP at oral dosages of 30 mg/d for 8 weeks (SOR: A).12 Azathioprine and cyclosporine have also been used successfully, but risk of renal dysfunction, hypertension, and increased viral and fungal infections make these agents third-line therapies (SOR: C).13-15
A good outcome for our patient
Our patient applied clobetasol ointment 0.05% to the affected areas twice daily until the lesions went away (approximately 2 months later).
CORRESPONDENCE
Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/SGOMD/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; wiscoderm@yahoo.com
A 56-year-old woman came into our medical center complaining of multiple pruritic, slowly growing scaly nodules over her right ankle (FIGURE 1A AND 1B). She indicated that the lesions started as small pink “bumps” at the staple sites of an open reduction and internal fixation surgery of her talus that she’d had 8 years ago.
There were no lesions elsewhere on her body and her past medical history was otherwise unremarkable.
FIGURE 1
Multiple pruritic, scaly nodules
What is your diagnosis?
How would you manage this condition?
Diagnosis: Hypertrophic lichen planus
Hypertrophic lichen planus (HLP), a variant of lichen planus (LP), is a lesion that is usually found on the distal extremities. HLP plaques evolve from initial characteristic LP lesions (purple, planar, pruritic, polygonal papules or plaque) to form reddish-brown to violaceous, hypertrophic, verrucous round-to-elongated plaques. Primary lesions may be spread by scratching or other trauma and often develop dark brown hyperpigmentation over several years. Like other variants of LP, HLP most commonly affects adults 30 to 60 years of age, with a slight female predominance.1
HLP may be idiopathic, drug induced, or associated with a systemic disease. Although many drugs have been linked to this lesion, the most commonly reported medications are gold salts, beta-blockers, antimalarials, thiazides, furosemide, and penicillamines. If your patient has HLP and is taking one of these medications, you should consider discontinuing the medication.1 As with other forms of LP, HLP has been associated with hepatitis C. Consider transaminases and a hepatitis panel for all patients with HLP. Other HLP-associated conditions include venous insufficiency, herpes simplex virus, and varicella-zoster virus.1
When the history confuses the diagnosis
When there are surrounding classic LP lesions, the diagnosis of HLP is fairly straightforward. However, when the patient has a history of surgery or trauma preceding the lesions and no surrounding classic LP lesions, the diagnosis may be less clear-cut. In such cases, the differential diagnosis includes lichen simplex chronicus, mycetoma, chromoblastomycosis, and squamous cell carcinoma.
Lichen simplex chronicus can be distinguished from HLP by reviewing the patient’s history. Patients who describe habitual rubbing or scratching of the area are likely to have lichen simplex chronicus. On exam, lichen simplex chronicus lesions are slightly erythematous, scaly, well-demarcated, and firm. There are rough plaques with exaggerated skin lines (lichenification) rather than the verrucous surface typically seen with HLP lesions. Wickham’s striae (seen in LP) are not seen with lichen simplex chronicus, and the lesions are localized only to easily reached areas.2
Mycetoma is a tumor-like lesion produced by a fungus (eumycetoma) or bacteria (actinomycetoma), typically encountered in arid areas rather than humid environments.3 These chronic, localized, nonpainful subcutaneous nodules develop on the foot and lower extremity after traumatic inoculation with the bacteria or fungus. Mycetomas persist for many years and classically present with a triad of tumefaction, draining sinus tracts, and “sulfur grains” that distinguish it from the dry, hyperkeratotic lesions of HLP. Diagnosis requires biopsy for histologic examination and both fungal and bacterial culture in order to choose the appropriate therapy.
Chromoblastomycosis is a deep fungal infection most commonly caused by the pigmented fungus Phialophora verrucosa found in tropical climates.4 The fungi enter the skin of the lower extremity after minor trauma, resulting in a gradually expanding verrucous nodule or plaque. The nodular variant is often pedunculated with classic pigmented cauliflower-like florets. While the nodular variant is localized, the plaque variant may spread laterally, possibly metastasizing through lymphatic channels with a concomitant bacterial infection. There is also a characteristic unpleasant odor with lymph stasis.
On potassium hydroxide (KOH) mounts or histologic examination, the thick-walled cells (muriform bodies) of chromoblastomycosis are diagnostic. Patients with chromoblastomycosis have seen response rates >60% with 10 to 24 months of daily itraconazole (200 mg) therapy.5
Squamous cell carcinoma (SCC) is the second most common skin cancer and affects more than 250,000 Americans each year. While associated with sun exposure, it has also been linked to ionizing radiation, arsenic, human papilloma virus, cigarette smoking, and chronic nonhealing wounds and scars such as Marjolin’s ulcer.1
Marjolin’s ulcer usually appears as a triad of nodule formation, induration, and ulceration at a scar site and thus may be confused with HLP. It is more common than sun-induced SCC in Asian and dark-skinned individuals.6 Marjolin’s ulcer will usually present in the fifth decade, years after the initial insult. Diagnosis is supported by the clinical appearance and history of a preceding scar at the site. Marjolin’s ulcer has a higher rate of recurrence and metastasis than other forms of SCC, and thus should be treated aggressively.7,8
A biopsy may be needed
A drop of immersion oil can confirm your HLP suspicions by revealing the white, lacy reticular network of Wickham’s striae.1 Other clinical clues to the diagnosis of LP or one of its variants include a white reticular, erythematous, or ulcerative appearance on the buccal mucosa in addition to a dorsal pterygium and/or diffuse pitting on the nails.
A deep shave or punch biopsy may be necessary, however, when the clinical diagnosis is unclear. Histological findings demonstrate focal hyperorthokeratosis, saw-toothed rete ridges, vacuolar change at the basal layer, and a band-like lymphocytic infiltrate.
Corticosteroids are the treatment of choice
There have been few large-scale prospective studies exploring the treatment of HLP. However, treatment for HLP is similar to that of LP and typically begins with topical class I or II glucocorticoids or intralesional injections of triamcinolone. Narrow-band ultraviolet-B (UVB) markedly reduces pruritus and flattens plaques, and is considered second-line treatment (strength of recommendation [SOR]: C).9-11 The retinoid acitretin may be effective for severe HLP at oral dosages of 30 mg/d for 8 weeks (SOR: A).12 Azathioprine and cyclosporine have also been used successfully, but risk of renal dysfunction, hypertension, and increased viral and fungal infections make these agents third-line therapies (SOR: C).13-15
A good outcome for our patient
Our patient applied clobetasol ointment 0.05% to the affected areas twice daily until the lesions went away (approximately 2 months later).
CORRESPONDENCE
Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/SGOMD/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; wiscoderm@yahoo.com
1. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
2. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia, Pa: Mosby; 2004:65.
3. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2301.
4. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2375.
5. Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31(3 Pt 2):S91-S102.
6. Chuang TY, Reizner GT, Elpern DJ, et al. Nonmelanoma skin cancer in Japanese ethnic Hawaiians in Kauai, Hawaii: an incidence report. J Am Acad Dermatol. 1995;33:422-426.
7. Treves N, Pack GT. The development of cancer in burn scars. An analysis and report of thirty-four cases. Surg Gynecol Obstet. 1930;51:749.-
8. Lifeso RM, Rooney RJ, el-Shaker M. Post-traumatic squamous-cell carcinoma. J Bone Joint Surg. 1990;72:12-18.
9. Gambichler T, Breuckmann F, Boms S, et al. Narrow-band UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
10. Saricaoglu H, Karadogan SK, Baskan EB. Narrowband UVB therapy in the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2003;19:265-267.
11. Taneja A, Taylor CR. Narrow-band UVB for lichen planus treatment. Int J Dermatol. 2002;41:282-283.
12. Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin: a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol 1991;24:434-437.
13. Kossard S, Artemi P. Acitretin for hypertrophic lichen planus-like reaction in a burn scar. Arch Dermatol. 2000;136:591-594.
14. Lear JT, English JS. Erosive and generalized lichen planus responsive to azathioprine. Clin Exp Dermatol. 1996;21:56-57.
15. Ho VC, Gupta AK, Ellis CN, et al. Treatment of severe lichen planus with cyclosporine. J Am Acad Dermatol. 1990;22:64-68.
1. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
2. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia, Pa: Mosby; 2004:65.
3. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2301.
4. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2375.
5. Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31(3 Pt 2):S91-S102.
6. Chuang TY, Reizner GT, Elpern DJ, et al. Nonmelanoma skin cancer in Japanese ethnic Hawaiians in Kauai, Hawaii: an incidence report. J Am Acad Dermatol. 1995;33:422-426.
7. Treves N, Pack GT. The development of cancer in burn scars. An analysis and report of thirty-four cases. Surg Gynecol Obstet. 1930;51:749.-
8. Lifeso RM, Rooney RJ, el-Shaker M. Post-traumatic squamous-cell carcinoma. J Bone Joint Surg. 1990;72:12-18.
9. Gambichler T, Breuckmann F, Boms S, et al. Narrow-band UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
10. Saricaoglu H, Karadogan SK, Baskan EB. Narrowband UVB therapy in the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2003;19:265-267.
11. Taneja A, Taylor CR. Narrow-band UVB for lichen planus treatment. Int J Dermatol. 2002;41:282-283.
12. Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin: a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol 1991;24:434-437.
13. Kossard S, Artemi P. Acitretin for hypertrophic lichen planus-like reaction in a burn scar. Arch Dermatol. 2000;136:591-594.
14. Lear JT, English JS. Erosive and generalized lichen planus responsive to azathioprine. Clin Exp Dermatol. 1996;21:56-57.
15. Ho VC, Gupta AK, Ellis CN, et al. Treatment of severe lichen planus with cyclosporine. J Am Acad Dermatol. 1990;22:64-68.