User login
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States, with an estimated 52,550 deaths in 2023.1 However, the disease burden varies among different segments of the population.2 While both CRC incidence and mortality have been decreasing due to screening and advances in treatment, there are disparities in incidence and mortality across the sociodemographic spectrum including race, ethnicity, education, and income.1-4 While CRC incidence is decreasing for older adults, it is increasing among those aged < 55 years.5 The incidence of CRC in adults aged 40 to 54 years has increased by 0.5% to 1.3% annually since the mid-1990s.6 The US Preventive Services Task Force now recommends starting CRC screening at age 45 years for asymptomatic adults with average risk.7
Disparities also exist across geographical boundaries and living environment. Rural Americans faces additional challenges in health and lifestyle that can affect CRC outcomes. Compared to their urban counterparts, rural residents are more likely to be older, have lower levels of education, higher levels of poverty, lack health insurance, and less access to health care practitioners (HCPs).8-10 Geographic proximity, defined as travel time or physical distance to a health facility, has been recognized as a predictor of inferior outcomes.11 These aspects of rural living may pose challenges for accessing care for CRC screening and treatment.11-13 National and local studies have shown disparities in CRC screening rates, incidence, and mortality between rural and urban populations.14-16
It is unclear whether rural/urban disparities persist under the Veterans Health Administration (VHA) health care delivery model. This study examined differences in baseline characteristics and mortality between rural and urban veterans newly diagnosed with CRC. We also focused on a subpopulation aged ≤ 45 years.
Methods
This study extracted national data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) hosted in the VA Informatics and Computing Infrastructure (VINCI) environment. VINCI is an initiative to improve access to VA data and facilitate the analysis of these data while ensuring veterans’ privacy and data security.17 CDW is the VHA business intelligence information repository, which extracts data from clinical and nonclinical sources following prescribed and validated protocols. Data extracted included demographics, diagnosis, and procedure codes for both inpatient and outpatient encounters, vital signs, and vital status. This study used data previously extracted from a national cohort of veterans that encompassed all patients who received a group of commonly prescribed medications, such as statins, proton pump inhibitors, histamine-2 blockers, acetaminophen-containing products, and hydrocortisone-containing skin applications. This cohort encompassed 8,648,754 veterans, from whom 2,460,727 had encounters during fiscal years (FY) 2016 to 2021 (study period). The cohort was used to ensure that subjects were VHA patients, allowing them to adequately capture their clinical profiles.
Patients were identified as rural or urban based on their residence address at the date of their first diagnosis of CRC. The Geospatial Service Support Center (GSSC) aggregates and updates veterans’ residence address records for all enrolled veterans from the National Change of Address database. The data contain 1 record per enrollee. GSSC Geocoded Enrollee File contains enrollee addresses and their rurality indicators, categorized as urban, rural, or highly rural.18 Rurality is defined by the Rural Urban Commuting Area (RUCA) categories developed by the Department of Agriculture and the Health Resources and Services Administration of the US Department of Health and Human Services.19 Urban areas had RUCA codes of 1.0 to 1.1, and highly rural areas had RUCA scores of 10.0. All other areas were classified as rural. Since the proportion of veterans from highly rural areas was small, we included residents from highly rural areas in the rural residents’ group.
Inclusion and Exclusion Criteria
All veterans newly diagnosed with CRC from FY 2016 to 2021 were included. We used the ninth and tenth clinical modification revisions of the International Classification of Diseases (ICD-9-CM and ICD-10-CM) to define CRC diagnosis (Supplemental materials).4,20 To ensure that patients were newly diagnosed with CRC, this study excluded patients with a previous ICD-9-CM code for CRC diagnosis since FY 2003.
Comorbidities were identified using diagnosis and procedure codes from inpatient and outpatient encounters, which were used to calculate the Charlson Comorbidity Index (CCI) at the time of CRC diagnosis using the weighted method described by Schneeweiss et al.21 We defined CRC high-risk conditions and CRC screening tests, including flexible sigmoidoscopy and stool tests, as described in previous studies (Supplemental materials).20
The main outcome was total mortality. The date of death was extracted from the VHA Death Ascertainment File, which contains mortality data from the Master Person Index file in CDW and the Social Security Administration Death Master File. We used the date of death from any cause, as cause of death was not available.
A propensity score (PS) was created to match rural (including highly rural) and urban residents at a ratio of 1:1. Using a standard procedure described in prior publications, multivariable logistic regression used all baseline characteristics to estimate the PS and perform nearest-number matching without replacement.22,23 A caliper of 0.01 maximized the matched cohort size and achieved balance (Supplemental materials). We then examined the balance of baseline characteristics between PS-matched groups.
Analyses
Cox proportional hazards regression analysis estimated the hazard ratio (HR) of death in rural residents compared to urban residents in the PS-matched cohort. The outcome event was the date of death during the study’s follow-up period (defined as period from first CRC diagnosis to death or study end), with censoring at the study’s end date (September 30, 2021). The proportional hazards assumption was assessed by inspecting the Kaplan-Meier curves. Multiple analyses examined the HR of total mortality in the PS-matched cohort, stratified by sex, race, and ethnicity. We also examined the HR of total mortality stratified by duration of follow-up.
Another PS-matching analysis among veterans aged ≤ 45 years was performed using the same techniques described earlier in this article. We performed a Cox proportional hazards regression analysis to compare mortality in PS-matched urban and rural veterans aged ≤ 45 years. The HR of death in all veterans aged ≤ 45 years (before PS-matching) was estimated using Cox proportional hazard regression analysis, adjusting for PS.
Dichotomous variables were compared using X2 tests and continuous variables were compared using t tests. Baseline characteristics with missing values were converted into categorical variables and the proportion of subjects with missing values was equalized between treatment groups after PS-matching. For subgroup analysis, we examined the HR of total mortality in each subgroup using separate Cox proportional hazards regression models similar to the primary analysis but adjusted for PS. Due to multiple comparisons in the subgroup analysis, the findings should be considered exploratory. Statistical tests were 2-tailed, and significance was defined as P < .05. Data management and statistical analyses were conducted from June 2022 to January 2023 using STATA, Version 17. The VA Orlando Healthcare System Institutional Review Board approved the study and waived requirements for informed consent because only deidentified data were used.
Results
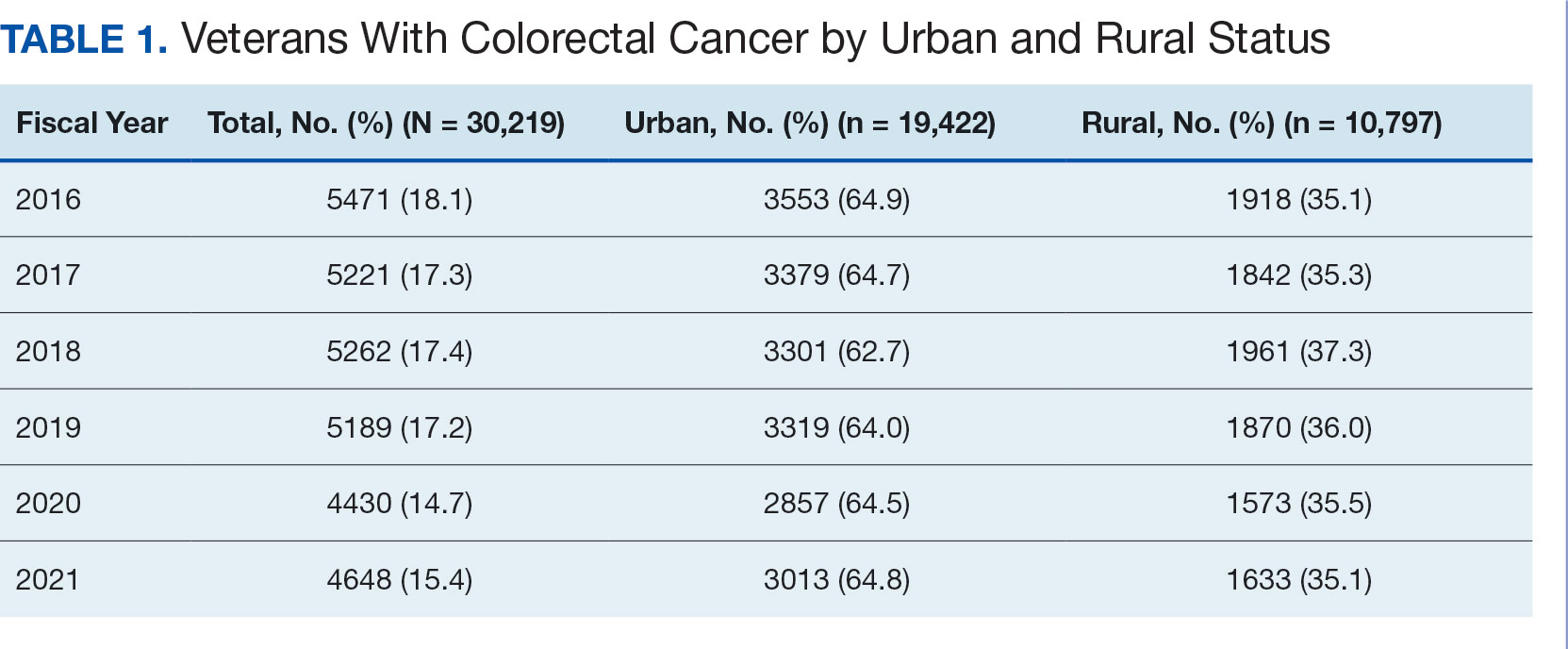
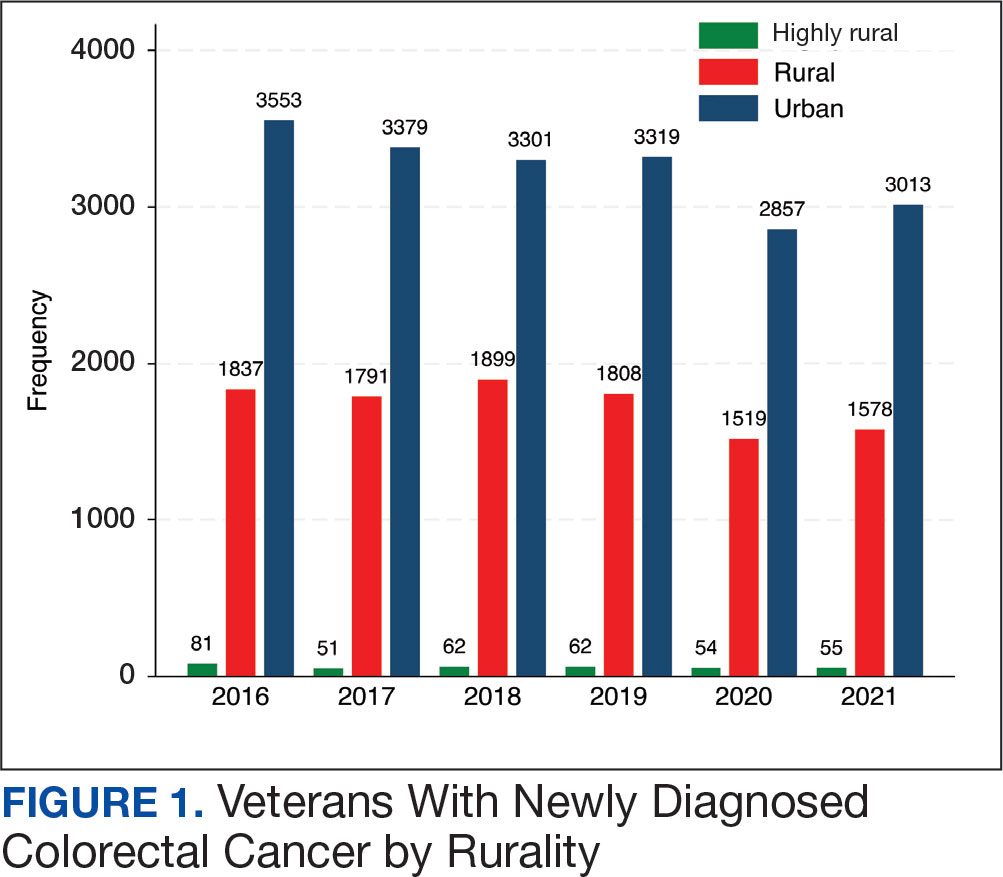
After excluding 49 patients (Supplemental materials, available at doi:10.12788/fp.0560), we identified 30,219 veterans with newly diagnosed CRC between FY 2016 to 2021 (Table 1). Of these, 19,422 (64.3%) resided in urban areas and 10,797 (35.7%) resided in rural areas (Table 2). The mean (SD) duration from the first CRC diagnosis to death or study end was 832 (640) days, and the median (IQR) was 723 (246–1330) days. Overall, incident CRC diagnoses were numerically highest in FY 2016 and lowest in FY 2020 (Figure 1). Patients with CRC in rural areas vs urban areas were significantly older (mean, 71.2 years vs 70.8 years, respectively; P < .001), more likely to be male (96.7% vs 95.7%, respectively; P < .001), more likely to be White (83.6% vs 67.8%, respectively; P < .001) and more likely to be non-Hispanic (92.2% vs 87.5%, respectively; P < .001). In terms of general health, rural veterans with CRC were more likely to be overweight or obese (81.5% rural vs 78.5% urban; P < .001) but had fewer mean comorbidities as measured by CCI (5.66 rural vs 5.90 urban; P < .001). A higher proportion of rural veterans with CRC had received stool-based (fecal occult blood test or fecal immunochemical test) CRC screening tests (61.6% rural vs 57.2% urban; P < .001). Fewer rural patients presented with systemic symptoms or signs within 1 year of CRC diagnosis (54.4% rural vs 57.5% urban, P < .001). Among urban patients with CRC, 6959 (35.8%) deaths were observed, compared with 3766 (34.9%) among rural patients (P = .10).
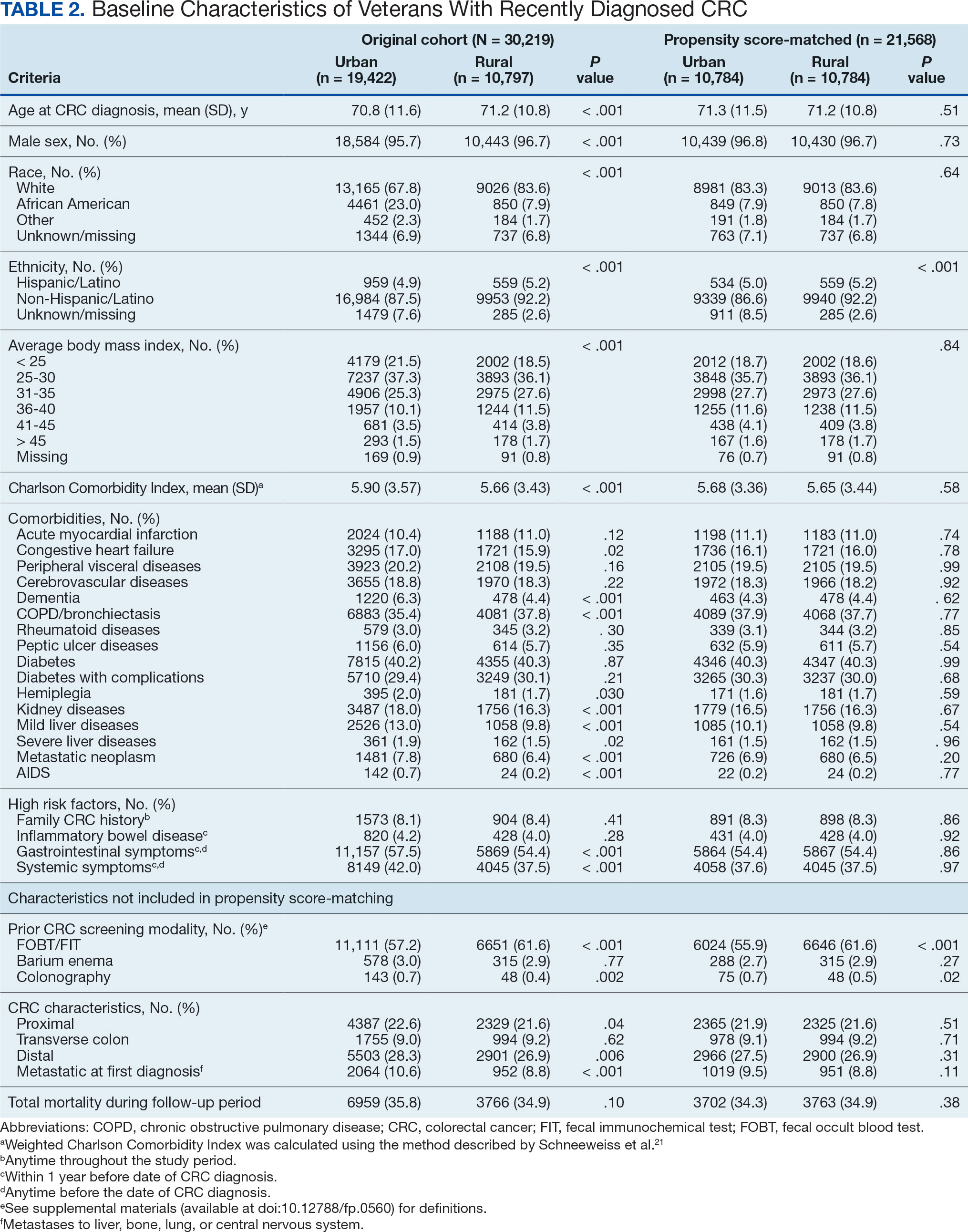
There were 21,568 PS-matched veterans: 10,784 in each group. In the PS-matched cohort, baseline characteristics were similar between veterans in urban and rural communities, including age, sex, race/ethnicity, body mass index, and comorbidities. Among rural patients with CRC, 3763 deaths (34.9%) were observed compared with 3702 (34.3%) among urban veterans. There was no significant difference in the HR of mortality between rural and urban CRC residents (HR, 1.01; 95% CI, 0.97-1.06; P = .53) (Figure 2).
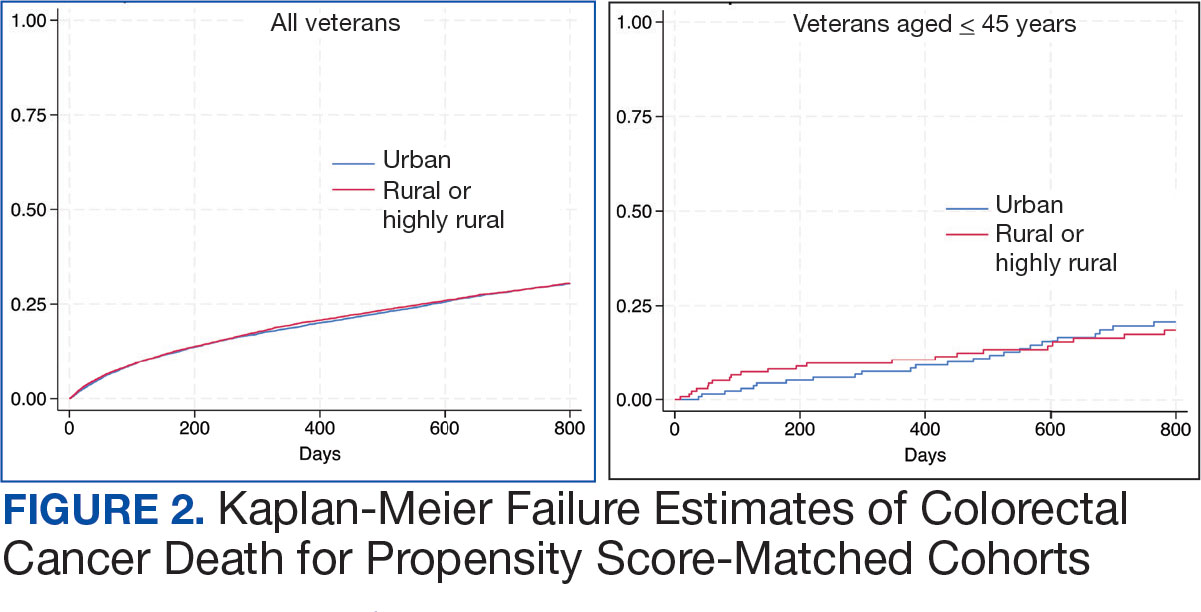
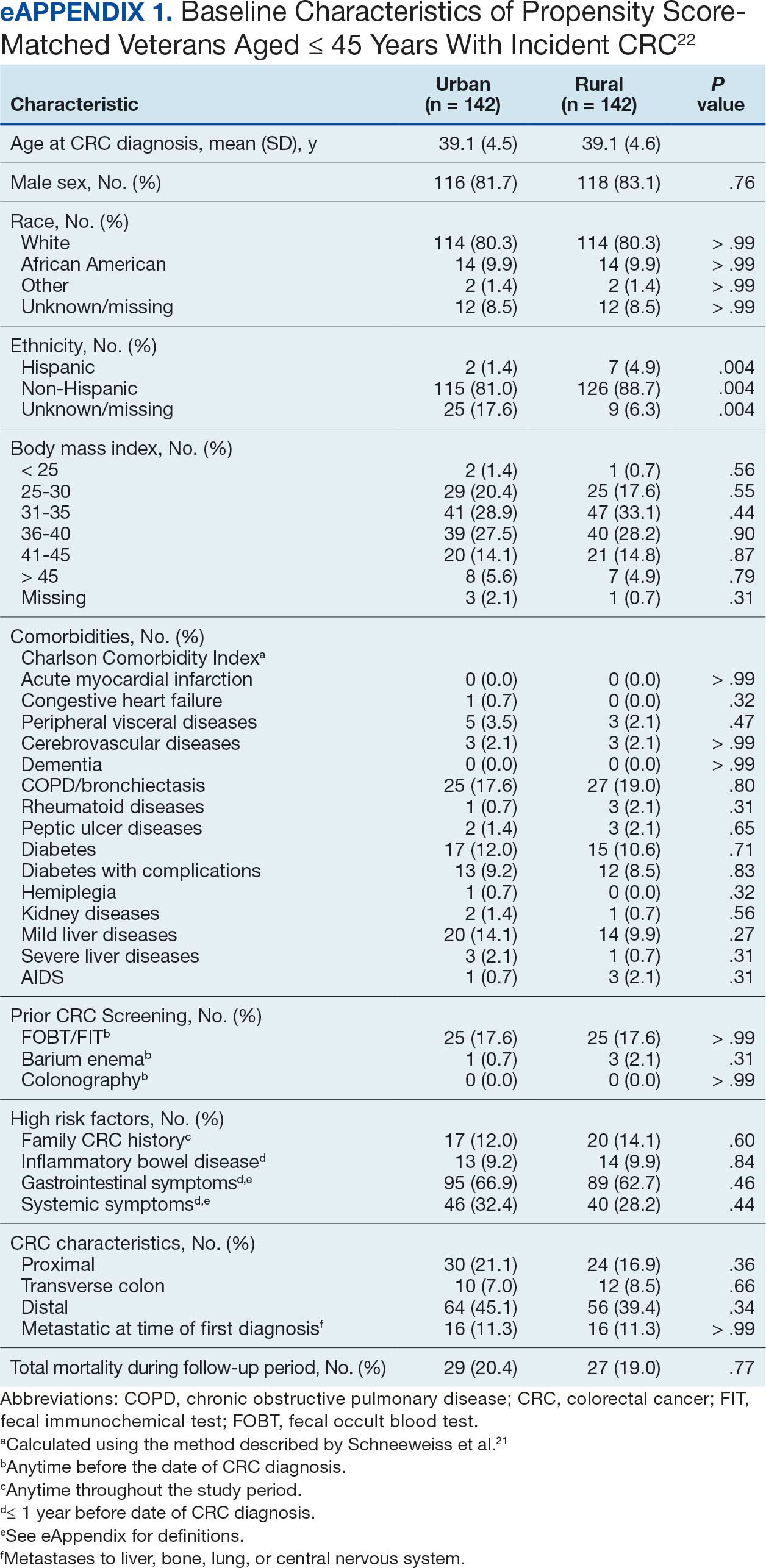
Among veterans aged ≤ 45 years, 551 were diagnosed with CRC (391 urban and 160 rural). We PS-matched 142 pairs of urban and rural veterans without residual differences in baseline characteristics (eAppendix 1). There was no significant difference in the HR of mortality between rural and urban veterans aged ≤ 45 years (HR, 0.97; 95% CI, 0.57-1.63; P = .90) (Figure 2). Similarly, no difference in mortality was observed adjusting for PS between all rural and urban veterans aged ≤ 45 years (HR, 1.03; 95% CI, 0.67-1.59; P = .88).
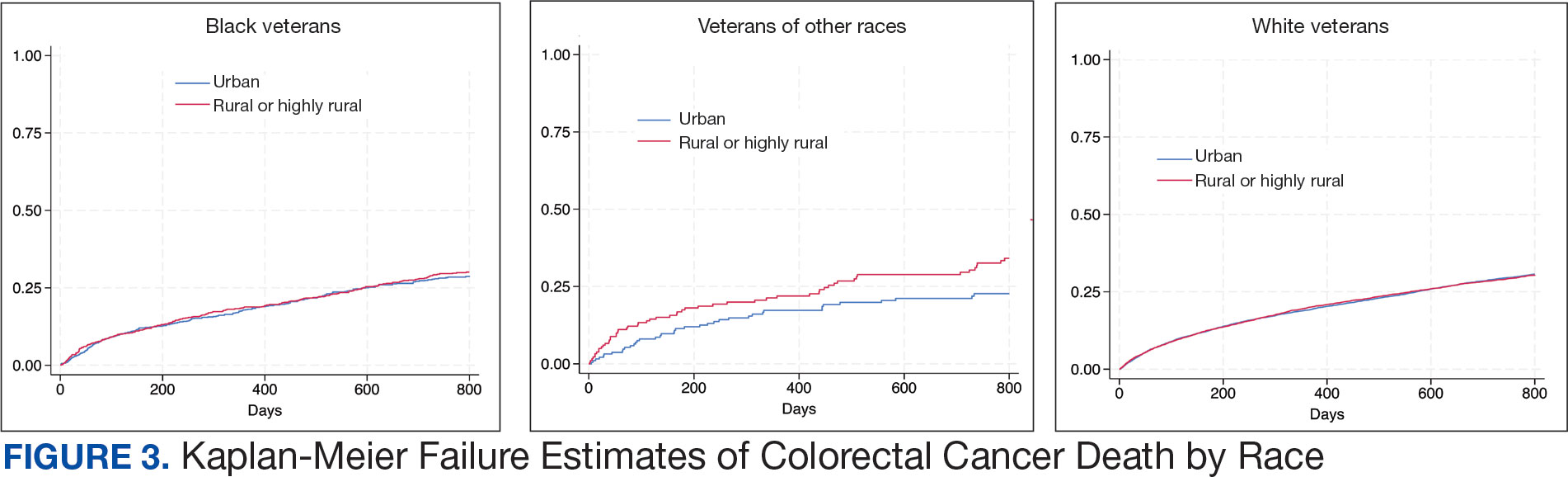
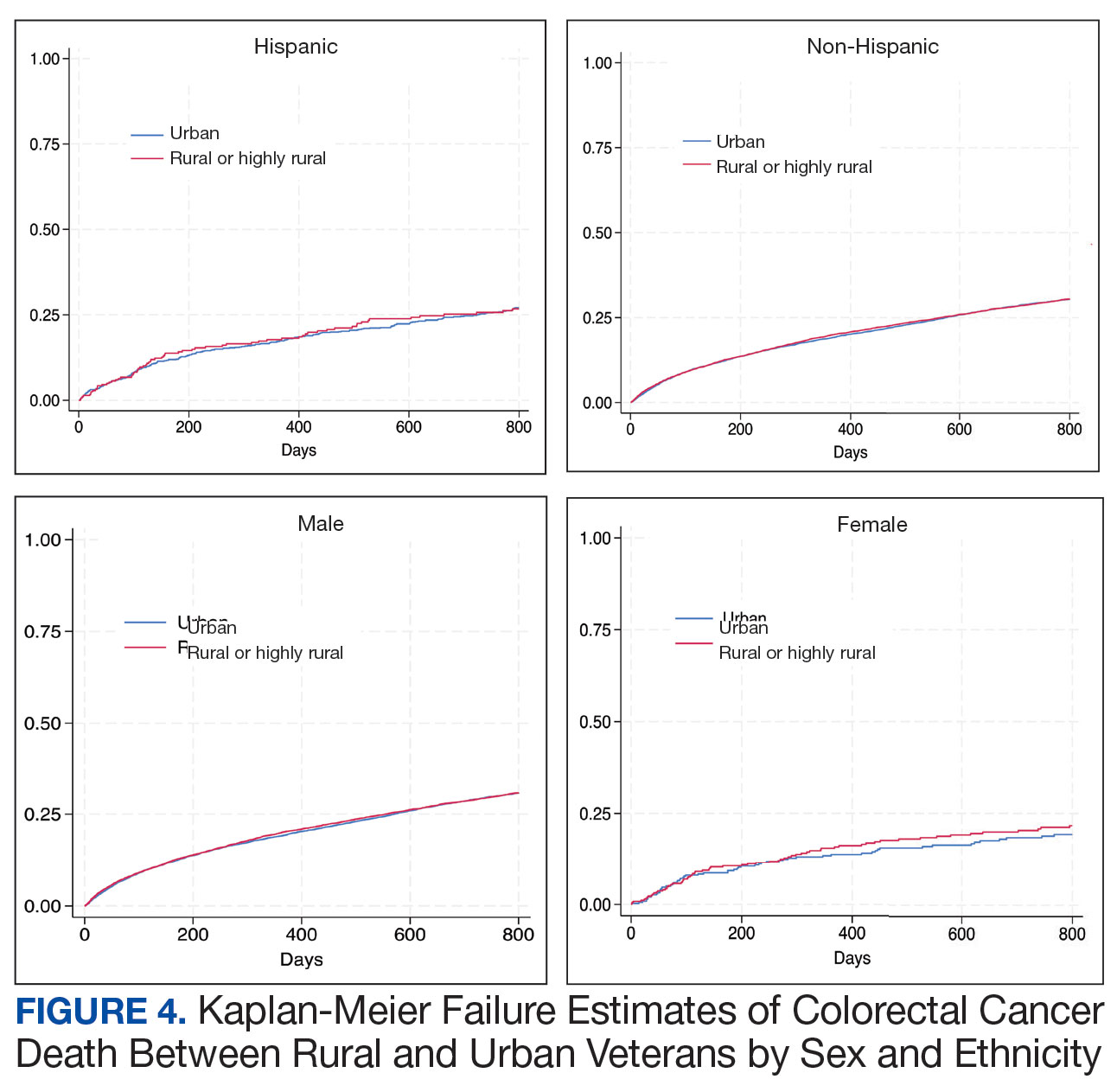
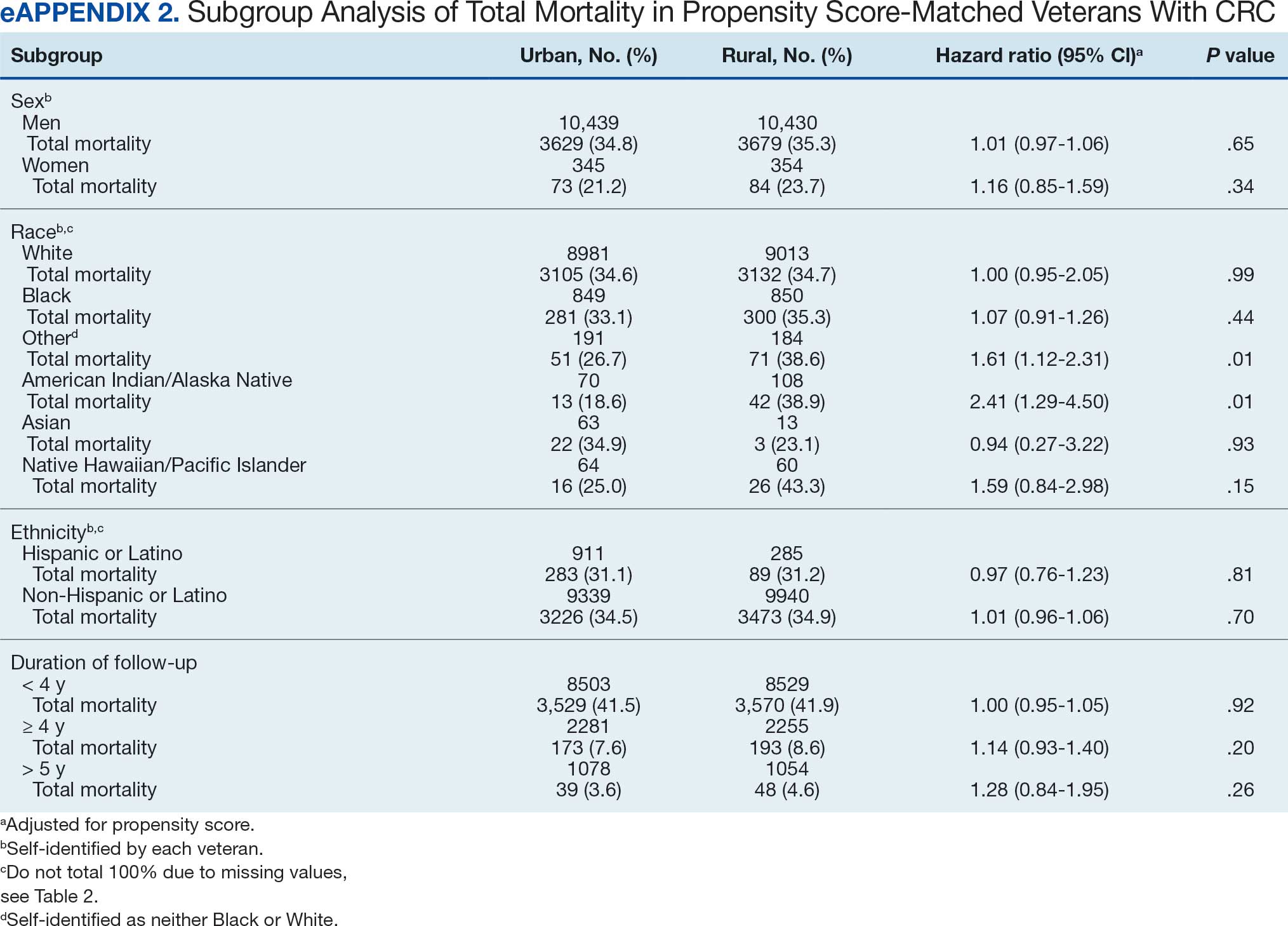
There was no difference in total mortality between rural and urban veterans in any subgroup except for American Indian or Alaska Native veterans (HR, 2.41; 95% CI, 1.29-4.50; P = .006) (eAppendix 2).
Discussion
This study examined characteristics of patients with CRC between urban and rural areas among veterans who were VHA patients. Similar to other studies, rural veterans with CRC were older, more likely to be White, and were obese, but exhibited fewer comorbidities (lower CCI and lower incidence of congestive heart failure, dementia, hemiplegia, kidney diseases, liver diseases and AIDS, but higher incidence of chronic obstructive lung disease).8,16 The incidence of CRC in this study population was lowest in FY 2020, which was reported by the Centers for Disease Control and Prevention and is attributed to COVID-19 pandemic disruption of health services.24 The overall mortality in this study was similar to rates reported in other studies from the VA Central Cancer Registry.4 In the PS-matched cohort, where baseline characteristics were similar between urban and rural patients with CRC, we found no disparities in CRC-specific mortality between veterans in rural and urban areas. Additionally, when analysis was restricted to veterans aged ≤ 45 years, the results remained consistent.
Subgroup analyses showed no significant difference in mortality between rural and urban areas by sex, race or ethnicity, except rural American Indian or Alaska Native veterans who had double the mortality of their urban counterparts (HR, 2.41; 95% CI, 1.29-4.50; P = .006). This finding is difficult to interpret due to the small number of events and the wide CI. While with a Bonferroni correction the adjusted P value was .08, which is not statistically significant, a previous study found that although CRC incidence was lower overall in American Indian or Alaska Native populations compared to non-Hispanic White populations, CRC incidence was higher among American Indian or Alaska Native individuals in some areas such as Alaska and the Northern Plains.25,26 Studies have noted that rural American Indian/Alaska Native populations experience greater poverty, less access to broadband internet, and limited access to care, contributing to poorer cancer outcomes and lower survival.27 Thus, the finding of disparity in mortality between rural and urban American Indian or Alaska Native veterans warrants further study.
Other studies have raised concerns that CRC disproportionately affects adults in rural areas with higher mortality rates.14-16 These disparities arise from sociodemographic factors and modifiable risk factors, including physical activity, dietary patterns, access to cancer screening, and gaps in quality treatment resources.16,28 These factors operate at multiple levels: from individual, local health system, to community and policy.2,27 For example, a South Carolina study (1996–2016) found that residents in rural areas were more likely to be diagnosed with advanced CRC, possibly indicating lower rates of CRC screening in rural areas. They also had higher likelihood of death from CRC.15 However, the study did not include any clinical parameters, such as comorbidities or obesity. A statewide, population-based study in Utah showed that rural men experienced a lower CRC survival in their unadjusted analysis.16 However, the study was small, with only 3948 urban and 712 rural residents. Additionally, there was no difference in total mortality in the whole cohort (HR, 0.96; 95% CI, 0.86-1.07) or in CRC-specific death (HR, 0.93; 95% CI, 0.81-1.08). A nationwide study also showed that CRC mortality rates were 8% higher in nonmetropolitan or rural areas than in the most urbanized areas containing large metropolitan counties.29 However, this study did not include descriptions of clinical confounders, such as comorbidities, making it difficult to ascertain whether the difference in CRC mortality was due to rurality or differences in baseline risk characteristics.
In this study, the lack of CRC-specific mortality disparities may be attributed to the structures and practices of VHA health care. Recent studies have noted that mortality of several chronic medical conditions treated at the VHA was lower than at non-VHA hospitals.30,31 One study that measured the quality of nonmetastatic CRC care based on National Comprehensive Cancer Network guidelines showed that > 72% of VHA patients received guideline-concordant care for each diagnostic and therapeutic measure, except for follow-up colonoscopy timing, which appear to be similar or superior to that of the private sector.30,32,33 Some of the VA initiative for CRC screening may bypass the urban-rurality divide such as the mailed fecal immunochemical test program for CRC. This program was implemented at the onset of the COVID-19 pandemic to avoid disruptions of medical care.34 Rural patients are more likely to undergo fecal immunochemical testing when compared to urban patients in this data. Beyond clinical care, the VHA uses processes to tackle social determinants of health such as housing, food security, and transportation, promoting equal access to health care, and promoting cultural competency among HCPs.35-37
The results suggest that solutions to CRC disparities between rural and urban areas need to consider known barriers to rural health care, including transportation, diminished rural health care workforce, and other social determinants of health.9,10,27,38 VHA makes considerable efforts to provide equitable care to all enrolled veterans, including specific programs for rural veterans, including ongoing outreach.39 This study demonstrated lack of disparity in CRC-specific mortality in veterans receiving VHA care, highlighting the importance of these efforts.
Strengths and Limitations
This study used the VHA cohort to compare patient characteristics and mortality between patients with CRC residing in rural and urban areas. The study provides nationwide perspectives on CRC across the geographical spectrum and used a longitudinal cohort with prolonged follow-up to account for comorbidities.
However, the study compared a cohort of rural and urban veterans enrolled in the VHA; hence, the results may not reflect CRC outcomes in veterans without access to VHA care. Rurality has been independently associated with decreased likelihood of meeting CRC screening guidelines among veterans and military service members.38 This study lacked sufficient information to compare CRC staging or treatment modalities among veterans. Although the data cannot identify CRC stage, the proportions of patients with metastatic CRC at diagnosis and CRC location were similar between groups. The study did not have information on their care outside of VHA setting.
This study could not ascertain whether disparities existed in CRC treatment modality since rural residence may result in referral to community-based CRC care, which did not appear in the data. To address these limitations, we used death from any cause as the primary outcome, since death is a hard outcome and is not subject to ascertainment bias. The relatively short follow-up time is another limitation, though subgroup analysis by follow-up did not show significant differences. Despite PS matching, residual unmeasured confounding may exist between urban and rural groups. The predominantly White, male VHA population with high CCI may limit the generalizability of the results.
Conclusions
Rural VHA enrollees had similar survival rates after CRC diagnosis compared to their urban counterparts in a PS-matched analysis. The VHA models of care—including mailed CRC screening tools, several socioeconomic determinants of health (housing, food security, and transportation), and promoting equal access to health care, as well as cultural competency among HCPs—HCPs—may help alleviate disparities across the rural-urban spectrum. The VHA should continue efforts to enroll veterans and provide comprehensive coordinated care in community partnerships.
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi:10.1053/j.gastro.2019.10.029
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668-75. doi:10.1002/ijc.25481
- Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs central cancer registry. Clin Colorectal Cancer. 2016;15(4):e199-e204. doi:10.1016/j.clcc.2016.04.005
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force. 2021. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews:Chapter 1. Agency for Healthcare Research and Quality (US); 2021. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK570917/
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw322
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
- Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63-e71. doi:10.2105/AJPH.2013.301572
- Cauwels J. The many barriers to high-quality rural health care. 2022;(9):1-32. NEJM Catal Innov Care Deliv. Accessed April 24, 2025. https://catalyst.nejm.org/doi/pdf/10.1056/CAT.22.0254
- Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Aff (Millwood);38(12):2003-2010. doi:10.1377/hlthaff.2019.00722
- Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg. 2014;149(6):537-543. doi:10.1001/jamasurg.2013.5062
- Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131-138. doi:10.1016/s1040-8428(01)00139-1
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341-378. doi:10.1146/annurev.publhealth.18.1.341
- Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
- Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996 - 2016. J Rural Health. 2022;38(1):34-39. doi:10.1111/jrh.12580
- Rogers CR, Blackburn BE, Huntington M, et al. Rural- urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes Control. 2020;31(3):241-253. doi:10.1007/s10552-020-01268-2
- US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. https://vincicentral.vinci.med.va.gov [Source not verified]
- US Department of Veterans Affairs Information Resource Center. VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition. US Department of Veterans Affairs, Health Services Research & Development Service, Information Resource Center; May. 2016. [source not verified]
- Goldsmith HF, Puskin DS, Stiles DJ. Improving the operational definition of “rural areas” for federal programs. US Department of Health and Human Services; 1993. Accessed February 27, 2025. https://www.ruralhealthinfo.org/pdf/improving-the-operational-definition-of-rural-areas.pdf
- Adams MA, Kerr EA, Dominitz JA, et al. Development and validation of a new ICD-10-based screening colonoscopy overuse measure in a large integrated healthcare system: a retrospective observational study. BMJ Qual Saf. 2023;32(7):414-424. doi:10.1136/bmjqs-2021-014236
- Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103-1120. doi:10.1111/1475-6773.00165
- Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2(4):358-377.
- Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical software components. Revised February 1, 2018. Accessed February 27, 2025. https://ideas.repec.org/c/boc/bocode/s432001.html.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool. Centers for Disease Control and Prevention. June 2024. Accessed February 27, 2025. https://www.cdc.gov/cancer/dataviz
- Cao J, Zhang S. Multiple Comparison Procedures. JAMA. 2014;312(5):543-544. doi:10.1001/jama.2014.9440
- Gopalani SV, Janitz AE, Martinez SA, et al. Trends in cancer incidence among American Indians and Alaska Natives and Non-Hispanic Whites in the United States, 1999-2015. Epidemiology. 2020;31(2):205-213. doi:10.1097/EDE.0000000000001140
- Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4). doi:10.3390/ijerph18041384
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi:10.1158/1055-9965.EPI-17-0092
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part iicolorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi:10.1155/2011/107497
- Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181. doi:10.1200/JCO.2009.26.7948
- Yoon J, Phibbs CS, Ong MK, et al. Outcomes of veterans treated in Veterans Affairs hospitals vs non-Veterans Affairs hospitals. JAMA Netw Open. 2023;6(12):e2345898. doi:10.1001/jamanetworkopen.2023.45898
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626-634. doi:10.1200/JCO.2005.03.3365
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595. doi:10.1053/j.gastro.2008.02.002
- Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4). doi:10.1136/bmjoq-2022-001927
- Yehia BR, Greenstone CL, Hosenfeld CB, Matthews KL, Zephyrin LC. The role of VA community care in addressing health and health care disparities. Med Care. 2017;55(Suppl 9 suppl 2):S4-S5. doi:10.1097/MLR.0000000000000768
- Wright BN, MacDermid Wadsworth S, Wellnitz A, Eicher- Miller HA. Reaching rural veterans: a new mechanism to connect rural, low-income US Veterans with resources and improve food security. J Public Health (Oxf). 2019;41(4):714-723. doi:10.1093/pubmed/fdy203
- Nelson RE, Byrne TH, Suo Y, et al. Association of temporary financial assistance with housing stability among US veterans in the supportive services for veteran families program. JAMA Netw Open. 2021;4(2):e2037047. doi:10.1001/jamanetworkopen.2020.37047
- McDaniel JT, Albright D, Lee HY, et al. Rural–urban disparities in colorectal cancer screening among military service members and Veterans. J Mil Veteran Fam Health. 2019;5(1):40-48. doi:10.3138/jmvfh.2018-0013
- US Department of Veterans Affairs, Office of Rural Health. The rural veteran outreach toolkit. Updated February 12, 2025. Accessed February 18, 2025. https://www.ruralhealth.va.gov/partners/toolkit.asp
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States, with an estimated 52,550 deaths in 2023.1 However, the disease burden varies among different segments of the population.2 While both CRC incidence and mortality have been decreasing due to screening and advances in treatment, there are disparities in incidence and mortality across the sociodemographic spectrum including race, ethnicity, education, and income.1-4 While CRC incidence is decreasing for older adults, it is increasing among those aged < 55 years.5 The incidence of CRC in adults aged 40 to 54 years has increased by 0.5% to 1.3% annually since the mid-1990s.6 The US Preventive Services Task Force now recommends starting CRC screening at age 45 years for asymptomatic adults with average risk.7
Disparities also exist across geographical boundaries and living environment. Rural Americans faces additional challenges in health and lifestyle that can affect CRC outcomes. Compared to their urban counterparts, rural residents are more likely to be older, have lower levels of education, higher levels of poverty, lack health insurance, and less access to health care practitioners (HCPs).8-10 Geographic proximity, defined as travel time or physical distance to a health facility, has been recognized as a predictor of inferior outcomes.11 These aspects of rural living may pose challenges for accessing care for CRC screening and treatment.11-13 National and local studies have shown disparities in CRC screening rates, incidence, and mortality between rural and urban populations.14-16
It is unclear whether rural/urban disparities persist under the Veterans Health Administration (VHA) health care delivery model. This study examined differences in baseline characteristics and mortality between rural and urban veterans newly diagnosed with CRC. We also focused on a subpopulation aged ≤ 45 years.
Methods
This study extracted national data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) hosted in the VA Informatics and Computing Infrastructure (VINCI) environment. VINCI is an initiative to improve access to VA data and facilitate the analysis of these data while ensuring veterans’ privacy and data security.17 CDW is the VHA business intelligence information repository, which extracts data from clinical and nonclinical sources following prescribed and validated protocols. Data extracted included demographics, diagnosis, and procedure codes for both inpatient and outpatient encounters, vital signs, and vital status. This study used data previously extracted from a national cohort of veterans that encompassed all patients who received a group of commonly prescribed medications, such as statins, proton pump inhibitors, histamine-2 blockers, acetaminophen-containing products, and hydrocortisone-containing skin applications. This cohort encompassed 8,648,754 veterans, from whom 2,460,727 had encounters during fiscal years (FY) 2016 to 2021 (study period). The cohort was used to ensure that subjects were VHA patients, allowing them to adequately capture their clinical profiles.
Patients were identified as rural or urban based on their residence address at the date of their first diagnosis of CRC. The Geospatial Service Support Center (GSSC) aggregates and updates veterans’ residence address records for all enrolled veterans from the National Change of Address database. The data contain 1 record per enrollee. GSSC Geocoded Enrollee File contains enrollee addresses and their rurality indicators, categorized as urban, rural, or highly rural.18 Rurality is defined by the Rural Urban Commuting Area (RUCA) categories developed by the Department of Agriculture and the Health Resources and Services Administration of the US Department of Health and Human Services.19 Urban areas had RUCA codes of 1.0 to 1.1, and highly rural areas had RUCA scores of 10.0. All other areas were classified as rural. Since the proportion of veterans from highly rural areas was small, we included residents from highly rural areas in the rural residents’ group.
Inclusion and Exclusion Criteria
All veterans newly diagnosed with CRC from FY 2016 to 2021 were included. We used the ninth and tenth clinical modification revisions of the International Classification of Diseases (ICD-9-CM and ICD-10-CM) to define CRC diagnosis (Supplemental materials).4,20 To ensure that patients were newly diagnosed with CRC, this study excluded patients with a previous ICD-9-CM code for CRC diagnosis since FY 2003.
Comorbidities were identified using diagnosis and procedure codes from inpatient and outpatient encounters, which were used to calculate the Charlson Comorbidity Index (CCI) at the time of CRC diagnosis using the weighted method described by Schneeweiss et al.21 We defined CRC high-risk conditions and CRC screening tests, including flexible sigmoidoscopy and stool tests, as described in previous studies (Supplemental materials).20
The main outcome was total mortality. The date of death was extracted from the VHA Death Ascertainment File, which contains mortality data from the Master Person Index file in CDW and the Social Security Administration Death Master File. We used the date of death from any cause, as cause of death was not available.
A propensity score (PS) was created to match rural (including highly rural) and urban residents at a ratio of 1:1. Using a standard procedure described in prior publications, multivariable logistic regression used all baseline characteristics to estimate the PS and perform nearest-number matching without replacement.22,23 A caliper of 0.01 maximized the matched cohort size and achieved balance (Supplemental materials). We then examined the balance of baseline characteristics between PS-matched groups.
Analyses
Cox proportional hazards regression analysis estimated the hazard ratio (HR) of death in rural residents compared to urban residents in the PS-matched cohort. The outcome event was the date of death during the study’s follow-up period (defined as period from first CRC diagnosis to death or study end), with censoring at the study’s end date (September 30, 2021). The proportional hazards assumption was assessed by inspecting the Kaplan-Meier curves. Multiple analyses examined the HR of total mortality in the PS-matched cohort, stratified by sex, race, and ethnicity. We also examined the HR of total mortality stratified by duration of follow-up.
Another PS-matching analysis among veterans aged ≤ 45 years was performed using the same techniques described earlier in this article. We performed a Cox proportional hazards regression analysis to compare mortality in PS-matched urban and rural veterans aged ≤ 45 years. The HR of death in all veterans aged ≤ 45 years (before PS-matching) was estimated using Cox proportional hazard regression analysis, adjusting for PS.
Dichotomous variables were compared using X2 tests and continuous variables were compared using t tests. Baseline characteristics with missing values were converted into categorical variables and the proportion of subjects with missing values was equalized between treatment groups after PS-matching. For subgroup analysis, we examined the HR of total mortality in each subgroup using separate Cox proportional hazards regression models similar to the primary analysis but adjusted for PS. Due to multiple comparisons in the subgroup analysis, the findings should be considered exploratory. Statistical tests were 2-tailed, and significance was defined as P < .05. Data management and statistical analyses were conducted from June 2022 to January 2023 using STATA, Version 17. The VA Orlando Healthcare System Institutional Review Board approved the study and waived requirements for informed consent because only deidentified data were used.
Results
After excluding 49 patients (Supplemental materials, available at doi:10.12788/fp.0560), we identified 30,219 veterans with newly diagnosed CRC between FY 2016 to 2021 (Table 1). Of these, 19,422 (64.3%) resided in urban areas and 10,797 (35.7%) resided in rural areas (Table 2). The mean (SD) duration from the first CRC diagnosis to death or study end was 832 (640) days, and the median (IQR) was 723 (246–1330) days. Overall, incident CRC diagnoses were numerically highest in FY 2016 and lowest in FY 2020 (Figure 1). Patients with CRC in rural areas vs urban areas were significantly older (mean, 71.2 years vs 70.8 years, respectively; P < .001), more likely to be male (96.7% vs 95.7%, respectively; P < .001), more likely to be White (83.6% vs 67.8%, respectively; P < .001) and more likely to be non-Hispanic (92.2% vs 87.5%, respectively; P < .001). In terms of general health, rural veterans with CRC were more likely to be overweight or obese (81.5% rural vs 78.5% urban; P < .001) but had fewer mean comorbidities as measured by CCI (5.66 rural vs 5.90 urban; P < .001). A higher proportion of rural veterans with CRC had received stool-based (fecal occult blood test or fecal immunochemical test) CRC screening tests (61.6% rural vs 57.2% urban; P < .001). Fewer rural patients presented with systemic symptoms or signs within 1 year of CRC diagnosis (54.4% rural vs 57.5% urban, P < .001). Among urban patients with CRC, 6959 (35.8%) deaths were observed, compared with 3766 (34.9%) among rural patients (P = .10).



There were 21,568 PS-matched veterans: 10,784 in each group. In the PS-matched cohort, baseline characteristics were similar between veterans in urban and rural communities, including age, sex, race/ethnicity, body mass index, and comorbidities. Among rural patients with CRC, 3763 deaths (34.9%) were observed compared with 3702 (34.3%) among urban veterans. There was no significant difference in the HR of mortality between rural and urban CRC residents (HR, 1.01; 95% CI, 0.97-1.06; P = .53) (Figure 2).



Among veterans aged ≤ 45 years, 551 were diagnosed with CRC (391 urban and 160 rural). We PS-matched 142 pairs of urban and rural veterans without residual differences in baseline characteristics (eAppendix 1). There was no significant difference in the HR of mortality between rural and urban veterans aged ≤ 45 years (HR, 0.97; 95% CI, 0.57-1.63; P = .90) (Figure 2). Similarly, no difference in mortality was observed adjusting for PS between all rural and urban veterans aged ≤ 45 years (HR, 1.03; 95% CI, 0.67-1.59; P = .88).

There was no difference in total mortality between rural and urban veterans in any subgroup except for American Indian or Alaska Native veterans (HR, 2.41; 95% CI, 1.29-4.50; P = .006) (eAppendix 2).

Discussion
This study examined characteristics of patients with CRC between urban and rural areas among veterans who were VHA patients. Similar to other studies, rural veterans with CRC were older, more likely to be White, and were obese, but exhibited fewer comorbidities (lower CCI and lower incidence of congestive heart failure, dementia, hemiplegia, kidney diseases, liver diseases and AIDS, but higher incidence of chronic obstructive lung disease).8,16 The incidence of CRC in this study population was lowest in FY 2020, which was reported by the Centers for Disease Control and Prevention and is attributed to COVID-19 pandemic disruption of health services.24 The overall mortality in this study was similar to rates reported in other studies from the VA Central Cancer Registry.4 In the PS-matched cohort, where baseline characteristics were similar between urban and rural patients with CRC, we found no disparities in CRC-specific mortality between veterans in rural and urban areas. Additionally, when analysis was restricted to veterans aged ≤ 45 years, the results remained consistent.
Subgroup analyses showed no significant difference in mortality between rural and urban areas by sex, race or ethnicity, except rural American Indian or Alaska Native veterans who had double the mortality of their urban counterparts (HR, 2.41; 95% CI, 1.29-4.50; P = .006). This finding is difficult to interpret due to the small number of events and the wide CI. While with a Bonferroni correction the adjusted P value was .08, which is not statistically significant, a previous study found that although CRC incidence was lower overall in American Indian or Alaska Native populations compared to non-Hispanic White populations, CRC incidence was higher among American Indian or Alaska Native individuals in some areas such as Alaska and the Northern Plains.25,26 Studies have noted that rural American Indian/Alaska Native populations experience greater poverty, less access to broadband internet, and limited access to care, contributing to poorer cancer outcomes and lower survival.27 Thus, the finding of disparity in mortality between rural and urban American Indian or Alaska Native veterans warrants further study.
Other studies have raised concerns that CRC disproportionately affects adults in rural areas with higher mortality rates.14-16 These disparities arise from sociodemographic factors and modifiable risk factors, including physical activity, dietary patterns, access to cancer screening, and gaps in quality treatment resources.16,28 These factors operate at multiple levels: from individual, local health system, to community and policy.2,27 For example, a South Carolina study (1996–2016) found that residents in rural areas were more likely to be diagnosed with advanced CRC, possibly indicating lower rates of CRC screening in rural areas. They also had higher likelihood of death from CRC.15 However, the study did not include any clinical parameters, such as comorbidities or obesity. A statewide, population-based study in Utah showed that rural men experienced a lower CRC survival in their unadjusted analysis.16 However, the study was small, with only 3948 urban and 712 rural residents. Additionally, there was no difference in total mortality in the whole cohort (HR, 0.96; 95% CI, 0.86-1.07) or in CRC-specific death (HR, 0.93; 95% CI, 0.81-1.08). A nationwide study also showed that CRC mortality rates were 8% higher in nonmetropolitan or rural areas than in the most urbanized areas containing large metropolitan counties.29 However, this study did not include descriptions of clinical confounders, such as comorbidities, making it difficult to ascertain whether the difference in CRC mortality was due to rurality or differences in baseline risk characteristics.
In this study, the lack of CRC-specific mortality disparities may be attributed to the structures and practices of VHA health care. Recent studies have noted that mortality of several chronic medical conditions treated at the VHA was lower than at non-VHA hospitals.30,31 One study that measured the quality of nonmetastatic CRC care based on National Comprehensive Cancer Network guidelines showed that > 72% of VHA patients received guideline-concordant care for each diagnostic and therapeutic measure, except for follow-up colonoscopy timing, which appear to be similar or superior to that of the private sector.30,32,33 Some of the VA initiative for CRC screening may bypass the urban-rurality divide such as the mailed fecal immunochemical test program for CRC. This program was implemented at the onset of the COVID-19 pandemic to avoid disruptions of medical care.34 Rural patients are more likely to undergo fecal immunochemical testing when compared to urban patients in this data. Beyond clinical care, the VHA uses processes to tackle social determinants of health such as housing, food security, and transportation, promoting equal access to health care, and promoting cultural competency among HCPs.35-37
The results suggest that solutions to CRC disparities between rural and urban areas need to consider known barriers to rural health care, including transportation, diminished rural health care workforce, and other social determinants of health.9,10,27,38 VHA makes considerable efforts to provide equitable care to all enrolled veterans, including specific programs for rural veterans, including ongoing outreach.39 This study demonstrated lack of disparity in CRC-specific mortality in veterans receiving VHA care, highlighting the importance of these efforts.
Strengths and Limitations
This study used the VHA cohort to compare patient characteristics and mortality between patients with CRC residing in rural and urban areas. The study provides nationwide perspectives on CRC across the geographical spectrum and used a longitudinal cohort with prolonged follow-up to account for comorbidities.
However, the study compared a cohort of rural and urban veterans enrolled in the VHA; hence, the results may not reflect CRC outcomes in veterans without access to VHA care. Rurality has been independently associated with decreased likelihood of meeting CRC screening guidelines among veterans and military service members.38 This study lacked sufficient information to compare CRC staging or treatment modalities among veterans. Although the data cannot identify CRC stage, the proportions of patients with metastatic CRC at diagnosis and CRC location were similar between groups. The study did not have information on their care outside of VHA setting.
This study could not ascertain whether disparities existed in CRC treatment modality since rural residence may result in referral to community-based CRC care, which did not appear in the data. To address these limitations, we used death from any cause as the primary outcome, since death is a hard outcome and is not subject to ascertainment bias. The relatively short follow-up time is another limitation, though subgroup analysis by follow-up did not show significant differences. Despite PS matching, residual unmeasured confounding may exist between urban and rural groups. The predominantly White, male VHA population with high CCI may limit the generalizability of the results.
Conclusions
Rural VHA enrollees had similar survival rates after CRC diagnosis compared to their urban counterparts in a PS-matched analysis. The VHA models of care—including mailed CRC screening tools, several socioeconomic determinants of health (housing, food security, and transportation), and promoting equal access to health care, as well as cultural competency among HCPs—HCPs—may help alleviate disparities across the rural-urban spectrum. The VHA should continue efforts to enroll veterans and provide comprehensive coordinated care in community partnerships.
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States, with an estimated 52,550 deaths in 2023.1 However, the disease burden varies among different segments of the population.2 While both CRC incidence and mortality have been decreasing due to screening and advances in treatment, there are disparities in incidence and mortality across the sociodemographic spectrum including race, ethnicity, education, and income.1-4 While CRC incidence is decreasing for older adults, it is increasing among those aged < 55 years.5 The incidence of CRC in adults aged 40 to 54 years has increased by 0.5% to 1.3% annually since the mid-1990s.6 The US Preventive Services Task Force now recommends starting CRC screening at age 45 years for asymptomatic adults with average risk.7
Disparities also exist across geographical boundaries and living environment. Rural Americans faces additional challenges in health and lifestyle that can affect CRC outcomes. Compared to their urban counterparts, rural residents are more likely to be older, have lower levels of education, higher levels of poverty, lack health insurance, and less access to health care practitioners (HCPs).8-10 Geographic proximity, defined as travel time or physical distance to a health facility, has been recognized as a predictor of inferior outcomes.11 These aspects of rural living may pose challenges for accessing care for CRC screening and treatment.11-13 National and local studies have shown disparities in CRC screening rates, incidence, and mortality between rural and urban populations.14-16
It is unclear whether rural/urban disparities persist under the Veterans Health Administration (VHA) health care delivery model. This study examined differences in baseline characteristics and mortality between rural and urban veterans newly diagnosed with CRC. We also focused on a subpopulation aged ≤ 45 years.
Methods
This study extracted national data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) hosted in the VA Informatics and Computing Infrastructure (VINCI) environment. VINCI is an initiative to improve access to VA data and facilitate the analysis of these data while ensuring veterans’ privacy and data security.17 CDW is the VHA business intelligence information repository, which extracts data from clinical and nonclinical sources following prescribed and validated protocols. Data extracted included demographics, diagnosis, and procedure codes for both inpatient and outpatient encounters, vital signs, and vital status. This study used data previously extracted from a national cohort of veterans that encompassed all patients who received a group of commonly prescribed medications, such as statins, proton pump inhibitors, histamine-2 blockers, acetaminophen-containing products, and hydrocortisone-containing skin applications. This cohort encompassed 8,648,754 veterans, from whom 2,460,727 had encounters during fiscal years (FY) 2016 to 2021 (study period). The cohort was used to ensure that subjects were VHA patients, allowing them to adequately capture their clinical profiles.
Patients were identified as rural or urban based on their residence address at the date of their first diagnosis of CRC. The Geospatial Service Support Center (GSSC) aggregates and updates veterans’ residence address records for all enrolled veterans from the National Change of Address database. The data contain 1 record per enrollee. GSSC Geocoded Enrollee File contains enrollee addresses and their rurality indicators, categorized as urban, rural, or highly rural.18 Rurality is defined by the Rural Urban Commuting Area (RUCA) categories developed by the Department of Agriculture and the Health Resources and Services Administration of the US Department of Health and Human Services.19 Urban areas had RUCA codes of 1.0 to 1.1, and highly rural areas had RUCA scores of 10.0. All other areas were classified as rural. Since the proportion of veterans from highly rural areas was small, we included residents from highly rural areas in the rural residents’ group.
Inclusion and Exclusion Criteria
All veterans newly diagnosed with CRC from FY 2016 to 2021 were included. We used the ninth and tenth clinical modification revisions of the International Classification of Diseases (ICD-9-CM and ICD-10-CM) to define CRC diagnosis (Supplemental materials).4,20 To ensure that patients were newly diagnosed with CRC, this study excluded patients with a previous ICD-9-CM code for CRC diagnosis since FY 2003.
Comorbidities were identified using diagnosis and procedure codes from inpatient and outpatient encounters, which were used to calculate the Charlson Comorbidity Index (CCI) at the time of CRC diagnosis using the weighted method described by Schneeweiss et al.21 We defined CRC high-risk conditions and CRC screening tests, including flexible sigmoidoscopy and stool tests, as described in previous studies (Supplemental materials).20
The main outcome was total mortality. The date of death was extracted from the VHA Death Ascertainment File, which contains mortality data from the Master Person Index file in CDW and the Social Security Administration Death Master File. We used the date of death from any cause, as cause of death was not available.
A propensity score (PS) was created to match rural (including highly rural) and urban residents at a ratio of 1:1. Using a standard procedure described in prior publications, multivariable logistic regression used all baseline characteristics to estimate the PS and perform nearest-number matching without replacement.22,23 A caliper of 0.01 maximized the matched cohort size and achieved balance (Supplemental materials). We then examined the balance of baseline characteristics between PS-matched groups.
Analyses
Cox proportional hazards regression analysis estimated the hazard ratio (HR) of death in rural residents compared to urban residents in the PS-matched cohort. The outcome event was the date of death during the study’s follow-up period (defined as period from first CRC diagnosis to death or study end), with censoring at the study’s end date (September 30, 2021). The proportional hazards assumption was assessed by inspecting the Kaplan-Meier curves. Multiple analyses examined the HR of total mortality in the PS-matched cohort, stratified by sex, race, and ethnicity. We also examined the HR of total mortality stratified by duration of follow-up.
Another PS-matching analysis among veterans aged ≤ 45 years was performed using the same techniques described earlier in this article. We performed a Cox proportional hazards regression analysis to compare mortality in PS-matched urban and rural veterans aged ≤ 45 years. The HR of death in all veterans aged ≤ 45 years (before PS-matching) was estimated using Cox proportional hazard regression analysis, adjusting for PS.
Dichotomous variables were compared using X2 tests and continuous variables were compared using t tests. Baseline characteristics with missing values were converted into categorical variables and the proportion of subjects with missing values was equalized between treatment groups after PS-matching. For subgroup analysis, we examined the HR of total mortality in each subgroup using separate Cox proportional hazards regression models similar to the primary analysis but adjusted for PS. Due to multiple comparisons in the subgroup analysis, the findings should be considered exploratory. Statistical tests were 2-tailed, and significance was defined as P < .05. Data management and statistical analyses were conducted from June 2022 to January 2023 using STATA, Version 17. The VA Orlando Healthcare System Institutional Review Board approved the study and waived requirements for informed consent because only deidentified data were used.
Results
After excluding 49 patients (Supplemental materials, available at doi:10.12788/fp.0560), we identified 30,219 veterans with newly diagnosed CRC between FY 2016 to 2021 (Table 1). Of these, 19,422 (64.3%) resided in urban areas and 10,797 (35.7%) resided in rural areas (Table 2). The mean (SD) duration from the first CRC diagnosis to death or study end was 832 (640) days, and the median (IQR) was 723 (246–1330) days. Overall, incident CRC diagnoses were numerically highest in FY 2016 and lowest in FY 2020 (Figure 1). Patients with CRC in rural areas vs urban areas were significantly older (mean, 71.2 years vs 70.8 years, respectively; P < .001), more likely to be male (96.7% vs 95.7%, respectively; P < .001), more likely to be White (83.6% vs 67.8%, respectively; P < .001) and more likely to be non-Hispanic (92.2% vs 87.5%, respectively; P < .001). In terms of general health, rural veterans with CRC were more likely to be overweight or obese (81.5% rural vs 78.5% urban; P < .001) but had fewer mean comorbidities as measured by CCI (5.66 rural vs 5.90 urban; P < .001). A higher proportion of rural veterans with CRC had received stool-based (fecal occult blood test or fecal immunochemical test) CRC screening tests (61.6% rural vs 57.2% urban; P < .001). Fewer rural patients presented with systemic symptoms or signs within 1 year of CRC diagnosis (54.4% rural vs 57.5% urban, P < .001). Among urban patients with CRC, 6959 (35.8%) deaths were observed, compared with 3766 (34.9%) among rural patients (P = .10).



There were 21,568 PS-matched veterans: 10,784 in each group. In the PS-matched cohort, baseline characteristics were similar between veterans in urban and rural communities, including age, sex, race/ethnicity, body mass index, and comorbidities. Among rural patients with CRC, 3763 deaths (34.9%) were observed compared with 3702 (34.3%) among urban veterans. There was no significant difference in the HR of mortality between rural and urban CRC residents (HR, 1.01; 95% CI, 0.97-1.06; P = .53) (Figure 2).



Among veterans aged ≤ 45 years, 551 were diagnosed with CRC (391 urban and 160 rural). We PS-matched 142 pairs of urban and rural veterans without residual differences in baseline characteristics (eAppendix 1). There was no significant difference in the HR of mortality between rural and urban veterans aged ≤ 45 years (HR, 0.97; 95% CI, 0.57-1.63; P = .90) (Figure 2). Similarly, no difference in mortality was observed adjusting for PS between all rural and urban veterans aged ≤ 45 years (HR, 1.03; 95% CI, 0.67-1.59; P = .88).

There was no difference in total mortality between rural and urban veterans in any subgroup except for American Indian or Alaska Native veterans (HR, 2.41; 95% CI, 1.29-4.50; P = .006) (eAppendix 2).

Discussion
This study examined characteristics of patients with CRC between urban and rural areas among veterans who were VHA patients. Similar to other studies, rural veterans with CRC were older, more likely to be White, and were obese, but exhibited fewer comorbidities (lower CCI and lower incidence of congestive heart failure, dementia, hemiplegia, kidney diseases, liver diseases and AIDS, but higher incidence of chronic obstructive lung disease).8,16 The incidence of CRC in this study population was lowest in FY 2020, which was reported by the Centers for Disease Control and Prevention and is attributed to COVID-19 pandemic disruption of health services.24 The overall mortality in this study was similar to rates reported in other studies from the VA Central Cancer Registry.4 In the PS-matched cohort, where baseline characteristics were similar between urban and rural patients with CRC, we found no disparities in CRC-specific mortality between veterans in rural and urban areas. Additionally, when analysis was restricted to veterans aged ≤ 45 years, the results remained consistent.
Subgroup analyses showed no significant difference in mortality between rural and urban areas by sex, race or ethnicity, except rural American Indian or Alaska Native veterans who had double the mortality of their urban counterparts (HR, 2.41; 95% CI, 1.29-4.50; P = .006). This finding is difficult to interpret due to the small number of events and the wide CI. While with a Bonferroni correction the adjusted P value was .08, which is not statistically significant, a previous study found that although CRC incidence was lower overall in American Indian or Alaska Native populations compared to non-Hispanic White populations, CRC incidence was higher among American Indian or Alaska Native individuals in some areas such as Alaska and the Northern Plains.25,26 Studies have noted that rural American Indian/Alaska Native populations experience greater poverty, less access to broadband internet, and limited access to care, contributing to poorer cancer outcomes and lower survival.27 Thus, the finding of disparity in mortality between rural and urban American Indian or Alaska Native veterans warrants further study.
Other studies have raised concerns that CRC disproportionately affects adults in rural areas with higher mortality rates.14-16 These disparities arise from sociodemographic factors and modifiable risk factors, including physical activity, dietary patterns, access to cancer screening, and gaps in quality treatment resources.16,28 These factors operate at multiple levels: from individual, local health system, to community and policy.2,27 For example, a South Carolina study (1996–2016) found that residents in rural areas were more likely to be diagnosed with advanced CRC, possibly indicating lower rates of CRC screening in rural areas. They also had higher likelihood of death from CRC.15 However, the study did not include any clinical parameters, such as comorbidities or obesity. A statewide, population-based study in Utah showed that rural men experienced a lower CRC survival in their unadjusted analysis.16 However, the study was small, with only 3948 urban and 712 rural residents. Additionally, there was no difference in total mortality in the whole cohort (HR, 0.96; 95% CI, 0.86-1.07) or in CRC-specific death (HR, 0.93; 95% CI, 0.81-1.08). A nationwide study also showed that CRC mortality rates were 8% higher in nonmetropolitan or rural areas than in the most urbanized areas containing large metropolitan counties.29 However, this study did not include descriptions of clinical confounders, such as comorbidities, making it difficult to ascertain whether the difference in CRC mortality was due to rurality or differences in baseline risk characteristics.
In this study, the lack of CRC-specific mortality disparities may be attributed to the structures and practices of VHA health care. Recent studies have noted that mortality of several chronic medical conditions treated at the VHA was lower than at non-VHA hospitals.30,31 One study that measured the quality of nonmetastatic CRC care based on National Comprehensive Cancer Network guidelines showed that > 72% of VHA patients received guideline-concordant care for each diagnostic and therapeutic measure, except for follow-up colonoscopy timing, which appear to be similar or superior to that of the private sector.30,32,33 Some of the VA initiative for CRC screening may bypass the urban-rurality divide such as the mailed fecal immunochemical test program for CRC. This program was implemented at the onset of the COVID-19 pandemic to avoid disruptions of medical care.34 Rural patients are more likely to undergo fecal immunochemical testing when compared to urban patients in this data. Beyond clinical care, the VHA uses processes to tackle social determinants of health such as housing, food security, and transportation, promoting equal access to health care, and promoting cultural competency among HCPs.35-37
The results suggest that solutions to CRC disparities between rural and urban areas need to consider known barriers to rural health care, including transportation, diminished rural health care workforce, and other social determinants of health.9,10,27,38 VHA makes considerable efforts to provide equitable care to all enrolled veterans, including specific programs for rural veterans, including ongoing outreach.39 This study demonstrated lack of disparity in CRC-specific mortality in veterans receiving VHA care, highlighting the importance of these efforts.
Strengths and Limitations
This study used the VHA cohort to compare patient characteristics and mortality between patients with CRC residing in rural and urban areas. The study provides nationwide perspectives on CRC across the geographical spectrum and used a longitudinal cohort with prolonged follow-up to account for comorbidities.
However, the study compared a cohort of rural and urban veterans enrolled in the VHA; hence, the results may not reflect CRC outcomes in veterans without access to VHA care. Rurality has been independently associated with decreased likelihood of meeting CRC screening guidelines among veterans and military service members.38 This study lacked sufficient information to compare CRC staging or treatment modalities among veterans. Although the data cannot identify CRC stage, the proportions of patients with metastatic CRC at diagnosis and CRC location were similar between groups. The study did not have information on their care outside of VHA setting.
This study could not ascertain whether disparities existed in CRC treatment modality since rural residence may result in referral to community-based CRC care, which did not appear in the data. To address these limitations, we used death from any cause as the primary outcome, since death is a hard outcome and is not subject to ascertainment bias. The relatively short follow-up time is another limitation, though subgroup analysis by follow-up did not show significant differences. Despite PS matching, residual unmeasured confounding may exist between urban and rural groups. The predominantly White, male VHA population with high CCI may limit the generalizability of the results.
Conclusions
Rural VHA enrollees had similar survival rates after CRC diagnosis compared to their urban counterparts in a PS-matched analysis. The VHA models of care—including mailed CRC screening tools, several socioeconomic determinants of health (housing, food security, and transportation), and promoting equal access to health care, as well as cultural competency among HCPs—HCPs—may help alleviate disparities across the rural-urban spectrum. The VHA should continue efforts to enroll veterans and provide comprehensive coordinated care in community partnerships.
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi:10.1053/j.gastro.2019.10.029
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668-75. doi:10.1002/ijc.25481
- Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs central cancer registry. Clin Colorectal Cancer. 2016;15(4):e199-e204. doi:10.1016/j.clcc.2016.04.005
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force. 2021. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews:Chapter 1. Agency for Healthcare Research and Quality (US); 2021. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK570917/
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw322
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
- Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63-e71. doi:10.2105/AJPH.2013.301572
- Cauwels J. The many barriers to high-quality rural health care. 2022;(9):1-32. NEJM Catal Innov Care Deliv. Accessed April 24, 2025. https://catalyst.nejm.org/doi/pdf/10.1056/CAT.22.0254
- Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Aff (Millwood);38(12):2003-2010. doi:10.1377/hlthaff.2019.00722
- Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg. 2014;149(6):537-543. doi:10.1001/jamasurg.2013.5062
- Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131-138. doi:10.1016/s1040-8428(01)00139-1
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341-378. doi:10.1146/annurev.publhealth.18.1.341
- Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
- Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996 - 2016. J Rural Health. 2022;38(1):34-39. doi:10.1111/jrh.12580
- Rogers CR, Blackburn BE, Huntington M, et al. Rural- urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes Control. 2020;31(3):241-253. doi:10.1007/s10552-020-01268-2
- US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. https://vincicentral.vinci.med.va.gov [Source not verified]
- US Department of Veterans Affairs Information Resource Center. VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition. US Department of Veterans Affairs, Health Services Research & Development Service, Information Resource Center; May. 2016. [source not verified]
- Goldsmith HF, Puskin DS, Stiles DJ. Improving the operational definition of “rural areas” for federal programs. US Department of Health and Human Services; 1993. Accessed February 27, 2025. https://www.ruralhealthinfo.org/pdf/improving-the-operational-definition-of-rural-areas.pdf
- Adams MA, Kerr EA, Dominitz JA, et al. Development and validation of a new ICD-10-based screening colonoscopy overuse measure in a large integrated healthcare system: a retrospective observational study. BMJ Qual Saf. 2023;32(7):414-424. doi:10.1136/bmjqs-2021-014236
- Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103-1120. doi:10.1111/1475-6773.00165
- Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2(4):358-377.
- Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical software components. Revised February 1, 2018. Accessed February 27, 2025. https://ideas.repec.org/c/boc/bocode/s432001.html.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool. Centers for Disease Control and Prevention. June 2024. Accessed February 27, 2025. https://www.cdc.gov/cancer/dataviz
- Cao J, Zhang S. Multiple Comparison Procedures. JAMA. 2014;312(5):543-544. doi:10.1001/jama.2014.9440
- Gopalani SV, Janitz AE, Martinez SA, et al. Trends in cancer incidence among American Indians and Alaska Natives and Non-Hispanic Whites in the United States, 1999-2015. Epidemiology. 2020;31(2):205-213. doi:10.1097/EDE.0000000000001140
- Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4). doi:10.3390/ijerph18041384
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi:10.1158/1055-9965.EPI-17-0092
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part iicolorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi:10.1155/2011/107497
- Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181. doi:10.1200/JCO.2009.26.7948
- Yoon J, Phibbs CS, Ong MK, et al. Outcomes of veterans treated in Veterans Affairs hospitals vs non-Veterans Affairs hospitals. JAMA Netw Open. 2023;6(12):e2345898. doi:10.1001/jamanetworkopen.2023.45898
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626-634. doi:10.1200/JCO.2005.03.3365
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595. doi:10.1053/j.gastro.2008.02.002
- Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4). doi:10.1136/bmjoq-2022-001927
- Yehia BR, Greenstone CL, Hosenfeld CB, Matthews KL, Zephyrin LC. The role of VA community care in addressing health and health care disparities. Med Care. 2017;55(Suppl 9 suppl 2):S4-S5. doi:10.1097/MLR.0000000000000768
- Wright BN, MacDermid Wadsworth S, Wellnitz A, Eicher- Miller HA. Reaching rural veterans: a new mechanism to connect rural, low-income US Veterans with resources and improve food security. J Public Health (Oxf). 2019;41(4):714-723. doi:10.1093/pubmed/fdy203
- Nelson RE, Byrne TH, Suo Y, et al. Association of temporary financial assistance with housing stability among US veterans in the supportive services for veteran families program. JAMA Netw Open. 2021;4(2):e2037047. doi:10.1001/jamanetworkopen.2020.37047
- McDaniel JT, Albright D, Lee HY, et al. Rural–urban disparities in colorectal cancer screening among military service members and Veterans. J Mil Veteran Fam Health. 2019;5(1):40-48. doi:10.3138/jmvfh.2018-0013
- US Department of Veterans Affairs, Office of Rural Health. The rural veteran outreach toolkit. Updated February 12, 2025. Accessed February 18, 2025. https://www.ruralhealth.va.gov/partners/toolkit.asp
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi:10.1053/j.gastro.2019.10.029
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668-75. doi:10.1002/ijc.25481
- Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs central cancer registry. Clin Colorectal Cancer. 2016;15(4):e199-e204. doi:10.1016/j.clcc.2016.04.005
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force. 2021. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews:Chapter 1. Agency for Healthcare Research and Quality (US); 2021. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK570917/
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw322
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
- Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63-e71. doi:10.2105/AJPH.2013.301572
- Cauwels J. The many barriers to high-quality rural health care. 2022;(9):1-32. NEJM Catal Innov Care Deliv. Accessed April 24, 2025. https://catalyst.nejm.org/doi/pdf/10.1056/CAT.22.0254
- Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Aff (Millwood);38(12):2003-2010. doi:10.1377/hlthaff.2019.00722
- Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg. 2014;149(6):537-543. doi:10.1001/jamasurg.2013.5062
- Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131-138. doi:10.1016/s1040-8428(01)00139-1
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341-378. doi:10.1146/annurev.publhealth.18.1.341
- Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
- Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996 - 2016. J Rural Health. 2022;38(1):34-39. doi:10.1111/jrh.12580
- Rogers CR, Blackburn BE, Huntington M, et al. Rural- urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes Control. 2020;31(3):241-253. doi:10.1007/s10552-020-01268-2
- US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. https://vincicentral.vinci.med.va.gov [Source not verified]
- US Department of Veterans Affairs Information Resource Center. VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition. US Department of Veterans Affairs, Health Services Research & Development Service, Information Resource Center; May. 2016. [source not verified]
- Goldsmith HF, Puskin DS, Stiles DJ. Improving the operational definition of “rural areas” for federal programs. US Department of Health and Human Services; 1993. Accessed February 27, 2025. https://www.ruralhealthinfo.org/pdf/improving-the-operational-definition-of-rural-areas.pdf
- Adams MA, Kerr EA, Dominitz JA, et al. Development and validation of a new ICD-10-based screening colonoscopy overuse measure in a large integrated healthcare system: a retrospective observational study. BMJ Qual Saf. 2023;32(7):414-424. doi:10.1136/bmjqs-2021-014236
- Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103-1120. doi:10.1111/1475-6773.00165
- Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2(4):358-377.
- Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical software components. Revised February 1, 2018. Accessed February 27, 2025. https://ideas.repec.org/c/boc/bocode/s432001.html.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool. Centers for Disease Control and Prevention. June 2024. Accessed February 27, 2025. https://www.cdc.gov/cancer/dataviz
- Cao J, Zhang S. Multiple Comparison Procedures. JAMA. 2014;312(5):543-544. doi:10.1001/jama.2014.9440
- Gopalani SV, Janitz AE, Martinez SA, et al. Trends in cancer incidence among American Indians and Alaska Natives and Non-Hispanic Whites in the United States, 1999-2015. Epidemiology. 2020;31(2):205-213. doi:10.1097/EDE.0000000000001140
- Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4). doi:10.3390/ijerph18041384
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi:10.1158/1055-9965.EPI-17-0092
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part iicolorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi:10.1155/2011/107497
- Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181. doi:10.1200/JCO.2009.26.7948
- Yoon J, Phibbs CS, Ong MK, et al. Outcomes of veterans treated in Veterans Affairs hospitals vs non-Veterans Affairs hospitals. JAMA Netw Open. 2023;6(12):e2345898. doi:10.1001/jamanetworkopen.2023.45898
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626-634. doi:10.1200/JCO.2005.03.3365
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595. doi:10.1053/j.gastro.2008.02.002
- Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4). doi:10.1136/bmjoq-2022-001927
- Yehia BR, Greenstone CL, Hosenfeld CB, Matthews KL, Zephyrin LC. The role of VA community care in addressing health and health care disparities. Med Care. 2017;55(Suppl 9 suppl 2):S4-S5. doi:10.1097/MLR.0000000000000768
- Wright BN, MacDermid Wadsworth S, Wellnitz A, Eicher- Miller HA. Reaching rural veterans: a new mechanism to connect rural, low-income US Veterans with resources and improve food security. J Public Health (Oxf). 2019;41(4):714-723. doi:10.1093/pubmed/fdy203
- Nelson RE, Byrne TH, Suo Y, et al. Association of temporary financial assistance with housing stability among US veterans in the supportive services for veteran families program. JAMA Netw Open. 2021;4(2):e2037047. doi:10.1001/jamanetworkopen.2020.37047
- McDaniel JT, Albright D, Lee HY, et al. Rural–urban disparities in colorectal cancer screening among military service members and Veterans. J Mil Veteran Fam Health. 2019;5(1):40-48. doi:10.3138/jmvfh.2018-0013
- US Department of Veterans Affairs, Office of Rural Health. The rural veteran outreach toolkit. Updated February 12, 2025. Accessed February 18, 2025. https://www.ruralhealth.va.gov/partners/toolkit.asp
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Utilization and Cost of Veterans Health Administration Referrals to Community Care-Based Physical Therapy
Utilization and Cost of Veterans Health Administration Referrals to Community Care-Based Physical Therapy
The Veterans Health Administration (VHA) is the largest US integrated health system, providing care to veterans through VHA and non-VHA practitioners and facilities.1,2 Providing high-quality, timely, and veteran-centric care remains a priority for the VHA. Legislative efforts have expanded opportunities for eligible veterans to receive care in the community purchased by VHA, known as community care (CC).1 The Veterans Access, Choice, and Accountability Act of 2014 came in response to reports of long wait times and drive times for patients.3-5 The MISSION Act of 2018 expanded access to CC by streamlining it and broadening eligibility criteria, especially for veterans in rural communities who often experience more barriers in accessing care than veterans living in urban communities.1,6-10 Since the implementation of the Choice and MISSION Acts, > 2.7 million veterans have received care through community practitioners within the VHA CC network.11
Background
Increased access to CC could benefit veterans living in rural communities by increasing care options and circumventing challenges to accessing VHA care (ie, geographic, transportation, and distance barriers, practitioner and specialist shortages, and hospital closures). 5,9,10,12,13 However, health care system deficits in rural areas could also limit CC effectiveness for veterans living in those communities. 3 Other challenges posed by using CC include care coordination, information sharing, care continuity, delayed payments to CC practitioners, and mixed findings regarding CC quality.5,8,13,14 VHA practitioners are specifically trained to meet the multifaceted needs unique to veterans’ health and subculture, training CC practitioners may not receive.5,15
CC offers services for primary care and a broad range of specialties, including rehabilitation services such as physical therapy (PT).6 PT is used for the effective treatment of various conditions veterans experience and promote wellbeing and independence.16 US Department of Veterans Affairs (VA) databases reveal a high prevalence of veterans receiving PT services through CC; PT is one of the most frequently used CC outpatient specialty services by veterans living in rural communities.14,17
Telerehabitltation Enterprisewide Initiative
VHA has greatly invested in delivering care virtually, especially for veterans living in rural communities.18 In 2017, the VHA Office of Rural Health funded the Telerehabilitation Enterprise-Wide Initiative (TR-EWI) in partnership with the Physical Medicine and Rehabilitation Services national program office to increase access to specialized rehabilitation services for veterans living in rural communities by leveraging telehealth technologies.18-21 This alternative mode of health care delivery allows clinicians to overcome access barriers by delivering rehabilitation therapies directly to veterans' homes or nearby community-based outpatient clinics. TR-EWI was conceived as a hub-and-spoke model, where rehabilitation expertise at the hub was virtually delivered to spoke sites that did not have in-house expertise. In subsequent years, the TR-EWI also evolved to provide targeted telerehabilitation programs within rural-serving community-based outpatient clinics, including PT as a predominant service.19,20
As TR-EWI progressed—and in conjunction with the uptake of telehealth across VHA during the COVID-19 pandemic—there has been increased focus on PT telerehabilitation, especially for the 4.6 million veterans in rural communities.18,22,23 Because health care delivery system deficits in rural areas could limit the effective use of CC, many TR-EWI sites hope to reduce their CC referrals by providing telehealth PT services to veterans who might otherwise need to be referred to CC. This strategy aligns with VHA goals of providing high-quality and timely care. To better understand opportunities for programs like TR-EWI to provide rehabilitation services for veterans and reduce care sent to the community, research that examines CC referral trends for PT over time is warranted.
This study examines CC from a rehabilitation perspective with a focus on CC referral trends for PT, specifically for Veterans Integrated Service Networks (VISNs) where TREWI sites are located. The study’s objectives were to describe rehabilitation PT services being referred to CC and examine associated CC costs for PT services. Two research questions guided the study. First, what are the utilization trends for CC PT referrals from fiscal year (FY) 2019 to FY 2022? Secondly, what is the cost breakdown of CC for PT referrals from FY 2020 to FY 2022?
Methods
This study was conducted by a multidisciplinary team comprised of public health, disability, rehabilitation counseling, and PT professionals. It was deemed a quality improvement project under VA guidance and followed the SQUIRE guidelines for quality improvement reporting.24,25 The study used the VA Common Operating Platform (Palantir) to obtain individual-level CC referral data from the HealthShare Referral Manager (HSRM) database and consult data from the Computerized Patient Record System. Palantir is used to store and integrate VA data derived from the VA Corporate Data Warehouse and VHA Support Service Center. Referrals are authorizations for care to be delivered by a CC practitioner.
TR-EWI is comprised of 7 sites: VISN 2, VISN 4, VISN 8, VISN 12, VISN 15, VISN 19, and VISN 22. Each site provides telerehabilitation services with an emphasis on reaching veterans living in rural communities. We joined the referrals and consults cubes in Palantir to extract PT referrals for FY 2019 to FY 2022 for the 7 VISNs with TR-EWI sites and obtain referral-specific information and demographic characteristics. 26 Data were extracted in October 2022.
The VHA Community Care Referral Dashboard (CC Dashboard) provided nonindividual level CC cost data.27 The CC Dashboard provides insights into the costs of CC services for VHA enrollees by category of care, standardized episode of care, and eligibility. Data are based on nationallevel HSRM referrals that are not suspended or linked to a canceled or discontinued consult. Data were aggregated by VISN. The dashboard only includes referrals dating back to FY 2020; therefore, PT data from FY 2020 through FY 2022 for VISNs with TR-EWI sites were collected. Data were extracted in December 2022.
This study examined CC referrals, station name, eligibility types, clinical diagnoses (International Classification of Diseases, Tenth Revision codes), and demographic information in the Palantir dataset. Six eligibility criteria can qualify a veteran to receive CC.28 Within clinical diagnoses, the variable of interest was the provisional diagnosis. Patient demographics included age, gender, and rurality of residence, as determined by the Rural-Urban Commuting Area system.29,30 Rural and highly rural categories were combined for analysis. For the CC cost dataset, this study examined CC referrals, referral cost, and eligibility type.
Analysis
For the first research question, we examined referral data from FY 2019 to FY 2022 using the Palantir dataset, performed descriptive statistical analysis for all variables, and analyzed data to identify trends. Descriptive statistics were completed using IBM SPSS Statistics for Windows Version 29.0.0.0.
A qualitative analysis of provisional diagnosis data revealed what is being referred to CC for PT. A preliminary overview of provisional diagnosis data was conducted to familiarize coders with the data. We developed a coding framework to categorize diagnoses based on anatomical location, body structure, and clinical areas of interest. Data were reviewed individually and grouped into categories within the coding framework before meeting as a team to achieve group consensus on categorization. We then totaled the frequency of occurrence for provisional diagnoses within each category. Qualitative analyses were completed using Microsoft Excel.
For the second research question, the study used the CC cost dataset to examine the cost breakdown of CC PT referrals from FY 2020 to FY 2022. We calculated the number and cost of PT referrals across eligibility groups for each FY and VISN. Data were analyzed using SPSS to identify cost trends.
Results
There were 344,406 referrals to CC for PT from FY 2019 to FY 2022 for the 7 VISNs analyzed (Table 1). Of these, 22.5% were from FY 2019, 19.1% from FY 2020, 28.2% from FY 2021, and 30.3% from FY 2022. VISN 8 and VISN 22 reported the most overall PT referrals, with VISN 8 comprising 22.2% and VISN 22 comprising 18.1% of all referrals. VISN 2 reported the least overall referrals (3.7%). VISN 4 and VISN 12 had decreases in referrals over time. VISN 2 and VISN 15 had decreases in referrals from FY 2019 to FY 2021 and slight increases from FY 2021 to FY 2022. VISN 19 and VISN 22 both saw slight increases from FY 2019 to FY 2020 and substantial increases from FY 2020 to FY 2022, with FY 2022 accounting for 40.0% and 42.3% of all referrals for VISN 19 and VISN 20, respectively (Figure 1).
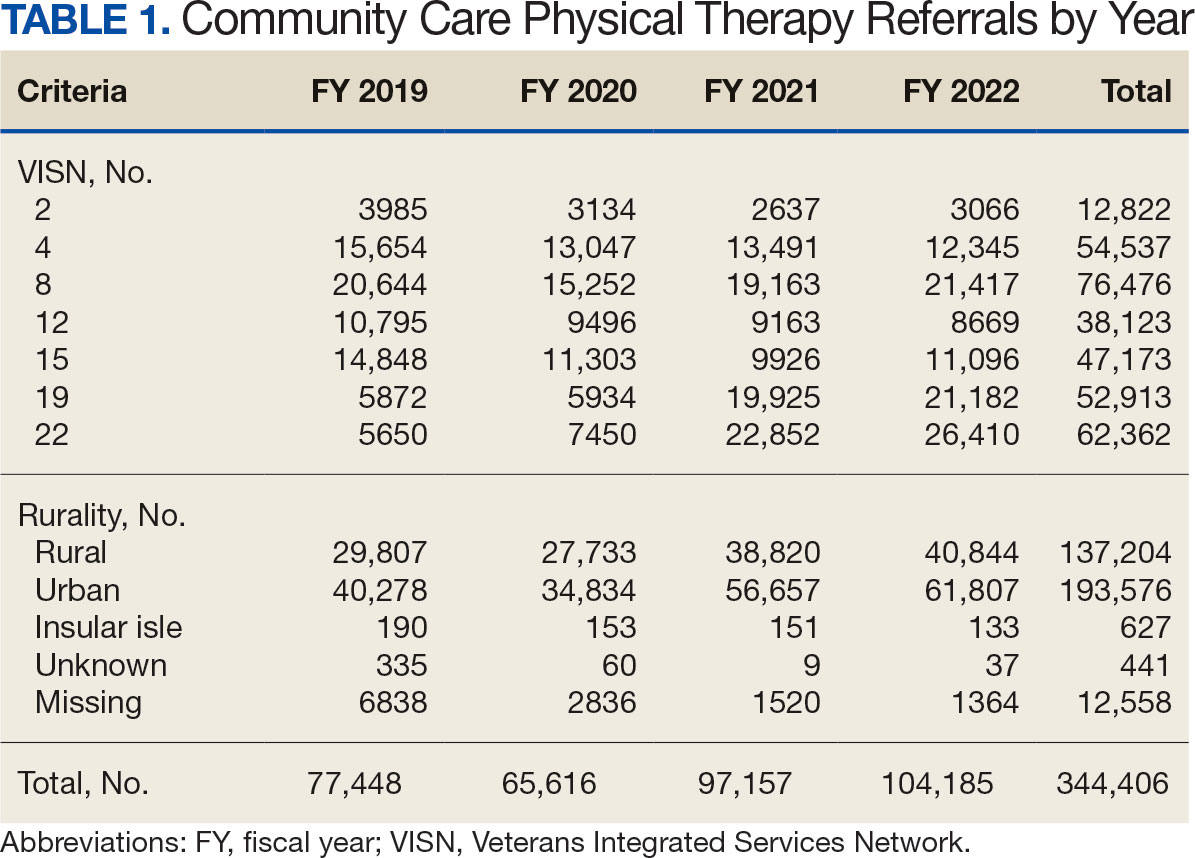
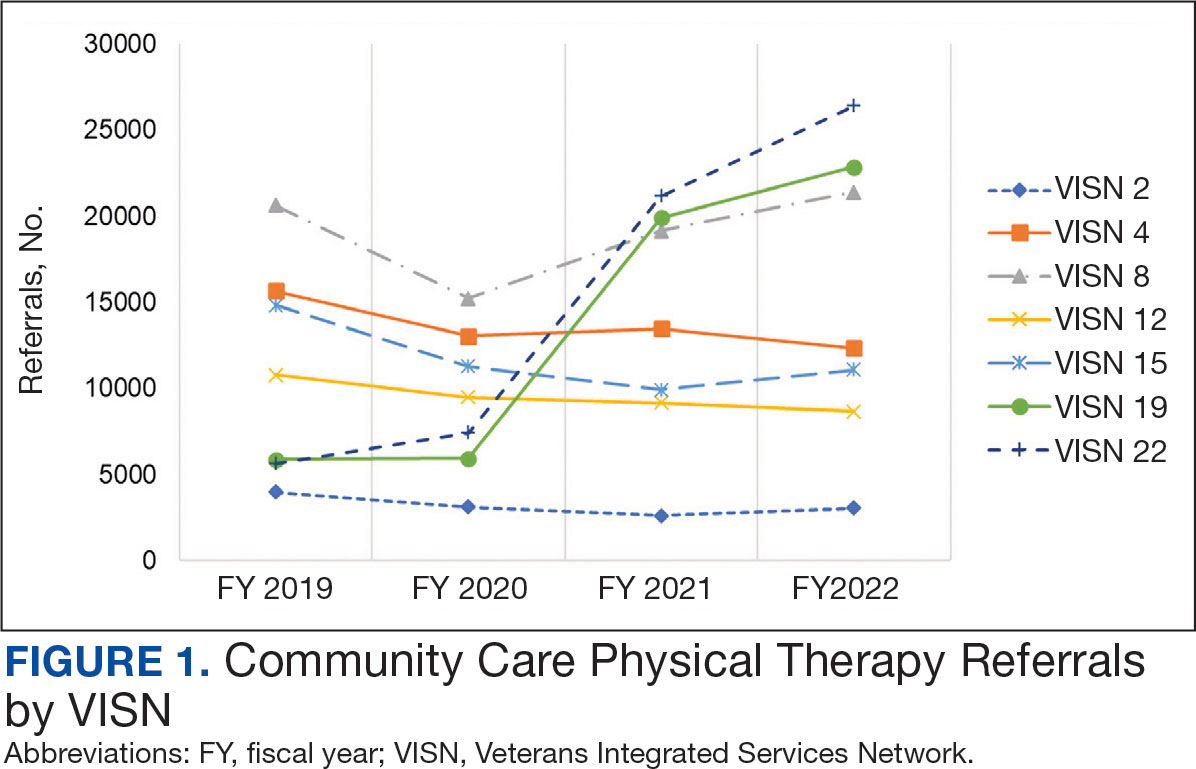
For FY 2019 and FY 2020, VISN 8 had the highest percentage of referrals (26.7% and 23.2%, respectively), whereas VISN 22 was among the lowest (7.3% and 11.4%, respectively). However, for FY 2021 and FY 2022, VISN 22 reported the highest percentage of referrals (23.5% and 25.3%, respectively) compared to all other VISNs. VISN 2 consistently reported the lowest percentage of referrals across all years.
There were 56 stations analyzed across the 7 VISNs (Appendix 1). Nine stations each accounted for ≥ 3.0% of the total PT referrals and only 2 stations accounted for > 5.0% of referrals. Orlando, Florida (6.0%), Philadelphia, Pennsylvania (5.2%), Tampa, Florida (4.9%), Aurora, Colorado (4.9%), and Gainesville, Florida (4.4%) reported the top 5 highest referrals, with 3 being from VISN 8 (Orlando, Tampa, Gainesville). Stations with the lowest reported referrals were all in VISN 2 in New York: The Bronx, (0%), New York Harbor (0%), Hudson Valley (0.1%) and Finger Lakes (0.2%).
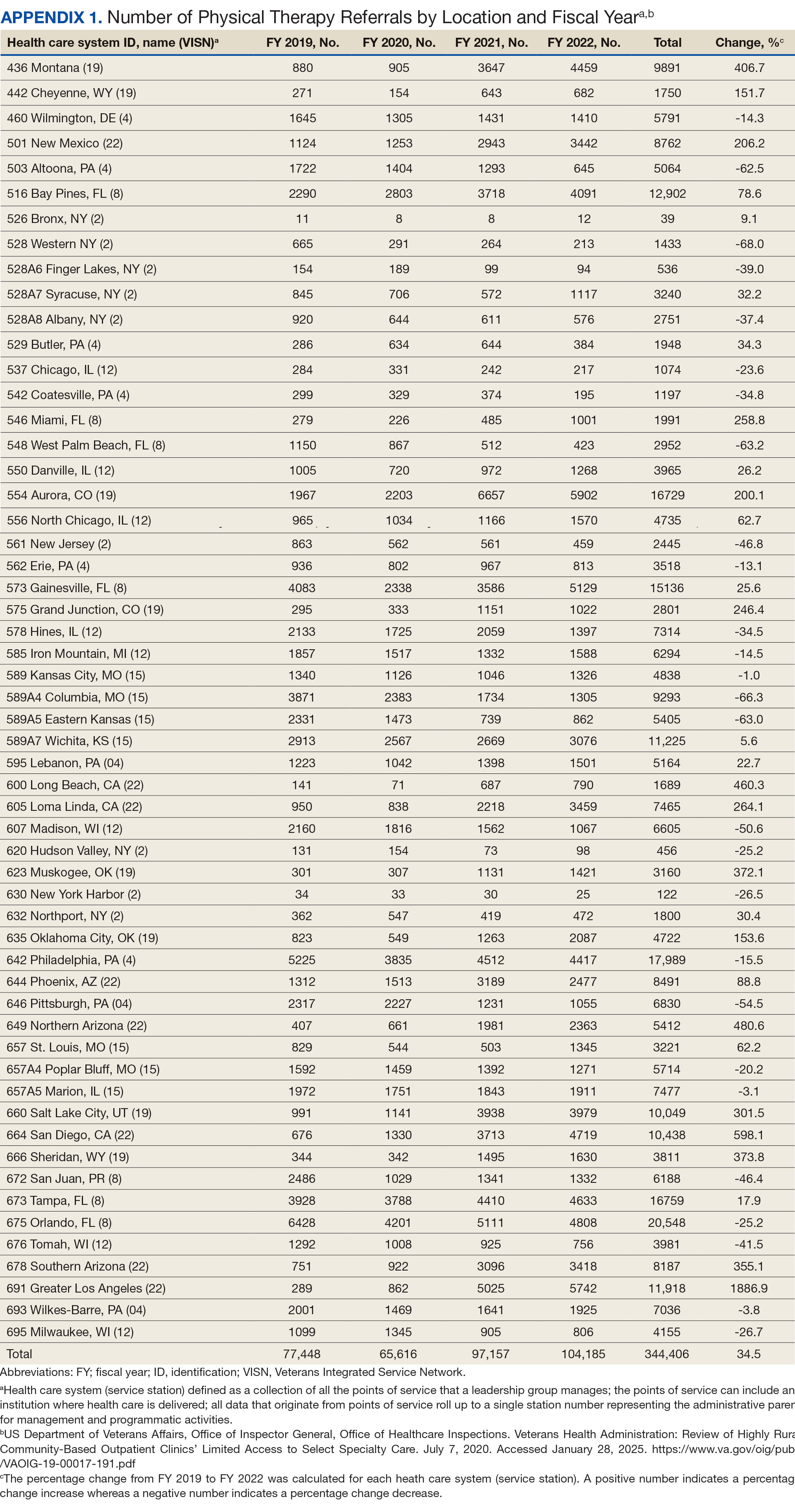
Rurality
Urban stations comprised 56.2% and rural stations comprised 39.8% of PT CC referrals, while 0.2% of referrals were from insular isle US territories: Guam, American Samoa, Northern Marianas, and the Virgin Islands. The sample had missing or unknown data for 3.8% of referrals. FY 2022 had the largest difference in rural and urban referrals. Additionally, there was an overall trend of more referrals over time for rural and urban, with a large increase in rural (+40.0%) and urban (+62.7%) referrals from FY 2020 to FY 2021 and a modest increase from FY 2021 to FY 2022 (+5.2% for rural and +9.1% for urban). There was a decrease in rural (-7.0%) and urban (-3.5%) referrals from FY 2019 to FY 2020 (Figure 2).
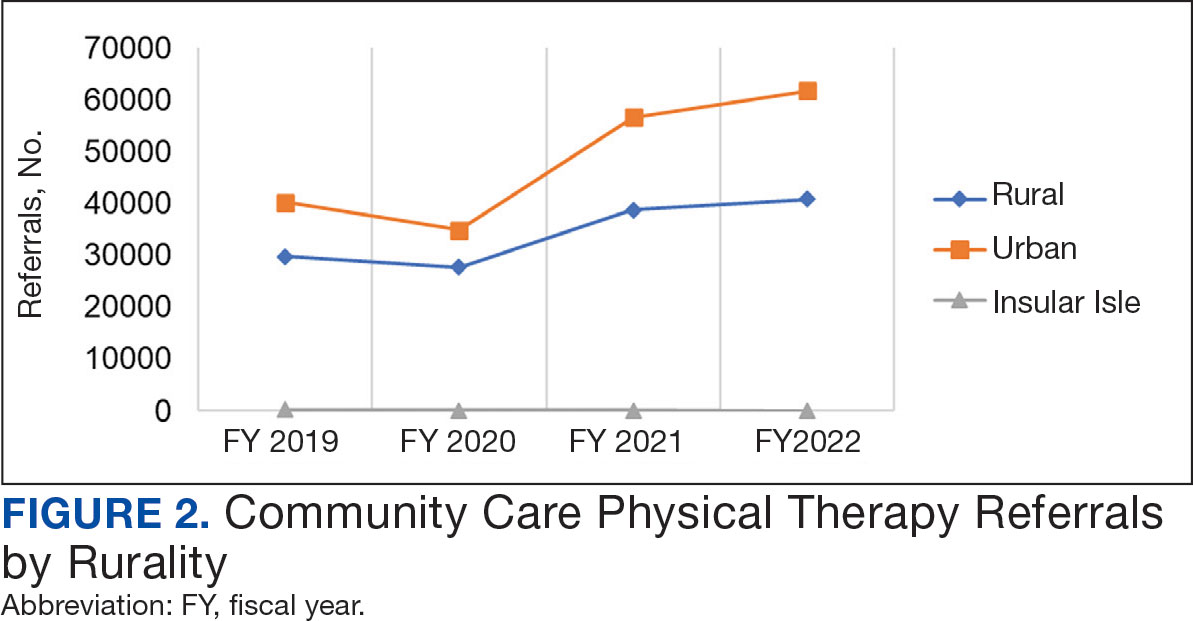
There were differences in referrals by rurality and VISN (Table 2). VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals, whereas VISN 4, VISN 8, and VISN 22 reported more urban than rural referrals. VISN 2 reported similar numbers for both, with slightly more urban than rural referrals. When reviewing trends over time for each FY, VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals and VISN 4, VISN 8, and VISN 22 had more urban than rural referrals. In FY 2019 and FY 2020, VISN 2 reported slightly more urban than rural referrals but almost the same number of referrals in FY 2021 and FY 2022 (Appendix 2).
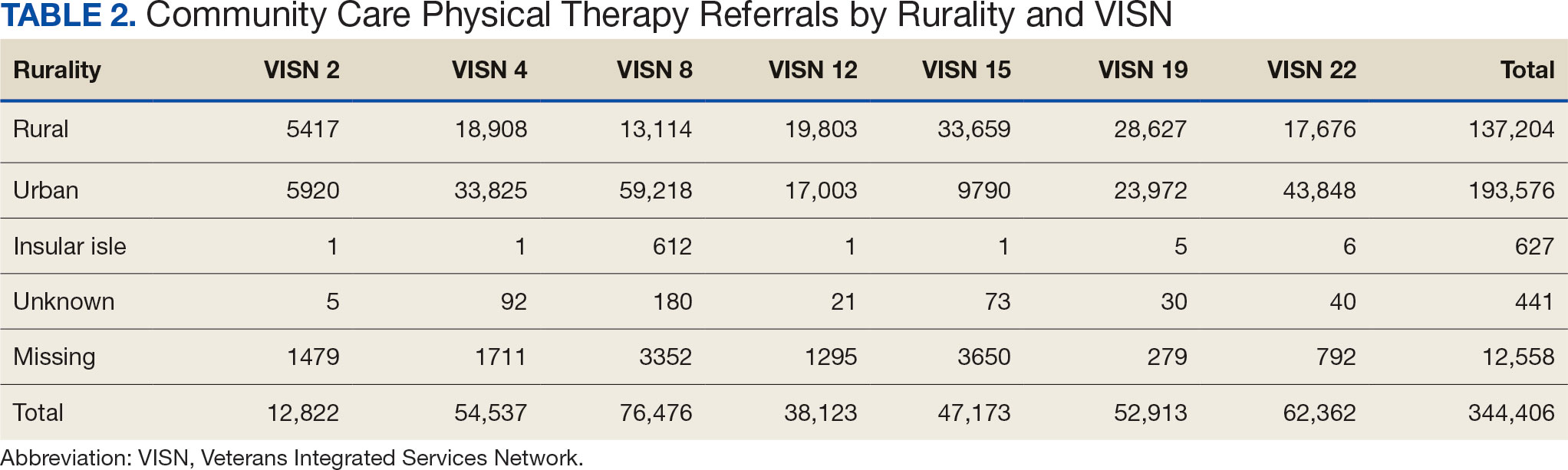
Demographics
The mean (SD) age was 61.2 (15.8) years (range, 20-105). Most PT CC referrals were for veterans aged 70 to 79 years (26.9%), followed by 60 to 69 years (20.7%), and 50 to 59 years (16.4%) (Appendix 3). Trends were consistent across VISNs. There was less of a difference between rural and urban referral percentages as the population aged. Veterans aged < 49 years residing in more urban areas accounted for more referrals to CC compared to their rural counterparts. This difference was less apparent in the 70 to 79 years and 80 to 89 years age brackets.
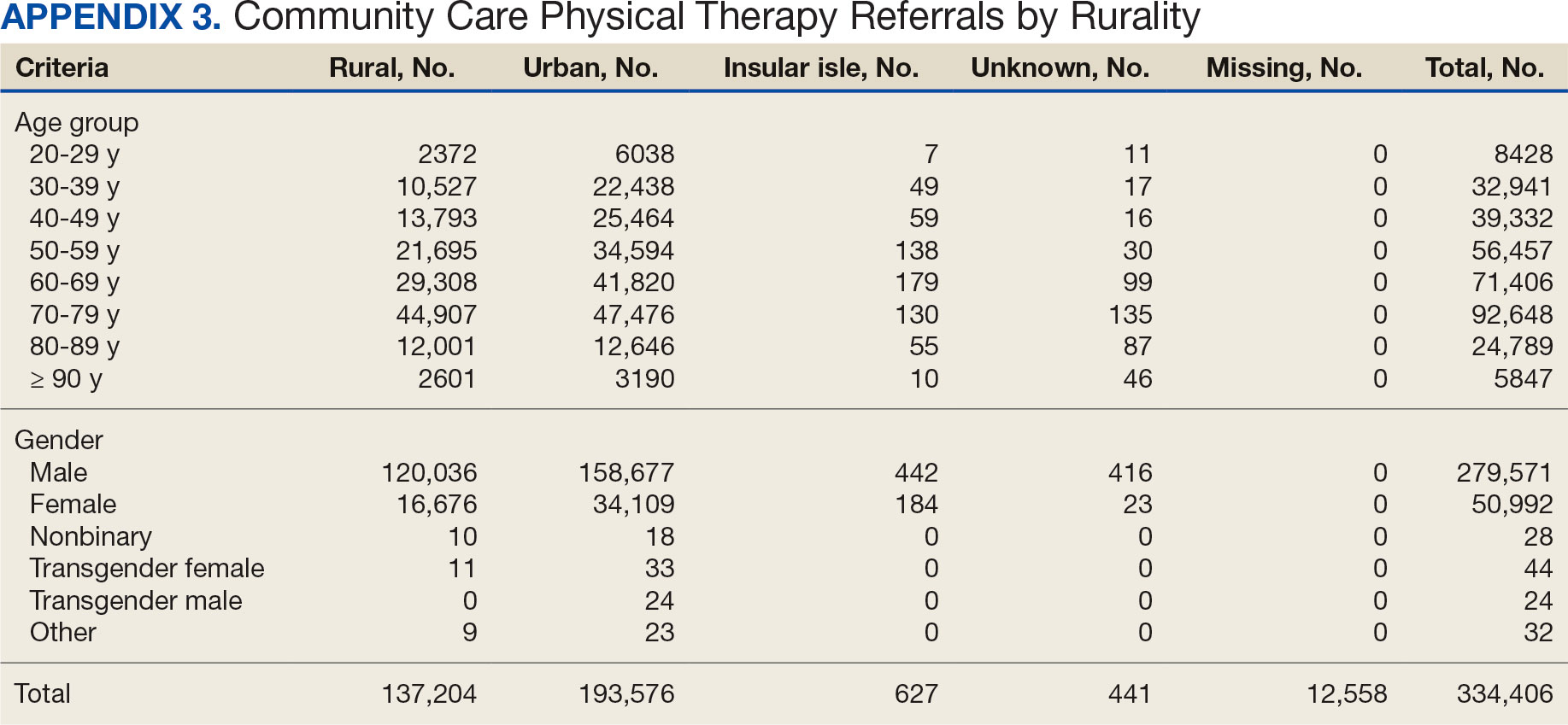
Most PT CC referrals (81.2%) were male and 14.8% were female. About 3.6% of referral data were missing sex information, and there was a smaller difference between male veterans living in rural communities and male veterans living in urban communities compared with female veterans. A total of 42.9% of male veterans resided in rural areas compared to 56.8% in urban areas; 32.7% of female veterans resided in rural areas compared to 66.9% in urban areas (Appendix 3).
Other Criteria
Of the 334,406 referrals, 114,983 (34.4%) had eligibility data, mostly from FY 2021 and FY 2022 (Table 3). Available eligibility data were likely affected by the MISSION Act and new regulations for reporting CC eligibility. Distance (33.4%) was the most common eligibility criteria, followed by timeliness of care (28.8%), and best medical interest (19.8%); 40.4% were rural and 59.5% were urban. Distance (55.4%) was most common for rural veterans, while timeliness of care (39.7%) was most common for urban veterans. For both groups, the second most common eligibility reason was best medical interest (Appendix 4).
Bone, joint, or soft tissue disorders were common diagnoses, with 25.2% located in the lower back, 14.7% in the shoulder, and 12.8% in the knee (Appendix 5). Amputations of the upper and lower limbs, fractures, cancer-related diagnoses, integumentary system disorders, thoracic and abdominal injuries and disorders, and other medical and mental health conditions each accounted for < 1% of the total diagnoses.
Costs
At time of analysis, the CC Dashboard had cost data available for 200,204 CC PT referrals from FY 2020 to FY 2022. The difference in referral numbers for the 2 datasets is likely attributed to several factors: CC cost data is exclusively from the HSRM, whereas Palantir includes other data sources; how VA cleans data pulled into Palantir; how the CC Dashboard algorithm populates data; and variances based on timing of reporting and/or if referrals are eventually canceled.
The total cost of PT CC referrals from FY 2020 to FY 2022 in selected VISNs was about $220,615,399 (Appendix 6). Appendix 7 details the methodology for determining the average standardized episode- of-care cost by VISN and how referral costs are calculated. Data show a continuous increase in total estimated cost from $46.8 million in FY 2020 to $92.1 million in FY 2022. From FY 2020 to FY 2022, aggregate costs ranged from $6,758,053 in VISN 2 to $47,209,162 in VISN 8 (Figure 3). The total referral cost for PT was highest at VISN 4 in FY 2020 ($10,447,140) and highest at VISN 22 in FY 2021 ($18,835,657) and FY 2022 ($22,962,438) (Figure 4). For referral costs from FY 2020 to FY 2022, distance accounted for $75,561,948 (34.3%), timeliness of care accounted for $60,413,496 (27.3%), and best medical interest accounted for $46,291,390 (21.0%) (Table 4).
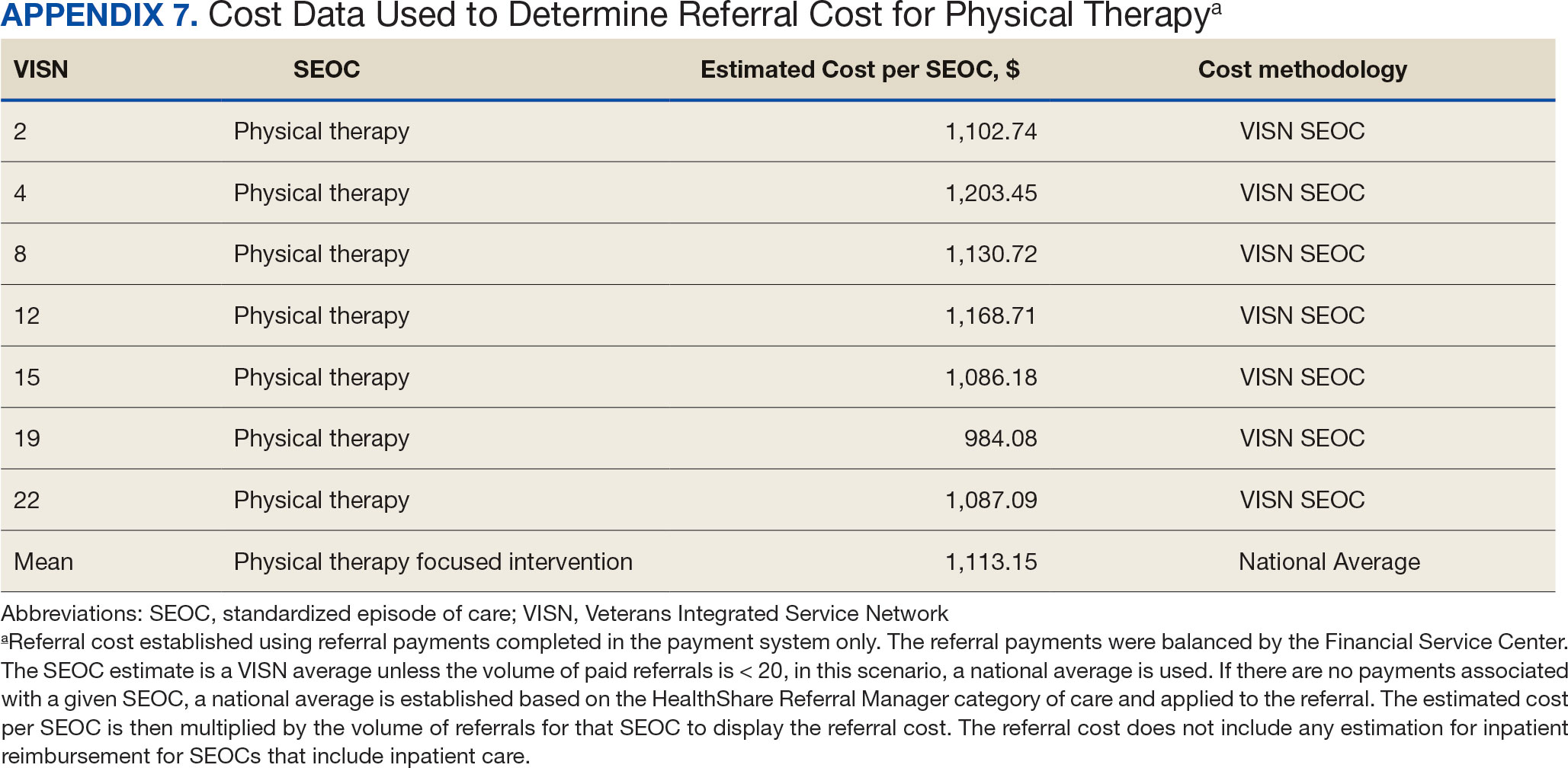
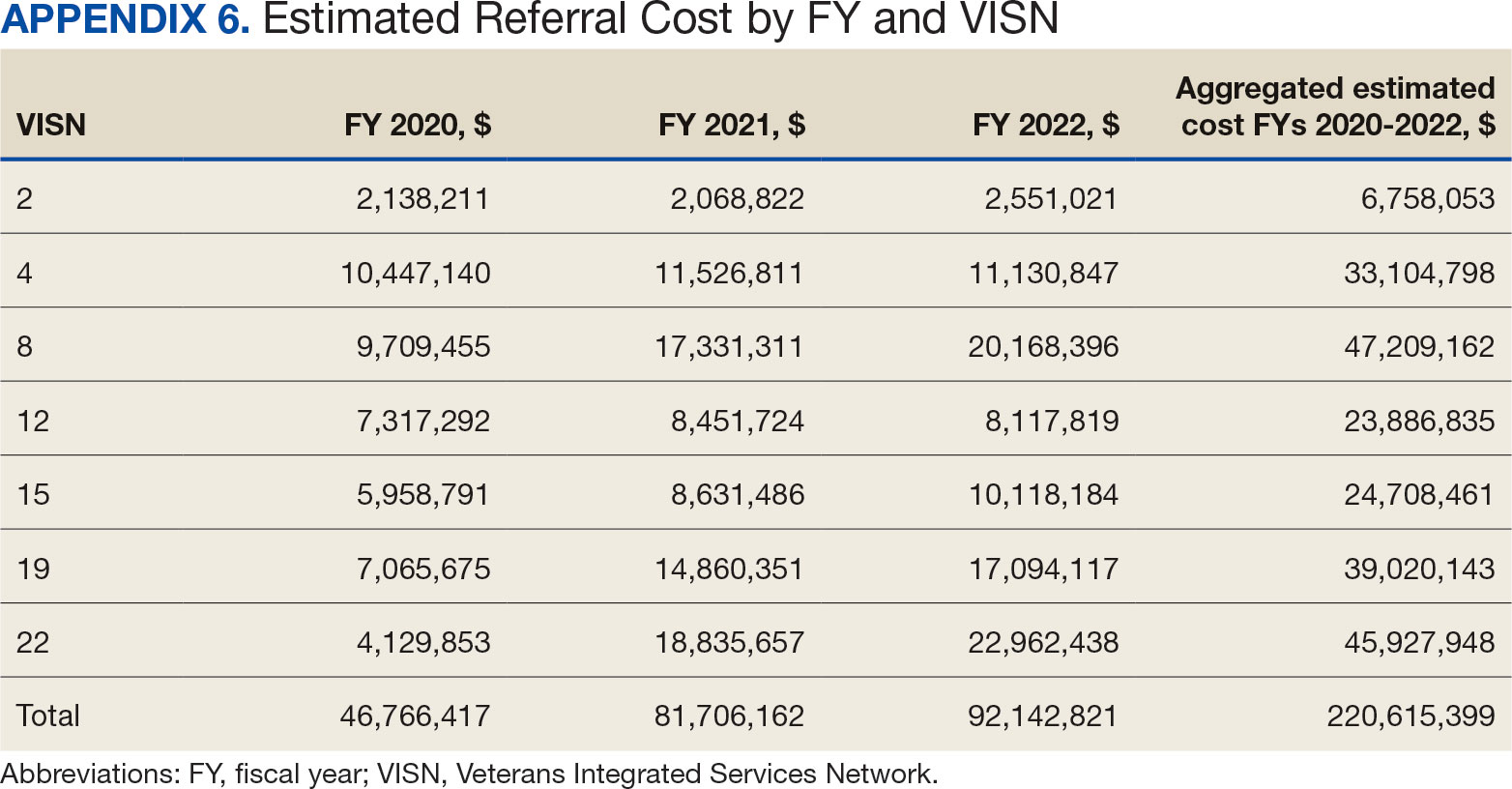
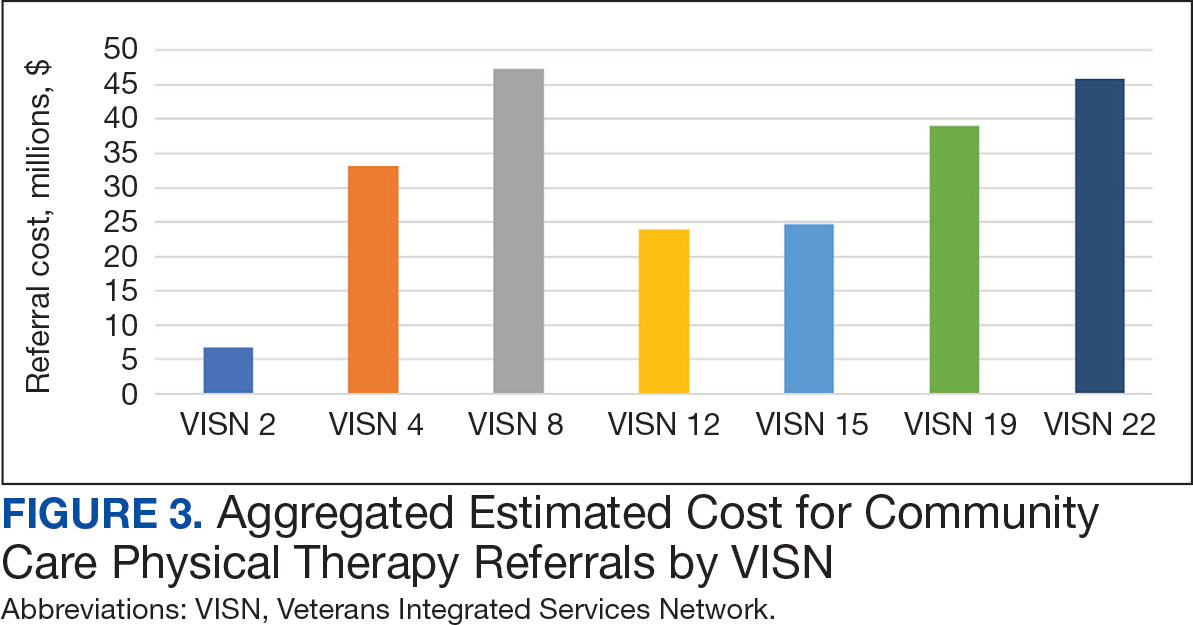
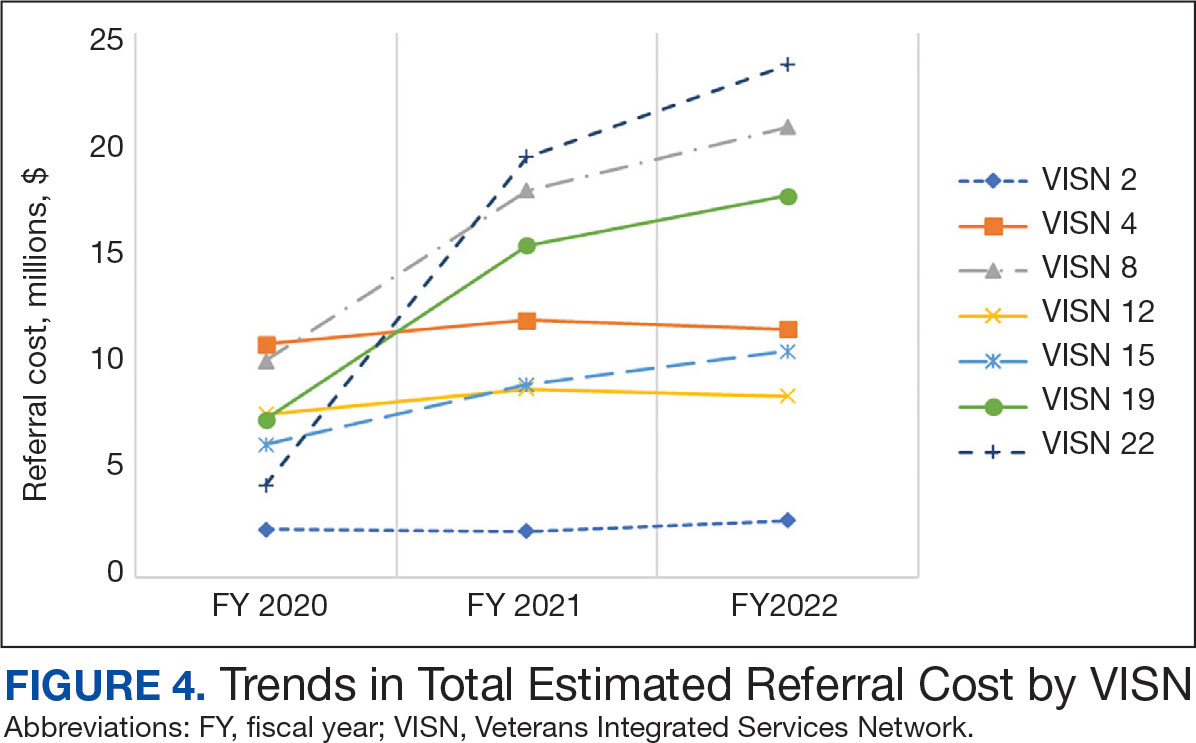
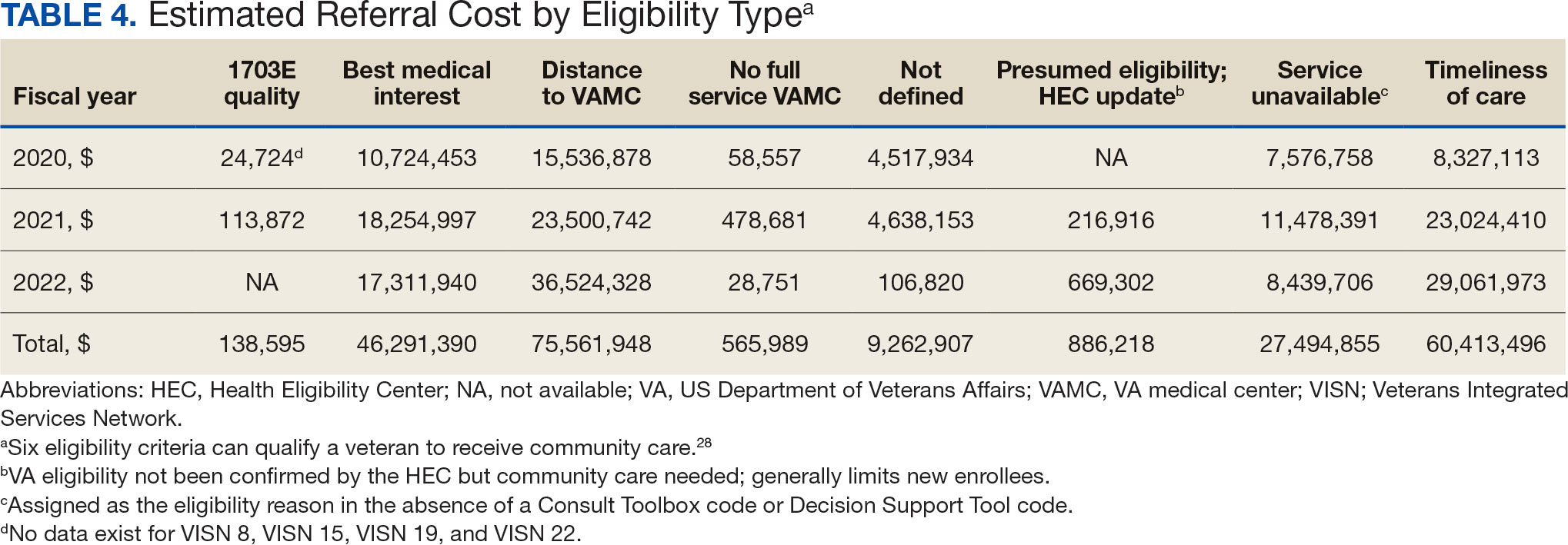
Overall costs were primarily driven by specific VISNs within each eligibility type (Appendix 8; Figure 5). VISN 19, VISN 22, and VISN 15 accounted for the highest referral costs for distance; VISN 22, VISN 8, and VISN 19 accounted for the secondhighest referral cost, timeliness of care; and VISN 4, VISN 8, and VISN 12 accounted for the third-highest referral cost, best medical interest (Figure 5). VISN 2, VISN 4, VISN 12, VISN 15, and VISN 22 had service unavailable as an eligibility type with 1 of the top 3 associated referral costs, which was higher in cost than timeliness of care for VISN 2, VISN 4, VISN 12, and VISN 15.
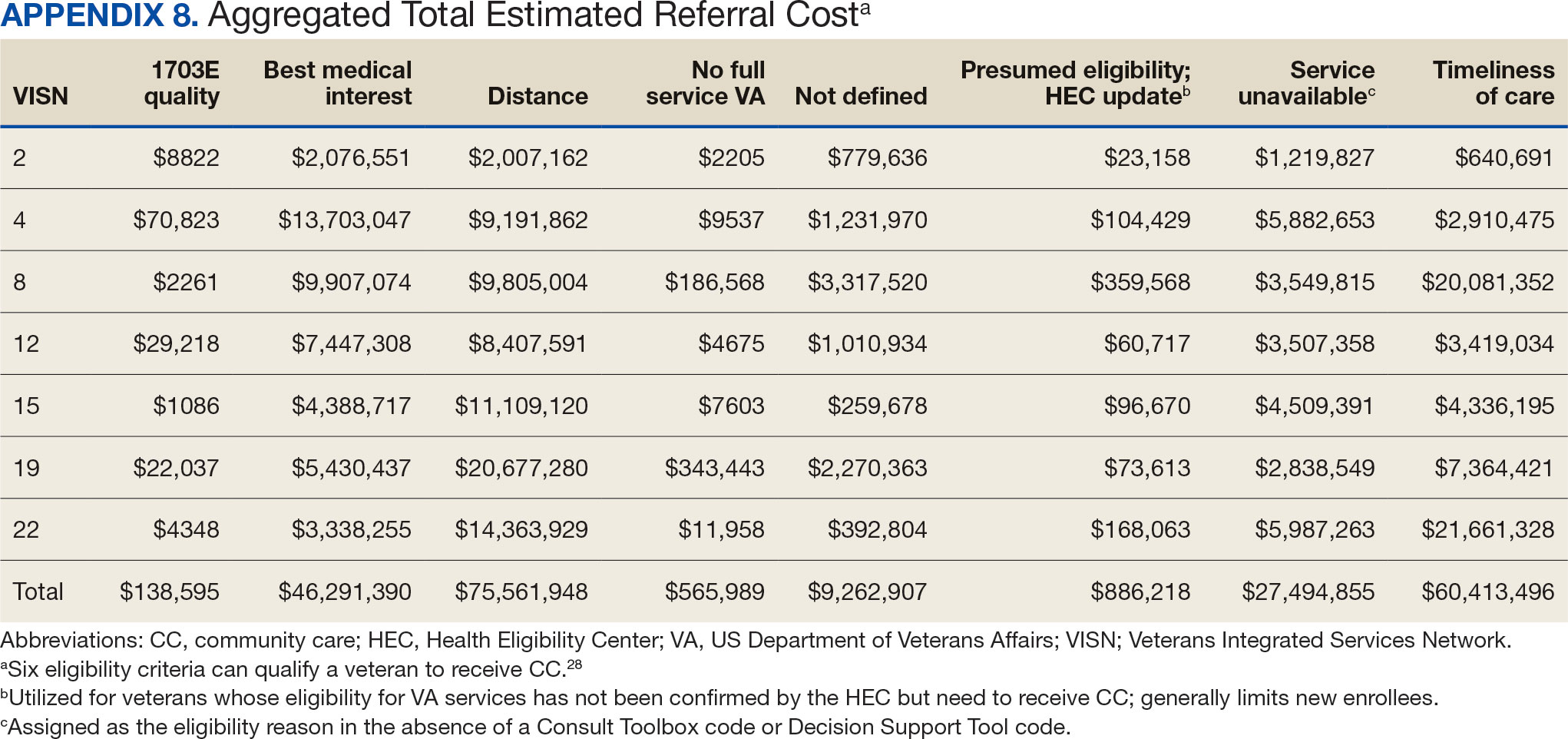
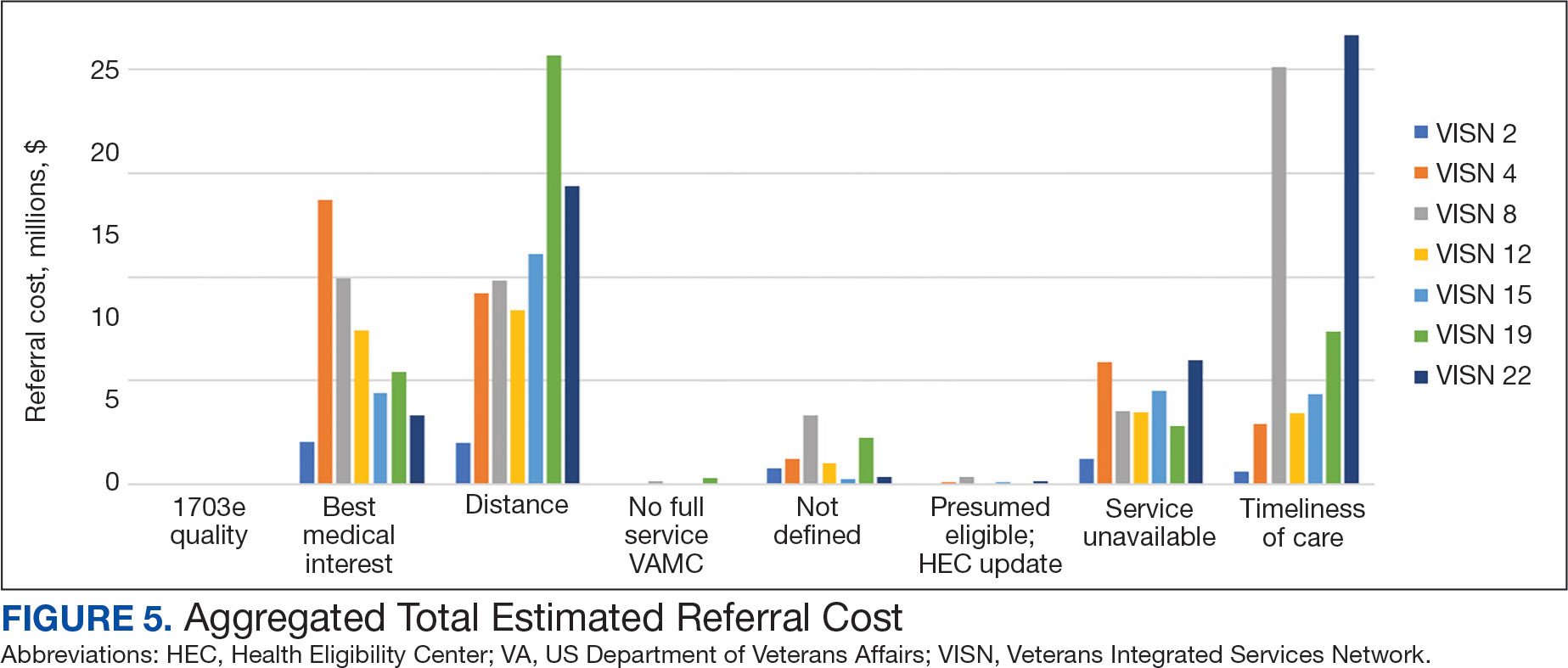
Discussion
This study examines the referral of rehabilitation PT services to CC, evaluates CC costs for PT services, and analyzes utilization and cost trends among veterans within the VHA. Utilization data demonstrated a decrease in referrals from FY 2019 to FY 2020 and increases in referrals from FY 2020 to FY 2022 for most variables of interest, with cost data exhibiting similar trends. Results highlight the need for further investigation to address variations in PT referrals and costs across VISNs and eligibility reasons for CC referral.
Results demonstrated a noteworthy increase in PT CC referrals over time. The largest increase occurred from FY 2020 to FY 2021, with a smaller increase from FY 2021 to FY 2022. During this period, total enrollee numbers decreased by 3.0% across the 7 VISNs included in this analysis and by 1.6% across all VISNs, a trend that illustrates an overall decrease in enrollees as CC use increased. Results align with the implementation of the MISSION Act of 2018, which further expanded veterans’ options to use CC.1,6,7 Results also align with the onset of the COVID-19 pandemic, which disrupted care access for many veterans, placed a larger emphasis on the use of telehealth, and increased opportunities to stay within the VA for care by rapidly shifting to telehealth and leveraging telerehabilitation investments and initiatives (such as TR-EWI).20,31
VISN 8, VISN 19, and VISN 22, accounted for more than half of PT referrals. These VISNs had higher enrollee counts compared to the other VISNs.32 VISN 8 consistently had high levels of referrals, whereas VISN 19 and VISN 22 saw dramatic increases in FY 2021 and FY 2022. In contrast, VISN 4 and VISN 12 gradually decreased referrals during the study. VISN 2 had the lowest referral numbers during the study period, and all stations with the lowest individual referral numbers were located within VISN 2. Of the VISNs included in this study, VISN 2 had the second lowest number of enrollees (324,042).32 Reasons for increases and decreases over time could not be determined based on data collected in this study.
There were more urban than rural PT CC referrals; however, both exhibited an increase in referrals over time. This is consistent with population trends showing that most VHA patients (62.6%) and veterans (75.9%) reside in urban areas, which could explain some of the trends in this study.33 Some VISNs have larger urban catchment areas (eg, VISN 8 and VISN 22), and some have larger rural catchment areas (eg, VISN 15 and VISN 19), which could partially explain the rural-urban differences by VISN.32 Rural-urban referral trends might also reflect existing health care delivery system deficits in rural areas and known challenges associated with accessing health care for veterans living in rural communities.8,9
This study found larger differences in rural and urban PT CC referrals for younger age groups, with more than twice as many urban referrals in veterans aged 20 to 29 years and aged 30 to 39 years, and roughly 1.8 times as many urban referrals in veterans aged 40 to 49 years. However, there were similar numbers of rural and urban referrals in those aged 70 to 79 years and aged 80 to 89 years. These trends are consistent with data showing veterans residing in rural communities are older than their urban counterparts.23,34 Data suggest that older veteran populations might seek PT at higher rates than younger veteran populations. Moreover, data suggest there could be differences in PT-seeking rates for younger veteran populations who reside in rural vs urban areas. Additional research is needed to understand these trends.
Distance and timeliness of care were the predominant reasons for referral among eligibility groups, which is consistent with the MISSION Act goals.1,6,7 The most common eligibility reason for rural referrals was distance; timeliness of care was most common for urban referrals. This finding is expected, as veterans living in rural communities are farther away from VHA facilities and have longer drive times, whereas veterans living in urban communities might live closer, yet experience longer wait times due to services and/or appointment availability. Best medical interest accounted for almost 20% of referrals, which does not provide detailed insights into why those veterans were referred to CC.
The top PT diagnoses referred to CC were related to bone, joint, or soft tissue disorders of the lower back, shoulder, and knee. This suggests that musculoskeletal-related issues are prevalent among veterans seeking PT care, which is consistent with research that found > 50% of veterans receiving VHA care have musculoskeletal disorders.35 The probability of experiencing musculoskeletal problems increases with age, as does the need for PT services. Amputations and fractures accounted for < 1% of CC referrals, which is consistent with the historic provision of VHA clinical specialized care to conditions prevalent among veterans. It may also represent VHA efforts to internally provide care for complex conditions requiring more extensive interdisciplinary coordination.
The total cost of referrals over time was about $221 million. VISN 8 accounted for the highest overall cost; VISN 2 had the lowest, mirroring referral utilization trends and aligning with VISN enrollee numbers. VISN 19 and VISN 22 reported large cost increases from FY 2020 to FY 2021. Total referral costs increased by $34.9 million from FY 2020 to FY 2021, which may be due to health care inflation (2.9% during FY 2019 to FY 2022), increased awareness of CC services, or increased VHA wait times.36 Additionally, there were limitations in care provided across health care systems during the COVID-19 pandemic, including the VA.5 The increase from FY 2020 to FY 2021 may reflect a rebound from restrictions in appointments across VA, CC, and the private sector.
While the increase in total referral cost may be partly attributed to inflation, the cost effectiveness and efficiency of referring veterans to CC vs keeping veterans within VHA care is an ongoing debate.5 Examining and addressing cost drivers within the top eligibility types and their respective VISNs is necessary to determine resource allocation and improve quality of care. This study found that best medical interest and unavailable services accounted for 33.4% of the total cost of CC referrals, highlighting the need for policies that strengthen in-house competencies and recruit personnel to provide PT services currently unavailable within the VA.
Future Directions
The VHA should explore opportunities for in-house care, especially for services appropriate for telehealth.18,20,37 Data indicated a smaller cost increase from FY 2021 to FY 2022 compared to the relatively large increase from FY 2020 to FY 2021. The increased telehealth usage across VHA by TR-EWI and non—TR-EWI sites within selected VISNs may have contributed to limiting the increase in CC costs. Future studies should investigate contextual factors of increased telehealth usage, which would offer guidance for implementation to optimize the integration of telehealth with PT rehabilitation provided in-house. Additionally, future studies can examine potential limitations experienced during PT telehealth visits, such as the inability to conduct hands-on assessments, challenges in viewing the quality of patient movement, ensuring patient safety in the remote environment, and the lack of PT equipment in homes for telehealth visits, and how these challenges are being addressed.38,39 Research is also needed to understand tradeoffs of CC vs VHA care and the potential and cost benefits of keeping veterans within VHA using programs like TR-EWI.5 Veterans living in rural communities may especially benefit from this as expanding telehealth options can provide access to PT care that may not be readily available, enabling them to stay connected and engaged in their care.18,40
Future studies could examine contributory factors to rising costs, such as demographic shifts, changes in PT service utilization, and policy. Researchers might also consider qualitative studies with clinicians and veterans within each VISN, which may provide insights into how local factors impact PT referral to the community.
Limitations
Due to its descriptive nature, this study can only speculate about factors influencing trends. Limitations include the inability to link the Palantir and CC Dashboard datasets for cost comparisons and potential data change over time on Palantir due to platform updates. The focus on VISNs with TREWI sites limited generalizability and this study did not compare CC PT vs VHA PT. Finally, there may have been cost drivers not identified in this study.
Conclusions
This descriptive study provides insights into the utilization and cost of PT CC referrals for selected VISNs. Cost trends underscore the financial commitment to providing PT services to veterans. Understanding what factors are driving this cost is necessary for VHA to optimally provide and manage the rehabilitation resources needed to serve veterans through traditional in-person care, telehealth, and CC options while ensuring timely, highquality care.
- Congressional Budget Office. The Veterans Community Care Program: Background and Early Effects. October 26, 2021. Accessed September 23, 2024. https://www.cbo.gov/publication/57257
- US Dept of Veterans Affairs. Providing Health Care for Veterans. Updated September 10, 2024. Accessed September 23, 2024. https://www.va.gov/health/
- Davila H, Rosen AK, Beilstein-Wedel E, Shwartz M, Chatelain LJ, Gurewich D. Rural veterans’ experiences with outpatient care in the Veterans Health Administration versus community care. Med Care. 2021;59(Suppl 3):S286-S291. doi:10.1097/MLR.0000000000001552
- Vanneman ME, Wagner TH, Shwartz M, et al. Veterans’ experiences with outpatient care: comparing the Veterans Affairs system with community-based care. Health Aff (Millwood). 2020;39(8):1368-1376. doi:10.1377/hlthaff.2019.01375
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10(3):9.
- Feyman Y, Legler A, Griffith KN. Appointment wait time data for primary & specialty care in veterans health administration facilities vs. community medical centers. Data Brief. 2021;36:107134. doi:10.1016/j.dib.2021.107134
- Kelley AT, Greenstone CL, Kirsh SR. Defining access and the role of community care in the Veterans Health Administration. J Gen Intern Med. 2020;35(5):1584-1585. doi:10.1007/s11606-019-05358-z
- Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(Suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
- US Dept of Veterans Affairs, Office of Rural Health. Rural Veterans: Rural Veteran Health Care Challenges. Updated May 14, 2024. Accessed September 23, 2024. https:// www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Ohl ME, Carrell M, Thurman A, et al. “Availability of healthcare providers for rural veterans eligible for purchased care under the veterans choice act.” BMC Health Serv Res. 2018;18(1):315. doi:10.1186/s12913-018-3108-8
- Mattocks KM, Cunningham KJ, Greenstone C, Atkins D, Rosen AK, Upton M. Innovations in community care programs, policies, and research. Med Care. 2021;59(Suppl 3):S229-S231. doi:10.1097/MLR.0000000000001550
- Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52 Suppl 1(Suppl 1):459-480. doi:10.1111/1475-6773.12633
- Patzel M, Barnes C, Ramalingam N, et al. Jumping through hoops: community care clinician and staff experiences providing primary care to rural veterans. J Gen Intern Med. 2023;38(Suppl 3):821-828. doi:10.1007/s11606-023-08126-2
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Campbell P, Pope R, Simas V, Canetti E, Schram B, Orr R. The effects of early physiotherapy treatment on musculoskeletal injury outcomes in military personnel: a narrative review. Int J Environ Res Public Health. 2022;19(20):13416. doi:10.3390/ijerph192013416
- Gurewich D, Shwartz M, Beilstein-Wedel E, Davila H, Rosen AK. Did access to care improve since passage of the veterans choice act? Differences between rural and urban veterans. Med Care. 2021;59(Suppl 3):S270-S278. doi:10.1097/MLR.0000000000001490
- Myers US, Birks A, Grubaugh AL, Axon RN. Flattening the curve by getting ahead of it: how the VA healthcare system is leveraging telehealth to provide continued access to care for rural veterans. J Rural Health. 2021;37(1):194-196. doi:10.1111/jrh.12449
- Hale-Gallardo JL, Kreider CM, Jia H, et al. Telerehabilitation for rural veterans: a qualitative assessment of barriers and facilitators to implementation. J Multidiscip Healthc. 2020;13:559-570. doi:10.2147/JMDH.S247267
- Kreider CM, Hale-Gallardo J, Kramer JC, et al. Providers’ shift to telerehabilitation at the U.S. Veterans Health Administration during COVID-19: practical applications. Front Public Health. 2022;10:831762. doi:10.3389/fpubh.2022.831762
- Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019;36(3):122-128.
- US Dept of Veterans Affairs, Office of Rural Health. VHA Office of Rural Health. Updated August 30, 2024. Accessed September 23, 2024. https://www.ruralhealth.va.gov/index.asp
- National Center for Veterans Analysis and Statistics. Rural Veterans: 2021-2023. April 2023. Accessed September 23, 2024. https://www.datahub.va.gov/stories/s/Rural-Veterans-FY2021-2023/kkh2-eymp/
- U.S. Department of Veterans Affairs, Office of Research & Development. Program Guide: 1200.21, VHA Operations Activities That May Constitute Research. January 9, 2019. https://www.research.va.gov/resources/policies/ProgramGuide-1200-21-VHA-Operations-Activities.pdf
- Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Nurs Care Qual. 2016;31(1):1-8. doi:10.1097/NCQ.0000000000000153
- US Dept of Veterans Affairs. Veterans Health Administration: Veterans Integrated Service Networks (VISNs). Updated January 29, 2024. Accessed September 23, 2024. https://www.va.gov/HEALTH/visns.asp
- Stomberg C, Frost A, Becker C, Stang H, Windschitl M, Carrier E. Community Care referral dashboard [Data dashboard]. https://app.powerbigov.us/groups/me/reports/090d22a7-0e1f-4cc5-bea8-0a1b87aa0bd9/ReportSectionacfd03cdebd76ffca9ec [Source not verified]
- US Dept of Veterans Affairs. Eligibility for community care outside VA. Updated May 30, 2024. Accessed September 23, 2024. https://www.va.gov/COMMUNITYCARE/programs/veterans/General_Care.asp
- US Department of Veterans Affairs, Office of Rural Health. How to define rurality fact sheet. Updated December 2023. Accessed January 28, 2025. https://www.ruralhealth.va.gov/docs/ORH_RuralityFactSheet_508.pdf
- Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed September 23, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx
- Gurewich D, Beilstein-Wedel E, Shwartz M, Davila H, Rosen AK. Disparities in wait times for care among US veterans by race and ethnici t y. JAMA Netw Open. 2023;6(1):e2252061. doi:10.1001/jamanetworkopen.2022.52061
- U.S. Department of Veterans Affairs, VA Office of Rural Health, Veterans Rural Health Resource Center-Gainesville, GeoSpatial Outcomes Division. VA and Community Healthcare, and VHA Rurality web map application. Published 2023. https://portal.vhagis.inv.vaec.va.gov/arcgis/apps/webappbuilder/index.html [source not verified]
- Chartbook on Healthcare for Veterans: National Healthcare Quality and Disparities Report. Agency for Healthcare Research and Quality; November 2020. Accessed September 23, 2024. https://www.ahrq.gov/research/findings/nhqrdr/chartbooks/veterans/index.html
- Lum HD, Nearing K, Pimentel CB, Levy CR, Hung WW. Anywhere to anywhere: use of telehealth to increase health care access for older, rural veterans. Public Policy Aging Rep. 2020;30(1):12-18. doi:10.1093/ppar/prz030
- Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696-1703. doi:10.1097/j.pain.0000000000000567
- US Inflation Calculator. Health Care Inflation in the United States (1948-2024). Accessed September 23, 2024. https://www.usinflationcalculator.com/inflation/health-care-inflation-in-the-united-states/
- Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638. doi:10.1177/0269215516645148
- Elor A, Conde S, Powel l M, Robbins A, Chen NN, Kurniawan S. Physical therapist impressions of telehealth and virtual reality needs amidst a pandemic. Front Virtual Real. 2022;3. doi:10.3389/frvir.2022.915332
- Lee AC, Harada N. Telehealth as a means of health care delivery for physical therapist practice. Phys Ther. 2012;92(3):463-468. doi:10.2522/ptj.20110100
- Hynes DM, Edwards S, Hickok A, et al. Veterans’ use of Veterans Health Administration primary care in an era of expanding choice. Med Care. 2021;59(Suppl 3):S292- S300. doi:10.1097/MLR.0000000000001554
The Veterans Health Administration (VHA) is the largest US integrated health system, providing care to veterans through VHA and non-VHA practitioners and facilities.1,2 Providing high-quality, timely, and veteran-centric care remains a priority for the VHA. Legislative efforts have expanded opportunities for eligible veterans to receive care in the community purchased by VHA, known as community care (CC).1 The Veterans Access, Choice, and Accountability Act of 2014 came in response to reports of long wait times and drive times for patients.3-5 The MISSION Act of 2018 expanded access to CC by streamlining it and broadening eligibility criteria, especially for veterans in rural communities who often experience more barriers in accessing care than veterans living in urban communities.1,6-10 Since the implementation of the Choice and MISSION Acts, > 2.7 million veterans have received care through community practitioners within the VHA CC network.11
Background
Increased access to CC could benefit veterans living in rural communities by increasing care options and circumventing challenges to accessing VHA care (ie, geographic, transportation, and distance barriers, practitioner and specialist shortages, and hospital closures). 5,9,10,12,13 However, health care system deficits in rural areas could also limit CC effectiveness for veterans living in those communities. 3 Other challenges posed by using CC include care coordination, information sharing, care continuity, delayed payments to CC practitioners, and mixed findings regarding CC quality.5,8,13,14 VHA practitioners are specifically trained to meet the multifaceted needs unique to veterans’ health and subculture, training CC practitioners may not receive.5,15
CC offers services for primary care and a broad range of specialties, including rehabilitation services such as physical therapy (PT).6 PT is used for the effective treatment of various conditions veterans experience and promote wellbeing and independence.16 US Department of Veterans Affairs (VA) databases reveal a high prevalence of veterans receiving PT services through CC; PT is one of the most frequently used CC outpatient specialty services by veterans living in rural communities.14,17
Telerehabitltation Enterprisewide Initiative
VHA has greatly invested in delivering care virtually, especially for veterans living in rural communities.18 In 2017, the VHA Office of Rural Health funded the Telerehabilitation Enterprise-Wide Initiative (TR-EWI) in partnership with the Physical Medicine and Rehabilitation Services national program office to increase access to specialized rehabilitation services for veterans living in rural communities by leveraging telehealth technologies.18-21 This alternative mode of health care delivery allows clinicians to overcome access barriers by delivering rehabilitation therapies directly to veterans' homes or nearby community-based outpatient clinics. TR-EWI was conceived as a hub-and-spoke model, where rehabilitation expertise at the hub was virtually delivered to spoke sites that did not have in-house expertise. In subsequent years, the TR-EWI also evolved to provide targeted telerehabilitation programs within rural-serving community-based outpatient clinics, including PT as a predominant service.19,20
As TR-EWI progressed—and in conjunction with the uptake of telehealth across VHA during the COVID-19 pandemic—there has been increased focus on PT telerehabilitation, especially for the 4.6 million veterans in rural communities.18,22,23 Because health care delivery system deficits in rural areas could limit the effective use of CC, many TR-EWI sites hope to reduce their CC referrals by providing telehealth PT services to veterans who might otherwise need to be referred to CC. This strategy aligns with VHA goals of providing high-quality and timely care. To better understand opportunities for programs like TR-EWI to provide rehabilitation services for veterans and reduce care sent to the community, research that examines CC referral trends for PT over time is warranted.
This study examines CC from a rehabilitation perspective with a focus on CC referral trends for PT, specifically for Veterans Integrated Service Networks (VISNs) where TREWI sites are located. The study’s objectives were to describe rehabilitation PT services being referred to CC and examine associated CC costs for PT services. Two research questions guided the study. First, what are the utilization trends for CC PT referrals from fiscal year (FY) 2019 to FY 2022? Secondly, what is the cost breakdown of CC for PT referrals from FY 2020 to FY 2022?
Methods
This study was conducted by a multidisciplinary team comprised of public health, disability, rehabilitation counseling, and PT professionals. It was deemed a quality improvement project under VA guidance and followed the SQUIRE guidelines for quality improvement reporting.24,25 The study used the VA Common Operating Platform (Palantir) to obtain individual-level CC referral data from the HealthShare Referral Manager (HSRM) database and consult data from the Computerized Patient Record System. Palantir is used to store and integrate VA data derived from the VA Corporate Data Warehouse and VHA Support Service Center. Referrals are authorizations for care to be delivered by a CC practitioner.
TR-EWI is comprised of 7 sites: VISN 2, VISN 4, VISN 8, VISN 12, VISN 15, VISN 19, and VISN 22. Each site provides telerehabilitation services with an emphasis on reaching veterans living in rural communities. We joined the referrals and consults cubes in Palantir to extract PT referrals for FY 2019 to FY 2022 for the 7 VISNs with TR-EWI sites and obtain referral-specific information and demographic characteristics. 26 Data were extracted in October 2022.
The VHA Community Care Referral Dashboard (CC Dashboard) provided nonindividual level CC cost data.27 The CC Dashboard provides insights into the costs of CC services for VHA enrollees by category of care, standardized episode of care, and eligibility. Data are based on nationallevel HSRM referrals that are not suspended or linked to a canceled or discontinued consult. Data were aggregated by VISN. The dashboard only includes referrals dating back to FY 2020; therefore, PT data from FY 2020 through FY 2022 for VISNs with TR-EWI sites were collected. Data were extracted in December 2022.
This study examined CC referrals, station name, eligibility types, clinical diagnoses (International Classification of Diseases, Tenth Revision codes), and demographic information in the Palantir dataset. Six eligibility criteria can qualify a veteran to receive CC.28 Within clinical diagnoses, the variable of interest was the provisional diagnosis. Patient demographics included age, gender, and rurality of residence, as determined by the Rural-Urban Commuting Area system.29,30 Rural and highly rural categories were combined for analysis. For the CC cost dataset, this study examined CC referrals, referral cost, and eligibility type.
Analysis
For the first research question, we examined referral data from FY 2019 to FY 2022 using the Palantir dataset, performed descriptive statistical analysis for all variables, and analyzed data to identify trends. Descriptive statistics were completed using IBM SPSS Statistics for Windows Version 29.0.0.0.
A qualitative analysis of provisional diagnosis data revealed what is being referred to CC for PT. A preliminary overview of provisional diagnosis data was conducted to familiarize coders with the data. We developed a coding framework to categorize diagnoses based on anatomical location, body structure, and clinical areas of interest. Data were reviewed individually and grouped into categories within the coding framework before meeting as a team to achieve group consensus on categorization. We then totaled the frequency of occurrence for provisional diagnoses within each category. Qualitative analyses were completed using Microsoft Excel.
For the second research question, the study used the CC cost dataset to examine the cost breakdown of CC PT referrals from FY 2020 to FY 2022. We calculated the number and cost of PT referrals across eligibility groups for each FY and VISN. Data were analyzed using SPSS to identify cost trends.
Results
There were 344,406 referrals to CC for PT from FY 2019 to FY 2022 for the 7 VISNs analyzed (Table 1). Of these, 22.5% were from FY 2019, 19.1% from FY 2020, 28.2% from FY 2021, and 30.3% from FY 2022. VISN 8 and VISN 22 reported the most overall PT referrals, with VISN 8 comprising 22.2% and VISN 22 comprising 18.1% of all referrals. VISN 2 reported the least overall referrals (3.7%). VISN 4 and VISN 12 had decreases in referrals over time. VISN 2 and VISN 15 had decreases in referrals from FY 2019 to FY 2021 and slight increases from FY 2021 to FY 2022. VISN 19 and VISN 22 both saw slight increases from FY 2019 to FY 2020 and substantial increases from FY 2020 to FY 2022, with FY 2022 accounting for 40.0% and 42.3% of all referrals for VISN 19 and VISN 20, respectively (Figure 1).


For FY 2019 and FY 2020, VISN 8 had the highest percentage of referrals (26.7% and 23.2%, respectively), whereas VISN 22 was among the lowest (7.3% and 11.4%, respectively). However, for FY 2021 and FY 2022, VISN 22 reported the highest percentage of referrals (23.5% and 25.3%, respectively) compared to all other VISNs. VISN 2 consistently reported the lowest percentage of referrals across all years.
There were 56 stations analyzed across the 7 VISNs (Appendix 1). Nine stations each accounted for ≥ 3.0% of the total PT referrals and only 2 stations accounted for > 5.0% of referrals. Orlando, Florida (6.0%), Philadelphia, Pennsylvania (5.2%), Tampa, Florida (4.9%), Aurora, Colorado (4.9%), and Gainesville, Florida (4.4%) reported the top 5 highest referrals, with 3 being from VISN 8 (Orlando, Tampa, Gainesville). Stations with the lowest reported referrals were all in VISN 2 in New York: The Bronx, (0%), New York Harbor (0%), Hudson Valley (0.1%) and Finger Lakes (0.2%).

Rurality
Urban stations comprised 56.2% and rural stations comprised 39.8% of PT CC referrals, while 0.2% of referrals were from insular isle US territories: Guam, American Samoa, Northern Marianas, and the Virgin Islands. The sample had missing or unknown data for 3.8% of referrals. FY 2022 had the largest difference in rural and urban referrals. Additionally, there was an overall trend of more referrals over time for rural and urban, with a large increase in rural (+40.0%) and urban (+62.7%) referrals from FY 2020 to FY 2021 and a modest increase from FY 2021 to FY 2022 (+5.2% for rural and +9.1% for urban). There was a decrease in rural (-7.0%) and urban (-3.5%) referrals from FY 2019 to FY 2020 (Figure 2).

There were differences in referrals by rurality and VISN (Table 2). VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals, whereas VISN 4, VISN 8, and VISN 22 reported more urban than rural referrals. VISN 2 reported similar numbers for both, with slightly more urban than rural referrals. When reviewing trends over time for each FY, VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals and VISN 4, VISN 8, and VISN 22 had more urban than rural referrals. In FY 2019 and FY 2020, VISN 2 reported slightly more urban than rural referrals but almost the same number of referrals in FY 2021 and FY 2022 (Appendix 2).


Demographics
The mean (SD) age was 61.2 (15.8) years (range, 20-105). Most PT CC referrals were for veterans aged 70 to 79 years (26.9%), followed by 60 to 69 years (20.7%), and 50 to 59 years (16.4%) (Appendix 3). Trends were consistent across VISNs. There was less of a difference between rural and urban referral percentages as the population aged. Veterans aged < 49 years residing in more urban areas accounted for more referrals to CC compared to their rural counterparts. This difference was less apparent in the 70 to 79 years and 80 to 89 years age brackets.

Most PT CC referrals (81.2%) were male and 14.8% were female. About 3.6% of referral data were missing sex information, and there was a smaller difference between male veterans living in rural communities and male veterans living in urban communities compared with female veterans. A total of 42.9% of male veterans resided in rural areas compared to 56.8% in urban areas; 32.7% of female veterans resided in rural areas compared to 66.9% in urban areas (Appendix 3).
Other Criteria
Of the 334,406 referrals, 114,983 (34.4%) had eligibility data, mostly from FY 2021 and FY 2022 (Table 3). Available eligibility data were likely affected by the MISSION Act and new regulations for reporting CC eligibility. Distance (33.4%) was the most common eligibility criteria, followed by timeliness of care (28.8%), and best medical interest (19.8%); 40.4% were rural and 59.5% were urban. Distance (55.4%) was most common for rural veterans, while timeliness of care (39.7%) was most common for urban veterans. For both groups, the second most common eligibility reason was best medical interest (Appendix 4).


Bone, joint, or soft tissue disorders were common diagnoses, with 25.2% located in the lower back, 14.7% in the shoulder, and 12.8% in the knee (Appendix 5). Amputations of the upper and lower limbs, fractures, cancer-related diagnoses, integumentary system disorders, thoracic and abdominal injuries and disorders, and other medical and mental health conditions each accounted for < 1% of the total diagnoses.

Costs
At time of analysis, the CC Dashboard had cost data available for 200,204 CC PT referrals from FY 2020 to FY 2022. The difference in referral numbers for the 2 datasets is likely attributed to several factors: CC cost data is exclusively from the HSRM, whereas Palantir includes other data sources; how VA cleans data pulled into Palantir; how the CC Dashboard algorithm populates data; and variances based on timing of reporting and/or if referrals are eventually canceled.
The total cost of PT CC referrals from FY 2020 to FY 2022 in selected VISNs was about $220,615,399 (Appendix 6). Appendix 7 details the methodology for determining the average standardized episode- of-care cost by VISN and how referral costs are calculated. Data show a continuous increase in total estimated cost from $46.8 million in FY 2020 to $92.1 million in FY 2022. From FY 2020 to FY 2022, aggregate costs ranged from $6,758,053 in VISN 2 to $47,209,162 in VISN 8 (Figure 3). The total referral cost for PT was highest at VISN 4 in FY 2020 ($10,447,140) and highest at VISN 22 in FY 2021 ($18,835,657) and FY 2022 ($22,962,438) (Figure 4). For referral costs from FY 2020 to FY 2022, distance accounted for $75,561,948 (34.3%), timeliness of care accounted for $60,413,496 (27.3%), and best medical interest accounted for $46,291,390 (21.0%) (Table 4).





Overall costs were primarily driven by specific VISNs within each eligibility type (Appendix 8; Figure 5). VISN 19, VISN 22, and VISN 15 accounted for the highest referral costs for distance; VISN 22, VISN 8, and VISN 19 accounted for the secondhighest referral cost, timeliness of care; and VISN 4, VISN 8, and VISN 12 accounted for the third-highest referral cost, best medical interest (Figure 5). VISN 2, VISN 4, VISN 12, VISN 15, and VISN 22 had service unavailable as an eligibility type with 1 of the top 3 associated referral costs, which was higher in cost than timeliness of care for VISN 2, VISN 4, VISN 12, and VISN 15.


Discussion
This study examines the referral of rehabilitation PT services to CC, evaluates CC costs for PT services, and analyzes utilization and cost trends among veterans within the VHA. Utilization data demonstrated a decrease in referrals from FY 2019 to FY 2020 and increases in referrals from FY 2020 to FY 2022 for most variables of interest, with cost data exhibiting similar trends. Results highlight the need for further investigation to address variations in PT referrals and costs across VISNs and eligibility reasons for CC referral.
Results demonstrated a noteworthy increase in PT CC referrals over time. The largest increase occurred from FY 2020 to FY 2021, with a smaller increase from FY 2021 to FY 2022. During this period, total enrollee numbers decreased by 3.0% across the 7 VISNs included in this analysis and by 1.6% across all VISNs, a trend that illustrates an overall decrease in enrollees as CC use increased. Results align with the implementation of the MISSION Act of 2018, which further expanded veterans’ options to use CC.1,6,7 Results also align with the onset of the COVID-19 pandemic, which disrupted care access for many veterans, placed a larger emphasis on the use of telehealth, and increased opportunities to stay within the VA for care by rapidly shifting to telehealth and leveraging telerehabilitation investments and initiatives (such as TR-EWI).20,31
VISN 8, VISN 19, and VISN 22, accounted for more than half of PT referrals. These VISNs had higher enrollee counts compared to the other VISNs.32 VISN 8 consistently had high levels of referrals, whereas VISN 19 and VISN 22 saw dramatic increases in FY 2021 and FY 2022. In contrast, VISN 4 and VISN 12 gradually decreased referrals during the study. VISN 2 had the lowest referral numbers during the study period, and all stations with the lowest individual referral numbers were located within VISN 2. Of the VISNs included in this study, VISN 2 had the second lowest number of enrollees (324,042).32 Reasons for increases and decreases over time could not be determined based on data collected in this study.
There were more urban than rural PT CC referrals; however, both exhibited an increase in referrals over time. This is consistent with population trends showing that most VHA patients (62.6%) and veterans (75.9%) reside in urban areas, which could explain some of the trends in this study.33 Some VISNs have larger urban catchment areas (eg, VISN 8 and VISN 22), and some have larger rural catchment areas (eg, VISN 15 and VISN 19), which could partially explain the rural-urban differences by VISN.32 Rural-urban referral trends might also reflect existing health care delivery system deficits in rural areas and known challenges associated with accessing health care for veterans living in rural communities.8,9
This study found larger differences in rural and urban PT CC referrals for younger age groups, with more than twice as many urban referrals in veterans aged 20 to 29 years and aged 30 to 39 years, and roughly 1.8 times as many urban referrals in veterans aged 40 to 49 years. However, there were similar numbers of rural and urban referrals in those aged 70 to 79 years and aged 80 to 89 years. These trends are consistent with data showing veterans residing in rural communities are older than their urban counterparts.23,34 Data suggest that older veteran populations might seek PT at higher rates than younger veteran populations. Moreover, data suggest there could be differences in PT-seeking rates for younger veteran populations who reside in rural vs urban areas. Additional research is needed to understand these trends.
Distance and timeliness of care were the predominant reasons for referral among eligibility groups, which is consistent with the MISSION Act goals.1,6,7 The most common eligibility reason for rural referrals was distance; timeliness of care was most common for urban referrals. This finding is expected, as veterans living in rural communities are farther away from VHA facilities and have longer drive times, whereas veterans living in urban communities might live closer, yet experience longer wait times due to services and/or appointment availability. Best medical interest accounted for almost 20% of referrals, which does not provide detailed insights into why those veterans were referred to CC.
The top PT diagnoses referred to CC were related to bone, joint, or soft tissue disorders of the lower back, shoulder, and knee. This suggests that musculoskeletal-related issues are prevalent among veterans seeking PT care, which is consistent with research that found > 50% of veterans receiving VHA care have musculoskeletal disorders.35 The probability of experiencing musculoskeletal problems increases with age, as does the need for PT services. Amputations and fractures accounted for < 1% of CC referrals, which is consistent with the historic provision of VHA clinical specialized care to conditions prevalent among veterans. It may also represent VHA efforts to internally provide care for complex conditions requiring more extensive interdisciplinary coordination.
The total cost of referrals over time was about $221 million. VISN 8 accounted for the highest overall cost; VISN 2 had the lowest, mirroring referral utilization trends and aligning with VISN enrollee numbers. VISN 19 and VISN 22 reported large cost increases from FY 2020 to FY 2021. Total referral costs increased by $34.9 million from FY 2020 to FY 2021, which may be due to health care inflation (2.9% during FY 2019 to FY 2022), increased awareness of CC services, or increased VHA wait times.36 Additionally, there were limitations in care provided across health care systems during the COVID-19 pandemic, including the VA.5 The increase from FY 2020 to FY 2021 may reflect a rebound from restrictions in appointments across VA, CC, and the private sector.
While the increase in total referral cost may be partly attributed to inflation, the cost effectiveness and efficiency of referring veterans to CC vs keeping veterans within VHA care is an ongoing debate.5 Examining and addressing cost drivers within the top eligibility types and their respective VISNs is necessary to determine resource allocation and improve quality of care. This study found that best medical interest and unavailable services accounted for 33.4% of the total cost of CC referrals, highlighting the need for policies that strengthen in-house competencies and recruit personnel to provide PT services currently unavailable within the VA.
Future Directions
The VHA should explore opportunities for in-house care, especially for services appropriate for telehealth.18,20,37 Data indicated a smaller cost increase from FY 2021 to FY 2022 compared to the relatively large increase from FY 2020 to FY 2021. The increased telehealth usage across VHA by TR-EWI and non—TR-EWI sites within selected VISNs may have contributed to limiting the increase in CC costs. Future studies should investigate contextual factors of increased telehealth usage, which would offer guidance for implementation to optimize the integration of telehealth with PT rehabilitation provided in-house. Additionally, future studies can examine potential limitations experienced during PT telehealth visits, such as the inability to conduct hands-on assessments, challenges in viewing the quality of patient movement, ensuring patient safety in the remote environment, and the lack of PT equipment in homes for telehealth visits, and how these challenges are being addressed.38,39 Research is also needed to understand tradeoffs of CC vs VHA care and the potential and cost benefits of keeping veterans within VHA using programs like TR-EWI.5 Veterans living in rural communities may especially benefit from this as expanding telehealth options can provide access to PT care that may not be readily available, enabling them to stay connected and engaged in their care.18,40
Future studies could examine contributory factors to rising costs, such as demographic shifts, changes in PT service utilization, and policy. Researchers might also consider qualitative studies with clinicians and veterans within each VISN, which may provide insights into how local factors impact PT referral to the community.
Limitations
Due to its descriptive nature, this study can only speculate about factors influencing trends. Limitations include the inability to link the Palantir and CC Dashboard datasets for cost comparisons and potential data change over time on Palantir due to platform updates. The focus on VISNs with TREWI sites limited generalizability and this study did not compare CC PT vs VHA PT. Finally, there may have been cost drivers not identified in this study.
Conclusions
This descriptive study provides insights into the utilization and cost of PT CC referrals for selected VISNs. Cost trends underscore the financial commitment to providing PT services to veterans. Understanding what factors are driving this cost is necessary for VHA to optimally provide and manage the rehabilitation resources needed to serve veterans through traditional in-person care, telehealth, and CC options while ensuring timely, highquality care.
The Veterans Health Administration (VHA) is the largest US integrated health system, providing care to veterans through VHA and non-VHA practitioners and facilities.1,2 Providing high-quality, timely, and veteran-centric care remains a priority for the VHA. Legislative efforts have expanded opportunities for eligible veterans to receive care in the community purchased by VHA, known as community care (CC).1 The Veterans Access, Choice, and Accountability Act of 2014 came in response to reports of long wait times and drive times for patients.3-5 The MISSION Act of 2018 expanded access to CC by streamlining it and broadening eligibility criteria, especially for veterans in rural communities who often experience more barriers in accessing care than veterans living in urban communities.1,6-10 Since the implementation of the Choice and MISSION Acts, > 2.7 million veterans have received care through community practitioners within the VHA CC network.11
Background
Increased access to CC could benefit veterans living in rural communities by increasing care options and circumventing challenges to accessing VHA care (ie, geographic, transportation, and distance barriers, practitioner and specialist shortages, and hospital closures). 5,9,10,12,13 However, health care system deficits in rural areas could also limit CC effectiveness for veterans living in those communities. 3 Other challenges posed by using CC include care coordination, information sharing, care continuity, delayed payments to CC practitioners, and mixed findings regarding CC quality.5,8,13,14 VHA practitioners are specifically trained to meet the multifaceted needs unique to veterans’ health and subculture, training CC practitioners may not receive.5,15
CC offers services for primary care and a broad range of specialties, including rehabilitation services such as physical therapy (PT).6 PT is used for the effective treatment of various conditions veterans experience and promote wellbeing and independence.16 US Department of Veterans Affairs (VA) databases reveal a high prevalence of veterans receiving PT services through CC; PT is one of the most frequently used CC outpatient specialty services by veterans living in rural communities.14,17
Telerehabitltation Enterprisewide Initiative
VHA has greatly invested in delivering care virtually, especially for veterans living in rural communities.18 In 2017, the VHA Office of Rural Health funded the Telerehabilitation Enterprise-Wide Initiative (TR-EWI) in partnership with the Physical Medicine and Rehabilitation Services national program office to increase access to specialized rehabilitation services for veterans living in rural communities by leveraging telehealth technologies.18-21 This alternative mode of health care delivery allows clinicians to overcome access barriers by delivering rehabilitation therapies directly to veterans' homes or nearby community-based outpatient clinics. TR-EWI was conceived as a hub-and-spoke model, where rehabilitation expertise at the hub was virtually delivered to spoke sites that did not have in-house expertise. In subsequent years, the TR-EWI also evolved to provide targeted telerehabilitation programs within rural-serving community-based outpatient clinics, including PT as a predominant service.19,20
As TR-EWI progressed—and in conjunction with the uptake of telehealth across VHA during the COVID-19 pandemic—there has been increased focus on PT telerehabilitation, especially for the 4.6 million veterans in rural communities.18,22,23 Because health care delivery system deficits in rural areas could limit the effective use of CC, many TR-EWI sites hope to reduce their CC referrals by providing telehealth PT services to veterans who might otherwise need to be referred to CC. This strategy aligns with VHA goals of providing high-quality and timely care. To better understand opportunities for programs like TR-EWI to provide rehabilitation services for veterans and reduce care sent to the community, research that examines CC referral trends for PT over time is warranted.
This study examines CC from a rehabilitation perspective with a focus on CC referral trends for PT, specifically for Veterans Integrated Service Networks (VISNs) where TREWI sites are located. The study’s objectives were to describe rehabilitation PT services being referred to CC and examine associated CC costs for PT services. Two research questions guided the study. First, what are the utilization trends for CC PT referrals from fiscal year (FY) 2019 to FY 2022? Secondly, what is the cost breakdown of CC for PT referrals from FY 2020 to FY 2022?
Methods
This study was conducted by a multidisciplinary team comprised of public health, disability, rehabilitation counseling, and PT professionals. It was deemed a quality improvement project under VA guidance and followed the SQUIRE guidelines for quality improvement reporting.24,25 The study used the VA Common Operating Platform (Palantir) to obtain individual-level CC referral data from the HealthShare Referral Manager (HSRM) database and consult data from the Computerized Patient Record System. Palantir is used to store and integrate VA data derived from the VA Corporate Data Warehouse and VHA Support Service Center. Referrals are authorizations for care to be delivered by a CC practitioner.
TR-EWI is comprised of 7 sites: VISN 2, VISN 4, VISN 8, VISN 12, VISN 15, VISN 19, and VISN 22. Each site provides telerehabilitation services with an emphasis on reaching veterans living in rural communities. We joined the referrals and consults cubes in Palantir to extract PT referrals for FY 2019 to FY 2022 for the 7 VISNs with TR-EWI sites and obtain referral-specific information and demographic characteristics. 26 Data were extracted in October 2022.
The VHA Community Care Referral Dashboard (CC Dashboard) provided nonindividual level CC cost data.27 The CC Dashboard provides insights into the costs of CC services for VHA enrollees by category of care, standardized episode of care, and eligibility. Data are based on nationallevel HSRM referrals that are not suspended or linked to a canceled or discontinued consult. Data were aggregated by VISN. The dashboard only includes referrals dating back to FY 2020; therefore, PT data from FY 2020 through FY 2022 for VISNs with TR-EWI sites were collected. Data were extracted in December 2022.
This study examined CC referrals, station name, eligibility types, clinical diagnoses (International Classification of Diseases, Tenth Revision codes), and demographic information in the Palantir dataset. Six eligibility criteria can qualify a veteran to receive CC.28 Within clinical diagnoses, the variable of interest was the provisional diagnosis. Patient demographics included age, gender, and rurality of residence, as determined by the Rural-Urban Commuting Area system.29,30 Rural and highly rural categories were combined for analysis. For the CC cost dataset, this study examined CC referrals, referral cost, and eligibility type.
Analysis
For the first research question, we examined referral data from FY 2019 to FY 2022 using the Palantir dataset, performed descriptive statistical analysis for all variables, and analyzed data to identify trends. Descriptive statistics were completed using IBM SPSS Statistics for Windows Version 29.0.0.0.
A qualitative analysis of provisional diagnosis data revealed what is being referred to CC for PT. A preliminary overview of provisional diagnosis data was conducted to familiarize coders with the data. We developed a coding framework to categorize diagnoses based on anatomical location, body structure, and clinical areas of interest. Data were reviewed individually and grouped into categories within the coding framework before meeting as a team to achieve group consensus on categorization. We then totaled the frequency of occurrence for provisional diagnoses within each category. Qualitative analyses were completed using Microsoft Excel.
For the second research question, the study used the CC cost dataset to examine the cost breakdown of CC PT referrals from FY 2020 to FY 2022. We calculated the number and cost of PT referrals across eligibility groups for each FY and VISN. Data were analyzed using SPSS to identify cost trends.
Results
There were 344,406 referrals to CC for PT from FY 2019 to FY 2022 for the 7 VISNs analyzed (Table 1). Of these, 22.5% were from FY 2019, 19.1% from FY 2020, 28.2% from FY 2021, and 30.3% from FY 2022. VISN 8 and VISN 22 reported the most overall PT referrals, with VISN 8 comprising 22.2% and VISN 22 comprising 18.1% of all referrals. VISN 2 reported the least overall referrals (3.7%). VISN 4 and VISN 12 had decreases in referrals over time. VISN 2 and VISN 15 had decreases in referrals from FY 2019 to FY 2021 and slight increases from FY 2021 to FY 2022. VISN 19 and VISN 22 both saw slight increases from FY 2019 to FY 2020 and substantial increases from FY 2020 to FY 2022, with FY 2022 accounting for 40.0% and 42.3% of all referrals for VISN 19 and VISN 20, respectively (Figure 1).


For FY 2019 and FY 2020, VISN 8 had the highest percentage of referrals (26.7% and 23.2%, respectively), whereas VISN 22 was among the lowest (7.3% and 11.4%, respectively). However, for FY 2021 and FY 2022, VISN 22 reported the highest percentage of referrals (23.5% and 25.3%, respectively) compared to all other VISNs. VISN 2 consistently reported the lowest percentage of referrals across all years.
There were 56 stations analyzed across the 7 VISNs (Appendix 1). Nine stations each accounted for ≥ 3.0% of the total PT referrals and only 2 stations accounted for > 5.0% of referrals. Orlando, Florida (6.0%), Philadelphia, Pennsylvania (5.2%), Tampa, Florida (4.9%), Aurora, Colorado (4.9%), and Gainesville, Florida (4.4%) reported the top 5 highest referrals, with 3 being from VISN 8 (Orlando, Tampa, Gainesville). Stations with the lowest reported referrals were all in VISN 2 in New York: The Bronx, (0%), New York Harbor (0%), Hudson Valley (0.1%) and Finger Lakes (0.2%).

Rurality
Urban stations comprised 56.2% and rural stations comprised 39.8% of PT CC referrals, while 0.2% of referrals were from insular isle US territories: Guam, American Samoa, Northern Marianas, and the Virgin Islands. The sample had missing or unknown data for 3.8% of referrals. FY 2022 had the largest difference in rural and urban referrals. Additionally, there was an overall trend of more referrals over time for rural and urban, with a large increase in rural (+40.0%) and urban (+62.7%) referrals from FY 2020 to FY 2021 and a modest increase from FY 2021 to FY 2022 (+5.2% for rural and +9.1% for urban). There was a decrease in rural (-7.0%) and urban (-3.5%) referrals from FY 2019 to FY 2020 (Figure 2).

There were differences in referrals by rurality and VISN (Table 2). VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals, whereas VISN 4, VISN 8, and VISN 22 reported more urban than rural referrals. VISN 2 reported similar numbers for both, with slightly more urban than rural referrals. When reviewing trends over time for each FY, VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals and VISN 4, VISN 8, and VISN 22 had more urban than rural referrals. In FY 2019 and FY 2020, VISN 2 reported slightly more urban than rural referrals but almost the same number of referrals in FY 2021 and FY 2022 (Appendix 2).


Demographics
The mean (SD) age was 61.2 (15.8) years (range, 20-105). Most PT CC referrals were for veterans aged 70 to 79 years (26.9%), followed by 60 to 69 years (20.7%), and 50 to 59 years (16.4%) (Appendix 3). Trends were consistent across VISNs. There was less of a difference between rural and urban referral percentages as the population aged. Veterans aged < 49 years residing in more urban areas accounted for more referrals to CC compared to their rural counterparts. This difference was less apparent in the 70 to 79 years and 80 to 89 years age brackets.

Most PT CC referrals (81.2%) were male and 14.8% were female. About 3.6% of referral data were missing sex information, and there was a smaller difference between male veterans living in rural communities and male veterans living in urban communities compared with female veterans. A total of 42.9% of male veterans resided in rural areas compared to 56.8% in urban areas; 32.7% of female veterans resided in rural areas compared to 66.9% in urban areas (Appendix 3).
Other Criteria
Of the 334,406 referrals, 114,983 (34.4%) had eligibility data, mostly from FY 2021 and FY 2022 (Table 3). Available eligibility data were likely affected by the MISSION Act and new regulations for reporting CC eligibility. Distance (33.4%) was the most common eligibility criteria, followed by timeliness of care (28.8%), and best medical interest (19.8%); 40.4% were rural and 59.5% were urban. Distance (55.4%) was most common for rural veterans, while timeliness of care (39.7%) was most common for urban veterans. For both groups, the second most common eligibility reason was best medical interest (Appendix 4).


Bone, joint, or soft tissue disorders were common diagnoses, with 25.2% located in the lower back, 14.7% in the shoulder, and 12.8% in the knee (Appendix 5). Amputations of the upper and lower limbs, fractures, cancer-related diagnoses, integumentary system disorders, thoracic and abdominal injuries and disorders, and other medical and mental health conditions each accounted for < 1% of the total diagnoses.

Costs
At time of analysis, the CC Dashboard had cost data available for 200,204 CC PT referrals from FY 2020 to FY 2022. The difference in referral numbers for the 2 datasets is likely attributed to several factors: CC cost data is exclusively from the HSRM, whereas Palantir includes other data sources; how VA cleans data pulled into Palantir; how the CC Dashboard algorithm populates data; and variances based on timing of reporting and/or if referrals are eventually canceled.
The total cost of PT CC referrals from FY 2020 to FY 2022 in selected VISNs was about $220,615,399 (Appendix 6). Appendix 7 details the methodology for determining the average standardized episode- of-care cost by VISN and how referral costs are calculated. Data show a continuous increase in total estimated cost from $46.8 million in FY 2020 to $92.1 million in FY 2022. From FY 2020 to FY 2022, aggregate costs ranged from $6,758,053 in VISN 2 to $47,209,162 in VISN 8 (Figure 3). The total referral cost for PT was highest at VISN 4 in FY 2020 ($10,447,140) and highest at VISN 22 in FY 2021 ($18,835,657) and FY 2022 ($22,962,438) (Figure 4). For referral costs from FY 2020 to FY 2022, distance accounted for $75,561,948 (34.3%), timeliness of care accounted for $60,413,496 (27.3%), and best medical interest accounted for $46,291,390 (21.0%) (Table 4).





Overall costs were primarily driven by specific VISNs within each eligibility type (Appendix 8; Figure 5). VISN 19, VISN 22, and VISN 15 accounted for the highest referral costs for distance; VISN 22, VISN 8, and VISN 19 accounted for the secondhighest referral cost, timeliness of care; and VISN 4, VISN 8, and VISN 12 accounted for the third-highest referral cost, best medical interest (Figure 5). VISN 2, VISN 4, VISN 12, VISN 15, and VISN 22 had service unavailable as an eligibility type with 1 of the top 3 associated referral costs, which was higher in cost than timeliness of care for VISN 2, VISN 4, VISN 12, and VISN 15.


Discussion
This study examines the referral of rehabilitation PT services to CC, evaluates CC costs for PT services, and analyzes utilization and cost trends among veterans within the VHA. Utilization data demonstrated a decrease in referrals from FY 2019 to FY 2020 and increases in referrals from FY 2020 to FY 2022 for most variables of interest, with cost data exhibiting similar trends. Results highlight the need for further investigation to address variations in PT referrals and costs across VISNs and eligibility reasons for CC referral.
Results demonstrated a noteworthy increase in PT CC referrals over time. The largest increase occurred from FY 2020 to FY 2021, with a smaller increase from FY 2021 to FY 2022. During this period, total enrollee numbers decreased by 3.0% across the 7 VISNs included in this analysis and by 1.6% across all VISNs, a trend that illustrates an overall decrease in enrollees as CC use increased. Results align with the implementation of the MISSION Act of 2018, which further expanded veterans’ options to use CC.1,6,7 Results also align with the onset of the COVID-19 pandemic, which disrupted care access for many veterans, placed a larger emphasis on the use of telehealth, and increased opportunities to stay within the VA for care by rapidly shifting to telehealth and leveraging telerehabilitation investments and initiatives (such as TR-EWI).20,31
VISN 8, VISN 19, and VISN 22, accounted for more than half of PT referrals. These VISNs had higher enrollee counts compared to the other VISNs.32 VISN 8 consistently had high levels of referrals, whereas VISN 19 and VISN 22 saw dramatic increases in FY 2021 and FY 2022. In contrast, VISN 4 and VISN 12 gradually decreased referrals during the study. VISN 2 had the lowest referral numbers during the study period, and all stations with the lowest individual referral numbers were located within VISN 2. Of the VISNs included in this study, VISN 2 had the second lowest number of enrollees (324,042).32 Reasons for increases and decreases over time could not be determined based on data collected in this study.
There were more urban than rural PT CC referrals; however, both exhibited an increase in referrals over time. This is consistent with population trends showing that most VHA patients (62.6%) and veterans (75.9%) reside in urban areas, which could explain some of the trends in this study.33 Some VISNs have larger urban catchment areas (eg, VISN 8 and VISN 22), and some have larger rural catchment areas (eg, VISN 15 and VISN 19), which could partially explain the rural-urban differences by VISN.32 Rural-urban referral trends might also reflect existing health care delivery system deficits in rural areas and known challenges associated with accessing health care for veterans living in rural communities.8,9
This study found larger differences in rural and urban PT CC referrals for younger age groups, with more than twice as many urban referrals in veterans aged 20 to 29 years and aged 30 to 39 years, and roughly 1.8 times as many urban referrals in veterans aged 40 to 49 years. However, there were similar numbers of rural and urban referrals in those aged 70 to 79 years and aged 80 to 89 years. These trends are consistent with data showing veterans residing in rural communities are older than their urban counterparts.23,34 Data suggest that older veteran populations might seek PT at higher rates than younger veteran populations. Moreover, data suggest there could be differences in PT-seeking rates for younger veteran populations who reside in rural vs urban areas. Additional research is needed to understand these trends.
Distance and timeliness of care were the predominant reasons for referral among eligibility groups, which is consistent with the MISSION Act goals.1,6,7 The most common eligibility reason for rural referrals was distance; timeliness of care was most common for urban referrals. This finding is expected, as veterans living in rural communities are farther away from VHA facilities and have longer drive times, whereas veterans living in urban communities might live closer, yet experience longer wait times due to services and/or appointment availability. Best medical interest accounted for almost 20% of referrals, which does not provide detailed insights into why those veterans were referred to CC.
The top PT diagnoses referred to CC were related to bone, joint, or soft tissue disorders of the lower back, shoulder, and knee. This suggests that musculoskeletal-related issues are prevalent among veterans seeking PT care, which is consistent with research that found > 50% of veterans receiving VHA care have musculoskeletal disorders.35 The probability of experiencing musculoskeletal problems increases with age, as does the need for PT services. Amputations and fractures accounted for < 1% of CC referrals, which is consistent with the historic provision of VHA clinical specialized care to conditions prevalent among veterans. It may also represent VHA efforts to internally provide care for complex conditions requiring more extensive interdisciplinary coordination.
The total cost of referrals over time was about $221 million. VISN 8 accounted for the highest overall cost; VISN 2 had the lowest, mirroring referral utilization trends and aligning with VISN enrollee numbers. VISN 19 and VISN 22 reported large cost increases from FY 2020 to FY 2021. Total referral costs increased by $34.9 million from FY 2020 to FY 2021, which may be due to health care inflation (2.9% during FY 2019 to FY 2022), increased awareness of CC services, or increased VHA wait times.36 Additionally, there were limitations in care provided across health care systems during the COVID-19 pandemic, including the VA.5 The increase from FY 2020 to FY 2021 may reflect a rebound from restrictions in appointments across VA, CC, and the private sector.
While the increase in total referral cost may be partly attributed to inflation, the cost effectiveness and efficiency of referring veterans to CC vs keeping veterans within VHA care is an ongoing debate.5 Examining and addressing cost drivers within the top eligibility types and their respective VISNs is necessary to determine resource allocation and improve quality of care. This study found that best medical interest and unavailable services accounted for 33.4% of the total cost of CC referrals, highlighting the need for policies that strengthen in-house competencies and recruit personnel to provide PT services currently unavailable within the VA.
Future Directions
The VHA should explore opportunities for in-house care, especially for services appropriate for telehealth.18,20,37 Data indicated a smaller cost increase from FY 2021 to FY 2022 compared to the relatively large increase from FY 2020 to FY 2021. The increased telehealth usage across VHA by TR-EWI and non—TR-EWI sites within selected VISNs may have contributed to limiting the increase in CC costs. Future studies should investigate contextual factors of increased telehealth usage, which would offer guidance for implementation to optimize the integration of telehealth with PT rehabilitation provided in-house. Additionally, future studies can examine potential limitations experienced during PT telehealth visits, such as the inability to conduct hands-on assessments, challenges in viewing the quality of patient movement, ensuring patient safety in the remote environment, and the lack of PT equipment in homes for telehealth visits, and how these challenges are being addressed.38,39 Research is also needed to understand tradeoffs of CC vs VHA care and the potential and cost benefits of keeping veterans within VHA using programs like TR-EWI.5 Veterans living in rural communities may especially benefit from this as expanding telehealth options can provide access to PT care that may not be readily available, enabling them to stay connected and engaged in their care.18,40
Future studies could examine contributory factors to rising costs, such as demographic shifts, changes in PT service utilization, and policy. Researchers might also consider qualitative studies with clinicians and veterans within each VISN, which may provide insights into how local factors impact PT referral to the community.
Limitations
Due to its descriptive nature, this study can only speculate about factors influencing trends. Limitations include the inability to link the Palantir and CC Dashboard datasets for cost comparisons and potential data change over time on Palantir due to platform updates. The focus on VISNs with TREWI sites limited generalizability and this study did not compare CC PT vs VHA PT. Finally, there may have been cost drivers not identified in this study.
Conclusions
This descriptive study provides insights into the utilization and cost of PT CC referrals for selected VISNs. Cost trends underscore the financial commitment to providing PT services to veterans. Understanding what factors are driving this cost is necessary for VHA to optimally provide and manage the rehabilitation resources needed to serve veterans through traditional in-person care, telehealth, and CC options while ensuring timely, highquality care.
- Congressional Budget Office. The Veterans Community Care Program: Background and Early Effects. October 26, 2021. Accessed September 23, 2024. https://www.cbo.gov/publication/57257
- US Dept of Veterans Affairs. Providing Health Care for Veterans. Updated September 10, 2024. Accessed September 23, 2024. https://www.va.gov/health/
- Davila H, Rosen AK, Beilstein-Wedel E, Shwartz M, Chatelain LJ, Gurewich D. Rural veterans’ experiences with outpatient care in the Veterans Health Administration versus community care. Med Care. 2021;59(Suppl 3):S286-S291. doi:10.1097/MLR.0000000000001552
- Vanneman ME, Wagner TH, Shwartz M, et al. Veterans’ experiences with outpatient care: comparing the Veterans Affairs system with community-based care. Health Aff (Millwood). 2020;39(8):1368-1376. doi:10.1377/hlthaff.2019.01375
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10(3):9.
- Feyman Y, Legler A, Griffith KN. Appointment wait time data for primary & specialty care in veterans health administration facilities vs. community medical centers. Data Brief. 2021;36:107134. doi:10.1016/j.dib.2021.107134
- Kelley AT, Greenstone CL, Kirsh SR. Defining access and the role of community care in the Veterans Health Administration. J Gen Intern Med. 2020;35(5):1584-1585. doi:10.1007/s11606-019-05358-z
- Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(Suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
- US Dept of Veterans Affairs, Office of Rural Health. Rural Veterans: Rural Veteran Health Care Challenges. Updated May 14, 2024. Accessed September 23, 2024. https:// www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Ohl ME, Carrell M, Thurman A, et al. “Availability of healthcare providers for rural veterans eligible for purchased care under the veterans choice act.” BMC Health Serv Res. 2018;18(1):315. doi:10.1186/s12913-018-3108-8
- Mattocks KM, Cunningham KJ, Greenstone C, Atkins D, Rosen AK, Upton M. Innovations in community care programs, policies, and research. Med Care. 2021;59(Suppl 3):S229-S231. doi:10.1097/MLR.0000000000001550
- Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52 Suppl 1(Suppl 1):459-480. doi:10.1111/1475-6773.12633
- Patzel M, Barnes C, Ramalingam N, et al. Jumping through hoops: community care clinician and staff experiences providing primary care to rural veterans. J Gen Intern Med. 2023;38(Suppl 3):821-828. doi:10.1007/s11606-023-08126-2
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Campbell P, Pope R, Simas V, Canetti E, Schram B, Orr R. The effects of early physiotherapy treatment on musculoskeletal injury outcomes in military personnel: a narrative review. Int J Environ Res Public Health. 2022;19(20):13416. doi:10.3390/ijerph192013416
- Gurewich D, Shwartz M, Beilstein-Wedel E, Davila H, Rosen AK. Did access to care improve since passage of the veterans choice act? Differences between rural and urban veterans. Med Care. 2021;59(Suppl 3):S270-S278. doi:10.1097/MLR.0000000000001490
- Myers US, Birks A, Grubaugh AL, Axon RN. Flattening the curve by getting ahead of it: how the VA healthcare system is leveraging telehealth to provide continued access to care for rural veterans. J Rural Health. 2021;37(1):194-196. doi:10.1111/jrh.12449
- Hale-Gallardo JL, Kreider CM, Jia H, et al. Telerehabilitation for rural veterans: a qualitative assessment of barriers and facilitators to implementation. J Multidiscip Healthc. 2020;13:559-570. doi:10.2147/JMDH.S247267
- Kreider CM, Hale-Gallardo J, Kramer JC, et al. Providers’ shift to telerehabilitation at the U.S. Veterans Health Administration during COVID-19: practical applications. Front Public Health. 2022;10:831762. doi:10.3389/fpubh.2022.831762
- Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019;36(3):122-128.
- US Dept of Veterans Affairs, Office of Rural Health. VHA Office of Rural Health. Updated August 30, 2024. Accessed September 23, 2024. https://www.ruralhealth.va.gov/index.asp
- National Center for Veterans Analysis and Statistics. Rural Veterans: 2021-2023. April 2023. Accessed September 23, 2024. https://www.datahub.va.gov/stories/s/Rural-Veterans-FY2021-2023/kkh2-eymp/
- U.S. Department of Veterans Affairs, Office of Research & Development. Program Guide: 1200.21, VHA Operations Activities That May Constitute Research. January 9, 2019. https://www.research.va.gov/resources/policies/ProgramGuide-1200-21-VHA-Operations-Activities.pdf
- Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Nurs Care Qual. 2016;31(1):1-8. doi:10.1097/NCQ.0000000000000153
- US Dept of Veterans Affairs. Veterans Health Administration: Veterans Integrated Service Networks (VISNs). Updated January 29, 2024. Accessed September 23, 2024. https://www.va.gov/HEALTH/visns.asp
- Stomberg C, Frost A, Becker C, Stang H, Windschitl M, Carrier E. Community Care referral dashboard [Data dashboard]. https://app.powerbigov.us/groups/me/reports/090d22a7-0e1f-4cc5-bea8-0a1b87aa0bd9/ReportSectionacfd03cdebd76ffca9ec [Source not verified]
- US Dept of Veterans Affairs. Eligibility for community care outside VA. Updated May 30, 2024. Accessed September 23, 2024. https://www.va.gov/COMMUNITYCARE/programs/veterans/General_Care.asp
- US Department of Veterans Affairs, Office of Rural Health. How to define rurality fact sheet. Updated December 2023. Accessed January 28, 2025. https://www.ruralhealth.va.gov/docs/ORH_RuralityFactSheet_508.pdf
- Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed September 23, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx
- Gurewich D, Beilstein-Wedel E, Shwartz M, Davila H, Rosen AK. Disparities in wait times for care among US veterans by race and ethnici t y. JAMA Netw Open. 2023;6(1):e2252061. doi:10.1001/jamanetworkopen.2022.52061
- U.S. Department of Veterans Affairs, VA Office of Rural Health, Veterans Rural Health Resource Center-Gainesville, GeoSpatial Outcomes Division. VA and Community Healthcare, and VHA Rurality web map application. Published 2023. https://portal.vhagis.inv.vaec.va.gov/arcgis/apps/webappbuilder/index.html [source not verified]
- Chartbook on Healthcare for Veterans: National Healthcare Quality and Disparities Report. Agency for Healthcare Research and Quality; November 2020. Accessed September 23, 2024. https://www.ahrq.gov/research/findings/nhqrdr/chartbooks/veterans/index.html
- Lum HD, Nearing K, Pimentel CB, Levy CR, Hung WW. Anywhere to anywhere: use of telehealth to increase health care access for older, rural veterans. Public Policy Aging Rep. 2020;30(1):12-18. doi:10.1093/ppar/prz030
- Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696-1703. doi:10.1097/j.pain.0000000000000567
- US Inflation Calculator. Health Care Inflation in the United States (1948-2024). Accessed September 23, 2024. https://www.usinflationcalculator.com/inflation/health-care-inflation-in-the-united-states/
- Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638. doi:10.1177/0269215516645148
- Elor A, Conde S, Powel l M, Robbins A, Chen NN, Kurniawan S. Physical therapist impressions of telehealth and virtual reality needs amidst a pandemic. Front Virtual Real. 2022;3. doi:10.3389/frvir.2022.915332
- Lee AC, Harada N. Telehealth as a means of health care delivery for physical therapist practice. Phys Ther. 2012;92(3):463-468. doi:10.2522/ptj.20110100
- Hynes DM, Edwards S, Hickok A, et al. Veterans’ use of Veterans Health Administration primary care in an era of expanding choice. Med Care. 2021;59(Suppl 3):S292- S300. doi:10.1097/MLR.0000000000001554
- Congressional Budget Office. The Veterans Community Care Program: Background and Early Effects. October 26, 2021. Accessed September 23, 2024. https://www.cbo.gov/publication/57257
- US Dept of Veterans Affairs. Providing Health Care for Veterans. Updated September 10, 2024. Accessed September 23, 2024. https://www.va.gov/health/
- Davila H, Rosen AK, Beilstein-Wedel E, Shwartz M, Chatelain LJ, Gurewich D. Rural veterans’ experiences with outpatient care in the Veterans Health Administration versus community care. Med Care. 2021;59(Suppl 3):S286-S291. doi:10.1097/MLR.0000000000001552
- Vanneman ME, Wagner TH, Shwartz M, et al. Veterans’ experiences with outpatient care: comparing the Veterans Affairs system with community-based care. Health Aff (Millwood). 2020;39(8):1368-1376. doi:10.1377/hlthaff.2019.01375
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10(3):9.
- Feyman Y, Legler A, Griffith KN. Appointment wait time data for primary & specialty care in veterans health administration facilities vs. community medical centers. Data Brief. 2021;36:107134. doi:10.1016/j.dib.2021.107134
- Kelley AT, Greenstone CL, Kirsh SR. Defining access and the role of community care in the Veterans Health Administration. J Gen Intern Med. 2020;35(5):1584-1585. doi:10.1007/s11606-019-05358-z
- Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(Suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
- US Dept of Veterans Affairs, Office of Rural Health. Rural Veterans: Rural Veteran Health Care Challenges. Updated May 14, 2024. Accessed September 23, 2024. https:// www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Ohl ME, Carrell M, Thurman A, et al. “Availability of healthcare providers for rural veterans eligible for purchased care under the veterans choice act.” BMC Health Serv Res. 2018;18(1):315. doi:10.1186/s12913-018-3108-8
- Mattocks KM, Cunningham KJ, Greenstone C, Atkins D, Rosen AK, Upton M. Innovations in community care programs, policies, and research. Med Care. 2021;59(Suppl 3):S229-S231. doi:10.1097/MLR.0000000000001550
- Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52 Suppl 1(Suppl 1):459-480. doi:10.1111/1475-6773.12633
- Patzel M, Barnes C, Ramalingam N, et al. Jumping through hoops: community care clinician and staff experiences providing primary care to rural veterans. J Gen Intern Med. 2023;38(Suppl 3):821-828. doi:10.1007/s11606-023-08126-2
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Campbell P, Pope R, Simas V, Canetti E, Schram B, Orr R. The effects of early physiotherapy treatment on musculoskeletal injury outcomes in military personnel: a narrative review. Int J Environ Res Public Health. 2022;19(20):13416. doi:10.3390/ijerph192013416
- Gurewich D, Shwartz M, Beilstein-Wedel E, Davila H, Rosen AK. Did access to care improve since passage of the veterans choice act? Differences between rural and urban veterans. Med Care. 2021;59(Suppl 3):S270-S278. doi:10.1097/MLR.0000000000001490
- Myers US, Birks A, Grubaugh AL, Axon RN. Flattening the curve by getting ahead of it: how the VA healthcare system is leveraging telehealth to provide continued access to care for rural veterans. J Rural Health. 2021;37(1):194-196. doi:10.1111/jrh.12449
- Hale-Gallardo JL, Kreider CM, Jia H, et al. Telerehabilitation for rural veterans: a qualitative assessment of barriers and facilitators to implementation. J Multidiscip Healthc. 2020;13:559-570. doi:10.2147/JMDH.S247267
- Kreider CM, Hale-Gallardo J, Kramer JC, et al. Providers’ shift to telerehabilitation at the U.S. Veterans Health Administration during COVID-19: practical applications. Front Public Health. 2022;10:831762. doi:10.3389/fpubh.2022.831762
- Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019;36(3):122-128.
- US Dept of Veterans Affairs, Office of Rural Health. VHA Office of Rural Health. Updated August 30, 2024. Accessed September 23, 2024. https://www.ruralhealth.va.gov/index.asp
- National Center for Veterans Analysis and Statistics. Rural Veterans: 2021-2023. April 2023. Accessed September 23, 2024. https://www.datahub.va.gov/stories/s/Rural-Veterans-FY2021-2023/kkh2-eymp/
- U.S. Department of Veterans Affairs, Office of Research & Development. Program Guide: 1200.21, VHA Operations Activities That May Constitute Research. January 9, 2019. https://www.research.va.gov/resources/policies/ProgramGuide-1200-21-VHA-Operations-Activities.pdf
- Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Nurs Care Qual. 2016;31(1):1-8. doi:10.1097/NCQ.0000000000000153
- US Dept of Veterans Affairs. Veterans Health Administration: Veterans Integrated Service Networks (VISNs). Updated January 29, 2024. Accessed September 23, 2024. https://www.va.gov/HEALTH/visns.asp
- Stomberg C, Frost A, Becker C, Stang H, Windschitl M, Carrier E. Community Care referral dashboard [Data dashboard]. https://app.powerbigov.us/groups/me/reports/090d22a7-0e1f-4cc5-bea8-0a1b87aa0bd9/ReportSectionacfd03cdebd76ffca9ec [Source not verified]
- US Dept of Veterans Affairs. Eligibility for community care outside VA. Updated May 30, 2024. Accessed September 23, 2024. https://www.va.gov/COMMUNITYCARE/programs/veterans/General_Care.asp
- US Department of Veterans Affairs, Office of Rural Health. How to define rurality fact sheet. Updated December 2023. Accessed January 28, 2025. https://www.ruralhealth.va.gov/docs/ORH_RuralityFactSheet_508.pdf
- Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed September 23, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx
- Gurewich D, Beilstein-Wedel E, Shwartz M, Davila H, Rosen AK. Disparities in wait times for care among US veterans by race and ethnici t y. JAMA Netw Open. 2023;6(1):e2252061. doi:10.1001/jamanetworkopen.2022.52061
- U.S. Department of Veterans Affairs, VA Office of Rural Health, Veterans Rural Health Resource Center-Gainesville, GeoSpatial Outcomes Division. VA and Community Healthcare, and VHA Rurality web map application. Published 2023. https://portal.vhagis.inv.vaec.va.gov/arcgis/apps/webappbuilder/index.html [source not verified]
- Chartbook on Healthcare for Veterans: National Healthcare Quality and Disparities Report. Agency for Healthcare Research and Quality; November 2020. Accessed September 23, 2024. https://www.ahrq.gov/research/findings/nhqrdr/chartbooks/veterans/index.html
- Lum HD, Nearing K, Pimentel CB, Levy CR, Hung WW. Anywhere to anywhere: use of telehealth to increase health care access for older, rural veterans. Public Policy Aging Rep. 2020;30(1):12-18. doi:10.1093/ppar/prz030
- Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696-1703. doi:10.1097/j.pain.0000000000000567
- US Inflation Calculator. Health Care Inflation in the United States (1948-2024). Accessed September 23, 2024. https://www.usinflationcalculator.com/inflation/health-care-inflation-in-the-united-states/
- Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638. doi:10.1177/0269215516645148
- Elor A, Conde S, Powel l M, Robbins A, Chen NN, Kurniawan S. Physical therapist impressions of telehealth and virtual reality needs amidst a pandemic. Front Virtual Real. 2022;3. doi:10.3389/frvir.2022.915332
- Lee AC, Harada N. Telehealth as a means of health care delivery for physical therapist practice. Phys Ther. 2012;92(3):463-468. doi:10.2522/ptj.20110100
- Hynes DM, Edwards S, Hickok A, et al. Veterans’ use of Veterans Health Administration primary care in an era of expanding choice. Med Care. 2021;59(Suppl 3):S292- S300. doi:10.1097/MLR.0000000000001554
Utilization and Cost of Veterans Health Administration Referrals to Community Care-Based Physical Therapy
Utilization and Cost of Veterans Health Administration Referrals to Community Care-Based Physical Therapy
Drug Overdose and Suicide Among Veteran Enrollees in the VHA: Comparison Among Local, Regional, and National Data
Suicide is the 10th leading cause of death in the US. In 2017, there were 47,173 deaths by suicide (14 deaths per 100,000 people), representing a 33% increase from 1999.1 In 2017 veterans accounted for 13.5% of all suicide deaths among US adults, although veterans comprised only 7.9% of the adult population; the age- and sex-adjusted suicide rate was 1.5 times higher for veterans than that of nonveteran adults.2,3
Among veteran users of Veterans Health Administration (VHA) services, mental health and substance use disorders, chronic medical conditions, and chronic pain are associated with an increased risk for suicide.3 About one-half of VHA veterans have been diagnosed with chronic pain.4 A chronic pain diagnosis (eg, back pain, migraine, and psychogenic pain) increased the risk of death by suicide even after adjusting for comorbid psychiatric diagnoses, according to a study on pain and suicide among US veterans.5
One-quarter of veterans received an opioid prescription during VHA outpatient care in 2012.4 Increased prescribing of opioid medications has been associated with opioid overdose and suicides.6-10 Opioids are the most common drugs found in suicide by overdose.11 The rate of opioid-related suicide deaths is 13 times higher among individuals with opioid use disorder (OUD) than it is for those without OUD.12 The rate of OUD diagnosis among VHA users was 7 times higher than that for non-VHA users.13
In the US the age-adjusted rate of drug overdose deaths increased from 6 per 100,000 persons in 1999 to 22 per 100,000 in 2017.14 Drug overdoses accounted for 52,404 US deaths in 2015; 33,091 (63.1%) were from opioids.15 In 2017, there were 70,237 drug overdose deaths; 67.8% involved opioids (ie, 5 per 100,000 population represent prescription opioids).16
The VHA is committed to reducing opioid use and veteran suicide prevention. In 2013 the VHA launched the Opioid Safety Initiative employing 4 strategies: education, pain management, risk management, and addiction treatment.17 To address the opioid epidemic, the North Florida/South Georgia Veteran Health System (NF/SGVHS) developed and implemented a multispecialty Opioid Risk Reduction Program that is fully integrated with mental health and addiction services. The purpose of the NF/SGVHS one-stop pain addiction clinic is to provide a treatment program for chronic pain and addiction. The program includes elements of a whole health approach to pain care, including battlefield and traditional acupuncture. The focus went beyond replacing pharmacologic treatments with a complementary integrative health approach to helping veterans regain control of their lives through empowerment, skill building, shared goal setting, and reinforcing self-management.
The self-management programs include a pain school for patient education, a pain psychology program, and a yoga program, all stressing self-management offered onsite and via telehealth. Special effort was directed to identify patients with OUD and opioid dependence. Many of these patients were transitioned to buprenorphine, a potent analgesic that suppresses opioid cravings and withdrawal symptoms associated with stopping opioids. The clinic was structured so that patients could be seen often for follow-up and support. In addition, open lines of communication and referral were set up between this clinic, the interventional pain clinic, and the physical medicine and rehabilitation service. A detailed description of this program has been published elsewhere.18
The number of veterans receiving opioid prescription across the VHA system decreased by 172,000 prescriptions quarterly between 2012 and 2016.19 Fewer veterans were prescribed high doses of opioids or concomitant interacting medicines and more veterans were receiving nonopioid therapies.19 The prescription reduction across the VHA has varied. For example, from 2012 to 2017 the NF/SGVHS reported an 87% reduction of opioid prescriptions (≥ 100 mg morphine equivalents/d), compared with the VHA national average reduction of 49%.18
Vigorous opioid reduction is controversial. In a systematic review on opioid reduction, Frank and colleagues reported some beneficial effects of opioid reduction, such as increased health-related quality of life.20 However, another study suggested a risk of increased pain with opioid tapering.21 The literature findings on the association between prescription opioid use and suicide are mixed. The VHA Office of Mental Health and Suicide Prevention literature review reported that veterans were at increased risk of committing suicide within the first 6 months of discontinuing opioid therapy.22 Another study reported that veterans who discontinued long-term opioid treatment had an increased risk for suicidal ideation.23 However, higher doses of opioids were associated with an increased risk for suicide among individuals with chronic pain.10 The link between opioid tapering and the risk of suicide or overdose is uncertain.
Bohnert and Ilgen suggested that discontinuing prescription opioids leads to suicide without examining the risk factors that influenced discontinuation is ill-informed.7 Strong evidence about the association or relationship among opioid use, overdose, and suicide is needed. To increase our understanding of that association, Bohnert and Ilgen argued for multifaceted interventions that simultaneously address the shared causes and risk factors for OUD,7 such as the multispecialty Opioid Risk Reduction Program at NF/SGVHS.
Because of the reported association between robust integrated mental health and addiction, primary care pain clinic intervention, and the higher rate of opioid tapering in NF/SGVHS,18 this study aims to describe the pattern of overdose diagnosis (opioid overdose and nonopioid overdose) and pattern of suicide rates among veterans enrolled in NF/SGVHS, Veterans Integrated Service Network (VISN) 8, and the entire VA health care system during 2012 to 2016.The study reviewed and compared overdose diagnosis and suicide rates among veterans across NF/SGVHS and 2 other levels of the VA health care system to determine whether there were variances in the pattern of overdose/suicide rates and to explore these differences.
Methods
In this retrospective study, aggregate data were obtained from several sources. First, the drug overdose data were extracted from the VA Support Service Center (VSSC) medical diagnosis cube. We reviewed the literature for opioid codes reported in the literature and compared these reported opioid International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, 10th Revision (ICD-10) codes with the local facility patient-level comprehensive overdose diagnosis codes. Based on the comparison, we found 98 ICD-9 and ICD-10 overdose diagnosis codes and ran the modified codes against the VSSC national database. Overdose data were aggregated by facility and fiscal year, and the overdose rates (per 1,000) were calculated for unique veteran users at the 3 levels (NF/SGVHS, VISN 8, and VA national) as the denominator.
Each of the 18 VISNs comprise multiple VAMCs and clinics within a geographic region. VISN 8 encompasses most of Florida and portions of southern Georgia and the Caribbean (Puerto Rico, US Virgin Islands), including NF/SGVHS.
In this study, drug overdose refers to the overdose or poisoning from all drugs (ie, opioids, cocaine, amphetamines, sedatives, etc) and defined as any unintentional (accidental), deliberate, or intent undetermined drug poisoning.24 The suicide data for this study were drawn from the VA Suicide Prevention Program at 3 different levels: NF/SGVHS, VISN 8, and VHA national. Suicide is death caused by an intentional act of injuring oneself with the intent to die.25
This descriptive study compared the rate of annual drug overdoses (per 1,000 enrollees) between NF/SGVHS, VISN 8, and VHA national from 2012 to 2016. It also compared the annual rate of suicide per 100,000 enrollees across these 3 levels of the VHA. The overdose and suicide rates and numbers are mutually exclusive, meaning the VISN 8 data do not include the NF/SGVHS information, and the national data excluded data from VISN 8 and NF/SGVHS. This approach helped improve the quality of multiple level comparisons for different levels of the VHA system.
Results
Figure 1 shows the pattern of overdose diagnosis by rates (per 1,000) across the study period (2012 to 2016) and compares patterns at 3 levels of VHA (NF/SGVHS, VISN 8, and VHA national). The average annual rate of overdose diagnoses for NF/SGVHS during the study was slightly higher (16.8 per 1,000) than that of VISN 8 (16 per 1,000) and VHA national (15.3 per 1,000), but by the end of the study period the NF/SGVHS rate (18.6 per 1,000) nearly matched the national rate (18.2 per 1,000) and was lower than the VISN 8 rate (20.4 per 1,000). Additionally, NF/SGVHS had less variability (SD, 1.34) in yearly average overdose rates compared with VISN 8 (SD, 2.96), and VHA national (SD, 1.69).
From 2013 to 2014 the overdose diagnosis rate for NF/SGVHS remained the same (17.1 per 1,000). A similar pattern was observed for the VHA national data, whereas the VISN 8 data showed a steady increase during the same period. In 2015, the NF/SGVHS had 0.7 per 1,000 decrease in overdose diagnosis rate, whereas VISN 8 and VHA national data showed 1.7 per 1,000 and 0.9 per 1,000 increases, respectively. During the last year of the study (2016), there was a dramatic increase in overdose diagnosis for all the health care systems, ranging from 2.2 per 1,000 for NF/SGVHS to 3.3 per 1,000 for VISN 8.
Figure 2 shows the annual rates (per 100,000 individuals) of suicide for NF/SGVHS, VISN 8, and VHA national. The suicide pattern for VISN 8 shows a cyclical acceleration and deceleration trend across the study period. From 2012 to 2014, the VHA national data show a steady increase of about 1 per 100,000 from year to year. On the contrary, NF/SGVHS shows a low suicide rate from year to year within the same period with a rate of 10 per 100,000 in 2013 compared with the previous year. Although the NF/SGVHS suicide rate increased in 2016 (10.4 per 100,000), it remained lower than that of VISN 8 (10.7 per 100,00) and VHA national (38.2 per 100,000).
This study shows that NF/SGVHS had the lowest average annual rate of suicide (9.1 per 100,000) during the study period, which was 4 times lower than that of VHA national and 2.6 times lower than VISN 8.
Discussion
This study described and compared the distribution pattern of overdose (nonopioid and opioid) and suicide rates at different levels of the VHA system. Although VHA implemented systemwide opioid tapering in 2013, little is known about the association between opioid tapering and overdose and suicide. We believe a retrospective examination regarding overdose and suicide among VHA users at 3 different levels of the system from 2012 to 2016 could contribute to the discussion regarding the potential risks and benefits of discontinuing opioids.
First, the average annual rate of overdose diagnosis for NF/SGVHS during the study period was slightly higher (16.8 per 1,000) compared with those of VISN 8 (16.0 per 1,000) and VHA national (15.3 per 1,000) with a general pattern of increase and minimum variations in the rates observed during the study period among the 3 levels of the system. These increased overdose patterns are consistent with other reports in the literature.14 By the end of the study period, the NF/SGVHS rate (18.6 per 1,000) nearly matched the national rate (18.2 per 1,000) and was lower than VISN 8 (20.4 per 1,000). During the last year of the study period (2016), there was a dramatic increase in overdose diagnosis for all health care systems ranging from 2.2 per 1,000 for NF/SGVHS to 3.3 per 1,000 for VISN 8, which might be because of the VHA systemwide change of diagnosis code from ICD-9 to ICD-10, which includes more detailed diagnosis codes.
Second, our results showed that NF/SGVHS had the lowest average annual suicide rate (9.1 per 100,000) during the study period, which is one-fourth the VHA national rate and 2.6 per 100,000 lower than the VISN 8 rate. According to Bohnert and Ilgen,programs that improve the quality of pain care, expand access to psychotherapy, and increase access to medication-assisted treatment for OUDs could reduce suicide by drug overdose.7 We suggest that the low suicide rate at NF/SGVHS and the difference in the suicide rates between the NF/SGVHS and VISN 8 and VHA national data might be associated with the practice-based biopsychosocial interventions implemented at NF/SGVHS.
Our data showed a rise in the incidence of suicide at the NF/SGVHS in 2016. We are not aware of a local change in conditions, policy, and practice that would account for this increase. Suicide is variable, and data are likely to show spikes and valleys. Based on the available data, although the incidence of suicides at the NF/SGVHS in 2016 was higher, it remained below the VISN 8 and national VHA rate. This study seems to support the practice of tapering or stopping opioids within the context of a multidisciplinary approach that offers frequent follow-up, nonopioid options, and treatment of opioid addiction/dependence.
Limitations
The research findings of this study are limited by the retrospective and descriptive nature of its design. However, the findings might provide important information for understanding variations of overdose and suicide among VHA enrollees. Studies that use more robust methodologies are warranted to clinically investigate the impact of a multispecialty opioid risk reduction program targeting chronic pain and addiction management and identify best practices of opioid reduction and any unintended consequences that might arise from opioid tapering.26 Further, we did not have access to the VA national overdose and suicide data after 2016. Similar to most retrospective data studies, ours might be limited by availability of national overdose and suicide data after 2016. It is important for future studies to cross-validate our study findings.
Conclusions
The NF/SGVHS developed and implemented a biopsychosocial model of pain treatment that includes multicomponent primary care integrated with mental health and addiction services as well as the interventional pain and physical medicine and rehabilitation services. The presence of this program, during a period when the facility was tapering opioids is likely to account for at least part of the relative reduction in suicide.
1. American Foundation for Suicide Prevention. Suicide statistics. https://afsp.org/about-suicide/suicide-statistics. Updated 2019. Accessed September 2, 2020.
2. Shane L 3rd. New veteran suicide numbers raise concerns among experts hoping for positive news. https://www.militarytimes.com/news/pentagon-congress/2019/10/09/new-veteran-suicide-numbers-raise-concerns-among-experts-hoping-for-positive-news. Published October 9, 2019. Accessed July 23, 2020.
3. Veterans Health Administration, Office of Mental Health and Suicide Prevention. Veteran suicide data report, 2005–2017. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 2019. Accessed July 20, 2020.
4. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
5. Ilgen MA, Kleinberg F, Ignacio RV, et al. Noncancer pain conditions and risk of suicide. JAMA Psychiatry. 2013;70(7):692-697. doi:10.1001/jamapsychiatry.2013.908
6. Frenk SM, Porter KS, Paulozzi LJ. Prescription opioid analgesic use among adults: United States, 1999-2012. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/products/databriefs/db189.htm. Published February 25, 2015. Accessed July 20, 2020.
7. Bohnert ASB, Ilgen MA. Understanding links among opioid use, overdose, and suicide. N Engl J Med. 2019;380(14):71-79. doi:10.1056/NEJMc1901540
8. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi:10.7326/0003-4819-152-2-201001190-00006
9. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691. doi:10.1001/archinternmed.2011.117
10. Ilgen MA, Bohnert AS, Ganoczy D, Bair MJ, McCarthy JF, Blow FC. Opioid dose and risk of suicide. Pain. 2016;157(5):1079-1084. doi:10.1097/j.pain.0000000000000484
11. Sinyor M, Howlett A, Cheung AH, Schaffer A. Substances used in completed suicide by overdose in Toronto: an observational study of coroner’s data. Can J Psychiatry. 2012;57(3):184-191. doi:10.1177/070674371205700308
12. Wilcox HC, Conner KR, Caine ED. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug Alcohol Depend. 2004;76(suppl):S11-S19 doi:10.1016/j.drugalcdep.2004.08.003.
13. Baser OL, Mardekian XJ, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans Health Administration. Pain Pract. 2014;14(5):437-445. doi:10.1111/papr.12097
14. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the united states, 1999-2015. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/data/databriefs/db273.pdf. Published February 2017. Accessed July 20, 2020.
15. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
16. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019,67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
17. US Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for opioid therapy for chronic pain version 3.0. https://www.healthquality.va.gov/guidelines/pain/cot. Updated March 1, 2018. Accessed July 20, 2020.
18. Vaughn IA, Beyth RJ, Ayers ML, et al. Multispecialty opioid risk reduction program targeting chronic pain and addiction management in veterans. Fed Pract. 2019;36(9):406-411.
19. Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: lessons from the Department of Veterans Affairs. JAMA Intern Med. 2017;177(5):611-612. doi:10.1001/jamainternmed.2017.0147
20. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167(3):181-191. doi:10.7326/M17-0598
21. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90(6):828-842. doi:10.1016/j.mayocp.2015.04.003
22. Veterans Health Administration, Office of Mental Health and Suicide Prevention. Opioid use and suicide risk. https://www.mentalhealth.va.gov/suicide_prevention/docs/Literature_Review_Opioid_Use_and_Suicide_Risk_508_FINAL_04-26-2019.pdf. Published April 26, 2019. Accessed July 20, 2020.
23. Demidenko MI, Dobscha SK, Morasco BJ, Meath THA, Ilgen MA, Lovejoy TI. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35. doi:10.1016/j.genhosppsych.2017.04.011
24. National Institute on Drug Abuse. Intentional versus unintentional overdose deaths. https://www.drugabuse.gov/related-topics/treatment/intentional-vs-unintentional-overdose-deaths. Updated February 13, 2017. Accessed July 20, 2020.
25. Centers for Disease Control and Prevention. Preventing suicide. https://www.cdc.gov/violenceprevention/pdf/suicide-factsheet.pdf. Published 2018. Accessed July 20, 2020.
26. Webster LR. Pain and suicide: the other side of the opioid story. Pain Med. 2014;15(3):345-346. doi:10.1111/pme.12398
Suicide is the 10th leading cause of death in the US. In 2017, there were 47,173 deaths by suicide (14 deaths per 100,000 people), representing a 33% increase from 1999.1 In 2017 veterans accounted for 13.5% of all suicide deaths among US adults, although veterans comprised only 7.9% of the adult population; the age- and sex-adjusted suicide rate was 1.5 times higher for veterans than that of nonveteran adults.2,3
Among veteran users of Veterans Health Administration (VHA) services, mental health and substance use disorders, chronic medical conditions, and chronic pain are associated with an increased risk for suicide.3 About one-half of VHA veterans have been diagnosed with chronic pain.4 A chronic pain diagnosis (eg, back pain, migraine, and psychogenic pain) increased the risk of death by suicide even after adjusting for comorbid psychiatric diagnoses, according to a study on pain and suicide among US veterans.5
One-quarter of veterans received an opioid prescription during VHA outpatient care in 2012.4 Increased prescribing of opioid medications has been associated with opioid overdose and suicides.6-10 Opioids are the most common drugs found in suicide by overdose.11 The rate of opioid-related suicide deaths is 13 times higher among individuals with opioid use disorder (OUD) than it is for those without OUD.12 The rate of OUD diagnosis among VHA users was 7 times higher than that for non-VHA users.13
In the US the age-adjusted rate of drug overdose deaths increased from 6 per 100,000 persons in 1999 to 22 per 100,000 in 2017.14 Drug overdoses accounted for 52,404 US deaths in 2015; 33,091 (63.1%) were from opioids.15 In 2017, there were 70,237 drug overdose deaths; 67.8% involved opioids (ie, 5 per 100,000 population represent prescription opioids).16
The VHA is committed to reducing opioid use and veteran suicide prevention. In 2013 the VHA launched the Opioid Safety Initiative employing 4 strategies: education, pain management, risk management, and addiction treatment.17 To address the opioid epidemic, the North Florida/South Georgia Veteran Health System (NF/SGVHS) developed and implemented a multispecialty Opioid Risk Reduction Program that is fully integrated with mental health and addiction services. The purpose of the NF/SGVHS one-stop pain addiction clinic is to provide a treatment program for chronic pain and addiction. The program includes elements of a whole health approach to pain care, including battlefield and traditional acupuncture. The focus went beyond replacing pharmacologic treatments with a complementary integrative health approach to helping veterans regain control of their lives through empowerment, skill building, shared goal setting, and reinforcing self-management.
The self-management programs include a pain school for patient education, a pain psychology program, and a yoga program, all stressing self-management offered onsite and via telehealth. Special effort was directed to identify patients with OUD and opioid dependence. Many of these patients were transitioned to buprenorphine, a potent analgesic that suppresses opioid cravings and withdrawal symptoms associated with stopping opioids. The clinic was structured so that patients could be seen often for follow-up and support. In addition, open lines of communication and referral were set up between this clinic, the interventional pain clinic, and the physical medicine and rehabilitation service. A detailed description of this program has been published elsewhere.18
The number of veterans receiving opioid prescription across the VHA system decreased by 172,000 prescriptions quarterly between 2012 and 2016.19 Fewer veterans were prescribed high doses of opioids or concomitant interacting medicines and more veterans were receiving nonopioid therapies.19 The prescription reduction across the VHA has varied. For example, from 2012 to 2017 the NF/SGVHS reported an 87% reduction of opioid prescriptions (≥ 100 mg morphine equivalents/d), compared with the VHA national average reduction of 49%.18
Vigorous opioid reduction is controversial. In a systematic review on opioid reduction, Frank and colleagues reported some beneficial effects of opioid reduction, such as increased health-related quality of life.20 However, another study suggested a risk of increased pain with opioid tapering.21 The literature findings on the association between prescription opioid use and suicide are mixed. The VHA Office of Mental Health and Suicide Prevention literature review reported that veterans were at increased risk of committing suicide within the first 6 months of discontinuing opioid therapy.22 Another study reported that veterans who discontinued long-term opioid treatment had an increased risk for suicidal ideation.23 However, higher doses of opioids were associated with an increased risk for suicide among individuals with chronic pain.10 The link between opioid tapering and the risk of suicide or overdose is uncertain.
Bohnert and Ilgen suggested that discontinuing prescription opioids leads to suicide without examining the risk factors that influenced discontinuation is ill-informed.7 Strong evidence about the association or relationship among opioid use, overdose, and suicide is needed. To increase our understanding of that association, Bohnert and Ilgen argued for multifaceted interventions that simultaneously address the shared causes and risk factors for OUD,7 such as the multispecialty Opioid Risk Reduction Program at NF/SGVHS.
Because of the reported association between robust integrated mental health and addiction, primary care pain clinic intervention, and the higher rate of opioid tapering in NF/SGVHS,18 this study aims to describe the pattern of overdose diagnosis (opioid overdose and nonopioid overdose) and pattern of suicide rates among veterans enrolled in NF/SGVHS, Veterans Integrated Service Network (VISN) 8, and the entire VA health care system during 2012 to 2016.The study reviewed and compared overdose diagnosis and suicide rates among veterans across NF/SGVHS and 2 other levels of the VA health care system to determine whether there were variances in the pattern of overdose/suicide rates and to explore these differences.
Methods
In this retrospective study, aggregate data were obtained from several sources. First, the drug overdose data were extracted from the VA Support Service Center (VSSC) medical diagnosis cube. We reviewed the literature for opioid codes reported in the literature and compared these reported opioid International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, 10th Revision (ICD-10) codes with the local facility patient-level comprehensive overdose diagnosis codes. Based on the comparison, we found 98 ICD-9 and ICD-10 overdose diagnosis codes and ran the modified codes against the VSSC national database. Overdose data were aggregated by facility and fiscal year, and the overdose rates (per 1,000) were calculated for unique veteran users at the 3 levels (NF/SGVHS, VISN 8, and VA national) as the denominator.
Each of the 18 VISNs comprise multiple VAMCs and clinics within a geographic region. VISN 8 encompasses most of Florida and portions of southern Georgia and the Caribbean (Puerto Rico, US Virgin Islands), including NF/SGVHS.
In this study, drug overdose refers to the overdose or poisoning from all drugs (ie, opioids, cocaine, amphetamines, sedatives, etc) and defined as any unintentional (accidental), deliberate, or intent undetermined drug poisoning.24 The suicide data for this study were drawn from the VA Suicide Prevention Program at 3 different levels: NF/SGVHS, VISN 8, and VHA national. Suicide is death caused by an intentional act of injuring oneself with the intent to die.25
This descriptive study compared the rate of annual drug overdoses (per 1,000 enrollees) between NF/SGVHS, VISN 8, and VHA national from 2012 to 2016. It also compared the annual rate of suicide per 100,000 enrollees across these 3 levels of the VHA. The overdose and suicide rates and numbers are mutually exclusive, meaning the VISN 8 data do not include the NF/SGVHS information, and the national data excluded data from VISN 8 and NF/SGVHS. This approach helped improve the quality of multiple level comparisons for different levels of the VHA system.
Results
Figure 1 shows the pattern of overdose diagnosis by rates (per 1,000) across the study period (2012 to 2016) and compares patterns at 3 levels of VHA (NF/SGVHS, VISN 8, and VHA national). The average annual rate of overdose diagnoses for NF/SGVHS during the study was slightly higher (16.8 per 1,000) than that of VISN 8 (16 per 1,000) and VHA national (15.3 per 1,000), but by the end of the study period the NF/SGVHS rate (18.6 per 1,000) nearly matched the national rate (18.2 per 1,000) and was lower than the VISN 8 rate (20.4 per 1,000). Additionally, NF/SGVHS had less variability (SD, 1.34) in yearly average overdose rates compared with VISN 8 (SD, 2.96), and VHA national (SD, 1.69).
From 2013 to 2014 the overdose diagnosis rate for NF/SGVHS remained the same (17.1 per 1,000). A similar pattern was observed for the VHA national data, whereas the VISN 8 data showed a steady increase during the same period. In 2015, the NF/SGVHS had 0.7 per 1,000 decrease in overdose diagnosis rate, whereas VISN 8 and VHA national data showed 1.7 per 1,000 and 0.9 per 1,000 increases, respectively. During the last year of the study (2016), there was a dramatic increase in overdose diagnosis for all the health care systems, ranging from 2.2 per 1,000 for NF/SGVHS to 3.3 per 1,000 for VISN 8.
Figure 2 shows the annual rates (per 100,000 individuals) of suicide for NF/SGVHS, VISN 8, and VHA national. The suicide pattern for VISN 8 shows a cyclical acceleration and deceleration trend across the study period. From 2012 to 2014, the VHA national data show a steady increase of about 1 per 100,000 from year to year. On the contrary, NF/SGVHS shows a low suicide rate from year to year within the same period with a rate of 10 per 100,000 in 2013 compared with the previous year. Although the NF/SGVHS suicide rate increased in 2016 (10.4 per 100,000), it remained lower than that of VISN 8 (10.7 per 100,00) and VHA national (38.2 per 100,000).
This study shows that NF/SGVHS had the lowest average annual rate of suicide (9.1 per 100,000) during the study period, which was 4 times lower than that of VHA national and 2.6 times lower than VISN 8.
Discussion
This study described and compared the distribution pattern of overdose (nonopioid and opioid) and suicide rates at different levels of the VHA system. Although VHA implemented systemwide opioid tapering in 2013, little is known about the association between opioid tapering and overdose and suicide. We believe a retrospective examination regarding overdose and suicide among VHA users at 3 different levels of the system from 2012 to 2016 could contribute to the discussion regarding the potential risks and benefits of discontinuing opioids.
First, the average annual rate of overdose diagnosis for NF/SGVHS during the study period was slightly higher (16.8 per 1,000) compared with those of VISN 8 (16.0 per 1,000) and VHA national (15.3 per 1,000) with a general pattern of increase and minimum variations in the rates observed during the study period among the 3 levels of the system. These increased overdose patterns are consistent with other reports in the literature.14 By the end of the study period, the NF/SGVHS rate (18.6 per 1,000) nearly matched the national rate (18.2 per 1,000) and was lower than VISN 8 (20.4 per 1,000). During the last year of the study period (2016), there was a dramatic increase in overdose diagnosis for all health care systems ranging from 2.2 per 1,000 for NF/SGVHS to 3.3 per 1,000 for VISN 8, which might be because of the VHA systemwide change of diagnosis code from ICD-9 to ICD-10, which includes more detailed diagnosis codes.
Second, our results showed that NF/SGVHS had the lowest average annual suicide rate (9.1 per 100,000) during the study period, which is one-fourth the VHA national rate and 2.6 per 100,000 lower than the VISN 8 rate. According to Bohnert and Ilgen,programs that improve the quality of pain care, expand access to psychotherapy, and increase access to medication-assisted treatment for OUDs could reduce suicide by drug overdose.7 We suggest that the low suicide rate at NF/SGVHS and the difference in the suicide rates between the NF/SGVHS and VISN 8 and VHA national data might be associated with the practice-based biopsychosocial interventions implemented at NF/SGVHS.
Our data showed a rise in the incidence of suicide at the NF/SGVHS in 2016. We are not aware of a local change in conditions, policy, and practice that would account for this increase. Suicide is variable, and data are likely to show spikes and valleys. Based on the available data, although the incidence of suicides at the NF/SGVHS in 2016 was higher, it remained below the VISN 8 and national VHA rate. This study seems to support the practice of tapering or stopping opioids within the context of a multidisciplinary approach that offers frequent follow-up, nonopioid options, and treatment of opioid addiction/dependence.
Limitations
The research findings of this study are limited by the retrospective and descriptive nature of its design. However, the findings might provide important information for understanding variations of overdose and suicide among VHA enrollees. Studies that use more robust methodologies are warranted to clinically investigate the impact of a multispecialty opioid risk reduction program targeting chronic pain and addiction management and identify best practices of opioid reduction and any unintended consequences that might arise from opioid tapering.26 Further, we did not have access to the VA national overdose and suicide data after 2016. Similar to most retrospective data studies, ours might be limited by availability of national overdose and suicide data after 2016. It is important for future studies to cross-validate our study findings.
Conclusions
The NF/SGVHS developed and implemented a biopsychosocial model of pain treatment that includes multicomponent primary care integrated with mental health and addiction services as well as the interventional pain and physical medicine and rehabilitation services. The presence of this program, during a period when the facility was tapering opioids is likely to account for at least part of the relative reduction in suicide.
Suicide is the 10th leading cause of death in the US. In 2017, there were 47,173 deaths by suicide (14 deaths per 100,000 people), representing a 33% increase from 1999.1 In 2017 veterans accounted for 13.5% of all suicide deaths among US adults, although veterans comprised only 7.9% of the adult population; the age- and sex-adjusted suicide rate was 1.5 times higher for veterans than that of nonveteran adults.2,3
Among veteran users of Veterans Health Administration (VHA) services, mental health and substance use disorders, chronic medical conditions, and chronic pain are associated with an increased risk for suicide.3 About one-half of VHA veterans have been diagnosed with chronic pain.4 A chronic pain diagnosis (eg, back pain, migraine, and psychogenic pain) increased the risk of death by suicide even after adjusting for comorbid psychiatric diagnoses, according to a study on pain and suicide among US veterans.5
One-quarter of veterans received an opioid prescription during VHA outpatient care in 2012.4 Increased prescribing of opioid medications has been associated with opioid overdose and suicides.6-10 Opioids are the most common drugs found in suicide by overdose.11 The rate of opioid-related suicide deaths is 13 times higher among individuals with opioid use disorder (OUD) than it is for those without OUD.12 The rate of OUD diagnosis among VHA users was 7 times higher than that for non-VHA users.13
In the US the age-adjusted rate of drug overdose deaths increased from 6 per 100,000 persons in 1999 to 22 per 100,000 in 2017.14 Drug overdoses accounted for 52,404 US deaths in 2015; 33,091 (63.1%) were from opioids.15 In 2017, there were 70,237 drug overdose deaths; 67.8% involved opioids (ie, 5 per 100,000 population represent prescription opioids).16
The VHA is committed to reducing opioid use and veteran suicide prevention. In 2013 the VHA launched the Opioid Safety Initiative employing 4 strategies: education, pain management, risk management, and addiction treatment.17 To address the opioid epidemic, the North Florida/South Georgia Veteran Health System (NF/SGVHS) developed and implemented a multispecialty Opioid Risk Reduction Program that is fully integrated with mental health and addiction services. The purpose of the NF/SGVHS one-stop pain addiction clinic is to provide a treatment program for chronic pain and addiction. The program includes elements of a whole health approach to pain care, including battlefield and traditional acupuncture. The focus went beyond replacing pharmacologic treatments with a complementary integrative health approach to helping veterans regain control of their lives through empowerment, skill building, shared goal setting, and reinforcing self-management.
The self-management programs include a pain school for patient education, a pain psychology program, and a yoga program, all stressing self-management offered onsite and via telehealth. Special effort was directed to identify patients with OUD and opioid dependence. Many of these patients were transitioned to buprenorphine, a potent analgesic that suppresses opioid cravings and withdrawal symptoms associated with stopping opioids. The clinic was structured so that patients could be seen often for follow-up and support. In addition, open lines of communication and referral were set up between this clinic, the interventional pain clinic, and the physical medicine and rehabilitation service. A detailed description of this program has been published elsewhere.18
The number of veterans receiving opioid prescription across the VHA system decreased by 172,000 prescriptions quarterly between 2012 and 2016.19 Fewer veterans were prescribed high doses of opioids or concomitant interacting medicines and more veterans were receiving nonopioid therapies.19 The prescription reduction across the VHA has varied. For example, from 2012 to 2017 the NF/SGVHS reported an 87% reduction of opioid prescriptions (≥ 100 mg morphine equivalents/d), compared with the VHA national average reduction of 49%.18
Vigorous opioid reduction is controversial. In a systematic review on opioid reduction, Frank and colleagues reported some beneficial effects of opioid reduction, such as increased health-related quality of life.20 However, another study suggested a risk of increased pain with opioid tapering.21 The literature findings on the association between prescription opioid use and suicide are mixed. The VHA Office of Mental Health and Suicide Prevention literature review reported that veterans were at increased risk of committing suicide within the first 6 months of discontinuing opioid therapy.22 Another study reported that veterans who discontinued long-term opioid treatment had an increased risk for suicidal ideation.23 However, higher doses of opioids were associated with an increased risk for suicide among individuals with chronic pain.10 The link between opioid tapering and the risk of suicide or overdose is uncertain.
Bohnert and Ilgen suggested that discontinuing prescription opioids leads to suicide without examining the risk factors that influenced discontinuation is ill-informed.7 Strong evidence about the association or relationship among opioid use, overdose, and suicide is needed. To increase our understanding of that association, Bohnert and Ilgen argued for multifaceted interventions that simultaneously address the shared causes and risk factors for OUD,7 such as the multispecialty Opioid Risk Reduction Program at NF/SGVHS.
Because of the reported association between robust integrated mental health and addiction, primary care pain clinic intervention, and the higher rate of opioid tapering in NF/SGVHS,18 this study aims to describe the pattern of overdose diagnosis (opioid overdose and nonopioid overdose) and pattern of suicide rates among veterans enrolled in NF/SGVHS, Veterans Integrated Service Network (VISN) 8, and the entire VA health care system during 2012 to 2016.The study reviewed and compared overdose diagnosis and suicide rates among veterans across NF/SGVHS and 2 other levels of the VA health care system to determine whether there were variances in the pattern of overdose/suicide rates and to explore these differences.
Methods
In this retrospective study, aggregate data were obtained from several sources. First, the drug overdose data were extracted from the VA Support Service Center (VSSC) medical diagnosis cube. We reviewed the literature for opioid codes reported in the literature and compared these reported opioid International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, 10th Revision (ICD-10) codes with the local facility patient-level comprehensive overdose diagnosis codes. Based on the comparison, we found 98 ICD-9 and ICD-10 overdose diagnosis codes and ran the modified codes against the VSSC national database. Overdose data were aggregated by facility and fiscal year, and the overdose rates (per 1,000) were calculated for unique veteran users at the 3 levels (NF/SGVHS, VISN 8, and VA national) as the denominator.
Each of the 18 VISNs comprise multiple VAMCs and clinics within a geographic region. VISN 8 encompasses most of Florida and portions of southern Georgia and the Caribbean (Puerto Rico, US Virgin Islands), including NF/SGVHS.
In this study, drug overdose refers to the overdose or poisoning from all drugs (ie, opioids, cocaine, amphetamines, sedatives, etc) and defined as any unintentional (accidental), deliberate, or intent undetermined drug poisoning.24 The suicide data for this study were drawn from the VA Suicide Prevention Program at 3 different levels: NF/SGVHS, VISN 8, and VHA national. Suicide is death caused by an intentional act of injuring oneself with the intent to die.25
This descriptive study compared the rate of annual drug overdoses (per 1,000 enrollees) between NF/SGVHS, VISN 8, and VHA national from 2012 to 2016. It also compared the annual rate of suicide per 100,000 enrollees across these 3 levels of the VHA. The overdose and suicide rates and numbers are mutually exclusive, meaning the VISN 8 data do not include the NF/SGVHS information, and the national data excluded data from VISN 8 and NF/SGVHS. This approach helped improve the quality of multiple level comparisons for different levels of the VHA system.
Results
Figure 1 shows the pattern of overdose diagnosis by rates (per 1,000) across the study period (2012 to 2016) and compares patterns at 3 levels of VHA (NF/SGVHS, VISN 8, and VHA national). The average annual rate of overdose diagnoses for NF/SGVHS during the study was slightly higher (16.8 per 1,000) than that of VISN 8 (16 per 1,000) and VHA national (15.3 per 1,000), but by the end of the study period the NF/SGVHS rate (18.6 per 1,000) nearly matched the national rate (18.2 per 1,000) and was lower than the VISN 8 rate (20.4 per 1,000). Additionally, NF/SGVHS had less variability (SD, 1.34) in yearly average overdose rates compared with VISN 8 (SD, 2.96), and VHA national (SD, 1.69).
From 2013 to 2014 the overdose diagnosis rate for NF/SGVHS remained the same (17.1 per 1,000). A similar pattern was observed for the VHA national data, whereas the VISN 8 data showed a steady increase during the same period. In 2015, the NF/SGVHS had 0.7 per 1,000 decrease in overdose diagnosis rate, whereas VISN 8 and VHA national data showed 1.7 per 1,000 and 0.9 per 1,000 increases, respectively. During the last year of the study (2016), there was a dramatic increase in overdose diagnosis for all the health care systems, ranging from 2.2 per 1,000 for NF/SGVHS to 3.3 per 1,000 for VISN 8.
Figure 2 shows the annual rates (per 100,000 individuals) of suicide for NF/SGVHS, VISN 8, and VHA national. The suicide pattern for VISN 8 shows a cyclical acceleration and deceleration trend across the study period. From 2012 to 2014, the VHA national data show a steady increase of about 1 per 100,000 from year to year. On the contrary, NF/SGVHS shows a low suicide rate from year to year within the same period with a rate of 10 per 100,000 in 2013 compared with the previous year. Although the NF/SGVHS suicide rate increased in 2016 (10.4 per 100,000), it remained lower than that of VISN 8 (10.7 per 100,00) and VHA national (38.2 per 100,000).
This study shows that NF/SGVHS had the lowest average annual rate of suicide (9.1 per 100,000) during the study period, which was 4 times lower than that of VHA national and 2.6 times lower than VISN 8.
Discussion
This study described and compared the distribution pattern of overdose (nonopioid and opioid) and suicide rates at different levels of the VHA system. Although VHA implemented systemwide opioid tapering in 2013, little is known about the association between opioid tapering and overdose and suicide. We believe a retrospective examination regarding overdose and suicide among VHA users at 3 different levels of the system from 2012 to 2016 could contribute to the discussion regarding the potential risks and benefits of discontinuing opioids.
First, the average annual rate of overdose diagnosis for NF/SGVHS during the study period was slightly higher (16.8 per 1,000) compared with those of VISN 8 (16.0 per 1,000) and VHA national (15.3 per 1,000) with a general pattern of increase and minimum variations in the rates observed during the study period among the 3 levels of the system. These increased overdose patterns are consistent with other reports in the literature.14 By the end of the study period, the NF/SGVHS rate (18.6 per 1,000) nearly matched the national rate (18.2 per 1,000) and was lower than VISN 8 (20.4 per 1,000). During the last year of the study period (2016), there was a dramatic increase in overdose diagnosis for all health care systems ranging from 2.2 per 1,000 for NF/SGVHS to 3.3 per 1,000 for VISN 8, which might be because of the VHA systemwide change of diagnosis code from ICD-9 to ICD-10, which includes more detailed diagnosis codes.
Second, our results showed that NF/SGVHS had the lowest average annual suicide rate (9.1 per 100,000) during the study period, which is one-fourth the VHA national rate and 2.6 per 100,000 lower than the VISN 8 rate. According to Bohnert and Ilgen,programs that improve the quality of pain care, expand access to psychotherapy, and increase access to medication-assisted treatment for OUDs could reduce suicide by drug overdose.7 We suggest that the low suicide rate at NF/SGVHS and the difference in the suicide rates between the NF/SGVHS and VISN 8 and VHA national data might be associated with the practice-based biopsychosocial interventions implemented at NF/SGVHS.
Our data showed a rise in the incidence of suicide at the NF/SGVHS in 2016. We are not aware of a local change in conditions, policy, and practice that would account for this increase. Suicide is variable, and data are likely to show spikes and valleys. Based on the available data, although the incidence of suicides at the NF/SGVHS in 2016 was higher, it remained below the VISN 8 and national VHA rate. This study seems to support the practice of tapering or stopping opioids within the context of a multidisciplinary approach that offers frequent follow-up, nonopioid options, and treatment of opioid addiction/dependence.
Limitations
The research findings of this study are limited by the retrospective and descriptive nature of its design. However, the findings might provide important information for understanding variations of overdose and suicide among VHA enrollees. Studies that use more robust methodologies are warranted to clinically investigate the impact of a multispecialty opioid risk reduction program targeting chronic pain and addiction management and identify best practices of opioid reduction and any unintended consequences that might arise from opioid tapering.26 Further, we did not have access to the VA national overdose and suicide data after 2016. Similar to most retrospective data studies, ours might be limited by availability of national overdose and suicide data after 2016. It is important for future studies to cross-validate our study findings.
Conclusions
The NF/SGVHS developed and implemented a biopsychosocial model of pain treatment that includes multicomponent primary care integrated with mental health and addiction services as well as the interventional pain and physical medicine and rehabilitation services. The presence of this program, during a period when the facility was tapering opioids is likely to account for at least part of the relative reduction in suicide.
1. American Foundation for Suicide Prevention. Suicide statistics. https://afsp.org/about-suicide/suicide-statistics. Updated 2019. Accessed September 2, 2020.
2. Shane L 3rd. New veteran suicide numbers raise concerns among experts hoping for positive news. https://www.militarytimes.com/news/pentagon-congress/2019/10/09/new-veteran-suicide-numbers-raise-concerns-among-experts-hoping-for-positive-news. Published October 9, 2019. Accessed July 23, 2020.
3. Veterans Health Administration, Office of Mental Health and Suicide Prevention. Veteran suicide data report, 2005–2017. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 2019. Accessed July 20, 2020.
4. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
5. Ilgen MA, Kleinberg F, Ignacio RV, et al. Noncancer pain conditions and risk of suicide. JAMA Psychiatry. 2013;70(7):692-697. doi:10.1001/jamapsychiatry.2013.908
6. Frenk SM, Porter KS, Paulozzi LJ. Prescription opioid analgesic use among adults: United States, 1999-2012. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/products/databriefs/db189.htm. Published February 25, 2015. Accessed July 20, 2020.
7. Bohnert ASB, Ilgen MA. Understanding links among opioid use, overdose, and suicide. N Engl J Med. 2019;380(14):71-79. doi:10.1056/NEJMc1901540
8. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi:10.7326/0003-4819-152-2-201001190-00006
9. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691. doi:10.1001/archinternmed.2011.117
10. Ilgen MA, Bohnert AS, Ganoczy D, Bair MJ, McCarthy JF, Blow FC. Opioid dose and risk of suicide. Pain. 2016;157(5):1079-1084. doi:10.1097/j.pain.0000000000000484
11. Sinyor M, Howlett A, Cheung AH, Schaffer A. Substances used in completed suicide by overdose in Toronto: an observational study of coroner’s data. Can J Psychiatry. 2012;57(3):184-191. doi:10.1177/070674371205700308
12. Wilcox HC, Conner KR, Caine ED. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug Alcohol Depend. 2004;76(suppl):S11-S19 doi:10.1016/j.drugalcdep.2004.08.003.
13. Baser OL, Mardekian XJ, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans Health Administration. Pain Pract. 2014;14(5):437-445. doi:10.1111/papr.12097
14. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the united states, 1999-2015. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/data/databriefs/db273.pdf. Published February 2017. Accessed July 20, 2020.
15. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
16. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019,67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
17. US Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for opioid therapy for chronic pain version 3.0. https://www.healthquality.va.gov/guidelines/pain/cot. Updated March 1, 2018. Accessed July 20, 2020.
18. Vaughn IA, Beyth RJ, Ayers ML, et al. Multispecialty opioid risk reduction program targeting chronic pain and addiction management in veterans. Fed Pract. 2019;36(9):406-411.
19. Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: lessons from the Department of Veterans Affairs. JAMA Intern Med. 2017;177(5):611-612. doi:10.1001/jamainternmed.2017.0147
20. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167(3):181-191. doi:10.7326/M17-0598
21. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90(6):828-842. doi:10.1016/j.mayocp.2015.04.003
22. Veterans Health Administration, Office of Mental Health and Suicide Prevention. Opioid use and suicide risk. https://www.mentalhealth.va.gov/suicide_prevention/docs/Literature_Review_Opioid_Use_and_Suicide_Risk_508_FINAL_04-26-2019.pdf. Published April 26, 2019. Accessed July 20, 2020.
23. Demidenko MI, Dobscha SK, Morasco BJ, Meath THA, Ilgen MA, Lovejoy TI. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35. doi:10.1016/j.genhosppsych.2017.04.011
24. National Institute on Drug Abuse. Intentional versus unintentional overdose deaths. https://www.drugabuse.gov/related-topics/treatment/intentional-vs-unintentional-overdose-deaths. Updated February 13, 2017. Accessed July 20, 2020.
25. Centers for Disease Control and Prevention. Preventing suicide. https://www.cdc.gov/violenceprevention/pdf/suicide-factsheet.pdf. Published 2018. Accessed July 20, 2020.
26. Webster LR. Pain and suicide: the other side of the opioid story. Pain Med. 2014;15(3):345-346. doi:10.1111/pme.12398
1. American Foundation for Suicide Prevention. Suicide statistics. https://afsp.org/about-suicide/suicide-statistics. Updated 2019. Accessed September 2, 2020.
2. Shane L 3rd. New veteran suicide numbers raise concerns among experts hoping for positive news. https://www.militarytimes.com/news/pentagon-congress/2019/10/09/new-veteran-suicide-numbers-raise-concerns-among-experts-hoping-for-positive-news. Published October 9, 2019. Accessed July 23, 2020.
3. Veterans Health Administration, Office of Mental Health and Suicide Prevention. Veteran suicide data report, 2005–2017. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 2019. Accessed July 20, 2020.
4. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
5. Ilgen MA, Kleinberg F, Ignacio RV, et al. Noncancer pain conditions and risk of suicide. JAMA Psychiatry. 2013;70(7):692-697. doi:10.1001/jamapsychiatry.2013.908
6. Frenk SM, Porter KS, Paulozzi LJ. Prescription opioid analgesic use among adults: United States, 1999-2012. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/products/databriefs/db189.htm. Published February 25, 2015. Accessed July 20, 2020.
7. Bohnert ASB, Ilgen MA. Understanding links among opioid use, overdose, and suicide. N Engl J Med. 2019;380(14):71-79. doi:10.1056/NEJMc1901540
8. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi:10.7326/0003-4819-152-2-201001190-00006
9. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691. doi:10.1001/archinternmed.2011.117
10. Ilgen MA, Bohnert AS, Ganoczy D, Bair MJ, McCarthy JF, Blow FC. Opioid dose and risk of suicide. Pain. 2016;157(5):1079-1084. doi:10.1097/j.pain.0000000000000484
11. Sinyor M, Howlett A, Cheung AH, Schaffer A. Substances used in completed suicide by overdose in Toronto: an observational study of coroner’s data. Can J Psychiatry. 2012;57(3):184-191. doi:10.1177/070674371205700308
12. Wilcox HC, Conner KR, Caine ED. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug Alcohol Depend. 2004;76(suppl):S11-S19 doi:10.1016/j.drugalcdep.2004.08.003.
13. Baser OL, Mardekian XJ, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans Health Administration. Pain Pract. 2014;14(5):437-445. doi:10.1111/papr.12097
14. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the united states, 1999-2015. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/data/databriefs/db273.pdf. Published February 2017. Accessed July 20, 2020.
15. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
16. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019,67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
17. US Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for opioid therapy for chronic pain version 3.0. https://www.healthquality.va.gov/guidelines/pain/cot. Updated March 1, 2018. Accessed July 20, 2020.
18. Vaughn IA, Beyth RJ, Ayers ML, et al. Multispecialty opioid risk reduction program targeting chronic pain and addiction management in veterans. Fed Pract. 2019;36(9):406-411.
19. Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: lessons from the Department of Veterans Affairs. JAMA Intern Med. 2017;177(5):611-612. doi:10.1001/jamainternmed.2017.0147
20. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167(3):181-191. doi:10.7326/M17-0598
21. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90(6):828-842. doi:10.1016/j.mayocp.2015.04.003
22. Veterans Health Administration, Office of Mental Health and Suicide Prevention. Opioid use and suicide risk. https://www.mentalhealth.va.gov/suicide_prevention/docs/Literature_Review_Opioid_Use_and_Suicide_Risk_508_FINAL_04-26-2019.pdf. Published April 26, 2019. Accessed July 20, 2020.
23. Demidenko MI, Dobscha SK, Morasco BJ, Meath THA, Ilgen MA, Lovejoy TI. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35. doi:10.1016/j.genhosppsych.2017.04.011
24. National Institute on Drug Abuse. Intentional versus unintentional overdose deaths. https://www.drugabuse.gov/related-topics/treatment/intentional-vs-unintentional-overdose-deaths. Updated February 13, 2017. Accessed July 20, 2020.
25. Centers for Disease Control and Prevention. Preventing suicide. https://www.cdc.gov/violenceprevention/pdf/suicide-factsheet.pdf. Published 2018. Accessed July 20, 2020.
26. Webster LR. Pain and suicide: the other side of the opioid story. Pain Med. 2014;15(3):345-346. doi:10.1111/pme.12398
Trends in VA Telerehabilitation Patients and Encounters Over Time and by Rurality
Historically, the Veterans Health Administration (VHA) has excelled at improving veterans’ access to health care and enhancing foundational services, such as prosthetics and other veteran-centric services, and this continues to be the VHA’s top priority.1 Travel distance and time are often barriers to accessing health care for many veterans.2-11 For veterans with disabilities who must overcome additional physical, cognitive, and emotional obstacles to access vital rehabilitation services, these geographic obstacles are magnified. Further compounding the challenge is that rehabilitation therapies frequently require multiple encounters. Telerehabilitation is a promising solution for veterans in need of rehabilitation to regain optimal functioning. This alternative mode of service delivery can help veterans overcome geographic access barriers by delivering health care directly to veterans in their homes or nearby community-based outpatient clinics.12,13
A growing body of evidence supports telerehabilitation. In a 2017 systematic review and meta-analysis, Cottrell and colleagues reviewed and analyzed data from 13 studies that met their inclusion criteria; specifically, their meta-analytic sample comprised adults aged ≥ 18 years presenting with any diagnosed primary musculoskeletal condition; treatment interventions via a real-time telerehabilitation medium, trials that had a comparison group with the same condition; provided clinical outcomes data, and included published randomized and nonrandomized controlled trials.14 Based on their aggregated results, they concluded that real-time telerehabilitation was effective in improving physical function (standardized mean difference [SMD], 0.63; 95% CI, 0.92-2.33; I2, 93%), and reducing pain (SMD, 0.66; 95% CI, −0.27- .60; I2, 96%) in patients with any diagnosed primary musculoskeletal condition.14
Two other systematic reviews conducted by Pietrzak and colleagues and Agostini and colleagues also demonstrated the clinical effectiveness of telerehabilitation.15,16 Clinical effectiveness was defined as changes in health, functional status, and satisfaction with the telerehabilitation services delivered. The studies examined in the review included those that provided online self-management and education in addition to exercise via teleconferencing in real time.
Pietrzak and colleagues found that Internet-based osteoarthritis self-management interventions significantly improved 4 of 6 health status measures reviewed (ie, pain, fatigue, activity limitation, health distress, disability, and self‐reported global health).15 User acceptance and satisfaction were high (≥ 70% satisfied) in all studies meeting the inclusion criteria.
Agostini and colleagues found that telerehabilitation was more effective than other modes of delivering rehabilitation to regain motor function in cardiac (SMD, 0.24; 95% CI, 0.04-0.43) and total knee arthroplasty (Timed Up and Go test: SMD, −5.17; 95% CI, −9.79- −0.55) patients.16 Some evidence from VHA and non-VHA studies also support the use of telerehabilitation to reduce health care costs,17-19 improve treatment adherence,12,20 and enhance patient physical, cognitive and mobility function, as well as patient satisfaction and health-related quality of life.13,21-24
Since the first recorded use of telehealth in 1959, the application of technology to deliver health care, including rehabilitation services, has increased exponentially.14 In fiscal year (FY) 2017 alone, the VA provided > 2 million episodes of care for > 700,000 veterans using telehealth services.2
Although the process for accessing telerehabilitation may vary throughout the VA, typically a few common factors make a veteran eligible for this mode of rehabilitation care delivery: Veterans must meet criteria for a specific program (eg, amputation, occupational therapy, and physical therapy) and receive VA care from a VA medical facility or clinic that offers telehealth services. Care providers must believe that the veteran would benefit from telerehabilitation (eg, limited mobility and long-distance travel to the facility) and that they would be able to receive an appropriate consult. The veteran must meet the following requirements: (1) willingness to consent to a visit via telehealth; (2) access to required equipment/e-mail; and (3) a caregiver to assist if they are unable to complete a visit independently.
In this article, we provide an overview of the growth of telerehabilitation in the VHA. Data are presented for specific telerehabilitation programs over time and by rurality.
Methods
The VHA Support Service Center works with VHA program offices and field users to provide field-focused business, clinical, and special topic reports. An online portal provides access to these customizable reports organized as data cubes, which represent data dimensions (ie, clinic type) and measures (ie, number of unique patients). For this study, we used the Connected Care, Telehealth, Call Centers Clinical Video Telehealth/Store and Forward Telehealth data cube clinical stop codes to identify the numbers of telerehabilitation veteran users and encounters across time. The following telerehabilitation clinic-stop codes were selected: 197 (polytrauma/traumatic brain injury [TBI]–individuals), 201 (Physical Medicine and Rehabilitation [PM&R] Service), 205 (physical therapy), 206 (occupational therapy), 211 (PM&R amputation clinic), 418 (amputation clinic), 214 (kinesiotherapy), and 240 (PM&R assistive technology clinic). Data for total unique patients served and the total number of encounters were extracted at the national level and by rurality from FY 2012 to FY 2017, providing the past 5 years of VHA telerehabilitation data.
It is important to note that in FY 2015, the VHA changed its definition of rurality to a rural-urban commuting areas (RUCA)-based system (www.ruralhealth.va.gov/rural-definition.asp). Prior to FY 2015, the VHA used the US Census Bureau (CB) urbanized area definitions. According to CB, an urbanized area contains a central city and surrounding area that totals > 50,000 in population. It also includes places outside of urbanized areas with populations > 2,500. Rural areas are defined as all other areas. VHA added a third category, highly rural, which is defined as areas that had < 7 people per square mile. In the RUCA system, each census tract defined by the CB is given a score. The VHA definitions are as follows:
- Urban (U)—census tracts with RUCA scores of 1.0 or 1.1. These tracts are determined by the CB as being in an urban core and having the majority of their workers commute within that same core (1.0). If 30% to 49% commute to an even larger urban core, then the code is 1.1;
- Rural (R)—all tracts not receiving scores in the urban or highly rural tiers; and
- Highly rural (H)—tracts with a RUCA score of 10.0. These are the most remote occupied land areas. Less than 10% of workers travel to CB-defined urbanized areas or urban clusters.
In addition, VHA recently added an “I” category to complement “U,” “R,” and “H.” The “I” value is assigned to veterans living on the US insular islands (ie, territories): Guam, American Samoa, Northern Marianas, and US Virgin Islands. For the analysis by rurality in this study, we excluded veterans living in the insular islands and those of unknown rurality (< 1.0% of patients and encounters). Further, because the numbers of highly rural veterans were relatively small (< 2% of patients and encounters), the rural and highly rural categories were combined and compared with urban-dwelling veterans.
Results
Overall, the workload for telerehabilitation nearly quadrupled over the 5-year period (Table 1 and Figure 1).
Interesting trends were seen by clinic type. Some clinics increased substantially, whereas others showed only moderate increases, and in 1 case (PM&R Service), a decrease. For example, there is significant growth in the number of patients and encounters involving physical therapy through telerehabilitation. This telerehabilitation clinic increased its workload from 1,676 patients with 3,016 encounters in FY 2012 to 9,136 patients with 11,834 encounters in FY 2017, accounting for 62.6% of total growth in patients and 56.8% of total growth in encounters.
Other clinics showing substantial growth over time included occupational therapy and polytrauma/TBI-individual secondary evaluation. Kinesiotherapy telerehabilitation was almost nonexistent in the VHA during FY 2012, with only 23 patients having 23 encounters. By FY 2017, there were 563 patients with 624 kinesiotherapy telerehabilitation encounters, equating to staggering increases in 5 years: 2,348% for patients and 2,613% for encounters. Similarly, the Physical Medicine and Rehabilitation Assistive Technology clinics had very low numbers in FY 2012 (patients, 2; encounters, 3) and increased over time; albeit, at a slow rate.
Trends by Rurality
Trends by rural location of patients and encounters must be interpreted with caution because of the changing rural definition between FY 2014 and FY 2015 (Tables 2 and 3; Figures 3 and 4).
The increased total number of patients seen between FY 2012 and FY 2014 (old definition) was 225% for rural veterans vs 134% for urban veterans. Between FY 2015 and FY 2017 (new definition), the increase was lower for both groups (rural, 13.4%; urban, 7.3%), but rural veterans still increased at a higher rate than did urban dwellers.
Discussion
Our primary aim was to provide data on the growth of telerehabilitation in the VHA over the past 5 years. Our secondary aim was to examine growth in the use of telerehabilitation by rurality. Specifically, we provided an overview of telerehabilitation growth in terms of unique patients and overall encounters in the VHA by rurality from FY 2012 to FY 2014 and FY 2015 to FY 2017 using the following programs: Polytrauma/TBI, PM&R Service, physical therapy, occupational therapy, PM&R amputation clinic, amputation clinic, kinesiotherapy, and PM&R assistive technology clinic. Our findings demonstrated a noteworthy increase in telerehabilitation encounters and unique patients over time for these programs. These findings were consistent with the overall trend of continued growth and expansion of telehealth within the VHA.
Our findings reveal an upward trend in the total number of rural encounters and rural unique patients despite the change in the VA’s definition of rurality in FY 2015. To our knowledge, urban and rural use of telerehabilitation has not been examined previously. Under both definitions of rurality, encounters and unique patients show an important increase over time, and by year-end 2017, more than half of all patients and encounters were attributed to rural patients (53.7% and 53.9%, respectively). Indeed, the upward trend may have been more pronounced if the rural definition had not changed in FY 2015. Our early VHA stroke patients study on the difference between rural-urban patients and taxonomies showed that the RUCA definition was more likely to reduce the number of rural patients by 8.5% than the early definition used by the VHA.26
It is notable that although the use of tele-delivery of rehabilitation has continually increased, the rate of this increase was steeper from FY 2012 to FY 2014 than FY 2015 to FY 2017. For the programs under consideration in this study, the total number of rural patients/encounters increased throughout the observed periods. However, urban patients and encounters increased through FY 2016 and experienced a slight decrease in FY 2017.
The appearance of a slower rate of increase may be due to a rapid initial rate of increase through early adopters and “crossing the diffusion chasm,” a well-documented process of slower diffusion between the time of invention to penetration that often characterizes the spread of successful telehealth innovations
With an emphasis on increasing access to rehabilitation services, the VHA can expect to see a continuing increase in both the number and the percentage of telerehabilitation rural patients and encounters. The VHA has several telerehabilitation initiatives underway through the VHA’s Physical Medicine and Rehabilitation Telerehabilitation Enterprise Wide Initiative (TREWI) and Rural Veterans Telerehabilitation Initiative. These projects demonstrate the feasibility of this delivery approach and facilitate integration of this modality in clinical workflows. However, to sustain these efforts, facilities will need more infrastructure and personnel resources dedicated to the delivery of services.
In an ongoing evaluation of the TREWI, several factors seem to influence the uptake of the VHA Office of Rural Health TREWI programs. These factors are the presence or absence of a local site champion; the quality of hospital leadership support; the quality of past relationships between telerehabilitation sending sites and receiving sites; barriers to getting a telehealth service agreement in place; the availability of space; administrative know-how on setting up clinics appropriately; time involved to bring on staff; contracting issues; equipment availability and installation; cultural issues in embracing technologic innovation; training burden; hassle factors; and limited funds. Although early adopters may be able to negotiate and push through many of the barriers associated with the diffusion of telerehabilitation, the numerous barriers may slow its larger systemwide diffusion.
Telerehabilitation is a promising mode to deliver care to rural veterans who otherwise may not have access to this type of specialty care. Therefore, the identification of elements that foster telerehabilitation growth in future investigations can assist policy makers and key stakeholders in optimally leveraging program resources for maximal productivity. Future studies investigating the drivers of increases in telerehabilitation growth by rurality are warranted. Furthermore, more research is needed to examine telerehabilitation growth quality of care outcomes (eg, patient and provider satisfaction) to ensure that care is not only timely and accessible, but of high quality.
Conclusion
Disparities between rural and urban veterans compel a mode of expanding delivery of care. The VHA has embraced the use of telehealth modalities to extend its reach of rehabilitation services to veterans with disability and rehabilitation needs. Growth in telerehabilitation rural patient encounters increases access to rehabilitative care, reduces patient and caregiver travel burden, and helps ensure treatment adherence. Telerehabilitation utilization (unique patients and total encounters) is growing more rapidly for rural veterans than for their urban counterparts. Overall, telerehabilitation is filling a gap for rural veterans, as well as veterans in general with challenges in accessibility to health care. In order to make full use of the telerehabilitation services across its health care system, VA health care facilities may need to expand their effort in telerehabilitation dissemination and education among providers and veterans, particularly among providers who are less familiar with telerehabilitation services and among veterans who live in rural or highly rural areas and need special rehabilitation care.
1. Shane L. What’s in the VA secretary’s 10-point plan to reform his department? https://rebootcamp.militarytimes.com/news/pentagon-congress/2017/02/28/what-s-in-the-va-secretary-s-10-point-plan-to-reform-his-department. Published February 28, 2017. Accessed November 21, 2018.
2. Burgess JF, DeFiore DA. The effect of distance to a VA facility on the choice and level of utilization of VA outpatient services. Soc Science Med. 1994;39(1):95-104.
3. LaVela SL, Smith B, Weaver FM, Miskevics SA. Geographical proximity and health care utilization in veterans with SCI&D in the USA. Soc Science Med. 2004;59:2387-2399.
4. Piette JD, Moos RH. The influence of distance on ambulatory care use, death, and readmission following a myocardial infarction. Health Serv Res. 1996;31(5):573-591.
5. Schmitt SK, Phibbs CS, Piette JD. The influence of distance on utilization of outpatient mental health aftercare following inpatient substance abuse treatment. Addictive Behav. 2003;28(6):1183-1192.
6. Fortney JC, Booth BM, Blow FC, Bunn JY. The effects of travel barriers and age on the utilization of alcoholism treatment aftercare. Am J Drug Alcohol Abuse. 1995;21(3):391-406.
7. McCarthy JF, Blow FC, Valenstein M, et al. Veterans Affairs Health System and mental health treatment retention among patients with serious mental illness: evaluating accessibility and availability barriers. Health Serv Res. 2007;42(3):1042-1060.
8. Mooney C, Zwanziger J, Phibbs CS, Schmitt S. Is travel distance a barrier to veterans’ use of VA hospitals for medical surgical care? Soc Sci Med. 2000;50(12):1743-1755.
9. Friedman SA, Frayne SM, Berg E, et al. Travel time and attrition from VHA care among women veterans: how far is too far? Med Care. 2015;53(4)(suppl 1):S15-S22.
10. Buzza C, Ono SS, Turvey C, et al. Distance is relative: unpacking a principal barrier in rural healthcare. J Gen Intern Med. 2011;26(suppl 2):648-654.
11. Goins RT, Williams KA, Carter MW, Spencer SM, Solovieva T. Perceived barriers to health care access among rural older adults: a qualitative study. J Rural Health. 2005;21(3):206-213.
12. Kairy D, Lehoux P, Vincent C, Visintin M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil Rehabil. 2009;31(6):427-447.
13. McCue M, Fairman A, Pramuka M. Enhancing quality of life through telerehabilitation. Phys Med Rehabil Clin N Am. 2010;21(1):195-205.
14. Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638.
15. Pietrzak E, Cotea C, Pullman S, Nasveld P. Self-management and rehabilitation in osteoarthritis: is there a place for internet-based interventions? Telemed J E Health. 2013;19(10):800-805.
16. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213.
17. Kortke H, Stromeyer H, Zittermann A, et al. New East-Westfalian Postoperative Therapy Concept: A telemedicine guide for the study of ambulatory rehabilitation of patients after cardiac surgery. Telemed J E-Health. 2006;12(4):475-483.
18. Tousignant M, Boissy P, Corriveau H, Moffet H. In home telerehabilitation for older adults after discharge from an acute hospital or rehabilitation unit: A proof-of- concept study and costs estimation. Disabil Rehabil Assist Technol. 2006;1(4):209-216.
19. Sanford JA, Griffiths PC, Richardson P, et al. The effects of in-home rehabilitation on task self-efficacy in mobility-impaired adults: a randomized clinical trial. J Am Geriatr Soc. 2006;54(11):1641-1648.
20. Nakamura K, Takano T, Akao C. The effectiveness of videophones in home healthcare for the elderly. Med Care. 1999;37(2):117-125.
21. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370.
22. Guilfoyle C, Wootton R, Hassall S, et al. User satisfaction with allied health services delivered to residential facilities via videoconferencing. J Telemed Telecare. 2003;9(1):S52-S54.23. Mair F, Whitten P. Systematic review of studies of patient satisfaction with telemedicine. BMJ. 2000;320(7248):1517-1520.
24. Williams T L, May C R, Esmail A. Limitations of patient satisfaction studies in telehealthcare: a systematic review of the literature. Telemed J E-Health. 2001;7(4):293-316.
25. US Department of Veterans Affairs, Office of Telehealth Services. http://vaww.telehealth.va.gov/quality/data/index.asp. Accessed June 1, 2018. [Nonpublic document; source not verified.]
26. Jia H, Cowper D, Tang Y, et al. Post-acute stroke rehabilitation utilization: Are there difference between rural-urban patients and taxonomies? J Rural Health. 2012;28(3):242-247.
27. Cho S, Mathiassen L, Gallivan M. Crossing the chasm: from adoption to diffusion of a telehealth innovation. In: León G, Bernardos AM, Casar JR, Kautz K, De Gross JI, eds. Open IT-Based Innovation: Moving Towards Cooperative IT Transfer and Knowledge Diffusion. Boston, MA: Springer; 2008.
28. Broderick A, Lindeman D. Scaling telehealth programs: lessons from early adopters. https://www.commonwealthfund.org/publications/case-study/2013/jan/scaling-telehealth-programs-lessons-early-adopters. Published January 2013. Accessed June 1, 2018.
Historically, the Veterans Health Administration (VHA) has excelled at improving veterans’ access to health care and enhancing foundational services, such as prosthetics and other veteran-centric services, and this continues to be the VHA’s top priority.1 Travel distance and time are often barriers to accessing health care for many veterans.2-11 For veterans with disabilities who must overcome additional physical, cognitive, and emotional obstacles to access vital rehabilitation services, these geographic obstacles are magnified. Further compounding the challenge is that rehabilitation therapies frequently require multiple encounters. Telerehabilitation is a promising solution for veterans in need of rehabilitation to regain optimal functioning. This alternative mode of service delivery can help veterans overcome geographic access barriers by delivering health care directly to veterans in their homes or nearby community-based outpatient clinics.12,13
A growing body of evidence supports telerehabilitation. In a 2017 systematic review and meta-analysis, Cottrell and colleagues reviewed and analyzed data from 13 studies that met their inclusion criteria; specifically, their meta-analytic sample comprised adults aged ≥ 18 years presenting with any diagnosed primary musculoskeletal condition; treatment interventions via a real-time telerehabilitation medium, trials that had a comparison group with the same condition; provided clinical outcomes data, and included published randomized and nonrandomized controlled trials.14 Based on their aggregated results, they concluded that real-time telerehabilitation was effective in improving physical function (standardized mean difference [SMD], 0.63; 95% CI, 0.92-2.33; I2, 93%), and reducing pain (SMD, 0.66; 95% CI, −0.27- .60; I2, 96%) in patients with any diagnosed primary musculoskeletal condition.14
Two other systematic reviews conducted by Pietrzak and colleagues and Agostini and colleagues also demonstrated the clinical effectiveness of telerehabilitation.15,16 Clinical effectiveness was defined as changes in health, functional status, and satisfaction with the telerehabilitation services delivered. The studies examined in the review included those that provided online self-management and education in addition to exercise via teleconferencing in real time.
Pietrzak and colleagues found that Internet-based osteoarthritis self-management interventions significantly improved 4 of 6 health status measures reviewed (ie, pain, fatigue, activity limitation, health distress, disability, and self‐reported global health).15 User acceptance and satisfaction were high (≥ 70% satisfied) in all studies meeting the inclusion criteria.
Agostini and colleagues found that telerehabilitation was more effective than other modes of delivering rehabilitation to regain motor function in cardiac (SMD, 0.24; 95% CI, 0.04-0.43) and total knee arthroplasty (Timed Up and Go test: SMD, −5.17; 95% CI, −9.79- −0.55) patients.16 Some evidence from VHA and non-VHA studies also support the use of telerehabilitation to reduce health care costs,17-19 improve treatment adherence,12,20 and enhance patient physical, cognitive and mobility function, as well as patient satisfaction and health-related quality of life.13,21-24
Since the first recorded use of telehealth in 1959, the application of technology to deliver health care, including rehabilitation services, has increased exponentially.14 In fiscal year (FY) 2017 alone, the VA provided > 2 million episodes of care for > 700,000 veterans using telehealth services.2
Although the process for accessing telerehabilitation may vary throughout the VA, typically a few common factors make a veteran eligible for this mode of rehabilitation care delivery: Veterans must meet criteria for a specific program (eg, amputation, occupational therapy, and physical therapy) and receive VA care from a VA medical facility or clinic that offers telehealth services. Care providers must believe that the veteran would benefit from telerehabilitation (eg, limited mobility and long-distance travel to the facility) and that they would be able to receive an appropriate consult. The veteran must meet the following requirements: (1) willingness to consent to a visit via telehealth; (2) access to required equipment/e-mail; and (3) a caregiver to assist if they are unable to complete a visit independently.
In this article, we provide an overview of the growth of telerehabilitation in the VHA. Data are presented for specific telerehabilitation programs over time and by rurality.
Methods
The VHA Support Service Center works with VHA program offices and field users to provide field-focused business, clinical, and special topic reports. An online portal provides access to these customizable reports organized as data cubes, which represent data dimensions (ie, clinic type) and measures (ie, number of unique patients). For this study, we used the Connected Care, Telehealth, Call Centers Clinical Video Telehealth/Store and Forward Telehealth data cube clinical stop codes to identify the numbers of telerehabilitation veteran users and encounters across time. The following telerehabilitation clinic-stop codes were selected: 197 (polytrauma/traumatic brain injury [TBI]–individuals), 201 (Physical Medicine and Rehabilitation [PM&R] Service), 205 (physical therapy), 206 (occupational therapy), 211 (PM&R amputation clinic), 418 (amputation clinic), 214 (kinesiotherapy), and 240 (PM&R assistive technology clinic). Data for total unique patients served and the total number of encounters were extracted at the national level and by rurality from FY 2012 to FY 2017, providing the past 5 years of VHA telerehabilitation data.
It is important to note that in FY 2015, the VHA changed its definition of rurality to a rural-urban commuting areas (RUCA)-based system (www.ruralhealth.va.gov/rural-definition.asp). Prior to FY 2015, the VHA used the US Census Bureau (CB) urbanized area definitions. According to CB, an urbanized area contains a central city and surrounding area that totals > 50,000 in population. It also includes places outside of urbanized areas with populations > 2,500. Rural areas are defined as all other areas. VHA added a third category, highly rural, which is defined as areas that had < 7 people per square mile. In the RUCA system, each census tract defined by the CB is given a score. The VHA definitions are as follows:
- Urban (U)—census tracts with RUCA scores of 1.0 or 1.1. These tracts are determined by the CB as being in an urban core and having the majority of their workers commute within that same core (1.0). If 30% to 49% commute to an even larger urban core, then the code is 1.1;
- Rural (R)—all tracts not receiving scores in the urban or highly rural tiers; and
- Highly rural (H)—tracts with a RUCA score of 10.0. These are the most remote occupied land areas. Less than 10% of workers travel to CB-defined urbanized areas or urban clusters.
In addition, VHA recently added an “I” category to complement “U,” “R,” and “H.” The “I” value is assigned to veterans living on the US insular islands (ie, territories): Guam, American Samoa, Northern Marianas, and US Virgin Islands. For the analysis by rurality in this study, we excluded veterans living in the insular islands and those of unknown rurality (< 1.0% of patients and encounters). Further, because the numbers of highly rural veterans were relatively small (< 2% of patients and encounters), the rural and highly rural categories were combined and compared with urban-dwelling veterans.
Results
Overall, the workload for telerehabilitation nearly quadrupled over the 5-year period (Table 1 and Figure 1).
Interesting trends were seen by clinic type. Some clinics increased substantially, whereas others showed only moderate increases, and in 1 case (PM&R Service), a decrease. For example, there is significant growth in the number of patients and encounters involving physical therapy through telerehabilitation. This telerehabilitation clinic increased its workload from 1,676 patients with 3,016 encounters in FY 2012 to 9,136 patients with 11,834 encounters in FY 2017, accounting for 62.6% of total growth in patients and 56.8% of total growth in encounters.
Other clinics showing substantial growth over time included occupational therapy and polytrauma/TBI-individual secondary evaluation. Kinesiotherapy telerehabilitation was almost nonexistent in the VHA during FY 2012, with only 23 patients having 23 encounters. By FY 2017, there were 563 patients with 624 kinesiotherapy telerehabilitation encounters, equating to staggering increases in 5 years: 2,348% for patients and 2,613% for encounters. Similarly, the Physical Medicine and Rehabilitation Assistive Technology clinics had very low numbers in FY 2012 (patients, 2; encounters, 3) and increased over time; albeit, at a slow rate.
Trends by Rurality
Trends by rural location of patients and encounters must be interpreted with caution because of the changing rural definition between FY 2014 and FY 2015 (Tables 2 and 3; Figures 3 and 4).
The increased total number of patients seen between FY 2012 and FY 2014 (old definition) was 225% for rural veterans vs 134% for urban veterans. Between FY 2015 and FY 2017 (new definition), the increase was lower for both groups (rural, 13.4%; urban, 7.3%), but rural veterans still increased at a higher rate than did urban dwellers.
Discussion
Our primary aim was to provide data on the growth of telerehabilitation in the VHA over the past 5 years. Our secondary aim was to examine growth in the use of telerehabilitation by rurality. Specifically, we provided an overview of telerehabilitation growth in terms of unique patients and overall encounters in the VHA by rurality from FY 2012 to FY 2014 and FY 2015 to FY 2017 using the following programs: Polytrauma/TBI, PM&R Service, physical therapy, occupational therapy, PM&R amputation clinic, amputation clinic, kinesiotherapy, and PM&R assistive technology clinic. Our findings demonstrated a noteworthy increase in telerehabilitation encounters and unique patients over time for these programs. These findings were consistent with the overall trend of continued growth and expansion of telehealth within the VHA.
Our findings reveal an upward trend in the total number of rural encounters and rural unique patients despite the change in the VA’s definition of rurality in FY 2015. To our knowledge, urban and rural use of telerehabilitation has not been examined previously. Under both definitions of rurality, encounters and unique patients show an important increase over time, and by year-end 2017, more than half of all patients and encounters were attributed to rural patients (53.7% and 53.9%, respectively). Indeed, the upward trend may have been more pronounced if the rural definition had not changed in FY 2015. Our early VHA stroke patients study on the difference between rural-urban patients and taxonomies showed that the RUCA definition was more likely to reduce the number of rural patients by 8.5% than the early definition used by the VHA.26
It is notable that although the use of tele-delivery of rehabilitation has continually increased, the rate of this increase was steeper from FY 2012 to FY 2014 than FY 2015 to FY 2017. For the programs under consideration in this study, the total number of rural patients/encounters increased throughout the observed periods. However, urban patients and encounters increased through FY 2016 and experienced a slight decrease in FY 2017.
The appearance of a slower rate of increase may be due to a rapid initial rate of increase through early adopters and “crossing the diffusion chasm,” a well-documented process of slower diffusion between the time of invention to penetration that often characterizes the spread of successful telehealth innovations
With an emphasis on increasing access to rehabilitation services, the VHA can expect to see a continuing increase in both the number and the percentage of telerehabilitation rural patients and encounters. The VHA has several telerehabilitation initiatives underway through the VHA’s Physical Medicine and Rehabilitation Telerehabilitation Enterprise Wide Initiative (TREWI) and Rural Veterans Telerehabilitation Initiative. These projects demonstrate the feasibility of this delivery approach and facilitate integration of this modality in clinical workflows. However, to sustain these efforts, facilities will need more infrastructure and personnel resources dedicated to the delivery of services.
In an ongoing evaluation of the TREWI, several factors seem to influence the uptake of the VHA Office of Rural Health TREWI programs. These factors are the presence or absence of a local site champion; the quality of hospital leadership support; the quality of past relationships between telerehabilitation sending sites and receiving sites; barriers to getting a telehealth service agreement in place; the availability of space; administrative know-how on setting up clinics appropriately; time involved to bring on staff; contracting issues; equipment availability and installation; cultural issues in embracing technologic innovation; training burden; hassle factors; and limited funds. Although early adopters may be able to negotiate and push through many of the barriers associated with the diffusion of telerehabilitation, the numerous barriers may slow its larger systemwide diffusion.
Telerehabilitation is a promising mode to deliver care to rural veterans who otherwise may not have access to this type of specialty care. Therefore, the identification of elements that foster telerehabilitation growth in future investigations can assist policy makers and key stakeholders in optimally leveraging program resources for maximal productivity. Future studies investigating the drivers of increases in telerehabilitation growth by rurality are warranted. Furthermore, more research is needed to examine telerehabilitation growth quality of care outcomes (eg, patient and provider satisfaction) to ensure that care is not only timely and accessible, but of high quality.
Conclusion
Disparities between rural and urban veterans compel a mode of expanding delivery of care. The VHA has embraced the use of telehealth modalities to extend its reach of rehabilitation services to veterans with disability and rehabilitation needs. Growth in telerehabilitation rural patient encounters increases access to rehabilitative care, reduces patient and caregiver travel burden, and helps ensure treatment adherence. Telerehabilitation utilization (unique patients and total encounters) is growing more rapidly for rural veterans than for their urban counterparts. Overall, telerehabilitation is filling a gap for rural veterans, as well as veterans in general with challenges in accessibility to health care. In order to make full use of the telerehabilitation services across its health care system, VA health care facilities may need to expand their effort in telerehabilitation dissemination and education among providers and veterans, particularly among providers who are less familiar with telerehabilitation services and among veterans who live in rural or highly rural areas and need special rehabilitation care.
Historically, the Veterans Health Administration (VHA) has excelled at improving veterans’ access to health care and enhancing foundational services, such as prosthetics and other veteran-centric services, and this continues to be the VHA’s top priority.1 Travel distance and time are often barriers to accessing health care for many veterans.2-11 For veterans with disabilities who must overcome additional physical, cognitive, and emotional obstacles to access vital rehabilitation services, these geographic obstacles are magnified. Further compounding the challenge is that rehabilitation therapies frequently require multiple encounters. Telerehabilitation is a promising solution for veterans in need of rehabilitation to regain optimal functioning. This alternative mode of service delivery can help veterans overcome geographic access barriers by delivering health care directly to veterans in their homes or nearby community-based outpatient clinics.12,13
A growing body of evidence supports telerehabilitation. In a 2017 systematic review and meta-analysis, Cottrell and colleagues reviewed and analyzed data from 13 studies that met their inclusion criteria; specifically, their meta-analytic sample comprised adults aged ≥ 18 years presenting with any diagnosed primary musculoskeletal condition; treatment interventions via a real-time telerehabilitation medium, trials that had a comparison group with the same condition; provided clinical outcomes data, and included published randomized and nonrandomized controlled trials.14 Based on their aggregated results, they concluded that real-time telerehabilitation was effective in improving physical function (standardized mean difference [SMD], 0.63; 95% CI, 0.92-2.33; I2, 93%), and reducing pain (SMD, 0.66; 95% CI, −0.27- .60; I2, 96%) in patients with any diagnosed primary musculoskeletal condition.14
Two other systematic reviews conducted by Pietrzak and colleagues and Agostini and colleagues also demonstrated the clinical effectiveness of telerehabilitation.15,16 Clinical effectiveness was defined as changes in health, functional status, and satisfaction with the telerehabilitation services delivered. The studies examined in the review included those that provided online self-management and education in addition to exercise via teleconferencing in real time.
Pietrzak and colleagues found that Internet-based osteoarthritis self-management interventions significantly improved 4 of 6 health status measures reviewed (ie, pain, fatigue, activity limitation, health distress, disability, and self‐reported global health).15 User acceptance and satisfaction were high (≥ 70% satisfied) in all studies meeting the inclusion criteria.
Agostini and colleagues found that telerehabilitation was more effective than other modes of delivering rehabilitation to regain motor function in cardiac (SMD, 0.24; 95% CI, 0.04-0.43) and total knee arthroplasty (Timed Up and Go test: SMD, −5.17; 95% CI, −9.79- −0.55) patients.16 Some evidence from VHA and non-VHA studies also support the use of telerehabilitation to reduce health care costs,17-19 improve treatment adherence,12,20 and enhance patient physical, cognitive and mobility function, as well as patient satisfaction and health-related quality of life.13,21-24
Since the first recorded use of telehealth in 1959, the application of technology to deliver health care, including rehabilitation services, has increased exponentially.14 In fiscal year (FY) 2017 alone, the VA provided > 2 million episodes of care for > 700,000 veterans using telehealth services.2
Although the process for accessing telerehabilitation may vary throughout the VA, typically a few common factors make a veteran eligible for this mode of rehabilitation care delivery: Veterans must meet criteria for a specific program (eg, amputation, occupational therapy, and physical therapy) and receive VA care from a VA medical facility or clinic that offers telehealth services. Care providers must believe that the veteran would benefit from telerehabilitation (eg, limited mobility and long-distance travel to the facility) and that they would be able to receive an appropriate consult. The veteran must meet the following requirements: (1) willingness to consent to a visit via telehealth; (2) access to required equipment/e-mail; and (3) a caregiver to assist if they are unable to complete a visit independently.
In this article, we provide an overview of the growth of telerehabilitation in the VHA. Data are presented for specific telerehabilitation programs over time and by rurality.
Methods
The VHA Support Service Center works with VHA program offices and field users to provide field-focused business, clinical, and special topic reports. An online portal provides access to these customizable reports organized as data cubes, which represent data dimensions (ie, clinic type) and measures (ie, number of unique patients). For this study, we used the Connected Care, Telehealth, Call Centers Clinical Video Telehealth/Store and Forward Telehealth data cube clinical stop codes to identify the numbers of telerehabilitation veteran users and encounters across time. The following telerehabilitation clinic-stop codes were selected: 197 (polytrauma/traumatic brain injury [TBI]–individuals), 201 (Physical Medicine and Rehabilitation [PM&R] Service), 205 (physical therapy), 206 (occupational therapy), 211 (PM&R amputation clinic), 418 (amputation clinic), 214 (kinesiotherapy), and 240 (PM&R assistive technology clinic). Data for total unique patients served and the total number of encounters were extracted at the national level and by rurality from FY 2012 to FY 2017, providing the past 5 years of VHA telerehabilitation data.
It is important to note that in FY 2015, the VHA changed its definition of rurality to a rural-urban commuting areas (RUCA)-based system (www.ruralhealth.va.gov/rural-definition.asp). Prior to FY 2015, the VHA used the US Census Bureau (CB) urbanized area definitions. According to CB, an urbanized area contains a central city and surrounding area that totals > 50,000 in population. It also includes places outside of urbanized areas with populations > 2,500. Rural areas are defined as all other areas. VHA added a third category, highly rural, which is defined as areas that had < 7 people per square mile. In the RUCA system, each census tract defined by the CB is given a score. The VHA definitions are as follows:
- Urban (U)—census tracts with RUCA scores of 1.0 or 1.1. These tracts are determined by the CB as being in an urban core and having the majority of their workers commute within that same core (1.0). If 30% to 49% commute to an even larger urban core, then the code is 1.1;
- Rural (R)—all tracts not receiving scores in the urban or highly rural tiers; and
- Highly rural (H)—tracts with a RUCA score of 10.0. These are the most remote occupied land areas. Less than 10% of workers travel to CB-defined urbanized areas or urban clusters.
In addition, VHA recently added an “I” category to complement “U,” “R,” and “H.” The “I” value is assigned to veterans living on the US insular islands (ie, territories): Guam, American Samoa, Northern Marianas, and US Virgin Islands. For the analysis by rurality in this study, we excluded veterans living in the insular islands and those of unknown rurality (< 1.0% of patients and encounters). Further, because the numbers of highly rural veterans were relatively small (< 2% of patients and encounters), the rural and highly rural categories were combined and compared with urban-dwelling veterans.
Results
Overall, the workload for telerehabilitation nearly quadrupled over the 5-year period (Table 1 and Figure 1).
Interesting trends were seen by clinic type. Some clinics increased substantially, whereas others showed only moderate increases, and in 1 case (PM&R Service), a decrease. For example, there is significant growth in the number of patients and encounters involving physical therapy through telerehabilitation. This telerehabilitation clinic increased its workload from 1,676 patients with 3,016 encounters in FY 2012 to 9,136 patients with 11,834 encounters in FY 2017, accounting for 62.6% of total growth in patients and 56.8% of total growth in encounters.
Other clinics showing substantial growth over time included occupational therapy and polytrauma/TBI-individual secondary evaluation. Kinesiotherapy telerehabilitation was almost nonexistent in the VHA during FY 2012, with only 23 patients having 23 encounters. By FY 2017, there were 563 patients with 624 kinesiotherapy telerehabilitation encounters, equating to staggering increases in 5 years: 2,348% for patients and 2,613% for encounters. Similarly, the Physical Medicine and Rehabilitation Assistive Technology clinics had very low numbers in FY 2012 (patients, 2; encounters, 3) and increased over time; albeit, at a slow rate.
Trends by Rurality
Trends by rural location of patients and encounters must be interpreted with caution because of the changing rural definition between FY 2014 and FY 2015 (Tables 2 and 3; Figures 3 and 4).
The increased total number of patients seen between FY 2012 and FY 2014 (old definition) was 225% for rural veterans vs 134% for urban veterans. Between FY 2015 and FY 2017 (new definition), the increase was lower for both groups (rural, 13.4%; urban, 7.3%), but rural veterans still increased at a higher rate than did urban dwellers.
Discussion
Our primary aim was to provide data on the growth of telerehabilitation in the VHA over the past 5 years. Our secondary aim was to examine growth in the use of telerehabilitation by rurality. Specifically, we provided an overview of telerehabilitation growth in terms of unique patients and overall encounters in the VHA by rurality from FY 2012 to FY 2014 and FY 2015 to FY 2017 using the following programs: Polytrauma/TBI, PM&R Service, physical therapy, occupational therapy, PM&R amputation clinic, amputation clinic, kinesiotherapy, and PM&R assistive technology clinic. Our findings demonstrated a noteworthy increase in telerehabilitation encounters and unique patients over time for these programs. These findings were consistent with the overall trend of continued growth and expansion of telehealth within the VHA.
Our findings reveal an upward trend in the total number of rural encounters and rural unique patients despite the change in the VA’s definition of rurality in FY 2015. To our knowledge, urban and rural use of telerehabilitation has not been examined previously. Under both definitions of rurality, encounters and unique patients show an important increase over time, and by year-end 2017, more than half of all patients and encounters were attributed to rural patients (53.7% and 53.9%, respectively). Indeed, the upward trend may have been more pronounced if the rural definition had not changed in FY 2015. Our early VHA stroke patients study on the difference between rural-urban patients and taxonomies showed that the RUCA definition was more likely to reduce the number of rural patients by 8.5% than the early definition used by the VHA.26
It is notable that although the use of tele-delivery of rehabilitation has continually increased, the rate of this increase was steeper from FY 2012 to FY 2014 than FY 2015 to FY 2017. For the programs under consideration in this study, the total number of rural patients/encounters increased throughout the observed periods. However, urban patients and encounters increased through FY 2016 and experienced a slight decrease in FY 2017.
The appearance of a slower rate of increase may be due to a rapid initial rate of increase through early adopters and “crossing the diffusion chasm,” a well-documented process of slower diffusion between the time of invention to penetration that often characterizes the spread of successful telehealth innovations
With an emphasis on increasing access to rehabilitation services, the VHA can expect to see a continuing increase in both the number and the percentage of telerehabilitation rural patients and encounters. The VHA has several telerehabilitation initiatives underway through the VHA’s Physical Medicine and Rehabilitation Telerehabilitation Enterprise Wide Initiative (TREWI) and Rural Veterans Telerehabilitation Initiative. These projects demonstrate the feasibility of this delivery approach and facilitate integration of this modality in clinical workflows. However, to sustain these efforts, facilities will need more infrastructure and personnel resources dedicated to the delivery of services.
In an ongoing evaluation of the TREWI, several factors seem to influence the uptake of the VHA Office of Rural Health TREWI programs. These factors are the presence or absence of a local site champion; the quality of hospital leadership support; the quality of past relationships between telerehabilitation sending sites and receiving sites; barriers to getting a telehealth service agreement in place; the availability of space; administrative know-how on setting up clinics appropriately; time involved to bring on staff; contracting issues; equipment availability and installation; cultural issues in embracing technologic innovation; training burden; hassle factors; and limited funds. Although early adopters may be able to negotiate and push through many of the barriers associated with the diffusion of telerehabilitation, the numerous barriers may slow its larger systemwide diffusion.
Telerehabilitation is a promising mode to deliver care to rural veterans who otherwise may not have access to this type of specialty care. Therefore, the identification of elements that foster telerehabilitation growth in future investigations can assist policy makers and key stakeholders in optimally leveraging program resources for maximal productivity. Future studies investigating the drivers of increases in telerehabilitation growth by rurality are warranted. Furthermore, more research is needed to examine telerehabilitation growth quality of care outcomes (eg, patient and provider satisfaction) to ensure that care is not only timely and accessible, but of high quality.
Conclusion
Disparities between rural and urban veterans compel a mode of expanding delivery of care. The VHA has embraced the use of telehealth modalities to extend its reach of rehabilitation services to veterans with disability and rehabilitation needs. Growth in telerehabilitation rural patient encounters increases access to rehabilitative care, reduces patient and caregiver travel burden, and helps ensure treatment adherence. Telerehabilitation utilization (unique patients and total encounters) is growing more rapidly for rural veterans than for their urban counterparts. Overall, telerehabilitation is filling a gap for rural veterans, as well as veterans in general with challenges in accessibility to health care. In order to make full use of the telerehabilitation services across its health care system, VA health care facilities may need to expand their effort in telerehabilitation dissemination and education among providers and veterans, particularly among providers who are less familiar with telerehabilitation services and among veterans who live in rural or highly rural areas and need special rehabilitation care.
1. Shane L. What’s in the VA secretary’s 10-point plan to reform his department? https://rebootcamp.militarytimes.com/news/pentagon-congress/2017/02/28/what-s-in-the-va-secretary-s-10-point-plan-to-reform-his-department. Published February 28, 2017. Accessed November 21, 2018.
2. Burgess JF, DeFiore DA. The effect of distance to a VA facility on the choice and level of utilization of VA outpatient services. Soc Science Med. 1994;39(1):95-104.
3. LaVela SL, Smith B, Weaver FM, Miskevics SA. Geographical proximity and health care utilization in veterans with SCI&D in the USA. Soc Science Med. 2004;59:2387-2399.
4. Piette JD, Moos RH. The influence of distance on ambulatory care use, death, and readmission following a myocardial infarction. Health Serv Res. 1996;31(5):573-591.
5. Schmitt SK, Phibbs CS, Piette JD. The influence of distance on utilization of outpatient mental health aftercare following inpatient substance abuse treatment. Addictive Behav. 2003;28(6):1183-1192.
6. Fortney JC, Booth BM, Blow FC, Bunn JY. The effects of travel barriers and age on the utilization of alcoholism treatment aftercare. Am J Drug Alcohol Abuse. 1995;21(3):391-406.
7. McCarthy JF, Blow FC, Valenstein M, et al. Veterans Affairs Health System and mental health treatment retention among patients with serious mental illness: evaluating accessibility and availability barriers. Health Serv Res. 2007;42(3):1042-1060.
8. Mooney C, Zwanziger J, Phibbs CS, Schmitt S. Is travel distance a barrier to veterans’ use of VA hospitals for medical surgical care? Soc Sci Med. 2000;50(12):1743-1755.
9. Friedman SA, Frayne SM, Berg E, et al. Travel time and attrition from VHA care among women veterans: how far is too far? Med Care. 2015;53(4)(suppl 1):S15-S22.
10. Buzza C, Ono SS, Turvey C, et al. Distance is relative: unpacking a principal barrier in rural healthcare. J Gen Intern Med. 2011;26(suppl 2):648-654.
11. Goins RT, Williams KA, Carter MW, Spencer SM, Solovieva T. Perceived barriers to health care access among rural older adults: a qualitative study. J Rural Health. 2005;21(3):206-213.
12. Kairy D, Lehoux P, Vincent C, Visintin M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil Rehabil. 2009;31(6):427-447.
13. McCue M, Fairman A, Pramuka M. Enhancing quality of life through telerehabilitation. Phys Med Rehabil Clin N Am. 2010;21(1):195-205.
14. Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638.
15. Pietrzak E, Cotea C, Pullman S, Nasveld P. Self-management and rehabilitation in osteoarthritis: is there a place for internet-based interventions? Telemed J E Health. 2013;19(10):800-805.
16. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213.
17. Kortke H, Stromeyer H, Zittermann A, et al. New East-Westfalian Postoperative Therapy Concept: A telemedicine guide for the study of ambulatory rehabilitation of patients after cardiac surgery. Telemed J E-Health. 2006;12(4):475-483.
18. Tousignant M, Boissy P, Corriveau H, Moffet H. In home telerehabilitation for older adults after discharge from an acute hospital or rehabilitation unit: A proof-of- concept study and costs estimation. Disabil Rehabil Assist Technol. 2006;1(4):209-216.
19. Sanford JA, Griffiths PC, Richardson P, et al. The effects of in-home rehabilitation on task self-efficacy in mobility-impaired adults: a randomized clinical trial. J Am Geriatr Soc. 2006;54(11):1641-1648.
20. Nakamura K, Takano T, Akao C. The effectiveness of videophones in home healthcare for the elderly. Med Care. 1999;37(2):117-125.
21. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370.
22. Guilfoyle C, Wootton R, Hassall S, et al. User satisfaction with allied health services delivered to residential facilities via videoconferencing. J Telemed Telecare. 2003;9(1):S52-S54.23. Mair F, Whitten P. Systematic review of studies of patient satisfaction with telemedicine. BMJ. 2000;320(7248):1517-1520.
24. Williams T L, May C R, Esmail A. Limitations of patient satisfaction studies in telehealthcare: a systematic review of the literature. Telemed J E-Health. 2001;7(4):293-316.
25. US Department of Veterans Affairs, Office of Telehealth Services. http://vaww.telehealth.va.gov/quality/data/index.asp. Accessed June 1, 2018. [Nonpublic document; source not verified.]
26. Jia H, Cowper D, Tang Y, et al. Post-acute stroke rehabilitation utilization: Are there difference between rural-urban patients and taxonomies? J Rural Health. 2012;28(3):242-247.
27. Cho S, Mathiassen L, Gallivan M. Crossing the chasm: from adoption to diffusion of a telehealth innovation. In: León G, Bernardos AM, Casar JR, Kautz K, De Gross JI, eds. Open IT-Based Innovation: Moving Towards Cooperative IT Transfer and Knowledge Diffusion. Boston, MA: Springer; 2008.
28. Broderick A, Lindeman D. Scaling telehealth programs: lessons from early adopters. https://www.commonwealthfund.org/publications/case-study/2013/jan/scaling-telehealth-programs-lessons-early-adopters. Published January 2013. Accessed June 1, 2018.
1. Shane L. What’s in the VA secretary’s 10-point plan to reform his department? https://rebootcamp.militarytimes.com/news/pentagon-congress/2017/02/28/what-s-in-the-va-secretary-s-10-point-plan-to-reform-his-department. Published February 28, 2017. Accessed November 21, 2018.
2. Burgess JF, DeFiore DA. The effect of distance to a VA facility on the choice and level of utilization of VA outpatient services. Soc Science Med. 1994;39(1):95-104.
3. LaVela SL, Smith B, Weaver FM, Miskevics SA. Geographical proximity and health care utilization in veterans with SCI&D in the USA. Soc Science Med. 2004;59:2387-2399.
4. Piette JD, Moos RH. The influence of distance on ambulatory care use, death, and readmission following a myocardial infarction. Health Serv Res. 1996;31(5):573-591.
5. Schmitt SK, Phibbs CS, Piette JD. The influence of distance on utilization of outpatient mental health aftercare following inpatient substance abuse treatment. Addictive Behav. 2003;28(6):1183-1192.
6. Fortney JC, Booth BM, Blow FC, Bunn JY. The effects of travel barriers and age on the utilization of alcoholism treatment aftercare. Am J Drug Alcohol Abuse. 1995;21(3):391-406.
7. McCarthy JF, Blow FC, Valenstein M, et al. Veterans Affairs Health System and mental health treatment retention among patients with serious mental illness: evaluating accessibility and availability barriers. Health Serv Res. 2007;42(3):1042-1060.
8. Mooney C, Zwanziger J, Phibbs CS, Schmitt S. Is travel distance a barrier to veterans’ use of VA hospitals for medical surgical care? Soc Sci Med. 2000;50(12):1743-1755.
9. Friedman SA, Frayne SM, Berg E, et al. Travel time and attrition from VHA care among women veterans: how far is too far? Med Care. 2015;53(4)(suppl 1):S15-S22.
10. Buzza C, Ono SS, Turvey C, et al. Distance is relative: unpacking a principal barrier in rural healthcare. J Gen Intern Med. 2011;26(suppl 2):648-654.
11. Goins RT, Williams KA, Carter MW, Spencer SM, Solovieva T. Perceived barriers to health care access among rural older adults: a qualitative study. J Rural Health. 2005;21(3):206-213.
12. Kairy D, Lehoux P, Vincent C, Visintin M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil Rehabil. 2009;31(6):427-447.
13. McCue M, Fairman A, Pramuka M. Enhancing quality of life through telerehabilitation. Phys Med Rehabil Clin N Am. 2010;21(1):195-205.
14. Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638.
15. Pietrzak E, Cotea C, Pullman S, Nasveld P. Self-management and rehabilitation in osteoarthritis: is there a place for internet-based interventions? Telemed J E Health. 2013;19(10):800-805.
16. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213.
17. Kortke H, Stromeyer H, Zittermann A, et al. New East-Westfalian Postoperative Therapy Concept: A telemedicine guide for the study of ambulatory rehabilitation of patients after cardiac surgery. Telemed J E-Health. 2006;12(4):475-483.
18. Tousignant M, Boissy P, Corriveau H, Moffet H. In home telerehabilitation for older adults after discharge from an acute hospital or rehabilitation unit: A proof-of- concept study and costs estimation. Disabil Rehabil Assist Technol. 2006;1(4):209-216.
19. Sanford JA, Griffiths PC, Richardson P, et al. The effects of in-home rehabilitation on task self-efficacy in mobility-impaired adults: a randomized clinical trial. J Am Geriatr Soc. 2006;54(11):1641-1648.
20. Nakamura K, Takano T, Akao C. The effectiveness of videophones in home healthcare for the elderly. Med Care. 1999;37(2):117-125.
21. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370.
22. Guilfoyle C, Wootton R, Hassall S, et al. User satisfaction with allied health services delivered to residential facilities via videoconferencing. J Telemed Telecare. 2003;9(1):S52-S54.23. Mair F, Whitten P. Systematic review of studies of patient satisfaction with telemedicine. BMJ. 2000;320(7248):1517-1520.
24. Williams T L, May C R, Esmail A. Limitations of patient satisfaction studies in telehealthcare: a systematic review of the literature. Telemed J E-Health. 2001;7(4):293-316.
25. US Department of Veterans Affairs, Office of Telehealth Services. http://vaww.telehealth.va.gov/quality/data/index.asp. Accessed June 1, 2018. [Nonpublic document; source not verified.]
26. Jia H, Cowper D, Tang Y, et al. Post-acute stroke rehabilitation utilization: Are there difference between rural-urban patients and taxonomies? J Rural Health. 2012;28(3):242-247.
27. Cho S, Mathiassen L, Gallivan M. Crossing the chasm: from adoption to diffusion of a telehealth innovation. In: León G, Bernardos AM, Casar JR, Kautz K, De Gross JI, eds. Open IT-Based Innovation: Moving Towards Cooperative IT Transfer and Knowledge Diffusion. Boston, MA: Springer; 2008.
28. Broderick A, Lindeman D. Scaling telehealth programs: lessons from early adopters. https://www.commonwealthfund.org/publications/case-study/2013/jan/scaling-telehealth-programs-lessons-early-adopters. Published January 2013. Accessed June 1, 2018.