User login
Alopecia areata: Positive results reported for two investigational JAK inhibitors
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Study identifies skin biomarkers that predict newborn eczema risk
It might be possible to develop a simple test to identify newborn children who are at risk of later developing atopic dermatitis (AD), according to findings from a Danish prospective birth cohort study.
but also for having more severe disease.
“We are able to identify predictive immune biomarkers of atopic dermatitis using a noninvasive method that was not associated with any pain,” one of the study’s investigators, Anne-Sofie Halling, MD, said at a press briefing at the annual congress of the European Academy of Dermatology and Venereology.
“Importantly, we were able to predict atopic dermatitis occurring months after [sample] collection,” said Dr. Halling, who works at Bispebjerg Hospital and is a PhD student at the University of Copenhagen.
These findings could hopefully be used to help identify children “so that preventive strategies can target these children ... and decrease the incidence of this common disease,” she added.
AD is caused “by a complex interplay between skin barrier dysfunction and immune dysregulation,” Dr. Halling said, and it is “the first step in the so-called atopic march, where children also develop food allergy, asthma, and rhinitis.” Almost all cases of AD begin during the first years of life. Approximately 15%-20% of children can be affected, she noted, emphasizing the high burden of the disease and pointing out that strategies are shifting toward trying to prevent the disease in those at risk.
Copenhagen BABY cohort
This is where the BABY study comes in, Dr. Halling said. The study enrolled 450 children at birth and followed them until age 2 years. Gene mutation testing was performed at enrollment. All children underwent skin examination, and skin samples were taken using tape strips. Tape strips were applied to the back of the hand of children born at term and between the shoulder blades on the back of children who were premature.
Skin examinations were repeated, and skin samples were obtained again at age 2 months. They were taken again only if there were any signs of AD. For those diagnosed with AD, disease severity was assessed using the Eczema Area and Severity Index (EASI) by the treating physician. Children were excluded if they had AD at the time the tape strip testing was due to be performed.
Comparing term and preterm children
Dr. Halling noted that analyses were performed separately for the 300 children born at term and for the 150 who were preterm.
The prevalence of AD was higher among children born at term than among the preterm children (34.6% vs. 21.2%), and the median time to onset was shorter (6 months vs. 8 months). There were also differences in the EASI scores among those who developed AD; median scores were higher in the children born at term than in the preterm children (4.1 vs. 1.6).
More children born at term than preterm children had moderate to severe AD (23.3% vs. 8%), Dr. Halling reported.
TARC, IL-8, and IL-18 predictive of AD
Multiple immune biomarkers were tested, including various cytokines and filaggrin degradation products. On examination of skin samples collected at birth, no particular biomarkers were found at higher levels among children who developed AD in comparison with those who did not develop AD.
With regard to biomarkers examined in skin samples at 2 months of age, however, the results were different, Dr. Halling said. One particular cytokine, thymus and activation-regulated chemokine (TARC), was seen to double the risk of AD in the first 2 years of a child’s life.
This doubled risk was seen not only among the children born at term but also among those born preterm, although the data were only significant with regard to the children born at term.
The unadjusted hazard ratios and adjusted HRs (adjusted for parental atopy and filaggrin gene mutations) in term children were 2.11 (95% confidence interval, 1.36-3.26; P = .0008) and 1.85 (95% CI, 1.18-2.89; P = .007), respectively.
For preterm children, the HRs were 2.23 (95% CI, 0.85-5.86; P = .1) and 2.60 (95% CI, 0.98-6.85; P =.05), respectively.
These findings were in line with findings of other studies, Dr. Halling said. “It is well recognized that TARC is currently the best biomarker in patients with established atopic dermatitis.” Moreover, she reported that TARC was associated with a cumulative increase in the risk for AD and that levels were found to be higher in children in whom onset occurred at a later age than among those diagnosed before 6 months of age.
“This is important, as these findings shows that TARC levels predict atopic dermatitis that occurred many months later,” Dr. Halling said.
And, in term-born children at least, TARC upped the chances that the severity of AD would be greater than had it not been present (adjusted HR, 4.65; 95% CI, 1.91-11.31; P = .0007).
Increased levels of interleukin-8 (IL-8) and IL-18 at 2 months of age were also found to be predictive of having moderate to severe AD. The risk was more than double in comparison with those in whom levels were not increased, again only in term-born children.
‘Stimulating and interesting findings’
These data are “very stimulating and interesting,” Dedee Murrell, MD, professor and head of the department of dermatology at St. George Hospital, University of New South Wales, Sydney, observed at the press briefing.
“You found this significant association mainly in the newborn children born at term, and the association in the preterm babies wasn’t as high. Is that anything to do with how they were taken care of in the hospital?” Dr. Murrell asked.
“That’s a really good question,” Dr. Halling said. “Maybe they need to be exposed for a month or two before we are actually able to identify which children will develop atopic dermatitis.”
The study was funded by the Lundbeck Foundation. Dr. Halling has acted as a consultant for Coloplast and as a speaker for Leo Pharma. Dr. Murrell has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It might be possible to develop a simple test to identify newborn children who are at risk of later developing atopic dermatitis (AD), according to findings from a Danish prospective birth cohort study.
but also for having more severe disease.
“We are able to identify predictive immune biomarkers of atopic dermatitis using a noninvasive method that was not associated with any pain,” one of the study’s investigators, Anne-Sofie Halling, MD, said at a press briefing at the annual congress of the European Academy of Dermatology and Venereology.
“Importantly, we were able to predict atopic dermatitis occurring months after [sample] collection,” said Dr. Halling, who works at Bispebjerg Hospital and is a PhD student at the University of Copenhagen.
These findings could hopefully be used to help identify children “so that preventive strategies can target these children ... and decrease the incidence of this common disease,” she added.
AD is caused “by a complex interplay between skin barrier dysfunction and immune dysregulation,” Dr. Halling said, and it is “the first step in the so-called atopic march, where children also develop food allergy, asthma, and rhinitis.” Almost all cases of AD begin during the first years of life. Approximately 15%-20% of children can be affected, she noted, emphasizing the high burden of the disease and pointing out that strategies are shifting toward trying to prevent the disease in those at risk.
Copenhagen BABY cohort
This is where the BABY study comes in, Dr. Halling said. The study enrolled 450 children at birth and followed them until age 2 years. Gene mutation testing was performed at enrollment. All children underwent skin examination, and skin samples were taken using tape strips. Tape strips were applied to the back of the hand of children born at term and between the shoulder blades on the back of children who were premature.
Skin examinations were repeated, and skin samples were obtained again at age 2 months. They were taken again only if there were any signs of AD. For those diagnosed with AD, disease severity was assessed using the Eczema Area and Severity Index (EASI) by the treating physician. Children were excluded if they had AD at the time the tape strip testing was due to be performed.
Comparing term and preterm children
Dr. Halling noted that analyses were performed separately for the 300 children born at term and for the 150 who were preterm.
The prevalence of AD was higher among children born at term than among the preterm children (34.6% vs. 21.2%), and the median time to onset was shorter (6 months vs. 8 months). There were also differences in the EASI scores among those who developed AD; median scores were higher in the children born at term than in the preterm children (4.1 vs. 1.6).
More children born at term than preterm children had moderate to severe AD (23.3% vs. 8%), Dr. Halling reported.
TARC, IL-8, and IL-18 predictive of AD
Multiple immune biomarkers were tested, including various cytokines and filaggrin degradation products. On examination of skin samples collected at birth, no particular biomarkers were found at higher levels among children who developed AD in comparison with those who did not develop AD.
With regard to biomarkers examined in skin samples at 2 months of age, however, the results were different, Dr. Halling said. One particular cytokine, thymus and activation-regulated chemokine (TARC), was seen to double the risk of AD in the first 2 years of a child’s life.
This doubled risk was seen not only among the children born at term but also among those born preterm, although the data were only significant with regard to the children born at term.
The unadjusted hazard ratios and adjusted HRs (adjusted for parental atopy and filaggrin gene mutations) in term children were 2.11 (95% confidence interval, 1.36-3.26; P = .0008) and 1.85 (95% CI, 1.18-2.89; P = .007), respectively.
For preterm children, the HRs were 2.23 (95% CI, 0.85-5.86; P = .1) and 2.60 (95% CI, 0.98-6.85; P =.05), respectively.
These findings were in line with findings of other studies, Dr. Halling said. “It is well recognized that TARC is currently the best biomarker in patients with established atopic dermatitis.” Moreover, she reported that TARC was associated with a cumulative increase in the risk for AD and that levels were found to be higher in children in whom onset occurred at a later age than among those diagnosed before 6 months of age.
“This is important, as these findings shows that TARC levels predict atopic dermatitis that occurred many months later,” Dr. Halling said.
And, in term-born children at least, TARC upped the chances that the severity of AD would be greater than had it not been present (adjusted HR, 4.65; 95% CI, 1.91-11.31; P = .0007).
Increased levels of interleukin-8 (IL-8) and IL-18 at 2 months of age were also found to be predictive of having moderate to severe AD. The risk was more than double in comparison with those in whom levels were not increased, again only in term-born children.
‘Stimulating and interesting findings’
These data are “very stimulating and interesting,” Dedee Murrell, MD, professor and head of the department of dermatology at St. George Hospital, University of New South Wales, Sydney, observed at the press briefing.
“You found this significant association mainly in the newborn children born at term, and the association in the preterm babies wasn’t as high. Is that anything to do with how they were taken care of in the hospital?” Dr. Murrell asked.
“That’s a really good question,” Dr. Halling said. “Maybe they need to be exposed for a month or two before we are actually able to identify which children will develop atopic dermatitis.”
The study was funded by the Lundbeck Foundation. Dr. Halling has acted as a consultant for Coloplast and as a speaker for Leo Pharma. Dr. Murrell has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It might be possible to develop a simple test to identify newborn children who are at risk of later developing atopic dermatitis (AD), according to findings from a Danish prospective birth cohort study.
but also for having more severe disease.
“We are able to identify predictive immune biomarkers of atopic dermatitis using a noninvasive method that was not associated with any pain,” one of the study’s investigators, Anne-Sofie Halling, MD, said at a press briefing at the annual congress of the European Academy of Dermatology and Venereology.
“Importantly, we were able to predict atopic dermatitis occurring months after [sample] collection,” said Dr. Halling, who works at Bispebjerg Hospital and is a PhD student at the University of Copenhagen.
These findings could hopefully be used to help identify children “so that preventive strategies can target these children ... and decrease the incidence of this common disease,” she added.
AD is caused “by a complex interplay between skin barrier dysfunction and immune dysregulation,” Dr. Halling said, and it is “the first step in the so-called atopic march, where children also develop food allergy, asthma, and rhinitis.” Almost all cases of AD begin during the first years of life. Approximately 15%-20% of children can be affected, she noted, emphasizing the high burden of the disease and pointing out that strategies are shifting toward trying to prevent the disease in those at risk.
Copenhagen BABY cohort
This is where the BABY study comes in, Dr. Halling said. The study enrolled 450 children at birth and followed them until age 2 years. Gene mutation testing was performed at enrollment. All children underwent skin examination, and skin samples were taken using tape strips. Tape strips were applied to the back of the hand of children born at term and between the shoulder blades on the back of children who were premature.
Skin examinations were repeated, and skin samples were obtained again at age 2 months. They were taken again only if there were any signs of AD. For those diagnosed with AD, disease severity was assessed using the Eczema Area and Severity Index (EASI) by the treating physician. Children were excluded if they had AD at the time the tape strip testing was due to be performed.
Comparing term and preterm children
Dr. Halling noted that analyses were performed separately for the 300 children born at term and for the 150 who were preterm.
The prevalence of AD was higher among children born at term than among the preterm children (34.6% vs. 21.2%), and the median time to onset was shorter (6 months vs. 8 months). There were also differences in the EASI scores among those who developed AD; median scores were higher in the children born at term than in the preterm children (4.1 vs. 1.6).
More children born at term than preterm children had moderate to severe AD (23.3% vs. 8%), Dr. Halling reported.
TARC, IL-8, and IL-18 predictive of AD
Multiple immune biomarkers were tested, including various cytokines and filaggrin degradation products. On examination of skin samples collected at birth, no particular biomarkers were found at higher levels among children who developed AD in comparison with those who did not develop AD.
With regard to biomarkers examined in skin samples at 2 months of age, however, the results were different, Dr. Halling said. One particular cytokine, thymus and activation-regulated chemokine (TARC), was seen to double the risk of AD in the first 2 years of a child’s life.
This doubled risk was seen not only among the children born at term but also among those born preterm, although the data were only significant with regard to the children born at term.
The unadjusted hazard ratios and adjusted HRs (adjusted for parental atopy and filaggrin gene mutations) in term children were 2.11 (95% confidence interval, 1.36-3.26; P = .0008) and 1.85 (95% CI, 1.18-2.89; P = .007), respectively.
For preterm children, the HRs were 2.23 (95% CI, 0.85-5.86; P = .1) and 2.60 (95% CI, 0.98-6.85; P =.05), respectively.
These findings were in line with findings of other studies, Dr. Halling said. “It is well recognized that TARC is currently the best biomarker in patients with established atopic dermatitis.” Moreover, she reported that TARC was associated with a cumulative increase in the risk for AD and that levels were found to be higher in children in whom onset occurred at a later age than among those diagnosed before 6 months of age.
“This is important, as these findings shows that TARC levels predict atopic dermatitis that occurred many months later,” Dr. Halling said.
And, in term-born children at least, TARC upped the chances that the severity of AD would be greater than had it not been present (adjusted HR, 4.65; 95% CI, 1.91-11.31; P = .0007).
Increased levels of interleukin-8 (IL-8) and IL-18 at 2 months of age were also found to be predictive of having moderate to severe AD. The risk was more than double in comparison with those in whom levels were not increased, again only in term-born children.
‘Stimulating and interesting findings’
These data are “very stimulating and interesting,” Dedee Murrell, MD, professor and head of the department of dermatology at St. George Hospital, University of New South Wales, Sydney, observed at the press briefing.
“You found this significant association mainly in the newborn children born at term, and the association in the preterm babies wasn’t as high. Is that anything to do with how they were taken care of in the hospital?” Dr. Murrell asked.
“That’s a really good question,” Dr. Halling said. “Maybe they need to be exposed for a month or two before we are actually able to identify which children will develop atopic dermatitis.”
The study was funded by the Lundbeck Foundation. Dr. Halling has acted as a consultant for Coloplast and as a speaker for Leo Pharma. Dr. Murrell has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
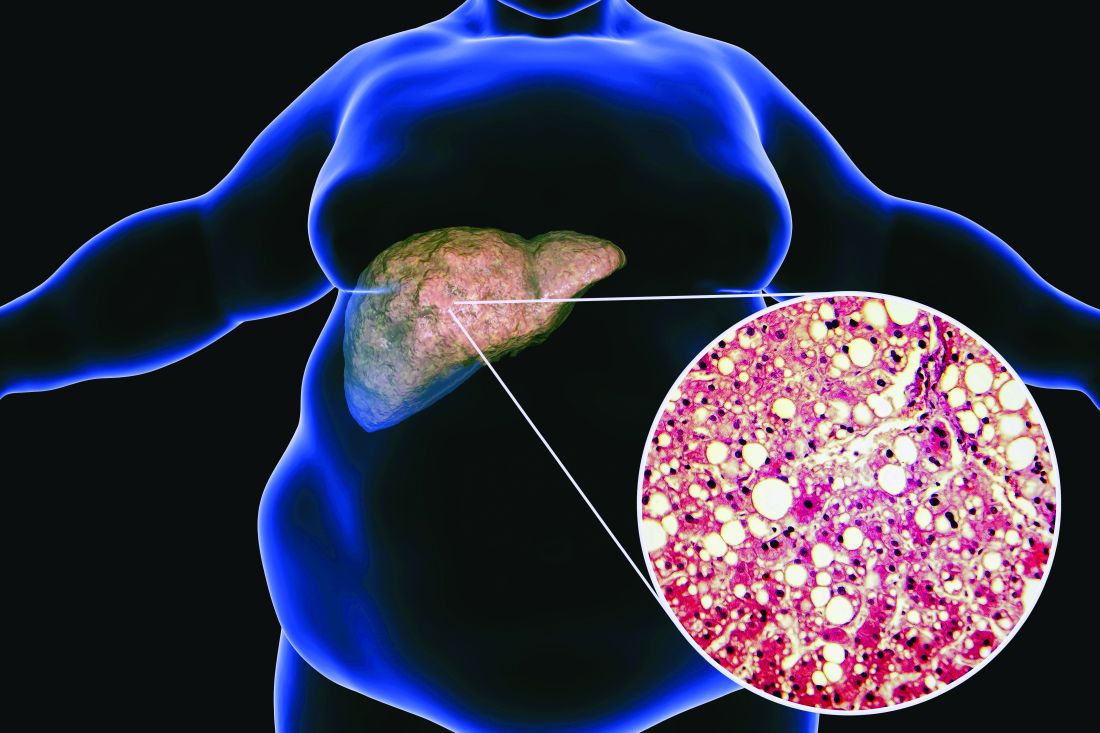
Ezetimibe-statin combo lowers liver fat in open-label trial
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
FROM EASD 2022
Early age at hysterectomy ups type 2 diabetes risk
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
FROM EASD 2022
Mothers’ diabetes linked to ADHD in their children
Children born to women who develop diabetes either before or during their pregnancy could be at risk for developing attention-deficit/hyperactivity disorder, data from a large multinational cohort study appear to show.
Considering more than 4.5 million mother-child pairs, it was found that children whose mothers had diabetes around the time of their pregnancy were 16% more likely to have ADHD diagnosed than were those whose mothers did not.
An increased risk was seen regardless of the type of diabetes, and regardless of whether or not the diabetes was present before or appeared during the pregnancy.
“We found a small increased risk of ADHD in children born to mothers with diabetes, including pregestational diabetes and gestational diabetes,” Carolyn Cesta, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Cesta, a postdoctoral researcher in the Centre for Pharmacoepidemiology at the Karolinska Institutet in Stockholm noted that the effect sizes seen were lower than had been reported previously.
“This may be because we adjusted for a large number of covariates, including maternal ADHD and psychiatric disorders,” Dr. Cesta said.
ADHD and diabetes
“Previous studies have reported an increase in the risk of ADHD in children born to mothers with diabetes,” explained Dr. Cesta.
However, “these studies have been limited by the use of self-reported data, small sample sizes, lack of adjustment for important confounders, and they’re often limited to [White] populations,” she added. “There’s a lot of heterogeneity between these studies,” she said.
To try to iron out the differences seen in the prior studies, Dr. Cesta and associates looked at data from several databases based in Hong Kong (Clinical Data Analysis and Reporting System), four Nordic countries (Population Health Registers for Finland, Iceland, Norway, and Sweden), and Taiwan (National Health Insurance Database).
To create the matched mother-child pairs, the databases were searched to find women who had children born between 2001 and 2018, and who had follow-up data available up to 2020 on not only their diabetes status and child’s ADHD status, but also other parameters, such as other maternal diagnoses, maternal medications, and a host of sociodemographic factors.
More than 24 potentially confounding or covariates were considered in the analysis, which used Cox proportional hazard regression modeling and propensity score analysis to calculate hazard ratios with 95% confidence intervals.
“We looked at whether [mothers] had a diagnosis of ADHD themselves, or other psychiatric disorders, because there is high heritability for these disorders,” Dr. Cesta said, indicating that all bases had endeavored to be covered.
Main findings
Results showed some differences in the prevalence of diabetes and ADHD between the three cohorts used in the analysis. The prevalence of any maternal diabetes ranged from 8.8% in the Hong Kong cohort to 3.3% in the Taiwan cohort, with a prevalence of 6.8% for the Nordic cohort.
Rates of pregestational diabetes were lowest in the Taiwan and Hong Kong cohorts, at 0.2% and 0.5%, respectively, and 2.2% in the Nordic cohort. Gestational diabetes rates were a respective 3.1%, 7.8%, and 4.6%.
The highest rate of ADHD in children was seen in the Taiwan cohort, at 9.6%, followed by 4.2% for the Hong Kong cohort, and 2.6% for the Nordic cohort.
The hazard ratio for having childhood ADHD was 1.16 when comparing any maternal diabetes to no maternal diabetes, 1.40 comparing mothers with and without pregestational diabetes, and a respective 1.36 and 1.37 comparing those with and without type 1 diabetes, and those with and without type 2 diabetes.
The HR for childhood ADHD comparing mothers with and without gestational diabetes was 1.13.
“Within the analysis for gestational diabetes, we had enough numbers to look at siblings that are discordant for maternal gestational diabetes,” Dr. Cesta said. Essentially “we’re comparing two siblings from the same mother, one that was exposed to gestational diabetes, one that wasn’t,” she explained.
Interestingly there was no association between ADHD and maternal gestational diabetes in the sibling analysis (HR, 1.0).
“When it comes to gestational diabetes, the evidence from our sibling analysis indicate that the association may actually be confounded by shared genetics and environmental factors,” said Dr. Cesta.
“So, future studies should explore the role of specific genetic factors in glycemic control during pregnancy and the relationship between maternal diabetes and ADHD.”
Answering long-standing questions
These data will help a lot in answering questions that clinicians have been asking themselves a long time, commented Jardena Puder, MD, who chaired the session.
“It still remains a bit puzzling that genetic and environmental factors could be responsible, if you see the same effect in type 1 [diabetes], and in type 2 [diabetes], and gestational diabetes,” said Dr. Puder, who is an endocrinologist and diabetologist at the woman-mother-child department at the Vaud University Hospital Center, Lausanne, Switzerland.
Type 1 and type 2 are “very distinct” in terms of the genetic and environmental factors involved, “so, the fact that you see [the effect] in both remains a bit puzzling,” said Dr. Puder.
“I wish we had the numbers to be able to do the sibling analysis for type 1 and type 2, just to see if we could tease anything out,” said Dr. Cesta.
“I do think this is part of the bigger question of what the relationship is between, like, metabolic disorders and psychiatric disorders, because even outside of pregnancy, we see that there’s often a comorbidity with them. So, it’s a good point.”
The next step is to look at the role of treatment and what effects glycemic control might have on the small, but still apparent, association between maternal diabetes and ADHD.
The study had multiple funders including the Hong Kong Research Grant Council, NordForsk, the Research Council of Norway, the Norwegian ADHD Research Network, the Hong Kong Innovation and Technology Commission, and European Horizon 2020.
Dr. Cesta had no conflicts of interest to disclose. Dr. Puder chaired the session in which the findings were presented and made no specific disclosures.
Children born to women who develop diabetes either before or during their pregnancy could be at risk for developing attention-deficit/hyperactivity disorder, data from a large multinational cohort study appear to show.
Considering more than 4.5 million mother-child pairs, it was found that children whose mothers had diabetes around the time of their pregnancy were 16% more likely to have ADHD diagnosed than were those whose mothers did not.
An increased risk was seen regardless of the type of diabetes, and regardless of whether or not the diabetes was present before or appeared during the pregnancy.
“We found a small increased risk of ADHD in children born to mothers with diabetes, including pregestational diabetes and gestational diabetes,” Carolyn Cesta, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Cesta, a postdoctoral researcher in the Centre for Pharmacoepidemiology at the Karolinska Institutet in Stockholm noted that the effect sizes seen were lower than had been reported previously.
“This may be because we adjusted for a large number of covariates, including maternal ADHD and psychiatric disorders,” Dr. Cesta said.
ADHD and diabetes
“Previous studies have reported an increase in the risk of ADHD in children born to mothers with diabetes,” explained Dr. Cesta.
However, “these studies have been limited by the use of self-reported data, small sample sizes, lack of adjustment for important confounders, and they’re often limited to [White] populations,” she added. “There’s a lot of heterogeneity between these studies,” she said.
To try to iron out the differences seen in the prior studies, Dr. Cesta and associates looked at data from several databases based in Hong Kong (Clinical Data Analysis and Reporting System), four Nordic countries (Population Health Registers for Finland, Iceland, Norway, and Sweden), and Taiwan (National Health Insurance Database).
To create the matched mother-child pairs, the databases were searched to find women who had children born between 2001 and 2018, and who had follow-up data available up to 2020 on not only their diabetes status and child’s ADHD status, but also other parameters, such as other maternal diagnoses, maternal medications, and a host of sociodemographic factors.
More than 24 potentially confounding or covariates were considered in the analysis, which used Cox proportional hazard regression modeling and propensity score analysis to calculate hazard ratios with 95% confidence intervals.
“We looked at whether [mothers] had a diagnosis of ADHD themselves, or other psychiatric disorders, because there is high heritability for these disorders,” Dr. Cesta said, indicating that all bases had endeavored to be covered.
Main findings
Results showed some differences in the prevalence of diabetes and ADHD between the three cohorts used in the analysis. The prevalence of any maternal diabetes ranged from 8.8% in the Hong Kong cohort to 3.3% in the Taiwan cohort, with a prevalence of 6.8% for the Nordic cohort.
Rates of pregestational diabetes were lowest in the Taiwan and Hong Kong cohorts, at 0.2% and 0.5%, respectively, and 2.2% in the Nordic cohort. Gestational diabetes rates were a respective 3.1%, 7.8%, and 4.6%.
The highest rate of ADHD in children was seen in the Taiwan cohort, at 9.6%, followed by 4.2% for the Hong Kong cohort, and 2.6% for the Nordic cohort.
The hazard ratio for having childhood ADHD was 1.16 when comparing any maternal diabetes to no maternal diabetes, 1.40 comparing mothers with and without pregestational diabetes, and a respective 1.36 and 1.37 comparing those with and without type 1 diabetes, and those with and without type 2 diabetes.
The HR for childhood ADHD comparing mothers with and without gestational diabetes was 1.13.
“Within the analysis for gestational diabetes, we had enough numbers to look at siblings that are discordant for maternal gestational diabetes,” Dr. Cesta said. Essentially “we’re comparing two siblings from the same mother, one that was exposed to gestational diabetes, one that wasn’t,” she explained.
Interestingly there was no association between ADHD and maternal gestational diabetes in the sibling analysis (HR, 1.0).
“When it comes to gestational diabetes, the evidence from our sibling analysis indicate that the association may actually be confounded by shared genetics and environmental factors,” said Dr. Cesta.
“So, future studies should explore the role of specific genetic factors in glycemic control during pregnancy and the relationship between maternal diabetes and ADHD.”
Answering long-standing questions
These data will help a lot in answering questions that clinicians have been asking themselves a long time, commented Jardena Puder, MD, who chaired the session.
“It still remains a bit puzzling that genetic and environmental factors could be responsible, if you see the same effect in type 1 [diabetes], and in type 2 [diabetes], and gestational diabetes,” said Dr. Puder, who is an endocrinologist and diabetologist at the woman-mother-child department at the Vaud University Hospital Center, Lausanne, Switzerland.
Type 1 and type 2 are “very distinct” in terms of the genetic and environmental factors involved, “so, the fact that you see [the effect] in both remains a bit puzzling,” said Dr. Puder.
“I wish we had the numbers to be able to do the sibling analysis for type 1 and type 2, just to see if we could tease anything out,” said Dr. Cesta.
“I do think this is part of the bigger question of what the relationship is between, like, metabolic disorders and psychiatric disorders, because even outside of pregnancy, we see that there’s often a comorbidity with them. So, it’s a good point.”
The next step is to look at the role of treatment and what effects glycemic control might have on the small, but still apparent, association between maternal diabetes and ADHD.
The study had multiple funders including the Hong Kong Research Grant Council, NordForsk, the Research Council of Norway, the Norwegian ADHD Research Network, the Hong Kong Innovation and Technology Commission, and European Horizon 2020.
Dr. Cesta had no conflicts of interest to disclose. Dr. Puder chaired the session in which the findings were presented and made no specific disclosures.
Children born to women who develop diabetes either before or during their pregnancy could be at risk for developing attention-deficit/hyperactivity disorder, data from a large multinational cohort study appear to show.
Considering more than 4.5 million mother-child pairs, it was found that children whose mothers had diabetes around the time of their pregnancy were 16% more likely to have ADHD diagnosed than were those whose mothers did not.
An increased risk was seen regardless of the type of diabetes, and regardless of whether or not the diabetes was present before or appeared during the pregnancy.
“We found a small increased risk of ADHD in children born to mothers with diabetes, including pregestational diabetes and gestational diabetes,” Carolyn Cesta, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Cesta, a postdoctoral researcher in the Centre for Pharmacoepidemiology at the Karolinska Institutet in Stockholm noted that the effect sizes seen were lower than had been reported previously.
“This may be because we adjusted for a large number of covariates, including maternal ADHD and psychiatric disorders,” Dr. Cesta said.
ADHD and diabetes
“Previous studies have reported an increase in the risk of ADHD in children born to mothers with diabetes,” explained Dr. Cesta.
However, “these studies have been limited by the use of self-reported data, small sample sizes, lack of adjustment for important confounders, and they’re often limited to [White] populations,” she added. “There’s a lot of heterogeneity between these studies,” she said.
To try to iron out the differences seen in the prior studies, Dr. Cesta and associates looked at data from several databases based in Hong Kong (Clinical Data Analysis and Reporting System), four Nordic countries (Population Health Registers for Finland, Iceland, Norway, and Sweden), and Taiwan (National Health Insurance Database).
To create the matched mother-child pairs, the databases were searched to find women who had children born between 2001 and 2018, and who had follow-up data available up to 2020 on not only their diabetes status and child’s ADHD status, but also other parameters, such as other maternal diagnoses, maternal medications, and a host of sociodemographic factors.
More than 24 potentially confounding or covariates were considered in the analysis, which used Cox proportional hazard regression modeling and propensity score analysis to calculate hazard ratios with 95% confidence intervals.
“We looked at whether [mothers] had a diagnosis of ADHD themselves, or other psychiatric disorders, because there is high heritability for these disorders,” Dr. Cesta said, indicating that all bases had endeavored to be covered.
Main findings
Results showed some differences in the prevalence of diabetes and ADHD between the three cohorts used in the analysis. The prevalence of any maternal diabetes ranged from 8.8% in the Hong Kong cohort to 3.3% in the Taiwan cohort, with a prevalence of 6.8% for the Nordic cohort.
Rates of pregestational diabetes were lowest in the Taiwan and Hong Kong cohorts, at 0.2% and 0.5%, respectively, and 2.2% in the Nordic cohort. Gestational diabetes rates were a respective 3.1%, 7.8%, and 4.6%.
The highest rate of ADHD in children was seen in the Taiwan cohort, at 9.6%, followed by 4.2% for the Hong Kong cohort, and 2.6% for the Nordic cohort.
The hazard ratio for having childhood ADHD was 1.16 when comparing any maternal diabetes to no maternal diabetes, 1.40 comparing mothers with and without pregestational diabetes, and a respective 1.36 and 1.37 comparing those with and without type 1 diabetes, and those with and without type 2 diabetes.
The HR for childhood ADHD comparing mothers with and without gestational diabetes was 1.13.
“Within the analysis for gestational diabetes, we had enough numbers to look at siblings that are discordant for maternal gestational diabetes,” Dr. Cesta said. Essentially “we’re comparing two siblings from the same mother, one that was exposed to gestational diabetes, one that wasn’t,” she explained.
Interestingly there was no association between ADHD and maternal gestational diabetes in the sibling analysis (HR, 1.0).
“When it comes to gestational diabetes, the evidence from our sibling analysis indicate that the association may actually be confounded by shared genetics and environmental factors,” said Dr. Cesta.
“So, future studies should explore the role of specific genetic factors in glycemic control during pregnancy and the relationship between maternal diabetes and ADHD.”
Answering long-standing questions
These data will help a lot in answering questions that clinicians have been asking themselves a long time, commented Jardena Puder, MD, who chaired the session.
“It still remains a bit puzzling that genetic and environmental factors could be responsible, if you see the same effect in type 1 [diabetes], and in type 2 [diabetes], and gestational diabetes,” said Dr. Puder, who is an endocrinologist and diabetologist at the woman-mother-child department at the Vaud University Hospital Center, Lausanne, Switzerland.
Type 1 and type 2 are “very distinct” in terms of the genetic and environmental factors involved, “so, the fact that you see [the effect] in both remains a bit puzzling,” said Dr. Puder.
“I wish we had the numbers to be able to do the sibling analysis for type 1 and type 2, just to see if we could tease anything out,” said Dr. Cesta.
“I do think this is part of the bigger question of what the relationship is between, like, metabolic disorders and psychiatric disorders, because even outside of pregnancy, we see that there’s often a comorbidity with them. So, it’s a good point.”
The next step is to look at the role of treatment and what effects glycemic control might have on the small, but still apparent, association between maternal diabetes and ADHD.
The study had multiple funders including the Hong Kong Research Grant Council, NordForsk, the Research Council of Norway, the Norwegian ADHD Research Network, the Hong Kong Innovation and Technology Commission, and European Horizon 2020.
Dr. Cesta had no conflicts of interest to disclose. Dr. Puder chaired the session in which the findings were presented and made no specific disclosures.
FROM EASD 2022
Apremilast alleviates severe psoriasis in some children, data show
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
FROM EADV 2022
Hope shines bright for hidradenitis suppurativa treatments
in separate trials.
Around 40%-50% of patients exhibited a clinical response to these agents at 16 weeks, a leading HS expert reported at the annual congress of the European Academy of Dermatology and Venereology.
Time in the spotlight for HS
Research into HS is “an incredibly active field at this moment,” said Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, and president and chief executive officer of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center, Boston.
It’s “been great for advancing our understanding of the biology and the treatments that we will be able to use,” she said.
During the late-breaking sessions at the annual EADV Congress, Dr. Kimball presented data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety and tolerability of the interleukin (IL) 17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS.
“This is only the second phase 3 program we have ever seen in HS and the first one since 2016,” Dr. Kimball said of the trials. It’s also the largest trial program in HS conducted to date, she added, “so it really is a milestone.”
The last big development was when adalimumab, a tumor necrosis factor (TNF) blocker, gained regulatory approval for HS in 2016, observed Neil Patel, PhD, MRCP, who leads the HS service at Imperial College Healthcare NHS Trust in London.
“Adalimumab has been very helpful for many patients, but not all patients respond, and others may respond initially but then the treatment starts to fail after a year or 2,” Dr. Patel said in an interview with this news organization.
“There is definitely a huge need for alternative medication for this condition, which still has a lack of effective treatment options,” added Dr. Patel, who was not involved in either of the studies.
“One major upside for secukinumab is that its safety profile is generally very good and familiarity in the dermatologic community is already well established,” Christopher Sayed, MD, said in a separate interview.
“This will make most providers very comfortable offering it as a potential treatment option sooner rather than later given that its efficacy has now been demonstrated in phase 3 trials,” added Dr. Sayed, associate professor of dermatology at the University of North Carolina at Chapel Hill.
Two identically designed trials
Altogether, SUNSHINE and SUNRISE enrolled just over 1,000 patients at 219 sites in 33 countries. Both trials were identical in their design: A 4-week run-in phase before a randomized, double-blind treatment phase that tested two dosing regimens of secukinumab (300 mg administered subcutaneously) every 2 or 4 weeks vs. placebo for 16 weeks. The trial continued after this time, with patients in the placebo arm re–randomly assigned to treatment with one of the two secukinumab regimens out to a year.
The primary endpoint was the percentage of patients achieving a Hidradenitis Suppurativa Clinical Response (HiSCR) after 16 weeks of treatment, with key secondary endpoints, which were abscess and inflammatory nodule (AN) count, occurrence of flares, and at least a 30% reduction in Patient’s Global Assessment of Skin Pain assessed using a numeric rating scale (NPRS30).
Secukinumab superior to placebo
The HiSCR is defined as at least a 50% decrease in AN count with no increase in the number of abscesses or in the number of draining fistulas relative to baseline. This was achieved by about 42%-45% of patients who received secukinumab every 2 weeks, about 42%-46% of those who received secukinumab every 4 weeks, and about 31%-33% of those on placebo in both studies.
Of note, fewer patients treated with secukinumab (about 15%-20% among those treated every 2 weeks, and about 15% to 23% among those treated every 4 weeks) than those on placebo (27%-29%) experienced flares, defined as at least a 25% increase in AN count and at least a two-point increase relative to baseline values.
Improvement in HS pain can be a difficult parameter to meet, Dr. Kimball noted. “Pain is such an important feature of this disease as it so debilitating for the patients.” More than one-third (almost 36%-39%) of patients given secukinumab vs. just over a quarter (26.9%) given placebo achieved at least a 30% reduction in NPRS30 ratings, she reported. The difference between active and placebo treatment was significant only when secukinumab was given every 2 weeks, however.
“The placebo rates that we see in these studies are exactly parallel to what we saw in other studies, and other disease states when we had a 50% bar of improvement,” Dr. Kimball said when questioned about these results.
“HS is a highly variable disease; it’s maybe not so much the placebo rate or the scoring system used but maybe the 50% bar set for improvement is too low. It’s likely, as data start to mature and a 75% HiSCR can be calculated, that the placebo rates will drop,” she said.
There were no surprises when it came to the safety of secukinumab, being an old player in a new game, she noted. It was “well tolerated” and tolerability was “consistent with the known safety profile,” Dr. Kimball said, “so we expect it to be a new, safe, and effective add to our armamentarium in treating this disease.”
This research involves “basically borrowing drugs from other areas and trying them in HS to see what effect they may have,” Dr. Patel said, noting that drugs such as adalimumab and secukinumab already had a proven track record in other diseases, such as psoriasis. “These early data for secukinumab definitely are very exciting, but we would need to see real-life results” in patients with HS who are not enrolled in trials to see the benefits, he added.
‘Tipping point’ for HS research
“I think we will look back on this meeting and realize that it was an incredibly important tipping point for the treatment of this incredibly debilitating disease,” Dr. Kimball said.
Elsewhere at the meeting, she had presented findings from a phase 2a study that pitted three different kinase inhibitors with different modes of action against each other and compared them with placebo.
The three agents evaluated are an IL-1 receptor–associated kinase 4 inhibitor known as PF-06650833, a tyrosine kinase 2 (TYK2) JAK1 inhibitor brepocitinib, and the TYK2 inhibitor PF-06826647.
“This technique has been used in oncology,” Dr. Kimball said, noting that the ability to test multiple drugs at the same time “means we can really much more efficiently test two different things at the same time, and also put fewer patients at risk for potential problems if drugs don’t work.”
Positive signs for brepocitinib, not the other kinases tested
The results showed that though brepocitinib worked in HS, the other two novel compounds did not appear to have beneficial effects. Just over half (52%) of the 52 patients treated with brepocitinib achieved an HiSCR at 16 weeks, compared with around one-third of those given placebo, PF-06650833, or PF-06826647.
A similar benefit was seen in terms of reduction in flares for brepocitinib but not the other agents, although there was no difference between them all in terms of NPRS30 pain reduction.
“We’ve been able to test three different modalities. This tells us some things about the pathophysiology for HS, which is a very profoundly intensive inflammatory process,” which, Dr. Kimball said, “may require multiple modalities of action to get it under control.” In addition, these “general modalities seem to safe and well tolerated,” she added.
Take-homes for practice and future research
“While it is disappointing that two of the drugs tested did not clearly demonstrate efficacy, it is very possible that these mechanisms of action may be successful targets in the future as new dosing strategies and drugs targeting these pathways are developed,” Dr. Sayed said.
A case in point, he added, was that “adalimumab did not meet treatment endpoints at a dose of 40 mg every other week, but clearly has made a major impact at 40 mg weekly.”
The bottom line is that “both secukinumab and beprocitinib demonstrated efficacy over placebo and are likely to be helpful for a significant number of patients with HS,” Dr. Sayed said. “Hopefully, we’ll see head-to-head trials and more data regarding proportions of patients with deeper responses using criteria such as HiSCR75 and HiSCR90.”
Moreover, “having a larger number of drugs with a range of mechanisms of action is extraordinarily helpful given how difficult the disease can be to manage. We will hopefully continue to see creative approaches and further successes in the current wave of phase 1, 2, and 3 trials that are already underway.”
The SUNSHINE and SUNRISE studies were funded by Novartis Pharma AG, Basel, Switzerland. The phase 2A study Dr. Kimball presented was sponsored by Pfizer.
Dr. Kimball disclosed ties to both Novartis and Pfizer and acts as a consultant and investigator to AbbVie, Bristol-Myers Squibb, Janssen, Eli Lilly, Novartis, and UCB. She is an investigator for Incyte and AnaptysBio; acts as a consultant to Bayer, Boehringer Ingelheim, Ventyz, Moonlake, Lily, Concert, EvoImmune, Sonoma Bio, and Sanofi; receives fellowship funding from Janssen, and serves on the Board of Directors for Almirall.
Dr. Patel had no conflicts of interest to disclose. Dr. Sayed is the director of the HS Foundation, a nonprofit organization, and has acted as an adviser or consultant to, speaker for, and received research funding from multiple drug companies including AbbVie, ChemoCentryx, Incyte, InflaRx, Novartis, and UCB.
A version of this article first appeared on Medscape.com.
in separate trials.
Around 40%-50% of patients exhibited a clinical response to these agents at 16 weeks, a leading HS expert reported at the annual congress of the European Academy of Dermatology and Venereology.
Time in the spotlight for HS
Research into HS is “an incredibly active field at this moment,” said Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, and president and chief executive officer of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center, Boston.
It’s “been great for advancing our understanding of the biology and the treatments that we will be able to use,” she said.
During the late-breaking sessions at the annual EADV Congress, Dr. Kimball presented data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety and tolerability of the interleukin (IL) 17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS.
“This is only the second phase 3 program we have ever seen in HS and the first one since 2016,” Dr. Kimball said of the trials. It’s also the largest trial program in HS conducted to date, she added, “so it really is a milestone.”
The last big development was when adalimumab, a tumor necrosis factor (TNF) blocker, gained regulatory approval for HS in 2016, observed Neil Patel, PhD, MRCP, who leads the HS service at Imperial College Healthcare NHS Trust in London.
“Adalimumab has been very helpful for many patients, but not all patients respond, and others may respond initially but then the treatment starts to fail after a year or 2,” Dr. Patel said in an interview with this news organization.
“There is definitely a huge need for alternative medication for this condition, which still has a lack of effective treatment options,” added Dr. Patel, who was not involved in either of the studies.
“One major upside for secukinumab is that its safety profile is generally very good and familiarity in the dermatologic community is already well established,” Christopher Sayed, MD, said in a separate interview.
“This will make most providers very comfortable offering it as a potential treatment option sooner rather than later given that its efficacy has now been demonstrated in phase 3 trials,” added Dr. Sayed, associate professor of dermatology at the University of North Carolina at Chapel Hill.
Two identically designed trials
Altogether, SUNSHINE and SUNRISE enrolled just over 1,000 patients at 219 sites in 33 countries. Both trials were identical in their design: A 4-week run-in phase before a randomized, double-blind treatment phase that tested two dosing regimens of secukinumab (300 mg administered subcutaneously) every 2 or 4 weeks vs. placebo for 16 weeks. The trial continued after this time, with patients in the placebo arm re–randomly assigned to treatment with one of the two secukinumab regimens out to a year.
The primary endpoint was the percentage of patients achieving a Hidradenitis Suppurativa Clinical Response (HiSCR) after 16 weeks of treatment, with key secondary endpoints, which were abscess and inflammatory nodule (AN) count, occurrence of flares, and at least a 30% reduction in Patient’s Global Assessment of Skin Pain assessed using a numeric rating scale (NPRS30).
Secukinumab superior to placebo
The HiSCR is defined as at least a 50% decrease in AN count with no increase in the number of abscesses or in the number of draining fistulas relative to baseline. This was achieved by about 42%-45% of patients who received secukinumab every 2 weeks, about 42%-46% of those who received secukinumab every 4 weeks, and about 31%-33% of those on placebo in both studies.
Of note, fewer patients treated with secukinumab (about 15%-20% among those treated every 2 weeks, and about 15% to 23% among those treated every 4 weeks) than those on placebo (27%-29%) experienced flares, defined as at least a 25% increase in AN count and at least a two-point increase relative to baseline values.
Improvement in HS pain can be a difficult parameter to meet, Dr. Kimball noted. “Pain is such an important feature of this disease as it so debilitating for the patients.” More than one-third (almost 36%-39%) of patients given secukinumab vs. just over a quarter (26.9%) given placebo achieved at least a 30% reduction in NPRS30 ratings, she reported. The difference between active and placebo treatment was significant only when secukinumab was given every 2 weeks, however.
“The placebo rates that we see in these studies are exactly parallel to what we saw in other studies, and other disease states when we had a 50% bar of improvement,” Dr. Kimball said when questioned about these results.
“HS is a highly variable disease; it’s maybe not so much the placebo rate or the scoring system used but maybe the 50% bar set for improvement is too low. It’s likely, as data start to mature and a 75% HiSCR can be calculated, that the placebo rates will drop,” she said.
There were no surprises when it came to the safety of secukinumab, being an old player in a new game, she noted. It was “well tolerated” and tolerability was “consistent with the known safety profile,” Dr. Kimball said, “so we expect it to be a new, safe, and effective add to our armamentarium in treating this disease.”
This research involves “basically borrowing drugs from other areas and trying them in HS to see what effect they may have,” Dr. Patel said, noting that drugs such as adalimumab and secukinumab already had a proven track record in other diseases, such as psoriasis. “These early data for secukinumab definitely are very exciting, but we would need to see real-life results” in patients with HS who are not enrolled in trials to see the benefits, he added.
‘Tipping point’ for HS research
“I think we will look back on this meeting and realize that it was an incredibly important tipping point for the treatment of this incredibly debilitating disease,” Dr. Kimball said.
Elsewhere at the meeting, she had presented findings from a phase 2a study that pitted three different kinase inhibitors with different modes of action against each other and compared them with placebo.
The three agents evaluated are an IL-1 receptor–associated kinase 4 inhibitor known as PF-06650833, a tyrosine kinase 2 (TYK2) JAK1 inhibitor brepocitinib, and the TYK2 inhibitor PF-06826647.
“This technique has been used in oncology,” Dr. Kimball said, noting that the ability to test multiple drugs at the same time “means we can really much more efficiently test two different things at the same time, and also put fewer patients at risk for potential problems if drugs don’t work.”
Positive signs for brepocitinib, not the other kinases tested
The results showed that though brepocitinib worked in HS, the other two novel compounds did not appear to have beneficial effects. Just over half (52%) of the 52 patients treated with brepocitinib achieved an HiSCR at 16 weeks, compared with around one-third of those given placebo, PF-06650833, or PF-06826647.
A similar benefit was seen in terms of reduction in flares for brepocitinib but not the other agents, although there was no difference between them all in terms of NPRS30 pain reduction.
“We’ve been able to test three different modalities. This tells us some things about the pathophysiology for HS, which is a very profoundly intensive inflammatory process,” which, Dr. Kimball said, “may require multiple modalities of action to get it under control.” In addition, these “general modalities seem to safe and well tolerated,” she added.
Take-homes for practice and future research
“While it is disappointing that two of the drugs tested did not clearly demonstrate efficacy, it is very possible that these mechanisms of action may be successful targets in the future as new dosing strategies and drugs targeting these pathways are developed,” Dr. Sayed said.
A case in point, he added, was that “adalimumab did not meet treatment endpoints at a dose of 40 mg every other week, but clearly has made a major impact at 40 mg weekly.”
The bottom line is that “both secukinumab and beprocitinib demonstrated efficacy over placebo and are likely to be helpful for a significant number of patients with HS,” Dr. Sayed said. “Hopefully, we’ll see head-to-head trials and more data regarding proportions of patients with deeper responses using criteria such as HiSCR75 and HiSCR90.”
Moreover, “having a larger number of drugs with a range of mechanisms of action is extraordinarily helpful given how difficult the disease can be to manage. We will hopefully continue to see creative approaches and further successes in the current wave of phase 1, 2, and 3 trials that are already underway.”
The SUNSHINE and SUNRISE studies were funded by Novartis Pharma AG, Basel, Switzerland. The phase 2A study Dr. Kimball presented was sponsored by Pfizer.
Dr. Kimball disclosed ties to both Novartis and Pfizer and acts as a consultant and investigator to AbbVie, Bristol-Myers Squibb, Janssen, Eli Lilly, Novartis, and UCB. She is an investigator for Incyte and AnaptysBio; acts as a consultant to Bayer, Boehringer Ingelheim, Ventyz, Moonlake, Lily, Concert, EvoImmune, Sonoma Bio, and Sanofi; receives fellowship funding from Janssen, and serves on the Board of Directors for Almirall.
Dr. Patel had no conflicts of interest to disclose. Dr. Sayed is the director of the HS Foundation, a nonprofit organization, and has acted as an adviser or consultant to, speaker for, and received research funding from multiple drug companies including AbbVie, ChemoCentryx, Incyte, InflaRx, Novartis, and UCB.
A version of this article first appeared on Medscape.com.
in separate trials.
Around 40%-50% of patients exhibited a clinical response to these agents at 16 weeks, a leading HS expert reported at the annual congress of the European Academy of Dermatology and Venereology.
Time in the spotlight for HS
Research into HS is “an incredibly active field at this moment,” said Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, and president and chief executive officer of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center, Boston.
It’s “been great for advancing our understanding of the biology and the treatments that we will be able to use,” she said.
During the late-breaking sessions at the annual EADV Congress, Dr. Kimball presented data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety and tolerability of the interleukin (IL) 17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS.
“This is only the second phase 3 program we have ever seen in HS and the first one since 2016,” Dr. Kimball said of the trials. It’s also the largest trial program in HS conducted to date, she added, “so it really is a milestone.”
The last big development was when adalimumab, a tumor necrosis factor (TNF) blocker, gained regulatory approval for HS in 2016, observed Neil Patel, PhD, MRCP, who leads the HS service at Imperial College Healthcare NHS Trust in London.
“Adalimumab has been very helpful for many patients, but not all patients respond, and others may respond initially but then the treatment starts to fail after a year or 2,” Dr. Patel said in an interview with this news organization.
“There is definitely a huge need for alternative medication for this condition, which still has a lack of effective treatment options,” added Dr. Patel, who was not involved in either of the studies.
“One major upside for secukinumab is that its safety profile is generally very good and familiarity in the dermatologic community is already well established,” Christopher Sayed, MD, said in a separate interview.
“This will make most providers very comfortable offering it as a potential treatment option sooner rather than later given that its efficacy has now been demonstrated in phase 3 trials,” added Dr. Sayed, associate professor of dermatology at the University of North Carolina at Chapel Hill.
Two identically designed trials
Altogether, SUNSHINE and SUNRISE enrolled just over 1,000 patients at 219 sites in 33 countries. Both trials were identical in their design: A 4-week run-in phase before a randomized, double-blind treatment phase that tested two dosing regimens of secukinumab (300 mg administered subcutaneously) every 2 or 4 weeks vs. placebo for 16 weeks. The trial continued after this time, with patients in the placebo arm re–randomly assigned to treatment with one of the two secukinumab regimens out to a year.
The primary endpoint was the percentage of patients achieving a Hidradenitis Suppurativa Clinical Response (HiSCR) after 16 weeks of treatment, with key secondary endpoints, which were abscess and inflammatory nodule (AN) count, occurrence of flares, and at least a 30% reduction in Patient’s Global Assessment of Skin Pain assessed using a numeric rating scale (NPRS30).
Secukinumab superior to placebo
The HiSCR is defined as at least a 50% decrease in AN count with no increase in the number of abscesses or in the number of draining fistulas relative to baseline. This was achieved by about 42%-45% of patients who received secukinumab every 2 weeks, about 42%-46% of those who received secukinumab every 4 weeks, and about 31%-33% of those on placebo in both studies.
Of note, fewer patients treated with secukinumab (about 15%-20% among those treated every 2 weeks, and about 15% to 23% among those treated every 4 weeks) than those on placebo (27%-29%) experienced flares, defined as at least a 25% increase in AN count and at least a two-point increase relative to baseline values.
Improvement in HS pain can be a difficult parameter to meet, Dr. Kimball noted. “Pain is such an important feature of this disease as it so debilitating for the patients.” More than one-third (almost 36%-39%) of patients given secukinumab vs. just over a quarter (26.9%) given placebo achieved at least a 30% reduction in NPRS30 ratings, she reported. The difference between active and placebo treatment was significant only when secukinumab was given every 2 weeks, however.
“The placebo rates that we see in these studies are exactly parallel to what we saw in other studies, and other disease states when we had a 50% bar of improvement,” Dr. Kimball said when questioned about these results.
“HS is a highly variable disease; it’s maybe not so much the placebo rate or the scoring system used but maybe the 50% bar set for improvement is too low. It’s likely, as data start to mature and a 75% HiSCR can be calculated, that the placebo rates will drop,” she said.
There were no surprises when it came to the safety of secukinumab, being an old player in a new game, she noted. It was “well tolerated” and tolerability was “consistent with the known safety profile,” Dr. Kimball said, “so we expect it to be a new, safe, and effective add to our armamentarium in treating this disease.”
This research involves “basically borrowing drugs from other areas and trying them in HS to see what effect they may have,” Dr. Patel said, noting that drugs such as adalimumab and secukinumab already had a proven track record in other diseases, such as psoriasis. “These early data for secukinumab definitely are very exciting, but we would need to see real-life results” in patients with HS who are not enrolled in trials to see the benefits, he added.
‘Tipping point’ for HS research
“I think we will look back on this meeting and realize that it was an incredibly important tipping point for the treatment of this incredibly debilitating disease,” Dr. Kimball said.
Elsewhere at the meeting, she had presented findings from a phase 2a study that pitted three different kinase inhibitors with different modes of action against each other and compared them with placebo.
The three agents evaluated are an IL-1 receptor–associated kinase 4 inhibitor known as PF-06650833, a tyrosine kinase 2 (TYK2) JAK1 inhibitor brepocitinib, and the TYK2 inhibitor PF-06826647.
“This technique has been used in oncology,” Dr. Kimball said, noting that the ability to test multiple drugs at the same time “means we can really much more efficiently test two different things at the same time, and also put fewer patients at risk for potential problems if drugs don’t work.”
Positive signs for brepocitinib, not the other kinases tested
The results showed that though brepocitinib worked in HS, the other two novel compounds did not appear to have beneficial effects. Just over half (52%) of the 52 patients treated with brepocitinib achieved an HiSCR at 16 weeks, compared with around one-third of those given placebo, PF-06650833, or PF-06826647.
A similar benefit was seen in terms of reduction in flares for brepocitinib but not the other agents, although there was no difference between them all in terms of NPRS30 pain reduction.
“We’ve been able to test three different modalities. This tells us some things about the pathophysiology for HS, which is a very profoundly intensive inflammatory process,” which, Dr. Kimball said, “may require multiple modalities of action to get it under control.” In addition, these “general modalities seem to safe and well tolerated,” she added.
Take-homes for practice and future research
“While it is disappointing that two of the drugs tested did not clearly demonstrate efficacy, it is very possible that these mechanisms of action may be successful targets in the future as new dosing strategies and drugs targeting these pathways are developed,” Dr. Sayed said.
A case in point, he added, was that “adalimumab did not meet treatment endpoints at a dose of 40 mg every other week, but clearly has made a major impact at 40 mg weekly.”
The bottom line is that “both secukinumab and beprocitinib demonstrated efficacy over placebo and are likely to be helpful for a significant number of patients with HS,” Dr. Sayed said. “Hopefully, we’ll see head-to-head trials and more data regarding proportions of patients with deeper responses using criteria such as HiSCR75 and HiSCR90.”
Moreover, “having a larger number of drugs with a range of mechanisms of action is extraordinarily helpful given how difficult the disease can be to manage. We will hopefully continue to see creative approaches and further successes in the current wave of phase 1, 2, and 3 trials that are already underway.”
The SUNSHINE and SUNRISE studies were funded by Novartis Pharma AG, Basel, Switzerland. The phase 2A study Dr. Kimball presented was sponsored by Pfizer.
Dr. Kimball disclosed ties to both Novartis and Pfizer and acts as a consultant and investigator to AbbVie, Bristol-Myers Squibb, Janssen, Eli Lilly, Novartis, and UCB. She is an investigator for Incyte and AnaptysBio; acts as a consultant to Bayer, Boehringer Ingelheim, Ventyz, Moonlake, Lily, Concert, EvoImmune, Sonoma Bio, and Sanofi; receives fellowship funding from Janssen, and serves on the Board of Directors for Almirall.
Dr. Patel had no conflicts of interest to disclose. Dr. Sayed is the director of the HS Foundation, a nonprofit organization, and has acted as an adviser or consultant to, speaker for, and received research funding from multiple drug companies including AbbVie, ChemoCentryx, Incyte, InflaRx, Novartis, and UCB.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Experts express caution over type 2 diabetes/tea-drinking claim
type 2 diabetes/tea-drinking claim
A claim that drinking tea might protect people against developing type 2 diabetes has been met with caution from multiple experts ahead of the annual meeting of the European Association for the Study of Diabetes.
The claim is that people who drink four or more cups of tea every day – specifically green, Oolong, or black tea – are 17% less likely to develop type 2 diabetes than those who do not drink tea. Drinking fewer cups of tea per day was not found to confer any benefit.
“Our results are exciting because they suggest that people can do something as simple as drinking four cups of tea a day to potentially lessen their risk of developing type 2 diabetes,” Xiaying Li of Wuhan (China) University of Science and Technology is quoted as saying in an official EASD press release.
“It is possible that particular components in tea, such as polyphenols, may reduce blood glucose levels, but a sufficient amount of these bioactive compounds may be needed to be effective,” Dr. Li added.
“The words ‘suggest’ and ‘potentially’ are crucial here,” said Kevin McConway, PhD, MSc, MBA, emeritus professor of applied statistics at The Open University, said in a separate statement to the press that reeled in Dr. Li’s enthusiasm.
“Tea drinking would only be useful for reducing diabetes risk if the tea drinking causes reductions in risk, that is, if the risk is reduced if you drink the tea and not if you don’t – and this study simply can’t show whether it does this or not,” Dr. Conway stressed.
Naveed Sattar, FMedSci FRCPath FRCPGlas FRSE, professor of metabolic medicine at the University of Glasgow, was also cautiously critical. “There is no good trial evidence whatsoever that the chemicals in tea prevent diabetes,” he observed separately.
“So, I suspect its more about tea being healthier (less calorific) than many alternative drinks or tea drinkers leading healthier lives more generally.”
Dr. Sattar added that it could be that people who drink tea might also be avoiding drinking more harmful sugary drinks and have other health behaviors that might lead them to have a lower risk for type 2 diabetes.
Time for tea?
Dr. Li will present the findings of two analyses on Sept. 21 at the EASD meeting: the first a large observational cohort study and the second an updated systematic review and meta-analysis.
For the cohort study, Dr. Li and her coauthors took data on more than 5,100 adults who had participated in the long-running and ongoing China Health and Nutrition Survey (CHNS). Information on tea drinking behavior was extracted from questionnaires that had been filled out at two time points – 1997 and 2009 – and they determined whether people had developed type 2 diabetes according to American Diabetes Association criteria.
Nearly half, 45.8%, were found to be tea drinkers, and 10% of the population they sampled had developed type 2 diabetes. No association between tea drinking and type 2 diabetes development was found, however, with the hazard ratio comparing tea drinkers and non–tea drinkers sitting firmly at 1.02. Moreover, a sensitivity analysis that excluded participants who had developed type 2 diabetes in the first 3 years of follow-up did not change the result.
Things were slightly different when Dr. Li and associates performed their meta-analysis that involved analyzing data on more than 1 million participants in 19 studies conducted in eight countries that had been published up to September 2021.
Here, they found there was a significant (P < .003) linear association between tea consumption and having type 2 diabetes, with the relative risk of developing type 2 diabetes decreasing by 0.986 for every additional cup of tea that was drunk.
HRs for the development of type 2 diabetes in tea drinkers versus non–tea drinkers were 1.00 for those who drank less than one cup per day, 0.96 for those who had one to two cups, and 0.84 for those who drank four or more cups.
“While more research needs to be done to determine the exact dosage and mechanisms behind these observations, our findings suggest that drinking tea is beneficial in reducing the risk of type 2 diabetes, but only at high doses (at least 4 cups a day)”, said Dr. Li.
Perhaps, “we did not find an association between tea drinking and type 2 diabetes in our cohort study because we did not look at higher tea consumption,” she added.
Tempest in a teacup
“This is large, observational data. It’s not a randomized controlled trial so there’s plenty of room for data to be misunderstood,” warned Matt Sydes, MSc, professor of clinical trials & methodology at the MRC Clinical Trials Unit, University College London.
“Everyone drinks fluids. If there is an effect here (and that’s a big if), it might be not about the tea they drink, but about what they don’t drink. One can’t tell at the moment. It seems unlikely that a large randomized controlled trial could be done to disambiguate” added Dr. Sydes
“Being only a conference abstract, it is difficult to assess the quality of this research,” Baptiste Leurent, PhD, a medical statistician also working at University College London, said. Not only was the cohort study observational, so were all the other studies included in the meta-analysis, he pointed out.
“Therefore, no cause-effect conclusions can be drawn. The association could simply be due to other factors, such as those drinking more tea having a healthier lifestyle. It does not seem that the authors tried to control for confounders, which is usually difficult in meta-analysis,” Dr. Leurent said.
“There is reason to be a bit skeptical at this point; we really need to have the full details to assess it properly,” said Jonathan Cook of the Centre for Statistics in Medicine at the University of Oxford (England). “It’s a fair attempt to look at this, but not cutting edge, [using] fairly standard approaches.”
Similar studies have shown a reduced risk associated with coffee drinking, noted Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University in Birmingham.
“The important take-home message is that lifestyle is important in managing risk of developing type 2 diabetes,” Dr. Mellor said.
“That includes choosing low-calorie drinks including mainly water as well as unsweetened tea and coffee as your drinks of choice as part of a healthy lifestyle.”
The study was funded by the Young Talents Project of Hubei Provincial Health Commission, the Science and Technology Research Key Project of Education Department of Hubei Province, the Sanuo Diabetes Charity Foundation, and the Xiangyang Science and Technology Plan Project, all based in China. Dr. Li had no conflicts of interest to disclose. Dr. McConway is a Trustee and on the advisory committee of The Science Media Centre. Dr. Sattar has consulted for many companies that make diabetes and cardiovascular drugs and has been involved in multiple trials of lifestyle approaches for the prevention and remission of diabetes. Dr. Sydes, Dr. Leurent, Dr. Cook, and Dr. Mellor had no conflicts of interest to report.
A claim that drinking tea might protect people against developing type 2 diabetes has been met with caution from multiple experts ahead of the annual meeting of the European Association for the Study of Diabetes.
The claim is that people who drink four or more cups of tea every day – specifically green, Oolong, or black tea – are 17% less likely to develop type 2 diabetes than those who do not drink tea. Drinking fewer cups of tea per day was not found to confer any benefit.
“Our results are exciting because they suggest that people can do something as simple as drinking four cups of tea a day to potentially lessen their risk of developing type 2 diabetes,” Xiaying Li of Wuhan (China) University of Science and Technology is quoted as saying in an official EASD press release.
“It is possible that particular components in tea, such as polyphenols, may reduce blood glucose levels, but a sufficient amount of these bioactive compounds may be needed to be effective,” Dr. Li added.
“The words ‘suggest’ and ‘potentially’ are crucial here,” said Kevin McConway, PhD, MSc, MBA, emeritus professor of applied statistics at The Open University, said in a separate statement to the press that reeled in Dr. Li’s enthusiasm.
“Tea drinking would only be useful for reducing diabetes risk if the tea drinking causes reductions in risk, that is, if the risk is reduced if you drink the tea and not if you don’t – and this study simply can’t show whether it does this or not,” Dr. Conway stressed.
Naveed Sattar, FMedSci FRCPath FRCPGlas FRSE, professor of metabolic medicine at the University of Glasgow, was also cautiously critical. “There is no good trial evidence whatsoever that the chemicals in tea prevent diabetes,” he observed separately.
“So, I suspect its more about tea being healthier (less calorific) than many alternative drinks or tea drinkers leading healthier lives more generally.”
Dr. Sattar added that it could be that people who drink tea might also be avoiding drinking more harmful sugary drinks and have other health behaviors that might lead them to have a lower risk for type 2 diabetes.
Time for tea?
Dr. Li will present the findings of two analyses on Sept. 21 at the EASD meeting: the first a large observational cohort study and the second an updated systematic review and meta-analysis.
For the cohort study, Dr. Li and her coauthors took data on more than 5,100 adults who had participated in the long-running and ongoing China Health and Nutrition Survey (CHNS). Information on tea drinking behavior was extracted from questionnaires that had been filled out at two time points – 1997 and 2009 – and they determined whether people had developed type 2 diabetes according to American Diabetes Association criteria.
Nearly half, 45.8%, were found to be tea drinkers, and 10% of the population they sampled had developed type 2 diabetes. No association between tea drinking and type 2 diabetes development was found, however, with the hazard ratio comparing tea drinkers and non–tea drinkers sitting firmly at 1.02. Moreover, a sensitivity analysis that excluded participants who had developed type 2 diabetes in the first 3 years of follow-up did not change the result.
Things were slightly different when Dr. Li and associates performed their meta-analysis that involved analyzing data on more than 1 million participants in 19 studies conducted in eight countries that had been published up to September 2021.
Here, they found there was a significant (P < .003) linear association between tea consumption and having type 2 diabetes, with the relative risk of developing type 2 diabetes decreasing by 0.986 for every additional cup of tea that was drunk.
HRs for the development of type 2 diabetes in tea drinkers versus non–tea drinkers were 1.00 for those who drank less than one cup per day, 0.96 for those who had one to two cups, and 0.84 for those who drank four or more cups.
“While more research needs to be done to determine the exact dosage and mechanisms behind these observations, our findings suggest that drinking tea is beneficial in reducing the risk of type 2 diabetes, but only at high doses (at least 4 cups a day)”, said Dr. Li.
Perhaps, “we did not find an association between tea drinking and type 2 diabetes in our cohort study because we did not look at higher tea consumption,” she added.
Tempest in a teacup
“This is large, observational data. It’s not a randomized controlled trial so there’s plenty of room for data to be misunderstood,” warned Matt Sydes, MSc, professor of clinical trials & methodology at the MRC Clinical Trials Unit, University College London.
“Everyone drinks fluids. If there is an effect here (and that’s a big if), it might be not about the tea they drink, but about what they don’t drink. One can’t tell at the moment. It seems unlikely that a large randomized controlled trial could be done to disambiguate” added Dr. Sydes
“Being only a conference abstract, it is difficult to assess the quality of this research,” Baptiste Leurent, PhD, a medical statistician also working at University College London, said. Not only was the cohort study observational, so were all the other studies included in the meta-analysis, he pointed out.
“Therefore, no cause-effect conclusions can be drawn. The association could simply be due to other factors, such as those drinking more tea having a healthier lifestyle. It does not seem that the authors tried to control for confounders, which is usually difficult in meta-analysis,” Dr. Leurent said.
“There is reason to be a bit skeptical at this point; we really need to have the full details to assess it properly,” said Jonathan Cook of the Centre for Statistics in Medicine at the University of Oxford (England). “It’s a fair attempt to look at this, but not cutting edge, [using] fairly standard approaches.”
Similar studies have shown a reduced risk associated with coffee drinking, noted Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University in Birmingham.
“The important take-home message is that lifestyle is important in managing risk of developing type 2 diabetes,” Dr. Mellor said.
“That includes choosing low-calorie drinks including mainly water as well as unsweetened tea and coffee as your drinks of choice as part of a healthy lifestyle.”
The study was funded by the Young Talents Project of Hubei Provincial Health Commission, the Science and Technology Research Key Project of Education Department of Hubei Province, the Sanuo Diabetes Charity Foundation, and the Xiangyang Science and Technology Plan Project, all based in China. Dr. Li had no conflicts of interest to disclose. Dr. McConway is a Trustee and on the advisory committee of The Science Media Centre. Dr. Sattar has consulted for many companies that make diabetes and cardiovascular drugs and has been involved in multiple trials of lifestyle approaches for the prevention and remission of diabetes. Dr. Sydes, Dr. Leurent, Dr. Cook, and Dr. Mellor had no conflicts of interest to report.
A claim that drinking tea might protect people against developing type 2 diabetes has been met with caution from multiple experts ahead of the annual meeting of the European Association for the Study of Diabetes.
The claim is that people who drink four or more cups of tea every day – specifically green, Oolong, or black tea – are 17% less likely to develop type 2 diabetes than those who do not drink tea. Drinking fewer cups of tea per day was not found to confer any benefit.
“Our results are exciting because they suggest that people can do something as simple as drinking four cups of tea a day to potentially lessen their risk of developing type 2 diabetes,” Xiaying Li of Wuhan (China) University of Science and Technology is quoted as saying in an official EASD press release.
“It is possible that particular components in tea, such as polyphenols, may reduce blood glucose levels, but a sufficient amount of these bioactive compounds may be needed to be effective,” Dr. Li added.
“The words ‘suggest’ and ‘potentially’ are crucial here,” said Kevin McConway, PhD, MSc, MBA, emeritus professor of applied statistics at The Open University, said in a separate statement to the press that reeled in Dr. Li’s enthusiasm.
“Tea drinking would only be useful for reducing diabetes risk if the tea drinking causes reductions in risk, that is, if the risk is reduced if you drink the tea and not if you don’t – and this study simply can’t show whether it does this or not,” Dr. Conway stressed.
Naveed Sattar, FMedSci FRCPath FRCPGlas FRSE, professor of metabolic medicine at the University of Glasgow, was also cautiously critical. “There is no good trial evidence whatsoever that the chemicals in tea prevent diabetes,” he observed separately.
“So, I suspect its more about tea being healthier (less calorific) than many alternative drinks or tea drinkers leading healthier lives more generally.”
Dr. Sattar added that it could be that people who drink tea might also be avoiding drinking more harmful sugary drinks and have other health behaviors that might lead them to have a lower risk for type 2 diabetes.
Time for tea?
Dr. Li will present the findings of two analyses on Sept. 21 at the EASD meeting: the first a large observational cohort study and the second an updated systematic review and meta-analysis.
For the cohort study, Dr. Li and her coauthors took data on more than 5,100 adults who had participated in the long-running and ongoing China Health and Nutrition Survey (CHNS). Information on tea drinking behavior was extracted from questionnaires that had been filled out at two time points – 1997 and 2009 – and they determined whether people had developed type 2 diabetes according to American Diabetes Association criteria.
Nearly half, 45.8%, were found to be tea drinkers, and 10% of the population they sampled had developed type 2 diabetes. No association between tea drinking and type 2 diabetes development was found, however, with the hazard ratio comparing tea drinkers and non–tea drinkers sitting firmly at 1.02. Moreover, a sensitivity analysis that excluded participants who had developed type 2 diabetes in the first 3 years of follow-up did not change the result.
Things were slightly different when Dr. Li and associates performed their meta-analysis that involved analyzing data on more than 1 million participants in 19 studies conducted in eight countries that had been published up to September 2021.
Here, they found there was a significant (P < .003) linear association between tea consumption and having type 2 diabetes, with the relative risk of developing type 2 diabetes decreasing by 0.986 for every additional cup of tea that was drunk.
HRs for the development of type 2 diabetes in tea drinkers versus non–tea drinkers were 1.00 for those who drank less than one cup per day, 0.96 for those who had one to two cups, and 0.84 for those who drank four or more cups.
“While more research needs to be done to determine the exact dosage and mechanisms behind these observations, our findings suggest that drinking tea is beneficial in reducing the risk of type 2 diabetes, but only at high doses (at least 4 cups a day)”, said Dr. Li.
Perhaps, “we did not find an association between tea drinking and type 2 diabetes in our cohort study because we did not look at higher tea consumption,” she added.
Tempest in a teacup
“This is large, observational data. It’s not a randomized controlled trial so there’s plenty of room for data to be misunderstood,” warned Matt Sydes, MSc, professor of clinical trials & methodology at the MRC Clinical Trials Unit, University College London.
“Everyone drinks fluids. If there is an effect here (and that’s a big if), it might be not about the tea they drink, but about what they don’t drink. One can’t tell at the moment. It seems unlikely that a large randomized controlled trial could be done to disambiguate” added Dr. Sydes
“Being only a conference abstract, it is difficult to assess the quality of this research,” Baptiste Leurent, PhD, a medical statistician also working at University College London, said. Not only was the cohort study observational, so were all the other studies included in the meta-analysis, he pointed out.
“Therefore, no cause-effect conclusions can be drawn. The association could simply be due to other factors, such as those drinking more tea having a healthier lifestyle. It does not seem that the authors tried to control for confounders, which is usually difficult in meta-analysis,” Dr. Leurent said.
“There is reason to be a bit skeptical at this point; we really need to have the full details to assess it properly,” said Jonathan Cook of the Centre for Statistics in Medicine at the University of Oxford (England). “It’s a fair attempt to look at this, but not cutting edge, [using] fairly standard approaches.”
Similar studies have shown a reduced risk associated with coffee drinking, noted Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University in Birmingham.
“The important take-home message is that lifestyle is important in managing risk of developing type 2 diabetes,” Dr. Mellor said.
“That includes choosing low-calorie drinks including mainly water as well as unsweetened tea and coffee as your drinks of choice as part of a healthy lifestyle.”
The study was funded by the Young Talents Project of Hubei Provincial Health Commission, the Science and Technology Research Key Project of Education Department of Hubei Province, the Sanuo Diabetes Charity Foundation, and the Xiangyang Science and Technology Plan Project, all based in China. Dr. Li had no conflicts of interest to disclose. Dr. McConway is a Trustee and on the advisory committee of The Science Media Centre. Dr. Sattar has consulted for many companies that make diabetes and cardiovascular drugs and has been involved in multiple trials of lifestyle approaches for the prevention and remission of diabetes. Dr. Sydes, Dr. Leurent, Dr. Cook, and Dr. Mellor had no conflicts of interest to report.
type 2 diabetes/tea-drinking claim
type 2 diabetes/tea-drinking claim
FROM EASD 2022
Roflumilast foam effectively eases seborrheic dermatitis
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Overall survival dips with vitamin D deficiency in melanoma
, according to research presented at the annual congress of the European Academy of Dermatology and Venereology.
Whereas the 5-year overall survival was 90% when vitamin D serum levels were above a 10 ng/mL threshold, it was 84% when levels fell below it. Notably, the gap in overall survival between those above and below the threshold appeared to widen as time went on.
The research adds to existing evidence that “vitamin D levels can play an important and independent role in patients’ survival outcomes,” study investigator Inés Gracia-Darder, MD, told this news organization. “The important application in clinical practice would be to know if vitamin D supplementation influences the survival of melanoma patients,” said Dr. Gracia-Darder, a clinical specialist in dermatology at the Hospital Universitari Son Espases, Mallorca, Spain.
Known association, but not much data
“It is not a new finding,” but there are limited data, especially in melanoma, said Julie De Smedt, MD, of KU Leuven, Belgium, who was asked to comment on the results. Other groups have shown, certainly for cancer in general, that vitamin D can have an effect on overall survival.
“Low levels of vitamin D are associated with the pathological parameters of the melanoma, such as the thickness of the tumor,” Dr. De Smedt said in an interview, indicating that it’s not just overall survival that might be affected.
“So we assume that also has an effect on melanoma-specific survival,” she added.
That assumption, however, is not supported by the data Dr. Gracia-Darder presented, as there was no difference in melanoma-specific survival among the two groups of patients that had been studied.
Retrospective cohort analysis
Vitamin D levels had been studied in 264 patients who were included in the retrospective cohort analysis. All had invasive melanomas, and all had been seen at the Hospital Clinic of Barcelona between January 1998 and June 2021. Their mean age was 57 years, and the median follow-up was 6.7 years.
For inclusion, all patients had to have had their vitamin D levels measured after being diagnosed with melanoma; those with a 25-hydroxyvitamin D3 serum level of less than 10 ng/mL were deemed to be vitamin D deficient, whereas those with levels of 10 ng/mL and above were deemed normal or insufficient.
A measurement less than 10 ng/mL is considered vitamin D deficiency, Dr. De Smedt said. “But there is a difference between countries, and there’s also a difference between societies,” noting the cut-off used in the lab where she works is 20 ng/mL. This makes it difficult to compare studies, she said.
Independent association with overall survival
Seasonal variation in vitamin D levels were considered as a possible confounding factor, but Dr. Gracia-Darder noted that there was a similar distribution of measurements taken between October to March and April to September.
Univariate and multivariate analyses established vitamin D deficiency as being independently associated with overall survival with hazard ratios of 2.34 and 2.45, respectively.
Other predictive factors were having a higher Breslow index, as well as older age and gender.
Time to recommend vitamin D supplementation?
So should patients with melanoma have their vitamin D levels routinely checked? And what about advising them to take vitamin D supplements?
“In our practice, we analyze the vitamin D levels of our patients,” Dr. Gracia-Darder said. Patients are told to limit their exposure to the sun because of their skin cancer, so they are very likely to become vitamin D deficient.
While dietary changes or supplements might be suggested, there’s no real evidence to support upping vitamin D levels to date, so “future prospective studies are needed,” Dr. Gracia-Darder added.
Such studies have already started, including one in Italy, one in Australia, and another study that Dr. De Smedt has been involved with for the past few years.
Called the ViDMe study, it’s a multicenter, randomized, double-blind trial in which patients are being given a high-dose oral vitamin D supplement or placebo once a month for at least 1 year. About 430 patients with a first cutaneous malignant melanoma have been included in the trial, which started in December 2012.
It is hoped that the results will show that the supplementation will have had a protective effect on the risk of relapse and that there will be a correlation between vitamin D levels in the blood and vitamin D receptor immunoreactivity in the tumor.
“The study is still blinded,” Dr. De Smedt said. “We will unblind in the coming months and then at the end of the year, maybe next year, we will have the results.”
The study reported by Dr. Gracia-Darder did not receive any specific funding. Dr. Gracia-Darder disclosed that the melanoma unit where the study was performed receives many grants and funds to carry out research. She reported no other relevant financial relationships. Dr. De Smedt had no relevant financial relationships. The ViDMe study is sponsored by the Universitaire Ziekenhuizen Leuven.
A version of this article first appeared on Medscape.com.
, according to research presented at the annual congress of the European Academy of Dermatology and Venereology.
Whereas the 5-year overall survival was 90% when vitamin D serum levels were above a 10 ng/mL threshold, it was 84% when levels fell below it. Notably, the gap in overall survival between those above and below the threshold appeared to widen as time went on.
The research adds to existing evidence that “vitamin D levels can play an important and independent role in patients’ survival outcomes,” study investigator Inés Gracia-Darder, MD, told this news organization. “The important application in clinical practice would be to know if vitamin D supplementation influences the survival of melanoma patients,” said Dr. Gracia-Darder, a clinical specialist in dermatology at the Hospital Universitari Son Espases, Mallorca, Spain.
Known association, but not much data
“It is not a new finding,” but there are limited data, especially in melanoma, said Julie De Smedt, MD, of KU Leuven, Belgium, who was asked to comment on the results. Other groups have shown, certainly for cancer in general, that vitamin D can have an effect on overall survival.
“Low levels of vitamin D are associated with the pathological parameters of the melanoma, such as the thickness of the tumor,” Dr. De Smedt said in an interview, indicating that it’s not just overall survival that might be affected.
“So we assume that also has an effect on melanoma-specific survival,” she added.
That assumption, however, is not supported by the data Dr. Gracia-Darder presented, as there was no difference in melanoma-specific survival among the two groups of patients that had been studied.
Retrospective cohort analysis
Vitamin D levels had been studied in 264 patients who were included in the retrospective cohort analysis. All had invasive melanomas, and all had been seen at the Hospital Clinic of Barcelona between January 1998 and June 2021. Their mean age was 57 years, and the median follow-up was 6.7 years.
For inclusion, all patients had to have had their vitamin D levels measured after being diagnosed with melanoma; those with a 25-hydroxyvitamin D3 serum level of less than 10 ng/mL were deemed to be vitamin D deficient, whereas those with levels of 10 ng/mL and above were deemed normal or insufficient.
A measurement less than 10 ng/mL is considered vitamin D deficiency, Dr. De Smedt said. “But there is a difference between countries, and there’s also a difference between societies,” noting the cut-off used in the lab where she works is 20 ng/mL. This makes it difficult to compare studies, she said.
Independent association with overall survival
Seasonal variation in vitamin D levels were considered as a possible confounding factor, but Dr. Gracia-Darder noted that there was a similar distribution of measurements taken between October to March and April to September.
Univariate and multivariate analyses established vitamin D deficiency as being independently associated with overall survival with hazard ratios of 2.34 and 2.45, respectively.
Other predictive factors were having a higher Breslow index, as well as older age and gender.
Time to recommend vitamin D supplementation?
So should patients with melanoma have their vitamin D levels routinely checked? And what about advising them to take vitamin D supplements?
“In our practice, we analyze the vitamin D levels of our patients,” Dr. Gracia-Darder said. Patients are told to limit their exposure to the sun because of their skin cancer, so they are very likely to become vitamin D deficient.
While dietary changes or supplements might be suggested, there’s no real evidence to support upping vitamin D levels to date, so “future prospective studies are needed,” Dr. Gracia-Darder added.
Such studies have already started, including one in Italy, one in Australia, and another study that Dr. De Smedt has been involved with for the past few years.
Called the ViDMe study, it’s a multicenter, randomized, double-blind trial in which patients are being given a high-dose oral vitamin D supplement or placebo once a month for at least 1 year. About 430 patients with a first cutaneous malignant melanoma have been included in the trial, which started in December 2012.
It is hoped that the results will show that the supplementation will have had a protective effect on the risk of relapse and that there will be a correlation between vitamin D levels in the blood and vitamin D receptor immunoreactivity in the tumor.
“The study is still blinded,” Dr. De Smedt said. “We will unblind in the coming months and then at the end of the year, maybe next year, we will have the results.”
The study reported by Dr. Gracia-Darder did not receive any specific funding. Dr. Gracia-Darder disclosed that the melanoma unit where the study was performed receives many grants and funds to carry out research. She reported no other relevant financial relationships. Dr. De Smedt had no relevant financial relationships. The ViDMe study is sponsored by the Universitaire Ziekenhuizen Leuven.
A version of this article first appeared on Medscape.com.
, according to research presented at the annual congress of the European Academy of Dermatology and Venereology.
Whereas the 5-year overall survival was 90% when vitamin D serum levels were above a 10 ng/mL threshold, it was 84% when levels fell below it. Notably, the gap in overall survival between those above and below the threshold appeared to widen as time went on.
The research adds to existing evidence that “vitamin D levels can play an important and independent role in patients’ survival outcomes,” study investigator Inés Gracia-Darder, MD, told this news organization. “The important application in clinical practice would be to know if vitamin D supplementation influences the survival of melanoma patients,” said Dr. Gracia-Darder, a clinical specialist in dermatology at the Hospital Universitari Son Espases, Mallorca, Spain.
Known association, but not much data
“It is not a new finding,” but there are limited data, especially in melanoma, said Julie De Smedt, MD, of KU Leuven, Belgium, who was asked to comment on the results. Other groups have shown, certainly for cancer in general, that vitamin D can have an effect on overall survival.
“Low levels of vitamin D are associated with the pathological parameters of the melanoma, such as the thickness of the tumor,” Dr. De Smedt said in an interview, indicating that it’s not just overall survival that might be affected.
“So we assume that also has an effect on melanoma-specific survival,” she added.
That assumption, however, is not supported by the data Dr. Gracia-Darder presented, as there was no difference in melanoma-specific survival among the two groups of patients that had been studied.
Retrospective cohort analysis
Vitamin D levels had been studied in 264 patients who were included in the retrospective cohort analysis. All had invasive melanomas, and all had been seen at the Hospital Clinic of Barcelona between January 1998 and June 2021. Their mean age was 57 years, and the median follow-up was 6.7 years.
For inclusion, all patients had to have had their vitamin D levels measured after being diagnosed with melanoma; those with a 25-hydroxyvitamin D3 serum level of less than 10 ng/mL were deemed to be vitamin D deficient, whereas those with levels of 10 ng/mL and above were deemed normal or insufficient.
A measurement less than 10 ng/mL is considered vitamin D deficiency, Dr. De Smedt said. “But there is a difference between countries, and there’s also a difference between societies,” noting the cut-off used in the lab where she works is 20 ng/mL. This makes it difficult to compare studies, she said.
Independent association with overall survival
Seasonal variation in vitamin D levels were considered as a possible confounding factor, but Dr. Gracia-Darder noted that there was a similar distribution of measurements taken between October to March and April to September.
Univariate and multivariate analyses established vitamin D deficiency as being independently associated with overall survival with hazard ratios of 2.34 and 2.45, respectively.
Other predictive factors were having a higher Breslow index, as well as older age and gender.
Time to recommend vitamin D supplementation?
So should patients with melanoma have their vitamin D levels routinely checked? And what about advising them to take vitamin D supplements?
“In our practice, we analyze the vitamin D levels of our patients,” Dr. Gracia-Darder said. Patients are told to limit their exposure to the sun because of their skin cancer, so they are very likely to become vitamin D deficient.
While dietary changes or supplements might be suggested, there’s no real evidence to support upping vitamin D levels to date, so “future prospective studies are needed,” Dr. Gracia-Darder added.
Such studies have already started, including one in Italy, one in Australia, and another study that Dr. De Smedt has been involved with for the past few years.
Called the ViDMe study, it’s a multicenter, randomized, double-blind trial in which patients are being given a high-dose oral vitamin D supplement or placebo once a month for at least 1 year. About 430 patients with a first cutaneous malignant melanoma have been included in the trial, which started in December 2012.
It is hoped that the results will show that the supplementation will have had a protective effect on the risk of relapse and that there will be a correlation between vitamin D levels in the blood and vitamin D receptor immunoreactivity in the tumor.
“The study is still blinded,” Dr. De Smedt said. “We will unblind in the coming months and then at the end of the year, maybe next year, we will have the results.”
The study reported by Dr. Gracia-Darder did not receive any specific funding. Dr. Gracia-Darder disclosed that the melanoma unit where the study was performed receives many grants and funds to carry out research. She reported no other relevant financial relationships. Dr. De Smedt had no relevant financial relationships. The ViDMe study is sponsored by the Universitaire Ziekenhuizen Leuven.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS