User login
Fountains of Wayne, and a hospitalist’s first day, remembered
Like many in the health care field, I have found it hard to watch the news over these past couple of months when it seems that almost every story is about COVID-19 or its repercussions. Luckily, I have two young daughters who “encourage” me to listen to the Frozen 2 soundtrack instead of putting on the evening news when I get home from work. Still, news manages to seep through my defenses. As I scrolled through some headlines recently, I learned of the death of musician Adam Schlesinger from COVID-19. He wasn’t a household name, but his death still hit me in unexpected ways.
I started internship in late June 2005, in a city (Portland, Ore.) about as different from my previous home (Dallas) as any two places can possibly be. I think the day before internship started still ranks as the most nervous of my life. I’m not sure how I slept at all that night, but somehow I did and arrived at the Portland Veterans Affairs Hospital the following morning to start my new career.
And then … nothing happened. Early on that first day, the electronic medical records crashed, and no patients were admitted during our time on “short call.” My upper level resident took care of the one or two established patients on the team (both discharged), so I ended the day with records that would not be broken during the remainder of my residency: 0 notes written, 0 patients seen. Perhaps the most successful first day that any intern, anywhere has ever had, although it prepared me quite poorly for all the subsequent days.
Since I had some time on my hands, I made the 20-minute walk to one of my new hometown’s record stores where Fountains of Wayne (FOW) was playing an acoustic in-store set. Their album from a few years prior, “Welcome Interstate Managers,” was in heavy rotation when I made the drive from Dallas to Portland. It was (and is) a great album for long drives – melodic, catchy, and (mostly) up-tempo. Adam and the band’s singer, Chris Collingwood, played several songs that night on the store’s stage. Then they headed out to the next city, and I headed back home and on to many far-busier days of residency.
We would cross paths again a decade later. I moved back to Texas and became a hospitalist. It turns out that, if you have enough hospitalists of a certain age and if enough of those hospitalists have unearned confidence in their musical ability, then a covers band will undoubtedly be formed. And so, it happened here in San Antonio. We were not selective in our song choices – we played songs from every decade of the last 50 years, bands as popular as the Beatles and as indie as the Rentals. And we played some FOW.
Our band (which will go nameless here so that our YouTube recordings are more difficult to find) played a grand total of one gig during our years of intermittent practicing. That one gig was my wedding rehearsal dinner and the penultimate song we played was “Stacy’s Mom,” which is notable for being both FOW’s biggest hit and a completely inappropriate song to play at a wedding rehearsal dinner. The crowd was probably around the same size as the one that had seen Adam and Chris play in Portland 10 years prior. I don’t think the applause we received was quite as genuine or deserved, though.
After Adam and Chris played their gig, there was an autograph session and I took home a signed poster. Last year, I decided to take it out of storage and hang it in my office. The date of the show and the first day of my physician career, a date now nearly 15 years ago, is written in psychedelic typography at the bottom. The store that I went to that day is no longer there, a victim of progress like so many other record stores across the country. Another location of the same store is still open in Portland. I hope that it and all the other small book and music stores across the country can survive this current crisis, but I know that many will not.
So, here’s to you Adam, and to all the others who have lost their lives to this terrible illness. As a small token of remembrance, I’ll be playing some Fountains of Wayne on the drive home tonight. It’s not quite the same as playing it on a cross-country drive, but hopefully, we will all be able to do that again soon.
Dr. Sehgal is a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. He is a member of the editorial advisory board for The Hospitalist.
Like many in the health care field, I have found it hard to watch the news over these past couple of months when it seems that almost every story is about COVID-19 or its repercussions. Luckily, I have two young daughters who “encourage” me to listen to the Frozen 2 soundtrack instead of putting on the evening news when I get home from work. Still, news manages to seep through my defenses. As I scrolled through some headlines recently, I learned of the death of musician Adam Schlesinger from COVID-19. He wasn’t a household name, but his death still hit me in unexpected ways.
I started internship in late June 2005, in a city (Portland, Ore.) about as different from my previous home (Dallas) as any two places can possibly be. I think the day before internship started still ranks as the most nervous of my life. I’m not sure how I slept at all that night, but somehow I did and arrived at the Portland Veterans Affairs Hospital the following morning to start my new career.
And then … nothing happened. Early on that first day, the electronic medical records crashed, and no patients were admitted during our time on “short call.” My upper level resident took care of the one or two established patients on the team (both discharged), so I ended the day with records that would not be broken during the remainder of my residency: 0 notes written, 0 patients seen. Perhaps the most successful first day that any intern, anywhere has ever had, although it prepared me quite poorly for all the subsequent days.
Since I had some time on my hands, I made the 20-minute walk to one of my new hometown’s record stores where Fountains of Wayne (FOW) was playing an acoustic in-store set. Their album from a few years prior, “Welcome Interstate Managers,” was in heavy rotation when I made the drive from Dallas to Portland. It was (and is) a great album for long drives – melodic, catchy, and (mostly) up-tempo. Adam and the band’s singer, Chris Collingwood, played several songs that night on the store’s stage. Then they headed out to the next city, and I headed back home and on to many far-busier days of residency.
We would cross paths again a decade later. I moved back to Texas and became a hospitalist. It turns out that, if you have enough hospitalists of a certain age and if enough of those hospitalists have unearned confidence in their musical ability, then a covers band will undoubtedly be formed. And so, it happened here in San Antonio. We were not selective in our song choices – we played songs from every decade of the last 50 years, bands as popular as the Beatles and as indie as the Rentals. And we played some FOW.
Our band (which will go nameless here so that our YouTube recordings are more difficult to find) played a grand total of one gig during our years of intermittent practicing. That one gig was my wedding rehearsal dinner and the penultimate song we played was “Stacy’s Mom,” which is notable for being both FOW’s biggest hit and a completely inappropriate song to play at a wedding rehearsal dinner. The crowd was probably around the same size as the one that had seen Adam and Chris play in Portland 10 years prior. I don’t think the applause we received was quite as genuine or deserved, though.
After Adam and Chris played their gig, there was an autograph session and I took home a signed poster. Last year, I decided to take it out of storage and hang it in my office. The date of the show and the first day of my physician career, a date now nearly 15 years ago, is written in psychedelic typography at the bottom. The store that I went to that day is no longer there, a victim of progress like so many other record stores across the country. Another location of the same store is still open in Portland. I hope that it and all the other small book and music stores across the country can survive this current crisis, but I know that many will not.
So, here’s to you Adam, and to all the others who have lost their lives to this terrible illness. As a small token of remembrance, I’ll be playing some Fountains of Wayne on the drive home tonight. It’s not quite the same as playing it on a cross-country drive, but hopefully, we will all be able to do that again soon.
Dr. Sehgal is a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. He is a member of the editorial advisory board for The Hospitalist.
Like many in the health care field, I have found it hard to watch the news over these past couple of months when it seems that almost every story is about COVID-19 or its repercussions. Luckily, I have two young daughters who “encourage” me to listen to the Frozen 2 soundtrack instead of putting on the evening news when I get home from work. Still, news manages to seep through my defenses. As I scrolled through some headlines recently, I learned of the death of musician Adam Schlesinger from COVID-19. He wasn’t a household name, but his death still hit me in unexpected ways.
I started internship in late June 2005, in a city (Portland, Ore.) about as different from my previous home (Dallas) as any two places can possibly be. I think the day before internship started still ranks as the most nervous of my life. I’m not sure how I slept at all that night, but somehow I did and arrived at the Portland Veterans Affairs Hospital the following morning to start my new career.
And then … nothing happened. Early on that first day, the electronic medical records crashed, and no patients were admitted during our time on “short call.” My upper level resident took care of the one or two established patients on the team (both discharged), so I ended the day with records that would not be broken during the remainder of my residency: 0 notes written, 0 patients seen. Perhaps the most successful first day that any intern, anywhere has ever had, although it prepared me quite poorly for all the subsequent days.
Since I had some time on my hands, I made the 20-minute walk to one of my new hometown’s record stores where Fountains of Wayne (FOW) was playing an acoustic in-store set. Their album from a few years prior, “Welcome Interstate Managers,” was in heavy rotation when I made the drive from Dallas to Portland. It was (and is) a great album for long drives – melodic, catchy, and (mostly) up-tempo. Adam and the band’s singer, Chris Collingwood, played several songs that night on the store’s stage. Then they headed out to the next city, and I headed back home and on to many far-busier days of residency.
We would cross paths again a decade later. I moved back to Texas and became a hospitalist. It turns out that, if you have enough hospitalists of a certain age and if enough of those hospitalists have unearned confidence in their musical ability, then a covers band will undoubtedly be formed. And so, it happened here in San Antonio. We were not selective in our song choices – we played songs from every decade of the last 50 years, bands as popular as the Beatles and as indie as the Rentals. And we played some FOW.
Our band (which will go nameless here so that our YouTube recordings are more difficult to find) played a grand total of one gig during our years of intermittent practicing. That one gig was my wedding rehearsal dinner and the penultimate song we played was “Stacy’s Mom,” which is notable for being both FOW’s biggest hit and a completely inappropriate song to play at a wedding rehearsal dinner. The crowd was probably around the same size as the one that had seen Adam and Chris play in Portland 10 years prior. I don’t think the applause we received was quite as genuine or deserved, though.
After Adam and Chris played their gig, there was an autograph session and I took home a signed poster. Last year, I decided to take it out of storage and hang it in my office. The date of the show and the first day of my physician career, a date now nearly 15 years ago, is written in psychedelic typography at the bottom. The store that I went to that day is no longer there, a victim of progress like so many other record stores across the country. Another location of the same store is still open in Portland. I hope that it and all the other small book and music stores across the country can survive this current crisis, but I know that many will not.
So, here’s to you Adam, and to all the others who have lost their lives to this terrible illness. As a small token of remembrance, I’ll be playing some Fountains of Wayne on the drive home tonight. It’s not quite the same as playing it on a cross-country drive, but hopefully, we will all be able to do that again soon.
Dr. Sehgal is a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. He is a member of the editorial advisory board for The Hospitalist.
How should anticoagulation be managed in a patient with cirrhosis?
DOACs may be a practical option for some CLD patients
Case
A 60-year-old man with cirrhosis is admitted to the hospital with concern for spontaneous bacterial peritonitis. His body mass index is 35 kg/m2. He is severely deconditioned and largely bed bound. His admission labs show thrombocytopenia (platelets 65,000/mcL) and an elevated international normalized ratio (INR) of 1.6. Should this patient be placed on venous thromboembolism (VTE) prophylaxis on admission?
Brief overview
Patients with chronic liver disease (CLD) have previously been considered “auto-anticoagulated” because of markers of increased bleeding risk, including a decreased platelet count and elevated INR, prothrombin time, and activated partial thromboplastin time. It is being increasingly recognized, however, that CLD often represents a hypercoagulable state despite these abnormalities.1
While cirrhotic patients produce less of several procoagulant substances (such as factors II, V, VII, X, XI, XII, XIII, and fibrinogen), they are also deficient in multiple anticoagulant factors (such as proteins C and S and antithrombin) and fibrinolytics (plasminogen). While the prothrombin time and activated partial thromboplastin time are sensitive to levels of procoagulant proteins in plasma, they do not measure response to the natural anticoagulants and therefore do not reflect an accurate picture of a cirrhotic patient’s risk of developing thrombosis. In addition, cirrhotic patients have many other risk factors for thrombosis, including poor functional status, frequent hospitalization, and elevated estrogen levels.
Overview of the data
VTE incidence among patients with CLD has varied across studies, ranging from 0.5% to 6.3%.2 A systemic review of VTE risk in cirrhotic patients concluded that they “have a significant risk of VTE, if not higher than noncirrhotic patients and this risk cannot be trivialized or ignored.”2
In a nationwide Danish case-control study, patients with cirrhosis had a 1.7 times increased risk of VTE, compared with the general population.3 Hypoalbuminemia appears to be one of the strongest associated risk factors for VTE in these patients, likely as a reflection of the degree of liver synthetic dysfunction (and therefore decreased synthesis of anticoagulant factors). One study showed that patients with an albumin of less than 1.9 g/dL had a VTE risk five times higher than patients with an albumin of 2.8 g/dL or higher.4
Prophylaxis
Given the increased risks of bleeding and thrombosis in patients with cirrhosis, how should VTE prophylaxis be managed in hospitalized patients? While current guidelines do not specifically address the use of pharmacologic prophylaxis in cirrhotic patients, the Padua Predictor Score, which is used to assess VTE risk in the general hospital population, has also been shown to be helpful in the subpopulation of patients with CLD (Table 1).
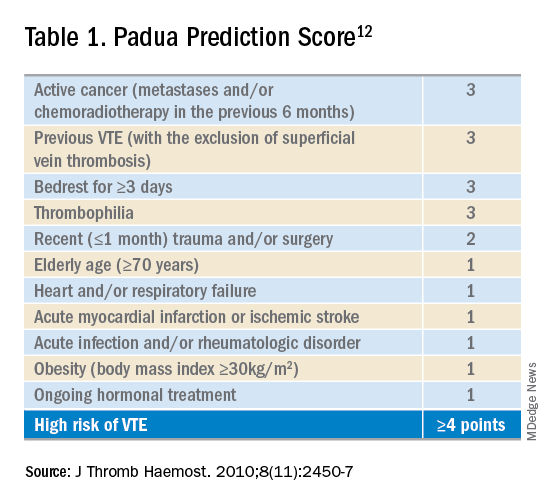
In one study, cirrhotic patients who were “high risk” by Padua Predictor score were over 12 times more likely to develop VTE than those who were “low risk.”5 Bleeding risk appears to be fairly low, and similar to those patients not receiving prophylactic anticoagulation. One retrospective case series of hospitalized cirrhotic patients receiving thromboprophylaxis showed a rate of GI bleeding of 2.5% (9 of 355 patients); the rate of major bleeding was less than 1%.6
Selection of anticoagulant for VTE prophylaxis should be similar to non-CLD patients. The choice of agent (low-molecular-weight heparin (LMWH) or unfractionated heparin) and dosing depends on factors including renal function and bodyweight. If anticoagulation is contraindicated (because of thrombocytopenia, for example), then mechanical prophylaxis should be considered.7
Treatment
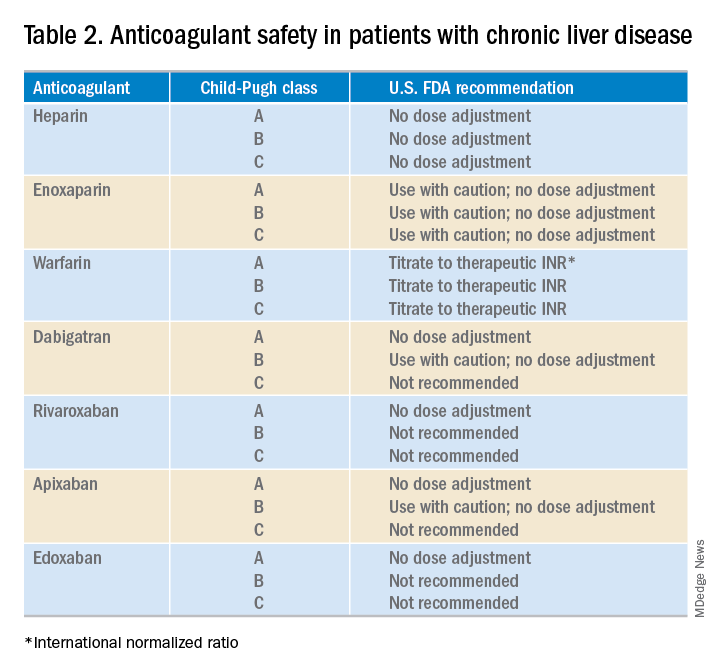
What about anticoagulation in patients with a known VTE? Food and Drug Administration safety recommendations are based on Child-Pugh class, although the current data on the safety and efficacy of full dose anticoagulation therapy for VTE in patients with cirrhosis are limited (Table 2). At this point, LMWH is often the preferred choice for anticoagulation in CLD patients. However, some limitations exist including the need for frequent subcutaneous injections and limited reliability of anti–factor Xa levels.
Cirrhotic patients often fail to achieve target anti–factor Xa levels on standard prophylactic and therapeutic doses of enoxaparin. This, however, is likely a lab anomaly as in vitro studies have shown that cirrhotic patients may show an increased response to LMWH despite reduced anti–factor Xa levels.8 Thus, LMWH remains the standard of care for many CLD patients.
The use of vitamin K antagonists (VKAs) such as warfarin for VTE treatment can be difficult to manage. Traditionally CLD patients have been started on lower doses of warfarin but given their already elevated INR, this may lead to a subtherapeutic dose of VKAs. A recent study of 23 patients with cirrhosis demonstrated that a target INR of 2-3 can be reached with VKA doses similar to those in noncirrhotic patients.9 These data support the practice of using the same VKA dosing strategies for CLD patients, and selecting a starting dose based on patient parameters such as age and weight.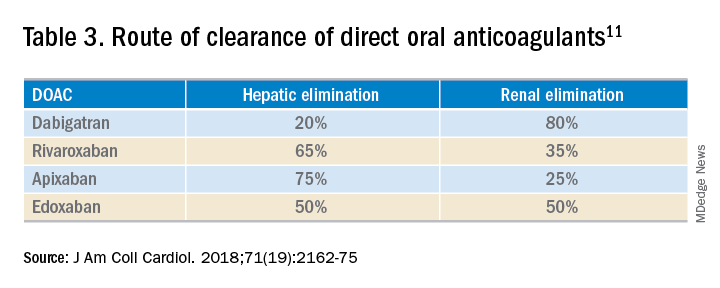
While the use of direct oral anticoagulants (DOACs) for this patient population is still not a common practice, they may be a practical option for some CLD patients. A meta-analysis found that the currently used DOACs have no significant risk of drug-induced hepatic injury.10 Currently, only observational data are available to assess the benefits and risks of bleeding with DOACs in this patient population, as patients with significant liver disease were excluded from the major randomized trials.11 DOACs may also represent a complicated choice for some patients given the effect of liver injury on their metabolism (Table 3).
Application of data to the original case
This patient should be assessed for both risk of VTE and risk of bleeding during the hospital admission. CLD patients likely have a risk of VTE similar to (or even greater than) that of general medical patients. The Padua score for this patient is 4 (bed rest, body mass index) indicating that he is at high risk of VTE. While he is thrombocytopenic, he is not below the threshold for receiving anticoagulation. His INR is elevated but this does not confer any reduced risk of VTE.
Bottom line
This patient should receive VTE prophylaxis with either subcutaneous heparin or LMWH during his hospital admission.
References
1. Khoury T et al. The complex role of anticoagulation in cirrhosis: An updated review of where we are and where we are going. Digestion. 2016 Mar;93(2):149-59.
2. Aggarwal A. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: Systematic review. World J Gastroenterol. 2014 May 21;20(19):5737-45.
3. Søgaard KK et al. Risk of venous thromboembolism in patients with liver disease: A nationwide population-based case-control study. Am J Gastroenterol. 2009 Jan;104(1):96-101.
4. Walsh KA et al. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann Pharmacother. 2013;47(3):333-9.
5. Bogari H et al. Risk-assessment and pharmacological prophylaxis of venous thromboembolism in hospitalized patients with chronic liver disease. Thromb Res. 2014 Dec;134(6):1220-3.
6. Intagliata NM et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014 Jan;34(1):26-32.
7. Pincus KJ et al. Risk of venous thromboembolism in patients with chronic liver disease and the utility of venous thromboembolism prophylaxis. Ann Pharmacother. 2012 Jun;46(6):873-8.
8. Lishman T et al. Established and new-generation antithrombotic drugs in patients with cirrhosis – possibilities and caveats. J Hepatol. 2013 Aug;59(2):358-66.
9. Tripodi A et al. Coagulation parameters in patients with cirrhosis and portal vein thrombosis treated sequentially with low molecular weight heparin and vitamin K antagonists. Dig Liver Dis. 2016 Oct;48(10):1208-13.
10. Caldeira D et al. Risk of drug-induced liver injury with the new oral anticoagulants: Systematic review and meta-analysis. Heart. 2014 Apr;100(7):550-6.
11. Qamar A et al. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018 May 15;71(19):2162-75.
12. Barbar S et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua prediction score. J Thromb Haemost. 2010 Nov;8(11):2450-7.
DOACs may be a practical option for some CLD patients
DOACs may be a practical option for some CLD patients
Case
A 60-year-old man with cirrhosis is admitted to the hospital with concern for spontaneous bacterial peritonitis. His body mass index is 35 kg/m2. He is severely deconditioned and largely bed bound. His admission labs show thrombocytopenia (platelets 65,000/mcL) and an elevated international normalized ratio (INR) of 1.6. Should this patient be placed on venous thromboembolism (VTE) prophylaxis on admission?
Brief overview
Patients with chronic liver disease (CLD) have previously been considered “auto-anticoagulated” because of markers of increased bleeding risk, including a decreased platelet count and elevated INR, prothrombin time, and activated partial thromboplastin time. It is being increasingly recognized, however, that CLD often represents a hypercoagulable state despite these abnormalities.1
While cirrhotic patients produce less of several procoagulant substances (such as factors II, V, VII, X, XI, XII, XIII, and fibrinogen), they are also deficient in multiple anticoagulant factors (such as proteins C and S and antithrombin) and fibrinolytics (plasminogen). While the prothrombin time and activated partial thromboplastin time are sensitive to levels of procoagulant proteins in plasma, they do not measure response to the natural anticoagulants and therefore do not reflect an accurate picture of a cirrhotic patient’s risk of developing thrombosis. In addition, cirrhotic patients have many other risk factors for thrombosis, including poor functional status, frequent hospitalization, and elevated estrogen levels.
Overview of the data
VTE incidence among patients with CLD has varied across studies, ranging from 0.5% to 6.3%.2 A systemic review of VTE risk in cirrhotic patients concluded that they “have a significant risk of VTE, if not higher than noncirrhotic patients and this risk cannot be trivialized or ignored.”2
In a nationwide Danish case-control study, patients with cirrhosis had a 1.7 times increased risk of VTE, compared with the general population.3 Hypoalbuminemia appears to be one of the strongest associated risk factors for VTE in these patients, likely as a reflection of the degree of liver synthetic dysfunction (and therefore decreased synthesis of anticoagulant factors). One study showed that patients with an albumin of less than 1.9 g/dL had a VTE risk five times higher than patients with an albumin of 2.8 g/dL or higher.4
Prophylaxis
Given the increased risks of bleeding and thrombosis in patients with cirrhosis, how should VTE prophylaxis be managed in hospitalized patients? While current guidelines do not specifically address the use of pharmacologic prophylaxis in cirrhotic patients, the Padua Predictor Score, which is used to assess VTE risk in the general hospital population, has also been shown to be helpful in the subpopulation of patients with CLD (Table 1).

In one study, cirrhotic patients who were “high risk” by Padua Predictor score were over 12 times more likely to develop VTE than those who were “low risk.”5 Bleeding risk appears to be fairly low, and similar to those patients not receiving prophylactic anticoagulation. One retrospective case series of hospitalized cirrhotic patients receiving thromboprophylaxis showed a rate of GI bleeding of 2.5% (9 of 355 patients); the rate of major bleeding was less than 1%.6
Selection of anticoagulant for VTE prophylaxis should be similar to non-CLD patients. The choice of agent (low-molecular-weight heparin (LMWH) or unfractionated heparin) and dosing depends on factors including renal function and bodyweight. If anticoagulation is contraindicated (because of thrombocytopenia, for example), then mechanical prophylaxis should be considered.7
Treatment

What about anticoagulation in patients with a known VTE? Food and Drug Administration safety recommendations are based on Child-Pugh class, although the current data on the safety and efficacy of full dose anticoagulation therapy for VTE in patients with cirrhosis are limited (Table 2). At this point, LMWH is often the preferred choice for anticoagulation in CLD patients. However, some limitations exist including the need for frequent subcutaneous injections and limited reliability of anti–factor Xa levels.
Cirrhotic patients often fail to achieve target anti–factor Xa levels on standard prophylactic and therapeutic doses of enoxaparin. This, however, is likely a lab anomaly as in vitro studies have shown that cirrhotic patients may show an increased response to LMWH despite reduced anti–factor Xa levels.8 Thus, LMWH remains the standard of care for many CLD patients.
The use of vitamin K antagonists (VKAs) such as warfarin for VTE treatment can be difficult to manage. Traditionally CLD patients have been started on lower doses of warfarin but given their already elevated INR, this may lead to a subtherapeutic dose of VKAs. A recent study of 23 patients with cirrhosis demonstrated that a target INR of 2-3 can be reached with VKA doses similar to those in noncirrhotic patients.9 These data support the practice of using the same VKA dosing strategies for CLD patients, and selecting a starting dose based on patient parameters such as age and weight.
While the use of direct oral anticoagulants (DOACs) for this patient population is still not a common practice, they may be a practical option for some CLD patients. A meta-analysis found that the currently used DOACs have no significant risk of drug-induced hepatic injury.10 Currently, only observational data are available to assess the benefits and risks of bleeding with DOACs in this patient population, as patients with significant liver disease were excluded from the major randomized trials.11 DOACs may also represent a complicated choice for some patients given the effect of liver injury on their metabolism (Table 3).
Application of data to the original case
This patient should be assessed for both risk of VTE and risk of bleeding during the hospital admission. CLD patients likely have a risk of VTE similar to (or even greater than) that of general medical patients. The Padua score for this patient is 4 (bed rest, body mass index) indicating that he is at high risk of VTE. While he is thrombocytopenic, he is not below the threshold for receiving anticoagulation. His INR is elevated but this does not confer any reduced risk of VTE.
Bottom line
This patient should receive VTE prophylaxis with either subcutaneous heparin or LMWH during his hospital admission.
References
1. Khoury T et al. The complex role of anticoagulation in cirrhosis: An updated review of where we are and where we are going. Digestion. 2016 Mar;93(2):149-59.
2. Aggarwal A. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: Systematic review. World J Gastroenterol. 2014 May 21;20(19):5737-45.
3. Søgaard KK et al. Risk of venous thromboembolism in patients with liver disease: A nationwide population-based case-control study. Am J Gastroenterol. 2009 Jan;104(1):96-101.
4. Walsh KA et al. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann Pharmacother. 2013;47(3):333-9.
5. Bogari H et al. Risk-assessment and pharmacological prophylaxis of venous thromboembolism in hospitalized patients with chronic liver disease. Thromb Res. 2014 Dec;134(6):1220-3.
6. Intagliata NM et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014 Jan;34(1):26-32.
7. Pincus KJ et al. Risk of venous thromboembolism in patients with chronic liver disease and the utility of venous thromboembolism prophylaxis. Ann Pharmacother. 2012 Jun;46(6):873-8.
8. Lishman T et al. Established and new-generation antithrombotic drugs in patients with cirrhosis – possibilities and caveats. J Hepatol. 2013 Aug;59(2):358-66.
9. Tripodi A et al. Coagulation parameters in patients with cirrhosis and portal vein thrombosis treated sequentially with low molecular weight heparin and vitamin K antagonists. Dig Liver Dis. 2016 Oct;48(10):1208-13.
10. Caldeira D et al. Risk of drug-induced liver injury with the new oral anticoagulants: Systematic review and meta-analysis. Heart. 2014 Apr;100(7):550-6.
11. Qamar A et al. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018 May 15;71(19):2162-75.
12. Barbar S et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua prediction score. J Thromb Haemost. 2010 Nov;8(11):2450-7.
Case
A 60-year-old man with cirrhosis is admitted to the hospital with concern for spontaneous bacterial peritonitis. His body mass index is 35 kg/m2. He is severely deconditioned and largely bed bound. His admission labs show thrombocytopenia (platelets 65,000/mcL) and an elevated international normalized ratio (INR) of 1.6. Should this patient be placed on venous thromboembolism (VTE) prophylaxis on admission?
Brief overview
Patients with chronic liver disease (CLD) have previously been considered “auto-anticoagulated” because of markers of increased bleeding risk, including a decreased platelet count and elevated INR, prothrombin time, and activated partial thromboplastin time. It is being increasingly recognized, however, that CLD often represents a hypercoagulable state despite these abnormalities.1
While cirrhotic patients produce less of several procoagulant substances (such as factors II, V, VII, X, XI, XII, XIII, and fibrinogen), they are also deficient in multiple anticoagulant factors (such as proteins C and S and antithrombin) and fibrinolytics (plasminogen). While the prothrombin time and activated partial thromboplastin time are sensitive to levels of procoagulant proteins in plasma, they do not measure response to the natural anticoagulants and therefore do not reflect an accurate picture of a cirrhotic patient’s risk of developing thrombosis. In addition, cirrhotic patients have many other risk factors for thrombosis, including poor functional status, frequent hospitalization, and elevated estrogen levels.
Overview of the data
VTE incidence among patients with CLD has varied across studies, ranging from 0.5% to 6.3%.2 A systemic review of VTE risk in cirrhotic patients concluded that they “have a significant risk of VTE, if not higher than noncirrhotic patients and this risk cannot be trivialized or ignored.”2
In a nationwide Danish case-control study, patients with cirrhosis had a 1.7 times increased risk of VTE, compared with the general population.3 Hypoalbuminemia appears to be one of the strongest associated risk factors for VTE in these patients, likely as a reflection of the degree of liver synthetic dysfunction (and therefore decreased synthesis of anticoagulant factors). One study showed that patients with an albumin of less than 1.9 g/dL had a VTE risk five times higher than patients with an albumin of 2.8 g/dL or higher.4
Prophylaxis
Given the increased risks of bleeding and thrombosis in patients with cirrhosis, how should VTE prophylaxis be managed in hospitalized patients? While current guidelines do not specifically address the use of pharmacologic prophylaxis in cirrhotic patients, the Padua Predictor Score, which is used to assess VTE risk in the general hospital population, has also been shown to be helpful in the subpopulation of patients with CLD (Table 1).

In one study, cirrhotic patients who were “high risk” by Padua Predictor score were over 12 times more likely to develop VTE than those who were “low risk.”5 Bleeding risk appears to be fairly low, and similar to those patients not receiving prophylactic anticoagulation. One retrospective case series of hospitalized cirrhotic patients receiving thromboprophylaxis showed a rate of GI bleeding of 2.5% (9 of 355 patients); the rate of major bleeding was less than 1%.6
Selection of anticoagulant for VTE prophylaxis should be similar to non-CLD patients. The choice of agent (low-molecular-weight heparin (LMWH) or unfractionated heparin) and dosing depends on factors including renal function and bodyweight. If anticoagulation is contraindicated (because of thrombocytopenia, for example), then mechanical prophylaxis should be considered.7
Treatment

What about anticoagulation in patients with a known VTE? Food and Drug Administration safety recommendations are based on Child-Pugh class, although the current data on the safety and efficacy of full dose anticoagulation therapy for VTE in patients with cirrhosis are limited (Table 2). At this point, LMWH is often the preferred choice for anticoagulation in CLD patients. However, some limitations exist including the need for frequent subcutaneous injections and limited reliability of anti–factor Xa levels.
Cirrhotic patients often fail to achieve target anti–factor Xa levels on standard prophylactic and therapeutic doses of enoxaparin. This, however, is likely a lab anomaly as in vitro studies have shown that cirrhotic patients may show an increased response to LMWH despite reduced anti–factor Xa levels.8 Thus, LMWH remains the standard of care for many CLD patients.
The use of vitamin K antagonists (VKAs) such as warfarin for VTE treatment can be difficult to manage. Traditionally CLD patients have been started on lower doses of warfarin but given their already elevated INR, this may lead to a subtherapeutic dose of VKAs. A recent study of 23 patients with cirrhosis demonstrated that a target INR of 2-3 can be reached with VKA doses similar to those in noncirrhotic patients.9 These data support the practice of using the same VKA dosing strategies for CLD patients, and selecting a starting dose based on patient parameters such as age and weight.
While the use of direct oral anticoagulants (DOACs) for this patient population is still not a common practice, they may be a practical option for some CLD patients. A meta-analysis found that the currently used DOACs have no significant risk of drug-induced hepatic injury.10 Currently, only observational data are available to assess the benefits and risks of bleeding with DOACs in this patient population, as patients with significant liver disease were excluded from the major randomized trials.11 DOACs may also represent a complicated choice for some patients given the effect of liver injury on their metabolism (Table 3).
Application of data to the original case
This patient should be assessed for both risk of VTE and risk of bleeding during the hospital admission. CLD patients likely have a risk of VTE similar to (or even greater than) that of general medical patients. The Padua score for this patient is 4 (bed rest, body mass index) indicating that he is at high risk of VTE. While he is thrombocytopenic, he is not below the threshold for receiving anticoagulation. His INR is elevated but this does not confer any reduced risk of VTE.
Bottom line
This patient should receive VTE prophylaxis with either subcutaneous heparin or LMWH during his hospital admission.
References
1. Khoury T et al. The complex role of anticoagulation in cirrhosis: An updated review of where we are and where we are going. Digestion. 2016 Mar;93(2):149-59.
2. Aggarwal A. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: Systematic review. World J Gastroenterol. 2014 May 21;20(19):5737-45.
3. Søgaard KK et al. Risk of venous thromboembolism in patients with liver disease: A nationwide population-based case-control study. Am J Gastroenterol. 2009 Jan;104(1):96-101.
4. Walsh KA et al. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann Pharmacother. 2013;47(3):333-9.
5. Bogari H et al. Risk-assessment and pharmacological prophylaxis of venous thromboembolism in hospitalized patients with chronic liver disease. Thromb Res. 2014 Dec;134(6):1220-3.
6. Intagliata NM et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014 Jan;34(1):26-32.
7. Pincus KJ et al. Risk of venous thromboembolism in patients with chronic liver disease and the utility of venous thromboembolism prophylaxis. Ann Pharmacother. 2012 Jun;46(6):873-8.
8. Lishman T et al. Established and new-generation antithrombotic drugs in patients with cirrhosis – possibilities and caveats. J Hepatol. 2013 Aug;59(2):358-66.
9. Tripodi A et al. Coagulation parameters in patients with cirrhosis and portal vein thrombosis treated sequentially with low molecular weight heparin and vitamin K antagonists. Dig Liver Dis. 2016 Oct;48(10):1208-13.
10. Caldeira D et al. Risk of drug-induced liver injury with the new oral anticoagulants: Systematic review and meta-analysis. Heart. 2014 Apr;100(7):550-6.
11. Qamar A et al. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018 May 15;71(19):2162-75.
12. Barbar S et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua prediction score. J Thromb Haemost. 2010 Nov;8(11):2450-7.
Challenging Dogma: The banana bag
Necessary, or just another pretty fluid?
The dogma
Patients with alcohol use disorders (AUD) are at risk for nutritional and vitamin deficiencies and may suffer from linked disease states, including Wernicke’s encephalopathy. These conditions may be underrecognized; for instance, an autopsy study suggests that Wernicke’s encephalopathy may have a prevalence rate of 12.5% among alcoholics.1
When patients with AUD are hospitalized, they have often already received a standard IV solution (100 mg of thiamine, 1 mg of folate, 1-2 g of magnesium, and a multivitamin dissolved in saline or dextrose). The practice is common enough that the solution is informally referred to as a “banana bag,” due to the yellow hue imparted by thiamine and multivitamin. These fluids might then be readministered daily during the inpatient stay. But what is the evidence supporting this widespread practice?
The evidence
While the banana bag (or “rally pack”, as it’s also colloquially known) hanging at the patient’s side may look cool, it may not be helping her. Let’s break down the ingredients:
- Folate: Patients with alcohol use disorder are at higher risk for folate deficiency (attributable to poor intake and decreased absorption), but overall rates of folate deficiency are still quite low.2 In addition, most oral and parenteral multivitamins already contain at least 400 mcg folate – the benefit of adding further intravenous folate is not clear.
- Magnesium. Patients with AUD are also at higher risk for magnesium deficiency attributable to increased excretion. While decreased magnesium levels could theoretically increase the risk of alcohol withdrawal symptoms, a Cochrane review found no evidence to support routine supplementation.3
- Multivitamin. Despite theoretical advantages in these (often) malnourished patients, there are no published studies on the benefit or harm of administering a “pan-vitamin” injection. The standard IV formulation is slightly different than an oral vitamin (the IV contains vitamin K, for instance, and lacks calcium), but the bioavailability should be roughly the same, except in rare patients with intestinal malabsorption.4
- IV fluids. Pharmacies typically mix these ingredients in a liter of normal saline or 5% dextrose. Once again, though, individual patients will have different needs. A dehydrated patient would benefit more from normal saline, a patient with alcoholic ketoacidosis would benefit more from dextrose, and a patient with alcohol-related cardiomyopathy likely shouldn’t be getting large volume IV fluids at all.
- Thiamine. Thiamine deficiency is likely the most common and most concerning vitamin deficiency in this patient population. The typical banana bag contains 100 mg of thiamine, which has been the traditional recommended daily amount for Wernicke’s treatment. This dosage, however, was apparently chosen arbitrarily in the 1950s (based on what the authors considered to be a high dose) and current recommendations suggest higher doses given more frequently because of the relatively short half-life of parenteral thiamine.5
Takeaway
The banana bag is a “one-size-fits-all” approach that offers too much of some of its ingredients and not enough of others. It’s better to individualize treatment based on a patient’s needs and consider high-dose thiamine (500 mg one to three times daily) for those at risk for, or showing signs of, Wernicke’s encephalopathy.
Dr. Sehgal and Dr. Hanson are clinical associate professors of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. Dr. Sehgal (@rtsehgal) is a member of the editorial advisory board for The Hospitalist.
References
1. Torvik A et al. Brain lesions in alcoholics: a neuropathological study with clinical correlation. J Neurol Sci. 1982 Nov;56(2-3):233-48.
2. Schwab RA et al. Prevalence in folate deficiency in emergency department patients with alcohol-related illness or injury. Am J Emerg Med. 1992 May;10(3):203-7.
3. Sarai M et al. Magnesium for alcohol withdrawal. Cochrane Database Syst Rev. 2013 Jun 5;(6):CD008358.
4. Krishel S et al. Intravenous vitamins for alcoholics in the emergency department: a review. J Emerg Med. 1998 May-Jun;16(3):419-24.
5. Donnino MW et al. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50(6): 715-21.
Necessary, or just another pretty fluid?
Necessary, or just another pretty fluid?
The dogma
Patients with alcohol use disorders (AUD) are at risk for nutritional and vitamin deficiencies and may suffer from linked disease states, including Wernicke’s encephalopathy. These conditions may be underrecognized; for instance, an autopsy study suggests that Wernicke’s encephalopathy may have a prevalence rate of 12.5% among alcoholics.1
When patients with AUD are hospitalized, they have often already received a standard IV solution (100 mg of thiamine, 1 mg of folate, 1-2 g of magnesium, and a multivitamin dissolved in saline or dextrose). The practice is common enough that the solution is informally referred to as a “banana bag,” due to the yellow hue imparted by thiamine and multivitamin. These fluids might then be readministered daily during the inpatient stay. But what is the evidence supporting this widespread practice?
The evidence
While the banana bag (or “rally pack”, as it’s also colloquially known) hanging at the patient’s side may look cool, it may not be helping her. Let’s break down the ingredients:
- Folate: Patients with alcohol use disorder are at higher risk for folate deficiency (attributable to poor intake and decreased absorption), but overall rates of folate deficiency are still quite low.2 In addition, most oral and parenteral multivitamins already contain at least 400 mcg folate – the benefit of adding further intravenous folate is not clear.
- Magnesium. Patients with AUD are also at higher risk for magnesium deficiency attributable to increased excretion. While decreased magnesium levels could theoretically increase the risk of alcohol withdrawal symptoms, a Cochrane review found no evidence to support routine supplementation.3
- Multivitamin. Despite theoretical advantages in these (often) malnourished patients, there are no published studies on the benefit or harm of administering a “pan-vitamin” injection. The standard IV formulation is slightly different than an oral vitamin (the IV contains vitamin K, for instance, and lacks calcium), but the bioavailability should be roughly the same, except in rare patients with intestinal malabsorption.4
- IV fluids. Pharmacies typically mix these ingredients in a liter of normal saline or 5% dextrose. Once again, though, individual patients will have different needs. A dehydrated patient would benefit more from normal saline, a patient with alcoholic ketoacidosis would benefit more from dextrose, and a patient with alcohol-related cardiomyopathy likely shouldn’t be getting large volume IV fluids at all.
- Thiamine. Thiamine deficiency is likely the most common and most concerning vitamin deficiency in this patient population. The typical banana bag contains 100 mg of thiamine, which has been the traditional recommended daily amount for Wernicke’s treatment. This dosage, however, was apparently chosen arbitrarily in the 1950s (based on what the authors considered to be a high dose) and current recommendations suggest higher doses given more frequently because of the relatively short half-life of parenteral thiamine.5
Takeaway
The banana bag is a “one-size-fits-all” approach that offers too much of some of its ingredients and not enough of others. It’s better to individualize treatment based on a patient’s needs and consider high-dose thiamine (500 mg one to three times daily) for those at risk for, or showing signs of, Wernicke’s encephalopathy.
Dr. Sehgal and Dr. Hanson are clinical associate professors of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. Dr. Sehgal (@rtsehgal) is a member of the editorial advisory board for The Hospitalist.
References
1. Torvik A et al. Brain lesions in alcoholics: a neuropathological study with clinical correlation. J Neurol Sci. 1982 Nov;56(2-3):233-48.
2. Schwab RA et al. Prevalence in folate deficiency in emergency department patients with alcohol-related illness or injury. Am J Emerg Med. 1992 May;10(3):203-7.
3. Sarai M et al. Magnesium for alcohol withdrawal. Cochrane Database Syst Rev. 2013 Jun 5;(6):CD008358.
4. Krishel S et al. Intravenous vitamins for alcoholics in the emergency department: a review. J Emerg Med. 1998 May-Jun;16(3):419-24.
5. Donnino MW et al. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50(6): 715-21.
The dogma
Patients with alcohol use disorders (AUD) are at risk for nutritional and vitamin deficiencies and may suffer from linked disease states, including Wernicke’s encephalopathy. These conditions may be underrecognized; for instance, an autopsy study suggests that Wernicke’s encephalopathy may have a prevalence rate of 12.5% among alcoholics.1
When patients with AUD are hospitalized, they have often already received a standard IV solution (100 mg of thiamine, 1 mg of folate, 1-2 g of magnesium, and a multivitamin dissolved in saline or dextrose). The practice is common enough that the solution is informally referred to as a “banana bag,” due to the yellow hue imparted by thiamine and multivitamin. These fluids might then be readministered daily during the inpatient stay. But what is the evidence supporting this widespread practice?
The evidence
While the banana bag (or “rally pack”, as it’s also colloquially known) hanging at the patient’s side may look cool, it may not be helping her. Let’s break down the ingredients:
- Folate: Patients with alcohol use disorder are at higher risk for folate deficiency (attributable to poor intake and decreased absorption), but overall rates of folate deficiency are still quite low.2 In addition, most oral and parenteral multivitamins already contain at least 400 mcg folate – the benefit of adding further intravenous folate is not clear.
- Magnesium. Patients with AUD are also at higher risk for magnesium deficiency attributable to increased excretion. While decreased magnesium levels could theoretically increase the risk of alcohol withdrawal symptoms, a Cochrane review found no evidence to support routine supplementation.3
- Multivitamin. Despite theoretical advantages in these (often) malnourished patients, there are no published studies on the benefit or harm of administering a “pan-vitamin” injection. The standard IV formulation is slightly different than an oral vitamin (the IV contains vitamin K, for instance, and lacks calcium), but the bioavailability should be roughly the same, except in rare patients with intestinal malabsorption.4
- IV fluids. Pharmacies typically mix these ingredients in a liter of normal saline or 5% dextrose. Once again, though, individual patients will have different needs. A dehydrated patient would benefit more from normal saline, a patient with alcoholic ketoacidosis would benefit more from dextrose, and a patient with alcohol-related cardiomyopathy likely shouldn’t be getting large volume IV fluids at all.
- Thiamine. Thiamine deficiency is likely the most common and most concerning vitamin deficiency in this patient population. The typical banana bag contains 100 mg of thiamine, which has been the traditional recommended daily amount for Wernicke’s treatment. This dosage, however, was apparently chosen arbitrarily in the 1950s (based on what the authors considered to be a high dose) and current recommendations suggest higher doses given more frequently because of the relatively short half-life of parenteral thiamine.5
Takeaway
The banana bag is a “one-size-fits-all” approach that offers too much of some of its ingredients and not enough of others. It’s better to individualize treatment based on a patient’s needs and consider high-dose thiamine (500 mg one to three times daily) for those at risk for, or showing signs of, Wernicke’s encephalopathy.
Dr. Sehgal and Dr. Hanson are clinical associate professors of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. Dr. Sehgal (@rtsehgal) is a member of the editorial advisory board for The Hospitalist.
References
1. Torvik A et al. Brain lesions in alcoholics: a neuropathological study with clinical correlation. J Neurol Sci. 1982 Nov;56(2-3):233-48.
2. Schwab RA et al. Prevalence in folate deficiency in emergency department patients with alcohol-related illness or injury. Am J Emerg Med. 1992 May;10(3):203-7.
3. Sarai M et al. Magnesium for alcohol withdrawal. Cochrane Database Syst Rev. 2013 Jun 5;(6):CD008358.
4. Krishel S et al. Intravenous vitamins for alcoholics in the emergency department: a review. J Emerg Med. 1998 May-Jun;16(3):419-24.
5. Donnino MW et al. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50(6): 715-21.
Challenging dogma: Postop fever
The dogma
During our medical school and residency years, many of us learned the “Rule of W” as a helpful mnemonic for causes of postoperative fever: Wind (pulmonary causes, including atelectasis), Water (urinary tract infection), Wound (infection), Walking (deep venous thrombosis), and Wonder Drugs (drug fever). Classic teaching has been that noninfectious causes predominate during the first 48 hours post op, with infectious diseases taking over after that. Atelectasis is also very common in the immediate postoperative period, seen in up to 90% of patients by postoperative day 3, and is often taught as the primary cause of fever in the immediate postoperative period.1,2 But is this backed up by the evidence?
The evidence
A 2011 systematic review looked at the association between atelectasis and fever. Eight studies involving 998 postoperative patients were included, with the majority of cases being postcardiac or abdominal surgeries. Seven of the eight studies failed to show a significant association between early postoperative fever (EPF) and atelectasis; in the one “positive” study, atelectasis was assessed only once on postop day 4. The authors of the review concluded that “there is no clinical evidence suggesting that atelectasis is a major cause of early EPF”.3 A subsequent study of postoperative fever in pediatric patients showed similar negative results.4 This begs the question – does atelectasis cause fever at all? Likely not. In an animal study from 1963, experimentally induced atelectasis resulted in fever, but the fever appeared secondary to infectious causes (i.e. pneumonia in the affected lung) and resolved with antibiotic administration.5 It seems more likely that EPF is due to other factors, such as the increase in pyrogenic cytokines seen in the postoperative period.3
Takeaway
Atelectasis and early postoperative fever are both commonly seen after surgery, but the relationship appears to be simply an association, not causal. The “Rule of W” can be an effective mnemonic for the causes of postop fever – just make sure you use the updated version.
Dr. Sehgal is clinical associate professor of medicine, division of hospital medicine, South Texas Veterans Health Care System and University of Texas Health Sciences Center at San Antonio. He is a member of the editorial advisory board for The Hospitalist.
References
1. Carter AR, et al. Thoracic Alterations After Cardiac Surgery. AJR. 1983;140(3):475-81.
2. Chu DI, Agarwal S. Postoperative Complications. In: Doherty GM. eds. CURRENT Diagnosis & Treatment: Surgery, 14e New York, NY: McGraw-Hill; 2014.
3. Mavros MN, Velmahos GC, Falagas ME. Atelectasis as a Cause of Postoperative Fever. Chest. 2011;140(2):418-24. doi: 10.1378/chest.11-0127.
4. Kane JM, Friedman M, Mitchell JB, Wang D, Huang Z, Backer CL. Association Between Postoperative Fever and Atelectasis in Pediatric Patients. World J Pediatr Congenit Heart Surg. 2011;2(3):359-63. doi: 10.1177/2150135111403778.
5. Lansing AM, Jamieson WG. Mechanisms of fever in pulmonary atelectasis. Arch Surg. 1963;87:168-74.
6. Hyder JA, Wakeam E, Arora V, Hevelone ND, Lipsitz SR, Nguyen LL. Investigating the “Rule of W,” a Mnemonic for Teaching on Postoperative Complications. J Surg Educ. 2015;72(3):430-7. doi: 10.1016/j.jsurg.2014.11.004.
The dogma
During our medical school and residency years, many of us learned the “Rule of W” as a helpful mnemonic for causes of postoperative fever: Wind (pulmonary causes, including atelectasis), Water (urinary tract infection), Wound (infection), Walking (deep venous thrombosis), and Wonder Drugs (drug fever). Classic teaching has been that noninfectious causes predominate during the first 48 hours post op, with infectious diseases taking over after that. Atelectasis is also very common in the immediate postoperative period, seen in up to 90% of patients by postoperative day 3, and is often taught as the primary cause of fever in the immediate postoperative period.1,2 But is this backed up by the evidence?
The evidence
A 2011 systematic review looked at the association between atelectasis and fever. Eight studies involving 998 postoperative patients were included, with the majority of cases being postcardiac or abdominal surgeries. Seven of the eight studies failed to show a significant association between early postoperative fever (EPF) and atelectasis; in the one “positive” study, atelectasis was assessed only once on postop day 4. The authors of the review concluded that “there is no clinical evidence suggesting that atelectasis is a major cause of early EPF”.3 A subsequent study of postoperative fever in pediatric patients showed similar negative results.4 This begs the question – does atelectasis cause fever at all? Likely not. In an animal study from 1963, experimentally induced atelectasis resulted in fever, but the fever appeared secondary to infectious causes (i.e. pneumonia in the affected lung) and resolved with antibiotic administration.5 It seems more likely that EPF is due to other factors, such as the increase in pyrogenic cytokines seen in the postoperative period.3
Takeaway
Atelectasis and early postoperative fever are both commonly seen after surgery, but the relationship appears to be simply an association, not causal. The “Rule of W” can be an effective mnemonic for the causes of postop fever – just make sure you use the updated version.
Dr. Sehgal is clinical associate professor of medicine, division of hospital medicine, South Texas Veterans Health Care System and University of Texas Health Sciences Center at San Antonio. He is a member of the editorial advisory board for The Hospitalist.
References
1. Carter AR, et al. Thoracic Alterations After Cardiac Surgery. AJR. 1983;140(3):475-81.
2. Chu DI, Agarwal S. Postoperative Complications. In: Doherty GM. eds. CURRENT Diagnosis & Treatment: Surgery, 14e New York, NY: McGraw-Hill; 2014.
3. Mavros MN, Velmahos GC, Falagas ME. Atelectasis as a Cause of Postoperative Fever. Chest. 2011;140(2):418-24. doi: 10.1378/chest.11-0127.
4. Kane JM, Friedman M, Mitchell JB, Wang D, Huang Z, Backer CL. Association Between Postoperative Fever and Atelectasis in Pediatric Patients. World J Pediatr Congenit Heart Surg. 2011;2(3):359-63. doi: 10.1177/2150135111403778.
5. Lansing AM, Jamieson WG. Mechanisms of fever in pulmonary atelectasis. Arch Surg. 1963;87:168-74.
6. Hyder JA, Wakeam E, Arora V, Hevelone ND, Lipsitz SR, Nguyen LL. Investigating the “Rule of W,” a Mnemonic for Teaching on Postoperative Complications. J Surg Educ. 2015;72(3):430-7. doi: 10.1016/j.jsurg.2014.11.004.
The dogma
During our medical school and residency years, many of us learned the “Rule of W” as a helpful mnemonic for causes of postoperative fever: Wind (pulmonary causes, including atelectasis), Water (urinary tract infection), Wound (infection), Walking (deep venous thrombosis), and Wonder Drugs (drug fever). Classic teaching has been that noninfectious causes predominate during the first 48 hours post op, with infectious diseases taking over after that. Atelectasis is also very common in the immediate postoperative period, seen in up to 90% of patients by postoperative day 3, and is often taught as the primary cause of fever in the immediate postoperative period.1,2 But is this backed up by the evidence?
The evidence
A 2011 systematic review looked at the association between atelectasis and fever. Eight studies involving 998 postoperative patients were included, with the majority of cases being postcardiac or abdominal surgeries. Seven of the eight studies failed to show a significant association between early postoperative fever (EPF) and atelectasis; in the one “positive” study, atelectasis was assessed only once on postop day 4. The authors of the review concluded that “there is no clinical evidence suggesting that atelectasis is a major cause of early EPF”.3 A subsequent study of postoperative fever in pediatric patients showed similar negative results.4 This begs the question – does atelectasis cause fever at all? Likely not. In an animal study from 1963, experimentally induced atelectasis resulted in fever, but the fever appeared secondary to infectious causes (i.e. pneumonia in the affected lung) and resolved with antibiotic administration.5 It seems more likely that EPF is due to other factors, such as the increase in pyrogenic cytokines seen in the postoperative period.3
Takeaway
Atelectasis and early postoperative fever are both commonly seen after surgery, but the relationship appears to be simply an association, not causal. The “Rule of W” can be an effective mnemonic for the causes of postop fever – just make sure you use the updated version.
Dr. Sehgal is clinical associate professor of medicine, division of hospital medicine, South Texas Veterans Health Care System and University of Texas Health Sciences Center at San Antonio. He is a member of the editorial advisory board for The Hospitalist.
References
1. Carter AR, et al. Thoracic Alterations After Cardiac Surgery. AJR. 1983;140(3):475-81.
2. Chu DI, Agarwal S. Postoperative Complications. In: Doherty GM. eds. CURRENT Diagnosis & Treatment: Surgery, 14e New York, NY: McGraw-Hill; 2014.
3. Mavros MN, Velmahos GC, Falagas ME. Atelectasis as a Cause of Postoperative Fever. Chest. 2011;140(2):418-24. doi: 10.1378/chest.11-0127.
4. Kane JM, Friedman M, Mitchell JB, Wang D, Huang Z, Backer CL. Association Between Postoperative Fever and Atelectasis in Pediatric Patients. World J Pediatr Congenit Heart Surg. 2011;2(3):359-63. doi: 10.1177/2150135111403778.
5. Lansing AM, Jamieson WG. Mechanisms of fever in pulmonary atelectasis. Arch Surg. 1963;87:168-74.
6. Hyder JA, Wakeam E, Arora V, Hevelone ND, Lipsitz SR, Nguyen LL. Investigating the “Rule of W,” a Mnemonic for Teaching on Postoperative Complications. J Surg Educ. 2015;72(3):430-7. doi: 10.1016/j.jsurg.2014.11.004.





