User login
Post-Ebola mortality five times higher than general population
Survivors of the 2013-2016 Ebola epidemic in West Africa had lingering health effects of the disease. These patients had a much greater mortality in the first year after discharge, compared with the general population. Among those survivors who died, the majority appear to have expired because of renal failure, according to the results of an assessment the by the Guinean national survivors’ monitoring program.
The Surveillance Active en Ceinture obtained data on 1,130 (89%) of survivors of Ebola virus disease who were discharged from Ebola treatment units in Guinea. Compared with the general Guinean population, survivors of Ebola virus showed a five times increased risk of mortality within a year of follow-up after discharge, according to a survey of patients’ medical records and patients’ relatives, reported researchers Mory Keita, MD, and colleagues.
After 1 year, the difference in mortality between Ebola survivors and the general population had disappeared, according to the study published online in the Lancet Infectious Diseases.
A total of 59 deaths were reported among the discharged survivors available for follow-up. Renal failure was the assumed cause in 37 (63%) of these patients based on a description of reported anuria. The exact date of death was unknown for 43 of the 59 deaths. Of the 16 initial survivors for whom an exact date of death was available, 5 died within a month of discharge from Ebola treatment units, an additional 3 died within 3 months of discharge, 4 died 3-12 months after discharge, and 4 died more than a year after discharge (up to 21 months).
Age and area of residence (urban vs. nonurban area) were independently and significantly associated with mortality, with patients of older age (55 years or greater) and those from nonurban areas being at greater risk. Patient sex was not associated with survival.
Those survivors who were hospitalized for 12 days or more had more than double the risk of death than did those hospitalized less than 12 days, which was a statistically significant association.
“Survivors’ monitoring programs should be strengthened and should not focus exclusively on testing of bodily fluids,” the authors advised. “Furthermore, our study provides preliminary evidence that survivors hospitalized for longer than 12 days with Ebola virus disease could be at particularly high risk of mortality and should be specifically targeted, and perhaps also evidence that renal function should be monitored,” Dr. Keita and colleagues concluded.
The study was funded by the World Health Organization, International Medical Corps, and the Guinean Red Cross. The authors reported that they had no conflicts
SOURCE: Keita M et al. Lancet Infect Dis 2019 Sept 4. doi: 10.1016/S1473-3099(19)30313-5.
Survivors of the 2013-2016 Ebola epidemic in West Africa had lingering health effects of the disease. These patients had a much greater mortality in the first year after discharge, compared with the general population. Among those survivors who died, the majority appear to have expired because of renal failure, according to the results of an assessment the by the Guinean national survivors’ monitoring program.
The Surveillance Active en Ceinture obtained data on 1,130 (89%) of survivors of Ebola virus disease who were discharged from Ebola treatment units in Guinea. Compared with the general Guinean population, survivors of Ebola virus showed a five times increased risk of mortality within a year of follow-up after discharge, according to a survey of patients’ medical records and patients’ relatives, reported researchers Mory Keita, MD, and colleagues.
After 1 year, the difference in mortality between Ebola survivors and the general population had disappeared, according to the study published online in the Lancet Infectious Diseases.
A total of 59 deaths were reported among the discharged survivors available for follow-up. Renal failure was the assumed cause in 37 (63%) of these patients based on a description of reported anuria. The exact date of death was unknown for 43 of the 59 deaths. Of the 16 initial survivors for whom an exact date of death was available, 5 died within a month of discharge from Ebola treatment units, an additional 3 died within 3 months of discharge, 4 died 3-12 months after discharge, and 4 died more than a year after discharge (up to 21 months).
Age and area of residence (urban vs. nonurban area) were independently and significantly associated with mortality, with patients of older age (55 years or greater) and those from nonurban areas being at greater risk. Patient sex was not associated with survival.
Those survivors who were hospitalized for 12 days or more had more than double the risk of death than did those hospitalized less than 12 days, which was a statistically significant association.
“Survivors’ monitoring programs should be strengthened and should not focus exclusively on testing of bodily fluids,” the authors advised. “Furthermore, our study provides preliminary evidence that survivors hospitalized for longer than 12 days with Ebola virus disease could be at particularly high risk of mortality and should be specifically targeted, and perhaps also evidence that renal function should be monitored,” Dr. Keita and colleagues concluded.
The study was funded by the World Health Organization, International Medical Corps, and the Guinean Red Cross. The authors reported that they had no conflicts
SOURCE: Keita M et al. Lancet Infect Dis 2019 Sept 4. doi: 10.1016/S1473-3099(19)30313-5.
Survivors of the 2013-2016 Ebola epidemic in West Africa had lingering health effects of the disease. These patients had a much greater mortality in the first year after discharge, compared with the general population. Among those survivors who died, the majority appear to have expired because of renal failure, according to the results of an assessment the by the Guinean national survivors’ monitoring program.
The Surveillance Active en Ceinture obtained data on 1,130 (89%) of survivors of Ebola virus disease who were discharged from Ebola treatment units in Guinea. Compared with the general Guinean population, survivors of Ebola virus showed a five times increased risk of mortality within a year of follow-up after discharge, according to a survey of patients’ medical records and patients’ relatives, reported researchers Mory Keita, MD, and colleagues.
After 1 year, the difference in mortality between Ebola survivors and the general population had disappeared, according to the study published online in the Lancet Infectious Diseases.
A total of 59 deaths were reported among the discharged survivors available for follow-up. Renal failure was the assumed cause in 37 (63%) of these patients based on a description of reported anuria. The exact date of death was unknown for 43 of the 59 deaths. Of the 16 initial survivors for whom an exact date of death was available, 5 died within a month of discharge from Ebola treatment units, an additional 3 died within 3 months of discharge, 4 died 3-12 months after discharge, and 4 died more than a year after discharge (up to 21 months).
Age and area of residence (urban vs. nonurban area) were independently and significantly associated with mortality, with patients of older age (55 years or greater) and those from nonurban areas being at greater risk. Patient sex was not associated with survival.
Those survivors who were hospitalized for 12 days or more had more than double the risk of death than did those hospitalized less than 12 days, which was a statistically significant association.
“Survivors’ monitoring programs should be strengthened and should not focus exclusively on testing of bodily fluids,” the authors advised. “Furthermore, our study provides preliminary evidence that survivors hospitalized for longer than 12 days with Ebola virus disease could be at particularly high risk of mortality and should be specifically targeted, and perhaps also evidence that renal function should be monitored,” Dr. Keita and colleagues concluded.
The study was funded by the World Health Organization, International Medical Corps, and the Guinean Red Cross. The authors reported that they had no conflicts
SOURCE: Keita M et al. Lancet Infect Dis 2019 Sept 4. doi: 10.1016/S1473-3099(19)30313-5.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point: Renal failure was the assumed cause of death in 63% of the survivors based on reported anuria.
Major finding:
Study details: A postdischarge survey of 1,130 (89%) of the Ebola survivors and their relations in Guinea.
Disclosures: The study was funded by the World Health Organization, International Medical Corps, and the Guinean Red Cross. The authors reported that they had no conflicts.
Source: Keita M et al. Lancet Infect Dis. 2019 Sept 4. doi: 10.1016/S1473-3099(19)30313-5.
ID Blog: The story of syphilis, part I
Rise of a global scourge
The Great Pox, the French Disease, Cupid’s Disease – syphilis has had many names throughout history.
Why should we care about the history of syphilis? Surely syphilis has reached the status of a nonentity disease – in-and-out of the doctor’s office with a course of antibiotics and farewell to the problem. And on the surface, that is certainly true. For now. In the developed world. For those with access to reasonable health care.
But that is all the shiny surface of modern medical triumph. Despite successes in prevention throughout the late 20th and early 21st century, syphilis is making comeback. A growing reservoir of syphilis, often untreated, lies hidden by the invisibility of poorer nations and increasingly in the lower economic strata of the developed world. And the danger is increased by the rise of antibiotic-resistant strains of the disease.
Over the last decade, the European Union and several other high-income countries observed an increasing syphilis trend, according to a recent report by the European Centre for Disease Prevention and Control. And in the United States, the Centers for Disease Control and Prevention has expressed concern over “the rising tide of syphilis” and a “devastating surge in congenital syphilis.” Many reasons have been suggested for this resurgence of syphilis, including the prevalence of unprotected sex and the overall increase in multiple sexual partners in the sexually active population. This trend has been ascribed to a reduced fear of acquiring HIV from condomless sex because of the rise of antiretroviral therapies, which make HIV infection no longer a death sentence for those who have access to and can afford the drugs.
Men who have sex with men are the most affected population cited, which may in part be related to the trend in unprotected sex that has accompanied the decreasing fear of HIV. But in some countries, syphilis rates among heterosexual populations are on the increase as well. Even more troubling were the increases in syphilis diagnosed among pregnant women that were reported in high-income settings outside of the European Union, which led to increases in congenital syphilis infections.
According to a 2018 update on the global epidemiology of syphilis, each year an estimated 6 million new cases are diagnosed in people aged 15-49 years, with more than 300,000 fetal and neonatal deaths attributed to the disease. An additional 215,000 infants are at increased risk of early death because of prenatal infection.
For syphilis is indeed a nasty disease. But a remarkable one as well. It presents an almost textbook example of disease evolution and adaptation writ large. It is also a disease with equally remarkable properties – acute, systemic, latent, eruptive, and congenital in its various manifestations. As Sir William Osler, one of the brightest lights of medical education of his time, said in 1897: “I often tell my students that it [syphilis] is the only disease which they require to know thoroughly. Know syphilis in all its manifestations and relations, and all other things clinical will be added unto you.”
Syphilis is caused by the spirochete Treponema pallidum subspecies pallidum and is generally acquired by sexual contact. Congenital syphilis infection occurs by transplacental transmission.
In its modern manifestation, the disease evolves through several stages – primary, secondary, and tertiary. Primary, noncongenital infection is characterized by a lesion. This chancre, as it is called, occurs at the original site of infection, typically between 10 days and 3 months after exposure. The chancre usually appears on the genitals, but given the variety of sexual behaviors, chancres can also occur on the rectum, tongue, pharynx, breast, and so on. The myth of only choosing “a clean partner,” one without visible lesions, is misleading because vaginal and rectal lesions may not be easy to spot yet still remain profoundly infectious.
The secondary stage of an untreated infection occurs 2-3 months after the onset of chancre, and results in multisystem involvement as the spirochetes spread through the bloodstream. Symptoms include skin rash (involving the palms and the soles of the feet) and potentially a variety of other dermatologic manifestations. Fever and swollen lymph nodes may also be present before the disease moves into a latent stage, in which no clinical symptoms are evident. Following this, tertiary syphilis can occur 10-30 years after the initial infection in about 30% of the untreated population, resulting in neurosyphilis, cardiovascular syphilis, or late benign syphilis. Disease progression in tertiary syphilis can lead to dementia, disfigurement, and death.
Sounds bad, doesn’t it? But what we’ve just recounted is the relatively benign disease that modern syphilis has become. Syphilis began as a sweeping, lethal epidemic in the late 15th century spreading dread across the world from the Americas to Europe and then to Asia at a speed equal to the fastest sailing ships of the era.
Syphilis first appeared in Naples in its epidemic form in 1495. Recent anthropological and historical consensus has suggested that syphilis, as we know it today, like tobacco, potatoes, and maize is a product of the Americas that was brought to the Old World by the intrepid exploits of one Christopher Columbus in 1493. Just as the Spanish inadvertently brought smallpox to devastate the population of the New World, Christopher Columbus appears to have brought epidemic syphilis to the Old World in an ironic twist of fate.
Ruy Diaz de Isla, one of two Spanish physicians present when Christopher Columbus returned from his first voyage to America, wrote in a manuscript that Pinzon de Palos, the pilot of Columbus, and also other members of the crew already suffered from symptoms of what was likely syphilis on their return from the New World
Although there has been some controversy regarding the origin of the syphilis epidemic, a recent molecular study using a large collection of pathogenic Treponema strains indicated that venereal syphilis arose relatively recently in human history, and that the closest related syphilis-causing strains were found in South America, providing support for the Columbian theory of syphilis’s origin.
Syphilis flamed across Europe like wildfire, lit by a series of small wars that started shortly after Columbus’s return. Soldiers throughout history have indulged themselves in activities well primed for the spread of venereal disease, and the doughty warriors of the late 15th century were no exception. And throughout the next 500-plus years, syphilis and war rode across the world in tandem, like the white and red horsemen of the Apocalypse.
In its initial launch, syphilis had the help of Charles VIII, the King of France, who had invaded Italy in early 1495 with an army of more than 30,000 mercenaries recruited from across Europe. His forces conquered Naples, which was primarily defended by Spanish mercenaries.
When Charles VIII broke up his army, “mercenaries, infected with a mysterious, serious disease, returned to their native lands or moved elsewhere to wage war, spreading the disease across Europe.” The “Great Pox” initially struck Italy, France, Germany, and Switzerland in 1495; then Holland and Greece in the following year, reaching England and Scotland by 1497; and then Hungary, Poland, and the Scandinavian countries by 1500.
As this period was the Age of Exploration, French, Dutch, and English sailors soon carried syphilis across the rest of an unsuspecting world, with the disease reaching India in 1498 before moving also to Africa and then throughout the rest of Asia in the early 16th century.
And yet, one of the most remarkable parts of the story is the rapid transformation of syphilis from a deadly virulent epidemic to a (comparatively) benign endemic status. Which will be the subject of my next posting.

Mark Lesney is the managing editor of MDedge.com/IDPractioner . He has a PhD in Plant Virology and a PhD in the History of Science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor at the Georgetown University School of Medicine, Department of Biochemistry and Molecular & Cellular Biology, Washington, DC.
Rise of a global scourge
Rise of a global scourge
The Great Pox, the French Disease, Cupid’s Disease – syphilis has had many names throughout history.
Why should we care about the history of syphilis? Surely syphilis has reached the status of a nonentity disease – in-and-out of the doctor’s office with a course of antibiotics and farewell to the problem. And on the surface, that is certainly true. For now. In the developed world. For those with access to reasonable health care.
But that is all the shiny surface of modern medical triumph. Despite successes in prevention throughout the late 20th and early 21st century, syphilis is making comeback. A growing reservoir of syphilis, often untreated, lies hidden by the invisibility of poorer nations and increasingly in the lower economic strata of the developed world. And the danger is increased by the rise of antibiotic-resistant strains of the disease.
Over the last decade, the European Union and several other high-income countries observed an increasing syphilis trend, according to a recent report by the European Centre for Disease Prevention and Control. And in the United States, the Centers for Disease Control and Prevention has expressed concern over “the rising tide of syphilis” and a “devastating surge in congenital syphilis.” Many reasons have been suggested for this resurgence of syphilis, including the prevalence of unprotected sex and the overall increase in multiple sexual partners in the sexually active population. This trend has been ascribed to a reduced fear of acquiring HIV from condomless sex because of the rise of antiretroviral therapies, which make HIV infection no longer a death sentence for those who have access to and can afford the drugs.
Men who have sex with men are the most affected population cited, which may in part be related to the trend in unprotected sex that has accompanied the decreasing fear of HIV. But in some countries, syphilis rates among heterosexual populations are on the increase as well. Even more troubling were the increases in syphilis diagnosed among pregnant women that were reported in high-income settings outside of the European Union, which led to increases in congenital syphilis infections.
According to a 2018 update on the global epidemiology of syphilis, each year an estimated 6 million new cases are diagnosed in people aged 15-49 years, with more than 300,000 fetal and neonatal deaths attributed to the disease. An additional 215,000 infants are at increased risk of early death because of prenatal infection.
For syphilis is indeed a nasty disease. But a remarkable one as well. It presents an almost textbook example of disease evolution and adaptation writ large. It is also a disease with equally remarkable properties – acute, systemic, latent, eruptive, and congenital in its various manifestations. As Sir William Osler, one of the brightest lights of medical education of his time, said in 1897: “I often tell my students that it [syphilis] is the only disease which they require to know thoroughly. Know syphilis in all its manifestations and relations, and all other things clinical will be added unto you.”
Syphilis is caused by the spirochete Treponema pallidum subspecies pallidum and is generally acquired by sexual contact. Congenital syphilis infection occurs by transplacental transmission.
In its modern manifestation, the disease evolves through several stages – primary, secondary, and tertiary. Primary, noncongenital infection is characterized by a lesion. This chancre, as it is called, occurs at the original site of infection, typically between 10 days and 3 months after exposure. The chancre usually appears on the genitals, but given the variety of sexual behaviors, chancres can also occur on the rectum, tongue, pharynx, breast, and so on. The myth of only choosing “a clean partner,” one without visible lesions, is misleading because vaginal and rectal lesions may not be easy to spot yet still remain profoundly infectious.
The secondary stage of an untreated infection occurs 2-3 months after the onset of chancre, and results in multisystem involvement as the spirochetes spread through the bloodstream. Symptoms include skin rash (involving the palms and the soles of the feet) and potentially a variety of other dermatologic manifestations. Fever and swollen lymph nodes may also be present before the disease moves into a latent stage, in which no clinical symptoms are evident. Following this, tertiary syphilis can occur 10-30 years after the initial infection in about 30% of the untreated population, resulting in neurosyphilis, cardiovascular syphilis, or late benign syphilis. Disease progression in tertiary syphilis can lead to dementia, disfigurement, and death.
Sounds bad, doesn’t it? But what we’ve just recounted is the relatively benign disease that modern syphilis has become. Syphilis began as a sweeping, lethal epidemic in the late 15th century spreading dread across the world from the Americas to Europe and then to Asia at a speed equal to the fastest sailing ships of the era.
Syphilis first appeared in Naples in its epidemic form in 1495. Recent anthropological and historical consensus has suggested that syphilis, as we know it today, like tobacco, potatoes, and maize is a product of the Americas that was brought to the Old World by the intrepid exploits of one Christopher Columbus in 1493. Just as the Spanish inadvertently brought smallpox to devastate the population of the New World, Christopher Columbus appears to have brought epidemic syphilis to the Old World in an ironic twist of fate.
Ruy Diaz de Isla, one of two Spanish physicians present when Christopher Columbus returned from his first voyage to America, wrote in a manuscript that Pinzon de Palos, the pilot of Columbus, and also other members of the crew already suffered from symptoms of what was likely syphilis on their return from the New World
Although there has been some controversy regarding the origin of the syphilis epidemic, a recent molecular study using a large collection of pathogenic Treponema strains indicated that venereal syphilis arose relatively recently in human history, and that the closest related syphilis-causing strains were found in South America, providing support for the Columbian theory of syphilis’s origin.
Syphilis flamed across Europe like wildfire, lit by a series of small wars that started shortly after Columbus’s return. Soldiers throughout history have indulged themselves in activities well primed for the spread of venereal disease, and the doughty warriors of the late 15th century were no exception. And throughout the next 500-plus years, syphilis and war rode across the world in tandem, like the white and red horsemen of the Apocalypse.
In its initial launch, syphilis had the help of Charles VIII, the King of France, who had invaded Italy in early 1495 with an army of more than 30,000 mercenaries recruited from across Europe. His forces conquered Naples, which was primarily defended by Spanish mercenaries.
When Charles VIII broke up his army, “mercenaries, infected with a mysterious, serious disease, returned to their native lands or moved elsewhere to wage war, spreading the disease across Europe.” The “Great Pox” initially struck Italy, France, Germany, and Switzerland in 1495; then Holland and Greece in the following year, reaching England and Scotland by 1497; and then Hungary, Poland, and the Scandinavian countries by 1500.
As this period was the Age of Exploration, French, Dutch, and English sailors soon carried syphilis across the rest of an unsuspecting world, with the disease reaching India in 1498 before moving also to Africa and then throughout the rest of Asia in the early 16th century.
And yet, one of the most remarkable parts of the story is the rapid transformation of syphilis from a deadly virulent epidemic to a (comparatively) benign endemic status. Which will be the subject of my next posting.

Mark Lesney is the managing editor of MDedge.com/IDPractioner . He has a PhD in Plant Virology and a PhD in the History of Science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor at the Georgetown University School of Medicine, Department of Biochemistry and Molecular & Cellular Biology, Washington, DC.
The Great Pox, the French Disease, Cupid’s Disease – syphilis has had many names throughout history.
Why should we care about the history of syphilis? Surely syphilis has reached the status of a nonentity disease – in-and-out of the doctor’s office with a course of antibiotics and farewell to the problem. And on the surface, that is certainly true. For now. In the developed world. For those with access to reasonable health care.
But that is all the shiny surface of modern medical triumph. Despite successes in prevention throughout the late 20th and early 21st century, syphilis is making comeback. A growing reservoir of syphilis, often untreated, lies hidden by the invisibility of poorer nations and increasingly in the lower economic strata of the developed world. And the danger is increased by the rise of antibiotic-resistant strains of the disease.
Over the last decade, the European Union and several other high-income countries observed an increasing syphilis trend, according to a recent report by the European Centre for Disease Prevention and Control. And in the United States, the Centers for Disease Control and Prevention has expressed concern over “the rising tide of syphilis” and a “devastating surge in congenital syphilis.” Many reasons have been suggested for this resurgence of syphilis, including the prevalence of unprotected sex and the overall increase in multiple sexual partners in the sexually active population. This trend has been ascribed to a reduced fear of acquiring HIV from condomless sex because of the rise of antiretroviral therapies, which make HIV infection no longer a death sentence for those who have access to and can afford the drugs.
Men who have sex with men are the most affected population cited, which may in part be related to the trend in unprotected sex that has accompanied the decreasing fear of HIV. But in some countries, syphilis rates among heterosexual populations are on the increase as well. Even more troubling were the increases in syphilis diagnosed among pregnant women that were reported in high-income settings outside of the European Union, which led to increases in congenital syphilis infections.
According to a 2018 update on the global epidemiology of syphilis, each year an estimated 6 million new cases are diagnosed in people aged 15-49 years, with more than 300,000 fetal and neonatal deaths attributed to the disease. An additional 215,000 infants are at increased risk of early death because of prenatal infection.
For syphilis is indeed a nasty disease. But a remarkable one as well. It presents an almost textbook example of disease evolution and adaptation writ large. It is also a disease with equally remarkable properties – acute, systemic, latent, eruptive, and congenital in its various manifestations. As Sir William Osler, one of the brightest lights of medical education of his time, said in 1897: “I often tell my students that it [syphilis] is the only disease which they require to know thoroughly. Know syphilis in all its manifestations and relations, and all other things clinical will be added unto you.”
Syphilis is caused by the spirochete Treponema pallidum subspecies pallidum and is generally acquired by sexual contact. Congenital syphilis infection occurs by transplacental transmission.
In its modern manifestation, the disease evolves through several stages – primary, secondary, and tertiary. Primary, noncongenital infection is characterized by a lesion. This chancre, as it is called, occurs at the original site of infection, typically between 10 days and 3 months after exposure. The chancre usually appears on the genitals, but given the variety of sexual behaviors, chancres can also occur on the rectum, tongue, pharynx, breast, and so on. The myth of only choosing “a clean partner,” one without visible lesions, is misleading because vaginal and rectal lesions may not be easy to spot yet still remain profoundly infectious.
The secondary stage of an untreated infection occurs 2-3 months after the onset of chancre, and results in multisystem involvement as the spirochetes spread through the bloodstream. Symptoms include skin rash (involving the palms and the soles of the feet) and potentially a variety of other dermatologic manifestations. Fever and swollen lymph nodes may also be present before the disease moves into a latent stage, in which no clinical symptoms are evident. Following this, tertiary syphilis can occur 10-30 years after the initial infection in about 30% of the untreated population, resulting in neurosyphilis, cardiovascular syphilis, or late benign syphilis. Disease progression in tertiary syphilis can lead to dementia, disfigurement, and death.
Sounds bad, doesn’t it? But what we’ve just recounted is the relatively benign disease that modern syphilis has become. Syphilis began as a sweeping, lethal epidemic in the late 15th century spreading dread across the world from the Americas to Europe and then to Asia at a speed equal to the fastest sailing ships of the era.
Syphilis first appeared in Naples in its epidemic form in 1495. Recent anthropological and historical consensus has suggested that syphilis, as we know it today, like tobacco, potatoes, and maize is a product of the Americas that was brought to the Old World by the intrepid exploits of one Christopher Columbus in 1493. Just as the Spanish inadvertently brought smallpox to devastate the population of the New World, Christopher Columbus appears to have brought epidemic syphilis to the Old World in an ironic twist of fate.
Ruy Diaz de Isla, one of two Spanish physicians present when Christopher Columbus returned from his first voyage to America, wrote in a manuscript that Pinzon de Palos, the pilot of Columbus, and also other members of the crew already suffered from symptoms of what was likely syphilis on their return from the New World
Although there has been some controversy regarding the origin of the syphilis epidemic, a recent molecular study using a large collection of pathogenic Treponema strains indicated that venereal syphilis arose relatively recently in human history, and that the closest related syphilis-causing strains were found in South America, providing support for the Columbian theory of syphilis’s origin.
Syphilis flamed across Europe like wildfire, lit by a series of small wars that started shortly after Columbus’s return. Soldiers throughout history have indulged themselves in activities well primed for the spread of venereal disease, and the doughty warriors of the late 15th century were no exception. And throughout the next 500-plus years, syphilis and war rode across the world in tandem, like the white and red horsemen of the Apocalypse.
In its initial launch, syphilis had the help of Charles VIII, the King of France, who had invaded Italy in early 1495 with an army of more than 30,000 mercenaries recruited from across Europe. His forces conquered Naples, which was primarily defended by Spanish mercenaries.
When Charles VIII broke up his army, “mercenaries, infected with a mysterious, serious disease, returned to their native lands or moved elsewhere to wage war, spreading the disease across Europe.” The “Great Pox” initially struck Italy, France, Germany, and Switzerland in 1495; then Holland and Greece in the following year, reaching England and Scotland by 1497; and then Hungary, Poland, and the Scandinavian countries by 1500.
As this period was the Age of Exploration, French, Dutch, and English sailors soon carried syphilis across the rest of an unsuspecting world, with the disease reaching India in 1498 before moving also to Africa and then throughout the rest of Asia in the early 16th century.
And yet, one of the most remarkable parts of the story is the rapid transformation of syphilis from a deadly virulent epidemic to a (comparatively) benign endemic status. Which will be the subject of my next posting.

Mark Lesney is the managing editor of MDedge.com/IDPractioner . He has a PhD in Plant Virology and a PhD in the History of Science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor at the Georgetown University School of Medicine, Department of Biochemistry and Molecular & Cellular Biology, Washington, DC.
HCV coinfection adds to cardiovascular risk in HIV-infected patients
Hepatitis C virus (HCV) coinfection, as well as an accumulation of viral and bacterial infections, was independently associated with the risk of developing a cardiovascular event in HIV-infected patients, according to the results of a large retrospective analysis.
The study comprised 823 patients at a single institution during 1982-2018. The researchers assessed those patients who had at least two visits to the HIV clinic, data concerning herpes varicella zoster virus (VZV) reactivation, and bacterial infections. Data on HCV coinfection status (as determined by HCV antibodies and qualitative HCV-PCR) were also available, according to Miguel Genebat, MD, of Virgen del Rocío University Hospital, Seville, Spain, and colleagues.
During the observational period, 58 patients (7%) experienced a cardiovascular event at a median age of 47 years. Most of these patients (50, 86%) had effective HIV treatment, with their viral load being persistently undetectable.
In terms of standard cardiovascular disease (CVD) risk factors, hypercholesterolemia was present in 31 patients (53%) and only 11 subjects (19%) had diabetes. This left 24 “low-risk” subjects, 5 of whom (21%) developed recurrent CVD and 8 of whom (33%) died after the development of cardiovascular disease.
The most frequent cardiovascular event was acute coronary syndrome (ACS), developed by 38 patients, with 14 (24%) of these individuals having recurrent CVD events. Among the 58 patients who experienced a cardiovascular event, 21 (36%) died, 17 from cardiovascular disease, 2 from cancer, and 2 each from acute bacterial infection and end-stage liver disease.
The researchers examined other variables potentially associated with the development of cardiovascular disease. They performed a multivariate analysis considering the added burden of infections and found that advanced age at HIV-1 diagnosis (OR, 1.07), a T-CD4 nadir of less than 200 cells/mcL (OR, 2.01), a diagnosis of HIV prior to combined antiretroviral therapy availability in 1996 (OR, 2.35), and cumulative infections greater than 2 (OR, 3.63), were all significantly and independently associated with the risk of developing a cardiovascular event.
They also found that HCV coinfection (OR, 2.84) on its own in simple multivariate analysis increased the risk of developing a CVD event in HIV-infected subjects. There was insufficient power to tease out the individual risk of other infections, such as herpes zoster virus and bacterial infections, hence the use of cumulative infections reported above.
The researchers concluded that potential strategies to minimize cardiovascular risk in these subjects could be treating HCV coinfection in all subjects independently of liver fibrosis stage, starting cART as soon as possible, and immunizing for those infections for which effective vaccine are available.
The authors reported that they had no conflicts of interest.
SOURCE: Genebat M. et al. Antiviral Res. 2019 Sep;169:104527.
Hepatitis C virus (HCV) coinfection, as well as an accumulation of viral and bacterial infections, was independently associated with the risk of developing a cardiovascular event in HIV-infected patients, according to the results of a large retrospective analysis.
The study comprised 823 patients at a single institution during 1982-2018. The researchers assessed those patients who had at least two visits to the HIV clinic, data concerning herpes varicella zoster virus (VZV) reactivation, and bacterial infections. Data on HCV coinfection status (as determined by HCV antibodies and qualitative HCV-PCR) were also available, according to Miguel Genebat, MD, of Virgen del Rocío University Hospital, Seville, Spain, and colleagues.
During the observational period, 58 patients (7%) experienced a cardiovascular event at a median age of 47 years. Most of these patients (50, 86%) had effective HIV treatment, with their viral load being persistently undetectable.
In terms of standard cardiovascular disease (CVD) risk factors, hypercholesterolemia was present in 31 patients (53%) and only 11 subjects (19%) had diabetes. This left 24 “low-risk” subjects, 5 of whom (21%) developed recurrent CVD and 8 of whom (33%) died after the development of cardiovascular disease.
The most frequent cardiovascular event was acute coronary syndrome (ACS), developed by 38 patients, with 14 (24%) of these individuals having recurrent CVD events. Among the 58 patients who experienced a cardiovascular event, 21 (36%) died, 17 from cardiovascular disease, 2 from cancer, and 2 each from acute bacterial infection and end-stage liver disease.
The researchers examined other variables potentially associated with the development of cardiovascular disease. They performed a multivariate analysis considering the added burden of infections and found that advanced age at HIV-1 diagnosis (OR, 1.07), a T-CD4 nadir of less than 200 cells/mcL (OR, 2.01), a diagnosis of HIV prior to combined antiretroviral therapy availability in 1996 (OR, 2.35), and cumulative infections greater than 2 (OR, 3.63), were all significantly and independently associated with the risk of developing a cardiovascular event.
They also found that HCV coinfection (OR, 2.84) on its own in simple multivariate analysis increased the risk of developing a CVD event in HIV-infected subjects. There was insufficient power to tease out the individual risk of other infections, such as herpes zoster virus and bacterial infections, hence the use of cumulative infections reported above.
The researchers concluded that potential strategies to minimize cardiovascular risk in these subjects could be treating HCV coinfection in all subjects independently of liver fibrosis stage, starting cART as soon as possible, and immunizing for those infections for which effective vaccine are available.
The authors reported that they had no conflicts of interest.
SOURCE: Genebat M. et al. Antiviral Res. 2019 Sep;169:104527.
Hepatitis C virus (HCV) coinfection, as well as an accumulation of viral and bacterial infections, was independently associated with the risk of developing a cardiovascular event in HIV-infected patients, according to the results of a large retrospective analysis.
The study comprised 823 patients at a single institution during 1982-2018. The researchers assessed those patients who had at least two visits to the HIV clinic, data concerning herpes varicella zoster virus (VZV) reactivation, and bacterial infections. Data on HCV coinfection status (as determined by HCV antibodies and qualitative HCV-PCR) were also available, according to Miguel Genebat, MD, of Virgen del Rocío University Hospital, Seville, Spain, and colleagues.
During the observational period, 58 patients (7%) experienced a cardiovascular event at a median age of 47 years. Most of these patients (50, 86%) had effective HIV treatment, with their viral load being persistently undetectable.
In terms of standard cardiovascular disease (CVD) risk factors, hypercholesterolemia was present in 31 patients (53%) and only 11 subjects (19%) had diabetes. This left 24 “low-risk” subjects, 5 of whom (21%) developed recurrent CVD and 8 of whom (33%) died after the development of cardiovascular disease.
The most frequent cardiovascular event was acute coronary syndrome (ACS), developed by 38 patients, with 14 (24%) of these individuals having recurrent CVD events. Among the 58 patients who experienced a cardiovascular event, 21 (36%) died, 17 from cardiovascular disease, 2 from cancer, and 2 each from acute bacterial infection and end-stage liver disease.
The researchers examined other variables potentially associated with the development of cardiovascular disease. They performed a multivariate analysis considering the added burden of infections and found that advanced age at HIV-1 diagnosis (OR, 1.07), a T-CD4 nadir of less than 200 cells/mcL (OR, 2.01), a diagnosis of HIV prior to combined antiretroviral therapy availability in 1996 (OR, 2.35), and cumulative infections greater than 2 (OR, 3.63), were all significantly and independently associated with the risk of developing a cardiovascular event.
They also found that HCV coinfection (OR, 2.84) on its own in simple multivariate analysis increased the risk of developing a CVD event in HIV-infected subjects. There was insufficient power to tease out the individual risk of other infections, such as herpes zoster virus and bacterial infections, hence the use of cumulative infections reported above.
The researchers concluded that potential strategies to minimize cardiovascular risk in these subjects could be treating HCV coinfection in all subjects independently of liver fibrosis stage, starting cART as soon as possible, and immunizing for those infections for which effective vaccine are available.
The authors reported that they had no conflicts of interest.
SOURCE: Genebat M. et al. Antiviral Res. 2019 Sep;169:104527.
FROM ANTIVIRAL RESEARCH
FDA approves drug combo to treat highly resistant TB
The U.S. Food and Drug Administration granted special approval to a new drug combo intended for the treatment of “a limited and specific population of adult patients with extensively drug resistant, treatment-intolerant or nonresponsive multidrug-resistant pulmonary” tuberculosis, according to an FDA news release.
The effectiveness of the combination treatment of pretomanid tablets with bedaquiline and linezolid was shown in a clinical study of patients with extensively drug-resistant, treatment-intolerant, or nonresponsive multidrug-resistant pulmonary tuberculosis of the lungs. Of 107 infected patients who were evaluated 6 months after the end of therapy, 95 (89%) were deemed successes, which significantly exceeded the historical success rates for treatment of extensively drug-resistant TB, the FDA reported. The trial is sponsored by the Global Alliance for TB Drug Development.
The most common adverse effects reported included peripheral neuropathy, anemia, nausea, vomiting, headache, increased liver enzymes, dyspepsia, rash, visual impairment, low blood sugar, and diarrhea, according to the release.
“Multidrug-resistant TB and extensively drug-resistant TB are public health threats due to limited treatment options. New treatments are important to meet patient national and global health needs,” stated FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD, in the release. She also explained that the approval marked the second time a drug was approved under the “Limited Population Pathway for Antibacterial and Antifungal Drugs, a pathway advanced by Congress to spur development of drugs targeting infections that lack effective therapies.”
In 2016, the World Health Organization reported that there were an estimated 490,000 new cases of multidrug-resistant TB worldwide, with a smaller portion of cases of extensively drug-resistant TB, according to the release, demonstrating the need for new therapeutics.
SOURCE: U.S. Food and Drug Administration. Aug. 14, 2019. News release.
The U.S. Food and Drug Administration granted special approval to a new drug combo intended for the treatment of “a limited and specific population of adult patients with extensively drug resistant, treatment-intolerant or nonresponsive multidrug-resistant pulmonary” tuberculosis, according to an FDA news release.
The effectiveness of the combination treatment of pretomanid tablets with bedaquiline and linezolid was shown in a clinical study of patients with extensively drug-resistant, treatment-intolerant, or nonresponsive multidrug-resistant pulmonary tuberculosis of the lungs. Of 107 infected patients who were evaluated 6 months after the end of therapy, 95 (89%) were deemed successes, which significantly exceeded the historical success rates for treatment of extensively drug-resistant TB, the FDA reported. The trial is sponsored by the Global Alliance for TB Drug Development.
The most common adverse effects reported included peripheral neuropathy, anemia, nausea, vomiting, headache, increased liver enzymes, dyspepsia, rash, visual impairment, low blood sugar, and diarrhea, according to the release.
“Multidrug-resistant TB and extensively drug-resistant TB are public health threats due to limited treatment options. New treatments are important to meet patient national and global health needs,” stated FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD, in the release. She also explained that the approval marked the second time a drug was approved under the “Limited Population Pathway for Antibacterial and Antifungal Drugs, a pathway advanced by Congress to spur development of drugs targeting infections that lack effective therapies.”
In 2016, the World Health Organization reported that there were an estimated 490,000 new cases of multidrug-resistant TB worldwide, with a smaller portion of cases of extensively drug-resistant TB, according to the release, demonstrating the need for new therapeutics.
SOURCE: U.S. Food and Drug Administration. Aug. 14, 2019. News release.
The U.S. Food and Drug Administration granted special approval to a new drug combo intended for the treatment of “a limited and specific population of adult patients with extensively drug resistant, treatment-intolerant or nonresponsive multidrug-resistant pulmonary” tuberculosis, according to an FDA news release.
The effectiveness of the combination treatment of pretomanid tablets with bedaquiline and linezolid was shown in a clinical study of patients with extensively drug-resistant, treatment-intolerant, or nonresponsive multidrug-resistant pulmonary tuberculosis of the lungs. Of 107 infected patients who were evaluated 6 months after the end of therapy, 95 (89%) were deemed successes, which significantly exceeded the historical success rates for treatment of extensively drug-resistant TB, the FDA reported. The trial is sponsored by the Global Alliance for TB Drug Development.
The most common adverse effects reported included peripheral neuropathy, anemia, nausea, vomiting, headache, increased liver enzymes, dyspepsia, rash, visual impairment, low blood sugar, and diarrhea, according to the release.
“Multidrug-resistant TB and extensively drug-resistant TB are public health threats due to limited treatment options. New treatments are important to meet patient national and global health needs,” stated FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD, in the release. She also explained that the approval marked the second time a drug was approved under the “Limited Population Pathway for Antibacterial and Antifungal Drugs, a pathway advanced by Congress to spur development of drugs targeting infections that lack effective therapies.”
In 2016, the World Health Organization reported that there were an estimated 490,000 new cases of multidrug-resistant TB worldwide, with a smaller portion of cases of extensively drug-resistant TB, according to the release, demonstrating the need for new therapeutics.
SOURCE: U.S. Food and Drug Administration. Aug. 14, 2019. News release.
NEWS FROM THE FDA
Possible role of enterovirus infection in acute flaccid myelitis cases detected
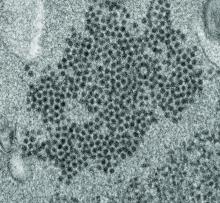
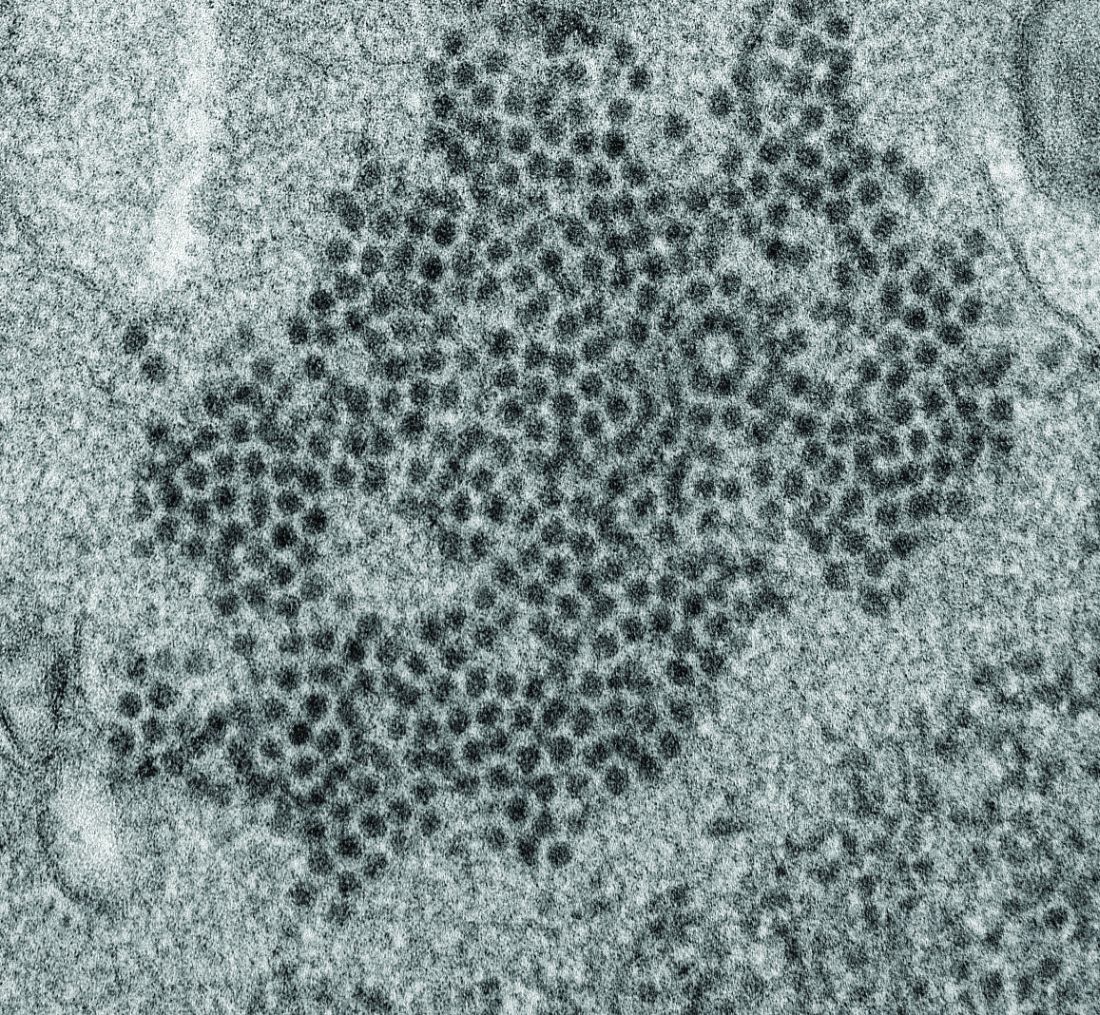
High levels of enterovirus (EV) peptides were found in the cerebrospinal fluid (CSF) and serum samples of individuals with acute flaccid myelitis (AFM) that were not present in a variety of control individuals, according to the results of a small study of patients with and without AFM published online in mBio.
In 2018, CSF samples from AFM patients were investigated by viral-capture high-throughput sequencing. These CSF and serum samples, as well as those from multiple controls, were tested for antibodies to human EVs using peptide microarrays, according to Nischay Mishra, PhD, of Columbia University, New York, and colleagues.
Although EV RNA was confirmed in CSF from only 1 adult AFM case and 1 non-AFM case, antibodies to EV peptides were present in 11 of 14 AFM patients (79%), which was a significantly higher rate than in control groups, including non-AFM patients (1 of 5, or 20%), children with Kawasaki disease (0 of 10), and adults with non-AFM CNS diseases (2 of 11, 18%), according to the authors.
In addition, 6 of 14 (43%) CSF samples and 8 of 11 (73%) serum samples from AFM patients were immunoreactive to an EV-D68–specific peptide, whereas samples from the three control groups were not immunoreactive in either CSF or sera. Previous studies have suggested that infection with EV-D68 and EV-A71 may contribute to AFM.
“There have been 570 confirmed cases since CDC began tracking AFM in August 2014. AFM outbreaks were reported to the CDC in 2014, 2016, and 2018. AFM affects the spinal cord and is characterized by the sudden onset of muscle weakness in one or more limbs. Spikes in AFM cases, primarily in children, have coincided in time and location with outbreaks of EV-D68 and a related enterovirus, EV-A71,” according to an NIH media advisory discussing the article.
In particular, as the study authors point out, a potential link to EV-D68 has also been based on the presence of viral RNA in some respiratory and stool specimens and the observation that EV-D68 infection can result in spinal cord infection.
“While other etiologies of AFM continue to be investigated, our study provides further evidence that EV infection may be a factor in AFM. In the absence of direct detection of a pathogen, antibody evidence of pathogen exposure within the CNS can be an important indicator of the underlying cause of disease,” Dr. Mishra and his colleagues added.
“These initial results may provide avenues to further explore how exposure to EV may contribute to AFM as well as the development of diagnostic tools and treatments,” the researchers concluded.
The study was funded by the National Institutes of Health. The authors reported that they had no competing financial interests.
SOURCE: Mishra N et al. mBio. 2019 Aug;10(4):e01903-19.
High levels of enterovirus (EV) peptides were found in the cerebrospinal fluid (CSF) and serum samples of individuals with acute flaccid myelitis (AFM) that were not present in a variety of control individuals, according to the results of a small study of patients with and without AFM published online in mBio.
In 2018, CSF samples from AFM patients were investigated by viral-capture high-throughput sequencing. These CSF and serum samples, as well as those from multiple controls, were tested for antibodies to human EVs using peptide microarrays, according to Nischay Mishra, PhD, of Columbia University, New York, and colleagues.
Although EV RNA was confirmed in CSF from only 1 adult AFM case and 1 non-AFM case, antibodies to EV peptides were present in 11 of 14 AFM patients (79%), which was a significantly higher rate than in control groups, including non-AFM patients (1 of 5, or 20%), children with Kawasaki disease (0 of 10), and adults with non-AFM CNS diseases (2 of 11, 18%), according to the authors.
In addition, 6 of 14 (43%) CSF samples and 8 of 11 (73%) serum samples from AFM patients were immunoreactive to an EV-D68–specific peptide, whereas samples from the three control groups were not immunoreactive in either CSF or sera. Previous studies have suggested that infection with EV-D68 and EV-A71 may contribute to AFM.
“There have been 570 confirmed cases since CDC began tracking AFM in August 2014. AFM outbreaks were reported to the CDC in 2014, 2016, and 2018. AFM affects the spinal cord and is characterized by the sudden onset of muscle weakness in one or more limbs. Spikes in AFM cases, primarily in children, have coincided in time and location with outbreaks of EV-D68 and a related enterovirus, EV-A71,” according to an NIH media advisory discussing the article.
In particular, as the study authors point out, a potential link to EV-D68 has also been based on the presence of viral RNA in some respiratory and stool specimens and the observation that EV-D68 infection can result in spinal cord infection.
“While other etiologies of AFM continue to be investigated, our study provides further evidence that EV infection may be a factor in AFM. In the absence of direct detection of a pathogen, antibody evidence of pathogen exposure within the CNS can be an important indicator of the underlying cause of disease,” Dr. Mishra and his colleagues added.
“These initial results may provide avenues to further explore how exposure to EV may contribute to AFM as well as the development of diagnostic tools and treatments,” the researchers concluded.
The study was funded by the National Institutes of Health. The authors reported that they had no competing financial interests.
SOURCE: Mishra N et al. mBio. 2019 Aug;10(4):e01903-19.
High levels of enterovirus (EV) peptides were found in the cerebrospinal fluid (CSF) and serum samples of individuals with acute flaccid myelitis (AFM) that were not present in a variety of control individuals, according to the results of a small study of patients with and without AFM published online in mBio.
In 2018, CSF samples from AFM patients were investigated by viral-capture high-throughput sequencing. These CSF and serum samples, as well as those from multiple controls, were tested for antibodies to human EVs using peptide microarrays, according to Nischay Mishra, PhD, of Columbia University, New York, and colleagues.
Although EV RNA was confirmed in CSF from only 1 adult AFM case and 1 non-AFM case, antibodies to EV peptides were present in 11 of 14 AFM patients (79%), which was a significantly higher rate than in control groups, including non-AFM patients (1 of 5, or 20%), children with Kawasaki disease (0 of 10), and adults with non-AFM CNS diseases (2 of 11, 18%), according to the authors.
In addition, 6 of 14 (43%) CSF samples and 8 of 11 (73%) serum samples from AFM patients were immunoreactive to an EV-D68–specific peptide, whereas samples from the three control groups were not immunoreactive in either CSF or sera. Previous studies have suggested that infection with EV-D68 and EV-A71 may contribute to AFM.
“There have been 570 confirmed cases since CDC began tracking AFM in August 2014. AFM outbreaks were reported to the CDC in 2014, 2016, and 2018. AFM affects the spinal cord and is characterized by the sudden onset of muscle weakness in one or more limbs. Spikes in AFM cases, primarily in children, have coincided in time and location with outbreaks of EV-D68 and a related enterovirus, EV-A71,” according to an NIH media advisory discussing the article.
In particular, as the study authors point out, a potential link to EV-D68 has also been based on the presence of viral RNA in some respiratory and stool specimens and the observation that EV-D68 infection can result in spinal cord infection.
“While other etiologies of AFM continue to be investigated, our study provides further evidence that EV infection may be a factor in AFM. In the absence of direct detection of a pathogen, antibody evidence of pathogen exposure within the CNS can be an important indicator of the underlying cause of disease,” Dr. Mishra and his colleagues added.
“These initial results may provide avenues to further explore how exposure to EV may contribute to AFM as well as the development of diagnostic tools and treatments,” the researchers concluded.
The study was funded by the National Institutes of Health. The authors reported that they had no competing financial interests.
SOURCE: Mishra N et al. mBio. 2019 Aug;10(4):e01903-19.
FROM MBIO
Key clinical point:
Major finding: EV peptide antibodies were present in 11 of 14 AFM patients (79%), significantly higher than in controls.
Study details: A peptide microarray analysis was performed on CSF and sera from 14 AFM patients, as well as three control groups of 5 pediatric and adult patients with a non-AFM CNS diseases, 10 children with Kawasaki disease, and 10 adult patients with non-AFM CNS diseases.
Disclosures: The study was funded by the National Institutes of Health. The authors reported that they had no conflicts.
Source: Mishra N et al. mBio. 2019 Aug;10(4):e01903-19.
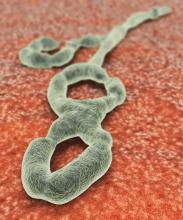
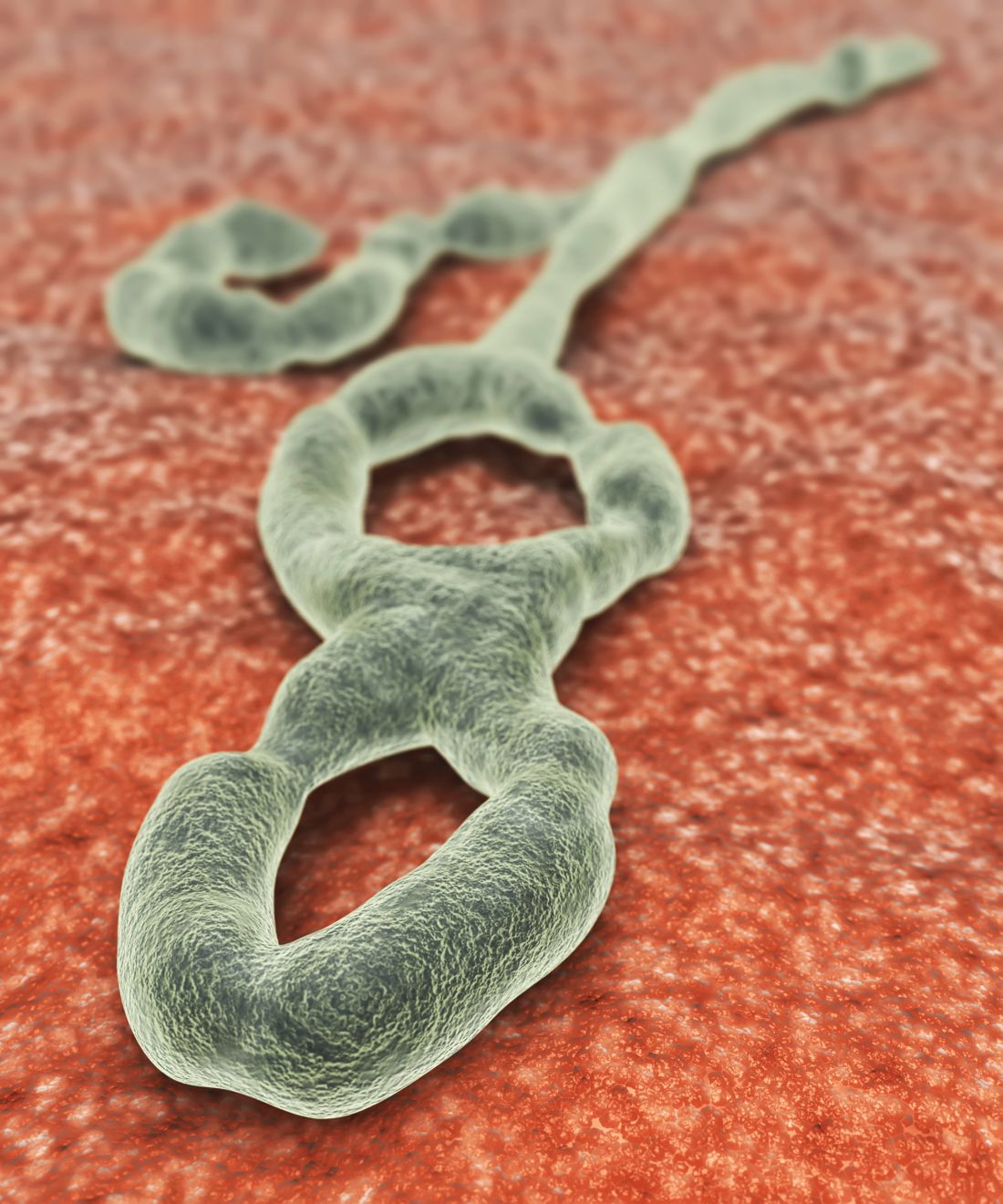
Favorable Ebola results lead to drug trial termination, new focus
An investigational agent known as REGN-EB3 has met an early stopping criterion in the protocol of an Ebola therapeutics trial, according to a National Institutes of Health media advisory.
Preliminary results in 499 study participants showed that individuals receiving either of two treatments, REGN-EB3 or mAb114, had a greater chance of survival, compared with participants in the other two study arms.
The randomized, controlled Pamoja Tulinde Maisha (PALM) study, which began Nov. 20, 2018, was designed to evaluate four investigational agents (ZMapp, remdesivir, mAb114, and REGN-EB3) for the treatment of patients with Ebola virus disease in the Democratic Republic of the Congo (DRC) as part of the emergency response to an ongoing outbreak in the North Kivu and Ituri provinces.
As of Aug. 9, 2019, the trial had enrolled 681 patients at four Ebola treatment centers in live outbreak regions of the DRC, with the goal of enrolling 725 patients in total.
The trial investigators and study cosponsors accepted the recommendation for early termination, and staff at the trial sites in the DRC were promptly informed, according to the media advisory. Additional patient randomizations in the now-revised trial will be limited to treatment either with REGN-EB3 or mAb114. Patients randomized to the ZMapp or remdesivir arms in the last 10 days of the original trial will be given the option, at the discretion of their treating physician, to receive either of the two more effective treatments, according to the NIH.
“While the final analysis of the data can occur only after all the data are generated and collected (likely late September/early October 2019), the DSMB [Data and Safety Monitoring Board] and the study leadership felt the preliminary analysis of the existing data was compelling enough to recommend and implement these changes in the trial immediately. The complete results will be submitted for publication in the peer-reviewed medical literature as soon as possible,” the NIH stated.
The study is cosponsored and funded by the NIH, carried out by an international research consortium coordinated by the World Health Organization, and supported by four pharmaceutical companies (MappBio, Gilead, Regeneron, and Ridgeback Biotherapeutics).
An investigational agent known as REGN-EB3 has met an early stopping criterion in the protocol of an Ebola therapeutics trial, according to a National Institutes of Health media advisory.
Preliminary results in 499 study participants showed that individuals receiving either of two treatments, REGN-EB3 or mAb114, had a greater chance of survival, compared with participants in the other two study arms.
The randomized, controlled Pamoja Tulinde Maisha (PALM) study, which began Nov. 20, 2018, was designed to evaluate four investigational agents (ZMapp, remdesivir, mAb114, and REGN-EB3) for the treatment of patients with Ebola virus disease in the Democratic Republic of the Congo (DRC) as part of the emergency response to an ongoing outbreak in the North Kivu and Ituri provinces.
As of Aug. 9, 2019, the trial had enrolled 681 patients at four Ebola treatment centers in live outbreak regions of the DRC, with the goal of enrolling 725 patients in total.
The trial investigators and study cosponsors accepted the recommendation for early termination, and staff at the trial sites in the DRC were promptly informed, according to the media advisory. Additional patient randomizations in the now-revised trial will be limited to treatment either with REGN-EB3 or mAb114. Patients randomized to the ZMapp or remdesivir arms in the last 10 days of the original trial will be given the option, at the discretion of their treating physician, to receive either of the two more effective treatments, according to the NIH.
“While the final analysis of the data can occur only after all the data are generated and collected (likely late September/early October 2019), the DSMB [Data and Safety Monitoring Board] and the study leadership felt the preliminary analysis of the existing data was compelling enough to recommend and implement these changes in the trial immediately. The complete results will be submitted for publication in the peer-reviewed medical literature as soon as possible,” the NIH stated.
The study is cosponsored and funded by the NIH, carried out by an international research consortium coordinated by the World Health Organization, and supported by four pharmaceutical companies (MappBio, Gilead, Regeneron, and Ridgeback Biotherapeutics).
An investigational agent known as REGN-EB3 has met an early stopping criterion in the protocol of an Ebola therapeutics trial, according to a National Institutes of Health media advisory.
Preliminary results in 499 study participants showed that individuals receiving either of two treatments, REGN-EB3 or mAb114, had a greater chance of survival, compared with participants in the other two study arms.
The randomized, controlled Pamoja Tulinde Maisha (PALM) study, which began Nov. 20, 2018, was designed to evaluate four investigational agents (ZMapp, remdesivir, mAb114, and REGN-EB3) for the treatment of patients with Ebola virus disease in the Democratic Republic of the Congo (DRC) as part of the emergency response to an ongoing outbreak in the North Kivu and Ituri provinces.
As of Aug. 9, 2019, the trial had enrolled 681 patients at four Ebola treatment centers in live outbreak regions of the DRC, with the goal of enrolling 725 patients in total.
The trial investigators and study cosponsors accepted the recommendation for early termination, and staff at the trial sites in the DRC were promptly informed, according to the media advisory. Additional patient randomizations in the now-revised trial will be limited to treatment either with REGN-EB3 or mAb114. Patients randomized to the ZMapp or remdesivir arms in the last 10 days of the original trial will be given the option, at the discretion of their treating physician, to receive either of the two more effective treatments, according to the NIH.
“While the final analysis of the data can occur only after all the data are generated and collected (likely late September/early October 2019), the DSMB [Data and Safety Monitoring Board] and the study leadership felt the preliminary analysis of the existing data was compelling enough to recommend and implement these changes in the trial immediately. The complete results will be submitted for publication in the peer-reviewed medical literature as soon as possible,” the NIH stated.
The study is cosponsored and funded by the NIH, carried out by an international research consortium coordinated by the World Health Organization, and supported by four pharmaceutical companies (MappBio, Gilead, Regeneron, and Ridgeback Biotherapeutics).
AHA highlights limitations of perfusion testing for critical limb ischemia
A new assessment statement from the American Heart Association reviewed the strengths and limitations of current imaging techniques for critical limb ischemia (CLI), the severest form of peripheral arterial disease (PAD).
The main techniques discussed were the ankle-brachial index (ABI), toe-brachial index (TBI), toe systolic pressure, transcutaneous oximetry (TcPO2) and skin perfusion pressure (SPP). The literature review also identified what the authors saw as opportunities for technology improvement.
“No single vascular test has been identified as the most important predictor of wound healing or major amputation for the threatened limb,” wrote Sanjay Misra, MD, of the Mayo Clinic in Rochester, Minn., and colleagues, on behalf of the American Heart Association Council on Peripheral Vascular Disease, the Council on Clinical Cardiology, and the Council on Cardiovascular and Stroke Nursing.
Of particular concern were limitations seen in the use of ABI, the most widely used assessment method. “Although ABI was first described to diagnose PAD, it has not been shown to be an accurate predictor of wound healing or major adverse limb events. Clearly, the ABI provides important prognostic information, including the risk of death, myocardial infarction, and stroke ... and should be performed in all patients suspected of having PAD,” but in about 30% of patients with angiographically documented CLI, the ABI is normal or noncompressible, the authors wrote.
And, although recent data indicate that toe pressure may be a better predictor of major adverse limb events and tibial disease in patients with CLI, especially among those with isolated below-knee disease, there was no solid evidence that ABI or TBI have the sensitivity or specificity to be used as perfusion tools to assess wound healing or limb salvage, the authors stated.
However, there may be some technological improvements on the horizon for assessing limb perfusion that might provide eventual benefits, according to the reviewers. These include the use of indigo carmine angiography to evaluate microcirculation and angiosomal revascularization, the use of CT perfusion or MRI to quantify perfusion and monitor treatment response, the use of contrast-enhanced ultrasound to assess calf muscle perfusion, and hyperspectral imaging.
Among the other issues of concern raised in the AHA statement were the significant demographic disparities that occur in detection and treatment of CLI. The authors noted differences in how CLI is diagnosed, the coexisting conditions that were present, and the disparities in treatment given based on sex and racial differences. For example, women were more likely to experience emergency hospitalization, have differences in blood flow, and have higher disability and death rates.
As for racial disparities, the reviewers found that black and Hispanic patients with CLI were more likely to have diabetes and chronic kidney disease, and were more likely to develop gangrene, compared with white patients, who were more likely to have ulcers and pain in their legs while at rest.
In terms of treatment, black patients were 78% more likely to receive lower extremity amputation for CLI, compared with their white peers, even after adjustment for socioeconomic status, access to facilities with revascularization services, and other factors, according to the report, which was published online in Circulation.
“CLI is a complex disease process with great morbidity. This statement highlights the importance of incorporating perfusion assessment into the care of CLI patients. Despite the high prevalence of CLI, strategies for perfusion assessment remain limited. New technologies offer potential opportunities to improve the precision and quality of CLI management,” the researchers concluded.
Dr. Misra and the majority of the authors reported having no relevant disclosures. Several authors reported receiving funding from the pharmaceutical and medical device industries.
SOURCE: Chandra S. et al. Circulation. 2019;140:00-00. doi: 10.1161/CIR.0000000000000708.
A new assessment statement from the American Heart Association reviewed the strengths and limitations of current imaging techniques for critical limb ischemia (CLI), the severest form of peripheral arterial disease (PAD).
The main techniques discussed were the ankle-brachial index (ABI), toe-brachial index (TBI), toe systolic pressure, transcutaneous oximetry (TcPO2) and skin perfusion pressure (SPP). The literature review also identified what the authors saw as opportunities for technology improvement.
“No single vascular test has been identified as the most important predictor of wound healing or major amputation for the threatened limb,” wrote Sanjay Misra, MD, of the Mayo Clinic in Rochester, Minn., and colleagues, on behalf of the American Heart Association Council on Peripheral Vascular Disease, the Council on Clinical Cardiology, and the Council on Cardiovascular and Stroke Nursing.
Of particular concern were limitations seen in the use of ABI, the most widely used assessment method. “Although ABI was first described to diagnose PAD, it has not been shown to be an accurate predictor of wound healing or major adverse limb events. Clearly, the ABI provides important prognostic information, including the risk of death, myocardial infarction, and stroke ... and should be performed in all patients suspected of having PAD,” but in about 30% of patients with angiographically documented CLI, the ABI is normal or noncompressible, the authors wrote.
And, although recent data indicate that toe pressure may be a better predictor of major adverse limb events and tibial disease in patients with CLI, especially among those with isolated below-knee disease, there was no solid evidence that ABI or TBI have the sensitivity or specificity to be used as perfusion tools to assess wound healing or limb salvage, the authors stated.
However, there may be some technological improvements on the horizon for assessing limb perfusion that might provide eventual benefits, according to the reviewers. These include the use of indigo carmine angiography to evaluate microcirculation and angiosomal revascularization, the use of CT perfusion or MRI to quantify perfusion and monitor treatment response, the use of contrast-enhanced ultrasound to assess calf muscle perfusion, and hyperspectral imaging.
Among the other issues of concern raised in the AHA statement were the significant demographic disparities that occur in detection and treatment of CLI. The authors noted differences in how CLI is diagnosed, the coexisting conditions that were present, and the disparities in treatment given based on sex and racial differences. For example, women were more likely to experience emergency hospitalization, have differences in blood flow, and have higher disability and death rates.
As for racial disparities, the reviewers found that black and Hispanic patients with CLI were more likely to have diabetes and chronic kidney disease, and were more likely to develop gangrene, compared with white patients, who were more likely to have ulcers and pain in their legs while at rest.
In terms of treatment, black patients were 78% more likely to receive lower extremity amputation for CLI, compared with their white peers, even after adjustment for socioeconomic status, access to facilities with revascularization services, and other factors, according to the report, which was published online in Circulation.
“CLI is a complex disease process with great morbidity. This statement highlights the importance of incorporating perfusion assessment into the care of CLI patients. Despite the high prevalence of CLI, strategies for perfusion assessment remain limited. New technologies offer potential opportunities to improve the precision and quality of CLI management,” the researchers concluded.
Dr. Misra and the majority of the authors reported having no relevant disclosures. Several authors reported receiving funding from the pharmaceutical and medical device industries.
SOURCE: Chandra S. et al. Circulation. 2019;140:00-00. doi: 10.1161/CIR.0000000000000708.
A new assessment statement from the American Heart Association reviewed the strengths and limitations of current imaging techniques for critical limb ischemia (CLI), the severest form of peripheral arterial disease (PAD).
The main techniques discussed were the ankle-brachial index (ABI), toe-brachial index (TBI), toe systolic pressure, transcutaneous oximetry (TcPO2) and skin perfusion pressure (SPP). The literature review also identified what the authors saw as opportunities for technology improvement.
“No single vascular test has been identified as the most important predictor of wound healing or major amputation for the threatened limb,” wrote Sanjay Misra, MD, of the Mayo Clinic in Rochester, Minn., and colleagues, on behalf of the American Heart Association Council on Peripheral Vascular Disease, the Council on Clinical Cardiology, and the Council on Cardiovascular and Stroke Nursing.
Of particular concern were limitations seen in the use of ABI, the most widely used assessment method. “Although ABI was first described to diagnose PAD, it has not been shown to be an accurate predictor of wound healing or major adverse limb events. Clearly, the ABI provides important prognostic information, including the risk of death, myocardial infarction, and stroke ... and should be performed in all patients suspected of having PAD,” but in about 30% of patients with angiographically documented CLI, the ABI is normal or noncompressible, the authors wrote.
And, although recent data indicate that toe pressure may be a better predictor of major adverse limb events and tibial disease in patients with CLI, especially among those with isolated below-knee disease, there was no solid evidence that ABI or TBI have the sensitivity or specificity to be used as perfusion tools to assess wound healing or limb salvage, the authors stated.
However, there may be some technological improvements on the horizon for assessing limb perfusion that might provide eventual benefits, according to the reviewers. These include the use of indigo carmine angiography to evaluate microcirculation and angiosomal revascularization, the use of CT perfusion or MRI to quantify perfusion and monitor treatment response, the use of contrast-enhanced ultrasound to assess calf muscle perfusion, and hyperspectral imaging.
Among the other issues of concern raised in the AHA statement were the significant demographic disparities that occur in detection and treatment of CLI. The authors noted differences in how CLI is diagnosed, the coexisting conditions that were present, and the disparities in treatment given based on sex and racial differences. For example, women were more likely to experience emergency hospitalization, have differences in blood flow, and have higher disability and death rates.
As for racial disparities, the reviewers found that black and Hispanic patients with CLI were more likely to have diabetes and chronic kidney disease, and were more likely to develop gangrene, compared with white patients, who were more likely to have ulcers and pain in their legs while at rest.
In terms of treatment, black patients were 78% more likely to receive lower extremity amputation for CLI, compared with their white peers, even after adjustment for socioeconomic status, access to facilities with revascularization services, and other factors, according to the report, which was published online in Circulation.
“CLI is a complex disease process with great morbidity. This statement highlights the importance of incorporating perfusion assessment into the care of CLI patients. Despite the high prevalence of CLI, strategies for perfusion assessment remain limited. New technologies offer potential opportunities to improve the precision and quality of CLI management,” the researchers concluded.
Dr. Misra and the majority of the authors reported having no relevant disclosures. Several authors reported receiving funding from the pharmaceutical and medical device industries.
SOURCE: Chandra S. et al. Circulation. 2019;140:00-00. doi: 10.1161/CIR.0000000000000708.
FROM CIRCULATION
HCV-infected people who inject drugs also have substantial alcohol use
Curing hepatitis C virus (HCV) infection without addressing the high rate of alcohol use disorder in many patients may undermine the benefits of treatment to long-term liver health, according to the results of a large cohort study.
Because excess alcohol use is known to accelerate liver disease progression, researchers Risha Irvin, MD, and her colleagues from Johns Hopkins University, Baltimore, examined the prevalence of alcohol use in HCV-infected people who inject drugs (PWID). Their study examined the prevalence and associated correlates of alcohol use (Addictive Behaviors 2019;96:56-61).
They followed a large cohort of 1,623 HCV-antibody positive PWID from 2005 to 2013 from the AIDS Linked to the Intravenous Experience (ALIVE) study. They characterized alcohol use with the Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire. Multivariable logistic regression with generalized estimated equations was used to examine sociodemographic, clinical, and substance use correlates of alcohol use.
At baseline, the median age was 47 years, 67% were men, 81% were black, and 34% were HIV positive. The majority (60%) reported injection drug use in the prior 6 months, while 46% reported noninjection cocaine or heroin, 31% reported street-acquired prescription drugs, and 22% reported marijuana use in the same time period. According to the AUDIT-C results, 41% of the patients reported no alcohol use, 21% reported moderate alcohol use, and 38% reported heavy alcohol use at their baseline visit.
The factors that were significantly associated with heavy alcohol use included male sex, black race, income of $5,000 or less, a Center for Epidemiologic Studies Depression Scale (range 0-60) score of 23 or greater, being homeless, being incarcerated, marijuana use, use of street-acquired prescription drugs, noninjection cocaine/heroin, injection drug use, and cigarette smoking. In a model that included the composite summary variable for substance use intensity, one drug type (adjusted odds ratio, 1.92), two drug types (AOR, 2.93), and three drug types (AOR, 3.65) were significantly associated with heavy alcohol use.
“While clinicians are undoubtedly concerned about any level of alcohol use in the setting of HCV infection due to the acceleration of liver fibrosis, there is particular concern for individuals with heavy alcohol use and their increased risk for cirrhosis and liver failure even after HCV cure. Without intervention, alcohol use will persist after HCV is cured with the potential to undermine the benefit of HCV cure. Therefore, our data point to the need to invest in and develop programs that effectively address alcohol use and co-occurring substance use in this population of PWID with HCV,” the researchers concluded.
The study was supported by the U.S. National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, and the National Institute on Alcohol Abuse and Alcoholism. The authors declared that they had no conflicts.
SOURCE: Irvin R et al. Addictive Behaviors. 2019;96:56-61.
Curing hepatitis C virus (HCV) infection without addressing the high rate of alcohol use disorder in many patients may undermine the benefits of treatment to long-term liver health, according to the results of a large cohort study.
Because excess alcohol use is known to accelerate liver disease progression, researchers Risha Irvin, MD, and her colleagues from Johns Hopkins University, Baltimore, examined the prevalence of alcohol use in HCV-infected people who inject drugs (PWID). Their study examined the prevalence and associated correlates of alcohol use (Addictive Behaviors 2019;96:56-61).
They followed a large cohort of 1,623 HCV-antibody positive PWID from 2005 to 2013 from the AIDS Linked to the Intravenous Experience (ALIVE) study. They characterized alcohol use with the Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire. Multivariable logistic regression with generalized estimated equations was used to examine sociodemographic, clinical, and substance use correlates of alcohol use.
At baseline, the median age was 47 years, 67% were men, 81% were black, and 34% were HIV positive. The majority (60%) reported injection drug use in the prior 6 months, while 46% reported noninjection cocaine or heroin, 31% reported street-acquired prescription drugs, and 22% reported marijuana use in the same time period. According to the AUDIT-C results, 41% of the patients reported no alcohol use, 21% reported moderate alcohol use, and 38% reported heavy alcohol use at their baseline visit.
The factors that were significantly associated with heavy alcohol use included male sex, black race, income of $5,000 or less, a Center for Epidemiologic Studies Depression Scale (range 0-60) score of 23 or greater, being homeless, being incarcerated, marijuana use, use of street-acquired prescription drugs, noninjection cocaine/heroin, injection drug use, and cigarette smoking. In a model that included the composite summary variable for substance use intensity, one drug type (adjusted odds ratio, 1.92), two drug types (AOR, 2.93), and three drug types (AOR, 3.65) were significantly associated with heavy alcohol use.
“While clinicians are undoubtedly concerned about any level of alcohol use in the setting of HCV infection due to the acceleration of liver fibrosis, there is particular concern for individuals with heavy alcohol use and their increased risk for cirrhosis and liver failure even after HCV cure. Without intervention, alcohol use will persist after HCV is cured with the potential to undermine the benefit of HCV cure. Therefore, our data point to the need to invest in and develop programs that effectively address alcohol use and co-occurring substance use in this population of PWID with HCV,” the researchers concluded.
The study was supported by the U.S. National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, and the National Institute on Alcohol Abuse and Alcoholism. The authors declared that they had no conflicts.
SOURCE: Irvin R et al. Addictive Behaviors. 2019;96:56-61.
Curing hepatitis C virus (HCV) infection without addressing the high rate of alcohol use disorder in many patients may undermine the benefits of treatment to long-term liver health, according to the results of a large cohort study.
Because excess alcohol use is known to accelerate liver disease progression, researchers Risha Irvin, MD, and her colleagues from Johns Hopkins University, Baltimore, examined the prevalence of alcohol use in HCV-infected people who inject drugs (PWID). Their study examined the prevalence and associated correlates of alcohol use (Addictive Behaviors 2019;96:56-61).
They followed a large cohort of 1,623 HCV-antibody positive PWID from 2005 to 2013 from the AIDS Linked to the Intravenous Experience (ALIVE) study. They characterized alcohol use with the Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire. Multivariable logistic regression with generalized estimated equations was used to examine sociodemographic, clinical, and substance use correlates of alcohol use.
At baseline, the median age was 47 years, 67% were men, 81% were black, and 34% were HIV positive. The majority (60%) reported injection drug use in the prior 6 months, while 46% reported noninjection cocaine or heroin, 31% reported street-acquired prescription drugs, and 22% reported marijuana use in the same time period. According to the AUDIT-C results, 41% of the patients reported no alcohol use, 21% reported moderate alcohol use, and 38% reported heavy alcohol use at their baseline visit.
The factors that were significantly associated with heavy alcohol use included male sex, black race, income of $5,000 or less, a Center for Epidemiologic Studies Depression Scale (range 0-60) score of 23 or greater, being homeless, being incarcerated, marijuana use, use of street-acquired prescription drugs, noninjection cocaine/heroin, injection drug use, and cigarette smoking. In a model that included the composite summary variable for substance use intensity, one drug type (adjusted odds ratio, 1.92), two drug types (AOR, 2.93), and three drug types (AOR, 3.65) were significantly associated with heavy alcohol use.
“While clinicians are undoubtedly concerned about any level of alcohol use in the setting of HCV infection due to the acceleration of liver fibrosis, there is particular concern for individuals with heavy alcohol use and their increased risk for cirrhosis and liver failure even after HCV cure. Without intervention, alcohol use will persist after HCV is cured with the potential to undermine the benefit of HCV cure. Therefore, our data point to the need to invest in and develop programs that effectively address alcohol use and co-occurring substance use in this population of PWID with HCV,” the researchers concluded.
The study was supported by the U.S. National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, and the National Institute on Alcohol Abuse and Alcoholism. The authors declared that they had no conflicts.
SOURCE: Irvin R et al. Addictive Behaviors. 2019;96:56-61.
FROM ADDICTIVE BEHAVIORS
DRC Ebola epidemic continues unabated despite international response
, “currently the outbreak continues at the same pace, so we don’t see evidence of slowing,” according to Henry Walke, MD, director of the Division of Preparedness and Emerging Infections and Incident Manager, 2018 CDC Ebola Response, Centers for Disease Control and Prevention.
He added that new cases of Ebola have been seen in Goma, which is outside the initial outbreak area. Goma is the largest city in the eastern part of the DRC and a major trading port.
Dr. Walke made his remarks in a telephone media briefing Aug. 1 by the U. S. Department of Health and Human Services outlining the current state of the U.S. response to the outbreak.
He described the efforts of the CDC to provide support to the DRC both from Atlanta and in the field. These efforts included support for vaccination activities in DRC’s North Kivu and Ituri provinces for the population and for at-risk health-care workers in the DRC and neighboring countries. In addition, the United States is involved in the testing of experimental therapeutics and vaccines in the DRC in an effort to aid in this and future outbreaks.
“There are no cases of Ebola in the United States,” said Dr. Walke, and the CDC believes the risk to the United States from the outbreak is low. He cited the limited number of travelers from DRC. “There [are] about 16,000 from the DRC to the U.S. on an annual basis, and only about 100 from Goma itself. There aren’t direct flights and we have at the Goma airport both entry and exit screening.”
According to a World Health Organization report, this Ebola outbreak is the second deadliest on record and has killed 1,750 people out of around 2,518 confirmed cases as of July 23.
Efforts to control the epidemic are severely hampered by civil unrest in the area, public mistrust of the government and health care workers, and a comparative lack of international aid compared to previous Ebola outbreaks.
, “currently the outbreak continues at the same pace, so we don’t see evidence of slowing,” according to Henry Walke, MD, director of the Division of Preparedness and Emerging Infections and Incident Manager, 2018 CDC Ebola Response, Centers for Disease Control and Prevention.
He added that new cases of Ebola have been seen in Goma, which is outside the initial outbreak area. Goma is the largest city in the eastern part of the DRC and a major trading port.
Dr. Walke made his remarks in a telephone media briefing Aug. 1 by the U. S. Department of Health and Human Services outlining the current state of the U.S. response to the outbreak.
He described the efforts of the CDC to provide support to the DRC both from Atlanta and in the field. These efforts included support for vaccination activities in DRC’s North Kivu and Ituri provinces for the population and for at-risk health-care workers in the DRC and neighboring countries. In addition, the United States is involved in the testing of experimental therapeutics and vaccines in the DRC in an effort to aid in this and future outbreaks.
“There are no cases of Ebola in the United States,” said Dr. Walke, and the CDC believes the risk to the United States from the outbreak is low. He cited the limited number of travelers from DRC. “There [are] about 16,000 from the DRC to the U.S. on an annual basis, and only about 100 from Goma itself. There aren’t direct flights and we have at the Goma airport both entry and exit screening.”
According to a World Health Organization report, this Ebola outbreak is the second deadliest on record and has killed 1,750 people out of around 2,518 confirmed cases as of July 23.
Efforts to control the epidemic are severely hampered by civil unrest in the area, public mistrust of the government and health care workers, and a comparative lack of international aid compared to previous Ebola outbreaks.
, “currently the outbreak continues at the same pace, so we don’t see evidence of slowing,” according to Henry Walke, MD, director of the Division of Preparedness and Emerging Infections and Incident Manager, 2018 CDC Ebola Response, Centers for Disease Control and Prevention.
He added that new cases of Ebola have been seen in Goma, which is outside the initial outbreak area. Goma is the largest city in the eastern part of the DRC and a major trading port.
Dr. Walke made his remarks in a telephone media briefing Aug. 1 by the U. S. Department of Health and Human Services outlining the current state of the U.S. response to the outbreak.
He described the efforts of the CDC to provide support to the DRC both from Atlanta and in the field. These efforts included support for vaccination activities in DRC’s North Kivu and Ituri provinces for the population and for at-risk health-care workers in the DRC and neighboring countries. In addition, the United States is involved in the testing of experimental therapeutics and vaccines in the DRC in an effort to aid in this and future outbreaks.
“There are no cases of Ebola in the United States,” said Dr. Walke, and the CDC believes the risk to the United States from the outbreak is low. He cited the limited number of travelers from DRC. “There [are] about 16,000 from the DRC to the U.S. on an annual basis, and only about 100 from Goma itself. There aren’t direct flights and we have at the Goma airport both entry and exit screening.”
According to a World Health Organization report, this Ebola outbreak is the second deadliest on record and has killed 1,750 people out of around 2,518 confirmed cases as of July 23.
Efforts to control the epidemic are severely hampered by civil unrest in the area, public mistrust of the government and health care workers, and a comparative lack of international aid compared to previous Ebola outbreaks.
REPORTING FROM A MEDIA BRIEFING BY HHS
Spleen/liver stiffness ratio differentiates HCV, ALD
The spleen stiffness (SS) to liver stiffness (LS) ratio was significantly higher in patients with hepatitis C virus infection (HCV) than in patients with alcohol-related liver disease (ALD), according to the results of a multicenter prospective study. In addition, long-term outcome and complications differed dramatically between HCV and ALD. Variceal bleeding was the most common sign of decompensation and cause of death in patients with HCV, while jaundice was the most common sign of decompensation in patients with ALD.
Omar Elshaarawy, MSc, of the University of Heidelberg (Germany) and colleagues reported on their prospective study of 411 patients with HCV (220 patients) or ALD (191 patients) that were assessed for both LS and SS using the Fibroscan device. They also discussed their retrospective analysis of LS and spleen length (SL) from a separate, retrospective cohort of 449 patients (267 with HCV, 182 with ALD) for whom long-term data on decompensation/death were available.
The researchers found that SS was significantly higher in HCV patients, compared with those with ALD (42.0 vs. 32.6 kPa; P less than .0001), as was SL (15.6 vs. 11.9 cm, P less than .0001); this was despite a lower mean LS in HCV. As a result, the SS/LS ratio and the SL/LS ratio were both significantly higher in HCV (3.8 vs. 1.72 and 1.46 vs. 0.86, P less than .0001) through all fibrosis stages.
They also found that patients with ALD had higher LS values (30.5 vs. 21.3 kPa) and predominantly presented with jaundice (65.2%), with liver failure as the major cause of death (P less than .01). In contrast, in HCV, spleens were larger (17.6 vs. 12.1 cm) while variceal bleeding was the major cause of decompensation (73.2%) and death (P less than .001).
“We have demonstrated the disease-specific differences in SS/LS and SL/LS ratio between HCV and ALD. They underscore the role of the intrahepatic histological site of inflammation/fibrosis. We suggest that the SS/LS ratio could be used to confirm the disease etiology and predict disease-specific complications,” the researchers concluded.
The study was supported by the Dietmar Hopp Foundation. The authors reported they had no conflicts.
SOURCE: Elshaarawy O et al. J Hepatol Reports. 2019 Jun 20. doi: 10.1016/j.jhepr.2019.05.003.
The spleen stiffness (SS) to liver stiffness (LS) ratio was significantly higher in patients with hepatitis C virus infection (HCV) than in patients with alcohol-related liver disease (ALD), according to the results of a multicenter prospective study. In addition, long-term outcome and complications differed dramatically between HCV and ALD. Variceal bleeding was the most common sign of decompensation and cause of death in patients with HCV, while jaundice was the most common sign of decompensation in patients with ALD.
Omar Elshaarawy, MSc, of the University of Heidelberg (Germany) and colleagues reported on their prospective study of 411 patients with HCV (220 patients) or ALD (191 patients) that were assessed for both LS and SS using the Fibroscan device. They also discussed their retrospective analysis of LS and spleen length (SL) from a separate, retrospective cohort of 449 patients (267 with HCV, 182 with ALD) for whom long-term data on decompensation/death were available.
The researchers found that SS was significantly higher in HCV patients, compared with those with ALD (42.0 vs. 32.6 kPa; P less than .0001), as was SL (15.6 vs. 11.9 cm, P less than .0001); this was despite a lower mean LS in HCV. As a result, the SS/LS ratio and the SL/LS ratio were both significantly higher in HCV (3.8 vs. 1.72 and 1.46 vs. 0.86, P less than .0001) through all fibrosis stages.
They also found that patients with ALD had higher LS values (30.5 vs. 21.3 kPa) and predominantly presented with jaundice (65.2%), with liver failure as the major cause of death (P less than .01). In contrast, in HCV, spleens were larger (17.6 vs. 12.1 cm) while variceal bleeding was the major cause of decompensation (73.2%) and death (P less than .001).
“We have demonstrated the disease-specific differences in SS/LS and SL/LS ratio between HCV and ALD. They underscore the role of the intrahepatic histological site of inflammation/fibrosis. We suggest that the SS/LS ratio could be used to confirm the disease etiology and predict disease-specific complications,” the researchers concluded.
The study was supported by the Dietmar Hopp Foundation. The authors reported they had no conflicts.
SOURCE: Elshaarawy O et al. J Hepatol Reports. 2019 Jun 20. doi: 10.1016/j.jhepr.2019.05.003.
The spleen stiffness (SS) to liver stiffness (LS) ratio was significantly higher in patients with hepatitis C virus infection (HCV) than in patients with alcohol-related liver disease (ALD), according to the results of a multicenter prospective study. In addition, long-term outcome and complications differed dramatically between HCV and ALD. Variceal bleeding was the most common sign of decompensation and cause of death in patients with HCV, while jaundice was the most common sign of decompensation in patients with ALD.
Omar Elshaarawy, MSc, of the University of Heidelberg (Germany) and colleagues reported on their prospective study of 411 patients with HCV (220 patients) or ALD (191 patients) that were assessed for both LS and SS using the Fibroscan device. They also discussed their retrospective analysis of LS and spleen length (SL) from a separate, retrospective cohort of 449 patients (267 with HCV, 182 with ALD) for whom long-term data on decompensation/death were available.
The researchers found that SS was significantly higher in HCV patients, compared with those with ALD (42.0 vs. 32.6 kPa; P less than .0001), as was SL (15.6 vs. 11.9 cm, P less than .0001); this was despite a lower mean LS in HCV. As a result, the SS/LS ratio and the SL/LS ratio were both significantly higher in HCV (3.8 vs. 1.72 and 1.46 vs. 0.86, P less than .0001) through all fibrosis stages.
They also found that patients with ALD had higher LS values (30.5 vs. 21.3 kPa) and predominantly presented with jaundice (65.2%), with liver failure as the major cause of death (P less than .01). In contrast, in HCV, spleens were larger (17.6 vs. 12.1 cm) while variceal bleeding was the major cause of decompensation (73.2%) and death (P less than .001).
“We have demonstrated the disease-specific differences in SS/LS and SL/LS ratio between HCV and ALD. They underscore the role of the intrahepatic histological site of inflammation/fibrosis. We suggest that the SS/LS ratio could be used to confirm the disease etiology and predict disease-specific complications,” the researchers concluded.
The study was supported by the Dietmar Hopp Foundation. The authors reported they had no conflicts.
SOURCE: Elshaarawy O et al. J Hepatol Reports. 2019 Jun 20. doi: 10.1016/j.jhepr.2019.05.003.
FROM JHEP REPORTS