User login
Small study suggests natural HCV clearance is caused by AR3-antibody response
Individuals who spontaneously cleared their primary hepatitis C virus (HCV) infection or reinfection had significantly more antibodies that recognized multiple HCV genotypes beyond the initial infection, compared with chronically infected individuals, according to a small molecular study of immortalized cultured B cells from patient.
In a study published in the Journal of Hepatology, Sabrina J. Merat of AIMM Therapeutics and colleagues classified patients into two groups based on the outcome of their HCV infection: individuals who became chronically infected (CHRs; n = 5) either after primary infection or after HCV reinfection and individuals who cleared one or more HCV infections and were HCV RNA negative at the end of follow-up (CLs; n = 8). The researchers considered that all CLs who cleared the infection were presumably re-exposed to HCV as they continued injecting drugs for a median of 5.9 years after primary infection. The median follow-up time of individuals after primary HCV infection was 17.5 years.
Although the frequency of total antibodies did not differ between the two groups, the antibodies from CHRs were mainly genotype specific and directed against the genotype of the ongoing infection. Antibodies from CLs showed a much broader reactivity than CHR-derived antibodies, with the absolute number of antibodies recognizing at least three or more genotypes was significantly higher in CLs than in CHRs (13 vs. 0, respectively; P = .03).
In addition, in order to determine which epitopes were being targeted in the CL patients, the researchers tested the antibodies secreted in the B-cell supernatant for binding to E2 alanine mutants in the four epitopes known to be recognized by broadly neutralizing HCV antibodies. They found that the majority of the cross-genotype antibodies (82/113; 73%) were specific for AR3 because they bound to the AR3-specific mutants.
“In chronically infected individuals, AR3-specific antibody responses may be too weak and/or may develop too late to prevent chronic infection. If confirmed, this means that a strong and broadly neutralizing antibody response should be established very early after infection in order to confer protection,” the researchers concluded.
This study was supported by the Virgo consortium, funded by the Dutch government. Sabrina Merat and several coauthors are employees of AIMM Therapeutics, as well as shareholders.
SOURCE: Merat SJ et al. J Hepatol 2019;71:14-24.
Individuals who spontaneously cleared their primary hepatitis C virus (HCV) infection or reinfection had significantly more antibodies that recognized multiple HCV genotypes beyond the initial infection, compared with chronically infected individuals, according to a small molecular study of immortalized cultured B cells from patient.
In a study published in the Journal of Hepatology, Sabrina J. Merat of AIMM Therapeutics and colleagues classified patients into two groups based on the outcome of their HCV infection: individuals who became chronically infected (CHRs; n = 5) either after primary infection or after HCV reinfection and individuals who cleared one or more HCV infections and were HCV RNA negative at the end of follow-up (CLs; n = 8). The researchers considered that all CLs who cleared the infection were presumably re-exposed to HCV as they continued injecting drugs for a median of 5.9 years after primary infection. The median follow-up time of individuals after primary HCV infection was 17.5 years.
Although the frequency of total antibodies did not differ between the two groups, the antibodies from CHRs were mainly genotype specific and directed against the genotype of the ongoing infection. Antibodies from CLs showed a much broader reactivity than CHR-derived antibodies, with the absolute number of antibodies recognizing at least three or more genotypes was significantly higher in CLs than in CHRs (13 vs. 0, respectively; P = .03).
In addition, in order to determine which epitopes were being targeted in the CL patients, the researchers tested the antibodies secreted in the B-cell supernatant for binding to E2 alanine mutants in the four epitopes known to be recognized by broadly neutralizing HCV antibodies. They found that the majority of the cross-genotype antibodies (82/113; 73%) were specific for AR3 because they bound to the AR3-specific mutants.
“In chronically infected individuals, AR3-specific antibody responses may be too weak and/or may develop too late to prevent chronic infection. If confirmed, this means that a strong and broadly neutralizing antibody response should be established very early after infection in order to confer protection,” the researchers concluded.
This study was supported by the Virgo consortium, funded by the Dutch government. Sabrina Merat and several coauthors are employees of AIMM Therapeutics, as well as shareholders.
SOURCE: Merat SJ et al. J Hepatol 2019;71:14-24.
Individuals who spontaneously cleared their primary hepatitis C virus (HCV) infection or reinfection had significantly more antibodies that recognized multiple HCV genotypes beyond the initial infection, compared with chronically infected individuals, according to a small molecular study of immortalized cultured B cells from patient.
In a study published in the Journal of Hepatology, Sabrina J. Merat of AIMM Therapeutics and colleagues classified patients into two groups based on the outcome of their HCV infection: individuals who became chronically infected (CHRs; n = 5) either after primary infection or after HCV reinfection and individuals who cleared one or more HCV infections and were HCV RNA negative at the end of follow-up (CLs; n = 8). The researchers considered that all CLs who cleared the infection were presumably re-exposed to HCV as they continued injecting drugs for a median of 5.9 years after primary infection. The median follow-up time of individuals after primary HCV infection was 17.5 years.
Although the frequency of total antibodies did not differ between the two groups, the antibodies from CHRs were mainly genotype specific and directed against the genotype of the ongoing infection. Antibodies from CLs showed a much broader reactivity than CHR-derived antibodies, with the absolute number of antibodies recognizing at least three or more genotypes was significantly higher in CLs than in CHRs (13 vs. 0, respectively; P = .03).
In addition, in order to determine which epitopes were being targeted in the CL patients, the researchers tested the antibodies secreted in the B-cell supernatant for binding to E2 alanine mutants in the four epitopes known to be recognized by broadly neutralizing HCV antibodies. They found that the majority of the cross-genotype antibodies (82/113; 73%) were specific for AR3 because they bound to the AR3-specific mutants.
“In chronically infected individuals, AR3-specific antibody responses may be too weak and/or may develop too late to prevent chronic infection. If confirmed, this means that a strong and broadly neutralizing antibody response should be established very early after infection in order to confer protection,” the researchers concluded.
This study was supported by the Virgo consortium, funded by the Dutch government. Sabrina Merat and several coauthors are employees of AIMM Therapeutics, as well as shareholders.
SOURCE: Merat SJ et al. J Hepatol 2019;71:14-24.
FROM THE JOURNAL OF HEPATOLOGY
Ebola outbreak: WHO/OCHA call for more aid, better security
The continuing outbreak of Ebola in the Democratic Republic of the Congo (DRC) was the subject of a special United Nations high-level event organized by the World Health Organization (WHO) and the United Nations Office for the Coordination of Humanitarian Affairs (OCHA). It was comoderated by Tedros Adhanom Ghebreyesus, Director-General of the World Health Organization, and Mark Lowcock, the UN Under-Secretary-General for Humanitarian Affairs and Emergency Relief Coordinator.

The DRC Ebola outbreak has drawn continuing concern and was highlighted by an even greater feeling of urgency as “on the eve of the conference,” according to one speaker.
That same infected individual – a priest arriving by bus to the city from an affected area – died of the disease the day after the conference concluded, according to the DRC authorities.
In his opening remarks, Mr. Lowcock stressed the importance of coordinating international efforts with the on-the-ground responses being carried out under the direction of Oly Ilunga Kalenga, MD, the DRC’s Minister of Public Health, who was also present and spoke at the meeting. Dr. Kalenga resigned his post on July 22, 2019.*
Mr. Lowcock stressed three points in particular that make for unique changes to the current response, compared with the earlier outbreak of 2014-2016.
First, in the previous outbreak in West Africa, “we didn’t have the vaccine and we didn’t have some of the successful treatments” that are currently available. Furthermore, more than 160,000 people have now been vaccinated and “the vaccine has a high degree of effectiveness.” This is an asset compared to the previous situation, he stated.
In his second point, he warned that the outbreak in the DRC “is taking place in an insecure and complex area with multiple armed groups present and large-scale preexisting humanitarian needs. Special interests distort the context. A history of disaffection with national authorities and foreigners generates distrust and makes the response more complicated. And one manifestation of that is attacks against health facilities and health care workers.” He added that “two more of our colleagues, trying to be part of the solution,” were killed in the past few days before the meeting. “Therefore, security for the response is of absolutely paramount importance, and we are trying to strengthen the way the UN family supports the government’s own security.”
The third major difference from the West Africa outbreak, Mr. Lowcock pointed out, was the issue of money. There was more than $2 billion in international support available for that earlier response. However, “what we have available for us in the DRC is just a small fraction of that. Donors released funds early on ... but much more is needed.” He warned that the cost of reaching zero cases must not be underestimated, and that the fourth strategic response plan for this outbreak, currently under development, “will be budgeted at a much higher level than the previous three plans, and that’s because it’s our assessment that we need a bigger, more comprehensive response if we’re to get to zero cases than we’ve had hitherto.”
In fact, he said, “unless there is a big scale up in the response, we’re unlikely to get to zero cases.”
The meeting also featured speakers outlining more local aspects of the response and discussing how international workers were coordinating more and more with local authorities and health practitioners in order to deliver health care on the ground while attempting to avoid the distrust created in the past, while still ensuring security for foreign personnel.
Commenting on the issue of security, Rory Stewart, the United Kingdom’s Secretary of State for International Development, described how a major DRC Ebola treatment center was attacked and burned by military insurgents, but is now rebuilt. He said that, while there have been some improvements and reasons to be hopeful, “this isn’t a moment for complacency; [the situation] is literally on the knife-edge.” He added: “If you go into that treatment center now, you will see that, although there are very good medical procedures, there are really, really worrying security procedures. The entire protection for the medical staff consists of a small square of sandbags about the height of this table [he raised his hand just above the standard conference table he was sitting at], behind which the doctors and nurses are supposed to hide if armed men get into the compound.”
In his presentation, David Gressly, the UN Emergency Ebola Response Coordinator (speaking by video from the DRC), stressed the need to cooperate with local authorities and to build local trust, stating that “the UN is putting together a tight, disciplined, coordinated system for rapid response and operational adjustments so that we can shift from chasing the disease to getting ahead of it. We need to quickly detect cases ... that have moved into areas of risk to stop the transmission early.”
Matshidiso Moeti, MD, WHO Regional Director for Africa, added in her presentation: “We’ve identified nine high-risk countries. Among those, Burundi, South Sudan, and Uganda face the highest risk and require our concerted and continued efforts.” She said that more than 10,000 health care workers in areas of high risk have so far been vaccinated against the disease.
In his concluding statement, Dr. Kalenga described the current state of affairs in his country with a modicum of hope. “A community that has been told it has a case of Ebola is a community that is traumatized by the very announcement of this epidemic. With time, the community has learned to face up to this epidemic differently. In some villages we are given a very different welcome than before. ... The villagers ask: ‘What should we do to make sure the Ebola case is the only one? The first and the last one?’ Throughout this epidemic, we have seen the people become more aware, and a certain acceptance of the very difficult and lethal diagnosis. ... So we have seen that work in the community has been maturing and bearing fruit.”
However, he pointed out, “there is a whole debate around the area of vaccinations, and we do need to close down this debate. At this point in time, we have a vaccine that is highly effective, a vaccine that is accepted by the population, after whole periods of mistrust. So we’ve come to a point in time when the population is accepting a vaccine, a vaccine that works, so we decided to no longer open the debate on vaccines and vaccination. ....We don’t want contradictory messages going out here, we don’t want different schemes going out. ...We have an effective weapon, we have an effective molecule. Let’s focus on that. Let’s all go in the same direction,” he concluded.
On July 11, an announcement by DRC officials stated that Merck’s rVSV-ZEBOV would be the only vaccine that will be used during the current Ebola outbreak in North Kivu and Ituri provinces, and that no other clinical vaccine trials to be allowed in the country so as not to confuse the population.
In that same announcement, the DRC reported that, since the beginning of the epidemic, the cumulative number of Ebola cases was 2,451, of which 2,357 were confirmed and 94 probable. There were 1,647 deaths (1,553 confirmed and 94 probable) and 683 people who survived. An additional 364 suspected cases were under investigation.
*Updated Aug. 1, 2019.
SOURCE: United Nations WHO/OCHA Webcast and Media Stakeout. July 15, 2019.
The continuing outbreak of Ebola in the Democratic Republic of the Congo (DRC) was the subject of a special United Nations high-level event organized by the World Health Organization (WHO) and the United Nations Office for the Coordination of Humanitarian Affairs (OCHA). It was comoderated by Tedros Adhanom Ghebreyesus, Director-General of the World Health Organization, and Mark Lowcock, the UN Under-Secretary-General for Humanitarian Affairs and Emergency Relief Coordinator.

The DRC Ebola outbreak has drawn continuing concern and was highlighted by an even greater feeling of urgency as “on the eve of the conference,” according to one speaker.
That same infected individual – a priest arriving by bus to the city from an affected area – died of the disease the day after the conference concluded, according to the DRC authorities.
In his opening remarks, Mr. Lowcock stressed the importance of coordinating international efforts with the on-the-ground responses being carried out under the direction of Oly Ilunga Kalenga, MD, the DRC’s Minister of Public Health, who was also present and spoke at the meeting. Dr. Kalenga resigned his post on July 22, 2019.*
Mr. Lowcock stressed three points in particular that make for unique changes to the current response, compared with the earlier outbreak of 2014-2016.
First, in the previous outbreak in West Africa, “we didn’t have the vaccine and we didn’t have some of the successful treatments” that are currently available. Furthermore, more than 160,000 people have now been vaccinated and “the vaccine has a high degree of effectiveness.” This is an asset compared to the previous situation, he stated.
In his second point, he warned that the outbreak in the DRC “is taking place in an insecure and complex area with multiple armed groups present and large-scale preexisting humanitarian needs. Special interests distort the context. A history of disaffection with national authorities and foreigners generates distrust and makes the response more complicated. And one manifestation of that is attacks against health facilities and health care workers.” He added that “two more of our colleagues, trying to be part of the solution,” were killed in the past few days before the meeting. “Therefore, security for the response is of absolutely paramount importance, and we are trying to strengthen the way the UN family supports the government’s own security.”
The third major difference from the West Africa outbreak, Mr. Lowcock pointed out, was the issue of money. There was more than $2 billion in international support available for that earlier response. However, “what we have available for us in the DRC is just a small fraction of that. Donors released funds early on ... but much more is needed.” He warned that the cost of reaching zero cases must not be underestimated, and that the fourth strategic response plan for this outbreak, currently under development, “will be budgeted at a much higher level than the previous three plans, and that’s because it’s our assessment that we need a bigger, more comprehensive response if we’re to get to zero cases than we’ve had hitherto.”
In fact, he said, “unless there is a big scale up in the response, we’re unlikely to get to zero cases.”
The meeting also featured speakers outlining more local aspects of the response and discussing how international workers were coordinating more and more with local authorities and health practitioners in order to deliver health care on the ground while attempting to avoid the distrust created in the past, while still ensuring security for foreign personnel.
Commenting on the issue of security, Rory Stewart, the United Kingdom’s Secretary of State for International Development, described how a major DRC Ebola treatment center was attacked and burned by military insurgents, but is now rebuilt. He said that, while there have been some improvements and reasons to be hopeful, “this isn’t a moment for complacency; [the situation] is literally on the knife-edge.” He added: “If you go into that treatment center now, you will see that, although there are very good medical procedures, there are really, really worrying security procedures. The entire protection for the medical staff consists of a small square of sandbags about the height of this table [he raised his hand just above the standard conference table he was sitting at], behind which the doctors and nurses are supposed to hide if armed men get into the compound.”
In his presentation, David Gressly, the UN Emergency Ebola Response Coordinator (speaking by video from the DRC), stressed the need to cooperate with local authorities and to build local trust, stating that “the UN is putting together a tight, disciplined, coordinated system for rapid response and operational adjustments so that we can shift from chasing the disease to getting ahead of it. We need to quickly detect cases ... that have moved into areas of risk to stop the transmission early.”
Matshidiso Moeti, MD, WHO Regional Director for Africa, added in her presentation: “We’ve identified nine high-risk countries. Among those, Burundi, South Sudan, and Uganda face the highest risk and require our concerted and continued efforts.” She said that more than 10,000 health care workers in areas of high risk have so far been vaccinated against the disease.
In his concluding statement, Dr. Kalenga described the current state of affairs in his country with a modicum of hope. “A community that has been told it has a case of Ebola is a community that is traumatized by the very announcement of this epidemic. With time, the community has learned to face up to this epidemic differently. In some villages we are given a very different welcome than before. ... The villagers ask: ‘What should we do to make sure the Ebola case is the only one? The first and the last one?’ Throughout this epidemic, we have seen the people become more aware, and a certain acceptance of the very difficult and lethal diagnosis. ... So we have seen that work in the community has been maturing and bearing fruit.”
However, he pointed out, “there is a whole debate around the area of vaccinations, and we do need to close down this debate. At this point in time, we have a vaccine that is highly effective, a vaccine that is accepted by the population, after whole periods of mistrust. So we’ve come to a point in time when the population is accepting a vaccine, a vaccine that works, so we decided to no longer open the debate on vaccines and vaccination. ....We don’t want contradictory messages going out here, we don’t want different schemes going out. ...We have an effective weapon, we have an effective molecule. Let’s focus on that. Let’s all go in the same direction,” he concluded.
On July 11, an announcement by DRC officials stated that Merck’s rVSV-ZEBOV would be the only vaccine that will be used during the current Ebola outbreak in North Kivu and Ituri provinces, and that no other clinical vaccine trials to be allowed in the country so as not to confuse the population.
In that same announcement, the DRC reported that, since the beginning of the epidemic, the cumulative number of Ebola cases was 2,451, of which 2,357 were confirmed and 94 probable. There were 1,647 deaths (1,553 confirmed and 94 probable) and 683 people who survived. An additional 364 suspected cases were under investigation.
*Updated Aug. 1, 2019.
SOURCE: United Nations WHO/OCHA Webcast and Media Stakeout. July 15, 2019.
The continuing outbreak of Ebola in the Democratic Republic of the Congo (DRC) was the subject of a special United Nations high-level event organized by the World Health Organization (WHO) and the United Nations Office for the Coordination of Humanitarian Affairs (OCHA). It was comoderated by Tedros Adhanom Ghebreyesus, Director-General of the World Health Organization, and Mark Lowcock, the UN Under-Secretary-General for Humanitarian Affairs and Emergency Relief Coordinator.

The DRC Ebola outbreak has drawn continuing concern and was highlighted by an even greater feeling of urgency as “on the eve of the conference,” according to one speaker.
That same infected individual – a priest arriving by bus to the city from an affected area – died of the disease the day after the conference concluded, according to the DRC authorities.
In his opening remarks, Mr. Lowcock stressed the importance of coordinating international efforts with the on-the-ground responses being carried out under the direction of Oly Ilunga Kalenga, MD, the DRC’s Minister of Public Health, who was also present and spoke at the meeting. Dr. Kalenga resigned his post on July 22, 2019.*
Mr. Lowcock stressed three points in particular that make for unique changes to the current response, compared with the earlier outbreak of 2014-2016.
First, in the previous outbreak in West Africa, “we didn’t have the vaccine and we didn’t have some of the successful treatments” that are currently available. Furthermore, more than 160,000 people have now been vaccinated and “the vaccine has a high degree of effectiveness.” This is an asset compared to the previous situation, he stated.
In his second point, he warned that the outbreak in the DRC “is taking place in an insecure and complex area with multiple armed groups present and large-scale preexisting humanitarian needs. Special interests distort the context. A history of disaffection with national authorities and foreigners generates distrust and makes the response more complicated. And one manifestation of that is attacks against health facilities and health care workers.” He added that “two more of our colleagues, trying to be part of the solution,” were killed in the past few days before the meeting. “Therefore, security for the response is of absolutely paramount importance, and we are trying to strengthen the way the UN family supports the government’s own security.”
The third major difference from the West Africa outbreak, Mr. Lowcock pointed out, was the issue of money. There was more than $2 billion in international support available for that earlier response. However, “what we have available for us in the DRC is just a small fraction of that. Donors released funds early on ... but much more is needed.” He warned that the cost of reaching zero cases must not be underestimated, and that the fourth strategic response plan for this outbreak, currently under development, “will be budgeted at a much higher level than the previous three plans, and that’s because it’s our assessment that we need a bigger, more comprehensive response if we’re to get to zero cases than we’ve had hitherto.”
In fact, he said, “unless there is a big scale up in the response, we’re unlikely to get to zero cases.”
The meeting also featured speakers outlining more local aspects of the response and discussing how international workers were coordinating more and more with local authorities and health practitioners in order to deliver health care on the ground while attempting to avoid the distrust created in the past, while still ensuring security for foreign personnel.
Commenting on the issue of security, Rory Stewart, the United Kingdom’s Secretary of State for International Development, described how a major DRC Ebola treatment center was attacked and burned by military insurgents, but is now rebuilt. He said that, while there have been some improvements and reasons to be hopeful, “this isn’t a moment for complacency; [the situation] is literally on the knife-edge.” He added: “If you go into that treatment center now, you will see that, although there are very good medical procedures, there are really, really worrying security procedures. The entire protection for the medical staff consists of a small square of sandbags about the height of this table [he raised his hand just above the standard conference table he was sitting at], behind which the doctors and nurses are supposed to hide if armed men get into the compound.”
In his presentation, David Gressly, the UN Emergency Ebola Response Coordinator (speaking by video from the DRC), stressed the need to cooperate with local authorities and to build local trust, stating that “the UN is putting together a tight, disciplined, coordinated system for rapid response and operational adjustments so that we can shift from chasing the disease to getting ahead of it. We need to quickly detect cases ... that have moved into areas of risk to stop the transmission early.”
Matshidiso Moeti, MD, WHO Regional Director for Africa, added in her presentation: “We’ve identified nine high-risk countries. Among those, Burundi, South Sudan, and Uganda face the highest risk and require our concerted and continued efforts.” She said that more than 10,000 health care workers in areas of high risk have so far been vaccinated against the disease.
In his concluding statement, Dr. Kalenga described the current state of affairs in his country with a modicum of hope. “A community that has been told it has a case of Ebola is a community that is traumatized by the very announcement of this epidemic. With time, the community has learned to face up to this epidemic differently. In some villages we are given a very different welcome than before. ... The villagers ask: ‘What should we do to make sure the Ebola case is the only one? The first and the last one?’ Throughout this epidemic, we have seen the people become more aware, and a certain acceptance of the very difficult and lethal diagnosis. ... So we have seen that work in the community has been maturing and bearing fruit.”
However, he pointed out, “there is a whole debate around the area of vaccinations, and we do need to close down this debate. At this point in time, we have a vaccine that is highly effective, a vaccine that is accepted by the population, after whole periods of mistrust. So we’ve come to a point in time when the population is accepting a vaccine, a vaccine that works, so we decided to no longer open the debate on vaccines and vaccination. ....We don’t want contradictory messages going out here, we don’t want different schemes going out. ...We have an effective weapon, we have an effective molecule. Let’s focus on that. Let’s all go in the same direction,” he concluded.
On July 11, an announcement by DRC officials stated that Merck’s rVSV-ZEBOV would be the only vaccine that will be used during the current Ebola outbreak in North Kivu and Ituri provinces, and that no other clinical vaccine trials to be allowed in the country so as not to confuse the population.
In that same announcement, the DRC reported that, since the beginning of the epidemic, the cumulative number of Ebola cases was 2,451, of which 2,357 were confirmed and 94 probable. There were 1,647 deaths (1,553 confirmed and 94 probable) and 683 people who survived. An additional 364 suspected cases were under investigation.
*Updated Aug. 1, 2019.
SOURCE: United Nations WHO/OCHA Webcast and Media Stakeout. July 15, 2019.
REPORTING FROM A UN MEETING LIVE WEBCAST
Patients with COPD at heightened risk for community-acquired pneumonia requiring hospitalization
Patients with chronic obstructive pulmonary disease are at a significantly increased risk for hospitalization for community-acquired pneumonia (CAP), compared with patients without COPD, a large prospective study has found.
Jose Bordon, MD, and colleagues aimed to define incidence and outcomes of COPD patients hospitalized with pneumonia in the city of Louisville, Ky., and to extrapolate the burden of disease in the U.S. population. They conducted a secondary analysis of data from the University of Louisville Pneumonia Study, a prospective population-based cohort study of all hospitalized adults with CAP who were residents in the city of Louisville, Ky., from June 1, 2014, to May 31, 2016.
COPD prevalence in the city of Louisville was derived via data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) as well as from the 2014 National Health Interview Survey (NHIS). In addition, the researchers analyzed clinical outcomes including time to clinical stability (TCS), length of hospital stay (LOS), and mortality, according to Dr. Bordon, an infectious disease specialist at Providence Health Center, Washington, and colleagues on behalf of the University of Louisville Pneumonia Study Group.
The researchers found an 18-fold greater incidence of community-acquired pneumonia in patients with COPD, compared with non-COPD patients.
A total of 18,246 individuals aged 40 and older with COPD were estimated to live in Louisville, Ky. The researchers found that 3,419 COPD patients were hospitalized due to CAP in Louisville during the 2-year study period. COPD patients, compared with non-COPD patients, were more likely to have a history of heart failure, more ICU admissions, and use of mechanical ventilation, compared with patients without COPD. The two groups had similar pneumonia severity index scores, and 17% received oral steroids prior to admission. COPD patients had more pneumococcal pneumonia, despite receiving pneumococcal vaccine significantly more often than non-COPD patients.
The annual incidence of hospitalized CAP was 9,369 cases per 100,000 COPD patients in the city of Louisville. In the same period, the incidence of CAP in patients without COPD was 509 per 100,000, a more than 18-fold difference.
Although the incidence of CAP in COPD patients was much higher than in those without, the difference didn’t appear to have an impact on clinical outcomes. There were no clinical differences among patients with vs. without COPD in regard to time to reach clinical improvement and time of hospital discharge, and in-hospital mortality was not statistically significantly different between the groups, the authors reported. The mortality of COPD patients during hospitalization, at 30 days, at 6 months, and at 1 year was 5.6% of patients, 11.9%, 24.3%, and 33.0%, respectively vs. 6.6%, 14.2%, 24.2%, and 30.1% in non-COPD patients. However, 1-year all-cause mortality was a significant 25% greater among COPD patients, as might be expected by the progression and effects of the underlying disease.
“[Our] observations mean that nearly 1 in 10 persons with COPD will be hospitalized annually due to CAP. This translates into approximately 500,000 COPD patients hospitalized with CAP every year in the U.S., resulting in a substantial burden of approximately 5 billion U.S. dollars in hospitalization costs,” the researchers stated.
“Modifiable factors associated with CAP such as tobacco smoking and immunizations should be health interventions to prevent the burden of CAP in COPD patients,” even though “pneumococcal vaccination was used more often in the COPD population than in other CAP patients, but pneumococcal pneumonia still occurred at a numerically higher rate,” they noted.
The study was supported by the University of Louisville, Ky., with partial support from Pfizer. The authors reported having no conflicts.
SOURCE: Bordon JM et al. Clin Microbiol Infect. 2019 Jun 26; doi: 10.1016/j.cmi.2019.06.025.
Patients with chronic obstructive pulmonary disease are at a significantly increased risk for hospitalization for community-acquired pneumonia (CAP), compared with patients without COPD, a large prospective study has found.
Jose Bordon, MD, and colleagues aimed to define incidence and outcomes of COPD patients hospitalized with pneumonia in the city of Louisville, Ky., and to extrapolate the burden of disease in the U.S. population. They conducted a secondary analysis of data from the University of Louisville Pneumonia Study, a prospective population-based cohort study of all hospitalized adults with CAP who were residents in the city of Louisville, Ky., from June 1, 2014, to May 31, 2016.
COPD prevalence in the city of Louisville was derived via data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) as well as from the 2014 National Health Interview Survey (NHIS). In addition, the researchers analyzed clinical outcomes including time to clinical stability (TCS), length of hospital stay (LOS), and mortality, according to Dr. Bordon, an infectious disease specialist at Providence Health Center, Washington, and colleagues on behalf of the University of Louisville Pneumonia Study Group.
The researchers found an 18-fold greater incidence of community-acquired pneumonia in patients with COPD, compared with non-COPD patients.
A total of 18,246 individuals aged 40 and older with COPD were estimated to live in Louisville, Ky. The researchers found that 3,419 COPD patients were hospitalized due to CAP in Louisville during the 2-year study period. COPD patients, compared with non-COPD patients, were more likely to have a history of heart failure, more ICU admissions, and use of mechanical ventilation, compared with patients without COPD. The two groups had similar pneumonia severity index scores, and 17% received oral steroids prior to admission. COPD patients had more pneumococcal pneumonia, despite receiving pneumococcal vaccine significantly more often than non-COPD patients.
The annual incidence of hospitalized CAP was 9,369 cases per 100,000 COPD patients in the city of Louisville. In the same period, the incidence of CAP in patients without COPD was 509 per 100,000, a more than 18-fold difference.
Although the incidence of CAP in COPD patients was much higher than in those without, the difference didn’t appear to have an impact on clinical outcomes. There were no clinical differences among patients with vs. without COPD in regard to time to reach clinical improvement and time of hospital discharge, and in-hospital mortality was not statistically significantly different between the groups, the authors reported. The mortality of COPD patients during hospitalization, at 30 days, at 6 months, and at 1 year was 5.6% of patients, 11.9%, 24.3%, and 33.0%, respectively vs. 6.6%, 14.2%, 24.2%, and 30.1% in non-COPD patients. However, 1-year all-cause mortality was a significant 25% greater among COPD patients, as might be expected by the progression and effects of the underlying disease.
“[Our] observations mean that nearly 1 in 10 persons with COPD will be hospitalized annually due to CAP. This translates into approximately 500,000 COPD patients hospitalized with CAP every year in the U.S., resulting in a substantial burden of approximately 5 billion U.S. dollars in hospitalization costs,” the researchers stated.
“Modifiable factors associated with CAP such as tobacco smoking and immunizations should be health interventions to prevent the burden of CAP in COPD patients,” even though “pneumococcal vaccination was used more often in the COPD population than in other CAP patients, but pneumococcal pneumonia still occurred at a numerically higher rate,” they noted.
The study was supported by the University of Louisville, Ky., with partial support from Pfizer. The authors reported having no conflicts.
SOURCE: Bordon JM et al. Clin Microbiol Infect. 2019 Jun 26; doi: 10.1016/j.cmi.2019.06.025.
Patients with chronic obstructive pulmonary disease are at a significantly increased risk for hospitalization for community-acquired pneumonia (CAP), compared with patients without COPD, a large prospective study has found.
Jose Bordon, MD, and colleagues aimed to define incidence and outcomes of COPD patients hospitalized with pneumonia in the city of Louisville, Ky., and to extrapolate the burden of disease in the U.S. population. They conducted a secondary analysis of data from the University of Louisville Pneumonia Study, a prospective population-based cohort study of all hospitalized adults with CAP who were residents in the city of Louisville, Ky., from June 1, 2014, to May 31, 2016.
COPD prevalence in the city of Louisville was derived via data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) as well as from the 2014 National Health Interview Survey (NHIS). In addition, the researchers analyzed clinical outcomes including time to clinical stability (TCS), length of hospital stay (LOS), and mortality, according to Dr. Bordon, an infectious disease specialist at Providence Health Center, Washington, and colleagues on behalf of the University of Louisville Pneumonia Study Group.
The researchers found an 18-fold greater incidence of community-acquired pneumonia in patients with COPD, compared with non-COPD patients.
A total of 18,246 individuals aged 40 and older with COPD were estimated to live in Louisville, Ky. The researchers found that 3,419 COPD patients were hospitalized due to CAP in Louisville during the 2-year study period. COPD patients, compared with non-COPD patients, were more likely to have a history of heart failure, more ICU admissions, and use of mechanical ventilation, compared with patients without COPD. The two groups had similar pneumonia severity index scores, and 17% received oral steroids prior to admission. COPD patients had more pneumococcal pneumonia, despite receiving pneumococcal vaccine significantly more often than non-COPD patients.
The annual incidence of hospitalized CAP was 9,369 cases per 100,000 COPD patients in the city of Louisville. In the same period, the incidence of CAP in patients without COPD was 509 per 100,000, a more than 18-fold difference.
Although the incidence of CAP in COPD patients was much higher than in those without, the difference didn’t appear to have an impact on clinical outcomes. There were no clinical differences among patients with vs. without COPD in regard to time to reach clinical improvement and time of hospital discharge, and in-hospital mortality was not statistically significantly different between the groups, the authors reported. The mortality of COPD patients during hospitalization, at 30 days, at 6 months, and at 1 year was 5.6% of patients, 11.9%, 24.3%, and 33.0%, respectively vs. 6.6%, 14.2%, 24.2%, and 30.1% in non-COPD patients. However, 1-year all-cause mortality was a significant 25% greater among COPD patients, as might be expected by the progression and effects of the underlying disease.
“[Our] observations mean that nearly 1 in 10 persons with COPD will be hospitalized annually due to CAP. This translates into approximately 500,000 COPD patients hospitalized with CAP every year in the U.S., resulting in a substantial burden of approximately 5 billion U.S. dollars in hospitalization costs,” the researchers stated.
“Modifiable factors associated with CAP such as tobacco smoking and immunizations should be health interventions to prevent the burden of CAP in COPD patients,” even though “pneumococcal vaccination was used more often in the COPD population than in other CAP patients, but pneumococcal pneumonia still occurred at a numerically higher rate,” they noted.
The study was supported by the University of Louisville, Ky., with partial support from Pfizer. The authors reported having no conflicts.
SOURCE: Bordon JM et al. Clin Microbiol Infect. 2019 Jun 26; doi: 10.1016/j.cmi.2019.06.025.
FROM CLINICAL MICROBIOLOGY AND INFECTION
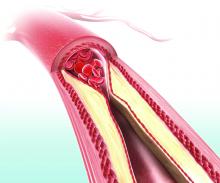
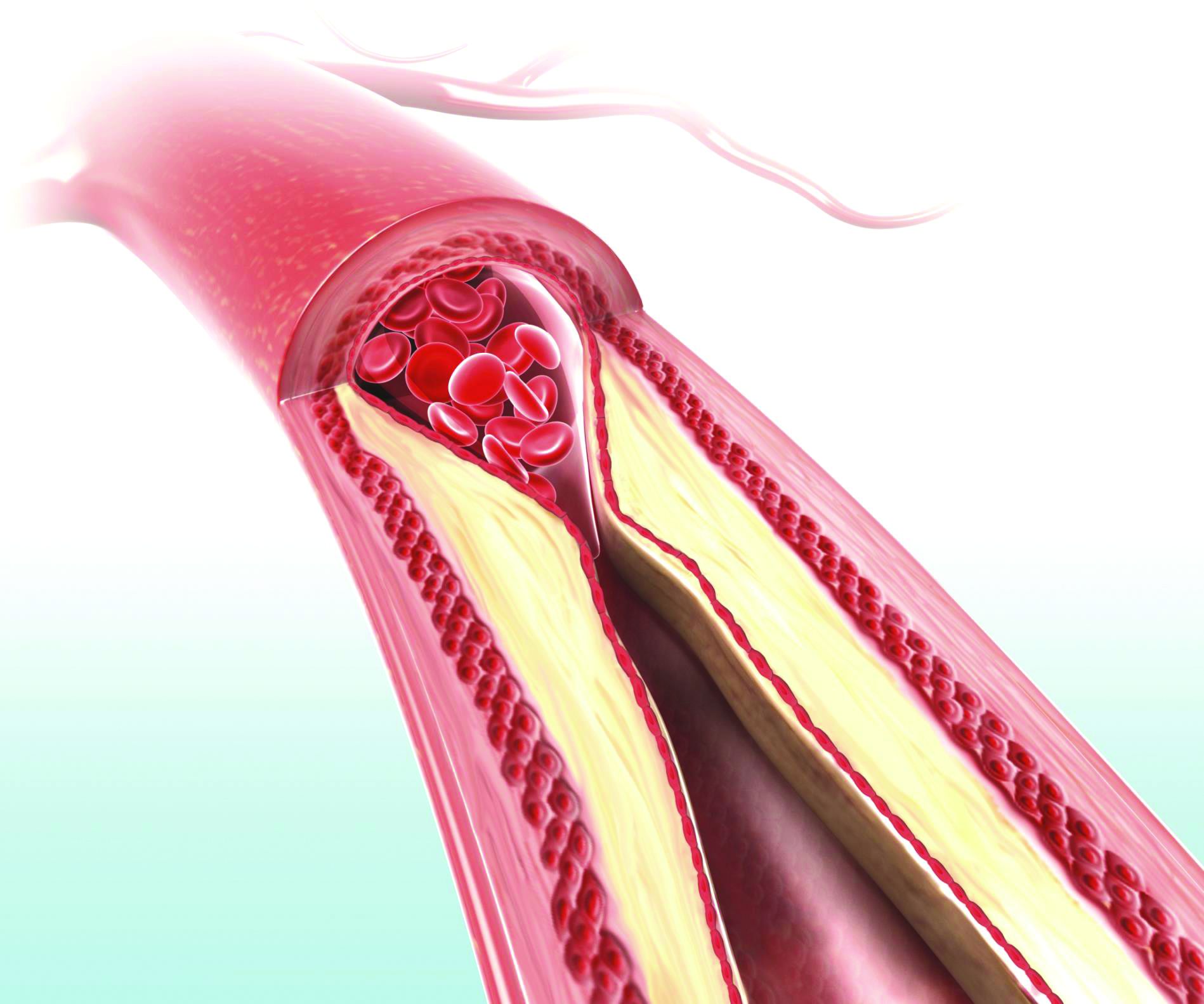
Microvascular disease: An independent and exacerbating risk factor for amputation
Individuals with microvascular disease (MVD) showed a significantly increased risk of lower limb amputation in the absence of peripheral artery disease (PAD), according to the results of a large database analysis published online in Circulation.
Furthermore, those who had both MVD and PAD had a greater than 20-fold increased risk of amputation than if they had either PAD or MVD alone, according to Joshua A. Beckman, MD, of Vanderbilt University, Nashville, Tenn., and colleagues.
“The novelty of these findings becomes clear when put into the current framework of critical limb ischemia,” they wrote.
“In a recent state of the art review of [critical limb ischemia], MVD as a whole or its components did not receive a single mention. Our work shows that MVD helps identify a population not previously considered at particularly high risk for amputation and, when added to PAD, identify a group of patients at very high risk for amputation,” they continued.
Dr. Beckman and colleagues assessed individuals in the Veterans Aging Cohort Study (VACS), a prospective longitudinal cohort of veterans. They included all VACS participants who were alive as of April 1, 2003 with the baseline as a participant’s first clinic visit on or after this date. Participants were followed from baseline to the minimum of: date of lower extremity amputation, death, or Dec. 31, 2014.
They assessed four levels of vascular involvement: neither MVD nor PAD, MVD alone, PAD alone, and MVD plus PAD, with the primary outcome being lower limb amputation, all based on a variety of measures including appropriate ICD-9 or CPT codes.
The rate of incident amputation over a median of 9.3 years of follow-up was 1.16 per 1000 person-years. At the time of amputation, retinopathy was present in 69%, nephropathy in 67%, and neuropathy in 78% of participants.
After multivariable adjustment for 216 demographic characteristics, cardiovascular disease risk factors, and other potential confounders, they found that, compared with participants without either vascular disease, the presence of MVD alone was associated with a 3.7-fold increased risk of amputation, PAD alone conferred a 13.9-fold elevated risk of amputation, and the combination of PAD and MVD was associated with a 22.7-fold increased risk of amputation.
They also found that the location of amputation also varied depending on the type of vascular disease at the time of amputation.
Participants with MVD alone accounted for 18% of all amputations, 21% of below ankle amputations, 15% of below knee amputations, and 6% of all above knee amputations. Participants with PAD alone accounted for 22% of all amputations, 17% of below ankle, 25% of below knee, and 39% of above knee amputations. The combination of MVD and PAD accounted for 45% of all amputation and caused the most amputation at all limb levels. In addition, they found a statistically significant variation in vascular involvement and level of amputation, with MVD more likely to cause a below-ankle amputation and PAD more likely to cause below- and above-knee amputations (P less than .001)
“MVD likely participates importantly in the development of adverse limb events in PAD and suggests additional patient populations who may benefit from greater foot surveillance to minimize amputation,” the researchers concluded.
The study was supported by grants from the American Heart Association. Dr. Beckman reported consulting for multiple pharmaceutical companies and serving on the DSMC for Bayer and Novartis.
SOURCE: Beckman JA et al. Circulation. 2019. doi: 10.1161/CIRCULATIONAHA.119.040672.
Individuals with microvascular disease (MVD) showed a significantly increased risk of lower limb amputation in the absence of peripheral artery disease (PAD), according to the results of a large database analysis published online in Circulation.
Furthermore, those who had both MVD and PAD had a greater than 20-fold increased risk of amputation than if they had either PAD or MVD alone, according to Joshua A. Beckman, MD, of Vanderbilt University, Nashville, Tenn., and colleagues.
“The novelty of these findings becomes clear when put into the current framework of critical limb ischemia,” they wrote.
“In a recent state of the art review of [critical limb ischemia], MVD as a whole or its components did not receive a single mention. Our work shows that MVD helps identify a population not previously considered at particularly high risk for amputation and, when added to PAD, identify a group of patients at very high risk for amputation,” they continued.
Dr. Beckman and colleagues assessed individuals in the Veterans Aging Cohort Study (VACS), a prospective longitudinal cohort of veterans. They included all VACS participants who were alive as of April 1, 2003 with the baseline as a participant’s first clinic visit on or after this date. Participants were followed from baseline to the minimum of: date of lower extremity amputation, death, or Dec. 31, 2014.
They assessed four levels of vascular involvement: neither MVD nor PAD, MVD alone, PAD alone, and MVD plus PAD, with the primary outcome being lower limb amputation, all based on a variety of measures including appropriate ICD-9 or CPT codes.
The rate of incident amputation over a median of 9.3 years of follow-up was 1.16 per 1000 person-years. At the time of amputation, retinopathy was present in 69%, nephropathy in 67%, and neuropathy in 78% of participants.
After multivariable adjustment for 216 demographic characteristics, cardiovascular disease risk factors, and other potential confounders, they found that, compared with participants without either vascular disease, the presence of MVD alone was associated with a 3.7-fold increased risk of amputation, PAD alone conferred a 13.9-fold elevated risk of amputation, and the combination of PAD and MVD was associated with a 22.7-fold increased risk of amputation.
They also found that the location of amputation also varied depending on the type of vascular disease at the time of amputation.
Participants with MVD alone accounted for 18% of all amputations, 21% of below ankle amputations, 15% of below knee amputations, and 6% of all above knee amputations. Participants with PAD alone accounted for 22% of all amputations, 17% of below ankle, 25% of below knee, and 39% of above knee amputations. The combination of MVD and PAD accounted for 45% of all amputation and caused the most amputation at all limb levels. In addition, they found a statistically significant variation in vascular involvement and level of amputation, with MVD more likely to cause a below-ankle amputation and PAD more likely to cause below- and above-knee amputations (P less than .001)
“MVD likely participates importantly in the development of adverse limb events in PAD and suggests additional patient populations who may benefit from greater foot surveillance to minimize amputation,” the researchers concluded.
The study was supported by grants from the American Heart Association. Dr. Beckman reported consulting for multiple pharmaceutical companies and serving on the DSMC for Bayer and Novartis.
SOURCE: Beckman JA et al. Circulation. 2019. doi: 10.1161/CIRCULATIONAHA.119.040672.
Individuals with microvascular disease (MVD) showed a significantly increased risk of lower limb amputation in the absence of peripheral artery disease (PAD), according to the results of a large database analysis published online in Circulation.
Furthermore, those who had both MVD and PAD had a greater than 20-fold increased risk of amputation than if they had either PAD or MVD alone, according to Joshua A. Beckman, MD, of Vanderbilt University, Nashville, Tenn., and colleagues.
“The novelty of these findings becomes clear when put into the current framework of critical limb ischemia,” they wrote.
“In a recent state of the art review of [critical limb ischemia], MVD as a whole or its components did not receive a single mention. Our work shows that MVD helps identify a population not previously considered at particularly high risk for amputation and, when added to PAD, identify a group of patients at very high risk for amputation,” they continued.
Dr. Beckman and colleagues assessed individuals in the Veterans Aging Cohort Study (VACS), a prospective longitudinal cohort of veterans. They included all VACS participants who were alive as of April 1, 2003 with the baseline as a participant’s first clinic visit on or after this date. Participants were followed from baseline to the minimum of: date of lower extremity amputation, death, or Dec. 31, 2014.
They assessed four levels of vascular involvement: neither MVD nor PAD, MVD alone, PAD alone, and MVD plus PAD, with the primary outcome being lower limb amputation, all based on a variety of measures including appropriate ICD-9 or CPT codes.
The rate of incident amputation over a median of 9.3 years of follow-up was 1.16 per 1000 person-years. At the time of amputation, retinopathy was present in 69%, nephropathy in 67%, and neuropathy in 78% of participants.
After multivariable adjustment for 216 demographic characteristics, cardiovascular disease risk factors, and other potential confounders, they found that, compared with participants without either vascular disease, the presence of MVD alone was associated with a 3.7-fold increased risk of amputation, PAD alone conferred a 13.9-fold elevated risk of amputation, and the combination of PAD and MVD was associated with a 22.7-fold increased risk of amputation.
They also found that the location of amputation also varied depending on the type of vascular disease at the time of amputation.
Participants with MVD alone accounted for 18% of all amputations, 21% of below ankle amputations, 15% of below knee amputations, and 6% of all above knee amputations. Participants with PAD alone accounted for 22% of all amputations, 17% of below ankle, 25% of below knee, and 39% of above knee amputations. The combination of MVD and PAD accounted for 45% of all amputation and caused the most amputation at all limb levels. In addition, they found a statistically significant variation in vascular involvement and level of amputation, with MVD more likely to cause a below-ankle amputation and PAD more likely to cause below- and above-knee amputations (P less than .001)
“MVD likely participates importantly in the development of adverse limb events in PAD and suggests additional patient populations who may benefit from greater foot surveillance to minimize amputation,” the researchers concluded.
The study was supported by grants from the American Heart Association. Dr. Beckman reported consulting for multiple pharmaceutical companies and serving on the DSMC for Bayer and Novartis.
SOURCE: Beckman JA et al. Circulation. 2019. doi: 10.1161/CIRCULATIONAHA.119.040672.
FROM CIRCULATION
Key clinical point: Microvascular disease yielded a 3.7-fold increased risk of lower limb amputation.
Major finding:
Study details: Database analysis of 125,674 participants in the Veterans Aging Cohort Study from April 2003 through December 2014.
Disclosures: The study was supported by grants from the American Heart Association. Dr. Beckman reported consulting for multiple pharmaceutical companies and serving on the DSMC for Bayer and Novartis.
Source: Beckman JA et al. Circulation. 2019. doi: 10.1161/CIRCULATIONAHA.119.040672.
FDA panel: Continue using paclitaxel-eluting PAD devices, with caveats
GAITHERSBURG, MD. – There was sufficient evidence of a late mortality signal seen at 2-5 years post procedurally for paclitaxel-eluting stents and coated balloons to warrant a label change for the devices, the Food and Drug Administration’s Circulatory System Devices Panel unanimously agreed after 2 days of deliberation.
That signal was brought to light in a meta-analysis published last December by Konstantinos Katsanos, MD, of Patras University Hospital, Rion, Greece, and colleagues (J Am Heart Assoc. 2018;7:e011245). Although there were concerns about the quality of the industry data used in the study, the caliber of the analysis itself and the subsequent data presented by the FDA to the panel were deemed sufficient to recommend a warning of concern to patients and providers.
Much of the new data from industry and large database registries presented to the panel, which was chaired by Richard A. Lange, MD, indicated a lessening to no evidence of the mortality effect. But this evidence was deemed insufficient to counter the evidence of the randomized controlled trials individually and collectively as presented in the Katsanos meta-analysis and subsequent information presented by the FDA that examined various parameters in a variety of sensitivity analyses that confirmed the late mortality signal. There was also concern that the industry and the registry analyses presented were not peer reviewed.
However, the panel also determined that it would be inappropriate to pull the devices from the market and from general use for several reasons.
One key reason was that, according to the panel, there was no mechanistic cause apparent for the late mortality. In addition, no convincing dose-response data could be teased from the preclinical and clinical trials studied because of their variability of devices, application methods, and lack of appropriate tissue analysis across studies.
Finally, the industry data used to create the meta-analysis were considered to be fundamentally flawed: in blinding, in the relatively small numbers of patients, and in the large percentage of patients lost to follow-up. The latter could have dramatically influenced the perceived results, especially as the studies were not powered or designed to follow mortality over such a period of time, according to the panel.
These limitations to the signal were especially important to the panel because of the obvious benefits with regard to quality of life provided to patients from these devices, which were attested to during the 2-day meeting by numerous presenters from industry, medical organizations – including societies and nonprofits – and providers.
In responding to FDA requests on a variety of concerns, the panel reiterated that there was a credible mortality signal, but that they could not be confident about the magnitude and whether it was caused by the paclitaxel treatment or some factor in the design or conduct of the studies. In addition, the panel members felt that they could neither confirm nor eliminate a class effect, given the fact that the information was based on a meta-analysis and thus none of the included devices could safely be removed from consideration.
They suggested that further safety information should be obtained, potentially by assessing and perhaps altering data collection in 29 ongoing studies over the next 5 years or so in more than 10,000 patients.
In addition, several of the panel members felt that additional animal studies might be performed including the use of older rat models; and using animal models that mimicked the kind of comorbidities present in the treated population, such as diabetes and atherosclerosis. They suggested cross-company industry cooperation with the FDA on these models, including looking at drug interactions and mimicking the dose application of stents/balloons.
Both the FDA representative and the panel were especially concerned with the benefit/risk profile.
The recommendation to still market the devices with a label warning was warranted, according to many members of the panel. They pointed to the clear benefits in quality of life and the lowered need for revascularization despite the evidence of the mortality signal, which, while statistically significant, could not be pinned town with regard to mechanisms or specific causes of death.
Overall, there was a concern that there should be a dialogue between patients and their doctors to discuss clear short-term benefits with unknown long-term risk, and that the label should support this by clearly mentioning the mortality signal that was found, although there was no attempt to develop exact wording.
Panel member Joaquin E. Cigarroa, MD, head of cardiovascular medicine at Oregon Health & Science University, Portland, suggested with regard to labeling that a statement “that ‘there may be’ – not ‘there is’ – a late mortality signal, should be included.”
Panel member John C. Somberg, MD, program director of clinical research and bioinformatics at Rush University in Lake Bluff, Ill., stated: “The label should say something like, ‘when looking at a meta-analysis that combined all studies with stents and balloons that carried paclitaxel, there may be a late mortality, which must be balanced against an early and sustained benefit in terms of pain on walking and potential loss of circulation to your extremity.’ ”
Other panel members thought that the meta-analysis should not be privileged and that somehow the totality of the evidence should somehow be distilled down into the label, including the evidence against the signal.
“We’re meeting because of a signal, of a concern – an honest, well-meaning concern – of increased mortality. And my opinion is that the patients need to be informed of it,” said Dr. Lange, president, Texas Tech University, El Paso.
Some members of the panel felt that it may not be justifiable to use these devices in patients with low intrinsic risk and low recurrence risk, and the whole spectrum of patients may need to be considered in further studies to figure out the subgroups that have more benefits and more risks, and also to consider how to mitigate risks in patients who receive the device, whether through medical therapy or lifestyle modification.
In particular, Frank W. LoGerfo, MD, the William V. McDermott Distinguished Professor of Surgery at Harvard Medical School, Boston, stated: “Interventions for claudication should be extremely rare. It rarely progresses, and the pain should be worked through by exercise with low risk of limb loss.” He added that intervention with these devices “takes away options. Trading life for something that is not limb-threatening is something we should not be considering.”
There was no firm consensus on whether new randomized trials should be done, although they were of course the ideal solution. Kevin E. Kip, PhD, Distinguished USF Health Professor at the University of South Florida, Tampa, and others argued that, whether new trials were necessary or not, to deal with the safety question in a timely fashion, existing trials have to capture as much of the missing data as possible, and carry out follow-up out further.
FDA representative Bram Zuckerman, MD, director of the Office of Cardiovascular Devices at the Center for Devices and Radiological Health, indicated that those things might not be easily be accomplished due to regulatory constraints and the financial costs, and that to do so there would be need for community effort among stakeholders, including collaborative efforts with existing prospective registries such as that run by the Vascular Quality Initiative.
One overall conclusion by both the FDA and panel members was that the quality of these and other such studies going forward must improve, by standardizing definitions and data forms to make studies more uniform across the industry. They reemphasized the need to work with the registries to get common data included, and to incorporate of insurance provider and Social Security death data as much as possible to help alleviate the lost follow-up problem.
SOURCE: Webcasts of the complete 2 days of the FDA panel meeting are available online.
GAITHERSBURG, MD. – There was sufficient evidence of a late mortality signal seen at 2-5 years post procedurally for paclitaxel-eluting stents and coated balloons to warrant a label change for the devices, the Food and Drug Administration’s Circulatory System Devices Panel unanimously agreed after 2 days of deliberation.
That signal was brought to light in a meta-analysis published last December by Konstantinos Katsanos, MD, of Patras University Hospital, Rion, Greece, and colleagues (J Am Heart Assoc. 2018;7:e011245). Although there were concerns about the quality of the industry data used in the study, the caliber of the analysis itself and the subsequent data presented by the FDA to the panel were deemed sufficient to recommend a warning of concern to patients and providers.
Much of the new data from industry and large database registries presented to the panel, which was chaired by Richard A. Lange, MD, indicated a lessening to no evidence of the mortality effect. But this evidence was deemed insufficient to counter the evidence of the randomized controlled trials individually and collectively as presented in the Katsanos meta-analysis and subsequent information presented by the FDA that examined various parameters in a variety of sensitivity analyses that confirmed the late mortality signal. There was also concern that the industry and the registry analyses presented were not peer reviewed.
However, the panel also determined that it would be inappropriate to pull the devices from the market and from general use for several reasons.
One key reason was that, according to the panel, there was no mechanistic cause apparent for the late mortality. In addition, no convincing dose-response data could be teased from the preclinical and clinical trials studied because of their variability of devices, application methods, and lack of appropriate tissue analysis across studies.
Finally, the industry data used to create the meta-analysis were considered to be fundamentally flawed: in blinding, in the relatively small numbers of patients, and in the large percentage of patients lost to follow-up. The latter could have dramatically influenced the perceived results, especially as the studies were not powered or designed to follow mortality over such a period of time, according to the panel.
These limitations to the signal were especially important to the panel because of the obvious benefits with regard to quality of life provided to patients from these devices, which were attested to during the 2-day meeting by numerous presenters from industry, medical organizations – including societies and nonprofits – and providers.
In responding to FDA requests on a variety of concerns, the panel reiterated that there was a credible mortality signal, but that they could not be confident about the magnitude and whether it was caused by the paclitaxel treatment or some factor in the design or conduct of the studies. In addition, the panel members felt that they could neither confirm nor eliminate a class effect, given the fact that the information was based on a meta-analysis and thus none of the included devices could safely be removed from consideration.
They suggested that further safety information should be obtained, potentially by assessing and perhaps altering data collection in 29 ongoing studies over the next 5 years or so in more than 10,000 patients.
In addition, several of the panel members felt that additional animal studies might be performed including the use of older rat models; and using animal models that mimicked the kind of comorbidities present in the treated population, such as diabetes and atherosclerosis. They suggested cross-company industry cooperation with the FDA on these models, including looking at drug interactions and mimicking the dose application of stents/balloons.
Both the FDA representative and the panel were especially concerned with the benefit/risk profile.
The recommendation to still market the devices with a label warning was warranted, according to many members of the panel. They pointed to the clear benefits in quality of life and the lowered need for revascularization despite the evidence of the mortality signal, which, while statistically significant, could not be pinned town with regard to mechanisms or specific causes of death.
Overall, there was a concern that there should be a dialogue between patients and their doctors to discuss clear short-term benefits with unknown long-term risk, and that the label should support this by clearly mentioning the mortality signal that was found, although there was no attempt to develop exact wording.
Panel member Joaquin E. Cigarroa, MD, head of cardiovascular medicine at Oregon Health & Science University, Portland, suggested with regard to labeling that a statement “that ‘there may be’ – not ‘there is’ – a late mortality signal, should be included.”
Panel member John C. Somberg, MD, program director of clinical research and bioinformatics at Rush University in Lake Bluff, Ill., stated: “The label should say something like, ‘when looking at a meta-analysis that combined all studies with stents and balloons that carried paclitaxel, there may be a late mortality, which must be balanced against an early and sustained benefit in terms of pain on walking and potential loss of circulation to your extremity.’ ”
Other panel members thought that the meta-analysis should not be privileged and that somehow the totality of the evidence should somehow be distilled down into the label, including the evidence against the signal.
“We’re meeting because of a signal, of a concern – an honest, well-meaning concern – of increased mortality. And my opinion is that the patients need to be informed of it,” said Dr. Lange, president, Texas Tech University, El Paso.
Some members of the panel felt that it may not be justifiable to use these devices in patients with low intrinsic risk and low recurrence risk, and the whole spectrum of patients may need to be considered in further studies to figure out the subgroups that have more benefits and more risks, and also to consider how to mitigate risks in patients who receive the device, whether through medical therapy or lifestyle modification.
In particular, Frank W. LoGerfo, MD, the William V. McDermott Distinguished Professor of Surgery at Harvard Medical School, Boston, stated: “Interventions for claudication should be extremely rare. It rarely progresses, and the pain should be worked through by exercise with low risk of limb loss.” He added that intervention with these devices “takes away options. Trading life for something that is not limb-threatening is something we should not be considering.”
There was no firm consensus on whether new randomized trials should be done, although they were of course the ideal solution. Kevin E. Kip, PhD, Distinguished USF Health Professor at the University of South Florida, Tampa, and others argued that, whether new trials were necessary or not, to deal with the safety question in a timely fashion, existing trials have to capture as much of the missing data as possible, and carry out follow-up out further.
FDA representative Bram Zuckerman, MD, director of the Office of Cardiovascular Devices at the Center for Devices and Radiological Health, indicated that those things might not be easily be accomplished due to regulatory constraints and the financial costs, and that to do so there would be need for community effort among stakeholders, including collaborative efforts with existing prospective registries such as that run by the Vascular Quality Initiative.
One overall conclusion by both the FDA and panel members was that the quality of these and other such studies going forward must improve, by standardizing definitions and data forms to make studies more uniform across the industry. They reemphasized the need to work with the registries to get common data included, and to incorporate of insurance provider and Social Security death data as much as possible to help alleviate the lost follow-up problem.
SOURCE: Webcasts of the complete 2 days of the FDA panel meeting are available online.
GAITHERSBURG, MD. – There was sufficient evidence of a late mortality signal seen at 2-5 years post procedurally for paclitaxel-eluting stents and coated balloons to warrant a label change for the devices, the Food and Drug Administration’s Circulatory System Devices Panel unanimously agreed after 2 days of deliberation.
That signal was brought to light in a meta-analysis published last December by Konstantinos Katsanos, MD, of Patras University Hospital, Rion, Greece, and colleagues (J Am Heart Assoc. 2018;7:e011245). Although there were concerns about the quality of the industry data used in the study, the caliber of the analysis itself and the subsequent data presented by the FDA to the panel were deemed sufficient to recommend a warning of concern to patients and providers.
Much of the new data from industry and large database registries presented to the panel, which was chaired by Richard A. Lange, MD, indicated a lessening to no evidence of the mortality effect. But this evidence was deemed insufficient to counter the evidence of the randomized controlled trials individually and collectively as presented in the Katsanos meta-analysis and subsequent information presented by the FDA that examined various parameters in a variety of sensitivity analyses that confirmed the late mortality signal. There was also concern that the industry and the registry analyses presented were not peer reviewed.
However, the panel also determined that it would be inappropriate to pull the devices from the market and from general use for several reasons.
One key reason was that, according to the panel, there was no mechanistic cause apparent for the late mortality. In addition, no convincing dose-response data could be teased from the preclinical and clinical trials studied because of their variability of devices, application methods, and lack of appropriate tissue analysis across studies.
Finally, the industry data used to create the meta-analysis were considered to be fundamentally flawed: in blinding, in the relatively small numbers of patients, and in the large percentage of patients lost to follow-up. The latter could have dramatically influenced the perceived results, especially as the studies were not powered or designed to follow mortality over such a period of time, according to the panel.
These limitations to the signal were especially important to the panel because of the obvious benefits with regard to quality of life provided to patients from these devices, which were attested to during the 2-day meeting by numerous presenters from industry, medical organizations – including societies and nonprofits – and providers.
In responding to FDA requests on a variety of concerns, the panel reiterated that there was a credible mortality signal, but that they could not be confident about the magnitude and whether it was caused by the paclitaxel treatment or some factor in the design or conduct of the studies. In addition, the panel members felt that they could neither confirm nor eliminate a class effect, given the fact that the information was based on a meta-analysis and thus none of the included devices could safely be removed from consideration.
They suggested that further safety information should be obtained, potentially by assessing and perhaps altering data collection in 29 ongoing studies over the next 5 years or so in more than 10,000 patients.
In addition, several of the panel members felt that additional animal studies might be performed including the use of older rat models; and using animal models that mimicked the kind of comorbidities present in the treated population, such as diabetes and atherosclerosis. They suggested cross-company industry cooperation with the FDA on these models, including looking at drug interactions and mimicking the dose application of stents/balloons.
Both the FDA representative and the panel were especially concerned with the benefit/risk profile.
The recommendation to still market the devices with a label warning was warranted, according to many members of the panel. They pointed to the clear benefits in quality of life and the lowered need for revascularization despite the evidence of the mortality signal, which, while statistically significant, could not be pinned town with regard to mechanisms or specific causes of death.
Overall, there was a concern that there should be a dialogue between patients and their doctors to discuss clear short-term benefits with unknown long-term risk, and that the label should support this by clearly mentioning the mortality signal that was found, although there was no attempt to develop exact wording.
Panel member Joaquin E. Cigarroa, MD, head of cardiovascular medicine at Oregon Health & Science University, Portland, suggested with regard to labeling that a statement “that ‘there may be’ – not ‘there is’ – a late mortality signal, should be included.”
Panel member John C. Somberg, MD, program director of clinical research and bioinformatics at Rush University in Lake Bluff, Ill., stated: “The label should say something like, ‘when looking at a meta-analysis that combined all studies with stents and balloons that carried paclitaxel, there may be a late mortality, which must be balanced against an early and sustained benefit in terms of pain on walking and potential loss of circulation to your extremity.’ ”
Other panel members thought that the meta-analysis should not be privileged and that somehow the totality of the evidence should somehow be distilled down into the label, including the evidence against the signal.
“We’re meeting because of a signal, of a concern – an honest, well-meaning concern – of increased mortality. And my opinion is that the patients need to be informed of it,” said Dr. Lange, president, Texas Tech University, El Paso.
Some members of the panel felt that it may not be justifiable to use these devices in patients with low intrinsic risk and low recurrence risk, and the whole spectrum of patients may need to be considered in further studies to figure out the subgroups that have more benefits and more risks, and also to consider how to mitigate risks in patients who receive the device, whether through medical therapy or lifestyle modification.
In particular, Frank W. LoGerfo, MD, the William V. McDermott Distinguished Professor of Surgery at Harvard Medical School, Boston, stated: “Interventions for claudication should be extremely rare. It rarely progresses, and the pain should be worked through by exercise with low risk of limb loss.” He added that intervention with these devices “takes away options. Trading life for something that is not limb-threatening is something we should not be considering.”
There was no firm consensus on whether new randomized trials should be done, although they were of course the ideal solution. Kevin E. Kip, PhD, Distinguished USF Health Professor at the University of South Florida, Tampa, and others argued that, whether new trials were necessary or not, to deal with the safety question in a timely fashion, existing trials have to capture as much of the missing data as possible, and carry out follow-up out further.
FDA representative Bram Zuckerman, MD, director of the Office of Cardiovascular Devices at the Center for Devices and Radiological Health, indicated that those things might not be easily be accomplished due to regulatory constraints and the financial costs, and that to do so there would be need for community effort among stakeholders, including collaborative efforts with existing prospective registries such as that run by the Vascular Quality Initiative.
One overall conclusion by both the FDA and panel members was that the quality of these and other such studies going forward must improve, by standardizing definitions and data forms to make studies more uniform across the industry. They reemphasized the need to work with the registries to get common data included, and to incorporate of insurance provider and Social Security death data as much as possible to help alleviate the lost follow-up problem.
SOURCE: Webcasts of the complete 2 days of the FDA panel meeting are available online.
REPORTING FROM AN FDA PANEL MEETING
FDA panel to reassess the fate of paclitaxel-coated PAD devices
The Food and Drug Administration (FDA) announced that the Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet June 19-20, 2019, at the Gaithersburg Holiday Inn, Gaithersburg, Md., to “discuss and make recommendations on information related to recent observations of increased compared to patients treated with uncoated comparator devices.”
The FDA is requesting the panel’s input “regarding the presence and magnitude of the signal and potential causes.” In addition, the FDA will seek input “regarding appropriate regulatory actions associated with the findings.”
In a Letter to Healthcare Providers issued March 15, the FDA reported that their preliminary review of these data found “a potentially concerning signal of increased long-term mortality in study subjects treated with paclitaxel-coated products, compared to patients treated with uncoated devices.”
Their recommendation was: “Alternative treatment options should generally be used for most patients,” rather than paclitaxel-coated balloons and stents for peripheral arterial disease (PAD), pending the above announced ongoing safety review.
The FDA intends to make background material available to the public no later than 2 business days before the meeting on its website at the appropriate advisory committee meeting link.
The Food and Drug Administration (FDA) announced that the Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet June 19-20, 2019, at the Gaithersburg Holiday Inn, Gaithersburg, Md., to “discuss and make recommendations on information related to recent observations of increased compared to patients treated with uncoated comparator devices.”
The FDA is requesting the panel’s input “regarding the presence and magnitude of the signal and potential causes.” In addition, the FDA will seek input “regarding appropriate regulatory actions associated with the findings.”
In a Letter to Healthcare Providers issued March 15, the FDA reported that their preliminary review of these data found “a potentially concerning signal of increased long-term mortality in study subjects treated with paclitaxel-coated products, compared to patients treated with uncoated devices.”
Their recommendation was: “Alternative treatment options should generally be used for most patients,” rather than paclitaxel-coated balloons and stents for peripheral arterial disease (PAD), pending the above announced ongoing safety review.
The FDA intends to make background material available to the public no later than 2 business days before the meeting on its website at the appropriate advisory committee meeting link.
The Food and Drug Administration (FDA) announced that the Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet June 19-20, 2019, at the Gaithersburg Holiday Inn, Gaithersburg, Md., to “discuss and make recommendations on information related to recent observations of increased compared to patients treated with uncoated comparator devices.”
The FDA is requesting the panel’s input “regarding the presence and magnitude of the signal and potential causes.” In addition, the FDA will seek input “regarding appropriate regulatory actions associated with the findings.”
In a Letter to Healthcare Providers issued March 15, the FDA reported that their preliminary review of these data found “a potentially concerning signal of increased long-term mortality in study subjects treated with paclitaxel-coated products, compared to patients treated with uncoated devices.”
Their recommendation was: “Alternative treatment options should generally be used for most patients,” rather than paclitaxel-coated balloons and stents for peripheral arterial disease (PAD), pending the above announced ongoing safety review.
The FDA intends to make background material available to the public no later than 2 business days before the meeting on its website at the appropriate advisory committee meeting link.
Despite HCV cure, liver cancer-associated genetic changes persist
A new study showed that liver tissue from hepatitis C virus (HCV)–infected humans with and without sustained virologic response found epigenetic and gene expression alterations associated with the risk for hepatocellular carcinoma (HCC), according to Nourdine Hamdane, PHD, of the Institut de Recherche sur les Maladies Virales et Hépatiques, Strasbourg, France, and colleagues.
The researchers analyzed liver tissue from 6 noninfected control patients, 18 patients with chronic HCV infection, 21 patients with cured chronic HCV, 4 patients with hepatitis B virus (HBV) infection, and 7 patients with nonalcoholic steatohepatitis (NASH), as well as 8 paired HCC samples with HCV-induced liver disease (Gastroenterology 2019;156:2313–29).
They found that several altered pathways related to carcinogenesis persisted after cure, including TNF-alpha signaling, inflammatory response, G2M checkpoint, epithelial–mesenchymal transition, and phosphoinositide 3-kinase, Akt, and mammalian target of rapamycin.
They also observed lower levels of H3K27ac mapping to genes related to oxidative phosphorylation pathways, providing evidence supporting a functional role for H3K27ac changes in establishing gene expression patterns that persist after cure and contribute to carcinogenesis, according to the authors.
“Our study exposes a previously undiscovered paradigm showing that chronic HCV infection induces H3K27ac modifications that are associated with HCC risk and that persist after HCV cure,” the authors wrote. “[This study] provides a unique opportunity to uncover novel biomarkers for HCC risk, that is, from plasma through the detection of epigenetic changes of histones bound to circulating DNA complexes. Furthermore, by uncovering virus-induced epigenetic changes as therapeutic targets, our findings offer novel perspectives for HCC prevention – a key unmet medical need,” the researchers concluded.
The authors declared that they had no conflicts.
SOURCE: Hamdane N, et al. 2019; Gastroenterology 156:2313–29.
A new study showed that liver tissue from hepatitis C virus (HCV)–infected humans with and without sustained virologic response found epigenetic and gene expression alterations associated with the risk for hepatocellular carcinoma (HCC), according to Nourdine Hamdane, PHD, of the Institut de Recherche sur les Maladies Virales et Hépatiques, Strasbourg, France, and colleagues.
The researchers analyzed liver tissue from 6 noninfected control patients, 18 patients with chronic HCV infection, 21 patients with cured chronic HCV, 4 patients with hepatitis B virus (HBV) infection, and 7 patients with nonalcoholic steatohepatitis (NASH), as well as 8 paired HCC samples with HCV-induced liver disease (Gastroenterology 2019;156:2313–29).
They found that several altered pathways related to carcinogenesis persisted after cure, including TNF-alpha signaling, inflammatory response, G2M checkpoint, epithelial–mesenchymal transition, and phosphoinositide 3-kinase, Akt, and mammalian target of rapamycin.
They also observed lower levels of H3K27ac mapping to genes related to oxidative phosphorylation pathways, providing evidence supporting a functional role for H3K27ac changes in establishing gene expression patterns that persist after cure and contribute to carcinogenesis, according to the authors.
“Our study exposes a previously undiscovered paradigm showing that chronic HCV infection induces H3K27ac modifications that are associated with HCC risk and that persist after HCV cure,” the authors wrote. “[This study] provides a unique opportunity to uncover novel biomarkers for HCC risk, that is, from plasma through the detection of epigenetic changes of histones bound to circulating DNA complexes. Furthermore, by uncovering virus-induced epigenetic changes as therapeutic targets, our findings offer novel perspectives for HCC prevention – a key unmet medical need,” the researchers concluded.
The authors declared that they had no conflicts.
SOURCE: Hamdane N, et al. 2019; Gastroenterology 156:2313–29.
A new study showed that liver tissue from hepatitis C virus (HCV)–infected humans with and without sustained virologic response found epigenetic and gene expression alterations associated with the risk for hepatocellular carcinoma (HCC), according to Nourdine Hamdane, PHD, of the Institut de Recherche sur les Maladies Virales et Hépatiques, Strasbourg, France, and colleagues.
The researchers analyzed liver tissue from 6 noninfected control patients, 18 patients with chronic HCV infection, 21 patients with cured chronic HCV, 4 patients with hepatitis B virus (HBV) infection, and 7 patients with nonalcoholic steatohepatitis (NASH), as well as 8 paired HCC samples with HCV-induced liver disease (Gastroenterology 2019;156:2313–29).
They found that several altered pathways related to carcinogenesis persisted after cure, including TNF-alpha signaling, inflammatory response, G2M checkpoint, epithelial–mesenchymal transition, and phosphoinositide 3-kinase, Akt, and mammalian target of rapamycin.
They also observed lower levels of H3K27ac mapping to genes related to oxidative phosphorylation pathways, providing evidence supporting a functional role for H3K27ac changes in establishing gene expression patterns that persist after cure and contribute to carcinogenesis, according to the authors.
“Our study exposes a previously undiscovered paradigm showing that chronic HCV infection induces H3K27ac modifications that are associated with HCC risk and that persist after HCV cure,” the authors wrote. “[This study] provides a unique opportunity to uncover novel biomarkers for HCC risk, that is, from plasma through the detection of epigenetic changes of histones bound to circulating DNA complexes. Furthermore, by uncovering virus-induced epigenetic changes as therapeutic targets, our findings offer novel perspectives for HCC prevention – a key unmet medical need,” the researchers concluded.
The authors declared that they had no conflicts.
SOURCE: Hamdane N, et al. 2019; Gastroenterology 156:2313–29.
FROM GASTROENTEROLOGY
Respiratory effects may account for worse survival in women undergoing DTA and TAAA repair
Women undergoing open descending thoracic aortic aneurysm (DTA) and open thoracoabdominal aortic aneurysm (TAAA) repair are not at greater risk for operative mortality than their male counterparts. However, they are at significantly greater risk for major adverse events and have significantly lower 5-year survival, according to the results of a single institution database review of 738 surgery patients.
From May 1997 to June 2017, there were 462 men (59%) and 321 women (41%) who underwent open repair of DTA or TAAA, according to Leonard N. Girardi, MD, and colleagues from Weill Cornell Medicine, New York, who performed the study published in the Journal of Vascular Surgery. The researchers used logistic regression and Cox regression analyses to assess the effect of sex on perioperative and long-term outcomes.
Demographically, women were significantly older (67.6 years vs. 62.6 years), with a significantly higher incidence of chronic obstructive pulmonary disease (47.0% vs. 35.7%) and a significantly greater percentage of patients with a forced expiratory volume in 1 second less than 50% (28.3% vs 18.2%). Degenerative aneurysms were significantly more common in women (61.7% vs. 41.6%), whereas chronic dissections significantly predominated in men (42.4% vs. 23.1%). Operative mortality was not significantly different between women and men (5.6% vs. 6.2%); however, women were significantly more likely to require a tracheostomy after surgery (10.6% vs. 5.0%).
Logistic regression found that being a woman was an independent risk factor for a composite of major adverse events (odds ratio, 2.68) and need for tracheostomy (OR, 3.73). In addition, women had significantly worse 5-year survival than men undergoing DTA or TAAA repair (59.7% vs. 66.2%, P =.025). There was no difference in overall survival between 1997-2007 and 2008-2017.
“Women and men undergoing TAAA repair have significant and consistent differences in preoperative characteristics. Despite these differences, operative mortality is similar between the two groups. However, women are at significantly increased risk of [major adverse events], especially respiratory failure, because of those differences in risk factors, including age, pulmonary function, and aneurysm etiology,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Girardi LN et al. J Vasc Surg 2019;69:1028-35.
Women undergoing open descending thoracic aortic aneurysm (DTA) and open thoracoabdominal aortic aneurysm (TAAA) repair are not at greater risk for operative mortality than their male counterparts. However, they are at significantly greater risk for major adverse events and have significantly lower 5-year survival, according to the results of a single institution database review of 738 surgery patients.
From May 1997 to June 2017, there were 462 men (59%) and 321 women (41%) who underwent open repair of DTA or TAAA, according to Leonard N. Girardi, MD, and colleagues from Weill Cornell Medicine, New York, who performed the study published in the Journal of Vascular Surgery. The researchers used logistic regression and Cox regression analyses to assess the effect of sex on perioperative and long-term outcomes.
Demographically, women were significantly older (67.6 years vs. 62.6 years), with a significantly higher incidence of chronic obstructive pulmonary disease (47.0% vs. 35.7%) and a significantly greater percentage of patients with a forced expiratory volume in 1 second less than 50% (28.3% vs 18.2%). Degenerative aneurysms were significantly more common in women (61.7% vs. 41.6%), whereas chronic dissections significantly predominated in men (42.4% vs. 23.1%). Operative mortality was not significantly different between women and men (5.6% vs. 6.2%); however, women were significantly more likely to require a tracheostomy after surgery (10.6% vs. 5.0%).
Logistic regression found that being a woman was an independent risk factor for a composite of major adverse events (odds ratio, 2.68) and need for tracheostomy (OR, 3.73). In addition, women had significantly worse 5-year survival than men undergoing DTA or TAAA repair (59.7% vs. 66.2%, P =.025). There was no difference in overall survival between 1997-2007 and 2008-2017.
“Women and men undergoing TAAA repair have significant and consistent differences in preoperative characteristics. Despite these differences, operative mortality is similar between the two groups. However, women are at significantly increased risk of [major adverse events], especially respiratory failure, because of those differences in risk factors, including age, pulmonary function, and aneurysm etiology,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Girardi LN et al. J Vasc Surg 2019;69:1028-35.
Women undergoing open descending thoracic aortic aneurysm (DTA) and open thoracoabdominal aortic aneurysm (TAAA) repair are not at greater risk for operative mortality than their male counterparts. However, they are at significantly greater risk for major adverse events and have significantly lower 5-year survival, according to the results of a single institution database review of 738 surgery patients.
From May 1997 to June 2017, there were 462 men (59%) and 321 women (41%) who underwent open repair of DTA or TAAA, according to Leonard N. Girardi, MD, and colleagues from Weill Cornell Medicine, New York, who performed the study published in the Journal of Vascular Surgery. The researchers used logistic regression and Cox regression analyses to assess the effect of sex on perioperative and long-term outcomes.
Demographically, women were significantly older (67.6 years vs. 62.6 years), with a significantly higher incidence of chronic obstructive pulmonary disease (47.0% vs. 35.7%) and a significantly greater percentage of patients with a forced expiratory volume in 1 second less than 50% (28.3% vs 18.2%). Degenerative aneurysms were significantly more common in women (61.7% vs. 41.6%), whereas chronic dissections significantly predominated in men (42.4% vs. 23.1%). Operative mortality was not significantly different between women and men (5.6% vs. 6.2%); however, women were significantly more likely to require a tracheostomy after surgery (10.6% vs. 5.0%).
Logistic regression found that being a woman was an independent risk factor for a composite of major adverse events (odds ratio, 2.68) and need for tracheostomy (OR, 3.73). In addition, women had significantly worse 5-year survival than men undergoing DTA or TAAA repair (59.7% vs. 66.2%, P =.025). There was no difference in overall survival between 1997-2007 and 2008-2017.
“Women and men undergoing TAAA repair have significant and consistent differences in preoperative characteristics. Despite these differences, operative mortality is similar between the two groups. However, women are at significantly increased risk of [major adverse events], especially respiratory failure, because of those differences in risk factors, including age, pulmonary function, and aneurysm etiology,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Girardi LN et al. J Vasc Surg 2019;69:1028-35.
FROM THE JOURNAL OF VASCULAR SURGERY
DOPPS participation associated with lower HCV rates in dialysis patients
Dialysis patients are commonly infected with hepatitis C virus (HCV), and such infections are associated with increased morbidity and mortality. A team of international researchers assessed trends in the prevalence, incidence, and risk factors for HCV infection among more than 82,000 dialysis patients as defined by a documented diagnosis or antibody positivity using the Dialysis Outcomes and Practice Patterns Study.
They found that overall, among prevalent hemodialysis patients, HCV prevalence was nearly 10% during 2012-2015. The prevalence ranged from a low of 4% in Belgium to as high as 20% in the Middle East, with intermediate prevalence in China, Japan, Italy, Spain, and Russia. However, the prevalence of HCV decreased over time in most countries participating in more than one phase of DOPPS, and prevalence was around 5% among patients who had initiated dialysis within less than 4 months.
The incidence of . Although most units reported no seroconversions, 10% of units experienced three or more cases over a median of 1.1 years.
The researchers also found that high HCV prevalence in the hemodialysis unit was a powerful facility-level risk factor for seroconversion, but the use of isolation stations for HCV-positive patients was not associated with significantly lower seroconversion rates.
“Overall, despite a trend toward lower HCV prevalence among hemodialysis patients, the prevalence of HCV infection remains higher than in the general population,” wrote Michel Jadoul, MD, Cliniques universitaires Saint-Luc, Université Catholique de Louvain, Brussels, and colleagues.
Their report, sponsored by Merck, appeared in Kidney International. A number of the authors reported being speakers or consultants for a variety of pharmaceutical companies; two of the authors are employees of Merck. Support for the ongoing DOPPS Program is provided without restriction on publications.
SOURCE: Jadoul M et al. Kidney Int. 2019;95:939-47.
Dialysis patients are commonly infected with hepatitis C virus (HCV), and such infections are associated with increased morbidity and mortality. A team of international researchers assessed trends in the prevalence, incidence, and risk factors for HCV infection among more than 82,000 dialysis patients as defined by a documented diagnosis or antibody positivity using the Dialysis Outcomes and Practice Patterns Study.

They found that overall, among prevalent hemodialysis patients, HCV prevalence was nearly 10% during 2012-2015. The prevalence ranged from a low of 4% in Belgium to as high as 20% in the Middle East, with intermediate prevalence in China, Japan, Italy, Spain, and Russia. However, the prevalence of HCV decreased over time in most countries participating in more than one phase of DOPPS, and prevalence was around 5% among patients who had initiated dialysis within less than 4 months.
The incidence of . Although most units reported no seroconversions, 10% of units experienced three or more cases over a median of 1.1 years.
The researchers also found that high HCV prevalence in the hemodialysis unit was a powerful facility-level risk factor for seroconversion, but the use of isolation stations for HCV-positive patients was not associated with significantly lower seroconversion rates.
“Overall, despite a trend toward lower HCV prevalence among hemodialysis patients, the prevalence of HCV infection remains higher than in the general population,” wrote Michel Jadoul, MD, Cliniques universitaires Saint-Luc, Université Catholique de Louvain, Brussels, and colleagues.
Their report, sponsored by Merck, appeared in Kidney International. A number of the authors reported being speakers or consultants for a variety of pharmaceutical companies; two of the authors are employees of Merck. Support for the ongoing DOPPS Program is provided without restriction on publications.
SOURCE: Jadoul M et al. Kidney Int. 2019;95:939-47.
Dialysis patients are commonly infected with hepatitis C virus (HCV), and such infections are associated with increased morbidity and mortality. A team of international researchers assessed trends in the prevalence, incidence, and risk factors for HCV infection among more than 82,000 dialysis patients as defined by a documented diagnosis or antibody positivity using the Dialysis Outcomes and Practice Patterns Study.

They found that overall, among prevalent hemodialysis patients, HCV prevalence was nearly 10% during 2012-2015. The prevalence ranged from a low of 4% in Belgium to as high as 20% in the Middle East, with intermediate prevalence in China, Japan, Italy, Spain, and Russia. However, the prevalence of HCV decreased over time in most countries participating in more than one phase of DOPPS, and prevalence was around 5% among patients who had initiated dialysis within less than 4 months.
The incidence of . Although most units reported no seroconversions, 10% of units experienced three or more cases over a median of 1.1 years.
The researchers also found that high HCV prevalence in the hemodialysis unit was a powerful facility-level risk factor for seroconversion, but the use of isolation stations for HCV-positive patients was not associated with significantly lower seroconversion rates.
“Overall, despite a trend toward lower HCV prevalence among hemodialysis patients, the prevalence of HCV infection remains higher than in the general population,” wrote Michel Jadoul, MD, Cliniques universitaires Saint-Luc, Université Catholique de Louvain, Brussels, and colleagues.
Their report, sponsored by Merck, appeared in Kidney International. A number of the authors reported being speakers or consultants for a variety of pharmaceutical companies; two of the authors are employees of Merck. Support for the ongoing DOPPS Program is provided without restriction on publications.
SOURCE: Jadoul M et al. Kidney Int. 2019;95:939-47.
FROM KIDNEY INTERNATIONAL
Higher serum leptin levels associated with PAD
The adipokine hormones leptin, adiponectin, and resistin are produced by adipocytes and have been implicated in the causal pathway of atherosclerosis. Researchers examined the association between adipokine levels and peripheral artery disease (PAD) in a cross-sectional sample of 179 vascular surgery outpatients (97% of whom were men) with PAD recruited from the San Francisco Veterans Affairs Medical Center.
In an analysis adjusting for body mass index and atherosclerotic risk factors, higher serum leptin was associated with PAD (odds ratio, 2.54; P = .03), whereas high molecular weight adiponectin and resistin were not significantly associated with PAD (J Surg Res. 2019;238:48-56). “Our results indicate that after adjusting for BMI or fat mass, , whereas high molecular weight adiponectin might be inversely associated. Using a more representative, nonveteran sample, further investigations should focus on the potential role of adipokines in the pathophysiology of PAD as well as determine whether leptin levels have clinical utility in predicting PAD outcomes,” wrote Greg J. Zahner, MSc, University of California, San Francisco, and colleagues.
They reported that they had no relevant disclosures.
The adipokine hormones leptin, adiponectin, and resistin are produced by adipocytes and have been implicated in the causal pathway of atherosclerosis. Researchers examined the association between adipokine levels and peripheral artery disease (PAD) in a cross-sectional sample of 179 vascular surgery outpatients (97% of whom were men) with PAD recruited from the San Francisco Veterans Affairs Medical Center.
In an analysis adjusting for body mass index and atherosclerotic risk factors, higher serum leptin was associated with PAD (odds ratio, 2.54; P = .03), whereas high molecular weight adiponectin and resistin were not significantly associated with PAD (J Surg Res. 2019;238:48-56). “Our results indicate that after adjusting for BMI or fat mass, , whereas high molecular weight adiponectin might be inversely associated. Using a more representative, nonveteran sample, further investigations should focus on the potential role of adipokines in the pathophysiology of PAD as well as determine whether leptin levels have clinical utility in predicting PAD outcomes,” wrote Greg J. Zahner, MSc, University of California, San Francisco, and colleagues.
They reported that they had no relevant disclosures.
The adipokine hormones leptin, adiponectin, and resistin are produced by adipocytes and have been implicated in the causal pathway of atherosclerosis. Researchers examined the association between adipokine levels and peripheral artery disease (PAD) in a cross-sectional sample of 179 vascular surgery outpatients (97% of whom were men) with PAD recruited from the San Francisco Veterans Affairs Medical Center.
In an analysis adjusting for body mass index and atherosclerotic risk factors, higher serum leptin was associated with PAD (odds ratio, 2.54; P = .03), whereas high molecular weight adiponectin and resistin were not significantly associated with PAD (J Surg Res. 2019;238:48-56). “Our results indicate that after adjusting for BMI or fat mass, , whereas high molecular weight adiponectin might be inversely associated. Using a more representative, nonveteran sample, further investigations should focus on the potential role of adipokines in the pathophysiology of PAD as well as determine whether leptin levels have clinical utility in predicting PAD outcomes,” wrote Greg J. Zahner, MSc, University of California, San Francisco, and colleagues.
They reported that they had no relevant disclosures.
FROM THE JOURNAL OF SURGICAL RESEARCH