User login
HCV remodels lipid metabolism in infected cells
Hepatitis C virus infection often is associated with the accumulation of fat in hepatocytes, which shows a connection between the virus and the lipid metabolism of the liver, according to Sarah Hoffman, MD, of the Leibniz Institute for Experimental Virology, Hamburg, Germany, and her colleagues. “Our study provides a detailed analysis of the changes in the lipid composition in HCV-infected cells that revealed dependency on FA [fatty acid] elongation and desaturation for effective viral replication and virion production,” they reported.
Dr. Hoffman and her colleagues assessed lipid composition of infected cells in an in vitro study of cell lines, which were assessed 8-11 days post infection, according to the report published in BBA: Molecular and Cell Biology of Lipids. They determined the abundance of each major lipid class and compared the pattern of HCV-infected cells with that of controls.
The researchers found that HCV caused an accumulation of membrane phosopholipids but not neutral lipids and that cholesterol accumulated in the perinuclear region of HCV-infected cells. In addition, lipid species with longer fatty acyl chains were more abundant in HCV-infected cells and free polyunsaturated fatty acid (PUFA) levels were greatly increased.
In further confirmation of the critical role of lipid metabolism in HCV replication, they found that knockdown of fatty acid elongases and desaturases disrupted HCV replication, while overexpression of these enzymes showed a proviral effect.
“We identified several lipid-remodeling pathways that are required for distinct steps in viral infection. Future studies have to address the molecular function of longer fatty acyl chains in HCV RNA replication and why PUFAs are needed for HCV particle production,” the researchers concluded.
The authors reported government and institutional-only funding and no personal disclosures.
SOURCE: Hoffman S et al. Biochim Biophys Acta. 2018 Jun 6;1863(9):1041-56.
Hepatitis C virus infection often is associated with the accumulation of fat in hepatocytes, which shows a connection between the virus and the lipid metabolism of the liver, according to Sarah Hoffman, MD, of the Leibniz Institute for Experimental Virology, Hamburg, Germany, and her colleagues. “Our study provides a detailed analysis of the changes in the lipid composition in HCV-infected cells that revealed dependency on FA [fatty acid] elongation and desaturation for effective viral replication and virion production,” they reported.
Dr. Hoffman and her colleagues assessed lipid composition of infected cells in an in vitro study of cell lines, which were assessed 8-11 days post infection, according to the report published in BBA: Molecular and Cell Biology of Lipids. They determined the abundance of each major lipid class and compared the pattern of HCV-infected cells with that of controls.
The researchers found that HCV caused an accumulation of membrane phosopholipids but not neutral lipids and that cholesterol accumulated in the perinuclear region of HCV-infected cells. In addition, lipid species with longer fatty acyl chains were more abundant in HCV-infected cells and free polyunsaturated fatty acid (PUFA) levels were greatly increased.
In further confirmation of the critical role of lipid metabolism in HCV replication, they found that knockdown of fatty acid elongases and desaturases disrupted HCV replication, while overexpression of these enzymes showed a proviral effect.
“We identified several lipid-remodeling pathways that are required for distinct steps in viral infection. Future studies have to address the molecular function of longer fatty acyl chains in HCV RNA replication and why PUFAs are needed for HCV particle production,” the researchers concluded.
The authors reported government and institutional-only funding and no personal disclosures.
SOURCE: Hoffman S et al. Biochim Biophys Acta. 2018 Jun 6;1863(9):1041-56.
Hepatitis C virus infection often is associated with the accumulation of fat in hepatocytes, which shows a connection between the virus and the lipid metabolism of the liver, according to Sarah Hoffman, MD, of the Leibniz Institute for Experimental Virology, Hamburg, Germany, and her colleagues. “Our study provides a detailed analysis of the changes in the lipid composition in HCV-infected cells that revealed dependency on FA [fatty acid] elongation and desaturation for effective viral replication and virion production,” they reported.
Dr. Hoffman and her colleagues assessed lipid composition of infected cells in an in vitro study of cell lines, which were assessed 8-11 days post infection, according to the report published in BBA: Molecular and Cell Biology of Lipids. They determined the abundance of each major lipid class and compared the pattern of HCV-infected cells with that of controls.
The researchers found that HCV caused an accumulation of membrane phosopholipids but not neutral lipids and that cholesterol accumulated in the perinuclear region of HCV-infected cells. In addition, lipid species with longer fatty acyl chains were more abundant in HCV-infected cells and free polyunsaturated fatty acid (PUFA) levels were greatly increased.
In further confirmation of the critical role of lipid metabolism in HCV replication, they found that knockdown of fatty acid elongases and desaturases disrupted HCV replication, while overexpression of these enzymes showed a proviral effect.
“We identified several lipid-remodeling pathways that are required for distinct steps in viral infection. Future studies have to address the molecular function of longer fatty acyl chains in HCV RNA replication and why PUFAs are needed for HCV particle production,” the researchers concluded.
The authors reported government and institutional-only funding and no personal disclosures.
SOURCE: Hoffman S et al. Biochim Biophys Acta. 2018 Jun 6;1863(9):1041-56.
FROM BBA: MOLECULAR AND CELL BIOLOGY OF LIPIDS
Cigarette smoking epidemic among HCV-infected individuals
There is a cigarette smoking epidemic embedded within the hepatitis C virus epidemic in the United States, according to the results of an analysis of data between 1999 and 2014 from the National Health and Nutrition Examination Survey (NHANES).
Smoking and hepatitis C information were available for 90.1% of the NHANES adult population. Of the 39,472 individuals evaluated, 1.3% were hepatitis C+ and 22.3% were current smokers. Hepatitis C+ individuals were almost three times as likely to be smokers as were those who were hepatitis C– (62.4% vs. 22.9%, respectively), according to the report, published in The American Journal of Medicine (Am J Med. 2018 Jun;131[6]:699-75).
Ryung S. Kim, PhD, of Albert Einstein College of Medicine, New York, and his colleagues also found that hepatitis C+ smokers were more likely to be older, male, black, less educated, poor, and uninsured compared with their hepatitis C– smoking counterparts. They also were more likely to use drugs, including heroin, and to be depressed.
Multivariate analysis showed a significant association of both hepatitis C infection and smoking with current depression and hypertension, Dr. Kim and his colleagues wrote.
“It is public health folly to spend tens of millions of dollars annually” on treatment of hepatitis C patients, “and ignore the lethal addiction affecting more than 60% of them. As we enter a new era of hepatitis C treatment, it is a public health imperative to research, develop, and implement tobacco treatments for the hepatitis C+ community,” Dr. Kim and his colleagues concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Kim RS et al. Am J Med. 2018Jun;131[6]:669-75).
There is a cigarette smoking epidemic embedded within the hepatitis C virus epidemic in the United States, according to the results of an analysis of data between 1999 and 2014 from the National Health and Nutrition Examination Survey (NHANES).
Smoking and hepatitis C information were available for 90.1% of the NHANES adult population. Of the 39,472 individuals evaluated, 1.3% were hepatitis C+ and 22.3% were current smokers. Hepatitis C+ individuals were almost three times as likely to be smokers as were those who were hepatitis C– (62.4% vs. 22.9%, respectively), according to the report, published in The American Journal of Medicine (Am J Med. 2018 Jun;131[6]:699-75).
Ryung S. Kim, PhD, of Albert Einstein College of Medicine, New York, and his colleagues also found that hepatitis C+ smokers were more likely to be older, male, black, less educated, poor, and uninsured compared with their hepatitis C– smoking counterparts. They also were more likely to use drugs, including heroin, and to be depressed.
Multivariate analysis showed a significant association of both hepatitis C infection and smoking with current depression and hypertension, Dr. Kim and his colleagues wrote.
“It is public health folly to spend tens of millions of dollars annually” on treatment of hepatitis C patients, “and ignore the lethal addiction affecting more than 60% of them. As we enter a new era of hepatitis C treatment, it is a public health imperative to research, develop, and implement tobacco treatments for the hepatitis C+ community,” Dr. Kim and his colleagues concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Kim RS et al. Am J Med. 2018Jun;131[6]:669-75).
There is a cigarette smoking epidemic embedded within the hepatitis C virus epidemic in the United States, according to the results of an analysis of data between 1999 and 2014 from the National Health and Nutrition Examination Survey (NHANES).
Smoking and hepatitis C information were available for 90.1% of the NHANES adult population. Of the 39,472 individuals evaluated, 1.3% were hepatitis C+ and 22.3% were current smokers. Hepatitis C+ individuals were almost three times as likely to be smokers as were those who were hepatitis C– (62.4% vs. 22.9%, respectively), according to the report, published in The American Journal of Medicine (Am J Med. 2018 Jun;131[6]:699-75).
Ryung S. Kim, PhD, of Albert Einstein College of Medicine, New York, and his colleagues also found that hepatitis C+ smokers were more likely to be older, male, black, less educated, poor, and uninsured compared with their hepatitis C– smoking counterparts. They also were more likely to use drugs, including heroin, and to be depressed.
Multivariate analysis showed a significant association of both hepatitis C infection and smoking with current depression and hypertension, Dr. Kim and his colleagues wrote.
“It is public health folly to spend tens of millions of dollars annually” on treatment of hepatitis C patients, “and ignore the lethal addiction affecting more than 60% of them. As we enter a new era of hepatitis C treatment, it is a public health imperative to research, develop, and implement tobacco treatments for the hepatitis C+ community,” Dr. Kim and his colleagues concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Kim RS et al. Am J Med. 2018Jun;131[6]:669-75).
FROM THE AMERICAN JOURNAL OF MEDICINE
HCV incidence is elevated in HIV-positive MSM
The incidence rate of HCV among HIV-positive men who have sex with men (MSM) was significantly higher than that found in HIV-negative MSM and the general population, according to a study published in Digestive and Liver Disease.
Sexual transmission of hepatitis C virus (HCV) is uncommon in the general population, but evidence indicates that its rate is higher in people living with HIV and that HIV-positive MSM who practice condomless sex have been shown to be at increased risk for sexually-acquired HCV, according to Gianluca Cuomo, MD, and his colleagues at the Azienda Ospedaliero-Universitaria di Modena (Italy), Infectious Diseases Clinic.
Dr. Cuomo and his colleagues assessed 442 HIV-positive MSM outpatients who were antibody negative to HCV-Ab at first observation who were entered into a Kaplan-Meier model in order to assess the HCV infection incidence rate. Prevalence analysis was performed with HIV-positive MSM who were on follow-up at 2016. An HIV-negative MSM population served as a control.
“Our study indicates an incidence rate of HCV among MSM living with HIV of 0.44 cases per 100 patient-years with a global prevalence of 9%, both of which are significantly higher than that of non-HIV MSM and the general population,” Dr. Cuomo and his colleagues found (Digestive and Liver Disease; 2018, [doi.org/10.1016/j.dld.2018.05.021]).
“Early management and treatment of HCV infection and behavioral interventions could reduce HCV transmission. Annual screenings for HCV and other sexually transmitted diseases should be performed for HIV MSM patients,” the researchers concluded.
Dr. Cuomo and his colleagues reported that they had no disclosures.
SOURCE: Cuomo, G., et al. Digestive and Liver Disease (2018), [doi.org/10.1016/j.dld.2018.05.021].
The incidence rate of HCV among HIV-positive men who have sex with men (MSM) was significantly higher than that found in HIV-negative MSM and the general population, according to a study published in Digestive and Liver Disease.
Sexual transmission of hepatitis C virus (HCV) is uncommon in the general population, but evidence indicates that its rate is higher in people living with HIV and that HIV-positive MSM who practice condomless sex have been shown to be at increased risk for sexually-acquired HCV, according to Gianluca Cuomo, MD, and his colleagues at the Azienda Ospedaliero-Universitaria di Modena (Italy), Infectious Diseases Clinic.
Dr. Cuomo and his colleagues assessed 442 HIV-positive MSM outpatients who were antibody negative to HCV-Ab at first observation who were entered into a Kaplan-Meier model in order to assess the HCV infection incidence rate. Prevalence analysis was performed with HIV-positive MSM who were on follow-up at 2016. An HIV-negative MSM population served as a control.
“Our study indicates an incidence rate of HCV among MSM living with HIV of 0.44 cases per 100 patient-years with a global prevalence of 9%, both of which are significantly higher than that of non-HIV MSM and the general population,” Dr. Cuomo and his colleagues found (Digestive and Liver Disease; 2018, [doi.org/10.1016/j.dld.2018.05.021]).
“Early management and treatment of HCV infection and behavioral interventions could reduce HCV transmission. Annual screenings for HCV and other sexually transmitted diseases should be performed for HIV MSM patients,” the researchers concluded.
Dr. Cuomo and his colleagues reported that they had no disclosures.
SOURCE: Cuomo, G., et al. Digestive and Liver Disease (2018), [doi.org/10.1016/j.dld.2018.05.021].
The incidence rate of HCV among HIV-positive men who have sex with men (MSM) was significantly higher than that found in HIV-negative MSM and the general population, according to a study published in Digestive and Liver Disease.
Sexual transmission of hepatitis C virus (HCV) is uncommon in the general population, but evidence indicates that its rate is higher in people living with HIV and that HIV-positive MSM who practice condomless sex have been shown to be at increased risk for sexually-acquired HCV, according to Gianluca Cuomo, MD, and his colleagues at the Azienda Ospedaliero-Universitaria di Modena (Italy), Infectious Diseases Clinic.
Dr. Cuomo and his colleagues assessed 442 HIV-positive MSM outpatients who were antibody negative to HCV-Ab at first observation who were entered into a Kaplan-Meier model in order to assess the HCV infection incidence rate. Prevalence analysis was performed with HIV-positive MSM who were on follow-up at 2016. An HIV-negative MSM population served as a control.
“Our study indicates an incidence rate of HCV among MSM living with HIV of 0.44 cases per 100 patient-years with a global prevalence of 9%, both of which are significantly higher than that of non-HIV MSM and the general population,” Dr. Cuomo and his colleagues found (Digestive and Liver Disease; 2018, [doi.org/10.1016/j.dld.2018.05.021]).
“Early management and treatment of HCV infection and behavioral interventions could reduce HCV transmission. Annual screenings for HCV and other sexually transmitted diseases should be performed for HIV MSM patients,” the researchers concluded.
Dr. Cuomo and his colleagues reported that they had no disclosures.
SOURCE: Cuomo, G., et al. Digestive and Liver Disease (2018), [doi.org/10.1016/j.dld.2018.05.021].
FROM DIGESTIVE AND LIVER DISEASE
HCV and alcohol use disorder – bad news for the liver
Patients infected with both hepatitis C virus (HCV) and alcohol use disorder (AUD) were twice as likely to present with advanced liver fibrosis at hospital admission, according to the results of a database study published in Drug and Alcohol Dependence (2018;188:180-6).
The study population consisted of 1,313 patients (80% men). Median age at admission was 45 years and the median alcohol consumption was 200 g/day. HCV infection was present in 236 patients (18%), according to Arantza Sanvisens, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain, and her colleagues.
After adjustment by sex, age and quantity of alcohol consumption, patients with HCV infection were two times more likely to have advanced liver fibrosis (odds ratio = 2.1, 95% confidence ratio,1.5–3.1).
“Successful evaluation of liver damage in this population includes the management of both excessive alcohol consumption and chronic HCV-related disease,” according to Dr. Sanvisens and her colleagues. “Furthermore, current guidelines from the American Association for the Study of Liver Disease, the European Association for the Study of the Liver, and the World Health Organization already recommend treatment of HCV infection in individuals with substance use disorder,” they concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Sanvisens, A et al., Drug and Alcohol Dependence (2018;188:180-6).
Patients infected with both hepatitis C virus (HCV) and alcohol use disorder (AUD) were twice as likely to present with advanced liver fibrosis at hospital admission, according to the results of a database study published in Drug and Alcohol Dependence (2018;188:180-6).
The study population consisted of 1,313 patients (80% men). Median age at admission was 45 years and the median alcohol consumption was 200 g/day. HCV infection was present in 236 patients (18%), according to Arantza Sanvisens, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain, and her colleagues.
After adjustment by sex, age and quantity of alcohol consumption, patients with HCV infection were two times more likely to have advanced liver fibrosis (odds ratio = 2.1, 95% confidence ratio,1.5–3.1).
“Successful evaluation of liver damage in this population includes the management of both excessive alcohol consumption and chronic HCV-related disease,” according to Dr. Sanvisens and her colleagues. “Furthermore, current guidelines from the American Association for the Study of Liver Disease, the European Association for the Study of the Liver, and the World Health Organization already recommend treatment of HCV infection in individuals with substance use disorder,” they concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Sanvisens, A et al., Drug and Alcohol Dependence (2018;188:180-6).
Patients infected with both hepatitis C virus (HCV) and alcohol use disorder (AUD) were twice as likely to present with advanced liver fibrosis at hospital admission, according to the results of a database study published in Drug and Alcohol Dependence (2018;188:180-6).
The study population consisted of 1,313 patients (80% men). Median age at admission was 45 years and the median alcohol consumption was 200 g/day. HCV infection was present in 236 patients (18%), according to Arantza Sanvisens, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain, and her colleagues.
After adjustment by sex, age and quantity of alcohol consumption, patients with HCV infection were two times more likely to have advanced liver fibrosis (odds ratio = 2.1, 95% confidence ratio,1.5–3.1).
“Successful evaluation of liver damage in this population includes the management of both excessive alcohol consumption and chronic HCV-related disease,” according to Dr. Sanvisens and her colleagues. “Furthermore, current guidelines from the American Association for the Study of Liver Disease, the European Association for the Study of the Liver, and the World Health Organization already recommend treatment of HCV infection in individuals with substance use disorder,” they concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Sanvisens, A et al., Drug and Alcohol Dependence (2018;188:180-6).
FROM DRUG AND ALCOHOL DEPENDENCE
FDA issues Ebola preparedness statement
In response to the Ebola outbreak in the Democratic Republic of Congo (DRC), the Food and Drug Administration has announced steps the agency is taking to make diagnostic and medical products available as part of critical response efforts.
In addition to providing scientific and regulatory advice to medical product developers, the FDA is using its authorities to ensure Merck’s investigational Ebola Zaire vaccine is made available appropriately to vaccinate high-risk populations in the DRC. Additionally, the FDA also is committed to facilitating the development of investigational drugs for the treatment of Ebola virus and supporting access to these products under appropriate regulatory pathways, FDA Commissioner Scott Gottlieb, MD, said in a statement.
Clinical trials that are adaptive to the circumstances of an outbreak are essential, he added. “During the 2014-2015 Ebola outbreak, the FDA recognized that some of the medical products that initially appeared to show great promise sometimes, when subjected to objective testing, were not effective or may have done more harm than good.”
Further, as there are no approved treatments or vaccines for Ebola, the agency will be monitoring for false product claims to protect consumers from fraudulent products claiming to prevent, treat, or cure the disease.
“The FDA knows that it takes a sustained, robust, and globally coordinated effort to best protect our nation from various infectious disease threats,” Dr. Gottlieb wrote. “We’re committed to supporting the people of the DRC and preventing a worsening circumstance during the current outbreak. And we remain highly engaged in the international response efforts.”
In response to the Ebola outbreak in the Democratic Republic of Congo (DRC), the Food and Drug Administration has announced steps the agency is taking to make diagnostic and medical products available as part of critical response efforts.
In addition to providing scientific and regulatory advice to medical product developers, the FDA is using its authorities to ensure Merck’s investigational Ebola Zaire vaccine is made available appropriately to vaccinate high-risk populations in the DRC. Additionally, the FDA also is committed to facilitating the development of investigational drugs for the treatment of Ebola virus and supporting access to these products under appropriate regulatory pathways, FDA Commissioner Scott Gottlieb, MD, said in a statement.
Clinical trials that are adaptive to the circumstances of an outbreak are essential, he added. “During the 2014-2015 Ebola outbreak, the FDA recognized that some of the medical products that initially appeared to show great promise sometimes, when subjected to objective testing, were not effective or may have done more harm than good.”
Further, as there are no approved treatments or vaccines for Ebola, the agency will be monitoring for false product claims to protect consumers from fraudulent products claiming to prevent, treat, or cure the disease.
“The FDA knows that it takes a sustained, robust, and globally coordinated effort to best protect our nation from various infectious disease threats,” Dr. Gottlieb wrote. “We’re committed to supporting the people of the DRC and preventing a worsening circumstance during the current outbreak. And we remain highly engaged in the international response efforts.”
In response to the Ebola outbreak in the Democratic Republic of Congo (DRC), the Food and Drug Administration has announced steps the agency is taking to make diagnostic and medical products available as part of critical response efforts.
In addition to providing scientific and regulatory advice to medical product developers, the FDA is using its authorities to ensure Merck’s investigational Ebola Zaire vaccine is made available appropriately to vaccinate high-risk populations in the DRC. Additionally, the FDA also is committed to facilitating the development of investigational drugs for the treatment of Ebola virus and supporting access to these products under appropriate regulatory pathways, FDA Commissioner Scott Gottlieb, MD, said in a statement.
Clinical trials that are adaptive to the circumstances of an outbreak are essential, he added. “During the 2014-2015 Ebola outbreak, the FDA recognized that some of the medical products that initially appeared to show great promise sometimes, when subjected to objective testing, were not effective or may have done more harm than good.”
Further, as there are no approved treatments or vaccines for Ebola, the agency will be monitoring for false product claims to protect consumers from fraudulent products claiming to prevent, treat, or cure the disease.
“The FDA knows that it takes a sustained, robust, and globally coordinated effort to best protect our nation from various infectious disease threats,” Dr. Gottlieb wrote. “We’re committed to supporting the people of the DRC and preventing a worsening circumstance during the current outbreak. And we remain highly engaged in the international response efforts.”
NIH launches early Ebola treatment trial
A study of a potential new Ebola treatment has begun at the National Institutes of Health Clinical Center in Bethesda, Md. The small phase 1 clinical trial will examine the safety and tolerability of a single monoclonal antibody (mAb114), which was developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) and their collaborators. Investigators plan to enroll between 18 and 30 healthy volunteers aged 18-60. The trial will not expose participants to Ebola virus, according to the NIH announcement.
MAb114 is a monoclonal antibody – a protein that binds to a single target on a pathogen — isolated from a human survivor of the 1995 Ebola outbreak in Kikwit, Democratic Republic of the Congo. Nancy Sullivan, PhD, chief of the Biodefense Research Section in NIAID’s Vaccine Research Center, and her team, in collaboration with researchers from the National Institute of Biomedical Research in the Democratic Republic of the Congo and the Institute for Biomedical Research in Switzerland, discovered that the survivor retained antibodies against Ebola 11 years after infection. They isolated and tested the antibodies and selected mAb114 as the most promising.
Although rVSV-ZEBOV, an experimental vaccine, is now available and in use in Africa during the current outbreak, specific treatment modalities are lacking.
More information can be found at www.clinicaltrials.gov, trial # NCT03478891.
A study of a potential new Ebola treatment has begun at the National Institutes of Health Clinical Center in Bethesda, Md. The small phase 1 clinical trial will examine the safety and tolerability of a single monoclonal antibody (mAb114), which was developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) and their collaborators. Investigators plan to enroll between 18 and 30 healthy volunteers aged 18-60. The trial will not expose participants to Ebola virus, according to the NIH announcement.
MAb114 is a monoclonal antibody – a protein that binds to a single target on a pathogen — isolated from a human survivor of the 1995 Ebola outbreak in Kikwit, Democratic Republic of the Congo. Nancy Sullivan, PhD, chief of the Biodefense Research Section in NIAID’s Vaccine Research Center, and her team, in collaboration with researchers from the National Institute of Biomedical Research in the Democratic Republic of the Congo and the Institute for Biomedical Research in Switzerland, discovered that the survivor retained antibodies against Ebola 11 years after infection. They isolated and tested the antibodies and selected mAb114 as the most promising.
Although rVSV-ZEBOV, an experimental vaccine, is now available and in use in Africa during the current outbreak, specific treatment modalities are lacking.
More information can be found at www.clinicaltrials.gov, trial # NCT03478891.
A study of a potential new Ebola treatment has begun at the National Institutes of Health Clinical Center in Bethesda, Md. The small phase 1 clinical trial will examine the safety and tolerability of a single monoclonal antibody (mAb114), which was developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) and their collaborators. Investigators plan to enroll between 18 and 30 healthy volunteers aged 18-60. The trial will not expose participants to Ebola virus, according to the NIH announcement.
MAb114 is a monoclonal antibody – a protein that binds to a single target on a pathogen — isolated from a human survivor of the 1995 Ebola outbreak in Kikwit, Democratic Republic of the Congo. Nancy Sullivan, PhD, chief of the Biodefense Research Section in NIAID’s Vaccine Research Center, and her team, in collaboration with researchers from the National Institute of Biomedical Research in the Democratic Republic of the Congo and the Institute for Biomedical Research in Switzerland, discovered that the survivor retained antibodies against Ebola 11 years after infection. They isolated and tested the antibodies and selected mAb114 as the most promising.
Although rVSV-ZEBOV, an experimental vaccine, is now available and in use in Africa during the current outbreak, specific treatment modalities are lacking.
More information can be found at www.clinicaltrials.gov, trial # NCT03478891.
Alcohol abuse untreated in HCV patients, including HIV coinfected
Nearly 4% of Veterans Affairs patients who screened positive for unhealthy alcohol use were infected with hepatitis C virus, and 64% of these patients were diagnosed with alcohol use disorder, according to the results of a large database analysis.
Despite the fact that alcohol use at all levels can compound the adverse effects of HCV and lead to heightened risks of mortality, particularly among those coinfected with HIV, the majority of these patients did not receive specialty addiction treatment, according to Mandy D. Owens, PhD, and her colleagues at the VA Puget Sound Health Care System, Seattle.
In their study, published in Drug and Alcohol Dependence, the researchers queried the national VA health care system database, which is made up of 139 large facilities and more than 900 clinics throughout the United States, for all patients with a documented outpatient appointment between October 2009 and May 2013 to identify those with one or more with positive screens on the AUDIT-C (Alcohol Use Disorders Identification Test-Consumption) questionnaire. Those with AUDIT-C scores greater than or equal to 5 were considered positive, and each positive screen was tracked for up to 1 year to assess alcohol-related care outcomes. The four alcohol-related care outcomes measured were: receipt of brief intervention, specialty addiction treatment, alcohol use disorder (AUD) pharmacotherapy, and a composite measure of receiving any of these services.
Patients also were compared across HCV status in the entire sample of patients with positive screening as well as in the subsample with a clinically documented AUD.
During the study period, 830,825 VA patients screened positive for unhealthy alcohol use. Among those, 31,841 (3.8%) patients had a documented diagnosis for HCV, and of these 20,320 (64%) had an AUD. Two-thirds of these AUD patients did not receive specialty addiction treatment, and more than 90% did not receive pharmacotherapy that is approved by the Food and Drug Administration to treat AUD, according to the researchers. “These rates are concerning given the negative impact alcohol use can have on HCV,” they wrote.
They reiterated the importance of the 2016 change in policy adopted by the VA Health System, which updated its treatment guidelines to recommend that all patients with HCV be considered for treatment, regardless of substance use, and explicitly stated that alcohol use and length of abstinence should not be disqualifiers for receiving HCV treatment.
“All patients with HCV should be receiving evidence-based alcohol-related care given the risks of alcohol use in this population, particularly among those coinfected with HIV,” the researchers concluded.
The research was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism. The authors reported that they had no conflicts of interest.
SOURCE: Owens MD et al. Drug Alcohol Depend. 2018;188:79-85.
Nearly 4% of Veterans Affairs patients who screened positive for unhealthy alcohol use were infected with hepatitis C virus, and 64% of these patients were diagnosed with alcohol use disorder, according to the results of a large database analysis.
Despite the fact that alcohol use at all levels can compound the adverse effects of HCV and lead to heightened risks of mortality, particularly among those coinfected with HIV, the majority of these patients did not receive specialty addiction treatment, according to Mandy D. Owens, PhD, and her colleagues at the VA Puget Sound Health Care System, Seattle.
In their study, published in Drug and Alcohol Dependence, the researchers queried the national VA health care system database, which is made up of 139 large facilities and more than 900 clinics throughout the United States, for all patients with a documented outpatient appointment between October 2009 and May 2013 to identify those with one or more with positive screens on the AUDIT-C (Alcohol Use Disorders Identification Test-Consumption) questionnaire. Those with AUDIT-C scores greater than or equal to 5 were considered positive, and each positive screen was tracked for up to 1 year to assess alcohol-related care outcomes. The four alcohol-related care outcomes measured were: receipt of brief intervention, specialty addiction treatment, alcohol use disorder (AUD) pharmacotherapy, and a composite measure of receiving any of these services.
Patients also were compared across HCV status in the entire sample of patients with positive screening as well as in the subsample with a clinically documented AUD.
During the study period, 830,825 VA patients screened positive for unhealthy alcohol use. Among those, 31,841 (3.8%) patients had a documented diagnosis for HCV, and of these 20,320 (64%) had an AUD. Two-thirds of these AUD patients did not receive specialty addiction treatment, and more than 90% did not receive pharmacotherapy that is approved by the Food and Drug Administration to treat AUD, according to the researchers. “These rates are concerning given the negative impact alcohol use can have on HCV,” they wrote.
They reiterated the importance of the 2016 change in policy adopted by the VA Health System, which updated its treatment guidelines to recommend that all patients with HCV be considered for treatment, regardless of substance use, and explicitly stated that alcohol use and length of abstinence should not be disqualifiers for receiving HCV treatment.
“All patients with HCV should be receiving evidence-based alcohol-related care given the risks of alcohol use in this population, particularly among those coinfected with HIV,” the researchers concluded.
The research was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism. The authors reported that they had no conflicts of interest.
SOURCE: Owens MD et al. Drug Alcohol Depend. 2018;188:79-85.
Nearly 4% of Veterans Affairs patients who screened positive for unhealthy alcohol use were infected with hepatitis C virus, and 64% of these patients were diagnosed with alcohol use disorder, according to the results of a large database analysis.
Despite the fact that alcohol use at all levels can compound the adverse effects of HCV and lead to heightened risks of mortality, particularly among those coinfected with HIV, the majority of these patients did not receive specialty addiction treatment, according to Mandy D. Owens, PhD, and her colleagues at the VA Puget Sound Health Care System, Seattle.
In their study, published in Drug and Alcohol Dependence, the researchers queried the national VA health care system database, which is made up of 139 large facilities and more than 900 clinics throughout the United States, for all patients with a documented outpatient appointment between October 2009 and May 2013 to identify those with one or more with positive screens on the AUDIT-C (Alcohol Use Disorders Identification Test-Consumption) questionnaire. Those with AUDIT-C scores greater than or equal to 5 were considered positive, and each positive screen was tracked for up to 1 year to assess alcohol-related care outcomes. The four alcohol-related care outcomes measured were: receipt of brief intervention, specialty addiction treatment, alcohol use disorder (AUD) pharmacotherapy, and a composite measure of receiving any of these services.
Patients also were compared across HCV status in the entire sample of patients with positive screening as well as in the subsample with a clinically documented AUD.
During the study period, 830,825 VA patients screened positive for unhealthy alcohol use. Among those, 31,841 (3.8%) patients had a documented diagnosis for HCV, and of these 20,320 (64%) had an AUD. Two-thirds of these AUD patients did not receive specialty addiction treatment, and more than 90% did not receive pharmacotherapy that is approved by the Food and Drug Administration to treat AUD, according to the researchers. “These rates are concerning given the negative impact alcohol use can have on HCV,” they wrote.
They reiterated the importance of the 2016 change in policy adopted by the VA Health System, which updated its treatment guidelines to recommend that all patients with HCV be considered for treatment, regardless of substance use, and explicitly stated that alcohol use and length of abstinence should not be disqualifiers for receiving HCV treatment.
“All patients with HCV should be receiving evidence-based alcohol-related care given the risks of alcohol use in this population, particularly among those coinfected with HIV,” the researchers concluded.
The research was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism. The authors reported that they had no conflicts of interest.
SOURCE: Owens MD et al. Drug Alcohol Depend. 2018;188:79-85.
FROM DRUG AND ALCOHOL DEPENDENCE
Key clinical point: Alcohol-use disorder therapy is underdelivered to patients with HCV who would benefit.
Major finding: Only 27% of patients with HCV plus alcohol-abuse disorder received AUD therapy.
Study details: National VA health care system database of 830,825 patients who screened positive for unhealthy alcohol use.
Disclosures: The research was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism. The authors reported that they had no conflicts of interest.
Source: Owens MD et al. Drug Alcohol Depend. 2018;188:79-85.
FDA warns of birth defect risks from dolutegravir
The Food and Drug Administration has issued a Drug Safety Communication alert that serious cases of neural tube birth defects have been reported in babies born to women treated with dolutegravir for human immunodeficiency virus. Reported defects have involved the brain, spine, and spinal cord.
To date, in this observational study, there are no reported cases of babies born with neural tube defects to women starting dolutegravir later in pregnancy, according to the FDA. “We are investigating this new safety issue and will update the public when we have more information,” the alert stated.
Dolutegravir is an FDA-approved antiretroviral used to treat HIV. The drug is available as a single ingredient under the brand name Tivicay and as a combination tablet with other HIV medicines under the brand names Juluca and Triumeq.
Medwatch also released an alert for medical practitioners to report adverse events related to this issue.
The Food and Drug Administration has issued a Drug Safety Communication alert that serious cases of neural tube birth defects have been reported in babies born to women treated with dolutegravir for human immunodeficiency virus. Reported defects have involved the brain, spine, and spinal cord.
To date, in this observational study, there are no reported cases of babies born with neural tube defects to women starting dolutegravir later in pregnancy, according to the FDA. “We are investigating this new safety issue and will update the public when we have more information,” the alert stated.
Dolutegravir is an FDA-approved antiretroviral used to treat HIV. The drug is available as a single ingredient under the brand name Tivicay and as a combination tablet with other HIV medicines under the brand names Juluca and Triumeq.
Medwatch also released an alert for medical practitioners to report adverse events related to this issue.
The Food and Drug Administration has issued a Drug Safety Communication alert that serious cases of neural tube birth defects have been reported in babies born to women treated with dolutegravir for human immunodeficiency virus. Reported defects have involved the brain, spine, and spinal cord.
To date, in this observational study, there are no reported cases of babies born with neural tube defects to women starting dolutegravir later in pregnancy, according to the FDA. “We are investigating this new safety issue and will update the public when we have more information,” the alert stated.
Dolutegravir is an FDA-approved antiretroviral used to treat HIV. The drug is available as a single ingredient under the brand name Tivicay and as a combination tablet with other HIV medicines under the brand names Juluca and Triumeq.
Medwatch also released an alert for medical practitioners to report adverse events related to this issue.
FDA seeks comments on pediatric HIV product development
The U.S. Food and Drug Administration announced a draft guidance for industry entitled “Pediatric HIV Infection: Drug Development for Treatment.” (from birth to younger than 17 years of age).
According to the FDA announcement, the draft includes recommendations on when sponsors should initiate pediatric formulation development and when to begin pediatric studies to evaluate antiretroviral drug products for the treatment of HIV infection.
SOURCE: Federal Register May 14. Pediatric HIV Infection: Drug Development for Treatment; Draft Guidance for Industry; Availability.
The U.S. Food and Drug Administration announced a draft guidance for industry entitled “Pediatric HIV Infection: Drug Development for Treatment.” (from birth to younger than 17 years of age).
According to the FDA announcement, the draft includes recommendations on when sponsors should initiate pediatric formulation development and when to begin pediatric studies to evaluate antiretroviral drug products for the treatment of HIV infection.
SOURCE: Federal Register May 14. Pediatric HIV Infection: Drug Development for Treatment; Draft Guidance for Industry; Availability.
The U.S. Food and Drug Administration announced a draft guidance for industry entitled “Pediatric HIV Infection: Drug Development for Treatment.” (from birth to younger than 17 years of age).
According to the FDA announcement, the draft includes recommendations on when sponsors should initiate pediatric formulation development and when to begin pediatric studies to evaluate antiretroviral drug products for the treatment of HIV infection.
SOURCE: Federal Register May 14. Pediatric HIV Infection: Drug Development for Treatment; Draft Guidance for Industry; Availability.
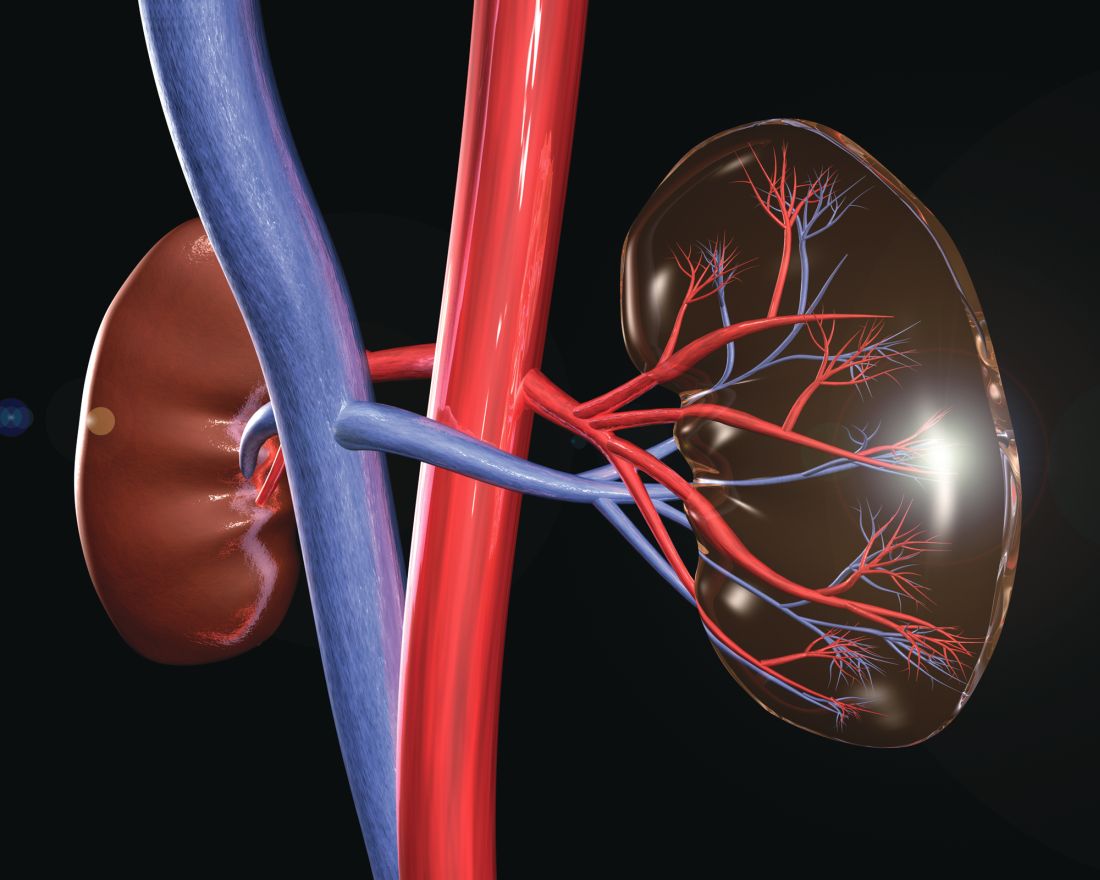
New trial to assess HIV+/HIV+ kidney transplants
The HOPE in Action Multicenter Kidney Study, the first large-scale clinical trial to study kidney transplantations between people with HIV, has begun at clinical centers across the United States, according to an announcement by the National Institutes of Health. The study, sponsored by the National Institute of Allergy and Infectious Diseases and NIH, will assess the safety of HIV-positive donor to HIV-positive recipient kidney transplantation. This form of transplantation was made legal by the passage of the HOPE (HIV Organ Policy Equity) Act, which permits the transplantation of organs from donors with HIV into qualified HIV-positive recipients with end-stage organ failure.
Outcomes to be studied in the multicenter trial will include potential transplant-related and HIV-related complications following surgery, as well as organ rejection, organ failure, and failure of previously effective HIV medications. The study is currently enrolling and is expected to include 360 participants, with an estimated completion date of Aug. 1, 2022.
“The HOPE Act of 2013 opened the door for researchers to explore a potential new source of donor organs for those living with HIV – a population with a significant and growing need for transplants. This study offers a chance to improve the health of those living with HIV, and increase the overall supply of transplantable organs,” said NIAID Director Anthony S. Fauci, MD, in the NIH news release. Transplantation of organs from HIV-positive donors to HIV-negative recipients remains illegal in the United States.
More information about the study can be found at ClinicalTrials.gov using the identifier NCT03500315. A similar trial to examine liver transplantation, the HOPE in Action Multicenter Liver Study, is expected to launch later in 2018.
The HOPE in Action Multicenter Kidney Study, the first large-scale clinical trial to study kidney transplantations between people with HIV, has begun at clinical centers across the United States, according to an announcement by the National Institutes of Health. The study, sponsored by the National Institute of Allergy and Infectious Diseases and NIH, will assess the safety of HIV-positive donor to HIV-positive recipient kidney transplantation. This form of transplantation was made legal by the passage of the HOPE (HIV Organ Policy Equity) Act, which permits the transplantation of organs from donors with HIV into qualified HIV-positive recipients with end-stage organ failure.
Outcomes to be studied in the multicenter trial will include potential transplant-related and HIV-related complications following surgery, as well as organ rejection, organ failure, and failure of previously effective HIV medications. The study is currently enrolling and is expected to include 360 participants, with an estimated completion date of Aug. 1, 2022.
“The HOPE Act of 2013 opened the door for researchers to explore a potential new source of donor organs for those living with HIV – a population with a significant and growing need for transplants. This study offers a chance to improve the health of those living with HIV, and increase the overall supply of transplantable organs,” said NIAID Director Anthony S. Fauci, MD, in the NIH news release. Transplantation of organs from HIV-positive donors to HIV-negative recipients remains illegal in the United States.
More information about the study can be found at ClinicalTrials.gov using the identifier NCT03500315. A similar trial to examine liver transplantation, the HOPE in Action Multicenter Liver Study, is expected to launch later in 2018.
The HOPE in Action Multicenter Kidney Study, the first large-scale clinical trial to study kidney transplantations between people with HIV, has begun at clinical centers across the United States, according to an announcement by the National Institutes of Health. The study, sponsored by the National Institute of Allergy and Infectious Diseases and NIH, will assess the safety of HIV-positive donor to HIV-positive recipient kidney transplantation. This form of transplantation was made legal by the passage of the HOPE (HIV Organ Policy Equity) Act, which permits the transplantation of organs from donors with HIV into qualified HIV-positive recipients with end-stage organ failure.
Outcomes to be studied in the multicenter trial will include potential transplant-related and HIV-related complications following surgery, as well as organ rejection, organ failure, and failure of previously effective HIV medications. The study is currently enrolling and is expected to include 360 participants, with an estimated completion date of Aug. 1, 2022.
“The HOPE Act of 2013 opened the door for researchers to explore a potential new source of donor organs for those living with HIV – a population with a significant and growing need for transplants. This study offers a chance to improve the health of those living with HIV, and increase the overall supply of transplantable organs,” said NIAID Director Anthony S. Fauci, MD, in the NIH news release. Transplantation of organs from HIV-positive donors to HIV-negative recipients remains illegal in the United States.
More information about the study can be found at ClinicalTrials.gov using the identifier NCT03500315. A similar trial to examine liver transplantation, the HOPE in Action Multicenter Liver Study, is expected to launch later in 2018.