User login
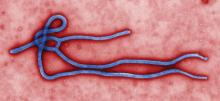
A study of a potential new Ebola treatment has begun at the National Institutes of Health Clinical Center in Bethesda, Md. The small phase 1 clinical trial will examine the safety and tolerability of a single monoclonal antibody (mAb114), which was developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) and their collaborators. Investigators plan to enroll between 18 and 30 healthy volunteers aged 18-60. The trial will not expose participants to Ebola virus, according to the NIH announcement.
MAb114 is a monoclonal antibody – a protein that binds to a single target on a pathogen — isolated from a human survivor of the 1995 Ebola outbreak in Kikwit, Democratic Republic of the Congo. Nancy Sullivan, PhD, chief of the Biodefense Research Section in NIAID’s Vaccine Research Center, and her team, in collaboration with researchers from the National Institute of Biomedical Research in the Democratic Republic of the Congo and the Institute for Biomedical Research in Switzerland, discovered that the survivor retained antibodies against Ebola 11 years after infection. They isolated and tested the antibodies and selected mAb114 as the most promising.
Although rVSV-ZEBOV, an experimental vaccine, is now available and in use in Africa during the current outbreak, specific treatment modalities are lacking.
More information can be found at www.clinicaltrials.gov, trial # NCT03478891.
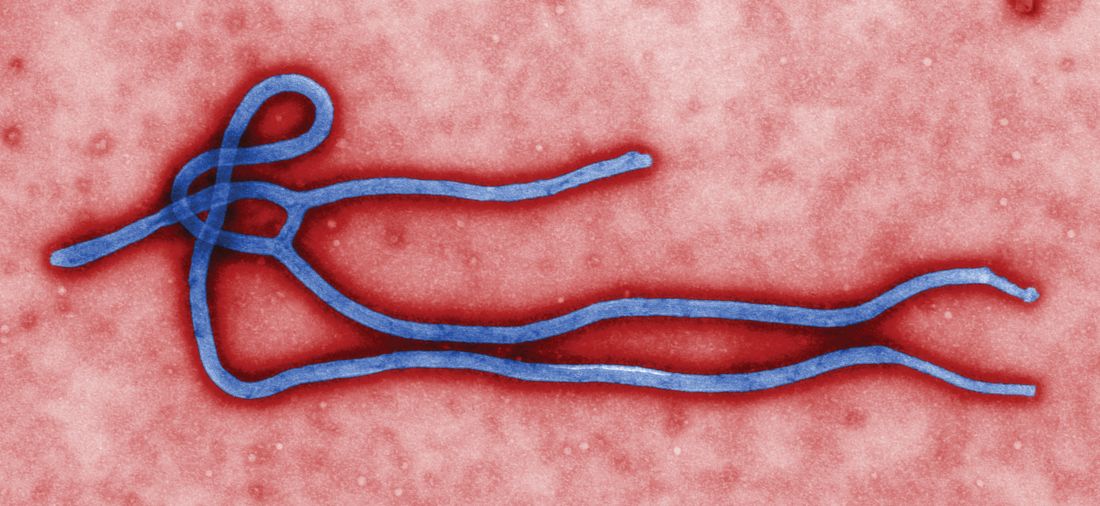
A study of a potential new Ebola treatment has begun at the National Institutes of Health Clinical Center in Bethesda, Md. The small phase 1 clinical trial will examine the safety and tolerability of a single monoclonal antibody (mAb114), which was developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) and their collaborators. Investigators plan to enroll between 18 and 30 healthy volunteers aged 18-60. The trial will not expose participants to Ebola virus, according to the NIH announcement.
MAb114 is a monoclonal antibody – a protein that binds to a single target on a pathogen — isolated from a human survivor of the 1995 Ebola outbreak in Kikwit, Democratic Republic of the Congo. Nancy Sullivan, PhD, chief of the Biodefense Research Section in NIAID’s Vaccine Research Center, and her team, in collaboration with researchers from the National Institute of Biomedical Research in the Democratic Republic of the Congo and the Institute for Biomedical Research in Switzerland, discovered that the survivor retained antibodies against Ebola 11 years after infection. They isolated and tested the antibodies and selected mAb114 as the most promising.
Although rVSV-ZEBOV, an experimental vaccine, is now available and in use in Africa during the current outbreak, specific treatment modalities are lacking.
More information can be found at www.clinicaltrials.gov, trial # NCT03478891.
A study of a potential new Ebola treatment has begun at the National Institutes of Health Clinical Center in Bethesda, Md. The small phase 1 clinical trial will examine the safety and tolerability of a single monoclonal antibody (mAb114), which was developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) and their collaborators. Investigators plan to enroll between 18 and 30 healthy volunteers aged 18-60. The trial will not expose participants to Ebola virus, according to the NIH announcement.
MAb114 is a monoclonal antibody – a protein that binds to a single target on a pathogen — isolated from a human survivor of the 1995 Ebola outbreak in Kikwit, Democratic Republic of the Congo. Nancy Sullivan, PhD, chief of the Biodefense Research Section in NIAID’s Vaccine Research Center, and her team, in collaboration with researchers from the National Institute of Biomedical Research in the Democratic Republic of the Congo and the Institute for Biomedical Research in Switzerland, discovered that the survivor retained antibodies against Ebola 11 years after infection. They isolated and tested the antibodies and selected mAb114 as the most promising.
Although rVSV-ZEBOV, an experimental vaccine, is now available and in use in Africa during the current outbreak, specific treatment modalities are lacking.
More information can be found at www.clinicaltrials.gov, trial # NCT03478891.