User login
Exploring options for POP treatment: Patient selection, surgical approaches, and ways to manage risks
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.
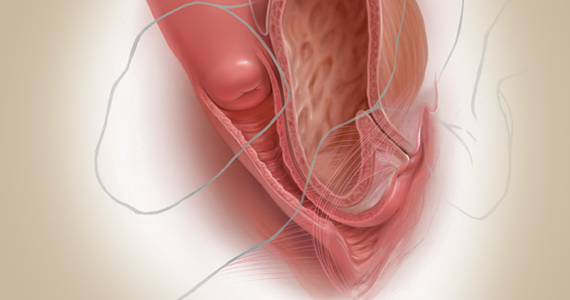
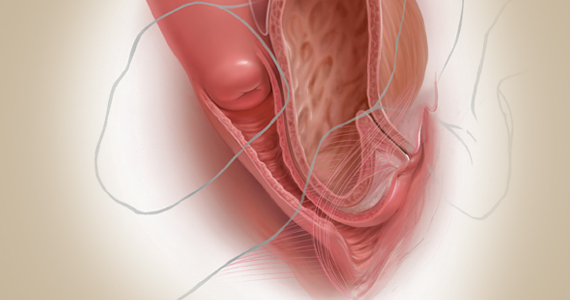
Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.

Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.

Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
Uterosacral ligament colpopexy: The way we do it
The way we do it

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
The way we do it
The way we do it
This video is brought to you by
Which sling for which SUI patient?
- Placement of TVT-O transobturator tape
- Monarc transobturator procedure
- TVT Exact retropubic sling operation
These videos were selected by Mark D. Walters, MD, and presented courtesy of the International Academy of Pelvic Surgery (IAPS).
Only 15 years ago, when surgery was recommended for patients who had bothersome stress urinary incontinence (SUI), they were offered operations such as suburethral (Kelly) plication, needle urethropexy, open or laparoscopic Burch procedure, and pubovaginal fascial sling procedure. Today, virtually all of these operations have been replaced in general practice by retropubic or transobturator (TOT) midurethral synthetic slings.
Although Burch colposuspension and the pubovaginal fascial sling procedure are effective for both primary and recurrent SUI, they are more invasive than midurethral slings, cause more voiding dysfunction, and have significantly longer recovery times, making them less attractive for most primary and recurrent cases of SUI.
The evolution of SUI surgeries has shifted so far toward midurethral slings that Burch colposuspension and the pubovaginal sling procedure are rarely performed or taught in obstetrics and gynecology or urology residency programs; these procedures are now mostly done in fellowship programs by specialists in female pelvic medicine and reconstructive surgery.
In this article, we describe how an ObGyn generalist can approach the surgical treatment of women who have either primary or recurrent SUI. Using evidence-based principles, when available, we also discuss how different clinical characteristics—as well as the characteristics of the available slings—affect the suitability of the sling for individual patients.
One caveat: This article assumes that the surgeon knows how to, and is able to, perform retropubic and TOT sling procedures equally well. However, when this is not the case, the surgeon should perform the sling procedure that she or he does best, assuming that it is appropriate for that particular patient.
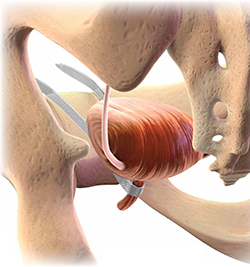
Almost all surgical procedures for stress urinary incontinence performed today involve placement of a retropubic or transobturator midurethral synthetic sling.
Illustration: Craig Zuckerman for OBG Management
CASE: SUI and Stage II anterior vaginal prolapse
A healthy 45-year-old G2P2 woman complains of a 5-year history of worsening SUI symptoms, mostly occurring during activities such as coughing, laughing, and running. The incontinence has become so severe that she requires several pads daily. She is able to void without difficulty or pain, and her bowel movements are normal. She has regular menses, has had a tubal ligation, and is sexually active.
She reports that she has been performing daily Kegel pelvic muscle exercises, without improvement.
On physical examination, she is found to have Stage II anterior vaginal prolapse and urethral hypermobility, with normal uterine and posterior vaginal support. The uterus and ovaries are of normal size.
A full bladder stress test in the office reveals immediate loss of urine from the urethra upon coughing in a semi-sitting position. She voids 325 mL after the examination and has a post-void residual urine volume, as measured by ultrasonography (US), of 25 mL. Urinalysis is negative.
When discussing her goals, the patient expresses a desire for a cure of her urinary incontinence, if possible.
What further testing and treatment options do you offer to her?
If you and the patient agree that surgery is warranted, which procedure do you recommend?
Recommended assessment of women who report SUI
Women who have bothersome urine loss during activities such as exercise, coughing, or laughing should undergo a history, physical examination, and urinalysis. During the pelvic examination, it is important to assess pelvic organ support defects, especially those involving the anterior vagina and urethra. Also note levator ani muscle contraction and strength. In addition, you can use this time to discuss whether the patient is doing, or has done, pelvic muscle (Kegel) exercises; teach the exercises, if necessary; and encourage her to do them in the future.
If the patient has no urinary infection, has performed Kegel exercises without further benefit, and wishes to consider surgical treatment, basic assessment of lower urinary function is indicated. Basic office urodynamic testing includes:
- a measured void
- measurement of post-void residual volume (by catheter or US)
- assessment of bladder sensation and capacity
- provocation for overactive bladder
- a full-bladder cough stress test (a positive test is direct observation of urethral loss of urine upon coughing).
Patients who have a complex history or mixed symptoms, previous failed surgery, or other characteristics that suggest a diagnosis other than simple SUI should undergo formal electronic urodynamic testing.1
Patient selection criteria
Primary sling surgery is an option for patients who have:
- no urinary infection
- normal voiding and bladder-filling function
- urethral hypermobility on examination
- SUI on a cough-stress test
- failure to improve sufficiently with pelvic muscle exercises.
Suburethral slings were initially developed as a treatment for recurrent, urodynamically confirmed SUI, particularly SUI caused by intrinsic sphincter deficiency (ISD). Pubovaginal slings, usually consisting of autologous fascia, were placed at the bladder neck to both support and slightly compress the proximal urethra. Compared with synthetic slings, fascial slings are effective but take longer to place and have a higher rate of surgical morbidity and more postoperative voiding dysfunction. They are now mostly indicated for complex recurrent SUI, usually managed by specialists in female pelvic medicine and reconstructive surgery.
Current slings are lightweight polypropylene mesh
Most slings today are tension-free midurethral slings consisting of synthetic, large-pore polypropylene mesh; they are sold in kits available from several different companies. Sling procedures can also be performed using hand-cut polypropylene mesh and a reusable needle passer.
These slings are placed at the midurethra and work by mechanical kinking or folding of the urethra over the sling, with an increase in intra-abdominal pressure. Ideally, the midurethral sling will not compress the urethra at rest and have no effect on the normal voiding mechanism.
Three main techniques are used to place synthetic midurethral slings:
- the retropubic approach
- the TOT approach
- variations of single-incision “mini-sling” procedures.
Early studies of mini-slings showed few complications but lower effectiveness, compared with retropubic and TOT midurethral slings, according to short-term follow-up data.2-4 A mini-sling might be an option for some patients in whom surgical complications must be kept to a minimum; otherwise, they will not be discussed further.
Retropubic midurethral slings
The tension-free vaginal tape (TVT) procedure described by Petros and Ulmsten was the first synthetic midurethral sling.5 This ambulatory procedure aims to restore the pubourethral ligament and suburethral vaginal hammock by using specially designed needles attached to synthetic sling material.
The synthetic sling consists of polypropylene, approximately 1 cm wide and 40 cm long. The sling material is attached to two stainless steel needles that are passed from a vaginal incision made at the level of the midurethra, through the retropubic space, and exiting at a previously created mark or stab incision in the suprapubic area (FIGURE 1).
Variations of the retropubic midurethral sling have been developed, with sling passers going from the vagina upward (“bottom to top”) and also from the suprapubic area downward (“top to bottom”). A recent Cochrane review reported that the bottom-to-top variation is slightly more effective.6
FIGURE 1 Retropubic sling
Placement of the tension-free vaginal tape trocar into the retropubic space.
Illustration: Craig Zuckerman for OBG Management
Transobturator midurethral slings
The TOT sling has become one of the most popular and effective surgical treatments for female SUI worldwide (Video 1 and Video 2). It is a relatively rapid and low-risk surgery that is comparable to other surgical options in effectiveness while avoiding an abdominal incision and the passage of a needle or trocar through the space of Retzius.
The TOT sling lies flatter under the urethra and carries a lower risk of urethral obstruction, urinary retention, and subsequent need for sling release, compared with retropubic slings.7-9 Compared with the retropubic TVT, the TOT sling produces similar rates of cure, with fewer bladder perforations and less postoperative irritative voiding symptoms.6,10-12 It nearly eliminates the rare but catastrophic risk of bowel or major vessel perforation. The trade-off is that patients experience more complications referable to the groin (pain and leg weakness or numbness) with the TOT approach.9,13
All TOT slings on the market consist of a large-pore, lightweight, polypropylene mesh strip, usually covered with a plastic sheath. Various devices are used to place the sling, but most of them involve a helical trocar that curves around the ischiopubic ramus, passing through the inner thigh and obturator membrane to a space created in the ipsilateral peri-urethral tissues.
TOT slings can be placed outside-to-inside or inside-to-outside (FIGURE 2), and the indications, effectiveness, and frequency of complications seem to be similar between these two approaches.12 One study found a higher frequency of new sexual dysfunction (tender, palpable sling; penile pain in male partner) in women after the “outside-in” approach,14 but this clinical issue has not been observed in all studies.15,16
FIGURE 2 TOT sling variations
Placement of the transobturator (TOT) sling helical trocar using the (A) “outside-in” variation and (B) “inside-out” variation.
Illustration: Craig Zuckerman for OBG Management
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography
© 2005-2012. All Rights Reserved.
Success rates are similar for retropubic and TOT slings
Despite differences in technique and brand of mesh used, treatment success rates for uncomplicated primary SUI are similar for the retropubic (Video 3) and TOT tension-free slings.6-8,10-12,17 The percentage of patients treated successfully depends on the definition used, ranging from a high of 96% to a low of 60%. When the definition of success is restricted to stress incontinence symptoms, especially over a short period of time, the reported effectiveness is high.
In contrast, when the definition of success includes incontinence of any type, the reported effectiveness is lower. For example, in the study that reported 60% effectiveness, success was defined as no incontinence symptoms of any type, a negative cough stress test, and no retreatment for stress incontinence or postoperative urinary retention.11
Retropubic slings, especially TVT, may be somewhat more effective for ISD,18-20 although this conclusion must be tempered by the small number of studies addressing the issue and differences in the diagnosis of ISD.21
Some studies have reported good success in treating mixed urinary incontinence with the retropubic and TOT slings,2,8 although other studies have reported that the initial benefit for urgency or urge incontinence is not sustained over time, compared with the benefit for stress incontinence.22 It is important to counsel patients before surgery that improvement in stress incontinence symptoms and general satisfaction is highly likely, but perfect bladder function is not.
Serious complications are uncommon
Complications are common after both retropubic and TOT slings, although serious complications are uncommon. Cystitis and temporary voiding difficulties are the most common problems after a sling procedure. If the patient is unable to void on the day of surgery, it is reasonable to discharge her with a Foley catheter in place for a few days or teach her to perform intermittent self-catheterization at home. In most cases, normal voiding will resume within a few days. Cystitis is at least partially related to the surgery itself and the duration of postoperative catheterization.
The frequency of some complications differs between the retropubic and TOT approaches to midurethral slings. For example, some literature suggests that irritative voiding symptoms such as urgency or voiding difficulty are somewhat less common after TOT slings, compared with retropubic slings. However, symptoms referable to the groin (pain and leg weakness or numbness) occur more commonly with the TOT approach.6
After placement of a TOT sling, 10% to 15% of women experience temporary inner thigh or groin pain or leg weakness and are usually managed conservatively with nonsteroidal anti-inflammatory drugs and physical therapy. Long-term or severe complications related to TOT sling passage are rare.
Major intraoperative complications are rare
The rate of these complications does not differ between retropubic and TOT approaches. Minor intraoperative complications—primarily, bladder perforation—occur more commonly with the retropubic approach.6
Bladder perforation with the TVT occurs in 4% to 7% of patients. However, the clinical significance of bladder perforation is minimal as long as the surgeon performs careful cystoscopy, recognizes bladder perforation, and repositions the trocar and mesh outside the bladder lumen. Bladder perforation caused by the trocar usually does not require specific treatment (except repositioning of the trocar outside the bladder lumen) and rarely results in later problems.
Mesh exposures occur with similar frequency for the different sling types as long as large-pore lightweight polypropylene is used. Dehiscence of the suburethral incision (mesh exposure) is uncommon with midurethral slings, occurring in 1% to 2% of patients. Dehiscence can be managed with estrogen cream or trimming of the exposed portion of the sling in the office. If symptoms or signs persist, removal of the exposed segment or the entire central portion of the sling, with closure of the vaginal epithelium, is indicated to allow for healing and resolution of symptoms. However, removal may lead to recurrence of the original SUI symptoms.
Retropubic hematomas occur in 1% to 2% of patients after placement of a retropubic sling, but major vascular injuries are rare—occurring in, perhaps, 3 in every 1,000 cases.
Bowel perforations are very rare but serious complications. A retropubic sling should be placed with caution or avoided in women who have a history of peritonitis, bowel surgery, ruptured appendix, or known extensive pelvic adhesions.
Major vascular injuries are also rare with TOT slings, occurring in approximately 1 to 2 cases in every 1,000.
Bladder injury occurs much less frequently after placement of a TOT sling, compared with the retropubic approach, although one study reported bladder injury in 2% of TOT cases.17 Although bladder injury is uncommon with the TOT approach, the morbidity associated with delayed detection of bladder injury is much higher than the morbidity associated with intraoperative detection and management. Therefore, we believe that cystoscopy should be performed in all TOT and retropubic sling procedures to either exclude bladder damage or detect and appropriately manage it.
For reassurance that intraoperative and postoperative blood loss is not excessive, it is reasonable to check one hemoglobin level before discharge, if desired.
How to individualize the choice of sling
Patients who have primary SUI: Retropubic or TOT sling. Objective and subjective success rates are similar, regardless of approach, and serious complications are infrequent. The retropubic approach has longer-term evidence of sustained benefit, compared with the newer TOT approach. We tend to treat younger patients with TVT and older patients with TOT. Surgeon experience and informed patient preferences may dictate the choice of sling (TABLE).
What we recommend surgically for our patients who have SUI—and why
| Clinical problem and patient characteristics | Surgery | Rationale |
|---|---|---|
| Primary SUI with urethral hypermobility—young patient | TVT | TVT has similar effectiveness and more long-term data than TOT; TVT may result in less sexual pain than TOT |
| Primary SUI with urethral hypermobility—older patient; leak point pressure >60 cmH20 | TOT | Similar effectiveness, fewer complications with TOT |
| Recurrent SUI with urethral hypermobility—any age; leak point pressure >60 cmH20 | TVT | Limited data suggest effectiveness of TVT after TOT failure |
| Recurrent SUI with urethral hypermobility—leak point pressure <60 cmH20 (ISD) | TVT or pubovaginal fascial sling | Some but not all data indicate that TVT is more effective for ISD; fascial slings in expert hands are effective, based on cohort studies |
| Recurrent SUI with nonmobile bladder neck; any leak point pressure | Urethral bulking | All sling procedures have lowered effectiveness when the bladder neck is immobile |
| SUI mixed with dominant urgency or voiding dysfunction | TOT | TOT improves or does not exacerbate mixed urinary symptoms to the extent that TVT may |
| SUI with prolapse and planned vaginal prolapse repair | TVT or TOT | Limited data support similar effectiveness for either approach |
| “Occult” SUI with prolapse reduced and planned vaginal prolapse repair | TOT or “wait and see” | TOT has a lower chance of creating new irritative voiding symptoms; “wait-and-see” approach allows treatment of SUI if it develops after prolapse repair |
| Recurrent SUI with previous synthetic sling mesh complication (or patients who desire treatment without mesh) | Pubovaginal fascial sling or Burch colposuspension | These nonmesh options are effective for recurrent SUI, but have higher surgical morbidity |
| ISD = intrinsic sphincter deficiency; SUI = stress urinary incontinence; TVT = tension-free vaginal tape or similar retropubic midurethral sling; TOT = transobturator sling placed either by outside-in or inside-out variations | ||
Patients who have recurrent SUI: Retropubic sling. Comparative data are limited regarding the retropubic and TOT approaches for recurrent SUI that does not involve ISD. One case series reported good results with the use of retropubic TVT for recurrent SUI after an initial TOT approach.23
Patients who have ISD: Retropubic sling (synthetic midurethral sling or fascial sling placed at the bladder neck). A few studies suggest that patients with ISD have better outcomes with the retropubic approach.19,20 However, with differing definitions of ISD and relatively few patients with ISD included in these trials, it is not possible to conclude definitively that the retropubic approach is more effective than the TOT approach for patients who have SUI and ISD. However, the retropubic approach has longer-term data to support its effectiveness; therefore, with some but not all evidence suggesting its superiority for ISD, it is reasonable to choose the retropubic midurethral approach.
A pubovaginal fascial sling placed at the proximal urethra is also an effective option, based on numerous cohort studies.12
Patients who have recurrent SUI or ISD, or both, with a non-mobile bladder neck: Urethral bulking. Although data are scant, urethral injection therapy is beneficial for SUI in the short-term, but long-term studies are lacking. Bulking agents include silicone particles, calcium hydroxylapatite, and carbon spheres; studies have not shown one to be more or less efficacious than the others.24
It is reasonable to use urethral bulking first in these patients as the morbidity is very low and some patients become continent. A retropubic sling can be performed if urethral bulking fails to adequately improve symptoms, although the effectiveness is lower in this population than in women with SUI and urethral hypermobility.
Patients who have mixed stress and urge incontinence or voiding dysfunction: TOT sling. Limited data suggest that the TOT approach improves symptoms of mixed incontinence—or, at least, exacerbates them to a lesser degree than the retropubic approach. Rarely is a sling release needed to treat obstructive urinary symptoms after the TOT approach.
Patients who have prolapse and SUI: Retropubic or TOT sling. When a sling procedure is performed at the same time as reconstructive surgery for prolapse, it has similar effectiveness, regardless of whether the retropubic or TOT approach is selected.8,11 A sling placed during prolapse surgery (placed through a separate midurethral incision) appears to be as effective as a sling placed as a sole procedure.
Patients who have prolapse and occult SUI: TOT sling. If you recommend a sling to prevent SUI after prolapse surgery by any route, pick the sling with acceptable efficacy and the lowest rates of complications and voiding dysfunction. Patients are especially intolerant of complications from a sling to prevent SUI. Given the ease of placement and low morbidity of a later outpatient sling procedure, it is also reasonable to offer patients the “wait-and-see” alternative to see if SUI develops after prolapse surgery and only then proceeding with sling surgery. In this way, overtreatment is avoided, and any complications that occur after sling surgery for SUI treatment may be better tolerated by the patient. The preferences of an informed patient may guide decisions in this setting.
Patients who have recurrent SUI with mesh complication: Pubovaginal fascial sling or Burch colposuspension. These non-mesh options are effective for recurrent SUI and can be performed at the same time as mesh removal. They carry higher surgical morbidity, longer operative time, and greater postoperative voiding dysfunction.
An informed patient can help guide the approach
The retropubic and TOT approaches to tension-free midurethral slings are similar in effectiveness. Most women experience significant improvement of SUI symptoms after sling placement, although many women continue to have some urinary symptoms.
Depending on their training, experience, and personal results—as well as the preferences of an informed patient—surgeons may recommend one approach over the other. In addition, certain clinical situations may favor one sling over another. Studies with longer-term follow-up in different patient subgroups are needed to adequately counsel women about the durability of results.
CASE: Resolved
After discussing the options with your patient, she opts to undergo anterior prolapse repair with concurrent placement of a TOT sling. The surgery is completed without complication. She is discharged later that day without a catheter after demonstrating normal voiding with low residual urine volume. Postoperatively, she reports mild pain referred to the groin. You instruct her to take nonsteroidal anti-inflammatory drugs for pain relief. On her postoperative visit, she reports that the pain is gone and the SUI has almost completely resolved.
We want to hear from you! Tell us what you think.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #63: Urinary incontinence in women. Obstet Gynecol. 2005;105(6):1533-1545.
2. Abdel-Fattah M, Ford JA, Lim CP, Madhuvrata P. Single-incision mini-slings versus standard midurethral slings in surgical management of female stress urinary incontinence: a meta-analysis of effectiveness and complications. Eur Urol. 2011;60(3):468-480.
3. Hinoul P, Vervest HA, den Boon J, et al. A randomized, controlled trial comparing an innovative single-incision sling with an established transobturator sling to treat female stress urinary incontinence. J Urol. 2011;185(4):1356-1362.
4. Barber MD, Weidner AC, Sokol AI, et al. Foundation for Female Health Awareness Research Network. Single-incision mini-sling compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2012;119(2 pt 1):328-337.
5. Petros P, Ulmsten U. Intravaginal sling plasty (IVS): An ambulatory surgical procedure for treatment of female urinary stress incontinence. Scand J Urol Nephrol. 1995;29(1):75-82.
6. Ogah J, Cody DJ, Rogerson L. Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women: a short version Cochrane review. Neurourol Urodyn. 2011;30(3):284-291.
7. Meschia M, Bertozzi R, Pifarotti P, et al. Perioperative morbidity and early results of a randomised trial comparing TVT and TVT-O. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(11):1257-1261.
8. Richter HE, Albo ME, Zyczynski HM, et al. Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362(22):2066-2076.
9. Brubaker L, Norton PA, Albo ME, et al. Urinary Incontinence Treatment Network. Adverse events over two years after retropubic or transobturator midurethral sling surgery: findings from the Trial of Midurethral Slings (TOMUS) study. Am J Obstet Gynecol. 2011;205(5):498.e1-6.
10. Sung VW, Schleinitz MD, Rardin CR, Ward RM, Myers DL. Comparison of retropubic vs transobturator approach to midurethral slings: a systematic review and meta-analysis. Am J Obstet Gynecol. 2007;197(1):3-11.
11. Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2008;111(3):611-621.
12. Novara G, Artibani W, Barber MD, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2010;58(2):218-238.
13. Ross S, Robert M, Swaby C, et al. Transobturator tape compared with tension-free vaginal tape for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2009;114(6):1287-1294.
14. Scheiner DA, Betschart C, Wiederkehr S, Seifert B, Fink D, Perucchini D. Twelve months effect on voiding function of retropubic compared with outside-in and inside-out transobturator midurethral slings. Int Urogynecol J. 2012;23(2):197-206.
15. De Souza A, Dwyer PL, Rosamilia A, et al. Sexual function following retropubic TVT and transobturator Monarc sling in women with intrinsic sphincter deficiency: a multicenter prospective study. Int Urogynecol J. 2012;23(2):153-158.
16. Sentilhes L, Berthier A, Loisel C, Descamps P, Marpeau L, Grise P. Female sexual function following surgery for stress urinary incontinence: tension-free vaginal versus transobturator tape procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(4):393-399.
17. Deffieux X, Daher N, Mansoor A, Debodinance P, Muhlstein J, Fernandez H. Transobturator TVT-O versus retropubic TVT: results of a multicenter randomized controlled trial at 24 months follow-up. Int Urogynecol J. 2010;21(11):1337-1345.
18. Schierlitz L, Dwyer PL, Rosamilia A, et al. Effectiveness of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency: a randomized controlled trial. Obstet Gynecol. 2008;112(6):1253-1261.
19. Rechberger T, Futyma K, Jankiewicz K, Adamiak A, Skorupski P. The clinical effectiveness of retropubic (IVS-02) and transobturator (IVS-04) midurethral slings: randomized trial. Eur Urol. 2009;56(1):24-30.
20. Schierlitz L, Dwyer PL, Rosamilia AN, et al. Three-year follow-up of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency. Obstet Gynecol. 2012;119(2 pt 1):321-327.
21. Nager C, Siris L, Litman HJ, et al. Urinary Incontinence Treatment Network. Baseline urodynamic predictors of treatment failure 1 year after midurethral sling surgery. J Urol. 2011;186(2):597-603.
22. Jain P, Jirschele K, Botros SM, Latthe PM. Effectiveness of midurethral slings in mixed urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J. 2011;22(8):923-932.
23. Sabadell J, Poza JL, Esgueva A, Morales JC, Sanchez-Iglesias JL, Xercavins J. Usefulness of retropubic tape for recurrent stress incontinence after transobturator tape failure. Int Urogynecol J. 2011;22(12):1543-1547.
24. Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2012; Feb 15;2:CD003881.-
- Placement of TVT-O transobturator tape
- Monarc transobturator procedure
- TVT Exact retropubic sling operation
These videos were selected by Mark D. Walters, MD, and presented courtesy of the International Academy of Pelvic Surgery (IAPS).
Only 15 years ago, when surgery was recommended for patients who had bothersome stress urinary incontinence (SUI), they were offered operations such as suburethral (Kelly) plication, needle urethropexy, open or laparoscopic Burch procedure, and pubovaginal fascial sling procedure. Today, virtually all of these operations have been replaced in general practice by retropubic or transobturator (TOT) midurethral synthetic slings.
Although Burch colposuspension and the pubovaginal fascial sling procedure are effective for both primary and recurrent SUI, they are more invasive than midurethral slings, cause more voiding dysfunction, and have significantly longer recovery times, making them less attractive for most primary and recurrent cases of SUI.
The evolution of SUI surgeries has shifted so far toward midurethral slings that Burch colposuspension and the pubovaginal sling procedure are rarely performed or taught in obstetrics and gynecology or urology residency programs; these procedures are now mostly done in fellowship programs by specialists in female pelvic medicine and reconstructive surgery.
In this article, we describe how an ObGyn generalist can approach the surgical treatment of women who have either primary or recurrent SUI. Using evidence-based principles, when available, we also discuss how different clinical characteristics—as well as the characteristics of the available slings—affect the suitability of the sling for individual patients.
One caveat: This article assumes that the surgeon knows how to, and is able to, perform retropubic and TOT sling procedures equally well. However, when this is not the case, the surgeon should perform the sling procedure that she or he does best, assuming that it is appropriate for that particular patient.

Almost all surgical procedures for stress urinary incontinence performed today involve placement of a retropubic or transobturator midurethral synthetic sling.
Illustration: Craig Zuckerman for OBG Management
CASE: SUI and Stage II anterior vaginal prolapse
A healthy 45-year-old G2P2 woman complains of a 5-year history of worsening SUI symptoms, mostly occurring during activities such as coughing, laughing, and running. The incontinence has become so severe that she requires several pads daily. She is able to void without difficulty or pain, and her bowel movements are normal. She has regular menses, has had a tubal ligation, and is sexually active.
She reports that she has been performing daily Kegel pelvic muscle exercises, without improvement.
On physical examination, she is found to have Stage II anterior vaginal prolapse and urethral hypermobility, with normal uterine and posterior vaginal support. The uterus and ovaries are of normal size.
A full bladder stress test in the office reveals immediate loss of urine from the urethra upon coughing in a semi-sitting position. She voids 325 mL after the examination and has a post-void residual urine volume, as measured by ultrasonography (US), of 25 mL. Urinalysis is negative.
When discussing her goals, the patient expresses a desire for a cure of her urinary incontinence, if possible.
What further testing and treatment options do you offer to her?
If you and the patient agree that surgery is warranted, which procedure do you recommend?
Recommended assessment of women who report SUI
Women who have bothersome urine loss during activities such as exercise, coughing, or laughing should undergo a history, physical examination, and urinalysis. During the pelvic examination, it is important to assess pelvic organ support defects, especially those involving the anterior vagina and urethra. Also note levator ani muscle contraction and strength. In addition, you can use this time to discuss whether the patient is doing, or has done, pelvic muscle (Kegel) exercises; teach the exercises, if necessary; and encourage her to do them in the future.
If the patient has no urinary infection, has performed Kegel exercises without further benefit, and wishes to consider surgical treatment, basic assessment of lower urinary function is indicated. Basic office urodynamic testing includes:
- a measured void
- measurement of post-void residual volume (by catheter or US)
- assessment of bladder sensation and capacity
- provocation for overactive bladder
- a full-bladder cough stress test (a positive test is direct observation of urethral loss of urine upon coughing).
Patients who have a complex history or mixed symptoms, previous failed surgery, or other characteristics that suggest a diagnosis other than simple SUI should undergo formal electronic urodynamic testing.1
Patient selection criteria
Primary sling surgery is an option for patients who have:
- no urinary infection
- normal voiding and bladder-filling function
- urethral hypermobility on examination
- SUI on a cough-stress test
- failure to improve sufficiently with pelvic muscle exercises.
Suburethral slings were initially developed as a treatment for recurrent, urodynamically confirmed SUI, particularly SUI caused by intrinsic sphincter deficiency (ISD). Pubovaginal slings, usually consisting of autologous fascia, were placed at the bladder neck to both support and slightly compress the proximal urethra. Compared with synthetic slings, fascial slings are effective but take longer to place and have a higher rate of surgical morbidity and more postoperative voiding dysfunction. They are now mostly indicated for complex recurrent SUI, usually managed by specialists in female pelvic medicine and reconstructive surgery.
Current slings are lightweight polypropylene mesh
Most slings today are tension-free midurethral slings consisting of synthetic, large-pore polypropylene mesh; they are sold in kits available from several different companies. Sling procedures can also be performed using hand-cut polypropylene mesh and a reusable needle passer.
These slings are placed at the midurethra and work by mechanical kinking or folding of the urethra over the sling, with an increase in intra-abdominal pressure. Ideally, the midurethral sling will not compress the urethra at rest and have no effect on the normal voiding mechanism.
Three main techniques are used to place synthetic midurethral slings:
- the retropubic approach
- the TOT approach
- variations of single-incision “mini-sling” procedures.
Early studies of mini-slings showed few complications but lower effectiveness, compared with retropubic and TOT midurethral slings, according to short-term follow-up data.2-4 A mini-sling might be an option for some patients in whom surgical complications must be kept to a minimum; otherwise, they will not be discussed further.
Retropubic midurethral slings
The tension-free vaginal tape (TVT) procedure described by Petros and Ulmsten was the first synthetic midurethral sling.5 This ambulatory procedure aims to restore the pubourethral ligament and suburethral vaginal hammock by using specially designed needles attached to synthetic sling material.
The synthetic sling consists of polypropylene, approximately 1 cm wide and 40 cm long. The sling material is attached to two stainless steel needles that are passed from a vaginal incision made at the level of the midurethra, through the retropubic space, and exiting at a previously created mark or stab incision in the suprapubic area (FIGURE 1).
Variations of the retropubic midurethral sling have been developed, with sling passers going from the vagina upward (“bottom to top”) and also from the suprapubic area downward (“top to bottom”). A recent Cochrane review reported that the bottom-to-top variation is slightly more effective.6

FIGURE 1 Retropubic sling
Placement of the tension-free vaginal tape trocar into the retropubic space.
Illustration: Craig Zuckerman for OBG Management
Transobturator midurethral slings
The TOT sling has become one of the most popular and effective surgical treatments for female SUI worldwide (Video 1 and Video 2). It is a relatively rapid and low-risk surgery that is comparable to other surgical options in effectiveness while avoiding an abdominal incision and the passage of a needle or trocar through the space of Retzius.
The TOT sling lies flatter under the urethra and carries a lower risk of urethral obstruction, urinary retention, and subsequent need for sling release, compared with retropubic slings.7-9 Compared with the retropubic TVT, the TOT sling produces similar rates of cure, with fewer bladder perforations and less postoperative irritative voiding symptoms.6,10-12 It nearly eliminates the rare but catastrophic risk of bowel or major vessel perforation. The trade-off is that patients experience more complications referable to the groin (pain and leg weakness or numbness) with the TOT approach.9,13
All TOT slings on the market consist of a large-pore, lightweight, polypropylene mesh strip, usually covered with a plastic sheath. Various devices are used to place the sling, but most of them involve a helical trocar that curves around the ischiopubic ramus, passing through the inner thigh and obturator membrane to a space created in the ipsilateral peri-urethral tissues.
TOT slings can be placed outside-to-inside or inside-to-outside (FIGURE 2), and the indications, effectiveness, and frequency of complications seem to be similar between these two approaches.12 One study found a higher frequency of new sexual dysfunction (tender, palpable sling; penile pain in male partner) in women after the “outside-in” approach,14 but this clinical issue has not been observed in all studies.15,16

FIGURE 2 TOT sling variations
Placement of the transobturator (TOT) sling helical trocar using the (A) “outside-in” variation and (B) “inside-out” variation.
Illustration: Craig Zuckerman for OBG Management
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography
© 2005-2012. All Rights Reserved.
Success rates are similar for retropubic and TOT slings
Despite differences in technique and brand of mesh used, treatment success rates for uncomplicated primary SUI are similar for the retropubic (Video 3) and TOT tension-free slings.6-8,10-12,17 The percentage of patients treated successfully depends on the definition used, ranging from a high of 96% to a low of 60%. When the definition of success is restricted to stress incontinence symptoms, especially over a short period of time, the reported effectiveness is high.
In contrast, when the definition of success includes incontinence of any type, the reported effectiveness is lower. For example, in the study that reported 60% effectiveness, success was defined as no incontinence symptoms of any type, a negative cough stress test, and no retreatment for stress incontinence or postoperative urinary retention.11
Retropubic slings, especially TVT, may be somewhat more effective for ISD,18-20 although this conclusion must be tempered by the small number of studies addressing the issue and differences in the diagnosis of ISD.21
Some studies have reported good success in treating mixed urinary incontinence with the retropubic and TOT slings,2,8 although other studies have reported that the initial benefit for urgency or urge incontinence is not sustained over time, compared with the benefit for stress incontinence.22 It is important to counsel patients before surgery that improvement in stress incontinence symptoms and general satisfaction is highly likely, but perfect bladder function is not.
Serious complications are uncommon
Complications are common after both retropubic and TOT slings, although serious complications are uncommon. Cystitis and temporary voiding difficulties are the most common problems after a sling procedure. If the patient is unable to void on the day of surgery, it is reasonable to discharge her with a Foley catheter in place for a few days or teach her to perform intermittent self-catheterization at home. In most cases, normal voiding will resume within a few days. Cystitis is at least partially related to the surgery itself and the duration of postoperative catheterization.
The frequency of some complications differs between the retropubic and TOT approaches to midurethral slings. For example, some literature suggests that irritative voiding symptoms such as urgency or voiding difficulty are somewhat less common after TOT slings, compared with retropubic slings. However, symptoms referable to the groin (pain and leg weakness or numbness) occur more commonly with the TOT approach.6
After placement of a TOT sling, 10% to 15% of women experience temporary inner thigh or groin pain or leg weakness and are usually managed conservatively with nonsteroidal anti-inflammatory drugs and physical therapy. Long-term or severe complications related to TOT sling passage are rare.
Major intraoperative complications are rare
The rate of these complications does not differ between retropubic and TOT approaches. Minor intraoperative complications—primarily, bladder perforation—occur more commonly with the retropubic approach.6
Bladder perforation with the TVT occurs in 4% to 7% of patients. However, the clinical significance of bladder perforation is minimal as long as the surgeon performs careful cystoscopy, recognizes bladder perforation, and repositions the trocar and mesh outside the bladder lumen. Bladder perforation caused by the trocar usually does not require specific treatment (except repositioning of the trocar outside the bladder lumen) and rarely results in later problems.
Mesh exposures occur with similar frequency for the different sling types as long as large-pore lightweight polypropylene is used. Dehiscence of the suburethral incision (mesh exposure) is uncommon with midurethral slings, occurring in 1% to 2% of patients. Dehiscence can be managed with estrogen cream or trimming of the exposed portion of the sling in the office. If symptoms or signs persist, removal of the exposed segment or the entire central portion of the sling, with closure of the vaginal epithelium, is indicated to allow for healing and resolution of symptoms. However, removal may lead to recurrence of the original SUI symptoms.
Retropubic hematomas occur in 1% to 2% of patients after placement of a retropubic sling, but major vascular injuries are rare—occurring in, perhaps, 3 in every 1,000 cases.
Bowel perforations are very rare but serious complications. A retropubic sling should be placed with caution or avoided in women who have a history of peritonitis, bowel surgery, ruptured appendix, or known extensive pelvic adhesions.
Major vascular injuries are also rare with TOT slings, occurring in approximately 1 to 2 cases in every 1,000.
Bladder injury occurs much less frequently after placement of a TOT sling, compared with the retropubic approach, although one study reported bladder injury in 2% of TOT cases.17 Although bladder injury is uncommon with the TOT approach, the morbidity associated with delayed detection of bladder injury is much higher than the morbidity associated with intraoperative detection and management. Therefore, we believe that cystoscopy should be performed in all TOT and retropubic sling procedures to either exclude bladder damage or detect and appropriately manage it.
For reassurance that intraoperative and postoperative blood loss is not excessive, it is reasonable to check one hemoglobin level before discharge, if desired.
How to individualize the choice of sling
Patients who have primary SUI: Retropubic or TOT sling. Objective and subjective success rates are similar, regardless of approach, and serious complications are infrequent. The retropubic approach has longer-term evidence of sustained benefit, compared with the newer TOT approach. We tend to treat younger patients with TVT and older patients with TOT. Surgeon experience and informed patient preferences may dictate the choice of sling (TABLE).
What we recommend surgically for our patients who have SUI—and why
| Clinical problem and patient characteristics | Surgery | Rationale |
|---|---|---|
| Primary SUI with urethral hypermobility—young patient | TVT | TVT has similar effectiveness and more long-term data than TOT; TVT may result in less sexual pain than TOT |
| Primary SUI with urethral hypermobility—older patient; leak point pressure >60 cmH20 | TOT | Similar effectiveness, fewer complications with TOT |
| Recurrent SUI with urethral hypermobility—any age; leak point pressure >60 cmH20 | TVT | Limited data suggest effectiveness of TVT after TOT failure |
| Recurrent SUI with urethral hypermobility—leak point pressure <60 cmH20 (ISD) | TVT or pubovaginal fascial sling | Some but not all data indicate that TVT is more effective for ISD; fascial slings in expert hands are effective, based on cohort studies |
| Recurrent SUI with nonmobile bladder neck; any leak point pressure | Urethral bulking | All sling procedures have lowered effectiveness when the bladder neck is immobile |
| SUI mixed with dominant urgency or voiding dysfunction | TOT | TOT improves or does not exacerbate mixed urinary symptoms to the extent that TVT may |
| SUI with prolapse and planned vaginal prolapse repair | TVT or TOT | Limited data support similar effectiveness for either approach |
| “Occult” SUI with prolapse reduced and planned vaginal prolapse repair | TOT or “wait and see” | TOT has a lower chance of creating new irritative voiding symptoms; “wait-and-see” approach allows treatment of SUI if it develops after prolapse repair |
| Recurrent SUI with previous synthetic sling mesh complication (or patients who desire treatment without mesh) | Pubovaginal fascial sling or Burch colposuspension | These nonmesh options are effective for recurrent SUI, but have higher surgical morbidity |
| ISD = intrinsic sphincter deficiency; SUI = stress urinary incontinence; TVT = tension-free vaginal tape or similar retropubic midurethral sling; TOT = transobturator sling placed either by outside-in or inside-out variations | ||
Patients who have recurrent SUI: Retropubic sling. Comparative data are limited regarding the retropubic and TOT approaches for recurrent SUI that does not involve ISD. One case series reported good results with the use of retropubic TVT for recurrent SUI after an initial TOT approach.23
Patients who have ISD: Retropubic sling (synthetic midurethral sling or fascial sling placed at the bladder neck). A few studies suggest that patients with ISD have better outcomes with the retropubic approach.19,20 However, with differing definitions of ISD and relatively few patients with ISD included in these trials, it is not possible to conclude definitively that the retropubic approach is more effective than the TOT approach for patients who have SUI and ISD. However, the retropubic approach has longer-term data to support its effectiveness; therefore, with some but not all evidence suggesting its superiority for ISD, it is reasonable to choose the retropubic midurethral approach.
A pubovaginal fascial sling placed at the proximal urethra is also an effective option, based on numerous cohort studies.12
Patients who have recurrent SUI or ISD, or both, with a non-mobile bladder neck: Urethral bulking. Although data are scant, urethral injection therapy is beneficial for SUI in the short-term, but long-term studies are lacking. Bulking agents include silicone particles, calcium hydroxylapatite, and carbon spheres; studies have not shown one to be more or less efficacious than the others.24
It is reasonable to use urethral bulking first in these patients as the morbidity is very low and some patients become continent. A retropubic sling can be performed if urethral bulking fails to adequately improve symptoms, although the effectiveness is lower in this population than in women with SUI and urethral hypermobility.
Patients who have mixed stress and urge incontinence or voiding dysfunction: TOT sling. Limited data suggest that the TOT approach improves symptoms of mixed incontinence—or, at least, exacerbates them to a lesser degree than the retropubic approach. Rarely is a sling release needed to treat obstructive urinary symptoms after the TOT approach.
Patients who have prolapse and SUI: Retropubic or TOT sling. When a sling procedure is performed at the same time as reconstructive surgery for prolapse, it has similar effectiveness, regardless of whether the retropubic or TOT approach is selected.8,11 A sling placed during prolapse surgery (placed through a separate midurethral incision) appears to be as effective as a sling placed as a sole procedure.
Patients who have prolapse and occult SUI: TOT sling. If you recommend a sling to prevent SUI after prolapse surgery by any route, pick the sling with acceptable efficacy and the lowest rates of complications and voiding dysfunction. Patients are especially intolerant of complications from a sling to prevent SUI. Given the ease of placement and low morbidity of a later outpatient sling procedure, it is also reasonable to offer patients the “wait-and-see” alternative to see if SUI develops after prolapse surgery and only then proceeding with sling surgery. In this way, overtreatment is avoided, and any complications that occur after sling surgery for SUI treatment may be better tolerated by the patient. The preferences of an informed patient may guide decisions in this setting.
Patients who have recurrent SUI with mesh complication: Pubovaginal fascial sling or Burch colposuspension. These non-mesh options are effective for recurrent SUI and can be performed at the same time as mesh removal. They carry higher surgical morbidity, longer operative time, and greater postoperative voiding dysfunction.
An informed patient can help guide the approach
The retropubic and TOT approaches to tension-free midurethral slings are similar in effectiveness. Most women experience significant improvement of SUI symptoms after sling placement, although many women continue to have some urinary symptoms.
Depending on their training, experience, and personal results—as well as the preferences of an informed patient—surgeons may recommend one approach over the other. In addition, certain clinical situations may favor one sling over another. Studies with longer-term follow-up in different patient subgroups are needed to adequately counsel women about the durability of results.
CASE: Resolved
After discussing the options with your patient, she opts to undergo anterior prolapse repair with concurrent placement of a TOT sling. The surgery is completed without complication. She is discharged later that day without a catheter after demonstrating normal voiding with low residual urine volume. Postoperatively, she reports mild pain referred to the groin. You instruct her to take nonsteroidal anti-inflammatory drugs for pain relief. On her postoperative visit, she reports that the pain is gone and the SUI has almost completely resolved.
We want to hear from you! Tell us what you think.
- Placement of TVT-O transobturator tape
- Monarc transobturator procedure
- TVT Exact retropubic sling operation
These videos were selected by Mark D. Walters, MD, and presented courtesy of the International Academy of Pelvic Surgery (IAPS).
Only 15 years ago, when surgery was recommended for patients who had bothersome stress urinary incontinence (SUI), they were offered operations such as suburethral (Kelly) plication, needle urethropexy, open or laparoscopic Burch procedure, and pubovaginal fascial sling procedure. Today, virtually all of these operations have been replaced in general practice by retropubic or transobturator (TOT) midurethral synthetic slings.
Although Burch colposuspension and the pubovaginal fascial sling procedure are effective for both primary and recurrent SUI, they are more invasive than midurethral slings, cause more voiding dysfunction, and have significantly longer recovery times, making them less attractive for most primary and recurrent cases of SUI.
The evolution of SUI surgeries has shifted so far toward midurethral slings that Burch colposuspension and the pubovaginal sling procedure are rarely performed or taught in obstetrics and gynecology or urology residency programs; these procedures are now mostly done in fellowship programs by specialists in female pelvic medicine and reconstructive surgery.
In this article, we describe how an ObGyn generalist can approach the surgical treatment of women who have either primary or recurrent SUI. Using evidence-based principles, when available, we also discuss how different clinical characteristics—as well as the characteristics of the available slings—affect the suitability of the sling for individual patients.
One caveat: This article assumes that the surgeon knows how to, and is able to, perform retropubic and TOT sling procedures equally well. However, when this is not the case, the surgeon should perform the sling procedure that she or he does best, assuming that it is appropriate for that particular patient.

Almost all surgical procedures for stress urinary incontinence performed today involve placement of a retropubic or transobturator midurethral synthetic sling.
Illustration: Craig Zuckerman for OBG Management
CASE: SUI and Stage II anterior vaginal prolapse
A healthy 45-year-old G2P2 woman complains of a 5-year history of worsening SUI symptoms, mostly occurring during activities such as coughing, laughing, and running. The incontinence has become so severe that she requires several pads daily. She is able to void without difficulty or pain, and her bowel movements are normal. She has regular menses, has had a tubal ligation, and is sexually active.
She reports that she has been performing daily Kegel pelvic muscle exercises, without improvement.
On physical examination, she is found to have Stage II anterior vaginal prolapse and urethral hypermobility, with normal uterine and posterior vaginal support. The uterus and ovaries are of normal size.
A full bladder stress test in the office reveals immediate loss of urine from the urethra upon coughing in a semi-sitting position. She voids 325 mL after the examination and has a post-void residual urine volume, as measured by ultrasonography (US), of 25 mL. Urinalysis is negative.
When discussing her goals, the patient expresses a desire for a cure of her urinary incontinence, if possible.
What further testing and treatment options do you offer to her?
If you and the patient agree that surgery is warranted, which procedure do you recommend?
Recommended assessment of women who report SUI
Women who have bothersome urine loss during activities such as exercise, coughing, or laughing should undergo a history, physical examination, and urinalysis. During the pelvic examination, it is important to assess pelvic organ support defects, especially those involving the anterior vagina and urethra. Also note levator ani muscle contraction and strength. In addition, you can use this time to discuss whether the patient is doing, or has done, pelvic muscle (Kegel) exercises; teach the exercises, if necessary; and encourage her to do them in the future.
If the patient has no urinary infection, has performed Kegel exercises without further benefit, and wishes to consider surgical treatment, basic assessment of lower urinary function is indicated. Basic office urodynamic testing includes:
- a measured void
- measurement of post-void residual volume (by catheter or US)
- assessment of bladder sensation and capacity
- provocation for overactive bladder
- a full-bladder cough stress test (a positive test is direct observation of urethral loss of urine upon coughing).
Patients who have a complex history or mixed symptoms, previous failed surgery, or other characteristics that suggest a diagnosis other than simple SUI should undergo formal electronic urodynamic testing.1
Patient selection criteria
Primary sling surgery is an option for patients who have:
- no urinary infection
- normal voiding and bladder-filling function
- urethral hypermobility on examination
- SUI on a cough-stress test
- failure to improve sufficiently with pelvic muscle exercises.
Suburethral slings were initially developed as a treatment for recurrent, urodynamically confirmed SUI, particularly SUI caused by intrinsic sphincter deficiency (ISD). Pubovaginal slings, usually consisting of autologous fascia, were placed at the bladder neck to both support and slightly compress the proximal urethra. Compared with synthetic slings, fascial slings are effective but take longer to place and have a higher rate of surgical morbidity and more postoperative voiding dysfunction. They are now mostly indicated for complex recurrent SUI, usually managed by specialists in female pelvic medicine and reconstructive surgery.
Current slings are lightweight polypropylene mesh
Most slings today are tension-free midurethral slings consisting of synthetic, large-pore polypropylene mesh; they are sold in kits available from several different companies. Sling procedures can also be performed using hand-cut polypropylene mesh and a reusable needle passer.
These slings are placed at the midurethra and work by mechanical kinking or folding of the urethra over the sling, with an increase in intra-abdominal pressure. Ideally, the midurethral sling will not compress the urethra at rest and have no effect on the normal voiding mechanism.
Three main techniques are used to place synthetic midurethral slings:
- the retropubic approach
- the TOT approach
- variations of single-incision “mini-sling” procedures.
Early studies of mini-slings showed few complications but lower effectiveness, compared with retropubic and TOT midurethral slings, according to short-term follow-up data.2-4 A mini-sling might be an option for some patients in whom surgical complications must be kept to a minimum; otherwise, they will not be discussed further.
Retropubic midurethral slings
The tension-free vaginal tape (TVT) procedure described by Petros and Ulmsten was the first synthetic midurethral sling.5 This ambulatory procedure aims to restore the pubourethral ligament and suburethral vaginal hammock by using specially designed needles attached to synthetic sling material.
The synthetic sling consists of polypropylene, approximately 1 cm wide and 40 cm long. The sling material is attached to two stainless steel needles that are passed from a vaginal incision made at the level of the midurethra, through the retropubic space, and exiting at a previously created mark or stab incision in the suprapubic area (FIGURE 1).
Variations of the retropubic midurethral sling have been developed, with sling passers going from the vagina upward (“bottom to top”) and also from the suprapubic area downward (“top to bottom”). A recent Cochrane review reported that the bottom-to-top variation is slightly more effective.6

FIGURE 1 Retropubic sling
Placement of the tension-free vaginal tape trocar into the retropubic space.
Illustration: Craig Zuckerman for OBG Management
Transobturator midurethral slings
The TOT sling has become one of the most popular and effective surgical treatments for female SUI worldwide (Video 1 and Video 2). It is a relatively rapid and low-risk surgery that is comparable to other surgical options in effectiveness while avoiding an abdominal incision and the passage of a needle or trocar through the space of Retzius.
The TOT sling lies flatter under the urethra and carries a lower risk of urethral obstruction, urinary retention, and subsequent need for sling release, compared with retropubic slings.7-9 Compared with the retropubic TVT, the TOT sling produces similar rates of cure, with fewer bladder perforations and less postoperative irritative voiding symptoms.6,10-12 It nearly eliminates the rare but catastrophic risk of bowel or major vessel perforation. The trade-off is that patients experience more complications referable to the groin (pain and leg weakness or numbness) with the TOT approach.9,13
All TOT slings on the market consist of a large-pore, lightweight, polypropylene mesh strip, usually covered with a plastic sheath. Various devices are used to place the sling, but most of them involve a helical trocar that curves around the ischiopubic ramus, passing through the inner thigh and obturator membrane to a space created in the ipsilateral peri-urethral tissues.
TOT slings can be placed outside-to-inside or inside-to-outside (FIGURE 2), and the indications, effectiveness, and frequency of complications seem to be similar between these two approaches.12 One study found a higher frequency of new sexual dysfunction (tender, palpable sling; penile pain in male partner) in women after the “outside-in” approach,14 but this clinical issue has not been observed in all studies.15,16

FIGURE 2 TOT sling variations
Placement of the transobturator (TOT) sling helical trocar using the (A) “outside-in” variation and (B) “inside-out” variation.
Illustration: Craig Zuckerman for OBG Management
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography
© 2005-2012. All Rights Reserved.
Success rates are similar for retropubic and TOT slings
Despite differences in technique and brand of mesh used, treatment success rates for uncomplicated primary SUI are similar for the retropubic (Video 3) and TOT tension-free slings.6-8,10-12,17 The percentage of patients treated successfully depends on the definition used, ranging from a high of 96% to a low of 60%. When the definition of success is restricted to stress incontinence symptoms, especially over a short period of time, the reported effectiveness is high.
In contrast, when the definition of success includes incontinence of any type, the reported effectiveness is lower. For example, in the study that reported 60% effectiveness, success was defined as no incontinence symptoms of any type, a negative cough stress test, and no retreatment for stress incontinence or postoperative urinary retention.11
Retropubic slings, especially TVT, may be somewhat more effective for ISD,18-20 although this conclusion must be tempered by the small number of studies addressing the issue and differences in the diagnosis of ISD.21
Some studies have reported good success in treating mixed urinary incontinence with the retropubic and TOT slings,2,8 although other studies have reported that the initial benefit for urgency or urge incontinence is not sustained over time, compared with the benefit for stress incontinence.22 It is important to counsel patients before surgery that improvement in stress incontinence symptoms and general satisfaction is highly likely, but perfect bladder function is not.
Serious complications are uncommon
Complications are common after both retropubic and TOT slings, although serious complications are uncommon. Cystitis and temporary voiding difficulties are the most common problems after a sling procedure. If the patient is unable to void on the day of surgery, it is reasonable to discharge her with a Foley catheter in place for a few days or teach her to perform intermittent self-catheterization at home. In most cases, normal voiding will resume within a few days. Cystitis is at least partially related to the surgery itself and the duration of postoperative catheterization.
The frequency of some complications differs between the retropubic and TOT approaches to midurethral slings. For example, some literature suggests that irritative voiding symptoms such as urgency or voiding difficulty are somewhat less common after TOT slings, compared with retropubic slings. However, symptoms referable to the groin (pain and leg weakness or numbness) occur more commonly with the TOT approach.6
After placement of a TOT sling, 10% to 15% of women experience temporary inner thigh or groin pain or leg weakness and are usually managed conservatively with nonsteroidal anti-inflammatory drugs and physical therapy. Long-term or severe complications related to TOT sling passage are rare.
Major intraoperative complications are rare
The rate of these complications does not differ between retropubic and TOT approaches. Minor intraoperative complications—primarily, bladder perforation—occur more commonly with the retropubic approach.6
Bladder perforation with the TVT occurs in 4% to 7% of patients. However, the clinical significance of bladder perforation is minimal as long as the surgeon performs careful cystoscopy, recognizes bladder perforation, and repositions the trocar and mesh outside the bladder lumen. Bladder perforation caused by the trocar usually does not require specific treatment (except repositioning of the trocar outside the bladder lumen) and rarely results in later problems.
Mesh exposures occur with similar frequency for the different sling types as long as large-pore lightweight polypropylene is used. Dehiscence of the suburethral incision (mesh exposure) is uncommon with midurethral slings, occurring in 1% to 2% of patients. Dehiscence can be managed with estrogen cream or trimming of the exposed portion of the sling in the office. If symptoms or signs persist, removal of the exposed segment or the entire central portion of the sling, with closure of the vaginal epithelium, is indicated to allow for healing and resolution of symptoms. However, removal may lead to recurrence of the original SUI symptoms.
Retropubic hematomas occur in 1% to 2% of patients after placement of a retropubic sling, but major vascular injuries are rare—occurring in, perhaps, 3 in every 1,000 cases.
Bowel perforations are very rare but serious complications. A retropubic sling should be placed with caution or avoided in women who have a history of peritonitis, bowel surgery, ruptured appendix, or known extensive pelvic adhesions.
Major vascular injuries are also rare with TOT slings, occurring in approximately 1 to 2 cases in every 1,000.
Bladder injury occurs much less frequently after placement of a TOT sling, compared with the retropubic approach, although one study reported bladder injury in 2% of TOT cases.17 Although bladder injury is uncommon with the TOT approach, the morbidity associated with delayed detection of bladder injury is much higher than the morbidity associated with intraoperative detection and management. Therefore, we believe that cystoscopy should be performed in all TOT and retropubic sling procedures to either exclude bladder damage or detect and appropriately manage it.
For reassurance that intraoperative and postoperative blood loss is not excessive, it is reasonable to check one hemoglobin level before discharge, if desired.
How to individualize the choice of sling
Patients who have primary SUI: Retropubic or TOT sling. Objective and subjective success rates are similar, regardless of approach, and serious complications are infrequent. The retropubic approach has longer-term evidence of sustained benefit, compared with the newer TOT approach. We tend to treat younger patients with TVT and older patients with TOT. Surgeon experience and informed patient preferences may dictate the choice of sling (TABLE).
What we recommend surgically for our patients who have SUI—and why
| Clinical problem and patient characteristics | Surgery | Rationale |
|---|---|---|
| Primary SUI with urethral hypermobility—young patient | TVT | TVT has similar effectiveness and more long-term data than TOT; TVT may result in less sexual pain than TOT |
| Primary SUI with urethral hypermobility—older patient; leak point pressure >60 cmH20 | TOT | Similar effectiveness, fewer complications with TOT |
| Recurrent SUI with urethral hypermobility—any age; leak point pressure >60 cmH20 | TVT | Limited data suggest effectiveness of TVT after TOT failure |
| Recurrent SUI with urethral hypermobility—leak point pressure <60 cmH20 (ISD) | TVT or pubovaginal fascial sling | Some but not all data indicate that TVT is more effective for ISD; fascial slings in expert hands are effective, based on cohort studies |
| Recurrent SUI with nonmobile bladder neck; any leak point pressure | Urethral bulking | All sling procedures have lowered effectiveness when the bladder neck is immobile |
| SUI mixed with dominant urgency or voiding dysfunction | TOT | TOT improves or does not exacerbate mixed urinary symptoms to the extent that TVT may |
| SUI with prolapse and planned vaginal prolapse repair | TVT or TOT | Limited data support similar effectiveness for either approach |
| “Occult” SUI with prolapse reduced and planned vaginal prolapse repair | TOT or “wait and see” | TOT has a lower chance of creating new irritative voiding symptoms; “wait-and-see” approach allows treatment of SUI if it develops after prolapse repair |
| Recurrent SUI with previous synthetic sling mesh complication (or patients who desire treatment without mesh) | Pubovaginal fascial sling or Burch colposuspension | These nonmesh options are effective for recurrent SUI, but have higher surgical morbidity |
| ISD = intrinsic sphincter deficiency; SUI = stress urinary incontinence; TVT = tension-free vaginal tape or similar retropubic midurethral sling; TOT = transobturator sling placed either by outside-in or inside-out variations | ||
Patients who have recurrent SUI: Retropubic sling. Comparative data are limited regarding the retropubic and TOT approaches for recurrent SUI that does not involve ISD. One case series reported good results with the use of retropubic TVT for recurrent SUI after an initial TOT approach.23
Patients who have ISD: Retropubic sling (synthetic midurethral sling or fascial sling placed at the bladder neck). A few studies suggest that patients with ISD have better outcomes with the retropubic approach.19,20 However, with differing definitions of ISD and relatively few patients with ISD included in these trials, it is not possible to conclude definitively that the retropubic approach is more effective than the TOT approach for patients who have SUI and ISD. However, the retropubic approach has longer-term data to support its effectiveness; therefore, with some but not all evidence suggesting its superiority for ISD, it is reasonable to choose the retropubic midurethral approach.
A pubovaginal fascial sling placed at the proximal urethra is also an effective option, based on numerous cohort studies.12
Patients who have recurrent SUI or ISD, or both, with a non-mobile bladder neck: Urethral bulking. Although data are scant, urethral injection therapy is beneficial for SUI in the short-term, but long-term studies are lacking. Bulking agents include silicone particles, calcium hydroxylapatite, and carbon spheres; studies have not shown one to be more or less efficacious than the others.24
It is reasonable to use urethral bulking first in these patients as the morbidity is very low and some patients become continent. A retropubic sling can be performed if urethral bulking fails to adequately improve symptoms, although the effectiveness is lower in this population than in women with SUI and urethral hypermobility.
Patients who have mixed stress and urge incontinence or voiding dysfunction: TOT sling. Limited data suggest that the TOT approach improves symptoms of mixed incontinence—or, at least, exacerbates them to a lesser degree than the retropubic approach. Rarely is a sling release needed to treat obstructive urinary symptoms after the TOT approach.
Patients who have prolapse and SUI: Retropubic or TOT sling. When a sling procedure is performed at the same time as reconstructive surgery for prolapse, it has similar effectiveness, regardless of whether the retropubic or TOT approach is selected.8,11 A sling placed during prolapse surgery (placed through a separate midurethral incision) appears to be as effective as a sling placed as a sole procedure.
Patients who have prolapse and occult SUI: TOT sling. If you recommend a sling to prevent SUI after prolapse surgery by any route, pick the sling with acceptable efficacy and the lowest rates of complications and voiding dysfunction. Patients are especially intolerant of complications from a sling to prevent SUI. Given the ease of placement and low morbidity of a later outpatient sling procedure, it is also reasonable to offer patients the “wait-and-see” alternative to see if SUI develops after prolapse surgery and only then proceeding with sling surgery. In this way, overtreatment is avoided, and any complications that occur after sling surgery for SUI treatment may be better tolerated by the patient. The preferences of an informed patient may guide decisions in this setting.
Patients who have recurrent SUI with mesh complication: Pubovaginal fascial sling or Burch colposuspension. These non-mesh options are effective for recurrent SUI and can be performed at the same time as mesh removal. They carry higher surgical morbidity, longer operative time, and greater postoperative voiding dysfunction.
An informed patient can help guide the approach
The retropubic and TOT approaches to tension-free midurethral slings are similar in effectiveness. Most women experience significant improvement of SUI symptoms after sling placement, although many women continue to have some urinary symptoms.
Depending on their training, experience, and personal results—as well as the preferences of an informed patient—surgeons may recommend one approach over the other. In addition, certain clinical situations may favor one sling over another. Studies with longer-term follow-up in different patient subgroups are needed to adequately counsel women about the durability of results.
CASE: Resolved
After discussing the options with your patient, she opts to undergo anterior prolapse repair with concurrent placement of a TOT sling. The surgery is completed without complication. She is discharged later that day without a catheter after demonstrating normal voiding with low residual urine volume. Postoperatively, she reports mild pain referred to the groin. You instruct her to take nonsteroidal anti-inflammatory drugs for pain relief. On her postoperative visit, she reports that the pain is gone and the SUI has almost completely resolved.
We want to hear from you! Tell us what you think.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #63: Urinary incontinence in women. Obstet Gynecol. 2005;105(6):1533-1545.
2. Abdel-Fattah M, Ford JA, Lim CP, Madhuvrata P. Single-incision mini-slings versus standard midurethral slings in surgical management of female stress urinary incontinence: a meta-analysis of effectiveness and complications. Eur Urol. 2011;60(3):468-480.
3. Hinoul P, Vervest HA, den Boon J, et al. A randomized, controlled trial comparing an innovative single-incision sling with an established transobturator sling to treat female stress urinary incontinence. J Urol. 2011;185(4):1356-1362.
4. Barber MD, Weidner AC, Sokol AI, et al. Foundation for Female Health Awareness Research Network. Single-incision mini-sling compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2012;119(2 pt 1):328-337.
5. Petros P, Ulmsten U. Intravaginal sling plasty (IVS): An ambulatory surgical procedure for treatment of female urinary stress incontinence. Scand J Urol Nephrol. 1995;29(1):75-82.
6. Ogah J, Cody DJ, Rogerson L. Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women: a short version Cochrane review. Neurourol Urodyn. 2011;30(3):284-291.
7. Meschia M, Bertozzi R, Pifarotti P, et al. Perioperative morbidity and early results of a randomised trial comparing TVT and TVT-O. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(11):1257-1261.
8. Richter HE, Albo ME, Zyczynski HM, et al. Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362(22):2066-2076.
9. Brubaker L, Norton PA, Albo ME, et al. Urinary Incontinence Treatment Network. Adverse events over two years after retropubic or transobturator midurethral sling surgery: findings from the Trial of Midurethral Slings (TOMUS) study. Am J Obstet Gynecol. 2011;205(5):498.e1-6.
10. Sung VW, Schleinitz MD, Rardin CR, Ward RM, Myers DL. Comparison of retropubic vs transobturator approach to midurethral slings: a systematic review and meta-analysis. Am J Obstet Gynecol. 2007;197(1):3-11.
11. Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2008;111(3):611-621.
12. Novara G, Artibani W, Barber MD, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2010;58(2):218-238.
13. Ross S, Robert M, Swaby C, et al. Transobturator tape compared with tension-free vaginal tape for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2009;114(6):1287-1294.
14. Scheiner DA, Betschart C, Wiederkehr S, Seifert B, Fink D, Perucchini D. Twelve months effect on voiding function of retropubic compared with outside-in and inside-out transobturator midurethral slings. Int Urogynecol J. 2012;23(2):197-206.
15. De Souza A, Dwyer PL, Rosamilia A, et al. Sexual function following retropubic TVT and transobturator Monarc sling in women with intrinsic sphincter deficiency: a multicenter prospective study. Int Urogynecol J. 2012;23(2):153-158.
16. Sentilhes L, Berthier A, Loisel C, Descamps P, Marpeau L, Grise P. Female sexual function following surgery for stress urinary incontinence: tension-free vaginal versus transobturator tape procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(4):393-399.
17. Deffieux X, Daher N, Mansoor A, Debodinance P, Muhlstein J, Fernandez H. Transobturator TVT-O versus retropubic TVT: results of a multicenter randomized controlled trial at 24 months follow-up. Int Urogynecol J. 2010;21(11):1337-1345.
18. Schierlitz L, Dwyer PL, Rosamilia A, et al. Effectiveness of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency: a randomized controlled trial. Obstet Gynecol. 2008;112(6):1253-1261.
19. Rechberger T, Futyma K, Jankiewicz K, Adamiak A, Skorupski P. The clinical effectiveness of retropubic (IVS-02) and transobturator (IVS-04) midurethral slings: randomized trial. Eur Urol. 2009;56(1):24-30.
20. Schierlitz L, Dwyer PL, Rosamilia AN, et al. Three-year follow-up of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency. Obstet Gynecol. 2012;119(2 pt 1):321-327.
21. Nager C, Siris L, Litman HJ, et al. Urinary Incontinence Treatment Network. Baseline urodynamic predictors of treatment failure 1 year after midurethral sling surgery. J Urol. 2011;186(2):597-603.
22. Jain P, Jirschele K, Botros SM, Latthe PM. Effectiveness of midurethral slings in mixed urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J. 2011;22(8):923-932.
23. Sabadell J, Poza JL, Esgueva A, Morales JC, Sanchez-Iglesias JL, Xercavins J. Usefulness of retropubic tape for recurrent stress incontinence after transobturator tape failure. Int Urogynecol J. 2011;22(12):1543-1547.
24. Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2012; Feb 15;2:CD003881.-
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #63: Urinary incontinence in women. Obstet Gynecol. 2005;105(6):1533-1545.
2. Abdel-Fattah M, Ford JA, Lim CP, Madhuvrata P. Single-incision mini-slings versus standard midurethral slings in surgical management of female stress urinary incontinence: a meta-analysis of effectiveness and complications. Eur Urol. 2011;60(3):468-480.
3. Hinoul P, Vervest HA, den Boon J, et al. A randomized, controlled trial comparing an innovative single-incision sling with an established transobturator sling to treat female stress urinary incontinence. J Urol. 2011;185(4):1356-1362.
4. Barber MD, Weidner AC, Sokol AI, et al. Foundation for Female Health Awareness Research Network. Single-incision mini-sling compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2012;119(2 pt 1):328-337.
5. Petros P, Ulmsten U. Intravaginal sling plasty (IVS): An ambulatory surgical procedure for treatment of female urinary stress incontinence. Scand J Urol Nephrol. 1995;29(1):75-82.
6. Ogah J, Cody DJ, Rogerson L. Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women: a short version Cochrane review. Neurourol Urodyn. 2011;30(3):284-291.
7. Meschia M, Bertozzi R, Pifarotti P, et al. Perioperative morbidity and early results of a randomised trial comparing TVT and TVT-O. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(11):1257-1261.
8. Richter HE, Albo ME, Zyczynski HM, et al. Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362(22):2066-2076.
9. Brubaker L, Norton PA, Albo ME, et al. Urinary Incontinence Treatment Network. Adverse events over two years after retropubic or transobturator midurethral sling surgery: findings from the Trial of Midurethral Slings (TOMUS) study. Am J Obstet Gynecol. 2011;205(5):498.e1-6.
10. Sung VW, Schleinitz MD, Rardin CR, Ward RM, Myers DL. Comparison of retropubic vs transobturator approach to midurethral slings: a systematic review and meta-analysis. Am J Obstet Gynecol. 2007;197(1):3-11.
11. Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2008;111(3):611-621.
12. Novara G, Artibani W, Barber MD, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2010;58(2):218-238.
13. Ross S, Robert M, Swaby C, et al. Transobturator tape compared with tension-free vaginal tape for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2009;114(6):1287-1294.
14. Scheiner DA, Betschart C, Wiederkehr S, Seifert B, Fink D, Perucchini D. Twelve months effect on voiding function of retropubic compared with outside-in and inside-out transobturator midurethral slings. Int Urogynecol J. 2012;23(2):197-206.
15. De Souza A, Dwyer PL, Rosamilia A, et al. Sexual function following retropubic TVT and transobturator Monarc sling in women with intrinsic sphincter deficiency: a multicenter prospective study. Int Urogynecol J. 2012;23(2):153-158.
16. Sentilhes L, Berthier A, Loisel C, Descamps P, Marpeau L, Grise P. Female sexual function following surgery for stress urinary incontinence: tension-free vaginal versus transobturator tape procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(4):393-399.
17. Deffieux X, Daher N, Mansoor A, Debodinance P, Muhlstein J, Fernandez H. Transobturator TVT-O versus retropubic TVT: results of a multicenter randomized controlled trial at 24 months follow-up. Int Urogynecol J. 2010;21(11):1337-1345.
18. Schierlitz L, Dwyer PL, Rosamilia A, et al. Effectiveness of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency: a randomized controlled trial. Obstet Gynecol. 2008;112(6):1253-1261.
19. Rechberger T, Futyma K, Jankiewicz K, Adamiak A, Skorupski P. The clinical effectiveness of retropubic (IVS-02) and transobturator (IVS-04) midurethral slings: randomized trial. Eur Urol. 2009;56(1):24-30.
20. Schierlitz L, Dwyer PL, Rosamilia AN, et al. Three-year follow-up of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency. Obstet Gynecol. 2012;119(2 pt 1):321-327.
21. Nager C, Siris L, Litman HJ, et al. Urinary Incontinence Treatment Network. Baseline urodynamic predictors of treatment failure 1 year after midurethral sling surgery. J Urol. 2011;186(2):597-603.
22. Jain P, Jirschele K, Botros SM, Latthe PM. Effectiveness of midurethral slings in mixed urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J. 2011;22(8):923-932.
23. Sabadell J, Poza JL, Esgueva A, Morales JC, Sanchez-Iglesias JL, Xercavins J. Usefulness of retropubic tape for recurrent stress incontinence after transobturator tape failure. Int Urogynecol J. 2011;22(12):1543-1547.
24. Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2012; Feb 15;2:CD003881.-
10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
- Update on contraception
Rachel B. Rapkin, MD; Mitchell D. Creinin, MD (August 2011) - An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate!
Andrew M. Kaunitz, MD; David A. Grimes, MD (August 2011) - Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other?
Robert L. Barbieri, MD (Editorial; March 2011) - IUD use in nulliparous and adolescent women
Jennefer A. Russo, MD; Mitchell D. Creinin, MD (Update on Contraception, August 2010)
EDITOR’S NOTE: Brand names are given parenthetically in some places in the text solely to provide better recognition of methods discussed.
As other articles in this issue of OBG Management attest, medical science continues to focus attention on improving methods of family planning. That emphasis has meant a regular flow of new reports, studies, and guidelines for you to absorb and translate into better practice—no easy task.
Here is help: 10 (+ 1) practical, sensible recommendations for improving contraceptive care that have emerged from recent evidence and that are reasonably easy to incorporate into the care you provide. As with previous installments of this occasional “recommendations” series, we include a brief discussion and pertinent references for each tip.
1. Pelvic exam? It isn’t mandatory.
Do not require pelvic examination before you prescribe an oral contraceptive.
Henderson JT, Sawaya GF, Blum M, Stratton L, Harper CC. Pelvic examinations and access to oral hormonal contraception. Obstet Gynecol. 2010;116(6):1257–1264.
The World Health Organization and ACOG recommend that you consider pelvic examination optional before prescribing an oral contraceptive (OC). Recent evidence indicates, however, that many health-care providers don’t follow that recommendation. Avoiding an unnecessary pelvic exam is a plus for a patient who may fear the procedure; following this guidance therefore removes a potential barrier to care and saves time in a busy practice.
2. Provide more, not less
Prescribe (when possible, dispense) 6 to 12 months of an OC at office visits.
Potter JE, McKinnon S, Hopkins K, et al. Continuation of prescribed compared with over-the-counter oral contraceptives. Obstet Gynecol. 2011;117(3):551–557.
Foster DG, Hulett D, Bradsberry M, Darney P, Policar M. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117(3):566–572.
Studies show that 1) women who are given six or more pill packages at a clinic visit have a lower discontinuation rate than women given one to five packs and 2) prescribing a 1-year supply of OC pill packages (as opposed to one to three packs) is associated with a 30% reduction in the odds of conceiving an unplanned pregnancy and a 46% reduction in the odds of having an abortion.
3. Make the case for long-acting reversibles
Use intrauterine devices and subdermal implants as first-line contraception more often.
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 59: Intrauterine device. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2005;105(1):223–232.
Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117(5):1105–1113.
Long-acting methods such as the copper intrauterine device (IUD) (Paragard) and the levonorgestrel intrauterine system (LNG-IUS) (Mirena) are the most effective reversible contraceptives because they eliminate the difference between perfect and typical use. A woman at low risk does not need to have a negative cervical culture before having an IUD or the LNG-IUS inserted, and a woman does not need to be on her menses at the time of insertion. In addition, antibiotic prophylaxis is not recommended before or at the time of insertion.
IUDs—and this applies to the subdermal contraceptive implant (Implanon), too—also have the highest rates of satisfaction and 12-month continuation.
4. Take advantage of broader benefits
Use hormonal contraceptives for noncontraceptive indications.
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 110: Noncontraceptive uses of hormonal contraceptives. Obstet Gynecol. 2010;115(6):206–218.
Jensen JT, Parke S, Mellinger U, Machlitt A, Fraser IS. Effective treatment of heavy menstrual bleeding with estradiol valerate and dienogest: a randomized controlled trial. Obstet Gynecol. 2011;117(4):777–787.
Kaunitz AM, Bissonnette F, Monteiro I, Lukkari-Lax E, Muysers C, Jensen JT. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
Hormonal contraceptives have long been used for such indications as cycle control and treatment of acne. The LNG-IUS and OCs are highly effective, compared with placebo, for treating heavy menstrual bleeding in the absence of organic pathology.* As a potential alternative to surgical treatment of menorrhagia, OCs offer even broader benefit for many women.
*Mirena is approved by the Food and Drug Administration for treating heavy menstrual bleeding.
5. To encourage continuation, begin now
Get a “quick start” to improve adherence to oral contraceptives.
Westhoff C, Kerns J, Morroni C, et al. Quick start: novel oral contraceptive initiation method. Contraception. 2002;66(3):141–145.
Starting OC pills immediately—instead of waiting for the Sunday after the next menses—can improve the short-term continuation rate for women patients who choose an OC.
6. Move away from every-day regimens
Consider a nondaily combined method, such as the transdermal patch or the vaginal ring, for current OC users.
Creinin MD, Meyn LA, Borgatta L, et al. Multicenter comparison of the contraceptive ring and patch: a randomized controlled trial. Obstet Gynecol. 2008;111(2 pt 1):267–277.
Many women who use an OC are satisfied with the positive effect the method has on menses and acne but find that they miss taking a pill some days; they might benefit from a method that involves nondaily administration. Studies show that switching from oral contraception to the transdermal patch (OrthoEvra) or vaginal ring (Nuvaring), for example, is acceptable to many women.
7. Preemptive prescribing
Prescribe emergency contraception before your patient needs it.
Jackson RA, Schwarz EB, Freedman L, Darney P. Advance supply of emergency contraception: effect on use and usual contraception—a randomized trial. Obstet Gynecol. 2003;102(1):8–16.
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 112: Emergency contraception. Obstet Gynecol. 2010;115(5):1100–1109.
Consider giving every sexually active woman a prescription for emergency contraception before she leaves your office. She can fill the prescription and keep it at home in case of an emergency.
8. Get to know ella
Become familiar with ulipristal acetate (ella) for emergency contraception.
Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555–562.
Barbieri RL. Levonorgestrel or ulipristal: is one a better emergency contraceptive than the other? OBG Manage. 2011;23(3):8–11.
This new FDA-approved agent for emergency contraception is effective for as long as 5 days after intercourse and results in fewer pregnancies than levonorgestrel does. It is available by prescription only, however, and is more expensive than levonorgestrel.
9. Pursue two urogenital pathogens
When you are not performing a speculum examination, screen for N. gonorrhoeae and C. trachomatis with a vaginal swab specimen or urine-based specimen.
Johnson RE, Newhall WJ, Papp JR, et al. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections–2002. MMWR Recomm Rep. 2002;51(RR-15):1–38; quiz CE1–4.
Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis. 2005;32(12):725–728.
Under new screening guidelines for cervical cancer, Pap smears are not required for women who are younger than 21 years. Gonorrhea and chlamydial infection screening is still important in this population, however, and can be done without a speculum exam.The patient or provider collects a specimen for testing with a vaginal swab, or the patient submits a urine specimen.
10. Now not later: An IUD, post-evacuation
Consider immediate, rather than delayed, IUD insertion after uterine evacuation for spontaneous or elective abortion in women who desire this form of contraception.
Bednarek PH, Creinin MD, Reeves MF, et al. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364:2208-2217.
A recent clinical trial enrolled 575 women who underwent uterine aspiration for induced or spontaneous abortion at 5 to 12 weeks’ gestation and who desired an IUD. Subjects were randomized to IUD insertion immediately after the procedure or 2 to 6 weeks later. The 6-month expulsion rate was 5.0% after immediate insertion; 2.7%, after delayed insertion (P = NS). There were no differences in the rates of other adverse events. Only 71% of patients returned for their “delayed” IUD placement; five pregnancies occurred among these women. No pregnancies occurred in the immediate-insertion group.
Offer office-based hysteroscopic sterilization.
Levie M, Weiss G, Kaiser B, Daif J, Chudnoff SG. Analysis of pain and satisfaction with office-based hysteroscopic sterilization. Fertil Steril. 2010;94(4):1189–1194.
Office hysteroscopy is well tolerated. Two hysteroscopic sterilization systems, Essure and Adiana, are available for use in the office. The systems are especially valuable in women who are poor surgical candidates or who want to avoid the inconvenience, or the risks, of a more major surgical procedure.
We want to hear from you! Tell us what you think.
- 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai, MD (June 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011) - 10 practical, evidence-based suggestions to improve your gyn practice now
Mark D. Walters, MD (January 2011) - 10 practical recommendations to improve maternal and perinatal outcomes in patients with eclampsia
Baha M. Sibai, MD
- Update on contraception
Rachel B. Rapkin, MD; Mitchell D. Creinin, MD (August 2011) - An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate!
Andrew M. Kaunitz, MD; David A. Grimes, MD (August 2011) - Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other?
Robert L. Barbieri, MD (Editorial; March 2011) - IUD use in nulliparous and adolescent women
Jennefer A. Russo, MD; Mitchell D. Creinin, MD (Update on Contraception, August 2010)
EDITOR’S NOTE: Brand names are given parenthetically in some places in the text solely to provide better recognition of methods discussed.
As other articles in this issue of OBG Management attest, medical science continues to focus attention on improving methods of family planning. That emphasis has meant a regular flow of new reports, studies, and guidelines for you to absorb and translate into better practice—no easy task.
Here is help: 10 (+ 1) practical, sensible recommendations for improving contraceptive care that have emerged from recent evidence and that are reasonably easy to incorporate into the care you provide. As with previous installments of this occasional “recommendations” series, we include a brief discussion and pertinent references for each tip.
1. Pelvic exam? It isn’t mandatory.
Do not require pelvic examination before you prescribe an oral contraceptive.
Henderson JT, Sawaya GF, Blum M, Stratton L, Harper CC. Pelvic examinations and access to oral hormonal contraception. Obstet Gynecol. 2010;116(6):1257–1264.
The World Health Organization and ACOG recommend that you consider pelvic examination optional before prescribing an oral contraceptive (OC). Recent evidence indicates, however, that many health-care providers don’t follow that recommendation. Avoiding an unnecessary pelvic exam is a plus for a patient who may fear the procedure; following this guidance therefore removes a potential barrier to care and saves time in a busy practice.
2. Provide more, not less
Prescribe (when possible, dispense) 6 to 12 months of an OC at office visits.
Potter JE, McKinnon S, Hopkins K, et al. Continuation of prescribed compared with over-the-counter oral contraceptives. Obstet Gynecol. 2011;117(3):551–557.
Foster DG, Hulett D, Bradsberry M, Darney P, Policar M. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117(3):566–572.
Studies show that 1) women who are given six or more pill packages at a clinic visit have a lower discontinuation rate than women given one to five packs and 2) prescribing a 1-year supply of OC pill packages (as opposed to one to three packs) is associated with a 30% reduction in the odds of conceiving an unplanned pregnancy and a 46% reduction in the odds of having an abortion.
3. Make the case for long-acting reversibles
Use intrauterine devices and subdermal implants as first-line contraception more often.
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 59: Intrauterine device. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2005;105(1):223–232.
Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117(5):1105–1113.
Long-acting methods such as the copper intrauterine device (IUD) (Paragard) and the levonorgestrel intrauterine system (LNG-IUS) (Mirena) are the most effective reversible contraceptives because they eliminate the difference between perfect and typical use. A woman at low risk does not need to have a negative cervical culture before having an IUD or the LNG-IUS inserted, and a woman does not need to be on her menses at the time of insertion. In addition, antibiotic prophylaxis is not recommended before or at the time of insertion.
IUDs—and this applies to the subdermal contraceptive implant (Implanon), too—also have the highest rates of satisfaction and 12-month continuation.
4. Take advantage of broader benefits
Use hormonal contraceptives for noncontraceptive indications.
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 110: Noncontraceptive uses of hormonal contraceptives. Obstet Gynecol. 2010;115(6):206–218.
Jensen JT, Parke S, Mellinger U, Machlitt A, Fraser IS. Effective treatment of heavy menstrual bleeding with estradiol valerate and dienogest: a randomized controlled trial. Obstet Gynecol. 2011;117(4):777–787.
Kaunitz AM, Bissonnette F, Monteiro I, Lukkari-Lax E, Muysers C, Jensen JT. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
Hormonal contraceptives have long been used for such indications as cycle control and treatment of acne. The LNG-IUS and OCs are highly effective, compared with placebo, for treating heavy menstrual bleeding in the absence of organic pathology.* As a potential alternative to surgical treatment of menorrhagia, OCs offer even broader benefit for many women.
*Mirena is approved by the Food and Drug Administration for treating heavy menstrual bleeding.
5. To encourage continuation, begin now
Get a “quick start” to improve adherence to oral contraceptives.
Westhoff C, Kerns J, Morroni C, et al. Quick start: novel oral contraceptive initiation method. Contraception. 2002;66(3):141–145.
Starting OC pills immediately—instead of waiting for the Sunday after the next menses—can improve the short-term continuation rate for women patients who choose an OC.
6. Move away from every-day regimens
Consider a nondaily combined method, such as the transdermal patch or the vaginal ring, for current OC users.
Creinin MD, Meyn LA, Borgatta L, et al. Multicenter comparison of the contraceptive ring and patch: a randomized controlled trial. Obstet Gynecol. 2008;111(2 pt 1):267–277.
Many women who use an OC are satisfied with the positive effect the method has on menses and acne but find that they miss taking a pill some days; they might benefit from a method that involves nondaily administration. Studies show that switching from oral contraception to the transdermal patch (OrthoEvra) or vaginal ring (Nuvaring), for example, is acceptable to many women.
7. Preemptive prescribing
Prescribe emergency contraception before your patient needs it.
Jackson RA, Schwarz EB, Freedman L, Darney P. Advance supply of emergency contraception: effect on use and usual contraception—a randomized trial. Obstet Gynecol. 2003;102(1):8–16.
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 112: Emergency contraception. Obstet Gynecol. 2010;115(5):1100–1109.
Consider giving every sexually active woman a prescription for emergency contraception before she leaves your office. She can fill the prescription and keep it at home in case of an emergency.
8. Get to know ella
Become familiar with ulipristal acetate (ella) for emergency contraception.
Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555–562.
Barbieri RL. Levonorgestrel or ulipristal: is one a better emergency contraceptive than the other? OBG Manage. 2011;23(3):8–11.
This new FDA-approved agent for emergency contraception is effective for as long as 5 days after intercourse and results in fewer pregnancies than levonorgestrel does. It is available by prescription only, however, and is more expensive than levonorgestrel.
9. Pursue two urogenital pathogens
When you are not performing a speculum examination, screen for N. gonorrhoeae and C. trachomatis with a vaginal swab specimen or urine-based specimen.
Johnson RE, Newhall WJ, Papp JR, et al. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections–2002. MMWR Recomm Rep. 2002;51(RR-15):1–38; quiz CE1–4.
Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis. 2005;32(12):725–728.
Under new screening guidelines for cervical cancer, Pap smears are not required for women who are younger than 21 years. Gonorrhea and chlamydial infection screening is still important in this population, however, and can be done without a speculum exam.The patient or provider collects a specimen for testing with a vaginal swab, or the patient submits a urine specimen.
10. Now not later: An IUD, post-evacuation
Consider immediate, rather than delayed, IUD insertion after uterine evacuation for spontaneous or elective abortion in women who desire this form of contraception.
Bednarek PH, Creinin MD, Reeves MF, et al. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364:2208-2217.
A recent clinical trial enrolled 575 women who underwent uterine aspiration for induced or spontaneous abortion at 5 to 12 weeks’ gestation and who desired an IUD. Subjects were randomized to IUD insertion immediately after the procedure or 2 to 6 weeks later. The 6-month expulsion rate was 5.0% after immediate insertion; 2.7%, after delayed insertion (P = NS). There were no differences in the rates of other adverse events. Only 71% of patients returned for their “delayed” IUD placement; five pregnancies occurred among these women. No pregnancies occurred in the immediate-insertion group.
Offer office-based hysteroscopic sterilization.
Levie M, Weiss G, Kaiser B, Daif J, Chudnoff SG. Analysis of pain and satisfaction with office-based hysteroscopic sterilization. Fertil Steril. 2010;94(4):1189–1194.
Office hysteroscopy is well tolerated. Two hysteroscopic sterilization systems, Essure and Adiana, are available for use in the office. The systems are especially valuable in women who are poor surgical candidates or who want to avoid the inconvenience, or the risks, of a more major surgical procedure.
We want to hear from you! Tell us what you think.
- 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai, MD (June 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011) - 10 practical, evidence-based suggestions to improve your gyn practice now
Mark D. Walters, MD (January 2011) - 10 practical recommendations to improve maternal and perinatal outcomes in patients with eclampsia
Baha M. Sibai, MD
- Update on contraception
Rachel B. Rapkin, MD; Mitchell D. Creinin, MD (August 2011) - An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate!
Andrew M. Kaunitz, MD; David A. Grimes, MD (August 2011) - Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other?
Robert L. Barbieri, MD (Editorial; March 2011) - IUD use in nulliparous and adolescent women
Jennefer A. Russo, MD; Mitchell D. Creinin, MD (Update on Contraception, August 2010)
EDITOR’S NOTE: Brand names are given parenthetically in some places in the text solely to provide better recognition of methods discussed.
As other articles in this issue of OBG Management attest, medical science continues to focus attention on improving methods of family planning. That emphasis has meant a regular flow of new reports, studies, and guidelines for you to absorb and translate into better practice—no easy task.
Here is help: 10 (+ 1) practical, sensible recommendations for improving contraceptive care that have emerged from recent evidence and that are reasonably easy to incorporate into the care you provide. As with previous installments of this occasional “recommendations” series, we include a brief discussion and pertinent references for each tip.
1. Pelvic exam? It isn’t mandatory.
Do not require pelvic examination before you prescribe an oral contraceptive.
Henderson JT, Sawaya GF, Blum M, Stratton L, Harper CC. Pelvic examinations and access to oral hormonal contraception. Obstet Gynecol. 2010;116(6):1257–1264.
The World Health Organization and ACOG recommend that you consider pelvic examination optional before prescribing an oral contraceptive (OC). Recent evidence indicates, however, that many health-care providers don’t follow that recommendation. Avoiding an unnecessary pelvic exam is a plus for a patient who may fear the procedure; following this guidance therefore removes a potential barrier to care and saves time in a busy practice.
2. Provide more, not less
Prescribe (when possible, dispense) 6 to 12 months of an OC at office visits.
Potter JE, McKinnon S, Hopkins K, et al. Continuation of prescribed compared with over-the-counter oral contraceptives. Obstet Gynecol. 2011;117(3):551–557.
Foster DG, Hulett D, Bradsberry M, Darney P, Policar M. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117(3):566–572.
Studies show that 1) women who are given six or more pill packages at a clinic visit have a lower discontinuation rate than women given one to five packs and 2) prescribing a 1-year supply of OC pill packages (as opposed to one to three packs) is associated with a 30% reduction in the odds of conceiving an unplanned pregnancy and a 46% reduction in the odds of having an abortion.
3. Make the case for long-acting reversibles
Use intrauterine devices and subdermal implants as first-line contraception more often.
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 59: Intrauterine device. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2005;105(1):223–232.
Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117(5):1105–1113.
Long-acting methods such as the copper intrauterine device (IUD) (Paragard) and the levonorgestrel intrauterine system (LNG-IUS) (Mirena) are the most effective reversible contraceptives because they eliminate the difference between perfect and typical use. A woman at low risk does not need to have a negative cervical culture before having an IUD or the LNG-IUS inserted, and a woman does not need to be on her menses at the time of insertion. In addition, antibiotic prophylaxis is not recommended before or at the time of insertion.
IUDs—and this applies to the subdermal contraceptive implant (Implanon), too—also have the highest rates of satisfaction and 12-month continuation.
4. Take advantage of broader benefits
Use hormonal contraceptives for noncontraceptive indications.
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 110: Noncontraceptive uses of hormonal contraceptives. Obstet Gynecol. 2010;115(6):206–218.
Jensen JT, Parke S, Mellinger U, Machlitt A, Fraser IS. Effective treatment of heavy menstrual bleeding with estradiol valerate and dienogest: a randomized controlled trial. Obstet Gynecol. 2011;117(4):777–787.
Kaunitz AM, Bissonnette F, Monteiro I, Lukkari-Lax E, Muysers C, Jensen JT. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
Hormonal contraceptives have long been used for such indications as cycle control and treatment of acne. The LNG-IUS and OCs are highly effective, compared with placebo, for treating heavy menstrual bleeding in the absence of organic pathology.* As a potential alternative to surgical treatment of menorrhagia, OCs offer even broader benefit for many women.
*Mirena is approved by the Food and Drug Administration for treating heavy menstrual bleeding.
5. To encourage continuation, begin now
Get a “quick start” to improve adherence to oral contraceptives.
Westhoff C, Kerns J, Morroni C, et al. Quick start: novel oral contraceptive initiation method. Contraception. 2002;66(3):141–145.
Starting OC pills immediately—instead of waiting for the Sunday after the next menses—can improve the short-term continuation rate for women patients who choose an OC.
6. Move away from every-day regimens
Consider a nondaily combined method, such as the transdermal patch or the vaginal ring, for current OC users.
Creinin MD, Meyn LA, Borgatta L, et al. Multicenter comparison of the contraceptive ring and patch: a randomized controlled trial. Obstet Gynecol. 2008;111(2 pt 1):267–277.
Many women who use an OC are satisfied with the positive effect the method has on menses and acne but find that they miss taking a pill some days; they might benefit from a method that involves nondaily administration. Studies show that switching from oral contraception to the transdermal patch (OrthoEvra) or vaginal ring (Nuvaring), for example, is acceptable to many women.
7. Preemptive prescribing
Prescribe emergency contraception before your patient needs it.
Jackson RA, Schwarz EB, Freedman L, Darney P. Advance supply of emergency contraception: effect on use and usual contraception—a randomized trial. Obstet Gynecol. 2003;102(1):8–16.
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 112: Emergency contraception. Obstet Gynecol. 2010;115(5):1100–1109.
Consider giving every sexually active woman a prescription for emergency contraception before she leaves your office. She can fill the prescription and keep it at home in case of an emergency.
8. Get to know ella
Become familiar with ulipristal acetate (ella) for emergency contraception.
Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555–562.
Barbieri RL. Levonorgestrel or ulipristal: is one a better emergency contraceptive than the other? OBG Manage. 2011;23(3):8–11.
This new FDA-approved agent for emergency contraception is effective for as long as 5 days after intercourse and results in fewer pregnancies than levonorgestrel does. It is available by prescription only, however, and is more expensive than levonorgestrel.
9. Pursue two urogenital pathogens
When you are not performing a speculum examination, screen for N. gonorrhoeae and C. trachomatis with a vaginal swab specimen or urine-based specimen.
Johnson RE, Newhall WJ, Papp JR, et al. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections–2002. MMWR Recomm Rep. 2002;51(RR-15):1–38; quiz CE1–4.
Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis. 2005;32(12):725–728.
Under new screening guidelines for cervical cancer, Pap smears are not required for women who are younger than 21 years. Gonorrhea and chlamydial infection screening is still important in this population, however, and can be done without a speculum exam.The patient or provider collects a specimen for testing with a vaginal swab, or the patient submits a urine specimen.
10. Now not later: An IUD, post-evacuation
Consider immediate, rather than delayed, IUD insertion after uterine evacuation for spontaneous or elective abortion in women who desire this form of contraception.
Bednarek PH, Creinin MD, Reeves MF, et al. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364:2208-2217.
A recent clinical trial enrolled 575 women who underwent uterine aspiration for induced or spontaneous abortion at 5 to 12 weeks’ gestation and who desired an IUD. Subjects were randomized to IUD insertion immediately after the procedure or 2 to 6 weeks later. The 6-month expulsion rate was 5.0% after immediate insertion; 2.7%, after delayed insertion (P = NS). There were no differences in the rates of other adverse events. Only 71% of patients returned for their “delayed” IUD placement; five pregnancies occurred among these women. No pregnancies occurred in the immediate-insertion group.
Offer office-based hysteroscopic sterilization.
Levie M, Weiss G, Kaiser B, Daif J, Chudnoff SG. Analysis of pain and satisfaction with office-based hysteroscopic sterilization. Fertil Steril. 2010;94(4):1189–1194.
Office hysteroscopy is well tolerated. Two hysteroscopic sterilization systems, Essure and Adiana, are available for use in the office. The systems are especially valuable in women who are poor surgical candidates or who want to avoid the inconvenience, or the risks, of a more major surgical procedure.
We want to hear from you! Tell us what you think.
- 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai, MD (June 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011) - 10 practical, evidence-based suggestions to improve your gyn practice now
Mark D. Walters, MD (January 2011) - 10 practical recommendations to improve maternal and perinatal outcomes in patients with eclampsia
Baha M. Sibai, MD
10 practice, evidence-based suggestions to improve your gyn practice now
As a new year, and a new decade, open, attention to evidence-based guidelines and to outcomes for individual patients continues to drive medicine. As a busy practicing ObGyn, you’re challenged to sort through the great, and growing, mass of medical information so you can focus on key issues that improve care.
Faced with such a task, it’s not surprising that physicians are notoriously slow to make changes to their practice in response to new evidence.
Here is help. In this article, I present 10 succinct clinical topics and offer medical and surgical suggestions that are easy to put into practice and backed by evidence. If you haven’t already done so, implementing some of these ideas can immediately improve the quality of your care. For each suggestion, I’ve included the pertinent reference.
1. Banish error
Embrace, and immediately implement, surgical time-out and safety checklists for all your surgeries and office procedures.
Altpeter T, Luckhardt K, Lewis JN, Harken AH, Polk HC Jr. Expanded surgical time out: a key to real-time data collection and quality improvement. J Am Coll Surg. 2007;204(4):527–532.
Surgical errors are rare, but we need to continually aim for “zero-error” conditions. These two quality assurance tools may help our efforts.
2. Route of hysterectomy
Make an effort to perform more vaginal hysterectomies and fewer open abdominal hysterectomies.
ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
In cases when vaginal hysterectomy isn’t feasible, consider laparoscopic (or, perhaps, robotic) hysterectomy more often. The role of robotic and single-port hysterectomies continues to evolve.
3. Cystoscopy
Learn, get credentialed for, and perform more diagnostic cystoscopies after certain gynecologic procedures.
ACOG Committee Opinion No. 372, July 2007. The role of cystourethroscopy in the generalist obstetrician-gynecologist practice. Obstet Gynecol. 2007;110(1):221–224.
Cystoscopy is appropriate when a gyn procedure carries at least a 1% risk of lower urinary tract injury. At most centers, that probably includes laparoscopic and robotic hysterectomy and colpopexy.
Simple cystoscopy at hysterectomy,
by Brent E. Seibel, MD
4. Oophorectomy
Perform fewer prophylactic oophorectomies at hysterectomy in low-risk women whose ovaries are normal. When you do remove ovaries as a preventive measure, do so with careful thought and informed consent—especially when the patient is premenopausal.
Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037.
In general, we probably perform too many “throw-in” oophorectomies at hysterectomy, with the aim of preventing cancer. But women who keep their ovaries may, in fact, live longer.
Give appropriate and properly timed (within 60 minutes of skin incision) antibiotic prophylaxis for hysterectomy, urogynecology procedures, hysterosalpingography or chromotubation, and surgical abortion.
ACOG Practice Bulletin No. 104: Antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113(5):1180–1189.
A single-dose antibiotic is adequate before hysterectomy. Give a second dose when surgery will take more than 3 hours or, perhaps, blood loss is expected to be >1,500 mL. Subsequent doses are not indicated for prophylaxis alone. Antibiotic prophylaxis is unnecessary before diagnostic laparoscopy (level-A evidence), hysteroscopic surgery (level-B), or exploratory laparotomy (level-C).
6. Blind biopsy
For evaluation of abnormal uterine bleeding, perform fewer blind endometrial biopsies and more imaging studies, such as transvaginal ultrasonography or saline infusion sonography, or office hysteroscopy.
Goldstein SR. Modern evaluation of the endometrium. Obstet Gynecol. 2010;116(1):168–176.
Blind endometrial biopsy alone yields an unacceptably high rate of false-negative results when utilized to determine the cause of abnormal uterine bleeding.
Pretreat with misoprostol before hysteroscopic procedures.
Choksuchat C. Clinical use of misoprostol in non-pregnant women: review article. J Minim Invasive Gynecol. 2010;17(4):449–455.
Misoprostol eases the force of cervical dilation, results in fewer cervical lacerations and less fluid absorption, and may lead to fewer uterine perforations. The dosage: 200–400 μg orally or intravaginally, at bedtime, for 1 or 2 days before the procedure.
8. Vaginal estrogen
Use intravaginal estrogen therapy as first-line treatment for postmenopausal women who experience vaginal irritation, dyspareunia, and frequent cystitis.
Krause M, Wheeler TL, Richter HE, Snyder TE. Systemic effects of vaginally administered estrogen therapy: a review. Female Pelvic Med Reconstr Surg. 2010;16(3):188–195.
Vaginal estrogen has many clear quality-of-life benefits and carries relatively low risk; risk is probably much lower than what is reported with systemic hormone replacement therapy. Delivery can be by cream, suppository, or vaginal ring. The relatively high cost of vaginal estrogen therapy remains a problem for some women.
9. Vaginal apical support
Consider adding or augmenting some vaginal apical support to help your cystocele repairs.
Summers A, Winkel LA, Hussain HK, DeLancey JOL. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438– 1443.
In women who have anterior vaginal prolapse (cystocele), approximately half of the prolapse might be explained by a defect in apical attachment. In addition, consider performing prophylactic McCall’s culdoplasty or uterosacral cuff suspension at the time of all hysterectomies, by all routes—even in the absence of prolapse.
Perform fewer, but better-timed, Pap tests.
Sawaya GF. Cervical-cancer screening—new guidelines and the balance between benefits and harms. N Engl J Med. 2009;361(26):2503–2305.
ACOG Practice Bulletin No. 109, December 2009. Cervical cytology screening. Obstet Gynecol. 2009;114(6):1409–1420.
New guidelines for cervical cancer screening that were issued by ACOG in November 2009 are meant to maximize benefit and minimize harm. The guidelines are summarized in the TABLE.
ACOG’s 2009 guidelines for cervical cytology screening
| Age (y) | Recommendation |
|---|---|
| Under 21 | Avoid screening |
| 21–29 | Screen every 2 y |
| 30–65 (or –70) | May screen every 3 y* |
| 65–70 | May discontinue screening† |
| *Applies only to women who have had three consecutive negative cytology tests; exceptions include women who have human immunodeficiency virus infection, compromised immunity, a history of grade-2 or -3 cervical intraepithelial neoplasia, or were exposed in utero to diethylstilbestrol. | |
| †Applies only to women who have had three or more consecutive negative cytology tests and no abnormal tests in the preceding 10 years; exceptions include women who have multiple sexual partners. | |
| Discontinue routine cytology in women who have had a total hysterectomy for benign indications and who do not have a history of high-grade cervical intraepithelial neoplasia (CIN). | |
We want to hear from you! Tell us what you think.
As a new year, and a new decade, open, attention to evidence-based guidelines and to outcomes for individual patients continues to drive medicine. As a busy practicing ObGyn, you’re challenged to sort through the great, and growing, mass of medical information so you can focus on key issues that improve care.
Faced with such a task, it’s not surprising that physicians are notoriously slow to make changes to their practice in response to new evidence.
Here is help. In this article, I present 10 succinct clinical topics and offer medical and surgical suggestions that are easy to put into practice and backed by evidence. If you haven’t already done so, implementing some of these ideas can immediately improve the quality of your care. For each suggestion, I’ve included the pertinent reference.
1. Banish error
Embrace, and immediately implement, surgical time-out and safety checklists for all your surgeries and office procedures.
Altpeter T, Luckhardt K, Lewis JN, Harken AH, Polk HC Jr. Expanded surgical time out: a key to real-time data collection and quality improvement. J Am Coll Surg. 2007;204(4):527–532.
Surgical errors are rare, but we need to continually aim for “zero-error” conditions. These two quality assurance tools may help our efforts.
2. Route of hysterectomy
Make an effort to perform more vaginal hysterectomies and fewer open abdominal hysterectomies.
ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
In cases when vaginal hysterectomy isn’t feasible, consider laparoscopic (or, perhaps, robotic) hysterectomy more often. The role of robotic and single-port hysterectomies continues to evolve.
3. Cystoscopy
Learn, get credentialed for, and perform more diagnostic cystoscopies after certain gynecologic procedures.
ACOG Committee Opinion No. 372, July 2007. The role of cystourethroscopy in the generalist obstetrician-gynecologist practice. Obstet Gynecol. 2007;110(1):221–224.
Cystoscopy is appropriate when a gyn procedure carries at least a 1% risk of lower urinary tract injury. At most centers, that probably includes laparoscopic and robotic hysterectomy and colpopexy.
Simple cystoscopy at hysterectomy,
by Brent E. Seibel, MD
4. Oophorectomy
Perform fewer prophylactic oophorectomies at hysterectomy in low-risk women whose ovaries are normal. When you do remove ovaries as a preventive measure, do so with careful thought and informed consent—especially when the patient is premenopausal.
Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037.
In general, we probably perform too many “throw-in” oophorectomies at hysterectomy, with the aim of preventing cancer. But women who keep their ovaries may, in fact, live longer.
Give appropriate and properly timed (within 60 minutes of skin incision) antibiotic prophylaxis for hysterectomy, urogynecology procedures, hysterosalpingography or chromotubation, and surgical abortion.
ACOG Practice Bulletin No. 104: Antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113(5):1180–1189.
A single-dose antibiotic is adequate before hysterectomy. Give a second dose when surgery will take more than 3 hours or, perhaps, blood loss is expected to be >1,500 mL. Subsequent doses are not indicated for prophylaxis alone. Antibiotic prophylaxis is unnecessary before diagnostic laparoscopy (level-A evidence), hysteroscopic surgery (level-B), or exploratory laparotomy (level-C).
6. Blind biopsy
For evaluation of abnormal uterine bleeding, perform fewer blind endometrial biopsies and more imaging studies, such as transvaginal ultrasonography or saline infusion sonography, or office hysteroscopy.
Goldstein SR. Modern evaluation of the endometrium. Obstet Gynecol. 2010;116(1):168–176.
Blind endometrial biopsy alone yields an unacceptably high rate of false-negative results when utilized to determine the cause of abnormal uterine bleeding.
Pretreat with misoprostol before hysteroscopic procedures.
Choksuchat C. Clinical use of misoprostol in non-pregnant women: review article. J Minim Invasive Gynecol. 2010;17(4):449–455.
Misoprostol eases the force of cervical dilation, results in fewer cervical lacerations and less fluid absorption, and may lead to fewer uterine perforations. The dosage: 200–400 μg orally or intravaginally, at bedtime, for 1 or 2 days before the procedure.
8. Vaginal estrogen
Use intravaginal estrogen therapy as first-line treatment for postmenopausal women who experience vaginal irritation, dyspareunia, and frequent cystitis.
Krause M, Wheeler TL, Richter HE, Snyder TE. Systemic effects of vaginally administered estrogen therapy: a review. Female Pelvic Med Reconstr Surg. 2010;16(3):188–195.
Vaginal estrogen has many clear quality-of-life benefits and carries relatively low risk; risk is probably much lower than what is reported with systemic hormone replacement therapy. Delivery can be by cream, suppository, or vaginal ring. The relatively high cost of vaginal estrogen therapy remains a problem for some women.
9. Vaginal apical support
Consider adding or augmenting some vaginal apical support to help your cystocele repairs.
Summers A, Winkel LA, Hussain HK, DeLancey JOL. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438– 1443.
In women who have anterior vaginal prolapse (cystocele), approximately half of the prolapse might be explained by a defect in apical attachment. In addition, consider performing prophylactic McCall’s culdoplasty or uterosacral cuff suspension at the time of all hysterectomies, by all routes—even in the absence of prolapse.
Perform fewer, but better-timed, Pap tests.
Sawaya GF. Cervical-cancer screening—new guidelines and the balance between benefits and harms. N Engl J Med. 2009;361(26):2503–2305.
ACOG Practice Bulletin No. 109, December 2009. Cervical cytology screening. Obstet Gynecol. 2009;114(6):1409–1420.
New guidelines for cervical cancer screening that were issued by ACOG in November 2009 are meant to maximize benefit and minimize harm. The guidelines are summarized in the TABLE.
ACOG’s 2009 guidelines for cervical cytology screening
| Age (y) | Recommendation |
|---|---|
| Under 21 | Avoid screening |
| 21–29 | Screen every 2 y |
| 30–65 (or –70) | May screen every 3 y* |
| 65–70 | May discontinue screening† |
| *Applies only to women who have had three consecutive negative cytology tests; exceptions include women who have human immunodeficiency virus infection, compromised immunity, a history of grade-2 or -3 cervical intraepithelial neoplasia, or were exposed in utero to diethylstilbestrol. | |
| †Applies only to women who have had three or more consecutive negative cytology tests and no abnormal tests in the preceding 10 years; exceptions include women who have multiple sexual partners. | |
| Discontinue routine cytology in women who have had a total hysterectomy for benign indications and who do not have a history of high-grade cervical intraepithelial neoplasia (CIN). | |
We want to hear from you! Tell us what you think.
As a new year, and a new decade, open, attention to evidence-based guidelines and to outcomes for individual patients continues to drive medicine. As a busy practicing ObGyn, you’re challenged to sort through the great, and growing, mass of medical information so you can focus on key issues that improve care.
Faced with such a task, it’s not surprising that physicians are notoriously slow to make changes to their practice in response to new evidence.
Here is help. In this article, I present 10 succinct clinical topics and offer medical and surgical suggestions that are easy to put into practice and backed by evidence. If you haven’t already done so, implementing some of these ideas can immediately improve the quality of your care. For each suggestion, I’ve included the pertinent reference.
1. Banish error
Embrace, and immediately implement, surgical time-out and safety checklists for all your surgeries and office procedures.
Altpeter T, Luckhardt K, Lewis JN, Harken AH, Polk HC Jr. Expanded surgical time out: a key to real-time data collection and quality improvement. J Am Coll Surg. 2007;204(4):527–532.
Surgical errors are rare, but we need to continually aim for “zero-error” conditions. These two quality assurance tools may help our efforts.
2. Route of hysterectomy
Make an effort to perform more vaginal hysterectomies and fewer open abdominal hysterectomies.
ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
In cases when vaginal hysterectomy isn’t feasible, consider laparoscopic (or, perhaps, robotic) hysterectomy more often. The role of robotic and single-port hysterectomies continues to evolve.
3. Cystoscopy
Learn, get credentialed for, and perform more diagnostic cystoscopies after certain gynecologic procedures.
ACOG Committee Opinion No. 372, July 2007. The role of cystourethroscopy in the generalist obstetrician-gynecologist practice. Obstet Gynecol. 2007;110(1):221–224.
Cystoscopy is appropriate when a gyn procedure carries at least a 1% risk of lower urinary tract injury. At most centers, that probably includes laparoscopic and robotic hysterectomy and colpopexy.
Simple cystoscopy at hysterectomy,
by Brent E. Seibel, MD
4. Oophorectomy
Perform fewer prophylactic oophorectomies at hysterectomy in low-risk women whose ovaries are normal. When you do remove ovaries as a preventive measure, do so with careful thought and informed consent—especially when the patient is premenopausal.
Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037.
In general, we probably perform too many “throw-in” oophorectomies at hysterectomy, with the aim of preventing cancer. But women who keep their ovaries may, in fact, live longer.
Give appropriate and properly timed (within 60 minutes of skin incision) antibiotic prophylaxis for hysterectomy, urogynecology procedures, hysterosalpingography or chromotubation, and surgical abortion.
ACOG Practice Bulletin No. 104: Antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113(5):1180–1189.
A single-dose antibiotic is adequate before hysterectomy. Give a second dose when surgery will take more than 3 hours or, perhaps, blood loss is expected to be >1,500 mL. Subsequent doses are not indicated for prophylaxis alone. Antibiotic prophylaxis is unnecessary before diagnostic laparoscopy (level-A evidence), hysteroscopic surgery (level-B), or exploratory laparotomy (level-C).
6. Blind biopsy
For evaluation of abnormal uterine bleeding, perform fewer blind endometrial biopsies and more imaging studies, such as transvaginal ultrasonography or saline infusion sonography, or office hysteroscopy.
Goldstein SR. Modern evaluation of the endometrium. Obstet Gynecol. 2010;116(1):168–176.
Blind endometrial biopsy alone yields an unacceptably high rate of false-negative results when utilized to determine the cause of abnormal uterine bleeding.
Pretreat with misoprostol before hysteroscopic procedures.
Choksuchat C. Clinical use of misoprostol in non-pregnant women: review article. J Minim Invasive Gynecol. 2010;17(4):449–455.
Misoprostol eases the force of cervical dilation, results in fewer cervical lacerations and less fluid absorption, and may lead to fewer uterine perforations. The dosage: 200–400 μg orally or intravaginally, at bedtime, for 1 or 2 days before the procedure.
8. Vaginal estrogen
Use intravaginal estrogen therapy as first-line treatment for postmenopausal women who experience vaginal irritation, dyspareunia, and frequent cystitis.
Krause M, Wheeler TL, Richter HE, Snyder TE. Systemic effects of vaginally administered estrogen therapy: a review. Female Pelvic Med Reconstr Surg. 2010;16(3):188–195.
Vaginal estrogen has many clear quality-of-life benefits and carries relatively low risk; risk is probably much lower than what is reported with systemic hormone replacement therapy. Delivery can be by cream, suppository, or vaginal ring. The relatively high cost of vaginal estrogen therapy remains a problem for some women.
9. Vaginal apical support
Consider adding or augmenting some vaginal apical support to help your cystocele repairs.
Summers A, Winkel LA, Hussain HK, DeLancey JOL. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438– 1443.
In women who have anterior vaginal prolapse (cystocele), approximately half of the prolapse might be explained by a defect in apical attachment. In addition, consider performing prophylactic McCall’s culdoplasty or uterosacral cuff suspension at the time of all hysterectomies, by all routes—even in the absence of prolapse.
Perform fewer, but better-timed, Pap tests.
Sawaya GF. Cervical-cancer screening—new guidelines and the balance between benefits and harms. N Engl J Med. 2009;361(26):2503–2305.
ACOG Practice Bulletin No. 109, December 2009. Cervical cytology screening. Obstet Gynecol. 2009;114(6):1409–1420.
New guidelines for cervical cancer screening that were issued by ACOG in November 2009 are meant to maximize benefit and minimize harm. The guidelines are summarized in the TABLE.
ACOG’s 2009 guidelines for cervical cytology screening
| Age (y) | Recommendation |
|---|---|
| Under 21 | Avoid screening |
| 21–29 | Screen every 2 y |
| 30–65 (or –70) | May screen every 3 y* |
| 65–70 | May discontinue screening† |
| *Applies only to women who have had three consecutive negative cytology tests; exceptions include women who have human immunodeficiency virus infection, compromised immunity, a history of grade-2 or -3 cervical intraepithelial neoplasia, or were exposed in utero to diethylstilbestrol. | |
| †Applies only to women who have had three or more consecutive negative cytology tests and no abnormal tests in the preceding 10 years; exceptions include women who have multiple sexual partners. | |
| Discontinue routine cytology in women who have had a total hysterectomy for benign indications and who do not have a history of high-grade cervical intraepithelial neoplasia (CIN). | |
We want to hear from you! Tell us what you think.