User login
ACR: Rheumatologists help reduce ED, hospitalization costs
Rheumatology care can save health systems more than $2,700 per patient per year, according to a new report from the American College of Rheumatology.
In a white paper and corresponding position statement, the organization outlined how rheumatology care delivers financial benefits for health systems.
The work also highlighted prior research on the positive outcomes associated with rheumatology care, including a decline in hip and knee replacements for patients with rheumatoid arthritis after the introduction of biologics, while the total number of hip and knee replacements for patients with osteoarthritis increased, as well as lower 30-day readmission rates among patients with systemic lupus erythematosus with access to a rheumatology clinic post discharge.
“Many rheumatologists can attest to the value they bring to the care team at a health care system,” said Christina Downey, MD, an assistant professor of medicine at Loma Linda (Calif.) University, in a press release. She is the lead author of the white paper and chair of the ACR’s Government Affairs Committee. “Our goal with the paper and position statement is to emphasize what that value looks like from a preventive and financial perspective. A rheumatologist on the care team benefits patients, practices, and the economy.”
The analysis used adjusted claims insurance data to compare markets with a high vs. low supply of rheumatologists. A high supply was defined as at least 1.5 rheumatologists per 100,000 population, whereas a low supply was less than this amount. On average, markets with a high supply of rheumatologists had lower emergency department (ED) and hospitalization costs per patient per year.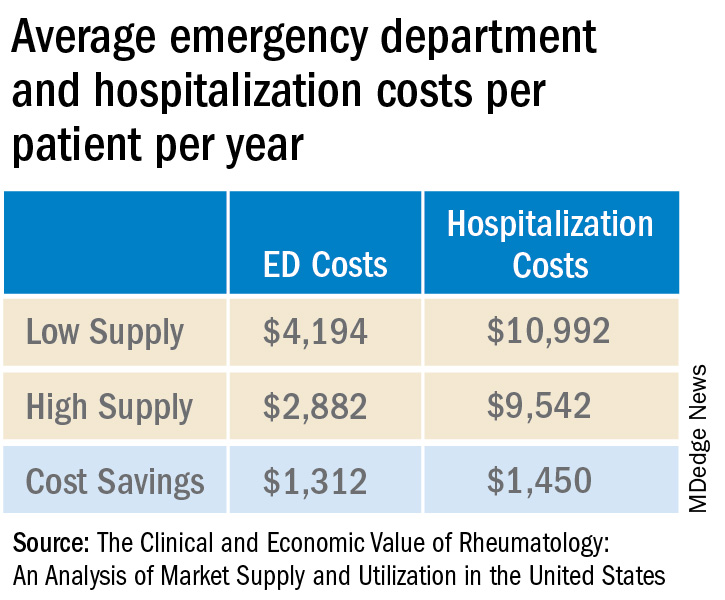
Added together, high-supply rheumatology markets save on average $2,762 in ED visit and hospitalization costs per patient per year.
Dr. Downey and colleagues also tallied the direct and downstream billings associated with rheumatologists, including office visits, consultations, lab testing, and radiology services. The average revenue generated per rheumatologist was $3.5 million per year.
“Emphasizing the impact rheumatologists have on the entire medical community is more important than ever, especially as we contend with an impending rheumatology workforce shortage coupled with an expected increase in patient demand for rheumatologic care,” Dr. Downey said. “This paper supports our recruitment and sustainability efforts for the specialty by spotlighting the significant contributions we make every day and every year to patient outcomes, hospitals, and other health care practices.”
A version of this article first appeared on Medscape.com.
Rheumatology care can save health systems more than $2,700 per patient per year, according to a new report from the American College of Rheumatology.
In a white paper and corresponding position statement, the organization outlined how rheumatology care delivers financial benefits for health systems.
The work also highlighted prior research on the positive outcomes associated with rheumatology care, including a decline in hip and knee replacements for patients with rheumatoid arthritis after the introduction of biologics, while the total number of hip and knee replacements for patients with osteoarthritis increased, as well as lower 30-day readmission rates among patients with systemic lupus erythematosus with access to a rheumatology clinic post discharge.
“Many rheumatologists can attest to the value they bring to the care team at a health care system,” said Christina Downey, MD, an assistant professor of medicine at Loma Linda (Calif.) University, in a press release. She is the lead author of the white paper and chair of the ACR’s Government Affairs Committee. “Our goal with the paper and position statement is to emphasize what that value looks like from a preventive and financial perspective. A rheumatologist on the care team benefits patients, practices, and the economy.”
The analysis used adjusted claims insurance data to compare markets with a high vs. low supply of rheumatologists. A high supply was defined as at least 1.5 rheumatologists per 100,000 population, whereas a low supply was less than this amount. On average, markets with a high supply of rheumatologists had lower emergency department (ED) and hospitalization costs per patient per year.
Added together, high-supply rheumatology markets save on average $2,762 in ED visit and hospitalization costs per patient per year.
Dr. Downey and colleagues also tallied the direct and downstream billings associated with rheumatologists, including office visits, consultations, lab testing, and radiology services. The average revenue generated per rheumatologist was $3.5 million per year.
“Emphasizing the impact rheumatologists have on the entire medical community is more important than ever, especially as we contend with an impending rheumatology workforce shortage coupled with an expected increase in patient demand for rheumatologic care,” Dr. Downey said. “This paper supports our recruitment and sustainability efforts for the specialty by spotlighting the significant contributions we make every day and every year to patient outcomes, hospitals, and other health care practices.”
A version of this article first appeared on Medscape.com.
Rheumatology care can save health systems more than $2,700 per patient per year, according to a new report from the American College of Rheumatology.
In a white paper and corresponding position statement, the organization outlined how rheumatology care delivers financial benefits for health systems.
The work also highlighted prior research on the positive outcomes associated with rheumatology care, including a decline in hip and knee replacements for patients with rheumatoid arthritis after the introduction of biologics, while the total number of hip and knee replacements for patients with osteoarthritis increased, as well as lower 30-day readmission rates among patients with systemic lupus erythematosus with access to a rheumatology clinic post discharge.
“Many rheumatologists can attest to the value they bring to the care team at a health care system,” said Christina Downey, MD, an assistant professor of medicine at Loma Linda (Calif.) University, in a press release. She is the lead author of the white paper and chair of the ACR’s Government Affairs Committee. “Our goal with the paper and position statement is to emphasize what that value looks like from a preventive and financial perspective. A rheumatologist on the care team benefits patients, practices, and the economy.”
The analysis used adjusted claims insurance data to compare markets with a high vs. low supply of rheumatologists. A high supply was defined as at least 1.5 rheumatologists per 100,000 population, whereas a low supply was less than this amount. On average, markets with a high supply of rheumatologists had lower emergency department (ED) and hospitalization costs per patient per year.
Added together, high-supply rheumatology markets save on average $2,762 in ED visit and hospitalization costs per patient per year.
Dr. Downey and colleagues also tallied the direct and downstream billings associated with rheumatologists, including office visits, consultations, lab testing, and radiology services. The average revenue generated per rheumatologist was $3.5 million per year.
“Emphasizing the impact rheumatologists have on the entire medical community is more important than ever, especially as we contend with an impending rheumatology workforce shortage coupled with an expected increase in patient demand for rheumatologic care,” Dr. Downey said. “This paper supports our recruitment and sustainability efforts for the specialty by spotlighting the significant contributions we make every day and every year to patient outcomes, hospitals, and other health care practices.”
A version of this article first appeared on Medscape.com.
Industry funding falls for rheumatology research
Industry-sponsored research funding has fallen by more than 20% from 2014 to 2022, according to a new analysis.
“Despite the growing partnerships and networks between rheumatologists, the public sector, and the health care industry to optimize research funding allocations, the declining trend in industry-sponsored research payments is a concerning sign for all rheumatologists,” writes study author Anju Murayama, an undergraduate medical student at the Tohoku University School of Medicine in Sendai City, Japan. The data suggest that “more and more rheumatologists are facing difficulties in obtaining research funding from the health care industry.”
Dr. Murayama used the Open Payments Database, which contains records of payments made by drug and pharmaceutical companies to health care providers. The analysis included research payments provided directly to rheumatologists (direct-research payments) and payments given to clinicians or health care organizations related to research whose principal investigator was a rheumatologist (associated-research payments). These associated payments included costs for study enrollment and screening, safety monitoring committees, research publication, and more.
The research was published August 15 in The Journal of Rheumatology .
In 2014, the total direct payments to rheumatologists from industry were $1.4 million. These payments jumped to nearly $4.6 million in 2016 but have declined since. In 2022, there were $976,481 in total payments, a 31% drop from 9 years before.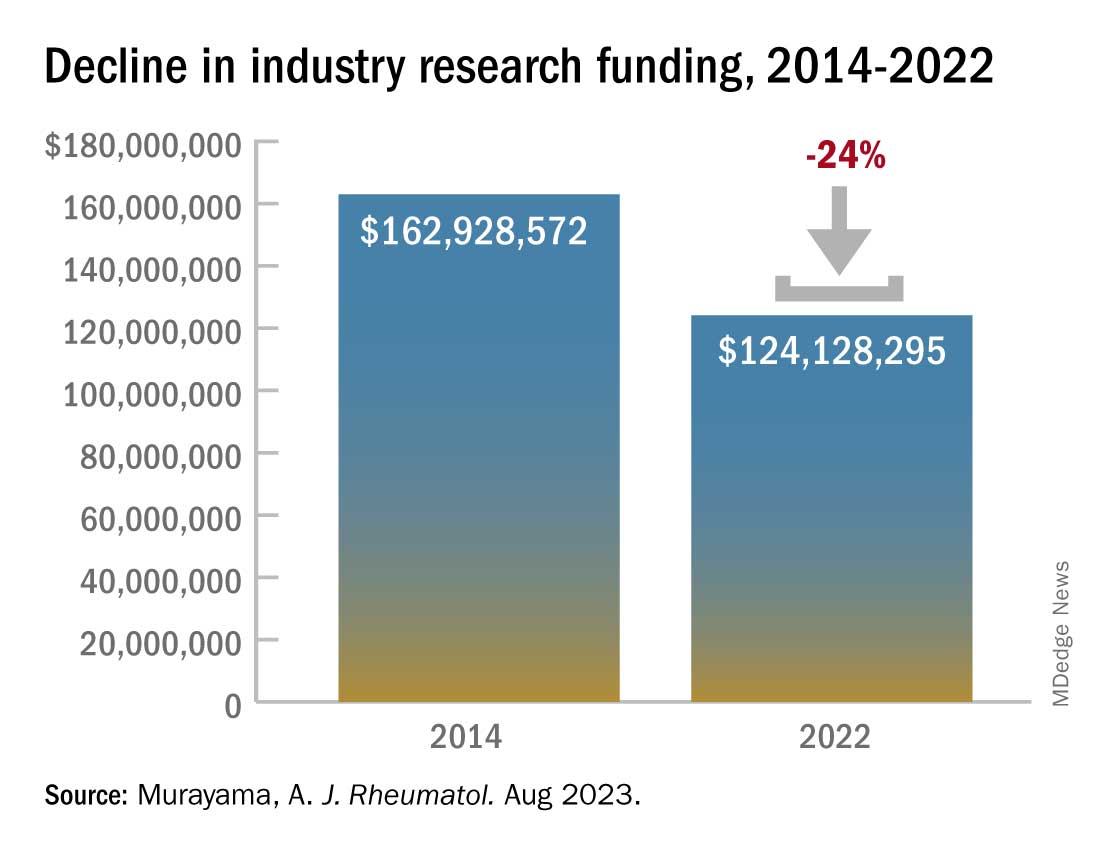
This decline comes after an observed drop in research funding from the public sector. From 2014 to 2017, public-sector research funding to members of the American College of Rheumatology fell by 7.5%. Timothy Niewold, MD, a rheumatologist and vice chair for research in the department of medicine at Hospital for Special Surgery, New York, said that he and colleagues have felt the funding squeeze from both public and industry sectors. “The budgets for trials have seemed tight,” he told this news organization. With the overhead and cost of doing a trial at an academic institution like HSS, “sometimes you can’t make the budget work,” and researchers must pass on industry-funded trials.
The analysis also found a larger discrepancy between average and median associated-research payments. Of the $1.4 billion in associated-research payments combined over the 9-year period, the median payments per physician ($173,022) were much smaller than the mean payments ($989,753), which indicates that “only a very small number of rheumatologists received substantial amounts of research funding from the industry,” Dr. Murayama wrote in an email to this news organization. “This finding might support statements published by Scher and Schett in Nature Review Rheumatology suggesting that many industry-initiated clinical trials are conducted and authored by a small number of influential rheumatologists, often referred to as key opinion leaders.”
The analysis also found that of all associated payments, less than 3% ($39.2 million) went to funding preclinical research, which is “more disappointing than surprising,” Dr. Niewold said. Though clinical trials are expensive and require larger amounts of investment, industry partnerships at preclinical phases of research are important for devising novel solutions for these complex rheumatic diseases, he noted. “The clinical trials are one piece,” he added, “but you need the whole [research] continuum.”
Dr. Niewold reports receiving research grants from EMD Serono and Zenas Biopharma and consulting for Thermo Fisher Scientific, Progentec Diagnostics, Roivant Sciences, Ventus, S3 Connected Health, AstraZeneca, and Inova. Dr. Murayama reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Industry-sponsored research funding has fallen by more than 20% from 2014 to 2022, according to a new analysis.
“Despite the growing partnerships and networks between rheumatologists, the public sector, and the health care industry to optimize research funding allocations, the declining trend in industry-sponsored research payments is a concerning sign for all rheumatologists,” writes study author Anju Murayama, an undergraduate medical student at the Tohoku University School of Medicine in Sendai City, Japan. The data suggest that “more and more rheumatologists are facing difficulties in obtaining research funding from the health care industry.”
Dr. Murayama used the Open Payments Database, which contains records of payments made by drug and pharmaceutical companies to health care providers. The analysis included research payments provided directly to rheumatologists (direct-research payments) and payments given to clinicians or health care organizations related to research whose principal investigator was a rheumatologist (associated-research payments). These associated payments included costs for study enrollment and screening, safety monitoring committees, research publication, and more.
The research was published August 15 in The Journal of Rheumatology .
In 2014, the total direct payments to rheumatologists from industry were $1.4 million. These payments jumped to nearly $4.6 million in 2016 but have declined since. In 2022, there were $976,481 in total payments, a 31% drop from 9 years before.
This decline comes after an observed drop in research funding from the public sector. From 2014 to 2017, public-sector research funding to members of the American College of Rheumatology fell by 7.5%. Timothy Niewold, MD, a rheumatologist and vice chair for research in the department of medicine at Hospital for Special Surgery, New York, said that he and colleagues have felt the funding squeeze from both public and industry sectors. “The budgets for trials have seemed tight,” he told this news organization. With the overhead and cost of doing a trial at an academic institution like HSS, “sometimes you can’t make the budget work,” and researchers must pass on industry-funded trials.
The analysis also found a larger discrepancy between average and median associated-research payments. Of the $1.4 billion in associated-research payments combined over the 9-year period, the median payments per physician ($173,022) were much smaller than the mean payments ($989,753), which indicates that “only a very small number of rheumatologists received substantial amounts of research funding from the industry,” Dr. Murayama wrote in an email to this news organization. “This finding might support statements published by Scher and Schett in Nature Review Rheumatology suggesting that many industry-initiated clinical trials are conducted and authored by a small number of influential rheumatologists, often referred to as key opinion leaders.”
The analysis also found that of all associated payments, less than 3% ($39.2 million) went to funding preclinical research, which is “more disappointing than surprising,” Dr. Niewold said. Though clinical trials are expensive and require larger amounts of investment, industry partnerships at preclinical phases of research are important for devising novel solutions for these complex rheumatic diseases, he noted. “The clinical trials are one piece,” he added, “but you need the whole [research] continuum.”
Dr. Niewold reports receiving research grants from EMD Serono and Zenas Biopharma and consulting for Thermo Fisher Scientific, Progentec Diagnostics, Roivant Sciences, Ventus, S3 Connected Health, AstraZeneca, and Inova. Dr. Murayama reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Industry-sponsored research funding has fallen by more than 20% from 2014 to 2022, according to a new analysis.
“Despite the growing partnerships and networks between rheumatologists, the public sector, and the health care industry to optimize research funding allocations, the declining trend in industry-sponsored research payments is a concerning sign for all rheumatologists,” writes study author Anju Murayama, an undergraduate medical student at the Tohoku University School of Medicine in Sendai City, Japan. The data suggest that “more and more rheumatologists are facing difficulties in obtaining research funding from the health care industry.”
Dr. Murayama used the Open Payments Database, which contains records of payments made by drug and pharmaceutical companies to health care providers. The analysis included research payments provided directly to rheumatologists (direct-research payments) and payments given to clinicians or health care organizations related to research whose principal investigator was a rheumatologist (associated-research payments). These associated payments included costs for study enrollment and screening, safety monitoring committees, research publication, and more.
The research was published August 15 in The Journal of Rheumatology .
In 2014, the total direct payments to rheumatologists from industry were $1.4 million. These payments jumped to nearly $4.6 million in 2016 but have declined since. In 2022, there were $976,481 in total payments, a 31% drop from 9 years before.
This decline comes after an observed drop in research funding from the public sector. From 2014 to 2017, public-sector research funding to members of the American College of Rheumatology fell by 7.5%. Timothy Niewold, MD, a rheumatologist and vice chair for research in the department of medicine at Hospital for Special Surgery, New York, said that he and colleagues have felt the funding squeeze from both public and industry sectors. “The budgets for trials have seemed tight,” he told this news organization. With the overhead and cost of doing a trial at an academic institution like HSS, “sometimes you can’t make the budget work,” and researchers must pass on industry-funded trials.
The analysis also found a larger discrepancy between average and median associated-research payments. Of the $1.4 billion in associated-research payments combined over the 9-year period, the median payments per physician ($173,022) were much smaller than the mean payments ($989,753), which indicates that “only a very small number of rheumatologists received substantial amounts of research funding from the industry,” Dr. Murayama wrote in an email to this news organization. “This finding might support statements published by Scher and Schett in Nature Review Rheumatology suggesting that many industry-initiated clinical trials are conducted and authored by a small number of influential rheumatologists, often referred to as key opinion leaders.”
The analysis also found that of all associated payments, less than 3% ($39.2 million) went to funding preclinical research, which is “more disappointing than surprising,” Dr. Niewold said. Though clinical trials are expensive and require larger amounts of investment, industry partnerships at preclinical phases of research are important for devising novel solutions for these complex rheumatic diseases, he noted. “The clinical trials are one piece,” he added, “but you need the whole [research] continuum.”
Dr. Niewold reports receiving research grants from EMD Serono and Zenas Biopharma and consulting for Thermo Fisher Scientific, Progentec Diagnostics, Roivant Sciences, Ventus, S3 Connected Health, AstraZeneca, and Inova. Dr. Murayama reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF RHEUMATOLOGY
FDA approves canakinumab for gout flares
The U.S. Food and Drug Administration has approved canakinumab (Ilaris) for the treatment of gout flares in adults who cannot be treated with NSAIDs, colchicine, or repeated courses of corticosteroids. The drug is also indicated for people who could not tolerate or had an inadequate response to NSAIDs or colchicine.
The drug, a humanized anti–interleukin-1 beta monoclonal antibody, is the first and only biologic approved in the United States for the treatment of gout flares, according to Novartis. It is administered in a single, subcutaneous injection of 150 mg.
“At Novartis, we are committed to bringing medicines that address high unmet needs to patients. We are proud to receive approval on our eighth indication for Ilaris in the U.S. and provide the first biologic medicine option for people with gout flares to help treat this painful and debilitating condition,” the company said in a statement to this news organization.
Canakinumab was first approved in the United States in 2009 for the treatment of children and adults with cryopyrin-associated periodic syndrome (CAPS). Since then, it has been approved for the treatment of several other autoinflammatory diseases, including Still’s disease and recurrent fever syndromes.
In 2011, an FDA advisory panel voted against the approval of canakinumab to treat acute gout flares refractory to NSAIDs, colchicine, or repeated courses of corticosteroids, while in 2013, the European Medicine Agency approved the drug for this treatment indication.
Since that FDA advisory committee meeting and the FDA’s subsequent rejection letter, “[Novartis] has conducted additional studies in patients with gout flares and other related populations to further characterize the short- and long-term safety of canakinumab supporting the current application. To further support the benefit-risk [profile of the drug], the indication is for a more restricted population than initially proposed in 2011,” the FDA’s Center for Drug Evaluation and Research said in a statement to this news organization. “Given these considerations and the available safety information, the Agency determined that canakinumab, at the recommended dosage, has a favorable risk-benefit profile” in the specified patient population.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved canakinumab (Ilaris) for the treatment of gout flares in adults who cannot be treated with NSAIDs, colchicine, or repeated courses of corticosteroids. The drug is also indicated for people who could not tolerate or had an inadequate response to NSAIDs or colchicine.
The drug, a humanized anti–interleukin-1 beta monoclonal antibody, is the first and only biologic approved in the United States for the treatment of gout flares, according to Novartis. It is administered in a single, subcutaneous injection of 150 mg.
“At Novartis, we are committed to bringing medicines that address high unmet needs to patients. We are proud to receive approval on our eighth indication for Ilaris in the U.S. and provide the first biologic medicine option for people with gout flares to help treat this painful and debilitating condition,” the company said in a statement to this news organization.
Canakinumab was first approved in the United States in 2009 for the treatment of children and adults with cryopyrin-associated periodic syndrome (CAPS). Since then, it has been approved for the treatment of several other autoinflammatory diseases, including Still’s disease and recurrent fever syndromes.
In 2011, an FDA advisory panel voted against the approval of canakinumab to treat acute gout flares refractory to NSAIDs, colchicine, or repeated courses of corticosteroids, while in 2013, the European Medicine Agency approved the drug for this treatment indication.
Since that FDA advisory committee meeting and the FDA’s subsequent rejection letter, “[Novartis] has conducted additional studies in patients with gout flares and other related populations to further characterize the short- and long-term safety of canakinumab supporting the current application. To further support the benefit-risk [profile of the drug], the indication is for a more restricted population than initially proposed in 2011,” the FDA’s Center for Drug Evaluation and Research said in a statement to this news organization. “Given these considerations and the available safety information, the Agency determined that canakinumab, at the recommended dosage, has a favorable risk-benefit profile” in the specified patient population.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved canakinumab (Ilaris) for the treatment of gout flares in adults who cannot be treated with NSAIDs, colchicine, or repeated courses of corticosteroids. The drug is also indicated for people who could not tolerate or had an inadequate response to NSAIDs or colchicine.
The drug, a humanized anti–interleukin-1 beta monoclonal antibody, is the first and only biologic approved in the United States for the treatment of gout flares, according to Novartis. It is administered in a single, subcutaneous injection of 150 mg.
“At Novartis, we are committed to bringing medicines that address high unmet needs to patients. We are proud to receive approval on our eighth indication for Ilaris in the U.S. and provide the first biologic medicine option for people with gout flares to help treat this painful and debilitating condition,” the company said in a statement to this news organization.
Canakinumab was first approved in the United States in 2009 for the treatment of children and adults with cryopyrin-associated periodic syndrome (CAPS). Since then, it has been approved for the treatment of several other autoinflammatory diseases, including Still’s disease and recurrent fever syndromes.
In 2011, an FDA advisory panel voted against the approval of canakinumab to treat acute gout flares refractory to NSAIDs, colchicine, or repeated courses of corticosteroids, while in 2013, the European Medicine Agency approved the drug for this treatment indication.
Since that FDA advisory committee meeting and the FDA’s subsequent rejection letter, “[Novartis] has conducted additional studies in patients with gout flares and other related populations to further characterize the short- and long-term safety of canakinumab supporting the current application. To further support the benefit-risk [profile of the drug], the indication is for a more restricted population than initially proposed in 2011,” the FDA’s Center for Drug Evaluation and Research said in a statement to this news organization. “Given these considerations and the available safety information, the Agency determined that canakinumab, at the recommended dosage, has a favorable risk-benefit profile” in the specified patient population.
A version of this article first appeared on Medscape.com.
ACR releases guideline for managing ILD in patients with rheumatic disease
The American College of Rheumatology has released a summary of upcoming guidelines on screening, monitoring, and treatment for interstitial lung disease (ILD) in patients with systemic autoimmune rheumatic disease.
The recommendations apply to adults with rheumatic diseases at greater risk for ILD: rheumatoid arthritis, systemic sclerosis (SSc), mixed connective tissue disease (MCTD), Sjögren’s disease (SjD), and idiopathic inflammatory myopathies (IIM).
“Interstitial lung disease is a major cause of morbidity and mortality across several systemic autoimmune rheumatic diseases,” Sindhu R. Johnson, MD, PhD, lead author of the new guidelines and director of the clinical epidemiology and health care research program at the University of Toronto, said in an ACR press release. “Guidance was needed for which tests to use for screening and monitoring this particular disease.”
The two documents are summaries of part of a larger manuscript currently awaiting peer review, according to the ACR, and the final guidelines are anticipated to be published by early 2024.
The recommendations were developed using “the best available evidence and consensus across a range of expert opinions and incorporated patient values and preferences,” according to the press release.
Highlights of recommendations for screening and monitoring ILD are:
- Providers can screen patients at higher risk for ILD with pulmonary function tests (PFTs) and high-resolution CT of the chest.
- PFTs, chest high-resolution CT, and ambulatory desaturation testing are conditionally recommended for monitoring ILD progression.
- It is conditionally recommended that providers do not use 6-minute walk test distance, chest radiography, or bronchoscopy for screening or monitoring disease.
- It is suggested that patients with IIM-ILD and SSc-ILD receive PFTs for monitoring every 3-6 months during the first year, then less frequently once stable.
- It is suggested that patients with RA-ILD, SjD-ILD, and MCTD-ILD receive PFTs every 3-12 months for the first year, then less frequently once stable.
Suggestions on how often to screen for ILD were not present in the summary documents, but will be made available in the larger manuscript, said Elana Bernstein, MD, director of the Columbia University Medical Center/New York–Presbyterian Hospital scleroderma program, New York. She is co–first author of the guidelines.
Nearly all recommendations are conditional, primarily because the certainty of evidence behind many of these recommendations is low or very low, she said in an interview. More clinical data on ILD in patients with rheumatic disease would help strengthen evidence, she said, particularly for best practices in frequency of testing. “We need more research on how often patients should be screened for ILD and how often they should be monitored for ILD progression,” she said. “That would enable us to provide recommendations, rather than just suggestions.”
Highlights of recommendations for ILD treatment are:
- The guidelines strongly recommend against using glucocorticoids for first-line ILD treatment in patients with SSc-ILD.
- Short-term glucocorticoids are conditionally recommended as a first-line ILD treatment for patients with systemic autoimmune rheumatic disease–related ILD (SARD-ILD), excluding SSc-ILD.
- Mycophenolate, azathioprine, rituximab, and cyclophosphamide are all potential first-line ILD treatment options for patients with SARD-ILD.
- It is conditionally recommended that patients with SARD-ILD do not receive leflunomide, methotrexate, tumor necrosis factor inhibitors, or abatacept as first-line ILD treatment.
- If SARD-ILD progresses despite first-line therapy, mycophenolate, rituximab, cyclophosphamide, and nintedanib are potential secondary treatment options.
- If RA-ILD progresses following initial therapy, pirfenidone is a treatment option.
- The guidelines conditionally recommend against pirfenidone as a secondary treatment option for SARD-ILD other than RA-ILD.
These summary guidelines appear “comprehensive,” but there has yet to be information published on the basis of these recommendations, Elizabeth Volkmann, MD, said in an interview.
“It’s important to understand that we don’t know whether most of these recommendations were just driven by expert opinion versus actual evidence from randomized, controlled clinical trials,” said Dr. Volkmann, who codirects the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles. She was not involved with creating the guidelines.
She expects that many of the recommendations for first- and second-line ILD treatment options were based on expert opinion, as there have been no randomized clinical trials looking at that specific topic, she said. For example, nintedanib is conditionally recommended as a first-line treatment option for SSc-ILD, but as a second-line treatment for SjD-ILD, IIM-ILD, and MCTD-ILD. “There’s no literature to support one or the other – whether nintedanib is first-line or second-line [treatment].”
The decision to publish the summary recommendations online prior to peer review is unusual, she said, as these recommendations could be altered during that process; however, Dr. Bernstein noted that was not likely.
By releasing the summary guideline now, the ACR can “get the needed information to clinicians earlier as the manuscript goes through its remaining stages and is finalized,” an ACR representative explained.
Prior to the expected publication of these guidelines in early 2024, Dr. Volkmann noted that the American Thoracic Society will be publishing guidelines on the treatment of SSc-ILD in the American Journal of Respiratory and Critical Care Medicine in September.
Dr. Bernstein reported grants/contracts with the Department of Defense, the Scleroderma Research Foundation, the National Institutes of Health, Eicos, Boehringer Ingelheim, Kadmon, and Pfizer. Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and GlaxoSmithKline and institutional support for performing studies on systemic sclerosis for Kadmon, Boehringer Ingelheim, Horizon, and Prometheus.
A version of this article first appeared on Medscape.com.
The American College of Rheumatology has released a summary of upcoming guidelines on screening, monitoring, and treatment for interstitial lung disease (ILD) in patients with systemic autoimmune rheumatic disease.
The recommendations apply to adults with rheumatic diseases at greater risk for ILD: rheumatoid arthritis, systemic sclerosis (SSc), mixed connective tissue disease (MCTD), Sjögren’s disease (SjD), and idiopathic inflammatory myopathies (IIM).
“Interstitial lung disease is a major cause of morbidity and mortality across several systemic autoimmune rheumatic diseases,” Sindhu R. Johnson, MD, PhD, lead author of the new guidelines and director of the clinical epidemiology and health care research program at the University of Toronto, said in an ACR press release. “Guidance was needed for which tests to use for screening and monitoring this particular disease.”
The two documents are summaries of part of a larger manuscript currently awaiting peer review, according to the ACR, and the final guidelines are anticipated to be published by early 2024.
The recommendations were developed using “the best available evidence and consensus across a range of expert opinions and incorporated patient values and preferences,” according to the press release.
Highlights of recommendations for screening and monitoring ILD are:
- Providers can screen patients at higher risk for ILD with pulmonary function tests (PFTs) and high-resolution CT of the chest.
- PFTs, chest high-resolution CT, and ambulatory desaturation testing are conditionally recommended for monitoring ILD progression.
- It is conditionally recommended that providers do not use 6-minute walk test distance, chest radiography, or bronchoscopy for screening or monitoring disease.
- It is suggested that patients with IIM-ILD and SSc-ILD receive PFTs for monitoring every 3-6 months during the first year, then less frequently once stable.
- It is suggested that patients with RA-ILD, SjD-ILD, and MCTD-ILD receive PFTs every 3-12 months for the first year, then less frequently once stable.
Suggestions on how often to screen for ILD were not present in the summary documents, but will be made available in the larger manuscript, said Elana Bernstein, MD, director of the Columbia University Medical Center/New York–Presbyterian Hospital scleroderma program, New York. She is co–first author of the guidelines.
Nearly all recommendations are conditional, primarily because the certainty of evidence behind many of these recommendations is low or very low, she said in an interview. More clinical data on ILD in patients with rheumatic disease would help strengthen evidence, she said, particularly for best practices in frequency of testing. “We need more research on how often patients should be screened for ILD and how often they should be monitored for ILD progression,” she said. “That would enable us to provide recommendations, rather than just suggestions.”
Highlights of recommendations for ILD treatment are:
- The guidelines strongly recommend against using glucocorticoids for first-line ILD treatment in patients with SSc-ILD.
- Short-term glucocorticoids are conditionally recommended as a first-line ILD treatment for patients with systemic autoimmune rheumatic disease–related ILD (SARD-ILD), excluding SSc-ILD.
- Mycophenolate, azathioprine, rituximab, and cyclophosphamide are all potential first-line ILD treatment options for patients with SARD-ILD.
- It is conditionally recommended that patients with SARD-ILD do not receive leflunomide, methotrexate, tumor necrosis factor inhibitors, or abatacept as first-line ILD treatment.
- If SARD-ILD progresses despite first-line therapy, mycophenolate, rituximab, cyclophosphamide, and nintedanib are potential secondary treatment options.
- If RA-ILD progresses following initial therapy, pirfenidone is a treatment option.
- The guidelines conditionally recommend against pirfenidone as a secondary treatment option for SARD-ILD other than RA-ILD.
These summary guidelines appear “comprehensive,” but there has yet to be information published on the basis of these recommendations, Elizabeth Volkmann, MD, said in an interview.
“It’s important to understand that we don’t know whether most of these recommendations were just driven by expert opinion versus actual evidence from randomized, controlled clinical trials,” said Dr. Volkmann, who codirects the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles. She was not involved with creating the guidelines.
She expects that many of the recommendations for first- and second-line ILD treatment options were based on expert opinion, as there have been no randomized clinical trials looking at that specific topic, she said. For example, nintedanib is conditionally recommended as a first-line treatment option for SSc-ILD, but as a second-line treatment for SjD-ILD, IIM-ILD, and MCTD-ILD. “There’s no literature to support one or the other – whether nintedanib is first-line or second-line [treatment].”
The decision to publish the summary recommendations online prior to peer review is unusual, she said, as these recommendations could be altered during that process; however, Dr. Bernstein noted that was not likely.
By releasing the summary guideline now, the ACR can “get the needed information to clinicians earlier as the manuscript goes through its remaining stages and is finalized,” an ACR representative explained.
Prior to the expected publication of these guidelines in early 2024, Dr. Volkmann noted that the American Thoracic Society will be publishing guidelines on the treatment of SSc-ILD in the American Journal of Respiratory and Critical Care Medicine in September.
Dr. Bernstein reported grants/contracts with the Department of Defense, the Scleroderma Research Foundation, the National Institutes of Health, Eicos, Boehringer Ingelheim, Kadmon, and Pfizer. Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and GlaxoSmithKline and institutional support for performing studies on systemic sclerosis for Kadmon, Boehringer Ingelheim, Horizon, and Prometheus.
A version of this article first appeared on Medscape.com.
The American College of Rheumatology has released a summary of upcoming guidelines on screening, monitoring, and treatment for interstitial lung disease (ILD) in patients with systemic autoimmune rheumatic disease.
The recommendations apply to adults with rheumatic diseases at greater risk for ILD: rheumatoid arthritis, systemic sclerosis (SSc), mixed connective tissue disease (MCTD), Sjögren’s disease (SjD), and idiopathic inflammatory myopathies (IIM).
“Interstitial lung disease is a major cause of morbidity and mortality across several systemic autoimmune rheumatic diseases,” Sindhu R. Johnson, MD, PhD, lead author of the new guidelines and director of the clinical epidemiology and health care research program at the University of Toronto, said in an ACR press release. “Guidance was needed for which tests to use for screening and monitoring this particular disease.”
The two documents are summaries of part of a larger manuscript currently awaiting peer review, according to the ACR, and the final guidelines are anticipated to be published by early 2024.
The recommendations were developed using “the best available evidence and consensus across a range of expert opinions and incorporated patient values and preferences,” according to the press release.
Highlights of recommendations for screening and monitoring ILD are:
- Providers can screen patients at higher risk for ILD with pulmonary function tests (PFTs) and high-resolution CT of the chest.
- PFTs, chest high-resolution CT, and ambulatory desaturation testing are conditionally recommended for monitoring ILD progression.
- It is conditionally recommended that providers do not use 6-minute walk test distance, chest radiography, or bronchoscopy for screening or monitoring disease.
- It is suggested that patients with IIM-ILD and SSc-ILD receive PFTs for monitoring every 3-6 months during the first year, then less frequently once stable.
- It is suggested that patients with RA-ILD, SjD-ILD, and MCTD-ILD receive PFTs every 3-12 months for the first year, then less frequently once stable.
Suggestions on how often to screen for ILD were not present in the summary documents, but will be made available in the larger manuscript, said Elana Bernstein, MD, director of the Columbia University Medical Center/New York–Presbyterian Hospital scleroderma program, New York. She is co–first author of the guidelines.
Nearly all recommendations are conditional, primarily because the certainty of evidence behind many of these recommendations is low or very low, she said in an interview. More clinical data on ILD in patients with rheumatic disease would help strengthen evidence, she said, particularly for best practices in frequency of testing. “We need more research on how often patients should be screened for ILD and how often they should be monitored for ILD progression,” she said. “That would enable us to provide recommendations, rather than just suggestions.”
Highlights of recommendations for ILD treatment are:
- The guidelines strongly recommend against using glucocorticoids for first-line ILD treatment in patients with SSc-ILD.
- Short-term glucocorticoids are conditionally recommended as a first-line ILD treatment for patients with systemic autoimmune rheumatic disease–related ILD (SARD-ILD), excluding SSc-ILD.
- Mycophenolate, azathioprine, rituximab, and cyclophosphamide are all potential first-line ILD treatment options for patients with SARD-ILD.
- It is conditionally recommended that patients with SARD-ILD do not receive leflunomide, methotrexate, tumor necrosis factor inhibitors, or abatacept as first-line ILD treatment.
- If SARD-ILD progresses despite first-line therapy, mycophenolate, rituximab, cyclophosphamide, and nintedanib are potential secondary treatment options.
- If RA-ILD progresses following initial therapy, pirfenidone is a treatment option.
- The guidelines conditionally recommend against pirfenidone as a secondary treatment option for SARD-ILD other than RA-ILD.
These summary guidelines appear “comprehensive,” but there has yet to be information published on the basis of these recommendations, Elizabeth Volkmann, MD, said in an interview.
“It’s important to understand that we don’t know whether most of these recommendations were just driven by expert opinion versus actual evidence from randomized, controlled clinical trials,” said Dr. Volkmann, who codirects the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles. She was not involved with creating the guidelines.
She expects that many of the recommendations for first- and second-line ILD treatment options were based on expert opinion, as there have been no randomized clinical trials looking at that specific topic, she said. For example, nintedanib is conditionally recommended as a first-line treatment option for SSc-ILD, but as a second-line treatment for SjD-ILD, IIM-ILD, and MCTD-ILD. “There’s no literature to support one or the other – whether nintedanib is first-line or second-line [treatment].”
The decision to publish the summary recommendations online prior to peer review is unusual, she said, as these recommendations could be altered during that process; however, Dr. Bernstein noted that was not likely.
By releasing the summary guideline now, the ACR can “get the needed information to clinicians earlier as the manuscript goes through its remaining stages and is finalized,” an ACR representative explained.
Prior to the expected publication of these guidelines in early 2024, Dr. Volkmann noted that the American Thoracic Society will be publishing guidelines on the treatment of SSc-ILD in the American Journal of Respiratory and Critical Care Medicine in September.
Dr. Bernstein reported grants/contracts with the Department of Defense, the Scleroderma Research Foundation, the National Institutes of Health, Eicos, Boehringer Ingelheim, Kadmon, and Pfizer. Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and GlaxoSmithKline and institutional support for performing studies on systemic sclerosis for Kadmon, Boehringer Ingelheim, Horizon, and Prometheus.
A version of this article first appeared on Medscape.com.
FDA warns AstraZeneca over ‘misleading claims’ about COPD drug
Promotional materials for the drug Breztri (budesonide/formoterol fumarate/glycopyrrolate inhaled) suggest that the drug has a positive effect on all-cause mortality for COPD patients, but the referenced clinical trial does not support that claim, the FDA letter states.
The FDA issued the warning letter on Aug. 4 and published the letter online on Aug. 15.
The sales aid highlights a 49% observed relative difference in time to all-cause mortality (ACM) over 1 year between Breztri and long-acting muscarinic antagonist/long-acting beta agonist (LAMA/LABA) inhalers.
Because of “statistical testing hierarchy failure” as well as confounding factors such as the removal of patients from inhaled corticosteroids (ICS) prior to entering the treatment arm of the trial, “no conclusions about the effect of Breztri on ACM can be drawn from the [clinical] trial,” the FDA wrote. “To date, no drug has been shown to improve ACM in COPD.”
The Breztri sales aid also states that there was a 20% reduction of severe exacerbations in patients using Breztri compared with patients using ICS/LABA. However, in the cited clinical trial, “the reduction in severe exacerbations was not statistically significant for patients treated with Breztri relative to comparator groups,” according to the FDA.
AstraZeneca has 15 working days from the receipt of the letter to respond in writing with “any plan for discontinuing use of such communications, or for ceasing distribution of Breztri,” the agency wrote.
A version of this article appeared on Medscape.com.
Promotional materials for the drug Breztri (budesonide/formoterol fumarate/glycopyrrolate inhaled) suggest that the drug has a positive effect on all-cause mortality for COPD patients, but the referenced clinical trial does not support that claim, the FDA letter states.
The FDA issued the warning letter on Aug. 4 and published the letter online on Aug. 15.
The sales aid highlights a 49% observed relative difference in time to all-cause mortality (ACM) over 1 year between Breztri and long-acting muscarinic antagonist/long-acting beta agonist (LAMA/LABA) inhalers.
Because of “statistical testing hierarchy failure” as well as confounding factors such as the removal of patients from inhaled corticosteroids (ICS) prior to entering the treatment arm of the trial, “no conclusions about the effect of Breztri on ACM can be drawn from the [clinical] trial,” the FDA wrote. “To date, no drug has been shown to improve ACM in COPD.”
The Breztri sales aid also states that there was a 20% reduction of severe exacerbations in patients using Breztri compared with patients using ICS/LABA. However, in the cited clinical trial, “the reduction in severe exacerbations was not statistically significant for patients treated with Breztri relative to comparator groups,” according to the FDA.
AstraZeneca has 15 working days from the receipt of the letter to respond in writing with “any plan for discontinuing use of such communications, or for ceasing distribution of Breztri,” the agency wrote.
A version of this article appeared on Medscape.com.
Promotional materials for the drug Breztri (budesonide/formoterol fumarate/glycopyrrolate inhaled) suggest that the drug has a positive effect on all-cause mortality for COPD patients, but the referenced clinical trial does not support that claim, the FDA letter states.
The FDA issued the warning letter on Aug. 4 and published the letter online on Aug. 15.
The sales aid highlights a 49% observed relative difference in time to all-cause mortality (ACM) over 1 year between Breztri and long-acting muscarinic antagonist/long-acting beta agonist (LAMA/LABA) inhalers.
Because of “statistical testing hierarchy failure” as well as confounding factors such as the removal of patients from inhaled corticosteroids (ICS) prior to entering the treatment arm of the trial, “no conclusions about the effect of Breztri on ACM can be drawn from the [clinical] trial,” the FDA wrote. “To date, no drug has been shown to improve ACM in COPD.”
The Breztri sales aid also states that there was a 20% reduction of severe exacerbations in patients using Breztri compared with patients using ICS/LABA. However, in the cited clinical trial, “the reduction in severe exacerbations was not statistically significant for patients treated with Breztri relative to comparator groups,” according to the FDA.
AstraZeneca has 15 working days from the receipt of the letter to respond in writing with “any plan for discontinuing use of such communications, or for ceasing distribution of Breztri,” the agency wrote.
A version of this article appeared on Medscape.com.
Autoantibody against enteric nervous system protein linked to GI dysfunction in systemic sclerosis
(SSc), new research suggests. Researchers also found that gephyrin is expressed in the patient’s enteric nervous system (ENS), which regulates gut motility.
“While there are many antibodies that are helpful in identifying patients at risk for extraintestinal complications of this disease, markers that identify patients at higher risk for gastrointestinal complications are limited. Furthermore, the biological mechanisms that cause and perpetuate the progression of gastrointestinal disease in scleroderma are not well understood, making it challenging to distinguish between patients whose gastrointestinal disease will progress from those whose GI disease will remain stable/mild,” Zsuzsanna H. McMahan, MD, MHS, told this news organization in an email. Dr. McMahan is co–first author on the study along with Subhash Kulkarni, PhD. They conducted the research with colleagues when they both worked at Johns Hopkins University in Baltimore, Md.
When asked for comment, Kimberly Lakin, MD, MS, assistant professor of medicine at Weill Cornell Medicine and a rheumatologist at Hospital for Special Surgery, New York, called the study “interesting and novel.”
“Not only did [antigephyrin antibodies] correlate with the presence of lower GI symptoms, but also higher levels of antibodies correlated with worse lower GI symptoms. This suggests that not only could this antibody be used to predict who may have constipation and potentially need more aggressive GI interventions, but it may also be useful in quantifying GI severity in systemic sclerosis, although more research is still needed,” said Dr. Lakin, who was not involved with the research.
The study was published online in Arthritis & Rheumatology.
In the cross-sectional study, researchers identified gephyrin as an autoantigen in sera from a single patient with SSc by isolating it from immunoprecipitations performed with murine myenteric plexus neuron lysates, and then characterizing it by mass spectrometry and validating it in further assays. That patient had GI dysfunction but no defined SSc-associated autoantibodies.
Dr. McMahan and colleagues then investigated the prevalence of the autoantibody by screening the sera of 188 patients with SSc who presented consecutively to the Johns Hopkins Scleroderma Center between April 2016 and August 2017, as well as 40 controls, and compared GI symptom severity between antibody-positive and antibody-negative patients with SSc.
A total of 16 (8.5%) of the 188 patients with SSc had antigephyrin antibodies, compared with none of the controls. Of these 16 patients, 4 had no other defined SSc antibodies. In the SSc cohort, severe constipation was more common in antigephyrin antibody–positive patients, compared with antibody-negative patients (46% vs. 15%). Antibody-positive patients also had higher constipation scores, and severe distension and bloating occurred in the antibody-positive group more than twice as often (54% vs. 25%).
Patients with severe constipation, distention, and bloating had higher antigephyrin antibody levels. After adjusting for confounders such as disease duration, patients with severe constipation were nearly five times as likely (odds ratio, 4.74; P = .010) to be antigephyrin antibody–positive, and patients with severe distention and bloating were nearly four times as likely (OR, 3.71; P = .027) to be antibody-positive.
Last, the authors showed via immunohistochemistry that gephyrin is expressed in the myenteric ganglia of human GI tissue.
“Gastrointestinal function is highly regulated by the ENS, so it is interesting that antibodies that target a protein expressed by ENS cells (gephyrin) were identified in patients with scleroderma who have severe lower bowel dysfunction,” said Dr. McMahan, who is associate professor in the division of rheumatology and codirector of the scleroderma program at the University of Texas Health Science Center at Houston. “Gephyrin is a key mediator of normal communications between nerves in the gut, so it is tantalizing to speculate that autoimmune-mediated disruption (e.g., an inhibitory or blocking antibody) in neural (ENS) communications in the gut might lead to impaired bowel transit and prominent constipation.”
The study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and other NIH grants, as well as the Scleroderma Research Foundation, Rheumatology Research Foundation, Jerome L. Greene Foundation, Martha McCrory Professorship, and Chresanthe Stauraluakis Memorial Discovery Fund. The study authors and Dr. Lakin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SSc), new research suggests. Researchers also found that gephyrin is expressed in the patient’s enteric nervous system (ENS), which regulates gut motility.
“While there are many antibodies that are helpful in identifying patients at risk for extraintestinal complications of this disease, markers that identify patients at higher risk for gastrointestinal complications are limited. Furthermore, the biological mechanisms that cause and perpetuate the progression of gastrointestinal disease in scleroderma are not well understood, making it challenging to distinguish between patients whose gastrointestinal disease will progress from those whose GI disease will remain stable/mild,” Zsuzsanna H. McMahan, MD, MHS, told this news organization in an email. Dr. McMahan is co–first author on the study along with Subhash Kulkarni, PhD. They conducted the research with colleagues when they both worked at Johns Hopkins University in Baltimore, Md.
When asked for comment, Kimberly Lakin, MD, MS, assistant professor of medicine at Weill Cornell Medicine and a rheumatologist at Hospital for Special Surgery, New York, called the study “interesting and novel.”
“Not only did [antigephyrin antibodies] correlate with the presence of lower GI symptoms, but also higher levels of antibodies correlated with worse lower GI symptoms. This suggests that not only could this antibody be used to predict who may have constipation and potentially need more aggressive GI interventions, but it may also be useful in quantifying GI severity in systemic sclerosis, although more research is still needed,” said Dr. Lakin, who was not involved with the research.
The study was published online in Arthritis & Rheumatology.
In the cross-sectional study, researchers identified gephyrin as an autoantigen in sera from a single patient with SSc by isolating it from immunoprecipitations performed with murine myenteric plexus neuron lysates, and then characterizing it by mass spectrometry and validating it in further assays. That patient had GI dysfunction but no defined SSc-associated autoantibodies.
Dr. McMahan and colleagues then investigated the prevalence of the autoantibody by screening the sera of 188 patients with SSc who presented consecutively to the Johns Hopkins Scleroderma Center between April 2016 and August 2017, as well as 40 controls, and compared GI symptom severity between antibody-positive and antibody-negative patients with SSc.
A total of 16 (8.5%) of the 188 patients with SSc had antigephyrin antibodies, compared with none of the controls. Of these 16 patients, 4 had no other defined SSc antibodies. In the SSc cohort, severe constipation was more common in antigephyrin antibody–positive patients, compared with antibody-negative patients (46% vs. 15%). Antibody-positive patients also had higher constipation scores, and severe distension and bloating occurred in the antibody-positive group more than twice as often (54% vs. 25%).
Patients with severe constipation, distention, and bloating had higher antigephyrin antibody levels. After adjusting for confounders such as disease duration, patients with severe constipation were nearly five times as likely (odds ratio, 4.74; P = .010) to be antigephyrin antibody–positive, and patients with severe distention and bloating were nearly four times as likely (OR, 3.71; P = .027) to be antibody-positive.
Last, the authors showed via immunohistochemistry that gephyrin is expressed in the myenteric ganglia of human GI tissue.
“Gastrointestinal function is highly regulated by the ENS, so it is interesting that antibodies that target a protein expressed by ENS cells (gephyrin) were identified in patients with scleroderma who have severe lower bowel dysfunction,” said Dr. McMahan, who is associate professor in the division of rheumatology and codirector of the scleroderma program at the University of Texas Health Science Center at Houston. “Gephyrin is a key mediator of normal communications between nerves in the gut, so it is tantalizing to speculate that autoimmune-mediated disruption (e.g., an inhibitory or blocking antibody) in neural (ENS) communications in the gut might lead to impaired bowel transit and prominent constipation.”
The study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and other NIH grants, as well as the Scleroderma Research Foundation, Rheumatology Research Foundation, Jerome L. Greene Foundation, Martha McCrory Professorship, and Chresanthe Stauraluakis Memorial Discovery Fund. The study authors and Dr. Lakin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SSc), new research suggests. Researchers also found that gephyrin is expressed in the patient’s enteric nervous system (ENS), which regulates gut motility.
“While there are many antibodies that are helpful in identifying patients at risk for extraintestinal complications of this disease, markers that identify patients at higher risk for gastrointestinal complications are limited. Furthermore, the biological mechanisms that cause and perpetuate the progression of gastrointestinal disease in scleroderma are not well understood, making it challenging to distinguish between patients whose gastrointestinal disease will progress from those whose GI disease will remain stable/mild,” Zsuzsanna H. McMahan, MD, MHS, told this news organization in an email. Dr. McMahan is co–first author on the study along with Subhash Kulkarni, PhD. They conducted the research with colleagues when they both worked at Johns Hopkins University in Baltimore, Md.
When asked for comment, Kimberly Lakin, MD, MS, assistant professor of medicine at Weill Cornell Medicine and a rheumatologist at Hospital for Special Surgery, New York, called the study “interesting and novel.”
“Not only did [antigephyrin antibodies] correlate with the presence of lower GI symptoms, but also higher levels of antibodies correlated with worse lower GI symptoms. This suggests that not only could this antibody be used to predict who may have constipation and potentially need more aggressive GI interventions, but it may also be useful in quantifying GI severity in systemic sclerosis, although more research is still needed,” said Dr. Lakin, who was not involved with the research.
The study was published online in Arthritis & Rheumatology.
In the cross-sectional study, researchers identified gephyrin as an autoantigen in sera from a single patient with SSc by isolating it from immunoprecipitations performed with murine myenteric plexus neuron lysates, and then characterizing it by mass spectrometry and validating it in further assays. That patient had GI dysfunction but no defined SSc-associated autoantibodies.
Dr. McMahan and colleagues then investigated the prevalence of the autoantibody by screening the sera of 188 patients with SSc who presented consecutively to the Johns Hopkins Scleroderma Center between April 2016 and August 2017, as well as 40 controls, and compared GI symptom severity between antibody-positive and antibody-negative patients with SSc.
A total of 16 (8.5%) of the 188 patients with SSc had antigephyrin antibodies, compared with none of the controls. Of these 16 patients, 4 had no other defined SSc antibodies. In the SSc cohort, severe constipation was more common in antigephyrin antibody–positive patients, compared with antibody-negative patients (46% vs. 15%). Antibody-positive patients also had higher constipation scores, and severe distension and bloating occurred in the antibody-positive group more than twice as often (54% vs. 25%).
Patients with severe constipation, distention, and bloating had higher antigephyrin antibody levels. After adjusting for confounders such as disease duration, patients with severe constipation were nearly five times as likely (odds ratio, 4.74; P = .010) to be antigephyrin antibody–positive, and patients with severe distention and bloating were nearly four times as likely (OR, 3.71; P = .027) to be antibody-positive.
Last, the authors showed via immunohistochemistry that gephyrin is expressed in the myenteric ganglia of human GI tissue.
“Gastrointestinal function is highly regulated by the ENS, so it is interesting that antibodies that target a protein expressed by ENS cells (gephyrin) were identified in patients with scleroderma who have severe lower bowel dysfunction,” said Dr. McMahan, who is associate professor in the division of rheumatology and codirector of the scleroderma program at the University of Texas Health Science Center at Houston. “Gephyrin is a key mediator of normal communications between nerves in the gut, so it is tantalizing to speculate that autoimmune-mediated disruption (e.g., an inhibitory or blocking antibody) in neural (ENS) communications in the gut might lead to impaired bowel transit and prominent constipation.”
The study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and other NIH grants, as well as the Scleroderma Research Foundation, Rheumatology Research Foundation, Jerome L. Greene Foundation, Martha McCrory Professorship, and Chresanthe Stauraluakis Memorial Discovery Fund. The study authors and Dr. Lakin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Low-dose steroids may not increase cardiovascular risk in rheumatoid arthritis
A daily prednisolone dose of 5 mg or higher is associated with increased risk for major adverse cardiovascular events (MACE) among patients with rheumatoid arthritis (RA), data suggest. Patients taking daily doses below this threshold did not appear to have an increased risk of MACE, compared with those not taking glucocorticoids (GCs).
Other studies of GCs and CV risk among RA patients have yielded conflicting results, especially for low-dose GCs. Findings from a 2020 study published in PLOS Medicine suggested that patients who had several immune-mediated inflammatory diseases – including RA – and who took less than a 5-mg prednisolone-equivalent dose daily had 74% higher risk for all-cause CVD, compared with nonusers. But results from a 2021 study published in Annals of the Rheumatic Diseases suggested that a daily prednisone dose of 4 mg or less did not increase cardiovascular events over a period of 6 months to 1 year.
These contradictory results were “primarily due to incomplete control of confounding variables, such as failure to adjust for C-reactive protein (CRP) levels,” Dr. Tam said. “Our study aimed to use a big data analytical approach to determine the effect of systemic GC dose and duration on the risk of major adverse cardiovascular events in patients with RA, while controlling for systemic inflammation, traditional CV risk factors, and other therapies.”
Is there a ‘safe’ dose for glucocorticoids?
To analyze this relationship, Dr. Lam and colleagues used the Hospital Authority Data Collaboration Laboratory, a citywide health care database. The investigators recruited patients with RA who had no history of MACE from 2006 to 2015 and followed them until the end of 2018. The primary outcome was the first occurrence of a MACE, defined as a composite of myocardial infarction (MI), unstable angina, ischemic or hemorrhagic cerebrovascular accident, transient ischemic attack, and CV death.
The study was published in Annals of the Rheumatic Diseases.
The analysis included 12,233 patients with RA and had over 105,826 person-years of follow-up. The average follow-up time was 8.7 years. During the study period, 860 patients had their first MACE. After controlling for confounding factors, a daily prednisolone dose of 5 mg or higher doubled the risk for MACE, compared with GC nonusers. MACE risk increased by 7% per month.
Long-term glucocorticoid use discouraged
Daily doses of less than 5 mg were not associated with higher MACE risk, but more research is necessary to understand whether these low doses are clinically efficacious, Dr. Tam said. “The study results suggest that a very-low-dose GC (less than 5 mg prednisolone daily) may be cardiovascular risk–neutral. However, further evaluation is needed to determine whether this dose is therapeutic. Other potential side effects, such as bone loss, increased infection risk, dyslipidemia, and hyperglycemia, should also be considered.”
Both the American College of Rheumatology and the European Alliance of Associations for Rheumatology acknowledge that short-term GCs may be necessary for some RA patients, but they emphasize using the smallest necessary dose for the shortest period possible because of the known toxicity of GCs.
“We recommend stopping GCs as soon as it is clinically feasible, in line with previous recommendations, until these issues are investigated further,” Dr. Tam added.
Dr. Bartels agreed that long-term use of GCs should be avoided if possible, even at lower doses, because although CV risk may be less of an issue, studies have shown an increased risk for infection even at GC doses of less than 5 mg a day.
How might risk increase with dose?
While the study showed a distinct difference in risk with doses of prednisolone higher and lower than 5 mg, more information on how risk increases with dose could be useful, said Beth Wallace, MD, an assistant professor in internal medicine at the University of Michigan, Ann Arbor, and a staff rheumatologist at the VA Ann Arbor Healthcare Center. She was also unaffiliated with the research. “If someone is on 5-10 mg ... how much better is that than being on 10-20 mg or being on 20-30 mg?” she asked. While these study findings are “very important,” she said, it would be useful to know the risk associated with 7.5 mg vs. a higher dose.
But even in this relatively healthy population in Hong Kong, “taking more than 5 mg of prednisolone doubles the risk of cardiovascular disease,” Dr. Wallace added. This is important for clinicians to know, especially if they are more cautious about prescribing steroids to older or sicker patients but are “using [the drugs] a little more indiscriminately in younger people and healthier people.”
The study did not receive outside funding. Dr. Tam and Dr. Bartels report no relevant financial relationships. Dr. Wallace has received a grant from the Department of Veterans Affairs Administration to study steroid tapering in RA.
A version of this article first appeared on Medscape.com.
A daily prednisolone dose of 5 mg or higher is associated with increased risk for major adverse cardiovascular events (MACE) among patients with rheumatoid arthritis (RA), data suggest. Patients taking daily doses below this threshold did not appear to have an increased risk of MACE, compared with those not taking glucocorticoids (GCs).
Other studies of GCs and CV risk among RA patients have yielded conflicting results, especially for low-dose GCs. Findings from a 2020 study published in PLOS Medicine suggested that patients who had several immune-mediated inflammatory diseases – including RA – and who took less than a 5-mg prednisolone-equivalent dose daily had 74% higher risk for all-cause CVD, compared with nonusers. But results from a 2021 study published in Annals of the Rheumatic Diseases suggested that a daily prednisone dose of 4 mg or less did not increase cardiovascular events over a period of 6 months to 1 year.
These contradictory results were “primarily due to incomplete control of confounding variables, such as failure to adjust for C-reactive protein (CRP) levels,” Dr. Tam said. “Our study aimed to use a big data analytical approach to determine the effect of systemic GC dose and duration on the risk of major adverse cardiovascular events in patients with RA, while controlling for systemic inflammation, traditional CV risk factors, and other therapies.”
Is there a ‘safe’ dose for glucocorticoids?
To analyze this relationship, Dr. Lam and colleagues used the Hospital Authority Data Collaboration Laboratory, a citywide health care database. The investigators recruited patients with RA who had no history of MACE from 2006 to 2015 and followed them until the end of 2018. The primary outcome was the first occurrence of a MACE, defined as a composite of myocardial infarction (MI), unstable angina, ischemic or hemorrhagic cerebrovascular accident, transient ischemic attack, and CV death.
The study was published in Annals of the Rheumatic Diseases.
The analysis included 12,233 patients with RA and had over 105,826 person-years of follow-up. The average follow-up time was 8.7 years. During the study period, 860 patients had their first MACE. After controlling for confounding factors, a daily prednisolone dose of 5 mg or higher doubled the risk for MACE, compared with GC nonusers. MACE risk increased by 7% per month.
Long-term glucocorticoid use discouraged
Daily doses of less than 5 mg were not associated with higher MACE risk, but more research is necessary to understand whether these low doses are clinically efficacious, Dr. Tam said. “The study results suggest that a very-low-dose GC (less than 5 mg prednisolone daily) may be cardiovascular risk–neutral. However, further evaluation is needed to determine whether this dose is therapeutic. Other potential side effects, such as bone loss, increased infection risk, dyslipidemia, and hyperglycemia, should also be considered.”
Both the American College of Rheumatology and the European Alliance of Associations for Rheumatology acknowledge that short-term GCs may be necessary for some RA patients, but they emphasize using the smallest necessary dose for the shortest period possible because of the known toxicity of GCs.
“We recommend stopping GCs as soon as it is clinically feasible, in line with previous recommendations, until these issues are investigated further,” Dr. Tam added.
Dr. Bartels agreed that long-term use of GCs should be avoided if possible, even at lower doses, because although CV risk may be less of an issue, studies have shown an increased risk for infection even at GC doses of less than 5 mg a day.
How might risk increase with dose?
While the study showed a distinct difference in risk with doses of prednisolone higher and lower than 5 mg, more information on how risk increases with dose could be useful, said Beth Wallace, MD, an assistant professor in internal medicine at the University of Michigan, Ann Arbor, and a staff rheumatologist at the VA Ann Arbor Healthcare Center. She was also unaffiliated with the research. “If someone is on 5-10 mg ... how much better is that than being on 10-20 mg or being on 20-30 mg?” she asked. While these study findings are “very important,” she said, it would be useful to know the risk associated with 7.5 mg vs. a higher dose.
But even in this relatively healthy population in Hong Kong, “taking more than 5 mg of prednisolone doubles the risk of cardiovascular disease,” Dr. Wallace added. This is important for clinicians to know, especially if they are more cautious about prescribing steroids to older or sicker patients but are “using [the drugs] a little more indiscriminately in younger people and healthier people.”
The study did not receive outside funding. Dr. Tam and Dr. Bartels report no relevant financial relationships. Dr. Wallace has received a grant from the Department of Veterans Affairs Administration to study steroid tapering in RA.
A version of this article first appeared on Medscape.com.
A daily prednisolone dose of 5 mg or higher is associated with increased risk for major adverse cardiovascular events (MACE) among patients with rheumatoid arthritis (RA), data suggest. Patients taking daily doses below this threshold did not appear to have an increased risk of MACE, compared with those not taking glucocorticoids (GCs).
Other studies of GCs and CV risk among RA patients have yielded conflicting results, especially for low-dose GCs. Findings from a 2020 study published in PLOS Medicine suggested that patients who had several immune-mediated inflammatory diseases – including RA – and who took less than a 5-mg prednisolone-equivalent dose daily had 74% higher risk for all-cause CVD, compared with nonusers. But results from a 2021 study published in Annals of the Rheumatic Diseases suggested that a daily prednisone dose of 4 mg or less did not increase cardiovascular events over a period of 6 months to 1 year.
These contradictory results were “primarily due to incomplete control of confounding variables, such as failure to adjust for C-reactive protein (CRP) levels,” Dr. Tam said. “Our study aimed to use a big data analytical approach to determine the effect of systemic GC dose and duration on the risk of major adverse cardiovascular events in patients with RA, while controlling for systemic inflammation, traditional CV risk factors, and other therapies.”
Is there a ‘safe’ dose for glucocorticoids?
To analyze this relationship, Dr. Lam and colleagues used the Hospital Authority Data Collaboration Laboratory, a citywide health care database. The investigators recruited patients with RA who had no history of MACE from 2006 to 2015 and followed them until the end of 2018. The primary outcome was the first occurrence of a MACE, defined as a composite of myocardial infarction (MI), unstable angina, ischemic or hemorrhagic cerebrovascular accident, transient ischemic attack, and CV death.
The study was published in Annals of the Rheumatic Diseases.
The analysis included 12,233 patients with RA and had over 105,826 person-years of follow-up. The average follow-up time was 8.7 years. During the study period, 860 patients had their first MACE. After controlling for confounding factors, a daily prednisolone dose of 5 mg or higher doubled the risk for MACE, compared with GC nonusers. MACE risk increased by 7% per month.
Long-term glucocorticoid use discouraged
Daily doses of less than 5 mg were not associated with higher MACE risk, but more research is necessary to understand whether these low doses are clinically efficacious, Dr. Tam said. “The study results suggest that a very-low-dose GC (less than 5 mg prednisolone daily) may be cardiovascular risk–neutral. However, further evaluation is needed to determine whether this dose is therapeutic. Other potential side effects, such as bone loss, increased infection risk, dyslipidemia, and hyperglycemia, should also be considered.”
Both the American College of Rheumatology and the European Alliance of Associations for Rheumatology acknowledge that short-term GCs may be necessary for some RA patients, but they emphasize using the smallest necessary dose for the shortest period possible because of the known toxicity of GCs.
“We recommend stopping GCs as soon as it is clinically feasible, in line with previous recommendations, until these issues are investigated further,” Dr. Tam added.
Dr. Bartels agreed that long-term use of GCs should be avoided if possible, even at lower doses, because although CV risk may be less of an issue, studies have shown an increased risk for infection even at GC doses of less than 5 mg a day.
How might risk increase with dose?
While the study showed a distinct difference in risk with doses of prednisolone higher and lower than 5 mg, more information on how risk increases with dose could be useful, said Beth Wallace, MD, an assistant professor in internal medicine at the University of Michigan, Ann Arbor, and a staff rheumatologist at the VA Ann Arbor Healthcare Center. She was also unaffiliated with the research. “If someone is on 5-10 mg ... how much better is that than being on 10-20 mg or being on 20-30 mg?” she asked. While these study findings are “very important,” she said, it would be useful to know the risk associated with 7.5 mg vs. a higher dose.
But even in this relatively healthy population in Hong Kong, “taking more than 5 mg of prednisolone doubles the risk of cardiovascular disease,” Dr. Wallace added. This is important for clinicians to know, especially if they are more cautious about prescribing steroids to older or sicker patients but are “using [the drugs] a little more indiscriminately in younger people and healthier people.”
The study did not receive outside funding. Dr. Tam and Dr. Bartels report no relevant financial relationships. Dr. Wallace has received a grant from the Department of Veterans Affairs Administration to study steroid tapering in RA.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
Disclosure inaccuracies common in top rheumatology journals
TOPLINE:
Conflicts of interests in rheumatology research – particularly for clinical trials – are often incorrectly reported, according to a new analysis.
METHODOLOGY:
- Researchers reviewed the first 50 clinical research reports, reviews, and editorials published in 2019 by Arthritis & Rheumatology, Arthritis Care & Research, and Seminars in Arthritis & Rheumatism.
- They cross-checked disclosures from the first, second, and last authors of each paper (150 total) with payment reports from the Open Payments Database (OPD).
- Payment reports captured consulting fees, honoraria, and speaker/faculty compensation in the 36 months prior to an article’s publication.
TAKEAWAY:
- A total of 87% of the 135 authors with potential conflicts of interest (PCOI) inaccurately reported their disclosures.
- All authors of the included 14 clinical trial publications either did not report or underreported PCOI.
- The total nondisclosed dollar amount was $5,190,901, and the total underdisclosed amount was $4,135,126.
IN PRACTICE:
“Improved community education and firmer expectations would permit readers to better assess any possible impact of PCOI on publications,” the authors wrote.
STUDY DETAILS:
Mary Guan, MD, of the NYU Grossman School of Medicine, New York, led the research. The study was published online in Arthritis Care & Research on July 31, 2023.
LIMITATIONS:
The OPD does not include non–U.S.-based authors and authors without a medical degree, and there are no data on the accuracy of the database. The analysis does not provide insight into why these discrepancies occurred and if they were unintentional errors.
DISCLOSURES:
One coauthor reported consulting fees from Federation Bio, Fortress Biotech, Horizon, and Sobi and receiving grants or contracts paid to his institution from Horizon and Hikma. Dr. Guan and senior author Aryeh Abeles, MD, reported no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Conflicts of interests in rheumatology research – particularly for clinical trials – are often incorrectly reported, according to a new analysis.
METHODOLOGY:
- Researchers reviewed the first 50 clinical research reports, reviews, and editorials published in 2019 by Arthritis & Rheumatology, Arthritis Care & Research, and Seminars in Arthritis & Rheumatism.
- They cross-checked disclosures from the first, second, and last authors of each paper (150 total) with payment reports from the Open Payments Database (OPD).
- Payment reports captured consulting fees, honoraria, and speaker/faculty compensation in the 36 months prior to an article’s publication.
TAKEAWAY:
- A total of 87% of the 135 authors with potential conflicts of interest (PCOI) inaccurately reported their disclosures.
- All authors of the included 14 clinical trial publications either did not report or underreported PCOI.
- The total nondisclosed dollar amount was $5,190,901, and the total underdisclosed amount was $4,135,126.
IN PRACTICE:
“Improved community education and firmer expectations would permit readers to better assess any possible impact of PCOI on publications,” the authors wrote.
STUDY DETAILS:
Mary Guan, MD, of the NYU Grossman School of Medicine, New York, led the research. The study was published online in Arthritis Care & Research on July 31, 2023.
LIMITATIONS:
The OPD does not include non–U.S.-based authors and authors without a medical degree, and there are no data on the accuracy of the database. The analysis does not provide insight into why these discrepancies occurred and if they were unintentional errors.
DISCLOSURES:
One coauthor reported consulting fees from Federation Bio, Fortress Biotech, Horizon, and Sobi and receiving grants or contracts paid to his institution from Horizon and Hikma. Dr. Guan and senior author Aryeh Abeles, MD, reported no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Conflicts of interests in rheumatology research – particularly for clinical trials – are often incorrectly reported, according to a new analysis.
METHODOLOGY:
- Researchers reviewed the first 50 clinical research reports, reviews, and editorials published in 2019 by Arthritis & Rheumatology, Arthritis Care & Research, and Seminars in Arthritis & Rheumatism.
- They cross-checked disclosures from the first, second, and last authors of each paper (150 total) with payment reports from the Open Payments Database (OPD).
- Payment reports captured consulting fees, honoraria, and speaker/faculty compensation in the 36 months prior to an article’s publication.
TAKEAWAY:
- A total of 87% of the 135 authors with potential conflicts of interest (PCOI) inaccurately reported their disclosures.
- All authors of the included 14 clinical trial publications either did not report or underreported PCOI.
- The total nondisclosed dollar amount was $5,190,901, and the total underdisclosed amount was $4,135,126.
IN PRACTICE:
“Improved community education and firmer expectations would permit readers to better assess any possible impact of PCOI on publications,” the authors wrote.
STUDY DETAILS:
Mary Guan, MD, of the NYU Grossman School of Medicine, New York, led the research. The study was published online in Arthritis Care & Research on July 31, 2023.
LIMITATIONS:
The OPD does not include non–U.S.-based authors and authors without a medical degree, and there are no data on the accuracy of the database. The analysis does not provide insight into why these discrepancies occurred and if they were unintentional errors.
DISCLOSURES:
One coauthor reported consulting fees from Federation Bio, Fortress Biotech, Horizon, and Sobi and receiving grants or contracts paid to his institution from Horizon and Hikma. Dr. Guan and senior author Aryeh Abeles, MD, reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ARTHRITIS CARE & RESEARCH
Autoantibodies could help predict cancer risk in scleroderma
TOPLINE:
METHODOLOGY:
- Included patients from the Johns Hopkins Scleroderma Center Research Registry and the University of Pittsburgh Scleroderma Center, Pittsburgh.
- A total of 676 patients with scleroderma and a history of cancer were compared with 687 control patients with scleroderma but without a history of cancer.
- Serum tested via line blot and enzyme-linked immunosorbent assay for an array of scleroderma autoantibodies.
- Examined association between autoantibodies and overall cancer risk.
TAKEAWAYS:
- Anti-POLR3 and monospecific anti-Ro52 were associated with significantly increased overall cancer risk.
- Anti-centromere and anti-U1RNP were associated with a decreased cancer risk.
- These associations remained when looking specifically at cancer-associated scleroderma.
- Patients positive for anti-Ro52 in combination with either anti-U1RNP or anti-Th/To had a decreased risk of cancer, compared with those who had anti-Ro52 alone.
IN PRACTICE:
This study is too preliminary to have practice application.
SOURCE:
Ji Soo Kim, PhD, of John Hopkins University, Baltimore, was the first author of the study, published in Arthritis & Rheumatology on July 24, 2023. Fellow Johns Hopkins researchers Livia Casciola-Rosen, PhD, and Ami A. Shah, MD, were joint senior authors.
DISCLOSURES:
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Donald B. and Dorothy L. Stabler Foundation, the Jerome L. Greene Foundation, the Chresanthe Staurulakis Memorial Discovery Fund, the Martha McCrory Professorship, and the Johns Hopkins inHealth initiative. The authors disclosed the following patents or patent applications: Autoimmune Antigens and Cancer, Materials and Methods for Assessing Cancer Risk and Treating Cancer.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Included patients from the Johns Hopkins Scleroderma Center Research Registry and the University of Pittsburgh Scleroderma Center, Pittsburgh.
- A total of 676 patients with scleroderma and a history of cancer were compared with 687 control patients with scleroderma but without a history of cancer.
- Serum tested via line blot and enzyme-linked immunosorbent assay for an array of scleroderma autoantibodies.
- Examined association between autoantibodies and overall cancer risk.
TAKEAWAYS:
- Anti-POLR3 and monospecific anti-Ro52 were associated with significantly increased overall cancer risk.
- Anti-centromere and anti-U1RNP were associated with a decreased cancer risk.
- These associations remained when looking specifically at cancer-associated scleroderma.
- Patients positive for anti-Ro52 in combination with either anti-U1RNP or anti-Th/To had a decreased risk of cancer, compared with those who had anti-Ro52 alone.
IN PRACTICE:
This study is too preliminary to have practice application.
SOURCE:
Ji Soo Kim, PhD, of John Hopkins University, Baltimore, was the first author of the study, published in Arthritis & Rheumatology on July 24, 2023. Fellow Johns Hopkins researchers Livia Casciola-Rosen, PhD, and Ami A. Shah, MD, were joint senior authors.
DISCLOSURES:
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Donald B. and Dorothy L. Stabler Foundation, the Jerome L. Greene Foundation, the Chresanthe Staurulakis Memorial Discovery Fund, the Martha McCrory Professorship, and the Johns Hopkins inHealth initiative. The authors disclosed the following patents or patent applications: Autoimmune Antigens and Cancer, Materials and Methods for Assessing Cancer Risk and Treating Cancer.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Included patients from the Johns Hopkins Scleroderma Center Research Registry and the University of Pittsburgh Scleroderma Center, Pittsburgh.
- A total of 676 patients with scleroderma and a history of cancer were compared with 687 control patients with scleroderma but without a history of cancer.
- Serum tested via line blot and enzyme-linked immunosorbent assay for an array of scleroderma autoantibodies.
- Examined association between autoantibodies and overall cancer risk.
TAKEAWAYS:
- Anti-POLR3 and monospecific anti-Ro52 were associated with significantly increased overall cancer risk.
- Anti-centromere and anti-U1RNP were associated with a decreased cancer risk.
- These associations remained when looking specifically at cancer-associated scleroderma.
- Patients positive for anti-Ro52 in combination with either anti-U1RNP or anti-Th/To had a decreased risk of cancer, compared with those who had anti-Ro52 alone.
IN PRACTICE:
This study is too preliminary to have practice application.
SOURCE:
Ji Soo Kim, PhD, of John Hopkins University, Baltimore, was the first author of the study, published in Arthritis & Rheumatology on July 24, 2023. Fellow Johns Hopkins researchers Livia Casciola-Rosen, PhD, and Ami A. Shah, MD, were joint senior authors.
DISCLOSURES:
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Donald B. and Dorothy L. Stabler Foundation, the Jerome L. Greene Foundation, the Chresanthe Staurulakis Memorial Discovery Fund, the Martha McCrory Professorship, and the Johns Hopkins inHealth initiative. The authors disclosed the following patents or patent applications: Autoimmune Antigens and Cancer, Materials and Methods for Assessing Cancer Risk and Treating Cancer.
A version of this article appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Tools may predict inflammatory arthritis in at-risk patients
, according to new research from England.
If validated in further studies, a new simple score using common biomarkers may help identify individuals who can be managed in primary care as well as higher-risk patients who should be referred to a rheumatologist.
The researchers designed a second comprehensive score adding genetics and ultrasound as a tool to identify patients at highest risk for IA for intervention studies and to guide clinical monitoring and care by specialists.
Though there are blood markers and early symptoms in patients that may signal a higher risk for IA, “we don’t know what to do with those people yet,” said Kevin D. Deane, MD, PhD, associate professor of medicine and chair in rheumatology research at the University of Colorado at Denver, Aurora.
“Understanding how to assess these people and predict who’s going to go on to get full blown IA that we should actually treat is very beneficial to the field,” Dr. Deane said. He was not involved with the research but had reviewed an early draft of the paper.
Study seeks to stratify at-risk population
For the study, researchers recruited 455 participants from June 2008 to November 2021, primarily through the UK Primary Care Clinical Research Network. All individuals had new musculoskeletal symptoms, a positive test for anticitrullinated protein antibodies (anti-CCP), and no clinical synovitis.
The researchers selected for anti-CCP positivity because these antibodies are associated with a more aggressive arthritis phenotype. Interventional trials have also found that these anti-CCP–positive individuals are the most responsive to disease-modifying antirheumatic therapy prior to IA development. Patients were followed for at least 48 weeks or until an IA diagnosis.
Using data from this cohort, the team ran statistical analyses guided by potential clinical impact. For the simple score, they aimed to ensure that most people who would go on to develop IA would be identified earlier in clinical practice. For the comprehensive score, they wanted to balance the potential harm of giving preventive treatment to someone who would not develop IA with failing to provide preventive treatment to someone who would develop IA, the authors write.
They developed two scores: a simple score to identify people at lower risk for IA and a comprehensive score to stratify high-risk individuals. The simple score used anti-CCP level, rheumatoid factor value, early morning stiffness, and erythrocyte sedimentation rate to calculate risk.
In addition to these factors, the comprehensive score added smoking history, ultrasound abnormalities, genetic markers for the rheumatoid arthritis shared epitope, as well as patient-reported outcomes from the Health Assessment Questionnaire and the visual analogue scale for global pain.
The study was published in the Annals of Internal Medicine.
Simple score rates more than half as low risk
The simple score identified 249 low-risk individuals, defined as having a less than 10% chance of developing IA within 1 year, with a 5% false-negative rate. This score can help determine which individuals do not need to be referred to a specialist even though they have some known risk factors, said Paul Emery, MD, director of the Leeds Biomedical Research Centre and clinical professor at the University of Leeds in England. He is a co–senior author of the research.
“If you had unlimited resources, you’d refer everyone. But in the real world, we would be overloaded in secondary care, and it just wouldn’t work,” he said. “This is a way of making sure the right people are referred into secondary care.”
The comprehensive score identified 119 high-risk individuals, defined as having a 50% chance or greater of developing IA in 5 years, with a false-positive rate of 29%. Of this high-risk group, 40% developed IA within 1 year, and 71% developed IA in 5 years.
Beyond identifying those who should be referred to specialist care, Dr. Emery noted, this score could be used in research studies to find patients for experimental clinical trials aimed at delaying or preventing the onset of IA.
Both Dr. Emery and Dr. Deane agreed that further research is needed to validate these findings in different patient populations as well as to understand how the scores could be integrated into clinical practice.
What is the role of anti-CCP tests in primary care?
The study also brings up additional questions about the use of anti-CCP tests in primary care, Dr. Deane said. Though previously considered a “specialty test” 10-15 years ago, “now, we really want primary care to do this test along with the rheumatoid factor test,” he noted.
Because the study only included individuals with anti-CCP antibodies, it did not touch on which patients should be getting tested in the first place. Would all patients coming into primary care with joint pain benefit from these blood-marker tests, Deane asked, or would only certain patients qualify? “I think that’s uncertain, and we need to learn more,” he said.
An additional caveat is that the researchers used abnormal ultrasound findings as a predictor of future IA in the comprehensive model, but many clinicians already use ultrasound to identify arthritis, Dr. Deane said.
“If a rheumatologist sees power Doppler signal or erosions, even if the physical examination of a joint didn’t find swelling or inflammation, then they are likely to say that this person has IA now,” and will start treatment, he said. “Because of that, it could be challenging to use ultrasound as a ‘predictive’ marker in clinical practice,” he added, but additional research could help elucidate when to wait on treatment even with abnormal ultrasound findings.
This study was funded by the UK National Institute for Health and Care Research Leeds Biomedical Research Centre. Dr. Emery disclosed financial relationships with AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Novartis, and Samsung Bioepis. Dr. Deane reports receiving consulting fees from Werfen.
A version of this article appeared on Medscape.com.
, according to new research from England.
If validated in further studies, a new simple score using common biomarkers may help identify individuals who can be managed in primary care as well as higher-risk patients who should be referred to a rheumatologist.
The researchers designed a second comprehensive score adding genetics and ultrasound as a tool to identify patients at highest risk for IA for intervention studies and to guide clinical monitoring and care by specialists.
Though there are blood markers and early symptoms in patients that may signal a higher risk for IA, “we don’t know what to do with those people yet,” said Kevin D. Deane, MD, PhD, associate professor of medicine and chair in rheumatology research at the University of Colorado at Denver, Aurora.
“Understanding how to assess these people and predict who’s going to go on to get full blown IA that we should actually treat is very beneficial to the field,” Dr. Deane said. He was not involved with the research but had reviewed an early draft of the paper.
Study seeks to stratify at-risk population
For the study, researchers recruited 455 participants from June 2008 to November 2021, primarily through the UK Primary Care Clinical Research Network. All individuals had new musculoskeletal symptoms, a positive test for anticitrullinated protein antibodies (anti-CCP), and no clinical synovitis.
The researchers selected for anti-CCP positivity because these antibodies are associated with a more aggressive arthritis phenotype. Interventional trials have also found that these anti-CCP–positive individuals are the most responsive to disease-modifying antirheumatic therapy prior to IA development. Patients were followed for at least 48 weeks or until an IA diagnosis.
Using data from this cohort, the team ran statistical analyses guided by potential clinical impact. For the simple score, they aimed to ensure that most people who would go on to develop IA would be identified earlier in clinical practice. For the comprehensive score, they wanted to balance the potential harm of giving preventive treatment to someone who would not develop IA with failing to provide preventive treatment to someone who would develop IA, the authors write.
They developed two scores: a simple score to identify people at lower risk for IA and a comprehensive score to stratify high-risk individuals. The simple score used anti-CCP level, rheumatoid factor value, early morning stiffness, and erythrocyte sedimentation rate to calculate risk.
In addition to these factors, the comprehensive score added smoking history, ultrasound abnormalities, genetic markers for the rheumatoid arthritis shared epitope, as well as patient-reported outcomes from the Health Assessment Questionnaire and the visual analogue scale for global pain.
The study was published in the Annals of Internal Medicine.
Simple score rates more than half as low risk
The simple score identified 249 low-risk individuals, defined as having a less than 10% chance of developing IA within 1 year, with a 5% false-negative rate. This score can help determine which individuals do not need to be referred to a specialist even though they have some known risk factors, said Paul Emery, MD, director of the Leeds Biomedical Research Centre and clinical professor at the University of Leeds in England. He is a co–senior author of the research.
“If you had unlimited resources, you’d refer everyone. But in the real world, we would be overloaded in secondary care, and it just wouldn’t work,” he said. “This is a way of making sure the right people are referred into secondary care.”
The comprehensive score identified 119 high-risk individuals, defined as having a 50% chance or greater of developing IA in 5 years, with a false-positive rate of 29%. Of this high-risk group, 40% developed IA within 1 year, and 71% developed IA in 5 years.
Beyond identifying those who should be referred to specialist care, Dr. Emery noted, this score could be used in research studies to find patients for experimental clinical trials aimed at delaying or preventing the onset of IA.
Both Dr. Emery and Dr. Deane agreed that further research is needed to validate these findings in different patient populations as well as to understand how the scores could be integrated into clinical practice.
What is the role of anti-CCP tests in primary care?
The study also brings up additional questions about the use of anti-CCP tests in primary care, Dr. Deane said. Though previously considered a “specialty test” 10-15 years ago, “now, we really want primary care to do this test along with the rheumatoid factor test,” he noted.
Because the study only included individuals with anti-CCP antibodies, it did not touch on which patients should be getting tested in the first place. Would all patients coming into primary care with joint pain benefit from these blood-marker tests, Deane asked, or would only certain patients qualify? “I think that’s uncertain, and we need to learn more,” he said.
An additional caveat is that the researchers used abnormal ultrasound findings as a predictor of future IA in the comprehensive model, but many clinicians already use ultrasound to identify arthritis, Dr. Deane said.
“If a rheumatologist sees power Doppler signal or erosions, even if the physical examination of a joint didn’t find swelling or inflammation, then they are likely to say that this person has IA now,” and will start treatment, he said. “Because of that, it could be challenging to use ultrasound as a ‘predictive’ marker in clinical practice,” he added, but additional research could help elucidate when to wait on treatment even with abnormal ultrasound findings.
This study was funded by the UK National Institute for Health and Care Research Leeds Biomedical Research Centre. Dr. Emery disclosed financial relationships with AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Novartis, and Samsung Bioepis. Dr. Deane reports receiving consulting fees from Werfen.
A version of this article appeared on Medscape.com.
, according to new research from England.
If validated in further studies, a new simple score using common biomarkers may help identify individuals who can be managed in primary care as well as higher-risk patients who should be referred to a rheumatologist.
The researchers designed a second comprehensive score adding genetics and ultrasound as a tool to identify patients at highest risk for IA for intervention studies and to guide clinical monitoring and care by specialists.
Though there are blood markers and early symptoms in patients that may signal a higher risk for IA, “we don’t know what to do with those people yet,” said Kevin D. Deane, MD, PhD, associate professor of medicine and chair in rheumatology research at the University of Colorado at Denver, Aurora.
“Understanding how to assess these people and predict who’s going to go on to get full blown IA that we should actually treat is very beneficial to the field,” Dr. Deane said. He was not involved with the research but had reviewed an early draft of the paper.
Study seeks to stratify at-risk population
For the study, researchers recruited 455 participants from June 2008 to November 2021, primarily through the UK Primary Care Clinical Research Network. All individuals had new musculoskeletal symptoms, a positive test for anticitrullinated protein antibodies (anti-CCP), and no clinical synovitis.
The researchers selected for anti-CCP positivity because these antibodies are associated with a more aggressive arthritis phenotype. Interventional trials have also found that these anti-CCP–positive individuals are the most responsive to disease-modifying antirheumatic therapy prior to IA development. Patients were followed for at least 48 weeks or until an IA diagnosis.
Using data from this cohort, the team ran statistical analyses guided by potential clinical impact. For the simple score, they aimed to ensure that most people who would go on to develop IA would be identified earlier in clinical practice. For the comprehensive score, they wanted to balance the potential harm of giving preventive treatment to someone who would not develop IA with failing to provide preventive treatment to someone who would develop IA, the authors write.
They developed two scores: a simple score to identify people at lower risk for IA and a comprehensive score to stratify high-risk individuals. The simple score used anti-CCP level, rheumatoid factor value, early morning stiffness, and erythrocyte sedimentation rate to calculate risk.
In addition to these factors, the comprehensive score added smoking history, ultrasound abnormalities, genetic markers for the rheumatoid arthritis shared epitope, as well as patient-reported outcomes from the Health Assessment Questionnaire and the visual analogue scale for global pain.
The study was published in the Annals of Internal Medicine.
Simple score rates more than half as low risk
The simple score identified 249 low-risk individuals, defined as having a less than 10% chance of developing IA within 1 year, with a 5% false-negative rate. This score can help determine which individuals do not need to be referred to a specialist even though they have some known risk factors, said Paul Emery, MD, director of the Leeds Biomedical Research Centre and clinical professor at the University of Leeds in England. He is a co–senior author of the research.
“If you had unlimited resources, you’d refer everyone. But in the real world, we would be overloaded in secondary care, and it just wouldn’t work,” he said. “This is a way of making sure the right people are referred into secondary care.”
The comprehensive score identified 119 high-risk individuals, defined as having a 50% chance or greater of developing IA in 5 years, with a false-positive rate of 29%. Of this high-risk group, 40% developed IA within 1 year, and 71% developed IA in 5 years.
Beyond identifying those who should be referred to specialist care, Dr. Emery noted, this score could be used in research studies to find patients for experimental clinical trials aimed at delaying or preventing the onset of IA.
Both Dr. Emery and Dr. Deane agreed that further research is needed to validate these findings in different patient populations as well as to understand how the scores could be integrated into clinical practice.
What is the role of anti-CCP tests in primary care?
The study also brings up additional questions about the use of anti-CCP tests in primary care, Dr. Deane said. Though previously considered a “specialty test” 10-15 years ago, “now, we really want primary care to do this test along with the rheumatoid factor test,” he noted.
Because the study only included individuals with anti-CCP antibodies, it did not touch on which patients should be getting tested in the first place. Would all patients coming into primary care with joint pain benefit from these blood-marker tests, Deane asked, or would only certain patients qualify? “I think that’s uncertain, and we need to learn more,” he said.
An additional caveat is that the researchers used abnormal ultrasound findings as a predictor of future IA in the comprehensive model, but many clinicians already use ultrasound to identify arthritis, Dr. Deane said.
“If a rheumatologist sees power Doppler signal or erosions, even if the physical examination of a joint didn’t find swelling or inflammation, then they are likely to say that this person has IA now,” and will start treatment, he said. “Because of that, it could be challenging to use ultrasound as a ‘predictive’ marker in clinical practice,” he added, but additional research could help elucidate when to wait on treatment even with abnormal ultrasound findings.
This study was funded by the UK National Institute for Health and Care Research Leeds Biomedical Research Centre. Dr. Emery disclosed financial relationships with AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Novartis, and Samsung Bioepis. Dr. Deane reports receiving consulting fees from Werfen.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE












