User login
Johns Hopkins retains title as best hospital for rheumatology
For the sixth year in a row, Johns Hopkins Hospital in Baltimore has been named the top hospital for rheumatology by U.S. News & World Report.
The No. 2 slot went to the Hospital for Special Surgery (HSS), New York. The Cleveland Clinic took third place. The magazine announced the 2023-2024 rankings on Aug. 1.
Most specialty rankings are determined through data on patient outcomes and hospital performance, but rheumatology rankings, as well as those for ophthalmology and psychiatry, were determined through expert opinion. For these three specialties, most care is delivered in outpatient settings, according to U.S. News & World Report, and “the number of outpatients who die in these specialties is so low that risk-adjusted mortality rates ... are not significantly tied to the quality of care.” Thus, the rankings are based on specialist responses to U.S. News surveys from the past 3 years.
The rankings for 11 rheumatology hospitals are as follows:
- 1. Johns Hopkins Hospital
- 2. HSS, New York–Presbyterian University Hospital of Columbia and Cornell
- 3. Cleveland Clinic
- 4. Mayo Clinic, Rochester, Minn.
- 5. Brigham and Women’s Hospital, Boston
- 6. Massachusetts General Hospital, Boston
- 7. UCSF Health-UCSF Medical Center, San Francisco
- 8. NYU Langone Hospitals, New York
- 9. UCLA Medical Center, Los Angeles
- 10. University of Alabama at Birmingham Hospital
- 11. University of Michigan Health–Ann Arbor
Nearly all hospitals on this list also made the Best Hospitals Honor Roll for 2023-2024. These Honor Roll hospitals excelled in care across multiple specialties. The University of Alabama at Birmingham Hospital was not on the honor roll but was ranked among the nation’s top 50 hospitals in cardiology, diabetes & endocrinology, gastroenterology, geriatrics, and obstetrics & gynecology.
A version of this article appeared on Medscape.com.
For the sixth year in a row, Johns Hopkins Hospital in Baltimore has been named the top hospital for rheumatology by U.S. News & World Report.
The No. 2 slot went to the Hospital for Special Surgery (HSS), New York. The Cleveland Clinic took third place. The magazine announced the 2023-2024 rankings on Aug. 1.
Most specialty rankings are determined through data on patient outcomes and hospital performance, but rheumatology rankings, as well as those for ophthalmology and psychiatry, were determined through expert opinion. For these three specialties, most care is delivered in outpatient settings, according to U.S. News & World Report, and “the number of outpatients who die in these specialties is so low that risk-adjusted mortality rates ... are not significantly tied to the quality of care.” Thus, the rankings are based on specialist responses to U.S. News surveys from the past 3 years.
The rankings for 11 rheumatology hospitals are as follows:
- 1. Johns Hopkins Hospital
- 2. HSS, New York–Presbyterian University Hospital of Columbia and Cornell
- 3. Cleveland Clinic
- 4. Mayo Clinic, Rochester, Minn.
- 5. Brigham and Women’s Hospital, Boston
- 6. Massachusetts General Hospital, Boston
- 7. UCSF Health-UCSF Medical Center, San Francisco
- 8. NYU Langone Hospitals, New York
- 9. UCLA Medical Center, Los Angeles
- 10. University of Alabama at Birmingham Hospital
- 11. University of Michigan Health–Ann Arbor
Nearly all hospitals on this list also made the Best Hospitals Honor Roll for 2023-2024. These Honor Roll hospitals excelled in care across multiple specialties. The University of Alabama at Birmingham Hospital was not on the honor roll but was ranked among the nation’s top 50 hospitals in cardiology, diabetes & endocrinology, gastroenterology, geriatrics, and obstetrics & gynecology.
A version of this article appeared on Medscape.com.
For the sixth year in a row, Johns Hopkins Hospital in Baltimore has been named the top hospital for rheumatology by U.S. News & World Report.
The No. 2 slot went to the Hospital for Special Surgery (HSS), New York. The Cleveland Clinic took third place. The magazine announced the 2023-2024 rankings on Aug. 1.
Most specialty rankings are determined through data on patient outcomes and hospital performance, but rheumatology rankings, as well as those for ophthalmology and psychiatry, were determined through expert opinion. For these three specialties, most care is delivered in outpatient settings, according to U.S. News & World Report, and “the number of outpatients who die in these specialties is so low that risk-adjusted mortality rates ... are not significantly tied to the quality of care.” Thus, the rankings are based on specialist responses to U.S. News surveys from the past 3 years.
The rankings for 11 rheumatology hospitals are as follows:
- 1. Johns Hopkins Hospital
- 2. HSS, New York–Presbyterian University Hospital of Columbia and Cornell
- 3. Cleveland Clinic
- 4. Mayo Clinic, Rochester, Minn.
- 5. Brigham and Women’s Hospital, Boston
- 6. Massachusetts General Hospital, Boston
- 7. UCSF Health-UCSF Medical Center, San Francisco
- 8. NYU Langone Hospitals, New York
- 9. UCLA Medical Center, Los Angeles
- 10. University of Alabama at Birmingham Hospital
- 11. University of Michigan Health–Ann Arbor
Nearly all hospitals on this list also made the Best Hospitals Honor Roll for 2023-2024. These Honor Roll hospitals excelled in care across multiple specialties. The University of Alabama at Birmingham Hospital was not on the honor roll but was ranked among the nation’s top 50 hospitals in cardiology, diabetes & endocrinology, gastroenterology, geriatrics, and obstetrics & gynecology.
A version of this article appeared on Medscape.com.
Could risk stratifying methotrexate users lead to less frequent testing?
A new model can predict which patients are more likely to experience side effects from long-term methotrexate (MTX) use, research suggests. Patients with a lower risk profile may benefit from less frequent testing, the authors hypothesize.
Most recommendations advise that patients initiating MTX therapy should get blood testing every 2-4 weeks to monitor for full blood count, liver function, urea electrolytes, and creatinine. After 6 months taking MTX, monitoring can be tapered to every 3 months. But Abhishek Abhishek, MD, PhD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust and colleagues argue that abnormal results after the initial 6 months of treatment are “infrequent,” and patients may benefit from fewer tests throughout the year.
“Unnecessary blood tests waste patients’ time and health care resources, including the time of general practitioners and phlebotomists,” Dr. Abhishek and associates write. “It would be beneficial to predict the risk of clinically significant abnormal blood test results during long-term methotrexate treatment to inform the frequency of testing for individuals.”
Stratifying risk
In the study, published in the BMJ, researchers used the UK’s Clinical Practice Research Datalink (CPRD) to identify the electronic medical records of over 37,000 adult patients with an immune-mediated inflammatory disease who were prescribed MTX during 2007-2019. All included patients were prescribed MTX for at least 6 months. The main outcome was discontinuation of methotrexate because of abnormal blood test results. Around 62% of patients had rheumatoid arthritis and 22% had psoriasis or psoriatic arthritis.
Using these anonymized data, the group developed a risk stratification model using 11 clinical predictors. “The factors that went in the model are simple things that most patients can self-report or doctors can get from their patient’s medical records,” Dr. Abhishek told this news organization, including methotrexate dose, age, sex, and comorbidities. Dr. Abhishek emphasized that the model should be used only in patients who have continued taking MTX for at least 6 months and have already undergone more frequent initial testing.
The strongest individual predictors were diabetes (hazard ratio, 1.25), chronic kidney disease stage 3 (HR, 2.01), and previous cytopenia or raised liver enzyme levels during the first 6 months of MTX therapy (HR, 2.97). However, Dr. Abhishek emphasized that the individual factors were less important, noting that the model sums the risks to predict outcomes more accurately. Most patients (68.4%) were sorted into the low-risk cohort, with a less than 10% estimated risk of discontinuing MTX over the next 5 years. About one-fifth (20.9%) were categorized as moderate risk (10%-20% estimated risk over 5 years), and 10.7% were high risk, with a greater than 20% estimated risk of discontinuing the drug over 5 years.
The authors argue that low-risk patients could receive less frequent testing – perhaps every 6 months or annually, while moderate-risk patients would continue to be tested every 3 months. High-risk patients could potentially be tested with even greater frequently.
More research needed
The research involved “incredibly sophisticated statistical analysis,” said Daniel E. Furst, MD, professor emeritus of medicine at the University of California, Los Angeles, who was not involved with the study. However, the data do not yet support altering blood testing frequency based on this model.
“The hypothesis that not all patients have to be examined so frequently is a very reasonable hypothesis,” Dr. Furst said in an interview, and additional research is needed to corroborate it. The model also needs to be validated in patient populations outside of the United Kingdom, he added.
Dr. Abhishek agreed that validating the model in other patient populations is an important next step. “When we develop a tool [using] a one-nation data set, we want other researchers to then validate it in other countries’ data sets to make sure there is nothing odd about patients in the U.K. that makes the tool work well here but not in [the] U.S., Europe, or Asia, for example,” he said. Doing so should be relatively easy, he said, as the model is publicly available, and the information required is routinely collected during clinic visits.
To understand if less frequent testing might be appropriate for some patients, researchers would need to look at data registries like the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS) registry or CorEvitas registries “where the testing is done in a very regular way over the long haul,” Dr. Furst said. Analyzing these datasets, researchers could determine the testing intervals that would be most efficient for low- and high-risk patients.
A word of caution
While less frequent testing for long-term MTX therapy could likely have benefits, there is still some risk involved, cautioned Prabha Ranganathan, MD, professor of medicine at Washington University in St. Louis.
“Although most methotrexate toxicity occurs within the first 6 months of starting treatment, rare idiosyncratic toxicity can occur that does not correlate with the dose, duration, or method of how methotrexate is administered,” she wrote in an accompanying editorial. “Most rheumatologists can identify a handful of patients who receive methotrexate in their practice who develop sudden leukopenia or thrombocytopenia or transaminitis that is severe enough to warrant drug discontinuation.” While tools like this prediction model can be useful, clinicians need to consider each patient individually and use shared decision-making when monitoring for MTX toxicity, she advised.
“As in most of areas of medicine, the one-size-fits-all approach does not work for methotrexate users,” she noted.
This study was funded by the U.K. National Institute for Health and Care Research and Health Technology Assessment. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadila Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Furst and Dr. Ranganathan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new model can predict which patients are more likely to experience side effects from long-term methotrexate (MTX) use, research suggests. Patients with a lower risk profile may benefit from less frequent testing, the authors hypothesize.
Most recommendations advise that patients initiating MTX therapy should get blood testing every 2-4 weeks to monitor for full blood count, liver function, urea electrolytes, and creatinine. After 6 months taking MTX, monitoring can be tapered to every 3 months. But Abhishek Abhishek, MD, PhD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust and colleagues argue that abnormal results after the initial 6 months of treatment are “infrequent,” and patients may benefit from fewer tests throughout the year.
“Unnecessary blood tests waste patients’ time and health care resources, including the time of general practitioners and phlebotomists,” Dr. Abhishek and associates write. “It would be beneficial to predict the risk of clinically significant abnormal blood test results during long-term methotrexate treatment to inform the frequency of testing for individuals.”
Stratifying risk
In the study, published in the BMJ, researchers used the UK’s Clinical Practice Research Datalink (CPRD) to identify the electronic medical records of over 37,000 adult patients with an immune-mediated inflammatory disease who were prescribed MTX during 2007-2019. All included patients were prescribed MTX for at least 6 months. The main outcome was discontinuation of methotrexate because of abnormal blood test results. Around 62% of patients had rheumatoid arthritis and 22% had psoriasis or psoriatic arthritis.
Using these anonymized data, the group developed a risk stratification model using 11 clinical predictors. “The factors that went in the model are simple things that most patients can self-report or doctors can get from their patient’s medical records,” Dr. Abhishek told this news organization, including methotrexate dose, age, sex, and comorbidities. Dr. Abhishek emphasized that the model should be used only in patients who have continued taking MTX for at least 6 months and have already undergone more frequent initial testing.
The strongest individual predictors were diabetes (hazard ratio, 1.25), chronic kidney disease stage 3 (HR, 2.01), and previous cytopenia or raised liver enzyme levels during the first 6 months of MTX therapy (HR, 2.97). However, Dr. Abhishek emphasized that the individual factors were less important, noting that the model sums the risks to predict outcomes more accurately. Most patients (68.4%) were sorted into the low-risk cohort, with a less than 10% estimated risk of discontinuing MTX over the next 5 years. About one-fifth (20.9%) were categorized as moderate risk (10%-20% estimated risk over 5 years), and 10.7% were high risk, with a greater than 20% estimated risk of discontinuing the drug over 5 years.
The authors argue that low-risk patients could receive less frequent testing – perhaps every 6 months or annually, while moderate-risk patients would continue to be tested every 3 months. High-risk patients could potentially be tested with even greater frequently.
More research needed
The research involved “incredibly sophisticated statistical analysis,” said Daniel E. Furst, MD, professor emeritus of medicine at the University of California, Los Angeles, who was not involved with the study. However, the data do not yet support altering blood testing frequency based on this model.
“The hypothesis that not all patients have to be examined so frequently is a very reasonable hypothesis,” Dr. Furst said in an interview, and additional research is needed to corroborate it. The model also needs to be validated in patient populations outside of the United Kingdom, he added.
Dr. Abhishek agreed that validating the model in other patient populations is an important next step. “When we develop a tool [using] a one-nation data set, we want other researchers to then validate it in other countries’ data sets to make sure there is nothing odd about patients in the U.K. that makes the tool work well here but not in [the] U.S., Europe, or Asia, for example,” he said. Doing so should be relatively easy, he said, as the model is publicly available, and the information required is routinely collected during clinic visits.
To understand if less frequent testing might be appropriate for some patients, researchers would need to look at data registries like the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS) registry or CorEvitas registries “where the testing is done in a very regular way over the long haul,” Dr. Furst said. Analyzing these datasets, researchers could determine the testing intervals that would be most efficient for low- and high-risk patients.
A word of caution
While less frequent testing for long-term MTX therapy could likely have benefits, there is still some risk involved, cautioned Prabha Ranganathan, MD, professor of medicine at Washington University in St. Louis.
“Although most methotrexate toxicity occurs within the first 6 months of starting treatment, rare idiosyncratic toxicity can occur that does not correlate with the dose, duration, or method of how methotrexate is administered,” she wrote in an accompanying editorial. “Most rheumatologists can identify a handful of patients who receive methotrexate in their practice who develop sudden leukopenia or thrombocytopenia or transaminitis that is severe enough to warrant drug discontinuation.” While tools like this prediction model can be useful, clinicians need to consider each patient individually and use shared decision-making when monitoring for MTX toxicity, she advised.
“As in most of areas of medicine, the one-size-fits-all approach does not work for methotrexate users,” she noted.
This study was funded by the U.K. National Institute for Health and Care Research and Health Technology Assessment. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadila Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Furst and Dr. Ranganathan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new model can predict which patients are more likely to experience side effects from long-term methotrexate (MTX) use, research suggests. Patients with a lower risk profile may benefit from less frequent testing, the authors hypothesize.
Most recommendations advise that patients initiating MTX therapy should get blood testing every 2-4 weeks to monitor for full blood count, liver function, urea electrolytes, and creatinine. After 6 months taking MTX, monitoring can be tapered to every 3 months. But Abhishek Abhishek, MD, PhD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust and colleagues argue that abnormal results after the initial 6 months of treatment are “infrequent,” and patients may benefit from fewer tests throughout the year.
“Unnecessary blood tests waste patients’ time and health care resources, including the time of general practitioners and phlebotomists,” Dr. Abhishek and associates write. “It would be beneficial to predict the risk of clinically significant abnormal blood test results during long-term methotrexate treatment to inform the frequency of testing for individuals.”
Stratifying risk
In the study, published in the BMJ, researchers used the UK’s Clinical Practice Research Datalink (CPRD) to identify the electronic medical records of over 37,000 adult patients with an immune-mediated inflammatory disease who were prescribed MTX during 2007-2019. All included patients were prescribed MTX for at least 6 months. The main outcome was discontinuation of methotrexate because of abnormal blood test results. Around 62% of patients had rheumatoid arthritis and 22% had psoriasis or psoriatic arthritis.
Using these anonymized data, the group developed a risk stratification model using 11 clinical predictors. “The factors that went in the model are simple things that most patients can self-report or doctors can get from their patient’s medical records,” Dr. Abhishek told this news organization, including methotrexate dose, age, sex, and comorbidities. Dr. Abhishek emphasized that the model should be used only in patients who have continued taking MTX for at least 6 months and have already undergone more frequent initial testing.
The strongest individual predictors were diabetes (hazard ratio, 1.25), chronic kidney disease stage 3 (HR, 2.01), and previous cytopenia or raised liver enzyme levels during the first 6 months of MTX therapy (HR, 2.97). However, Dr. Abhishek emphasized that the individual factors were less important, noting that the model sums the risks to predict outcomes more accurately. Most patients (68.4%) were sorted into the low-risk cohort, with a less than 10% estimated risk of discontinuing MTX over the next 5 years. About one-fifth (20.9%) were categorized as moderate risk (10%-20% estimated risk over 5 years), and 10.7% were high risk, with a greater than 20% estimated risk of discontinuing the drug over 5 years.
The authors argue that low-risk patients could receive less frequent testing – perhaps every 6 months or annually, while moderate-risk patients would continue to be tested every 3 months. High-risk patients could potentially be tested with even greater frequently.
More research needed
The research involved “incredibly sophisticated statistical analysis,” said Daniel E. Furst, MD, professor emeritus of medicine at the University of California, Los Angeles, who was not involved with the study. However, the data do not yet support altering blood testing frequency based on this model.
“The hypothesis that not all patients have to be examined so frequently is a very reasonable hypothesis,” Dr. Furst said in an interview, and additional research is needed to corroborate it. The model also needs to be validated in patient populations outside of the United Kingdom, he added.
Dr. Abhishek agreed that validating the model in other patient populations is an important next step. “When we develop a tool [using] a one-nation data set, we want other researchers to then validate it in other countries’ data sets to make sure there is nothing odd about patients in the U.K. that makes the tool work well here but not in [the] U.S., Europe, or Asia, for example,” he said. Doing so should be relatively easy, he said, as the model is publicly available, and the information required is routinely collected during clinic visits.
To understand if less frequent testing might be appropriate for some patients, researchers would need to look at data registries like the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS) registry or CorEvitas registries “where the testing is done in a very regular way over the long haul,” Dr. Furst said. Analyzing these datasets, researchers could determine the testing intervals that would be most efficient for low- and high-risk patients.
A word of caution
While less frequent testing for long-term MTX therapy could likely have benefits, there is still some risk involved, cautioned Prabha Ranganathan, MD, professor of medicine at Washington University in St. Louis.
“Although most methotrexate toxicity occurs within the first 6 months of starting treatment, rare idiosyncratic toxicity can occur that does not correlate with the dose, duration, or method of how methotrexate is administered,” she wrote in an accompanying editorial. “Most rheumatologists can identify a handful of patients who receive methotrexate in their practice who develop sudden leukopenia or thrombocytopenia or transaminitis that is severe enough to warrant drug discontinuation.” While tools like this prediction model can be useful, clinicians need to consider each patient individually and use shared decision-making when monitoring for MTX toxicity, she advised.
“As in most of areas of medicine, the one-size-fits-all approach does not work for methotrexate users,” she noted.
This study was funded by the U.K. National Institute for Health and Care Research and Health Technology Assessment. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadila Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Furst and Dr. Ranganathan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
Transcutaneous vagus nerve stimulation on the ear proves ineffective in RA treatment
Noninvasive auricular vagus nerve stimulation (VNS) is no more effective than placebo at controlling symptoms of rheumatoid arthritis, according to a new study. But experts emphasize that these results do not mean trials of different forms of VNS will have the same fate.
VNS offers a potential additional therapy for autoimmune disease beyond disease-modifying antirheumatic drugs, explained first author Matthew Baker, MD, clinical chief, division of immunology and rheumatology, Stanford (Calif.) University, and colleagues.
“The principle of VNS is based upon the inflammatory reflex, which describes a primitive connection between the nervous system and immune system,” the authors write. Signals sent down the vagus nerve to the splenic nerve stimulate immune cells in the spleen, which ultimately results in blocking production of inflammatory cytokines such as tumor necrosis factor. “It is hypothesized that this reduction in systemic inflammation can be harnessed for the treatment of diseases such as RA,” they continue, and smaller studies suggest this treatment could benefit patients.
In a previous 12-week, open-label trial, 17 patients with RA who were implanted with a VNS device on the left cervical vagus nerve saw improvement in RA symptoms, as well as a decrease in TNF production. Noninvasive devices that stimulate the auricular branch of the vagus nerve have also shown some promise. A sham-controlled study of 18 patients with systemic lupus erythematosus (SLE) found that patients who received transcutaneous auricular VNS reported reduced musculoskeletal pain over just 4 days. An open-label study of 30 patients with RA showed clinically significant reductions in disease activity score of 28 joints with C-reactive protein (DAS28-CRP) and clinical improvement in American College of Rheumatology responses over 12 weeks. Additional trials have also demonstrated this positive effect of noninvasive VNS on RA symptoms, but all studies conducted thus far have been relatively small or uncontrolled, Dr. Baker said.
Results of latest trial
In this new trial, published online in Arthritis & Rheumatology, researchers enrolled 113 patients with active RA who had inadequate responses or intolerance to conventional synthetic DMARDs and were naïve to biologic or targeted synthetic DMARDs. All patients were given an auricular vagus nerve stimulator via a custom-molded earpiece that was controlled by a smartphone app. Patients wore the device for 15 minutes each day. When worn and turned on, the device generated electrical signals delivered transcutaneously to the cymba concha, a region of the ear connected to the auricular branch of the vagus nerve. This stimulation is imperceptible to patients, Dr. Baker explained. “For the sham arm, we simply did not turn the device on at all,” he said. A subject in the sham arm would use the same device on a 15-minute timer, but no stimulation was given.
After 12 weeks, researchers found no statistically significant difference between the treatment and sham arms in achieving 20% improvement in ACR response criteria or mean change in DAS28-CRP. A total of 17 patients, including 12 in the treatment arm, reported adverse events during the study, and all events were categorized as mild to moderate.
While the research team was “obviously disappointed” about the results, Dr. Baker said, negative findings in trials also are important. “The real value of our study is pointing out the need for large controlled, sham-controlled studies,” he said, especially for potential treatments with a lot of enthusiasm behind them.
Results don’t seal the fate of other VNS approaches
“As a properly controlled trial, the results are impressively negative,” writes Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, and codirector, Metroplex Clinical Research Center, both in Dallas, in an editorial about the study. Many of the previous studies looking at this therapy in RA were open label, which could bias the results, he argued. The biggest question, he noted, is if other blinded, sham-controlled trials looking at VNS devices will show similar results.
By itself, this finding does not imply that other VNS devices will be unsuccessful, argued Jonathan Kay, MD, the Timothy S. and Elaine L. Peterson chair in rheumatology, and professor of medicine and population and quantitative health sciences, UMass Chan Medical School and UMass Memorial Medical Center, both in Worcester, Mass. He is also an investigator for the RESET-RA trial, a randomized, sham-controlled trial that will assess the safety and efficacy of an implantable VNS device in an estimated 250 patients with RA. He was not involved with Dr. Baker’s work.
“Auricular VNS is delivered more distally than cervical or splenic nerve stimulation,” Dr. Kay said, and the potential effect of these other forms of VNS may have different outcomes.
Cynthia Aranow, MD, rheumatologist and director of the Clinical Autoimmunity Center of Excellence at Feinstein Institutes for Medical Research, Manhasset, New York, agreed with Dr. Kay, noting that direct VNS stimulation via implantable device and transcutaneous stimulation through the skin are not comparable. She also is unaffiliated with the study.
“This group conducted a well-designed, sham-controlled study of a reasonable number of patients and over a reasonable period of time and observed no significant differences between those participants receiving true and those participants receiving sham stimulation,” she wrote in an email. “However, it’s important to point out that the stimulation settings used in this study were kHz (kilohertz) which is 1,000 times greater than the settings used in multiple other studies in which transauricular VNS has been shown to be clinically effective, including studies in long COVID, tinnitus, SLE, cluster headaches, erosive hand osteoarthritis, pediatric kidney disease, among others,” she said.
The role for VNS treatment, whether direct stimulation via implantable device or transcutaneous, in autoimmune and inflammatory diseases “remains to be determined by future studies,” she said.
The study was funded by Nesos. Dr. Baker received personal fees from Nesos during the study. Dr. Kay has received consulting fees from AbbVie, Boehringer Ingelheim, Celltrion Healthcare, and several other pharmaceutical companies. Dr. Aranow reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Noninvasive auricular vagus nerve stimulation (VNS) is no more effective than placebo at controlling symptoms of rheumatoid arthritis, according to a new study. But experts emphasize that these results do not mean trials of different forms of VNS will have the same fate.
VNS offers a potential additional therapy for autoimmune disease beyond disease-modifying antirheumatic drugs, explained first author Matthew Baker, MD, clinical chief, division of immunology and rheumatology, Stanford (Calif.) University, and colleagues.
“The principle of VNS is based upon the inflammatory reflex, which describes a primitive connection between the nervous system and immune system,” the authors write. Signals sent down the vagus nerve to the splenic nerve stimulate immune cells in the spleen, which ultimately results in blocking production of inflammatory cytokines such as tumor necrosis factor. “It is hypothesized that this reduction in systemic inflammation can be harnessed for the treatment of diseases such as RA,” they continue, and smaller studies suggest this treatment could benefit patients.
In a previous 12-week, open-label trial, 17 patients with RA who were implanted with a VNS device on the left cervical vagus nerve saw improvement in RA symptoms, as well as a decrease in TNF production. Noninvasive devices that stimulate the auricular branch of the vagus nerve have also shown some promise. A sham-controlled study of 18 patients with systemic lupus erythematosus (SLE) found that patients who received transcutaneous auricular VNS reported reduced musculoskeletal pain over just 4 days. An open-label study of 30 patients with RA showed clinically significant reductions in disease activity score of 28 joints with C-reactive protein (DAS28-CRP) and clinical improvement in American College of Rheumatology responses over 12 weeks. Additional trials have also demonstrated this positive effect of noninvasive VNS on RA symptoms, but all studies conducted thus far have been relatively small or uncontrolled, Dr. Baker said.
Results of latest trial
In this new trial, published online in Arthritis & Rheumatology, researchers enrolled 113 patients with active RA who had inadequate responses or intolerance to conventional synthetic DMARDs and were naïve to biologic or targeted synthetic DMARDs. All patients were given an auricular vagus nerve stimulator via a custom-molded earpiece that was controlled by a smartphone app. Patients wore the device for 15 minutes each day. When worn and turned on, the device generated electrical signals delivered transcutaneously to the cymba concha, a region of the ear connected to the auricular branch of the vagus nerve. This stimulation is imperceptible to patients, Dr. Baker explained. “For the sham arm, we simply did not turn the device on at all,” he said. A subject in the sham arm would use the same device on a 15-minute timer, but no stimulation was given.
After 12 weeks, researchers found no statistically significant difference between the treatment and sham arms in achieving 20% improvement in ACR response criteria or mean change in DAS28-CRP. A total of 17 patients, including 12 in the treatment arm, reported adverse events during the study, and all events were categorized as mild to moderate.
While the research team was “obviously disappointed” about the results, Dr. Baker said, negative findings in trials also are important. “The real value of our study is pointing out the need for large controlled, sham-controlled studies,” he said, especially for potential treatments with a lot of enthusiasm behind them.
Results don’t seal the fate of other VNS approaches
“As a properly controlled trial, the results are impressively negative,” writes Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, and codirector, Metroplex Clinical Research Center, both in Dallas, in an editorial about the study. Many of the previous studies looking at this therapy in RA were open label, which could bias the results, he argued. The biggest question, he noted, is if other blinded, sham-controlled trials looking at VNS devices will show similar results.
By itself, this finding does not imply that other VNS devices will be unsuccessful, argued Jonathan Kay, MD, the Timothy S. and Elaine L. Peterson chair in rheumatology, and professor of medicine and population and quantitative health sciences, UMass Chan Medical School and UMass Memorial Medical Center, both in Worcester, Mass. He is also an investigator for the RESET-RA trial, a randomized, sham-controlled trial that will assess the safety and efficacy of an implantable VNS device in an estimated 250 patients with RA. He was not involved with Dr. Baker’s work.
“Auricular VNS is delivered more distally than cervical or splenic nerve stimulation,” Dr. Kay said, and the potential effect of these other forms of VNS may have different outcomes.
Cynthia Aranow, MD, rheumatologist and director of the Clinical Autoimmunity Center of Excellence at Feinstein Institutes for Medical Research, Manhasset, New York, agreed with Dr. Kay, noting that direct VNS stimulation via implantable device and transcutaneous stimulation through the skin are not comparable. She also is unaffiliated with the study.
“This group conducted a well-designed, sham-controlled study of a reasonable number of patients and over a reasonable period of time and observed no significant differences between those participants receiving true and those participants receiving sham stimulation,” she wrote in an email. “However, it’s important to point out that the stimulation settings used in this study were kHz (kilohertz) which is 1,000 times greater than the settings used in multiple other studies in which transauricular VNS has been shown to be clinically effective, including studies in long COVID, tinnitus, SLE, cluster headaches, erosive hand osteoarthritis, pediatric kidney disease, among others,” she said.
The role for VNS treatment, whether direct stimulation via implantable device or transcutaneous, in autoimmune and inflammatory diseases “remains to be determined by future studies,” she said.
The study was funded by Nesos. Dr. Baker received personal fees from Nesos during the study. Dr. Kay has received consulting fees from AbbVie, Boehringer Ingelheim, Celltrion Healthcare, and several other pharmaceutical companies. Dr. Aranow reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Noninvasive auricular vagus nerve stimulation (VNS) is no more effective than placebo at controlling symptoms of rheumatoid arthritis, according to a new study. But experts emphasize that these results do not mean trials of different forms of VNS will have the same fate.
VNS offers a potential additional therapy for autoimmune disease beyond disease-modifying antirheumatic drugs, explained first author Matthew Baker, MD, clinical chief, division of immunology and rheumatology, Stanford (Calif.) University, and colleagues.
“The principle of VNS is based upon the inflammatory reflex, which describes a primitive connection between the nervous system and immune system,” the authors write. Signals sent down the vagus nerve to the splenic nerve stimulate immune cells in the spleen, which ultimately results in blocking production of inflammatory cytokines such as tumor necrosis factor. “It is hypothesized that this reduction in systemic inflammation can be harnessed for the treatment of diseases such as RA,” they continue, and smaller studies suggest this treatment could benefit patients.
In a previous 12-week, open-label trial, 17 patients with RA who were implanted with a VNS device on the left cervical vagus nerve saw improvement in RA symptoms, as well as a decrease in TNF production. Noninvasive devices that stimulate the auricular branch of the vagus nerve have also shown some promise. A sham-controlled study of 18 patients with systemic lupus erythematosus (SLE) found that patients who received transcutaneous auricular VNS reported reduced musculoskeletal pain over just 4 days. An open-label study of 30 patients with RA showed clinically significant reductions in disease activity score of 28 joints with C-reactive protein (DAS28-CRP) and clinical improvement in American College of Rheumatology responses over 12 weeks. Additional trials have also demonstrated this positive effect of noninvasive VNS on RA symptoms, but all studies conducted thus far have been relatively small or uncontrolled, Dr. Baker said.
Results of latest trial
In this new trial, published online in Arthritis & Rheumatology, researchers enrolled 113 patients with active RA who had inadequate responses or intolerance to conventional synthetic DMARDs and were naïve to biologic or targeted synthetic DMARDs. All patients were given an auricular vagus nerve stimulator via a custom-molded earpiece that was controlled by a smartphone app. Patients wore the device for 15 minutes each day. When worn and turned on, the device generated electrical signals delivered transcutaneously to the cymba concha, a region of the ear connected to the auricular branch of the vagus nerve. This stimulation is imperceptible to patients, Dr. Baker explained. “For the sham arm, we simply did not turn the device on at all,” he said. A subject in the sham arm would use the same device on a 15-minute timer, but no stimulation was given.
After 12 weeks, researchers found no statistically significant difference between the treatment and sham arms in achieving 20% improvement in ACR response criteria or mean change in DAS28-CRP. A total of 17 patients, including 12 in the treatment arm, reported adverse events during the study, and all events were categorized as mild to moderate.
While the research team was “obviously disappointed” about the results, Dr. Baker said, negative findings in trials also are important. “The real value of our study is pointing out the need for large controlled, sham-controlled studies,” he said, especially for potential treatments with a lot of enthusiasm behind them.
Results don’t seal the fate of other VNS approaches
“As a properly controlled trial, the results are impressively negative,” writes Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, and codirector, Metroplex Clinical Research Center, both in Dallas, in an editorial about the study. Many of the previous studies looking at this therapy in RA were open label, which could bias the results, he argued. The biggest question, he noted, is if other blinded, sham-controlled trials looking at VNS devices will show similar results.
By itself, this finding does not imply that other VNS devices will be unsuccessful, argued Jonathan Kay, MD, the Timothy S. and Elaine L. Peterson chair in rheumatology, and professor of medicine and population and quantitative health sciences, UMass Chan Medical School and UMass Memorial Medical Center, both in Worcester, Mass. He is also an investigator for the RESET-RA trial, a randomized, sham-controlled trial that will assess the safety and efficacy of an implantable VNS device in an estimated 250 patients with RA. He was not involved with Dr. Baker’s work.
“Auricular VNS is delivered more distally than cervical or splenic nerve stimulation,” Dr. Kay said, and the potential effect of these other forms of VNS may have different outcomes.
Cynthia Aranow, MD, rheumatologist and director of the Clinical Autoimmunity Center of Excellence at Feinstein Institutes for Medical Research, Manhasset, New York, agreed with Dr. Kay, noting that direct VNS stimulation via implantable device and transcutaneous stimulation through the skin are not comparable. She also is unaffiliated with the study.
“This group conducted a well-designed, sham-controlled study of a reasonable number of patients and over a reasonable period of time and observed no significant differences between those participants receiving true and those participants receiving sham stimulation,” she wrote in an email. “However, it’s important to point out that the stimulation settings used in this study were kHz (kilohertz) which is 1,000 times greater than the settings used in multiple other studies in which transauricular VNS has been shown to be clinically effective, including studies in long COVID, tinnitus, SLE, cluster headaches, erosive hand osteoarthritis, pediatric kidney disease, among others,” she said.
The role for VNS treatment, whether direct stimulation via implantable device or transcutaneous, in autoimmune and inflammatory diseases “remains to be determined by future studies,” she said.
The study was funded by Nesos. Dr. Baker received personal fees from Nesos during the study. Dr. Kay has received consulting fees from AbbVie, Boehringer Ingelheim, Celltrion Healthcare, and several other pharmaceutical companies. Dr. Aranow reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Humira biosimilars: Five things to know
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.
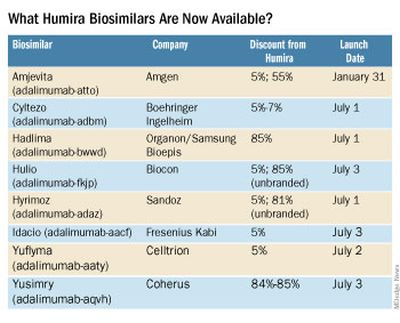
Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
FDA rejects NASH drug for the second time
In response, the company has decided to discontinue all NASH-related investment.
Intercept first sought FDA approval for OCA in treatment of NASH in 2019 and received a complete response letter. The company refiled for a new drug application in December 2022. A second resubmission would require a completion of the long-term outcomes phase of an ongoing clinical trial, according to an Intercept press release.
The FDA decision follows the recommendation from May’s FDA Gastrointestinal Drugs Advisory Committee meeting. During the meeting, members voted 15 to 1 to advise deferring approval until clinical outcome data became available. Intercept’s clinical trial data demonstrated that OCA showed moderate benefit over placebo in improving fibrosis in NASH patients, but “there is uncertainty how the magnitude of changes in these surrogate endpoints may translate to meaningful changes in clinical outcomes,” an FDA meeting briefing document stated. There were also notable safety concerns including an increased risk for drug-induced liver injury.
An estimated 16.8 million Americans have NASH, and there are no FDA-approved medications for the condition.
Intercept plans to promptly begin closing out their NASH clinical trial and restructuring to focus on rare and serious liver diseases.
“While this is clearly not the outcome that we have worked toward, I’m proud of the impact that Intercept has made to move the science of NASH forward and bring the field closer to a treatment option,” said Jerry Durso, the president and CEO of Intercept, in a statement. “Intercept thanks the scientists, clinicians, and patients whose contributions to the clinical development of OCA in NASH have significantly advanced the understanding of this deadly disease.”
A version of this article originally appeared on Medscape.com.
In response, the company has decided to discontinue all NASH-related investment.
Intercept first sought FDA approval for OCA in treatment of NASH in 2019 and received a complete response letter. The company refiled for a new drug application in December 2022. A second resubmission would require a completion of the long-term outcomes phase of an ongoing clinical trial, according to an Intercept press release.
The FDA decision follows the recommendation from May’s FDA Gastrointestinal Drugs Advisory Committee meeting. During the meeting, members voted 15 to 1 to advise deferring approval until clinical outcome data became available. Intercept’s clinical trial data demonstrated that OCA showed moderate benefit over placebo in improving fibrosis in NASH patients, but “there is uncertainty how the magnitude of changes in these surrogate endpoints may translate to meaningful changes in clinical outcomes,” an FDA meeting briefing document stated. There were also notable safety concerns including an increased risk for drug-induced liver injury.
An estimated 16.8 million Americans have NASH, and there are no FDA-approved medications for the condition.
Intercept plans to promptly begin closing out their NASH clinical trial and restructuring to focus on rare and serious liver diseases.
“While this is clearly not the outcome that we have worked toward, I’m proud of the impact that Intercept has made to move the science of NASH forward and bring the field closer to a treatment option,” said Jerry Durso, the president and CEO of Intercept, in a statement. “Intercept thanks the scientists, clinicians, and patients whose contributions to the clinical development of OCA in NASH have significantly advanced the understanding of this deadly disease.”
A version of this article originally appeared on Medscape.com.
In response, the company has decided to discontinue all NASH-related investment.
Intercept first sought FDA approval for OCA in treatment of NASH in 2019 and received a complete response letter. The company refiled for a new drug application in December 2022. A second resubmission would require a completion of the long-term outcomes phase of an ongoing clinical trial, according to an Intercept press release.
The FDA decision follows the recommendation from May’s FDA Gastrointestinal Drugs Advisory Committee meeting. During the meeting, members voted 15 to 1 to advise deferring approval until clinical outcome data became available. Intercept’s clinical trial data demonstrated that OCA showed moderate benefit over placebo in improving fibrosis in NASH patients, but “there is uncertainty how the magnitude of changes in these surrogate endpoints may translate to meaningful changes in clinical outcomes,” an FDA meeting briefing document stated. There were also notable safety concerns including an increased risk for drug-induced liver injury.
An estimated 16.8 million Americans have NASH, and there are no FDA-approved medications for the condition.
Intercept plans to promptly begin closing out their NASH clinical trial and restructuring to focus on rare and serious liver diseases.
“While this is clearly not the outcome that we have worked toward, I’m proud of the impact that Intercept has made to move the science of NASH forward and bring the field closer to a treatment option,” said Jerry Durso, the president and CEO of Intercept, in a statement. “Intercept thanks the scientists, clinicians, and patients whose contributions to the clinical development of OCA in NASH have significantly advanced the understanding of this deadly disease.”
A version of this article originally appeared on Medscape.com.
Systemic JIA and AOSD are the same disease, EULAR says
Systemic juvenile idiopathic arthritis (sJIA) and adult-onset Still’s disease (AOSD) should be grouped into one disease, Still’s disease, according to new diagnosis and treatment recommendations presented at the annual European Congress of Rheumatology.
The recommendations, made in collaboration with EULAR and the Pediatric Rheumatology European Society, emphasized that the ultimate treatment target for Still’s disease should be drug-free remission in all patients and that macrophage activation syndrome (MAS) should be identified and treated as soon as possible.
The task force focused on MAS because despite effective, innovative therapies, “we continued to see MAS,” said presenter Bruno Fautrel, MD, Pitié-Salpêtrière University Hospital, Paris. “We have to be very concerned about this potential complication.”
Dr. Fautrel copresented the recommendations with Fabrizio De Benedetti, MD, PhD, head of the division of rheumatology, Bambino Gesù Hospital, Rome.
Diagnosis
Dr. Fautrel noted that the cutoff age of 16 that differentiates sJIA and AOSD is “arbitrary.” There are some differences in age: The frequency of the disease is higher in young children, but it plateaus in young adults. Children under 18 months old are also far more likely to develop MAS.
To diagnose and treat Still’s disease, the recommendations state that clinicians should consider four criteria:
- A fever spiking at or above 39° C (102.2° F) for at least 7 days.
- A transient rash, preferentially on the trunk, that coincides with fever spikes, rash is typically erythematous but other rashes, like urticaria, can be consistent with the diagnosis.
- Some musculoskeletal involvement is common, involving arthralgia/myalgia.
- High levels of inflammation identified by neutrophilic leukocytosis, increased serum C-reactive protein (CRP), and ferritin.
Dr. Fautrel noted that, while arthritis can be present, it is not necessary to make a diagnosis. In pediatrics, “arthritis is likely to happen after a few weeks of the evolution of the disease,” and waiting for arthritis to develop can lead to diagnostic delay, “which is a problem.”
For individuals with suspected Still’s disease, NSAIDs can be used as a “bridging therapy” before the diagnosis is confirmed.
Treatment
The recommendations emphasized that treatment and therapeutic strategy “should be based on shared decision-making between the parents/patients and the treating team,” with the ultimate goal of drug-free remission.
To achieve this goal, the document outlines time-based targets for clinically inactive disease (CID). At 4 weeks, patients should have no fever, reduction of active or swollen joint count by more than 50%, a normal CRP level, and a rating of less than 20 on a visual analog scale of 0-100. At 3 months, patients should maintain clinically inactive disease with a glucocorticoid dose of less than 0.1 or 0.2 mg/kg per day. At 6 months, CID should be maintained without glucocorticoids.
While the authors of the recommendations noted that glucocorticoids are efficacious, their long-term use should be avoided because of safety issues. An interleukin-1 or IL-6 inhibitor should be prioritized and initiated as soon as possible after diagnosis.
Patients should maintain CID between 3 and 6 months before tapering off biologics.
The recommendations are congruent with the 2021 American College of Rheumatology’s guidelines for sJIA, noted Karen Onel, MD, pediatric rheumatologist, Hospital for Special Surgery, New York, and the principle investigator for the ACR guidelines. One main difference is that the EULAR recommendations included time lines for treatment targets, while the ACR’s did not.
“It’s great to have these time lines in there,” she said in an interview, though there are still some unknowns. “We don’t actually know what the tapering frequency should be,” she said, “but these are definitely goals that we need to explore and see how they evolve.”
MAS and lung complications
The EULAR recommendations also touched on two concerning complications, particularly in children: MAS and lung disease. According to the document, MAS should be considered in patients with Still’s disease with these symptoms: fever, splenomegaly, elevated serum ferritin, low cell counts, abnormal liver function tests, elevated serum triglycerides, and intravascular activation of coagulation. The MAS 2016 criteria can also be used to facilitate diagnosis.
“MAS treatment must include high-dose glucocorticoids,” the document states. “In addition, treatments including anakinra, ciclosporin, and/or interferon-gamma inhibitors should be considered as a part of initial therapy.”
The recommendations also addressed the risk for lung disease, “which is an emerging issue, particularly in children, that the physician should be very well aware of,” Dr. De Benedetti said. This complication can arise at any time point of the disease, he added.
The document advised actively screening for lung disease by searching for clinical symptoms such as digital clubbing, persistent cough, and shortness of breath. Pulmonary function tests like pulse oximetry and diffusing capacity of the lungs for carbon monoxide may also be used, but these standard lung function tests are very difficult to do in children under 6 years old, Dr. De Benedetti noted. The recommendations advise performing high-resolution CT in “any patients with clinical concerns.”
“We have lowered the threshold for CT scan because of the emerging features of this lung disease that may actually be lethal and therefore require prompt attention,” Dr. De Benedetti noted.
The recommendations for lung disease are “broad,” as there is still much to learn about the risk for lung disease in a small portion of sJIA patients, Dr. Onel said.
“There’s a lot that we are trying to work out about this; exactly how to screen, who to screen, what to do, who to treat, and how to treat really remains unclear,” she said. “We absolutely agree that this is a major, major issue that we need to come to some sort of agreement upon, but we’re just not there yet.”
Dr. De Benedetti, Dr. Fautrel, and Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Systemic juvenile idiopathic arthritis (sJIA) and adult-onset Still’s disease (AOSD) should be grouped into one disease, Still’s disease, according to new diagnosis and treatment recommendations presented at the annual European Congress of Rheumatology.
The recommendations, made in collaboration with EULAR and the Pediatric Rheumatology European Society, emphasized that the ultimate treatment target for Still’s disease should be drug-free remission in all patients and that macrophage activation syndrome (MAS) should be identified and treated as soon as possible.
The task force focused on MAS because despite effective, innovative therapies, “we continued to see MAS,” said presenter Bruno Fautrel, MD, Pitié-Salpêtrière University Hospital, Paris. “We have to be very concerned about this potential complication.”
Dr. Fautrel copresented the recommendations with Fabrizio De Benedetti, MD, PhD, head of the division of rheumatology, Bambino Gesù Hospital, Rome.
Diagnosis
Dr. Fautrel noted that the cutoff age of 16 that differentiates sJIA and AOSD is “arbitrary.” There are some differences in age: The frequency of the disease is higher in young children, but it plateaus in young adults. Children under 18 months old are also far more likely to develop MAS.
To diagnose and treat Still’s disease, the recommendations state that clinicians should consider four criteria:
- A fever spiking at or above 39° C (102.2° F) for at least 7 days.
- A transient rash, preferentially on the trunk, that coincides with fever spikes, rash is typically erythematous but other rashes, like urticaria, can be consistent with the diagnosis.
- Some musculoskeletal involvement is common, involving arthralgia/myalgia.
- High levels of inflammation identified by neutrophilic leukocytosis, increased serum C-reactive protein (CRP), and ferritin.
Dr. Fautrel noted that, while arthritis can be present, it is not necessary to make a diagnosis. In pediatrics, “arthritis is likely to happen after a few weeks of the evolution of the disease,” and waiting for arthritis to develop can lead to diagnostic delay, “which is a problem.”
For individuals with suspected Still’s disease, NSAIDs can be used as a “bridging therapy” before the diagnosis is confirmed.
Treatment
The recommendations emphasized that treatment and therapeutic strategy “should be based on shared decision-making between the parents/patients and the treating team,” with the ultimate goal of drug-free remission.
To achieve this goal, the document outlines time-based targets for clinically inactive disease (CID). At 4 weeks, patients should have no fever, reduction of active or swollen joint count by more than 50%, a normal CRP level, and a rating of less than 20 on a visual analog scale of 0-100. At 3 months, patients should maintain clinically inactive disease with a glucocorticoid dose of less than 0.1 or 0.2 mg/kg per day. At 6 months, CID should be maintained without glucocorticoids.
While the authors of the recommendations noted that glucocorticoids are efficacious, their long-term use should be avoided because of safety issues. An interleukin-1 or IL-6 inhibitor should be prioritized and initiated as soon as possible after diagnosis.
Patients should maintain CID between 3 and 6 months before tapering off biologics.
The recommendations are congruent with the 2021 American College of Rheumatology’s guidelines for sJIA, noted Karen Onel, MD, pediatric rheumatologist, Hospital for Special Surgery, New York, and the principle investigator for the ACR guidelines. One main difference is that the EULAR recommendations included time lines for treatment targets, while the ACR’s did not.
“It’s great to have these time lines in there,” she said in an interview, though there are still some unknowns. “We don’t actually know what the tapering frequency should be,” she said, “but these are definitely goals that we need to explore and see how they evolve.”
MAS and lung complications
The EULAR recommendations also touched on two concerning complications, particularly in children: MAS and lung disease. According to the document, MAS should be considered in patients with Still’s disease with these symptoms: fever, splenomegaly, elevated serum ferritin, low cell counts, abnormal liver function tests, elevated serum triglycerides, and intravascular activation of coagulation. The MAS 2016 criteria can also be used to facilitate diagnosis.
“MAS treatment must include high-dose glucocorticoids,” the document states. “In addition, treatments including anakinra, ciclosporin, and/or interferon-gamma inhibitors should be considered as a part of initial therapy.”
The recommendations also addressed the risk for lung disease, “which is an emerging issue, particularly in children, that the physician should be very well aware of,” Dr. De Benedetti said. This complication can arise at any time point of the disease, he added.
The document advised actively screening for lung disease by searching for clinical symptoms such as digital clubbing, persistent cough, and shortness of breath. Pulmonary function tests like pulse oximetry and diffusing capacity of the lungs for carbon monoxide may also be used, but these standard lung function tests are very difficult to do in children under 6 years old, Dr. De Benedetti noted. The recommendations advise performing high-resolution CT in “any patients with clinical concerns.”
“We have lowered the threshold for CT scan because of the emerging features of this lung disease that may actually be lethal and therefore require prompt attention,” Dr. De Benedetti noted.
The recommendations for lung disease are “broad,” as there is still much to learn about the risk for lung disease in a small portion of sJIA patients, Dr. Onel said.
“There’s a lot that we are trying to work out about this; exactly how to screen, who to screen, what to do, who to treat, and how to treat really remains unclear,” she said. “We absolutely agree that this is a major, major issue that we need to come to some sort of agreement upon, but we’re just not there yet.”
Dr. De Benedetti, Dr. Fautrel, and Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Systemic juvenile idiopathic arthritis (sJIA) and adult-onset Still’s disease (AOSD) should be grouped into one disease, Still’s disease, according to new diagnosis and treatment recommendations presented at the annual European Congress of Rheumatology.
The recommendations, made in collaboration with EULAR and the Pediatric Rheumatology European Society, emphasized that the ultimate treatment target for Still’s disease should be drug-free remission in all patients and that macrophage activation syndrome (MAS) should be identified and treated as soon as possible.
The task force focused on MAS because despite effective, innovative therapies, “we continued to see MAS,” said presenter Bruno Fautrel, MD, Pitié-Salpêtrière University Hospital, Paris. “We have to be very concerned about this potential complication.”
Dr. Fautrel copresented the recommendations with Fabrizio De Benedetti, MD, PhD, head of the division of rheumatology, Bambino Gesù Hospital, Rome.
Diagnosis
Dr. Fautrel noted that the cutoff age of 16 that differentiates sJIA and AOSD is “arbitrary.” There are some differences in age: The frequency of the disease is higher in young children, but it plateaus in young adults. Children under 18 months old are also far more likely to develop MAS.
To diagnose and treat Still’s disease, the recommendations state that clinicians should consider four criteria:
- A fever spiking at or above 39° C (102.2° F) for at least 7 days.
- A transient rash, preferentially on the trunk, that coincides with fever spikes, rash is typically erythematous but other rashes, like urticaria, can be consistent with the diagnosis.
- Some musculoskeletal involvement is common, involving arthralgia/myalgia.
- High levels of inflammation identified by neutrophilic leukocytosis, increased serum C-reactive protein (CRP), and ferritin.
Dr. Fautrel noted that, while arthritis can be present, it is not necessary to make a diagnosis. In pediatrics, “arthritis is likely to happen after a few weeks of the evolution of the disease,” and waiting for arthritis to develop can lead to diagnostic delay, “which is a problem.”
For individuals with suspected Still’s disease, NSAIDs can be used as a “bridging therapy” before the diagnosis is confirmed.
Treatment
The recommendations emphasized that treatment and therapeutic strategy “should be based on shared decision-making between the parents/patients and the treating team,” with the ultimate goal of drug-free remission.
To achieve this goal, the document outlines time-based targets for clinically inactive disease (CID). At 4 weeks, patients should have no fever, reduction of active or swollen joint count by more than 50%, a normal CRP level, and a rating of less than 20 on a visual analog scale of 0-100. At 3 months, patients should maintain clinically inactive disease with a glucocorticoid dose of less than 0.1 or 0.2 mg/kg per day. At 6 months, CID should be maintained without glucocorticoids.
While the authors of the recommendations noted that glucocorticoids are efficacious, their long-term use should be avoided because of safety issues. An interleukin-1 or IL-6 inhibitor should be prioritized and initiated as soon as possible after diagnosis.
Patients should maintain CID between 3 and 6 months before tapering off biologics.
The recommendations are congruent with the 2021 American College of Rheumatology’s guidelines for sJIA, noted Karen Onel, MD, pediatric rheumatologist, Hospital for Special Surgery, New York, and the principle investigator for the ACR guidelines. One main difference is that the EULAR recommendations included time lines for treatment targets, while the ACR’s did not.
“It’s great to have these time lines in there,” she said in an interview, though there are still some unknowns. “We don’t actually know what the tapering frequency should be,” she said, “but these are definitely goals that we need to explore and see how they evolve.”
MAS and lung complications
The EULAR recommendations also touched on two concerning complications, particularly in children: MAS and lung disease. According to the document, MAS should be considered in patients with Still’s disease with these symptoms: fever, splenomegaly, elevated serum ferritin, low cell counts, abnormal liver function tests, elevated serum triglycerides, and intravascular activation of coagulation. The MAS 2016 criteria can also be used to facilitate diagnosis.
“MAS treatment must include high-dose glucocorticoids,” the document states. “In addition, treatments including anakinra, ciclosporin, and/or interferon-gamma inhibitors should be considered as a part of initial therapy.”
The recommendations also addressed the risk for lung disease, “which is an emerging issue, particularly in children, that the physician should be very well aware of,” Dr. De Benedetti said. This complication can arise at any time point of the disease, he added.
The document advised actively screening for lung disease by searching for clinical symptoms such as digital clubbing, persistent cough, and shortness of breath. Pulmonary function tests like pulse oximetry and diffusing capacity of the lungs for carbon monoxide may also be used, but these standard lung function tests are very difficult to do in children under 6 years old, Dr. De Benedetti noted. The recommendations advise performing high-resolution CT in “any patients with clinical concerns.”
“We have lowered the threshold for CT scan because of the emerging features of this lung disease that may actually be lethal and therefore require prompt attention,” Dr. De Benedetti noted.
The recommendations for lung disease are “broad,” as there is still much to learn about the risk for lung disease in a small portion of sJIA patients, Dr. Onel said.
“There’s a lot that we are trying to work out about this; exactly how to screen, who to screen, what to do, who to treat, and how to treat really remains unclear,” she said. “We absolutely agree that this is a major, major issue that we need to come to some sort of agreement upon, but we’re just not there yet.”
Dr. De Benedetti, Dr. Fautrel, and Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EULAR 2023
EULAR issues imaging recommendations for crystal-induced arthropathies
A European Alliance of Associations for Rheumatology task force has released new guidance on imaging of crystal-induced arthropathies (CiA). The document provides recommendations for using imaging for diagnosis and monitoring of these types of diseases.
“These are the first-ever EULAR recommendations on imaging in this group of diseases. In fact, we are not aware of any similar international recommendations which provide guidance on which imaging technique, when, and how [they] should be used for crystal-induced arthropathies,” lead author Peter Mandl, MD, PhD, of the division of rheumatology at the Medical University of Vienna, told this news organization. Dr. Mandl presented the new recommendations at the annual European Congress of Rheumatology.
While some rheumatologists very familiar with crystal-induced arthropathies already regularly use imaging with these patients, these formal recommendations could highlight to wider audiences that “these imaging modalities can be very sensitive and specific for CiA,” said Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School and head of crystal-induced arthritis diseases at Brigham and Women’s Hospital in Boston. She was not involved with the work.
The document included general recommendations for imaging in CiA as well as specific recommendations for gout, basic calcium phosphate deposition disease (BCPD), and calcium pyrophosphate deposition disease (CPPD). Across all disease types, performing imaging on symptomatic areas as well as disease-specific target sites should be considered, the recommendations state. This includes the first metatarsophalangeal joint in gout, the wrist and knee in CPPD, and the shoulder in BCPD.
Both ultrasound (US) and dual-energy CT (DECT) are the recommended imaging modalities in gout. If imaging reveals characteristic features of monosodium urate (MSU) crystal deposition, synovial fluid analysis is not necessary to confirm a gout diagnosis. The volume of MSU crystals on imaging can also be used to predict future disease flares.
Showing imaging and explaining imaging findings may help patients understand their condition and adhere to treatment regimens, the recommendations state. “I think it’s a very powerful way to counsel patients,” Dr. Tedeschi said in an interview.
Imaging is necessary in the diagnosis of BCPD, and clinicians should use either conventional radiography or US. These imagining modalities are recommended for CPPD, and clinicians can use CT if they suspect axial involvement. The document does not recommend serial imaging for either BCPD or CPPD unless there has been an “unsuspected change in clinical characteristics.”
These recommendations highlight how imaging can have a “powerful impact on patient counseling and diagnosis,” said Dr. Tedeschi. She emphasized the importance of US training in rheumatology fellowship programs.
During his presentation at EULAR 2023, Dr. Mandl also highlighted a robust research agenda to further investigate how imaging can aid in the diagnosis and treatment of CiA. “It would be great to have an imaging modality someday that would help us differentiate between various types of calcium crystal,” he said.
Dr. Mandl has financial relationships with AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Roche, and UCB. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article first appeared on Medscape.com.
A European Alliance of Associations for Rheumatology task force has released new guidance on imaging of crystal-induced arthropathies (CiA). The document provides recommendations for using imaging for diagnosis and monitoring of these types of diseases.
“These are the first-ever EULAR recommendations on imaging in this group of diseases. In fact, we are not aware of any similar international recommendations which provide guidance on which imaging technique, when, and how [they] should be used for crystal-induced arthropathies,” lead author Peter Mandl, MD, PhD, of the division of rheumatology at the Medical University of Vienna, told this news organization. Dr. Mandl presented the new recommendations at the annual European Congress of Rheumatology.
While some rheumatologists very familiar with crystal-induced arthropathies already regularly use imaging with these patients, these formal recommendations could highlight to wider audiences that “these imaging modalities can be very sensitive and specific for CiA,” said Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School and head of crystal-induced arthritis diseases at Brigham and Women’s Hospital in Boston. She was not involved with the work.
The document included general recommendations for imaging in CiA as well as specific recommendations for gout, basic calcium phosphate deposition disease (BCPD), and calcium pyrophosphate deposition disease (CPPD). Across all disease types, performing imaging on symptomatic areas as well as disease-specific target sites should be considered, the recommendations state. This includes the first metatarsophalangeal joint in gout, the wrist and knee in CPPD, and the shoulder in BCPD.
Both ultrasound (US) and dual-energy CT (DECT) are the recommended imaging modalities in gout. If imaging reveals characteristic features of monosodium urate (MSU) crystal deposition, synovial fluid analysis is not necessary to confirm a gout diagnosis. The volume of MSU crystals on imaging can also be used to predict future disease flares.
Showing imaging and explaining imaging findings may help patients understand their condition and adhere to treatment regimens, the recommendations state. “I think it’s a very powerful way to counsel patients,” Dr. Tedeschi said in an interview.
Imaging is necessary in the diagnosis of BCPD, and clinicians should use either conventional radiography or US. These imagining modalities are recommended for CPPD, and clinicians can use CT if they suspect axial involvement. The document does not recommend serial imaging for either BCPD or CPPD unless there has been an “unsuspected change in clinical characteristics.”
These recommendations highlight how imaging can have a “powerful impact on patient counseling and diagnosis,” said Dr. Tedeschi. She emphasized the importance of US training in rheumatology fellowship programs.
During his presentation at EULAR 2023, Dr. Mandl also highlighted a robust research agenda to further investigate how imaging can aid in the diagnosis and treatment of CiA. “It would be great to have an imaging modality someday that would help us differentiate between various types of calcium crystal,” he said.
Dr. Mandl has financial relationships with AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Roche, and UCB. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article first appeared on Medscape.com.
A European Alliance of Associations for Rheumatology task force has released new guidance on imaging of crystal-induced arthropathies (CiA). The document provides recommendations for using imaging for diagnosis and monitoring of these types of diseases.
“These are the first-ever EULAR recommendations on imaging in this group of diseases. In fact, we are not aware of any similar international recommendations which provide guidance on which imaging technique, when, and how [they] should be used for crystal-induced arthropathies,” lead author Peter Mandl, MD, PhD, of the division of rheumatology at the Medical University of Vienna, told this news organization. Dr. Mandl presented the new recommendations at the annual European Congress of Rheumatology.
While some rheumatologists very familiar with crystal-induced arthropathies already regularly use imaging with these patients, these formal recommendations could highlight to wider audiences that “these imaging modalities can be very sensitive and specific for CiA,” said Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School and head of crystal-induced arthritis diseases at Brigham and Women’s Hospital in Boston. She was not involved with the work.
The document included general recommendations for imaging in CiA as well as specific recommendations for gout, basic calcium phosphate deposition disease (BCPD), and calcium pyrophosphate deposition disease (CPPD). Across all disease types, performing imaging on symptomatic areas as well as disease-specific target sites should be considered, the recommendations state. This includes the first metatarsophalangeal joint in gout, the wrist and knee in CPPD, and the shoulder in BCPD.
Both ultrasound (US) and dual-energy CT (DECT) are the recommended imaging modalities in gout. If imaging reveals characteristic features of monosodium urate (MSU) crystal deposition, synovial fluid analysis is not necessary to confirm a gout diagnosis. The volume of MSU crystals on imaging can also be used to predict future disease flares.
Showing imaging and explaining imaging findings may help patients understand their condition and adhere to treatment regimens, the recommendations state. “I think it’s a very powerful way to counsel patients,” Dr. Tedeschi said in an interview.
Imaging is necessary in the diagnosis of BCPD, and clinicians should use either conventional radiography or US. These imagining modalities are recommended for CPPD, and clinicians can use CT if they suspect axial involvement. The document does not recommend serial imaging for either BCPD or CPPD unless there has been an “unsuspected change in clinical characteristics.”
These recommendations highlight how imaging can have a “powerful impact on patient counseling and diagnosis,” said Dr. Tedeschi. She emphasized the importance of US training in rheumatology fellowship programs.
During his presentation at EULAR 2023, Dr. Mandl also highlighted a robust research agenda to further investigate how imaging can aid in the diagnosis and treatment of CiA. “It would be great to have an imaging modality someday that would help us differentiate between various types of calcium crystal,” he said.
Dr. Mandl has financial relationships with AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Roche, and UCB. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article first appeared on Medscape.com.
FROM EULAR 2023
Methotrexate does not impair sperm quality, small study finds
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
Why not both? Dual biologics for treatment-resistant RA and PsA
The introduction of tumor necrosis factor (TNF) inhibitors in the late 1990s revolutionized treatment of rheumatic diseases, such as rheumatoid arthritis (RA) and psoriatic arthritis (PsA), providing patients with another treatment option when conventional therapies were ineffective. However, when these diseases don’t respond to anti-TNF therapy, it is still difficult to determine the next best course of action.
“One of the big challenges we have in treatment of psoriatic arthritis, and I would say rheumatoid arthritis was well, is how to handle patients who have failed their first biologic therapy,” Christopher T. Ritchlin, MD, MPH, professor of allergy, immunology, and rheumatology at the University of Rochester (N.Y.), told this news organization. “In the case of both RA and PsA, that’s quite frequently an anti-TNF agent.”
For an estimated 30% to 40% of patients, TNF inhibitor therapy is discontinued because of nonresponse or intolerance. Clinicians can switch to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD) or add another conventional DMARD, such as methotrexate. Now, several case studies as well as promising findings from phase 2 clinical trials suggest that combining two biologics could be an alternative strategy to improve patient response to treatment. However, concerns about safety and higher costs remain.
Targeting multiple mechanisms of action
Rheumatic conditions affect multiple areas of the body and involve different signaling pathways, said Dr. Ritchlin, who heads the Clinical Immunology Research Unit at the University of Rochester. PsA, for example, affects the skin, peripheral joints, the axial skeleton, and the entheses.
“The question is, Are these various manifestations – of which multiple [ones] are often seen in one patient – likely to respond to one therapy that targets one single pathway?” he said.
Combination therapies have been effective in treating leukemia and lymphoma as well as infection with HIV, Melek Yalçin Mutlu, MD, and colleagues from Friedrich Alexander University Erlangen-Nuremberg and the University Clinic Erlangen (Germany), wrote in a review about combining biologic DMARDs in the treatment of RA and PsA. The review was published in Joint Bone Spine.
“Cumulative evidence on the success of combination therapies in various diseases supports an akin approach in rheumatology, and simultaneous or sequential blockade of multiple mechanisms that generate or propagate arthritis could theoretically enhance efficacy,” the authors wrote. “On the other hand, intervening on multiple targets in the immune system brings about a risk of adverse events, among which infection is a major concern.”
Failed clinical trials
Clinical trials of combination biologic therapies for rheumatic disease have been tried before, but these combinations did not show superior efficacy, and they increased patients’ risk for infection. One study published in 2004 compared monotherapy with the TNF inhibitor etanercept (Enbrel) to the combination of etanercept and anakinra (Kineret), an interleukin-1 (IL-1) antagonist, in 244 patients with active RA despite methotrexate therapy. Researchers found no statistically significant difference in achieving 20% improvement in modified American College of Rheumatology response criteria (ACR20), ACR50, or ACR70 between the groups that received etanercept and anakinra and those that received etanercept alone. There were nine serious infections among patients given etanercept and anakinra, including one death due to pneumonia. There were no serious infections in the etanercept monotherapy group.
In another RA trial, 121 patients were given etanercept 25 mg twice weekly and were randomly assigned to also receive a placebo or low-dose abatacept (Orencia), a T-cell co-stimulation inhibitor. There was no significant difference in disease improvement between the two groups, although the rate of serious adverse events was nearly six times higher in the etanercept-abatacept group (16.5% vs. 2.8%).
These studies had a “chilling effect on the whole field for some years,” Brian G. Feagan, MD, the senior scientific director of the gastrointestinal contract research firm Alimentiv in London, Ontario, told this news organization. People were reluctant to try new biologic combinations, owing to the fear that these safety issues would plague subsequent trials.
Promising combinations
But a recent phase 2 trial, led by Dr. Feagan, suggests that certain combinations can be effective. In the Janssen-sponsored VEGA trial, researchers found that a combination of guselkumab (Tremfya), an IL-23 inhibitor, and golimumab (Simponi), an anti-TNF agent, was more effective than either drug used as monotherapy for initial induction treatment for moderate to severe ulcerative colitis. Importantly, there was no difference in adverse events between any of the groups. This same combination therapy is now being tried for patients with active PsA in Janssen’s AFFINITY trial, for which Dr. Ritchlin is one of the lead investigators.
Other trials have also delivered promising results. One study enrolled 51 adults with active RA who were all receiving stable doses of both a TNF inhibitor – either etanercept or adalimumab (Humira) – and methotrexate. Patients were randomly assigned to receive one course of rituximab (Rituxan) or placebo. The researchers found that the safety profile of this TNF inhibitor/methotrexate/rituximab combination was “consistent” with the safety profiles of previous studies of methotrexate/rituximab dual combinations with no TNF inhibitor; there were no new safety signals. At 24 weeks, 30% of the group that received rituximab reached ACR20, compared with 17% of the group that was given placebo. Twelve percent of the rituximab group achieved ACR50, compared with 6% of the group that received placebo.
“B-cell depletion is fundamentally different from cytokine inhibition and even from co-stimulation blockade, making an additive effect more likely,” Dr. Mutlu and colleagues wrote in their review. Reports have also suggested possible benefits of combining a TNF inhibitor and an IL-17 inhibitor in the treatment of RA and PsA, as well as the combination of a TNF inhibitor and an IL-23 antagonist for PsA.
While these combinations require controlled clinical trials, “there’s some smoke signals out there that this might be an effective strategy for some patients,” Dr. Ritchlin said.
In addition to the AFFINITY trial, two clinical trials are underway in France. The first, CRI-RA, is evaluating the combination of baricitinib (Olumiant), a Janus kinase (JAK) inhibitor, and adalimumab. Although baricitinib is not a biologic, as a targeted synthetic DMARD, the therapy is more potent than conventional DMARDs, and the same potential safety concerns apply. However, use of a combination of tofacitinib (Xeljanz) and different biologics for RA patients has been reported; no serious side effects were reported over 11 months of therapy. The randomized, placebo-controlled trial began in July 2021 and will enroll 178 patients. The estimated study completion date is July 2025.
“Of note, baricitinib does not directly block signaling downstream of TNF, even if an indirect effect on TNF production is likely to occur,” the CRI-RA entry on clinicaltrials.gov reads. “Targeting multiple inflammatory cytokines in combination may lead to more effective treatment and enhanced clinical responses in patients with RA compared to the current second-line strategies.”
The second trial, SEQUENS-RA, is evaluating the use of TNF inhibitors followed by abatacept for patients with RA who test positive for anticitrullinated protein autoantibodies (ACPAs). In the past, the combination of a TNF inhibitor and abatacept did not lead to promising results, but in this trial, the drugs will be administered sequentially.
“Although abatacept has shown a very good tolerance profile that might be superior to other bDMARDs [biologic DMARDs], rheumatologists might be reluctant to use it as a first line bDMARD as there is a belief of a slower efficacy compared to other bDMARDs or JAK inhibitors,” according to the clinical trial’s description. “Investigators have hypothesized that first rapidly controlling the inflammation phase, using TNF inhibitors, followed by abatacept to induce an immunological remission, would optimize response and tolerance of ACPA-positive patients with RA.”
The randomized trial of 220 participants began in November 2022. The estimated completion date for the study is November 2025.
Finding the right patients
Though these studies have had some promising results, the difference in efficacy between biologic monotherapy and dual therapy has been mostly moderate, Dr. Mutlu and coauthors wrote. Identifying disease subtypes for which there might be a higher likelihood of response to dual biologic treatment, especially multidrug-resistant types, could improve efficacies in future trials, they argued. “The good effects of bDMARD combinations in resistant patients in fact point into this direction, though they were observed in uncontrolled studies,” the authors noted.
Insurance coverage remains a “huge challenge” for these dual therapies because of the higher expense, noted Dr. Ritchlin. Better targeting therapies could help convince these companies to pay for these therapies.
“I would say that if we were able to demonstrate a phenotype of a patient that would respond to biologics and not monotherapies, [then] many companies would be amenable to this kind of approach,” he said.
Dr. Ritchlin reports financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Pfizer, Eli Lilly, Novartis, and UCB. Dr. Feagan reports financial relationships with AbbVie, Amgen, Janssen, Pfizer, Takeda, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
The introduction of tumor necrosis factor (TNF) inhibitors in the late 1990s revolutionized treatment of rheumatic diseases, such as rheumatoid arthritis (RA) and psoriatic arthritis (PsA), providing patients with another treatment option when conventional therapies were ineffective. However, when these diseases don’t respond to anti-TNF therapy, it is still difficult to determine the next best course of action.
“One of the big challenges we have in treatment of psoriatic arthritis, and I would say rheumatoid arthritis was well, is how to handle patients who have failed their first biologic therapy,” Christopher T. Ritchlin, MD, MPH, professor of allergy, immunology, and rheumatology at the University of Rochester (N.Y.), told this news organization. “In the case of both RA and PsA, that’s quite frequently an anti-TNF agent.”
For an estimated 30% to 40% of patients, TNF inhibitor therapy is discontinued because of nonresponse or intolerance. Clinicians can switch to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD) or add another conventional DMARD, such as methotrexate. Now, several case studies as well as promising findings from phase 2 clinical trials suggest that combining two biologics could be an alternative strategy to improve patient response to treatment. However, concerns about safety and higher costs remain.
Targeting multiple mechanisms of action
Rheumatic conditions affect multiple areas of the body and involve different signaling pathways, said Dr. Ritchlin, who heads the Clinical Immunology Research Unit at the University of Rochester. PsA, for example, affects the skin, peripheral joints, the axial skeleton, and the entheses.
“The question is, Are these various manifestations – of which multiple [ones] are often seen in one patient – likely to respond to one therapy that targets one single pathway?” he said.
Combination therapies have been effective in treating leukemia and lymphoma as well as infection with HIV, Melek Yalçin Mutlu, MD, and colleagues from Friedrich Alexander University Erlangen-Nuremberg and the University Clinic Erlangen (Germany), wrote in a review about combining biologic DMARDs in the treatment of RA and PsA. The review was published in Joint Bone Spine.
“Cumulative evidence on the success of combination therapies in various diseases supports an akin approach in rheumatology, and simultaneous or sequential blockade of multiple mechanisms that generate or propagate arthritis could theoretically enhance efficacy,” the authors wrote. “On the other hand, intervening on multiple targets in the immune system brings about a risk of adverse events, among which infection is a major concern.”
Failed clinical trials
Clinical trials of combination biologic therapies for rheumatic disease have been tried before, but these combinations did not show superior efficacy, and they increased patients’ risk for infection. One study published in 2004 compared monotherapy with the TNF inhibitor etanercept (Enbrel) to the combination of etanercept and anakinra (Kineret), an interleukin-1 (IL-1) antagonist, in 244 patients with active RA despite methotrexate therapy. Researchers found no statistically significant difference in achieving 20% improvement in modified American College of Rheumatology response criteria (ACR20), ACR50, or ACR70 between the groups that received etanercept and anakinra and those that received etanercept alone. There were nine serious infections among patients given etanercept and anakinra, including one death due to pneumonia. There were no serious infections in the etanercept monotherapy group.
In another RA trial, 121 patients were given etanercept 25 mg twice weekly and were randomly assigned to also receive a placebo or low-dose abatacept (Orencia), a T-cell co-stimulation inhibitor. There was no significant difference in disease improvement between the two groups, although the rate of serious adverse events was nearly six times higher in the etanercept-abatacept group (16.5% vs. 2.8%).
These studies had a “chilling effect on the whole field for some years,” Brian G. Feagan, MD, the senior scientific director of the gastrointestinal contract research firm Alimentiv in London, Ontario, told this news organization. People were reluctant to try new biologic combinations, owing to the fear that these safety issues would plague subsequent trials.
Promising combinations
But a recent phase 2 trial, led by Dr. Feagan, suggests that certain combinations can be effective. In the Janssen-sponsored VEGA trial, researchers found that a combination of guselkumab (Tremfya), an IL-23 inhibitor, and golimumab (Simponi), an anti-TNF agent, was more effective than either drug used as monotherapy for initial induction treatment for moderate to severe ulcerative colitis. Importantly, there was no difference in adverse events between any of the groups. This same combination therapy is now being tried for patients with active PsA in Janssen’s AFFINITY trial, for which Dr. Ritchlin is one of the lead investigators.
Other trials have also delivered promising results. One study enrolled 51 adults with active RA who were all receiving stable doses of both a TNF inhibitor – either etanercept or adalimumab (Humira) – and methotrexate. Patients were randomly assigned to receive one course of rituximab (Rituxan) or placebo. The researchers found that the safety profile of this TNF inhibitor/methotrexate/rituximab combination was “consistent” with the safety profiles of previous studies of methotrexate/rituximab dual combinations with no TNF inhibitor; there were no new safety signals. At 24 weeks, 30% of the group that received rituximab reached ACR20, compared with 17% of the group that was given placebo. Twelve percent of the rituximab group achieved ACR50, compared with 6% of the group that received placebo.
“B-cell depletion is fundamentally different from cytokine inhibition and even from co-stimulation blockade, making an additive effect more likely,” Dr. Mutlu and colleagues wrote in their review. Reports have also suggested possible benefits of combining a TNF inhibitor and an IL-17 inhibitor in the treatment of RA and PsA, as well as the combination of a TNF inhibitor and an IL-23 antagonist for PsA.
While these combinations require controlled clinical trials, “there’s some smoke signals out there that this might be an effective strategy for some patients,” Dr. Ritchlin said.
In addition to the AFFINITY trial, two clinical trials are underway in France. The first, CRI-RA, is evaluating the combination of baricitinib (Olumiant), a Janus kinase (JAK) inhibitor, and adalimumab. Although baricitinib is not a biologic, as a targeted synthetic DMARD, the therapy is more potent than conventional DMARDs, and the same potential safety concerns apply. However, use of a combination of tofacitinib (Xeljanz) and different biologics for RA patients has been reported; no serious side effects were reported over 11 months of therapy. The randomized, placebo-controlled trial began in July 2021 and will enroll 178 patients. The estimated study completion date is July 2025.
“Of note, baricitinib does not directly block signaling downstream of TNF, even if an indirect effect on TNF production is likely to occur,” the CRI-RA entry on clinicaltrials.gov reads. “Targeting multiple inflammatory cytokines in combination may lead to more effective treatment and enhanced clinical responses in patients with RA compared to the current second-line strategies.”
The second trial, SEQUENS-RA, is evaluating the use of TNF inhibitors followed by abatacept for patients with RA who test positive for anticitrullinated protein autoantibodies (ACPAs). In the past, the combination of a TNF inhibitor and abatacept did not lead to promising results, but in this trial, the drugs will be administered sequentially.
“Although abatacept has shown a very good tolerance profile that might be superior to other bDMARDs [biologic DMARDs], rheumatologists might be reluctant to use it as a first line bDMARD as there is a belief of a slower efficacy compared to other bDMARDs or JAK inhibitors,” according to the clinical trial’s description. “Investigators have hypothesized that first rapidly controlling the inflammation phase, using TNF inhibitors, followed by abatacept to induce an immunological remission, would optimize response and tolerance of ACPA-positive patients with RA.”
The randomized trial of 220 participants began in November 2022. The estimated completion date for the study is November 2025.
Finding the right patients
Though these studies have had some promising results, the difference in efficacy between biologic monotherapy and dual therapy has been mostly moderate, Dr. Mutlu and coauthors wrote. Identifying disease subtypes for which there might be a higher likelihood of response to dual biologic treatment, especially multidrug-resistant types, could improve efficacies in future trials, they argued. “The good effects of bDMARD combinations in resistant patients in fact point into this direction, though they were observed in uncontrolled studies,” the authors noted.
Insurance coverage remains a “huge challenge” for these dual therapies because of the higher expense, noted Dr. Ritchlin. Better targeting therapies could help convince these companies to pay for these therapies.
“I would say that if we were able to demonstrate a phenotype of a patient that would respond to biologics and not monotherapies, [then] many companies would be amenable to this kind of approach,” he said.
Dr. Ritchlin reports financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Pfizer, Eli Lilly, Novartis, and UCB. Dr. Feagan reports financial relationships with AbbVie, Amgen, Janssen, Pfizer, Takeda, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
The introduction of tumor necrosis factor (TNF) inhibitors in the late 1990s revolutionized treatment of rheumatic diseases, such as rheumatoid arthritis (RA) and psoriatic arthritis (PsA), providing patients with another treatment option when conventional therapies were ineffective. However, when these diseases don’t respond to anti-TNF therapy, it is still difficult to determine the next best course of action.
“One of the big challenges we have in treatment of psoriatic arthritis, and I would say rheumatoid arthritis was well, is how to handle patients who have failed their first biologic therapy,” Christopher T. Ritchlin, MD, MPH, professor of allergy, immunology, and rheumatology at the University of Rochester (N.Y.), told this news organization. “In the case of both RA and PsA, that’s quite frequently an anti-TNF agent.”
For an estimated 30% to 40% of patients, TNF inhibitor therapy is discontinued because of nonresponse or intolerance. Clinicians can switch to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD) or add another conventional DMARD, such as methotrexate. Now, several case studies as well as promising findings from phase 2 clinical trials suggest that combining two biologics could be an alternative strategy to improve patient response to treatment. However, concerns about safety and higher costs remain.
Targeting multiple mechanisms of action
Rheumatic conditions affect multiple areas of the body and involve different signaling pathways, said Dr. Ritchlin, who heads the Clinical Immunology Research Unit at the University of Rochester. PsA, for example, affects the skin, peripheral joints, the axial skeleton, and the entheses.
“The question is, Are these various manifestations – of which multiple [ones] are often seen in one patient – likely to respond to one therapy that targets one single pathway?” he said.
Combination therapies have been effective in treating leukemia and lymphoma as well as infection with HIV, Melek Yalçin Mutlu, MD, and colleagues from Friedrich Alexander University Erlangen-Nuremberg and the University Clinic Erlangen (Germany), wrote in a review about combining biologic DMARDs in the treatment of RA and PsA. The review was published in Joint Bone Spine.
“Cumulative evidence on the success of combination therapies in various diseases supports an akin approach in rheumatology, and simultaneous or sequential blockade of multiple mechanisms that generate or propagate arthritis could theoretically enhance efficacy,” the authors wrote. “On the other hand, intervening on multiple targets in the immune system brings about a risk of adverse events, among which infection is a major concern.”
Failed clinical trials
Clinical trials of combination biologic therapies for rheumatic disease have been tried before, but these combinations did not show superior efficacy, and they increased patients’ risk for infection. One study published in 2004 compared monotherapy with the TNF inhibitor etanercept (Enbrel) to the combination of etanercept and anakinra (Kineret), an interleukin-1 (IL-1) antagonist, in 244 patients with active RA despite methotrexate therapy. Researchers found no statistically significant difference in achieving 20% improvement in modified American College of Rheumatology response criteria (ACR20), ACR50, or ACR70 between the groups that received etanercept and anakinra and those that received etanercept alone. There were nine serious infections among patients given etanercept and anakinra, including one death due to pneumonia. There were no serious infections in the etanercept monotherapy group.
In another RA trial, 121 patients were given etanercept 25 mg twice weekly and were randomly assigned to also receive a placebo or low-dose abatacept (Orencia), a T-cell co-stimulation inhibitor. There was no significant difference in disease improvement between the two groups, although the rate of serious adverse events was nearly six times higher in the etanercept-abatacept group (16.5% vs. 2.8%).
These studies had a “chilling effect on the whole field for some years,” Brian G. Feagan, MD, the senior scientific director of the gastrointestinal contract research firm Alimentiv in London, Ontario, told this news organization. People were reluctant to try new biologic combinations, owing to the fear that these safety issues would plague subsequent trials.
Promising combinations
But a recent phase 2 trial, led by Dr. Feagan, suggests that certain combinations can be effective. In the Janssen-sponsored VEGA trial, researchers found that a combination of guselkumab (Tremfya), an IL-23 inhibitor, and golimumab (Simponi), an anti-TNF agent, was more effective than either drug used as monotherapy for initial induction treatment for moderate to severe ulcerative colitis. Importantly, there was no difference in adverse events between any of the groups. This same combination therapy is now being tried for patients with active PsA in Janssen’s AFFINITY trial, for which Dr. Ritchlin is one of the lead investigators.
Other trials have also delivered promising results. One study enrolled 51 adults with active RA who were all receiving stable doses of both a TNF inhibitor – either etanercept or adalimumab (Humira) – and methotrexate. Patients were randomly assigned to receive one course of rituximab (Rituxan) or placebo. The researchers found that the safety profile of this TNF inhibitor/methotrexate/rituximab combination was “consistent” with the safety profiles of previous studies of methotrexate/rituximab dual combinations with no TNF inhibitor; there were no new safety signals. At 24 weeks, 30% of the group that received rituximab reached ACR20, compared with 17% of the group that was given placebo. Twelve percent of the rituximab group achieved ACR50, compared with 6% of the group that received placebo.
“B-cell depletion is fundamentally different from cytokine inhibition and even from co-stimulation blockade, making an additive effect more likely,” Dr. Mutlu and colleagues wrote in their review. Reports have also suggested possible benefits of combining a TNF inhibitor and an IL-17 inhibitor in the treatment of RA and PsA, as well as the combination of a TNF inhibitor and an IL-23 antagonist for PsA.
While these combinations require controlled clinical trials, “there’s some smoke signals out there that this might be an effective strategy for some patients,” Dr. Ritchlin said.
In addition to the AFFINITY trial, two clinical trials are underway in France. The first, CRI-RA, is evaluating the combination of baricitinib (Olumiant), a Janus kinase (JAK) inhibitor, and adalimumab. Although baricitinib is not a biologic, as a targeted synthetic DMARD, the therapy is more potent than conventional DMARDs, and the same potential safety concerns apply. However, use of a combination of tofacitinib (Xeljanz) and different biologics for RA patients has been reported; no serious side effects were reported over 11 months of therapy. The randomized, placebo-controlled trial began in July 2021 and will enroll 178 patients. The estimated study completion date is July 2025.
“Of note, baricitinib does not directly block signaling downstream of TNF, even if an indirect effect on TNF production is likely to occur,” the CRI-RA entry on clinicaltrials.gov reads. “Targeting multiple inflammatory cytokines in combination may lead to more effective treatment and enhanced clinical responses in patients with RA compared to the current second-line strategies.”
The second trial, SEQUENS-RA, is evaluating the use of TNF inhibitors followed by abatacept for patients with RA who test positive for anticitrullinated protein autoantibodies (ACPAs). In the past, the combination of a TNF inhibitor and abatacept did not lead to promising results, but in this trial, the drugs will be administered sequentially.
“Although abatacept has shown a very good tolerance profile that might be superior to other bDMARDs [biologic DMARDs], rheumatologists might be reluctant to use it as a first line bDMARD as there is a belief of a slower efficacy compared to other bDMARDs or JAK inhibitors,” according to the clinical trial’s description. “Investigators have hypothesized that first rapidly controlling the inflammation phase, using TNF inhibitors, followed by abatacept to induce an immunological remission, would optimize response and tolerance of ACPA-positive patients with RA.”
The randomized trial of 220 participants began in November 2022. The estimated completion date for the study is November 2025.
Finding the right patients
Though these studies have had some promising results, the difference in efficacy between biologic monotherapy and dual therapy has been mostly moderate, Dr. Mutlu and coauthors wrote. Identifying disease subtypes for which there might be a higher likelihood of response to dual biologic treatment, especially multidrug-resistant types, could improve efficacies in future trials, they argued. “The good effects of bDMARD combinations in resistant patients in fact point into this direction, though they were observed in uncontrolled studies,” the authors noted.
Insurance coverage remains a “huge challenge” for these dual therapies because of the higher expense, noted Dr. Ritchlin. Better targeting therapies could help convince these companies to pay for these therapies.
“I would say that if we were able to demonstrate a phenotype of a patient that would respond to biologics and not monotherapies, [then] many companies would be amenable to this kind of approach,” he said.
Dr. Ritchlin reports financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Pfizer, Eli Lilly, Novartis, and UCB. Dr. Feagan reports financial relationships with AbbVie, Amgen, Janssen, Pfizer, Takeda, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Is ChatGPT a friend or foe of medical publishing?
. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and much more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora. It is also not totally clear how information is stored and processed in these kinds of tools, and who has access to that information, he noted.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said. “I don’t think there’s a way out of that at this point.”
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
“We think that’s not appropriate, because coauthorship means that you are taking responsibility for the analysis and the generation of data that are included in a manuscript. A machine that is dictated by AI can’t take responsibility,” said Daniel Solomon, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, and the editor in chief of the ACR journal Arthritis & Rheumatology.
Issues with AI
One of the big concerns around using AI in writing is that it can generate text that seems plausible but is untrue or not supported by data. For example, Dr. Greene and colleague Milton Pividori, PhD, also of the University of Colorado, were writing a journal article about new software they developed that uses a large language model to revise scientific manuscripts.
“We used the same software to revise that article and at one point, it added a line that noted that the large language model had been fine-tuned on a data set of manuscripts from within the same field. This makes a lot of sense, and is absolutely something you could do, but was not something that we did,” Dr. Greene said. “Without a really careful review of the content, it becomes possible to invent things that were not actually done.”
In another case, ChatGPT falsely stated that a prominent law professor had been accused of sexual assault, citing a Washington Post article that did not exist.
“We live in a society where we are extremely concerned about fake news,” Dr. Pividori added, “and [these kinds of errors] could certainly exacerbate that in the scientific community, which is very concerning because science informs public policy.”
Another issue is the lack of transparency around how large language models like ChatGPT process and store data used to make queries.
“We have no idea how they are recording all the prompts and things that we input into ChatGPT and their systems,” Dr. Pividori said.
OpenAI recently addressed some privacy concerns by allowing users to turn off their chat history with the AI chatbot, so conversations cannot be used to train or improve the company’s models. But Dr. Greene noted that the terms of service “still remain pretty nebulous.”
Dr. Solomon is also concerned with researchers using these AI tools in authoring without knowing how they work. “The thing we are really concerned about is that fact that [LLMs] are a bit of a black box – people don’t really understand the methodologies,” he said.
A positive tool?
But despite these concerns, many think that these types of AI-assisted tools could have a positive impact on medical publishing, particularly for researchers for whom English is not their first language, noted Catherine Gao, MD, a pulmonary and critical care instructor at Northwestern University, Chicago. She recently led research comparing scientific abstracts written by ChatGPT and real abstracts and discovered that reviewers found it “surprisingly difficult” to differentiate the two.
“The majority of research is published in English,” she said in an email. “Responsible use of LLMs can potentially reduce the burden of writing for busy scientists and improve equity for those who are not native English speakers.”
Dr. Pividori agreed, adding that as a non-native English speaker, he spends much more time working on the structure and grammar of sentences when authoring a manuscript, compared with people who speak English as a first language. He noted that these tools can also be used to automate some of the more monotonous tasks that come along with writing manuscripts and allow researchers to focus on the more creative aspects.
In the future, “I want to focus more on the things that only a human can do and let these tools do all the rest of it,” he said.
New rules
But despite how individual researchers feel about LLMs, they agree that these AI tools are here to stay.
“I think that we should anticipate that they will become part of the medical research establishment over time, when we figure out how to use them appropriately,” Dr. Solomon said.
While the debate of how to best use AI in medical publications will continue, journal editors agree that all authors of a manuscript are solely responsible for content in articles that used AI-assisted technology.
“Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased,” the ICMJE guidelines state. “Authors should be able to assert that there is no plagiarism in their paper, including in text and images produced by the AI.” This includes appropriate attribution of all cited materials.
The committee also recommends that authors write in both the cover letter and submitted work how AI was used in the manuscript writing process. Recently updated guidelines from the World Association of Medical Editors recommend that all prompts used to generate new text or analytical work should be provided in submitted work. Dr. Greene also noted that if authors used an AI tool to revise their work, they can include a version of the manuscript untouched by LLMs.
It is similar to a preprint, he said, but rather than publishing a version of a paper prior to peer review, someone is showing a version of a manuscript before it was reviewed and revised by AI. “This type of practice could be a path that lets us benefit from these models,” he said, “without having the drawbacks that many are concerned about.”
Dr. Solomon has financial relationships with AbbVie, Amgen, Janssen, CorEvitas, and Moderna. Both Dr. Greene and Dr. Pividori are inventors in the U.S. Provisional Patent Application No. 63/486,706 that the University of Colorado has filed for the “Publishing Infrastructure For AI-Assisted Academic Authoring” invention with the U.S. Patent and Trademark Office. Dr. Greene and Dr. Pividori also received a grant from the Alfred P. Sloan Foundation to improve their AI-based manuscript revision tool. Dr. Gao reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and much more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora. It is also not totally clear how information is stored and processed in these kinds of tools, and who has access to that information, he noted.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said. “I don’t think there’s a way out of that at this point.”
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
“We think that’s not appropriate, because coauthorship means that you are taking responsibility for the analysis and the generation of data that are included in a manuscript. A machine that is dictated by AI can’t take responsibility,” said Daniel Solomon, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, and the editor in chief of the ACR journal Arthritis & Rheumatology.
Issues with AI
One of the big concerns around using AI in writing is that it can generate text that seems plausible but is untrue or not supported by data. For example, Dr. Greene and colleague Milton Pividori, PhD, also of the University of Colorado, were writing a journal article about new software they developed that uses a large language model to revise scientific manuscripts.
“We used the same software to revise that article and at one point, it added a line that noted that the large language model had been fine-tuned on a data set of manuscripts from within the same field. This makes a lot of sense, and is absolutely something you could do, but was not something that we did,” Dr. Greene said. “Without a really careful review of the content, it becomes possible to invent things that were not actually done.”
In another case, ChatGPT falsely stated that a prominent law professor had been accused of sexual assault, citing a Washington Post article that did not exist.
“We live in a society where we are extremely concerned about fake news,” Dr. Pividori added, “and [these kinds of errors] could certainly exacerbate that in the scientific community, which is very concerning because science informs public policy.”
Another issue is the lack of transparency around how large language models like ChatGPT process and store data used to make queries.
“We have no idea how they are recording all the prompts and things that we input into ChatGPT and their systems,” Dr. Pividori said.
OpenAI recently addressed some privacy concerns by allowing users to turn off their chat history with the AI chatbot, so conversations cannot be used to train or improve the company’s models. But Dr. Greene noted that the terms of service “still remain pretty nebulous.”
Dr. Solomon is also concerned with researchers using these AI tools in authoring without knowing how they work. “The thing we are really concerned about is that fact that [LLMs] are a bit of a black box – people don’t really understand the methodologies,” he said.
A positive tool?
But despite these concerns, many think that these types of AI-assisted tools could have a positive impact on medical publishing, particularly for researchers for whom English is not their first language, noted Catherine Gao, MD, a pulmonary and critical care instructor at Northwestern University, Chicago. She recently led research comparing scientific abstracts written by ChatGPT and real abstracts and discovered that reviewers found it “surprisingly difficult” to differentiate the two.
“The majority of research is published in English,” she said in an email. “Responsible use of LLMs can potentially reduce the burden of writing for busy scientists and improve equity for those who are not native English speakers.”
Dr. Pividori agreed, adding that as a non-native English speaker, he spends much more time working on the structure and grammar of sentences when authoring a manuscript, compared with people who speak English as a first language. He noted that these tools can also be used to automate some of the more monotonous tasks that come along with writing manuscripts and allow researchers to focus on the more creative aspects.
In the future, “I want to focus more on the things that only a human can do and let these tools do all the rest of it,” he said.
New rules
But despite how individual researchers feel about LLMs, they agree that these AI tools are here to stay.
“I think that we should anticipate that they will become part of the medical research establishment over time, when we figure out how to use them appropriately,” Dr. Solomon said.
While the debate of how to best use AI in medical publications will continue, journal editors agree that all authors of a manuscript are solely responsible for content in articles that used AI-assisted technology.
“Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased,” the ICMJE guidelines state. “Authors should be able to assert that there is no plagiarism in their paper, including in text and images produced by the AI.” This includes appropriate attribution of all cited materials.
The committee also recommends that authors write in both the cover letter and submitted work how AI was used in the manuscript writing process. Recently updated guidelines from the World Association of Medical Editors recommend that all prompts used to generate new text or analytical work should be provided in submitted work. Dr. Greene also noted that if authors used an AI tool to revise their work, they can include a version of the manuscript untouched by LLMs.
It is similar to a preprint, he said, but rather than publishing a version of a paper prior to peer review, someone is showing a version of a manuscript before it was reviewed and revised by AI. “This type of practice could be a path that lets us benefit from these models,” he said, “without having the drawbacks that many are concerned about.”
Dr. Solomon has financial relationships with AbbVie, Amgen, Janssen, CorEvitas, and Moderna. Both Dr. Greene and Dr. Pividori are inventors in the U.S. Provisional Patent Application No. 63/486,706 that the University of Colorado has filed for the “Publishing Infrastructure For AI-Assisted Academic Authoring” invention with the U.S. Patent and Trademark Office. Dr. Greene and Dr. Pividori also received a grant from the Alfred P. Sloan Foundation to improve their AI-based manuscript revision tool. Dr. Gao reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and much more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora. It is also not totally clear how information is stored and processed in these kinds of tools, and who has access to that information, he noted.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said. “I don’t think there’s a way out of that at this point.”
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
“We think that’s not appropriate, because coauthorship means that you are taking responsibility for the analysis and the generation of data that are included in a manuscript. A machine that is dictated by AI can’t take responsibility,” said Daniel Solomon, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, and the editor in chief of the ACR journal Arthritis & Rheumatology.
Issues with AI
One of the big concerns around using AI in writing is that it can generate text that seems plausible but is untrue or not supported by data. For example, Dr. Greene and colleague Milton Pividori, PhD, also of the University of Colorado, were writing a journal article about new software they developed that uses a large language model to revise scientific manuscripts.
“We used the same software to revise that article and at one point, it added a line that noted that the large language model had been fine-tuned on a data set of manuscripts from within the same field. This makes a lot of sense, and is absolutely something you could do, but was not something that we did,” Dr. Greene said. “Without a really careful review of the content, it becomes possible to invent things that were not actually done.”
In another case, ChatGPT falsely stated that a prominent law professor had been accused of sexual assault, citing a Washington Post article that did not exist.
“We live in a society where we are extremely concerned about fake news,” Dr. Pividori added, “and [these kinds of errors] could certainly exacerbate that in the scientific community, which is very concerning because science informs public policy.”
Another issue is the lack of transparency around how large language models like ChatGPT process and store data used to make queries.
“We have no idea how they are recording all the prompts and things that we input into ChatGPT and their systems,” Dr. Pividori said.
OpenAI recently addressed some privacy concerns by allowing users to turn off their chat history with the AI chatbot, so conversations cannot be used to train or improve the company’s models. But Dr. Greene noted that the terms of service “still remain pretty nebulous.”
Dr. Solomon is also concerned with researchers using these AI tools in authoring without knowing how they work. “The thing we are really concerned about is that fact that [LLMs] are a bit of a black box – people don’t really understand the methodologies,” he said.
A positive tool?
But despite these concerns, many think that these types of AI-assisted tools could have a positive impact on medical publishing, particularly for researchers for whom English is not their first language, noted Catherine Gao, MD, a pulmonary and critical care instructor at Northwestern University, Chicago. She recently led research comparing scientific abstracts written by ChatGPT and real abstracts and discovered that reviewers found it “surprisingly difficult” to differentiate the two.
“The majority of research is published in English,” she said in an email. “Responsible use of LLMs can potentially reduce the burden of writing for busy scientists and improve equity for those who are not native English speakers.”
Dr. Pividori agreed, adding that as a non-native English speaker, he spends much more time working on the structure and grammar of sentences when authoring a manuscript, compared with people who speak English as a first language. He noted that these tools can also be used to automate some of the more monotonous tasks that come along with writing manuscripts and allow researchers to focus on the more creative aspects.
In the future, “I want to focus more on the things that only a human can do and let these tools do all the rest of it,” he said.
New rules
But despite how individual researchers feel about LLMs, they agree that these AI tools are here to stay.
“I think that we should anticipate that they will become part of the medical research establishment over time, when we figure out how to use them appropriately,” Dr. Solomon said.
While the debate of how to best use AI in medical publications will continue, journal editors agree that all authors of a manuscript are solely responsible for content in articles that used AI-assisted technology.
“Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased,” the ICMJE guidelines state. “Authors should be able to assert that there is no plagiarism in their paper, including in text and images produced by the AI.” This includes appropriate attribution of all cited materials.
The committee also recommends that authors write in both the cover letter and submitted work how AI was used in the manuscript writing process. Recently updated guidelines from the World Association of Medical Editors recommend that all prompts used to generate new text or analytical work should be provided in submitted work. Dr. Greene also noted that if authors used an AI tool to revise their work, they can include a version of the manuscript untouched by LLMs.
It is similar to a preprint, he said, but rather than publishing a version of a paper prior to peer review, someone is showing a version of a manuscript before it was reviewed and revised by AI. “This type of practice could be a path that lets us benefit from these models,” he said, “without having the drawbacks that many are concerned about.”
Dr. Solomon has financial relationships with AbbVie, Amgen, Janssen, CorEvitas, and Moderna. Both Dr. Greene and Dr. Pividori are inventors in the U.S. Provisional Patent Application No. 63/486,706 that the University of Colorado has filed for the “Publishing Infrastructure For AI-Assisted Academic Authoring” invention with the U.S. Patent and Trademark Office. Dr. Greene and Dr. Pividori also received a grant from the Alfred P. Sloan Foundation to improve their AI-based manuscript revision tool. Dr. Gao reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.