User login
New trial data show hair growth in more alopecia areata patients
BOSTON – according to updated results from two phase 3 trials presented at the annual meeting of the American Academy of Dermatology.
The results indicate improved response rates and hair growth among trial participants, said Brett King, MD, PhD, an associate professor of dermatology at Yale University, New Haven, Conn. He is the lead author of the analyses and presented the research.
Dr. King presented 36-week results from the clinical trials at the 2021 annual meeting of the European Academy of Dermatology and Venereology. The same results were also published March 26, 2022, in the New England Journal of Medicine.
“Every bit of data we’ve had is hugely important,” Dr. King said in an interview. “Every time we add 16 weeks of data across hundreds of patients, we are making a huge step forward toward the goal of [Food and Drug Administration approval for a medication for alopecia areata.”
All patients enrolled in the two trials, called BRAVE-AA1 and BRAVE-AA2, had severe alopecia areata, defined as a Severity of Alopecia Tool (SALT) score of at least 50, meaning 50% or less scalp coverage. The score ranges from 0 (no hair loss) to 100 (complete hair loss). The primary endpoint was a SALT score of 20 or less (80% scalp hair coverage).
The researchers pooled data from both clinical trials, with a combined enrollment of 1,200, for the 52-week results presented at the meeting. The placebo group stopped at 36 weeks, and these patients were randomly reassigned to either the 4-mg or 2-mg once-daily baricitinib treatment groups.
At baseline, patients enrolled in the trial had a mean SALT score of 85.5. After 52 weeks, 39.0% of patients who received 4 mg of baricitinib had at least 80% scalp coverage. Of this group, nearly three out of four (74.1%) had at least 90% scalp coverage, or a SALT score of 10 or less.
In patients who received 2 mg of baricitinib, 22.6% had a SALT score of 20 or less 20 (at least 80% scalp hair coverage) at 52 weeks, and two-thirds of that group (67.5%) had at least 90% scalp hair coverage at 52 weeks.
Comparatively, at 36 weeks, 35.2% of participants in BRAVE-AA1 and 32.5% of participants in BRAVE-AA2 receiving 4 mg of baricitinib had at least 80% scalp coverage. In the group taking the lower dose, 21.7% and 17.3% of patients in the BRAVE-AA1 and BRAVE-AA2 trials, respectively, had achieved at least 80% scalp coverage at 36 weeks. (These percentages differ slightly from the NEJM article because of a different analysis of missing data, Dr. King said. For comparison of both 36- and 52-week results, the percentages from the EADV are used above.)
The results indicate that 5% more patients reached the primary endpoint in the additional 16 weeks of the trial, Dr. King said.
Alopecia areata is an autoimmune condition where immune cells attack hair follicles, causing the hair to fall out, and is associated with emotional and psychological distress. Any hair follicle can be attacked, but they are rarely destroyed, so hair can regrow.
"Many underestimate the impact of this autoimmune hair loss condition," Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, told this news organization. He was not involved with the trial. "The burden of the disease, which certainly is an emotional but also a physical one, definitely needs to be addressed with indicated FDA-approved drugs," he noted, which is the goal of these trials.
The BRAVE-AA1 and BRAVE-AA2 trials focused on scalp hair regrowth.
Eyebrow and eyelash growth, secondary outcomes, also improved between 36 and 52 weeks in both groups, calculated using the proportion of participants who had achieved full regrowth or regrowth with minimal gaps. At 36 weeks, about 31%-35% of patients who received 4 mg of baricitinib regrew eyebrow and eyelash hair. By 52 weeks, more than two out of five patients regrew eyebrow (44.1%) and eyelash (45.3%) hair.
“It’s a fantastic achievement and a major step forward in alopecia areata, especially for patients with the most severe and refractory cases,” said Arash Mostaghimi, MD, MPH, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. Dr. Mostaghimi is on the advisory board for Eli Lilly, which manufactures baricitinib, and Brigham and Women’s was one of the clinical sites of the trial.
While dermatologists have been aware of how JAK inhibitors can affect hair regrowth in alopecia patients, they have been using these drugs off label, Dr. Friedman said. Therefore, these drugs are expensive and more difficult to access. These trials provide "data that proves the efficacy and safety of [baricitinib] under the umbrella of the FDA portal," he added, which will hopefully lead to an approved indication for alopecia areata, so it can be more accessible to patients.
Adverse events at 52 weeks were consistent with data from 36 weeks, which found that none of these adverse events occurred in more than 10% of participants. The most common adverse events were headache, acne, and increases in muscle-related blood markers. The most common infections reported were pneumonia, herpes zoster, and urinary tract infection.
In February 2022, the FDA granted priority review for baricitinib for the treatment of severe alopecia areata. Lilly expects a regulatory decision by the end of 2022, they said in a press release.
Lilly provided funding for the BRAVE-AA1 and BRAVE-AA2 trials. Dr. King reported financial relationships with Aclaris, Arena Pharmaceuticals, Bristol-Myers Squibb, Concert Pharmaceutics, Dermavant, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Viela Bio. Dr. Mostaghimi has reported serving on an advisory board for Lilly. Dr. Friedman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*This article was updated on 3/28/2022 to include Dr. Friedman's comments, and on 3/31/2022 to correct the statement regarding adverse events reported in the study
BOSTON – according to updated results from two phase 3 trials presented at the annual meeting of the American Academy of Dermatology.
The results indicate improved response rates and hair growth among trial participants, said Brett King, MD, PhD, an associate professor of dermatology at Yale University, New Haven, Conn. He is the lead author of the analyses and presented the research.
Dr. King presented 36-week results from the clinical trials at the 2021 annual meeting of the European Academy of Dermatology and Venereology. The same results were also published March 26, 2022, in the New England Journal of Medicine.
“Every bit of data we’ve had is hugely important,” Dr. King said in an interview. “Every time we add 16 weeks of data across hundreds of patients, we are making a huge step forward toward the goal of [Food and Drug Administration approval for a medication for alopecia areata.”
All patients enrolled in the two trials, called BRAVE-AA1 and BRAVE-AA2, had severe alopecia areata, defined as a Severity of Alopecia Tool (SALT) score of at least 50, meaning 50% or less scalp coverage. The score ranges from 0 (no hair loss) to 100 (complete hair loss). The primary endpoint was a SALT score of 20 or less (80% scalp hair coverage).
The researchers pooled data from both clinical trials, with a combined enrollment of 1,200, for the 52-week results presented at the meeting. The placebo group stopped at 36 weeks, and these patients were randomly reassigned to either the 4-mg or 2-mg once-daily baricitinib treatment groups.
At baseline, patients enrolled in the trial had a mean SALT score of 85.5. After 52 weeks, 39.0% of patients who received 4 mg of baricitinib had at least 80% scalp coverage. Of this group, nearly three out of four (74.1%) had at least 90% scalp coverage, or a SALT score of 10 or less.
In patients who received 2 mg of baricitinib, 22.6% had a SALT score of 20 or less 20 (at least 80% scalp hair coverage) at 52 weeks, and two-thirds of that group (67.5%) had at least 90% scalp hair coverage at 52 weeks.
Comparatively, at 36 weeks, 35.2% of participants in BRAVE-AA1 and 32.5% of participants in BRAVE-AA2 receiving 4 mg of baricitinib had at least 80% scalp coverage. In the group taking the lower dose, 21.7% and 17.3% of patients in the BRAVE-AA1 and BRAVE-AA2 trials, respectively, had achieved at least 80% scalp coverage at 36 weeks. (These percentages differ slightly from the NEJM article because of a different analysis of missing data, Dr. King said. For comparison of both 36- and 52-week results, the percentages from the EADV are used above.)
The results indicate that 5% more patients reached the primary endpoint in the additional 16 weeks of the trial, Dr. King said.
Alopecia areata is an autoimmune condition where immune cells attack hair follicles, causing the hair to fall out, and is associated with emotional and psychological distress. Any hair follicle can be attacked, but they are rarely destroyed, so hair can regrow.
"Many underestimate the impact of this autoimmune hair loss condition," Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, told this news organization. He was not involved with the trial. "The burden of the disease, which certainly is an emotional but also a physical one, definitely needs to be addressed with indicated FDA-approved drugs," he noted, which is the goal of these trials.
The BRAVE-AA1 and BRAVE-AA2 trials focused on scalp hair regrowth.
Eyebrow and eyelash growth, secondary outcomes, also improved between 36 and 52 weeks in both groups, calculated using the proportion of participants who had achieved full regrowth or regrowth with minimal gaps. At 36 weeks, about 31%-35% of patients who received 4 mg of baricitinib regrew eyebrow and eyelash hair. By 52 weeks, more than two out of five patients regrew eyebrow (44.1%) and eyelash (45.3%) hair.
“It’s a fantastic achievement and a major step forward in alopecia areata, especially for patients with the most severe and refractory cases,” said Arash Mostaghimi, MD, MPH, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. Dr. Mostaghimi is on the advisory board for Eli Lilly, which manufactures baricitinib, and Brigham and Women’s was one of the clinical sites of the trial.
While dermatologists have been aware of how JAK inhibitors can affect hair regrowth in alopecia patients, they have been using these drugs off label, Dr. Friedman said. Therefore, these drugs are expensive and more difficult to access. These trials provide "data that proves the efficacy and safety of [baricitinib] under the umbrella of the FDA portal," he added, which will hopefully lead to an approved indication for alopecia areata, so it can be more accessible to patients.
Adverse events at 52 weeks were consistent with data from 36 weeks, which found that none of these adverse events occurred in more than 10% of participants. The most common adverse events were headache, acne, and increases in muscle-related blood markers. The most common infections reported were pneumonia, herpes zoster, and urinary tract infection.
In February 2022, the FDA granted priority review for baricitinib for the treatment of severe alopecia areata. Lilly expects a regulatory decision by the end of 2022, they said in a press release.
Lilly provided funding for the BRAVE-AA1 and BRAVE-AA2 trials. Dr. King reported financial relationships with Aclaris, Arena Pharmaceuticals, Bristol-Myers Squibb, Concert Pharmaceutics, Dermavant, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Viela Bio. Dr. Mostaghimi has reported serving on an advisory board for Lilly. Dr. Friedman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*This article was updated on 3/28/2022 to include Dr. Friedman's comments, and on 3/31/2022 to correct the statement regarding adverse events reported in the study
BOSTON – according to updated results from two phase 3 trials presented at the annual meeting of the American Academy of Dermatology.
The results indicate improved response rates and hair growth among trial participants, said Brett King, MD, PhD, an associate professor of dermatology at Yale University, New Haven, Conn. He is the lead author of the analyses and presented the research.
Dr. King presented 36-week results from the clinical trials at the 2021 annual meeting of the European Academy of Dermatology and Venereology. The same results were also published March 26, 2022, in the New England Journal of Medicine.
“Every bit of data we’ve had is hugely important,” Dr. King said in an interview. “Every time we add 16 weeks of data across hundreds of patients, we are making a huge step forward toward the goal of [Food and Drug Administration approval for a medication for alopecia areata.”
All patients enrolled in the two trials, called BRAVE-AA1 and BRAVE-AA2, had severe alopecia areata, defined as a Severity of Alopecia Tool (SALT) score of at least 50, meaning 50% or less scalp coverage. The score ranges from 0 (no hair loss) to 100 (complete hair loss). The primary endpoint was a SALT score of 20 or less (80% scalp hair coverage).
The researchers pooled data from both clinical trials, with a combined enrollment of 1,200, for the 52-week results presented at the meeting. The placebo group stopped at 36 weeks, and these patients were randomly reassigned to either the 4-mg or 2-mg once-daily baricitinib treatment groups.
At baseline, patients enrolled in the trial had a mean SALT score of 85.5. After 52 weeks, 39.0% of patients who received 4 mg of baricitinib had at least 80% scalp coverage. Of this group, nearly three out of four (74.1%) had at least 90% scalp coverage, or a SALT score of 10 or less.
In patients who received 2 mg of baricitinib, 22.6% had a SALT score of 20 or less 20 (at least 80% scalp hair coverage) at 52 weeks, and two-thirds of that group (67.5%) had at least 90% scalp hair coverage at 52 weeks.
Comparatively, at 36 weeks, 35.2% of participants in BRAVE-AA1 and 32.5% of participants in BRAVE-AA2 receiving 4 mg of baricitinib had at least 80% scalp coverage. In the group taking the lower dose, 21.7% and 17.3% of patients in the BRAVE-AA1 and BRAVE-AA2 trials, respectively, had achieved at least 80% scalp coverage at 36 weeks. (These percentages differ slightly from the NEJM article because of a different analysis of missing data, Dr. King said. For comparison of both 36- and 52-week results, the percentages from the EADV are used above.)
The results indicate that 5% more patients reached the primary endpoint in the additional 16 weeks of the trial, Dr. King said.
Alopecia areata is an autoimmune condition where immune cells attack hair follicles, causing the hair to fall out, and is associated with emotional and psychological distress. Any hair follicle can be attacked, but they are rarely destroyed, so hair can regrow.
"Many underestimate the impact of this autoimmune hair loss condition," Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, told this news organization. He was not involved with the trial. "The burden of the disease, which certainly is an emotional but also a physical one, definitely needs to be addressed with indicated FDA-approved drugs," he noted, which is the goal of these trials.
The BRAVE-AA1 and BRAVE-AA2 trials focused on scalp hair regrowth.
Eyebrow and eyelash growth, secondary outcomes, also improved between 36 and 52 weeks in both groups, calculated using the proportion of participants who had achieved full regrowth or regrowth with minimal gaps. At 36 weeks, about 31%-35% of patients who received 4 mg of baricitinib regrew eyebrow and eyelash hair. By 52 weeks, more than two out of five patients regrew eyebrow (44.1%) and eyelash (45.3%) hair.
“It’s a fantastic achievement and a major step forward in alopecia areata, especially for patients with the most severe and refractory cases,” said Arash Mostaghimi, MD, MPH, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. Dr. Mostaghimi is on the advisory board for Eli Lilly, which manufactures baricitinib, and Brigham and Women’s was one of the clinical sites of the trial.
While dermatologists have been aware of how JAK inhibitors can affect hair regrowth in alopecia patients, they have been using these drugs off label, Dr. Friedman said. Therefore, these drugs are expensive and more difficult to access. These trials provide "data that proves the efficacy and safety of [baricitinib] under the umbrella of the FDA portal," he added, which will hopefully lead to an approved indication for alopecia areata, so it can be more accessible to patients.
Adverse events at 52 weeks were consistent with data from 36 weeks, which found that none of these adverse events occurred in more than 10% of participants. The most common adverse events were headache, acne, and increases in muscle-related blood markers. The most common infections reported were pneumonia, herpes zoster, and urinary tract infection.
In February 2022, the FDA granted priority review for baricitinib for the treatment of severe alopecia areata. Lilly expects a regulatory decision by the end of 2022, they said in a press release.
Lilly provided funding for the BRAVE-AA1 and BRAVE-AA2 trials. Dr. King reported financial relationships with Aclaris, Arena Pharmaceuticals, Bristol-Myers Squibb, Concert Pharmaceutics, Dermavant, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Viela Bio. Dr. Mostaghimi has reported serving on an advisory board for Lilly. Dr. Friedman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*This article was updated on 3/28/2022 to include Dr. Friedman's comments, and on 3/31/2022 to correct the statement regarding adverse events reported in the study
AT AAD 2022
Aluminum named allergen of the year
BOSTON – The . Aluminum salts, which are the major cause of allergic reactions, are “ubiquitous,” Donald Belsito, MD, professor of dermatology at Columbia University, New York, said at the annual meeting of the American Contact Dermatitis Society.
These salts can be found in sunscreen, cosmetics, dental restorations, and food, to name a few, though the most commonly identified reactions are from aluminum hydroxide, which can be found in some vaccines or preparations for allergen-specific immunotherapy. “It’s the aluminum hydroxide that seems to be more allergenic than other aluminum salts,” Dr. Belsito said in an interview.
“It’s not a dangerous allergy; It’s not a threat,” he said, “but it’s something that dermatologists need to be aware of.”
These reactions normally present as itchy nodules that can last for months and even years, like some reactions from patch testing. “We’re not talking about a vaccine allergy in such a way where people are getting anaphylaxis,” JiaDe Yu, MD, a pediatric dermatologist specializing in allergic contact dermatitis at Massachusetts General Hospital, Boston, said in an interview. “An itchy rash is what we tend to see.”
There have also been occasional reports of atopic dermatitis from aluminum in antiperspirants, astringents, as well as from the metallic aluminum.
Dr. Yu noted that aluminum allergies are not thought to be very common, but the overall prevalence is not known. Studies do suggest, however, that the allergy may be more prevalent in children. In one recent study in Sweden, 5% of children and 0.9% of adults who underwent patch testing had an aluminum contact allergy.
Recommendations for testing
Aluminum is not included in baseline patch testing in the United States, though a recent report about the allergen in the journal Dermatitis argued for its inclusion for pediatric patch testing. Both Dr. Belsito and Dr. Yu agreed that the best approach is to do targeted testing. “If there is a suspicion for it, absolutely test for it,” Dr. Yu said, but if a patient comes in with something like eyelid dermatitis or a rash after a hair care appointment, an aluminum allergy is not very likely.
Because aluminum is also present in Finn Chambers for patch testing, Dr. Belsito advised using plastic chambers in people suspected of having an aluminum allergy. He now uses only plastic chambers in children, he said, as some patients have had reactions to the Finn Chambers even if they have no history of reactions to vaccines or other aluminum-containing products.
While aluminum chloride hexahydrate (ACH) 2% in petrolatum is the commercially available preparation in patch testing, a preparation with ACH 10% is more sensitive, Dr. Belsito said. If a physician strongly suspects an aluminum allergy in a patient but the test with the ACH 2% is negative, he or she should then try a 10% solution, he noted, adding that 7-day readings are also necessary to maximize accuracy.
Vaccine safety
One of the concerns about naming aluminum as the allergen of the year is the potential to cause anxiety around vaccines. “We want to make sure that we’re not giving more fuel to people who have an excuse not to get a vaccine,” Dr. Yu said. “We certainly want to reinforce that fact that it is safe.” Dr. Belsito noted that COVID-19 vaccines do not contain aluminum.
Even on the rare chance that a patient does have a reaction to an aluminum-containing vaccine, these subcutaneous nodules resolve over time, Dr. Belsito said. In his own clinical experience, “99.99% of the time they resolve and there is no residual.” He did add that overreacting to the rash by prescribing injectable steroids can lead to steroid atrophy. In these cases, a topical steroid may be more appropriate.
All unexpected or clinically significant vaccine reactions should be reported to the Vaccine Adverse Event Reporting System, cosponsored by the Centers for Disease Control and Prevention and the Food and Drug Administration. The Clinical Immunization Project Safety Assessment Project, from the CDC, also can provide expertise and advice on aluminum-free alternatives for some vaccines.
Dr. Belsito and Dr. Yu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – The . Aluminum salts, which are the major cause of allergic reactions, are “ubiquitous,” Donald Belsito, MD, professor of dermatology at Columbia University, New York, said at the annual meeting of the American Contact Dermatitis Society.
These salts can be found in sunscreen, cosmetics, dental restorations, and food, to name a few, though the most commonly identified reactions are from aluminum hydroxide, which can be found in some vaccines or preparations for allergen-specific immunotherapy. “It’s the aluminum hydroxide that seems to be more allergenic than other aluminum salts,” Dr. Belsito said in an interview.
“It’s not a dangerous allergy; It’s not a threat,” he said, “but it’s something that dermatologists need to be aware of.”
These reactions normally present as itchy nodules that can last for months and even years, like some reactions from patch testing. “We’re not talking about a vaccine allergy in such a way where people are getting anaphylaxis,” JiaDe Yu, MD, a pediatric dermatologist specializing in allergic contact dermatitis at Massachusetts General Hospital, Boston, said in an interview. “An itchy rash is what we tend to see.”
There have also been occasional reports of atopic dermatitis from aluminum in antiperspirants, astringents, as well as from the metallic aluminum.
Dr. Yu noted that aluminum allergies are not thought to be very common, but the overall prevalence is not known. Studies do suggest, however, that the allergy may be more prevalent in children. In one recent study in Sweden, 5% of children and 0.9% of adults who underwent patch testing had an aluminum contact allergy.
Recommendations for testing
Aluminum is not included in baseline patch testing in the United States, though a recent report about the allergen in the journal Dermatitis argued for its inclusion for pediatric patch testing. Both Dr. Belsito and Dr. Yu agreed that the best approach is to do targeted testing. “If there is a suspicion for it, absolutely test for it,” Dr. Yu said, but if a patient comes in with something like eyelid dermatitis or a rash after a hair care appointment, an aluminum allergy is not very likely.
Because aluminum is also present in Finn Chambers for patch testing, Dr. Belsito advised using plastic chambers in people suspected of having an aluminum allergy. He now uses only plastic chambers in children, he said, as some patients have had reactions to the Finn Chambers even if they have no history of reactions to vaccines or other aluminum-containing products.
While aluminum chloride hexahydrate (ACH) 2% in petrolatum is the commercially available preparation in patch testing, a preparation with ACH 10% is more sensitive, Dr. Belsito said. If a physician strongly suspects an aluminum allergy in a patient but the test with the ACH 2% is negative, he or she should then try a 10% solution, he noted, adding that 7-day readings are also necessary to maximize accuracy.
Vaccine safety
One of the concerns about naming aluminum as the allergen of the year is the potential to cause anxiety around vaccines. “We want to make sure that we’re not giving more fuel to people who have an excuse not to get a vaccine,” Dr. Yu said. “We certainly want to reinforce that fact that it is safe.” Dr. Belsito noted that COVID-19 vaccines do not contain aluminum.
Even on the rare chance that a patient does have a reaction to an aluminum-containing vaccine, these subcutaneous nodules resolve over time, Dr. Belsito said. In his own clinical experience, “99.99% of the time they resolve and there is no residual.” He did add that overreacting to the rash by prescribing injectable steroids can lead to steroid atrophy. In these cases, a topical steroid may be more appropriate.
All unexpected or clinically significant vaccine reactions should be reported to the Vaccine Adverse Event Reporting System, cosponsored by the Centers for Disease Control and Prevention and the Food and Drug Administration. The Clinical Immunization Project Safety Assessment Project, from the CDC, also can provide expertise and advice on aluminum-free alternatives for some vaccines.
Dr. Belsito and Dr. Yu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – The . Aluminum salts, which are the major cause of allergic reactions, are “ubiquitous,” Donald Belsito, MD, professor of dermatology at Columbia University, New York, said at the annual meeting of the American Contact Dermatitis Society.
These salts can be found in sunscreen, cosmetics, dental restorations, and food, to name a few, though the most commonly identified reactions are from aluminum hydroxide, which can be found in some vaccines or preparations for allergen-specific immunotherapy. “It’s the aluminum hydroxide that seems to be more allergenic than other aluminum salts,” Dr. Belsito said in an interview.
“It’s not a dangerous allergy; It’s not a threat,” he said, “but it’s something that dermatologists need to be aware of.”
These reactions normally present as itchy nodules that can last for months and even years, like some reactions from patch testing. “We’re not talking about a vaccine allergy in such a way where people are getting anaphylaxis,” JiaDe Yu, MD, a pediatric dermatologist specializing in allergic contact dermatitis at Massachusetts General Hospital, Boston, said in an interview. “An itchy rash is what we tend to see.”
There have also been occasional reports of atopic dermatitis from aluminum in antiperspirants, astringents, as well as from the metallic aluminum.
Dr. Yu noted that aluminum allergies are not thought to be very common, but the overall prevalence is not known. Studies do suggest, however, that the allergy may be more prevalent in children. In one recent study in Sweden, 5% of children and 0.9% of adults who underwent patch testing had an aluminum contact allergy.
Recommendations for testing
Aluminum is not included in baseline patch testing in the United States, though a recent report about the allergen in the journal Dermatitis argued for its inclusion for pediatric patch testing. Both Dr. Belsito and Dr. Yu agreed that the best approach is to do targeted testing. “If there is a suspicion for it, absolutely test for it,” Dr. Yu said, but if a patient comes in with something like eyelid dermatitis or a rash after a hair care appointment, an aluminum allergy is not very likely.
Because aluminum is also present in Finn Chambers for patch testing, Dr. Belsito advised using plastic chambers in people suspected of having an aluminum allergy. He now uses only plastic chambers in children, he said, as some patients have had reactions to the Finn Chambers even if they have no history of reactions to vaccines or other aluminum-containing products.
While aluminum chloride hexahydrate (ACH) 2% in petrolatum is the commercially available preparation in patch testing, a preparation with ACH 10% is more sensitive, Dr. Belsito said. If a physician strongly suspects an aluminum allergy in a patient but the test with the ACH 2% is negative, he or she should then try a 10% solution, he noted, adding that 7-day readings are also necessary to maximize accuracy.
Vaccine safety
One of the concerns about naming aluminum as the allergen of the year is the potential to cause anxiety around vaccines. “We want to make sure that we’re not giving more fuel to people who have an excuse not to get a vaccine,” Dr. Yu said. “We certainly want to reinforce that fact that it is safe.” Dr. Belsito noted that COVID-19 vaccines do not contain aluminum.
Even on the rare chance that a patient does have a reaction to an aluminum-containing vaccine, these subcutaneous nodules resolve over time, Dr. Belsito said. In his own clinical experience, “99.99% of the time they resolve and there is no residual.” He did add that overreacting to the rash by prescribing injectable steroids can lead to steroid atrophy. In these cases, a topical steroid may be more appropriate.
All unexpected or clinically significant vaccine reactions should be reported to the Vaccine Adverse Event Reporting System, cosponsored by the Centers for Disease Control and Prevention and the Food and Drug Administration. The Clinical Immunization Project Safety Assessment Project, from the CDC, also can provide expertise and advice on aluminum-free alternatives for some vaccines.
Dr. Belsito and Dr. Yu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACDS 2022
More questions than answers when managing HIV and menopause
Note: In this article, “women” refers to ciswomen – those who identify as women and were assigned female sex at birth. Menopause also affects transmen and nonbinary people, but published research on the menopause experience has included only ciswomen participants.
Gina Brown was boarding an early morning flight in 2016 when suddenly she started to overheat. “As soon as I stepped on the plane, I immediately was drenched in sweat,” she said. Not knowing what to do, she stood still until a fellow female passenger noticed her alarm and asked a flight attendant to grab her a cup of ice. “Is this the first time this has happened to you?” the woman asked, and Ms. Brown nodded. “It’s called a hot flash,” the woman continued, “and you’re going to be okay.”
As soon as Ms. Brown returned from her trip, she visited her doctor for blood work and learned that her hormone levels were decreasing. “I knew something was going on, but [my provider and I] didn’t have a conversation about menopause,” she said. Ms. Brown, who is 56 years old, has been living with HIV for nearly 28 years, and is part of a growing group of women with HIV now entering menopause.
In 1996, a person diagnosed with HIV at 20 years of age could expect to live only to age 39. Because of antiretroviral therapy (ART), an HIV diagnosis is not nearly so dire. Now, someone with HIV who adheres to the ART regimen is estimated to have a lifespan close to that of the general population.
For women with HIV, this means going through menopause. Though this transition can be challenging for any woman, experiencing menopause with HIV adds another level of complication. On top of adhering to daily ART regimens, the woman must also deal with the hormonal changes of menopause and the symptoms that come with it. And the limited research in this area suggests that women with HIV and their clinicians may not be prepared.
“Those of us long-term survivors who have been around for a while never expected to be here, and I don’t think providers or the health care system expected us to be here,” said Vickie A. Lynn, PhD, 56, who has been living with HIV for 37 years and received an AIDS diagnosis in 1991. Her work focuses on health care interventions for people with HIV. “So now that we’re here, I don’t know that we have enough information or research to inform some of our treatment options.” Instead, these women are met with a series of unknowns due to limited studies and conflicting findings.
Earlier menopause?
The onset of menopause can be difficult to determine in women living with HIV, said Sara Looby, PhD, ANP-BC, a researcher at Massachusetts General Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Her research focuses on metabolic disorders, including bone loss, cardiovascular disease risk, and menopause in women living with HIV. This population is at an increased risk for amenorrhea, due to both behavioral and clinical factors, and sometimes this amenorrhea is mistakenly assumed to be menopause, she explained. A history of smoking, low weight, methadone use, or use of other psychotropic medications are common in women with HIV and can lead to missed periods. Some factors specific to HIV – including a low CD4 count and a history of an AIDS diagnosis – have also been linked to amenorrhea.
This is likely why research studies on the age of onset of menopause with women with HIV can reach conflicting conclusions. Some studies suggest that women with HIV tend to go through menopause 3-5 years earlier than women without HIV. Other studies suggest no difference in the age of onset in menopause between women living with and without HIV. But how menopause status has been accessed can vary from study to study, Dr. Looby said. Future research needs to consider participants’ complete menstrual and reproductive history, as well as relevant medical, social, and behavioral factors, she added, so that the findings are reliably capturing the age of onset of menopause rather than amenorrhea from other causes.
If menopause does occur earlier in women with HIV, there could be additional health implications. Estrogen regulates bone mass, and some research suggests the hormone may be cardioprotective. Estrogen is also thought to increase production of the neurotransmitter serotonin, which could affect mood and cognition. Women with HIV are already at higher risk for bone loss, cardiovascular disease, and depressed mood compared to women without HIV, Dr. Looby said, and as estrogen levels fall during menopause, these conditions may be deleteriously affected.
“If it is determined that women with HIV experience menopause at an earlier age, maybe early to mid-40s instead of 51 and older, they may be at increased risk for cardiovascular and bone conditions as well as mood symptoms associated with estrogen loss at an earlier age than women without HIV, which could be highly detrimental to their physical and mental health,” Dr. Looby said.
More frequent and severe menopausal symptoms?
Women with HIV may not only go through menopause earlier than women without HIV, but their symptoms may also be more frequent and more severe. In a 2017 study of both HIV-positive and HIV-negative Nigerian women, participants with HIV had more menopause symptoms overall and were three times as likely to report severe symptoms compared to women without HIV. A 2005 study conducted in New York found HIV-positive women were 24% more likely to report menopause symptoms compared to HIV-negative women in the study.
Looby’s own research has also found a similar pattern. In a study comparing 33 women with HIV to 33 women without HIV – all were close to menopause and matched for age, race, body mass index, and menstrual patterns – women with HIV reported more severe hot flashes and more days with hot flashes. These women also reported that their hot flashes interfered to a much greater degree with daily activities and quality of life compared to participants without HIV.
But studies of women with HIV who are entering menopause are rare, and most include only small numbers of women. As a result, many women with HIV do not know what to expect entering menopause. “I always say, I wish somebody would do some real research on HIV and menopause, because I want to know if it is worse for us or if it is the same,” said Ms. Brown, who works as the director of strategic partnership and community engagement at the Southern Aids Coalition in Powder Springs, Ga. “I would think it’s worse for me.”
More frequent and severe symptoms can have downstream effects, with some evidence suggesting that women with HIV who experience severe menopause symptoms are less likely to stick to their ART regimen. “There’s a clear picture emerging that menopausal symptoms in this group really matter,” said Shema Tariq, PhD, FRCP, an HIV physician-scientist at the University College London Institute for Global Health in England. “They really impact women’s well-being, as well as impacting their ability to look after their long-term condition.”
Providers wary of treating menopause symptoms in women with HIV
The little research we do have about women with HIV experiencing menopause suggests that this population could greatly benefit from treatment prescribed in women without HIV for menopause symptoms and conditions, including hormone replacement therapy (HRT). Women with HIV regularly experience night sweats and hot flashes during the menopause transition and may have more severe symptoms than women not living with the virus. If women with HIV also frequently enter early menopause (entering menopause before the age of 45), then this group meets two indications for hormone replacement therapy.
Despite the potential benefits of HRT in this population, some studies suggest this intervention is underutilized. In Dr. Tariq’s Positive Transitions through Menopause (PRIME) study, which explores how menopause affects more than 800 women living with HIV, only 8% of respondents reported using HRT. In a Canadian study that has not yet gone through peer review, 11.8% of perimenopausal and postmenopausal women reported ever using HRT, about half the rate of women in North America without HIV.
Provider discomfort with managing menopause-related care in women with HIV is one reason for such low HRT use in this population, Dr. Tariq said. In a survey of 88 general practitioners in the United Kingdom, nearly all (> 95%) respondents said they were comfortable managing menopause in a general population, but just 46% said they felt comfortable managing menopause in women with HIV. Their top concerns included the potential for drug-to-drug interactions between ART and HRT, missing an HIV-related diagnosis, and risks of menopausal hormone therapy in HIV. Nearly half of respondents (46%) said only specialists should be providing menopause-related care for women with HIV.
But specialists may also feel conflicted about managing menopause-related care in women with HIV, said Dr. Tariq. “If you’re looking at people who manage HIV, you’re looking primarily at infectious disease physicians and HIV physicians. We’re not trained as gynecologists. We’re not used to prescribing HRT,” she said. “And the problem is gynecologists aren’t used to managing HIV. They get nervous about prescribing anything when they see antiretroviral medication because all that people think of is a drug-drug interaction.”
This leaves women with HIV seeking care and treatment for menopause in a difficult situation, where they are “just being ping-ponged around between different health care providers,” said Susan Cole-Haley, 53, an HIV-activist in London who has been living with the virus for 23 years. “So many women with HIV have multiple health conditions and multiple health care providers, which can just make it really problematic and really exhausting in terms of getting help.”
Many unknowns
Providers may also be uncomfortable with prescribing hormone therapy because of alarming research in the early 2000s, which found that hormone replacement therapy increased the risk of breast cancer and cardiovascular disease. Later analyses have found no increased cardiovascular disease risk in women who were younger than 60 or were less than 10 years beyond the onset of menopause. Still, the “media frenzy” around the initial findings “has put off a whole load of patients and a whole load of clinicians from even thinking of HRT,” Dr. Tariq said.
Providers may be even more hesitant because people with HIV already have a higher risk for heart disease, due to behaviors such as smoking and HIV-specific factors. (Research has yet to tease out whether these cardiovascular effects are a result of the virus, a result of the antiretroviral therapy, or a result of both factors.) In addition, there have been no prospective studies looking directly at the efficacy and safety of hormone replacement therapy in women with HIV, so providers generally rely on the guidelines for the use of menopausal hormone therapy for women without HIV. While researchers from Canada and the United Kingdom have compiled recommendations for HRT in women with HIV, there is great need for a large-scale clinical trial to establish consistent guidelines for the use of HRT for women with HIV globally, Dr. Looby said.
There are also hormonal preparations and drug-to-drug interactions to consider, though none of the interactions identified so far rise to the level of contraindications. Because of how the liver metabolizes ART and HRT, hormone doses may need to be adjusted, or perhaps administered transdermally via a patch versus a pill form. (Estrogen delivered via skin patch may have reduced cardiovascular disease risk compared to other methods of delivery, some studies in women without HIV suggest.) These expected interactions are based on data from contraceptives, noted Elizabeth King, MD, whose research at the Women’s Health Research Institute at BC Women’s Hospital in Vancouver, B.C., focuses on menopause and HIV. Studies have not been done on drug-drug interactions between ART and HRT specifically, she said, and formulations for HRT are a bit different from contraceptives.
While these unknowns do need to be discussed in shared decision-making around starting HRT in women with HIV, they should not dissuade providers from considering the treatment, Dr. King said. “If women are having extremely troublesome symptoms, then withholding therapy that is potentially beneficial because of worries about some of the things we do not know – I don’t know if that is any better,” she said.
Many women with HIV may not want to start HRT – as was the case for Dr. Lynn. “I’ve taken a lot of medication in my time, and I really try to avoid it as much as possible,” she said. Uncertainties around drug interactions were the main concern for Dawn Averitt, 53, founder of the Well Project, an HIV nonprofit focused on women and girls. Ms. Averitt has lived with HIV for 34 years. “What if some of the things that I’m dealing with could be managed by HRT?” she said. “Or what if taking it exacerbates problems in a way that nobody knows to look for?” In this case, providers may work with patients to discuss nonhormonal treatment options for menopause symptom management.
While some women with HIV may not want HRT, “It’s important that women have that option, and from what we are seeing right now, not a lot of women are even being offered the therapy,” Dr. King said.
There are other nonhormonal treatments available for managing menopause symptoms, including selective serotonin reuptake inhibitors (SSRIs) as well as nonmedicinal interventions such as cognitive behavioral therapy, but these also have not been studied specifically in women with HIV.
The path forward
Dr. Tariq and Dr. Looby agreed the next step in expanding our knowledge around HIV and menopause should be to better engage women with HIV in research and clinical care around their experience with menopause. This includes studies on the symptoms they regularly experience and how these symptoms affect their quality of life, including their physical, psychological, cognitive, and social health. These studies could also help researchers and clinicians understand what these women with HIV want for their menopause care, whether that be medication, psychotherapy, and/or peer support groups. These interventions, whether pharmaceutical based or not, can then be assessed based on outcomes in women with HIV, Dr. Tariq noted.
Another important factor is increasing education, on both the patient and provider side, Dr. Looby said. Many women may not know what menopause is, what symptoms look like, and how these hormonal changes can affect their health. If providers keep an open dialogue with female patients around menopause throughout their adult care, that can better prepare women for the menopause transition and alert them to common symptoms they may experience. There also is a great need for provider education, Dr. Looby added. Infectious disease specialists may need further education on menopause management, while women’s health specialists may need additional training for managing care for patients with HIV. Ideally, this information could be shared among a team of providers, including infectious disease, primary care, and women’s health specialists, so that clinicians can collaborate in prescribing treatment for women with HIV, Dr. Looby said.
Lastly, there needs to be more research funding allocated toward answering questions related to menopause and HIV, including the age of onset of menopause in women with HIV, the severity of symptoms, how HIV may influence the menopause transition and vice versa, and regarding the effectiveness of treatment – pharmaceutical and nonpharmaceutical – for women with HIV going through the menopause transition. “If we don’t have funding for these studies, then we won’t have answers to establish clinical care guidelines necessary to support the health, well-being, and quality of life of women with HIV,” Dr. Looby said.
And the number of women living with HIV entering menopause is expected to keep growing, Dr. King added. “It was only a couple of decades ago when women were being told they wouldn’t even live to experience menopause, and now we are at a point where this is the highest proportion of menopausal women ever that we have seen in our HIV clinics,” she said. “It speaks to the success of antiretrovirals,” Dr. King acknowledged, but that also means identifying new challenges and addressing recognized gaps in care.
“We are charting a new course, in some ways,” she added. “There is a lot of work to be done.”
A version of this article first appeared on Medscape.com.
Note: In this article, “women” refers to ciswomen – those who identify as women and were assigned female sex at birth. Menopause also affects transmen and nonbinary people, but published research on the menopause experience has included only ciswomen participants.
Gina Brown was boarding an early morning flight in 2016 when suddenly she started to overheat. “As soon as I stepped on the plane, I immediately was drenched in sweat,” she said. Not knowing what to do, she stood still until a fellow female passenger noticed her alarm and asked a flight attendant to grab her a cup of ice. “Is this the first time this has happened to you?” the woman asked, and Ms. Brown nodded. “It’s called a hot flash,” the woman continued, “and you’re going to be okay.”
As soon as Ms. Brown returned from her trip, she visited her doctor for blood work and learned that her hormone levels were decreasing. “I knew something was going on, but [my provider and I] didn’t have a conversation about menopause,” she said. Ms. Brown, who is 56 years old, has been living with HIV for nearly 28 years, and is part of a growing group of women with HIV now entering menopause.
In 1996, a person diagnosed with HIV at 20 years of age could expect to live only to age 39. Because of antiretroviral therapy (ART), an HIV diagnosis is not nearly so dire. Now, someone with HIV who adheres to the ART regimen is estimated to have a lifespan close to that of the general population.
For women with HIV, this means going through menopause. Though this transition can be challenging for any woman, experiencing menopause with HIV adds another level of complication. On top of adhering to daily ART regimens, the woman must also deal with the hormonal changes of menopause and the symptoms that come with it. And the limited research in this area suggests that women with HIV and their clinicians may not be prepared.
“Those of us long-term survivors who have been around for a while never expected to be here, and I don’t think providers or the health care system expected us to be here,” said Vickie A. Lynn, PhD, 56, who has been living with HIV for 37 years and received an AIDS diagnosis in 1991. Her work focuses on health care interventions for people with HIV. “So now that we’re here, I don’t know that we have enough information or research to inform some of our treatment options.” Instead, these women are met with a series of unknowns due to limited studies and conflicting findings.
Earlier menopause?
The onset of menopause can be difficult to determine in women living with HIV, said Sara Looby, PhD, ANP-BC, a researcher at Massachusetts General Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Her research focuses on metabolic disorders, including bone loss, cardiovascular disease risk, and menopause in women living with HIV. This population is at an increased risk for amenorrhea, due to both behavioral and clinical factors, and sometimes this amenorrhea is mistakenly assumed to be menopause, she explained. A history of smoking, low weight, methadone use, or use of other psychotropic medications are common in women with HIV and can lead to missed periods. Some factors specific to HIV – including a low CD4 count and a history of an AIDS diagnosis – have also been linked to amenorrhea.
This is likely why research studies on the age of onset of menopause with women with HIV can reach conflicting conclusions. Some studies suggest that women with HIV tend to go through menopause 3-5 years earlier than women without HIV. Other studies suggest no difference in the age of onset in menopause between women living with and without HIV. But how menopause status has been accessed can vary from study to study, Dr. Looby said. Future research needs to consider participants’ complete menstrual and reproductive history, as well as relevant medical, social, and behavioral factors, she added, so that the findings are reliably capturing the age of onset of menopause rather than amenorrhea from other causes.
If menopause does occur earlier in women with HIV, there could be additional health implications. Estrogen regulates bone mass, and some research suggests the hormone may be cardioprotective. Estrogen is also thought to increase production of the neurotransmitter serotonin, which could affect mood and cognition. Women with HIV are already at higher risk for bone loss, cardiovascular disease, and depressed mood compared to women without HIV, Dr. Looby said, and as estrogen levels fall during menopause, these conditions may be deleteriously affected.
“If it is determined that women with HIV experience menopause at an earlier age, maybe early to mid-40s instead of 51 and older, they may be at increased risk for cardiovascular and bone conditions as well as mood symptoms associated with estrogen loss at an earlier age than women without HIV, which could be highly detrimental to their physical and mental health,” Dr. Looby said.
More frequent and severe menopausal symptoms?
Women with HIV may not only go through menopause earlier than women without HIV, but their symptoms may also be more frequent and more severe. In a 2017 study of both HIV-positive and HIV-negative Nigerian women, participants with HIV had more menopause symptoms overall and were three times as likely to report severe symptoms compared to women without HIV. A 2005 study conducted in New York found HIV-positive women were 24% more likely to report menopause symptoms compared to HIV-negative women in the study.
Looby’s own research has also found a similar pattern. In a study comparing 33 women with HIV to 33 women without HIV – all were close to menopause and matched for age, race, body mass index, and menstrual patterns – women with HIV reported more severe hot flashes and more days with hot flashes. These women also reported that their hot flashes interfered to a much greater degree with daily activities and quality of life compared to participants without HIV.
But studies of women with HIV who are entering menopause are rare, and most include only small numbers of women. As a result, many women with HIV do not know what to expect entering menopause. “I always say, I wish somebody would do some real research on HIV and menopause, because I want to know if it is worse for us or if it is the same,” said Ms. Brown, who works as the director of strategic partnership and community engagement at the Southern Aids Coalition in Powder Springs, Ga. “I would think it’s worse for me.”
More frequent and severe symptoms can have downstream effects, with some evidence suggesting that women with HIV who experience severe menopause symptoms are less likely to stick to their ART regimen. “There’s a clear picture emerging that menopausal symptoms in this group really matter,” said Shema Tariq, PhD, FRCP, an HIV physician-scientist at the University College London Institute for Global Health in England. “They really impact women’s well-being, as well as impacting their ability to look after their long-term condition.”
Providers wary of treating menopause symptoms in women with HIV
The little research we do have about women with HIV experiencing menopause suggests that this population could greatly benefit from treatment prescribed in women without HIV for menopause symptoms and conditions, including hormone replacement therapy (HRT). Women with HIV regularly experience night sweats and hot flashes during the menopause transition and may have more severe symptoms than women not living with the virus. If women with HIV also frequently enter early menopause (entering menopause before the age of 45), then this group meets two indications for hormone replacement therapy.
Despite the potential benefits of HRT in this population, some studies suggest this intervention is underutilized. In Dr. Tariq’s Positive Transitions through Menopause (PRIME) study, which explores how menopause affects more than 800 women living with HIV, only 8% of respondents reported using HRT. In a Canadian study that has not yet gone through peer review, 11.8% of perimenopausal and postmenopausal women reported ever using HRT, about half the rate of women in North America without HIV.
Provider discomfort with managing menopause-related care in women with HIV is one reason for such low HRT use in this population, Dr. Tariq said. In a survey of 88 general practitioners in the United Kingdom, nearly all (> 95%) respondents said they were comfortable managing menopause in a general population, but just 46% said they felt comfortable managing menopause in women with HIV. Their top concerns included the potential for drug-to-drug interactions between ART and HRT, missing an HIV-related diagnosis, and risks of menopausal hormone therapy in HIV. Nearly half of respondents (46%) said only specialists should be providing menopause-related care for women with HIV.
But specialists may also feel conflicted about managing menopause-related care in women with HIV, said Dr. Tariq. “If you’re looking at people who manage HIV, you’re looking primarily at infectious disease physicians and HIV physicians. We’re not trained as gynecologists. We’re not used to prescribing HRT,” she said. “And the problem is gynecologists aren’t used to managing HIV. They get nervous about prescribing anything when they see antiretroviral medication because all that people think of is a drug-drug interaction.”
This leaves women with HIV seeking care and treatment for menopause in a difficult situation, where they are “just being ping-ponged around between different health care providers,” said Susan Cole-Haley, 53, an HIV-activist in London who has been living with the virus for 23 years. “So many women with HIV have multiple health conditions and multiple health care providers, which can just make it really problematic and really exhausting in terms of getting help.”
Many unknowns
Providers may also be uncomfortable with prescribing hormone therapy because of alarming research in the early 2000s, which found that hormone replacement therapy increased the risk of breast cancer and cardiovascular disease. Later analyses have found no increased cardiovascular disease risk in women who were younger than 60 or were less than 10 years beyond the onset of menopause. Still, the “media frenzy” around the initial findings “has put off a whole load of patients and a whole load of clinicians from even thinking of HRT,” Dr. Tariq said.
Providers may be even more hesitant because people with HIV already have a higher risk for heart disease, due to behaviors such as smoking and HIV-specific factors. (Research has yet to tease out whether these cardiovascular effects are a result of the virus, a result of the antiretroviral therapy, or a result of both factors.) In addition, there have been no prospective studies looking directly at the efficacy and safety of hormone replacement therapy in women with HIV, so providers generally rely on the guidelines for the use of menopausal hormone therapy for women without HIV. While researchers from Canada and the United Kingdom have compiled recommendations for HRT in women with HIV, there is great need for a large-scale clinical trial to establish consistent guidelines for the use of HRT for women with HIV globally, Dr. Looby said.
There are also hormonal preparations and drug-to-drug interactions to consider, though none of the interactions identified so far rise to the level of contraindications. Because of how the liver metabolizes ART and HRT, hormone doses may need to be adjusted, or perhaps administered transdermally via a patch versus a pill form. (Estrogen delivered via skin patch may have reduced cardiovascular disease risk compared to other methods of delivery, some studies in women without HIV suggest.) These expected interactions are based on data from contraceptives, noted Elizabeth King, MD, whose research at the Women’s Health Research Institute at BC Women’s Hospital in Vancouver, B.C., focuses on menopause and HIV. Studies have not been done on drug-drug interactions between ART and HRT specifically, she said, and formulations for HRT are a bit different from contraceptives.
While these unknowns do need to be discussed in shared decision-making around starting HRT in women with HIV, they should not dissuade providers from considering the treatment, Dr. King said. “If women are having extremely troublesome symptoms, then withholding therapy that is potentially beneficial because of worries about some of the things we do not know – I don’t know if that is any better,” she said.
Many women with HIV may not want to start HRT – as was the case for Dr. Lynn. “I’ve taken a lot of medication in my time, and I really try to avoid it as much as possible,” she said. Uncertainties around drug interactions were the main concern for Dawn Averitt, 53, founder of the Well Project, an HIV nonprofit focused on women and girls. Ms. Averitt has lived with HIV for 34 years. “What if some of the things that I’m dealing with could be managed by HRT?” she said. “Or what if taking it exacerbates problems in a way that nobody knows to look for?” In this case, providers may work with patients to discuss nonhormonal treatment options for menopause symptom management.
While some women with HIV may not want HRT, “It’s important that women have that option, and from what we are seeing right now, not a lot of women are even being offered the therapy,” Dr. King said.
There are other nonhormonal treatments available for managing menopause symptoms, including selective serotonin reuptake inhibitors (SSRIs) as well as nonmedicinal interventions such as cognitive behavioral therapy, but these also have not been studied specifically in women with HIV.
The path forward
Dr. Tariq and Dr. Looby agreed the next step in expanding our knowledge around HIV and menopause should be to better engage women with HIV in research and clinical care around their experience with menopause. This includes studies on the symptoms they regularly experience and how these symptoms affect their quality of life, including their physical, psychological, cognitive, and social health. These studies could also help researchers and clinicians understand what these women with HIV want for their menopause care, whether that be medication, psychotherapy, and/or peer support groups. These interventions, whether pharmaceutical based or not, can then be assessed based on outcomes in women with HIV, Dr. Tariq noted.
Another important factor is increasing education, on both the patient and provider side, Dr. Looby said. Many women may not know what menopause is, what symptoms look like, and how these hormonal changes can affect their health. If providers keep an open dialogue with female patients around menopause throughout their adult care, that can better prepare women for the menopause transition and alert them to common symptoms they may experience. There also is a great need for provider education, Dr. Looby added. Infectious disease specialists may need further education on menopause management, while women’s health specialists may need additional training for managing care for patients with HIV. Ideally, this information could be shared among a team of providers, including infectious disease, primary care, and women’s health specialists, so that clinicians can collaborate in prescribing treatment for women with HIV, Dr. Looby said.
Lastly, there needs to be more research funding allocated toward answering questions related to menopause and HIV, including the age of onset of menopause in women with HIV, the severity of symptoms, how HIV may influence the menopause transition and vice versa, and regarding the effectiveness of treatment – pharmaceutical and nonpharmaceutical – for women with HIV going through the menopause transition. “If we don’t have funding for these studies, then we won’t have answers to establish clinical care guidelines necessary to support the health, well-being, and quality of life of women with HIV,” Dr. Looby said.
And the number of women living with HIV entering menopause is expected to keep growing, Dr. King added. “It was only a couple of decades ago when women were being told they wouldn’t even live to experience menopause, and now we are at a point where this is the highest proportion of menopausal women ever that we have seen in our HIV clinics,” she said. “It speaks to the success of antiretrovirals,” Dr. King acknowledged, but that also means identifying new challenges and addressing recognized gaps in care.
“We are charting a new course, in some ways,” she added. “There is a lot of work to be done.”
A version of this article first appeared on Medscape.com.
Note: In this article, “women” refers to ciswomen – those who identify as women and were assigned female sex at birth. Menopause also affects transmen and nonbinary people, but published research on the menopause experience has included only ciswomen participants.
Gina Brown was boarding an early morning flight in 2016 when suddenly she started to overheat. “As soon as I stepped on the plane, I immediately was drenched in sweat,” she said. Not knowing what to do, she stood still until a fellow female passenger noticed her alarm and asked a flight attendant to grab her a cup of ice. “Is this the first time this has happened to you?” the woman asked, and Ms. Brown nodded. “It’s called a hot flash,” the woman continued, “and you’re going to be okay.”
As soon as Ms. Brown returned from her trip, she visited her doctor for blood work and learned that her hormone levels were decreasing. “I knew something was going on, but [my provider and I] didn’t have a conversation about menopause,” she said. Ms. Brown, who is 56 years old, has been living with HIV for nearly 28 years, and is part of a growing group of women with HIV now entering menopause.
In 1996, a person diagnosed with HIV at 20 years of age could expect to live only to age 39. Because of antiretroviral therapy (ART), an HIV diagnosis is not nearly so dire. Now, someone with HIV who adheres to the ART regimen is estimated to have a lifespan close to that of the general population.
For women with HIV, this means going through menopause. Though this transition can be challenging for any woman, experiencing menopause with HIV adds another level of complication. On top of adhering to daily ART regimens, the woman must also deal with the hormonal changes of menopause and the symptoms that come with it. And the limited research in this area suggests that women with HIV and their clinicians may not be prepared.
“Those of us long-term survivors who have been around for a while never expected to be here, and I don’t think providers or the health care system expected us to be here,” said Vickie A. Lynn, PhD, 56, who has been living with HIV for 37 years and received an AIDS diagnosis in 1991. Her work focuses on health care interventions for people with HIV. “So now that we’re here, I don’t know that we have enough information or research to inform some of our treatment options.” Instead, these women are met with a series of unknowns due to limited studies and conflicting findings.
Earlier menopause?
The onset of menopause can be difficult to determine in women living with HIV, said Sara Looby, PhD, ANP-BC, a researcher at Massachusetts General Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Her research focuses on metabolic disorders, including bone loss, cardiovascular disease risk, and menopause in women living with HIV. This population is at an increased risk for amenorrhea, due to both behavioral and clinical factors, and sometimes this amenorrhea is mistakenly assumed to be menopause, she explained. A history of smoking, low weight, methadone use, or use of other psychotropic medications are common in women with HIV and can lead to missed periods. Some factors specific to HIV – including a low CD4 count and a history of an AIDS diagnosis – have also been linked to amenorrhea.
This is likely why research studies on the age of onset of menopause with women with HIV can reach conflicting conclusions. Some studies suggest that women with HIV tend to go through menopause 3-5 years earlier than women without HIV. Other studies suggest no difference in the age of onset in menopause between women living with and without HIV. But how menopause status has been accessed can vary from study to study, Dr. Looby said. Future research needs to consider participants’ complete menstrual and reproductive history, as well as relevant medical, social, and behavioral factors, she added, so that the findings are reliably capturing the age of onset of menopause rather than amenorrhea from other causes.
If menopause does occur earlier in women with HIV, there could be additional health implications. Estrogen regulates bone mass, and some research suggests the hormone may be cardioprotective. Estrogen is also thought to increase production of the neurotransmitter serotonin, which could affect mood and cognition. Women with HIV are already at higher risk for bone loss, cardiovascular disease, and depressed mood compared to women without HIV, Dr. Looby said, and as estrogen levels fall during menopause, these conditions may be deleteriously affected.
“If it is determined that women with HIV experience menopause at an earlier age, maybe early to mid-40s instead of 51 and older, they may be at increased risk for cardiovascular and bone conditions as well as mood symptoms associated with estrogen loss at an earlier age than women without HIV, which could be highly detrimental to their physical and mental health,” Dr. Looby said.
More frequent and severe menopausal symptoms?
Women with HIV may not only go through menopause earlier than women without HIV, but their symptoms may also be more frequent and more severe. In a 2017 study of both HIV-positive and HIV-negative Nigerian women, participants with HIV had more menopause symptoms overall and were three times as likely to report severe symptoms compared to women without HIV. A 2005 study conducted in New York found HIV-positive women were 24% more likely to report menopause symptoms compared to HIV-negative women in the study.
Looby’s own research has also found a similar pattern. In a study comparing 33 women with HIV to 33 women without HIV – all were close to menopause and matched for age, race, body mass index, and menstrual patterns – women with HIV reported more severe hot flashes and more days with hot flashes. These women also reported that their hot flashes interfered to a much greater degree with daily activities and quality of life compared to participants without HIV.
But studies of women with HIV who are entering menopause are rare, and most include only small numbers of women. As a result, many women with HIV do not know what to expect entering menopause. “I always say, I wish somebody would do some real research on HIV and menopause, because I want to know if it is worse for us or if it is the same,” said Ms. Brown, who works as the director of strategic partnership and community engagement at the Southern Aids Coalition in Powder Springs, Ga. “I would think it’s worse for me.”
More frequent and severe symptoms can have downstream effects, with some evidence suggesting that women with HIV who experience severe menopause symptoms are less likely to stick to their ART regimen. “There’s a clear picture emerging that menopausal symptoms in this group really matter,” said Shema Tariq, PhD, FRCP, an HIV physician-scientist at the University College London Institute for Global Health in England. “They really impact women’s well-being, as well as impacting their ability to look after their long-term condition.”
Providers wary of treating menopause symptoms in women with HIV
The little research we do have about women with HIV experiencing menopause suggests that this population could greatly benefit from treatment prescribed in women without HIV for menopause symptoms and conditions, including hormone replacement therapy (HRT). Women with HIV regularly experience night sweats and hot flashes during the menopause transition and may have more severe symptoms than women not living with the virus. If women with HIV also frequently enter early menopause (entering menopause before the age of 45), then this group meets two indications for hormone replacement therapy.
Despite the potential benefits of HRT in this population, some studies suggest this intervention is underutilized. In Dr. Tariq’s Positive Transitions through Menopause (PRIME) study, which explores how menopause affects more than 800 women living with HIV, only 8% of respondents reported using HRT. In a Canadian study that has not yet gone through peer review, 11.8% of perimenopausal and postmenopausal women reported ever using HRT, about half the rate of women in North America without HIV.
Provider discomfort with managing menopause-related care in women with HIV is one reason for such low HRT use in this population, Dr. Tariq said. In a survey of 88 general practitioners in the United Kingdom, nearly all (> 95%) respondents said they were comfortable managing menopause in a general population, but just 46% said they felt comfortable managing menopause in women with HIV. Their top concerns included the potential for drug-to-drug interactions between ART and HRT, missing an HIV-related diagnosis, and risks of menopausal hormone therapy in HIV. Nearly half of respondents (46%) said only specialists should be providing menopause-related care for women with HIV.
But specialists may also feel conflicted about managing menopause-related care in women with HIV, said Dr. Tariq. “If you’re looking at people who manage HIV, you’re looking primarily at infectious disease physicians and HIV physicians. We’re not trained as gynecologists. We’re not used to prescribing HRT,” she said. “And the problem is gynecologists aren’t used to managing HIV. They get nervous about prescribing anything when they see antiretroviral medication because all that people think of is a drug-drug interaction.”
This leaves women with HIV seeking care and treatment for menopause in a difficult situation, where they are “just being ping-ponged around between different health care providers,” said Susan Cole-Haley, 53, an HIV-activist in London who has been living with the virus for 23 years. “So many women with HIV have multiple health conditions and multiple health care providers, which can just make it really problematic and really exhausting in terms of getting help.”
Many unknowns
Providers may also be uncomfortable with prescribing hormone therapy because of alarming research in the early 2000s, which found that hormone replacement therapy increased the risk of breast cancer and cardiovascular disease. Later analyses have found no increased cardiovascular disease risk in women who were younger than 60 or were less than 10 years beyond the onset of menopause. Still, the “media frenzy” around the initial findings “has put off a whole load of patients and a whole load of clinicians from even thinking of HRT,” Dr. Tariq said.
Providers may be even more hesitant because people with HIV already have a higher risk for heart disease, due to behaviors such as smoking and HIV-specific factors. (Research has yet to tease out whether these cardiovascular effects are a result of the virus, a result of the antiretroviral therapy, or a result of both factors.) In addition, there have been no prospective studies looking directly at the efficacy and safety of hormone replacement therapy in women with HIV, so providers generally rely on the guidelines for the use of menopausal hormone therapy for women without HIV. While researchers from Canada and the United Kingdom have compiled recommendations for HRT in women with HIV, there is great need for a large-scale clinical trial to establish consistent guidelines for the use of HRT for women with HIV globally, Dr. Looby said.
There are also hormonal preparations and drug-to-drug interactions to consider, though none of the interactions identified so far rise to the level of contraindications. Because of how the liver metabolizes ART and HRT, hormone doses may need to be adjusted, or perhaps administered transdermally via a patch versus a pill form. (Estrogen delivered via skin patch may have reduced cardiovascular disease risk compared to other methods of delivery, some studies in women without HIV suggest.) These expected interactions are based on data from contraceptives, noted Elizabeth King, MD, whose research at the Women’s Health Research Institute at BC Women’s Hospital in Vancouver, B.C., focuses on menopause and HIV. Studies have not been done on drug-drug interactions between ART and HRT specifically, she said, and formulations for HRT are a bit different from contraceptives.
While these unknowns do need to be discussed in shared decision-making around starting HRT in women with HIV, they should not dissuade providers from considering the treatment, Dr. King said. “If women are having extremely troublesome symptoms, then withholding therapy that is potentially beneficial because of worries about some of the things we do not know – I don’t know if that is any better,” she said.
Many women with HIV may not want to start HRT – as was the case for Dr. Lynn. “I’ve taken a lot of medication in my time, and I really try to avoid it as much as possible,” she said. Uncertainties around drug interactions were the main concern for Dawn Averitt, 53, founder of the Well Project, an HIV nonprofit focused on women and girls. Ms. Averitt has lived with HIV for 34 years. “What if some of the things that I’m dealing with could be managed by HRT?” she said. “Or what if taking it exacerbates problems in a way that nobody knows to look for?” In this case, providers may work with patients to discuss nonhormonal treatment options for menopause symptom management.
While some women with HIV may not want HRT, “It’s important that women have that option, and from what we are seeing right now, not a lot of women are even being offered the therapy,” Dr. King said.
There are other nonhormonal treatments available for managing menopause symptoms, including selective serotonin reuptake inhibitors (SSRIs) as well as nonmedicinal interventions such as cognitive behavioral therapy, but these also have not been studied specifically in women with HIV.
The path forward
Dr. Tariq and Dr. Looby agreed the next step in expanding our knowledge around HIV and menopause should be to better engage women with HIV in research and clinical care around their experience with menopause. This includes studies on the symptoms they regularly experience and how these symptoms affect their quality of life, including their physical, psychological, cognitive, and social health. These studies could also help researchers and clinicians understand what these women with HIV want for their menopause care, whether that be medication, psychotherapy, and/or peer support groups. These interventions, whether pharmaceutical based or not, can then be assessed based on outcomes in women with HIV, Dr. Tariq noted.
Another important factor is increasing education, on both the patient and provider side, Dr. Looby said. Many women may not know what menopause is, what symptoms look like, and how these hormonal changes can affect their health. If providers keep an open dialogue with female patients around menopause throughout their adult care, that can better prepare women for the menopause transition and alert them to common symptoms they may experience. There also is a great need for provider education, Dr. Looby added. Infectious disease specialists may need further education on menopause management, while women’s health specialists may need additional training for managing care for patients with HIV. Ideally, this information could be shared among a team of providers, including infectious disease, primary care, and women’s health specialists, so that clinicians can collaborate in prescribing treatment for women with HIV, Dr. Looby said.
Lastly, there needs to be more research funding allocated toward answering questions related to menopause and HIV, including the age of onset of menopause in women with HIV, the severity of symptoms, how HIV may influence the menopause transition and vice versa, and regarding the effectiveness of treatment – pharmaceutical and nonpharmaceutical – for women with HIV going through the menopause transition. “If we don’t have funding for these studies, then we won’t have answers to establish clinical care guidelines necessary to support the health, well-being, and quality of life of women with HIV,” Dr. Looby said.
And the number of women living with HIV entering menopause is expected to keep growing, Dr. King added. “It was only a couple of decades ago when women were being told they wouldn’t even live to experience menopause, and now we are at a point where this is the highest proportion of menopausal women ever that we have seen in our HIV clinics,” she said. “It speaks to the success of antiretrovirals,” Dr. King acknowledged, but that also means identifying new challenges and addressing recognized gaps in care.
“We are charting a new course, in some ways,” she added. “There is a lot of work to be done.”
A version of this article first appeared on Medscape.com.
WHO issues new TB guidelines for children and adolescents
The World Health Organization now recommends shortened treatment for children with mild tuberculosis, as well as two oral TB treatments (bedaquiline and delamanid) for use in children of all ages. The updated guidelines for TB management in children and adolescents were announced March 21 ahead of World Tuberculosis Day on March 24.
The agency also called for increased investment in global TB programs, noting that in 2020, TB deaths increased for the first time in over a decade. “We cannot falter in our commitment to reach and save every man, woman, child, family, and community impacted by this deadly disease,” said Tereza Kasaeva, MD, PhD, director of the WHO Global Tuberculosis Programme during a press conference.
TB is the 13th-leading cause of death and the second top infectious killer after COVID-19, with more than 4,100 people dying from TB every day. WHO estimates that 1.1 million children fall ill with TB each year.
Calls for investment
The increase in TB deaths from 1.4 million in 2019 to 1.5 million in 2020 was coupled with a decrease in funding. From 2019-2020, global spending for TB diagnostic, treatment, and prevention services fell from $5.8 billion to $5.3 billion. This is less than half of the $13 billion target funding amount for 2022, Dr. Kasaeva said.
Efforts to expand access to TB care have fallen short mainly because of this lack of funding, especially for children. In 2020, about 63% of children under 15 years of age with TB either did not receive or were not reported to have access to TB diagnosis and treatment services, which rose to 72% in children under age 5. Almost two-thirds of children under age 5 also did not receive TB preventive treatment in 2022, according to WHO statistics.
The socioeconomic ramifications of the COVID-19 pandemic as well as ongoing conflict in Eastern Europe, Africa, and the Middle East have “further exacerbated the situation,” Dr. Kasaeva said. “This conveys the urgent need to dramatically increase investments to ramp up the fight against TB and achieve commitments to end TB made by global leaders.”
Dr. Kasaeva laid out WHO’s main points for global investment in TB care:
- Increase domestic and international funding to close gaps in TB research and program implementation. For countries with smaller economies, increased international investment will be necessary in the short or medium term to help regain progress.
- Double funding for TB research, including vaccines.
- Invest in sustaining TB programs and services during the COVID-19 pandemic and ongoing crises so care is not disrupted.
New guidelines
Dr. Kasaeva also noted that adoption of WHO’s new guidelines for children and adolescents should be fast-tracked to improve access to and quality of care. The updates include:
- Rapid molecular tests called Xpert Ultra should be used as the initial test for TB in children and adolescents.
- Diagnostic testing can now include noninvasive specimens, like stool samples.
- Children with mild TB can be treated with a , rather than 6 months. This shortened regimen will allow children to return to school faster and save money for families and the health care system, said Kerri Viney, MD, PhD, a team lead for the WHO Tuberculosis Programme, with a focus on vulnerable populations, including children. She presented the new guidelines during the WHO press conference.
- The recommended treatment regimen for TB meningitis has also been shortened from 12 to 6 months.
Two oral medications for drug-resistant TB (bedaquiline and delamanid) are now recommended for use in children of all ages. “There is no longer a need for painful injections that can have serious side effects, including deafness,” Dr. Viney said.
Health systems should develop new models of decentralized and integrated TB care to bring TB care closer to where children live.
The guidelines are available on the WHO website.
“The WHO guidelines issued today are a game changer for children and adolescents with TB,” Dr. Kasaeva said. The next step is assisting countries in implementing these updates so that children and adolescents globally have access to high quality TB care,” Dr. Viney added. “We have the policy recommendations. We have the implementation guidance, we have child-friendly formulations of TB medicines,” she said. “Let us not wait any longer. Let us invest to end TB in children and adolescents.”
A version of this article first appeared on Medscape.com.
The World Health Organization now recommends shortened treatment for children with mild tuberculosis, as well as two oral TB treatments (bedaquiline and delamanid) for use in children of all ages. The updated guidelines for TB management in children and adolescents were announced March 21 ahead of World Tuberculosis Day on March 24.
The agency also called for increased investment in global TB programs, noting that in 2020, TB deaths increased for the first time in over a decade. “We cannot falter in our commitment to reach and save every man, woman, child, family, and community impacted by this deadly disease,” said Tereza Kasaeva, MD, PhD, director of the WHO Global Tuberculosis Programme during a press conference.
TB is the 13th-leading cause of death and the second top infectious killer after COVID-19, with more than 4,100 people dying from TB every day. WHO estimates that 1.1 million children fall ill with TB each year.
Calls for investment
The increase in TB deaths from 1.4 million in 2019 to 1.5 million in 2020 was coupled with a decrease in funding. From 2019-2020, global spending for TB diagnostic, treatment, and prevention services fell from $5.8 billion to $5.3 billion. This is less than half of the $13 billion target funding amount for 2022, Dr. Kasaeva said.
Efforts to expand access to TB care have fallen short mainly because of this lack of funding, especially for children. In 2020, about 63% of children under 15 years of age with TB either did not receive or were not reported to have access to TB diagnosis and treatment services, which rose to 72% in children under age 5. Almost two-thirds of children under age 5 also did not receive TB preventive treatment in 2022, according to WHO statistics.
The socioeconomic ramifications of the COVID-19 pandemic as well as ongoing conflict in Eastern Europe, Africa, and the Middle East have “further exacerbated the situation,” Dr. Kasaeva said. “This conveys the urgent need to dramatically increase investments to ramp up the fight against TB and achieve commitments to end TB made by global leaders.”
Dr. Kasaeva laid out WHO’s main points for global investment in TB care:
- Increase domestic and international funding to close gaps in TB research and program implementation. For countries with smaller economies, increased international investment will be necessary in the short or medium term to help regain progress.
- Double funding for TB research, including vaccines.
- Invest in sustaining TB programs and services during the COVID-19 pandemic and ongoing crises so care is not disrupted.
New guidelines
Dr. Kasaeva also noted that adoption of WHO’s new guidelines for children and adolescents should be fast-tracked to improve access to and quality of care. The updates include:
- Rapid molecular tests called Xpert Ultra should be used as the initial test for TB in children and adolescents.
- Diagnostic testing can now include noninvasive specimens, like stool samples.
- Children with mild TB can be treated with a , rather than 6 months. This shortened regimen will allow children to return to school faster and save money for families and the health care system, said Kerri Viney, MD, PhD, a team lead for the WHO Tuberculosis Programme, with a focus on vulnerable populations, including children. She presented the new guidelines during the WHO press conference.
- The recommended treatment regimen for TB meningitis has also been shortened from 12 to 6 months.
Two oral medications for drug-resistant TB (bedaquiline and delamanid) are now recommended for use in children of all ages. “There is no longer a need for painful injections that can have serious side effects, including deafness,” Dr. Viney said.
Health systems should develop new models of decentralized and integrated TB care to bring TB care closer to where children live.
The guidelines are available on the WHO website.
“The WHO guidelines issued today are a game changer for children and adolescents with TB,” Dr. Kasaeva said. The next step is assisting countries in implementing these updates so that children and adolescents globally have access to high quality TB care,” Dr. Viney added. “We have the policy recommendations. We have the implementation guidance, we have child-friendly formulations of TB medicines,” she said. “Let us not wait any longer. Let us invest to end TB in children and adolescents.”
A version of this article first appeared on Medscape.com.
The World Health Organization now recommends shortened treatment for children with mild tuberculosis, as well as two oral TB treatments (bedaquiline and delamanid) for use in children of all ages. The updated guidelines for TB management in children and adolescents were announced March 21 ahead of World Tuberculosis Day on March 24.
The agency also called for increased investment in global TB programs, noting that in 2020, TB deaths increased for the first time in over a decade. “We cannot falter in our commitment to reach and save every man, woman, child, family, and community impacted by this deadly disease,” said Tereza Kasaeva, MD, PhD, director of the WHO Global Tuberculosis Programme during a press conference.
TB is the 13th-leading cause of death and the second top infectious killer after COVID-19, with more than 4,100 people dying from TB every day. WHO estimates that 1.1 million children fall ill with TB each year.
Calls for investment
The increase in TB deaths from 1.4 million in 2019 to 1.5 million in 2020 was coupled with a decrease in funding. From 2019-2020, global spending for TB diagnostic, treatment, and prevention services fell from $5.8 billion to $5.3 billion. This is less than half of the $13 billion target funding amount for 2022, Dr. Kasaeva said.
Efforts to expand access to TB care have fallen short mainly because of this lack of funding, especially for children. In 2020, about 63% of children under 15 years of age with TB either did not receive or were not reported to have access to TB diagnosis and treatment services, which rose to 72% in children under age 5. Almost two-thirds of children under age 5 also did not receive TB preventive treatment in 2022, according to WHO statistics.
The socioeconomic ramifications of the COVID-19 pandemic as well as ongoing conflict in Eastern Europe, Africa, and the Middle East have “further exacerbated the situation,” Dr. Kasaeva said. “This conveys the urgent need to dramatically increase investments to ramp up the fight against TB and achieve commitments to end TB made by global leaders.”
Dr. Kasaeva laid out WHO’s main points for global investment in TB care:
- Increase domestic and international funding to close gaps in TB research and program implementation. For countries with smaller economies, increased international investment will be necessary in the short or medium term to help regain progress.
- Double funding for TB research, including vaccines.
- Invest in sustaining TB programs and services during the COVID-19 pandemic and ongoing crises so care is not disrupted.
New guidelines
Dr. Kasaeva also noted that adoption of WHO’s new guidelines for children and adolescents should be fast-tracked to improve access to and quality of care. The updates include:
- Rapid molecular tests called Xpert Ultra should be used as the initial test for TB in children and adolescents.
- Diagnostic testing can now include noninvasive specimens, like stool samples.
- Children with mild TB can be treated with a , rather than 6 months. This shortened regimen will allow children to return to school faster and save money for families and the health care system, said Kerri Viney, MD, PhD, a team lead for the WHO Tuberculosis Programme, with a focus on vulnerable populations, including children. She presented the new guidelines during the WHO press conference.
- The recommended treatment regimen for TB meningitis has also been shortened from 12 to 6 months.
Two oral medications for drug-resistant TB (bedaquiline and delamanid) are now recommended for use in children of all ages. “There is no longer a need for painful injections that can have serious side effects, including deafness,” Dr. Viney said.
Health systems should develop new models of decentralized and integrated TB care to bring TB care closer to where children live.
The guidelines are available on the WHO website.
“The WHO guidelines issued today are a game changer for children and adolescents with TB,” Dr. Kasaeva said. The next step is assisting countries in implementing these updates so that children and adolescents globally have access to high quality TB care,” Dr. Viney added. “We have the policy recommendations. We have the implementation guidance, we have child-friendly formulations of TB medicines,” she said. “Let us not wait any longer. Let us invest to end TB in children and adolescents.”
A version of this article first appeared on Medscape.com.
FDA committee recommends 2022-2023 influenza vaccine strains
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee has chosen the influenza vaccine strains for the 2022-2023 season in the northern hemisphere, which begins in the fall of 2022.
On March 3, the committee unanimously voted to endorse the World Health Organization’s recommendations as to which influenza strains to include for coverage by vaccines for the upcoming flu season. Two of the four recommended strains are different from last season.
The committee also heard updates on flu activity this season. So far, data from the U.S. Flu Vaccine Effectiveness (VE) network, which consists of seven study sites, have not shown that the vaccine is protective against influenza A. “We can say that it is not highly effective,” Brendan Flannery, PhD, who leads the U.S. Flu VE network for the Centers for Disease Control and Prevention, said in an interview. He was not involved with the advisory committee meeting. Flu activity this season has been low, he explained, so there are fewer cases his team can use to estimate vaccine efficacy. “If there’s some benefit, it’s hard for us to show that now,” he said.
Vaccine strains
The panel voted to include a A/Darwin/9/2021-like strain for the H3N2 component of the vaccine; this is changed from A/Cambodia/e0826360/2020. For the influenza B Victoria lineage component, the committee voted to include a B/Austria/1359417/2021-like virus, a swap from this year’s B/Washington/02/2019-like virus. These changes apply to the egg-based, cell-culture, and recombinant vaccines. Both new strains were included in WHO’s 2022 influenza vaccine strain recommendations for the southern hemisphere.
For the influenza A H1N1 component, the group also agreed to include a A/Victoria/2570/2019 (H1N1) pdm09-like virus for the egg-based vaccine and the A/Wisconsin/588/2019 (H1N1) pdm09-like virus for cell culture or recombinant vaccines. These strains were included for the 2021-2022 season. The panel also voted for the inclusion of a B/Phuket/3073/2013-like virus (B/Yamagata lineage) as the second influenza B strain for the quadrivalent egg-based, cell culture, or recombinant vaccines, which is unchanged from this flu season.
‘Sporadic’ flu activity
While there was an uptick in influenza activity this year compared to the 2020-2021 season, hospitalization rates are lower than in the four seasons preceding the pandemic (from 2016-2017 to 2019-2020). As of Feb. 26, the cumulative hospitalization rate for this flu season was 5.2 hospitalizations per 100,000 individuals. There have been eight pediatric deaths due to influenza so far this season, compared to one pediatric death reported to the CDC during the 2020-2021 flu season.
About 4.1% of specimens tested at clinical laboratories were positive for flu. Since Oct. 30, 2.7% of specimens have been positive for influenza this season. Nearly all viruses detected (97.7%) have been influenza A.
Lisa Grohskopf, MD, MPH, a medical officer in the influenza division at the CDC who presented the data at the meeting, described flu activity this season as “sporadic” and noted that activity is increasing in some areas of the country. According to CDC’s weekly influenza surveillance report, most states had minimal influenza-like illness (ILI) activity, although Arkansas, Idaho, Iowa, Kansas, Minnesota, and Utah had slightly higher ILI activity as of Feb. 26. Champaign-Urbana, Illinois; St. Cloud, Minnesota; and Brownwood, Texas, had the highest levels of flu activity in the country.
Low vaccine effectiveness
As of Jan. 22, results from the U.S. Flu VE network do not show statistically significant evidence that the flu vaccine is effective. Currently, the vaccine is estimated to be 8% effective against preventing influenza A infection (95% confidence interval, –31% to 36%) and 14% effective against preventing A/H3N2 infection (95% CI, –28% to 43%) for people aged 6 months and older.
The network did not have enough data to provide age-specific VE estimates or estimates of effectiveness against influenza B. This could be due to low flu activity relative to prepandemic years, Dr. Flannery said. Of the 2,758 individuals enrolled in the VE flu network this season, just 147 (5%) tested positive for the flu this season. This is the lowest positivity rate observed in the Flu VE network participants with respiratory illness over the past 10 flu seasons, Dr. Grohskopf noted. In comparison, estimates from the 2019 to 2020 season included 4,112 individuals, and 1,060 tested positive for flu.
“We are really at the bare minimum of what we can use for a flu vaccine effectiveness estimate,” Dr. Flannery said about the more recent data. The network was not able to produce any estimates about flu vaccine effectiveness for the 2020-2021 season because of historically low flu activity.
The Department of Defense also presented vaccine efficacy estimates for the 2021–2022 season. The vaccine has been 36% effective (95% CI, 28%-44%) against all strains of the virus, 33% effective against influenza A (95% CI, 24%-41%), 32% effective against A/H3N2 (95% CI, 3%-53%), and 59% effective against influenza B (95% CI, 42%-71%). These results are from a young, healthy adult population, Lieutenant Commander Courtney Gustin, DrPH, MSN, told the panel, and they may not be reflective of efficacy rates across all age groups.
Though these findings suggest there is low to no measurable benefit against influenza A, Dr. Flannery said the CDC still recommends getting the flu vaccine, as it can be protective against other circulating flu strains. “We have been able to demonstrate protection against other H3 [viruses], B viruses, and H1 viruses in the past,” he said. And as these results only show protection against mild disease, “there is still possibility that there’s benefit against more severe disease,” he added. Studies measuring effectiveness against more severe outcomes are not yet available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee has chosen the influenza vaccine strains for the 2022-2023 season in the northern hemisphere, which begins in the fall of 2022.
On March 3, the committee unanimously voted to endorse the World Health Organization’s recommendations as to which influenza strains to include for coverage by vaccines for the upcoming flu season. Two of the four recommended strains are different from last season.
The committee also heard updates on flu activity this season. So far, data from the U.S. Flu Vaccine Effectiveness (VE) network, which consists of seven study sites, have not shown that the vaccine is protective against influenza A. “We can say that it is not highly effective,” Brendan Flannery, PhD, who leads the U.S. Flu VE network for the Centers for Disease Control and Prevention, said in an interview. He was not involved with the advisory committee meeting. Flu activity this season has been low, he explained, so there are fewer cases his team can use to estimate vaccine efficacy. “If there’s some benefit, it’s hard for us to show that now,” he said.
Vaccine strains
The panel voted to include a A/Darwin/9/2021-like strain for the H3N2 component of the vaccine; this is changed from A/Cambodia/e0826360/2020. For the influenza B Victoria lineage component, the committee voted to include a B/Austria/1359417/2021-like virus, a swap from this year’s B/Washington/02/2019-like virus. These changes apply to the egg-based, cell-culture, and recombinant vaccines. Both new strains were included in WHO’s 2022 influenza vaccine strain recommendations for the southern hemisphere.
For the influenza A H1N1 component, the group also agreed to include a A/Victoria/2570/2019 (H1N1) pdm09-like virus for the egg-based vaccine and the A/Wisconsin/588/2019 (H1N1) pdm09-like virus for cell culture or recombinant vaccines. These strains were included for the 2021-2022 season. The panel also voted for the inclusion of a B/Phuket/3073/2013-like virus (B/Yamagata lineage) as the second influenza B strain for the quadrivalent egg-based, cell culture, or recombinant vaccines, which is unchanged from this flu season.
‘Sporadic’ flu activity
While there was an uptick in influenza activity this year compared to the 2020-2021 season, hospitalization rates are lower than in the four seasons preceding the pandemic (from 2016-2017 to 2019-2020). As of Feb. 26, the cumulative hospitalization rate for this flu season was 5.2 hospitalizations per 100,000 individuals. There have been eight pediatric deaths due to influenza so far this season, compared to one pediatric death reported to the CDC during the 2020-2021 flu season.
About 4.1% of specimens tested at clinical laboratories were positive for flu. Since Oct. 30, 2.7% of specimens have been positive for influenza this season. Nearly all viruses detected (97.7%) have been influenza A.
Lisa Grohskopf, MD, MPH, a medical officer in the influenza division at the CDC who presented the data at the meeting, described flu activity this season as “sporadic” and noted that activity is increasing in some areas of the country. According to CDC’s weekly influenza surveillance report, most states had minimal influenza-like illness (ILI) activity, although Arkansas, Idaho, Iowa, Kansas, Minnesota, and Utah had slightly higher ILI activity as of Feb. 26. Champaign-Urbana, Illinois; St. Cloud, Minnesota; and Brownwood, Texas, had the highest levels of flu activity in the country.
Low vaccine effectiveness
As of Jan. 22, results from the U.S. Flu VE network do not show statistically significant evidence that the flu vaccine is effective. Currently, the vaccine is estimated to be 8% effective against preventing influenza A infection (95% confidence interval, –31% to 36%) and 14% effective against preventing A/H3N2 infection (95% CI, –28% to 43%) for people aged 6 months and older.
The network did not have enough data to provide age-specific VE estimates or estimates of effectiveness against influenza B. This could be due to low flu activity relative to prepandemic years, Dr. Flannery said. Of the 2,758 individuals enrolled in the VE flu network this season, just 147 (5%) tested positive for the flu this season. This is the lowest positivity rate observed in the Flu VE network participants with respiratory illness over the past 10 flu seasons, Dr. Grohskopf noted. In comparison, estimates from the 2019 to 2020 season included 4,112 individuals, and 1,060 tested positive for flu.
“We are really at the bare minimum of what we can use for a flu vaccine effectiveness estimate,” Dr. Flannery said about the more recent data. The network was not able to produce any estimates about flu vaccine effectiveness for the 2020-2021 season because of historically low flu activity.
The Department of Defense also presented vaccine efficacy estimates for the 2021–2022 season. The vaccine has been 36% effective (95% CI, 28%-44%) against all strains of the virus, 33% effective against influenza A (95% CI, 24%-41%), 32% effective against A/H3N2 (95% CI, 3%-53%), and 59% effective against influenza B (95% CI, 42%-71%). These results are from a young, healthy adult population, Lieutenant Commander Courtney Gustin, DrPH, MSN, told the panel, and they may not be reflective of efficacy rates across all age groups.
Though these findings suggest there is low to no measurable benefit against influenza A, Dr. Flannery said the CDC still recommends getting the flu vaccine, as it can be protective against other circulating flu strains. “We have been able to demonstrate protection against other H3 [viruses], B viruses, and H1 viruses in the past,” he said. And as these results only show protection against mild disease, “there is still possibility that there’s benefit against more severe disease,” he added. Studies measuring effectiveness against more severe outcomes are not yet available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee has chosen the influenza vaccine strains for the 2022-2023 season in the northern hemisphere, which begins in the fall of 2022.
On March 3, the committee unanimously voted to endorse the World Health Organization’s recommendations as to which influenza strains to include for coverage by vaccines for the upcoming flu season. Two of the four recommended strains are different from last season.
The committee also heard updates on flu activity this season. So far, data from the U.S. Flu Vaccine Effectiveness (VE) network, which consists of seven study sites, have not shown that the vaccine is protective against influenza A. “We can say that it is not highly effective,” Brendan Flannery, PhD, who leads the U.S. Flu VE network for the Centers for Disease Control and Prevention, said in an interview. He was not involved with the advisory committee meeting. Flu activity this season has been low, he explained, so there are fewer cases his team can use to estimate vaccine efficacy. “If there’s some benefit, it’s hard for us to show that now,” he said.
Vaccine strains
The panel voted to include a A/Darwin/9/2021-like strain for the H3N2 component of the vaccine; this is changed from A/Cambodia/e0826360/2020. For the influenza B Victoria lineage component, the committee voted to include a B/Austria/1359417/2021-like virus, a swap from this year’s B/Washington/02/2019-like virus. These changes apply to the egg-based, cell-culture, and recombinant vaccines. Both new strains were included in WHO’s 2022 influenza vaccine strain recommendations for the southern hemisphere.
For the influenza A H1N1 component, the group also agreed to include a A/Victoria/2570/2019 (H1N1) pdm09-like virus for the egg-based vaccine and the A/Wisconsin/588/2019 (H1N1) pdm09-like virus for cell culture or recombinant vaccines. These strains were included for the 2021-2022 season. The panel also voted for the inclusion of a B/Phuket/3073/2013-like virus (B/Yamagata lineage) as the second influenza B strain for the quadrivalent egg-based, cell culture, or recombinant vaccines, which is unchanged from this flu season.
‘Sporadic’ flu activity
While there was an uptick in influenza activity this year compared to the 2020-2021 season, hospitalization rates are lower than in the four seasons preceding the pandemic (from 2016-2017 to 2019-2020). As of Feb. 26, the cumulative hospitalization rate for this flu season was 5.2 hospitalizations per 100,000 individuals. There have been eight pediatric deaths due to influenza so far this season, compared to one pediatric death reported to the CDC during the 2020-2021 flu season.
About 4.1% of specimens tested at clinical laboratories were positive for flu. Since Oct. 30, 2.7% of specimens have been positive for influenza this season. Nearly all viruses detected (97.7%) have been influenza A.
Lisa Grohskopf, MD, MPH, a medical officer in the influenza division at the CDC who presented the data at the meeting, described flu activity this season as “sporadic” and noted that activity is increasing in some areas of the country. According to CDC’s weekly influenza surveillance report, most states had minimal influenza-like illness (ILI) activity, although Arkansas, Idaho, Iowa, Kansas, Minnesota, and Utah had slightly higher ILI activity as of Feb. 26. Champaign-Urbana, Illinois; St. Cloud, Minnesota; and Brownwood, Texas, had the highest levels of flu activity in the country.
Low vaccine effectiveness
As of Jan. 22, results from the U.S. Flu VE network do not show statistically significant evidence that the flu vaccine is effective. Currently, the vaccine is estimated to be 8% effective against preventing influenza A infection (95% confidence interval, –31% to 36%) and 14% effective against preventing A/H3N2 infection (95% CI, –28% to 43%) for people aged 6 months and older.
The network did not have enough data to provide age-specific VE estimates or estimates of effectiveness against influenza B. This could be due to low flu activity relative to prepandemic years, Dr. Flannery said. Of the 2,758 individuals enrolled in the VE flu network this season, just 147 (5%) tested positive for the flu this season. This is the lowest positivity rate observed in the Flu VE network participants with respiratory illness over the past 10 flu seasons, Dr. Grohskopf noted. In comparison, estimates from the 2019 to 2020 season included 4,112 individuals, and 1,060 tested positive for flu.
“We are really at the bare minimum of what we can use for a flu vaccine effectiveness estimate,” Dr. Flannery said about the more recent data. The network was not able to produce any estimates about flu vaccine effectiveness for the 2020-2021 season because of historically low flu activity.
The Department of Defense also presented vaccine efficacy estimates for the 2021–2022 season. The vaccine has been 36% effective (95% CI, 28%-44%) against all strains of the virus, 33% effective against influenza A (95% CI, 24%-41%), 32% effective against A/H3N2 (95% CI, 3%-53%), and 59% effective against influenza B (95% CI, 42%-71%). These results are from a young, healthy adult population, Lieutenant Commander Courtney Gustin, DrPH, MSN, told the panel, and they may not be reflective of efficacy rates across all age groups.
Though these findings suggest there is low to no measurable benefit against influenza A, Dr. Flannery said the CDC still recommends getting the flu vaccine, as it can be protective against other circulating flu strains. “We have been able to demonstrate protection against other H3 [viruses], B viruses, and H1 viruses in the past,” he said. And as these results only show protection against mild disease, “there is still possibility that there’s benefit against more severe disease,” he added. Studies measuring effectiveness against more severe outcomes are not yet available.
A version of this article first appeared on Medscape.com.
Hernia recurrence has improved only slightly
, according to a new research letter published March 1 in JAMA. Patients who underwent minimally invasive hernia repair had a higher incidence of reoperation than those who underwent open repairs.
In the United States, surgeons perform more than 1 million hernia repairs each year, according to the U.S. Food and Drug Administration. Despite hernias being such a common condition, it is “not at the forefront of many research agendas,” senior author Dana Telem, MD, an associate professor and section chief of general surgery at University of Michigan Health in Ann Arbor, said in an interview
While many surgical outcomes are measured within 30 days of operation, recurrences generally happen within 2 to 5 years after repair, she said. The last study that looked at reoperations for hernia repair at 10 years was published in 2003 and found that about 20% of patients needed surgery for reoccurrence over a decade. “We don’t really have a good understanding of what happened after these operations,” she explained. “Without knowing that piece, it is hard to go back retrospectively and understand what is the right operation for the right person at the right time.”
To understand rates of reoperation for hernia reoccurrence in today’s U.S. population of older adults, Dr. Telem and colleagues sorted through Medicare claims data to find adult patients who had undergone ventral or incisional and umbilical hernia repair from January 1, 2007 through December 31, 2018. They identified a total of 175,735 patients, 162,292 that underwent ventral or incisional hernia repair and 13,443 that underwent umbilical hernia repair. The average age of patients was 68.9 years and 39.2% were men. Most patients were White (87.2%), 8.1% were Black, 1.9% were Hispanic, and 0.5% were Asian. Median follow-up was 5.3 years.
Over the 10-year study period, 25,061 patients required reoperation for hernia recurrence with an adjusted cumulative incidence of 16.1% (95% CI, 16.1% - 16.2%). Patients who underwent open repair had a lower incidence of recurrence over 10 years than those who underwent minimally invasive repair for all hernia types (Table 1).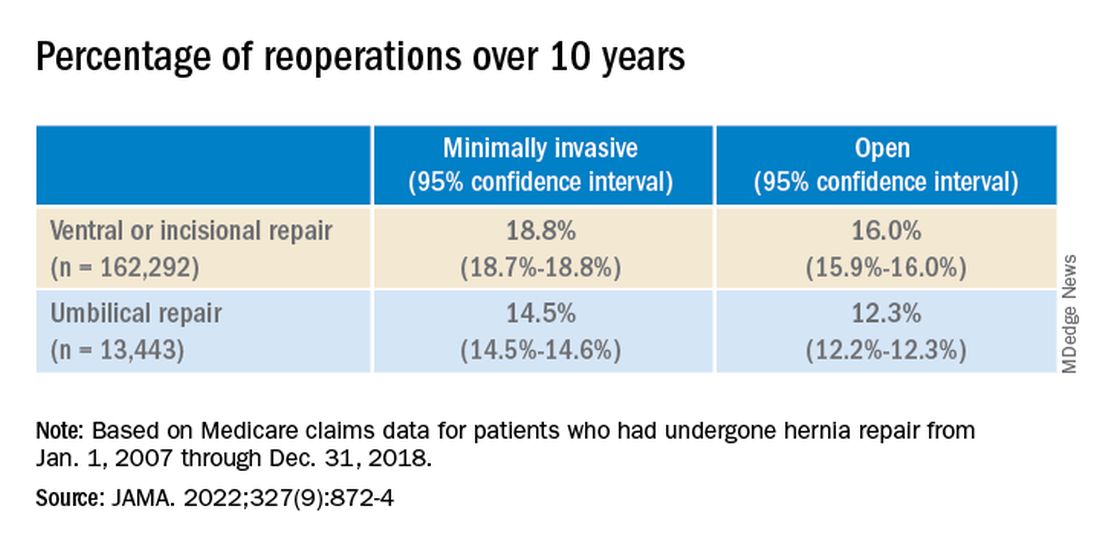
While it appears that hernia recurrence and reoperation have only marginally improved from 2003 to today, Vedra Augenstein, MD, an associate professor of surgery at the Atrium Health General & Complex Abdominal Surgery facility in Charlotte, N.C., suspects there is more to the story. “I think the reason it hasn’t gotten a whole lot better is just because we are operating on much tougher cases than we used to,” she said in an interview. “The way we are fixing hernias has changed and patients are being optimized differently.” Dr. Augenstein was not involved with the research.
To better understand how recurrence has changed over time, there needs to be more data about the comorbidities of patients, the techniques employed, and the meshes used in these surgeries, she said. Those numbers are not available in the published JAMA research letter, but Dr. Telem and colleagues will be submitting an article about this work with greater details.
Dr. Augenstein was also surprised that minimally invasive surgeries had higher incidences of reoperation for recurrence compared to open hernia surgeries. “I would think that patients who had minimally invasive repairs would actually have a lower chance of having postoperative complications because of wound issues,” she said. “Literature has shown that the recurrence rate is lower [in minimally invasive surgeries] because of fewer surgical site infections.”
While Dr. Telem also considers this research letter to be the first step in understanding modern hernia surgery outcomes, it is also a reminder that there is room for improvement in hernia repair surgeries. This includes advising patients on risk factors that may make them more likely to have a hernia recurrence, such as obesity, smoking, and diabetes, she added. “If we know it’s not a perfect science, then we have to do everything that we can upfront to help those numbers.”
Dr. Telem has reported receiving grants from the Agency for Healthcare Research and Quality and consulting fees from Medtronic. Dr. Augenstein has reported consulting for Intuitive Surgical, Medtronic, Allergan, Acelity, Vicarious Surgical, and Bard Pharmaceuticals and has received honoraria for speaking from Medtronic, Allergan, Intuitive Surgical, Acelity, and WL Gore.
A version of this article first appeared on Medscape.com.
, according to a new research letter published March 1 in JAMA. Patients who underwent minimally invasive hernia repair had a higher incidence of reoperation than those who underwent open repairs.
In the United States, surgeons perform more than 1 million hernia repairs each year, according to the U.S. Food and Drug Administration. Despite hernias being such a common condition, it is “not at the forefront of many research agendas,” senior author Dana Telem, MD, an associate professor and section chief of general surgery at University of Michigan Health in Ann Arbor, said in an interview
While many surgical outcomes are measured within 30 days of operation, recurrences generally happen within 2 to 5 years after repair, she said. The last study that looked at reoperations for hernia repair at 10 years was published in 2003 and found that about 20% of patients needed surgery for reoccurrence over a decade. “We don’t really have a good understanding of what happened after these operations,” she explained. “Without knowing that piece, it is hard to go back retrospectively and understand what is the right operation for the right person at the right time.”
To understand rates of reoperation for hernia reoccurrence in today’s U.S. population of older adults, Dr. Telem and colleagues sorted through Medicare claims data to find adult patients who had undergone ventral or incisional and umbilical hernia repair from January 1, 2007 through December 31, 2018. They identified a total of 175,735 patients, 162,292 that underwent ventral or incisional hernia repair and 13,443 that underwent umbilical hernia repair. The average age of patients was 68.9 years and 39.2% were men. Most patients were White (87.2%), 8.1% were Black, 1.9% were Hispanic, and 0.5% were Asian. Median follow-up was 5.3 years.
Over the 10-year study period, 25,061 patients required reoperation for hernia recurrence with an adjusted cumulative incidence of 16.1% (95% CI, 16.1% - 16.2%). Patients who underwent open repair had a lower incidence of recurrence over 10 years than those who underwent minimally invasive repair for all hernia types (Table 1).
While it appears that hernia recurrence and reoperation have only marginally improved from 2003 to today, Vedra Augenstein, MD, an associate professor of surgery at the Atrium Health General & Complex Abdominal Surgery facility in Charlotte, N.C., suspects there is more to the story. “I think the reason it hasn’t gotten a whole lot better is just because we are operating on much tougher cases than we used to,” she said in an interview. “The way we are fixing hernias has changed and patients are being optimized differently.” Dr. Augenstein was not involved with the research.
To better understand how recurrence has changed over time, there needs to be more data about the comorbidities of patients, the techniques employed, and the meshes used in these surgeries, she said. Those numbers are not available in the published JAMA research letter, but Dr. Telem and colleagues will be submitting an article about this work with greater details.
Dr. Augenstein was also surprised that minimally invasive surgeries had higher incidences of reoperation for recurrence compared to open hernia surgeries. “I would think that patients who had minimally invasive repairs would actually have a lower chance of having postoperative complications because of wound issues,” she said. “Literature has shown that the recurrence rate is lower [in minimally invasive surgeries] because of fewer surgical site infections.”
While Dr. Telem also considers this research letter to be the first step in understanding modern hernia surgery outcomes, it is also a reminder that there is room for improvement in hernia repair surgeries. This includes advising patients on risk factors that may make them more likely to have a hernia recurrence, such as obesity, smoking, and diabetes, she added. “If we know it’s not a perfect science, then we have to do everything that we can upfront to help those numbers.”
Dr. Telem has reported receiving grants from the Agency for Healthcare Research and Quality and consulting fees from Medtronic. Dr. Augenstein has reported consulting for Intuitive Surgical, Medtronic, Allergan, Acelity, Vicarious Surgical, and Bard Pharmaceuticals and has received honoraria for speaking from Medtronic, Allergan, Intuitive Surgical, Acelity, and WL Gore.
A version of this article first appeared on Medscape.com.
, according to a new research letter published March 1 in JAMA. Patients who underwent minimally invasive hernia repair had a higher incidence of reoperation than those who underwent open repairs.
In the United States, surgeons perform more than 1 million hernia repairs each year, according to the U.S. Food and Drug Administration. Despite hernias being such a common condition, it is “not at the forefront of many research agendas,” senior author Dana Telem, MD, an associate professor and section chief of general surgery at University of Michigan Health in Ann Arbor, said in an interview
While many surgical outcomes are measured within 30 days of operation, recurrences generally happen within 2 to 5 years after repair, she said. The last study that looked at reoperations for hernia repair at 10 years was published in 2003 and found that about 20% of patients needed surgery for reoccurrence over a decade. “We don’t really have a good understanding of what happened after these operations,” she explained. “Without knowing that piece, it is hard to go back retrospectively and understand what is the right operation for the right person at the right time.”
To understand rates of reoperation for hernia reoccurrence in today’s U.S. population of older adults, Dr. Telem and colleagues sorted through Medicare claims data to find adult patients who had undergone ventral or incisional and umbilical hernia repair from January 1, 2007 through December 31, 2018. They identified a total of 175,735 patients, 162,292 that underwent ventral or incisional hernia repair and 13,443 that underwent umbilical hernia repair. The average age of patients was 68.9 years and 39.2% were men. Most patients were White (87.2%), 8.1% were Black, 1.9% were Hispanic, and 0.5% were Asian. Median follow-up was 5.3 years.
Over the 10-year study period, 25,061 patients required reoperation for hernia recurrence with an adjusted cumulative incidence of 16.1% (95% CI, 16.1% - 16.2%). Patients who underwent open repair had a lower incidence of recurrence over 10 years than those who underwent minimally invasive repair for all hernia types (Table 1).
While it appears that hernia recurrence and reoperation have only marginally improved from 2003 to today, Vedra Augenstein, MD, an associate professor of surgery at the Atrium Health General & Complex Abdominal Surgery facility in Charlotte, N.C., suspects there is more to the story. “I think the reason it hasn’t gotten a whole lot better is just because we are operating on much tougher cases than we used to,” she said in an interview. “The way we are fixing hernias has changed and patients are being optimized differently.” Dr. Augenstein was not involved with the research.
To better understand how recurrence has changed over time, there needs to be more data about the comorbidities of patients, the techniques employed, and the meshes used in these surgeries, she said. Those numbers are not available in the published JAMA research letter, but Dr. Telem and colleagues will be submitting an article about this work with greater details.
Dr. Augenstein was also surprised that minimally invasive surgeries had higher incidences of reoperation for recurrence compared to open hernia surgeries. “I would think that patients who had minimally invasive repairs would actually have a lower chance of having postoperative complications because of wound issues,” she said. “Literature has shown that the recurrence rate is lower [in minimally invasive surgeries] because of fewer surgical site infections.”
While Dr. Telem also considers this research letter to be the first step in understanding modern hernia surgery outcomes, it is also a reminder that there is room for improvement in hernia repair surgeries. This includes advising patients on risk factors that may make them more likely to have a hernia recurrence, such as obesity, smoking, and diabetes, she added. “If we know it’s not a perfect science, then we have to do everything that we can upfront to help those numbers.”
Dr. Telem has reported receiving grants from the Agency for Healthcare Research and Quality and consulting fees from Medtronic. Dr. Augenstein has reported consulting for Intuitive Surgical, Medtronic, Allergan, Acelity, Vicarious Surgical, and Bard Pharmaceuticals and has received honoraria for speaking from Medtronic, Allergan, Intuitive Surgical, Acelity, and WL Gore.
A version of this article first appeared on Medscape.com.
FROM JAMA
EMA recommends PreHevbri hepatitis B vaccine for approval
The European Medicines Agency’s (EMA’s) human medicines committee has recommended approval of a hepatitis B vaccine for adults.
The agency’s Committee for Medicinal Products for Human Use (CHMP) granted a positive opinion for PreHevbri on Feb. 24 for active immunization against hepatitis B virus (HBV) infection. PreHevbri (PreHevBrio in the United States and Sci-B-Vac in Israel) received approval from the Food and Drug Administration on Nov. 30, 2021. The vaccine is produced by VBI Vaccines (Delaware) Inc., based in Cambridge, Mass.
The World Health Organization estimates that more than 290 million people globally are infected with HBV. HBV is the leading cause of liver disease, and an estimated 900,000 people die every year from complications from chronic HBV infection, according to a VBI Vaccine press release. A 2019 report from the European Centre for Disease Prevention and Control found that adults in the European Union aged 35-44 had the highest rates of acute infections with HBV, and people aged 25-34 had the highest rate of chronic HBV infections. Vaccination programs are key interventions in preventing transmission of the virus, the report noted.
PreHevbri is a hepatitis B vaccine composed of three surface antigens of the hepatitis B virus. The vaccine is administered via injection in three doses on a 0-, 1-, and 6-month schedule and is indicated for use in adults aged 18 years and older.
The CHMP recommendation was based on data from a safety and immunogenicity study, which included 1,607 participants aged 18 and older, and a lot-to-lot study, which included 2,838 adults aged 18-45, according the VBI vaccine press release.
The recommendation will now be reviewed by the European Commission. If approved, PreHevbri will be the only three-antigen HBV vaccine for adults approved in the European Union.
A version of this article first appeared on Medscape.com.
The European Medicines Agency’s (EMA’s) human medicines committee has recommended approval of a hepatitis B vaccine for adults.
The agency’s Committee for Medicinal Products for Human Use (CHMP) granted a positive opinion for PreHevbri on Feb. 24 for active immunization against hepatitis B virus (HBV) infection. PreHevbri (PreHevBrio in the United States and Sci-B-Vac in Israel) received approval from the Food and Drug Administration on Nov. 30, 2021. The vaccine is produced by VBI Vaccines (Delaware) Inc., based in Cambridge, Mass.
The World Health Organization estimates that more than 290 million people globally are infected with HBV. HBV is the leading cause of liver disease, and an estimated 900,000 people die every year from complications from chronic HBV infection, according to a VBI Vaccine press release. A 2019 report from the European Centre for Disease Prevention and Control found that adults in the European Union aged 35-44 had the highest rates of acute infections with HBV, and people aged 25-34 had the highest rate of chronic HBV infections. Vaccination programs are key interventions in preventing transmission of the virus, the report noted.
PreHevbri is a hepatitis B vaccine composed of three surface antigens of the hepatitis B virus. The vaccine is administered via injection in three doses on a 0-, 1-, and 6-month schedule and is indicated for use in adults aged 18 years and older.
The CHMP recommendation was based on data from a safety and immunogenicity study, which included 1,607 participants aged 18 and older, and a lot-to-lot study, which included 2,838 adults aged 18-45, according the VBI vaccine press release.
The recommendation will now be reviewed by the European Commission. If approved, PreHevbri will be the only three-antigen HBV vaccine for adults approved in the European Union.
A version of this article first appeared on Medscape.com.
The European Medicines Agency’s (EMA’s) human medicines committee has recommended approval of a hepatitis B vaccine for adults.
The agency’s Committee for Medicinal Products for Human Use (CHMP) granted a positive opinion for PreHevbri on Feb. 24 for active immunization against hepatitis B virus (HBV) infection. PreHevbri (PreHevBrio in the United States and Sci-B-Vac in Israel) received approval from the Food and Drug Administration on Nov. 30, 2021. The vaccine is produced by VBI Vaccines (Delaware) Inc., based in Cambridge, Mass.
The World Health Organization estimates that more than 290 million people globally are infected with HBV. HBV is the leading cause of liver disease, and an estimated 900,000 people die every year from complications from chronic HBV infection, according to a VBI Vaccine press release. A 2019 report from the European Centre for Disease Prevention and Control found that adults in the European Union aged 35-44 had the highest rates of acute infections with HBV, and people aged 25-34 had the highest rate of chronic HBV infections. Vaccination programs are key interventions in preventing transmission of the virus, the report noted.
PreHevbri is a hepatitis B vaccine composed of three surface antigens of the hepatitis B virus. The vaccine is administered via injection in three doses on a 0-, 1-, and 6-month schedule and is indicated for use in adults aged 18 years and older.
The CHMP recommendation was based on data from a safety and immunogenicity study, which included 1,607 participants aged 18 and older, and a lot-to-lot study, which included 2,838 adults aged 18-45, according the VBI vaccine press release.
The recommendation will now be reviewed by the European Commission. If approved, PreHevbri will be the only three-antigen HBV vaccine for adults approved in the European Union.
A version of this article first appeared on Medscape.com.
Elective surgery should be delayed 7 weeks after COVID-19 infection for unvaccinated patients, statement recommends
.
For patients fully vaccinated against COVID-19 with breakthrough infections, there is no consensus on how vaccination affects the time between COVID-19 infection and elective surgery. Clinicians should use their clinical judgment to schedule procedures, said Randall M. Clark, MD, president of the American Society of Anesthesiologists (ASA). “We need all physicians, anesthesiologists, surgeons, and others to base their decision to go ahead with elective surgery on the patient’s symptoms, their need for the procedure, and whether delays could cause other problems with their health,” he said in an interview.
Prior to these updated recommendations, which were published Feb. 22, the ASA and the APSF recommended a 4-week gap between COVID-19 diagnosis and elective surgery for asymptomatic or mild cases, regardless of a patient’s vaccination status.
Extending the wait time from 4 to 7 weeks was based on a multination study conducted in October 2020 following more than 140,000 surgical patients. Patients with previous COVID-19 infection had an increased risk for complications and death in elective surgery for up to 6 weeks following their diagnosis, compared with patients without COVID-19. Additional research in the United States found that patients with a preoperative COVID diagnosis were at higher risk for postoperative complications of respiratory failure for up to 4 weeks after diagnosis and postoperative pneumonia complications for up to 8 weeks after diagnosis.
Because these studies were conducted in unvaccinated populations or those with low vaccination rates, and preliminary data suggest vaccinated patients with breakthrough infections may have a lower risk for complications and death postinfection, “we felt that it was prudent to just make recommendations specific to unvaccinated patients,” Dr. Clark added.
Although this guidance is “very helpful” in that it summarizes the currently available research to give evidence-based recommendations, the 7-week wait time is a “very conservative estimate,” Brent Matthews, MD, surgeon-in-chief of the surgery care division of Atrium Health, Charlotte, N.C., told this news organization. At Atrium Health, surgery is scheduled at least 21 days after a patient’s COVID-19 diagnosis, regardless of their vaccination status, Dr. Matthews said.
The studies currently available were conducted earlier in the pandemic, when a different variant was prevalent, Dr. Matthews explained. The Omicron variant is currently the most prevalent COVID-19 variant and is less virulent than earlier strains of the virus. The joint statement does note that there is currently “no robust data” on patients infected with the Delta or Omicron variants of COVID-19, and that “the Omicron variant causes less severe disease and is more likely to reside in the oro- and nasopharynx without infiltration and damage to the lungs.”
Still, the new recommendations are a reminder to re-evaluate the potential complications from surgery for previously infected patients and to consider what comorbidities might make them more vulnerable, Dr. Matthews said. “The real power of the joint statement is to get people to ensure that they make an assessment of every patient that comes in front of them who has had a recent positive COVID test.”
A version of this article first appeared on Medscape.com.
.
For patients fully vaccinated against COVID-19 with breakthrough infections, there is no consensus on how vaccination affects the time between COVID-19 infection and elective surgery. Clinicians should use their clinical judgment to schedule procedures, said Randall M. Clark, MD, president of the American Society of Anesthesiologists (ASA). “We need all physicians, anesthesiologists, surgeons, and others to base their decision to go ahead with elective surgery on the patient’s symptoms, their need for the procedure, and whether delays could cause other problems with their health,” he said in an interview.
Prior to these updated recommendations, which were published Feb. 22, the ASA and the APSF recommended a 4-week gap between COVID-19 diagnosis and elective surgery for asymptomatic or mild cases, regardless of a patient’s vaccination status.
Extending the wait time from 4 to 7 weeks was based on a multination study conducted in October 2020 following more than 140,000 surgical patients. Patients with previous COVID-19 infection had an increased risk for complications and death in elective surgery for up to 6 weeks following their diagnosis, compared with patients without COVID-19. Additional research in the United States found that patients with a preoperative COVID diagnosis were at higher risk for postoperative complications of respiratory failure for up to 4 weeks after diagnosis and postoperative pneumonia complications for up to 8 weeks after diagnosis.
Because these studies were conducted in unvaccinated populations or those with low vaccination rates, and preliminary data suggest vaccinated patients with breakthrough infections may have a lower risk for complications and death postinfection, “we felt that it was prudent to just make recommendations specific to unvaccinated patients,” Dr. Clark added.
Although this guidance is “very helpful” in that it summarizes the currently available research to give evidence-based recommendations, the 7-week wait time is a “very conservative estimate,” Brent Matthews, MD, surgeon-in-chief of the surgery care division of Atrium Health, Charlotte, N.C., told this news organization. At Atrium Health, surgery is scheduled at least 21 days after a patient’s COVID-19 diagnosis, regardless of their vaccination status, Dr. Matthews said.
The studies currently available were conducted earlier in the pandemic, when a different variant was prevalent, Dr. Matthews explained. The Omicron variant is currently the most prevalent COVID-19 variant and is less virulent than earlier strains of the virus. The joint statement does note that there is currently “no robust data” on patients infected with the Delta or Omicron variants of COVID-19, and that “the Omicron variant causes less severe disease and is more likely to reside in the oro- and nasopharynx without infiltration and damage to the lungs.”
Still, the new recommendations are a reminder to re-evaluate the potential complications from surgery for previously infected patients and to consider what comorbidities might make them more vulnerable, Dr. Matthews said. “The real power of the joint statement is to get people to ensure that they make an assessment of every patient that comes in front of them who has had a recent positive COVID test.”
A version of this article first appeared on Medscape.com.
.
For patients fully vaccinated against COVID-19 with breakthrough infections, there is no consensus on how vaccination affects the time between COVID-19 infection and elective surgery. Clinicians should use their clinical judgment to schedule procedures, said Randall M. Clark, MD, president of the American Society of Anesthesiologists (ASA). “We need all physicians, anesthesiologists, surgeons, and others to base their decision to go ahead with elective surgery on the patient’s symptoms, their need for the procedure, and whether delays could cause other problems with their health,” he said in an interview.
Prior to these updated recommendations, which were published Feb. 22, the ASA and the APSF recommended a 4-week gap between COVID-19 diagnosis and elective surgery for asymptomatic or mild cases, regardless of a patient’s vaccination status.
Extending the wait time from 4 to 7 weeks was based on a multination study conducted in October 2020 following more than 140,000 surgical patients. Patients with previous COVID-19 infection had an increased risk for complications and death in elective surgery for up to 6 weeks following their diagnosis, compared with patients without COVID-19. Additional research in the United States found that patients with a preoperative COVID diagnosis were at higher risk for postoperative complications of respiratory failure for up to 4 weeks after diagnosis and postoperative pneumonia complications for up to 8 weeks after diagnosis.
Because these studies were conducted in unvaccinated populations or those with low vaccination rates, and preliminary data suggest vaccinated patients with breakthrough infections may have a lower risk for complications and death postinfection, “we felt that it was prudent to just make recommendations specific to unvaccinated patients,” Dr. Clark added.
Although this guidance is “very helpful” in that it summarizes the currently available research to give evidence-based recommendations, the 7-week wait time is a “very conservative estimate,” Brent Matthews, MD, surgeon-in-chief of the surgery care division of Atrium Health, Charlotte, N.C., told this news organization. At Atrium Health, surgery is scheduled at least 21 days after a patient’s COVID-19 diagnosis, regardless of their vaccination status, Dr. Matthews said.
The studies currently available were conducted earlier in the pandemic, when a different variant was prevalent, Dr. Matthews explained. The Omicron variant is currently the most prevalent COVID-19 variant and is less virulent than earlier strains of the virus. The joint statement does note that there is currently “no robust data” on patients infected with the Delta or Omicron variants of COVID-19, and that “the Omicron variant causes less severe disease and is more likely to reside in the oro- and nasopharynx without infiltration and damage to the lungs.”
Still, the new recommendations are a reminder to re-evaluate the potential complications from surgery for previously infected patients and to consider what comorbidities might make them more vulnerable, Dr. Matthews said. “The real power of the joint statement is to get people to ensure that they make an assessment of every patient that comes in front of them who has had a recent positive COVID test.”
A version of this article first appeared on Medscape.com.
FDA approves 2-month dosing of injectable HIV drug Cabenuva
Cabenuva was first approved by the FDA in January 2021 to be administered once monthly to treat HIV-1 infection in virologically suppressed adults. The medication was the first injectable complete antiretroviral regimen approved by the FDA.
Cabenuva can replace a current treatment in virologically suppressed adults on a stable antiretroviral regimen with no history of treatment failure and no known or suspected resistance to rilpivirine and cabotegravir, the Janssen Pharmaceutical Companies of Johnson & Johnson said in a press release. Janssen and ViiV Healthcare codeveloped the injectable antiretroviral medication Cabenuva.
The expanded label approval “marks an important step forward in advancing the treatment landscape for people living with HIV,” said Candice Long, the president of infectious diseases and vaccines at Janssen Therapeutics, in a Feb. 1 press release. “With this milestone, adults living with HIV have a treatment option that further reduces the frequency of medication.”
This expanded approval was based on global clinical trial of 1,045 adults with HIV-1, which found Cabenuva administered every 8 weeks (3 mL dose of both cabotegravir and rilpivirine) to be noninferior to the 4-week regimen (2 mL dose of both medicines). At week 48 of the trial, the proportion of participants with viral loads above 50 copies per milliliter was 1.7% in the 2-month arm and 1.0% in the 1-month arm. The study found that rates of virological suppression were similar for both the 1-month and 2-month regimens (93.5% and 94.3%, respectively).
The most common side effects were injection site reactions, pyrexia, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. Adverse reactions reported in individuals receiving the regimen every 2 months or once monthly were similar. Cabenuva is contraindicated for patients with a hypersensitivity reaction to cabotegravir or rilpivirine or for those receiving carbamazepine, oxcarbazepine, phenobarbital, phenytoin, rifabutin, rifampin, rifapentine, St. John’s wort, and more than one dose of systemic dexamethasone.
A version of this article first appeared on Medscape.com.
Cabenuva was first approved by the FDA in January 2021 to be administered once monthly to treat HIV-1 infection in virologically suppressed adults. The medication was the first injectable complete antiretroviral regimen approved by the FDA.
Cabenuva can replace a current treatment in virologically suppressed adults on a stable antiretroviral regimen with no history of treatment failure and no known or suspected resistance to rilpivirine and cabotegravir, the Janssen Pharmaceutical Companies of Johnson & Johnson said in a press release. Janssen and ViiV Healthcare codeveloped the injectable antiretroviral medication Cabenuva.
The expanded label approval “marks an important step forward in advancing the treatment landscape for people living with HIV,” said Candice Long, the president of infectious diseases and vaccines at Janssen Therapeutics, in a Feb. 1 press release. “With this milestone, adults living with HIV have a treatment option that further reduces the frequency of medication.”
This expanded approval was based on global clinical trial of 1,045 adults with HIV-1, which found Cabenuva administered every 8 weeks (3 mL dose of both cabotegravir and rilpivirine) to be noninferior to the 4-week regimen (2 mL dose of both medicines). At week 48 of the trial, the proportion of participants with viral loads above 50 copies per milliliter was 1.7% in the 2-month arm and 1.0% in the 1-month arm. The study found that rates of virological suppression were similar for both the 1-month and 2-month regimens (93.5% and 94.3%, respectively).
The most common side effects were injection site reactions, pyrexia, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. Adverse reactions reported in individuals receiving the regimen every 2 months or once monthly were similar. Cabenuva is contraindicated for patients with a hypersensitivity reaction to cabotegravir or rilpivirine or for those receiving carbamazepine, oxcarbazepine, phenobarbital, phenytoin, rifabutin, rifampin, rifapentine, St. John’s wort, and more than one dose of systemic dexamethasone.
A version of this article first appeared on Medscape.com.
Cabenuva was first approved by the FDA in January 2021 to be administered once monthly to treat HIV-1 infection in virologically suppressed adults. The medication was the first injectable complete antiretroviral regimen approved by the FDA.
Cabenuva can replace a current treatment in virologically suppressed adults on a stable antiretroviral regimen with no history of treatment failure and no known or suspected resistance to rilpivirine and cabotegravir, the Janssen Pharmaceutical Companies of Johnson & Johnson said in a press release. Janssen and ViiV Healthcare codeveloped the injectable antiretroviral medication Cabenuva.
The expanded label approval “marks an important step forward in advancing the treatment landscape for people living with HIV,” said Candice Long, the president of infectious diseases and vaccines at Janssen Therapeutics, in a Feb. 1 press release. “With this milestone, adults living with HIV have a treatment option that further reduces the frequency of medication.”
This expanded approval was based on global clinical trial of 1,045 adults with HIV-1, which found Cabenuva administered every 8 weeks (3 mL dose of both cabotegravir and rilpivirine) to be noninferior to the 4-week regimen (2 mL dose of both medicines). At week 48 of the trial, the proportion of participants with viral loads above 50 copies per milliliter was 1.7% in the 2-month arm and 1.0% in the 1-month arm. The study found that rates of virological suppression were similar for both the 1-month and 2-month regimens (93.5% and 94.3%, respectively).
The most common side effects were injection site reactions, pyrexia, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. Adverse reactions reported in individuals receiving the regimen every 2 months or once monthly were similar. Cabenuva is contraindicated for patients with a hypersensitivity reaction to cabotegravir or rilpivirine or for those receiving carbamazepine, oxcarbazepine, phenobarbital, phenytoin, rifabutin, rifampin, rifapentine, St. John’s wort, and more than one dose of systemic dexamethasone.
A version of this article first appeared on Medscape.com.
Orthopedists rank third in malpractice suits, survey finds
according to the Medscape Orthopedist Malpractice Report 2021.
Orthopedists ranked third among specialists most likely to be sued, surpassed only by plastic surgeons and general surgeons (both 83%). In comparison, just over half of physicians across all specialties (51%) reported being named in lawsuit. More than one-third of orthopedists (34%) said they had been individually named in a suit, whereas just 14% of all specialists were named individually.
More than half (54%) of orthopedists said they were sued over complications from treatment or surgery. The second-most common reason orthopedists were sued was poor outcome/disease progression (30%), followed by failure to diagnose/delayed diagnosis (21%), failure to treat/delayed treatment (13%), and abnormal injury (9%).
This new report was compiled from an online survey including more than 4,300 physicians from 29 specialties. The survey was available from May 21 to Aug. 28, 2021, and included 250 orthopedists and orthopedic surgeons. Most respondents (62%) had practiced orthopedics for more than 25 years and 60% were aged 60 years or older.
Orthopedists tended to pay more for malpractice insurance than do other specialists. Less than one-third of orthopedists (31%) reported a premium under $20,000 per year, compared with 52% of all specialists. The most common premium for orthopedists was $30,000 or more (29%), whereas only 11% of all specialists reported paying a similar premium.
Nearly 9 out of 10 (89%) of orthopedists said they were “very surprised” or “somewhat surprised” by the malpractice suit. In some of these cases, the physician never personally treated the patient. Wrote one respondent: “I was part of a group of physicians and got dragged into the suit.” The vast majority of orthopedists (82%) said the suit was not warranted, which was similar to responses for physicians as a whole (83%).
Most commonly, orthopedists said lawsuits were settled before trial (34%). The second-most common outcome was the judge and jury deciding in the respondent’s favor (16%), followed by the plaintiff voluntarily dismissing the suit prior to trial (8%), and the respondent being dismissed from the suit in the first few months (8%). Very few (2%) said the judge or jury ruled in the patient’s favor, and 9% of respondents said the case was ongoing.
Most orthopedists reported that cases lasted between 1 and 2 years (41%) and 29% said a lawsuit took 3-5 years. If the plaintiff did receive a monetary award, 42% of physicians reported paying under $100,000, and 30% paid less than $500,000. This is similar to reports from other specialties, though more patients in orthopedic cases received payments under $1 million, compared with other specialties (21% vs. 15%).
More than three-quarters of orthopedists (76%) said that the lawsuit did not negatively affect their career, and more than half (52%) said they did not undergo any attitude or career changes after the suit. More orthopedists than other specialists (31% vs. 24%) did say that they trusted patients less.
When asked if they would do anything differently, one-third (33%) of orthopedists said their actions would remain the same, compared with 43% of the general physician pool. One-quarter of orthopedists said they would have not taken on the patient in the first place, and 14% noted they would have referred to another physician.
A version of this article first appeared on Medscape.com.
according to the Medscape Orthopedist Malpractice Report 2021.
Orthopedists ranked third among specialists most likely to be sued, surpassed only by plastic surgeons and general surgeons (both 83%). In comparison, just over half of physicians across all specialties (51%) reported being named in lawsuit. More than one-third of orthopedists (34%) said they had been individually named in a suit, whereas just 14% of all specialists were named individually.
More than half (54%) of orthopedists said they were sued over complications from treatment or surgery. The second-most common reason orthopedists were sued was poor outcome/disease progression (30%), followed by failure to diagnose/delayed diagnosis (21%), failure to treat/delayed treatment (13%), and abnormal injury (9%).
This new report was compiled from an online survey including more than 4,300 physicians from 29 specialties. The survey was available from May 21 to Aug. 28, 2021, and included 250 orthopedists and orthopedic surgeons. Most respondents (62%) had practiced orthopedics for more than 25 years and 60% were aged 60 years or older.
Orthopedists tended to pay more for malpractice insurance than do other specialists. Less than one-third of orthopedists (31%) reported a premium under $20,000 per year, compared with 52% of all specialists. The most common premium for orthopedists was $30,000 or more (29%), whereas only 11% of all specialists reported paying a similar premium.
Nearly 9 out of 10 (89%) of orthopedists said they were “very surprised” or “somewhat surprised” by the malpractice suit. In some of these cases, the physician never personally treated the patient. Wrote one respondent: “I was part of a group of physicians and got dragged into the suit.” The vast majority of orthopedists (82%) said the suit was not warranted, which was similar to responses for physicians as a whole (83%).
Most commonly, orthopedists said lawsuits were settled before trial (34%). The second-most common outcome was the judge and jury deciding in the respondent’s favor (16%), followed by the plaintiff voluntarily dismissing the suit prior to trial (8%), and the respondent being dismissed from the suit in the first few months (8%). Very few (2%) said the judge or jury ruled in the patient’s favor, and 9% of respondents said the case was ongoing.
Most orthopedists reported that cases lasted between 1 and 2 years (41%) and 29% said a lawsuit took 3-5 years. If the plaintiff did receive a monetary award, 42% of physicians reported paying under $100,000, and 30% paid less than $500,000. This is similar to reports from other specialties, though more patients in orthopedic cases received payments under $1 million, compared with other specialties (21% vs. 15%).
More than three-quarters of orthopedists (76%) said that the lawsuit did not negatively affect their career, and more than half (52%) said they did not undergo any attitude or career changes after the suit. More orthopedists than other specialists (31% vs. 24%) did say that they trusted patients less.
When asked if they would do anything differently, one-third (33%) of orthopedists said their actions would remain the same, compared with 43% of the general physician pool. One-quarter of orthopedists said they would have not taken on the patient in the first place, and 14% noted they would have referred to another physician.
A version of this article first appeared on Medscape.com.
according to the Medscape Orthopedist Malpractice Report 2021.
Orthopedists ranked third among specialists most likely to be sued, surpassed only by plastic surgeons and general surgeons (both 83%). In comparison, just over half of physicians across all specialties (51%) reported being named in lawsuit. More than one-third of orthopedists (34%) said they had been individually named in a suit, whereas just 14% of all specialists were named individually.
More than half (54%) of orthopedists said they were sued over complications from treatment or surgery. The second-most common reason orthopedists were sued was poor outcome/disease progression (30%), followed by failure to diagnose/delayed diagnosis (21%), failure to treat/delayed treatment (13%), and abnormal injury (9%).
This new report was compiled from an online survey including more than 4,300 physicians from 29 specialties. The survey was available from May 21 to Aug. 28, 2021, and included 250 orthopedists and orthopedic surgeons. Most respondents (62%) had practiced orthopedics for more than 25 years and 60% were aged 60 years or older.
Orthopedists tended to pay more for malpractice insurance than do other specialists. Less than one-third of orthopedists (31%) reported a premium under $20,000 per year, compared with 52% of all specialists. The most common premium for orthopedists was $30,000 or more (29%), whereas only 11% of all specialists reported paying a similar premium.
Nearly 9 out of 10 (89%) of orthopedists said they were “very surprised” or “somewhat surprised” by the malpractice suit. In some of these cases, the physician never personally treated the patient. Wrote one respondent: “I was part of a group of physicians and got dragged into the suit.” The vast majority of orthopedists (82%) said the suit was not warranted, which was similar to responses for physicians as a whole (83%).
Most commonly, orthopedists said lawsuits were settled before trial (34%). The second-most common outcome was the judge and jury deciding in the respondent’s favor (16%), followed by the plaintiff voluntarily dismissing the suit prior to trial (8%), and the respondent being dismissed from the suit in the first few months (8%). Very few (2%) said the judge or jury ruled in the patient’s favor, and 9% of respondents said the case was ongoing.
Most orthopedists reported that cases lasted between 1 and 2 years (41%) and 29% said a lawsuit took 3-5 years. If the plaintiff did receive a monetary award, 42% of physicians reported paying under $100,000, and 30% paid less than $500,000. This is similar to reports from other specialties, though more patients in orthopedic cases received payments under $1 million, compared with other specialties (21% vs. 15%).
More than three-quarters of orthopedists (76%) said that the lawsuit did not negatively affect their career, and more than half (52%) said they did not undergo any attitude or career changes after the suit. More orthopedists than other specialists (31% vs. 24%) did say that they trusted patients less.
When asked if they would do anything differently, one-third (33%) of orthopedists said their actions would remain the same, compared with 43% of the general physician pool. One-quarter of orthopedists said they would have not taken on the patient in the first place, and 14% noted they would have referred to another physician.
A version of this article first appeared on Medscape.com.