User login
New secondary fracture–prevention recommendations carry simple messages
Ensuring that older adults who have experienced a hip or vertebral fracture understand they likely have osteoporosis, and offering prompt drug treatment for the condition, are among five fundamental
The recommendations were announced at the annual meeting of the American Society for Bone and Mineral Research (ASBMR) in Montreal. The 40-member group that developed the recommendations, called the ASBMR Secondary Fracture Prevention Initiative Coalition, includes the American Association of Clinical Endocrinologists/American College of Endocrinology, the Endocrine Society, the American College of Rheumatology, the American College of Physicians, the American Geriatrics Society, the American Academy of Physical Medicine and Rehabilitation, and the American Academy of Orthopaedic Surgeons.
Additional fundamental recommendations from the Coalition advised ensuring that patients’ primary health care providers are aware of the fracture, regularly assessing the risk of falls, and routinely reevaluating patients who are being treated for osteoporosis. These suggestions were developed in response to growing evidence of a rising trend in osteoporosis patients not being prescribed appropriate medications or not taking them, the ASBMR said.
“The very simple message is if you’ve got somebody who has had a hip fracture or a vertebral fracture, that needs secondary prevention just like somebody who’s had an MI needs to be on a statin and a beta blocker,” said coalition cochair Sundeep Khosla, MD, a past president of the ASBMR and director of the Center for Clinical and Translational Science at the Mayo Clinic, Rochester, Minn. “You can’t ignore the fracture because it’s not immediately life-threatening. Down the road they’re going to have another hip fracture if nothing is done.”
Only 23% of elderly patients who have a hip fracture receive osteoporosis medication to reduce future fracture risk, according to the ASBMR. A 30-year downward trend in the number of hip fractures in the United States has recently plateaued, raising concerns this may have been caused by doctors and patients not following diagnostic and treatment guidelines, the organization noted.
The reasons for the plateau are uncertain, Dr. Khosla said, but could include a reluctance by patients to take bisphosphonates following some reports of relatively rare side effects, such as atypical femoral fractures and osteonecrosis of the jaw. In addition, he said, reimbursement for dual-energy x-ray absorptiometry (DEXA) scans to measure bone mineral density has gone down, which has led to fewer osteoporosis diagnoses. But fracture prevention is important, he said. Of the 300,000 hip fractures in the United States each year, one of every two patients never regains their previous functioning. In addition, one of every four hip fracture patients ends up in a nursing home or dies within a year, according to the ASBMR.
The recommendations and more data about osteoporosis treatment are available on the coalition’s website, www.secondaryfractures.org. An action plan for clinicians should be added to the site sometime this fall, Dr. Khosla said.
There are five fundamental recommendations:
First, communicate three simple messages to patients and their family/caregivers throughout the fracture care and healing process. These include: Their broken bone likely means they have osteoporosis and are at high risk for breaking more bones; breaking bones means they may have to use a walker, cane, or wheelchair or move from their home to a residential facility and will be at higher risk for premature death; and there are actions they can take to reduce their risk.
This is key, said coalition cochair Douglas P. Kiel, MD, a past president of ASBMR, the director of the Musculoskeletal Research Center at the Institute for Aging Research at Hebrew SeniorLife in Boston, and a professor of medicine at Harvard Medical School, also in Boston.
“If you talk to people who have had a broken bone, they view this as an accident and not that they have anything wrong with them,” he said. “The communication should be that if you broke something, it is not a random, chance event. You have osteoporosis, and if you don’t do anything about it, you’re going to be at great risk of a life-threatening, independence-threatening fracture in the future.”
Second, ensure the patient’s primary health care provider is made aware of the occurrence of the fracture. Take action to be sure the communication is made.
Third, regularly assess the risk of falling in women and men age 65 years or older who have had a hip or vertebral fracture. At minimum, take a history of falls within the last year, minimize the use of medications associated with increased risk for falls, evaluate patients for conditions associated with an increased risk for falls, and strongly consider referring patients to physical and/or occupational therapy or a physiatrist for evaluation and interventions to improve impairments in mobility, gait, and balance to reduce the risk for falls.
Fourth, reduce the risk of additional fractures by offering pharmacologic therapy for osteoporosis to women and men age 65 years or older who have had a hip or vertebral fracture. This can begin in the hospital and be included in discharge orders. Do not delay initiation of therapy for bone mineral density (BMD) testing. Consider patients’ oral health before starting therapy with bisphosphonates or denosumab (Prolia).
Most hip fracture patients leave the hospital without osteoporosis medications, Dr. Kiel said. It could be that hospital-based physicians are concerned patients are still unsteady such that they may not want to start patients on a new medication when they’re discharging them. Physicians in rehabilitation units may not prescribe these medications because they feel they have the patients for a short time, so by the time the patient returns to their primary care provider, the patient may have the same mistaken impression the fracture was an accident.
“We’re advocating not to delay treatment for any of these care transitions or because you think they need a BMD test,” Dr. Kiel said. “Just get them treated like they do with heart attacks.”
Finally, follow and reevaluate women and men age 65 years or older who have had a hip or vertebral fracture and are being treated for osteoporosis because it is a life-long chronic condition. This can help reinforce key messages about osteoporosis and associated fractures, identify any barriers to treatment adherence, assess the risk of falls, evaluate the effectiveness of a treatment plan, monitor for adverse effects, and determine whether any changes in treatment should be made, including whether any osteoporosis pharmacotherapy should be changed or discontinued.
Ideally, patients should be managed in the context of a multidisciplinary clinical system that includes case management, such as a fracture liaison service, according to the recommendations.
Besides the fundamental five recommendations, the documents lists another seven that deal with referring patients, prescribing vitamin D, counseling on lifestyle and diet, discussing pharmacotherapy benefits and risks, weighing first-line therapy options, and determining the duration of pharmacotherapy.
Ensuring that older adults who have experienced a hip or vertebral fracture understand they likely have osteoporosis, and offering prompt drug treatment for the condition, are among five fundamental
The recommendations were announced at the annual meeting of the American Society for Bone and Mineral Research (ASBMR) in Montreal. The 40-member group that developed the recommendations, called the ASBMR Secondary Fracture Prevention Initiative Coalition, includes the American Association of Clinical Endocrinologists/American College of Endocrinology, the Endocrine Society, the American College of Rheumatology, the American College of Physicians, the American Geriatrics Society, the American Academy of Physical Medicine and Rehabilitation, and the American Academy of Orthopaedic Surgeons.
Additional fundamental recommendations from the Coalition advised ensuring that patients’ primary health care providers are aware of the fracture, regularly assessing the risk of falls, and routinely reevaluating patients who are being treated for osteoporosis. These suggestions were developed in response to growing evidence of a rising trend in osteoporosis patients not being prescribed appropriate medications or not taking them, the ASBMR said.
“The very simple message is if you’ve got somebody who has had a hip fracture or a vertebral fracture, that needs secondary prevention just like somebody who’s had an MI needs to be on a statin and a beta blocker,” said coalition cochair Sundeep Khosla, MD, a past president of the ASBMR and director of the Center for Clinical and Translational Science at the Mayo Clinic, Rochester, Minn. “You can’t ignore the fracture because it’s not immediately life-threatening. Down the road they’re going to have another hip fracture if nothing is done.”
Only 23% of elderly patients who have a hip fracture receive osteoporosis medication to reduce future fracture risk, according to the ASBMR. A 30-year downward trend in the number of hip fractures in the United States has recently plateaued, raising concerns this may have been caused by doctors and patients not following diagnostic and treatment guidelines, the organization noted.
The reasons for the plateau are uncertain, Dr. Khosla said, but could include a reluctance by patients to take bisphosphonates following some reports of relatively rare side effects, such as atypical femoral fractures and osteonecrosis of the jaw. In addition, he said, reimbursement for dual-energy x-ray absorptiometry (DEXA) scans to measure bone mineral density has gone down, which has led to fewer osteoporosis diagnoses. But fracture prevention is important, he said. Of the 300,000 hip fractures in the United States each year, one of every two patients never regains their previous functioning. In addition, one of every four hip fracture patients ends up in a nursing home or dies within a year, according to the ASBMR.
The recommendations and more data about osteoporosis treatment are available on the coalition’s website, www.secondaryfractures.org. An action plan for clinicians should be added to the site sometime this fall, Dr. Khosla said.
There are five fundamental recommendations:
First, communicate three simple messages to patients and their family/caregivers throughout the fracture care and healing process. These include: Their broken bone likely means they have osteoporosis and are at high risk for breaking more bones; breaking bones means they may have to use a walker, cane, or wheelchair or move from their home to a residential facility and will be at higher risk for premature death; and there are actions they can take to reduce their risk.
This is key, said coalition cochair Douglas P. Kiel, MD, a past president of ASBMR, the director of the Musculoskeletal Research Center at the Institute for Aging Research at Hebrew SeniorLife in Boston, and a professor of medicine at Harvard Medical School, also in Boston.
“If you talk to people who have had a broken bone, they view this as an accident and not that they have anything wrong with them,” he said. “The communication should be that if you broke something, it is not a random, chance event. You have osteoporosis, and if you don’t do anything about it, you’re going to be at great risk of a life-threatening, independence-threatening fracture in the future.”
Second, ensure the patient’s primary health care provider is made aware of the occurrence of the fracture. Take action to be sure the communication is made.
Third, regularly assess the risk of falling in women and men age 65 years or older who have had a hip or vertebral fracture. At minimum, take a history of falls within the last year, minimize the use of medications associated with increased risk for falls, evaluate patients for conditions associated with an increased risk for falls, and strongly consider referring patients to physical and/or occupational therapy or a physiatrist for evaluation and interventions to improve impairments in mobility, gait, and balance to reduce the risk for falls.
Fourth, reduce the risk of additional fractures by offering pharmacologic therapy for osteoporosis to women and men age 65 years or older who have had a hip or vertebral fracture. This can begin in the hospital and be included in discharge orders. Do not delay initiation of therapy for bone mineral density (BMD) testing. Consider patients’ oral health before starting therapy with bisphosphonates or denosumab (Prolia).
Most hip fracture patients leave the hospital without osteoporosis medications, Dr. Kiel said. It could be that hospital-based physicians are concerned patients are still unsteady such that they may not want to start patients on a new medication when they’re discharging them. Physicians in rehabilitation units may not prescribe these medications because they feel they have the patients for a short time, so by the time the patient returns to their primary care provider, the patient may have the same mistaken impression the fracture was an accident.
“We’re advocating not to delay treatment for any of these care transitions or because you think they need a BMD test,” Dr. Kiel said. “Just get them treated like they do with heart attacks.”
Finally, follow and reevaluate women and men age 65 years or older who have had a hip or vertebral fracture and are being treated for osteoporosis because it is a life-long chronic condition. This can help reinforce key messages about osteoporosis and associated fractures, identify any barriers to treatment adherence, assess the risk of falls, evaluate the effectiveness of a treatment plan, monitor for adverse effects, and determine whether any changes in treatment should be made, including whether any osteoporosis pharmacotherapy should be changed or discontinued.
Ideally, patients should be managed in the context of a multidisciplinary clinical system that includes case management, such as a fracture liaison service, according to the recommendations.
Besides the fundamental five recommendations, the documents lists another seven that deal with referring patients, prescribing vitamin D, counseling on lifestyle and diet, discussing pharmacotherapy benefits and risks, weighing first-line therapy options, and determining the duration of pharmacotherapy.
Ensuring that older adults who have experienced a hip or vertebral fracture understand they likely have osteoporosis, and offering prompt drug treatment for the condition, are among five fundamental
The recommendations were announced at the annual meeting of the American Society for Bone and Mineral Research (ASBMR) in Montreal. The 40-member group that developed the recommendations, called the ASBMR Secondary Fracture Prevention Initiative Coalition, includes the American Association of Clinical Endocrinologists/American College of Endocrinology, the Endocrine Society, the American College of Rheumatology, the American College of Physicians, the American Geriatrics Society, the American Academy of Physical Medicine and Rehabilitation, and the American Academy of Orthopaedic Surgeons.
Additional fundamental recommendations from the Coalition advised ensuring that patients’ primary health care providers are aware of the fracture, regularly assessing the risk of falls, and routinely reevaluating patients who are being treated for osteoporosis. These suggestions were developed in response to growing evidence of a rising trend in osteoporosis patients not being prescribed appropriate medications or not taking them, the ASBMR said.
“The very simple message is if you’ve got somebody who has had a hip fracture or a vertebral fracture, that needs secondary prevention just like somebody who’s had an MI needs to be on a statin and a beta blocker,” said coalition cochair Sundeep Khosla, MD, a past president of the ASBMR and director of the Center for Clinical and Translational Science at the Mayo Clinic, Rochester, Minn. “You can’t ignore the fracture because it’s not immediately life-threatening. Down the road they’re going to have another hip fracture if nothing is done.”
Only 23% of elderly patients who have a hip fracture receive osteoporosis medication to reduce future fracture risk, according to the ASBMR. A 30-year downward trend in the number of hip fractures in the United States has recently plateaued, raising concerns this may have been caused by doctors and patients not following diagnostic and treatment guidelines, the organization noted.
The reasons for the plateau are uncertain, Dr. Khosla said, but could include a reluctance by patients to take bisphosphonates following some reports of relatively rare side effects, such as atypical femoral fractures and osteonecrosis of the jaw. In addition, he said, reimbursement for dual-energy x-ray absorptiometry (DEXA) scans to measure bone mineral density has gone down, which has led to fewer osteoporosis diagnoses. But fracture prevention is important, he said. Of the 300,000 hip fractures in the United States each year, one of every two patients never regains their previous functioning. In addition, one of every four hip fracture patients ends up in a nursing home or dies within a year, according to the ASBMR.
The recommendations and more data about osteoporosis treatment are available on the coalition’s website, www.secondaryfractures.org. An action plan for clinicians should be added to the site sometime this fall, Dr. Khosla said.
There are five fundamental recommendations:
First, communicate three simple messages to patients and their family/caregivers throughout the fracture care and healing process. These include: Their broken bone likely means they have osteoporosis and are at high risk for breaking more bones; breaking bones means they may have to use a walker, cane, or wheelchair or move from their home to a residential facility and will be at higher risk for premature death; and there are actions they can take to reduce their risk.
This is key, said coalition cochair Douglas P. Kiel, MD, a past president of ASBMR, the director of the Musculoskeletal Research Center at the Institute for Aging Research at Hebrew SeniorLife in Boston, and a professor of medicine at Harvard Medical School, also in Boston.
“If you talk to people who have had a broken bone, they view this as an accident and not that they have anything wrong with them,” he said. “The communication should be that if you broke something, it is not a random, chance event. You have osteoporosis, and if you don’t do anything about it, you’re going to be at great risk of a life-threatening, independence-threatening fracture in the future.”
Second, ensure the patient’s primary health care provider is made aware of the occurrence of the fracture. Take action to be sure the communication is made.
Third, regularly assess the risk of falling in women and men age 65 years or older who have had a hip or vertebral fracture. At minimum, take a history of falls within the last year, minimize the use of medications associated with increased risk for falls, evaluate patients for conditions associated with an increased risk for falls, and strongly consider referring patients to physical and/or occupational therapy or a physiatrist for evaluation and interventions to improve impairments in mobility, gait, and balance to reduce the risk for falls.
Fourth, reduce the risk of additional fractures by offering pharmacologic therapy for osteoporosis to women and men age 65 years or older who have had a hip or vertebral fracture. This can begin in the hospital and be included in discharge orders. Do not delay initiation of therapy for bone mineral density (BMD) testing. Consider patients’ oral health before starting therapy with bisphosphonates or denosumab (Prolia).
Most hip fracture patients leave the hospital without osteoporosis medications, Dr. Kiel said. It could be that hospital-based physicians are concerned patients are still unsteady such that they may not want to start patients on a new medication when they’re discharging them. Physicians in rehabilitation units may not prescribe these medications because they feel they have the patients for a short time, so by the time the patient returns to their primary care provider, the patient may have the same mistaken impression the fracture was an accident.
“We’re advocating not to delay treatment for any of these care transitions or because you think they need a BMD test,” Dr. Kiel said. “Just get them treated like they do with heart attacks.”
Finally, follow and reevaluate women and men age 65 years or older who have had a hip or vertebral fracture and are being treated for osteoporosis because it is a life-long chronic condition. This can help reinforce key messages about osteoporosis and associated fractures, identify any barriers to treatment adherence, assess the risk of falls, evaluate the effectiveness of a treatment plan, monitor for adverse effects, and determine whether any changes in treatment should be made, including whether any osteoporosis pharmacotherapy should be changed or discontinued.
Ideally, patients should be managed in the context of a multidisciplinary clinical system that includes case management, such as a fracture liaison service, according to the recommendations.
Besides the fundamental five recommendations, the documents lists another seven that deal with referring patients, prescribing vitamin D, counseling on lifestyle and diet, discussing pharmacotherapy benefits and risks, weighing first-line therapy options, and determining the duration of pharmacotherapy.
EXPERT ANALYSIS FROM ASBMR 2018
Secondary fractures in older men spike soon after first, but exercise may help
Older men have a higher risk than women of sustaining secondary fractures within a few years of their first fracture, but moderate physical activity may improve bone strength, potentially reducing their risk of fractures, according to two studies presented at the annual meeting of the American Society for Bone and Mineral Research in Montreal.
The first study, a matched historical cohort of 57,783 people aged 50 or older (40,062 women and 17,721 men) in Manitoba, Canada, found that men had a threefold higher risk of sustaining a secondary major osteoporotic fracture (MOF) within 1 year of a first fracture, compared with healthy controls. The risk for women, by comparison, was 1.8 times higher than in age-matched controls who did not experience a fracture. These risks declined over time but remained elevated even as much as 15-25 years after the index fracture, according to primary investigator Suzanne N. Morin, MD, of the department of medicine at McGill University in Montreal.
“Often, men and clinicians don’t think men have skeletal fragility – everybody thinks it’s a women’s disease,” Dr. Morin said. “It’s true that it’s more frequent in women, but men do have osteoporosis, and often when they have it, they tend to have more serious complications following the fractures.” This includes higher risk of subsequent fractures and higher mortality, she said. “If you see an older gentleman with a fracture, it really should be some kind of an alarm signal.”
Using administrative health care databases, Dr. Morin and her colleagues reviewed records of patients who had an index MOF between 1989 and 2006. They compared rates of subsequent MOFs until 2016 with those of age- and sex-matched controls (n = 165,965), allowing for between 10 and 25 years of follow-up.
Researchers identified 29,694 index MOF cases (11,028 to the wrist, 9,313 to the hip, 5,799 to the humerus, and 3,554 to the spine). The annual crude rate of subsequent MOFs per 1,000 person-years was 18.5 in men (95% confidence interval, 17.3-19.8) and 29.6 in women (95% CI, 28.8-30.4). The cumulative incidence of subsequent MOFs up to 25 years later was higher in cases versus controls for both sexes and across all ages except those over 80.
Hazard ratios for subsequent MOFs were higher in men than women, particularly in the first year following the index fracture and remained very high for men during the first 3 years of follow-up. Across all follow-up years, men who had fractures were 2.5 times more likely to experience a secondary MOF (95% CI, 2.3-2.7) and women who had fractures were 1.6 times more likely to experience a secondary MOF (95% CI, 1.6-1.7), compared with controls.
To prevent fractures, clinicians should consider gait or balance training for older men and women, especially those who already have experienced a fracture, Dr. Morin said. Physicians also should note any medications such as sedatives that put patients at higher risk for falls and consider medications like bisphosphonates to reduce fracture risk. Additionally, they should ensure there are no underlying causes for skeletal fragility, such as severe vitamin D deficiency or a hormonal imbalance, she said.
Physical activity could reduce risk
In a second, unrelated study, researchers found that moderate physical activity may have a modest effect on bone strength in older men, accounting for up to a 20% lower fracture risk, according to Lisa Langsetmo, PhD, primary investigator and a senior research associate at the University of Minnesota, Minneapolis. She and her colleagues studied physical activity and bone strength in 994 older men (mean age 83.9) participating in the Osteoporotic Fractures in Men (MrOS) Study, a longitudinal, observational study of musculoskeletal health in older American men that initially enrolled about 6,000 participants.
Participants wore armband activity monitors for 5 days during their year-7 and year-14 assessments; investigators averaged their physical activity over the two time points and used armband data along with factors like height, weight, and smoking status to estimate total energy expenditure (TEE), total steps per day, and level of activity, from sedentary to at least moderate. The men also underwent bone microarchitecture assessments of the distal radius and tibia using high-resolution peripheral quantitative computed tomography (HR-pQCT), a technique that produces detailed pictures of the bones. Investigators used mathematical models to predict failure load, or the force required to break a bone – a predictor of osteoporotic fractures in men. They also computed total, cortical, and trabecular volumetric bone mineral density (BMD).
Overall, researchers found that time spent doing at least moderate activity versus time spent in sedentary activity was related to better bone strength at both sites, whereas time spent in light activity was not. The results suggest that at least moderate physical activity such as vigorous walking averaged over a period of time may have a modest effect on bone strength among older men, Dr. Langsetmo said.
“This is important for older men,” she said. “They may not be able to jog any more but they may be able to do more moderate activity.” Physicians should ask older male patients about their activity levels and any barriers to activity, or consider a referral to a physical therapist to keep them active, she said.
Higher TEE, step count, and peak 30-minute cadence (P30MC), a measure of vigorous activity, were each associated with higher failure load of the distal radius (effect size 0.08-0.13) but not higher volumetric or compartment-specific BMD. These measures also were associated with higher failure load of the distal tibia (effect size 0.19-0.21), higher volumetric BMD (effect size 0.08-0.15), higher trabecular BMD (effect size 0.07-0.11), and higher cortical BMD (0.09-0.13).
The first study was funded internally; Manitoba Health provided the data. The second study was funded by the National Institutes of Health. Dr. Morin and Dr. Langsetmo reported no relevant financial disclosures.
Older men have a higher risk than women of sustaining secondary fractures within a few years of their first fracture, but moderate physical activity may improve bone strength, potentially reducing their risk of fractures, according to two studies presented at the annual meeting of the American Society for Bone and Mineral Research in Montreal.
The first study, a matched historical cohort of 57,783 people aged 50 or older (40,062 women and 17,721 men) in Manitoba, Canada, found that men had a threefold higher risk of sustaining a secondary major osteoporotic fracture (MOF) within 1 year of a first fracture, compared with healthy controls. The risk for women, by comparison, was 1.8 times higher than in age-matched controls who did not experience a fracture. These risks declined over time but remained elevated even as much as 15-25 years after the index fracture, according to primary investigator Suzanne N. Morin, MD, of the department of medicine at McGill University in Montreal.
“Often, men and clinicians don’t think men have skeletal fragility – everybody thinks it’s a women’s disease,” Dr. Morin said. “It’s true that it’s more frequent in women, but men do have osteoporosis, and often when they have it, they tend to have more serious complications following the fractures.” This includes higher risk of subsequent fractures and higher mortality, she said. “If you see an older gentleman with a fracture, it really should be some kind of an alarm signal.”
Using administrative health care databases, Dr. Morin and her colleagues reviewed records of patients who had an index MOF between 1989 and 2006. They compared rates of subsequent MOFs until 2016 with those of age- and sex-matched controls (n = 165,965), allowing for between 10 and 25 years of follow-up.
Researchers identified 29,694 index MOF cases (11,028 to the wrist, 9,313 to the hip, 5,799 to the humerus, and 3,554 to the spine). The annual crude rate of subsequent MOFs per 1,000 person-years was 18.5 in men (95% confidence interval, 17.3-19.8) and 29.6 in women (95% CI, 28.8-30.4). The cumulative incidence of subsequent MOFs up to 25 years later was higher in cases versus controls for both sexes and across all ages except those over 80.
Hazard ratios for subsequent MOFs were higher in men than women, particularly in the first year following the index fracture and remained very high for men during the first 3 years of follow-up. Across all follow-up years, men who had fractures were 2.5 times more likely to experience a secondary MOF (95% CI, 2.3-2.7) and women who had fractures were 1.6 times more likely to experience a secondary MOF (95% CI, 1.6-1.7), compared with controls.
To prevent fractures, clinicians should consider gait or balance training for older men and women, especially those who already have experienced a fracture, Dr. Morin said. Physicians also should note any medications such as sedatives that put patients at higher risk for falls and consider medications like bisphosphonates to reduce fracture risk. Additionally, they should ensure there are no underlying causes for skeletal fragility, such as severe vitamin D deficiency or a hormonal imbalance, she said.
Physical activity could reduce risk
In a second, unrelated study, researchers found that moderate physical activity may have a modest effect on bone strength in older men, accounting for up to a 20% lower fracture risk, according to Lisa Langsetmo, PhD, primary investigator and a senior research associate at the University of Minnesota, Minneapolis. She and her colleagues studied physical activity and bone strength in 994 older men (mean age 83.9) participating in the Osteoporotic Fractures in Men (MrOS) Study, a longitudinal, observational study of musculoskeletal health in older American men that initially enrolled about 6,000 participants.
Participants wore armband activity monitors for 5 days during their year-7 and year-14 assessments; investigators averaged their physical activity over the two time points and used armband data along with factors like height, weight, and smoking status to estimate total energy expenditure (TEE), total steps per day, and level of activity, from sedentary to at least moderate. The men also underwent bone microarchitecture assessments of the distal radius and tibia using high-resolution peripheral quantitative computed tomography (HR-pQCT), a technique that produces detailed pictures of the bones. Investigators used mathematical models to predict failure load, or the force required to break a bone – a predictor of osteoporotic fractures in men. They also computed total, cortical, and trabecular volumetric bone mineral density (BMD).
Overall, researchers found that time spent doing at least moderate activity versus time spent in sedentary activity was related to better bone strength at both sites, whereas time spent in light activity was not. The results suggest that at least moderate physical activity such as vigorous walking averaged over a period of time may have a modest effect on bone strength among older men, Dr. Langsetmo said.
“This is important for older men,” she said. “They may not be able to jog any more but they may be able to do more moderate activity.” Physicians should ask older male patients about their activity levels and any barriers to activity, or consider a referral to a physical therapist to keep them active, she said.
Higher TEE, step count, and peak 30-minute cadence (P30MC), a measure of vigorous activity, were each associated with higher failure load of the distal radius (effect size 0.08-0.13) but not higher volumetric or compartment-specific BMD. These measures also were associated with higher failure load of the distal tibia (effect size 0.19-0.21), higher volumetric BMD (effect size 0.08-0.15), higher trabecular BMD (effect size 0.07-0.11), and higher cortical BMD (0.09-0.13).
The first study was funded internally; Manitoba Health provided the data. The second study was funded by the National Institutes of Health. Dr. Morin and Dr. Langsetmo reported no relevant financial disclosures.
Older men have a higher risk than women of sustaining secondary fractures within a few years of their first fracture, but moderate physical activity may improve bone strength, potentially reducing their risk of fractures, according to two studies presented at the annual meeting of the American Society for Bone and Mineral Research in Montreal.
The first study, a matched historical cohort of 57,783 people aged 50 or older (40,062 women and 17,721 men) in Manitoba, Canada, found that men had a threefold higher risk of sustaining a secondary major osteoporotic fracture (MOF) within 1 year of a first fracture, compared with healthy controls. The risk for women, by comparison, was 1.8 times higher than in age-matched controls who did not experience a fracture. These risks declined over time but remained elevated even as much as 15-25 years after the index fracture, according to primary investigator Suzanne N. Morin, MD, of the department of medicine at McGill University in Montreal.
“Often, men and clinicians don’t think men have skeletal fragility – everybody thinks it’s a women’s disease,” Dr. Morin said. “It’s true that it’s more frequent in women, but men do have osteoporosis, and often when they have it, they tend to have more serious complications following the fractures.” This includes higher risk of subsequent fractures and higher mortality, she said. “If you see an older gentleman with a fracture, it really should be some kind of an alarm signal.”
Using administrative health care databases, Dr. Morin and her colleagues reviewed records of patients who had an index MOF between 1989 and 2006. They compared rates of subsequent MOFs until 2016 with those of age- and sex-matched controls (n = 165,965), allowing for between 10 and 25 years of follow-up.
Researchers identified 29,694 index MOF cases (11,028 to the wrist, 9,313 to the hip, 5,799 to the humerus, and 3,554 to the spine). The annual crude rate of subsequent MOFs per 1,000 person-years was 18.5 in men (95% confidence interval, 17.3-19.8) and 29.6 in women (95% CI, 28.8-30.4). The cumulative incidence of subsequent MOFs up to 25 years later was higher in cases versus controls for both sexes and across all ages except those over 80.
Hazard ratios for subsequent MOFs were higher in men than women, particularly in the first year following the index fracture and remained very high for men during the first 3 years of follow-up. Across all follow-up years, men who had fractures were 2.5 times more likely to experience a secondary MOF (95% CI, 2.3-2.7) and women who had fractures were 1.6 times more likely to experience a secondary MOF (95% CI, 1.6-1.7), compared with controls.
To prevent fractures, clinicians should consider gait or balance training for older men and women, especially those who already have experienced a fracture, Dr. Morin said. Physicians also should note any medications such as sedatives that put patients at higher risk for falls and consider medications like bisphosphonates to reduce fracture risk. Additionally, they should ensure there are no underlying causes for skeletal fragility, such as severe vitamin D deficiency or a hormonal imbalance, she said.
Physical activity could reduce risk
In a second, unrelated study, researchers found that moderate physical activity may have a modest effect on bone strength in older men, accounting for up to a 20% lower fracture risk, according to Lisa Langsetmo, PhD, primary investigator and a senior research associate at the University of Minnesota, Minneapolis. She and her colleagues studied physical activity and bone strength in 994 older men (mean age 83.9) participating in the Osteoporotic Fractures in Men (MrOS) Study, a longitudinal, observational study of musculoskeletal health in older American men that initially enrolled about 6,000 participants.
Participants wore armband activity monitors for 5 days during their year-7 and year-14 assessments; investigators averaged their physical activity over the two time points and used armband data along with factors like height, weight, and smoking status to estimate total energy expenditure (TEE), total steps per day, and level of activity, from sedentary to at least moderate. The men also underwent bone microarchitecture assessments of the distal radius and tibia using high-resolution peripheral quantitative computed tomography (HR-pQCT), a technique that produces detailed pictures of the bones. Investigators used mathematical models to predict failure load, or the force required to break a bone – a predictor of osteoporotic fractures in men. They also computed total, cortical, and trabecular volumetric bone mineral density (BMD).
Overall, researchers found that time spent doing at least moderate activity versus time spent in sedentary activity was related to better bone strength at both sites, whereas time spent in light activity was not. The results suggest that at least moderate physical activity such as vigorous walking averaged over a period of time may have a modest effect on bone strength among older men, Dr. Langsetmo said.
“This is important for older men,” she said. “They may not be able to jog any more but they may be able to do more moderate activity.” Physicians should ask older male patients about their activity levels and any barriers to activity, or consider a referral to a physical therapist to keep them active, she said.
Higher TEE, step count, and peak 30-minute cadence (P30MC), a measure of vigorous activity, were each associated with higher failure load of the distal radius (effect size 0.08-0.13) but not higher volumetric or compartment-specific BMD. These measures also were associated with higher failure load of the distal tibia (effect size 0.19-0.21), higher volumetric BMD (effect size 0.08-0.15), higher trabecular BMD (effect size 0.07-0.11), and higher cortical BMD (0.09-0.13).
The first study was funded internally; Manitoba Health provided the data. The second study was funded by the National Institutes of Health. Dr. Morin and Dr. Langsetmo reported no relevant financial disclosures.
FROM ASBMR 2018
Update on AGA-Medtronic Research and Development Pilot Award in Technology
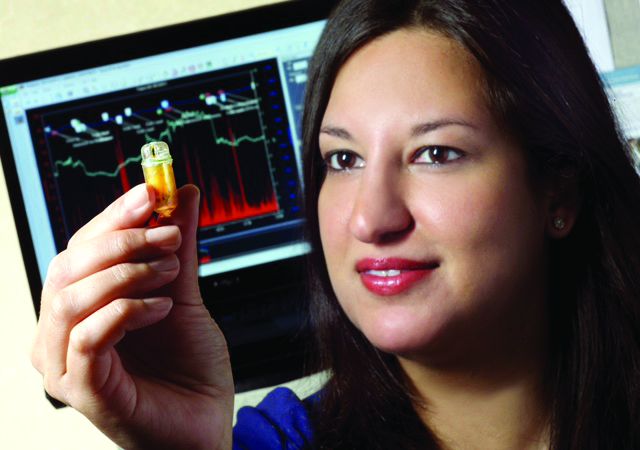
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research and Development Pilot Award in Technology, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and functioning. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington, D.C., as a poster of distinction.
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiologic mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using the Wireless Motility Capsule (WMC, SmartPill) to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO – just the sort of innovative technology that the AGA Center for GI Innovation and Technology (CGIT) has fostered. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with IBS and SIBO have increased expression of pro-inflammatory markers compared to those with only IBS; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries, without understanding the etiology for this syndrome,” said Dr. Roland, director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
In data to be presented in the DDW poster, Dr. Roland’s team used the SmartPill to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared to patients with IBS without evidence of SIBO. “Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as TNF (tumor necrosis factor)-alpha and IL (interleukin)-6, to patients with IBS alone. They will use their data to apply for future funding.
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research and Development Pilot Award in Technology, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and functioning. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington, D.C., as a poster of distinction.
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiologic mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using the Wireless Motility Capsule (WMC, SmartPill) to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO – just the sort of innovative technology that the AGA Center for GI Innovation and Technology (CGIT) has fostered. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with IBS and SIBO have increased expression of pro-inflammatory markers compared to those with only IBS; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries, without understanding the etiology for this syndrome,” said Dr. Roland, director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
In data to be presented in the DDW poster, Dr. Roland’s team used the SmartPill to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared to patients with IBS without evidence of SIBO. “Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as TNF (tumor necrosis factor)-alpha and IL (interleukin)-6, to patients with IBS alone. They will use their data to apply for future funding.
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research and Development Pilot Award in Technology, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and functioning. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington, D.C., as a poster of distinction.
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiologic mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using the Wireless Motility Capsule (WMC, SmartPill) to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO – just the sort of innovative technology that the AGA Center for GI Innovation and Technology (CGIT) has fostered. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with IBS and SIBO have increased expression of pro-inflammatory markers compared to those with only IBS; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries, without understanding the etiology for this syndrome,” said Dr. Roland, director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
In data to be presented in the DDW poster, Dr. Roland’s team used the SmartPill to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared to patients with IBS without evidence of SIBO. “Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as TNF (tumor necrosis factor)-alpha and IL (interleukin)-6, to patients with IBS alone. They will use their data to apply for future funding.
2018 AGA TECH SUMMIT
New consensus on inpatient opioid use
SHM’s new recommendations to improve opioid prescribing for acute, noncancer pain in hospitalized adults will be the focus of Monday (April 9) morning’s session, “Opioids: What Now?”
Many patients who wind up on opioids for chronic pain start on the medications in an acute pain setting, said presenter Shoshana J. Herzig, MD, MPH, director of hospital medicine research at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, both in Boston.
“Our prescribing patterns in the setting of acute pain meaningfully impact downstream outcomes and prescribing practices,” she said. “The degree of importance related to this topic often is underestimated by hospitalists, because we think of it as a more straightforward situation – prescribing for acute pain. In reality, there are nuances to it, and we have data to show that it’s not done well a lot of the time. It’s a big problem.”
During the session, “we’re going to do a case-based review that highlights the main points of the SHM consensus statement, just published in the Journal of Hospital Medicine [April issue],” Dr. Herzig said. She led the working group that developed the consensus statement. It features 16 suggestions to help hospital-based physicians appropriately employ opioids as part of their acute pain management strategies.
The copresenter will be Teryl K. Nuckols, MD, FHM, associate professor of medicine at the University of California, Los Angeles, and director of the division of internal medicine and associate professor of medicine at Cedars-Sinai Medical Center, Los Angeles. Dr. Nuckols was senior author of the JHM articles.
The presentation will assess the state of opioid prescribing in hospitalized patients and the challenges to acute pain management in hospitalized adults and explain how to improve prescribing practices to prevent opioid-related adverse events, opioid-use disorder, and long-term opioid use. Dr. Herzig and Dr. Nuckols will discuss how their group developed the new consensus statement by culling the key points from other physician group guidelines and present several case studies for interactive discussion to showcase the consensus statement suggestions. They also will go over topics in need of future research.
“We hope that attending the session and reading over the consensus statement will help to improve the appropriateness as well as the safety of opioid prescribing in the setting of acute pain in the hospital and help physicians recognize common pitfalls,” Dr. Herzig said. These include not remembering to combine opioids with nonopioid-based pharmacologic therapy; inappropriately continuing a patient on intravenous opioids when oral opioids, which have a lower risk of adverse outcomes, would suffice; and being able to identify patients at increased risk for opioid-related adverse events for whom a dose reduction or increased monitoring may be warranted.
Hospitalists should continue to strive to achieve a proper balance with opioids between offering adequate analgesia for their patients and the risk of adverse events, she said. “The need for judicious prescribing is our main take-home message. I hope that people leave with a better understanding of what a reasonable amount of opioids to prescribe on discharge looks like.”
Dr. Herzig receives financial compensation from SHM for her role as senior deputy editor of the Journal of Hospital Medicine (unrelated to the present work).
Opioids: What Now?
Monday, April 10:35-11:35 a.m.
Crystal Ballroom G1/A&B
SHM’s new recommendations to improve opioid prescribing for acute, noncancer pain in hospitalized adults will be the focus of Monday (April 9) morning’s session, “Opioids: What Now?”
Many patients who wind up on opioids for chronic pain start on the medications in an acute pain setting, said presenter Shoshana J. Herzig, MD, MPH, director of hospital medicine research at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, both in Boston.
“Our prescribing patterns in the setting of acute pain meaningfully impact downstream outcomes and prescribing practices,” she said. “The degree of importance related to this topic often is underestimated by hospitalists, because we think of it as a more straightforward situation – prescribing for acute pain. In reality, there are nuances to it, and we have data to show that it’s not done well a lot of the time. It’s a big problem.”
During the session, “we’re going to do a case-based review that highlights the main points of the SHM consensus statement, just published in the Journal of Hospital Medicine [April issue],” Dr. Herzig said. She led the working group that developed the consensus statement. It features 16 suggestions to help hospital-based physicians appropriately employ opioids as part of their acute pain management strategies.
The copresenter will be Teryl K. Nuckols, MD, FHM, associate professor of medicine at the University of California, Los Angeles, and director of the division of internal medicine and associate professor of medicine at Cedars-Sinai Medical Center, Los Angeles. Dr. Nuckols was senior author of the JHM articles.
The presentation will assess the state of opioid prescribing in hospitalized patients and the challenges to acute pain management in hospitalized adults and explain how to improve prescribing practices to prevent opioid-related adverse events, opioid-use disorder, and long-term opioid use. Dr. Herzig and Dr. Nuckols will discuss how their group developed the new consensus statement by culling the key points from other physician group guidelines and present several case studies for interactive discussion to showcase the consensus statement suggestions. They also will go over topics in need of future research.
“We hope that attending the session and reading over the consensus statement will help to improve the appropriateness as well as the safety of opioid prescribing in the setting of acute pain in the hospital and help physicians recognize common pitfalls,” Dr. Herzig said. These include not remembering to combine opioids with nonopioid-based pharmacologic therapy; inappropriately continuing a patient on intravenous opioids when oral opioids, which have a lower risk of adverse outcomes, would suffice; and being able to identify patients at increased risk for opioid-related adverse events for whom a dose reduction or increased monitoring may be warranted.
Hospitalists should continue to strive to achieve a proper balance with opioids between offering adequate analgesia for their patients and the risk of adverse events, she said. “The need for judicious prescribing is our main take-home message. I hope that people leave with a better understanding of what a reasonable amount of opioids to prescribe on discharge looks like.”
Dr. Herzig receives financial compensation from SHM for her role as senior deputy editor of the Journal of Hospital Medicine (unrelated to the present work).
Opioids: What Now?
Monday, April 10:35-11:35 a.m.
Crystal Ballroom G1/A&B
SHM’s new recommendations to improve opioid prescribing for acute, noncancer pain in hospitalized adults will be the focus of Monday (April 9) morning’s session, “Opioids: What Now?”
Many patients who wind up on opioids for chronic pain start on the medications in an acute pain setting, said presenter Shoshana J. Herzig, MD, MPH, director of hospital medicine research at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, both in Boston.
“Our prescribing patterns in the setting of acute pain meaningfully impact downstream outcomes and prescribing practices,” she said. “The degree of importance related to this topic often is underestimated by hospitalists, because we think of it as a more straightforward situation – prescribing for acute pain. In reality, there are nuances to it, and we have data to show that it’s not done well a lot of the time. It’s a big problem.”
During the session, “we’re going to do a case-based review that highlights the main points of the SHM consensus statement, just published in the Journal of Hospital Medicine [April issue],” Dr. Herzig said. She led the working group that developed the consensus statement. It features 16 suggestions to help hospital-based physicians appropriately employ opioids as part of their acute pain management strategies.
The copresenter will be Teryl K. Nuckols, MD, FHM, associate professor of medicine at the University of California, Los Angeles, and director of the division of internal medicine and associate professor of medicine at Cedars-Sinai Medical Center, Los Angeles. Dr. Nuckols was senior author of the JHM articles.
The presentation will assess the state of opioid prescribing in hospitalized patients and the challenges to acute pain management in hospitalized adults and explain how to improve prescribing practices to prevent opioid-related adverse events, opioid-use disorder, and long-term opioid use. Dr. Herzig and Dr. Nuckols will discuss how their group developed the new consensus statement by culling the key points from other physician group guidelines and present several case studies for interactive discussion to showcase the consensus statement suggestions. They also will go over topics in need of future research.
“We hope that attending the session and reading over the consensus statement will help to improve the appropriateness as well as the safety of opioid prescribing in the setting of acute pain in the hospital and help physicians recognize common pitfalls,” Dr. Herzig said. These include not remembering to combine opioids with nonopioid-based pharmacologic therapy; inappropriately continuing a patient on intravenous opioids when oral opioids, which have a lower risk of adverse outcomes, would suffice; and being able to identify patients at increased risk for opioid-related adverse events for whom a dose reduction or increased monitoring may be warranted.
Hospitalists should continue to strive to achieve a proper balance with opioids between offering adequate analgesia for their patients and the risk of adverse events, she said. “The need for judicious prescribing is our main take-home message. I hope that people leave with a better understanding of what a reasonable amount of opioids to prescribe on discharge looks like.”
Dr. Herzig receives financial compensation from SHM for her role as senior deputy editor of the Journal of Hospital Medicine (unrelated to the present work).
Opioids: What Now?
Monday, April 10:35-11:35 a.m.
Crystal Ballroom G1/A&B
Update on AGA-Medtronic Research & Development Pilot Award in Technology
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research & Development Pilot Award in Technology by the AGA Research Foundation, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and its function. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington as a poster of distinction.
“Dr. Roland’s research is innovative and clinically relevant. It’s great to see the progress her team has made since receiving this grant from the AGA Research Foundation,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “I want to thank Medtronic for their partnership on this award and their shared commitment to funding innovative research projects.”
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiological mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using a wireless motility capsule (PillCam) – just the kind of technology fostered by the AGA GI Center for Innovation and Technology – to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with both IBS and SIBO have increased expression of proinflammatory markers, compared with those with IBS only; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries without understanding the etiology for this syndrome,” said Dr. Roland, the director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
To capture the data to be presented in the DDW poster, Dr. Roland’s team used the wireless motility capsule to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared with patients who have IBS without evidence of SIBO
“Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as tumor necrosis factor–alpha and interleukin 6, with those in patients who have only IBS. They will use their data to apply for future funding.
Since 2014, the AGA Research Foundation has partnered with medical technology companies such as Medtronic to provide a total of over $450,000 in research grants to six investigators working on novel and innovative technology projects. The AGA Research Foundation will begin accepting applications for the next round of research grants in summer 2018. Stay tuned to www.gastro.org/research-funding.
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research & Development Pilot Award in Technology by the AGA Research Foundation, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and its function. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington as a poster of distinction.
“Dr. Roland’s research is innovative and clinically relevant. It’s great to see the progress her team has made since receiving this grant from the AGA Research Foundation,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “I want to thank Medtronic for their partnership on this award and their shared commitment to funding innovative research projects.”
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiological mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using a wireless motility capsule (PillCam) – just the kind of technology fostered by the AGA GI Center for Innovation and Technology – to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with both IBS and SIBO have increased expression of proinflammatory markers, compared with those with IBS only; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries without understanding the etiology for this syndrome,” said Dr. Roland, the director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
To capture the data to be presented in the DDW poster, Dr. Roland’s team used the wireless motility capsule to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared with patients who have IBS without evidence of SIBO
“Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as tumor necrosis factor–alpha and interleukin 6, with those in patients who have only IBS. They will use their data to apply for future funding.
Since 2014, the AGA Research Foundation has partnered with medical technology companies such as Medtronic to provide a total of over $450,000 in research grants to six investigators working on novel and innovative technology projects. The AGA Research Foundation will begin accepting applications for the next round of research grants in summer 2018. Stay tuned to www.gastro.org/research-funding.
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research & Development Pilot Award in Technology by the AGA Research Foundation, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and its function. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington as a poster of distinction.
“Dr. Roland’s research is innovative and clinically relevant. It’s great to see the progress her team has made since receiving this grant from the AGA Research Foundation,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “I want to thank Medtronic for their partnership on this award and their shared commitment to funding innovative research projects.”
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiological mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using a wireless motility capsule (PillCam) – just the kind of technology fostered by the AGA GI Center for Innovation and Technology – to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with both IBS and SIBO have increased expression of proinflammatory markers, compared with those with IBS only; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries without understanding the etiology for this syndrome,” said Dr. Roland, the director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
To capture the data to be presented in the DDW poster, Dr. Roland’s team used the wireless motility capsule to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared with patients who have IBS without evidence of SIBO
“Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as tumor necrosis factor–alpha and interleukin 6, with those in patients who have only IBS. They will use their data to apply for future funding.
Since 2014, the AGA Research Foundation has partnered with medical technology companies such as Medtronic to provide a total of over $450,000 in research grants to six investigators working on novel and innovative technology projects. The AGA Research Foundation will begin accepting applications for the next round of research grants in summer 2018. Stay tuned to www.gastro.org/research-funding.
REPORTING FROM 2018 AGA TECH SUMMIT
Innovation as a key to patient outcomes
For about 10 years, the AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology, has brought together physician innovators, entrepreneurs, and industry representatives to facilitate innovation in gastroenterology practice, and this year is no exception.
Attendees will get a “comprehensive, almost immersive experience to understand what’s hot in gastroenterology and innovation in 2018, where the gaps are, and where we need future innovators to be successful to improve patient outcomes,” said Srinadh (Sri) Komanduri, MD, the medical director of the GI laboratory and director of interventional endoscopy at Northwestern University in Chicago, as well as one of the meeting’s organizers.
The meeting is designed to aid entrepreneurs, as well as physician innovators, who have ideas how to improve the field of gastroenterology but have found it daunting to take their ideas and commercialize them, added meeting organizer V. Raman Muthusamy, MD, the director of endoscopy for the University of California, Los Angeles, Health System. Dr. Muthusamy and Dr. Komanduri serve as cochairs of the AGA Center for GI Innovation and Technology executive committee.
“Innovation is key to evolving and thriving in any field, and we’re trying to foster that in gastroenterology,” Dr. Muthusamy said. “There are many good ideas in GI that probably never see the light of day simply because of real and perceived barriers to successful innovation. We’re trying to demystify and simplify that process.”
New this year will be a Wednesday preconference entrepreneur and innovator package session, in which innovators can meet one-on-one with experts – including intellectual property attorneys, physician innovators, venture capitalists, and payers – for personalized advice on how to move their products forward. An evening dinner reception for summit sponsors will feature a talk by Kevin Volpp, MD, PhD, founding director of the Center for Health Incentives and Behavior Economics at the University of Pennsylvania, Philadelphia, about the psychology of purchasing and how economic decisions are made in health care.
Also new for 2018 is a Thursday afternoon session on the evolving role of digital health in GI diseases. “We’ll look at where medicine is headed in terms of artificial intelligence, patient-driven mobile applications, and everything becoming digitally balanced,” said Dr. Komanduri, who will moderate the session. “We need to understand the space and how to utilize it on a more global aspect when it comes to innovation because there is a need for further education.”
The main summit will kick off Thursday with an address by Vadim Backman, PhD, the Walter Dill Scott Professor of Biomedical Engineering at Northwestern University, about nanotechnology and how it applies to gastroenterology. “We have a lot of entrepreneurs wondering what’s the next thing,” Dr. Muthusamy said. “I think Vadim is going to open up some new horizons and get us thinking about things that we haven’t traditionally thought of.”
The morning will focus on avoiding critical mistakes in startups and on medical device development, including lessons learned about developing a product and practical approaches to getting funded. Sessions scheduled for after lunch will highlight how to achieve physician adoption, including how to obtain a CPT code and physician barriers to incorporating new technology.
Friday’s sessions are unofficially titled “the next generation of endoscopy,” Dr. Muthusamy said. The day will begin with a look at bariatric endoscopy and surgical techniques and at the challenges and unmet needs of bariatric procedures, as well as organ-sparing resection techniques. Following the Shark Tank presentations, in which small companies can present their ideas to an expert panel, and lunch, the afternoon program will feature discussions on the reprocessing of duodenoscopes and quality in endoscopy.
“Our goal is not just to have these sessions be an interesting 90 minutes but to inspire a group of people with passion and interest in these areas to guide the field over the next 5-10 years in terms of where our energies should be focused, what’s needed, and what trials should be done so we can achieve the best results the quickest to achieve adoption of novel technologies,” Dr. Muthusamy said.
Dr. Komanduri is a consultant for Boston Scientific, Cook Medical, Olympus and Medtronic. Dr. Muthusamy disclosed financial relationships with CapsoVision, Boston Scientific and Medtronic.
For about 10 years, the AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology, has brought together physician innovators, entrepreneurs, and industry representatives to facilitate innovation in gastroenterology practice, and this year is no exception.
Attendees will get a “comprehensive, almost immersive experience to understand what’s hot in gastroenterology and innovation in 2018, where the gaps are, and where we need future innovators to be successful to improve patient outcomes,” said Srinadh (Sri) Komanduri, MD, the medical director of the GI laboratory and director of interventional endoscopy at Northwestern University in Chicago, as well as one of the meeting’s organizers.
The meeting is designed to aid entrepreneurs, as well as physician innovators, who have ideas how to improve the field of gastroenterology but have found it daunting to take their ideas and commercialize them, added meeting organizer V. Raman Muthusamy, MD, the director of endoscopy for the University of California, Los Angeles, Health System. Dr. Muthusamy and Dr. Komanduri serve as cochairs of the AGA Center for GI Innovation and Technology executive committee.
“Innovation is key to evolving and thriving in any field, and we’re trying to foster that in gastroenterology,” Dr. Muthusamy said. “There are many good ideas in GI that probably never see the light of day simply because of real and perceived barriers to successful innovation. We’re trying to demystify and simplify that process.”
New this year will be a Wednesday preconference entrepreneur and innovator package session, in which innovators can meet one-on-one with experts – including intellectual property attorneys, physician innovators, venture capitalists, and payers – for personalized advice on how to move their products forward. An evening dinner reception for summit sponsors will feature a talk by Kevin Volpp, MD, PhD, founding director of the Center for Health Incentives and Behavior Economics at the University of Pennsylvania, Philadelphia, about the psychology of purchasing and how economic decisions are made in health care.
Also new for 2018 is a Thursday afternoon session on the evolving role of digital health in GI diseases. “We’ll look at where medicine is headed in terms of artificial intelligence, patient-driven mobile applications, and everything becoming digitally balanced,” said Dr. Komanduri, who will moderate the session. “We need to understand the space and how to utilize it on a more global aspect when it comes to innovation because there is a need for further education.”
The main summit will kick off Thursday with an address by Vadim Backman, PhD, the Walter Dill Scott Professor of Biomedical Engineering at Northwestern University, about nanotechnology and how it applies to gastroenterology. “We have a lot of entrepreneurs wondering what’s the next thing,” Dr. Muthusamy said. “I think Vadim is going to open up some new horizons and get us thinking about things that we haven’t traditionally thought of.”
The morning will focus on avoiding critical mistakes in startups and on medical device development, including lessons learned about developing a product and practical approaches to getting funded. Sessions scheduled for after lunch will highlight how to achieve physician adoption, including how to obtain a CPT code and physician barriers to incorporating new technology.
Friday’s sessions are unofficially titled “the next generation of endoscopy,” Dr. Muthusamy said. The day will begin with a look at bariatric endoscopy and surgical techniques and at the challenges and unmet needs of bariatric procedures, as well as organ-sparing resection techniques. Following the Shark Tank presentations, in which small companies can present their ideas to an expert panel, and lunch, the afternoon program will feature discussions on the reprocessing of duodenoscopes and quality in endoscopy.
“Our goal is not just to have these sessions be an interesting 90 minutes but to inspire a group of people with passion and interest in these areas to guide the field over the next 5-10 years in terms of where our energies should be focused, what’s needed, and what trials should be done so we can achieve the best results the quickest to achieve adoption of novel technologies,” Dr. Muthusamy said.
Dr. Komanduri is a consultant for Boston Scientific, Cook Medical, Olympus and Medtronic. Dr. Muthusamy disclosed financial relationships with CapsoVision, Boston Scientific and Medtronic.
For about 10 years, the AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology, has brought together physician innovators, entrepreneurs, and industry representatives to facilitate innovation in gastroenterology practice, and this year is no exception.
Attendees will get a “comprehensive, almost immersive experience to understand what’s hot in gastroenterology and innovation in 2018, where the gaps are, and where we need future innovators to be successful to improve patient outcomes,” said Srinadh (Sri) Komanduri, MD, the medical director of the GI laboratory and director of interventional endoscopy at Northwestern University in Chicago, as well as one of the meeting’s organizers.
The meeting is designed to aid entrepreneurs, as well as physician innovators, who have ideas how to improve the field of gastroenterology but have found it daunting to take their ideas and commercialize them, added meeting organizer V. Raman Muthusamy, MD, the director of endoscopy for the University of California, Los Angeles, Health System. Dr. Muthusamy and Dr. Komanduri serve as cochairs of the AGA Center for GI Innovation and Technology executive committee.
“Innovation is key to evolving and thriving in any field, and we’re trying to foster that in gastroenterology,” Dr. Muthusamy said. “There are many good ideas in GI that probably never see the light of day simply because of real and perceived barriers to successful innovation. We’re trying to demystify and simplify that process.”
New this year will be a Wednesday preconference entrepreneur and innovator package session, in which innovators can meet one-on-one with experts – including intellectual property attorneys, physician innovators, venture capitalists, and payers – for personalized advice on how to move their products forward. An evening dinner reception for summit sponsors will feature a talk by Kevin Volpp, MD, PhD, founding director of the Center for Health Incentives and Behavior Economics at the University of Pennsylvania, Philadelphia, about the psychology of purchasing and how economic decisions are made in health care.
Also new for 2018 is a Thursday afternoon session on the evolving role of digital health in GI diseases. “We’ll look at where medicine is headed in terms of artificial intelligence, patient-driven mobile applications, and everything becoming digitally balanced,” said Dr. Komanduri, who will moderate the session. “We need to understand the space and how to utilize it on a more global aspect when it comes to innovation because there is a need for further education.”
The main summit will kick off Thursday with an address by Vadim Backman, PhD, the Walter Dill Scott Professor of Biomedical Engineering at Northwestern University, about nanotechnology and how it applies to gastroenterology. “We have a lot of entrepreneurs wondering what’s the next thing,” Dr. Muthusamy said. “I think Vadim is going to open up some new horizons and get us thinking about things that we haven’t traditionally thought of.”
The morning will focus on avoiding critical mistakes in startups and on medical device development, including lessons learned about developing a product and practical approaches to getting funded. Sessions scheduled for after lunch will highlight how to achieve physician adoption, including how to obtain a CPT code and physician barriers to incorporating new technology.
Friday’s sessions are unofficially titled “the next generation of endoscopy,” Dr. Muthusamy said. The day will begin with a look at bariatric endoscopy and surgical techniques and at the challenges and unmet needs of bariatric procedures, as well as organ-sparing resection techniques. Following the Shark Tank presentations, in which small companies can present their ideas to an expert panel, and lunch, the afternoon program will feature discussions on the reprocessing of duodenoscopes and quality in endoscopy.
“Our goal is not just to have these sessions be an interesting 90 minutes but to inspire a group of people with passion and interest in these areas to guide the field over the next 5-10 years in terms of where our energies should be focused, what’s needed, and what trials should be done so we can achieve the best results the quickest to achieve adoption of novel technologies,” Dr. Muthusamy said.
Dr. Komanduri is a consultant for Boston Scientific, Cook Medical, Olympus and Medtronic. Dr. Muthusamy disclosed financial relationships with CapsoVision, Boston Scientific and Medtronic.
Sprifermin shows cartilage-building potential in knee OA patients
in the initial 2-year results of the ongoing FORWARD trial.
“Sprifermin appears to be the first investigational medicinal product to show dose-dependent prevention of cartilage loss and an increase in cartilage thickness, not only in the total tibiofemoral joint [TFJ] but also in both the medial and lateral compartments, including the central medial femorotibial region,” said Marc H. Hochberg, MD, primary investigator in the trial and division head of rheumatology and clinical immunology at the University of Maryland, Baltimore. “The recommendation is that these findings should be further evaluated in phase 3 clinical trials.”
He and his colleagues randomized 549 osteoarthritis (OA) patients to double-blind treatment with one of four different dosing regimens of sprifermin or placebo. These patients were aged 40-85 with symptomatic radiographic primary femorotibial knee OA measuring grade 2 or 3 on the Kellgren-Lawrence scale and a medial minimum joint space width (mJSW) 2.5 mm or greater.
At 2 years, researchers observed a significant dose-dependent relationship between the amount of sprifermin given and the increase in total TFJ cartilage thickness. Patients who received three 100-mcg intra-articular injections of sprifermin every 6 months (group 1) showed a gain in TFJ cartilage thickness of 0.03 mm as seen on MRI, while those who received three 100-mcg injections of sprifermin every 12 months (group 2) had a gain of 0.02 mm, Dr. Hochberg said during a late-breaking abstract session at the annual meeting of the American College of Rheumatology. By contrast, those who received placebo had a loss in TFJ cartilage thickness of 0.02 mm (P less than .001). The other two groups received 30 mcg of sprifermin in three weekly injections every 6 months (group 3) or every 12 months (group 4), and these had TFJ cartilage thickness losses of about 0.01 mm or less.
Similar dose-dependent relationships were observed for some of the secondary endpoints, which included changes in cartilage thickness seen in the medial and lateral compartments, changes in cartilage thickness in the compartments’ subregions, and changes in mJSW. Significant differences in cartilage thickness were observed between sprifermin treatment groups and placebo in the medial (group 1, gain of 0.02 mm vs. loss of 0.03 mm; P less than .001) and lateral (groups 1 and 2, gain of 0.04 mm vs. loss of 0.01 mm; P less than .001) TFJ compartments, and in central medial and lateral TFJ subregions.
Changes in mJSW as seen on x-ray between those in group 1 and those on placebo were significant for the lateral compartment, with an increase in mJSW at the higher doses and a decline in the placebo group, but not for the medial compartment.
There were no significant differences in Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores among the treatment groups. Dr. Hochberg noted that patients were permitted to take pain medications during the study, which could have affected this result.
The most frequently reported adverse events were musculoskeletal and connective tissue disorders, specifically arthralgias and back pain, Dr. Hochberg said. The incidence of acute inflammatory reactions was higher with sprifermin, compared with placebo, but the increase was only significant after the first injection cycle, he said.
Merck KGaA and the EMD Serono Research Institute funded the study. Dr. Hochberg reported receiving consulting fees from numerous companies that market or are developing OA drugs, including EMD Serono.
in the initial 2-year results of the ongoing FORWARD trial.
“Sprifermin appears to be the first investigational medicinal product to show dose-dependent prevention of cartilage loss and an increase in cartilage thickness, not only in the total tibiofemoral joint [TFJ] but also in both the medial and lateral compartments, including the central medial femorotibial region,” said Marc H. Hochberg, MD, primary investigator in the trial and division head of rheumatology and clinical immunology at the University of Maryland, Baltimore. “The recommendation is that these findings should be further evaluated in phase 3 clinical trials.”
He and his colleagues randomized 549 osteoarthritis (OA) patients to double-blind treatment with one of four different dosing regimens of sprifermin or placebo. These patients were aged 40-85 with symptomatic radiographic primary femorotibial knee OA measuring grade 2 or 3 on the Kellgren-Lawrence scale and a medial minimum joint space width (mJSW) 2.5 mm or greater.
At 2 years, researchers observed a significant dose-dependent relationship between the amount of sprifermin given and the increase in total TFJ cartilage thickness. Patients who received three 100-mcg intra-articular injections of sprifermin every 6 months (group 1) showed a gain in TFJ cartilage thickness of 0.03 mm as seen on MRI, while those who received three 100-mcg injections of sprifermin every 12 months (group 2) had a gain of 0.02 mm, Dr. Hochberg said during a late-breaking abstract session at the annual meeting of the American College of Rheumatology. By contrast, those who received placebo had a loss in TFJ cartilage thickness of 0.02 mm (P less than .001). The other two groups received 30 mcg of sprifermin in three weekly injections every 6 months (group 3) or every 12 months (group 4), and these had TFJ cartilage thickness losses of about 0.01 mm or less.
Similar dose-dependent relationships were observed for some of the secondary endpoints, which included changes in cartilage thickness seen in the medial and lateral compartments, changes in cartilage thickness in the compartments’ subregions, and changes in mJSW. Significant differences in cartilage thickness were observed between sprifermin treatment groups and placebo in the medial (group 1, gain of 0.02 mm vs. loss of 0.03 mm; P less than .001) and lateral (groups 1 and 2, gain of 0.04 mm vs. loss of 0.01 mm; P less than .001) TFJ compartments, and in central medial and lateral TFJ subregions.
Changes in mJSW as seen on x-ray between those in group 1 and those on placebo were significant for the lateral compartment, with an increase in mJSW at the higher doses and a decline in the placebo group, but not for the medial compartment.
There were no significant differences in Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores among the treatment groups. Dr. Hochberg noted that patients were permitted to take pain medications during the study, which could have affected this result.
The most frequently reported adverse events were musculoskeletal and connective tissue disorders, specifically arthralgias and back pain, Dr. Hochberg said. The incidence of acute inflammatory reactions was higher with sprifermin, compared with placebo, but the increase was only significant after the first injection cycle, he said.
Merck KGaA and the EMD Serono Research Institute funded the study. Dr. Hochberg reported receiving consulting fees from numerous companies that market or are developing OA drugs, including EMD Serono.
in the initial 2-year results of the ongoing FORWARD trial.
“Sprifermin appears to be the first investigational medicinal product to show dose-dependent prevention of cartilage loss and an increase in cartilage thickness, not only in the total tibiofemoral joint [TFJ] but also in both the medial and lateral compartments, including the central medial femorotibial region,” said Marc H. Hochberg, MD, primary investigator in the trial and division head of rheumatology and clinical immunology at the University of Maryland, Baltimore. “The recommendation is that these findings should be further evaluated in phase 3 clinical trials.”
He and his colleagues randomized 549 osteoarthritis (OA) patients to double-blind treatment with one of four different dosing regimens of sprifermin or placebo. These patients were aged 40-85 with symptomatic radiographic primary femorotibial knee OA measuring grade 2 or 3 on the Kellgren-Lawrence scale and a medial minimum joint space width (mJSW) 2.5 mm or greater.
At 2 years, researchers observed a significant dose-dependent relationship between the amount of sprifermin given and the increase in total TFJ cartilage thickness. Patients who received three 100-mcg intra-articular injections of sprifermin every 6 months (group 1) showed a gain in TFJ cartilage thickness of 0.03 mm as seen on MRI, while those who received three 100-mcg injections of sprifermin every 12 months (group 2) had a gain of 0.02 mm, Dr. Hochberg said during a late-breaking abstract session at the annual meeting of the American College of Rheumatology. By contrast, those who received placebo had a loss in TFJ cartilage thickness of 0.02 mm (P less than .001). The other two groups received 30 mcg of sprifermin in three weekly injections every 6 months (group 3) or every 12 months (group 4), and these had TFJ cartilage thickness losses of about 0.01 mm or less.
Similar dose-dependent relationships were observed for some of the secondary endpoints, which included changes in cartilage thickness seen in the medial and lateral compartments, changes in cartilage thickness in the compartments’ subregions, and changes in mJSW. Significant differences in cartilage thickness were observed between sprifermin treatment groups and placebo in the medial (group 1, gain of 0.02 mm vs. loss of 0.03 mm; P less than .001) and lateral (groups 1 and 2, gain of 0.04 mm vs. loss of 0.01 mm; P less than .001) TFJ compartments, and in central medial and lateral TFJ subregions.
Changes in mJSW as seen on x-ray between those in group 1 and those on placebo were significant for the lateral compartment, with an increase in mJSW at the higher doses and a decline in the placebo group, but not for the medial compartment.
There were no significant differences in Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores among the treatment groups. Dr. Hochberg noted that patients were permitted to take pain medications during the study, which could have affected this result.
The most frequently reported adverse events were musculoskeletal and connective tissue disorders, specifically arthralgias and back pain, Dr. Hochberg said. The incidence of acute inflammatory reactions was higher with sprifermin, compared with placebo, but the increase was only significant after the first injection cycle, he said.
Merck KGaA and the EMD Serono Research Institute funded the study. Dr. Hochberg reported receiving consulting fees from numerous companies that market or are developing OA drugs, including EMD Serono.
REPORTING FROM ACR 2017
Key clinical point: Sprifermin may help build knee joint cartilage in patients with OA.
Major finding: Patients taking sprifermin 100 mcg three times a week every 6 months or every 12 months had gains in tibiofemoral joint cartilage thickness of 0.03 mm and 0.02 mm, respectively, over a 2-year period.
Study details: A study of 549 patients with symptomatic knee OA randomized to receive either 30 mcg or 100 mcg of sprifermin three times a week every 6 or every 12 months, or placebo.
Disclosures: Merck KGaA and the EMD Serono Research Institute funded the study. The presenter reported receiving consulting fees from numerous companies that market or are developing OA drugs, including EMD Serono.
Source: Hochberg M et al. ACR 2017 abstract 1L.
‘Multimorbidities’ in RA make impact on treatment efficacy, disease activity
While clinicians are accustomed to managing patients with rheumatoid arthritis and comorbidities, RA patients more frequently have “multimorbidities” – the simultaneous presence of two or more chronic conditions.
“For anyone who sees patients, this is the reality of our lives,” said Jeffrey R. Curtis, MD, at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. Some conditions are “bystanders” that don’t impact RA, some are risk factors for RA, and some can be caused by RA treatments, said Dr. Curtis, professor of medicine and William J. Koopman Professor in Rheumatology and Immunology at the University of Alabama at Birmingham. He discussed several comorbid conditions and their interactions with RA.
Obesity’s toll on disease activity
It’s long been recognized that obesity along with RA makes things worse for patients, he said: “If you have a patient who is obese, with a BMI [body mass index] over 30-35, they’re less likely clinically to achieve low disease activity or remission.”
Obesity also affects biomarkers, he said. The multibiomarker disease activity test, marketed as Vectra DA, incorporates several adipokines in its score, but given any level of RA disease, that score is roughly 5 units higher if someone is obese (Sem Arthritis Rheum. 2017 Aug 2. doi: 10.1016/j.semarthrit.2017.07.010). “The company that manufactures the test is working on an adjustment factor to optimize the role of obesity and what those adipokines are doing to better predict x-ray progression to take this influence into account,” he said. “How we measure obesity and how we’re measuring RA are probably very much affected by this issue.”
RA and the somatization comorbidity phenotype
A second common co-occurrence is what Dr. Curtis calls a somatization comorbidity phenotype (SCP), defined as RA with several other ailments such as fibromyalgia, depression, anxiety, sleep apnea, or neuropathy. “The troubling thing about this is I could cure this patient’s RA tomorrow, but they actually wouldn’t feel better in their overall health because of other things dragging down their function,” he said, noting patients are more concerned about their fatigue and pain than their swollen joint count or DAS28 score.
Dr. Curtis and his colleagues completed a recent analysis that’s in press in Arthritis Research & Therapy of about 800 RA patients in the United States starting certolizumab pegol (Cimzia) to look for how well patients with this phenotype responded to a tumor necrosis factor (TNF) inhibitor. The percentage of patients with RA plus SCP who achieved American College of Rheumatology (ACR) 20/50/70 scores were all about 10% lower than for patients with RA alone, and about 15% fewer patients achieved remission using a score of less than 2.6 on the DAS28, based on erythrocyte sedimentation rate. There was about a 10% higher withdrawal rate among the RA plus SCP group, compared with those with RA alone, mainly for lack of efficacy, and the RA plus SCP patients had three times the rate of serious infections and twice the rate of nonserious infections of typical RA patients.
Impact on choice of therapy
Clinicians have questioned whether multimorbidities should affect the choice of RA therapy, Dr. Curtis said. One of the most vexing scenarios has been in patients with a history of cancer, he noted. There have been five studies worldwide looking at RA therapy in this population. One from Sweden (Ann Rheum Dis. 2015 Dec;74[12]:2137-43), of 240 breast cancer survivors, found no difference in the occurrence or hazard ratio of recurrent breast cancer between those treated with TNF inhibitors versus those who were not. Another study coauthored by Dr. Curtis found similar results (Arthritis Rheumatol. 2016 Dec;68[10]:2403–11).
According to the ACR’s 2015 treatment guidelines, “If your RA patient has a previously treated, solid organ malignancy, there’s no restrictions on what you and I should use,” he said. “Basically, treat them like they hadn’t had a history of cancer.” The exception is for patients with previously treated nonmelanoma skin cancer or melanoma, in whom conventional synthetic DMARDs are preferred over biologics or tofacitinib (Xeljanz). In addition, patients with previously treated lymphoproliferative disorders should be given rituximab (Rituxan) – or DMARDs, abatacept (Orencia), or tocilizumab (Actemra) – over TNF inhibitors. “This is good news,” Dr. Curtis said. “It really expands the range of options for patients, now supported by data, who have a history of cancer and bad RA.”
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Curtis receives research support or serves as a consultant for Amgen, Bristol-Myers Squibb, Corrona, Lilly, Janssen, Myriad, Pfizer, and Sanofi/Regeneron.
While clinicians are accustomed to managing patients with rheumatoid arthritis and comorbidities, RA patients more frequently have “multimorbidities” – the simultaneous presence of two or more chronic conditions.
“For anyone who sees patients, this is the reality of our lives,” said Jeffrey R. Curtis, MD, at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. Some conditions are “bystanders” that don’t impact RA, some are risk factors for RA, and some can be caused by RA treatments, said Dr. Curtis, professor of medicine and William J. Koopman Professor in Rheumatology and Immunology at the University of Alabama at Birmingham. He discussed several comorbid conditions and their interactions with RA.
Obesity’s toll on disease activity
It’s long been recognized that obesity along with RA makes things worse for patients, he said: “If you have a patient who is obese, with a BMI [body mass index] over 30-35, they’re less likely clinically to achieve low disease activity or remission.”
Obesity also affects biomarkers, he said. The multibiomarker disease activity test, marketed as Vectra DA, incorporates several adipokines in its score, but given any level of RA disease, that score is roughly 5 units higher if someone is obese (Sem Arthritis Rheum. 2017 Aug 2. doi: 10.1016/j.semarthrit.2017.07.010). “The company that manufactures the test is working on an adjustment factor to optimize the role of obesity and what those adipokines are doing to better predict x-ray progression to take this influence into account,” he said. “How we measure obesity and how we’re measuring RA are probably very much affected by this issue.”
RA and the somatization comorbidity phenotype
A second common co-occurrence is what Dr. Curtis calls a somatization comorbidity phenotype (SCP), defined as RA with several other ailments such as fibromyalgia, depression, anxiety, sleep apnea, or neuropathy. “The troubling thing about this is I could cure this patient’s RA tomorrow, but they actually wouldn’t feel better in their overall health because of other things dragging down their function,” he said, noting patients are more concerned about their fatigue and pain than their swollen joint count or DAS28 score.
Dr. Curtis and his colleagues completed a recent analysis that’s in press in Arthritis Research & Therapy of about 800 RA patients in the United States starting certolizumab pegol (Cimzia) to look for how well patients with this phenotype responded to a tumor necrosis factor (TNF) inhibitor. The percentage of patients with RA plus SCP who achieved American College of Rheumatology (ACR) 20/50/70 scores were all about 10% lower than for patients with RA alone, and about 15% fewer patients achieved remission using a score of less than 2.6 on the DAS28, based on erythrocyte sedimentation rate. There was about a 10% higher withdrawal rate among the RA plus SCP group, compared with those with RA alone, mainly for lack of efficacy, and the RA plus SCP patients had three times the rate of serious infections and twice the rate of nonserious infections of typical RA patients.
Impact on choice of therapy
Clinicians have questioned whether multimorbidities should affect the choice of RA therapy, Dr. Curtis said. One of the most vexing scenarios has been in patients with a history of cancer, he noted. There have been five studies worldwide looking at RA therapy in this population. One from Sweden (Ann Rheum Dis. 2015 Dec;74[12]:2137-43), of 240 breast cancer survivors, found no difference in the occurrence or hazard ratio of recurrent breast cancer between those treated with TNF inhibitors versus those who were not. Another study coauthored by Dr. Curtis found similar results (Arthritis Rheumatol. 2016 Dec;68[10]:2403–11).
According to the ACR’s 2015 treatment guidelines, “If your RA patient has a previously treated, solid organ malignancy, there’s no restrictions on what you and I should use,” he said. “Basically, treat them like they hadn’t had a history of cancer.” The exception is for patients with previously treated nonmelanoma skin cancer or melanoma, in whom conventional synthetic DMARDs are preferred over biologics or tofacitinib (Xeljanz). In addition, patients with previously treated lymphoproliferative disorders should be given rituximab (Rituxan) – or DMARDs, abatacept (Orencia), or tocilizumab (Actemra) – over TNF inhibitors. “This is good news,” Dr. Curtis said. “It really expands the range of options for patients, now supported by data, who have a history of cancer and bad RA.”
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Curtis receives research support or serves as a consultant for Amgen, Bristol-Myers Squibb, Corrona, Lilly, Janssen, Myriad, Pfizer, and Sanofi/Regeneron.
While clinicians are accustomed to managing patients with rheumatoid arthritis and comorbidities, RA patients more frequently have “multimorbidities” – the simultaneous presence of two or more chronic conditions.
“For anyone who sees patients, this is the reality of our lives,” said Jeffrey R. Curtis, MD, at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. Some conditions are “bystanders” that don’t impact RA, some are risk factors for RA, and some can be caused by RA treatments, said Dr. Curtis, professor of medicine and William J. Koopman Professor in Rheumatology and Immunology at the University of Alabama at Birmingham. He discussed several comorbid conditions and their interactions with RA.
Obesity’s toll on disease activity
It’s long been recognized that obesity along with RA makes things worse for patients, he said: “If you have a patient who is obese, with a BMI [body mass index] over 30-35, they’re less likely clinically to achieve low disease activity or remission.”
Obesity also affects biomarkers, he said. The multibiomarker disease activity test, marketed as Vectra DA, incorporates several adipokines in its score, but given any level of RA disease, that score is roughly 5 units higher if someone is obese (Sem Arthritis Rheum. 2017 Aug 2. doi: 10.1016/j.semarthrit.2017.07.010). “The company that manufactures the test is working on an adjustment factor to optimize the role of obesity and what those adipokines are doing to better predict x-ray progression to take this influence into account,” he said. “How we measure obesity and how we’re measuring RA are probably very much affected by this issue.”
RA and the somatization comorbidity phenotype
A second common co-occurrence is what Dr. Curtis calls a somatization comorbidity phenotype (SCP), defined as RA with several other ailments such as fibromyalgia, depression, anxiety, sleep apnea, or neuropathy. “The troubling thing about this is I could cure this patient’s RA tomorrow, but they actually wouldn’t feel better in their overall health because of other things dragging down their function,” he said, noting patients are more concerned about their fatigue and pain than their swollen joint count or DAS28 score.
Dr. Curtis and his colleagues completed a recent analysis that’s in press in Arthritis Research & Therapy of about 800 RA patients in the United States starting certolizumab pegol (Cimzia) to look for how well patients with this phenotype responded to a tumor necrosis factor (TNF) inhibitor. The percentage of patients with RA plus SCP who achieved American College of Rheumatology (ACR) 20/50/70 scores were all about 10% lower than for patients with RA alone, and about 15% fewer patients achieved remission using a score of less than 2.6 on the DAS28, based on erythrocyte sedimentation rate. There was about a 10% higher withdrawal rate among the RA plus SCP group, compared with those with RA alone, mainly for lack of efficacy, and the RA plus SCP patients had three times the rate of serious infections and twice the rate of nonserious infections of typical RA patients.
Impact on choice of therapy
Clinicians have questioned whether multimorbidities should affect the choice of RA therapy, Dr. Curtis said. One of the most vexing scenarios has been in patients with a history of cancer, he noted. There have been five studies worldwide looking at RA therapy in this population. One from Sweden (Ann Rheum Dis. 2015 Dec;74[12]:2137-43), of 240 breast cancer survivors, found no difference in the occurrence or hazard ratio of recurrent breast cancer between those treated with TNF inhibitors versus those who were not. Another study coauthored by Dr. Curtis found similar results (Arthritis Rheumatol. 2016 Dec;68[10]:2403–11).
According to the ACR’s 2015 treatment guidelines, “If your RA patient has a previously treated, solid organ malignancy, there’s no restrictions on what you and I should use,” he said. “Basically, treat them like they hadn’t had a history of cancer.” The exception is for patients with previously treated nonmelanoma skin cancer or melanoma, in whom conventional synthetic DMARDs are preferred over biologics or tofacitinib (Xeljanz). In addition, patients with previously treated lymphoproliferative disorders should be given rituximab (Rituxan) – or DMARDs, abatacept (Orencia), or tocilizumab (Actemra) – over TNF inhibitors. “This is good news,” Dr. Curtis said. “It really expands the range of options for patients, now supported by data, who have a history of cancer and bad RA.”
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Curtis receives research support or serves as a consultant for Amgen, Bristol-Myers Squibb, Corrona, Lilly, Janssen, Myriad, Pfizer, and Sanofi/Regeneron.
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Novel med-rec program keys on timely, accurate patient histories
A novel medication reconciliation program that hospitalists might adopt in their own centers will be featured Wednesday during a 4:15 p.m. session, “Medication Reconciliation Implementation: A Hospital Case.”
Dr. Shabbir’s hospital was one of six that participated in the nationwide Multi-Center Medication Reconciliation Quality Improvement Study (MARQUIS), an effort to develop a more accurate and safe med-rec process when patients enter and leave the hospital. Among other interventions, the hospitals employed pharmacy technicians in the newly created role of “medication reconciliation assistants,” responsible for taking a best possible medication history. These technicians had expertise in recognizing frequently prescribed pills and verified patient medication lists with at least two sources (i.e., local pharmacies, nursing homes, doctor’s offices). They entered the information in patients’ electronic health records and, if they noticed discrepancies, alerted the hospitalist attending.
The service “gave me, as a physician, a sigh of relief and a confidence in that history,” Dr. Shabbir said, noting that it also saved time. “In the age of polypharmacy and medication complexity, this is really new work that we didn’t have anybody to do until we had this program.”
A med-rec assistant program is worth considering, Dr. Shabbir said, as it has “the potential to impact the quadruple aim – patient outcomes, patient experience, joy in work, and lower costs.”
“Hospitalists may not appreciate the extent to which quality is often lacking in medication reconciliation,” he said. “We will teach hospitalists how to make a case for a good medication reconciliation program at their own medical centers. Not having a good history as a hospitalist – and trying to obtain it when a patient is moved up to the floor [and] the family has left with the medications, if they even were brought in – and having out-of-date medication lists is really troubling. This is something that affects anyone who’s admitting, following, or discharging a patient.
“It’s a big deal. This program perhaps will give some ideas and guidance. Hopefully, if it’s replicated, it can prevent harm to patients. There’s a very compelling case to be made for these programs from a business point of view.”
Medication Reconciliation Implementation: A Hospital Case
Wednesday, 4:15–5:20 p.m.
A novel medication reconciliation program that hospitalists might adopt in their own centers will be featured Wednesday during a 4:15 p.m. session, “Medication Reconciliation Implementation: A Hospital Case.”
Dr. Shabbir’s hospital was one of six that participated in the nationwide Multi-Center Medication Reconciliation Quality Improvement Study (MARQUIS), an effort to develop a more accurate and safe med-rec process when patients enter and leave the hospital. Among other interventions, the hospitals employed pharmacy technicians in the newly created role of “medication reconciliation assistants,” responsible for taking a best possible medication history. These technicians had expertise in recognizing frequently prescribed pills and verified patient medication lists with at least two sources (i.e., local pharmacies, nursing homes, doctor’s offices). They entered the information in patients’ electronic health records and, if they noticed discrepancies, alerted the hospitalist attending.
The service “gave me, as a physician, a sigh of relief and a confidence in that history,” Dr. Shabbir said, noting that it also saved time. “In the age of polypharmacy and medication complexity, this is really new work that we didn’t have anybody to do until we had this program.”
A med-rec assistant program is worth considering, Dr. Shabbir said, as it has “the potential to impact the quadruple aim – patient outcomes, patient experience, joy in work, and lower costs.”
“Hospitalists may not appreciate the extent to which quality is often lacking in medication reconciliation,” he said. “We will teach hospitalists how to make a case for a good medication reconciliation program at their own medical centers. Not having a good history as a hospitalist – and trying to obtain it when a patient is moved up to the floor [and] the family has left with the medications, if they even were brought in – and having out-of-date medication lists is really troubling. This is something that affects anyone who’s admitting, following, or discharging a patient.
“It’s a big deal. This program perhaps will give some ideas and guidance. Hopefully, if it’s replicated, it can prevent harm to patients. There’s a very compelling case to be made for these programs from a business point of view.”
Medication Reconciliation Implementation: A Hospital Case
Wednesday, 4:15–5:20 p.m.
A novel medication reconciliation program that hospitalists might adopt in their own centers will be featured Wednesday during a 4:15 p.m. session, “Medication Reconciliation Implementation: A Hospital Case.”
Dr. Shabbir’s hospital was one of six that participated in the nationwide Multi-Center Medication Reconciliation Quality Improvement Study (MARQUIS), an effort to develop a more accurate and safe med-rec process when patients enter and leave the hospital. Among other interventions, the hospitals employed pharmacy technicians in the newly created role of “medication reconciliation assistants,” responsible for taking a best possible medication history. These technicians had expertise in recognizing frequently prescribed pills and verified patient medication lists with at least two sources (i.e., local pharmacies, nursing homes, doctor’s offices). They entered the information in patients’ electronic health records and, if they noticed discrepancies, alerted the hospitalist attending.
The service “gave me, as a physician, a sigh of relief and a confidence in that history,” Dr. Shabbir said, noting that it also saved time. “In the age of polypharmacy and medication complexity, this is really new work that we didn’t have anybody to do until we had this program.”
A med-rec assistant program is worth considering, Dr. Shabbir said, as it has “the potential to impact the quadruple aim – patient outcomes, patient experience, joy in work, and lower costs.”
“Hospitalists may not appreciate the extent to which quality is often lacking in medication reconciliation,” he said. “We will teach hospitalists how to make a case for a good medication reconciliation program at their own medical centers. Not having a good history as a hospitalist – and trying to obtain it when a patient is moved up to the floor [and] the family has left with the medications, if they even were brought in – and having out-of-date medication lists is really troubling. This is something that affects anyone who’s admitting, following, or discharging a patient.
“It’s a big deal. This program perhaps will give some ideas and guidance. Hopefully, if it’s replicated, it can prevent harm to patients. There’s a very compelling case to be made for these programs from a business point of view.”
Medication Reconciliation Implementation: A Hospital Case
Wednesday, 4:15–5:20 p.m.
Exploring issues at academic centers affiliated with HM
How hospitalists at community hospitals can leverage resources from their larger, affiliated academic centers will be discussed Wednesday at 4:20 p.m. at “Hospitalists Affiliated with Academic Centers: Challenges & How to Address Them.”
“In a lot of community hospitals, pediatric units tend to be fairly small,” said copresenter James O’Callaghan, MD, FAAP, SFHM, lead pediatric hospitalist at a community hospital affiliated with Seattle Children’s Hospital. “You may feel you’re a little bit isolated from the main center and a very small piece of the pie. There are sometimes difficulties getting resources or quality work done, partly because, at a community site, you can’t justify spending money for QI [quality improvement] resources or helping the pediatric unit do chart review when they’ve got much bigger fish to fry in the adult world.”
During a bed crunch at the main hospital over the winter, he and his colleagues worked to accept some patients from the main hospital because the community site had some open beds.
“It was a win-win arrangement,” he said. “The community site got more patients and filled more beds, and the main hospital was able to free up beds for more patients who were postsurgical or acutely ill.”
The talk also will explore benefits such as resources, academic research experience, and the potential to recruit skilled professionals. Dr. Alvarez said, “Affiliated community-based pediatric hospitalist programs have a significant resource advantage for quality and safety initiatives, stemming from the direct affiliation with their tertiary institution that they can use to make significant care improvement within their community.”
“If you are at a single community-based hospital without any larger affiliation, the challenges are bigger because you don’t have extra resources to draw on,” he said.
Community Hospitalists Affiliated with Academic Centers: Challenges & How to Address Them
Wednesday, 4:20–5:20 p.m.
Available via HM17 On Demand
How hospitalists at community hospitals can leverage resources from their larger, affiliated academic centers will be discussed Wednesday at 4:20 p.m. at “Hospitalists Affiliated with Academic Centers: Challenges & How to Address Them.”
“In a lot of community hospitals, pediatric units tend to be fairly small,” said copresenter James O’Callaghan, MD, FAAP, SFHM, lead pediatric hospitalist at a community hospital affiliated with Seattle Children’s Hospital. “You may feel you’re a little bit isolated from the main center and a very small piece of the pie. There are sometimes difficulties getting resources or quality work done, partly because, at a community site, you can’t justify spending money for QI [quality improvement] resources or helping the pediatric unit do chart review when they’ve got much bigger fish to fry in the adult world.”
During a bed crunch at the main hospital over the winter, he and his colleagues worked to accept some patients from the main hospital because the community site had some open beds.
“It was a win-win arrangement,” he said. “The community site got more patients and filled more beds, and the main hospital was able to free up beds for more patients who were postsurgical or acutely ill.”
The talk also will explore benefits such as resources, academic research experience, and the potential to recruit skilled professionals. Dr. Alvarez said, “Affiliated community-based pediatric hospitalist programs have a significant resource advantage for quality and safety initiatives, stemming from the direct affiliation with their tertiary institution that they can use to make significant care improvement within their community.”
“If you are at a single community-based hospital without any larger affiliation, the challenges are bigger because you don’t have extra resources to draw on,” he said.
Community Hospitalists Affiliated with Academic Centers: Challenges & How to Address Them
Wednesday, 4:20–5:20 p.m.
Available via HM17 On Demand
How hospitalists at community hospitals can leverage resources from their larger, affiliated academic centers will be discussed Wednesday at 4:20 p.m. at “Hospitalists Affiliated with Academic Centers: Challenges & How to Address Them.”
“In a lot of community hospitals, pediatric units tend to be fairly small,” said copresenter James O’Callaghan, MD, FAAP, SFHM, lead pediatric hospitalist at a community hospital affiliated with Seattle Children’s Hospital. “You may feel you’re a little bit isolated from the main center and a very small piece of the pie. There are sometimes difficulties getting resources or quality work done, partly because, at a community site, you can’t justify spending money for QI [quality improvement] resources or helping the pediatric unit do chart review when they’ve got much bigger fish to fry in the adult world.”
During a bed crunch at the main hospital over the winter, he and his colleagues worked to accept some patients from the main hospital because the community site had some open beds.
“It was a win-win arrangement,” he said. “The community site got more patients and filled more beds, and the main hospital was able to free up beds for more patients who were postsurgical or acutely ill.”
The talk also will explore benefits such as resources, academic research experience, and the potential to recruit skilled professionals. Dr. Alvarez said, “Affiliated community-based pediatric hospitalist programs have a significant resource advantage for quality and safety initiatives, stemming from the direct affiliation with their tertiary institution that they can use to make significant care improvement within their community.”
“If you are at a single community-based hospital without any larger affiliation, the challenges are bigger because you don’t have extra resources to draw on,” he said.
Community Hospitalists Affiliated with Academic Centers: Challenges & How to Address Them
Wednesday, 4:20–5:20 p.m.
Available via HM17 On Demand