User login
FDA expands fingolimod's indications to treat relapsing MS in pediatric patients
Pediatric patients with
The Food and Drug Administration approved on May 11 an expanded indication of the drug to allow it to be used to treat relapsing MS in children and adolescents age 10 and older. Gilenya was first approved by FDA to treat adults with relapsing MS in 2010.
“For the first time, we have an FDA-approved treatment specifically for children and adolescents with multiple sclerosis,” Billy Dunn, MD, director of the Division of Neurology Products in the FDA Center for Drug Evaluation and Research, said in a statement. “This represents an important and needed advance in the care of pediatric patients with multiple sclerosis.”
Side effects for fingolimod in pediatric trial participants were similar to those experienced in adults, the most common of which include headache, liver enzyme elevation, diarrhea, cough, flu, sinusitis, back pain, abdominal pain, and pain in extremities. The drug must be dispensed with a medication guide that describes the product’s more serious risks.
The FDA, which granted the product a priority review and breakthrough designation for this indication, noted that 2%-5% of people with MS have symptoms onset before age 18 and suggested that 8,000-10,000 children and adolescents in the United States suffer from the disease.
The clinical trial evaluating the drug included 214 patients aged 10-17 years and compared fingolimod with interferon beta-1a. In that study, the FDA stated that 86% of patients receiving fingolimod remained relapse-free after 24 months of treatment, compared with 46% of those treated with interferon beta-1a.
Pediatric patients with
The Food and Drug Administration approved on May 11 an expanded indication of the drug to allow it to be used to treat relapsing MS in children and adolescents age 10 and older. Gilenya was first approved by FDA to treat adults with relapsing MS in 2010.
“For the first time, we have an FDA-approved treatment specifically for children and adolescents with multiple sclerosis,” Billy Dunn, MD, director of the Division of Neurology Products in the FDA Center for Drug Evaluation and Research, said in a statement. “This represents an important and needed advance in the care of pediatric patients with multiple sclerosis.”
Side effects for fingolimod in pediatric trial participants were similar to those experienced in adults, the most common of which include headache, liver enzyme elevation, diarrhea, cough, flu, sinusitis, back pain, abdominal pain, and pain in extremities. The drug must be dispensed with a medication guide that describes the product’s more serious risks.
The FDA, which granted the product a priority review and breakthrough designation for this indication, noted that 2%-5% of people with MS have symptoms onset before age 18 and suggested that 8,000-10,000 children and adolescents in the United States suffer from the disease.
The clinical trial evaluating the drug included 214 patients aged 10-17 years and compared fingolimod with interferon beta-1a. In that study, the FDA stated that 86% of patients receiving fingolimod remained relapse-free after 24 months of treatment, compared with 46% of those treated with interferon beta-1a.
Pediatric patients with
The Food and Drug Administration approved on May 11 an expanded indication of the drug to allow it to be used to treat relapsing MS in children and adolescents age 10 and older. Gilenya was first approved by FDA to treat adults with relapsing MS in 2010.
“For the first time, we have an FDA-approved treatment specifically for children and adolescents with multiple sclerosis,” Billy Dunn, MD, director of the Division of Neurology Products in the FDA Center for Drug Evaluation and Research, said in a statement. “This represents an important and needed advance in the care of pediatric patients with multiple sclerosis.”
Side effects for fingolimod in pediatric trial participants were similar to those experienced in adults, the most common of which include headache, liver enzyme elevation, diarrhea, cough, flu, sinusitis, back pain, abdominal pain, and pain in extremities. The drug must be dispensed with a medication guide that describes the product’s more serious risks.
The FDA, which granted the product a priority review and breakthrough designation for this indication, noted that 2%-5% of people with MS have symptoms onset before age 18 and suggested that 8,000-10,000 children and adolescents in the United States suffer from the disease.
The clinical trial evaluating the drug included 214 patients aged 10-17 years and compared fingolimod with interferon beta-1a. In that study, the FDA stated that 86% of patients receiving fingolimod remained relapse-free after 24 months of treatment, compared with 46% of those treated with interferon beta-1a.
White House targets CHIP, CMMI for budget cuts
The package also includes an $800 million cut to the Centers for Medicare & Medicaid Innovation (CMMI), a policy incubator that the Centers for Medicare & Medicaid Services uses to test innovative payment approaches.
These funds were a one-time, additional appropriation “to reimburse states for eligible CHIP expenses.” According to the rescission request, the authority “to obligate these funds to states expired on Sept. 30, 2017, and the remaining funding is no longer needed.”
A second request would rescind nearly $1.9 billion from the Child Enrollment Contingency Fund, which provides payments to states that experience shortfalls from higher-than-expected enrollment. The White House notes that there were $2.4 billion available as of March 23, 2018, and that CMS “does not expect that any state would require a contingency fund payment in fiscal year 2018; therefore, this funding is not needed.”
If enacted, these rescissions “would have no programmatic impact,” according to the White House.
A broad coalition of physicians’ organizations and other advocates protested the proposed cuts in a May 9 letter to Congress.
“While White House officials insist that the CHIP cuts would not harm access to care for children and families, that is simply not the case,” according to the letter signed by more than 500 organizations. “Our nation is facing an increasing number of natural disasters ... [and] each catastrophe leaves families more vulnerable and more likely to qualify for CHIP. In addition to natural disasters, the Child Enrollment Contingency Fund provides states needed protection and security should their CHIP enrollment suddenly spike due to an economic recession or a public health crisis.”
Regarding the CMMI cut, the $800,000 would be rescinded from funds appropriated through fiscal year 2019. The White House notes that $3.5 billion was available as of Oct. 1, 2017.
The rescission represents funds that “are in excess of amounts needed to carry out the Innovation Center’s planned activities in fiscal 2018 and fiscal 2019, and the Innovation Center will receive new mandatory appropriation in fiscal 2020. Enacting this rescission would allow the Innovation Center to continue its current activity, initiate new activity, and continue to pay for its administrative costs,” according to the rescission request.
CMMI “is our best hope to innovate & achieve better health outcomes at lower costs,” Patrick Conway, MD, former deputy administrator and chief medical officer at CMS said in a May 8 tweet. “Cutting $800M or any amount would be a bad decision. CMS spends over $1 trillion per year; over $100M/hour. CMMI is key to better system.”
American Academy of Family Physicians President Michael Munger, MD, agreed.
“We’re also concerned about the $800 million rescission in funding for the CMS Innovation Center,” he said. “This initiative has been at the forefront of innovative demonstrations that can pave the way for a health care system that improves the quality of care and cuts health care spending.”
Congress has 45 days to act on the White House’s rescission proposal. If it takes no action, the proposal expires and the funds cannot be targeted by the same mechanism in the future. If Congress addresses the rescission request, it would need just a simple majority to pass each chamber.
Despite slashing more than $15 billion from the budget, the White House notes that the rescission request would reduce spending by only $3 billion.
The package also includes an $800 million cut to the Centers for Medicare & Medicaid Innovation (CMMI), a policy incubator that the Centers for Medicare & Medicaid Services uses to test innovative payment approaches.
These funds were a one-time, additional appropriation “to reimburse states for eligible CHIP expenses.” According to the rescission request, the authority “to obligate these funds to states expired on Sept. 30, 2017, and the remaining funding is no longer needed.”
A second request would rescind nearly $1.9 billion from the Child Enrollment Contingency Fund, which provides payments to states that experience shortfalls from higher-than-expected enrollment. The White House notes that there were $2.4 billion available as of March 23, 2018, and that CMS “does not expect that any state would require a contingency fund payment in fiscal year 2018; therefore, this funding is not needed.”
If enacted, these rescissions “would have no programmatic impact,” according to the White House.
A broad coalition of physicians’ organizations and other advocates protested the proposed cuts in a May 9 letter to Congress.
“While White House officials insist that the CHIP cuts would not harm access to care for children and families, that is simply not the case,” according to the letter signed by more than 500 organizations. “Our nation is facing an increasing number of natural disasters ... [and] each catastrophe leaves families more vulnerable and more likely to qualify for CHIP. In addition to natural disasters, the Child Enrollment Contingency Fund provides states needed protection and security should their CHIP enrollment suddenly spike due to an economic recession or a public health crisis.”
Regarding the CMMI cut, the $800,000 would be rescinded from funds appropriated through fiscal year 2019. The White House notes that $3.5 billion was available as of Oct. 1, 2017.
The rescission represents funds that “are in excess of amounts needed to carry out the Innovation Center’s planned activities in fiscal 2018 and fiscal 2019, and the Innovation Center will receive new mandatory appropriation in fiscal 2020. Enacting this rescission would allow the Innovation Center to continue its current activity, initiate new activity, and continue to pay for its administrative costs,” according to the rescission request.
CMMI “is our best hope to innovate & achieve better health outcomes at lower costs,” Patrick Conway, MD, former deputy administrator and chief medical officer at CMS said in a May 8 tweet. “Cutting $800M or any amount would be a bad decision. CMS spends over $1 trillion per year; over $100M/hour. CMMI is key to better system.”
American Academy of Family Physicians President Michael Munger, MD, agreed.
“We’re also concerned about the $800 million rescission in funding for the CMS Innovation Center,” he said. “This initiative has been at the forefront of innovative demonstrations that can pave the way for a health care system that improves the quality of care and cuts health care spending.”
Congress has 45 days to act on the White House’s rescission proposal. If it takes no action, the proposal expires and the funds cannot be targeted by the same mechanism in the future. If Congress addresses the rescission request, it would need just a simple majority to pass each chamber.
Despite slashing more than $15 billion from the budget, the White House notes that the rescission request would reduce spending by only $3 billion.
The package also includes an $800 million cut to the Centers for Medicare & Medicaid Innovation (CMMI), a policy incubator that the Centers for Medicare & Medicaid Services uses to test innovative payment approaches.
These funds were a one-time, additional appropriation “to reimburse states for eligible CHIP expenses.” According to the rescission request, the authority “to obligate these funds to states expired on Sept. 30, 2017, and the remaining funding is no longer needed.”
A second request would rescind nearly $1.9 billion from the Child Enrollment Contingency Fund, which provides payments to states that experience shortfalls from higher-than-expected enrollment. The White House notes that there were $2.4 billion available as of March 23, 2018, and that CMS “does not expect that any state would require a contingency fund payment in fiscal year 2018; therefore, this funding is not needed.”
If enacted, these rescissions “would have no programmatic impact,” according to the White House.
A broad coalition of physicians’ organizations and other advocates protested the proposed cuts in a May 9 letter to Congress.
“While White House officials insist that the CHIP cuts would not harm access to care for children and families, that is simply not the case,” according to the letter signed by more than 500 organizations. “Our nation is facing an increasing number of natural disasters ... [and] each catastrophe leaves families more vulnerable and more likely to qualify for CHIP. In addition to natural disasters, the Child Enrollment Contingency Fund provides states needed protection and security should their CHIP enrollment suddenly spike due to an economic recession or a public health crisis.”
Regarding the CMMI cut, the $800,000 would be rescinded from funds appropriated through fiscal year 2019. The White House notes that $3.5 billion was available as of Oct. 1, 2017.
The rescission represents funds that “are in excess of amounts needed to carry out the Innovation Center’s planned activities in fiscal 2018 and fiscal 2019, and the Innovation Center will receive new mandatory appropriation in fiscal 2020. Enacting this rescission would allow the Innovation Center to continue its current activity, initiate new activity, and continue to pay for its administrative costs,” according to the rescission request.
CMMI “is our best hope to innovate & achieve better health outcomes at lower costs,” Patrick Conway, MD, former deputy administrator and chief medical officer at CMS said in a May 8 tweet. “Cutting $800M or any amount would be a bad decision. CMS spends over $1 trillion per year; over $100M/hour. CMMI is key to better system.”
American Academy of Family Physicians President Michael Munger, MD, agreed.
“We’re also concerned about the $800 million rescission in funding for the CMS Innovation Center,” he said. “This initiative has been at the forefront of innovative demonstrations that can pave the way for a health care system that improves the quality of care and cuts health care spending.”
Congress has 45 days to act on the White House’s rescission proposal. If it takes no action, the proposal expires and the funds cannot be targeted by the same mechanism in the future. If Congress addresses the rescission request, it would need just a simple majority to pass each chamber.
Despite slashing more than $15 billion from the budget, the White House notes that the rescission request would reduce spending by only $3 billion.
CMS floats Medicare direct provider contracting
Under a direct provider contracting (DPC) arrangement, Medicare could pay physicians or physician groups a monthly fee to deliver a specific set of services to beneficiaries, who would gain greater access to the physicians. The physicians would be accountable for those Medicare patients’ costs and care quality.
CMS is looking at how to incorporate this concept into the Medicare ranks. On April 23, CMS issued a request for information (RFI) seeking input across a wide range of topics, including provider/state participation, beneficiary participation, payment, general model design, program integrity and beneficiary protection, and how such models would fit within the existing accountable care organization framework.
The RFI offered one possible vision on how a direct provider contracting model could work.
“Under a primary care–focused DPC model, CMS could enter into arrangements with primary care practices under which CMS would pay these participating practices a fixed per beneficiary per month (PBPM) payment to cover the primary care services the practice would be expected to furnish under the model, which may include office visits, certain office-based procedures, and other non–visit-based services covered under the physician fee schedule, and flexibility in how otherwise billable services are delivered,” the RFI states.
Physicians could also earn performance bonuses, depending on how the DPC is structured, through “performance-based incentives for total cost of care and quality.”
CMS noted it also “could test ways to reduce administrative burden though innovative changes to claims submission processes for services included in the PBPM payment under these models.”
The direct provider contracting idea grew out of a previous RFI issued in 2017 by CMS’s Center for Medicare and Medicaid Innovation to collect ideas on new ways to deliver patient-centered care. The agency released the more than 1,000 comments received from that request on the same day it issued the RFI on direct provider contracting.
In those comments, a number of physician groups offered support for a direct-contracting approach.
For example, the American Academy of Family Physicians wrote that it “sees continued growth and interest in family physicians adopting this practice model in all settings types, including rural and underserved communities.” And the AAFP suggested that the innovation center should work with DPC organizations to learn more about them.
The American College of Physicians reiterated its previous position that it “supports physician and patient choice of practice and delivery models that are accessible, ethical, and viable and that strengthen the patient-physician relationship.” But the ACP raised a number of issues that could impede access to care or result in lower quality care.
The American Medical Association offered support for “testing of models in which physicians have the ability to deliver more or different services to patients who need them and to be paid more for doing so.”
The AMA suggested that some of the models to be tested include allowing patients to contract directly with physicians, with Medicare paying its fee schedule rates and patients paying the difference; allowing patients to receive their care from DPC practices and get reimbursed by Medicare; or allowing “physicians to define a team of providers who will provide all of the treatment needed for an acute condition or management of a chronic condition, and then allowing patients who select the team to receive all of the services related to their condition from the team in return for a single predefined cost-sharing amount.”
Comments on the RFI are due May 25.
Under a direct provider contracting (DPC) arrangement, Medicare could pay physicians or physician groups a monthly fee to deliver a specific set of services to beneficiaries, who would gain greater access to the physicians. The physicians would be accountable for those Medicare patients’ costs and care quality.
CMS is looking at how to incorporate this concept into the Medicare ranks. On April 23, CMS issued a request for information (RFI) seeking input across a wide range of topics, including provider/state participation, beneficiary participation, payment, general model design, program integrity and beneficiary protection, and how such models would fit within the existing accountable care organization framework.
The RFI offered one possible vision on how a direct provider contracting model could work.
“Under a primary care–focused DPC model, CMS could enter into arrangements with primary care practices under which CMS would pay these participating practices a fixed per beneficiary per month (PBPM) payment to cover the primary care services the practice would be expected to furnish under the model, which may include office visits, certain office-based procedures, and other non–visit-based services covered under the physician fee schedule, and flexibility in how otherwise billable services are delivered,” the RFI states.
Physicians could also earn performance bonuses, depending on how the DPC is structured, through “performance-based incentives for total cost of care and quality.”
CMS noted it also “could test ways to reduce administrative burden though innovative changes to claims submission processes for services included in the PBPM payment under these models.”
The direct provider contracting idea grew out of a previous RFI issued in 2017 by CMS’s Center for Medicare and Medicaid Innovation to collect ideas on new ways to deliver patient-centered care. The agency released the more than 1,000 comments received from that request on the same day it issued the RFI on direct provider contracting.
In those comments, a number of physician groups offered support for a direct-contracting approach.
For example, the American Academy of Family Physicians wrote that it “sees continued growth and interest in family physicians adopting this practice model in all settings types, including rural and underserved communities.” And the AAFP suggested that the innovation center should work with DPC organizations to learn more about them.
The American College of Physicians reiterated its previous position that it “supports physician and patient choice of practice and delivery models that are accessible, ethical, and viable and that strengthen the patient-physician relationship.” But the ACP raised a number of issues that could impede access to care or result in lower quality care.
The American Medical Association offered support for “testing of models in which physicians have the ability to deliver more or different services to patients who need them and to be paid more for doing so.”
The AMA suggested that some of the models to be tested include allowing patients to contract directly with physicians, with Medicare paying its fee schedule rates and patients paying the difference; allowing patients to receive their care from DPC practices and get reimbursed by Medicare; or allowing “physicians to define a team of providers who will provide all of the treatment needed for an acute condition or management of a chronic condition, and then allowing patients who select the team to receive all of the services related to their condition from the team in return for a single predefined cost-sharing amount.”
Comments on the RFI are due May 25.
Under a direct provider contracting (DPC) arrangement, Medicare could pay physicians or physician groups a monthly fee to deliver a specific set of services to beneficiaries, who would gain greater access to the physicians. The physicians would be accountable for those Medicare patients’ costs and care quality.
CMS is looking at how to incorporate this concept into the Medicare ranks. On April 23, CMS issued a request for information (RFI) seeking input across a wide range of topics, including provider/state participation, beneficiary participation, payment, general model design, program integrity and beneficiary protection, and how such models would fit within the existing accountable care organization framework.
The RFI offered one possible vision on how a direct provider contracting model could work.
“Under a primary care–focused DPC model, CMS could enter into arrangements with primary care practices under which CMS would pay these participating practices a fixed per beneficiary per month (PBPM) payment to cover the primary care services the practice would be expected to furnish under the model, which may include office visits, certain office-based procedures, and other non–visit-based services covered under the physician fee schedule, and flexibility in how otherwise billable services are delivered,” the RFI states.
Physicians could also earn performance bonuses, depending on how the DPC is structured, through “performance-based incentives for total cost of care and quality.”
CMS noted it also “could test ways to reduce administrative burden though innovative changes to claims submission processes for services included in the PBPM payment under these models.”
The direct provider contracting idea grew out of a previous RFI issued in 2017 by CMS’s Center for Medicare and Medicaid Innovation to collect ideas on new ways to deliver patient-centered care. The agency released the more than 1,000 comments received from that request on the same day it issued the RFI on direct provider contracting.
In those comments, a number of physician groups offered support for a direct-contracting approach.
For example, the American Academy of Family Physicians wrote that it “sees continued growth and interest in family physicians adopting this practice model in all settings types, including rural and underserved communities.” And the AAFP suggested that the innovation center should work with DPC organizations to learn more about them.
The American College of Physicians reiterated its previous position that it “supports physician and patient choice of practice and delivery models that are accessible, ethical, and viable and that strengthen the patient-physician relationship.” But the ACP raised a number of issues that could impede access to care or result in lower quality care.
The American Medical Association offered support for “testing of models in which physicians have the ability to deliver more or different services to patients who need them and to be paid more for doing so.”
The AMA suggested that some of the models to be tested include allowing patients to contract directly with physicians, with Medicare paying its fee schedule rates and patients paying the difference; allowing patients to receive their care from DPC practices and get reimbursed by Medicare; or allowing “physicians to define a team of providers who will provide all of the treatment needed for an acute condition or management of a chronic condition, and then allowing patients who select the team to receive all of the services related to their condition from the team in return for a single predefined cost-sharing amount.”
Comments on the RFI are due May 25.
CMS will release Medicare Advantage claims data to researchers
WASHINGTON – Centers for Medicare & Medicaid Services Administrator Seema Verma announced.
“We recognize that the Medicare Advantage data are not perfect, but we have determined that the quality of the available data is adequate enough to support research,” Ms. Verma told attendees April 26 at an annual conference on health data and innovation.
CMS is starting with the Medicare managed care plans’ encounter data from 2015, and Ms. Verma said the data will be updated annually.
In addition, she announced that in 2019 CMS will make Medicaid and Children’s Health Insurance Program data available. That will give researchers access to data from another 70 million patients.
Ms. Verma noted that the Medicaid population includes a range of people, including people with disabilities, pregnant women, children, and low-income adults. Those low-income adults “often experience multiple health issues and face challenges managing their care,” Ms. Verma noted. “Our hope is that these data will be used for critical research on this vulnerable population.”
CMS also will look to the health information technology developer community to create open application program interface tools “to modernize how we share data with our partners,” she said. That push is part of the overall MyHealthEData initiative to improve patient access to their health data and become more informed about their own health care.
“Who knows what knowledge, treatments, and cures are hidden in the reams of CMS data?” Ms. Verma said. “Help us use it securely. After all, this is knowledge that could change the life of a patient or the trajectory of a health care system.”
WASHINGTON – Centers for Medicare & Medicaid Services Administrator Seema Verma announced.
“We recognize that the Medicare Advantage data are not perfect, but we have determined that the quality of the available data is adequate enough to support research,” Ms. Verma told attendees April 26 at an annual conference on health data and innovation.
CMS is starting with the Medicare managed care plans’ encounter data from 2015, and Ms. Verma said the data will be updated annually.
In addition, she announced that in 2019 CMS will make Medicaid and Children’s Health Insurance Program data available. That will give researchers access to data from another 70 million patients.
Ms. Verma noted that the Medicaid population includes a range of people, including people with disabilities, pregnant women, children, and low-income adults. Those low-income adults “often experience multiple health issues and face challenges managing their care,” Ms. Verma noted. “Our hope is that these data will be used for critical research on this vulnerable population.”
CMS also will look to the health information technology developer community to create open application program interface tools “to modernize how we share data with our partners,” she said. That push is part of the overall MyHealthEData initiative to improve patient access to their health data and become more informed about their own health care.
“Who knows what knowledge, treatments, and cures are hidden in the reams of CMS data?” Ms. Verma said. “Help us use it securely. After all, this is knowledge that could change the life of a patient or the trajectory of a health care system.”
WASHINGTON – Centers for Medicare & Medicaid Services Administrator Seema Verma announced.
“We recognize that the Medicare Advantage data are not perfect, but we have determined that the quality of the available data is adequate enough to support research,” Ms. Verma told attendees April 26 at an annual conference on health data and innovation.
CMS is starting with the Medicare managed care plans’ encounter data from 2015, and Ms. Verma said the data will be updated annually.
In addition, she announced that in 2019 CMS will make Medicaid and Children’s Health Insurance Program data available. That will give researchers access to data from another 70 million patients.
Ms. Verma noted that the Medicaid population includes a range of people, including people with disabilities, pregnant women, children, and low-income adults. Those low-income adults “often experience multiple health issues and face challenges managing their care,” Ms. Verma noted. “Our hope is that these data will be used for critical research on this vulnerable population.”
CMS also will look to the health information technology developer community to create open application program interface tools “to modernize how we share data with our partners,” she said. That push is part of the overall MyHealthEData initiative to improve patient access to their health data and become more informed about their own health care.
“Who knows what knowledge, treatments, and cures are hidden in the reams of CMS data?” Ms. Verma said. “Help us use it securely. After all, this is knowledge that could change the life of a patient or the trajectory of a health care system.”
REPORTING FROM HEALTH DATAPALOOZA 2018
What’s in a name? Get ready for the feds’ Promoting Interoperability program
The federal EHR Incentive Program – a program most doctors love to hate – is getting a new name to better reflect a focus on interoperability and improved patient access to their health care data, the Centers for Medicare & Medicaid Services announced.
For clinicians who participate in fee-for-service Medicare, eligible hospitals, and critical access hospitals, the new name of the program will be the Promoting Interoperability Program. For those participating in the Merit-Based Incentive Payment System (MIPS) track of the Quality Payment Program (QPP), the Advancing Care Information performance category will be rebranded the Promoting Interoperability performance category.
The first steps of these changes were announced April 25 in the 2019 proposed rule for the inpatient prospective payment system and the Long-Term Care Hospital Prospective Payment System.
CMS notes in the proposed rule that the name EHR Incentive Program “does not adequately reflect the current status of the programs, as the incentive payments under Medicare generally have ended.” Eligible medical professionals and hospitals have received Medicare bonuses for adopting and demonstrating meaningful use of EHRs. Penalties for not doing so are still in place.
The federal EHR Incentive Program – a program most doctors love to hate – is getting a new name to better reflect a focus on interoperability and improved patient access to their health care data, the Centers for Medicare & Medicaid Services announced.
For clinicians who participate in fee-for-service Medicare, eligible hospitals, and critical access hospitals, the new name of the program will be the Promoting Interoperability Program. For those participating in the Merit-Based Incentive Payment System (MIPS) track of the Quality Payment Program (QPP), the Advancing Care Information performance category will be rebranded the Promoting Interoperability performance category.
The first steps of these changes were announced April 25 in the 2019 proposed rule for the inpatient prospective payment system and the Long-Term Care Hospital Prospective Payment System.
CMS notes in the proposed rule that the name EHR Incentive Program “does not adequately reflect the current status of the programs, as the incentive payments under Medicare generally have ended.” Eligible medical professionals and hospitals have received Medicare bonuses for adopting and demonstrating meaningful use of EHRs. Penalties for not doing so are still in place.
The federal EHR Incentive Program – a program most doctors love to hate – is getting a new name to better reflect a focus on interoperability and improved patient access to their health care data, the Centers for Medicare & Medicaid Services announced.
For clinicians who participate in fee-for-service Medicare, eligible hospitals, and critical access hospitals, the new name of the program will be the Promoting Interoperability Program. For those participating in the Merit-Based Incentive Payment System (MIPS) track of the Quality Payment Program (QPP), the Advancing Care Information performance category will be rebranded the Promoting Interoperability performance category.
The first steps of these changes were announced April 25 in the 2019 proposed rule for the inpatient prospective payment system and the Long-Term Care Hospital Prospective Payment System.
CMS notes in the proposed rule that the name EHR Incentive Program “does not adequately reflect the current status of the programs, as the incentive payments under Medicare generally have ended.” Eligible medical professionals and hospitals have received Medicare bonuses for adopting and demonstrating meaningful use of EHRs. Penalties for not doing so are still in place.
Will patients get on board with CMS’s new health data approach?
CMS is turning to patients to help drive health information technology toward greater interoperability, accessibility, and usability – elusive goals that have not been reached by working with health care professionals and IT vendors alone.
“MyHealthEData makes it clear that patients should have access and control to share their data with whomever they want, making the patient the center of our health system,” Seema Verma, administrator of the Centers for Medicare & Medicaid Services, said at the annual HIMSS conference. “Patients need to be able to control their information and know that it is secure and private. Having access to their medical information will help them make decisions about their care and have a better understanding of their health.”
She added that “once information is freely flowing from the patient to the provider, the advances in coordinated, value-based care and patient-centric care will be even greater than anything we can imagine today.”
Patients could share their data with applications designed to help them make more informed health care decisions of with their providers and caregivers to help better manage their care,” Ms. Verma said in an interview.
For example, the initiative could lead to the development of products such as the following:
- Mobile apps to help patients manage medications and medical appointments.
- Simple processes that carry patients’ data when they switch providers or health insurance plans.
- Wearables, such as step trackers or glucose monitors, that are linked to patients’ clinical record.
To get to this future health IT nirvana, CMS will need to address the ongoing interoperability issues that continue to plague EHRs. And that’s where CMS is turning its efforts back to helping clinicians.
The agency “will be announcing a complete overhaul of the meaningful use program for hospitals and the advancing care information performance category of the Quality Payment Program,” Ms. Verma announced at HIMSS.
A complete overhaul is “the appropriate approach,” and one that doctors could support, according to a spokesman for the American Medical Association. “We are not talking about painting the walls. We are talking about stripping things down to the bare studs and coming back to this and looking at it from a fresh start.
“We have a perfect storm of opportunity here to really rethink the program,” the spokesman said. “It’s not just rethinking the program from the standpoint of burden; it’s rethinking CMS programs from the standpoint of collapsing some of the reporting into to one holistic picture where the doctor is participating with their patients in chronic care or different care teams or the patient-centered medical home.”
And fixing those regulations is going to go a long way in helping patients to actually interact with their data and to really put them in the center of their care; this is especially important since EHRs are currently built to comply with reporting requirements and not necessarily to help improve care, the AMA spokesman noted. And while current patient portal requirements promote engagement, the information is not necessarily useful for patients.
“What we see as the next step is an opportunity here to reduce some of the prescriptive nature of the regulation on the design and use of EHRs,” the spokesman said. “It is an interesting way to look at it because, while we want to encourage patients to access their complete record set, the front-to-back side of their entire medical record is not always available due to EHR design.”
Indeed, at HIMSS Ms. Verma said that “for those of you that still subscribe to the outdated idea that you can deny patients’ access to their health records, I encourage you, in the strongest way, to change course and accept that those practices will come to an end.”
CMS currently does not have a specific timeline for all the aspects of the MyHealthEData initiative, but Ms. Verma did note that the agency already is working with vendors on one aspect: Blue Button 2.0, a developer-friendly, standards-based developer tool that will allow the creation of apps to connect Medicare claims data to programs that can help create context and make informed medical decisions easier.
In 2019, new EHR requirements will follow that will require data to be shareable in a more standardized manner.
“Through the MyHealthEData initiative, this administration is focused on putting patients first, truly first, by empowering them to make cost and quality decisions, and giving them the information they need to prevent disease and improve their health,” Ms. Verma said.
CMS is turning to patients to help drive health information technology toward greater interoperability, accessibility, and usability – elusive goals that have not been reached by working with health care professionals and IT vendors alone.
“MyHealthEData makes it clear that patients should have access and control to share their data with whomever they want, making the patient the center of our health system,” Seema Verma, administrator of the Centers for Medicare & Medicaid Services, said at the annual HIMSS conference. “Patients need to be able to control their information and know that it is secure and private. Having access to their medical information will help them make decisions about their care and have a better understanding of their health.”
She added that “once information is freely flowing from the patient to the provider, the advances in coordinated, value-based care and patient-centric care will be even greater than anything we can imagine today.”
Patients could share their data with applications designed to help them make more informed health care decisions of with their providers and caregivers to help better manage their care,” Ms. Verma said in an interview.
For example, the initiative could lead to the development of products such as the following:
- Mobile apps to help patients manage medications and medical appointments.
- Simple processes that carry patients’ data when they switch providers or health insurance plans.
- Wearables, such as step trackers or glucose monitors, that are linked to patients’ clinical record.
To get to this future health IT nirvana, CMS will need to address the ongoing interoperability issues that continue to plague EHRs. And that’s where CMS is turning its efforts back to helping clinicians.
The agency “will be announcing a complete overhaul of the meaningful use program for hospitals and the advancing care information performance category of the Quality Payment Program,” Ms. Verma announced at HIMSS.
A complete overhaul is “the appropriate approach,” and one that doctors could support, according to a spokesman for the American Medical Association. “We are not talking about painting the walls. We are talking about stripping things down to the bare studs and coming back to this and looking at it from a fresh start.
“We have a perfect storm of opportunity here to really rethink the program,” the spokesman said. “It’s not just rethinking the program from the standpoint of burden; it’s rethinking CMS programs from the standpoint of collapsing some of the reporting into to one holistic picture where the doctor is participating with their patients in chronic care or different care teams or the patient-centered medical home.”
And fixing those regulations is going to go a long way in helping patients to actually interact with their data and to really put them in the center of their care; this is especially important since EHRs are currently built to comply with reporting requirements and not necessarily to help improve care, the AMA spokesman noted. And while current patient portal requirements promote engagement, the information is not necessarily useful for patients.
“What we see as the next step is an opportunity here to reduce some of the prescriptive nature of the regulation on the design and use of EHRs,” the spokesman said. “It is an interesting way to look at it because, while we want to encourage patients to access their complete record set, the front-to-back side of their entire medical record is not always available due to EHR design.”
Indeed, at HIMSS Ms. Verma said that “for those of you that still subscribe to the outdated idea that you can deny patients’ access to their health records, I encourage you, in the strongest way, to change course and accept that those practices will come to an end.”
CMS currently does not have a specific timeline for all the aspects of the MyHealthEData initiative, but Ms. Verma did note that the agency already is working with vendors on one aspect: Blue Button 2.0, a developer-friendly, standards-based developer tool that will allow the creation of apps to connect Medicare claims data to programs that can help create context and make informed medical decisions easier.
In 2019, new EHR requirements will follow that will require data to be shareable in a more standardized manner.
“Through the MyHealthEData initiative, this administration is focused on putting patients first, truly first, by empowering them to make cost and quality decisions, and giving them the information they need to prevent disease and improve their health,” Ms. Verma said.
CMS is turning to patients to help drive health information technology toward greater interoperability, accessibility, and usability – elusive goals that have not been reached by working with health care professionals and IT vendors alone.
“MyHealthEData makes it clear that patients should have access and control to share their data with whomever they want, making the patient the center of our health system,” Seema Verma, administrator of the Centers for Medicare & Medicaid Services, said at the annual HIMSS conference. “Patients need to be able to control their information and know that it is secure and private. Having access to their medical information will help them make decisions about their care and have a better understanding of their health.”
She added that “once information is freely flowing from the patient to the provider, the advances in coordinated, value-based care and patient-centric care will be even greater than anything we can imagine today.”
Patients could share their data with applications designed to help them make more informed health care decisions of with their providers and caregivers to help better manage their care,” Ms. Verma said in an interview.
For example, the initiative could lead to the development of products such as the following:
- Mobile apps to help patients manage medications and medical appointments.
- Simple processes that carry patients’ data when they switch providers or health insurance plans.
- Wearables, such as step trackers or glucose monitors, that are linked to patients’ clinical record.
To get to this future health IT nirvana, CMS will need to address the ongoing interoperability issues that continue to plague EHRs. And that’s where CMS is turning its efforts back to helping clinicians.
The agency “will be announcing a complete overhaul of the meaningful use program for hospitals and the advancing care information performance category of the Quality Payment Program,” Ms. Verma announced at HIMSS.
A complete overhaul is “the appropriate approach,” and one that doctors could support, according to a spokesman for the American Medical Association. “We are not talking about painting the walls. We are talking about stripping things down to the bare studs and coming back to this and looking at it from a fresh start.
“We have a perfect storm of opportunity here to really rethink the program,” the spokesman said. “It’s not just rethinking the program from the standpoint of burden; it’s rethinking CMS programs from the standpoint of collapsing some of the reporting into to one holistic picture where the doctor is participating with their patients in chronic care or different care teams or the patient-centered medical home.”
And fixing those regulations is going to go a long way in helping patients to actually interact with their data and to really put them in the center of their care; this is especially important since EHRs are currently built to comply with reporting requirements and not necessarily to help improve care, the AMA spokesman noted. And while current patient portal requirements promote engagement, the information is not necessarily useful for patients.
“What we see as the next step is an opportunity here to reduce some of the prescriptive nature of the regulation on the design and use of EHRs,” the spokesman said. “It is an interesting way to look at it because, while we want to encourage patients to access their complete record set, the front-to-back side of their entire medical record is not always available due to EHR design.”
Indeed, at HIMSS Ms. Verma said that “for those of you that still subscribe to the outdated idea that you can deny patients’ access to their health records, I encourage you, in the strongest way, to change course and accept that those practices will come to an end.”
CMS currently does not have a specific timeline for all the aspects of the MyHealthEData initiative, but Ms. Verma did note that the agency already is working with vendors on one aspect: Blue Button 2.0, a developer-friendly, standards-based developer tool that will allow the creation of apps to connect Medicare claims data to programs that can help create context and make informed medical decisions easier.
In 2019, new EHR requirements will follow that will require data to be shareable in a more standardized manner.
“Through the MyHealthEData initiative, this administration is focused on putting patients first, truly first, by empowering them to make cost and quality decisions, and giving them the information they need to prevent disease and improve their health,” Ms. Verma said.
Doctors call for a pause to rethink MIPS measures
A “time-out” is needed to reevaluate how quality measures are used as part of Medicare’s Merit-based Incentive Payment System (MIPS), according to officials at the American College of Physicians.
according to ACP criteria.
The quality measures were assessed regarding importance, appropriate care, clinical evidence base, measure specifications, and measure feasibility and applicability.
“We also determined that the proportion of the measures that had been developed by the National Committee for Quality Assurance [NCQA] or endorsed by the National Quality Forum [NQF] that were rated as valid by our method,” Dr. MacLean and colleagues wrote. “As compared with measures that were not endorsed by these organizations, greater percentages of NCQA-developed and NQF-endorsed measures were deemed valid [59% and 48%, respectively, vs. 27% for nonendorsed measures], and smaller percentages were deemed not valid [7% and 22% vs. 49% for nonendorsed measures].”
The lack of measures that were found to be valid for primary care is frustrating for doctors and could cause harm to patients, according to the authors.
“We need a time-out during which to assess and revise our approach to physician performance measurement,” they wrote.
The ACP recommends that “physicians with expertise in clinical medicine and research develop measures using clinically relevant methodology,” President Jack Ende said in a statement. “Performance measures should be fully integrated into care delivery so they can help address the most pressing performance gaps and direct quality improvement.”
The time-out call comes amidst differing opinions on how to proceed with the MIPS track of the Quality Payment Program. The Medicare Payment Advisory Commission has recommended to Congress that MIPS be repealed and replaced, while health care experts and physician associations believe the program should stay the course, given the investments that have been made already to accommodate the program’s reporting requirements.
SOURCE: MacLean CH et al. New Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMp1802595.
A “time-out” is needed to reevaluate how quality measures are used as part of Medicare’s Merit-based Incentive Payment System (MIPS), according to officials at the American College of Physicians.
according to ACP criteria.
The quality measures were assessed regarding importance, appropriate care, clinical evidence base, measure specifications, and measure feasibility and applicability.
“We also determined that the proportion of the measures that had been developed by the National Committee for Quality Assurance [NCQA] or endorsed by the National Quality Forum [NQF] that were rated as valid by our method,” Dr. MacLean and colleagues wrote. “As compared with measures that were not endorsed by these organizations, greater percentages of NCQA-developed and NQF-endorsed measures were deemed valid [59% and 48%, respectively, vs. 27% for nonendorsed measures], and smaller percentages were deemed not valid [7% and 22% vs. 49% for nonendorsed measures].”
The lack of measures that were found to be valid for primary care is frustrating for doctors and could cause harm to patients, according to the authors.
“We need a time-out during which to assess and revise our approach to physician performance measurement,” they wrote.
The ACP recommends that “physicians with expertise in clinical medicine and research develop measures using clinically relevant methodology,” President Jack Ende said in a statement. “Performance measures should be fully integrated into care delivery so they can help address the most pressing performance gaps and direct quality improvement.”
The time-out call comes amidst differing opinions on how to proceed with the MIPS track of the Quality Payment Program. The Medicare Payment Advisory Commission has recommended to Congress that MIPS be repealed and replaced, while health care experts and physician associations believe the program should stay the course, given the investments that have been made already to accommodate the program’s reporting requirements.
SOURCE: MacLean CH et al. New Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMp1802595.
A “time-out” is needed to reevaluate how quality measures are used as part of Medicare’s Merit-based Incentive Payment System (MIPS), according to officials at the American College of Physicians.
according to ACP criteria.
The quality measures were assessed regarding importance, appropriate care, clinical evidence base, measure specifications, and measure feasibility and applicability.
“We also determined that the proportion of the measures that had been developed by the National Committee for Quality Assurance [NCQA] or endorsed by the National Quality Forum [NQF] that were rated as valid by our method,” Dr. MacLean and colleagues wrote. “As compared with measures that were not endorsed by these organizations, greater percentages of NCQA-developed and NQF-endorsed measures were deemed valid [59% and 48%, respectively, vs. 27% for nonendorsed measures], and smaller percentages were deemed not valid [7% and 22% vs. 49% for nonendorsed measures].”
The lack of measures that were found to be valid for primary care is frustrating for doctors and could cause harm to patients, according to the authors.
“We need a time-out during which to assess and revise our approach to physician performance measurement,” they wrote.
The ACP recommends that “physicians with expertise in clinical medicine and research develop measures using clinically relevant methodology,” President Jack Ende said in a statement. “Performance measures should be fully integrated into care delivery so they can help address the most pressing performance gaps and direct quality improvement.”
The time-out call comes amidst differing opinions on how to proceed with the MIPS track of the Quality Payment Program. The Medicare Payment Advisory Commission has recommended to Congress that MIPS be repealed and replaced, while health care experts and physician associations believe the program should stay the course, given the investments that have been made already to accommodate the program’s reporting requirements.
SOURCE: MacLean CH et al. New Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMp1802595.
FROM NEW ENGLAND JOURNAL OF MEDICINE
MedPAC urges CMS to curb low-value care
according to a staff presentation at a meeting of the Medicare Payment Advisory Commission.
At the commission’s April meeting, MedPAC staff presented data from various literature searches, noting a “substantial use of low-value services in Medicare.” For example, they found that about 20% of Virginians, across all payers, received a low-value service in 2014, while 15% of Medicaid patients and 11% of commercially insured patients in Oregon received a low-value service in 2013.
“It is very hard to know at the beginning of coverage that something is going to be low value,” MedPAC commission member Kathy Buto, former vice president of global health policy at Johnson & Johnson, noted. “It may be covered for something narrow for which it is high value and then spreads. It’s important to have those kinds of tools once technologies and procedures are covered to be able to actually monitor what is going on and assess.”
But, she added, the Centers for Medicare & Medicaid Services needs to do more to routinely reexamine its coverage decisions.
Ms. Buto noted that noncoverage decisions are rarely issued and suggested that “there is an opportunity for us to take a look at whether we would advise CMS to take a look at using more of those tools more aggressively.”
Paul Ginsburg, PhD, commissioner and senior fellow in economic studies at the Brookings Institution, Washington, suggested that, for any new procedure or drug, initial coverage is always provisional for a certain length of time, which would force CMS to revisit coverage decisions.
“If there is no evidence, the coverage ends,” Dr Ginsburg said. “If there is positive evidence, the coverage proceeds.”
However, as commissioner Rita Redberg, MD, of the University of California, San Francisco, said of CMS, even with tools to cut coverage, its hands may be tied by outside forces. “CMS needs a lot more political cover.”
Dr. Redberg noted that there was extensive lobbying of local carriers, and within 6 months, despite the lack of evidence, everyone was covering cardiac CT.
“A few years later, CMS tried to walk back the coverage because it was just hemorrhaging money for cardiac CT, but there was no chance because it was a capital investment,” she added. “Even when there are restrictions on coverage, CMS doesn’t enforce them.”
Ms. Buto also raised the issue of how much influence CMS has over Medicare Part D prescription drug plan sponsors’ coverage decision policies, but suggested CMS could play a larger role in that.
Commissioner Amy Bricker, vice president of supply chain strategy at Express Scripts, St. Louis, suggested that, “We need to do more from a Part D perspective to allow plans to manage drug coverage more aggressively and in line with the commercial space. CMS has handcuffed them,” noting that FDA approval, regardless of value, generally means Medicare coverage.
Commissioner Jack Hoadley, PhD, of Georgetown University in Washington, cautioned that any discussion on these or possibly other tools needs to take into account the needs of those who will legitimately benefit from some of the low-value services so they do not inadvertently prevent access for those patients.
At press time, MedPAC had not scheduled a vote on specific recommendations to address low-value care.
according to a staff presentation at a meeting of the Medicare Payment Advisory Commission.
At the commission’s April meeting, MedPAC staff presented data from various literature searches, noting a “substantial use of low-value services in Medicare.” For example, they found that about 20% of Virginians, across all payers, received a low-value service in 2014, while 15% of Medicaid patients and 11% of commercially insured patients in Oregon received a low-value service in 2013.
“It is very hard to know at the beginning of coverage that something is going to be low value,” MedPAC commission member Kathy Buto, former vice president of global health policy at Johnson & Johnson, noted. “It may be covered for something narrow for which it is high value and then spreads. It’s important to have those kinds of tools once technologies and procedures are covered to be able to actually monitor what is going on and assess.”
But, she added, the Centers for Medicare & Medicaid Services needs to do more to routinely reexamine its coverage decisions.
Ms. Buto noted that noncoverage decisions are rarely issued and suggested that “there is an opportunity for us to take a look at whether we would advise CMS to take a look at using more of those tools more aggressively.”
Paul Ginsburg, PhD, commissioner and senior fellow in economic studies at the Brookings Institution, Washington, suggested that, for any new procedure or drug, initial coverage is always provisional for a certain length of time, which would force CMS to revisit coverage decisions.
“If there is no evidence, the coverage ends,” Dr Ginsburg said. “If there is positive evidence, the coverage proceeds.”
However, as commissioner Rita Redberg, MD, of the University of California, San Francisco, said of CMS, even with tools to cut coverage, its hands may be tied by outside forces. “CMS needs a lot more political cover.”
Dr. Redberg noted that there was extensive lobbying of local carriers, and within 6 months, despite the lack of evidence, everyone was covering cardiac CT.
“A few years later, CMS tried to walk back the coverage because it was just hemorrhaging money for cardiac CT, but there was no chance because it was a capital investment,” she added. “Even when there are restrictions on coverage, CMS doesn’t enforce them.”
Ms. Buto also raised the issue of how much influence CMS has over Medicare Part D prescription drug plan sponsors’ coverage decision policies, but suggested CMS could play a larger role in that.
Commissioner Amy Bricker, vice president of supply chain strategy at Express Scripts, St. Louis, suggested that, “We need to do more from a Part D perspective to allow plans to manage drug coverage more aggressively and in line with the commercial space. CMS has handcuffed them,” noting that FDA approval, regardless of value, generally means Medicare coverage.
Commissioner Jack Hoadley, PhD, of Georgetown University in Washington, cautioned that any discussion on these or possibly other tools needs to take into account the needs of those who will legitimately benefit from some of the low-value services so they do not inadvertently prevent access for those patients.
At press time, MedPAC had not scheduled a vote on specific recommendations to address low-value care.
according to a staff presentation at a meeting of the Medicare Payment Advisory Commission.
At the commission’s April meeting, MedPAC staff presented data from various literature searches, noting a “substantial use of low-value services in Medicare.” For example, they found that about 20% of Virginians, across all payers, received a low-value service in 2014, while 15% of Medicaid patients and 11% of commercially insured patients in Oregon received a low-value service in 2013.
“It is very hard to know at the beginning of coverage that something is going to be low value,” MedPAC commission member Kathy Buto, former vice president of global health policy at Johnson & Johnson, noted. “It may be covered for something narrow for which it is high value and then spreads. It’s important to have those kinds of tools once technologies and procedures are covered to be able to actually monitor what is going on and assess.”
But, she added, the Centers for Medicare & Medicaid Services needs to do more to routinely reexamine its coverage decisions.
Ms. Buto noted that noncoverage decisions are rarely issued and suggested that “there is an opportunity for us to take a look at whether we would advise CMS to take a look at using more of those tools more aggressively.”
Paul Ginsburg, PhD, commissioner and senior fellow in economic studies at the Brookings Institution, Washington, suggested that, for any new procedure or drug, initial coverage is always provisional for a certain length of time, which would force CMS to revisit coverage decisions.
“If there is no evidence, the coverage ends,” Dr Ginsburg said. “If there is positive evidence, the coverage proceeds.”
However, as commissioner Rita Redberg, MD, of the University of California, San Francisco, said of CMS, even with tools to cut coverage, its hands may be tied by outside forces. “CMS needs a lot more political cover.”
Dr. Redberg noted that there was extensive lobbying of local carriers, and within 6 months, despite the lack of evidence, everyone was covering cardiac CT.
“A few years later, CMS tried to walk back the coverage because it was just hemorrhaging money for cardiac CT, but there was no chance because it was a capital investment,” she added. “Even when there are restrictions on coverage, CMS doesn’t enforce them.”
Ms. Buto also raised the issue of how much influence CMS has over Medicare Part D prescription drug plan sponsors’ coverage decision policies, but suggested CMS could play a larger role in that.
Commissioner Amy Bricker, vice president of supply chain strategy at Express Scripts, St. Louis, suggested that, “We need to do more from a Part D perspective to allow plans to manage drug coverage more aggressively and in line with the commercial space. CMS has handcuffed them,” noting that FDA approval, regardless of value, generally means Medicare coverage.
Commissioner Jack Hoadley, PhD, of Georgetown University in Washington, cautioned that any discussion on these or possibly other tools needs to take into account the needs of those who will legitimately benefit from some of the low-value services so they do not inadvertently prevent access for those patients.
At press time, MedPAC had not scheduled a vote on specific recommendations to address low-value care.
REPORTING FROM MEDPAC
Building a career in quality improvement
As quality continues to take on a more important role in the health care delivery system, new opportunities for employment are taking shape.
For the hospitalist, that can mean a number of different career paths. What those paths could look like is the subject of the session “Making a Career Out of Quality” at 11:00 a.m. Tuesday.
“Because [quality] is a growing field and because many of the roles that are being created are often new within health systems,” the options hospitalists might have – or the highest value–added roles they might create – are not obvious, said Paul W. Helgerson, MD, SFHM, of the University of Virginia, Charlottesville, a presenter at the session.
Shedding light on those opportunities is the overall objective of this session, he said.
“One objective is to display for attendees what the diversity of roles looks like in this space,” Dr. Helgerson said. “If I am interested in spending part of my time or part of my career in quality, ‘What do people do?’ The truth is there is a whole array of different things.”
The second objective addresses the training that is required for some of these career options.
“We want to be able to represent what the whole breadth of the training experience could look like, from individual mentorship at the local level to formal training for a few days or even a few months nationwide,” he said.
Finally, the session will provide some boots-on-the-ground insight from clinicians at various stages in this space. Dr. Helgerson wants to highlight effective strategies to develop one’s career efficiently and effectively, to align with institutional leadership to create high-impact projects, and to look outside institutions for mentors to help make the career path successful.
The panel features three different perspectives. Dr. Helgerson will talk about his role at the University of Virginia and the work he has done in faculty development and interdisciplinary team development. Read G. Pierce, MD, of the University of Colorado at Denver, Aurora, is heavily involved in leadership training, and Nazima Allaudeen, MD, of the VA Palo Alto (Calif.) Health Care System, will talk about quality outcomes improvement on an operational level.
“My specific section is about different training in QI and that takes a lot of different shapes,” Dr. Allaudeen said. “There is a lot out there that people may not know about, and we will help explain how to match people with the right type of training that will be of the most value.”
“A hospitalist will be able to walk out of this session with a plan,” Dr. Helgerson said.
Making a Career Out of Quality
Tuesday, 11 a.m.-Noon
Crystal Ballroom G1/A&B
As quality continues to take on a more important role in the health care delivery system, new opportunities for employment are taking shape.
For the hospitalist, that can mean a number of different career paths. What those paths could look like is the subject of the session “Making a Career Out of Quality” at 11:00 a.m. Tuesday.
“Because [quality] is a growing field and because many of the roles that are being created are often new within health systems,” the options hospitalists might have – or the highest value–added roles they might create – are not obvious, said Paul W. Helgerson, MD, SFHM, of the University of Virginia, Charlottesville, a presenter at the session.
Shedding light on those opportunities is the overall objective of this session, he said.
“One objective is to display for attendees what the diversity of roles looks like in this space,” Dr. Helgerson said. “If I am interested in spending part of my time or part of my career in quality, ‘What do people do?’ The truth is there is a whole array of different things.”
The second objective addresses the training that is required for some of these career options.
“We want to be able to represent what the whole breadth of the training experience could look like, from individual mentorship at the local level to formal training for a few days or even a few months nationwide,” he said.
Finally, the session will provide some boots-on-the-ground insight from clinicians at various stages in this space. Dr. Helgerson wants to highlight effective strategies to develop one’s career efficiently and effectively, to align with institutional leadership to create high-impact projects, and to look outside institutions for mentors to help make the career path successful.
The panel features three different perspectives. Dr. Helgerson will talk about his role at the University of Virginia and the work he has done in faculty development and interdisciplinary team development. Read G. Pierce, MD, of the University of Colorado at Denver, Aurora, is heavily involved in leadership training, and Nazima Allaudeen, MD, of the VA Palo Alto (Calif.) Health Care System, will talk about quality outcomes improvement on an operational level.
“My specific section is about different training in QI and that takes a lot of different shapes,” Dr. Allaudeen said. “There is a lot out there that people may not know about, and we will help explain how to match people with the right type of training that will be of the most value.”
“A hospitalist will be able to walk out of this session with a plan,” Dr. Helgerson said.
Making a Career Out of Quality
Tuesday, 11 a.m.-Noon
Crystal Ballroom G1/A&B
As quality continues to take on a more important role in the health care delivery system, new opportunities for employment are taking shape.
For the hospitalist, that can mean a number of different career paths. What those paths could look like is the subject of the session “Making a Career Out of Quality” at 11:00 a.m. Tuesday.
“Because [quality] is a growing field and because many of the roles that are being created are often new within health systems,” the options hospitalists might have – or the highest value–added roles they might create – are not obvious, said Paul W. Helgerson, MD, SFHM, of the University of Virginia, Charlottesville, a presenter at the session.
Shedding light on those opportunities is the overall objective of this session, he said.
“One objective is to display for attendees what the diversity of roles looks like in this space,” Dr. Helgerson said. “If I am interested in spending part of my time or part of my career in quality, ‘What do people do?’ The truth is there is a whole array of different things.”
The second objective addresses the training that is required for some of these career options.
“We want to be able to represent what the whole breadth of the training experience could look like, from individual mentorship at the local level to formal training for a few days or even a few months nationwide,” he said.
Finally, the session will provide some boots-on-the-ground insight from clinicians at various stages in this space. Dr. Helgerson wants to highlight effective strategies to develop one’s career efficiently and effectively, to align with institutional leadership to create high-impact projects, and to look outside institutions for mentors to help make the career path successful.
The panel features three different perspectives. Dr. Helgerson will talk about his role at the University of Virginia and the work he has done in faculty development and interdisciplinary team development. Read G. Pierce, MD, of the University of Colorado at Denver, Aurora, is heavily involved in leadership training, and Nazima Allaudeen, MD, of the VA Palo Alto (Calif.) Health Care System, will talk about quality outcomes improvement on an operational level.
“My specific section is about different training in QI and that takes a lot of different shapes,” Dr. Allaudeen said. “There is a lot out there that people may not know about, and we will help explain how to match people with the right type of training that will be of the most value.”
“A hospitalist will be able to walk out of this session with a plan,” Dr. Helgerson said.
Making a Career Out of Quality
Tuesday, 11 a.m.-Noon
Crystal Ballroom G1/A&B
CMS finalizes measures to help combat opioid crisis
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.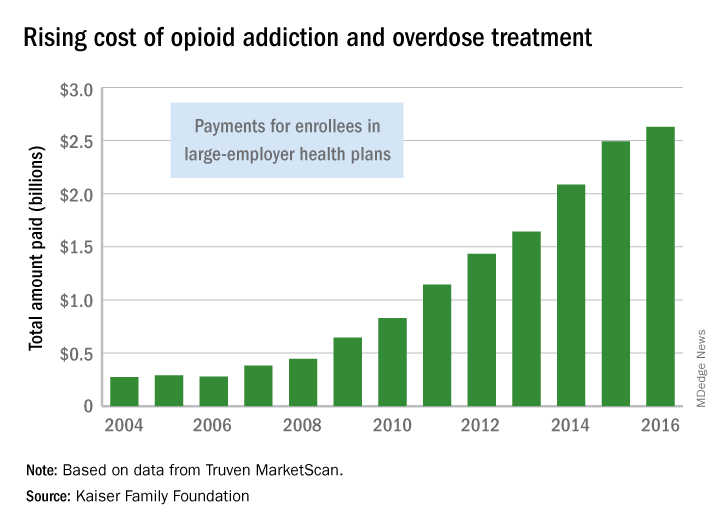
The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.
For chronic opioid users, CMS expects “all sponsors to implement an opioid care coordination edit at 90 morphine milligram equivalent (MME) per day. This formulary-level safety edit should trigger when a beneficiary’s cumulative MME per day across their opioid prescriptions reaches or exceeds 90 MME.”
There is flexibility: Pharmacists may contact prescribers and confirm that more pain medication is needed and then override the 90 MME/day threshold, if appropriate.
Finally, CMS expects “sponsors to implement additional soft safety edits to alert the pharmacist about duplicative opioid therapy and concurrent use of opioids and benzodiazepines,” according to the guidance.
The executive and legislative branches also are taking action.
President Trump in March detailed initiatives to address the opioid crisis across three domains: reducing demand through education, awareness, and prevention of overprescribing; reducing illicit drug importation and distribution; and expanding opportunities for proven treatment options.
For prescribers, the president’s plan calls for a nationwide reduction in opioid prescriptions filled by one-third within 3 years. It also looks aims to ensure that 75% of opioid prescriptions paid for by the government are “issued using best practices within 3 years, and 95% within 5 years,” a White House fact sheet notes.
When it comes to federal health care providers, those standards are to be met by half within 2 years and all within 5.
The White House also is calling on states to transition to a nationally interoperable Prescription Drug Monitoring Program (PDMP) network.
The president’s plan also calls for ensuring first-responder access to naloxone, improving overdose tracking systems, and expanding access to medication-assisted treatment. It also aims to change the Medicaid law that prohibits reimbursement of residential treatment at certain facilities with more than 16 beds.
On the legislative side, the House Energy and Commerce Committee is in the process of hosting a series of hearings and is expected to introduce a comprehensive package of bills aimed at various aspects of the opioid crisis. Across the four hearings, more than 30 pieces of individual legislation have been examined.
In the Senate, the Health, Education, Labor and Pensions Committee on April 4 released a discussion draft of the Opioid Crisis Response Act of 2018 and has scheduled a hearing for April 11 to discuss it.
The bill would spur development of nonaddictive pain killers, clarify FDA authority on small-quantity blister packs for opioids, provide states with better PDMP support; increase access to mental health services in schools, and improve substance use disorder treatment in underserved areas.
Also on April 4, the National Institutes of Health launched the HEAL Initiative (Helping to End Addiction Long-Term) to increase research to help prevent addiction through advanced pain management, and improve treatments for opioid misuse disorder and addiction. NIH is nearly doubling funding into opioid misuse/addiction research from $600 million in fiscal 2016 to $1.1 billion in fiscal 2018.
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.
The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.
For chronic opioid users, CMS expects “all sponsors to implement an opioid care coordination edit at 90 morphine milligram equivalent (MME) per day. This formulary-level safety edit should trigger when a beneficiary’s cumulative MME per day across their opioid prescriptions reaches or exceeds 90 MME.”
There is flexibility: Pharmacists may contact prescribers and confirm that more pain medication is needed and then override the 90 MME/day threshold, if appropriate.
Finally, CMS expects “sponsors to implement additional soft safety edits to alert the pharmacist about duplicative opioid therapy and concurrent use of opioids and benzodiazepines,” according to the guidance.
The executive and legislative branches also are taking action.
President Trump in March detailed initiatives to address the opioid crisis across three domains: reducing demand through education, awareness, and prevention of overprescribing; reducing illicit drug importation and distribution; and expanding opportunities for proven treatment options.
For prescribers, the president’s plan calls for a nationwide reduction in opioid prescriptions filled by one-third within 3 years. It also looks aims to ensure that 75% of opioid prescriptions paid for by the government are “issued using best practices within 3 years, and 95% within 5 years,” a White House fact sheet notes.
When it comes to federal health care providers, those standards are to be met by half within 2 years and all within 5.
The White House also is calling on states to transition to a nationally interoperable Prescription Drug Monitoring Program (PDMP) network.
The president’s plan also calls for ensuring first-responder access to naloxone, improving overdose tracking systems, and expanding access to medication-assisted treatment. It also aims to change the Medicaid law that prohibits reimbursement of residential treatment at certain facilities with more than 16 beds.
On the legislative side, the House Energy and Commerce Committee is in the process of hosting a series of hearings and is expected to introduce a comprehensive package of bills aimed at various aspects of the opioid crisis. Across the four hearings, more than 30 pieces of individual legislation have been examined.
In the Senate, the Health, Education, Labor and Pensions Committee on April 4 released a discussion draft of the Opioid Crisis Response Act of 2018 and has scheduled a hearing for April 11 to discuss it.
The bill would spur development of nonaddictive pain killers, clarify FDA authority on small-quantity blister packs for opioids, provide states with better PDMP support; increase access to mental health services in schools, and improve substance use disorder treatment in underserved areas.
Also on April 4, the National Institutes of Health launched the HEAL Initiative (Helping to End Addiction Long-Term) to increase research to help prevent addiction through advanced pain management, and improve treatments for opioid misuse disorder and addiction. NIH is nearly doubling funding into opioid misuse/addiction research from $600 million in fiscal 2016 to $1.1 billion in fiscal 2018.
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.
The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.
For chronic opioid users, CMS expects “all sponsors to implement an opioid care coordination edit at 90 morphine milligram equivalent (MME) per day. This formulary-level safety edit should trigger when a beneficiary’s cumulative MME per day across their opioid prescriptions reaches or exceeds 90 MME.”
There is flexibility: Pharmacists may contact prescribers and confirm that more pain medication is needed and then override the 90 MME/day threshold, if appropriate.
Finally, CMS expects “sponsors to implement additional soft safety edits to alert the pharmacist about duplicative opioid therapy and concurrent use of opioids and benzodiazepines,” according to the guidance.
The executive and legislative branches also are taking action.
President Trump in March detailed initiatives to address the opioid crisis across three domains: reducing demand through education, awareness, and prevention of overprescribing; reducing illicit drug importation and distribution; and expanding opportunities for proven treatment options.
For prescribers, the president’s plan calls for a nationwide reduction in opioid prescriptions filled by one-third within 3 years. It also looks aims to ensure that 75% of opioid prescriptions paid for by the government are “issued using best practices within 3 years, and 95% within 5 years,” a White House fact sheet notes.
When it comes to federal health care providers, those standards are to be met by half within 2 years and all within 5.
The White House also is calling on states to transition to a nationally interoperable Prescription Drug Monitoring Program (PDMP) network.
The president’s plan also calls for ensuring first-responder access to naloxone, improving overdose tracking systems, and expanding access to medication-assisted treatment. It also aims to change the Medicaid law that prohibits reimbursement of residential treatment at certain facilities with more than 16 beds.
On the legislative side, the House Energy and Commerce Committee is in the process of hosting a series of hearings and is expected to introduce a comprehensive package of bills aimed at various aspects of the opioid crisis. Across the four hearings, more than 30 pieces of individual legislation have been examined.
In the Senate, the Health, Education, Labor and Pensions Committee on April 4 released a discussion draft of the Opioid Crisis Response Act of 2018 and has scheduled a hearing for April 11 to discuss it.
The bill would spur development of nonaddictive pain killers, clarify FDA authority on small-quantity blister packs for opioids, provide states with better PDMP support; increase access to mental health services in schools, and improve substance use disorder treatment in underserved areas.
Also on April 4, the National Institutes of Health launched the HEAL Initiative (Helping to End Addiction Long-Term) to increase research to help prevent addiction through advanced pain management, and improve treatments for opioid misuse disorder and addiction. NIH is nearly doubling funding into opioid misuse/addiction research from $600 million in fiscal 2016 to $1.1 billion in fiscal 2018.





















