User login
How to proceed when it comes to vitamin D
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.
Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.
What should the family physician do?
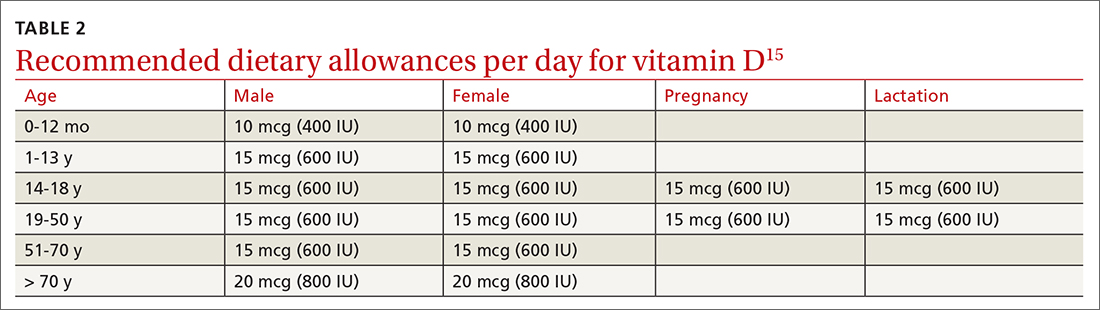
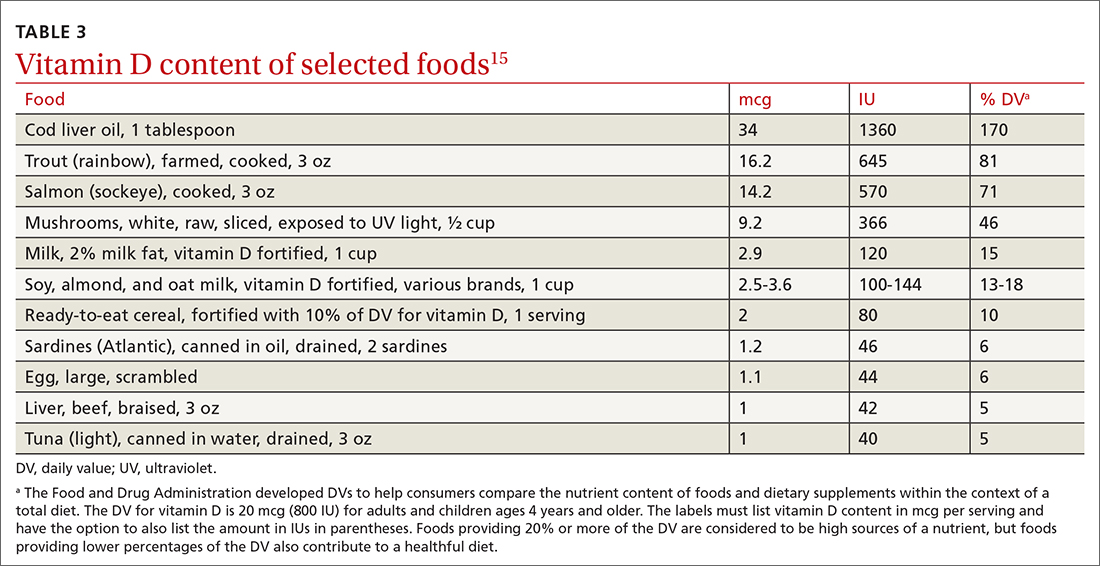
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15
Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.
Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.
What should the family physician do?
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15
Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.
Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.
What should the family physician do?
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15
Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
Healthy weight gain in pregnancy: What the USPSTF recommends
REFERENCES
- US Preventive Services Task Force. Behavioral counseling interventions for healthy weight and weight gain in pregnancy: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:2087-2092. doi:10.1001/jama.2021.6949
- Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; 2009. doi: 10.17226/12584
REFERENCES
- US Preventive Services Task Force. Behavioral counseling interventions for healthy weight and weight gain in pregnancy: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:2087-2092. doi:10.1001/jama.2021.6949
- Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; 2009. doi: 10.17226/12584
REFERENCES
- US Preventive Services Task Force. Behavioral counseling interventions for healthy weight and weight gain in pregnancy: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:2087-2092. doi:10.1001/jama.2021.6949
- Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; 2009. doi: 10.17226/12584
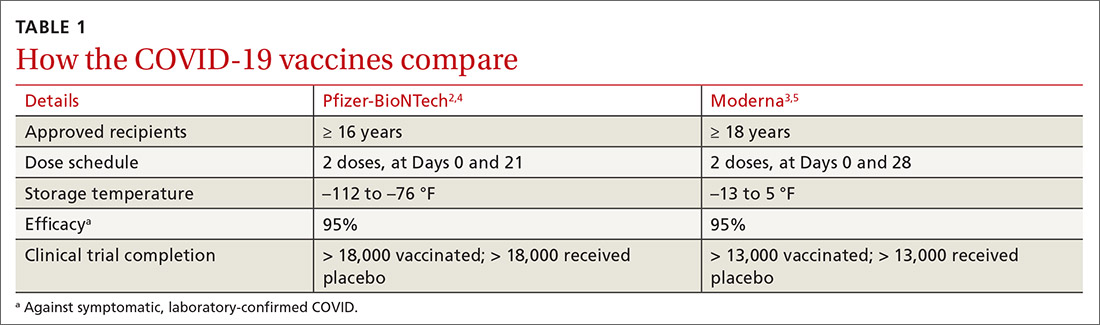
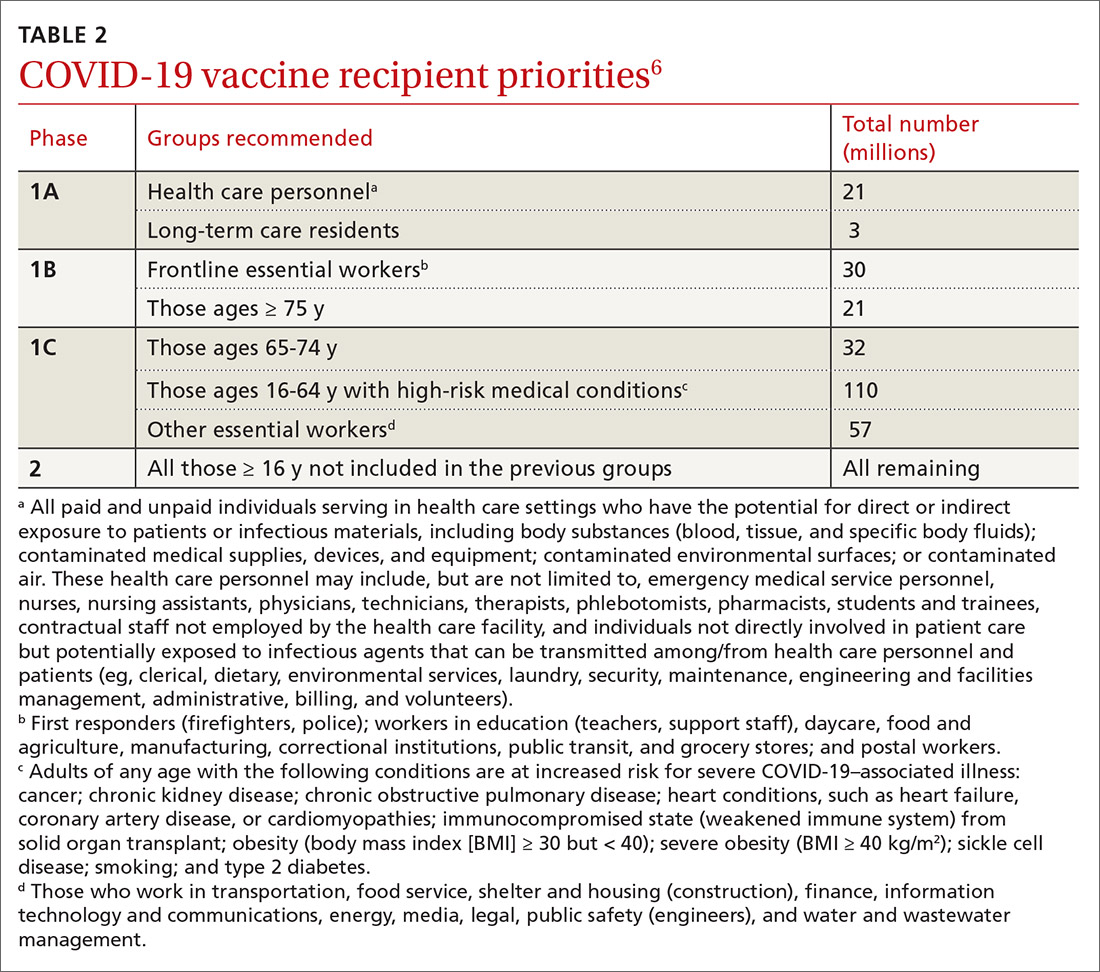
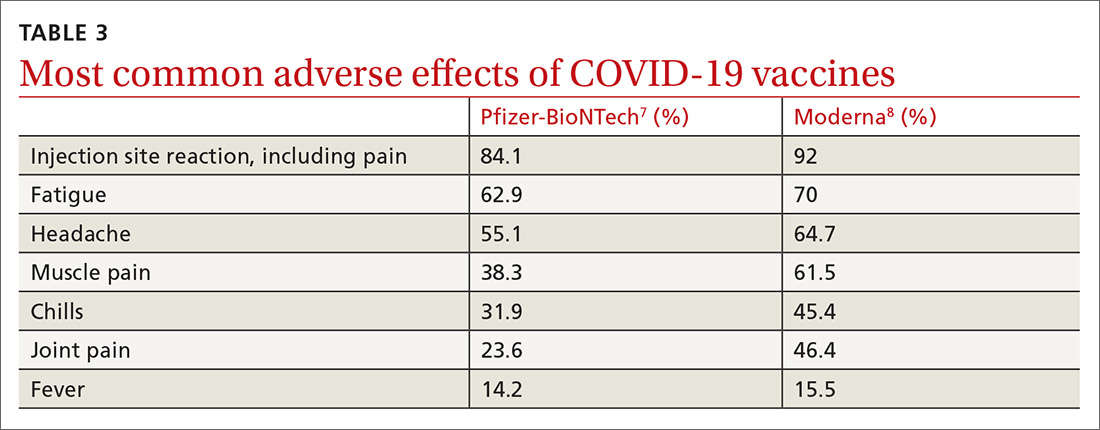
COVID-19 vaccine update: Uptake, effectiveness, and safety concerns
REFERENCES
- CDC. COVID Data Tracker. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- WHO Coronavirus (COVID-19) Dashboard. Accessed June 3, 2021. https://covid19.who.int/
- CDC. Demographic trends of people receiving COVID-19 vaccinations in the United States. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends
- Shimabukuro T. Update: thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf
- Fleming-Dutra K. CDC COVID-19 vaccine effectiveness studies. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/09-COVID-Fleming-Dutra-508.pdf
- Scobie H. Update on emerging SARS-CoV-2 variants and vaccine considerations. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/10-COVID-Scobie-508.pdf
REFERENCES
- CDC. COVID Data Tracker. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- WHO Coronavirus (COVID-19) Dashboard. Accessed June 3, 2021. https://covid19.who.int/
- CDC. Demographic trends of people receiving COVID-19 vaccinations in the United States. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends
- Shimabukuro T. Update: thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf
- Fleming-Dutra K. CDC COVID-19 vaccine effectiveness studies. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/09-COVID-Fleming-Dutra-508.pdf
- Scobie H. Update on emerging SARS-CoV-2 variants and vaccine considerations. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/10-COVID-Scobie-508.pdf
REFERENCES
- CDC. COVID Data Tracker. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- WHO Coronavirus (COVID-19) Dashboard. Accessed June 3, 2021. https://covid19.who.int/
- CDC. Demographic trends of people receiving COVID-19 vaccinations in the United States. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends
- Shimabukuro T. Update: thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf
- Fleming-Dutra K. CDC COVID-19 vaccine effectiveness studies. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/09-COVID-Fleming-Dutra-508.pdf
- Scobie H. Update on emerging SARS-CoV-2 variants and vaccine considerations. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/10-COVID-Scobie-508.pdf
A review of the latest USPSTF recommendations
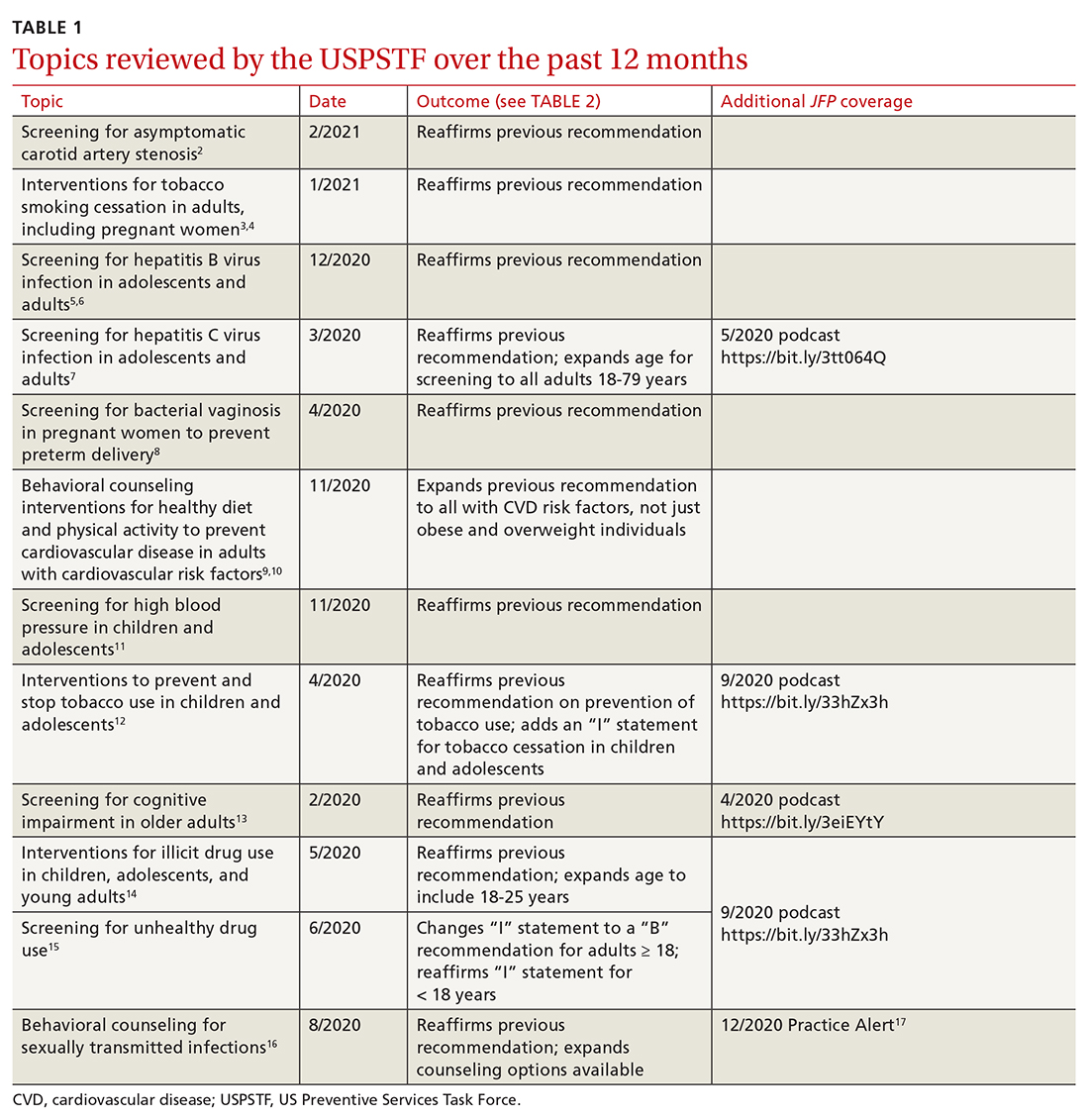
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.
Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.
Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.
Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
USPSTF makes 2 major changes to its lung cancer screening recs
REFERENCES
- American Academy of Family Physicians. Lung cancer: lung cancer screening in adults. AAFP Clinical Preventive Service Recommendations. Accessed April 26, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/all-clinical-recommendations/lung-cancer.html
- USPSTF. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:962-970. doi:10.1001/jama.2021.1117
- Jonas DE, Reuland DS, Reddy, SM, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:971-987. doi:10.1001/jama.2021.0377
- Henderson LM, Rivera MP, Basch E. Broadened eligibility for lung cancer screening: challenges and uncertainty for implementation and equity. JAMA. 2021;325:939-941. doi:10.1001/jama.2020.26422
- Meza R, Jeon J, Toumazis I, et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: modeling study for the US Preventive Services Task Force. JAMA. 2021;325:988-997. doi:10.1001/jama.2021.1077
REFERENCES
- American Academy of Family Physicians. Lung cancer: lung cancer screening in adults. AAFP Clinical Preventive Service Recommendations. Accessed April 26, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/all-clinical-recommendations/lung-cancer.html
- USPSTF. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:962-970. doi:10.1001/jama.2021.1117
- Jonas DE, Reuland DS, Reddy, SM, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:971-987. doi:10.1001/jama.2021.0377
- Henderson LM, Rivera MP, Basch E. Broadened eligibility for lung cancer screening: challenges and uncertainty for implementation and equity. JAMA. 2021;325:939-941. doi:10.1001/jama.2020.26422
- Meza R, Jeon J, Toumazis I, et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: modeling study for the US Preventive Services Task Force. JAMA. 2021;325:988-997. doi:10.1001/jama.2021.1077
REFERENCES
- American Academy of Family Physicians. Lung cancer: lung cancer screening in adults. AAFP Clinical Preventive Service Recommendations. Accessed April 26, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/all-clinical-recommendations/lung-cancer.html
- USPSTF. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:962-970. doi:10.1001/jama.2021.1117
- Jonas DE, Reuland DS, Reddy, SM, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:971-987. doi:10.1001/jama.2021.0377
- Henderson LM, Rivera MP, Basch E. Broadened eligibility for lung cancer screening: challenges and uncertainty for implementation and equity. JAMA. 2021;325:939-941. doi:10.1001/jama.2020.26422
- Meza R, Jeon J, Toumazis I, et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: modeling study for the US Preventive Services Task Force. JAMA. 2021;325:988-997. doi:10.1001/jama.2021.1077
CDC’s new gonorrhea treatment recs: What’s changed, and when to retest
REFERENCES
- CDC. Expedited partner therapy. Accessed March 15, 2021. www.cdc.gov/std/ept/default.htm
- St. Cyr S, Barbee L, Workowski KA, et al. Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916. Accessed March 15, 2021. https://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm
- CDC. Gonococcal infections. Accessed March 15, 2021. www.cdc.gov/std/tg2015/gonorrhea.htm
REFERENCES
- CDC. Expedited partner therapy. Accessed March 15, 2021. www.cdc.gov/std/ept/default.htm
- St. Cyr S, Barbee L, Workowski KA, et al. Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916. Accessed March 15, 2021. https://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm
- CDC. Gonococcal infections. Accessed March 15, 2021. www.cdc.gov/std/tg2015/gonorrhea.htm
REFERENCES
- CDC. Expedited partner therapy. Accessed March 15, 2021. www.cdc.gov/std/ept/default.htm
- St. Cyr S, Barbee L, Workowski KA, et al. Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916. Accessed March 15, 2021. https://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm
- CDC. Gonococcal infections. Accessed March 15, 2021. www.cdc.gov/std/tg2015/gonorrhea.htm
ACIP recommendations for COVID-19 vaccines—and more
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).
In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).
Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.
Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.
The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9
Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).
In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).
Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.
Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.
The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9
Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).
In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).
Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.
Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.
The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9
Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
AT PRESS TIME
The US Food and Drug Administration issued an Emergency Use Authorization for a third COVID-19 vaccine. The single-dose vaccine was developed by the Janssen Pharmaceutical Companies of Johnson & Johnson. For more information, go to www.mdedge.com/familymedicine
COVID-19 vaccines: New candidates & answers to commonly asked questions
REFERENCES
- CDC. COVID-19 vaccination. Accessed February 22, 2021.
- CDC. COVID data tracker. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed February 22, 2021.
- Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morbid Mortal Wkly Rep. ePub: February 19, 2021. Accessed February 22, 2021.
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125-129. Accessed February 25, 2021.
REFERENCES
- CDC. COVID-19 vaccination. Accessed February 22, 2021.
- CDC. COVID data tracker. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed February 22, 2021.
- Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morbid Mortal Wkly Rep. ePub: February 19, 2021. Accessed February 22, 2021.
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125-129. Accessed February 25, 2021.
REFERENCES
- CDC. COVID-19 vaccination. Accessed February 22, 2021.
- CDC. COVID data tracker. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed February 22, 2021.
- Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morbid Mortal Wkly Rep. ePub: February 19, 2021. Accessed February 22, 2021.
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125-129. Accessed February 25, 2021.
COVID-19 vaccines: The rollout, the risks, and the reason to still wear a mask
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
USPSTF update on sexually transmitted infections
In August 2020, the US Preventive Services Task Force published an update of its recommendation on preventing sexually transmitted infections (STIs) with behavioral counseling interventions.1
Whom to counsel. The USPSTF continues to recommend behavioral counseling for all sexually active adolescents and for adults at increased risk for STIs. Adults at increased risk include those who have been diagnosed with an STI in the past year, those with multiple sex partners or a sex partner at high risk for an STI, those not using condoms consistently, and those belonging to populations with high prevalence rates of STIs. These populations with high prevalence rates include1
- individuals seeking care at STI clinics,
- sexual and gender minorities, and
- those who are positive for human immunodeficiency virus (HIV), use injection drugs, exchange sex for drugs or money, or have recently been in a correctional facility.
Features of effective counseling. The Task Force recommends that primary care clinicians provide behavioral counseling or refer to counseling services or suggest media-based interventions. The most effective counseling interventions are those that span more than 120 minutes over several sessions. But the Task Force also states that counseling lasting about 30 minutes in a single session can also be effective. Counseling should include information about common STIs and their modes of transmission; encouragement in the use of safer sex practices; and training in proper condom use, how to communicate with partners about safer sex practices, and problem-solving. Various approaches to this counseling can be found at https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
This updated recommendation is timely because most STIs in the United States have been increasing in incidence for the past decade or longer.2 Per 100,000 population, the total number of chlamydia cases since 2000 has risen from 251.4 to 539.9 (115%);gonorrhea cases since 2009 have risen from 98.1 to 179.1 (83%).3 And since 2000, the total number of reported syphilis cases per 100,000 has risen from 2.1 to 10.8 (414%).3
Chlamydia affects primarily those ages 15 to 24 years, with highest rates occurring in females (FIGURE 1).2 Gonorrhea affects women and men fairly evenly with slightly higher rates in men; the highest rates are seen in those ages 20 to 29 (FIGURE 2).2 Syphilis predominantly affects men who have sex with men, and the highest rates are in those ages 20 to 34 (FIGURE 3).2 In contrast to these upward trends, the number of HIV cases diagnosed has been relatively steady, with a slight downward trend over the past decade.4Other STIs that can be prevented through behavioral counseling include herpes simplex, human papillomavirus (HPV), hepatitis B virus (HBV) and trichomonas vaginalis.
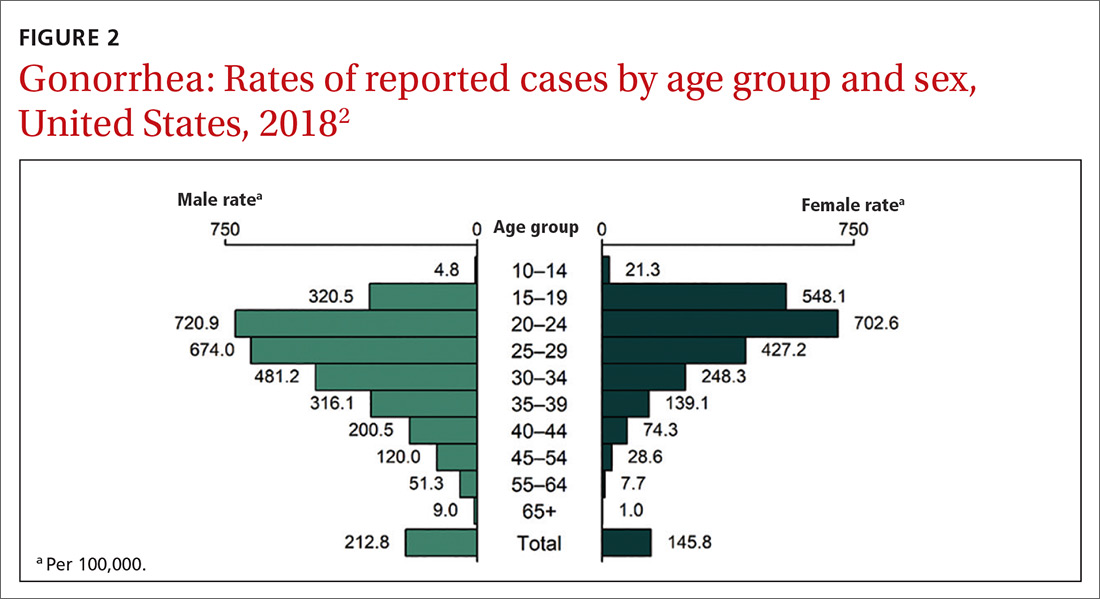
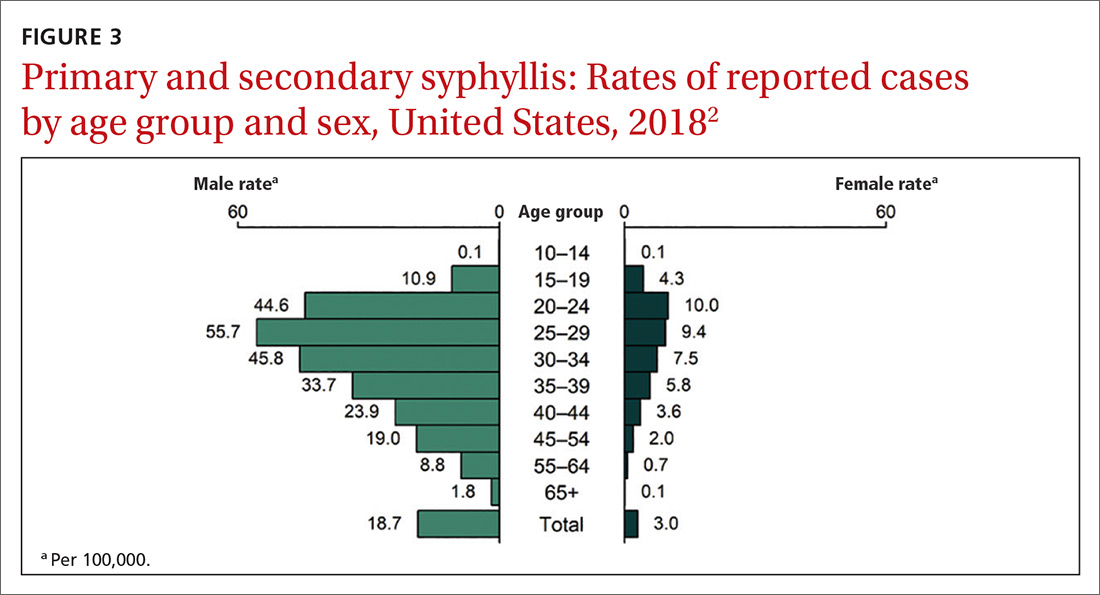
Continue to: How to integrate STI preventioninto the primary care encounter
How to integrate STI preventioninto the primary care encounter
A key resource for learning to recognize the signs and symptoms of STIs, to correctly diagnose them, and to treat them according to CDC guidelines can be found at www.cdc.gov/std/tg2015/default.htm.5 Equally important is to integrate the prevention of STIs into the clinical routine by using a 4-step approach: risk assessment, risk reduction (counseling and chemoprevention), screening, and vaccination.
Risk assessment. The first step in prevention is taking a sexual history to accurately assess a patient’s risk for STIs. The CDC provides a tool (www.cdc.gov/std/products/provider-pocket-guides.htm) that can assist in gathering information in a nonjudgmental fashion about 5 Ps: partners, practices, protection from STIs, past history of STIs, and prevention of pregnancy.
Risk reduction. Following STI risk assessment, recommend risk-reduction interventions, as appropriate. Notable in the new Task Force recommendation are behavioral counseling methods that work. Additionally, when needed, pre-exposure prophylaxis with effective antiretroviral agents can be offered to those at high risk of HIV.6
Screening. Task Force recommendations for STI screening are described in the TABLE.7-12 Screening for HIV, chlamydia, gonorrhea, syphilis, and HBV are also recommended for pregnant women. And, although pregnant women are not specifically mentioned in the recommendation on chlamydia screening, it is reasonable to include it in prenatal care testing for STIs.
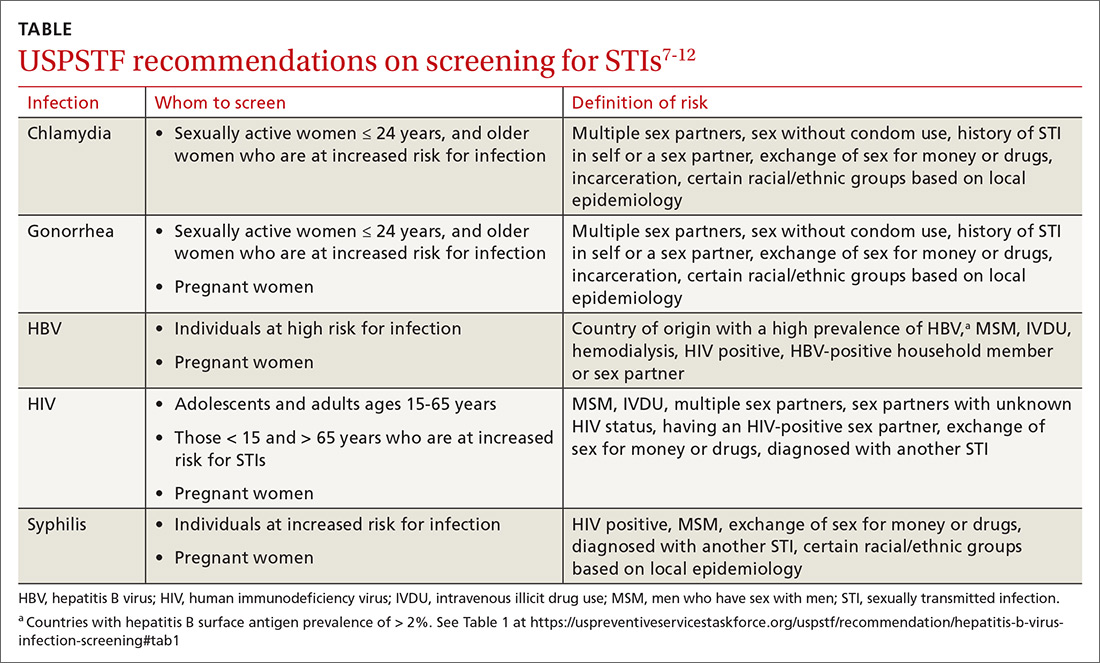
The Task Force has made an “I” statement regarding screening for gonorrhea and chlamydia in males. This does not mean that screening should be avoided, but only that there is insufficient evidence to support a firm statement regarding the harms and benefits in males. Keep in mind that this applies to asymptomatic males, and that testing and preventive treatment are warranted after documented exposure to either infection.
The Task Force recommends against screening for genital herpes, including in pregnant women, because of a lack of evidence of benefit from such screening, the high rate of false-positive tests, and the potential to cause anxiety and harm to personal relationships.
Continue to: Although hepatitis C virus...
Although hepatitis C virus (HCV) is transmitted mainly through intravenous drug use, it can also be transmitted sexually. The Task Force recommends screening for HCV in all adults ages 18 to 79 years.13
Vaccination. Two STIs can be prevented by immunizations: HPV and HBV. The current recommendations by the Advisory Committee on Immunization Practices (ACIP) are to vaccinate all infants with HBV vaccine and all unvaccinated children and adolescents through age 18.14 Unvaccinated adults who are at risk for HBV infection, including those at risk through sexual practices, should also be vaccinated.14
ACIP recommends routine HPV vaccination at age 11 or 12 years, but it can be started as early as 9 years.15 Catch-up vaccination is recommended for males and females through age 26 years.15 The vaccine is approved for use in individuals ages 27 through 45 years, but ACIP has not recommended it for routine use in this age group, and has instead recommended shared clinical decision-making to evaluate whether there is potential individual benefit from the vaccine.15
Public health implications
All STIs are reportable to local or state health departments. This is important for tracking community infection trends and, if resources are available, for contact notification and testing. In most jurisdictions, local health department resources are limited and contact tracing may be restricted to syphilis and HIV infections. When this is the case, it is especially important to instruct patients in whom STIs have been detected to notify their recent sex partners and advise them to be tested or preventively treated.
Expedited partner therapy (EPT)—providing treatment for exposed sexual contacts without a clinical encounter—is allowed in some states and is a tool that can prevent re-infection in the treated patient and suppress spread in the community. This is most useful for partners of those with gonorrhea, chlamydia, or trichomonas. The CDC has published guidance on how to implement EPT in a clinical setting if state law allows it.16
1. Henderson JT, Senger CA, Henninger M, et al. Behavioral counseling interventions to prevent sexually transmitted infections. JAMA. 2020;324:682-699.
2. CDC. Sexually transmitted disease surveillance, 2018. www.cdc.gov/std/stats18/slides.htm. Accessed November 25, 2020.
3. CDC. Sexually transmitted disease surveillance 2018. www.cdc.gov/std/stats18/tables/1.htm. Accessed November 25, 2020.
4. CDC. Estimated HIV incidence and prevalence in the United States (2010-2018). www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-surveillance-epidemiology-2018.pdf. Accessed November 25, 2020.
5. CDC. 2015 sexually transmitted disease treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed November 25, 2020.
6. USPSTF. Prevention of human immunodeficiency (HIV) infection: pre-exposure prophylaxis. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Accessed November 25, 2020.
7. LeFevre ML, U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:902-910. 8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed November 25, 2020.
9. Curry SJ, Krist AH, Owens DK, et al. Screening for syphilis in pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2018;320:911-917.
10. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336.
11. USPSTF. US Preventive Services Task Force issues draft recommendation statement on screening for hepatitis B virus infection in adolescents and adults. www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/hepatitis-b-nonpregnant-adults-draft-rs-bulletin.pdf. Accessed November 25, 2020.
12. Owens DK, Davidson KW, Krist AH, et al. Screening for Hepatitis B Virus Infection in Pregnant Women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;322:349-354.
13. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening. Accessed November 25, 2020. 14. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67;1-31.
15. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
16. CDC. Expedited partner therapy in the management of sexually transmitted diseases. www.cdc.gov/std/treatment/eptfinalreport2006.pdf. Accessed November 25, 2020.
In August 2020, the US Preventive Services Task Force published an update of its recommendation on preventing sexually transmitted infections (STIs) with behavioral counseling interventions.1
Whom to counsel. The USPSTF continues to recommend behavioral counseling for all sexually active adolescents and for adults at increased risk for STIs. Adults at increased risk include those who have been diagnosed with an STI in the past year, those with multiple sex partners or a sex partner at high risk for an STI, those not using condoms consistently, and those belonging to populations with high prevalence rates of STIs. These populations with high prevalence rates include1
- individuals seeking care at STI clinics,
- sexual and gender minorities, and
- those who are positive for human immunodeficiency virus (HIV), use injection drugs, exchange sex for drugs or money, or have recently been in a correctional facility.
Features of effective counseling. The Task Force recommends that primary care clinicians provide behavioral counseling or refer to counseling services or suggest media-based interventions. The most effective counseling interventions are those that span more than 120 minutes over several sessions. But the Task Force also states that counseling lasting about 30 minutes in a single session can also be effective. Counseling should include information about common STIs and their modes of transmission; encouragement in the use of safer sex practices; and training in proper condom use, how to communicate with partners about safer sex practices, and problem-solving. Various approaches to this counseling can be found at https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
This updated recommendation is timely because most STIs in the United States have been increasing in incidence for the past decade or longer.2 Per 100,000 population, the total number of chlamydia cases since 2000 has risen from 251.4 to 539.9 (115%);gonorrhea cases since 2009 have risen from 98.1 to 179.1 (83%).3 And since 2000, the total number of reported syphilis cases per 100,000 has risen from 2.1 to 10.8 (414%).3
Chlamydia affects primarily those ages 15 to 24 years, with highest rates occurring in females (FIGURE 1).2 Gonorrhea affects women and men fairly evenly with slightly higher rates in men; the highest rates are seen in those ages 20 to 29 (FIGURE 2).2 Syphilis predominantly affects men who have sex with men, and the highest rates are in those ages 20 to 34 (FIGURE 3).2 In contrast to these upward trends, the number of HIV cases diagnosed has been relatively steady, with a slight downward trend over the past decade.4Other STIs that can be prevented through behavioral counseling include herpes simplex, human papillomavirus (HPV), hepatitis B virus (HBV) and trichomonas vaginalis.



Continue to: How to integrate STI preventioninto the primary care encounter
How to integrate STI preventioninto the primary care encounter
A key resource for learning to recognize the signs and symptoms of STIs, to correctly diagnose them, and to treat them according to CDC guidelines can be found at www.cdc.gov/std/tg2015/default.htm.5 Equally important is to integrate the prevention of STIs into the clinical routine by using a 4-step approach: risk assessment, risk reduction (counseling and chemoprevention), screening, and vaccination.
Risk assessment. The first step in prevention is taking a sexual history to accurately assess a patient’s risk for STIs. The CDC provides a tool (www.cdc.gov/std/products/provider-pocket-guides.htm) that can assist in gathering information in a nonjudgmental fashion about 5 Ps: partners, practices, protection from STIs, past history of STIs, and prevention of pregnancy.
Risk reduction. Following STI risk assessment, recommend risk-reduction interventions, as appropriate. Notable in the new Task Force recommendation are behavioral counseling methods that work. Additionally, when needed, pre-exposure prophylaxis with effective antiretroviral agents can be offered to those at high risk of HIV.6
Screening. Task Force recommendations for STI screening are described in the TABLE.7-12 Screening for HIV, chlamydia, gonorrhea, syphilis, and HBV are also recommended for pregnant women. And, although pregnant women are not specifically mentioned in the recommendation on chlamydia screening, it is reasonable to include it in prenatal care testing for STIs.

The Task Force has made an “I” statement regarding screening for gonorrhea and chlamydia in males. This does not mean that screening should be avoided, but only that there is insufficient evidence to support a firm statement regarding the harms and benefits in males. Keep in mind that this applies to asymptomatic males, and that testing and preventive treatment are warranted after documented exposure to either infection.
The Task Force recommends against screening for genital herpes, including in pregnant women, because of a lack of evidence of benefit from such screening, the high rate of false-positive tests, and the potential to cause anxiety and harm to personal relationships.
Continue to: Although hepatitis C virus...
Although hepatitis C virus (HCV) is transmitted mainly through intravenous drug use, it can also be transmitted sexually. The Task Force recommends screening for HCV in all adults ages 18 to 79 years.13
Vaccination. Two STIs can be prevented by immunizations: HPV and HBV. The current recommendations by the Advisory Committee on Immunization Practices (ACIP) are to vaccinate all infants with HBV vaccine and all unvaccinated children and adolescents through age 18.14 Unvaccinated adults who are at risk for HBV infection, including those at risk through sexual practices, should also be vaccinated.14
ACIP recommends routine HPV vaccination at age 11 or 12 years, but it can be started as early as 9 years.15 Catch-up vaccination is recommended for males and females through age 26 years.15 The vaccine is approved for use in individuals ages 27 through 45 years, but ACIP has not recommended it for routine use in this age group, and has instead recommended shared clinical decision-making to evaluate whether there is potential individual benefit from the vaccine.15
Public health implications
All STIs are reportable to local or state health departments. This is important for tracking community infection trends and, if resources are available, for contact notification and testing. In most jurisdictions, local health department resources are limited and contact tracing may be restricted to syphilis and HIV infections. When this is the case, it is especially important to instruct patients in whom STIs have been detected to notify their recent sex partners and advise them to be tested or preventively treated.
Expedited partner therapy (EPT)—providing treatment for exposed sexual contacts without a clinical encounter—is allowed in some states and is a tool that can prevent re-infection in the treated patient and suppress spread in the community. This is most useful for partners of those with gonorrhea, chlamydia, or trichomonas. The CDC has published guidance on how to implement EPT in a clinical setting if state law allows it.16
In August 2020, the US Preventive Services Task Force published an update of its recommendation on preventing sexually transmitted infections (STIs) with behavioral counseling interventions.1
Whom to counsel. The USPSTF continues to recommend behavioral counseling for all sexually active adolescents and for adults at increased risk for STIs. Adults at increased risk include those who have been diagnosed with an STI in the past year, those with multiple sex partners or a sex partner at high risk for an STI, those not using condoms consistently, and those belonging to populations with high prevalence rates of STIs. These populations with high prevalence rates include1
- individuals seeking care at STI clinics,
- sexual and gender minorities, and
- those who are positive for human immunodeficiency virus (HIV), use injection drugs, exchange sex for drugs or money, or have recently been in a correctional facility.
Features of effective counseling. The Task Force recommends that primary care clinicians provide behavioral counseling or refer to counseling services or suggest media-based interventions. The most effective counseling interventions are those that span more than 120 minutes over several sessions. But the Task Force also states that counseling lasting about 30 minutes in a single session can also be effective. Counseling should include information about common STIs and their modes of transmission; encouragement in the use of safer sex practices; and training in proper condom use, how to communicate with partners about safer sex practices, and problem-solving. Various approaches to this counseling can be found at https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
This updated recommendation is timely because most STIs in the United States have been increasing in incidence for the past decade or longer.2 Per 100,000 population, the total number of chlamydia cases since 2000 has risen from 251.4 to 539.9 (115%);gonorrhea cases since 2009 have risen from 98.1 to 179.1 (83%).3 And since 2000, the total number of reported syphilis cases per 100,000 has risen from 2.1 to 10.8 (414%).3
Chlamydia affects primarily those ages 15 to 24 years, with highest rates occurring in females (FIGURE 1).2 Gonorrhea affects women and men fairly evenly with slightly higher rates in men; the highest rates are seen in those ages 20 to 29 (FIGURE 2).2 Syphilis predominantly affects men who have sex with men, and the highest rates are in those ages 20 to 34 (FIGURE 3).2 In contrast to these upward trends, the number of HIV cases diagnosed has been relatively steady, with a slight downward trend over the past decade.4Other STIs that can be prevented through behavioral counseling include herpes simplex, human papillomavirus (HPV), hepatitis B virus (HBV) and trichomonas vaginalis.



Continue to: How to integrate STI preventioninto the primary care encounter
How to integrate STI preventioninto the primary care encounter
A key resource for learning to recognize the signs and symptoms of STIs, to correctly diagnose them, and to treat them according to CDC guidelines can be found at www.cdc.gov/std/tg2015/default.htm.5 Equally important is to integrate the prevention of STIs into the clinical routine by using a 4-step approach: risk assessment, risk reduction (counseling and chemoprevention), screening, and vaccination.
Risk assessment. The first step in prevention is taking a sexual history to accurately assess a patient’s risk for STIs. The CDC provides a tool (www.cdc.gov/std/products/provider-pocket-guides.htm) that can assist in gathering information in a nonjudgmental fashion about 5 Ps: partners, practices, protection from STIs, past history of STIs, and prevention of pregnancy.
Risk reduction. Following STI risk assessment, recommend risk-reduction interventions, as appropriate. Notable in the new Task Force recommendation are behavioral counseling methods that work. Additionally, when needed, pre-exposure prophylaxis with effective antiretroviral agents can be offered to those at high risk of HIV.6
Screening. Task Force recommendations for STI screening are described in the TABLE.7-12 Screening for HIV, chlamydia, gonorrhea, syphilis, and HBV are also recommended for pregnant women. And, although pregnant women are not specifically mentioned in the recommendation on chlamydia screening, it is reasonable to include it in prenatal care testing for STIs.

The Task Force has made an “I” statement regarding screening for gonorrhea and chlamydia in males. This does not mean that screening should be avoided, but only that there is insufficient evidence to support a firm statement regarding the harms and benefits in males. Keep in mind that this applies to asymptomatic males, and that testing and preventive treatment are warranted after documented exposure to either infection.
The Task Force recommends against screening for genital herpes, including in pregnant women, because of a lack of evidence of benefit from such screening, the high rate of false-positive tests, and the potential to cause anxiety and harm to personal relationships.
Continue to: Although hepatitis C virus...
Although hepatitis C virus (HCV) is transmitted mainly through intravenous drug use, it can also be transmitted sexually. The Task Force recommends screening for HCV in all adults ages 18 to 79 years.13
Vaccination. Two STIs can be prevented by immunizations: HPV and HBV. The current recommendations by the Advisory Committee on Immunization Practices (ACIP) are to vaccinate all infants with HBV vaccine and all unvaccinated children and adolescents through age 18.14 Unvaccinated adults who are at risk for HBV infection, including those at risk through sexual practices, should also be vaccinated.14
ACIP recommends routine HPV vaccination at age 11 or 12 years, but it can be started as early as 9 years.15 Catch-up vaccination is recommended for males and females through age 26 years.15 The vaccine is approved for use in individuals ages 27 through 45 years, but ACIP has not recommended it for routine use in this age group, and has instead recommended shared clinical decision-making to evaluate whether there is potential individual benefit from the vaccine.15
Public health implications
All STIs are reportable to local or state health departments. This is important for tracking community infection trends and, if resources are available, for contact notification and testing. In most jurisdictions, local health department resources are limited and contact tracing may be restricted to syphilis and HIV infections. When this is the case, it is especially important to instruct patients in whom STIs have been detected to notify their recent sex partners and advise them to be tested or preventively treated.
Expedited partner therapy (EPT)—providing treatment for exposed sexual contacts without a clinical encounter—is allowed in some states and is a tool that can prevent re-infection in the treated patient and suppress spread in the community. This is most useful for partners of those with gonorrhea, chlamydia, or trichomonas. The CDC has published guidance on how to implement EPT in a clinical setting if state law allows it.16
1. Henderson JT, Senger CA, Henninger M, et al. Behavioral counseling interventions to prevent sexually transmitted infections. JAMA. 2020;324:682-699.
2. CDC. Sexually transmitted disease surveillance, 2018. www.cdc.gov/std/stats18/slides.htm. Accessed November 25, 2020.
3. CDC. Sexually transmitted disease surveillance 2018. www.cdc.gov/std/stats18/tables/1.htm. Accessed November 25, 2020.
4. CDC. Estimated HIV incidence and prevalence in the United States (2010-2018). www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-surveillance-epidemiology-2018.pdf. Accessed November 25, 2020.
5. CDC. 2015 sexually transmitted disease treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed November 25, 2020.
6. USPSTF. Prevention of human immunodeficiency (HIV) infection: pre-exposure prophylaxis. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Accessed November 25, 2020.
7. LeFevre ML, U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:902-910. 8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed November 25, 2020.
9. Curry SJ, Krist AH, Owens DK, et al. Screening for syphilis in pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2018;320:911-917.
10. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336.
11. USPSTF. US Preventive Services Task Force issues draft recommendation statement on screening for hepatitis B virus infection in adolescents and adults. www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/hepatitis-b-nonpregnant-adults-draft-rs-bulletin.pdf. Accessed November 25, 2020.
12. Owens DK, Davidson KW, Krist AH, et al. Screening for Hepatitis B Virus Infection in Pregnant Women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;322:349-354.
13. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening. Accessed November 25, 2020. 14. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67;1-31.
15. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
16. CDC. Expedited partner therapy in the management of sexually transmitted diseases. www.cdc.gov/std/treatment/eptfinalreport2006.pdf. Accessed November 25, 2020.
1. Henderson JT, Senger CA, Henninger M, et al. Behavioral counseling interventions to prevent sexually transmitted infections. JAMA. 2020;324:682-699.
2. CDC. Sexually transmitted disease surveillance, 2018. www.cdc.gov/std/stats18/slides.htm. Accessed November 25, 2020.
3. CDC. Sexually transmitted disease surveillance 2018. www.cdc.gov/std/stats18/tables/1.htm. Accessed November 25, 2020.
4. CDC. Estimated HIV incidence and prevalence in the United States (2010-2018). www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-surveillance-epidemiology-2018.pdf. Accessed November 25, 2020.
5. CDC. 2015 sexually transmitted disease treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed November 25, 2020.
6. USPSTF. Prevention of human immunodeficiency (HIV) infection: pre-exposure prophylaxis. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Accessed November 25, 2020.
7. LeFevre ML, U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:902-910. 8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed November 25, 2020.
9. Curry SJ, Krist AH, Owens DK, et al. Screening for syphilis in pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2018;320:911-917.
10. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336.
11. USPSTF. US Preventive Services Task Force issues draft recommendation statement on screening for hepatitis B virus infection in adolescents and adults. www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/hepatitis-b-nonpregnant-adults-draft-rs-bulletin.pdf. Accessed November 25, 2020.
12. Owens DK, Davidson KW, Krist AH, et al. Screening for Hepatitis B Virus Infection in Pregnant Women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;322:349-354.
13. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening. Accessed November 25, 2020. 14. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67;1-31.
15. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
16. CDC. Expedited partner therapy in the management of sexually transmitted diseases. www.cdc.gov/std/treatment/eptfinalreport2006.pdf. Accessed November 25, 2020.