User login
Colorectal cancer screening: The new draft recs & the cost to screen
References
- US Preventive Services Task Force. Colorectal cancer: screening [draft recommendation statement]. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/colorectal-cancer-screening3. Published October 27, 2020. Accessed November 23, 2020.
References
- US Preventive Services Task Force. Colorectal cancer: screening [draft recommendation statement]. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/colorectal-cancer-screening3. Published October 27, 2020. Accessed November 23, 2020.
References
- US Preventive Services Task Force. Colorectal cancer: screening [draft recommendation statement]. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/colorectal-cancer-screening3. Published October 27, 2020. Accessed November 23, 2020.
COVID-19 treatment: What the NIH recommends
References
- National Institute of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. www.covid19treatmentguidelines.nih.gov/. Updated October 22, 2020. Accessed October 28, 2020.
References
- National Institute of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. www.covid19treatmentguidelines.nih.gov/. Updated October 22, 2020. Accessed October 28, 2020.
References
- National Institute of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. www.covid19treatmentguidelines.nih.gov/. Updated October 22, 2020. Accessed October 28, 2020.
Prospects and challenges for the upcoming influenza season
The 2020-2021 influenza season is shaping up to be challenging. Its likely concurrence with the ongoing severe acute respiratory syndrome-coronavirus 2 (SARS-coV-2) pandemic (COVID-19) will pose diagnostic and therapeutic dilemmas and could overload the hospital system. But there could also be potential synergies in preventing morbidity and mortality from each disease.
A consistent pattern overthe past few influenza seasons
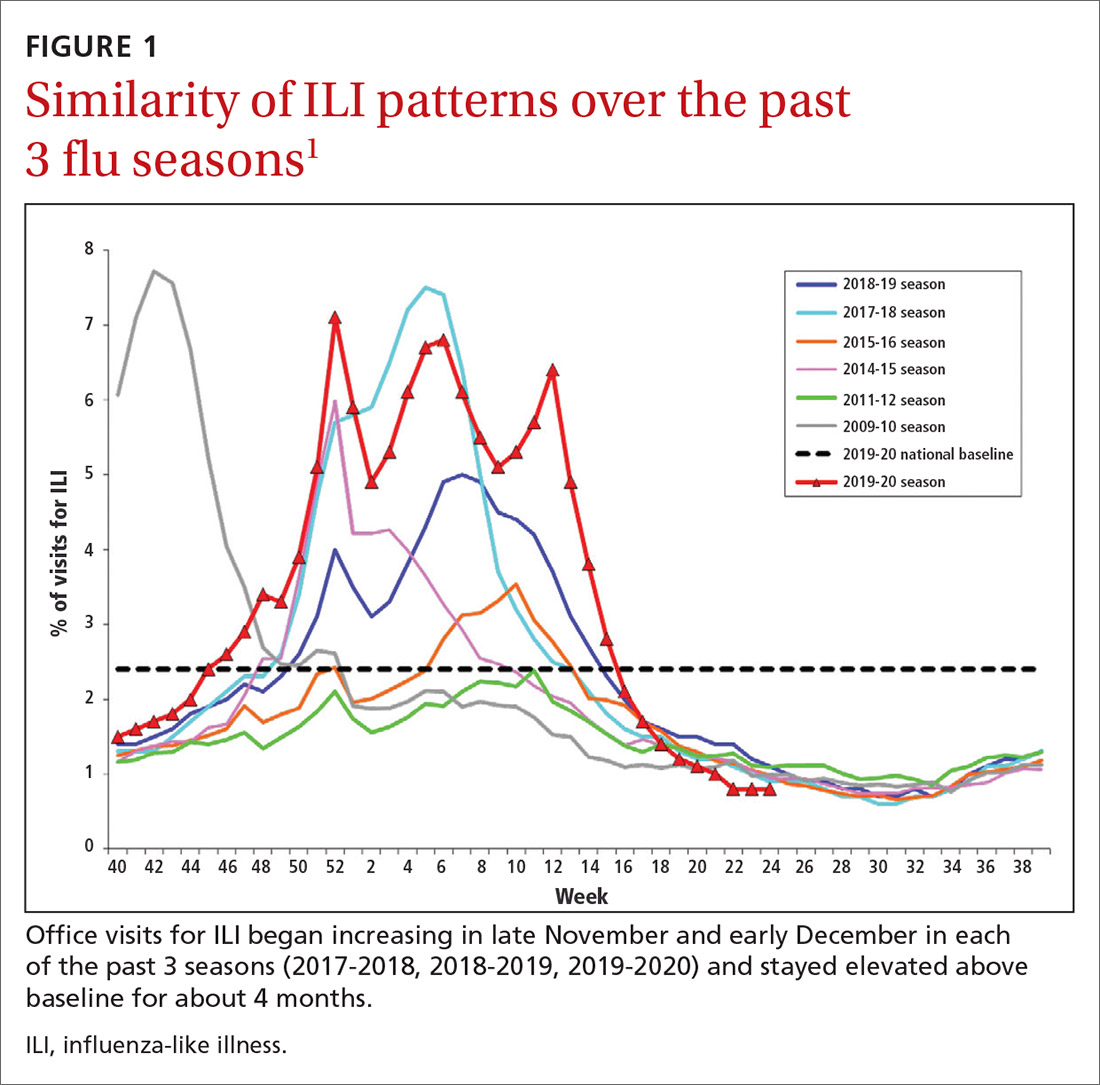
During the 2019-2020 flu season, there were an estimated 410,000 to 740,000 hospitalizations and 24,000 to 62,000 deaths attributed to influenza.1 As seen in FIGURE 1, office visits for influenza-like illness (ILI) began to increase in late November and early December in each of the last 3 years (2017-2018, 2018-2019, 2019-2020) and stayed elevated above baseline for about 4 months each season.1
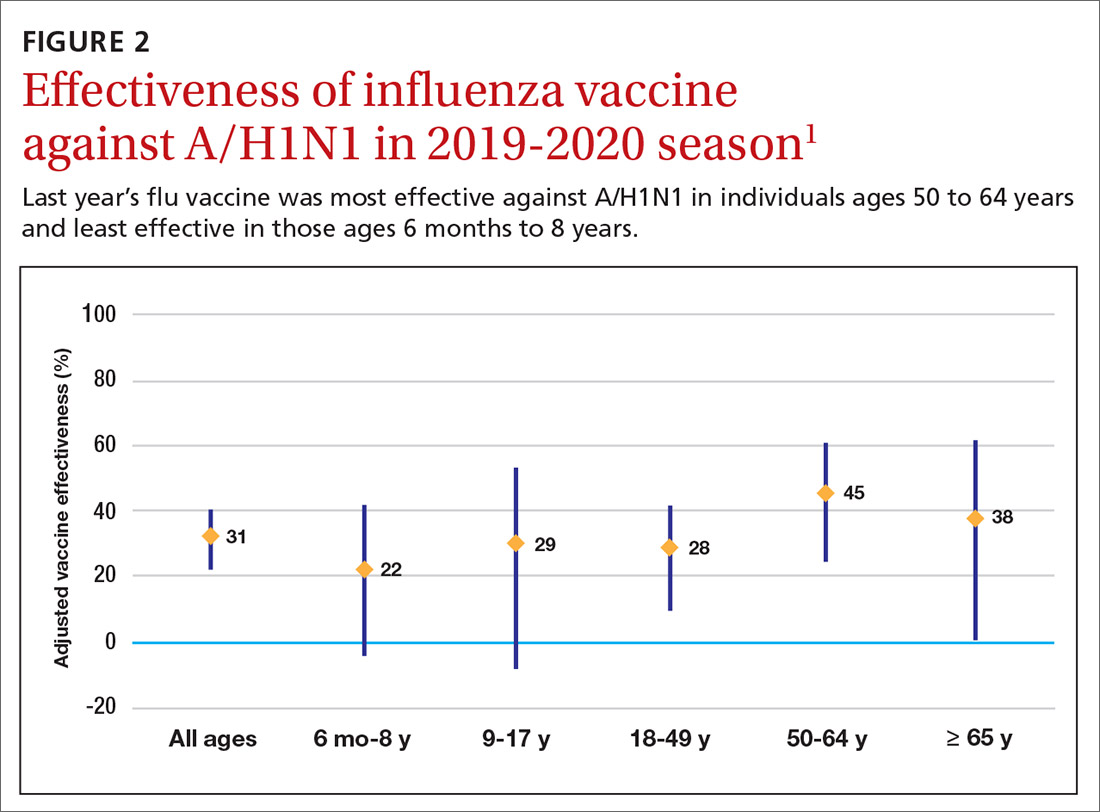
The effectiveness of influenza vaccine during the 2019-2020 season is being estimated using the US Flu Vaccine Effectiveness Network, which has close to 9000 enrollees. Overall, it appears the vaccine was 39% effective against medically attended influenza, with a higher effectiveness against influenza B (44%) than against A/H1N1 (31%). Effectiveness against influenza B was similar in all age groups, but effectiveness against A/H1N1 was highest for those ages 50 to 64 years (45%) and lowest for those ages 6 months through 8 years (22%), although 95% confidence intervals overlapped for all age groups (FIGURE 2). These preliminary effectiveness rates were presented at the summer meeting of the Advisory Committee on Immunization Practices (ACIP).1
Influenza vaccine safety data for 2019-2020 were based on the Vaccine Adverse Event Reporting System (VAERS), a passive surveillance system, and on the Vaccine Safety Datalink (VSD) system, an active surveillance system involving close to 6 million doses administered at VSD sites. No safety concerns were identified for any of the different vaccine types. Both the VAERS and VSD surveillance systems have been described in more detail in a previous Practice Alert.2
Recommendations for 2020-2021
The composition of the influenza vaccines for this year’s flu season will be different for 3 of the 4 antigens: A/H1N1, A/H2N2 and B/Victoria.3 The antigens included in the influenza vaccines each year are decided on in the spring, based on surveillance of circulating strains around the world. The effectiveness of the vaccine each year largely depends on how well the strains included in the vaccine match those circulating in the United States during the influenza season.
The main immunization recommendation for preventing morbidity and mortality from influenza has not changed: All individuals ages 6 months and older without a contraindication should receive an influenza vaccine.4 The Centers for Disease Control and Prevention (CDC) recommends that patients receive the vaccine by the end of October.4 This includes the second dose for those children younger than 9 years who need 2 doses—ie, those who have received fewer than 2 doses of influenza vaccine prior to July 2020. Vaccination should continue through the end of the season for anyone who has not received a 2020-2021 influenza vaccine.
Two new influenza vaccine products are available for use in those ages 65 years and older: Fluzone high-dose quadrivalent and Fluad Quadrivalent (adjuvanted).4 Both of these products were available last year as trivalent options. Currently no specific vaccine product is listed as preferred by ACIP for those ages 65 and older.
Continue to: New vaccine contraindications
New vaccine contraindications. Four medical conditions have been added to the list of contraindications for quadrivalent live, attenuated influenza vaccine (LAIV4): cochlear implant, cerebrospinal fluid leak, asplenia (anatomic and functional), and sickle cell anemia.4 In addition, those who receive LAIV4 should not be prescribed an influenza antiviral until 2 weeks after receiving the vaccine. And the vaccine should not be administered for 48 hours after receipt of oseltamivir or zanamivir, 5 days after peramivir, and 17 days after baloxavir marboxil.4 This is to prevent possible antiviral inactivation of the live attenuated influenza viruses in the vaccine.
For those who have a history of severe allergic reaction to eggs, there are now 2 egg-free options: cell-culture-based inactivated vaccine (ccIIV4) and recombinant influenza vaccine (RIV4).3,4 Urticaria alone is not considered a severe reaction. If neither of these egg-free options is available, a vaccine may still be administered in a medical setting supervised by a provider who is able to manage a severe allergic reaction (which rarely occurs).
All vaccine products available for the upcoming influenza season are listed and described on the CDC Web site, as is a summary of related recommendations.4 Particular attention should be paid to the dose of vaccine administered, as it differs by product for those ages 6 through 35 months of age and those ages 65 years and older.
Use of antiviral medications
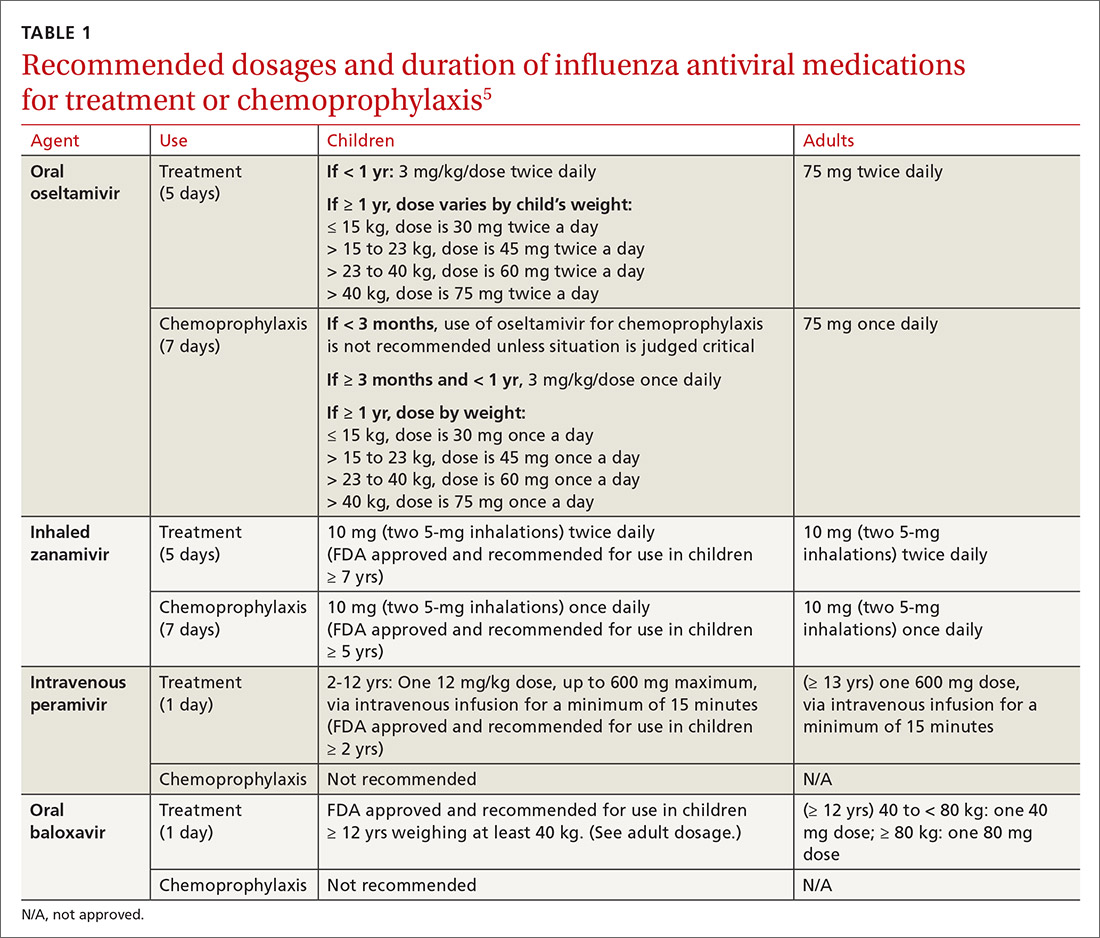
Four antiviral medications are now available for treating influenza (3 neuraminidase inhibitors and 1 endonuclease inhibitor), and there are 2 agents for preventing influenza, both neuraminidase inhibitors (TABLE 1).5 The CDC recommends treating with antivirals as soon as possible if individuals with confirmed or suspected influenza require hospitalization; have severe, complicated, or progressive illness; or are at high risk for complications. Use antivirals based on clinical judgment if previously healthy individuals do not have severe complications and are not at increased risk for complications, and only if the medication can be started within 48 hours of symptom onset.
The CDC discourages widespread use of antivirals to prevent influenza, either pre- or postexposure, although it specifies certain situations in which usage would be acceptable (TABLE 2).5 There is some concern that widespread use could lead to the emergence of drug-resistant strains and that using postexposure dosing could lead to suboptimal treatment if influenza infection occurred before the start of prophylaxis. If postexposure antivirals are prescribed, they should be started within 48 hours of exposure and continued for 7 days after the last exposure.
Continue to: A potential perfect storm
A potential perfect storm: Concurrence of influenza and SARS-coV-19
While we have vaccines and antivirals to prevent influenza, and have effective antivirals for treatment, no prevention or treatment options exist for COVID-19, except, possibly, dexamethasone to reduce mortality among those seriously ill.6 The concurrence of influenza and COVID-19 will present unique challenges for the health care system.
Action steps. Keep abreast of the incidences of circulating SARS-coV-19 and influenza viruses in your community. The similar signs and symptoms of these 2 infectious agents will complicate diagnosis. Rapid, or point-of-care, tests for influenza are widely available, but their accuracy varies and not all tests detect both influenza A and B. The CDC lists approved point-of-care tests at www.cdc.gov/flu/professionals/diagnosis/table-ridt.html and advises on how to interpret these test results when influenza is and is not circulating in the community, at www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm.
Clinical practice advice for both conditions should be implemented when any patient presents with ILI:7
- Most patients who are not seriously ill and have no conditions that place them at high risk for adverse outcomes can be treated symptomatically at home.
- Those with ILI should be tested for both influenza virus and SARS-CoV-2 if testing is available. It is possible to be co-infected.
- Sick patients should self-isolate at home for the duration of their symptoms.
- If others live in the house, the sick person should stay in a separate room and wear a mask. Everyone in the house should cover coughs and sneezes (if not wearing a mask), dispose of used tissues in a trash can (rather than leaving them on night stands and countertops), and wash hands frequently.
- All household members should be vaccinated against influenza. Those who are unvaccinated, and those at high risk who have been recently vaccinated, can consider influenza antiviral prophylaxis. If the sick family member is confirmed to have COVID-19, with no co-existing influenza, anti-influenza antiviral prophylaxis may be discontinued.
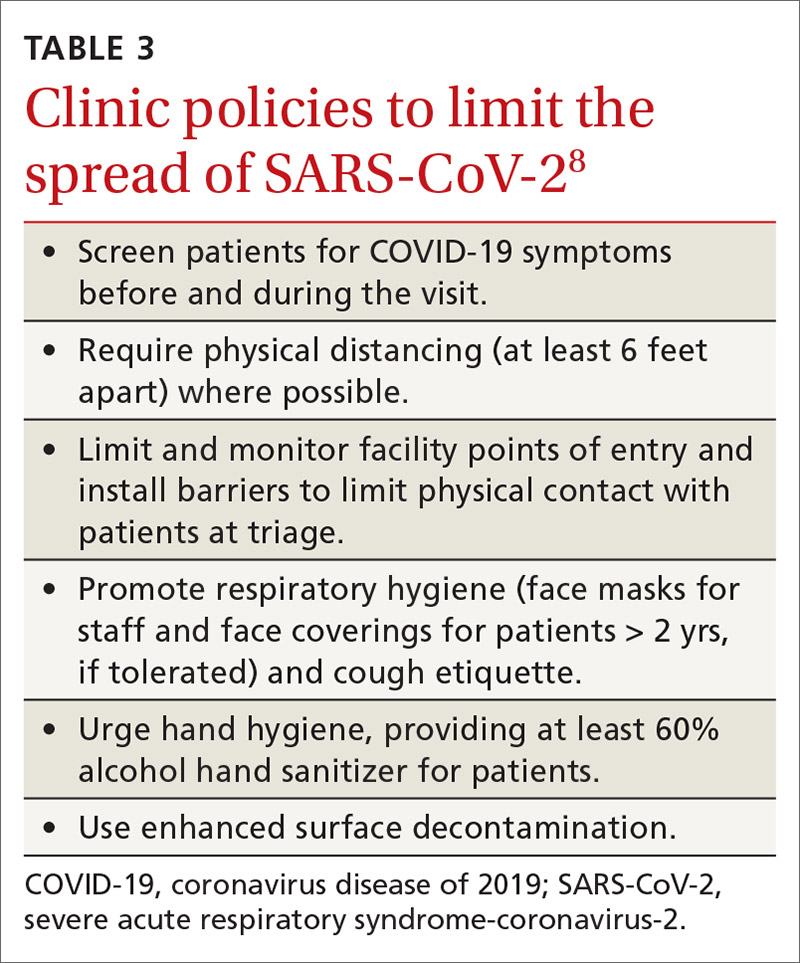
- Clinical infection control practices should be the same for anyone presenting with ILI.7 Enhanced clinic-based infection control practices to prevent spread of SARS-CoV-2 are listed in TABLE 3.8
Since there currently are no preventive medications proven to work for COVID-19, the main clinical decision physicians will have to make when a patient presents with ILI is whether to use antivirals to treat those who are at risk for complications based on the result of rapid, on-site influenza testing, or clinical presentation, or both. In this situation, knowledge of which viruses are circulating at high rates in the community could be valuable.
Milder season or perfect storm? The society-wide interventions that have been encouraged (although not mandated everywhere) to prevent community spread of SARS-CoV-2 should help prevent the community spread of influenza as well, and, if adhered to, may lead to a milder influenza season than would otherwise have occurred. However, given the uncertainties, the combination of influenza and coronavirus could present a perfect storm for the health care system and result in higher-than-normal morbidity and mortality from ILI and pneumonia overall.
Continue to: The possibility that one or more vaccines...
The possibility that one or more vaccines to prevent COVID-19 may be available in late 2020 or early 2021 offers hope. However, in current testing, the vaccine is not being given simultaneously with the influenza vaccine. If the potential for adverse interaction exists between the vaccines, it is important that influenza vaccine be given by mid- to late-October to avoid such an interaction if and when the new SARS-CoV-2 vaccine becomes available. Individuals who have symptoms of COVID-19 should not be vaccinated with influenza vaccine until they are considered noninfectious.
Encourage influenza vaccination. The COVID-19 pandemic may make it difficult to achieve desired community influenza vaccine levels because of decreased visits to medical facilities for preventive care, possible lower insurance coverage due to loss of employment, and a decrease in worksite mass vaccination programs. This makes it important for family physicians to encourage and offer influenza vaccines at their clinical sites.
Several evidence-based practices have been shown to improve vaccine uptake. Examples of such practices include patient reminder and recall systems that provide feedback to clinicians about rates of vaccination among patients, and establishing standing orders for vaccine administration that allow other health care providers to assess a patient’s immunization status and administer vaccinations according to a protocol.9 Finally, the CDC provides a video on how to recommend influenza vaccine to those who may be resistant (www.cdc.gov/vaccines/howirecommend/adult-vacc-videos.html).
SIDEBAR
CDC influenza resources
Point-of-care tests that detect both influenza A and B viruses approved by the CDC
www.cdc.gov/flu/professionals/diagnosis/table-ridt.html
Advice on how to interpret the test results
www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm
How to recommend influenza vaccine to reluctant patients
www.cdc.gov/vaccines/howirecommend/adult-vacc-videos.html
CDC, Centers for Disease Control and Prevention.
1. Grohskopf L. Influenza work groups: updates, considerations, and proposed recommendations for the 2020-2021 season. Presented at the ACIP meeting June 24, 2020. www.youtube.com/watch?v=W1SV2DSJsaQ&list=PLvrp9iOILTQb6D9e1YZWpbUvzfptNMKx2&index=8&t=0s. [Time stamp: 1:26:48] Accessed Septemeber 29, 2020.
2. Campos-Outcalt D. Facts to help you keep pace with the vaccine conversation. J Fam Pract. 2019;68:341-346.
3. Grohskopf L, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020-21 Influenza Season. MMWR Recomm Rep. 2020;69:1-24.
4. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2020-21 Summary of Recommendations. www.cdc.gov/flu/pdf/professionals/acip/acip-2020-21-summary-of-recommendations.pdf. Accessed September 29, 2020.
5. CDC. Influenza antiviral medications: summary for clinicians. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed September 29, 2020.
6. NIH. COVID-19 treatment guidelines. Corticosteroids. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 29, 2020.
7. CDC. Infection control. www.cdc.gov/infectioncontrol/. Accessed September 29, 2020.
8. CDC. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Accessed September 29, 2020.
9. HHS. CPSTF findings for increasing vaccination. www.thecommunityguide.org/content/task-force-findings-increasing-vaccination. Accessed September 29, 2020.
The 2020-2021 influenza season is shaping up to be challenging. Its likely concurrence with the ongoing severe acute respiratory syndrome-coronavirus 2 (SARS-coV-2) pandemic (COVID-19) will pose diagnostic and therapeutic dilemmas and could overload the hospital system. But there could also be potential synergies in preventing morbidity and mortality from each disease.
A consistent pattern overthe past few influenza seasons
During the 2019-2020 flu season, there were an estimated 410,000 to 740,000 hospitalizations and 24,000 to 62,000 deaths attributed to influenza.1 As seen in FIGURE 1, office visits for influenza-like illness (ILI) began to increase in late November and early December in each of the last 3 years (2017-2018, 2018-2019, 2019-2020) and stayed elevated above baseline for about 4 months each season.1
The effectiveness of influenza vaccine during the 2019-2020 season is being estimated using the US Flu Vaccine Effectiveness Network, which has close to 9000 enrollees. Overall, it appears the vaccine was 39% effective against medically attended influenza, with a higher effectiveness against influenza B (44%) than against A/H1N1 (31%). Effectiveness against influenza B was similar in all age groups, but effectiveness against A/H1N1 was highest for those ages 50 to 64 years (45%) and lowest for those ages 6 months through 8 years (22%), although 95% confidence intervals overlapped for all age groups (FIGURE 2). These preliminary effectiveness rates were presented at the summer meeting of the Advisory Committee on Immunization Practices (ACIP).1
Influenza vaccine safety data for 2019-2020 were based on the Vaccine Adverse Event Reporting System (VAERS), a passive surveillance system, and on the Vaccine Safety Datalink (VSD) system, an active surveillance system involving close to 6 million doses administered at VSD sites. No safety concerns were identified for any of the different vaccine types. Both the VAERS and VSD surveillance systems have been described in more detail in a previous Practice Alert.2
Recommendations for 2020-2021
The composition of the influenza vaccines for this year’s flu season will be different for 3 of the 4 antigens: A/H1N1, A/H2N2 and B/Victoria.3 The antigens included in the influenza vaccines each year are decided on in the spring, based on surveillance of circulating strains around the world. The effectiveness of the vaccine each year largely depends on how well the strains included in the vaccine match those circulating in the United States during the influenza season.
The main immunization recommendation for preventing morbidity and mortality from influenza has not changed: All individuals ages 6 months and older without a contraindication should receive an influenza vaccine.4 The Centers for Disease Control and Prevention (CDC) recommends that patients receive the vaccine by the end of October.4 This includes the second dose for those children younger than 9 years who need 2 doses—ie, those who have received fewer than 2 doses of influenza vaccine prior to July 2020. Vaccination should continue through the end of the season for anyone who has not received a 2020-2021 influenza vaccine.
Two new influenza vaccine products are available for use in those ages 65 years and older: Fluzone high-dose quadrivalent and Fluad Quadrivalent (adjuvanted).4 Both of these products were available last year as trivalent options. Currently no specific vaccine product is listed as preferred by ACIP for those ages 65 and older.
Continue to: New vaccine contraindications
New vaccine contraindications. Four medical conditions have been added to the list of contraindications for quadrivalent live, attenuated influenza vaccine (LAIV4): cochlear implant, cerebrospinal fluid leak, asplenia (anatomic and functional), and sickle cell anemia.4 In addition, those who receive LAIV4 should not be prescribed an influenza antiviral until 2 weeks after receiving the vaccine. And the vaccine should not be administered for 48 hours after receipt of oseltamivir or zanamivir, 5 days after peramivir, and 17 days after baloxavir marboxil.4 This is to prevent possible antiviral inactivation of the live attenuated influenza viruses in the vaccine.
For those who have a history of severe allergic reaction to eggs, there are now 2 egg-free options: cell-culture-based inactivated vaccine (ccIIV4) and recombinant influenza vaccine (RIV4).3,4 Urticaria alone is not considered a severe reaction. If neither of these egg-free options is available, a vaccine may still be administered in a medical setting supervised by a provider who is able to manage a severe allergic reaction (which rarely occurs).
All vaccine products available for the upcoming influenza season are listed and described on the CDC Web site, as is a summary of related recommendations.4 Particular attention should be paid to the dose of vaccine administered, as it differs by product for those ages 6 through 35 months of age and those ages 65 years and older.
Use of antiviral medications
Four antiviral medications are now available for treating influenza (3 neuraminidase inhibitors and 1 endonuclease inhibitor), and there are 2 agents for preventing influenza, both neuraminidase inhibitors (TABLE 1).5 The CDC recommends treating with antivirals as soon as possible if individuals with confirmed or suspected influenza require hospitalization; have severe, complicated, or progressive illness; or are at high risk for complications. Use antivirals based on clinical judgment if previously healthy individuals do not have severe complications and are not at increased risk for complications, and only if the medication can be started within 48 hours of symptom onset.
The CDC discourages widespread use of antivirals to prevent influenza, either pre- or postexposure, although it specifies certain situations in which usage would be acceptable (TABLE 2).5 There is some concern that widespread use could lead to the emergence of drug-resistant strains and that using postexposure dosing could lead to suboptimal treatment if influenza infection occurred before the start of prophylaxis. If postexposure antivirals are prescribed, they should be started within 48 hours of exposure and continued for 7 days after the last exposure.
Continue to: A potential perfect storm
A potential perfect storm: Concurrence of influenza and SARS-coV-19
While we have vaccines and antivirals to prevent influenza, and have effective antivirals for treatment, no prevention or treatment options exist for COVID-19, except, possibly, dexamethasone to reduce mortality among those seriously ill.6 The concurrence of influenza and COVID-19 will present unique challenges for the health care system.
Action steps. Keep abreast of the incidences of circulating SARS-coV-19 and influenza viruses in your community. The similar signs and symptoms of these 2 infectious agents will complicate diagnosis. Rapid, or point-of-care, tests for influenza are widely available, but their accuracy varies and not all tests detect both influenza A and B. The CDC lists approved point-of-care tests at www.cdc.gov/flu/professionals/diagnosis/table-ridt.html and advises on how to interpret these test results when influenza is and is not circulating in the community, at www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm.
Clinical practice advice for both conditions should be implemented when any patient presents with ILI:7
- Most patients who are not seriously ill and have no conditions that place them at high risk for adverse outcomes can be treated symptomatically at home.
- Those with ILI should be tested for both influenza virus and SARS-CoV-2 if testing is available. It is possible to be co-infected.
- Sick patients should self-isolate at home for the duration of their symptoms.
- If others live in the house, the sick person should stay in a separate room and wear a mask. Everyone in the house should cover coughs and sneezes (if not wearing a mask), dispose of used tissues in a trash can (rather than leaving them on night stands and countertops), and wash hands frequently.
- All household members should be vaccinated against influenza. Those who are unvaccinated, and those at high risk who have been recently vaccinated, can consider influenza antiviral prophylaxis. If the sick family member is confirmed to have COVID-19, with no co-existing influenza, anti-influenza antiviral prophylaxis may be discontinued.
- Clinical infection control practices should be the same for anyone presenting with ILI.7 Enhanced clinic-based infection control practices to prevent spread of SARS-CoV-2 are listed in TABLE 3.8
Since there currently are no preventive medications proven to work for COVID-19, the main clinical decision physicians will have to make when a patient presents with ILI is whether to use antivirals to treat those who are at risk for complications based on the result of rapid, on-site influenza testing, or clinical presentation, or both. In this situation, knowledge of which viruses are circulating at high rates in the community could be valuable.
Milder season or perfect storm? The society-wide interventions that have been encouraged (although not mandated everywhere) to prevent community spread of SARS-CoV-2 should help prevent the community spread of influenza as well, and, if adhered to, may lead to a milder influenza season than would otherwise have occurred. However, given the uncertainties, the combination of influenza and coronavirus could present a perfect storm for the health care system and result in higher-than-normal morbidity and mortality from ILI and pneumonia overall.
Continue to: The possibility that one or more vaccines...
The possibility that one or more vaccines to prevent COVID-19 may be available in late 2020 or early 2021 offers hope. However, in current testing, the vaccine is not being given simultaneously with the influenza vaccine. If the potential for adverse interaction exists between the vaccines, it is important that influenza vaccine be given by mid- to late-October to avoid such an interaction if and when the new SARS-CoV-2 vaccine becomes available. Individuals who have symptoms of COVID-19 should not be vaccinated with influenza vaccine until they are considered noninfectious.
Encourage influenza vaccination. The COVID-19 pandemic may make it difficult to achieve desired community influenza vaccine levels because of decreased visits to medical facilities for preventive care, possible lower insurance coverage due to loss of employment, and a decrease in worksite mass vaccination programs. This makes it important for family physicians to encourage and offer influenza vaccines at their clinical sites.
Several evidence-based practices have been shown to improve vaccine uptake. Examples of such practices include patient reminder and recall systems that provide feedback to clinicians about rates of vaccination among patients, and establishing standing orders for vaccine administration that allow other health care providers to assess a patient’s immunization status and administer vaccinations according to a protocol.9 Finally, the CDC provides a video on how to recommend influenza vaccine to those who may be resistant (www.cdc.gov/vaccines/howirecommend/adult-vacc-videos.html).
SIDEBAR
CDC influenza resources
Point-of-care tests that detect both influenza A and B viruses approved by the CDC
www.cdc.gov/flu/professionals/diagnosis/table-ridt.html
Advice on how to interpret the test results
www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm
How to recommend influenza vaccine to reluctant patients
www.cdc.gov/vaccines/howirecommend/adult-vacc-videos.html
CDC, Centers for Disease Control and Prevention.
The 2020-2021 influenza season is shaping up to be challenging. Its likely concurrence with the ongoing severe acute respiratory syndrome-coronavirus 2 (SARS-coV-2) pandemic (COVID-19) will pose diagnostic and therapeutic dilemmas and could overload the hospital system. But there could also be potential synergies in preventing morbidity and mortality from each disease.
A consistent pattern overthe past few influenza seasons
During the 2019-2020 flu season, there were an estimated 410,000 to 740,000 hospitalizations and 24,000 to 62,000 deaths attributed to influenza.1 As seen in FIGURE 1, office visits for influenza-like illness (ILI) began to increase in late November and early December in each of the last 3 years (2017-2018, 2018-2019, 2019-2020) and stayed elevated above baseline for about 4 months each season.1
The effectiveness of influenza vaccine during the 2019-2020 season is being estimated using the US Flu Vaccine Effectiveness Network, which has close to 9000 enrollees. Overall, it appears the vaccine was 39% effective against medically attended influenza, with a higher effectiveness against influenza B (44%) than against A/H1N1 (31%). Effectiveness against influenza B was similar in all age groups, but effectiveness against A/H1N1 was highest for those ages 50 to 64 years (45%) and lowest for those ages 6 months through 8 years (22%), although 95% confidence intervals overlapped for all age groups (FIGURE 2). These preliminary effectiveness rates were presented at the summer meeting of the Advisory Committee on Immunization Practices (ACIP).1
Influenza vaccine safety data for 2019-2020 were based on the Vaccine Adverse Event Reporting System (VAERS), a passive surveillance system, and on the Vaccine Safety Datalink (VSD) system, an active surveillance system involving close to 6 million doses administered at VSD sites. No safety concerns were identified for any of the different vaccine types. Both the VAERS and VSD surveillance systems have been described in more detail in a previous Practice Alert.2
Recommendations for 2020-2021
The composition of the influenza vaccines for this year’s flu season will be different for 3 of the 4 antigens: A/H1N1, A/H2N2 and B/Victoria.3 The antigens included in the influenza vaccines each year are decided on in the spring, based on surveillance of circulating strains around the world. The effectiveness of the vaccine each year largely depends on how well the strains included in the vaccine match those circulating in the United States during the influenza season.
The main immunization recommendation for preventing morbidity and mortality from influenza has not changed: All individuals ages 6 months and older without a contraindication should receive an influenza vaccine.4 The Centers for Disease Control and Prevention (CDC) recommends that patients receive the vaccine by the end of October.4 This includes the second dose for those children younger than 9 years who need 2 doses—ie, those who have received fewer than 2 doses of influenza vaccine prior to July 2020. Vaccination should continue through the end of the season for anyone who has not received a 2020-2021 influenza vaccine.
Two new influenza vaccine products are available for use in those ages 65 years and older: Fluzone high-dose quadrivalent and Fluad Quadrivalent (adjuvanted).4 Both of these products were available last year as trivalent options. Currently no specific vaccine product is listed as preferred by ACIP for those ages 65 and older.
Continue to: New vaccine contraindications
New vaccine contraindications. Four medical conditions have been added to the list of contraindications for quadrivalent live, attenuated influenza vaccine (LAIV4): cochlear implant, cerebrospinal fluid leak, asplenia (anatomic and functional), and sickle cell anemia.4 In addition, those who receive LAIV4 should not be prescribed an influenza antiviral until 2 weeks after receiving the vaccine. And the vaccine should not be administered for 48 hours after receipt of oseltamivir or zanamivir, 5 days after peramivir, and 17 days after baloxavir marboxil.4 This is to prevent possible antiviral inactivation of the live attenuated influenza viruses in the vaccine.
For those who have a history of severe allergic reaction to eggs, there are now 2 egg-free options: cell-culture-based inactivated vaccine (ccIIV4) and recombinant influenza vaccine (RIV4).3,4 Urticaria alone is not considered a severe reaction. If neither of these egg-free options is available, a vaccine may still be administered in a medical setting supervised by a provider who is able to manage a severe allergic reaction (which rarely occurs).
All vaccine products available for the upcoming influenza season are listed and described on the CDC Web site, as is a summary of related recommendations.4 Particular attention should be paid to the dose of vaccine administered, as it differs by product for those ages 6 through 35 months of age and those ages 65 years and older.
Use of antiviral medications
Four antiviral medications are now available for treating influenza (3 neuraminidase inhibitors and 1 endonuclease inhibitor), and there are 2 agents for preventing influenza, both neuraminidase inhibitors (TABLE 1).5 The CDC recommends treating with antivirals as soon as possible if individuals with confirmed or suspected influenza require hospitalization; have severe, complicated, or progressive illness; or are at high risk for complications. Use antivirals based on clinical judgment if previously healthy individuals do not have severe complications and are not at increased risk for complications, and only if the medication can be started within 48 hours of symptom onset.
The CDC discourages widespread use of antivirals to prevent influenza, either pre- or postexposure, although it specifies certain situations in which usage would be acceptable (TABLE 2).5 There is some concern that widespread use could lead to the emergence of drug-resistant strains and that using postexposure dosing could lead to suboptimal treatment if influenza infection occurred before the start of prophylaxis. If postexposure antivirals are prescribed, they should be started within 48 hours of exposure and continued for 7 days after the last exposure.
Continue to: A potential perfect storm
A potential perfect storm: Concurrence of influenza and SARS-coV-19
While we have vaccines and antivirals to prevent influenza, and have effective antivirals for treatment, no prevention or treatment options exist for COVID-19, except, possibly, dexamethasone to reduce mortality among those seriously ill.6 The concurrence of influenza and COVID-19 will present unique challenges for the health care system.
Action steps. Keep abreast of the incidences of circulating SARS-coV-19 and influenza viruses in your community. The similar signs and symptoms of these 2 infectious agents will complicate diagnosis. Rapid, or point-of-care, tests for influenza are widely available, but their accuracy varies and not all tests detect both influenza A and B. The CDC lists approved point-of-care tests at www.cdc.gov/flu/professionals/diagnosis/table-ridt.html and advises on how to interpret these test results when influenza is and is not circulating in the community, at www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm.
Clinical practice advice for both conditions should be implemented when any patient presents with ILI:7
- Most patients who are not seriously ill and have no conditions that place them at high risk for adverse outcomes can be treated symptomatically at home.
- Those with ILI should be tested for both influenza virus and SARS-CoV-2 if testing is available. It is possible to be co-infected.
- Sick patients should self-isolate at home for the duration of their symptoms.
- If others live in the house, the sick person should stay in a separate room and wear a mask. Everyone in the house should cover coughs and sneezes (if not wearing a mask), dispose of used tissues in a trash can (rather than leaving them on night stands and countertops), and wash hands frequently.
- All household members should be vaccinated against influenza. Those who are unvaccinated, and those at high risk who have been recently vaccinated, can consider influenza antiviral prophylaxis. If the sick family member is confirmed to have COVID-19, with no co-existing influenza, anti-influenza antiviral prophylaxis may be discontinued.
- Clinical infection control practices should be the same for anyone presenting with ILI.7 Enhanced clinic-based infection control practices to prevent spread of SARS-CoV-2 are listed in TABLE 3.8
Since there currently are no preventive medications proven to work for COVID-19, the main clinical decision physicians will have to make when a patient presents with ILI is whether to use antivirals to treat those who are at risk for complications based on the result of rapid, on-site influenza testing, or clinical presentation, or both. In this situation, knowledge of which viruses are circulating at high rates in the community could be valuable.
Milder season or perfect storm? The society-wide interventions that have been encouraged (although not mandated everywhere) to prevent community spread of SARS-CoV-2 should help prevent the community spread of influenza as well, and, if adhered to, may lead to a milder influenza season than would otherwise have occurred. However, given the uncertainties, the combination of influenza and coronavirus could present a perfect storm for the health care system and result in higher-than-normal morbidity and mortality from ILI and pneumonia overall.
Continue to: The possibility that one or more vaccines...
The possibility that one or more vaccines to prevent COVID-19 may be available in late 2020 or early 2021 offers hope. However, in current testing, the vaccine is not being given simultaneously with the influenza vaccine. If the potential for adverse interaction exists between the vaccines, it is important that influenza vaccine be given by mid- to late-October to avoid such an interaction if and when the new SARS-CoV-2 vaccine becomes available. Individuals who have symptoms of COVID-19 should not be vaccinated with influenza vaccine until they are considered noninfectious.
Encourage influenza vaccination. The COVID-19 pandemic may make it difficult to achieve desired community influenza vaccine levels because of decreased visits to medical facilities for preventive care, possible lower insurance coverage due to loss of employment, and a decrease in worksite mass vaccination programs. This makes it important for family physicians to encourage and offer influenza vaccines at their clinical sites.
Several evidence-based practices have been shown to improve vaccine uptake. Examples of such practices include patient reminder and recall systems that provide feedback to clinicians about rates of vaccination among patients, and establishing standing orders for vaccine administration that allow other health care providers to assess a patient’s immunization status and administer vaccinations according to a protocol.9 Finally, the CDC provides a video on how to recommend influenza vaccine to those who may be resistant (www.cdc.gov/vaccines/howirecommend/adult-vacc-videos.html).
SIDEBAR
CDC influenza resources
Point-of-care tests that detect both influenza A and B viruses approved by the CDC
www.cdc.gov/flu/professionals/diagnosis/table-ridt.html
Advice on how to interpret the test results
www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm
How to recommend influenza vaccine to reluctant patients
www.cdc.gov/vaccines/howirecommend/adult-vacc-videos.html
CDC, Centers for Disease Control and Prevention.
1. Grohskopf L. Influenza work groups: updates, considerations, and proposed recommendations for the 2020-2021 season. Presented at the ACIP meeting June 24, 2020. www.youtube.com/watch?v=W1SV2DSJsaQ&list=PLvrp9iOILTQb6D9e1YZWpbUvzfptNMKx2&index=8&t=0s. [Time stamp: 1:26:48] Accessed Septemeber 29, 2020.
2. Campos-Outcalt D. Facts to help you keep pace with the vaccine conversation. J Fam Pract. 2019;68:341-346.
3. Grohskopf L, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020-21 Influenza Season. MMWR Recomm Rep. 2020;69:1-24.
4. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2020-21 Summary of Recommendations. www.cdc.gov/flu/pdf/professionals/acip/acip-2020-21-summary-of-recommendations.pdf. Accessed September 29, 2020.
5. CDC. Influenza antiviral medications: summary for clinicians. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed September 29, 2020.
6. NIH. COVID-19 treatment guidelines. Corticosteroids. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 29, 2020.
7. CDC. Infection control. www.cdc.gov/infectioncontrol/. Accessed September 29, 2020.
8. CDC. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Accessed September 29, 2020.
9. HHS. CPSTF findings for increasing vaccination. www.thecommunityguide.org/content/task-force-findings-increasing-vaccination. Accessed September 29, 2020.
1. Grohskopf L. Influenza work groups: updates, considerations, and proposed recommendations for the 2020-2021 season. Presented at the ACIP meeting June 24, 2020. www.youtube.com/watch?v=W1SV2DSJsaQ&list=PLvrp9iOILTQb6D9e1YZWpbUvzfptNMKx2&index=8&t=0s. [Time stamp: 1:26:48] Accessed Septemeber 29, 2020.
2. Campos-Outcalt D. Facts to help you keep pace with the vaccine conversation. J Fam Pract. 2019;68:341-346.
3. Grohskopf L, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020-21 Influenza Season. MMWR Recomm Rep. 2020;69:1-24.
4. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2020-21 Summary of Recommendations. www.cdc.gov/flu/pdf/professionals/acip/acip-2020-21-summary-of-recommendations.pdf. Accessed September 29, 2020.
5. CDC. Influenza antiviral medications: summary for clinicians. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed September 29, 2020.
6. NIH. COVID-19 treatment guidelines. Corticosteroids. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 29, 2020.
7. CDC. Infection control. www.cdc.gov/infectioncontrol/. Accessed September 29, 2020.
8. CDC. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Accessed September 29, 2020.
9. HHS. CPSTF findings for increasing vaccination. www.thecommunityguide.org/content/task-force-findings-increasing-vaccination. Accessed September 29, 2020.
Study offers reassurance to postmenopausal women taking hormone therapy
References
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;32:369-380.
- The North American Menopause Society. Menopause Guidebook. 8th ed. www.menopause.org/publications/consumer-publications/-em-menopause-guidebook-em-8th-edition. Accessed September 25, 2020.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;318:2224-2233.
References
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;32:369-380.
- The North American Menopause Society. Menopause Guidebook. 8th ed. www.menopause.org/publications/consumer-publications/-em-menopause-guidebook-em-8th-edition. Accessed September 25, 2020.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;318:2224-2233.
References
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;32:369-380.
- The North American Menopause Society. Menopause Guidebook. 8th ed. www.menopause.org/publications/consumer-publications/-em-menopause-guidebook-em-8th-edition. Accessed September 25, 2020.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;318:2224-2233.
Coronavirus vaccine: The contenders, the potential controversy
References
- CDC. Coronavirus Disease 2019 (COVID-19): Cases in the US. www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed August 18, 2020.
- US Department of Health and Human Services. Fact Sheet: explaining Operation Warp Speed. www.hhs.gov/coronavirus/explaining-operation-warp-speed/index.html. Accessed August 18, 2020.
- O’Callahan KP, Blatz AM, Offit PA. Developing a SARS-CoV-2 vaccine at warp speed. JAMA. 2020;324:437-438.
- Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279.
- Lurie N, Sharfstein JM, Goodman JL. The development of COVID-19 vaccines: safeguards needed [commentary]. JAMA. 2020;324:439-440.
- Salman DA, Akhtar A, Mergler MJ, et al; H1N1 Working Group of Federal Immunization Safety Task Force. Immunization safety monitoring systems for the 2009 H1N1 monovalent influenza vaccination program. Pediatrics. 2011;127(suppl 1):S78-S86.
References
- CDC. Coronavirus Disease 2019 (COVID-19): Cases in the US. www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed August 18, 2020.
- US Department of Health and Human Services. Fact Sheet: explaining Operation Warp Speed. www.hhs.gov/coronavirus/explaining-operation-warp-speed/index.html. Accessed August 18, 2020.
- O’Callahan KP, Blatz AM, Offit PA. Developing a SARS-CoV-2 vaccine at warp speed. JAMA. 2020;324:437-438.
- Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279.
- Lurie N, Sharfstein JM, Goodman JL. The development of COVID-19 vaccines: safeguards needed [commentary]. JAMA. 2020;324:439-440.
- Salman DA, Akhtar A, Mergler MJ, et al; H1N1 Working Group of Federal Immunization Safety Task Force. Immunization safety monitoring systems for the 2009 H1N1 monovalent influenza vaccination program. Pediatrics. 2011;127(suppl 1):S78-S86.
References
- CDC. Coronavirus Disease 2019 (COVID-19): Cases in the US. www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed August 18, 2020.
- US Department of Health and Human Services. Fact Sheet: explaining Operation Warp Speed. www.hhs.gov/coronavirus/explaining-operation-warp-speed/index.html. Accessed August 18, 2020.
- O’Callahan KP, Blatz AM, Offit PA. Developing a SARS-CoV-2 vaccine at warp speed. JAMA. 2020;324:437-438.
- Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279.
- Lurie N, Sharfstein JM, Goodman JL. The development of COVID-19 vaccines: safeguards needed [commentary]. JAMA. 2020;324:439-440.
- Salman DA, Akhtar A, Mergler MJ, et al; H1N1 Working Group of Federal Immunization Safety Task Force. Immunization safety monitoring systems for the 2009 H1N1 monovalent influenza vaccination program. Pediatrics. 2011;127(suppl 1):S78-S86.
Tackling unhealthy substance use using USPSTF guidance and a 1-question tool
References
- US Preventive Services Task Force. Unhealthy drug use: screening [final recommendation statement]. Published June 9, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening. Accessed July 28, 2020.
- US Preventive Services Task Force. Illicit drug use in children, adolescents, and young adults: primary care-based interventions [final recommendation statement]. Published May 26, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents. Accessed July 28, 2020.
- US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions [final recommendation statement]. Published April 28, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed July 28, 2020.
- National Institute on Drug Abuse. NIDA Quick Screen v 1.0. www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf. Accessed July 28, 2020.
- US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions [update in progress]. Published September 21, 2015. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed July 28, 2020.
References
- US Preventive Services Task Force. Unhealthy drug use: screening [final recommendation statement]. Published June 9, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening. Accessed July 28, 2020.
- US Preventive Services Task Force. Illicit drug use in children, adolescents, and young adults: primary care-based interventions [final recommendation statement]. Published May 26, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents. Accessed July 28, 2020.
- US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions [final recommendation statement]. Published April 28, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed July 28, 2020.
- National Institute on Drug Abuse. NIDA Quick Screen v 1.0. www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf. Accessed July 28, 2020.
- US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions [update in progress]. Published September 21, 2015. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed July 28, 2020.
References
- US Preventive Services Task Force. Unhealthy drug use: screening [final recommendation statement]. Published June 9, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening. Accessed July 28, 2020.
- US Preventive Services Task Force. Illicit drug use in children, adolescents, and young adults: primary care-based interventions [final recommendation statement]. Published May 26, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents. Accessed July 28, 2020.
- US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions [final recommendation statement]. Published April 28, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed July 28, 2020.
- National Institute on Drug Abuse. NIDA Quick Screen v 1.0. www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf. Accessed July 28, 2020.
- US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions [update in progress]. Published September 21, 2015. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed July 28, 2020.
Taking steps to slow the upswing in oral and pharyngeal cancers
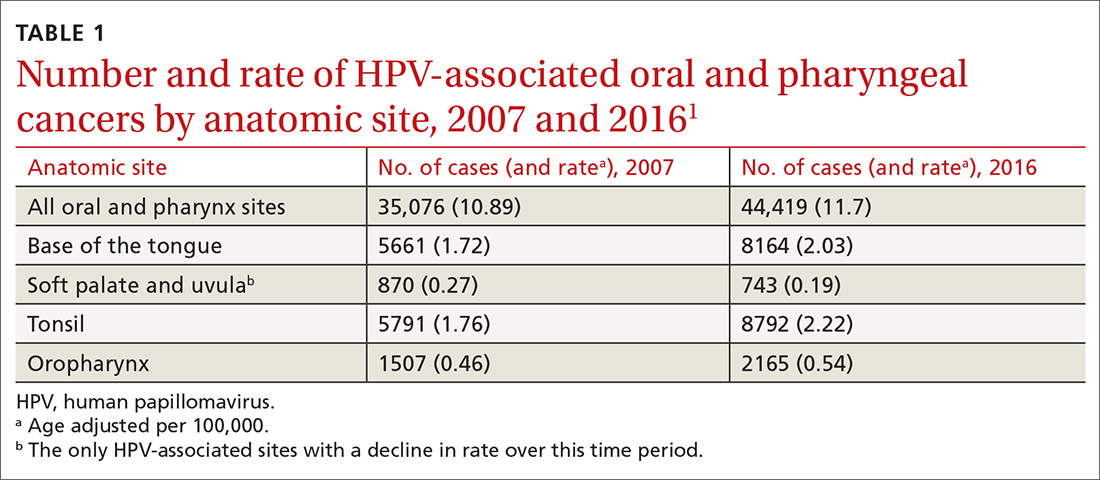
A recent report by the Centers for Disease Control and Prevention (CDC) documents the trends in oral and pharyngeal cancers (OPC) in the United States over a 10-year period, 2007-2016.1 The rate of OPC began to increase in 1999 and has been increasing ever since. The age-adjusted rate in 2007 was 10.89/100,000 compared with 11.7/100,000 in 2016 (TABLE 11). This is an annual relative increase of about 6% per year. In absolute numbers, there were 35,076 cases in 2007 and 44,419 in 2016.1 The trends in incidence of OPC vary by anatomical site, with some increasing and others declining.
There are 3 known causal factors related to OPC: tobacco use, alcohol use, and human papillomavirus (HPV) infection. The CDC estimates that, overall, 70% of OPCs are caused by HPV.2 However, while cancers at some oropharyngeal sites are likely related to HPV infection, cancers at other sites are not. The rising overall incidence of OPC is being driven by increases in HPV-related cancers at an average rate of 2.1% per year, while the rates at non-HPV-associated sites have been declining by 0.4% per year.1 It is also important to appreciate that HPV causes cancer at other anatomical sites (TABLE 22) and is responsible for an estimated 35,000 cancers per year.2
Other trends of note in all OPCs combined are increasing rates among non-Hispanic whites and Asian-Pacific Islanders; decreasing rates among Hispanics and African Americans; increasing rates among males with no real change in rates among females; increasing rates in those 50 to 79 years of age; decreasing rates among those 40 to 49 years of age; and unchanged rates in other age groups.1
The role of the family physician
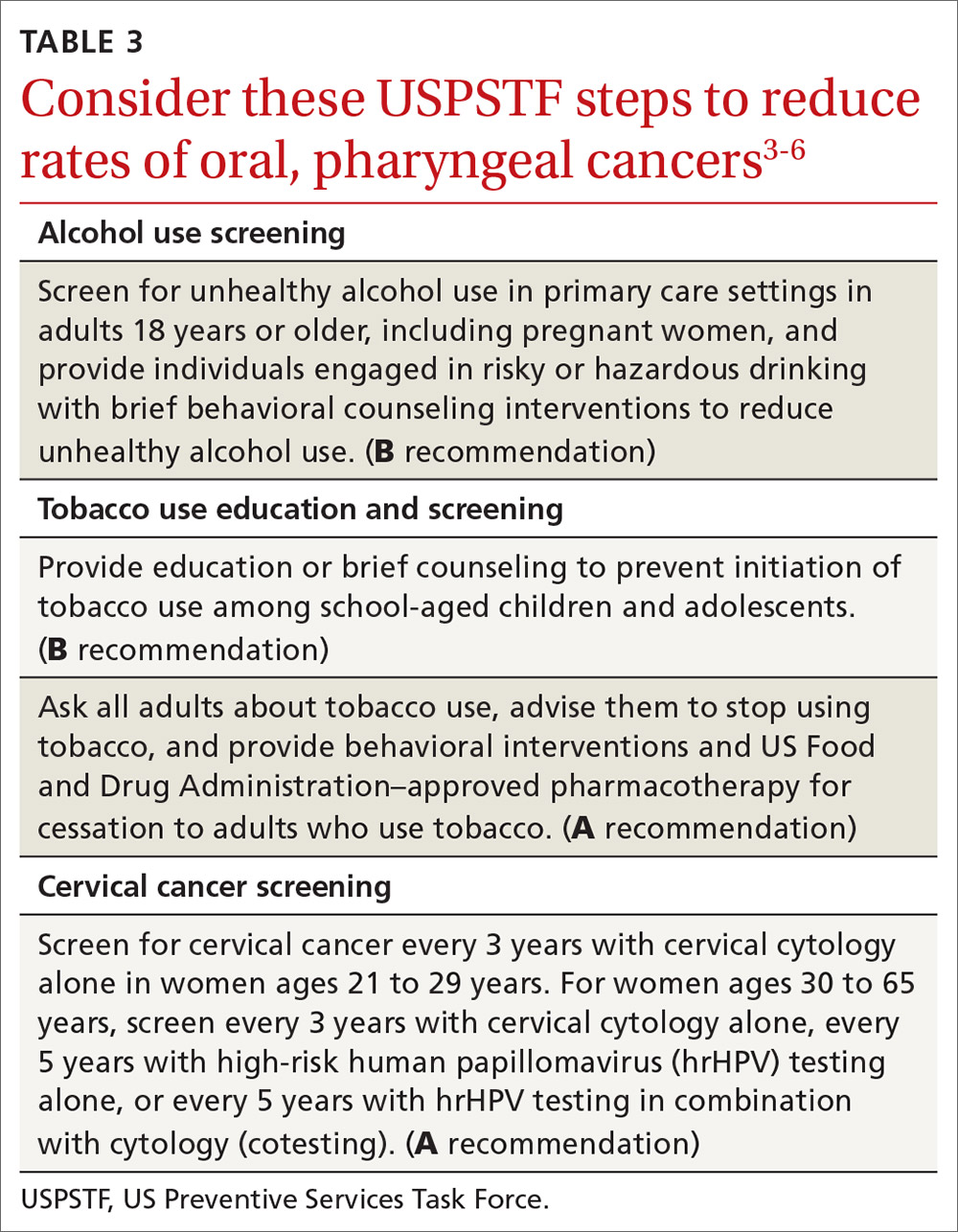
Preventing OPC and all HPV-related cancers begins by encouraging patients to reduce alcohol and tobacco use and by emphasizing the importance of HPV vaccination. Educate teens and parents/guardians about HPV vaccine and its safety. Screen for tobacco and alcohol use, and offer brief clinical interventions as needed to decrease usage.
Recommendations by the US Preventive Services Task Force regarding screening for, and reducing use of, tobacco and alcohol, as well as screening for cervical cancer, are listed in TABLE 3.3-6 Remember that cervical cancer screening is both a primary and secondary intervention: It can reduce mortality by preventing cervical cancer (via treatment of precancerous lesions) and by detecting cervical cancer early at more treatable stages.
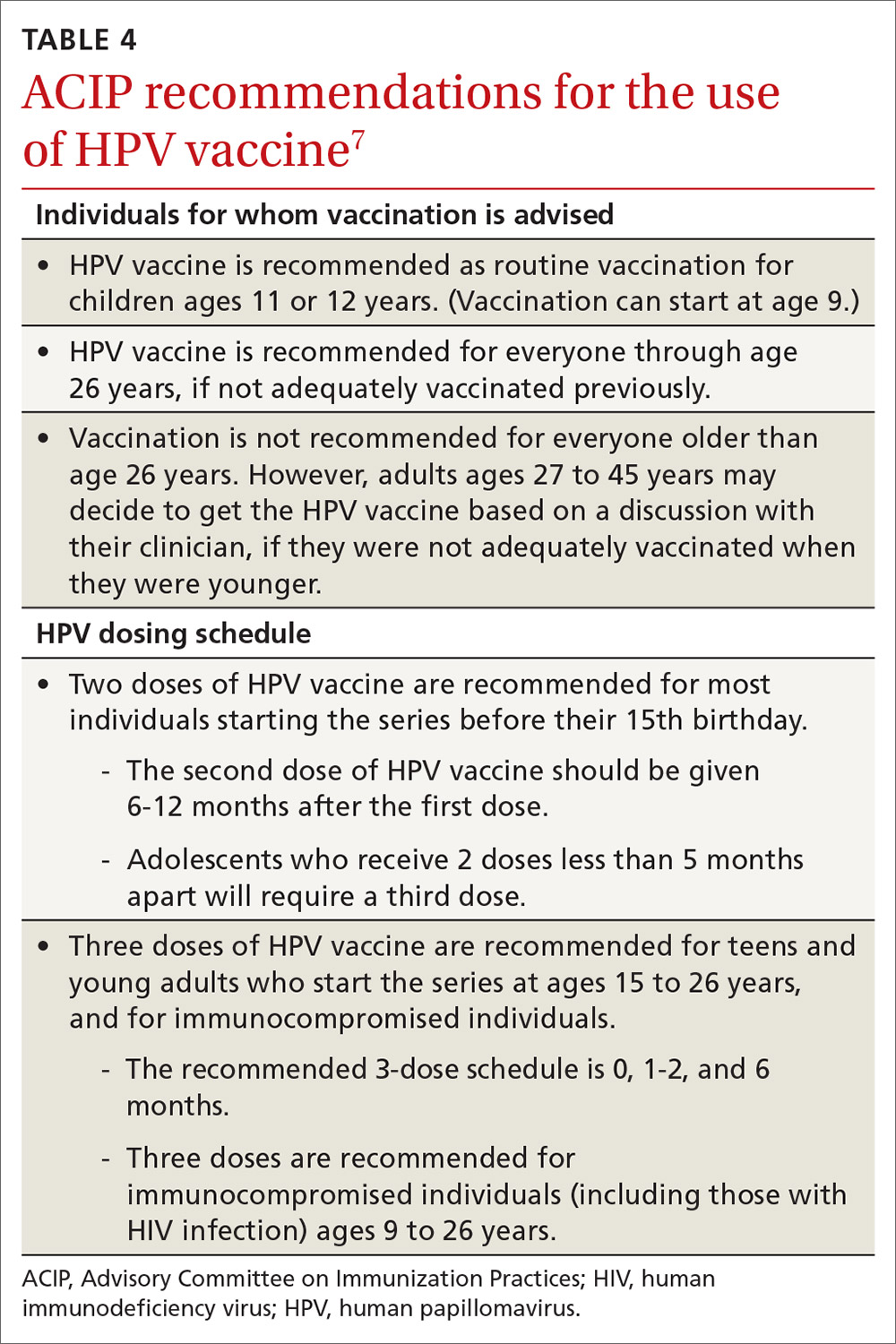
HPV vaccination essentials. CDC recommendations for the use of HPV vaccine and the vaccine dosing schedule appear in TABLE 4.7 While it is true that the best evidence for HPV vaccine’s prevention of cancer comes from the study of cervical and anal cancers, it is reasonable to expect that it will also be proven over time to prevent other HPV-caused cancers as the rate of HPV infections declines.
HPV vaccine is underused. In a 2018 survey, only 68.1% of adolescents had received 1 or more doses of HPV vaccine, and only 51.1% were up to date.8 In contrast, 86.6% had received 1 or more doses of quadrivalent meningococcal vaccine; 88.9% had received 1 or more doses of tetanus, diphtheria & acellular pertussis vaccine; 91.9% were up to date with 2 or more doses of measles, mumps & rubella vaccine; and 92.1% were up to date with hepatitis B vaccine, with 3 or more doses.8
Continue to: Address parental concerns, including these 5 false beliefs
Address parental concerns, including these 5 false beliefs
One study found 5 major false beliefs parents hold about HPV vaccine9:
- Vaccination is not effective at preventing cancer.
- Pap smears are sufficient to prevent cervical cancer.
- HPV vaccination is not safe.
- HPV vaccination is not needed since most infections are naturally cleared by the immune system.
- Eleven to 12 years of age is too young to vaccinate.
There is some evidence that if clinicians actively engage with parents about these concerns and address them head on, same-day vaccination rates can improve.10
We can expect to see HPV-associated OPC decline in the coming years due to the delayed effects on cancer incidence by the HPV vaccine. These anticipated declines will be more dramatic if we can increase the uptake of the HPV vaccine.
1. Ellington TD, Henley SJ, Senkomago V, et al. Trends in the incidence of cancers of the oral cavity and pharynx—United States 2007-2016. MMWR Morb Mortal Wkly Rep. 2020;69:433-438.
2. CDC. HPV and cancer. 2019. https://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed June 29, 2020.
3. USPSTF. Unhealthy alcohol use in adolescents and adults: screening and behavioral counseling interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/unhealthy-alcohol-use-in-adolescents-and-adults-screening-and-behavioral-counseling-interventions. Accessed June 29, 2020.
4. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed June 29, 2020.
5. USPSTF. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed June 29, 2020.
6. USPSTF. Cervical cancer: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening. Accessed June 29, 2020.
7. CDC. Vaccines and preventable diseases. HPV vaccine recommendations. 2020. www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed June 29, 2020.
8. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years-United States, 2018. MMWR Morb Mortal Wkly Rep. 2019:68:718-723.
9. Bednarczyk RA. Addressing HPV vaccine myths: practical information for healthcare providers. Hum Vaccin Immunother. 2019;15:1628-1638.
10. Shay LA, Baldwin AS, Betts AC, et al. Parent-provider communication of HPV vaccine hesitancy. Pediatrics 2018;141:e20172312.
A recent report by the Centers for Disease Control and Prevention (CDC) documents the trends in oral and pharyngeal cancers (OPC) in the United States over a 10-year period, 2007-2016.1 The rate of OPC began to increase in 1999 and has been increasing ever since. The age-adjusted rate in 2007 was 10.89/100,000 compared with 11.7/100,000 in 2016 (TABLE 11). This is an annual relative increase of about 6% per year. In absolute numbers, there were 35,076 cases in 2007 and 44,419 in 2016.1 The trends in incidence of OPC vary by anatomical site, with some increasing and others declining.
There are 3 known causal factors related to OPC: tobacco use, alcohol use, and human papillomavirus (HPV) infection. The CDC estimates that, overall, 70% of OPCs are caused by HPV.2 However, while cancers at some oropharyngeal sites are likely related to HPV infection, cancers at other sites are not. The rising overall incidence of OPC is being driven by increases in HPV-related cancers at an average rate of 2.1% per year, while the rates at non-HPV-associated sites have been declining by 0.4% per year.1 It is also important to appreciate that HPV causes cancer at other anatomical sites (TABLE 22) and is responsible for an estimated 35,000 cancers per year.2
Other trends of note in all OPCs combined are increasing rates among non-Hispanic whites and Asian-Pacific Islanders; decreasing rates among Hispanics and African Americans; increasing rates among males with no real change in rates among females; increasing rates in those 50 to 79 years of age; decreasing rates among those 40 to 49 years of age; and unchanged rates in other age groups.1
The role of the family physician
Preventing OPC and all HPV-related cancers begins by encouraging patients to reduce alcohol and tobacco use and by emphasizing the importance of HPV vaccination. Educate teens and parents/guardians about HPV vaccine and its safety. Screen for tobacco and alcohol use, and offer brief clinical interventions as needed to decrease usage.
Recommendations by the US Preventive Services Task Force regarding screening for, and reducing use of, tobacco and alcohol, as well as screening for cervical cancer, are listed in TABLE 3.3-6 Remember that cervical cancer screening is both a primary and secondary intervention: It can reduce mortality by preventing cervical cancer (via treatment of precancerous lesions) and by detecting cervical cancer early at more treatable stages.
HPV vaccination essentials. CDC recommendations for the use of HPV vaccine and the vaccine dosing schedule appear in TABLE 4.7 While it is true that the best evidence for HPV vaccine’s prevention of cancer comes from the study of cervical and anal cancers, it is reasonable to expect that it will also be proven over time to prevent other HPV-caused cancers as the rate of HPV infections declines.
HPV vaccine is underused. In a 2018 survey, only 68.1% of adolescents had received 1 or more doses of HPV vaccine, and only 51.1% were up to date.8 In contrast, 86.6% had received 1 or more doses of quadrivalent meningococcal vaccine; 88.9% had received 1 or more doses of tetanus, diphtheria & acellular pertussis vaccine; 91.9% were up to date with 2 or more doses of measles, mumps & rubella vaccine; and 92.1% were up to date with hepatitis B vaccine, with 3 or more doses.8
Continue to: Address parental concerns, including these 5 false beliefs
Address parental concerns, including these 5 false beliefs
One study found 5 major false beliefs parents hold about HPV vaccine9:
- Vaccination is not effective at preventing cancer.
- Pap smears are sufficient to prevent cervical cancer.
- HPV vaccination is not safe.
- HPV vaccination is not needed since most infections are naturally cleared by the immune system.
- Eleven to 12 years of age is too young to vaccinate.
There is some evidence that if clinicians actively engage with parents about these concerns and address them head on, same-day vaccination rates can improve.10
We can expect to see HPV-associated OPC decline in the coming years due to the delayed effects on cancer incidence by the HPV vaccine. These anticipated declines will be more dramatic if we can increase the uptake of the HPV vaccine.
A recent report by the Centers for Disease Control and Prevention (CDC) documents the trends in oral and pharyngeal cancers (OPC) in the United States over a 10-year period, 2007-2016.1 The rate of OPC began to increase in 1999 and has been increasing ever since. The age-adjusted rate in 2007 was 10.89/100,000 compared with 11.7/100,000 in 2016 (TABLE 11). This is an annual relative increase of about 6% per year. In absolute numbers, there were 35,076 cases in 2007 and 44,419 in 2016.1 The trends in incidence of OPC vary by anatomical site, with some increasing and others declining.
There are 3 known causal factors related to OPC: tobacco use, alcohol use, and human papillomavirus (HPV) infection. The CDC estimates that, overall, 70% of OPCs are caused by HPV.2 However, while cancers at some oropharyngeal sites are likely related to HPV infection, cancers at other sites are not. The rising overall incidence of OPC is being driven by increases in HPV-related cancers at an average rate of 2.1% per year, while the rates at non-HPV-associated sites have been declining by 0.4% per year.1 It is also important to appreciate that HPV causes cancer at other anatomical sites (TABLE 22) and is responsible for an estimated 35,000 cancers per year.2
Other trends of note in all OPCs combined are increasing rates among non-Hispanic whites and Asian-Pacific Islanders; decreasing rates among Hispanics and African Americans; increasing rates among males with no real change in rates among females; increasing rates in those 50 to 79 years of age; decreasing rates among those 40 to 49 years of age; and unchanged rates in other age groups.1
The role of the family physician
Preventing OPC and all HPV-related cancers begins by encouraging patients to reduce alcohol and tobacco use and by emphasizing the importance of HPV vaccination. Educate teens and parents/guardians about HPV vaccine and its safety. Screen for tobacco and alcohol use, and offer brief clinical interventions as needed to decrease usage.
Recommendations by the US Preventive Services Task Force regarding screening for, and reducing use of, tobacco and alcohol, as well as screening for cervical cancer, are listed in TABLE 3.3-6 Remember that cervical cancer screening is both a primary and secondary intervention: It can reduce mortality by preventing cervical cancer (via treatment of precancerous lesions) and by detecting cervical cancer early at more treatable stages.
HPV vaccination essentials. CDC recommendations for the use of HPV vaccine and the vaccine dosing schedule appear in TABLE 4.7 While it is true that the best evidence for HPV vaccine’s prevention of cancer comes from the study of cervical and anal cancers, it is reasonable to expect that it will also be proven over time to prevent other HPV-caused cancers as the rate of HPV infections declines.
HPV vaccine is underused. In a 2018 survey, only 68.1% of adolescents had received 1 or more doses of HPV vaccine, and only 51.1% were up to date.8 In contrast, 86.6% had received 1 or more doses of quadrivalent meningococcal vaccine; 88.9% had received 1 or more doses of tetanus, diphtheria & acellular pertussis vaccine; 91.9% were up to date with 2 or more doses of measles, mumps & rubella vaccine; and 92.1% were up to date with hepatitis B vaccine, with 3 or more doses.8
Continue to: Address parental concerns, including these 5 false beliefs
Address parental concerns, including these 5 false beliefs
One study found 5 major false beliefs parents hold about HPV vaccine9:
- Vaccination is not effective at preventing cancer.
- Pap smears are sufficient to prevent cervical cancer.
- HPV vaccination is not safe.
- HPV vaccination is not needed since most infections are naturally cleared by the immune system.
- Eleven to 12 years of age is too young to vaccinate.
There is some evidence that if clinicians actively engage with parents about these concerns and address them head on, same-day vaccination rates can improve.10
We can expect to see HPV-associated OPC decline in the coming years due to the delayed effects on cancer incidence by the HPV vaccine. These anticipated declines will be more dramatic if we can increase the uptake of the HPV vaccine.
1. Ellington TD, Henley SJ, Senkomago V, et al. Trends in the incidence of cancers of the oral cavity and pharynx—United States 2007-2016. MMWR Morb Mortal Wkly Rep. 2020;69:433-438.
2. CDC. HPV and cancer. 2019. https://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed June 29, 2020.
3. USPSTF. Unhealthy alcohol use in adolescents and adults: screening and behavioral counseling interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/unhealthy-alcohol-use-in-adolescents-and-adults-screening-and-behavioral-counseling-interventions. Accessed June 29, 2020.
4. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed June 29, 2020.
5. USPSTF. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed June 29, 2020.
6. USPSTF. Cervical cancer: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening. Accessed June 29, 2020.
7. CDC. Vaccines and preventable diseases. HPV vaccine recommendations. 2020. www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed June 29, 2020.
8. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years-United States, 2018. MMWR Morb Mortal Wkly Rep. 2019:68:718-723.
9. Bednarczyk RA. Addressing HPV vaccine myths: practical information for healthcare providers. Hum Vaccin Immunother. 2019;15:1628-1638.
10. Shay LA, Baldwin AS, Betts AC, et al. Parent-provider communication of HPV vaccine hesitancy. Pediatrics 2018;141:e20172312.
1. Ellington TD, Henley SJ, Senkomago V, et al. Trends in the incidence of cancers of the oral cavity and pharynx—United States 2007-2016. MMWR Morb Mortal Wkly Rep. 2020;69:433-438.
2. CDC. HPV and cancer. 2019. https://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed June 29, 2020.
3. USPSTF. Unhealthy alcohol use in adolescents and adults: screening and behavioral counseling interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/unhealthy-alcohol-use-in-adolescents-and-adults-screening-and-behavioral-counseling-interventions. Accessed June 29, 2020.
4. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed June 29, 2020.
5. USPSTF. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed June 29, 2020.
6. USPSTF. Cervical cancer: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening. Accessed June 29, 2020.
7. CDC. Vaccines and preventable diseases. HPV vaccine recommendations. 2020. www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed June 29, 2020.
8. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years-United States, 2018. MMWR Morb Mortal Wkly Rep. 2019:68:718-723.
9. Bednarczyk RA. Addressing HPV vaccine myths: practical information for healthcare providers. Hum Vaccin Immunother. 2019;15:1628-1638.
10. Shay LA, Baldwin AS, Betts AC, et al. Parent-provider communication of HPV vaccine hesitancy. Pediatrics 2018;141:e20172312.
Medication use & COVID-19: Unwarranted concerns, evidence-based approaches
References
- National Institute of Health. COVID-19 treatment guidelines: what’s new in the guidelines? Updated June 25, 2020. www.covid19treatmentguidelines.nih.gov/whats-new/. Accessed June 26, 2020.
- National Institute for Health Care and Excellence. COVID-19 rapid evidence summary: Remdesivir for treating hospitalised patients with suspected or confirmed COVID-19. Evidence summary [ES27]. Published June 5, 2020. www.nice.org.uk/advice/es27/chapter/Key-messages. Accessed June 26, 2020.
- National Institute for Health Care and Excellence. COVID-19 rapid evidence summary: angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) in people with or at risk of COVID-19. Evidence summary [ES24]. Published May 21, 2020. www.nice.org.uk/advice/es24/chapter/Key-messages. Accessed June 26, 2020.
- National Institute for Health Care and Excellence. COVID-19 rapid evidence summary: Long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) for people with or at risk of COVID-19. Evidence summary [ES25]. Published May 21, 2020. www.nice.org.uk/advice/es25/chapter/Key-messages. Accessed June 26, 2020.
- Hernandez AV, Roman YM, Pasupuleti V, et al. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 2020 May 27. doi: 10.7326/M20-2496. Online ahead of print.
References
- National Institute of Health. COVID-19 treatment guidelines: what’s new in the guidelines? Updated June 25, 2020. www.covid19treatmentguidelines.nih.gov/whats-new/. Accessed June 26, 2020.
- National Institute for Health Care and Excellence. COVID-19 rapid evidence summary: Remdesivir for treating hospitalised patients with suspected or confirmed COVID-19. Evidence summary [ES27]. Published June 5, 2020. www.nice.org.uk/advice/es27/chapter/Key-messages. Accessed June 26, 2020.
- National Institute for Health Care and Excellence. COVID-19 rapid evidence summary: angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) in people with or at risk of COVID-19. Evidence summary [ES24]. Published May 21, 2020. www.nice.org.uk/advice/es24/chapter/Key-messages. Accessed June 26, 2020.
- National Institute for Health Care and Excellence. COVID-19 rapid evidence summary: Long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) for people with or at risk of COVID-19. Evidence summary [ES25]. Published May 21, 2020. www.nice.org.uk/advice/es25/chapter/Key-messages. Accessed June 26, 2020.
- Hernandez AV, Roman YM, Pasupuleti V, et al. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 2020 May 27. doi: 10.7326/M20-2496. Online ahead of print.
References
- National Institute of Health. COVID-19 treatment guidelines: what’s new in the guidelines? Updated June 25, 2020. www.covid19treatmentguidelines.nih.gov/whats-new/. Accessed June 26, 2020.
- National Institute for Health Care and Excellence. COVID-19 rapid evidence summary: Remdesivir for treating hospitalised patients with suspected or confirmed COVID-19. Evidence summary [ES27]. Published June 5, 2020. www.nice.org.uk/advice/es27/chapter/Key-messages. Accessed June 26, 2020.
- National Institute for Health Care and Excellence. COVID-19 rapid evidence summary: angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) in people with or at risk of COVID-19. Evidence summary [ES24]. Published May 21, 2020. www.nice.org.uk/advice/es24/chapter/Key-messages. Accessed June 26, 2020.
- National Institute for Health Care and Excellence. COVID-19 rapid evidence summary: Long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) for people with or at risk of COVID-19. Evidence summary [ES25]. Published May 21, 2020. www.nice.org.uk/advice/es25/chapter/Key-messages. Accessed June 26, 2020.
- Hernandez AV, Roman YM, Pasupuleti V, et al. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 2020 May 27. doi: 10.7326/M20-2496. Online ahead of print.
3 new latent TB preventive regimens dramatically cut Tx time
References
- Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tubercular infection: recommendations from the National Tuberculosis Controllers Association and the CDC, 2020. MMWR Recomm Rep. 2020;69:1-11.
- USPSTF. Latent tuberculosis screening [final recommendation statement]. Published September 6, 2016. www.uspreventiveservicestaskforce.org/uspstf/recommendation/latent-tuberculosis-infection-screening. Accessed May 19, 2020.
- CDC. Tuberculosis (TB): data and statistics. Updated September 6, 2019. www.cdc.gov/tb/statistics/default.htm. Accessed May 19, 2020.
References
- Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tubercular infection: recommendations from the National Tuberculosis Controllers Association and the CDC, 2020. MMWR Recomm Rep. 2020;69:1-11.
- USPSTF. Latent tuberculosis screening [final recommendation statement]. Published September 6, 2016. www.uspreventiveservicestaskforce.org/uspstf/recommendation/latent-tuberculosis-infection-screening. Accessed May 19, 2020.
- CDC. Tuberculosis (TB): data and statistics. Updated September 6, 2019. www.cdc.gov/tb/statistics/default.htm. Accessed May 19, 2020.
References
- Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tubercular infection: recommendations from the National Tuberculosis Controllers Association and the CDC, 2020. MMWR Recomm Rep. 2020;69:1-11.
- USPSTF. Latent tuberculosis screening [final recommendation statement]. Published September 6, 2016. www.uspreventiveservicestaskforce.org/uspstf/recommendation/latent-tuberculosis-infection-screening. Accessed May 19, 2020.
- CDC. Tuberculosis (TB): data and statistics. Updated September 6, 2019. www.cdc.gov/tb/statistics/default.htm. Accessed May 19, 2020.
USPSTF round-up
In 2019, the US Preventive Services Task Force published 19 recommendation statements on 11 topics. Two of the topics are new; 9 are topics the Task Force had previously reviewed and has updated (TABLE 1). Three of these topics have been covered in Practice Alert podcasts (mdedge.com/familymedicine) and will not be discussed here: risk assessment, genetic counseling, and genetic testing for breast cancer susceptibility gene mutations (October 2019); medications to reduce the risk of breast cancer (December 2019); and preexposure prophylaxis to prevent HIV infections (January 2020).
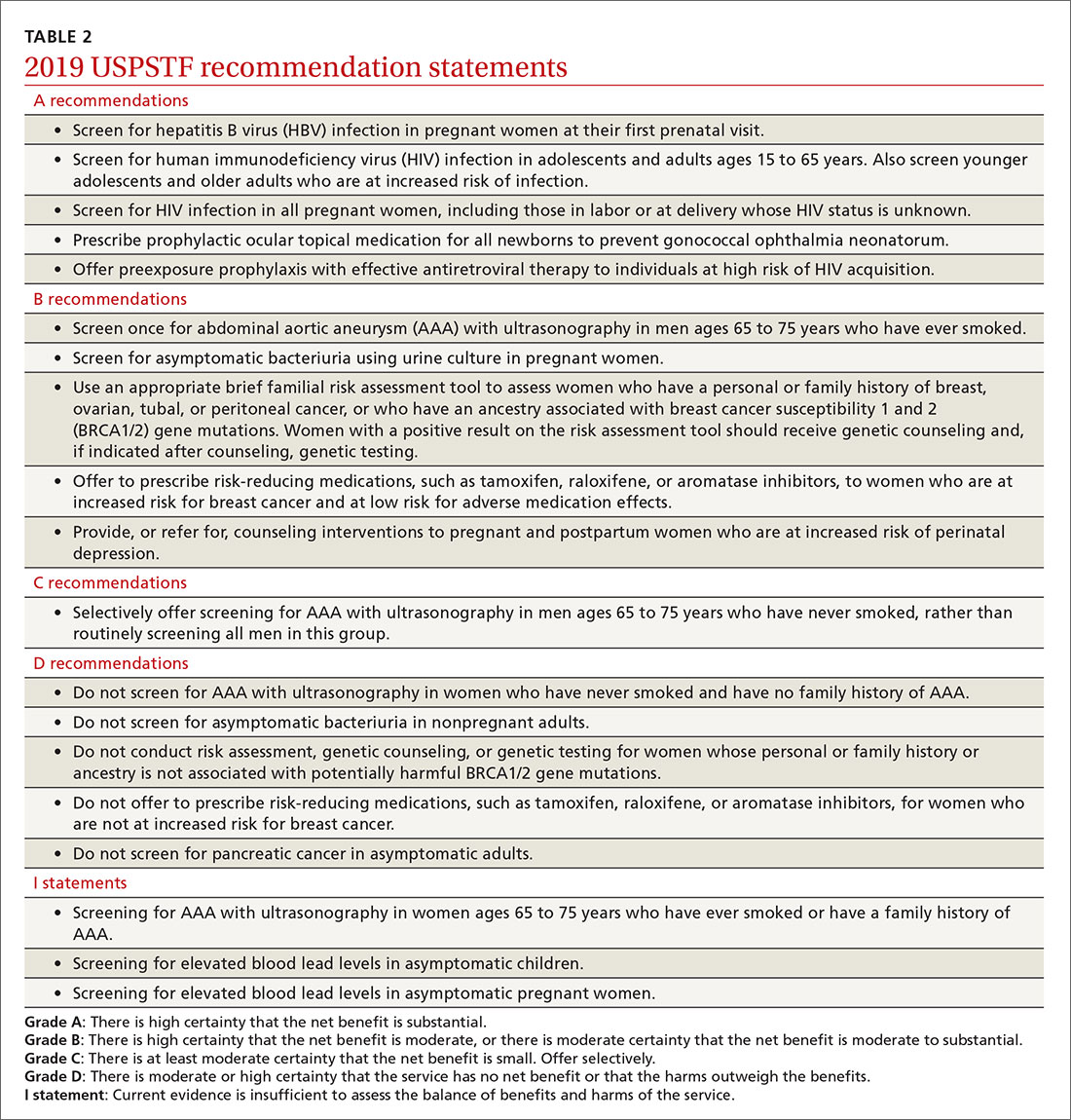
Of the 19 recommendation statements made in 2019 (TABLE 2), 5 were rated “A” and 5 were “B,” meaning the evidence shows that benefits outweigh harms and these interventions should be offered in primary care practice. There were 5 “D” recommendations for interventions that should not be offered because they are either ineffective or harms exceed benefits. There were 3 “I” statements on interventions having insufficient evidence on benefits or harms to warrant a recommendation. Only 1 recommendation was rated “C” (selectively offer based on individual factors); this assessment is the hardest one to interpret and implement. Keep in mind that all “A” and “B” recommendations must be covered by commercial health plans with no out-of-pocket cost to the patient (ie, no co-pay or deductible).
New recommendation on preventing perinatal depression
One of 2 new topics reviewed in 2019 was the prevention of perinatal depression. (As noted, the other on preexposure prophylaxis to prevent HIV infection has already been covered in a Practice Alert podcast.) The Task Force found that the prevalence of depression is estimated at 8.9% among pregnant women and 37% at any point in the first year postpartum.1
Depression during pregnancy and the postpartum period is associated with adverse effects on the mother and infant, including higher rates of suicide and suicidal ideation and thoughts of harming the infant.1 Women with perinatal depression are also more likely to exhibit significantly lower levels of positive maternal behaviors, such as praising and playing with their child,2 and higher rates of negative maternal behaviors.2 Perinatal depression is also associated with increased rates of preterm birth and low birth weight.3
Mothers with postpartum depression have higher rates of early termination of breast feeding and lower adherence for recommended child preventive services including vaccination.1 Children of mothers with perinatal depression develop more behavior problems, have lower cognitive functioning, and have an increased risk of psychiatric disorders than do children of mothers without this condition.4,5
A number of risk factors are associated with perinatal depression, but no screening tool was found to have enough predictive value to be recommended. In deciding who should receive an offer or referral for counseling, the Task Force recommends as a practical approach providing “counseling interventions to women with 1 or more of the following: a history of depression, current depressive symptoms (that do not reach a diagnostic threshold), certain socioeconomic risk factors such as low income or adolescent or single parenthood, recent intimate partner violence, or mental health-related factors such as elevated anxiety symptoms or a history of significant negative life events.”1
There is no conclusive evidence to guide timing of counseling interventions, but most studies reviewed started them in the second trimester. These studies included cognitive behavioral therapy and interpersonal therapy and involved counseling sessions that ranged from 4 to 20 sessions and lasted for 4 to 70 weeks. They involved group and individual sessions, mostly in-person visits, and were provided by a variety of health professionals.6
Continue to: The studies reviewed showed...
The studies reviewed showed that counseling interventions reduced the likelihood of developing depression symptoms by 39%, with a number needed to treat of 13.5.6 Studies that looked at pregnancy and maternal and infant clinical outcomes were mixed but usually found little to no difference with counseling.6 Even so, the Task Force felt that a reduction in depression itself was enough to warrant a “B” recommendation.
Screening for abdominal aortic aneurisms
Ultrasound is underused in screening for abdominal aortic aneurisms (AAA) and preventing death from their rupture. (See “Whom should you screen for abdominal aortic aneurysm?”) The prevalence of AAA is the United States is unknown; in other western countries it varies from 1.2% to 3.3% in men and is declining due to decreased rates of smoking, the primary risk factor.
The risk of AAA rupture is related to the size of the aneurism, and surgical repair (either endovascular or open repair) is usually reserved for lesions > 5.5 cm in diameter or for smaller ones that are rapidly increasing in size. The standard of care for most aneurysms < 5.5 cm is to periodically monitor growth using ultrasound.
The 2019 recommendations on AAA screening are essentially the same as those made in 2004; evaluation of new evidence supported the previous recommendations. The Task Force recommends one-time screening for men ages 65 to 75 years who have ever smoked (B recommendation). Selective screening is recommended for men in this age group who have never smoked, based mainly on personal factors such as a family history of AAA, the presence of other arterial aneurisms, and the number of risk factors for cardiovascular disease (C recommendation).
The Task Force recommends against screening women ages 65 to 75 years with no history of smoking or family history of AAA, while the evidence was felt to be insufficient to make a recommendation for women in this age range who have either risk factor. This is problematic for family physicians since women with these risk factors are at increased risk of AAA compared with women without risk factors.8 And aneurisms in women appear to rupture more frequently at smaller sizes, although at a later age than in men.8 Operative mortality is also higher in women than in men8 and there is no direct evidence that screening improves outcomes for women.
Continue to: Screening for asymptomatic bacteriuria
Screening for asymptomatic bacteriuria
The Task Force re-examined and reconfirmed its previous recommendations on screening for asymptomatic bacteriuria in adults. It recommends in favor of it for pregnant women, using a urine culture to screen, and against it for all other adults. There is good evidence that treating screen-detected asymptomatic bacteriuria in pregnant women reduces the incidence of pyelonephritis in pregnancy.
The Task Force made this a “B” recommendation based on a lower prevalence of pyelonephritis found in more recent studies, making the overall magnitude of benefits moderate. There is also good evidence that treating asymptomatic bacteriuria in nonpregnant adults offers no benefits.9 The Task Force has re-examined this topic 5 times since 1996 with essentially the same results.
Screening for elevated lead levels in children and pregnant women
In 2019 the Task Force changed its 2006 recommendation on screening for elevated lead levels. The earlier recommendation advised against screening both children ages 1 to 5 years and pregnant women at average risk for elevated blood lead levels. In 2006 the Task Force also felt that evidence was insufficient to make a recommendation regarding children ages 1 to 5 years at elevated risk.
The Task Force now believes the evidence is insufficient to make a recommendation for all children ages 1 to 5 years and for pregnant women, thus moving from a “D” to an “I” recommendation for children and pregnant women with average risk. Even though there is little evidence to support screening for elevated lead levels in children ages 1 to 5 years and in pregnant women, the Task Force apparently did not feel comfortable recommending against testing, given that the cutoff for elevated blood lead levels has been lowered from 10 to 5 mcg/dL and that other sources of lead may now be more prevalent than in 2006.10
Remember that the Medicaid Early and Periodic Screening, Diagnostic, and Treatment program requires that all children receive a blood lead test twice, at ages 12 and 24 months, and that previously unscreened children ages 36 to 72 months must be tested once.
Continue to: Additional updates with no recommendation changes
Additional updates with no recommendation changes
Four other topics were re-examined by the Task Force in 2019, resulting in no significant changes to recommendations (TABLE 2):
- Screen for hepatitis B infection in pregnant women at the first prenatal visit (A recommendation; updated from 2009).
- Screen for HIV infection in adolescents and adults ages 15 to 65 years, and in those younger and older who are at high risk, and during pregnancy (A recommendation; updated from 2013).
- Provide topical medication for all newborns to prevent gonococcal ophthalmia neonatorum (A recommendation; first recommendation in 1996, updated in 2005 and 2011).
- Avoid screening for pancreatic cancer in asymptomatic adults (D recommendation; updated from 2004).
Affirmation of USPSTF’s value
In only 1 out of 9 reassessments of past topics did the Task Force modify its previous recommendations in any significant way. This demonstrates that recommendations will usually stand the test of time if they are made using robust, evidence-based methods (that consider both benefits and harms) and they are not made when evidence is insufficient. That only 2 new topics could be addressed in 2019 may reflect a need for more resources for the Task Force.
1. USPSTF. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendation statement. 2019;321:580-587.
2. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592.
3. Szegda K, Markenson G, Bertone-Johnson ER, et al. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. J Matern Fetal Neonatal Med. 2014;27:960-967.
4. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12:12-20.
5. Santos IS, Matijasevich A, Barros AJ, et al. Antenatal and postnatal maternal mood symptoms and psychiatric disorders in pre-school children from the 2004 Pelotas Birth Cohort. J Affect Disord. 2014;164:112-117.
6. O’Connor E, Senger CA, Henniger ML, et al. Interventions to prevent perinatal depression. Evidence report and systematic review for the US preventive services task force. JAMA. 2019;321:588-601.
7. USPSTF. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. 2019;322:2211-2218.
8. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238.
9. USPSTF. Owens DK, Davidson KW, Krist AH, et al. Screening for asymptomatic bacteriuria in adults: US Preventive Services Task Force recommendation statement. 2019;322:1188-1194.
10. USPSTF. Screening for elevated blood lead levels in children and pregnant women: US Preventive Services Task Force recommendation statement. 2019;321:1502-1509.
In 2019, the US Preventive Services Task Force published 19 recommendation statements on 11 topics. Two of the topics are new; 9 are topics the Task Force had previously reviewed and has updated (TABLE 1). Three of these topics have been covered in Practice Alert podcasts (mdedge.com/familymedicine) and will not be discussed here: risk assessment, genetic counseling, and genetic testing for breast cancer susceptibility gene mutations (October 2019); medications to reduce the risk of breast cancer (December 2019); and preexposure prophylaxis to prevent HIV infections (January 2020).
Of the 19 recommendation statements made in 2019 (TABLE 2), 5 were rated “A” and 5 were “B,” meaning the evidence shows that benefits outweigh harms and these interventions should be offered in primary care practice. There were 5 “D” recommendations for interventions that should not be offered because they are either ineffective or harms exceed benefits. There were 3 “I” statements on interventions having insufficient evidence on benefits or harms to warrant a recommendation. Only 1 recommendation was rated “C” (selectively offer based on individual factors); this assessment is the hardest one to interpret and implement. Keep in mind that all “A” and “B” recommendations must be covered by commercial health plans with no out-of-pocket cost to the patient (ie, no co-pay or deductible).
New recommendation on preventing perinatal depression
One of 2 new topics reviewed in 2019 was the prevention of perinatal depression. (As noted, the other on preexposure prophylaxis to prevent HIV infection has already been covered in a Practice Alert podcast.) The Task Force found that the prevalence of depression is estimated at 8.9% among pregnant women and 37% at any point in the first year postpartum.1
Depression during pregnancy and the postpartum period is associated with adverse effects on the mother and infant, including higher rates of suicide and suicidal ideation and thoughts of harming the infant.1 Women with perinatal depression are also more likely to exhibit significantly lower levels of positive maternal behaviors, such as praising and playing with their child,2 and higher rates of negative maternal behaviors.2 Perinatal depression is also associated with increased rates of preterm birth and low birth weight.3
Mothers with postpartum depression have higher rates of early termination of breast feeding and lower adherence for recommended child preventive services including vaccination.1 Children of mothers with perinatal depression develop more behavior problems, have lower cognitive functioning, and have an increased risk of psychiatric disorders than do children of mothers without this condition.4,5
A number of risk factors are associated with perinatal depression, but no screening tool was found to have enough predictive value to be recommended. In deciding who should receive an offer or referral for counseling, the Task Force recommends as a practical approach providing “counseling interventions to women with 1 or more of the following: a history of depression, current depressive symptoms (that do not reach a diagnostic threshold), certain socioeconomic risk factors such as low income or adolescent or single parenthood, recent intimate partner violence, or mental health-related factors such as elevated anxiety symptoms or a history of significant negative life events.”1
There is no conclusive evidence to guide timing of counseling interventions, but most studies reviewed started them in the second trimester. These studies included cognitive behavioral therapy and interpersonal therapy and involved counseling sessions that ranged from 4 to 20 sessions and lasted for 4 to 70 weeks. They involved group and individual sessions, mostly in-person visits, and were provided by a variety of health professionals.6
Continue to: The studies reviewed showed...
The studies reviewed showed that counseling interventions reduced the likelihood of developing depression symptoms by 39%, with a number needed to treat of 13.5.6 Studies that looked at pregnancy and maternal and infant clinical outcomes were mixed but usually found little to no difference with counseling.6 Even so, the Task Force felt that a reduction in depression itself was enough to warrant a “B” recommendation.
Screening for abdominal aortic aneurisms
Ultrasound is underused in screening for abdominal aortic aneurisms (AAA) and preventing death from their rupture. (See “Whom should you screen for abdominal aortic aneurysm?”) The prevalence of AAA is the United States is unknown; in other western countries it varies from 1.2% to 3.3% in men and is declining due to decreased rates of smoking, the primary risk factor.
The risk of AAA rupture is related to the size of the aneurism, and surgical repair (either endovascular or open repair) is usually reserved for lesions > 5.5 cm in diameter or for smaller ones that are rapidly increasing in size. The standard of care for most aneurysms < 5.5 cm is to periodically monitor growth using ultrasound.
The 2019 recommendations on AAA screening are essentially the same as those made in 2004; evaluation of new evidence supported the previous recommendations. The Task Force recommends one-time screening for men ages 65 to 75 years who have ever smoked (B recommendation). Selective screening is recommended for men in this age group who have never smoked, based mainly on personal factors such as a family history of AAA, the presence of other arterial aneurisms, and the number of risk factors for cardiovascular disease (C recommendation).
The Task Force recommends against screening women ages 65 to 75 years with no history of smoking or family history of AAA, while the evidence was felt to be insufficient to make a recommendation for women in this age range who have either risk factor. This is problematic for family physicians since women with these risk factors are at increased risk of AAA compared with women without risk factors.8 And aneurisms in women appear to rupture more frequently at smaller sizes, although at a later age than in men.8 Operative mortality is also higher in women than in men8 and there is no direct evidence that screening improves outcomes for women.
Continue to: Screening for asymptomatic bacteriuria
Screening for asymptomatic bacteriuria
The Task Force re-examined and reconfirmed its previous recommendations on screening for asymptomatic bacteriuria in adults. It recommends in favor of it for pregnant women, using a urine culture to screen, and against it for all other adults. There is good evidence that treating screen-detected asymptomatic bacteriuria in pregnant women reduces the incidence of pyelonephritis in pregnancy.
The Task Force made this a “B” recommendation based on a lower prevalence of pyelonephritis found in more recent studies, making the overall magnitude of benefits moderate. There is also good evidence that treating asymptomatic bacteriuria in nonpregnant adults offers no benefits.9 The Task Force has re-examined this topic 5 times since 1996 with essentially the same results.
Screening for elevated lead levels in children and pregnant women
In 2019 the Task Force changed its 2006 recommendation on screening for elevated lead levels. The earlier recommendation advised against screening both children ages 1 to 5 years and pregnant women at average risk for elevated blood lead levels. In 2006 the Task Force also felt that evidence was insufficient to make a recommendation regarding children ages 1 to 5 years at elevated risk.
The Task Force now believes the evidence is insufficient to make a recommendation for all children ages 1 to 5 years and for pregnant women, thus moving from a “D” to an “I” recommendation for children and pregnant women with average risk. Even though there is little evidence to support screening for elevated lead levels in children ages 1 to 5 years and in pregnant women, the Task Force apparently did not feel comfortable recommending against testing, given that the cutoff for elevated blood lead levels has been lowered from 10 to 5 mcg/dL and that other sources of lead may now be more prevalent than in 2006.10
Remember that the Medicaid Early and Periodic Screening, Diagnostic, and Treatment program requires that all children receive a blood lead test twice, at ages 12 and 24 months, and that previously unscreened children ages 36 to 72 months must be tested once.
Continue to: Additional updates with no recommendation changes
Additional updates with no recommendation changes
Four other topics were re-examined by the Task Force in 2019, resulting in no significant changes to recommendations (TABLE 2):
- Screen for hepatitis B infection in pregnant women at the first prenatal visit (A recommendation; updated from 2009).
- Screen for HIV infection in adolescents and adults ages 15 to 65 years, and in those younger and older who are at high risk, and during pregnancy (A recommendation; updated from 2013).
- Provide topical medication for all newborns to prevent gonococcal ophthalmia neonatorum (A recommendation; first recommendation in 1996, updated in 2005 and 2011).
- Avoid screening for pancreatic cancer in asymptomatic adults (D recommendation; updated from 2004).
Affirmation of USPSTF’s value
In only 1 out of 9 reassessments of past topics did the Task Force modify its previous recommendations in any significant way. This demonstrates that recommendations will usually stand the test of time if they are made using robust, evidence-based methods (that consider both benefits and harms) and they are not made when evidence is insufficient. That only 2 new topics could be addressed in 2019 may reflect a need for more resources for the Task Force.
In 2019, the US Preventive Services Task Force published 19 recommendation statements on 11 topics. Two of the topics are new; 9 are topics the Task Force had previously reviewed and has updated (TABLE 1). Three of these topics have been covered in Practice Alert podcasts (mdedge.com/familymedicine) and will not be discussed here: risk assessment, genetic counseling, and genetic testing for breast cancer susceptibility gene mutations (October 2019); medications to reduce the risk of breast cancer (December 2019); and preexposure prophylaxis to prevent HIV infections (January 2020).
Of the 19 recommendation statements made in 2019 (TABLE 2), 5 were rated “A” and 5 were “B,” meaning the evidence shows that benefits outweigh harms and these interventions should be offered in primary care practice. There were 5 “D” recommendations for interventions that should not be offered because they are either ineffective or harms exceed benefits. There were 3 “I” statements on interventions having insufficient evidence on benefits or harms to warrant a recommendation. Only 1 recommendation was rated “C” (selectively offer based on individual factors); this assessment is the hardest one to interpret and implement. Keep in mind that all “A” and “B” recommendations must be covered by commercial health plans with no out-of-pocket cost to the patient (ie, no co-pay or deductible).
New recommendation on preventing perinatal depression
One of 2 new topics reviewed in 2019 was the prevention of perinatal depression. (As noted, the other on preexposure prophylaxis to prevent HIV infection has already been covered in a Practice Alert podcast.) The Task Force found that the prevalence of depression is estimated at 8.9% among pregnant women and 37% at any point in the first year postpartum.1
Depression during pregnancy and the postpartum period is associated with adverse effects on the mother and infant, including higher rates of suicide and suicidal ideation and thoughts of harming the infant.1 Women with perinatal depression are also more likely to exhibit significantly lower levels of positive maternal behaviors, such as praising and playing with their child,2 and higher rates of negative maternal behaviors.2 Perinatal depression is also associated with increased rates of preterm birth and low birth weight.3
Mothers with postpartum depression have higher rates of early termination of breast feeding and lower adherence for recommended child preventive services including vaccination.1 Children of mothers with perinatal depression develop more behavior problems, have lower cognitive functioning, and have an increased risk of psychiatric disorders than do children of mothers without this condition.4,5
A number of risk factors are associated with perinatal depression, but no screening tool was found to have enough predictive value to be recommended. In deciding who should receive an offer or referral for counseling, the Task Force recommends as a practical approach providing “counseling interventions to women with 1 or more of the following: a history of depression, current depressive symptoms (that do not reach a diagnostic threshold), certain socioeconomic risk factors such as low income or adolescent or single parenthood, recent intimate partner violence, or mental health-related factors such as elevated anxiety symptoms or a history of significant negative life events.”1
There is no conclusive evidence to guide timing of counseling interventions, but most studies reviewed started them in the second trimester. These studies included cognitive behavioral therapy and interpersonal therapy and involved counseling sessions that ranged from 4 to 20 sessions and lasted for 4 to 70 weeks. They involved group and individual sessions, mostly in-person visits, and were provided by a variety of health professionals.6
Continue to: The studies reviewed showed...
The studies reviewed showed that counseling interventions reduced the likelihood of developing depression symptoms by 39%, with a number needed to treat of 13.5.6 Studies that looked at pregnancy and maternal and infant clinical outcomes were mixed but usually found little to no difference with counseling.6 Even so, the Task Force felt that a reduction in depression itself was enough to warrant a “B” recommendation.
Screening for abdominal aortic aneurisms
Ultrasound is underused in screening for abdominal aortic aneurisms (AAA) and preventing death from their rupture. (See “Whom should you screen for abdominal aortic aneurysm?”) The prevalence of AAA is the United States is unknown; in other western countries it varies from 1.2% to 3.3% in men and is declining due to decreased rates of smoking, the primary risk factor.
The risk of AAA rupture is related to the size of the aneurism, and surgical repair (either endovascular or open repair) is usually reserved for lesions > 5.5 cm in diameter or for smaller ones that are rapidly increasing in size. The standard of care for most aneurysms < 5.5 cm is to periodically monitor growth using ultrasound.
The 2019 recommendations on AAA screening are essentially the same as those made in 2004; evaluation of new evidence supported the previous recommendations. The Task Force recommends one-time screening for men ages 65 to 75 years who have ever smoked (B recommendation). Selective screening is recommended for men in this age group who have never smoked, based mainly on personal factors such as a family history of AAA, the presence of other arterial aneurisms, and the number of risk factors for cardiovascular disease (C recommendation).
The Task Force recommends against screening women ages 65 to 75 years with no history of smoking or family history of AAA, while the evidence was felt to be insufficient to make a recommendation for women in this age range who have either risk factor. This is problematic for family physicians since women with these risk factors are at increased risk of AAA compared with women without risk factors.8 And aneurisms in women appear to rupture more frequently at smaller sizes, although at a later age than in men.8 Operative mortality is also higher in women than in men8 and there is no direct evidence that screening improves outcomes for women.
Continue to: Screening for asymptomatic bacteriuria
Screening for asymptomatic bacteriuria
The Task Force re-examined and reconfirmed its previous recommendations on screening for asymptomatic bacteriuria in adults. It recommends in favor of it for pregnant women, using a urine culture to screen, and against it for all other adults. There is good evidence that treating screen-detected asymptomatic bacteriuria in pregnant women reduces the incidence of pyelonephritis in pregnancy.
The Task Force made this a “B” recommendation based on a lower prevalence of pyelonephritis found in more recent studies, making the overall magnitude of benefits moderate. There is also good evidence that treating asymptomatic bacteriuria in nonpregnant adults offers no benefits.9 The Task Force has re-examined this topic 5 times since 1996 with essentially the same results.
Screening for elevated lead levels in children and pregnant women
In 2019 the Task Force changed its 2006 recommendation on screening for elevated lead levels. The earlier recommendation advised against screening both children ages 1 to 5 years and pregnant women at average risk for elevated blood lead levels. In 2006 the Task Force also felt that evidence was insufficient to make a recommendation regarding children ages 1 to 5 years at elevated risk.
The Task Force now believes the evidence is insufficient to make a recommendation for all children ages 1 to 5 years and for pregnant women, thus moving from a “D” to an “I” recommendation for children and pregnant women with average risk. Even though there is little evidence to support screening for elevated lead levels in children ages 1 to 5 years and in pregnant women, the Task Force apparently did not feel comfortable recommending against testing, given that the cutoff for elevated blood lead levels has been lowered from 10 to 5 mcg/dL and that other sources of lead may now be more prevalent than in 2006.10
Remember that the Medicaid Early and Periodic Screening, Diagnostic, and Treatment program requires that all children receive a blood lead test twice, at ages 12 and 24 months, and that previously unscreened children ages 36 to 72 months must be tested once.
Continue to: Additional updates with no recommendation changes
Additional updates with no recommendation changes
Four other topics were re-examined by the Task Force in 2019, resulting in no significant changes to recommendations (TABLE 2):
- Screen for hepatitis B infection in pregnant women at the first prenatal visit (A recommendation; updated from 2009).
- Screen for HIV infection in adolescents and adults ages 15 to 65 years, and in those younger and older who are at high risk, and during pregnancy (A recommendation; updated from 2013).
- Provide topical medication for all newborns to prevent gonococcal ophthalmia neonatorum (A recommendation; first recommendation in 1996, updated in 2005 and 2011).
- Avoid screening for pancreatic cancer in asymptomatic adults (D recommendation; updated from 2004).
Affirmation of USPSTF’s value
In only 1 out of 9 reassessments of past topics did the Task Force modify its previous recommendations in any significant way. This demonstrates that recommendations will usually stand the test of time if they are made using robust, evidence-based methods (that consider both benefits and harms) and they are not made when evidence is insufficient. That only 2 new topics could be addressed in 2019 may reflect a need for more resources for the Task Force.
1. USPSTF. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendation statement. 2019;321:580-587.
2. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592.
3. Szegda K, Markenson G, Bertone-Johnson ER, et al. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. J Matern Fetal Neonatal Med. 2014;27:960-967.
4. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12:12-20.
5. Santos IS, Matijasevich A, Barros AJ, et al. Antenatal and postnatal maternal mood symptoms and psychiatric disorders in pre-school children from the 2004 Pelotas Birth Cohort. J Affect Disord. 2014;164:112-117.
6. O’Connor E, Senger CA, Henniger ML, et al. Interventions to prevent perinatal depression. Evidence report and systematic review for the US preventive services task force. JAMA. 2019;321:588-601.
7. USPSTF. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. 2019;322:2211-2218.
8. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238.
9. USPSTF. Owens DK, Davidson KW, Krist AH, et al. Screening for asymptomatic bacteriuria in adults: US Preventive Services Task Force recommendation statement. 2019;322:1188-1194.
10. USPSTF. Screening for elevated blood lead levels in children and pregnant women: US Preventive Services Task Force recommendation statement. 2019;321:1502-1509.
1. USPSTF. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendation statement. 2019;321:580-587.
2. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592.
3. Szegda K, Markenson G, Bertone-Johnson ER, et al. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. J Matern Fetal Neonatal Med. 2014;27:960-967.
4. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12:12-20.
5. Santos IS, Matijasevich A, Barros AJ, et al. Antenatal and postnatal maternal mood symptoms and psychiatric disorders in pre-school children from the 2004 Pelotas Birth Cohort. J Affect Disord. 2014;164:112-117.
6. O’Connor E, Senger CA, Henniger ML, et al. Interventions to prevent perinatal depression. Evidence report and systematic review for the US preventive services task force. JAMA. 2019;321:588-601.
7. USPSTF. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. 2019;322:2211-2218.
8. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238.
9. USPSTF. Owens DK, Davidson KW, Krist AH, et al. Screening for asymptomatic bacteriuria in adults: US Preventive Services Task Force recommendation statement. 2019;322:1188-1194.
10. USPSTF. Screening for elevated blood lead levels in children and pregnant women: US Preventive Services Task Force recommendation statement. 2019;321:1502-1509.