User login
What should I address at follow-up of patients who survive critical illness?
Patients who survive critical illness such as shock or respiratory failure warranting admission to an intensive care unit (ICU) often develop a constellation of chronic symptoms including cognitive decline, psychiatric disturbances, and physical weakness. These changes can prevent patients from returning to their former level of function and often necessitate significant support for patients and their caregivers.1
With growing awareness of the unique needs of ICU survivors, multidisciplinary PICS clinics have emerged. However, access to these clinics is limited, and most patients discharged from the ICU eventually follow up with their primary care provider. Primary care physicians who recognize PICS, understand its prognosis and its burden on caregivers, and are aware of tools that have shown promise in its management will be well prepared to address the needs of these patients.
COGNITIVE DECLINE
Several studies have shown that survivors of critical illness suffer from long-term impairment of multiple domains of cognition, including executive function. In one study, 40% of ICU survivors had global cognition scores at 1 year after discharge that were worse than those seen in moderate traumatic brain injury, and over 25% had scores similar to those seen in Alzheimer dementia.2 Age had poor correlation with the incidence of long-term cognitive impairment. Cognitive impairment may not be recognized in younger patients without a high index of suspicion and directed cognitive screening. Well-known cognitive impairment screening tests such as the Montreal Cognitive Assessment may help in the evaluation of PICS.
No treatment has been shown to improve long-term cognitive impairment from any cause. The most important intervention is to recognize it and to consider how impaired executive function may interfere with other aspects of treatment, such as participation in physical therapy and adherence to medication regimens.
Evidence is also emerging that patients are often inappropriately discharged on psychoactive medications (including atypical antipsychotic drugs and sedatives) that were started in the inpatient setting.7 These medications increase the risk of accidents, arrhythmia, and infection, as well as add to the overall cost of postdischarge care, and they do not improve the prolonged confusion and cognitive impairment associated with PICS.8 Psychoactive medications should be discontinued once delirium-associated behavior has resolved, as recommended in the American Geriatrics Society guideline on postoperative delirium.9 Further, patients and caregivers should be counseled so that they have reasonable expectations regarding the timing of cognitive recovery, which may be prolonged and incomplete.
PHYSICAL WEAKNESS
Prolonged physical weakness may affect up to one-third of patients who survive critical illness, and it may persist for years, severely compromising quality of life.10 In addition to deconditioning due to bedrest and illness, ICU patients often develop critical illness myopathy and critical illness polyneuropathy.
Although the mechanisms and risk factors for injury to muscles and peripheral nerves are not completely understood, the severity has been well described and ranges from proximal muscle weakness to complete quadriparesis, with inability to wean from mechanical ventilation. There is also an association with the severity of sepsis and the use of glucocorticoids and paralytics.10
Physical weakness can be readily apparent on routine history and physical examination. Differentiating critical illness myopathy from critical illness polyneuropathy requires invasive testing, including electromyography, but the results may not change management in the outpatient setting, making it unnecessary for most patients.
Physical weakness places a heavy burden on patients and their family and caregivers. As a result, most ICU patients suffer loss of employment and require supportive services on discharge, including home health aides and even institutionalization.
Physical therapy and occupational therapy are effective in reducing weakness and improving physical functioning; starting physical therapy in the outpatient setting may be as effective as early intervention in the ICU.11 Given the high prevalence of respiratory and cardiovascular disease in patients after ICU discharge, referral for pulmonary or cardiovascular rehabilitation is recommended. Because of the possible link between glucocorticoids and critical illness myopathy, these drugs should be decreased or discontinued as soon as possible.
PSYCHIATRIC DISTURBANCES
Mental health impairments in ICU survivors are common, severe, debilitating, and unfortunately, commonly overlooked. A recent study found a 37% incidence of depression and a 40% incidence of anxiety; further, 22% of patients met criteria for posttraumatic stress disorder.12 Patients with critical illness are also more likely to have had untreated mental health illness before hospitalization. Anxiety may present with poor sleep, irritability, and fatigue. Posttraumatic stress disorder may manifest as flashbacks or as a severe cognitive or behavioral response to provocation. All of these may be assessed using standard screening questionnaires, including the Posttraumatic Stress Disorder Checklist, the 2-item Patient Health Questionnaire (PHQ-2) for depression, and the 7-item Generalized Anxiety Disorder Screen (GAD-7).
Many primary care physicians are comfortable treating some of the psychiatric disturbances associated with PICS, such as depression, but may be challenged by the spectrum and complexity of mental illness of ICU survivors. Early referral to a mental health professional ensures optimal psychiatric care and allows more time to focus on the patient’s medical comorbidities.
SOCIAL SUPPORT
The cognitive, physical, and mental health complications coupled with other medical and psychiatric comorbidities result in serious social and financial stress on patients and their families. Long-term follow-up studies show that only half of patients return to work within 1 year of critical illness and that nearly one-fourth require continued assistance with activities of daily living.13 Reassuringly, however, most patients in 1 study had returned to work by 2 years from discharge.3
The immense burden on caregivers, the decrease in income, and increased expenditures in providing care result in increased stress on families. The incidence of depression, anxiety, and posttraumatic stress disorder is similar among patients and their caregivers.11 The frequency of emotional morbidity and the severity of the caregiver burden associated with caring for ICU survivors led to the description of a new entity: post-intensive care syndrome-family, or PICS-F.
Because of these stresses, patients often benefit from referral to a social worker. Patients should also be encouraged to bring their caregivers to physician appointments, and family members should be encouraged to discuss their perspectives in the context of a dedicated appointment. Family members should also be screened and treated for their own medical and mental health challenges. A dedicated ICU survivorship clinic may help facilitate this holistic approach and provide complementary services to the primary care provider.
CRITICAL CARE RECOVERY
As survival rates after critical illness continue to improve and clinicians encounter more patients with PICS, it is essential to appreciate the extent of associated physical, emotional, and financial hardship and to recognize when cognitive impairment may interfere with treatment. Early and accurate recognition of these challenges can help the primary care physician arrange and coordinate recovery services that ICU survivors require. Including family members in follow-up appointments can help overcome challenges in adherence to treatment plans, uncover gaps in social support, and identify signs of caregiver distress.
A thorough physical assessment and a thoughtful reconciliation of medications are critical, as is engaging the assistance of physical and occupational therapists, mental health professionals, and social workers.
Risk factors for the illness that necessitated the ICU stay such as uncontrolled diabetes, chronic obstructive pulmonary disease, and substance abuse, as well as medical sequelae such as chronic respiratory failure and heart failure, must be considered and addressed by the primary care physician, with referral to medical specialists if necessary.
Referral to an ICU survivorship center, if locally available, could help the physician manage the patient’s complex and multidisciplinary physical and neuropsychiatric needs. The Society of Critical Care Medicine maintains a resource for survivors and families at www.myicucare.org/thrive/pages/find-in-person-support-groups.aspx.
- Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40(2):502–509. doi:10.1097/CCM.0b013e318232da75
- Pandharipande PP, Girard TD, Jackson JC, et al; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316. doi:10.1056/NEJMoa1301372
- Herridge MS, Tansey CM, Matte A, et al; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364(14):1293–1304. doi:10.1056/NEJMoa1011802
- Rothenhäusler H-B, Ehrentraut S, Stoll C, Schelling G, Kapfhammer H-P. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry 2001; 23(2):90–96. pmid:11313077
- Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016; 43:23–29. doi:10.1016/j.genhosppsych.2016.08.005
- Jackson JC, Pandharipande PP, Girard TD, et al; Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med 2014; 2(5):369–379. doi:10.1016/S2213-2600(14)70051-7
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc 2013; 61(7):1128–1134. doi:10.1111/jgs.12329
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63(1):142–150. doi:10.1111/jgs.13281
- Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care 2015; 19:274. doi:10.1186/s13054-015-0993-7
- Calvo-Ayala E, Khan BA, Farber MO, Ely EW, Boustani MA. Interventions to improve the physical function of ICU survivors: a systematic review. Chest 2013; 144(5):1469–1480. doi:10.1378/chest.13-0779
- Wang S, Allen D, Kheir YN, Campbell N, Khan B. Aging and post-intensive care syndrome: a critical need for geriatric psychiatry. Am J Geriatr Psychiatry 2018; 26(2):212–221. doi:10.1016/j.jagp.2017.05.016
- Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med 2010; 38(7):1554–1561. doi:10.1097/CCM.0b013e3181e2c8b1
- van Beusekom I, Bakhshi-Raiez F, de Keizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care 2016; 20:16. doi:10.1186/s13054-016-1185-9
Patients who survive critical illness such as shock or respiratory failure warranting admission to an intensive care unit (ICU) often develop a constellation of chronic symptoms including cognitive decline, psychiatric disturbances, and physical weakness. These changes can prevent patients from returning to their former level of function and often necessitate significant support for patients and their caregivers.1
With growing awareness of the unique needs of ICU survivors, multidisciplinary PICS clinics have emerged. However, access to these clinics is limited, and most patients discharged from the ICU eventually follow up with their primary care provider. Primary care physicians who recognize PICS, understand its prognosis and its burden on caregivers, and are aware of tools that have shown promise in its management will be well prepared to address the needs of these patients.
COGNITIVE DECLINE
Several studies have shown that survivors of critical illness suffer from long-term impairment of multiple domains of cognition, including executive function. In one study, 40% of ICU survivors had global cognition scores at 1 year after discharge that were worse than those seen in moderate traumatic brain injury, and over 25% had scores similar to those seen in Alzheimer dementia.2 Age had poor correlation with the incidence of long-term cognitive impairment. Cognitive impairment may not be recognized in younger patients without a high index of suspicion and directed cognitive screening. Well-known cognitive impairment screening tests such as the Montreal Cognitive Assessment may help in the evaluation of PICS.
No treatment has been shown to improve long-term cognitive impairment from any cause. The most important intervention is to recognize it and to consider how impaired executive function may interfere with other aspects of treatment, such as participation in physical therapy and adherence to medication regimens.
Evidence is also emerging that patients are often inappropriately discharged on psychoactive medications (including atypical antipsychotic drugs and sedatives) that were started in the inpatient setting.7 These medications increase the risk of accidents, arrhythmia, and infection, as well as add to the overall cost of postdischarge care, and they do not improve the prolonged confusion and cognitive impairment associated with PICS.8 Psychoactive medications should be discontinued once delirium-associated behavior has resolved, as recommended in the American Geriatrics Society guideline on postoperative delirium.9 Further, patients and caregivers should be counseled so that they have reasonable expectations regarding the timing of cognitive recovery, which may be prolonged and incomplete.
PHYSICAL WEAKNESS
Prolonged physical weakness may affect up to one-third of patients who survive critical illness, and it may persist for years, severely compromising quality of life.10 In addition to deconditioning due to bedrest and illness, ICU patients often develop critical illness myopathy and critical illness polyneuropathy.
Although the mechanisms and risk factors for injury to muscles and peripheral nerves are not completely understood, the severity has been well described and ranges from proximal muscle weakness to complete quadriparesis, with inability to wean from mechanical ventilation. There is also an association with the severity of sepsis and the use of glucocorticoids and paralytics.10
Physical weakness can be readily apparent on routine history and physical examination. Differentiating critical illness myopathy from critical illness polyneuropathy requires invasive testing, including electromyography, but the results may not change management in the outpatient setting, making it unnecessary for most patients.
Physical weakness places a heavy burden on patients and their family and caregivers. As a result, most ICU patients suffer loss of employment and require supportive services on discharge, including home health aides and even institutionalization.
Physical therapy and occupational therapy are effective in reducing weakness and improving physical functioning; starting physical therapy in the outpatient setting may be as effective as early intervention in the ICU.11 Given the high prevalence of respiratory and cardiovascular disease in patients after ICU discharge, referral for pulmonary or cardiovascular rehabilitation is recommended. Because of the possible link between glucocorticoids and critical illness myopathy, these drugs should be decreased or discontinued as soon as possible.
PSYCHIATRIC DISTURBANCES
Mental health impairments in ICU survivors are common, severe, debilitating, and unfortunately, commonly overlooked. A recent study found a 37% incidence of depression and a 40% incidence of anxiety; further, 22% of patients met criteria for posttraumatic stress disorder.12 Patients with critical illness are also more likely to have had untreated mental health illness before hospitalization. Anxiety may present with poor sleep, irritability, and fatigue. Posttraumatic stress disorder may manifest as flashbacks or as a severe cognitive or behavioral response to provocation. All of these may be assessed using standard screening questionnaires, including the Posttraumatic Stress Disorder Checklist, the 2-item Patient Health Questionnaire (PHQ-2) for depression, and the 7-item Generalized Anxiety Disorder Screen (GAD-7).
Many primary care physicians are comfortable treating some of the psychiatric disturbances associated with PICS, such as depression, but may be challenged by the spectrum and complexity of mental illness of ICU survivors. Early referral to a mental health professional ensures optimal psychiatric care and allows more time to focus on the patient’s medical comorbidities.
SOCIAL SUPPORT
The cognitive, physical, and mental health complications coupled with other medical and psychiatric comorbidities result in serious social and financial stress on patients and their families. Long-term follow-up studies show that only half of patients return to work within 1 year of critical illness and that nearly one-fourth require continued assistance with activities of daily living.13 Reassuringly, however, most patients in 1 study had returned to work by 2 years from discharge.3
The immense burden on caregivers, the decrease in income, and increased expenditures in providing care result in increased stress on families. The incidence of depression, anxiety, and posttraumatic stress disorder is similar among patients and their caregivers.11 The frequency of emotional morbidity and the severity of the caregiver burden associated with caring for ICU survivors led to the description of a new entity: post-intensive care syndrome-family, or PICS-F.
Because of these stresses, patients often benefit from referral to a social worker. Patients should also be encouraged to bring their caregivers to physician appointments, and family members should be encouraged to discuss their perspectives in the context of a dedicated appointment. Family members should also be screened and treated for their own medical and mental health challenges. A dedicated ICU survivorship clinic may help facilitate this holistic approach and provide complementary services to the primary care provider.
CRITICAL CARE RECOVERY
As survival rates after critical illness continue to improve and clinicians encounter more patients with PICS, it is essential to appreciate the extent of associated physical, emotional, and financial hardship and to recognize when cognitive impairment may interfere with treatment. Early and accurate recognition of these challenges can help the primary care physician arrange and coordinate recovery services that ICU survivors require. Including family members in follow-up appointments can help overcome challenges in adherence to treatment plans, uncover gaps in social support, and identify signs of caregiver distress.
A thorough physical assessment and a thoughtful reconciliation of medications are critical, as is engaging the assistance of physical and occupational therapists, mental health professionals, and social workers.
Risk factors for the illness that necessitated the ICU stay such as uncontrolled diabetes, chronic obstructive pulmonary disease, and substance abuse, as well as medical sequelae such as chronic respiratory failure and heart failure, must be considered and addressed by the primary care physician, with referral to medical specialists if necessary.
Referral to an ICU survivorship center, if locally available, could help the physician manage the patient’s complex and multidisciplinary physical and neuropsychiatric needs. The Society of Critical Care Medicine maintains a resource for survivors and families at www.myicucare.org/thrive/pages/find-in-person-support-groups.aspx.
Patients who survive critical illness such as shock or respiratory failure warranting admission to an intensive care unit (ICU) often develop a constellation of chronic symptoms including cognitive decline, psychiatric disturbances, and physical weakness. These changes can prevent patients from returning to their former level of function and often necessitate significant support for patients and their caregivers.1
With growing awareness of the unique needs of ICU survivors, multidisciplinary PICS clinics have emerged. However, access to these clinics is limited, and most patients discharged from the ICU eventually follow up with their primary care provider. Primary care physicians who recognize PICS, understand its prognosis and its burden on caregivers, and are aware of tools that have shown promise in its management will be well prepared to address the needs of these patients.
COGNITIVE DECLINE
Several studies have shown that survivors of critical illness suffer from long-term impairment of multiple domains of cognition, including executive function. In one study, 40% of ICU survivors had global cognition scores at 1 year after discharge that were worse than those seen in moderate traumatic brain injury, and over 25% had scores similar to those seen in Alzheimer dementia.2 Age had poor correlation with the incidence of long-term cognitive impairment. Cognitive impairment may not be recognized in younger patients without a high index of suspicion and directed cognitive screening. Well-known cognitive impairment screening tests such as the Montreal Cognitive Assessment may help in the evaluation of PICS.
No treatment has been shown to improve long-term cognitive impairment from any cause. The most important intervention is to recognize it and to consider how impaired executive function may interfere with other aspects of treatment, such as participation in physical therapy and adherence to medication regimens.
Evidence is also emerging that patients are often inappropriately discharged on psychoactive medications (including atypical antipsychotic drugs and sedatives) that were started in the inpatient setting.7 These medications increase the risk of accidents, arrhythmia, and infection, as well as add to the overall cost of postdischarge care, and they do not improve the prolonged confusion and cognitive impairment associated with PICS.8 Psychoactive medications should be discontinued once delirium-associated behavior has resolved, as recommended in the American Geriatrics Society guideline on postoperative delirium.9 Further, patients and caregivers should be counseled so that they have reasonable expectations regarding the timing of cognitive recovery, which may be prolonged and incomplete.
PHYSICAL WEAKNESS
Prolonged physical weakness may affect up to one-third of patients who survive critical illness, and it may persist for years, severely compromising quality of life.10 In addition to deconditioning due to bedrest and illness, ICU patients often develop critical illness myopathy and critical illness polyneuropathy.
Although the mechanisms and risk factors for injury to muscles and peripheral nerves are not completely understood, the severity has been well described and ranges from proximal muscle weakness to complete quadriparesis, with inability to wean from mechanical ventilation. There is also an association with the severity of sepsis and the use of glucocorticoids and paralytics.10
Physical weakness can be readily apparent on routine history and physical examination. Differentiating critical illness myopathy from critical illness polyneuropathy requires invasive testing, including electromyography, but the results may not change management in the outpatient setting, making it unnecessary for most patients.
Physical weakness places a heavy burden on patients and their family and caregivers. As a result, most ICU patients suffer loss of employment and require supportive services on discharge, including home health aides and even institutionalization.
Physical therapy and occupational therapy are effective in reducing weakness and improving physical functioning; starting physical therapy in the outpatient setting may be as effective as early intervention in the ICU.11 Given the high prevalence of respiratory and cardiovascular disease in patients after ICU discharge, referral for pulmonary or cardiovascular rehabilitation is recommended. Because of the possible link between glucocorticoids and critical illness myopathy, these drugs should be decreased or discontinued as soon as possible.
PSYCHIATRIC DISTURBANCES
Mental health impairments in ICU survivors are common, severe, debilitating, and unfortunately, commonly overlooked. A recent study found a 37% incidence of depression and a 40% incidence of anxiety; further, 22% of patients met criteria for posttraumatic stress disorder.12 Patients with critical illness are also more likely to have had untreated mental health illness before hospitalization. Anxiety may present with poor sleep, irritability, and fatigue. Posttraumatic stress disorder may manifest as flashbacks or as a severe cognitive or behavioral response to provocation. All of these may be assessed using standard screening questionnaires, including the Posttraumatic Stress Disorder Checklist, the 2-item Patient Health Questionnaire (PHQ-2) for depression, and the 7-item Generalized Anxiety Disorder Screen (GAD-7).
Many primary care physicians are comfortable treating some of the psychiatric disturbances associated with PICS, such as depression, but may be challenged by the spectrum and complexity of mental illness of ICU survivors. Early referral to a mental health professional ensures optimal psychiatric care and allows more time to focus on the patient’s medical comorbidities.
SOCIAL SUPPORT
The cognitive, physical, and mental health complications coupled with other medical and psychiatric comorbidities result in serious social and financial stress on patients and their families. Long-term follow-up studies show that only half of patients return to work within 1 year of critical illness and that nearly one-fourth require continued assistance with activities of daily living.13 Reassuringly, however, most patients in 1 study had returned to work by 2 years from discharge.3
The immense burden on caregivers, the decrease in income, and increased expenditures in providing care result in increased stress on families. The incidence of depression, anxiety, and posttraumatic stress disorder is similar among patients and their caregivers.11 The frequency of emotional morbidity and the severity of the caregiver burden associated with caring for ICU survivors led to the description of a new entity: post-intensive care syndrome-family, or PICS-F.
Because of these stresses, patients often benefit from referral to a social worker. Patients should also be encouraged to bring their caregivers to physician appointments, and family members should be encouraged to discuss their perspectives in the context of a dedicated appointment. Family members should also be screened and treated for their own medical and mental health challenges. A dedicated ICU survivorship clinic may help facilitate this holistic approach and provide complementary services to the primary care provider.
CRITICAL CARE RECOVERY
As survival rates after critical illness continue to improve and clinicians encounter more patients with PICS, it is essential to appreciate the extent of associated physical, emotional, and financial hardship and to recognize when cognitive impairment may interfere with treatment. Early and accurate recognition of these challenges can help the primary care physician arrange and coordinate recovery services that ICU survivors require. Including family members in follow-up appointments can help overcome challenges in adherence to treatment plans, uncover gaps in social support, and identify signs of caregiver distress.
A thorough physical assessment and a thoughtful reconciliation of medications are critical, as is engaging the assistance of physical and occupational therapists, mental health professionals, and social workers.
Risk factors for the illness that necessitated the ICU stay such as uncontrolled diabetes, chronic obstructive pulmonary disease, and substance abuse, as well as medical sequelae such as chronic respiratory failure and heart failure, must be considered and addressed by the primary care physician, with referral to medical specialists if necessary.
Referral to an ICU survivorship center, if locally available, could help the physician manage the patient’s complex and multidisciplinary physical and neuropsychiatric needs. The Society of Critical Care Medicine maintains a resource for survivors and families at www.myicucare.org/thrive/pages/find-in-person-support-groups.aspx.
- Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40(2):502–509. doi:10.1097/CCM.0b013e318232da75
- Pandharipande PP, Girard TD, Jackson JC, et al; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316. doi:10.1056/NEJMoa1301372
- Herridge MS, Tansey CM, Matte A, et al; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364(14):1293–1304. doi:10.1056/NEJMoa1011802
- Rothenhäusler H-B, Ehrentraut S, Stoll C, Schelling G, Kapfhammer H-P. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry 2001; 23(2):90–96. pmid:11313077
- Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016; 43:23–29. doi:10.1016/j.genhosppsych.2016.08.005
- Jackson JC, Pandharipande PP, Girard TD, et al; Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med 2014; 2(5):369–379. doi:10.1016/S2213-2600(14)70051-7
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc 2013; 61(7):1128–1134. doi:10.1111/jgs.12329
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63(1):142–150. doi:10.1111/jgs.13281
- Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care 2015; 19:274. doi:10.1186/s13054-015-0993-7
- Calvo-Ayala E, Khan BA, Farber MO, Ely EW, Boustani MA. Interventions to improve the physical function of ICU survivors: a systematic review. Chest 2013; 144(5):1469–1480. doi:10.1378/chest.13-0779
- Wang S, Allen D, Kheir YN, Campbell N, Khan B. Aging and post-intensive care syndrome: a critical need for geriatric psychiatry. Am J Geriatr Psychiatry 2018; 26(2):212–221. doi:10.1016/j.jagp.2017.05.016
- Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med 2010; 38(7):1554–1561. doi:10.1097/CCM.0b013e3181e2c8b1
- van Beusekom I, Bakhshi-Raiez F, de Keizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care 2016; 20:16. doi:10.1186/s13054-016-1185-9
- Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40(2):502–509. doi:10.1097/CCM.0b013e318232da75
- Pandharipande PP, Girard TD, Jackson JC, et al; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316. doi:10.1056/NEJMoa1301372
- Herridge MS, Tansey CM, Matte A, et al; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364(14):1293–1304. doi:10.1056/NEJMoa1011802
- Rothenhäusler H-B, Ehrentraut S, Stoll C, Schelling G, Kapfhammer H-P. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry 2001; 23(2):90–96. pmid:11313077
- Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016; 43:23–29. doi:10.1016/j.genhosppsych.2016.08.005
- Jackson JC, Pandharipande PP, Girard TD, et al; Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med 2014; 2(5):369–379. doi:10.1016/S2213-2600(14)70051-7
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc 2013; 61(7):1128–1134. doi:10.1111/jgs.12329
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63(1):142–150. doi:10.1111/jgs.13281
- Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care 2015; 19:274. doi:10.1186/s13054-015-0993-7
- Calvo-Ayala E, Khan BA, Farber MO, Ely EW, Boustani MA. Interventions to improve the physical function of ICU survivors: a systematic review. Chest 2013; 144(5):1469–1480. doi:10.1378/chest.13-0779
- Wang S, Allen D, Kheir YN, Campbell N, Khan B. Aging and post-intensive care syndrome: a critical need for geriatric psychiatry. Am J Geriatr Psychiatry 2018; 26(2):212–221. doi:10.1016/j.jagp.2017.05.016
- Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med 2010; 38(7):1554–1561. doi:10.1097/CCM.0b013e3181e2c8b1
- van Beusekom I, Bakhshi-Raiez F, de Keizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care 2016; 20:16. doi:10.1186/s13054-016-1185-9
Prevention and treatment of influenza in the primary care office
Every year, 5% to 20% of US residents contract the flu, 200,000 are hospitalized for it, and 36,000 die of influenza-related complications. The economic impact, including direct medical costs and lost earnings, exceeds $87 billion.1 Despite this, less than half of eligible US residents were vaccinated in the 2012–2013 season, with uninsured people more than twice as likely to forgo vaccination.2,3
Several studies have shown that influenza vaccination reduces the need for outpatient encounters and hospitalizations and lowers the incidence of death from acute myocardial infarction, the rate of all-cause mortality, and even the incidence of therapies administered by implantable defibrillators.4–6 In the 2012–2013 influenza season, vaccination prevented an estimated 3.2 million medically attended illnesses and almost 80,000 hospitalizations; 70% of hospitalizations prevented were in children age 6 months to 4 years and in adults over age 65.7
After the 2009 H1N1 pandemic, which disproportionately killed previously healthy adults, the US Centers for Disease Control and Prevention (CDC) expanded its vaccination recommendations to include everyone above the age of 6 months, with few contraindications.8
In addition, recent years have seen a great expansion in vaccine options, changes in the at-risk demographics, and continued widespread resistance to certain antiviral agents, with implications for practice in primary care.
Here, we review the barriers and the new options for treatment and prevention of influenza.
HEMAGGLUTININ AND NEURAMINIDASE
Influenza infection is caused by one of the circulating strains of influenza virus A or B.
The major viral surface glycoproteins are hemagglutinin and neuraminidase. Hemagglutinin plays an important role in viral attachment to host cells and is the major immunogen in the influenza vaccine. Neuraminidase contains an active enzymatic site that cleaves the newly formed budding influenza viruses from host-cell sialic acid residues and allows them to be released from the cell membrane to infect other respiratory epithelial cells. It is the target of currently recommended antiviral drugs.
VACCINE PRODUCTION
Throughout the year, 130 influenza centers around the world sample circulating strains and share their data with five World Health Organization (WHO) Collaborating Centers for Reference and Research on Influenza. The WHO analyzes the circulation patterns, predicts the strains most likely to be circulating in the next influenza season, and shares these strains with manufacturers of the vaccine.
Pharmaceutical companies then begin an elaborate process of producing and distributing hundreds of millions of doses of vaccine worldwide. The production traditionally uses millions of fertilized chicken eggs to produce strain-specific influenza hemagglutinin. Individual vaccine strains are combined into the final product after being inactivated by chemical or physical splitting of the viral envelope with or without subsequent purification of the hemagglutinin particles.
Before 2013, the WHO’s yearly recommendations included two strains of influenza A and a single strain of influenza B. In 2013, new quadrivalent vaccines that include protection against a second strain of influenza B were approved.
The WHO strain-selection process allows manufacturers about 6 months to produce the vaccine. In a typical year, the worldwide demand is about 400 million doses. The theoretical maximal annual worldwide capacity, given current techniques, is fewer than 1 billion doses, which is well short of the 10 billion doses necessary to allow for the double vaccination needed in a pandemic.9 Newly approved recombinant manufacturing techniques offer greater production efficiency, while novel methods of intradermal administration increase vaccine immunogenicity, decreasing the amount of viral antigens used per dose.
INACTIVATED VS LIVE-ATTENUATED
In addition to intramuscular inactivated influenza vaccine, a live-attenuated vaccine in the form of an intranasal spray (FluMist) became available in 2003. This form is generally favored in children, as it avoids the discomfort of an injection. It contains live, weakened, cold-adapted influenza strains that reproduce in the relatively colder temperatures of the exterior nares but cannot survive in the warmer temperatures of the lung and proximal airways. It is approved for healthy people 2 to 49 years of age, and some evidence suggests that it may be more effective than inactivated influenza vaccine in children,10 although its utility is limited by multiple contraindications (see below).
INFLUENZA VACCINE INDICATIONS AND CONTRAINDICATIONS
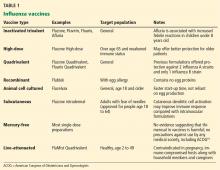
Vaccination for influenza is recommended for all persons 6 months of age and older, an expansion from pre-2009 guidelines that did not recommend vaccination for healthy adults age 19 to 49 who were not in contact with people at high risk of influenza-related complications.8 Many new vaccine formulations have become available in recent years, each with specific benefits, risks, and target populations (Table 1).
Contraindications to inactivated vaccine
The only firm contraindication to inactivated influenza vaccine is previous severe allergic reaction to influenza vaccine or any of its components. Those with moderate to severe acute illness are advised to wait until their condition improves before being vaccinated. People who have had Guillain-Barré syndrome and those with egg allergy are discussed in MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION, below. There is no risk of influenza infection from inactivated influenza vaccine.
Contraindications to live-attenuated influenza vaccine
Unlike inactivated influenza vaccine, the live-attenuated vaccine does result in shedding of vaccine-strain virus from the vaccinated host, with the theoretical potential for transmission of the virus from the vaccine recipient to other people, as well as the potential for influenza-like illness in vaccine recipients.11,12 Based on reported events, the former is estimated to occur in 10 to 20 per 1 million vaccinations, although these cases have never been proven to be caused by a cold-adapted vaccine-strain rather than by coincidental transmission of circulating wild-type viral strains.13
Despite this exceedingly small risk of viral transmission, live-attenuated influenza vaccine has multiple contraindications, including age less than 2 years and more than 49 years, disease- or drug-related compromised immune status, pregnancy, egg allergy, and history of allergic reaction to the formulation. These limit its use and are important to review in detail before prescribing.14
Use of neuraminidase inhibitors within 2 days before or 2 weeks after receiving live-attenuated influenza vaccine may interfere with replication of the cold-adapted strain and decrease the vaccine’s effectiveness.14
EFFECTIVENESS OF INFLUENZA VACCINATION IN OLDER ADULTS
The effectiveness of influenza vaccination depends on the age and health status of the person being vaccinated, as well as on the quality of the match between the vaccine and the circulating influenza viruses.
In the 2012–2013 season, the adjusted vaccine effectiveness was 56% overall, 47% for influenza A H3N2, and 67% for influenza B. However, in people age 65 and older, the overall adjusted vaccine effectiveness was 27%, and only 9% for influenza A H3N2.15 Thus, even though the vaccine-virus match was considered good, the vaccine was suboptimally effective in the older group. This may be an argument for using the recently approved high-dose vaccine in that age group. Although the high-dose vaccine has been shown to be significantly more immunogenic in older adults, it is too early to know if it is clinically more effective in preventing influenza in this age group.
Despite the lower-than-expected effectiveness in preventing influenza in the 2012–2013 season in people age 65 and older, several well-designed studies found that influenza vaccination prevented severe disease, including one study that found vaccination to be 89% effective in reducing influenza-associated hospitalizations in the 2010–2011 flu season.4,16
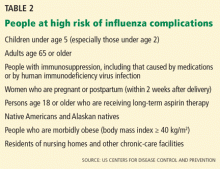
The limited effectiveness of vaccination in the older age group reminds us of the importance of early recognition and treatment of patients at high risk of influenza-related complications (see Table 2). It is also a call for greater compliance with vaccination in younger people, with a goal of achieving the 80% vaccination rate that has been calculated as adequate to achieve herd immunity.17
MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION
Concern about potential adverse effects is the most common reason for refusing influenza vaccination, even among health care workers.18 However, the only commonly encountered adverse effect of the intramuscular inactivated influenza vaccine is injection-site pain.
‘Catching the flu from a flu shot’
Many people think that they can “catch the flu from a flu shot” (or think that they actually did), but vaccine-acquired influenza is not possible with the inactivated influenza vaccine,19 and it is only a theoretical, undocumented consideration with the live-attenuated vaccine.
Various respiratory viruses other than influenza also cause viral upper-respiratory infections during the influenza season. These infections may coincide with influenza vaccination and are frequently misconstrued as a side effect of the influenza vaccine or as evidence of vaccine ineffectiveness.
Unnecessary concerns about simultaneous vaccinations
Patients and doctors are often concerned about simultaneous administration of multiple vaccines and choose to spread out indicated vaccinations over multiple visits. This practice increases patients’ risk of illness from vaccine-preventable diseases. Research shows that simultaneous administration does not alter the safety or effectiveness of vaccination.20–22 The CDC recommends simultaneous administration of all indicated live and inactivated vaccinations in order to reduce barriers to vaccination.20
Fear of Guillain-Barré syndrome
Guillain-Barré syndrome, an acute ascending polyneuropathy, has been blamed on influenza vaccination in cases that developed after the 1976 influenza A (H1N1) epidemic.
Most cases are self-limiting but require intensive treatment and supportive care. Full recovery occurs in 60% of cases, though some people experience persistent symptoms. The mortality rate is less than 5%.23
After the 1976 influenza pandemic, approximately 400 cases of Guillain-Barré syndrome arose in 45 million vaccine recipients, or about 1 case per 100,000 people.24 Multiple subsequent population analyses concluded that the actual incidence of Guillain-Barré syndrome attributable to influenza vaccination is negligible, at less than 1 case in 1 million vaccinations. Against this, we should compare the real risk of illness and death from influenza infection, which itself is a risk factor for Guillain-Barré syndrome.25
Should a person with a history of Guillain-Barré syndrome be revaccinated against influenza? The risk was evaluated in a large retrospective analysis of cases identified in the Kaiser Permanente Northern California Database from 1995 to 2006.26 Five hundred fifty cases of Guillain-Barré syndrome were identified, of which 18 had arisen within 6 weeks of the patient receiving a flu shot. Four hundred five doses of inactivated influenza vaccine were subsequently given to 105 patients who had a history of Guillain-Barré syndrome, two of whom had developed the syndrome within 6 weeks of receiving the shot. There were no documented episodes of recurrent Guillain-Barré syndrome in any of these patients. Only 6 of 550 patients with a history of the disease developed it again; none of these 6 had received the influenza vaccine in the preceding 2 months, and only 1 had been exposed to the measles-mumps-rubella vaccine in the 4 months before vaccination.
Nevertheless, expert opinion recommends lifelong avoidance of any immunization that had been given within 6 weeks before the onset of symptoms of Guillain-Barré syndrome.27
Overstated concern about egg allergy
Anaphylactic reactions can occur after influenza vaccination in people who have severe egg allergy, and concern about these reactions unfortunately prevents many otherwise eligible people with mild allergy from being vaccinated.
These reactions are much less common than feared. In a well-designed prospective cohort study of 367 patients with a history of egg allergy and positive skin-prick tests, including 132 with a history of severe allergy and 4 with a history of mild allergic symptoms arising in response to previous influenza vaccinations, none developed anaphylaxis.28
The same authors reviewed 26 studies in more than 4,000 egg-allergic patients, of whom more than 500 had a history of severe egg-associated reactions, and likewise found no cases of influenza vaccine-associated anaphylaxis. They concluded that the inactivated influenza vaccine is safer than the egg-derived mumps-measles-rubella vaccine, for which precautions for egg allergy no longer exist.28
People with a history of more serious reactions, ranging from stomach upset to anaphylaxis, can be safely vaccinated with a recombinant vaccine or referred to an allergist for further testing. People who experience hives as their only reaction to egg exposure should receive full-dose vaccination but then be observed for a half hour afterward.
The recombinant trivalent influenza vaccine Flublok was approved in 2013 for people age 18 to 49. It is the first commercially available influenza vaccine produced in a continuous insect cell line using a baculovirus vector. No eggs are used in its production, and it is approved for use in patients with egg allergy of any severity.
People who have a history of more serious reactions, including abdominal pain, nausea, vomiting, dizziness, or wheezing can be vaccinated with the recombinant vaccine or referred to an allergy specialist.
Despite this new option, understanding of alternative immunization guidelines for people with egg allergies, available on the CDC website29 remains important, as the availability of the recombinant trivalent influenza vaccine remains limited in the 2013–2014 influenza season.
Misconception about mercury toxicity
Thimerosal is an ethylmercury-containing preservative used in multidose antiviral vaccines, including some influenza vaccines.30 It is designed to prevent bacterial and fungal colonization of the vaccine vial while not reducing vaccine effectiveness or causing toxicity.
Contemporary understanding of mercury neurotoxicity is based largely on studies of methylmercury, including long-term, low-dose exposure in remote communities in the Faroe Islands and the Seychelles through regular consumption of fish and whale meat.31,32 These exposure studies had conflicting results: those in the Faroe Islands demonstrated toxicity, but the Seychelles studies actually showed better neurologic test scores at higher mercury levels, a trend the authors attributed to the beneficial effects of maternal fish consumption.
The results of the methylmercury studies have been extrapolated to ethylmercury (contained in thimerosal), although the two chemicals have vastly different pharmacologic properties. For example, methylmercury has a longer half-life and greater transport across the blood-brain barrier.33 A direct comparison found that ethylmercury is less toxic than methylmercury, although an increase in ethylmercury concentration of only 20% resulted in similar toxicity profiles.34 These studies were performed at concentrations of mercury thousands of times higher than those resulting from vaccination: nearly 150,000 times greater than those in an average adult or 15,000 times greater than those in a 1-year-old child from the typical 25-μg thimerosal dose allowed in contemporary influenza vaccines.
Despite much negative publicity, no link has been shown between thimerosal and autism.30 Multiple regulatory, scientific, and medical organizations including the US Food and Drug Administration (FDA), the WHO, the National Institutes of Health, the CDC, the American Academy of Pediatrics, and the American Congress of Obstetricians and Gynecologists (ACOG) have evaluated the data on the safety of thimerosal in vaccines and have agreed that it is safe. However, most of them urged vaccine manufacturers to eliminate mercury from vaccines as a precaution.30,35 Thimerosal has subsequently been eliminated from all childhood vaccines except for influenza vaccine, with no resulting decrease in childhood autism diagnoses.36
Considering that no harm from thimerosal at FDA-approved doses has been documented, and considering the real risk of influenza-related complications, particularly in young children and pregnant women, we recommend vaccination using whatever vaccine formulation is locally available for all patients, including children age 6 months and older and pregnant women. Nevertheless, given that mercury is being eliminated from childhood vaccines and that preservative-free single-dose vials are increasingly available in the United States, it seems reasonable to use thimerosal-free formulations for children, expectant mothers, and patients concerned about exposure if these formulations are readily available. Influenza vaccination should not be delayed if a thimerosal-free formulation is not readily available.
NEW VACCINE FORMULATIONS
Recent years have seen a dramatic expansion in influenza vaccine options (Table 1).
Quadrivalent vaccines
Quadrivalent vaccines protect against two strains of influenza A and two strains of influenza B, whereas earlier formulations included only one influenza B strain. Vaccination against either influenza B strain offers only limited cross-protection against the other B strain, and previous formulations involved assumptions about which strain would predominate in any given year. The CDC estimates that switching to quadrivalent vaccines will prevent up to 970,000 cases of influenza, 8,200 hospitalizations, and 485 deaths per year.37
Intradermal vaccine
The newly available Fluzone Intradermal vaccine contains smaller doses of hemagglutinin but is still effective because antigen-presenting dendritic cells in the skin reduce the required amount of vaccine antigen necessary for inducing protection.38 This may provide an advantage in the event of vaccine shortage. Also, since it is given in needles only 1.5 mm long, it may appeal to people who are afraid of needles.
The stronger immune reaction with intradermal administration causes more redness, induration, and tenderness at the injection site than with intramuscular administration.39 Patients should not be surprised by this reaction and can be advised to apply ice packs for symptomatic relief.
High-dose vaccine
A high-dose vaccine was approved in 2009 for use in adults age 65 and older. It contains 60 μg of hemagglutinin, compared with 15 μg in standard-dose vaccines, and has been shown to improve seroconversion rates. It remains to be seen if this translates into better clinical outcomes in older adults.40 Further studies will be necessary before we can recommend high-dose vaccines to other people with weakened immune response, such as those undergoing chemotherapy or those infected with human immunodeficiency virus (HIV).
Cell-based vaccines
Flucelvax was the first cell-based influenza vaccine. However, unlike the recombinant trivalent influenza vaccine, which uses no eggs in its manufacturing process, Flucelvax production starts with egg-derived influenza strains that are subsequently propagated in liquid culture of animal cells. It may therefore contain traces of egg protein, and it has not been studied in people with egg allergy.41
An advantage of the cell-based production technique is the use of fewer or no eggs at all, which may result in greater manufacturing efficiency. Also, it is a closed process that reduces the risk of bacterial contamination as well as reliance on antibiotics or preservatives, such as thimerosal, in the manufacturing process.42
CHEMOPROPHYLAXIS WITH NEURAMINIDASE INHIBITORS
The mainstays of influenza prevention are seasonal vaccination and appropriate infection-prevention practices. In addition, in patients at high risk of influenza-related complications (Table 2),43 postexposure chemoprophylaxis with a neuraminidase inhibitor, ie, oseltamivir (Tamiflu) or zanamivir (Relenza), is an effective preventive strategy, especially in years when the match between vaccine and circulating virus strains is suboptimal.44,45
Neuraminidase inhibitors are competitive inhibitors of the active site of the influenza glycoprotein neuraminidase, responsible for viral release from infected respiratory epithelial cells. Rates of resistance to neuraminidase inhibitors have been less than 1% in the United States in recent years, while resistance to the adamantanes amantadine (Symmetrel) and rimantadine (Flumadine) can be as high as 92%, depending on the virus isolate. Thus, their use for treatment or prophylaxis of influenza is not currently recommended by the CDC.46
Chemoprophylaxis with any agent may promote emergence of resistant strains, can cause adverse reactions, and should never be considered a substitute for vaccination.
ANTI-INFLUENZA AGENTS
Two neuraminidase inhibitors, oseltamivir and zanamivir, are approved by the FDA for preventing and treating uncomplicated influenza. Treatment must be instituted within 2 days of onset of symptoms to be effective.
Oseltamivir is available as an oral capsule or powder for liquid suspension. Its most common adverse effects are gastrointestinal upset including diarrhea, nausea, and vomiting.44
Zanamivir is only available in the form of a dry powder inhaler because of the drug’s poor oral bioavailability, and only 4% to 17% of the inhaled dose is systemically absorbed.45 There is a theoretical benefit in targeted delivery of zanamivir to the primary organ affected by influenza, and gastrointestinal side effects are less common with this drug.44,45 Unfortunately, the zanamivir inhaler requires complicated assembly and dexterity for administration (see the video on YouTube47), which may make it unreliable in certain patient groups, especially handicapped and elderly patients. Administration has been associated with bronchospasm, resulting in a more than 20% reduction in the forced expiratory volume in 1 second, and it is contraindicated in patients with underlying reactive airway disease such as chronic obstructive pulmonary disease or asthma.45
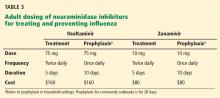
Table 3 lists the doses and duration of therapy for oseltamivir and zanamivir in adults with normal renal function, as well as approximate costs. No generic formulations of neuraminidase inhibitors are currently available, and outpatient use may not be covered by medical insurance. Several other neuraminidase inhibitors are either under development or at various stages in the FDA approval process.
EFFECTIVENESS OF ANTI-INFLUENZA DRUGS
Treatment with oseltamivir has been shown to reduce the duration of symptoms by approximately 1 day if initiated within 36 hours of onset of illness and 1.5 to 2 days if initiated within 24 hours.48,49 Trials and meta-analyses of zanamivir show similar effectiveness, though some suggest that symptoms were alleviated as much as 3 days sooner than in controls in a subgroup of patients who were febrile at presentation.50,51 Dual neuraminidase inhibitor therapy in an attempt to prevent emergence of resistance seems logical but was actually found to be less effective than monotherapy, according to a 2010 study.52
The effectiveness of neuraminidase inhibitors in reducing influenza-related complications and mortality rates has been controversial in recent years, as these outcomes were not addressed in initial studies that secured FDA approval. Several meta-analyses differ in their assessments of available data quality and conclusions. A 2009 Cochrane review questioned the completeness and the veracity of the data from manufacturer-funded trial data, much of which was unpublished and not made available to reviewers, and it concluded that a reduction of complications could not be supported by the available data.53 Hernán and Lipsitch,54 in a 2011 review, calculated that oseltamivir reduces the risk of lower respiratory tract complications by 28% in patients with influenza-like symptoms and by 37% in patients with confirmed influenza infection.
Additional trials and better access to available data are needed to settle the question of the effectiveness of neuraminidase inhibitors in reducing complications of influenza. Meanwhile, they remain strongly recommended by major health organizations, including the CDC and the WHO, which lists oseltamivir on its “model list of essential medicines.”
VIRAL RESISTANCE TO NEURAMINIDASE INHIBITORS
Viral resistance to neuraminidase inhibitors occurs through multiple mechanisms and may arise without selective pressure from exposure to these drugs.55
Oseltamivir possesses a hydrophobic moiety that requires viral neuraminidase to undergo a complex reconfiguration to expose the active site prior to binding. Any mutation affecting its ability to undergo this structural rearrangement can promote resistance by decreased oseltamivir access to the active site.
Zanamivir has a structural homology to the neuraminidase active site and requires no such reconfiguration. Additionally, mutations promoting resistance to zanamivir may actually decrease viral fitness; thus, resistance to zanamivir is significantly less common than to oseltamivir.55
About 2,000 influenza virus isolates currently circulating in the United States were tested for resistance; only 1% of the 2009 influenza A H1N1 isolates demonstrated resistance to oseltamivir, and none to zanamivir.56
The CDC regularly updates the resistance patterns of circulating influenza strains at www.cdc.gov/flu/weekly/index.htm.
SPECIAL CONSIDERATIONS
Pregnancy
Pregnant women may be at higher risk of severe influenza complications. This was especially true during the 2009 H1N1 pandemic, when pregnant women had a five times higher risk of death from influenza-related complications. Additionally, fever during pregnancy is an independent risk factor for adverse outcomes in the offspring.57 Maternal vaccination against influenza effectively protects the infant for the first 6 months of life, when vaccination is not recommended because of a poor immune response.58
Live-attenuated influenza vaccine is contraindicated during pregnancy. Given the documented risks to the mother from influenza and no documented harm from preservatives in multiuse vaccine vials, the Advisory Committee on Immunization Practices (ACIP) and ACOG do not state a preference for thimerosal-containing or thimerosal-free vaccine for any group, including pregnant women. Pregnant women should be vaccinated with whatever inactivated influenza vaccine formulation is available at the earliest opportunity in the beginning of the influenza season, regardless of the trimester of pregnancy.
Pregnant women are at high risk of influenza-related complications and should be considered for postexposure antiviral prophylaxis or early treatment with a neuraminidase inhibitor. However, both of the approved neuraminidase inhibitors are in pregnancy safety category C, indicating possible adverse effects in animal studies and a lack of safety data in pregnant humans. As with all category C medications, the risks and benefits must be considered, taking into account maternal comorbidities, vaccination status, effectiveness of the season’s influenza vaccine, and the virulence of circulating influenza strains.
As oseltamivir is associated with nausea and gastrointestinal side effects and as zanamivir has less systemic absorption, it may be reasonable to prescribe zanamivir for women already experiencing severe pregnancy-related nausea.
Immunocompromised people
Inactivated influenza vaccine is recommended and live-attenuated influenza vaccine is contraindicated for all immunocompromised people. Generally speaking, any form of immune compromise will decrease the immunogenicity of the vaccine. Additional considerations vary depending on the cause and severity of the immunocompromised status.
HIV-infected patients have higher seroconversion rates when vaccinated with the high-dose vaccine than with the standard-dose vaccine; however, as in adults over age 65, the clinical benefit has yet to be evaluated.59 The efficacy of vaccination is predictably related to the CD4 cell count, as T cells are necessary to mount a response.60 No documented benefit is gained from booster influenza vaccination in this group of patients.
Cancer patients should receive inactivated influenza vaccine every year. Postexposure chemoprophylaxis should be considered, and early treatment with a neuraminidase inhibitor is recommended in patients undergoing chemotherapy.
Solid-organ transplant recipients face a risk of organ rejection if they contract influenza infection, in addition to a higher risk of influenza-related complications.61 Transplant recipients should receive inactivated influenza vaccine as soon as it becomes available at the beginning of every influenza season. Additional research is necessary to evaluate the safety and effectiveness of the high-dose influenza vaccine in this patient group.
MORE OPTIONS, GREAT BENEFIT
Influenza remains a significant source of morbidity and mortality in the United States, and emerging pandemic strains as well as the aging population pose the risk of increased disease burden. New vaccine options offer hope of greater safety, improved efficacy, and higher vaccination rates though broader appeal to individuals. The actual differences in protection between various vaccine options are insignificant relative to the overall benefit of vaccination.
Health care providers should inquire about patients’ understanding and address their concerns about vaccination. Giving an available influenza vaccine within approved indications should not be delayed if alternative vaccine options are not readily available.
In addition to vaccination, patients at high risk of complications should be advised early in the influenza season to inform their doctors about potential exposure to influenza or the development of flu-like symptoms for consideration of early treatment or postexposure prophylaxis with a neuraminidase inhibitor.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–5096.
- Soni A. Influenza Immunization Rates for Selected at Risk Populations among the US Adult Civilian Noninstitutionalized Population, 2006. Statistical Brief #226. December 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://meps.ahrq.gov/data_files/publications/st226/stat226.pdf. Accessed January 31, 2014.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2012–13 Influenza Season. http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm - age-group-adults. Accessed January 31, 2014.
- Castilla J, Godoy P, Domínguez A, et al; CIBERESP Cases and Controls in Influenza Working Group Spain. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis 2013; 57:167–175.
- Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011–2012 influenza season. Clin Infect Dis 2013; 56:1774–1777.
- Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013; 310:1711–1720.
- Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by influenza vaccination—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2013; 62:997–1000.
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013; 62:1–43.
- Friede M. Snapshot of influenza vaccine manufacturing capacity worldwide and summary of WHO-HHS activities to promote technology transfer. World Health Organization Global Action Plan for Influenza II Meeting 2011. www.who.int/phi/Session1B_Current_Manufacturing_Capacity_Worldwide_Friede.pdf. Accessed February 5, 2014.
- Ashkenazi S, Vertruyen A, Arístegui J, et al., CAIV-T Study Group. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879.
- Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA 2005; 294:2720–2725.
- Vesikari T, Karvonen A, Korhonen T, et al; CAIV-T Transmission Study Group. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 2006; 25:590–595.
- Kamboj M, Sepkowitz KA. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect Control Hosp Epidemiol 2007; 28:702–707.
- Centers for Disease Control and Prevention (CDC). Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). http://www.cdc.gov/flu/about/qa/nasalspray.htm. Accessed February 3, 2014.
- Centers for Disease Control and Prevention (CDC). Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep 2013; 62:119–123.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med 2012; 55:72–77.
- Aziz NA, Muhamad S, Manaf MR, Hamid MZ. Factors Influencing H1N1 vaccination among primary health care workers: a cross-sectional study. Int J Prev Med 2013; 4:664–670.
- Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch Intern Med 1996; 156:1546–1550.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60( 2):1–64.
- Tseng HF, Smith N, Sy LS, Jacobsen LJ. Evaluation of the incidence of herpes zoster after concomitant administration of zoster vaccine and polysaccharide pneumococcal vaccine. Vaccine 2011; 29:3628–3632.
- Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics 2002; 109:124–129.
- Rajabally YA, Uncini A. Outcome and its predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 2012; 83:711–718.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976—1977. Am J Epidemiol 1979; 110:105–123.
- Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect Dis 2010; 10:643–651.
- Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP, Network C. Recurrent Guillain-Barré syndrome following vaccination. Clin Infect Dis 2012; 54:800–804.
- Hughes RA, Wijdicks EF, Benson E, et al. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol 2005; 62:1194–1198.
- Des Roches A, Paradis L, Gagnon R, et al. Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol 2012; 130:1213–1216.e1.
- US Centers for Disease Control and Prevention. Influenza vaccination of people with a history of egg allergy. www.immunize.org/catg.d/p3094.pdf. Accessed February 3, 2014.
- US Food Drug Administration. Thimerosal in vaccines. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed February 3, 2014.
- Davidson PW, Kost J, Myers GJ, Cox C, Clarkson TW, Shamlaye CF. Methylmercury and neurodevelopment: reanalysis of the Seychelles Child Development Study outcomes at 66 months of age. JAMA 2001; 285:1291–1293.
- Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997; 19:417–428.
- Nelson KB, Bauman ML. Thimerosal and autism? Pediatrics 2003; 111:674–679.
- Magos L, Brown AW, Sparrow S, Bailey E, Snowden RT, Skipp WR. The comparative toxicology of ethyl- and methylmercury. Arch Toxicol 1985; 57:260–267.
- American Congress of Obstetricians and Gynecologists. Influenza vaccination during pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Influenza_Vaccination_During_Pregnancy. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. Understanding thimerosal, mercury, and vaccine safety. www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-thimerosal-color-office.pdf. Accessed February 3, 2014.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Tsang P, Gorse GJ, Strout CB, et al. Immunogenicity and safety of Fluzone intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine 2013. doi: 10.1016/j.vaccine.2013.09.074. [Epub ahead of print]
- Sanofi Pasteur. Fluzone package insert. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM305080.pdf. Accessed February 3, 2014.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food Drug Administration. Flucelvax FDA application. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM332069.pdf. Accessed February 3, 2014.
- Novartis. Flucelvax (influenza virus vaccine) fact sheet. www.novartis-vaccines.com/downloads/flucelvax/Flucelvax_Fact_Sheet.pdf. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. People at high risk for developing flu-related complications. www.cdc.gov/flu/about/disease/high_risk.htm. Accessed February 3, 2014.
- Roche Pharmaceuticals. Tamiflu package insert. http://www.gene.com/download/pdf/tamiflu_prescribing.pdf. Accessed February 3, 2014.
- GlaxoSmithKline. Relenza package insert. http://us.gsk.com/products/assets/us_relenza.pdf. Accessed February 3, 2014.
- Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60:1–24.
- Administration technique for zanamivir (Relenza) Diskhaler. YouTube. 2009. www.youtube.com/watch?v=sQI0a0ToSPo. Accessed February 6, 2014.
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355:1845–1850.
- Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283:1016–1624.
- Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003; 326:1235.
- Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med 1997; 337:874–880.
- Duval X, van der Werf S, Blanchon T, et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med 2010; 7:e1000362.
- Jefferson T, Jones M, Doshi P, Del Mar C. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ 2009; 339:b5106.
- Hernán MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 2011; 53:277–279.
- Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 2013; 98:174–185.
- US Centers for Disease Control and Prevention. FluView. www.cdc.gov/flu/weekly. Accessed February 3, 2014.
- Acs N, Bánhidy F, Puhó E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol 2005; 73:989–996.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564.
- McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med 2013; 158:19–26.
- Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994; 8:469–476.
- Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2:287–291.
Every year, 5% to 20% of US residents contract the flu, 200,000 are hospitalized for it, and 36,000 die of influenza-related complications. The economic impact, including direct medical costs and lost earnings, exceeds $87 billion.1 Despite this, less than half of eligible US residents were vaccinated in the 2012–2013 season, with uninsured people more than twice as likely to forgo vaccination.2,3
Several studies have shown that influenza vaccination reduces the need for outpatient encounters and hospitalizations and lowers the incidence of death from acute myocardial infarction, the rate of all-cause mortality, and even the incidence of therapies administered by implantable defibrillators.4–6 In the 2012–2013 influenza season, vaccination prevented an estimated 3.2 million medically attended illnesses and almost 80,000 hospitalizations; 70% of hospitalizations prevented were in children age 6 months to 4 years and in adults over age 65.7
After the 2009 H1N1 pandemic, which disproportionately killed previously healthy adults, the US Centers for Disease Control and Prevention (CDC) expanded its vaccination recommendations to include everyone above the age of 6 months, with few contraindications.8
In addition, recent years have seen a great expansion in vaccine options, changes in the at-risk demographics, and continued widespread resistance to certain antiviral agents, with implications for practice in primary care.
Here, we review the barriers and the new options for treatment and prevention of influenza.
HEMAGGLUTININ AND NEURAMINIDASE
Influenza infection is caused by one of the circulating strains of influenza virus A or B.
The major viral surface glycoproteins are hemagglutinin and neuraminidase. Hemagglutinin plays an important role in viral attachment to host cells and is the major immunogen in the influenza vaccine. Neuraminidase contains an active enzymatic site that cleaves the newly formed budding influenza viruses from host-cell sialic acid residues and allows them to be released from the cell membrane to infect other respiratory epithelial cells. It is the target of currently recommended antiviral drugs.
VACCINE PRODUCTION
Throughout the year, 130 influenza centers around the world sample circulating strains and share their data with five World Health Organization (WHO) Collaborating Centers for Reference and Research on Influenza. The WHO analyzes the circulation patterns, predicts the strains most likely to be circulating in the next influenza season, and shares these strains with manufacturers of the vaccine.
Pharmaceutical companies then begin an elaborate process of producing and distributing hundreds of millions of doses of vaccine worldwide. The production traditionally uses millions of fertilized chicken eggs to produce strain-specific influenza hemagglutinin. Individual vaccine strains are combined into the final product after being inactivated by chemical or physical splitting of the viral envelope with or without subsequent purification of the hemagglutinin particles.
Before 2013, the WHO’s yearly recommendations included two strains of influenza A and a single strain of influenza B. In 2013, new quadrivalent vaccines that include protection against a second strain of influenza B were approved.
The WHO strain-selection process allows manufacturers about 6 months to produce the vaccine. In a typical year, the worldwide demand is about 400 million doses. The theoretical maximal annual worldwide capacity, given current techniques, is fewer than 1 billion doses, which is well short of the 10 billion doses necessary to allow for the double vaccination needed in a pandemic.9 Newly approved recombinant manufacturing techniques offer greater production efficiency, while novel methods of intradermal administration increase vaccine immunogenicity, decreasing the amount of viral antigens used per dose.
INACTIVATED VS LIVE-ATTENUATED
In addition to intramuscular inactivated influenza vaccine, a live-attenuated vaccine in the form of an intranasal spray (FluMist) became available in 2003. This form is generally favored in children, as it avoids the discomfort of an injection. It contains live, weakened, cold-adapted influenza strains that reproduce in the relatively colder temperatures of the exterior nares but cannot survive in the warmer temperatures of the lung and proximal airways. It is approved for healthy people 2 to 49 years of age, and some evidence suggests that it may be more effective than inactivated influenza vaccine in children,10 although its utility is limited by multiple contraindications (see below).
INFLUENZA VACCINE INDICATIONS AND CONTRAINDICATIONS
Vaccination for influenza is recommended for all persons 6 months of age and older, an expansion from pre-2009 guidelines that did not recommend vaccination for healthy adults age 19 to 49 who were not in contact with people at high risk of influenza-related complications.8 Many new vaccine formulations have become available in recent years, each with specific benefits, risks, and target populations (Table 1).
Contraindications to inactivated vaccine
The only firm contraindication to inactivated influenza vaccine is previous severe allergic reaction to influenza vaccine or any of its components. Those with moderate to severe acute illness are advised to wait until their condition improves before being vaccinated. People who have had Guillain-Barré syndrome and those with egg allergy are discussed in MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION, below. There is no risk of influenza infection from inactivated influenza vaccine.
Contraindications to live-attenuated influenza vaccine
Unlike inactivated influenza vaccine, the live-attenuated vaccine does result in shedding of vaccine-strain virus from the vaccinated host, with the theoretical potential for transmission of the virus from the vaccine recipient to other people, as well as the potential for influenza-like illness in vaccine recipients.11,12 Based on reported events, the former is estimated to occur in 10 to 20 per 1 million vaccinations, although these cases have never been proven to be caused by a cold-adapted vaccine-strain rather than by coincidental transmission of circulating wild-type viral strains.13
Despite this exceedingly small risk of viral transmission, live-attenuated influenza vaccine has multiple contraindications, including age less than 2 years and more than 49 years, disease- or drug-related compromised immune status, pregnancy, egg allergy, and history of allergic reaction to the formulation. These limit its use and are important to review in detail before prescribing.14
Use of neuraminidase inhibitors within 2 days before or 2 weeks after receiving live-attenuated influenza vaccine may interfere with replication of the cold-adapted strain and decrease the vaccine’s effectiveness.14
EFFECTIVENESS OF INFLUENZA VACCINATION IN OLDER ADULTS
The effectiveness of influenza vaccination depends on the age and health status of the person being vaccinated, as well as on the quality of the match between the vaccine and the circulating influenza viruses.
In the 2012–2013 season, the adjusted vaccine effectiveness was 56% overall, 47% for influenza A H3N2, and 67% for influenza B. However, in people age 65 and older, the overall adjusted vaccine effectiveness was 27%, and only 9% for influenza A H3N2.15 Thus, even though the vaccine-virus match was considered good, the vaccine was suboptimally effective in the older group. This may be an argument for using the recently approved high-dose vaccine in that age group. Although the high-dose vaccine has been shown to be significantly more immunogenic in older adults, it is too early to know if it is clinically more effective in preventing influenza in this age group.
Despite the lower-than-expected effectiveness in preventing influenza in the 2012–2013 season in people age 65 and older, several well-designed studies found that influenza vaccination prevented severe disease, including one study that found vaccination to be 89% effective in reducing influenza-associated hospitalizations in the 2010–2011 flu season.4,16
The limited effectiveness of vaccination in the older age group reminds us of the importance of early recognition and treatment of patients at high risk of influenza-related complications (see Table 2). It is also a call for greater compliance with vaccination in younger people, with a goal of achieving the 80% vaccination rate that has been calculated as adequate to achieve herd immunity.17
MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION
Concern about potential adverse effects is the most common reason for refusing influenza vaccination, even among health care workers.18 However, the only commonly encountered adverse effect of the intramuscular inactivated influenza vaccine is injection-site pain.
‘Catching the flu from a flu shot’
Many people think that they can “catch the flu from a flu shot” (or think that they actually did), but vaccine-acquired influenza is not possible with the inactivated influenza vaccine,19 and it is only a theoretical, undocumented consideration with the live-attenuated vaccine.
Various respiratory viruses other than influenza also cause viral upper-respiratory infections during the influenza season. These infections may coincide with influenza vaccination and are frequently misconstrued as a side effect of the influenza vaccine or as evidence of vaccine ineffectiveness.
Unnecessary concerns about simultaneous vaccinations
Patients and doctors are often concerned about simultaneous administration of multiple vaccines and choose to spread out indicated vaccinations over multiple visits. This practice increases patients’ risk of illness from vaccine-preventable diseases. Research shows that simultaneous administration does not alter the safety or effectiveness of vaccination.20–22 The CDC recommends simultaneous administration of all indicated live and inactivated vaccinations in order to reduce barriers to vaccination.20
Fear of Guillain-Barré syndrome
Guillain-Barré syndrome, an acute ascending polyneuropathy, has been blamed on influenza vaccination in cases that developed after the 1976 influenza A (H1N1) epidemic.
Most cases are self-limiting but require intensive treatment and supportive care. Full recovery occurs in 60% of cases, though some people experience persistent symptoms. The mortality rate is less than 5%.23
After the 1976 influenza pandemic, approximately 400 cases of Guillain-Barré syndrome arose in 45 million vaccine recipients, or about 1 case per 100,000 people.24 Multiple subsequent population analyses concluded that the actual incidence of Guillain-Barré syndrome attributable to influenza vaccination is negligible, at less than 1 case in 1 million vaccinations. Against this, we should compare the real risk of illness and death from influenza infection, which itself is a risk factor for Guillain-Barré syndrome.25
Should a person with a history of Guillain-Barré syndrome be revaccinated against influenza? The risk was evaluated in a large retrospective analysis of cases identified in the Kaiser Permanente Northern California Database from 1995 to 2006.26 Five hundred fifty cases of Guillain-Barré syndrome were identified, of which 18 had arisen within 6 weeks of the patient receiving a flu shot. Four hundred five doses of inactivated influenza vaccine were subsequently given to 105 patients who had a history of Guillain-Barré syndrome, two of whom had developed the syndrome within 6 weeks of receiving the shot. There were no documented episodes of recurrent Guillain-Barré syndrome in any of these patients. Only 6 of 550 patients with a history of the disease developed it again; none of these 6 had received the influenza vaccine in the preceding 2 months, and only 1 had been exposed to the measles-mumps-rubella vaccine in the 4 months before vaccination.
Nevertheless, expert opinion recommends lifelong avoidance of any immunization that had been given within 6 weeks before the onset of symptoms of Guillain-Barré syndrome.27
Overstated concern about egg allergy
Anaphylactic reactions can occur after influenza vaccination in people who have severe egg allergy, and concern about these reactions unfortunately prevents many otherwise eligible people with mild allergy from being vaccinated.
These reactions are much less common than feared. In a well-designed prospective cohort study of 367 patients with a history of egg allergy and positive skin-prick tests, including 132 with a history of severe allergy and 4 with a history of mild allergic symptoms arising in response to previous influenza vaccinations, none developed anaphylaxis.28
The same authors reviewed 26 studies in more than 4,000 egg-allergic patients, of whom more than 500 had a history of severe egg-associated reactions, and likewise found no cases of influenza vaccine-associated anaphylaxis. They concluded that the inactivated influenza vaccine is safer than the egg-derived mumps-measles-rubella vaccine, for which precautions for egg allergy no longer exist.28
People with a history of more serious reactions, ranging from stomach upset to anaphylaxis, can be safely vaccinated with a recombinant vaccine or referred to an allergist for further testing. People who experience hives as their only reaction to egg exposure should receive full-dose vaccination but then be observed for a half hour afterward.
The recombinant trivalent influenza vaccine Flublok was approved in 2013 for people age 18 to 49. It is the first commercially available influenza vaccine produced in a continuous insect cell line using a baculovirus vector. No eggs are used in its production, and it is approved for use in patients with egg allergy of any severity.
People who have a history of more serious reactions, including abdominal pain, nausea, vomiting, dizziness, or wheezing can be vaccinated with the recombinant vaccine or referred to an allergy specialist.
Despite this new option, understanding of alternative immunization guidelines for people with egg allergies, available on the CDC website29 remains important, as the availability of the recombinant trivalent influenza vaccine remains limited in the 2013–2014 influenza season.
Misconception about mercury toxicity
Thimerosal is an ethylmercury-containing preservative used in multidose antiviral vaccines, including some influenza vaccines.30 It is designed to prevent bacterial and fungal colonization of the vaccine vial while not reducing vaccine effectiveness or causing toxicity.
Contemporary understanding of mercury neurotoxicity is based largely on studies of methylmercury, including long-term, low-dose exposure in remote communities in the Faroe Islands and the Seychelles through regular consumption of fish and whale meat.31,32 These exposure studies had conflicting results: those in the Faroe Islands demonstrated toxicity, but the Seychelles studies actually showed better neurologic test scores at higher mercury levels, a trend the authors attributed to the beneficial effects of maternal fish consumption.
The results of the methylmercury studies have been extrapolated to ethylmercury (contained in thimerosal), although the two chemicals have vastly different pharmacologic properties. For example, methylmercury has a longer half-life and greater transport across the blood-brain barrier.33 A direct comparison found that ethylmercury is less toxic than methylmercury, although an increase in ethylmercury concentration of only 20% resulted in similar toxicity profiles.34 These studies were performed at concentrations of mercury thousands of times higher than those resulting from vaccination: nearly 150,000 times greater than those in an average adult or 15,000 times greater than those in a 1-year-old child from the typical 25-μg thimerosal dose allowed in contemporary influenza vaccines.
Despite much negative publicity, no link has been shown between thimerosal and autism.30 Multiple regulatory, scientific, and medical organizations including the US Food and Drug Administration (FDA), the WHO, the National Institutes of Health, the CDC, the American Academy of Pediatrics, and the American Congress of Obstetricians and Gynecologists (ACOG) have evaluated the data on the safety of thimerosal in vaccines and have agreed that it is safe. However, most of them urged vaccine manufacturers to eliminate mercury from vaccines as a precaution.30,35 Thimerosal has subsequently been eliminated from all childhood vaccines except for influenza vaccine, with no resulting decrease in childhood autism diagnoses.36
Considering that no harm from thimerosal at FDA-approved doses has been documented, and considering the real risk of influenza-related complications, particularly in young children and pregnant women, we recommend vaccination using whatever vaccine formulation is locally available for all patients, including children age 6 months and older and pregnant women. Nevertheless, given that mercury is being eliminated from childhood vaccines and that preservative-free single-dose vials are increasingly available in the United States, it seems reasonable to use thimerosal-free formulations for children, expectant mothers, and patients concerned about exposure if these formulations are readily available. Influenza vaccination should not be delayed if a thimerosal-free formulation is not readily available.
NEW VACCINE FORMULATIONS
Recent years have seen a dramatic expansion in influenza vaccine options (Table 1).
Quadrivalent vaccines
Quadrivalent vaccines protect against two strains of influenza A and two strains of influenza B, whereas earlier formulations included only one influenza B strain. Vaccination against either influenza B strain offers only limited cross-protection against the other B strain, and previous formulations involved assumptions about which strain would predominate in any given year. The CDC estimates that switching to quadrivalent vaccines will prevent up to 970,000 cases of influenza, 8,200 hospitalizations, and 485 deaths per year.37
Intradermal vaccine
The newly available Fluzone Intradermal vaccine contains smaller doses of hemagglutinin but is still effective because antigen-presenting dendritic cells in the skin reduce the required amount of vaccine antigen necessary for inducing protection.38 This may provide an advantage in the event of vaccine shortage. Also, since it is given in needles only 1.5 mm long, it may appeal to people who are afraid of needles.
The stronger immune reaction with intradermal administration causes more redness, induration, and tenderness at the injection site than with intramuscular administration.39 Patients should not be surprised by this reaction and can be advised to apply ice packs for symptomatic relief.
High-dose vaccine
A high-dose vaccine was approved in 2009 for use in adults age 65 and older. It contains 60 μg of hemagglutinin, compared with 15 μg in standard-dose vaccines, and has been shown to improve seroconversion rates. It remains to be seen if this translates into better clinical outcomes in older adults.40 Further studies will be necessary before we can recommend high-dose vaccines to other people with weakened immune response, such as those undergoing chemotherapy or those infected with human immunodeficiency virus (HIV).
Cell-based vaccines
Flucelvax was the first cell-based influenza vaccine. However, unlike the recombinant trivalent influenza vaccine, which uses no eggs in its manufacturing process, Flucelvax production starts with egg-derived influenza strains that are subsequently propagated in liquid culture of animal cells. It may therefore contain traces of egg protein, and it has not been studied in people with egg allergy.41
An advantage of the cell-based production technique is the use of fewer or no eggs at all, which may result in greater manufacturing efficiency. Also, it is a closed process that reduces the risk of bacterial contamination as well as reliance on antibiotics or preservatives, such as thimerosal, in the manufacturing process.42
CHEMOPROPHYLAXIS WITH NEURAMINIDASE INHIBITORS
The mainstays of influenza prevention are seasonal vaccination and appropriate infection-prevention practices. In addition, in patients at high risk of influenza-related complications (Table 2),43 postexposure chemoprophylaxis with a neuraminidase inhibitor, ie, oseltamivir (Tamiflu) or zanamivir (Relenza), is an effective preventive strategy, especially in years when the match between vaccine and circulating virus strains is suboptimal.44,45
Neuraminidase inhibitors are competitive inhibitors of the active site of the influenza glycoprotein neuraminidase, responsible for viral release from infected respiratory epithelial cells. Rates of resistance to neuraminidase inhibitors have been less than 1% in the United States in recent years, while resistance to the adamantanes amantadine (Symmetrel) and rimantadine (Flumadine) can be as high as 92%, depending on the virus isolate. Thus, their use for treatment or prophylaxis of influenza is not currently recommended by the CDC.46
Chemoprophylaxis with any agent may promote emergence of resistant strains, can cause adverse reactions, and should never be considered a substitute for vaccination.
ANTI-INFLUENZA AGENTS
Two neuraminidase inhibitors, oseltamivir and zanamivir, are approved by the FDA for preventing and treating uncomplicated influenza. Treatment must be instituted within 2 days of onset of symptoms to be effective.
Oseltamivir is available as an oral capsule or powder for liquid suspension. Its most common adverse effects are gastrointestinal upset including diarrhea, nausea, and vomiting.44
Zanamivir is only available in the form of a dry powder inhaler because of the drug’s poor oral bioavailability, and only 4% to 17% of the inhaled dose is systemically absorbed.45 There is a theoretical benefit in targeted delivery of zanamivir to the primary organ affected by influenza, and gastrointestinal side effects are less common with this drug.44,45 Unfortunately, the zanamivir inhaler requires complicated assembly and dexterity for administration (see the video on YouTube47), which may make it unreliable in certain patient groups, especially handicapped and elderly patients. Administration has been associated with bronchospasm, resulting in a more than 20% reduction in the forced expiratory volume in 1 second, and it is contraindicated in patients with underlying reactive airway disease such as chronic obstructive pulmonary disease or asthma.45
Table 3 lists the doses and duration of therapy for oseltamivir and zanamivir in adults with normal renal function, as well as approximate costs. No generic formulations of neuraminidase inhibitors are currently available, and outpatient use may not be covered by medical insurance. Several other neuraminidase inhibitors are either under development or at various stages in the FDA approval process.
EFFECTIVENESS OF ANTI-INFLUENZA DRUGS
Treatment with oseltamivir has been shown to reduce the duration of symptoms by approximately 1 day if initiated within 36 hours of onset of illness and 1.5 to 2 days if initiated within 24 hours.48,49 Trials and meta-analyses of zanamivir show similar effectiveness, though some suggest that symptoms were alleviated as much as 3 days sooner than in controls in a subgroup of patients who were febrile at presentation.50,51 Dual neuraminidase inhibitor therapy in an attempt to prevent emergence of resistance seems logical but was actually found to be less effective than monotherapy, according to a 2010 study.52
The effectiveness of neuraminidase inhibitors in reducing influenza-related complications and mortality rates has been controversial in recent years, as these outcomes were not addressed in initial studies that secured FDA approval. Several meta-analyses differ in their assessments of available data quality and conclusions. A 2009 Cochrane review questioned the completeness and the veracity of the data from manufacturer-funded trial data, much of which was unpublished and not made available to reviewers, and it concluded that a reduction of complications could not be supported by the available data.53 Hernán and Lipsitch,54 in a 2011 review, calculated that oseltamivir reduces the risk of lower respiratory tract complications by 28% in patients with influenza-like symptoms and by 37% in patients with confirmed influenza infection.
Additional trials and better access to available data are needed to settle the question of the effectiveness of neuraminidase inhibitors in reducing complications of influenza. Meanwhile, they remain strongly recommended by major health organizations, including the CDC and the WHO, which lists oseltamivir on its “model list of essential medicines.”
VIRAL RESISTANCE TO NEURAMINIDASE INHIBITORS
Viral resistance to neuraminidase inhibitors occurs through multiple mechanisms and may arise without selective pressure from exposure to these drugs.55
Oseltamivir possesses a hydrophobic moiety that requires viral neuraminidase to undergo a complex reconfiguration to expose the active site prior to binding. Any mutation affecting its ability to undergo this structural rearrangement can promote resistance by decreased oseltamivir access to the active site.
Zanamivir has a structural homology to the neuraminidase active site and requires no such reconfiguration. Additionally, mutations promoting resistance to zanamivir may actually decrease viral fitness; thus, resistance to zanamivir is significantly less common than to oseltamivir.55
About 2,000 influenza virus isolates currently circulating in the United States were tested for resistance; only 1% of the 2009 influenza A H1N1 isolates demonstrated resistance to oseltamivir, and none to zanamivir.56
The CDC regularly updates the resistance patterns of circulating influenza strains at www.cdc.gov/flu/weekly/index.htm.
SPECIAL CONSIDERATIONS
Pregnancy
Pregnant women may be at higher risk of severe influenza complications. This was especially true during the 2009 H1N1 pandemic, when pregnant women had a five times higher risk of death from influenza-related complications. Additionally, fever during pregnancy is an independent risk factor for adverse outcomes in the offspring.57 Maternal vaccination against influenza effectively protects the infant for the first 6 months of life, when vaccination is not recommended because of a poor immune response.58
Live-attenuated influenza vaccine is contraindicated during pregnancy. Given the documented risks to the mother from influenza and no documented harm from preservatives in multiuse vaccine vials, the Advisory Committee on Immunization Practices (ACIP) and ACOG do not state a preference for thimerosal-containing or thimerosal-free vaccine for any group, including pregnant women. Pregnant women should be vaccinated with whatever inactivated influenza vaccine formulation is available at the earliest opportunity in the beginning of the influenza season, regardless of the trimester of pregnancy.
Pregnant women are at high risk of influenza-related complications and should be considered for postexposure antiviral prophylaxis or early treatment with a neuraminidase inhibitor. However, both of the approved neuraminidase inhibitors are in pregnancy safety category C, indicating possible adverse effects in animal studies and a lack of safety data in pregnant humans. As with all category C medications, the risks and benefits must be considered, taking into account maternal comorbidities, vaccination status, effectiveness of the season’s influenza vaccine, and the virulence of circulating influenza strains.
As oseltamivir is associated with nausea and gastrointestinal side effects and as zanamivir has less systemic absorption, it may be reasonable to prescribe zanamivir for women already experiencing severe pregnancy-related nausea.
Immunocompromised people
Inactivated influenza vaccine is recommended and live-attenuated influenza vaccine is contraindicated for all immunocompromised people. Generally speaking, any form of immune compromise will decrease the immunogenicity of the vaccine. Additional considerations vary depending on the cause and severity of the immunocompromised status.
HIV-infected patients have higher seroconversion rates when vaccinated with the high-dose vaccine than with the standard-dose vaccine; however, as in adults over age 65, the clinical benefit has yet to be evaluated.59 The efficacy of vaccination is predictably related to the CD4 cell count, as T cells are necessary to mount a response.60 No documented benefit is gained from booster influenza vaccination in this group of patients.
Cancer patients should receive inactivated influenza vaccine every year. Postexposure chemoprophylaxis should be considered, and early treatment with a neuraminidase inhibitor is recommended in patients undergoing chemotherapy.
Solid-organ transplant recipients face a risk of organ rejection if they contract influenza infection, in addition to a higher risk of influenza-related complications.61 Transplant recipients should receive inactivated influenza vaccine as soon as it becomes available at the beginning of every influenza season. Additional research is necessary to evaluate the safety and effectiveness of the high-dose influenza vaccine in this patient group.
MORE OPTIONS, GREAT BENEFIT
Influenza remains a significant source of morbidity and mortality in the United States, and emerging pandemic strains as well as the aging population pose the risk of increased disease burden. New vaccine options offer hope of greater safety, improved efficacy, and higher vaccination rates though broader appeal to individuals. The actual differences in protection between various vaccine options are insignificant relative to the overall benefit of vaccination.
Health care providers should inquire about patients’ understanding and address their concerns about vaccination. Giving an available influenza vaccine within approved indications should not be delayed if alternative vaccine options are not readily available.
In addition to vaccination, patients at high risk of complications should be advised early in the influenza season to inform their doctors about potential exposure to influenza or the development of flu-like symptoms for consideration of early treatment or postexposure prophylaxis with a neuraminidase inhibitor.
Every year, 5% to 20% of US residents contract the flu, 200,000 are hospitalized for it, and 36,000 die of influenza-related complications. The economic impact, including direct medical costs and lost earnings, exceeds $87 billion.1 Despite this, less than half of eligible US residents were vaccinated in the 2012–2013 season, with uninsured people more than twice as likely to forgo vaccination.2,3
Several studies have shown that influenza vaccination reduces the need for outpatient encounters and hospitalizations and lowers the incidence of death from acute myocardial infarction, the rate of all-cause mortality, and even the incidence of therapies administered by implantable defibrillators.4–6 In the 2012–2013 influenza season, vaccination prevented an estimated 3.2 million medically attended illnesses and almost 80,000 hospitalizations; 70% of hospitalizations prevented were in children age 6 months to 4 years and in adults over age 65.7
After the 2009 H1N1 pandemic, which disproportionately killed previously healthy adults, the US Centers for Disease Control and Prevention (CDC) expanded its vaccination recommendations to include everyone above the age of 6 months, with few contraindications.8
In addition, recent years have seen a great expansion in vaccine options, changes in the at-risk demographics, and continued widespread resistance to certain antiviral agents, with implications for practice in primary care.
Here, we review the barriers and the new options for treatment and prevention of influenza.
HEMAGGLUTININ AND NEURAMINIDASE
Influenza infection is caused by one of the circulating strains of influenza virus A or B.
The major viral surface glycoproteins are hemagglutinin and neuraminidase. Hemagglutinin plays an important role in viral attachment to host cells and is the major immunogen in the influenza vaccine. Neuraminidase contains an active enzymatic site that cleaves the newly formed budding influenza viruses from host-cell sialic acid residues and allows them to be released from the cell membrane to infect other respiratory epithelial cells. It is the target of currently recommended antiviral drugs.
VACCINE PRODUCTION
Throughout the year, 130 influenza centers around the world sample circulating strains and share their data with five World Health Organization (WHO) Collaborating Centers for Reference and Research on Influenza. The WHO analyzes the circulation patterns, predicts the strains most likely to be circulating in the next influenza season, and shares these strains with manufacturers of the vaccine.
Pharmaceutical companies then begin an elaborate process of producing and distributing hundreds of millions of doses of vaccine worldwide. The production traditionally uses millions of fertilized chicken eggs to produce strain-specific influenza hemagglutinin. Individual vaccine strains are combined into the final product after being inactivated by chemical or physical splitting of the viral envelope with or without subsequent purification of the hemagglutinin particles.
Before 2013, the WHO’s yearly recommendations included two strains of influenza A and a single strain of influenza B. In 2013, new quadrivalent vaccines that include protection against a second strain of influenza B were approved.
The WHO strain-selection process allows manufacturers about 6 months to produce the vaccine. In a typical year, the worldwide demand is about 400 million doses. The theoretical maximal annual worldwide capacity, given current techniques, is fewer than 1 billion doses, which is well short of the 10 billion doses necessary to allow for the double vaccination needed in a pandemic.9 Newly approved recombinant manufacturing techniques offer greater production efficiency, while novel methods of intradermal administration increase vaccine immunogenicity, decreasing the amount of viral antigens used per dose.
INACTIVATED VS LIVE-ATTENUATED
In addition to intramuscular inactivated influenza vaccine, a live-attenuated vaccine in the form of an intranasal spray (FluMist) became available in 2003. This form is generally favored in children, as it avoids the discomfort of an injection. It contains live, weakened, cold-adapted influenza strains that reproduce in the relatively colder temperatures of the exterior nares but cannot survive in the warmer temperatures of the lung and proximal airways. It is approved for healthy people 2 to 49 years of age, and some evidence suggests that it may be more effective than inactivated influenza vaccine in children,10 although its utility is limited by multiple contraindications (see below).
INFLUENZA VACCINE INDICATIONS AND CONTRAINDICATIONS
Vaccination for influenza is recommended for all persons 6 months of age and older, an expansion from pre-2009 guidelines that did not recommend vaccination for healthy adults age 19 to 49 who were not in contact with people at high risk of influenza-related complications.8 Many new vaccine formulations have become available in recent years, each with specific benefits, risks, and target populations (Table 1).
Contraindications to inactivated vaccine
The only firm contraindication to inactivated influenza vaccine is previous severe allergic reaction to influenza vaccine or any of its components. Those with moderate to severe acute illness are advised to wait until their condition improves before being vaccinated. People who have had Guillain-Barré syndrome and those with egg allergy are discussed in MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION, below. There is no risk of influenza infection from inactivated influenza vaccine.
Contraindications to live-attenuated influenza vaccine
Unlike inactivated influenza vaccine, the live-attenuated vaccine does result in shedding of vaccine-strain virus from the vaccinated host, with the theoretical potential for transmission of the virus from the vaccine recipient to other people, as well as the potential for influenza-like illness in vaccine recipients.11,12 Based on reported events, the former is estimated to occur in 10 to 20 per 1 million vaccinations, although these cases have never been proven to be caused by a cold-adapted vaccine-strain rather than by coincidental transmission of circulating wild-type viral strains.13
Despite this exceedingly small risk of viral transmission, live-attenuated influenza vaccine has multiple contraindications, including age less than 2 years and more than 49 years, disease- or drug-related compromised immune status, pregnancy, egg allergy, and history of allergic reaction to the formulation. These limit its use and are important to review in detail before prescribing.14
Use of neuraminidase inhibitors within 2 days before or 2 weeks after receiving live-attenuated influenza vaccine may interfere with replication of the cold-adapted strain and decrease the vaccine’s effectiveness.14
EFFECTIVENESS OF INFLUENZA VACCINATION IN OLDER ADULTS
The effectiveness of influenza vaccination depends on the age and health status of the person being vaccinated, as well as on the quality of the match between the vaccine and the circulating influenza viruses.
In the 2012–2013 season, the adjusted vaccine effectiveness was 56% overall, 47% for influenza A H3N2, and 67% for influenza B. However, in people age 65 and older, the overall adjusted vaccine effectiveness was 27%, and only 9% for influenza A H3N2.15 Thus, even though the vaccine-virus match was considered good, the vaccine was suboptimally effective in the older group. This may be an argument for using the recently approved high-dose vaccine in that age group. Although the high-dose vaccine has been shown to be significantly more immunogenic in older adults, it is too early to know if it is clinically more effective in preventing influenza in this age group.
Despite the lower-than-expected effectiveness in preventing influenza in the 2012–2013 season in people age 65 and older, several well-designed studies found that influenza vaccination prevented severe disease, including one study that found vaccination to be 89% effective in reducing influenza-associated hospitalizations in the 2010–2011 flu season.4,16
The limited effectiveness of vaccination in the older age group reminds us of the importance of early recognition and treatment of patients at high risk of influenza-related complications (see Table 2). It is also a call for greater compliance with vaccination in younger people, with a goal of achieving the 80% vaccination rate that has been calculated as adequate to achieve herd immunity.17
MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION
Concern about potential adverse effects is the most common reason for refusing influenza vaccination, even among health care workers.18 However, the only commonly encountered adverse effect of the intramuscular inactivated influenza vaccine is injection-site pain.
‘Catching the flu from a flu shot’
Many people think that they can “catch the flu from a flu shot” (or think that they actually did), but vaccine-acquired influenza is not possible with the inactivated influenza vaccine,19 and it is only a theoretical, undocumented consideration with the live-attenuated vaccine.
Various respiratory viruses other than influenza also cause viral upper-respiratory infections during the influenza season. These infections may coincide with influenza vaccination and are frequently misconstrued as a side effect of the influenza vaccine or as evidence of vaccine ineffectiveness.
Unnecessary concerns about simultaneous vaccinations
Patients and doctors are often concerned about simultaneous administration of multiple vaccines and choose to spread out indicated vaccinations over multiple visits. This practice increases patients’ risk of illness from vaccine-preventable diseases. Research shows that simultaneous administration does not alter the safety or effectiveness of vaccination.20–22 The CDC recommends simultaneous administration of all indicated live and inactivated vaccinations in order to reduce barriers to vaccination.20
Fear of Guillain-Barré syndrome
Guillain-Barré syndrome, an acute ascending polyneuropathy, has been blamed on influenza vaccination in cases that developed after the 1976 influenza A (H1N1) epidemic.
Most cases are self-limiting but require intensive treatment and supportive care. Full recovery occurs in 60% of cases, though some people experience persistent symptoms. The mortality rate is less than 5%.23
After the 1976 influenza pandemic, approximately 400 cases of Guillain-Barré syndrome arose in 45 million vaccine recipients, or about 1 case per 100,000 people.24 Multiple subsequent population analyses concluded that the actual incidence of Guillain-Barré syndrome attributable to influenza vaccination is negligible, at less than 1 case in 1 million vaccinations. Against this, we should compare the real risk of illness and death from influenza infection, which itself is a risk factor for Guillain-Barré syndrome.25
Should a person with a history of Guillain-Barré syndrome be revaccinated against influenza? The risk was evaluated in a large retrospective analysis of cases identified in the Kaiser Permanente Northern California Database from 1995 to 2006.26 Five hundred fifty cases of Guillain-Barré syndrome were identified, of which 18 had arisen within 6 weeks of the patient receiving a flu shot. Four hundred five doses of inactivated influenza vaccine were subsequently given to 105 patients who had a history of Guillain-Barré syndrome, two of whom had developed the syndrome within 6 weeks of receiving the shot. There were no documented episodes of recurrent Guillain-Barré syndrome in any of these patients. Only 6 of 550 patients with a history of the disease developed it again; none of these 6 had received the influenza vaccine in the preceding 2 months, and only 1 had been exposed to the measles-mumps-rubella vaccine in the 4 months before vaccination.
Nevertheless, expert opinion recommends lifelong avoidance of any immunization that had been given within 6 weeks before the onset of symptoms of Guillain-Barré syndrome.27
Overstated concern about egg allergy
Anaphylactic reactions can occur after influenza vaccination in people who have severe egg allergy, and concern about these reactions unfortunately prevents many otherwise eligible people with mild allergy from being vaccinated.
These reactions are much less common than feared. In a well-designed prospective cohort study of 367 patients with a history of egg allergy and positive skin-prick tests, including 132 with a history of severe allergy and 4 with a history of mild allergic symptoms arising in response to previous influenza vaccinations, none developed anaphylaxis.28
The same authors reviewed 26 studies in more than 4,000 egg-allergic patients, of whom more than 500 had a history of severe egg-associated reactions, and likewise found no cases of influenza vaccine-associated anaphylaxis. They concluded that the inactivated influenza vaccine is safer than the egg-derived mumps-measles-rubella vaccine, for which precautions for egg allergy no longer exist.28
People with a history of more serious reactions, ranging from stomach upset to anaphylaxis, can be safely vaccinated with a recombinant vaccine or referred to an allergist for further testing. People who experience hives as their only reaction to egg exposure should receive full-dose vaccination but then be observed for a half hour afterward.
The recombinant trivalent influenza vaccine Flublok was approved in 2013 for people age 18 to 49. It is the first commercially available influenza vaccine produced in a continuous insect cell line using a baculovirus vector. No eggs are used in its production, and it is approved for use in patients with egg allergy of any severity.
People who have a history of more serious reactions, including abdominal pain, nausea, vomiting, dizziness, or wheezing can be vaccinated with the recombinant vaccine or referred to an allergy specialist.
Despite this new option, understanding of alternative immunization guidelines for people with egg allergies, available on the CDC website29 remains important, as the availability of the recombinant trivalent influenza vaccine remains limited in the 2013–2014 influenza season.
Misconception about mercury toxicity
Thimerosal is an ethylmercury-containing preservative used in multidose antiviral vaccines, including some influenza vaccines.30 It is designed to prevent bacterial and fungal colonization of the vaccine vial while not reducing vaccine effectiveness or causing toxicity.
Contemporary understanding of mercury neurotoxicity is based largely on studies of methylmercury, including long-term, low-dose exposure in remote communities in the Faroe Islands and the Seychelles through regular consumption of fish and whale meat.31,32 These exposure studies had conflicting results: those in the Faroe Islands demonstrated toxicity, but the Seychelles studies actually showed better neurologic test scores at higher mercury levels, a trend the authors attributed to the beneficial effects of maternal fish consumption.
The results of the methylmercury studies have been extrapolated to ethylmercury (contained in thimerosal), although the two chemicals have vastly different pharmacologic properties. For example, methylmercury has a longer half-life and greater transport across the blood-brain barrier.33 A direct comparison found that ethylmercury is less toxic than methylmercury, although an increase in ethylmercury concentration of only 20% resulted in similar toxicity profiles.34 These studies were performed at concentrations of mercury thousands of times higher than those resulting from vaccination: nearly 150,000 times greater than those in an average adult or 15,000 times greater than those in a 1-year-old child from the typical 25-μg thimerosal dose allowed in contemporary influenza vaccines.
Despite much negative publicity, no link has been shown between thimerosal and autism.30 Multiple regulatory, scientific, and medical organizations including the US Food and Drug Administration (FDA), the WHO, the National Institutes of Health, the CDC, the American Academy of Pediatrics, and the American Congress of Obstetricians and Gynecologists (ACOG) have evaluated the data on the safety of thimerosal in vaccines and have agreed that it is safe. However, most of them urged vaccine manufacturers to eliminate mercury from vaccines as a precaution.30,35 Thimerosal has subsequently been eliminated from all childhood vaccines except for influenza vaccine, with no resulting decrease in childhood autism diagnoses.36
Considering that no harm from thimerosal at FDA-approved doses has been documented, and considering the real risk of influenza-related complications, particularly in young children and pregnant women, we recommend vaccination using whatever vaccine formulation is locally available for all patients, including children age 6 months and older and pregnant women. Nevertheless, given that mercury is being eliminated from childhood vaccines and that preservative-free single-dose vials are increasingly available in the United States, it seems reasonable to use thimerosal-free formulations for children, expectant mothers, and patients concerned about exposure if these formulations are readily available. Influenza vaccination should not be delayed if a thimerosal-free formulation is not readily available.
NEW VACCINE FORMULATIONS
Recent years have seen a dramatic expansion in influenza vaccine options (Table 1).
Quadrivalent vaccines
Quadrivalent vaccines protect against two strains of influenza A and two strains of influenza B, whereas earlier formulations included only one influenza B strain. Vaccination against either influenza B strain offers only limited cross-protection against the other B strain, and previous formulations involved assumptions about which strain would predominate in any given year. The CDC estimates that switching to quadrivalent vaccines will prevent up to 970,000 cases of influenza, 8,200 hospitalizations, and 485 deaths per year.37
Intradermal vaccine
The newly available Fluzone Intradermal vaccine contains smaller doses of hemagglutinin but is still effective because antigen-presenting dendritic cells in the skin reduce the required amount of vaccine antigen necessary for inducing protection.38 This may provide an advantage in the event of vaccine shortage. Also, since it is given in needles only 1.5 mm long, it may appeal to people who are afraid of needles.
The stronger immune reaction with intradermal administration causes more redness, induration, and tenderness at the injection site than with intramuscular administration.39 Patients should not be surprised by this reaction and can be advised to apply ice packs for symptomatic relief.
High-dose vaccine
A high-dose vaccine was approved in 2009 for use in adults age 65 and older. It contains 60 μg of hemagglutinin, compared with 15 μg in standard-dose vaccines, and has been shown to improve seroconversion rates. It remains to be seen if this translates into better clinical outcomes in older adults.40 Further studies will be necessary before we can recommend high-dose vaccines to other people with weakened immune response, such as those undergoing chemotherapy or those infected with human immunodeficiency virus (HIV).
Cell-based vaccines
Flucelvax was the first cell-based influenza vaccine. However, unlike the recombinant trivalent influenza vaccine, which uses no eggs in its manufacturing process, Flucelvax production starts with egg-derived influenza strains that are subsequently propagated in liquid culture of animal cells. It may therefore contain traces of egg protein, and it has not been studied in people with egg allergy.41
An advantage of the cell-based production technique is the use of fewer or no eggs at all, which may result in greater manufacturing efficiency. Also, it is a closed process that reduces the risk of bacterial contamination as well as reliance on antibiotics or preservatives, such as thimerosal, in the manufacturing process.42
CHEMOPROPHYLAXIS WITH NEURAMINIDASE INHIBITORS
The mainstays of influenza prevention are seasonal vaccination and appropriate infection-prevention practices. In addition, in patients at high risk of influenza-related complications (Table 2),43 postexposure chemoprophylaxis with a neuraminidase inhibitor, ie, oseltamivir (Tamiflu) or zanamivir (Relenza), is an effective preventive strategy, especially in years when the match between vaccine and circulating virus strains is suboptimal.44,45
Neuraminidase inhibitors are competitive inhibitors of the active site of the influenza glycoprotein neuraminidase, responsible for viral release from infected respiratory epithelial cells. Rates of resistance to neuraminidase inhibitors have been less than 1% in the United States in recent years, while resistance to the adamantanes amantadine (Symmetrel) and rimantadine (Flumadine) can be as high as 92%, depending on the virus isolate. Thus, their use for treatment or prophylaxis of influenza is not currently recommended by the CDC.46
Chemoprophylaxis with any agent may promote emergence of resistant strains, can cause adverse reactions, and should never be considered a substitute for vaccination.
ANTI-INFLUENZA AGENTS
Two neuraminidase inhibitors, oseltamivir and zanamivir, are approved by the FDA for preventing and treating uncomplicated influenza. Treatment must be instituted within 2 days of onset of symptoms to be effective.
Oseltamivir is available as an oral capsule or powder for liquid suspension. Its most common adverse effects are gastrointestinal upset including diarrhea, nausea, and vomiting.44
Zanamivir is only available in the form of a dry powder inhaler because of the drug’s poor oral bioavailability, and only 4% to 17% of the inhaled dose is systemically absorbed.45 There is a theoretical benefit in targeted delivery of zanamivir to the primary organ affected by influenza, and gastrointestinal side effects are less common with this drug.44,45 Unfortunately, the zanamivir inhaler requires complicated assembly and dexterity for administration (see the video on YouTube47), which may make it unreliable in certain patient groups, especially handicapped and elderly patients. Administration has been associated with bronchospasm, resulting in a more than 20% reduction in the forced expiratory volume in 1 second, and it is contraindicated in patients with underlying reactive airway disease such as chronic obstructive pulmonary disease or asthma.45
Table 3 lists the doses and duration of therapy for oseltamivir and zanamivir in adults with normal renal function, as well as approximate costs. No generic formulations of neuraminidase inhibitors are currently available, and outpatient use may not be covered by medical insurance. Several other neuraminidase inhibitors are either under development or at various stages in the FDA approval process.
EFFECTIVENESS OF ANTI-INFLUENZA DRUGS
Treatment with oseltamivir has been shown to reduce the duration of symptoms by approximately 1 day if initiated within 36 hours of onset of illness and 1.5 to 2 days if initiated within 24 hours.48,49 Trials and meta-analyses of zanamivir show similar effectiveness, though some suggest that symptoms were alleviated as much as 3 days sooner than in controls in a subgroup of patients who were febrile at presentation.50,51 Dual neuraminidase inhibitor therapy in an attempt to prevent emergence of resistance seems logical but was actually found to be less effective than monotherapy, according to a 2010 study.52
The effectiveness of neuraminidase inhibitors in reducing influenza-related complications and mortality rates has been controversial in recent years, as these outcomes were not addressed in initial studies that secured FDA approval. Several meta-analyses differ in their assessments of available data quality and conclusions. A 2009 Cochrane review questioned the completeness and the veracity of the data from manufacturer-funded trial data, much of which was unpublished and not made available to reviewers, and it concluded that a reduction of complications could not be supported by the available data.53 Hernán and Lipsitch,54 in a 2011 review, calculated that oseltamivir reduces the risk of lower respiratory tract complications by 28% in patients with influenza-like symptoms and by 37% in patients with confirmed influenza infection.
Additional trials and better access to available data are needed to settle the question of the effectiveness of neuraminidase inhibitors in reducing complications of influenza. Meanwhile, they remain strongly recommended by major health organizations, including the CDC and the WHO, which lists oseltamivir on its “model list of essential medicines.”
VIRAL RESISTANCE TO NEURAMINIDASE INHIBITORS
Viral resistance to neuraminidase inhibitors occurs through multiple mechanisms and may arise without selective pressure from exposure to these drugs.55
Oseltamivir possesses a hydrophobic moiety that requires viral neuraminidase to undergo a complex reconfiguration to expose the active site prior to binding. Any mutation affecting its ability to undergo this structural rearrangement can promote resistance by decreased oseltamivir access to the active site.
Zanamivir has a structural homology to the neuraminidase active site and requires no such reconfiguration. Additionally, mutations promoting resistance to zanamivir may actually decrease viral fitness; thus, resistance to zanamivir is significantly less common than to oseltamivir.55
About 2,000 influenza virus isolates currently circulating in the United States were tested for resistance; only 1% of the 2009 influenza A H1N1 isolates demonstrated resistance to oseltamivir, and none to zanamivir.56
The CDC regularly updates the resistance patterns of circulating influenza strains at www.cdc.gov/flu/weekly/index.htm.
SPECIAL CONSIDERATIONS
Pregnancy
Pregnant women may be at higher risk of severe influenza complications. This was especially true during the 2009 H1N1 pandemic, when pregnant women had a five times higher risk of death from influenza-related complications. Additionally, fever during pregnancy is an independent risk factor for adverse outcomes in the offspring.57 Maternal vaccination against influenza effectively protects the infant for the first 6 months of life, when vaccination is not recommended because of a poor immune response.58
Live-attenuated influenza vaccine is contraindicated during pregnancy. Given the documented risks to the mother from influenza and no documented harm from preservatives in multiuse vaccine vials, the Advisory Committee on Immunization Practices (ACIP) and ACOG do not state a preference for thimerosal-containing or thimerosal-free vaccine for any group, including pregnant women. Pregnant women should be vaccinated with whatever inactivated influenza vaccine formulation is available at the earliest opportunity in the beginning of the influenza season, regardless of the trimester of pregnancy.
Pregnant women are at high risk of influenza-related complications and should be considered for postexposure antiviral prophylaxis or early treatment with a neuraminidase inhibitor. However, both of the approved neuraminidase inhibitors are in pregnancy safety category C, indicating possible adverse effects in animal studies and a lack of safety data in pregnant humans. As with all category C medications, the risks and benefits must be considered, taking into account maternal comorbidities, vaccination status, effectiveness of the season’s influenza vaccine, and the virulence of circulating influenza strains.
As oseltamivir is associated with nausea and gastrointestinal side effects and as zanamivir has less systemic absorption, it may be reasonable to prescribe zanamivir for women already experiencing severe pregnancy-related nausea.
Immunocompromised people
Inactivated influenza vaccine is recommended and live-attenuated influenza vaccine is contraindicated for all immunocompromised people. Generally speaking, any form of immune compromise will decrease the immunogenicity of the vaccine. Additional considerations vary depending on the cause and severity of the immunocompromised status.
HIV-infected patients have higher seroconversion rates when vaccinated with the high-dose vaccine than with the standard-dose vaccine; however, as in adults over age 65, the clinical benefit has yet to be evaluated.59 The efficacy of vaccination is predictably related to the CD4 cell count, as T cells are necessary to mount a response.60 No documented benefit is gained from booster influenza vaccination in this group of patients.
Cancer patients should receive inactivated influenza vaccine every year. Postexposure chemoprophylaxis should be considered, and early treatment with a neuraminidase inhibitor is recommended in patients undergoing chemotherapy.
Solid-organ transplant recipients face a risk of organ rejection if they contract influenza infection, in addition to a higher risk of influenza-related complications.61 Transplant recipients should receive inactivated influenza vaccine as soon as it becomes available at the beginning of every influenza season. Additional research is necessary to evaluate the safety and effectiveness of the high-dose influenza vaccine in this patient group.
MORE OPTIONS, GREAT BENEFIT
Influenza remains a significant source of morbidity and mortality in the United States, and emerging pandemic strains as well as the aging population pose the risk of increased disease burden. New vaccine options offer hope of greater safety, improved efficacy, and higher vaccination rates though broader appeal to individuals. The actual differences in protection between various vaccine options are insignificant relative to the overall benefit of vaccination.
Health care providers should inquire about patients’ understanding and address their concerns about vaccination. Giving an available influenza vaccine within approved indications should not be delayed if alternative vaccine options are not readily available.
In addition to vaccination, patients at high risk of complications should be advised early in the influenza season to inform their doctors about potential exposure to influenza or the development of flu-like symptoms for consideration of early treatment or postexposure prophylaxis with a neuraminidase inhibitor.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–5096.
- Soni A. Influenza Immunization Rates for Selected at Risk Populations among the US Adult Civilian Noninstitutionalized Population, 2006. Statistical Brief #226. December 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://meps.ahrq.gov/data_files/publications/st226/stat226.pdf. Accessed January 31, 2014.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2012–13 Influenza Season. http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm - age-group-adults. Accessed January 31, 2014.
- Castilla J, Godoy P, Domínguez A, et al; CIBERESP Cases and Controls in Influenza Working Group Spain. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis 2013; 57:167–175.
- Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011–2012 influenza season. Clin Infect Dis 2013; 56:1774–1777.
- Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013; 310:1711–1720.
- Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by influenza vaccination—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2013; 62:997–1000.
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013; 62:1–43.
- Friede M. Snapshot of influenza vaccine manufacturing capacity worldwide and summary of WHO-HHS activities to promote technology transfer. World Health Organization Global Action Plan for Influenza II Meeting 2011. www.who.int/phi/Session1B_Current_Manufacturing_Capacity_Worldwide_Friede.pdf. Accessed February 5, 2014.
- Ashkenazi S, Vertruyen A, Arístegui J, et al., CAIV-T Study Group. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879.
- Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA 2005; 294:2720–2725.
- Vesikari T, Karvonen A, Korhonen T, et al; CAIV-T Transmission Study Group. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 2006; 25:590–595.
- Kamboj M, Sepkowitz KA. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect Control Hosp Epidemiol 2007; 28:702–707.
- Centers for Disease Control and Prevention (CDC). Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). http://www.cdc.gov/flu/about/qa/nasalspray.htm. Accessed February 3, 2014.
- Centers for Disease Control and Prevention (CDC). Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep 2013; 62:119–123.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med 2012; 55:72–77.
- Aziz NA, Muhamad S, Manaf MR, Hamid MZ. Factors Influencing H1N1 vaccination among primary health care workers: a cross-sectional study. Int J Prev Med 2013; 4:664–670.
- Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch Intern Med 1996; 156:1546–1550.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60( 2):1–64.
- Tseng HF, Smith N, Sy LS, Jacobsen LJ. Evaluation of the incidence of herpes zoster after concomitant administration of zoster vaccine and polysaccharide pneumococcal vaccine. Vaccine 2011; 29:3628–3632.
- Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics 2002; 109:124–129.
- Rajabally YA, Uncini A. Outcome and its predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 2012; 83:711–718.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976—1977. Am J Epidemiol 1979; 110:105–123.
- Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect Dis 2010; 10:643–651.
- Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP, Network C. Recurrent Guillain-Barré syndrome following vaccination. Clin Infect Dis 2012; 54:800–804.
- Hughes RA, Wijdicks EF, Benson E, et al. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol 2005; 62:1194–1198.
- Des Roches A, Paradis L, Gagnon R, et al. Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol 2012; 130:1213–1216.e1.
- US Centers for Disease Control and Prevention. Influenza vaccination of people with a history of egg allergy. www.immunize.org/catg.d/p3094.pdf. Accessed February 3, 2014.
- US Food Drug Administration. Thimerosal in vaccines. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed February 3, 2014.
- Davidson PW, Kost J, Myers GJ, Cox C, Clarkson TW, Shamlaye CF. Methylmercury and neurodevelopment: reanalysis of the Seychelles Child Development Study outcomes at 66 months of age. JAMA 2001; 285:1291–1293.
- Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997; 19:417–428.
- Nelson KB, Bauman ML. Thimerosal and autism? Pediatrics 2003; 111:674–679.
- Magos L, Brown AW, Sparrow S, Bailey E, Snowden RT, Skipp WR. The comparative toxicology of ethyl- and methylmercury. Arch Toxicol 1985; 57:260–267.
- American Congress of Obstetricians and Gynecologists. Influenza vaccination during pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Influenza_Vaccination_During_Pregnancy. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. Understanding thimerosal, mercury, and vaccine safety. www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-thimerosal-color-office.pdf. Accessed February 3, 2014.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Tsang P, Gorse GJ, Strout CB, et al. Immunogenicity and safety of Fluzone intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine 2013. doi: 10.1016/j.vaccine.2013.09.074. [Epub ahead of print]
- Sanofi Pasteur. Fluzone package insert. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM305080.pdf. Accessed February 3, 2014.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food Drug Administration. Flucelvax FDA application. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM332069.pdf. Accessed February 3, 2014.
- Novartis. Flucelvax (influenza virus vaccine) fact sheet. www.novartis-vaccines.com/downloads/flucelvax/Flucelvax_Fact_Sheet.pdf. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. People at high risk for developing flu-related complications. www.cdc.gov/flu/about/disease/high_risk.htm. Accessed February 3, 2014.
- Roche Pharmaceuticals. Tamiflu package insert. http://www.gene.com/download/pdf/tamiflu_prescribing.pdf. Accessed February 3, 2014.
- GlaxoSmithKline. Relenza package insert. http://us.gsk.com/products/assets/us_relenza.pdf. Accessed February 3, 2014.
- Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60:1–24.
- Administration technique for zanamivir (Relenza) Diskhaler. YouTube. 2009. www.youtube.com/watch?v=sQI0a0ToSPo. Accessed February 6, 2014.
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355:1845–1850.
- Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283:1016–1624.
- Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003; 326:1235.
- Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med 1997; 337:874–880.
- Duval X, van der Werf S, Blanchon T, et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med 2010; 7:e1000362.
- Jefferson T, Jones M, Doshi P, Del Mar C. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ 2009; 339:b5106.
- Hernán MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 2011; 53:277–279.
- Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 2013; 98:174–185.
- US Centers for Disease Control and Prevention. FluView. www.cdc.gov/flu/weekly. Accessed February 3, 2014.
- Acs N, Bánhidy F, Puhó E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol 2005; 73:989–996.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564.
- McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med 2013; 158:19–26.
- Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994; 8:469–476.
- Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2:287–291.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–5096.
- Soni A. Influenza Immunization Rates for Selected at Risk Populations among the US Adult Civilian Noninstitutionalized Population, 2006. Statistical Brief #226. December 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://meps.ahrq.gov/data_files/publications/st226/stat226.pdf. Accessed January 31, 2014.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2012–13 Influenza Season. http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm - age-group-adults. Accessed January 31, 2014.
- Castilla J, Godoy P, Domínguez A, et al; CIBERESP Cases and Controls in Influenza Working Group Spain. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis 2013; 57:167–175.
- Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011–2012 influenza season. Clin Infect Dis 2013; 56:1774–1777.
- Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013; 310:1711–1720.
- Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by influenza vaccination—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2013; 62:997–1000.
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013; 62:1–43.
- Friede M. Snapshot of influenza vaccine manufacturing capacity worldwide and summary of WHO-HHS activities to promote technology transfer. World Health Organization Global Action Plan for Influenza II Meeting 2011. www.who.int/phi/Session1B_Current_Manufacturing_Capacity_Worldwide_Friede.pdf. Accessed February 5, 2014.
- Ashkenazi S, Vertruyen A, Arístegui J, et al., CAIV-T Study Group. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879.
- Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA 2005; 294:2720–2725.
- Vesikari T, Karvonen A, Korhonen T, et al; CAIV-T Transmission Study Group. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 2006; 25:590–595.
- Kamboj M, Sepkowitz KA. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect Control Hosp Epidemiol 2007; 28:702–707.
- Centers for Disease Control and Prevention (CDC). Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). http://www.cdc.gov/flu/about/qa/nasalspray.htm. Accessed February 3, 2014.
- Centers for Disease Control and Prevention (CDC). Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep 2013; 62:119–123.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med 2012; 55:72–77.
- Aziz NA, Muhamad S, Manaf MR, Hamid MZ. Factors Influencing H1N1 vaccination among primary health care workers: a cross-sectional study. Int J Prev Med 2013; 4:664–670.
- Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch Intern Med 1996; 156:1546–1550.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60( 2):1–64.
- Tseng HF, Smith N, Sy LS, Jacobsen LJ. Evaluation of the incidence of herpes zoster after concomitant administration of zoster vaccine and polysaccharide pneumococcal vaccine. Vaccine 2011; 29:3628–3632.
- Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics 2002; 109:124–129.
- Rajabally YA, Uncini A. Outcome and its predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 2012; 83:711–718.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976—1977. Am J Epidemiol 1979; 110:105–123.
- Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect Dis 2010; 10:643–651.
- Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP, Network C. Recurrent Guillain-Barré syndrome following vaccination. Clin Infect Dis 2012; 54:800–804.
- Hughes RA, Wijdicks EF, Benson E, et al. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol 2005; 62:1194–1198.
- Des Roches A, Paradis L, Gagnon R, et al. Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol 2012; 130:1213–1216.e1.
- US Centers for Disease Control and Prevention. Influenza vaccination of people with a history of egg allergy. www.immunize.org/catg.d/p3094.pdf. Accessed February 3, 2014.
- US Food Drug Administration. Thimerosal in vaccines. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed February 3, 2014.
- Davidson PW, Kost J, Myers GJ, Cox C, Clarkson TW, Shamlaye CF. Methylmercury and neurodevelopment: reanalysis of the Seychelles Child Development Study outcomes at 66 months of age. JAMA 2001; 285:1291–1293.
- Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997; 19:417–428.
- Nelson KB, Bauman ML. Thimerosal and autism? Pediatrics 2003; 111:674–679.
- Magos L, Brown AW, Sparrow S, Bailey E, Snowden RT, Skipp WR. The comparative toxicology of ethyl- and methylmercury. Arch Toxicol 1985; 57:260–267.
- American Congress of Obstetricians and Gynecologists. Influenza vaccination during pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Influenza_Vaccination_During_Pregnancy. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. Understanding thimerosal, mercury, and vaccine safety. www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-thimerosal-color-office.pdf. Accessed February 3, 2014.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Tsang P, Gorse GJ, Strout CB, et al. Immunogenicity and safety of Fluzone intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine 2013. doi: 10.1016/j.vaccine.2013.09.074. [Epub ahead of print]
- Sanofi Pasteur. Fluzone package insert. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM305080.pdf. Accessed February 3, 2014.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food Drug Administration. Flucelvax FDA application. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM332069.pdf. Accessed February 3, 2014.
- Novartis. Flucelvax (influenza virus vaccine) fact sheet. www.novartis-vaccines.com/downloads/flucelvax/Flucelvax_Fact_Sheet.pdf. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. People at high risk for developing flu-related complications. www.cdc.gov/flu/about/disease/high_risk.htm. Accessed February 3, 2014.
- Roche Pharmaceuticals. Tamiflu package insert. http://www.gene.com/download/pdf/tamiflu_prescribing.pdf. Accessed February 3, 2014.
- GlaxoSmithKline. Relenza package insert. http://us.gsk.com/products/assets/us_relenza.pdf. Accessed February 3, 2014.
- Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60:1–24.
- Administration technique for zanamivir (Relenza) Diskhaler. YouTube. 2009. www.youtube.com/watch?v=sQI0a0ToSPo. Accessed February 6, 2014.
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355:1845–1850.
- Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283:1016–1624.
- Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003; 326:1235.
- Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med 1997; 337:874–880.
- Duval X, van der Werf S, Blanchon T, et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med 2010; 7:e1000362.
- Jefferson T, Jones M, Doshi P, Del Mar C. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ 2009; 339:b5106.
- Hernán MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 2011; 53:277–279.
- Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 2013; 98:174–185.
- US Centers for Disease Control and Prevention. FluView. www.cdc.gov/flu/weekly. Accessed February 3, 2014.
- Acs N, Bánhidy F, Puhó E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol 2005; 73:989–996.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564.
- McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med 2013; 158:19–26.
- Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994; 8:469–476.
- Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2:287–291.
KEY POINTS
- Influenza vaccination is effective at preventing influenza-associated disease.
- Influenza vaccine is safe in people with a history of mild egg allergy.
- Many new vaccine formulations exist and may offer benefits to different patient groups.
- Neuraminidase inhibitors are recommended for treatment and postexposure prophylaxis in patients at high risk of influenza-related complications; however, they are not a substitute for vaccination.