User login
Catherine Cooper Nellist is editor of Pediatric News and Ob. Gyn. News. She has more than 30 years of experience reporting, writing, and editing stories about clinical medicine and the U.S. health care industry. Prior to taking the helm of these award-winning publications, Catherine covered major medical research meetings throughout the United States and Canada, and had been editor of Clinical Psychiatry News, and Dermatology News. She joined the company in 1984 after graduating magna cum laude from Dickinson College, Carlisle, Pa., with a BA in English.
FDA: No more codeine or hydrocodone cold medicines for children
New safety labeling changes for prescription cough and cold medicines containing codeine or hydrocodone limit their use to adults 18 years or older.
The Food and Drug Administration took this action after “conducting an extensive review and convening a panel of outside experts,” which determined that the risks of these medicines outweigh their benefits in children younger than 18 years. The agency also is requiring companies to add a boxed warning to drug labels for prescription cough and cold medicines containing codeine or hydrocodone about the “risks of misuse, abuse, addiction, overdose, death, and slowed or difficult breathing,” according to an FDA safety announcement.
Common side effects of opioids include drowsiness, dizziness, nausea, vomiting, constipation, shortness of breath, and headache, according to the press release.
Reassure parents that cough because of a cold or upper respiratory infection is self-limited and generally does not need to be treated, the FDA advised. If children do need cough treatment, there are over-the-counter products such as dextromethorphan, as well as prescription benzonatate products, the FDA said. Encourage parents to check labels of nonprescription cough and cold products.
In a few states, some codeine cough medicines are available OTC. The FDA is considering regulatory action for these products, according to the safety announcement.
New safety labeling changes for prescription cough and cold medicines containing codeine or hydrocodone limit their use to adults 18 years or older.
The Food and Drug Administration took this action after “conducting an extensive review and convening a panel of outside experts,” which determined that the risks of these medicines outweigh their benefits in children younger than 18 years. The agency also is requiring companies to add a boxed warning to drug labels for prescription cough and cold medicines containing codeine or hydrocodone about the “risks of misuse, abuse, addiction, overdose, death, and slowed or difficult breathing,” according to an FDA safety announcement.
Common side effects of opioids include drowsiness, dizziness, nausea, vomiting, constipation, shortness of breath, and headache, according to the press release.
Reassure parents that cough because of a cold or upper respiratory infection is self-limited and generally does not need to be treated, the FDA advised. If children do need cough treatment, there are over-the-counter products such as dextromethorphan, as well as prescription benzonatate products, the FDA said. Encourage parents to check labels of nonprescription cough and cold products.
In a few states, some codeine cough medicines are available OTC. The FDA is considering regulatory action for these products, according to the safety announcement.
New safety labeling changes for prescription cough and cold medicines containing codeine or hydrocodone limit their use to adults 18 years or older.
The Food and Drug Administration took this action after “conducting an extensive review and convening a panel of outside experts,” which determined that the risks of these medicines outweigh their benefits in children younger than 18 years. The agency also is requiring companies to add a boxed warning to drug labels for prescription cough and cold medicines containing codeine or hydrocodone about the “risks of misuse, abuse, addiction, overdose, death, and slowed or difficult breathing,” according to an FDA safety announcement.
Common side effects of opioids include drowsiness, dizziness, nausea, vomiting, constipation, shortness of breath, and headache, according to the press release.
Reassure parents that cough because of a cold or upper respiratory infection is self-limited and generally does not need to be treated, the FDA advised. If children do need cough treatment, there are over-the-counter products such as dextromethorphan, as well as prescription benzonatate products, the FDA said. Encourage parents to check labels of nonprescription cough and cold products.
In a few states, some codeine cough medicines are available OTC. The FDA is considering regulatory action for these products, according to the safety announcement.
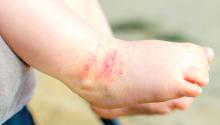
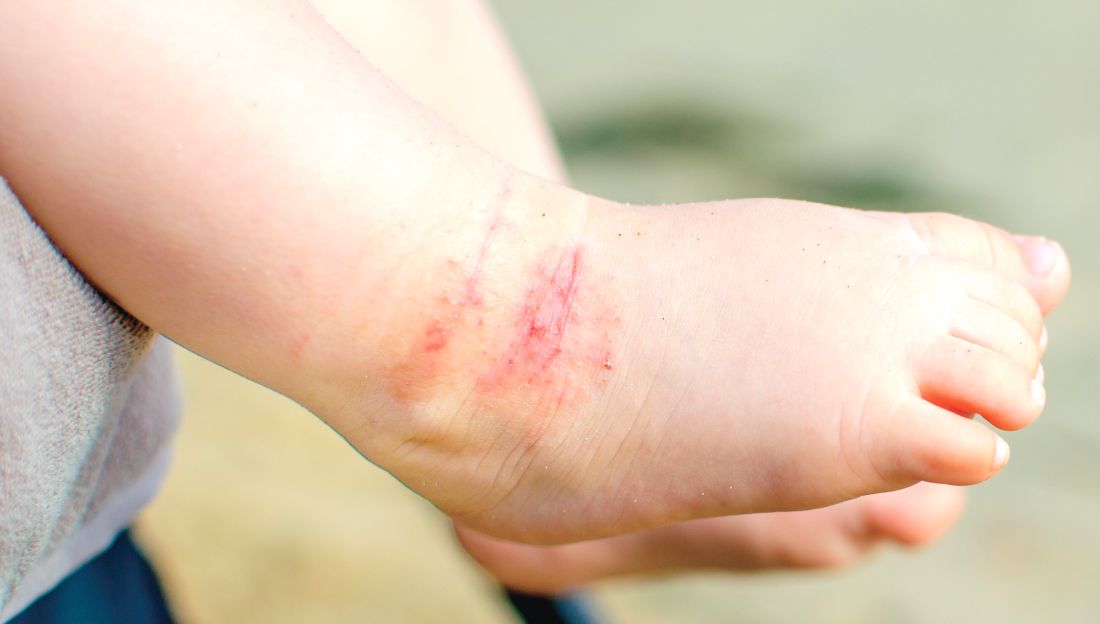
Study: Atopic dermatitis subgroups identified in children
Identification of subphenotypes of atopic dermatitis (AD) in children, with differing risk factors, prognoses, and comorbidities, could lead to a stratified approach to managing pediatric AD, said Lavinia Paternoster, PhD, of the University of Bristol, England, and her associates.
The study identified six classes of AD in these children. Early-onset/early-resolving AD, occurring in 13%-15% of the children, was most prevalent and was associated with male gender. Children in this class had a favorable prognosis, and there was only a very weak association with asthma in later life.
Two classes of persistent disease were identified: early-onset persistent AD (rash occurring in most of this class by 30 months and resolving in half by 16.5 years) and early-onset/late-resolving AD (rash occurring in most by 30 months and resolving in most by 16.5 years). These classes, occurring in about 7% of the children, had the strongest association with an AD genetic risk score; a strong link with personal and parental history of atopic disease; and a strong tie to asthma.
An unrecognized class of mid-onset-resolving AD, occurring in 7% of children, was not significantly linked to FLG mutations but was tied to asthma. In those children, AD prevalence rose sharply from 2.5 years of age and peaked at about 6 years, Dr. Paternoster and her associates said.
The investigators also found an unaffected/transient AD class in which children either had never reported rash; had one or two isolated occasions of rash; or reported a rash consistent with AD at 6-18 months that declined with age. In the two cohorts, 58%-63% children fell into this class. Late-onset-resolving AD occurred in 7%-8% of children, with most developing rash by 12 years and declining by 16.5 years.
There was a preponderance of females in the early-onset persistent AD and the late onset classes, and more males in the early-onset resolving class. “The associations with asthma at ages 7 and 11-13 years were strongest with the persistent class, but all AD classes showed evidence of some increased risk of asthma at these ages,” Dr. Paternoster and her associates wrote.
“There was evidence that FLG null mutations were associated with all classes, however ... the association was strongest in the group with early-onset-persistent disease,” the researchers said. The “heterogeneity of effect of genetic variants on different disease profiles, emphasizes the need for patient stratification in future genetic studies. Stratification may be used to increase the power to detect variants associated with specific classes; stratification could also allow the identification of phenotype-specific mechanistic pathways as future therapeutic targets.”
Read more at (J Allerg Clin Immunol. 2017 Nov 10. doi: 10.1016/j.jaci.2017.09.044).
Identification of subphenotypes of atopic dermatitis (AD) in children, with differing risk factors, prognoses, and comorbidities, could lead to a stratified approach to managing pediatric AD, said Lavinia Paternoster, PhD, of the University of Bristol, England, and her associates.
The study identified six classes of AD in these children. Early-onset/early-resolving AD, occurring in 13%-15% of the children, was most prevalent and was associated with male gender. Children in this class had a favorable prognosis, and there was only a very weak association with asthma in later life.
Two classes of persistent disease were identified: early-onset persistent AD (rash occurring in most of this class by 30 months and resolving in half by 16.5 years) and early-onset/late-resolving AD (rash occurring in most by 30 months and resolving in most by 16.5 years). These classes, occurring in about 7% of the children, had the strongest association with an AD genetic risk score; a strong link with personal and parental history of atopic disease; and a strong tie to asthma.
An unrecognized class of mid-onset-resolving AD, occurring in 7% of children, was not significantly linked to FLG mutations but was tied to asthma. In those children, AD prevalence rose sharply from 2.5 years of age and peaked at about 6 years, Dr. Paternoster and her associates said.
The investigators also found an unaffected/transient AD class in which children either had never reported rash; had one or two isolated occasions of rash; or reported a rash consistent with AD at 6-18 months that declined with age. In the two cohorts, 58%-63% children fell into this class. Late-onset-resolving AD occurred in 7%-8% of children, with most developing rash by 12 years and declining by 16.5 years.
There was a preponderance of females in the early-onset persistent AD and the late onset classes, and more males in the early-onset resolving class. “The associations with asthma at ages 7 and 11-13 years were strongest with the persistent class, but all AD classes showed evidence of some increased risk of asthma at these ages,” Dr. Paternoster and her associates wrote.
“There was evidence that FLG null mutations were associated with all classes, however ... the association was strongest in the group with early-onset-persistent disease,” the researchers said. The “heterogeneity of effect of genetic variants on different disease profiles, emphasizes the need for patient stratification in future genetic studies. Stratification may be used to increase the power to detect variants associated with specific classes; stratification could also allow the identification of phenotype-specific mechanistic pathways as future therapeutic targets.”
Read more at (J Allerg Clin Immunol. 2017 Nov 10. doi: 10.1016/j.jaci.2017.09.044).
Identification of subphenotypes of atopic dermatitis (AD) in children, with differing risk factors, prognoses, and comorbidities, could lead to a stratified approach to managing pediatric AD, said Lavinia Paternoster, PhD, of the University of Bristol, England, and her associates.
The study identified six classes of AD in these children. Early-onset/early-resolving AD, occurring in 13%-15% of the children, was most prevalent and was associated with male gender. Children in this class had a favorable prognosis, and there was only a very weak association with asthma in later life.
Two classes of persistent disease were identified: early-onset persistent AD (rash occurring in most of this class by 30 months and resolving in half by 16.5 years) and early-onset/late-resolving AD (rash occurring in most by 30 months and resolving in most by 16.5 years). These classes, occurring in about 7% of the children, had the strongest association with an AD genetic risk score; a strong link with personal and parental history of atopic disease; and a strong tie to asthma.
An unrecognized class of mid-onset-resolving AD, occurring in 7% of children, was not significantly linked to FLG mutations but was tied to asthma. In those children, AD prevalence rose sharply from 2.5 years of age and peaked at about 6 years, Dr. Paternoster and her associates said.
The investigators also found an unaffected/transient AD class in which children either had never reported rash; had one or two isolated occasions of rash; or reported a rash consistent with AD at 6-18 months that declined with age. In the two cohorts, 58%-63% children fell into this class. Late-onset-resolving AD occurred in 7%-8% of children, with most developing rash by 12 years and declining by 16.5 years.
There was a preponderance of females in the early-onset persistent AD and the late onset classes, and more males in the early-onset resolving class. “The associations with asthma at ages 7 and 11-13 years were strongest with the persistent class, but all AD classes showed evidence of some increased risk of asthma at these ages,” Dr. Paternoster and her associates wrote.
“There was evidence that FLG null mutations were associated with all classes, however ... the association was strongest in the group with early-onset-persistent disease,” the researchers said. The “heterogeneity of effect of genetic variants on different disease profiles, emphasizes the need for patient stratification in future genetic studies. Stratification may be used to increase the power to detect variants associated with specific classes; stratification could also allow the identification of phenotype-specific mechanistic pathways as future therapeutic targets.”
Read more at (J Allerg Clin Immunol. 2017 Nov 10. doi: 10.1016/j.jaci.2017.09.044).
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
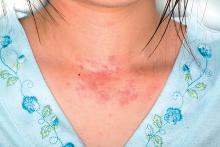
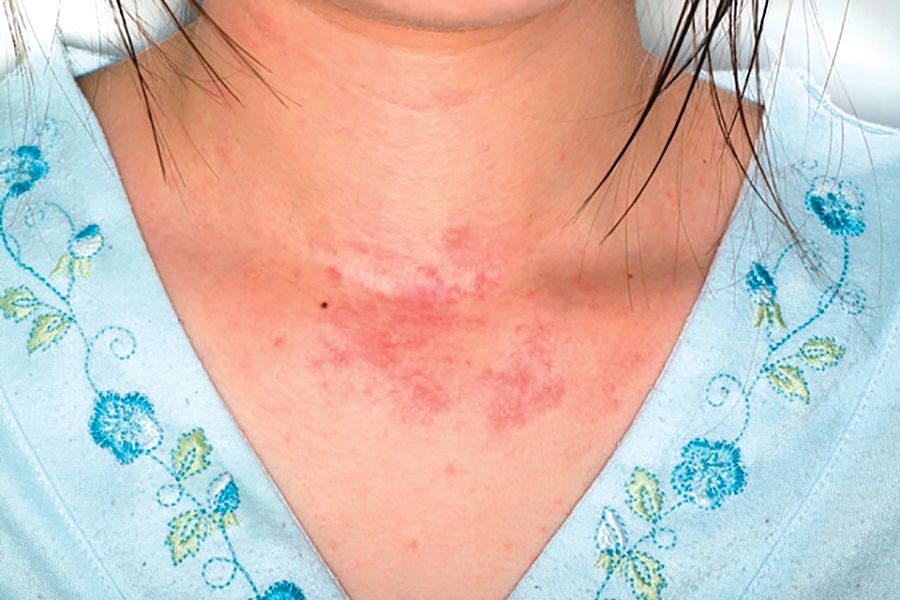
Gene mutations tied to viral skin infection in pediatric AD patients
Filaggrin (FLG) polymorphism appears to be linked with Molluscum contagiosum virus skin infection in children with atopic dermatitis (AD), reported Sara Manti, MD, of the University of Messina (Italy), and her associates.
“To the best of our knowledge, this is the first study” to describe this association, they said.
In an Italian study of 100 children with AD and 97 healthy children who served as controls, both clinical and laboratory data showed that FLG gene mutations were linked to early AD onset, a more severe clinical course of disease, and a significantly higher risk of Molluscum contagiosum virus (MCV)–associated skin infection.
The data “suggest that FLG gene may serve as a potential biomarker to screen the atopic population, identifying patients at high risk of AD, to adopt preventive measures that can restore the barrier function of the skin and reduce patients’ susceptibility to recurrent skin infection,” they wrote. “Our findings also indicate that the identified SNPs [single-nucleotide polymorphisms] might have clinically relevant implications with respect to a major MCV [M. contagiosum virus] colonization of skin, also permitting the identification of a new AD phenotype in children. However, to date, because of the existence of controversial data, we believe that further and needed insights into AD pathophysiology may also be gained by studying people with the FLG gene who do not have atopic predisposition and/or AD. Thus, a large population-based study is necessary to further validate these early interpretations and to gain detailed information about the role of FLG variants,” Dr. Manti and her coinvestigators concluded.
Read more in Manti S et al. Ann Allergy Asthma Immunol. 2017;119:446-51.
cnellist@frontlinemedcom.com
Filaggrin (FLG) polymorphism appears to be linked with Molluscum contagiosum virus skin infection in children with atopic dermatitis (AD), reported Sara Manti, MD, of the University of Messina (Italy), and her associates.
“To the best of our knowledge, this is the first study” to describe this association, they said.
In an Italian study of 100 children with AD and 97 healthy children who served as controls, both clinical and laboratory data showed that FLG gene mutations were linked to early AD onset, a more severe clinical course of disease, and a significantly higher risk of Molluscum contagiosum virus (MCV)–associated skin infection.
The data “suggest that FLG gene may serve as a potential biomarker to screen the atopic population, identifying patients at high risk of AD, to adopt preventive measures that can restore the barrier function of the skin and reduce patients’ susceptibility to recurrent skin infection,” they wrote. “Our findings also indicate that the identified SNPs [single-nucleotide polymorphisms] might have clinically relevant implications with respect to a major MCV [M. contagiosum virus] colonization of skin, also permitting the identification of a new AD phenotype in children. However, to date, because of the existence of controversial data, we believe that further and needed insights into AD pathophysiology may also be gained by studying people with the FLG gene who do not have atopic predisposition and/or AD. Thus, a large population-based study is necessary to further validate these early interpretations and to gain detailed information about the role of FLG variants,” Dr. Manti and her coinvestigators concluded.
Read more in Manti S et al. Ann Allergy Asthma Immunol. 2017;119:446-51.
cnellist@frontlinemedcom.com
Filaggrin (FLG) polymorphism appears to be linked with Molluscum contagiosum virus skin infection in children with atopic dermatitis (AD), reported Sara Manti, MD, of the University of Messina (Italy), and her associates.
“To the best of our knowledge, this is the first study” to describe this association, they said.
In an Italian study of 100 children with AD and 97 healthy children who served as controls, both clinical and laboratory data showed that FLG gene mutations were linked to early AD onset, a more severe clinical course of disease, and a significantly higher risk of Molluscum contagiosum virus (MCV)–associated skin infection.
The data “suggest that FLG gene may serve as a potential biomarker to screen the atopic population, identifying patients at high risk of AD, to adopt preventive measures that can restore the barrier function of the skin and reduce patients’ susceptibility to recurrent skin infection,” they wrote. “Our findings also indicate that the identified SNPs [single-nucleotide polymorphisms] might have clinically relevant implications with respect to a major MCV [M. contagiosum virus] colonization of skin, also permitting the identification of a new AD phenotype in children. However, to date, because of the existence of controversial data, we believe that further and needed insights into AD pathophysiology may also be gained by studying people with the FLG gene who do not have atopic predisposition and/or AD. Thus, a large population-based study is necessary to further validate these early interpretations and to gain detailed information about the role of FLG variants,” Dr. Manti and her coinvestigators concluded.
Read more in Manti S et al. Ann Allergy Asthma Immunol. 2017;119:446-51.
cnellist@frontlinemedcom.com
FROM THE ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY
Practice changing events of 2017
Members of the Pediatric News Editorial Advisory Board share some of the events and findings of 2017 that they believe have or will have the most impact on pediatric practice.
Francis Rushton Jr., MD, practiced pediatrics in Beaufort, S.C. for 32 years, and currently is the medical director of S.C. Quality through Technology and Innovation in Pediatrics (QTIP), funded by the South Carolina Department of Health and Human Services.
Dropping the human papillomavirus (HPV) regimen to two shots from three shots, as recommended by the Centers for Disease Control and Prevention, appears to have really improved uptake of HPV immunization.
Preventive oral health in the pediatrician’s office is not really a new recommendation from 2017; we have been talking about fluoride varnish for over a decade. What is new is that we gradually are seeing fluoride varnish move into practice, up from 1,000 applications in pediatric offices in 2011 to close to 20,000 applications in South Carolina alone.
Pediatricians are being asked to screen more and more. We’re asked to do developmental screening, postpartum depression screening, autism screening, behavioral health screening, social determinants of health screening, parental concerns screening, etc. As a result, we now have multiple different screens with different schedules. The Survey of Well-Being of Young Children screening tool does it all – one screen at each preschool well visit from birth to age 5 years.
A different approach is to use CHADIS (Child Health and Development Interactive System), a for-profit venture where all the screens are loaded electronically.
Howard Smart, MD, is chairman of pediatrics at Sharp Rees-Stealy Medical Group, San Diego.
The switch to a two-dose schedule for HPV vaccination has improved both acceptance of the vaccine and the likelihood of timely completion of the HPV series.
Kelly Curran, MD, MA, is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, practicing adolescent medicine.
The news from Australia is that the older meningitis B vaccine (MeNZB) provides some protection against Neisseria gonorrhoeae, as reported in the Lancet (2017 July 10. doi: 10.1016/S0140-6736[17]31449-6)! The newer version of the meningitis B vaccine Bexsero also contains the same outer membrane vesicle antigen. Given increasing bacterial resistance – and pan-resistant gonorrhea organisms already in some parts of the world – this is exciting news for the future!
The increased use of the reverse screening algorithm for syphilis is exciting. Although this has been “available” for several years, increasingly more physicians/laboratories are using this in practice. Our academic center – in a relatively high prevalence area for syphilis – recently switched to this screening method.
M. Susan Jay, MD, is a professor of pediatrics and section chief of adolescent medicine at the Medical College of Wisconsin and program director of adolescent health and medicine at the Children’s Hospital of Wisconsin, both in Milwaukee.
In adolescent medicine, the addition of long-acting reversible contraceptives has been wonderful as an aid to both menstrual management and contraception. Specifically, Liletta, a new IUD that is smaller in size and remains in place for 5 years as well as being considerably more cost effective, has changed care for adolescent females.
Suzanne C. Boulter, MD, is adjunct professor of pediatrics and community and family medicine at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
I was very impressed with the recent American Academy of Pediatrics policy on human trafficking published in Pediatrics (2017 November. doi: 10.1542/peds.2017-3138), and think this is a new area of knowledge of which pediatricians need to be aware.
With all the news and social media about sexual misconduct by persons in power, I’m a bit concerned that there could be a fallout on pediatricians performing appropriate examinations on their patients that could be interpreted as something else.
Timothy J. Joos, MD, MPH, is a practicing clinician in combined internal medicine/pediatrics in Seattle. For the last decade, he has worked at a federally qualified community health center in Seattle serving a largely low-income and immigrant population.
With regard to practice-changing events for 2017, I don’t wish to downplay the numerous research advances over the year, but the advances cannot be made without funding, and they are not going to be practice-changing if they can’t reach the patients. We can’t ignore the uncertainty that the current political situation in 2017 has caused for our patients and their families, as well as for research and for the health care industry in general.
It is impossible to deny the important role government health care programs play in the health of our own patients and the health of the whole country. According to numbers from the Kaiser Family Foundation website, currently 38% of the estimated 74 million kids in this country are covered by Medicaid and CHIP programs. The numbers of uninsured children are at all-time lows at 5% (adults 10%). The current uncertainty of government funding is felt strongly by safety net providers such as community health centers that have traditionally seen the uninsured patients. The community health center where I work went from 35% of its patients being uninsured before the Affordable Care Act to about 15% now.
Efforts to dismantle the Affordable Care Act and reverse Medicaid expansions, as well as delays on funding to the CHIP program, have created uncertainty and anxiety across health care from the administrators and insurance companies to us – the providers – and the families we take care of. In addition, National Institutes of Health funding is threatened to be cut by 20%. 2017 will go down in history as the year of health care toxic stress (that is, unless 2018 is worse). As we celebrate the end of the year, we all deserve a Xanax and a Zantac.
Members of the Pediatric News Editorial Advisory Board share some of the events and findings of 2017 that they believe have or will have the most impact on pediatric practice.
Francis Rushton Jr., MD, practiced pediatrics in Beaufort, S.C. for 32 years, and currently is the medical director of S.C. Quality through Technology and Innovation in Pediatrics (QTIP), funded by the South Carolina Department of Health and Human Services.
Dropping the human papillomavirus (HPV) regimen to two shots from three shots, as recommended by the Centers for Disease Control and Prevention, appears to have really improved uptake of HPV immunization.
Preventive oral health in the pediatrician’s office is not really a new recommendation from 2017; we have been talking about fluoride varnish for over a decade. What is new is that we gradually are seeing fluoride varnish move into practice, up from 1,000 applications in pediatric offices in 2011 to close to 20,000 applications in South Carolina alone.
Pediatricians are being asked to screen more and more. We’re asked to do developmental screening, postpartum depression screening, autism screening, behavioral health screening, social determinants of health screening, parental concerns screening, etc. As a result, we now have multiple different screens with different schedules. The Survey of Well-Being of Young Children screening tool does it all – one screen at each preschool well visit from birth to age 5 years.
A different approach is to use CHADIS (Child Health and Development Interactive System), a for-profit venture where all the screens are loaded electronically.
Howard Smart, MD, is chairman of pediatrics at Sharp Rees-Stealy Medical Group, San Diego.
The switch to a two-dose schedule for HPV vaccination has improved both acceptance of the vaccine and the likelihood of timely completion of the HPV series.
Kelly Curran, MD, MA, is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, practicing adolescent medicine.
The news from Australia is that the older meningitis B vaccine (MeNZB) provides some protection against Neisseria gonorrhoeae, as reported in the Lancet (2017 July 10. doi: 10.1016/S0140-6736[17]31449-6)! The newer version of the meningitis B vaccine Bexsero also contains the same outer membrane vesicle antigen. Given increasing bacterial resistance – and pan-resistant gonorrhea organisms already in some parts of the world – this is exciting news for the future!
The increased use of the reverse screening algorithm for syphilis is exciting. Although this has been “available” for several years, increasingly more physicians/laboratories are using this in practice. Our academic center – in a relatively high prevalence area for syphilis – recently switched to this screening method.
M. Susan Jay, MD, is a professor of pediatrics and section chief of adolescent medicine at the Medical College of Wisconsin and program director of adolescent health and medicine at the Children’s Hospital of Wisconsin, both in Milwaukee.
In adolescent medicine, the addition of long-acting reversible contraceptives has been wonderful as an aid to both menstrual management and contraception. Specifically, Liletta, a new IUD that is smaller in size and remains in place for 5 years as well as being considerably more cost effective, has changed care for adolescent females.
Suzanne C. Boulter, MD, is adjunct professor of pediatrics and community and family medicine at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
I was very impressed with the recent American Academy of Pediatrics policy on human trafficking published in Pediatrics (2017 November. doi: 10.1542/peds.2017-3138), and think this is a new area of knowledge of which pediatricians need to be aware.
With all the news and social media about sexual misconduct by persons in power, I’m a bit concerned that there could be a fallout on pediatricians performing appropriate examinations on their patients that could be interpreted as something else.
Timothy J. Joos, MD, MPH, is a practicing clinician in combined internal medicine/pediatrics in Seattle. For the last decade, he has worked at a federally qualified community health center in Seattle serving a largely low-income and immigrant population.
With regard to practice-changing events for 2017, I don’t wish to downplay the numerous research advances over the year, but the advances cannot be made without funding, and they are not going to be practice-changing if they can’t reach the patients. We can’t ignore the uncertainty that the current political situation in 2017 has caused for our patients and their families, as well as for research and for the health care industry in general.
It is impossible to deny the important role government health care programs play in the health of our own patients and the health of the whole country. According to numbers from the Kaiser Family Foundation website, currently 38% of the estimated 74 million kids in this country are covered by Medicaid and CHIP programs. The numbers of uninsured children are at all-time lows at 5% (adults 10%). The current uncertainty of government funding is felt strongly by safety net providers such as community health centers that have traditionally seen the uninsured patients. The community health center where I work went from 35% of its patients being uninsured before the Affordable Care Act to about 15% now.
Efforts to dismantle the Affordable Care Act and reverse Medicaid expansions, as well as delays on funding to the CHIP program, have created uncertainty and anxiety across health care from the administrators and insurance companies to us – the providers – and the families we take care of. In addition, National Institutes of Health funding is threatened to be cut by 20%. 2017 will go down in history as the year of health care toxic stress (that is, unless 2018 is worse). As we celebrate the end of the year, we all deserve a Xanax and a Zantac.
Members of the Pediatric News Editorial Advisory Board share some of the events and findings of 2017 that they believe have or will have the most impact on pediatric practice.
Francis Rushton Jr., MD, practiced pediatrics in Beaufort, S.C. for 32 years, and currently is the medical director of S.C. Quality through Technology and Innovation in Pediatrics (QTIP), funded by the South Carolina Department of Health and Human Services.
Dropping the human papillomavirus (HPV) regimen to two shots from three shots, as recommended by the Centers for Disease Control and Prevention, appears to have really improved uptake of HPV immunization.
Preventive oral health in the pediatrician’s office is not really a new recommendation from 2017; we have been talking about fluoride varnish for over a decade. What is new is that we gradually are seeing fluoride varnish move into practice, up from 1,000 applications in pediatric offices in 2011 to close to 20,000 applications in South Carolina alone.
Pediatricians are being asked to screen more and more. We’re asked to do developmental screening, postpartum depression screening, autism screening, behavioral health screening, social determinants of health screening, parental concerns screening, etc. As a result, we now have multiple different screens with different schedules. The Survey of Well-Being of Young Children screening tool does it all – one screen at each preschool well visit from birth to age 5 years.
A different approach is to use CHADIS (Child Health and Development Interactive System), a for-profit venture where all the screens are loaded electronically.
Howard Smart, MD, is chairman of pediatrics at Sharp Rees-Stealy Medical Group, San Diego.
The switch to a two-dose schedule for HPV vaccination has improved both acceptance of the vaccine and the likelihood of timely completion of the HPV series.
Kelly Curran, MD, MA, is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, practicing adolescent medicine.
The news from Australia is that the older meningitis B vaccine (MeNZB) provides some protection against Neisseria gonorrhoeae, as reported in the Lancet (2017 July 10. doi: 10.1016/S0140-6736[17]31449-6)! The newer version of the meningitis B vaccine Bexsero also contains the same outer membrane vesicle antigen. Given increasing bacterial resistance – and pan-resistant gonorrhea organisms already in some parts of the world – this is exciting news for the future!
The increased use of the reverse screening algorithm for syphilis is exciting. Although this has been “available” for several years, increasingly more physicians/laboratories are using this in practice. Our academic center – in a relatively high prevalence area for syphilis – recently switched to this screening method.
M. Susan Jay, MD, is a professor of pediatrics and section chief of adolescent medicine at the Medical College of Wisconsin and program director of adolescent health and medicine at the Children’s Hospital of Wisconsin, both in Milwaukee.
In adolescent medicine, the addition of long-acting reversible contraceptives has been wonderful as an aid to both menstrual management and contraception. Specifically, Liletta, a new IUD that is smaller in size and remains in place for 5 years as well as being considerably more cost effective, has changed care for adolescent females.
Suzanne C. Boulter, MD, is adjunct professor of pediatrics and community and family medicine at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
I was very impressed with the recent American Academy of Pediatrics policy on human trafficking published in Pediatrics (2017 November. doi: 10.1542/peds.2017-3138), and think this is a new area of knowledge of which pediatricians need to be aware.
With all the news and social media about sexual misconduct by persons in power, I’m a bit concerned that there could be a fallout on pediatricians performing appropriate examinations on their patients that could be interpreted as something else.
Timothy J. Joos, MD, MPH, is a practicing clinician in combined internal medicine/pediatrics in Seattle. For the last decade, he has worked at a federally qualified community health center in Seattle serving a largely low-income and immigrant population.
With regard to practice-changing events for 2017, I don’t wish to downplay the numerous research advances over the year, but the advances cannot be made without funding, and they are not going to be practice-changing if they can’t reach the patients. We can’t ignore the uncertainty that the current political situation in 2017 has caused for our patients and their families, as well as for research and for the health care industry in general.
It is impossible to deny the important role government health care programs play in the health of our own patients and the health of the whole country. According to numbers from the Kaiser Family Foundation website, currently 38% of the estimated 74 million kids in this country are covered by Medicaid and CHIP programs. The numbers of uninsured children are at all-time lows at 5% (adults 10%). The current uncertainty of government funding is felt strongly by safety net providers such as community health centers that have traditionally seen the uninsured patients. The community health center where I work went from 35% of its patients being uninsured before the Affordable Care Act to about 15% now.
Efforts to dismantle the Affordable Care Act and reverse Medicaid expansions, as well as delays on funding to the CHIP program, have created uncertainty and anxiety across health care from the administrators and insurance companies to us – the providers – and the families we take care of. In addition, National Institutes of Health funding is threatened to be cut by 20%. 2017 will go down in history as the year of health care toxic stress (that is, unless 2018 is worse). As we celebrate the end of the year, we all deserve a Xanax and a Zantac.
MMRV vaccine cut chickenpox hospitalizations in young Brazilian children
(VZV) in children aged 1-4 years, said Marcelo Comerlato Scotta and associates at Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil.
Of the 69,791 admissions caused by VZV in Brazilian patients younger than 20 years, the rate of such hospitalizations for children aged 1-4 years significantly decreased from 27 cases per 100,000 children per year to 14 cases per 100,000 children per year after the vaccine was introduced, a reduction of 48% (P < .001). Changes in other age groups were not significant. That decrease in the rate of VZV admissions remained statistically significant in the vaccinated group after adjusting for seasonality (P < .001).
Direct costs of VZV-related admissions dropped 38% after introducing the MMRV vaccine, the researchers said. “Further studies are needed to evaluate long-term direct and indirect impact on the epidemiology of VZV infections.”
Read more in Vaccine (2017 Dec 1. doi: 10.1016/j.vaccine.2017.11.057.)
(VZV) in children aged 1-4 years, said Marcelo Comerlato Scotta and associates at Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil.
Of the 69,791 admissions caused by VZV in Brazilian patients younger than 20 years, the rate of such hospitalizations for children aged 1-4 years significantly decreased from 27 cases per 100,000 children per year to 14 cases per 100,000 children per year after the vaccine was introduced, a reduction of 48% (P < .001). Changes in other age groups were not significant. That decrease in the rate of VZV admissions remained statistically significant in the vaccinated group after adjusting for seasonality (P < .001).
Direct costs of VZV-related admissions dropped 38% after introducing the MMRV vaccine, the researchers said. “Further studies are needed to evaluate long-term direct and indirect impact on the epidemiology of VZV infections.”
Read more in Vaccine (2017 Dec 1. doi: 10.1016/j.vaccine.2017.11.057.)
(VZV) in children aged 1-4 years, said Marcelo Comerlato Scotta and associates at Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil.
Of the 69,791 admissions caused by VZV in Brazilian patients younger than 20 years, the rate of such hospitalizations for children aged 1-4 years significantly decreased from 27 cases per 100,000 children per year to 14 cases per 100,000 children per year after the vaccine was introduced, a reduction of 48% (P < .001). Changes in other age groups were not significant. That decrease in the rate of VZV admissions remained statistically significant in the vaccinated group after adjusting for seasonality (P < .001).
Direct costs of VZV-related admissions dropped 38% after introducing the MMRV vaccine, the researchers said. “Further studies are needed to evaluate long-term direct and indirect impact on the epidemiology of VZV infections.”
Read more in Vaccine (2017 Dec 1. doi: 10.1016/j.vaccine.2017.11.057.)
FROM VACCINE
PCVs reduced CAP hospitalizations in young children but not other age groups
, but there was no clear impact apparent in other age groups, reported Annemarie van Deursen, MD, of the University Medical Centre (the Netherlands) Utrecht, and her associates.
In the Netherlands, the 7-valent pneumococcal conjugate vaccine (PCV7) was added to the national infant immunization program in 2006; in 2011, PCV7 was replaced by the 10-valent vaccine (PCV10). The investigators undertook a population-based retrospective study during 1999-2014 on all-cause CAP hospitalizations in all ages, identifying 155,994 CAP hospitalizations.
In children aged 0-6 months, the CAP hospitalization rate ratio (RR) was significant from 2012 onward, with an overall post-PCV RR of 0.62 and a RR of 0.19 at the end of the study period in December 2014. In children aged 6 months-1 year, the RR was statistically significant directly after the introduction of PCV, with an overall post-PCV RR of 0.67 and a RR of 0.47 in December 2014, the investigators wrote.
In none of the other age groups did the overall post-PCV hospitalization RR reach statistical significance.
The association of reductions in CAP hospitalizations in children up to 2 years with the introduction of PCV7 “supports the interpretation for a direct causal effect of PCV7, in line with IPD [invasive pneumococcal disease] results that showed a sustained overall IPD reduction in children,” the investigators said. “Furthermore, [during] each subsequent year of the post-PCV period, the reduction in CAP hospitalization rates increased in line with progressive vaccine-type–IPD reduction in the population and limited replacement by nonvaccine type in childhood IPD.”
Read more in Vaccine (2017 Nov 13. doi: 10.1016/j.vaccine.2017.10.090).
, but there was no clear impact apparent in other age groups, reported Annemarie van Deursen, MD, of the University Medical Centre (the Netherlands) Utrecht, and her associates.
In the Netherlands, the 7-valent pneumococcal conjugate vaccine (PCV7) was added to the national infant immunization program in 2006; in 2011, PCV7 was replaced by the 10-valent vaccine (PCV10). The investigators undertook a population-based retrospective study during 1999-2014 on all-cause CAP hospitalizations in all ages, identifying 155,994 CAP hospitalizations.
In children aged 0-6 months, the CAP hospitalization rate ratio (RR) was significant from 2012 onward, with an overall post-PCV RR of 0.62 and a RR of 0.19 at the end of the study period in December 2014. In children aged 6 months-1 year, the RR was statistically significant directly after the introduction of PCV, with an overall post-PCV RR of 0.67 and a RR of 0.47 in December 2014, the investigators wrote.
In none of the other age groups did the overall post-PCV hospitalization RR reach statistical significance.
The association of reductions in CAP hospitalizations in children up to 2 years with the introduction of PCV7 “supports the interpretation for a direct causal effect of PCV7, in line with IPD [invasive pneumococcal disease] results that showed a sustained overall IPD reduction in children,” the investigators said. “Furthermore, [during] each subsequent year of the post-PCV period, the reduction in CAP hospitalization rates increased in line with progressive vaccine-type–IPD reduction in the population and limited replacement by nonvaccine type in childhood IPD.”
Read more in Vaccine (2017 Nov 13. doi: 10.1016/j.vaccine.2017.10.090).
, but there was no clear impact apparent in other age groups, reported Annemarie van Deursen, MD, of the University Medical Centre (the Netherlands) Utrecht, and her associates.
In the Netherlands, the 7-valent pneumococcal conjugate vaccine (PCV7) was added to the national infant immunization program in 2006; in 2011, PCV7 was replaced by the 10-valent vaccine (PCV10). The investigators undertook a population-based retrospective study during 1999-2014 on all-cause CAP hospitalizations in all ages, identifying 155,994 CAP hospitalizations.
In children aged 0-6 months, the CAP hospitalization rate ratio (RR) was significant from 2012 onward, with an overall post-PCV RR of 0.62 and a RR of 0.19 at the end of the study period in December 2014. In children aged 6 months-1 year, the RR was statistically significant directly after the introduction of PCV, with an overall post-PCV RR of 0.67 and a RR of 0.47 in December 2014, the investigators wrote.
In none of the other age groups did the overall post-PCV hospitalization RR reach statistical significance.
The association of reductions in CAP hospitalizations in children up to 2 years with the introduction of PCV7 “supports the interpretation for a direct causal effect of PCV7, in line with IPD [invasive pneumococcal disease] results that showed a sustained overall IPD reduction in children,” the investigators said. “Furthermore, [during] each subsequent year of the post-PCV period, the reduction in CAP hospitalization rates increased in line with progressive vaccine-type–IPD reduction in the population and limited replacement by nonvaccine type in childhood IPD.”
Read more in Vaccine (2017 Nov 13. doi: 10.1016/j.vaccine.2017.10.090).
FROM VACCINE
Flu vaccine did not protect children with acute leukemia
said April Sykes of St. Jude Children’s Research Hospital in Carmel, Ind., and her associates.
Patients aged 1-21 years being treated for acute leukemia during three successive influenza seasons (2011-2012, 2012-2013, and 2013-2014) were identified by a retrospective review of EHRs; of those patients, 354 (71%) patients received TIV, and 98 (20%) received a booster dose of flu vaccine.
Also, whether the children and youth received one or two doses of flu vaccine made no difference in the rates of influenza (0.60 vs. 1.02; P = .107), the investigators reported.
These data suggest “that influenza vaccine may be ineffective in children receiving therapy for acute leukemia and that routine administration of TIV may not reflect high-value care,” the researchers said. “Until more immunogenic and protective vaccines are developed, efforts to prevent influenza in high-risk populations should focus on more general strategies, such as avoiding ill persons and practicing good respiratory hygiene in households and health care facilities.”
Read more in the Journal of Pediatrics (2017 Nov 21. doi: 10.1016/j.jpeds.2017.08.071).
said April Sykes of St. Jude Children’s Research Hospital in Carmel, Ind., and her associates.
Patients aged 1-21 years being treated for acute leukemia during three successive influenza seasons (2011-2012, 2012-2013, and 2013-2014) were identified by a retrospective review of EHRs; of those patients, 354 (71%) patients received TIV, and 98 (20%) received a booster dose of flu vaccine.
Also, whether the children and youth received one or two doses of flu vaccine made no difference in the rates of influenza (0.60 vs. 1.02; P = .107), the investigators reported.
These data suggest “that influenza vaccine may be ineffective in children receiving therapy for acute leukemia and that routine administration of TIV may not reflect high-value care,” the researchers said. “Until more immunogenic and protective vaccines are developed, efforts to prevent influenza in high-risk populations should focus on more general strategies, such as avoiding ill persons and practicing good respiratory hygiene in households and health care facilities.”
Read more in the Journal of Pediatrics (2017 Nov 21. doi: 10.1016/j.jpeds.2017.08.071).
said April Sykes of St. Jude Children’s Research Hospital in Carmel, Ind., and her associates.
Patients aged 1-21 years being treated for acute leukemia during three successive influenza seasons (2011-2012, 2012-2013, and 2013-2014) were identified by a retrospective review of EHRs; of those patients, 354 (71%) patients received TIV, and 98 (20%) received a booster dose of flu vaccine.
Also, whether the children and youth received one or two doses of flu vaccine made no difference in the rates of influenza (0.60 vs. 1.02; P = .107), the investigators reported.
These data suggest “that influenza vaccine may be ineffective in children receiving therapy for acute leukemia and that routine administration of TIV may not reflect high-value care,” the researchers said. “Until more immunogenic and protective vaccines are developed, efforts to prevent influenza in high-risk populations should focus on more general strategies, such as avoiding ill persons and practicing good respiratory hygiene in households and health care facilities.”
Read more in the Journal of Pediatrics (2017 Nov 21. doi: 10.1016/j.jpeds.2017.08.071).
FROM THE JOURNAL OF PEDIATRICS
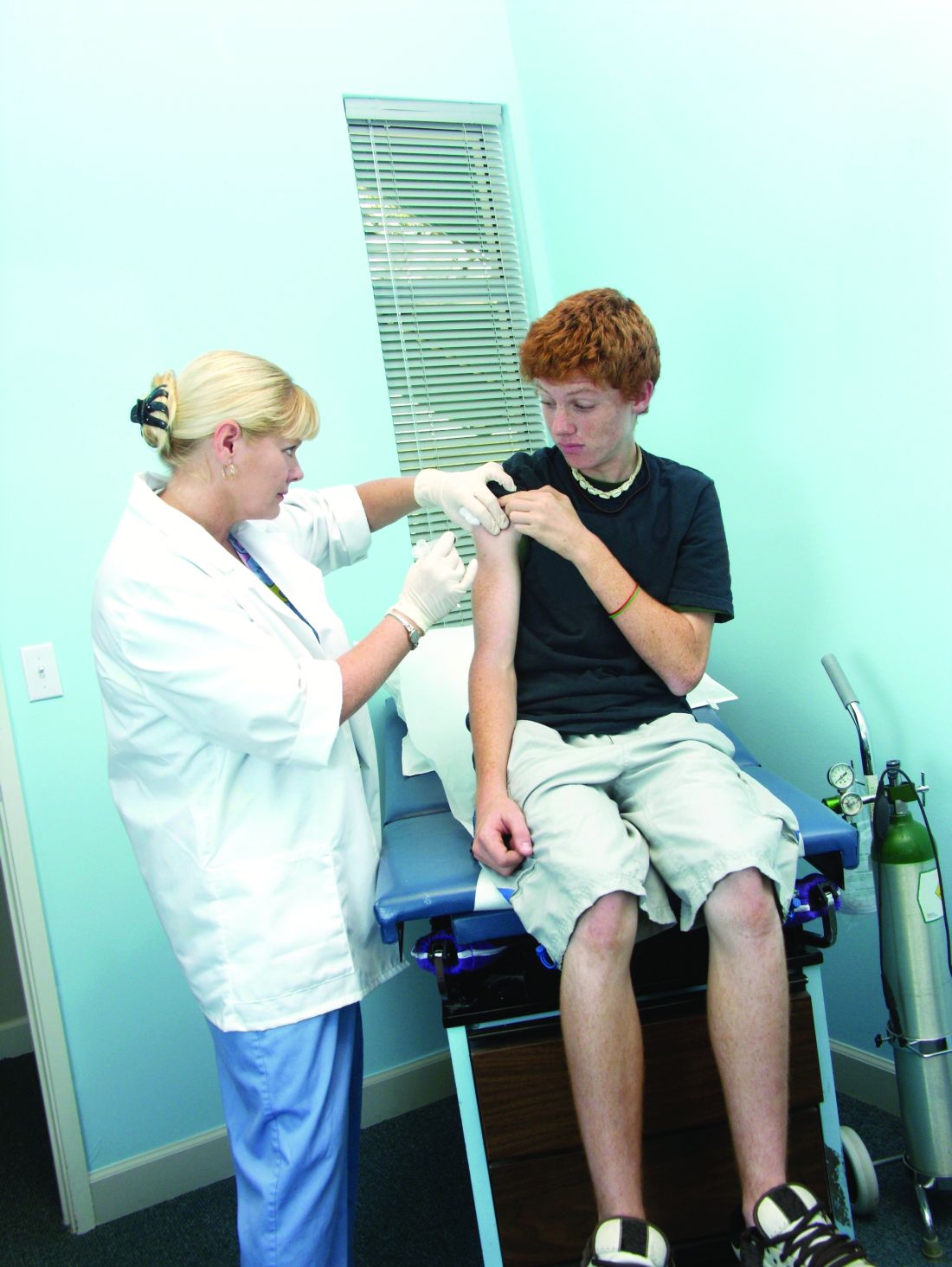
More states allowing pharmacists to administer vaccines to younger patients
.
Since the 1990s, states have made laws to increase access to immunization services by giving pharmacists authority to give vaccines, said Cason D. Schmit, JD, of Texas A&M University at College Station, and Matthew S. Penn, JD, director of the Public Health Law Program at the Centers for Disease Control and Prevention, Atlanta. This has the advantage of pharmacies being open longer hours than most physicians’ offices, in addition to the opportunities for immunizing people in rural locations as well as those people without a regular physician.
Yet barriers to pharmacists’ providing immunization services remain because of some state laws. Laws in nine states that prevent pharmacists from vaccinating patients younger than age 18 years keep pharmacists from administering any of the vaccines listed in the 2016 Advisory Committee of Immunization Practices child immunization schedule. The two states in which pharmacists can vaccinate patients as young as 14 years allow the pharmacists to administer only the recommended booster for meningococcal vaccine and annual influenza vaccines for children. And the 15 states with minimum patient age restrictions for 7- to 12-year-oldss allow pharmacists to administer only the four vaccines ACIP recommends on the 2016 schedule for children (meningococcal, Tdap, human papillomavirus, and annual influenza vaccines).
Read more in the Journal of the American Pharmacists Association (2017 Nov-Dec;57[6]:661-9).
.
Since the 1990s, states have made laws to increase access to immunization services by giving pharmacists authority to give vaccines, said Cason D. Schmit, JD, of Texas A&M University at College Station, and Matthew S. Penn, JD, director of the Public Health Law Program at the Centers for Disease Control and Prevention, Atlanta. This has the advantage of pharmacies being open longer hours than most physicians’ offices, in addition to the opportunities for immunizing people in rural locations as well as those people without a regular physician.
Yet barriers to pharmacists’ providing immunization services remain because of some state laws. Laws in nine states that prevent pharmacists from vaccinating patients younger than age 18 years keep pharmacists from administering any of the vaccines listed in the 2016 Advisory Committee of Immunization Practices child immunization schedule. The two states in which pharmacists can vaccinate patients as young as 14 years allow the pharmacists to administer only the recommended booster for meningococcal vaccine and annual influenza vaccines for children. And the 15 states with minimum patient age restrictions for 7- to 12-year-oldss allow pharmacists to administer only the four vaccines ACIP recommends on the 2016 schedule for children (meningococcal, Tdap, human papillomavirus, and annual influenza vaccines).
Read more in the Journal of the American Pharmacists Association (2017 Nov-Dec;57[6]:661-9).
.
Since the 1990s, states have made laws to increase access to immunization services by giving pharmacists authority to give vaccines, said Cason D. Schmit, JD, of Texas A&M University at College Station, and Matthew S. Penn, JD, director of the Public Health Law Program at the Centers for Disease Control and Prevention, Atlanta. This has the advantage of pharmacies being open longer hours than most physicians’ offices, in addition to the opportunities for immunizing people in rural locations as well as those people without a regular physician.
Yet barriers to pharmacists’ providing immunization services remain because of some state laws. Laws in nine states that prevent pharmacists from vaccinating patients younger than age 18 years keep pharmacists from administering any of the vaccines listed in the 2016 Advisory Committee of Immunization Practices child immunization schedule. The two states in which pharmacists can vaccinate patients as young as 14 years allow the pharmacists to administer only the recommended booster for meningococcal vaccine and annual influenza vaccines for children. And the 15 states with minimum patient age restrictions for 7- to 12-year-oldss allow pharmacists to administer only the four vaccines ACIP recommends on the 2016 schedule for children (meningococcal, Tdap, human papillomavirus, and annual influenza vaccines).
Read more in the Journal of the American Pharmacists Association (2017 Nov-Dec;57[6]:661-9).
FROM THE JOURNAL OF THE AMERICAN PHARMACISTS ASSOCIATION
Parents taking photos of kids’ lesions for telederm diagnosis looks promising
, said Daniel M. O’Connor, MD, of the Children’s Hospital of Philadelphia, and his associates.
Skin conditions make up 10%-30% of the approximately 200 million pediatric outpatient visits each year, Dr. O’Connor and his colleagues said. But there are fewer than 300 board-certified U.S. pediatric dermatologists for the nation’s nearly 75 million children. So, the possibility of using photos taken by parents for distant pediatric dermatologists to assess is an attractive one.
Concordance between photograph-based vs. in-person diagnosis was 83%. In three cases, diagnoses could not be made by the remote dermatologist because of poor photograph quality. When those cases were excluded, concordance was 89% between photograph-based vs. in-person diagnosis. Concordance for birthmarks was 100%, 92% for rashes, and 64% for alopecia-related diagnoses. Of four cases that were misdiagnosed, there were three cases of alopecia and one nodule.
Half the parents received a simple, three-step instruction sheet on smartphone photography. There was no statistical difference in diagnostic concordance between the parents who received the instruction sheet and those who didn’t.
“When dealing with categories with low concordance, such as alopecia and nodules and tumors, teledermatology practitioners may need to be cautious about attempting definitive diagnoses in some cases, and may need to refer patients for in-person consultation,” Dr. O’Connor and his associates wrote. “For these cases, teledermatology may still serve as a triage tool. For example, patients with suspicious nodules could be referred for expedited appointments in specialty clinics, whereas patients with isolated alopecia could be scheduled for routine visits. Conversely, in diagnostic categories with high concordance, such as birthmarks and rashes, certain cases could be definitively diagnosed and treated exclusively using teledermatology (for example, mild acne).”
Read more in JAMA Dermatology (2017 Nov 15. doi: 10.1001/jamadermatol.2017.4280).
, said Daniel M. O’Connor, MD, of the Children’s Hospital of Philadelphia, and his associates.
Skin conditions make up 10%-30% of the approximately 200 million pediatric outpatient visits each year, Dr. O’Connor and his colleagues said. But there are fewer than 300 board-certified U.S. pediatric dermatologists for the nation’s nearly 75 million children. So, the possibility of using photos taken by parents for distant pediatric dermatologists to assess is an attractive one.
Concordance between photograph-based vs. in-person diagnosis was 83%. In three cases, diagnoses could not be made by the remote dermatologist because of poor photograph quality. When those cases were excluded, concordance was 89% between photograph-based vs. in-person diagnosis. Concordance for birthmarks was 100%, 92% for rashes, and 64% for alopecia-related diagnoses. Of four cases that were misdiagnosed, there were three cases of alopecia and one nodule.
Half the parents received a simple, three-step instruction sheet on smartphone photography. There was no statistical difference in diagnostic concordance between the parents who received the instruction sheet and those who didn’t.
“When dealing with categories with low concordance, such as alopecia and nodules and tumors, teledermatology practitioners may need to be cautious about attempting definitive diagnoses in some cases, and may need to refer patients for in-person consultation,” Dr. O’Connor and his associates wrote. “For these cases, teledermatology may still serve as a triage tool. For example, patients with suspicious nodules could be referred for expedited appointments in specialty clinics, whereas patients with isolated alopecia could be scheduled for routine visits. Conversely, in diagnostic categories with high concordance, such as birthmarks and rashes, certain cases could be definitively diagnosed and treated exclusively using teledermatology (for example, mild acne).”
Read more in JAMA Dermatology (2017 Nov 15. doi: 10.1001/jamadermatol.2017.4280).
, said Daniel M. O’Connor, MD, of the Children’s Hospital of Philadelphia, and his associates.
Skin conditions make up 10%-30% of the approximately 200 million pediatric outpatient visits each year, Dr. O’Connor and his colleagues said. But there are fewer than 300 board-certified U.S. pediatric dermatologists for the nation’s nearly 75 million children. So, the possibility of using photos taken by parents for distant pediatric dermatologists to assess is an attractive one.
Concordance between photograph-based vs. in-person diagnosis was 83%. In three cases, diagnoses could not be made by the remote dermatologist because of poor photograph quality. When those cases were excluded, concordance was 89% between photograph-based vs. in-person diagnosis. Concordance for birthmarks was 100%, 92% for rashes, and 64% for alopecia-related diagnoses. Of four cases that were misdiagnosed, there were three cases of alopecia and one nodule.
Half the parents received a simple, three-step instruction sheet on smartphone photography. There was no statistical difference in diagnostic concordance between the parents who received the instruction sheet and those who didn’t.
“When dealing with categories with low concordance, such as alopecia and nodules and tumors, teledermatology practitioners may need to be cautious about attempting definitive diagnoses in some cases, and may need to refer patients for in-person consultation,” Dr. O’Connor and his associates wrote. “For these cases, teledermatology may still serve as a triage tool. For example, patients with suspicious nodules could be referred for expedited appointments in specialty clinics, whereas patients with isolated alopecia could be scheduled for routine visits. Conversely, in diagnostic categories with high concordance, such as birthmarks and rashes, certain cases could be definitively diagnosed and treated exclusively using teledermatology (for example, mild acne).”
Read more in JAMA Dermatology (2017 Nov 15. doi: 10.1001/jamadermatol.2017.4280).
FROM JAMA DERMATOLOGY
Adolescents with chronic health conditions often undervaccinated
said Annika M. Hofstetter, MD, PhD, of Columbia University, New York, and her associates.
The National Health Interview Survey on Disability in 1994-1995 estimated that chronic conditions of any type affected 15%-18% of U.S. children and adolescents. The Advisory Committee on Immunization Practices recommends that all adolescents, whether or not they have chronic medical condition, be vaccinated with human papillomavirus (HPV), Tdap, meningococcal, and flu vaccines.
Fewer adolescents with CMCs had received one more doses of HPV (81%), than did those without CMCs (85%; P less than .01). Fewer adolescents with epilepsy (63%), mental retardation (58%), cerebral palsy (54%), and autism spectrum disorder (46%) had started HPV vaccination, compared with those without each of these conditions (84%; all comparisons, P less than .001). No differences were seen for asthma or congenital heart disease, the investigators said.
More adolescents with CMCs had gotten their flu shot than did those without CMCs during the 2011-2012 season (67% vs. 50%; P less than .001) or during the 2012-2013 season (74% vs. 65%; P less than .001). More adolescents with asthma got their flu shot than did those without asthma during the 2011-2012 season (69% vs. 51%; P less than .001) or during the 2012-2013 season (74% vs. 65%; P less than .001). No differences were seen for the other common CMCs.
Nonetheless, the mean number of missed opportunities was significantly higher among unvaccinated adolescents with CMCs, compared with those without CMCs, for the first HPV vaccination, meningococcal vaccination, and influenza vaccination in both seasons measured (P less than .001 for all).
“Missed opportunities for the third HPV vaccine dose or Tdap did not differ by CMC status,” Dr. Hofstetter and her associates said.
Read more in the American Journal of Preventive Medicine (2017 Nov;53[5]:680-8).
said Annika M. Hofstetter, MD, PhD, of Columbia University, New York, and her associates.
The National Health Interview Survey on Disability in 1994-1995 estimated that chronic conditions of any type affected 15%-18% of U.S. children and adolescents. The Advisory Committee on Immunization Practices recommends that all adolescents, whether or not they have chronic medical condition, be vaccinated with human papillomavirus (HPV), Tdap, meningococcal, and flu vaccines.
Fewer adolescents with CMCs had received one more doses of HPV (81%), than did those without CMCs (85%; P less than .01). Fewer adolescents with epilepsy (63%), mental retardation (58%), cerebral palsy (54%), and autism spectrum disorder (46%) had started HPV vaccination, compared with those without each of these conditions (84%; all comparisons, P less than .001). No differences were seen for asthma or congenital heart disease, the investigators said.
More adolescents with CMCs had gotten their flu shot than did those without CMCs during the 2011-2012 season (67% vs. 50%; P less than .001) or during the 2012-2013 season (74% vs. 65%; P less than .001). More adolescents with asthma got their flu shot than did those without asthma during the 2011-2012 season (69% vs. 51%; P less than .001) or during the 2012-2013 season (74% vs. 65%; P less than .001). No differences were seen for the other common CMCs.
Nonetheless, the mean number of missed opportunities was significantly higher among unvaccinated adolescents with CMCs, compared with those without CMCs, for the first HPV vaccination, meningococcal vaccination, and influenza vaccination in both seasons measured (P less than .001 for all).
“Missed opportunities for the third HPV vaccine dose or Tdap did not differ by CMC status,” Dr. Hofstetter and her associates said.
Read more in the American Journal of Preventive Medicine (2017 Nov;53[5]:680-8).
said Annika M. Hofstetter, MD, PhD, of Columbia University, New York, and her associates.
The National Health Interview Survey on Disability in 1994-1995 estimated that chronic conditions of any type affected 15%-18% of U.S. children and adolescents. The Advisory Committee on Immunization Practices recommends that all adolescents, whether or not they have chronic medical condition, be vaccinated with human papillomavirus (HPV), Tdap, meningococcal, and flu vaccines.
Fewer adolescents with CMCs had received one more doses of HPV (81%), than did those without CMCs (85%; P less than .01). Fewer adolescents with epilepsy (63%), mental retardation (58%), cerebral palsy (54%), and autism spectrum disorder (46%) had started HPV vaccination, compared with those without each of these conditions (84%; all comparisons, P less than .001). No differences were seen for asthma or congenital heart disease, the investigators said.
More adolescents with CMCs had gotten their flu shot than did those without CMCs during the 2011-2012 season (67% vs. 50%; P less than .001) or during the 2012-2013 season (74% vs. 65%; P less than .001). More adolescents with asthma got their flu shot than did those without asthma during the 2011-2012 season (69% vs. 51%; P less than .001) or during the 2012-2013 season (74% vs. 65%; P less than .001). No differences were seen for the other common CMCs.
Nonetheless, the mean number of missed opportunities was significantly higher among unvaccinated adolescents with CMCs, compared with those without CMCs, for the first HPV vaccination, meningococcal vaccination, and influenza vaccination in both seasons measured (P less than .001 for all).
“Missed opportunities for the third HPV vaccine dose or Tdap did not differ by CMC status,” Dr. Hofstetter and her associates said.
Read more in the American Journal of Preventive Medicine (2017 Nov;53[5]:680-8).
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE