User login
Radiation-associated childhood cancer quantified in congenital heart disease
Children with congenital heart disease exposed to low-dose ionizing radiation from cardiac procedures had a cancer risk more than triple that of pediatric congenital heart disease (CHD) patients without such exposures, according to a large Canadian nested case-control study presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
This cancer risk was dose dependent. It rose stepwise with the number of cardiac procedures involving exposure to low-dose ionizing radiation (LDIR) and the total radiation dose. Moreover, roughly 80% of the cancers were of types known to be associated with radiation exposure in children, reported Elie Ganni, a medical student at McGill University, Montreal, working with MAUDE, the McGill Adult Unit for Congenital Heart Disease.
The MAUDE group previously published the first large, population-based study analyzing the association between LDIR from cardiac procedures and incident cancer in adults with CHD. The study, which included nearly 25,000 adult CHD patients aged 18-64 years with more than 250,000 person-years of follow-up, concluded that individuals with LDIR exposure from six or more cardiac procedures had a 140% greater cancer incidence than those with no or one exposure (Circulation. 2018 Mar 27;137[13]:1334-45).
Because children are considered to be more sensitive to the carcinogenic effects of LDIR than adults, the MAUDE group next did a similar study in a pediatric CHD population included in the Quebec Congenital Heart Disease Database. This nested case-control study included 232 children with CHD who were first diagnosed with cancer at a median age of 3.9 years and 8,160 pediatric CHD controls matched for gender and birth year. About 76% of cancers were diagnosed before age 7, 20% at ages 7-12 years, and the remaining 4% at ages 13-18. Hematologic malignancies accounted for 61% of the pediatric cancers, CNS cancers for another 12.5%, and thyroid cancers 6.6%; all three types of cancer are associated with radiation exposure.
After excluding all cardiac procedures involving LDIR performed within 6 months prior to cancer diagnosis, the risk of developing a pediatric cancer was 230% greater in children with LDIR exposure from cardiac procedures than in CHD patients without such exposure. For every 4 mSv in estimated LDIR exposure from cardiac procedures, the risk of cancer rose by 15.5%. In contrast, in the earlier study in adults with CHD, cancer risk climbed by 10% per 10 mSv. Patients with six or more LDIR cardiac procedures – not at all unusual in contemporary practice – were 2.4 times more likely to have cancer than those with no or one such radiation exposure.
Current ACC guidelines on radiation exposure from cardiac procedures recommend calculating an individual’s lifetime attributable cancer incidence and mortality risks, as well as adhering to the time-honored principle of ensuring that radiation exposure is as low as reasonably achievable without sacrificing quality of care.
“Our findings strongly support these ACC recommendations and moreover suggest that radiation surveillance for patients with congenital heart disease should be considered using radiation badges. Also, cancer surveillance guidelines should be considered for CHD patients exposed to LDIR,” Mr. Ganni said.
These suggestions for creation of patient radiation passports and cancer surveillance guidelines take on greater weight in light of two trends: the increasing life expectancy of children with CHD during the past 3 decades as a result of procedural advances that entail LDIR exposure, mostly for imaging, and the growing number of such procedures performed per patient earlier and earlier in life.
He and the MAUDE group plan to confirm their latest findings in other, larger data sets and hope to identify threshold effects for LDIR for specific cancers, with hematologic malignancies as the top priority.
Mr. Ganni reported having no financial conflicts regarding his study, funded by the Heart and Stroke Foundation of Canada, the Quebec Foundation for Health Research, and the Canadian Institutes for Health Research.
Children with congenital heart disease exposed to low-dose ionizing radiation from cardiac procedures had a cancer risk more than triple that of pediatric congenital heart disease (CHD) patients without such exposures, according to a large Canadian nested case-control study presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
This cancer risk was dose dependent. It rose stepwise with the number of cardiac procedures involving exposure to low-dose ionizing radiation (LDIR) and the total radiation dose. Moreover, roughly 80% of the cancers were of types known to be associated with radiation exposure in children, reported Elie Ganni, a medical student at McGill University, Montreal, working with MAUDE, the McGill Adult Unit for Congenital Heart Disease.
The MAUDE group previously published the first large, population-based study analyzing the association between LDIR from cardiac procedures and incident cancer in adults with CHD. The study, which included nearly 25,000 adult CHD patients aged 18-64 years with more than 250,000 person-years of follow-up, concluded that individuals with LDIR exposure from six or more cardiac procedures had a 140% greater cancer incidence than those with no or one exposure (Circulation. 2018 Mar 27;137[13]:1334-45).
Because children are considered to be more sensitive to the carcinogenic effects of LDIR than adults, the MAUDE group next did a similar study in a pediatric CHD population included in the Quebec Congenital Heart Disease Database. This nested case-control study included 232 children with CHD who were first diagnosed with cancer at a median age of 3.9 years and 8,160 pediatric CHD controls matched for gender and birth year. About 76% of cancers were diagnosed before age 7, 20% at ages 7-12 years, and the remaining 4% at ages 13-18. Hematologic malignancies accounted for 61% of the pediatric cancers, CNS cancers for another 12.5%, and thyroid cancers 6.6%; all three types of cancer are associated with radiation exposure.
After excluding all cardiac procedures involving LDIR performed within 6 months prior to cancer diagnosis, the risk of developing a pediatric cancer was 230% greater in children with LDIR exposure from cardiac procedures than in CHD patients without such exposure. For every 4 mSv in estimated LDIR exposure from cardiac procedures, the risk of cancer rose by 15.5%. In contrast, in the earlier study in adults with CHD, cancer risk climbed by 10% per 10 mSv. Patients with six or more LDIR cardiac procedures – not at all unusual in contemporary practice – were 2.4 times more likely to have cancer than those with no or one such radiation exposure.
Current ACC guidelines on radiation exposure from cardiac procedures recommend calculating an individual’s lifetime attributable cancer incidence and mortality risks, as well as adhering to the time-honored principle of ensuring that radiation exposure is as low as reasonably achievable without sacrificing quality of care.
“Our findings strongly support these ACC recommendations and moreover suggest that radiation surveillance for patients with congenital heart disease should be considered using radiation badges. Also, cancer surveillance guidelines should be considered for CHD patients exposed to LDIR,” Mr. Ganni said.
These suggestions for creation of patient radiation passports and cancer surveillance guidelines take on greater weight in light of two trends: the increasing life expectancy of children with CHD during the past 3 decades as a result of procedural advances that entail LDIR exposure, mostly for imaging, and the growing number of such procedures performed per patient earlier and earlier in life.
He and the MAUDE group plan to confirm their latest findings in other, larger data sets and hope to identify threshold effects for LDIR for specific cancers, with hematologic malignancies as the top priority.
Mr. Ganni reported having no financial conflicts regarding his study, funded by the Heart and Stroke Foundation of Canada, the Quebec Foundation for Health Research, and the Canadian Institutes for Health Research.
Children with congenital heart disease exposed to low-dose ionizing radiation from cardiac procedures had a cancer risk more than triple that of pediatric congenital heart disease (CHD) patients without such exposures, according to a large Canadian nested case-control study presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
This cancer risk was dose dependent. It rose stepwise with the number of cardiac procedures involving exposure to low-dose ionizing radiation (LDIR) and the total radiation dose. Moreover, roughly 80% of the cancers were of types known to be associated with radiation exposure in children, reported Elie Ganni, a medical student at McGill University, Montreal, working with MAUDE, the McGill Adult Unit for Congenital Heart Disease.
The MAUDE group previously published the first large, population-based study analyzing the association between LDIR from cardiac procedures and incident cancer in adults with CHD. The study, which included nearly 25,000 adult CHD patients aged 18-64 years with more than 250,000 person-years of follow-up, concluded that individuals with LDIR exposure from six or more cardiac procedures had a 140% greater cancer incidence than those with no or one exposure (Circulation. 2018 Mar 27;137[13]:1334-45).
Because children are considered to be more sensitive to the carcinogenic effects of LDIR than adults, the MAUDE group next did a similar study in a pediatric CHD population included in the Quebec Congenital Heart Disease Database. This nested case-control study included 232 children with CHD who were first diagnosed with cancer at a median age of 3.9 years and 8,160 pediatric CHD controls matched for gender and birth year. About 76% of cancers were diagnosed before age 7, 20% at ages 7-12 years, and the remaining 4% at ages 13-18. Hematologic malignancies accounted for 61% of the pediatric cancers, CNS cancers for another 12.5%, and thyroid cancers 6.6%; all three types of cancer are associated with radiation exposure.
After excluding all cardiac procedures involving LDIR performed within 6 months prior to cancer diagnosis, the risk of developing a pediatric cancer was 230% greater in children with LDIR exposure from cardiac procedures than in CHD patients without such exposure. For every 4 mSv in estimated LDIR exposure from cardiac procedures, the risk of cancer rose by 15.5%. In contrast, in the earlier study in adults with CHD, cancer risk climbed by 10% per 10 mSv. Patients with six or more LDIR cardiac procedures – not at all unusual in contemporary practice – were 2.4 times more likely to have cancer than those with no or one such radiation exposure.
Current ACC guidelines on radiation exposure from cardiac procedures recommend calculating an individual’s lifetime attributable cancer incidence and mortality risks, as well as adhering to the time-honored principle of ensuring that radiation exposure is as low as reasonably achievable without sacrificing quality of care.
“Our findings strongly support these ACC recommendations and moreover suggest that radiation surveillance for patients with congenital heart disease should be considered using radiation badges. Also, cancer surveillance guidelines should be considered for CHD patients exposed to LDIR,” Mr. Ganni said.
These suggestions for creation of patient radiation passports and cancer surveillance guidelines take on greater weight in light of two trends: the increasing life expectancy of children with CHD during the past 3 decades as a result of procedural advances that entail LDIR exposure, mostly for imaging, and the growing number of such procedures performed per patient earlier and earlier in life.
He and the MAUDE group plan to confirm their latest findings in other, larger data sets and hope to identify threshold effects for LDIR for specific cancers, with hematologic malignancies as the top priority.
Mr. Ganni reported having no financial conflicts regarding his study, funded by the Heart and Stroke Foundation of Canada, the Quebec Foundation for Health Research, and the Canadian Institutes for Health Research.
FROM ACC 2020
Bariatric surgery in advanced heart failure wins transplant eligibility
Bariatric surgery is a safe and effective means for obese patients with advanced heart failure supported by a left ventricular assist device to qualify for heart transplantation, Praneet Wander, MD, reported in an abstract released as part of the annual Digestive Disease Week®.
She presented a systematic review and meta-analysis of nine retrospective or cross-sectional cohort studies totaling
Of the 86 patients, 50 (58%) were able to drop their BMI below 35, a requirement for inclusion on the heart transplant waiting list, noted Dr. Wander, a gastroenterology fellow at Hofstra University, Hempstead, N.Y., and North Shore LIJ Hospital in Manhasset, N.Y.
“A lot of bariatric surgeons don’t feel comfortable operating on patients who have a low ejection fraction,” she explained in an interview. “This study should encourage bariatric surgeons to do procedures even in patients with advanced heart failure so they can meet the BMI requirement for heart transplantation.”
Even if patients don’t actually undergo heart transplantation because of the perpetual donor organ shortage or inability to meet non–BMI-related eligibility criteria, they gain other major benefits from bariatric surgery: Their blood pressure goes down, their diabetes improves, and they become better able to engage in physical activity, she added.
Of the 86 patients in the meta-analysis, 84 underwent laparoscopic sleeve gastrectomy. That’s the preferred bariatric operation in patients with advanced heart failure at the Mayo Clinic as well, according to Andres J. Acosta, MD, PhD, a gastroenterologist at the medical center in Rochester, Minn.
There’s less weight loss achieved than with an open Roux-en-Y gastric bypass, but it’s a simpler operation in these high-risk patients, who typically have multiple comorbid conditions, he explained.
He predicted that Dr. Wander’s study will indeed influence bariatric surgeons at tertiary medical centers around the country to become more willing to consider weight-loss surgery in patients with advanced heart failure, while those in community practice will likely continue to be most comfortable operating on more stable patients with minimal comorbidities aside from their obesity.
“Data such as [these] will be reassuring to bariatric surgery programs such as ours, where we’re able to say: ‘Yes, there are risks, but these patients will benefit in the long term if we assume those risks,’ ” Dr. Acosta said.
He’s confident that, in the near future, the preferred form of bariatric surgery in patients with advanced heart failure will be a minimally invasive procedure performed endoscopically by gastroenterologists. He and his Mayo Clinic colleagues have already established a track record of success with endoscopic sleeve gastrectomy in patients with advanced kidney, liver, or lung disease in order to make them eligible for transplantation, as well as for the ancillary benefits provided by massive weight loss.
“There’s a little less weight loss than with laparoscopic sleeve gastrectomy, but it’s a significantly less risky operation. Shorter operative time, shorter hospital length of stay, less risk of infections and leaks,” he said in an interview. “We haven’t done it yet in heart disease, but I think based on this study this should be the next step at Mayo.”
Radha Gopalan, MD, director of heart failure and transplantation at Banner–University Medical Center in Phoenix, pronounced Dr. Wander’s meta-analysis “a positive study that’s very supportive of what we’re doing at our center.
“At a busy heart transplant center like ours, we are comfortable managing these patients, so the bariatric surgeons are reassured that the heart failure team is behind them. The risk of the procedure is mitigated by the availability of the multidisciplinary team to get the patient with obesity and heart failure through the surgery,” he explained.
Dr. Gopalan heads a novel bariatric heart failure program at Banner. While Dr. Wander’s meta-analysis focused on bariatric surgery in heart failure patients on LVAD circulatory support, Dr. Gopalan and colleagues are moving the intervention upstream. Roughly roughly 80% of patients in his bariatric heart failure program who meet criteria for LVAD implantation are now offered bariatric surgery before an LVAD is put in.
“I am moving away from putting the LVAD in first and then doing bariatric surgery. We have gotten comfortable taking these patients for bariatric surgery with inotropic support before going to the LVAD, which has the potential to even eliminate the requirement for an LVAD. Some patients get so much better that they become transplant ineligible,” Dr. Gopalan said.
Dr. Wander reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Wander P. DDW 2020 Abstract, #Mo2010.
Bariatric surgery is a safe and effective means for obese patients with advanced heart failure supported by a left ventricular assist device to qualify for heart transplantation, Praneet Wander, MD, reported in an abstract released as part of the annual Digestive Disease Week®.
She presented a systematic review and meta-analysis of nine retrospective or cross-sectional cohort studies totaling
Of the 86 patients, 50 (58%) were able to drop their BMI below 35, a requirement for inclusion on the heart transplant waiting list, noted Dr. Wander, a gastroenterology fellow at Hofstra University, Hempstead, N.Y., and North Shore LIJ Hospital in Manhasset, N.Y.
“A lot of bariatric surgeons don’t feel comfortable operating on patients who have a low ejection fraction,” she explained in an interview. “This study should encourage bariatric surgeons to do procedures even in patients with advanced heart failure so they can meet the BMI requirement for heart transplantation.”
Even if patients don’t actually undergo heart transplantation because of the perpetual donor organ shortage or inability to meet non–BMI-related eligibility criteria, they gain other major benefits from bariatric surgery: Their blood pressure goes down, their diabetes improves, and they become better able to engage in physical activity, she added.
Of the 86 patients in the meta-analysis, 84 underwent laparoscopic sleeve gastrectomy. That’s the preferred bariatric operation in patients with advanced heart failure at the Mayo Clinic as well, according to Andres J. Acosta, MD, PhD, a gastroenterologist at the medical center in Rochester, Minn.
There’s less weight loss achieved than with an open Roux-en-Y gastric bypass, but it’s a simpler operation in these high-risk patients, who typically have multiple comorbid conditions, he explained.
He predicted that Dr. Wander’s study will indeed influence bariatric surgeons at tertiary medical centers around the country to become more willing to consider weight-loss surgery in patients with advanced heart failure, while those in community practice will likely continue to be most comfortable operating on more stable patients with minimal comorbidities aside from their obesity.
“Data such as [these] will be reassuring to bariatric surgery programs such as ours, where we’re able to say: ‘Yes, there are risks, but these patients will benefit in the long term if we assume those risks,’ ” Dr. Acosta said.
He’s confident that, in the near future, the preferred form of bariatric surgery in patients with advanced heart failure will be a minimally invasive procedure performed endoscopically by gastroenterologists. He and his Mayo Clinic colleagues have already established a track record of success with endoscopic sleeve gastrectomy in patients with advanced kidney, liver, or lung disease in order to make them eligible for transplantation, as well as for the ancillary benefits provided by massive weight loss.
“There’s a little less weight loss than with laparoscopic sleeve gastrectomy, but it’s a significantly less risky operation. Shorter operative time, shorter hospital length of stay, less risk of infections and leaks,” he said in an interview. “We haven’t done it yet in heart disease, but I think based on this study this should be the next step at Mayo.”
Radha Gopalan, MD, director of heart failure and transplantation at Banner–University Medical Center in Phoenix, pronounced Dr. Wander’s meta-analysis “a positive study that’s very supportive of what we’re doing at our center.
“At a busy heart transplant center like ours, we are comfortable managing these patients, so the bariatric surgeons are reassured that the heart failure team is behind them. The risk of the procedure is mitigated by the availability of the multidisciplinary team to get the patient with obesity and heart failure through the surgery,” he explained.
Dr. Gopalan heads a novel bariatric heart failure program at Banner. While Dr. Wander’s meta-analysis focused on bariatric surgery in heart failure patients on LVAD circulatory support, Dr. Gopalan and colleagues are moving the intervention upstream. Roughly roughly 80% of patients in his bariatric heart failure program who meet criteria for LVAD implantation are now offered bariatric surgery before an LVAD is put in.
“I am moving away from putting the LVAD in first and then doing bariatric surgery. We have gotten comfortable taking these patients for bariatric surgery with inotropic support before going to the LVAD, which has the potential to even eliminate the requirement for an LVAD. Some patients get so much better that they become transplant ineligible,” Dr. Gopalan said.
Dr. Wander reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Wander P. DDW 2020 Abstract, #Mo2010.
Bariatric surgery is a safe and effective means for obese patients with advanced heart failure supported by a left ventricular assist device to qualify for heart transplantation, Praneet Wander, MD, reported in an abstract released as part of the annual Digestive Disease Week®.
She presented a systematic review and meta-analysis of nine retrospective or cross-sectional cohort studies totaling
Of the 86 patients, 50 (58%) were able to drop their BMI below 35, a requirement for inclusion on the heart transplant waiting list, noted Dr. Wander, a gastroenterology fellow at Hofstra University, Hempstead, N.Y., and North Shore LIJ Hospital in Manhasset, N.Y.
“A lot of bariatric surgeons don’t feel comfortable operating on patients who have a low ejection fraction,” she explained in an interview. “This study should encourage bariatric surgeons to do procedures even in patients with advanced heart failure so they can meet the BMI requirement for heart transplantation.”
Even if patients don’t actually undergo heart transplantation because of the perpetual donor organ shortage or inability to meet non–BMI-related eligibility criteria, they gain other major benefits from bariatric surgery: Their blood pressure goes down, their diabetes improves, and they become better able to engage in physical activity, she added.
Of the 86 patients in the meta-analysis, 84 underwent laparoscopic sleeve gastrectomy. That’s the preferred bariatric operation in patients with advanced heart failure at the Mayo Clinic as well, according to Andres J. Acosta, MD, PhD, a gastroenterologist at the medical center in Rochester, Minn.
There’s less weight loss achieved than with an open Roux-en-Y gastric bypass, but it’s a simpler operation in these high-risk patients, who typically have multiple comorbid conditions, he explained.
He predicted that Dr. Wander’s study will indeed influence bariatric surgeons at tertiary medical centers around the country to become more willing to consider weight-loss surgery in patients with advanced heart failure, while those in community practice will likely continue to be most comfortable operating on more stable patients with minimal comorbidities aside from their obesity.
“Data such as [these] will be reassuring to bariatric surgery programs such as ours, where we’re able to say: ‘Yes, there are risks, but these patients will benefit in the long term if we assume those risks,’ ” Dr. Acosta said.
He’s confident that, in the near future, the preferred form of bariatric surgery in patients with advanced heart failure will be a minimally invasive procedure performed endoscopically by gastroenterologists. He and his Mayo Clinic colleagues have already established a track record of success with endoscopic sleeve gastrectomy in patients with advanced kidney, liver, or lung disease in order to make them eligible for transplantation, as well as for the ancillary benefits provided by massive weight loss.
“There’s a little less weight loss than with laparoscopic sleeve gastrectomy, but it’s a significantly less risky operation. Shorter operative time, shorter hospital length of stay, less risk of infections and leaks,” he said in an interview. “We haven’t done it yet in heart disease, but I think based on this study this should be the next step at Mayo.”
Radha Gopalan, MD, director of heart failure and transplantation at Banner–University Medical Center in Phoenix, pronounced Dr. Wander’s meta-analysis “a positive study that’s very supportive of what we’re doing at our center.
“At a busy heart transplant center like ours, we are comfortable managing these patients, so the bariatric surgeons are reassured that the heart failure team is behind them. The risk of the procedure is mitigated by the availability of the multidisciplinary team to get the patient with obesity and heart failure through the surgery,” he explained.
Dr. Gopalan heads a novel bariatric heart failure program at Banner. While Dr. Wander’s meta-analysis focused on bariatric surgery in heart failure patients on LVAD circulatory support, Dr. Gopalan and colleagues are moving the intervention upstream. Roughly roughly 80% of patients in his bariatric heart failure program who meet criteria for LVAD implantation are now offered bariatric surgery before an LVAD is put in.
“I am moving away from putting the LVAD in first and then doing bariatric surgery. We have gotten comfortable taking these patients for bariatric surgery with inotropic support before going to the LVAD, which has the potential to even eliminate the requirement for an LVAD. Some patients get so much better that they become transplant ineligible,” Dr. Gopalan said.
Dr. Wander reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Wander P. DDW 2020 Abstract, #Mo2010.
FROM DDW 2020
ER docs ask, “Where are our patients?”
according to an expert panel on unanticipated consequences of pandemic care hosted by the presidents of the Society of Critical Care Medicine and the American College of Emergency Physicians.*
“At the peak of exposure to COVID-19 illness or infection, ED volumes in my system, which are really not much different from others across the country, were cut in half, if not more. And those changes happened across virtually every form of ED presentation, from the highest acuity to the lowest. We’re now beyond our highest level of exposure to COVID-19 clinically symptomatic patients in western Pennsylvania, but that recovery in volume hasn’t occurred yet, although there are some embers,” explained Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh.
He and other panelists also addressed some of the other unanticipated developments in the COVID-19 pandemic, including a recently recognized childhood manifestation called for now COVID-associated pediatric multisystem inflammatory syndrome, an anticipated massive second wave of non-COVID patients expected to present late to EDs and primary care clinics after having avoided needed medical care out of fear of infection, and the pandemic’s negative impact upon medical education.
Who’s not showing up in the ED
Dr. Yealy said that across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more.
“You might say, if I was designing from the insurance side, this is exactly what I’d hope for. I’ve heard that some people on the insurance-only side of the business really are experiencing a pretty good deal right now: They’re collecting premiums and not having to pay out on the ED or hospital side,” he said.
Tweaking the public health message on seeking medical care
“One of the unanticipated casualties of the pandemic are the patients who don’t have it. It will take a whole lot of work and coordinated effort to re-engage with those patients,” predicted SCCM President Lewis J. Kaplan, MD, professor of surgery at the University of Pennsylvania, Philadelphia.
Evie G. Marcolini, MD, described what she believes is necessary now: “We need to have a big focus on getting the word out to the public that acute MI, stroke, and other acute injuries are still a time-sensitive problem and they warrant at least a call to their physician or consideration of coming in to the ED.
“I think when we started out, we were telling people, ‘Don’t come in.’ Now we’re trying to dial it back a little bit and say, ‘Listen, there are things you really do need to come in for. And we will keep you safe,’” said Dr. Marcolini, an emergency medicine and neurocritical care specialist at Dartmouth-Hitchcock Medical Center, Hanover, N.H.
“It is safe,” Dr. Yealy agreed. “The safest place in the world to be right now is the ED. Everybody’s cordoned off. There’s way more PPE [personal protective equipment]. There’s a level of precision now that should have existed but never did in our previous influenza seasons. So we have something very unique to offer, and we can put people’s minds at rest.”
He spoke of a coming “tsunami of untreated illness.”
“My concern is there is a significant subset of people who are not only eschewing ED care but staying away from their primary care provider. My fear is that we’re not as well aware of this,” he said. “Together with our primary care partners, we have to figure out ways to reach the people who are ignoring illnesses and injuries that they’re making long-term decisions about without realizing it. We have to find a way to reach those people and say it’s okay to reach for care.”
SCCM Immediate Past President Heatherlee Bailey, MD, also sees a problematic looming wave.
“I’m quite concerned about the coming second wave of non-COVID patients who’ve sat home with their worsening renal failure that’s gone from 2 to 5 because they’ve been taking a lot of NSAIDs, or the individual who’s had several TIAs that self-resolved, and we’ve missed an opportunity to prevent some significant disease. At some point they’re going to come back, and we need to figure out how to get these individuals hooked up with care, either through the ED or with their primary care provider, to prevent these potential bad outcomes,” said Dr. Bailey of the Durham (N.C.) Veterans Affairs Medical Center.
Interim guidance for pediatricians on an alarming new syndrome
Edward E. Conway Jr., MD, recalled that early in the U.S. pandemic, pediatricians felt a sense of relief that children appeared to be spared from severe COVID-19 disease. But, in just the past few weeks, a new syndrome has emerged. New York City has recorded more than 100 cases of what’s provisionally being called COVID-associated pediatric multisystem inflammatory syndrome. Dr. Conway and others are working with the Centers for Disease Control and Prevention to develop a case definition for the syndrome, first reported by pediatricians in Italy and the United Kingdom.
“We’re trying to get the word out to general pediatricians as to the common signs and symptoms that should prompt parents to bring their children in for medical care,” according to Dr. Conway, chief of pediatric critical care medicine and vice-chair of pediatrics at Jacobi Medical Center in New York.
Ninety percent of affected children have abdominal symptoms early on, including abdominal pain, diarrhea, emesis, or enteritis upon imaging. A nondescript rash, headache, conjunctivitis, and irritability are common, cough much less so – under 25%.
“The thought is that if any one of these is associated with a fever lasting more than 4 days, we suggest these children be brought in and seen by a pediatrician. We don’t have a formal guideline – we’re working on that – but basically the current recommendation is to screen them initially with a CBC with differential, a chem 10, and liver function tests, but also to look for inflammatory markers that we see in our COVID patients. We’ve been quite surprised: These patients have C-reactive proteins of about 240 mg/L on average, ferritin is quite high at around 1,200 ng/mL, and d-dimers of 2,300 ng/mL. We’ve also found very high brain natriuretic peptides and troponins in these patients,” according to Dr. Conway.
Analogies have been made between this COVID-19 pediatric syndrome and Kawasaki disease. Dr. Conway is unconvinced.
“This is quite different from Kawasaki in that these children are usually thrombocytopenic and usually present with DIC [disseminated intravascular coagulation], and the d-dimers are extraordinarily high, compared to what we’re used to seeing in pediatric patients,” he said.
Symptomatic children with laboratory red flags should be hospitalized. Most of the affected New York City children have recovered after 5 or 6 days in the pediatric ICU with empiric treatment using intravenous immunoglobulin (IVIG), corticosteroids, and/or interleukin-6 inhibitors. However, five recent deaths are now under study.
Dr. Yealy commented that this new pediatric syndrome is “really interesting,” but to date, it affects only a very small percentage of children, and children overall have been much less affected by the pandemic than are adults.
“The populations being disproportionately impacted are the elderly, the elderly, the elderly, and then other vulnerable populations, particularly congregants and the poor,” he said. “At my site, three-quarters of the patients coming in are either patients at assisted-living facilities or work at one of those congregant facilities.”
The pandemic’s impact on medical education
In many hospitals, grand rounds are being done virtually via videoconferencing, often with attendant challenges in asking and answering questions. Hospital patient volumes are diminished. Medical students aren’t coming in to do clinical rotations. Medical students and residents can’t travel to interview for future residencies or jobs.
“It’s affecting education across all of the components of medicine. It’s hard to say how long this pandemic is going to last. We’re all trying to be innovative in using online tools, but I believe it’s going to have a long-lasting effect on our education system,” Dr. Marcolini predicted.
Remote interface while working from home has become frustrating, especially during peak Internet use hours.
“It’s staggering how slow my home system has become in comparison to what’s wired at work. Now many times when you try to get into your work system from home, you time out while you’re waiting for the next piece of information to come across,” Dr. Kaplan commented.
All panel participants reported having no financial conflicts of interest.
*Correction, 5/15/20: An earlier version of this article misstated the name of the American College of Emergency Physicians.)
according to an expert panel on unanticipated consequences of pandemic care hosted by the presidents of the Society of Critical Care Medicine and the American College of Emergency Physicians.*
“At the peak of exposure to COVID-19 illness or infection, ED volumes in my system, which are really not much different from others across the country, were cut in half, if not more. And those changes happened across virtually every form of ED presentation, from the highest acuity to the lowest. We’re now beyond our highest level of exposure to COVID-19 clinically symptomatic patients in western Pennsylvania, but that recovery in volume hasn’t occurred yet, although there are some embers,” explained Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh.
He and other panelists also addressed some of the other unanticipated developments in the COVID-19 pandemic, including a recently recognized childhood manifestation called for now COVID-associated pediatric multisystem inflammatory syndrome, an anticipated massive second wave of non-COVID patients expected to present late to EDs and primary care clinics after having avoided needed medical care out of fear of infection, and the pandemic’s negative impact upon medical education.
Who’s not showing up in the ED
Dr. Yealy said that across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more.
“You might say, if I was designing from the insurance side, this is exactly what I’d hope for. I’ve heard that some people on the insurance-only side of the business really are experiencing a pretty good deal right now: They’re collecting premiums and not having to pay out on the ED or hospital side,” he said.
Tweaking the public health message on seeking medical care
“One of the unanticipated casualties of the pandemic are the patients who don’t have it. It will take a whole lot of work and coordinated effort to re-engage with those patients,” predicted SCCM President Lewis J. Kaplan, MD, professor of surgery at the University of Pennsylvania, Philadelphia.
Evie G. Marcolini, MD, described what she believes is necessary now: “We need to have a big focus on getting the word out to the public that acute MI, stroke, and other acute injuries are still a time-sensitive problem and they warrant at least a call to their physician or consideration of coming in to the ED.
“I think when we started out, we were telling people, ‘Don’t come in.’ Now we’re trying to dial it back a little bit and say, ‘Listen, there are things you really do need to come in for. And we will keep you safe,’” said Dr. Marcolini, an emergency medicine and neurocritical care specialist at Dartmouth-Hitchcock Medical Center, Hanover, N.H.
“It is safe,” Dr. Yealy agreed. “The safest place in the world to be right now is the ED. Everybody’s cordoned off. There’s way more PPE [personal protective equipment]. There’s a level of precision now that should have existed but never did in our previous influenza seasons. So we have something very unique to offer, and we can put people’s minds at rest.”
He spoke of a coming “tsunami of untreated illness.”
“My concern is there is a significant subset of people who are not only eschewing ED care but staying away from their primary care provider. My fear is that we’re not as well aware of this,” he said. “Together with our primary care partners, we have to figure out ways to reach the people who are ignoring illnesses and injuries that they’re making long-term decisions about without realizing it. We have to find a way to reach those people and say it’s okay to reach for care.”
SCCM Immediate Past President Heatherlee Bailey, MD, also sees a problematic looming wave.
“I’m quite concerned about the coming second wave of non-COVID patients who’ve sat home with their worsening renal failure that’s gone from 2 to 5 because they’ve been taking a lot of NSAIDs, or the individual who’s had several TIAs that self-resolved, and we’ve missed an opportunity to prevent some significant disease. At some point they’re going to come back, and we need to figure out how to get these individuals hooked up with care, either through the ED or with their primary care provider, to prevent these potential bad outcomes,” said Dr. Bailey of the Durham (N.C.) Veterans Affairs Medical Center.
Interim guidance for pediatricians on an alarming new syndrome
Edward E. Conway Jr., MD, recalled that early in the U.S. pandemic, pediatricians felt a sense of relief that children appeared to be spared from severe COVID-19 disease. But, in just the past few weeks, a new syndrome has emerged. New York City has recorded more than 100 cases of what’s provisionally being called COVID-associated pediatric multisystem inflammatory syndrome. Dr. Conway and others are working with the Centers for Disease Control and Prevention to develop a case definition for the syndrome, first reported by pediatricians in Italy and the United Kingdom.
“We’re trying to get the word out to general pediatricians as to the common signs and symptoms that should prompt parents to bring their children in for medical care,” according to Dr. Conway, chief of pediatric critical care medicine and vice-chair of pediatrics at Jacobi Medical Center in New York.
Ninety percent of affected children have abdominal symptoms early on, including abdominal pain, diarrhea, emesis, or enteritis upon imaging. A nondescript rash, headache, conjunctivitis, and irritability are common, cough much less so – under 25%.
“The thought is that if any one of these is associated with a fever lasting more than 4 days, we suggest these children be brought in and seen by a pediatrician. We don’t have a formal guideline – we’re working on that – but basically the current recommendation is to screen them initially with a CBC with differential, a chem 10, and liver function tests, but also to look for inflammatory markers that we see in our COVID patients. We’ve been quite surprised: These patients have C-reactive proteins of about 240 mg/L on average, ferritin is quite high at around 1,200 ng/mL, and d-dimers of 2,300 ng/mL. We’ve also found very high brain natriuretic peptides and troponins in these patients,” according to Dr. Conway.
Analogies have been made between this COVID-19 pediatric syndrome and Kawasaki disease. Dr. Conway is unconvinced.
“This is quite different from Kawasaki in that these children are usually thrombocytopenic and usually present with DIC [disseminated intravascular coagulation], and the d-dimers are extraordinarily high, compared to what we’re used to seeing in pediatric patients,” he said.
Symptomatic children with laboratory red flags should be hospitalized. Most of the affected New York City children have recovered after 5 or 6 days in the pediatric ICU with empiric treatment using intravenous immunoglobulin (IVIG), corticosteroids, and/or interleukin-6 inhibitors. However, five recent deaths are now under study.
Dr. Yealy commented that this new pediatric syndrome is “really interesting,” but to date, it affects only a very small percentage of children, and children overall have been much less affected by the pandemic than are adults.
“The populations being disproportionately impacted are the elderly, the elderly, the elderly, and then other vulnerable populations, particularly congregants and the poor,” he said. “At my site, three-quarters of the patients coming in are either patients at assisted-living facilities or work at one of those congregant facilities.”
The pandemic’s impact on medical education
In many hospitals, grand rounds are being done virtually via videoconferencing, often with attendant challenges in asking and answering questions. Hospital patient volumes are diminished. Medical students aren’t coming in to do clinical rotations. Medical students and residents can’t travel to interview for future residencies or jobs.
“It’s affecting education across all of the components of medicine. It’s hard to say how long this pandemic is going to last. We’re all trying to be innovative in using online tools, but I believe it’s going to have a long-lasting effect on our education system,” Dr. Marcolini predicted.
Remote interface while working from home has become frustrating, especially during peak Internet use hours.
“It’s staggering how slow my home system has become in comparison to what’s wired at work. Now many times when you try to get into your work system from home, you time out while you’re waiting for the next piece of information to come across,” Dr. Kaplan commented.
All panel participants reported having no financial conflicts of interest.
*Correction, 5/15/20: An earlier version of this article misstated the name of the American College of Emergency Physicians.)
according to an expert panel on unanticipated consequences of pandemic care hosted by the presidents of the Society of Critical Care Medicine and the American College of Emergency Physicians.*
“At the peak of exposure to COVID-19 illness or infection, ED volumes in my system, which are really not much different from others across the country, were cut in half, if not more. And those changes happened across virtually every form of ED presentation, from the highest acuity to the lowest. We’re now beyond our highest level of exposure to COVID-19 clinically symptomatic patients in western Pennsylvania, but that recovery in volume hasn’t occurred yet, although there are some embers,” explained Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh.
He and other panelists also addressed some of the other unanticipated developments in the COVID-19 pandemic, including a recently recognized childhood manifestation called for now COVID-associated pediatric multisystem inflammatory syndrome, an anticipated massive second wave of non-COVID patients expected to present late to EDs and primary care clinics after having avoided needed medical care out of fear of infection, and the pandemic’s negative impact upon medical education.
Who’s not showing up in the ED
Dr. Yealy said that across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more.
“You might say, if I was designing from the insurance side, this is exactly what I’d hope for. I’ve heard that some people on the insurance-only side of the business really are experiencing a pretty good deal right now: They’re collecting premiums and not having to pay out on the ED or hospital side,” he said.
Tweaking the public health message on seeking medical care
“One of the unanticipated casualties of the pandemic are the patients who don’t have it. It will take a whole lot of work and coordinated effort to re-engage with those patients,” predicted SCCM President Lewis J. Kaplan, MD, professor of surgery at the University of Pennsylvania, Philadelphia.
Evie G. Marcolini, MD, described what she believes is necessary now: “We need to have a big focus on getting the word out to the public that acute MI, stroke, and other acute injuries are still a time-sensitive problem and they warrant at least a call to their physician or consideration of coming in to the ED.
“I think when we started out, we were telling people, ‘Don’t come in.’ Now we’re trying to dial it back a little bit and say, ‘Listen, there are things you really do need to come in for. And we will keep you safe,’” said Dr. Marcolini, an emergency medicine and neurocritical care specialist at Dartmouth-Hitchcock Medical Center, Hanover, N.H.
“It is safe,” Dr. Yealy agreed. “The safest place in the world to be right now is the ED. Everybody’s cordoned off. There’s way more PPE [personal protective equipment]. There’s a level of precision now that should have existed but never did in our previous influenza seasons. So we have something very unique to offer, and we can put people’s minds at rest.”
He spoke of a coming “tsunami of untreated illness.”
“My concern is there is a significant subset of people who are not only eschewing ED care but staying away from their primary care provider. My fear is that we’re not as well aware of this,” he said. “Together with our primary care partners, we have to figure out ways to reach the people who are ignoring illnesses and injuries that they’re making long-term decisions about without realizing it. We have to find a way to reach those people and say it’s okay to reach for care.”
SCCM Immediate Past President Heatherlee Bailey, MD, also sees a problematic looming wave.
“I’m quite concerned about the coming second wave of non-COVID patients who’ve sat home with their worsening renal failure that’s gone from 2 to 5 because they’ve been taking a lot of NSAIDs, or the individual who’s had several TIAs that self-resolved, and we’ve missed an opportunity to prevent some significant disease. At some point they’re going to come back, and we need to figure out how to get these individuals hooked up with care, either through the ED or with their primary care provider, to prevent these potential bad outcomes,” said Dr. Bailey of the Durham (N.C.) Veterans Affairs Medical Center.
Interim guidance for pediatricians on an alarming new syndrome
Edward E. Conway Jr., MD, recalled that early in the U.S. pandemic, pediatricians felt a sense of relief that children appeared to be spared from severe COVID-19 disease. But, in just the past few weeks, a new syndrome has emerged. New York City has recorded more than 100 cases of what’s provisionally being called COVID-associated pediatric multisystem inflammatory syndrome. Dr. Conway and others are working with the Centers for Disease Control and Prevention to develop a case definition for the syndrome, first reported by pediatricians in Italy and the United Kingdom.
“We’re trying to get the word out to general pediatricians as to the common signs and symptoms that should prompt parents to bring their children in for medical care,” according to Dr. Conway, chief of pediatric critical care medicine and vice-chair of pediatrics at Jacobi Medical Center in New York.
Ninety percent of affected children have abdominal symptoms early on, including abdominal pain, diarrhea, emesis, or enteritis upon imaging. A nondescript rash, headache, conjunctivitis, and irritability are common, cough much less so – under 25%.
“The thought is that if any one of these is associated with a fever lasting more than 4 days, we suggest these children be brought in and seen by a pediatrician. We don’t have a formal guideline – we’re working on that – but basically the current recommendation is to screen them initially with a CBC with differential, a chem 10, and liver function tests, but also to look for inflammatory markers that we see in our COVID patients. We’ve been quite surprised: These patients have C-reactive proteins of about 240 mg/L on average, ferritin is quite high at around 1,200 ng/mL, and d-dimers of 2,300 ng/mL. We’ve also found very high brain natriuretic peptides and troponins in these patients,” according to Dr. Conway.
Analogies have been made between this COVID-19 pediatric syndrome and Kawasaki disease. Dr. Conway is unconvinced.
“This is quite different from Kawasaki in that these children are usually thrombocytopenic and usually present with DIC [disseminated intravascular coagulation], and the d-dimers are extraordinarily high, compared to what we’re used to seeing in pediatric patients,” he said.
Symptomatic children with laboratory red flags should be hospitalized. Most of the affected New York City children have recovered after 5 or 6 days in the pediatric ICU with empiric treatment using intravenous immunoglobulin (IVIG), corticosteroids, and/or interleukin-6 inhibitors. However, five recent deaths are now under study.
Dr. Yealy commented that this new pediatric syndrome is “really interesting,” but to date, it affects only a very small percentage of children, and children overall have been much less affected by the pandemic than are adults.
“The populations being disproportionately impacted are the elderly, the elderly, the elderly, and then other vulnerable populations, particularly congregants and the poor,” he said. “At my site, three-quarters of the patients coming in are either patients at assisted-living facilities or work at one of those congregant facilities.”
The pandemic’s impact on medical education
In many hospitals, grand rounds are being done virtually via videoconferencing, often with attendant challenges in asking and answering questions. Hospital patient volumes are diminished. Medical students aren’t coming in to do clinical rotations. Medical students and residents can’t travel to interview for future residencies or jobs.
“It’s affecting education across all of the components of medicine. It’s hard to say how long this pandemic is going to last. We’re all trying to be innovative in using online tools, but I believe it’s going to have a long-lasting effect on our education system,” Dr. Marcolini predicted.
Remote interface while working from home has become frustrating, especially during peak Internet use hours.
“It’s staggering how slow my home system has become in comparison to what’s wired at work. Now many times when you try to get into your work system from home, you time out while you’re waiting for the next piece of information to come across,” Dr. Kaplan commented.
All panel participants reported having no financial conflicts of interest.
*Correction, 5/15/20: An earlier version of this article misstated the name of the American College of Emergency Physicians.)
Yoga is a good adjunct to migraine therapy
in Neurology.
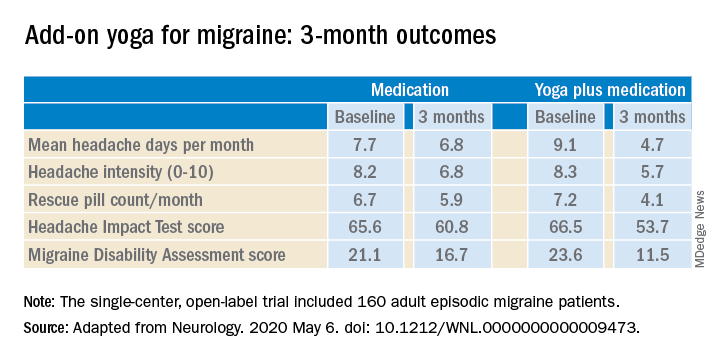
The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
in Neurology.

The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
in Neurology.

The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
FROM NEUROLOGY
Evolocumab safe, well-tolerated in HIV+ patients
Evolocumab proved effective, well tolerated, and safe for the treatment of refractory dyslipidemia in persons living with HIV in the phase 3, randomized, double-blind BEIJERINCK study.
At 24 weeks, nearly three-quarters of patients randomized to evolocumab (Repatha) achieved at least a 50% reduction in LDL cholesterol while on maximally tolerated background lipid lowering with a statin and/or other drugs. This was accompanied by significant reductions in other atherogenic lipids, Franck Boccara, MD, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Evolocumab thus shows the potential to help fill a major unmet need for more effective treatment of dyslipidemia in HIV-positive patients, who number an estimated 38 million worldwide, including 1.1 million in the United States. Access to highly active antiretroviral therapies has transformed HIV infection into a chronic manageable disease, but this major advance has been accompanied by a rate of premature atherosclerotic cardiovascular disease that’s nearly twice that of the general population, observed Dr. Boccara, a cardiologist at Sorbonne University, Paris.
The BEIJERINCK study included 464 HIV-infected patients in the United States and 14 other countries on five continents. Participants had a mean baseline LDL cholesterol of 133 mg/dL and triglycerides of about 190 mg/dL while on maximally tolerated lipid-lowering therapy. They had been diagnosed with HIV an average of 18 years earlier. One-third of them had known atherosclerotic cardiovascular disease. More than one-quarter of participants were cigarette smokers. Patients were randomized 2:1 to 24 weeks of double-blind subcutaneous evolocumab at 420 mg once monthly or placebo, then an additional 24 weeks of open-label evolocumab for all.
The primary endpoint was change in LDL from baseline to week 24: a 56.2% reduction in the evolocumab group and a 0.7% increase with placebo. About 73% of patients on evolocumab achieved at least a 50% reduction in LDL cholesterol, as did less than 1% of controls. Likewise, 73% of the evolocumab group got their LDL cholesterol below 70 mg/dL, compared with 7.9% with placebo.
The evolocumab group also experienced favorable placebo-subtracted differences from baseline of 23% in triglycerides, 27% in lipoprotein(a), and 22% in very-low-density lipoprotein cholesterol.
As was the case in the earlier, much larger landmark clinical trials, evolocumab was well tolerated in BEIJERINCK, with a side effect profile similar to placebo. Notably, there was no increase in liver abnormalities in evolocumab-treated patients on highly active antiretroviral therapy, and no one developed evolocumab neutralizing antibodies.
Dr. Boccara reported receiving a research grant from Amgen, the study sponsor, as well as lecture fees from several other pharmaceutical companies.
Simultaneous with the presentation at ACC 2020, the primary results of the BEIJERINCK study were published online (J Am Coll Cardiol. 2020 Mar 19. doi: 10.1016/j.jacc.2020.03.025).
Evolocumab proved effective, well tolerated, and safe for the treatment of refractory dyslipidemia in persons living with HIV in the phase 3, randomized, double-blind BEIJERINCK study.
At 24 weeks, nearly three-quarters of patients randomized to evolocumab (Repatha) achieved at least a 50% reduction in LDL cholesterol while on maximally tolerated background lipid lowering with a statin and/or other drugs. This was accompanied by significant reductions in other atherogenic lipids, Franck Boccara, MD, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Evolocumab thus shows the potential to help fill a major unmet need for more effective treatment of dyslipidemia in HIV-positive patients, who number an estimated 38 million worldwide, including 1.1 million in the United States. Access to highly active antiretroviral therapies has transformed HIV infection into a chronic manageable disease, but this major advance has been accompanied by a rate of premature atherosclerotic cardiovascular disease that’s nearly twice that of the general population, observed Dr. Boccara, a cardiologist at Sorbonne University, Paris.
The BEIJERINCK study included 464 HIV-infected patients in the United States and 14 other countries on five continents. Participants had a mean baseline LDL cholesterol of 133 mg/dL and triglycerides of about 190 mg/dL while on maximally tolerated lipid-lowering therapy. They had been diagnosed with HIV an average of 18 years earlier. One-third of them had known atherosclerotic cardiovascular disease. More than one-quarter of participants were cigarette smokers. Patients were randomized 2:1 to 24 weeks of double-blind subcutaneous evolocumab at 420 mg once monthly or placebo, then an additional 24 weeks of open-label evolocumab for all.
The primary endpoint was change in LDL from baseline to week 24: a 56.2% reduction in the evolocumab group and a 0.7% increase with placebo. About 73% of patients on evolocumab achieved at least a 50% reduction in LDL cholesterol, as did less than 1% of controls. Likewise, 73% of the evolocumab group got their LDL cholesterol below 70 mg/dL, compared with 7.9% with placebo.
The evolocumab group also experienced favorable placebo-subtracted differences from baseline of 23% in triglycerides, 27% in lipoprotein(a), and 22% in very-low-density lipoprotein cholesterol.
As was the case in the earlier, much larger landmark clinical trials, evolocumab was well tolerated in BEIJERINCK, with a side effect profile similar to placebo. Notably, there was no increase in liver abnormalities in evolocumab-treated patients on highly active antiretroviral therapy, and no one developed evolocumab neutralizing antibodies.
Dr. Boccara reported receiving a research grant from Amgen, the study sponsor, as well as lecture fees from several other pharmaceutical companies.
Simultaneous with the presentation at ACC 2020, the primary results of the BEIJERINCK study were published online (J Am Coll Cardiol. 2020 Mar 19. doi: 10.1016/j.jacc.2020.03.025).
Evolocumab proved effective, well tolerated, and safe for the treatment of refractory dyslipidemia in persons living with HIV in the phase 3, randomized, double-blind BEIJERINCK study.
At 24 weeks, nearly three-quarters of patients randomized to evolocumab (Repatha) achieved at least a 50% reduction in LDL cholesterol while on maximally tolerated background lipid lowering with a statin and/or other drugs. This was accompanied by significant reductions in other atherogenic lipids, Franck Boccara, MD, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Evolocumab thus shows the potential to help fill a major unmet need for more effective treatment of dyslipidemia in HIV-positive patients, who number an estimated 38 million worldwide, including 1.1 million in the United States. Access to highly active antiretroviral therapies has transformed HIV infection into a chronic manageable disease, but this major advance has been accompanied by a rate of premature atherosclerotic cardiovascular disease that’s nearly twice that of the general population, observed Dr. Boccara, a cardiologist at Sorbonne University, Paris.
The BEIJERINCK study included 464 HIV-infected patients in the United States and 14 other countries on five continents. Participants had a mean baseline LDL cholesterol of 133 mg/dL and triglycerides of about 190 mg/dL while on maximally tolerated lipid-lowering therapy. They had been diagnosed with HIV an average of 18 years earlier. One-third of them had known atherosclerotic cardiovascular disease. More than one-quarter of participants were cigarette smokers. Patients were randomized 2:1 to 24 weeks of double-blind subcutaneous evolocumab at 420 mg once monthly or placebo, then an additional 24 weeks of open-label evolocumab for all.
The primary endpoint was change in LDL from baseline to week 24: a 56.2% reduction in the evolocumab group and a 0.7% increase with placebo. About 73% of patients on evolocumab achieved at least a 50% reduction in LDL cholesterol, as did less than 1% of controls. Likewise, 73% of the evolocumab group got their LDL cholesterol below 70 mg/dL, compared with 7.9% with placebo.
The evolocumab group also experienced favorable placebo-subtracted differences from baseline of 23% in triglycerides, 27% in lipoprotein(a), and 22% in very-low-density lipoprotein cholesterol.
As was the case in the earlier, much larger landmark clinical trials, evolocumab was well tolerated in BEIJERINCK, with a side effect profile similar to placebo. Notably, there was no increase in liver abnormalities in evolocumab-treated patients on highly active antiretroviral therapy, and no one developed evolocumab neutralizing antibodies.
Dr. Boccara reported receiving a research grant from Amgen, the study sponsor, as well as lecture fees from several other pharmaceutical companies.
Simultaneous with the presentation at ACC 2020, the primary results of the BEIJERINCK study were published online (J Am Coll Cardiol. 2020 Mar 19. doi: 10.1016/j.jacc.2020.03.025).
FROM ACC 2020
Anti-Ro52 autoantibodies signal interstitial lung disease in juvenile dermatomyositis teaser
MAUI, HAWAII – , Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
And in a recent potential treatment advance, Janus kinase inhibition shows promise as a novel therapy for ILD in patients with juvenile dermatomyositis (JDM), added Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 3 (12%) of 25 patients with juvenile polymyositis and in 8 (18%) of 44 youths with juvenile connective tissue disease–myositis overlap. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: namely, 9% of the cohort were positive for anti–melanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl-tRNA synthetase (anti-Jo-1) autoantibodies were present in 4%.
Thirty-three of the 371 juvenile myositis patients had ILD based upon CT imaging, chest x-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo1. After controlling for the presence of these other myositis-specific autoantibodies, anti-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52 and anti-Jo1 positive (Ann Rheum Dis. 2019 Jul;78[7]:988-95).
Patients with anti-Ro autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, IVIG, and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation–syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody–positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody–positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals (Brain. 2019 Nov 1;142[11]:e59).
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody–positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms (Rheumatology [Oxford]. 2019 Nov 22. doi: 10.1093/rheumatology/kez525. [Epub ahead of print]).
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – , Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
And in a recent potential treatment advance, Janus kinase inhibition shows promise as a novel therapy for ILD in patients with juvenile dermatomyositis (JDM), added Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 3 (12%) of 25 patients with juvenile polymyositis and in 8 (18%) of 44 youths with juvenile connective tissue disease–myositis overlap. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: namely, 9% of the cohort were positive for anti–melanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl-tRNA synthetase (anti-Jo-1) autoantibodies were present in 4%.
Thirty-three of the 371 juvenile myositis patients had ILD based upon CT imaging, chest x-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo1. After controlling for the presence of these other myositis-specific autoantibodies, anti-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52 and anti-Jo1 positive (Ann Rheum Dis. 2019 Jul;78[7]:988-95).
Patients with anti-Ro autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, IVIG, and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation–syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody–positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody–positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals (Brain. 2019 Nov 1;142[11]:e59).
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody–positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms (Rheumatology [Oxford]. 2019 Nov 22. doi: 10.1093/rheumatology/kez525. [Epub ahead of print]).
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – , Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
And in a recent potential treatment advance, Janus kinase inhibition shows promise as a novel therapy for ILD in patients with juvenile dermatomyositis (JDM), added Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 3 (12%) of 25 patients with juvenile polymyositis and in 8 (18%) of 44 youths with juvenile connective tissue disease–myositis overlap. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: namely, 9% of the cohort were positive for anti–melanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl-tRNA synthetase (anti-Jo-1) autoantibodies were present in 4%.
Thirty-three of the 371 juvenile myositis patients had ILD based upon CT imaging, chest x-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo1. After controlling for the presence of these other myositis-specific autoantibodies, anti-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52 and anti-Jo1 positive (Ann Rheum Dis. 2019 Jul;78[7]:988-95).
Patients with anti-Ro autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, IVIG, and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation–syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody–positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody–positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals (Brain. 2019 Nov 1;142[11]:e59).
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody–positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms (Rheumatology [Oxford]. 2019 Nov 22. doi: 10.1093/rheumatology/kez525. [Epub ahead of print]).
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
REPORTING FROM RWCS 2020
Coronary CT angiography gives superior MI risk prediction
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
FROM ACC 2020
Expert discusses red flags for interstitial lung disease in pediatric rheumatology
MAUI, HAWAII – Anti-Ro52 autoantibodies are the latest and most potent of the autoantibody predictors of interstitial lung disease (ILD) discovered in patients with juvenile dermatomyositis, Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
In addition to detailing the autoantibody red flags for ILD in juvenile dermatomyositis (JDM), she called for “hypervigilance” in patients with systemic juvenile idiopathic arthritis (SJIA) who exhibit any of a series of risk factors for ILD.
“Most of the lung disease in kids with systemic JIA is asymptomatic until very late, but it can be reversible if we treat it. So it’s worth finding and monitoring and giving everyone PCP [pneumocystis pneumonia] prophylaxis, because they have a high incidence of PCP if they have any of those risk factors,” observed Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 12% of 25 patients with juvenile polymyositis and in 18% of 44 youths with an overlap of juvenile connective tissue disease and myositis. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: Namely, 9% of the cohort were positive for antimelanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl tRNA synthestase (anti-Jo-1) autoantibodies were present in 4%.
A total of 33 of the 371 juvenile myositis patients had ILD based upon CT imaging, chest X-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo-1. After controlling for the presence of these other myositis-specific autoantibodies, auto-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52- and anti-Jo-1 positive.
Patients with anti-Ro52 autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Novel potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, intravenous immunoglobulin (IVIG), and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody-positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody-positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near-resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals.
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than did the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms.
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Risk factors for ILD in SJIA
In the past half-dozen years or so, pediatric rheumatologists have become increasingly aware of and concerned about a new development in SJIA: the occurrence of comorbid ILD. This is a poor-prognosis disease: In a cohort from the United Kingdom, 5-year mortality from the time of diagnosis was 41%, fully 40-fold higher than in patients with SJIA only.
Patient cohorts with SJIA and ILD have unusual clinical and laboratory features that aren’t part of the typical picture in SJIA. These include acute clubbing, lymphopenia, a fixed pruritic rash, unexplained abdominal pain, peripheral eosinophilia, facial swelling, and an increased ferritin level, a hallmark of acute macrophage activation syndrome. Onset of SJIA before 2 years of age is another red flag associated with increased risk for ILD. So is trisomy 21, which is up to 50 times more prevalent in patients with SJIA and ILD than in the general population or in patients with SJIA only. Another clue is an adverse reaction to tocilizumab (Actemra).
Any of these findings warrant hypervigilance: “Be on high alert and monitor these patients for ILD much more closely,” Dr. Stevens advised.
This means ordering a CT scan, prescribing PCP prophylaxis, and regularly measuring pulmonary function, admittedly a challenge in children under 7 years old. In these younger kids, practical solutions include measuring their oxygen saturation before and after running around the room to see if it drops. A 6-minute walk test and sleep oximetry are other options.
The explanation for the abrupt arrival of ILD as part of the picture in SJIA during the past decade remains unclear. The timing coincides with a major advance in the treatment of SJIA: the arrival of biologic agents blocking interleukin-1 and -6. Could this be a serious treatment side effect?
“It’s all association so far, and we’re not really sure why we’re seeing this association. Is it because we’re using a lot [fewer] corticosteroids now, and maybe those were preventing lung disease in the past?” Dr. Stevens speculated.
At this point, she and her fellow pediatric rheumatologists are awaiting further evidence before discussing a curb in their use of IL-1 or -6 inhibitors in patients with SJIA.
“These drugs have turned around the lives of kids with SJIA. They used to suffer through all our ineffective treatments for years, with terrible joint destruction and a pretty high mortality rate. These are great drugs for this disease, and we certainly don’t want to limit them,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Anti-Ro52 autoantibodies are the latest and most potent of the autoantibody predictors of interstitial lung disease (ILD) discovered in patients with juvenile dermatomyositis, Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
In addition to detailing the autoantibody red flags for ILD in juvenile dermatomyositis (JDM), she called for “hypervigilance” in patients with systemic juvenile idiopathic arthritis (SJIA) who exhibit any of a series of risk factors for ILD.
“Most of the lung disease in kids with systemic JIA is asymptomatic until very late, but it can be reversible if we treat it. So it’s worth finding and monitoring and giving everyone PCP [pneumocystis pneumonia] prophylaxis, because they have a high incidence of PCP if they have any of those risk factors,” observed Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 12% of 25 patients with juvenile polymyositis and in 18% of 44 youths with an overlap of juvenile connective tissue disease and myositis. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: Namely, 9% of the cohort were positive for antimelanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl tRNA synthestase (anti-Jo-1) autoantibodies were present in 4%.
A total of 33 of the 371 juvenile myositis patients had ILD based upon CT imaging, chest X-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo-1. After controlling for the presence of these other myositis-specific autoantibodies, auto-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52- and anti-Jo-1 positive.
Patients with anti-Ro52 autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Novel potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, intravenous immunoglobulin (IVIG), and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody-positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody-positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near-resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals.
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than did the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms.
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Risk factors for ILD in SJIA
In the past half-dozen years or so, pediatric rheumatologists have become increasingly aware of and concerned about a new development in SJIA: the occurrence of comorbid ILD. This is a poor-prognosis disease: In a cohort from the United Kingdom, 5-year mortality from the time of diagnosis was 41%, fully 40-fold higher than in patients with SJIA only.
Patient cohorts with SJIA and ILD have unusual clinical and laboratory features that aren’t part of the typical picture in SJIA. These include acute clubbing, lymphopenia, a fixed pruritic rash, unexplained abdominal pain, peripheral eosinophilia, facial swelling, and an increased ferritin level, a hallmark of acute macrophage activation syndrome. Onset of SJIA before 2 years of age is another red flag associated with increased risk for ILD. So is trisomy 21, which is up to 50 times more prevalent in patients with SJIA and ILD than in the general population or in patients with SJIA only. Another clue is an adverse reaction to tocilizumab (Actemra).
Any of these findings warrant hypervigilance: “Be on high alert and monitor these patients for ILD much more closely,” Dr. Stevens advised.
This means ordering a CT scan, prescribing PCP prophylaxis, and regularly measuring pulmonary function, admittedly a challenge in children under 7 years old. In these younger kids, practical solutions include measuring their oxygen saturation before and after running around the room to see if it drops. A 6-minute walk test and sleep oximetry are other options.
The explanation for the abrupt arrival of ILD as part of the picture in SJIA during the past decade remains unclear. The timing coincides with a major advance in the treatment of SJIA: the arrival of biologic agents blocking interleukin-1 and -6. Could this be a serious treatment side effect?
“It’s all association so far, and we’re not really sure why we’re seeing this association. Is it because we’re using a lot [fewer] corticosteroids now, and maybe those were preventing lung disease in the past?” Dr. Stevens speculated.
At this point, she and her fellow pediatric rheumatologists are awaiting further evidence before discussing a curb in their use of IL-1 or -6 inhibitors in patients with SJIA.
“These drugs have turned around the lives of kids with SJIA. They used to suffer through all our ineffective treatments for years, with terrible joint destruction and a pretty high mortality rate. These are great drugs for this disease, and we certainly don’t want to limit them,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Anti-Ro52 autoantibodies are the latest and most potent of the autoantibody predictors of interstitial lung disease (ILD) discovered in patients with juvenile dermatomyositis, Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
In addition to detailing the autoantibody red flags for ILD in juvenile dermatomyositis (JDM), she called for “hypervigilance” in patients with systemic juvenile idiopathic arthritis (SJIA) who exhibit any of a series of risk factors for ILD.
“Most of the lung disease in kids with systemic JIA is asymptomatic until very late, but it can be reversible if we treat it. So it’s worth finding and monitoring and giving everyone PCP [pneumocystis pneumonia] prophylaxis, because they have a high incidence of PCP if they have any of those risk factors,” observed Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 12% of 25 patients with juvenile polymyositis and in 18% of 44 youths with an overlap of juvenile connective tissue disease and myositis. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: Namely, 9% of the cohort were positive for antimelanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl tRNA synthestase (anti-Jo-1) autoantibodies were present in 4%.
A total of 33 of the 371 juvenile myositis patients had ILD based upon CT imaging, chest X-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo-1. After controlling for the presence of these other myositis-specific autoantibodies, auto-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52- and anti-Jo-1 positive.
Patients with anti-Ro52 autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Novel potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, intravenous immunoglobulin (IVIG), and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody-positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody-positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near-resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals.
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than did the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms.
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Risk factors for ILD in SJIA
In the past half-dozen years or so, pediatric rheumatologists have become increasingly aware of and concerned about a new development in SJIA: the occurrence of comorbid ILD. This is a poor-prognosis disease: In a cohort from the United Kingdom, 5-year mortality from the time of diagnosis was 41%, fully 40-fold higher than in patients with SJIA only.
Patient cohorts with SJIA and ILD have unusual clinical and laboratory features that aren’t part of the typical picture in SJIA. These include acute clubbing, lymphopenia, a fixed pruritic rash, unexplained abdominal pain, peripheral eosinophilia, facial swelling, and an increased ferritin level, a hallmark of acute macrophage activation syndrome. Onset of SJIA before 2 years of age is another red flag associated with increased risk for ILD. So is trisomy 21, which is up to 50 times more prevalent in patients with SJIA and ILD than in the general population or in patients with SJIA only. Another clue is an adverse reaction to tocilizumab (Actemra).
Any of these findings warrant hypervigilance: “Be on high alert and monitor these patients for ILD much more closely,” Dr. Stevens advised.
This means ordering a CT scan, prescribing PCP prophylaxis, and regularly measuring pulmonary function, admittedly a challenge in children under 7 years old. In these younger kids, practical solutions include measuring their oxygen saturation before and after running around the room to see if it drops. A 6-minute walk test and sleep oximetry are other options.
The explanation for the abrupt arrival of ILD as part of the picture in SJIA during the past decade remains unclear. The timing coincides with a major advance in the treatment of SJIA: the arrival of biologic agents blocking interleukin-1 and -6. Could this be a serious treatment side effect?
“It’s all association so far, and we’re not really sure why we’re seeing this association. Is it because we’re using a lot [fewer] corticosteroids now, and maybe those were preventing lung disease in the past?” Dr. Stevens speculated.
At this point, she and her fellow pediatric rheumatologists are awaiting further evidence before discussing a curb in their use of IL-1 or -6 inhibitors in patients with SJIA.
“These drugs have turned around the lives of kids with SJIA. They used to suffer through all our ineffective treatments for years, with terrible joint destruction and a pretty high mortality rate. These are great drugs for this disease, and we certainly don’t want to limit them,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
REPORTING FROM RWCS 2020
Multiple atopic dermatitis therapies completed or close to completing phase 3 studies
Major , Jonathan I. Silverberg, MD, PhD, said during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
“In the next 2-3 years, we may have nine new treatments approved for atopic dermatitis,” said Dr. Silverberg, director of clinical research and contact dermatitis at the University.
All nine medications he discussed are either in ongoing pivotal phase 3 randomized controlled trials or have completed their phase 3 developmental programs. “This is not theoretical; these are things you’re going to be using in your toolbox imminently,” he stressed.
Oral JAK inhibitors
The Janus kinase (JAK) pathway is the intracellular signaling mediator that interacts with extracellular inflammatory cytokines, including interleukin-4, -13, and -31, which are familiar to dermatologists because they’re targeted by potent biologic monoclonal antibody therapies. For example, IL-4 goes through JAK1 and 3, while IL-31 signals through JAK1 and 2.
“You really need to know the key JAK and STAT pathways involved in atopic dermatitis because it will help you determine the selectivity of the agents you’re going to be using,” the dermatologist advised.
Three oral, once-daily JAK inhibitors – abrocitinib, upadacitinib, and baricitinib – are in an advanced stage of development.
“Upadacitinib and abrocitinib may be the two most potent options coming to market soon for us to be thinking about,” Dr. Silverberg said.
Abrocitinib: Three positive phase 3 studies featuring this selective JAK1 inhibitor have been completed in adults with moderate to severe atopic dermatitis (AD). The most recent, JADE COMPARE, featured a head-to-head randomized comparison of abrocitinib and the injectable IL-4/IL-13 inhibitor dupilumab. The results of this 837-patient study haven’t yet been formally presented at a conference because of the COVID-19 pandemic. However, Pfizer recently announced that abrocitinib at 200 mg/day achieved significantly greater improvements than dupilumab (Dupixent) in the coprimary endpoints of skin clearance as reflected in an Investigator’s Global Assessment (IGA) score of 0 or 1 (that is, clear or almost clear) and disease extent based upon 75% reduction from baseline in Eczema Area and Severity Index (EASI 75) at 12 weeks. The same was true at 16 weeks.
Also, a significantly larger proportion of abrocitinib-treated patients achieved at least a 4-point reduction in itch severity as measured using the Peak Pruritus Numerical Rating Scale at week 2. The company plans to file for regulatory approval later this year.
The JADE COMPARE data are exciting because of a pressing unmet need for treatment options that are even more powerful than dupilumab, Dr. Silverberg said.
Upadacitinib: This is selective JAK1 inhibitor is not as far along in the developmental pipeline as abrocitinib, but the efficacy appears to be comparable. In a phase 2 study of 126 adults with moderate to severe AD, upadacitinib at the top dose of 30 mg/day achieved efficacy results Dr. Silverberg deemed “quite extraordinary,” with a rate of IGA score of 0 or 1 of 50% at 16 weeks and an EASI 75 response rate of 69%. Those findings numerically eclipsed results seen in an earlier phase 3 pivotal trial for dupilumab, in which the IGA 0/1 rate was 37% and EASI 75 was 48%, albeit with the caveat that cross-trial comparisons must be taken with a large grain of salt.
Baricitinib: Multiple phase 3 studies of this JAK 1/2 inhibitor have reported positive results. At the top dose of 4 mg/day, baricitinib appears to be less effective than dupilumab in its earlier pivotal trials.
“This may be a good oral option for our patients. It could be similar to the Otezla [apremilast] story in psoriasis: It’s perhaps not as effective as a lot of the biologics, but patients often prefer an oral option,” Dr. Silverberg said.
Of note, in one large, placebo-controlled, phase 3 study of baricitinib on top of background low- or medium-potency topical steroids, the IGA 0/1 rate at 16 weeks with placebo plus topical steroids was a modest 14.7%, which underscores that this long-time workhorse topical therapy is objectively less effective than most physicians think. In contrast, the IGA 0/1 rate with baricitinib at 4 mg/day plus topical steroids was a more respectable 30.6%.
All three oral JAK inhibitors have rapid onset of efficacy, a key advantage over the biologic agents.
“The issue you have to keep in mind is safety. The safety in the atopic dermatitis population was overall quite good for all three drugs. However, safety concerns have come up with JAK inhibitors in rheumatoid arthritis. I think that’s the part we watch the most in this. The efficacy has become clear. Now the question is where does the safety take us,” he said.
Novel injectable biologics
Nemolizumab: This humanized monoclonal antibody inhibits IL-31 receptor alpha. Mounting evidence implicates IL-31 as both a proinflammatory and immunomodulatory cytokine linking the immune and neural systems.
Early on, most researchers pigeonholed IL-31 as being a key player only in the itch factor in AD. Not so. Indeed, Dr. Silverberg was the lead investigator in a recent phase 2b study of nemolizumab that demonstrated the biologic is also effective at rapidly clearing AD lesions. The study, which evaluated three different doses in 226 adults with moderate to severe AD and severe pruritus who were on background topical corticosteroids, showed that nemolizumab at 30 mg every 4 weeks trounced placebo in terms of itch reduction: The 69% drop from baseline in Peak Pruritus Numeric Rating Scale at week 16 was twice that in controls, with a significant difference apparent even at week 1.
But in addition, the 33% IGA 0/1 rate at the same time point bested the 12% rate in controls. The EASI 75 response rate was significantly higher as well – 49% versus 19% – as was the EASI 90 response of 33%, compared with 9% in controls. Moreover, nemolizumab-treated patients used close to 40% less topical steroids during the study (J Allergy Clin Immunol. 2020 Jan;145[1]:173-82).
“This is something that’s fascinating. The study gets into the idea that a subset of atopic dermatitis patients have the itch that rashes, and perhaps if you break the itch/scratch cycle you can modify the lesions. Or the effect may even be due to the direct anti-inflammatory action of IL-31 blockade,” Dr. Silverberg observed.
It appeared that a plateau hadn’t been reached for some endpoints out at week 24, when the study ended. Japanese phase 3 studies have been completed, with what he called “great results,” and others are ongoing in the United States.
Tralokinumab: This fully human monoclonal antibody binds to IL-13, but unlike dupilumab, it doesn’t also inhibit IL-4. Tralokinumab met all primary and secondary endpoints in three pivotal phase 3 clinical trials, known as ECZTRA 1-3, that assessed it as treatment for moderate to severe AD in adults and showed an overall adverse event rate comparable with placebo. Leo Pharma, the Danish company developing the biologic, has announced it will file for marketing approval before the end of 2020. Phase 3 data would have been presented at the annual meeting of the American Academy of Dermatology in Denver, had it not been canceled. Dr. Silverberg said that, based upon phase 2 results, it appears tralokinumab may not be quite as effective as dupilumab in the overall AD population, but he predicted the newcomer will still play a useful role.
“The complexities of the immune system are such that some patients will respond better to one drug than another. I think we still have a lot to learn about who the patients are for these novel assets,” he said.
Lebrikizumab: This is another selective IL-13 inhibitor, but this one binds to IL-13 in a slightly different way than tralokinumab. The Food and Drug Administration granted it Fast Track status in December 2019. Twin placebo-controlled phase 3 studies of lebrikizumab as monotherapy for moderate to severe AD are ongoing, and another phase 3 trial of the biologic in combination with topical steroids is planned. Based upon the results of a phase 2b study, the highest dose studied – 250 mg every 2 weeks – appears to be at least as effective as dupilumab.
Nonsteroidal topical agents
These three late-stage topical creams – ruxolitinib, delgocitinib, and tapinarof – have previously received considerable coverage in Dermatology News. Ruxolitinib, a selective JAK1/2 inhibitor, has completed a positive phase 3 trial in adolescents and adults with mild to moderate AD. Delgocitinib, a pan-JAK1/2/3 and Tyrosine kinase 2 inhibitor, is already approved in an ointment formulation in Japan, and the cream formulation is in phase 2 studies in the United States and Europe. Tapinarof has a unique mechanism of action – it’s an aryl hydrocarbon receptor modulator – and is now in phase 3 in adolescents and adults with moderate to severe AD.
These three drugs appear to offer efficacy that’s comparable to or even better than medium-potency topical steroids, and without the notorious steroidal side effects that have caused widespread parental steroid-phobia. Potential applications for other inflammatory diseases, including vitiligo and psoriasis, are under study.
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to those pharmaceutical companies and more than a dozen others.
Major , Jonathan I. Silverberg, MD, PhD, said during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
“In the next 2-3 years, we may have nine new treatments approved for atopic dermatitis,” said Dr. Silverberg, director of clinical research and contact dermatitis at the University.
All nine medications he discussed are either in ongoing pivotal phase 3 randomized controlled trials or have completed their phase 3 developmental programs. “This is not theoretical; these are things you’re going to be using in your toolbox imminently,” he stressed.
Oral JAK inhibitors
The Janus kinase (JAK) pathway is the intracellular signaling mediator that interacts with extracellular inflammatory cytokines, including interleukin-4, -13, and -31, which are familiar to dermatologists because they’re targeted by potent biologic monoclonal antibody therapies. For example, IL-4 goes through JAK1 and 3, while IL-31 signals through JAK1 and 2.
“You really need to know the key JAK and STAT pathways involved in atopic dermatitis because it will help you determine the selectivity of the agents you’re going to be using,” the dermatologist advised.
Three oral, once-daily JAK inhibitors – abrocitinib, upadacitinib, and baricitinib – are in an advanced stage of development.
“Upadacitinib and abrocitinib may be the two most potent options coming to market soon for us to be thinking about,” Dr. Silverberg said.
Abrocitinib: Three positive phase 3 studies featuring this selective JAK1 inhibitor have been completed in adults with moderate to severe atopic dermatitis (AD). The most recent, JADE COMPARE, featured a head-to-head randomized comparison of abrocitinib and the injectable IL-4/IL-13 inhibitor dupilumab. The results of this 837-patient study haven’t yet been formally presented at a conference because of the COVID-19 pandemic. However, Pfizer recently announced that abrocitinib at 200 mg/day achieved significantly greater improvements than dupilumab (Dupixent) in the coprimary endpoints of skin clearance as reflected in an Investigator’s Global Assessment (IGA) score of 0 or 1 (that is, clear or almost clear) and disease extent based upon 75% reduction from baseline in Eczema Area and Severity Index (EASI 75) at 12 weeks. The same was true at 16 weeks.
Also, a significantly larger proportion of abrocitinib-treated patients achieved at least a 4-point reduction in itch severity as measured using the Peak Pruritus Numerical Rating Scale at week 2. The company plans to file for regulatory approval later this year.
The JADE COMPARE data are exciting because of a pressing unmet need for treatment options that are even more powerful than dupilumab, Dr. Silverberg said.
Upadacitinib: This is selective JAK1 inhibitor is not as far along in the developmental pipeline as abrocitinib, but the efficacy appears to be comparable. In a phase 2 study of 126 adults with moderate to severe AD, upadacitinib at the top dose of 30 mg/day achieved efficacy results Dr. Silverberg deemed “quite extraordinary,” with a rate of IGA score of 0 or 1 of 50% at 16 weeks and an EASI 75 response rate of 69%. Those findings numerically eclipsed results seen in an earlier phase 3 pivotal trial for dupilumab, in which the IGA 0/1 rate was 37% and EASI 75 was 48%, albeit with the caveat that cross-trial comparisons must be taken with a large grain of salt.
Baricitinib: Multiple phase 3 studies of this JAK 1/2 inhibitor have reported positive results. At the top dose of 4 mg/day, baricitinib appears to be less effective than dupilumab in its earlier pivotal trials.
“This may be a good oral option for our patients. It could be similar to the Otezla [apremilast] story in psoriasis: It’s perhaps not as effective as a lot of the biologics, but patients often prefer an oral option,” Dr. Silverberg said.
Of note, in one large, placebo-controlled, phase 3 study of baricitinib on top of background low- or medium-potency topical steroids, the IGA 0/1 rate at 16 weeks with placebo plus topical steroids was a modest 14.7%, which underscores that this long-time workhorse topical therapy is objectively less effective than most physicians think. In contrast, the IGA 0/1 rate with baricitinib at 4 mg/day plus topical steroids was a more respectable 30.6%.
All three oral JAK inhibitors have rapid onset of efficacy, a key advantage over the biologic agents.
“The issue you have to keep in mind is safety. The safety in the atopic dermatitis population was overall quite good for all three drugs. However, safety concerns have come up with JAK inhibitors in rheumatoid arthritis. I think that’s the part we watch the most in this. The efficacy has become clear. Now the question is where does the safety take us,” he said.
Novel injectable biologics
Nemolizumab: This humanized monoclonal antibody inhibits IL-31 receptor alpha. Mounting evidence implicates IL-31 as both a proinflammatory and immunomodulatory cytokine linking the immune and neural systems.
Early on, most researchers pigeonholed IL-31 as being a key player only in the itch factor in AD. Not so. Indeed, Dr. Silverberg was the lead investigator in a recent phase 2b study of nemolizumab that demonstrated the biologic is also effective at rapidly clearing AD lesions. The study, which evaluated three different doses in 226 adults with moderate to severe AD and severe pruritus who were on background topical corticosteroids, showed that nemolizumab at 30 mg every 4 weeks trounced placebo in terms of itch reduction: The 69% drop from baseline in Peak Pruritus Numeric Rating Scale at week 16 was twice that in controls, with a significant difference apparent even at week 1.
But in addition, the 33% IGA 0/1 rate at the same time point bested the 12% rate in controls. The EASI 75 response rate was significantly higher as well – 49% versus 19% – as was the EASI 90 response of 33%, compared with 9% in controls. Moreover, nemolizumab-treated patients used close to 40% less topical steroids during the study (J Allergy Clin Immunol. 2020 Jan;145[1]:173-82).
“This is something that’s fascinating. The study gets into the idea that a subset of atopic dermatitis patients have the itch that rashes, and perhaps if you break the itch/scratch cycle you can modify the lesions. Or the effect may even be due to the direct anti-inflammatory action of IL-31 blockade,” Dr. Silverberg observed.
It appeared that a plateau hadn’t been reached for some endpoints out at week 24, when the study ended. Japanese phase 3 studies have been completed, with what he called “great results,” and others are ongoing in the United States.
Tralokinumab: This fully human monoclonal antibody binds to IL-13, but unlike dupilumab, it doesn’t also inhibit IL-4. Tralokinumab met all primary and secondary endpoints in three pivotal phase 3 clinical trials, known as ECZTRA 1-3, that assessed it as treatment for moderate to severe AD in adults and showed an overall adverse event rate comparable with placebo. Leo Pharma, the Danish company developing the biologic, has announced it will file for marketing approval before the end of 2020. Phase 3 data would have been presented at the annual meeting of the American Academy of Dermatology in Denver, had it not been canceled. Dr. Silverberg said that, based upon phase 2 results, it appears tralokinumab may not be quite as effective as dupilumab in the overall AD population, but he predicted the newcomer will still play a useful role.
“The complexities of the immune system are such that some patients will respond better to one drug than another. I think we still have a lot to learn about who the patients are for these novel assets,” he said.
Lebrikizumab: This is another selective IL-13 inhibitor, but this one binds to IL-13 in a slightly different way than tralokinumab. The Food and Drug Administration granted it Fast Track status in December 2019. Twin placebo-controlled phase 3 studies of lebrikizumab as monotherapy for moderate to severe AD are ongoing, and another phase 3 trial of the biologic in combination with topical steroids is planned. Based upon the results of a phase 2b study, the highest dose studied – 250 mg every 2 weeks – appears to be at least as effective as dupilumab.
Nonsteroidal topical agents
These three late-stage topical creams – ruxolitinib, delgocitinib, and tapinarof – have previously received considerable coverage in Dermatology News. Ruxolitinib, a selective JAK1/2 inhibitor, has completed a positive phase 3 trial in adolescents and adults with mild to moderate AD. Delgocitinib, a pan-JAK1/2/3 and Tyrosine kinase 2 inhibitor, is already approved in an ointment formulation in Japan, and the cream formulation is in phase 2 studies in the United States and Europe. Tapinarof has a unique mechanism of action – it’s an aryl hydrocarbon receptor modulator – and is now in phase 3 in adolescents and adults with moderate to severe AD.
These three drugs appear to offer efficacy that’s comparable to or even better than medium-potency topical steroids, and without the notorious steroidal side effects that have caused widespread parental steroid-phobia. Potential applications for other inflammatory diseases, including vitiligo and psoriasis, are under study.
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to those pharmaceutical companies and more than a dozen others.
Major , Jonathan I. Silverberg, MD, PhD, said during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
“In the next 2-3 years, we may have nine new treatments approved for atopic dermatitis,” said Dr. Silverberg, director of clinical research and contact dermatitis at the University.
All nine medications he discussed are either in ongoing pivotal phase 3 randomized controlled trials or have completed their phase 3 developmental programs. “This is not theoretical; these are things you’re going to be using in your toolbox imminently,” he stressed.
Oral JAK inhibitors
The Janus kinase (JAK) pathway is the intracellular signaling mediator that interacts with extracellular inflammatory cytokines, including interleukin-4, -13, and -31, which are familiar to dermatologists because they’re targeted by potent biologic monoclonal antibody therapies. For example, IL-4 goes through JAK1 and 3, while IL-31 signals through JAK1 and 2.
“You really need to know the key JAK and STAT pathways involved in atopic dermatitis because it will help you determine the selectivity of the agents you’re going to be using,” the dermatologist advised.
Three oral, once-daily JAK inhibitors – abrocitinib, upadacitinib, and baricitinib – are in an advanced stage of development.
“Upadacitinib and abrocitinib may be the two most potent options coming to market soon for us to be thinking about,” Dr. Silverberg said.
Abrocitinib: Three positive phase 3 studies featuring this selective JAK1 inhibitor have been completed in adults with moderate to severe atopic dermatitis (AD). The most recent, JADE COMPARE, featured a head-to-head randomized comparison of abrocitinib and the injectable IL-4/IL-13 inhibitor dupilumab. The results of this 837-patient study haven’t yet been formally presented at a conference because of the COVID-19 pandemic. However, Pfizer recently announced that abrocitinib at 200 mg/day achieved significantly greater improvements than dupilumab (Dupixent) in the coprimary endpoints of skin clearance as reflected in an Investigator’s Global Assessment (IGA) score of 0 or 1 (that is, clear or almost clear) and disease extent based upon 75% reduction from baseline in Eczema Area and Severity Index (EASI 75) at 12 weeks. The same was true at 16 weeks.
Also, a significantly larger proportion of abrocitinib-treated patients achieved at least a 4-point reduction in itch severity as measured using the Peak Pruritus Numerical Rating Scale at week 2. The company plans to file for regulatory approval later this year.
The JADE COMPARE data are exciting because of a pressing unmet need for treatment options that are even more powerful than dupilumab, Dr. Silverberg said.
Upadacitinib: This is selective JAK1 inhibitor is not as far along in the developmental pipeline as abrocitinib, but the efficacy appears to be comparable. In a phase 2 study of 126 adults with moderate to severe AD, upadacitinib at the top dose of 30 mg/day achieved efficacy results Dr. Silverberg deemed “quite extraordinary,” with a rate of IGA score of 0 or 1 of 50% at 16 weeks and an EASI 75 response rate of 69%. Those findings numerically eclipsed results seen in an earlier phase 3 pivotal trial for dupilumab, in which the IGA 0/1 rate was 37% and EASI 75 was 48%, albeit with the caveat that cross-trial comparisons must be taken with a large grain of salt.
Baricitinib: Multiple phase 3 studies of this JAK 1/2 inhibitor have reported positive results. At the top dose of 4 mg/day, baricitinib appears to be less effective than dupilumab in its earlier pivotal trials.
“This may be a good oral option for our patients. It could be similar to the Otezla [apremilast] story in psoriasis: It’s perhaps not as effective as a lot of the biologics, but patients often prefer an oral option,” Dr. Silverberg said.
Of note, in one large, placebo-controlled, phase 3 study of baricitinib on top of background low- or medium-potency topical steroids, the IGA 0/1 rate at 16 weeks with placebo plus topical steroids was a modest 14.7%, which underscores that this long-time workhorse topical therapy is objectively less effective than most physicians think. In contrast, the IGA 0/1 rate with baricitinib at 4 mg/day plus topical steroids was a more respectable 30.6%.
All three oral JAK inhibitors have rapid onset of efficacy, a key advantage over the biologic agents.
“The issue you have to keep in mind is safety. The safety in the atopic dermatitis population was overall quite good for all three drugs. However, safety concerns have come up with JAK inhibitors in rheumatoid arthritis. I think that’s the part we watch the most in this. The efficacy has become clear. Now the question is where does the safety take us,” he said.
Novel injectable biologics
Nemolizumab: This humanized monoclonal antibody inhibits IL-31 receptor alpha. Mounting evidence implicates IL-31 as both a proinflammatory and immunomodulatory cytokine linking the immune and neural systems.
Early on, most researchers pigeonholed IL-31 as being a key player only in the itch factor in AD. Not so. Indeed, Dr. Silverberg was the lead investigator in a recent phase 2b study of nemolizumab that demonstrated the biologic is also effective at rapidly clearing AD lesions. The study, which evaluated three different doses in 226 adults with moderate to severe AD and severe pruritus who were on background topical corticosteroids, showed that nemolizumab at 30 mg every 4 weeks trounced placebo in terms of itch reduction: The 69% drop from baseline in Peak Pruritus Numeric Rating Scale at week 16 was twice that in controls, with a significant difference apparent even at week 1.
But in addition, the 33% IGA 0/1 rate at the same time point bested the 12% rate in controls. The EASI 75 response rate was significantly higher as well – 49% versus 19% – as was the EASI 90 response of 33%, compared with 9% in controls. Moreover, nemolizumab-treated patients used close to 40% less topical steroids during the study (J Allergy Clin Immunol. 2020 Jan;145[1]:173-82).
“This is something that’s fascinating. The study gets into the idea that a subset of atopic dermatitis patients have the itch that rashes, and perhaps if you break the itch/scratch cycle you can modify the lesions. Or the effect may even be due to the direct anti-inflammatory action of IL-31 blockade,” Dr. Silverberg observed.
It appeared that a plateau hadn’t been reached for some endpoints out at week 24, when the study ended. Japanese phase 3 studies have been completed, with what he called “great results,” and others are ongoing in the United States.
Tralokinumab: This fully human monoclonal antibody binds to IL-13, but unlike dupilumab, it doesn’t also inhibit IL-4. Tralokinumab met all primary and secondary endpoints in three pivotal phase 3 clinical trials, known as ECZTRA 1-3, that assessed it as treatment for moderate to severe AD in adults and showed an overall adverse event rate comparable with placebo. Leo Pharma, the Danish company developing the biologic, has announced it will file for marketing approval before the end of 2020. Phase 3 data would have been presented at the annual meeting of the American Academy of Dermatology in Denver, had it not been canceled. Dr. Silverberg said that, based upon phase 2 results, it appears tralokinumab may not be quite as effective as dupilumab in the overall AD population, but he predicted the newcomer will still play a useful role.
“The complexities of the immune system are such that some patients will respond better to one drug than another. I think we still have a lot to learn about who the patients are for these novel assets,” he said.
Lebrikizumab: This is another selective IL-13 inhibitor, but this one binds to IL-13 in a slightly different way than tralokinumab. The Food and Drug Administration granted it Fast Track status in December 2019. Twin placebo-controlled phase 3 studies of lebrikizumab as monotherapy for moderate to severe AD are ongoing, and another phase 3 trial of the biologic in combination with topical steroids is planned. Based upon the results of a phase 2b study, the highest dose studied – 250 mg every 2 weeks – appears to be at least as effective as dupilumab.
Nonsteroidal topical agents
These three late-stage topical creams – ruxolitinib, delgocitinib, and tapinarof – have previously received considerable coverage in Dermatology News. Ruxolitinib, a selective JAK1/2 inhibitor, has completed a positive phase 3 trial in adolescents and adults with mild to moderate AD. Delgocitinib, a pan-JAK1/2/3 and Tyrosine kinase 2 inhibitor, is already approved in an ointment formulation in Japan, and the cream formulation is in phase 2 studies in the United States and Europe. Tapinarof has a unique mechanism of action – it’s an aryl hydrocarbon receptor modulator – and is now in phase 3 in adolescents and adults with moderate to severe AD.
These three drugs appear to offer efficacy that’s comparable to or even better than medium-potency topical steroids, and without the notorious steroidal side effects that have caused widespread parental steroid-phobia. Potential applications for other inflammatory diseases, including vitiligo and psoriasis, are under study.
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to those pharmaceutical companies and more than a dozen others.
Cautionary findings on acquired immunodeficiency from anti-CD20 MS therapy
, Brandi L. Vollmer, MPH, reported online as part of the 2020 American Academy of Neurology Science Highlights.
The hypogammaglobulinemia was preceded by an IgM of 40 mg/dL or less in 35% of cases and was accompanied by concurrent development of low IgM in another 39%, added Ms. Vollmer, a professional research assistant at the Rocky Mountain Multiple Sclerosis Center at Anschutz Medical Campus, University of Colorado, Denver.
She presented a retrospective study of 527 randomly selected MS patients and another 17 with neuromyelitis optica spectrum disorder who averaged 44 years of age and a 9.2-year disease duration upon commencing rituximab (Rituxan) with close laboratory monitoring. Their mean cumulative rituximab dose during a mean 30.2 months of therapy was 3,312 mg. Ninety-six MS patients eventually switched to ocrelizumab (Ocrevus), accumulating a total dose of 1,175 mg of that anti-CD20 humanized monoclonal antibody.
Absolute lymphocyte count dropped to 500 cells/mm3 or lower in 10.4% of patients at a mean of 11.3 months into anti-CD20 therapy. Low immunoglobulins came later: The mean time to onset of low IgM in affected patients was 19.7 months, and hypogammaglobulinemia, as defined by an IgG of 500 mg/dL or less, occurred at a mean of 36.1 months. Higher cumulative doses of anti-CD20 agents were associated with increased likelihood of hypogammaglobulinemia.
Asked to comment on the research findings, neurologist Nida Laurin, MD, said the Colorado study provides helpful insights into the timing of onset of acquired immunodeficiency in patients on B-cell-targeted therapy.
“This paper informs us that we should monitor our patients much closer for signs of hypogammaglobulinemia and lymphopenia starting with year 2 on therapy, and switch treatment when the threshold is reached. I do expect production of gamma globulins and lymphocytes to recover with discontinuation of anti-CD20 therapy, maybe over a period of 6-10 months. It might also recover with lower-dose therapy because the effect on B cells is dose-dependent,” observed Dr. Laurin, an MS specialist at the Banner Health–University Medicine Neuroscience Institute in Phoenix and the University of Arizona in Tucson.
Her colleague Barry Hendin, MD, noted that there is no consensus regarding the best response to all these changes.
“Some clinicians add IVIG, some change therapies, and some observe only,” said Dr. Hendin, a neurologist at Banner Health–University Medical Center, Phoenix, and clinical professor of neurology at the University of Arizona in Tucson.
However, Dr. Laurin asserted that it would be a mistake for physicians and patients to shrug off anti-CD20 therapy–induced lymphopenia in light of studies demonstrating that lymphopenia and older age are two main risk factors for progressive multifocal leukoencephalopathy in patients on disease-modifying therapies.
“More cases of PML can be expected with continuous use of anti-CD20 therapies if lymphopenia is ignored,” she cautioned.
Depressed levels of IgM and IgG have been associated with increased risk of serious infections. In light of the COVID-19 pandemic and the eventual prospect of a vaccine, it is especially important to avoid putting patients with MS in harm’s way via treatment-induced acquired immunodeficiency, Dr. Laurin said.
Ms. Vollmer reported having no financial conflicts regarding her study. Dr. Laurin reported serving as a speaker or consultant for Alexion, Allergan, Biogen, Bristol-Myers Squibb, EMD Serono, Genentech, Lundbeck, and Sanofi Genzyme. Dr. Hendin serves as a consultant to Biogen, Genentech, Genzyme, EMD Serono, Novartis, and Bristol-Myers Squibb.
SOURCE: Vollmer BL et al. AAN 2020. Abstract S29.002.
, Brandi L. Vollmer, MPH, reported online as part of the 2020 American Academy of Neurology Science Highlights.
The hypogammaglobulinemia was preceded by an IgM of 40 mg/dL or less in 35% of cases and was accompanied by concurrent development of low IgM in another 39%, added Ms. Vollmer, a professional research assistant at the Rocky Mountain Multiple Sclerosis Center at Anschutz Medical Campus, University of Colorado, Denver.
She presented a retrospective study of 527 randomly selected MS patients and another 17 with neuromyelitis optica spectrum disorder who averaged 44 years of age and a 9.2-year disease duration upon commencing rituximab (Rituxan) with close laboratory monitoring. Their mean cumulative rituximab dose during a mean 30.2 months of therapy was 3,312 mg. Ninety-six MS patients eventually switched to ocrelizumab (Ocrevus), accumulating a total dose of 1,175 mg of that anti-CD20 humanized monoclonal antibody.
Absolute lymphocyte count dropped to 500 cells/mm3 or lower in 10.4% of patients at a mean of 11.3 months into anti-CD20 therapy. Low immunoglobulins came later: The mean time to onset of low IgM in affected patients was 19.7 months, and hypogammaglobulinemia, as defined by an IgG of 500 mg/dL or less, occurred at a mean of 36.1 months. Higher cumulative doses of anti-CD20 agents were associated with increased likelihood of hypogammaglobulinemia.
Asked to comment on the research findings, neurologist Nida Laurin, MD, said the Colorado study provides helpful insights into the timing of onset of acquired immunodeficiency in patients on B-cell-targeted therapy.
“This paper informs us that we should monitor our patients much closer for signs of hypogammaglobulinemia and lymphopenia starting with year 2 on therapy, and switch treatment when the threshold is reached. I do expect production of gamma globulins and lymphocytes to recover with discontinuation of anti-CD20 therapy, maybe over a period of 6-10 months. It might also recover with lower-dose therapy because the effect on B cells is dose-dependent,” observed Dr. Laurin, an MS specialist at the Banner Health–University Medicine Neuroscience Institute in Phoenix and the University of Arizona in Tucson.
Her colleague Barry Hendin, MD, noted that there is no consensus regarding the best response to all these changes.
“Some clinicians add IVIG, some change therapies, and some observe only,” said Dr. Hendin, a neurologist at Banner Health–University Medical Center, Phoenix, and clinical professor of neurology at the University of Arizona in Tucson.
However, Dr. Laurin asserted that it would be a mistake for physicians and patients to shrug off anti-CD20 therapy–induced lymphopenia in light of studies demonstrating that lymphopenia and older age are two main risk factors for progressive multifocal leukoencephalopathy in patients on disease-modifying therapies.
“More cases of PML can be expected with continuous use of anti-CD20 therapies if lymphopenia is ignored,” she cautioned.
Depressed levels of IgM and IgG have been associated with increased risk of serious infections. In light of the COVID-19 pandemic and the eventual prospect of a vaccine, it is especially important to avoid putting patients with MS in harm’s way via treatment-induced acquired immunodeficiency, Dr. Laurin said.
Ms. Vollmer reported having no financial conflicts regarding her study. Dr. Laurin reported serving as a speaker or consultant for Alexion, Allergan, Biogen, Bristol-Myers Squibb, EMD Serono, Genentech, Lundbeck, and Sanofi Genzyme. Dr. Hendin serves as a consultant to Biogen, Genentech, Genzyme, EMD Serono, Novartis, and Bristol-Myers Squibb.
SOURCE: Vollmer BL et al. AAN 2020. Abstract S29.002.
, Brandi L. Vollmer, MPH, reported online as part of the 2020 American Academy of Neurology Science Highlights.
The hypogammaglobulinemia was preceded by an IgM of 40 mg/dL or less in 35% of cases and was accompanied by concurrent development of low IgM in another 39%, added Ms. Vollmer, a professional research assistant at the Rocky Mountain Multiple Sclerosis Center at Anschutz Medical Campus, University of Colorado, Denver.
She presented a retrospective study of 527 randomly selected MS patients and another 17 with neuromyelitis optica spectrum disorder who averaged 44 years of age and a 9.2-year disease duration upon commencing rituximab (Rituxan) with close laboratory monitoring. Their mean cumulative rituximab dose during a mean 30.2 months of therapy was 3,312 mg. Ninety-six MS patients eventually switched to ocrelizumab (Ocrevus), accumulating a total dose of 1,175 mg of that anti-CD20 humanized monoclonal antibody.
Absolute lymphocyte count dropped to 500 cells/mm3 or lower in 10.4% of patients at a mean of 11.3 months into anti-CD20 therapy. Low immunoglobulins came later: The mean time to onset of low IgM in affected patients was 19.7 months, and hypogammaglobulinemia, as defined by an IgG of 500 mg/dL or less, occurred at a mean of 36.1 months. Higher cumulative doses of anti-CD20 agents were associated with increased likelihood of hypogammaglobulinemia.
Asked to comment on the research findings, neurologist Nida Laurin, MD, said the Colorado study provides helpful insights into the timing of onset of acquired immunodeficiency in patients on B-cell-targeted therapy.
“This paper informs us that we should monitor our patients much closer for signs of hypogammaglobulinemia and lymphopenia starting with year 2 on therapy, and switch treatment when the threshold is reached. I do expect production of gamma globulins and lymphocytes to recover with discontinuation of anti-CD20 therapy, maybe over a period of 6-10 months. It might also recover with lower-dose therapy because the effect on B cells is dose-dependent,” observed Dr. Laurin, an MS specialist at the Banner Health–University Medicine Neuroscience Institute in Phoenix and the University of Arizona in Tucson.
Her colleague Barry Hendin, MD, noted that there is no consensus regarding the best response to all these changes.
“Some clinicians add IVIG, some change therapies, and some observe only,” said Dr. Hendin, a neurologist at Banner Health–University Medical Center, Phoenix, and clinical professor of neurology at the University of Arizona in Tucson.
However, Dr. Laurin asserted that it would be a mistake for physicians and patients to shrug off anti-CD20 therapy–induced lymphopenia in light of studies demonstrating that lymphopenia and older age are two main risk factors for progressive multifocal leukoencephalopathy in patients on disease-modifying therapies.
“More cases of PML can be expected with continuous use of anti-CD20 therapies if lymphopenia is ignored,” she cautioned.
Depressed levels of IgM and IgG have been associated with increased risk of serious infections. In light of the COVID-19 pandemic and the eventual prospect of a vaccine, it is especially important to avoid putting patients with MS in harm’s way via treatment-induced acquired immunodeficiency, Dr. Laurin said.
Ms. Vollmer reported having no financial conflicts regarding her study. Dr. Laurin reported serving as a speaker or consultant for Alexion, Allergan, Biogen, Bristol-Myers Squibb, EMD Serono, Genentech, Lundbeck, and Sanofi Genzyme. Dr. Hendin serves as a consultant to Biogen, Genentech, Genzyme, EMD Serono, Novartis, and Bristol-Myers Squibb.
SOURCE: Vollmer BL et al. AAN 2020. Abstract S29.002.
REPORTING FROM AAN 2020









