User login
New-onset psychosis while being treated for coronavirus
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.
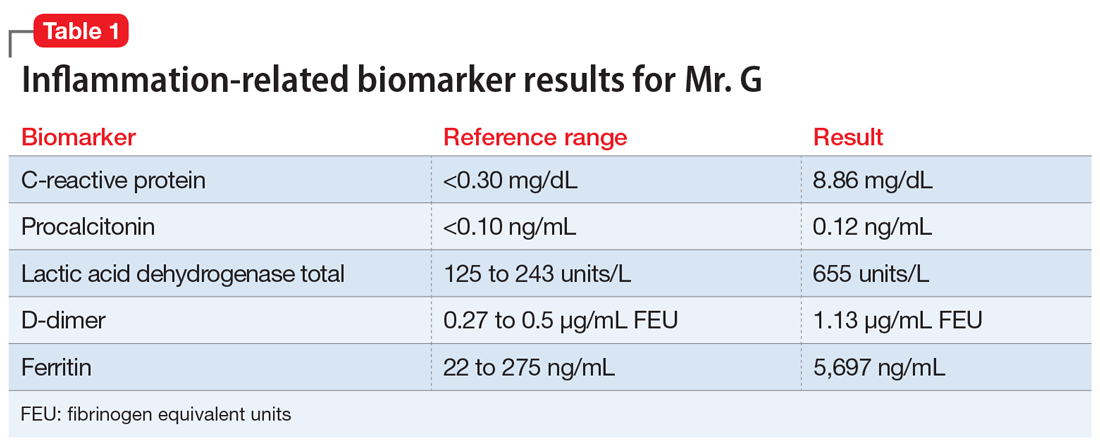
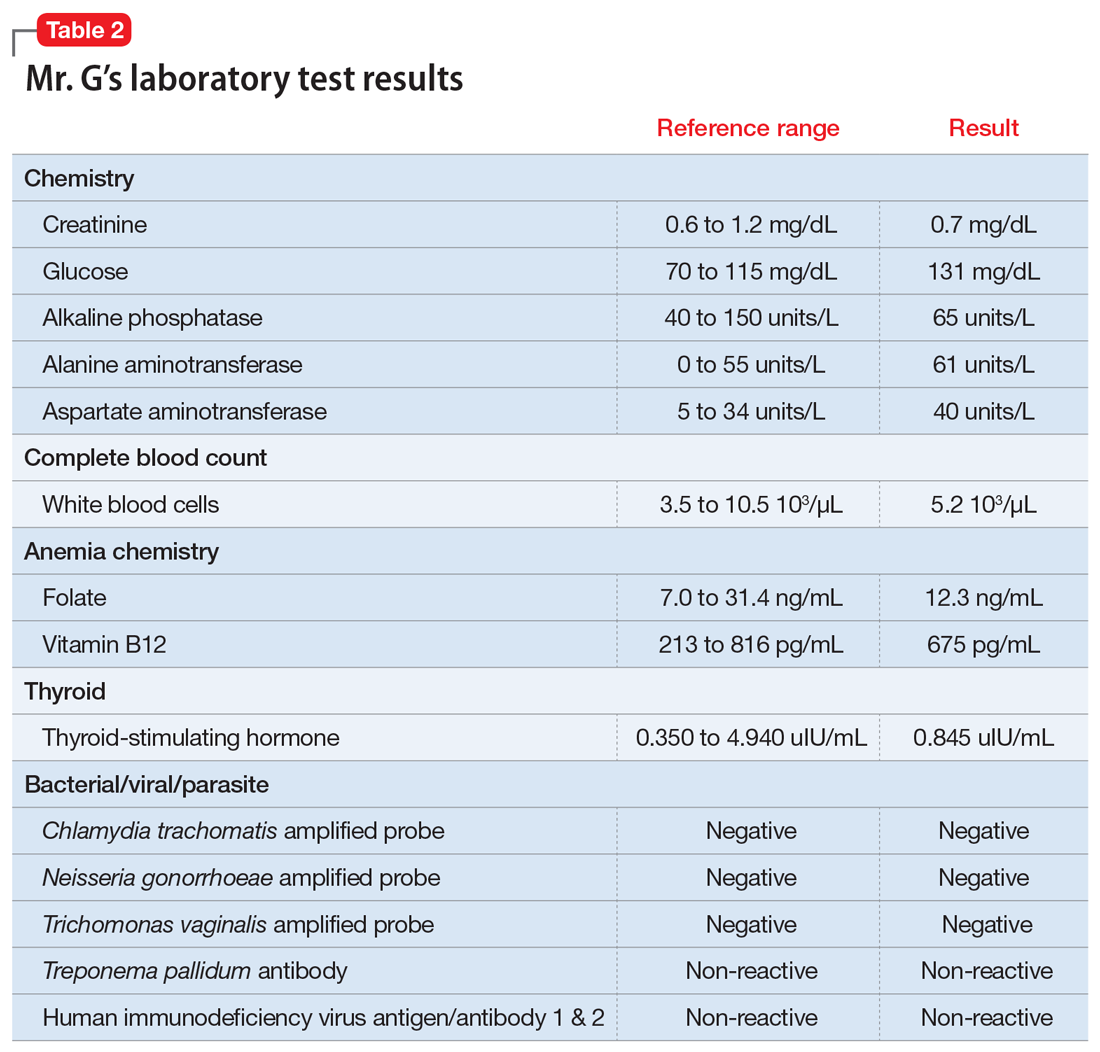
Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.
Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.

Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.

Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.

Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.

Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
The psychiatrist’s role in liver transplantation
Since the first liver transplant (LT) was performed in 1963 by Starzl et al, there have been considerable advances in the field, with improvements in post-transplant survival.1 There are multiple indications for LT, including acute liver failure and index complications of cirrhosis such as ascites, encephalopathy, and hepatopulmonary syndrome.2 Once a patient develops one of these conditions, he/she is evaluated for LT, even as the complications of liver failure are being managed.
Although the number of LTs has risen, the demand for transplant continues to exceed availability. In 2015, chronic liver disease and cirrhosis was the 12th leading cause of death in the United States.3 In 2016, approximately 50% of waitlisted candidates received a transplant.4 There is also a donor shortage. In part, this shortage may be due to longer life spans and the subsequent increase in the age of the potential donor.5 In light of this shortage and increased demand, the pre-LT workup is comprehensive. The pre-transplant assessment typically consists of cardiology, surgery, hepatology, and psychosocial evaluations, and hence requires a team of experts to determine who is an ideal candidate for transplant.
Psychiatrists play a key role in the pre-transplant psychosocial evaluations. This article describes the elements of these evaluations, and what psychiatrists can do to help patients both before and after they undergo LT.
Elements of the pre-transplant evaluation
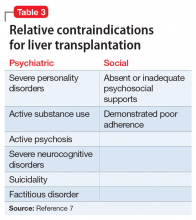
The psychosocial evaluation is a critical component of the pre-transplant assessment. As part of the evaluation, patients are screened for psychosocial limitations that may complicate transplantation, such as demonstrated noncompliance, ongoing alcohol or drug use, and lack of social support (Table 12 ). Other goals of the psychosocial evaluation are to identify in the pre-transplant period patients with possible risk factors, such as substance use or psychiatric disorders, and develop treatment plans to optimize transplant outcomes (Table 26). There are relative contraindications to LT (Table 37) but no absolute psychiatric contraindications, according to the 2013 American Association for the Study of Liver Diseases (AASLD) practice guideline for transplantation.2
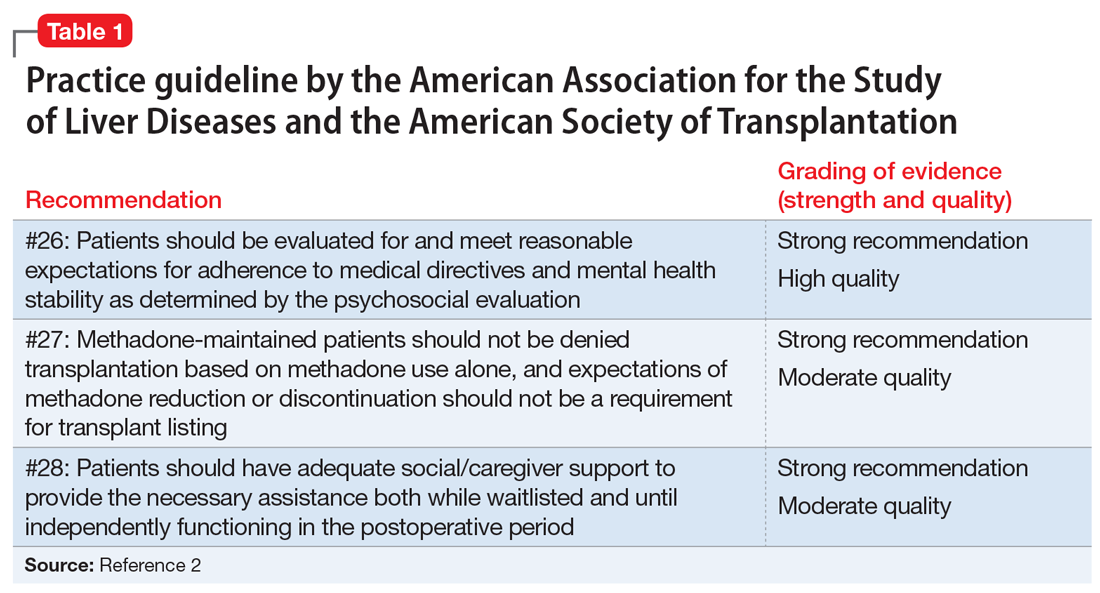
Adherence. The 2013 AASLD practice guideline states that patients “should be evaluated for and meet reasonable expectations for adherence to medical directives and mental health stability as determined by the psychosocial evaluation.”2 In the transplant setting, adherence is complex. It requires compliance with complicated medication regimens and laboratory testing, frequent follow-up appointments, and close, prompt communication of concerns to the health care team. Patient adherence to medication regimens plays an important role in transplant outcomes.8 In fact, in patients who have undergone renal transplant, nonadherence to therapy is considered the leading cause of avoidable graft failure.9
A retrospective study of adult LT recipients found that pre-transplant chart evidence of nonadherence, such as missed laboratory testing and clinic visits, was a significant predictor of post-transplant nonadherence with immunosuppressant therapy. Pre-transplant unemployment status and a history of substance abuse also were associated with nonadherence.9
Dobbels et al10 found that patients with a self-reported history of pre-transplant non-adherence had a higher risk of being nonadherent with their immunosuppressive therapy after transplant (odd ratio [OR]: 7.9). Their self-report adherence questionnaire included questions that addressed pre-transplant smoking status, alcohol use, and adherence with medication. In this prospective study, researchers also found that patients with a low “conscientiousness” score were at a higher risk for post-transplant medication nonadherence (OR: 0.8).
Continue to: Studies have also found...
Studies have also found that patients with higher education are more at risk for post-transplant medication nonadherence. Higher education may be associated with higher employment status resulting in a busier lifestyle, a known risk factor that may prevent patients from regular medication adherence.11,12 Alternatively, it is possible that higher educated patients are “decisive” nonadherers who prefer independent decision-making regarding their disease and treatment.13
Substance use. The 2013 AASLD practice guideline lists “ongoing alcohol or illicit substance abuse” as one of the contraindications to LT.2 In guidelines from the Austrian Society for Gastroenterology and Hepatology, Graziadei et al14 listed “alcohol addiction without motivation for alcohol abstinence and untreated/ongoing substance abuse” as absolute contraindications and “untreated alcohol abuse and other drug-related addiction” as relative contraindications. Hence, the pre-transplant evaluation should include a thorough substance use history, including duration, amount, previous attempts to quit, and motivation for abstinence.
Substance use history is especially important because alcoholic liver disease is the second most common indication for LT.2 Most LT programs require 6 months of abstinence before a patient can be considered for transplant.15 The 6-month period was based on studies demonstrating that pre-transplant abstinence from alcohol for <6 months is a risk factor for relapse.15 However, this guideline remains controversial because the transplant referral and workup may be delayed as the patient’s liver disease worsens. Other risk factors for substance relapse should also be taken into consideration, such as depression, personality disorders, lack of social support, severity of alcohol use, and family history of alcoholism.16 Lee and Leggio16 developed the Sustained Alcohol Use Post-Liver Transplant (SALT) score to identify patients who were at risk for sustained alcohol use posttransplant. The 4 SALT criteria are:
- >10 drinks per day at initial hospitalization (+4 points)
- multiple prior rehabilitation attempts (+4 points)
- prior alcohol‐related legal issues (+2 points), and
- prior illicit substance abuse (+1 point).
A SALT score can range from 0 to 11. Lee et al17 found a SALT score ≥5 had a 25% positive predictive value (95% confidence interval [CI]: 10% to 47%) and a SALT score of <5 had a 95% negative predictive value (95% CI: 89% to 98%) for sustained alcohol use post‐LT. Thus, the 2013 AASLD guideline cautions against delaying evaluation based on the 6-month abstinence rule, and instead recommends early transplant referral for patients with alcoholic liver disease to encourage such patients to begin addiction treatment.2
As part of the substance use history, it is important to ask about the patient’s smoking history. Approximately 60% of LT candidates have a history of smoking cigarettes.18 Tobacco use history is associated with increased post-transplant vascular complications, such as hepatic artery thrombosis or stenosis, portal vein thrombosis, and deep vein thrombosis.19 The 2013 AASLD guideline recommends that tobacco use should be prohibited in LT candidates.2 Pungpapong et al19 reported that smoking cessation for at least 2 years prior to transplant led to a significantly decreased risk of developing arterial complications, with an absolute risk reduction of approximately 16%.
Continue to: Liver cirrhosis due to...
Liver cirrhosis due to chronic hepatitis C virus (HCV) infection is one of the leading causes for LT. In the United States, HCV is commonly transmitted during injection drug use. According to the 2013 AASLD guideline, ongoing illicit substance use is a relative contraindication to LT.2 It is important to note, however, that methadone maintenance therapy (MMT) is not a contraindication to LT. In fact, the 2013 AASLD guideline recommends that patients receiving MMT should not be required to reduce or stop therapy in order to be listed for transplant.2 Studies have shown that in 80% of patients, tapering MMT leads to illicit opiate relapse.20 Currently, there is no evidence that patients receiving MMT have poorer post-transplant outcomes compared with patients not receiving MMT.21
Whether cannabis use is a relative contraindication to LT remains controversial.22 Possible adverse effects of cannabis use in transplant patients include drug–drug interactions and infections. Hézode et al23 reported that daily cannabis use is significantly associated with an increased fibrosis progression rate in patients with chronic HCV infection. Another recent study found that a history of cannabis use was not associated with worse outcomes among patients on the LT waitlist.24 With the increased legalization of cannabis, more studies are needed to assess ongoing cannabis use in patients on the LT waitlist and post-LT outcomes.
Psychiatric history. When assessing a patient for possible LT, no psychiatric disorder is considered an absolute contraindication. Patients with a serious mental illness, such as schizophrenia, and those with intellectual disability can have successful, long-term outcomes with proper evaluation and preparation, including social support. However, empirical literature regarding transplant outcomes and predictive factors in patients with serious mental illness is scarce.2
Studies examining the predictive value of pre-transplant depression on post-transplant outcomes have had mixed results.25 Depression may predict lower post-transplant quality of life. Pre-LT suicidal thoughts (as noted on the Beck Depression Inventory, for example) are associated with post-LT depression.25 In contrast, available data show no significant effect of pre-transplant anxiety on post-LT outcomes. Similarly, pre-transplant cognitive performance appears not to predict survival or other post-transplant outcomes, but may predict poorer quality of life after transplant.25
A few psychiatric factors are considered relative contraindications for LT. These include severe personality disorders, active substance use with no motivation for treatment or abstinence, active psychosis, severe neurocognitive disorders, suicidality, and factitious disorder.7
Continue to: Social support
Social support. Assessing a pre-LT patient’s level of social support is an essential part of the psychosocial evaluation. According to the 2013 AASLD guideline, patients should have “adequate” social support both during the waitlist and post-operative periods.2 Lack of partnership is a significant predictor of poor post-transplant outcomes, such as late graft loss.10 Satapathy and Sanyal26 reported that among patients who receive an LT for alcoholic liver disease, those with immediate family support were less likely to relapse to using alcohol after transplant. Poor social support was also a predictor of post-transplant medication nonadherence.10 Thus, the patient needs enough social support to engage in the pre-transplant health care requirements and to participate in post-transplant recommendations until he/she is functioning independently post-transplant.
Screening tools
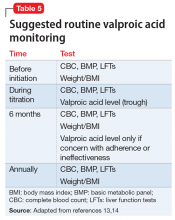
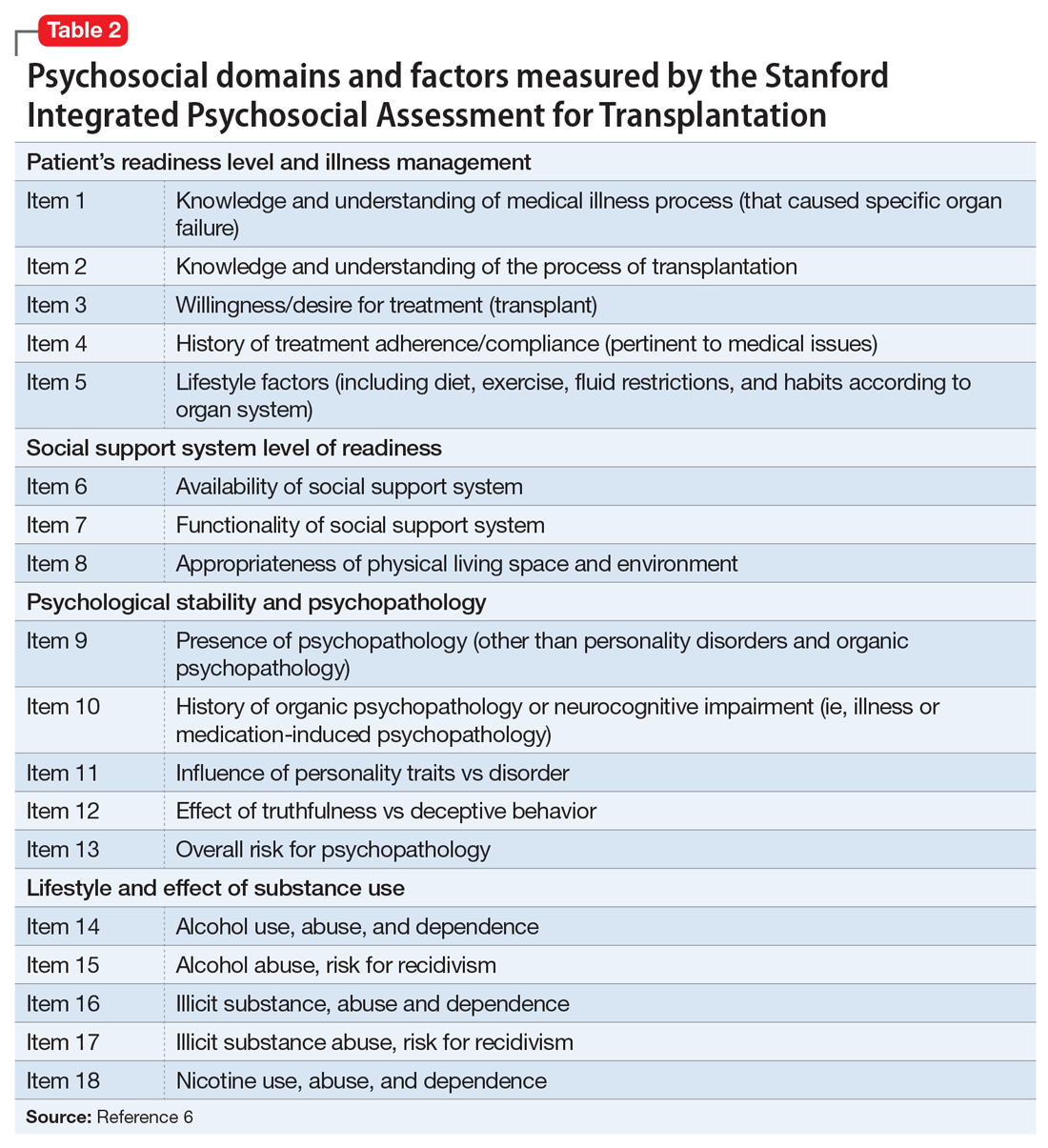
Various screening tools may be useful in a pre-LT evaluation. Three standardized assessment tools available specifically for pre-transplant psychosocial assessments are the Stanford Integrated Psychosocial Assessment for Transplantation (Table 26), the Psychosocial Assessment of Candidates for Transplantation,27 and the Transplant Evaluation Rating Scale.28 Instruments to aid in the assessment of depression, anxiety, and delirium,29-31 a structured personality assessment,32 coping inventories,33 neuropsychological batteries,34 and others also have been used to evaluate patients before LT. The self-rated Beck Depression Inventory and the clinician-rated Hamilton Depression Rating Scale are commonly used.7 Other tools, such as the LEIPAD quality of life instrument and the Brief Symptom Inventory (BSI), have been used to assess for perceived quality of life and psychological distress, respectively.35 These screening tools can be helpful as aids for the pre-LT evaluation; however, diagnoses and treatment plan recommendations require a psychiatric evaluation conducted by a trained clinician.
Treatment after liver transplant
Psychiatric issues. After LT, various psychiatric complications may arise, including (but not limited to) delirium7 and “paradoxical psychiatric syndrome” (PPS).36 Delirium can be managed by administering low-dose antipsychotic medications, limiting the use of benzodiazepines and medications with anticholinergic effects, implementing behavioral interventions (frequent orientation, maintaining sleep/wake cycle, limiting noise, presence of a family member or a sitter at bedside),37 and addressing the underlying etiology. Paradoxical psychiatric syndrome is defined as psychiatric symptoms that occur despite a successful LT. It develops within the first year of transplantation and is characterized by recipients having strong guilt feelings toward their donors.38
Drug interactions. In the post-transplant period, antipsychotics are used for management of delirium and psychosis, antidepressants for anxiety and depression, and benzodiazepines for anxiety and sleep problems.7 Drug–drug interactions between psychotropic medications and the immunosuppressants required after LT must be closely monitored. First-generation antipsychotics should be avoided in post-transplant patients taking tacrolimus due to the increased risk of QTc prolongation. Tacrolimus can also increase the risk of nephrotoxicity when co-administered with lithium. Post-LT patients taking steroids and bupropion have an increased risk of seizure. Carbamazepine may decrease blood levels of cyclosporine due to the induction of hepatic metabolism.39,40 The psychiatrist should review and update the patient’s complete medication list at each visit, checking for possible medication interactions.
Quality of life. In the first 6 months post-transplant, patients typically experience improved quality of life in both physical and psychological domains. However, this improvement vacillates as the patient adjusts to post-transplant life. A reduction in BSI score 1 year after transplant has been reported. The BSI evaluates psychopathological symptoms, which are early indicators of psychological discomfort. One study noted a reduction in the LEIPAD quality of life score, which measures overall quality of life, 2 years after transplant.35 This decline may reflect the difficulties associated with the new challenges after transplant. Patients may endure both physical changes due to medical complications as well as psychological problems as they adjust to their new bodily integrity, their dependence on medications and medical staff, and other changes in function. Three to 5 years after transplant, patients reached a new psychological stability, with reported improvements in quality of life and decreased psychological distress.35
Continue to: Special populations
Special populations
HCV infection. Recurrent HCV infection and liver disease after transplantation are associated with psychological distress. This is particularly evident in patients 6 months after transplantation. Depression and psychological distress have been reported in male patients with recurrent HCV infection within the first year after transplantation.35
Acetaminophen overdose. Patients who receive a transplant for acetaminophen-induced acute liver failure (ALF) had a greater prevalence of psychiatric comorbidity as reflected by predefined diagnoses, medication, and previous suicide attempts.41 Despite this, outcomes for patients transplanted emergently for acetaminophen-induced ALF were comparable to those transplanted for non-acetaminophen-induced ALF and for chronic liver disease. Multidisciplinary approaches with long-term psychiatric follow-up may contribute to low post-transplant suicide rates and low rates of graft loss because of noncompliance.41
CASE REPORT
A complicated presentation
Ms. A, age 45, a married woman with history of chronic back pain and self-reported bipolar disorder, presented to our hospital with acute liver failure secondary to acetaminophen overdose. Her Model for End-Stage Liver Disease (MELD) score on presentation was 38 (range: 0 to 40 with higher scores indicating increased likelihood of mortality). Her urine drug screen was positive for benzodiazepines and opiates. On hospital Day 2, the primary team consulted psychiatry for a pre-transplant evaluation and consideration of suicidality. Hepatology, toxicology, and transplant surgery services also were consulted.
Because Ms. A was intubated for acute respiratory failure, the initial history was gathered from family, a review of the medical record, consultation with her pharmacy, and collateral from her outpatient physician. Ms. A had been taking
Four days before presenting with acute liver failure, Ms. A had visited another hospital for lethargy. Benzodiazepines and opiates were stopped abruptly, and she was discharged with the recommendation to take acetaminophen for her pain. Approximately 24 hours after returning home, Ms. A began having auditory and visual hallucinations, and she did not sleep for days. She continued to complain of pain and was taking acetaminophen as recommended by the outside hospital. Her husband notes that she was intermittently confused. He was unsure how much acetaminophen she was taking.
Continue to: Her family noted...
Her family noted Ms. A had been diagnosed with bipolar disorder “years ago” but was unable to describe any manic episodes, and Ms. A had been treated only with an antidepressant from her primary care physician. She had persistent low mood and increased sleep since developing chronic back pain that severely limited her functioning. Ms. A attempted suicide once years ago by cutting her wrists. She had 2 prior psychiatric hospitalizations for suicidal ideation and the suicide attempt; however, she had not recently voiced suicidal ideation to her husband or family. She was adherent to psychotropic medications and follow-up appointments. Ms. A is a current smoker. She had used marijuana in the past, but her family denies current use, as well as any alcohol use or illicit substance use.
Ms. A’s diagnosis was consistent with tobacco use disorder and major depressive disorder (MDD). She likely developed withdrawal after abrupt cessation of diazepam, which she had been taking as prescribed for years. There was no evidence at the time of her initial psychiatric evaluation that the acetaminophen overdose was a suicide attempt; however, because Ms. A was intubated and sedated at that time, the consultation team recommended direct observation until she could participate in a risk assessment.
For the pre-transplant psychiatric evaluation, our consultation-liaison team noted Ms. A’s history of MDD, with recent active symptoms, chronic pain, and a past suicide attempt. She was a current tobacco smoker, which increases the risk of post-transplant vascular problems. However, she had been adherent to medications and follow-up, had very close family support, and there was no clear evidence that this acetaminophen ingestion was a suicide attempt. We noted that outpatient psychiatric follow-up and better chronic pain management would be helpful post-transplant. We would have to re-evaluate Ms. A when she was medically stable enough to communicate before making any further recommendations. Due to medical complications that developed after our evaluation, the transplant team noted Ms. A was no longer a transplant candidate.
Fortunately, Ms. A recovered with medical management over the next 2 weeks. She denied any suicidal ideation throughout her hospitalization. She was restarted on an antidepressant and received supportive therapy until discharge. Outpatient psychiatry follow-up and pain management was set up before Ms. A was discharged. Inpatient psychiatric hospitalization was not recommended. Per available records, Ms. A followed up with all outpatient appointments, including with her psychiatrist, since discharge.
Avoiding problems, maximizing outcomes
In addition to medical factors, psychosocial factors may affect the success of LT, although empirical data regarding which factors are most predictive of post-transplant outcomes is lacking, especially in patients with serious mental illness. The goals of a psychosocial pre-transplant evaluation are to promote fairness and equal access to care, maximize optimal outcomes, wisely use scarce resources, and ensure that the potential for benefits outweigh surgical risks to the patient. Identifying potential complicating factors (ie, substance abuse, nonadherence, serious psychopathology) can help guide the medical and psychiatric treatment plan and help minimize preventable problems both before and after transplant.42
Continue to: In patients who have...
In patients who have a history of alcohol use and alcohol liver disease, relapse to alcohol is a significant problem. Relapse rates vary from 10% to 30%.7 The duration of abstinence before LT appears to be a poor predictor of abstinence after LT.43 Polysubstance use also adversely affects outcomes in patients with alcohol liver disease. Approximately one-third of patients with polysubstance use who receive a LT relapse to substance use.44 Coffman et al45 showed that the presence of antisocial behavior and eating disorders may increase the risk of relapse after LT.
The psychiatrist’s role in the setting of LT spans from the pre-transplant assessment to post-transplant management and follow-up. Clarifying specific psychiatric diagnoses, psychosocial factors that need to be addressed before transplant, and substance use diagnoses and treatment recommendations can help the transplant team clearly identify modifiable factors that can affect transplant outcomes.
Bottom Line
Psychiatrists can help patients who are candidates for liver transplantation (LT) by performing a pre-transplant psychosocial assessment to identity factors that might complicate transplantation or recovery. After LT, patients require careful monitoring for psychiatric comorbidities, drug interactions, and other factors that can affect quality of life.
Related Resources
- Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018;53(2):135-144.
- Marcangelo MJ, Crone C. Tools, techniques to assess organ transplant candidates. Current Psychiatry. 2007;6(9):56-66.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Hydromorphone • Dilaudid
Lithium • Eskalith, Lithobid
Tacrolimus • Astagraf XL, Envarsus XR
1. Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives [Article in English, Portuguese]. Einstein (São Paulo). 2015;13(1):149-152.
2. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144-1165.
3. Centers for Disease Control and Prevention. QuickStats: number of deaths from 10 leading causes,* by sex—National Vital Statistics System, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(15):413.
4. Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31(1):84-89.
5. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59.
6. Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123-132.
7. Grover S, Sarkar S. Liver transplant—psychiatric and psychosocial aspects. J Clin Exp Hepatol. 2012;2(4):382-392.
8. Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760-770.
9. Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824-834.
10. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497-1504.
11. De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323.
12. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172-183.
13. Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718-1726.
14. Graziadei I, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 2016;128(19):679-690.
15. Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981-987.
16. Lee MR, Leggio L. Management of alcohol use disorder in patients requiring liver transplant. Am J Psychiatry. 2015;172(12):1182-1189.
17. Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the Sustained Alcohol Use Post-Liver Transplant score. Hepatology. 2019;69(4):1477-1487.
18. DiMartini A, Crone C, Dew MA. Alcohol and substance use in liver transplant patients. Clinics in Liver Disease. 2011;15(4):727-751.
19. Pungpapong S, Manzarbeitia C, Ortiz J, et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582-587.
20. Kreek MJ. Pharmacotherapy of opioid dependence: rationale and update. Regulatory Peptides. 1994;53(suppl 1):S255-S256.
21. Jiao M, Greanya ED, Haque M, et al. Methadone maintenance therapy in liver transplantation. Prog Transplant. 2010;20(3):209-214; quiz 215.
22. Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91.
23. Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63-71.
24. Kotwani P, Saxena V, Dodge JL, et al. History of marijuana use does not affect outcomes on the liver transplant waitlist. Transplantation. 2018;102(5):794-802.
25. Fineberg SK, West A, Na PJ, et al. Utility of pretransplant psychological measures to predict posttransplant outcomes in liver transplant patients: a systematic review. Gen Hospl Psychiatry. 2016;40:4-11.
26. Satapathy S, Sanyal A. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
27. Olbrisch ME, Levenson JL, Hamer R. The PACT: a rating scale for the study of clinical decision making in psychosocial screening of organ transplant candidates. Clin Transplant. 1989;3:164-169.
28. Twillman RK, Manetto C, Wellisch DK, et al. Transplant Evaluation Rating Scale: a revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153.
29. Goodier J. Evaluating Stress:97496. In: Zalaquett CP, Wood RJ, eds. Evaluating stress: a book of resources. Lanham, MD: Scarecrow Press; 1997:29-29.
30. Beck AT, Steer RA, Carbin, MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8(1):77-100.
31. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242.
32. Cottle WC. The MMPI: a review. Lawrence, KS: University of Kansas; 1953.
33. Addison CC, Campbell-Jenkins BW, Sarpong DF, et al. Psychometric Evaluation of a Coping Strategies Inventory Short-Form (CSI-SF) in the Jackson Heart Study Cohort. Int J Environ Res Public Health. 2007;4(4):289-295.
34. Mooney S, Hasssanein T, Hilsabeck R, et al. Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175-186.
35. De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33(4):609-615.
36. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42(4):337-343.
37. Landefeld CS, Palme, RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344.
38. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric problems in living-related transplantation (II): the association between paradoxical psychiatric syndrome and guilt feelings in adult recipients after living donor liver transplantation. Transplantation Proceedings. 2002;34(7):2632-2633.
39. Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet. 1996;30(2):141-179.
40. Ozkanlar Y, Nishijima Y, Cunha DD, et al. Acute effects of tacrolimus (FK506) on left ventricular mechanics. Pharmacol Res. 2005;52(4):307-312.
41. Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826-833.
42. Maldonado J R. I have been asked to work up a patient who requires a liver transplant how should I proceed? FOCUS. 2009;7(3):332-335.
43. Mccallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol and Alcoholism. 2006;41(4):358-363.
44. Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680-685.
45. Coffman KL, Hoffman A, Sher L, et al. Treatment of the postoperative alcoholic liver transplant recipient with other addictions. Liver Transpl Surg. 1997;3(3):322-327.
Since the first liver transplant (LT) was performed in 1963 by Starzl et al, there have been considerable advances in the field, with improvements in post-transplant survival.1 There are multiple indications for LT, including acute liver failure and index complications of cirrhosis such as ascites, encephalopathy, and hepatopulmonary syndrome.2 Once a patient develops one of these conditions, he/she is evaluated for LT, even as the complications of liver failure are being managed.
Although the number of LTs has risen, the demand for transplant continues to exceed availability. In 2015, chronic liver disease and cirrhosis was the 12th leading cause of death in the United States.3 In 2016, approximately 50% of waitlisted candidates received a transplant.4 There is also a donor shortage. In part, this shortage may be due to longer life spans and the subsequent increase in the age of the potential donor.5 In light of this shortage and increased demand, the pre-LT workup is comprehensive. The pre-transplant assessment typically consists of cardiology, surgery, hepatology, and psychosocial evaluations, and hence requires a team of experts to determine who is an ideal candidate for transplant.
Psychiatrists play a key role in the pre-transplant psychosocial evaluations. This article describes the elements of these evaluations, and what psychiatrists can do to help patients both before and after they undergo LT.
Elements of the pre-transplant evaluation
The psychosocial evaluation is a critical component of the pre-transplant assessment. As part of the evaluation, patients are screened for psychosocial limitations that may complicate transplantation, such as demonstrated noncompliance, ongoing alcohol or drug use, and lack of social support (Table 12 ). Other goals of the psychosocial evaluation are to identify in the pre-transplant period patients with possible risk factors, such as substance use or psychiatric disorders, and develop treatment plans to optimize transplant outcomes (Table 26). There are relative contraindications to LT (Table 37) but no absolute psychiatric contraindications, according to the 2013 American Association for the Study of Liver Diseases (AASLD) practice guideline for transplantation.2

Adherence. The 2013 AASLD practice guideline states that patients “should be evaluated for and meet reasonable expectations for adherence to medical directives and mental health stability as determined by the psychosocial evaluation.”2 In the transplant setting, adherence is complex. It requires compliance with complicated medication regimens and laboratory testing, frequent follow-up appointments, and close, prompt communication of concerns to the health care team. Patient adherence to medication regimens plays an important role in transplant outcomes.8 In fact, in patients who have undergone renal transplant, nonadherence to therapy is considered the leading cause of avoidable graft failure.9

A retrospective study of adult LT recipients found that pre-transplant chart evidence of nonadherence, such as missed laboratory testing and clinic visits, was a significant predictor of post-transplant nonadherence with immunosuppressant therapy. Pre-transplant unemployment status and a history of substance abuse also were associated with nonadherence.9
Dobbels et al10 found that patients with a self-reported history of pre-transplant non-adherence had a higher risk of being nonadherent with their immunosuppressive therapy after transplant (odd ratio [OR]: 7.9). Their self-report adherence questionnaire included questions that addressed pre-transplant smoking status, alcohol use, and adherence with medication. In this prospective study, researchers also found that patients with a low “conscientiousness” score were at a higher risk for post-transplant medication nonadherence (OR: 0.8).
Continue to: Studies have also found...
Studies have also found that patients with higher education are more at risk for post-transplant medication nonadherence. Higher education may be associated with higher employment status resulting in a busier lifestyle, a known risk factor that may prevent patients from regular medication adherence.11,12 Alternatively, it is possible that higher educated patients are “decisive” nonadherers who prefer independent decision-making regarding their disease and treatment.13
Substance use. The 2013 AASLD practice guideline lists “ongoing alcohol or illicit substance abuse” as one of the contraindications to LT.2 In guidelines from the Austrian Society for Gastroenterology and Hepatology, Graziadei et al14 listed “alcohol addiction without motivation for alcohol abstinence and untreated/ongoing substance abuse” as absolute contraindications and “untreated alcohol abuse and other drug-related addiction” as relative contraindications. Hence, the pre-transplant evaluation should include a thorough substance use history, including duration, amount, previous attempts to quit, and motivation for abstinence.
Substance use history is especially important because alcoholic liver disease is the second most common indication for LT.2 Most LT programs require 6 months of abstinence before a patient can be considered for transplant.15 The 6-month period was based on studies demonstrating that pre-transplant abstinence from alcohol for <6 months is a risk factor for relapse.15 However, this guideline remains controversial because the transplant referral and workup may be delayed as the patient’s liver disease worsens. Other risk factors for substance relapse should also be taken into consideration, such as depression, personality disorders, lack of social support, severity of alcohol use, and family history of alcoholism.16 Lee and Leggio16 developed the Sustained Alcohol Use Post-Liver Transplant (SALT) score to identify patients who were at risk for sustained alcohol use posttransplant. The 4 SALT criteria are:
- >10 drinks per day at initial hospitalization (+4 points)
- multiple prior rehabilitation attempts (+4 points)
- prior alcohol‐related legal issues (+2 points), and
- prior illicit substance abuse (+1 point).
A SALT score can range from 0 to 11. Lee et al17 found a SALT score ≥5 had a 25% positive predictive value (95% confidence interval [CI]: 10% to 47%) and a SALT score of <5 had a 95% negative predictive value (95% CI: 89% to 98%) for sustained alcohol use post‐LT. Thus, the 2013 AASLD guideline cautions against delaying evaluation based on the 6-month abstinence rule, and instead recommends early transplant referral for patients with alcoholic liver disease to encourage such patients to begin addiction treatment.2
As part of the substance use history, it is important to ask about the patient’s smoking history. Approximately 60% of LT candidates have a history of smoking cigarettes.18 Tobacco use history is associated with increased post-transplant vascular complications, such as hepatic artery thrombosis or stenosis, portal vein thrombosis, and deep vein thrombosis.19 The 2013 AASLD guideline recommends that tobacco use should be prohibited in LT candidates.2 Pungpapong et al19 reported that smoking cessation for at least 2 years prior to transplant led to a significantly decreased risk of developing arterial complications, with an absolute risk reduction of approximately 16%.
Continue to: Liver cirrhosis due to...
Liver cirrhosis due to chronic hepatitis C virus (HCV) infection is one of the leading causes for LT. In the United States, HCV is commonly transmitted during injection drug use. According to the 2013 AASLD guideline, ongoing illicit substance use is a relative contraindication to LT.2 It is important to note, however, that methadone maintenance therapy (MMT) is not a contraindication to LT. In fact, the 2013 AASLD guideline recommends that patients receiving MMT should not be required to reduce or stop therapy in order to be listed for transplant.2 Studies have shown that in 80% of patients, tapering MMT leads to illicit opiate relapse.20 Currently, there is no evidence that patients receiving MMT have poorer post-transplant outcomes compared with patients not receiving MMT.21
Whether cannabis use is a relative contraindication to LT remains controversial.22 Possible adverse effects of cannabis use in transplant patients include drug–drug interactions and infections. Hézode et al23 reported that daily cannabis use is significantly associated with an increased fibrosis progression rate in patients with chronic HCV infection. Another recent study found that a history of cannabis use was not associated with worse outcomes among patients on the LT waitlist.24 With the increased legalization of cannabis, more studies are needed to assess ongoing cannabis use in patients on the LT waitlist and post-LT outcomes.
Psychiatric history. When assessing a patient for possible LT, no psychiatric disorder is considered an absolute contraindication. Patients with a serious mental illness, such as schizophrenia, and those with intellectual disability can have successful, long-term outcomes with proper evaluation and preparation, including social support. However, empirical literature regarding transplant outcomes and predictive factors in patients with serious mental illness is scarce.2
Studies examining the predictive value of pre-transplant depression on post-transplant outcomes have had mixed results.25 Depression may predict lower post-transplant quality of life. Pre-LT suicidal thoughts (as noted on the Beck Depression Inventory, for example) are associated with post-LT depression.25 In contrast, available data show no significant effect of pre-transplant anxiety on post-LT outcomes. Similarly, pre-transplant cognitive performance appears not to predict survival or other post-transplant outcomes, but may predict poorer quality of life after transplant.25
A few psychiatric factors are considered relative contraindications for LT. These include severe personality disorders, active substance use with no motivation for treatment or abstinence, active psychosis, severe neurocognitive disorders, suicidality, and factitious disorder.7
Continue to: Social support
Social support. Assessing a pre-LT patient’s level of social support is an essential part of the psychosocial evaluation. According to the 2013 AASLD guideline, patients should have “adequate” social support both during the waitlist and post-operative periods.2 Lack of partnership is a significant predictor of poor post-transplant outcomes, such as late graft loss.10 Satapathy and Sanyal26 reported that among patients who receive an LT for alcoholic liver disease, those with immediate family support were less likely to relapse to using alcohol after transplant. Poor social support was also a predictor of post-transplant medication nonadherence.10 Thus, the patient needs enough social support to engage in the pre-transplant health care requirements and to participate in post-transplant recommendations until he/she is functioning independently post-transplant.
Screening tools
Various screening tools may be useful in a pre-LT evaluation. Three standardized assessment tools available specifically for pre-transplant psychosocial assessments are the Stanford Integrated Psychosocial Assessment for Transplantation (Table 26), the Psychosocial Assessment of Candidates for Transplantation,27 and the Transplant Evaluation Rating Scale.28 Instruments to aid in the assessment of depression, anxiety, and delirium,29-31 a structured personality assessment,32 coping inventories,33 neuropsychological batteries,34 and others also have been used to evaluate patients before LT. The self-rated Beck Depression Inventory and the clinician-rated Hamilton Depression Rating Scale are commonly used.7 Other tools, such as the LEIPAD quality of life instrument and the Brief Symptom Inventory (BSI), have been used to assess for perceived quality of life and psychological distress, respectively.35 These screening tools can be helpful as aids for the pre-LT evaluation; however, diagnoses and treatment plan recommendations require a psychiatric evaluation conducted by a trained clinician.
Treatment after liver transplant
Psychiatric issues. After LT, various psychiatric complications may arise, including (but not limited to) delirium7 and “paradoxical psychiatric syndrome” (PPS).36 Delirium can be managed by administering low-dose antipsychotic medications, limiting the use of benzodiazepines and medications with anticholinergic effects, implementing behavioral interventions (frequent orientation, maintaining sleep/wake cycle, limiting noise, presence of a family member or a sitter at bedside),37 and addressing the underlying etiology. Paradoxical psychiatric syndrome is defined as psychiatric symptoms that occur despite a successful LT. It develops within the first year of transplantation and is characterized by recipients having strong guilt feelings toward their donors.38
Drug interactions. In the post-transplant period, antipsychotics are used for management of delirium and psychosis, antidepressants for anxiety and depression, and benzodiazepines for anxiety and sleep problems.7 Drug–drug interactions between psychotropic medications and the immunosuppressants required after LT must be closely monitored. First-generation antipsychotics should be avoided in post-transplant patients taking tacrolimus due to the increased risk of QTc prolongation. Tacrolimus can also increase the risk of nephrotoxicity when co-administered with lithium. Post-LT patients taking steroids and bupropion have an increased risk of seizure. Carbamazepine may decrease blood levels of cyclosporine due to the induction of hepatic metabolism.39,40 The psychiatrist should review and update the patient’s complete medication list at each visit, checking for possible medication interactions.
Quality of life. In the first 6 months post-transplant, patients typically experience improved quality of life in both physical and psychological domains. However, this improvement vacillates as the patient adjusts to post-transplant life. A reduction in BSI score 1 year after transplant has been reported. The BSI evaluates psychopathological symptoms, which are early indicators of psychological discomfort. One study noted a reduction in the LEIPAD quality of life score, which measures overall quality of life, 2 years after transplant.35 This decline may reflect the difficulties associated with the new challenges after transplant. Patients may endure both physical changes due to medical complications as well as psychological problems as they adjust to their new bodily integrity, their dependence on medications and medical staff, and other changes in function. Three to 5 years after transplant, patients reached a new psychological stability, with reported improvements in quality of life and decreased psychological distress.35
Continue to: Special populations
Special populations
HCV infection. Recurrent HCV infection and liver disease after transplantation are associated with psychological distress. This is particularly evident in patients 6 months after transplantation. Depression and psychological distress have been reported in male patients with recurrent HCV infection within the first year after transplantation.35
Acetaminophen overdose. Patients who receive a transplant for acetaminophen-induced acute liver failure (ALF) had a greater prevalence of psychiatric comorbidity as reflected by predefined diagnoses, medication, and previous suicide attempts.41 Despite this, outcomes for patients transplanted emergently for acetaminophen-induced ALF were comparable to those transplanted for non-acetaminophen-induced ALF and for chronic liver disease. Multidisciplinary approaches with long-term psychiatric follow-up may contribute to low post-transplant suicide rates and low rates of graft loss because of noncompliance.41
CASE REPORT
A complicated presentation
Ms. A, age 45, a married woman with history of chronic back pain and self-reported bipolar disorder, presented to our hospital with acute liver failure secondary to acetaminophen overdose. Her Model for End-Stage Liver Disease (MELD) score on presentation was 38 (range: 0 to 40 with higher scores indicating increased likelihood of mortality). Her urine drug screen was positive for benzodiazepines and opiates. On hospital Day 2, the primary team consulted psychiatry for a pre-transplant evaluation and consideration of suicidality. Hepatology, toxicology, and transplant surgery services also were consulted.
Because Ms. A was intubated for acute respiratory failure, the initial history was gathered from family, a review of the medical record, consultation with her pharmacy, and collateral from her outpatient physician. Ms. A had been taking
Four days before presenting with acute liver failure, Ms. A had visited another hospital for lethargy. Benzodiazepines and opiates were stopped abruptly, and she was discharged with the recommendation to take acetaminophen for her pain. Approximately 24 hours after returning home, Ms. A began having auditory and visual hallucinations, and she did not sleep for days. She continued to complain of pain and was taking acetaminophen as recommended by the outside hospital. Her husband notes that she was intermittently confused. He was unsure how much acetaminophen she was taking.
Continue to: Her family noted...
Her family noted Ms. A had been diagnosed with bipolar disorder “years ago” but was unable to describe any manic episodes, and Ms. A had been treated only with an antidepressant from her primary care physician. She had persistent low mood and increased sleep since developing chronic back pain that severely limited her functioning. Ms. A attempted suicide once years ago by cutting her wrists. She had 2 prior psychiatric hospitalizations for suicidal ideation and the suicide attempt; however, she had not recently voiced suicidal ideation to her husband or family. She was adherent to psychotropic medications and follow-up appointments. Ms. A is a current smoker. She had used marijuana in the past, but her family denies current use, as well as any alcohol use or illicit substance use.
Ms. A’s diagnosis was consistent with tobacco use disorder and major depressive disorder (MDD). She likely developed withdrawal after abrupt cessation of diazepam, which she had been taking as prescribed for years. There was no evidence at the time of her initial psychiatric evaluation that the acetaminophen overdose was a suicide attempt; however, because Ms. A was intubated and sedated at that time, the consultation team recommended direct observation until she could participate in a risk assessment.
For the pre-transplant psychiatric evaluation, our consultation-liaison team noted Ms. A’s history of MDD, with recent active symptoms, chronic pain, and a past suicide attempt. She was a current tobacco smoker, which increases the risk of post-transplant vascular problems. However, she had been adherent to medications and follow-up, had very close family support, and there was no clear evidence that this acetaminophen ingestion was a suicide attempt. We noted that outpatient psychiatric follow-up and better chronic pain management would be helpful post-transplant. We would have to re-evaluate Ms. A when she was medically stable enough to communicate before making any further recommendations. Due to medical complications that developed after our evaluation, the transplant team noted Ms. A was no longer a transplant candidate.
Fortunately, Ms. A recovered with medical management over the next 2 weeks. She denied any suicidal ideation throughout her hospitalization. She was restarted on an antidepressant and received supportive therapy until discharge. Outpatient psychiatry follow-up and pain management was set up before Ms. A was discharged. Inpatient psychiatric hospitalization was not recommended. Per available records, Ms. A followed up with all outpatient appointments, including with her psychiatrist, since discharge.
Avoiding problems, maximizing outcomes
In addition to medical factors, psychosocial factors may affect the success of LT, although empirical data regarding which factors are most predictive of post-transplant outcomes is lacking, especially in patients with serious mental illness. The goals of a psychosocial pre-transplant evaluation are to promote fairness and equal access to care, maximize optimal outcomes, wisely use scarce resources, and ensure that the potential for benefits outweigh surgical risks to the patient. Identifying potential complicating factors (ie, substance abuse, nonadherence, serious psychopathology) can help guide the medical and psychiatric treatment plan and help minimize preventable problems both before and after transplant.42
Continue to: In patients who have...
In patients who have a history of alcohol use and alcohol liver disease, relapse to alcohol is a significant problem. Relapse rates vary from 10% to 30%.7 The duration of abstinence before LT appears to be a poor predictor of abstinence after LT.43 Polysubstance use also adversely affects outcomes in patients with alcohol liver disease. Approximately one-third of patients with polysubstance use who receive a LT relapse to substance use.44 Coffman et al45 showed that the presence of antisocial behavior and eating disorders may increase the risk of relapse after LT.
The psychiatrist’s role in the setting of LT spans from the pre-transplant assessment to post-transplant management and follow-up. Clarifying specific psychiatric diagnoses, psychosocial factors that need to be addressed before transplant, and substance use diagnoses and treatment recommendations can help the transplant team clearly identify modifiable factors that can affect transplant outcomes.
Bottom Line
Psychiatrists can help patients who are candidates for liver transplantation (LT) by performing a pre-transplant psychosocial assessment to identity factors that might complicate transplantation or recovery. After LT, patients require careful monitoring for psychiatric comorbidities, drug interactions, and other factors that can affect quality of life.
Related Resources
- Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018;53(2):135-144.
- Marcangelo MJ, Crone C. Tools, techniques to assess organ transplant candidates. Current Psychiatry. 2007;6(9):56-66.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Hydromorphone • Dilaudid
Lithium • Eskalith, Lithobid
Tacrolimus • Astagraf XL, Envarsus XR
Since the first liver transplant (LT) was performed in 1963 by Starzl et al, there have been considerable advances in the field, with improvements in post-transplant survival.1 There are multiple indications for LT, including acute liver failure and index complications of cirrhosis such as ascites, encephalopathy, and hepatopulmonary syndrome.2 Once a patient develops one of these conditions, he/she is evaluated for LT, even as the complications of liver failure are being managed.
Although the number of LTs has risen, the demand for transplant continues to exceed availability. In 2015, chronic liver disease and cirrhosis was the 12th leading cause of death in the United States.3 In 2016, approximately 50% of waitlisted candidates received a transplant.4 There is also a donor shortage. In part, this shortage may be due to longer life spans and the subsequent increase in the age of the potential donor.5 In light of this shortage and increased demand, the pre-LT workup is comprehensive. The pre-transplant assessment typically consists of cardiology, surgery, hepatology, and psychosocial evaluations, and hence requires a team of experts to determine who is an ideal candidate for transplant.
Psychiatrists play a key role in the pre-transplant psychosocial evaluations. This article describes the elements of these evaluations, and what psychiatrists can do to help patients both before and after they undergo LT.
Elements of the pre-transplant evaluation
The psychosocial evaluation is a critical component of the pre-transplant assessment. As part of the evaluation, patients are screened for psychosocial limitations that may complicate transplantation, such as demonstrated noncompliance, ongoing alcohol or drug use, and lack of social support (Table 12 ). Other goals of the psychosocial evaluation are to identify in the pre-transplant period patients with possible risk factors, such as substance use or psychiatric disorders, and develop treatment plans to optimize transplant outcomes (Table 26). There are relative contraindications to LT (Table 37) but no absolute psychiatric contraindications, according to the 2013 American Association for the Study of Liver Diseases (AASLD) practice guideline for transplantation.2

Adherence. The 2013 AASLD practice guideline states that patients “should be evaluated for and meet reasonable expectations for adherence to medical directives and mental health stability as determined by the psychosocial evaluation.”2 In the transplant setting, adherence is complex. It requires compliance with complicated medication regimens and laboratory testing, frequent follow-up appointments, and close, prompt communication of concerns to the health care team. Patient adherence to medication regimens plays an important role in transplant outcomes.8 In fact, in patients who have undergone renal transplant, nonadherence to therapy is considered the leading cause of avoidable graft failure.9

A retrospective study of adult LT recipients found that pre-transplant chart evidence of nonadherence, such as missed laboratory testing and clinic visits, was a significant predictor of post-transplant nonadherence with immunosuppressant therapy. Pre-transplant unemployment status and a history of substance abuse also were associated with nonadherence.9
Dobbels et al10 found that patients with a self-reported history of pre-transplant non-adherence had a higher risk of being nonadherent with their immunosuppressive therapy after transplant (odd ratio [OR]: 7.9). Their self-report adherence questionnaire included questions that addressed pre-transplant smoking status, alcohol use, and adherence with medication. In this prospective study, researchers also found that patients with a low “conscientiousness” score were at a higher risk for post-transplant medication nonadherence (OR: 0.8).
Continue to: Studies have also found...
Studies have also found that patients with higher education are more at risk for post-transplant medication nonadherence. Higher education may be associated with higher employment status resulting in a busier lifestyle, a known risk factor that may prevent patients from regular medication adherence.11,12 Alternatively, it is possible that higher educated patients are “decisive” nonadherers who prefer independent decision-making regarding their disease and treatment.13
Substance use. The 2013 AASLD practice guideline lists “ongoing alcohol or illicit substance abuse” as one of the contraindications to LT.2 In guidelines from the Austrian Society for Gastroenterology and Hepatology, Graziadei et al14 listed “alcohol addiction without motivation for alcohol abstinence and untreated/ongoing substance abuse” as absolute contraindications and “untreated alcohol abuse and other drug-related addiction” as relative contraindications. Hence, the pre-transplant evaluation should include a thorough substance use history, including duration, amount, previous attempts to quit, and motivation for abstinence.
Substance use history is especially important because alcoholic liver disease is the second most common indication for LT.2 Most LT programs require 6 months of abstinence before a patient can be considered for transplant.15 The 6-month period was based on studies demonstrating that pre-transplant abstinence from alcohol for <6 months is a risk factor for relapse.15 However, this guideline remains controversial because the transplant referral and workup may be delayed as the patient’s liver disease worsens. Other risk factors for substance relapse should also be taken into consideration, such as depression, personality disorders, lack of social support, severity of alcohol use, and family history of alcoholism.16 Lee and Leggio16 developed the Sustained Alcohol Use Post-Liver Transplant (SALT) score to identify patients who were at risk for sustained alcohol use posttransplant. The 4 SALT criteria are:
- >10 drinks per day at initial hospitalization (+4 points)
- multiple prior rehabilitation attempts (+4 points)
- prior alcohol‐related legal issues (+2 points), and
- prior illicit substance abuse (+1 point).
A SALT score can range from 0 to 11. Lee et al17 found a SALT score ≥5 had a 25% positive predictive value (95% confidence interval [CI]: 10% to 47%) and a SALT score of <5 had a 95% negative predictive value (95% CI: 89% to 98%) for sustained alcohol use post‐LT. Thus, the 2013 AASLD guideline cautions against delaying evaluation based on the 6-month abstinence rule, and instead recommends early transplant referral for patients with alcoholic liver disease to encourage such patients to begin addiction treatment.2
As part of the substance use history, it is important to ask about the patient’s smoking history. Approximately 60% of LT candidates have a history of smoking cigarettes.18 Tobacco use history is associated with increased post-transplant vascular complications, such as hepatic artery thrombosis or stenosis, portal vein thrombosis, and deep vein thrombosis.19 The 2013 AASLD guideline recommends that tobacco use should be prohibited in LT candidates.2 Pungpapong et al19 reported that smoking cessation for at least 2 years prior to transplant led to a significantly decreased risk of developing arterial complications, with an absolute risk reduction of approximately 16%.
Continue to: Liver cirrhosis due to...
Liver cirrhosis due to chronic hepatitis C virus (HCV) infection is one of the leading causes for LT. In the United States, HCV is commonly transmitted during injection drug use. According to the 2013 AASLD guideline, ongoing illicit substance use is a relative contraindication to LT.2 It is important to note, however, that methadone maintenance therapy (MMT) is not a contraindication to LT. In fact, the 2013 AASLD guideline recommends that patients receiving MMT should not be required to reduce or stop therapy in order to be listed for transplant.2 Studies have shown that in 80% of patients, tapering MMT leads to illicit opiate relapse.20 Currently, there is no evidence that patients receiving MMT have poorer post-transplant outcomes compared with patients not receiving MMT.21
Whether cannabis use is a relative contraindication to LT remains controversial.22 Possible adverse effects of cannabis use in transplant patients include drug–drug interactions and infections. Hézode et al23 reported that daily cannabis use is significantly associated with an increased fibrosis progression rate in patients with chronic HCV infection. Another recent study found that a history of cannabis use was not associated with worse outcomes among patients on the LT waitlist.24 With the increased legalization of cannabis, more studies are needed to assess ongoing cannabis use in patients on the LT waitlist and post-LT outcomes.
Psychiatric history. When assessing a patient for possible LT, no psychiatric disorder is considered an absolute contraindication. Patients with a serious mental illness, such as schizophrenia, and those with intellectual disability can have successful, long-term outcomes with proper evaluation and preparation, including social support. However, empirical literature regarding transplant outcomes and predictive factors in patients with serious mental illness is scarce.2
Studies examining the predictive value of pre-transplant depression on post-transplant outcomes have had mixed results.25 Depression may predict lower post-transplant quality of life. Pre-LT suicidal thoughts (as noted on the Beck Depression Inventory, for example) are associated with post-LT depression.25 In contrast, available data show no significant effect of pre-transplant anxiety on post-LT outcomes. Similarly, pre-transplant cognitive performance appears not to predict survival or other post-transplant outcomes, but may predict poorer quality of life after transplant.25
A few psychiatric factors are considered relative contraindications for LT. These include severe personality disorders, active substance use with no motivation for treatment or abstinence, active psychosis, severe neurocognitive disorders, suicidality, and factitious disorder.7
Continue to: Social support
Social support. Assessing a pre-LT patient’s level of social support is an essential part of the psychosocial evaluation. According to the 2013 AASLD guideline, patients should have “adequate” social support both during the waitlist and post-operative periods.2 Lack of partnership is a significant predictor of poor post-transplant outcomes, such as late graft loss.10 Satapathy and Sanyal26 reported that among patients who receive an LT for alcoholic liver disease, those with immediate family support were less likely to relapse to using alcohol after transplant. Poor social support was also a predictor of post-transplant medication nonadherence.10 Thus, the patient needs enough social support to engage in the pre-transplant health care requirements and to participate in post-transplant recommendations until he/she is functioning independently post-transplant.
Screening tools
Various screening tools may be useful in a pre-LT evaluation. Three standardized assessment tools available specifically for pre-transplant psychosocial assessments are the Stanford Integrated Psychosocial Assessment for Transplantation (Table 26), the Psychosocial Assessment of Candidates for Transplantation,27 and the Transplant Evaluation Rating Scale.28 Instruments to aid in the assessment of depression, anxiety, and delirium,29-31 a structured personality assessment,32 coping inventories,33 neuropsychological batteries,34 and others also have been used to evaluate patients before LT. The self-rated Beck Depression Inventory and the clinician-rated Hamilton Depression Rating Scale are commonly used.7 Other tools, such as the LEIPAD quality of life instrument and the Brief Symptom Inventory (BSI), have been used to assess for perceived quality of life and psychological distress, respectively.35 These screening tools can be helpful as aids for the pre-LT evaluation; however, diagnoses and treatment plan recommendations require a psychiatric evaluation conducted by a trained clinician.
Treatment after liver transplant
Psychiatric issues. After LT, various psychiatric complications may arise, including (but not limited to) delirium7 and “paradoxical psychiatric syndrome” (PPS).36 Delirium can be managed by administering low-dose antipsychotic medications, limiting the use of benzodiazepines and medications with anticholinergic effects, implementing behavioral interventions (frequent orientation, maintaining sleep/wake cycle, limiting noise, presence of a family member or a sitter at bedside),37 and addressing the underlying etiology. Paradoxical psychiatric syndrome is defined as psychiatric symptoms that occur despite a successful LT. It develops within the first year of transplantation and is characterized by recipients having strong guilt feelings toward their donors.38
Drug interactions. In the post-transplant period, antipsychotics are used for management of delirium and psychosis, antidepressants for anxiety and depression, and benzodiazepines for anxiety and sleep problems.7 Drug–drug interactions between psychotropic medications and the immunosuppressants required after LT must be closely monitored. First-generation antipsychotics should be avoided in post-transplant patients taking tacrolimus due to the increased risk of QTc prolongation. Tacrolimus can also increase the risk of nephrotoxicity when co-administered with lithium. Post-LT patients taking steroids and bupropion have an increased risk of seizure. Carbamazepine may decrease blood levels of cyclosporine due to the induction of hepatic metabolism.39,40 The psychiatrist should review and update the patient’s complete medication list at each visit, checking for possible medication interactions.
Quality of life. In the first 6 months post-transplant, patients typically experience improved quality of life in both physical and psychological domains. However, this improvement vacillates as the patient adjusts to post-transplant life. A reduction in BSI score 1 year after transplant has been reported. The BSI evaluates psychopathological symptoms, which are early indicators of psychological discomfort. One study noted a reduction in the LEIPAD quality of life score, which measures overall quality of life, 2 years after transplant.35 This decline may reflect the difficulties associated with the new challenges after transplant. Patients may endure both physical changes due to medical complications as well as psychological problems as they adjust to their new bodily integrity, their dependence on medications and medical staff, and other changes in function. Three to 5 years after transplant, patients reached a new psychological stability, with reported improvements in quality of life and decreased psychological distress.35
Continue to: Special populations
Special populations
HCV infection. Recurrent HCV infection and liver disease after transplantation are associated with psychological distress. This is particularly evident in patients 6 months after transplantation. Depression and psychological distress have been reported in male patients with recurrent HCV infection within the first year after transplantation.35
Acetaminophen overdose. Patients who receive a transplant for acetaminophen-induced acute liver failure (ALF) had a greater prevalence of psychiatric comorbidity as reflected by predefined diagnoses, medication, and previous suicide attempts.41 Despite this, outcomes for patients transplanted emergently for acetaminophen-induced ALF were comparable to those transplanted for non-acetaminophen-induced ALF and for chronic liver disease. Multidisciplinary approaches with long-term psychiatric follow-up may contribute to low post-transplant suicide rates and low rates of graft loss because of noncompliance.41
CASE REPORT
A complicated presentation
Ms. A, age 45, a married woman with history of chronic back pain and self-reported bipolar disorder, presented to our hospital with acute liver failure secondary to acetaminophen overdose. Her Model for End-Stage Liver Disease (MELD) score on presentation was 38 (range: 0 to 40 with higher scores indicating increased likelihood of mortality). Her urine drug screen was positive for benzodiazepines and opiates. On hospital Day 2, the primary team consulted psychiatry for a pre-transplant evaluation and consideration of suicidality. Hepatology, toxicology, and transplant surgery services also were consulted.
Because Ms. A was intubated for acute respiratory failure, the initial history was gathered from family, a review of the medical record, consultation with her pharmacy, and collateral from her outpatient physician. Ms. A had been taking
Four days before presenting with acute liver failure, Ms. A had visited another hospital for lethargy. Benzodiazepines and opiates were stopped abruptly, and she was discharged with the recommendation to take acetaminophen for her pain. Approximately 24 hours after returning home, Ms. A began having auditory and visual hallucinations, and she did not sleep for days. She continued to complain of pain and was taking acetaminophen as recommended by the outside hospital. Her husband notes that she was intermittently confused. He was unsure how much acetaminophen she was taking.
Continue to: Her family noted...
Her family noted Ms. A had been diagnosed with bipolar disorder “years ago” but was unable to describe any manic episodes, and Ms. A had been treated only with an antidepressant from her primary care physician. She had persistent low mood and increased sleep since developing chronic back pain that severely limited her functioning. Ms. A attempted suicide once years ago by cutting her wrists. She had 2 prior psychiatric hospitalizations for suicidal ideation and the suicide attempt; however, she had not recently voiced suicidal ideation to her husband or family. She was adherent to psychotropic medications and follow-up appointments. Ms. A is a current smoker. She had used marijuana in the past, but her family denies current use, as well as any alcohol use or illicit substance use.
Ms. A’s diagnosis was consistent with tobacco use disorder and major depressive disorder (MDD). She likely developed withdrawal after abrupt cessation of diazepam, which she had been taking as prescribed for years. There was no evidence at the time of her initial psychiatric evaluation that the acetaminophen overdose was a suicide attempt; however, because Ms. A was intubated and sedated at that time, the consultation team recommended direct observation until she could participate in a risk assessment.
For the pre-transplant psychiatric evaluation, our consultation-liaison team noted Ms. A’s history of MDD, with recent active symptoms, chronic pain, and a past suicide attempt. She was a current tobacco smoker, which increases the risk of post-transplant vascular problems. However, she had been adherent to medications and follow-up, had very close family support, and there was no clear evidence that this acetaminophen ingestion was a suicide attempt. We noted that outpatient psychiatric follow-up and better chronic pain management would be helpful post-transplant. We would have to re-evaluate Ms. A when she was medically stable enough to communicate before making any further recommendations. Due to medical complications that developed after our evaluation, the transplant team noted Ms. A was no longer a transplant candidate.
Fortunately, Ms. A recovered with medical management over the next 2 weeks. She denied any suicidal ideation throughout her hospitalization. She was restarted on an antidepressant and received supportive therapy until discharge. Outpatient psychiatry follow-up and pain management was set up before Ms. A was discharged. Inpatient psychiatric hospitalization was not recommended. Per available records, Ms. A followed up with all outpatient appointments, including with her psychiatrist, since discharge.
Avoiding problems, maximizing outcomes
In addition to medical factors, psychosocial factors may affect the success of LT, although empirical data regarding which factors are most predictive of post-transplant outcomes is lacking, especially in patients with serious mental illness. The goals of a psychosocial pre-transplant evaluation are to promote fairness and equal access to care, maximize optimal outcomes, wisely use scarce resources, and ensure that the potential for benefits outweigh surgical risks to the patient. Identifying potential complicating factors (ie, substance abuse, nonadherence, serious psychopathology) can help guide the medical and psychiatric treatment plan and help minimize preventable problems both before and after transplant.42
Continue to: In patients who have...
In patients who have a history of alcohol use and alcohol liver disease, relapse to alcohol is a significant problem. Relapse rates vary from 10% to 30%.7 The duration of abstinence before LT appears to be a poor predictor of abstinence after LT.43 Polysubstance use also adversely affects outcomes in patients with alcohol liver disease. Approximately one-third of patients with polysubstance use who receive a LT relapse to substance use.44 Coffman et al45 showed that the presence of antisocial behavior and eating disorders may increase the risk of relapse after LT.
The psychiatrist’s role in the setting of LT spans from the pre-transplant assessment to post-transplant management and follow-up. Clarifying specific psychiatric diagnoses, psychosocial factors that need to be addressed before transplant, and substance use diagnoses and treatment recommendations can help the transplant team clearly identify modifiable factors that can affect transplant outcomes.
Bottom Line
Psychiatrists can help patients who are candidates for liver transplantation (LT) by performing a pre-transplant psychosocial assessment to identity factors that might complicate transplantation or recovery. After LT, patients require careful monitoring for psychiatric comorbidities, drug interactions, and other factors that can affect quality of life.
Related Resources
- Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018;53(2):135-144.
- Marcangelo MJ, Crone C. Tools, techniques to assess organ transplant candidates. Current Psychiatry. 2007;6(9):56-66.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Hydromorphone • Dilaudid
Lithium • Eskalith, Lithobid
Tacrolimus • Astagraf XL, Envarsus XR
1. Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives [Article in English, Portuguese]. Einstein (São Paulo). 2015;13(1):149-152.
2. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144-1165.
3. Centers for Disease Control and Prevention. QuickStats: number of deaths from 10 leading causes,* by sex—National Vital Statistics System, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(15):413.
4. Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31(1):84-89.
5. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59.
6. Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123-132.
7. Grover S, Sarkar S. Liver transplant—psychiatric and psychosocial aspects. J Clin Exp Hepatol. 2012;2(4):382-392.
8. Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760-770.
9. Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824-834.
10. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497-1504.
11. De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323.
12. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172-183.
13. Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718-1726.
14. Graziadei I, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 2016;128(19):679-690.
15. Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981-987.
16. Lee MR, Leggio L. Management of alcohol use disorder in patients requiring liver transplant. Am J Psychiatry. 2015;172(12):1182-1189.
17. Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the Sustained Alcohol Use Post-Liver Transplant score. Hepatology. 2019;69(4):1477-1487.
18. DiMartini A, Crone C, Dew MA. Alcohol and substance use in liver transplant patients. Clinics in Liver Disease. 2011;15(4):727-751.
19. Pungpapong S, Manzarbeitia C, Ortiz J, et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582-587.
20. Kreek MJ. Pharmacotherapy of opioid dependence: rationale and update. Regulatory Peptides. 1994;53(suppl 1):S255-S256.
21. Jiao M, Greanya ED, Haque M, et al. Methadone maintenance therapy in liver transplantation. Prog Transplant. 2010;20(3):209-214; quiz 215.
22. Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91.
23. Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63-71.
24. Kotwani P, Saxena V, Dodge JL, et al. History of marijuana use does not affect outcomes on the liver transplant waitlist. Transplantation. 2018;102(5):794-802.
25. Fineberg SK, West A, Na PJ, et al. Utility of pretransplant psychological measures to predict posttransplant outcomes in liver transplant patients: a systematic review. Gen Hospl Psychiatry. 2016;40:4-11.
26. Satapathy S, Sanyal A. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
27. Olbrisch ME, Levenson JL, Hamer R. The PACT: a rating scale for the study of clinical decision making in psychosocial screening of organ transplant candidates. Clin Transplant. 1989;3:164-169.
28. Twillman RK, Manetto C, Wellisch DK, et al. Transplant Evaluation Rating Scale: a revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153.
29. Goodier J. Evaluating Stress:97496. In: Zalaquett CP, Wood RJ, eds. Evaluating stress: a book of resources. Lanham, MD: Scarecrow Press; 1997:29-29.
30. Beck AT, Steer RA, Carbin, MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8(1):77-100.
31. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242.
32. Cottle WC. The MMPI: a review. Lawrence, KS: University of Kansas; 1953.
33. Addison CC, Campbell-Jenkins BW, Sarpong DF, et al. Psychometric Evaluation of a Coping Strategies Inventory Short-Form (CSI-SF) in the Jackson Heart Study Cohort. Int J Environ Res Public Health. 2007;4(4):289-295.
34. Mooney S, Hasssanein T, Hilsabeck R, et al. Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175-186.
35. De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33(4):609-615.
36. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42(4):337-343.
37. Landefeld CS, Palme, RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344.
38. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric problems in living-related transplantation (II): the association between paradoxical psychiatric syndrome and guilt feelings in adult recipients after living donor liver transplantation. Transplantation Proceedings. 2002;34(7):2632-2633.
39. Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet. 1996;30(2):141-179.
40. Ozkanlar Y, Nishijima Y, Cunha DD, et al. Acute effects of tacrolimus (FK506) on left ventricular mechanics. Pharmacol Res. 2005;52(4):307-312.
41. Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826-833.
42. Maldonado J R. I have been asked to work up a patient who requires a liver transplant how should I proceed? FOCUS. 2009;7(3):332-335.
43. Mccallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol and Alcoholism. 2006;41(4):358-363.
44. Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680-685.
45. Coffman KL, Hoffman A, Sher L, et al. Treatment of the postoperative alcoholic liver transplant recipient with other addictions. Liver Transpl Surg. 1997;3(3):322-327.
1. Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives [Article in English, Portuguese]. Einstein (São Paulo). 2015;13(1):149-152.
2. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144-1165.
3. Centers for Disease Control and Prevention. QuickStats: number of deaths from 10 leading causes,* by sex—National Vital Statistics System, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(15):413.
4. Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31(1):84-89.
5. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59.
6. Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123-132.
7. Grover S, Sarkar S. Liver transplant—psychiatric and psychosocial aspects. J Clin Exp Hepatol. 2012;2(4):382-392.
8. Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760-770.
9. Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824-834.
10. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497-1504.
11. De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323.
12. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172-183.
13. Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718-1726.
14. Graziadei I, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 2016;128(19):679-690.
15. Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981-987.
16. Lee MR, Leggio L. Management of alcohol use disorder in patients requiring liver transplant. Am J Psychiatry. 2015;172(12):1182-1189.
17. Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the Sustained Alcohol Use Post-Liver Transplant score. Hepatology. 2019;69(4):1477-1487.
18. DiMartini A, Crone C, Dew MA. Alcohol and substance use in liver transplant patients. Clinics in Liver Disease. 2011;15(4):727-751.
19. Pungpapong S, Manzarbeitia C, Ortiz J, et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582-587.
20. Kreek MJ. Pharmacotherapy of opioid dependence: rationale and update. Regulatory Peptides. 1994;53(suppl 1):S255-S256.
21. Jiao M, Greanya ED, Haque M, et al. Methadone maintenance therapy in liver transplantation. Prog Transplant. 2010;20(3):209-214; quiz 215.
22. Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91.
23. Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63-71.
24. Kotwani P, Saxena V, Dodge JL, et al. History of marijuana use does not affect outcomes on the liver transplant waitlist. Transplantation. 2018;102(5):794-802.
25. Fineberg SK, West A, Na PJ, et al. Utility of pretransplant psychological measures to predict posttransplant outcomes in liver transplant patients: a systematic review. Gen Hospl Psychiatry. 2016;40:4-11.
26. Satapathy S, Sanyal A. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
27. Olbrisch ME, Levenson JL, Hamer R. The PACT: a rating scale for the study of clinical decision making in psychosocial screening of organ transplant candidates. Clin Transplant. 1989;3:164-169.
28. Twillman RK, Manetto C, Wellisch DK, et al. Transplant Evaluation Rating Scale: a revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153.
29. Goodier J. Evaluating Stress:97496. In: Zalaquett CP, Wood RJ, eds. Evaluating stress: a book of resources. Lanham, MD: Scarecrow Press; 1997:29-29.
30. Beck AT, Steer RA, Carbin, MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8(1):77-100.
31. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242.
32. Cottle WC. The MMPI: a review. Lawrence, KS: University of Kansas; 1953.
33. Addison CC, Campbell-Jenkins BW, Sarpong DF, et al. Psychometric Evaluation of a Coping Strategies Inventory Short-Form (CSI-SF) in the Jackson Heart Study Cohort. Int J Environ Res Public Health. 2007;4(4):289-295.
34. Mooney S, Hasssanein T, Hilsabeck R, et al. Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175-186.
35. De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33(4):609-615.
36. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42(4):337-343.
37. Landefeld CS, Palme, RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344.
38. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric problems in living-related transplantation (II): the association between paradoxical psychiatric syndrome and guilt feelings in adult recipients after living donor liver transplantation. Transplantation Proceedings. 2002;34(7):2632-2633.
39. Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet. 1996;30(2):141-179.
40. Ozkanlar Y, Nishijima Y, Cunha DD, et al. Acute effects of tacrolimus (FK506) on left ventricular mechanics. Pharmacol Res. 2005;52(4):307-312.
41. Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826-833.
42. Maldonado J R. I have been asked to work up a patient who requires a liver transplant how should I proceed? FOCUS. 2009;7(3):332-335.
43. Mccallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol and Alcoholism. 2006;41(4):358-363.
44. Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680-685.
45. Coffman KL, Hoffman A, Sher L, et al. Treatment of the postoperative alcoholic liver transplant recipient with other addictions. Liver Transpl Surg. 1997;3(3):322-327.
Young, angry, and in need of a liver transplant
CASE Rash, fever, extreme lethargy; multiple hospital visits
Ms. L, age 21, a single woman with a history of major depressive disorder (MDD), is directly admitted from an outside community hospital to our tertiary care academic hospital with acute liver failure.
One month earlier, Ms. L had an argument with her family and punched a wall, fracturing her hand. Following the episode, Ms. L’s primary care physician (PCP) prescribed valproic acid, 500 mg/d, to address “mood swings,” which included angry outbursts and irritability. According to her PCP, no baseline laboratory tests were ordered for Ms. L when she started valproic acid because she was young and otherwise healthy.
After Ms. L had been taking valproic acid for approximately 2 weeks, her mother noticed she became extremely lethargic and took her to the emergency department (ED) of a community hospital (Visit 1) (Table 1). At this time, her laboratory results were notable for an aspartate aminotransferase (AST) level of 303 IU/L (reference range: 8 to 40 IU/L) and an alanine aminotransferase (ALT) level of 241 IU/L (reference range: 20 to 60 IU/L). She also underwent a liver ultrasound, urine toxicology screen, blood alcohol level, and acetaminophen level; the results of all of these tests were unremarkable. Her valproic acid level was within therapeutic limits, consistent with patient adherence; her ammonia level was within normal limits. At Visit 1, Ms. L’s transaminitis was presumed to be secondary to valproic acid. The ED clinicians told her to stop taking valproic acid and discharged her. Her PCP did not give her any follow-up instructions for further laboratory tests or any other recommendations.
During the next week, even though she stopped taking the valproic acid as instructed, Ms. L developed a rash and fever, and continued to have lethargy and general malaise. When she returned to the ED (Visit 2) (Table 1), she was febrile, tachycardic, and hypotensive, with an elevated white blood cell count, eosinophilia, low platelets, and elevated liver function tests. At Visit 2, she was alert and oriented to person, place, time, and situation. Ms. L insisted that she had not overdosed on any medications, or used illicit drugs or alcohol. A test for hepatitis C was negative. Her ammonia level was 58 µmol/L (reference range: 11 to 32 µmol/L). Ms. L received N-acetylcysteine (NAC), prednisone, diphenhydramine, famotidine, and ibuprofen before she was transferred to our tertiary care hospital.
When she arrives at our facility (Visit 3) (Table 1), Ms. L is admitted with acute liver failure. She has an ALT level of 4,091 IU/L, and an AST level of 2,049 IU/L. Ms. L’s mother says that her daughter had been taking sertraline for depression for “some time” with no adverse effects, although she is not clear on the dose or frequency. Her mother says that Ms. L generally likes to spend most of her time at home, and does not believe her daughter is a danger to herself or others. Ms. L’s mother could not describe any episodes of mania or recurrent, dangerous anger episodes. Ms. L has no other medical history and has otherwise been healthy.
On hospital Day 2, Ms. L’s ammonia level is 72 µmol/L, which is slightly elevated. The hepatology team confirms that Ms. L may require a liver transplantation. The primary team consults the inpatient psychiatry consultation-liaison (C-L) team for a pre-transplant psychiatric evaluation.
[polldaddy:10307646]
The authors’ observations
The differential diagnosis for Ms. L was broad and included both accidental and intentional medication overdose. The primary team consulted the inpatient psychiatry C-L team not only for a pre-transplant evaluation, but also to assess for possible overdose.
Continue to: A review of the records...
A review of the records from Visit 1 and Visit 2 at the outside hospital found no acetaminophen in Ms. L’s system and verified that there was no evidence of a current valproic acid overdose. Ms. L had stated that she had not overdosed on any other medications or used any illicit drugs or alcohol. Ms. L’s complex symptoms—namely fever, acute liver failure, and rash—were more consistent with an adverse effect of valproic acid or possibly an inherent autoimmune process.
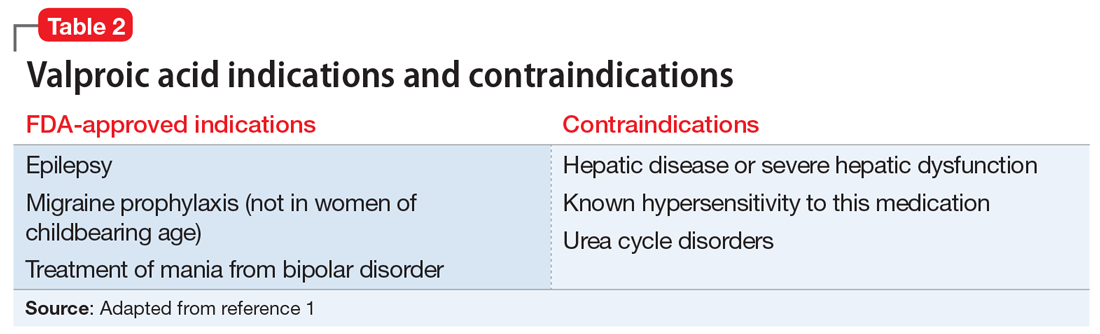
Liver damage from valproic acid
Valproic acid is FDA-approved for treating bipolar disorder, epilepsy, and migraine headaches1 (Table 21). Common adverse effects include nausea, vomiting, sleepiness, and dry mouth. Rarely, valproic acid can impair liver function. While receiving valproic acid, 5% to 10% of patients develop elevated ALT levels, but most are asymptomatic and resolve with time, even if the patient continues taking valproic acid.2 Valproic acid hepatotoxicity resulting in liver transplantation for a healthy patient is extremely rare (Table 31). Liver failure, both fatal and non-fatal, is more prevalent in patients concurrently taking other medications, such as antiepileptics, benzodiazepines, and antipsychotics, as compared with patients receiving only valproic acid.3
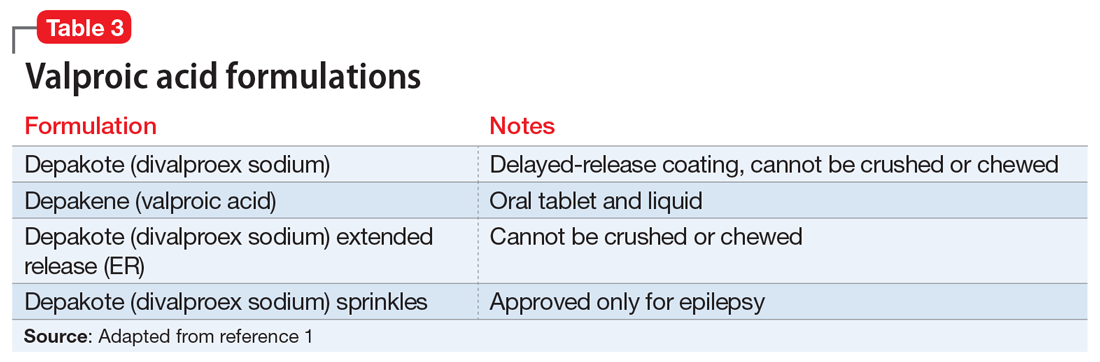
There are 3 clinically distinguishable forms of hepatotoxicity due to valproic acid2:
- hyperammonemia
- acute liver failure and jaundice
- Myriad ProReye-like syndrome, which is generally seen in children.
In case reports of hyperammonemia due to valproic acid, previously healthy patients experience confusion, lethargy, and eventual coma in the context of elevated serum ammonia levels; these symptoms resolved upon discontinuing valproic acid.4,5 Liver function remained normal, with normal to near-normal liver enzymes and bilirubin.3 Hyperammonemia and resulting encephalopathy generally occurred within 1 to 3 weeks after initiation of valproate therapy, with resolution of hyperammonemia and resulting symptoms within a few days after stopping valproic acid.2-4
At Visit 2, Ms. L’s presentation was not initially consistent with hepatic encephalopathy. She was alert and oriented to person, place, time, and situation. Additionally, Ms. L’s presenting problem was elevated liver function tests, not elevated ammonia levels. At Visit 2, her ammonia level was 58 µmol/L; on Day 2 (Visit 3) of her hospital stay, her ammonia level was 72 µmol/L (slightly elevated).
Continue to: At Visit 2 in the ED...
At Visit 2 in the ED, Ms. L was started on NAC because the team suspected she was experiencing drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. This syndrome is characterized by extensive rash, fever, and involvement of at least 1 internal organ. It is a variation of a drug-induced hypersensitivity syndrome. Ms. L’s unremarkable valproic acid levels combined with the psychiatry assessment ruled out valproic hepatotoxicity due to overdose, either intentional or accidental.
In case reports, patients who developed acute liver failure due to valproic acid typically had a hepatitis-like syndrome consisting of moderate elevation in liver enzymes, jaundice, and liver failure necessitating transplantation after at least 1 month of treatment with valproic acid.2 In addition to the typical hepatitis-like syndrome resulting from valproic acid, case reports have also linked treatment with valproic acid to DRESS syndrome.2 This syndrome is known to occur with anticonvulsants such as phenobarbital, lamotrigine, and phenytoin, but there are only a few reported cases of DRESS syndrome due to valproic acid therapy alone.6 Drug rash with eosinophilia and systemic symptoms syndrome differs from other acute liver failure cases in that patients also develop lymphadenopathy, fever, and rash.2,6,7 Patients with DRESS syndrome typically respond to corticosteroid therapy and discontinuation of valproic acid, and the liver damage resolves after several weeks, without a need for transplantation.2,6,7
Ms. L seemed to have similarities to DRESS syndrome. However, the severity of her liver damage, which might require transplantation even after only 2 weeks of valproic acid therapy, initially led the hepatology and C-L teams to consider her presentation similar to severe hepatitis-like cases.
EVALUATION Consent for transplantation
As an inpatient, Ms. L undergoes further laboratory testing. Her hepatic function panel demonstrates a total protein level of 4.8 g/dL, an albumin level of 2.0 g/dL, total bilirubin level of 12.2 mg/dL, and alkaline phosphatase of 166 IU/L. Her laboratory results indicate a prothrombin time (PT) of 77.4 seconds, partial thromboplastin time of 61.5 seconds, and PT international normalized ratio (INR) of 9.6. Ms. L’s basic metabolic panel is within normal limits except for a blood urea nitrogen level of 6 mg/dL, glucose level of 136 mg/dL, and calcium level of 7.0 mg/dL. Her complete blood count indicates a white blood cell count of 12.1, hemoglobin of 10.3 g/dL, hematocrit of 30.4%, mean corpuscular volume of 85.9 fL, and platelet count of 84. Her lipase level is normal at 49 U/L. Her serum acetaminophen concentration is <3.0 mcg/mL, valproic acid level was <2 µg/mL, and she is negative for hepatitis A, B, and C. A urine toxicology screen and testing for herpes simplex, rapid plasma reagin, and human immunodeficiency virus are all negative. Results from several auto-antibodies tests are negative and within normal limits, except filamentous actin (F-actin) antibody, which is slightly higher than normal at 21.4 ELISA units. Based on these results, Ms. L’s liver failure seemed most likely secondary to a reaction to valproic acid.
During her pre-transplant psychiatric evaluation, Ms. L is found to be a poor historian with minimal speech production, flat affect, and clouded sensorium. She denies overdosing on her prescribed valproic acid or sertraline, reports no current suicidal ideation, and does not want to die. She accurately recalls her correct daily dosing of each medication, and verifies that she stopped taking valproic acid 2 weeks ago after being advised to do so by the ED clinicians at Visit 2. She continued to take sertraline until Visit 2. She denied any past or present episodes consistent with mania, which was consistent with her mother’s report.
Continue to: Ms. L becomes agitated...
Ms. L becomes agitated upon further questioning, and requests immediate discharge so that she can return to her family. The evaluation is postponed briefly.
When they reconvene, the C-L team performs a decision-making capacity evaluation, which reveals that Ms. L’s mood and affect are consistent with fear of her impending liver transplant and being alone and approximately 2 hours from her family. This is likely complicated by delirium due to hepatotoxicity. Further discussion between Ms. L and the multidisciplinary team focuses on the risks, benefits, adverse effects of, and alternatives to her current treatment; the possibility of needing a liver transplantation; and how to help her family with transportation to the hospital. Following the discussion, Ms. L is fully cooperative with further treatment, and the pre-transplant psychiatric evaluation is completed.
On physical examination, Ms. L is noted to have a widespread morbilliform rash covering 50% to 60% of her body.
[polldaddy:10307651]
The authors’ observations
L-carnitine supplementation
Multiple studies have shown that supplementation with L-carnitine may increase survival from severe hepatotoxicity due to valproic acid.8,9 Valproic acid may contribute to carnitine deficiency due to its inhibition of carnitine biosynthesis via a decrease in alpha-ketoglutarate concentration.8 Hepatotoxicity or hyperammonemia due to valproic acid may be potentiated by a carnitine deficiency, either pre-existing or resulting from valproic acid.8 L-carnitine supplementation has hastened the decrease of valproic acid–induced ammonemia in a dose-dependent manner,10 and it is currently recommended in cases of valproic acid toxicity, especially in children.8 Children at high risk for developing carnitine deficiency who need to receive valproic acid can be given carnitine supplementation.11 It is not known whether L-carnitine is clinically effective in protecting the liver or hastening liver recovery,8 but it is believed that it might prevent adverse effects of hepatotoxicity and hyperammonemia, especially in patients who receive long-term valproic acid therapy.12
TREATMENT Decompensation and transplantation
Ms. L’s treatment regimen includes NAC, lactulose, and L-carnitine supplementation. During the course of Ms. L’s hospital stay, her liver enzymes begin to trend downward, but her INR and PT remain elevated.
Continue to: On hospital Day 6...
On hospital Day 6, she develops more severe symptoms of hepatic encephalopathy, with significant altered mental status and inattention. Ms. L is transferred to the ICU, intubated, and placed on the liver transplant list.
On hospital Day 9, she undergoes a liver transplantation.
[polldaddy:10307652]
The authors’ observations
Baseline laboratory testing should have been conducted prior to initiating valproic acid. As Ms. L’s symptoms worsened, better communication with her PCP and closer monitoring after starting valproic acid might have resulted in more immediate care. Early recognition of her symptoms and decompensation may have triggered earlier inpatient admission and/or transfer to a tertiary care facility for observation and treatment. Additionally, repeat laboratory testing and instructions on when to return to the ED should have been provided at Visit 1.
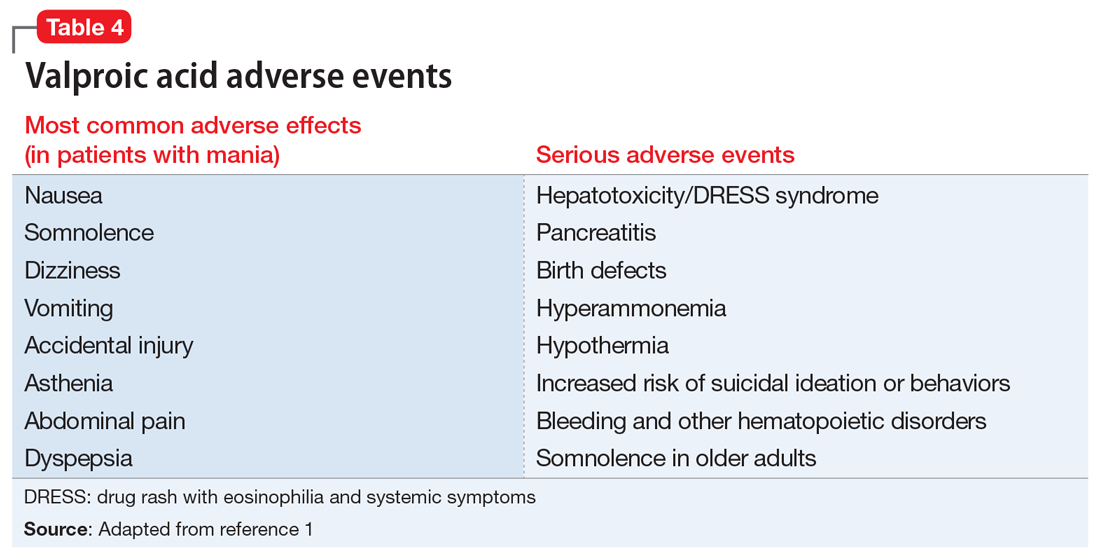
This case demonstrates the need for all clinicians who prescribe valproic acid to remain diligent about the accurate diagnosis of mood and behavioral symptoms, knowing when psychotropic medications are indicated, and carefully considering and discussing even rare, potentially life-threatening adverse effects of all medications with patients.
Although rare, after starting valproic acid, a patient may experience a rapid decompensation and life-threatening illness. Ideally, clinicians should closely monitor patients after initiating valproic acid (Table 41). Clinicians must have a clear knowledge of the recommended monitoring and indications for hospitalization and treatment when they note adverse effects such as elevated liver enzymes or transaminitis (Table 513,14). Even after stopping valproic acid, patients who have experienced adverse events should be closely monitored to ensure complete resolution.
Continue to: Consider patient-specific factors
Consider patient-specific factors
Consider the mental state, intellectual capacity, and social support of each patient before initiating valproic acid. Its use as a mood stabilizer for “mood swings” outside of the context of bipolar disorder is questionable. Valproic acid is FDA-approved for treating bipolar disorder and seizures, but not for anger outbursts/irritability. Prior to starting valproic acid, Ms. L may have benefited from alternative nonpharmacologic treatments, such as psychotherapy, for her anger outbursts and poor coping skills. Therapeutic techniques that focused on helping her acquire better coping mechanisms may have been useful, especially because her mood symptoms did not meet criteria for bipolar disorder, and her depression had long been controlled with sertraline monotherapy.
OUTCOME Discharged after 20 days
Ms. L stays in the hospital for 10 days after receiving her liver transplant. She has low appetite and some difficulty with sleep after the transplant; therefore, the C-L team recommends mirtazapine, 15 mg/d. She has no behavioral problems during her stay, and is set up with home health, case management, and psychiatry follow-up. On hospital Day 20, she is discharged.
Bottom Line
Use caution when prescribing valproic acid, even in young, otherwise healthy patients. Although rare, some patients may experience a rapid decompensation and life-threatening illness after starting valproic acid. When prescribing valproic acid, ensure close follow-up after initiation, including mental status examinations, physical examinations, and laboratory testing.
Related Resource
- Doroudgar S, Chou TI. How to modify psychotropic therapy for patients who have liver dysfunction. Current Psychiatry. 2014;13(12):46-49.
Drug Brand Names
Diphenhydramine • Benadryl
Famotidine • Fluxid, Pepcid
Lamotrigine • Lamictal
Mirtazapine • Remeron
N-acetylcysteine • Mucomyst
Phenobarbital • Luminal
Phenytoin • Dilantin
Prednisone • Cortan, Deltasone
Sertraline • Zoloft
Valproic acid • Depakene
1. Depakote [package insert]. North Chicago, IL: AbbVie, Inc.; 2019.
2. National Institutes of Health. U.S. Department of Health and Human Services. Drug Record: Valproate. https://livertox.nlm.nih.gov/Valproate.htm. Updated October 30, 2018. Accessed March 21, 2019.
3. Schmid MM, Freudenmann RW, Keller F, et al. Non-fatal and fatal liver failure associated with valproic acid. Pharmacopsychiatry. 2013;46(2):63-68.
4. Patel N, Landry KB, Fargason RE, et al. Reversible encephalopathy due to valproic acid induced hyperammonemia in a patient with Bipolar I disorder: a cautionary report. Psychopharmacol Bull. 2017;47(1):40-44.
5. Eze E, Workman M, Donley B. Hyperammonemia and coma developed by a woman treated with valproic acid for affective disorder. Psychiatr Serv. 1998;49(10):1358-1359.
6. Darban M and Bagheri B. Drug reaction with eosinophilia and systemic symptoms induced by valproic acid: a case report. Iran Red Crescent Med J. 2016;18(9): e35825.
7. van Zoelen MA, de Graaf M, van Dijk MR, et al. Valproic acid-induced DRESS syndrome with acute liver failure. Neth J Med. 2012;70(3):155.
8. Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila). 2009;47(2):101-111.
9. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56(10):1405-1409.
10. Böhles H, Sewell AC, Wenzel D. The effect of carnitine supplementation in valproate-induced hyperammonaemia. Acta Paediatr. 1996;85(4):446-449.
11. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638.
12. Romero-Falcón A, de la Santa-Belda E, García-Contreras R, et al. A case of valproate-associated hepatotoxicity treated with L-carnitine. Eur J Intern Med. 2003;14(5):338-340.
13. National Institute for Health and Clinical Excellence. Bipolar disorder: the management of bipolar disorder in adults, children, and adolescents, in primary and secondary care. https://www.nice.org.uk/guidance/cg185. Updated April 2018. Accessed March 21, 2019.
14 . Hirschfeld RMA, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with biopolar disorder: second edition. American Psychiatric Association. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2002. Accessed March 21, 2019.
CASE Rash, fever, extreme lethargy; multiple hospital visits
Ms. L, age 21, a single woman with a history of major depressive disorder (MDD), is directly admitted from an outside community hospital to our tertiary care academic hospital with acute liver failure.
One month earlier, Ms. L had an argument with her family and punched a wall, fracturing her hand. Following the episode, Ms. L’s primary care physician (PCP) prescribed valproic acid, 500 mg/d, to address “mood swings,” which included angry outbursts and irritability. According to her PCP, no baseline laboratory tests were ordered for Ms. L when she started valproic acid because she was young and otherwise healthy.
After Ms. L had been taking valproic acid for approximately 2 weeks, her mother noticed she became extremely lethargic and took her to the emergency department (ED) of a community hospital (Visit 1) (Table 1). At this time, her laboratory results were notable for an aspartate aminotransferase (AST) level of 303 IU/L (reference range: 8 to 40 IU/L) and an alanine aminotransferase (ALT) level of 241 IU/L (reference range: 20 to 60 IU/L). She also underwent a liver ultrasound, urine toxicology screen, blood alcohol level, and acetaminophen level; the results of all of these tests were unremarkable. Her valproic acid level was within therapeutic limits, consistent with patient adherence; her ammonia level was within normal limits. At Visit 1, Ms. L’s transaminitis was presumed to be secondary to valproic acid. The ED clinicians told her to stop taking valproic acid and discharged her. Her PCP did not give her any follow-up instructions for further laboratory tests or any other recommendations.

During the next week, even though she stopped taking the valproic acid as instructed, Ms. L developed a rash and fever, and continued to have lethargy and general malaise. When she returned to the ED (Visit 2) (Table 1), she was febrile, tachycardic, and hypotensive, with an elevated white blood cell count, eosinophilia, low platelets, and elevated liver function tests. At Visit 2, she was alert and oriented to person, place, time, and situation. Ms. L insisted that she had not overdosed on any medications, or used illicit drugs or alcohol. A test for hepatitis C was negative. Her ammonia level was 58 µmol/L (reference range: 11 to 32 µmol/L). Ms. L received N-acetylcysteine (NAC), prednisone, diphenhydramine, famotidine, and ibuprofen before she was transferred to our tertiary care hospital.
When she arrives at our facility (Visit 3) (Table 1), Ms. L is admitted with acute liver failure. She has an ALT level of 4,091 IU/L, and an AST level of 2,049 IU/L. Ms. L’s mother says that her daughter had been taking sertraline for depression for “some time” with no adverse effects, although she is not clear on the dose or frequency. Her mother says that Ms. L generally likes to spend most of her time at home, and does not believe her daughter is a danger to herself or others. Ms. L’s mother could not describe any episodes of mania or recurrent, dangerous anger episodes. Ms. L has no other medical history and has otherwise been healthy.
On hospital Day 2, Ms. L’s ammonia level is 72 µmol/L, which is slightly elevated. The hepatology team confirms that Ms. L may require a liver transplantation. The primary team consults the inpatient psychiatry consultation-liaison (C-L) team for a pre-transplant psychiatric evaluation.
[polldaddy:10307646]
The authors’ observations
The differential diagnosis for Ms. L was broad and included both accidental and intentional medication overdose. The primary team consulted the inpatient psychiatry C-L team not only for a pre-transplant evaluation, but also to assess for possible overdose.
Continue to: A review of the records...
A review of the records from Visit 1 and Visit 2 at the outside hospital found no acetaminophen in Ms. L’s system and verified that there was no evidence of a current valproic acid overdose. Ms. L had stated that she had not overdosed on any other medications or used any illicit drugs or alcohol. Ms. L’s complex symptoms—namely fever, acute liver failure, and rash—were more consistent with an adverse effect of valproic acid or possibly an inherent autoimmune process.

Liver damage from valproic acid
Valproic acid is FDA-approved for treating bipolar disorder, epilepsy, and migraine headaches1 (Table 21). Common adverse effects include nausea, vomiting, sleepiness, and dry mouth. Rarely, valproic acid can impair liver function. While receiving valproic acid, 5% to 10% of patients develop elevated ALT levels, but most are asymptomatic and resolve with time, even if the patient continues taking valproic acid.2 Valproic acid hepatotoxicity resulting in liver transplantation for a healthy patient is extremely rare (Table 31). Liver failure, both fatal and non-fatal, is more prevalent in patients concurrently taking other medications, such as antiepileptics, benzodiazepines, and antipsychotics, as compared with patients receiving only valproic acid.3

There are 3 clinically distinguishable forms of hepatotoxicity due to valproic acid2:
- hyperammonemia
- acute liver failure and jaundice
- Myriad ProReye-like syndrome, which is generally seen in children.
In case reports of hyperammonemia due to valproic acid, previously healthy patients experience confusion, lethargy, and eventual coma in the context of elevated serum ammonia levels; these symptoms resolved upon discontinuing valproic acid.4,5 Liver function remained normal, with normal to near-normal liver enzymes and bilirubin.3 Hyperammonemia and resulting encephalopathy generally occurred within 1 to 3 weeks after initiation of valproate therapy, with resolution of hyperammonemia and resulting symptoms within a few days after stopping valproic acid.2-4
At Visit 2, Ms. L’s presentation was not initially consistent with hepatic encephalopathy. She was alert and oriented to person, place, time, and situation. Additionally, Ms. L’s presenting problem was elevated liver function tests, not elevated ammonia levels. At Visit 2, her ammonia level was 58 µmol/L; on Day 2 (Visit 3) of her hospital stay, her ammonia level was 72 µmol/L (slightly elevated).
Continue to: At Visit 2 in the ED...
At Visit 2 in the ED, Ms. L was started on NAC because the team suspected she was experiencing drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. This syndrome is characterized by extensive rash, fever, and involvement of at least 1 internal organ. It is a variation of a drug-induced hypersensitivity syndrome. Ms. L’s unremarkable valproic acid levels combined with the psychiatry assessment ruled out valproic hepatotoxicity due to overdose, either intentional or accidental.
In case reports, patients who developed acute liver failure due to valproic acid typically had a hepatitis-like syndrome consisting of moderate elevation in liver enzymes, jaundice, and liver failure necessitating transplantation after at least 1 month of treatment with valproic acid.2 In addition to the typical hepatitis-like syndrome resulting from valproic acid, case reports have also linked treatment with valproic acid to DRESS syndrome.2 This syndrome is known to occur with anticonvulsants such as phenobarbital, lamotrigine, and phenytoin, but there are only a few reported cases of DRESS syndrome due to valproic acid therapy alone.6 Drug rash with eosinophilia and systemic symptoms syndrome differs from other acute liver failure cases in that patients also develop lymphadenopathy, fever, and rash.2,6,7 Patients with DRESS syndrome typically respond to corticosteroid therapy and discontinuation of valproic acid, and the liver damage resolves after several weeks, without a need for transplantation.2,6,7
Ms. L seemed to have similarities to DRESS syndrome. However, the severity of her liver damage, which might require transplantation even after only 2 weeks of valproic acid therapy, initially led the hepatology and C-L teams to consider her presentation similar to severe hepatitis-like cases.
EVALUATION Consent for transplantation
As an inpatient, Ms. L undergoes further laboratory testing. Her hepatic function panel demonstrates a total protein level of 4.8 g/dL, an albumin level of 2.0 g/dL, total bilirubin level of 12.2 mg/dL, and alkaline phosphatase of 166 IU/L. Her laboratory results indicate a prothrombin time (PT) of 77.4 seconds, partial thromboplastin time of 61.5 seconds, and PT international normalized ratio (INR) of 9.6. Ms. L’s basic metabolic panel is within normal limits except for a blood urea nitrogen level of 6 mg/dL, glucose level of 136 mg/dL, and calcium level of 7.0 mg/dL. Her complete blood count indicates a white blood cell count of 12.1, hemoglobin of 10.3 g/dL, hematocrit of 30.4%, mean corpuscular volume of 85.9 fL, and platelet count of 84. Her lipase level is normal at 49 U/L. Her serum acetaminophen concentration is <3.0 mcg/mL, valproic acid level was <2 µg/mL, and she is negative for hepatitis A, B, and C. A urine toxicology screen and testing for herpes simplex, rapid plasma reagin, and human immunodeficiency virus are all negative. Results from several auto-antibodies tests are negative and within normal limits, except filamentous actin (F-actin) antibody, which is slightly higher than normal at 21.4 ELISA units. Based on these results, Ms. L’s liver failure seemed most likely secondary to a reaction to valproic acid.
During her pre-transplant psychiatric evaluation, Ms. L is found to be a poor historian with minimal speech production, flat affect, and clouded sensorium. She denies overdosing on her prescribed valproic acid or sertraline, reports no current suicidal ideation, and does not want to die. She accurately recalls her correct daily dosing of each medication, and verifies that she stopped taking valproic acid 2 weeks ago after being advised to do so by the ED clinicians at Visit 2. She continued to take sertraline until Visit 2. She denied any past or present episodes consistent with mania, which was consistent with her mother’s report.
Continue to: Ms. L becomes agitated...
Ms. L becomes agitated upon further questioning, and requests immediate discharge so that she can return to her family. The evaluation is postponed briefly.
When they reconvene, the C-L team performs a decision-making capacity evaluation, which reveals that Ms. L’s mood and affect are consistent with fear of her impending liver transplant and being alone and approximately 2 hours from her family. This is likely complicated by delirium due to hepatotoxicity. Further discussion between Ms. L and the multidisciplinary team focuses on the risks, benefits, adverse effects of, and alternatives to her current treatment; the possibility of needing a liver transplantation; and how to help her family with transportation to the hospital. Following the discussion, Ms. L is fully cooperative with further treatment, and the pre-transplant psychiatric evaluation is completed.
On physical examination, Ms. L is noted to have a widespread morbilliform rash covering 50% to 60% of her body.
[polldaddy:10307651]
The authors’ observations
L-carnitine supplementation
Multiple studies have shown that supplementation with L-carnitine may increase survival from severe hepatotoxicity due to valproic acid.8,9 Valproic acid may contribute to carnitine deficiency due to its inhibition of carnitine biosynthesis via a decrease in alpha-ketoglutarate concentration.8 Hepatotoxicity or hyperammonemia due to valproic acid may be potentiated by a carnitine deficiency, either pre-existing or resulting from valproic acid.8 L-carnitine supplementation has hastened the decrease of valproic acid–induced ammonemia in a dose-dependent manner,10 and it is currently recommended in cases of valproic acid toxicity, especially in children.8 Children at high risk for developing carnitine deficiency who need to receive valproic acid can be given carnitine supplementation.11 It is not known whether L-carnitine is clinically effective in protecting the liver or hastening liver recovery,8 but it is believed that it might prevent adverse effects of hepatotoxicity and hyperammonemia, especially in patients who receive long-term valproic acid therapy.12
TREATMENT Decompensation and transplantation
Ms. L’s treatment regimen includes NAC, lactulose, and L-carnitine supplementation. During the course of Ms. L’s hospital stay, her liver enzymes begin to trend downward, but her INR and PT remain elevated.
Continue to: On hospital Day 6...
On hospital Day 6, she develops more severe symptoms of hepatic encephalopathy, with significant altered mental status and inattention. Ms. L is transferred to the ICU, intubated, and placed on the liver transplant list.
On hospital Day 9, she undergoes a liver transplantation.
[polldaddy:10307652]
The authors’ observations
Baseline laboratory testing should have been conducted prior to initiating valproic acid. As Ms. L’s symptoms worsened, better communication with her PCP and closer monitoring after starting valproic acid might have resulted in more immediate care. Early recognition of her symptoms and decompensation may have triggered earlier inpatient admission and/or transfer to a tertiary care facility for observation and treatment. Additionally, repeat laboratory testing and instructions on when to return to the ED should have been provided at Visit 1.

This case demonstrates the need for all clinicians who prescribe valproic acid to remain diligent about the accurate diagnosis of mood and behavioral symptoms, knowing when psychotropic medications are indicated, and carefully considering and discussing even rare, potentially life-threatening adverse effects of all medications with patients.
Although rare, after starting valproic acid, a patient may experience a rapid decompensation and life-threatening illness. Ideally, clinicians should closely monitor patients after initiating valproic acid (Table 41). Clinicians must have a clear knowledge of the recommended monitoring and indications for hospitalization and treatment when they note adverse effects such as elevated liver enzymes or transaminitis (Table 513,14). Even after stopping valproic acid, patients who have experienced adverse events should be closely monitored to ensure complete resolution.
Continue to: Consider patient-specific factors
Consider patient-specific factors
Consider the mental state, intellectual capacity, and social support of each patient before initiating valproic acid. Its use as a mood stabilizer for “mood swings” outside of the context of bipolar disorder is questionable. Valproic acid is FDA-approved for treating bipolar disorder and seizures, but not for anger outbursts/irritability. Prior to starting valproic acid, Ms. L may have benefited from alternative nonpharmacologic treatments, such as psychotherapy, for her anger outbursts and poor coping skills. Therapeutic techniques that focused on helping her acquire better coping mechanisms may have been useful, especially because her mood symptoms did not meet criteria for bipolar disorder, and her depression had long been controlled with sertraline monotherapy.
OUTCOME Discharged after 20 days
Ms. L stays in the hospital for 10 days after receiving her liver transplant. She has low appetite and some difficulty with sleep after the transplant; therefore, the C-L team recommends mirtazapine, 15 mg/d. She has no behavioral problems during her stay, and is set up with home health, case management, and psychiatry follow-up. On hospital Day 20, she is discharged.
Bottom Line
Use caution when prescribing valproic acid, even in young, otherwise healthy patients. Although rare, some patients may experience a rapid decompensation and life-threatening illness after starting valproic acid. When prescribing valproic acid, ensure close follow-up after initiation, including mental status examinations, physical examinations, and laboratory testing.
Related Resource
- Doroudgar S, Chou TI. How to modify psychotropic therapy for patients who have liver dysfunction. Current Psychiatry. 2014;13(12):46-49.
Drug Brand Names
Diphenhydramine • Benadryl
Famotidine • Fluxid, Pepcid
Lamotrigine • Lamictal
Mirtazapine • Remeron
N-acetylcysteine • Mucomyst
Phenobarbital • Luminal
Phenytoin • Dilantin
Prednisone • Cortan, Deltasone
Sertraline • Zoloft
Valproic acid • Depakene
CASE Rash, fever, extreme lethargy; multiple hospital visits
Ms. L, age 21, a single woman with a history of major depressive disorder (MDD), is directly admitted from an outside community hospital to our tertiary care academic hospital with acute liver failure.
One month earlier, Ms. L had an argument with her family and punched a wall, fracturing her hand. Following the episode, Ms. L’s primary care physician (PCP) prescribed valproic acid, 500 mg/d, to address “mood swings,” which included angry outbursts and irritability. According to her PCP, no baseline laboratory tests were ordered for Ms. L when she started valproic acid because she was young and otherwise healthy.
After Ms. L had been taking valproic acid for approximately 2 weeks, her mother noticed she became extremely lethargic and took her to the emergency department (ED) of a community hospital (Visit 1) (Table 1). At this time, her laboratory results were notable for an aspartate aminotransferase (AST) level of 303 IU/L (reference range: 8 to 40 IU/L) and an alanine aminotransferase (ALT) level of 241 IU/L (reference range: 20 to 60 IU/L). She also underwent a liver ultrasound, urine toxicology screen, blood alcohol level, and acetaminophen level; the results of all of these tests were unremarkable. Her valproic acid level was within therapeutic limits, consistent with patient adherence; her ammonia level was within normal limits. At Visit 1, Ms. L’s transaminitis was presumed to be secondary to valproic acid. The ED clinicians told her to stop taking valproic acid and discharged her. Her PCP did not give her any follow-up instructions for further laboratory tests or any other recommendations.

During the next week, even though she stopped taking the valproic acid as instructed, Ms. L developed a rash and fever, and continued to have lethargy and general malaise. When she returned to the ED (Visit 2) (Table 1), she was febrile, tachycardic, and hypotensive, with an elevated white blood cell count, eosinophilia, low platelets, and elevated liver function tests. At Visit 2, she was alert and oriented to person, place, time, and situation. Ms. L insisted that she had not overdosed on any medications, or used illicit drugs or alcohol. A test for hepatitis C was negative. Her ammonia level was 58 µmol/L (reference range: 11 to 32 µmol/L). Ms. L received N-acetylcysteine (NAC), prednisone, diphenhydramine, famotidine, and ibuprofen before she was transferred to our tertiary care hospital.
When she arrives at our facility (Visit 3) (Table 1), Ms. L is admitted with acute liver failure. She has an ALT level of 4,091 IU/L, and an AST level of 2,049 IU/L. Ms. L’s mother says that her daughter had been taking sertraline for depression for “some time” with no adverse effects, although she is not clear on the dose or frequency. Her mother says that Ms. L generally likes to spend most of her time at home, and does not believe her daughter is a danger to herself or others. Ms. L’s mother could not describe any episodes of mania or recurrent, dangerous anger episodes. Ms. L has no other medical history and has otherwise been healthy.
On hospital Day 2, Ms. L’s ammonia level is 72 µmol/L, which is slightly elevated. The hepatology team confirms that Ms. L may require a liver transplantation. The primary team consults the inpatient psychiatry consultation-liaison (C-L) team for a pre-transplant psychiatric evaluation.
[polldaddy:10307646]
The authors’ observations
The differential diagnosis for Ms. L was broad and included both accidental and intentional medication overdose. The primary team consulted the inpatient psychiatry C-L team not only for a pre-transplant evaluation, but also to assess for possible overdose.
Continue to: A review of the records...
A review of the records from Visit 1 and Visit 2 at the outside hospital found no acetaminophen in Ms. L’s system and verified that there was no evidence of a current valproic acid overdose. Ms. L had stated that she had not overdosed on any other medications or used any illicit drugs or alcohol. Ms. L’s complex symptoms—namely fever, acute liver failure, and rash—were more consistent with an adverse effect of valproic acid or possibly an inherent autoimmune process.

Liver damage from valproic acid
Valproic acid is FDA-approved for treating bipolar disorder, epilepsy, and migraine headaches1 (Table 21). Common adverse effects include nausea, vomiting, sleepiness, and dry mouth. Rarely, valproic acid can impair liver function. While receiving valproic acid, 5% to 10% of patients develop elevated ALT levels, but most are asymptomatic and resolve with time, even if the patient continues taking valproic acid.2 Valproic acid hepatotoxicity resulting in liver transplantation for a healthy patient is extremely rare (Table 31). Liver failure, both fatal and non-fatal, is more prevalent in patients concurrently taking other medications, such as antiepileptics, benzodiazepines, and antipsychotics, as compared with patients receiving only valproic acid.3

There are 3 clinically distinguishable forms of hepatotoxicity due to valproic acid2:
- hyperammonemia
- acute liver failure and jaundice
- Myriad ProReye-like syndrome, which is generally seen in children.
In case reports of hyperammonemia due to valproic acid, previously healthy patients experience confusion, lethargy, and eventual coma in the context of elevated serum ammonia levels; these symptoms resolved upon discontinuing valproic acid.4,5 Liver function remained normal, with normal to near-normal liver enzymes and bilirubin.3 Hyperammonemia and resulting encephalopathy generally occurred within 1 to 3 weeks after initiation of valproate therapy, with resolution of hyperammonemia and resulting symptoms within a few days after stopping valproic acid.2-4
At Visit 2, Ms. L’s presentation was not initially consistent with hepatic encephalopathy. She was alert and oriented to person, place, time, and situation. Additionally, Ms. L’s presenting problem was elevated liver function tests, not elevated ammonia levels. At Visit 2, her ammonia level was 58 µmol/L; on Day 2 (Visit 3) of her hospital stay, her ammonia level was 72 µmol/L (slightly elevated).
Continue to: At Visit 2 in the ED...
At Visit 2 in the ED, Ms. L was started on NAC because the team suspected she was experiencing drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. This syndrome is characterized by extensive rash, fever, and involvement of at least 1 internal organ. It is a variation of a drug-induced hypersensitivity syndrome. Ms. L’s unremarkable valproic acid levels combined with the psychiatry assessment ruled out valproic hepatotoxicity due to overdose, either intentional or accidental.
In case reports, patients who developed acute liver failure due to valproic acid typically had a hepatitis-like syndrome consisting of moderate elevation in liver enzymes, jaundice, and liver failure necessitating transplantation after at least 1 month of treatment with valproic acid.2 In addition to the typical hepatitis-like syndrome resulting from valproic acid, case reports have also linked treatment with valproic acid to DRESS syndrome.2 This syndrome is known to occur with anticonvulsants such as phenobarbital, lamotrigine, and phenytoin, but there are only a few reported cases of DRESS syndrome due to valproic acid therapy alone.6 Drug rash with eosinophilia and systemic symptoms syndrome differs from other acute liver failure cases in that patients also develop lymphadenopathy, fever, and rash.2,6,7 Patients with DRESS syndrome typically respond to corticosteroid therapy and discontinuation of valproic acid, and the liver damage resolves after several weeks, without a need for transplantation.2,6,7
Ms. L seemed to have similarities to DRESS syndrome. However, the severity of her liver damage, which might require transplantation even after only 2 weeks of valproic acid therapy, initially led the hepatology and C-L teams to consider her presentation similar to severe hepatitis-like cases.
EVALUATION Consent for transplantation
As an inpatient, Ms. L undergoes further laboratory testing. Her hepatic function panel demonstrates a total protein level of 4.8 g/dL, an albumin level of 2.0 g/dL, total bilirubin level of 12.2 mg/dL, and alkaline phosphatase of 166 IU/L. Her laboratory results indicate a prothrombin time (PT) of 77.4 seconds, partial thromboplastin time of 61.5 seconds, and PT international normalized ratio (INR) of 9.6. Ms. L’s basic metabolic panel is within normal limits except for a blood urea nitrogen level of 6 mg/dL, glucose level of 136 mg/dL, and calcium level of 7.0 mg/dL. Her complete blood count indicates a white blood cell count of 12.1, hemoglobin of 10.3 g/dL, hematocrit of 30.4%, mean corpuscular volume of 85.9 fL, and platelet count of 84. Her lipase level is normal at 49 U/L. Her serum acetaminophen concentration is <3.0 mcg/mL, valproic acid level was <2 µg/mL, and she is negative for hepatitis A, B, and C. A urine toxicology screen and testing for herpes simplex, rapid plasma reagin, and human immunodeficiency virus are all negative. Results from several auto-antibodies tests are negative and within normal limits, except filamentous actin (F-actin) antibody, which is slightly higher than normal at 21.4 ELISA units. Based on these results, Ms. L’s liver failure seemed most likely secondary to a reaction to valproic acid.
During her pre-transplant psychiatric evaluation, Ms. L is found to be a poor historian with minimal speech production, flat affect, and clouded sensorium. She denies overdosing on her prescribed valproic acid or sertraline, reports no current suicidal ideation, and does not want to die. She accurately recalls her correct daily dosing of each medication, and verifies that she stopped taking valproic acid 2 weeks ago after being advised to do so by the ED clinicians at Visit 2. She continued to take sertraline until Visit 2. She denied any past or present episodes consistent with mania, which was consistent with her mother’s report.
Continue to: Ms. L becomes agitated...
Ms. L becomes agitated upon further questioning, and requests immediate discharge so that she can return to her family. The evaluation is postponed briefly.
When they reconvene, the C-L team performs a decision-making capacity evaluation, which reveals that Ms. L’s mood and affect are consistent with fear of her impending liver transplant and being alone and approximately 2 hours from her family. This is likely complicated by delirium due to hepatotoxicity. Further discussion between Ms. L and the multidisciplinary team focuses on the risks, benefits, adverse effects of, and alternatives to her current treatment; the possibility of needing a liver transplantation; and how to help her family with transportation to the hospital. Following the discussion, Ms. L is fully cooperative with further treatment, and the pre-transplant psychiatric evaluation is completed.
On physical examination, Ms. L is noted to have a widespread morbilliform rash covering 50% to 60% of her body.
[polldaddy:10307651]
The authors’ observations
L-carnitine supplementation
Multiple studies have shown that supplementation with L-carnitine may increase survival from severe hepatotoxicity due to valproic acid.8,9 Valproic acid may contribute to carnitine deficiency due to its inhibition of carnitine biosynthesis via a decrease in alpha-ketoglutarate concentration.8 Hepatotoxicity or hyperammonemia due to valproic acid may be potentiated by a carnitine deficiency, either pre-existing or resulting from valproic acid.8 L-carnitine supplementation has hastened the decrease of valproic acid–induced ammonemia in a dose-dependent manner,10 and it is currently recommended in cases of valproic acid toxicity, especially in children.8 Children at high risk for developing carnitine deficiency who need to receive valproic acid can be given carnitine supplementation.11 It is not known whether L-carnitine is clinically effective in protecting the liver or hastening liver recovery,8 but it is believed that it might prevent adverse effects of hepatotoxicity and hyperammonemia, especially in patients who receive long-term valproic acid therapy.12
TREATMENT Decompensation and transplantation
Ms. L’s treatment regimen includes NAC, lactulose, and L-carnitine supplementation. During the course of Ms. L’s hospital stay, her liver enzymes begin to trend downward, but her INR and PT remain elevated.
Continue to: On hospital Day 6...
On hospital Day 6, she develops more severe symptoms of hepatic encephalopathy, with significant altered mental status and inattention. Ms. L is transferred to the ICU, intubated, and placed on the liver transplant list.
On hospital Day 9, she undergoes a liver transplantation.
[polldaddy:10307652]
The authors’ observations
Baseline laboratory testing should have been conducted prior to initiating valproic acid. As Ms. L’s symptoms worsened, better communication with her PCP and closer monitoring after starting valproic acid might have resulted in more immediate care. Early recognition of her symptoms and decompensation may have triggered earlier inpatient admission and/or transfer to a tertiary care facility for observation and treatment. Additionally, repeat laboratory testing and instructions on when to return to the ED should have been provided at Visit 1.

This case demonstrates the need for all clinicians who prescribe valproic acid to remain diligent about the accurate diagnosis of mood and behavioral symptoms, knowing when psychotropic medications are indicated, and carefully considering and discussing even rare, potentially life-threatening adverse effects of all medications with patients.
Although rare, after starting valproic acid, a patient may experience a rapid decompensation and life-threatening illness. Ideally, clinicians should closely monitor patients after initiating valproic acid (Table 41). Clinicians must have a clear knowledge of the recommended monitoring and indications for hospitalization and treatment when they note adverse effects such as elevated liver enzymes or transaminitis (Table 513,14). Even after stopping valproic acid, patients who have experienced adverse events should be closely monitored to ensure complete resolution.
Continue to: Consider patient-specific factors
Consider patient-specific factors
Consider the mental state, intellectual capacity, and social support of each patient before initiating valproic acid. Its use as a mood stabilizer for “mood swings” outside of the context of bipolar disorder is questionable. Valproic acid is FDA-approved for treating bipolar disorder and seizures, but not for anger outbursts/irritability. Prior to starting valproic acid, Ms. L may have benefited from alternative nonpharmacologic treatments, such as psychotherapy, for her anger outbursts and poor coping skills. Therapeutic techniques that focused on helping her acquire better coping mechanisms may have been useful, especially because her mood symptoms did not meet criteria for bipolar disorder, and her depression had long been controlled with sertraline monotherapy.
OUTCOME Discharged after 20 days
Ms. L stays in the hospital for 10 days after receiving her liver transplant. She has low appetite and some difficulty with sleep after the transplant; therefore, the C-L team recommends mirtazapine, 15 mg/d. She has no behavioral problems during her stay, and is set up with home health, case management, and psychiatry follow-up. On hospital Day 20, she is discharged.
Bottom Line
Use caution when prescribing valproic acid, even in young, otherwise healthy patients. Although rare, some patients may experience a rapid decompensation and life-threatening illness after starting valproic acid. When prescribing valproic acid, ensure close follow-up after initiation, including mental status examinations, physical examinations, and laboratory testing.
Related Resource
- Doroudgar S, Chou TI. How to modify psychotropic therapy for patients who have liver dysfunction. Current Psychiatry. 2014;13(12):46-49.
Drug Brand Names
Diphenhydramine • Benadryl
Famotidine • Fluxid, Pepcid
Lamotrigine • Lamictal
Mirtazapine • Remeron
N-acetylcysteine • Mucomyst
Phenobarbital • Luminal
Phenytoin • Dilantin
Prednisone • Cortan, Deltasone
Sertraline • Zoloft
Valproic acid • Depakene
1. Depakote [package insert]. North Chicago, IL: AbbVie, Inc.; 2019.
2. National Institutes of Health. U.S. Department of Health and Human Services. Drug Record: Valproate. https://livertox.nlm.nih.gov/Valproate.htm. Updated October 30, 2018. Accessed March 21, 2019.
3. Schmid MM, Freudenmann RW, Keller F, et al. Non-fatal and fatal liver failure associated with valproic acid. Pharmacopsychiatry. 2013;46(2):63-68.
4. Patel N, Landry KB, Fargason RE, et al. Reversible encephalopathy due to valproic acid induced hyperammonemia in a patient with Bipolar I disorder: a cautionary report. Psychopharmacol Bull. 2017;47(1):40-44.
5. Eze E, Workman M, Donley B. Hyperammonemia and coma developed by a woman treated with valproic acid for affective disorder. Psychiatr Serv. 1998;49(10):1358-1359.
6. Darban M and Bagheri B. Drug reaction with eosinophilia and systemic symptoms induced by valproic acid: a case report. Iran Red Crescent Med J. 2016;18(9): e35825.
7. van Zoelen MA, de Graaf M, van Dijk MR, et al. Valproic acid-induced DRESS syndrome with acute liver failure. Neth J Med. 2012;70(3):155.
8. Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila). 2009;47(2):101-111.
9. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56(10):1405-1409.
10. Böhles H, Sewell AC, Wenzel D. The effect of carnitine supplementation in valproate-induced hyperammonaemia. Acta Paediatr. 1996;85(4):446-449.
11. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638.
12. Romero-Falcón A, de la Santa-Belda E, García-Contreras R, et al. A case of valproate-associated hepatotoxicity treated with L-carnitine. Eur J Intern Med. 2003;14(5):338-340.
13. National Institute for Health and Clinical Excellence. Bipolar disorder: the management of bipolar disorder in adults, children, and adolescents, in primary and secondary care. https://www.nice.org.uk/guidance/cg185. Updated April 2018. Accessed March 21, 2019.
14 . Hirschfeld RMA, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with biopolar disorder: second edition. American Psychiatric Association. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2002. Accessed March 21, 2019.
1. Depakote [package insert]. North Chicago, IL: AbbVie, Inc.; 2019.
2. National Institutes of Health. U.S. Department of Health and Human Services. Drug Record: Valproate. https://livertox.nlm.nih.gov/Valproate.htm. Updated October 30, 2018. Accessed March 21, 2019.
3. Schmid MM, Freudenmann RW, Keller F, et al. Non-fatal and fatal liver failure associated with valproic acid. Pharmacopsychiatry. 2013;46(2):63-68.
4. Patel N, Landry KB, Fargason RE, et al. Reversible encephalopathy due to valproic acid induced hyperammonemia in a patient with Bipolar I disorder: a cautionary report. Psychopharmacol Bull. 2017;47(1):40-44.
5. Eze E, Workman M, Donley B. Hyperammonemia and coma developed by a woman treated with valproic acid for affective disorder. Psychiatr Serv. 1998;49(10):1358-1359.
6. Darban M and Bagheri B. Drug reaction with eosinophilia and systemic symptoms induced by valproic acid: a case report. Iran Red Crescent Med J. 2016;18(9): e35825.
7. van Zoelen MA, de Graaf M, van Dijk MR, et al. Valproic acid-induced DRESS syndrome with acute liver failure. Neth J Med. 2012;70(3):155.
8. Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila). 2009;47(2):101-111.
9. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56(10):1405-1409.
10. Böhles H, Sewell AC, Wenzel D. The effect of carnitine supplementation in valproate-induced hyperammonaemia. Acta Paediatr. 1996;85(4):446-449.
11. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638.
12. Romero-Falcón A, de la Santa-Belda E, García-Contreras R, et al. A case of valproate-associated hepatotoxicity treated with L-carnitine. Eur J Intern Med. 2003;14(5):338-340.
13. National Institute for Health and Clinical Excellence. Bipolar disorder: the management of bipolar disorder in adults, children, and adolescents, in primary and secondary care. https://www.nice.org.uk/guidance/cg185. Updated April 2018. Accessed March 21, 2019.
14 . Hirschfeld RMA, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with biopolar disorder: second edition. American Psychiatric Association. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2002. Accessed March 21, 2019.
Rediscovering clozapine: Clinically relevant off-label uses
Clozapine has been available for decades, but relatively little has been published regarding its off-label uses. This data shortage likely is due in part to clozapine’s strict monitoring requirements, and we suspect off-label use is more commonplace than the literature reflects.
Refractory schizophrenia and reduction in suicidal behavior in schizophrenia or schizoaffective disorder are clozapine’s 2 FDA-approved indications. Clozapine also may be prescribed for other indications, and off-label uses have varying degrees of scientific support.
Our goal in “Rediscovering clozapine” has been to deepen clinicians’ appreciation for this unique medication and provide practical clinical guidance for its safe and effective use.1,2 This final segment reviews representative literature regarding clozapine’s off-label use for bipolar disorder and other indications (Table).
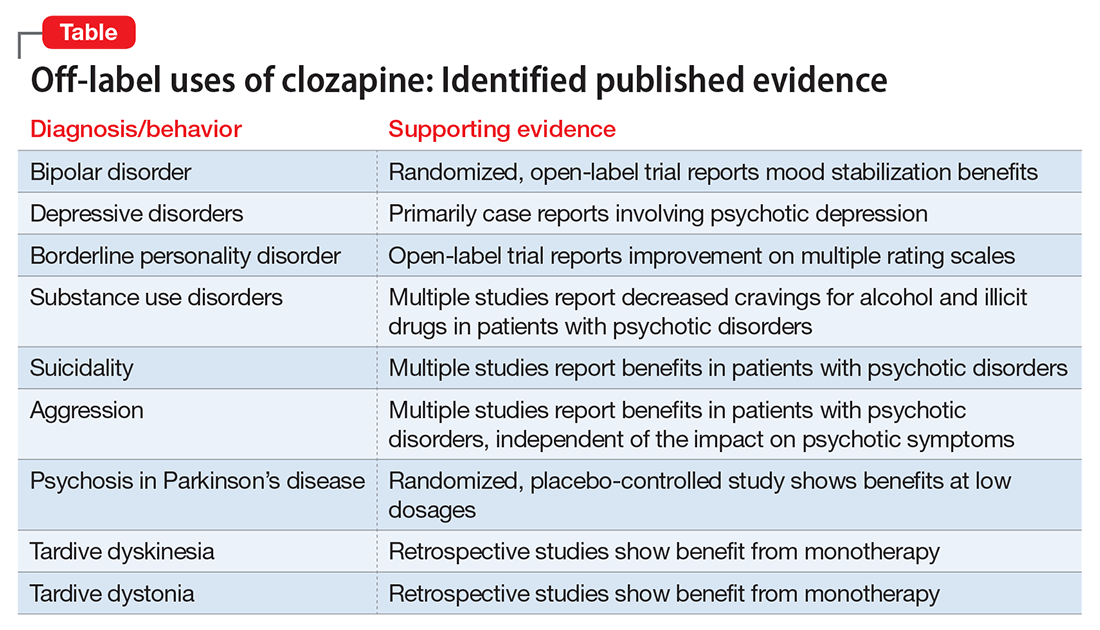
At this point, clozapine still is generally most appropriate for use in refractory cases, regardless of the primary condition being treated. We suggest, however, that physicians should at least consider, “Why is clozapine NOT appropriate for this refractory patient?”
7 Steps define off-label use
Seven steps are useful to consider when prescribing a medication off-label (Figure).3 Off-label prescribing is common in medicine and remains an important component of clinical practice. Sixty percent of antipsychotic prescriptions are written off-label,4 and physicians can prescribe any available medication to any patient for any purpose.
The FDA endorses off-label prescribing: “Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices according to their best knowledge and judgment.”5 Published case reports and case series provide guidance about the scientific support behind specific off-label indications.
Prescribing off-label based on clinical experience alone is legal, and 1 study reported that 73% of off-label prescriptions written by office-based physicians had little or no scientific support.6 From a medicolegal perspective, prescribing off-label with scientific support is preferred.
Bipolar disorder
Clozapine clearly is established as the most effective antipsychotic for treating refractory schizophrenia. A growing body of evidence supports the off-label use of clozapine for patients with bipolar disorder as well. This literature includes:
- a randomized, open-label trial of maintenance treatment of refractory bipolar disorder7
- 2 studies of treatment of acute mania8,9
- a case series of 3 patients with refractory bipolar disorder and psychotic features who were effectively treated during acute manic episodes with ultra-rapid dose titrations of clozapine.10
In China, clozapine commonly is used to treat bipolar disorder. Results have been positive, and some clinicians there consider clozapine a first-line treatment for this indication.11
In the largest published study of clozapine’s benefits for bipolar disorder, a Danish group presented a retrospective analysis of 326 patients with bipolar disorder (and no history of a schizophrenia-spectrum disorder) treated with clozapine between 1996 and 2007. The study group displayed a significant and clinically relevant reduction in psychiatric hospitalizations, polypharmacy, and self-harm. The authors concluded that clozapine appeared to be an appropriate choice for refractory bipolar disorder and encouraged future investigators to consider randomized controlled studies.12
Major depressive disorder
Published evidence supporting clozapine’s use for refractory unipolar depression is less robust than the evidence for refractory bipolar disorder. One retrospective analysis comparing clozapine treatment for bipolar disorder and unipolar depression concluded that patients with bipolar disorder responded better overall.13
Most case reports involve psychotic depression. One case series discussed clozapine treatment of 3 patients with psychotic depression and reported significant improvement in both depressive and psychotic symptoms.14 Other case reports also described patients with refractory psychotic depression.15,16
We located only 1 case report about using clozapine for depressive symptoms absent psychosis. This case involved a patient who developed recurrent depression, hypersomnia, and behavioral disturbances at age 13 after a viral febrile infection. At age 27, she was hospitalized during an episode and started on low-dose clozapine. After discharge, she remained symptom-free for 30 months on clozapine, 50 to 100 mg/d. Although her symptoms included recurrent depression, her overall clinical picture seemed most consistent with Kleine-Levin syndrome (also known as “Sleeping Beauty” syndrome) rather than a primary mood disorder.17
Borderline personality disorder
Psychotherapy is the mainstay for treating borderline personality disorder (BPD), with pharmacotherapy often added to target symptoms such as anger and impulsivity.18 Some small studies and case series have examined clozapine use for BPD.
An open-label study of 15 inpatients with BPD and psychotic disorder not otherwise specified showed improvement on multiple rating scales with clozapine dosages averaging 250 mg/d.19 In a case series of 22 female inpatients with a primary diagnosis of BPD, clozapine showed beneficial effects in several clinical domains, including symptom severity and frequency of aggressive incidents. The greatest improvement occurred within the first 6 months of treatment.20
Eight patients who continued clozapine after hospital discharge had fewer and shorter subsequent hospitalizations than others with BPD who were not prescribed clozapine at discharge.21 Individual case reports have discussed benefits of clozapine in challenging BPD cases.22-24
Substance use treatment
A growing body of literature suggests that clozapine may reduce cravings for alcohol and illicit drugs because of its unique receptor profile. Much of the data has been collected in dual diagnosis patients taking clozapine primarily to treat schizophrenia or schizoaffective disorder. Patients in 1 study showed a comparable response to clozapine therapy whether they had a history of substance abuse or not. The authors opined that their results demonstrated a more generalizable decrease in cravings and recommended further study.25
In a naturalistic study of 151 dual diagnosis patients with schizophrenia, alcohol use rates decreased significantly among those who received clozapine for psychiatric symptoms. After 3 years, 79% of patients treated with clozapine were in remission from alcohol use, compared with 33.7% of patients treated with other antipsychotics.26
Other studies have reported decreased alcohol and illicit drug use in patients with schizophrenia and concomitant substance use.27,28 Animal studies have displayed similar results, showing decreased alcohol intake with clozapine.29,30
Compelling results have been shown in patients with schizophrenia and Cannabis use disorder. A small randomized trial compared clozapine with other antipsychotics in individuals with schizophrenia and Cannabis use disorder. Clozapine was associated with significantly decreased Cannabis use, independent of overall symptom response or level of functioning.31 An animal study demonstrated an attenuated development of conditioned place preference (classical conditioning) to cocaine. The authors suggested that clozapine should be considered as a future pharmacotherapy to treat cocaine use.32
The literature does not support prescribing clozapine solely for alcohol or illicit drug use, but clozapine merits consideration in patients with schizophrenia and comorbid substance use. This approach may be most beneficial in controlled environments, such as inpatient or residential facilities.
Suicidality
The 2-year International Suicide Prevention Trial (InterSePT) was the first to support clozapine’s efficacy in reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder.33 InterSePT data were in line with earlier observations, including improvement in reported depression and hopelessness in patients with primary psychotic disorders.34,35 Clozapine’s action at serotonin receptors (in addition to dopamine receptors) may explain the benefits, based on the suspected link between suicide risk and serotonin.34,36
Most published reports regarding clozapine for suicidality involve patients with schizophrenia or schizoaffective disorder. We found only 1 published case report describing clozapine’s use for recurrent suicidality in a patient with bipolar disorder. The authors described a dramatic reduction in suicidal ideation, suicide attempts, and hospitalizations after other attempted interventions—including electroconvulsive therapy—had been ineffective.37
Aggression
In the absence of FDA-approved treatments for long-term management of aggression, many clinicians prescribe atypical antipsychotics. With the exception of clozapine, the demonstrated benefits of these medications for reducing aggression are equivocal. Clozapine is thought to be superior among atypical antipsychotics for addressing aggression because of its unique and broad combination of dopaminergic and serotonergic activity. Its effects on the D1-dopamine receptor likely target aggression, and its effects on the serotonin 2A receptor (5-HT2A) likely target the impulsivity commonly associated with aggression.38,39
Clozapine has been shown to reduce long-term aggression in patients with psychotic disorders.40-44 Most reports involve individuals with schizophrenia or schizoaffective disorder because this population is most commonly treated with clozapine. However, clozapine’s anti-aggressive benefits appear not to be solely related to sedation or improvement in psychosis.42,45
What is known about clozapine’s mechanism suggests that its anti-aggressive benefits would extend beyond patients with schizophrenia and schizoaffective disorder. In a case series of 7 nonpsychotic patients with antisocial personality disorder and psychopathic traits, all displayed benefits with clozapine—particularly in domains of impulsive behavioral dyscontrol and anger.46
Self-injurious behaviors (SIB) and aggression in 2 patients with profound mental retardation were reduced significantly after treatment was switched from risperidone to clozapine.47 In a similar case, SIB and aggression improved in a man with cognitive impairment.48 The case of Mr. C recounts our experience with using clozapine in a patient with cognitive impairment.
CASE REPORT
Daily assaults keep patient hospitalized
Mr. C, age 19 at the end of treatment, had moderate intellectual disability and an extensive history of violence. He grew up in group homes and long-term psychiatric facilities. Immediately after turning 18, he was transferred from an adolescent facility to an adult psychiatric hospital.
Our treatment team tried various combinations of benzodiazepines, mood stabilizers, and antipsychotics, but Mr. C consistently assaulted 1 or 2 peers daily without clear provocation. Eventually we started him on clozapine, which we titrated to an effective dose (based on a therapeutic serum level). We also added a therapeutic dosage of lithium to address his residual aggression. With the regimen of clozapine and lithium, Mr. C’s assaultive behavior improved dramatically. After going more than 1 year without assaulting a peer, he was placed in the community.
Movement disorders
Parkinson’s disease. The most extensive evidence for treating movement disorders with clozapine involves patients with Parkinson’s disease (PD). Geriatric psychiatrists commonly use clozapine, particularly at low doses, to treat psychotic symptoms in patients with PD. Because of a relatively low likelihood of extrapyramidal side effects, clozapine and quetiapine are the 2 antipsychotics most often used to treat dopamimetic psychosis in PD.49 In a randomized, placebo-controlled study, low-dose clozapine showed benefits in treating dopamimetic psychosis in PD, without worsening overall motor function.50 (The recent approval of pimavanserin for PD psychosis likely will impact off-label use of clozapine for this condition.)
A retrospective review of patients with PD and Lewy body dementia described benefits of treating psychosis with clozapine.51 Benefits also have been reported in using clozapine to address levodopa-induced dyskinesia (LID) absent psychotic symptoms. In an evidence-based review, the Movement Disorder Society described clozapine for LID as “efficacious and possibly useful.”52
Tardive syndromes. In a retrospective review of clozapine use for tardive dyskinesia, 43% of the 30 patients showed improvement, particularly those with concomitant dystonia.53 Another retrospective analysis reported similar outcomes for 48 patients with tardive dyskinesia treated with clozapine.54 Case series and case reports show support for clozapine as monotherapy for tardive dystonia.55
Huntington’s disease. A randomized, double-blind study found little benefit in using clozapine for patients with Huntington’s disease. The authors concluded that, although individual patients may be able to tolerate sufficiently high dosages to improve chorea, clinicians should use restraint when considering clozapine for this population.56
Precautions in older patients. Caution is advised when using clozapine for movement disorders in older individuals, particularly those with concurrent dementia. All antipsychotics, including clozapine,57 carry a “black-box” warning of increased mortality in older adults with dementia.
We hope that this series, “Rediscovering clozapine,” has helped you get reacquainted with this effective medication, employ appropriate caution, and explore off-label uses.
1. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
2. Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop—what should you do now? Current Psychiatry. 2016;15(8):40-46,48-49.
3. Newman WJ, Xiong GL, Barnhorst AV. Beta-blockers: off-label use in psychiatric disorders. Psychopharm Review. 2013;48(10):73-80.
4. Stafford RS. Regulating off-label drug use—rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
5. U.S. Food and Drug Administration. “Off-label” and investigational use of marketed drugs, biologics, and medical devices—information sheet. http://www.fda.gov/RegulatoryInformation/Guidances/ucm126486.htm. Updated January 25, 2016. Accessed November 24, 2015.
6. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026.
7. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156(8):1164-1169.
8. Barbini B, Scherillo P, Benedetti F, et al. Response to clozapine in acute mania is more rapid than that of chlorpromazine. Int Clin Psychopharmacol. 1997;12(2):109-112.
9. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry. 2000;157(6):982-986.
10. Aksoy-Poyraz C, Turan ¸S, Demirel ÖF, et al. Effectiveness of ultra-rapid dose titration of clozapine for treatment-resistant bipolar mania: case series. Ther Adv Psychopharmacol. 2015;5(4):237-242.
11. Li XB, Tang YL, Wang CY, et al. Clozapine for treatment-resistant bipolar disorder: a systematic review. Bipolar Disord. 2015;17(3):235-247.
12. Nielsen J, Kane JM, Correll CU. Real-world effectiveness of clozapine in patients with bipolar disorder: results from a 2-year mirror-image study. Bipolar Disord. 2012;14(8):863-869.
13. Banov MD, Zarate CA Jr, Tohen M, et al. Clozapine therapy in refractory affective disorders: polarity predicts response in long-term follow-up. J Clin Psychiatry. 1994;55(7):295-300.
14. Ranjan R, Meltzer HY. Acute and long-term effectiveness of clozapine in treatment-resistant psychotic depression. Biol Psychiatry. 1996;40(4):253-258.
15. Dassa D, Kaladjian A, Azorin JM, et al. Clozapine in the treatment of psychotic refractory depression. Br J Psychiatry. 1993;163:822-824.
16. Jeyapaul P, Vieweg R. A case study evaluating the use of clozapine in depression with psychotic features. Ann Gen Psychiatry. 2006;5:20.
17. Havaki-Kontaxaki BJ, Ferentinos PP, Kontaxakis VP, et al. Low-dose clozapine monotherapy for recurring episodes of depression, hypersomnia and behavioural disturbances: a case report. Acta Neuropsychiatr. 2011;23(4):191-193.
18. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653. doi: 10.1002/14651858.CD005653.pub2.
19. Frankenburg FR, Zanarini MC. Clozapine treatment of borderline patients: a preliminary study. Compr Psychiatry. 1993;34(6):402-405.
20. Frogley C, Anagnostakis K, Mitchell S, et al. A case series of clozapine for borderline personality disorder. Ann Clin Psychiatry. 2013;25(2):125-134.
21. Parker GF. Clozapine and borderline personality disorder. Psychiatr Serv. 2002;53(3):348-349.
22. Chengappa KNR, Baker RW, Sirri C. The successful use of clozapine in ameliorating severe self mutilation in a patient with borderline personality disorder. J Pers Disord. 1995;9(1):76-82.
23. Rutledge E, O’Regan M, Mohan D. Borderline personality disorder and clozapine. Ir J Psychol Med. 2007;24(1):40-41.
24. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
25. Buckley P, Thompson P, Way L, et al. Substance abuse among patients with treatment-resistant schizophrenia: characteristics and implications for clozapine therapy. Am J Psychiatry. 1994;151(3):385-389.
26. Drake RE, Xie H, McHugo GJ, et al. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26(2):441-449.
27. Zimmet SV, Strous RD, Burgess ES, et al. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. J Clin Psychopharmacol. 2000;20(1):94-98.
28. Green AI, Noordsy DL, Brunette MF, et al. Substance abuse and schizophrenia: pharmacotherapeutic intervention. J Subst Abuse Treat. 2008;34(1):61-71.
29. Green AI, Chau DT, Keung WM, et al. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res. 2004;128(1):9-20.
30. Chau DT, Gulick D, Xie H, et al. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58(2):351-356.
31. Brunette MF, Dawson R, O’Keefe CD, et al. A randomized trial of clozapine vs. other antipsychotics for cannabis use disorder in patients with schizophrenia. J Dual Diagn. 2011;7(1-2):50-63.
32. Kosten TA, Nestler EJ. Clozapine attenuates cocaine conditioned place preference. Life Sci. 1994;55(1):9-14.
33. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735]. Arch Gen Psychiatry. 2003;60(1):82-91.
34. Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry. 1995;152(2):183-190.
35. Sernyak MJ, Desai R, Stolar M, et al. Impact of clozapine on completed suicide. Am J Psychiatry. 2001;158(6):931-937.
36. Nordström P, Asberg M. Suicide risk and serotonin. Int Clin Psychopharmacol. 1992;6(suppl 6):12-21.
37. Vangala VR, Brown ES, Suppes T. Clozapine associated with decreased suicidality in bipolar disorder: a case report. Bipolar Disord. 1999;1(2):123-124.
38. Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17(2):263-287.
39. Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(suppl B):47-52.
40. Rabinowitz J, Avnon M, Rosenberg V. Effect of clozapine on physical and verbal aggression. Schizophr Res. 1996;22(3):249-255.
41. Spivak B, Roitman S, Vered Y, et al. Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic-resistant schizophrenic patients maintained on clozapine. Clin Neuropharmacol. 1998;21(4):245-250.
42. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv. 2001;52(11):1510-1514.
43. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24(2):225-228.
44. Krakowski MI, Czobar P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
45. Chiles JA, Davidson P, McBride D. Effects of clozapine on use of seclusion and restraint at a state hospital. Hosp Community Psychiatry. 1994;45(3):269-271.
46. Brown D, Larkin F, Sengupta S, et al. Clozapine: an effective treatment for seriously violent and psychopathic men with antisocial personality disorder in a UK high-security hospital. CNS Spectr. 2014;19(5):391-402.
47. Hammock R, Levine WR, Schroeder SR. Brief report: effects of clozapine on self-injurious behavior of two risperidone nonresponders with mental retardation. J Autism Dev Disord. 2001;31(1):109-113.
48. Hammock RG, Schroeder SR, Levine WR. The effect of clozapine on self-injurious behavior. J Autism Dev Disord. 1995;25(6):611-626.
49. Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis [Erratum in: Clin Neuropharmacol. 2004;27(5):256]. Clin Neuropharmacol. 2004;27(4):153-156.
50. Pollak P, Tison F, Rascol O. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689-695.
51. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
52. Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: treatment for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(suppl 3):S2-S41.
53. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
54. Naber D, Leppig M, Grohmann R, et al. Efficacy and adverse effects of clozapine in the treatment of schizophrenia and tardive dyskinesia—a retrospective study. Psychopharmacology (Berl). 1989;99(suppl):S73-S76.
55. Pinninti NR, Faden J, Adityanjee A. Are second-generation antipsychotics useful in tardive dystonia? Clin Neuropharmacol. 2015;38(5):183-197.
56. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomised comparative study. J Neurol Neurosurg Psychiatry. 1997;63(1):35-39.
57. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed September 2, 2016.
Clozapine has been available for decades, but relatively little has been published regarding its off-label uses. This data shortage likely is due in part to clozapine’s strict monitoring requirements, and we suspect off-label use is more commonplace than the literature reflects.
Refractory schizophrenia and reduction in suicidal behavior in schizophrenia or schizoaffective disorder are clozapine’s 2 FDA-approved indications. Clozapine also may be prescribed for other indications, and off-label uses have varying degrees of scientific support.
Our goal in “Rediscovering clozapine” has been to deepen clinicians’ appreciation for this unique medication and provide practical clinical guidance for its safe and effective use.1,2 This final segment reviews representative literature regarding clozapine’s off-label use for bipolar disorder and other indications (Table).

At this point, clozapine still is generally most appropriate for use in refractory cases, regardless of the primary condition being treated. We suggest, however, that physicians should at least consider, “Why is clozapine NOT appropriate for this refractory patient?”
7 Steps define off-label use
Seven steps are useful to consider when prescribing a medication off-label (Figure).3 Off-label prescribing is common in medicine and remains an important component of clinical practice. Sixty percent of antipsychotic prescriptions are written off-label,4 and physicians can prescribe any available medication to any patient for any purpose.
The FDA endorses off-label prescribing: “Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices according to their best knowledge and judgment.”5 Published case reports and case series provide guidance about the scientific support behind specific off-label indications.
Prescribing off-label based on clinical experience alone is legal, and 1 study reported that 73% of off-label prescriptions written by office-based physicians had little or no scientific support.6 From a medicolegal perspective, prescribing off-label with scientific support is preferred.
Bipolar disorder
Clozapine clearly is established as the most effective antipsychotic for treating refractory schizophrenia. A growing body of evidence supports the off-label use of clozapine for patients with bipolar disorder as well. This literature includes:
- a randomized, open-label trial of maintenance treatment of refractory bipolar disorder7
- 2 studies of treatment of acute mania8,9
- a case series of 3 patients with refractory bipolar disorder and psychotic features who were effectively treated during acute manic episodes with ultra-rapid dose titrations of clozapine.10
In China, clozapine commonly is used to treat bipolar disorder. Results have been positive, and some clinicians there consider clozapine a first-line treatment for this indication.11
In the largest published study of clozapine’s benefits for bipolar disorder, a Danish group presented a retrospective analysis of 326 patients with bipolar disorder (and no history of a schizophrenia-spectrum disorder) treated with clozapine between 1996 and 2007. The study group displayed a significant and clinically relevant reduction in psychiatric hospitalizations, polypharmacy, and self-harm. The authors concluded that clozapine appeared to be an appropriate choice for refractory bipolar disorder and encouraged future investigators to consider randomized controlled studies.12
Major depressive disorder
Published evidence supporting clozapine’s use for refractory unipolar depression is less robust than the evidence for refractory bipolar disorder. One retrospective analysis comparing clozapine treatment for bipolar disorder and unipolar depression concluded that patients with bipolar disorder responded better overall.13
Most case reports involve psychotic depression. One case series discussed clozapine treatment of 3 patients with psychotic depression and reported significant improvement in both depressive and psychotic symptoms.14 Other case reports also described patients with refractory psychotic depression.15,16
We located only 1 case report about using clozapine for depressive symptoms absent psychosis. This case involved a patient who developed recurrent depression, hypersomnia, and behavioral disturbances at age 13 after a viral febrile infection. At age 27, she was hospitalized during an episode and started on low-dose clozapine. After discharge, she remained symptom-free for 30 months on clozapine, 50 to 100 mg/d. Although her symptoms included recurrent depression, her overall clinical picture seemed most consistent with Kleine-Levin syndrome (also known as “Sleeping Beauty” syndrome) rather than a primary mood disorder.17
Borderline personality disorder
Psychotherapy is the mainstay for treating borderline personality disorder (BPD), with pharmacotherapy often added to target symptoms such as anger and impulsivity.18 Some small studies and case series have examined clozapine use for BPD.
An open-label study of 15 inpatients with BPD and psychotic disorder not otherwise specified showed improvement on multiple rating scales with clozapine dosages averaging 250 mg/d.19 In a case series of 22 female inpatients with a primary diagnosis of BPD, clozapine showed beneficial effects in several clinical domains, including symptom severity and frequency of aggressive incidents. The greatest improvement occurred within the first 6 months of treatment.20
Eight patients who continued clozapine after hospital discharge had fewer and shorter subsequent hospitalizations than others with BPD who were not prescribed clozapine at discharge.21 Individual case reports have discussed benefits of clozapine in challenging BPD cases.22-24
Substance use treatment
A growing body of literature suggests that clozapine may reduce cravings for alcohol and illicit drugs because of its unique receptor profile. Much of the data has been collected in dual diagnosis patients taking clozapine primarily to treat schizophrenia or schizoaffective disorder. Patients in 1 study showed a comparable response to clozapine therapy whether they had a history of substance abuse or not. The authors opined that their results demonstrated a more generalizable decrease in cravings and recommended further study.25
In a naturalistic study of 151 dual diagnosis patients with schizophrenia, alcohol use rates decreased significantly among those who received clozapine for psychiatric symptoms. After 3 years, 79% of patients treated with clozapine were in remission from alcohol use, compared with 33.7% of patients treated with other antipsychotics.26
Other studies have reported decreased alcohol and illicit drug use in patients with schizophrenia and concomitant substance use.27,28 Animal studies have displayed similar results, showing decreased alcohol intake with clozapine.29,30
Compelling results have been shown in patients with schizophrenia and Cannabis use disorder. A small randomized trial compared clozapine with other antipsychotics in individuals with schizophrenia and Cannabis use disorder. Clozapine was associated with significantly decreased Cannabis use, independent of overall symptom response or level of functioning.31 An animal study demonstrated an attenuated development of conditioned place preference (classical conditioning) to cocaine. The authors suggested that clozapine should be considered as a future pharmacotherapy to treat cocaine use.32
The literature does not support prescribing clozapine solely for alcohol or illicit drug use, but clozapine merits consideration in patients with schizophrenia and comorbid substance use. This approach may be most beneficial in controlled environments, such as inpatient or residential facilities.
Suicidality
The 2-year International Suicide Prevention Trial (InterSePT) was the first to support clozapine’s efficacy in reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder.33 InterSePT data were in line with earlier observations, including improvement in reported depression and hopelessness in patients with primary psychotic disorders.34,35 Clozapine’s action at serotonin receptors (in addition to dopamine receptors) may explain the benefits, based on the suspected link between suicide risk and serotonin.34,36
Most published reports regarding clozapine for suicidality involve patients with schizophrenia or schizoaffective disorder. We found only 1 published case report describing clozapine’s use for recurrent suicidality in a patient with bipolar disorder. The authors described a dramatic reduction in suicidal ideation, suicide attempts, and hospitalizations after other attempted interventions—including electroconvulsive therapy—had been ineffective.37
Aggression
In the absence of FDA-approved treatments for long-term management of aggression, many clinicians prescribe atypical antipsychotics. With the exception of clozapine, the demonstrated benefits of these medications for reducing aggression are equivocal. Clozapine is thought to be superior among atypical antipsychotics for addressing aggression because of its unique and broad combination of dopaminergic and serotonergic activity. Its effects on the D1-dopamine receptor likely target aggression, and its effects on the serotonin 2A receptor (5-HT2A) likely target the impulsivity commonly associated with aggression.38,39
Clozapine has been shown to reduce long-term aggression in patients with psychotic disorders.40-44 Most reports involve individuals with schizophrenia or schizoaffective disorder because this population is most commonly treated with clozapine. However, clozapine’s anti-aggressive benefits appear not to be solely related to sedation or improvement in psychosis.42,45
What is known about clozapine’s mechanism suggests that its anti-aggressive benefits would extend beyond patients with schizophrenia and schizoaffective disorder. In a case series of 7 nonpsychotic patients with antisocial personality disorder and psychopathic traits, all displayed benefits with clozapine—particularly in domains of impulsive behavioral dyscontrol and anger.46
Self-injurious behaviors (SIB) and aggression in 2 patients with profound mental retardation were reduced significantly after treatment was switched from risperidone to clozapine.47 In a similar case, SIB and aggression improved in a man with cognitive impairment.48 The case of Mr. C recounts our experience with using clozapine in a patient with cognitive impairment.
CASE REPORT
Daily assaults keep patient hospitalized
Mr. C, age 19 at the end of treatment, had moderate intellectual disability and an extensive history of violence. He grew up in group homes and long-term psychiatric facilities. Immediately after turning 18, he was transferred from an adolescent facility to an adult psychiatric hospital.
Our treatment team tried various combinations of benzodiazepines, mood stabilizers, and antipsychotics, but Mr. C consistently assaulted 1 or 2 peers daily without clear provocation. Eventually we started him on clozapine, which we titrated to an effective dose (based on a therapeutic serum level). We also added a therapeutic dosage of lithium to address his residual aggression. With the regimen of clozapine and lithium, Mr. C’s assaultive behavior improved dramatically. After going more than 1 year without assaulting a peer, he was placed in the community.
Movement disorders
Parkinson’s disease. The most extensive evidence for treating movement disorders with clozapine involves patients with Parkinson’s disease (PD). Geriatric psychiatrists commonly use clozapine, particularly at low doses, to treat psychotic symptoms in patients with PD. Because of a relatively low likelihood of extrapyramidal side effects, clozapine and quetiapine are the 2 antipsychotics most often used to treat dopamimetic psychosis in PD.49 In a randomized, placebo-controlled study, low-dose clozapine showed benefits in treating dopamimetic psychosis in PD, without worsening overall motor function.50 (The recent approval of pimavanserin for PD psychosis likely will impact off-label use of clozapine for this condition.)
A retrospective review of patients with PD and Lewy body dementia described benefits of treating psychosis with clozapine.51 Benefits also have been reported in using clozapine to address levodopa-induced dyskinesia (LID) absent psychotic symptoms. In an evidence-based review, the Movement Disorder Society described clozapine for LID as “efficacious and possibly useful.”52
Tardive syndromes. In a retrospective review of clozapine use for tardive dyskinesia, 43% of the 30 patients showed improvement, particularly those with concomitant dystonia.53 Another retrospective analysis reported similar outcomes for 48 patients with tardive dyskinesia treated with clozapine.54 Case series and case reports show support for clozapine as monotherapy for tardive dystonia.55
Huntington’s disease. A randomized, double-blind study found little benefit in using clozapine for patients with Huntington’s disease. The authors concluded that, although individual patients may be able to tolerate sufficiently high dosages to improve chorea, clinicians should use restraint when considering clozapine for this population.56
Precautions in older patients. Caution is advised when using clozapine for movement disorders in older individuals, particularly those with concurrent dementia. All antipsychotics, including clozapine,57 carry a “black-box” warning of increased mortality in older adults with dementia.
We hope that this series, “Rediscovering clozapine,” has helped you get reacquainted with this effective medication, employ appropriate caution, and explore off-label uses.
Clozapine has been available for decades, but relatively little has been published regarding its off-label uses. This data shortage likely is due in part to clozapine’s strict monitoring requirements, and we suspect off-label use is more commonplace than the literature reflects.
Refractory schizophrenia and reduction in suicidal behavior in schizophrenia or schizoaffective disorder are clozapine’s 2 FDA-approved indications. Clozapine also may be prescribed for other indications, and off-label uses have varying degrees of scientific support.
Our goal in “Rediscovering clozapine” has been to deepen clinicians’ appreciation for this unique medication and provide practical clinical guidance for its safe and effective use.1,2 This final segment reviews representative literature regarding clozapine’s off-label use for bipolar disorder and other indications (Table).

At this point, clozapine still is generally most appropriate for use in refractory cases, regardless of the primary condition being treated. We suggest, however, that physicians should at least consider, “Why is clozapine NOT appropriate for this refractory patient?”
7 Steps define off-label use
Seven steps are useful to consider when prescribing a medication off-label (Figure).3 Off-label prescribing is common in medicine and remains an important component of clinical practice. Sixty percent of antipsychotic prescriptions are written off-label,4 and physicians can prescribe any available medication to any patient for any purpose.
The FDA endorses off-label prescribing: “Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices according to their best knowledge and judgment.”5 Published case reports and case series provide guidance about the scientific support behind specific off-label indications.
Prescribing off-label based on clinical experience alone is legal, and 1 study reported that 73% of off-label prescriptions written by office-based physicians had little or no scientific support.6 From a medicolegal perspective, prescribing off-label with scientific support is preferred.
Bipolar disorder
Clozapine clearly is established as the most effective antipsychotic for treating refractory schizophrenia. A growing body of evidence supports the off-label use of clozapine for patients with bipolar disorder as well. This literature includes:
- a randomized, open-label trial of maintenance treatment of refractory bipolar disorder7
- 2 studies of treatment of acute mania8,9
- a case series of 3 patients with refractory bipolar disorder and psychotic features who were effectively treated during acute manic episodes with ultra-rapid dose titrations of clozapine.10
In China, clozapine commonly is used to treat bipolar disorder. Results have been positive, and some clinicians there consider clozapine a first-line treatment for this indication.11
In the largest published study of clozapine’s benefits for bipolar disorder, a Danish group presented a retrospective analysis of 326 patients with bipolar disorder (and no history of a schizophrenia-spectrum disorder) treated with clozapine between 1996 and 2007. The study group displayed a significant and clinically relevant reduction in psychiatric hospitalizations, polypharmacy, and self-harm. The authors concluded that clozapine appeared to be an appropriate choice for refractory bipolar disorder and encouraged future investigators to consider randomized controlled studies.12
Major depressive disorder
Published evidence supporting clozapine’s use for refractory unipolar depression is less robust than the evidence for refractory bipolar disorder. One retrospective analysis comparing clozapine treatment for bipolar disorder and unipolar depression concluded that patients with bipolar disorder responded better overall.13
Most case reports involve psychotic depression. One case series discussed clozapine treatment of 3 patients with psychotic depression and reported significant improvement in both depressive and psychotic symptoms.14 Other case reports also described patients with refractory psychotic depression.15,16
We located only 1 case report about using clozapine for depressive symptoms absent psychosis. This case involved a patient who developed recurrent depression, hypersomnia, and behavioral disturbances at age 13 after a viral febrile infection. At age 27, she was hospitalized during an episode and started on low-dose clozapine. After discharge, she remained symptom-free for 30 months on clozapine, 50 to 100 mg/d. Although her symptoms included recurrent depression, her overall clinical picture seemed most consistent with Kleine-Levin syndrome (also known as “Sleeping Beauty” syndrome) rather than a primary mood disorder.17
Borderline personality disorder
Psychotherapy is the mainstay for treating borderline personality disorder (BPD), with pharmacotherapy often added to target symptoms such as anger and impulsivity.18 Some small studies and case series have examined clozapine use for BPD.
An open-label study of 15 inpatients with BPD and psychotic disorder not otherwise specified showed improvement on multiple rating scales with clozapine dosages averaging 250 mg/d.19 In a case series of 22 female inpatients with a primary diagnosis of BPD, clozapine showed beneficial effects in several clinical domains, including symptom severity and frequency of aggressive incidents. The greatest improvement occurred within the first 6 months of treatment.20
Eight patients who continued clozapine after hospital discharge had fewer and shorter subsequent hospitalizations than others with BPD who were not prescribed clozapine at discharge.21 Individual case reports have discussed benefits of clozapine in challenging BPD cases.22-24
Substance use treatment
A growing body of literature suggests that clozapine may reduce cravings for alcohol and illicit drugs because of its unique receptor profile. Much of the data has been collected in dual diagnosis patients taking clozapine primarily to treat schizophrenia or schizoaffective disorder. Patients in 1 study showed a comparable response to clozapine therapy whether they had a history of substance abuse or not. The authors opined that their results demonstrated a more generalizable decrease in cravings and recommended further study.25
In a naturalistic study of 151 dual diagnosis patients with schizophrenia, alcohol use rates decreased significantly among those who received clozapine for psychiatric symptoms. After 3 years, 79% of patients treated with clozapine were in remission from alcohol use, compared with 33.7% of patients treated with other antipsychotics.26
Other studies have reported decreased alcohol and illicit drug use in patients with schizophrenia and concomitant substance use.27,28 Animal studies have displayed similar results, showing decreased alcohol intake with clozapine.29,30
Compelling results have been shown in patients with schizophrenia and Cannabis use disorder. A small randomized trial compared clozapine with other antipsychotics in individuals with schizophrenia and Cannabis use disorder. Clozapine was associated with significantly decreased Cannabis use, independent of overall symptom response or level of functioning.31 An animal study demonstrated an attenuated development of conditioned place preference (classical conditioning) to cocaine. The authors suggested that clozapine should be considered as a future pharmacotherapy to treat cocaine use.32
The literature does not support prescribing clozapine solely for alcohol or illicit drug use, but clozapine merits consideration in patients with schizophrenia and comorbid substance use. This approach may be most beneficial in controlled environments, such as inpatient or residential facilities.
Suicidality
The 2-year International Suicide Prevention Trial (InterSePT) was the first to support clozapine’s efficacy in reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder.33 InterSePT data were in line with earlier observations, including improvement in reported depression and hopelessness in patients with primary psychotic disorders.34,35 Clozapine’s action at serotonin receptors (in addition to dopamine receptors) may explain the benefits, based on the suspected link between suicide risk and serotonin.34,36
Most published reports regarding clozapine for suicidality involve patients with schizophrenia or schizoaffective disorder. We found only 1 published case report describing clozapine’s use for recurrent suicidality in a patient with bipolar disorder. The authors described a dramatic reduction in suicidal ideation, suicide attempts, and hospitalizations after other attempted interventions—including electroconvulsive therapy—had been ineffective.37
Aggression
In the absence of FDA-approved treatments for long-term management of aggression, many clinicians prescribe atypical antipsychotics. With the exception of clozapine, the demonstrated benefits of these medications for reducing aggression are equivocal. Clozapine is thought to be superior among atypical antipsychotics for addressing aggression because of its unique and broad combination of dopaminergic and serotonergic activity. Its effects on the D1-dopamine receptor likely target aggression, and its effects on the serotonin 2A receptor (5-HT2A) likely target the impulsivity commonly associated with aggression.38,39
Clozapine has been shown to reduce long-term aggression in patients with psychotic disorders.40-44 Most reports involve individuals with schizophrenia or schizoaffective disorder because this population is most commonly treated with clozapine. However, clozapine’s anti-aggressive benefits appear not to be solely related to sedation or improvement in psychosis.42,45
What is known about clozapine’s mechanism suggests that its anti-aggressive benefits would extend beyond patients with schizophrenia and schizoaffective disorder. In a case series of 7 nonpsychotic patients with antisocial personality disorder and psychopathic traits, all displayed benefits with clozapine—particularly in domains of impulsive behavioral dyscontrol and anger.46
Self-injurious behaviors (SIB) and aggression in 2 patients with profound mental retardation were reduced significantly after treatment was switched from risperidone to clozapine.47 In a similar case, SIB and aggression improved in a man with cognitive impairment.48 The case of Mr. C recounts our experience with using clozapine in a patient with cognitive impairment.
CASE REPORT
Daily assaults keep patient hospitalized
Mr. C, age 19 at the end of treatment, had moderate intellectual disability and an extensive history of violence. He grew up in group homes and long-term psychiatric facilities. Immediately after turning 18, he was transferred from an adolescent facility to an adult psychiatric hospital.
Our treatment team tried various combinations of benzodiazepines, mood stabilizers, and antipsychotics, but Mr. C consistently assaulted 1 or 2 peers daily without clear provocation. Eventually we started him on clozapine, which we titrated to an effective dose (based on a therapeutic serum level). We also added a therapeutic dosage of lithium to address his residual aggression. With the regimen of clozapine and lithium, Mr. C’s assaultive behavior improved dramatically. After going more than 1 year without assaulting a peer, he was placed in the community.
Movement disorders
Parkinson’s disease. The most extensive evidence for treating movement disorders with clozapine involves patients with Parkinson’s disease (PD). Geriatric psychiatrists commonly use clozapine, particularly at low doses, to treat psychotic symptoms in patients with PD. Because of a relatively low likelihood of extrapyramidal side effects, clozapine and quetiapine are the 2 antipsychotics most often used to treat dopamimetic psychosis in PD.49 In a randomized, placebo-controlled study, low-dose clozapine showed benefits in treating dopamimetic psychosis in PD, without worsening overall motor function.50 (The recent approval of pimavanserin for PD psychosis likely will impact off-label use of clozapine for this condition.)
A retrospective review of patients with PD and Lewy body dementia described benefits of treating psychosis with clozapine.51 Benefits also have been reported in using clozapine to address levodopa-induced dyskinesia (LID) absent psychotic symptoms. In an evidence-based review, the Movement Disorder Society described clozapine for LID as “efficacious and possibly useful.”52
Tardive syndromes. In a retrospective review of clozapine use for tardive dyskinesia, 43% of the 30 patients showed improvement, particularly those with concomitant dystonia.53 Another retrospective analysis reported similar outcomes for 48 patients with tardive dyskinesia treated with clozapine.54 Case series and case reports show support for clozapine as monotherapy for tardive dystonia.55
Huntington’s disease. A randomized, double-blind study found little benefit in using clozapine for patients with Huntington’s disease. The authors concluded that, although individual patients may be able to tolerate sufficiently high dosages to improve chorea, clinicians should use restraint when considering clozapine for this population.56
Precautions in older patients. Caution is advised when using clozapine for movement disorders in older individuals, particularly those with concurrent dementia. All antipsychotics, including clozapine,57 carry a “black-box” warning of increased mortality in older adults with dementia.
We hope that this series, “Rediscovering clozapine,” has helped you get reacquainted with this effective medication, employ appropriate caution, and explore off-label uses.
1. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
2. Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop—what should you do now? Current Psychiatry. 2016;15(8):40-46,48-49.
3. Newman WJ, Xiong GL, Barnhorst AV. Beta-blockers: off-label use in psychiatric disorders. Psychopharm Review. 2013;48(10):73-80.
4. Stafford RS. Regulating off-label drug use—rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
5. U.S. Food and Drug Administration. “Off-label” and investigational use of marketed drugs, biologics, and medical devices—information sheet. http://www.fda.gov/RegulatoryInformation/Guidances/ucm126486.htm. Updated January 25, 2016. Accessed November 24, 2015.
6. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026.
7. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156(8):1164-1169.
8. Barbini B, Scherillo P, Benedetti F, et al. Response to clozapine in acute mania is more rapid than that of chlorpromazine. Int Clin Psychopharmacol. 1997;12(2):109-112.
9. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry. 2000;157(6):982-986.
10. Aksoy-Poyraz C, Turan ¸S, Demirel ÖF, et al. Effectiveness of ultra-rapid dose titration of clozapine for treatment-resistant bipolar mania: case series. Ther Adv Psychopharmacol. 2015;5(4):237-242.
11. Li XB, Tang YL, Wang CY, et al. Clozapine for treatment-resistant bipolar disorder: a systematic review. Bipolar Disord. 2015;17(3):235-247.
12. Nielsen J, Kane JM, Correll CU. Real-world effectiveness of clozapine in patients with bipolar disorder: results from a 2-year mirror-image study. Bipolar Disord. 2012;14(8):863-869.
13. Banov MD, Zarate CA Jr, Tohen M, et al. Clozapine therapy in refractory affective disorders: polarity predicts response in long-term follow-up. J Clin Psychiatry. 1994;55(7):295-300.
14. Ranjan R, Meltzer HY. Acute and long-term effectiveness of clozapine in treatment-resistant psychotic depression. Biol Psychiatry. 1996;40(4):253-258.
15. Dassa D, Kaladjian A, Azorin JM, et al. Clozapine in the treatment of psychotic refractory depression. Br J Psychiatry. 1993;163:822-824.
16. Jeyapaul P, Vieweg R. A case study evaluating the use of clozapine in depression with psychotic features. Ann Gen Psychiatry. 2006;5:20.
17. Havaki-Kontaxaki BJ, Ferentinos PP, Kontaxakis VP, et al. Low-dose clozapine monotherapy for recurring episodes of depression, hypersomnia and behavioural disturbances: a case report. Acta Neuropsychiatr. 2011;23(4):191-193.
18. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653. doi: 10.1002/14651858.CD005653.pub2.
19. Frankenburg FR, Zanarini MC. Clozapine treatment of borderline patients: a preliminary study. Compr Psychiatry. 1993;34(6):402-405.
20. Frogley C, Anagnostakis K, Mitchell S, et al. A case series of clozapine for borderline personality disorder. Ann Clin Psychiatry. 2013;25(2):125-134.
21. Parker GF. Clozapine and borderline personality disorder. Psychiatr Serv. 2002;53(3):348-349.
22. Chengappa KNR, Baker RW, Sirri C. The successful use of clozapine in ameliorating severe self mutilation in a patient with borderline personality disorder. J Pers Disord. 1995;9(1):76-82.
23. Rutledge E, O’Regan M, Mohan D. Borderline personality disorder and clozapine. Ir J Psychol Med. 2007;24(1):40-41.
24. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
25. Buckley P, Thompson P, Way L, et al. Substance abuse among patients with treatment-resistant schizophrenia: characteristics and implications for clozapine therapy. Am J Psychiatry. 1994;151(3):385-389.
26. Drake RE, Xie H, McHugo GJ, et al. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26(2):441-449.
27. Zimmet SV, Strous RD, Burgess ES, et al. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. J Clin Psychopharmacol. 2000;20(1):94-98.
28. Green AI, Noordsy DL, Brunette MF, et al. Substance abuse and schizophrenia: pharmacotherapeutic intervention. J Subst Abuse Treat. 2008;34(1):61-71.
29. Green AI, Chau DT, Keung WM, et al. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res. 2004;128(1):9-20.
30. Chau DT, Gulick D, Xie H, et al. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58(2):351-356.
31. Brunette MF, Dawson R, O’Keefe CD, et al. A randomized trial of clozapine vs. other antipsychotics for cannabis use disorder in patients with schizophrenia. J Dual Diagn. 2011;7(1-2):50-63.
32. Kosten TA, Nestler EJ. Clozapine attenuates cocaine conditioned place preference. Life Sci. 1994;55(1):9-14.
33. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735]. Arch Gen Psychiatry. 2003;60(1):82-91.
34. Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry. 1995;152(2):183-190.
35. Sernyak MJ, Desai R, Stolar M, et al. Impact of clozapine on completed suicide. Am J Psychiatry. 2001;158(6):931-937.
36. Nordström P, Asberg M. Suicide risk and serotonin. Int Clin Psychopharmacol. 1992;6(suppl 6):12-21.
37. Vangala VR, Brown ES, Suppes T. Clozapine associated with decreased suicidality in bipolar disorder: a case report. Bipolar Disord. 1999;1(2):123-124.
38. Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17(2):263-287.
39. Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(suppl B):47-52.
40. Rabinowitz J, Avnon M, Rosenberg V. Effect of clozapine on physical and verbal aggression. Schizophr Res. 1996;22(3):249-255.
41. Spivak B, Roitman S, Vered Y, et al. Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic-resistant schizophrenic patients maintained on clozapine. Clin Neuropharmacol. 1998;21(4):245-250.
42. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv. 2001;52(11):1510-1514.
43. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24(2):225-228.
44. Krakowski MI, Czobar P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
45. Chiles JA, Davidson P, McBride D. Effects of clozapine on use of seclusion and restraint at a state hospital. Hosp Community Psychiatry. 1994;45(3):269-271.
46. Brown D, Larkin F, Sengupta S, et al. Clozapine: an effective treatment for seriously violent and psychopathic men with antisocial personality disorder in a UK high-security hospital. CNS Spectr. 2014;19(5):391-402.
47. Hammock R, Levine WR, Schroeder SR. Brief report: effects of clozapine on self-injurious behavior of two risperidone nonresponders with mental retardation. J Autism Dev Disord. 2001;31(1):109-113.
48. Hammock RG, Schroeder SR, Levine WR. The effect of clozapine on self-injurious behavior. J Autism Dev Disord. 1995;25(6):611-626.
49. Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis [Erratum in: Clin Neuropharmacol. 2004;27(5):256]. Clin Neuropharmacol. 2004;27(4):153-156.
50. Pollak P, Tison F, Rascol O. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689-695.
51. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
52. Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: treatment for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(suppl 3):S2-S41.
53. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
54. Naber D, Leppig M, Grohmann R, et al. Efficacy and adverse effects of clozapine in the treatment of schizophrenia and tardive dyskinesia—a retrospective study. Psychopharmacology (Berl). 1989;99(suppl):S73-S76.
55. Pinninti NR, Faden J, Adityanjee A. Are second-generation antipsychotics useful in tardive dystonia? Clin Neuropharmacol. 2015;38(5):183-197.
56. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomised comparative study. J Neurol Neurosurg Psychiatry. 1997;63(1):35-39.
57. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed September 2, 2016.
1. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
2. Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop—what should you do now? Current Psychiatry. 2016;15(8):40-46,48-49.
3. Newman WJ, Xiong GL, Barnhorst AV. Beta-blockers: off-label use in psychiatric disorders. Psychopharm Review. 2013;48(10):73-80.
4. Stafford RS. Regulating off-label drug use—rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
5. U.S. Food and Drug Administration. “Off-label” and investigational use of marketed drugs, biologics, and medical devices—information sheet. http://www.fda.gov/RegulatoryInformation/Guidances/ucm126486.htm. Updated January 25, 2016. Accessed November 24, 2015.
6. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026.
7. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156(8):1164-1169.
8. Barbini B, Scherillo P, Benedetti F, et al. Response to clozapine in acute mania is more rapid than that of chlorpromazine. Int Clin Psychopharmacol. 1997;12(2):109-112.
9. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry. 2000;157(6):982-986.
10. Aksoy-Poyraz C, Turan ¸S, Demirel ÖF, et al. Effectiveness of ultra-rapid dose titration of clozapine for treatment-resistant bipolar mania: case series. Ther Adv Psychopharmacol. 2015;5(4):237-242.
11. Li XB, Tang YL, Wang CY, et al. Clozapine for treatment-resistant bipolar disorder: a systematic review. Bipolar Disord. 2015;17(3):235-247.
12. Nielsen J, Kane JM, Correll CU. Real-world effectiveness of clozapine in patients with bipolar disorder: results from a 2-year mirror-image study. Bipolar Disord. 2012;14(8):863-869.
13. Banov MD, Zarate CA Jr, Tohen M, et al. Clozapine therapy in refractory affective disorders: polarity predicts response in long-term follow-up. J Clin Psychiatry. 1994;55(7):295-300.
14. Ranjan R, Meltzer HY. Acute and long-term effectiveness of clozapine in treatment-resistant psychotic depression. Biol Psychiatry. 1996;40(4):253-258.
15. Dassa D, Kaladjian A, Azorin JM, et al. Clozapine in the treatment of psychotic refractory depression. Br J Psychiatry. 1993;163:822-824.
16. Jeyapaul P, Vieweg R. A case study evaluating the use of clozapine in depression with psychotic features. Ann Gen Psychiatry. 2006;5:20.
17. Havaki-Kontaxaki BJ, Ferentinos PP, Kontaxakis VP, et al. Low-dose clozapine monotherapy for recurring episodes of depression, hypersomnia and behavioural disturbances: a case report. Acta Neuropsychiatr. 2011;23(4):191-193.
18. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653. doi: 10.1002/14651858.CD005653.pub2.
19. Frankenburg FR, Zanarini MC. Clozapine treatment of borderline patients: a preliminary study. Compr Psychiatry. 1993;34(6):402-405.
20. Frogley C, Anagnostakis K, Mitchell S, et al. A case series of clozapine for borderline personality disorder. Ann Clin Psychiatry. 2013;25(2):125-134.
21. Parker GF. Clozapine and borderline personality disorder. Psychiatr Serv. 2002;53(3):348-349.
22. Chengappa KNR, Baker RW, Sirri C. The successful use of clozapine in ameliorating severe self mutilation in a patient with borderline personality disorder. J Pers Disord. 1995;9(1):76-82.
23. Rutledge E, O’Regan M, Mohan D. Borderline personality disorder and clozapine. Ir J Psychol Med. 2007;24(1):40-41.
24. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
25. Buckley P, Thompson P, Way L, et al. Substance abuse among patients with treatment-resistant schizophrenia: characteristics and implications for clozapine therapy. Am J Psychiatry. 1994;151(3):385-389.
26. Drake RE, Xie H, McHugo GJ, et al. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26(2):441-449.
27. Zimmet SV, Strous RD, Burgess ES, et al. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. J Clin Psychopharmacol. 2000;20(1):94-98.
28. Green AI, Noordsy DL, Brunette MF, et al. Substance abuse and schizophrenia: pharmacotherapeutic intervention. J Subst Abuse Treat. 2008;34(1):61-71.
29. Green AI, Chau DT, Keung WM, et al. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res. 2004;128(1):9-20.
30. Chau DT, Gulick D, Xie H, et al. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58(2):351-356.
31. Brunette MF, Dawson R, O’Keefe CD, et al. A randomized trial of clozapine vs. other antipsychotics for cannabis use disorder in patients with schizophrenia. J Dual Diagn. 2011;7(1-2):50-63.
32. Kosten TA, Nestler EJ. Clozapine attenuates cocaine conditioned place preference. Life Sci. 1994;55(1):9-14.
33. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735]. Arch Gen Psychiatry. 2003;60(1):82-91.
34. Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry. 1995;152(2):183-190.
35. Sernyak MJ, Desai R, Stolar M, et al. Impact of clozapine on completed suicide. Am J Psychiatry. 2001;158(6):931-937.
36. Nordström P, Asberg M. Suicide risk and serotonin. Int Clin Psychopharmacol. 1992;6(suppl 6):12-21.
37. Vangala VR, Brown ES, Suppes T. Clozapine associated with decreased suicidality in bipolar disorder: a case report. Bipolar Disord. 1999;1(2):123-124.
38. Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17(2):263-287.
39. Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(suppl B):47-52.
40. Rabinowitz J, Avnon M, Rosenberg V. Effect of clozapine on physical and verbal aggression. Schizophr Res. 1996;22(3):249-255.
41. Spivak B, Roitman S, Vered Y, et al. Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic-resistant schizophrenic patients maintained on clozapine. Clin Neuropharmacol. 1998;21(4):245-250.
42. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv. 2001;52(11):1510-1514.
43. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24(2):225-228.
44. Krakowski MI, Czobar P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
45. Chiles JA, Davidson P, McBride D. Effects of clozapine on use of seclusion and restraint at a state hospital. Hosp Community Psychiatry. 1994;45(3):269-271.
46. Brown D, Larkin F, Sengupta S, et al. Clozapine: an effective treatment for seriously violent and psychopathic men with antisocial personality disorder in a UK high-security hospital. CNS Spectr. 2014;19(5):391-402.
47. Hammock R, Levine WR, Schroeder SR. Brief report: effects of clozapine on self-injurious behavior of two risperidone nonresponders with mental retardation. J Autism Dev Disord. 2001;31(1):109-113.
48. Hammock RG, Schroeder SR, Levine WR. The effect of clozapine on self-injurious behavior. J Autism Dev Disord. 1995;25(6):611-626.
49. Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis [Erratum in: Clin Neuropharmacol. 2004;27(5):256]. Clin Neuropharmacol. 2004;27(4):153-156.
50. Pollak P, Tison F, Rascol O. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689-695.
51. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
52. Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: treatment for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(suppl 3):S2-S41.
53. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
54. Naber D, Leppig M, Grohmann R, et al. Efficacy and adverse effects of clozapine in the treatment of schizophrenia and tardive dyskinesia—a retrospective study. Psychopharmacology (Berl). 1989;99(suppl):S73-S76.
55. Pinninti NR, Faden J, Adityanjee A. Are second-generation antipsychotics useful in tardive dystonia? Clin Neuropharmacol. 2015;38(5):183-197.
56. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomised comparative study. J Neurol Neurosurg Psychiatry. 1997;63(1):35-39.
57. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed September 2, 2016.
Rediscovering clozapine: Adverse effects develop—what should you do now?
Clozapine is a highly effective antipsychotic with superior efficacy in treatment-resistant schizophrenia, but its side effect profile is daunting (Figure 1).1 Adverse reactions lead to approximately 17% of patients who take clozapine eventually discontinuing the medication.1 As we noted in Part 1 of this 3-part series,2 clozapine remains the most efficacious, but most tedious, antipsychotic available to psychiatrists because of its monitoring requirements and potential side effects.
A powerful rationale for prescribing clozapine, despite its drawbacks, is its association with a reduced risk of all-cause mortality.3,4 People with serious mental illness, including schizophrenia, have a median 10-year shorter life expectancy than the general population.5
A recent cohort study6 examined electronic health records to test whether intensive monitoring or lower suicide risk might account for the reduced mortality with clozapine. The authors found that the reduced mortality rate was not directly related to clozapine’s clinical monitoring or other confounding factors. They did find an association between clozapine use and reduced risk of death from both natural and unnatural causes.
This second article in our series examines clozapine’s adverse effects from a systems perspective. Severe neutropenia, myocarditis, sedation, weight gain, orthostatic hypotension, and sialorrhea appear to be the most studied adverse effects, but myriad others can occur.7 We offer guidance to help the astute clinician continue this effective antipsychotic by monitoring carefully, treating side effects early, and managing potential drug interactions (Table 1).8
Hematologic eventsSevere neutropenia, defined as absolute neutrophil count (ANC) <500/µL, is a well-known adverse effect of clozapine that requires specific clinical monitoring, a requirement that was updated by the FDA in 2015.2 The incidence of severe neutropenia peaks in the first 2 months of clozapine therapy and tapers after 6 months, but some risk always remains.
Older efficacy studies in the United States gauged the 1-year cumulative incidence of severe clozapine-induced neutropenia to be 2%.9 A 1998 study of the effects of using a clozapine registry reported a lower incidence—0.38%—than the 2% noted above.10 Early recognition and recommended interventions can improve clinical outcomes.2
Drug interactions and neutropenia. A retrospective study of mental health inpatients taking clozapine concurrently with oseltamivir during an influenza outbreak found a statistically significant—but not clinically significant—change in ANC values.11 The authors noted that viral infection might lead to blood dyscrasia early in illness, and that oseltamivir has been associated with a small incidence of blood dyscrasia.11-13 This information might be useful when treating influenza in patients taking clozapine, although no specific change in management is recommended.
Similarly, concomitant treatment with clozapine and lithium can affect both white blood cell and ANC values.14,15 Lithium-treated patients often demonstrate increased circulating neutrophils via enhancement of granulocyte-colony stimulating factor.16 Case studies describe how initiating lithium treatment enabled some patients to continue clozapine after developing neutropenia.14,17 Leukocytosis can affect blood monitoring, possibly masking other blood dyscrasias, when lithium is used concurrently with clozapine.
Eosinophilia (blood eosinophil count >700/µL) occurs in approximately 1% of clozapine users, usually in the first 4 weeks of treatment.18 It can be benign and transient or a harbinger of a more rare adverse reaction such as myocarditis, pancreatitis, hepatitis, colitis, or nephritis.19 If a patient taking clozapine develops eosinophilia, clozapine’s package insert recommends that you:
- evaluate promptly for other systemic involvement (rash, other evidence of allergic reaction, myocarditis, other organ-specific disease)
- stop clozapine immediately if any of these are found.
If other causes of eosinophilia are identified (asthma, allergies, collagen vascular disease, parasitic infection, neoplasm), treat these and continue clozapine.
The manufacturer also mentions the occurrence of clozapine-related eosinophilia without organ involvement that can resolve without intervention, with careful monitoring over several weeks.8 In this scenario, there is flexibility to judge whether clozapine should be stopped or re-challenged, or if close monitoring is adequate. Consulting with an internal medicine or hematology specialist might be helpful.
Cardiovascular side effectsMost common events. Three of the 10 most common clozapine side effects are cardiac: tachycardia, hypotension, and hypertension (Figure 1).1 Orthostatic hypotension, bradycardia, and syncope also can occur, especially with rapid clozapine titration. Baseline electrocardiogram (ECG) can help differentiate whether abnormalities are clozapine-induced or related to a preexisting condition.
Reducing the dosage of clozapine or slowing titration could reverse cardiac side effects.8 If dosage reduction is not an option or is ineffective, first consider treating the side effect rather than discontinuing clozapine.20
Sinus tachycardia is one of the most common side effects of clozapine. First, rule out serious conditions—myocarditis, cardiomyopathy, neuroleptic malignant syndrome (NMS)—then consider waiting and monitoring for the first few months of clozapine treatment. If tachycardia continues, consider dosage reduction. Slower titration, or treatment with a cardio-selective beta blocker such as atenolol.21,22 Note that a recent Cochrane Review concluded that there is not enough randomized evidence to support any particular treatment for clozapine-induced tachycardia; the prescriber must therefore make a case-by-case clinical judgment.22
Similarly, orthostatic hypotension can be managed with a reduced dosage of clozapine or slower titration. Increased fluid intake, compression stockings, and, if necessary, fludrocortisone also can be initiated.20
Rare, potentially fatal events. Myocarditis, pericarditis, and cardiomyopathy are among the rare but potentially fatal adverse effects of clozapine. A recent study reported the incidence of myocarditis with clozapine at a range of 0.015% to 1.3%; cardiomyopathy was even more rare.23 Pulmonary embolism and deep venous thrombosis also are very rare possibilities; keep them in mind, however, when patients taking clozapine report new cardiovascular symptoms.
Patients with clozapine-induced cardiovascular effects most commonly report shortness of breath (60%), palpitations (36%), cough (16%), fatigue (16%), and chest pain (8%).7,24
Clozapine’s “black-box” warning specifically recommends discontinuing clozapine and consulting cardiology when myocarditis or cardiomyopathy is suspected. In 50% of cases, myocarditis symptoms present in the first few weeks of clozapine treatment.23 The manufacturer states that myocarditis usually presents in the first 2 months, and cardiomyopathy after 8 weeks of treatment; however, either can present at any time.8Figure 2 provides a clinical reference for monitoring a clozapine patient for cardiomyopathy.24
Laboratory findings that support a diagnosis of clozapine-related myocarditis include:
- elevated C-reactive protein
- elevated troponin I or T
- elevated creatine kinase-MB
- peripheral eosinophilia.8,25
ECG, echocardiography, and cardiac MRI can be helpful in diagnosis, in consultation with a cardiologist.
Neurologic side effectsSeizures are listed in the “black-box” warning for clozapine. Seizure incidence with clozapine is 5% per year, with higher incidence at dosages ≥600 mg/d.8 Because clozapine-induced seizures are dosage-dependent, slow titration can mitigate this risk. Tonic-clonic seizures are the most common type associated with clozapine.
The manufacturer recommends caution when using clozapine in patients with a known seizure disorder, alcohol use disorder, or other CNS pathology.8 Patients with a seizure disorder may be at increased risk of experiencing clozapine-induced seizures, but this is not an absolute contraindication.26 Smoking cessation increases clozapine blood levels by an average of 57.4%, further increasing seizure risk.26,27
Discontinuing clozapine is unnecessary when a patient experiences a seizure. Instead, you can:
- halve the dosage prescribed at the time of the seizure (or at least reduce to the last seizure-free dosage)
- consider any medications or medical problems that might have contributed to a lower seizure threshold
- consider prophylaxis with an antiepileptic medication (eg, valproic acid has efficacy for both myoclonic and tonic-clonic seizures).20,26
Sedation is the most common side effect of clozapine.1 Patients experiencing severe sedation should not drive or operate heavy machinery. To reduce sedation, consider instructing the patient to take all or most of the clozapine dosage at bedtime. A critical review of modafinil for sedation caused by antipsychotics in schizophrenia found only 1 open-label study that showed any positive effects; the authors concluded that further study is needed.28
Cognitive and motor slowing are possible neurologic side effects of clozapine. Caution patients about the risk of participating in activities that require cognitive or motor performance until the individual effects of clozapine are known.8
Tardive dyskinesia. Clozapine carries some risk of tardive dyskinesia, although that risk is lower than with other antipsychotics. Similarly, all antipsychotics including clozapine are associated with a risk of NMS. In the rare case of clozapine-induced NMS, stop clozapine immediately and initiate supportive therapy. Clozapine-induced NMS is not an absolute contraindication to re-challenging a patient with clozapine, however, if doing so is clinically appropriate.20
Cerebrovascular events. In older people with dementia, the use of antipsychotics—including clozapine—has been shown to increase the risk of cerebrovascular events. Because most antipsychotics are not FDA-approved for treating psychosis associated with dementia (only pimavanserin is FDA-approved for symptoms of psychosis in Parkinson’s disease), a risk-benefit analysis should be documented when prescribing any antipsychotic in this population. In practice, clozapine’s benefits may outweigh the mortality risks in specific situations.29,30
CASE Sialorrhea puts progress at risk
Ms. B, age 40, has a history of treatment-resistant schizophrenia and is starting clozapine because of residual psychosis during trials of other antipsychotics. She develops severe persistent drooling, mostly at night, during clozapine titration. Sugar-free candy, multiple bed pillows, and changing the dosing schedule do not significantly improve the sialorrhea.
As a result, Ms. B is embarrassed to continue her usual activities. She asks to stop clozapine, even though her psychotic symptoms have improved and she is functioning at her highest level in years.
Ms. B already is taking trihexyphenidyl, 5 mg, 3 times daily, to manage extrapyramidal symptoms related to haloperidol decanoate treatment. After discussing other medication options for sialorrhea, she agrees to a trial of glycopyrrolate, 1 mg, twice daily. She experiences significant improvement and continues taking clozapine.
Sialorrhea develops in 13% of patients taking clozapine.1 As in Ms. B’s case, this side effect can be embarrassing, can limit social or occupational functioning, and might lead patients to discontinue clozapine treatment despite efficacy. Nonpharmacotherapeutic options include covering the pillow with a towel, lowering the clozapine dosage or titrating slowly (or both), and using sugarless gum or candy to increase swallowing.
If the benefits of additional medications targeting side effects outweigh the risks, pharmacotherapeutic intervention may be appropriate. Options include the tricyclic antidepressant amitriptyline31; alpha-adrenergic agonists or antagonists (clonidine, terazosin); and anti-muscarinic medications (benztropine, atropine, trihexyphenidyl, glycopyrrolate) (Table 231). Scopolamine transdermal patch is another possible treatment strategy; however, the scopolamine patch was used for clozapine-induced sialorrhea in only a few case reports, and it is not considered a first-line treatment choice.30
When prescribing, consider the possibility of combined side effects with clozapine and adjunct medications having antimuscarinic or alpha-adrenergic activity, or both. Even atropine ophthalmic drops, administered sublingually, are readily absorbed and cross the blood–brain barrier.31 Another antimuscarinic agent, glycopyrrolate, is less likely to cross the blood–brain barrier and therefore is less likely to cause cognitive side effects. Glycopyrrolate is 5 times more potent at blocking the muscarinic receptor than atropine.31,32 Ipratropium bromide, another nonselective muscarinic receptor antagonist, has less systemic absorption than atropine drops, with less anticholinergic side effects when administered sublingually.
Limited evidence supports the efficacy of alpha-adrenergic medications for managing clozapine-induced sialorrhea. Monitor blood pressure when prescribing terazosin or clonidine, which could potentiate clozapine’s hypotensive effects.
Endocrine side effectsAmong antipsychotics, clozapine is associated with the greatest weight gain—averaging nearly 10% of body weight.33,34 Similarly, the risk of new-onset diabetes mellitus is highest with clozapine in relation to other antipsychotics: 43% reported in a 10-year naturalistic study.35 The risk of hyperlipidemia also increases with clozapine treatment.36 These metabolic changes increase the risk of cardiovascular-related death, with a 10-year mortality rate from cardiovascular disease reported at 9% in clozapine-treated patients.35
Despite clozapine’s metabolic side effects, patients with schizophrenia who are treated with clozapine show a significant reduction in overall mortality compared with patients not treated with clozapine.6 Effective identification and management of metabolic side effects can prevent the need to discontinue clozapine.
Behavioral weight management and exercise are recommended as initial therapy.20 If, based on clinical judgment, these alone are insufficient, data support the use of pharmacotherapeutic interventions. Metformin demonstrates a positive effect on body weight, insulin resistance, and lipids, making it the first choice for adjunctive treatment of clozapine-induced metabolic side effects.37-39
Gastrointestinal side effectsClozapine’s anticholinergic activity can lead to serious gastrointestinal (GI) side effects, including constipation, intestinal obstruction, fecal impaction, and paralytic ileus.8 Ileus has produced more fatal adverse reactions with clozapine than has severe neutropenia.20,40 Co-administered anticholinergic medications could increase the risk of ileus. Obtaining a GI review of systems and monitoring bowel movements (in inpatient or residential facilities) can aid in early identification and limit morbidity and mortality from GI adverse events. A high-fiber diet, adequate hydration, bulk laxatives in patients who can reliably maintain hydration, and GI consultation (if needed) may help manage GI side effects.20
Constitutional side effectsFever can occur with clozapine, most often in the first month of treatment, but the incidence is quite variable (0.5% to 55%).20,41 Although benign fever is common, agranulocytosis with infection, NMS, and other systemic illness must be ruled out. The recommended workup when a patient develops fever while taking clozapine includes physical examination and relevant testing (urinalysis, measurement of ANC and serum creatine kinase, chest radiograph, ECG, and, possibly, blood cultures).41
If evidence supports a serious adverse reaction, stop clozapine immediately.20 If benign clozapine-related fever is suspected, acetaminophen or another antipyretic might provide symptomatic relief; discontinuing clozapine is then unnecessary.41
Pregnancy. When a patient with schizophrenia requires clozapine treatment during pregnancy, reliable clinical guidance is limited. The American College of Obstetricians and Gynecologists Practice Bulletin on the use of psychiatric medications during pregnancy and lactation can be a useful resource.42
Be aware that the FDA very recently made major changes to the format and content of pregnancy and lactation labeling, removing the letter categories that have been used for medications approved on or after June 30, 2001. The manufacturers of medications (such as clozapine) that were approved before June 30, 2001, have 3 years to comply with new requirements.43
The FDA had rated clozapine a pregnancy risk category B medication, meaning no evidence of risk in humans. In 2011, the FDA issued a general warning that antipsychotic use in pregnancy can cause extrapyramidal symptoms and discontinuation symptoms in newborns.44,45
A 2015 review of psychotropic medications and pregnancy noted that approximately 60% of women with schizophrenia became pregnant, with an increased incidence of unplanned pregnancy. A high risk of psychotic relapse (65%) during pregnancy and in the postpartum period may lead to insufficient prenatal care, drug use, and obstetric complications.45 Some data suggest low fetal birth weight and an increased rate of therapeutic abortions in women with schizophrenia.42,46
When treating a pregnant patient, weigh the benefits of clozapine against the risks of adverse events, and clearly document the analysis. Clozapine treatment is not recommended during breast-feeding because of the risk of side effects for newborns.8
We highly recommend keeping updated on the literature regarding pregnancy and lactation information with antipsychotics, including clozapine, because prescribing information will likely be updated in the near future to comply with recent FDA labeling changes.
Final installment: Using clozapine off-labelClozapine is FDA-approved for refractory schizophrenia and for reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder. In Part 3 of this series, we review off-label uses—such as managing bipolar disorder, borderline personality disorder, and aggressive behavior—that have varying degrees of scientific support.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
3. Walker AM, Lanza LL, Arellano F, et al. Mortality in current and former users of clozapine. Epidemiology. 1997;8(6):671-677.
4. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
5. Walker E, McGee RE, Druss BG. Mortality in mental disorders and global disease burden Implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
6. Hayes RD, Downs J, Chang CK, et al. The effect of clozapine on premature mortality: an assessment of clinical monitoring and other potential confounders. Schizophr Bull. 2015;41(3):644-655.
7. De Fazio P, Gaetano R, Caroleo M, et al. Rare and very rare adverse effects of clozapine. Neuropsychiatr Dis Treat. 2015;11:1995-2003.
8. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed June 29, 2016.
9. Lieberman JA, Johns CA, Kane JM, et al. Clozapine-induced agranulocytosis: non-cross-reactivity with other psychotropic drugs. J Clin Psychiatry. 1988;49(7):271-277.
10. Honigfeld G, Arellano F, Sethi J, et al. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
11. Demler TL, Trigoboff E. Are clozapine patients at risk for blood dyscrasias with concomitant tamiflu use? Psychiatry (Edgmont). 2009;6(11):29-33.
12. Karalakulasingam R, Schacht RA, Lansing AM, et al. Influenza virus pneumonia after renal transplant. Postgrad Med. 1977;62(2):164-167.
13. Hoffman-La Roche Limited. Product monograph: Tamiflu. http://www.rochecanada.com/content/dam/roche_canada/en_CA/documents/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/Tamiflu/Tamiflu_PM_E.pdf. Updated January 26, 2015. Accessed November 28, 2015.
14. Whiskey E, Taylor D. Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs. 2007;21(1):25-35.
15. Palominao A, Kukoyi O, Xiong GL. Leukocytosis after lithium and clozapine combination therapy. Ann Clin Psychiatry. 2010;22(3):205-206.
16. Focosi D, Azzarà A, Kast RE, et al. Lithium and hematology: established and proposed uses. J Leukoc Biol. 2009;85(1):20-28.
17. Papetti F, Darcourt G, Giordana JY, et al. Treatment of clozapine-induced granulocytopenia with lithium (two observations) [in French]. Encephale. 2004;30(6):578-582.
18. Hummer M, Sperner-Unterweger B, Kemmler G, et al. Does eosinophilia predict clozapine induced neutropenia? Psychopharmacology (Berl). 1996;124(1-2):201-204.
19. Aneja J, Sharma N, Mahajan S, et al. Eosinophilia induced by clozapine: a report of two cases and review of the literature. J Family Med Prim Care. 2015;4(1):127-129.
20. Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
21. Stryjer R, Timinsky I, Reznik, I, et al. Beta-adrenergic antagonists for the treatment of clozapine-induced sinus tachycardia: a retrospective study. Clin Neuropharmacol. 2009;32(5):290-292.
22. Lally J, Docherty MJ, MacCabe JH. Pharmacological interventions for clozapine-induced sinus tachycardia. Cochrane Database Syst Rev. 2016;9(6):CD011566.
23. Kamphuis H, Arends J, Timmerman L, et al. Myocarditis and cardiomyopathy: underestimated complications resulting from clozapine therapy [in Dutch]. Tijdschr Psychiatr. 2010;52(4):223-233.
24. Alawami M, Wasywich C, Cicovic A, et al. A systematic review of clozapine induced cardiomyopathy. Int J Cardiol. 2014;176(2):315-320.
25. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458-465.
26. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
27. Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569-574.
28. Saavedra-Velez C, Yusim A, Anbarasan D, et al. Modafinil as an adjunctive treatment of sedation, negative symptoms, and cognition in schizophrenia: a critical review. J Clin Psychiatry. 2009;70(1):104-112.
29. Klein C, Gordon J, Pollak L, et al. Clozapine in Parkinson’s disease psychosis: 5-year follow-up review. Clin Neuropharmacol. 2003;26(1):8-11.
30. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
31. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45(5):667-675.
32. Duggal HS. Glycopyrrolate for clozapine-induced sialorrhea. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1546-1547.
33. Leadbetter R, Shutty M, Pavalonis D, et al. Clozapine-induced weight gain: prevalence and clinical relevance. Am J Psychiatry. 1992;149(1):68-72.
34. Lundblad W, Azzam PN, Gopalan, et al. Medical management of patients on clozapine: a guide for internists. J Hosp Med. 2015;10(8):537-543.
35. Henderson DC, Nguyen DD, Copeland PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66(9):1116-1121.
36. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
37. Carrizo E, Fernández V, Connell L, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. 2009;113(1):19-26.
38. Chen CH, Huang MC, Kao CF, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(5):e424-e430.
39. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
40. Nielsen J, Meyer JM. Risk factors for ileus in patients with schizophrenia. Schizophr Bull. 2012;38(3):592-598.
41. Lowe CM, Grube RR, Scates AC. Characterization and clinical management of clozapine-induced fever. Ann Pharmacother. 2007;41(10):1700-1704.
42. ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020.
43. U.S. Food and Drug Administration. Pregnancy and Lactation Labeling (Drugs) Final Rule. https://s3.amazonaws.com/public-inspection.federalregister.gov/2014-28241.pdf. Published December 4, 2014. Accessed July 6, 2016.
44. Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. 9th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2011.
45. Larsen ER, Damkier P, Pedersen LH, et al; Danish Psychiatric Society; Danish Society of Obstetrics and Gynecology; Danish Paediatric Society; Danish Society of Clinical Pharmacology. Use of psychotropic drugs during pregnancy and breast-feeding. Acta Psychiatr Scand Suppl. 2015;(445):1-28.
46. McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4):444-449.
Clozapine is a highly effective antipsychotic with superior efficacy in treatment-resistant schizophrenia, but its side effect profile is daunting (Figure 1).1 Adverse reactions lead to approximately 17% of patients who take clozapine eventually discontinuing the medication.1 As we noted in Part 1 of this 3-part series,2 clozapine remains the most efficacious, but most tedious, antipsychotic available to psychiatrists because of its monitoring requirements and potential side effects.
A powerful rationale for prescribing clozapine, despite its drawbacks, is its association with a reduced risk of all-cause mortality.3,4 People with serious mental illness, including schizophrenia, have a median 10-year shorter life expectancy than the general population.5
A recent cohort study6 examined electronic health records to test whether intensive monitoring or lower suicide risk might account for the reduced mortality with clozapine. The authors found that the reduced mortality rate was not directly related to clozapine’s clinical monitoring or other confounding factors. They did find an association between clozapine use and reduced risk of death from both natural and unnatural causes.
This second article in our series examines clozapine’s adverse effects from a systems perspective. Severe neutropenia, myocarditis, sedation, weight gain, orthostatic hypotension, and sialorrhea appear to be the most studied adverse effects, but myriad others can occur.7 We offer guidance to help the astute clinician continue this effective antipsychotic by monitoring carefully, treating side effects early, and managing potential drug interactions (Table 1).8
Hematologic eventsSevere neutropenia, defined as absolute neutrophil count (ANC) <500/µL, is a well-known adverse effect of clozapine that requires specific clinical monitoring, a requirement that was updated by the FDA in 2015.2 The incidence of severe neutropenia peaks in the first 2 months of clozapine therapy and tapers after 6 months, but some risk always remains.
Older efficacy studies in the United States gauged the 1-year cumulative incidence of severe clozapine-induced neutropenia to be 2%.9 A 1998 study of the effects of using a clozapine registry reported a lower incidence—0.38%—than the 2% noted above.10 Early recognition and recommended interventions can improve clinical outcomes.2
Drug interactions and neutropenia. A retrospective study of mental health inpatients taking clozapine concurrently with oseltamivir during an influenza outbreak found a statistically significant—but not clinically significant—change in ANC values.11 The authors noted that viral infection might lead to blood dyscrasia early in illness, and that oseltamivir has been associated with a small incidence of blood dyscrasia.11-13 This information might be useful when treating influenza in patients taking clozapine, although no specific change in management is recommended.
Similarly, concomitant treatment with clozapine and lithium can affect both white blood cell and ANC values.14,15 Lithium-treated patients often demonstrate increased circulating neutrophils via enhancement of granulocyte-colony stimulating factor.16 Case studies describe how initiating lithium treatment enabled some patients to continue clozapine after developing neutropenia.14,17 Leukocytosis can affect blood monitoring, possibly masking other blood dyscrasias, when lithium is used concurrently with clozapine.
Eosinophilia (blood eosinophil count >700/µL) occurs in approximately 1% of clozapine users, usually in the first 4 weeks of treatment.18 It can be benign and transient or a harbinger of a more rare adverse reaction such as myocarditis, pancreatitis, hepatitis, colitis, or nephritis.19 If a patient taking clozapine develops eosinophilia, clozapine’s package insert recommends that you:
- evaluate promptly for other systemic involvement (rash, other evidence of allergic reaction, myocarditis, other organ-specific disease)
- stop clozapine immediately if any of these are found.
If other causes of eosinophilia are identified (asthma, allergies, collagen vascular disease, parasitic infection, neoplasm), treat these and continue clozapine.
The manufacturer also mentions the occurrence of clozapine-related eosinophilia without organ involvement that can resolve without intervention, with careful monitoring over several weeks.8 In this scenario, there is flexibility to judge whether clozapine should be stopped or re-challenged, or if close monitoring is adequate. Consulting with an internal medicine or hematology specialist might be helpful.
Cardiovascular side effectsMost common events. Three of the 10 most common clozapine side effects are cardiac: tachycardia, hypotension, and hypertension (Figure 1).1 Orthostatic hypotension, bradycardia, and syncope also can occur, especially with rapid clozapine titration. Baseline electrocardiogram (ECG) can help differentiate whether abnormalities are clozapine-induced or related to a preexisting condition.
Reducing the dosage of clozapine or slowing titration could reverse cardiac side effects.8 If dosage reduction is not an option or is ineffective, first consider treating the side effect rather than discontinuing clozapine.20
Sinus tachycardia is one of the most common side effects of clozapine. First, rule out serious conditions—myocarditis, cardiomyopathy, neuroleptic malignant syndrome (NMS)—then consider waiting and monitoring for the first few months of clozapine treatment. If tachycardia continues, consider dosage reduction. Slower titration, or treatment with a cardio-selective beta blocker such as atenolol.21,22 Note that a recent Cochrane Review concluded that there is not enough randomized evidence to support any particular treatment for clozapine-induced tachycardia; the prescriber must therefore make a case-by-case clinical judgment.22
Similarly, orthostatic hypotension can be managed with a reduced dosage of clozapine or slower titration. Increased fluid intake, compression stockings, and, if necessary, fludrocortisone also can be initiated.20
Rare, potentially fatal events. Myocarditis, pericarditis, and cardiomyopathy are among the rare but potentially fatal adverse effects of clozapine. A recent study reported the incidence of myocarditis with clozapine at a range of 0.015% to 1.3%; cardiomyopathy was even more rare.23 Pulmonary embolism and deep venous thrombosis also are very rare possibilities; keep them in mind, however, when patients taking clozapine report new cardiovascular symptoms.
Patients with clozapine-induced cardiovascular effects most commonly report shortness of breath (60%), palpitations (36%), cough (16%), fatigue (16%), and chest pain (8%).7,24
Clozapine’s “black-box” warning specifically recommends discontinuing clozapine and consulting cardiology when myocarditis or cardiomyopathy is suspected. In 50% of cases, myocarditis symptoms present in the first few weeks of clozapine treatment.23 The manufacturer states that myocarditis usually presents in the first 2 months, and cardiomyopathy after 8 weeks of treatment; however, either can present at any time.8Figure 2 provides a clinical reference for monitoring a clozapine patient for cardiomyopathy.24
Laboratory findings that support a diagnosis of clozapine-related myocarditis include:
- elevated C-reactive protein
- elevated troponin I or T
- elevated creatine kinase-MB
- peripheral eosinophilia.8,25
ECG, echocardiography, and cardiac MRI can be helpful in diagnosis, in consultation with a cardiologist.
Neurologic side effectsSeizures are listed in the “black-box” warning for clozapine. Seizure incidence with clozapine is 5% per year, with higher incidence at dosages ≥600 mg/d.8 Because clozapine-induced seizures are dosage-dependent, slow titration can mitigate this risk. Tonic-clonic seizures are the most common type associated with clozapine.
The manufacturer recommends caution when using clozapine in patients with a known seizure disorder, alcohol use disorder, or other CNS pathology.8 Patients with a seizure disorder may be at increased risk of experiencing clozapine-induced seizures, but this is not an absolute contraindication.26 Smoking cessation increases clozapine blood levels by an average of 57.4%, further increasing seizure risk.26,27
Discontinuing clozapine is unnecessary when a patient experiences a seizure. Instead, you can:
- halve the dosage prescribed at the time of the seizure (or at least reduce to the last seizure-free dosage)
- consider any medications or medical problems that might have contributed to a lower seizure threshold
- consider prophylaxis with an antiepileptic medication (eg, valproic acid has efficacy for both myoclonic and tonic-clonic seizures).20,26
Sedation is the most common side effect of clozapine.1 Patients experiencing severe sedation should not drive or operate heavy machinery. To reduce sedation, consider instructing the patient to take all or most of the clozapine dosage at bedtime. A critical review of modafinil for sedation caused by antipsychotics in schizophrenia found only 1 open-label study that showed any positive effects; the authors concluded that further study is needed.28
Cognitive and motor slowing are possible neurologic side effects of clozapine. Caution patients about the risk of participating in activities that require cognitive or motor performance until the individual effects of clozapine are known.8
Tardive dyskinesia. Clozapine carries some risk of tardive dyskinesia, although that risk is lower than with other antipsychotics. Similarly, all antipsychotics including clozapine are associated with a risk of NMS. In the rare case of clozapine-induced NMS, stop clozapine immediately and initiate supportive therapy. Clozapine-induced NMS is not an absolute contraindication to re-challenging a patient with clozapine, however, if doing so is clinically appropriate.20
Cerebrovascular events. In older people with dementia, the use of antipsychotics—including clozapine—has been shown to increase the risk of cerebrovascular events. Because most antipsychotics are not FDA-approved for treating psychosis associated with dementia (only pimavanserin is FDA-approved for symptoms of psychosis in Parkinson’s disease), a risk-benefit analysis should be documented when prescribing any antipsychotic in this population. In practice, clozapine’s benefits may outweigh the mortality risks in specific situations.29,30
CASE Sialorrhea puts progress at risk
Ms. B, age 40, has a history of treatment-resistant schizophrenia and is starting clozapine because of residual psychosis during trials of other antipsychotics. She develops severe persistent drooling, mostly at night, during clozapine titration. Sugar-free candy, multiple bed pillows, and changing the dosing schedule do not significantly improve the sialorrhea.
As a result, Ms. B is embarrassed to continue her usual activities. She asks to stop clozapine, even though her psychotic symptoms have improved and she is functioning at her highest level in years.
Ms. B already is taking trihexyphenidyl, 5 mg, 3 times daily, to manage extrapyramidal symptoms related to haloperidol decanoate treatment. After discussing other medication options for sialorrhea, she agrees to a trial of glycopyrrolate, 1 mg, twice daily. She experiences significant improvement and continues taking clozapine.
Sialorrhea develops in 13% of patients taking clozapine.1 As in Ms. B’s case, this side effect can be embarrassing, can limit social or occupational functioning, and might lead patients to discontinue clozapine treatment despite efficacy. Nonpharmacotherapeutic options include covering the pillow with a towel, lowering the clozapine dosage or titrating slowly (or both), and using sugarless gum or candy to increase swallowing.
If the benefits of additional medications targeting side effects outweigh the risks, pharmacotherapeutic intervention may be appropriate. Options include the tricyclic antidepressant amitriptyline31; alpha-adrenergic agonists or antagonists (clonidine, terazosin); and anti-muscarinic medications (benztropine, atropine, trihexyphenidyl, glycopyrrolate) (Table 231). Scopolamine transdermal patch is another possible treatment strategy; however, the scopolamine patch was used for clozapine-induced sialorrhea in only a few case reports, and it is not considered a first-line treatment choice.30
When prescribing, consider the possibility of combined side effects with clozapine and adjunct medications having antimuscarinic or alpha-adrenergic activity, or both. Even atropine ophthalmic drops, administered sublingually, are readily absorbed and cross the blood–brain barrier.31 Another antimuscarinic agent, glycopyrrolate, is less likely to cross the blood–brain barrier and therefore is less likely to cause cognitive side effects. Glycopyrrolate is 5 times more potent at blocking the muscarinic receptor than atropine.31,32 Ipratropium bromide, another nonselective muscarinic receptor antagonist, has less systemic absorption than atropine drops, with less anticholinergic side effects when administered sublingually.
Limited evidence supports the efficacy of alpha-adrenergic medications for managing clozapine-induced sialorrhea. Monitor blood pressure when prescribing terazosin or clonidine, which could potentiate clozapine’s hypotensive effects.
Endocrine side effectsAmong antipsychotics, clozapine is associated with the greatest weight gain—averaging nearly 10% of body weight.33,34 Similarly, the risk of new-onset diabetes mellitus is highest with clozapine in relation to other antipsychotics: 43% reported in a 10-year naturalistic study.35 The risk of hyperlipidemia also increases with clozapine treatment.36 These metabolic changes increase the risk of cardiovascular-related death, with a 10-year mortality rate from cardiovascular disease reported at 9% in clozapine-treated patients.35
Despite clozapine’s metabolic side effects, patients with schizophrenia who are treated with clozapine show a significant reduction in overall mortality compared with patients not treated with clozapine.6 Effective identification and management of metabolic side effects can prevent the need to discontinue clozapine.
Behavioral weight management and exercise are recommended as initial therapy.20 If, based on clinical judgment, these alone are insufficient, data support the use of pharmacotherapeutic interventions. Metformin demonstrates a positive effect on body weight, insulin resistance, and lipids, making it the first choice for adjunctive treatment of clozapine-induced metabolic side effects.37-39
Gastrointestinal side effectsClozapine’s anticholinergic activity can lead to serious gastrointestinal (GI) side effects, including constipation, intestinal obstruction, fecal impaction, and paralytic ileus.8 Ileus has produced more fatal adverse reactions with clozapine than has severe neutropenia.20,40 Co-administered anticholinergic medications could increase the risk of ileus. Obtaining a GI review of systems and monitoring bowel movements (in inpatient or residential facilities) can aid in early identification and limit morbidity and mortality from GI adverse events. A high-fiber diet, adequate hydration, bulk laxatives in patients who can reliably maintain hydration, and GI consultation (if needed) may help manage GI side effects.20
Constitutional side effectsFever can occur with clozapine, most often in the first month of treatment, but the incidence is quite variable (0.5% to 55%).20,41 Although benign fever is common, agranulocytosis with infection, NMS, and other systemic illness must be ruled out. The recommended workup when a patient develops fever while taking clozapine includes physical examination and relevant testing (urinalysis, measurement of ANC and serum creatine kinase, chest radiograph, ECG, and, possibly, blood cultures).41
If evidence supports a serious adverse reaction, stop clozapine immediately.20 If benign clozapine-related fever is suspected, acetaminophen or another antipyretic might provide symptomatic relief; discontinuing clozapine is then unnecessary.41
Pregnancy. When a patient with schizophrenia requires clozapine treatment during pregnancy, reliable clinical guidance is limited. The American College of Obstetricians and Gynecologists Practice Bulletin on the use of psychiatric medications during pregnancy and lactation can be a useful resource.42
Be aware that the FDA very recently made major changes to the format and content of pregnancy and lactation labeling, removing the letter categories that have been used for medications approved on or after June 30, 2001. The manufacturers of medications (such as clozapine) that were approved before June 30, 2001, have 3 years to comply with new requirements.43
The FDA had rated clozapine a pregnancy risk category B medication, meaning no evidence of risk in humans. In 2011, the FDA issued a general warning that antipsychotic use in pregnancy can cause extrapyramidal symptoms and discontinuation symptoms in newborns.44,45
A 2015 review of psychotropic medications and pregnancy noted that approximately 60% of women with schizophrenia became pregnant, with an increased incidence of unplanned pregnancy. A high risk of psychotic relapse (65%) during pregnancy and in the postpartum period may lead to insufficient prenatal care, drug use, and obstetric complications.45 Some data suggest low fetal birth weight and an increased rate of therapeutic abortions in women with schizophrenia.42,46
When treating a pregnant patient, weigh the benefits of clozapine against the risks of adverse events, and clearly document the analysis. Clozapine treatment is not recommended during breast-feeding because of the risk of side effects for newborns.8
We highly recommend keeping updated on the literature regarding pregnancy and lactation information with antipsychotics, including clozapine, because prescribing information will likely be updated in the near future to comply with recent FDA labeling changes.
Final installment: Using clozapine off-labelClozapine is FDA-approved for refractory schizophrenia and for reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder. In Part 3 of this series, we review off-label uses—such as managing bipolar disorder, borderline personality disorder, and aggressive behavior—that have varying degrees of scientific support.
Clozapine is a highly effective antipsychotic with superior efficacy in treatment-resistant schizophrenia, but its side effect profile is daunting (Figure 1).1 Adverse reactions lead to approximately 17% of patients who take clozapine eventually discontinuing the medication.1 As we noted in Part 1 of this 3-part series,2 clozapine remains the most efficacious, but most tedious, antipsychotic available to psychiatrists because of its monitoring requirements and potential side effects.
A powerful rationale for prescribing clozapine, despite its drawbacks, is its association with a reduced risk of all-cause mortality.3,4 People with serious mental illness, including schizophrenia, have a median 10-year shorter life expectancy than the general population.5
A recent cohort study6 examined electronic health records to test whether intensive monitoring or lower suicide risk might account for the reduced mortality with clozapine. The authors found that the reduced mortality rate was not directly related to clozapine’s clinical monitoring or other confounding factors. They did find an association between clozapine use and reduced risk of death from both natural and unnatural causes.
This second article in our series examines clozapine’s adverse effects from a systems perspective. Severe neutropenia, myocarditis, sedation, weight gain, orthostatic hypotension, and sialorrhea appear to be the most studied adverse effects, but myriad others can occur.7 We offer guidance to help the astute clinician continue this effective antipsychotic by monitoring carefully, treating side effects early, and managing potential drug interactions (Table 1).8
Hematologic eventsSevere neutropenia, defined as absolute neutrophil count (ANC) <500/µL, is a well-known adverse effect of clozapine that requires specific clinical monitoring, a requirement that was updated by the FDA in 2015.2 The incidence of severe neutropenia peaks in the first 2 months of clozapine therapy and tapers after 6 months, but some risk always remains.
Older efficacy studies in the United States gauged the 1-year cumulative incidence of severe clozapine-induced neutropenia to be 2%.9 A 1998 study of the effects of using a clozapine registry reported a lower incidence—0.38%—than the 2% noted above.10 Early recognition and recommended interventions can improve clinical outcomes.2
Drug interactions and neutropenia. A retrospective study of mental health inpatients taking clozapine concurrently with oseltamivir during an influenza outbreak found a statistically significant—but not clinically significant—change in ANC values.11 The authors noted that viral infection might lead to blood dyscrasia early in illness, and that oseltamivir has been associated with a small incidence of blood dyscrasia.11-13 This information might be useful when treating influenza in patients taking clozapine, although no specific change in management is recommended.
Similarly, concomitant treatment with clozapine and lithium can affect both white blood cell and ANC values.14,15 Lithium-treated patients often demonstrate increased circulating neutrophils via enhancement of granulocyte-colony stimulating factor.16 Case studies describe how initiating lithium treatment enabled some patients to continue clozapine after developing neutropenia.14,17 Leukocytosis can affect blood monitoring, possibly masking other blood dyscrasias, when lithium is used concurrently with clozapine.
Eosinophilia (blood eosinophil count >700/µL) occurs in approximately 1% of clozapine users, usually in the first 4 weeks of treatment.18 It can be benign and transient or a harbinger of a more rare adverse reaction such as myocarditis, pancreatitis, hepatitis, colitis, or nephritis.19 If a patient taking clozapine develops eosinophilia, clozapine’s package insert recommends that you:
- evaluate promptly for other systemic involvement (rash, other evidence of allergic reaction, myocarditis, other organ-specific disease)
- stop clozapine immediately if any of these are found.
If other causes of eosinophilia are identified (asthma, allergies, collagen vascular disease, parasitic infection, neoplasm), treat these and continue clozapine.
The manufacturer also mentions the occurrence of clozapine-related eosinophilia without organ involvement that can resolve without intervention, with careful monitoring over several weeks.8 In this scenario, there is flexibility to judge whether clozapine should be stopped or re-challenged, or if close monitoring is adequate. Consulting with an internal medicine or hematology specialist might be helpful.
Cardiovascular side effectsMost common events. Three of the 10 most common clozapine side effects are cardiac: tachycardia, hypotension, and hypertension (Figure 1).1 Orthostatic hypotension, bradycardia, and syncope also can occur, especially with rapid clozapine titration. Baseline electrocardiogram (ECG) can help differentiate whether abnormalities are clozapine-induced or related to a preexisting condition.
Reducing the dosage of clozapine or slowing titration could reverse cardiac side effects.8 If dosage reduction is not an option or is ineffective, first consider treating the side effect rather than discontinuing clozapine.20
Sinus tachycardia is one of the most common side effects of clozapine. First, rule out serious conditions—myocarditis, cardiomyopathy, neuroleptic malignant syndrome (NMS)—then consider waiting and monitoring for the first few months of clozapine treatment. If tachycardia continues, consider dosage reduction. Slower titration, or treatment with a cardio-selective beta blocker such as atenolol.21,22 Note that a recent Cochrane Review concluded that there is not enough randomized evidence to support any particular treatment for clozapine-induced tachycardia; the prescriber must therefore make a case-by-case clinical judgment.22
Similarly, orthostatic hypotension can be managed with a reduced dosage of clozapine or slower titration. Increased fluid intake, compression stockings, and, if necessary, fludrocortisone also can be initiated.20
Rare, potentially fatal events. Myocarditis, pericarditis, and cardiomyopathy are among the rare but potentially fatal adverse effects of clozapine. A recent study reported the incidence of myocarditis with clozapine at a range of 0.015% to 1.3%; cardiomyopathy was even more rare.23 Pulmonary embolism and deep venous thrombosis also are very rare possibilities; keep them in mind, however, when patients taking clozapine report new cardiovascular symptoms.
Patients with clozapine-induced cardiovascular effects most commonly report shortness of breath (60%), palpitations (36%), cough (16%), fatigue (16%), and chest pain (8%).7,24
Clozapine’s “black-box” warning specifically recommends discontinuing clozapine and consulting cardiology when myocarditis or cardiomyopathy is suspected. In 50% of cases, myocarditis symptoms present in the first few weeks of clozapine treatment.23 The manufacturer states that myocarditis usually presents in the first 2 months, and cardiomyopathy after 8 weeks of treatment; however, either can present at any time.8Figure 2 provides a clinical reference for monitoring a clozapine patient for cardiomyopathy.24
Laboratory findings that support a diagnosis of clozapine-related myocarditis include:
- elevated C-reactive protein
- elevated troponin I or T
- elevated creatine kinase-MB
- peripheral eosinophilia.8,25
ECG, echocardiography, and cardiac MRI can be helpful in diagnosis, in consultation with a cardiologist.
Neurologic side effectsSeizures are listed in the “black-box” warning for clozapine. Seizure incidence with clozapine is 5% per year, with higher incidence at dosages ≥600 mg/d.8 Because clozapine-induced seizures are dosage-dependent, slow titration can mitigate this risk. Tonic-clonic seizures are the most common type associated with clozapine.
The manufacturer recommends caution when using clozapine in patients with a known seizure disorder, alcohol use disorder, or other CNS pathology.8 Patients with a seizure disorder may be at increased risk of experiencing clozapine-induced seizures, but this is not an absolute contraindication.26 Smoking cessation increases clozapine blood levels by an average of 57.4%, further increasing seizure risk.26,27
Discontinuing clozapine is unnecessary when a patient experiences a seizure. Instead, you can:
- halve the dosage prescribed at the time of the seizure (or at least reduce to the last seizure-free dosage)
- consider any medications or medical problems that might have contributed to a lower seizure threshold
- consider prophylaxis with an antiepileptic medication (eg, valproic acid has efficacy for both myoclonic and tonic-clonic seizures).20,26
Sedation is the most common side effect of clozapine.1 Patients experiencing severe sedation should not drive or operate heavy machinery. To reduce sedation, consider instructing the patient to take all or most of the clozapine dosage at bedtime. A critical review of modafinil for sedation caused by antipsychotics in schizophrenia found only 1 open-label study that showed any positive effects; the authors concluded that further study is needed.28
Cognitive and motor slowing are possible neurologic side effects of clozapine. Caution patients about the risk of participating in activities that require cognitive or motor performance until the individual effects of clozapine are known.8
Tardive dyskinesia. Clozapine carries some risk of tardive dyskinesia, although that risk is lower than with other antipsychotics. Similarly, all antipsychotics including clozapine are associated with a risk of NMS. In the rare case of clozapine-induced NMS, stop clozapine immediately and initiate supportive therapy. Clozapine-induced NMS is not an absolute contraindication to re-challenging a patient with clozapine, however, if doing so is clinically appropriate.20
Cerebrovascular events. In older people with dementia, the use of antipsychotics—including clozapine—has been shown to increase the risk of cerebrovascular events. Because most antipsychotics are not FDA-approved for treating psychosis associated with dementia (only pimavanserin is FDA-approved for symptoms of psychosis in Parkinson’s disease), a risk-benefit analysis should be documented when prescribing any antipsychotic in this population. In practice, clozapine’s benefits may outweigh the mortality risks in specific situations.29,30
CASE Sialorrhea puts progress at risk
Ms. B, age 40, has a history of treatment-resistant schizophrenia and is starting clozapine because of residual psychosis during trials of other antipsychotics. She develops severe persistent drooling, mostly at night, during clozapine titration. Sugar-free candy, multiple bed pillows, and changing the dosing schedule do not significantly improve the sialorrhea.
As a result, Ms. B is embarrassed to continue her usual activities. She asks to stop clozapine, even though her psychotic symptoms have improved and she is functioning at her highest level in years.
Ms. B already is taking trihexyphenidyl, 5 mg, 3 times daily, to manage extrapyramidal symptoms related to haloperidol decanoate treatment. After discussing other medication options for sialorrhea, she agrees to a trial of glycopyrrolate, 1 mg, twice daily. She experiences significant improvement and continues taking clozapine.
Sialorrhea develops in 13% of patients taking clozapine.1 As in Ms. B’s case, this side effect can be embarrassing, can limit social or occupational functioning, and might lead patients to discontinue clozapine treatment despite efficacy. Nonpharmacotherapeutic options include covering the pillow with a towel, lowering the clozapine dosage or titrating slowly (or both), and using sugarless gum or candy to increase swallowing.
If the benefits of additional medications targeting side effects outweigh the risks, pharmacotherapeutic intervention may be appropriate. Options include the tricyclic antidepressant amitriptyline31; alpha-adrenergic agonists or antagonists (clonidine, terazosin); and anti-muscarinic medications (benztropine, atropine, trihexyphenidyl, glycopyrrolate) (Table 231). Scopolamine transdermal patch is another possible treatment strategy; however, the scopolamine patch was used for clozapine-induced sialorrhea in only a few case reports, and it is not considered a first-line treatment choice.30
When prescribing, consider the possibility of combined side effects with clozapine and adjunct medications having antimuscarinic or alpha-adrenergic activity, or both. Even atropine ophthalmic drops, administered sublingually, are readily absorbed and cross the blood–brain barrier.31 Another antimuscarinic agent, glycopyrrolate, is less likely to cross the blood–brain barrier and therefore is less likely to cause cognitive side effects. Glycopyrrolate is 5 times more potent at blocking the muscarinic receptor than atropine.31,32 Ipratropium bromide, another nonselective muscarinic receptor antagonist, has less systemic absorption than atropine drops, with less anticholinergic side effects when administered sublingually.
Limited evidence supports the efficacy of alpha-adrenergic medications for managing clozapine-induced sialorrhea. Monitor blood pressure when prescribing terazosin or clonidine, which could potentiate clozapine’s hypotensive effects.
Endocrine side effectsAmong antipsychotics, clozapine is associated with the greatest weight gain—averaging nearly 10% of body weight.33,34 Similarly, the risk of new-onset diabetes mellitus is highest with clozapine in relation to other antipsychotics: 43% reported in a 10-year naturalistic study.35 The risk of hyperlipidemia also increases with clozapine treatment.36 These metabolic changes increase the risk of cardiovascular-related death, with a 10-year mortality rate from cardiovascular disease reported at 9% in clozapine-treated patients.35
Despite clozapine’s metabolic side effects, patients with schizophrenia who are treated with clozapine show a significant reduction in overall mortality compared with patients not treated with clozapine.6 Effective identification and management of metabolic side effects can prevent the need to discontinue clozapine.
Behavioral weight management and exercise are recommended as initial therapy.20 If, based on clinical judgment, these alone are insufficient, data support the use of pharmacotherapeutic interventions. Metformin demonstrates a positive effect on body weight, insulin resistance, and lipids, making it the first choice for adjunctive treatment of clozapine-induced metabolic side effects.37-39
Gastrointestinal side effectsClozapine’s anticholinergic activity can lead to serious gastrointestinal (GI) side effects, including constipation, intestinal obstruction, fecal impaction, and paralytic ileus.8 Ileus has produced more fatal adverse reactions with clozapine than has severe neutropenia.20,40 Co-administered anticholinergic medications could increase the risk of ileus. Obtaining a GI review of systems and monitoring bowel movements (in inpatient or residential facilities) can aid in early identification and limit morbidity and mortality from GI adverse events. A high-fiber diet, adequate hydration, bulk laxatives in patients who can reliably maintain hydration, and GI consultation (if needed) may help manage GI side effects.20
Constitutional side effectsFever can occur with clozapine, most often in the first month of treatment, but the incidence is quite variable (0.5% to 55%).20,41 Although benign fever is common, agranulocytosis with infection, NMS, and other systemic illness must be ruled out. The recommended workup when a patient develops fever while taking clozapine includes physical examination and relevant testing (urinalysis, measurement of ANC and serum creatine kinase, chest radiograph, ECG, and, possibly, blood cultures).41
If evidence supports a serious adverse reaction, stop clozapine immediately.20 If benign clozapine-related fever is suspected, acetaminophen or another antipyretic might provide symptomatic relief; discontinuing clozapine is then unnecessary.41
Pregnancy. When a patient with schizophrenia requires clozapine treatment during pregnancy, reliable clinical guidance is limited. The American College of Obstetricians and Gynecologists Practice Bulletin on the use of psychiatric medications during pregnancy and lactation can be a useful resource.42
Be aware that the FDA very recently made major changes to the format and content of pregnancy and lactation labeling, removing the letter categories that have been used for medications approved on or after June 30, 2001. The manufacturers of medications (such as clozapine) that were approved before June 30, 2001, have 3 years to comply with new requirements.43
The FDA had rated clozapine a pregnancy risk category B medication, meaning no evidence of risk in humans. In 2011, the FDA issued a general warning that antipsychotic use in pregnancy can cause extrapyramidal symptoms and discontinuation symptoms in newborns.44,45
A 2015 review of psychotropic medications and pregnancy noted that approximately 60% of women with schizophrenia became pregnant, with an increased incidence of unplanned pregnancy. A high risk of psychotic relapse (65%) during pregnancy and in the postpartum period may lead to insufficient prenatal care, drug use, and obstetric complications.45 Some data suggest low fetal birth weight and an increased rate of therapeutic abortions in women with schizophrenia.42,46
When treating a pregnant patient, weigh the benefits of clozapine against the risks of adverse events, and clearly document the analysis. Clozapine treatment is not recommended during breast-feeding because of the risk of side effects for newborns.8
We highly recommend keeping updated on the literature regarding pregnancy and lactation information with antipsychotics, including clozapine, because prescribing information will likely be updated in the near future to comply with recent FDA labeling changes.
Final installment: Using clozapine off-labelClozapine is FDA-approved for refractory schizophrenia and for reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder. In Part 3 of this series, we review off-label uses—such as managing bipolar disorder, borderline personality disorder, and aggressive behavior—that have varying degrees of scientific support.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
3. Walker AM, Lanza LL, Arellano F, et al. Mortality in current and former users of clozapine. Epidemiology. 1997;8(6):671-677.
4. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
5. Walker E, McGee RE, Druss BG. Mortality in mental disorders and global disease burden Implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
6. Hayes RD, Downs J, Chang CK, et al. The effect of clozapine on premature mortality: an assessment of clinical monitoring and other potential confounders. Schizophr Bull. 2015;41(3):644-655.
7. De Fazio P, Gaetano R, Caroleo M, et al. Rare and very rare adverse effects of clozapine. Neuropsychiatr Dis Treat. 2015;11:1995-2003.
8. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed June 29, 2016.
9. Lieberman JA, Johns CA, Kane JM, et al. Clozapine-induced agranulocytosis: non-cross-reactivity with other psychotropic drugs. J Clin Psychiatry. 1988;49(7):271-277.
10. Honigfeld G, Arellano F, Sethi J, et al. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
11. Demler TL, Trigoboff E. Are clozapine patients at risk for blood dyscrasias with concomitant tamiflu use? Psychiatry (Edgmont). 2009;6(11):29-33.
12. Karalakulasingam R, Schacht RA, Lansing AM, et al. Influenza virus pneumonia after renal transplant. Postgrad Med. 1977;62(2):164-167.
13. Hoffman-La Roche Limited. Product monograph: Tamiflu. http://www.rochecanada.com/content/dam/roche_canada/en_CA/documents/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/Tamiflu/Tamiflu_PM_E.pdf. Updated January 26, 2015. Accessed November 28, 2015.
14. Whiskey E, Taylor D. Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs. 2007;21(1):25-35.
15. Palominao A, Kukoyi O, Xiong GL. Leukocytosis after lithium and clozapine combination therapy. Ann Clin Psychiatry. 2010;22(3):205-206.
16. Focosi D, Azzarà A, Kast RE, et al. Lithium and hematology: established and proposed uses. J Leukoc Biol. 2009;85(1):20-28.
17. Papetti F, Darcourt G, Giordana JY, et al. Treatment of clozapine-induced granulocytopenia with lithium (two observations) [in French]. Encephale. 2004;30(6):578-582.
18. Hummer M, Sperner-Unterweger B, Kemmler G, et al. Does eosinophilia predict clozapine induced neutropenia? Psychopharmacology (Berl). 1996;124(1-2):201-204.
19. Aneja J, Sharma N, Mahajan S, et al. Eosinophilia induced by clozapine: a report of two cases and review of the literature. J Family Med Prim Care. 2015;4(1):127-129.
20. Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
21. Stryjer R, Timinsky I, Reznik, I, et al. Beta-adrenergic antagonists for the treatment of clozapine-induced sinus tachycardia: a retrospective study. Clin Neuropharmacol. 2009;32(5):290-292.
22. Lally J, Docherty MJ, MacCabe JH. Pharmacological interventions for clozapine-induced sinus tachycardia. Cochrane Database Syst Rev. 2016;9(6):CD011566.
23. Kamphuis H, Arends J, Timmerman L, et al. Myocarditis and cardiomyopathy: underestimated complications resulting from clozapine therapy [in Dutch]. Tijdschr Psychiatr. 2010;52(4):223-233.
24. Alawami M, Wasywich C, Cicovic A, et al. A systematic review of clozapine induced cardiomyopathy. Int J Cardiol. 2014;176(2):315-320.
25. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458-465.
26. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
27. Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569-574.
28. Saavedra-Velez C, Yusim A, Anbarasan D, et al. Modafinil as an adjunctive treatment of sedation, negative symptoms, and cognition in schizophrenia: a critical review. J Clin Psychiatry. 2009;70(1):104-112.
29. Klein C, Gordon J, Pollak L, et al. Clozapine in Parkinson’s disease psychosis: 5-year follow-up review. Clin Neuropharmacol. 2003;26(1):8-11.
30. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
31. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45(5):667-675.
32. Duggal HS. Glycopyrrolate for clozapine-induced sialorrhea. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1546-1547.
33. Leadbetter R, Shutty M, Pavalonis D, et al. Clozapine-induced weight gain: prevalence and clinical relevance. Am J Psychiatry. 1992;149(1):68-72.
34. Lundblad W, Azzam PN, Gopalan, et al. Medical management of patients on clozapine: a guide for internists. J Hosp Med. 2015;10(8):537-543.
35. Henderson DC, Nguyen DD, Copeland PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66(9):1116-1121.
36. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
37. Carrizo E, Fernández V, Connell L, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. 2009;113(1):19-26.
38. Chen CH, Huang MC, Kao CF, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(5):e424-e430.
39. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
40. Nielsen J, Meyer JM. Risk factors for ileus in patients with schizophrenia. Schizophr Bull. 2012;38(3):592-598.
41. Lowe CM, Grube RR, Scates AC. Characterization and clinical management of clozapine-induced fever. Ann Pharmacother. 2007;41(10):1700-1704.
42. ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020.
43. U.S. Food and Drug Administration. Pregnancy and Lactation Labeling (Drugs) Final Rule. https://s3.amazonaws.com/public-inspection.federalregister.gov/2014-28241.pdf. Published December 4, 2014. Accessed July 6, 2016.
44. Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. 9th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2011.
45. Larsen ER, Damkier P, Pedersen LH, et al; Danish Psychiatric Society; Danish Society of Obstetrics and Gynecology; Danish Paediatric Society; Danish Society of Clinical Pharmacology. Use of psychotropic drugs during pregnancy and breast-feeding. Acta Psychiatr Scand Suppl. 2015;(445):1-28.
46. McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4):444-449.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
3. Walker AM, Lanza LL, Arellano F, et al. Mortality in current and former users of clozapine. Epidemiology. 1997;8(6):671-677.
4. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
5. Walker E, McGee RE, Druss BG. Mortality in mental disorders and global disease burden Implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
6. Hayes RD, Downs J, Chang CK, et al. The effect of clozapine on premature mortality: an assessment of clinical monitoring and other potential confounders. Schizophr Bull. 2015;41(3):644-655.
7. De Fazio P, Gaetano R, Caroleo M, et al. Rare and very rare adverse effects of clozapine. Neuropsychiatr Dis Treat. 2015;11:1995-2003.
8. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed June 29, 2016.
9. Lieberman JA, Johns CA, Kane JM, et al. Clozapine-induced agranulocytosis: non-cross-reactivity with other psychotropic drugs. J Clin Psychiatry. 1988;49(7):271-277.
10. Honigfeld G, Arellano F, Sethi J, et al. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
11. Demler TL, Trigoboff E. Are clozapine patients at risk for blood dyscrasias with concomitant tamiflu use? Psychiatry (Edgmont). 2009;6(11):29-33.
12. Karalakulasingam R, Schacht RA, Lansing AM, et al. Influenza virus pneumonia after renal transplant. Postgrad Med. 1977;62(2):164-167.
13. Hoffman-La Roche Limited. Product monograph: Tamiflu. http://www.rochecanada.com/content/dam/roche_canada/en_CA/documents/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/Tamiflu/Tamiflu_PM_E.pdf. Updated January 26, 2015. Accessed November 28, 2015.
14. Whiskey E, Taylor D. Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs. 2007;21(1):25-35.
15. Palominao A, Kukoyi O, Xiong GL. Leukocytosis after lithium and clozapine combination therapy. Ann Clin Psychiatry. 2010;22(3):205-206.
16. Focosi D, Azzarà A, Kast RE, et al. Lithium and hematology: established and proposed uses. J Leukoc Biol. 2009;85(1):20-28.
17. Papetti F, Darcourt G, Giordana JY, et al. Treatment of clozapine-induced granulocytopenia with lithium (two observations) [in French]. Encephale. 2004;30(6):578-582.
18. Hummer M, Sperner-Unterweger B, Kemmler G, et al. Does eosinophilia predict clozapine induced neutropenia? Psychopharmacology (Berl). 1996;124(1-2):201-204.
19. Aneja J, Sharma N, Mahajan S, et al. Eosinophilia induced by clozapine: a report of two cases and review of the literature. J Family Med Prim Care. 2015;4(1):127-129.
20. Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
21. Stryjer R, Timinsky I, Reznik, I, et al. Beta-adrenergic antagonists for the treatment of clozapine-induced sinus tachycardia: a retrospective study. Clin Neuropharmacol. 2009;32(5):290-292.
22. Lally J, Docherty MJ, MacCabe JH. Pharmacological interventions for clozapine-induced sinus tachycardia. Cochrane Database Syst Rev. 2016;9(6):CD011566.
23. Kamphuis H, Arends J, Timmerman L, et al. Myocarditis and cardiomyopathy: underestimated complications resulting from clozapine therapy [in Dutch]. Tijdschr Psychiatr. 2010;52(4):223-233.
24. Alawami M, Wasywich C, Cicovic A, et al. A systematic review of clozapine induced cardiomyopathy. Int J Cardiol. 2014;176(2):315-320.
25. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458-465.
26. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
27. Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569-574.
28. Saavedra-Velez C, Yusim A, Anbarasan D, et al. Modafinil as an adjunctive treatment of sedation, negative symptoms, and cognition in schizophrenia: a critical review. J Clin Psychiatry. 2009;70(1):104-112.
29. Klein C, Gordon J, Pollak L, et al. Clozapine in Parkinson’s disease psychosis: 5-year follow-up review. Clin Neuropharmacol. 2003;26(1):8-11.
30. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
31. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45(5):667-675.
32. Duggal HS. Glycopyrrolate for clozapine-induced sialorrhea. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1546-1547.
33. Leadbetter R, Shutty M, Pavalonis D, et al. Clozapine-induced weight gain: prevalence and clinical relevance. Am J Psychiatry. 1992;149(1):68-72.
34. Lundblad W, Azzam PN, Gopalan, et al. Medical management of patients on clozapine: a guide for internists. J Hosp Med. 2015;10(8):537-543.
35. Henderson DC, Nguyen DD, Copeland PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66(9):1116-1121.
36. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
37. Carrizo E, Fernández V, Connell L, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. 2009;113(1):19-26.
38. Chen CH, Huang MC, Kao CF, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(5):e424-e430.
39. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
40. Nielsen J, Meyer JM. Risk factors for ileus in patients with schizophrenia. Schizophr Bull. 2012;38(3):592-598.
41. Lowe CM, Grube RR, Scates AC. Characterization and clinical management of clozapine-induced fever. Ann Pharmacother. 2007;41(10):1700-1704.
42. ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020.
43. U.S. Food and Drug Administration. Pregnancy and Lactation Labeling (Drugs) Final Rule. https://s3.amazonaws.com/public-inspection.federalregister.gov/2014-28241.pdf. Published December 4, 2014. Accessed July 6, 2016.
44. Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. 9th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2011.
45. Larsen ER, Damkier P, Pedersen LH, et al; Danish Psychiatric Society; Danish Society of Obstetrics and Gynecology; Danish Paediatric Society; Danish Society of Clinical Pharmacology. Use of psychotropic drugs during pregnancy and breast-feeding. Acta Psychiatr Scand Suppl. 2015;(445):1-28.
46. McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4):444-449.
Rediscovering clozapine
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Rediscovering clozapine: After a turbulent history, current guidance on initiating and monitoring
Although clozapine is the medication with the clearest benefits in treatment-resistant schizophrenia, many eligible patients never receive it. In the United States, 20% to 30% of patients with schizophrenia can be classified as treatment resistant, but clozapine accounts for <5% of antipsychotics prescribed.1,2 Clinicians worldwide tend to under-prescribe clozapine3—a reluctance one author coined as “clozaphobia.”4
Admittedly, clozapine has had a turbulent history—both lauded as a near-miracle drug and condemned as a deadly agent. The FDA has overhauled its prescribing and monitoring guidelines, however, offering psychiatrists a perfect opportunity to reacquaint themselves with this potentially life-changing intervention.
We begin this article with clozapine’s story, then spotlight new terrain the FDA created in 2015 when the agency introduced the Clozapine Risk Evaluation and Mitigation Strategy (REMS). Our goal in the 3 articles of this series is to deepen your appreciation for this tricyclic antipsychotic and provide practical clinical guidance for using it safely and effectively.
Setbacks, but the drug has an enduring presenceThe 1950s was an exciting era of exploration for new psychotropic medications. While searching for tricyclic antidepressants, Wander Laboratories discovered neuroleptic tricyclics, with clozapine identified in 1959 (Figure 1). Haloperidol’s development and release in the 1960s reinforced the prevailing dogma of the time that effective neuroleptics correlated with extrapyramidal symptoms, thus limiting interest in the newly discovered, but pharmacologically unique, clozapine. Throughout the 1960s, most research on clozapine was published in German, with less of an international presence.5
Agranulocytosis deaths. Clozapine earned its scarlet letter in 1975, when 8 patients in Finland died of agranulocytosis.6 Sandoz, its manufacturer, withdrew clozapine from the market and halted all clinical trials. The Finnish epidemic triggered detailed investigations into blood dyscrasias and early identification of agranulocytosis associated with clozapine and other antipsychotics.7
Clozapine endured only because of its unique efficacy. When psychiatrists witnessed relapses in patients who had to discontinue clozapine, some countries allowed its use with strict monitoring.5 The FDA kept clozapine minimally available in the United States by allowing so-called “compassionate need programs” to continue.7
New data, FDA approval. Two studies in 1987 and 1988 that compared clozapine with chlorpromazine for treatment-refractory schizophrenia demonstrated clozapine’s superior effect on both negative and positive symptoms.8,9 The FDA approved clozapine for refractory schizophrenia in 1989, and clozapine became clinically available in 1990.
Initially, the high annual cost of clozapine’s required “bundle” ($8,900 per patient for medication and monitoring) led to political outcry. As patients and their family struggled to afford the newly released medication, multiple states filed antitrust lawsuits. A federal court found both the manufacturer and individual states at fault and required expanded access to clozapine and its necessary monitoring. National clozapine registries were formed, and bundling was eliminated.7
The clozapine REMS programSix clozapine registries operated independently, each managed by a different manufacturer,10 until the FDA introduced REMS in September 2015. The REMS program created a centralized registry to monitor all U.S. patients treated with clozapine and made important changes to prescribing and monitoring guidelines.11,12 It also incorporated the National Non-Rechallenge Master File (NNRMF).
Initially, the REMS program was scheduled for rollout October 12, 2015, the closing date of the 6 registries. Since November 2015, pharmacies have been required to register with the program to dispense clozapine. A similar registration deadline for clozapine prescribers was extended indefinitely, however, because of technical problems. Once the deadline is finalized, all clozapine prescribers must complete 3 steps to be certified in the REMS program (Table 1).11
New requirements. Certified clozapine prescribers will have new responsibilities: enrolling patients and submitting lab results. They can designate someone else to perform these tasks on their behalf, but designees must enroll in the REMS program and the prescriber must confirm the designee. Pharmacists can no longer enroll patients for clozapine therapy unless they are confirmed as a prescriber designee. For outpatients, the absolute neutrophil count (ANC) must be reported before the pharmacy can dispense clozapine. For inpatients, the ANC must be reported within 7 days of the patient’s most recent blood draw.
Once the system is fully operational, Social Security numbers will no longer be used as patient identification for dispensing clozapine. Instead, outpatient pharmacies will obtain a predispense authorization, or PDA, from the REMS program. A person initiated on clozapine as an inpatient must be re-enrolled after discharge by their outpatient prescriber.
The REMS program includes information about clozapine patients who were maintained through the 6 registries, and these patients have been allowed to continue clozapine treatment. Data pertaining to patients last prescribed clozapine before October 1, 2012, did not transfer into the new system unless their name was on the NNRMF.
CASE
Is Mr. A a candidate for clozapine?Age 28, with schizophrenia, Mr. A is highly disorganized and psychotic when brought to the emergency room by police for inappropriate behavior. His family arrives and reports that similar events have occurred several times over the past few years. Mr. A’s outpatient psychiatrist has prescribed 3 different antipsychotic medications at adequate dosages, including 1 long-acting injectable, but Mr. A has remained consistently symptomatic.
Although disorganized and psychotic, Mr. A does not meet criteria for long-term involuntary hospitalization. His family wants to take him home, and the treatment team discusses clozapine as an antipsychotic option. Mr. A and his family agree to a trial of clozapine during voluntary hospitalization, but they would like him home within a week to attend his sister’s birthday party.
The treatment team decides to initiate clozapine and monitor his response in a controlled setting for a few days before transitioning him to outpatient care.
Initiating clozapine therapyThe case of Mr. A exemplifies a situation in which initiating clozapine is a reasonable clinical consideration. As the first step, we recommend checking baseline lab values and vital signs (Table 2), keeping in mind that the REMS program requires a baseline ANC within 7 days of initiating clozapine. When working with a highly disorganized or agitated patient, balance benefits of testing against the risk of harm to staff and patient.
REMS guidelines recommend a baseline ANC ≥1,500/µL for a new patient starting clozapine, except when benign ethnic neutropenia (BEN) has been confirmed. (Initiation guidelines for BEN are discussed later in this article.)
Dosing alternatives. We recommend following the manufacturer’s dosing guidelines when initiating clozapine (Figure 2).13,14 Three oral forms are available: tablet, disintegrating tablet, and suspension. All can be titrated using the schedule suggested with tablets. The disintegrating tablets or suspension might be beneficial for a patient with either:
- a history of “cheeking” or otherwise disposing of tablets
- a medical condition that affects swallowing or absorption.
The disintegrating tablet is available in 12.5-mg, 25-mg, 100-mg, 150-mg, and 200-mg doses. It dissolves without requiring additional liquids. Each mL of the suspension contains 50 mg of clozapine.
Rapid titration? One group, working in Romania, examined the safety and efficacy of rapid titration of clozapine in 111 inpatients with schizophrenia.15 In the absence of additional studies, we do not recommend routine rapid titration of clozapine.
Monitoring: Greater flexibilityUnder the REMS program, laboratory monitoring of clozapine treatment must continue indefinitely. If not, pharmacies cannot dispense clozapine. Fortunately, the ANC is the only lab value tracked by the registry, and the frequency of required blood draws decreases over time (Figure 3).
Other guideline changes provide clinicians with greater flexibility to make patient-specific treatment decisions; for example, the allowable ANC to continue clozapine therapy has decreased. Usually, clozapine therapy should be interrupted for an ANC <1,000/µL if the prescriber suspects clozapine-induced neutropenia. Even when the ANC drops below 1,000/µL, however, prescribers can now continue clozapine treatment if they consider the benefits to outweigh risks for a given patient.
Separate guidelines now exist for patients with BEN, most commonly observed in persons of certain ethnic groups. BEN typically is diagnosed based on repeated ANC values <1,500/µL over several months. Patients with BEN do not have an increased risk of oral or systemic infections, as occur with other congenital neutropenias.16 In patients with BEN, clozapine therapy:
- can be initiated only after at least 2 baseline ANC measurements ≥1,000/µL
- should be interrupted for an ANC <500/µL if the prescriber suspects clozapine-induced neutropenia.
Substantial drops in ANC no longer require action (repeat lab draws) unless the drop causes neutropenia. Prescribers will receive an automated notification any time a patient experiences neutropenia that is considered mild (ANC 1,000 to 1,499/µL), moderate (ANC 500 to 999/µL), or severe (ANC <500/µL).
The NNRMF list is no longer definitive. All patients are now eligible for rechallenge, assuming they meet the new clozapine initiation criteria.
Next, when rediscovering clozapine: Adverse effectsDespite an intimidating list of side effects and interactions, clozapine is associated with a significant reduction in patients’ risk of overall mortality. In Part 2 of this series in the August 2016 issue, we discuss early identification of clozapine’s adverse effects and provide guidance for management.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatr Serv. 2014;65(2):186-192.
2. Olfson M, Gerhard T, Crystal S, et al. Clozapine for schizophrenia: state variation in evidence-based practice. Psychiatr Serv. 2016;67(2):152.
3. Warnez S, Alessi-Severini S. Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry. 2014;14:102.
4. Cetin M. Clozaphobia: fear of prescribers of clozapine for treatment of schizophrenia. Klinik Psikofarmakol Bulteni. 2014;24(4):295-301.
5. Hippius H. A historical perspective of clozapine. J Clin Psychiatry. 1999;60(suppl 12):22-23.
6. Amsler HA, Teerenhovi L, Barth E, et al. Agranulocytosis in patients treated with clozapine. A study of the Finnish epidemic. Acta Psychiatr Scand. 1977;56(4):241-248.
7. Crilly J. The history of clozapine and its emergence in the U.S. market: a review and analysis. Hist Psychiatry. 2007;18(1):39-60.
8. Claghorn J, Honigfeld G, Abuzzahab FS, et al. The risks and benefits of clozapine versus chlorpromazine. J Clin Psychopharmacol. 1987;7(6):377-384.
9. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
10. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA modified monitoring for neutropenia associated with schizophrenia medicine clozapine; approves new shared REMS program for all clozapine medicines. http://www.fda.gov/Drugs/DrugSafety/ucm461853.htm. Published September 15, 2015. Accessed November 23, 2015.
11. Clozapine REMS Program. What’s new with clozapine: an overview. https://www.clozapinerems.com/CpmgClozapineUI/rems/pdf/WhatsNEWwithClozapine_An%20Overview.pdf. Published September 2015. Accessed November 23, 2015.
12. Clozapine REMS Program. Clozapine and the risk of neutropenia: a guide for healthcare providers. https://www.clozapinerems.com/CpmgClozapineUI/rems/pdf/resources/Clozapine_REMS_HCP_Guide.pdf. Published September 2015. Accessed November 23, 2015.
13. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed June 16, 2016.
14. Newman WJ. Psychopharmacologic management of aggression. Psychiatr Clin North Am. 2012;35(4):957-972.
15. Ifteni P, Nielsen J, Burtea V, et al. Effectiveness and safety of rapid clozapine titration in schizophrenia. Acta Psychiatr Scand. 2014;130(1):25-29.
16. Hsieh MM, Tisdale JF, Rodgers GP, et al. Neutrophil count in African Americans: lowering the target cutoff to initiate or resume chemotherapy? J Clin Oncol. 2010;28(10):1633-1637.
Although clozapine is the medication with the clearest benefits in treatment-resistant schizophrenia, many eligible patients never receive it. In the United States, 20% to 30% of patients with schizophrenia can be classified as treatment resistant, but clozapine accounts for <5% of antipsychotics prescribed.1,2 Clinicians worldwide tend to under-prescribe clozapine3—a reluctance one author coined as “clozaphobia.”4
Admittedly, clozapine has had a turbulent history—both lauded as a near-miracle drug and condemned as a deadly agent. The FDA has overhauled its prescribing and monitoring guidelines, however, offering psychiatrists a perfect opportunity to reacquaint themselves with this potentially life-changing intervention.
We begin this article with clozapine’s story, then spotlight new terrain the FDA created in 2015 when the agency introduced the Clozapine Risk Evaluation and Mitigation Strategy (REMS). Our goal in the 3 articles of this series is to deepen your appreciation for this tricyclic antipsychotic and provide practical clinical guidance for using it safely and effectively.
Setbacks, but the drug has an enduring presenceThe 1950s was an exciting era of exploration for new psychotropic medications. While searching for tricyclic antidepressants, Wander Laboratories discovered neuroleptic tricyclics, with clozapine identified in 1959 (Figure 1). Haloperidol’s development and release in the 1960s reinforced the prevailing dogma of the time that effective neuroleptics correlated with extrapyramidal symptoms, thus limiting interest in the newly discovered, but pharmacologically unique, clozapine. Throughout the 1960s, most research on clozapine was published in German, with less of an international presence.5
Agranulocytosis deaths. Clozapine earned its scarlet letter in 1975, when 8 patients in Finland died of agranulocytosis.6 Sandoz, its manufacturer, withdrew clozapine from the market and halted all clinical trials. The Finnish epidemic triggered detailed investigations into blood dyscrasias and early identification of agranulocytosis associated with clozapine and other antipsychotics.7
Clozapine endured only because of its unique efficacy. When psychiatrists witnessed relapses in patients who had to discontinue clozapine, some countries allowed its use with strict monitoring.5 The FDA kept clozapine minimally available in the United States by allowing so-called “compassionate need programs” to continue.7
New data, FDA approval. Two studies in 1987 and 1988 that compared clozapine with chlorpromazine for treatment-refractory schizophrenia demonstrated clozapine’s superior effect on both negative and positive symptoms.8,9 The FDA approved clozapine for refractory schizophrenia in 1989, and clozapine became clinically available in 1990.
Initially, the high annual cost of clozapine’s required “bundle” ($8,900 per patient for medication and monitoring) led to political outcry. As patients and their family struggled to afford the newly released medication, multiple states filed antitrust lawsuits. A federal court found both the manufacturer and individual states at fault and required expanded access to clozapine and its necessary monitoring. National clozapine registries were formed, and bundling was eliminated.7
The clozapine REMS programSix clozapine registries operated independently, each managed by a different manufacturer,10 until the FDA introduced REMS in September 2015. The REMS program created a centralized registry to monitor all U.S. patients treated with clozapine and made important changes to prescribing and monitoring guidelines.11,12 It also incorporated the National Non-Rechallenge Master File (NNRMF).
Initially, the REMS program was scheduled for rollout October 12, 2015, the closing date of the 6 registries. Since November 2015, pharmacies have been required to register with the program to dispense clozapine. A similar registration deadline for clozapine prescribers was extended indefinitely, however, because of technical problems. Once the deadline is finalized, all clozapine prescribers must complete 3 steps to be certified in the REMS program (Table 1).11
New requirements. Certified clozapine prescribers will have new responsibilities: enrolling patients and submitting lab results. They can designate someone else to perform these tasks on their behalf, but designees must enroll in the REMS program and the prescriber must confirm the designee. Pharmacists can no longer enroll patients for clozapine therapy unless they are confirmed as a prescriber designee. For outpatients, the absolute neutrophil count (ANC) must be reported before the pharmacy can dispense clozapine. For inpatients, the ANC must be reported within 7 days of the patient’s most recent blood draw.
Once the system is fully operational, Social Security numbers will no longer be used as patient identification for dispensing clozapine. Instead, outpatient pharmacies will obtain a predispense authorization, or PDA, from the REMS program. A person initiated on clozapine as an inpatient must be re-enrolled after discharge by their outpatient prescriber.
The REMS program includes information about clozapine patients who were maintained through the 6 registries, and these patients have been allowed to continue clozapine treatment. Data pertaining to patients last prescribed clozapine before October 1, 2012, did not transfer into the new system unless their name was on the NNRMF.
CASE
Is Mr. A a candidate for clozapine?Age 28, with schizophrenia, Mr. A is highly disorganized and psychotic when brought to the emergency room by police for inappropriate behavior. His family arrives and reports that similar events have occurred several times over the past few years. Mr. A’s outpatient psychiatrist has prescribed 3 different antipsychotic medications at adequate dosages, including 1 long-acting injectable, but Mr. A has remained consistently symptomatic.
Although disorganized and psychotic, Mr. A does not meet criteria for long-term involuntary hospitalization. His family wants to take him home, and the treatment team discusses clozapine as an antipsychotic option. Mr. A and his family agree to a trial of clozapine during voluntary hospitalization, but they would like him home within a week to attend his sister’s birthday party.
The treatment team decides to initiate clozapine and monitor his response in a controlled setting for a few days before transitioning him to outpatient care.
Initiating clozapine therapyThe case of Mr. A exemplifies a situation in which initiating clozapine is a reasonable clinical consideration. As the first step, we recommend checking baseline lab values and vital signs (Table 2), keeping in mind that the REMS program requires a baseline ANC within 7 days of initiating clozapine. When working with a highly disorganized or agitated patient, balance benefits of testing against the risk of harm to staff and patient.
REMS guidelines recommend a baseline ANC ≥1,500/µL for a new patient starting clozapine, except when benign ethnic neutropenia (BEN) has been confirmed. (Initiation guidelines for BEN are discussed later in this article.)
Dosing alternatives. We recommend following the manufacturer’s dosing guidelines when initiating clozapine (Figure 2).13,14 Three oral forms are available: tablet, disintegrating tablet, and suspension. All can be titrated using the schedule suggested with tablets. The disintegrating tablets or suspension might be beneficial for a patient with either:
- a history of “cheeking” or otherwise disposing of tablets
- a medical condition that affects swallowing or absorption.
The disintegrating tablet is available in 12.5-mg, 25-mg, 100-mg, 150-mg, and 200-mg doses. It dissolves without requiring additional liquids. Each mL of the suspension contains 50 mg of clozapine.
Rapid titration? One group, working in Romania, examined the safety and efficacy of rapid titration of clozapine in 111 inpatients with schizophrenia.15 In the absence of additional studies, we do not recommend routine rapid titration of clozapine.
Monitoring: Greater flexibilityUnder the REMS program, laboratory monitoring of clozapine treatment must continue indefinitely. If not, pharmacies cannot dispense clozapine. Fortunately, the ANC is the only lab value tracked by the registry, and the frequency of required blood draws decreases over time (Figure 3).
Other guideline changes provide clinicians with greater flexibility to make patient-specific treatment decisions; for example, the allowable ANC to continue clozapine therapy has decreased. Usually, clozapine therapy should be interrupted for an ANC <1,000/µL if the prescriber suspects clozapine-induced neutropenia. Even when the ANC drops below 1,000/µL, however, prescribers can now continue clozapine treatment if they consider the benefits to outweigh risks for a given patient.
Separate guidelines now exist for patients with BEN, most commonly observed in persons of certain ethnic groups. BEN typically is diagnosed based on repeated ANC values <1,500/µL over several months. Patients with BEN do not have an increased risk of oral or systemic infections, as occur with other congenital neutropenias.16 In patients with BEN, clozapine therapy:
- can be initiated only after at least 2 baseline ANC measurements ≥1,000/µL
- should be interrupted for an ANC <500/µL if the prescriber suspects clozapine-induced neutropenia.
Substantial drops in ANC no longer require action (repeat lab draws) unless the drop causes neutropenia. Prescribers will receive an automated notification any time a patient experiences neutropenia that is considered mild (ANC 1,000 to 1,499/µL), moderate (ANC 500 to 999/µL), or severe (ANC <500/µL).
The NNRMF list is no longer definitive. All patients are now eligible for rechallenge, assuming they meet the new clozapine initiation criteria.
Next, when rediscovering clozapine: Adverse effectsDespite an intimidating list of side effects and interactions, clozapine is associated with a significant reduction in patients’ risk of overall mortality. In Part 2 of this series in the August 2016 issue, we discuss early identification of clozapine’s adverse effects and provide guidance for management.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Although clozapine is the medication with the clearest benefits in treatment-resistant schizophrenia, many eligible patients never receive it. In the United States, 20% to 30% of patients with schizophrenia can be classified as treatment resistant, but clozapine accounts for <5% of antipsychotics prescribed.1,2 Clinicians worldwide tend to under-prescribe clozapine3—a reluctance one author coined as “clozaphobia.”4
Admittedly, clozapine has had a turbulent history—both lauded as a near-miracle drug and condemned as a deadly agent. The FDA has overhauled its prescribing and monitoring guidelines, however, offering psychiatrists a perfect opportunity to reacquaint themselves with this potentially life-changing intervention.
We begin this article with clozapine’s story, then spotlight new terrain the FDA created in 2015 when the agency introduced the Clozapine Risk Evaluation and Mitigation Strategy (REMS). Our goal in the 3 articles of this series is to deepen your appreciation for this tricyclic antipsychotic and provide practical clinical guidance for using it safely and effectively.
Setbacks, but the drug has an enduring presenceThe 1950s was an exciting era of exploration for new psychotropic medications. While searching for tricyclic antidepressants, Wander Laboratories discovered neuroleptic tricyclics, with clozapine identified in 1959 (Figure 1). Haloperidol’s development and release in the 1960s reinforced the prevailing dogma of the time that effective neuroleptics correlated with extrapyramidal symptoms, thus limiting interest in the newly discovered, but pharmacologically unique, clozapine. Throughout the 1960s, most research on clozapine was published in German, with less of an international presence.5
Agranulocytosis deaths. Clozapine earned its scarlet letter in 1975, when 8 patients in Finland died of agranulocytosis.6 Sandoz, its manufacturer, withdrew clozapine from the market and halted all clinical trials. The Finnish epidemic triggered detailed investigations into blood dyscrasias and early identification of agranulocytosis associated with clozapine and other antipsychotics.7
Clozapine endured only because of its unique efficacy. When psychiatrists witnessed relapses in patients who had to discontinue clozapine, some countries allowed its use with strict monitoring.5 The FDA kept clozapine minimally available in the United States by allowing so-called “compassionate need programs” to continue.7
New data, FDA approval. Two studies in 1987 and 1988 that compared clozapine with chlorpromazine for treatment-refractory schizophrenia demonstrated clozapine’s superior effect on both negative and positive symptoms.8,9 The FDA approved clozapine for refractory schizophrenia in 1989, and clozapine became clinically available in 1990.
Initially, the high annual cost of clozapine’s required “bundle” ($8,900 per patient for medication and monitoring) led to political outcry. As patients and their family struggled to afford the newly released medication, multiple states filed antitrust lawsuits. A federal court found both the manufacturer and individual states at fault and required expanded access to clozapine and its necessary monitoring. National clozapine registries were formed, and bundling was eliminated.7
The clozapine REMS programSix clozapine registries operated independently, each managed by a different manufacturer,10 until the FDA introduced REMS in September 2015. The REMS program created a centralized registry to monitor all U.S. patients treated with clozapine and made important changes to prescribing and monitoring guidelines.11,12 It also incorporated the National Non-Rechallenge Master File (NNRMF).
Initially, the REMS program was scheduled for rollout October 12, 2015, the closing date of the 6 registries. Since November 2015, pharmacies have been required to register with the program to dispense clozapine. A similar registration deadline for clozapine prescribers was extended indefinitely, however, because of technical problems. Once the deadline is finalized, all clozapine prescribers must complete 3 steps to be certified in the REMS program (Table 1).11
New requirements. Certified clozapine prescribers will have new responsibilities: enrolling patients and submitting lab results. They can designate someone else to perform these tasks on their behalf, but designees must enroll in the REMS program and the prescriber must confirm the designee. Pharmacists can no longer enroll patients for clozapine therapy unless they are confirmed as a prescriber designee. For outpatients, the absolute neutrophil count (ANC) must be reported before the pharmacy can dispense clozapine. For inpatients, the ANC must be reported within 7 days of the patient’s most recent blood draw.
Once the system is fully operational, Social Security numbers will no longer be used as patient identification for dispensing clozapine. Instead, outpatient pharmacies will obtain a predispense authorization, or PDA, from the REMS program. A person initiated on clozapine as an inpatient must be re-enrolled after discharge by their outpatient prescriber.
The REMS program includes information about clozapine patients who were maintained through the 6 registries, and these patients have been allowed to continue clozapine treatment. Data pertaining to patients last prescribed clozapine before October 1, 2012, did not transfer into the new system unless their name was on the NNRMF.
CASE
Is Mr. A a candidate for clozapine?Age 28, with schizophrenia, Mr. A is highly disorganized and psychotic when brought to the emergency room by police for inappropriate behavior. His family arrives and reports that similar events have occurred several times over the past few years. Mr. A’s outpatient psychiatrist has prescribed 3 different antipsychotic medications at adequate dosages, including 1 long-acting injectable, but Mr. A has remained consistently symptomatic.
Although disorganized and psychotic, Mr. A does not meet criteria for long-term involuntary hospitalization. His family wants to take him home, and the treatment team discusses clozapine as an antipsychotic option. Mr. A and his family agree to a trial of clozapine during voluntary hospitalization, but they would like him home within a week to attend his sister’s birthday party.
The treatment team decides to initiate clozapine and monitor his response in a controlled setting for a few days before transitioning him to outpatient care.
Initiating clozapine therapyThe case of Mr. A exemplifies a situation in which initiating clozapine is a reasonable clinical consideration. As the first step, we recommend checking baseline lab values and vital signs (Table 2), keeping in mind that the REMS program requires a baseline ANC within 7 days of initiating clozapine. When working with a highly disorganized or agitated patient, balance benefits of testing against the risk of harm to staff and patient.
REMS guidelines recommend a baseline ANC ≥1,500/µL for a new patient starting clozapine, except when benign ethnic neutropenia (BEN) has been confirmed. (Initiation guidelines for BEN are discussed later in this article.)
Dosing alternatives. We recommend following the manufacturer’s dosing guidelines when initiating clozapine (Figure 2).13,14 Three oral forms are available: tablet, disintegrating tablet, and suspension. All can be titrated using the schedule suggested with tablets. The disintegrating tablets or suspension might be beneficial for a patient with either:
- a history of “cheeking” or otherwise disposing of tablets
- a medical condition that affects swallowing or absorption.
The disintegrating tablet is available in 12.5-mg, 25-mg, 100-mg, 150-mg, and 200-mg doses. It dissolves without requiring additional liquids. Each mL of the suspension contains 50 mg of clozapine.
Rapid titration? One group, working in Romania, examined the safety and efficacy of rapid titration of clozapine in 111 inpatients with schizophrenia.15 In the absence of additional studies, we do not recommend routine rapid titration of clozapine.
Monitoring: Greater flexibilityUnder the REMS program, laboratory monitoring of clozapine treatment must continue indefinitely. If not, pharmacies cannot dispense clozapine. Fortunately, the ANC is the only lab value tracked by the registry, and the frequency of required blood draws decreases over time (Figure 3).
Other guideline changes provide clinicians with greater flexibility to make patient-specific treatment decisions; for example, the allowable ANC to continue clozapine therapy has decreased. Usually, clozapine therapy should be interrupted for an ANC <1,000/µL if the prescriber suspects clozapine-induced neutropenia. Even when the ANC drops below 1,000/µL, however, prescribers can now continue clozapine treatment if they consider the benefits to outweigh risks for a given patient.
Separate guidelines now exist for patients with BEN, most commonly observed in persons of certain ethnic groups. BEN typically is diagnosed based on repeated ANC values <1,500/µL over several months. Patients with BEN do not have an increased risk of oral or systemic infections, as occur with other congenital neutropenias.16 In patients with BEN, clozapine therapy:
- can be initiated only after at least 2 baseline ANC measurements ≥1,000/µL
- should be interrupted for an ANC <500/µL if the prescriber suspects clozapine-induced neutropenia.
Substantial drops in ANC no longer require action (repeat lab draws) unless the drop causes neutropenia. Prescribers will receive an automated notification any time a patient experiences neutropenia that is considered mild (ANC 1,000 to 1,499/µL), moderate (ANC 500 to 999/µL), or severe (ANC <500/µL).
The NNRMF list is no longer definitive. All patients are now eligible for rechallenge, assuming they meet the new clozapine initiation criteria.
Next, when rediscovering clozapine: Adverse effectsDespite an intimidating list of side effects and interactions, clozapine is associated with a significant reduction in patients’ risk of overall mortality. In Part 2 of this series in the August 2016 issue, we discuss early identification of clozapine’s adverse effects and provide guidance for management.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatr Serv. 2014;65(2):186-192.
2. Olfson M, Gerhard T, Crystal S, et al. Clozapine for schizophrenia: state variation in evidence-based practice. Psychiatr Serv. 2016;67(2):152.
3. Warnez S, Alessi-Severini S. Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry. 2014;14:102.
4. Cetin M. Clozaphobia: fear of prescribers of clozapine for treatment of schizophrenia. Klinik Psikofarmakol Bulteni. 2014;24(4):295-301.
5. Hippius H. A historical perspective of clozapine. J Clin Psychiatry. 1999;60(suppl 12):22-23.
6. Amsler HA, Teerenhovi L, Barth E, et al. Agranulocytosis in patients treated with clozapine. A study of the Finnish epidemic. Acta Psychiatr Scand. 1977;56(4):241-248.
7. Crilly J. The history of clozapine and its emergence in the U.S. market: a review and analysis. Hist Psychiatry. 2007;18(1):39-60.
8. Claghorn J, Honigfeld G, Abuzzahab FS, et al. The risks and benefits of clozapine versus chlorpromazine. J Clin Psychopharmacol. 1987;7(6):377-384.
9. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
10. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA modified monitoring for neutropenia associated with schizophrenia medicine clozapine; approves new shared REMS program for all clozapine medicines. http://www.fda.gov/Drugs/DrugSafety/ucm461853.htm. Published September 15, 2015. Accessed November 23, 2015.
11. Clozapine REMS Program. What’s new with clozapine: an overview. https://www.clozapinerems.com/CpmgClozapineUI/rems/pdf/WhatsNEWwithClozapine_An%20Overview.pdf. Published September 2015. Accessed November 23, 2015.
12. Clozapine REMS Program. Clozapine and the risk of neutropenia: a guide for healthcare providers. https://www.clozapinerems.com/CpmgClozapineUI/rems/pdf/resources/Clozapine_REMS_HCP_Guide.pdf. Published September 2015. Accessed November 23, 2015.
13. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed June 16, 2016.
14. Newman WJ. Psychopharmacologic management of aggression. Psychiatr Clin North Am. 2012;35(4):957-972.
15. Ifteni P, Nielsen J, Burtea V, et al. Effectiveness and safety of rapid clozapine titration in schizophrenia. Acta Psychiatr Scand. 2014;130(1):25-29.
16. Hsieh MM, Tisdale JF, Rodgers GP, et al. Neutrophil count in African Americans: lowering the target cutoff to initiate or resume chemotherapy? J Clin Oncol. 2010;28(10):1633-1637.
1. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatr Serv. 2014;65(2):186-192.
2. Olfson M, Gerhard T, Crystal S, et al. Clozapine for schizophrenia: state variation in evidence-based practice. Psychiatr Serv. 2016;67(2):152.
3. Warnez S, Alessi-Severini S. Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry. 2014;14:102.
4. Cetin M. Clozaphobia: fear of prescribers of clozapine for treatment of schizophrenia. Klinik Psikofarmakol Bulteni. 2014;24(4):295-301.
5. Hippius H. A historical perspective of clozapine. J Clin Psychiatry. 1999;60(suppl 12):22-23.
6. Amsler HA, Teerenhovi L, Barth E, et al. Agranulocytosis in patients treated with clozapine. A study of the Finnish epidemic. Acta Psychiatr Scand. 1977;56(4):241-248.
7. Crilly J. The history of clozapine and its emergence in the U.S. market: a review and analysis. Hist Psychiatry. 2007;18(1):39-60.
8. Claghorn J, Honigfeld G, Abuzzahab FS, et al. The risks and benefits of clozapine versus chlorpromazine. J Clin Psychopharmacol. 1987;7(6):377-384.
9. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
10. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA modified monitoring for neutropenia associated with schizophrenia medicine clozapine; approves new shared REMS program for all clozapine medicines. http://www.fda.gov/Drugs/DrugSafety/ucm461853.htm. Published September 15, 2015. Accessed November 23, 2015.
11. Clozapine REMS Program. What’s new with clozapine: an overview. https://www.clozapinerems.com/CpmgClozapineUI/rems/pdf/WhatsNEWwithClozapine_An%20Overview.pdf. Published September 2015. Accessed November 23, 2015.
12. Clozapine REMS Program. Clozapine and the risk of neutropenia: a guide for healthcare providers. https://www.clozapinerems.com/CpmgClozapineUI/rems/pdf/resources/Clozapine_REMS_HCP_Guide.pdf. Published September 2015. Accessed November 23, 2015.
13. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed June 16, 2016.
14. Newman WJ. Psychopharmacologic management of aggression. Psychiatr Clin North Am. 2012;35(4):957-972.
15. Ifteni P, Nielsen J, Burtea V, et al. Effectiveness and safety of rapid clozapine titration in schizophrenia. Acta Psychiatr Scand. 2014;130(1):25-29.
16. Hsieh MM, Tisdale JF, Rodgers GP, et al. Neutrophil count in African Americans: lowering the target cutoff to initiate or resume chemotherapy? J Clin Oncol. 2010;28(10):1633-1637.