User login
New-onset psychosis while being treated for coronavirus
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.
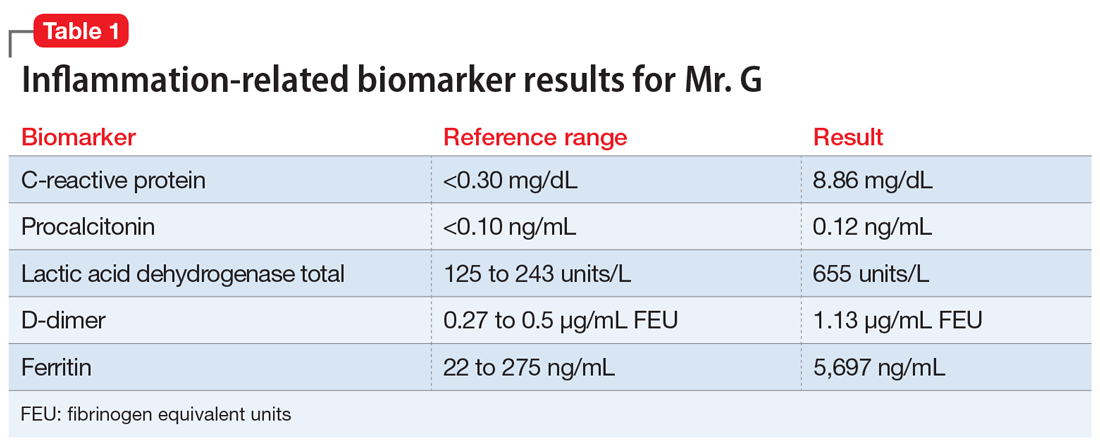
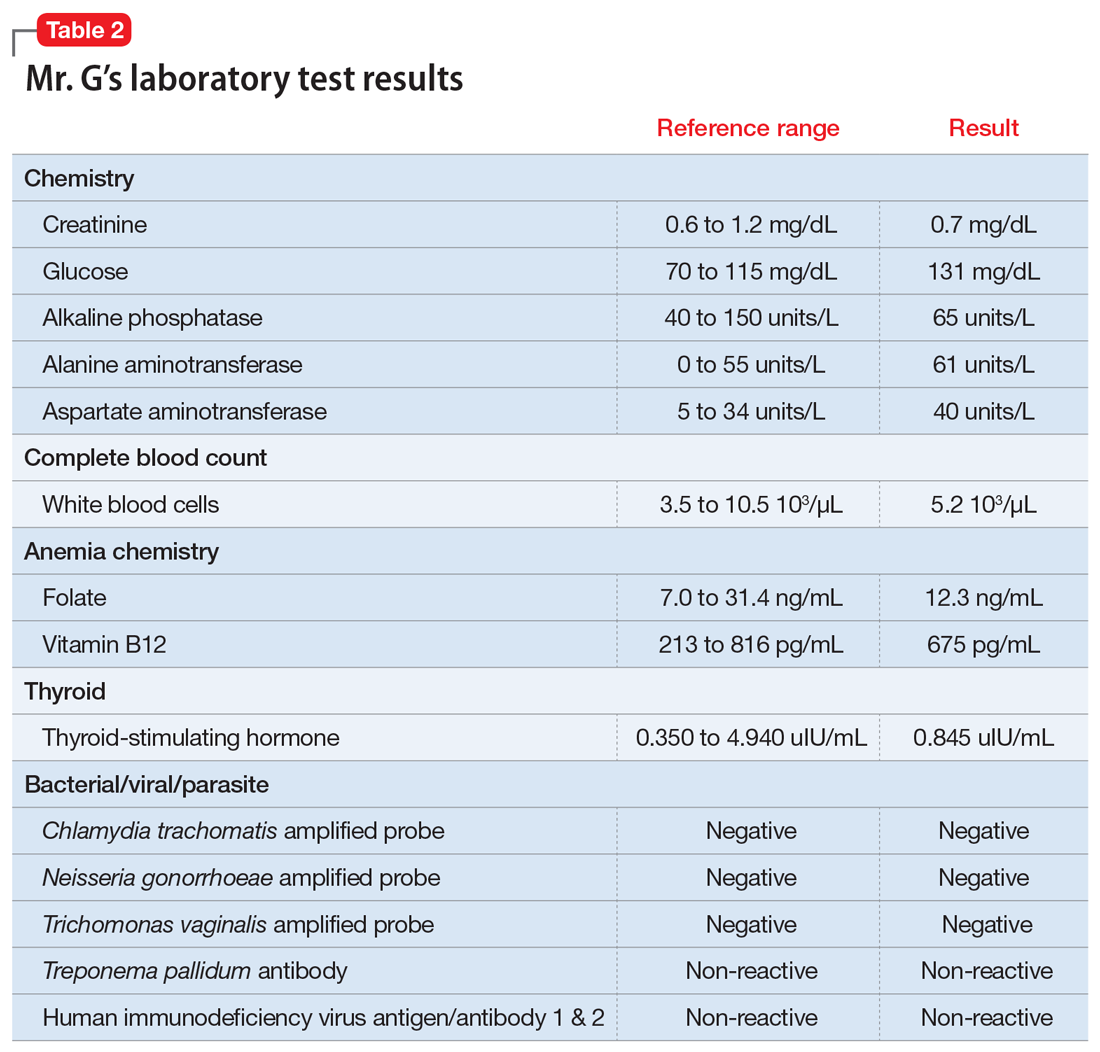
Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.
Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.
Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.
Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.
Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.
Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
The psychiatrist’s role in liver transplantation
Since the first liver transplant (LT) was performed in 1963 by Starzl et al, there have been considerable advances in the field, with improvements in post-transplant survival.1 There are multiple indications for LT, including acute liver failure and index complications of cirrhosis such as ascites, encephalopathy, and hepatopulmonary syndrome.2 Once a patient develops one of these conditions, he/she is evaluated for LT, even as the complications of liver failure are being managed.
Although the number of LTs has risen, the demand for transplant continues to exceed availability. In 2015, chronic liver disease and cirrhosis was the 12th leading cause of death in the United States.3 In 2016, approximately 50% of waitlisted candidates received a transplant.4 There is also a donor shortage. In part, this shortage may be due to longer life spans and the subsequent increase in the age of the potential donor.5 In light of this shortage and increased demand, the pre-LT workup is comprehensive. The pre-transplant assessment typically consists of cardiology, surgery, hepatology, and psychosocial evaluations, and hence requires a team of experts to determine who is an ideal candidate for transplant.
Psychiatrists play a key role in the pre-transplant psychosocial evaluations. This article describes the elements of these evaluations, and what psychiatrists can do to help patients both before and after they undergo LT.
Elements of the pre-transplant evaluation
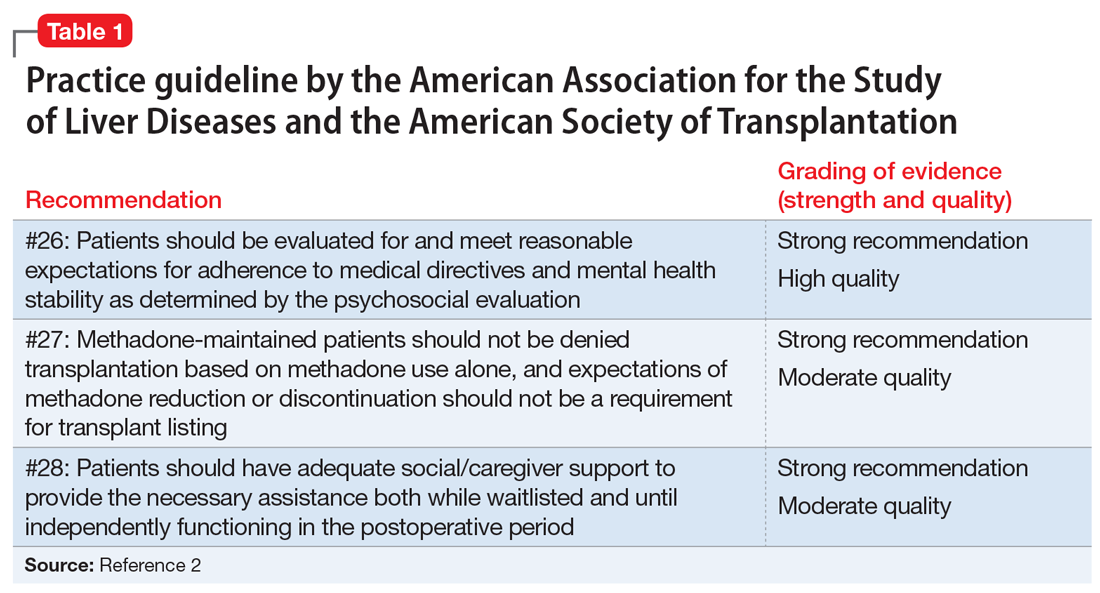
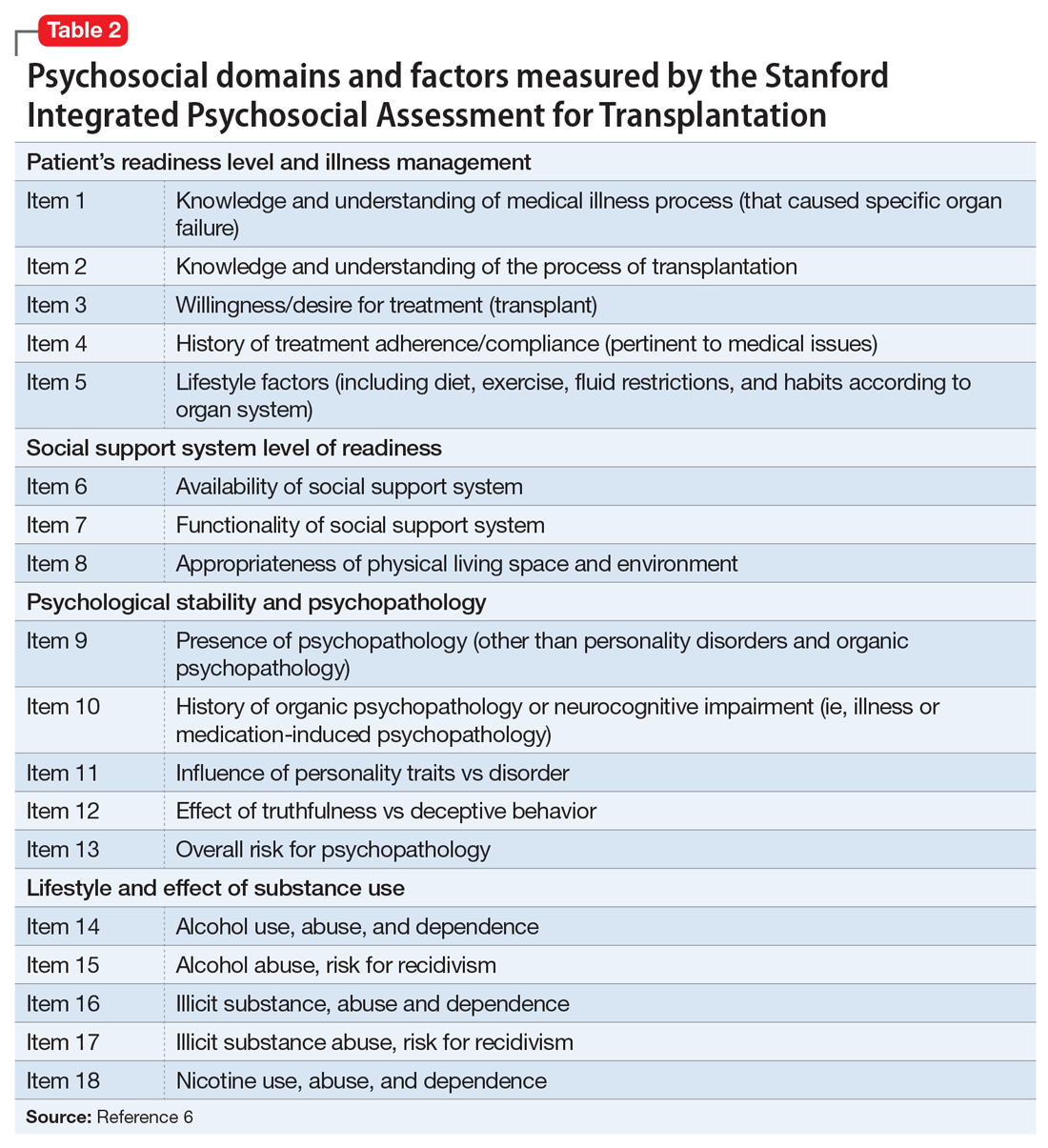
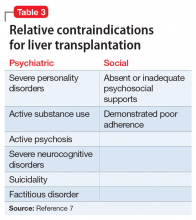
The psychosocial evaluation is a critical component of the pre-transplant assessment. As part of the evaluation, patients are screened for psychosocial limitations that may complicate transplantation, such as demonstrated noncompliance, ongoing alcohol or drug use, and lack of social support (Table 12 ). Other goals of the psychosocial evaluation are to identify in the pre-transplant period patients with possible risk factors, such as substance use or psychiatric disorders, and develop treatment plans to optimize transplant outcomes (Table 26). There are relative contraindications to LT (Table 37) but no absolute psychiatric contraindications, according to the 2013 American Association for the Study of Liver Diseases (AASLD) practice guideline for transplantation.2
Adherence. The 2013 AASLD practice guideline states that patients “should be evaluated for and meet reasonable expectations for adherence to medical directives and mental health stability as determined by the psychosocial evaluation.”2 In the transplant setting, adherence is complex. It requires compliance with complicated medication regimens and laboratory testing, frequent follow-up appointments, and close, prompt communication of concerns to the health care team. Patient adherence to medication regimens plays an important role in transplant outcomes.8 In fact, in patients who have undergone renal transplant, nonadherence to therapy is considered the leading cause of avoidable graft failure.9
A retrospective study of adult LT recipients found that pre-transplant chart evidence of nonadherence, such as missed laboratory testing and clinic visits, was a significant predictor of post-transplant nonadherence with immunosuppressant therapy. Pre-transplant unemployment status and a history of substance abuse also were associated with nonadherence.9
Dobbels et al10 found that patients with a self-reported history of pre-transplant non-adherence had a higher risk of being nonadherent with their immunosuppressive therapy after transplant (odd ratio [OR]: 7.9). Their self-report adherence questionnaire included questions that addressed pre-transplant smoking status, alcohol use, and adherence with medication. In this prospective study, researchers also found that patients with a low “conscientiousness” score were at a higher risk for post-transplant medication nonadherence (OR: 0.8).
Continue to: Studies have also found...
Studies have also found that patients with higher education are more at risk for post-transplant medication nonadherence. Higher education may be associated with higher employment status resulting in a busier lifestyle, a known risk factor that may prevent patients from regular medication adherence.11,12 Alternatively, it is possible that higher educated patients are “decisive” nonadherers who prefer independent decision-making regarding their disease and treatment.13
Substance use. The 2013 AASLD practice guideline lists “ongoing alcohol or illicit substance abuse” as one of the contraindications to LT.2 In guidelines from the Austrian Society for Gastroenterology and Hepatology, Graziadei et al14 listed “alcohol addiction without motivation for alcohol abstinence and untreated/ongoing substance abuse” as absolute contraindications and “untreated alcohol abuse and other drug-related addiction” as relative contraindications. Hence, the pre-transplant evaluation should include a thorough substance use history, including duration, amount, previous attempts to quit, and motivation for abstinence.
Substance use history is especially important because alcoholic liver disease is the second most common indication for LT.2 Most LT programs require 6 months of abstinence before a patient can be considered for transplant.15 The 6-month period was based on studies demonstrating that pre-transplant abstinence from alcohol for <6 months is a risk factor for relapse.15 However, this guideline remains controversial because the transplant referral and workup may be delayed as the patient’s liver disease worsens. Other risk factors for substance relapse should also be taken into consideration, such as depression, personality disorders, lack of social support, severity of alcohol use, and family history of alcoholism.16 Lee and Leggio16 developed the Sustained Alcohol Use Post-Liver Transplant (SALT) score to identify patients who were at risk for sustained alcohol use posttransplant. The 4 SALT criteria are:
- >10 drinks per day at initial hospitalization (+4 points)
- multiple prior rehabilitation attempts (+4 points)
- prior alcohol‐related legal issues (+2 points), and
- prior illicit substance abuse (+1 point).
A SALT score can range from 0 to 11. Lee et al17 found a SALT score ≥5 had a 25% positive predictive value (95% confidence interval [CI]: 10% to 47%) and a SALT score of <5 had a 95% negative predictive value (95% CI: 89% to 98%) for sustained alcohol use post‐LT. Thus, the 2013 AASLD guideline cautions against delaying evaluation based on the 6-month abstinence rule, and instead recommends early transplant referral for patients with alcoholic liver disease to encourage such patients to begin addiction treatment.2
As part of the substance use history, it is important to ask about the patient’s smoking history. Approximately 60% of LT candidates have a history of smoking cigarettes.18 Tobacco use history is associated with increased post-transplant vascular complications, such as hepatic artery thrombosis or stenosis, portal vein thrombosis, and deep vein thrombosis.19 The 2013 AASLD guideline recommends that tobacco use should be prohibited in LT candidates.2 Pungpapong et al19 reported that smoking cessation for at least 2 years prior to transplant led to a significantly decreased risk of developing arterial complications, with an absolute risk reduction of approximately 16%.
Continue to: Liver cirrhosis due to...
Liver cirrhosis due to chronic hepatitis C virus (HCV) infection is one of the leading causes for LT. In the United States, HCV is commonly transmitted during injection drug use. According to the 2013 AASLD guideline, ongoing illicit substance use is a relative contraindication to LT.2 It is important to note, however, that methadone maintenance therapy (MMT) is not a contraindication to LT. In fact, the 2013 AASLD guideline recommends that patients receiving MMT should not be required to reduce or stop therapy in order to be listed for transplant.2 Studies have shown that in 80% of patients, tapering MMT leads to illicit opiate relapse.20 Currently, there is no evidence that patients receiving MMT have poorer post-transplant outcomes compared with patients not receiving MMT.21
Whether cannabis use is a relative contraindication to LT remains controversial.22 Possible adverse effects of cannabis use in transplant patients include drug–drug interactions and infections. Hézode et al23 reported that daily cannabis use is significantly associated with an increased fibrosis progression rate in patients with chronic HCV infection. Another recent study found that a history of cannabis use was not associated with worse outcomes among patients on the LT waitlist.24 With the increased legalization of cannabis, more studies are needed to assess ongoing cannabis use in patients on the LT waitlist and post-LT outcomes.
Psychiatric history. When assessing a patient for possible LT, no psychiatric disorder is considered an absolute contraindication. Patients with a serious mental illness, such as schizophrenia, and those with intellectual disability can have successful, long-term outcomes with proper evaluation and preparation, including social support. However, empirical literature regarding transplant outcomes and predictive factors in patients with serious mental illness is scarce.2
Studies examining the predictive value of pre-transplant depression on post-transplant outcomes have had mixed results.25 Depression may predict lower post-transplant quality of life. Pre-LT suicidal thoughts (as noted on the Beck Depression Inventory, for example) are associated with post-LT depression.25 In contrast, available data show no significant effect of pre-transplant anxiety on post-LT outcomes. Similarly, pre-transplant cognitive performance appears not to predict survival or other post-transplant outcomes, but may predict poorer quality of life after transplant.25
A few psychiatric factors are considered relative contraindications for LT. These include severe personality disorders, active substance use with no motivation for treatment or abstinence, active psychosis, severe neurocognitive disorders, suicidality, and factitious disorder.7
Continue to: Social support
Social support. Assessing a pre-LT patient’s level of social support is an essential part of the psychosocial evaluation. According to the 2013 AASLD guideline, patients should have “adequate” social support both during the waitlist and post-operative periods.2 Lack of partnership is a significant predictor of poor post-transplant outcomes, such as late graft loss.10 Satapathy and Sanyal26 reported that among patients who receive an LT for alcoholic liver disease, those with immediate family support were less likely to relapse to using alcohol after transplant. Poor social support was also a predictor of post-transplant medication nonadherence.10 Thus, the patient needs enough social support to engage in the pre-transplant health care requirements and to participate in post-transplant recommendations until he/she is functioning independently post-transplant.
Screening tools
Various screening tools may be useful in a pre-LT evaluation. Three standardized assessment tools available specifically for pre-transplant psychosocial assessments are the Stanford Integrated Psychosocial Assessment for Transplantation (Table 26), the Psychosocial Assessment of Candidates for Transplantation,27 and the Transplant Evaluation Rating Scale.28 Instruments to aid in the assessment of depression, anxiety, and delirium,29-31 a structured personality assessment,32 coping inventories,33 neuropsychological batteries,34 and others also have been used to evaluate patients before LT. The self-rated Beck Depression Inventory and the clinician-rated Hamilton Depression Rating Scale are commonly used.7 Other tools, such as the LEIPAD quality of life instrument and the Brief Symptom Inventory (BSI), have been used to assess for perceived quality of life and psychological distress, respectively.35 These screening tools can be helpful as aids for the pre-LT evaluation; however, diagnoses and treatment plan recommendations require a psychiatric evaluation conducted by a trained clinician.
Treatment after liver transplant
Psychiatric issues. After LT, various psychiatric complications may arise, including (but not limited to) delirium7 and “paradoxical psychiatric syndrome” (PPS).36 Delirium can be managed by administering low-dose antipsychotic medications, limiting the use of benzodiazepines and medications with anticholinergic effects, implementing behavioral interventions (frequent orientation, maintaining sleep/wake cycle, limiting noise, presence of a family member or a sitter at bedside),37 and addressing the underlying etiology. Paradoxical psychiatric syndrome is defined as psychiatric symptoms that occur despite a successful LT. It develops within the first year of transplantation and is characterized by recipients having strong guilt feelings toward their donors.38
Drug interactions. In the post-transplant period, antipsychotics are used for management of delirium and psychosis, antidepressants for anxiety and depression, and benzodiazepines for anxiety and sleep problems.7 Drug–drug interactions between psychotropic medications and the immunosuppressants required after LT must be closely monitored. First-generation antipsychotics should be avoided in post-transplant patients taking tacrolimus due to the increased risk of QTc prolongation. Tacrolimus can also increase the risk of nephrotoxicity when co-administered with lithium. Post-LT patients taking steroids and bupropion have an increased risk of seizure. Carbamazepine may decrease blood levels of cyclosporine due to the induction of hepatic metabolism.39,40 The psychiatrist should review and update the patient’s complete medication list at each visit, checking for possible medication interactions.
Quality of life. In the first 6 months post-transplant, patients typically experience improved quality of life in both physical and psychological domains. However, this improvement vacillates as the patient adjusts to post-transplant life. A reduction in BSI score 1 year after transplant has been reported. The BSI evaluates psychopathological symptoms, which are early indicators of psychological discomfort. One study noted a reduction in the LEIPAD quality of life score, which measures overall quality of life, 2 years after transplant.35 This decline may reflect the difficulties associated with the new challenges after transplant. Patients may endure both physical changes due to medical complications as well as psychological problems as they adjust to their new bodily integrity, their dependence on medications and medical staff, and other changes in function. Three to 5 years after transplant, patients reached a new psychological stability, with reported improvements in quality of life and decreased psychological distress.35
Continue to: Special populations
Special populations
HCV infection. Recurrent HCV infection and liver disease after transplantation are associated with psychological distress. This is particularly evident in patients 6 months after transplantation. Depression and psychological distress have been reported in male patients with recurrent HCV infection within the first year after transplantation.35
Acetaminophen overdose. Patients who receive a transplant for acetaminophen-induced acute liver failure (ALF) had a greater prevalence of psychiatric comorbidity as reflected by predefined diagnoses, medication, and previous suicide attempts.41 Despite this, outcomes for patients transplanted emergently for acetaminophen-induced ALF were comparable to those transplanted for non-acetaminophen-induced ALF and for chronic liver disease. Multidisciplinary approaches with long-term psychiatric follow-up may contribute to low post-transplant suicide rates and low rates of graft loss because of noncompliance.41
CASE REPORT
A complicated presentation
Ms. A, age 45, a married woman with history of chronic back pain and self-reported bipolar disorder, presented to our hospital with acute liver failure secondary to acetaminophen overdose. Her Model for End-Stage Liver Disease (MELD) score on presentation was 38 (range: 0 to 40 with higher scores indicating increased likelihood of mortality). Her urine drug screen was positive for benzodiazepines and opiates. On hospital Day 2, the primary team consulted psychiatry for a pre-transplant evaluation and consideration of suicidality. Hepatology, toxicology, and transplant surgery services also were consulted.
Because Ms. A was intubated for acute respiratory failure, the initial history was gathered from family, a review of the medical record, consultation with her pharmacy, and collateral from her outpatient physician. Ms. A had been taking
Four days before presenting with acute liver failure, Ms. A had visited another hospital for lethargy. Benzodiazepines and opiates were stopped abruptly, and she was discharged with the recommendation to take acetaminophen for her pain. Approximately 24 hours after returning home, Ms. A began having auditory and visual hallucinations, and she did not sleep for days. She continued to complain of pain and was taking acetaminophen as recommended by the outside hospital. Her husband notes that she was intermittently confused. He was unsure how much acetaminophen she was taking.
Continue to: Her family noted...
Her family noted Ms. A had been diagnosed with bipolar disorder “years ago” but was unable to describe any manic episodes, and Ms. A had been treated only with an antidepressant from her primary care physician. She had persistent low mood and increased sleep since developing chronic back pain that severely limited her functioning. Ms. A attempted suicide once years ago by cutting her wrists. She had 2 prior psychiatric hospitalizations for suicidal ideation and the suicide attempt; however, she had not recently voiced suicidal ideation to her husband or family. She was adherent to psychotropic medications and follow-up appointments. Ms. A is a current smoker. She had used marijuana in the past, but her family denies current use, as well as any alcohol use or illicit substance use.
Ms. A’s diagnosis was consistent with tobacco use disorder and major depressive disorder (MDD). She likely developed withdrawal after abrupt cessation of diazepam, which she had been taking as prescribed for years. There was no evidence at the time of her initial psychiatric evaluation that the acetaminophen overdose was a suicide attempt; however, because Ms. A was intubated and sedated at that time, the consultation team recommended direct observation until she could participate in a risk assessment.
For the pre-transplant psychiatric evaluation, our consultation-liaison team noted Ms. A’s history of MDD, with recent active symptoms, chronic pain, and a past suicide attempt. She was a current tobacco smoker, which increases the risk of post-transplant vascular problems. However, she had been adherent to medications and follow-up, had very close family support, and there was no clear evidence that this acetaminophen ingestion was a suicide attempt. We noted that outpatient psychiatric follow-up and better chronic pain management would be helpful post-transplant. We would have to re-evaluate Ms. A when she was medically stable enough to communicate before making any further recommendations. Due to medical complications that developed after our evaluation, the transplant team noted Ms. A was no longer a transplant candidate.
Fortunately, Ms. A recovered with medical management over the next 2 weeks. She denied any suicidal ideation throughout her hospitalization. She was restarted on an antidepressant and received supportive therapy until discharge. Outpatient psychiatry follow-up and pain management was set up before Ms. A was discharged. Inpatient psychiatric hospitalization was not recommended. Per available records, Ms. A followed up with all outpatient appointments, including with her psychiatrist, since discharge.
Avoiding problems, maximizing outcomes
In addition to medical factors, psychosocial factors may affect the success of LT, although empirical data regarding which factors are most predictive of post-transplant outcomes is lacking, especially in patients with serious mental illness. The goals of a psychosocial pre-transplant evaluation are to promote fairness and equal access to care, maximize optimal outcomes, wisely use scarce resources, and ensure that the potential for benefits outweigh surgical risks to the patient. Identifying potential complicating factors (ie, substance abuse, nonadherence, serious psychopathology) can help guide the medical and psychiatric treatment plan and help minimize preventable problems both before and after transplant.42
Continue to: In patients who have...
In patients who have a history of alcohol use and alcohol liver disease, relapse to alcohol is a significant problem. Relapse rates vary from 10% to 30%.7 The duration of abstinence before LT appears to be a poor predictor of abstinence after LT.43 Polysubstance use also adversely affects outcomes in patients with alcohol liver disease. Approximately one-third of patients with polysubstance use who receive a LT relapse to substance use.44 Coffman et al45 showed that the presence of antisocial behavior and eating disorders may increase the risk of relapse after LT.
The psychiatrist’s role in the setting of LT spans from the pre-transplant assessment to post-transplant management and follow-up. Clarifying specific psychiatric diagnoses, psychosocial factors that need to be addressed before transplant, and substance use diagnoses and treatment recommendations can help the transplant team clearly identify modifiable factors that can affect transplant outcomes.
Bottom Line
Psychiatrists can help patients who are candidates for liver transplantation (LT) by performing a pre-transplant psychosocial assessment to identity factors that might complicate transplantation or recovery. After LT, patients require careful monitoring for psychiatric comorbidities, drug interactions, and other factors that can affect quality of life.
Related Resources
- Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018;53(2):135-144.
- Marcangelo MJ, Crone C. Tools, techniques to assess organ transplant candidates. Current Psychiatry. 2007;6(9):56-66.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Hydromorphone • Dilaudid
Lithium • Eskalith, Lithobid
Tacrolimus • Astagraf XL, Envarsus XR
1. Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives [Article in English, Portuguese]. Einstein (São Paulo). 2015;13(1):149-152.
2. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144-1165.
3. Centers for Disease Control and Prevention. QuickStats: number of deaths from 10 leading causes,* by sex—National Vital Statistics System, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(15):413.
4. Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31(1):84-89.
5. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59.
6. Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123-132.
7. Grover S, Sarkar S. Liver transplant—psychiatric and psychosocial aspects. J Clin Exp Hepatol. 2012;2(4):382-392.
8. Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760-770.
9. Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824-834.
10. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497-1504.
11. De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323.
12. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172-183.
13. Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718-1726.
14. Graziadei I, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 2016;128(19):679-690.
15. Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981-987.
16. Lee MR, Leggio L. Management of alcohol use disorder in patients requiring liver transplant. Am J Psychiatry. 2015;172(12):1182-1189.
17. Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the Sustained Alcohol Use Post-Liver Transplant score. Hepatology. 2019;69(4):1477-1487.
18. DiMartini A, Crone C, Dew MA. Alcohol and substance use in liver transplant patients. Clinics in Liver Disease. 2011;15(4):727-751.
19. Pungpapong S, Manzarbeitia C, Ortiz J, et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582-587.
20. Kreek MJ. Pharmacotherapy of opioid dependence: rationale and update. Regulatory Peptides. 1994;53(suppl 1):S255-S256.
21. Jiao M, Greanya ED, Haque M, et al. Methadone maintenance therapy in liver transplantation. Prog Transplant. 2010;20(3):209-214; quiz 215.
22. Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91.
23. Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63-71.
24. Kotwani P, Saxena V, Dodge JL, et al. History of marijuana use does not affect outcomes on the liver transplant waitlist. Transplantation. 2018;102(5):794-802.
25. Fineberg SK, West A, Na PJ, et al. Utility of pretransplant psychological measures to predict posttransplant outcomes in liver transplant patients: a systematic review. Gen Hospl Psychiatry. 2016;40:4-11.
26. Satapathy S, Sanyal A. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
27. Olbrisch ME, Levenson JL, Hamer R. The PACT: a rating scale for the study of clinical decision making in psychosocial screening of organ transplant candidates. Clin Transplant. 1989;3:164-169.
28. Twillman RK, Manetto C, Wellisch DK, et al. Transplant Evaluation Rating Scale: a revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153.
29. Goodier J. Evaluating Stress:97496. In: Zalaquett CP, Wood RJ, eds. Evaluating stress: a book of resources. Lanham, MD: Scarecrow Press; 1997:29-29.
30. Beck AT, Steer RA, Carbin, MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8(1):77-100.
31. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242.
32. Cottle WC. The MMPI: a review. Lawrence, KS: University of Kansas; 1953.
33. Addison CC, Campbell-Jenkins BW, Sarpong DF, et al. Psychometric Evaluation of a Coping Strategies Inventory Short-Form (CSI-SF) in the Jackson Heart Study Cohort. Int J Environ Res Public Health. 2007;4(4):289-295.
34. Mooney S, Hasssanein T, Hilsabeck R, et al. Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175-186.
35. De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33(4):609-615.
36. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42(4):337-343.
37. Landefeld CS, Palme, RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344.
38. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric problems in living-related transplantation (II): the association between paradoxical psychiatric syndrome and guilt feelings in adult recipients after living donor liver transplantation. Transplantation Proceedings. 2002;34(7):2632-2633.
39. Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet. 1996;30(2):141-179.
40. Ozkanlar Y, Nishijima Y, Cunha DD, et al. Acute effects of tacrolimus (FK506) on left ventricular mechanics. Pharmacol Res. 2005;52(4):307-312.
41. Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826-833.
42. Maldonado J R. I have been asked to work up a patient who requires a liver transplant how should I proceed? FOCUS. 2009;7(3):332-335.
43. Mccallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol and Alcoholism. 2006;41(4):358-363.
44. Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680-685.
45. Coffman KL, Hoffman A, Sher L, et al. Treatment of the postoperative alcoholic liver transplant recipient with other addictions. Liver Transpl Surg. 1997;3(3):322-327.
Since the first liver transplant (LT) was performed in 1963 by Starzl et al, there have been considerable advances in the field, with improvements in post-transplant survival.1 There are multiple indications for LT, including acute liver failure and index complications of cirrhosis such as ascites, encephalopathy, and hepatopulmonary syndrome.2 Once a patient develops one of these conditions, he/she is evaluated for LT, even as the complications of liver failure are being managed.
Although the number of LTs has risen, the demand for transplant continues to exceed availability. In 2015, chronic liver disease and cirrhosis was the 12th leading cause of death in the United States.3 In 2016, approximately 50% of waitlisted candidates received a transplant.4 There is also a donor shortage. In part, this shortage may be due to longer life spans and the subsequent increase in the age of the potential donor.5 In light of this shortage and increased demand, the pre-LT workup is comprehensive. The pre-transplant assessment typically consists of cardiology, surgery, hepatology, and psychosocial evaluations, and hence requires a team of experts to determine who is an ideal candidate for transplant.
Psychiatrists play a key role in the pre-transplant psychosocial evaluations. This article describes the elements of these evaluations, and what psychiatrists can do to help patients both before and after they undergo LT.
Elements of the pre-transplant evaluation
The psychosocial evaluation is a critical component of the pre-transplant assessment. As part of the evaluation, patients are screened for psychosocial limitations that may complicate transplantation, such as demonstrated noncompliance, ongoing alcohol or drug use, and lack of social support (Table 12 ). Other goals of the psychosocial evaluation are to identify in the pre-transplant period patients with possible risk factors, such as substance use or psychiatric disorders, and develop treatment plans to optimize transplant outcomes (Table 26). There are relative contraindications to LT (Table 37) but no absolute psychiatric contraindications, according to the 2013 American Association for the Study of Liver Diseases (AASLD) practice guideline for transplantation.2
Adherence. The 2013 AASLD practice guideline states that patients “should be evaluated for and meet reasonable expectations for adherence to medical directives and mental health stability as determined by the psychosocial evaluation.”2 In the transplant setting, adherence is complex. It requires compliance with complicated medication regimens and laboratory testing, frequent follow-up appointments, and close, prompt communication of concerns to the health care team. Patient adherence to medication regimens plays an important role in transplant outcomes.8 In fact, in patients who have undergone renal transplant, nonadherence to therapy is considered the leading cause of avoidable graft failure.9
A retrospective study of adult LT recipients found that pre-transplant chart evidence of nonadherence, such as missed laboratory testing and clinic visits, was a significant predictor of post-transplant nonadherence with immunosuppressant therapy. Pre-transplant unemployment status and a history of substance abuse also were associated with nonadherence.9
Dobbels et al10 found that patients with a self-reported history of pre-transplant non-adherence had a higher risk of being nonadherent with their immunosuppressive therapy after transplant (odd ratio [OR]: 7.9). Their self-report adherence questionnaire included questions that addressed pre-transplant smoking status, alcohol use, and adherence with medication. In this prospective study, researchers also found that patients with a low “conscientiousness” score were at a higher risk for post-transplant medication nonadherence (OR: 0.8).
Continue to: Studies have also found...
Studies have also found that patients with higher education are more at risk for post-transplant medication nonadherence. Higher education may be associated with higher employment status resulting in a busier lifestyle, a known risk factor that may prevent patients from regular medication adherence.11,12 Alternatively, it is possible that higher educated patients are “decisive” nonadherers who prefer independent decision-making regarding their disease and treatment.13
Substance use. The 2013 AASLD practice guideline lists “ongoing alcohol or illicit substance abuse” as one of the contraindications to LT.2 In guidelines from the Austrian Society for Gastroenterology and Hepatology, Graziadei et al14 listed “alcohol addiction without motivation for alcohol abstinence and untreated/ongoing substance abuse” as absolute contraindications and “untreated alcohol abuse and other drug-related addiction” as relative contraindications. Hence, the pre-transplant evaluation should include a thorough substance use history, including duration, amount, previous attempts to quit, and motivation for abstinence.
Substance use history is especially important because alcoholic liver disease is the second most common indication for LT.2 Most LT programs require 6 months of abstinence before a patient can be considered for transplant.15 The 6-month period was based on studies demonstrating that pre-transplant abstinence from alcohol for <6 months is a risk factor for relapse.15 However, this guideline remains controversial because the transplant referral and workup may be delayed as the patient’s liver disease worsens. Other risk factors for substance relapse should also be taken into consideration, such as depression, personality disorders, lack of social support, severity of alcohol use, and family history of alcoholism.16 Lee and Leggio16 developed the Sustained Alcohol Use Post-Liver Transplant (SALT) score to identify patients who were at risk for sustained alcohol use posttransplant. The 4 SALT criteria are:
- >10 drinks per day at initial hospitalization (+4 points)
- multiple prior rehabilitation attempts (+4 points)
- prior alcohol‐related legal issues (+2 points), and
- prior illicit substance abuse (+1 point).
A SALT score can range from 0 to 11. Lee et al17 found a SALT score ≥5 had a 25% positive predictive value (95% confidence interval [CI]: 10% to 47%) and a SALT score of <5 had a 95% negative predictive value (95% CI: 89% to 98%) for sustained alcohol use post‐LT. Thus, the 2013 AASLD guideline cautions against delaying evaluation based on the 6-month abstinence rule, and instead recommends early transplant referral for patients with alcoholic liver disease to encourage such patients to begin addiction treatment.2
As part of the substance use history, it is important to ask about the patient’s smoking history. Approximately 60% of LT candidates have a history of smoking cigarettes.18 Tobacco use history is associated with increased post-transplant vascular complications, such as hepatic artery thrombosis or stenosis, portal vein thrombosis, and deep vein thrombosis.19 The 2013 AASLD guideline recommends that tobacco use should be prohibited in LT candidates.2 Pungpapong et al19 reported that smoking cessation for at least 2 years prior to transplant led to a significantly decreased risk of developing arterial complications, with an absolute risk reduction of approximately 16%.
Continue to: Liver cirrhosis due to...
Liver cirrhosis due to chronic hepatitis C virus (HCV) infection is one of the leading causes for LT. In the United States, HCV is commonly transmitted during injection drug use. According to the 2013 AASLD guideline, ongoing illicit substance use is a relative contraindication to LT.2 It is important to note, however, that methadone maintenance therapy (MMT) is not a contraindication to LT. In fact, the 2013 AASLD guideline recommends that patients receiving MMT should not be required to reduce or stop therapy in order to be listed for transplant.2 Studies have shown that in 80% of patients, tapering MMT leads to illicit opiate relapse.20 Currently, there is no evidence that patients receiving MMT have poorer post-transplant outcomes compared with patients not receiving MMT.21
Whether cannabis use is a relative contraindication to LT remains controversial.22 Possible adverse effects of cannabis use in transplant patients include drug–drug interactions and infections. Hézode et al23 reported that daily cannabis use is significantly associated with an increased fibrosis progression rate in patients with chronic HCV infection. Another recent study found that a history of cannabis use was not associated with worse outcomes among patients on the LT waitlist.24 With the increased legalization of cannabis, more studies are needed to assess ongoing cannabis use in patients on the LT waitlist and post-LT outcomes.
Psychiatric history. When assessing a patient for possible LT, no psychiatric disorder is considered an absolute contraindication. Patients with a serious mental illness, such as schizophrenia, and those with intellectual disability can have successful, long-term outcomes with proper evaluation and preparation, including social support. However, empirical literature regarding transplant outcomes and predictive factors in patients with serious mental illness is scarce.2
Studies examining the predictive value of pre-transplant depression on post-transplant outcomes have had mixed results.25 Depression may predict lower post-transplant quality of life. Pre-LT suicidal thoughts (as noted on the Beck Depression Inventory, for example) are associated with post-LT depression.25 In contrast, available data show no significant effect of pre-transplant anxiety on post-LT outcomes. Similarly, pre-transplant cognitive performance appears not to predict survival or other post-transplant outcomes, but may predict poorer quality of life after transplant.25
A few psychiatric factors are considered relative contraindications for LT. These include severe personality disorders, active substance use with no motivation for treatment or abstinence, active psychosis, severe neurocognitive disorders, suicidality, and factitious disorder.7
Continue to: Social support
Social support. Assessing a pre-LT patient’s level of social support is an essential part of the psychosocial evaluation. According to the 2013 AASLD guideline, patients should have “adequate” social support both during the waitlist and post-operative periods.2 Lack of partnership is a significant predictor of poor post-transplant outcomes, such as late graft loss.10 Satapathy and Sanyal26 reported that among patients who receive an LT for alcoholic liver disease, those with immediate family support were less likely to relapse to using alcohol after transplant. Poor social support was also a predictor of post-transplant medication nonadherence.10 Thus, the patient needs enough social support to engage in the pre-transplant health care requirements and to participate in post-transplant recommendations until he/she is functioning independently post-transplant.
Screening tools
Various screening tools may be useful in a pre-LT evaluation. Three standardized assessment tools available specifically for pre-transplant psychosocial assessments are the Stanford Integrated Psychosocial Assessment for Transplantation (Table 26), the Psychosocial Assessment of Candidates for Transplantation,27 and the Transplant Evaluation Rating Scale.28 Instruments to aid in the assessment of depression, anxiety, and delirium,29-31 a structured personality assessment,32 coping inventories,33 neuropsychological batteries,34 and others also have been used to evaluate patients before LT. The self-rated Beck Depression Inventory and the clinician-rated Hamilton Depression Rating Scale are commonly used.7 Other tools, such as the LEIPAD quality of life instrument and the Brief Symptom Inventory (BSI), have been used to assess for perceived quality of life and psychological distress, respectively.35 These screening tools can be helpful as aids for the pre-LT evaluation; however, diagnoses and treatment plan recommendations require a psychiatric evaluation conducted by a trained clinician.
Treatment after liver transplant
Psychiatric issues. After LT, various psychiatric complications may arise, including (but not limited to) delirium7 and “paradoxical psychiatric syndrome” (PPS).36 Delirium can be managed by administering low-dose antipsychotic medications, limiting the use of benzodiazepines and medications with anticholinergic effects, implementing behavioral interventions (frequent orientation, maintaining sleep/wake cycle, limiting noise, presence of a family member or a sitter at bedside),37 and addressing the underlying etiology. Paradoxical psychiatric syndrome is defined as psychiatric symptoms that occur despite a successful LT. It develops within the first year of transplantation and is characterized by recipients having strong guilt feelings toward their donors.38
Drug interactions. In the post-transplant period, antipsychotics are used for management of delirium and psychosis, antidepressants for anxiety and depression, and benzodiazepines for anxiety and sleep problems.7 Drug–drug interactions between psychotropic medications and the immunosuppressants required after LT must be closely monitored. First-generation antipsychotics should be avoided in post-transplant patients taking tacrolimus due to the increased risk of QTc prolongation. Tacrolimus can also increase the risk of nephrotoxicity when co-administered with lithium. Post-LT patients taking steroids and bupropion have an increased risk of seizure. Carbamazepine may decrease blood levels of cyclosporine due to the induction of hepatic metabolism.39,40 The psychiatrist should review and update the patient’s complete medication list at each visit, checking for possible medication interactions.
Quality of life. In the first 6 months post-transplant, patients typically experience improved quality of life in both physical and psychological domains. However, this improvement vacillates as the patient adjusts to post-transplant life. A reduction in BSI score 1 year after transplant has been reported. The BSI evaluates psychopathological symptoms, which are early indicators of psychological discomfort. One study noted a reduction in the LEIPAD quality of life score, which measures overall quality of life, 2 years after transplant.35 This decline may reflect the difficulties associated with the new challenges after transplant. Patients may endure both physical changes due to medical complications as well as psychological problems as they adjust to their new bodily integrity, their dependence on medications and medical staff, and other changes in function. Three to 5 years after transplant, patients reached a new psychological stability, with reported improvements in quality of life and decreased psychological distress.35
Continue to: Special populations
Special populations
HCV infection. Recurrent HCV infection and liver disease after transplantation are associated with psychological distress. This is particularly evident in patients 6 months after transplantation. Depression and psychological distress have been reported in male patients with recurrent HCV infection within the first year after transplantation.35
Acetaminophen overdose. Patients who receive a transplant for acetaminophen-induced acute liver failure (ALF) had a greater prevalence of psychiatric comorbidity as reflected by predefined diagnoses, medication, and previous suicide attempts.41 Despite this, outcomes for patients transplanted emergently for acetaminophen-induced ALF were comparable to those transplanted for non-acetaminophen-induced ALF and for chronic liver disease. Multidisciplinary approaches with long-term psychiatric follow-up may contribute to low post-transplant suicide rates and low rates of graft loss because of noncompliance.41
CASE REPORT
A complicated presentation
Ms. A, age 45, a married woman with history of chronic back pain and self-reported bipolar disorder, presented to our hospital with acute liver failure secondary to acetaminophen overdose. Her Model for End-Stage Liver Disease (MELD) score on presentation was 38 (range: 0 to 40 with higher scores indicating increased likelihood of mortality). Her urine drug screen was positive for benzodiazepines and opiates. On hospital Day 2, the primary team consulted psychiatry for a pre-transplant evaluation and consideration of suicidality. Hepatology, toxicology, and transplant surgery services also were consulted.
Because Ms. A was intubated for acute respiratory failure, the initial history was gathered from family, a review of the medical record, consultation with her pharmacy, and collateral from her outpatient physician. Ms. A had been taking
Four days before presenting with acute liver failure, Ms. A had visited another hospital for lethargy. Benzodiazepines and opiates were stopped abruptly, and she was discharged with the recommendation to take acetaminophen for her pain. Approximately 24 hours after returning home, Ms. A began having auditory and visual hallucinations, and she did not sleep for days. She continued to complain of pain and was taking acetaminophen as recommended by the outside hospital. Her husband notes that she was intermittently confused. He was unsure how much acetaminophen she was taking.
Continue to: Her family noted...
Her family noted Ms. A had been diagnosed with bipolar disorder “years ago” but was unable to describe any manic episodes, and Ms. A had been treated only with an antidepressant from her primary care physician. She had persistent low mood and increased sleep since developing chronic back pain that severely limited her functioning. Ms. A attempted suicide once years ago by cutting her wrists. She had 2 prior psychiatric hospitalizations for suicidal ideation and the suicide attempt; however, she had not recently voiced suicidal ideation to her husband or family. She was adherent to psychotropic medications and follow-up appointments. Ms. A is a current smoker. She had used marijuana in the past, but her family denies current use, as well as any alcohol use or illicit substance use.
Ms. A’s diagnosis was consistent with tobacco use disorder and major depressive disorder (MDD). She likely developed withdrawal after abrupt cessation of diazepam, which she had been taking as prescribed for years. There was no evidence at the time of her initial psychiatric evaluation that the acetaminophen overdose was a suicide attempt; however, because Ms. A was intubated and sedated at that time, the consultation team recommended direct observation until she could participate in a risk assessment.
For the pre-transplant psychiatric evaluation, our consultation-liaison team noted Ms. A’s history of MDD, with recent active symptoms, chronic pain, and a past suicide attempt. She was a current tobacco smoker, which increases the risk of post-transplant vascular problems. However, she had been adherent to medications and follow-up, had very close family support, and there was no clear evidence that this acetaminophen ingestion was a suicide attempt. We noted that outpatient psychiatric follow-up and better chronic pain management would be helpful post-transplant. We would have to re-evaluate Ms. A when she was medically stable enough to communicate before making any further recommendations. Due to medical complications that developed after our evaluation, the transplant team noted Ms. A was no longer a transplant candidate.
Fortunately, Ms. A recovered with medical management over the next 2 weeks. She denied any suicidal ideation throughout her hospitalization. She was restarted on an antidepressant and received supportive therapy until discharge. Outpatient psychiatry follow-up and pain management was set up before Ms. A was discharged. Inpatient psychiatric hospitalization was not recommended. Per available records, Ms. A followed up with all outpatient appointments, including with her psychiatrist, since discharge.
Avoiding problems, maximizing outcomes
In addition to medical factors, psychosocial factors may affect the success of LT, although empirical data regarding which factors are most predictive of post-transplant outcomes is lacking, especially in patients with serious mental illness. The goals of a psychosocial pre-transplant evaluation are to promote fairness and equal access to care, maximize optimal outcomes, wisely use scarce resources, and ensure that the potential for benefits outweigh surgical risks to the patient. Identifying potential complicating factors (ie, substance abuse, nonadherence, serious psychopathology) can help guide the medical and psychiatric treatment plan and help minimize preventable problems both before and after transplant.42
Continue to: In patients who have...
In patients who have a history of alcohol use and alcohol liver disease, relapse to alcohol is a significant problem. Relapse rates vary from 10% to 30%.7 The duration of abstinence before LT appears to be a poor predictor of abstinence after LT.43 Polysubstance use also adversely affects outcomes in patients with alcohol liver disease. Approximately one-third of patients with polysubstance use who receive a LT relapse to substance use.44 Coffman et al45 showed that the presence of antisocial behavior and eating disorders may increase the risk of relapse after LT.
The psychiatrist’s role in the setting of LT spans from the pre-transplant assessment to post-transplant management and follow-up. Clarifying specific psychiatric diagnoses, psychosocial factors that need to be addressed before transplant, and substance use diagnoses and treatment recommendations can help the transplant team clearly identify modifiable factors that can affect transplant outcomes.
Bottom Line
Psychiatrists can help patients who are candidates for liver transplantation (LT) by performing a pre-transplant psychosocial assessment to identity factors that might complicate transplantation or recovery. After LT, patients require careful monitoring for psychiatric comorbidities, drug interactions, and other factors that can affect quality of life.
Related Resources
- Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018;53(2):135-144.
- Marcangelo MJ, Crone C. Tools, techniques to assess organ transplant candidates. Current Psychiatry. 2007;6(9):56-66.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Hydromorphone • Dilaudid
Lithium • Eskalith, Lithobid
Tacrolimus • Astagraf XL, Envarsus XR
Since the first liver transplant (LT) was performed in 1963 by Starzl et al, there have been considerable advances in the field, with improvements in post-transplant survival.1 There are multiple indications for LT, including acute liver failure and index complications of cirrhosis such as ascites, encephalopathy, and hepatopulmonary syndrome.2 Once a patient develops one of these conditions, he/she is evaluated for LT, even as the complications of liver failure are being managed.
Although the number of LTs has risen, the demand for transplant continues to exceed availability. In 2015, chronic liver disease and cirrhosis was the 12th leading cause of death in the United States.3 In 2016, approximately 50% of waitlisted candidates received a transplant.4 There is also a donor shortage. In part, this shortage may be due to longer life spans and the subsequent increase in the age of the potential donor.5 In light of this shortage and increased demand, the pre-LT workup is comprehensive. The pre-transplant assessment typically consists of cardiology, surgery, hepatology, and psychosocial evaluations, and hence requires a team of experts to determine who is an ideal candidate for transplant.
Psychiatrists play a key role in the pre-transplant psychosocial evaluations. This article describes the elements of these evaluations, and what psychiatrists can do to help patients both before and after they undergo LT.
Elements of the pre-transplant evaluation
The psychosocial evaluation is a critical component of the pre-transplant assessment. As part of the evaluation, patients are screened for psychosocial limitations that may complicate transplantation, such as demonstrated noncompliance, ongoing alcohol or drug use, and lack of social support (Table 12 ). Other goals of the psychosocial evaluation are to identify in the pre-transplant period patients with possible risk factors, such as substance use or psychiatric disorders, and develop treatment plans to optimize transplant outcomes (Table 26). There are relative contraindications to LT (Table 37) but no absolute psychiatric contraindications, according to the 2013 American Association for the Study of Liver Diseases (AASLD) practice guideline for transplantation.2
Adherence. The 2013 AASLD practice guideline states that patients “should be evaluated for and meet reasonable expectations for adherence to medical directives and mental health stability as determined by the psychosocial evaluation.”2 In the transplant setting, adherence is complex. It requires compliance with complicated medication regimens and laboratory testing, frequent follow-up appointments, and close, prompt communication of concerns to the health care team. Patient adherence to medication regimens plays an important role in transplant outcomes.8 In fact, in patients who have undergone renal transplant, nonadherence to therapy is considered the leading cause of avoidable graft failure.9
A retrospective study of adult LT recipients found that pre-transplant chart evidence of nonadherence, such as missed laboratory testing and clinic visits, was a significant predictor of post-transplant nonadherence with immunosuppressant therapy. Pre-transplant unemployment status and a history of substance abuse also were associated with nonadherence.9
Dobbels et al10 found that patients with a self-reported history of pre-transplant non-adherence had a higher risk of being nonadherent with their immunosuppressive therapy after transplant (odd ratio [OR]: 7.9). Their self-report adherence questionnaire included questions that addressed pre-transplant smoking status, alcohol use, and adherence with medication. In this prospective study, researchers also found that patients with a low “conscientiousness” score were at a higher risk for post-transplant medication nonadherence (OR: 0.8).
Continue to: Studies have also found...
Studies have also found that patients with higher education are more at risk for post-transplant medication nonadherence. Higher education may be associated with higher employment status resulting in a busier lifestyle, a known risk factor that may prevent patients from regular medication adherence.11,12 Alternatively, it is possible that higher educated patients are “decisive” nonadherers who prefer independent decision-making regarding their disease and treatment.13
Substance use. The 2013 AASLD practice guideline lists “ongoing alcohol or illicit substance abuse” as one of the contraindications to LT.2 In guidelines from the Austrian Society for Gastroenterology and Hepatology, Graziadei et al14 listed “alcohol addiction without motivation for alcohol abstinence and untreated/ongoing substance abuse” as absolute contraindications and “untreated alcohol abuse and other drug-related addiction” as relative contraindications. Hence, the pre-transplant evaluation should include a thorough substance use history, including duration, amount, previous attempts to quit, and motivation for abstinence.
Substance use history is especially important because alcoholic liver disease is the second most common indication for LT.2 Most LT programs require 6 months of abstinence before a patient can be considered for transplant.15 The 6-month period was based on studies demonstrating that pre-transplant abstinence from alcohol for <6 months is a risk factor for relapse.15 However, this guideline remains controversial because the transplant referral and workup may be delayed as the patient’s liver disease worsens. Other risk factors for substance relapse should also be taken into consideration, such as depression, personality disorders, lack of social support, severity of alcohol use, and family history of alcoholism.16 Lee and Leggio16 developed the Sustained Alcohol Use Post-Liver Transplant (SALT) score to identify patients who were at risk for sustained alcohol use posttransplant. The 4 SALT criteria are:
- >10 drinks per day at initial hospitalization (+4 points)
- multiple prior rehabilitation attempts (+4 points)
- prior alcohol‐related legal issues (+2 points), and
- prior illicit substance abuse (+1 point).
A SALT score can range from 0 to 11. Lee et al17 found a SALT score ≥5 had a 25% positive predictive value (95% confidence interval [CI]: 10% to 47%) and a SALT score of <5 had a 95% negative predictive value (95% CI: 89% to 98%) for sustained alcohol use post‐LT. Thus, the 2013 AASLD guideline cautions against delaying evaluation based on the 6-month abstinence rule, and instead recommends early transplant referral for patients with alcoholic liver disease to encourage such patients to begin addiction treatment.2
As part of the substance use history, it is important to ask about the patient’s smoking history. Approximately 60% of LT candidates have a history of smoking cigarettes.18 Tobacco use history is associated with increased post-transplant vascular complications, such as hepatic artery thrombosis or stenosis, portal vein thrombosis, and deep vein thrombosis.19 The 2013 AASLD guideline recommends that tobacco use should be prohibited in LT candidates.2 Pungpapong et al19 reported that smoking cessation for at least 2 years prior to transplant led to a significantly decreased risk of developing arterial complications, with an absolute risk reduction of approximately 16%.
Continue to: Liver cirrhosis due to...
Liver cirrhosis due to chronic hepatitis C virus (HCV) infection is one of the leading causes for LT. In the United States, HCV is commonly transmitted during injection drug use. According to the 2013 AASLD guideline, ongoing illicit substance use is a relative contraindication to LT.2 It is important to note, however, that methadone maintenance therapy (MMT) is not a contraindication to LT. In fact, the 2013 AASLD guideline recommends that patients receiving MMT should not be required to reduce or stop therapy in order to be listed for transplant.2 Studies have shown that in 80% of patients, tapering MMT leads to illicit opiate relapse.20 Currently, there is no evidence that patients receiving MMT have poorer post-transplant outcomes compared with patients not receiving MMT.21
Whether cannabis use is a relative contraindication to LT remains controversial.22 Possible adverse effects of cannabis use in transplant patients include drug–drug interactions and infections. Hézode et al23 reported that daily cannabis use is significantly associated with an increased fibrosis progression rate in patients with chronic HCV infection. Another recent study found that a history of cannabis use was not associated with worse outcomes among patients on the LT waitlist.24 With the increased legalization of cannabis, more studies are needed to assess ongoing cannabis use in patients on the LT waitlist and post-LT outcomes.
Psychiatric history. When assessing a patient for possible LT, no psychiatric disorder is considered an absolute contraindication. Patients with a serious mental illness, such as schizophrenia, and those with intellectual disability can have successful, long-term outcomes with proper evaluation and preparation, including social support. However, empirical literature regarding transplant outcomes and predictive factors in patients with serious mental illness is scarce.2
Studies examining the predictive value of pre-transplant depression on post-transplant outcomes have had mixed results.25 Depression may predict lower post-transplant quality of life. Pre-LT suicidal thoughts (as noted on the Beck Depression Inventory, for example) are associated with post-LT depression.25 In contrast, available data show no significant effect of pre-transplant anxiety on post-LT outcomes. Similarly, pre-transplant cognitive performance appears not to predict survival or other post-transplant outcomes, but may predict poorer quality of life after transplant.25
A few psychiatric factors are considered relative contraindications for LT. These include severe personality disorders, active substance use with no motivation for treatment or abstinence, active psychosis, severe neurocognitive disorders, suicidality, and factitious disorder.7
Continue to: Social support
Social support. Assessing a pre-LT patient’s level of social support is an essential part of the psychosocial evaluation. According to the 2013 AASLD guideline, patients should have “adequate” social support both during the waitlist and post-operative periods.2 Lack of partnership is a significant predictor of poor post-transplant outcomes, such as late graft loss.10 Satapathy and Sanyal26 reported that among patients who receive an LT for alcoholic liver disease, those with immediate family support were less likely to relapse to using alcohol after transplant. Poor social support was also a predictor of post-transplant medication nonadherence.10 Thus, the patient needs enough social support to engage in the pre-transplant health care requirements and to participate in post-transplant recommendations until he/she is functioning independently post-transplant.
Screening tools
Various screening tools may be useful in a pre-LT evaluation. Three standardized assessment tools available specifically for pre-transplant psychosocial assessments are the Stanford Integrated Psychosocial Assessment for Transplantation (Table 26), the Psychosocial Assessment of Candidates for Transplantation,27 and the Transplant Evaluation Rating Scale.28 Instruments to aid in the assessment of depression, anxiety, and delirium,29-31 a structured personality assessment,32 coping inventories,33 neuropsychological batteries,34 and others also have been used to evaluate patients before LT. The self-rated Beck Depression Inventory and the clinician-rated Hamilton Depression Rating Scale are commonly used.7 Other tools, such as the LEIPAD quality of life instrument and the Brief Symptom Inventory (BSI), have been used to assess for perceived quality of life and psychological distress, respectively.35 These screening tools can be helpful as aids for the pre-LT evaluation; however, diagnoses and treatment plan recommendations require a psychiatric evaluation conducted by a trained clinician.
Treatment after liver transplant
Psychiatric issues. After LT, various psychiatric complications may arise, including (but not limited to) delirium7 and “paradoxical psychiatric syndrome” (PPS).36 Delirium can be managed by administering low-dose antipsychotic medications, limiting the use of benzodiazepines and medications with anticholinergic effects, implementing behavioral interventions (frequent orientation, maintaining sleep/wake cycle, limiting noise, presence of a family member or a sitter at bedside),37 and addressing the underlying etiology. Paradoxical psychiatric syndrome is defined as psychiatric symptoms that occur despite a successful LT. It develops within the first year of transplantation and is characterized by recipients having strong guilt feelings toward their donors.38
Drug interactions. In the post-transplant period, antipsychotics are used for management of delirium and psychosis, antidepressants for anxiety and depression, and benzodiazepines for anxiety and sleep problems.7 Drug–drug interactions between psychotropic medications and the immunosuppressants required after LT must be closely monitored. First-generation antipsychotics should be avoided in post-transplant patients taking tacrolimus due to the increased risk of QTc prolongation. Tacrolimus can also increase the risk of nephrotoxicity when co-administered with lithium. Post-LT patients taking steroids and bupropion have an increased risk of seizure. Carbamazepine may decrease blood levels of cyclosporine due to the induction of hepatic metabolism.39,40 The psychiatrist should review and update the patient’s complete medication list at each visit, checking for possible medication interactions.
Quality of life. In the first 6 months post-transplant, patients typically experience improved quality of life in both physical and psychological domains. However, this improvement vacillates as the patient adjusts to post-transplant life. A reduction in BSI score 1 year after transplant has been reported. The BSI evaluates psychopathological symptoms, which are early indicators of psychological discomfort. One study noted a reduction in the LEIPAD quality of life score, which measures overall quality of life, 2 years after transplant.35 This decline may reflect the difficulties associated with the new challenges after transplant. Patients may endure both physical changes due to medical complications as well as psychological problems as they adjust to their new bodily integrity, their dependence on medications and medical staff, and other changes in function. Three to 5 years after transplant, patients reached a new psychological stability, with reported improvements in quality of life and decreased psychological distress.35
Continue to: Special populations
Special populations
HCV infection. Recurrent HCV infection and liver disease after transplantation are associated with psychological distress. This is particularly evident in patients 6 months after transplantation. Depression and psychological distress have been reported in male patients with recurrent HCV infection within the first year after transplantation.35
Acetaminophen overdose. Patients who receive a transplant for acetaminophen-induced acute liver failure (ALF) had a greater prevalence of psychiatric comorbidity as reflected by predefined diagnoses, medication, and previous suicide attempts.41 Despite this, outcomes for patients transplanted emergently for acetaminophen-induced ALF were comparable to those transplanted for non-acetaminophen-induced ALF and for chronic liver disease. Multidisciplinary approaches with long-term psychiatric follow-up may contribute to low post-transplant suicide rates and low rates of graft loss because of noncompliance.41
CASE REPORT
A complicated presentation
Ms. A, age 45, a married woman with history of chronic back pain and self-reported bipolar disorder, presented to our hospital with acute liver failure secondary to acetaminophen overdose. Her Model for End-Stage Liver Disease (MELD) score on presentation was 38 (range: 0 to 40 with higher scores indicating increased likelihood of mortality). Her urine drug screen was positive for benzodiazepines and opiates. On hospital Day 2, the primary team consulted psychiatry for a pre-transplant evaluation and consideration of suicidality. Hepatology, toxicology, and transplant surgery services also were consulted.
Because Ms. A was intubated for acute respiratory failure, the initial history was gathered from family, a review of the medical record, consultation with her pharmacy, and collateral from her outpatient physician. Ms. A had been taking
Four days before presenting with acute liver failure, Ms. A had visited another hospital for lethargy. Benzodiazepines and opiates were stopped abruptly, and she was discharged with the recommendation to take acetaminophen for her pain. Approximately 24 hours after returning home, Ms. A began having auditory and visual hallucinations, and she did not sleep for days. She continued to complain of pain and was taking acetaminophen as recommended by the outside hospital. Her husband notes that she was intermittently confused. He was unsure how much acetaminophen she was taking.
Continue to: Her family noted...
Her family noted Ms. A had been diagnosed with bipolar disorder “years ago” but was unable to describe any manic episodes, and Ms. A had been treated only with an antidepressant from her primary care physician. She had persistent low mood and increased sleep since developing chronic back pain that severely limited her functioning. Ms. A attempted suicide once years ago by cutting her wrists. She had 2 prior psychiatric hospitalizations for suicidal ideation and the suicide attempt; however, she had not recently voiced suicidal ideation to her husband or family. She was adherent to psychotropic medications and follow-up appointments. Ms. A is a current smoker. She had used marijuana in the past, but her family denies current use, as well as any alcohol use or illicit substance use.
Ms. A’s diagnosis was consistent with tobacco use disorder and major depressive disorder (MDD). She likely developed withdrawal after abrupt cessation of diazepam, which she had been taking as prescribed for years. There was no evidence at the time of her initial psychiatric evaluation that the acetaminophen overdose was a suicide attempt; however, because Ms. A was intubated and sedated at that time, the consultation team recommended direct observation until she could participate in a risk assessment.
For the pre-transplant psychiatric evaluation, our consultation-liaison team noted Ms. A’s history of MDD, with recent active symptoms, chronic pain, and a past suicide attempt. She was a current tobacco smoker, which increases the risk of post-transplant vascular problems. However, she had been adherent to medications and follow-up, had very close family support, and there was no clear evidence that this acetaminophen ingestion was a suicide attempt. We noted that outpatient psychiatric follow-up and better chronic pain management would be helpful post-transplant. We would have to re-evaluate Ms. A when she was medically stable enough to communicate before making any further recommendations. Due to medical complications that developed after our evaluation, the transplant team noted Ms. A was no longer a transplant candidate.
Fortunately, Ms. A recovered with medical management over the next 2 weeks. She denied any suicidal ideation throughout her hospitalization. She was restarted on an antidepressant and received supportive therapy until discharge. Outpatient psychiatry follow-up and pain management was set up before Ms. A was discharged. Inpatient psychiatric hospitalization was not recommended. Per available records, Ms. A followed up with all outpatient appointments, including with her psychiatrist, since discharge.
Avoiding problems, maximizing outcomes
In addition to medical factors, psychosocial factors may affect the success of LT, although empirical data regarding which factors are most predictive of post-transplant outcomes is lacking, especially in patients with serious mental illness. The goals of a psychosocial pre-transplant evaluation are to promote fairness and equal access to care, maximize optimal outcomes, wisely use scarce resources, and ensure that the potential for benefits outweigh surgical risks to the patient. Identifying potential complicating factors (ie, substance abuse, nonadherence, serious psychopathology) can help guide the medical and psychiatric treatment plan and help minimize preventable problems both before and after transplant.42
Continue to: In patients who have...
In patients who have a history of alcohol use and alcohol liver disease, relapse to alcohol is a significant problem. Relapse rates vary from 10% to 30%.7 The duration of abstinence before LT appears to be a poor predictor of abstinence after LT.43 Polysubstance use also adversely affects outcomes in patients with alcohol liver disease. Approximately one-third of patients with polysubstance use who receive a LT relapse to substance use.44 Coffman et al45 showed that the presence of antisocial behavior and eating disorders may increase the risk of relapse after LT.
The psychiatrist’s role in the setting of LT spans from the pre-transplant assessment to post-transplant management and follow-up. Clarifying specific psychiatric diagnoses, psychosocial factors that need to be addressed before transplant, and substance use diagnoses and treatment recommendations can help the transplant team clearly identify modifiable factors that can affect transplant outcomes.
Bottom Line
Psychiatrists can help patients who are candidates for liver transplantation (LT) by performing a pre-transplant psychosocial assessment to identity factors that might complicate transplantation or recovery. After LT, patients require careful monitoring for psychiatric comorbidities, drug interactions, and other factors that can affect quality of life.
Related Resources
- Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018;53(2):135-144.
- Marcangelo MJ, Crone C. Tools, techniques to assess organ transplant candidates. Current Psychiatry. 2007;6(9):56-66.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Hydromorphone • Dilaudid
Lithium • Eskalith, Lithobid
Tacrolimus • Astagraf XL, Envarsus XR
1. Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives [Article in English, Portuguese]. Einstein (São Paulo). 2015;13(1):149-152.
2. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144-1165.
3. Centers for Disease Control and Prevention. QuickStats: number of deaths from 10 leading causes,* by sex—National Vital Statistics System, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(15):413.
4. Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31(1):84-89.
5. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59.
6. Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123-132.
7. Grover S, Sarkar S. Liver transplant—psychiatric and psychosocial aspects. J Clin Exp Hepatol. 2012;2(4):382-392.
8. Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760-770.
9. Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824-834.
10. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497-1504.
11. De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323.
12. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172-183.
13. Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718-1726.
14. Graziadei I, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 2016;128(19):679-690.
15. Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981-987.
16. Lee MR, Leggio L. Management of alcohol use disorder in patients requiring liver transplant. Am J Psychiatry. 2015;172(12):1182-1189.
17. Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the Sustained Alcohol Use Post-Liver Transplant score. Hepatology. 2019;69(4):1477-1487.
18. DiMartini A, Crone C, Dew MA. Alcohol and substance use in liver transplant patients. Clinics in Liver Disease. 2011;15(4):727-751.
19. Pungpapong S, Manzarbeitia C, Ortiz J, et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582-587.
20. Kreek MJ. Pharmacotherapy of opioid dependence: rationale and update. Regulatory Peptides. 1994;53(suppl 1):S255-S256.
21. Jiao M, Greanya ED, Haque M, et al. Methadone maintenance therapy in liver transplantation. Prog Transplant. 2010;20(3):209-214; quiz 215.
22. Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91.
23. Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63-71.
24. Kotwani P, Saxena V, Dodge JL, et al. History of marijuana use does not affect outcomes on the liver transplant waitlist. Transplantation. 2018;102(5):794-802.
25. Fineberg SK, West A, Na PJ, et al. Utility of pretransplant psychological measures to predict posttransplant outcomes in liver transplant patients: a systematic review. Gen Hospl Psychiatry. 2016;40:4-11.
26. Satapathy S, Sanyal A. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
27. Olbrisch ME, Levenson JL, Hamer R. The PACT: a rating scale for the study of clinical decision making in psychosocial screening of organ transplant candidates. Clin Transplant. 1989;3:164-169.
28. Twillman RK, Manetto C, Wellisch DK, et al. Transplant Evaluation Rating Scale: a revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153.
29. Goodier J. Evaluating Stress:97496. In: Zalaquett CP, Wood RJ, eds. Evaluating stress: a book of resources. Lanham, MD: Scarecrow Press; 1997:29-29.
30. Beck AT, Steer RA, Carbin, MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8(1):77-100.
31. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242.
32. Cottle WC. The MMPI: a review. Lawrence, KS: University of Kansas; 1953.
33. Addison CC, Campbell-Jenkins BW, Sarpong DF, et al. Psychometric Evaluation of a Coping Strategies Inventory Short-Form (CSI-SF) in the Jackson Heart Study Cohort. Int J Environ Res Public Health. 2007;4(4):289-295.
34. Mooney S, Hasssanein T, Hilsabeck R, et al. Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175-186.
35. De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33(4):609-615.
36. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42(4):337-343.
37. Landefeld CS, Palme, RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344.
38. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric problems in living-related transplantation (II): the association between paradoxical psychiatric syndrome and guilt feelings in adult recipients after living donor liver transplantation. Transplantation Proceedings. 2002;34(7):2632-2633.
39. Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet. 1996;30(2):141-179.
40. Ozkanlar Y, Nishijima Y, Cunha DD, et al. Acute effects of tacrolimus (FK506) on left ventricular mechanics. Pharmacol Res. 2005;52(4):307-312.
41. Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826-833.
42. Maldonado J R. I have been asked to work up a patient who requires a liver transplant how should I proceed? FOCUS. 2009;7(3):332-335.
43. Mccallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol and Alcoholism. 2006;41(4):358-363.
44. Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680-685.
45. Coffman KL, Hoffman A, Sher L, et al. Treatment of the postoperative alcoholic liver transplant recipient with other addictions. Liver Transpl Surg. 1997;3(3):322-327.
1. Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives [Article in English, Portuguese]. Einstein (São Paulo). 2015;13(1):149-152.
2. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144-1165.
3. Centers for Disease Control and Prevention. QuickStats: number of deaths from 10 leading causes,* by sex—National Vital Statistics System, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(15):413.
4. Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31(1):84-89.
5. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59.
6. Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123-132.
7. Grover S, Sarkar S. Liver transplant—psychiatric and psychosocial aspects. J Clin Exp Hepatol. 2012;2(4):382-392.
8. Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760-770.
9. Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824-834.
10. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497-1504.
11. De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323.
12. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172-183.
13. Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718-1726.
14. Graziadei I, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 2016;128(19):679-690.
15. Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981-987.
16. Lee MR, Leggio L. Management of alcohol use disorder in patients requiring liver transplant. Am J Psychiatry. 2015;172(12):1182-1189.
17. Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the Sustained Alcohol Use Post-Liver Transplant score. Hepatology. 2019;69(4):1477-1487.
18. DiMartini A, Crone C, Dew MA. Alcohol and substance use in liver transplant patients. Clinics in Liver Disease. 2011;15(4):727-751.
19. Pungpapong S, Manzarbeitia C, Ortiz J, et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582-587.
20. Kreek MJ. Pharmacotherapy of opioid dependence: rationale and update. Regulatory Peptides. 1994;53(suppl 1):S255-S256.
21. Jiao M, Greanya ED, Haque M, et al. Methadone maintenance therapy in liver transplantation. Prog Transplant. 2010;20(3):209-214; quiz 215.
22. Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91.
23. Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63-71.
24. Kotwani P, Saxena V, Dodge JL, et al. History of marijuana use does not affect outcomes on the liver transplant waitlist. Transplantation. 2018;102(5):794-802.
25. Fineberg SK, West A, Na PJ, et al. Utility of pretransplant psychological measures to predict posttransplant outcomes in liver transplant patients: a systematic review. Gen Hospl Psychiatry. 2016;40:4-11.
26. Satapathy S, Sanyal A. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
27. Olbrisch ME, Levenson JL, Hamer R. The PACT: a rating scale for the study of clinical decision making in psychosocial screening of organ transplant candidates. Clin Transplant. 1989;3:164-169.
28. Twillman RK, Manetto C, Wellisch DK, et al. Transplant Evaluation Rating Scale: a revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153.
29. Goodier J. Evaluating Stress:97496. In: Zalaquett CP, Wood RJ, eds. Evaluating stress: a book of resources. Lanham, MD: Scarecrow Press; 1997:29-29.
30. Beck AT, Steer RA, Carbin, MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8(1):77-100.
31. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242.
32. Cottle WC. The MMPI: a review. Lawrence, KS: University of Kansas; 1953.
33. Addison CC, Campbell-Jenkins BW, Sarpong DF, et al. Psychometric Evaluation of a Coping Strategies Inventory Short-Form (CSI-SF) in the Jackson Heart Study Cohort. Int J Environ Res Public Health. 2007;4(4):289-295.
34. Mooney S, Hasssanein T, Hilsabeck R, et al. Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175-186.
35. De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33(4):609-615.
36. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42(4):337-343.
37. Landefeld CS, Palme, RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344.
38. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric problems in living-related transplantation (II): the association between paradoxical psychiatric syndrome and guilt feelings in adult recipients after living donor liver transplantation. Transplantation Proceedings. 2002;34(7):2632-2633.
39. Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet. 1996;30(2):141-179.
40. Ozkanlar Y, Nishijima Y, Cunha DD, et al. Acute effects of tacrolimus (FK506) on left ventricular mechanics. Pharmacol Res. 2005;52(4):307-312.
41. Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826-833.
42. Maldonado J R. I have been asked to work up a patient who requires a liver transplant how should I proceed? FOCUS. 2009;7(3):332-335.
43. Mccallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol and Alcoholism. 2006;41(4):358-363.
44. Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680-685.
45. Coffman KL, Hoffman A, Sher L, et al. Treatment of the postoperative alcoholic liver transplant recipient with other addictions. Liver Transpl Surg. 1997;3(3):322-327.