User login
A Checklist for Compounded Semaglutide or Tirzepatide
Consider this: If you’re taking your children to the beach, how do you protect them from drowning? You don’t tell them, “Don’t go into the ocean.” You teach them how to swim; you give them floaties; and you accompany them in the water and go in only when a lifeguard is present. In other words, you give them all the tools to protect themselves because you know they will go into the ocean anyway.
Patients are diving into the ocean. Patients with obesity, who know that a treatment for their disease exists but is inaccessible, are diving into the ocean. Unfortunately, they are diving in without floaties or a lifeguard, and well-meaning bystanders are simply telling them to not go.
Compounded peptides are an ocean of alternative therapies. Even though compounding pharmacists need specialized training, facilities and equipment need to be properly certified, and final dosage forms need extensive testing, pharmacies are not equal when it comes to sterile compounding. Regulatory agencies such as the US Food and Drug Administration (FDA) have expressed caution around compounded semaglutide. Professional societies such as the Obesity Medicine Association (OMA) advise against compounded peptides because they lack clinical trials that prove their safety and efficacy. Ask any individual doctor and you are likely to receive a range of opinions.
As an endocrinologist specializing in obesity, I practice evidence-based medicine as much as possible. However, I also recognize how today’s dysfunctional medical system compels patients to dive into that ocean in search of an alternative solution.
With the help of pharmacists, compounding pharmacists, researchers, and clinicians, here is a checklist for patients who seek compounded semaglutide or tirzepatide:
- Check the state licensing board website to see if there have been any complaints or disciplinary actions made against the pharmacy facility. These government-maintained websites vary in searchability and user-friendliness, but you are specifically looking for whether the FDA ever issued a 483 form.
- Ask for the pharmacy’s state board inspection report. There should be at least one of these reports, issued at the pharmacy’s founding, and there may be more depending on individual state regulations on frequencies of inspections.
- Ask if the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation is an extra optional step that some compounding pharmacies take to be legitimized by a third party.
- Ask if the pharmacy follows Current Good Manufacturing Practice (CGMP). CGMP is not required of 503a pharmacies, which are pharmacies that provide semaglutide or tirzepatide directly to patients, but following CGMP means an extra level of quality assurance. The bare minimum for anyone doing sterile compounding in the United States is to meet the standards found in the US Pharmacopeia, chapter <797>, Sterile Compounding.
- Ask your compounding pharmacy where they source the medication’s active pharmaceutical ingredient (API).
- Find out if this supplier is registered with the FDA by searching here or here.
- Confirm that semaglutide base, not semaglutide salt, is used in the compounding process.
- Request a certificate of analysis (COA) of the active pharmaceutical ingredient, which should be semaglutide base. This shows you whether the medication has impurities or byproducts due to its manufacturing process.
- Ask if they have third-party confirmation of potency, stability, and sterility testing of the final product.
In generating this guidance, I’m not endorsing compounded peptides, and in fact, I recognize that there is nothing keeping small-time compounding pharmacies from skirting some of these quality measures, falsifying records, and flying under the radar. However, I hope this checklist serves as a starting point for education and risk mitigation. If a compounder is unwilling or unable to answer these questions, consider it a red flag and look elsewhere.
In an ideal world, the state regulators or the FDA would proactively supervise instead of reactively monitor; trusted compounding pharmacies would be systematically activated to ease medication shortages; and patients with obesity would have access to safe and efficacious treatments for their disease. Until then, we as providers can acknowledge the disappointments of our healthcare system while still developing realistic and individualized solutions that prioritize patient care and safety.
Dr. Tchang is assistant professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine, and a physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York. She is an adviser for Novo Nordisk, which manufactures Wegovy, and an adviser for Ro, a telehealth company that offers compounded semaglutide, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Consider this: If you’re taking your children to the beach, how do you protect them from drowning? You don’t tell them, “Don’t go into the ocean.” You teach them how to swim; you give them floaties; and you accompany them in the water and go in only when a lifeguard is present. In other words, you give them all the tools to protect themselves because you know they will go into the ocean anyway.
Patients are diving into the ocean. Patients with obesity, who know that a treatment for their disease exists but is inaccessible, are diving into the ocean. Unfortunately, they are diving in without floaties or a lifeguard, and well-meaning bystanders are simply telling them to not go.
Compounded peptides are an ocean of alternative therapies. Even though compounding pharmacists need specialized training, facilities and equipment need to be properly certified, and final dosage forms need extensive testing, pharmacies are not equal when it comes to sterile compounding. Regulatory agencies such as the US Food and Drug Administration (FDA) have expressed caution around compounded semaglutide. Professional societies such as the Obesity Medicine Association (OMA) advise against compounded peptides because they lack clinical trials that prove their safety and efficacy. Ask any individual doctor and you are likely to receive a range of opinions.
As an endocrinologist specializing in obesity, I practice evidence-based medicine as much as possible. However, I also recognize how today’s dysfunctional medical system compels patients to dive into that ocean in search of an alternative solution.
With the help of pharmacists, compounding pharmacists, researchers, and clinicians, here is a checklist for patients who seek compounded semaglutide or tirzepatide:
- Check the state licensing board website to see if there have been any complaints or disciplinary actions made against the pharmacy facility. These government-maintained websites vary in searchability and user-friendliness, but you are specifically looking for whether the FDA ever issued a 483 form.
- Ask for the pharmacy’s state board inspection report. There should be at least one of these reports, issued at the pharmacy’s founding, and there may be more depending on individual state regulations on frequencies of inspections.
- Ask if the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation is an extra optional step that some compounding pharmacies take to be legitimized by a third party.
- Ask if the pharmacy follows Current Good Manufacturing Practice (CGMP). CGMP is not required of 503a pharmacies, which are pharmacies that provide semaglutide or tirzepatide directly to patients, but following CGMP means an extra level of quality assurance. The bare minimum for anyone doing sterile compounding in the United States is to meet the standards found in the US Pharmacopeia, chapter <797>, Sterile Compounding.
- Ask your compounding pharmacy where they source the medication’s active pharmaceutical ingredient (API).
- Find out if this supplier is registered with the FDA by searching here or here.
- Confirm that semaglutide base, not semaglutide salt, is used in the compounding process.
- Request a certificate of analysis (COA) of the active pharmaceutical ingredient, which should be semaglutide base. This shows you whether the medication has impurities or byproducts due to its manufacturing process.
- Ask if they have third-party confirmation of potency, stability, and sterility testing of the final product.
In generating this guidance, I’m not endorsing compounded peptides, and in fact, I recognize that there is nothing keeping small-time compounding pharmacies from skirting some of these quality measures, falsifying records, and flying under the radar. However, I hope this checklist serves as a starting point for education and risk mitigation. If a compounder is unwilling or unable to answer these questions, consider it a red flag and look elsewhere.
In an ideal world, the state regulators or the FDA would proactively supervise instead of reactively monitor; trusted compounding pharmacies would be systematically activated to ease medication shortages; and patients with obesity would have access to safe and efficacious treatments for their disease. Until then, we as providers can acknowledge the disappointments of our healthcare system while still developing realistic and individualized solutions that prioritize patient care and safety.
Dr. Tchang is assistant professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine, and a physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York. She is an adviser for Novo Nordisk, which manufactures Wegovy, and an adviser for Ro, a telehealth company that offers compounded semaglutide, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Consider this: If you’re taking your children to the beach, how do you protect them from drowning? You don’t tell them, “Don’t go into the ocean.” You teach them how to swim; you give them floaties; and you accompany them in the water and go in only when a lifeguard is present. In other words, you give them all the tools to protect themselves because you know they will go into the ocean anyway.
Patients are diving into the ocean. Patients with obesity, who know that a treatment for their disease exists but is inaccessible, are diving into the ocean. Unfortunately, they are diving in without floaties or a lifeguard, and well-meaning bystanders are simply telling them to not go.
Compounded peptides are an ocean of alternative therapies. Even though compounding pharmacists need specialized training, facilities and equipment need to be properly certified, and final dosage forms need extensive testing, pharmacies are not equal when it comes to sterile compounding. Regulatory agencies such as the US Food and Drug Administration (FDA) have expressed caution around compounded semaglutide. Professional societies such as the Obesity Medicine Association (OMA) advise against compounded peptides because they lack clinical trials that prove their safety and efficacy. Ask any individual doctor and you are likely to receive a range of opinions.
As an endocrinologist specializing in obesity, I practice evidence-based medicine as much as possible. However, I also recognize how today’s dysfunctional medical system compels patients to dive into that ocean in search of an alternative solution.
With the help of pharmacists, compounding pharmacists, researchers, and clinicians, here is a checklist for patients who seek compounded semaglutide or tirzepatide:
- Check the state licensing board website to see if there have been any complaints or disciplinary actions made against the pharmacy facility. These government-maintained websites vary in searchability and user-friendliness, but you are specifically looking for whether the FDA ever issued a 483 form.
- Ask for the pharmacy’s state board inspection report. There should be at least one of these reports, issued at the pharmacy’s founding, and there may be more depending on individual state regulations on frequencies of inspections.
- Ask if the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation is an extra optional step that some compounding pharmacies take to be legitimized by a third party.
- Ask if the pharmacy follows Current Good Manufacturing Practice (CGMP). CGMP is not required of 503a pharmacies, which are pharmacies that provide semaglutide or tirzepatide directly to patients, but following CGMP means an extra level of quality assurance. The bare minimum for anyone doing sterile compounding in the United States is to meet the standards found in the US Pharmacopeia, chapter <797>, Sterile Compounding.
- Ask your compounding pharmacy where they source the medication’s active pharmaceutical ingredient (API).
- Find out if this supplier is registered with the FDA by searching here or here.
- Confirm that semaglutide base, not semaglutide salt, is used in the compounding process.
- Request a certificate of analysis (COA) of the active pharmaceutical ingredient, which should be semaglutide base. This shows you whether the medication has impurities or byproducts due to its manufacturing process.
- Ask if they have third-party confirmation of potency, stability, and sterility testing of the final product.
In generating this guidance, I’m not endorsing compounded peptides, and in fact, I recognize that there is nothing keeping small-time compounding pharmacies from skirting some of these quality measures, falsifying records, and flying under the radar. However, I hope this checklist serves as a starting point for education and risk mitigation. If a compounder is unwilling or unable to answer these questions, consider it a red flag and look elsewhere.
In an ideal world, the state regulators or the FDA would proactively supervise instead of reactively monitor; trusted compounding pharmacies would be systematically activated to ease medication shortages; and patients with obesity would have access to safe and efficacious treatments for their disease. Until then, we as providers can acknowledge the disappointments of our healthcare system while still developing realistic and individualized solutions that prioritize patient care and safety.
Dr. Tchang is assistant professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine, and a physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York. She is an adviser for Novo Nordisk, which manufactures Wegovy, and an adviser for Ro, a telehealth company that offers compounded semaglutide, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk.
A version of this article first appeared on Medscape.com.
5 Things to Know About the Future of Obesity Medicine
As more and more treatments for obesity become available, what does the future hold for these patients? Here are five things that clinicians need to know.
1. Public health officials will prioritize dietary quality over quantity.
Dietitians, healthcare providers, and scientists are already prioritizing the quality of calories, and now policymakers are aligning with this goal, with calls for more research on ultraprocessed foods (UPFs) to answer the key question, “Why do UPFs cause people to eat 500 more calories per day compared with unprocessed foods?” The food industry has taken notice of the potential “Ozempic effect” associated with reduced spending on groceries and is responding with product lines “designed to complement” glucagon-like peptide-1 receptor agonists (GLP-1 RAs) while simultaneously lobbying against any UPF reform. However, with emerging data on how sugar taxes may reduce sales and Congress honing in on the diabetes epidemic, we are hopeful that change is coming in 2024.
2. Antiobesity medications will target fat loss instead of weight loss.
The focus on weight has been long-standing, but with highly effective medications like tirzepatide causing about 20% weight loss, attention is shifting to body composition — namely, how do we optimize fat loss while preserving muscle? We are seeing this transition in the research community. Bimagrumab, for example, a once-monthly injection that increases muscle mass and decreases fat mass, is being tested in a phase 3 clinical trial alongside semaglutide. Agents initially designed for spinal muscular atrophy, like apitegromab and taldefgrobep alfa, are being repurposed for obesity. Watch for results of these phase 2 trials in 2024.
3. Increasing energy expenditure is the holy grail of obesity research.
The success of GLP-1 RAs, and the even greater success of dual- and triple-target agents like tirzepatide and retatrutide, tells us that obesity is, indeed, a hormone problem. These medications primarily cause weight loss by suppressing appetite and reducing caloric intake. As scientists develop more therapeutics to normalize appetite regulation, attention will shift to optimizing energy expenditure. Researchers are already investigating brown fat, mitochondrial uncouplers, and skeletal muscle metabolism, but no agent thus far has been proven to be both safe and effective in increasing energy expenditure. Of these, keep an eye on clinical trials involving brown fat and the excitement over the anti-inflammatory cytokine growth differentiating factor 15 (GDF15).
4. Chronic disease without chronic medications.
Obesity is a chronic disease, just like hypertension or diabetes. Similarly, medications that treat chronic diseases are expected to be taken long-term because discontinuation often results in disease recurrence. However, obesity research is getting closer and closer to options that require less frequent administration. Bimagrumab, for example, is a once-monthly injection. In endocrinology, the premier example is osteoporosis: Osteoporosis can be treated with just 3 years of an annual injection and never require treatment again. In obesity, anticipate more basic science discoveries aimed at developing safe and specific treatments that are truly disease-modifying — ones that reverse appetitive dysregulation, reduce proinflammatory adiposity, and optimize anabolic metabolism.
5. Barriers to access are barriers to progress.
The biggest challenge to obesity treatment today is access: drug shortages, medication costs, and lack of obesity medicine providers. Shortages of medications like semaglutide 2.4 mg are being driven by high “demand”; in other words, manufacturers failed to anticipate the massive interest in antiobesity medications.
Medicare and most state Medicaid programs don’t cover these medications; commercial payers are refusing, reversing, or limiting coverage. An out-of-pocket monthly cost over $1000 limits affordability for the majority of Americans.
Seeking care from an obesity medicine doctor is a challenge as well. Over 40% of US adults have obesity, but less than 1% of doctors are certified in obesity medicine. Meanwhile, private equity is eager to address the lack of access through compounding pharmacies, medispas, or telemedicine services, but the quality of care varies greatly. Some companies purposely avoid the term “patient,” preferring ethics-free labels like “consumers” or “members.”
The $100 billion–dollar weight loss industry unfortunately has created financial incentives that drive obesity commerce over obesity care. Because of these barriers, the epidemic of obesity, with a prevalence projected to be 50% by 2030, will not be solved or slowed despite the scientific progress in obesity treatment. A single silver lining exists among policymakers who are aiming to correct our costly sick-care system in steps, starting with pharmacy benefit manager reform. Five of these bills are the ones to track in 2024: Pharmacy Benefit Manager Reform Act, Pharmacy Benefits Manager Accountability Act, Pharmacy Benefit Manager Sunshine and Accountability Act, Pharmacy Benefit Manager Transparency Act of 2023, and Lower Costs, More Transparency Act.
I believe that these five things will have the most impact on the treatment of our patients with obesity. Stay tuned throughout the year as I share updates in obesity research, pharmacotherapy, and public policy.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
As more and more treatments for obesity become available, what does the future hold for these patients? Here are five things that clinicians need to know.
1. Public health officials will prioritize dietary quality over quantity.
Dietitians, healthcare providers, and scientists are already prioritizing the quality of calories, and now policymakers are aligning with this goal, with calls for more research on ultraprocessed foods (UPFs) to answer the key question, “Why do UPFs cause people to eat 500 more calories per day compared with unprocessed foods?” The food industry has taken notice of the potential “Ozempic effect” associated with reduced spending on groceries and is responding with product lines “designed to complement” glucagon-like peptide-1 receptor agonists (GLP-1 RAs) while simultaneously lobbying against any UPF reform. However, with emerging data on how sugar taxes may reduce sales and Congress honing in on the diabetes epidemic, we are hopeful that change is coming in 2024.
2. Antiobesity medications will target fat loss instead of weight loss.
The focus on weight has been long-standing, but with highly effective medications like tirzepatide causing about 20% weight loss, attention is shifting to body composition — namely, how do we optimize fat loss while preserving muscle? We are seeing this transition in the research community. Bimagrumab, for example, a once-monthly injection that increases muscle mass and decreases fat mass, is being tested in a phase 3 clinical trial alongside semaglutide. Agents initially designed for spinal muscular atrophy, like apitegromab and taldefgrobep alfa, are being repurposed for obesity. Watch for results of these phase 2 trials in 2024.
3. Increasing energy expenditure is the holy grail of obesity research.
The success of GLP-1 RAs, and the even greater success of dual- and triple-target agents like tirzepatide and retatrutide, tells us that obesity is, indeed, a hormone problem. These medications primarily cause weight loss by suppressing appetite and reducing caloric intake. As scientists develop more therapeutics to normalize appetite regulation, attention will shift to optimizing energy expenditure. Researchers are already investigating brown fat, mitochondrial uncouplers, and skeletal muscle metabolism, but no agent thus far has been proven to be both safe and effective in increasing energy expenditure. Of these, keep an eye on clinical trials involving brown fat and the excitement over the anti-inflammatory cytokine growth differentiating factor 15 (GDF15).
4. Chronic disease without chronic medications.
Obesity is a chronic disease, just like hypertension or diabetes. Similarly, medications that treat chronic diseases are expected to be taken long-term because discontinuation often results in disease recurrence. However, obesity research is getting closer and closer to options that require less frequent administration. Bimagrumab, for example, is a once-monthly injection. In endocrinology, the premier example is osteoporosis: Osteoporosis can be treated with just 3 years of an annual injection and never require treatment again. In obesity, anticipate more basic science discoveries aimed at developing safe and specific treatments that are truly disease-modifying — ones that reverse appetitive dysregulation, reduce proinflammatory adiposity, and optimize anabolic metabolism.
5. Barriers to access are barriers to progress.
The biggest challenge to obesity treatment today is access: drug shortages, medication costs, and lack of obesity medicine providers. Shortages of medications like semaglutide 2.4 mg are being driven by high “demand”; in other words, manufacturers failed to anticipate the massive interest in antiobesity medications.
Medicare and most state Medicaid programs don’t cover these medications; commercial payers are refusing, reversing, or limiting coverage. An out-of-pocket monthly cost over $1000 limits affordability for the majority of Americans.
Seeking care from an obesity medicine doctor is a challenge as well. Over 40% of US adults have obesity, but less than 1% of doctors are certified in obesity medicine. Meanwhile, private equity is eager to address the lack of access through compounding pharmacies, medispas, or telemedicine services, but the quality of care varies greatly. Some companies purposely avoid the term “patient,” preferring ethics-free labels like “consumers” or “members.”
The $100 billion–dollar weight loss industry unfortunately has created financial incentives that drive obesity commerce over obesity care. Because of these barriers, the epidemic of obesity, with a prevalence projected to be 50% by 2030, will not be solved or slowed despite the scientific progress in obesity treatment. A single silver lining exists among policymakers who are aiming to correct our costly sick-care system in steps, starting with pharmacy benefit manager reform. Five of these bills are the ones to track in 2024: Pharmacy Benefit Manager Reform Act, Pharmacy Benefits Manager Accountability Act, Pharmacy Benefit Manager Sunshine and Accountability Act, Pharmacy Benefit Manager Transparency Act of 2023, and Lower Costs, More Transparency Act.
I believe that these five things will have the most impact on the treatment of our patients with obesity. Stay tuned throughout the year as I share updates in obesity research, pharmacotherapy, and public policy.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
As more and more treatments for obesity become available, what does the future hold for these patients? Here are five things that clinicians need to know.
1. Public health officials will prioritize dietary quality over quantity.
Dietitians, healthcare providers, and scientists are already prioritizing the quality of calories, and now policymakers are aligning with this goal, with calls for more research on ultraprocessed foods (UPFs) to answer the key question, “Why do UPFs cause people to eat 500 more calories per day compared with unprocessed foods?” The food industry has taken notice of the potential “Ozempic effect” associated with reduced spending on groceries and is responding with product lines “designed to complement” glucagon-like peptide-1 receptor agonists (GLP-1 RAs) while simultaneously lobbying against any UPF reform. However, with emerging data on how sugar taxes may reduce sales and Congress honing in on the diabetes epidemic, we are hopeful that change is coming in 2024.
2. Antiobesity medications will target fat loss instead of weight loss.
The focus on weight has been long-standing, but with highly effective medications like tirzepatide causing about 20% weight loss, attention is shifting to body composition — namely, how do we optimize fat loss while preserving muscle? We are seeing this transition in the research community. Bimagrumab, for example, a once-monthly injection that increases muscle mass and decreases fat mass, is being tested in a phase 3 clinical trial alongside semaglutide. Agents initially designed for spinal muscular atrophy, like apitegromab and taldefgrobep alfa, are being repurposed for obesity. Watch for results of these phase 2 trials in 2024.
3. Increasing energy expenditure is the holy grail of obesity research.
The success of GLP-1 RAs, and the even greater success of dual- and triple-target agents like tirzepatide and retatrutide, tells us that obesity is, indeed, a hormone problem. These medications primarily cause weight loss by suppressing appetite and reducing caloric intake. As scientists develop more therapeutics to normalize appetite regulation, attention will shift to optimizing energy expenditure. Researchers are already investigating brown fat, mitochondrial uncouplers, and skeletal muscle metabolism, but no agent thus far has been proven to be both safe and effective in increasing energy expenditure. Of these, keep an eye on clinical trials involving brown fat and the excitement over the anti-inflammatory cytokine growth differentiating factor 15 (GDF15).
4. Chronic disease without chronic medications.
Obesity is a chronic disease, just like hypertension or diabetes. Similarly, medications that treat chronic diseases are expected to be taken long-term because discontinuation often results in disease recurrence. However, obesity research is getting closer and closer to options that require less frequent administration. Bimagrumab, for example, is a once-monthly injection. In endocrinology, the premier example is osteoporosis: Osteoporosis can be treated with just 3 years of an annual injection and never require treatment again. In obesity, anticipate more basic science discoveries aimed at developing safe and specific treatments that are truly disease-modifying — ones that reverse appetitive dysregulation, reduce proinflammatory adiposity, and optimize anabolic metabolism.
5. Barriers to access are barriers to progress.
The biggest challenge to obesity treatment today is access: drug shortages, medication costs, and lack of obesity medicine providers. Shortages of medications like semaglutide 2.4 mg are being driven by high “demand”; in other words, manufacturers failed to anticipate the massive interest in antiobesity medications.
Medicare and most state Medicaid programs don’t cover these medications; commercial payers are refusing, reversing, or limiting coverage. An out-of-pocket monthly cost over $1000 limits affordability for the majority of Americans.
Seeking care from an obesity medicine doctor is a challenge as well. Over 40% of US adults have obesity, but less than 1% of doctors are certified in obesity medicine. Meanwhile, private equity is eager to address the lack of access through compounding pharmacies, medispas, or telemedicine services, but the quality of care varies greatly. Some companies purposely avoid the term “patient,” preferring ethics-free labels like “consumers” or “members.”
The $100 billion–dollar weight loss industry unfortunately has created financial incentives that drive obesity commerce over obesity care. Because of these barriers, the epidemic of obesity, with a prevalence projected to be 50% by 2030, will not be solved or slowed despite the scientific progress in obesity treatment. A single silver lining exists among policymakers who are aiming to correct our costly sick-care system in steps, starting with pharmacy benefit manager reform. Five of these bills are the ones to track in 2024: Pharmacy Benefit Manager Reform Act, Pharmacy Benefits Manager Accountability Act, Pharmacy Benefit Manager Sunshine and Accountability Act, Pharmacy Benefit Manager Transparency Act of 2023, and Lower Costs, More Transparency Act.
I believe that these five things will have the most impact on the treatment of our patients with obesity. Stay tuned throughout the year as I share updates in obesity research, pharmacotherapy, and public policy.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
‘Shower’ Me for My Medical Expertise
A recent Reuters article reported that the manufacturer of the leading antiobesity medication semaglutide (Wegovy) “showers money on U.S. obesity doctors.”
Hello. I’d like to be showered.
According to the article, Novo Nordisk, Wegovy/Ozempic manufacturer, paid medical professionals $25.8 million in “fees and expenses” over a decade.
I think all doctors — who spend a decade of their life in training only to emerge with thousands of dollars in debt — would like to be similarly compensated for their expertise.
Yet, many of us forgo lucrative private practices or industry jobs to work at academic medical centers because we wish to pursue our original mission: To provide the best patient care possible.
Fulfilling this mission in today’s “sickcare” system means being more than a clinician. We become researchers, educators, advocates, mentors, consultants, and advisors. We do so because the system compels us to find other ways to provide quality health care, outside of clinic walls. These ways often include:
- Educating the public through media, social media, or community events.
- Training students, residents, and fellows on the latest medical knowledge.
- Advising industry innovators and entrepreneurs who seek our expertise.
Personally, I engaged in these activities because working 8-5 seeing 20 patients a day wasn’t enough. I wanted to help more people, more quickly. When I was faced with these opportunities, I was excited to say “yes” and never thought to ask for compensation because I didn’t want to seem ungrateful.
Eventually, I learned to ask for compensation.
And then I learned to decide my value.
The Reuters article reports that obesity medicine experts Drs. Lee Kaplan, Donna Ryan, Ania Jastreboff, and Jamy Ard were paid thousands of dollars in consulting fees over a decade. This industry-to-physician relationship should be celebrated because:
- Industries should consult experts in the field.
- These leaders have dedicated decades of their lives to understanding and solving the obesity epidemic.
- This collaboration has resulted in a therapeutic option that is changing lives.
If there is anything to criticize, it should be:
- The expectation that medical expertise should be free.Wegovy’s manufacturer is worth $403 billion, and the experts are valued at less than 0.1% of that figure.
- The lack of context.
- Some celebrity doctors can earn $300,000 in just one consult. Other medical or surgical specialties are valued at 100 times more than obesity specialists.
- The false dichotomy.
- Just because money is involved, it doesn’t mean the product is invalid.
Industry and physician relationships have long been examined. Such financial relationships are always disclosed (eg, at conferences, in publications). The Sunshine Act of 2010 and Open Payments provide the necessary transparency for people to decide for themselves whether there were financial incentives or potential conflicts of interest.
We should also take it a step further and ask ourselves: Do conflicts of interest require us to dismiss the end result? In other words, just because the pharmaceutical industry pays these doctors for their time and expertise, does that mean their life’s work is wrong, or that the drug isn’t effective?
In the case of obesity, Wegovy speaks for itself. Remember that the manufacturer stopped advertising. When a disease finally has a treatment, it does not need promoters or salespersons. Just speak to any person with obesity.
Ultimately, I see three main takeaways from Reuters’ reporting:
- The weight loss industry needs more obesity medicine experts.
- We should value ourselves more.
Dr. Tchang received $5525 in 2022 in consulting fees from Novo Nordisk. She plans to ask for more. She also disclosed ties with Gelesis.
A version of this article appeared on Medscape.com.
A recent Reuters article reported that the manufacturer of the leading antiobesity medication semaglutide (Wegovy) “showers money on U.S. obesity doctors.”
Hello. I’d like to be showered.
According to the article, Novo Nordisk, Wegovy/Ozempic manufacturer, paid medical professionals $25.8 million in “fees and expenses” over a decade.
I think all doctors — who spend a decade of their life in training only to emerge with thousands of dollars in debt — would like to be similarly compensated for their expertise.
Yet, many of us forgo lucrative private practices or industry jobs to work at academic medical centers because we wish to pursue our original mission: To provide the best patient care possible.
Fulfilling this mission in today’s “sickcare” system means being more than a clinician. We become researchers, educators, advocates, mentors, consultants, and advisors. We do so because the system compels us to find other ways to provide quality health care, outside of clinic walls. These ways often include:
- Educating the public through media, social media, or community events.
- Training students, residents, and fellows on the latest medical knowledge.
- Advising industry innovators and entrepreneurs who seek our expertise.
Personally, I engaged in these activities because working 8-5 seeing 20 patients a day wasn’t enough. I wanted to help more people, more quickly. When I was faced with these opportunities, I was excited to say “yes” and never thought to ask for compensation because I didn’t want to seem ungrateful.
Eventually, I learned to ask for compensation.
And then I learned to decide my value.
The Reuters article reports that obesity medicine experts Drs. Lee Kaplan, Donna Ryan, Ania Jastreboff, and Jamy Ard were paid thousands of dollars in consulting fees over a decade. This industry-to-physician relationship should be celebrated because:
- Industries should consult experts in the field.
- These leaders have dedicated decades of their lives to understanding and solving the obesity epidemic.
- This collaboration has resulted in a therapeutic option that is changing lives.
If there is anything to criticize, it should be:
- The expectation that medical expertise should be free.Wegovy’s manufacturer is worth $403 billion, and the experts are valued at less than 0.1% of that figure.
- The lack of context.
- Some celebrity doctors can earn $300,000 in just one consult. Other medical or surgical specialties are valued at 100 times more than obesity specialists.
- The false dichotomy.
- Just because money is involved, it doesn’t mean the product is invalid.
Industry and physician relationships have long been examined. Such financial relationships are always disclosed (eg, at conferences, in publications). The Sunshine Act of 2010 and Open Payments provide the necessary transparency for people to decide for themselves whether there were financial incentives or potential conflicts of interest.
We should also take it a step further and ask ourselves: Do conflicts of interest require us to dismiss the end result? In other words, just because the pharmaceutical industry pays these doctors for their time and expertise, does that mean their life’s work is wrong, or that the drug isn’t effective?
In the case of obesity, Wegovy speaks for itself. Remember that the manufacturer stopped advertising. When a disease finally has a treatment, it does not need promoters or salespersons. Just speak to any person with obesity.
Ultimately, I see three main takeaways from Reuters’ reporting:
- The weight loss industry needs more obesity medicine experts.
- We should value ourselves more.
Dr. Tchang received $5525 in 2022 in consulting fees from Novo Nordisk. She plans to ask for more. She also disclosed ties with Gelesis.
A version of this article appeared on Medscape.com.
A recent Reuters article reported that the manufacturer of the leading antiobesity medication semaglutide (Wegovy) “showers money on U.S. obesity doctors.”
Hello. I’d like to be showered.
According to the article, Novo Nordisk, Wegovy/Ozempic manufacturer, paid medical professionals $25.8 million in “fees and expenses” over a decade.
I think all doctors — who spend a decade of their life in training only to emerge with thousands of dollars in debt — would like to be similarly compensated for their expertise.
Yet, many of us forgo lucrative private practices or industry jobs to work at academic medical centers because we wish to pursue our original mission: To provide the best patient care possible.
Fulfilling this mission in today’s “sickcare” system means being more than a clinician. We become researchers, educators, advocates, mentors, consultants, and advisors. We do so because the system compels us to find other ways to provide quality health care, outside of clinic walls. These ways often include:
- Educating the public through media, social media, or community events.
- Training students, residents, and fellows on the latest medical knowledge.
- Advising industry innovators and entrepreneurs who seek our expertise.
Personally, I engaged in these activities because working 8-5 seeing 20 patients a day wasn’t enough. I wanted to help more people, more quickly. When I was faced with these opportunities, I was excited to say “yes” and never thought to ask for compensation because I didn’t want to seem ungrateful.
Eventually, I learned to ask for compensation.
And then I learned to decide my value.
The Reuters article reports that obesity medicine experts Drs. Lee Kaplan, Donna Ryan, Ania Jastreboff, and Jamy Ard were paid thousands of dollars in consulting fees over a decade. This industry-to-physician relationship should be celebrated because:
- Industries should consult experts in the field.
- These leaders have dedicated decades of their lives to understanding and solving the obesity epidemic.
- This collaboration has resulted in a therapeutic option that is changing lives.
If there is anything to criticize, it should be:
- The expectation that medical expertise should be free.Wegovy’s manufacturer is worth $403 billion, and the experts are valued at less than 0.1% of that figure.
- The lack of context.
- Some celebrity doctors can earn $300,000 in just one consult. Other medical or surgical specialties are valued at 100 times more than obesity specialists.
- The false dichotomy.
- Just because money is involved, it doesn’t mean the product is invalid.
Industry and physician relationships have long been examined. Such financial relationships are always disclosed (eg, at conferences, in publications). The Sunshine Act of 2010 and Open Payments provide the necessary transparency for people to decide for themselves whether there were financial incentives or potential conflicts of interest.
We should also take it a step further and ask ourselves: Do conflicts of interest require us to dismiss the end result? In other words, just because the pharmaceutical industry pays these doctors for their time and expertise, does that mean their life’s work is wrong, or that the drug isn’t effective?
In the case of obesity, Wegovy speaks for itself. Remember that the manufacturer stopped advertising. When a disease finally has a treatment, it does not need promoters or salespersons. Just speak to any person with obesity.
Ultimately, I see three main takeaways from Reuters’ reporting:
- The weight loss industry needs more obesity medicine experts.
- We should value ourselves more.
Dr. Tchang received $5525 in 2022 in consulting fees from Novo Nordisk. She plans to ask for more. She also disclosed ties with Gelesis.
A version of this article appeared on Medscape.com.
Spending the Holidays With GLP-1 Receptor Agonists: 5 Things to Know
As an endocrinologist, I treat many patients who have diabetes, obesity, or both. Antiobesity medications, particularly the class of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), are our first support tools when nutrition and physical activity aren’t enough.
1. Be mindful of fullness cues.
GLP-1 RAs increase satiety; they help patients feel fuller sooner within a meal and longer in between meals. This means consuming the “usual” at a holiday gathering makes them feel as if they ate too much, and often this will result in more side effects, such as nausea and reflux.
Patient tip: A good rule of thumb is to anticipate feeling full with half of your usual portion. Start with half a plate and reassess your hunger level after finishing.
2. Distinguish between hunger and “food noise.”
Ask your patients, “Do you ever find yourself eating even when you’re not hungry?” Many people eat because of emotions (eg, stress, anxiety, happiness), social situations, or cultural expectations. This type of food consumption is what scientists call “hedonic food intake” and may be driven by the “food noise” that patients describe as persistent thoughts about food in the absence of physiologic hunger. Semaglutide (Ozempic, Wegovy) has been found to reduce cravings, though other research has shown that emotional eating may blunt the effect of GLP-1 RAs.
Patient tip: Recognize when you might be seeking food for reasons other than hunger, and try a different way to address the cue (eg, chat with a friend or family member, go for a walk).
3. Be careful with alcohol.
GLP-1 RAs are being researched as potential treatments for alcohol use disorder. Many patients report less interest in alcohol and a lower tolerance to alcohol when they are taking a GLP-1 RA. Additionally, GLP-1 RAs may be a risk factor for pancreatitis, which can be caused by consuming too much alcohol.
Patient tip: The standard recommendation remains true: If drinking alcohol, limit to one to two servings per day, but also know that reduced intake or interest is normal when taking a GLP-1 RA.
4. Be aware of sickness vs side effects.
With holiday travel and the winter season, it is common for people to catch a cold or a stomach bug. Symptoms of common illnesses might include fatigue, loss of appetite, or diarrhea. These symptoms overlap with side effects of antiobesity medications like semaglutide and tirzepatide.
Patient tip: If you are experiencing constitutional or gastrointestinal symptoms due to illness, speak with your board-certified obesity medicine doctor, who may recommend a temporary medication adjustment to avoid excess side effects.
5. Stay strong against weight stigma.
The holiday season can be a stressful time, especially as patients are reconnecting with people who have not been a part of their health or weight loss journey. Unfortunately, weight bias and weight stigma remain rampant. Many people don’t understand the biology of obesity and refuse to accept the necessity of medical treatment. They may be surrounded by opinions, often louder and less informed.
Patient tip: Remember that obesity is a medical disease. Tell your nosy cousin that it’s a private health matter and that your decisions are your own.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed financial relationships with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
As an endocrinologist, I treat many patients who have diabetes, obesity, or both. Antiobesity medications, particularly the class of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), are our first support tools when nutrition and physical activity aren’t enough.
1. Be mindful of fullness cues.
GLP-1 RAs increase satiety; they help patients feel fuller sooner within a meal and longer in between meals. This means consuming the “usual” at a holiday gathering makes them feel as if they ate too much, and often this will result in more side effects, such as nausea and reflux.
Patient tip: A good rule of thumb is to anticipate feeling full with half of your usual portion. Start with half a plate and reassess your hunger level after finishing.
2. Distinguish between hunger and “food noise.”
Ask your patients, “Do you ever find yourself eating even when you’re not hungry?” Many people eat because of emotions (eg, stress, anxiety, happiness), social situations, or cultural expectations. This type of food consumption is what scientists call “hedonic food intake” and may be driven by the “food noise” that patients describe as persistent thoughts about food in the absence of physiologic hunger. Semaglutide (Ozempic, Wegovy) has been found to reduce cravings, though other research has shown that emotional eating may blunt the effect of GLP-1 RAs.
Patient tip: Recognize when you might be seeking food for reasons other than hunger, and try a different way to address the cue (eg, chat with a friend or family member, go for a walk).
3. Be careful with alcohol.
GLP-1 RAs are being researched as potential treatments for alcohol use disorder. Many patients report less interest in alcohol and a lower tolerance to alcohol when they are taking a GLP-1 RA. Additionally, GLP-1 RAs may be a risk factor for pancreatitis, which can be caused by consuming too much alcohol.
Patient tip: The standard recommendation remains true: If drinking alcohol, limit to one to two servings per day, but also know that reduced intake or interest is normal when taking a GLP-1 RA.
4. Be aware of sickness vs side effects.
With holiday travel and the winter season, it is common for people to catch a cold or a stomach bug. Symptoms of common illnesses might include fatigue, loss of appetite, or diarrhea. These symptoms overlap with side effects of antiobesity medications like semaglutide and tirzepatide.
Patient tip: If you are experiencing constitutional or gastrointestinal symptoms due to illness, speak with your board-certified obesity medicine doctor, who may recommend a temporary medication adjustment to avoid excess side effects.
5. Stay strong against weight stigma.
The holiday season can be a stressful time, especially as patients are reconnecting with people who have not been a part of their health or weight loss journey. Unfortunately, weight bias and weight stigma remain rampant. Many people don’t understand the biology of obesity and refuse to accept the necessity of medical treatment. They may be surrounded by opinions, often louder and less informed.
Patient tip: Remember that obesity is a medical disease. Tell your nosy cousin that it’s a private health matter and that your decisions are your own.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed financial relationships with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
As an endocrinologist, I treat many patients who have diabetes, obesity, or both. Antiobesity medications, particularly the class of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), are our first support tools when nutrition and physical activity aren’t enough.
1. Be mindful of fullness cues.
GLP-1 RAs increase satiety; they help patients feel fuller sooner within a meal and longer in between meals. This means consuming the “usual” at a holiday gathering makes them feel as if they ate too much, and often this will result in more side effects, such as nausea and reflux.
Patient tip: A good rule of thumb is to anticipate feeling full with half of your usual portion. Start with half a plate and reassess your hunger level after finishing.
2. Distinguish between hunger and “food noise.”
Ask your patients, “Do you ever find yourself eating even when you’re not hungry?” Many people eat because of emotions (eg, stress, anxiety, happiness), social situations, or cultural expectations. This type of food consumption is what scientists call “hedonic food intake” and may be driven by the “food noise” that patients describe as persistent thoughts about food in the absence of physiologic hunger. Semaglutide (Ozempic, Wegovy) has been found to reduce cravings, though other research has shown that emotional eating may blunt the effect of GLP-1 RAs.
Patient tip: Recognize when you might be seeking food for reasons other than hunger, and try a different way to address the cue (eg, chat with a friend or family member, go for a walk).
3. Be careful with alcohol.
GLP-1 RAs are being researched as potential treatments for alcohol use disorder. Many patients report less interest in alcohol and a lower tolerance to alcohol when they are taking a GLP-1 RA. Additionally, GLP-1 RAs may be a risk factor for pancreatitis, which can be caused by consuming too much alcohol.
Patient tip: The standard recommendation remains true: If drinking alcohol, limit to one to two servings per day, but also know that reduced intake or interest is normal when taking a GLP-1 RA.
4. Be aware of sickness vs side effects.
With holiday travel and the winter season, it is common for people to catch a cold or a stomach bug. Symptoms of common illnesses might include fatigue, loss of appetite, or diarrhea. These symptoms overlap with side effects of antiobesity medications like semaglutide and tirzepatide.
Patient tip: If you are experiencing constitutional or gastrointestinal symptoms due to illness, speak with your board-certified obesity medicine doctor, who may recommend a temporary medication adjustment to avoid excess side effects.
5. Stay strong against weight stigma.
The holiday season can be a stressful time, especially as patients are reconnecting with people who have not been a part of their health or weight loss journey. Unfortunately, weight bias and weight stigma remain rampant. Many people don’t understand the biology of obesity and refuse to accept the necessity of medical treatment. They may be surrounded by opinions, often louder and less informed.
Patient tip: Remember that obesity is a medical disease. Tell your nosy cousin that it’s a private health matter and that your decisions are your own.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed financial relationships with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
How to prescribe Zepbound
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 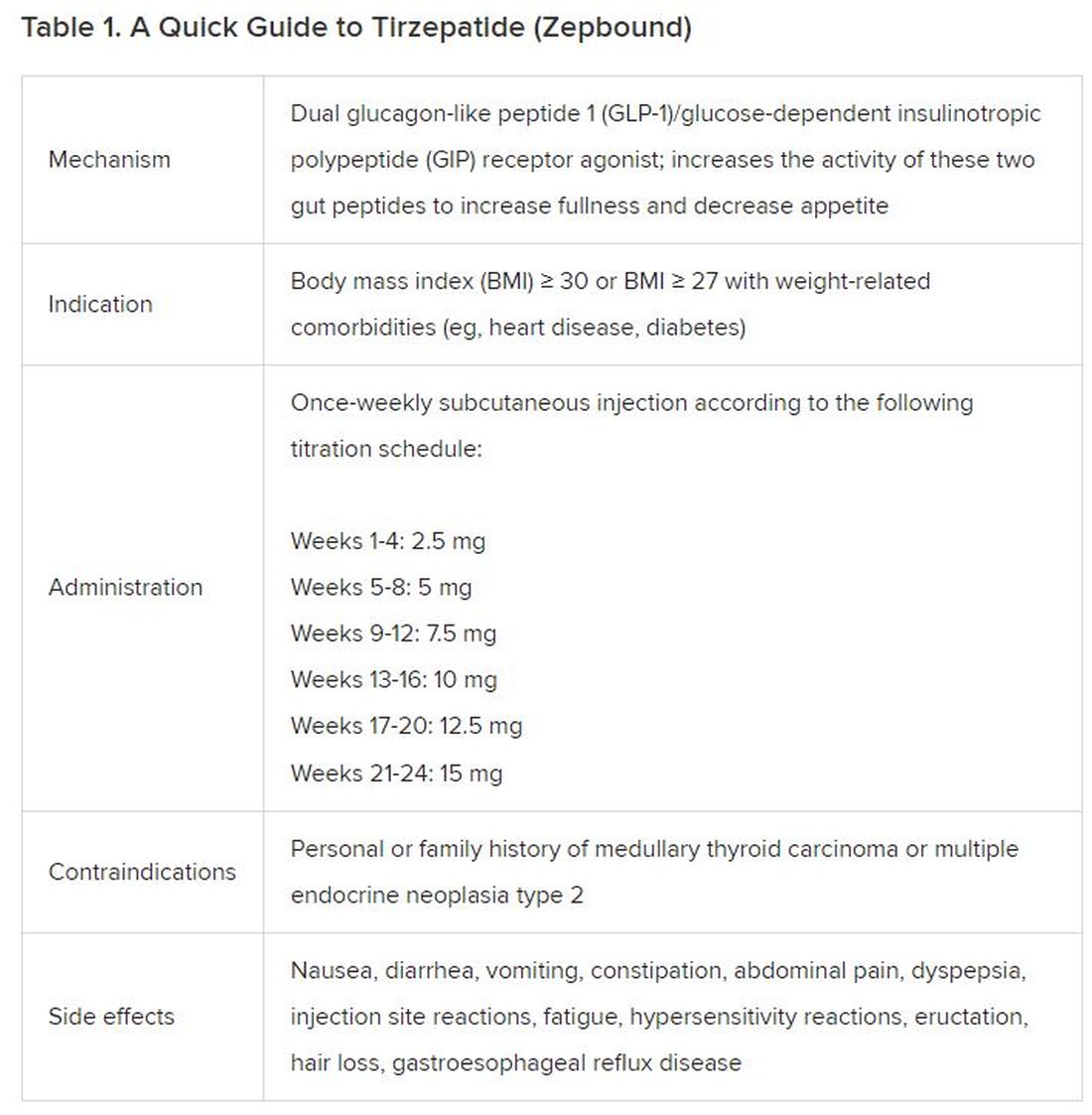
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).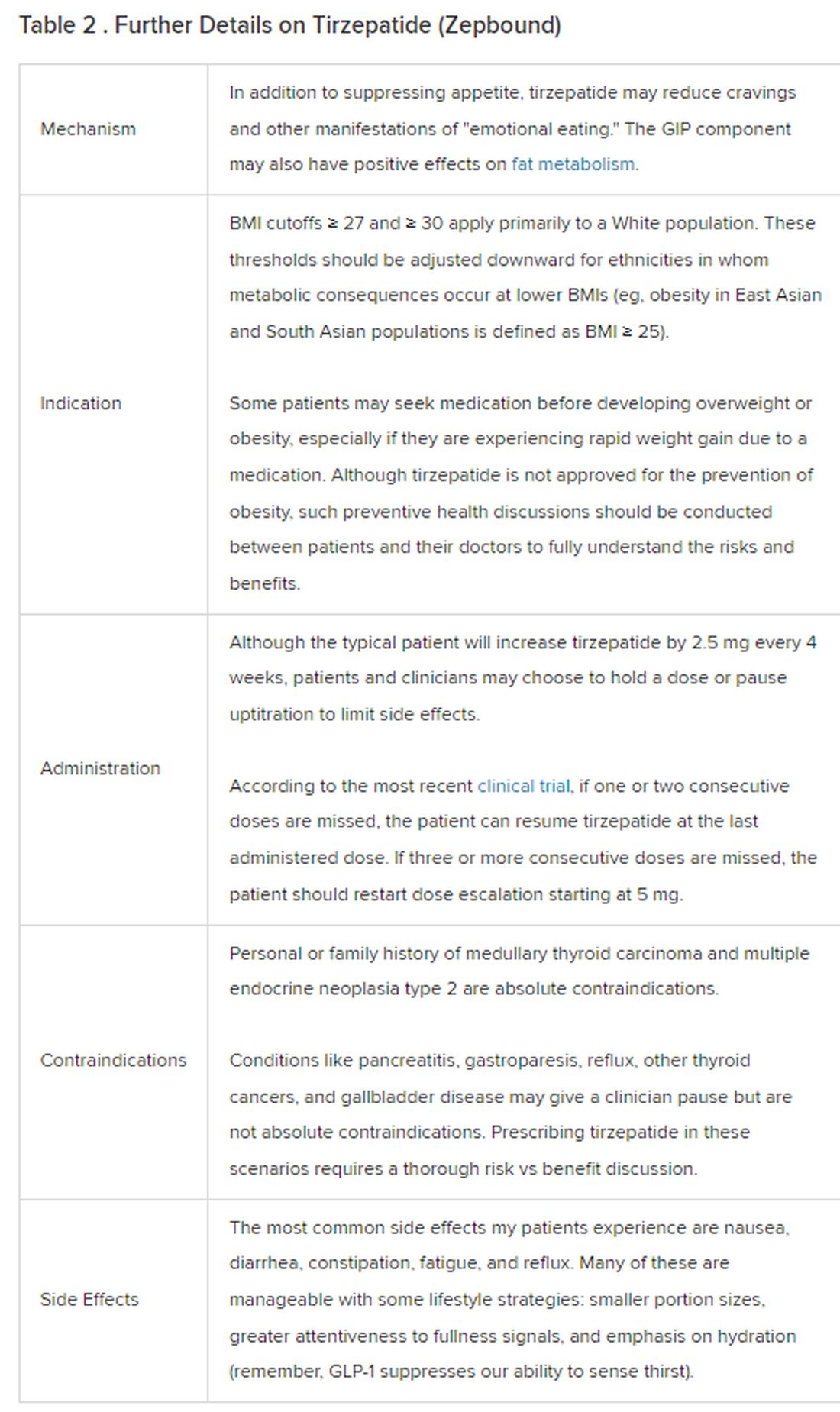
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
When to prescribe semaglutide?
A 36-year-old woman presents to your office for assistance with weight loss. She usually weighs around 150 lb, but she had two pregnancies in the past 4 years and has gained 70 lb. Her current weight is 220 lb with a body mass index (BMI) of 36.6 kg/m2, and she has been unable to lose any weight despite diet and exercise. She reports back pain and generalized fatigue but is primarily worried about developing type 2 diabetes, which runs in her family. Her insurance covers weight loss medications, but
More and more people are turning to “medical weight management” to drop pounds and improve their health. This is a strategy that adds pharmacotherapy to lifestyle modifications to treat the chronic disease of obesity, and it is analogous to the treatment of high blood pressure or high cholesterol with medications.
This patient meets the criteria set forth by the American Heart Association, American College of Cardiology, and The Obesity Society for the management of obesity with antiobesity medications:
- BMI ≥ 30 or BMI ≥ 27 with weight-related comorbidities and
- Has been unable to achieve ≥ 5% weight loss with lifestyle changes alone.
Several U.S. Food and Drug Administration–approved antiobesity medications have been proven to cause clinically significant weight loss:
- orlistat (Alli or Xenical).
- phentermine/topiramate (Qsymia).
- naltrexone/bupropion (Contrave).
- liraglutide 3.0 mg subcutaneously daily (Saxenda).
- semaglutide 2.4 mg subcutaneously weekly (Wegovy).
When considering an antiobesity medication for a patient, it’s important to discuss efficacy, side-effect profile, contraindications, cost and coverage, and long-term use.
In this commentary, we’ll specifically focus on semaglutide (Wegovy) as it is currently the most effective FDA-approved medication for weight loss.
Efficacy
In a phase 3 clinical trial, patients on semaglutide 2.4 mg weekly lost an average of 15% of their body weight at 68 weeks, or approximately 33 lb. It is important to note that there is variability in treatment response to semaglutide 2.4 mg, just like with any other medication. About 1 in 3 individuals lost ≥ 20% of their weight, but about 1 in every 10 patients did not lose any weight.
In this patient, who has a family history of type 2 diabetes, weight loss with semaglutide 2.4 mg will probably reduce her risk of developing diabetes. With just 5%-10% weight loss, she will see improvements in her blood glucose, blood pressure, and cholesterol. Even greater weight loss (≥ 10%) has been associated with resolution of fatty liver and sleep apnea.
Side effects
Before starting semaglutide, patients should be counseled about potential gastrointestinal side effects, including nausea, upset stomach, diarrhea, constipation, and reflux.
Side effects can be managed with dietary modifications, over-the-counter treatments, and slow dose escalation. Some common tips include:
- Eat slowly.
- Eat a bland diet.
- Avoid fatty or fried foods.
- Avoid lying down immediately after eating.
- Prioritize water and fiber intake to mitigate constipation.
- Use over-the-counter treatments as needed (for example, laxative for constipation).
Most of these side effects are present only during dose escalation and resolve once the patient is on a stable dose.
Patients should be counseled about the less than 1% risk for gallbladder issues or pancreatitis. They should be instructed to go to an urgent care or emergency room if they develop severe abdominal pain, recurrent vomiting, or the inability to eat or drink.
Contraindications
We don’t prescribe GLP-1 receptor agonists, including semaglutide 2.4 mg, in patients with a personal or family history of medullary thyroid cancer. GLP-1 agonists are contraindicated in patients with a history of pancreatitis or gastroparesis. All FDA-approved antiobesity medications are contraindicated in women who are breastfeeding or trying for pregnancy. If this patient would like to pursue pregnancy again, semaglutide 2.4 mg should be stopped 2 months prior to conception.
Access
In this case, the patient’s insurance covered semaglutide 2.4 mg with a copay of $25 per month. Without insurance, semaglutide 2.4 mg (Wegovy) costs about $1,400 per month, and semaglutide 2.0 mg (Ozempic), the formulation approved for type 2 diabetes, costs up to $1,000 per month. These price ranges are often cost-prohibitive and unsustainable, especially because these medications are intended for long-term use.
Currently, Medicare does not cover antiobesity medications nor do most state Medicaid plans. Therefore, these medications are usually not considered by patients who have Medicare or Medicaid insurance.
Because insurance coverage varies and out-of-pocket costs can be prohibitive, many individuals seek other ways of acquiring semaglutide. The off-label use of semaglutide 2.0 mg (Ozempic) for obesity is scientifically supported and safe, whereas the use of compounded semaglutide is risky due to lack of regulation.
Compounded semaglutide should be avoided, given that these products are not controlled by the FDA, and adverse events have been reported in connection with compounded semaglutide.
In our clinical practice, patients have reported advertisements for “generic semaglutide” compounded with vitamins like vitamin B12 or B6. This is a significant area of concern because some vitamins (for instance, vitamin B6) are toxic at high doses.
We discussed the dangers of compounded semaglutide with our patient and told her that this isn’t something we recommend prescribing. If the patient didn’t want to wait for semaglutide 2.4 mg to be available at her pharmacy, we discussed alternative medications used for the management of obesity, such as other FDA-approved GLP-1 agonists (that is, liraglutide 3.0 mg) and off-label medications. In this case, the patient opted to wait for semaglutide 2.4 mg because she preferred a weekly injectable medication, given her busy lifestyle as a new mom.
Dr. Schmitz, of Weill Cornell Medicine, New York, disclosed no relevant financial relationships. Dr. Tchang, of Weill Cornell Medicine and the Iris Cantor Women's Health Center, both in New York, serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk, and has received income from Gelesis.
A version of this article first appeared on Medscape.com.
A 36-year-old woman presents to your office for assistance with weight loss. She usually weighs around 150 lb, but she had two pregnancies in the past 4 years and has gained 70 lb. Her current weight is 220 lb with a body mass index (BMI) of 36.6 kg/m2, and she has been unable to lose any weight despite diet and exercise. She reports back pain and generalized fatigue but is primarily worried about developing type 2 diabetes, which runs in her family. Her insurance covers weight loss medications, but
More and more people are turning to “medical weight management” to drop pounds and improve their health. This is a strategy that adds pharmacotherapy to lifestyle modifications to treat the chronic disease of obesity, and it is analogous to the treatment of high blood pressure or high cholesterol with medications.
This patient meets the criteria set forth by the American Heart Association, American College of Cardiology, and The Obesity Society for the management of obesity with antiobesity medications:
- BMI ≥ 30 or BMI ≥ 27 with weight-related comorbidities and
- Has been unable to achieve ≥ 5% weight loss with lifestyle changes alone.
Several U.S. Food and Drug Administration–approved antiobesity medications have been proven to cause clinically significant weight loss:
- orlistat (Alli or Xenical).
- phentermine/topiramate (Qsymia).
- naltrexone/bupropion (Contrave).
- liraglutide 3.0 mg subcutaneously daily (Saxenda).
- semaglutide 2.4 mg subcutaneously weekly (Wegovy).
When considering an antiobesity medication for a patient, it’s important to discuss efficacy, side-effect profile, contraindications, cost and coverage, and long-term use.
In this commentary, we’ll specifically focus on semaglutide (Wegovy) as it is currently the most effective FDA-approved medication for weight loss.
Efficacy
In a phase 3 clinical trial, patients on semaglutide 2.4 mg weekly lost an average of 15% of their body weight at 68 weeks, or approximately 33 lb. It is important to note that there is variability in treatment response to semaglutide 2.4 mg, just like with any other medication. About 1 in 3 individuals lost ≥ 20% of their weight, but about 1 in every 10 patients did not lose any weight.
In this patient, who has a family history of type 2 diabetes, weight loss with semaglutide 2.4 mg will probably reduce her risk of developing diabetes. With just 5%-10% weight loss, she will see improvements in her blood glucose, blood pressure, and cholesterol. Even greater weight loss (≥ 10%) has been associated with resolution of fatty liver and sleep apnea.
Side effects
Before starting semaglutide, patients should be counseled about potential gastrointestinal side effects, including nausea, upset stomach, diarrhea, constipation, and reflux.
Side effects can be managed with dietary modifications, over-the-counter treatments, and slow dose escalation. Some common tips include:
- Eat slowly.
- Eat a bland diet.
- Avoid fatty or fried foods.
- Avoid lying down immediately after eating.
- Prioritize water and fiber intake to mitigate constipation.
- Use over-the-counter treatments as needed (for example, laxative for constipation).
Most of these side effects are present only during dose escalation and resolve once the patient is on a stable dose.
Patients should be counseled about the less than 1% risk for gallbladder issues or pancreatitis. They should be instructed to go to an urgent care or emergency room if they develop severe abdominal pain, recurrent vomiting, or the inability to eat or drink.
Contraindications
We don’t prescribe GLP-1 receptor agonists, including semaglutide 2.4 mg, in patients with a personal or family history of medullary thyroid cancer. GLP-1 agonists are contraindicated in patients with a history of pancreatitis or gastroparesis. All FDA-approved antiobesity medications are contraindicated in women who are breastfeeding or trying for pregnancy. If this patient would like to pursue pregnancy again, semaglutide 2.4 mg should be stopped 2 months prior to conception.
Access
In this case, the patient’s insurance covered semaglutide 2.4 mg with a copay of $25 per month. Without insurance, semaglutide 2.4 mg (Wegovy) costs about $1,400 per month, and semaglutide 2.0 mg (Ozempic), the formulation approved for type 2 diabetes, costs up to $1,000 per month. These price ranges are often cost-prohibitive and unsustainable, especially because these medications are intended for long-term use.
Currently, Medicare does not cover antiobesity medications nor do most state Medicaid plans. Therefore, these medications are usually not considered by patients who have Medicare or Medicaid insurance.
Because insurance coverage varies and out-of-pocket costs can be prohibitive, many individuals seek other ways of acquiring semaglutide. The off-label use of semaglutide 2.0 mg (Ozempic) for obesity is scientifically supported and safe, whereas the use of compounded semaglutide is risky due to lack of regulation.
Compounded semaglutide should be avoided, given that these products are not controlled by the FDA, and adverse events have been reported in connection with compounded semaglutide.
In our clinical practice, patients have reported advertisements for “generic semaglutide” compounded with vitamins like vitamin B12 or B6. This is a significant area of concern because some vitamins (for instance, vitamin B6) are toxic at high doses.
We discussed the dangers of compounded semaglutide with our patient and told her that this isn’t something we recommend prescribing. If the patient didn’t want to wait for semaglutide 2.4 mg to be available at her pharmacy, we discussed alternative medications used for the management of obesity, such as other FDA-approved GLP-1 agonists (that is, liraglutide 3.0 mg) and off-label medications. In this case, the patient opted to wait for semaglutide 2.4 mg because she preferred a weekly injectable medication, given her busy lifestyle as a new mom.
Dr. Schmitz, of Weill Cornell Medicine, New York, disclosed no relevant financial relationships. Dr. Tchang, of Weill Cornell Medicine and the Iris Cantor Women's Health Center, both in New York, serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk, and has received income from Gelesis.
A version of this article first appeared on Medscape.com.
A 36-year-old woman presents to your office for assistance with weight loss. She usually weighs around 150 lb, but she had two pregnancies in the past 4 years and has gained 70 lb. Her current weight is 220 lb with a body mass index (BMI) of 36.6 kg/m2, and she has been unable to lose any weight despite diet and exercise. She reports back pain and generalized fatigue but is primarily worried about developing type 2 diabetes, which runs in her family. Her insurance covers weight loss medications, but
More and more people are turning to “medical weight management” to drop pounds and improve their health. This is a strategy that adds pharmacotherapy to lifestyle modifications to treat the chronic disease of obesity, and it is analogous to the treatment of high blood pressure or high cholesterol with medications.
This patient meets the criteria set forth by the American Heart Association, American College of Cardiology, and The Obesity Society for the management of obesity with antiobesity medications:
- BMI ≥ 30 or BMI ≥ 27 with weight-related comorbidities and
- Has been unable to achieve ≥ 5% weight loss with lifestyle changes alone.
Several U.S. Food and Drug Administration–approved antiobesity medications have been proven to cause clinically significant weight loss:
- orlistat (Alli or Xenical).
- phentermine/topiramate (Qsymia).
- naltrexone/bupropion (Contrave).
- liraglutide 3.0 mg subcutaneously daily (Saxenda).
- semaglutide 2.4 mg subcutaneously weekly (Wegovy).
When considering an antiobesity medication for a patient, it’s important to discuss efficacy, side-effect profile, contraindications, cost and coverage, and long-term use.
In this commentary, we’ll specifically focus on semaglutide (Wegovy) as it is currently the most effective FDA-approved medication for weight loss.
Efficacy
In a phase 3 clinical trial, patients on semaglutide 2.4 mg weekly lost an average of 15% of their body weight at 68 weeks, or approximately 33 lb. It is important to note that there is variability in treatment response to semaglutide 2.4 mg, just like with any other medication. About 1 in 3 individuals lost ≥ 20% of their weight, but about 1 in every 10 patients did not lose any weight.
In this patient, who has a family history of type 2 diabetes, weight loss with semaglutide 2.4 mg will probably reduce her risk of developing diabetes. With just 5%-10% weight loss, she will see improvements in her blood glucose, blood pressure, and cholesterol. Even greater weight loss (≥ 10%) has been associated with resolution of fatty liver and sleep apnea.
Side effects
Before starting semaglutide, patients should be counseled about potential gastrointestinal side effects, including nausea, upset stomach, diarrhea, constipation, and reflux.
Side effects can be managed with dietary modifications, over-the-counter treatments, and slow dose escalation. Some common tips include:
- Eat slowly.
- Eat a bland diet.
- Avoid fatty or fried foods.
- Avoid lying down immediately after eating.
- Prioritize water and fiber intake to mitigate constipation.
- Use over-the-counter treatments as needed (for example, laxative for constipation).
Most of these side effects are present only during dose escalation and resolve once the patient is on a stable dose.
Patients should be counseled about the less than 1% risk for gallbladder issues or pancreatitis. They should be instructed to go to an urgent care or emergency room if they develop severe abdominal pain, recurrent vomiting, or the inability to eat or drink.
Contraindications
We don’t prescribe GLP-1 receptor agonists, including semaglutide 2.4 mg, in patients with a personal or family history of medullary thyroid cancer. GLP-1 agonists are contraindicated in patients with a history of pancreatitis or gastroparesis. All FDA-approved antiobesity medications are contraindicated in women who are breastfeeding or trying for pregnancy. If this patient would like to pursue pregnancy again, semaglutide 2.4 mg should be stopped 2 months prior to conception.
Access
In this case, the patient’s insurance covered semaglutide 2.4 mg with a copay of $25 per month. Without insurance, semaglutide 2.4 mg (Wegovy) costs about $1,400 per month, and semaglutide 2.0 mg (Ozempic), the formulation approved for type 2 diabetes, costs up to $1,000 per month. These price ranges are often cost-prohibitive and unsustainable, especially because these medications are intended for long-term use.
Currently, Medicare does not cover antiobesity medications nor do most state Medicaid plans. Therefore, these medications are usually not considered by patients who have Medicare or Medicaid insurance.
Because insurance coverage varies and out-of-pocket costs can be prohibitive, many individuals seek other ways of acquiring semaglutide. The off-label use of semaglutide 2.0 mg (Ozempic) for obesity is scientifically supported and safe, whereas the use of compounded semaglutide is risky due to lack of regulation.
Compounded semaglutide should be avoided, given that these products are not controlled by the FDA, and adverse events have been reported in connection with compounded semaglutide.
In our clinical practice, patients have reported advertisements for “generic semaglutide” compounded with vitamins like vitamin B12 or B6. This is a significant area of concern because some vitamins (for instance, vitamin B6) are toxic at high doses.
We discussed the dangers of compounded semaglutide with our patient and told her that this isn’t something we recommend prescribing. If the patient didn’t want to wait for semaglutide 2.4 mg to be available at her pharmacy, we discussed alternative medications used for the management of obesity, such as other FDA-approved GLP-1 agonists (that is, liraglutide 3.0 mg) and off-label medications. In this case, the patient opted to wait for semaglutide 2.4 mg because she preferred a weekly injectable medication, given her busy lifestyle as a new mom.
Dr. Schmitz, of Weill Cornell Medicine, New York, disclosed no relevant financial relationships. Dr. Tchang, of Weill Cornell Medicine and the Iris Cantor Women's Health Center, both in New York, serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk, and has received income from Gelesis.
A version of this article first appeared on Medscape.com.