User login
The Patient Portal That Patients Can’t Navigate
Beth Cavanaugh, 79, was starting a new medication when she ran into a modern hurdle: Her doctor’s office required all follow–up questions, even those about side effects of the drug, to go through the patient portal.
Cavanaugh said she did not know how to set up or use the system.
“I tried to explain that, but the receptionist said that was the only way to contact the doctor. I felt lost,” said Cavanaugh, a retired psychotherapist near Albany, New York.
Cavanaugh is far from alone. Many older people balk at the idea of communicating with their physicians over the internet. They may have limited digital skills, have physical challenges, or simply prefer human connection.
As medicine leans harder on electronic portals and telehealth, these patients are finding themselves shut out of their own care. Experts warn this approach deepens inequities in access to care and can worsen health outcomes.
Clinicians should “offer options for various types of communication, such as phone calls or texts, because whenever an older adult — or anyone, for that matter — is given a choice, they feel more empowered and more committed to their care,” said Susan Wehry, MD, associate clinical professor at the University of New England College of Osteopathic Medicine in Biddeford, Maine.
Tech Support
Use of medical communication tools varies among older adults. One study in JAMA Network Open found nearly two thirds of those older than 65 years who filled out surveys via phone or internet had used a patient portal, while a little under half used telehealth, and only 44% used a medical health application.
Older patients tend to fall into two camps, said Neela Patel, MD, MPH, CMD, chief of the Division of Geriatrics and Supportive Care at the UT Health San Antonio.
Her patients “are at two extremes of the spectrum — some technologically savvy and others with limited digital literacy or limited or no access to the Internet,” Patel, who is also the vice chair of the Health Systems Innovations and Technology Committee of the American Geriatric Society, said.
Patel’s practice has dedicated staff to help patients master certain technologies. For example, a pharmacist teaches patients how to use a glucometer and a blood pressure cuff. Other staff teach them how to use smartphone apps that track blood pressure or glucose.
She usually sees patients in person before offering telehealth as an option, ensuring the person has “enough digital literacy to utilize them and that the patient can see and hear the visit.”
If technological limitations impede a telehealth appointment, clinicians can help patients navigate their computer screen. Patel recounted the story of an older woman who was unable to come to the clinic in person, so had a telehealth visit instead.
“She had trouble hearing me, so I asked her to share her screen with me. I walked her through how to do that. Then I showed her where the ‘volume’ button was located. It turns out her volume was at zero,” Patel said. “Once that was adjusted, we were able to proceed with the appointment.”
Educating older adults on how to use health technology does not have to fall upon clinicians and their staff, according to Wehry. She routinely refers her patients to community resources to help them develop digital skills.
Local libraries and community centers often offer digital education. Some retirement communities and assisted living facilities also have tech support personnel or classes available to residents.
Wehry refers some of her patients to the National Digital Equity Center which teaches older adults how to hold a telehealth visit.
Roughly 90% of Patel’s patients are signed up for the patient portal, but they may not be operating the technology, she said. She advises these patients to ask their children or caregivers for help as appropriate.
Teaching patients to use the communication technology early on can also be helpful in other ways. If patients who have been technologically proficient start having difficulty, “it’s a clue there may be cognitive changes, and we follow up on those,” Patel said.
Additional resources to help older adults develop digital competence include Cyber Seniors, Older Adults Technology Services, AARP, AARP Find Digital Courses, Area Agencies on Aging, and Senior Navigator.
Human Touch
Some older adults may simply want a more traditional means of communicating with their clinician. A review of 29 papers, encompassing over 6200 adults older than 60 years, identified several domains affecting the adoption of healthcare technology, two of which were resistance to new technology and having family or friends that could help with.
Wehry said many older adults “don’t resist this technology because they’re unable to figure out how to use it. Instead, they see the technology as too impersonal.”
One study found many older adults fear technologies may end up replacing face-to-face contact.
“I’m beginning to encourage primary care providers to take a step back and refocus on the doctor-patient relationship. When communication is limited to the technological approach, it can erode trust in that relationship,” Wehry said.
The American Medical Association recommends clinicians “provide a method other than electronic communication for patients who are without technological proficiency or access.”
Some busy clinicians might be concerned phone calls will be too time-consuming, Wehry said. Patients should be informed of hours of phone availability, how much time is allotted to calls, and how many days or hours a response may take. Clinicians might also use tools that allow patients to use their cell phone to text their practice with medical questions.
Cavanaugh ended up finding technological help from a professional organizer whom she hired to help rearrange her closets.
“She’s knowledgeable and patient, and she’s helping me with the portal,” she said. “If I hadn’t serendipitously found the organizer, I’d still be struggling and unable to access proper medical care.”
Wehry and Patel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Beth Cavanaugh, 79, was starting a new medication when she ran into a modern hurdle: Her doctor’s office required all follow–up questions, even those about side effects of the drug, to go through the patient portal.
Cavanaugh said she did not know how to set up or use the system.
“I tried to explain that, but the receptionist said that was the only way to contact the doctor. I felt lost,” said Cavanaugh, a retired psychotherapist near Albany, New York.
Cavanaugh is far from alone. Many older people balk at the idea of communicating with their physicians over the internet. They may have limited digital skills, have physical challenges, or simply prefer human connection.
As medicine leans harder on electronic portals and telehealth, these patients are finding themselves shut out of their own care. Experts warn this approach deepens inequities in access to care and can worsen health outcomes.
Clinicians should “offer options for various types of communication, such as phone calls or texts, because whenever an older adult — or anyone, for that matter — is given a choice, they feel more empowered and more committed to their care,” said Susan Wehry, MD, associate clinical professor at the University of New England College of Osteopathic Medicine in Biddeford, Maine.
Tech Support
Use of medical communication tools varies among older adults. One study in JAMA Network Open found nearly two thirds of those older than 65 years who filled out surveys via phone or internet had used a patient portal, while a little under half used telehealth, and only 44% used a medical health application.
Older patients tend to fall into two camps, said Neela Patel, MD, MPH, CMD, chief of the Division of Geriatrics and Supportive Care at the UT Health San Antonio.
Her patients “are at two extremes of the spectrum — some technologically savvy and others with limited digital literacy or limited or no access to the Internet,” Patel, who is also the vice chair of the Health Systems Innovations and Technology Committee of the American Geriatric Society, said.
Patel’s practice has dedicated staff to help patients master certain technologies. For example, a pharmacist teaches patients how to use a glucometer and a blood pressure cuff. Other staff teach them how to use smartphone apps that track blood pressure or glucose.
She usually sees patients in person before offering telehealth as an option, ensuring the person has “enough digital literacy to utilize them and that the patient can see and hear the visit.”
If technological limitations impede a telehealth appointment, clinicians can help patients navigate their computer screen. Patel recounted the story of an older woman who was unable to come to the clinic in person, so had a telehealth visit instead.
“She had trouble hearing me, so I asked her to share her screen with me. I walked her through how to do that. Then I showed her where the ‘volume’ button was located. It turns out her volume was at zero,” Patel said. “Once that was adjusted, we were able to proceed with the appointment.”
Educating older adults on how to use health technology does not have to fall upon clinicians and their staff, according to Wehry. She routinely refers her patients to community resources to help them develop digital skills.
Local libraries and community centers often offer digital education. Some retirement communities and assisted living facilities also have tech support personnel or classes available to residents.
Wehry refers some of her patients to the National Digital Equity Center which teaches older adults how to hold a telehealth visit.
Roughly 90% of Patel’s patients are signed up for the patient portal, but they may not be operating the technology, she said. She advises these patients to ask their children or caregivers for help as appropriate.
Teaching patients to use the communication technology early on can also be helpful in other ways. If patients who have been technologically proficient start having difficulty, “it’s a clue there may be cognitive changes, and we follow up on those,” Patel said.
Additional resources to help older adults develop digital competence include Cyber Seniors, Older Adults Technology Services, AARP, AARP Find Digital Courses, Area Agencies on Aging, and Senior Navigator.
Human Touch
Some older adults may simply want a more traditional means of communicating with their clinician. A review of 29 papers, encompassing over 6200 adults older than 60 years, identified several domains affecting the adoption of healthcare technology, two of which were resistance to new technology and having family or friends that could help with.
Wehry said many older adults “don’t resist this technology because they’re unable to figure out how to use it. Instead, they see the technology as too impersonal.”
One study found many older adults fear technologies may end up replacing face-to-face contact.
“I’m beginning to encourage primary care providers to take a step back and refocus on the doctor-patient relationship. When communication is limited to the technological approach, it can erode trust in that relationship,” Wehry said.
The American Medical Association recommends clinicians “provide a method other than electronic communication for patients who are without technological proficiency or access.”
Some busy clinicians might be concerned phone calls will be too time-consuming, Wehry said. Patients should be informed of hours of phone availability, how much time is allotted to calls, and how many days or hours a response may take. Clinicians might also use tools that allow patients to use their cell phone to text their practice with medical questions.
Cavanaugh ended up finding technological help from a professional organizer whom she hired to help rearrange her closets.
“She’s knowledgeable and patient, and she’s helping me with the portal,” she said. “If I hadn’t serendipitously found the organizer, I’d still be struggling and unable to access proper medical care.”
Wehry and Patel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Beth Cavanaugh, 79, was starting a new medication when she ran into a modern hurdle: Her doctor’s office required all follow–up questions, even those about side effects of the drug, to go through the patient portal.
Cavanaugh said she did not know how to set up or use the system.
“I tried to explain that, but the receptionist said that was the only way to contact the doctor. I felt lost,” said Cavanaugh, a retired psychotherapist near Albany, New York.
Cavanaugh is far from alone. Many older people balk at the idea of communicating with their physicians over the internet. They may have limited digital skills, have physical challenges, or simply prefer human connection.
As medicine leans harder on electronic portals and telehealth, these patients are finding themselves shut out of their own care. Experts warn this approach deepens inequities in access to care and can worsen health outcomes.
Clinicians should “offer options for various types of communication, such as phone calls or texts, because whenever an older adult — or anyone, for that matter — is given a choice, they feel more empowered and more committed to their care,” said Susan Wehry, MD, associate clinical professor at the University of New England College of Osteopathic Medicine in Biddeford, Maine.
Tech Support
Use of medical communication tools varies among older adults. One study in JAMA Network Open found nearly two thirds of those older than 65 years who filled out surveys via phone or internet had used a patient portal, while a little under half used telehealth, and only 44% used a medical health application.
Older patients tend to fall into two camps, said Neela Patel, MD, MPH, CMD, chief of the Division of Geriatrics and Supportive Care at the UT Health San Antonio.
Her patients “are at two extremes of the spectrum — some technologically savvy and others with limited digital literacy or limited or no access to the Internet,” Patel, who is also the vice chair of the Health Systems Innovations and Technology Committee of the American Geriatric Society, said.
Patel’s practice has dedicated staff to help patients master certain technologies. For example, a pharmacist teaches patients how to use a glucometer and a blood pressure cuff. Other staff teach them how to use smartphone apps that track blood pressure or glucose.
She usually sees patients in person before offering telehealth as an option, ensuring the person has “enough digital literacy to utilize them and that the patient can see and hear the visit.”
If technological limitations impede a telehealth appointment, clinicians can help patients navigate their computer screen. Patel recounted the story of an older woman who was unable to come to the clinic in person, so had a telehealth visit instead.
“She had trouble hearing me, so I asked her to share her screen with me. I walked her through how to do that. Then I showed her where the ‘volume’ button was located. It turns out her volume was at zero,” Patel said. “Once that was adjusted, we were able to proceed with the appointment.”
Educating older adults on how to use health technology does not have to fall upon clinicians and their staff, according to Wehry. She routinely refers her patients to community resources to help them develop digital skills.
Local libraries and community centers often offer digital education. Some retirement communities and assisted living facilities also have tech support personnel or classes available to residents.
Wehry refers some of her patients to the National Digital Equity Center which teaches older adults how to hold a telehealth visit.
Roughly 90% of Patel’s patients are signed up for the patient portal, but they may not be operating the technology, she said. She advises these patients to ask their children or caregivers for help as appropriate.
Teaching patients to use the communication technology early on can also be helpful in other ways. If patients who have been technologically proficient start having difficulty, “it’s a clue there may be cognitive changes, and we follow up on those,” Patel said.
Additional resources to help older adults develop digital competence include Cyber Seniors, Older Adults Technology Services, AARP, AARP Find Digital Courses, Area Agencies on Aging, and Senior Navigator.
Human Touch
Some older adults may simply want a more traditional means of communicating with their clinician. A review of 29 papers, encompassing over 6200 adults older than 60 years, identified several domains affecting the adoption of healthcare technology, two of which were resistance to new technology and having family or friends that could help with.
Wehry said many older adults “don’t resist this technology because they’re unable to figure out how to use it. Instead, they see the technology as too impersonal.”
One study found many older adults fear technologies may end up replacing face-to-face contact.
“I’m beginning to encourage primary care providers to take a step back and refocus on the doctor-patient relationship. When communication is limited to the technological approach, it can erode trust in that relationship,” Wehry said.
The American Medical Association recommends clinicians “provide a method other than electronic communication for patients who are without technological proficiency or access.”
Some busy clinicians might be concerned phone calls will be too time-consuming, Wehry said. Patients should be informed of hours of phone availability, how much time is allotted to calls, and how many days or hours a response may take. Clinicians might also use tools that allow patients to use their cell phone to text their practice with medical questions.
Cavanaugh ended up finding technological help from a professional organizer whom she hired to help rearrange her closets.
“She’s knowledgeable and patient, and she’s helping me with the portal,” she said. “If I hadn’t serendipitously found the organizer, I’d still be struggling and unable to access proper medical care.”
Wehry and Patel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nutrition, Drugs, or Bariatric Surgery: What’s the Best Approach for Sustained Weight Loss?
Given that more than 100 million US adults have obesity, including 22 million with severe obesity, physicians regularly see patients with the condition in their practices.
Fortunately, doctors have more tools than ever to help their patients. But the question remains: Which method is the safest and most effective? Is it diet and lifestyle changes, one of the recently approved anti-obesity medications (AOMs), bariatric surgery, or a combination approach?
There are no head-to-head trials comparing these three approaches, said Vanita Rahman, MD, clinic director of the Barnard Medical Center, Washington, DC, at the International Conference on Nutrition in Medicine, sponsored by the Physicians Committee for Responsible Medicine.
Instead, doctors must evaluate the merits and drawbacks of each intervention and decide with their patients which treatment is best for them, she told Medscape Medical News. When she sees patients, Rahman shares the pertinent research with them, so they are able to make an informed choice.
Looking at the Options
In her presentation at the conference, Rahman summarized the guidelines issued by the American Heart Association/American College of Cardiology/The Obesity Society for Management of Overweight and Obesity in Adults and the American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care of Patients with Obesity, including lifestyle changes, AOMs, and bariatric surgery (Table 1).
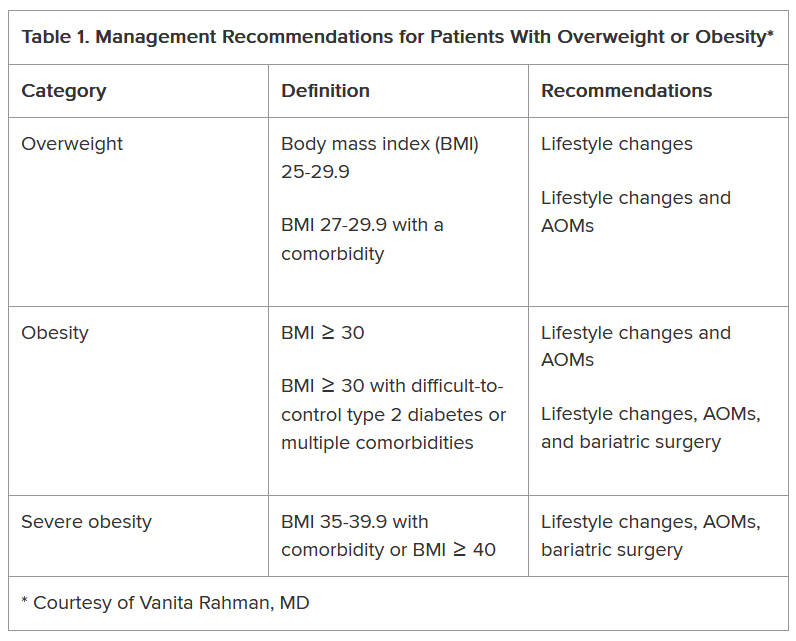
As shown, the current clinical guidelines offer recommendations that consider such factors as the patient’s BMI and presence of one or more comorbidities. Generally, they begin with lifestyle changes for people with overweight, the possibility of an AOM for those with obesity, and bariatric surgery as an option for those with severe obesity-related complications.
“In obesity, we traditionally thought the process was ‘either-or’ — either lifestyle or surgery or medication — and somehow lifestyle is better,” Sheethal Reddy, PhD, a psychologist at the Bariatric Center at Emory University Hospital Midtown, Atlanta, told Medscape Medical News.
Now physicians often use a combination of methods, but lifestyle is foundational to all of them, she said.
“If you don’t make lifestyle changes, none of the approaches will ultimately be effective,” said Reddy, who also is an assistant professor in the Division of General and GI Surgery at Emory School of Medicine, Atlanta.
Lifestyle changes don’t just involve diet and nutrition but include physical exercise.
“Being sedentary affects everything — sleep quality, appetite regulation, and metabolism. Without sufficient exercise, the body isn’t functioning well enough to have a healthy metabolism,” Reddy said.
How Durable Are the Interventions?
Although bariatric surgery has demonstrated effectiveness in helping patients lose weight, many of them regain some or most of it, Rahman said.
A systematic review and meta-analysis found weight regain in 49% of patients who underwent bariatric surgery patients, with the highest prevalence after Roux-en-Y gastric bypass.
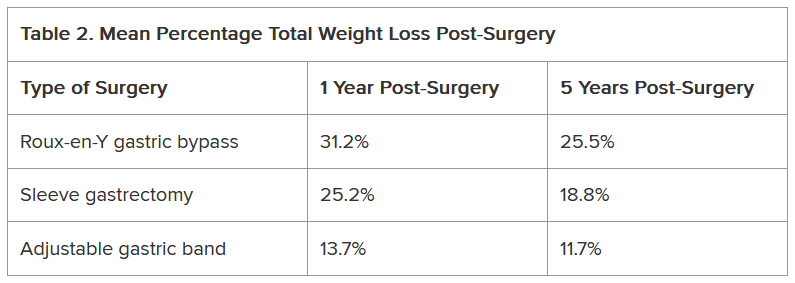
Another study of approximately 45,000 patients who underwent bariatric surgery found differences not only in the percentage of total weight loss among Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric band procedures but also in how much of that weight stayed off between 1 and 5 years following the procedure (Table 2).
Weight regain also is a risk with AOMs, if they’re discontinued.
The STEP 1 trial tested the effectiveness of semaglutide — a glucagon-like peptide 1 (GLP-1) receptor agonist — as an adjunct to lifestyle intervention for weight loss in patients with obesity or with overweight and at least one comorbidity but not diabetes. Mean weight loss with semaglutide was 17.3% but that figure dropped 11.6 percentage points after treatment was discontinued.
Other studies also have found that patients regain weight after GLP-1 discontinuation.
Tirzepatide, a GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) combination, has shown efficacy with weight reduction, but patients experienced some weight regain upon discontinuation. In one study, patients experienced a mean weight loss of 20.9% after 36 weeks of tirzepatide. In the study’s subsequent 52-week double-blind, placebo-controlled period, patients who stopped taking the medication experienced a weight regain of 14%, whereas those who remained on the medication lost an additional 5.5% of weight.
GLP-1 and GLP-1/GIP medications do not address the factors that contribute to overweight and obesity, Rahman said. “They simply suppress the appetite; therefore, weight gain occurs after stopping them.”
Patients may stop taking anti-obesity drugs for a variety of reasons, including side effects. Rahman noted that the common side effects include nausea, vomiting, and constipation, whereas rare side effects include gastroparesis, gallbladder and biliary disease, thyroid cancer, and suicidal thoughts. GLP-1 and GLP-1/GIP medications also carry a risk for non-arteritic anterior ischemic optic neuropathy, she said.
Moreover, health insurance does not always cover these medications, which likely affects patient access to the drugs and compliance rates.
“Given the side effects and frequent lack of insurance coverage, significant questions remain about long-term safety and feasibility of these agents,” Rahman said.
What About Nutritional Approaches?
The lifestyle interventions in the semaglutide and tirzepatide studies included 500 kcal/d deficit diets, which is difficult for people to maintain, noted Rahman, who is the author of the book Simply Plant Based: Fabulous Food for a Healthy Life.
Additionally, bariatric surgery has been associated with long-term micronutrient deficiencies, including deficiencies in vitamins A, D, E, K, B1, and B12, as well as folate, iron, zinc, copper, selenium, and calcium, she said.
The best approach to food from a patient compliance standpoint and to avoid nutrient deficiencies is a whole-food, plant-based diet, Rahman said. She advocates this nutritional approach, along with physical activity, for patients regardless of whether they’ve selected lifestyle intervention alone or combined with an AOM or bariatric surgery to address obesity.
Rahman cited a 5-year heart disease study comparing an intensive lifestyle program involving a vegetarian diet, aerobic exercise, stress management training, smoking cessation, and group psychosocial support to treatment as usual. Patients in the lifestyle group lost 10.9 kg at 1 year and sustained weight loss of 5.8 kg at 5 years, whereas weight in the control group remained relatively unchanged from baseline.
She also pointed to the findings of a study of patients with obesity or with overweight and at least one comorbidity that compared standard care with a low-fat, whole-food, plant-based diet with vitamin B12 supplementation. At 6 months, mean BMI reduction was greater in the intervention group than the standard care group (−4.4 vs −0.4).
In her practice, Rahman has seen the benefits of a whole-food, plant-based diet for patients with obesity.
If people are committed to this type of dietary approach and are given the tools and resources to do it effectively, “their thinking changes, their taste buds change, and they grow to enjoy this new way of eating,” she said. “They see results, and it’s a lifestyle that can be sustained long-term.”
Addressing Drivers of Weight Gain
Patients also need help addressing the various factors that may contribute to overweight and obesity, including overconsumption of ultra-processed foods, substandard nutritional quality of restaurant foods, increasing portion sizes, distraction during eating, emotional eating, late-night eating, and cultural/traditional values surrounding food, Rahman noted.
Supatra Tovar, PsyD, RD, a clinical psychologist with a practice in Pasadena, California, agreed that identifying the reasons for weight gain is critical for treatment.
“If you’re not addressing underlying issues, such as a person’s relationship with food, behaviors around food, the tendency to mindlessly eat or emotionally eat or eat to seek comfort, the person’s weight problems won’t ultimately be fully solved by any of the three approaches — dieting, medications, or bariatric surgery,” she said.
Some of her patients “engage in extreme dieting and deprivation, and many who use medications or have had bariatric surgery hardly eat and often develop nutritional deficiencies,” said Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life.
The key to healthy and sustained weight loss is to “become attuned to the body’s signals, learn how to honor hunger, stop eating when satisfied, and eat more healthful foods, such as fruits and vegetables, whole grains, lean proteins — especially plant-based proteins — and the body gives signals that this is what it wants,” she said.
Tovar doesn’t give her clients a specific diet or set of portions.
“I teach them to listen to their bodies,” she said. “They’ve lost significant amounts of weight and continued to keep it off because they’ve done this kind of work.”
When Lifestyle Changes Aren’t Enough
For many patients, lifestyle interventions are insufficient to address the degree of overweight and obesity and common comorbidities, said W. Timothy Garvey, MD, associate director and professor, Department of Nutrition Sciences, School of Health Professions, University of Alabama at Birmingham.
“Of course, nutritional approaches are very important, not only for weight but also for general health-related reasons,” said Garvey, lead author of the 2016 American Association of Clinical Endocrinologists obesity guidelines. “We’ve seen that the Mediterranean and some plant-based diets can prevent progression from prediabetes to diabetes and improve other parameters that reflect metabolic health.”
However, it’s “not common that patients can follow these diets, lose weight, and keep it off,” Garvey cautioned. Up to 50% of weight that’s lost through lifestyle changes is typically regained by 1-year follow-up, with almost all remaining lost weight subsequently regained in the majority of individuals because the person “has to fight against pathophysiological process that drive weight regain,” he noted.
Weight-loss medications can address these pathophysiologic processes by “addressing interactions of satiety hormones with feeding centers in the brain, suppressing the appetite, and making it easier for patients to adhere to a reduced-calorie diet.”
Garvey views the weight-loss medications in the same light as drugs for diabetes and hypertension, in that people need to keep taking them to sustain the benefit.
There’s still a role for bariatric surgery because not everyone can tolerate the AOMs or achieve sufficient weight loss.
“Patients with very high BMI who have trouble ambulating might benefit from a combination of bariatric surgery and medication,” Garvey said.
While some side effects are associated with AOMs, being an “alarmist” about them can be detrimental to patients, he warned.
Rahman and Tovar are authors of books about weight loss. Reddy reported no relevant financial relationships. Garvey is a consultant on advisory boards for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Pfizer, Fractyl Health, Alnylam Pharmaceuticals, Inogen, Zealand, Allurion, Carmot/Roche, Terns Pharmaceuticals, Neurocrine, Keros Therapeutics, and Regeneron. He is the site principal investigator for multi-centered clinical trials sponsored by his university and funded by Novo Nordisk, Eli Lilly, Epitomee, Neurovalens, and Pfizer. He serves as a consultant on the advisory board for the nonprofit Milken Foundation and is a member of the Data Monitoring Committee for phase 3 clinical trials conducted by Boehringer-Ingelheim and Eli Lilly.
A version of this article first appeared on Medscape.com.
Given that more than 100 million US adults have obesity, including 22 million with severe obesity, physicians regularly see patients with the condition in their practices.
Fortunately, doctors have more tools than ever to help their patients. But the question remains: Which method is the safest and most effective? Is it diet and lifestyle changes, one of the recently approved anti-obesity medications (AOMs), bariatric surgery, or a combination approach?
There are no head-to-head trials comparing these three approaches, said Vanita Rahman, MD, clinic director of the Barnard Medical Center, Washington, DC, at the International Conference on Nutrition in Medicine, sponsored by the Physicians Committee for Responsible Medicine.
Instead, doctors must evaluate the merits and drawbacks of each intervention and decide with their patients which treatment is best for them, she told Medscape Medical News. When she sees patients, Rahman shares the pertinent research with them, so they are able to make an informed choice.
Looking at the Options
In her presentation at the conference, Rahman summarized the guidelines issued by the American Heart Association/American College of Cardiology/The Obesity Society for Management of Overweight and Obesity in Adults and the American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care of Patients with Obesity, including lifestyle changes, AOMs, and bariatric surgery (Table 1).

As shown, the current clinical guidelines offer recommendations that consider such factors as the patient’s BMI and presence of one or more comorbidities. Generally, they begin with lifestyle changes for people with overweight, the possibility of an AOM for those with obesity, and bariatric surgery as an option for those with severe obesity-related complications.
“In obesity, we traditionally thought the process was ‘either-or’ — either lifestyle or surgery or medication — and somehow lifestyle is better,” Sheethal Reddy, PhD, a psychologist at the Bariatric Center at Emory University Hospital Midtown, Atlanta, told Medscape Medical News.
Now physicians often use a combination of methods, but lifestyle is foundational to all of them, she said.
“If you don’t make lifestyle changes, none of the approaches will ultimately be effective,” said Reddy, who also is an assistant professor in the Division of General and GI Surgery at Emory School of Medicine, Atlanta.
Lifestyle changes don’t just involve diet and nutrition but include physical exercise.
“Being sedentary affects everything — sleep quality, appetite regulation, and metabolism. Without sufficient exercise, the body isn’t functioning well enough to have a healthy metabolism,” Reddy said.
How Durable Are the Interventions?
Although bariatric surgery has demonstrated effectiveness in helping patients lose weight, many of them regain some or most of it, Rahman said.
A systematic review and meta-analysis found weight regain in 49% of patients who underwent bariatric surgery patients, with the highest prevalence after Roux-en-Y gastric bypass.
Another study of approximately 45,000 patients who underwent bariatric surgery found differences not only in the percentage of total weight loss among Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric band procedures but also in how much of that weight stayed off between 1 and 5 years following the procedure (Table 2).

Weight regain also is a risk with AOMs, if they’re discontinued.
The STEP 1 trial tested the effectiveness of semaglutide — a glucagon-like peptide 1 (GLP-1) receptor agonist — as an adjunct to lifestyle intervention for weight loss in patients with obesity or with overweight and at least one comorbidity but not diabetes. Mean weight loss with semaglutide was 17.3% but that figure dropped 11.6 percentage points after treatment was discontinued.
Other studies also have found that patients regain weight after GLP-1 discontinuation.
Tirzepatide, a GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) combination, has shown efficacy with weight reduction, but patients experienced some weight regain upon discontinuation. In one study, patients experienced a mean weight loss of 20.9% after 36 weeks of tirzepatide. In the study’s subsequent 52-week double-blind, placebo-controlled period, patients who stopped taking the medication experienced a weight regain of 14%, whereas those who remained on the medication lost an additional 5.5% of weight.
GLP-1 and GLP-1/GIP medications do not address the factors that contribute to overweight and obesity, Rahman said. “They simply suppress the appetite; therefore, weight gain occurs after stopping them.”
Patients may stop taking anti-obesity drugs for a variety of reasons, including side effects. Rahman noted that the common side effects include nausea, vomiting, and constipation, whereas rare side effects include gastroparesis, gallbladder and biliary disease, thyroid cancer, and suicidal thoughts. GLP-1 and GLP-1/GIP medications also carry a risk for non-arteritic anterior ischemic optic neuropathy, she said.
Moreover, health insurance does not always cover these medications, which likely affects patient access to the drugs and compliance rates.
“Given the side effects and frequent lack of insurance coverage, significant questions remain about long-term safety and feasibility of these agents,” Rahman said.
What About Nutritional Approaches?
The lifestyle interventions in the semaglutide and tirzepatide studies included 500 kcal/d deficit diets, which is difficult for people to maintain, noted Rahman, who is the author of the book Simply Plant Based: Fabulous Food for a Healthy Life.
Additionally, bariatric surgery has been associated with long-term micronutrient deficiencies, including deficiencies in vitamins A, D, E, K, B1, and B12, as well as folate, iron, zinc, copper, selenium, and calcium, she said.
The best approach to food from a patient compliance standpoint and to avoid nutrient deficiencies is a whole-food, plant-based diet, Rahman said. She advocates this nutritional approach, along with physical activity, for patients regardless of whether they’ve selected lifestyle intervention alone or combined with an AOM or bariatric surgery to address obesity.
Rahman cited a 5-year heart disease study comparing an intensive lifestyle program involving a vegetarian diet, aerobic exercise, stress management training, smoking cessation, and group psychosocial support to treatment as usual. Patients in the lifestyle group lost 10.9 kg at 1 year and sustained weight loss of 5.8 kg at 5 years, whereas weight in the control group remained relatively unchanged from baseline.
She also pointed to the findings of a study of patients with obesity or with overweight and at least one comorbidity that compared standard care with a low-fat, whole-food, plant-based diet with vitamin B12 supplementation. At 6 months, mean BMI reduction was greater in the intervention group than the standard care group (−4.4 vs −0.4).
In her practice, Rahman has seen the benefits of a whole-food, plant-based diet for patients with obesity.
If people are committed to this type of dietary approach and are given the tools and resources to do it effectively, “their thinking changes, their taste buds change, and they grow to enjoy this new way of eating,” she said. “They see results, and it’s a lifestyle that can be sustained long-term.”
Addressing Drivers of Weight Gain
Patients also need help addressing the various factors that may contribute to overweight and obesity, including overconsumption of ultra-processed foods, substandard nutritional quality of restaurant foods, increasing portion sizes, distraction during eating, emotional eating, late-night eating, and cultural/traditional values surrounding food, Rahman noted.
Supatra Tovar, PsyD, RD, a clinical psychologist with a practice in Pasadena, California, agreed that identifying the reasons for weight gain is critical for treatment.
“If you’re not addressing underlying issues, such as a person’s relationship with food, behaviors around food, the tendency to mindlessly eat or emotionally eat or eat to seek comfort, the person’s weight problems won’t ultimately be fully solved by any of the three approaches — dieting, medications, or bariatric surgery,” she said.
Some of her patients “engage in extreme dieting and deprivation, and many who use medications or have had bariatric surgery hardly eat and often develop nutritional deficiencies,” said Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life.
The key to healthy and sustained weight loss is to “become attuned to the body’s signals, learn how to honor hunger, stop eating when satisfied, and eat more healthful foods, such as fruits and vegetables, whole grains, lean proteins — especially plant-based proteins — and the body gives signals that this is what it wants,” she said.
Tovar doesn’t give her clients a specific diet or set of portions.
“I teach them to listen to their bodies,” she said. “They’ve lost significant amounts of weight and continued to keep it off because they’ve done this kind of work.”
When Lifestyle Changes Aren’t Enough
For many patients, lifestyle interventions are insufficient to address the degree of overweight and obesity and common comorbidities, said W. Timothy Garvey, MD, associate director and professor, Department of Nutrition Sciences, School of Health Professions, University of Alabama at Birmingham.
“Of course, nutritional approaches are very important, not only for weight but also for general health-related reasons,” said Garvey, lead author of the 2016 American Association of Clinical Endocrinologists obesity guidelines. “We’ve seen that the Mediterranean and some plant-based diets can prevent progression from prediabetes to diabetes and improve other parameters that reflect metabolic health.”
However, it’s “not common that patients can follow these diets, lose weight, and keep it off,” Garvey cautioned. Up to 50% of weight that’s lost through lifestyle changes is typically regained by 1-year follow-up, with almost all remaining lost weight subsequently regained in the majority of individuals because the person “has to fight against pathophysiological process that drive weight regain,” he noted.
Weight-loss medications can address these pathophysiologic processes by “addressing interactions of satiety hormones with feeding centers in the brain, suppressing the appetite, and making it easier for patients to adhere to a reduced-calorie diet.”
Garvey views the weight-loss medications in the same light as drugs for diabetes and hypertension, in that people need to keep taking them to sustain the benefit.
There’s still a role for bariatric surgery because not everyone can tolerate the AOMs or achieve sufficient weight loss.
“Patients with very high BMI who have trouble ambulating might benefit from a combination of bariatric surgery and medication,” Garvey said.
While some side effects are associated with AOMs, being an “alarmist” about them can be detrimental to patients, he warned.
Rahman and Tovar are authors of books about weight loss. Reddy reported no relevant financial relationships. Garvey is a consultant on advisory boards for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Pfizer, Fractyl Health, Alnylam Pharmaceuticals, Inogen, Zealand, Allurion, Carmot/Roche, Terns Pharmaceuticals, Neurocrine, Keros Therapeutics, and Regeneron. He is the site principal investigator for multi-centered clinical trials sponsored by his university and funded by Novo Nordisk, Eli Lilly, Epitomee, Neurovalens, and Pfizer. He serves as a consultant on the advisory board for the nonprofit Milken Foundation and is a member of the Data Monitoring Committee for phase 3 clinical trials conducted by Boehringer-Ingelheim and Eli Lilly.
A version of this article first appeared on Medscape.com.
Given that more than 100 million US adults have obesity, including 22 million with severe obesity, physicians regularly see patients with the condition in their practices.
Fortunately, doctors have more tools than ever to help their patients. But the question remains: Which method is the safest and most effective? Is it diet and lifestyle changes, one of the recently approved anti-obesity medications (AOMs), bariatric surgery, or a combination approach?
There are no head-to-head trials comparing these three approaches, said Vanita Rahman, MD, clinic director of the Barnard Medical Center, Washington, DC, at the International Conference on Nutrition in Medicine, sponsored by the Physicians Committee for Responsible Medicine.
Instead, doctors must evaluate the merits and drawbacks of each intervention and decide with their patients which treatment is best for them, she told Medscape Medical News. When she sees patients, Rahman shares the pertinent research with them, so they are able to make an informed choice.
Looking at the Options
In her presentation at the conference, Rahman summarized the guidelines issued by the American Heart Association/American College of Cardiology/The Obesity Society for Management of Overweight and Obesity in Adults and the American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care of Patients with Obesity, including lifestyle changes, AOMs, and bariatric surgery (Table 1).

As shown, the current clinical guidelines offer recommendations that consider such factors as the patient’s BMI and presence of one or more comorbidities. Generally, they begin with lifestyle changes for people with overweight, the possibility of an AOM for those with obesity, and bariatric surgery as an option for those with severe obesity-related complications.
“In obesity, we traditionally thought the process was ‘either-or’ — either lifestyle or surgery or medication — and somehow lifestyle is better,” Sheethal Reddy, PhD, a psychologist at the Bariatric Center at Emory University Hospital Midtown, Atlanta, told Medscape Medical News.
Now physicians often use a combination of methods, but lifestyle is foundational to all of them, she said.
“If you don’t make lifestyle changes, none of the approaches will ultimately be effective,” said Reddy, who also is an assistant professor in the Division of General and GI Surgery at Emory School of Medicine, Atlanta.
Lifestyle changes don’t just involve diet and nutrition but include physical exercise.
“Being sedentary affects everything — sleep quality, appetite regulation, and metabolism. Without sufficient exercise, the body isn’t functioning well enough to have a healthy metabolism,” Reddy said.
How Durable Are the Interventions?
Although bariatric surgery has demonstrated effectiveness in helping patients lose weight, many of them regain some or most of it, Rahman said.
A systematic review and meta-analysis found weight regain in 49% of patients who underwent bariatric surgery patients, with the highest prevalence after Roux-en-Y gastric bypass.
Another study of approximately 45,000 patients who underwent bariatric surgery found differences not only in the percentage of total weight loss among Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric band procedures but also in how much of that weight stayed off between 1 and 5 years following the procedure (Table 2).

Weight regain also is a risk with AOMs, if they’re discontinued.
The STEP 1 trial tested the effectiveness of semaglutide — a glucagon-like peptide 1 (GLP-1) receptor agonist — as an adjunct to lifestyle intervention for weight loss in patients with obesity or with overweight and at least one comorbidity but not diabetes. Mean weight loss with semaglutide was 17.3% but that figure dropped 11.6 percentage points after treatment was discontinued.
Other studies also have found that patients regain weight after GLP-1 discontinuation.
Tirzepatide, a GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) combination, has shown efficacy with weight reduction, but patients experienced some weight regain upon discontinuation. In one study, patients experienced a mean weight loss of 20.9% after 36 weeks of tirzepatide. In the study’s subsequent 52-week double-blind, placebo-controlled period, patients who stopped taking the medication experienced a weight regain of 14%, whereas those who remained on the medication lost an additional 5.5% of weight.
GLP-1 and GLP-1/GIP medications do not address the factors that contribute to overweight and obesity, Rahman said. “They simply suppress the appetite; therefore, weight gain occurs after stopping them.”
Patients may stop taking anti-obesity drugs for a variety of reasons, including side effects. Rahman noted that the common side effects include nausea, vomiting, and constipation, whereas rare side effects include gastroparesis, gallbladder and biliary disease, thyroid cancer, and suicidal thoughts. GLP-1 and GLP-1/GIP medications also carry a risk for non-arteritic anterior ischemic optic neuropathy, she said.
Moreover, health insurance does not always cover these medications, which likely affects patient access to the drugs and compliance rates.
“Given the side effects and frequent lack of insurance coverage, significant questions remain about long-term safety and feasibility of these agents,” Rahman said.
What About Nutritional Approaches?
The lifestyle interventions in the semaglutide and tirzepatide studies included 500 kcal/d deficit diets, which is difficult for people to maintain, noted Rahman, who is the author of the book Simply Plant Based: Fabulous Food for a Healthy Life.
Additionally, bariatric surgery has been associated with long-term micronutrient deficiencies, including deficiencies in vitamins A, D, E, K, B1, and B12, as well as folate, iron, zinc, copper, selenium, and calcium, she said.
The best approach to food from a patient compliance standpoint and to avoid nutrient deficiencies is a whole-food, plant-based diet, Rahman said. She advocates this nutritional approach, along with physical activity, for patients regardless of whether they’ve selected lifestyle intervention alone or combined with an AOM or bariatric surgery to address obesity.
Rahman cited a 5-year heart disease study comparing an intensive lifestyle program involving a vegetarian diet, aerobic exercise, stress management training, smoking cessation, and group psychosocial support to treatment as usual. Patients in the lifestyle group lost 10.9 kg at 1 year and sustained weight loss of 5.8 kg at 5 years, whereas weight in the control group remained relatively unchanged from baseline.
She also pointed to the findings of a study of patients with obesity or with overweight and at least one comorbidity that compared standard care with a low-fat, whole-food, plant-based diet with vitamin B12 supplementation. At 6 months, mean BMI reduction was greater in the intervention group than the standard care group (−4.4 vs −0.4).
In her practice, Rahman has seen the benefits of a whole-food, plant-based diet for patients with obesity.
If people are committed to this type of dietary approach and are given the tools and resources to do it effectively, “their thinking changes, their taste buds change, and they grow to enjoy this new way of eating,” she said. “They see results, and it’s a lifestyle that can be sustained long-term.”
Addressing Drivers of Weight Gain
Patients also need help addressing the various factors that may contribute to overweight and obesity, including overconsumption of ultra-processed foods, substandard nutritional quality of restaurant foods, increasing portion sizes, distraction during eating, emotional eating, late-night eating, and cultural/traditional values surrounding food, Rahman noted.
Supatra Tovar, PsyD, RD, a clinical psychologist with a practice in Pasadena, California, agreed that identifying the reasons for weight gain is critical for treatment.
“If you’re not addressing underlying issues, such as a person’s relationship with food, behaviors around food, the tendency to mindlessly eat or emotionally eat or eat to seek comfort, the person’s weight problems won’t ultimately be fully solved by any of the three approaches — dieting, medications, or bariatric surgery,” she said.
Some of her patients “engage in extreme dieting and deprivation, and many who use medications or have had bariatric surgery hardly eat and often develop nutritional deficiencies,” said Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life.
The key to healthy and sustained weight loss is to “become attuned to the body’s signals, learn how to honor hunger, stop eating when satisfied, and eat more healthful foods, such as fruits and vegetables, whole grains, lean proteins — especially plant-based proteins — and the body gives signals that this is what it wants,” she said.
Tovar doesn’t give her clients a specific diet or set of portions.
“I teach them to listen to their bodies,” she said. “They’ve lost significant amounts of weight and continued to keep it off because they’ve done this kind of work.”
When Lifestyle Changes Aren’t Enough
For many patients, lifestyle interventions are insufficient to address the degree of overweight and obesity and common comorbidities, said W. Timothy Garvey, MD, associate director and professor, Department of Nutrition Sciences, School of Health Professions, University of Alabama at Birmingham.
“Of course, nutritional approaches are very important, not only for weight but also for general health-related reasons,” said Garvey, lead author of the 2016 American Association of Clinical Endocrinologists obesity guidelines. “We’ve seen that the Mediterranean and some plant-based diets can prevent progression from prediabetes to diabetes and improve other parameters that reflect metabolic health.”
However, it’s “not common that patients can follow these diets, lose weight, and keep it off,” Garvey cautioned. Up to 50% of weight that’s lost through lifestyle changes is typically regained by 1-year follow-up, with almost all remaining lost weight subsequently regained in the majority of individuals because the person “has to fight against pathophysiological process that drive weight regain,” he noted.
Weight-loss medications can address these pathophysiologic processes by “addressing interactions of satiety hormones with feeding centers in the brain, suppressing the appetite, and making it easier for patients to adhere to a reduced-calorie diet.”
Garvey views the weight-loss medications in the same light as drugs for diabetes and hypertension, in that people need to keep taking them to sustain the benefit.
There’s still a role for bariatric surgery because not everyone can tolerate the AOMs or achieve sufficient weight loss.
“Patients with very high BMI who have trouble ambulating might benefit from a combination of bariatric surgery and medication,” Garvey said.
While some side effects are associated with AOMs, being an “alarmist” about them can be detrimental to patients, he warned.
Rahman and Tovar are authors of books about weight loss. Reddy reported no relevant financial relationships. Garvey is a consultant on advisory boards for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Pfizer, Fractyl Health, Alnylam Pharmaceuticals, Inogen, Zealand, Allurion, Carmot/Roche, Terns Pharmaceuticals, Neurocrine, Keros Therapeutics, and Regeneron. He is the site principal investigator for multi-centered clinical trials sponsored by his university and funded by Novo Nordisk, Eli Lilly, Epitomee, Neurovalens, and Pfizer. He serves as a consultant on the advisory board for the nonprofit Milken Foundation and is a member of the Data Monitoring Committee for phase 3 clinical trials conducted by Boehringer-Ingelheim and Eli Lilly.
A version of this article first appeared on Medscape.com.
Are Patients On GLP-1s Getting the Right Nutrients?
As the use of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) continues to exponentially expand obesity treatment, concerns have arisen regarding their impact on nutrition in people who take them.
While the medications’ dampening effects on appetite result in an average weight reduction ≥ 15%, they also pose a risk for malnutrition.
“It’s important to eat a balanced diet when taking these medications,” Deena Adimoolam, MD, an endocrinologist based in New York City and a member of the national advisory committees for the Endocrine Society and the American Diabetes Association, said in an interview.
The decreased caloric intake resulting from the use of GLP-1 RAs makes it essential for patients to consume nutrient-dense foods. Clinicians can help patients achieve a healthy diet by anticipating nutrition problems, advising them on recommended target ranges of nutrient intake, and referring them for appropriate counseling.
Where to Begin
The task begins with “setting the right expectations before the patient starts treatment,” said Scott Isaacs, MD, president-elect of the American Association of Clinical Endocrinology.
To that end, it’s important to explain to patients how the medications affect appetite and how to adapt. GLP-1 RAs don’t completely turn off the appetite, and the effect at the beginning will likely be very mild, Isaacs said in an interview.
Some patients don’t notice a change for 2-3 months, although others see an effect sooner.
“Typically, people will notice that the main impact is on satiation, meaning they’ll fill up more quickly,” said Isaacs, who is an adjunct associate professor at Emory University School of Medicine, Atlanta, Georgia. “It’s important to tell them to stop eating when they feel full because eating when full can increase the side effects, such as nausea, vomiting, diarrhea, and constipation.”
A review article, written by lead author Jaime Almandoz, MD, University of Texas Southwestern Medical Center, Dallas, in Obesity offers a “5 A’s model” as a guide on how to begin discussing overweight or obesity with patients. This involves asking for permission to discuss weight and asking about food and vitamin/supplement intake; assessing the patient’s medical history and root causes of obesity, and conducting a physical examination; advising the patient regarding treatment options and reasonable expectations; agreeing on treatment and lifestyle goals; and assisting the patient to address challenges, referring them as needed to for additional support (eg, a dietitian), as well as arranging for follow-up.
Impact of GLP-1 RAs on Food Preferences
Besides reducing hunger and increasing satiety, GLP-1 RAs may affect food preferences, according to a research review published in The International Journal of Obesity. It cites a 2014 study that found that people taking GLP-1 RAs displayed decreased neuronal responses to images of food measured by functional magnetic resonance imaging in the areas of brain associated with appetite and reward. This might affect taste preferences and food intake.
Additionally, a 2023 study suggested that during the weight-loss phase of treatment (as opposed to the maintenance phase), patients may experience reduced cravings for dairy and starchy food, less desire to eat salty or spicy foods, and less difficulty controlling eating and resisting cravings.
“Altered food preferences, decreased food cravings, and reduced food intake may contribute to long-term weight loss,” according to the research review. Tailored treatments focusing on the weight maintenance phase are needed, the authors wrote.
Are Patients Vulnerable to Malnutrition?
A recent review found that total caloric intake was reduced by 16%-39% in patients taking a GLP-1 RA or dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA, but few studies evaluated the composition of these patients’ diets. Research that examines the qualitative changes in macronutrient and micronutrient intake of patients on these medications is needed, the authors wrote.
They outlined several nutritional concerns, including whether GLP-1 RA or GIP/ GLP-1 RA use could result in protein intake insufficient for maintaining muscle strength, mass, and function or in inadequate dietary quality (ie, poor intake of micronutrients, fiber, and fluid).
“Although we don’t necessarily see ‘malnutrition’ in our practice, we do see patients who lose too much weight after months and months of treatment, patients who aren’t hungry and don’t eat all day and have one big meal at the end of the day because they don’t feel like eating, and people who continue to eat unhealthy foods,” Isaacs said.
Some patients, however, have medical histories placing them at a greater risk for malnutrition. “Identification of these individuals may help prevent more serious nutritional and medical complications that might occur with decreased food intake associated with AOMs [anti-obesity medications],” Almandoz and colleagues noted in their review.
What Should Patients Eat?
Nutritional needs vary based on the patient’s age, sex, body weight, physical activity, and other factors, Almandoz and colleagues wrote. For this reason, energy intake during weight loss should be “personalized.”
The authors also recommended specific sources of the various dietary components and noted red flags signaling potential deficiencies
Nutritional needs vary based on the degree of appetite suppression in the patient, Adimoolam said. “I recommend at least two servings of fruits and vegetables daily, and drinking plenty of water throughout the day,” she added.
Protein in particular is a “key macronutrient,” and insufficient intake can lead to a variety of adverse effects, including sarcopenia — which is already a concern in individuals being treated with GLP-1 RAs. Meal replacement products (eg, shakes or bars) can supplement diets to help meet protein needs, especially if appetite is significantly reduced.
“There are definitely concerns for sarcopenia, so we have our patients taking these drugs try to eat healthy lean proteins – 100 g/d — and exercise,” Isaacs said. Exercise, including resistance training, not only improves muscle mass but also potentiates the effects of the GLP-1 RAs in patients with obesity and type 2 diabetes.
Adequate hydration is essential for patients taking GLP-1 RAs. “One of the commonly described side effects is fatigue, but there’s no biological reason why these medications should cause fatigue. My opinion is that these patients are dehydrated, and that may be causing the fatigue,” Isaacs said.
Some patients taking GLP-1 RAs lose interest in food. Isaacs regarded this as an “adverse reaction to the medication, which necessitates either stopping it altogether, changing the dose, or adjusting the diet.” There are “many different solutions, and one size doesn’t fit all,” he said.
Dietary and Behavioral Counseling
The drugs don’t necessarily motivate a person to eat healthier food, only to eat less food, Isaacs noted.
“The person might be eating low-volume but high-calorie food, such as bag of chips or a cookie instead of an apple,” Isaacs said. Patients who are losing weight “may not realize that weight loss isn’t the only important outcome. Because they’re losing weight, they think it’s okay to eat junk food.”
Patients need education and guidance about how to eat while on these medications. Most patients find counseling about meal planning helpful, he said.
Isaacs gives nutritional guidance to his patients when he prescribes a weight loss medication. “But most physicians don’t have time to offer that type of specific counseling on an ongoing basis,” he said. Isaacs refers patients requiring more detailed and long-term guidance to a dietitian.
Patients with monotonous diets of poor quality are at increased risk for nutrition deficiencies, and counseling by a registered dietitian could help improve their dietary quality.
Registered dietitians can develop a multifaceted approach not only focusing on medication management but also on customizing the patient’s diet, assisting with lifestyle adjustments, and addressing the mental health issues surrounding obesity and its management.
People seeking obesity treatment often have psychiatric conditions, psychological distress, or disordered eating patterns, and questions and concerns have emerged about how GLP-1 RA use might affect existing mental health problems. For example, if the medication suppresses the feeling of gratification a person once got from eating high-energy dense foods, that individual may “seek rewards or pleasure elsewhere, and possibly from unhealthy sources.”
Psychological issues also may emerge as a result of weight loss, so it’s helpful to take a multidisciplinary approach that includes mental health practitioners to support patients who are being treated with GLP-1 RAs. Patients taking these agents should be monitored for the emergence or worsening of psychiatric conditions, such as depression and suicidal ideation.
Achieving significant weight loss may lead to “unexpected changes” in the dynamics of patients’ relationship with others, “which can be distressing.” Clinicians should be “sensitive to patients’ social and emotional needs” and provide support or refer patients for help with coping strategies.
GLP-1 RAs have enormous potential to improve health outcomes in patients with obesity. Careful patient selection, close monitoring, and support for patients with nutrition and other lifestyle issues can increase the chances that these agents will fulfill their potential.
Isaacs declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
As the use of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) continues to exponentially expand obesity treatment, concerns have arisen regarding their impact on nutrition in people who take them.
While the medications’ dampening effects on appetite result in an average weight reduction ≥ 15%, they also pose a risk for malnutrition.
“It’s important to eat a balanced diet when taking these medications,” Deena Adimoolam, MD, an endocrinologist based in New York City and a member of the national advisory committees for the Endocrine Society and the American Diabetes Association, said in an interview.
The decreased caloric intake resulting from the use of GLP-1 RAs makes it essential for patients to consume nutrient-dense foods. Clinicians can help patients achieve a healthy diet by anticipating nutrition problems, advising them on recommended target ranges of nutrient intake, and referring them for appropriate counseling.
Where to Begin
The task begins with “setting the right expectations before the patient starts treatment,” said Scott Isaacs, MD, president-elect of the American Association of Clinical Endocrinology.
To that end, it’s important to explain to patients how the medications affect appetite and how to adapt. GLP-1 RAs don’t completely turn off the appetite, and the effect at the beginning will likely be very mild, Isaacs said in an interview.
Some patients don’t notice a change for 2-3 months, although others see an effect sooner.
“Typically, people will notice that the main impact is on satiation, meaning they’ll fill up more quickly,” said Isaacs, who is an adjunct associate professor at Emory University School of Medicine, Atlanta, Georgia. “It’s important to tell them to stop eating when they feel full because eating when full can increase the side effects, such as nausea, vomiting, diarrhea, and constipation.”
A review article, written by lead author Jaime Almandoz, MD, University of Texas Southwestern Medical Center, Dallas, in Obesity offers a “5 A’s model” as a guide on how to begin discussing overweight or obesity with patients. This involves asking for permission to discuss weight and asking about food and vitamin/supplement intake; assessing the patient’s medical history and root causes of obesity, and conducting a physical examination; advising the patient regarding treatment options and reasonable expectations; agreeing on treatment and lifestyle goals; and assisting the patient to address challenges, referring them as needed to for additional support (eg, a dietitian), as well as arranging for follow-up.
Impact of GLP-1 RAs on Food Preferences
Besides reducing hunger and increasing satiety, GLP-1 RAs may affect food preferences, according to a research review published in The International Journal of Obesity. It cites a 2014 study that found that people taking GLP-1 RAs displayed decreased neuronal responses to images of food measured by functional magnetic resonance imaging in the areas of brain associated with appetite and reward. This might affect taste preferences and food intake.
Additionally, a 2023 study suggested that during the weight-loss phase of treatment (as opposed to the maintenance phase), patients may experience reduced cravings for dairy and starchy food, less desire to eat salty or spicy foods, and less difficulty controlling eating and resisting cravings.
“Altered food preferences, decreased food cravings, and reduced food intake may contribute to long-term weight loss,” according to the research review. Tailored treatments focusing on the weight maintenance phase are needed, the authors wrote.
Are Patients Vulnerable to Malnutrition?
A recent review found that total caloric intake was reduced by 16%-39% in patients taking a GLP-1 RA or dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA, but few studies evaluated the composition of these patients’ diets. Research that examines the qualitative changes in macronutrient and micronutrient intake of patients on these medications is needed, the authors wrote.
They outlined several nutritional concerns, including whether GLP-1 RA or GIP/ GLP-1 RA use could result in protein intake insufficient for maintaining muscle strength, mass, and function or in inadequate dietary quality (ie, poor intake of micronutrients, fiber, and fluid).
“Although we don’t necessarily see ‘malnutrition’ in our practice, we do see patients who lose too much weight after months and months of treatment, patients who aren’t hungry and don’t eat all day and have one big meal at the end of the day because they don’t feel like eating, and people who continue to eat unhealthy foods,” Isaacs said.
Some patients, however, have medical histories placing them at a greater risk for malnutrition. “Identification of these individuals may help prevent more serious nutritional and medical complications that might occur with decreased food intake associated with AOMs [anti-obesity medications],” Almandoz and colleagues noted in their review.
What Should Patients Eat?
Nutritional needs vary based on the patient’s age, sex, body weight, physical activity, and other factors, Almandoz and colleagues wrote. For this reason, energy intake during weight loss should be “personalized.”
The authors also recommended specific sources of the various dietary components and noted red flags signaling potential deficiencies
Nutritional needs vary based on the degree of appetite suppression in the patient, Adimoolam said. “I recommend at least two servings of fruits and vegetables daily, and drinking plenty of water throughout the day,” she added.
Protein in particular is a “key macronutrient,” and insufficient intake can lead to a variety of adverse effects, including sarcopenia — which is already a concern in individuals being treated with GLP-1 RAs. Meal replacement products (eg, shakes or bars) can supplement diets to help meet protein needs, especially if appetite is significantly reduced.
“There are definitely concerns for sarcopenia, so we have our patients taking these drugs try to eat healthy lean proteins – 100 g/d — and exercise,” Isaacs said. Exercise, including resistance training, not only improves muscle mass but also potentiates the effects of the GLP-1 RAs in patients with obesity and type 2 diabetes.
Adequate hydration is essential for patients taking GLP-1 RAs. “One of the commonly described side effects is fatigue, but there’s no biological reason why these medications should cause fatigue. My opinion is that these patients are dehydrated, and that may be causing the fatigue,” Isaacs said.
Some patients taking GLP-1 RAs lose interest in food. Isaacs regarded this as an “adverse reaction to the medication, which necessitates either stopping it altogether, changing the dose, or adjusting the diet.” There are “many different solutions, and one size doesn’t fit all,” he said.
Dietary and Behavioral Counseling
The drugs don’t necessarily motivate a person to eat healthier food, only to eat less food, Isaacs noted.
“The person might be eating low-volume but high-calorie food, such as bag of chips or a cookie instead of an apple,” Isaacs said. Patients who are losing weight “may not realize that weight loss isn’t the only important outcome. Because they’re losing weight, they think it’s okay to eat junk food.”
Patients need education and guidance about how to eat while on these medications. Most patients find counseling about meal planning helpful, he said.
Isaacs gives nutritional guidance to his patients when he prescribes a weight loss medication. “But most physicians don’t have time to offer that type of specific counseling on an ongoing basis,” he said. Isaacs refers patients requiring more detailed and long-term guidance to a dietitian.
Patients with monotonous diets of poor quality are at increased risk for nutrition deficiencies, and counseling by a registered dietitian could help improve their dietary quality.
Registered dietitians can develop a multifaceted approach not only focusing on medication management but also on customizing the patient’s diet, assisting with lifestyle adjustments, and addressing the mental health issues surrounding obesity and its management.
People seeking obesity treatment often have psychiatric conditions, psychological distress, or disordered eating patterns, and questions and concerns have emerged about how GLP-1 RA use might affect existing mental health problems. For example, if the medication suppresses the feeling of gratification a person once got from eating high-energy dense foods, that individual may “seek rewards or pleasure elsewhere, and possibly from unhealthy sources.”
Psychological issues also may emerge as a result of weight loss, so it’s helpful to take a multidisciplinary approach that includes mental health practitioners to support patients who are being treated with GLP-1 RAs. Patients taking these agents should be monitored for the emergence or worsening of psychiatric conditions, such as depression and suicidal ideation.
Achieving significant weight loss may lead to “unexpected changes” in the dynamics of patients’ relationship with others, “which can be distressing.” Clinicians should be “sensitive to patients’ social and emotional needs” and provide support or refer patients for help with coping strategies.
GLP-1 RAs have enormous potential to improve health outcomes in patients with obesity. Careful patient selection, close monitoring, and support for patients with nutrition and other lifestyle issues can increase the chances that these agents will fulfill their potential.
Isaacs declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
As the use of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) continues to exponentially expand obesity treatment, concerns have arisen regarding their impact on nutrition in people who take them.
While the medications’ dampening effects on appetite result in an average weight reduction ≥ 15%, they also pose a risk for malnutrition.
“It’s important to eat a balanced diet when taking these medications,” Deena Adimoolam, MD, an endocrinologist based in New York City and a member of the national advisory committees for the Endocrine Society and the American Diabetes Association, said in an interview.
The decreased caloric intake resulting from the use of GLP-1 RAs makes it essential for patients to consume nutrient-dense foods. Clinicians can help patients achieve a healthy diet by anticipating nutrition problems, advising them on recommended target ranges of nutrient intake, and referring them for appropriate counseling.
Where to Begin
The task begins with “setting the right expectations before the patient starts treatment,” said Scott Isaacs, MD, president-elect of the American Association of Clinical Endocrinology.
To that end, it’s important to explain to patients how the medications affect appetite and how to adapt. GLP-1 RAs don’t completely turn off the appetite, and the effect at the beginning will likely be very mild, Isaacs said in an interview.
Some patients don’t notice a change for 2-3 months, although others see an effect sooner.
“Typically, people will notice that the main impact is on satiation, meaning they’ll fill up more quickly,” said Isaacs, who is an adjunct associate professor at Emory University School of Medicine, Atlanta, Georgia. “It’s important to tell them to stop eating when they feel full because eating when full can increase the side effects, such as nausea, vomiting, diarrhea, and constipation.”
A review article, written by lead author Jaime Almandoz, MD, University of Texas Southwestern Medical Center, Dallas, in Obesity offers a “5 A’s model” as a guide on how to begin discussing overweight or obesity with patients. This involves asking for permission to discuss weight and asking about food and vitamin/supplement intake; assessing the patient’s medical history and root causes of obesity, and conducting a physical examination; advising the patient regarding treatment options and reasonable expectations; agreeing on treatment and lifestyle goals; and assisting the patient to address challenges, referring them as needed to for additional support (eg, a dietitian), as well as arranging for follow-up.
Impact of GLP-1 RAs on Food Preferences
Besides reducing hunger and increasing satiety, GLP-1 RAs may affect food preferences, according to a research review published in The International Journal of Obesity. It cites a 2014 study that found that people taking GLP-1 RAs displayed decreased neuronal responses to images of food measured by functional magnetic resonance imaging in the areas of brain associated with appetite and reward. This might affect taste preferences and food intake.
Additionally, a 2023 study suggested that during the weight-loss phase of treatment (as opposed to the maintenance phase), patients may experience reduced cravings for dairy and starchy food, less desire to eat salty or spicy foods, and less difficulty controlling eating and resisting cravings.
“Altered food preferences, decreased food cravings, and reduced food intake may contribute to long-term weight loss,” according to the research review. Tailored treatments focusing on the weight maintenance phase are needed, the authors wrote.
Are Patients Vulnerable to Malnutrition?
A recent review found that total caloric intake was reduced by 16%-39% in patients taking a GLP-1 RA or dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA, but few studies evaluated the composition of these patients’ diets. Research that examines the qualitative changes in macronutrient and micronutrient intake of patients on these medications is needed, the authors wrote.
They outlined several nutritional concerns, including whether GLP-1 RA or GIP/ GLP-1 RA use could result in protein intake insufficient for maintaining muscle strength, mass, and function or in inadequate dietary quality (ie, poor intake of micronutrients, fiber, and fluid).
“Although we don’t necessarily see ‘malnutrition’ in our practice, we do see patients who lose too much weight after months and months of treatment, patients who aren’t hungry and don’t eat all day and have one big meal at the end of the day because they don’t feel like eating, and people who continue to eat unhealthy foods,” Isaacs said.
Some patients, however, have medical histories placing them at a greater risk for malnutrition. “Identification of these individuals may help prevent more serious nutritional and medical complications that might occur with decreased food intake associated with AOMs [anti-obesity medications],” Almandoz and colleagues noted in their review.
What Should Patients Eat?
Nutritional needs vary based on the patient’s age, sex, body weight, physical activity, and other factors, Almandoz and colleagues wrote. For this reason, energy intake during weight loss should be “personalized.”
The authors also recommended specific sources of the various dietary components and noted red flags signaling potential deficiencies
Nutritional needs vary based on the degree of appetite suppression in the patient, Adimoolam said. “I recommend at least two servings of fruits and vegetables daily, and drinking plenty of water throughout the day,” she added.
Protein in particular is a “key macronutrient,” and insufficient intake can lead to a variety of adverse effects, including sarcopenia — which is already a concern in individuals being treated with GLP-1 RAs. Meal replacement products (eg, shakes or bars) can supplement diets to help meet protein needs, especially if appetite is significantly reduced.
“There are definitely concerns for sarcopenia, so we have our patients taking these drugs try to eat healthy lean proteins – 100 g/d — and exercise,” Isaacs said. Exercise, including resistance training, not only improves muscle mass but also potentiates the effects of the GLP-1 RAs in patients with obesity and type 2 diabetes.
Adequate hydration is essential for patients taking GLP-1 RAs. “One of the commonly described side effects is fatigue, but there’s no biological reason why these medications should cause fatigue. My opinion is that these patients are dehydrated, and that may be causing the fatigue,” Isaacs said.
Some patients taking GLP-1 RAs lose interest in food. Isaacs regarded this as an “adverse reaction to the medication, which necessitates either stopping it altogether, changing the dose, or adjusting the diet.” There are “many different solutions, and one size doesn’t fit all,” he said.
Dietary and Behavioral Counseling
The drugs don’t necessarily motivate a person to eat healthier food, only to eat less food, Isaacs noted.
“The person might be eating low-volume but high-calorie food, such as bag of chips or a cookie instead of an apple,” Isaacs said. Patients who are losing weight “may not realize that weight loss isn’t the only important outcome. Because they’re losing weight, they think it’s okay to eat junk food.”
Patients need education and guidance about how to eat while on these medications. Most patients find counseling about meal planning helpful, he said.
Isaacs gives nutritional guidance to his patients when he prescribes a weight loss medication. “But most physicians don’t have time to offer that type of specific counseling on an ongoing basis,” he said. Isaacs refers patients requiring more detailed and long-term guidance to a dietitian.
Patients with monotonous diets of poor quality are at increased risk for nutrition deficiencies, and counseling by a registered dietitian could help improve their dietary quality.
Registered dietitians can develop a multifaceted approach not only focusing on medication management but also on customizing the patient’s diet, assisting with lifestyle adjustments, and addressing the mental health issues surrounding obesity and its management.
People seeking obesity treatment often have psychiatric conditions, psychological distress, or disordered eating patterns, and questions and concerns have emerged about how GLP-1 RA use might affect existing mental health problems. For example, if the medication suppresses the feeling of gratification a person once got from eating high-energy dense foods, that individual may “seek rewards or pleasure elsewhere, and possibly from unhealthy sources.”
Psychological issues also may emerge as a result of weight loss, so it’s helpful to take a multidisciplinary approach that includes mental health practitioners to support patients who are being treated with GLP-1 RAs. Patients taking these agents should be monitored for the emergence or worsening of psychiatric conditions, such as depression and suicidal ideation.
Achieving significant weight loss may lead to “unexpected changes” in the dynamics of patients’ relationship with others, “which can be distressing.” Clinicians should be “sensitive to patients’ social and emotional needs” and provide support or refer patients for help with coping strategies.
GLP-1 RAs have enormous potential to improve health outcomes in patients with obesity. Careful patient selection, close monitoring, and support for patients with nutrition and other lifestyle issues can increase the chances that these agents will fulfill their potential.
Isaacs declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
Cultural Respect vs Individual Patient Autonomy: A Delicate Balancing Act
Cultural competency is one of the most important values in the practice of medicine. Defined as the “ability to collaborate effectively with individuals from different cultures,” this type of competence “improves healthcare experiences and outcomes.” But within the context of cultural familiarity, it’s equally important to “understand that each person is an individual and may or may not adhere to certain cultural beliefs or practices common in his or her culture,” according to the Agency for Healthcare Research and Quality’s (AHRQ’s) Health Literacy Universal Precautions Toolkit.
Sarah Candler, MD, MPH, an internal medicine physician specializing in primary care for older adults in Washington, DC, said that the medical code of ethics consists of several pillars, with patient autonomy as the “first and most primary of those pillars.” She calls the balance of patient autonomy and cultural respect a “complicated tightrope to walk,” but says that these ethical principles can inform medical decisions and the patient-physician relationship.
Cultural Familiarity
It’s important to be as familiar as possible with the patient’s culture, Santina Wheat, MD, program director, Northwestern McGaw Family Medicine Residency at Delnor Hospital, Geneva, told this news organization. “For example, we serve many Orthodox Jewish patients. We had a meeting with rabbis from the community to present to us what religious laws might affect our patients. Until recently, I was delivering babies, and there was always a 24-hour emergency rabbi on call if an Orthodox patient wanted the input of a rabbi into her decisions.”
Jay W. Lee, MD, MPH, a member of the board of directors of the American Academy of Family Physicians, also sets out to educate himself about the cultural norms of his patients if they come from populations he’s not familiar with. “For example, this comes up when a new refugee population comes to the United States — most recently, there was a population of Afghan refugees,” Lee told this news organization.
Lee spent “a lot of time trying to learn about their cultural norms,” which prepared him to “ask more targeted questions about the patient’s understanding of the tests we were ordering or treatment options we were bringing forward.”
Lee, also the medical director at Integrated Health Partners of Southern California and associate clinical professor of family medicine at the University of California, Irvine, said it might be best if the physician is “language congruent or culturally similar.” Lee is of Asian descent and also speaks Spanish fluently. “I enjoy cultural exchanges with my patients, and I encourage patients to find a physician who’s the best fit.” But being from the same culture isn’t absolutely necessary for building relationships with the patient. “The key is offering the patient autonomy” while understanding the cultural context.
Don’t Assume ... Always Ask
Cultural familiarity doesn’t equate with stereotyping, Wheat emphasized. “Proceeding without assumptions opens the opportunity to ask questions for clarification and understanding and to improve patient care,” said Lee.
Sara Glass, PhD, LCSW, agrees. She’s the clinical director of Soul Wellness NYC, New York City, a psychotherapy practice that specializes in treating trauma. Based on her own experiences, she knows that some physicians and other healthcare professionals confuse cultural sensitivity with cultural stereotyping.
Glass, formerly Hasidic and ultra-Orthodox, shared an example from her own life. During the delivery of her second child, she sustained a vaginal tear. At her 6-week postpartum visit, her ob/gyn said, “Just remind me when you’re in your ninth month next time, and I can sew it up right after you deliver.”
Much of this physician’s practice “consisted of Hasidic women who looked just like me, wearing the same garb — head coverings such as wigs and scarves and long skirts. Most women in that community have multiple pregnancies,” Glass told this news organization. “My sister has 10 children, and that’s not unusual. The doctor simply assumed I’d be going on to have more babies without asking if that’s what I wanted.”
Glass says she was also never given information by her physician about the range of available contraceptive options. The rabbis of the Hasidic sect to which Glass belonged allowed women to practice contraception for 6 months following childbirth, or for longer, in the setting of certain medical conditions, but only certain types of birth control were religiously permissible. Other options were not mentioned to her by her physician, and she didn’t know that they existed.
Making no assumptions applies not only to patients from other cultures but also to all patients — including members of “mainstream American culture.”
Candler recalls a young patient with a new baby, who shared “how exhausted she was and how much time, energy, and work it took to care for children,” Candler recounted. “To me, it sounded as though she didn’t want another child, and I was about to offer contraception when it occurred to me to first ask if she wanted to have more children.” Candler was surprised when the patient said that, although she wasn’t actively looking to become pregnant again, she didn’t want to take preventive measures. “I’m so glad I asked, rather than simply assuming.”
Culture Is Mutable
Important questions to ask patients include whether there are aspects of their culture or religion that might affect their care — which can include medications they may feel uncomfortable using — and what family members they want to have involved in clinical discussions and decisions, said Wheat.
Lee described treating a refugee from Afghanistan who was in her sixth month of pregnancy. “I quickly needed to learn about what her expectations were for her care and my presence as a male on her care team,” he recounted. Lee arranged for the patient to receive prenatal care from a different clinician and arranged for follow-up for her husband and children. “Everyone had good results.”
Candler noted that some patients choose their physician specifically because that practitioner is conversant with their culture and respectful of its mores — especially when physicians share the same culture as the patient. But that level of familiarity can make it easy to forget to ask questions about the experience of the individual patient within that culture.
Moreover, Glass suggested, some physicians who treat patients from a particular culture or religious group may be concerned about offending them or antagonizing religious leaders if they discuss medical options that aren’t accepted or practiced in that community or culture, such as vasectomy for male contraception. “But that deprives patients of knowing what choices are available and making truly informed decisions.”
This is especially important because “culture is mutable,” said Candler, and religious or cultural practices can “look one way on paper but be implemented, adopted, or executed in a completely different way by every human being who lives in that culture.” The best cultural competency “comes from continuing to build relationships with our patients. But even in a single visit, a single hospitalization, we should get to know patients as human beings, not just members of a given culture.”
There are cultures in which families want to be the liaison between the patient and the physician and to make decisions on the patient’s behalf. “I always ask patients what role they want their family members to play even if the cultural expectation is that the family will be heavily involved,” Candler said.
Sometimes, this can be awkward, and families might become upset. Candler described an elderly, frail patient who was diagnosed with end-stage cancer. She had always relied heavily on family to care for her. Concerned about overburdening them, she didn’t want them to know her diagnosis. The patient was mentally competent to make that decision.
“Usually, I would have had the family at the bedside so I could be sure everyone was appropriately informed and prepared for what lay ahead, but in this case, I couldn’t do so,” Candler said. “I had to inform her entire care team not to discuss the cancer diagnosis with any family members because this was the patient’s express wish. And when the family asked me if the diagnosis was cancer, I had to respond, ‘I’m so sorry, but your loved one doesn’t want us to discuss details of her diagnosis.’”
Other patients don’t want to know their own diagnosis and specifically ask Candler to inform a family member. “I’ve had patients request that I tell their children. They want their children to make decisions on their behalf.”
The main thing, Candler emphasized, is to “ask the patient, make sure the patient is competent to make that decision, thoroughly document the patient’s decision in the chart, and respect whatever that decision is.”
You Can Revisit the Questions
Having a longitudinal relationship means that the physician can revisit the same questions at different junctures because people’s perspectives sometimes change over time. “Discussing what a patient wants isn’t necessarily a one-time occurrence,” Wheat said. For example, “I’ve had situations where a patient has been a member of Jehovah’s Witnesses and won’t accept blood products — like transfusions — in treatment. I tell these patients that if an emergent situation arises, I would like to have the conversation again.”
Of course, sometimes patients are seen in the emergency department or in other situations where the physician has no prior relationship with them. “I always go into a room, especially with new patients, aiming to build rapport, communicate with a high level of respect, introduce myself, explain my approach, and understand the patient’s wishes,” Lee said. “As scenarios play out, I ask in multiple ways for the patient to confirm those wishes.”
He acknowledges that this can be time-consuming, “but it helps ensure the care that patient receives is complete, thorough, comprehensive, and respectful of the patient’s values and wishes.”
Candler disclosed paid part-time clinical work at CuraCapitol Primary Care Services, volunteer advocacy (reimbursed for travel) for the American College of Physicians, volunteer advocacy (reimbursed for travel) for the American Medical Association while serving on their Task Force to Preserve the Patient-Physician Relationship, and serving as a partner representative (reimbursed for time) for the AHRQ’s Person-Centered Care Planning Partnership, representing the American College of Physicians. Lee, Wheat, and Glass disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cultural competency is one of the most important values in the practice of medicine. Defined as the “ability to collaborate effectively with individuals from different cultures,” this type of competence “improves healthcare experiences and outcomes.” But within the context of cultural familiarity, it’s equally important to “understand that each person is an individual and may or may not adhere to certain cultural beliefs or practices common in his or her culture,” according to the Agency for Healthcare Research and Quality’s (AHRQ’s) Health Literacy Universal Precautions Toolkit.
Sarah Candler, MD, MPH, an internal medicine physician specializing in primary care for older adults in Washington, DC, said that the medical code of ethics consists of several pillars, with patient autonomy as the “first and most primary of those pillars.” She calls the balance of patient autonomy and cultural respect a “complicated tightrope to walk,” but says that these ethical principles can inform medical decisions and the patient-physician relationship.
Cultural Familiarity
It’s important to be as familiar as possible with the patient’s culture, Santina Wheat, MD, program director, Northwestern McGaw Family Medicine Residency at Delnor Hospital, Geneva, told this news organization. “For example, we serve many Orthodox Jewish patients. We had a meeting with rabbis from the community to present to us what religious laws might affect our patients. Until recently, I was delivering babies, and there was always a 24-hour emergency rabbi on call if an Orthodox patient wanted the input of a rabbi into her decisions.”
Jay W. Lee, MD, MPH, a member of the board of directors of the American Academy of Family Physicians, also sets out to educate himself about the cultural norms of his patients if they come from populations he’s not familiar with. “For example, this comes up when a new refugee population comes to the United States — most recently, there was a population of Afghan refugees,” Lee told this news organization.
Lee spent “a lot of time trying to learn about their cultural norms,” which prepared him to “ask more targeted questions about the patient’s understanding of the tests we were ordering or treatment options we were bringing forward.”
Lee, also the medical director at Integrated Health Partners of Southern California and associate clinical professor of family medicine at the University of California, Irvine, said it might be best if the physician is “language congruent or culturally similar.” Lee is of Asian descent and also speaks Spanish fluently. “I enjoy cultural exchanges with my patients, and I encourage patients to find a physician who’s the best fit.” But being from the same culture isn’t absolutely necessary for building relationships with the patient. “The key is offering the patient autonomy” while understanding the cultural context.
Don’t Assume ... Always Ask
Cultural familiarity doesn’t equate with stereotyping, Wheat emphasized. “Proceeding without assumptions opens the opportunity to ask questions for clarification and understanding and to improve patient care,” said Lee.
Sara Glass, PhD, LCSW, agrees. She’s the clinical director of Soul Wellness NYC, New York City, a psychotherapy practice that specializes in treating trauma. Based on her own experiences, she knows that some physicians and other healthcare professionals confuse cultural sensitivity with cultural stereotyping.
Glass, formerly Hasidic and ultra-Orthodox, shared an example from her own life. During the delivery of her second child, she sustained a vaginal tear. At her 6-week postpartum visit, her ob/gyn said, “Just remind me when you’re in your ninth month next time, and I can sew it up right after you deliver.”
Much of this physician’s practice “consisted of Hasidic women who looked just like me, wearing the same garb — head coverings such as wigs and scarves and long skirts. Most women in that community have multiple pregnancies,” Glass told this news organization. “My sister has 10 children, and that’s not unusual. The doctor simply assumed I’d be going on to have more babies without asking if that’s what I wanted.”
Glass says she was also never given information by her physician about the range of available contraceptive options. The rabbis of the Hasidic sect to which Glass belonged allowed women to practice contraception for 6 months following childbirth, or for longer, in the setting of certain medical conditions, but only certain types of birth control were religiously permissible. Other options were not mentioned to her by her physician, and she didn’t know that they existed.
Making no assumptions applies not only to patients from other cultures but also to all patients — including members of “mainstream American culture.”
Candler recalls a young patient with a new baby, who shared “how exhausted she was and how much time, energy, and work it took to care for children,” Candler recounted. “To me, it sounded as though she didn’t want another child, and I was about to offer contraception when it occurred to me to first ask if she wanted to have more children.” Candler was surprised when the patient said that, although she wasn’t actively looking to become pregnant again, she didn’t want to take preventive measures. “I’m so glad I asked, rather than simply assuming.”
Culture Is Mutable
Important questions to ask patients include whether there are aspects of their culture or religion that might affect their care — which can include medications they may feel uncomfortable using — and what family members they want to have involved in clinical discussions and decisions, said Wheat.
Lee described treating a refugee from Afghanistan who was in her sixth month of pregnancy. “I quickly needed to learn about what her expectations were for her care and my presence as a male on her care team,” he recounted. Lee arranged for the patient to receive prenatal care from a different clinician and arranged for follow-up for her husband and children. “Everyone had good results.”
Candler noted that some patients choose their physician specifically because that practitioner is conversant with their culture and respectful of its mores — especially when physicians share the same culture as the patient. But that level of familiarity can make it easy to forget to ask questions about the experience of the individual patient within that culture.
Moreover, Glass suggested, some physicians who treat patients from a particular culture or religious group may be concerned about offending them or antagonizing religious leaders if they discuss medical options that aren’t accepted or practiced in that community or culture, such as vasectomy for male contraception. “But that deprives patients of knowing what choices are available and making truly informed decisions.”
This is especially important because “culture is mutable,” said Candler, and religious or cultural practices can “look one way on paper but be implemented, adopted, or executed in a completely different way by every human being who lives in that culture.” The best cultural competency “comes from continuing to build relationships with our patients. But even in a single visit, a single hospitalization, we should get to know patients as human beings, not just members of a given culture.”
There are cultures in which families want to be the liaison between the patient and the physician and to make decisions on the patient’s behalf. “I always ask patients what role they want their family members to play even if the cultural expectation is that the family will be heavily involved,” Candler said.
Sometimes, this can be awkward, and families might become upset. Candler described an elderly, frail patient who was diagnosed with end-stage cancer. She had always relied heavily on family to care for her. Concerned about overburdening them, she didn’t want them to know her diagnosis. The patient was mentally competent to make that decision.
“Usually, I would have had the family at the bedside so I could be sure everyone was appropriately informed and prepared for what lay ahead, but in this case, I couldn’t do so,” Candler said. “I had to inform her entire care team not to discuss the cancer diagnosis with any family members because this was the patient’s express wish. And when the family asked me if the diagnosis was cancer, I had to respond, ‘I’m so sorry, but your loved one doesn’t want us to discuss details of her diagnosis.’”
Other patients don’t want to know their own diagnosis and specifically ask Candler to inform a family member. “I’ve had patients request that I tell their children. They want their children to make decisions on their behalf.”
The main thing, Candler emphasized, is to “ask the patient, make sure the patient is competent to make that decision, thoroughly document the patient’s decision in the chart, and respect whatever that decision is.”
You Can Revisit the Questions
Having a longitudinal relationship means that the physician can revisit the same questions at different junctures because people’s perspectives sometimes change over time. “Discussing what a patient wants isn’t necessarily a one-time occurrence,” Wheat said. For example, “I’ve had situations where a patient has been a member of Jehovah’s Witnesses and won’t accept blood products — like transfusions — in treatment. I tell these patients that if an emergent situation arises, I would like to have the conversation again.”
Of course, sometimes patients are seen in the emergency department or in other situations where the physician has no prior relationship with them. “I always go into a room, especially with new patients, aiming to build rapport, communicate with a high level of respect, introduce myself, explain my approach, and understand the patient’s wishes,” Lee said. “As scenarios play out, I ask in multiple ways for the patient to confirm those wishes.”
He acknowledges that this can be time-consuming, “but it helps ensure the care that patient receives is complete, thorough, comprehensive, and respectful of the patient’s values and wishes.”
Candler disclosed paid part-time clinical work at CuraCapitol Primary Care Services, volunteer advocacy (reimbursed for travel) for the American College of Physicians, volunteer advocacy (reimbursed for travel) for the American Medical Association while serving on their Task Force to Preserve the Patient-Physician Relationship, and serving as a partner representative (reimbursed for time) for the AHRQ’s Person-Centered Care Planning Partnership, representing the American College of Physicians. Lee, Wheat, and Glass disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cultural competency is one of the most important values in the practice of medicine. Defined as the “ability to collaborate effectively with individuals from different cultures,” this type of competence “improves healthcare experiences and outcomes.” But within the context of cultural familiarity, it’s equally important to “understand that each person is an individual and may or may not adhere to certain cultural beliefs or practices common in his or her culture,” according to the Agency for Healthcare Research and Quality’s (AHRQ’s) Health Literacy Universal Precautions Toolkit.
Sarah Candler, MD, MPH, an internal medicine physician specializing in primary care for older adults in Washington, DC, said that the medical code of ethics consists of several pillars, with patient autonomy as the “first and most primary of those pillars.” She calls the balance of patient autonomy and cultural respect a “complicated tightrope to walk,” but says that these ethical principles can inform medical decisions and the patient-physician relationship.
Cultural Familiarity
It’s important to be as familiar as possible with the patient’s culture, Santina Wheat, MD, program director, Northwestern McGaw Family Medicine Residency at Delnor Hospital, Geneva, told this news organization. “For example, we serve many Orthodox Jewish patients. We had a meeting with rabbis from the community to present to us what religious laws might affect our patients. Until recently, I was delivering babies, and there was always a 24-hour emergency rabbi on call if an Orthodox patient wanted the input of a rabbi into her decisions.”
Jay W. Lee, MD, MPH, a member of the board of directors of the American Academy of Family Physicians, also sets out to educate himself about the cultural norms of his patients if they come from populations he’s not familiar with. “For example, this comes up when a new refugee population comes to the United States — most recently, there was a population of Afghan refugees,” Lee told this news organization.
Lee spent “a lot of time trying to learn about their cultural norms,” which prepared him to “ask more targeted questions about the patient’s understanding of the tests we were ordering or treatment options we were bringing forward.”
Lee, also the medical director at Integrated Health Partners of Southern California and associate clinical professor of family medicine at the University of California, Irvine, said it might be best if the physician is “language congruent or culturally similar.” Lee is of Asian descent and also speaks Spanish fluently. “I enjoy cultural exchanges with my patients, and I encourage patients to find a physician who’s the best fit.” But being from the same culture isn’t absolutely necessary for building relationships with the patient. “The key is offering the patient autonomy” while understanding the cultural context.
Don’t Assume ... Always Ask
Cultural familiarity doesn’t equate with stereotyping, Wheat emphasized. “Proceeding without assumptions opens the opportunity to ask questions for clarification and understanding and to improve patient care,” said Lee.
Sara Glass, PhD, LCSW, agrees. She’s the clinical director of Soul Wellness NYC, New York City, a psychotherapy practice that specializes in treating trauma. Based on her own experiences, she knows that some physicians and other healthcare professionals confuse cultural sensitivity with cultural stereotyping.
Glass, formerly Hasidic and ultra-Orthodox, shared an example from her own life. During the delivery of her second child, she sustained a vaginal tear. At her 6-week postpartum visit, her ob/gyn said, “Just remind me when you’re in your ninth month next time, and I can sew it up right after you deliver.”
Much of this physician’s practice “consisted of Hasidic women who looked just like me, wearing the same garb — head coverings such as wigs and scarves and long skirts. Most women in that community have multiple pregnancies,” Glass told this news organization. “My sister has 10 children, and that’s not unusual. The doctor simply assumed I’d be going on to have more babies without asking if that’s what I wanted.”
Glass says she was also never given information by her physician about the range of available contraceptive options. The rabbis of the Hasidic sect to which Glass belonged allowed women to practice contraception for 6 months following childbirth, or for longer, in the setting of certain medical conditions, but only certain types of birth control were religiously permissible. Other options were not mentioned to her by her physician, and she didn’t know that they existed.
Making no assumptions applies not only to patients from other cultures but also to all patients — including members of “mainstream American culture.”
Candler recalls a young patient with a new baby, who shared “how exhausted she was and how much time, energy, and work it took to care for children,” Candler recounted. “To me, it sounded as though she didn’t want another child, and I was about to offer contraception when it occurred to me to first ask if she wanted to have more children.” Candler was surprised when the patient said that, although she wasn’t actively looking to become pregnant again, she didn’t want to take preventive measures. “I’m so glad I asked, rather than simply assuming.”
Culture Is Mutable
Important questions to ask patients include whether there are aspects of their culture or religion that might affect their care — which can include medications they may feel uncomfortable using — and what family members they want to have involved in clinical discussions and decisions, said Wheat.
Lee described treating a refugee from Afghanistan who was in her sixth month of pregnancy. “I quickly needed to learn about what her expectations were for her care and my presence as a male on her care team,” he recounted. Lee arranged for the patient to receive prenatal care from a different clinician and arranged for follow-up for her husband and children. “Everyone had good results.”
Candler noted that some patients choose their physician specifically because that practitioner is conversant with their culture and respectful of its mores — especially when physicians share the same culture as the patient. But that level of familiarity can make it easy to forget to ask questions about the experience of the individual patient within that culture.
Moreover, Glass suggested, some physicians who treat patients from a particular culture or religious group may be concerned about offending them or antagonizing religious leaders if they discuss medical options that aren’t accepted or practiced in that community or culture, such as vasectomy for male contraception. “But that deprives patients of knowing what choices are available and making truly informed decisions.”
This is especially important because “culture is mutable,” said Candler, and religious or cultural practices can “look one way on paper but be implemented, adopted, or executed in a completely different way by every human being who lives in that culture.” The best cultural competency “comes from continuing to build relationships with our patients. But even in a single visit, a single hospitalization, we should get to know patients as human beings, not just members of a given culture.”
There are cultures in which families want to be the liaison between the patient and the physician and to make decisions on the patient’s behalf. “I always ask patients what role they want their family members to play even if the cultural expectation is that the family will be heavily involved,” Candler said.
Sometimes, this can be awkward, and families might become upset. Candler described an elderly, frail patient who was diagnosed with end-stage cancer. She had always relied heavily on family to care for her. Concerned about overburdening them, she didn’t want them to know her diagnosis. The patient was mentally competent to make that decision.
“Usually, I would have had the family at the bedside so I could be sure everyone was appropriately informed and prepared for what lay ahead, but in this case, I couldn’t do so,” Candler said. “I had to inform her entire care team not to discuss the cancer diagnosis with any family members because this was the patient’s express wish. And when the family asked me if the diagnosis was cancer, I had to respond, ‘I’m so sorry, but your loved one doesn’t want us to discuss details of her diagnosis.’”
Other patients don’t want to know their own diagnosis and specifically ask Candler to inform a family member. “I’ve had patients request that I tell their children. They want their children to make decisions on their behalf.”
The main thing, Candler emphasized, is to “ask the patient, make sure the patient is competent to make that decision, thoroughly document the patient’s decision in the chart, and respect whatever that decision is.”
You Can Revisit the Questions
Having a longitudinal relationship means that the physician can revisit the same questions at different junctures because people’s perspectives sometimes change over time. “Discussing what a patient wants isn’t necessarily a one-time occurrence,” Wheat said. For example, “I’ve had situations where a patient has been a member of Jehovah’s Witnesses and won’t accept blood products — like transfusions — in treatment. I tell these patients that if an emergent situation arises, I would like to have the conversation again.”
Of course, sometimes patients are seen in the emergency department or in other situations where the physician has no prior relationship with them. “I always go into a room, especially with new patients, aiming to build rapport, communicate with a high level of respect, introduce myself, explain my approach, and understand the patient’s wishes,” Lee said. “As scenarios play out, I ask in multiple ways for the patient to confirm those wishes.”
He acknowledges that this can be time-consuming, “but it helps ensure the care that patient receives is complete, thorough, comprehensive, and respectful of the patient’s values and wishes.”
Candler disclosed paid part-time clinical work at CuraCapitol Primary Care Services, volunteer advocacy (reimbursed for travel) for the American College of Physicians, volunteer advocacy (reimbursed for travel) for the American Medical Association while serving on their Task Force to Preserve the Patient-Physician Relationship, and serving as a partner representative (reimbursed for time) for the AHRQ’s Person-Centered Care Planning Partnership, representing the American College of Physicians. Lee, Wheat, and Glass disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Food as Medicine: Diet’s Role in Parkinson’s Disease
For 15 years, John Duda, MD, national director of the VA Parkinson’s Disease Research, Education and Clinical Centers, has urged his patients to “keep waiting” for effective treatments to manage both motor and nonmotor symptoms of Parkinson’s disease.
However, Duda, who also serves as director of the Brain Wellness Clinic at the Corporal Michael J. Crescenz VA Medical Center in Philadelphia, Pennsylvania, recognized the persistent lack of effective drugs to address these symptoms. This prompted him to consider what other evidence-based strategies he could use to support his patients.
“I recognized that nutritional approaches within a broader program that includes medication review, stress management, social connections, adequate sleep, and physical exercise could make a real difference,” he said.
Observational studies have shown an inverse association between dietary patterns and Parkinson’s disease risk, age of onset, symptom severity, and mortality rates — particularly with the Mediterranean diet (MeDi) and the MIND diet, which combines elements of MeDi and the Dietary Approaches to Stop Hypertension (DASH) diet. Although randomized controlled trials are still limited, the epidemiologic evidence supporting dietary interventions is “compelling,” said Duda.
For example, a cross-sectional study comparing 167 participants with Parkinson’s disease vs 119 controls showed that later age of Parkinson’s disease onset correlated with adherence to the MIND diet in women, with a difference of up to 17.4 years (P < .001) between low and high dietary tertiles.
The MeDi was correlated with later onset in men, with differences of up to 8.4 years (P = .002). As previously reported, a healthy diet emphasizing vegetables, fruits, nuts, and grains was inversely associated with prodromal features of Parkinson’s disease, including constipation, excessive daytime sleepiness, and depression. In addition, lower rates of Parkinson’s disease have been shown in populations following vegetarian and vegan dietary patterns.
Does Parkinson’s disease Start in the Gut?
Parkinson’s disease is characterized by decreased short-chain fatty acid–producing bacteria and increased pro-inflammatory species linked to intestinal inflammation and alpha-synuclein aggregation. “There are reasons to believe that a-synuclein accumulation may start in the gut,” Duda noted.
Numerous studies implicate gut microbiome dysbiosis as a pathogenic mechanism in Parkinson’s disease, with gastrointestinal symptoms often predating motor symptoms. Dysbiosis might result in a pro-inflammatory state potentially linked to the recurrent gastrointestinal symptoms. Fecal microbiota transplant may restore a healthier gut environment and beneficially affect Parkinson’s disease symptoms, he said.
Some of the benefits conferred by the MeDi and other healthy diets may be mediated by improving the gut microbiome. Duda cited a study that showed that a 14-day ovo-lacto vegetarian diet intervention and a daily fecal enema for 8 days improved not only the microbiome but also Movement Disorder Society Unified Parkinson’s Disease Rating Scale—part III scores.
Duda also reviewed the role of dietary interventions in addressing common Parkinson’s disease symptoms, such as orthostatic hypotension. He recommended that Parkinson’s disease patients with this condition should avoid eating large meals, increase dietary salt intake, increase fluid intake, and decrease alcohol intake.
Malnutrition affects close to 25% of those with Parkinson’s disease, which is partially attributable to diminished olfaction. Because the experience of taste is largely driven by a sense of smell, patients may be less interested in eating. Duda recommended increasing herbs, spices, and other flavors in food. High caloric–density foods, including nuts, nut butters, and seeds, can boost weight, he said. However, he added, any patient with significant weight loss should consult a nutritionist.
Constipation is one of the most debilitating symptoms of Parkinson’s disease, affecting up to 66% of patients. Duda advised increasing fluid intake, exercise, and dietary fiber and use of stool softeners and laxatives. The MeDi may reduce symptoms of constipation and have a beneficial effect on gut microbiota.
Coffee may be helpful for sleepiness in Parkinson’s disease and may also confer neuroprotective, motor, and cognitive benefits. As an adjuvant treatment, caffeine may alter levodopa pharmacokinetics, reduce dyskinesia, improve gait in patients with freezing and may even reduce the risk of developing Parkinson’s disease, with a maximum benefit reached at approximately three cups of coffee daily.
Problematic Foods
There is also a growing body of evidence regarding the deleterious effects of ultraprocessed foods (UPFs), Duda said. He noted that a recent systematic review and meta-analysis of 28 studies showed that higher UPF intake was significantly associated with an enhanced risk for Parkinson’s disease (relative risk, 1.56; 95% CI, 1.21-2.02). As previously reported, UPFs have been tied to a host of adverse neurologic outcomes, including cognitive decline and stroke.
Although protein is a necessary nutrient, incorporating it into the diet of Parkinson’s disease patients taking levodopa is complicated. Levodopa, a large neutral amino acid (LNAA), competes with other LNAAs for transport to the brain from the small intestine, Duda explained.
“Some people notice that carbidopa-levodopa doesn’t work as well if taken with a high-protein meal.” He recommended taking carbidopa-levodopa 30 minutes before or 60 minutes after meals.
Rebecca Gilbert, MD, PhD, chief mission officer of the American Parkinson’s Disease Association, said that patients with Parkinson’s disease might want to avoid eating protein during the day, concentrating instead on carbohydrates and vegetables and saving the protein for the evening, which is closer to bedtime. Some evidence also supports the use of protein redistribution diets to enhance the clinical response to levodopa and reduce motor fluctuations.
What About Supplements?
It’s “hard to prove that one specific supplement can be protective against Parkinson’s disease because diet consists of many different components and the whole diet may be worth more than the sum of its parts,” Gilbert said. The evidence for individual supplements “isn’t robust enough to say they prevent or treat Parkinson’s disease.”
Research on the role of specific nutrients in Parkinson’s disease is conflicting, with no clear evidence supporting or refuting their benefits. For example, a study that followed participants for about 30 years showed no link between reduced Parkinson’s disease risk and vitamin B or folate intake.
On the other hand, there is research suggesting that certain vitamins may help reduce Parkinson’s disease risk, although these nutrients do not operate in isolation. For instance, one recent study showed a connection between vitamins C and E and reduced Parkinson’s disease risk, but factors such as body mass index and coffee consumption appeared to influence the strength of this association.
Consuming polyunsaturated fatty acids along with reducing saturated fatty acid intake has been tied to a reduced risk for Parkinson’s disease.
Additionally, certain foods may offer protective effects, including green and black tea, with consumption of three or more cups per day associated with a delay in motor symptom onset by 7.7 years. Foods high in nicotine content, such as those from the Solanaceae family — including peppers, tomatoes, tomato juice, and potatoes — have also been linked to potential protective benefits.
Diets rich in antioxidants, including carotenoids, lutein, and vitamins E and C, have been robustly linked to a reduced risk for parkinsonism and progression of parkinsonian symptoms in older adults.
Increasing the intake of dietary flavonoids, particularly tea, berry fruits, apples, red wine, and oranges or orange juice, can reduce Parkinson’s disease risk. One study showed that male participants in the highest quintile of total flavonoid consumption had a 40% lower Parkinson’s disease risk compared with those in the lowest quintile. Another study showed that flavonoid-rich foods were also associated with a lower risk for death in patients with Parkinson’s disease.
Food as Medicine
Although recent research shows that the drug development pipeline for Parkinson’s disease is robust, with a wide variety of approaches being developed and evaluated in phase 1 and 2, investigators note that only a limited number of disease-modifying treatments are transitioning to phase 3.
Duda noted that phytochemicals incorporated into the diet might target some of the same mechanisms that are targets of these drugs in development.
“Flavonoids have been shown to stabilize alpha-synuclein in vitro,” he said. “Caffeine, curcumin, resveratrol, and eliminating meat and dairy inhibit mTOR [mammalian target of rapamycin], and mTOR inhibition results in increased autophagy that may help clear alpha-synuclein. Genestein, an isoflavone in soybeans, protects dopaminergic neurons by inhibiting microglia activation. Flavonoids inhibit inflammation by inhibiting release of NO [nitric oxide] and pro-inflammatory cytokines,” he noted.
Ongoing studies of dietary interventions for Parkinson’s disease are exploring various areas, including the potential role of the ketogenic diet in protecting the gut microbiome, optimizing protein intake for muscle preservation and sleep, the effects of psyllium and wheat bran on weight and constipation, and the impact of a gluten-free diet.
Practical Tips for Healthy Eating
Gilbert emphasized that there are no medications or interventions currently available that can delay a Parkinson’s disease diagnosis by up to 17 years, as some dietary patterns have been shown to do, and she noted that it’s not possible to replicate the MeDi diet in a pill. However, she recommended a practical approach to eating that includes a diet low in ultraprocessed foods and high in beneficial nutrients. She encouraged people to shop for “real food” and enjoy a variety of colorful fruits and vegetables.
Duda acknowledged that motivating patients to follow a healthy diet can be difficult. As a result, the focus often shifts to making small adjustments and modifications. For example, he suggested that instead of pairing meat with French fries, people could opt for vegetables or add greens to their meals. Similarly, instead of having eggs and bacon for breakfast, they might choose oatmeal.
Preparing whole-food, plant-based meals may take more time than patients are accustomed to, so Duda suggests that, if possible, patients involve loved ones in both the meal preparation and the meal itself. He explained that a healthy meal can become an opportunity for bonding and that the key is educating them about new meal-related concepts.
Duda reported no relevant financial relationships with the pharmaceutical or food industries. He has received compensation from the Physicians Committee for Responsible Medicine for his lecture delivered at the conference and research grant support from the VA, the National Institutes of Health, the Michael J. Fox Foundation, and the Department of Defense unrelated to this topic. Gilbert reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
For 15 years, John Duda, MD, national director of the VA Parkinson’s Disease Research, Education and Clinical Centers, has urged his patients to “keep waiting” for effective treatments to manage both motor and nonmotor symptoms of Parkinson’s disease.
However, Duda, who also serves as director of the Brain Wellness Clinic at the Corporal Michael J. Crescenz VA Medical Center in Philadelphia, Pennsylvania, recognized the persistent lack of effective drugs to address these symptoms. This prompted him to consider what other evidence-based strategies he could use to support his patients.
“I recognized that nutritional approaches within a broader program that includes medication review, stress management, social connections, adequate sleep, and physical exercise could make a real difference,” he said.
Observational studies have shown an inverse association between dietary patterns and Parkinson’s disease risk, age of onset, symptom severity, and mortality rates — particularly with the Mediterranean diet (MeDi) and the MIND diet, which combines elements of MeDi and the Dietary Approaches to Stop Hypertension (DASH) diet. Although randomized controlled trials are still limited, the epidemiologic evidence supporting dietary interventions is “compelling,” said Duda.
For example, a cross-sectional study comparing 167 participants with Parkinson’s disease vs 119 controls showed that later age of Parkinson’s disease onset correlated with adherence to the MIND diet in women, with a difference of up to 17.4 years (P < .001) between low and high dietary tertiles.
The MeDi was correlated with later onset in men, with differences of up to 8.4 years (P = .002). As previously reported, a healthy diet emphasizing vegetables, fruits, nuts, and grains was inversely associated with prodromal features of Parkinson’s disease, including constipation, excessive daytime sleepiness, and depression. In addition, lower rates of Parkinson’s disease have been shown in populations following vegetarian and vegan dietary patterns.
Does Parkinson’s disease Start in the Gut?
Parkinson’s disease is characterized by decreased short-chain fatty acid–producing bacteria and increased pro-inflammatory species linked to intestinal inflammation and alpha-synuclein aggregation. “There are reasons to believe that a-synuclein accumulation may start in the gut,” Duda noted.
Numerous studies implicate gut microbiome dysbiosis as a pathogenic mechanism in Parkinson’s disease, with gastrointestinal symptoms often predating motor symptoms. Dysbiosis might result in a pro-inflammatory state potentially linked to the recurrent gastrointestinal symptoms. Fecal microbiota transplant may restore a healthier gut environment and beneficially affect Parkinson’s disease symptoms, he said.
Some of the benefits conferred by the MeDi and other healthy diets may be mediated by improving the gut microbiome. Duda cited a study that showed that a 14-day ovo-lacto vegetarian diet intervention and a daily fecal enema for 8 days improved not only the microbiome but also Movement Disorder Society Unified Parkinson’s Disease Rating Scale—part III scores.
Duda also reviewed the role of dietary interventions in addressing common Parkinson’s disease symptoms, such as orthostatic hypotension. He recommended that Parkinson’s disease patients with this condition should avoid eating large meals, increase dietary salt intake, increase fluid intake, and decrease alcohol intake.
Malnutrition affects close to 25% of those with Parkinson’s disease, which is partially attributable to diminished olfaction. Because the experience of taste is largely driven by a sense of smell, patients may be less interested in eating. Duda recommended increasing herbs, spices, and other flavors in food. High caloric–density foods, including nuts, nut butters, and seeds, can boost weight, he said. However, he added, any patient with significant weight loss should consult a nutritionist.
Constipation is one of the most debilitating symptoms of Parkinson’s disease, affecting up to 66% of patients. Duda advised increasing fluid intake, exercise, and dietary fiber and use of stool softeners and laxatives. The MeDi may reduce symptoms of constipation and have a beneficial effect on gut microbiota.
Coffee may be helpful for sleepiness in Parkinson’s disease and may also confer neuroprotective, motor, and cognitive benefits. As an adjuvant treatment, caffeine may alter levodopa pharmacokinetics, reduce dyskinesia, improve gait in patients with freezing and may even reduce the risk of developing Parkinson’s disease, with a maximum benefit reached at approximately three cups of coffee daily.
Problematic Foods
There is also a growing body of evidence regarding the deleterious effects of ultraprocessed foods (UPFs), Duda said. He noted that a recent systematic review and meta-analysis of 28 studies showed that higher UPF intake was significantly associated with an enhanced risk for Parkinson’s disease (relative risk, 1.56; 95% CI, 1.21-2.02). As previously reported, UPFs have been tied to a host of adverse neurologic outcomes, including cognitive decline and stroke.
Although protein is a necessary nutrient, incorporating it into the diet of Parkinson’s disease patients taking levodopa is complicated. Levodopa, a large neutral amino acid (LNAA), competes with other LNAAs for transport to the brain from the small intestine, Duda explained.
“Some people notice that carbidopa-levodopa doesn’t work as well if taken with a high-protein meal.” He recommended taking carbidopa-levodopa 30 minutes before or 60 minutes after meals.
Rebecca Gilbert, MD, PhD, chief mission officer of the American Parkinson’s Disease Association, said that patients with Parkinson’s disease might want to avoid eating protein during the day, concentrating instead on carbohydrates and vegetables and saving the protein for the evening, which is closer to bedtime. Some evidence also supports the use of protein redistribution diets to enhance the clinical response to levodopa and reduce motor fluctuations.
What About Supplements?
It’s “hard to prove that one specific supplement can be protective against Parkinson’s disease because diet consists of many different components and the whole diet may be worth more than the sum of its parts,” Gilbert said. The evidence for individual supplements “isn’t robust enough to say they prevent or treat Parkinson’s disease.”
Research on the role of specific nutrients in Parkinson’s disease is conflicting, with no clear evidence supporting or refuting their benefits. For example, a study that followed participants for about 30 years showed no link between reduced Parkinson’s disease risk and vitamin B or folate intake.
On the other hand, there is research suggesting that certain vitamins may help reduce Parkinson’s disease risk, although these nutrients do not operate in isolation. For instance, one recent study showed a connection between vitamins C and E and reduced Parkinson’s disease risk, but factors such as body mass index and coffee consumption appeared to influence the strength of this association.
Consuming polyunsaturated fatty acids along with reducing saturated fatty acid intake has been tied to a reduced risk for Parkinson’s disease.
Additionally, certain foods may offer protective effects, including green and black tea, with consumption of three or more cups per day associated with a delay in motor symptom onset by 7.7 years. Foods high in nicotine content, such as those from the Solanaceae family — including peppers, tomatoes, tomato juice, and potatoes — have also been linked to potential protective benefits.
Diets rich in antioxidants, including carotenoids, lutein, and vitamins E and C, have been robustly linked to a reduced risk for parkinsonism and progression of parkinsonian symptoms in older adults.
Increasing the intake of dietary flavonoids, particularly tea, berry fruits, apples, red wine, and oranges or orange juice, can reduce Parkinson’s disease risk. One study showed that male participants in the highest quintile of total flavonoid consumption had a 40% lower Parkinson’s disease risk compared with those in the lowest quintile. Another study showed that flavonoid-rich foods were also associated with a lower risk for death in patients with Parkinson’s disease.
Food as Medicine
Although recent research shows that the drug development pipeline for Parkinson’s disease is robust, with a wide variety of approaches being developed and evaluated in phase 1 and 2, investigators note that only a limited number of disease-modifying treatments are transitioning to phase 3.
Duda noted that phytochemicals incorporated into the diet might target some of the same mechanisms that are targets of these drugs in development.
“Flavonoids have been shown to stabilize alpha-synuclein in vitro,” he said. “Caffeine, curcumin, resveratrol, and eliminating meat and dairy inhibit mTOR [mammalian target of rapamycin], and mTOR inhibition results in increased autophagy that may help clear alpha-synuclein. Genestein, an isoflavone in soybeans, protects dopaminergic neurons by inhibiting microglia activation. Flavonoids inhibit inflammation by inhibiting release of NO [nitric oxide] and pro-inflammatory cytokines,” he noted.
Ongoing studies of dietary interventions for Parkinson’s disease are exploring various areas, including the potential role of the ketogenic diet in protecting the gut microbiome, optimizing protein intake for muscle preservation and sleep, the effects of psyllium and wheat bran on weight and constipation, and the impact of a gluten-free diet.
Practical Tips for Healthy Eating
Gilbert emphasized that there are no medications or interventions currently available that can delay a Parkinson’s disease diagnosis by up to 17 years, as some dietary patterns have been shown to do, and she noted that it’s not possible to replicate the MeDi diet in a pill. However, she recommended a practical approach to eating that includes a diet low in ultraprocessed foods and high in beneficial nutrients. She encouraged people to shop for “real food” and enjoy a variety of colorful fruits and vegetables.
Duda acknowledged that motivating patients to follow a healthy diet can be difficult. As a result, the focus often shifts to making small adjustments and modifications. For example, he suggested that instead of pairing meat with French fries, people could opt for vegetables or add greens to their meals. Similarly, instead of having eggs and bacon for breakfast, they might choose oatmeal.
Preparing whole-food, plant-based meals may take more time than patients are accustomed to, so Duda suggests that, if possible, patients involve loved ones in both the meal preparation and the meal itself. He explained that a healthy meal can become an opportunity for bonding and that the key is educating them about new meal-related concepts.
Duda reported no relevant financial relationships with the pharmaceutical or food industries. He has received compensation from the Physicians Committee for Responsible Medicine for his lecture delivered at the conference and research grant support from the VA, the National Institutes of Health, the Michael J. Fox Foundation, and the Department of Defense unrelated to this topic. Gilbert reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
For 15 years, John Duda, MD, national director of the VA Parkinson’s Disease Research, Education and Clinical Centers, has urged his patients to “keep waiting” for effective treatments to manage both motor and nonmotor symptoms of Parkinson’s disease.
However, Duda, who also serves as director of the Brain Wellness Clinic at the Corporal Michael J. Crescenz VA Medical Center in Philadelphia, Pennsylvania, recognized the persistent lack of effective drugs to address these symptoms. This prompted him to consider what other evidence-based strategies he could use to support his patients.
“I recognized that nutritional approaches within a broader program that includes medication review, stress management, social connections, adequate sleep, and physical exercise could make a real difference,” he said.
Observational studies have shown an inverse association between dietary patterns and Parkinson’s disease risk, age of onset, symptom severity, and mortality rates — particularly with the Mediterranean diet (MeDi) and the MIND diet, which combines elements of MeDi and the Dietary Approaches to Stop Hypertension (DASH) diet. Although randomized controlled trials are still limited, the epidemiologic evidence supporting dietary interventions is “compelling,” said Duda.
For example, a cross-sectional study comparing 167 participants with Parkinson’s disease vs 119 controls showed that later age of Parkinson’s disease onset correlated with adherence to the MIND diet in women, with a difference of up to 17.4 years (P < .001) between low and high dietary tertiles.
The MeDi was correlated with later onset in men, with differences of up to 8.4 years (P = .002). As previously reported, a healthy diet emphasizing vegetables, fruits, nuts, and grains was inversely associated with prodromal features of Parkinson’s disease, including constipation, excessive daytime sleepiness, and depression. In addition, lower rates of Parkinson’s disease have been shown in populations following vegetarian and vegan dietary patterns.
Does Parkinson’s disease Start in the Gut?
Parkinson’s disease is characterized by decreased short-chain fatty acid–producing bacteria and increased pro-inflammatory species linked to intestinal inflammation and alpha-synuclein aggregation. “There are reasons to believe that a-synuclein accumulation may start in the gut,” Duda noted.
Numerous studies implicate gut microbiome dysbiosis as a pathogenic mechanism in Parkinson’s disease, with gastrointestinal symptoms often predating motor symptoms. Dysbiosis might result in a pro-inflammatory state potentially linked to the recurrent gastrointestinal symptoms. Fecal microbiota transplant may restore a healthier gut environment and beneficially affect Parkinson’s disease symptoms, he said.
Some of the benefits conferred by the MeDi and other healthy diets may be mediated by improving the gut microbiome. Duda cited a study that showed that a 14-day ovo-lacto vegetarian diet intervention and a daily fecal enema for 8 days improved not only the microbiome but also Movement Disorder Society Unified Parkinson’s Disease Rating Scale—part III scores.
Duda also reviewed the role of dietary interventions in addressing common Parkinson’s disease symptoms, such as orthostatic hypotension. He recommended that Parkinson’s disease patients with this condition should avoid eating large meals, increase dietary salt intake, increase fluid intake, and decrease alcohol intake.
Malnutrition affects close to 25% of those with Parkinson’s disease, which is partially attributable to diminished olfaction. Because the experience of taste is largely driven by a sense of smell, patients may be less interested in eating. Duda recommended increasing herbs, spices, and other flavors in food. High caloric–density foods, including nuts, nut butters, and seeds, can boost weight, he said. However, he added, any patient with significant weight loss should consult a nutritionist.
Constipation is one of the most debilitating symptoms of Parkinson’s disease, affecting up to 66% of patients. Duda advised increasing fluid intake, exercise, and dietary fiber and use of stool softeners and laxatives. The MeDi may reduce symptoms of constipation and have a beneficial effect on gut microbiota.
Coffee may be helpful for sleepiness in Parkinson’s disease and may also confer neuroprotective, motor, and cognitive benefits. As an adjuvant treatment, caffeine may alter levodopa pharmacokinetics, reduce dyskinesia, improve gait in patients with freezing and may even reduce the risk of developing Parkinson’s disease, with a maximum benefit reached at approximately three cups of coffee daily.
Problematic Foods
There is also a growing body of evidence regarding the deleterious effects of ultraprocessed foods (UPFs), Duda said. He noted that a recent systematic review and meta-analysis of 28 studies showed that higher UPF intake was significantly associated with an enhanced risk for Parkinson’s disease (relative risk, 1.56; 95% CI, 1.21-2.02). As previously reported, UPFs have been tied to a host of adverse neurologic outcomes, including cognitive decline and stroke.
Although protein is a necessary nutrient, incorporating it into the diet of Parkinson’s disease patients taking levodopa is complicated. Levodopa, a large neutral amino acid (LNAA), competes with other LNAAs for transport to the brain from the small intestine, Duda explained.
“Some people notice that carbidopa-levodopa doesn’t work as well if taken with a high-protein meal.” He recommended taking carbidopa-levodopa 30 minutes before or 60 minutes after meals.
Rebecca Gilbert, MD, PhD, chief mission officer of the American Parkinson’s Disease Association, said that patients with Parkinson’s disease might want to avoid eating protein during the day, concentrating instead on carbohydrates and vegetables and saving the protein for the evening, which is closer to bedtime. Some evidence also supports the use of protein redistribution diets to enhance the clinical response to levodopa and reduce motor fluctuations.
What About Supplements?
It’s “hard to prove that one specific supplement can be protective against Parkinson’s disease because diet consists of many different components and the whole diet may be worth more than the sum of its parts,” Gilbert said. The evidence for individual supplements “isn’t robust enough to say they prevent or treat Parkinson’s disease.”
Research on the role of specific nutrients in Parkinson’s disease is conflicting, with no clear evidence supporting or refuting their benefits. For example, a study that followed participants for about 30 years showed no link between reduced Parkinson’s disease risk and vitamin B or folate intake.
On the other hand, there is research suggesting that certain vitamins may help reduce Parkinson’s disease risk, although these nutrients do not operate in isolation. For instance, one recent study showed a connection between vitamins C and E and reduced Parkinson’s disease risk, but factors such as body mass index and coffee consumption appeared to influence the strength of this association.
Consuming polyunsaturated fatty acids along with reducing saturated fatty acid intake has been tied to a reduced risk for Parkinson’s disease.
Additionally, certain foods may offer protective effects, including green and black tea, with consumption of three or more cups per day associated with a delay in motor symptom onset by 7.7 years. Foods high in nicotine content, such as those from the Solanaceae family — including peppers, tomatoes, tomato juice, and potatoes — have also been linked to potential protective benefits.
Diets rich in antioxidants, including carotenoids, lutein, and vitamins E and C, have been robustly linked to a reduced risk for parkinsonism and progression of parkinsonian symptoms in older adults.
Increasing the intake of dietary flavonoids, particularly tea, berry fruits, apples, red wine, and oranges or orange juice, can reduce Parkinson’s disease risk. One study showed that male participants in the highest quintile of total flavonoid consumption had a 40% lower Parkinson’s disease risk compared with those in the lowest quintile. Another study showed that flavonoid-rich foods were also associated with a lower risk for death in patients with Parkinson’s disease.
Food as Medicine
Although recent research shows that the drug development pipeline for Parkinson’s disease is robust, with a wide variety of approaches being developed and evaluated in phase 1 and 2, investigators note that only a limited number of disease-modifying treatments are transitioning to phase 3.
Duda noted that phytochemicals incorporated into the diet might target some of the same mechanisms that are targets of these drugs in development.
“Flavonoids have been shown to stabilize alpha-synuclein in vitro,” he said. “Caffeine, curcumin, resveratrol, and eliminating meat and dairy inhibit mTOR [mammalian target of rapamycin], and mTOR inhibition results in increased autophagy that may help clear alpha-synuclein. Genestein, an isoflavone in soybeans, protects dopaminergic neurons by inhibiting microglia activation. Flavonoids inhibit inflammation by inhibiting release of NO [nitric oxide] and pro-inflammatory cytokines,” he noted.
Ongoing studies of dietary interventions for Parkinson’s disease are exploring various areas, including the potential role of the ketogenic diet in protecting the gut microbiome, optimizing protein intake for muscle preservation and sleep, the effects of psyllium and wheat bran on weight and constipation, and the impact of a gluten-free diet.
Practical Tips for Healthy Eating
Gilbert emphasized that there are no medications or interventions currently available that can delay a Parkinson’s disease diagnosis by up to 17 years, as some dietary patterns have been shown to do, and she noted that it’s not possible to replicate the MeDi diet in a pill. However, she recommended a practical approach to eating that includes a diet low in ultraprocessed foods and high in beneficial nutrients. She encouraged people to shop for “real food” and enjoy a variety of colorful fruits and vegetables.
Duda acknowledged that motivating patients to follow a healthy diet can be difficult. As a result, the focus often shifts to making small adjustments and modifications. For example, he suggested that instead of pairing meat with French fries, people could opt for vegetables or add greens to their meals. Similarly, instead of having eggs and bacon for breakfast, they might choose oatmeal.
Preparing whole-food, plant-based meals may take more time than patients are accustomed to, so Duda suggests that, if possible, patients involve loved ones in both the meal preparation and the meal itself. He explained that a healthy meal can become an opportunity for bonding and that the key is educating them about new meal-related concepts.
Duda reported no relevant financial relationships with the pharmaceutical or food industries. He has received compensation from the Physicians Committee for Responsible Medicine for his lecture delivered at the conference and research grant support from the VA, the National Institutes of Health, the Michael J. Fox Foundation, and the Department of Defense unrelated to this topic. Gilbert reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
As GLP-1 Use Surges, Clinicians Weigh Benefits and Risks
Rates of overweight and obesity have more than doubled in the United States during the last three decades, according to a new analysis. By 2050, it’s anticipated that 213 million adults (age, > 25 years) and 43 million children and adolescents will have overweight or obesity. The results led authors of a study to describe obesity as having reached a “crisis point” requiring urgent action and interventions.
Are glucagon-like peptide 1 receptor agonists (GLP-1 RAs), originally developed and prescribed for diabetes and now approved for weight loss, the answer?
Their popularity is certainly surging. Between the last 6 months of 2022 vs the last 6 months of 2024, the number of patients prescribed GLP-1 RAs increased by 132.6%. This is also reflected in a shift in public awareness, with a recent survey of US adults finding that 32% of respondents had heard “a lot” about these drugs, up from 19% in 2023.
GLP-1 RAs (including tirzepatide, which targets not only the GLP-1 receptor but also the glucose-dependent insulinotropic polypeptide receptor) have shown efficacy in weight loss. A 2022 review and meta-analysis of 22 trials (17,183 patients) found that 50.2% and 17.5% of those treated with GLP-1 RAs had a ≥ 5% and ≥ 10% weight loss, respectively, compared with placebo. A 2023 review of 41 trials (15,135 patients) found that compared with controls, GLP-1 RAs significantly reduced body weight, body mass index, waist circumference, and waist-to-hip ratio.
“GLP-1 RAs are great medications,” Andres Acosta, MD, PhD, director of the Precision Medicine for Obesity Laboratory, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News. “We’ve been using them for almost two decades. But now there’s excitement about their utility in treating obesity.”
Treating the Four Categories of Obesity
Daniel Drucker, MD, senior investigator at the Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada, is a pioneer in diabetes treatment and particularly in the development of GLP-1 RAs. Drucker told Medscape Medical News that despite the efficacy and enormous potential of GLP-1 RAs, “we know some people don’t lose much weight when taking these medicines and others don’t feel well and can’t take them.”
The number of individuals who don’t respond to or aren’t able to tolerate GLP-1 RAs “might be small — less than 10% of people who try to take them — but we don’t fully understand the differences in response across different individuals,” Drucker said.
Acosta agreed, adding that it’s “essential for us to identify who will be the best responders, as we do with medications for other conditions, such as cancer and cardiovascular disease.”
Acosta’s group has spent more than a decade engaged in efforts to identify unique characteristics among patients with obesity and has succeeded in identifying four obesity phenotypes.
“What matters in the space of GLP-1 is that using this classification, we can identify the best responders and those who don’t respond.”
The first phenotype, described as “Hungry Gut” (HG), includes patients with abnormal postprandial satiety. “Although they may be satiated at the end of a meal, they have accelerated gastric emptying and therefore feel hungry between meals and want to keep eating,” he said.
There are also patients who experience abnormal satiety during meals. According to Acosta, these are the patients who will return to the table for second and third helpings. “They don’t feel full and continue to eat more and more in a single sitting” — a phenomenon referred to as “Hungry Brain.”
The third phenotype — “Emotional Hunger” — consists of people who are “hedonic” about food or engage in emotional eating behavior, whereas in the fourth group, people have “an abnormal metabolism in which they don’t burn enough calories. They have an inefficient metabolic rate.” This latter phenomenon is called “Slow Burn.”
Acosta and colleagues randomized 312 patients attending a weight management center to phenotype-guided or non–phenotype-guided treatment with anti-obesity medications (phentermine, phentermine/topiramate, bupropion/naltrexone, lorcaserin, and liraglutide). The phenotype-guided approach was associated with a 1.75-fold greater weight loss after 1 year than the non–phenotype-guided approach (mean weight loss, 15.9% vs 9.0%, respectively).
GLP-1 RAs: Not One-Size-Fits-All
Acosta’s group has developed a genetic test that uses patients’ saliva to identify their obesity phenotype, with the aim of predicting the best responders to GLP-1 RAs. The test, MyPhenome genetic obesity test, is licensed by Acosta’s lab and available through Phenomix Sciences.
Acosta and colleagues presented their findings at the American Gastroenterological Association’s 2024 annual meeting regarding a machine-learning gene risk score (ML-GRS) they developed to predict HG, based on saliva and blood samples. Their genetic studies generated a ML-GRS that classified participants with obesity along a continuum from “HG Positive” (HG+) to “HG Negative” (HG−). Compared with the HG− participants, those who were HG+ had superior total body weight loss with semaglutide at 9 and 12 months. When used to predict response, the ML-GRS had an area under the curve of 0.76 (P = .04) and a positive predictive value of 0.95.
According to Acosta, HG+ patients are “the best responders to the GLP-1 RAs, although we don’t yet understand the mechanism of why they have the phenomenon of abnormal postprandial satiety. It may be an abnormal genetic pathway or abnormal secretion of GLP-1. More studies are needed.”
He noted that GLP-1 RAs “might also be helpful with the second [Hungry Brain] category, but these patients do better with phentermine-topiramate,” as demonstrated in a 2023 study conducted by Acosta and colleagues.
His group has also studied which lifestyle interventions are most effective for each phenotype. “When a unique lifestyle intervention targeting each phenotype was applied, patients lost more weight and had greater metabolic improvement,” he reported.
“Treating obesity no longer needs to be trial-and-error, but should be done using precision medicine because one size doesn’t fit all,” Acosta said.
Concerning Side Effects
The popular media has featured stories about individuals who took GLP-1 RAs for weight loss and experienced serious side effects, including a recent account of a British nurse who died after taking tirzepatide. As reported by the BBC, the nurse’s death certificate listed multiple organ failure, septic shock, and pancreatitis as the immediate causes of death, with the “use of prescribed tirzepatide” recorded as a contributing factor. The report went on to note that there were 23 suspected deaths in the United Kingdom tied to semaglutide since 2019.
Beyond brand-name products, there are also risks associated with GLP-1 RAs manufactured by compounding pharmacies. In early November, CNN reported that compounded semaglutide has been linked to at least 10 deaths. Because of a prior shortage of tirzepatide, the US Food and Drug Administration (FDA) had allowed compounding pharmacies to manufacture the drug. In October, the FDA clarified that it won’t take legal action against compounders, even now that the shortage has been resolved.
A pharmacovigilance study using the FDA Adverse Event Reporting System identified “potential safety signals of increased mortality and serious adverse event reporting” associated with certain GLP-1 RAs — especially in younger patients and women (P < .0001 for both groups).
The most common side effects reported with GLP-1 RAs are gastrointestinal events, such as nausea, diarrhea, constipation, and vomiting. Most occur during dose initiation and escalation and wane over the following weeks. However, studies have also reported severe side effects, including a higher risk for pancreatitis, bowel obstruction, and gastroparesis, as well as a significantly higher risk for gallbladder and biliary diseases. In fact, according to one study, patients with diabetes taking GLP-1 RAs reported gastrointestinal-related issues as a “prominent factor” in their decision to discontinue taking these medications.
Several types of cancer are potentially associated with GLP-1 RAs, but findings regarding this potential link have been inconsistent. In a recent review article, Drucker noted there were only inconsistent data linking GLP-1 RAs with thyroid cancer and medullary thyroid cancer and that their potential association with pancreatic has “not been supported by results from randomized controlled trials or real-world data.”
Concerns have been raised about loss of lean mass and muscle strength and function, especially in older individuals with obesity and advanced liver, cardiovascular, or kidney disease. However, as Drucker pointed out in his review article, muscle function may not correlate with the loss of lean mass. In fact, there are “consistent reductions” in lean mass after bariatric surgery, but “little evidence to date for impairment of muscle function.” He added that newer GLP-1 agents under development for obesity treatment are focusing on “developing complementary therapies that preferentially reduce adipose tissue, while sparing lean mass.”
As covered by Medscape Medical News, there have been reports of potential suicidal ideation associated with GLP-1 RAs. This triggered a 2023 review from the European Medicines Agency. However, recent results from a cohort study and a post hoc analysis of randomized controlled trials concluded that there is no evidence that these drugs increase suicidal ideation or behavior.
In early November, the FDA updated the labels for the GLP-1 RAs to include a warning regarding pulmonary aspiration during general anesthesia or deep sedation. Guidance from a group of societies, led by the American Society of Anesthesiologists, contains recommendations regarding nuances of addressing this concern in surgical patients taking these agents.
Not a Standalone Treatment
Marc-Andre Cornier, MD, professor of medicine, James A. Keating Endowed Chair in Diabetes, and director of the Division of Endocrinology, Diabetes and Metabolic Diseases, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina, told Medscape Medical News that GLP-1 RAs should not be viewed as cosmetic interventions but rather as medical treatments, “not only for weight loss but to reverse obesity-associated complications.”
Moreover, they should be used “as an adjunct to lifestyle changes,” emphasized Cornier. “We want our patients to have a high-quality diet with high protein content, fluid, vitamins, and minerals, and we want them to exercise.” Especially with the concern of potential loss of muscle mass with these agents, “resistance exercise might help mitigate that concern.”
Recently published recommendations can assist clinicians in guiding patients taking GLP-1 RAs to optimize nutrition. The recommendations note that patients should be referred to a registered dietitian to “complement and support” treatment with anti-obesity medications.
What Do Patients Want?
Despite the ever-rising popularity of GLP-1 RAs, a new national survey of over 2200 US adults conducted by the Physicians Committee for Responsible Medicine suggests that most Americans don’t want to use them. Among those who wanted to lose weight, almost three-quarters “disagreed” or “strongly disagreed” with the idea of taking a weight-loss injectable, and 68% of those who wanted to lose weight “agreed” or “strongly agreed” that they would be willing to try a plant-based diet, if it could lead to significant weight loss.
Moreover, many individuals treated with GLP-1 RAs discontinue their use, despite the probability of regaining the weight, according to a report that found only 46.3% of GLP-1 users were still taking the medications at 6 months and only 32.3% at 1 year. The authors commented that their real-world findings show a “substantially lower” 1-year persistence rate, compared with the rate reported in clinical trials. They suggest that the financial burden (> $12,000/year) may contribute to discontinuation.
Discontinuation of GLP-1 RAs can lead to worsening cardiometabolic parameters, with a potential increased risk for adverse outcomes; moreover, weight cycling (“yo-yo dieting”) carries its own risks. In light of these concerns, it’s particularly important to select appropriate patients and to determine whether potential short-term therapy has any enduring benefit.
Acosta agreed. “It’s exciting when looking at the data on how to find the best responders and who should make the effort to take these medications — not only in terms of side effects but also in terms of cost and which patients will receive maximum benefits and should be covered by insurance.”
Drucker has served as a consultant or speaker for Altimmune, Amgen, AstraZeneca, Arrowhead, Boehringer Ingelheim, Kallyope, Merck Research Laboratories, Novo Nordisk, Pfizer, and Zealand Pharma. He holds nonexercised options in Kallyope. Mount Sinai Hospital receives research support for investigator-initiated studies in the Drucker laboratory from Amgen, Novo Nordisk, Pfizer, and Zealand Pharma. Gila Therapeutics and Phenomix Sciences have licensed Acosta’s research technologies from University of Florida and Mayo Clinic. Acosta received consultant fees in the last 5 years from Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, Regeneron Pharmaceuticals, Boehringer Ingelheim, Novo Nordisk, Currax, Nestlé, Phenomix Sciences, Bausch Health, and Rare Disease. He received funding support from the National Institutes of Health, Vivus Pharmaceuticals, Novo Nordisk, Apollo Endosurgery, Satiogen Pharmaceuticals, Spatz Medical, Rhythm Pharmaceuticals, Regeneron Pharmaceuticals, Boehringer Ingelheim, and Novo Nordisk. In the past, Cornier has served as a consultant for Novo Nordisk.
A version of this article first appeared on Medscape.com.
Rates of overweight and obesity have more than doubled in the United States during the last three decades, according to a new analysis. By 2050, it’s anticipated that 213 million adults (age, > 25 years) and 43 million children and adolescents will have overweight or obesity. The results led authors of a study to describe obesity as having reached a “crisis point” requiring urgent action and interventions.
Are glucagon-like peptide 1 receptor agonists (GLP-1 RAs), originally developed and prescribed for diabetes and now approved for weight loss, the answer?
Their popularity is certainly surging. Between the last 6 months of 2022 vs the last 6 months of 2024, the number of patients prescribed GLP-1 RAs increased by 132.6%. This is also reflected in a shift in public awareness, with a recent survey of US adults finding that 32% of respondents had heard “a lot” about these drugs, up from 19% in 2023.
GLP-1 RAs (including tirzepatide, which targets not only the GLP-1 receptor but also the glucose-dependent insulinotropic polypeptide receptor) have shown efficacy in weight loss. A 2022 review and meta-analysis of 22 trials (17,183 patients) found that 50.2% and 17.5% of those treated with GLP-1 RAs had a ≥ 5% and ≥ 10% weight loss, respectively, compared with placebo. A 2023 review of 41 trials (15,135 patients) found that compared with controls, GLP-1 RAs significantly reduced body weight, body mass index, waist circumference, and waist-to-hip ratio.
“GLP-1 RAs are great medications,” Andres Acosta, MD, PhD, director of the Precision Medicine for Obesity Laboratory, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News. “We’ve been using them for almost two decades. But now there’s excitement about their utility in treating obesity.”
Treating the Four Categories of Obesity
Daniel Drucker, MD, senior investigator at the Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada, is a pioneer in diabetes treatment and particularly in the development of GLP-1 RAs. Drucker told Medscape Medical News that despite the efficacy and enormous potential of GLP-1 RAs, “we know some people don’t lose much weight when taking these medicines and others don’t feel well and can’t take them.”
The number of individuals who don’t respond to or aren’t able to tolerate GLP-1 RAs “might be small — less than 10% of people who try to take them — but we don’t fully understand the differences in response across different individuals,” Drucker said.
Acosta agreed, adding that it’s “essential for us to identify who will be the best responders, as we do with medications for other conditions, such as cancer and cardiovascular disease.”
Acosta’s group has spent more than a decade engaged in efforts to identify unique characteristics among patients with obesity and has succeeded in identifying four obesity phenotypes.
“What matters in the space of GLP-1 is that using this classification, we can identify the best responders and those who don’t respond.”
The first phenotype, described as “Hungry Gut” (HG), includes patients with abnormal postprandial satiety. “Although they may be satiated at the end of a meal, they have accelerated gastric emptying and therefore feel hungry between meals and want to keep eating,” he said.
There are also patients who experience abnormal satiety during meals. According to Acosta, these are the patients who will return to the table for second and third helpings. “They don’t feel full and continue to eat more and more in a single sitting” — a phenomenon referred to as “Hungry Brain.”
The third phenotype — “Emotional Hunger” — consists of people who are “hedonic” about food or engage in emotional eating behavior, whereas in the fourth group, people have “an abnormal metabolism in which they don’t burn enough calories. They have an inefficient metabolic rate.” This latter phenomenon is called “Slow Burn.”
Acosta and colleagues randomized 312 patients attending a weight management center to phenotype-guided or non–phenotype-guided treatment with anti-obesity medications (phentermine, phentermine/topiramate, bupropion/naltrexone, lorcaserin, and liraglutide). The phenotype-guided approach was associated with a 1.75-fold greater weight loss after 1 year than the non–phenotype-guided approach (mean weight loss, 15.9% vs 9.0%, respectively).
GLP-1 RAs: Not One-Size-Fits-All
Acosta’s group has developed a genetic test that uses patients’ saliva to identify their obesity phenotype, with the aim of predicting the best responders to GLP-1 RAs. The test, MyPhenome genetic obesity test, is licensed by Acosta’s lab and available through Phenomix Sciences.
Acosta and colleagues presented their findings at the American Gastroenterological Association’s 2024 annual meeting regarding a machine-learning gene risk score (ML-GRS) they developed to predict HG, based on saliva and blood samples. Their genetic studies generated a ML-GRS that classified participants with obesity along a continuum from “HG Positive” (HG+) to “HG Negative” (HG−). Compared with the HG− participants, those who were HG+ had superior total body weight loss with semaglutide at 9 and 12 months. When used to predict response, the ML-GRS had an area under the curve of 0.76 (P = .04) and a positive predictive value of 0.95.
According to Acosta, HG+ patients are “the best responders to the GLP-1 RAs, although we don’t yet understand the mechanism of why they have the phenomenon of abnormal postprandial satiety. It may be an abnormal genetic pathway or abnormal secretion of GLP-1. More studies are needed.”
He noted that GLP-1 RAs “might also be helpful with the second [Hungry Brain] category, but these patients do better with phentermine-topiramate,” as demonstrated in a 2023 study conducted by Acosta and colleagues.
His group has also studied which lifestyle interventions are most effective for each phenotype. “When a unique lifestyle intervention targeting each phenotype was applied, patients lost more weight and had greater metabolic improvement,” he reported.
“Treating obesity no longer needs to be trial-and-error, but should be done using precision medicine because one size doesn’t fit all,” Acosta said.
Concerning Side Effects
The popular media has featured stories about individuals who took GLP-1 RAs for weight loss and experienced serious side effects, including a recent account of a British nurse who died after taking tirzepatide. As reported by the BBC, the nurse’s death certificate listed multiple organ failure, septic shock, and pancreatitis as the immediate causes of death, with the “use of prescribed tirzepatide” recorded as a contributing factor. The report went on to note that there were 23 suspected deaths in the United Kingdom tied to semaglutide since 2019.
Beyond brand-name products, there are also risks associated with GLP-1 RAs manufactured by compounding pharmacies. In early November, CNN reported that compounded semaglutide has been linked to at least 10 deaths. Because of a prior shortage of tirzepatide, the US Food and Drug Administration (FDA) had allowed compounding pharmacies to manufacture the drug. In October, the FDA clarified that it won’t take legal action against compounders, even now that the shortage has been resolved.
A pharmacovigilance study using the FDA Adverse Event Reporting System identified “potential safety signals of increased mortality and serious adverse event reporting” associated with certain GLP-1 RAs — especially in younger patients and women (P < .0001 for both groups).
The most common side effects reported with GLP-1 RAs are gastrointestinal events, such as nausea, diarrhea, constipation, and vomiting. Most occur during dose initiation and escalation and wane over the following weeks. However, studies have also reported severe side effects, including a higher risk for pancreatitis, bowel obstruction, and gastroparesis, as well as a significantly higher risk for gallbladder and biliary diseases. In fact, according to one study, patients with diabetes taking GLP-1 RAs reported gastrointestinal-related issues as a “prominent factor” in their decision to discontinue taking these medications.
Several types of cancer are potentially associated with GLP-1 RAs, but findings regarding this potential link have been inconsistent. In a recent review article, Drucker noted there were only inconsistent data linking GLP-1 RAs with thyroid cancer and medullary thyroid cancer and that their potential association with pancreatic has “not been supported by results from randomized controlled trials or real-world data.”
Concerns have been raised about loss of lean mass and muscle strength and function, especially in older individuals with obesity and advanced liver, cardiovascular, or kidney disease. However, as Drucker pointed out in his review article, muscle function may not correlate with the loss of lean mass. In fact, there are “consistent reductions” in lean mass after bariatric surgery, but “little evidence to date for impairment of muscle function.” He added that newer GLP-1 agents under development for obesity treatment are focusing on “developing complementary therapies that preferentially reduce adipose tissue, while sparing lean mass.”
As covered by Medscape Medical News, there have been reports of potential suicidal ideation associated with GLP-1 RAs. This triggered a 2023 review from the European Medicines Agency. However, recent results from a cohort study and a post hoc analysis of randomized controlled trials concluded that there is no evidence that these drugs increase suicidal ideation or behavior.
In early November, the FDA updated the labels for the GLP-1 RAs to include a warning regarding pulmonary aspiration during general anesthesia or deep sedation. Guidance from a group of societies, led by the American Society of Anesthesiologists, contains recommendations regarding nuances of addressing this concern in surgical patients taking these agents.
Not a Standalone Treatment
Marc-Andre Cornier, MD, professor of medicine, James A. Keating Endowed Chair in Diabetes, and director of the Division of Endocrinology, Diabetes and Metabolic Diseases, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina, told Medscape Medical News that GLP-1 RAs should not be viewed as cosmetic interventions but rather as medical treatments, “not only for weight loss but to reverse obesity-associated complications.”
Moreover, they should be used “as an adjunct to lifestyle changes,” emphasized Cornier. “We want our patients to have a high-quality diet with high protein content, fluid, vitamins, and minerals, and we want them to exercise.” Especially with the concern of potential loss of muscle mass with these agents, “resistance exercise might help mitigate that concern.”
Recently published recommendations can assist clinicians in guiding patients taking GLP-1 RAs to optimize nutrition. The recommendations note that patients should be referred to a registered dietitian to “complement and support” treatment with anti-obesity medications.
What Do Patients Want?
Despite the ever-rising popularity of GLP-1 RAs, a new national survey of over 2200 US adults conducted by the Physicians Committee for Responsible Medicine suggests that most Americans don’t want to use them. Among those who wanted to lose weight, almost three-quarters “disagreed” or “strongly disagreed” with the idea of taking a weight-loss injectable, and 68% of those who wanted to lose weight “agreed” or “strongly agreed” that they would be willing to try a plant-based diet, if it could lead to significant weight loss.
Moreover, many individuals treated with GLP-1 RAs discontinue their use, despite the probability of regaining the weight, according to a report that found only 46.3% of GLP-1 users were still taking the medications at 6 months and only 32.3% at 1 year. The authors commented that their real-world findings show a “substantially lower” 1-year persistence rate, compared with the rate reported in clinical trials. They suggest that the financial burden (> $12,000/year) may contribute to discontinuation.
Discontinuation of GLP-1 RAs can lead to worsening cardiometabolic parameters, with a potential increased risk for adverse outcomes; moreover, weight cycling (“yo-yo dieting”) carries its own risks. In light of these concerns, it’s particularly important to select appropriate patients and to determine whether potential short-term therapy has any enduring benefit.
Acosta agreed. “It’s exciting when looking at the data on how to find the best responders and who should make the effort to take these medications — not only in terms of side effects but also in terms of cost and which patients will receive maximum benefits and should be covered by insurance.”
Drucker has served as a consultant or speaker for Altimmune, Amgen, AstraZeneca, Arrowhead, Boehringer Ingelheim, Kallyope, Merck Research Laboratories, Novo Nordisk, Pfizer, and Zealand Pharma. He holds nonexercised options in Kallyope. Mount Sinai Hospital receives research support for investigator-initiated studies in the Drucker laboratory from Amgen, Novo Nordisk, Pfizer, and Zealand Pharma. Gila Therapeutics and Phenomix Sciences have licensed Acosta’s research technologies from University of Florida and Mayo Clinic. Acosta received consultant fees in the last 5 years from Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, Regeneron Pharmaceuticals, Boehringer Ingelheim, Novo Nordisk, Currax, Nestlé, Phenomix Sciences, Bausch Health, and Rare Disease. He received funding support from the National Institutes of Health, Vivus Pharmaceuticals, Novo Nordisk, Apollo Endosurgery, Satiogen Pharmaceuticals, Spatz Medical, Rhythm Pharmaceuticals, Regeneron Pharmaceuticals, Boehringer Ingelheim, and Novo Nordisk. In the past, Cornier has served as a consultant for Novo Nordisk.
A version of this article first appeared on Medscape.com.
Rates of overweight and obesity have more than doubled in the United States during the last three decades, according to a new analysis. By 2050, it’s anticipated that 213 million adults (age, > 25 years) and 43 million children and adolescents will have overweight or obesity. The results led authors of a study to describe obesity as having reached a “crisis point” requiring urgent action and interventions.
Are glucagon-like peptide 1 receptor agonists (GLP-1 RAs), originally developed and prescribed for diabetes and now approved for weight loss, the answer?
Their popularity is certainly surging. Between the last 6 months of 2022 vs the last 6 months of 2024, the number of patients prescribed GLP-1 RAs increased by 132.6%. This is also reflected in a shift in public awareness, with a recent survey of US adults finding that 32% of respondents had heard “a lot” about these drugs, up from 19% in 2023.
GLP-1 RAs (including tirzepatide, which targets not only the GLP-1 receptor but also the glucose-dependent insulinotropic polypeptide receptor) have shown efficacy in weight loss. A 2022 review and meta-analysis of 22 trials (17,183 patients) found that 50.2% and 17.5% of those treated with GLP-1 RAs had a ≥ 5% and ≥ 10% weight loss, respectively, compared with placebo. A 2023 review of 41 trials (15,135 patients) found that compared with controls, GLP-1 RAs significantly reduced body weight, body mass index, waist circumference, and waist-to-hip ratio.
“GLP-1 RAs are great medications,” Andres Acosta, MD, PhD, director of the Precision Medicine for Obesity Laboratory, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News. “We’ve been using them for almost two decades. But now there’s excitement about their utility in treating obesity.”
Treating the Four Categories of Obesity
Daniel Drucker, MD, senior investigator at the Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada, is a pioneer in diabetes treatment and particularly in the development of GLP-1 RAs. Drucker told Medscape Medical News that despite the efficacy and enormous potential of GLP-1 RAs, “we know some people don’t lose much weight when taking these medicines and others don’t feel well and can’t take them.”
The number of individuals who don’t respond to or aren’t able to tolerate GLP-1 RAs “might be small — less than 10% of people who try to take them — but we don’t fully understand the differences in response across different individuals,” Drucker said.
Acosta agreed, adding that it’s “essential for us to identify who will be the best responders, as we do with medications for other conditions, such as cancer and cardiovascular disease.”
Acosta’s group has spent more than a decade engaged in efforts to identify unique characteristics among patients with obesity and has succeeded in identifying four obesity phenotypes.
“What matters in the space of GLP-1 is that using this classification, we can identify the best responders and those who don’t respond.”
The first phenotype, described as “Hungry Gut” (HG), includes patients with abnormal postprandial satiety. “Although they may be satiated at the end of a meal, they have accelerated gastric emptying and therefore feel hungry between meals and want to keep eating,” he said.
There are also patients who experience abnormal satiety during meals. According to Acosta, these are the patients who will return to the table for second and third helpings. “They don’t feel full and continue to eat more and more in a single sitting” — a phenomenon referred to as “Hungry Brain.”
The third phenotype — “Emotional Hunger” — consists of people who are “hedonic” about food or engage in emotional eating behavior, whereas in the fourth group, people have “an abnormal metabolism in which they don’t burn enough calories. They have an inefficient metabolic rate.” This latter phenomenon is called “Slow Burn.”
Acosta and colleagues randomized 312 patients attending a weight management center to phenotype-guided or non–phenotype-guided treatment with anti-obesity medications (phentermine, phentermine/topiramate, bupropion/naltrexone, lorcaserin, and liraglutide). The phenotype-guided approach was associated with a 1.75-fold greater weight loss after 1 year than the non–phenotype-guided approach (mean weight loss, 15.9% vs 9.0%, respectively).
GLP-1 RAs: Not One-Size-Fits-All
Acosta’s group has developed a genetic test that uses patients’ saliva to identify their obesity phenotype, with the aim of predicting the best responders to GLP-1 RAs. The test, MyPhenome genetic obesity test, is licensed by Acosta’s lab and available through Phenomix Sciences.
Acosta and colleagues presented their findings at the American Gastroenterological Association’s 2024 annual meeting regarding a machine-learning gene risk score (ML-GRS) they developed to predict HG, based on saliva and blood samples. Their genetic studies generated a ML-GRS that classified participants with obesity along a continuum from “HG Positive” (HG+) to “HG Negative” (HG−). Compared with the HG− participants, those who were HG+ had superior total body weight loss with semaglutide at 9 and 12 months. When used to predict response, the ML-GRS had an area under the curve of 0.76 (P = .04) and a positive predictive value of 0.95.
According to Acosta, HG+ patients are “the best responders to the GLP-1 RAs, although we don’t yet understand the mechanism of why they have the phenomenon of abnormal postprandial satiety. It may be an abnormal genetic pathway or abnormal secretion of GLP-1. More studies are needed.”
He noted that GLP-1 RAs “might also be helpful with the second [Hungry Brain] category, but these patients do better with phentermine-topiramate,” as demonstrated in a 2023 study conducted by Acosta and colleagues.
His group has also studied which lifestyle interventions are most effective for each phenotype. “When a unique lifestyle intervention targeting each phenotype was applied, patients lost more weight and had greater metabolic improvement,” he reported.
“Treating obesity no longer needs to be trial-and-error, but should be done using precision medicine because one size doesn’t fit all,” Acosta said.
Concerning Side Effects
The popular media has featured stories about individuals who took GLP-1 RAs for weight loss and experienced serious side effects, including a recent account of a British nurse who died after taking tirzepatide. As reported by the BBC, the nurse’s death certificate listed multiple organ failure, septic shock, and pancreatitis as the immediate causes of death, with the “use of prescribed tirzepatide” recorded as a contributing factor. The report went on to note that there were 23 suspected deaths in the United Kingdom tied to semaglutide since 2019.
Beyond brand-name products, there are also risks associated with GLP-1 RAs manufactured by compounding pharmacies. In early November, CNN reported that compounded semaglutide has been linked to at least 10 deaths. Because of a prior shortage of tirzepatide, the US Food and Drug Administration (FDA) had allowed compounding pharmacies to manufacture the drug. In October, the FDA clarified that it won’t take legal action against compounders, even now that the shortage has been resolved.
A pharmacovigilance study using the FDA Adverse Event Reporting System identified “potential safety signals of increased mortality and serious adverse event reporting” associated with certain GLP-1 RAs — especially in younger patients and women (P < .0001 for both groups).
The most common side effects reported with GLP-1 RAs are gastrointestinal events, such as nausea, diarrhea, constipation, and vomiting. Most occur during dose initiation and escalation and wane over the following weeks. However, studies have also reported severe side effects, including a higher risk for pancreatitis, bowel obstruction, and gastroparesis, as well as a significantly higher risk for gallbladder and biliary diseases. In fact, according to one study, patients with diabetes taking GLP-1 RAs reported gastrointestinal-related issues as a “prominent factor” in their decision to discontinue taking these medications.
Several types of cancer are potentially associated with GLP-1 RAs, but findings regarding this potential link have been inconsistent. In a recent review article, Drucker noted there were only inconsistent data linking GLP-1 RAs with thyroid cancer and medullary thyroid cancer and that their potential association with pancreatic has “not been supported by results from randomized controlled trials or real-world data.”
Concerns have been raised about loss of lean mass and muscle strength and function, especially in older individuals with obesity and advanced liver, cardiovascular, or kidney disease. However, as Drucker pointed out in his review article, muscle function may not correlate with the loss of lean mass. In fact, there are “consistent reductions” in lean mass after bariatric surgery, but “little evidence to date for impairment of muscle function.” He added that newer GLP-1 agents under development for obesity treatment are focusing on “developing complementary therapies that preferentially reduce adipose tissue, while sparing lean mass.”
As covered by Medscape Medical News, there have been reports of potential suicidal ideation associated with GLP-1 RAs. This triggered a 2023 review from the European Medicines Agency. However, recent results from a cohort study and a post hoc analysis of randomized controlled trials concluded that there is no evidence that these drugs increase suicidal ideation or behavior.
In early November, the FDA updated the labels for the GLP-1 RAs to include a warning regarding pulmonary aspiration during general anesthesia or deep sedation. Guidance from a group of societies, led by the American Society of Anesthesiologists, contains recommendations regarding nuances of addressing this concern in surgical patients taking these agents.
Not a Standalone Treatment
Marc-Andre Cornier, MD, professor of medicine, James A. Keating Endowed Chair in Diabetes, and director of the Division of Endocrinology, Diabetes and Metabolic Diseases, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina, told Medscape Medical News that GLP-1 RAs should not be viewed as cosmetic interventions but rather as medical treatments, “not only for weight loss but to reverse obesity-associated complications.”
Moreover, they should be used “as an adjunct to lifestyle changes,” emphasized Cornier. “We want our patients to have a high-quality diet with high protein content, fluid, vitamins, and minerals, and we want them to exercise.” Especially with the concern of potential loss of muscle mass with these agents, “resistance exercise might help mitigate that concern.”
Recently published recommendations can assist clinicians in guiding patients taking GLP-1 RAs to optimize nutrition. The recommendations note that patients should be referred to a registered dietitian to “complement and support” treatment with anti-obesity medications.
What Do Patients Want?
Despite the ever-rising popularity of GLP-1 RAs, a new national survey of over 2200 US adults conducted by the Physicians Committee for Responsible Medicine suggests that most Americans don’t want to use them. Among those who wanted to lose weight, almost three-quarters “disagreed” or “strongly disagreed” with the idea of taking a weight-loss injectable, and 68% of those who wanted to lose weight “agreed” or “strongly agreed” that they would be willing to try a plant-based diet, if it could lead to significant weight loss.
Moreover, many individuals treated with GLP-1 RAs discontinue their use, despite the probability of regaining the weight, according to a report that found only 46.3% of GLP-1 users were still taking the medications at 6 months and only 32.3% at 1 year. The authors commented that their real-world findings show a “substantially lower” 1-year persistence rate, compared with the rate reported in clinical trials. They suggest that the financial burden (> $12,000/year) may contribute to discontinuation.
Discontinuation of GLP-1 RAs can lead to worsening cardiometabolic parameters, with a potential increased risk for adverse outcomes; moreover, weight cycling (“yo-yo dieting”) carries its own risks. In light of these concerns, it’s particularly important to select appropriate patients and to determine whether potential short-term therapy has any enduring benefit.
Acosta agreed. “It’s exciting when looking at the data on how to find the best responders and who should make the effort to take these medications — not only in terms of side effects but also in terms of cost and which patients will receive maximum benefits and should be covered by insurance.”
Drucker has served as a consultant or speaker for Altimmune, Amgen, AstraZeneca, Arrowhead, Boehringer Ingelheim, Kallyope, Merck Research Laboratories, Novo Nordisk, Pfizer, and Zealand Pharma. He holds nonexercised options in Kallyope. Mount Sinai Hospital receives research support for investigator-initiated studies in the Drucker laboratory from Amgen, Novo Nordisk, Pfizer, and Zealand Pharma. Gila Therapeutics and Phenomix Sciences have licensed Acosta’s research technologies from University of Florida and Mayo Clinic. Acosta received consultant fees in the last 5 years from Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, Regeneron Pharmaceuticals, Boehringer Ingelheim, Novo Nordisk, Currax, Nestlé, Phenomix Sciences, Bausch Health, and Rare Disease. He received funding support from the National Institutes of Health, Vivus Pharmaceuticals, Novo Nordisk, Apollo Endosurgery, Satiogen Pharmaceuticals, Spatz Medical, Rhythm Pharmaceuticals, Regeneron Pharmaceuticals, Boehringer Ingelheim, and Novo Nordisk. In the past, Cornier has served as a consultant for Novo Nordisk.
A version of this article first appeared on Medscape.com.
Interim guidance for CPR in patients with COVID-19
The American Heart Association (AHA) and seven other medical societies have issued interim guidance to inform treatment of victims of cardiac arrest with suspected or confirmed COVID-19, focusing on reducing provider exposure, and prioritizing oxygenation and ventilation strategies, goals of care, and appropriateness of resuscitation.
“We were very specific in calling this ‘interim guidance’ based on expert opinion because things are evolving so quickly and we are learning more and more every day as more and more patients with COVID-19 are taken care of,” corresponding author Comilla Sasson, MD, PhD, vice president, Emergency Cardiovascular Care (ECC) Science and Innovation, American Heart Association, told theheart.org | Medscape Cardiology.
“We wanted this to be a starting point for providing the clinical guidance that everyone is looking for and, as we collect more data, the guidance will change, as it has for CDC [Centers for Disease Control and Prevention] and WHO [World Health Organization],” she said.
“The guidance sought to balance the provision of timely, high-quality resuscitation to patients while simultaneously protecting rescuers,” she added.
The guidance was published online April 9 in Circulation. The AHA produced the guidelines in collaboration with the American Academy of Pediatrics, American Association for Respiratory Care, American College of Emergency Physicians, the Society of Critical Care Anesthesiologists, and the American Society of Anesthesiologists, with support from the American Association of Critical Care Nurses and National EMS Physicians.
Respiratory Etiologies
“We think of cardiac arrest in adults, especially as related to cardiac etiologies, but we are now thinking of it in COVID-19 more as hypoxemia or respiratory failure, which can predispose patients to cardiac arrest,” Sasson explained.
Healthcare workers are the “highest-risk profession” for contracting the COVID-19, with resuscitations carrying “added risk” for several reasons, the authors note.
Administering CPR involves performing numerous aerosol-generating procedures that can cause viral particles to remain suspended in the air and be inhaled by those nearby, with a half-life of approximately 1 hour, they point out.
Moreover, resuscitation efforts “require numerous providers to work in close proximity to one another and the patient,” and the high-stress emergent nature of these events may result in lapses in infection-control procedures.
The guidance is designed “to protect not only the patient but also the provider and involves strategies regarding oxygenation and ventilation that differ from what we’ve done in the past since we have a strong feeling that this is a different disease process that may require different approaches than what we’ve dealt with in the past,” Sasson commented.
Reducing Provider Exposure
Providers should don PPE to protect both themselves and their colleagues from unnecessary exposure, the authors advise, noting that recommendations for PPE standards may “vary considerably,” so health or emergency medical services (EMS) standards should be taken into account.
Moreover, it is important to allow only the most essential providers into the room or on the scene. In keeping with reducing the number of rescuers, the authors recommend replacing manual chest compressions with mechanical CPR devices for patients who meet height and weight criteria in settings with “protocols and expertise in place for their use.”
COVID-19 status should be communicated to any new providers prior to their arrival on the scene, the authors stress.
Oxygenation and Ventilation Strategies
“Reducing risk of aerosolization during the process of intubation is key,” Sasson emphasized.
For this reason, a high-efficiency particulate air HEPA filter (if available) should be attached to any manual or mechanical ventilation device, specifically in the path of exhaled gas, before any breaths are administered.
Moreover, it is important to intubate early with a cuffed tube and connect to a mechanical ventilator, if possible. The intubator should be engaged with the “highest chance of first-pass success,” and chest compression should be paused to intubate.
To further increase the chance of a successful first intubation, use of video laryngoscopy (if available) is helpful.
Additional guidance includes:
- Using a bag-mask device (or T-piece in neonates) with a HEPA filter and a tight seal prior to intubation
- Considering passive oxygenation with non-rebreathing face mask as an alternative to bag-mask device for short duration (in adults)
- Considering supraglottic airway if intubation is delayed
- Minimizing closed circuit disconnections.
Resuscitation Considerations
“One big take-home point of the guidance is to consider resuscitation appropriateness, starting with goals of care when the patient comes to us, and continuing or stopping resuscitation when needed, based on the discussion with the family as well as local protocol,” Sasson said.
A variety of factors need to be taken into account, including age, comorbidities, and illness severity to determine the appropriateness of resuscitation, and “the likelihood of success” must be balanced “against the risk to rescuers and patients from whom resources are being diverted,” the authors state.
An Array of Scenarios
“We divided bystander CPR into adults vs pediatrics and into those who are living with a person who is in cardiac arrest – because they have already been exposed [to COVID-19] – vs those who are not living with the patient,” Sasson reported. “We also addressed the role of lay bystanders.”
For lay rescuers:
- Household members should perform at least hands-only CPR, if willing and able to do so
- Use of a face mark or cloth covering of the mouth and nose of the rescuer and/or patient may reduce the risk of transmission to a nonhousehold member
- In children, lay rescuers should perform chest compressions and “consider mouth-to-mouth resuscitation,” especially if they are household members.
- If available, an automated external defibrillator should be used to assess and treat victims of out-of-hospital cardiac arrest (OHCA).
The authors offer additional guidance for in-hospital cardiac arrest (IHCA), including addressing advanced care directives, closing the door when possible to prevent airborne contamination of adjacent space, and considering leaving the patient on a mechanical ventilator with HEPA filter.
They additionally address the special needs of neonates, recommending the presence of a “skilled attendant prepared to resuscitate, irrespective of COVID-19 status,” and stressing the importance of PPE since the mother may be a “potential source of aerosolization for the neonatal team.” Additional measures include avoidance of routine airway suctioning and the use of endotracheal medications.
Critically ill pregnant women with COVID-19 are more vulnerable to acute decompensation because of the cardiopulmonary physiological changes associated with pregnancy, the authors note. Preparation for a potential perimortem delivery should take place after 4 minutes of resuscitation and be initiated early in the resuscitation algorithm so as to allow specialized obstetrical and neonatal teams with PPE to convene.
“We will be continually updating this guidance and we are encouraging people to ask questions,” Sasson summarized.
She noted that a hospital-based COVID-19 registry is being formed to collect “clinically relevant data” that will inform and update the current guidance.
Sasson reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper.
This article first appeared on Medscape.com.
The American Heart Association (AHA) and seven other medical societies have issued interim guidance to inform treatment of victims of cardiac arrest with suspected or confirmed COVID-19, focusing on reducing provider exposure, and prioritizing oxygenation and ventilation strategies, goals of care, and appropriateness of resuscitation.
“We were very specific in calling this ‘interim guidance’ based on expert opinion because things are evolving so quickly and we are learning more and more every day as more and more patients with COVID-19 are taken care of,” corresponding author Comilla Sasson, MD, PhD, vice president, Emergency Cardiovascular Care (ECC) Science and Innovation, American Heart Association, told theheart.org | Medscape Cardiology.
“We wanted this to be a starting point for providing the clinical guidance that everyone is looking for and, as we collect more data, the guidance will change, as it has for CDC [Centers for Disease Control and Prevention] and WHO [World Health Organization],” she said.
“The guidance sought to balance the provision of timely, high-quality resuscitation to patients while simultaneously protecting rescuers,” she added.
The guidance was published online April 9 in Circulation. The AHA produced the guidelines in collaboration with the American Academy of Pediatrics, American Association for Respiratory Care, American College of Emergency Physicians, the Society of Critical Care Anesthesiologists, and the American Society of Anesthesiologists, with support from the American Association of Critical Care Nurses and National EMS Physicians.
Respiratory Etiologies
“We think of cardiac arrest in adults, especially as related to cardiac etiologies, but we are now thinking of it in COVID-19 more as hypoxemia or respiratory failure, which can predispose patients to cardiac arrest,” Sasson explained.
Healthcare workers are the “highest-risk profession” for contracting the COVID-19, with resuscitations carrying “added risk” for several reasons, the authors note.
Administering CPR involves performing numerous aerosol-generating procedures that can cause viral particles to remain suspended in the air and be inhaled by those nearby, with a half-life of approximately 1 hour, they point out.
Moreover, resuscitation efforts “require numerous providers to work in close proximity to one another and the patient,” and the high-stress emergent nature of these events may result in lapses in infection-control procedures.
The guidance is designed “to protect not only the patient but also the provider and involves strategies regarding oxygenation and ventilation that differ from what we’ve done in the past since we have a strong feeling that this is a different disease process that may require different approaches than what we’ve dealt with in the past,” Sasson commented.
Reducing Provider Exposure
Providers should don PPE to protect both themselves and their colleagues from unnecessary exposure, the authors advise, noting that recommendations for PPE standards may “vary considerably,” so health or emergency medical services (EMS) standards should be taken into account.
Moreover, it is important to allow only the most essential providers into the room or on the scene. In keeping with reducing the number of rescuers, the authors recommend replacing manual chest compressions with mechanical CPR devices for patients who meet height and weight criteria in settings with “protocols and expertise in place for their use.”
COVID-19 status should be communicated to any new providers prior to their arrival on the scene, the authors stress.
Oxygenation and Ventilation Strategies
“Reducing risk of aerosolization during the process of intubation is key,” Sasson emphasized.
For this reason, a high-efficiency particulate air HEPA filter (if available) should be attached to any manual or mechanical ventilation device, specifically in the path of exhaled gas, before any breaths are administered.
Moreover, it is important to intubate early with a cuffed tube and connect to a mechanical ventilator, if possible. The intubator should be engaged with the “highest chance of first-pass success,” and chest compression should be paused to intubate.
To further increase the chance of a successful first intubation, use of video laryngoscopy (if available) is helpful.
Additional guidance includes:
- Using a bag-mask device (or T-piece in neonates) with a HEPA filter and a tight seal prior to intubation
- Considering passive oxygenation with non-rebreathing face mask as an alternative to bag-mask device for short duration (in adults)
- Considering supraglottic airway if intubation is delayed
- Minimizing closed circuit disconnections.
Resuscitation Considerations
“One big take-home point of the guidance is to consider resuscitation appropriateness, starting with goals of care when the patient comes to us, and continuing or stopping resuscitation when needed, based on the discussion with the family as well as local protocol,” Sasson said.
A variety of factors need to be taken into account, including age, comorbidities, and illness severity to determine the appropriateness of resuscitation, and “the likelihood of success” must be balanced “against the risk to rescuers and patients from whom resources are being diverted,” the authors state.
An Array of Scenarios
“We divided bystander CPR into adults vs pediatrics and into those who are living with a person who is in cardiac arrest – because they have already been exposed [to COVID-19] – vs those who are not living with the patient,” Sasson reported. “We also addressed the role of lay bystanders.”
For lay rescuers:
- Household members should perform at least hands-only CPR, if willing and able to do so
- Use of a face mark or cloth covering of the mouth and nose of the rescuer and/or patient may reduce the risk of transmission to a nonhousehold member
- In children, lay rescuers should perform chest compressions and “consider mouth-to-mouth resuscitation,” especially if they are household members.
- If available, an automated external defibrillator should be used to assess and treat victims of out-of-hospital cardiac arrest (OHCA).
The authors offer additional guidance for in-hospital cardiac arrest (IHCA), including addressing advanced care directives, closing the door when possible to prevent airborne contamination of adjacent space, and considering leaving the patient on a mechanical ventilator with HEPA filter.
They additionally address the special needs of neonates, recommending the presence of a “skilled attendant prepared to resuscitate, irrespective of COVID-19 status,” and stressing the importance of PPE since the mother may be a “potential source of aerosolization for the neonatal team.” Additional measures include avoidance of routine airway suctioning and the use of endotracheal medications.
Critically ill pregnant women with COVID-19 are more vulnerable to acute decompensation because of the cardiopulmonary physiological changes associated with pregnancy, the authors note. Preparation for a potential perimortem delivery should take place after 4 minutes of resuscitation and be initiated early in the resuscitation algorithm so as to allow specialized obstetrical and neonatal teams with PPE to convene.
“We will be continually updating this guidance and we are encouraging people to ask questions,” Sasson summarized.
She noted that a hospital-based COVID-19 registry is being formed to collect “clinically relevant data” that will inform and update the current guidance.
Sasson reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper.
This article first appeared on Medscape.com.
The American Heart Association (AHA) and seven other medical societies have issued interim guidance to inform treatment of victims of cardiac arrest with suspected or confirmed COVID-19, focusing on reducing provider exposure, and prioritizing oxygenation and ventilation strategies, goals of care, and appropriateness of resuscitation.
“We were very specific in calling this ‘interim guidance’ based on expert opinion because things are evolving so quickly and we are learning more and more every day as more and more patients with COVID-19 are taken care of,” corresponding author Comilla Sasson, MD, PhD, vice president, Emergency Cardiovascular Care (ECC) Science and Innovation, American Heart Association, told theheart.org | Medscape Cardiology.
“We wanted this to be a starting point for providing the clinical guidance that everyone is looking for and, as we collect more data, the guidance will change, as it has for CDC [Centers for Disease Control and Prevention] and WHO [World Health Organization],” she said.
“The guidance sought to balance the provision of timely, high-quality resuscitation to patients while simultaneously protecting rescuers,” she added.
The guidance was published online April 9 in Circulation. The AHA produced the guidelines in collaboration with the American Academy of Pediatrics, American Association for Respiratory Care, American College of Emergency Physicians, the Society of Critical Care Anesthesiologists, and the American Society of Anesthesiologists, with support from the American Association of Critical Care Nurses and National EMS Physicians.
Respiratory Etiologies
“We think of cardiac arrest in adults, especially as related to cardiac etiologies, but we are now thinking of it in COVID-19 more as hypoxemia or respiratory failure, which can predispose patients to cardiac arrest,” Sasson explained.
Healthcare workers are the “highest-risk profession” for contracting the COVID-19, with resuscitations carrying “added risk” for several reasons, the authors note.
Administering CPR involves performing numerous aerosol-generating procedures that can cause viral particles to remain suspended in the air and be inhaled by those nearby, with a half-life of approximately 1 hour, they point out.
Moreover, resuscitation efforts “require numerous providers to work in close proximity to one another and the patient,” and the high-stress emergent nature of these events may result in lapses in infection-control procedures.
The guidance is designed “to protect not only the patient but also the provider and involves strategies regarding oxygenation and ventilation that differ from what we’ve done in the past since we have a strong feeling that this is a different disease process that may require different approaches than what we’ve dealt with in the past,” Sasson commented.
Reducing Provider Exposure
Providers should don PPE to protect both themselves and their colleagues from unnecessary exposure, the authors advise, noting that recommendations for PPE standards may “vary considerably,” so health or emergency medical services (EMS) standards should be taken into account.
Moreover, it is important to allow only the most essential providers into the room or on the scene. In keeping with reducing the number of rescuers, the authors recommend replacing manual chest compressions with mechanical CPR devices for patients who meet height and weight criteria in settings with “protocols and expertise in place for their use.”
COVID-19 status should be communicated to any new providers prior to their arrival on the scene, the authors stress.
Oxygenation and Ventilation Strategies
“Reducing risk of aerosolization during the process of intubation is key,” Sasson emphasized.
For this reason, a high-efficiency particulate air HEPA filter (if available) should be attached to any manual or mechanical ventilation device, specifically in the path of exhaled gas, before any breaths are administered.
Moreover, it is important to intubate early with a cuffed tube and connect to a mechanical ventilator, if possible. The intubator should be engaged with the “highest chance of first-pass success,” and chest compression should be paused to intubate.
To further increase the chance of a successful first intubation, use of video laryngoscopy (if available) is helpful.
Additional guidance includes:
- Using a bag-mask device (or T-piece in neonates) with a HEPA filter and a tight seal prior to intubation
- Considering passive oxygenation with non-rebreathing face mask as an alternative to bag-mask device for short duration (in adults)
- Considering supraglottic airway if intubation is delayed
- Minimizing closed circuit disconnections.
Resuscitation Considerations
“One big take-home point of the guidance is to consider resuscitation appropriateness, starting with goals of care when the patient comes to us, and continuing or stopping resuscitation when needed, based on the discussion with the family as well as local protocol,” Sasson said.
A variety of factors need to be taken into account, including age, comorbidities, and illness severity to determine the appropriateness of resuscitation, and “the likelihood of success” must be balanced “against the risk to rescuers and patients from whom resources are being diverted,” the authors state.
An Array of Scenarios
“We divided bystander CPR into adults vs pediatrics and into those who are living with a person who is in cardiac arrest – because they have already been exposed [to COVID-19] – vs those who are not living with the patient,” Sasson reported. “We also addressed the role of lay bystanders.”
For lay rescuers:
- Household members should perform at least hands-only CPR, if willing and able to do so
- Use of a face mark or cloth covering of the mouth and nose of the rescuer and/or patient may reduce the risk of transmission to a nonhousehold member
- In children, lay rescuers should perform chest compressions and “consider mouth-to-mouth resuscitation,” especially if they are household members.
- If available, an automated external defibrillator should be used to assess and treat victims of out-of-hospital cardiac arrest (OHCA).
The authors offer additional guidance for in-hospital cardiac arrest (IHCA), including addressing advanced care directives, closing the door when possible to prevent airborne contamination of adjacent space, and considering leaving the patient on a mechanical ventilator with HEPA filter.
They additionally address the special needs of neonates, recommending the presence of a “skilled attendant prepared to resuscitate, irrespective of COVID-19 status,” and stressing the importance of PPE since the mother may be a “potential source of aerosolization for the neonatal team.” Additional measures include avoidance of routine airway suctioning and the use of endotracheal medications.
Critically ill pregnant women with COVID-19 are more vulnerable to acute decompensation because of the cardiopulmonary physiological changes associated with pregnancy, the authors note. Preparation for a potential perimortem delivery should take place after 4 minutes of resuscitation and be initiated early in the resuscitation algorithm so as to allow specialized obstetrical and neonatal teams with PPE to convene.
“We will be continually updating this guidance and we are encouraging people to ask questions,” Sasson summarized.
She noted that a hospital-based COVID-19 registry is being formed to collect “clinically relevant data” that will inform and update the current guidance.
Sasson reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper.
This article first appeared on Medscape.com.
More conflicting evidence on paclitaxel devices in PAD
The controversy regarding the safety of treating peripheral artery disease (PAD) with paclitaxel-coated devices has only deepened in the new year, with two recent studies suggesting opposite safety findings.
The debate began with a 2018 meta-analysis showing a late mortality signal associated with paclitaxel drug-coated balloons (DCBs) that sent reverberations through the interventional cardiology community (J Am Heart Assoc. 2018 Dec 18;7[24]:e011245).
Now, in a new meta-analysis involving eight randomized controlled trials (RCTs) and more than 1,400 patients with critical limb ischemia (CLI), the same researchers found significantly more early amputations and deaths in those treated with DCB below the knee, compared with conventional balloon angioplasty.
“The findings of our latest report add to previous evidence underpinning major safety concerns around use of paclitaxel in lower limb angioplasties – increased long-term patient mortality in cases of intermittent claudication,” lead author Konstantinos Katsanos MD, MSc, PhD, Patras University Hospital, Greece, said in an interview.
By contrast, a retrospective study of insurance claims in Germany showed no heightened mortality with paclitaxel-coated balloons and stents, compared with uncoated devices, in close to 38,000 patients with PAD.
On the contrary, use of paclitaxel-coated devices was associated with higher long-term survival, better amputation-free survival (AFS), and lower rates of major cardiovascular events in the treatment of chronic limb-threatening ischemia (CLTI).
These findings “emphasize the difference between population-based evidence and randomized trials,” lead author Christian-Alexander Behrendt, MD, University Medical Center Hamburg-Eppendorf, Germany, said in an interview.
Downstream “showers”
In the new meta-analysis led by Dr. Katsanos, published online Jan. 15, the 1,420 patients were treated with five different DCBs and 97% had CLI (J Vasc Intervent Radiol 2020 Feb;31[2]:202-12).
In up to 1-year follow-up, the paclitaxel DCB group had fewer target lesion revascularizations (TLR) than those of the uncoated device group (11.8% vs. 25.6%; risk ratio, 0.53; 95% confidence interval, 0.35-0.81) but worse AFS (13.7% vs. 9.4%; hazard ratio [HR], 1.52; 95% CI, 1.12-2.07).
The latter finding was driven by nonsignificant increased risks for all-cause death (odds ratio [OR], 1.39; 95% CI, 0.94-2.07) and major amputations (OR, 1.63; 95% CI, 0.92-2.90).
In dose-subgroup analyses, AFS was significantly worse in cases with high-dose (3.0-3.5 mcg/mm2) devices, but not in the single trial with a low-dose DCB (2.0 mcg/mm2).
“Considering the well-described downstream ‘showers’ of paclitaxel particles with current drug-coated balloons, we hypothesize that nontarget paclitaxel embolization is a plausible mechanism for distal foot and systemic toxicity,” Dr. Katsanos said.
Short time frame
Eric Secemsky, MD, of Harvard Medical School, and director of vascular intervention at Beth Israel Deaconess Medical Center, Boston, suggested in an interview that this theorized mechanism of harm in below-the-knee procedures could potentially shed light on a similar mechanism at play in above-the-knee procedures.
“We didn’t understand why people could potentially be dying in above-the-knee [procedures], and the suggestion here is that these devices might perhaps be causing particular embolization or maybe delayed wound healing,” Dr. Secemsky speculated.
However, “I don’t know that this is true, so I am cautious to say this is true,” he emphasized.
Dr. Secemsky said a strength of the Katsanos analysis is that the RCTs included more than 1,000 patients, but noted that it is hard to vet the quality and rigor of the data, as some of the studies have not yet been published. He also noted that paclitaxel-coated devices are not approved by the Food and Drug Administration in the United States for below-the-knee procedures.
Moreover, he continued, “two studies were driving the signal of harm: the IN.PACT DEEP, which included an iteration of their DCB that is no longer being tested; and the unpublished SINGA-PACLI trial. Those studies contributed most of the adverse events seen in this meta-analysis.”
In addition, the trials had different lengths of follow-up (6-12 months), he said. “Thus, the five trials with data available to 12 months are driving the 1-year findings, whereas three RCTs, including the primary RCT showing safety [Lutonix-BTK trial], only contribute data to 6 months.”
For this reason, “we are not too excited about this meta-analysis as of now, [because] all it tells us is that we need more data to support the safety of drug-coated devices in this population,” Dr. Secemsky said.
Dr. Katsanos explained that, “to address the differences in follow-up period and number of cases lost to follow-up, the primary endpoint was calculated on the log-hazard scale and expressed as a hazard ratio, as recommended for time-to-event outcomes.”
He highlighted that a short-term time frame of 6 months to 1 year was chosen “because it is clinically relevant to limb-threatening CLI.”
Sensitivity tests also “showed consistent direction and magnitude of the summary treatment effects in case of both AFS and freedom from TLR,” Dr. Katsanos emphasized.
Lower mortality, fewer amputations
The second study, published online Jan. 8, drew on health insurance claims in the German BARMER database to analyze 37,914 patients (mean age, 73.3 years, 49% female) and 21,546 propensity-score-matched patients with symptomatic CLTI or intermittent claudication (IC) with an index revascularization during 2010-2018 (Eur J Vasc Endovasc Surg. 2020 Jan 8. doi: 10.1016/j.ejvs.2019.12.034).
Patients were first stratified by CLTI or IC, and then by balloon vs. stent use. Paclitaxel-coated devices were then compared with uncoated devices within each stratum. The primary outcome was all-cause mortality at the end of follow-up.
From 2010 to 2018, the annual use of paclitaxel-coated devices increased dramatically from 3% to 39% in the CLTI group and from 4% to 48% in the IC group (P less than .001 for both).
A total of 2,454 deaths occurred within 5 years of follow-up (median, 2.7 years; longest, 8 years).
A Cox proportional hazards model (based on propensity-score-matched cohorts at 5 years) showed that, compared with uncoated devices, use of paclitaxel-coated devices in the CLTI group was associated with several improvements:
- Overall survival: HR, 0.83; 95% CI, 0.77-0.90.
- Amputation-free survival: HR, 0.85; 95% CI, 0.78-0.91.
- Major cardiovascular events: HR, 0.82; 95% CI, 0.77-0.88.
In the IC group, mortality was significantly better with DCB (HR, 0.87; 95% CI, 0.76-0.99) or a combination of DCB and drug-eluting stents (HR, 0.88; 95% CI, 0.80-0.98) than with uncoated devices, but similar for DES alone (HR, 0.91; 95% CI, 0.77-1.08).
No benefit was found for paclitaxel-coated devices in the IC group for AFS (HR, 0.91; 95% CI, 0.82-1.00) or major cardiovascular events (HR, 0.93; 95% CI, 0.87-1.00).
The authors acknowledge that “unmeasured confounding” may partly explain the results. It may be that patients revascularized with DCB or DES “are more likely to be treated in highly specialized trial centers with clear follow-up protocol.”
Moreover, these patients may have received “the best treatment,” including statin therapy, added Dr. Behrendt.
More evidence needed
Dr. Secemsky, who was not involved with either study, said the German investigators “did a wonderful job with this analysis in a large population of several thousand patients, showing nicely that after accounting for differences in comorbidities, the patients had no evidence of harm with [paclitaxel-coated] devices through 5 years.”
However, he cautioned, median follow-up time was just over 2 years. “Although the investigators had data all the way out to 5 years, over time, the number of patients contributing data became smaller, which results in more uncertainty with these longer-term findings,” he said. “As such, we still need to look at additional long-term data in this patient population to confirm the safety of these devices.”
At present, the “major consideration we want to address is whether it’s safe to use these devices, and we’re undertaking these analyses to examine safety, not to see if they improve mortality,” although the present study “has a suggestion of mortality benefit,” Dr. Secemsky said.
Dr. Katsanos added that paclitaxel-coated balloons “remain under investigation for below-knee arteries and critical limb ischemia,” with “a few randomized controlled trials on the way.”
“We need definitive evidence from high-quality multicenter controlled trials that these devices may improve wound healing and limb salvage without any systemic mortality risk,” he said.
Dr. Katsanos receives personal fees from Boston Scientific and Philips Healthcare. The study by Dr. Behrendt was part of the IDOMENEO project funded by the German Joint Federal Committee. Dr. Behrendt reports no relevant financial relationships. Dr. Secemsky reports institutional grants from Cook Medical, BD Bard, Medtronic, Beth Israel Deaconess Medical Center, and Boston Scientific, and reports consultancy for Cook Medical, BD Bard, and Medtronic.
This article first appeared on Medscape.com.
The controversy regarding the safety of treating peripheral artery disease (PAD) with paclitaxel-coated devices has only deepened in the new year, with two recent studies suggesting opposite safety findings.
The debate began with a 2018 meta-analysis showing a late mortality signal associated with paclitaxel drug-coated balloons (DCBs) that sent reverberations through the interventional cardiology community (J Am Heart Assoc. 2018 Dec 18;7[24]:e011245).
Now, in a new meta-analysis involving eight randomized controlled trials (RCTs) and more than 1,400 patients with critical limb ischemia (CLI), the same researchers found significantly more early amputations and deaths in those treated with DCB below the knee, compared with conventional balloon angioplasty.
“The findings of our latest report add to previous evidence underpinning major safety concerns around use of paclitaxel in lower limb angioplasties – increased long-term patient mortality in cases of intermittent claudication,” lead author Konstantinos Katsanos MD, MSc, PhD, Patras University Hospital, Greece, said in an interview.
By contrast, a retrospective study of insurance claims in Germany showed no heightened mortality with paclitaxel-coated balloons and stents, compared with uncoated devices, in close to 38,000 patients with PAD.
On the contrary, use of paclitaxel-coated devices was associated with higher long-term survival, better amputation-free survival (AFS), and lower rates of major cardiovascular events in the treatment of chronic limb-threatening ischemia (CLTI).
These findings “emphasize the difference between population-based evidence and randomized trials,” lead author Christian-Alexander Behrendt, MD, University Medical Center Hamburg-Eppendorf, Germany, said in an interview.
Downstream “showers”
In the new meta-analysis led by Dr. Katsanos, published online Jan. 15, the 1,420 patients were treated with five different DCBs and 97% had CLI (J Vasc Intervent Radiol 2020 Feb;31[2]:202-12).
In up to 1-year follow-up, the paclitaxel DCB group had fewer target lesion revascularizations (TLR) than those of the uncoated device group (11.8% vs. 25.6%; risk ratio, 0.53; 95% confidence interval, 0.35-0.81) but worse AFS (13.7% vs. 9.4%; hazard ratio [HR], 1.52; 95% CI, 1.12-2.07).
The latter finding was driven by nonsignificant increased risks for all-cause death (odds ratio [OR], 1.39; 95% CI, 0.94-2.07) and major amputations (OR, 1.63; 95% CI, 0.92-2.90).
In dose-subgroup analyses, AFS was significantly worse in cases with high-dose (3.0-3.5 mcg/mm2) devices, but not in the single trial with a low-dose DCB (2.0 mcg/mm2).
“Considering the well-described downstream ‘showers’ of paclitaxel particles with current drug-coated balloons, we hypothesize that nontarget paclitaxel embolization is a plausible mechanism for distal foot and systemic toxicity,” Dr. Katsanos said.
Short time frame
Eric Secemsky, MD, of Harvard Medical School, and director of vascular intervention at Beth Israel Deaconess Medical Center, Boston, suggested in an interview that this theorized mechanism of harm in below-the-knee procedures could potentially shed light on a similar mechanism at play in above-the-knee procedures.
“We didn’t understand why people could potentially be dying in above-the-knee [procedures], and the suggestion here is that these devices might perhaps be causing particular embolization or maybe delayed wound healing,” Dr. Secemsky speculated.
However, “I don’t know that this is true, so I am cautious to say this is true,” he emphasized.
Dr. Secemsky said a strength of the Katsanos analysis is that the RCTs included more than 1,000 patients, but noted that it is hard to vet the quality and rigor of the data, as some of the studies have not yet been published. He also noted that paclitaxel-coated devices are not approved by the Food and Drug Administration in the United States for below-the-knee procedures.
Moreover, he continued, “two studies were driving the signal of harm: the IN.PACT DEEP, which included an iteration of their DCB that is no longer being tested; and the unpublished SINGA-PACLI trial. Those studies contributed most of the adverse events seen in this meta-analysis.”
In addition, the trials had different lengths of follow-up (6-12 months), he said. “Thus, the five trials with data available to 12 months are driving the 1-year findings, whereas three RCTs, including the primary RCT showing safety [Lutonix-BTK trial], only contribute data to 6 months.”
For this reason, “we are not too excited about this meta-analysis as of now, [because] all it tells us is that we need more data to support the safety of drug-coated devices in this population,” Dr. Secemsky said.
Dr. Katsanos explained that, “to address the differences in follow-up period and number of cases lost to follow-up, the primary endpoint was calculated on the log-hazard scale and expressed as a hazard ratio, as recommended for time-to-event outcomes.”
He highlighted that a short-term time frame of 6 months to 1 year was chosen “because it is clinically relevant to limb-threatening CLI.”
Sensitivity tests also “showed consistent direction and magnitude of the summary treatment effects in case of both AFS and freedom from TLR,” Dr. Katsanos emphasized.
Lower mortality, fewer amputations
The second study, published online Jan. 8, drew on health insurance claims in the German BARMER database to analyze 37,914 patients (mean age, 73.3 years, 49% female) and 21,546 propensity-score-matched patients with symptomatic CLTI or intermittent claudication (IC) with an index revascularization during 2010-2018 (Eur J Vasc Endovasc Surg. 2020 Jan 8. doi: 10.1016/j.ejvs.2019.12.034).
Patients were first stratified by CLTI or IC, and then by balloon vs. stent use. Paclitaxel-coated devices were then compared with uncoated devices within each stratum. The primary outcome was all-cause mortality at the end of follow-up.
From 2010 to 2018, the annual use of paclitaxel-coated devices increased dramatically from 3% to 39% in the CLTI group and from 4% to 48% in the IC group (P less than .001 for both).
A total of 2,454 deaths occurred within 5 years of follow-up (median, 2.7 years; longest, 8 years).
A Cox proportional hazards model (based on propensity-score-matched cohorts at 5 years) showed that, compared with uncoated devices, use of paclitaxel-coated devices in the CLTI group was associated with several improvements:
- Overall survival: HR, 0.83; 95% CI, 0.77-0.90.
- Amputation-free survival: HR, 0.85; 95% CI, 0.78-0.91.
- Major cardiovascular events: HR, 0.82; 95% CI, 0.77-0.88.
In the IC group, mortality was significantly better with DCB (HR, 0.87; 95% CI, 0.76-0.99) or a combination of DCB and drug-eluting stents (HR, 0.88; 95% CI, 0.80-0.98) than with uncoated devices, but similar for DES alone (HR, 0.91; 95% CI, 0.77-1.08).
No benefit was found for paclitaxel-coated devices in the IC group for AFS (HR, 0.91; 95% CI, 0.82-1.00) or major cardiovascular events (HR, 0.93; 95% CI, 0.87-1.00).
The authors acknowledge that “unmeasured confounding” may partly explain the results. It may be that patients revascularized with DCB or DES “are more likely to be treated in highly specialized trial centers with clear follow-up protocol.”
Moreover, these patients may have received “the best treatment,” including statin therapy, added Dr. Behrendt.
More evidence needed
Dr. Secemsky, who was not involved with either study, said the German investigators “did a wonderful job with this analysis in a large population of several thousand patients, showing nicely that after accounting for differences in comorbidities, the patients had no evidence of harm with [paclitaxel-coated] devices through 5 years.”
However, he cautioned, median follow-up time was just over 2 years. “Although the investigators had data all the way out to 5 years, over time, the number of patients contributing data became smaller, which results in more uncertainty with these longer-term findings,” he said. “As such, we still need to look at additional long-term data in this patient population to confirm the safety of these devices.”
At present, the “major consideration we want to address is whether it’s safe to use these devices, and we’re undertaking these analyses to examine safety, not to see if they improve mortality,” although the present study “has a suggestion of mortality benefit,” Dr. Secemsky said.
Dr. Katsanos added that paclitaxel-coated balloons “remain under investigation for below-knee arteries and critical limb ischemia,” with “a few randomized controlled trials on the way.”
“We need definitive evidence from high-quality multicenter controlled trials that these devices may improve wound healing and limb salvage without any systemic mortality risk,” he said.
Dr. Katsanos receives personal fees from Boston Scientific and Philips Healthcare. The study by Dr. Behrendt was part of the IDOMENEO project funded by the German Joint Federal Committee. Dr. Behrendt reports no relevant financial relationships. Dr. Secemsky reports institutional grants from Cook Medical, BD Bard, Medtronic, Beth Israel Deaconess Medical Center, and Boston Scientific, and reports consultancy for Cook Medical, BD Bard, and Medtronic.
This article first appeared on Medscape.com.
The controversy regarding the safety of treating peripheral artery disease (PAD) with paclitaxel-coated devices has only deepened in the new year, with two recent studies suggesting opposite safety findings.
The debate began with a 2018 meta-analysis showing a late mortality signal associated with paclitaxel drug-coated balloons (DCBs) that sent reverberations through the interventional cardiology community (J Am Heart Assoc. 2018 Dec 18;7[24]:e011245).
Now, in a new meta-analysis involving eight randomized controlled trials (RCTs) and more than 1,400 patients with critical limb ischemia (CLI), the same researchers found significantly more early amputations and deaths in those treated with DCB below the knee, compared with conventional balloon angioplasty.
“The findings of our latest report add to previous evidence underpinning major safety concerns around use of paclitaxel in lower limb angioplasties – increased long-term patient mortality in cases of intermittent claudication,” lead author Konstantinos Katsanos MD, MSc, PhD, Patras University Hospital, Greece, said in an interview.
By contrast, a retrospective study of insurance claims in Germany showed no heightened mortality with paclitaxel-coated balloons and stents, compared with uncoated devices, in close to 38,000 patients with PAD.
On the contrary, use of paclitaxel-coated devices was associated with higher long-term survival, better amputation-free survival (AFS), and lower rates of major cardiovascular events in the treatment of chronic limb-threatening ischemia (CLTI).
These findings “emphasize the difference between population-based evidence and randomized trials,” lead author Christian-Alexander Behrendt, MD, University Medical Center Hamburg-Eppendorf, Germany, said in an interview.
Downstream “showers”
In the new meta-analysis led by Dr. Katsanos, published online Jan. 15, the 1,420 patients were treated with five different DCBs and 97% had CLI (J Vasc Intervent Radiol 2020 Feb;31[2]:202-12).
In up to 1-year follow-up, the paclitaxel DCB group had fewer target lesion revascularizations (TLR) than those of the uncoated device group (11.8% vs. 25.6%; risk ratio, 0.53; 95% confidence interval, 0.35-0.81) but worse AFS (13.7% vs. 9.4%; hazard ratio [HR], 1.52; 95% CI, 1.12-2.07).
The latter finding was driven by nonsignificant increased risks for all-cause death (odds ratio [OR], 1.39; 95% CI, 0.94-2.07) and major amputations (OR, 1.63; 95% CI, 0.92-2.90).
In dose-subgroup analyses, AFS was significantly worse in cases with high-dose (3.0-3.5 mcg/mm2) devices, but not in the single trial with a low-dose DCB (2.0 mcg/mm2).
“Considering the well-described downstream ‘showers’ of paclitaxel particles with current drug-coated balloons, we hypothesize that nontarget paclitaxel embolization is a plausible mechanism for distal foot and systemic toxicity,” Dr. Katsanos said.
Short time frame
Eric Secemsky, MD, of Harvard Medical School, and director of vascular intervention at Beth Israel Deaconess Medical Center, Boston, suggested in an interview that this theorized mechanism of harm in below-the-knee procedures could potentially shed light on a similar mechanism at play in above-the-knee procedures.
“We didn’t understand why people could potentially be dying in above-the-knee [procedures], and the suggestion here is that these devices might perhaps be causing particular embolization or maybe delayed wound healing,” Dr. Secemsky speculated.
However, “I don’t know that this is true, so I am cautious to say this is true,” he emphasized.
Dr. Secemsky said a strength of the Katsanos analysis is that the RCTs included more than 1,000 patients, but noted that it is hard to vet the quality and rigor of the data, as some of the studies have not yet been published. He also noted that paclitaxel-coated devices are not approved by the Food and Drug Administration in the United States for below-the-knee procedures.
Moreover, he continued, “two studies were driving the signal of harm: the IN.PACT DEEP, which included an iteration of their DCB that is no longer being tested; and the unpublished SINGA-PACLI trial. Those studies contributed most of the adverse events seen in this meta-analysis.”
In addition, the trials had different lengths of follow-up (6-12 months), he said. “Thus, the five trials with data available to 12 months are driving the 1-year findings, whereas three RCTs, including the primary RCT showing safety [Lutonix-BTK trial], only contribute data to 6 months.”
For this reason, “we are not too excited about this meta-analysis as of now, [because] all it tells us is that we need more data to support the safety of drug-coated devices in this population,” Dr. Secemsky said.
Dr. Katsanos explained that, “to address the differences in follow-up period and number of cases lost to follow-up, the primary endpoint was calculated on the log-hazard scale and expressed as a hazard ratio, as recommended for time-to-event outcomes.”
He highlighted that a short-term time frame of 6 months to 1 year was chosen “because it is clinically relevant to limb-threatening CLI.”
Sensitivity tests also “showed consistent direction and magnitude of the summary treatment effects in case of both AFS and freedom from TLR,” Dr. Katsanos emphasized.
Lower mortality, fewer amputations
The second study, published online Jan. 8, drew on health insurance claims in the German BARMER database to analyze 37,914 patients (mean age, 73.3 years, 49% female) and 21,546 propensity-score-matched patients with symptomatic CLTI or intermittent claudication (IC) with an index revascularization during 2010-2018 (Eur J Vasc Endovasc Surg. 2020 Jan 8. doi: 10.1016/j.ejvs.2019.12.034).
Patients were first stratified by CLTI or IC, and then by balloon vs. stent use. Paclitaxel-coated devices were then compared with uncoated devices within each stratum. The primary outcome was all-cause mortality at the end of follow-up.
From 2010 to 2018, the annual use of paclitaxel-coated devices increased dramatically from 3% to 39% in the CLTI group and from 4% to 48% in the IC group (P less than .001 for both).
A total of 2,454 deaths occurred within 5 years of follow-up (median, 2.7 years; longest, 8 years).
A Cox proportional hazards model (based on propensity-score-matched cohorts at 5 years) showed that, compared with uncoated devices, use of paclitaxel-coated devices in the CLTI group was associated with several improvements:
- Overall survival: HR, 0.83; 95% CI, 0.77-0.90.
- Amputation-free survival: HR, 0.85; 95% CI, 0.78-0.91.
- Major cardiovascular events: HR, 0.82; 95% CI, 0.77-0.88.
In the IC group, mortality was significantly better with DCB (HR, 0.87; 95% CI, 0.76-0.99) or a combination of DCB and drug-eluting stents (HR, 0.88; 95% CI, 0.80-0.98) than with uncoated devices, but similar for DES alone (HR, 0.91; 95% CI, 0.77-1.08).
No benefit was found for paclitaxel-coated devices in the IC group for AFS (HR, 0.91; 95% CI, 0.82-1.00) or major cardiovascular events (HR, 0.93; 95% CI, 0.87-1.00).
The authors acknowledge that “unmeasured confounding” may partly explain the results. It may be that patients revascularized with DCB or DES “are more likely to be treated in highly specialized trial centers with clear follow-up protocol.”
Moreover, these patients may have received “the best treatment,” including statin therapy, added Dr. Behrendt.
More evidence needed
Dr. Secemsky, who was not involved with either study, said the German investigators “did a wonderful job with this analysis in a large population of several thousand patients, showing nicely that after accounting for differences in comorbidities, the patients had no evidence of harm with [paclitaxel-coated] devices through 5 years.”
However, he cautioned, median follow-up time was just over 2 years. “Although the investigators had data all the way out to 5 years, over time, the number of patients contributing data became smaller, which results in more uncertainty with these longer-term findings,” he said. “As such, we still need to look at additional long-term data in this patient population to confirm the safety of these devices.”
At present, the “major consideration we want to address is whether it’s safe to use these devices, and we’re undertaking these analyses to examine safety, not to see if they improve mortality,” although the present study “has a suggestion of mortality benefit,” Dr. Secemsky said.
Dr. Katsanos added that paclitaxel-coated balloons “remain under investigation for below-knee arteries and critical limb ischemia,” with “a few randomized controlled trials on the way.”
“We need definitive evidence from high-quality multicenter controlled trials that these devices may improve wound healing and limb salvage without any systemic mortality risk,” he said.
Dr. Katsanos receives personal fees from Boston Scientific and Philips Healthcare. The study by Dr. Behrendt was part of the IDOMENEO project funded by the German Joint Federal Committee. Dr. Behrendt reports no relevant financial relationships. Dr. Secemsky reports institutional grants from Cook Medical, BD Bard, Medtronic, Beth Israel Deaconess Medical Center, and Boston Scientific, and reports consultancy for Cook Medical, BD Bard, and Medtronic.
This article first appeared on Medscape.com.