User login
Musculoskeletal ultrasonography has arrived
A 50-year-old woman with hypertension presents with a history of polyarticular small-joint pain for the last 3 months. Her pain is worse in the morning, and it affects her metacarpal, proximal, and distal phalangeal joints. She describes intermittent swelling of her hands and morning stiffness lasting 15 to 30 minutes.
Her physical examination is unremarkable, with no evidence of active inflammation (synovitis), joint tenderness, restrictions in movement, or deformity. Her description of her symptoms raises suspicion for an inflammatory arthritis, but her physical examination does not support this diagnosis.
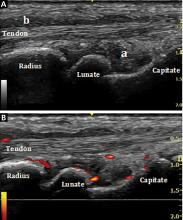
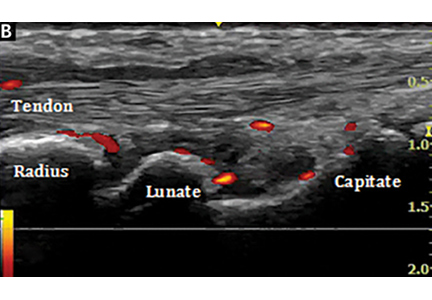
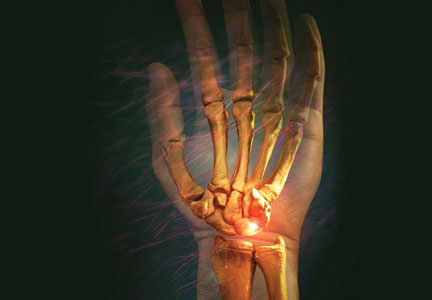
Bedside musculoskeletal ultrasonography of her wrists reveals synovial hypertrophy, and power Doppler shows active inflammation, findings consistent with synovitis (Figure 1).
This scenario illustrates how musculoskeletal ultrasonography can prevent delayed diagnosis, thus directing the ordering of appropriate laboratory studies and allowing treatment for pain relief to be started promptly.
ULTRASONOGRAPHY HAS GAINED A SOLID ROLE
Ultrasonography has gained a solid role in the care of patients with musculoskeletal conditions.
Using obtained images, as well as power Doppler to assess inflammation, the clinician can visualize superficial anatomic structures, including the skin, muscles, joints, nerves, and the cortical layer of bone. Combining the dynamic assessment with the clinical history and findings of the physical examination makes musculoskeletal ultrasonography a powerful tool for diagnosis and management.1
In this issue, Forney and Delzell2 review the clinical use of ultrasonography of the muscles and bones and its advantages and disadvantages compared with other imaging methods. They describe its gain in popularity over the last decade and its incorporation into clinical care in multiple medical subspecialties.
Musculoskeletal ultrasonography is performed and interpreted by specially trained sonographers. It should be viewed as a complementary procedure, not as a replacement for a thorough and systematic clinical examination.3
ADVANTAGES ARE MANY
A major advantage of musculoskeletal ultrasonography over other imaging techniques is its capacity to dynamically assess joint and tendon movements4 and to immediately interpret them in real time.
In rheumatology, where it has made the biggest impact, it can help evaluate inflammatory and noninflammatory rheumatic diseases, assess treatment response, and guide joint injections.1 It has been demonstrated to significantly improve timely diagnosis and management,5 decrease dependence on other imaging modalities, and reduce healthcare costs.6
With its easy portability, ultrasonography has also been integrated into orthopedics, podiatry, physical medicine and rehabilitation, sports medicine, and emergency medicine. Its role is expanding to include the assessment of the skin in systemic sclerosis, parotid and submandibular glands in Sjögren syndrome, nails in patients with psoriasis, and temporal arteries in giant cell arteritis.
A ROLE IN MEDICAL EDUCATION
Musculoskeletal ultrasonography has entered into medical education, with an increasing number of medical schools incorporating it into their curriculum over the last few years.7 It enhances student learning of anatomy, the physical examination, and pathologic findings of rheumatic diseases.7,8 Some internal medicine residency programs have added ultrasonography to help identify anatomic structures for invasive procedures, increasing patient safety and reducing procedural complications.9
It has been incorporated into the core curriculum in many rheumatology fellowship training programs.10 Rheumatologists can now also take additional courses to enhance their skills and become certified sonographers.
Musculoskeletal ultrasonography has proven to be a useful adjunct to the physical examination. With its many advantages, it has gained acceptance and is now a mainstay in many subspecialties.
- Cannella AC, Kissin EY, Torralba KD, Higgs JB, Kaeley GS. Evolution of musculoskeletal ultrasound in the United States: implementation and practice in rheumatology. Arthritis Care Res (Hoboken) 2014; 66(1):7–13. doi:10.1002/acr.22183
- Forney MC, Delzell PB. Musculoskeletal ultrasonography basics. Cleve Clin J Med 2018; 85(4):283–300. doi:10.3949/ccjm.85a.17014
- McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res (Hoboken) 2012; 64(11):1625–1640. doi:10.1002/acr.21836
- Backhaus M, Burmester GR, Gerber T, et al; Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001; 60(7):641–649.
- Micu MC, Alcalde M, Saenz JI, et al. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res (Hoboken) 2013; 65(4):615–621. doi:10.1002/acr.21853
- Kay JC, Higgs JB, Battafarano DF. Utility of musculoskeletal ultrasound in a Department of Defense rheumatology practice: a four-year retrospective experience. Arthritis Care Res (Hoboken) 2014; 66(1):14–18. doi:10.1002/acr.22127
- Dinh VA, Fu JY, Lu S, Chiem A, Fox JC, Blaivas M. Integration of ultrasound in medical education at United States medical schools. J Ultrasound Med 2016; 35(2):413–419. doi:10.7863/ultra.15.05073
- Wright SA, Bell AL. Enhancement of undergraduate rheumatology teaching through the use of musculoskeletal ultrasound. Rheumatology (Oxford) 2008; 47(10):1564–1566. doi:10.1093/rheumatology/ken324
- Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ 2011; 11:75. doi:0.1186/1472-6920-11-75
- Torralba K, Cannella AC, Kissin EY, et al. Musculoskeletal ultrasound instruction in adult rheumatology fellowship programs. Arthritis Care Res (Hoboken) 2017. Epub ahead of print. doi:10.1002/acr.23336
A 50-year-old woman with hypertension presents with a history of polyarticular small-joint pain for the last 3 months. Her pain is worse in the morning, and it affects her metacarpal, proximal, and distal phalangeal joints. She describes intermittent swelling of her hands and morning stiffness lasting 15 to 30 minutes.
Her physical examination is unremarkable, with no evidence of active inflammation (synovitis), joint tenderness, restrictions in movement, or deformity. Her description of her symptoms raises suspicion for an inflammatory arthritis, but her physical examination does not support this diagnosis.
Bedside musculoskeletal ultrasonography of her wrists reveals synovial hypertrophy, and power Doppler shows active inflammation, findings consistent with synovitis (Figure 1).
This scenario illustrates how musculoskeletal ultrasonography can prevent delayed diagnosis, thus directing the ordering of appropriate laboratory studies and allowing treatment for pain relief to be started promptly.
ULTRASONOGRAPHY HAS GAINED A SOLID ROLE
Ultrasonography has gained a solid role in the care of patients with musculoskeletal conditions.
Using obtained images, as well as power Doppler to assess inflammation, the clinician can visualize superficial anatomic structures, including the skin, muscles, joints, nerves, and the cortical layer of bone. Combining the dynamic assessment with the clinical history and findings of the physical examination makes musculoskeletal ultrasonography a powerful tool for diagnosis and management.1
In this issue, Forney and Delzell2 review the clinical use of ultrasonography of the muscles and bones and its advantages and disadvantages compared with other imaging methods. They describe its gain in popularity over the last decade and its incorporation into clinical care in multiple medical subspecialties.
Musculoskeletal ultrasonography is performed and interpreted by specially trained sonographers. It should be viewed as a complementary procedure, not as a replacement for a thorough and systematic clinical examination.3
ADVANTAGES ARE MANY
A major advantage of musculoskeletal ultrasonography over other imaging techniques is its capacity to dynamically assess joint and tendon movements4 and to immediately interpret them in real time.
In rheumatology, where it has made the biggest impact, it can help evaluate inflammatory and noninflammatory rheumatic diseases, assess treatment response, and guide joint injections.1 It has been demonstrated to significantly improve timely diagnosis and management,5 decrease dependence on other imaging modalities, and reduce healthcare costs.6
With its easy portability, ultrasonography has also been integrated into orthopedics, podiatry, physical medicine and rehabilitation, sports medicine, and emergency medicine. Its role is expanding to include the assessment of the skin in systemic sclerosis, parotid and submandibular glands in Sjögren syndrome, nails in patients with psoriasis, and temporal arteries in giant cell arteritis.
A ROLE IN MEDICAL EDUCATION
Musculoskeletal ultrasonography has entered into medical education, with an increasing number of medical schools incorporating it into their curriculum over the last few years.7 It enhances student learning of anatomy, the physical examination, and pathologic findings of rheumatic diseases.7,8 Some internal medicine residency programs have added ultrasonography to help identify anatomic structures for invasive procedures, increasing patient safety and reducing procedural complications.9
It has been incorporated into the core curriculum in many rheumatology fellowship training programs.10 Rheumatologists can now also take additional courses to enhance their skills and become certified sonographers.
Musculoskeletal ultrasonography has proven to be a useful adjunct to the physical examination. With its many advantages, it has gained acceptance and is now a mainstay in many subspecialties.
A 50-year-old woman with hypertension presents with a history of polyarticular small-joint pain for the last 3 months. Her pain is worse in the morning, and it affects her metacarpal, proximal, and distal phalangeal joints. She describes intermittent swelling of her hands and morning stiffness lasting 15 to 30 minutes.
Her physical examination is unremarkable, with no evidence of active inflammation (synovitis), joint tenderness, restrictions in movement, or deformity. Her description of her symptoms raises suspicion for an inflammatory arthritis, but her physical examination does not support this diagnosis.
Bedside musculoskeletal ultrasonography of her wrists reveals synovial hypertrophy, and power Doppler shows active inflammation, findings consistent with synovitis (Figure 1).
This scenario illustrates how musculoskeletal ultrasonography can prevent delayed diagnosis, thus directing the ordering of appropriate laboratory studies and allowing treatment for pain relief to be started promptly.
ULTRASONOGRAPHY HAS GAINED A SOLID ROLE
Ultrasonography has gained a solid role in the care of patients with musculoskeletal conditions.
Using obtained images, as well as power Doppler to assess inflammation, the clinician can visualize superficial anatomic structures, including the skin, muscles, joints, nerves, and the cortical layer of bone. Combining the dynamic assessment with the clinical history and findings of the physical examination makes musculoskeletal ultrasonography a powerful tool for diagnosis and management.1
In this issue, Forney and Delzell2 review the clinical use of ultrasonography of the muscles and bones and its advantages and disadvantages compared with other imaging methods. They describe its gain in popularity over the last decade and its incorporation into clinical care in multiple medical subspecialties.
Musculoskeletal ultrasonography is performed and interpreted by specially trained sonographers. It should be viewed as a complementary procedure, not as a replacement for a thorough and systematic clinical examination.3
ADVANTAGES ARE MANY
A major advantage of musculoskeletal ultrasonography over other imaging techniques is its capacity to dynamically assess joint and tendon movements4 and to immediately interpret them in real time.
In rheumatology, where it has made the biggest impact, it can help evaluate inflammatory and noninflammatory rheumatic diseases, assess treatment response, and guide joint injections.1 It has been demonstrated to significantly improve timely diagnosis and management,5 decrease dependence on other imaging modalities, and reduce healthcare costs.6
With its easy portability, ultrasonography has also been integrated into orthopedics, podiatry, physical medicine and rehabilitation, sports medicine, and emergency medicine. Its role is expanding to include the assessment of the skin in systemic sclerosis, parotid and submandibular glands in Sjögren syndrome, nails in patients with psoriasis, and temporal arteries in giant cell arteritis.
A ROLE IN MEDICAL EDUCATION
Musculoskeletal ultrasonography has entered into medical education, with an increasing number of medical schools incorporating it into their curriculum over the last few years.7 It enhances student learning of anatomy, the physical examination, and pathologic findings of rheumatic diseases.7,8 Some internal medicine residency programs have added ultrasonography to help identify anatomic structures for invasive procedures, increasing patient safety and reducing procedural complications.9
It has been incorporated into the core curriculum in many rheumatology fellowship training programs.10 Rheumatologists can now also take additional courses to enhance their skills and become certified sonographers.
Musculoskeletal ultrasonography has proven to be a useful adjunct to the physical examination. With its many advantages, it has gained acceptance and is now a mainstay in many subspecialties.
- Cannella AC, Kissin EY, Torralba KD, Higgs JB, Kaeley GS. Evolution of musculoskeletal ultrasound in the United States: implementation and practice in rheumatology. Arthritis Care Res (Hoboken) 2014; 66(1):7–13. doi:10.1002/acr.22183
- Forney MC, Delzell PB. Musculoskeletal ultrasonography basics. Cleve Clin J Med 2018; 85(4):283–300. doi:10.3949/ccjm.85a.17014
- McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res (Hoboken) 2012; 64(11):1625–1640. doi:10.1002/acr.21836
- Backhaus M, Burmester GR, Gerber T, et al; Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001; 60(7):641–649.
- Micu MC, Alcalde M, Saenz JI, et al. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res (Hoboken) 2013; 65(4):615–621. doi:10.1002/acr.21853
- Kay JC, Higgs JB, Battafarano DF. Utility of musculoskeletal ultrasound in a Department of Defense rheumatology practice: a four-year retrospective experience. Arthritis Care Res (Hoboken) 2014; 66(1):14–18. doi:10.1002/acr.22127
- Dinh VA, Fu JY, Lu S, Chiem A, Fox JC, Blaivas M. Integration of ultrasound in medical education at United States medical schools. J Ultrasound Med 2016; 35(2):413–419. doi:10.7863/ultra.15.05073
- Wright SA, Bell AL. Enhancement of undergraduate rheumatology teaching through the use of musculoskeletal ultrasound. Rheumatology (Oxford) 2008; 47(10):1564–1566. doi:10.1093/rheumatology/ken324
- Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ 2011; 11:75. doi:0.1186/1472-6920-11-75
- Torralba K, Cannella AC, Kissin EY, et al. Musculoskeletal ultrasound instruction in adult rheumatology fellowship programs. Arthritis Care Res (Hoboken) 2017. Epub ahead of print. doi:10.1002/acr.23336
- Cannella AC, Kissin EY, Torralba KD, Higgs JB, Kaeley GS. Evolution of musculoskeletal ultrasound in the United States: implementation and practice in rheumatology. Arthritis Care Res (Hoboken) 2014; 66(1):7–13. doi:10.1002/acr.22183
- Forney MC, Delzell PB. Musculoskeletal ultrasonography basics. Cleve Clin J Med 2018; 85(4):283–300. doi:10.3949/ccjm.85a.17014
- McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res (Hoboken) 2012; 64(11):1625–1640. doi:10.1002/acr.21836
- Backhaus M, Burmester GR, Gerber T, et al; Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001; 60(7):641–649.
- Micu MC, Alcalde M, Saenz JI, et al. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res (Hoboken) 2013; 65(4):615–621. doi:10.1002/acr.21853
- Kay JC, Higgs JB, Battafarano DF. Utility of musculoskeletal ultrasound in a Department of Defense rheumatology practice: a four-year retrospective experience. Arthritis Care Res (Hoboken) 2014; 66(1):14–18. doi:10.1002/acr.22127
- Dinh VA, Fu JY, Lu S, Chiem A, Fox JC, Blaivas M. Integration of ultrasound in medical education at United States medical schools. J Ultrasound Med 2016; 35(2):413–419. doi:10.7863/ultra.15.05073
- Wright SA, Bell AL. Enhancement of undergraduate rheumatology teaching through the use of musculoskeletal ultrasound. Rheumatology (Oxford) 2008; 47(10):1564–1566. doi:10.1093/rheumatology/ken324
- Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ 2011; 11:75. doi:0.1186/1472-6920-11-75
- Torralba K, Cannella AC, Kissin EY, et al. Musculoskeletal ultrasound instruction in adult rheumatology fellowship programs. Arthritis Care Res (Hoboken) 2017. Epub ahead of print. doi:10.1002/acr.23336
General Applications of Ultrasound in Rheumatology Practice
Over the past 2 decades, an increasing number of rheumatologists have progressively incorporated ultrasound (US) as an invaluable diagnostic and monitoring tool into their clinical and research practice.1,2 This imaging modality has become an established aid incorporated into the clinical evaluation of periarticular and articular structures involved in the diagnosis of several rheumatic disorders.
Ultrasound is a safe, noninvasive, patient-friendly imaging modality with a lack of contraindications and free of ionizing radiation. It allows real-time evaluation with dynamic assessment in a multiplanar view, assessment of multiple targets, and lower cost compared with magnetic resonance imaging (MRI) or computerized tomography scan. Above all, for the rheumatologist, US provides real-time scanning of all peripheral joints as many times as is required at the time of consultation. It is of great advantage in the assessment of a wide spectrum of abnormalities in rheumatic diseases with the potential of point-of-care imaging modality in the clinical evaluation and management of the patient. It facilitates a direct correlation between imaging findings and clinical data that improves the approach to a wide range of rheumatic diseases, from acute to chronic inflammatory arthritis, crystalline arthropathies, osteoarthritis (OA), spondyloarthropathies (SpA), vasculitis, and soft tissue syndromes. In addition, US is a bedside tool for performing accurate and safe diagnostic arthrocentesis, injections, and synovial biopsies.3,4
Recently, a gradual attempt has been made to incorporate US into rheumatology disease classification or diagnostic criteria for rheumatoid arthritis (RA), polymyalgia rheumatica, gout, calcium pyrophosphate deposition disease (CPPD), and Sjögren’s syndrome.5-10 Furthermore, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) have produced evidence and expert opinion-based recommendations on the use of US in the clinical management of rheumatic diseases.10-12 This article highlights the most common applications of US for assessment and management of different rheumatic diseases frequently encountered at the VAMC rheumatology inpatient and outpatient clinical service.
Evaluation of Inflammatory Arthritis
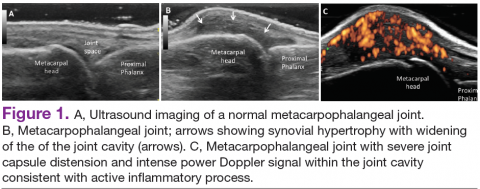
In RA and any other inflammatory arthritis, US has been used for the detection of joint effusions, synovitis, bone erosions, and tendon and enthesis involvement.11,12 Ultrasound B-mode and power Doppler (PD) techniques have demonstrated a consistent and relevant role in optimizing the diagnosis, assessing the inflammatory activity, monitoring response to therapy, and predicting the inflammatory arthritis outcomes (Figures 1-3).10-12 Ultrasound provides real-time information about the status of the synovial membrane, tendons, cartilage, bursae, and cortical bones, allowing an accurate assessment of the degree of inflammatory process in periarticular and articular tissues. Also, US can provide details about the characteristics of the collected fluid (ie, effusion or synovial hypertrophy), which is fundamental for the correct interpretation of the pathologic joint and/or soft tissue processes. The inflammatory process can be assessed by using PD mode, which detects and quantifies the vascular changes in the pannus due to vasodilation and the increased blood flow characteristic of active inflammation.13,14
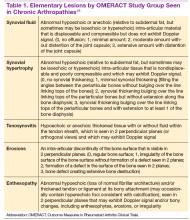
The Outcomes Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) study group developed standardized sonopathologic definitions and scanning methods to be used in the daily rheumatologic practice and clinical trials (Table 1).15 Furthermore, it developed a semiquantitative scale to assess the degree of synovitis in US B-mode and PD mode (Table 2).15
The use of US to find subclinical synovitis in patients with RA considered to be in clinical remission is a new issue.16 Some reports have demonstrated progressive joint damage in these patients with evidence of active inflammation on PDUS despite clinical remission.17,18 More prospective studies are required to provide a better understanding of the long-term effects of residual inflammation and the proper long-term treatment of these patients. Furthermore, the PD signal has been shown to be superior to the Disease Activity Score 28 (DAS-28) in evaluating disease activity, particularly in predicting joint damage.18
Ultrasound may be considered the gold standard imaging tool for the assessment of tendons in inflammatory arthritis and includes the detection of tenosynovitis and anatomical damage represented by the loss of the normal fibrillar echotexture and loss of definition of the tendon margins, which may occur in early disease.19,20 Tenosynovitis of the extensor carpi ulnaris (ECU) detected by US has been shown to be an independent predictive factor of erosive joint damage, suggesting that ECU tenosynovitis represents a useful ultrasonographic landmark in the diagnosis of early RA.21
The availability of new nonbiologic and biologic therapies for inflammatory arthritis has raised the importance of identifying early changes, such as the detection of early erosions, which portend a poor long-term prognosis. The capability of US in identifying this lesion at an earlier stage compared with conventional radiography (CR) has allowed the early diagnosis and treatment of these patients before irreversible joint destruction occurs.22 In spite of all the supportive evidence of US utility in RA, it is not considered among the mandatory diagnostic criteria in the ACR/EULAR classification criteria for RA.5 Still, the addition of US findings to these criteria has increased the number of patients who fulfilled the 1987 ACR classification criteria for RA after 18 months of follow-up.23 Despite extensive evidence of its utility in the diagnosis and monitoring of RA, further studies are still needed.
Spondyloarthritis
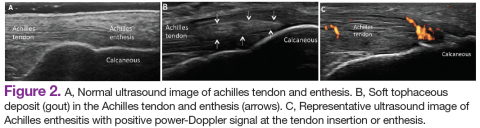
Similar to RA, SpA discloses sonographic findings of inflammatory arthritis; however, with more entheseal and tenosynovium involvement. Ultrasound has also been used in the early identification of characteristic changes of the skin and nail tissues, which can aid the global assessment of this heterogeneous disease, especially in psoriatic arthritis (PsA). The most common locations of enthesitis in SpA are the quadriceps and the Achilles enthesis.24,25
Although US offers detailed imaging for the assessment of both tendons and enthesis, there is a lack of literature evaluating dactylitis. The OMERACT group recently released a composite measure of activity and severity of US dactylitis, which included newly defined elementary US lesions that may discern dactylitis of a digit.26,27 Ultrasound has been compared with MRI in the detection of SpA-related synovitis of the hands and feet and has demonstrated competitive diagnostic sensitivity.28 Ultrasound also shows higher sensitivity in detecting synovitis of the hands and feet compared with clinical examination and CR in PsA.28,29 Unfortunately, there are no strongly validated US findings that can aid in the differential diagnosis of PsA against other chronic inflammatory arthritides. The presence of peritendinous extensor tendon inflammation was a highly specific sonographic feature of PsA, because it was present in 66% of metacarpophalangeal (MCP) joints as the only US sign of inflammation compared with patients with RA.30
Another application of US is in the evaluation of subclinical inflammation at the enthesis in patients with a history of psoriasis without prior history of PsA.27,31 In those patients with psoriatic nail changes, more subclinical enthesitis was found compared with patients with psoriasis without nail involvement.32 Furthermore, subclinical joint inflammation has also been described.33 These findings suggest a possible predictive value in patients with psoriasis who should be monitored on a regular basis, because they are at risk of developing PsA.
Subclinical enthesitis by US imaging has been described in patients with recurrent anterior uveitis and inflammatory bowel disease.34,35 In cases where SpA is suspected but diagnostic criteria are not fulfilled, the presence of one enthesis with increased PD signal highly predicts the eventual development of SpA.36 Therefore, B-mode and PD evaluations of the entheses are critical in the identification of patients who are at an increased risk of developing SpA.37 Treatment monitoring is performed by using a US scoring system in a follow-up evaluation of patients with PsA. Some of the scoring systems have evaluated changes in B-mode US lesions (enthesis and soft tissues, such as skin and nails), whereas others focus on changes in the PD signal.37,38
The Five Targets Power Doppler for Psoriatic Disease PD scoring system comprises the assessment of PD signal in the joint, tendon with synovial sheath, enthesis, skin, and nails. Each of the targets is scored from 0 to 3 points, with a maximum of 15 total points. Some studies have shown that PDUS can provide valuable information in the evaluation of psoriatic plaques and onychopathy in patients with psoriasis and PsA.39 The detection of a PD signal within the dermis and nail bed is equivalent to active inflammation in these sites.39-41 However, further studies with larger cohorts proving inter- and intra-observer reliability are necessary to consolidate these findings and comfortably apply them in clinical practice.
Osteoarthritis
Increasingly US is studied for its validity and reliability in evaluating periarticular soft tissue and cartilage changes in knee OA. The associated US findings include a high prevalence of synovitis with a low prevalence of a PD signal, the presence of osteophytes, and joint space narrowing.42,43 Increased PD signal, synovial hypertrophy, and joint effusion were observed in patients with radiographically erosive OA compared with those with radiographically nonerosive OA.44
Bone erosions and inflammatory changes are also frequently detected by US in both erosive and nodal hand OA.45 Compared with MRI, US has shown a good to excellent correlation in the assessment of osteophytes, bone erosions, synovitis, and tenosynovitis in erosive and hand nodal OA.46 In comparison with CR, US has shown to have a higher sensitivity in the assessment of bony erosions, osteophytes, and space narrowing.47 Ultrasound is able to detect changes in the earlier stages of cartilage erosion in OA, characterized by loss of the sharp contour and variations in the echogenicity of the cartilage matrix, asymmetric shrinkage, and ultimately the disappearance of the cartilaginous band, which is more evident in the later stages of OA.45
Similar to RA management, US has been used to monitor disease activity and response to OA treatment. Patients who received intra-articular hyaluronic acid or intramuscular methylprednisolone for OA treatment were found to have a decrease of PD signal intensity and synovial effusion posttreatment.48 One could extrapolate these findings and conclude that US could be an additional tool for monitoring disease activity and assessing response to local and systemic treatments in OA.
Crystalline Arthropathies
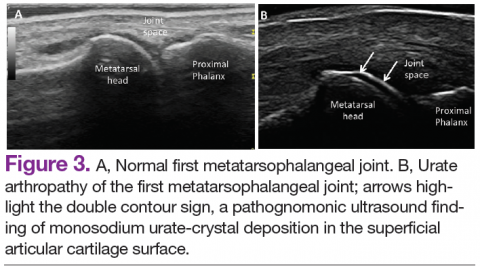
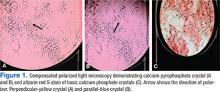
Ultrasound application to crystal diseases facilitates the identification of microcrystalline deposits within the synovial membrane (joints), cartilage (both hyaline cartilage and fibrocartilage), and periarticular tissues (tendons, bursae, and soft tissues). Crystals appear as hyperechogenic spots of different sizes and shapes that can be seen in both articular and periarticular tissues.49,50 The crystal deposition pattern on hyaline cartilage allows the differentiation between monosodium urate (MSU) and calcium pyrophosphate dehydrate (CPP) crystals. The MSU crystals are deposited at the chondrosynovial (or superficial) margin of the hyaline cartilage and described sonographically as the double contour sign in gout, whereas CPP crystals are deposited within the intermediate layer of the hyaline cartilage and are seen as hyperechoic spots frequently described as rosary beads on US.6,49,50
Other important sites that can be evaluated to determine the presence of CPP crystals include the menisci, symphysis pubis, and triangular fibrocartilage at the wrists, hips, and shoulders. Recent EULAR recommendations have incorporated US as part of the diagnostic imaging modality for the diagnosis of CPPD and more recently for gout.6,51 Tophi are seen as MSU precipitates deposited in the joint cavity, tendons, and/or periarticular tissues such as bursae. They can show different echogenic signal. Soft tophi can demonstrate high PD signal due to high vascularization. On the other hand, hard tophi are hyperechoic on B-mode due to the presence of calcification, which does not allow passage of US waves, creating postacoustic shadowing.8 Studies have evaluated the predictive role of US in evaluating patients with asymptomatic hyperuricemia without any prior history of crystal-related joint disease and found tophaceous deposits in the triceps and patellar and quadriceps tendons.52-55 Studies have also looked at using US in the assessment of treatment response to serum uratelowering therapy in patients with gout.56,57 These studies have noted an improvement in the double contour sign, hyperechoic spots, cloudy areas in the synovial fluid, and tophus diameter and size in those patients who achieved a treat-to-target with a serum uric acid level ≤ 6 mg/dL. Patients who did not reach this target had no changes in the gout US features.56-57 Larger cohort studies are needed to confirm these findings.
An active inflammatory process can be determined by using a PD signal in the acute gout setting with increased vascularization; however, an increased PD signal can also be seen in septic arthritis or tenosynovitis, which sometimes can coexist with crystal-induced arthritis. Therefore, diagnostic arthrocentesis, Gram stain, and culture, as well as evaluation of crystals under polarized microscopy, are still recommended.
Therapeutic Interventions
Real-time visualization of the injection needle by US allows reliable placement of the needle tip in the tissue or cavity of interest. Multiple studies have shown the low accuracy of palpation-guided injection for reaching the site of interest.58,59 Some studies have shown a higher response rate to US-guided injections compared with palpation-guided as well as a higher rate of successful aspirations and clinical outcomes. Meta-analyses have demonstrated improved treatment response with the use of US-guided procedures compared with blinded injections.60,61 Ultrasound-guided interventions are performed in both peripheral and axial joints.62 The most common US-guided procedures at the VA rheumatology clinic include arthrocentesis and intra-articular corticosteroid injections of small and medium-sized joints, such as MCP joints, elbows, wrists, and ankles.
Conclusions
Ultrasound is becoming a relevant part of rheumatology practice and research and can be regarded as a feasible and effective imaging technique that can allow real-time recognition of early anatomical changes, provide careful guidance for aspiration, and monitor local and/or systemic treatment response at the joint, tendon, enthesis, nail, and skin levels. Ultrasound is a user-friendly imaging modality readily applied at the bedside and considered an extension of the rheumatologist's physical examination.
The success of US depends on the individual operator. For this reason, structured educational programs during fellowship training programs and an efficient competency assessment system would facilitate proper implementation of US in rheumatology practice as performed by some but not all institutions.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Naredo E, D’Agostino MA, Conaghan PG, et al. Current state of musculoskeletal ultrasound training and implementation in Europe: results of a survey of experts and scientific societies. Rheumatology (Oxford). 2010;49(12):2438-2943.
2. Micu MC, Alcalde M, Sáenz JI, et al. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res (Hoboken). 2013;65(4):615-621.
3. Koski JM. Ultrasound guided injections in rheumatology. J Rheumatol. 2000;27(9):2131-2138.
4. Kelly S, Humby F, Filer A, et al. Ultrasound-guided synovial biopsy: a safe, well-tolerated and reliable technique for obtaining high-quality synovial tissue from both large and small joints in early arthritis patients. Ann Rheum Dis. 2015;74(3):611-617.
5. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum Dis. 2010;69(9):1580-1588.
6. Zhang W, Doherty M, Bardin T, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis. 2011;70(4):563-570.
7. Dasgupta B, Cimmino MA, Kremers HM, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/ American College of Rheumatology collaborative initiative. Arthritis Rheum. 2012;64(4):943-954.
8. Fodor D, Nestorova R, Vlad V, Micu M. The place of musculoskeletal ultrasonography in gout diagnosis. Med Ultrason. 2014;16(4):336-344.
9. Takagi Y, Sumi M, Nakamura H, et al. Ultrasonography as an additional item in the American College of Rheumatology classification of Sjögren’s syndrome. Rheumatology (Oxford). 2014;53(11):1977-1983.
10. Colebatch AN, Edwards CJ, Østergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72(6):804-814.
11. American College of Rheumatology Musculoskeletal Ultrasound Task Force. Ultrasound in American rheumatology practice: report of the American College of Rheumatology musculoskeletal ultrasound task force. Arthritis Care Res (Hoboken). 2010;62(9):1206-1219.
12. McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res (Hoboken). 2012;64(11):1625-1640.
13. Naredo E, Möller I, Cruz A, Carmona L, Garrido J. Power Doppler ultrasonographic monitoring of response to anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58(8):2248-2256.
14. Newman JS, Laing TJ, McCarthy CJ, Adler RS. Power Doppler sonography of synovitis: assessment of therapeutic response—preliminary observations. Radiology. 1996;198(2):582-584.
15. Wakefield RJ, Balint PV, Szkudlarek M, et al; OMERACT 7 Special Interest Group. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32(12):2485-2487.
16. Wakefield RJ, Green MJ, Marzo-Ortega H, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis. 2004;63(4):382-385.
17. Brown AK, Quinn MA, Karim Z, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54(12):3761-3773.
18. Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58(10):2958-2967.
19. Bruyn GA, Hanova P, Iagnocco A, et al; OMERACT Ultrasound Task Force. Ultrasound definition of tendon damage in patients with rheumatoid arthritis. Results of a OMERACT consensus-based ultrasound score focusing on the diagnostic reliability. Ann Rheum Dis. 2014;73(11):1929-1934.
20. Filippucci E, Gabba A, Di Geso L, Girolimetti R, Salaffi F, Grassi W. Hand tendon involvement in rheumatoid arthritis: an ultrasound study. Semin Arthritis Rheum. 2012;41(6):752-760.
21. Lillegraven S, Bøyesen P, Hammer HB, et al. Tenosynovitis of the extensor carpi ulnaris tendon predicts erosive progression in early rheumatoid arthritis. Ann Rheum Dis. 2011;70(11):2049-2050.
22. Baillet A, Gaujoux-Viala C, Mouterde G, et al. Comparison of the efficacy of sonography, magnetic resonance imaging and conventional radiography for the detection of bone erosions in rheumatoid arthritis patients: a systematic review and meta-analysis. Rheumatology (Oxford). 2011;50(6):1137-1147.
23. Filer A, de Pablo P, Allen G, et al. Utility of ultrasound joint counts in the prediction of rheumatoid arthritis in patients with very early synovitis. Ann Rheum Dis. 2011;70(3):500-507.
24. Frediani B, Falsetti P, Storri L, et al. Quadricepital tendon enthesitis in psoriatic arthritis and rheumatoid arthritis: ultrasound examinations and clinical correlations. J Rheumatol. 2001;28(11):2566-2568.
25. D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48(2):523-533.
26. Gisondi P, Tinazzi I, El-Dalati G, et al. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: a hospital-based case-control study. Ann Rheum Dis. 2008;67(1):26-30.
27. Gutierrez M, Filippucci E, De Angelis R, et al. Subclinical entheseal involvement
in patients with psoriasis: an ultrasound study. Semin Arthritis Rheum. 2011;40(5):407-412.
28. Weiner SM, Jurenz S, Uhl M, et al. Ultrasonography in the assessment of peripheral joint involvement in psoriatic arthritis: a comparison with radiography, MRI and scintigraphy. Clin Rheumatol. 2008;27(8):983-989.
29. Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61(10):905-910.
30. Gutierrez M, Filippucci E, Salaffi F, Di Geso L, Grassi W. Differential diagnosis
between rheumatoid arthritis and psoriatic arthritis: the value of ultrasound findings at metacarpophalangeal joints level. Ann Rheum Dis. 2011;70(6):1111-1114.
31. De Miguel E, Cobo T, Muñoz-Fernández S, et al. Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis. 2009;68(2):169-174.
32. Ash ZR, Tinazzi I, Gallego CC, et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis. 2012;71(4):553-556.
33. Naredo E, Möller I, de Miguel E, et al; Ultrasound School of the Spanish Society of Rheumatology and Spanish ECO-APs Group. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology (Oxford). 2011;50(10):1838-1848.
34. Muñoz-Fernández S, de Miguel E, Cobo-Ibáñez T, et al. Enthesis inflammation in recurrent acute anterior uveitis without spondylarthritis. Arthritis Rheum. 2009;60(7):1985-1990.
35. Bandinelli F, Milla M, Genise S, et al. Ultrasound discloses entheseal involvement
in inactive and low active inflammatory bowel disease without clinical signs and symptoms of spondyloarthropathy. Rheumatology (Oxford). 2011;50(7):1275-1279.
36. D’Agostino MA, Aegerter P, Bechara K, et al. How to diagnose spondyloarthritis early? Accuracy of peripheral enthesitis detection by power Doppler ultrasonography. Ann Rheum Dis. 2011;70(8):1433-1440.
37. Aydin SZ, Karadag O, Filippucci E, et al. Monitoring Achilles enthesitis in ankylosing spondylitis during TNF-alpha antagonist therapy: an ultrasound study. Rheumatology (Oxford). 2010;49(3):578-582.
38. Naredo E, Batlle-Gualda E, Garcia-Vivar ML, et al; Ultrasound Group of the Spanish Society of Rheumatology. Power Doppler ultrasonography assessment of entheses in spondyloarthropathies: response to therapy of entheseal abnormalities. J Rheumatol. 2010;37(10):2110-2117.
39. Gutierrez M, Di Geso L, Salaffi F, et al. Development of a preliminary US power Doppler composite score for monitoring treatment in PsA. Rheumatology (Oxford). 2012;51(7):1261-1268.
40. Gutierrez M, De Angelis R, Bernardini ML, et al. Clinical, power Doppler sonography and histological assessment of the psoriatic plaque: short-term monitoring in patients treated with etanercept. Br J Dermatol. 2011;164(1):33-37.
41. Gutierrez M, Filippucci E, Bertolazzi C, Grassi W. Sonographic monitoring of psoriatic plaque. J Rheumatol. 2009;36(4):850-851.
42. Keen HI, Wakefield RJ, Grainger AJ, Hensor EM, Emery P, Conaghan PG. An ultrasonographic study of osteoarthritis of the hand: synovitis and its relationship to structural pathology and symptoms. Arthritis Rheum. 2008;59(12):1756-1763.
43. Kortekaas MC, Kwok WY, Reijnierse M, Watt I, Huizinga TW, Kloppenburg M. Pain in hand osteoarthritis is associated with inflammation: the value of ultrasound. Ann Rheum Dis. 2010;69(7):1367-1369.
44. Mancarella L, Magnani M, Addimanda O, Pignotti E, Galletti S, Meliconi R. Ultrasound-detected synovitis with power Doppler signal is associated with severe radiographic damage and reduced cartilage thickness in hand osteoarthritis. Osteoarthritis Cartilage. 2010;18(10):1263-1268.
45. Möller I, Bong D, Naredo E, et al. Ultrasound in the study and monitoring of osteoarthritis. Osteoarthritis Cartilage. 2008;16(suppl 3):S4-S7.
46. Vlychou M, Koutroumpas A, Alexiou I, Fezoulidis I, Sakkas LI. High-resolution ultrasonography and 3.0 T magnetic resonance imaging in erosive and nodal hand osteoarthritis: high frequency of erosions in nodal osteoarthritis. Clin Rheumatol. 2013;32(6):755-762.
47. Keen HI, Wakefield RJ, Grainger AJ, Hensor EM, Emery P, Conaghan PG. Can ultrasonography improve on radiographic assessment in osteoarthritis of the hands? A comparison between radiographic and ultrasonographic detected pathology. Ann Rheum Dis. 2008;67(8):1116-1120.
48. Keen HI, Wakefield RJ, Hensor EM, Emery P, Conaghan PG. Response of symptoms
and synovitis to intra-muscular methylprednisolone in osteoarthritis of the hand: an ultrasonographic study. Rheumatology (Oxford). 2010;49(6):1093-1100.
49. Grassi W, Meenagh G, Pascual E, Filippucci E. “Crystal clear”-sonographic assessment of gout and calcium pyrophosphate deposition disease. Semin Arthritis Rheum. 2006;36(3):197-202.
50. Ciapetti A, Filippucci E, Gutierrez M, Grassi W. Calcium pyrophosphate dihydrate crystal deposition disease: sonographic findings. Clin Rheumatol. 2009;28(3):271-276.
51. Taylor WJ, Fransen J, Jansen TL, et al. Study for Updated Gout Classification Criteria (SUGAR): identification of features to classify gout. Arthritis Care Res (Hoboken). [Published online ahead of print March 16, 2015.]
52. Puig JG, de Miguel E, Castillo MC, Rocha AL, Martinez MA, Torres RJ. Asymptomatic hyperuricemia: impact of ultrasonography. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):592-595.
53. Pineda C, Amezcua-Guerra LM, Solano C, et al. Joint and tendon subclinical involvement suggestive of gouty arthritis in asymptomatic hyperuricemia: an ultrasound controlled study. Arthritis Res Ther. 2011;13(1):R4.
54. Naredo E, Uson J, Jiménez-Palop M, et al. Ultrasound-detected musculoskeletal urate crystal deposition: which joints and what findings should be assessed for diagnosing gout? Ann Rheum Dis. 2014;73(8):1522-1528.
55. De Miguel E, Puig JG, Castillo C, Peiteado D, Torres RJ, Martín-Mola E. Diagnosis of gout in patients with asymptomatic hyperuricaemia: a pilot ultrasound study. Ann Rheum Dis. 2012;71(1):157-158.
56. Perez-Ruiz F, Martin I, Canteli B. Ultrasonographic measurement of tophi as an outcome measure for chronic gout. J Rheumatol. 2007;34(9):1888-1893.
57. Thiele RG, Schlesinger N. Ultrasonography shows disappearance of monosodium urate crystal deposition on hyaline cartilage after sustained normouricemia is achieved. Rheumatol Int. 2010;30(4):495-503.
58. Balint PV, Kane D, Hunter J, McInnes IB, Field M, Sturrock RD. Ultrasound guided versus conventional joint and soft tissue fluid aspiration in rheumatology practice: a pilot study. J Rheumatol. 2002;29(10):2209-2213.
59. Raza K, Lee CY, Pilling D, et al. Ultrasound guidance allows accurate needle placement and aspiration from small joints in patients with early inflammatory arthritis. Rheumatology (Oxford). 2003;42(8):976-979.
60. Dubreuil M, Greger S, LaValley M, Cunnington J, Sibbitt WL Jr, Kissin EY. Improvement in wrist pain with ultrasound-guided glucocorticoid injections: a metaanalysis of individual patient data. Semin Arthritis Rheum. 2013;42(5):492-497.
61. Sage W, Pickup L, Smith TO, Denton ER, Toms AP. The clinical and functional outcomes of ultrasound-guided vs landmark-guided injections for adults with shoulder pathology—a systematic review and meta-analysis. Rheumatology (Oxford). 2013;52(4):743-751.
62. Darrieutort-Laffite C, Hamel O, Glémarec J, Maugars Y, Le Goff B. Ultrasonography of the lumbar spine: sonoanatomy and practical applications. Joint Bone Spine. 2014;81(2):130-136.
Over the past 2 decades, an increasing number of rheumatologists have progressively incorporated ultrasound (US) as an invaluable diagnostic and monitoring tool into their clinical and research practice.1,2 This imaging modality has become an established aid incorporated into the clinical evaluation of periarticular and articular structures involved in the diagnosis of several rheumatic disorders.
Ultrasound is a safe, noninvasive, patient-friendly imaging modality with a lack of contraindications and free of ionizing radiation. It allows real-time evaluation with dynamic assessment in a multiplanar view, assessment of multiple targets, and lower cost compared with magnetic resonance imaging (MRI) or computerized tomography scan. Above all, for the rheumatologist, US provides real-time scanning of all peripheral joints as many times as is required at the time of consultation. It is of great advantage in the assessment of a wide spectrum of abnormalities in rheumatic diseases with the potential of point-of-care imaging modality in the clinical evaluation and management of the patient. It facilitates a direct correlation between imaging findings and clinical data that improves the approach to a wide range of rheumatic diseases, from acute to chronic inflammatory arthritis, crystalline arthropathies, osteoarthritis (OA), spondyloarthropathies (SpA), vasculitis, and soft tissue syndromes. In addition, US is a bedside tool for performing accurate and safe diagnostic arthrocentesis, injections, and synovial biopsies.3,4
Recently, a gradual attempt has been made to incorporate US into rheumatology disease classification or diagnostic criteria for rheumatoid arthritis (RA), polymyalgia rheumatica, gout, calcium pyrophosphate deposition disease (CPPD), and Sjögren’s syndrome.5-10 Furthermore, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) have produced evidence and expert opinion-based recommendations on the use of US in the clinical management of rheumatic diseases.10-12 This article highlights the most common applications of US for assessment and management of different rheumatic diseases frequently encountered at the VAMC rheumatology inpatient and outpatient clinical service.
Evaluation of Inflammatory Arthritis
In RA and any other inflammatory arthritis, US has been used for the detection of joint effusions, synovitis, bone erosions, and tendon and enthesis involvement.11,12 Ultrasound B-mode and power Doppler (PD) techniques have demonstrated a consistent and relevant role in optimizing the diagnosis, assessing the inflammatory activity, monitoring response to therapy, and predicting the inflammatory arthritis outcomes (Figures 1-3).10-12 Ultrasound provides real-time information about the status of the synovial membrane, tendons, cartilage, bursae, and cortical bones, allowing an accurate assessment of the degree of inflammatory process in periarticular and articular tissues. Also, US can provide details about the characteristics of the collected fluid (ie, effusion or synovial hypertrophy), which is fundamental for the correct interpretation of the pathologic joint and/or soft tissue processes. The inflammatory process can be assessed by using PD mode, which detects and quantifies the vascular changes in the pannus due to vasodilation and the increased blood flow characteristic of active inflammation.13,14
The Outcomes Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) study group developed standardized sonopathologic definitions and scanning methods to be used in the daily rheumatologic practice and clinical trials (Table 1).15 Furthermore, it developed a semiquantitative scale to assess the degree of synovitis in US B-mode and PD mode (Table 2).15
The use of US to find subclinical synovitis in patients with RA considered to be in clinical remission is a new issue.16 Some reports have demonstrated progressive joint damage in these patients with evidence of active inflammation on PDUS despite clinical remission.17,18 More prospective studies are required to provide a better understanding of the long-term effects of residual inflammation and the proper long-term treatment of these patients. Furthermore, the PD signal has been shown to be superior to the Disease Activity Score 28 (DAS-28) in evaluating disease activity, particularly in predicting joint damage.18
Ultrasound may be considered the gold standard imaging tool for the assessment of tendons in inflammatory arthritis and includes the detection of tenosynovitis and anatomical damage represented by the loss of the normal fibrillar echotexture and loss of definition of the tendon margins, which may occur in early disease.19,20 Tenosynovitis of the extensor carpi ulnaris (ECU) detected by US has been shown to be an independent predictive factor of erosive joint damage, suggesting that ECU tenosynovitis represents a useful ultrasonographic landmark in the diagnosis of early RA.21
The availability of new nonbiologic and biologic therapies for inflammatory arthritis has raised the importance of identifying early changes, such as the detection of early erosions, which portend a poor long-term prognosis. The capability of US in identifying this lesion at an earlier stage compared with conventional radiography (CR) has allowed the early diagnosis and treatment of these patients before irreversible joint destruction occurs.22 In spite of all the supportive evidence of US utility in RA, it is not considered among the mandatory diagnostic criteria in the ACR/EULAR classification criteria for RA.5 Still, the addition of US findings to these criteria has increased the number of patients who fulfilled the 1987 ACR classification criteria for RA after 18 months of follow-up.23 Despite extensive evidence of its utility in the diagnosis and monitoring of RA, further studies are still needed.
Spondyloarthritis
Similar to RA, SpA discloses sonographic findings of inflammatory arthritis; however, with more entheseal and tenosynovium involvement. Ultrasound has also been used in the early identification of characteristic changes of the skin and nail tissues, which can aid the global assessment of this heterogeneous disease, especially in psoriatic arthritis (PsA). The most common locations of enthesitis in SpA are the quadriceps and the Achilles enthesis.24,25
Although US offers detailed imaging for the assessment of both tendons and enthesis, there is a lack of literature evaluating dactylitis. The OMERACT group recently released a composite measure of activity and severity of US dactylitis, which included newly defined elementary US lesions that may discern dactylitis of a digit.26,27 Ultrasound has been compared with MRI in the detection of SpA-related synovitis of the hands and feet and has demonstrated competitive diagnostic sensitivity.28 Ultrasound also shows higher sensitivity in detecting synovitis of the hands and feet compared with clinical examination and CR in PsA.28,29 Unfortunately, there are no strongly validated US findings that can aid in the differential diagnosis of PsA against other chronic inflammatory arthritides. The presence of peritendinous extensor tendon inflammation was a highly specific sonographic feature of PsA, because it was present in 66% of metacarpophalangeal (MCP) joints as the only US sign of inflammation compared with patients with RA.30
Another application of US is in the evaluation of subclinical inflammation at the enthesis in patients with a history of psoriasis without prior history of PsA.27,31 In those patients with psoriatic nail changes, more subclinical enthesitis was found compared with patients with psoriasis without nail involvement.32 Furthermore, subclinical joint inflammation has also been described.33 These findings suggest a possible predictive value in patients with psoriasis who should be monitored on a regular basis, because they are at risk of developing PsA.
Subclinical enthesitis by US imaging has been described in patients with recurrent anterior uveitis and inflammatory bowel disease.34,35 In cases where SpA is suspected but diagnostic criteria are not fulfilled, the presence of one enthesis with increased PD signal highly predicts the eventual development of SpA.36 Therefore, B-mode and PD evaluations of the entheses are critical in the identification of patients who are at an increased risk of developing SpA.37 Treatment monitoring is performed by using a US scoring system in a follow-up evaluation of patients with PsA. Some of the scoring systems have evaluated changes in B-mode US lesions (enthesis and soft tissues, such as skin and nails), whereas others focus on changes in the PD signal.37,38
The Five Targets Power Doppler for Psoriatic Disease PD scoring system comprises the assessment of PD signal in the joint, tendon with synovial sheath, enthesis, skin, and nails. Each of the targets is scored from 0 to 3 points, with a maximum of 15 total points. Some studies have shown that PDUS can provide valuable information in the evaluation of psoriatic plaques and onychopathy in patients with psoriasis and PsA.39 The detection of a PD signal within the dermis and nail bed is equivalent to active inflammation in these sites.39-41 However, further studies with larger cohorts proving inter- and intra-observer reliability are necessary to consolidate these findings and comfortably apply them in clinical practice.
Osteoarthritis
Increasingly US is studied for its validity and reliability in evaluating periarticular soft tissue and cartilage changes in knee OA. The associated US findings include a high prevalence of synovitis with a low prevalence of a PD signal, the presence of osteophytes, and joint space narrowing.42,43 Increased PD signal, synovial hypertrophy, and joint effusion were observed in patients with radiographically erosive OA compared with those with radiographically nonerosive OA.44
Bone erosions and inflammatory changes are also frequently detected by US in both erosive and nodal hand OA.45 Compared with MRI, US has shown a good to excellent correlation in the assessment of osteophytes, bone erosions, synovitis, and tenosynovitis in erosive and hand nodal OA.46 In comparison with CR, US has shown to have a higher sensitivity in the assessment of bony erosions, osteophytes, and space narrowing.47 Ultrasound is able to detect changes in the earlier stages of cartilage erosion in OA, characterized by loss of the sharp contour and variations in the echogenicity of the cartilage matrix, asymmetric shrinkage, and ultimately the disappearance of the cartilaginous band, which is more evident in the later stages of OA.45
Similar to RA management, US has been used to monitor disease activity and response to OA treatment. Patients who received intra-articular hyaluronic acid or intramuscular methylprednisolone for OA treatment were found to have a decrease of PD signal intensity and synovial effusion posttreatment.48 One could extrapolate these findings and conclude that US could be an additional tool for monitoring disease activity and assessing response to local and systemic treatments in OA.
Crystalline Arthropathies
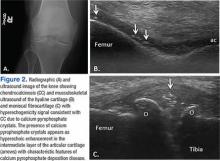
Ultrasound application to crystal diseases facilitates the identification of microcrystalline deposits within the synovial membrane (joints), cartilage (both hyaline cartilage and fibrocartilage), and periarticular tissues (tendons, bursae, and soft tissues). Crystals appear as hyperechogenic spots of different sizes and shapes that can be seen in both articular and periarticular tissues.49,50 The crystal deposition pattern on hyaline cartilage allows the differentiation between monosodium urate (MSU) and calcium pyrophosphate dehydrate (CPP) crystals. The MSU crystals are deposited at the chondrosynovial (or superficial) margin of the hyaline cartilage and described sonographically as the double contour sign in gout, whereas CPP crystals are deposited within the intermediate layer of the hyaline cartilage and are seen as hyperechoic spots frequently described as rosary beads on US.6,49,50
Other important sites that can be evaluated to determine the presence of CPP crystals include the menisci, symphysis pubis, and triangular fibrocartilage at the wrists, hips, and shoulders. Recent EULAR recommendations have incorporated US as part of the diagnostic imaging modality for the diagnosis of CPPD and more recently for gout.6,51 Tophi are seen as MSU precipitates deposited in the joint cavity, tendons, and/or periarticular tissues such as bursae. They can show different echogenic signal. Soft tophi can demonstrate high PD signal due to high vascularization. On the other hand, hard tophi are hyperechoic on B-mode due to the presence of calcification, which does not allow passage of US waves, creating postacoustic shadowing.8 Studies have evaluated the predictive role of US in evaluating patients with asymptomatic hyperuricemia without any prior history of crystal-related joint disease and found tophaceous deposits in the triceps and patellar and quadriceps tendons.52-55 Studies have also looked at using US in the assessment of treatment response to serum uratelowering therapy in patients with gout.56,57 These studies have noted an improvement in the double contour sign, hyperechoic spots, cloudy areas in the synovial fluid, and tophus diameter and size in those patients who achieved a treat-to-target with a serum uric acid level ≤ 6 mg/dL. Patients who did not reach this target had no changes in the gout US features.56-57 Larger cohort studies are needed to confirm these findings.
An active inflammatory process can be determined by using a PD signal in the acute gout setting with increased vascularization; however, an increased PD signal can also be seen in septic arthritis or tenosynovitis, which sometimes can coexist with crystal-induced arthritis. Therefore, diagnostic arthrocentesis, Gram stain, and culture, as well as evaluation of crystals under polarized microscopy, are still recommended.
Therapeutic Interventions
Real-time visualization of the injection needle by US allows reliable placement of the needle tip in the tissue or cavity of interest. Multiple studies have shown the low accuracy of palpation-guided injection for reaching the site of interest.58,59 Some studies have shown a higher response rate to US-guided injections compared with palpation-guided as well as a higher rate of successful aspirations and clinical outcomes. Meta-analyses have demonstrated improved treatment response with the use of US-guided procedures compared with blinded injections.60,61 Ultrasound-guided interventions are performed in both peripheral and axial joints.62 The most common US-guided procedures at the VA rheumatology clinic include arthrocentesis and intra-articular corticosteroid injections of small and medium-sized joints, such as MCP joints, elbows, wrists, and ankles.
Conclusions
Ultrasound is becoming a relevant part of rheumatology practice and research and can be regarded as a feasible and effective imaging technique that can allow real-time recognition of early anatomical changes, provide careful guidance for aspiration, and monitor local and/or systemic treatment response at the joint, tendon, enthesis, nail, and skin levels. Ultrasound is a user-friendly imaging modality readily applied at the bedside and considered an extension of the rheumatologist's physical examination.
The success of US depends on the individual operator. For this reason, structured educational programs during fellowship training programs and an efficient competency assessment system would facilitate proper implementation of US in rheumatology practice as performed by some but not all institutions.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Over the past 2 decades, an increasing number of rheumatologists have progressively incorporated ultrasound (US) as an invaluable diagnostic and monitoring tool into their clinical and research practice.1,2 This imaging modality has become an established aid incorporated into the clinical evaluation of periarticular and articular structures involved in the diagnosis of several rheumatic disorders.
Ultrasound is a safe, noninvasive, patient-friendly imaging modality with a lack of contraindications and free of ionizing radiation. It allows real-time evaluation with dynamic assessment in a multiplanar view, assessment of multiple targets, and lower cost compared with magnetic resonance imaging (MRI) or computerized tomography scan. Above all, for the rheumatologist, US provides real-time scanning of all peripheral joints as many times as is required at the time of consultation. It is of great advantage in the assessment of a wide spectrum of abnormalities in rheumatic diseases with the potential of point-of-care imaging modality in the clinical evaluation and management of the patient. It facilitates a direct correlation between imaging findings and clinical data that improves the approach to a wide range of rheumatic diseases, from acute to chronic inflammatory arthritis, crystalline arthropathies, osteoarthritis (OA), spondyloarthropathies (SpA), vasculitis, and soft tissue syndromes. In addition, US is a bedside tool for performing accurate and safe diagnostic arthrocentesis, injections, and synovial biopsies.3,4
Recently, a gradual attempt has been made to incorporate US into rheumatology disease classification or diagnostic criteria for rheumatoid arthritis (RA), polymyalgia rheumatica, gout, calcium pyrophosphate deposition disease (CPPD), and Sjögren’s syndrome.5-10 Furthermore, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) have produced evidence and expert opinion-based recommendations on the use of US in the clinical management of rheumatic diseases.10-12 This article highlights the most common applications of US for assessment and management of different rheumatic diseases frequently encountered at the VAMC rheumatology inpatient and outpatient clinical service.
Evaluation of Inflammatory Arthritis
In RA and any other inflammatory arthritis, US has been used for the detection of joint effusions, synovitis, bone erosions, and tendon and enthesis involvement.11,12 Ultrasound B-mode and power Doppler (PD) techniques have demonstrated a consistent and relevant role in optimizing the diagnosis, assessing the inflammatory activity, monitoring response to therapy, and predicting the inflammatory arthritis outcomes (Figures 1-3).10-12 Ultrasound provides real-time information about the status of the synovial membrane, tendons, cartilage, bursae, and cortical bones, allowing an accurate assessment of the degree of inflammatory process in periarticular and articular tissues. Also, US can provide details about the characteristics of the collected fluid (ie, effusion or synovial hypertrophy), which is fundamental for the correct interpretation of the pathologic joint and/or soft tissue processes. The inflammatory process can be assessed by using PD mode, which detects and quantifies the vascular changes in the pannus due to vasodilation and the increased blood flow characteristic of active inflammation.13,14
The Outcomes Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) study group developed standardized sonopathologic definitions and scanning methods to be used in the daily rheumatologic practice and clinical trials (Table 1).15 Furthermore, it developed a semiquantitative scale to assess the degree of synovitis in US B-mode and PD mode (Table 2).15
The use of US to find subclinical synovitis in patients with RA considered to be in clinical remission is a new issue.16 Some reports have demonstrated progressive joint damage in these patients with evidence of active inflammation on PDUS despite clinical remission.17,18 More prospective studies are required to provide a better understanding of the long-term effects of residual inflammation and the proper long-term treatment of these patients. Furthermore, the PD signal has been shown to be superior to the Disease Activity Score 28 (DAS-28) in evaluating disease activity, particularly in predicting joint damage.18
Ultrasound may be considered the gold standard imaging tool for the assessment of tendons in inflammatory arthritis and includes the detection of tenosynovitis and anatomical damage represented by the loss of the normal fibrillar echotexture and loss of definition of the tendon margins, which may occur in early disease.19,20 Tenosynovitis of the extensor carpi ulnaris (ECU) detected by US has been shown to be an independent predictive factor of erosive joint damage, suggesting that ECU tenosynovitis represents a useful ultrasonographic landmark in the diagnosis of early RA.21
The availability of new nonbiologic and biologic therapies for inflammatory arthritis has raised the importance of identifying early changes, such as the detection of early erosions, which portend a poor long-term prognosis. The capability of US in identifying this lesion at an earlier stage compared with conventional radiography (CR) has allowed the early diagnosis and treatment of these patients before irreversible joint destruction occurs.22 In spite of all the supportive evidence of US utility in RA, it is not considered among the mandatory diagnostic criteria in the ACR/EULAR classification criteria for RA.5 Still, the addition of US findings to these criteria has increased the number of patients who fulfilled the 1987 ACR classification criteria for RA after 18 months of follow-up.23 Despite extensive evidence of its utility in the diagnosis and monitoring of RA, further studies are still needed.
Spondyloarthritis
Similar to RA, SpA discloses sonographic findings of inflammatory arthritis; however, with more entheseal and tenosynovium involvement. Ultrasound has also been used in the early identification of characteristic changes of the skin and nail tissues, which can aid the global assessment of this heterogeneous disease, especially in psoriatic arthritis (PsA). The most common locations of enthesitis in SpA are the quadriceps and the Achilles enthesis.24,25
Although US offers detailed imaging for the assessment of both tendons and enthesis, there is a lack of literature evaluating dactylitis. The OMERACT group recently released a composite measure of activity and severity of US dactylitis, which included newly defined elementary US lesions that may discern dactylitis of a digit.26,27 Ultrasound has been compared with MRI in the detection of SpA-related synovitis of the hands and feet and has demonstrated competitive diagnostic sensitivity.28 Ultrasound also shows higher sensitivity in detecting synovitis of the hands and feet compared with clinical examination and CR in PsA.28,29 Unfortunately, there are no strongly validated US findings that can aid in the differential diagnosis of PsA against other chronic inflammatory arthritides. The presence of peritendinous extensor tendon inflammation was a highly specific sonographic feature of PsA, because it was present in 66% of metacarpophalangeal (MCP) joints as the only US sign of inflammation compared with patients with RA.30
Another application of US is in the evaluation of subclinical inflammation at the enthesis in patients with a history of psoriasis without prior history of PsA.27,31 In those patients with psoriatic nail changes, more subclinical enthesitis was found compared with patients with psoriasis without nail involvement.32 Furthermore, subclinical joint inflammation has also been described.33 These findings suggest a possible predictive value in patients with psoriasis who should be monitored on a regular basis, because they are at risk of developing PsA.
Subclinical enthesitis by US imaging has been described in patients with recurrent anterior uveitis and inflammatory bowel disease.34,35 In cases where SpA is suspected but diagnostic criteria are not fulfilled, the presence of one enthesis with increased PD signal highly predicts the eventual development of SpA.36 Therefore, B-mode and PD evaluations of the entheses are critical in the identification of patients who are at an increased risk of developing SpA.37 Treatment monitoring is performed by using a US scoring system in a follow-up evaluation of patients with PsA. Some of the scoring systems have evaluated changes in B-mode US lesions (enthesis and soft tissues, such as skin and nails), whereas others focus on changes in the PD signal.37,38
The Five Targets Power Doppler for Psoriatic Disease PD scoring system comprises the assessment of PD signal in the joint, tendon with synovial sheath, enthesis, skin, and nails. Each of the targets is scored from 0 to 3 points, with a maximum of 15 total points. Some studies have shown that PDUS can provide valuable information in the evaluation of psoriatic plaques and onychopathy in patients with psoriasis and PsA.39 The detection of a PD signal within the dermis and nail bed is equivalent to active inflammation in these sites.39-41 However, further studies with larger cohorts proving inter- and intra-observer reliability are necessary to consolidate these findings and comfortably apply them in clinical practice.
Osteoarthritis
Increasingly US is studied for its validity and reliability in evaluating periarticular soft tissue and cartilage changes in knee OA. The associated US findings include a high prevalence of synovitis with a low prevalence of a PD signal, the presence of osteophytes, and joint space narrowing.42,43 Increased PD signal, synovial hypertrophy, and joint effusion were observed in patients with radiographically erosive OA compared with those with radiographically nonerosive OA.44
Bone erosions and inflammatory changes are also frequently detected by US in both erosive and nodal hand OA.45 Compared with MRI, US has shown a good to excellent correlation in the assessment of osteophytes, bone erosions, synovitis, and tenosynovitis in erosive and hand nodal OA.46 In comparison with CR, US has shown to have a higher sensitivity in the assessment of bony erosions, osteophytes, and space narrowing.47 Ultrasound is able to detect changes in the earlier stages of cartilage erosion in OA, characterized by loss of the sharp contour and variations in the echogenicity of the cartilage matrix, asymmetric shrinkage, and ultimately the disappearance of the cartilaginous band, which is more evident in the later stages of OA.45
Similar to RA management, US has been used to monitor disease activity and response to OA treatment. Patients who received intra-articular hyaluronic acid or intramuscular methylprednisolone for OA treatment were found to have a decrease of PD signal intensity and synovial effusion posttreatment.48 One could extrapolate these findings and conclude that US could be an additional tool for monitoring disease activity and assessing response to local and systemic treatments in OA.
Crystalline Arthropathies
Ultrasound application to crystal diseases facilitates the identification of microcrystalline deposits within the synovial membrane (joints), cartilage (both hyaline cartilage and fibrocartilage), and periarticular tissues (tendons, bursae, and soft tissues). Crystals appear as hyperechogenic spots of different sizes and shapes that can be seen in both articular and periarticular tissues.49,50 The crystal deposition pattern on hyaline cartilage allows the differentiation between monosodium urate (MSU) and calcium pyrophosphate dehydrate (CPP) crystals. The MSU crystals are deposited at the chondrosynovial (or superficial) margin of the hyaline cartilage and described sonographically as the double contour sign in gout, whereas CPP crystals are deposited within the intermediate layer of the hyaline cartilage and are seen as hyperechoic spots frequently described as rosary beads on US.6,49,50
Other important sites that can be evaluated to determine the presence of CPP crystals include the menisci, symphysis pubis, and triangular fibrocartilage at the wrists, hips, and shoulders. Recent EULAR recommendations have incorporated US as part of the diagnostic imaging modality for the diagnosis of CPPD and more recently for gout.6,51 Tophi are seen as MSU precipitates deposited in the joint cavity, tendons, and/or periarticular tissues such as bursae. They can show different echogenic signal. Soft tophi can demonstrate high PD signal due to high vascularization. On the other hand, hard tophi are hyperechoic on B-mode due to the presence of calcification, which does not allow passage of US waves, creating postacoustic shadowing.8 Studies have evaluated the predictive role of US in evaluating patients with asymptomatic hyperuricemia without any prior history of crystal-related joint disease and found tophaceous deposits in the triceps and patellar and quadriceps tendons.52-55 Studies have also looked at using US in the assessment of treatment response to serum uratelowering therapy in patients with gout.56,57 These studies have noted an improvement in the double contour sign, hyperechoic spots, cloudy areas in the synovial fluid, and tophus diameter and size in those patients who achieved a treat-to-target with a serum uric acid level ≤ 6 mg/dL. Patients who did not reach this target had no changes in the gout US features.56-57 Larger cohort studies are needed to confirm these findings.
An active inflammatory process can be determined by using a PD signal in the acute gout setting with increased vascularization; however, an increased PD signal can also be seen in septic arthritis or tenosynovitis, which sometimes can coexist with crystal-induced arthritis. Therefore, diagnostic arthrocentesis, Gram stain, and culture, as well as evaluation of crystals under polarized microscopy, are still recommended.
Therapeutic Interventions
Real-time visualization of the injection needle by US allows reliable placement of the needle tip in the tissue or cavity of interest. Multiple studies have shown the low accuracy of palpation-guided injection for reaching the site of interest.58,59 Some studies have shown a higher response rate to US-guided injections compared with palpation-guided as well as a higher rate of successful aspirations and clinical outcomes. Meta-analyses have demonstrated improved treatment response with the use of US-guided procedures compared with blinded injections.60,61 Ultrasound-guided interventions are performed in both peripheral and axial joints.62 The most common US-guided procedures at the VA rheumatology clinic include arthrocentesis and intra-articular corticosteroid injections of small and medium-sized joints, such as MCP joints, elbows, wrists, and ankles.
Conclusions
Ultrasound is becoming a relevant part of rheumatology practice and research and can be regarded as a feasible and effective imaging technique that can allow real-time recognition of early anatomical changes, provide careful guidance for aspiration, and monitor local and/or systemic treatment response at the joint, tendon, enthesis, nail, and skin levels. Ultrasound is a user-friendly imaging modality readily applied at the bedside and considered an extension of the rheumatologist's physical examination.
The success of US depends on the individual operator. For this reason, structured educational programs during fellowship training programs and an efficient competency assessment system would facilitate proper implementation of US in rheumatology practice as performed by some but not all institutions.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Naredo E, D’Agostino MA, Conaghan PG, et al. Current state of musculoskeletal ultrasound training and implementation in Europe: results of a survey of experts and scientific societies. Rheumatology (Oxford). 2010;49(12):2438-2943.
2. Micu MC, Alcalde M, Sáenz JI, et al. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res (Hoboken). 2013;65(4):615-621.
3. Koski JM. Ultrasound guided injections in rheumatology. J Rheumatol. 2000;27(9):2131-2138.
4. Kelly S, Humby F, Filer A, et al. Ultrasound-guided synovial biopsy: a safe, well-tolerated and reliable technique for obtaining high-quality synovial tissue from both large and small joints in early arthritis patients. Ann Rheum Dis. 2015;74(3):611-617.
5. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum Dis. 2010;69(9):1580-1588.
6. Zhang W, Doherty M, Bardin T, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis. 2011;70(4):563-570.
7. Dasgupta B, Cimmino MA, Kremers HM, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/ American College of Rheumatology collaborative initiative. Arthritis Rheum. 2012;64(4):943-954.
8. Fodor D, Nestorova R, Vlad V, Micu M. The place of musculoskeletal ultrasonography in gout diagnosis. Med Ultrason. 2014;16(4):336-344.
9. Takagi Y, Sumi M, Nakamura H, et al. Ultrasonography as an additional item in the American College of Rheumatology classification of Sjögren’s syndrome. Rheumatology (Oxford). 2014;53(11):1977-1983.
10. Colebatch AN, Edwards CJ, Østergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72(6):804-814.
11. American College of Rheumatology Musculoskeletal Ultrasound Task Force. Ultrasound in American rheumatology practice: report of the American College of Rheumatology musculoskeletal ultrasound task force. Arthritis Care Res (Hoboken). 2010;62(9):1206-1219.
12. McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res (Hoboken). 2012;64(11):1625-1640.
13. Naredo E, Möller I, Cruz A, Carmona L, Garrido J. Power Doppler ultrasonographic monitoring of response to anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58(8):2248-2256.
14. Newman JS, Laing TJ, McCarthy CJ, Adler RS. Power Doppler sonography of synovitis: assessment of therapeutic response—preliminary observations. Radiology. 1996;198(2):582-584.
15. Wakefield RJ, Balint PV, Szkudlarek M, et al; OMERACT 7 Special Interest Group. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32(12):2485-2487.
16. Wakefield RJ, Green MJ, Marzo-Ortega H, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis. 2004;63(4):382-385.
17. Brown AK, Quinn MA, Karim Z, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54(12):3761-3773.
18. Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58(10):2958-2967.
19. Bruyn GA, Hanova P, Iagnocco A, et al; OMERACT Ultrasound Task Force. Ultrasound definition of tendon damage in patients with rheumatoid arthritis. Results of a OMERACT consensus-based ultrasound score focusing on the diagnostic reliability. Ann Rheum Dis. 2014;73(11):1929-1934.
20. Filippucci E, Gabba A, Di Geso L, Girolimetti R, Salaffi F, Grassi W. Hand tendon involvement in rheumatoid arthritis: an ultrasound study. Semin Arthritis Rheum. 2012;41(6):752-760.
21. Lillegraven S, Bøyesen P, Hammer HB, et al. Tenosynovitis of the extensor carpi ulnaris tendon predicts erosive progression in early rheumatoid arthritis. Ann Rheum Dis. 2011;70(11):2049-2050.
22. Baillet A, Gaujoux-Viala C, Mouterde G, et al. Comparison of the efficacy of sonography, magnetic resonance imaging and conventional radiography for the detection of bone erosions in rheumatoid arthritis patients: a systematic review and meta-analysis. Rheumatology (Oxford). 2011;50(6):1137-1147.
23. Filer A, de Pablo P, Allen G, et al. Utility of ultrasound joint counts in the prediction of rheumatoid arthritis in patients with very early synovitis. Ann Rheum Dis. 2011;70(3):500-507.
24. Frediani B, Falsetti P, Storri L, et al. Quadricepital tendon enthesitis in psoriatic arthritis and rheumatoid arthritis: ultrasound examinations and clinical correlations. J Rheumatol. 2001;28(11):2566-2568.
25. D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48(2):523-533.
26. Gisondi P, Tinazzi I, El-Dalati G, et al. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: a hospital-based case-control study. Ann Rheum Dis. 2008;67(1):26-30.
27. Gutierrez M, Filippucci E, De Angelis R, et al. Subclinical entheseal involvement
in patients with psoriasis: an ultrasound study. Semin Arthritis Rheum. 2011;40(5):407-412.
28. Weiner SM, Jurenz S, Uhl M, et al. Ultrasonography in the assessment of peripheral joint involvement in psoriatic arthritis: a comparison with radiography, MRI and scintigraphy. Clin Rheumatol. 2008;27(8):983-989.
29. Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61(10):905-910.
30. Gutierrez M, Filippucci E, Salaffi F, Di Geso L, Grassi W. Differential diagnosis
between rheumatoid arthritis and psoriatic arthritis: the value of ultrasound findings at metacarpophalangeal joints level. Ann Rheum Dis. 2011;70(6):1111-1114.
31. De Miguel E, Cobo T, Muñoz-Fernández S, et al. Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis. 2009;68(2):169-174.
32. Ash ZR, Tinazzi I, Gallego CC, et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis. 2012;71(4):553-556.
33. Naredo E, Möller I, de Miguel E, et al; Ultrasound School of the Spanish Society of Rheumatology and Spanish ECO-APs Group. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology (Oxford). 2011;50(10):1838-1848.
34. Muñoz-Fernández S, de Miguel E, Cobo-Ibáñez T, et al. Enthesis inflammation in recurrent acute anterior uveitis without spondylarthritis. Arthritis Rheum. 2009;60(7):1985-1990.
35. Bandinelli F, Milla M, Genise S, et al. Ultrasound discloses entheseal involvement
in inactive and low active inflammatory bowel disease without clinical signs and symptoms of spondyloarthropathy. Rheumatology (Oxford). 2011;50(7):1275-1279.
36. D’Agostino MA, Aegerter P, Bechara K, et al. How to diagnose spondyloarthritis early? Accuracy of peripheral enthesitis detection by power Doppler ultrasonography. Ann Rheum Dis. 2011;70(8):1433-1440.
37. Aydin SZ, Karadag O, Filippucci E, et al. Monitoring Achilles enthesitis in ankylosing spondylitis during TNF-alpha antagonist therapy: an ultrasound study. Rheumatology (Oxford). 2010;49(3):578-582.
38. Naredo E, Batlle-Gualda E, Garcia-Vivar ML, et al; Ultrasound Group of the Spanish Society of Rheumatology. Power Doppler ultrasonography assessment of entheses in spondyloarthropathies: response to therapy of entheseal abnormalities. J Rheumatol. 2010;37(10):2110-2117.
39. Gutierrez M, Di Geso L, Salaffi F, et al. Development of a preliminary US power Doppler composite score for monitoring treatment in PsA. Rheumatology (Oxford). 2012;51(7):1261-1268.
40. Gutierrez M, De Angelis R, Bernardini ML, et al. Clinical, power Doppler sonography and histological assessment of the psoriatic plaque: short-term monitoring in patients treated with etanercept. Br J Dermatol. 2011;164(1):33-37.
41. Gutierrez M, Filippucci E, Bertolazzi C, Grassi W. Sonographic monitoring of psoriatic plaque. J Rheumatol. 2009;36(4):850-851.
42. Keen HI, Wakefield RJ, Grainger AJ, Hensor EM, Emery P, Conaghan PG. An ultrasonographic study of osteoarthritis of the hand: synovitis and its relationship to structural pathology and symptoms. Arthritis Rheum. 2008;59(12):1756-1763.
43. Kortekaas MC, Kwok WY, Reijnierse M, Watt I, Huizinga TW, Kloppenburg M. Pain in hand osteoarthritis is associated with inflammation: the value of ultrasound. Ann Rheum Dis. 2010;69(7):1367-1369.
44. Mancarella L, Magnani M, Addimanda O, Pignotti E, Galletti S, Meliconi R. Ultrasound-detected synovitis with power Doppler signal is associated with severe radiographic damage and reduced cartilage thickness in hand osteoarthritis. Osteoarthritis Cartilage. 2010;18(10):1263-1268.
45. Möller I, Bong D, Naredo E, et al. Ultrasound in the study and monitoring of osteoarthritis. Osteoarthritis Cartilage. 2008;16(suppl 3):S4-S7.
46. Vlychou M, Koutroumpas A, Alexiou I, Fezoulidis I, Sakkas LI. High-resolution ultrasonography and 3.0 T magnetic resonance imaging in erosive and nodal hand osteoarthritis: high frequency of erosions in nodal osteoarthritis. Clin Rheumatol. 2013;32(6):755-762.
47. Keen HI, Wakefield RJ, Grainger AJ, Hensor EM, Emery P, Conaghan PG. Can ultrasonography improve on radiographic assessment in osteoarthritis of the hands? A comparison between radiographic and ultrasonographic detected pathology. Ann Rheum Dis. 2008;67(8):1116-1120.
48. Keen HI, Wakefield RJ, Hensor EM, Emery P, Conaghan PG. Response of symptoms
and synovitis to intra-muscular methylprednisolone in osteoarthritis of the hand: an ultrasonographic study. Rheumatology (Oxford). 2010;49(6):1093-1100.
49. Grassi W, Meenagh G, Pascual E, Filippucci E. “Crystal clear”-sonographic assessment of gout and calcium pyrophosphate deposition disease. Semin Arthritis Rheum. 2006;36(3):197-202.
50. Ciapetti A, Filippucci E, Gutierrez M, Grassi W. Calcium pyrophosphate dihydrate crystal deposition disease: sonographic findings. Clin Rheumatol. 2009;28(3):271-276.
51. Taylor WJ, Fransen J, Jansen TL, et al. Study for Updated Gout Classification Criteria (SUGAR): identification of features to classify gout. Arthritis Care Res (Hoboken). [Published online ahead of print March 16, 2015.]
52. Puig JG, de Miguel E, Castillo MC, Rocha AL, Martinez MA, Torres RJ. Asymptomatic hyperuricemia: impact of ultrasonography. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):592-595.
53. Pineda C, Amezcua-Guerra LM, Solano C, et al. Joint and tendon subclinical involvement suggestive of gouty arthritis in asymptomatic hyperuricemia: an ultrasound controlled study. Arthritis Res Ther. 2011;13(1):R4.
54. Naredo E, Uson J, Jiménez-Palop M, et al. Ultrasound-detected musculoskeletal urate crystal deposition: which joints and what findings should be assessed for diagnosing gout? Ann Rheum Dis. 2014;73(8):1522-1528.
55. De Miguel E, Puig JG, Castillo C, Peiteado D, Torres RJ, Martín-Mola E. Diagnosis of gout in patients with asymptomatic hyperuricaemia: a pilot ultrasound study. Ann Rheum Dis. 2012;71(1):157-158.
56. Perez-Ruiz F, Martin I, Canteli B. Ultrasonographic measurement of tophi as an outcome measure for chronic gout. J Rheumatol. 2007;34(9):1888-1893.
57. Thiele RG, Schlesinger N. Ultrasonography shows disappearance of monosodium urate crystal deposition on hyaline cartilage after sustained normouricemia is achieved. Rheumatol Int. 2010;30(4):495-503.
58. Balint PV, Kane D, Hunter J, McInnes IB, Field M, Sturrock RD. Ultrasound guided versus conventional joint and soft tissue fluid aspiration in rheumatology practice: a pilot study. J Rheumatol. 2002;29(10):2209-2213.
59. Raza K, Lee CY, Pilling D, et al. Ultrasound guidance allows accurate needle placement and aspiration from small joints in patients with early inflammatory arthritis. Rheumatology (Oxford). 2003;42(8):976-979.
60. Dubreuil M, Greger S, LaValley M, Cunnington J, Sibbitt WL Jr, Kissin EY. Improvement in wrist pain with ultrasound-guided glucocorticoid injections: a metaanalysis of individual patient data. Semin Arthritis Rheum. 2013;42(5):492-497.
61. Sage W, Pickup L, Smith TO, Denton ER, Toms AP. The clinical and functional outcomes of ultrasound-guided vs landmark-guided injections for adults with shoulder pathology—a systematic review and meta-analysis. Rheumatology (Oxford). 2013;52(4):743-751.
62. Darrieutort-Laffite C, Hamel O, Glémarec J, Maugars Y, Le Goff B. Ultrasonography of the lumbar spine: sonoanatomy and practical applications. Joint Bone Spine. 2014;81(2):130-136.
1. Naredo E, D’Agostino MA, Conaghan PG, et al. Current state of musculoskeletal ultrasound training and implementation in Europe: results of a survey of experts and scientific societies. Rheumatology (Oxford). 2010;49(12):2438-2943.
2. Micu MC, Alcalde M, Sáenz JI, et al. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res (Hoboken). 2013;65(4):615-621.
3. Koski JM. Ultrasound guided injections in rheumatology. J Rheumatol. 2000;27(9):2131-2138.
4. Kelly S, Humby F, Filer A, et al. Ultrasound-guided synovial biopsy: a safe, well-tolerated and reliable technique for obtaining high-quality synovial tissue from both large and small joints in early arthritis patients. Ann Rheum Dis. 2015;74(3):611-617.
5. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum Dis. 2010;69(9):1580-1588.
6. Zhang W, Doherty M, Bardin T, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis. 2011;70(4):563-570.
7. Dasgupta B, Cimmino MA, Kremers HM, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/ American College of Rheumatology collaborative initiative. Arthritis Rheum. 2012;64(4):943-954.
8. Fodor D, Nestorova R, Vlad V, Micu M. The place of musculoskeletal ultrasonography in gout diagnosis. Med Ultrason. 2014;16(4):336-344.
9. Takagi Y, Sumi M, Nakamura H, et al. Ultrasonography as an additional item in the American College of Rheumatology classification of Sjögren’s syndrome. Rheumatology (Oxford). 2014;53(11):1977-1983.
10. Colebatch AN, Edwards CJ, Østergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72(6):804-814.
11. American College of Rheumatology Musculoskeletal Ultrasound Task Force. Ultrasound in American rheumatology practice: report of the American College of Rheumatology musculoskeletal ultrasound task force. Arthritis Care Res (Hoboken). 2010;62(9):1206-1219.
12. McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res (Hoboken). 2012;64(11):1625-1640.
13. Naredo E, Möller I, Cruz A, Carmona L, Garrido J. Power Doppler ultrasonographic monitoring of response to anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58(8):2248-2256.
14. Newman JS, Laing TJ, McCarthy CJ, Adler RS. Power Doppler sonography of synovitis: assessment of therapeutic response—preliminary observations. Radiology. 1996;198(2):582-584.
15. Wakefield RJ, Balint PV, Szkudlarek M, et al; OMERACT 7 Special Interest Group. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32(12):2485-2487.
16. Wakefield RJ, Green MJ, Marzo-Ortega H, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis. 2004;63(4):382-385.
17. Brown AK, Quinn MA, Karim Z, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54(12):3761-3773.
18. Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58(10):2958-2967.
19. Bruyn GA, Hanova P, Iagnocco A, et al; OMERACT Ultrasound Task Force. Ultrasound definition of tendon damage in patients with rheumatoid arthritis. Results of a OMERACT consensus-based ultrasound score focusing on the diagnostic reliability. Ann Rheum Dis. 2014;73(11):1929-1934.
20. Filippucci E, Gabba A, Di Geso L, Girolimetti R, Salaffi F, Grassi W. Hand tendon involvement in rheumatoid arthritis: an ultrasound study. Semin Arthritis Rheum. 2012;41(6):752-760.
21. Lillegraven S, Bøyesen P, Hammer HB, et al. Tenosynovitis of the extensor carpi ulnaris tendon predicts erosive progression in early rheumatoid arthritis. Ann Rheum Dis. 2011;70(11):2049-2050.
22. Baillet A, Gaujoux-Viala C, Mouterde G, et al. Comparison of the efficacy of sonography, magnetic resonance imaging and conventional radiography for the detection of bone erosions in rheumatoid arthritis patients: a systematic review and meta-analysis. Rheumatology (Oxford). 2011;50(6):1137-1147.
23. Filer A, de Pablo P, Allen G, et al. Utility of ultrasound joint counts in the prediction of rheumatoid arthritis in patients with very early synovitis. Ann Rheum Dis. 2011;70(3):500-507.
24. Frediani B, Falsetti P, Storri L, et al. Quadricepital tendon enthesitis in psoriatic arthritis and rheumatoid arthritis: ultrasound examinations and clinical correlations. J Rheumatol. 2001;28(11):2566-2568.
25. D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48(2):523-533.
26. Gisondi P, Tinazzi I, El-Dalati G, et al. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: a hospital-based case-control study. Ann Rheum Dis. 2008;67(1):26-30.
27. Gutierrez M, Filippucci E, De Angelis R, et al. Subclinical entheseal involvement
in patients with psoriasis: an ultrasound study. Semin Arthritis Rheum. 2011;40(5):407-412.
28. Weiner SM, Jurenz S, Uhl M, et al. Ultrasonography in the assessment of peripheral joint involvement in psoriatic arthritis: a comparison with radiography, MRI and scintigraphy. Clin Rheumatol. 2008;27(8):983-989.
29. Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61(10):905-910.
30. Gutierrez M, Filippucci E, Salaffi F, Di Geso L, Grassi W. Differential diagnosis
between rheumatoid arthritis and psoriatic arthritis: the value of ultrasound findings at metacarpophalangeal joints level. Ann Rheum Dis. 2011;70(6):1111-1114.
31. De Miguel E, Cobo T, Muñoz-Fernández S, et al. Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis. 2009;68(2):169-174.
32. Ash ZR, Tinazzi I, Gallego CC, et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis. 2012;71(4):553-556.
33. Naredo E, Möller I, de Miguel E, et al; Ultrasound School of the Spanish Society of Rheumatology and Spanish ECO-APs Group. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology (Oxford). 2011;50(10):1838-1848.
34. Muñoz-Fernández S, de Miguel E, Cobo-Ibáñez T, et al. Enthesis inflammation in recurrent acute anterior uveitis without spondylarthritis. Arthritis Rheum. 2009;60(7):1985-1990.
35. Bandinelli F, Milla M, Genise S, et al. Ultrasound discloses entheseal involvement
in inactive and low active inflammatory bowel disease without clinical signs and symptoms of spondyloarthropathy. Rheumatology (Oxford). 2011;50(7):1275-1279.
36. D’Agostino MA, Aegerter P, Bechara K, et al. How to diagnose spondyloarthritis early? Accuracy of peripheral enthesitis detection by power Doppler ultrasonography. Ann Rheum Dis. 2011;70(8):1433-1440.
37. Aydin SZ, Karadag O, Filippucci E, et al. Monitoring Achilles enthesitis in ankylosing spondylitis during TNF-alpha antagonist therapy: an ultrasound study. Rheumatology (Oxford). 2010;49(3):578-582.
38. Naredo E, Batlle-Gualda E, Garcia-Vivar ML, et al; Ultrasound Group of the Spanish Society of Rheumatology. Power Doppler ultrasonography assessment of entheses in spondyloarthropathies: response to therapy of entheseal abnormalities. J Rheumatol. 2010;37(10):2110-2117.
39. Gutierrez M, Di Geso L, Salaffi F, et al. Development of a preliminary US power Doppler composite score for monitoring treatment in PsA. Rheumatology (Oxford). 2012;51(7):1261-1268.
40. Gutierrez M, De Angelis R, Bernardini ML, et al. Clinical, power Doppler sonography and histological assessment of the psoriatic plaque: short-term monitoring in patients treated with etanercept. Br J Dermatol. 2011;164(1):33-37.
41. Gutierrez M, Filippucci E, Bertolazzi C, Grassi W. Sonographic monitoring of psoriatic plaque. J Rheumatol. 2009;36(4):850-851.
42. Keen HI, Wakefield RJ, Grainger AJ, Hensor EM, Emery P, Conaghan PG. An ultrasonographic study of osteoarthritis of the hand: synovitis and its relationship to structural pathology and symptoms. Arthritis Rheum. 2008;59(12):1756-1763.
43. Kortekaas MC, Kwok WY, Reijnierse M, Watt I, Huizinga TW, Kloppenburg M. Pain in hand osteoarthritis is associated with inflammation: the value of ultrasound. Ann Rheum Dis. 2010;69(7):1367-1369.
44. Mancarella L, Magnani M, Addimanda O, Pignotti E, Galletti S, Meliconi R. Ultrasound-detected synovitis with power Doppler signal is associated with severe radiographic damage and reduced cartilage thickness in hand osteoarthritis. Osteoarthritis Cartilage. 2010;18(10):1263-1268.
45. Möller I, Bong D, Naredo E, et al. Ultrasound in the study and monitoring of osteoarthritis. Osteoarthritis Cartilage. 2008;16(suppl 3):S4-S7.
46. Vlychou M, Koutroumpas A, Alexiou I, Fezoulidis I, Sakkas LI. High-resolution ultrasonography and 3.0 T magnetic resonance imaging in erosive and nodal hand osteoarthritis: high frequency of erosions in nodal osteoarthritis. Clin Rheumatol. 2013;32(6):755-762.
47. Keen HI, Wakefield RJ, Grainger AJ, Hensor EM, Emery P, Conaghan PG. Can ultrasonography improve on radiographic assessment in osteoarthritis of the hands? A comparison between radiographic and ultrasonographic detected pathology. Ann Rheum Dis. 2008;67(8):1116-1120.
48. Keen HI, Wakefield RJ, Hensor EM, Emery P, Conaghan PG. Response of symptoms
and synovitis to intra-muscular methylprednisolone in osteoarthritis of the hand: an ultrasonographic study. Rheumatology (Oxford). 2010;49(6):1093-1100.
49. Grassi W, Meenagh G, Pascual E, Filippucci E. “Crystal clear”-sonographic assessment of gout and calcium pyrophosphate deposition disease. Semin Arthritis Rheum. 2006;36(3):197-202.
50. Ciapetti A, Filippucci E, Gutierrez M, Grassi W. Calcium pyrophosphate dihydrate crystal deposition disease: sonographic findings. Clin Rheumatol. 2009;28(3):271-276.
51. Taylor WJ, Fransen J, Jansen TL, et al. Study for Updated Gout Classification Criteria (SUGAR): identification of features to classify gout. Arthritis Care Res (Hoboken). [Published online ahead of print March 16, 2015.]
52. Puig JG, de Miguel E, Castillo MC, Rocha AL, Martinez MA, Torres RJ. Asymptomatic hyperuricemia: impact of ultrasonography. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):592-595.
53. Pineda C, Amezcua-Guerra LM, Solano C, et al. Joint and tendon subclinical involvement suggestive of gouty arthritis in asymptomatic hyperuricemia: an ultrasound controlled study. Arthritis Res Ther. 2011;13(1):R4.
54. Naredo E, Uson J, Jiménez-Palop M, et al. Ultrasound-detected musculoskeletal urate crystal deposition: which joints and what findings should be assessed for diagnosing gout? Ann Rheum Dis. 2014;73(8):1522-1528.
55. De Miguel E, Puig JG, Castillo C, Peiteado D, Torres RJ, Martín-Mola E. Diagnosis of gout in patients with asymptomatic hyperuricaemia: a pilot ultrasound study. Ann Rheum Dis. 2012;71(1):157-158.
56. Perez-Ruiz F, Martin I, Canteli B. Ultrasonographic measurement of tophi as an outcome measure for chronic gout. J Rheumatol. 2007;34(9):1888-1893.
57. Thiele RG, Schlesinger N. Ultrasonography shows disappearance of monosodium urate crystal deposition on hyaline cartilage after sustained normouricemia is achieved. Rheumatol Int. 2010;30(4):495-503.
58. Balint PV, Kane D, Hunter J, McInnes IB, Field M, Sturrock RD. Ultrasound guided versus conventional joint and soft tissue fluid aspiration in rheumatology practice: a pilot study. J Rheumatol. 2002;29(10):2209-2213.
59. Raza K, Lee CY, Pilling D, et al. Ultrasound guidance allows accurate needle placement and aspiration from small joints in patients with early inflammatory arthritis. Rheumatology (Oxford). 2003;42(8):976-979.
60. Dubreuil M, Greger S, LaValley M, Cunnington J, Sibbitt WL Jr, Kissin EY. Improvement in wrist pain with ultrasound-guided glucocorticoid injections: a metaanalysis of individual patient data. Semin Arthritis Rheum. 2013;42(5):492-497.
61. Sage W, Pickup L, Smith TO, Denton ER, Toms AP. The clinical and functional outcomes of ultrasound-guided vs landmark-guided injections for adults with shoulder pathology—a systematic review and meta-analysis. Rheumatology (Oxford). 2013;52(4):743-751.
62. Darrieutort-Laffite C, Hamel O, Glémarec J, Maugars Y, Le Goff B. Ultrasonography of the lumbar spine: sonoanatomy and practical applications. Joint Bone Spine. 2014;81(2):130-136.
Calcium-Containing Crystal-Associated Arthropathies in the Elderly
Calcium pyrophosphate (CPP) crystals may deposit in both articular tissues (predominantly hyaline cartilage and fibrocartilage) and periarticular soft tissues.1,2 Calcium pyrophosphate deposition disease (CPPD) may be asymptomatic or be associated with a spectrum of clinical syndromes, including both acute and chronic inflammatory arthritis.2
The European League Against Rheumatism (EULAR) recently suggested changes in CPPD terminology.2 According to the new EULAR classification, pseudogout, or CPPD, has been reclassified based on new key terms that include several of the previously described disease phenotypes: asymptomatic CPPD; acute CPP crystal arthritis (previously known as pseudogout); osteoarthritis (OA) with CPPD (previously, pseudo-OA); and the chronic CCP crystal inflammatory arthritis (previously, pseudorheumatoid arthritis). In similar fashion, chondrocalcinosis (CC) refers to calcification of the fibrocartilage and/or hyaline cartilage identified by imaging or histologic analysis. Although CC is most commonly seen in CPPD, it is not exclusive to this disease, as it can be seen in other crystal diseases (oxalosis, basic calcium phosphate [BCP]) and can appear as casual finding or coexist with OA.2
Clinical Manifestations
In clinical practice, CPPD may present with several phenotypic forms. In asymptomatic CPPD, CC is a common radiographic finding without clinical symptoms. Acute CPP arthritis always should be suspected in any patient aged > 65 years presenting with acute monoarticular or oligoarticular, migratory or additive, symmetrical, or polyarticular arthritis.3 Acute CCP arthritis is characterized by self-limited acute or subacute attacks of arthritis involving 1 or several extremity joints (knees, wrists, ankles; rarely affects large toe). Typically, the acute attacks last 7 to 10 days. Several unusual sites (eg, the hip joints, trochanteric bursa, and deep spinal joints) also may be affected. However, differences in pattern of joint involvement are insufficient to permit definitive diagnosis without demonstration of the specific crystal type in the inflammatory joint fluid.
Pseudogout attacks closely resemble gouty arthritis; CPPD presents as intermittent flares and often is asymptomatic between flares. Trauma, surgery, or severe medical illness frequently provokes attacks of monosodium urate (MSU) as well as acute CPP arthritis. Systemic findings, such as fever; leukocytosis with a left shift in the differential count; inflammatory markers, such as elevated sedimentation rate (ESR); or C-reactive protein, also can occur, resembling pyogenic arthritis, osteomyelitis, and/or systemic sepsis in the elderly patient.
Diagnosis must be confirmed with aspiration, Gram stain and cultures of the synovial fluid, and evaluation for the presence of CPP crystals under polarized light microscopy.2 The diagnosis can be difficult to confirm secondary to the weakly birefringent nature of CPP crystals.4 Coexistence of MSU and CPP crystals in a single inflammatory effusion is neither uncommon nor unexplained given increased frequencies of both hyperuricemia/gout and CC among elderly patients.5
Chronic CPP crystal inflammatory arthritis may present as a chronic, symmetrical, bilateral, and deforming polyarthritis. It frequently affects the wrists and metacarpophalangeal joints and tendon sheaths. Chronic CPP may resemble rheumatoid arthritis (RA) and produce wrist tenosynovitis, which may manifest as carpal tunnel syndrome and/or cubital tunnel syndrome. Calcium pyrophosphate deposition disease should be on the differential diagnosis in the elderly patient presenting with a clinical picture that resembles “seronegative” RA, with morning stiffness, synovial thickening, localized edema, and restricted motion due to active inflammation or flexion contracture of the hands/wrist. It may present with prominent systemic features, such as leukocytosis, fevers, mental confusion, and inflammatory oligoarthritis or polyarthritis. The diagnosis of CPPD still may be possible even though the rheumatoid factor (RF) is positive, given the increasing likelihood of elevated RF in the older population. In this setting, aspiration of joint fluid and radiography will assist in clarification of the diagnosis. Furthermore, CPPD typically does not cause the type of erosive disease that is often seen in RA.
Calcium pyrophosphate deposition disease also can mimic polymyalgia rheumatica (PMR). A direct comparison of a cohort of patients with pseudo-PMR (PMR/CPPD) with actual PMR patients found that increased age at diagnosis, presence of knee osteoarthritis, tendinous calcifications, and ankle arthritis carried the highest predictive value in patients with CPPD presenting with PMR-like symptoms.6 However, the PMR/CPPD variant can be difficult to distinguish, because both conditions can have elevated systemic inflammatory markers, and both are steroid responsive.
Calcium pyrophosphate deposition disease involving a single joint can rarely lead to extensive destruction—as with neuropathic joints in the absence of any neurologic deficits—and is extremely debilitating. This presentation is not well understood and does not have good treatment alternatives. Calcium pyrophosphate crystals often are associated with manifestations of OA.1,2 Indeed, up to 20% of OA joints have been found to be positive for CPP crystals in various studies. Given the extensive evidence supporting treatment of OA, usually they are treated in a similar fashion with good results. Occasionally, these will have unusual manifestations for typical OA, such as involvement of wrists and metacarpophalangeal joints; however, the presentation is often indolent like OA.
Calcium pyrophosphate crystal deposition involving the spine has been associated with a number of clinical manifestations. Spine stiffness, sometimes associated with bony ankylosis, can resemble ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis. Such symptoms are seen more commonly in familial CPPD rather than in the elderly. However, crystal deposition in the ligamentum flavum at the cervical spine levels has been associated with a condition called crowned dens syndrome.7 Although mostly asymptomatic, it may be present with acute neck pain, fever, and an increased ESR, sometimes mimicking PMR or giant cell arteritis or neurologic symptoms. Similarly, CPP crystal deposition in the posterior longitudinal ligament at the lower levels of the spine may lead to spinal cord compression syndromes or symptoms of either acute nerve compression or chronic spinal stenosis.8,9 Calcium pyrophosphate crystal deposition also can occur in other soft tissues, such as bursae, ligaments, and tendons and may be sufficient to cause local nerve compression, such as carpal or cubital tunnel syndrome.
Epidemiology
Radiographic surveys of the knees, hands, wrists, and pelvis and epidemiologic studies have demonstrated an age-related increase in the prevalence of CPPD: 15% prevalence in patients aged 65 to 74 years, 36% prevalence in patients aged 75 to 84 years, and 50% prevalence in patients aged > 84 years.10 In a recent radiographic study, 40% of patients with CPPD did not present with CC of the knee, and the study’s authors recommended additional radiographs of pelvis, wrists, or hands for accurate diagnosis of radiographic CC.11
Diagnosis
Accurate diagnosis should be achieved on the basis of the clinical picture and demonstration of CPP crystals in synovial fluid or tissue by compensated polarized light microscopy (Figures 1A and 1B).2 The sensitivity and specificity for CPP crystal detection has been shown to be 95.9% and 86.5%, respectively.12 However, the CPP crystal is more readily identified by a rheumatologist rather than in a standard hospital laboratory, which misses 30% of CPP crystals.13
Findings of CC on radiograph strengthens a CPPD diagnosis, but its absence does not rule it out (Figure 2A).2 More recently, the use of new imaging modalities, such as musculoskeletal ultrasound, provides the capacity to visualize crystal deposits within the joint structures, the hyaline cartilage, and/or fibrocartilage (Figure 2B and 2C).14 The presence of hyperechoic bands within the intermediate layer hyaline cartilage and hyperechoic spots in fibrocartilage are consistent with CPP crystal deposits.2,14 The use of computed tomography is the gold standard imaging modality for the identification of CPPD of the spine.15 There is not enough evidence to support the use of magnetic resonance imaging in CPPD, but it may play a role in rare complications.2
Treatment
The EULAR recently defined new guidelines for the management of CPPD.16 Asymptomatic CPPD needs no treatment.In other CPPD phenotypes, the goals are to attempt prompt resolution of the acute synovitis, reduction in chronic damage, and management of associated conditions.In acute attacks, treatment modalities used in gout are often required; however, data for CPPD treatment are limited (Table). Treatment relies on the use of colchicine and nonsteroidal anti-inflammatory drugs (NSAIDs), but toxicity and comorbidities in the elderly limit the usage of these drugs.
Given increased renal impairment, the loading dose of colchicine is not recommended.16 Colchicine has recently been shown to completely block crystal-induced maturation of IL-1β in vitro, indicating that the drug acts upstream of inflammasome activation.17 This is in addition to the well-known role of colchicine in inhibition of micro-tubule formation, which likely leads to prevention of cell migration, phagocytosis, and activation of inflammasome.18-20
Intra-articular injection of corticosteroid is an efficient and well-tolerated treatment alternative for monoarticular CPP flares. Oral or parenteral corticosteroids are frequently used for polyarticular flares in particular for those patients in which NSAIDs and colchicine are contraindicated.16 Parenteral adrenocorticotropic hormone has been used in patients with congestive heart failure, renal insufficiency, gastrointestinal bleeding, or resistance to NSAIDs.21 For prophylaxis of acute CPP crystal arthritis, a low dose of oral NSAIDs, oral colchicine, or prednisone may be used with good results.16 In chronic CPP arthritis, continuous use of colchicine, NSAIDs, or low-dose prednisone is often appropriate. If these interventions are ineffective or contraindicated, using hydroxychloroquine (HCQ) and methotrexate (MTX) have been successfully used to control chronic CPP crystal inflammation.22,23 Recent trials have raised questions about MTX, and further trials on HCQ usage are underway.24 Biologic agents targeting IL-1 are not currently approved for the treatment of CPPD, but there are suggestions that it may be effective in refractory cases and induce rapid stable remissions after 3 days of therapy.25
In contrast to gout, there is no specific target therapy for lowering CPP crystal load in the elderly. Crucial in the management of CPPD in the elderly is the search for associated diseases, such as hyperparathyroidism, hemochromatosis, hypomagnesemia, and hypophosphatemia, as well as avoidance of tacrolimus, which facilitates or causes CC.16 Correction of the underlying metabolic disorder, especially when undertaken early, may reduce the severity of CPPD. However, there is little evidence to suggest that treatment of associated disease results in resolution of CPPD—most famously, although therapeutic phlebotomy does not help in hemochromatosis for prevention of crystal disease, chelating agents do seem to be moderately effective.26 Only oral administration of magnesium has shown a reduction in meniscal CC in a patient with CPPD arthropathy.27 In addition, this was in the setting of familial hypomagnesemia associated CPPD. However, unlike uricosuric agents for gout, no pharmacologic treatments can prevent CPPD crystal formation and deposition in tissues.
Therapeutic Agents
Magnesium
Magnesium is a cofactor for the activity of pyrophosphatases that converts inorganic pyrophosphates (PPis) into orthophosphates. In addition, magnesium can increase the solubility of CPP crystals. Early detection and management of hypomagnesemia are recommended, because it occurs in patients who have well-defined conditions and situations: Gitelman syndrome, thiazide and loop diuretics use, tacrolimus use, familial forms of renal magnesium wasting or use of proton pump inhibitors, short bowel syndrome, and intestinal failure in patients receiving home parenteral nutrition. Long-term administration of magnesium in some patients with chronic hypomagnesemia decreased meniscal calcification.27-29
Dietary Calcium
Epidemiologic studies showed a lower incidence of CC in Chinese subjects. The authors of the study speculate that this lower prevalence of CPPD could result from high levels of calcium found in the drinking water in Beijing, which may affect parathyroid hormone secretion.30 Further studies are needed to confirm this hypothesis, as it could be a cheaper approach to pseudogout prevention.
Probenecid
Probenecid is an in vitro inhibitor of the transmembrane PPi transporter thought to possibly prevent extracellular PPi elaboration. However, this observation has not been confirmed by either case reports or clinical trials.31
Phosphocitrate
Phosphocitrate acts directly on preventing crystal deposition in tissues in CPPD as well as BCP based on in vitro evidence and mouse models.32,33
Hyaluronan
An amelioration of pain and increased range of motion were observed in radiographic CC with OA.34 However, it is associated with increased acute CPP arthritis.35
Radiosynovectomy
In a double-blind study of 15 patients with symmetrical CPPD arthropathy, the knee that underwent intra-articular injection of yttrium-90 (5 mCi) plus steroid had less pain, stiffness, joint line tenderness, and effusion compared with the contralateral control knee injected with saline and steroids.36
Precipitators of Acute Pseudogout
Diuretics are known to exacerbate gout, but they also can exacerbate pseudogout. A recent case-control study nested within a United Kingdom general practice database found that loop diuretics rather than hydrochlorothiazide was associated with increased risk of CPPD mediated primarily by magnesium reabsorption in the loop of Henle.28 Chronic kidney disease associated with secondary and tertiary hyperparathyroidism increases calcium or PPi concentration, which leads to CPP-crystal deposition.
In addition, multiple case reports have described acute pseudogout caused by bisphosphonate administration for osteoporosis or Paget disease—more likely in the elderly population. Intravenous pamidronate, oral etidronate, and alendronate therapy have all been described in the elderly.37 The overall mechanism behind this link is not completely understood, but bisphosphonates are structurally similar to PPi. Pseudogout attacks also have been described in neutropenic patients undergoing treatment with granulocyte-colony stimulating factor.38 In addition to pharmaceutical exacerbation of pseudogout, surgical procedures and trauma can precipitate attacks. Joint lavage has been described to increase the incidence of pseudogout.39 It was hypothesized that joint lavage with fluid induced “crystal shedding” from CPPD crystals imbedded in the joint tissue. Patients who underwent meniscectomy of the knee 20 years ago had a 20% incidence of CC in the knee that was operated compared with 4% CC in the contralateral nonoperated knee.40 Overall, the surgery most linked with a pseudogout attack, however, is parathyroidectomy.41
Basic Calcium Phosphate Crystals
Basic calcium phosphate crystals are common but rarely diagnosed due to the cumbersome and expensive methods required to identify these crystals.42
Basic calcium phosphate and CPPD crystals may coexist in synovial fluid. Similar to CPPD, BCP crystal disease is often concurrent with OA and can cause calcification of articular cartilage. Basic calcium phosphate is more common than CPP crystals with occurrence of 30% to 50% in OA synovial fluid.42 Additionally, BCP crystal disease has been linked to increased severity of OA. Basic calcium phosphate crystals in knee joints were found to have radiographically more severe arthritis with larger effusions.44,45 Similarly, BCP crystals in OA synovial fluid correlated with higher Kellgreen-Lawrence grade scores by radiography.42,46
It is currently believed that BCP crystals are continuously formed in the extracellular matrix, and their deposition is actively prevented by PPi present in the matrix.47 Elevated PPi levels, on the other hand, favor the formation of CPP crystals.48 The clinical upshot seems to be that although CPP crystals are almost universally intra-articular and released by chondrocytes, BCD crystals and deposits are more frequently present in soft tissues.
Acute Calcific Tendinitis
Typically, this type of tendinitis involves the shoulder joint and is extra-articular. Common treatments help, including NSAIDs, intra-articular steroids, ice, and rest. In addition, high-energy extracorporeal shock wave therapy has been shown to be effective when used with conscious sedation.49,50 Needling or barbotage in association with lavage and steroid injections also is effective and has occasionally been shown to reduce the size of the calcium deposit as well, often in combination with IV drugs like ethylenediaminetetraacetic acid.51-53
Acute calcific periarthritis of the hand presents similar to gout or pseudogout, affecting the wrist, usually in postmenopausal women.54 Basic calcium phosphate crystals are aspirated from the joint, and periarticular crystals may be subtle. Local steroid injections are beneficial.Milwaukee shoulder syndrome is an arthropathy associated with BCP crystals in the joint fluid and results in extensive destruction of shoulder articular cartilage and surrounding tissues. It is commonly bilateral and occurs in elderly women more often than it does in men.55 Aspiration of the shoulder joint typically reveals a serosanginous fluid. Fluid samples can be assessed for hydroxyapatite crystals by staining with alizarin red dye, which produces a characteristic “halo” or orange-red stain by light microscopy.43 Surgical treatment of Milwaukee shoulder syndrome is difficult due to increased age of the population affected and the severity of the shoulder destruction. Usually a conservative approach of analgesics, recurrent shoulder aspirations, and steroid injections is the best treatment option.
Conclusions
Calcium-containing crystal-associated arthropathies are a complex array of entities that target the veteran elderly population with increasing frequency. Challenges still remain in the diagnosis, crystal identification, and treatment due to coexisting comorbid conditions and polypharmacy commonly seen in veterans. Overall morbidity associated with calcium-containing crystal-associated arthropathies and the coexisting osteoarthritis is great, and focused identification of the disease process with tailored treatment can achieve the goal of decreasing symptoms and improving quality of life.
Acknowledgements
This work was supported by grant P20GM104937 (A.M.R.).
1. Guerne PA, Terkeltaub R. Clinical Features, Diagnosis, and Treatment of CPPD Crystal Arthropathy. In: Terkeltaub R, ed. Gout and Other Crystal Arthropathies. Philadelphia, PA: Saunders/Elsevier; 2012:249-265.
2. Zhang W, Doherty M, Bardin T, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis. 2011;70(4):563-570.
3. McCarty DJ. Calcium pyrophosphate dihydrate crystal deposition disease—1975. Arthritis Rheum. 1976;19(S3):275-285.
4. Ivorra J, Rosas J, Pascual E. Most calcium pyrophosphate crystals appear as non-birefringent. Ann Rheum Dis. 1999;58(9):582-584.
5. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
6. Pego-Reigosa JM, Rodriguez-Rodriguez M, Hurtado-Hernandez Z, et al. Calcium pyrophosphate deposition disease mimicking polymyalgia rheumatica: a prospective followup study of predictive factors for this condition in patients presenting with polymyalgia symptoms. Arthritis Rheum. 2005;53(6):931-938.
7. Bouvet JP, le Parc JM, Michalski B, Benlahrache C, Auquier L. Acute neck pain due to calcifications surrounding the odontoid process: the crowned dens syndrome. Arthritis Rheum. 1985;28(12):1417-1420.
8. Muthukumar N, Karuppaswamy U. Tumoral calcium pyrophosphate dihydrate deposition disease of the ligamentum flavum. Neurosurgery. 2003;53(1):103-109.
9. Armas JB, Couto AR, Bettencourt BF. Spondyloarthritis, diffuse idiopathic skeletal hyperostosis (DISH) and chondrocalcinosis. Adv in Exp Med Biol. 2009;649:37-56.
10. Abhishek A, Doherty M. Epidemiology of calcium pyrophosphate crystal arthritis and basic calcium phosphate crystal arthropathy. Rheum Dis Clin North Am. 2014;40(2):177-191.
11. Abhishek A, Doherty S, Maciewicz R, Muir K, Zhang W, Doherty M. Chondrocalcinosis is common in the absence of knee involvement. Arthritis Res Ther. 2012;14(5):R205.
12. Lumbreras B, Pascual E, Frasquet J, González-Salinas J, Rodríguez E, Hernández-Aguado I. Analysis for crystals in synovial fluid: training of the analysts results in high consistency. Ann Rheum Dis. 2005;64(4):612-615.
13. Szscygiel J, Reginato AM SS. Quality improvements in the identification of crystals from synovial fluid: hospital laboratory versus rheumatology department evaluation. Poster presented at: 2014 ACR/ARHP Annual Meeting; November 15, 2014; Boston, MA.
14. Grassi W, Meenagh G, Pascual E, Filippucci E. “Crystal clear”-sonographic assessment of gout and calcium pyrophosphate deposition disease. Semin Arthritis Rheum. 2006;36(3):197-202.
15. Scutellari PN, Galeotti R, Leprotti S, Ridolfi M, Franciosi R, Antinolfi G. The crowned dens syndrome. Evaluation with CT imaging. Radiol Med. 2007;112(2):195-207.
16. Zhang W, Doherty M, Pascual E, et al. EULAR recommendations for calcium pyrophosphate deposition. Part II: management. Ann Rheum Dis. 2011;70(4):571-575.
17. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237-241.
18. Nuki G. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep. 2008;10(3):218-227.
19. Borisy GG, Taylor EW. The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol. 1967;34(2):535-548.
20. Borisy GG, Taylor EW. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967;34(2):525-533.
21. Daoussis D, Antonopoulos I, Andonopoulos AP. ACTH as a treatment for acute crystal-induced arthritis: update on clinical evidence and mechanisms of action. Semin Arthritis Rheum. 2014;43(5):648-653.
22. Rothschild B, Yakubov LE. Prospective 6-month, double-blind trial of hydroxychloroquine treatment of CPDD. Compr Ther. 1997;23(5):327-331.
23. Chollet-Janin A, Finckh A, Dudler J, Guerne PA. Methotrexate as an alternative therapy for chronic calcium pyrophosphate deposition disease: an exploratory analysis. Arthritis Rheum. 2007;56(2):688-692.
24. Finckh A, Mc Carthy GM, Madigan A, et al. Methotrexate in chronic-recurrent calcium pyrophosphate deposition disease: no significant effect in a randomized crossover trial. Arthritis Res Ther. 2014;16(5):458.
25. Moltó A, Ea HK, Richette P, Bardin T, Lioté F. Efficacy of anakinra for refractory acute calcium pyrophosphate crystal arthritis. Joint Bone Spine. 2012;79(6):621-623.
26. Harty LC, Lai D, Connor S, et al. Prevalence and progress of joint symptoms in hereditary hemochromatosis and symptomatic response to venesection. J Clin Rheumatol. 2011;17(4):220-222.
27. Doherty M, Dieppe PA. Double blind, placebo controlled trial of magnesium carbonate in chronic pyrophosphate arthropathy. Ann Rheum Dis. 1983;42(suppl 1):106-107.
28. Rho YH, Zhu Y, Zhang Y, Reginato AM, Choi HK. Risk factors for pseudogout in the general population. Rheumatology (Oxford). 2012;51(11):2070-2074.
29. Park CH, Kim EH, Roh YH, Kim HY, Lee SK. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112558.
30. Zhang Y, Terkeltaub R, Nevitt M, et al. Lower prevalence of chondrocalcinosis in Chinese subjects in Beijing than in white subjects in the United States: the Beijing Osteoarthritis Study. Arthritis Rheum. 2006;54(11):3508-3512.
31. Rosenthal AK, Ryan LM. Probenecid inhibits transforming growth factor-beta 1 induced pyrophosphate elaboration by chondrocytes. J Rheumatol. 1994;21(5):896-900.
32. Cheung HS, Sallis JD, Demadis KD, Wierzbicki A. Phosphocitrate blocks calcification-induced articular joint degeneration in a guinea pig model. Arthritis Rheum. 2006;54(8):2452-2461.
33. Sun Y, Mauerhan DR, Honeycutt PR, et al. Calcium deposition in osteoarthritic meniscus and meniscal cell culture. Arthritis Res Ther. 2010;12(2):R56.
34. Daumen-Legre V, Pham T, Acquaviva PC, Lafforgue P. Evaluation of safety and efficacy of viscosupplementation in knee osteoarthritis with chondrocalcinosis. In: Arthritis and Rheumatism.Vol. 42. Lippincott Williams and Wilkins; 1999:S158-S158.
35. Disla E, Infante R, Fahmy A, Karten I, Cuppari GG. Recurrent acute calcium pyrophosphate dihydrate arthritis following intraarticular hyaluronate injection. Arthritis Rheum. 1999;42(6):1302-1303.
36. Doherty M, Dieppe PA. Effect of intra-articularYttrium-90 on chronic pyrophosphate arthropathy of the knee. Lancet. 1981;2(8258):1243-1246.
37. Wendling D, Tisserand G, Griffond V, Saccomani C, Toussirot E. Acute pseudogout after pamidronate infusion. Clin Rheumatol. 2008;27(9):1205-1206.
38. Ames PRJ, Rainey MG. Consecutive pseudogout attacks after repetitive granulocyte colony-stimulating factor administration for neutropenia. Mod Rheumatol. 2007;17(5):445-446.
39. Pasquetti P, Selvi E, Righeschi K, et al. Joint lavage and pseudogout. Ann Rheum Dis. 2004;63(11):1529-1530.
40. Doherty M, Watt I, Dieppe P. Localised chondrocalcinosis in post-meniscectomy knees. Lancet. 1982;1(8283):1207-1210.
41. Rubin MR, Silverberg SJ. Rheumatic manifestations of primary hyperparathyroidism and parathyroid hormone therapy. Curr Rheumatol Rep. 2002;4(2):179-185.
42. Ea HK, Lioté F. Diagnosis and clinical manifestations of calcium pyrophosphate and basic calcium phosphate crystal deposition diseases. Rheum Dis Clin North Am. 2014;40(2):207-229.
43. Paul H, Reginato AJ, Ralph Schumacher HR. Alizarin red s staining as a screening test to detect calcium compounds in synovial fluid. Arthritis Rheum. 1983;26(2):191-200.
44. Molloy ES, McCarthy GM. Basic calcium phosphate crystals: pathways to joint degeneration. Curr Opin Rheumatol. 2006;18(2):187-192.
45. Carroll GJ, Stuart RA, Armstrong JA, Breidahl PD, Laing BA. Hydroxyapatite crystals are a frequent finding in osteoarthritic synovial fluid, but are not related to increased concentrations of keratan sulfate or interleukin 1 beta. J Rheumatol. 1991;18(6):861-866.
46. Derfus BA, Kurian JB, Butler JJ, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29(3):570-574.
47. Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289(5477):265-270.
48. Macmullan P, McCarthy G. Treatment and management of pseudogout: insights for the clinician. Ther Adv Musculoskelet Dis. 2012;4(2):121-131.
49. Gerdesmeyer L, Wagenpfeil S, Haake M, et al. Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff: a randomized controlled trial. JAMA. 2003;290(19):2573-2580.
50. Lee SY, Cheng B, Grimmer-Somers K. The midterm effectiveness of extracorporeal shockwave therapy in the management of chronic calcific shoulder tendinitis. J Shoulder Elbow Surg. 2011;20(5):845-854.
51. Pfister J, Gerber H. Chronic calcifying tendinitis of the shoulder-therapy by percutaneous needle aspiration and lavage: a prospective open study of 62 shoulders. Clin Rheumatol. 1997;16(3):269-274.
52. del Cura JL, Torre I, Zabala R, Legórburu A. Sonographically guided percutaneous needle lavage in calcific tendinitis of the shoulder: short- and long-term results. AJR Am J Roentgenol. 2007;189(3):W128-W134.
53. Yoo JC, Koh KH, Park WH, Park JC, Kim SM, Yoon YC. The outcome of ultrasound-guided needle decompression and steroid injection in calcific tendinitis. J Shoulder Elbow Surg. 2010;19(4):596-600.
54. Wiper JD, Garrido A. Images in clinical medicine. Acute calcific tendinitis. N Engl J Med. 2008;359(23):2477.
55. Halverson PB, Carrera GF, McCarty DJ. Milwaukee shoulder syndrome. Arch Intern Med. 1990;150(3):677-682.
Calcium pyrophosphate (CPP) crystals may deposit in both articular tissues (predominantly hyaline cartilage and fibrocartilage) and periarticular soft tissues.1,2 Calcium pyrophosphate deposition disease (CPPD) may be asymptomatic or be associated with a spectrum of clinical syndromes, including both acute and chronic inflammatory arthritis.2
The European League Against Rheumatism (EULAR) recently suggested changes in CPPD terminology.2 According to the new EULAR classification, pseudogout, or CPPD, has been reclassified based on new key terms that include several of the previously described disease phenotypes: asymptomatic CPPD; acute CPP crystal arthritis (previously known as pseudogout); osteoarthritis (OA) with CPPD (previously, pseudo-OA); and the chronic CCP crystal inflammatory arthritis (previously, pseudorheumatoid arthritis). In similar fashion, chondrocalcinosis (CC) refers to calcification of the fibrocartilage and/or hyaline cartilage identified by imaging or histologic analysis. Although CC is most commonly seen in CPPD, it is not exclusive to this disease, as it can be seen in other crystal diseases (oxalosis, basic calcium phosphate [BCP]) and can appear as casual finding or coexist with OA.2
Clinical Manifestations
In clinical practice, CPPD may present with several phenotypic forms. In asymptomatic CPPD, CC is a common radiographic finding without clinical symptoms. Acute CPP arthritis always should be suspected in any patient aged > 65 years presenting with acute monoarticular or oligoarticular, migratory or additive, symmetrical, or polyarticular arthritis.3 Acute CCP arthritis is characterized by self-limited acute or subacute attacks of arthritis involving 1 or several extremity joints (knees, wrists, ankles; rarely affects large toe). Typically, the acute attacks last 7 to 10 days. Several unusual sites (eg, the hip joints, trochanteric bursa, and deep spinal joints) also may be affected. However, differences in pattern of joint involvement are insufficient to permit definitive diagnosis without demonstration of the specific crystal type in the inflammatory joint fluid.
Pseudogout attacks closely resemble gouty arthritis; CPPD presents as intermittent flares and often is asymptomatic between flares. Trauma, surgery, or severe medical illness frequently provokes attacks of monosodium urate (MSU) as well as acute CPP arthritis. Systemic findings, such as fever; leukocytosis with a left shift in the differential count; inflammatory markers, such as elevated sedimentation rate (ESR); or C-reactive protein, also can occur, resembling pyogenic arthritis, osteomyelitis, and/or systemic sepsis in the elderly patient.
Diagnosis must be confirmed with aspiration, Gram stain and cultures of the synovial fluid, and evaluation for the presence of CPP crystals under polarized light microscopy.2 The diagnosis can be difficult to confirm secondary to the weakly birefringent nature of CPP crystals.4 Coexistence of MSU and CPP crystals in a single inflammatory effusion is neither uncommon nor unexplained given increased frequencies of both hyperuricemia/gout and CC among elderly patients.5
Chronic CPP crystal inflammatory arthritis may present as a chronic, symmetrical, bilateral, and deforming polyarthritis. It frequently affects the wrists and metacarpophalangeal joints and tendon sheaths. Chronic CPP may resemble rheumatoid arthritis (RA) and produce wrist tenosynovitis, which may manifest as carpal tunnel syndrome and/or cubital tunnel syndrome. Calcium pyrophosphate deposition disease should be on the differential diagnosis in the elderly patient presenting with a clinical picture that resembles “seronegative” RA, with morning stiffness, synovial thickening, localized edema, and restricted motion due to active inflammation or flexion contracture of the hands/wrist. It may present with prominent systemic features, such as leukocytosis, fevers, mental confusion, and inflammatory oligoarthritis or polyarthritis. The diagnosis of CPPD still may be possible even though the rheumatoid factor (RF) is positive, given the increasing likelihood of elevated RF in the older population. In this setting, aspiration of joint fluid and radiography will assist in clarification of the diagnosis. Furthermore, CPPD typically does not cause the type of erosive disease that is often seen in RA.
Calcium pyrophosphate deposition disease also can mimic polymyalgia rheumatica (PMR). A direct comparison of a cohort of patients with pseudo-PMR (PMR/CPPD) with actual PMR patients found that increased age at diagnosis, presence of knee osteoarthritis, tendinous calcifications, and ankle arthritis carried the highest predictive value in patients with CPPD presenting with PMR-like symptoms.6 However, the PMR/CPPD variant can be difficult to distinguish, because both conditions can have elevated systemic inflammatory markers, and both are steroid responsive.
Calcium pyrophosphate deposition disease involving a single joint can rarely lead to extensive destruction—as with neuropathic joints in the absence of any neurologic deficits—and is extremely debilitating. This presentation is not well understood and does not have good treatment alternatives. Calcium pyrophosphate crystals often are associated with manifestations of OA.1,2 Indeed, up to 20% of OA joints have been found to be positive for CPP crystals in various studies. Given the extensive evidence supporting treatment of OA, usually they are treated in a similar fashion with good results. Occasionally, these will have unusual manifestations for typical OA, such as involvement of wrists and metacarpophalangeal joints; however, the presentation is often indolent like OA.
Calcium pyrophosphate crystal deposition involving the spine has been associated with a number of clinical manifestations. Spine stiffness, sometimes associated with bony ankylosis, can resemble ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis. Such symptoms are seen more commonly in familial CPPD rather than in the elderly. However, crystal deposition in the ligamentum flavum at the cervical spine levels has been associated with a condition called crowned dens syndrome.7 Although mostly asymptomatic, it may be present with acute neck pain, fever, and an increased ESR, sometimes mimicking PMR or giant cell arteritis or neurologic symptoms. Similarly, CPP crystal deposition in the posterior longitudinal ligament at the lower levels of the spine may lead to spinal cord compression syndromes or symptoms of either acute nerve compression or chronic spinal stenosis.8,9 Calcium pyrophosphate crystal deposition also can occur in other soft tissues, such as bursae, ligaments, and tendons and may be sufficient to cause local nerve compression, such as carpal or cubital tunnel syndrome.
Epidemiology
Radiographic surveys of the knees, hands, wrists, and pelvis and epidemiologic studies have demonstrated an age-related increase in the prevalence of CPPD: 15% prevalence in patients aged 65 to 74 years, 36% prevalence in patients aged 75 to 84 years, and 50% prevalence in patients aged > 84 years.10 In a recent radiographic study, 40% of patients with CPPD did not present with CC of the knee, and the study’s authors recommended additional radiographs of pelvis, wrists, or hands for accurate diagnosis of radiographic CC.11
Diagnosis
Accurate diagnosis should be achieved on the basis of the clinical picture and demonstration of CPP crystals in synovial fluid or tissue by compensated polarized light microscopy (Figures 1A and 1B).2 The sensitivity and specificity for CPP crystal detection has been shown to be 95.9% and 86.5%, respectively.12 However, the CPP crystal is more readily identified by a rheumatologist rather than in a standard hospital laboratory, which misses 30% of CPP crystals.13
Findings of CC on radiograph strengthens a CPPD diagnosis, but its absence does not rule it out (Figure 2A).2 More recently, the use of new imaging modalities, such as musculoskeletal ultrasound, provides the capacity to visualize crystal deposits within the joint structures, the hyaline cartilage, and/or fibrocartilage (Figure 2B and 2C).14 The presence of hyperechoic bands within the intermediate layer hyaline cartilage and hyperechoic spots in fibrocartilage are consistent with CPP crystal deposits.2,14 The use of computed tomography is the gold standard imaging modality for the identification of CPPD of the spine.15 There is not enough evidence to support the use of magnetic resonance imaging in CPPD, but it may play a role in rare complications.2
Treatment
The EULAR recently defined new guidelines for the management of CPPD.16 Asymptomatic CPPD needs no treatment.In other CPPD phenotypes, the goals are to attempt prompt resolution of the acute synovitis, reduction in chronic damage, and management of associated conditions.In acute attacks, treatment modalities used in gout are often required; however, data for CPPD treatment are limited (Table). Treatment relies on the use of colchicine and nonsteroidal anti-inflammatory drugs (NSAIDs), but toxicity and comorbidities in the elderly limit the usage of these drugs.
Given increased renal impairment, the loading dose of colchicine is not recommended.16 Colchicine has recently been shown to completely block crystal-induced maturation of IL-1β in vitro, indicating that the drug acts upstream of inflammasome activation.17 This is in addition to the well-known role of colchicine in inhibition of micro-tubule formation, which likely leads to prevention of cell migration, phagocytosis, and activation of inflammasome.18-20
Intra-articular injection of corticosteroid is an efficient and well-tolerated treatment alternative for monoarticular CPP flares. Oral or parenteral corticosteroids are frequently used for polyarticular flares in particular for those patients in which NSAIDs and colchicine are contraindicated.16 Parenteral adrenocorticotropic hormone has been used in patients with congestive heart failure, renal insufficiency, gastrointestinal bleeding, or resistance to NSAIDs.21 For prophylaxis of acute CPP crystal arthritis, a low dose of oral NSAIDs, oral colchicine, or prednisone may be used with good results.16 In chronic CPP arthritis, continuous use of colchicine, NSAIDs, or low-dose prednisone is often appropriate. If these interventions are ineffective or contraindicated, using hydroxychloroquine (HCQ) and methotrexate (MTX) have been successfully used to control chronic CPP crystal inflammation.22,23 Recent trials have raised questions about MTX, and further trials on HCQ usage are underway.24 Biologic agents targeting IL-1 are not currently approved for the treatment of CPPD, but there are suggestions that it may be effective in refractory cases and induce rapid stable remissions after 3 days of therapy.25
In contrast to gout, there is no specific target therapy for lowering CPP crystal load in the elderly. Crucial in the management of CPPD in the elderly is the search for associated diseases, such as hyperparathyroidism, hemochromatosis, hypomagnesemia, and hypophosphatemia, as well as avoidance of tacrolimus, which facilitates or causes CC.16 Correction of the underlying metabolic disorder, especially when undertaken early, may reduce the severity of CPPD. However, there is little evidence to suggest that treatment of associated disease results in resolution of CPPD—most famously, although therapeutic phlebotomy does not help in hemochromatosis for prevention of crystal disease, chelating agents do seem to be moderately effective.26 Only oral administration of magnesium has shown a reduction in meniscal CC in a patient with CPPD arthropathy.27 In addition, this was in the setting of familial hypomagnesemia associated CPPD. However, unlike uricosuric agents for gout, no pharmacologic treatments can prevent CPPD crystal formation and deposition in tissues.
Therapeutic Agents
Magnesium
Magnesium is a cofactor for the activity of pyrophosphatases that converts inorganic pyrophosphates (PPis) into orthophosphates. In addition, magnesium can increase the solubility of CPP crystals. Early detection and management of hypomagnesemia are recommended, because it occurs in patients who have well-defined conditions and situations: Gitelman syndrome, thiazide and loop diuretics use, tacrolimus use, familial forms of renal magnesium wasting or use of proton pump inhibitors, short bowel syndrome, and intestinal failure in patients receiving home parenteral nutrition. Long-term administration of magnesium in some patients with chronic hypomagnesemia decreased meniscal calcification.27-29
Dietary Calcium
Epidemiologic studies showed a lower incidence of CC in Chinese subjects. The authors of the study speculate that this lower prevalence of CPPD could result from high levels of calcium found in the drinking water in Beijing, which may affect parathyroid hormone secretion.30 Further studies are needed to confirm this hypothesis, as it could be a cheaper approach to pseudogout prevention.
Probenecid
Probenecid is an in vitro inhibitor of the transmembrane PPi transporter thought to possibly prevent extracellular PPi elaboration. However, this observation has not been confirmed by either case reports or clinical trials.31
Phosphocitrate
Phosphocitrate acts directly on preventing crystal deposition in tissues in CPPD as well as BCP based on in vitro evidence and mouse models.32,33
Hyaluronan
An amelioration of pain and increased range of motion were observed in radiographic CC with OA.34 However, it is associated with increased acute CPP arthritis.35
Radiosynovectomy
In a double-blind study of 15 patients with symmetrical CPPD arthropathy, the knee that underwent intra-articular injection of yttrium-90 (5 mCi) plus steroid had less pain, stiffness, joint line tenderness, and effusion compared with the contralateral control knee injected with saline and steroids.36
Precipitators of Acute Pseudogout
Diuretics are known to exacerbate gout, but they also can exacerbate pseudogout. A recent case-control study nested within a United Kingdom general practice database found that loop diuretics rather than hydrochlorothiazide was associated with increased risk of CPPD mediated primarily by magnesium reabsorption in the loop of Henle.28 Chronic kidney disease associated with secondary and tertiary hyperparathyroidism increases calcium or PPi concentration, which leads to CPP-crystal deposition.
In addition, multiple case reports have described acute pseudogout caused by bisphosphonate administration for osteoporosis or Paget disease—more likely in the elderly population. Intravenous pamidronate, oral etidronate, and alendronate therapy have all been described in the elderly.37 The overall mechanism behind this link is not completely understood, but bisphosphonates are structurally similar to PPi. Pseudogout attacks also have been described in neutropenic patients undergoing treatment with granulocyte-colony stimulating factor.38 In addition to pharmaceutical exacerbation of pseudogout, surgical procedures and trauma can precipitate attacks. Joint lavage has been described to increase the incidence of pseudogout.39 It was hypothesized that joint lavage with fluid induced “crystal shedding” from CPPD crystals imbedded in the joint tissue. Patients who underwent meniscectomy of the knee 20 years ago had a 20% incidence of CC in the knee that was operated compared with 4% CC in the contralateral nonoperated knee.40 Overall, the surgery most linked with a pseudogout attack, however, is parathyroidectomy.41
Basic Calcium Phosphate Crystals
Basic calcium phosphate crystals are common but rarely diagnosed due to the cumbersome and expensive methods required to identify these crystals.42
Basic calcium phosphate and CPPD crystals may coexist in synovial fluid. Similar to CPPD, BCP crystal disease is often concurrent with OA and can cause calcification of articular cartilage. Basic calcium phosphate is more common than CPP crystals with occurrence of 30% to 50% in OA synovial fluid.42 Additionally, BCP crystal disease has been linked to increased severity of OA. Basic calcium phosphate crystals in knee joints were found to have radiographically more severe arthritis with larger effusions.44,45 Similarly, BCP crystals in OA synovial fluid correlated with higher Kellgreen-Lawrence grade scores by radiography.42,46
It is currently believed that BCP crystals are continuously formed in the extracellular matrix, and their deposition is actively prevented by PPi present in the matrix.47 Elevated PPi levels, on the other hand, favor the formation of CPP crystals.48 The clinical upshot seems to be that although CPP crystals are almost universally intra-articular and released by chondrocytes, BCD crystals and deposits are more frequently present in soft tissues.
Acute Calcific Tendinitis
Typically, this type of tendinitis involves the shoulder joint and is extra-articular. Common treatments help, including NSAIDs, intra-articular steroids, ice, and rest. In addition, high-energy extracorporeal shock wave therapy has been shown to be effective when used with conscious sedation.49,50 Needling or barbotage in association with lavage and steroid injections also is effective and has occasionally been shown to reduce the size of the calcium deposit as well, often in combination with IV drugs like ethylenediaminetetraacetic acid.51-53
Acute calcific periarthritis of the hand presents similar to gout or pseudogout, affecting the wrist, usually in postmenopausal women.54 Basic calcium phosphate crystals are aspirated from the joint, and periarticular crystals may be subtle. Local steroid injections are beneficial.Milwaukee shoulder syndrome is an arthropathy associated with BCP crystals in the joint fluid and results in extensive destruction of shoulder articular cartilage and surrounding tissues. It is commonly bilateral and occurs in elderly women more often than it does in men.55 Aspiration of the shoulder joint typically reveals a serosanginous fluid. Fluid samples can be assessed for hydroxyapatite crystals by staining with alizarin red dye, which produces a characteristic “halo” or orange-red stain by light microscopy.43 Surgical treatment of Milwaukee shoulder syndrome is difficult due to increased age of the population affected and the severity of the shoulder destruction. Usually a conservative approach of analgesics, recurrent shoulder aspirations, and steroid injections is the best treatment option.
Conclusions
Calcium-containing crystal-associated arthropathies are a complex array of entities that target the veteran elderly population with increasing frequency. Challenges still remain in the diagnosis, crystal identification, and treatment due to coexisting comorbid conditions and polypharmacy commonly seen in veterans. Overall morbidity associated with calcium-containing crystal-associated arthropathies and the coexisting osteoarthritis is great, and focused identification of the disease process with tailored treatment can achieve the goal of decreasing symptoms and improving quality of life.
Acknowledgements
This work was supported by grant P20GM104937 (A.M.R.).
Calcium pyrophosphate (CPP) crystals may deposit in both articular tissues (predominantly hyaline cartilage and fibrocartilage) and periarticular soft tissues.1,2 Calcium pyrophosphate deposition disease (CPPD) may be asymptomatic or be associated with a spectrum of clinical syndromes, including both acute and chronic inflammatory arthritis.2
The European League Against Rheumatism (EULAR) recently suggested changes in CPPD terminology.2 According to the new EULAR classification, pseudogout, or CPPD, has been reclassified based on new key terms that include several of the previously described disease phenotypes: asymptomatic CPPD; acute CPP crystal arthritis (previously known as pseudogout); osteoarthritis (OA) with CPPD (previously, pseudo-OA); and the chronic CCP crystal inflammatory arthritis (previously, pseudorheumatoid arthritis). In similar fashion, chondrocalcinosis (CC) refers to calcification of the fibrocartilage and/or hyaline cartilage identified by imaging or histologic analysis. Although CC is most commonly seen in CPPD, it is not exclusive to this disease, as it can be seen in other crystal diseases (oxalosis, basic calcium phosphate [BCP]) and can appear as casual finding or coexist with OA.2
Clinical Manifestations
In clinical practice, CPPD may present with several phenotypic forms. In asymptomatic CPPD, CC is a common radiographic finding without clinical symptoms. Acute CPP arthritis always should be suspected in any patient aged > 65 years presenting with acute monoarticular or oligoarticular, migratory or additive, symmetrical, or polyarticular arthritis.3 Acute CCP arthritis is characterized by self-limited acute or subacute attacks of arthritis involving 1 or several extremity joints (knees, wrists, ankles; rarely affects large toe). Typically, the acute attacks last 7 to 10 days. Several unusual sites (eg, the hip joints, trochanteric bursa, and deep spinal joints) also may be affected. However, differences in pattern of joint involvement are insufficient to permit definitive diagnosis without demonstration of the specific crystal type in the inflammatory joint fluid.
Pseudogout attacks closely resemble gouty arthritis; CPPD presents as intermittent flares and often is asymptomatic between flares. Trauma, surgery, or severe medical illness frequently provokes attacks of monosodium urate (MSU) as well as acute CPP arthritis. Systemic findings, such as fever; leukocytosis with a left shift in the differential count; inflammatory markers, such as elevated sedimentation rate (ESR); or C-reactive protein, also can occur, resembling pyogenic arthritis, osteomyelitis, and/or systemic sepsis in the elderly patient.
Diagnosis must be confirmed with aspiration, Gram stain and cultures of the synovial fluid, and evaluation for the presence of CPP crystals under polarized light microscopy.2 The diagnosis can be difficult to confirm secondary to the weakly birefringent nature of CPP crystals.4 Coexistence of MSU and CPP crystals in a single inflammatory effusion is neither uncommon nor unexplained given increased frequencies of both hyperuricemia/gout and CC among elderly patients.5
Chronic CPP crystal inflammatory arthritis may present as a chronic, symmetrical, bilateral, and deforming polyarthritis. It frequently affects the wrists and metacarpophalangeal joints and tendon sheaths. Chronic CPP may resemble rheumatoid arthritis (RA) and produce wrist tenosynovitis, which may manifest as carpal tunnel syndrome and/or cubital tunnel syndrome. Calcium pyrophosphate deposition disease should be on the differential diagnosis in the elderly patient presenting with a clinical picture that resembles “seronegative” RA, with morning stiffness, synovial thickening, localized edema, and restricted motion due to active inflammation or flexion contracture of the hands/wrist. It may present with prominent systemic features, such as leukocytosis, fevers, mental confusion, and inflammatory oligoarthritis or polyarthritis. The diagnosis of CPPD still may be possible even though the rheumatoid factor (RF) is positive, given the increasing likelihood of elevated RF in the older population. In this setting, aspiration of joint fluid and radiography will assist in clarification of the diagnosis. Furthermore, CPPD typically does not cause the type of erosive disease that is often seen in RA.
Calcium pyrophosphate deposition disease also can mimic polymyalgia rheumatica (PMR). A direct comparison of a cohort of patients with pseudo-PMR (PMR/CPPD) with actual PMR patients found that increased age at diagnosis, presence of knee osteoarthritis, tendinous calcifications, and ankle arthritis carried the highest predictive value in patients with CPPD presenting with PMR-like symptoms.6 However, the PMR/CPPD variant can be difficult to distinguish, because both conditions can have elevated systemic inflammatory markers, and both are steroid responsive.
Calcium pyrophosphate deposition disease involving a single joint can rarely lead to extensive destruction—as with neuropathic joints in the absence of any neurologic deficits—and is extremely debilitating. This presentation is not well understood and does not have good treatment alternatives. Calcium pyrophosphate crystals often are associated with manifestations of OA.1,2 Indeed, up to 20% of OA joints have been found to be positive for CPP crystals in various studies. Given the extensive evidence supporting treatment of OA, usually they are treated in a similar fashion with good results. Occasionally, these will have unusual manifestations for typical OA, such as involvement of wrists and metacarpophalangeal joints; however, the presentation is often indolent like OA.
Calcium pyrophosphate crystal deposition involving the spine has been associated with a number of clinical manifestations. Spine stiffness, sometimes associated with bony ankylosis, can resemble ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis. Such symptoms are seen more commonly in familial CPPD rather than in the elderly. However, crystal deposition in the ligamentum flavum at the cervical spine levels has been associated with a condition called crowned dens syndrome.7 Although mostly asymptomatic, it may be present with acute neck pain, fever, and an increased ESR, sometimes mimicking PMR or giant cell arteritis or neurologic symptoms. Similarly, CPP crystal deposition in the posterior longitudinal ligament at the lower levels of the spine may lead to spinal cord compression syndromes or symptoms of either acute nerve compression or chronic spinal stenosis.8,9 Calcium pyrophosphate crystal deposition also can occur in other soft tissues, such as bursae, ligaments, and tendons and may be sufficient to cause local nerve compression, such as carpal or cubital tunnel syndrome.
Epidemiology
Radiographic surveys of the knees, hands, wrists, and pelvis and epidemiologic studies have demonstrated an age-related increase in the prevalence of CPPD: 15% prevalence in patients aged 65 to 74 years, 36% prevalence in patients aged 75 to 84 years, and 50% prevalence in patients aged > 84 years.10 In a recent radiographic study, 40% of patients with CPPD did not present with CC of the knee, and the study’s authors recommended additional radiographs of pelvis, wrists, or hands for accurate diagnosis of radiographic CC.11
Diagnosis
Accurate diagnosis should be achieved on the basis of the clinical picture and demonstration of CPP crystals in synovial fluid or tissue by compensated polarized light microscopy (Figures 1A and 1B).2 The sensitivity and specificity for CPP crystal detection has been shown to be 95.9% and 86.5%, respectively.12 However, the CPP crystal is more readily identified by a rheumatologist rather than in a standard hospital laboratory, which misses 30% of CPP crystals.13
Findings of CC on radiograph strengthens a CPPD diagnosis, but its absence does not rule it out (Figure 2A).2 More recently, the use of new imaging modalities, such as musculoskeletal ultrasound, provides the capacity to visualize crystal deposits within the joint structures, the hyaline cartilage, and/or fibrocartilage (Figure 2B and 2C).14 The presence of hyperechoic bands within the intermediate layer hyaline cartilage and hyperechoic spots in fibrocartilage are consistent with CPP crystal deposits.2,14 The use of computed tomography is the gold standard imaging modality for the identification of CPPD of the spine.15 There is not enough evidence to support the use of magnetic resonance imaging in CPPD, but it may play a role in rare complications.2
Treatment
The EULAR recently defined new guidelines for the management of CPPD.16 Asymptomatic CPPD needs no treatment.In other CPPD phenotypes, the goals are to attempt prompt resolution of the acute synovitis, reduction in chronic damage, and management of associated conditions.In acute attacks, treatment modalities used in gout are often required; however, data for CPPD treatment are limited (Table). Treatment relies on the use of colchicine and nonsteroidal anti-inflammatory drugs (NSAIDs), but toxicity and comorbidities in the elderly limit the usage of these drugs.
Given increased renal impairment, the loading dose of colchicine is not recommended.16 Colchicine has recently been shown to completely block crystal-induced maturation of IL-1β in vitro, indicating that the drug acts upstream of inflammasome activation.17 This is in addition to the well-known role of colchicine in inhibition of micro-tubule formation, which likely leads to prevention of cell migration, phagocytosis, and activation of inflammasome.18-20
Intra-articular injection of corticosteroid is an efficient and well-tolerated treatment alternative for monoarticular CPP flares. Oral or parenteral corticosteroids are frequently used for polyarticular flares in particular for those patients in which NSAIDs and colchicine are contraindicated.16 Parenteral adrenocorticotropic hormone has been used in patients with congestive heart failure, renal insufficiency, gastrointestinal bleeding, or resistance to NSAIDs.21 For prophylaxis of acute CPP crystal arthritis, a low dose of oral NSAIDs, oral colchicine, or prednisone may be used with good results.16 In chronic CPP arthritis, continuous use of colchicine, NSAIDs, or low-dose prednisone is often appropriate. If these interventions are ineffective or contraindicated, using hydroxychloroquine (HCQ) and methotrexate (MTX) have been successfully used to control chronic CPP crystal inflammation.22,23 Recent trials have raised questions about MTX, and further trials on HCQ usage are underway.24 Biologic agents targeting IL-1 are not currently approved for the treatment of CPPD, but there are suggestions that it may be effective in refractory cases and induce rapid stable remissions after 3 days of therapy.25
In contrast to gout, there is no specific target therapy for lowering CPP crystal load in the elderly. Crucial in the management of CPPD in the elderly is the search for associated diseases, such as hyperparathyroidism, hemochromatosis, hypomagnesemia, and hypophosphatemia, as well as avoidance of tacrolimus, which facilitates or causes CC.16 Correction of the underlying metabolic disorder, especially when undertaken early, may reduce the severity of CPPD. However, there is little evidence to suggest that treatment of associated disease results in resolution of CPPD—most famously, although therapeutic phlebotomy does not help in hemochromatosis for prevention of crystal disease, chelating agents do seem to be moderately effective.26 Only oral administration of magnesium has shown a reduction in meniscal CC in a patient with CPPD arthropathy.27 In addition, this was in the setting of familial hypomagnesemia associated CPPD. However, unlike uricosuric agents for gout, no pharmacologic treatments can prevent CPPD crystal formation and deposition in tissues.
Therapeutic Agents
Magnesium
Magnesium is a cofactor for the activity of pyrophosphatases that converts inorganic pyrophosphates (PPis) into orthophosphates. In addition, magnesium can increase the solubility of CPP crystals. Early detection and management of hypomagnesemia are recommended, because it occurs in patients who have well-defined conditions and situations: Gitelman syndrome, thiazide and loop diuretics use, tacrolimus use, familial forms of renal magnesium wasting or use of proton pump inhibitors, short bowel syndrome, and intestinal failure in patients receiving home parenteral nutrition. Long-term administration of magnesium in some patients with chronic hypomagnesemia decreased meniscal calcification.27-29
Dietary Calcium
Epidemiologic studies showed a lower incidence of CC in Chinese subjects. The authors of the study speculate that this lower prevalence of CPPD could result from high levels of calcium found in the drinking water in Beijing, which may affect parathyroid hormone secretion.30 Further studies are needed to confirm this hypothesis, as it could be a cheaper approach to pseudogout prevention.
Probenecid
Probenecid is an in vitro inhibitor of the transmembrane PPi transporter thought to possibly prevent extracellular PPi elaboration. However, this observation has not been confirmed by either case reports or clinical trials.31
Phosphocitrate
Phosphocitrate acts directly on preventing crystal deposition in tissues in CPPD as well as BCP based on in vitro evidence and mouse models.32,33
Hyaluronan
An amelioration of pain and increased range of motion were observed in radiographic CC with OA.34 However, it is associated with increased acute CPP arthritis.35
Radiosynovectomy
In a double-blind study of 15 patients with symmetrical CPPD arthropathy, the knee that underwent intra-articular injection of yttrium-90 (5 mCi) plus steroid had less pain, stiffness, joint line tenderness, and effusion compared with the contralateral control knee injected with saline and steroids.36
Precipitators of Acute Pseudogout
Diuretics are known to exacerbate gout, but they also can exacerbate pseudogout. A recent case-control study nested within a United Kingdom general practice database found that loop diuretics rather than hydrochlorothiazide was associated with increased risk of CPPD mediated primarily by magnesium reabsorption in the loop of Henle.28 Chronic kidney disease associated with secondary and tertiary hyperparathyroidism increases calcium or PPi concentration, which leads to CPP-crystal deposition.
In addition, multiple case reports have described acute pseudogout caused by bisphosphonate administration for osteoporosis or Paget disease—more likely in the elderly population. Intravenous pamidronate, oral etidronate, and alendronate therapy have all been described in the elderly.37 The overall mechanism behind this link is not completely understood, but bisphosphonates are structurally similar to PPi. Pseudogout attacks also have been described in neutropenic patients undergoing treatment with granulocyte-colony stimulating factor.38 In addition to pharmaceutical exacerbation of pseudogout, surgical procedures and trauma can precipitate attacks. Joint lavage has been described to increase the incidence of pseudogout.39 It was hypothesized that joint lavage with fluid induced “crystal shedding” from CPPD crystals imbedded in the joint tissue. Patients who underwent meniscectomy of the knee 20 years ago had a 20% incidence of CC in the knee that was operated compared with 4% CC in the contralateral nonoperated knee.40 Overall, the surgery most linked with a pseudogout attack, however, is parathyroidectomy.41
Basic Calcium Phosphate Crystals
Basic calcium phosphate crystals are common but rarely diagnosed due to the cumbersome and expensive methods required to identify these crystals.42
Basic calcium phosphate and CPPD crystals may coexist in synovial fluid. Similar to CPPD, BCP crystal disease is often concurrent with OA and can cause calcification of articular cartilage. Basic calcium phosphate is more common than CPP crystals with occurrence of 30% to 50% in OA synovial fluid.42 Additionally, BCP crystal disease has been linked to increased severity of OA. Basic calcium phosphate crystals in knee joints were found to have radiographically more severe arthritis with larger effusions.44,45 Similarly, BCP crystals in OA synovial fluid correlated with higher Kellgreen-Lawrence grade scores by radiography.42,46
It is currently believed that BCP crystals are continuously formed in the extracellular matrix, and their deposition is actively prevented by PPi present in the matrix.47 Elevated PPi levels, on the other hand, favor the formation of CPP crystals.48 The clinical upshot seems to be that although CPP crystals are almost universally intra-articular and released by chondrocytes, BCD crystals and deposits are more frequently present in soft tissues.
Acute Calcific Tendinitis
Typically, this type of tendinitis involves the shoulder joint and is extra-articular. Common treatments help, including NSAIDs, intra-articular steroids, ice, and rest. In addition, high-energy extracorporeal shock wave therapy has been shown to be effective when used with conscious sedation.49,50 Needling or barbotage in association with lavage and steroid injections also is effective and has occasionally been shown to reduce the size of the calcium deposit as well, often in combination with IV drugs like ethylenediaminetetraacetic acid.51-53
Acute calcific periarthritis of the hand presents similar to gout or pseudogout, affecting the wrist, usually in postmenopausal women.54 Basic calcium phosphate crystals are aspirated from the joint, and periarticular crystals may be subtle. Local steroid injections are beneficial.Milwaukee shoulder syndrome is an arthropathy associated with BCP crystals in the joint fluid and results in extensive destruction of shoulder articular cartilage and surrounding tissues. It is commonly bilateral and occurs in elderly women more often than it does in men.55 Aspiration of the shoulder joint typically reveals a serosanginous fluid. Fluid samples can be assessed for hydroxyapatite crystals by staining with alizarin red dye, which produces a characteristic “halo” or orange-red stain by light microscopy.43 Surgical treatment of Milwaukee shoulder syndrome is difficult due to increased age of the population affected and the severity of the shoulder destruction. Usually a conservative approach of analgesics, recurrent shoulder aspirations, and steroid injections is the best treatment option.
Conclusions
Calcium-containing crystal-associated arthropathies are a complex array of entities that target the veteran elderly population with increasing frequency. Challenges still remain in the diagnosis, crystal identification, and treatment due to coexisting comorbid conditions and polypharmacy commonly seen in veterans. Overall morbidity associated with calcium-containing crystal-associated arthropathies and the coexisting osteoarthritis is great, and focused identification of the disease process with tailored treatment can achieve the goal of decreasing symptoms and improving quality of life.
Acknowledgements
This work was supported by grant P20GM104937 (A.M.R.).
1. Guerne PA, Terkeltaub R. Clinical Features, Diagnosis, and Treatment of CPPD Crystal Arthropathy. In: Terkeltaub R, ed. Gout and Other Crystal Arthropathies. Philadelphia, PA: Saunders/Elsevier; 2012:249-265.
2. Zhang W, Doherty M, Bardin T, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis. 2011;70(4):563-570.
3. McCarty DJ. Calcium pyrophosphate dihydrate crystal deposition disease—1975. Arthritis Rheum. 1976;19(S3):275-285.
4. Ivorra J, Rosas J, Pascual E. Most calcium pyrophosphate crystals appear as non-birefringent. Ann Rheum Dis. 1999;58(9):582-584.
5. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
6. Pego-Reigosa JM, Rodriguez-Rodriguez M, Hurtado-Hernandez Z, et al. Calcium pyrophosphate deposition disease mimicking polymyalgia rheumatica: a prospective followup study of predictive factors for this condition in patients presenting with polymyalgia symptoms. Arthritis Rheum. 2005;53(6):931-938.
7. Bouvet JP, le Parc JM, Michalski B, Benlahrache C, Auquier L. Acute neck pain due to calcifications surrounding the odontoid process: the crowned dens syndrome. Arthritis Rheum. 1985;28(12):1417-1420.
8. Muthukumar N, Karuppaswamy U. Tumoral calcium pyrophosphate dihydrate deposition disease of the ligamentum flavum. Neurosurgery. 2003;53(1):103-109.
9. Armas JB, Couto AR, Bettencourt BF. Spondyloarthritis, diffuse idiopathic skeletal hyperostosis (DISH) and chondrocalcinosis. Adv in Exp Med Biol. 2009;649:37-56.
10. Abhishek A, Doherty M. Epidemiology of calcium pyrophosphate crystal arthritis and basic calcium phosphate crystal arthropathy. Rheum Dis Clin North Am. 2014;40(2):177-191.
11. Abhishek A, Doherty S, Maciewicz R, Muir K, Zhang W, Doherty M. Chondrocalcinosis is common in the absence of knee involvement. Arthritis Res Ther. 2012;14(5):R205.
12. Lumbreras B, Pascual E, Frasquet J, González-Salinas J, Rodríguez E, Hernández-Aguado I. Analysis for crystals in synovial fluid: training of the analysts results in high consistency. Ann Rheum Dis. 2005;64(4):612-615.
13. Szscygiel J, Reginato AM SS. Quality improvements in the identification of crystals from synovial fluid: hospital laboratory versus rheumatology department evaluation. Poster presented at: 2014 ACR/ARHP Annual Meeting; November 15, 2014; Boston, MA.
14. Grassi W, Meenagh G, Pascual E, Filippucci E. “Crystal clear”-sonographic assessment of gout and calcium pyrophosphate deposition disease. Semin Arthritis Rheum. 2006;36(3):197-202.
15. Scutellari PN, Galeotti R, Leprotti S, Ridolfi M, Franciosi R, Antinolfi G. The crowned dens syndrome. Evaluation with CT imaging. Radiol Med. 2007;112(2):195-207.
16. Zhang W, Doherty M, Pascual E, et al. EULAR recommendations for calcium pyrophosphate deposition. Part II: management. Ann Rheum Dis. 2011;70(4):571-575.
17. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237-241.
18. Nuki G. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep. 2008;10(3):218-227.
19. Borisy GG, Taylor EW. The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol. 1967;34(2):535-548.
20. Borisy GG, Taylor EW. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967;34(2):525-533.
21. Daoussis D, Antonopoulos I, Andonopoulos AP. ACTH as a treatment for acute crystal-induced arthritis: update on clinical evidence and mechanisms of action. Semin Arthritis Rheum. 2014;43(5):648-653.
22. Rothschild B, Yakubov LE. Prospective 6-month, double-blind trial of hydroxychloroquine treatment of CPDD. Compr Ther. 1997;23(5):327-331.
23. Chollet-Janin A, Finckh A, Dudler J, Guerne PA. Methotrexate as an alternative therapy for chronic calcium pyrophosphate deposition disease: an exploratory analysis. Arthritis Rheum. 2007;56(2):688-692.
24. Finckh A, Mc Carthy GM, Madigan A, et al. Methotrexate in chronic-recurrent calcium pyrophosphate deposition disease: no significant effect in a randomized crossover trial. Arthritis Res Ther. 2014;16(5):458.
25. Moltó A, Ea HK, Richette P, Bardin T, Lioté F. Efficacy of anakinra for refractory acute calcium pyrophosphate crystal arthritis. Joint Bone Spine. 2012;79(6):621-623.
26. Harty LC, Lai D, Connor S, et al. Prevalence and progress of joint symptoms in hereditary hemochromatosis and symptomatic response to venesection. J Clin Rheumatol. 2011;17(4):220-222.
27. Doherty M, Dieppe PA. Double blind, placebo controlled trial of magnesium carbonate in chronic pyrophosphate arthropathy. Ann Rheum Dis. 1983;42(suppl 1):106-107.
28. Rho YH, Zhu Y, Zhang Y, Reginato AM, Choi HK. Risk factors for pseudogout in the general population. Rheumatology (Oxford). 2012;51(11):2070-2074.
29. Park CH, Kim EH, Roh YH, Kim HY, Lee SK. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112558.
30. Zhang Y, Terkeltaub R, Nevitt M, et al. Lower prevalence of chondrocalcinosis in Chinese subjects in Beijing than in white subjects in the United States: the Beijing Osteoarthritis Study. Arthritis Rheum. 2006;54(11):3508-3512.
31. Rosenthal AK, Ryan LM. Probenecid inhibits transforming growth factor-beta 1 induced pyrophosphate elaboration by chondrocytes. J Rheumatol. 1994;21(5):896-900.
32. Cheung HS, Sallis JD, Demadis KD, Wierzbicki A. Phosphocitrate blocks calcification-induced articular joint degeneration in a guinea pig model. Arthritis Rheum. 2006;54(8):2452-2461.
33. Sun Y, Mauerhan DR, Honeycutt PR, et al. Calcium deposition in osteoarthritic meniscus and meniscal cell culture. Arthritis Res Ther. 2010;12(2):R56.
34. Daumen-Legre V, Pham T, Acquaviva PC, Lafforgue P. Evaluation of safety and efficacy of viscosupplementation in knee osteoarthritis with chondrocalcinosis. In: Arthritis and Rheumatism.Vol. 42. Lippincott Williams and Wilkins; 1999:S158-S158.
35. Disla E, Infante R, Fahmy A, Karten I, Cuppari GG. Recurrent acute calcium pyrophosphate dihydrate arthritis following intraarticular hyaluronate injection. Arthritis Rheum. 1999;42(6):1302-1303.
36. Doherty M, Dieppe PA. Effect of intra-articularYttrium-90 on chronic pyrophosphate arthropathy of the knee. Lancet. 1981;2(8258):1243-1246.
37. Wendling D, Tisserand G, Griffond V, Saccomani C, Toussirot E. Acute pseudogout after pamidronate infusion. Clin Rheumatol. 2008;27(9):1205-1206.
38. Ames PRJ, Rainey MG. Consecutive pseudogout attacks after repetitive granulocyte colony-stimulating factor administration for neutropenia. Mod Rheumatol. 2007;17(5):445-446.
39. Pasquetti P, Selvi E, Righeschi K, et al. Joint lavage and pseudogout. Ann Rheum Dis. 2004;63(11):1529-1530.
40. Doherty M, Watt I, Dieppe P. Localised chondrocalcinosis in post-meniscectomy knees. Lancet. 1982;1(8283):1207-1210.
41. Rubin MR, Silverberg SJ. Rheumatic manifestations of primary hyperparathyroidism and parathyroid hormone therapy. Curr Rheumatol Rep. 2002;4(2):179-185.
42. Ea HK, Lioté F. Diagnosis and clinical manifestations of calcium pyrophosphate and basic calcium phosphate crystal deposition diseases. Rheum Dis Clin North Am. 2014;40(2):207-229.
43. Paul H, Reginato AJ, Ralph Schumacher HR. Alizarin red s staining as a screening test to detect calcium compounds in synovial fluid. Arthritis Rheum. 1983;26(2):191-200.
44. Molloy ES, McCarthy GM. Basic calcium phosphate crystals: pathways to joint degeneration. Curr Opin Rheumatol. 2006;18(2):187-192.
45. Carroll GJ, Stuart RA, Armstrong JA, Breidahl PD, Laing BA. Hydroxyapatite crystals are a frequent finding in osteoarthritic synovial fluid, but are not related to increased concentrations of keratan sulfate or interleukin 1 beta. J Rheumatol. 1991;18(6):861-866.
46. Derfus BA, Kurian JB, Butler JJ, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29(3):570-574.
47. Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289(5477):265-270.
48. Macmullan P, McCarthy G. Treatment and management of pseudogout: insights for the clinician. Ther Adv Musculoskelet Dis. 2012;4(2):121-131.
49. Gerdesmeyer L, Wagenpfeil S, Haake M, et al. Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff: a randomized controlled trial. JAMA. 2003;290(19):2573-2580.
50. Lee SY, Cheng B, Grimmer-Somers K. The midterm effectiveness of extracorporeal shockwave therapy in the management of chronic calcific shoulder tendinitis. J Shoulder Elbow Surg. 2011;20(5):845-854.
51. Pfister J, Gerber H. Chronic calcifying tendinitis of the shoulder-therapy by percutaneous needle aspiration and lavage: a prospective open study of 62 shoulders. Clin Rheumatol. 1997;16(3):269-274.
52. del Cura JL, Torre I, Zabala R, Legórburu A. Sonographically guided percutaneous needle lavage in calcific tendinitis of the shoulder: short- and long-term results. AJR Am J Roentgenol. 2007;189(3):W128-W134.
53. Yoo JC, Koh KH, Park WH, Park JC, Kim SM, Yoon YC. The outcome of ultrasound-guided needle decompression and steroid injection in calcific tendinitis. J Shoulder Elbow Surg. 2010;19(4):596-600.
54. Wiper JD, Garrido A. Images in clinical medicine. Acute calcific tendinitis. N Engl J Med. 2008;359(23):2477.
55. Halverson PB, Carrera GF, McCarty DJ. Milwaukee shoulder syndrome. Arch Intern Med. 1990;150(3):677-682.
1. Guerne PA, Terkeltaub R. Clinical Features, Diagnosis, and Treatment of CPPD Crystal Arthropathy. In: Terkeltaub R, ed. Gout and Other Crystal Arthropathies. Philadelphia, PA: Saunders/Elsevier; 2012:249-265.
2. Zhang W, Doherty M, Bardin T, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis. 2011;70(4):563-570.
3. McCarty DJ. Calcium pyrophosphate dihydrate crystal deposition disease—1975. Arthritis Rheum. 1976;19(S3):275-285.
4. Ivorra J, Rosas J, Pascual E. Most calcium pyrophosphate crystals appear as non-birefringent. Ann Rheum Dis. 1999;58(9):582-584.
5. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
6. Pego-Reigosa JM, Rodriguez-Rodriguez M, Hurtado-Hernandez Z, et al. Calcium pyrophosphate deposition disease mimicking polymyalgia rheumatica: a prospective followup study of predictive factors for this condition in patients presenting with polymyalgia symptoms. Arthritis Rheum. 2005;53(6):931-938.
7. Bouvet JP, le Parc JM, Michalski B, Benlahrache C, Auquier L. Acute neck pain due to calcifications surrounding the odontoid process: the crowned dens syndrome. Arthritis Rheum. 1985;28(12):1417-1420.
8. Muthukumar N, Karuppaswamy U. Tumoral calcium pyrophosphate dihydrate deposition disease of the ligamentum flavum. Neurosurgery. 2003;53(1):103-109.
9. Armas JB, Couto AR, Bettencourt BF. Spondyloarthritis, diffuse idiopathic skeletal hyperostosis (DISH) and chondrocalcinosis. Adv in Exp Med Biol. 2009;649:37-56.
10. Abhishek A, Doherty M. Epidemiology of calcium pyrophosphate crystal arthritis and basic calcium phosphate crystal arthropathy. Rheum Dis Clin North Am. 2014;40(2):177-191.
11. Abhishek A, Doherty S, Maciewicz R, Muir K, Zhang W, Doherty M. Chondrocalcinosis is common in the absence of knee involvement. Arthritis Res Ther. 2012;14(5):R205.
12. Lumbreras B, Pascual E, Frasquet J, González-Salinas J, Rodríguez E, Hernández-Aguado I. Analysis for crystals in synovial fluid: training of the analysts results in high consistency. Ann Rheum Dis. 2005;64(4):612-615.
13. Szscygiel J, Reginato AM SS. Quality improvements in the identification of crystals from synovial fluid: hospital laboratory versus rheumatology department evaluation. Poster presented at: 2014 ACR/ARHP Annual Meeting; November 15, 2014; Boston, MA.
14. Grassi W, Meenagh G, Pascual E, Filippucci E. “Crystal clear”-sonographic assessment of gout and calcium pyrophosphate deposition disease. Semin Arthritis Rheum. 2006;36(3):197-202.
15. Scutellari PN, Galeotti R, Leprotti S, Ridolfi M, Franciosi R, Antinolfi G. The crowned dens syndrome. Evaluation with CT imaging. Radiol Med. 2007;112(2):195-207.
16. Zhang W, Doherty M, Pascual E, et al. EULAR recommendations for calcium pyrophosphate deposition. Part II: management. Ann Rheum Dis. 2011;70(4):571-575.
17. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237-241.
18. Nuki G. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep. 2008;10(3):218-227.
19. Borisy GG, Taylor EW. The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol. 1967;34(2):535-548.
20. Borisy GG, Taylor EW. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967;34(2):525-533.
21. Daoussis D, Antonopoulos I, Andonopoulos AP. ACTH as a treatment for acute crystal-induced arthritis: update on clinical evidence and mechanisms of action. Semin Arthritis Rheum. 2014;43(5):648-653.
22. Rothschild B, Yakubov LE. Prospective 6-month, double-blind trial of hydroxychloroquine treatment of CPDD. Compr Ther. 1997;23(5):327-331.
23. Chollet-Janin A, Finckh A, Dudler J, Guerne PA. Methotrexate as an alternative therapy for chronic calcium pyrophosphate deposition disease: an exploratory analysis. Arthritis Rheum. 2007;56(2):688-692.
24. Finckh A, Mc Carthy GM, Madigan A, et al. Methotrexate in chronic-recurrent calcium pyrophosphate deposition disease: no significant effect in a randomized crossover trial. Arthritis Res Ther. 2014;16(5):458.
25. Moltó A, Ea HK, Richette P, Bardin T, Lioté F. Efficacy of anakinra for refractory acute calcium pyrophosphate crystal arthritis. Joint Bone Spine. 2012;79(6):621-623.
26. Harty LC, Lai D, Connor S, et al. Prevalence and progress of joint symptoms in hereditary hemochromatosis and symptomatic response to venesection. J Clin Rheumatol. 2011;17(4):220-222.
27. Doherty M, Dieppe PA. Double blind, placebo controlled trial of magnesium carbonate in chronic pyrophosphate arthropathy. Ann Rheum Dis. 1983;42(suppl 1):106-107.
28. Rho YH, Zhu Y, Zhang Y, Reginato AM, Choi HK. Risk factors for pseudogout in the general population. Rheumatology (Oxford). 2012;51(11):2070-2074.
29. Park CH, Kim EH, Roh YH, Kim HY, Lee SK. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112558.
30. Zhang Y, Terkeltaub R, Nevitt M, et al. Lower prevalence of chondrocalcinosis in Chinese subjects in Beijing than in white subjects in the United States: the Beijing Osteoarthritis Study. Arthritis Rheum. 2006;54(11):3508-3512.
31. Rosenthal AK, Ryan LM. Probenecid inhibits transforming growth factor-beta 1 induced pyrophosphate elaboration by chondrocytes. J Rheumatol. 1994;21(5):896-900.
32. Cheung HS, Sallis JD, Demadis KD, Wierzbicki A. Phosphocitrate blocks calcification-induced articular joint degeneration in a guinea pig model. Arthritis Rheum. 2006;54(8):2452-2461.
33. Sun Y, Mauerhan DR, Honeycutt PR, et al. Calcium deposition in osteoarthritic meniscus and meniscal cell culture. Arthritis Res Ther. 2010;12(2):R56.
34. Daumen-Legre V, Pham T, Acquaviva PC, Lafforgue P. Evaluation of safety and efficacy of viscosupplementation in knee osteoarthritis with chondrocalcinosis. In: Arthritis and Rheumatism.Vol. 42. Lippincott Williams and Wilkins; 1999:S158-S158.
35. Disla E, Infante R, Fahmy A, Karten I, Cuppari GG. Recurrent acute calcium pyrophosphate dihydrate arthritis following intraarticular hyaluronate injection. Arthritis Rheum. 1999;42(6):1302-1303.
36. Doherty M, Dieppe PA. Effect of intra-articularYttrium-90 on chronic pyrophosphate arthropathy of the knee. Lancet. 1981;2(8258):1243-1246.
37. Wendling D, Tisserand G, Griffond V, Saccomani C, Toussirot E. Acute pseudogout after pamidronate infusion. Clin Rheumatol. 2008;27(9):1205-1206.
38. Ames PRJ, Rainey MG. Consecutive pseudogout attacks after repetitive granulocyte colony-stimulating factor administration for neutropenia. Mod Rheumatol. 2007;17(5):445-446.
39. Pasquetti P, Selvi E, Righeschi K, et al. Joint lavage and pseudogout. Ann Rheum Dis. 2004;63(11):1529-1530.
40. Doherty M, Watt I, Dieppe P. Localised chondrocalcinosis in post-meniscectomy knees. Lancet. 1982;1(8283):1207-1210.
41. Rubin MR, Silverberg SJ. Rheumatic manifestations of primary hyperparathyroidism and parathyroid hormone therapy. Curr Rheumatol Rep. 2002;4(2):179-185.
42. Ea HK, Lioté F. Diagnosis and clinical manifestations of calcium pyrophosphate and basic calcium phosphate crystal deposition diseases. Rheum Dis Clin North Am. 2014;40(2):207-229.
43. Paul H, Reginato AJ, Ralph Schumacher HR. Alizarin red s staining as a screening test to detect calcium compounds in synovial fluid. Arthritis Rheum. 1983;26(2):191-200.
44. Molloy ES, McCarthy GM. Basic calcium phosphate crystals: pathways to joint degeneration. Curr Opin Rheumatol. 2006;18(2):187-192.
45. Carroll GJ, Stuart RA, Armstrong JA, Breidahl PD, Laing BA. Hydroxyapatite crystals are a frequent finding in osteoarthritic synovial fluid, but are not related to increased concentrations of keratan sulfate or interleukin 1 beta. J Rheumatol. 1991;18(6):861-866.
46. Derfus BA, Kurian JB, Butler JJ, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29(3):570-574.
47. Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289(5477):265-270.
48. Macmullan P, McCarthy G. Treatment and management of pseudogout: insights for the clinician. Ther Adv Musculoskelet Dis. 2012;4(2):121-131.
49. Gerdesmeyer L, Wagenpfeil S, Haake M, et al. Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff: a randomized controlled trial. JAMA. 2003;290(19):2573-2580.
50. Lee SY, Cheng B, Grimmer-Somers K. The midterm effectiveness of extracorporeal shockwave therapy in the management of chronic calcific shoulder tendinitis. J Shoulder Elbow Surg. 2011;20(5):845-854.
51. Pfister J, Gerber H. Chronic calcifying tendinitis of the shoulder-therapy by percutaneous needle aspiration and lavage: a prospective open study of 62 shoulders. Clin Rheumatol. 1997;16(3):269-274.
52. del Cura JL, Torre I, Zabala R, Legórburu A. Sonographically guided percutaneous needle lavage in calcific tendinitis of the shoulder: short- and long-term results. AJR Am J Roentgenol. 2007;189(3):W128-W134.
53. Yoo JC, Koh KH, Park WH, Park JC, Kim SM, Yoon YC. The outcome of ultrasound-guided needle decompression and steroid injection in calcific tendinitis. J Shoulder Elbow Surg. 2010;19(4):596-600.
54. Wiper JD, Garrido A. Images in clinical medicine. Acute calcific tendinitis. N Engl J Med. 2008;359(23):2477.
55. Halverson PB, Carrera GF, McCarty DJ. Milwaukee shoulder syndrome. Arch Intern Med. 1990;150(3):677-682.