User login
Uterine adenomyosis: Noninvasive diagnosis
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s installment of Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of describing what adenomyosis will look like on transvaginal ultrasound
In my first book, entitled Endovaginal Ultrasound,1 I coined the phrase “sonomicoscopy.” I maintain that we are seeing things with transvaginal ultrasound that you could not see with your naked eye if you could hold the structure at arms length and squint at it.
Adenomyosis is defined as endometrial glands and stroma embedded within the myometrium. Literature has shown that if you do three sections on a routine hysterectomy specimen the incidence of adenomyosis is 31%; with six sections the incidence is 61%! In other words, it is a very prevalent occurrence.
There is no question that adenomyosis CAN be a source of uterine enlargement, pain, and bleeding. But it is such a prevalent finding that the real question is: What percent of women, especially parous women, will have sonographic evidence of adenomyosis but be totally asymptomatic? Such women represent the denominator while the symptomatic ones represent the numerator. I worry about labeling asymptomatic patients with this entity—when they become perimenopausal and oligo-ovulatory, and may have irregular bleeding—their symptoms can be judged to be FROM adenomyosis and surgical correction is offered.
An important part of successful ultrasound use is being sure that we redefine what is “normal” as we examine patients with this “low power microscope.” So, while transvaginal ultrasound CAN identify glands and stroma within the myometrium, we must be careful not to automatically label this finding as a “disease.”
Reference
1. Goldstein SR. Endovaginal Ultrasound. 2nd ed. John Wiley & Sons, Inc: Hoboken, NJ; January 1991.
Uterine adenomyosis: Noninvasive diagnosis
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
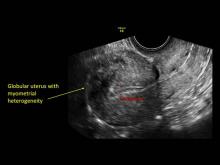
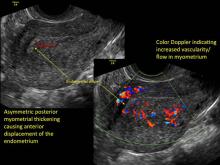
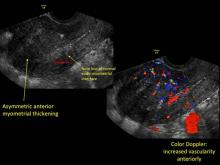
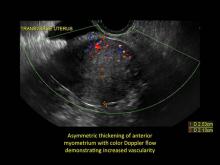
Uterine adenomyosis is a pathologic condition in which endometrial glands and stroma are present in the uterine myometrium. Uterine adenomyosis is common, and may coexist with leiomyomata or endometriosis. When present, it may cause dysmenorrhea and heavy menses.
Until recently, the best way to establish a diagnosis of uterine adenomyosis was through histologic examination of a hysterectomy specimen. However, transvaginal ultrasound and pelvic magnetic resonance imaging have been shown to be accurate for noninvasive diagnosis.
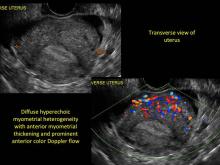
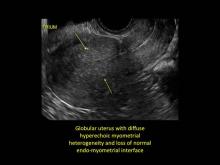
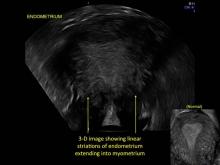
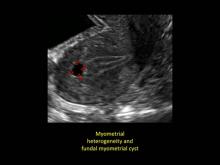
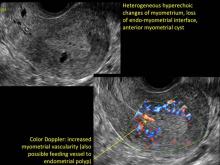
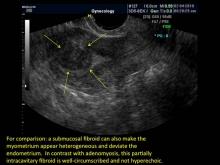
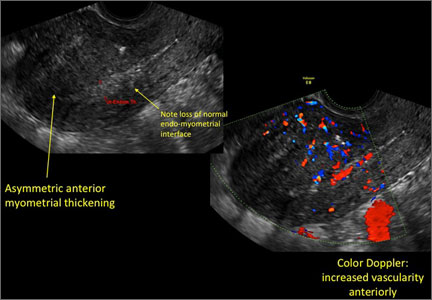
Signs on imaging include:
- Globular/bulky uterus
- Asymmetric thickening of myometrium
- Loss of clarity of endo-myometrial interface
- Diffuse heterogenous myometrial echogenicity
- Myometrial cysts
| Click to enlarge image |
1. Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstetricia et Gynecologica. 2010;89(11):1374-1384.
2. Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and meta-analysis. Am J Obstet Gynecol. 2009;201(1):107.e1-e6.
3. Munro MG. Update: abnormal uterine bleeding. OBG Manag. 2014;26(3):27-32.
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s installment of Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of describing what adenomyosis will look like on transvaginal ultrasound
In my first book, entitled Endovaginal Ultrasound,1 I coined the phrase “sonomicoscopy.” I maintain that we are seeing things with transvaginal ultrasound that you could not see with your naked eye if you could hold the structure at arms length and squint at it.
Adenomyosis is defined as endometrial glands and stroma embedded within the myometrium. Literature has shown that if you do three sections on a routine hysterectomy specimen the incidence of adenomyosis is 31%; with six sections the incidence is 61%! In other words, it is a very prevalent occurrence.
There is no question that adenomyosis CAN be a source of uterine enlargement, pain, and bleeding. But it is such a prevalent finding that the real question is: What percent of women, especially parous women, will have sonographic evidence of adenomyosis but be totally asymptomatic? Such women represent the denominator while the symptomatic ones represent the numerator. I worry about labeling asymptomatic patients with this entity—when they become perimenopausal and oligo-ovulatory, and may have irregular bleeding—their symptoms can be judged to be FROM adenomyosis and surgical correction is offered.
An important part of successful ultrasound use is being sure that we redefine what is “normal” as we examine patients with this “low power microscope.” So, while transvaginal ultrasound CAN identify glands and stroma within the myometrium, we must be careful not to automatically label this finding as a “disease.”
Reference
1. Goldstein SR. Endovaginal Ultrasound. 2nd ed. John Wiley & Sons, Inc: Hoboken, NJ; January 1991.
Uterine adenomyosis: Noninvasive diagnosis
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine adenomyosis is a pathologic condition in which endometrial glands and stroma are present in the uterine myometrium. Uterine adenomyosis is common, and may coexist with leiomyomata or endometriosis. When present, it may cause dysmenorrhea and heavy menses.
Until recently, the best way to establish a diagnosis of uterine adenomyosis was through histologic examination of a hysterectomy specimen. However, transvaginal ultrasound and pelvic magnetic resonance imaging have been shown to be accurate for noninvasive diagnosis.
Signs on imaging include:
- Globular/bulky uterus
- Asymmetric thickening of myometrium
- Loss of clarity of endo-myometrial interface
- Diffuse heterogenous myometrial echogenicity
- Myometrial cysts
| Click to enlarge image |
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s installment of Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of describing what adenomyosis will look like on transvaginal ultrasound
In my first book, entitled Endovaginal Ultrasound,1 I coined the phrase “sonomicoscopy.” I maintain that we are seeing things with transvaginal ultrasound that you could not see with your naked eye if you could hold the structure at arms length and squint at it.
Adenomyosis is defined as endometrial glands and stroma embedded within the myometrium. Literature has shown that if you do three sections on a routine hysterectomy specimen the incidence of adenomyosis is 31%; with six sections the incidence is 61%! In other words, it is a very prevalent occurrence.
There is no question that adenomyosis CAN be a source of uterine enlargement, pain, and bleeding. But it is such a prevalent finding that the real question is: What percent of women, especially parous women, will have sonographic evidence of adenomyosis but be totally asymptomatic? Such women represent the denominator while the symptomatic ones represent the numerator. I worry about labeling asymptomatic patients with this entity—when they become perimenopausal and oligo-ovulatory, and may have irregular bleeding—their symptoms can be judged to be FROM adenomyosis and surgical correction is offered.
An important part of successful ultrasound use is being sure that we redefine what is “normal” as we examine patients with this “low power microscope.” So, while transvaginal ultrasound CAN identify glands and stroma within the myometrium, we must be careful not to automatically label this finding as a “disease.”
Reference
1. Goldstein SR. Endovaginal Ultrasound. 2nd ed. John Wiley & Sons, Inc: Hoboken, NJ; January 1991.
Uterine adenomyosis: Noninvasive diagnosis
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine adenomyosis is a pathologic condition in which endometrial glands and stroma are present in the uterine myometrium. Uterine adenomyosis is common, and may coexist with leiomyomata or endometriosis. When present, it may cause dysmenorrhea and heavy menses.
Until recently, the best way to establish a diagnosis of uterine adenomyosis was through histologic examination of a hysterectomy specimen. However, transvaginal ultrasound and pelvic magnetic resonance imaging have been shown to be accurate for noninvasive diagnosis.
Signs on imaging include:
- Globular/bulky uterus
- Asymmetric thickening of myometrium
- Loss of clarity of endo-myometrial interface
- Diffuse heterogenous myometrial echogenicity
- Myometrial cysts
| Click to enlarge image |
1. Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstetricia et Gynecologica. 2010;89(11):1374-1384.
2. Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and meta-analysis. Am J Obstet Gynecol. 2009;201(1):107.e1-e6.
3. Munro MG. Update: abnormal uterine bleeding. OBG Manag. 2014;26(3):27-32.
1. Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstetricia et Gynecologica. 2010;89(11):1374-1384.
2. Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and meta-analysis. Am J Obstet Gynecol. 2009;201(1):107.e1-e6.
3. Munro MG. Update: abnormal uterine bleeding. OBG Manag. 2014;26(3):27-32.
What is the clinical and economic return on taxpayers’ $260M investment in the WHI?
The WHI estrogen plus progestin (EPT) clinical trial, a $260 million venture, is among the most expensive projects ever undertaken by the National Institutes of Health. Following 2002 publication of its initial findings, use of EPT and estrogen alone (ET) hormone therapy (HT) among US women plummeted. Investigators, including WHI leadership, estimated the clinical and economic impact of this trial from a payer perspective.
Details of the study
For the years 2003 to 2012, the authors used a disease-simulation model to evaluate the effect of the WHI EPT trial on women aged 50 to 79 with an intact uterus (women who were combined-HT [cHT], or EPT, eligible). They compared outcomes between a “WHI scenario,” in which the prevalence of cHT use was based on actual WHI findings, with a “no-WHI” scenario, in which pre-WHI trends in cHT use (from 1998 to 2002) were linearly extrapolated.
The simulation model predicted that 9.5 million women used cHT in the no-WHI scenario, 4.3 million more than actually used cHT in the WHI scenario. The authors estimated that, compared with the no-WHI scenario, 126,000, 76,000, and 80,000 fewer respective cases of breast cancer, cardiovascular disease (CVD), and venous thromboembolism occurred and that 263,000 and 15,000 more respective cases of fractures and colorectal cancer occurred among women as a result of the WHI.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; February 2014)
Regarding economic outcomes, the authors estimated that the WHI resulted in $35.2 billion in direct medical expenditure savings—principally from fewer prescriptions for EPT and associated office visits ($26.2 billion), but also from decreased breast cancer incidence ($4.5 billion) and decreased CVD incidence ($2.2 billion), among other savings, which offset increases in expenditures for greater fracture incidence ($4.8 billion) and colorectal cancer ($1.0 billion).
Related article: In the latest report from the WHI, the data contradict the conclusions. Holly Thacker, MD (Commentary; March 2014)
In addition, the investigators reported a tremendous gain in quality of life years (145,000) in the WHI versus the no-WHI scenario, attributing the difference to the greater health-related quality-of-life effect associated with decreased breast cancer and CVD incidence in the WHI scenario.
What this evidence means for practice
At first glance, the clinical and economic benefits of the WHI EPT trial appear enormous. However, the authors surprisingly failed to take into consideration relevant issues well known to women’s health clinicians: lower use of systemic HT (both in women with an intact uterus and those posthysterectomy) has resulted in many more women suffering from bothersome vasomotor and sleep-related menopausal symptoms, with resultant impairment of quality of life.
In addition, the authors did not account for the major reduction in use of ET after the 2002 WHI findings in women who have had a hysterectomy; given that ET reduces the incidence of breast cancer and cardiovascular disease, declines in ET use have resulted in increased morbidity and mortality from these conditions.1
Finally, as the profound declines in use of systemic HT have not been accompanied by a substantive increase in the use of vaginal estrogen, we have an epidemic of symptomatic vulvovaginal atrophy, with attendant sexual dysfunction and impaired quality of life.
Andrew M. Kaunitz, MD
TELL US WHAT YOU THINK!
Share your thoughts on this article. Send your Letter to the Editor: rbarbieri@frontlinemedcom.com
Reference
- Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59 years. Am J Public Health. 2013;103(9):1583–1588.
The WHI estrogen plus progestin (EPT) clinical trial, a $260 million venture, is among the most expensive projects ever undertaken by the National Institutes of Health. Following 2002 publication of its initial findings, use of EPT and estrogen alone (ET) hormone therapy (HT) among US women plummeted. Investigators, including WHI leadership, estimated the clinical and economic impact of this trial from a payer perspective.
Details of the study
For the years 2003 to 2012, the authors used a disease-simulation model to evaluate the effect of the WHI EPT trial on women aged 50 to 79 with an intact uterus (women who were combined-HT [cHT], or EPT, eligible). They compared outcomes between a “WHI scenario,” in which the prevalence of cHT use was based on actual WHI findings, with a “no-WHI” scenario, in which pre-WHI trends in cHT use (from 1998 to 2002) were linearly extrapolated.
The simulation model predicted that 9.5 million women used cHT in the no-WHI scenario, 4.3 million more than actually used cHT in the WHI scenario. The authors estimated that, compared with the no-WHI scenario, 126,000, 76,000, and 80,000 fewer respective cases of breast cancer, cardiovascular disease (CVD), and venous thromboembolism occurred and that 263,000 and 15,000 more respective cases of fractures and colorectal cancer occurred among women as a result of the WHI.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; February 2014)
Regarding economic outcomes, the authors estimated that the WHI resulted in $35.2 billion in direct medical expenditure savings—principally from fewer prescriptions for EPT and associated office visits ($26.2 billion), but also from decreased breast cancer incidence ($4.5 billion) and decreased CVD incidence ($2.2 billion), among other savings, which offset increases in expenditures for greater fracture incidence ($4.8 billion) and colorectal cancer ($1.0 billion).
Related article: In the latest report from the WHI, the data contradict the conclusions. Holly Thacker, MD (Commentary; March 2014)
In addition, the investigators reported a tremendous gain in quality of life years (145,000) in the WHI versus the no-WHI scenario, attributing the difference to the greater health-related quality-of-life effect associated with decreased breast cancer and CVD incidence in the WHI scenario.
What this evidence means for practice
At first glance, the clinical and economic benefits of the WHI EPT trial appear enormous. However, the authors surprisingly failed to take into consideration relevant issues well known to women’s health clinicians: lower use of systemic HT (both in women with an intact uterus and those posthysterectomy) has resulted in many more women suffering from bothersome vasomotor and sleep-related menopausal symptoms, with resultant impairment of quality of life.
In addition, the authors did not account for the major reduction in use of ET after the 2002 WHI findings in women who have had a hysterectomy; given that ET reduces the incidence of breast cancer and cardiovascular disease, declines in ET use have resulted in increased morbidity and mortality from these conditions.1
Finally, as the profound declines in use of systemic HT have not been accompanied by a substantive increase in the use of vaginal estrogen, we have an epidemic of symptomatic vulvovaginal atrophy, with attendant sexual dysfunction and impaired quality of life.
Andrew M. Kaunitz, MD
TELL US WHAT YOU THINK!
Share your thoughts on this article. Send your Letter to the Editor: rbarbieri@frontlinemedcom.com
The WHI estrogen plus progestin (EPT) clinical trial, a $260 million venture, is among the most expensive projects ever undertaken by the National Institutes of Health. Following 2002 publication of its initial findings, use of EPT and estrogen alone (ET) hormone therapy (HT) among US women plummeted. Investigators, including WHI leadership, estimated the clinical and economic impact of this trial from a payer perspective.
Details of the study
For the years 2003 to 2012, the authors used a disease-simulation model to evaluate the effect of the WHI EPT trial on women aged 50 to 79 with an intact uterus (women who were combined-HT [cHT], or EPT, eligible). They compared outcomes between a “WHI scenario,” in which the prevalence of cHT use was based on actual WHI findings, with a “no-WHI” scenario, in which pre-WHI trends in cHT use (from 1998 to 2002) were linearly extrapolated.
The simulation model predicted that 9.5 million women used cHT in the no-WHI scenario, 4.3 million more than actually used cHT in the WHI scenario. The authors estimated that, compared with the no-WHI scenario, 126,000, 76,000, and 80,000 fewer respective cases of breast cancer, cardiovascular disease (CVD), and venous thromboembolism occurred and that 263,000 and 15,000 more respective cases of fractures and colorectal cancer occurred among women as a result of the WHI.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; February 2014)
Regarding economic outcomes, the authors estimated that the WHI resulted in $35.2 billion in direct medical expenditure savings—principally from fewer prescriptions for EPT and associated office visits ($26.2 billion), but also from decreased breast cancer incidence ($4.5 billion) and decreased CVD incidence ($2.2 billion), among other savings, which offset increases in expenditures for greater fracture incidence ($4.8 billion) and colorectal cancer ($1.0 billion).
Related article: In the latest report from the WHI, the data contradict the conclusions. Holly Thacker, MD (Commentary; March 2014)
In addition, the investigators reported a tremendous gain in quality of life years (145,000) in the WHI versus the no-WHI scenario, attributing the difference to the greater health-related quality-of-life effect associated with decreased breast cancer and CVD incidence in the WHI scenario.
What this evidence means for practice
At first glance, the clinical and economic benefits of the WHI EPT trial appear enormous. However, the authors surprisingly failed to take into consideration relevant issues well known to women’s health clinicians: lower use of systemic HT (both in women with an intact uterus and those posthysterectomy) has resulted in many more women suffering from bothersome vasomotor and sleep-related menopausal symptoms, with resultant impairment of quality of life.
In addition, the authors did not account for the major reduction in use of ET after the 2002 WHI findings in women who have had a hysterectomy; given that ET reduces the incidence of breast cancer and cardiovascular disease, declines in ET use have resulted in increased morbidity and mortality from these conditions.1
Finally, as the profound declines in use of systemic HT have not been accompanied by a substantive increase in the use of vaginal estrogen, we have an epidemic of symptomatic vulvovaginal atrophy, with attendant sexual dysfunction and impaired quality of life.
Andrew M. Kaunitz, MD
TELL US WHAT YOU THINK!
Share your thoughts on this article. Send your Letter to the Editor: rbarbieri@frontlinemedcom.com
Reference
- Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59 years. Am J Public Health. 2013;103(9):1583–1588.
Reference
- Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59 years. Am J Public Health. 2013;103(9):1583–1588.
Hemorrhagic ovarian cysts: One entity with many appearances
FOREWARD
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
This is the inaugural offering in a new series, titled Images in Gyn Ultrasound. It is interesting and important that Dr. Michelle Stalnaker and Dr. Andrew Kaunitz have chosen hemorrhagic ovarian cysts as their debut topic.
Realize that since the vaginal probe was introduced in the 1980s, our entire specialty has had to undergo a learning curve--just as individuals will have a learning curve. In the early days of transvaginal ultrasound, an imager often provided a differential for such masses, along the lines of “compatible with hemorrhagic cyst, endometrioma, dermoid…cannot rule out neoplasia.” Today, however, with better understanding, and especially with the addition of color flow Doppler, very often a definitive diagnosis can be made.
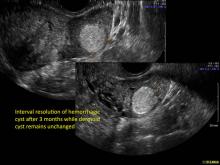
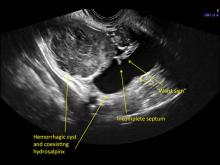
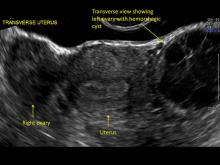
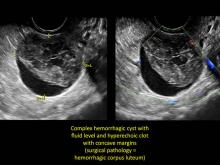
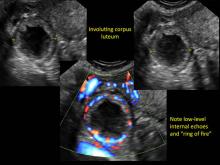
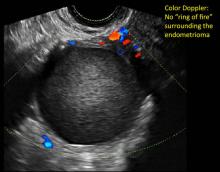
These “hemorrhagic cysts” are nothing more than bleeding into a corpus luteum at the time of ovulation−the more blood that collects before tamponade or clot stops its accumulation, the larger the “cyst” can become. As the cyst goes through a “maturation” process and undergoes clot retraction and clot lysis, the variable internal echo patterns presented in the following images are possible, but there will ALWAYS only be peripheral blood flow as evidenced by the morphologic appearance of the vascular distribution. See video.
Study these images carefully as they are very representative of the many faces of the hemorrhagic corpus luteum.
Hemorrhagic ovarian cysts: One entity with many appearances
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
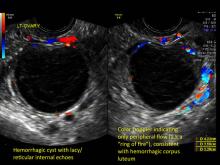
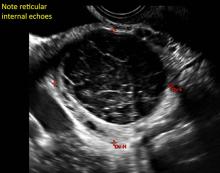
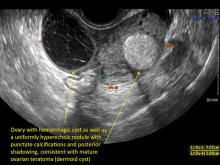
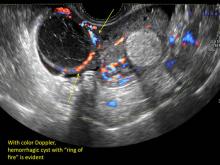
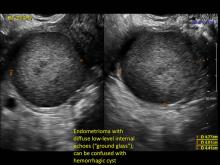
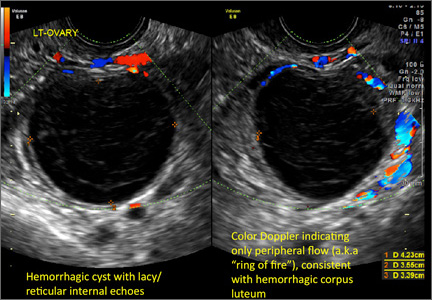
Hemorrhagic cysts are normal in ovulatory women, usually resolving within 8 weeks. They can be quite variable in appearance, however, and can be confused with ovarian endometriomae. Presenting characteristics can include:
- reticular (lacy, cobweb, fishnet) internal echoes due to fibrin strands
- a solid-appearing area with concave margins
- on Color Doppler: circumferential peripheral vascular flow (“ring of fire”), with no internal flow
Management. With respect to hemorrhagic cysts, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement indicates:
- For premenopausal women:
- No follow-up imaging needed unless there’s an uncertain diagnosis or if the cyst is larger than 5 cm
- Cyst size > 5 cm; short-interval follow-up ultrasound is indicated (6-12 weeks)
- For recently menopausal women:
- Follow-up ultrasound in 6 to 12 weeks to ensure resolution of the initial findings
- For later postmenopausal women:
- Cyst possibly neoplastic; consider surgical removal
FOREWARD
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
This is the inaugural offering in a new series, titled Images in Gyn Ultrasound. It is interesting and important that Dr. Michelle Stalnaker and Dr. Andrew Kaunitz have chosen hemorrhagic ovarian cysts as their debut topic.
Realize that since the vaginal probe was introduced in the 1980s, our entire specialty has had to undergo a learning curve--just as individuals will have a learning curve. In the early days of transvaginal ultrasound, an imager often provided a differential for such masses, along the lines of “compatible with hemorrhagic cyst, endometrioma, dermoid…cannot rule out neoplasia.” Today, however, with better understanding, and especially with the addition of color flow Doppler, very often a definitive diagnosis can be made.
These “hemorrhagic cysts” are nothing more than bleeding into a corpus luteum at the time of ovulation−the more blood that collects before tamponade or clot stops its accumulation, the larger the “cyst” can become. As the cyst goes through a “maturation” process and undergoes clot retraction and clot lysis, the variable internal echo patterns presented in the following images are possible, but there will ALWAYS only be peripheral blood flow as evidenced by the morphologic appearance of the vascular distribution. See video.
Study these images carefully as they are very representative of the many faces of the hemorrhagic corpus luteum.
Hemorrhagic ovarian cysts: One entity with many appearances
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Hemorrhagic cysts are normal in ovulatory women, usually resolving within 8 weeks. They can be quite variable in appearance, however, and can be confused with ovarian endometriomae. Presenting characteristics can include:
- reticular (lacy, cobweb, fishnet) internal echoes due to fibrin strands
- a solid-appearing area with concave margins
- on Color Doppler: circumferential peripheral vascular flow (“ring of fire”), with no internal flow
Management. With respect to hemorrhagic cysts, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement indicates:
- For premenopausal women:
- No follow-up imaging needed unless there’s an uncertain diagnosis or if the cyst is larger than 5 cm
- Cyst size > 5 cm; short-interval follow-up ultrasound is indicated (6-12 weeks)
- For recently menopausal women:
- Follow-up ultrasound in 6 to 12 weeks to ensure resolution of the initial findings
- For later postmenopausal women:
- Cyst possibly neoplastic; consider surgical removal
FOREWARD
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
This is the inaugural offering in a new series, titled Images in Gyn Ultrasound. It is interesting and important that Dr. Michelle Stalnaker and Dr. Andrew Kaunitz have chosen hemorrhagic ovarian cysts as their debut topic.
Realize that since the vaginal probe was introduced in the 1980s, our entire specialty has had to undergo a learning curve--just as individuals will have a learning curve. In the early days of transvaginal ultrasound, an imager often provided a differential for such masses, along the lines of “compatible with hemorrhagic cyst, endometrioma, dermoid…cannot rule out neoplasia.” Today, however, with better understanding, and especially with the addition of color flow Doppler, very often a definitive diagnosis can be made.
These “hemorrhagic cysts” are nothing more than bleeding into a corpus luteum at the time of ovulation−the more blood that collects before tamponade or clot stops its accumulation, the larger the “cyst” can become. As the cyst goes through a “maturation” process and undergoes clot retraction and clot lysis, the variable internal echo patterns presented in the following images are possible, but there will ALWAYS only be peripheral blood flow as evidenced by the morphologic appearance of the vascular distribution. See video.
Study these images carefully as they are very representative of the many faces of the hemorrhagic corpus luteum.
Hemorrhagic ovarian cysts: One entity with many appearances
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Hemorrhagic cysts are normal in ovulatory women, usually resolving within 8 weeks. They can be quite variable in appearance, however, and can be confused with ovarian endometriomae. Presenting characteristics can include:
- reticular (lacy, cobweb, fishnet) internal echoes due to fibrin strands
- a solid-appearing area with concave margins
- on Color Doppler: circumferential peripheral vascular flow (“ring of fire”), with no internal flow
Management. With respect to hemorrhagic cysts, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement indicates:
- For premenopausal women:
- No follow-up imaging needed unless there’s an uncertain diagnosis or if the cyst is larger than 5 cm
- Cyst size > 5 cm; short-interval follow-up ultrasound is indicated (6-12 weeks)
- For recently menopausal women:
- Follow-up ultrasound in 6 to 12 weeks to ensure resolution of the initial findings
- For later postmenopausal women:
- Cyst possibly neoplastic; consider surgical removal
When should a menopausal woman discontinue hormone therapy?
CASE: AFTER 3 YEARS OF HT, A PATIENT ASKS WHETHER IT’S TIME TO QUIT
My menopausal patient is a 57-year-old woman with a body mass index (BMI) of 21 kg/m2. Her mother, who also was slender, suffered a hip fracture at age 74.
When this patient was 53, approximately 8 months after her last menstrual period, she scheduled a problem visit to discuss bothersome hot flushes, which occurred primarily at night. These symptoms were associated with sleep disruption and irritability. At that problem visit, the patient and I discussed the benefits and risks of menopausal hormone therapy (HT), and she elected to initiate it, choosing transdermal estradiol using an 0.05-mg patch, combined with oral micronized progesterone 100-mg (one capsule) at bedtime. Two months later, she telephoned my office to report that she was experiencing only moderate relief of her symptoms. I increased the dose of estradiol to 0.075 mg. On her next well-woman visit, the patient remarked that her symptoms were largely resolved and said that she wished to continue the regimen.
Now, as she presents for her well-woman visit 3 years later, she asks how long she should continue the HT.
How would you counsel such a patient?
Although the duration of HT is still marked by controversy, clinicians often encounter the issue in practice. As the North American Menopause Society (NAMS) notes in a recent Practice Pearl and in its 2012 Position Statement on hormone therapy, the determination of the optimal duration of HT can be a challenge for clinicians and patients.1,2
In this article, I discuss indications for HT and consider variables that may influence its duration. I also offer practical guidance on therapeutic options for women who elect to use HT for an extended duration.
HOT FLUSHES CAN BE A LONG-TERM CONCERN
Moderate to severe vasomotor symptoms (VMS) are the most common indication for systemic combination estrogen-progestin or estrogen-only HT—and HT is the most effective treatment for VMS.2
Some experts have cautioned that “it remains prudent to keep the…duration of treatment short” or that HT “may serve a useful role in short-term symptom management.”3,4 However, for many menopausal women, VMS are a long-term concern. The Penn Ovarian Aging Study was conducted to estimate the duration of moderate-to-severe VMS and found a median duration of such symptoms of more than 10 years. In this landmark cohort study, the median duration of VMS, which began near the time of the menopausal transition, was almost 12 years.5
In a study of older menopausal women (mean age, 67 years; mean time since menopause, 19 years), 11.8% reported “clinically significant” hot flushes and “more than half of these women who complained of significant hot flushes at baseline continued to report bothersome symptoms after 3 years.”6
These observations underscore the fact that, in many women, short-term use (3–5 years) of HT will not be sufficient to control bothersome VMS.
SYSTEMIC HT ALSO BENEFITS BONE
The standard daily dose of HT (eg, conjugated equine estrogens [CEE], 0.625 mg; micronized estradiol, 1.0 mg; or transdermal estradiol, 0.05 mg) for relief of VMS also prevents osteoporosis,2 with many HT formulations approved for prevention of this condition. Randomized trial data from the Women’s Health Initiative (WHI) also have confirmed that a standard dose of HT prevents fractures in menopausal women.2
However, in menopausal women, doses of estrogen therapy substantially lower than those commonly used to treat VMS can still maintain or improve bone mineral density (BMD). For example, serum estradiol levels remain in the menopausal range during use of the weekly estradiol ultra-low-dose patch (0.014 mg).7 In a clinical trial of women (mean age, 66 years) with an intact uterus, use of this ultra-low-dose estradiol patch for 2 years without progestin did not increase the risk of endometrial hyperplasia7—although this patch does appear to increase the incidence of endometrial proliferation.8 For this reason, periodic endometrial monitoring may be appropriate in women using the 0.014-mg estradiol patch over the long term, including vaginal ultrasound assessment of endometrial thickness. Package labeling for this patch recommends that women with an intact uterus be given a progestogen for 14 days every 6 to 12 months.9
Related Article: Update on Osteoporosis Steven R. Goldstein, MD (December 2013)
Although the ultra-low-dose estradiol patch is approved for the prevention of osteoporosis, its efficacy in treating VMS is uncertain. For instance, in a study of this patch in women aged 60 to 80 years, with skeletal health and safety as the primary outcomes, 16% of participants reported VMS at baseline. The 0.014-mg estradiol patch did not reduce VMS more than placebo.10 However, in a trial of the 0.014-mg estradiol patch in which impact on VMS was the primary outcome, the ultra-low-dose patch did relieve VMS.11 (The ultra-low-dose patch currently is not approved to treat VMS.) Low-dose CEE (0.3 mg, 0.45 mg) and low-dose oral estradiol (0.5 mg) have been found to be effective in the treatment of VMS.12,13
Data on the risk of osteoporotic fractures among women using the ultra-low-dose estradiol patch are not available.
Use of HT to prevent osteoporosis is appropriate for women who have other indications for HT, such as VMS. For women using HT who no longer experience VMS, long-term use of HT for osteoporosis is controversial. However, it may be considered for women at elevated risk for osteoporosis when skeleton-specific treatments (eg, bisphosphonates) are not tolerated or when such women prefer not to use skeleton-specific therapy.
FDA package labeling for systemic HT indicates that, “When prescribing solely for the prevention of postmenopausal osteoporosis, therapy only should be considered for women at significant risk of osteoporosis, and non-estrogen medications should be carefully considered.”14
The NAMS 2012 Position Statement on HT states: “Provided that the woman is well aware of the potential benefits and risks and has clinical supervision, extending [estrogen-progestin therapy] use with the lowest effective dose is acceptable under some circumstances, including 1) for the woman who has determined that the benefits of menopause symptom relief outweigh risks, notably after failing an attempt to stop [estrogen-progestin therapy], and 2) for the woman at high risk of fracture for whom alternate therapies are not appropriate or cause unacceptable adverse effects.”2
A 2014 Practice Bulletin from the American College of Obstetricians and Gynecologists (ACOG) on the management of menopausal symptoms states: “The decision to continue HT should be individualized and be based on a woman’s symptoms and the risk–benefit ratio, regardless of age. Because some women aged 65 years and older may continue to need systemic HT for the management of vasomotor symptoms, ACOG recommends against routine discontinuation of systemic estrogen at age 65. As with younger women, use of HT and estrogen therapy should be individualized based on each woman’s risk–benefit ratio and clinical presentation.”15
As I have detailed, doses of HT that are lower than those used to treat VMS can prevent loss of BMD. Accordingly, clinicians prescribing HT for the sole indication of osteoporosis prevention should use doses lower than those for standard HT. Moreover, clinicians prescribing HT specifically to prevent osteoporosis should recognize the elevated risk of breast cancer with estrogen-progestin therapy. Extended use of estrogen-only therapy is more appropriate for this indication.
Related Article: Your menopausal patient's breast biopsy reveals atypical hyperplasia JoAnn V. Pinkerton, MD (Cases in Menopause, May 2013)
While estrogen-only therapy is common in women following hysterectomy, ultra-low-dose estrogen therapy with regular endometrial monitoring also can be considered in women with an intact uterus.
Also be aware that BMD declines rapidly after discontinuation of HT (in contrast with bisphosphonates), so alternative agents to maintain BMD should be considered when HT is stopped.16
HOW SAFE IS EXTENDED USE OF SYSTEMIC HT?
The incidence of breast cancer and mortality from breast cancer increase after 3 to 5 years of estrogen-progestin therapy, and the risk of stroke remains elevated throughout use of combination as well as estrogen-only HT.2 Women with an intact uterus who choose to extend the duration of combination therapy beyond 5 years for control of VMS or protection against osteoporosis, or both, need to be candidly counseled about these concerns. No increase in the risk of breast cancer was observed in the estrogen-only arm of the WHI randomized, clinical trial (mean duration of CEE therapy of 7.1 years).17
Related Article: Stop enforcing a 5-year rule for menopausal hormone therapy Robert L. Reid, MD (Stop/Start, December 2013)
Long-term risks of oral estrogen
Among women who initiate HT at the time of menopause, long-term use does not appear to increase the risk of coronary heart disease (CHD), although follow-up in clinical trials has not extended beyond 7 years for estrogen-progestin therapy, and midlife may bring increases in a woman’s baseline cardiovascular risk.2 However, in the WHI, women who initiated oral estrogen-only or estrogen-progestin therapy later in menopause experienced an increased risk of CHD,18 underscoring the need for caution and individualization in this patient population.
Oral HT increases the risk of venous thromboembolism (VTE) and stroke.2 In addition, age is an independent risk factor for these two outcomes. Observational studies suggest that, in contrast with oral estrogen, transdermal HT does not increase the risk of VTE.19–24 Randomized trial data are lacking.
Similarly, one observational study suggests that low-dose (≤0.05 mg) transdermal estradiol does not appear to increase the risk of stroke,25 but, again, clinical trial data are unavailable.
Given these apparent safety advantages, transdermal estrogen therapy would appear to be preferable to oral estrogen in older, long-term users, a perspective supported by ACOG.26
Related Article: Is one oral estrogen formulation safer than another for menopausal women? Andrew M. Kaunitz, MD (Examining the Evidence, January 2014)
In regard to VTE, oral estradiol appears to be safer than CEE.27 Accordingly, oral estradiol may be preferable for older long-term users who don’t tolerate transdermal estradiol due to local skin reactions or costs. Oral estradiol also is less expensive than CEE.
What to expect when your patient discontinues systemic HT
VMS may recur is as many as 50% of women after they discontinue HT. The likelihood of recurring VMS does not appear to vary between abrupt and tapered discontinuation.2
Some HT users may be reluctant to reduce their dose or discontinue HT, particularly those who experienced severe VMS originally. In my clinical experience, many of these women are receptive to a trial of lower-dose HT, especially when I advise them that they can resume their original (higher) dose should bothersome VMS recur.
Individualized assessment of HT benefits and risks and shared decision-making play important roles in the management of these patients. As the dose of HT declines, or systemic HT is discontinued, symptoms of genital atrophy may become more prominent and, in the absence of indications for systemic HT (bothersome VMS or prevention of osteoporosis), may best be addressed with vaginal estrogen therapy or ospemifene.
Extended use of vaginal estrogen
Unlike VMS, untreated genital atrophy may continue to progress as women age, sometimes necessitating use of vaginal estrogen. Because the clinical trials that served as the basis for FDA approval of vaginal estrogen formulations did not find an elevated risk of endometrial hyperplasia, routine use of a progestin to prevent endometrial proliferation in women with an intact uterus is not recommended.28 However, these trials were too limited in duration to assure long-term endometrial safety. All postmenopausal women using vaginal ET should be advised to report any vaginal bleeding, and that bleeding should be evaluated appropriately.
Although low-dose local or vaginal estrogen therapy has not been studied in clinical trials beyond 1 year, it is thought to carry significantly fewer risks than systemic HT.28 Several studies have confirmed that serum estrogen levels remain in the postmenopausal range in women using low-dose vaginal estrogen, specifically the 3-month estradiol ring (2 mg) or twice-weekly estradiol tablets (10 µg).28
Besides relieving vaginal dryness and dyspareunia, low-dose vaginal estrogen also may improve overactive bladder and reduce the incidence of recurrent urinary tract infection.29,30
CASE: RESOLVED
The 57-year-old patient has been essentially symptom-free for the past 3 years using an estradiol patch (0.075 mg) with progesterone (100 mg nightly). Now she asks how long she should continue HT, and I explain that the duration of bothersome VMS is different in each woman. I also counsel her that hot flushes do resolve over time in almost all women. When she asks how likely it is that bothersome VMS will recur if she simply stops HT, I explain that bothersome symptoms often last 10 years or longer, and I remind her that her VMS began some 4 years earlier.
I also briefly review HT benefits (treatment of VMS as well as prevention of vulvovaginal atrophy and osteoporosis) and risks (small increased risk of breast cancer and stroke). I suggest a reduction in her HT dose as a reasonable method to determine her ongoing need for HT, telling her that she should know within about 1 month how she feels on the lower dose (0.05-mg patch). I also advise her to call my office if bothersome VMS recur on the lower dose so that I can increase the dose back to its original level.
After her estradiol dose is reduced, the patient reports only minimal VMS, and she opts to continue the estradiol patch (0.05 mg) with nightly progesterone (100 mg) for another 2 years.
At age 59, during her well-woman visit, she decides to lower the estradiol further, transitioning to a 0.0375-mg patch but maintaining the nightly progesterone (100 mg). She reports no VMS on this new regimen.
At age 61, because of her maternal history of osteoporosis and her own low BMI, the patient undergoes BMD assessment with dual x-ray absorptiometry (DXA). In average-risk women, NAMS recommends that BMD assessment be performed at age 65.31 The results of BMD assessment are normal.
After further discussion, the patient agrees to an even lower dose of estradiol, switching to an 0.025-mg patch, with progesterone (100 mg nightly) administered for 2 weeks in every 3-month interval. She reports no VMS or vaginal bleeding on this lower-dose HT regimen.
After 12 months on this new regimen, the patient undergoes vaginal sonography, revealing an endometrial thickness of 3 mm. She continues this regimen, including annual vaginal ultrasound assessment of the endometrium, without problems until her well-woman visit at age 65.
At that visit, I explain that discontinuation of HT is unlikely to trigger recurred VMS but may cause her to lose BMD rapidly for several years, and may also result in unpleasant symptoms from vulvovaginal atrophy including sexual discomfort. She decides to switch to an 0.014-mg estradiol patch without progesterone, and to undergo ultrasound assessment of her endometrium every 1 to 2 years.
Do you have a troubling case in menopause?
Suggest it to our expert panel. They may address your management dilemma in a future issue!
Email us at obg@frontlinemedcom.com
BOTTOM LINE: INDIVIDUALIZE THE DURATION OF HT
Although published data on extended use of HT are few, many clinicians caring for menopausal women are asked to make a recommendation. Because extended use of estrogen-progestin HT increases the risk of breast cancer, estrogen-only HT has a more favorable benefit-risk ratio. If a patient uses estrogen-progestin HT for an extended duration, periodic discussions about the elevated risk of breast cancer are appropriate.
We lack randomized trial data on CHD and other risks in women who begin HT at the time of menopause and continue it for decades. In older women who use HT for an extended duration, transdermal estrogen may be safer in regard to the risk of VTE and stroke.
As the systemic estrogen dose is lowered, it is possible to reduce the dose of the progestin (the sole function of which is to protect the endometrium from estrogen stimulation). Intermittent dosing can be used, although we lack long-term safety data, and periodic endometrial evaluation should be considered.
Remember also that, with intermittent or daily dosing of a progestin, you are relying on the patient to take this medication to protect the endometrium.
Extended use of low-dose vaginal estrogen HT may be necessary to treat symptoms of vulvovaginal atrophy, which tend to worsen over time. Administration of a progestin is not currently recommended with use of vaginal estrogen, but long-term use may increase the risk of endometrial stimulation.
Read other CASES IN MENOPAUSE
Your postmenopausal patient reports a history of migraine James A. Simon, MD (October 2013)
Your menopausal patient's breast biopsy reveals atypical hyperplasia JoAnn V. Pinkerton, MD (May 2013)
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com
- Kaunitz AM. NAMS Practice Pearl: Extended duration use of menopausal hormone therapy [published online ahead of print January 6, 2014]. Menopause.
- North American Menopause Society. Position Statement: The 2012 hormone therapy position statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.
- Nelson HD. Postmenopausal estrogen for treatment of hot flashes: clinical applications. JAMA. 2004;291(13):1621–1625.
- Hulley SB, Grady D. The WHI estrogen-alone trial—do things look any better? JAMA. 2004;291(14):1769–1771.
- Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095–1104.
- Huang AJ, Grady D, Jacoby VL, Blackwell TL, Bauer DC, Sawaya GF. Persistent hot flushes in older postmenopausal women. Arch Intern Med. 2008;168(8):840–846.
- Ettinger B, Ensrud KE, Wallace R, Johnson KC, Cummings SR, Yankov V, Vittinghoff E, Grady D. Effects of ultralow-dose transdermal estradiol on bone mineral density: A randomized clinical trial. Obstet Gynecol. 2004;104(3):443–451.
- Johnson SR, Ettinger B, Macer JL, Ensrud KE, Quan J, Grady D. Uterine and vaginal effects of unopposed ultralow-dose transdermal estradiol. Obstet Gynecol. 2005;105(4):779–787.
- Menostar [package insert]. Wayne, NJ: Bayer; 2007.
- Diem S, Grady D, Quan J, Vittinghoff E, Wallace R, Hanes V, Ensrud K. Effects of ultralow-dose transdermal estradiol on postmenopausal symptoms in women aged 60 to 80 years. Menopause. 2006;13(1):130–138.
- Bachmann GA, Schaefers M, Uddin A, Utian WH. Lowest effective transdermal 17beta-estradiol dose for relief of hot flushes in postmenopausal women: A randomized controlled trial. Obstet Gynecol. 2007;110(4):771–779.
- Honjo H, Taketani Y. Low-dose estradiol for climacteric symptoms in Japanese women: A randomized, controlled trial. Climacteric. 2009;12(4):319–328.
- Utian WH, Shoupe D, Bachmann G, Pinkerton JV, Pickar JH. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril. 2001;75(6):1065–1079.
- Premarin [package insert]. Philadelphia, PA: Pfizer; 2012.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–216.
- National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2013.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;4:297(13):1465–1477.
- Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340–345.
- Sweetland S, Beral V, Balkwill A, et al; The Million Women Study Collaborators. Venous thromboembolism risk in relation to use of different types of postmenopausal hormone therapy in a large prospective study [published online ahead of print September 10, 2012]. J Thromb Haemost. 2012. doi:10.1111/j.1538-7836.2012.04919.x.
- Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, van Hylckama Vlieg A. The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy. J Thromb Haemost. 2013;11(1):124–131.
- Renoux C, Dell’Aniello S, Suissa S. Hormone replacement therapy and the risk of venous thromboembolism: A population-based study. J Thromb Haemost. 2010;8(5):979–986.
- Laliberté F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052–1059.
- Scarabin PY, Oger E, Plu-Bureau G; EStrogen and THromboEmbolism Risk Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362(9382):428–432.
- Renoux C, Dell’Aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: A nested case-control study. BMJ. 2010;340:c2519. doi:10.1136/bmj.c2519.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 556: Postmenopausal estrogen therapy: Route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887–890.
- Smith NL, Blondon M, Wiggins KL, Harrington LB, van Hylckama Vlieg A, Floyd JS. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;14(1):25–31.
- North American Menopause Society. 2013 Position Statement: Management of symptomatic vulvovaginal atrophy. Menopause. 2013;20(9):888–902.
- Nelken RS, Ozel BZ, Leegant AR, Felix JC, Mishell DR. Randomized trial of estradiol vaginal ring versus oral oxybutynin for the treatment of overactive bladder. Menopause. 2011;18(9):962–966.
- Eriksen BC. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180(5):1072–1079.
- North American Menopause Society. 2010 Position Statement: Management of osteoporosis in postmenopausal women. Menopause. 2010;17(1):25–54.
CASE: AFTER 3 YEARS OF HT, A PATIENT ASKS WHETHER IT’S TIME TO QUIT
My menopausal patient is a 57-year-old woman with a body mass index (BMI) of 21 kg/m2. Her mother, who also was slender, suffered a hip fracture at age 74.
When this patient was 53, approximately 8 months after her last menstrual period, she scheduled a problem visit to discuss bothersome hot flushes, which occurred primarily at night. These symptoms were associated with sleep disruption and irritability. At that problem visit, the patient and I discussed the benefits and risks of menopausal hormone therapy (HT), and she elected to initiate it, choosing transdermal estradiol using an 0.05-mg patch, combined with oral micronized progesterone 100-mg (one capsule) at bedtime. Two months later, she telephoned my office to report that she was experiencing only moderate relief of her symptoms. I increased the dose of estradiol to 0.075 mg. On her next well-woman visit, the patient remarked that her symptoms were largely resolved and said that she wished to continue the regimen.
Now, as she presents for her well-woman visit 3 years later, she asks how long she should continue the HT.
How would you counsel such a patient?
Although the duration of HT is still marked by controversy, clinicians often encounter the issue in practice. As the North American Menopause Society (NAMS) notes in a recent Practice Pearl and in its 2012 Position Statement on hormone therapy, the determination of the optimal duration of HT can be a challenge for clinicians and patients.1,2
In this article, I discuss indications for HT and consider variables that may influence its duration. I also offer practical guidance on therapeutic options for women who elect to use HT for an extended duration.
HOT FLUSHES CAN BE A LONG-TERM CONCERN
Moderate to severe vasomotor symptoms (VMS) are the most common indication for systemic combination estrogen-progestin or estrogen-only HT—and HT is the most effective treatment for VMS.2
Some experts have cautioned that “it remains prudent to keep the…duration of treatment short” or that HT “may serve a useful role in short-term symptom management.”3,4 However, for many menopausal women, VMS are a long-term concern. The Penn Ovarian Aging Study was conducted to estimate the duration of moderate-to-severe VMS and found a median duration of such symptoms of more than 10 years. In this landmark cohort study, the median duration of VMS, which began near the time of the menopausal transition, was almost 12 years.5
In a study of older menopausal women (mean age, 67 years; mean time since menopause, 19 years), 11.8% reported “clinically significant” hot flushes and “more than half of these women who complained of significant hot flushes at baseline continued to report bothersome symptoms after 3 years.”6
These observations underscore the fact that, in many women, short-term use (3–5 years) of HT will not be sufficient to control bothersome VMS.
SYSTEMIC HT ALSO BENEFITS BONE
The standard daily dose of HT (eg, conjugated equine estrogens [CEE], 0.625 mg; micronized estradiol, 1.0 mg; or transdermal estradiol, 0.05 mg) for relief of VMS also prevents osteoporosis,2 with many HT formulations approved for prevention of this condition. Randomized trial data from the Women’s Health Initiative (WHI) also have confirmed that a standard dose of HT prevents fractures in menopausal women.2
However, in menopausal women, doses of estrogen therapy substantially lower than those commonly used to treat VMS can still maintain or improve bone mineral density (BMD). For example, serum estradiol levels remain in the menopausal range during use of the weekly estradiol ultra-low-dose patch (0.014 mg).7 In a clinical trial of women (mean age, 66 years) with an intact uterus, use of this ultra-low-dose estradiol patch for 2 years without progestin did not increase the risk of endometrial hyperplasia7—although this patch does appear to increase the incidence of endometrial proliferation.8 For this reason, periodic endometrial monitoring may be appropriate in women using the 0.014-mg estradiol patch over the long term, including vaginal ultrasound assessment of endometrial thickness. Package labeling for this patch recommends that women with an intact uterus be given a progestogen for 14 days every 6 to 12 months.9
Related Article: Update on Osteoporosis Steven R. Goldstein, MD (December 2013)
Although the ultra-low-dose estradiol patch is approved for the prevention of osteoporosis, its efficacy in treating VMS is uncertain. For instance, in a study of this patch in women aged 60 to 80 years, with skeletal health and safety as the primary outcomes, 16% of participants reported VMS at baseline. The 0.014-mg estradiol patch did not reduce VMS more than placebo.10 However, in a trial of the 0.014-mg estradiol patch in which impact on VMS was the primary outcome, the ultra-low-dose patch did relieve VMS.11 (The ultra-low-dose patch currently is not approved to treat VMS.) Low-dose CEE (0.3 mg, 0.45 mg) and low-dose oral estradiol (0.5 mg) have been found to be effective in the treatment of VMS.12,13
Data on the risk of osteoporotic fractures among women using the ultra-low-dose estradiol patch are not available.
Use of HT to prevent osteoporosis is appropriate for women who have other indications for HT, such as VMS. For women using HT who no longer experience VMS, long-term use of HT for osteoporosis is controversial. However, it may be considered for women at elevated risk for osteoporosis when skeleton-specific treatments (eg, bisphosphonates) are not tolerated or when such women prefer not to use skeleton-specific therapy.
FDA package labeling for systemic HT indicates that, “When prescribing solely for the prevention of postmenopausal osteoporosis, therapy only should be considered for women at significant risk of osteoporosis, and non-estrogen medications should be carefully considered.”14
The NAMS 2012 Position Statement on HT states: “Provided that the woman is well aware of the potential benefits and risks and has clinical supervision, extending [estrogen-progestin therapy] use with the lowest effective dose is acceptable under some circumstances, including 1) for the woman who has determined that the benefits of menopause symptom relief outweigh risks, notably after failing an attempt to stop [estrogen-progestin therapy], and 2) for the woman at high risk of fracture for whom alternate therapies are not appropriate or cause unacceptable adverse effects.”2
A 2014 Practice Bulletin from the American College of Obstetricians and Gynecologists (ACOG) on the management of menopausal symptoms states: “The decision to continue HT should be individualized and be based on a woman’s symptoms and the risk–benefit ratio, regardless of age. Because some women aged 65 years and older may continue to need systemic HT for the management of vasomotor symptoms, ACOG recommends against routine discontinuation of systemic estrogen at age 65. As with younger women, use of HT and estrogen therapy should be individualized based on each woman’s risk–benefit ratio and clinical presentation.”15
As I have detailed, doses of HT that are lower than those used to treat VMS can prevent loss of BMD. Accordingly, clinicians prescribing HT for the sole indication of osteoporosis prevention should use doses lower than those for standard HT. Moreover, clinicians prescribing HT specifically to prevent osteoporosis should recognize the elevated risk of breast cancer with estrogen-progestin therapy. Extended use of estrogen-only therapy is more appropriate for this indication.
Related Article: Your menopausal patient's breast biopsy reveals atypical hyperplasia JoAnn V. Pinkerton, MD (Cases in Menopause, May 2013)
While estrogen-only therapy is common in women following hysterectomy, ultra-low-dose estrogen therapy with regular endometrial monitoring also can be considered in women with an intact uterus.
Also be aware that BMD declines rapidly after discontinuation of HT (in contrast with bisphosphonates), so alternative agents to maintain BMD should be considered when HT is stopped.16
HOW SAFE IS EXTENDED USE OF SYSTEMIC HT?
The incidence of breast cancer and mortality from breast cancer increase after 3 to 5 years of estrogen-progestin therapy, and the risk of stroke remains elevated throughout use of combination as well as estrogen-only HT.2 Women with an intact uterus who choose to extend the duration of combination therapy beyond 5 years for control of VMS or protection against osteoporosis, or both, need to be candidly counseled about these concerns. No increase in the risk of breast cancer was observed in the estrogen-only arm of the WHI randomized, clinical trial (mean duration of CEE therapy of 7.1 years).17
Related Article: Stop enforcing a 5-year rule for menopausal hormone therapy Robert L. Reid, MD (Stop/Start, December 2013)
Long-term risks of oral estrogen
Among women who initiate HT at the time of menopause, long-term use does not appear to increase the risk of coronary heart disease (CHD), although follow-up in clinical trials has not extended beyond 7 years for estrogen-progestin therapy, and midlife may bring increases in a woman’s baseline cardiovascular risk.2 However, in the WHI, women who initiated oral estrogen-only or estrogen-progestin therapy later in menopause experienced an increased risk of CHD,18 underscoring the need for caution and individualization in this patient population.
Oral HT increases the risk of venous thromboembolism (VTE) and stroke.2 In addition, age is an independent risk factor for these two outcomes. Observational studies suggest that, in contrast with oral estrogen, transdermal HT does not increase the risk of VTE.19–24 Randomized trial data are lacking.
Similarly, one observational study suggests that low-dose (≤0.05 mg) transdermal estradiol does not appear to increase the risk of stroke,25 but, again, clinical trial data are unavailable.
Given these apparent safety advantages, transdermal estrogen therapy would appear to be preferable to oral estrogen in older, long-term users, a perspective supported by ACOG.26
Related Article: Is one oral estrogen formulation safer than another for menopausal women? Andrew M. Kaunitz, MD (Examining the Evidence, January 2014)
In regard to VTE, oral estradiol appears to be safer than CEE.27 Accordingly, oral estradiol may be preferable for older long-term users who don’t tolerate transdermal estradiol due to local skin reactions or costs. Oral estradiol also is less expensive than CEE.
What to expect when your patient discontinues systemic HT
VMS may recur is as many as 50% of women after they discontinue HT. The likelihood of recurring VMS does not appear to vary between abrupt and tapered discontinuation.2
Some HT users may be reluctant to reduce their dose or discontinue HT, particularly those who experienced severe VMS originally. In my clinical experience, many of these women are receptive to a trial of lower-dose HT, especially when I advise them that they can resume their original (higher) dose should bothersome VMS recur.
Individualized assessment of HT benefits and risks and shared decision-making play important roles in the management of these patients. As the dose of HT declines, or systemic HT is discontinued, symptoms of genital atrophy may become more prominent and, in the absence of indications for systemic HT (bothersome VMS or prevention of osteoporosis), may best be addressed with vaginal estrogen therapy or ospemifene.
Extended use of vaginal estrogen
Unlike VMS, untreated genital atrophy may continue to progress as women age, sometimes necessitating use of vaginal estrogen. Because the clinical trials that served as the basis for FDA approval of vaginal estrogen formulations did not find an elevated risk of endometrial hyperplasia, routine use of a progestin to prevent endometrial proliferation in women with an intact uterus is not recommended.28 However, these trials were too limited in duration to assure long-term endometrial safety. All postmenopausal women using vaginal ET should be advised to report any vaginal bleeding, and that bleeding should be evaluated appropriately.
Although low-dose local or vaginal estrogen therapy has not been studied in clinical trials beyond 1 year, it is thought to carry significantly fewer risks than systemic HT.28 Several studies have confirmed that serum estrogen levels remain in the postmenopausal range in women using low-dose vaginal estrogen, specifically the 3-month estradiol ring (2 mg) or twice-weekly estradiol tablets (10 µg).28
Besides relieving vaginal dryness and dyspareunia, low-dose vaginal estrogen also may improve overactive bladder and reduce the incidence of recurrent urinary tract infection.29,30
CASE: RESOLVED
The 57-year-old patient has been essentially symptom-free for the past 3 years using an estradiol patch (0.075 mg) with progesterone (100 mg nightly). Now she asks how long she should continue HT, and I explain that the duration of bothersome VMS is different in each woman. I also counsel her that hot flushes do resolve over time in almost all women. When she asks how likely it is that bothersome VMS will recur if she simply stops HT, I explain that bothersome symptoms often last 10 years or longer, and I remind her that her VMS began some 4 years earlier.
I also briefly review HT benefits (treatment of VMS as well as prevention of vulvovaginal atrophy and osteoporosis) and risks (small increased risk of breast cancer and stroke). I suggest a reduction in her HT dose as a reasonable method to determine her ongoing need for HT, telling her that she should know within about 1 month how she feels on the lower dose (0.05-mg patch). I also advise her to call my office if bothersome VMS recur on the lower dose so that I can increase the dose back to its original level.
After her estradiol dose is reduced, the patient reports only minimal VMS, and she opts to continue the estradiol patch (0.05 mg) with nightly progesterone (100 mg) for another 2 years.
At age 59, during her well-woman visit, she decides to lower the estradiol further, transitioning to a 0.0375-mg patch but maintaining the nightly progesterone (100 mg). She reports no VMS on this new regimen.
At age 61, because of her maternal history of osteoporosis and her own low BMI, the patient undergoes BMD assessment with dual x-ray absorptiometry (DXA). In average-risk women, NAMS recommends that BMD assessment be performed at age 65.31 The results of BMD assessment are normal.
After further discussion, the patient agrees to an even lower dose of estradiol, switching to an 0.025-mg patch, with progesterone (100 mg nightly) administered for 2 weeks in every 3-month interval. She reports no VMS or vaginal bleeding on this lower-dose HT regimen.
After 12 months on this new regimen, the patient undergoes vaginal sonography, revealing an endometrial thickness of 3 mm. She continues this regimen, including annual vaginal ultrasound assessment of the endometrium, without problems until her well-woman visit at age 65.
At that visit, I explain that discontinuation of HT is unlikely to trigger recurred VMS but may cause her to lose BMD rapidly for several years, and may also result in unpleasant symptoms from vulvovaginal atrophy including sexual discomfort. She decides to switch to an 0.014-mg estradiol patch without progesterone, and to undergo ultrasound assessment of her endometrium every 1 to 2 years.
Do you have a troubling case in menopause?
Suggest it to our expert panel. They may address your management dilemma in a future issue!
Email us at obg@frontlinemedcom.com
BOTTOM LINE: INDIVIDUALIZE THE DURATION OF HT
Although published data on extended use of HT are few, many clinicians caring for menopausal women are asked to make a recommendation. Because extended use of estrogen-progestin HT increases the risk of breast cancer, estrogen-only HT has a more favorable benefit-risk ratio. If a patient uses estrogen-progestin HT for an extended duration, periodic discussions about the elevated risk of breast cancer are appropriate.
We lack randomized trial data on CHD and other risks in women who begin HT at the time of menopause and continue it for decades. In older women who use HT for an extended duration, transdermal estrogen may be safer in regard to the risk of VTE and stroke.
As the systemic estrogen dose is lowered, it is possible to reduce the dose of the progestin (the sole function of which is to protect the endometrium from estrogen stimulation). Intermittent dosing can be used, although we lack long-term safety data, and periodic endometrial evaluation should be considered.
Remember also that, with intermittent or daily dosing of a progestin, you are relying on the patient to take this medication to protect the endometrium.
Extended use of low-dose vaginal estrogen HT may be necessary to treat symptoms of vulvovaginal atrophy, which tend to worsen over time. Administration of a progestin is not currently recommended with use of vaginal estrogen, but long-term use may increase the risk of endometrial stimulation.
Read other CASES IN MENOPAUSE
Your postmenopausal patient reports a history of migraine James A. Simon, MD (October 2013)
Your menopausal patient's breast biopsy reveals atypical hyperplasia JoAnn V. Pinkerton, MD (May 2013)
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com
CASE: AFTER 3 YEARS OF HT, A PATIENT ASKS WHETHER IT’S TIME TO QUIT
My menopausal patient is a 57-year-old woman with a body mass index (BMI) of 21 kg/m2. Her mother, who also was slender, suffered a hip fracture at age 74.
When this patient was 53, approximately 8 months after her last menstrual period, she scheduled a problem visit to discuss bothersome hot flushes, which occurred primarily at night. These symptoms were associated with sleep disruption and irritability. At that problem visit, the patient and I discussed the benefits and risks of menopausal hormone therapy (HT), and she elected to initiate it, choosing transdermal estradiol using an 0.05-mg patch, combined with oral micronized progesterone 100-mg (one capsule) at bedtime. Two months later, she telephoned my office to report that she was experiencing only moderate relief of her symptoms. I increased the dose of estradiol to 0.075 mg. On her next well-woman visit, the patient remarked that her symptoms were largely resolved and said that she wished to continue the regimen.
Now, as she presents for her well-woman visit 3 years later, she asks how long she should continue the HT.
How would you counsel such a patient?
Although the duration of HT is still marked by controversy, clinicians often encounter the issue in practice. As the North American Menopause Society (NAMS) notes in a recent Practice Pearl and in its 2012 Position Statement on hormone therapy, the determination of the optimal duration of HT can be a challenge for clinicians and patients.1,2
In this article, I discuss indications for HT and consider variables that may influence its duration. I also offer practical guidance on therapeutic options for women who elect to use HT for an extended duration.
HOT FLUSHES CAN BE A LONG-TERM CONCERN
Moderate to severe vasomotor symptoms (VMS) are the most common indication for systemic combination estrogen-progestin or estrogen-only HT—and HT is the most effective treatment for VMS.2
Some experts have cautioned that “it remains prudent to keep the…duration of treatment short” or that HT “may serve a useful role in short-term symptom management.”3,4 However, for many menopausal women, VMS are a long-term concern. The Penn Ovarian Aging Study was conducted to estimate the duration of moderate-to-severe VMS and found a median duration of such symptoms of more than 10 years. In this landmark cohort study, the median duration of VMS, which began near the time of the menopausal transition, was almost 12 years.5
In a study of older menopausal women (mean age, 67 years; mean time since menopause, 19 years), 11.8% reported “clinically significant” hot flushes and “more than half of these women who complained of significant hot flushes at baseline continued to report bothersome symptoms after 3 years.”6
These observations underscore the fact that, in many women, short-term use (3–5 years) of HT will not be sufficient to control bothersome VMS.
SYSTEMIC HT ALSO BENEFITS BONE
The standard daily dose of HT (eg, conjugated equine estrogens [CEE], 0.625 mg; micronized estradiol, 1.0 mg; or transdermal estradiol, 0.05 mg) for relief of VMS also prevents osteoporosis,2 with many HT formulations approved for prevention of this condition. Randomized trial data from the Women’s Health Initiative (WHI) also have confirmed that a standard dose of HT prevents fractures in menopausal women.2
However, in menopausal women, doses of estrogen therapy substantially lower than those commonly used to treat VMS can still maintain or improve bone mineral density (BMD). For example, serum estradiol levels remain in the menopausal range during use of the weekly estradiol ultra-low-dose patch (0.014 mg).7 In a clinical trial of women (mean age, 66 years) with an intact uterus, use of this ultra-low-dose estradiol patch for 2 years without progestin did not increase the risk of endometrial hyperplasia7—although this patch does appear to increase the incidence of endometrial proliferation.8 For this reason, periodic endometrial monitoring may be appropriate in women using the 0.014-mg estradiol patch over the long term, including vaginal ultrasound assessment of endometrial thickness. Package labeling for this patch recommends that women with an intact uterus be given a progestogen for 14 days every 6 to 12 months.9
Related Article: Update on Osteoporosis Steven R. Goldstein, MD (December 2013)
Although the ultra-low-dose estradiol patch is approved for the prevention of osteoporosis, its efficacy in treating VMS is uncertain. For instance, in a study of this patch in women aged 60 to 80 years, with skeletal health and safety as the primary outcomes, 16% of participants reported VMS at baseline. The 0.014-mg estradiol patch did not reduce VMS more than placebo.10 However, in a trial of the 0.014-mg estradiol patch in which impact on VMS was the primary outcome, the ultra-low-dose patch did relieve VMS.11 (The ultra-low-dose patch currently is not approved to treat VMS.) Low-dose CEE (0.3 mg, 0.45 mg) and low-dose oral estradiol (0.5 mg) have been found to be effective in the treatment of VMS.12,13
Data on the risk of osteoporotic fractures among women using the ultra-low-dose estradiol patch are not available.
Use of HT to prevent osteoporosis is appropriate for women who have other indications for HT, such as VMS. For women using HT who no longer experience VMS, long-term use of HT for osteoporosis is controversial. However, it may be considered for women at elevated risk for osteoporosis when skeleton-specific treatments (eg, bisphosphonates) are not tolerated or when such women prefer not to use skeleton-specific therapy.
FDA package labeling for systemic HT indicates that, “When prescribing solely for the prevention of postmenopausal osteoporosis, therapy only should be considered for women at significant risk of osteoporosis, and non-estrogen medications should be carefully considered.”14
The NAMS 2012 Position Statement on HT states: “Provided that the woman is well aware of the potential benefits and risks and has clinical supervision, extending [estrogen-progestin therapy] use with the lowest effective dose is acceptable under some circumstances, including 1) for the woman who has determined that the benefits of menopause symptom relief outweigh risks, notably after failing an attempt to stop [estrogen-progestin therapy], and 2) for the woman at high risk of fracture for whom alternate therapies are not appropriate or cause unacceptable adverse effects.”2
A 2014 Practice Bulletin from the American College of Obstetricians and Gynecologists (ACOG) on the management of menopausal symptoms states: “The decision to continue HT should be individualized and be based on a woman’s symptoms and the risk–benefit ratio, regardless of age. Because some women aged 65 years and older may continue to need systemic HT for the management of vasomotor symptoms, ACOG recommends against routine discontinuation of systemic estrogen at age 65. As with younger women, use of HT and estrogen therapy should be individualized based on each woman’s risk–benefit ratio and clinical presentation.”15
As I have detailed, doses of HT that are lower than those used to treat VMS can prevent loss of BMD. Accordingly, clinicians prescribing HT for the sole indication of osteoporosis prevention should use doses lower than those for standard HT. Moreover, clinicians prescribing HT specifically to prevent osteoporosis should recognize the elevated risk of breast cancer with estrogen-progestin therapy. Extended use of estrogen-only therapy is more appropriate for this indication.
Related Article: Your menopausal patient's breast biopsy reveals atypical hyperplasia JoAnn V. Pinkerton, MD (Cases in Menopause, May 2013)
While estrogen-only therapy is common in women following hysterectomy, ultra-low-dose estrogen therapy with regular endometrial monitoring also can be considered in women with an intact uterus.
Also be aware that BMD declines rapidly after discontinuation of HT (in contrast with bisphosphonates), so alternative agents to maintain BMD should be considered when HT is stopped.16
HOW SAFE IS EXTENDED USE OF SYSTEMIC HT?
The incidence of breast cancer and mortality from breast cancer increase after 3 to 5 years of estrogen-progestin therapy, and the risk of stroke remains elevated throughout use of combination as well as estrogen-only HT.2 Women with an intact uterus who choose to extend the duration of combination therapy beyond 5 years for control of VMS or protection against osteoporosis, or both, need to be candidly counseled about these concerns. No increase in the risk of breast cancer was observed in the estrogen-only arm of the WHI randomized, clinical trial (mean duration of CEE therapy of 7.1 years).17
Related Article: Stop enforcing a 5-year rule for menopausal hormone therapy Robert L. Reid, MD (Stop/Start, December 2013)
Long-term risks of oral estrogen
Among women who initiate HT at the time of menopause, long-term use does not appear to increase the risk of coronary heart disease (CHD), although follow-up in clinical trials has not extended beyond 7 years for estrogen-progestin therapy, and midlife may bring increases in a woman’s baseline cardiovascular risk.2 However, in the WHI, women who initiated oral estrogen-only or estrogen-progestin therapy later in menopause experienced an increased risk of CHD,18 underscoring the need for caution and individualization in this patient population.
Oral HT increases the risk of venous thromboembolism (VTE) and stroke.2 In addition, age is an independent risk factor for these two outcomes. Observational studies suggest that, in contrast with oral estrogen, transdermal HT does not increase the risk of VTE.19–24 Randomized trial data are lacking.
Similarly, one observational study suggests that low-dose (≤0.05 mg) transdermal estradiol does not appear to increase the risk of stroke,25 but, again, clinical trial data are unavailable.
Given these apparent safety advantages, transdermal estrogen therapy would appear to be preferable to oral estrogen in older, long-term users, a perspective supported by ACOG.26
Related Article: Is one oral estrogen formulation safer than another for menopausal women? Andrew M. Kaunitz, MD (Examining the Evidence, January 2014)
In regard to VTE, oral estradiol appears to be safer than CEE.27 Accordingly, oral estradiol may be preferable for older long-term users who don’t tolerate transdermal estradiol due to local skin reactions or costs. Oral estradiol also is less expensive than CEE.
What to expect when your patient discontinues systemic HT
VMS may recur is as many as 50% of women after they discontinue HT. The likelihood of recurring VMS does not appear to vary between abrupt and tapered discontinuation.2
Some HT users may be reluctant to reduce their dose or discontinue HT, particularly those who experienced severe VMS originally. In my clinical experience, many of these women are receptive to a trial of lower-dose HT, especially when I advise them that they can resume their original (higher) dose should bothersome VMS recur.
Individualized assessment of HT benefits and risks and shared decision-making play important roles in the management of these patients. As the dose of HT declines, or systemic HT is discontinued, symptoms of genital atrophy may become more prominent and, in the absence of indications for systemic HT (bothersome VMS or prevention of osteoporosis), may best be addressed with vaginal estrogen therapy or ospemifene.
Extended use of vaginal estrogen
Unlike VMS, untreated genital atrophy may continue to progress as women age, sometimes necessitating use of vaginal estrogen. Because the clinical trials that served as the basis for FDA approval of vaginal estrogen formulations did not find an elevated risk of endometrial hyperplasia, routine use of a progestin to prevent endometrial proliferation in women with an intact uterus is not recommended.28 However, these trials were too limited in duration to assure long-term endometrial safety. All postmenopausal women using vaginal ET should be advised to report any vaginal bleeding, and that bleeding should be evaluated appropriately.
Although low-dose local or vaginal estrogen therapy has not been studied in clinical trials beyond 1 year, it is thought to carry significantly fewer risks than systemic HT.28 Several studies have confirmed that serum estrogen levels remain in the postmenopausal range in women using low-dose vaginal estrogen, specifically the 3-month estradiol ring (2 mg) or twice-weekly estradiol tablets (10 µg).28
Besides relieving vaginal dryness and dyspareunia, low-dose vaginal estrogen also may improve overactive bladder and reduce the incidence of recurrent urinary tract infection.29,30
CASE: RESOLVED
The 57-year-old patient has been essentially symptom-free for the past 3 years using an estradiol patch (0.075 mg) with progesterone (100 mg nightly). Now she asks how long she should continue HT, and I explain that the duration of bothersome VMS is different in each woman. I also counsel her that hot flushes do resolve over time in almost all women. When she asks how likely it is that bothersome VMS will recur if she simply stops HT, I explain that bothersome symptoms often last 10 years or longer, and I remind her that her VMS began some 4 years earlier.
I also briefly review HT benefits (treatment of VMS as well as prevention of vulvovaginal atrophy and osteoporosis) and risks (small increased risk of breast cancer and stroke). I suggest a reduction in her HT dose as a reasonable method to determine her ongoing need for HT, telling her that she should know within about 1 month how she feels on the lower dose (0.05-mg patch). I also advise her to call my office if bothersome VMS recur on the lower dose so that I can increase the dose back to its original level.
After her estradiol dose is reduced, the patient reports only minimal VMS, and she opts to continue the estradiol patch (0.05 mg) with nightly progesterone (100 mg) for another 2 years.
At age 59, during her well-woman visit, she decides to lower the estradiol further, transitioning to a 0.0375-mg patch but maintaining the nightly progesterone (100 mg). She reports no VMS on this new regimen.
At age 61, because of her maternal history of osteoporosis and her own low BMI, the patient undergoes BMD assessment with dual x-ray absorptiometry (DXA). In average-risk women, NAMS recommends that BMD assessment be performed at age 65.31 The results of BMD assessment are normal.
After further discussion, the patient agrees to an even lower dose of estradiol, switching to an 0.025-mg patch, with progesterone (100 mg nightly) administered for 2 weeks in every 3-month interval. She reports no VMS or vaginal bleeding on this lower-dose HT regimen.
After 12 months on this new regimen, the patient undergoes vaginal sonography, revealing an endometrial thickness of 3 mm. She continues this regimen, including annual vaginal ultrasound assessment of the endometrium, without problems until her well-woman visit at age 65.
At that visit, I explain that discontinuation of HT is unlikely to trigger recurred VMS but may cause her to lose BMD rapidly for several years, and may also result in unpleasant symptoms from vulvovaginal atrophy including sexual discomfort. She decides to switch to an 0.014-mg estradiol patch without progesterone, and to undergo ultrasound assessment of her endometrium every 1 to 2 years.
Do you have a troubling case in menopause?
Suggest it to our expert panel. They may address your management dilemma in a future issue!
Email us at obg@frontlinemedcom.com
BOTTOM LINE: INDIVIDUALIZE THE DURATION OF HT
Although published data on extended use of HT are few, many clinicians caring for menopausal women are asked to make a recommendation. Because extended use of estrogen-progestin HT increases the risk of breast cancer, estrogen-only HT has a more favorable benefit-risk ratio. If a patient uses estrogen-progestin HT for an extended duration, periodic discussions about the elevated risk of breast cancer are appropriate.
We lack randomized trial data on CHD and other risks in women who begin HT at the time of menopause and continue it for decades. In older women who use HT for an extended duration, transdermal estrogen may be safer in regard to the risk of VTE and stroke.
As the systemic estrogen dose is lowered, it is possible to reduce the dose of the progestin (the sole function of which is to protect the endometrium from estrogen stimulation). Intermittent dosing can be used, although we lack long-term safety data, and periodic endometrial evaluation should be considered.
Remember also that, with intermittent or daily dosing of a progestin, you are relying on the patient to take this medication to protect the endometrium.
Extended use of low-dose vaginal estrogen HT may be necessary to treat symptoms of vulvovaginal atrophy, which tend to worsen over time. Administration of a progestin is not currently recommended with use of vaginal estrogen, but long-term use may increase the risk of endometrial stimulation.
Read other CASES IN MENOPAUSE
Your postmenopausal patient reports a history of migraine James A. Simon, MD (October 2013)
Your menopausal patient's breast biopsy reveals atypical hyperplasia JoAnn V. Pinkerton, MD (May 2013)
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com
- Kaunitz AM. NAMS Practice Pearl: Extended duration use of menopausal hormone therapy [published online ahead of print January 6, 2014]. Menopause.
- North American Menopause Society. Position Statement: The 2012 hormone therapy position statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.
- Nelson HD. Postmenopausal estrogen for treatment of hot flashes: clinical applications. JAMA. 2004;291(13):1621–1625.
- Hulley SB, Grady D. The WHI estrogen-alone trial—do things look any better? JAMA. 2004;291(14):1769–1771.
- Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095–1104.
- Huang AJ, Grady D, Jacoby VL, Blackwell TL, Bauer DC, Sawaya GF. Persistent hot flushes in older postmenopausal women. Arch Intern Med. 2008;168(8):840–846.
- Ettinger B, Ensrud KE, Wallace R, Johnson KC, Cummings SR, Yankov V, Vittinghoff E, Grady D. Effects of ultralow-dose transdermal estradiol on bone mineral density: A randomized clinical trial. Obstet Gynecol. 2004;104(3):443–451.
- Johnson SR, Ettinger B, Macer JL, Ensrud KE, Quan J, Grady D. Uterine and vaginal effects of unopposed ultralow-dose transdermal estradiol. Obstet Gynecol. 2005;105(4):779–787.
- Menostar [package insert]. Wayne, NJ: Bayer; 2007.
- Diem S, Grady D, Quan J, Vittinghoff E, Wallace R, Hanes V, Ensrud K. Effects of ultralow-dose transdermal estradiol on postmenopausal symptoms in women aged 60 to 80 years. Menopause. 2006;13(1):130–138.
- Bachmann GA, Schaefers M, Uddin A, Utian WH. Lowest effective transdermal 17beta-estradiol dose for relief of hot flushes in postmenopausal women: A randomized controlled trial. Obstet Gynecol. 2007;110(4):771–779.
- Honjo H, Taketani Y. Low-dose estradiol for climacteric symptoms in Japanese women: A randomized, controlled trial. Climacteric. 2009;12(4):319–328.
- Utian WH, Shoupe D, Bachmann G, Pinkerton JV, Pickar JH. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril. 2001;75(6):1065–1079.
- Premarin [package insert]. Philadelphia, PA: Pfizer; 2012.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–216.
- National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2013.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;4:297(13):1465–1477.
- Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340–345.
- Sweetland S, Beral V, Balkwill A, et al; The Million Women Study Collaborators. Venous thromboembolism risk in relation to use of different types of postmenopausal hormone therapy in a large prospective study [published online ahead of print September 10, 2012]. J Thromb Haemost. 2012. doi:10.1111/j.1538-7836.2012.04919.x.
- Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, van Hylckama Vlieg A. The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy. J Thromb Haemost. 2013;11(1):124–131.
- Renoux C, Dell’Aniello S, Suissa S. Hormone replacement therapy and the risk of venous thromboembolism: A population-based study. J Thromb Haemost. 2010;8(5):979–986.
- Laliberté F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052–1059.
- Scarabin PY, Oger E, Plu-Bureau G; EStrogen and THromboEmbolism Risk Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362(9382):428–432.
- Renoux C, Dell’Aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: A nested case-control study. BMJ. 2010;340:c2519. doi:10.1136/bmj.c2519.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 556: Postmenopausal estrogen therapy: Route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887–890.
- Smith NL, Blondon M, Wiggins KL, Harrington LB, van Hylckama Vlieg A, Floyd JS. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;14(1):25–31.
- North American Menopause Society. 2013 Position Statement: Management of symptomatic vulvovaginal atrophy. Menopause. 2013;20(9):888–902.
- Nelken RS, Ozel BZ, Leegant AR, Felix JC, Mishell DR. Randomized trial of estradiol vaginal ring versus oral oxybutynin for the treatment of overactive bladder. Menopause. 2011;18(9):962–966.
- Eriksen BC. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180(5):1072–1079.
- North American Menopause Society. 2010 Position Statement: Management of osteoporosis in postmenopausal women. Menopause. 2010;17(1):25–54.
- Kaunitz AM. NAMS Practice Pearl: Extended duration use of menopausal hormone therapy [published online ahead of print January 6, 2014]. Menopause.
- North American Menopause Society. Position Statement: The 2012 hormone therapy position statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.
- Nelson HD. Postmenopausal estrogen for treatment of hot flashes: clinical applications. JAMA. 2004;291(13):1621–1625.
- Hulley SB, Grady D. The WHI estrogen-alone trial—do things look any better? JAMA. 2004;291(14):1769–1771.
- Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095–1104.
- Huang AJ, Grady D, Jacoby VL, Blackwell TL, Bauer DC, Sawaya GF. Persistent hot flushes in older postmenopausal women. Arch Intern Med. 2008;168(8):840–846.
- Ettinger B, Ensrud KE, Wallace R, Johnson KC, Cummings SR, Yankov V, Vittinghoff E, Grady D. Effects of ultralow-dose transdermal estradiol on bone mineral density: A randomized clinical trial. Obstet Gynecol. 2004;104(3):443–451.
- Johnson SR, Ettinger B, Macer JL, Ensrud KE, Quan J, Grady D. Uterine and vaginal effects of unopposed ultralow-dose transdermal estradiol. Obstet Gynecol. 2005;105(4):779–787.
- Menostar [package insert]. Wayne, NJ: Bayer; 2007.
- Diem S, Grady D, Quan J, Vittinghoff E, Wallace R, Hanes V, Ensrud K. Effects of ultralow-dose transdermal estradiol on postmenopausal symptoms in women aged 60 to 80 years. Menopause. 2006;13(1):130–138.
- Bachmann GA, Schaefers M, Uddin A, Utian WH. Lowest effective transdermal 17beta-estradiol dose for relief of hot flushes in postmenopausal women: A randomized controlled trial. Obstet Gynecol. 2007;110(4):771–779.
- Honjo H, Taketani Y. Low-dose estradiol for climacteric symptoms in Japanese women: A randomized, controlled trial. Climacteric. 2009;12(4):319–328.
- Utian WH, Shoupe D, Bachmann G, Pinkerton JV, Pickar JH. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril. 2001;75(6):1065–1079.
- Premarin [package insert]. Philadelphia, PA: Pfizer; 2012.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–216.
- National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2013.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;4:297(13):1465–1477.
- Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340–345.
- Sweetland S, Beral V, Balkwill A, et al; The Million Women Study Collaborators. Venous thromboembolism risk in relation to use of different types of postmenopausal hormone therapy in a large prospective study [published online ahead of print September 10, 2012]. J Thromb Haemost. 2012. doi:10.1111/j.1538-7836.2012.04919.x.
- Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, van Hylckama Vlieg A. The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy. J Thromb Haemost. 2013;11(1):124–131.
- Renoux C, Dell’Aniello S, Suissa S. Hormone replacement therapy and the risk of venous thromboembolism: A population-based study. J Thromb Haemost. 2010;8(5):979–986.
- Laliberté F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052–1059.
- Scarabin PY, Oger E, Plu-Bureau G; EStrogen and THromboEmbolism Risk Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362(9382):428–432.
- Renoux C, Dell’Aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: A nested case-control study. BMJ. 2010;340:c2519. doi:10.1136/bmj.c2519.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 556: Postmenopausal estrogen therapy: Route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887–890.
- Smith NL, Blondon M, Wiggins KL, Harrington LB, van Hylckama Vlieg A, Floyd JS. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;14(1):25–31.
- North American Menopause Society. 2013 Position Statement: Management of symptomatic vulvovaginal atrophy. Menopause. 2013;20(9):888–902.
- Nelken RS, Ozel BZ, Leegant AR, Felix JC, Mishell DR. Randomized trial of estradiol vaginal ring versus oral oxybutynin for the treatment of overactive bladder. Menopause. 2011;18(9):962–966.
- Eriksen BC. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180(5):1072–1079.
- North American Menopause Society. 2010 Position Statement: Management of osteoporosis in postmenopausal women. Menopause. 2010;17(1):25–54.
Is one oral estrogen formulation safer than another for menopausal women?
Although numerous investigators, including the Women’s Health Initiative (WHI) team, have compared cardiovascular risks in women using menopausal hormone therapy (HT) versus nonusers, few researchers have addressed the comparative safety of different oral estrogen formulations.
Related Article: Update on Menopause (Andrew M. Kaunitz, MD, June 2013)
In this case-control study, Smith and colleagues compared the safety of oral estradiol and CEE in menopausal members of a large US Health Maintenance Organization who were using these oral estrogens between 2003 and 2009.
Details of the study
Cases were women diagnosed with deep venous thrombosis, including pulmonary embolism; myocardial infarction; or ischemic stroke. Women in the control group had no history of cardiovascular events. The endogenous thrombin potential-based normalized activated protein C sensitivity ratio (nAPCsr), which has been shown to predict venous thromboembolism (VTE) in the setting of estrogen therapy, was measured in the control group.
Between 2003 and 2009, incident VTE, MI, and stroke were diagnosed in 68, 67, and 49 cases, respectively, and 201 controls were identified. Cases were more likely than controls to have cardiovascular risk factors.
More than 90% of participants were white, with a mean age ranging from 63.2 to 67.6 years.
Among women in the control group, those using oral estradiol had slightly more cardiovascular risk factors than those using CEE, although age, body mass index, and the recency of HT initiation were similar among women using the two oral estrogens.
Although the ORs for VTE and MI were elevated among CEE users, the risk for ischemic stroke was similar for estradiol and CEE users. Women using CEE had higher nAPCsrs (P <.001), however, suggesting a greater tendency to clot.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although the risk of VTE appears to be higher among users of oral estrogen than among those using a transdermal formulation,1 many menopausal women prefer oral estrogen for its convenience and because patch adherence can sometimes be an issue.
Oral estradiol and oral CEE appear to be equally effective in relieving menopausal symptoms. However, there is a significant cost differential: A 1-month supply of 1-mg estradiol tablets costs $4 at some chain pharmacies, whereas 0.625-mg tablets of CEE cost $84.92 (according to goodrx.com). Therefore, for menopausal women who elect to use an oral estrogen formulation, estradiol appears to be a wise choice for both safety and economy.
Andrew M. Kaunitz, MD
WE WANT TO HEAR FROM YOU. Tell us what you think.
Reference
- Postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Committee Opinion #556. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;121(4):887–890.
Although numerous investigators, including the Women’s Health Initiative (WHI) team, have compared cardiovascular risks in women using menopausal hormone therapy (HT) versus nonusers, few researchers have addressed the comparative safety of different oral estrogen formulations.
Related Article: Update on Menopause (Andrew M. Kaunitz, MD, June 2013)
In this case-control study, Smith and colleagues compared the safety of oral estradiol and CEE in menopausal members of a large US Health Maintenance Organization who were using these oral estrogens between 2003 and 2009.
Details of the study
Cases were women diagnosed with deep venous thrombosis, including pulmonary embolism; myocardial infarction; or ischemic stroke. Women in the control group had no history of cardiovascular events. The endogenous thrombin potential-based normalized activated protein C sensitivity ratio (nAPCsr), which has been shown to predict venous thromboembolism (VTE) in the setting of estrogen therapy, was measured in the control group.
Between 2003 and 2009, incident VTE, MI, and stroke were diagnosed in 68, 67, and 49 cases, respectively, and 201 controls were identified. Cases were more likely than controls to have cardiovascular risk factors.
More than 90% of participants were white, with a mean age ranging from 63.2 to 67.6 years.
Among women in the control group, those using oral estradiol had slightly more cardiovascular risk factors than those using CEE, although age, body mass index, and the recency of HT initiation were similar among women using the two oral estrogens.
Although the ORs for VTE and MI were elevated among CEE users, the risk for ischemic stroke was similar for estradiol and CEE users. Women using CEE had higher nAPCsrs (P <.001), however, suggesting a greater tendency to clot.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although the risk of VTE appears to be higher among users of oral estrogen than among those using a transdermal formulation,1 many menopausal women prefer oral estrogen for its convenience and because patch adherence can sometimes be an issue.
Oral estradiol and oral CEE appear to be equally effective in relieving menopausal symptoms. However, there is a significant cost differential: A 1-month supply of 1-mg estradiol tablets costs $4 at some chain pharmacies, whereas 0.625-mg tablets of CEE cost $84.92 (according to goodrx.com). Therefore, for menopausal women who elect to use an oral estrogen formulation, estradiol appears to be a wise choice for both safety and economy.
Andrew M. Kaunitz, MD
WE WANT TO HEAR FROM YOU. Tell us what you think.
Although numerous investigators, including the Women’s Health Initiative (WHI) team, have compared cardiovascular risks in women using menopausal hormone therapy (HT) versus nonusers, few researchers have addressed the comparative safety of different oral estrogen formulations.
Related Article: Update on Menopause (Andrew M. Kaunitz, MD, June 2013)
In this case-control study, Smith and colleagues compared the safety of oral estradiol and CEE in menopausal members of a large US Health Maintenance Organization who were using these oral estrogens between 2003 and 2009.
Details of the study
Cases were women diagnosed with deep venous thrombosis, including pulmonary embolism; myocardial infarction; or ischemic stroke. Women in the control group had no history of cardiovascular events. The endogenous thrombin potential-based normalized activated protein C sensitivity ratio (nAPCsr), which has been shown to predict venous thromboembolism (VTE) in the setting of estrogen therapy, was measured in the control group.
Between 2003 and 2009, incident VTE, MI, and stroke were diagnosed in 68, 67, and 49 cases, respectively, and 201 controls were identified. Cases were more likely than controls to have cardiovascular risk factors.
More than 90% of participants were white, with a mean age ranging from 63.2 to 67.6 years.
Among women in the control group, those using oral estradiol had slightly more cardiovascular risk factors than those using CEE, although age, body mass index, and the recency of HT initiation were similar among women using the two oral estrogens.
Although the ORs for VTE and MI were elevated among CEE users, the risk for ischemic stroke was similar for estradiol and CEE users. Women using CEE had higher nAPCsrs (P <.001), however, suggesting a greater tendency to clot.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although the risk of VTE appears to be higher among users of oral estrogen than among those using a transdermal formulation,1 many menopausal women prefer oral estrogen for its convenience and because patch adherence can sometimes be an issue.
Oral estradiol and oral CEE appear to be equally effective in relieving menopausal symptoms. However, there is a significant cost differential: A 1-month supply of 1-mg estradiol tablets costs $4 at some chain pharmacies, whereas 0.625-mg tablets of CEE cost $84.92 (according to goodrx.com). Therefore, for menopausal women who elect to use an oral estrogen formulation, estradiol appears to be a wise choice for both safety and economy.
Andrew M. Kaunitz, MD
WE WANT TO HEAR FROM YOU. Tell us what you think.
Reference
- Postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Committee Opinion #556. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;121(4):887–890.
Reference
- Postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Committee Opinion #556. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;121(4):887–890.
Best age to begin screening mammograms: How I manage my patients
Controversy has surrounded the utility of screening mammograms, particularly in women in their 40s. In 2009, the US Preventive Services Task Force recommended that screening mammography begin at age 50 and that women aged 50 to 74 receive a mammogram every 2 years.1 However, the American Cancer Society2 and other professional groups continue to recommend that annual screening begin at age 40, leading to controversy and confusion among women’s health clinicians and our patients.
In a recent study, Webb and colleagues3 used registry data based on a health plan in a single US city to assess the cause of death and mammogram history of 1,705 women who died following a diagnosis of invasive breast cancer from 1990 to 1999. They confirmed that 609 of these deaths were from breast cancer. How many of these patients were screened?
What did they find?
The investigators found that 29% of the 609 women who died from breast cancer had been screened for it—19% of the cancers that caused death were screen-detected and 10% were interval cancers. (Interval cancers were defined as symptomatic or palpable tumors that presented less than 2 years after the prior screening mammogram.) That means that 71% of 609 deaths from breast cancer were among unscreened women, with 6% of the fatal cancers diagnosed more than 2 years after the last mammogram and 65% never found upon screening because screening did not occur.
Among deaths caused (n = 609) and not caused (n = 905) by breast cancer, the median age at diagnosis was 49 and 72 years, respectively. Investigators concluded that regular screening of women younger than age 50 years would lower the death rate from breast cancer.
Related Article: Biennial vs annual mammography: How I manage my patients Andrew M. Kaunitz, MD (June 2013)
Let’s not jump to any conclusions
Although some may find the report by Webb and colleagues persuasive, I am concerned about this study’s limitations, of which there are a few. First, analyses that focus on women diagnosed with breast cancers do not allow comparison of outcomes among screened and unscreened populations.
Moreover, this report provides no information on treatment received by screened and unscreened women. It is likely that women who have never been screened, or who have been screened only infrequently, are considerably less affluent and less educated than women who are regularly screened. Accordingly, upon noting a palpable breast mass, unscreened women may be less likely to seek timely medical attention than regularly screened women, leading to differences in breast cancer outcomes, which are independent of screening history.
How I counsel my patients
For now, I will continue to be laissez-fare in my recommendations about screening mammograms for average-risk women in their 40s by supporting their individual preferences about when to initiate such screening.
- Screening for breast cancer, Topic Page. US Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Updated July 2010. Accessed October 28, 2013.
- American Cancer Society Guidelines for the Early Detection of Cancer: Breast cancer. American Cancer Society Web site. http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Updated May 3, 2013. Accessed October 28, 2013.
- Webb ML, Cady B, Michaelson JS, et al. A failure analysis of invasive breast cancer: Most deaths from disease occur in women not regularly screened [published online ahead of print September 9, 2013]. Cancer. doi:10.1002/cncr.28199.
Controversy has surrounded the utility of screening mammograms, particularly in women in their 40s. In 2009, the US Preventive Services Task Force recommended that screening mammography begin at age 50 and that women aged 50 to 74 receive a mammogram every 2 years.1 However, the American Cancer Society2 and other professional groups continue to recommend that annual screening begin at age 40, leading to controversy and confusion among women’s health clinicians and our patients.
In a recent study, Webb and colleagues3 used registry data based on a health plan in a single US city to assess the cause of death and mammogram history of 1,705 women who died following a diagnosis of invasive breast cancer from 1990 to 1999. They confirmed that 609 of these deaths were from breast cancer. How many of these patients were screened?
What did they find?
The investigators found that 29% of the 609 women who died from breast cancer had been screened for it—19% of the cancers that caused death were screen-detected and 10% were interval cancers. (Interval cancers were defined as symptomatic or palpable tumors that presented less than 2 years after the prior screening mammogram.) That means that 71% of 609 deaths from breast cancer were among unscreened women, with 6% of the fatal cancers diagnosed more than 2 years after the last mammogram and 65% never found upon screening because screening did not occur.
Among deaths caused (n = 609) and not caused (n = 905) by breast cancer, the median age at diagnosis was 49 and 72 years, respectively. Investigators concluded that regular screening of women younger than age 50 years would lower the death rate from breast cancer.
Related Article: Biennial vs annual mammography: How I manage my patients Andrew M. Kaunitz, MD (June 2013)
Let’s not jump to any conclusions
Although some may find the report by Webb and colleagues persuasive, I am concerned about this study’s limitations, of which there are a few. First, analyses that focus on women diagnosed with breast cancers do not allow comparison of outcomes among screened and unscreened populations.
Moreover, this report provides no information on treatment received by screened and unscreened women. It is likely that women who have never been screened, or who have been screened only infrequently, are considerably less affluent and less educated than women who are regularly screened. Accordingly, upon noting a palpable breast mass, unscreened women may be less likely to seek timely medical attention than regularly screened women, leading to differences in breast cancer outcomes, which are independent of screening history.
How I counsel my patients
For now, I will continue to be laissez-fare in my recommendations about screening mammograms for average-risk women in their 40s by supporting their individual preferences about when to initiate such screening.
Controversy has surrounded the utility of screening mammograms, particularly in women in their 40s. In 2009, the US Preventive Services Task Force recommended that screening mammography begin at age 50 and that women aged 50 to 74 receive a mammogram every 2 years.1 However, the American Cancer Society2 and other professional groups continue to recommend that annual screening begin at age 40, leading to controversy and confusion among women’s health clinicians and our patients.
In a recent study, Webb and colleagues3 used registry data based on a health plan in a single US city to assess the cause of death and mammogram history of 1,705 women who died following a diagnosis of invasive breast cancer from 1990 to 1999. They confirmed that 609 of these deaths were from breast cancer. How many of these patients were screened?
What did they find?
The investigators found that 29% of the 609 women who died from breast cancer had been screened for it—19% of the cancers that caused death were screen-detected and 10% were interval cancers. (Interval cancers were defined as symptomatic or palpable tumors that presented less than 2 years after the prior screening mammogram.) That means that 71% of 609 deaths from breast cancer were among unscreened women, with 6% of the fatal cancers diagnosed more than 2 years after the last mammogram and 65% never found upon screening because screening did not occur.
Among deaths caused (n = 609) and not caused (n = 905) by breast cancer, the median age at diagnosis was 49 and 72 years, respectively. Investigators concluded that regular screening of women younger than age 50 years would lower the death rate from breast cancer.
Related Article: Biennial vs annual mammography: How I manage my patients Andrew M. Kaunitz, MD (June 2013)
Let’s not jump to any conclusions
Although some may find the report by Webb and colleagues persuasive, I am concerned about this study’s limitations, of which there are a few. First, analyses that focus on women diagnosed with breast cancers do not allow comparison of outcomes among screened and unscreened populations.
Moreover, this report provides no information on treatment received by screened and unscreened women. It is likely that women who have never been screened, or who have been screened only infrequently, are considerably less affluent and less educated than women who are regularly screened. Accordingly, upon noting a palpable breast mass, unscreened women may be less likely to seek timely medical attention than regularly screened women, leading to differences in breast cancer outcomes, which are independent of screening history.
How I counsel my patients
For now, I will continue to be laissez-fare in my recommendations about screening mammograms for average-risk women in their 40s by supporting their individual preferences about when to initiate such screening.
- Screening for breast cancer, Topic Page. US Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Updated July 2010. Accessed October 28, 2013.
- American Cancer Society Guidelines for the Early Detection of Cancer: Breast cancer. American Cancer Society Web site. http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Updated May 3, 2013. Accessed October 28, 2013.
- Webb ML, Cady B, Michaelson JS, et al. A failure analysis of invasive breast cancer: Most deaths from disease occur in women not regularly screened [published online ahead of print September 9, 2013]. Cancer. doi:10.1002/cncr.28199.
- Screening for breast cancer, Topic Page. US Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Updated July 2010. Accessed October 28, 2013.
- American Cancer Society Guidelines for the Early Detection of Cancer: Breast cancer. American Cancer Society Web site. http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Updated May 3, 2013. Accessed October 28, 2013.
- Webb ML, Cady B, Michaelson JS, et al. A failure analysis of invasive breast cancer: Most deaths from disease occur in women not regularly screened [published online ahead of print September 9, 2013]. Cancer. doi:10.1002/cncr.28199.
In young hysterectomized women, does unopposed estrogen therapy increase overall survival?
During the 1990s, more than 90% of hysterectomized women aged 50 to 59 years used ET following the procedure. When the initial findings of the WHI were published in 2002, they prompted many women to refuse or discontinue ET—despite the fact that the initial findings concerned the use of estrogen and progestin in combination in women with an intact uterus. Today, only some 30% of women use ET after hysterectomy.
When findings from the WHI estrogen-only arm were eventually published, they revealed that ET reduces mortality among women 50 to 59 years old, compared with placebo. Although most of the reduction in mortality relates to fewer deaths from coronary heart disease, a decline in deaths from breast cancer also was seen.2,3
Sarrel and colleagues calculated the excess mortality among US women aged 50 to 59 that could have been prevented by ET during the decade from 2002 through 2011. Their estimates ranged from approximately 19,000 deaths to as many as 92,000 deaths.
By calling attention to the negative health consequences of estrogen avoidance in young hysterectomized women, Sarrel and colleagues have performed a valuable public service.
Plethora of WHI data may have contributed to confusion
The WHI clinical trials have produced numerous analyses in various subsets of women. The sheer volume of data may be daunting in some cases, and likely has led to a failure to distinguish between estrogen-only and estrogen-progestin therapy, which have very different safety profiles.
Further, some clinicians and many patients have overlooked the fact that the risk-benefit profile of hormone therapy (both estrogen-only and estrogen-progestin therapy) is more favorable in younger, recently menopausal women than it is in older women.
I encounter evidence of this unwarranted fear of ET in my practice, with highly symptomatic, recently menopausal women who are appropriate candidates for hormone therapy electing to refuse the most effective treatment for menopausal symptoms.
Of course, hormone therapy, like all medications, has risks as well as benefits. For example, oral ET increases the risk of venous thrombosis and stroke, and long-term use of estrogen-progestin therapy increases the risk of breast cancer. However, the overblown fears of estrogen therapy have caused many appropriate candidates to miss out on symptom relief, prevention of osteoporosis, and treatment of symptomatic genital atrophy.
What this evidence means for practice
This provocative report demonstrates that wholesale avoidance of hormone therapy can have important negative public health consequence.
--Andrew M. Kaunitz, MD
We want to hear from you! Tell us what you think.
1. Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
2. LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–1314.
3. Anderson GL, Chlebowski RI, Aragaki AK, et al. Conjugated equine estrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012; 13(5):476–486.
During the 1990s, more than 90% of hysterectomized women aged 50 to 59 years used ET following the procedure. When the initial findings of the WHI were published in 2002, they prompted many women to refuse or discontinue ET—despite the fact that the initial findings concerned the use of estrogen and progestin in combination in women with an intact uterus. Today, only some 30% of women use ET after hysterectomy.
When findings from the WHI estrogen-only arm were eventually published, they revealed that ET reduces mortality among women 50 to 59 years old, compared with placebo. Although most of the reduction in mortality relates to fewer deaths from coronary heart disease, a decline in deaths from breast cancer also was seen.2,3
Sarrel and colleagues calculated the excess mortality among US women aged 50 to 59 that could have been prevented by ET during the decade from 2002 through 2011. Their estimates ranged from approximately 19,000 deaths to as many as 92,000 deaths.
By calling attention to the negative health consequences of estrogen avoidance in young hysterectomized women, Sarrel and colleagues have performed a valuable public service.
Plethora of WHI data may have contributed to confusion
The WHI clinical trials have produced numerous analyses in various subsets of women. The sheer volume of data may be daunting in some cases, and likely has led to a failure to distinguish between estrogen-only and estrogen-progestin therapy, which have very different safety profiles.
Further, some clinicians and many patients have overlooked the fact that the risk-benefit profile of hormone therapy (both estrogen-only and estrogen-progestin therapy) is more favorable in younger, recently menopausal women than it is in older women.
I encounter evidence of this unwarranted fear of ET in my practice, with highly symptomatic, recently menopausal women who are appropriate candidates for hormone therapy electing to refuse the most effective treatment for menopausal symptoms.
Of course, hormone therapy, like all medications, has risks as well as benefits. For example, oral ET increases the risk of venous thrombosis and stroke, and long-term use of estrogen-progestin therapy increases the risk of breast cancer. However, the overblown fears of estrogen therapy have caused many appropriate candidates to miss out on symptom relief, prevention of osteoporosis, and treatment of symptomatic genital atrophy.
What this evidence means for practice
This provocative report demonstrates that wholesale avoidance of hormone therapy can have important negative public health consequence.
--Andrew M. Kaunitz, MD
We want to hear from you! Tell us what you think.
During the 1990s, more than 90% of hysterectomized women aged 50 to 59 years used ET following the procedure. When the initial findings of the WHI were published in 2002, they prompted many women to refuse or discontinue ET—despite the fact that the initial findings concerned the use of estrogen and progestin in combination in women with an intact uterus. Today, only some 30% of women use ET after hysterectomy.
When findings from the WHI estrogen-only arm were eventually published, they revealed that ET reduces mortality among women 50 to 59 years old, compared with placebo. Although most of the reduction in mortality relates to fewer deaths from coronary heart disease, a decline in deaths from breast cancer also was seen.2,3
Sarrel and colleagues calculated the excess mortality among US women aged 50 to 59 that could have been prevented by ET during the decade from 2002 through 2011. Their estimates ranged from approximately 19,000 deaths to as many as 92,000 deaths.
By calling attention to the negative health consequences of estrogen avoidance in young hysterectomized women, Sarrel and colleagues have performed a valuable public service.
Plethora of WHI data may have contributed to confusion
The WHI clinical trials have produced numerous analyses in various subsets of women. The sheer volume of data may be daunting in some cases, and likely has led to a failure to distinguish between estrogen-only and estrogen-progestin therapy, which have very different safety profiles.
Further, some clinicians and many patients have overlooked the fact that the risk-benefit profile of hormone therapy (both estrogen-only and estrogen-progestin therapy) is more favorable in younger, recently menopausal women than it is in older women.
I encounter evidence of this unwarranted fear of ET in my practice, with highly symptomatic, recently menopausal women who are appropriate candidates for hormone therapy electing to refuse the most effective treatment for menopausal symptoms.
Of course, hormone therapy, like all medications, has risks as well as benefits. For example, oral ET increases the risk of venous thrombosis and stroke, and long-term use of estrogen-progestin therapy increases the risk of breast cancer. However, the overblown fears of estrogen therapy have caused many appropriate candidates to miss out on symptom relief, prevention of osteoporosis, and treatment of symptomatic genital atrophy.
What this evidence means for practice
This provocative report demonstrates that wholesale avoidance of hormone therapy can have important negative public health consequence.
--Andrew M. Kaunitz, MD
We want to hear from you! Tell us what you think.
1. Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
2. LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–1314.
3. Anderson GL, Chlebowski RI, Aragaki AK, et al. Conjugated equine estrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012; 13(5):476–486.
1. Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
2. LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–1314.
3. Anderson GL, Chlebowski RI, Aragaki AK, et al. Conjugated equine estrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012; 13(5):476–486.
Battle of the Contraceptives
Because half of US pregnancies continue to be unintended, rates of induced abortion in our patients remain high. In addition, unintended pregnancies lead to negative health and social consequences for women and infants. A report from the Contraceptive Choice Project, spearheaded by Dr. Jeffrey Peipert from the Department of Obstetrics and Gynecology, Washington University School of Medicine, St. Louis, Missouri, and published in New England Journal of Medicine, underscores the high efficacy of long-acting reversible contraceptives, a term referring to intrauterine devices (IUDs) and the contraceptive implant, in preventing unintended pregnancy in a US population.
Details of the study
Eligibility criteria for the Project included age 14 to 45, residence in the St. Louis, Missouri, area, and the need for contraception. The woman’s contraceptive of choice was made available at no charge, with most women choosing a long-acting method. The published study includes outcomes for
7,486 women who used oral contraceptives (OCs), the patch, the ring, an IUD, an implant, or depot medroxyprogesterone acetate (DMPA) injections.
Among women using OCs, the patch, or the ring, the pregnancy rate was 4.55 per 100 participant-years. This rate was nearly 22-fold higher than that observed in women using IUDs or the implant (hazard ratio, 21.8): that rate was 0.27 per 100 participant-years. A similar low rate of pregnancy was noted among women who chose DMPA and returned every three months for follow-up injections.
Among women younger than 21 who used OCs, the patch, or the ring, the rate of unintended pregnancy was twice as high as in older women using these same methods. By contrast, regardless of age, pregnancy rates were uniformly low among women using long-acting methods.
Study limitations
The authors point out that their study design was not randomized—participants were at high risk for unintended pregnancy and willing to begin using a new contraceptive method, which could have resulted in higher adherence rates and lower failure rates.
Access is ongoing
barrier to use
These important data from the Contraceptive Choice Project clarify that long-acting reversible contraceptives represent powerful tools to help women minimize unintended pregnancy and induced abortion, and that women will choose these methods if they are accessible.
The findings in this report also make it clear that rates of unintended pregnancy are particularly high among adolescents using shorter-acting hor monal contraceptives (OCs, patch, or ring) and that longer-acting contraceptives are particularly useful in our younger patients. Other recent reports have provided clear evidence that immediately providing long-acting contraceptives after childbirth or induced abortion reduces unintended pregnancy in these settings.1,2
In the US, inadequate access to long-acting reversible contraceptives continues to constrain use. Accordingly, insurance pol icies that fully cover longer-acting contraceptives could go a long way toward reducing the rate of unintended pregnancies and induced abortions in our patients.
If long-acting reversible contraceptives become more widely available in the US, I look forward to a time when the great majority of our patients’ pregnancies are planned and when far fewer women will face the troubling prospect of an induced abortion.
References
1. Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481.e1–e7.
2. Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT; Post-Aspiration IUD Randomization (PAIR) Study Trial Group. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364(23):2208–2217.
Because half of US pregnancies continue to be unintended, rates of induced abortion in our patients remain high. In addition, unintended pregnancies lead to negative health and social consequences for women and infants. A report from the Contraceptive Choice Project, spearheaded by Dr. Jeffrey Peipert from the Department of Obstetrics and Gynecology, Washington University School of Medicine, St. Louis, Missouri, and published in New England Journal of Medicine, underscores the high efficacy of long-acting reversible contraceptives, a term referring to intrauterine devices (IUDs) and the contraceptive implant, in preventing unintended pregnancy in a US population.
Details of the study
Eligibility criteria for the Project included age 14 to 45, residence in the St. Louis, Missouri, area, and the need for contraception. The woman’s contraceptive of choice was made available at no charge, with most women choosing a long-acting method. The published study includes outcomes for
7,486 women who used oral contraceptives (OCs), the patch, the ring, an IUD, an implant, or depot medroxyprogesterone acetate (DMPA) injections.
Among women using OCs, the patch, or the ring, the pregnancy rate was 4.55 per 100 participant-years. This rate was nearly 22-fold higher than that observed in women using IUDs or the implant (hazard ratio, 21.8): that rate was 0.27 per 100 participant-years. A similar low rate of pregnancy was noted among women who chose DMPA and returned every three months for follow-up injections.
Among women younger than 21 who used OCs, the patch, or the ring, the rate of unintended pregnancy was twice as high as in older women using these same methods. By contrast, regardless of age, pregnancy rates were uniformly low among women using long-acting methods.
Study limitations
The authors point out that their study design was not randomized—participants were at high risk for unintended pregnancy and willing to begin using a new contraceptive method, which could have resulted in higher adherence rates and lower failure rates.
Access is ongoing
barrier to use
These important data from the Contraceptive Choice Project clarify that long-acting reversible contraceptives represent powerful tools to help women minimize unintended pregnancy and induced abortion, and that women will choose these methods if they are accessible.
The findings in this report also make it clear that rates of unintended pregnancy are particularly high among adolescents using shorter-acting hor monal contraceptives (OCs, patch, or ring) and that longer-acting contraceptives are particularly useful in our younger patients. Other recent reports have provided clear evidence that immediately providing long-acting contraceptives after childbirth or induced abortion reduces unintended pregnancy in these settings.1,2
In the US, inadequate access to long-acting reversible contraceptives continues to constrain use. Accordingly, insurance pol icies that fully cover longer-acting contraceptives could go a long way toward reducing the rate of unintended pregnancies and induced abortions in our patients.
If long-acting reversible contraceptives become more widely available in the US, I look forward to a time when the great majority of our patients’ pregnancies are planned and when far fewer women will face the troubling prospect of an induced abortion.
References
1. Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481.e1–e7.
2. Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT; Post-Aspiration IUD Randomization (PAIR) Study Trial Group. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364(23):2208–2217.
Because half of US pregnancies continue to be unintended, rates of induced abortion in our patients remain high. In addition, unintended pregnancies lead to negative health and social consequences for women and infants. A report from the Contraceptive Choice Project, spearheaded by Dr. Jeffrey Peipert from the Department of Obstetrics and Gynecology, Washington University School of Medicine, St. Louis, Missouri, and published in New England Journal of Medicine, underscores the high efficacy of long-acting reversible contraceptives, a term referring to intrauterine devices (IUDs) and the contraceptive implant, in preventing unintended pregnancy in a US population.
Details of the study
Eligibility criteria for the Project included age 14 to 45, residence in the St. Louis, Missouri, area, and the need for contraception. The woman’s contraceptive of choice was made available at no charge, with most women choosing a long-acting method. The published study includes outcomes for
7,486 women who used oral contraceptives (OCs), the patch, the ring, an IUD, an implant, or depot medroxyprogesterone acetate (DMPA) injections.
Among women using OCs, the patch, or the ring, the pregnancy rate was 4.55 per 100 participant-years. This rate was nearly 22-fold higher than that observed in women using IUDs or the implant (hazard ratio, 21.8): that rate was 0.27 per 100 participant-years. A similar low rate of pregnancy was noted among women who chose DMPA and returned every three months for follow-up injections.
Among women younger than 21 who used OCs, the patch, or the ring, the rate of unintended pregnancy was twice as high as in older women using these same methods. By contrast, regardless of age, pregnancy rates were uniformly low among women using long-acting methods.
Study limitations
The authors point out that their study design was not randomized—participants were at high risk for unintended pregnancy and willing to begin using a new contraceptive method, which could have resulted in higher adherence rates and lower failure rates.
Access is ongoing
barrier to use
These important data from the Contraceptive Choice Project clarify that long-acting reversible contraceptives represent powerful tools to help women minimize unintended pregnancy and induced abortion, and that women will choose these methods if they are accessible.
The findings in this report also make it clear that rates of unintended pregnancy are particularly high among adolescents using shorter-acting hor monal contraceptives (OCs, patch, or ring) and that longer-acting contraceptives are particularly useful in our younger patients. Other recent reports have provided clear evidence that immediately providing long-acting contraceptives after childbirth or induced abortion reduces unintended pregnancy in these settings.1,2
In the US, inadequate access to long-acting reversible contraceptives continues to constrain use. Accordingly, insurance pol icies that fully cover longer-acting contraceptives could go a long way toward reducing the rate of unintended pregnancies and induced abortions in our patients.
If long-acting reversible contraceptives become more widely available in the US, I look forward to a time when the great majority of our patients’ pregnancies are planned and when far fewer women will face the troubling prospect of an induced abortion.
References
1. Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481.e1–e7.
2. Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT; Post-Aspiration IUD Randomization (PAIR) Study Trial Group. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364(23):2208–2217.
Is there a link between impaired mobility and urinary incontinence in elderly, community-dwelling women?
Urinary incontinence affects more than one-third of women aged 70 years or older. As the authors of this study point out, urinary incontinence is comparable to other chronic conditions, such as hypertension and diabetes mellitus, in its impact on quality of life, and is a common reason for institutionalization.
The risk of urinary incontinence increases with age. In elderly women, it is often mixed (ie, having both urge- and stress-related components) and associated with functional impairments, including reduced mobility. It is thought that the association between incontinence and functional impairment is related primarily to the urge component:
- Women with impaired mobility take longer to reach the toilet, increasing the risk of leakage when the urge to urinate is strong
- Women with urge incontinence may be more likely to limit their activities so that they are always near a toilet.
Cognitive impairment may also play a role, affecting motor skills and bladder control.
To better understand why advancing age is linked with urinary incontinence, Fritel and colleagues studied a population of 1,942 urban-dwelling French women aged 75 to 85 years (mean age, 79.3 years; mean body mass index, 25.9 kg/m2).
Details of the study
Investigators assessed the frequency and quantity of urine leaks, the impact of urinary incontinence on daily life, and the participants’ mobility and balance. Data on urinary incontinence were collected via a self-administered questionnaire (the International Consultation on Incontinence Questionnaire–Short Form). Motor-related physical function was assessed using standardized balance and gait tests.
Urinary incontinence was reported by 42% of participants. Of these women, 57% reported daily urine leakage, with mixed incontinence found to be more prevalent than urge incontinence, which was more prevalent than stress incontinence. Overall, women with urinary incontinence reported that its impact on daily life was mild. Among those with mixed or urge incontinence, limitations in mobility and balance were correlated significantly with the severity of incontinence.
What this evidence means for practice
These findings support earlier data suggesting that impaired mobility can promote urge and mixed urinary incontinence. Because there also is a possibility that cerebral deterioration in aging women causes gait and balance problems that increase the likelihood of urge incontinence, the authors advise against the use of anticholinergic agents in elderly women, as these drugs can impair cognitive function. Another take-home message from this French report is that we should counsel elderly patients about the importance of maintaining balance and mobility through exercise, physical therapy, or other strategies.
Andrew M. Kaunitz, MD
Urinary incontinence affects more than one-third of women aged 70 years or older. As the authors of this study point out, urinary incontinence is comparable to other chronic conditions, such as hypertension and diabetes mellitus, in its impact on quality of life, and is a common reason for institutionalization.
The risk of urinary incontinence increases with age. In elderly women, it is often mixed (ie, having both urge- and stress-related components) and associated with functional impairments, including reduced mobility. It is thought that the association between incontinence and functional impairment is related primarily to the urge component:
- Women with impaired mobility take longer to reach the toilet, increasing the risk of leakage when the urge to urinate is strong
- Women with urge incontinence may be more likely to limit their activities so that they are always near a toilet.
Cognitive impairment may also play a role, affecting motor skills and bladder control.
To better understand why advancing age is linked with urinary incontinence, Fritel and colleagues studied a population of 1,942 urban-dwelling French women aged 75 to 85 years (mean age, 79.3 years; mean body mass index, 25.9 kg/m2).
Details of the study
Investigators assessed the frequency and quantity of urine leaks, the impact of urinary incontinence on daily life, and the participants’ mobility and balance. Data on urinary incontinence were collected via a self-administered questionnaire (the International Consultation on Incontinence Questionnaire–Short Form). Motor-related physical function was assessed using standardized balance and gait tests.
Urinary incontinence was reported by 42% of participants. Of these women, 57% reported daily urine leakage, with mixed incontinence found to be more prevalent than urge incontinence, which was more prevalent than stress incontinence. Overall, women with urinary incontinence reported that its impact on daily life was mild. Among those with mixed or urge incontinence, limitations in mobility and balance were correlated significantly with the severity of incontinence.
What this evidence means for practice
These findings support earlier data suggesting that impaired mobility can promote urge and mixed urinary incontinence. Because there also is a possibility that cerebral deterioration in aging women causes gait and balance problems that increase the likelihood of urge incontinence, the authors advise against the use of anticholinergic agents in elderly women, as these drugs can impair cognitive function. Another take-home message from this French report is that we should counsel elderly patients about the importance of maintaining balance and mobility through exercise, physical therapy, or other strategies.
Andrew M. Kaunitz, MD
Urinary incontinence affects more than one-third of women aged 70 years or older. As the authors of this study point out, urinary incontinence is comparable to other chronic conditions, such as hypertension and diabetes mellitus, in its impact on quality of life, and is a common reason for institutionalization.
The risk of urinary incontinence increases with age. In elderly women, it is often mixed (ie, having both urge- and stress-related components) and associated with functional impairments, including reduced mobility. It is thought that the association between incontinence and functional impairment is related primarily to the urge component:
- Women with impaired mobility take longer to reach the toilet, increasing the risk of leakage when the urge to urinate is strong
- Women with urge incontinence may be more likely to limit their activities so that they are always near a toilet.
Cognitive impairment may also play a role, affecting motor skills and bladder control.
To better understand why advancing age is linked with urinary incontinence, Fritel and colleagues studied a population of 1,942 urban-dwelling French women aged 75 to 85 years (mean age, 79.3 years; mean body mass index, 25.9 kg/m2).
Details of the study
Investigators assessed the frequency and quantity of urine leaks, the impact of urinary incontinence on daily life, and the participants’ mobility and balance. Data on urinary incontinence were collected via a self-administered questionnaire (the International Consultation on Incontinence Questionnaire–Short Form). Motor-related physical function was assessed using standardized balance and gait tests.
Urinary incontinence was reported by 42% of participants. Of these women, 57% reported daily urine leakage, with mixed incontinence found to be more prevalent than urge incontinence, which was more prevalent than stress incontinence. Overall, women with urinary incontinence reported that its impact on daily life was mild. Among those with mixed or urge incontinence, limitations in mobility and balance were correlated significantly with the severity of incontinence.
What this evidence means for practice
These findings support earlier data suggesting that impaired mobility can promote urge and mixed urinary incontinence. Because there also is a possibility that cerebral deterioration in aging women causes gait and balance problems that increase the likelihood of urge incontinence, the authors advise against the use of anticholinergic agents in elderly women, as these drugs can impair cognitive function. Another take-home message from this French report is that we should counsel elderly patients about the importance of maintaining balance and mobility through exercise, physical therapy, or other strategies.
Andrew M. Kaunitz, MD
Do cosmetic breast implants hinder the detection of malignancy and reduce breast cancer–specific survival?
Most epidemiologic studies have found no elevated risk of breast cancer among women who undergo cosmetic breast augmentation. However, there is concern that implants, which are radio-opaque, may limit our ability to diagnose malignancies at an early stage using screening mammography.
In this study, investigators compared the stage distribution of breast cancers at diagnosis and documented breast cancer–specific survival among women with and without cosmetic breast implants. Twelve cross-sectional studies published after 2000 in the United States had evaluated stage distribution of breast cancer among women with and without cosmetic implants. As stated above, investigators found an elevated risk of nonlocalized breast cancer among women with implants in their meta-analysis of these studies (OR, 1.26), but this elevated risk did not achieve statistical significance. A second analysis of five studies found an elevated risk of breast cancer–specific mortality (OR, 1.38), compared with the general population (no implants), which did achieve significance.
MRI may be helpful—but is the expense justified?
More than 300,000 women underwent cosmetic breast augmentation in 2011 in the United States, an increase of roughly 800% since the early 1990s. The impaired visualization of breast tissue via mammography in these women ranges from 22% to 83%. In addition, the implants limit compression of the breasts during mammography, and capsular contraction further contributes to this problem.
Magnetic resonance imaging (MRI) may be helpful in screening women with cosmetic breast implants, but this technology is expensive, and evidence supporting its routine use in this population is limited.
Some mammographers use special techniques to better visualize the breast tissue of women with implants. These techniques include displacing the implant posteriorly and pulling the breast tissue in front of it. However, even with such strategies, as much as one-third of the breast tissue may be inadequately assessed.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These findings underscore the importance of sharing the risks of nonlocalized breast malignancy and increased breast cancer mortality with patients who are considering cosmetic breast implants, as well as with women who have already undergone this common procedure. Future studies are needed to address relevant issues, including the role of 3-D (tomosynthesis) technology in screening women with breast implants and optimal screening intervals in this subgroup.
ANDREW M. KAUNITZ, MD
We want to hear from you. Tell us what you think.
Most epidemiologic studies have found no elevated risk of breast cancer among women who undergo cosmetic breast augmentation. However, there is concern that implants, which are radio-opaque, may limit our ability to diagnose malignancies at an early stage using screening mammography.
In this study, investigators compared the stage distribution of breast cancers at diagnosis and documented breast cancer–specific survival among women with and without cosmetic breast implants. Twelve cross-sectional studies published after 2000 in the United States had evaluated stage distribution of breast cancer among women with and without cosmetic implants. As stated above, investigators found an elevated risk of nonlocalized breast cancer among women with implants in their meta-analysis of these studies (OR, 1.26), but this elevated risk did not achieve statistical significance. A second analysis of five studies found an elevated risk of breast cancer–specific mortality (OR, 1.38), compared with the general population (no implants), which did achieve significance.
MRI may be helpful—but is the expense justified?
More than 300,000 women underwent cosmetic breast augmentation in 2011 in the United States, an increase of roughly 800% since the early 1990s. The impaired visualization of breast tissue via mammography in these women ranges from 22% to 83%. In addition, the implants limit compression of the breasts during mammography, and capsular contraction further contributes to this problem.
Magnetic resonance imaging (MRI) may be helpful in screening women with cosmetic breast implants, but this technology is expensive, and evidence supporting its routine use in this population is limited.
Some mammographers use special techniques to better visualize the breast tissue of women with implants. These techniques include displacing the implant posteriorly and pulling the breast tissue in front of it. However, even with such strategies, as much as one-third of the breast tissue may be inadequately assessed.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These findings underscore the importance of sharing the risks of nonlocalized breast malignancy and increased breast cancer mortality with patients who are considering cosmetic breast implants, as well as with women who have already undergone this common procedure. Future studies are needed to address relevant issues, including the role of 3-D (tomosynthesis) technology in screening women with breast implants and optimal screening intervals in this subgroup.
ANDREW M. KAUNITZ, MD
We want to hear from you. Tell us what you think.
Most epidemiologic studies have found no elevated risk of breast cancer among women who undergo cosmetic breast augmentation. However, there is concern that implants, which are radio-opaque, may limit our ability to diagnose malignancies at an early stage using screening mammography.
In this study, investigators compared the stage distribution of breast cancers at diagnosis and documented breast cancer–specific survival among women with and without cosmetic breast implants. Twelve cross-sectional studies published after 2000 in the United States had evaluated stage distribution of breast cancer among women with and without cosmetic implants. As stated above, investigators found an elevated risk of nonlocalized breast cancer among women with implants in their meta-analysis of these studies (OR, 1.26), but this elevated risk did not achieve statistical significance. A second analysis of five studies found an elevated risk of breast cancer–specific mortality (OR, 1.38), compared with the general population (no implants), which did achieve significance.
MRI may be helpful—but is the expense justified?
More than 300,000 women underwent cosmetic breast augmentation in 2011 in the United States, an increase of roughly 800% since the early 1990s. The impaired visualization of breast tissue via mammography in these women ranges from 22% to 83%. In addition, the implants limit compression of the breasts during mammography, and capsular contraction further contributes to this problem.
Magnetic resonance imaging (MRI) may be helpful in screening women with cosmetic breast implants, but this technology is expensive, and evidence supporting its routine use in this population is limited.
Some mammographers use special techniques to better visualize the breast tissue of women with implants. These techniques include displacing the implant posteriorly and pulling the breast tissue in front of it. However, even with such strategies, as much as one-third of the breast tissue may be inadequately assessed.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These findings underscore the importance of sharing the risks of nonlocalized breast malignancy and increased breast cancer mortality with patients who are considering cosmetic breast implants, as well as with women who have already undergone this common procedure. Future studies are needed to address relevant issues, including the role of 3-D (tomosynthesis) technology in screening women with breast implants and optimal screening intervals in this subgroup.
ANDREW M. KAUNITZ, MD
We want to hear from you. Tell us what you think.