User login
Treatment of Seborrheic Dermatitis in Black Patients
Treatment of Seborrheic Dermatitis in Black Patients
Seborrheic dermatitis (SD) is a common chronic inflammatory skin condition that predominantly affects areas with high concentrations of sebaceous glands such as the scalp and face. Up to 5% of the worldwide population is affected by SD each year, causing a major burden of disease for patients and the health care system.1 In 2023, the cost of medical treatment for SD in the United States was $300 million, with outpatient office visits alone costing $58 million and prescription drugs costing $109 million. Indirect costs of disease (eg, lost workdays) account for another $51 million.1 Since SD frequently manifests on the face, it tends to have negative effects on the patient’s quality of life, resulting in psychological distress and low self-esteem.2
Patients with SD may describe symptoms of excessive dandruff and itching along with hyperpigmentation or hypopigmentation of the skin; Black patients tend to present with the classic manifestations: a combination of scaling, flaking, and erythematous patches on the scalp, ears, and face, particularly around the eyebrows, eyelids, and nose. With SD being the second most common diagnosis in Black patients who seek care from a dermatologist, it is important to have effective treatment approaches for SD in this patient population.3
In this study, we aimed to evaluate medical and nonmedical treatment options for SD in Black patients by identifying common practices and products mentioned on consumer websites and in the medical literature.
Methods
A Google search was conducted during 2 time periods (September 2022—October 2022 and March 2023—April 2023) using the terms products for itchy scalp in Black patients, products for dandruff in Black patients, itchy scalp in Black women, itchy scalp in Black men, treatment for scalp itch in Black patients, and dry scalp in Black hair. Products that were recommended by at least 1 website on the first page of search results were included in our list of products, and the ingredients were reviewed by the authors. We excluded individual retailer websites as well as those that did not provide specific recommendations on products or ingredients to use when treating SD. To ensure reliability and standardization, we did not review products that were suggested by ads in the shopping section on the first page of search results.
We also evaluated medical treatments used for SD in dermatology literature. A PubMed search of articles indexed for MEDLINE using the terms seborrheic dermatitis treatment for Black patients, treatment for dandruff for Black patients, and seborrheic dermatitis and skin of color was conducted. We excluded articles that did not address treatment options for SD, were specific to treating SD in patient populations with specific comorbidities being studied, discussed SD in animals, or were published prior to 1990.
Results
We identified 16 unique consumer websites with product or ingredient recommendations for SD in Black patients, none of which were provided by authors with a medical or scientific background; however, 4 (25%) websites included insights from board-certified dermatologists. A total of 16 ingredients were recommended, 15 (94%) of which were mentioned at least twice in our search results (eTable 1).
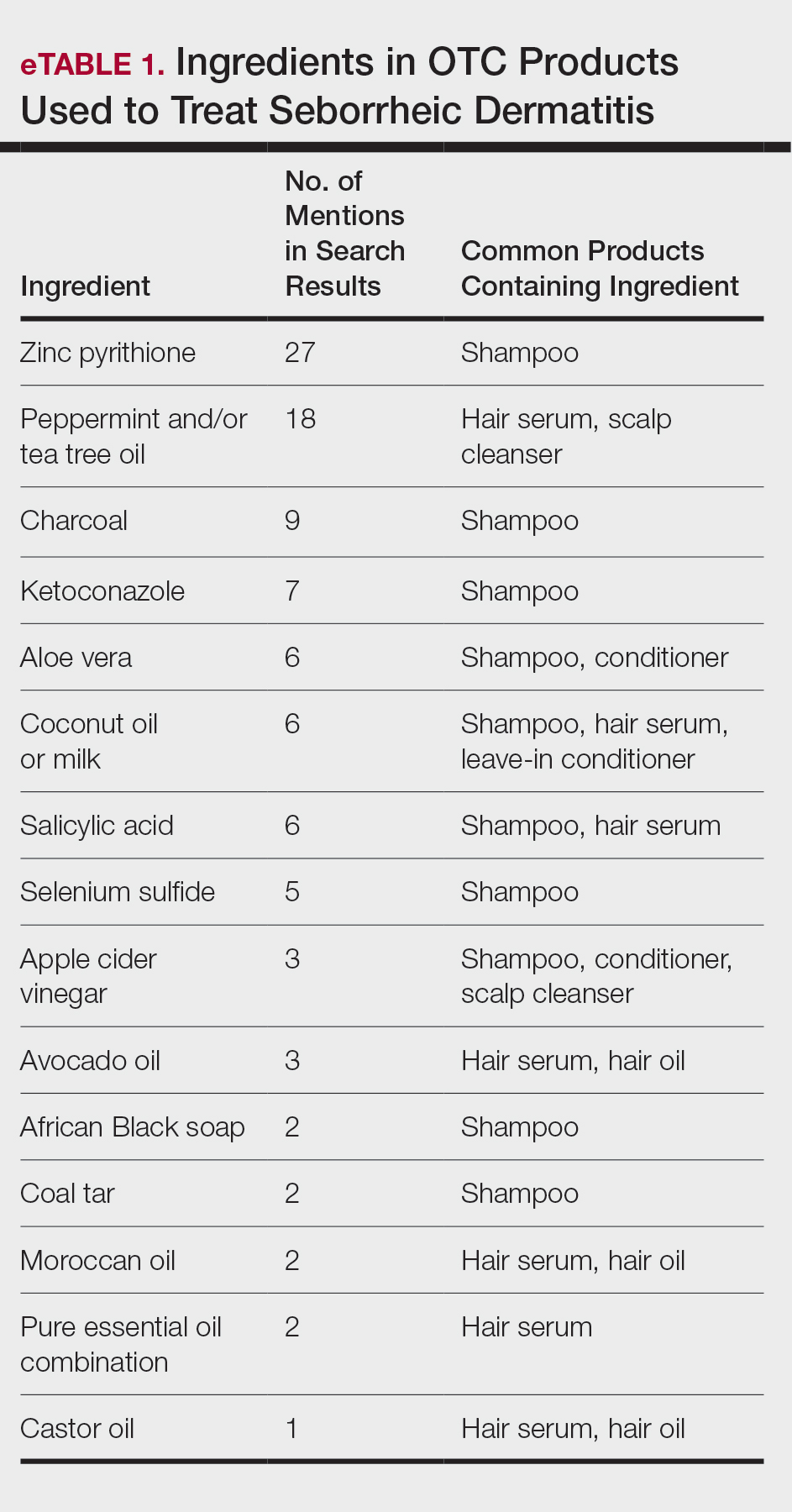
Overall, we noticed that ingredients labeled as natural or organic were common in over-the-counter (OTC) products, and ingredients such as sulfates and parabens were avoided. Common OTC ingredients for antidandruff and anti-itch shampoos and conditioners include zinc pyrithione, selenium sulfide, coal tar, salicylic acid, and citric acid. Additionally, coconut oil, tea tree oil, apple cider vinegar, and charcoal are common natural alternatives used to address SD symptoms.
Our review of the literature yielded limited recommendations tailored specifically to Black patients with SD. Of 108 abstracts, articles, or textbook chapters providing treatment recommendations for SD, 6 (6%) specifically discussed treatments for Black patients. All articles were written by authors with medical or scientific backgrounds. Of the treatment options discussed, topical antifungals generally were considered first-line for SD in all patients, with ketoconazole shampoo being a common first choice.4,5
Comment
Our study indicated that many consumer websites recommend unstudied nonmedical treatments for SD. Zinc pyrithione was one of the most commonly mentioned ingredients in OTC products to treat SD targeted toward Black patients, as its properties have contributed to ease of hair combing and less frizz.6 Zinc pyrithione has antifungal properties that reduce the proliferation of Malassezia furfur as well as anti-inflammatory properties that reduce irritation, pruritus, and erythema in areas affected by SD.7 Tea tree and peppermint oils also were commonly mentioned; the theory is that these oils mitigate SD by reducing yeast growth and soothing inflammation through antioxidant activity.8,9 Coal tar also is used due to its keratoplastic properties, which slow the growth of skin cells and ultimately reduce scaling and dryness.10 Yeast thrives in basic pH conditions; apple cider vinegar is used as an ingredient in OTC products for SD because its acidic pH creates a less favorable environment for yeast to grow.11 Although many of the ingredients found in OTC products we identified have not yet been studied, they have properties that theoretically would be helpful in treating SD.
Our review of the medical literature revealed that while there are treatments that are effective for SD, the recommended use may not consider the cultural differences that exist for Black patients. For instance, reports in the literature regarding ketoconazole shampoo revealed that ketoconazole increases the risk for hair shaft dryness, damage, and subsequent breakage, especially in Black women who also may be using heat styling or chemical relaxers.5 As a result, ketoconazole should be used with caution in Black women, with an emphasis on direct application to the scalp rather than the hair shafts.12 Additional options reported for Black patients include ciclopirox olamine and zinc pyrithione, which may have fewer risks.13
When prescribing medicated shampoos, traditional instructions regarding frequency of use to control symptoms of SD range from 2 to 3 times weekly to daily for a specified period of time determined by the dermatologist.14 However, frequency of hair washing varies greatly among Black patients, sometimes occurring only once monthly. The frequency also may change based on styling techniques (eg, braids, weaves, and wigs).15 Based on previous research underscoring the tendency for Black patients to use medicated shampoos less frequently than White patients, it is important for clinicians to understand that these cultural practices can undermine the effectiveness when medicated shampoos are prescribed for SD.16
Additionally, topical corticosteroids often are used in conjunction with antifungals to help decrease inflammation of the scalp.17 An option reported for Black patients is topical fluocinolone 0.01%; however, package instructions state to apply topically to the scalp nightly and wash the hair thoroughly each morning, which may not be feasible for Black patients based on previously mentioned differences in hair-washing techniques. An alternative option may be to apply the medication 3 to 4 times per week, washing the hair weekly rather than daily.18 Fluocinolone can be used as an ointment, solution, oil, or cream.19,20 When comparing treatment vehicles for SD, a study conducted by Chappell et al21 found that Black patients preferred using ointment or oil vehicles; White patients preferred foams and sprays, which may not be suitable for Afro hair patterns. As such, using less-drying modalities may increase compliance and treatment success in Black patients. For patients who may have involvement on the hairline, face, or ears along with hypopigmentation (which is a common skin concern associated with SD), calcineurin inhibitors can be used until resolution occurs.5,22 High et al15 found that twice-daily use of pimecrolimus rapidly normalized skin pigmentation during the first 2 weeks of use. Overall, personalization of treatment may not only avoid adverse effects but also ensure patient compliance, with the overall goal of treating to reduce yeast activity, pruritus, and dyschromia.22
Interestingly, after the website searches were completed for this study, the US Food and Drug Administration approved topical roflumilast foam for SD. In a phase III trial of 457 total patients, 36 Black patients were included.23 It was determined that 79.5% of patients overall throughout the trial achieved Investigator Global Assessment success (score of 0 [clear] or 1 [almost clear]) plus ≥2-point improvement from baseline (on a scale of 0 [clear] to 4 [severe]) at weeks 2, 4, and 8. Although there currently are no long-term studies, roflumilast may be a promising option for Black patients with SD.23
Aside from developing an individualized treatment approach for Black patients with SD, it is important to ask targeted questions during the clinical encounter to identify factors that may be exacerbating symptoms, especially due to the wide range of hair care practices used by the Black community (eTable 2). Asking targeted questions is especially important, as prior studies have shown that extensions, hair relaxers, and particular hair products can irritate the scalp and increase the likelihood of developing SD.21,24 Rucker Wright et al25 evaluated different hair care practices among young Black females and their association with the development of SD. The authors found that using hair extensions (either braided, cornrowed, or ponytails), chemical relaxers, and hair oils every 2 weeks was associated with SD. The study also found that SD rates were roughly 20% higher among Black girls with extensions compared to Black girls without extensions, regardless of how frequently hair was washed.25

Many Black patients grease the scalp with oils that are beneficial for lubrication and reduction of abrasive damage caused by grooming; however, they also may increase incidence of SD.26 Tight curls worn by Black patients also can impede sebum from traveling down the hair shaft, leading to oil buildup on the scalp. This is the ideal environment for increased Malassezia density and higher risk for SD development.27 To balance the beneficial effects of hair oils with the increased susceptibility for SD, providers should emphasize applying these oils only to distal hair shafts, which are more likely to be damaged, and avoiding application to the scalp.19
Conclusion
Given its long-term relapsing and remitting nature, SD can be distressing for Black patients, many of whom may seek additional treatment options aside from those recommended by health care professionals. In order to better educate patients, it is important for dermatologists to know not only the common ingredients that may be present in OTC products but also the thought process behind why patients use them. Additionally, prescription treatments for Black patients with SD may require nuanced alterations to the product instructions that may prevent health disparities and provide culturally sensitive care. Overall, the literature regarding treatment for Black patients with SD is limited, and more high-quality studies are needed.
- Tucker D, Masood S. Seborrheic dermatitis. StatPearls [Internet]. Updated March 1, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551707/
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3:10.13188 /2373-1044.1000019.
- American Academy of Dermatology. Seborrheic dermatitis by the numbers. American Academy of Dermatology Skin Disease Briefs. Updated May 5, 2018. Accessed November 22, 2024. https://www.aad.org/asset/49w949DPcF8RSJYIRHfDon
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158-169.
- Draelos ZD, Kenneally DC, Hodges LT, et al. A comparison of hair quality and cosmetic acceptance following the use of two anti-dandruff shampoos. J Investig Dermatol Symp Proc. 2005;10:201-214.
- Barak-Shinar D, Green LJ. Scalp seborrheic dermatitis and dandruff therapy using a herbal and zinc pyrithione-based therapy of shampoo and scalp lotion. J Clin Aesthet Dermatol. 2018;11:26-31.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of dandruff with 5% tea tree oil shampoo. J Am Acad Dermatol. 2002;47:852-855.
- Herro E, Jacob SE. Mentha piperita (peppermint). Dermatitis. 2010;21:327-329.
- Sanfilippo A, English JC. An overview of medicated shampoos used in dandruff treatment. Pharm Ther. 2006;31:396-400.
- Arun PVPS, Vineetha Y, Waheed M, et al. Quantification of the minimum amount of lemon juice and apple cider vinegar required for the growth inhibition of dandruff causing fungi Malassezia furfur. Int J Sci Res in Biological Sciences. 2019;6:144-147.
- Gao HY, Li Wan Po A. Topical formulations of fluocinolone acetonide. Are creams, gels and ointments bioequivalent and does dilution affect activity? Eur J Clin Pharmacol. 1994;46:71-75.
- Pauporte M, Maibach H, Lowe N, et al. Fluocinolone acetonide topical oil for scalp psoriasis. J Dermatolog Treat. 2004;15:360-364.
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- High WA, Pandya AG. Pilot trial of 1% pimecrolimus cream in the treatment of seborrheic dermatitis in African American adults with associated hypopigmentation. J Am Acad Dermatol. 2006;54:1083-1088.
- Hollins LC, Butt M, Hong J, et al. Research in brief: survey of hair care practices in various ethnic and racial pediatric populations. Pediatr Dermatol. 2022;39:494-496.
- Halder RM, Roberts CI, Nootheti PK. Cutaneous diseases in the black races. Dermatol Clin. 2003;21:679-687, ix.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Friedmann DP, Mishra V, Batty T. Progressive facial papules in an African- American patient: an atypical presentation of seborrheic dermatitis. J Clin Aesthet Dermatol. 2018;11:44-45.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Chappell J, Mattox A, Simonetta C, et al. Seborrheic dermatitis of the scalp in populations practicing less frequent hair washing: ketoconazole 2% foam versus ketoconazole 2% shampoo. three-year data. J Am Acad Dermatol. 2014;70:AB54.
- Dadzie OE, Salam A. The hair grooming practices of women of African descent in London, United Kingdom: findings of a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:1021-1024.
- Blauvelt A, Draelos ZD, Stein Gold L, et al. Roflumilast foam 0.3% for adolescent and adult patients with seborrheic dermatitis: a randomized, double-blinded, vehicle-controlled, phase 3 trial. J Am Acad Dermatol. 2024;90:986-993.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319.
- Mayo T, Dinkins J, Elewski B. Hair oils may worsen seborrheic dermatitis in Black patients. Skin Appendage Disord. 2023;9:151-152.
Seborrheic dermatitis (SD) is a common chronic inflammatory skin condition that predominantly affects areas with high concentrations of sebaceous glands such as the scalp and face. Up to 5% of the worldwide population is affected by SD each year, causing a major burden of disease for patients and the health care system.1 In 2023, the cost of medical treatment for SD in the United States was $300 million, with outpatient office visits alone costing $58 million and prescription drugs costing $109 million. Indirect costs of disease (eg, lost workdays) account for another $51 million.1 Since SD frequently manifests on the face, it tends to have negative effects on the patient’s quality of life, resulting in psychological distress and low self-esteem.2
Patients with SD may describe symptoms of excessive dandruff and itching along with hyperpigmentation or hypopigmentation of the skin; Black patients tend to present with the classic manifestations: a combination of scaling, flaking, and erythematous patches on the scalp, ears, and face, particularly around the eyebrows, eyelids, and nose. With SD being the second most common diagnosis in Black patients who seek care from a dermatologist, it is important to have effective treatment approaches for SD in this patient population.3
In this study, we aimed to evaluate medical and nonmedical treatment options for SD in Black patients by identifying common practices and products mentioned on consumer websites and in the medical literature.
Methods
A Google search was conducted during 2 time periods (September 2022—October 2022 and March 2023—April 2023) using the terms products for itchy scalp in Black patients, products for dandruff in Black patients, itchy scalp in Black women, itchy scalp in Black men, treatment for scalp itch in Black patients, and dry scalp in Black hair. Products that were recommended by at least 1 website on the first page of search results were included in our list of products, and the ingredients were reviewed by the authors. We excluded individual retailer websites as well as those that did not provide specific recommendations on products or ingredients to use when treating SD. To ensure reliability and standardization, we did not review products that were suggested by ads in the shopping section on the first page of search results.
We also evaluated medical treatments used for SD in dermatology literature. A PubMed search of articles indexed for MEDLINE using the terms seborrheic dermatitis treatment for Black patients, treatment for dandruff for Black patients, and seborrheic dermatitis and skin of color was conducted. We excluded articles that did not address treatment options for SD, were specific to treating SD in patient populations with specific comorbidities being studied, discussed SD in animals, or were published prior to 1990.
Results
We identified 16 unique consumer websites with product or ingredient recommendations for SD in Black patients, none of which were provided by authors with a medical or scientific background; however, 4 (25%) websites included insights from board-certified dermatologists. A total of 16 ingredients were recommended, 15 (94%) of which were mentioned at least twice in our search results (eTable 1).

Overall, we noticed that ingredients labeled as natural or organic were common in over-the-counter (OTC) products, and ingredients such as sulfates and parabens were avoided. Common OTC ingredients for antidandruff and anti-itch shampoos and conditioners include zinc pyrithione, selenium sulfide, coal tar, salicylic acid, and citric acid. Additionally, coconut oil, tea tree oil, apple cider vinegar, and charcoal are common natural alternatives used to address SD symptoms.
Our review of the literature yielded limited recommendations tailored specifically to Black patients with SD. Of 108 abstracts, articles, or textbook chapters providing treatment recommendations for SD, 6 (6%) specifically discussed treatments for Black patients. All articles were written by authors with medical or scientific backgrounds. Of the treatment options discussed, topical antifungals generally were considered first-line for SD in all patients, with ketoconazole shampoo being a common first choice.4,5
Comment
Our study indicated that many consumer websites recommend unstudied nonmedical treatments for SD. Zinc pyrithione was one of the most commonly mentioned ingredients in OTC products to treat SD targeted toward Black patients, as its properties have contributed to ease of hair combing and less frizz.6 Zinc pyrithione has antifungal properties that reduce the proliferation of Malassezia furfur as well as anti-inflammatory properties that reduce irritation, pruritus, and erythema in areas affected by SD.7 Tea tree and peppermint oils also were commonly mentioned; the theory is that these oils mitigate SD by reducing yeast growth and soothing inflammation through antioxidant activity.8,9 Coal tar also is used due to its keratoplastic properties, which slow the growth of skin cells and ultimately reduce scaling and dryness.10 Yeast thrives in basic pH conditions; apple cider vinegar is used as an ingredient in OTC products for SD because its acidic pH creates a less favorable environment for yeast to grow.11 Although many of the ingredients found in OTC products we identified have not yet been studied, they have properties that theoretically would be helpful in treating SD.
Our review of the medical literature revealed that while there are treatments that are effective for SD, the recommended use may not consider the cultural differences that exist for Black patients. For instance, reports in the literature regarding ketoconazole shampoo revealed that ketoconazole increases the risk for hair shaft dryness, damage, and subsequent breakage, especially in Black women who also may be using heat styling or chemical relaxers.5 As a result, ketoconazole should be used with caution in Black women, with an emphasis on direct application to the scalp rather than the hair shafts.12 Additional options reported for Black patients include ciclopirox olamine and zinc pyrithione, which may have fewer risks.13
When prescribing medicated shampoos, traditional instructions regarding frequency of use to control symptoms of SD range from 2 to 3 times weekly to daily for a specified period of time determined by the dermatologist.14 However, frequency of hair washing varies greatly among Black patients, sometimes occurring only once monthly. The frequency also may change based on styling techniques (eg, braids, weaves, and wigs).15 Based on previous research underscoring the tendency for Black patients to use medicated shampoos less frequently than White patients, it is important for clinicians to understand that these cultural practices can undermine the effectiveness when medicated shampoos are prescribed for SD.16
Additionally, topical corticosteroids often are used in conjunction with antifungals to help decrease inflammation of the scalp.17 An option reported for Black patients is topical fluocinolone 0.01%; however, package instructions state to apply topically to the scalp nightly and wash the hair thoroughly each morning, which may not be feasible for Black patients based on previously mentioned differences in hair-washing techniques. An alternative option may be to apply the medication 3 to 4 times per week, washing the hair weekly rather than daily.18 Fluocinolone can be used as an ointment, solution, oil, or cream.19,20 When comparing treatment vehicles for SD, a study conducted by Chappell et al21 found that Black patients preferred using ointment or oil vehicles; White patients preferred foams and sprays, which may not be suitable for Afro hair patterns. As such, using less-drying modalities may increase compliance and treatment success in Black patients. For patients who may have involvement on the hairline, face, or ears along with hypopigmentation (which is a common skin concern associated with SD), calcineurin inhibitors can be used until resolution occurs.5,22 High et al15 found that twice-daily use of pimecrolimus rapidly normalized skin pigmentation during the first 2 weeks of use. Overall, personalization of treatment may not only avoid adverse effects but also ensure patient compliance, with the overall goal of treating to reduce yeast activity, pruritus, and dyschromia.22
Interestingly, after the website searches were completed for this study, the US Food and Drug Administration approved topical roflumilast foam for SD. In a phase III trial of 457 total patients, 36 Black patients were included.23 It was determined that 79.5% of patients overall throughout the trial achieved Investigator Global Assessment success (score of 0 [clear] or 1 [almost clear]) plus ≥2-point improvement from baseline (on a scale of 0 [clear] to 4 [severe]) at weeks 2, 4, and 8. Although there currently are no long-term studies, roflumilast may be a promising option for Black patients with SD.23
Aside from developing an individualized treatment approach for Black patients with SD, it is important to ask targeted questions during the clinical encounter to identify factors that may be exacerbating symptoms, especially due to the wide range of hair care practices used by the Black community (eTable 2). Asking targeted questions is especially important, as prior studies have shown that extensions, hair relaxers, and particular hair products can irritate the scalp and increase the likelihood of developing SD.21,24 Rucker Wright et al25 evaluated different hair care practices among young Black females and their association with the development of SD. The authors found that using hair extensions (either braided, cornrowed, or ponytails), chemical relaxers, and hair oils every 2 weeks was associated with SD. The study also found that SD rates were roughly 20% higher among Black girls with extensions compared to Black girls without extensions, regardless of how frequently hair was washed.25

Many Black patients grease the scalp with oils that are beneficial for lubrication and reduction of abrasive damage caused by grooming; however, they also may increase incidence of SD.26 Tight curls worn by Black patients also can impede sebum from traveling down the hair shaft, leading to oil buildup on the scalp. This is the ideal environment for increased Malassezia density and higher risk for SD development.27 To balance the beneficial effects of hair oils with the increased susceptibility for SD, providers should emphasize applying these oils only to distal hair shafts, which are more likely to be damaged, and avoiding application to the scalp.19
Conclusion
Given its long-term relapsing and remitting nature, SD can be distressing for Black patients, many of whom may seek additional treatment options aside from those recommended by health care professionals. In order to better educate patients, it is important for dermatologists to know not only the common ingredients that may be present in OTC products but also the thought process behind why patients use them. Additionally, prescription treatments for Black patients with SD may require nuanced alterations to the product instructions that may prevent health disparities and provide culturally sensitive care. Overall, the literature regarding treatment for Black patients with SD is limited, and more high-quality studies are needed.
Seborrheic dermatitis (SD) is a common chronic inflammatory skin condition that predominantly affects areas with high concentrations of sebaceous glands such as the scalp and face. Up to 5% of the worldwide population is affected by SD each year, causing a major burden of disease for patients and the health care system.1 In 2023, the cost of medical treatment for SD in the United States was $300 million, with outpatient office visits alone costing $58 million and prescription drugs costing $109 million. Indirect costs of disease (eg, lost workdays) account for another $51 million.1 Since SD frequently manifests on the face, it tends to have negative effects on the patient’s quality of life, resulting in psychological distress and low self-esteem.2
Patients with SD may describe symptoms of excessive dandruff and itching along with hyperpigmentation or hypopigmentation of the skin; Black patients tend to present with the classic manifestations: a combination of scaling, flaking, and erythematous patches on the scalp, ears, and face, particularly around the eyebrows, eyelids, and nose. With SD being the second most common diagnosis in Black patients who seek care from a dermatologist, it is important to have effective treatment approaches for SD in this patient population.3
In this study, we aimed to evaluate medical and nonmedical treatment options for SD in Black patients by identifying common practices and products mentioned on consumer websites and in the medical literature.
Methods
A Google search was conducted during 2 time periods (September 2022—October 2022 and March 2023—April 2023) using the terms products for itchy scalp in Black patients, products for dandruff in Black patients, itchy scalp in Black women, itchy scalp in Black men, treatment for scalp itch in Black patients, and dry scalp in Black hair. Products that were recommended by at least 1 website on the first page of search results were included in our list of products, and the ingredients were reviewed by the authors. We excluded individual retailer websites as well as those that did not provide specific recommendations on products or ingredients to use when treating SD. To ensure reliability and standardization, we did not review products that were suggested by ads in the shopping section on the first page of search results.
We also evaluated medical treatments used for SD in dermatology literature. A PubMed search of articles indexed for MEDLINE using the terms seborrheic dermatitis treatment for Black patients, treatment for dandruff for Black patients, and seborrheic dermatitis and skin of color was conducted. We excluded articles that did not address treatment options for SD, were specific to treating SD in patient populations with specific comorbidities being studied, discussed SD in animals, or were published prior to 1990.
Results
We identified 16 unique consumer websites with product or ingredient recommendations for SD in Black patients, none of which were provided by authors with a medical or scientific background; however, 4 (25%) websites included insights from board-certified dermatologists. A total of 16 ingredients were recommended, 15 (94%) of which were mentioned at least twice in our search results (eTable 1).

Overall, we noticed that ingredients labeled as natural or organic were common in over-the-counter (OTC) products, and ingredients such as sulfates and parabens were avoided. Common OTC ingredients for antidandruff and anti-itch shampoos and conditioners include zinc pyrithione, selenium sulfide, coal tar, salicylic acid, and citric acid. Additionally, coconut oil, tea tree oil, apple cider vinegar, and charcoal are common natural alternatives used to address SD symptoms.
Our review of the literature yielded limited recommendations tailored specifically to Black patients with SD. Of 108 abstracts, articles, or textbook chapters providing treatment recommendations for SD, 6 (6%) specifically discussed treatments for Black patients. All articles were written by authors with medical or scientific backgrounds. Of the treatment options discussed, topical antifungals generally were considered first-line for SD in all patients, with ketoconazole shampoo being a common first choice.4,5
Comment
Our study indicated that many consumer websites recommend unstudied nonmedical treatments for SD. Zinc pyrithione was one of the most commonly mentioned ingredients in OTC products to treat SD targeted toward Black patients, as its properties have contributed to ease of hair combing and less frizz.6 Zinc pyrithione has antifungal properties that reduce the proliferation of Malassezia furfur as well as anti-inflammatory properties that reduce irritation, pruritus, and erythema in areas affected by SD.7 Tea tree and peppermint oils also were commonly mentioned; the theory is that these oils mitigate SD by reducing yeast growth and soothing inflammation through antioxidant activity.8,9 Coal tar also is used due to its keratoplastic properties, which slow the growth of skin cells and ultimately reduce scaling and dryness.10 Yeast thrives in basic pH conditions; apple cider vinegar is used as an ingredient in OTC products for SD because its acidic pH creates a less favorable environment for yeast to grow.11 Although many of the ingredients found in OTC products we identified have not yet been studied, they have properties that theoretically would be helpful in treating SD.
Our review of the medical literature revealed that while there are treatments that are effective for SD, the recommended use may not consider the cultural differences that exist for Black patients. For instance, reports in the literature regarding ketoconazole shampoo revealed that ketoconazole increases the risk for hair shaft dryness, damage, and subsequent breakage, especially in Black women who also may be using heat styling or chemical relaxers.5 As a result, ketoconazole should be used with caution in Black women, with an emphasis on direct application to the scalp rather than the hair shafts.12 Additional options reported for Black patients include ciclopirox olamine and zinc pyrithione, which may have fewer risks.13
When prescribing medicated shampoos, traditional instructions regarding frequency of use to control symptoms of SD range from 2 to 3 times weekly to daily for a specified period of time determined by the dermatologist.14 However, frequency of hair washing varies greatly among Black patients, sometimes occurring only once monthly. The frequency also may change based on styling techniques (eg, braids, weaves, and wigs).15 Based on previous research underscoring the tendency for Black patients to use medicated shampoos less frequently than White patients, it is important for clinicians to understand that these cultural practices can undermine the effectiveness when medicated shampoos are prescribed for SD.16
Additionally, topical corticosteroids often are used in conjunction with antifungals to help decrease inflammation of the scalp.17 An option reported for Black patients is topical fluocinolone 0.01%; however, package instructions state to apply topically to the scalp nightly and wash the hair thoroughly each morning, which may not be feasible for Black patients based on previously mentioned differences in hair-washing techniques. An alternative option may be to apply the medication 3 to 4 times per week, washing the hair weekly rather than daily.18 Fluocinolone can be used as an ointment, solution, oil, or cream.19,20 When comparing treatment vehicles for SD, a study conducted by Chappell et al21 found that Black patients preferred using ointment or oil vehicles; White patients preferred foams and sprays, which may not be suitable for Afro hair patterns. As such, using less-drying modalities may increase compliance and treatment success in Black patients. For patients who may have involvement on the hairline, face, or ears along with hypopigmentation (which is a common skin concern associated with SD), calcineurin inhibitors can be used until resolution occurs.5,22 High et al15 found that twice-daily use of pimecrolimus rapidly normalized skin pigmentation during the first 2 weeks of use. Overall, personalization of treatment may not only avoid adverse effects but also ensure patient compliance, with the overall goal of treating to reduce yeast activity, pruritus, and dyschromia.22
Interestingly, after the website searches were completed for this study, the US Food and Drug Administration approved topical roflumilast foam for SD. In a phase III trial of 457 total patients, 36 Black patients were included.23 It was determined that 79.5% of patients overall throughout the trial achieved Investigator Global Assessment success (score of 0 [clear] or 1 [almost clear]) plus ≥2-point improvement from baseline (on a scale of 0 [clear] to 4 [severe]) at weeks 2, 4, and 8. Although there currently are no long-term studies, roflumilast may be a promising option for Black patients with SD.23
Aside from developing an individualized treatment approach for Black patients with SD, it is important to ask targeted questions during the clinical encounter to identify factors that may be exacerbating symptoms, especially due to the wide range of hair care practices used by the Black community (eTable 2). Asking targeted questions is especially important, as prior studies have shown that extensions, hair relaxers, and particular hair products can irritate the scalp and increase the likelihood of developing SD.21,24 Rucker Wright et al25 evaluated different hair care practices among young Black females and their association with the development of SD. The authors found that using hair extensions (either braided, cornrowed, or ponytails), chemical relaxers, and hair oils every 2 weeks was associated with SD. The study also found that SD rates were roughly 20% higher among Black girls with extensions compared to Black girls without extensions, regardless of how frequently hair was washed.25

Many Black patients grease the scalp with oils that are beneficial for lubrication and reduction of abrasive damage caused by grooming; however, they also may increase incidence of SD.26 Tight curls worn by Black patients also can impede sebum from traveling down the hair shaft, leading to oil buildup on the scalp. This is the ideal environment for increased Malassezia density and higher risk for SD development.27 To balance the beneficial effects of hair oils with the increased susceptibility for SD, providers should emphasize applying these oils only to distal hair shafts, which are more likely to be damaged, and avoiding application to the scalp.19
Conclusion
Given its long-term relapsing and remitting nature, SD can be distressing for Black patients, many of whom may seek additional treatment options aside from those recommended by health care professionals. In order to better educate patients, it is important for dermatologists to know not only the common ingredients that may be present in OTC products but also the thought process behind why patients use them. Additionally, prescription treatments for Black patients with SD may require nuanced alterations to the product instructions that may prevent health disparities and provide culturally sensitive care. Overall, the literature regarding treatment for Black patients with SD is limited, and more high-quality studies are needed.
- Tucker D, Masood S. Seborrheic dermatitis. StatPearls [Internet]. Updated March 1, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551707/
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3:10.13188 /2373-1044.1000019.
- American Academy of Dermatology. Seborrheic dermatitis by the numbers. American Academy of Dermatology Skin Disease Briefs. Updated May 5, 2018. Accessed November 22, 2024. https://www.aad.org/asset/49w949DPcF8RSJYIRHfDon
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158-169.
- Draelos ZD, Kenneally DC, Hodges LT, et al. A comparison of hair quality and cosmetic acceptance following the use of two anti-dandruff shampoos. J Investig Dermatol Symp Proc. 2005;10:201-214.
- Barak-Shinar D, Green LJ. Scalp seborrheic dermatitis and dandruff therapy using a herbal and zinc pyrithione-based therapy of shampoo and scalp lotion. J Clin Aesthet Dermatol. 2018;11:26-31.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of dandruff with 5% tea tree oil shampoo. J Am Acad Dermatol. 2002;47:852-855.
- Herro E, Jacob SE. Mentha piperita (peppermint). Dermatitis. 2010;21:327-329.
- Sanfilippo A, English JC. An overview of medicated shampoos used in dandruff treatment. Pharm Ther. 2006;31:396-400.
- Arun PVPS, Vineetha Y, Waheed M, et al. Quantification of the minimum amount of lemon juice and apple cider vinegar required for the growth inhibition of dandruff causing fungi Malassezia furfur. Int J Sci Res in Biological Sciences. 2019;6:144-147.
- Gao HY, Li Wan Po A. Topical formulations of fluocinolone acetonide. Are creams, gels and ointments bioequivalent and does dilution affect activity? Eur J Clin Pharmacol. 1994;46:71-75.
- Pauporte M, Maibach H, Lowe N, et al. Fluocinolone acetonide topical oil for scalp psoriasis. J Dermatolog Treat. 2004;15:360-364.
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- High WA, Pandya AG. Pilot trial of 1% pimecrolimus cream in the treatment of seborrheic dermatitis in African American adults with associated hypopigmentation. J Am Acad Dermatol. 2006;54:1083-1088.
- Hollins LC, Butt M, Hong J, et al. Research in brief: survey of hair care practices in various ethnic and racial pediatric populations. Pediatr Dermatol. 2022;39:494-496.
- Halder RM, Roberts CI, Nootheti PK. Cutaneous diseases in the black races. Dermatol Clin. 2003;21:679-687, ix.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Friedmann DP, Mishra V, Batty T. Progressive facial papules in an African- American patient: an atypical presentation of seborrheic dermatitis. J Clin Aesthet Dermatol. 2018;11:44-45.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Chappell J, Mattox A, Simonetta C, et al. Seborrheic dermatitis of the scalp in populations practicing less frequent hair washing: ketoconazole 2% foam versus ketoconazole 2% shampoo. three-year data. J Am Acad Dermatol. 2014;70:AB54.
- Dadzie OE, Salam A. The hair grooming practices of women of African descent in London, United Kingdom: findings of a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:1021-1024.
- Blauvelt A, Draelos ZD, Stein Gold L, et al. Roflumilast foam 0.3% for adolescent and adult patients with seborrheic dermatitis: a randomized, double-blinded, vehicle-controlled, phase 3 trial. J Am Acad Dermatol. 2024;90:986-993.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319.
- Mayo T, Dinkins J, Elewski B. Hair oils may worsen seborrheic dermatitis in Black patients. Skin Appendage Disord. 2023;9:151-152.
- Tucker D, Masood S. Seborrheic dermatitis. StatPearls [Internet]. Updated March 1, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551707/
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3:10.13188 /2373-1044.1000019.
- American Academy of Dermatology. Seborrheic dermatitis by the numbers. American Academy of Dermatology Skin Disease Briefs. Updated May 5, 2018. Accessed November 22, 2024. https://www.aad.org/asset/49w949DPcF8RSJYIRHfDon
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158-169.
- Draelos ZD, Kenneally DC, Hodges LT, et al. A comparison of hair quality and cosmetic acceptance following the use of two anti-dandruff shampoos. J Investig Dermatol Symp Proc. 2005;10:201-214.
- Barak-Shinar D, Green LJ. Scalp seborrheic dermatitis and dandruff therapy using a herbal and zinc pyrithione-based therapy of shampoo and scalp lotion. J Clin Aesthet Dermatol. 2018;11:26-31.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of dandruff with 5% tea tree oil shampoo. J Am Acad Dermatol. 2002;47:852-855.
- Herro E, Jacob SE. Mentha piperita (peppermint). Dermatitis. 2010;21:327-329.
- Sanfilippo A, English JC. An overview of medicated shampoos used in dandruff treatment. Pharm Ther. 2006;31:396-400.
- Arun PVPS, Vineetha Y, Waheed M, et al. Quantification of the minimum amount of lemon juice and apple cider vinegar required for the growth inhibition of dandruff causing fungi Malassezia furfur. Int J Sci Res in Biological Sciences. 2019;6:144-147.
- Gao HY, Li Wan Po A. Topical formulations of fluocinolone acetonide. Are creams, gels and ointments bioequivalent and does dilution affect activity? Eur J Clin Pharmacol. 1994;46:71-75.
- Pauporte M, Maibach H, Lowe N, et al. Fluocinolone acetonide topical oil for scalp psoriasis. J Dermatolog Treat. 2004;15:360-364.
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- High WA, Pandya AG. Pilot trial of 1% pimecrolimus cream in the treatment of seborrheic dermatitis in African American adults with associated hypopigmentation. J Am Acad Dermatol. 2006;54:1083-1088.
- Hollins LC, Butt M, Hong J, et al. Research in brief: survey of hair care practices in various ethnic and racial pediatric populations. Pediatr Dermatol. 2022;39:494-496.
- Halder RM, Roberts CI, Nootheti PK. Cutaneous diseases in the black races. Dermatol Clin. 2003;21:679-687, ix.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Friedmann DP, Mishra V, Batty T. Progressive facial papules in an African- American patient: an atypical presentation of seborrheic dermatitis. J Clin Aesthet Dermatol. 2018;11:44-45.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Chappell J, Mattox A, Simonetta C, et al. Seborrheic dermatitis of the scalp in populations practicing less frequent hair washing: ketoconazole 2% foam versus ketoconazole 2% shampoo. three-year data. J Am Acad Dermatol. 2014;70:AB54.
- Dadzie OE, Salam A. The hair grooming practices of women of African descent in London, United Kingdom: findings of a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:1021-1024.
- Blauvelt A, Draelos ZD, Stein Gold L, et al. Roflumilast foam 0.3% for adolescent and adult patients with seborrheic dermatitis: a randomized, double-blinded, vehicle-controlled, phase 3 trial. J Am Acad Dermatol. 2024;90:986-993.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319.
- Mayo T, Dinkins J, Elewski B. Hair oils may worsen seborrheic dermatitis in Black patients. Skin Appendage Disord. 2023;9:151-152.
Treatment of Seborrheic Dermatitis in Black Patients
Treatment of Seborrheic Dermatitis in Black Patients
PRACTICE POINTS
- Cultural awareness when treating Black patients with seborrheic dermatitis is vital to providing appropriate care, as hair care practices may impact treatment options and regimen.
- Knowledge about over-the-counter products that are targeted toward Black patients and the ingredients they contain can assist in providing better counseling to patients and improve shared decision-making.
Debunking Dermatology Myths to Enhance Patient Care
Debunking Dermatology Myths to Enhance Patient Care
The advent of social media has revolutionized the way patients access and consume health information. While this increased access has its merits, it also has given rise to the proliferation of medical myths, which have considerable effects on patient-physician interactions.1 Myths are prevalent across all fields of health care, ranging from misconceptions about disease etiology and prevention to the efficacy and safety of treatments. This influx of misinformation can derail the clinical encounter, shifting the focus from evidence-based medicine to myth-busting.2 The COVID-19 pandemic exacerbated this issue, as widespread lockdowns and social distancing measures limited access to in-person medical consultations, prompting patients to increasingly turn to online sources for health information that often were unreliable, thereby bypassing professional medical advice.3 Herein, we highlight the challenges and implications of common dermatology myths and provide strategies for effectively debunking these myths to enhance patient care.
Common Dermatology Myths
In dermatology, where visible and often distressing conditions such as acne and hair loss are common, the impact of myths on patient perceptions and treatment outcomes can be particularly profound. Patients often arrive for consultations with preconceived notions that are not grounded in scientific evidence. Common dermatologic myths include eczema and the efficacy of topical corticosteroids, the causes and treatment of hair loss, and risk factors associated with skin cancer.
Eczema and Topical Corticosteroids—Topical corticosteroids for eczema are safe and effective, but nonadherence due to phobias stemming from misinformation online can impede treatment.4 Myths such as red skin syndrome and topical corticosteroid addiction are prevalent. Red skin syndrome refers to claims that prolonged use of topical corticosteroids causes severe redness and burning of the skin and worsening eczema symptoms upon withdrawal. Topical corticosteroid addiction suggests that patients become dependent on corticosteroids, requiring higher doses over time to maintain efficacy. These misconceptions contribute to fear and avoidance of prescribed treatments.
Eczema myths often divert focus from its true etiology as a genetic inflammatory skin disease, suggesting instead that it is caused by leaky gut or food intolerances.4 Risks such as skin thinning and stunted growth often are exaggerated on social media and other nonmedical platforms, though these adverse effects rarely are seen when topical corticosteroids are used appropriately under medical supervision. Misinformation often is linked to companies promoting unregulated consultations, tests, or supposedly natural treatments, including herbal remedies that may surreptitiously contain corticosteroids without clear labeling. This fosters distrust of US Food and Drug Administration– approved and dermatologist-prescribed treatments, as patients may cite concerns based on experiences with or claims about unapproved products.4
Sunscreen and Skin Cancer—In 2018, the American Academy of Dermatology prioritized skin cancer prevention due to suboptimal public adoption of photoprotection measures.5 However, the proliferation of misinformation regarding sunscreen and its potential to cause skin cancer is a more pressing issue. Myths range from claims that sunscreen is ineffective to warnings that it is dangerous, with some social media influencers even suggesting that sunscreen causes skin cancer due to toxic ingredients.6 Oxybenzone, typically found in chemical sunscreens, has been criticized by some advocacy groups and social media influencers as a potential hormone disruptor (ie, a chemical that could interfere with hormone production).7 However, no conclusive evidence has shown that oxybenzone is harmful to humans. Consumer concerns often are based on animal studies in which rats are fed oxybenzone, but mathematical modeling has indicated it would take 277 years of sunscreen use by humans to match the doses used in these studies.8 The false association between sunscreen use and skin cancer is based on flawed studies that found higher rates of skin cancer—including melanoma—in sunscreen users compared to those who did not use sunscreen. However, those using sunscreen also were more likely to travel to sunnier climates and engage in sunbathing, and it may have been this increased sun exposure that elevated their risk for skin cancer.7 It is imperative that the dermatology community counteract this type of misinformation with evidence-based advice.
Hair Loss—Some patients believe that hair loss is caused by wearing hats, frequent shampooing, or even stress in a way that oversimplifies complex physiological processes. Biotin, which commonly is added to supplements for hair, skin, and nails, has been linked to potential risks, such as interference with laboratory testing and false-positive or false-negative results in critical medical tests, which can lead to misdiagnosis or inappropriate treatment.9 Biotin interference can result in falsely low troponin readings, which are critical in diagnosing acute myocardial infarction. Tests for other hormones such as cortisol and parathyroid hormone also are affected, potentially impacting the evaluation and management of endocrine disorders. The US Food and Drug Administration has issued warnings for patients on this topic, emphasizing the importance of informing health care providers about any biotin supplementation prior to laboratory testing. Despite its popularity, there is no substantial scientific evidence to suggest that biotin supplementation promotes hair growth in anyone other than those with deficiency, which is quite rare.9
Myths and the Patient-Physician Relationship
The proliferation of medical myths and misinformation affects the dynamic between patients and dermatologists in several ways. Research across various medical fields has demonstrated that misinformation can substantially impact patient behavior and treatment adherence. Like many other specialists, dermatologists often spend considerable time during consultations with patients debunking myths and correcting misconceptions, which can detract from discussing more critical aspects of the patient’s condition and treatment plan and lead to frustration and anxiety among patients. It also can be challenging for physicians to have these conversations without alienating patients, who may distrust medical recommendations and believe that natural or alternative treatments are superior. This can lead to noncompliance with prescribed treatments, and patients may instead opt to try unproven remedies they encounter online, ultimately resulting in poorer health outcomes.
Strategies to Debunk Myths
By implementing the following strategies, dermatologists can combat the spread of myths, foster trust among patients, and promote adherence to evidence-based treatments:
- Provide educational outreach. Preemptively address myths by giving patients accurate and accessible resources. Including a dedicated section on your clinic’s website with articles, frequently asked questions, videos, and links to reputable sources can be effective. Sharing patient testimonials and before-and-after photographs to demonstrate the success of evidence-based treatments also is recommended, as real-life stories can be powerful tools in dispelling myths.
- Practice effective communication. Involve patients in the decision-making process by discussing their treatment goals, preferences, and concerns. It is important to present all options clearly, including the potential benefits and adverse effects. Discuss the expected outcomes and timelines, and be transparent about the limitations of certain treatment—honesty helps build trust and sets realistic expectations.
- Conduct structured consultations. Ensure that consultations with patients follow a structured format—history, physical examination, and discussion—to help keep the focus on evidence-based practice.
- Leverage technology. Guide patients toward reliable digital patient education tools to empower them with accurate information. Hosting live sessions on social media platforms during which patients can ask questions and receive evidence-based answers also can be beneficial.
Final Thoughts
In summary, the rise of medical myths poses a considerable challenge to dermatologic practice. By understanding the sources and impacts of these myths and employing strategies to dispel them, dermatologists can better navigate the complexities of modern patient interactions and ensure that care remains grounded in scientific evidence.
- Kessler SH, Bachmann E. Debunking health myths on the internet: the persuasive effect of (visual) online communication. Z Gesundheitswissenschaften J Public Health. 2022;30:1823-1835.
- Fridman I, Johnson S, Elston Lafata J. Health information and misinformation: a framework to guide research and practice. JMIR Med Educ. 2023;9:E38687.
- Di Novi C, Kovacic M, Orso CE. Online health information seeking behavior, healthcare access, and health status during exceptional times. J Econ Behav Organ. 2024;220:675-690.
- Finnegan P, Murphy M, O’Connor C. #corticophobia: a review on online misinformation related to topical steroids. Clin Exp Dermatol. 2023;48:112-115.
- Yang EJ, Beck KM, Maarouf M, et al. Truths and myths in sunscreen labeling. J Cosmet Dermatol. 2018;17:1288-1292.
- Hopkins C. What Gen Z gets wrong about sunscreen. New York Times. Published May 27, 2024. Accessed December 16, 2024. https://www.nytimes.com/2024/05/27/well/live/sunscreen-skin-cancer-gen-z.html
- Harvard Health Publishing. The science of sunscreen. Published February 15, 2021. Accessed December 9, 2024. https://www.health.harvard.edu/staying-healthy/the-science-of-sunscreen
- Lim HW, Arellano-Mendoza MI, Stengel F. Current challenges in photoprotection. J Am Acad Dermatol. 2017;76:S91-S99.
- Li D, Ferguson A, Cervinski MA, et al. AACC guidance document on biotin interference in laboratory tests. J Appl Lab Med. 2020; 5:575-587.
The advent of social media has revolutionized the way patients access and consume health information. While this increased access has its merits, it also has given rise to the proliferation of medical myths, which have considerable effects on patient-physician interactions.1 Myths are prevalent across all fields of health care, ranging from misconceptions about disease etiology and prevention to the efficacy and safety of treatments. This influx of misinformation can derail the clinical encounter, shifting the focus from evidence-based medicine to myth-busting.2 The COVID-19 pandemic exacerbated this issue, as widespread lockdowns and social distancing measures limited access to in-person medical consultations, prompting patients to increasingly turn to online sources for health information that often were unreliable, thereby bypassing professional medical advice.3 Herein, we highlight the challenges and implications of common dermatology myths and provide strategies for effectively debunking these myths to enhance patient care.
Common Dermatology Myths
In dermatology, where visible and often distressing conditions such as acne and hair loss are common, the impact of myths on patient perceptions and treatment outcomes can be particularly profound. Patients often arrive for consultations with preconceived notions that are not grounded in scientific evidence. Common dermatologic myths include eczema and the efficacy of topical corticosteroids, the causes and treatment of hair loss, and risk factors associated with skin cancer.
Eczema and Topical Corticosteroids—Topical corticosteroids for eczema are safe and effective, but nonadherence due to phobias stemming from misinformation online can impede treatment.4 Myths such as red skin syndrome and topical corticosteroid addiction are prevalent. Red skin syndrome refers to claims that prolonged use of topical corticosteroids causes severe redness and burning of the skin and worsening eczema symptoms upon withdrawal. Topical corticosteroid addiction suggests that patients become dependent on corticosteroids, requiring higher doses over time to maintain efficacy. These misconceptions contribute to fear and avoidance of prescribed treatments.
Eczema myths often divert focus from its true etiology as a genetic inflammatory skin disease, suggesting instead that it is caused by leaky gut or food intolerances.4 Risks such as skin thinning and stunted growth often are exaggerated on social media and other nonmedical platforms, though these adverse effects rarely are seen when topical corticosteroids are used appropriately under medical supervision. Misinformation often is linked to companies promoting unregulated consultations, tests, or supposedly natural treatments, including herbal remedies that may surreptitiously contain corticosteroids without clear labeling. This fosters distrust of US Food and Drug Administration– approved and dermatologist-prescribed treatments, as patients may cite concerns based on experiences with or claims about unapproved products.4
Sunscreen and Skin Cancer—In 2018, the American Academy of Dermatology prioritized skin cancer prevention due to suboptimal public adoption of photoprotection measures.5 However, the proliferation of misinformation regarding sunscreen and its potential to cause skin cancer is a more pressing issue. Myths range from claims that sunscreen is ineffective to warnings that it is dangerous, with some social media influencers even suggesting that sunscreen causes skin cancer due to toxic ingredients.6 Oxybenzone, typically found in chemical sunscreens, has been criticized by some advocacy groups and social media influencers as a potential hormone disruptor (ie, a chemical that could interfere with hormone production).7 However, no conclusive evidence has shown that oxybenzone is harmful to humans. Consumer concerns often are based on animal studies in which rats are fed oxybenzone, but mathematical modeling has indicated it would take 277 years of sunscreen use by humans to match the doses used in these studies.8 The false association between sunscreen use and skin cancer is based on flawed studies that found higher rates of skin cancer—including melanoma—in sunscreen users compared to those who did not use sunscreen. However, those using sunscreen also were more likely to travel to sunnier climates and engage in sunbathing, and it may have been this increased sun exposure that elevated their risk for skin cancer.7 It is imperative that the dermatology community counteract this type of misinformation with evidence-based advice.
Hair Loss—Some patients believe that hair loss is caused by wearing hats, frequent shampooing, or even stress in a way that oversimplifies complex physiological processes. Biotin, which commonly is added to supplements for hair, skin, and nails, has been linked to potential risks, such as interference with laboratory testing and false-positive or false-negative results in critical medical tests, which can lead to misdiagnosis or inappropriate treatment.9 Biotin interference can result in falsely low troponin readings, which are critical in diagnosing acute myocardial infarction. Tests for other hormones such as cortisol and parathyroid hormone also are affected, potentially impacting the evaluation and management of endocrine disorders. The US Food and Drug Administration has issued warnings for patients on this topic, emphasizing the importance of informing health care providers about any biotin supplementation prior to laboratory testing. Despite its popularity, there is no substantial scientific evidence to suggest that biotin supplementation promotes hair growth in anyone other than those with deficiency, which is quite rare.9
Myths and the Patient-Physician Relationship
The proliferation of medical myths and misinformation affects the dynamic between patients and dermatologists in several ways. Research across various medical fields has demonstrated that misinformation can substantially impact patient behavior and treatment adherence. Like many other specialists, dermatologists often spend considerable time during consultations with patients debunking myths and correcting misconceptions, which can detract from discussing more critical aspects of the patient’s condition and treatment plan and lead to frustration and anxiety among patients. It also can be challenging for physicians to have these conversations without alienating patients, who may distrust medical recommendations and believe that natural or alternative treatments are superior. This can lead to noncompliance with prescribed treatments, and patients may instead opt to try unproven remedies they encounter online, ultimately resulting in poorer health outcomes.
Strategies to Debunk Myths
By implementing the following strategies, dermatologists can combat the spread of myths, foster trust among patients, and promote adherence to evidence-based treatments:
- Provide educational outreach. Preemptively address myths by giving patients accurate and accessible resources. Including a dedicated section on your clinic’s website with articles, frequently asked questions, videos, and links to reputable sources can be effective. Sharing patient testimonials and before-and-after photographs to demonstrate the success of evidence-based treatments also is recommended, as real-life stories can be powerful tools in dispelling myths.
- Practice effective communication. Involve patients in the decision-making process by discussing their treatment goals, preferences, and concerns. It is important to present all options clearly, including the potential benefits and adverse effects. Discuss the expected outcomes and timelines, and be transparent about the limitations of certain treatment—honesty helps build trust and sets realistic expectations.
- Conduct structured consultations. Ensure that consultations with patients follow a structured format—history, physical examination, and discussion—to help keep the focus on evidence-based practice.
- Leverage technology. Guide patients toward reliable digital patient education tools to empower them with accurate information. Hosting live sessions on social media platforms during which patients can ask questions and receive evidence-based answers also can be beneficial.
Final Thoughts
In summary, the rise of medical myths poses a considerable challenge to dermatologic practice. By understanding the sources and impacts of these myths and employing strategies to dispel them, dermatologists can better navigate the complexities of modern patient interactions and ensure that care remains grounded in scientific evidence.
The advent of social media has revolutionized the way patients access and consume health information. While this increased access has its merits, it also has given rise to the proliferation of medical myths, which have considerable effects on patient-physician interactions.1 Myths are prevalent across all fields of health care, ranging from misconceptions about disease etiology and prevention to the efficacy and safety of treatments. This influx of misinformation can derail the clinical encounter, shifting the focus from evidence-based medicine to myth-busting.2 The COVID-19 pandemic exacerbated this issue, as widespread lockdowns and social distancing measures limited access to in-person medical consultations, prompting patients to increasingly turn to online sources for health information that often were unreliable, thereby bypassing professional medical advice.3 Herein, we highlight the challenges and implications of common dermatology myths and provide strategies for effectively debunking these myths to enhance patient care.
Common Dermatology Myths
In dermatology, where visible and often distressing conditions such as acne and hair loss are common, the impact of myths on patient perceptions and treatment outcomes can be particularly profound. Patients often arrive for consultations with preconceived notions that are not grounded in scientific evidence. Common dermatologic myths include eczema and the efficacy of topical corticosteroids, the causes and treatment of hair loss, and risk factors associated with skin cancer.
Eczema and Topical Corticosteroids—Topical corticosteroids for eczema are safe and effective, but nonadherence due to phobias stemming from misinformation online can impede treatment.4 Myths such as red skin syndrome and topical corticosteroid addiction are prevalent. Red skin syndrome refers to claims that prolonged use of topical corticosteroids causes severe redness and burning of the skin and worsening eczema symptoms upon withdrawal. Topical corticosteroid addiction suggests that patients become dependent on corticosteroids, requiring higher doses over time to maintain efficacy. These misconceptions contribute to fear and avoidance of prescribed treatments.
Eczema myths often divert focus from its true etiology as a genetic inflammatory skin disease, suggesting instead that it is caused by leaky gut or food intolerances.4 Risks such as skin thinning and stunted growth often are exaggerated on social media and other nonmedical platforms, though these adverse effects rarely are seen when topical corticosteroids are used appropriately under medical supervision. Misinformation often is linked to companies promoting unregulated consultations, tests, or supposedly natural treatments, including herbal remedies that may surreptitiously contain corticosteroids without clear labeling. This fosters distrust of US Food and Drug Administration– approved and dermatologist-prescribed treatments, as patients may cite concerns based on experiences with or claims about unapproved products.4
Sunscreen and Skin Cancer—In 2018, the American Academy of Dermatology prioritized skin cancer prevention due to suboptimal public adoption of photoprotection measures.5 However, the proliferation of misinformation regarding sunscreen and its potential to cause skin cancer is a more pressing issue. Myths range from claims that sunscreen is ineffective to warnings that it is dangerous, with some social media influencers even suggesting that sunscreen causes skin cancer due to toxic ingredients.6 Oxybenzone, typically found in chemical sunscreens, has been criticized by some advocacy groups and social media influencers as a potential hormone disruptor (ie, a chemical that could interfere with hormone production).7 However, no conclusive evidence has shown that oxybenzone is harmful to humans. Consumer concerns often are based on animal studies in which rats are fed oxybenzone, but mathematical modeling has indicated it would take 277 years of sunscreen use by humans to match the doses used in these studies.8 The false association between sunscreen use and skin cancer is based on flawed studies that found higher rates of skin cancer—including melanoma—in sunscreen users compared to those who did not use sunscreen. However, those using sunscreen also were more likely to travel to sunnier climates and engage in sunbathing, and it may have been this increased sun exposure that elevated their risk for skin cancer.7 It is imperative that the dermatology community counteract this type of misinformation with evidence-based advice.
Hair Loss—Some patients believe that hair loss is caused by wearing hats, frequent shampooing, or even stress in a way that oversimplifies complex physiological processes. Biotin, which commonly is added to supplements for hair, skin, and nails, has been linked to potential risks, such as interference with laboratory testing and false-positive or false-negative results in critical medical tests, which can lead to misdiagnosis or inappropriate treatment.9 Biotin interference can result in falsely low troponin readings, which are critical in diagnosing acute myocardial infarction. Tests for other hormones such as cortisol and parathyroid hormone also are affected, potentially impacting the evaluation and management of endocrine disorders. The US Food and Drug Administration has issued warnings for patients on this topic, emphasizing the importance of informing health care providers about any biotin supplementation prior to laboratory testing. Despite its popularity, there is no substantial scientific evidence to suggest that biotin supplementation promotes hair growth in anyone other than those with deficiency, which is quite rare.9
Myths and the Patient-Physician Relationship
The proliferation of medical myths and misinformation affects the dynamic between patients and dermatologists in several ways. Research across various medical fields has demonstrated that misinformation can substantially impact patient behavior and treatment adherence. Like many other specialists, dermatologists often spend considerable time during consultations with patients debunking myths and correcting misconceptions, which can detract from discussing more critical aspects of the patient’s condition and treatment plan and lead to frustration and anxiety among patients. It also can be challenging for physicians to have these conversations without alienating patients, who may distrust medical recommendations and believe that natural or alternative treatments are superior. This can lead to noncompliance with prescribed treatments, and patients may instead opt to try unproven remedies they encounter online, ultimately resulting in poorer health outcomes.
Strategies to Debunk Myths
By implementing the following strategies, dermatologists can combat the spread of myths, foster trust among patients, and promote adherence to evidence-based treatments:
- Provide educational outreach. Preemptively address myths by giving patients accurate and accessible resources. Including a dedicated section on your clinic’s website with articles, frequently asked questions, videos, and links to reputable sources can be effective. Sharing patient testimonials and before-and-after photographs to demonstrate the success of evidence-based treatments also is recommended, as real-life stories can be powerful tools in dispelling myths.
- Practice effective communication. Involve patients in the decision-making process by discussing their treatment goals, preferences, and concerns. It is important to present all options clearly, including the potential benefits and adverse effects. Discuss the expected outcomes and timelines, and be transparent about the limitations of certain treatment—honesty helps build trust and sets realistic expectations.
- Conduct structured consultations. Ensure that consultations with patients follow a structured format—history, physical examination, and discussion—to help keep the focus on evidence-based practice.
- Leverage technology. Guide patients toward reliable digital patient education tools to empower them with accurate information. Hosting live sessions on social media platforms during which patients can ask questions and receive evidence-based answers also can be beneficial.
Final Thoughts
In summary, the rise of medical myths poses a considerable challenge to dermatologic practice. By understanding the sources and impacts of these myths and employing strategies to dispel them, dermatologists can better navigate the complexities of modern patient interactions and ensure that care remains grounded in scientific evidence.
- Kessler SH, Bachmann E. Debunking health myths on the internet: the persuasive effect of (visual) online communication. Z Gesundheitswissenschaften J Public Health. 2022;30:1823-1835.
- Fridman I, Johnson S, Elston Lafata J. Health information and misinformation: a framework to guide research and practice. JMIR Med Educ. 2023;9:E38687.
- Di Novi C, Kovacic M, Orso CE. Online health information seeking behavior, healthcare access, and health status during exceptional times. J Econ Behav Organ. 2024;220:675-690.
- Finnegan P, Murphy M, O’Connor C. #corticophobia: a review on online misinformation related to topical steroids. Clin Exp Dermatol. 2023;48:112-115.
- Yang EJ, Beck KM, Maarouf M, et al. Truths and myths in sunscreen labeling. J Cosmet Dermatol. 2018;17:1288-1292.
- Hopkins C. What Gen Z gets wrong about sunscreen. New York Times. Published May 27, 2024. Accessed December 16, 2024. https://www.nytimes.com/2024/05/27/well/live/sunscreen-skin-cancer-gen-z.html
- Harvard Health Publishing. The science of sunscreen. Published February 15, 2021. Accessed December 9, 2024. https://www.health.harvard.edu/staying-healthy/the-science-of-sunscreen
- Lim HW, Arellano-Mendoza MI, Stengel F. Current challenges in photoprotection. J Am Acad Dermatol. 2017;76:S91-S99.
- Li D, Ferguson A, Cervinski MA, et al. AACC guidance document on biotin interference in laboratory tests. J Appl Lab Med. 2020; 5:575-587.
- Kessler SH, Bachmann E. Debunking health myths on the internet: the persuasive effect of (visual) online communication. Z Gesundheitswissenschaften J Public Health. 2022;30:1823-1835.
- Fridman I, Johnson S, Elston Lafata J. Health information and misinformation: a framework to guide research and practice. JMIR Med Educ. 2023;9:E38687.
- Di Novi C, Kovacic M, Orso CE. Online health information seeking behavior, healthcare access, and health status during exceptional times. J Econ Behav Organ. 2024;220:675-690.
- Finnegan P, Murphy M, O’Connor C. #corticophobia: a review on online misinformation related to topical steroids. Clin Exp Dermatol. 2023;48:112-115.
- Yang EJ, Beck KM, Maarouf M, et al. Truths and myths in sunscreen labeling. J Cosmet Dermatol. 2018;17:1288-1292.
- Hopkins C. What Gen Z gets wrong about sunscreen. New York Times. Published May 27, 2024. Accessed December 16, 2024. https://www.nytimes.com/2024/05/27/well/live/sunscreen-skin-cancer-gen-z.html
- Harvard Health Publishing. The science of sunscreen. Published February 15, 2021. Accessed December 9, 2024. https://www.health.harvard.edu/staying-healthy/the-science-of-sunscreen
- Lim HW, Arellano-Mendoza MI, Stengel F. Current challenges in photoprotection. J Am Acad Dermatol. 2017;76:S91-S99.
- Li D, Ferguson A, Cervinski MA, et al. AACC guidance document on biotin interference in laboratory tests. J Appl Lab Med. 2020; 5:575-587.
Debunking Dermatology Myths to Enhance Patient Care
Debunking Dermatology Myths to Enhance Patient Care
Hidden Risks of Formaldehyde in Hair-Straightening Products
Hidden Risks of Formaldehyde in Hair-Straightening Products
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
Hidden Risks of Formaldehyde in Hair-Straightening Products
Hidden Risks of Formaldehyde in Hair-Straightening Products

Depression As a Potential Contributing Factor in Hidradenitis Suppurativa and Associated Racial Gaps
Hidradenitis suppurativa (HS)—a chronic, relapsing, inflammatory disorder involving terminal hair follicles in apocrine gland–rich skin—manifests as tender inflamed nodules that transform into abscesses, sinus tracts, and scarring.1,2 The etiology of HS is multifactorial, encompassing lifestyle, microbiota, hormonal status, and genetic and environmental factors. These factors activate the immune system around the terminal hair follicles and lead to hyperkeratosis of the infundibulum of the hair follicles in intertriginous regions. This progresses to follicular occlusion, stasis, and eventual rupture. Bacterial multiplication within the plugged pilosebaceous units further boosts immune activation. Resident and migrated cells of the innate and adaptive immune system then release proinflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-17, which further enhance immune cell influx and inflammation.3,4 This aberrant immune response propagates the production of deep-seated inflammatory nodules and abscesses.3-8
The estimated prevalence of HS is 1% worldwide.9 It is more prevalent in female and Black patients (0.30%) than White patients (0.09%) and is intermediate in prevalence in the biracial population (0.22%).10 Hidradenitis suppurativa is thought to be associated with lower socioeconomic status (SES). In a retrospective analysis of HS patients (N=375), approximately one-third of patients were Black, had advanced disease, and had a notably lower SES.11 Furthermore, HS has been reported to be associated with systemic inflammation and comorbidities such as morbid obesity (38.3%) and hypertension (39.6%) as well as other metabolic syndrome–related disorders and depression (48.1%).1
Hidradenitis suppurativa may contribute to the risk for depression through its substantial impact on health-related quality of life, which culminates in social withdrawal, unemployment, and suicidal thoughts.12 The high prevalence of depression in individuals with HS1 and its association with systemic inflammation13 increases the likelihood that a common genetic predisposition also may exist between both conditions. Because depression frequently has been discovered as a concomitant diagnosis in patients with HS, we hypothesize that a shared genetic susceptibility also may exist between the 2 disorders. Our study sought to explore data on the co-occurrence of depression with HS, including its demographics and racial data.
Methods
We conducted a PubMed search of articles indexed for MEDLINE as well as Google Scholar using the terms depression and hidradenitis suppurativa to obtain all research articles published from 2000 to 2022. Articles were selected based on relevance to the topic of exploration. English-language articles that directly addressed the epidemiology, etiology, pathophysiology, and co-occurrence of both depression and HS with numerical data were included. Articles were excluded if they did not explore the information of interest on these 2 disorders or did not contain clear statistical data of patients with the 2 concurrent medical conditions.
Results
Twenty-two cross-sectional, prospective, and retrospective studies that fit the search criteria were identified and included in the analysis (eTable).1,14-34 Sixteen (72.7%) studies were cross-sectional, 5 (22.7%) were retrospective, and only 1 (4.5%) was a prospective study. Only 6 of the studies provided racial data,1,14,17,26,28,32 and of them, 4 had predominately White patients,1,14,26,32 whereas the other 2 had predominantly Black patients.17,28
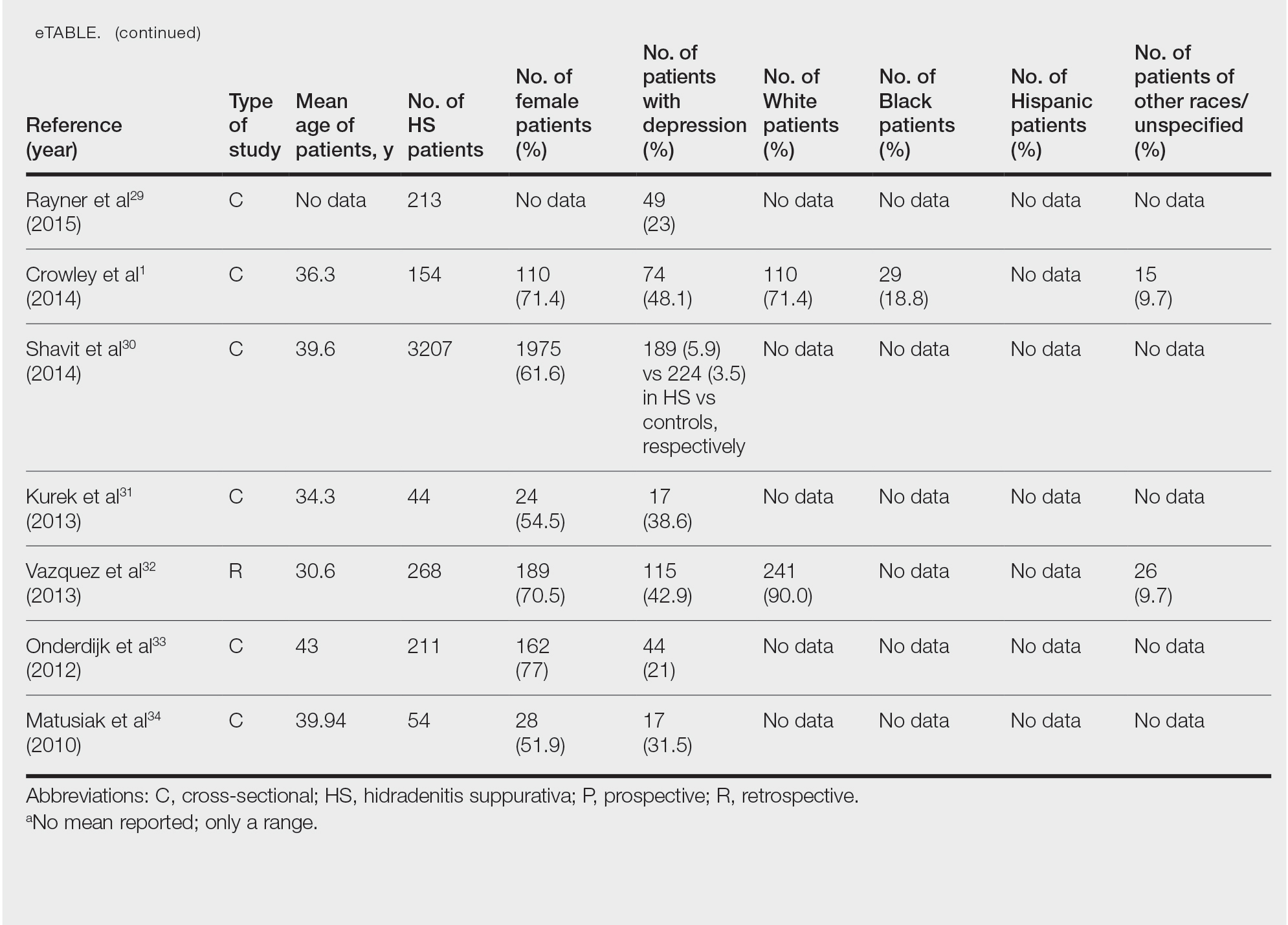
Hidradenitis suppurativa was found to coexist with depression in all the studies, with a prevalence of 1.2% to 48.1%. There also was a higher prevalence of depression in HS patients than in the control patients without HS. Furthermore, a recent study by Wright and colleagues14 stratified the depression prevalence data by age and found a higher prevalence of depression in adults vs children with HS (30% vs 12%).
Comment
Major depression—a chronic and debilitating illness—is the chief cause of disability globally and in the United States alone and has a global lifetime prevalence of 17%.35 In a study of 388 patients diagnosed with depression and 404 community-matched controls who were observed for 10 years, depressed patients had a two-thirds higher likelihood of developing a serious physical illness than controls. The depression-associated elevated risk for serious physical illness persisted after controlling for confounding variables such as alcohol abuse, smoking, and level of physical activity.36 Studies also have demonstrated that HS is more prevalent in Black individuals10 and in individuals of low SES,37 who are mostly the Black and Hispanic populations that experience the highest burden of racial microaggression38 and disparities in health access and outcomes.39,40 The severity and chronicity of major depressive disorder also is higher in Black patients compared with White patients (57% vs 39%).41 Because major depression and HS are most common among Black patients who experience the highest-burden negative financial and health disparities, there may be a shared genetic disposition to both medical conditions.
Moreover, the common detrimental lifestyle choices associated with patients with depression and HS also suggest the possibility of a collective genetic susceptibility. Patients with depression also report increased consumption of alcohol, tobacco, and illicit substances; sedentary lifestyle leading to obesity; and poor compliance with prescribed medical treatment.42 Smoking and obesity are known contributors to the pathogenesis of HS, and their modification also is known to positively impact the disease course. In a retrospective single-cohort study, 50% of obese HS patients (n=35) reported a substantial decrease in disease severity after a reduction of more than 15% in body mass index over 2 years following bariatric surgery (n=35).43 Patients with HS also have reported disease remission following extensive weight loss.44 In addition, evidence has supported smoking cessation in improving the disease course of HS.43 Because these detrimental lifestyle choices are prevalent in both patients with HS and those with depression, a co-genetic susceptibility also may exist.
Furthermore, depression is characterized by a persistent inflammatory state,13,45 similar to HS.46 Elevated levels of a variety of inflammatory markers, such as C-reactive protein (CRP), IL-6, and soluble intercellular adhesion molecule 1, have been reported in patients with depression compared with healthy controls.13,45 Further analysis found a positive correlation and a strong association between depression and these inflammatory markers.47 Moreover, adipokines regulate inflammatory responses, and adipokines play a role in the pathogenesis of HS. Adipokine levels such as elevated omentin-1 (a recently identified adipokine) were found to be altered in patients with HS compared with controls.48 Results from clinical studies and meta-analyses of patients with depression also have demonstrated that adipokines are dysregulated in this population,49,50 which may be another potential genetic link between depression and HS.
In addition, genetic susceptibility to depression and HS may be shared because the inflammatory markers that have a strong association with depression also have been found to play an important role in HS treatment and disease severity prediction. In a retrospective cohort study of 404 patients, CRP or IL-6 levels were found to be reliable predictors of HS disease severity, which may explain why anti–tumor necrosis factor antibody regimens such as adalimumab and infliximab have clinically ameliorated disease activity in several cases of HS.51 In a study evaluating these drugs, high baseline levels of high-sensitivity CRP and IL-6 were predictive of patient response to infliximab.52 In a meta-analysis evaluating 20,791 participants, an association was found between concurrent depression and CRP. Furthermore, inflammation measured by high levels of CRP or IL-6 was observed to predict future depression.53 If the same inflammatory markers—CRP and IL-6—both play a major role in the disease activity of depression and HS, then a concurrent genetic predisposition may exist.
Conclusion
Understanding the comorbidities, etiologies, and risk factors for the development and progression of HS is an important step toward improved disease management. Available studies on comorbid depression in HS largely involve White patients, and more studies are needed in patients with skin of color, particularly the Black population, who have the highest prevalence of HS.10 Given the evidence for an association between depression and HS, we suggest a large-scale investigation of this patient population that includes a complete medical history, onset of HS in comparison to the onset of depression, and specific measures of disease progress and lifetime management of depression, which may help to increase knowledge about the role of depression in HS and encourage more research in this area. If shared genetic susceptibility is established, aggressive management of depression in patients at risk for HS may reduce disease incidence and severity as well as the psychological burden on patients.
- Crowley JJ, Mekkes JR, Zouboulis CC, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561-1565.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:1-20.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-1239.
- von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537.
- Van Der Zee HH, De Ruiter L, Van Den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292-1298.
- Schlapbach C, Hänni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439.
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4-S7.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Soliman YS, Hoffman LK, Guzman AK, et al. African American patients with hidradenitis suppurativa have significant health care disparities: a retrospective study. J Cutan Med Surg. 2019;23:334-336.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101.
- Beatriz Currier M, Nemeroff CB. Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Antiinflamm Antiallergy Agents Med Chem. 2012;9:212-220.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol. 2022;86:55-60.
- Sampogna F, Fania L, Mastroeni S, et al. Correlation between depression, quality of life and clinical severity in patients with hidradenitis suppurativa. Acta Derm Venereol. 2020;100:1-6.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Senthilnathan A, Kolli SS, Cardwell LA, et al. Depression in hidradenitis suppurativa. Br J Dermatol. 2019;181:1087-1088.
- Pavon Blanco A, Turner MA, Petrof G, et al. To what extent do disease severity and illness perceptions explain depression, anxiety and quality of life in hidradenitis suppurativa? Br J Dermatol. 2019;180:338-345.
- Butt M, Sisic M, Silva C, et al. The associations of depression and coping methods on health-related quality of life for those with hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:1137-1139.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13:e0200683.
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924.
- Kimball AB, Sundaram M, Gauthier G, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther (Heidelb). 2018;8:557.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Tiri H, Jokelainen J, Timonen M, et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol. 2018;79:514-519.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Kirby JS, Butt M, Esmann S, et al. Association of resilience with depression and health-related quality of life for patients with hidradenitis suppurativa. JAMA Dermatol. 2017;153:1263.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429-434.
- Vangipuram R, Vaidya T, Jandarov R, et al. Factors contributing to depression and chronic pain in patients with hidradenitis suppurativa: results from a single-center retrospective review. Dermatology. 2016;232:692-695.
- Rayner L, Jackson K, Turner M, et al. Integrated mental health assessment in a tertiary medical dermatology service: feasibility and the prevalence of common mental disorder. Br J Dermatol. 2015;173:201.
- Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa [published online June 9, 2014]. J Eur Acad Dermatol Venereol. 2015;29:371-376.
- Kurek A, Johanne Peters EM, Sabat R, et al. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11:743-749.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97.
- Onderdijk AJ, Van Der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa [published online February 20, 2012]. J Eur Acad Dermatol Venereol. 2013;27:473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268.
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
- Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2009;123:222-229.
- Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755-759.e1.
- Williams MT, Skinta MD, Kanter JW, et al. A qualitative study of microaggressions against African Americans on predominantly White campuses. BMC Psychol. 2020;8:1-13.
- Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945-1952.
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407-411.
- Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305-315.
- Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506-511.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824.
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg . 2020;24:64-72.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519-525.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409.
- Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761.
- González-López MA, Ocejo-Viñals JG, Mata C, et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2021;38:450-454.
- Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048.
- Setayesh L, Ebrahimi R, Pooyan S, et al. The possible mediatory role of adipokines in the association between low carbohydrate diet and depressive symptoms among overweight and obese women. PLoS One. 2021;16:e0257275 .
- Andriano TM, Benesh G, Babbush KM, et al. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61:1270-1275.
- Montaudié H, Seitz-Polski B, Cornille A, et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;6:156-158.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940-948.
Hidradenitis suppurativa (HS)—a chronic, relapsing, inflammatory disorder involving terminal hair follicles in apocrine gland–rich skin—manifests as tender inflamed nodules that transform into abscesses, sinus tracts, and scarring.1,2 The etiology of HS is multifactorial, encompassing lifestyle, microbiota, hormonal status, and genetic and environmental factors. These factors activate the immune system around the terminal hair follicles and lead to hyperkeratosis of the infundibulum of the hair follicles in intertriginous regions. This progresses to follicular occlusion, stasis, and eventual rupture. Bacterial multiplication within the plugged pilosebaceous units further boosts immune activation. Resident and migrated cells of the innate and adaptive immune system then release proinflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-17, which further enhance immune cell influx and inflammation.3,4 This aberrant immune response propagates the production of deep-seated inflammatory nodules and abscesses.3-8
The estimated prevalence of HS is 1% worldwide.9 It is more prevalent in female and Black patients (0.30%) than White patients (0.09%) and is intermediate in prevalence in the biracial population (0.22%).10 Hidradenitis suppurativa is thought to be associated with lower socioeconomic status (SES). In a retrospective analysis of HS patients (N=375), approximately one-third of patients were Black, had advanced disease, and had a notably lower SES.11 Furthermore, HS has been reported to be associated with systemic inflammation and comorbidities such as morbid obesity (38.3%) and hypertension (39.6%) as well as other metabolic syndrome–related disorders and depression (48.1%).1
Hidradenitis suppurativa may contribute to the risk for depression through its substantial impact on health-related quality of life, which culminates in social withdrawal, unemployment, and suicidal thoughts.12 The high prevalence of depression in individuals with HS1 and its association with systemic inflammation13 increases the likelihood that a common genetic predisposition also may exist between both conditions. Because depression frequently has been discovered as a concomitant diagnosis in patients with HS, we hypothesize that a shared genetic susceptibility also may exist between the 2 disorders. Our study sought to explore data on the co-occurrence of depression with HS, including its demographics and racial data.
Methods
We conducted a PubMed search of articles indexed for MEDLINE as well as Google Scholar using the terms depression and hidradenitis suppurativa to obtain all research articles published from 2000 to 2022. Articles were selected based on relevance to the topic of exploration. English-language articles that directly addressed the epidemiology, etiology, pathophysiology, and co-occurrence of both depression and HS with numerical data were included. Articles were excluded if they did not explore the information of interest on these 2 disorders or did not contain clear statistical data of patients with the 2 concurrent medical conditions.
Results
Twenty-two cross-sectional, prospective, and retrospective studies that fit the search criteria were identified and included in the analysis (eTable).1,14-34 Sixteen (72.7%) studies were cross-sectional, 5 (22.7%) were retrospective, and only 1 (4.5%) was a prospective study. Only 6 of the studies provided racial data,1,14,17,26,28,32 and of them, 4 had predominately White patients,1,14,26,32 whereas the other 2 had predominantly Black patients.17,28


Hidradenitis suppurativa was found to coexist with depression in all the studies, with a prevalence of 1.2% to 48.1%. There also was a higher prevalence of depression in HS patients than in the control patients without HS. Furthermore, a recent study by Wright and colleagues14 stratified the depression prevalence data by age and found a higher prevalence of depression in adults vs children with HS (30% vs 12%).
Comment
Major depression—a chronic and debilitating illness—is the chief cause of disability globally and in the United States alone and has a global lifetime prevalence of 17%.35 In a study of 388 patients diagnosed with depression and 404 community-matched controls who were observed for 10 years, depressed patients had a two-thirds higher likelihood of developing a serious physical illness than controls. The depression-associated elevated risk for serious physical illness persisted after controlling for confounding variables such as alcohol abuse, smoking, and level of physical activity.36 Studies also have demonstrated that HS is more prevalent in Black individuals10 and in individuals of low SES,37 who are mostly the Black and Hispanic populations that experience the highest burden of racial microaggression38 and disparities in health access and outcomes.39,40 The severity and chronicity of major depressive disorder also is higher in Black patients compared with White patients (57% vs 39%).41 Because major depression and HS are most common among Black patients who experience the highest-burden negative financial and health disparities, there may be a shared genetic disposition to both medical conditions.
Moreover, the common detrimental lifestyle choices associated with patients with depression and HS also suggest the possibility of a collective genetic susceptibility. Patients with depression also report increased consumption of alcohol, tobacco, and illicit substances; sedentary lifestyle leading to obesity; and poor compliance with prescribed medical treatment.42 Smoking and obesity are known contributors to the pathogenesis of HS, and their modification also is known to positively impact the disease course. In a retrospective single-cohort study, 50% of obese HS patients (n=35) reported a substantial decrease in disease severity after a reduction of more than 15% in body mass index over 2 years following bariatric surgery (n=35).43 Patients with HS also have reported disease remission following extensive weight loss.44 In addition, evidence has supported smoking cessation in improving the disease course of HS.43 Because these detrimental lifestyle choices are prevalent in both patients with HS and those with depression, a co-genetic susceptibility also may exist.
Furthermore, depression is characterized by a persistent inflammatory state,13,45 similar to HS.46 Elevated levels of a variety of inflammatory markers, such as C-reactive protein (CRP), IL-6, and soluble intercellular adhesion molecule 1, have been reported in patients with depression compared with healthy controls.13,45 Further analysis found a positive correlation and a strong association between depression and these inflammatory markers.47 Moreover, adipokines regulate inflammatory responses, and adipokines play a role in the pathogenesis of HS. Adipokine levels such as elevated omentin-1 (a recently identified adipokine) were found to be altered in patients with HS compared with controls.48 Results from clinical studies and meta-analyses of patients with depression also have demonstrated that adipokines are dysregulated in this population,49,50 which may be another potential genetic link between depression and HS.
In addition, genetic susceptibility to depression and HS may be shared because the inflammatory markers that have a strong association with depression also have been found to play an important role in HS treatment and disease severity prediction. In a retrospective cohort study of 404 patients, CRP or IL-6 levels were found to be reliable predictors of HS disease severity, which may explain why anti–tumor necrosis factor antibody regimens such as adalimumab and infliximab have clinically ameliorated disease activity in several cases of HS.51 In a study evaluating these drugs, high baseline levels of high-sensitivity CRP and IL-6 were predictive of patient response to infliximab.52 In a meta-analysis evaluating 20,791 participants, an association was found between concurrent depression and CRP. Furthermore, inflammation measured by high levels of CRP or IL-6 was observed to predict future depression.53 If the same inflammatory markers—CRP and IL-6—both play a major role in the disease activity of depression and HS, then a concurrent genetic predisposition may exist.
Conclusion
Understanding the comorbidities, etiologies, and risk factors for the development and progression of HS is an important step toward improved disease management. Available studies on comorbid depression in HS largely involve White patients, and more studies are needed in patients with skin of color, particularly the Black population, who have the highest prevalence of HS.10 Given the evidence for an association between depression and HS, we suggest a large-scale investigation of this patient population that includes a complete medical history, onset of HS in comparison to the onset of depression, and specific measures of disease progress and lifetime management of depression, which may help to increase knowledge about the role of depression in HS and encourage more research in this area. If shared genetic susceptibility is established, aggressive management of depression in patients at risk for HS may reduce disease incidence and severity as well as the psychological burden on patients.
Hidradenitis suppurativa (HS)—a chronic, relapsing, inflammatory disorder involving terminal hair follicles in apocrine gland–rich skin—manifests as tender inflamed nodules that transform into abscesses, sinus tracts, and scarring.1,2 The etiology of HS is multifactorial, encompassing lifestyle, microbiota, hormonal status, and genetic and environmental factors. These factors activate the immune system around the terminal hair follicles and lead to hyperkeratosis of the infundibulum of the hair follicles in intertriginous regions. This progresses to follicular occlusion, stasis, and eventual rupture. Bacterial multiplication within the plugged pilosebaceous units further boosts immune activation. Resident and migrated cells of the innate and adaptive immune system then release proinflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-17, which further enhance immune cell influx and inflammation.3,4 This aberrant immune response propagates the production of deep-seated inflammatory nodules and abscesses.3-8
The estimated prevalence of HS is 1% worldwide.9 It is more prevalent in female and Black patients (0.30%) than White patients (0.09%) and is intermediate in prevalence in the biracial population (0.22%).10 Hidradenitis suppurativa is thought to be associated with lower socioeconomic status (SES). In a retrospective analysis of HS patients (N=375), approximately one-third of patients were Black, had advanced disease, and had a notably lower SES.11 Furthermore, HS has been reported to be associated with systemic inflammation and comorbidities such as morbid obesity (38.3%) and hypertension (39.6%) as well as other metabolic syndrome–related disorders and depression (48.1%).1
Hidradenitis suppurativa may contribute to the risk for depression through its substantial impact on health-related quality of life, which culminates in social withdrawal, unemployment, and suicidal thoughts.12 The high prevalence of depression in individuals with HS1 and its association with systemic inflammation13 increases the likelihood that a common genetic predisposition also may exist between both conditions. Because depression frequently has been discovered as a concomitant diagnosis in patients with HS, we hypothesize that a shared genetic susceptibility also may exist between the 2 disorders. Our study sought to explore data on the co-occurrence of depression with HS, including its demographics and racial data.
Methods
We conducted a PubMed search of articles indexed for MEDLINE as well as Google Scholar using the terms depression and hidradenitis suppurativa to obtain all research articles published from 2000 to 2022. Articles were selected based on relevance to the topic of exploration. English-language articles that directly addressed the epidemiology, etiology, pathophysiology, and co-occurrence of both depression and HS with numerical data were included. Articles were excluded if they did not explore the information of interest on these 2 disorders or did not contain clear statistical data of patients with the 2 concurrent medical conditions.
Results
Twenty-two cross-sectional, prospective, and retrospective studies that fit the search criteria were identified and included in the analysis (eTable).1,14-34 Sixteen (72.7%) studies were cross-sectional, 5 (22.7%) were retrospective, and only 1 (4.5%) was a prospective study. Only 6 of the studies provided racial data,1,14,17,26,28,32 and of them, 4 had predominately White patients,1,14,26,32 whereas the other 2 had predominantly Black patients.17,28


Hidradenitis suppurativa was found to coexist with depression in all the studies, with a prevalence of 1.2% to 48.1%. There also was a higher prevalence of depression in HS patients than in the control patients without HS. Furthermore, a recent study by Wright and colleagues14 stratified the depression prevalence data by age and found a higher prevalence of depression in adults vs children with HS (30% vs 12%).
Comment
Major depression—a chronic and debilitating illness—is the chief cause of disability globally and in the United States alone and has a global lifetime prevalence of 17%.35 In a study of 388 patients diagnosed with depression and 404 community-matched controls who were observed for 10 years, depressed patients had a two-thirds higher likelihood of developing a serious physical illness than controls. The depression-associated elevated risk for serious physical illness persisted after controlling for confounding variables such as alcohol abuse, smoking, and level of physical activity.36 Studies also have demonstrated that HS is more prevalent in Black individuals10 and in individuals of low SES,37 who are mostly the Black and Hispanic populations that experience the highest burden of racial microaggression38 and disparities in health access and outcomes.39,40 The severity and chronicity of major depressive disorder also is higher in Black patients compared with White patients (57% vs 39%).41 Because major depression and HS are most common among Black patients who experience the highest-burden negative financial and health disparities, there may be a shared genetic disposition to both medical conditions.
Moreover, the common detrimental lifestyle choices associated with patients with depression and HS also suggest the possibility of a collective genetic susceptibility. Patients with depression also report increased consumption of alcohol, tobacco, and illicit substances; sedentary lifestyle leading to obesity; and poor compliance with prescribed medical treatment.42 Smoking and obesity are known contributors to the pathogenesis of HS, and their modification also is known to positively impact the disease course. In a retrospective single-cohort study, 50% of obese HS patients (n=35) reported a substantial decrease in disease severity after a reduction of more than 15% in body mass index over 2 years following bariatric surgery (n=35).43 Patients with HS also have reported disease remission following extensive weight loss.44 In addition, evidence has supported smoking cessation in improving the disease course of HS.43 Because these detrimental lifestyle choices are prevalent in both patients with HS and those with depression, a co-genetic susceptibility also may exist.
Furthermore, depression is characterized by a persistent inflammatory state,13,45 similar to HS.46 Elevated levels of a variety of inflammatory markers, such as C-reactive protein (CRP), IL-6, and soluble intercellular adhesion molecule 1, have been reported in patients with depression compared with healthy controls.13,45 Further analysis found a positive correlation and a strong association between depression and these inflammatory markers.47 Moreover, adipokines regulate inflammatory responses, and adipokines play a role in the pathogenesis of HS. Adipokine levels such as elevated omentin-1 (a recently identified adipokine) were found to be altered in patients with HS compared with controls.48 Results from clinical studies and meta-analyses of patients with depression also have demonstrated that adipokines are dysregulated in this population,49,50 which may be another potential genetic link between depression and HS.
In addition, genetic susceptibility to depression and HS may be shared because the inflammatory markers that have a strong association with depression also have been found to play an important role in HS treatment and disease severity prediction. In a retrospective cohort study of 404 patients, CRP or IL-6 levels were found to be reliable predictors of HS disease severity, which may explain why anti–tumor necrosis factor antibody regimens such as adalimumab and infliximab have clinically ameliorated disease activity in several cases of HS.51 In a study evaluating these drugs, high baseline levels of high-sensitivity CRP and IL-6 were predictive of patient response to infliximab.52 In a meta-analysis evaluating 20,791 participants, an association was found between concurrent depression and CRP. Furthermore, inflammation measured by high levels of CRP or IL-6 was observed to predict future depression.53 If the same inflammatory markers—CRP and IL-6—both play a major role in the disease activity of depression and HS, then a concurrent genetic predisposition may exist.
Conclusion
Understanding the comorbidities, etiologies, and risk factors for the development and progression of HS is an important step toward improved disease management. Available studies on comorbid depression in HS largely involve White patients, and more studies are needed in patients with skin of color, particularly the Black population, who have the highest prevalence of HS.10 Given the evidence for an association between depression and HS, we suggest a large-scale investigation of this patient population that includes a complete medical history, onset of HS in comparison to the onset of depression, and specific measures of disease progress and lifetime management of depression, which may help to increase knowledge about the role of depression in HS and encourage more research in this area. If shared genetic susceptibility is established, aggressive management of depression in patients at risk for HS may reduce disease incidence and severity as well as the psychological burden on patients.
- Crowley JJ, Mekkes JR, Zouboulis CC, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561-1565.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:1-20.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-1239.
- von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537.
- Van Der Zee HH, De Ruiter L, Van Den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292-1298.
- Schlapbach C, Hänni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439.
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4-S7.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Soliman YS, Hoffman LK, Guzman AK, et al. African American patients with hidradenitis suppurativa have significant health care disparities: a retrospective study. J Cutan Med Surg. 2019;23:334-336.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101.
- Beatriz Currier M, Nemeroff CB. Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Antiinflamm Antiallergy Agents Med Chem. 2012;9:212-220.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol. 2022;86:55-60.
- Sampogna F, Fania L, Mastroeni S, et al. Correlation between depression, quality of life and clinical severity in patients with hidradenitis suppurativa. Acta Derm Venereol. 2020;100:1-6.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Senthilnathan A, Kolli SS, Cardwell LA, et al. Depression in hidradenitis suppurativa. Br J Dermatol. 2019;181:1087-1088.
- Pavon Blanco A, Turner MA, Petrof G, et al. To what extent do disease severity and illness perceptions explain depression, anxiety and quality of life in hidradenitis suppurativa? Br J Dermatol. 2019;180:338-345.
- Butt M, Sisic M, Silva C, et al. The associations of depression and coping methods on health-related quality of life for those with hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:1137-1139.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13:e0200683.
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924.
- Kimball AB, Sundaram M, Gauthier G, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther (Heidelb). 2018;8:557.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Tiri H, Jokelainen J, Timonen M, et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol. 2018;79:514-519.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Kirby JS, Butt M, Esmann S, et al. Association of resilience with depression and health-related quality of life for patients with hidradenitis suppurativa. JAMA Dermatol. 2017;153:1263.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429-434.
- Vangipuram R, Vaidya T, Jandarov R, et al. Factors contributing to depression and chronic pain in patients with hidradenitis suppurativa: results from a single-center retrospective review. Dermatology. 2016;232:692-695.
- Rayner L, Jackson K, Turner M, et al. Integrated mental health assessment in a tertiary medical dermatology service: feasibility and the prevalence of common mental disorder. Br J Dermatol. 2015;173:201.
- Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa [published online June 9, 2014]. J Eur Acad Dermatol Venereol. 2015;29:371-376.
- Kurek A, Johanne Peters EM, Sabat R, et al. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11:743-749.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97.
- Onderdijk AJ, Van Der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa [published online February 20, 2012]. J Eur Acad Dermatol Venereol. 2013;27:473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268.
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
- Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2009;123:222-229.
- Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755-759.e1.
- Williams MT, Skinta MD, Kanter JW, et al. A qualitative study of microaggressions against African Americans on predominantly White campuses. BMC Psychol. 2020;8:1-13.
- Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945-1952.
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407-411.
- Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305-315.
- Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506-511.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824.
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg . 2020;24:64-72.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519-525.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409.
- Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761.
- González-López MA, Ocejo-Viñals JG, Mata C, et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2021;38:450-454.
- Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048.
- Setayesh L, Ebrahimi R, Pooyan S, et al. The possible mediatory role of adipokines in the association between low carbohydrate diet and depressive symptoms among overweight and obese women. PLoS One. 2021;16:e0257275 .
- Andriano TM, Benesh G, Babbush KM, et al. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61:1270-1275.
- Montaudié H, Seitz-Polski B, Cornille A, et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;6:156-158.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940-948.
- Crowley JJ, Mekkes JR, Zouboulis CC, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561-1565.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:1-20.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-1239.
- von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537.
- Van Der Zee HH, De Ruiter L, Van Den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292-1298.
- Schlapbach C, Hänni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439.
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4-S7.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Soliman YS, Hoffman LK, Guzman AK, et al. African American patients with hidradenitis suppurativa have significant health care disparities: a retrospective study. J Cutan Med Surg. 2019;23:334-336.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101.
- Beatriz Currier M, Nemeroff CB. Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Antiinflamm Antiallergy Agents Med Chem. 2012;9:212-220.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol. 2022;86:55-60.
- Sampogna F, Fania L, Mastroeni S, et al. Correlation between depression, quality of life and clinical severity in patients with hidradenitis suppurativa. Acta Derm Venereol. 2020;100:1-6.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Senthilnathan A, Kolli SS, Cardwell LA, et al. Depression in hidradenitis suppurativa. Br J Dermatol. 2019;181:1087-1088.
- Pavon Blanco A, Turner MA, Petrof G, et al. To what extent do disease severity and illness perceptions explain depression, anxiety and quality of life in hidradenitis suppurativa? Br J Dermatol. 2019;180:338-345.
- Butt M, Sisic M, Silva C, et al. The associations of depression and coping methods on health-related quality of life for those with hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:1137-1139.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13:e0200683.
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924.
- Kimball AB, Sundaram M, Gauthier G, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther (Heidelb). 2018;8:557.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Tiri H, Jokelainen J, Timonen M, et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol. 2018;79:514-519.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Kirby JS, Butt M, Esmann S, et al. Association of resilience with depression and health-related quality of life for patients with hidradenitis suppurativa. JAMA Dermatol. 2017;153:1263.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429-434.
- Vangipuram R, Vaidya T, Jandarov R, et al. Factors contributing to depression and chronic pain in patients with hidradenitis suppurativa: results from a single-center retrospective review. Dermatology. 2016;232:692-695.
- Rayner L, Jackson K, Turner M, et al. Integrated mental health assessment in a tertiary medical dermatology service: feasibility and the prevalence of common mental disorder. Br J Dermatol. 2015;173:201.
- Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa [published online June 9, 2014]. J Eur Acad Dermatol Venereol. 2015;29:371-376.
- Kurek A, Johanne Peters EM, Sabat R, et al. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11:743-749.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97.
- Onderdijk AJ, Van Der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa [published online February 20, 2012]. J Eur Acad Dermatol Venereol. 2013;27:473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268.
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
- Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2009;123:222-229.
- Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755-759.e1.
- Williams MT, Skinta MD, Kanter JW, et al. A qualitative study of microaggressions against African Americans on predominantly White campuses. BMC Psychol. 2020;8:1-13.
- Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945-1952.
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407-411.
- Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305-315.
- Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506-511.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824.
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg . 2020;24:64-72.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519-525.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409.
- Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761.
- González-López MA, Ocejo-Viñals JG, Mata C, et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2021;38:450-454.
- Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048.
- Setayesh L, Ebrahimi R, Pooyan S, et al. The possible mediatory role of adipokines in the association between low carbohydrate diet and depressive symptoms among overweight and obese women. PLoS One. 2021;16:e0257275 .
- Andriano TM, Benesh G, Babbush KM, et al. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61:1270-1275.
- Montaudié H, Seitz-Polski B, Cornille A, et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;6:156-158.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940-948.
Practice Points
- Hidradenitis suppurativa (HS) is known to be associated with systemic inflammation and comorbidities, including depression.
- Depression may be a potential contributing factor to HS in affected patients, and studies on HS with comorbid depression in patients with skin of color are lacking.
Alopecia Areata in Skin of Color Patients: New Considerations Sparked by the Approval of Baricitinib
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
Deployed Airbag Causes Bullous Reaction Following a Motor Vehicle Accident
Airbags are lifesaving during motor vehicle accidents (MVAs), but their deployment has been associated with skin issues such as irritant dermatitis1; lacerations2; abrasions3; and thermal, friction, and chemical burns.4-6 Ocular issues such as alkaline chemical keratitis7 and ocular alkali injuries8 also have been described.
Airbag deployment is triggered by rapid deceleration and impact, which ignite a sodium azide cartridge, causing the woven nylon bag to inflate with hydrocarbon gases.8 This leads to release of sodium hydroxide, sodium bicarbonate, and metallic oxides in an aerosolized form. If a tear in the meshwork of the airbag occurs, exposure to an even larger amount of powder containing caustic alkali chemicals can occur.8
We describe a patient who developed a bullous reaction to airbag contents after he was involved in an MVA in which the airbag deployed.
Case Report
A 35-year-old man with a history of type 2 diabetes mellitus and chronic hepatitis B presented to the dermatology clinic for an evaluation of new-onset blisters. The rash occurred 1 day after the patient was involved in an MVA in which he was exposed to the airbag’s contents after it burst. He had been evaluated twice in the emergency department for the skin eruption before being referred to dermatology. He noted the lesions were pruritic and painful. Prior treatments included silver sulfadiazine cream 1% and clobetasol cream 0.05%, though he discontinued using the latter because of burning with application. Physical examination revealed tense vesicles and bullae on an erythematous base on the right lower flank, forearms, and legs, with the exception of the lower right leg where a cast had been from a prior injury (Figure 1).

Two punch biopsies of the left arm were performed and sent for hematoxylin and eosin staining and direct immunofluorescence to rule out bullous diseases, such as bullous pemphigoid, linear IgA, and bullous lupus. Hematoxylin and eosin staining revealed extensive spongiosis with blister formation and a dense perivascular infiltrate in the superficial and mid dermis composed of lymphocytes with numerous scattered eosinophils (Figures 2 and 3). Direct immunofluorescence studies were negative. Treatment with oral prednisone and oral antihistamines was initiated.
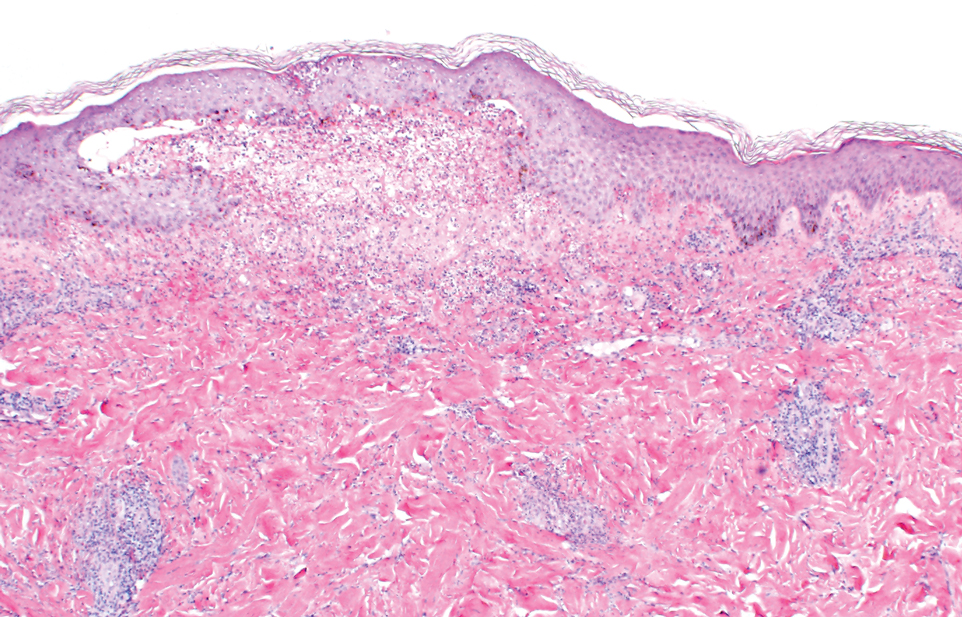
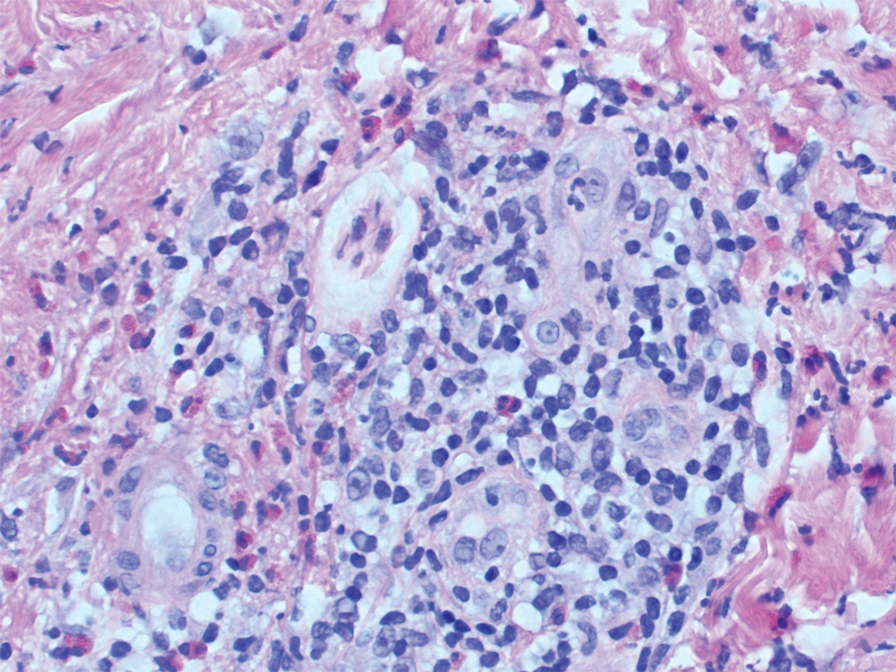
At 10-day follow-up, the patient had a few residual bullae; most lesions were demonstrating various stages of healing (Figure 4). The patient’s cast had been removed, and there were no lesions in this previously covered area. At 6-week follow-up he had continued healing of the bullae and erosions as well as postinflammatory hyperpigmentation (Figure 5).

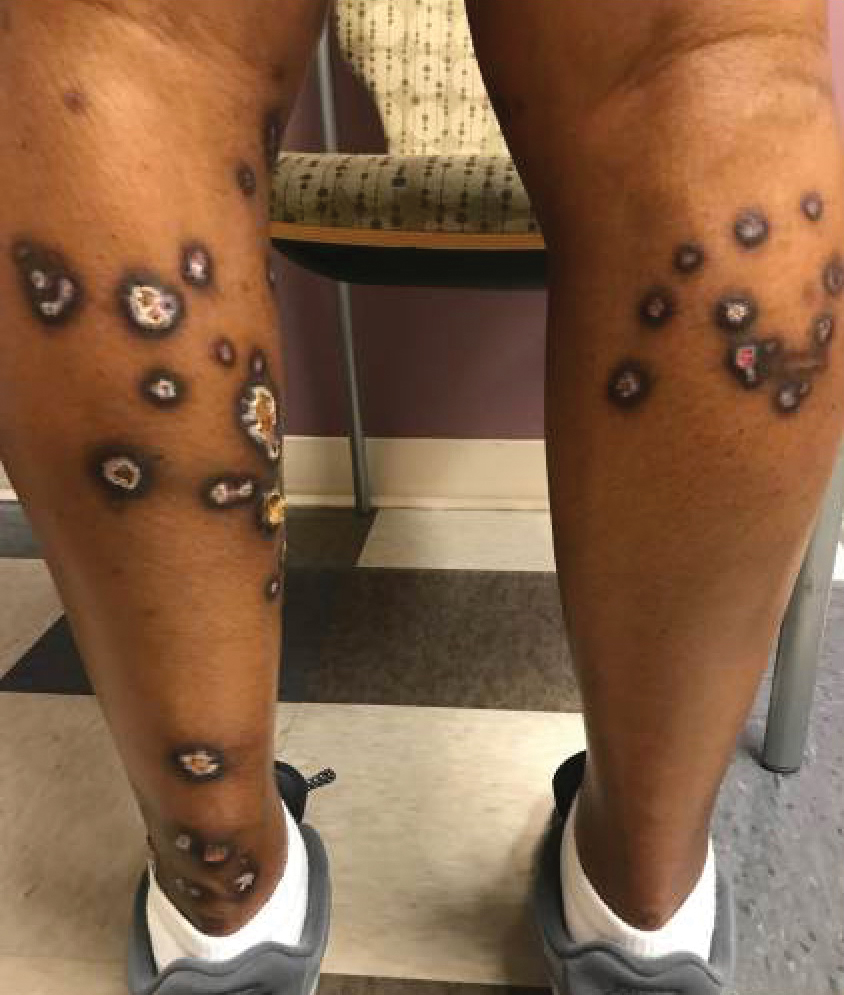
Comment
With the advent of airbags for safety purposes, these potentially lifesaving devices also have been known to cause injury.9 In 1998, the most commonly reported airbag injuries were ocular injuries.10 Cutaneous manifestations of airbag injury are less well known.11
Two cases of airbag deployment with skin blistering have been reported in the literature based on a PubMed search of articles indexed for MEDLINE using the terms airbag blistering or airbag bullae12,13; however, the blistering was described in the context of a burn. One case of the effects of airbag deployment residue highlights a patient arriving to the emergency department with erythema and blisters on the hands within 48 hours of airbag deployment in an MVA, and the treatment was standard burn therapy.12 Another case report described a patient with a second-degree burn with a 12-cm blister occurring on the radial side of the hand and distal forearm following an MVA and airbag deployment, which was treated conservatively.13 Cases of thermal burns, chemical burns, and irritant contact dermatitis after airbag deployment have been described in the literature.4-6,11,12,14,15 Our patient’s distal right lower leg was covered with a cast for osteomyelitis, and no blisters had developed in this area. It is likely that the transfer of airbag contents occurred during the process of unbuckling his seatbelt, which could explain the bullae that developed on the right flank. Per the Centers for Disease Control and Prevention, individuals should quickly remove clothing and wash their body with large amounts of soap and water following exposure to sodium azide.16
In 1989, the Federal Motor Vehicle Safety Standard No. 208 (occupant crash protection) became effective, stating all cars must have vehicle crash protection.12 Prior to 1993, it was reported that there had been no associated chemical injuries with airbag deployment. Subsequently, a 6-month retrospective study in 1993 showed that dermal injuries were found in connection with the presence of sodium hydroxide in automobile airbags.12 By 2004, it was known that airbags could cause chemical and thermal burns in addition to traumatic injuries from deployment.1 Since 2007, all motor vehicles have been required to have advanced airbags, which are engineered to sense the presence of passengers and determine if the airbag will deploy, and if so, how much to deploy to minimize airbag-related injury.3
The brand of car that our patient drove during the MVA is one with known airbag recalls due to safety defects; however, the year and actual model of the vehicle are not known, so specific information about the airbag in question is not available. It has been noted that some defective airbag inflators that were exposed to excess moisture during the manufacturing process could explode during deployment, causing shrapnel and airbag rupture, which has been linked to nearly 300 injuries worldwide.17
Conclusion
It is evident that the use of airbag devices reduces morbidity and mortality due to MVAs.9 It also had been reported that up to 96% of airbag-related injuries are relatively minor, which many would argue justifies their use.18 Furthermore, it has been reported that 99.8% of skin injuries following airbag deployment are minor.19 In the United States, it is mandated that every vehicle have functional airbags installed.8
This case highlights the potential for substantial airbag-induced skin reactions, specifically a bullous reaction, following airbag deployment. The persistent pruritus and lasting postinflammatory hyperpigmentation seen in this case were certainly worrisome for our patient. We also present this case to remind dermatology providers of possible treatment approaches to these skin reactions. Immediate cleansing of the affected areas of skin may help avoid such reactions.
- Corazza M, Trincone S, Zampino MR, et al. Air bags and the skin. Skinmed. 2004;3:256-258.
- Corazza M, Trincone S, Virgili A. Effects of airbag deployment: lesions, epidemiology, and management. Am J Clin Dermatol. 2004;5:295-300.
- Kuska TC. Air bag safety: an update. J Emerg Nurs. 2016;42:438-441.
- Ulrich D, Noah EM, Fuchs P, et al. Burn injuries caused by air bag deployment. Burns. 2001;27:196-199.
- Erpenbeck SP, Roy E, Ziembicki JA, et al. A systematic review on airbag-induced burns. J Burn Care Res. 2021;42:481-487.
- Skibba KEH, Cleveland CN, Bell DE. Airbag burns: an unfortunate consequence of motor vehicle safety. J Burn Care Res. 2021;42:71-73.
- Smally AJ, Binzer A, Dolin S, et al. Alkaline chemical keratitis: eye injury from airbags. Ann Emerg Med. 1992;21:1400-1402.
- Barnes SS, Wong W Jr, Affeldt JC. A case of severe airbag related ocular alkali injury. Hawaii J Med Public Health. 2012;71:229-231.
- Wallis LA, Greaves I. Injuries associated with airbag deployment. Emerg Med J. 2002;19:490-493.
- Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74:455-458.
- Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66:251-252.
- Swanson-Biearman B, Mrvos R, Dean BS, et al. Air bags: lifesaving with toxic potential? Am J Emerg Med. 1993;11:38-39.
- Roth T, Meredith P. Traumatic lesions caused by the “air-bag” system [in French]. Z Unfallchir Versicherungsmed. 1993;86:189-193.
- Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138:1383-1384.
- Vitello W, Kim M, Johnson RM, et al. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212-215.
- Centers for Disease Control and Prevention. Facts about sodium azide. Updated April 4, 2018. Accessed May 15, 2022. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp
- Shepardson D. Honda to recall 1.2 million vehicles in North America to replace Takata airbags. March 12, 2019. Accessed March 22, 2022. https://www.reuters.com/article/us-honda-takata-recall/honda-to-recall-1-2-million-vehicles-in-north-america-to-replace-takata-airbags-idUSKBN1QT1C9
- Gabauer DJ, Gabler HC. The effects of airbags and seatbelts on occupant injury in longitudinal barrier crashes. J Safety Res. 2010;41:9-15.
- Rath AL, Jernigan MV, Stitzel JD, et al. The effects of depowered airbags on skin injuries in frontal automobile crashes. Plast Reconstr Surg. 2005;115:428-435.
Airbags are lifesaving during motor vehicle accidents (MVAs), but their deployment has been associated with skin issues such as irritant dermatitis1; lacerations2; abrasions3; and thermal, friction, and chemical burns.4-6 Ocular issues such as alkaline chemical keratitis7 and ocular alkali injuries8 also have been described.
Airbag deployment is triggered by rapid deceleration and impact, which ignite a sodium azide cartridge, causing the woven nylon bag to inflate with hydrocarbon gases.8 This leads to release of sodium hydroxide, sodium bicarbonate, and metallic oxides in an aerosolized form. If a tear in the meshwork of the airbag occurs, exposure to an even larger amount of powder containing caustic alkali chemicals can occur.8
We describe a patient who developed a bullous reaction to airbag contents after he was involved in an MVA in which the airbag deployed.
Case Report
A 35-year-old man with a history of type 2 diabetes mellitus and chronic hepatitis B presented to the dermatology clinic for an evaluation of new-onset blisters. The rash occurred 1 day after the patient was involved in an MVA in which he was exposed to the airbag’s contents after it burst. He had been evaluated twice in the emergency department for the skin eruption before being referred to dermatology. He noted the lesions were pruritic and painful. Prior treatments included silver sulfadiazine cream 1% and clobetasol cream 0.05%, though he discontinued using the latter because of burning with application. Physical examination revealed tense vesicles and bullae on an erythematous base on the right lower flank, forearms, and legs, with the exception of the lower right leg where a cast had been from a prior injury (Figure 1).

Two punch biopsies of the left arm were performed and sent for hematoxylin and eosin staining and direct immunofluorescence to rule out bullous diseases, such as bullous pemphigoid, linear IgA, and bullous lupus. Hematoxylin and eosin staining revealed extensive spongiosis with blister formation and a dense perivascular infiltrate in the superficial and mid dermis composed of lymphocytes with numerous scattered eosinophils (Figures 2 and 3). Direct immunofluorescence studies were negative. Treatment with oral prednisone and oral antihistamines was initiated.


At 10-day follow-up, the patient had a few residual bullae; most lesions were demonstrating various stages of healing (Figure 4). The patient’s cast had been removed, and there were no lesions in this previously covered area. At 6-week follow-up he had continued healing of the bullae and erosions as well as postinflammatory hyperpigmentation (Figure 5).


Comment
With the advent of airbags for safety purposes, these potentially lifesaving devices also have been known to cause injury.9 In 1998, the most commonly reported airbag injuries were ocular injuries.10 Cutaneous manifestations of airbag injury are less well known.11
Two cases of airbag deployment with skin blistering have been reported in the literature based on a PubMed search of articles indexed for MEDLINE using the terms airbag blistering or airbag bullae12,13; however, the blistering was described in the context of a burn. One case of the effects of airbag deployment residue highlights a patient arriving to the emergency department with erythema and blisters on the hands within 48 hours of airbag deployment in an MVA, and the treatment was standard burn therapy.12 Another case report described a patient with a second-degree burn with a 12-cm blister occurring on the radial side of the hand and distal forearm following an MVA and airbag deployment, which was treated conservatively.13 Cases of thermal burns, chemical burns, and irritant contact dermatitis after airbag deployment have been described in the literature.4-6,11,12,14,15 Our patient’s distal right lower leg was covered with a cast for osteomyelitis, and no blisters had developed in this area. It is likely that the transfer of airbag contents occurred during the process of unbuckling his seatbelt, which could explain the bullae that developed on the right flank. Per the Centers for Disease Control and Prevention, individuals should quickly remove clothing and wash their body with large amounts of soap and water following exposure to sodium azide.16
In 1989, the Federal Motor Vehicle Safety Standard No. 208 (occupant crash protection) became effective, stating all cars must have vehicle crash protection.12 Prior to 1993, it was reported that there had been no associated chemical injuries with airbag deployment. Subsequently, a 6-month retrospective study in 1993 showed that dermal injuries were found in connection with the presence of sodium hydroxide in automobile airbags.12 By 2004, it was known that airbags could cause chemical and thermal burns in addition to traumatic injuries from deployment.1 Since 2007, all motor vehicles have been required to have advanced airbags, which are engineered to sense the presence of passengers and determine if the airbag will deploy, and if so, how much to deploy to minimize airbag-related injury.3
The brand of car that our patient drove during the MVA is one with known airbag recalls due to safety defects; however, the year and actual model of the vehicle are not known, so specific information about the airbag in question is not available. It has been noted that some defective airbag inflators that were exposed to excess moisture during the manufacturing process could explode during deployment, causing shrapnel and airbag rupture, which has been linked to nearly 300 injuries worldwide.17
Conclusion
It is evident that the use of airbag devices reduces morbidity and mortality due to MVAs.9 It also had been reported that up to 96% of airbag-related injuries are relatively minor, which many would argue justifies their use.18 Furthermore, it has been reported that 99.8% of skin injuries following airbag deployment are minor.19 In the United States, it is mandated that every vehicle have functional airbags installed.8
This case highlights the potential for substantial airbag-induced skin reactions, specifically a bullous reaction, following airbag deployment. The persistent pruritus and lasting postinflammatory hyperpigmentation seen in this case were certainly worrisome for our patient. We also present this case to remind dermatology providers of possible treatment approaches to these skin reactions. Immediate cleansing of the affected areas of skin may help avoid such reactions.
Airbags are lifesaving during motor vehicle accidents (MVAs), but their deployment has been associated with skin issues such as irritant dermatitis1; lacerations2; abrasions3; and thermal, friction, and chemical burns.4-6 Ocular issues such as alkaline chemical keratitis7 and ocular alkali injuries8 also have been described.
Airbag deployment is triggered by rapid deceleration and impact, which ignite a sodium azide cartridge, causing the woven nylon bag to inflate with hydrocarbon gases.8 This leads to release of sodium hydroxide, sodium bicarbonate, and metallic oxides in an aerosolized form. If a tear in the meshwork of the airbag occurs, exposure to an even larger amount of powder containing caustic alkali chemicals can occur.8
We describe a patient who developed a bullous reaction to airbag contents after he was involved in an MVA in which the airbag deployed.
Case Report
A 35-year-old man with a history of type 2 diabetes mellitus and chronic hepatitis B presented to the dermatology clinic for an evaluation of new-onset blisters. The rash occurred 1 day after the patient was involved in an MVA in which he was exposed to the airbag’s contents after it burst. He had been evaluated twice in the emergency department for the skin eruption before being referred to dermatology. He noted the lesions were pruritic and painful. Prior treatments included silver sulfadiazine cream 1% and clobetasol cream 0.05%, though he discontinued using the latter because of burning with application. Physical examination revealed tense vesicles and bullae on an erythematous base on the right lower flank, forearms, and legs, with the exception of the lower right leg where a cast had been from a prior injury (Figure 1).

Two punch biopsies of the left arm were performed and sent for hematoxylin and eosin staining and direct immunofluorescence to rule out bullous diseases, such as bullous pemphigoid, linear IgA, and bullous lupus. Hematoxylin and eosin staining revealed extensive spongiosis with blister formation and a dense perivascular infiltrate in the superficial and mid dermis composed of lymphocytes with numerous scattered eosinophils (Figures 2 and 3). Direct immunofluorescence studies were negative. Treatment with oral prednisone and oral antihistamines was initiated.


At 10-day follow-up, the patient had a few residual bullae; most lesions were demonstrating various stages of healing (Figure 4). The patient’s cast had been removed, and there were no lesions in this previously covered area. At 6-week follow-up he had continued healing of the bullae and erosions as well as postinflammatory hyperpigmentation (Figure 5).


Comment
With the advent of airbags for safety purposes, these potentially lifesaving devices also have been known to cause injury.9 In 1998, the most commonly reported airbag injuries were ocular injuries.10 Cutaneous manifestations of airbag injury are less well known.11
Two cases of airbag deployment with skin blistering have been reported in the literature based on a PubMed search of articles indexed for MEDLINE using the terms airbag blistering or airbag bullae12,13; however, the blistering was described in the context of a burn. One case of the effects of airbag deployment residue highlights a patient arriving to the emergency department with erythema and blisters on the hands within 48 hours of airbag deployment in an MVA, and the treatment was standard burn therapy.12 Another case report described a patient with a second-degree burn with a 12-cm blister occurring on the radial side of the hand and distal forearm following an MVA and airbag deployment, which was treated conservatively.13 Cases of thermal burns, chemical burns, and irritant contact dermatitis after airbag deployment have been described in the literature.4-6,11,12,14,15 Our patient’s distal right lower leg was covered with a cast for osteomyelitis, and no blisters had developed in this area. It is likely that the transfer of airbag contents occurred during the process of unbuckling his seatbelt, which could explain the bullae that developed on the right flank. Per the Centers for Disease Control and Prevention, individuals should quickly remove clothing and wash their body with large amounts of soap and water following exposure to sodium azide.16
In 1989, the Federal Motor Vehicle Safety Standard No. 208 (occupant crash protection) became effective, stating all cars must have vehicle crash protection.12 Prior to 1993, it was reported that there had been no associated chemical injuries with airbag deployment. Subsequently, a 6-month retrospective study in 1993 showed that dermal injuries were found in connection with the presence of sodium hydroxide in automobile airbags.12 By 2004, it was known that airbags could cause chemical and thermal burns in addition to traumatic injuries from deployment.1 Since 2007, all motor vehicles have been required to have advanced airbags, which are engineered to sense the presence of passengers and determine if the airbag will deploy, and if so, how much to deploy to minimize airbag-related injury.3
The brand of car that our patient drove during the MVA is one with known airbag recalls due to safety defects; however, the year and actual model of the vehicle are not known, so specific information about the airbag in question is not available. It has been noted that some defective airbag inflators that were exposed to excess moisture during the manufacturing process could explode during deployment, causing shrapnel and airbag rupture, which has been linked to nearly 300 injuries worldwide.17
Conclusion
It is evident that the use of airbag devices reduces morbidity and mortality due to MVAs.9 It also had been reported that up to 96% of airbag-related injuries are relatively minor, which many would argue justifies their use.18 Furthermore, it has been reported that 99.8% of skin injuries following airbag deployment are minor.19 In the United States, it is mandated that every vehicle have functional airbags installed.8
This case highlights the potential for substantial airbag-induced skin reactions, specifically a bullous reaction, following airbag deployment. The persistent pruritus and lasting postinflammatory hyperpigmentation seen in this case were certainly worrisome for our patient. We also present this case to remind dermatology providers of possible treatment approaches to these skin reactions. Immediate cleansing of the affected areas of skin may help avoid such reactions.
- Corazza M, Trincone S, Zampino MR, et al. Air bags and the skin. Skinmed. 2004;3:256-258.
- Corazza M, Trincone S, Virgili A. Effects of airbag deployment: lesions, epidemiology, and management. Am J Clin Dermatol. 2004;5:295-300.
- Kuska TC. Air bag safety: an update. J Emerg Nurs. 2016;42:438-441.
- Ulrich D, Noah EM, Fuchs P, et al. Burn injuries caused by air bag deployment. Burns. 2001;27:196-199.
- Erpenbeck SP, Roy E, Ziembicki JA, et al. A systematic review on airbag-induced burns. J Burn Care Res. 2021;42:481-487.
- Skibba KEH, Cleveland CN, Bell DE. Airbag burns: an unfortunate consequence of motor vehicle safety. J Burn Care Res. 2021;42:71-73.
- Smally AJ, Binzer A, Dolin S, et al. Alkaline chemical keratitis: eye injury from airbags. Ann Emerg Med. 1992;21:1400-1402.
- Barnes SS, Wong W Jr, Affeldt JC. A case of severe airbag related ocular alkali injury. Hawaii J Med Public Health. 2012;71:229-231.
- Wallis LA, Greaves I. Injuries associated with airbag deployment. Emerg Med J. 2002;19:490-493.
- Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74:455-458.
- Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66:251-252.
- Swanson-Biearman B, Mrvos R, Dean BS, et al. Air bags: lifesaving with toxic potential? Am J Emerg Med. 1993;11:38-39.
- Roth T, Meredith P. Traumatic lesions caused by the “air-bag” system [in French]. Z Unfallchir Versicherungsmed. 1993;86:189-193.
- Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138:1383-1384.
- Vitello W, Kim M, Johnson RM, et al. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212-215.
- Centers for Disease Control and Prevention. Facts about sodium azide. Updated April 4, 2018. Accessed May 15, 2022. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp
- Shepardson D. Honda to recall 1.2 million vehicles in North America to replace Takata airbags. March 12, 2019. Accessed March 22, 2022. https://www.reuters.com/article/us-honda-takata-recall/honda-to-recall-1-2-million-vehicles-in-north-america-to-replace-takata-airbags-idUSKBN1QT1C9
- Gabauer DJ, Gabler HC. The effects of airbags and seatbelts on occupant injury in longitudinal barrier crashes. J Safety Res. 2010;41:9-15.
- Rath AL, Jernigan MV, Stitzel JD, et al. The effects of depowered airbags on skin injuries in frontal automobile crashes. Plast Reconstr Surg. 2005;115:428-435.
- Corazza M, Trincone S, Zampino MR, et al. Air bags and the skin. Skinmed. 2004;3:256-258.
- Corazza M, Trincone S, Virgili A. Effects of airbag deployment: lesions, epidemiology, and management. Am J Clin Dermatol. 2004;5:295-300.
- Kuska TC. Air bag safety: an update. J Emerg Nurs. 2016;42:438-441.
- Ulrich D, Noah EM, Fuchs P, et al. Burn injuries caused by air bag deployment. Burns. 2001;27:196-199.
- Erpenbeck SP, Roy E, Ziembicki JA, et al. A systematic review on airbag-induced burns. J Burn Care Res. 2021;42:481-487.
- Skibba KEH, Cleveland CN, Bell DE. Airbag burns: an unfortunate consequence of motor vehicle safety. J Burn Care Res. 2021;42:71-73.
- Smally AJ, Binzer A, Dolin S, et al. Alkaline chemical keratitis: eye injury from airbags. Ann Emerg Med. 1992;21:1400-1402.
- Barnes SS, Wong W Jr, Affeldt JC. A case of severe airbag related ocular alkali injury. Hawaii J Med Public Health. 2012;71:229-231.
- Wallis LA, Greaves I. Injuries associated with airbag deployment. Emerg Med J. 2002;19:490-493.
- Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74:455-458.
- Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66:251-252.
- Swanson-Biearman B, Mrvos R, Dean BS, et al. Air bags: lifesaving with toxic potential? Am J Emerg Med. 1993;11:38-39.
- Roth T, Meredith P. Traumatic lesions caused by the “air-bag” system [in French]. Z Unfallchir Versicherungsmed. 1993;86:189-193.
- Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138:1383-1384.
- Vitello W, Kim M, Johnson RM, et al. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212-215.
- Centers for Disease Control and Prevention. Facts about sodium azide. Updated April 4, 2018. Accessed May 15, 2022. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp
- Shepardson D. Honda to recall 1.2 million vehicles in North America to replace Takata airbags. March 12, 2019. Accessed March 22, 2022. https://www.reuters.com/article/us-honda-takata-recall/honda-to-recall-1-2-million-vehicles-in-north-america-to-replace-takata-airbags-idUSKBN1QT1C9
- Gabauer DJ, Gabler HC. The effects of airbags and seatbelts on occupant injury in longitudinal barrier crashes. J Safety Res. 2010;41:9-15.
- Rath AL, Jernigan MV, Stitzel JD, et al. The effects of depowered airbags on skin injuries in frontal automobile crashes. Plast Reconstr Surg. 2005;115:428-435.
Practice Points
- This case highlights the potential for a bullous reaction following airbag deployment.
- After airbag deployment, it is important to immediately cleanse the affected areas of skin with soap and water.
Views and Beliefs of Vitiligo Patients in Online Discussion Forums: A Qualitative Study
Vitiligo is a chronic dermatologic condition that negatively affects quality of life (QOL), with substantial burden on the psychosocial well-being of patients.1 There is no cure, and current treatment modalities are aimed at controlling the chronic relapsing condition.1-3 Despite topical and cosmetic treatments for stabilization and repigmentation, vitiligo remains unpredictable.3
All genders, races, ethnicities, and socioeconomic classes are equally affected.4 The underlying etiology of vitiligo remains unknown to a great extent and is more poorly understood by the general public compared with other skin diseases (eg, acne).5 Patients with vitiligo experience social withdrawal, decreased sense of self-esteem, anxiety, depression, and suicidal ideation.5,6 Stigmatization has the greatest impact on QOL, with strong correlations between avoidance behaviors and lesion concealment.6-8 Although the condition is especially disfiguring for darker skin types, lighter skin types also are substantially affected, with similar overall self-reported stress.6,7
Individuals with chronic illnesses such as vitiligo turn to online communities for health information and social support, commiserating with others who have the same condition.9,10 Online forums are platforms for asynchronous peer-to-peer exchange of disease-related information for better management of long-term disease.11 Moreover, of all available internet resources, online forum posts are the most commonly accessed source of information (91%) for patients following visits with their doctors.12
Qualitative research involving chronic skin conditions and the information exchanged in online forums has been conducted for patients with acne, psoriasis, and atopic dermatitis, but not for patients with vitiligo.13-16 Although online questionnaires have been administered to patients with vitiligo, the content within online forums is not well characterized.2,17
The purpose of this qualitative study was to evaluate the online content exchanged by individuals with vitiligo to better understand the general attitudes and help-seeking behaviors in online forums.
Methods
Study Design—This qualitative study sought to investigate health beliefs and messages about vitiligo posted by users in US-based online discussion forums. An interpretive research paradigm was utilized so that all content collected in online forums were the views expressed by individuals.18-20 An integrated approach was used in the development of the coding manual, with pre-established major themes and subthemes as a guiding framework.16,21,22 We adhered to an inductive grounded method by means of de novo line-by-line coding, such that we had flexibility for new subthemes to emerge throughout the duration of the entire coding process.23
Individual posts and subsequent replies embedded within public online forums were used as the collected data source. Google was utilized as the primary search engine to identify forums pertaining to vitiligo, as 80% of US adults with chronic disease report that their inquiries for health information start with Google, Bing, or Yahoo.24 The institutional review board at the Wake Forest School of Medicine (Winston-Salem, North Carolina) granted approval of the study (IRB00063073). Online forums were considered “property” of the public domain and were accessible to all, eliminating the need for written informed consent.24-26
Search Criteria—We conducted our forum search in February 2020 with a systematic approach using predetermined phrases—online forum vitiligo support, vitiligo online message board, and vitiligo forums—which yielded more than 358,171 total results (eTable 1). Threads were identified in chronological order (from newest to oldest) based on how they appeared during each internet search, and all Google results for the respective search phrases were reviewed. Dates of selected threads ranged from 2005 to 2020. Only sites with US domains were included. Posts that either included views and understandings of vitiligo or belonged to a thread that contained a vitiligo discussion were deemed relevant for inclusion. Forums were excluded if registration or means of payment was required to view posts, if there were fewer than 2 user replies to a thread, if threads contained patient photographs, or if no posts had been made in the last 2 years (rendering the thread inactive). No social media platforms, such as Facebook, or formal online platforms, such as MyVitiligoTeam, were included in the search. A no-fee-for-access was chosen for this study, as the majority of those with a chronic condition who encounter a required paywall find the information elsewhere.25
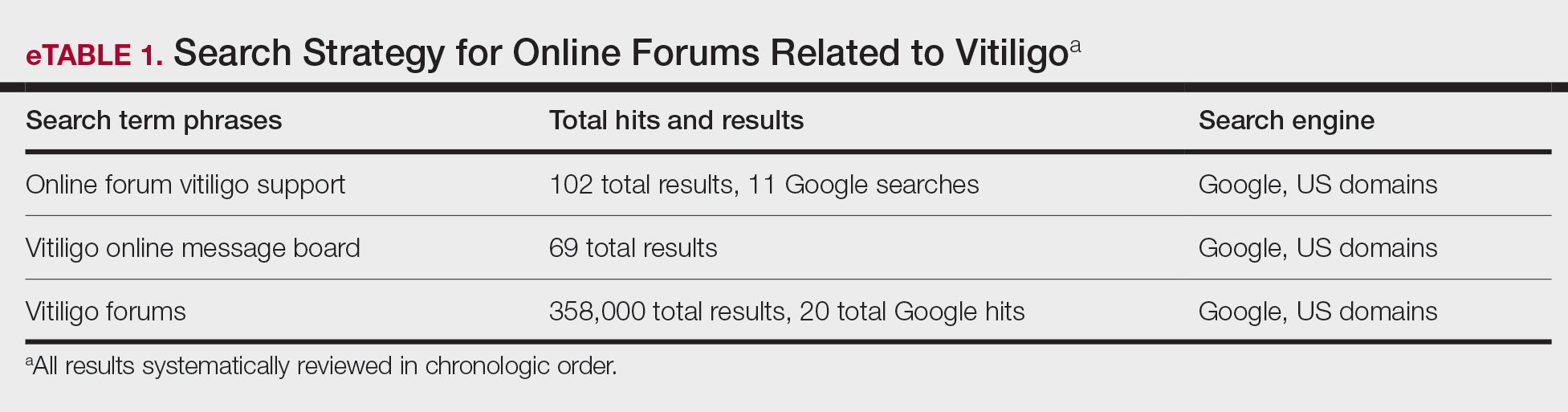
Data Analysis—A total of 39 online forums were deemed relevant to the topic of vitiligo; 9 of them met inclusion criteria (eTable 2). The messages within the forums were copied verbatim into a password-encrypted text document, and usernames in the threads were de-identified, ensuring user confidentiality.
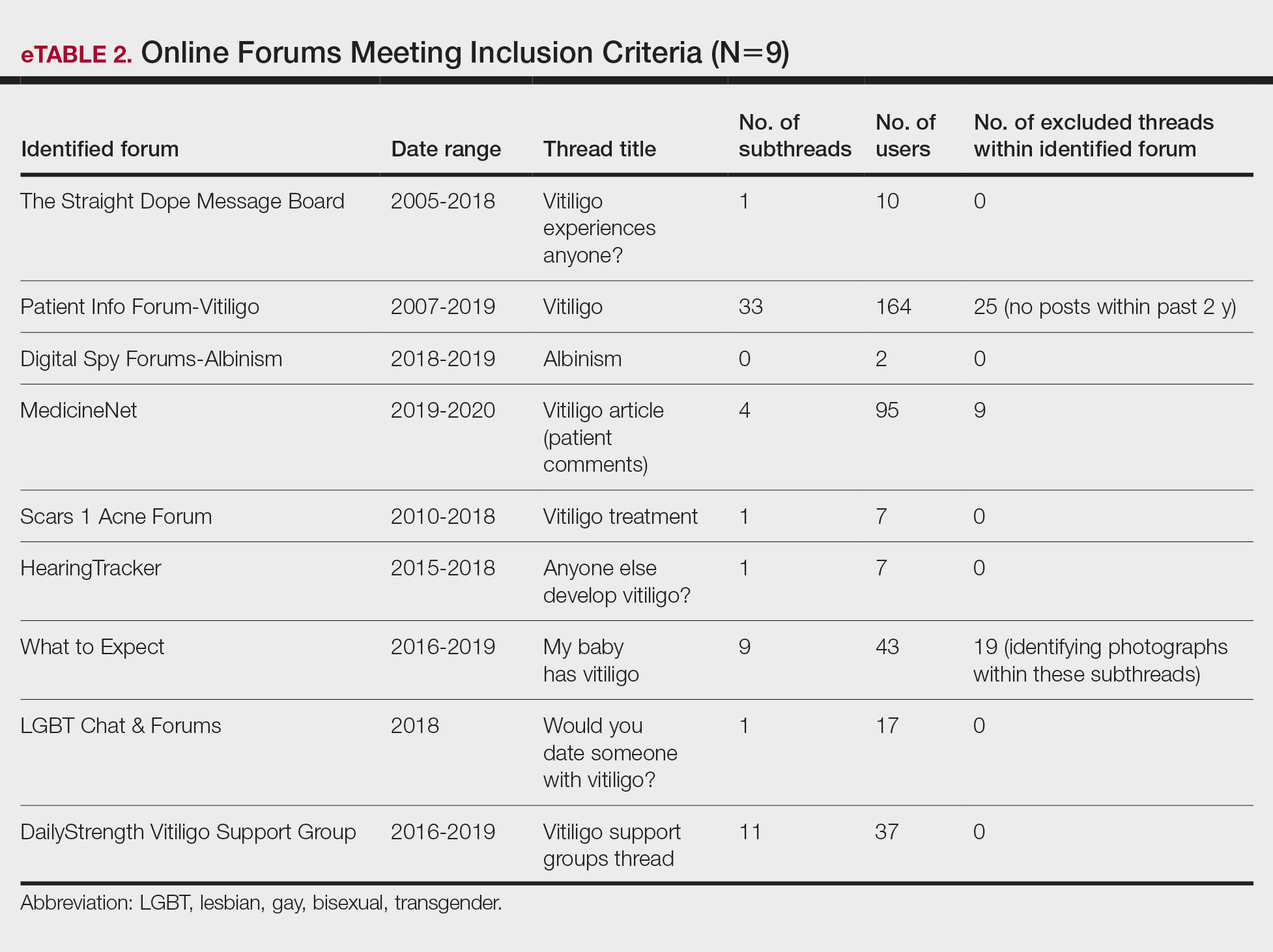
An inductive thematic analysis was utilized to explore the views and beliefs of online forum users discussing vitiligo. One author (M.B.G.) read the extracted message threads, developed an initial codebook, and established a finalized version with the agreement of another author (A.M.B.)(eTable 3). The forums were independently coded (M.B.G. and A.M.B.) in a line-by-line manner according to the codebook. Discrepancies were documented and resolved. Data saturation was adequately achieved, such that no new themes emerged during the iterative coding process. NVivo was used for qualitative analysis.

Results
Nine forums met inclusion criteria, comprising 105 pages of text. There were 61 total discussion threads, with 382 anonymous contributing users. Most users initiated a thread by posting either a question, an advice statement, or a request for help. The psychosocial impact of the disease permeated multiple domains,including personal relationships and daily life. Several threads discussed treatment, including effective camouflage and makeup, as well as peer validation of physician-prescribed treatments, along with threads dedicated to “cures” or homeopathy regimens. In several instances, commercial product endorsement, testimonials, and marketing links were reposted by the same user multiple times.
Inductive thematic analysis highlighted diverse themes and subthemes related to the beliefs and perspectives of users with vitiligo or with relatives or friends with vitiligo: psychosocial impact, disease management and camouflage/concealment, alternative medicine/homeopathy/cures, interactions with the public and health care providers, and skin tone and race. Quotes from individuals were included to demonstrate themes and subthemes.
Psychosocial Impact: QOL, Sources of Support, and Coping—There was a broad range of comments on how patients cope with and view their vitiligo. Some individuals felt vitiligo made them special, and others were at peace with and accepted their condition. In contrast, others reported the disease had devastated them and interfered with relationships. Individuals shared their stories of grief and hardships through childhood and adulthood and their concerns, especially on affected visible areas or the potential for disease progression. Users were vocal about how vitiligo affected their daily routines and lives, sharing how they felt uncomfortable outside the home, no longer engaged in swimming or exposing their legs, and preferred to stay inside instead. Some users adopted a “tough love” approach to coping, sharing how they have learned to either embrace their vitiligo or “live with it.” Some examples include:
“My best advice is go with the flow, vitiligo is not the worst thing that can happen.”
“I hate my life with vitiligo yet really I feel so selfish that there is much worse suffering in the world than a few white patches.”
Other advice was very practical:
“I hope it isn’t vanity that is tearing you apart because that is only skin deep. Make a fashion statement with hats.”
Some users acknowledged and adopted the mantra that vitiligo is not a somatic condition or “physical ailment,” while others emphasized its pervasive psychological burden:
“I still deal with this psychologically . . . You must keep a positive attitude and frame of mind . . . Vitiligo will not kill you, but you do need to stay strong and keep your head up emotionally.”
“I am just really thankful that I have a disease that will not kill me or that has [not] affected me physically at all. I consider myself lucky.”
Disease Management: Treatment, Vitiligo Course, Advice-Seeking, Camouflage—The range of information discussed for treatment was highly variable. There were many accounts in which users advised others to seek professional help, namely that of a dermatologist, for a formal assessment. Many expressed frustrations with treatments and their ineffectiveness, to which the majority of users said to consult with a professional and to remain patient and hopeful/optimistic:
“The best thing to do would be to take an appointment with a dermatologist and have the discoloration checked out. That’s the only way to know whether it is vitiligo or not.”
“My way of dealing with it is to gain control by camouflage.”
“The calming effect of being in control of my vitiligo, whether with concealers, self-tan or anything else, has stopped my feelings of despair.”
Beliefs on Alternative Medicine: Homeopathy and Alternative Regimens—Although some threads started with a post asking for the best treatments, others initiated a discussion by posting “best herbal treatments for cure” or “how to cure my vitiligo,” emphasizing the beliefs and wishes for a cure for vitiligo. Alternative therapies that users endorsed included apple cider vinegar, toothpaste, vitamins, and Ayurvedic treatment, among others. Dietary plans were popular, with users claiming success with dietary alterations in stopping and preventing lesion progression. For example, individuals felt that avoidance of sugar, meat, dairy, and citrus fruits or drinks and consumption of only filtered water were crucial to preventing further lesion spread and resulted in their “cure”:
“Don’t eat chocolate, wine (made of grapes), coffee, or tea if you don’t want to have vitiligo or let it get worse. Take Vitamin B, biotin, and nuts for Vitamin E.”
Other dangerous messages pitted treatments by health professionals against beliefs in homeopathy:
“I feel that vitiligo treatment is all in your diet and vitamins. All that medicine and UV lights is a no-no . . .w ith every medicine there is a side effect. The doctors could be healing your vitiligo and severely damaging you inside and out, and you won’t know until years later.”
There was a minor presence of users advising against homeopathy and the associated misinformation and inaccurate claims on curing vitiligo, though this group was small in comparison to the number of users posting outlandish claims on cure:
“There is no cure . . . It’s where your immune system attacks your skin cells causing loss of pigmentation. The skin that has lost the pigmentation can’t be reversed.”
Interactions With the Public and Health Care Providers—Those with vitiligo encounter unique situations in public and in their daily lives. Many of the accounts shared anecdotal stories on how patients have handled the stigma and discrimination faced:
“I have had to face discrimination at school, public places, college, functions, and every new person I have met has asked me this: ‘how did this happen?’”
Those with vitiligo even stated how they wished others would deal with their condition out in public, hoping that others would directly ask what the lesions were instead of the more hurtful staring. There were many stories in which users said others feel vitiligo was contagious or “dirty” and stressed that the condition is not infectious:
“I refer to myself as ‘camo-man’ and reassure people I come into contact with that it is not contagious.”
“Once I was eating at a restaurant . . . and a little girl said to her mom, ‘Look, Mom, that lady doesn’t wash her arms, look how dirty they are.’ That just broke my heart.”
Skin Tone and Implications—The belief that vitiligo lesions are less dramatic or less anxiety provoking for individuals with lighter skin was noted by users themselves and by health care providers in certain cases. Skin tone and its impact on QOL was confusing and contentious. Some users with fair skin stated their vitiligo was “less of an annoyance” or “less obvious” compared with individuals with darker complexions. Conversely, other accounts of self-reported White users vehemently stressed the anxieties felt by depigmented lesions, despite being “already white at baseline.”
“Was told by my dermatologist (upon diagnosis) that ‘You’re lucky you’re not African American—it shows up on them much worse. You’re so fair, it doesn’t really matter.’ ”
“You didn’t say what race you are. I could imagine it has a bigger impact if you are anything other than White.”
Comment
Patients Looking for Cures—The general attitude within the forums was uplifting and encouraging, with users detailing how they respond to others in public and sharing their personal perspectives. We found a mix of information regarding disease management and treatment of vitiligo. Overall, there was uncertainty about treatments, with individuals expressing concern that their treatments were ineffective or had failed or that better alternatives would be more suitable for their condition. We found many anecdotal endorsements of homeopathic remedies for vitiligo, with users boasting that their disease had not only been cured but had never returned. Some users completely denounced these statements, while other threads seemed to revolve completely around “cure” discussions with no dissenting voices. The number of discussions related to homeopathy was concerning. Furthermore, there often were no moderators within threads to remove cure-related content, whether commercially endorsed or anecdotal. It is plausible that supplements and vitamins recommended by some physicians may be incorrectly interpreted as a “cure” in online discussions. Our findings are consistent with prior reports that forums are a platform to express dissatisfaction with treatment and the need for additional treatment options.15,22
Concern Expressed by Health Care Providers—Prior qualitative research has described how patients with chronic dermatologic conditions believe that health care providers minimize patients’ psychological distress.27,28 We found several accounts in which an individual had explicitly stated their provider had “belittled” the extent and impact of vitiligo when comparing skin phototypes. This suggests either that physicians underestimate the impact of vitiligo on their patients or that physicians are not expressing enough empathic concern about the impact the condition has on those affected.
Cosmetic Aspects of Vitiligo—Few clinical trials have investigated QOL and cosmetic acceptability of treatments as outcome measures.29 We found several instances in which users with vitiligo had reported being dismissed as having a “cosmetic disease,” consistent with other work demonstrating the negative impact on such dismissals.22 Moreover, concealment and camouflage techniques frequently were discussed, demonstrating the relevance of cosmetic management as an important research topic.
Trustworthy Sources of Health Information—Patients still view physicians as trustworthy and a key source of health care information and advice.30-32 Patients with vitiligo who have been directed to reliable information sources often express gratitude22 and want health professionals to remain an important source in their health information-seeking.31 Given the range in information discussed online, it may be valuable to invite patients to share what information they have encountered online.
Our study highlights the conflicting health information and advice shared by users in online forums, complicating an already psychologically burdensome condition. Guiding patients to credible, moderated sites and resources that are accurate, understandable, and easy to access may help dispel the conflicting messages and stories discussed in the online community.
Study Strengths and Limitations—Limitations included reporting bias and reliance on self-reported information on the diagnosis and extent of individuals’ vitiligo. Excluding social media websites and platforms from the data collection is a limitation to comprehensively assessing the topic of internet users with vitiligo. Many social media platforms direct patients and their family members to support groups and therefore may have excluded these particular individuals. Social media platforms were excluded from our research owing to the prerequisite of creating user accounts or registering as an online member. Our inclusion criteria were specific to forums that did not require registering or creating an account and were therefore freely accessible to all internet viewers. There is an inherent lack of context present in online forums, preventing data collection on individuals’ demographics and socioeconomic backgrounds. However, anonymity may have allowed individuals to express their thoughts more freely.
An integrated approach, along with our sampling method of online forums not requiring registration, allows for greater transferability and understanding of the health needs of the general public with vitiligo.
Conclusion
Individuals with vitiligo continue to seek peer psychosocial support for the physical and emotional management of their disease. Counseling those with vitiligo about cosmetic concealment options, homeopathy, and treatment scams remains paramount. Directing patients to evidence-based resources, along with providing structured sources of support, may help to improve the psychosocial burden and QOL experienced by patients with vitiligo. Connecting patients with local and national support groups moderated by physicians, such as the Global Vitiligo Foundation (https://globalvitiligofoundation.org/), may provide benefit to patients with vitiligo.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883-885.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo—part 2—classification, histopathology and treatment. An Bras Dermatol. 2014;89:784-790.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383-392.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Grimes PE, Billips M. Childhood vitiligo: clinical spectrum and therapeutic approaches. In: Hann SK, Nordlund JJ, eds. Vitiligo: A Monograph on the Basic and Clinical Science. Blackwell Science; 2000.
- Sawant NS, Vanjari NA, Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019:6879412.
- Liu Y, Kornfield R, Shaw BR, et al. When support is needed: social support solicitation and provision in an online alcohol use disorder forum. Digit Health. 2017;3:2055207617704274.
- Health 2.0. The Economist. 2007;384:14.
- Fox S. Peer-to-peer health care. Pew Research Center. February 28, 2011. Accessed December 14, 2021. https://www.pewinternet.org/wp-content/uploads/sites/9/media/Files/Reports/2011/Pew_P2PHealthcare_2011.pdf
- Li N, Orrange S, Kravitz RL, et al. Reasons for and predictors of patients’ online health information seeking following a medical appointment. Fam Pract. 2014;31:550-556.
- Idriss SZ, Kvedar JC, Watson AJ. The role of online support communities: benefits of expanded social networks to patients with psoriasis. Arch Dermatol. 2009;145:46-51.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol 2017;176:1500-1507.
- Santer M, Chandler D, Lown M, et al. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol. 2017;177:751-757.
- Santer M, Burgess H, Yardley L, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62:e261-e267.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736-739.
- Guba EG, Lincoln YS. Competing paradigms in qualitative research. In: Denzin NK, Lincoln YS, eds. Handbook of Qualitative Research. Sage Publications, Inc; 1994:105-117.
- Lincoln YS. Emerging criteria for quality in qualitative and interpretive research. Qualitative Inquiry. 2016;1:275-289.
- O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol. 2017;176:1500-1507.
- Teasdale E, Muller I, Sani AA, et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open. 2018;8:e018652.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772.
- Hewson C, Buchanan T, Brown I, et al. Ethics Guidelines for Internet-mediated Research. The British Psychological Society; 2017.
- Coulson NS. Sharing, supporting and sobriety: a qualitative analysis of messages posted to alcohol-related online discussion forums in the United Kingdom. J Subst Use. 2014;19:176-180.
- Attard A, Coulson NS. A thematic analysis of patient communication in Parkinson’s disease online support group discussion forums. Comput Hum Behav. 2012;28:500-506.
- Nelson PA, Chew-Graham CA, Griffiths CE, et al. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol. 2013;168:354-361.
- Gore C, Johnson RJ, Caress AL, et al. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy. 2005;60:938-943.
- Eleftheriadou V, Thomas KS, Whitton ME, et al. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804-814.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
- Sillence E, Briggs P, Harris PR, et al. How do patients evaluate and make use of online health information? Soc Sci Med. 2007;64:1853-1862.
- Hay MC, Cadigan RJ, Khanna D, et al. Prepared patients: internet information seeking by new rheumatology patients. Arthritis Rheum. 2008;59:575-582.
Vitiligo is a chronic dermatologic condition that negatively affects quality of life (QOL), with substantial burden on the psychosocial well-being of patients.1 There is no cure, and current treatment modalities are aimed at controlling the chronic relapsing condition.1-3 Despite topical and cosmetic treatments for stabilization and repigmentation, vitiligo remains unpredictable.3
All genders, races, ethnicities, and socioeconomic classes are equally affected.4 The underlying etiology of vitiligo remains unknown to a great extent and is more poorly understood by the general public compared with other skin diseases (eg, acne).5 Patients with vitiligo experience social withdrawal, decreased sense of self-esteem, anxiety, depression, and suicidal ideation.5,6 Stigmatization has the greatest impact on QOL, with strong correlations between avoidance behaviors and lesion concealment.6-8 Although the condition is especially disfiguring for darker skin types, lighter skin types also are substantially affected, with similar overall self-reported stress.6,7
Individuals with chronic illnesses such as vitiligo turn to online communities for health information and social support, commiserating with others who have the same condition.9,10 Online forums are platforms for asynchronous peer-to-peer exchange of disease-related information for better management of long-term disease.11 Moreover, of all available internet resources, online forum posts are the most commonly accessed source of information (91%) for patients following visits with their doctors.12
Qualitative research involving chronic skin conditions and the information exchanged in online forums has been conducted for patients with acne, psoriasis, and atopic dermatitis, but not for patients with vitiligo.13-16 Although online questionnaires have been administered to patients with vitiligo, the content within online forums is not well characterized.2,17
The purpose of this qualitative study was to evaluate the online content exchanged by individuals with vitiligo to better understand the general attitudes and help-seeking behaviors in online forums.
Methods
Study Design—This qualitative study sought to investigate health beliefs and messages about vitiligo posted by users in US-based online discussion forums. An interpretive research paradigm was utilized so that all content collected in online forums were the views expressed by individuals.18-20 An integrated approach was used in the development of the coding manual, with pre-established major themes and subthemes as a guiding framework.16,21,22 We adhered to an inductive grounded method by means of de novo line-by-line coding, such that we had flexibility for new subthemes to emerge throughout the duration of the entire coding process.23
Individual posts and subsequent replies embedded within public online forums were used as the collected data source. Google was utilized as the primary search engine to identify forums pertaining to vitiligo, as 80% of US adults with chronic disease report that their inquiries for health information start with Google, Bing, or Yahoo.24 The institutional review board at the Wake Forest School of Medicine (Winston-Salem, North Carolina) granted approval of the study (IRB00063073). Online forums were considered “property” of the public domain and were accessible to all, eliminating the need for written informed consent.24-26
Search Criteria—We conducted our forum search in February 2020 with a systematic approach using predetermined phrases—online forum vitiligo support, vitiligo online message board, and vitiligo forums—which yielded more than 358,171 total results (eTable 1). Threads were identified in chronological order (from newest to oldest) based on how they appeared during each internet search, and all Google results for the respective search phrases were reviewed. Dates of selected threads ranged from 2005 to 2020. Only sites with US domains were included. Posts that either included views and understandings of vitiligo or belonged to a thread that contained a vitiligo discussion were deemed relevant for inclusion. Forums were excluded if registration or means of payment was required to view posts, if there were fewer than 2 user replies to a thread, if threads contained patient photographs, or if no posts had been made in the last 2 years (rendering the thread inactive). No social media platforms, such as Facebook, or formal online platforms, such as MyVitiligoTeam, were included in the search. A no-fee-for-access was chosen for this study, as the majority of those with a chronic condition who encounter a required paywall find the information elsewhere.25

Data Analysis—A total of 39 online forums were deemed relevant to the topic of vitiligo; 9 of them met inclusion criteria (eTable 2). The messages within the forums were copied verbatim into a password-encrypted text document, and usernames in the threads were de-identified, ensuring user confidentiality.

An inductive thematic analysis was utilized to explore the views and beliefs of online forum users discussing vitiligo. One author (M.B.G.) read the extracted message threads, developed an initial codebook, and established a finalized version with the agreement of another author (A.M.B.)(eTable 3). The forums were independently coded (M.B.G. and A.M.B.) in a line-by-line manner according to the codebook. Discrepancies were documented and resolved. Data saturation was adequately achieved, such that no new themes emerged during the iterative coding process. NVivo was used for qualitative analysis.

Results
Nine forums met inclusion criteria, comprising 105 pages of text. There were 61 total discussion threads, with 382 anonymous contributing users. Most users initiated a thread by posting either a question, an advice statement, or a request for help. The psychosocial impact of the disease permeated multiple domains,including personal relationships and daily life. Several threads discussed treatment, including effective camouflage and makeup, as well as peer validation of physician-prescribed treatments, along with threads dedicated to “cures” or homeopathy regimens. In several instances, commercial product endorsement, testimonials, and marketing links were reposted by the same user multiple times.
Inductive thematic analysis highlighted diverse themes and subthemes related to the beliefs and perspectives of users with vitiligo or with relatives or friends with vitiligo: psychosocial impact, disease management and camouflage/concealment, alternative medicine/homeopathy/cures, interactions with the public and health care providers, and skin tone and race. Quotes from individuals were included to demonstrate themes and subthemes.
Psychosocial Impact: QOL, Sources of Support, and Coping—There was a broad range of comments on how patients cope with and view their vitiligo. Some individuals felt vitiligo made them special, and others were at peace with and accepted their condition. In contrast, others reported the disease had devastated them and interfered with relationships. Individuals shared their stories of grief and hardships through childhood and adulthood and their concerns, especially on affected visible areas or the potential for disease progression. Users were vocal about how vitiligo affected their daily routines and lives, sharing how they felt uncomfortable outside the home, no longer engaged in swimming or exposing their legs, and preferred to stay inside instead. Some users adopted a “tough love” approach to coping, sharing how they have learned to either embrace their vitiligo or “live with it.” Some examples include:
“My best advice is go with the flow, vitiligo is not the worst thing that can happen.”
“I hate my life with vitiligo yet really I feel so selfish that there is much worse suffering in the world than a few white patches.”
Other advice was very practical:
“I hope it isn’t vanity that is tearing you apart because that is only skin deep. Make a fashion statement with hats.”
Some users acknowledged and adopted the mantra that vitiligo is not a somatic condition or “physical ailment,” while others emphasized its pervasive psychological burden:
“I still deal with this psychologically . . . You must keep a positive attitude and frame of mind . . . Vitiligo will not kill you, but you do need to stay strong and keep your head up emotionally.”
“I am just really thankful that I have a disease that will not kill me or that has [not] affected me physically at all. I consider myself lucky.”
Disease Management: Treatment, Vitiligo Course, Advice-Seeking, Camouflage—The range of information discussed for treatment was highly variable. There were many accounts in which users advised others to seek professional help, namely that of a dermatologist, for a formal assessment. Many expressed frustrations with treatments and their ineffectiveness, to which the majority of users said to consult with a professional and to remain patient and hopeful/optimistic:
“The best thing to do would be to take an appointment with a dermatologist and have the discoloration checked out. That’s the only way to know whether it is vitiligo or not.”
“My way of dealing with it is to gain control by camouflage.”
“The calming effect of being in control of my vitiligo, whether with concealers, self-tan or anything else, has stopped my feelings of despair.”
Beliefs on Alternative Medicine: Homeopathy and Alternative Regimens—Although some threads started with a post asking for the best treatments, others initiated a discussion by posting “best herbal treatments for cure” or “how to cure my vitiligo,” emphasizing the beliefs and wishes for a cure for vitiligo. Alternative therapies that users endorsed included apple cider vinegar, toothpaste, vitamins, and Ayurvedic treatment, among others. Dietary plans were popular, with users claiming success with dietary alterations in stopping and preventing lesion progression. For example, individuals felt that avoidance of sugar, meat, dairy, and citrus fruits or drinks and consumption of only filtered water were crucial to preventing further lesion spread and resulted in their “cure”:
“Don’t eat chocolate, wine (made of grapes), coffee, or tea if you don’t want to have vitiligo or let it get worse. Take Vitamin B, biotin, and nuts for Vitamin E.”
Other dangerous messages pitted treatments by health professionals against beliefs in homeopathy:
“I feel that vitiligo treatment is all in your diet and vitamins. All that medicine and UV lights is a no-no . . .w ith every medicine there is a side effect. The doctors could be healing your vitiligo and severely damaging you inside and out, and you won’t know until years later.”
There was a minor presence of users advising against homeopathy and the associated misinformation and inaccurate claims on curing vitiligo, though this group was small in comparison to the number of users posting outlandish claims on cure:
“There is no cure . . . It’s where your immune system attacks your skin cells causing loss of pigmentation. The skin that has lost the pigmentation can’t be reversed.”
Interactions With the Public and Health Care Providers—Those with vitiligo encounter unique situations in public and in their daily lives. Many of the accounts shared anecdotal stories on how patients have handled the stigma and discrimination faced:
“I have had to face discrimination at school, public places, college, functions, and every new person I have met has asked me this: ‘how did this happen?’”
Those with vitiligo even stated how they wished others would deal with their condition out in public, hoping that others would directly ask what the lesions were instead of the more hurtful staring. There were many stories in which users said others feel vitiligo was contagious or “dirty” and stressed that the condition is not infectious:
“I refer to myself as ‘camo-man’ and reassure people I come into contact with that it is not contagious.”
“Once I was eating at a restaurant . . . and a little girl said to her mom, ‘Look, Mom, that lady doesn’t wash her arms, look how dirty they are.’ That just broke my heart.”
Skin Tone and Implications—The belief that vitiligo lesions are less dramatic or less anxiety provoking for individuals with lighter skin was noted by users themselves and by health care providers in certain cases. Skin tone and its impact on QOL was confusing and contentious. Some users with fair skin stated their vitiligo was “less of an annoyance” or “less obvious” compared with individuals with darker complexions. Conversely, other accounts of self-reported White users vehemently stressed the anxieties felt by depigmented lesions, despite being “already white at baseline.”
“Was told by my dermatologist (upon diagnosis) that ‘You’re lucky you’re not African American—it shows up on them much worse. You’re so fair, it doesn’t really matter.’ ”
“You didn’t say what race you are. I could imagine it has a bigger impact if you are anything other than White.”
Comment
Patients Looking for Cures—The general attitude within the forums was uplifting and encouraging, with users detailing how they respond to others in public and sharing their personal perspectives. We found a mix of information regarding disease management and treatment of vitiligo. Overall, there was uncertainty about treatments, with individuals expressing concern that their treatments were ineffective or had failed or that better alternatives would be more suitable for their condition. We found many anecdotal endorsements of homeopathic remedies for vitiligo, with users boasting that their disease had not only been cured but had never returned. Some users completely denounced these statements, while other threads seemed to revolve completely around “cure” discussions with no dissenting voices. The number of discussions related to homeopathy was concerning. Furthermore, there often were no moderators within threads to remove cure-related content, whether commercially endorsed or anecdotal. It is plausible that supplements and vitamins recommended by some physicians may be incorrectly interpreted as a “cure” in online discussions. Our findings are consistent with prior reports that forums are a platform to express dissatisfaction with treatment and the need for additional treatment options.15,22
Concern Expressed by Health Care Providers—Prior qualitative research has described how patients with chronic dermatologic conditions believe that health care providers minimize patients’ psychological distress.27,28 We found several accounts in which an individual had explicitly stated their provider had “belittled” the extent and impact of vitiligo when comparing skin phototypes. This suggests either that physicians underestimate the impact of vitiligo on their patients or that physicians are not expressing enough empathic concern about the impact the condition has on those affected.
Cosmetic Aspects of Vitiligo—Few clinical trials have investigated QOL and cosmetic acceptability of treatments as outcome measures.29 We found several instances in which users with vitiligo had reported being dismissed as having a “cosmetic disease,” consistent with other work demonstrating the negative impact on such dismissals.22 Moreover, concealment and camouflage techniques frequently were discussed, demonstrating the relevance of cosmetic management as an important research topic.
Trustworthy Sources of Health Information—Patients still view physicians as trustworthy and a key source of health care information and advice.30-32 Patients with vitiligo who have been directed to reliable information sources often express gratitude22 and want health professionals to remain an important source in their health information-seeking.31 Given the range in information discussed online, it may be valuable to invite patients to share what information they have encountered online.
Our study highlights the conflicting health information and advice shared by users in online forums, complicating an already psychologically burdensome condition. Guiding patients to credible, moderated sites and resources that are accurate, understandable, and easy to access may help dispel the conflicting messages and stories discussed in the online community.
Study Strengths and Limitations—Limitations included reporting bias and reliance on self-reported information on the diagnosis and extent of individuals’ vitiligo. Excluding social media websites and platforms from the data collection is a limitation to comprehensively assessing the topic of internet users with vitiligo. Many social media platforms direct patients and their family members to support groups and therefore may have excluded these particular individuals. Social media platforms were excluded from our research owing to the prerequisite of creating user accounts or registering as an online member. Our inclusion criteria were specific to forums that did not require registering or creating an account and were therefore freely accessible to all internet viewers. There is an inherent lack of context present in online forums, preventing data collection on individuals’ demographics and socioeconomic backgrounds. However, anonymity may have allowed individuals to express their thoughts more freely.
An integrated approach, along with our sampling method of online forums not requiring registration, allows for greater transferability and understanding of the health needs of the general public with vitiligo.
Conclusion
Individuals with vitiligo continue to seek peer psychosocial support for the physical and emotional management of their disease. Counseling those with vitiligo about cosmetic concealment options, homeopathy, and treatment scams remains paramount. Directing patients to evidence-based resources, along with providing structured sources of support, may help to improve the psychosocial burden and QOL experienced by patients with vitiligo. Connecting patients with local and national support groups moderated by physicians, such as the Global Vitiligo Foundation (https://globalvitiligofoundation.org/), may provide benefit to patients with vitiligo.
Vitiligo is a chronic dermatologic condition that negatively affects quality of life (QOL), with substantial burden on the psychosocial well-being of patients.1 There is no cure, and current treatment modalities are aimed at controlling the chronic relapsing condition.1-3 Despite topical and cosmetic treatments for stabilization and repigmentation, vitiligo remains unpredictable.3
All genders, races, ethnicities, and socioeconomic classes are equally affected.4 The underlying etiology of vitiligo remains unknown to a great extent and is more poorly understood by the general public compared with other skin diseases (eg, acne).5 Patients with vitiligo experience social withdrawal, decreased sense of self-esteem, anxiety, depression, and suicidal ideation.5,6 Stigmatization has the greatest impact on QOL, with strong correlations between avoidance behaviors and lesion concealment.6-8 Although the condition is especially disfiguring for darker skin types, lighter skin types also are substantially affected, with similar overall self-reported stress.6,7
Individuals with chronic illnesses such as vitiligo turn to online communities for health information and social support, commiserating with others who have the same condition.9,10 Online forums are platforms for asynchronous peer-to-peer exchange of disease-related information for better management of long-term disease.11 Moreover, of all available internet resources, online forum posts are the most commonly accessed source of information (91%) for patients following visits with their doctors.12
Qualitative research involving chronic skin conditions and the information exchanged in online forums has been conducted for patients with acne, psoriasis, and atopic dermatitis, but not for patients with vitiligo.13-16 Although online questionnaires have been administered to patients with vitiligo, the content within online forums is not well characterized.2,17
The purpose of this qualitative study was to evaluate the online content exchanged by individuals with vitiligo to better understand the general attitudes and help-seeking behaviors in online forums.
Methods
Study Design—This qualitative study sought to investigate health beliefs and messages about vitiligo posted by users in US-based online discussion forums. An interpretive research paradigm was utilized so that all content collected in online forums were the views expressed by individuals.18-20 An integrated approach was used in the development of the coding manual, with pre-established major themes and subthemes as a guiding framework.16,21,22 We adhered to an inductive grounded method by means of de novo line-by-line coding, such that we had flexibility for new subthemes to emerge throughout the duration of the entire coding process.23
Individual posts and subsequent replies embedded within public online forums were used as the collected data source. Google was utilized as the primary search engine to identify forums pertaining to vitiligo, as 80% of US adults with chronic disease report that their inquiries for health information start with Google, Bing, or Yahoo.24 The institutional review board at the Wake Forest School of Medicine (Winston-Salem, North Carolina) granted approval of the study (IRB00063073). Online forums were considered “property” of the public domain and were accessible to all, eliminating the need for written informed consent.24-26
Search Criteria—We conducted our forum search in February 2020 with a systematic approach using predetermined phrases—online forum vitiligo support, vitiligo online message board, and vitiligo forums—which yielded more than 358,171 total results (eTable 1). Threads were identified in chronological order (from newest to oldest) based on how they appeared during each internet search, and all Google results for the respective search phrases were reviewed. Dates of selected threads ranged from 2005 to 2020. Only sites with US domains were included. Posts that either included views and understandings of vitiligo or belonged to a thread that contained a vitiligo discussion were deemed relevant for inclusion. Forums were excluded if registration or means of payment was required to view posts, if there were fewer than 2 user replies to a thread, if threads contained patient photographs, or if no posts had been made in the last 2 years (rendering the thread inactive). No social media platforms, such as Facebook, or formal online platforms, such as MyVitiligoTeam, were included in the search. A no-fee-for-access was chosen for this study, as the majority of those with a chronic condition who encounter a required paywall find the information elsewhere.25

Data Analysis—A total of 39 online forums were deemed relevant to the topic of vitiligo; 9 of them met inclusion criteria (eTable 2). The messages within the forums were copied verbatim into a password-encrypted text document, and usernames in the threads were de-identified, ensuring user confidentiality.

An inductive thematic analysis was utilized to explore the views and beliefs of online forum users discussing vitiligo. One author (M.B.G.) read the extracted message threads, developed an initial codebook, and established a finalized version with the agreement of another author (A.M.B.)(eTable 3). The forums were independently coded (M.B.G. and A.M.B.) in a line-by-line manner according to the codebook. Discrepancies were documented and resolved. Data saturation was adequately achieved, such that no new themes emerged during the iterative coding process. NVivo was used for qualitative analysis.

Results
Nine forums met inclusion criteria, comprising 105 pages of text. There were 61 total discussion threads, with 382 anonymous contributing users. Most users initiated a thread by posting either a question, an advice statement, or a request for help. The psychosocial impact of the disease permeated multiple domains,including personal relationships and daily life. Several threads discussed treatment, including effective camouflage and makeup, as well as peer validation of physician-prescribed treatments, along with threads dedicated to “cures” or homeopathy regimens. In several instances, commercial product endorsement, testimonials, and marketing links were reposted by the same user multiple times.
Inductive thematic analysis highlighted diverse themes and subthemes related to the beliefs and perspectives of users with vitiligo or with relatives or friends with vitiligo: psychosocial impact, disease management and camouflage/concealment, alternative medicine/homeopathy/cures, interactions with the public and health care providers, and skin tone and race. Quotes from individuals were included to demonstrate themes and subthemes.
Psychosocial Impact: QOL, Sources of Support, and Coping—There was a broad range of comments on how patients cope with and view their vitiligo. Some individuals felt vitiligo made them special, and others were at peace with and accepted their condition. In contrast, others reported the disease had devastated them and interfered with relationships. Individuals shared their stories of grief and hardships through childhood and adulthood and their concerns, especially on affected visible areas or the potential for disease progression. Users were vocal about how vitiligo affected their daily routines and lives, sharing how they felt uncomfortable outside the home, no longer engaged in swimming or exposing their legs, and preferred to stay inside instead. Some users adopted a “tough love” approach to coping, sharing how they have learned to either embrace their vitiligo or “live with it.” Some examples include:
“My best advice is go with the flow, vitiligo is not the worst thing that can happen.”
“I hate my life with vitiligo yet really I feel so selfish that there is much worse suffering in the world than a few white patches.”
Other advice was very practical:
“I hope it isn’t vanity that is tearing you apart because that is only skin deep. Make a fashion statement with hats.”
Some users acknowledged and adopted the mantra that vitiligo is not a somatic condition or “physical ailment,” while others emphasized its pervasive psychological burden:
“I still deal with this psychologically . . . You must keep a positive attitude and frame of mind . . . Vitiligo will not kill you, but you do need to stay strong and keep your head up emotionally.”
“I am just really thankful that I have a disease that will not kill me or that has [not] affected me physically at all. I consider myself lucky.”
Disease Management: Treatment, Vitiligo Course, Advice-Seeking, Camouflage—The range of information discussed for treatment was highly variable. There were many accounts in which users advised others to seek professional help, namely that of a dermatologist, for a formal assessment. Many expressed frustrations with treatments and their ineffectiveness, to which the majority of users said to consult with a professional and to remain patient and hopeful/optimistic:
“The best thing to do would be to take an appointment with a dermatologist and have the discoloration checked out. That’s the only way to know whether it is vitiligo or not.”
“My way of dealing with it is to gain control by camouflage.”
“The calming effect of being in control of my vitiligo, whether with concealers, self-tan or anything else, has stopped my feelings of despair.”
Beliefs on Alternative Medicine: Homeopathy and Alternative Regimens—Although some threads started with a post asking for the best treatments, others initiated a discussion by posting “best herbal treatments for cure” or “how to cure my vitiligo,” emphasizing the beliefs and wishes for a cure for vitiligo. Alternative therapies that users endorsed included apple cider vinegar, toothpaste, vitamins, and Ayurvedic treatment, among others. Dietary plans were popular, with users claiming success with dietary alterations in stopping and preventing lesion progression. For example, individuals felt that avoidance of sugar, meat, dairy, and citrus fruits or drinks and consumption of only filtered water were crucial to preventing further lesion spread and resulted in their “cure”:
“Don’t eat chocolate, wine (made of grapes), coffee, or tea if you don’t want to have vitiligo or let it get worse. Take Vitamin B, biotin, and nuts for Vitamin E.”
Other dangerous messages pitted treatments by health professionals against beliefs in homeopathy:
“I feel that vitiligo treatment is all in your diet and vitamins. All that medicine and UV lights is a no-no . . .w ith every medicine there is a side effect. The doctors could be healing your vitiligo and severely damaging you inside and out, and you won’t know until years later.”
There was a minor presence of users advising against homeopathy and the associated misinformation and inaccurate claims on curing vitiligo, though this group was small in comparison to the number of users posting outlandish claims on cure:
“There is no cure . . . It’s where your immune system attacks your skin cells causing loss of pigmentation. The skin that has lost the pigmentation can’t be reversed.”
Interactions With the Public and Health Care Providers—Those with vitiligo encounter unique situations in public and in their daily lives. Many of the accounts shared anecdotal stories on how patients have handled the stigma and discrimination faced:
“I have had to face discrimination at school, public places, college, functions, and every new person I have met has asked me this: ‘how did this happen?’”
Those with vitiligo even stated how they wished others would deal with their condition out in public, hoping that others would directly ask what the lesions were instead of the more hurtful staring. There were many stories in which users said others feel vitiligo was contagious or “dirty” and stressed that the condition is not infectious:
“I refer to myself as ‘camo-man’ and reassure people I come into contact with that it is not contagious.”
“Once I was eating at a restaurant . . . and a little girl said to her mom, ‘Look, Mom, that lady doesn’t wash her arms, look how dirty they are.’ That just broke my heart.”
Skin Tone and Implications—The belief that vitiligo lesions are less dramatic or less anxiety provoking for individuals with lighter skin was noted by users themselves and by health care providers in certain cases. Skin tone and its impact on QOL was confusing and contentious. Some users with fair skin stated their vitiligo was “less of an annoyance” or “less obvious” compared with individuals with darker complexions. Conversely, other accounts of self-reported White users vehemently stressed the anxieties felt by depigmented lesions, despite being “already white at baseline.”
“Was told by my dermatologist (upon diagnosis) that ‘You’re lucky you’re not African American—it shows up on them much worse. You’re so fair, it doesn’t really matter.’ ”
“You didn’t say what race you are. I could imagine it has a bigger impact if you are anything other than White.”
Comment
Patients Looking for Cures—The general attitude within the forums was uplifting and encouraging, with users detailing how they respond to others in public and sharing their personal perspectives. We found a mix of information regarding disease management and treatment of vitiligo. Overall, there was uncertainty about treatments, with individuals expressing concern that their treatments were ineffective or had failed or that better alternatives would be more suitable for their condition. We found many anecdotal endorsements of homeopathic remedies for vitiligo, with users boasting that their disease had not only been cured but had never returned. Some users completely denounced these statements, while other threads seemed to revolve completely around “cure” discussions with no dissenting voices. The number of discussions related to homeopathy was concerning. Furthermore, there often were no moderators within threads to remove cure-related content, whether commercially endorsed or anecdotal. It is plausible that supplements and vitamins recommended by some physicians may be incorrectly interpreted as a “cure” in online discussions. Our findings are consistent with prior reports that forums are a platform to express dissatisfaction with treatment and the need for additional treatment options.15,22
Concern Expressed by Health Care Providers—Prior qualitative research has described how patients with chronic dermatologic conditions believe that health care providers minimize patients’ psychological distress.27,28 We found several accounts in which an individual had explicitly stated their provider had “belittled” the extent and impact of vitiligo when comparing skin phototypes. This suggests either that physicians underestimate the impact of vitiligo on their patients or that physicians are not expressing enough empathic concern about the impact the condition has on those affected.
Cosmetic Aspects of Vitiligo—Few clinical trials have investigated QOL and cosmetic acceptability of treatments as outcome measures.29 We found several instances in which users with vitiligo had reported being dismissed as having a “cosmetic disease,” consistent with other work demonstrating the negative impact on such dismissals.22 Moreover, concealment and camouflage techniques frequently were discussed, demonstrating the relevance of cosmetic management as an important research topic.
Trustworthy Sources of Health Information—Patients still view physicians as trustworthy and a key source of health care information and advice.30-32 Patients with vitiligo who have been directed to reliable information sources often express gratitude22 and want health professionals to remain an important source in their health information-seeking.31 Given the range in information discussed online, it may be valuable to invite patients to share what information they have encountered online.
Our study highlights the conflicting health information and advice shared by users in online forums, complicating an already psychologically burdensome condition. Guiding patients to credible, moderated sites and resources that are accurate, understandable, and easy to access may help dispel the conflicting messages and stories discussed in the online community.
Study Strengths and Limitations—Limitations included reporting bias and reliance on self-reported information on the diagnosis and extent of individuals’ vitiligo. Excluding social media websites and platforms from the data collection is a limitation to comprehensively assessing the topic of internet users with vitiligo. Many social media platforms direct patients and their family members to support groups and therefore may have excluded these particular individuals. Social media platforms were excluded from our research owing to the prerequisite of creating user accounts or registering as an online member. Our inclusion criteria were specific to forums that did not require registering or creating an account and were therefore freely accessible to all internet viewers. There is an inherent lack of context present in online forums, preventing data collection on individuals’ demographics and socioeconomic backgrounds. However, anonymity may have allowed individuals to express their thoughts more freely.
An integrated approach, along with our sampling method of online forums not requiring registration, allows for greater transferability and understanding of the health needs of the general public with vitiligo.
Conclusion
Individuals with vitiligo continue to seek peer psychosocial support for the physical and emotional management of their disease. Counseling those with vitiligo about cosmetic concealment options, homeopathy, and treatment scams remains paramount. Directing patients to evidence-based resources, along with providing structured sources of support, may help to improve the psychosocial burden and QOL experienced by patients with vitiligo. Connecting patients with local and national support groups moderated by physicians, such as the Global Vitiligo Foundation (https://globalvitiligofoundation.org/), may provide benefit to patients with vitiligo.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883-885.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo—part 2—classification, histopathology and treatment. An Bras Dermatol. 2014;89:784-790.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383-392.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Grimes PE, Billips M. Childhood vitiligo: clinical spectrum and therapeutic approaches. In: Hann SK, Nordlund JJ, eds. Vitiligo: A Monograph on the Basic and Clinical Science. Blackwell Science; 2000.
- Sawant NS, Vanjari NA, Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019:6879412.
- Liu Y, Kornfield R, Shaw BR, et al. When support is needed: social support solicitation and provision in an online alcohol use disorder forum. Digit Health. 2017;3:2055207617704274.
- Health 2.0. The Economist. 2007;384:14.
- Fox S. Peer-to-peer health care. Pew Research Center. February 28, 2011. Accessed December 14, 2021. https://www.pewinternet.org/wp-content/uploads/sites/9/media/Files/Reports/2011/Pew_P2PHealthcare_2011.pdf
- Li N, Orrange S, Kravitz RL, et al. Reasons for and predictors of patients’ online health information seeking following a medical appointment. Fam Pract. 2014;31:550-556.
- Idriss SZ, Kvedar JC, Watson AJ. The role of online support communities: benefits of expanded social networks to patients with psoriasis. Arch Dermatol. 2009;145:46-51.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol 2017;176:1500-1507.
- Santer M, Chandler D, Lown M, et al. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol. 2017;177:751-757.
- Santer M, Burgess H, Yardley L, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62:e261-e267.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736-739.
- Guba EG, Lincoln YS. Competing paradigms in qualitative research. In: Denzin NK, Lincoln YS, eds. Handbook of Qualitative Research. Sage Publications, Inc; 1994:105-117.
- Lincoln YS. Emerging criteria for quality in qualitative and interpretive research. Qualitative Inquiry. 2016;1:275-289.
- O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol. 2017;176:1500-1507.
- Teasdale E, Muller I, Sani AA, et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open. 2018;8:e018652.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772.
- Hewson C, Buchanan T, Brown I, et al. Ethics Guidelines for Internet-mediated Research. The British Psychological Society; 2017.
- Coulson NS. Sharing, supporting and sobriety: a qualitative analysis of messages posted to alcohol-related online discussion forums in the United Kingdom. J Subst Use. 2014;19:176-180.
- Attard A, Coulson NS. A thematic analysis of patient communication in Parkinson’s disease online support group discussion forums. Comput Hum Behav. 2012;28:500-506.
- Nelson PA, Chew-Graham CA, Griffiths CE, et al. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol. 2013;168:354-361.
- Gore C, Johnson RJ, Caress AL, et al. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy. 2005;60:938-943.
- Eleftheriadou V, Thomas KS, Whitton ME, et al. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804-814.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
- Sillence E, Briggs P, Harris PR, et al. How do patients evaluate and make use of online health information? Soc Sci Med. 2007;64:1853-1862.
- Hay MC, Cadigan RJ, Khanna D, et al. Prepared patients: internet information seeking by new rheumatology patients. Arthritis Rheum. 2008;59:575-582.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883-885.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo—part 2—classification, histopathology and treatment. An Bras Dermatol. 2014;89:784-790.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383-392.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Grimes PE, Billips M. Childhood vitiligo: clinical spectrum and therapeutic approaches. In: Hann SK, Nordlund JJ, eds. Vitiligo: A Monograph on the Basic and Clinical Science. Blackwell Science; 2000.
- Sawant NS, Vanjari NA, Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019:6879412.
- Liu Y, Kornfield R, Shaw BR, et al. When support is needed: social support solicitation and provision in an online alcohol use disorder forum. Digit Health. 2017;3:2055207617704274.
- Health 2.0. The Economist. 2007;384:14.
- Fox S. Peer-to-peer health care. Pew Research Center. February 28, 2011. Accessed December 14, 2021. https://www.pewinternet.org/wp-content/uploads/sites/9/media/Files/Reports/2011/Pew_P2PHealthcare_2011.pdf
- Li N, Orrange S, Kravitz RL, et al. Reasons for and predictors of patients’ online health information seeking following a medical appointment. Fam Pract. 2014;31:550-556.
- Idriss SZ, Kvedar JC, Watson AJ. The role of online support communities: benefits of expanded social networks to patients with psoriasis. Arch Dermatol. 2009;145:46-51.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol 2017;176:1500-1507.
- Santer M, Chandler D, Lown M, et al. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol. 2017;177:751-757.
- Santer M, Burgess H, Yardley L, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62:e261-e267.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736-739.
- Guba EG, Lincoln YS. Competing paradigms in qualitative research. In: Denzin NK, Lincoln YS, eds. Handbook of Qualitative Research. Sage Publications, Inc; 1994:105-117.
- Lincoln YS. Emerging criteria for quality in qualitative and interpretive research. Qualitative Inquiry. 2016;1:275-289.
- O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol. 2017;176:1500-1507.
- Teasdale E, Muller I, Sani AA, et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open. 2018;8:e018652.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772.
- Hewson C, Buchanan T, Brown I, et al. Ethics Guidelines for Internet-mediated Research. The British Psychological Society; 2017.
- Coulson NS. Sharing, supporting and sobriety: a qualitative analysis of messages posted to alcohol-related online discussion forums in the United Kingdom. J Subst Use. 2014;19:176-180.
- Attard A, Coulson NS. A thematic analysis of patient communication in Parkinson’s disease online support group discussion forums. Comput Hum Behav. 2012;28:500-506.
- Nelson PA, Chew-Graham CA, Griffiths CE, et al. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol. 2013;168:354-361.
- Gore C, Johnson RJ, Caress AL, et al. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy. 2005;60:938-943.
- Eleftheriadou V, Thomas KS, Whitton ME, et al. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804-814.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
- Sillence E, Briggs P, Harris PR, et al. How do patients evaluate and make use of online health information? Soc Sci Med. 2007;64:1853-1862.
- Hay MC, Cadigan RJ, Khanna D, et al. Prepared patients: internet information seeking by new rheumatology patients. Arthritis Rheum. 2008;59:575-582.
Practice Points
- Online forums provide invaluable insight on vitiligo disease management, psychosocial impact, and burden on quality of life. Patient care can be improved by inquiring where patients seek information and whether online forums are utilized.
- Commonly discussed topics in online forums were cosmetic concealment of vitiligo lesions and homeopathy or “cure” discussions. Health care providers can engage in honest conversations about evidence-based medical treatments for vitiligo. The interest in cosmetic management highlights a relevant research area in this field.
- Health care providers can better serve patients with vitiligo by providing online resources that are reputable and can help guide patients to credible internet sources such as the Global Vitiligo Foundation.
Microaggressions in Medicine
As manifestations of overt racism and macroaggressions have gained increased visibility, there is a need for discussion of another expression of racism: microaggressions. Although racism classically is viewed as blatant structural, attitudinal, and behavioral prejudice, experts pose that the face of racism has evolved into a more covert insidious form. This form of racism was originally coined racial microaggressions by psychiatrist Chester M. Pierce, MD, 50 years ago.1,2 Since that time, microaggressions have further expanded to describe “brief and commonplace daily verbal, behavioral, and environmental indignities, whether intentional or unintentional, that communicate hostile, derogatory, or negative racial, gender, sexual-orientation, and religious slights and insults to the target person or group.” 3 This article aims to define and depict examples of microaggressions in medicine, discuss the resulting harmful effects, and offer strategies to minimize and counter these negative ramifications.
What are microaggressions?
Microaggressions are behaviors that stem from implicit bias and occur at an interpersonal level. Implicit bias refers to unconscious stereotypes, assumptions, and beliefs held about an individual’s identity. One of the earliest microaggressions—invisibility—was characterized by Ralph Ellison in his novel Invisible Man. Ellison states, “I am invisible, understand, simply because people refuse to see me . . . When they approach me they see only my surroundings, themselves, or figments of their imagination—indeed, everything and anything except me.”4 This concept of invisibility is a primary microaggression faced by people of color.
In medicine, microaggressions and implicit bias may be encountered throughout medical training and clinical practice in interactions with colleagues, superiors, patients, and patients’ families.5,6 Examples of microaggressions in medicine include demeaning comments, nonverbal disrespect, generalizations of social identity, assumption of nonphysician status, role- or credential-questioning behavior, explicit epithets, rejection of care, questioning or inquiries of ethnic/racial origin, and sexual harassment.7
An example of microaggressions in medicine was fully displayed when physician Tamika Cross described her experience of being turned away from helping an unresponsive passenger during a flight emergency.
[T]he flight attendant yells “call overhead for a physician on board.” I raised my hand to grab her attention. She said to me “oh no sweetie put [your] hand down, we are looking for actual physicians or nurses or some type of medical personnel, we don’t have time to talk to you” . . . Another “seasoned” white male approaches the row and says he is a physician as well. She says to me “thanks for your help but he can help us, and he has his credentials.”8
What are the effects of microaggressions?
Although microaggressions may be unconscious and unintentional by the offender, the negative ramifications are notable. Recent studies report that women and underrepresented minority (URM) medical students, residents, and physicians experience microaggressions and implicit bias at a higher prevalence and frequency compared with their male and non-URM counterparts.7,9 Repetitive microaggressions are harmful to the health and safety of women and URM medical students, residents, physicians, other providers, and patients. The Table provides example scenarios of microaggressions in medicine categorized according to Berk.10
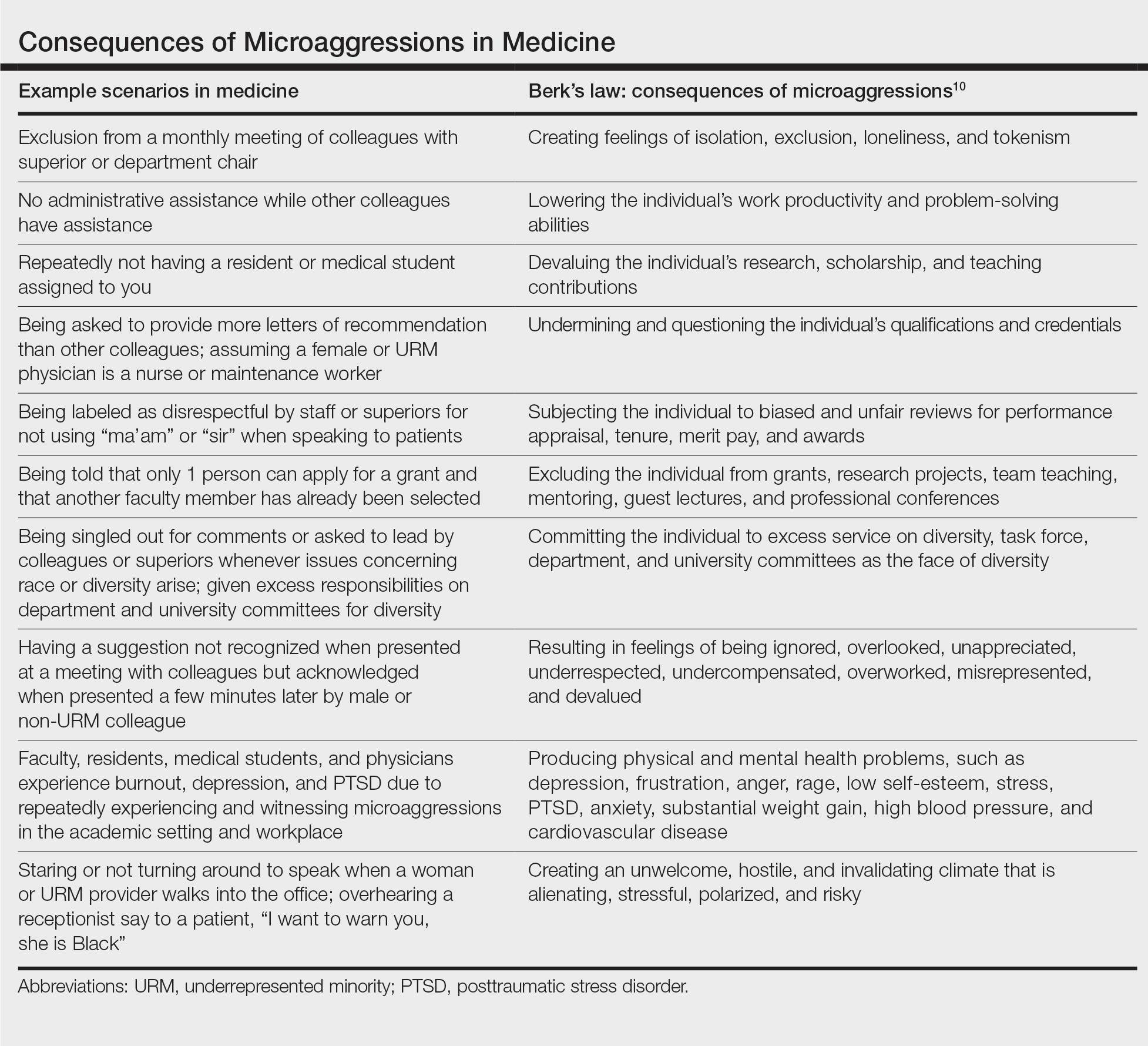
Microaggressions negatively impact physical, mental, and emotional well-being. Current data support that medical students and residents who experience microaggressions are more likely to report associated symptoms of burnout, depression, and suicidal thoughts.11,12 Subjection to persistent bias can lead to minority status stress and racial battle fatigue, creating feelings of invisibility, isolation, exclusion, and loneliness for those impacted.13,14
In the book Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine, Damon Tweedy, MD, reflects on race in medicine. Tweedy notes his experience as a medical student when a professor mistakenly assumed he was a maintenance worker in the classroom. Tweedy describes how he internalized the exchange and, despite his success throughout the course of his medical training, combatted feelings of anxiety, self-doubt, and implied inferiority.15
Although microaggressions are harmful to one’s health, they also undermine the learning and teaching experience for students, residents, and faculty, and they detract from the larger goal of providing care for patients.11 Frequent devaluing and questioning of an individual’s contributions, qualifications, and credentials based on identity can lower productivity and problem-solving abilities. These behaviors cultivate an unwelcome and hostile work/learning environment that is stressful and polarizing for the recipient.
Despite the heavy burden of microaggressions, most students, residents, and faculty physicians do not report incidents to their institutions and feel that training, resources, and policies to respond to bias adequately are lacking.7 As a result of implicit bias and microaggressions, women and URM medical students and providers are unable to focus solely on the practice of medicine. They are tasked with the additional burden of shouldering the emotional and cognitive complexities that microaggressions produce.16
What are strategies to reduce microaggressions in medicine?
To minimize the harmful effects of microaggressions, intervention strategies must be implemented that reduce the likelihood of the occurrence of microaggressions and challenge the stereotypes that undergird implicit bias. These strategies include cultivating allies, followed by demanding structural accountability. Allies are members of the majority group who collectively collaborate with members of the nonmajority group to effect change through the promotion of diversity, equity, and inclusion efforts.17 Cultivating allies involves building a network of collaboration among these groups and emphasizes education. Education is critical for allies to address microaggressions at the interpersonal level. This process of education involves personal reflection and self-awareness in exploring one’s biases, fears, and assumptions. Integral to this step is broadening one’s acceptance of different cultures, racial/ethnic groups, and identities. There must be a willingness to engage in difficult or uncomfortable conversations and a readiness to actively listen to concerns rather than perpetuating further harm through avoidance and dismissive or defensive behavior.18
Demanding structural accountability facilitates deconstruction of bias and microaggression at the larger systemic level. This strategy involves implicit bias and antiracism training, development of retention plans, and identification of mentors for women and URM providers and students. Implicit bias and microaggression training and policies should be incorporated into medical education and resident curriculums. Similarly, educational resources and training must be made available to practicing physicians, faculty, and other providers through their institutions and places of employment. Equipping students and providers with the tools needed when microaggressions are witnessed or experienced demonstrates systemic-level accountability and communicates the importance of the issue. Furthermore, the development of retention plans and identification of mentors provide a support system and foster a culture of inclusion where recipients of microaggressions feel protected and valued. Increased feelings of inclusivity and belonging help bridge the gap created through microaggressions and implicit bias.
Final Thoughts
Despite an often covert nature, the detrimental effects of microaggressions are tangible and far reaching. As providers, we must strive to understand all categories of racism and expose the many ways prejudice manifests within medical training and clinical practice. It is our obligation to undertake the challenge of “making the ‘invisible’ visible” as we confront microaggressions and implicit bias to promote a safer and more inclusive medical community and workforce.19
- Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154:868-872. doi:10.1001/jamasurg.2019.1648
- Williams MT. Microaggressions: clarification, evidence, and impact. Perspect Psychol Sci. 2020;15:3-26. doi:10.1177/1745691619827499
- Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Wiley; 2010.
- Ellison R. Invisible Man. Random House; 1952.
- Molina MF, Landry AI, Chary AN, et al. Addressing the elephant in the room: microaggressions in medicine. Ann Emerg Med. 2020;76:387-391. doi:10.1016/j.annemergmed.2020.04.009
- Overland MK, Zumsteg JM, Lindo EG, et al. Microaggressions in clinical training and practice. PM R. 2019;11:1004-1012. doi:10.1002/pmrj.12229
- de Bourmont SS, Burra A, Nouri SS, et al. Resident physician experiences with and responses to biased patients. JAMA Netw Open. 2020;3:e2021769. doi:10.1001/jamanetworkopen.2020.21769
- TK Cross Facebook page. October 9, 2016. Accessed April 19, 2021. https://www.facebook.com/tamika.cross.52/posts/658443077654049
- Periyakoil VS, Chaudron L, Hill EV, et al. Common types of gender-based microaggressions in medicine. Acad Med. 2020;95:450-457. doi:10.1097/ACM.0000000000003057
- Berk RA. Microaggressions trilogy: part 1. why do microaggressions matter? J Fac Dev. 2017;31:63-73.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2020:S0027-9684(20)30428-4. doi:10.1016/j.jnma.2020.11.009
- Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381:1741-1752. doi:10.1056/NEJMsa1903759
- Acholonu RG, Oyeku SO. Addressing microaggressions in the health care workforce-a path toward achieving equity and inclusion. JAMA Netw Open. 2020;3:E2021770. doi:10.1001/jamanetworkopen.2020.21770
- O’Keefe VM, Wingate LR, Cole AB, et al. Seemingly harmless racial communications are not so harmless: racial microaggressions lead to suicidal ideation by way of depression symptoms. Suicide Life Threat Behav. 2015;45:567-576. doi:10.1111/sltb.12150
- Tweedy D. Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine. Picador; 2016.
- Osseo-Asare A, Balasuriya L, Huot SJ, et al. Minority resident physicians’ views on the role of race/ethnicity in their training experiences in the workplace. JAMA Netw Open. 2018;1:E182723. doi: 10.1001/jamanetworkopen.2018.2723
- Melaku TM, Beeman A, Smith DG, et al. Be a better ally. Harvard Business Review. Published November-December 2020. Accessed April 23, 2021. https://hbr.org/2020/11/be-a-better-ally
- Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62:271-286. doi:10.1037/0003-066X.62.4.271
- Sue DW. Whiteness and ethnocentric monoculturalism: making the “invisible” visible. Am Psychol. 2004;59:761-769. doi:10.1037/0003-066X.59.8.761
As manifestations of overt racism and macroaggressions have gained increased visibility, there is a need for discussion of another expression of racism: microaggressions. Although racism classically is viewed as blatant structural, attitudinal, and behavioral prejudice, experts pose that the face of racism has evolved into a more covert insidious form. This form of racism was originally coined racial microaggressions by psychiatrist Chester M. Pierce, MD, 50 years ago.1,2 Since that time, microaggressions have further expanded to describe “brief and commonplace daily verbal, behavioral, and environmental indignities, whether intentional or unintentional, that communicate hostile, derogatory, or negative racial, gender, sexual-orientation, and religious slights and insults to the target person or group.” 3 This article aims to define and depict examples of microaggressions in medicine, discuss the resulting harmful effects, and offer strategies to minimize and counter these negative ramifications.
What are microaggressions?
Microaggressions are behaviors that stem from implicit bias and occur at an interpersonal level. Implicit bias refers to unconscious stereotypes, assumptions, and beliefs held about an individual’s identity. One of the earliest microaggressions—invisibility—was characterized by Ralph Ellison in his novel Invisible Man. Ellison states, “I am invisible, understand, simply because people refuse to see me . . . When they approach me they see only my surroundings, themselves, or figments of their imagination—indeed, everything and anything except me.”4 This concept of invisibility is a primary microaggression faced by people of color.
In medicine, microaggressions and implicit bias may be encountered throughout medical training and clinical practice in interactions with colleagues, superiors, patients, and patients’ families.5,6 Examples of microaggressions in medicine include demeaning comments, nonverbal disrespect, generalizations of social identity, assumption of nonphysician status, role- or credential-questioning behavior, explicit epithets, rejection of care, questioning or inquiries of ethnic/racial origin, and sexual harassment.7
An example of microaggressions in medicine was fully displayed when physician Tamika Cross described her experience of being turned away from helping an unresponsive passenger during a flight emergency.
[T]he flight attendant yells “call overhead for a physician on board.” I raised my hand to grab her attention. She said to me “oh no sweetie put [your] hand down, we are looking for actual physicians or nurses or some type of medical personnel, we don’t have time to talk to you” . . . Another “seasoned” white male approaches the row and says he is a physician as well. She says to me “thanks for your help but he can help us, and he has his credentials.”8
What are the effects of microaggressions?
Although microaggressions may be unconscious and unintentional by the offender, the negative ramifications are notable. Recent studies report that women and underrepresented minority (URM) medical students, residents, and physicians experience microaggressions and implicit bias at a higher prevalence and frequency compared with their male and non-URM counterparts.7,9 Repetitive microaggressions are harmful to the health and safety of women and URM medical students, residents, physicians, other providers, and patients. The Table provides example scenarios of microaggressions in medicine categorized according to Berk.10

Microaggressions negatively impact physical, mental, and emotional well-being. Current data support that medical students and residents who experience microaggressions are more likely to report associated symptoms of burnout, depression, and suicidal thoughts.11,12 Subjection to persistent bias can lead to minority status stress and racial battle fatigue, creating feelings of invisibility, isolation, exclusion, and loneliness for those impacted.13,14
In the book Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine, Damon Tweedy, MD, reflects on race in medicine. Tweedy notes his experience as a medical student when a professor mistakenly assumed he was a maintenance worker in the classroom. Tweedy describes how he internalized the exchange and, despite his success throughout the course of his medical training, combatted feelings of anxiety, self-doubt, and implied inferiority.15
Although microaggressions are harmful to one’s health, they also undermine the learning and teaching experience for students, residents, and faculty, and they detract from the larger goal of providing care for patients.11 Frequent devaluing and questioning of an individual’s contributions, qualifications, and credentials based on identity can lower productivity and problem-solving abilities. These behaviors cultivate an unwelcome and hostile work/learning environment that is stressful and polarizing for the recipient.
Despite the heavy burden of microaggressions, most students, residents, and faculty physicians do not report incidents to their institutions and feel that training, resources, and policies to respond to bias adequately are lacking.7 As a result of implicit bias and microaggressions, women and URM medical students and providers are unable to focus solely on the practice of medicine. They are tasked with the additional burden of shouldering the emotional and cognitive complexities that microaggressions produce.16
What are strategies to reduce microaggressions in medicine?
To minimize the harmful effects of microaggressions, intervention strategies must be implemented that reduce the likelihood of the occurrence of microaggressions and challenge the stereotypes that undergird implicit bias. These strategies include cultivating allies, followed by demanding structural accountability. Allies are members of the majority group who collectively collaborate with members of the nonmajority group to effect change through the promotion of diversity, equity, and inclusion efforts.17 Cultivating allies involves building a network of collaboration among these groups and emphasizes education. Education is critical for allies to address microaggressions at the interpersonal level. This process of education involves personal reflection and self-awareness in exploring one’s biases, fears, and assumptions. Integral to this step is broadening one’s acceptance of different cultures, racial/ethnic groups, and identities. There must be a willingness to engage in difficult or uncomfortable conversations and a readiness to actively listen to concerns rather than perpetuating further harm through avoidance and dismissive or defensive behavior.18
Demanding structural accountability facilitates deconstruction of bias and microaggression at the larger systemic level. This strategy involves implicit bias and antiracism training, development of retention plans, and identification of mentors for women and URM providers and students. Implicit bias and microaggression training and policies should be incorporated into medical education and resident curriculums. Similarly, educational resources and training must be made available to practicing physicians, faculty, and other providers through their institutions and places of employment. Equipping students and providers with the tools needed when microaggressions are witnessed or experienced demonstrates systemic-level accountability and communicates the importance of the issue. Furthermore, the development of retention plans and identification of mentors provide a support system and foster a culture of inclusion where recipients of microaggressions feel protected and valued. Increased feelings of inclusivity and belonging help bridge the gap created through microaggressions and implicit bias.
Final Thoughts
Despite an often covert nature, the detrimental effects of microaggressions are tangible and far reaching. As providers, we must strive to understand all categories of racism and expose the many ways prejudice manifests within medical training and clinical practice. It is our obligation to undertake the challenge of “making the ‘invisible’ visible” as we confront microaggressions and implicit bias to promote a safer and more inclusive medical community and workforce.19
As manifestations of overt racism and macroaggressions have gained increased visibility, there is a need for discussion of another expression of racism: microaggressions. Although racism classically is viewed as blatant structural, attitudinal, and behavioral prejudice, experts pose that the face of racism has evolved into a more covert insidious form. This form of racism was originally coined racial microaggressions by psychiatrist Chester M. Pierce, MD, 50 years ago.1,2 Since that time, microaggressions have further expanded to describe “brief and commonplace daily verbal, behavioral, and environmental indignities, whether intentional or unintentional, that communicate hostile, derogatory, or negative racial, gender, sexual-orientation, and religious slights and insults to the target person or group.” 3 This article aims to define and depict examples of microaggressions in medicine, discuss the resulting harmful effects, and offer strategies to minimize and counter these negative ramifications.
What are microaggressions?
Microaggressions are behaviors that stem from implicit bias and occur at an interpersonal level. Implicit bias refers to unconscious stereotypes, assumptions, and beliefs held about an individual’s identity. One of the earliest microaggressions—invisibility—was characterized by Ralph Ellison in his novel Invisible Man. Ellison states, “I am invisible, understand, simply because people refuse to see me . . . When they approach me they see only my surroundings, themselves, or figments of their imagination—indeed, everything and anything except me.”4 This concept of invisibility is a primary microaggression faced by people of color.
In medicine, microaggressions and implicit bias may be encountered throughout medical training and clinical practice in interactions with colleagues, superiors, patients, and patients’ families.5,6 Examples of microaggressions in medicine include demeaning comments, nonverbal disrespect, generalizations of social identity, assumption of nonphysician status, role- or credential-questioning behavior, explicit epithets, rejection of care, questioning or inquiries of ethnic/racial origin, and sexual harassment.7
An example of microaggressions in medicine was fully displayed when physician Tamika Cross described her experience of being turned away from helping an unresponsive passenger during a flight emergency.
[T]he flight attendant yells “call overhead for a physician on board.” I raised my hand to grab her attention. She said to me “oh no sweetie put [your] hand down, we are looking for actual physicians or nurses or some type of medical personnel, we don’t have time to talk to you” . . . Another “seasoned” white male approaches the row and says he is a physician as well. She says to me “thanks for your help but he can help us, and he has his credentials.”8
What are the effects of microaggressions?
Although microaggressions may be unconscious and unintentional by the offender, the negative ramifications are notable. Recent studies report that women and underrepresented minority (URM) medical students, residents, and physicians experience microaggressions and implicit bias at a higher prevalence and frequency compared with their male and non-URM counterparts.7,9 Repetitive microaggressions are harmful to the health and safety of women and URM medical students, residents, physicians, other providers, and patients. The Table provides example scenarios of microaggressions in medicine categorized according to Berk.10

Microaggressions negatively impact physical, mental, and emotional well-being. Current data support that medical students and residents who experience microaggressions are more likely to report associated symptoms of burnout, depression, and suicidal thoughts.11,12 Subjection to persistent bias can lead to minority status stress and racial battle fatigue, creating feelings of invisibility, isolation, exclusion, and loneliness for those impacted.13,14
In the book Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine, Damon Tweedy, MD, reflects on race in medicine. Tweedy notes his experience as a medical student when a professor mistakenly assumed he was a maintenance worker in the classroom. Tweedy describes how he internalized the exchange and, despite his success throughout the course of his medical training, combatted feelings of anxiety, self-doubt, and implied inferiority.15
Although microaggressions are harmful to one’s health, they also undermine the learning and teaching experience for students, residents, and faculty, and they detract from the larger goal of providing care for patients.11 Frequent devaluing and questioning of an individual’s contributions, qualifications, and credentials based on identity can lower productivity and problem-solving abilities. These behaviors cultivate an unwelcome and hostile work/learning environment that is stressful and polarizing for the recipient.
Despite the heavy burden of microaggressions, most students, residents, and faculty physicians do not report incidents to their institutions and feel that training, resources, and policies to respond to bias adequately are lacking.7 As a result of implicit bias and microaggressions, women and URM medical students and providers are unable to focus solely on the practice of medicine. They are tasked with the additional burden of shouldering the emotional and cognitive complexities that microaggressions produce.16
What are strategies to reduce microaggressions in medicine?
To minimize the harmful effects of microaggressions, intervention strategies must be implemented that reduce the likelihood of the occurrence of microaggressions and challenge the stereotypes that undergird implicit bias. These strategies include cultivating allies, followed by demanding structural accountability. Allies are members of the majority group who collectively collaborate with members of the nonmajority group to effect change through the promotion of diversity, equity, and inclusion efforts.17 Cultivating allies involves building a network of collaboration among these groups and emphasizes education. Education is critical for allies to address microaggressions at the interpersonal level. This process of education involves personal reflection and self-awareness in exploring one’s biases, fears, and assumptions. Integral to this step is broadening one’s acceptance of different cultures, racial/ethnic groups, and identities. There must be a willingness to engage in difficult or uncomfortable conversations and a readiness to actively listen to concerns rather than perpetuating further harm through avoidance and dismissive or defensive behavior.18
Demanding structural accountability facilitates deconstruction of bias and microaggression at the larger systemic level. This strategy involves implicit bias and antiracism training, development of retention plans, and identification of mentors for women and URM providers and students. Implicit bias and microaggression training and policies should be incorporated into medical education and resident curriculums. Similarly, educational resources and training must be made available to practicing physicians, faculty, and other providers through their institutions and places of employment. Equipping students and providers with the tools needed when microaggressions are witnessed or experienced demonstrates systemic-level accountability and communicates the importance of the issue. Furthermore, the development of retention plans and identification of mentors provide a support system and foster a culture of inclusion where recipients of microaggressions feel protected and valued. Increased feelings of inclusivity and belonging help bridge the gap created through microaggressions and implicit bias.
Final Thoughts
Despite an often covert nature, the detrimental effects of microaggressions are tangible and far reaching. As providers, we must strive to understand all categories of racism and expose the many ways prejudice manifests within medical training and clinical practice. It is our obligation to undertake the challenge of “making the ‘invisible’ visible” as we confront microaggressions and implicit bias to promote a safer and more inclusive medical community and workforce.19
- Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154:868-872. doi:10.1001/jamasurg.2019.1648
- Williams MT. Microaggressions: clarification, evidence, and impact. Perspect Psychol Sci. 2020;15:3-26. doi:10.1177/1745691619827499
- Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Wiley; 2010.
- Ellison R. Invisible Man. Random House; 1952.
- Molina MF, Landry AI, Chary AN, et al. Addressing the elephant in the room: microaggressions in medicine. Ann Emerg Med. 2020;76:387-391. doi:10.1016/j.annemergmed.2020.04.009
- Overland MK, Zumsteg JM, Lindo EG, et al. Microaggressions in clinical training and practice. PM R. 2019;11:1004-1012. doi:10.1002/pmrj.12229
- de Bourmont SS, Burra A, Nouri SS, et al. Resident physician experiences with and responses to biased patients. JAMA Netw Open. 2020;3:e2021769. doi:10.1001/jamanetworkopen.2020.21769
- TK Cross Facebook page. October 9, 2016. Accessed April 19, 2021. https://www.facebook.com/tamika.cross.52/posts/658443077654049
- Periyakoil VS, Chaudron L, Hill EV, et al. Common types of gender-based microaggressions in medicine. Acad Med. 2020;95:450-457. doi:10.1097/ACM.0000000000003057
- Berk RA. Microaggressions trilogy: part 1. why do microaggressions matter? J Fac Dev. 2017;31:63-73.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2020:S0027-9684(20)30428-4. doi:10.1016/j.jnma.2020.11.009
- Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381:1741-1752. doi:10.1056/NEJMsa1903759
- Acholonu RG, Oyeku SO. Addressing microaggressions in the health care workforce-a path toward achieving equity and inclusion. JAMA Netw Open. 2020;3:E2021770. doi:10.1001/jamanetworkopen.2020.21770
- O’Keefe VM, Wingate LR, Cole AB, et al. Seemingly harmless racial communications are not so harmless: racial microaggressions lead to suicidal ideation by way of depression symptoms. Suicide Life Threat Behav. 2015;45:567-576. doi:10.1111/sltb.12150
- Tweedy D. Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine. Picador; 2016.
- Osseo-Asare A, Balasuriya L, Huot SJ, et al. Minority resident physicians’ views on the role of race/ethnicity in their training experiences in the workplace. JAMA Netw Open. 2018;1:E182723. doi: 10.1001/jamanetworkopen.2018.2723
- Melaku TM, Beeman A, Smith DG, et al. Be a better ally. Harvard Business Review. Published November-December 2020. Accessed April 23, 2021. https://hbr.org/2020/11/be-a-better-ally
- Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62:271-286. doi:10.1037/0003-066X.62.4.271
- Sue DW. Whiteness and ethnocentric monoculturalism: making the “invisible” visible. Am Psychol. 2004;59:761-769. doi:10.1037/0003-066X.59.8.761
- Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154:868-872. doi:10.1001/jamasurg.2019.1648
- Williams MT. Microaggressions: clarification, evidence, and impact. Perspect Psychol Sci. 2020;15:3-26. doi:10.1177/1745691619827499
- Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Wiley; 2010.
- Ellison R. Invisible Man. Random House; 1952.
- Molina MF, Landry AI, Chary AN, et al. Addressing the elephant in the room: microaggressions in medicine. Ann Emerg Med. 2020;76:387-391. doi:10.1016/j.annemergmed.2020.04.009
- Overland MK, Zumsteg JM, Lindo EG, et al. Microaggressions in clinical training and practice. PM R. 2019;11:1004-1012. doi:10.1002/pmrj.12229
- de Bourmont SS, Burra A, Nouri SS, et al. Resident physician experiences with and responses to biased patients. JAMA Netw Open. 2020;3:e2021769. doi:10.1001/jamanetworkopen.2020.21769
- TK Cross Facebook page. October 9, 2016. Accessed April 19, 2021. https://www.facebook.com/tamika.cross.52/posts/658443077654049
- Periyakoil VS, Chaudron L, Hill EV, et al. Common types of gender-based microaggressions in medicine. Acad Med. 2020;95:450-457. doi:10.1097/ACM.0000000000003057
- Berk RA. Microaggressions trilogy: part 1. why do microaggressions matter? J Fac Dev. 2017;31:63-73.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2020:S0027-9684(20)30428-4. doi:10.1016/j.jnma.2020.11.009
- Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381:1741-1752. doi:10.1056/NEJMsa1903759
- Acholonu RG, Oyeku SO. Addressing microaggressions in the health care workforce-a path toward achieving equity and inclusion. JAMA Netw Open. 2020;3:E2021770. doi:10.1001/jamanetworkopen.2020.21770
- O’Keefe VM, Wingate LR, Cole AB, et al. Seemingly harmless racial communications are not so harmless: racial microaggressions lead to suicidal ideation by way of depression symptoms. Suicide Life Threat Behav. 2015;45:567-576. doi:10.1111/sltb.12150
- Tweedy D. Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine. Picador; 2016.
- Osseo-Asare A, Balasuriya L, Huot SJ, et al. Minority resident physicians’ views on the role of race/ethnicity in their training experiences in the workplace. JAMA Netw Open. 2018;1:E182723. doi: 10.1001/jamanetworkopen.2018.2723
- Melaku TM, Beeman A, Smith DG, et al. Be a better ally. Harvard Business Review. Published November-December 2020. Accessed April 23, 2021. https://hbr.org/2020/11/be-a-better-ally
- Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62:271-286. doi:10.1037/0003-066X.62.4.271
- Sue DW. Whiteness and ethnocentric monoculturalism: making the “invisible” visible. Am Psychol. 2004;59:761-769. doi:10.1037/0003-066X.59.8.761
Practice Points
- As providers, we must strive to understand all categories of racism and expose the many ways prejudice manifests within medical training and clinical practice.
- Intervention strategies must be implemented to reduce the likelihood of the occurrence of microaggressions in medicine and challenge the stereotypes that undergird implicit bias.
- It is important to promote collaboration in diversity, equity, and inclusion efforts to demonstrate support for women and underrepresented minority medical students, residents, physicians, providers, and patients.
Multiethnic Training in Residency: A Survey of Dermatology Residents
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
Practice Points
- To treat the ever-changing demographics of patients in the United States, dermatologists must receive adequate exposure and education regarding dermatologic conditions in patients from various ethnic backgrounds.
- Dermatology residents from less diverse regions are more likely to agree that dedicated clinics and rotations are important to gain competence compared to those from more diverse regions.
- In areas with less diversity, dedicated multiethnic skin clinics and faculty may be more important for assuring an adequate residency experience.
Atopic Dermatitis in Adolescents With Skin of Color
Data are limited on the management of atopic dermatitis (AD) in adolescents, particularly in patients with skin of color, making it important to identify factors that may improve AD management in this population. Comorbid conditions (eg, acne, postinflammatory hyperpigmentation [PIH]), extracurricular activities (eg, athletics), and experimentation with cosmetics in adolescents, all of which can undermine treatment efficacy and medication adherence, make it particularly challenging to devise a therapeutic regimen in this patient population. We review the management of AD in black adolescents, with special consideration of concomitant treatment of acne vulgaris (AV) as well as lifestyle and social choices (Table).

Prevalence and Epidemiology
Atopic dermatitis affects 13% to 25% of children and 2% to 10% of adults.1,2 Population‐based studies in the United States show a higher prevalence of AD in black children (19.3%) compared to European American (EA) children (16.1%).3,4
AD in Black Adolescents
Atopic dermatitis is a common skin condition that is defined as a chronic, pruritic, inflammatory dermatosis with recurrent scaling, papules, and plaques (Figure) that usually develop during infancy and early childhood.3 Although AD severity improves for some patients in adolescence, it can be a lifelong issue affecting performance in academic and occupational settings.5 One US study of 8015 children found that there are racial and ethnic disparities in school absences among children (age range, 2–17 years) with AD, with children with skin of color being absent more often than white children.6 The same study noted that black children had a 1.5-fold higher chance of being absent 6 days over a 6-month school period compared to white children. It is postulated that AD has a greater impact on quality of life (QOL) in children with skin of color, resulting in the increased number of school absences in this population.6
The origin of AD currently is thought to be complex and can involve skin barrier dysfunction, environmental factors, microbiome effects, genetic predisposition, and immune dysregulation.1,4 Atopic dermatitis is a heterogeneous disease with variations in the prevalence, genetic background, and immune activation patterns across racial groups.4 It is now understood to be an immune-mediated disease with multiple inflammatory pathways, with type 2–associated inflammation being a primary pathway. Patients with AD have strong helper T cell (TH2) activation, and black patients with AD have higher IgE serum levels as well as absent TH17/TH1 activation.4
Atopic dermatitis currently is seen as a defect of the epidermal barrier, with variable clinical manifestations and expressivity.7 Filaggrin is an epidermal barrier protein, encoded by the FLG gene, and plays a major role in barrier function by regulating pH and promoting hydration of the skin.4 Loss of function of the FLG gene is the most well-studied genetic risk factor for developing AD, and this mutation is seen in patients with more severe and persistent AD in addition to patients with more skin infections and allergic sensitizations.3,4 However, in the skin of color population, FLG mutations are 6 times less common than in the EA population, despite the fact that AD is more prevalent in patients of African descent.4 Therefore, the role of the FLG loss-of-function mutation and AD is not as well defined in black patients, and some researchers have found no association.3 The FLG loss-of-function mutation seems to play a smaller role in black patients than in EA patients, and other genes may be involved in skin barrier dysfunction.3,4 In a small study of patients with mild AD compared to nonaffected patients, those with AD had lower total ceramide levels in the stratum corneum of affected sites than normal skin sites in healthy individuals.8
Particular disturbances in the gut microbiome have the possibility of impacting the development of AD.9 Additionally, the development of AD may be influenced by the skin microbiome, which can change depending on body site, with fungal organisms thought to make up a large proportion of the microbiome of patients with AD. In patients with AD, there is a lack of microbial diversity and an overgrowth of Staphylococcus aureus.9
Diagnosis
Clinicians diagnose AD based on clinical characteristics, and the lack of objective criteria can hinder diagnosis.1 Thus, diagnosing AD in children with dark skin can pose a particular challenge given the varied clinical presentation of AD across skin types. Severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin types.10 Furthermore, clinical erythema scores among black children may be “strongly” underestimated using scoring systems such as Eczema Area and Severity Index and SCORing Atopic Dermatitis.4 It is estimated that the risk for severe AD may be 6 times higher in black children compared to white children.10 Additionally, patients with skin of color can present with more treatment-resistant AD.4
Treatment of AD
Current treatment is focused on restoring epidermal barrier function, often with topical agents, such as moisturizers containing different amounts of emollients, occlusives, and humectants; corticosteroids; calcineurin inhibitors; and antimicrobials. Emollients such as glycol stearate, glyceryl stearate, and soy sterols function as lubricants, softening the skin. Occlusive agents include petrolatum, dimethicone, and mineral oil; they act by forming a layer to slow evaporation of water. Humectants including glycerol, lactic acid, and urea function by promoting water retention.11 For acute flares, mid- to high-potency topical corticosteroids are recommended. Also, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used alone or in combination with topical steroids. Finally, bleach baths and topical mupirocin applied to the nares also have proved helpful in moderate to severe AD with secondary bacterial infections.11 Phototherapy can be used in adult and pediatric patients with acute and chronic AD if traditional treatments have failed.2
Systemic agents are indicated and recommended for the subset of adult and pediatric patients in whom optimized topical regimens and/or phototherapy do not adequately provide disease control or when QOL is substantially impacted. The systemic agents effective in the pediatric population include cyclosporine, azathioprine, mycophenolate mofetil, and possibly methotrexate.11 Dupilumab recently was approved by the US Food and Drug Administration for patients 12 years and older with moderate to severe AD whose disease is not well controlled with topical medications.
Patients with AD are predisposed to secondary bacterial and viral infections because of their dysfunctional skin barrier; these infections most commonly are caused by S aureus and herpes simplex virus, respectively.2 Systemic antibiotics are only recommended for patients with AD when there is clinical evidence of bacterial infection. In patients with evidence of eczema herpeticum, systemic antiviral agents should be used to treat the underlying herpes simplex virus infection.2 Atopic dermatitis typically has been studied in white patients; however, patients with skin of color have higher frequencies of treatment-resistant AD. Further research on treatment efficacy for AD in this patient population is needed, as data are limited.4
Treatment of AV in Patients With AD
Two of the most prevalent skin diseases affecting the pediatric population are AD and AV, and both can remarkably impact QOL.12 Acne is one of the most common reasons for adolescent patients to seek dermatologic care, including patients with skin of color (Fitzpatrick skin types IV to VI).13 Thus, it is to be expected that many black adolescents with AD also will have AV. For mild to moderate acne in patients with skin of color, topical retinoids and benzoyl peroxide typically are first line.13 These medications can be problematic for patients with AD, as retinoids and many other acne treatments can cause dryness, which may exacerbate AD.
Moisturizers containing ceramide can be a helpful adjunctive therapy in treating acne,14 especially in patients with AD. Modifications to application of acne medications, such as using topical retinoids every other night or mixing them with moisturizers to minimize dryness, may be beneficial to these patients. Dapsone gel 7.5% used daily also may be an option for adolescents with AD and AV. A double-blind, vehicle-controlled study demonstrated that dapsone is safe and effective for patients 12 years and older with moderate acne, and patients with Fitzpatrick skin types IV to VI rated local scaling, erythema, dryness, and stinging/burning as “none” in the study.15 Another potentially helpful topical agent in patients with AD and AV is sulfacetamide, as it is not likely to cause dryness of the skin. In a small study, sodium sulfacetamide 10% and sulfur 5% in an emollient foam vehicle showed no residual film or sulfur smell and resulted in acne reduction of 50%.16
Patients with skin of color often experience PIH in AD and acne or hypopigmentation from inflammatory dermatoses including AD.17,18 In addition to the dryness from AD and topical retinoid use, patients with skin of color may develop irritant contact dermatitis, thus leading to PIH.13 Dryness and irritant contact dermatitis also can be seen with the use of benzoyl peroxide in black patients. Because of these effects, gentle moisturizers are recommended, and both benzoyl peroxide and retinoids should be initiated at lower doses in patients with skin of color.13
For patients with severe nodulocystic acne, isotretinoin is the treatment of choice in patients with skin of color,13 but there is a dearth of clinical studies addressing complications seen in black adolescents on this treatment, especially with respect to those with AD. Of note, systemic antibiotics typically are initiated before isotretinoin; however, this strategy is falling out of favor due to concern for antibiotic resistance with long-term use.19
Impact of Athletics on AD in Black Adolescents
Because of the exacerbating effects of perspiration and heat causing itch and irritation in patients with AD, it is frequently advised that pediatric patients limit their participation in athletics because of the exacerbating effects of strenuous physical exercise on their disease.12 In one study, 429 pediatric patients or their parents/guardians completed QOL questionnaires; 89% of patients 15 years and younger with severe AD reported that their disease was impacted by athletics and outdoor activities, and 86% of these pediatric patients with severe AD responded that their social lives and leisure activities were impacted.20 Because adolescents often are involved in athletics or have mandatory physical education classes, AD may be isolating and may have a severe impact on self-esteem.
Aggressive treatment of AD with topical and systemic medications may be helpful in adolescents who may be reluctant to participate in sports because of teasing, bullying, or worsening of symptoms with heat or sweating.21 Now that dupilumab is available for adolescents, there is a chance that patients with severe and/or recalcitrant disease managed on this medication can achieve better control of their symptoms without the laboratory requirement of methotrexate and the difficulties of topical medication application, allowing them to engage in mandatory athletic classes as well as desired organized sports.
Use of Cosmetics for AD
Many adolescents experiment with cosmetics, and those with AD may use cosmetic products to cover hyperpigmented or hypopigmented lesions.18 In patients with active AD or increased sensitivity to allergens in cosmetic products, use of makeup can be a contributing factor for AD flares. Acne associated with cosmetics is especially important to consider in darker-skinned patients who may use makeup that is opaque and contains oil to conceal acne or PIH.
Allergens can be present in both cosmetics and pharmaceutical topical agents, and a Brazilian study found that approximately 89% of 813 prescription and nonprescription products (eg, topical drugs, sunscreens, moisturizers, soaps, cleansing lotions, shampoos, cosmeceuticals) contained allergens.22 Patients with AD have a higher prevalence of contact sensitization to fragrances, including balsam of Peru.23 Some AD treatments that contain fragrances have caused further skin issues in a few patients. In one case series, 3 pediatric patients developed allergic contact dermatitis to Myroxylon pereirae (balsam of Peru) when using topical treatments for their AD, and their symptoms of scalp inflammation and alopecia resolved with discontinuation.23
In a Dutch study, sensitization to Fragrance Mix I and M pereirae as well as other ingredients (eg, lanolin alcohol, Amerchol™ L 101 [a lanolin product]) was notably more common in pediatric patients with AD than in patients without AD; however, no data on patients with skin of color were included in this study.24
Because of the increased risk of sensitization to fragrances and other ingredients in patients with AD as well as the high percentage of allergens in prescription and nonprescription products, it is important to discuss all personal care products that patients may be using, not just their cosmetic products. Also, patch testing may be helpful in determining true allergens in some patients. Patch testing is recommended for patients with treatment-resistant AD, and a recent study suggested it should be done prior to long-term use of immunosuppressive agents.25 Increased steroid phobia and a push toward alternative medicines are leading both patients with AD and guardians of children with AD to look for other forms of moisturization, such as olive oil, coconut oil, sunflower seed oil, and shea butter, to decrease transepidermal water loss.26,27 An important factor in AD treatment efficacy is patient acceptability in using what is recommended.27 One study showed there was no difference in efficacy or acceptability in using a cream containing shea butter extract vs the ceramide-precursor product.27 Current data show olive oil may exacerbate dry skin and AD,26 and recommendation of any over-the-counter oils and butters in patients with AD should be made with great caution, as many of these products contain fragrances and other potential allergens.
Alternative Therapies for AD
Patients with AD often seek alternative or integrative treatment options, including dietary modifications and holistic remedies. Studies investigating the role of vitamins and supplements in treating AD are limited by sample size.28 However, there is some evidence that may support supplementation with vitamins D and E in addressing AD symptoms. The use of probiotics in treating AD is controversial, but there are studies suggesting that the use of probiotics may prove beneficial in preventing infantile AD.28 Additionally, findings from an ex vivo and in vitro study show that some conditions, including AD and acne, may benefit from the same probiotics, despite the differences in these two diseases. Both AD and acne have inflammatory and skin dysbiosis characteristics, which may be the common thread leading to both conditions potentially responding to treatment with probiotics.29
Preliminary evidence indicates that supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of AD.28 In a 20-week, randomized, single-blind, crossover study published in 2005, dietary hemp seed oil showed an improvement of clinical symptoms, including dry skin and itchiness, in patients with AD.30
In light of recent legalization in several states, patients may turn to use of cannabinoid products to manage AD. In a systematic review, cannabinoid use was reportedly a therapeutic option in the treatment of AD and AV; however, the data are based on preclinical work, and there are no randomized, placebo-controlled studies to support the use of cannabinoids.31 Furthermore, there is great concern that use of these products in adolescents is an even larger unknown.
Final Thoughts
Eighty percent of children diagnosed with AD experience symptom improvement before their early teens32; for those with AD during their preteen and teenage years, there can be psychological ramifications, as teenagers with AD report having fewer friends, are less socially involved, participate in fewer sports, and are absent from classes more often than their peers.5 In black patients with AD, school absences are even more common.6 Given the social and emotional impact of AD on patients with skin of color, it is imperative to treat the condition appropriately.33 There are areas of opportunity for further research on alternate dosing of existing treatments for AV in patients with AD, further recommendations for adolescent athletes with AD, and which cosmetic and alternative medicine products may be beneficial for this population to improve their QOL.
Providers should discuss medical management in a broader context considering patients’ extracurricular activities, treatment vehicle preferences, expectations, and personal care habits. It also is important to address the many possible factors that may influence treatment adherence early on, particularly in adolescents, as these could be barriers to treatment. This article highlights considerations for treating AD and comorbid conditions that may further complicate treatment in adolescent patients with skin of color. The information provided should serve as a guide in initial counseling and management of AD in adolescents with skin of color.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83-93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357.
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-455.
- Vivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. Int J Womens Dermatol. 2018;4:27-31.
- Wan J, Margolis DJ, Mitra N, et al. Racial and ethnic differences in atopic dermatitis–related school absences among US children [published online May 22, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.0597.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514.
- Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. 2019;31:418-425.
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33:129-135.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Lynde CW, Andriessen A, Barankin B, et al. Moisturizers and ceramide-containing moisturizers may offer concomitant therapy with benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- Taylor SC, Cook-Bolden FE, McMichael A, et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% for treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17:160-167.
- Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-236.
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402.
- Heath CR. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis. 2018;102:71-73.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Paller AS, McAlister RO, Doyle JJ, et al. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41:323-332.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35:303-316.
- Rocha VB, Machado CJ, Bittencourt FV. Presence of allergens in the vehicles of Brazilian dermatological products. Contact Dermatitis. 2017;76:126-128.
- Admani S, Goldenberg A, Jacob SE. Contact alopecia: improvement of alopecia with discontinuation of fluocinolone oil in individuals allergic to balsam fragrance. Pediatr Dermatol. 2017;34:e57-e60.
- Uter W, Werfel T, White IR, et al. Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. 2018;15:E1108.
- López-Jiménez EC, Marrero-Alemán G, Borrego L. One-third of patients with therapy-resistant atopic dermatitis may benefit after patch testing [published online May 13, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15672.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- Hon KL, Tsang YC, Pong NH, et al. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-425.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review [published online March 20, 2019]. Int J Dermatol. doi:10.1111/ijd.14404.
- Mottin VHM, Suyenaga ES. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol. 2018;57:1425-1432.
- Callaway J, Schwab U, Harvima I, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat. 2005;16:87-94.
- Eagleston LRM, Kalani NK, Patel RR, et al. Cannabinoids in dermatology: a scoping review [published June 15, 2018]. Dermatol Online J. 2018;24.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-687.e611.
- de María Díaz Granados L, Quijano MA, Ramírez PA, et al. Quality assessment of atopic dermatitis clinical practice guidelines in ≤ 18 years. Arch Dermatol Res. 2018;310:29-37.
Data are limited on the management of atopic dermatitis (AD) in adolescents, particularly in patients with skin of color, making it important to identify factors that may improve AD management in this population. Comorbid conditions (eg, acne, postinflammatory hyperpigmentation [PIH]), extracurricular activities (eg, athletics), and experimentation with cosmetics in adolescents, all of which can undermine treatment efficacy and medication adherence, make it particularly challenging to devise a therapeutic regimen in this patient population. We review the management of AD in black adolescents, with special consideration of concomitant treatment of acne vulgaris (AV) as well as lifestyle and social choices (Table).

Prevalence and Epidemiology
Atopic dermatitis affects 13% to 25% of children and 2% to 10% of adults.1,2 Population‐based studies in the United States show a higher prevalence of AD in black children (19.3%) compared to European American (EA) children (16.1%).3,4
AD in Black Adolescents
Atopic dermatitis is a common skin condition that is defined as a chronic, pruritic, inflammatory dermatosis with recurrent scaling, papules, and plaques (Figure) that usually develop during infancy and early childhood.3 Although AD severity improves for some patients in adolescence, it can be a lifelong issue affecting performance in academic and occupational settings.5 One US study of 8015 children found that there are racial and ethnic disparities in school absences among children (age range, 2–17 years) with AD, with children with skin of color being absent more often than white children.6 The same study noted that black children had a 1.5-fold higher chance of being absent 6 days over a 6-month school period compared to white children. It is postulated that AD has a greater impact on quality of life (QOL) in children with skin of color, resulting in the increased number of school absences in this population.6

The origin of AD currently is thought to be complex and can involve skin barrier dysfunction, environmental factors, microbiome effects, genetic predisposition, and immune dysregulation.1,4 Atopic dermatitis is a heterogeneous disease with variations in the prevalence, genetic background, and immune activation patterns across racial groups.4 It is now understood to be an immune-mediated disease with multiple inflammatory pathways, with type 2–associated inflammation being a primary pathway. Patients with AD have strong helper T cell (TH2) activation, and black patients with AD have higher IgE serum levels as well as absent TH17/TH1 activation.4
Atopic dermatitis currently is seen as a defect of the epidermal barrier, with variable clinical manifestations and expressivity.7 Filaggrin is an epidermal barrier protein, encoded by the FLG gene, and plays a major role in barrier function by regulating pH and promoting hydration of the skin.4 Loss of function of the FLG gene is the most well-studied genetic risk factor for developing AD, and this mutation is seen in patients with more severe and persistent AD in addition to patients with more skin infections and allergic sensitizations.3,4 However, in the skin of color population, FLG mutations are 6 times less common than in the EA population, despite the fact that AD is more prevalent in patients of African descent.4 Therefore, the role of the FLG loss-of-function mutation and AD is not as well defined in black patients, and some researchers have found no association.3 The FLG loss-of-function mutation seems to play a smaller role in black patients than in EA patients, and other genes may be involved in skin barrier dysfunction.3,4 In a small study of patients with mild AD compared to nonaffected patients, those with AD had lower total ceramide levels in the stratum corneum of affected sites than normal skin sites in healthy individuals.8
Particular disturbances in the gut microbiome have the possibility of impacting the development of AD.9 Additionally, the development of AD may be influenced by the skin microbiome, which can change depending on body site, with fungal organisms thought to make up a large proportion of the microbiome of patients with AD. In patients with AD, there is a lack of microbial diversity and an overgrowth of Staphylococcus aureus.9
Diagnosis
Clinicians diagnose AD based on clinical characteristics, and the lack of objective criteria can hinder diagnosis.1 Thus, diagnosing AD in children with dark skin can pose a particular challenge given the varied clinical presentation of AD across skin types. Severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin types.10 Furthermore, clinical erythema scores among black children may be “strongly” underestimated using scoring systems such as Eczema Area and Severity Index and SCORing Atopic Dermatitis.4 It is estimated that the risk for severe AD may be 6 times higher in black children compared to white children.10 Additionally, patients with skin of color can present with more treatment-resistant AD.4
Treatment of AD
Current treatment is focused on restoring epidermal barrier function, often with topical agents, such as moisturizers containing different amounts of emollients, occlusives, and humectants; corticosteroids; calcineurin inhibitors; and antimicrobials. Emollients such as glycol stearate, glyceryl stearate, and soy sterols function as lubricants, softening the skin. Occlusive agents include petrolatum, dimethicone, and mineral oil; they act by forming a layer to slow evaporation of water. Humectants including glycerol, lactic acid, and urea function by promoting water retention.11 For acute flares, mid- to high-potency topical corticosteroids are recommended. Also, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used alone or in combination with topical steroids. Finally, bleach baths and topical mupirocin applied to the nares also have proved helpful in moderate to severe AD with secondary bacterial infections.11 Phototherapy can be used in adult and pediatric patients with acute and chronic AD if traditional treatments have failed.2
Systemic agents are indicated and recommended for the subset of adult and pediatric patients in whom optimized topical regimens and/or phototherapy do not adequately provide disease control or when QOL is substantially impacted. The systemic agents effective in the pediatric population include cyclosporine, azathioprine, mycophenolate mofetil, and possibly methotrexate.11 Dupilumab recently was approved by the US Food and Drug Administration for patients 12 years and older with moderate to severe AD whose disease is not well controlled with topical medications.
Patients with AD are predisposed to secondary bacterial and viral infections because of their dysfunctional skin barrier; these infections most commonly are caused by S aureus and herpes simplex virus, respectively.2 Systemic antibiotics are only recommended for patients with AD when there is clinical evidence of bacterial infection. In patients with evidence of eczema herpeticum, systemic antiviral agents should be used to treat the underlying herpes simplex virus infection.2 Atopic dermatitis typically has been studied in white patients; however, patients with skin of color have higher frequencies of treatment-resistant AD. Further research on treatment efficacy for AD in this patient population is needed, as data are limited.4
Treatment of AV in Patients With AD
Two of the most prevalent skin diseases affecting the pediatric population are AD and AV, and both can remarkably impact QOL.12 Acne is one of the most common reasons for adolescent patients to seek dermatologic care, including patients with skin of color (Fitzpatrick skin types IV to VI).13 Thus, it is to be expected that many black adolescents with AD also will have AV. For mild to moderate acne in patients with skin of color, topical retinoids and benzoyl peroxide typically are first line.13 These medications can be problematic for patients with AD, as retinoids and many other acne treatments can cause dryness, which may exacerbate AD.
Moisturizers containing ceramide can be a helpful adjunctive therapy in treating acne,14 especially in patients with AD. Modifications to application of acne medications, such as using topical retinoids every other night or mixing them with moisturizers to minimize dryness, may be beneficial to these patients. Dapsone gel 7.5% used daily also may be an option for adolescents with AD and AV. A double-blind, vehicle-controlled study demonstrated that dapsone is safe and effective for patients 12 years and older with moderate acne, and patients with Fitzpatrick skin types IV to VI rated local scaling, erythema, dryness, and stinging/burning as “none” in the study.15 Another potentially helpful topical agent in patients with AD and AV is sulfacetamide, as it is not likely to cause dryness of the skin. In a small study, sodium sulfacetamide 10% and sulfur 5% in an emollient foam vehicle showed no residual film or sulfur smell and resulted in acne reduction of 50%.16
Patients with skin of color often experience PIH in AD and acne or hypopigmentation from inflammatory dermatoses including AD.17,18 In addition to the dryness from AD and topical retinoid use, patients with skin of color may develop irritant contact dermatitis, thus leading to PIH.13 Dryness and irritant contact dermatitis also can be seen with the use of benzoyl peroxide in black patients. Because of these effects, gentle moisturizers are recommended, and both benzoyl peroxide and retinoids should be initiated at lower doses in patients with skin of color.13
For patients with severe nodulocystic acne, isotretinoin is the treatment of choice in patients with skin of color,13 but there is a dearth of clinical studies addressing complications seen in black adolescents on this treatment, especially with respect to those with AD. Of note, systemic antibiotics typically are initiated before isotretinoin; however, this strategy is falling out of favor due to concern for antibiotic resistance with long-term use.19
Impact of Athletics on AD in Black Adolescents
Because of the exacerbating effects of perspiration and heat causing itch and irritation in patients with AD, it is frequently advised that pediatric patients limit their participation in athletics because of the exacerbating effects of strenuous physical exercise on their disease.12 In one study, 429 pediatric patients or their parents/guardians completed QOL questionnaires; 89% of patients 15 years and younger with severe AD reported that their disease was impacted by athletics and outdoor activities, and 86% of these pediatric patients with severe AD responded that their social lives and leisure activities were impacted.20 Because adolescents often are involved in athletics or have mandatory physical education classes, AD may be isolating and may have a severe impact on self-esteem.
Aggressive treatment of AD with topical and systemic medications may be helpful in adolescents who may be reluctant to participate in sports because of teasing, bullying, or worsening of symptoms with heat or sweating.21 Now that dupilumab is available for adolescents, there is a chance that patients with severe and/or recalcitrant disease managed on this medication can achieve better control of their symptoms without the laboratory requirement of methotrexate and the difficulties of topical medication application, allowing them to engage in mandatory athletic classes as well as desired organized sports.
Use of Cosmetics for AD
Many adolescents experiment with cosmetics, and those with AD may use cosmetic products to cover hyperpigmented or hypopigmented lesions.18 In patients with active AD or increased sensitivity to allergens in cosmetic products, use of makeup can be a contributing factor for AD flares. Acne associated with cosmetics is especially important to consider in darker-skinned patients who may use makeup that is opaque and contains oil to conceal acne or PIH.
Allergens can be present in both cosmetics and pharmaceutical topical agents, and a Brazilian study found that approximately 89% of 813 prescription and nonprescription products (eg, topical drugs, sunscreens, moisturizers, soaps, cleansing lotions, shampoos, cosmeceuticals) contained allergens.22 Patients with AD have a higher prevalence of contact sensitization to fragrances, including balsam of Peru.23 Some AD treatments that contain fragrances have caused further skin issues in a few patients. In one case series, 3 pediatric patients developed allergic contact dermatitis to Myroxylon pereirae (balsam of Peru) when using topical treatments for their AD, and their symptoms of scalp inflammation and alopecia resolved with discontinuation.23
In a Dutch study, sensitization to Fragrance Mix I and M pereirae as well as other ingredients (eg, lanolin alcohol, Amerchol™ L 101 [a lanolin product]) was notably more common in pediatric patients with AD than in patients without AD; however, no data on patients with skin of color were included in this study.24
Because of the increased risk of sensitization to fragrances and other ingredients in patients with AD as well as the high percentage of allergens in prescription and nonprescription products, it is important to discuss all personal care products that patients may be using, not just their cosmetic products. Also, patch testing may be helpful in determining true allergens in some patients. Patch testing is recommended for patients with treatment-resistant AD, and a recent study suggested it should be done prior to long-term use of immunosuppressive agents.25 Increased steroid phobia and a push toward alternative medicines are leading both patients with AD and guardians of children with AD to look for other forms of moisturization, such as olive oil, coconut oil, sunflower seed oil, and shea butter, to decrease transepidermal water loss.26,27 An important factor in AD treatment efficacy is patient acceptability in using what is recommended.27 One study showed there was no difference in efficacy or acceptability in using a cream containing shea butter extract vs the ceramide-precursor product.27 Current data show olive oil may exacerbate dry skin and AD,26 and recommendation of any over-the-counter oils and butters in patients with AD should be made with great caution, as many of these products contain fragrances and other potential allergens.
Alternative Therapies for AD
Patients with AD often seek alternative or integrative treatment options, including dietary modifications and holistic remedies. Studies investigating the role of vitamins and supplements in treating AD are limited by sample size.28 However, there is some evidence that may support supplementation with vitamins D and E in addressing AD symptoms. The use of probiotics in treating AD is controversial, but there are studies suggesting that the use of probiotics may prove beneficial in preventing infantile AD.28 Additionally, findings from an ex vivo and in vitro study show that some conditions, including AD and acne, may benefit from the same probiotics, despite the differences in these two diseases. Both AD and acne have inflammatory and skin dysbiosis characteristics, which may be the common thread leading to both conditions potentially responding to treatment with probiotics.29
Preliminary evidence indicates that supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of AD.28 In a 20-week, randomized, single-blind, crossover study published in 2005, dietary hemp seed oil showed an improvement of clinical symptoms, including dry skin and itchiness, in patients with AD.30
In light of recent legalization in several states, patients may turn to use of cannabinoid products to manage AD. In a systematic review, cannabinoid use was reportedly a therapeutic option in the treatment of AD and AV; however, the data are based on preclinical work, and there are no randomized, placebo-controlled studies to support the use of cannabinoids.31 Furthermore, there is great concern that use of these products in adolescents is an even larger unknown.
Final Thoughts
Eighty percent of children diagnosed with AD experience symptom improvement before their early teens32; for those with AD during their preteen and teenage years, there can be psychological ramifications, as teenagers with AD report having fewer friends, are less socially involved, participate in fewer sports, and are absent from classes more often than their peers.5 In black patients with AD, school absences are even more common.6 Given the social and emotional impact of AD on patients with skin of color, it is imperative to treat the condition appropriately.33 There are areas of opportunity for further research on alternate dosing of existing treatments for AV in patients with AD, further recommendations for adolescent athletes with AD, and which cosmetic and alternative medicine products may be beneficial for this population to improve their QOL.
Providers should discuss medical management in a broader context considering patients’ extracurricular activities, treatment vehicle preferences, expectations, and personal care habits. It also is important to address the many possible factors that may influence treatment adherence early on, particularly in adolescents, as these could be barriers to treatment. This article highlights considerations for treating AD and comorbid conditions that may further complicate treatment in adolescent patients with skin of color. The information provided should serve as a guide in initial counseling and management of AD in adolescents with skin of color.
Data are limited on the management of atopic dermatitis (AD) in adolescents, particularly in patients with skin of color, making it important to identify factors that may improve AD management in this population. Comorbid conditions (eg, acne, postinflammatory hyperpigmentation [PIH]), extracurricular activities (eg, athletics), and experimentation with cosmetics in adolescents, all of which can undermine treatment efficacy and medication adherence, make it particularly challenging to devise a therapeutic regimen in this patient population. We review the management of AD in black adolescents, with special consideration of concomitant treatment of acne vulgaris (AV) as well as lifestyle and social choices (Table).

Prevalence and Epidemiology
Atopic dermatitis affects 13% to 25% of children and 2% to 10% of adults.1,2 Population‐based studies in the United States show a higher prevalence of AD in black children (19.3%) compared to European American (EA) children (16.1%).3,4
AD in Black Adolescents
Atopic dermatitis is a common skin condition that is defined as a chronic, pruritic, inflammatory dermatosis with recurrent scaling, papules, and plaques (Figure) that usually develop during infancy and early childhood.3 Although AD severity improves for some patients in adolescence, it can be a lifelong issue affecting performance in academic and occupational settings.5 One US study of 8015 children found that there are racial and ethnic disparities in school absences among children (age range, 2–17 years) with AD, with children with skin of color being absent more often than white children.6 The same study noted that black children had a 1.5-fold higher chance of being absent 6 days over a 6-month school period compared to white children. It is postulated that AD has a greater impact on quality of life (QOL) in children with skin of color, resulting in the increased number of school absences in this population.6

The origin of AD currently is thought to be complex and can involve skin barrier dysfunction, environmental factors, microbiome effects, genetic predisposition, and immune dysregulation.1,4 Atopic dermatitis is a heterogeneous disease with variations in the prevalence, genetic background, and immune activation patterns across racial groups.4 It is now understood to be an immune-mediated disease with multiple inflammatory pathways, with type 2–associated inflammation being a primary pathway. Patients with AD have strong helper T cell (TH2) activation, and black patients with AD have higher IgE serum levels as well as absent TH17/TH1 activation.4
Atopic dermatitis currently is seen as a defect of the epidermal barrier, with variable clinical manifestations and expressivity.7 Filaggrin is an epidermal barrier protein, encoded by the FLG gene, and plays a major role in barrier function by regulating pH and promoting hydration of the skin.4 Loss of function of the FLG gene is the most well-studied genetic risk factor for developing AD, and this mutation is seen in patients with more severe and persistent AD in addition to patients with more skin infections and allergic sensitizations.3,4 However, in the skin of color population, FLG mutations are 6 times less common than in the EA population, despite the fact that AD is more prevalent in patients of African descent.4 Therefore, the role of the FLG loss-of-function mutation and AD is not as well defined in black patients, and some researchers have found no association.3 The FLG loss-of-function mutation seems to play a smaller role in black patients than in EA patients, and other genes may be involved in skin barrier dysfunction.3,4 In a small study of patients with mild AD compared to nonaffected patients, those with AD had lower total ceramide levels in the stratum corneum of affected sites than normal skin sites in healthy individuals.8
Particular disturbances in the gut microbiome have the possibility of impacting the development of AD.9 Additionally, the development of AD may be influenced by the skin microbiome, which can change depending on body site, with fungal organisms thought to make up a large proportion of the microbiome of patients with AD. In patients with AD, there is a lack of microbial diversity and an overgrowth of Staphylococcus aureus.9
Diagnosis
Clinicians diagnose AD based on clinical characteristics, and the lack of objective criteria can hinder diagnosis.1 Thus, diagnosing AD in children with dark skin can pose a particular challenge given the varied clinical presentation of AD across skin types. Severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin types.10 Furthermore, clinical erythema scores among black children may be “strongly” underestimated using scoring systems such as Eczema Area and Severity Index and SCORing Atopic Dermatitis.4 It is estimated that the risk for severe AD may be 6 times higher in black children compared to white children.10 Additionally, patients with skin of color can present with more treatment-resistant AD.4
Treatment of AD
Current treatment is focused on restoring epidermal barrier function, often with topical agents, such as moisturizers containing different amounts of emollients, occlusives, and humectants; corticosteroids; calcineurin inhibitors; and antimicrobials. Emollients such as glycol stearate, glyceryl stearate, and soy sterols function as lubricants, softening the skin. Occlusive agents include petrolatum, dimethicone, and mineral oil; they act by forming a layer to slow evaporation of water. Humectants including glycerol, lactic acid, and urea function by promoting water retention.11 For acute flares, mid- to high-potency topical corticosteroids are recommended. Also, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used alone or in combination with topical steroids. Finally, bleach baths and topical mupirocin applied to the nares also have proved helpful in moderate to severe AD with secondary bacterial infections.11 Phototherapy can be used in adult and pediatric patients with acute and chronic AD if traditional treatments have failed.2
Systemic agents are indicated and recommended for the subset of adult and pediatric patients in whom optimized topical regimens and/or phototherapy do not adequately provide disease control or when QOL is substantially impacted. The systemic agents effective in the pediatric population include cyclosporine, azathioprine, mycophenolate mofetil, and possibly methotrexate.11 Dupilumab recently was approved by the US Food and Drug Administration for patients 12 years and older with moderate to severe AD whose disease is not well controlled with topical medications.
Patients with AD are predisposed to secondary bacterial and viral infections because of their dysfunctional skin barrier; these infections most commonly are caused by S aureus and herpes simplex virus, respectively.2 Systemic antibiotics are only recommended for patients with AD when there is clinical evidence of bacterial infection. In patients with evidence of eczema herpeticum, systemic antiviral agents should be used to treat the underlying herpes simplex virus infection.2 Atopic dermatitis typically has been studied in white patients; however, patients with skin of color have higher frequencies of treatment-resistant AD. Further research on treatment efficacy for AD in this patient population is needed, as data are limited.4
Treatment of AV in Patients With AD
Two of the most prevalent skin diseases affecting the pediatric population are AD and AV, and both can remarkably impact QOL.12 Acne is one of the most common reasons for adolescent patients to seek dermatologic care, including patients with skin of color (Fitzpatrick skin types IV to VI).13 Thus, it is to be expected that many black adolescents with AD also will have AV. For mild to moderate acne in patients with skin of color, topical retinoids and benzoyl peroxide typically are first line.13 These medications can be problematic for patients with AD, as retinoids and many other acne treatments can cause dryness, which may exacerbate AD.
Moisturizers containing ceramide can be a helpful adjunctive therapy in treating acne,14 especially in patients with AD. Modifications to application of acne medications, such as using topical retinoids every other night or mixing them with moisturizers to minimize dryness, may be beneficial to these patients. Dapsone gel 7.5% used daily also may be an option for adolescents with AD and AV. A double-blind, vehicle-controlled study demonstrated that dapsone is safe and effective for patients 12 years and older with moderate acne, and patients with Fitzpatrick skin types IV to VI rated local scaling, erythema, dryness, and stinging/burning as “none” in the study.15 Another potentially helpful topical agent in patients with AD and AV is sulfacetamide, as it is not likely to cause dryness of the skin. In a small study, sodium sulfacetamide 10% and sulfur 5% in an emollient foam vehicle showed no residual film or sulfur smell and resulted in acne reduction of 50%.16
Patients with skin of color often experience PIH in AD and acne or hypopigmentation from inflammatory dermatoses including AD.17,18 In addition to the dryness from AD and topical retinoid use, patients with skin of color may develop irritant contact dermatitis, thus leading to PIH.13 Dryness and irritant contact dermatitis also can be seen with the use of benzoyl peroxide in black patients. Because of these effects, gentle moisturizers are recommended, and both benzoyl peroxide and retinoids should be initiated at lower doses in patients with skin of color.13
For patients with severe nodulocystic acne, isotretinoin is the treatment of choice in patients with skin of color,13 but there is a dearth of clinical studies addressing complications seen in black adolescents on this treatment, especially with respect to those with AD. Of note, systemic antibiotics typically are initiated before isotretinoin; however, this strategy is falling out of favor due to concern for antibiotic resistance with long-term use.19
Impact of Athletics on AD in Black Adolescents
Because of the exacerbating effects of perspiration and heat causing itch and irritation in patients with AD, it is frequently advised that pediatric patients limit their participation in athletics because of the exacerbating effects of strenuous physical exercise on their disease.12 In one study, 429 pediatric patients or their parents/guardians completed QOL questionnaires; 89% of patients 15 years and younger with severe AD reported that their disease was impacted by athletics and outdoor activities, and 86% of these pediatric patients with severe AD responded that their social lives and leisure activities were impacted.20 Because adolescents often are involved in athletics or have mandatory physical education classes, AD may be isolating and may have a severe impact on self-esteem.
Aggressive treatment of AD with topical and systemic medications may be helpful in adolescents who may be reluctant to participate in sports because of teasing, bullying, or worsening of symptoms with heat or sweating.21 Now that dupilumab is available for adolescents, there is a chance that patients with severe and/or recalcitrant disease managed on this medication can achieve better control of their symptoms without the laboratory requirement of methotrexate and the difficulties of topical medication application, allowing them to engage in mandatory athletic classes as well as desired organized sports.
Use of Cosmetics for AD
Many adolescents experiment with cosmetics, and those with AD may use cosmetic products to cover hyperpigmented or hypopigmented lesions.18 In patients with active AD or increased sensitivity to allergens in cosmetic products, use of makeup can be a contributing factor for AD flares. Acne associated with cosmetics is especially important to consider in darker-skinned patients who may use makeup that is opaque and contains oil to conceal acne or PIH.
Allergens can be present in both cosmetics and pharmaceutical topical agents, and a Brazilian study found that approximately 89% of 813 prescription and nonprescription products (eg, topical drugs, sunscreens, moisturizers, soaps, cleansing lotions, shampoos, cosmeceuticals) contained allergens.22 Patients with AD have a higher prevalence of contact sensitization to fragrances, including balsam of Peru.23 Some AD treatments that contain fragrances have caused further skin issues in a few patients. In one case series, 3 pediatric patients developed allergic contact dermatitis to Myroxylon pereirae (balsam of Peru) when using topical treatments for their AD, and their symptoms of scalp inflammation and alopecia resolved with discontinuation.23
In a Dutch study, sensitization to Fragrance Mix I and M pereirae as well as other ingredients (eg, lanolin alcohol, Amerchol™ L 101 [a lanolin product]) was notably more common in pediatric patients with AD than in patients without AD; however, no data on patients with skin of color were included in this study.24
Because of the increased risk of sensitization to fragrances and other ingredients in patients with AD as well as the high percentage of allergens in prescription and nonprescription products, it is important to discuss all personal care products that patients may be using, not just their cosmetic products. Also, patch testing may be helpful in determining true allergens in some patients. Patch testing is recommended for patients with treatment-resistant AD, and a recent study suggested it should be done prior to long-term use of immunosuppressive agents.25 Increased steroid phobia and a push toward alternative medicines are leading both patients with AD and guardians of children with AD to look for other forms of moisturization, such as olive oil, coconut oil, sunflower seed oil, and shea butter, to decrease transepidermal water loss.26,27 An important factor in AD treatment efficacy is patient acceptability in using what is recommended.27 One study showed there was no difference in efficacy or acceptability in using a cream containing shea butter extract vs the ceramide-precursor product.27 Current data show olive oil may exacerbate dry skin and AD,26 and recommendation of any over-the-counter oils and butters in patients with AD should be made with great caution, as many of these products contain fragrances and other potential allergens.
Alternative Therapies for AD
Patients with AD often seek alternative or integrative treatment options, including dietary modifications and holistic remedies. Studies investigating the role of vitamins and supplements in treating AD are limited by sample size.28 However, there is some evidence that may support supplementation with vitamins D and E in addressing AD symptoms. The use of probiotics in treating AD is controversial, but there are studies suggesting that the use of probiotics may prove beneficial in preventing infantile AD.28 Additionally, findings from an ex vivo and in vitro study show that some conditions, including AD and acne, may benefit from the same probiotics, despite the differences in these two diseases. Both AD and acne have inflammatory and skin dysbiosis characteristics, which may be the common thread leading to both conditions potentially responding to treatment with probiotics.29
Preliminary evidence indicates that supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of AD.28 In a 20-week, randomized, single-blind, crossover study published in 2005, dietary hemp seed oil showed an improvement of clinical symptoms, including dry skin and itchiness, in patients with AD.30
In light of recent legalization in several states, patients may turn to use of cannabinoid products to manage AD. In a systematic review, cannabinoid use was reportedly a therapeutic option in the treatment of AD and AV; however, the data are based on preclinical work, and there are no randomized, placebo-controlled studies to support the use of cannabinoids.31 Furthermore, there is great concern that use of these products in adolescents is an even larger unknown.
Final Thoughts
Eighty percent of children diagnosed with AD experience symptom improvement before their early teens32; for those with AD during their preteen and teenage years, there can be psychological ramifications, as teenagers with AD report having fewer friends, are less socially involved, participate in fewer sports, and are absent from classes more often than their peers.5 In black patients with AD, school absences are even more common.6 Given the social and emotional impact of AD on patients with skin of color, it is imperative to treat the condition appropriately.33 There are areas of opportunity for further research on alternate dosing of existing treatments for AV in patients with AD, further recommendations for adolescent athletes with AD, and which cosmetic and alternative medicine products may be beneficial for this population to improve their QOL.
Providers should discuss medical management in a broader context considering patients’ extracurricular activities, treatment vehicle preferences, expectations, and personal care habits. It also is important to address the many possible factors that may influence treatment adherence early on, particularly in adolescents, as these could be barriers to treatment. This article highlights considerations for treating AD and comorbid conditions that may further complicate treatment in adolescent patients with skin of color. The information provided should serve as a guide in initial counseling and management of AD in adolescents with skin of color.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83-93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357.
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-455.
- Vivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. Int J Womens Dermatol. 2018;4:27-31.
- Wan J, Margolis DJ, Mitra N, et al. Racial and ethnic differences in atopic dermatitis–related school absences among US children [published online May 22, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.0597.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514.
- Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. 2019;31:418-425.
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33:129-135.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Lynde CW, Andriessen A, Barankin B, et al. Moisturizers and ceramide-containing moisturizers may offer concomitant therapy with benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- Taylor SC, Cook-Bolden FE, McMichael A, et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% for treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17:160-167.
- Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-236.
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402.
- Heath CR. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis. 2018;102:71-73.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Paller AS, McAlister RO, Doyle JJ, et al. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41:323-332.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35:303-316.
- Rocha VB, Machado CJ, Bittencourt FV. Presence of allergens in the vehicles of Brazilian dermatological products. Contact Dermatitis. 2017;76:126-128.
- Admani S, Goldenberg A, Jacob SE. Contact alopecia: improvement of alopecia with discontinuation of fluocinolone oil in individuals allergic to balsam fragrance. Pediatr Dermatol. 2017;34:e57-e60.
- Uter W, Werfel T, White IR, et al. Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. 2018;15:E1108.
- López-Jiménez EC, Marrero-Alemán G, Borrego L. One-third of patients with therapy-resistant atopic dermatitis may benefit after patch testing [published online May 13, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15672.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- Hon KL, Tsang YC, Pong NH, et al. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-425.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review [published online March 20, 2019]. Int J Dermatol. doi:10.1111/ijd.14404.
- Mottin VHM, Suyenaga ES. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol. 2018;57:1425-1432.
- Callaway J, Schwab U, Harvima I, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat. 2005;16:87-94.
- Eagleston LRM, Kalani NK, Patel RR, et al. Cannabinoids in dermatology: a scoping review [published June 15, 2018]. Dermatol Online J. 2018;24.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-687.e611.
- de María Díaz Granados L, Quijano MA, Ramírez PA, et al. Quality assessment of atopic dermatitis clinical practice guidelines in ≤ 18 years. Arch Dermatol Res. 2018;310:29-37.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83-93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357.
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-455.
- Vivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. Int J Womens Dermatol. 2018;4:27-31.
- Wan J, Margolis DJ, Mitra N, et al. Racial and ethnic differences in atopic dermatitis–related school absences among US children [published online May 22, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.0597.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514.
- Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. 2019;31:418-425.
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33:129-135.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Lynde CW, Andriessen A, Barankin B, et al. Moisturizers and ceramide-containing moisturizers may offer concomitant therapy with benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- Taylor SC, Cook-Bolden FE, McMichael A, et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% for treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17:160-167.
- Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-236.
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402.
- Heath CR. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis. 2018;102:71-73.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Paller AS, McAlister RO, Doyle JJ, et al. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41:323-332.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35:303-316.
- Rocha VB, Machado CJ, Bittencourt FV. Presence of allergens in the vehicles of Brazilian dermatological products. Contact Dermatitis. 2017;76:126-128.
- Admani S, Goldenberg A, Jacob SE. Contact alopecia: improvement of alopecia with discontinuation of fluocinolone oil in individuals allergic to balsam fragrance. Pediatr Dermatol. 2017;34:e57-e60.
- Uter W, Werfel T, White IR, et al. Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. 2018;15:E1108.
- López-Jiménez EC, Marrero-Alemán G, Borrego L. One-third of patients with therapy-resistant atopic dermatitis may benefit after patch testing [published online May 13, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15672.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- Hon KL, Tsang YC, Pong NH, et al. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-425.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review [published online March 20, 2019]. Int J Dermatol. doi:10.1111/ijd.14404.
- Mottin VHM, Suyenaga ES. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol. 2018;57:1425-1432.
- Callaway J, Schwab U, Harvima I, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat. 2005;16:87-94.
- Eagleston LRM, Kalani NK, Patel RR, et al. Cannabinoids in dermatology: a scoping review [published June 15, 2018]. Dermatol Online J. 2018;24.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-687.e611.
- de María Díaz Granados L, Quijano MA, Ramírez PA, et al. Quality assessment of atopic dermatitis clinical practice guidelines in ≤ 18 years. Arch Dermatol Res. 2018;310:29-37.
Practice Points
- Atopic dermatitis (AD) can be a lifelong issue that affects academic and occupational performance, with higher rates of absenteeism seen in black patients.
- The FLG loss-of-function mutation seems to play a smaller role in black patients, and other genes may be involved in skin barrier dysfunction, which could be why there is a higher rate of skin of color patients with treatment-resistant AD.
- Diagnosing AD in skin of color patients can pose a particular challenge, and severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin tones.
- There are several areas of opportunity for further research to better treat AD in this patient population and improve
quality of life.