User login
A Case of Duodenocaval Fistula in the Setting of Respiratory Failure Initially Confused for Transfusion-Related Acute Lung Injury
A duodenocaval fistula (DCF) is seen when a connection exists between the duodenum and the inferior vena cava. It is a rare entity that is commonly missed and presents a diagnostic challenge due to its nonspecific presenting symptoms.1,2 Patients commonly present with gastrointestinal (GI) bleeding or sepsis. Here we present a case of a 37-year-old man who presented to the hospital for a workup related to melena but went into cardiac arrest prior to an esophagogastroduodenoscopy. Unfortunately, on autopsy, the patient was found to have a DCF. We highlight the diagnostic challenge associated with DCF and how in this case the presentation was confused by a diagnosis of possible transfusion-related acute lung injury (TRALI). To the best of our knowledge, this is also the first description of a case of DCF associated with food embolism to the lungs causing respiratory failure.
Case Presentation
A 37-year-old man with a history significant for bulimia presented to the hospital with a 3-day history of melena and reports of dizziness. The patient did not report being on any prescribed medications but noted that he took 4 aspirin daily to “calm his nerves.” The rest of the patient’s history was unremarkable aside from a reported history of induced emesis 3 to 4 times per week for an extended period up until 2 weeks before admission.
On admission, his vital signs demonstrated tachycardia and orthostatic hypotension. Pertinent findings on physical examination were skin pallor, a normal lung examination, mild epigastric tenderness, and guaiac-positive stools. He was alert and oriented to person, place, and time with no focal deficits. His admission laboratory tests were notable for a hemoglobin (Hb) level of 4.6 g/dL (reference range, 14-17.9), a white blood cell count of 13.5 K/cm (reference range, 4.5-11), an international normalized ratio of 1.21, a blood urea nitrogen of 61 mg/dL (reference range, 10-20), and a creatinine of 2.3 mg/dL (reference range, 0.8-1.4). The patient was placed on 2 L of oxygen via nasal cannula for comfort rather than true hypoxia. A chest X-ray on admission was negative with no signs of infiltrate, edema, or widened mediastinum. An abdominal X-ray was significant for a dilated stomach consistent with bulimia with no abdominal free air or signs of obstruction. The case was discussed with the gastroenterology service who felt that the patient needed to be more hemodynamically stable before pursuing endoscopic evaluation.
He was admitted to the intensive care unit and give a transfusion of 4 units of fresh frozen plasma and 2 packed red blood cells (PRBCs) without any issues. During the infusion of a third PRBC, he developed chills, tachycardia, and hypertension with accompanying respiratory distress characterized by wheezing, decreased breath sounds bilaterally, and a decrease in oxygen saturation to 70% on 2 L supplemental oxygen. He responded to treatment with meperidine, methylprednisolone sodium succinate, albuterol nebulizer, and acetaminophen. A new chest X-ray was read as “development of pulmonary edema vs bilateral pneumonitis.” A transfusion reaction was reported to the blood bank and a diagnosis of TRALI was considered. That evening, he completed a dose of platelets and another PRBC without difficulty after he was premedicated with meperidine, methylprednisolone sodium succinate, and acetaminophen. During the night, the patient spiked a temperature of 40.3 °C that was successfully treated with a cooling blanket and acetaminophen.
The following morning the patient was found to be tachypneic and tachycardic with his face mask off. His symptoms were corrected by replacing his face mask. He claimed he felt anxious about getting more transfusions and that he had breathing problems like this at home in the recent past. The patient requested an aspirin to calm his nerves. Over the course of the day, his Hb level dropped from 6.6 g/dL to 5.9 g/dL, and 2 washed leukopoor PRBCs were ordered.
The first unit was infused uneventfully, but after 125 cc of the second unit, the patient developed respiratory distress, rigors, and hypotension to 70/58 mm Hg despite premedication. He again was treated successfully with increased face mask support. A few rales were noted, but his fluid balance was even. A second transfusion reaction was filed with the blood bank and based on the 2 transfusion-associated events with no other clear explanation for his symptoms, the clinical team favored the TRALI diagnosis. However, the blood bank was suspicious this might not be TRALI as the previous night the patient had 2 episodes of respiratory distress with drops in oxygen saturation unassociated with transfusions. The patient was clinically stable for the remainder of the night.
Early the following morning the patient was scheduled for an esophagogastroduodenoscopy to evaluate for a source of his bleeding. At the beginning of the procedure, a unit of washed leukoreduced PRBCs was hung for a Hb level of 6.9 g/dL. No bleeding source was noted in the stomach, but as the endoscope was passed into the duodenum, and after an infusion of only 25 cc of RBCs, the patient became cyanotic and went into cardiac arrest. Despite advanced resuscitation efforts over 90 minutes, the patient could not be successfully resuscitated and died while in the endoscopy suite. A transfusion reaction workup was initiated but was unremarkable. The transfusion medicine staff was suspicious that something other than TRALI was the cause of the patient’s respiratory distress as he had respiratory distress remote to the transfusions and the unit was prepared correctly before administration. The patient’s family agreed to an autopsy.
Pathology
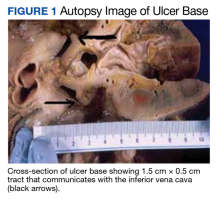
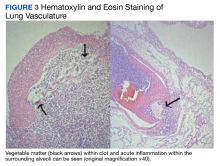
A full autopsy was performed 22 hours after the patient died. The lungs were congested and of increased weight: The right lung was 800 g, and the left was 750 g. The right lower lobe had a wedge-shaped infarction measuring 6 cm × 5 cm fed by a thrombosed vessel. Multiple small hemorrhagic wedge-shaped areas were noted in the left lung. An ulcer measuring 6 cm × 5 cm was noted just distal to the pylorus. At the base of this ulcer was a 1.5 cm × 0.5 cm tract that communicated with the inferior vena cava (Figure 1).
A postmortem blood culture was positive for Clostridium perfringens (C perfringens) and Candida albicans (C Albicans). Interestingly, one of the collected blood culture vials exploded en route to the laboratory, presumably due to the presence of many gas-forming C perfringens bacteria.
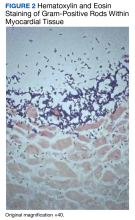
On microscopic examination of the autopsy samples, gram-positive rods were observed in the tissue of multiple organs, including the heart, lungs, liver, and kidneys (Figure 2).
Serology
Fourteen days after the patient’s death, both PRBC units infused during transfusion reactions were positive for granulocyte antibodies by immunofluorescence and agglutination techniques. Human leukocyte antigen antibody testing was also sent but was not found in either the donor or patient.
Discussion
Our case illustrates the unique and challenging diagnosis of DCF given the rarity of presentation and how quickly patients may clinically decompensate. After an extensive search of the medical literature, we were only able to identify about 40 previous cases of DCF, of which 37 were described in one review.1 DCF, although rare, should be considered at risk for forming in the following settings: migrating inferior vena cava filter, right nephrectomy and radiotherapy, duodenal peptic ulcer, abdominal trauma, and oncologic settings involving metastatic malignancy requiring radiation and/or surgical grafting of the inferior vena cava.1-4 When the diagnosis is considered, computed tomography (CT) is the best initial imaging modality as it allows for noninvasive evaluation of both the inferior vena cava and nonadjacent structures. A commonality of our case and those described in the literature is the diagnostic mystery and nonspecific symptoms patients present with, thus making CT an appropriate diagnostic modality. Endoscopy is useful for the further workup of GI bleeding and the diagnosis of peptic ulcer disease.5 In our case, given the patient’s autopsy findings and history of extensive nonsteroidal anti-inflammatory drug use, the duodenal peptic ulcer was likely the precipitating factor for his DCF.
The most challenging aspect in diagnosing DCF is that many times patients present with nonspecific symptoms, and given its rarity it is not something that is usually at the forefront of most differentials.2 This diagnostic difficulty may elucidate why there is such a relatively high mortality rate—nearly 40%—associated with DCF and why many times accurate diagnosis is not made until autopsy.1,3 The most common presenting manifestations are sepsis and/or GI bleeding; in less than half the cases described in the literature patients had both sepsis and GI bleeding. In our case, the patient had signs of melena but was not felt to be septic as his presenting signs were felt to be in the setting of blood loss and dehydration (given his history of bulimia), not an acute infectious source.
In retrospect, one of the more confounding aspects of this case is the clinical picture concerning for TRALI. The patient required supplemental oxygen throughout his hospitalization and decompensated while or after receiving a transfusion, thus having TRALI on the differential was not felt inappropriate at that time. However, this case also illustrates the power of an anchoring bias, and perhaps the clinical team anchored on the diagnosis of TRALI too quickly before considering other possible etiologies for the patient’s respiratory distress. TRALI can be one of the most challenging diagnoses to make in the field of transfusion medicine as there are no definitive diagnostic criteria.6 It is felt to be a clinical diagnosis of exclusion as there is no pathognomonic sign or diagnostic test to confirm it as the cause of the patient’s respiratory distress, though anti–human leukocyte antigen antibodies commonly are present.6,7 Considering how quickly the patient decompensated on day 2 of hospitalization and the presence of C perfringens bacteremia, which
Conclusions
Our investigation reports a case of a DCF in the setting of significant duodenal peptic ulcer disease. We highlight the diagnostic challenge that this commonly lethal etiology presents. We believe ours is the first case in which it was confused for TRALI and associated with food embolism to the lungs causing hypoxic respiratory failure. We want to highlight that DCF, though rare, should be considered for patients who present with GI bleeding and hypoxic respiratory failure.
1. Guillem PG, Binot D, Dupuy-Cuny J, et al. Duodenocaval fistula: a life-threatening condition of various origins. J Vasc Surg. 2001;33(3):643-645. doi:10.1067/mva.2001.111741
2. Ippolito D, Querques G, Drago SG, Bonaffini PA, Sironi S. Duodenocaval fistula in a patient with inferior vena cava leiomyosarcoma treated by surgical resection and caval polytetrafluoroethylene prosthesis. Case Rep Radiol. 2015;2015:1-5. doi:10.1155/2015/575961
3. Guo Y, Zhang YQ, Lin W. Radiological diagnosis of duodenocaval fistula: a case report and literature review. World J Gastroenterol. 2010;16(18):2314-2316. doi:10.3748/wjg.v16.i18.2314
4. Perera GB, Wilson SE, Barie PS, Butler JA. Duodenocaval fistula: A late complication of retroperitoneal irradiation and vena cava replacement. Ann Vasc Surg. 2004;18(1):52-58. doi:10.1007/s10016-003-0097-8
5. Addeo P, Rosso E, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg. 2012;55(1):226-229. doi:10.1016/j.jvs.2011.05.118
6. Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. doi:10.1111/j.1537-2995.2008.01948.x
7. Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36(1):53-58.
8. Yang C-C, Hsu P-C, Chang H-J, Cheng C-W, Lee M-H. Clinical significance and outcomes of clostridium perfringens bacteremia—a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e9of55-e960. doi:10.1016/j.ijid.2013.03.001
A duodenocaval fistula (DCF) is seen when a connection exists between the duodenum and the inferior vena cava. It is a rare entity that is commonly missed and presents a diagnostic challenge due to its nonspecific presenting symptoms.1,2 Patients commonly present with gastrointestinal (GI) bleeding or sepsis. Here we present a case of a 37-year-old man who presented to the hospital for a workup related to melena but went into cardiac arrest prior to an esophagogastroduodenoscopy. Unfortunately, on autopsy, the patient was found to have a DCF. We highlight the diagnostic challenge associated with DCF and how in this case the presentation was confused by a diagnosis of possible transfusion-related acute lung injury (TRALI). To the best of our knowledge, this is also the first description of a case of DCF associated with food embolism to the lungs causing respiratory failure.
Case Presentation
A 37-year-old man with a history significant for bulimia presented to the hospital with a 3-day history of melena and reports of dizziness. The patient did not report being on any prescribed medications but noted that he took 4 aspirin daily to “calm his nerves.” The rest of the patient’s history was unremarkable aside from a reported history of induced emesis 3 to 4 times per week for an extended period up until 2 weeks before admission.
On admission, his vital signs demonstrated tachycardia and orthostatic hypotension. Pertinent findings on physical examination were skin pallor, a normal lung examination, mild epigastric tenderness, and guaiac-positive stools. He was alert and oriented to person, place, and time with no focal deficits. His admission laboratory tests were notable for a hemoglobin (Hb) level of 4.6 g/dL (reference range, 14-17.9), a white blood cell count of 13.5 K/cm (reference range, 4.5-11), an international normalized ratio of 1.21, a blood urea nitrogen of 61 mg/dL (reference range, 10-20), and a creatinine of 2.3 mg/dL (reference range, 0.8-1.4). The patient was placed on 2 L of oxygen via nasal cannula for comfort rather than true hypoxia. A chest X-ray on admission was negative with no signs of infiltrate, edema, or widened mediastinum. An abdominal X-ray was significant for a dilated stomach consistent with bulimia with no abdominal free air or signs of obstruction. The case was discussed with the gastroenterology service who felt that the patient needed to be more hemodynamically stable before pursuing endoscopic evaluation.
He was admitted to the intensive care unit and give a transfusion of 4 units of fresh frozen plasma and 2 packed red blood cells (PRBCs) without any issues. During the infusion of a third PRBC, he developed chills, tachycardia, and hypertension with accompanying respiratory distress characterized by wheezing, decreased breath sounds bilaterally, and a decrease in oxygen saturation to 70% on 2 L supplemental oxygen. He responded to treatment with meperidine, methylprednisolone sodium succinate, albuterol nebulizer, and acetaminophen. A new chest X-ray was read as “development of pulmonary edema vs bilateral pneumonitis.” A transfusion reaction was reported to the blood bank and a diagnosis of TRALI was considered. That evening, he completed a dose of platelets and another PRBC without difficulty after he was premedicated with meperidine, methylprednisolone sodium succinate, and acetaminophen. During the night, the patient spiked a temperature of 40.3 °C that was successfully treated with a cooling blanket and acetaminophen.
The following morning the patient was found to be tachypneic and tachycardic with his face mask off. His symptoms were corrected by replacing his face mask. He claimed he felt anxious about getting more transfusions and that he had breathing problems like this at home in the recent past. The patient requested an aspirin to calm his nerves. Over the course of the day, his Hb level dropped from 6.6 g/dL to 5.9 g/dL, and 2 washed leukopoor PRBCs were ordered.
The first unit was infused uneventfully, but after 125 cc of the second unit, the patient developed respiratory distress, rigors, and hypotension to 70/58 mm Hg despite premedication. He again was treated successfully with increased face mask support. A few rales were noted, but his fluid balance was even. A second transfusion reaction was filed with the blood bank and based on the 2 transfusion-associated events with no other clear explanation for his symptoms, the clinical team favored the TRALI diagnosis. However, the blood bank was suspicious this might not be TRALI as the previous night the patient had 2 episodes of respiratory distress with drops in oxygen saturation unassociated with transfusions. The patient was clinically stable for the remainder of the night.
Early the following morning the patient was scheduled for an esophagogastroduodenoscopy to evaluate for a source of his bleeding. At the beginning of the procedure, a unit of washed leukoreduced PRBCs was hung for a Hb level of 6.9 g/dL. No bleeding source was noted in the stomach, but as the endoscope was passed into the duodenum, and after an infusion of only 25 cc of RBCs, the patient became cyanotic and went into cardiac arrest. Despite advanced resuscitation efforts over 90 minutes, the patient could not be successfully resuscitated and died while in the endoscopy suite. A transfusion reaction workup was initiated but was unremarkable. The transfusion medicine staff was suspicious that something other than TRALI was the cause of the patient’s respiratory distress as he had respiratory distress remote to the transfusions and the unit was prepared correctly before administration. The patient’s family agreed to an autopsy.
Pathology
A full autopsy was performed 22 hours after the patient died. The lungs were congested and of increased weight: The right lung was 800 g, and the left was 750 g. The right lower lobe had a wedge-shaped infarction measuring 6 cm × 5 cm fed by a thrombosed vessel. Multiple small hemorrhagic wedge-shaped areas were noted in the left lung. An ulcer measuring 6 cm × 5 cm was noted just distal to the pylorus. At the base of this ulcer was a 1.5 cm × 0.5 cm tract that communicated with the inferior vena cava (Figure 1).
A postmortem blood culture was positive for Clostridium perfringens (C perfringens) and Candida albicans (C Albicans). Interestingly, one of the collected blood culture vials exploded en route to the laboratory, presumably due to the presence of many gas-forming C perfringens bacteria.
On microscopic examination of the autopsy samples, gram-positive rods were observed in the tissue of multiple organs, including the heart, lungs, liver, and kidneys (Figure 2).
Serology
Fourteen days after the patient’s death, both PRBC units infused during transfusion reactions were positive for granulocyte antibodies by immunofluorescence and agglutination techniques. Human leukocyte antigen antibody testing was also sent but was not found in either the donor or patient.
Discussion
Our case illustrates the unique and challenging diagnosis of DCF given the rarity of presentation and how quickly patients may clinically decompensate. After an extensive search of the medical literature, we were only able to identify about 40 previous cases of DCF, of which 37 were described in one review.1 DCF, although rare, should be considered at risk for forming in the following settings: migrating inferior vena cava filter, right nephrectomy and radiotherapy, duodenal peptic ulcer, abdominal trauma, and oncologic settings involving metastatic malignancy requiring radiation and/or surgical grafting of the inferior vena cava.1-4 When the diagnosis is considered, computed tomography (CT) is the best initial imaging modality as it allows for noninvasive evaluation of both the inferior vena cava and nonadjacent structures. A commonality of our case and those described in the literature is the diagnostic mystery and nonspecific symptoms patients present with, thus making CT an appropriate diagnostic modality. Endoscopy is useful for the further workup of GI bleeding and the diagnosis of peptic ulcer disease.5 In our case, given the patient’s autopsy findings and history of extensive nonsteroidal anti-inflammatory drug use, the duodenal peptic ulcer was likely the precipitating factor for his DCF.
The most challenging aspect in diagnosing DCF is that many times patients present with nonspecific symptoms, and given its rarity it is not something that is usually at the forefront of most differentials.2 This diagnostic difficulty may elucidate why there is such a relatively high mortality rate—nearly 40%—associated with DCF and why many times accurate diagnosis is not made until autopsy.1,3 The most common presenting manifestations are sepsis and/or GI bleeding; in less than half the cases described in the literature patients had both sepsis and GI bleeding. In our case, the patient had signs of melena but was not felt to be septic as his presenting signs were felt to be in the setting of blood loss and dehydration (given his history of bulimia), not an acute infectious source.
In retrospect, one of the more confounding aspects of this case is the clinical picture concerning for TRALI. The patient required supplemental oxygen throughout his hospitalization and decompensated while or after receiving a transfusion, thus having TRALI on the differential was not felt inappropriate at that time. However, this case also illustrates the power of an anchoring bias, and perhaps the clinical team anchored on the diagnosis of TRALI too quickly before considering other possible etiologies for the patient’s respiratory distress. TRALI can be one of the most challenging diagnoses to make in the field of transfusion medicine as there are no definitive diagnostic criteria.6 It is felt to be a clinical diagnosis of exclusion as there is no pathognomonic sign or diagnostic test to confirm it as the cause of the patient’s respiratory distress, though anti–human leukocyte antigen antibodies commonly are present.6,7 Considering how quickly the patient decompensated on day 2 of hospitalization and the presence of C perfringens bacteremia, which
Conclusions
Our investigation reports a case of a DCF in the setting of significant duodenal peptic ulcer disease. We highlight the diagnostic challenge that this commonly lethal etiology presents. We believe ours is the first case in which it was confused for TRALI and associated with food embolism to the lungs causing hypoxic respiratory failure. We want to highlight that DCF, though rare, should be considered for patients who present with GI bleeding and hypoxic respiratory failure.
A duodenocaval fistula (DCF) is seen when a connection exists between the duodenum and the inferior vena cava. It is a rare entity that is commonly missed and presents a diagnostic challenge due to its nonspecific presenting symptoms.1,2 Patients commonly present with gastrointestinal (GI) bleeding or sepsis. Here we present a case of a 37-year-old man who presented to the hospital for a workup related to melena but went into cardiac arrest prior to an esophagogastroduodenoscopy. Unfortunately, on autopsy, the patient was found to have a DCF. We highlight the diagnostic challenge associated with DCF and how in this case the presentation was confused by a diagnosis of possible transfusion-related acute lung injury (TRALI). To the best of our knowledge, this is also the first description of a case of DCF associated with food embolism to the lungs causing respiratory failure.
Case Presentation
A 37-year-old man with a history significant for bulimia presented to the hospital with a 3-day history of melena and reports of dizziness. The patient did not report being on any prescribed medications but noted that he took 4 aspirin daily to “calm his nerves.” The rest of the patient’s history was unremarkable aside from a reported history of induced emesis 3 to 4 times per week for an extended period up until 2 weeks before admission.
On admission, his vital signs demonstrated tachycardia and orthostatic hypotension. Pertinent findings on physical examination were skin pallor, a normal lung examination, mild epigastric tenderness, and guaiac-positive stools. He was alert and oriented to person, place, and time with no focal deficits. His admission laboratory tests were notable for a hemoglobin (Hb) level of 4.6 g/dL (reference range, 14-17.9), a white blood cell count of 13.5 K/cm (reference range, 4.5-11), an international normalized ratio of 1.21, a blood urea nitrogen of 61 mg/dL (reference range, 10-20), and a creatinine of 2.3 mg/dL (reference range, 0.8-1.4). The patient was placed on 2 L of oxygen via nasal cannula for comfort rather than true hypoxia. A chest X-ray on admission was negative with no signs of infiltrate, edema, or widened mediastinum. An abdominal X-ray was significant for a dilated stomach consistent with bulimia with no abdominal free air or signs of obstruction. The case was discussed with the gastroenterology service who felt that the patient needed to be more hemodynamically stable before pursuing endoscopic evaluation.
He was admitted to the intensive care unit and give a transfusion of 4 units of fresh frozen plasma and 2 packed red blood cells (PRBCs) without any issues. During the infusion of a third PRBC, he developed chills, tachycardia, and hypertension with accompanying respiratory distress characterized by wheezing, decreased breath sounds bilaterally, and a decrease in oxygen saturation to 70% on 2 L supplemental oxygen. He responded to treatment with meperidine, methylprednisolone sodium succinate, albuterol nebulizer, and acetaminophen. A new chest X-ray was read as “development of pulmonary edema vs bilateral pneumonitis.” A transfusion reaction was reported to the blood bank and a diagnosis of TRALI was considered. That evening, he completed a dose of platelets and another PRBC without difficulty after he was premedicated with meperidine, methylprednisolone sodium succinate, and acetaminophen. During the night, the patient spiked a temperature of 40.3 °C that was successfully treated with a cooling blanket and acetaminophen.
The following morning the patient was found to be tachypneic and tachycardic with his face mask off. His symptoms were corrected by replacing his face mask. He claimed he felt anxious about getting more transfusions and that he had breathing problems like this at home in the recent past. The patient requested an aspirin to calm his nerves. Over the course of the day, his Hb level dropped from 6.6 g/dL to 5.9 g/dL, and 2 washed leukopoor PRBCs were ordered.
The first unit was infused uneventfully, but after 125 cc of the second unit, the patient developed respiratory distress, rigors, and hypotension to 70/58 mm Hg despite premedication. He again was treated successfully with increased face mask support. A few rales were noted, but his fluid balance was even. A second transfusion reaction was filed with the blood bank and based on the 2 transfusion-associated events with no other clear explanation for his symptoms, the clinical team favored the TRALI diagnosis. However, the blood bank was suspicious this might not be TRALI as the previous night the patient had 2 episodes of respiratory distress with drops in oxygen saturation unassociated with transfusions. The patient was clinically stable for the remainder of the night.
Early the following morning the patient was scheduled for an esophagogastroduodenoscopy to evaluate for a source of his bleeding. At the beginning of the procedure, a unit of washed leukoreduced PRBCs was hung for a Hb level of 6.9 g/dL. No bleeding source was noted in the stomach, but as the endoscope was passed into the duodenum, and after an infusion of only 25 cc of RBCs, the patient became cyanotic and went into cardiac arrest. Despite advanced resuscitation efforts over 90 minutes, the patient could not be successfully resuscitated and died while in the endoscopy suite. A transfusion reaction workup was initiated but was unremarkable. The transfusion medicine staff was suspicious that something other than TRALI was the cause of the patient’s respiratory distress as he had respiratory distress remote to the transfusions and the unit was prepared correctly before administration. The patient’s family agreed to an autopsy.
Pathology
A full autopsy was performed 22 hours after the patient died. The lungs were congested and of increased weight: The right lung was 800 g, and the left was 750 g. The right lower lobe had a wedge-shaped infarction measuring 6 cm × 5 cm fed by a thrombosed vessel. Multiple small hemorrhagic wedge-shaped areas were noted in the left lung. An ulcer measuring 6 cm × 5 cm was noted just distal to the pylorus. At the base of this ulcer was a 1.5 cm × 0.5 cm tract that communicated with the inferior vena cava (Figure 1).
A postmortem blood culture was positive for Clostridium perfringens (C perfringens) and Candida albicans (C Albicans). Interestingly, one of the collected blood culture vials exploded en route to the laboratory, presumably due to the presence of many gas-forming C perfringens bacteria.
On microscopic examination of the autopsy samples, gram-positive rods were observed in the tissue of multiple organs, including the heart, lungs, liver, and kidneys (Figure 2).
Serology
Fourteen days after the patient’s death, both PRBC units infused during transfusion reactions were positive for granulocyte antibodies by immunofluorescence and agglutination techniques. Human leukocyte antigen antibody testing was also sent but was not found in either the donor or patient.
Discussion
Our case illustrates the unique and challenging diagnosis of DCF given the rarity of presentation and how quickly patients may clinically decompensate. After an extensive search of the medical literature, we were only able to identify about 40 previous cases of DCF, of which 37 were described in one review.1 DCF, although rare, should be considered at risk for forming in the following settings: migrating inferior vena cava filter, right nephrectomy and radiotherapy, duodenal peptic ulcer, abdominal trauma, and oncologic settings involving metastatic malignancy requiring radiation and/or surgical grafting of the inferior vena cava.1-4 When the diagnosis is considered, computed tomography (CT) is the best initial imaging modality as it allows for noninvasive evaluation of both the inferior vena cava and nonadjacent structures. A commonality of our case and those described in the literature is the diagnostic mystery and nonspecific symptoms patients present with, thus making CT an appropriate diagnostic modality. Endoscopy is useful for the further workup of GI bleeding and the diagnosis of peptic ulcer disease.5 In our case, given the patient’s autopsy findings and history of extensive nonsteroidal anti-inflammatory drug use, the duodenal peptic ulcer was likely the precipitating factor for his DCF.
The most challenging aspect in diagnosing DCF is that many times patients present with nonspecific symptoms, and given its rarity it is not something that is usually at the forefront of most differentials.2 This diagnostic difficulty may elucidate why there is such a relatively high mortality rate—nearly 40%—associated with DCF and why many times accurate diagnosis is not made until autopsy.1,3 The most common presenting manifestations are sepsis and/or GI bleeding; in less than half the cases described in the literature patients had both sepsis and GI bleeding. In our case, the patient had signs of melena but was not felt to be septic as his presenting signs were felt to be in the setting of blood loss and dehydration (given his history of bulimia), not an acute infectious source.
In retrospect, one of the more confounding aspects of this case is the clinical picture concerning for TRALI. The patient required supplemental oxygen throughout his hospitalization and decompensated while or after receiving a transfusion, thus having TRALI on the differential was not felt inappropriate at that time. However, this case also illustrates the power of an anchoring bias, and perhaps the clinical team anchored on the diagnosis of TRALI too quickly before considering other possible etiologies for the patient’s respiratory distress. TRALI can be one of the most challenging diagnoses to make in the field of transfusion medicine as there are no definitive diagnostic criteria.6 It is felt to be a clinical diagnosis of exclusion as there is no pathognomonic sign or diagnostic test to confirm it as the cause of the patient’s respiratory distress, though anti–human leukocyte antigen antibodies commonly are present.6,7 Considering how quickly the patient decompensated on day 2 of hospitalization and the presence of C perfringens bacteremia, which
Conclusions
Our investigation reports a case of a DCF in the setting of significant duodenal peptic ulcer disease. We highlight the diagnostic challenge that this commonly lethal etiology presents. We believe ours is the first case in which it was confused for TRALI and associated with food embolism to the lungs causing hypoxic respiratory failure. We want to highlight that DCF, though rare, should be considered for patients who present with GI bleeding and hypoxic respiratory failure.
1. Guillem PG, Binot D, Dupuy-Cuny J, et al. Duodenocaval fistula: a life-threatening condition of various origins. J Vasc Surg. 2001;33(3):643-645. doi:10.1067/mva.2001.111741
2. Ippolito D, Querques G, Drago SG, Bonaffini PA, Sironi S. Duodenocaval fistula in a patient with inferior vena cava leiomyosarcoma treated by surgical resection and caval polytetrafluoroethylene prosthesis. Case Rep Radiol. 2015;2015:1-5. doi:10.1155/2015/575961
3. Guo Y, Zhang YQ, Lin W. Radiological diagnosis of duodenocaval fistula: a case report and literature review. World J Gastroenterol. 2010;16(18):2314-2316. doi:10.3748/wjg.v16.i18.2314
4. Perera GB, Wilson SE, Barie PS, Butler JA. Duodenocaval fistula: A late complication of retroperitoneal irradiation and vena cava replacement. Ann Vasc Surg. 2004;18(1):52-58. doi:10.1007/s10016-003-0097-8
5. Addeo P, Rosso E, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg. 2012;55(1):226-229. doi:10.1016/j.jvs.2011.05.118
6. Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. doi:10.1111/j.1537-2995.2008.01948.x
7. Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36(1):53-58.
8. Yang C-C, Hsu P-C, Chang H-J, Cheng C-W, Lee M-H. Clinical significance and outcomes of clostridium perfringens bacteremia—a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e9of55-e960. doi:10.1016/j.ijid.2013.03.001
1. Guillem PG, Binot D, Dupuy-Cuny J, et al. Duodenocaval fistula: a life-threatening condition of various origins. J Vasc Surg. 2001;33(3):643-645. doi:10.1067/mva.2001.111741
2. Ippolito D, Querques G, Drago SG, Bonaffini PA, Sironi S. Duodenocaval fistula in a patient with inferior vena cava leiomyosarcoma treated by surgical resection and caval polytetrafluoroethylene prosthesis. Case Rep Radiol. 2015;2015:1-5. doi:10.1155/2015/575961
3. Guo Y, Zhang YQ, Lin W. Radiological diagnosis of duodenocaval fistula: a case report and literature review. World J Gastroenterol. 2010;16(18):2314-2316. doi:10.3748/wjg.v16.i18.2314
4. Perera GB, Wilson SE, Barie PS, Butler JA. Duodenocaval fistula: A late complication of retroperitoneal irradiation and vena cava replacement. Ann Vasc Surg. 2004;18(1):52-58. doi:10.1007/s10016-003-0097-8
5. Addeo P, Rosso E, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg. 2012;55(1):226-229. doi:10.1016/j.jvs.2011.05.118
6. Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. doi:10.1111/j.1537-2995.2008.01948.x
7. Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36(1):53-58.
8. Yang C-C, Hsu P-C, Chang H-J, Cheng C-W, Lee M-H. Clinical significance and outcomes of clostridium perfringens bacteremia—a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e9of55-e960. doi:10.1016/j.ijid.2013.03.001
Oral Therapy for Aerococcus urinae Bacteremia and Thoracic Spondylodiscitis of Presumed Urinary Origin
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6 A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
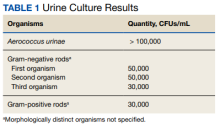
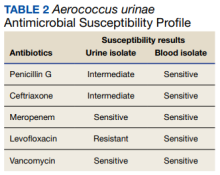
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6 A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6 A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
SARS-CoV-2: A Novel Precipitant of Ischemic Priapism
Priapism is a disorder that occurs when the penis maintains a prolonged erection in the absence of appropriate stimulation. The disorder is typically divided into subgroups based on arterial flow: low flow (ischemic) and high flow (nonischemic). Ischemic priapism is the most common form and results from venous congestion due to obstructed outflow and inability of cavernous smooth muscle to contract, resulting in compartment syndrome, tissue hypoxia, hypercapnia, and acidosis.1 Conditions that result in hypercoagulable states and hyperviscosity are associated with ischemic priapism. COVID-19 is well known to cause an acute respiratory illness and systemic inflammatory response and has been increasingly associated with coagulopathy. Studies have shown that 20% to 55% of patients admitted to the hospital for COVID-19 show objective laboratory evidence of a hypercoagulable state.2
To date, there are 6 reported cases of priapism occurring in the setting of COVID-19 with all cases demonstrating the ischemic subtype. The onset of priapism from the beginning of infectious symptoms ranged from 2 days to more than a month. Five of the cases occurred in patients with critical COVID-19 and 1 in the setting of mild disease.3-8 Two critically ill patients did not receive treatment for their ischemic priapism as they were transitioned to expectant management and/or comfort measures.Most were treated with cavernosal blood aspiration and intracavernosal injections of phenylephrine or ethylephrine. Some patients were managed with prophylactic doses of anticoagulation after the identification of priapism; others were transitioned to therapeutic doses. Two patients were followed postdischarge; one patient reported normal nighttime erections with sexual desire 2 weeks postdischarge, and another patient, who underwent a bilateral T-shunt procedure after unsuccessful phenylephrine injections, reported complete erectile dysfunction at 3 months postdischarge.4,7 There was a potentially confounding variable in 2 cases in which propofol infusions were used for sedation management in the setting of mechanical ventilation.6,8 Propofol has been linked to priapism through its blockade of sympathetic activation resulting in persistent relaxation of cavernosal smooth muscle.9 We present a unique case of COVID-19–associated ischemic priapism as our patient had moderate rather than critical COVID-19.
Case Presentation
A 67-year-old male patient presented to the emergency department for a painful erection of 34-hour duration. The patient had been exposed to COVID-19 roughly 2 months prior. Since the exposure, he had experienced headache, nonproductive cough, sore throat, and decreased appetite with weight loss. His medical history included hypertension, thoracic aortic aneurysm, B-cell type chronic lymphocytic leukemia (CLL), and obstructive sleep apnea. Daily outpatient medications included atenolol 100 mg, hydrochlorothiazide 25 mg, and omeprazole 20 mg. The patient stopped tobacco use about 30 years previously. He reported no alcohol consumption or illicit drug use and had no previous episodes of prolonged erection.
The patient was afebrile, hemodynamically stable, and had an oxygen saturation of 92% on room air. Physical examination revealed clear breath sounds and an erect circumcised penis without any lesions, discoloration, or skin necrosis. Laboratory data were remarkable for the following values: 125,660 cells/μL white blood cells (WBCs), 13.82 × 103/ μL neutrophils, 110.58 × 103/μL lymphocytes, 1.26 × 103/μL monocytes, no blasts, 9.4 gm/dL hemoglobin, 100.3 fl mean corpuscular volume, 417,000 cells/μL platelets, 23,671 ng/mL D-dimer, 29.6 seconds activated partial thromboplastin time (aPTT), 16.3 seconds prothrombin time, 743 mg/dL fibrinogen, 474 U/L lactate dehydrogenase, and 202.1 mg/dL haptoglobin. A nasopharyngeal reverse transcription polymerase chain reaction test resulted positive for the SARS-CoV-2 virus, and subsequent chest X-ray revealed bilateral, hazy opacities predominantly in a peripheral distribution. Computed tomography (CT) angiogram of the chest did not reveal pulmonary emboli, pneumothorax, effusions, or lobar consolidation. However, it displayed bilateral ground-glass opacities with interstitial consolidation worst in the upper lobes. Corporal aspiration and blood gas analysis revealed a pH of 7.05, P
Differential Diagnosis
The first consideration in the differential diagnosis of priapism is to differentiate between ischemic and nonischemic. Based on the abnormal blood gas results above, this case clearly falls within the ischemic spectrum. Ischemic priapism secondary to CLL-induced hyperleukocytosis was considered. It has been noted that up to 20% of priapism cases in adults are related to hematologic disorders.10 While it is not uncommon to see hyperleukocytosis (total WBC count > 100 × 109/L) in CLL, leukostasis is rare with most reports demonstrating WBC counts > 1000 × 109/L.11 Hematology, vascular surgery, and urology services were consulted and agreed that ischemic priapism was due to microthrombi or pelvic vein thrombosis secondary to COVID-19–associated coagulopathy (CAC) was the most likely etiology.
Treatment
After corporal aspiration, intracorporal phenylephrine was administered. Diluted phenylephrine (100 ug/mL) was injected every 5 to 10 minutes while intermittently aspirating and irrigating multiple sites along the lateral length of the penile shaft. This initial procedure reduced the erection from 100% to 30% rigidity, with repeat blood gas analysis revealing minimal improvement. CT of the abdomen and pelvis with IV contrast revealed no evidence of pelvic thrombi. A second round of phenylephrine injections were administered, resulting in detumescence. The patient was treated with 2 to 3 L/min of oxygen supplementation via nasal cannula, a 5-day course of remdesivir and low-intensity heparin drip. Following the initial low-intensity heparin drip, the patient transitioned to therapeutic enoxaparin and subsequently was discharged on apixaban for a 3-month course. Since discharge, the patient followed up with hematology. He tolerated and completed the anticoagulation regimen without any recurrences of priapism or residual deficits.
Discussion
Recent studies have overwhelmingly analyzed the incidence and presentation of thrombotic complications in critically ill patients with COVID-19. CAC has been postulated to result from endotheliopathy along with immune cell activation and propagation of coagulation. While COVID-19 has been noted to create lung injury through binding angiotensin-converting enzyme 2 receptors expressed on alveolar pneumocytes, it increasingly has been found to affect endothelial cells throughout the body. Recent postmortem analyses have demonstrated direct viral infection of endothelial cells with consequent diffuse endothelial inflammation, as evidenced by viral inclusions, sequestered immune cells, and endothelial apoptosis.12,13 Manifestations of this endotheliopathy have been delineated through various studies.
An early retrospective study in Wuhan, China, illustrated that 36% of the first 99 patients hospitalized with COVID-19 demonstrated an elevated D-dimer, 6% an elevated aPTT, and 5% an elevated prothrombin time.14 Another retrospective study conducted in Wuhan found a 25% incidence of venous thromboembolic complications in critically ill patients with severe COVID-19.15 In the Netherlands, a study reported the incidence of arterial and venous thrombotic complications to be 31% in 184 critically ill patients with COVID-19, with 81% of these cases involving pulmonary emboli.16
To our knowledge, our patient is the seventh reported case of ischemic priapism occurring in the setting of a COVID-19 infection, and the first to have occurred in its moderate form. Ischemic priapism is often a consequence of penile venous outflow obstruction and resultant stasis of hypoxic blood.7 The prothrombotic state induced by CAC has been proposed to cause the obstruction of small emissary veins in the subtunical space and in turn lead to venous stasis, which propagates the formation of ischemic priapism.8 Furthermore, 4 of the previously reported cases shared laboratory data on their patients, and all demonstrated elevated D-dimer and fibrinogen levels, which strengthens this hypothesis.3,5,7,8 CLL presents a potential confounding variable in this case; however, as we have reviewed earlier, the risk of leukostasis at WBC counts < 1000 × 109/L is very low.11 It is also probable that the patient had some level of immune dysregulation secondary to CLL, leading to his prolonged course and slow clearance of the virus.
Conclusions
Although only a handful of CAC cases leading to ischemic priapism have been reported, the true incidence may be much higher. While our case highlights the importance of considering COVID-19 infection in the differential diagnosis of ischemic priapism, more research is needed to understand incidence and definitively establish a causative relationship.
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1(1):116-120. doi:10.1111/j.1743-6109.2004.10117.x
2. Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21):E583. doi:10.1503/cmaj.200685
3. Lam G, McCarthy R, Haider R. A peculiar case of priapism: the hypercoagulable state in patients with severe COVID-19 infection. Eur J Case Rep Intern Med. 2020;7(8):001779. doi:10.12890/2020_001779
4. Addar A, Al Fraidi O, Nazer A, Althonayan N, Ghazwani Y. Priapism for 10 days in a patient with SARS-CoV-2 pneumonia: a case report. J Surg Case Rep. 2021;2021(4):rjab020. doi:10.1093/jscr/rjab020
5. Lamamri M, Chebbi A, Mamane J, et al. Priapism in a patient with coronavirus disease 2019 (COVID-19). Am J Emerg Med. 2021;39:251.e5-251.e7. doi:10.1016/j.ajem.2020.06.027
6. Silverman ML, VanDerVeer SJ, Donnelly TJ. Priapism in COVID-19: a thromboembolic complication. Am J Emerg Med. 2021;45:686.e5-686.e6. doi:10.1016/j.ajem.2020.12.072
7. Giuliano AFM, Vulpi M, Passerini F, et al. SARS-CoV-2 infection as a determining factor to the precipitation of ischemic priapism in a young patient with asymptomatic COVID-19. Case Rep Urol. 2021;2021:9936891. doi:10.1155/2021/9936891
8. Carreno BD, Perez CP, Vasquez D, Oyola JA, Suarez O, Bedoya C. Veno-occlusive priapism in COVID-19 disease. Urol Int. 2021;105(9-10):916-919. doi:10.1159/000514421
9. Senthilkumaran S, Shah S, Ganapathysubramanian, Balamurgan N, Thirumalaikolundusubramanian P. Propofol and priapism. Indian J Pharmacol. 2010;42(4):238-239. doi:10.4103/0253-7613.68430
10. Qu M, Lu X, Wang L, Liu Z, Sun Y, Gao X. Priapism secondary to chronic myeloid leukemia treated by a surgical cavernosa-corpus spongiosum shunt: case report. Asian J Urol. 2019;6(4):373-376. doi:10.1016/j.ajur.2018.12.004
11. Singh N, Singh Lubana S, Dabrowski L, Sidhu G. Leukostasis in chronic lymphocytic leukemia. Am J Case Rep. 2020;21:e924798. doi:10.12659/AJCR.924798
12. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. doi:10.1016/S0140-6736(20)30937-5
13. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. doi:10.1182/blood.2020006000
14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
15. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. doi:10.1111/jth.14830
16. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
Priapism is a disorder that occurs when the penis maintains a prolonged erection in the absence of appropriate stimulation. The disorder is typically divided into subgroups based on arterial flow: low flow (ischemic) and high flow (nonischemic). Ischemic priapism is the most common form and results from venous congestion due to obstructed outflow and inability of cavernous smooth muscle to contract, resulting in compartment syndrome, tissue hypoxia, hypercapnia, and acidosis.1 Conditions that result in hypercoagulable states and hyperviscosity are associated with ischemic priapism. COVID-19 is well known to cause an acute respiratory illness and systemic inflammatory response and has been increasingly associated with coagulopathy. Studies have shown that 20% to 55% of patients admitted to the hospital for COVID-19 show objective laboratory evidence of a hypercoagulable state.2
To date, there are 6 reported cases of priapism occurring in the setting of COVID-19 with all cases demonstrating the ischemic subtype. The onset of priapism from the beginning of infectious symptoms ranged from 2 days to more than a month. Five of the cases occurred in patients with critical COVID-19 and 1 in the setting of mild disease.3-8 Two critically ill patients did not receive treatment for their ischemic priapism as they were transitioned to expectant management and/or comfort measures.Most were treated with cavernosal blood aspiration and intracavernosal injections of phenylephrine or ethylephrine. Some patients were managed with prophylactic doses of anticoagulation after the identification of priapism; others were transitioned to therapeutic doses. Two patients were followed postdischarge; one patient reported normal nighttime erections with sexual desire 2 weeks postdischarge, and another patient, who underwent a bilateral T-shunt procedure after unsuccessful phenylephrine injections, reported complete erectile dysfunction at 3 months postdischarge.4,7 There was a potentially confounding variable in 2 cases in which propofol infusions were used for sedation management in the setting of mechanical ventilation.6,8 Propofol has been linked to priapism through its blockade of sympathetic activation resulting in persistent relaxation of cavernosal smooth muscle.9 We present a unique case of COVID-19–associated ischemic priapism as our patient had moderate rather than critical COVID-19.
Case Presentation
A 67-year-old male patient presented to the emergency department for a painful erection of 34-hour duration. The patient had been exposed to COVID-19 roughly 2 months prior. Since the exposure, he had experienced headache, nonproductive cough, sore throat, and decreased appetite with weight loss. His medical history included hypertension, thoracic aortic aneurysm, B-cell type chronic lymphocytic leukemia (CLL), and obstructive sleep apnea. Daily outpatient medications included atenolol 100 mg, hydrochlorothiazide 25 mg, and omeprazole 20 mg. The patient stopped tobacco use about 30 years previously. He reported no alcohol consumption or illicit drug use and had no previous episodes of prolonged erection.
The patient was afebrile, hemodynamically stable, and had an oxygen saturation of 92% on room air. Physical examination revealed clear breath sounds and an erect circumcised penis without any lesions, discoloration, or skin necrosis. Laboratory data were remarkable for the following values: 125,660 cells/μL white blood cells (WBCs), 13.82 × 103/ μL neutrophils, 110.58 × 103/μL lymphocytes, 1.26 × 103/μL monocytes, no blasts, 9.4 gm/dL hemoglobin, 100.3 fl mean corpuscular volume, 417,000 cells/μL platelets, 23,671 ng/mL D-dimer, 29.6 seconds activated partial thromboplastin time (aPTT), 16.3 seconds prothrombin time, 743 mg/dL fibrinogen, 474 U/L lactate dehydrogenase, and 202.1 mg/dL haptoglobin. A nasopharyngeal reverse transcription polymerase chain reaction test resulted positive for the SARS-CoV-2 virus, and subsequent chest X-ray revealed bilateral, hazy opacities predominantly in a peripheral distribution. Computed tomography (CT) angiogram of the chest did not reveal pulmonary emboli, pneumothorax, effusions, or lobar consolidation. However, it displayed bilateral ground-glass opacities with interstitial consolidation worst in the upper lobes. Corporal aspiration and blood gas analysis revealed a pH of 7.05, P
Differential Diagnosis
The first consideration in the differential diagnosis of priapism is to differentiate between ischemic and nonischemic. Based on the abnormal blood gas results above, this case clearly falls within the ischemic spectrum. Ischemic priapism secondary to CLL-induced hyperleukocytosis was considered. It has been noted that up to 20% of priapism cases in adults are related to hematologic disorders.10 While it is not uncommon to see hyperleukocytosis (total WBC count > 100 × 109/L) in CLL, leukostasis is rare with most reports demonstrating WBC counts > 1000 × 109/L.11 Hematology, vascular surgery, and urology services were consulted and agreed that ischemic priapism was due to microthrombi or pelvic vein thrombosis secondary to COVID-19–associated coagulopathy (CAC) was the most likely etiology.
Treatment
After corporal aspiration, intracorporal phenylephrine was administered. Diluted phenylephrine (100 ug/mL) was injected every 5 to 10 minutes while intermittently aspirating and irrigating multiple sites along the lateral length of the penile shaft. This initial procedure reduced the erection from 100% to 30% rigidity, with repeat blood gas analysis revealing minimal improvement. CT of the abdomen and pelvis with IV contrast revealed no evidence of pelvic thrombi. A second round of phenylephrine injections were administered, resulting in detumescence. The patient was treated with 2 to 3 L/min of oxygen supplementation via nasal cannula, a 5-day course of remdesivir and low-intensity heparin drip. Following the initial low-intensity heparin drip, the patient transitioned to therapeutic enoxaparin and subsequently was discharged on apixaban for a 3-month course. Since discharge, the patient followed up with hematology. He tolerated and completed the anticoagulation regimen without any recurrences of priapism or residual deficits.
Discussion
Recent studies have overwhelmingly analyzed the incidence and presentation of thrombotic complications in critically ill patients with COVID-19. CAC has been postulated to result from endotheliopathy along with immune cell activation and propagation of coagulation. While COVID-19 has been noted to create lung injury through binding angiotensin-converting enzyme 2 receptors expressed on alveolar pneumocytes, it increasingly has been found to affect endothelial cells throughout the body. Recent postmortem analyses have demonstrated direct viral infection of endothelial cells with consequent diffuse endothelial inflammation, as evidenced by viral inclusions, sequestered immune cells, and endothelial apoptosis.12,13 Manifestations of this endotheliopathy have been delineated through various studies.
An early retrospective study in Wuhan, China, illustrated that 36% of the first 99 patients hospitalized with COVID-19 demonstrated an elevated D-dimer, 6% an elevated aPTT, and 5% an elevated prothrombin time.14 Another retrospective study conducted in Wuhan found a 25% incidence of venous thromboembolic complications in critically ill patients with severe COVID-19.15 In the Netherlands, a study reported the incidence of arterial and venous thrombotic complications to be 31% in 184 critically ill patients with COVID-19, with 81% of these cases involving pulmonary emboli.16
To our knowledge, our patient is the seventh reported case of ischemic priapism occurring in the setting of a COVID-19 infection, and the first to have occurred in its moderate form. Ischemic priapism is often a consequence of penile venous outflow obstruction and resultant stasis of hypoxic blood.7 The prothrombotic state induced by CAC has been proposed to cause the obstruction of small emissary veins in the subtunical space and in turn lead to venous stasis, which propagates the formation of ischemic priapism.8 Furthermore, 4 of the previously reported cases shared laboratory data on their patients, and all demonstrated elevated D-dimer and fibrinogen levels, which strengthens this hypothesis.3,5,7,8 CLL presents a potential confounding variable in this case; however, as we have reviewed earlier, the risk of leukostasis at WBC counts < 1000 × 109/L is very low.11 It is also probable that the patient had some level of immune dysregulation secondary to CLL, leading to his prolonged course and slow clearance of the virus.
Conclusions
Although only a handful of CAC cases leading to ischemic priapism have been reported, the true incidence may be much higher. While our case highlights the importance of considering COVID-19 infection in the differential diagnosis of ischemic priapism, more research is needed to understand incidence and definitively establish a causative relationship.
Priapism is a disorder that occurs when the penis maintains a prolonged erection in the absence of appropriate stimulation. The disorder is typically divided into subgroups based on arterial flow: low flow (ischemic) and high flow (nonischemic). Ischemic priapism is the most common form and results from venous congestion due to obstructed outflow and inability of cavernous smooth muscle to contract, resulting in compartment syndrome, tissue hypoxia, hypercapnia, and acidosis.1 Conditions that result in hypercoagulable states and hyperviscosity are associated with ischemic priapism. COVID-19 is well known to cause an acute respiratory illness and systemic inflammatory response and has been increasingly associated with coagulopathy. Studies have shown that 20% to 55% of patients admitted to the hospital for COVID-19 show objective laboratory evidence of a hypercoagulable state.2
To date, there are 6 reported cases of priapism occurring in the setting of COVID-19 with all cases demonstrating the ischemic subtype. The onset of priapism from the beginning of infectious symptoms ranged from 2 days to more than a month. Five of the cases occurred in patients with critical COVID-19 and 1 in the setting of mild disease.3-8 Two critically ill patients did not receive treatment for their ischemic priapism as they were transitioned to expectant management and/or comfort measures.Most were treated with cavernosal blood aspiration and intracavernosal injections of phenylephrine or ethylephrine. Some patients were managed with prophylactic doses of anticoagulation after the identification of priapism; others were transitioned to therapeutic doses. Two patients were followed postdischarge; one patient reported normal nighttime erections with sexual desire 2 weeks postdischarge, and another patient, who underwent a bilateral T-shunt procedure after unsuccessful phenylephrine injections, reported complete erectile dysfunction at 3 months postdischarge.4,7 There was a potentially confounding variable in 2 cases in which propofol infusions were used for sedation management in the setting of mechanical ventilation.6,8 Propofol has been linked to priapism through its blockade of sympathetic activation resulting in persistent relaxation of cavernosal smooth muscle.9 We present a unique case of COVID-19–associated ischemic priapism as our patient had moderate rather than critical COVID-19.
Case Presentation
A 67-year-old male patient presented to the emergency department for a painful erection of 34-hour duration. The patient had been exposed to COVID-19 roughly 2 months prior. Since the exposure, he had experienced headache, nonproductive cough, sore throat, and decreased appetite with weight loss. His medical history included hypertension, thoracic aortic aneurysm, B-cell type chronic lymphocytic leukemia (CLL), and obstructive sleep apnea. Daily outpatient medications included atenolol 100 mg, hydrochlorothiazide 25 mg, and omeprazole 20 mg. The patient stopped tobacco use about 30 years previously. He reported no alcohol consumption or illicit drug use and had no previous episodes of prolonged erection.
The patient was afebrile, hemodynamically stable, and had an oxygen saturation of 92% on room air. Physical examination revealed clear breath sounds and an erect circumcised penis without any lesions, discoloration, or skin necrosis. Laboratory data were remarkable for the following values: 125,660 cells/μL white blood cells (WBCs), 13.82 × 103/ μL neutrophils, 110.58 × 103/μL lymphocytes, 1.26 × 103/μL monocytes, no blasts, 9.4 gm/dL hemoglobin, 100.3 fl mean corpuscular volume, 417,000 cells/μL platelets, 23,671 ng/mL D-dimer, 29.6 seconds activated partial thromboplastin time (aPTT), 16.3 seconds prothrombin time, 743 mg/dL fibrinogen, 474 U/L lactate dehydrogenase, and 202.1 mg/dL haptoglobin. A nasopharyngeal reverse transcription polymerase chain reaction test resulted positive for the SARS-CoV-2 virus, and subsequent chest X-ray revealed bilateral, hazy opacities predominantly in a peripheral distribution. Computed tomography (CT) angiogram of the chest did not reveal pulmonary emboli, pneumothorax, effusions, or lobar consolidation. However, it displayed bilateral ground-glass opacities with interstitial consolidation worst in the upper lobes. Corporal aspiration and blood gas analysis revealed a pH of 7.05, P
Differential Diagnosis
The first consideration in the differential diagnosis of priapism is to differentiate between ischemic and nonischemic. Based on the abnormal blood gas results above, this case clearly falls within the ischemic spectrum. Ischemic priapism secondary to CLL-induced hyperleukocytosis was considered. It has been noted that up to 20% of priapism cases in adults are related to hematologic disorders.10 While it is not uncommon to see hyperleukocytosis (total WBC count > 100 × 109/L) in CLL, leukostasis is rare with most reports demonstrating WBC counts > 1000 × 109/L.11 Hematology, vascular surgery, and urology services were consulted and agreed that ischemic priapism was due to microthrombi or pelvic vein thrombosis secondary to COVID-19–associated coagulopathy (CAC) was the most likely etiology.
Treatment
After corporal aspiration, intracorporal phenylephrine was administered. Diluted phenylephrine (100 ug/mL) was injected every 5 to 10 minutes while intermittently aspirating and irrigating multiple sites along the lateral length of the penile shaft. This initial procedure reduced the erection from 100% to 30% rigidity, with repeat blood gas analysis revealing minimal improvement. CT of the abdomen and pelvis with IV contrast revealed no evidence of pelvic thrombi. A second round of phenylephrine injections were administered, resulting in detumescence. The patient was treated with 2 to 3 L/min of oxygen supplementation via nasal cannula, a 5-day course of remdesivir and low-intensity heparin drip. Following the initial low-intensity heparin drip, the patient transitioned to therapeutic enoxaparin and subsequently was discharged on apixaban for a 3-month course. Since discharge, the patient followed up with hematology. He tolerated and completed the anticoagulation regimen without any recurrences of priapism or residual deficits.
Discussion
Recent studies have overwhelmingly analyzed the incidence and presentation of thrombotic complications in critically ill patients with COVID-19. CAC has been postulated to result from endotheliopathy along with immune cell activation and propagation of coagulation. While COVID-19 has been noted to create lung injury through binding angiotensin-converting enzyme 2 receptors expressed on alveolar pneumocytes, it increasingly has been found to affect endothelial cells throughout the body. Recent postmortem analyses have demonstrated direct viral infection of endothelial cells with consequent diffuse endothelial inflammation, as evidenced by viral inclusions, sequestered immune cells, and endothelial apoptosis.12,13 Manifestations of this endotheliopathy have been delineated through various studies.
An early retrospective study in Wuhan, China, illustrated that 36% of the first 99 patients hospitalized with COVID-19 demonstrated an elevated D-dimer, 6% an elevated aPTT, and 5% an elevated prothrombin time.14 Another retrospective study conducted in Wuhan found a 25% incidence of venous thromboembolic complications in critically ill patients with severe COVID-19.15 In the Netherlands, a study reported the incidence of arterial and venous thrombotic complications to be 31% in 184 critically ill patients with COVID-19, with 81% of these cases involving pulmonary emboli.16
To our knowledge, our patient is the seventh reported case of ischemic priapism occurring in the setting of a COVID-19 infection, and the first to have occurred in its moderate form. Ischemic priapism is often a consequence of penile venous outflow obstruction and resultant stasis of hypoxic blood.7 The prothrombotic state induced by CAC has been proposed to cause the obstruction of small emissary veins in the subtunical space and in turn lead to venous stasis, which propagates the formation of ischemic priapism.8 Furthermore, 4 of the previously reported cases shared laboratory data on their patients, and all demonstrated elevated D-dimer and fibrinogen levels, which strengthens this hypothesis.3,5,7,8 CLL presents a potential confounding variable in this case; however, as we have reviewed earlier, the risk of leukostasis at WBC counts < 1000 × 109/L is very low.11 It is also probable that the patient had some level of immune dysregulation secondary to CLL, leading to his prolonged course and slow clearance of the virus.
Conclusions
Although only a handful of CAC cases leading to ischemic priapism have been reported, the true incidence may be much higher. While our case highlights the importance of considering COVID-19 infection in the differential diagnosis of ischemic priapism, more research is needed to understand incidence and definitively establish a causative relationship.
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1(1):116-120. doi:10.1111/j.1743-6109.2004.10117.x
2. Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21):E583. doi:10.1503/cmaj.200685
3. Lam G, McCarthy R, Haider R. A peculiar case of priapism: the hypercoagulable state in patients with severe COVID-19 infection. Eur J Case Rep Intern Med. 2020;7(8):001779. doi:10.12890/2020_001779
4. Addar A, Al Fraidi O, Nazer A, Althonayan N, Ghazwani Y. Priapism for 10 days in a patient with SARS-CoV-2 pneumonia: a case report. J Surg Case Rep. 2021;2021(4):rjab020. doi:10.1093/jscr/rjab020
5. Lamamri M, Chebbi A, Mamane J, et al. Priapism in a patient with coronavirus disease 2019 (COVID-19). Am J Emerg Med. 2021;39:251.e5-251.e7. doi:10.1016/j.ajem.2020.06.027
6. Silverman ML, VanDerVeer SJ, Donnelly TJ. Priapism in COVID-19: a thromboembolic complication. Am J Emerg Med. 2021;45:686.e5-686.e6. doi:10.1016/j.ajem.2020.12.072
7. Giuliano AFM, Vulpi M, Passerini F, et al. SARS-CoV-2 infection as a determining factor to the precipitation of ischemic priapism in a young patient with asymptomatic COVID-19. Case Rep Urol. 2021;2021:9936891. doi:10.1155/2021/9936891
8. Carreno BD, Perez CP, Vasquez D, Oyola JA, Suarez O, Bedoya C. Veno-occlusive priapism in COVID-19 disease. Urol Int. 2021;105(9-10):916-919. doi:10.1159/000514421
9. Senthilkumaran S, Shah S, Ganapathysubramanian, Balamurgan N, Thirumalaikolundusubramanian P. Propofol and priapism. Indian J Pharmacol. 2010;42(4):238-239. doi:10.4103/0253-7613.68430
10. Qu M, Lu X, Wang L, Liu Z, Sun Y, Gao X. Priapism secondary to chronic myeloid leukemia treated by a surgical cavernosa-corpus spongiosum shunt: case report. Asian J Urol. 2019;6(4):373-376. doi:10.1016/j.ajur.2018.12.004
11. Singh N, Singh Lubana S, Dabrowski L, Sidhu G. Leukostasis in chronic lymphocytic leukemia. Am J Case Rep. 2020;21:e924798. doi:10.12659/AJCR.924798
12. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. doi:10.1016/S0140-6736(20)30937-5
13. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. doi:10.1182/blood.2020006000
14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
15. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. doi:10.1111/jth.14830
16. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1(1):116-120. doi:10.1111/j.1743-6109.2004.10117.x
2. Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21):E583. doi:10.1503/cmaj.200685
3. Lam G, McCarthy R, Haider R. A peculiar case of priapism: the hypercoagulable state in patients with severe COVID-19 infection. Eur J Case Rep Intern Med. 2020;7(8):001779. doi:10.12890/2020_001779
4. Addar A, Al Fraidi O, Nazer A, Althonayan N, Ghazwani Y. Priapism for 10 days in a patient with SARS-CoV-2 pneumonia: a case report. J Surg Case Rep. 2021;2021(4):rjab020. doi:10.1093/jscr/rjab020
5. Lamamri M, Chebbi A, Mamane J, et al. Priapism in a patient with coronavirus disease 2019 (COVID-19). Am J Emerg Med. 2021;39:251.e5-251.e7. doi:10.1016/j.ajem.2020.06.027
6. Silverman ML, VanDerVeer SJ, Donnelly TJ. Priapism in COVID-19: a thromboembolic complication. Am J Emerg Med. 2021;45:686.e5-686.e6. doi:10.1016/j.ajem.2020.12.072
7. Giuliano AFM, Vulpi M, Passerini F, et al. SARS-CoV-2 infection as a determining factor to the precipitation of ischemic priapism in a young patient with asymptomatic COVID-19. Case Rep Urol. 2021;2021:9936891. doi:10.1155/2021/9936891
8. Carreno BD, Perez CP, Vasquez D, Oyola JA, Suarez O, Bedoya C. Veno-occlusive priapism in COVID-19 disease. Urol Int. 2021;105(9-10):916-919. doi:10.1159/000514421
9. Senthilkumaran S, Shah S, Ganapathysubramanian, Balamurgan N, Thirumalaikolundusubramanian P. Propofol and priapism. Indian J Pharmacol. 2010;42(4):238-239. doi:10.4103/0253-7613.68430
10. Qu M, Lu X, Wang L, Liu Z, Sun Y, Gao X. Priapism secondary to chronic myeloid leukemia treated by a surgical cavernosa-corpus spongiosum shunt: case report. Asian J Urol. 2019;6(4):373-376. doi:10.1016/j.ajur.2018.12.004
11. Singh N, Singh Lubana S, Dabrowski L, Sidhu G. Leukostasis in chronic lymphocytic leukemia. Am J Case Rep. 2020;21:e924798. doi:10.12659/AJCR.924798
12. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. doi:10.1016/S0140-6736(20)30937-5
13. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. doi:10.1182/blood.2020006000
14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
15. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. doi:10.1111/jth.14830
16. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013