User login
A Case of Duodenocaval Fistula in the Setting of Respiratory Failure Initially Confused for Transfusion-Related Acute Lung Injury
A duodenocaval fistula (DCF) is seen when a connection exists between the duodenum and the inferior vena cava. It is a rare entity that is commonly missed and presents a diagnostic challenge due to its nonspecific presenting symptoms.1,2 Patients commonly present with gastrointestinal (GI) bleeding or sepsis. Here we present a case of a 37-year-old man who presented to the hospital for a workup related to melena but went into cardiac arrest prior to an esophagogastroduodenoscopy. Unfortunately, on autopsy, the patient was found to have a DCF. We highlight the diagnostic challenge associated with DCF and how in this case the presentation was confused by a diagnosis of possible transfusion-related acute lung injury (TRALI). To the best of our knowledge, this is also the first description of a case of DCF associated with food embolism to the lungs causing respiratory failure.
Case Presentation
A 37-year-old man with a history significant for bulimia presented to the hospital with a 3-day history of melena and reports of dizziness. The patient did not report being on any prescribed medications but noted that he took 4 aspirin daily to “calm his nerves.” The rest of the patient’s history was unremarkable aside from a reported history of induced emesis 3 to 4 times per week for an extended period up until 2 weeks before admission.
On admission, his vital signs demonstrated tachycardia and orthostatic hypotension. Pertinent findings on physical examination were skin pallor, a normal lung examination, mild epigastric tenderness, and guaiac-positive stools. He was alert and oriented to person, place, and time with no focal deficits. His admission laboratory tests were notable for a hemoglobin (Hb) level of 4.6 g/dL (reference range, 14-17.9), a white blood cell count of 13.5 K/cm (reference range, 4.5-11), an international normalized ratio of 1.21, a blood urea nitrogen of 61 mg/dL (reference range, 10-20), and a creatinine of 2.3 mg/dL (reference range, 0.8-1.4). The patient was placed on 2 L of oxygen via nasal cannula for comfort rather than true hypoxia. A chest X-ray on admission was negative with no signs of infiltrate, edema, or widened mediastinum. An abdominal X-ray was significant for a dilated stomach consistent with bulimia with no abdominal free air or signs of obstruction. The case was discussed with the gastroenterology service who felt that the patient needed to be more hemodynamically stable before pursuing endoscopic evaluation.
He was admitted to the intensive care unit and give a transfusion of 4 units of fresh frozen plasma and 2 packed red blood cells (PRBCs) without any issues. During the infusion of a third PRBC, he developed chills, tachycardia, and hypertension with accompanying respiratory distress characterized by wheezing, decreased breath sounds bilaterally, and a decrease in oxygen saturation to 70% on 2 L supplemental oxygen. He responded to treatment with meperidine, methylprednisolone sodium succinate, albuterol nebulizer, and acetaminophen. A new chest X-ray was read as “development of pulmonary edema vs bilateral pneumonitis.” A transfusion reaction was reported to the blood bank and a diagnosis of TRALI was considered. That evening, he completed a dose of platelets and another PRBC without difficulty after he was premedicated with meperidine, methylprednisolone sodium succinate, and acetaminophen. During the night, the patient spiked a temperature of 40.3 °C that was successfully treated with a cooling blanket and acetaminophen.
The following morning the patient was found to be tachypneic and tachycardic with his face mask off. His symptoms were corrected by replacing his face mask. He claimed he felt anxious about getting more transfusions and that he had breathing problems like this at home in the recent past. The patient requested an aspirin to calm his nerves. Over the course of the day, his Hb level dropped from 6.6 g/dL to 5.9 g/dL, and 2 washed leukopoor PRBCs were ordered.
The first unit was infused uneventfully, but after 125 cc of the second unit, the patient developed respiratory distress, rigors, and hypotension to 70/58 mm Hg despite premedication. He again was treated successfully with increased face mask support. A few rales were noted, but his fluid balance was even. A second transfusion reaction was filed with the blood bank and based on the 2 transfusion-associated events with no other clear explanation for his symptoms, the clinical team favored the TRALI diagnosis. However, the blood bank was suspicious this might not be TRALI as the previous night the patient had 2 episodes of respiratory distress with drops in oxygen saturation unassociated with transfusions. The patient was clinically stable for the remainder of the night.
Early the following morning the patient was scheduled for an esophagogastroduodenoscopy to evaluate for a source of his bleeding. At the beginning of the procedure, a unit of washed leukoreduced PRBCs was hung for a Hb level of 6.9 g/dL. No bleeding source was noted in the stomach, but as the endoscope was passed into the duodenum, and after an infusion of only 25 cc of RBCs, the patient became cyanotic and went into cardiac arrest. Despite advanced resuscitation efforts over 90 minutes, the patient could not be successfully resuscitated and died while in the endoscopy suite. A transfusion reaction workup was initiated but was unremarkable. The transfusion medicine staff was suspicious that something other than TRALI was the cause of the patient’s respiratory distress as he had respiratory distress remote to the transfusions and the unit was prepared correctly before administration. The patient’s family agreed to an autopsy.
Pathology
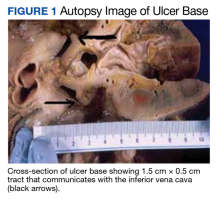
A full autopsy was performed 22 hours after the patient died. The lungs were congested and of increased weight: The right lung was 800 g, and the left was 750 g. The right lower lobe had a wedge-shaped infarction measuring 6 cm × 5 cm fed by a thrombosed vessel. Multiple small hemorrhagic wedge-shaped areas were noted in the left lung. An ulcer measuring 6 cm × 5 cm was noted just distal to the pylorus. At the base of this ulcer was a 1.5 cm × 0.5 cm tract that communicated with the inferior vena cava (Figure 1).
A postmortem blood culture was positive for Clostridium perfringens (C perfringens) and Candida albicans (C Albicans). Interestingly, one of the collected blood culture vials exploded en route to the laboratory, presumably due to the presence of many gas-forming C perfringens bacteria.
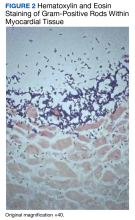
On microscopic examination of the autopsy samples, gram-positive rods were observed in the tissue of multiple organs, including the heart, lungs, liver, and kidneys (Figure 2).
Serology
Fourteen days after the patient’s death, both PRBC units infused during transfusion reactions were positive for granulocyte antibodies by immunofluorescence and agglutination techniques. Human leukocyte antigen antibody testing was also sent but was not found in either the donor or patient.
Discussion
Our case illustrates the unique and challenging diagnosis of DCF given the rarity of presentation and how quickly patients may clinically decompensate. After an extensive search of the medical literature, we were only able to identify about 40 previous cases of DCF, of which 37 were described in one review.1 DCF, although rare, should be considered at risk for forming in the following settings: migrating inferior vena cava filter, right nephrectomy and radiotherapy, duodenal peptic ulcer, abdominal trauma, and oncologic settings involving metastatic malignancy requiring radiation and/or surgical grafting of the inferior vena cava.1-4 When the diagnosis is considered, computed tomography (CT) is the best initial imaging modality as it allows for noninvasive evaluation of both the inferior vena cava and nonadjacent structures. A commonality of our case and those described in the literature is the diagnostic mystery and nonspecific symptoms patients present with, thus making CT an appropriate diagnostic modality. Endoscopy is useful for the further workup of GI bleeding and the diagnosis of peptic ulcer disease.5 In our case, given the patient’s autopsy findings and history of extensive nonsteroidal anti-inflammatory drug use, the duodenal peptic ulcer was likely the precipitating factor for his DCF.
The most challenging aspect in diagnosing DCF is that many times patients present with nonspecific symptoms, and given its rarity it is not something that is usually at the forefront of most differentials.2 This diagnostic difficulty may elucidate why there is such a relatively high mortality rate—nearly 40%—associated with DCF and why many times accurate diagnosis is not made until autopsy.1,3 The most common presenting manifestations are sepsis and/or GI bleeding; in less than half the cases described in the literature patients had both sepsis and GI bleeding. In our case, the patient had signs of melena but was not felt to be septic as his presenting signs were felt to be in the setting of blood loss and dehydration (given his history of bulimia), not an acute infectious source.
In retrospect, one of the more confounding aspects of this case is the clinical picture concerning for TRALI. The patient required supplemental oxygen throughout his hospitalization and decompensated while or after receiving a transfusion, thus having TRALI on the differential was not felt inappropriate at that time. However, this case also illustrates the power of an anchoring bias, and perhaps the clinical team anchored on the diagnosis of TRALI too quickly before considering other possible etiologies for the patient’s respiratory distress. TRALI can be one of the most challenging diagnoses to make in the field of transfusion medicine as there are no definitive diagnostic criteria.6 It is felt to be a clinical diagnosis of exclusion as there is no pathognomonic sign or diagnostic test to confirm it as the cause of the patient’s respiratory distress, though anti–human leukocyte antigen antibodies commonly are present.6,7 Considering how quickly the patient decompensated on day 2 of hospitalization and the presence of C perfringens bacteremia, which
Conclusions
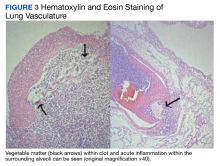
Our investigation reports a case of a DCF in the setting of significant duodenal peptic ulcer disease. We highlight the diagnostic challenge that this commonly lethal etiology presents. We believe ours is the first case in which it was confused for TRALI and associated with food embolism to the lungs causing hypoxic respiratory failure. We want to highlight that DCF, though rare, should be considered for patients who present with GI bleeding and hypoxic respiratory failure.
1. Guillem PG, Binot D, Dupuy-Cuny J, et al. Duodenocaval fistula: a life-threatening condition of various origins. J Vasc Surg. 2001;33(3):643-645. doi:10.1067/mva.2001.111741
2. Ippolito D, Querques G, Drago SG, Bonaffini PA, Sironi S. Duodenocaval fistula in a patient with inferior vena cava leiomyosarcoma treated by surgical resection and caval polytetrafluoroethylene prosthesis. Case Rep Radiol. 2015;2015:1-5. doi:10.1155/2015/575961
3. Guo Y, Zhang YQ, Lin W. Radiological diagnosis of duodenocaval fistula: a case report and literature review. World J Gastroenterol. 2010;16(18):2314-2316. doi:10.3748/wjg.v16.i18.2314
4. Perera GB, Wilson SE, Barie PS, Butler JA. Duodenocaval fistula: A late complication of retroperitoneal irradiation and vena cava replacement. Ann Vasc Surg. 2004;18(1):52-58. doi:10.1007/s10016-003-0097-8
5. Addeo P, Rosso E, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg. 2012;55(1):226-229. doi:10.1016/j.jvs.2011.05.118
6. Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. doi:10.1111/j.1537-2995.2008.01948.x
7. Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36(1):53-58.
8. Yang C-C, Hsu P-C, Chang H-J, Cheng C-W, Lee M-H. Clinical significance and outcomes of clostridium perfringens bacteremia—a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e9of55-e960. doi:10.1016/j.ijid.2013.03.001
A duodenocaval fistula (DCF) is seen when a connection exists between the duodenum and the inferior vena cava. It is a rare entity that is commonly missed and presents a diagnostic challenge due to its nonspecific presenting symptoms.1,2 Patients commonly present with gastrointestinal (GI) bleeding or sepsis. Here we present a case of a 37-year-old man who presented to the hospital for a workup related to melena but went into cardiac arrest prior to an esophagogastroduodenoscopy. Unfortunately, on autopsy, the patient was found to have a DCF. We highlight the diagnostic challenge associated with DCF and how in this case the presentation was confused by a diagnosis of possible transfusion-related acute lung injury (TRALI). To the best of our knowledge, this is also the first description of a case of DCF associated with food embolism to the lungs causing respiratory failure.
Case Presentation
A 37-year-old man with a history significant for bulimia presented to the hospital with a 3-day history of melena and reports of dizziness. The patient did not report being on any prescribed medications but noted that he took 4 aspirin daily to “calm his nerves.” The rest of the patient’s history was unremarkable aside from a reported history of induced emesis 3 to 4 times per week for an extended period up until 2 weeks before admission.
On admission, his vital signs demonstrated tachycardia and orthostatic hypotension. Pertinent findings on physical examination were skin pallor, a normal lung examination, mild epigastric tenderness, and guaiac-positive stools. He was alert and oriented to person, place, and time with no focal deficits. His admission laboratory tests were notable for a hemoglobin (Hb) level of 4.6 g/dL (reference range, 14-17.9), a white blood cell count of 13.5 K/cm (reference range, 4.5-11), an international normalized ratio of 1.21, a blood urea nitrogen of 61 mg/dL (reference range, 10-20), and a creatinine of 2.3 mg/dL (reference range, 0.8-1.4). The patient was placed on 2 L of oxygen via nasal cannula for comfort rather than true hypoxia. A chest X-ray on admission was negative with no signs of infiltrate, edema, or widened mediastinum. An abdominal X-ray was significant for a dilated stomach consistent with bulimia with no abdominal free air or signs of obstruction. The case was discussed with the gastroenterology service who felt that the patient needed to be more hemodynamically stable before pursuing endoscopic evaluation.
He was admitted to the intensive care unit and give a transfusion of 4 units of fresh frozen plasma and 2 packed red blood cells (PRBCs) without any issues. During the infusion of a third PRBC, he developed chills, tachycardia, and hypertension with accompanying respiratory distress characterized by wheezing, decreased breath sounds bilaterally, and a decrease in oxygen saturation to 70% on 2 L supplemental oxygen. He responded to treatment with meperidine, methylprednisolone sodium succinate, albuterol nebulizer, and acetaminophen. A new chest X-ray was read as “development of pulmonary edema vs bilateral pneumonitis.” A transfusion reaction was reported to the blood bank and a diagnosis of TRALI was considered. That evening, he completed a dose of platelets and another PRBC without difficulty after he was premedicated with meperidine, methylprednisolone sodium succinate, and acetaminophen. During the night, the patient spiked a temperature of 40.3 °C that was successfully treated with a cooling blanket and acetaminophen.
The following morning the patient was found to be tachypneic and tachycardic with his face mask off. His symptoms were corrected by replacing his face mask. He claimed he felt anxious about getting more transfusions and that he had breathing problems like this at home in the recent past. The patient requested an aspirin to calm his nerves. Over the course of the day, his Hb level dropped from 6.6 g/dL to 5.9 g/dL, and 2 washed leukopoor PRBCs were ordered.
The first unit was infused uneventfully, but after 125 cc of the second unit, the patient developed respiratory distress, rigors, and hypotension to 70/58 mm Hg despite premedication. He again was treated successfully with increased face mask support. A few rales were noted, but his fluid balance was even. A second transfusion reaction was filed with the blood bank and based on the 2 transfusion-associated events with no other clear explanation for his symptoms, the clinical team favored the TRALI diagnosis. However, the blood bank was suspicious this might not be TRALI as the previous night the patient had 2 episodes of respiratory distress with drops in oxygen saturation unassociated with transfusions. The patient was clinically stable for the remainder of the night.
Early the following morning the patient was scheduled for an esophagogastroduodenoscopy to evaluate for a source of his bleeding. At the beginning of the procedure, a unit of washed leukoreduced PRBCs was hung for a Hb level of 6.9 g/dL. No bleeding source was noted in the stomach, but as the endoscope was passed into the duodenum, and after an infusion of only 25 cc of RBCs, the patient became cyanotic and went into cardiac arrest. Despite advanced resuscitation efforts over 90 minutes, the patient could not be successfully resuscitated and died while in the endoscopy suite. A transfusion reaction workup was initiated but was unremarkable. The transfusion medicine staff was suspicious that something other than TRALI was the cause of the patient’s respiratory distress as he had respiratory distress remote to the transfusions and the unit was prepared correctly before administration. The patient’s family agreed to an autopsy.
Pathology
A full autopsy was performed 22 hours after the patient died. The lungs were congested and of increased weight: The right lung was 800 g, and the left was 750 g. The right lower lobe had a wedge-shaped infarction measuring 6 cm × 5 cm fed by a thrombosed vessel. Multiple small hemorrhagic wedge-shaped areas were noted in the left lung. An ulcer measuring 6 cm × 5 cm was noted just distal to the pylorus. At the base of this ulcer was a 1.5 cm × 0.5 cm tract that communicated with the inferior vena cava (Figure 1).
A postmortem blood culture was positive for Clostridium perfringens (C perfringens) and Candida albicans (C Albicans). Interestingly, one of the collected blood culture vials exploded en route to the laboratory, presumably due to the presence of many gas-forming C perfringens bacteria.
On microscopic examination of the autopsy samples, gram-positive rods were observed in the tissue of multiple organs, including the heart, lungs, liver, and kidneys (Figure 2).
Serology
Fourteen days after the patient’s death, both PRBC units infused during transfusion reactions were positive for granulocyte antibodies by immunofluorescence and agglutination techniques. Human leukocyte antigen antibody testing was also sent but was not found in either the donor or patient.
Discussion
Our case illustrates the unique and challenging diagnosis of DCF given the rarity of presentation and how quickly patients may clinically decompensate. After an extensive search of the medical literature, we were only able to identify about 40 previous cases of DCF, of which 37 were described in one review.1 DCF, although rare, should be considered at risk for forming in the following settings: migrating inferior vena cava filter, right nephrectomy and radiotherapy, duodenal peptic ulcer, abdominal trauma, and oncologic settings involving metastatic malignancy requiring radiation and/or surgical grafting of the inferior vena cava.1-4 When the diagnosis is considered, computed tomography (CT) is the best initial imaging modality as it allows for noninvasive evaluation of both the inferior vena cava and nonadjacent structures. A commonality of our case and those described in the literature is the diagnostic mystery and nonspecific symptoms patients present with, thus making CT an appropriate diagnostic modality. Endoscopy is useful for the further workup of GI bleeding and the diagnosis of peptic ulcer disease.5 In our case, given the patient’s autopsy findings and history of extensive nonsteroidal anti-inflammatory drug use, the duodenal peptic ulcer was likely the precipitating factor for his DCF.
The most challenging aspect in diagnosing DCF is that many times patients present with nonspecific symptoms, and given its rarity it is not something that is usually at the forefront of most differentials.2 This diagnostic difficulty may elucidate why there is such a relatively high mortality rate—nearly 40%—associated with DCF and why many times accurate diagnosis is not made until autopsy.1,3 The most common presenting manifestations are sepsis and/or GI bleeding; in less than half the cases described in the literature patients had both sepsis and GI bleeding. In our case, the patient had signs of melena but was not felt to be septic as his presenting signs were felt to be in the setting of blood loss and dehydration (given his history of bulimia), not an acute infectious source.
In retrospect, one of the more confounding aspects of this case is the clinical picture concerning for TRALI. The patient required supplemental oxygen throughout his hospitalization and decompensated while or after receiving a transfusion, thus having TRALI on the differential was not felt inappropriate at that time. However, this case also illustrates the power of an anchoring bias, and perhaps the clinical team anchored on the diagnosis of TRALI too quickly before considering other possible etiologies for the patient’s respiratory distress. TRALI can be one of the most challenging diagnoses to make in the field of transfusion medicine as there are no definitive diagnostic criteria.6 It is felt to be a clinical diagnosis of exclusion as there is no pathognomonic sign or diagnostic test to confirm it as the cause of the patient’s respiratory distress, though anti–human leukocyte antigen antibodies commonly are present.6,7 Considering how quickly the patient decompensated on day 2 of hospitalization and the presence of C perfringens bacteremia, which
Conclusions
Our investigation reports a case of a DCF in the setting of significant duodenal peptic ulcer disease. We highlight the diagnostic challenge that this commonly lethal etiology presents. We believe ours is the first case in which it was confused for TRALI and associated with food embolism to the lungs causing hypoxic respiratory failure. We want to highlight that DCF, though rare, should be considered for patients who present with GI bleeding and hypoxic respiratory failure.
A duodenocaval fistula (DCF) is seen when a connection exists between the duodenum and the inferior vena cava. It is a rare entity that is commonly missed and presents a diagnostic challenge due to its nonspecific presenting symptoms.1,2 Patients commonly present with gastrointestinal (GI) bleeding or sepsis. Here we present a case of a 37-year-old man who presented to the hospital for a workup related to melena but went into cardiac arrest prior to an esophagogastroduodenoscopy. Unfortunately, on autopsy, the patient was found to have a DCF. We highlight the diagnostic challenge associated with DCF and how in this case the presentation was confused by a diagnosis of possible transfusion-related acute lung injury (TRALI). To the best of our knowledge, this is also the first description of a case of DCF associated with food embolism to the lungs causing respiratory failure.
Case Presentation
A 37-year-old man with a history significant for bulimia presented to the hospital with a 3-day history of melena and reports of dizziness. The patient did not report being on any prescribed medications but noted that he took 4 aspirin daily to “calm his nerves.” The rest of the patient’s history was unremarkable aside from a reported history of induced emesis 3 to 4 times per week for an extended period up until 2 weeks before admission.
On admission, his vital signs demonstrated tachycardia and orthostatic hypotension. Pertinent findings on physical examination were skin pallor, a normal lung examination, mild epigastric tenderness, and guaiac-positive stools. He was alert and oriented to person, place, and time with no focal deficits. His admission laboratory tests were notable for a hemoglobin (Hb) level of 4.6 g/dL (reference range, 14-17.9), a white blood cell count of 13.5 K/cm (reference range, 4.5-11), an international normalized ratio of 1.21, a blood urea nitrogen of 61 mg/dL (reference range, 10-20), and a creatinine of 2.3 mg/dL (reference range, 0.8-1.4). The patient was placed on 2 L of oxygen via nasal cannula for comfort rather than true hypoxia. A chest X-ray on admission was negative with no signs of infiltrate, edema, or widened mediastinum. An abdominal X-ray was significant for a dilated stomach consistent with bulimia with no abdominal free air or signs of obstruction. The case was discussed with the gastroenterology service who felt that the patient needed to be more hemodynamically stable before pursuing endoscopic evaluation.
He was admitted to the intensive care unit and give a transfusion of 4 units of fresh frozen plasma and 2 packed red blood cells (PRBCs) without any issues. During the infusion of a third PRBC, he developed chills, tachycardia, and hypertension with accompanying respiratory distress characterized by wheezing, decreased breath sounds bilaterally, and a decrease in oxygen saturation to 70% on 2 L supplemental oxygen. He responded to treatment with meperidine, methylprednisolone sodium succinate, albuterol nebulizer, and acetaminophen. A new chest X-ray was read as “development of pulmonary edema vs bilateral pneumonitis.” A transfusion reaction was reported to the blood bank and a diagnosis of TRALI was considered. That evening, he completed a dose of platelets and another PRBC without difficulty after he was premedicated with meperidine, methylprednisolone sodium succinate, and acetaminophen. During the night, the patient spiked a temperature of 40.3 °C that was successfully treated with a cooling blanket and acetaminophen.
The following morning the patient was found to be tachypneic and tachycardic with his face mask off. His symptoms were corrected by replacing his face mask. He claimed he felt anxious about getting more transfusions and that he had breathing problems like this at home in the recent past. The patient requested an aspirin to calm his nerves. Over the course of the day, his Hb level dropped from 6.6 g/dL to 5.9 g/dL, and 2 washed leukopoor PRBCs were ordered.
The first unit was infused uneventfully, but after 125 cc of the second unit, the patient developed respiratory distress, rigors, and hypotension to 70/58 mm Hg despite premedication. He again was treated successfully with increased face mask support. A few rales were noted, but his fluid balance was even. A second transfusion reaction was filed with the blood bank and based on the 2 transfusion-associated events with no other clear explanation for his symptoms, the clinical team favored the TRALI diagnosis. However, the blood bank was suspicious this might not be TRALI as the previous night the patient had 2 episodes of respiratory distress with drops in oxygen saturation unassociated with transfusions. The patient was clinically stable for the remainder of the night.
Early the following morning the patient was scheduled for an esophagogastroduodenoscopy to evaluate for a source of his bleeding. At the beginning of the procedure, a unit of washed leukoreduced PRBCs was hung for a Hb level of 6.9 g/dL. No bleeding source was noted in the stomach, but as the endoscope was passed into the duodenum, and after an infusion of only 25 cc of RBCs, the patient became cyanotic and went into cardiac arrest. Despite advanced resuscitation efforts over 90 minutes, the patient could not be successfully resuscitated and died while in the endoscopy suite. A transfusion reaction workup was initiated but was unremarkable. The transfusion medicine staff was suspicious that something other than TRALI was the cause of the patient’s respiratory distress as he had respiratory distress remote to the transfusions and the unit was prepared correctly before administration. The patient’s family agreed to an autopsy.
Pathology
A full autopsy was performed 22 hours after the patient died. The lungs were congested and of increased weight: The right lung was 800 g, and the left was 750 g. The right lower lobe had a wedge-shaped infarction measuring 6 cm × 5 cm fed by a thrombosed vessel. Multiple small hemorrhagic wedge-shaped areas were noted in the left lung. An ulcer measuring 6 cm × 5 cm was noted just distal to the pylorus. At the base of this ulcer was a 1.5 cm × 0.5 cm tract that communicated with the inferior vena cava (Figure 1).
A postmortem blood culture was positive for Clostridium perfringens (C perfringens) and Candida albicans (C Albicans). Interestingly, one of the collected blood culture vials exploded en route to the laboratory, presumably due to the presence of many gas-forming C perfringens bacteria.
On microscopic examination of the autopsy samples, gram-positive rods were observed in the tissue of multiple organs, including the heart, lungs, liver, and kidneys (Figure 2).
Serology
Fourteen days after the patient’s death, both PRBC units infused during transfusion reactions were positive for granulocyte antibodies by immunofluorescence and agglutination techniques. Human leukocyte antigen antibody testing was also sent but was not found in either the donor or patient.
Discussion
Our case illustrates the unique and challenging diagnosis of DCF given the rarity of presentation and how quickly patients may clinically decompensate. After an extensive search of the medical literature, we were only able to identify about 40 previous cases of DCF, of which 37 were described in one review.1 DCF, although rare, should be considered at risk for forming in the following settings: migrating inferior vena cava filter, right nephrectomy and radiotherapy, duodenal peptic ulcer, abdominal trauma, and oncologic settings involving metastatic malignancy requiring radiation and/or surgical grafting of the inferior vena cava.1-4 When the diagnosis is considered, computed tomography (CT) is the best initial imaging modality as it allows for noninvasive evaluation of both the inferior vena cava and nonadjacent structures. A commonality of our case and those described in the literature is the diagnostic mystery and nonspecific symptoms patients present with, thus making CT an appropriate diagnostic modality. Endoscopy is useful for the further workup of GI bleeding and the diagnosis of peptic ulcer disease.5 In our case, given the patient’s autopsy findings and history of extensive nonsteroidal anti-inflammatory drug use, the duodenal peptic ulcer was likely the precipitating factor for his DCF.
The most challenging aspect in diagnosing DCF is that many times patients present with nonspecific symptoms, and given its rarity it is not something that is usually at the forefront of most differentials.2 This diagnostic difficulty may elucidate why there is such a relatively high mortality rate—nearly 40%—associated with DCF and why many times accurate diagnosis is not made until autopsy.1,3 The most common presenting manifestations are sepsis and/or GI bleeding; in less than half the cases described in the literature patients had both sepsis and GI bleeding. In our case, the patient had signs of melena but was not felt to be septic as his presenting signs were felt to be in the setting of blood loss and dehydration (given his history of bulimia), not an acute infectious source.
In retrospect, one of the more confounding aspects of this case is the clinical picture concerning for TRALI. The patient required supplemental oxygen throughout his hospitalization and decompensated while or after receiving a transfusion, thus having TRALI on the differential was not felt inappropriate at that time. However, this case also illustrates the power of an anchoring bias, and perhaps the clinical team anchored on the diagnosis of TRALI too quickly before considering other possible etiologies for the patient’s respiratory distress. TRALI can be one of the most challenging diagnoses to make in the field of transfusion medicine as there are no definitive diagnostic criteria.6 It is felt to be a clinical diagnosis of exclusion as there is no pathognomonic sign or diagnostic test to confirm it as the cause of the patient’s respiratory distress, though anti–human leukocyte antigen antibodies commonly are present.6,7 Considering how quickly the patient decompensated on day 2 of hospitalization and the presence of C perfringens bacteremia, which
Conclusions
Our investigation reports a case of a DCF in the setting of significant duodenal peptic ulcer disease. We highlight the diagnostic challenge that this commonly lethal etiology presents. We believe ours is the first case in which it was confused for TRALI and associated with food embolism to the lungs causing hypoxic respiratory failure. We want to highlight that DCF, though rare, should be considered for patients who present with GI bleeding and hypoxic respiratory failure.
1. Guillem PG, Binot D, Dupuy-Cuny J, et al. Duodenocaval fistula: a life-threatening condition of various origins. J Vasc Surg. 2001;33(3):643-645. doi:10.1067/mva.2001.111741
2. Ippolito D, Querques G, Drago SG, Bonaffini PA, Sironi S. Duodenocaval fistula in a patient with inferior vena cava leiomyosarcoma treated by surgical resection and caval polytetrafluoroethylene prosthesis. Case Rep Radiol. 2015;2015:1-5. doi:10.1155/2015/575961
3. Guo Y, Zhang YQ, Lin W. Radiological diagnosis of duodenocaval fistula: a case report and literature review. World J Gastroenterol. 2010;16(18):2314-2316. doi:10.3748/wjg.v16.i18.2314
4. Perera GB, Wilson SE, Barie PS, Butler JA. Duodenocaval fistula: A late complication of retroperitoneal irradiation and vena cava replacement. Ann Vasc Surg. 2004;18(1):52-58. doi:10.1007/s10016-003-0097-8
5. Addeo P, Rosso E, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg. 2012;55(1):226-229. doi:10.1016/j.jvs.2011.05.118
6. Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. doi:10.1111/j.1537-2995.2008.01948.x
7. Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36(1):53-58.
8. Yang C-C, Hsu P-C, Chang H-J, Cheng C-W, Lee M-H. Clinical significance and outcomes of clostridium perfringens bacteremia—a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e9of55-e960. doi:10.1016/j.ijid.2013.03.001
1. Guillem PG, Binot D, Dupuy-Cuny J, et al. Duodenocaval fistula: a life-threatening condition of various origins. J Vasc Surg. 2001;33(3):643-645. doi:10.1067/mva.2001.111741
2. Ippolito D, Querques G, Drago SG, Bonaffini PA, Sironi S. Duodenocaval fistula in a patient with inferior vena cava leiomyosarcoma treated by surgical resection and caval polytetrafluoroethylene prosthesis. Case Rep Radiol. 2015;2015:1-5. doi:10.1155/2015/575961
3. Guo Y, Zhang YQ, Lin W. Radiological diagnosis of duodenocaval fistula: a case report and literature review. World J Gastroenterol. 2010;16(18):2314-2316. doi:10.3748/wjg.v16.i18.2314
4. Perera GB, Wilson SE, Barie PS, Butler JA. Duodenocaval fistula: A late complication of retroperitoneal irradiation and vena cava replacement. Ann Vasc Surg. 2004;18(1):52-58. doi:10.1007/s10016-003-0097-8
5. Addeo P, Rosso E, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg. 2012;55(1):226-229. doi:10.1016/j.jvs.2011.05.118
6. Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. doi:10.1111/j.1537-2995.2008.01948.x
7. Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36(1):53-58.
8. Yang C-C, Hsu P-C, Chang H-J, Cheng C-W, Lee M-H. Clinical significance and outcomes of clostridium perfringens bacteremia—a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e9of55-e960. doi:10.1016/j.ijid.2013.03.001