User login
Medical Liability for the Gastroenterologist
While nearly 75% of physicians in low-risk specialties and 99% of physicians in high-risk specialties may face a malpractice claim in their careers,1 malpractice is rarely discussed openly in medical school, residency, fellowship, or even amongst colleagues. Indeed, one study suggested that more than 10% of practicing gastroenterologists may face a malpractice claim,2 with gastroenterologists expected to spend 10-15% of their careers with an outstanding malpractice claim3 as cases may take 27-29 months to resolve on average.4
Believing that if a physician is sued, one must have done something “wrong” or that speaking about one’s experience may implicate a colleague, creates an intense stigma and isolation that only serves to aggravate the “second victim syndrome” (SVS) that is well documented in the surgical literature.2 Herein,
What is Malpractice? Why Do Physicians Get Sued?
Malpractice is defined as negligence on the part of a physician which causes physical or emotional damage to the patient. This requires a variety of legal issues to be evaluated (e.g. breach of duty between the physicians and patient, breach of standard of care), that often center around the question: would a “reasonable, careful, and prudent” doctor behave in the same manner in the same circumstance?
While some fields of medicine lend themselves better to algorithmic applications of highly evidence-based guidelines, many aspects of GI care and endoscopic practice are highly physician/patient-specific, dependent on local expertise, and based on low-quality evidence. As a result, an assessment of negligence may be quite subjective, depending on the expert retained by a plaintiff. Conflicting expert testimony on what professional custom is and whether practice deviates may hinge on technical details that may or may not be appreciated by a lay jury.
Plaintiffs must prove both that they have sustained an injury and that the injury (emotional or physical) was due to the physician’s negligence. While this may be straightforward in a “slip-and-fall” tort claim, medical malpractice claims usually involve sick patients with multiple comorbidities, where assigning causality to a single intervention/misinterpretation/missed opportunity is difficult to weigh against competing causes of adverse outcomes. Assessing a specific liability requires that the plaintiff prove this to a “more likely than not” standard which may be part of the reason why only 30% of cases are closed with indemnity payments, a figure that has not changed significantly in the past decade.4
While the perception amongst physicians is that tort legislation is ever increasing, data from the National Practitioner Data Bank (NPDB) demonstrates that the number of paid claims against physicians has decreased by 75% in the last 20 years.5 This may reflect a progressive improvement in the quality of care delivered or success of “tort reform” on the state level to limit damages and “nuisance” lawsuits. However, another more problematic possibility is that with the corporatization of medicine, an untold number of physicians may be removed from litigation as a named party, with their institution shielding them from reporting. While the number of cases may or may not be declining, the average indemnity payment appears to be rising to $330,000 on average,4 with one study suggesting a significant growth in paid claims in gastroenterology.6
Historically, studies of closed malpractice claims have demonstrated that 59% involved diagnostic errors involving a cancer diagnosis,7 though why this actually happens may be for a wide variety of reasons including errors in the development of a differential diagnosis, ordering of an appropriate diagnostic test, interpretation of the diagnostic test, or follow-up of an abnormality identified.
What are the Intended/Unintended Consequences of Litigation?
The objective of our tort system is to compensate patients for economic damages (medical costs and lost wages) and non-economic damages (pain and suffering), and to ideally deter negligent behavior of providers. Interestingly, data from the NPDB have suggested that approximately 1% of all physicians account for 32% of all paid claims, with the same study showing that among physicians with paid claims, 4% had at least 3.8
While certain fields are obviously more prone to litigation, the risk of additional claims on a physician with 3 prior claims was more than 3 times that of physicians with 1 lifetime claim. One would assume that the system was built to drive out a small proportion of “bad actors.” Indeed, similar data from the NPDB has demonstrated that the number of claims against physicians was associated both with their leaving the practice of medicine and relocating to smaller practice settings.9
Another frequent question is whether the threat of litigation drives “defensive medicine” (i.e. medical care that is not beneficial) or avoidance medicine (i.e. excluding high risk patients and procedures from ones’ practice). These behaviors have been well documented in physicians around the world,10 as well as several surveys of gastroenterologists specifically suggesting regular ordering of unnecessary imaging/endoscopy and referrals of patients to specialists that may not be necessary.11,12
However, does defensive medicine work: does spending more prevent you from being the target of a lawsuit? In an observational study in Florida from 2000-2009, researchers demonstrated that across specialties, greater average spending by physicians was associated with a reduced risk of incurring a malpractice claim. Indeed, the likelihood of a top quintile spending internist having a malpractice incident vs a bottom quintile spending internist was 0.3% vs 1.5%.13
Approximately 10.4-43.3% of physicians may experience SVS, experiencing trauma after an adverse patient event/medical error, manifesting with psychological trauma (shame, guilt, anxiety) and cognitive limitations (burnout, stress).2 Significant emotional consequences are common on the part of the physician and have well-documented stages to recovery,14 which if ignored may lead to long-term detrimental mental/emotional health of the physician and their future patients.
Specifically, in one study, 80.8% of physicians who had a closed malpractice claim reported significant emotional distress (regardless of the legal outcome), with frequent reports of mood symptoms that affected professional conduct.15 Recognizing these effects and implementing peer counseling and institutional support may help to expedite recovery and mitigate future adverse career outcomes.14
Anatomy/Timeline of a Liability Lawsuit
Medical malpractice cases are heard in state courts, in the jurisdictions where the care was provided. From the time an event occurs to when a jury verdict may be rendered may take 4-5 years or more depending on the local statute of limitations, discovery process, backlog of the local case docket, and specific circumstances of the case. The length of time is important to consider given the likelihood that a physician may advance in training or move practice locations during the course of litigation. Several common myths surrounding this process are summarized in the accompanying box, titled “Myths Surrounding Medical Liability Litigation.”
The plaintiff faces a statute of limitations to file a lawsuit that may range from 1-6 years depending on the state. The first indication that legal action may be pending will generally be a plaintiff’s formal request for medical records. After these records are reviewed, the plaintiff’s attorney will consult one or more experts (often credentialed in the same specialty) to assess if the case is viable and to ultimately form the basis of an affidavit of merit from a plaintiff expert.
Once the lawsuit is filed, the physician(s) named will be assigned an attorney by their employer/insurance company. A state medical board malpractice questionnaire will generally follow that will seek to independently evaluate the alleged malpractice with interrogatives to determine if censure is warranted. There is a formal response to the plaintiff’s petition by the defense and then the discovery phase begins where both sides depose the defendants/plaintiffs and retain medical experts that are favorable to their arguments.
In choosing potential “experts,” physicians must ensure that they are willing/able to be present for a potential trial, do not have any personal/professional/academic conflicts with the defendants, and are willing to provide compelling testimony to a jury. A pre-trial conference and trial date is set which may be >12 months away depending on the local docket. While the amount of time a trial may take is variable, it may be up to 5-7 days that the defendants are expected to be in court in addition to days where depositions are being taken.
During the discovery process, dismissal of the physician from the lawsuit is pursued. In addition, settlement negotiations generally proceed in parallel with discovery process and may result in a pre-trial/pre-verdict settlement. Once a verdict is reached, whether for the plaintiff or the defendant, the case may be appealed, and the trial preparation process may be repeated.
Conclusions
Awareness of the medical liability process is critical for trainees and attendings alike, given the high likelihood of litigation in a gastroenterologist’s career. Specific considerations like local tort law and malpractice coverage are important to be familiar. Ongoing health services research help to shape our understanding on the intended and unintended consequences of litigation on medicine, though detailed data on outcomes/settlements are limited by confidentiality agreements, which may hamper efforts to improve patient safety.
Dr. Das is associate professor of medicine in the Division of Gastroenterology at Washington University School of Medicine, St. Louis, Missouri. He has served as a consultant for Olympus, but has no other relevant conflicts.
References
1. Jena AB, et al. Malpractice Risk According to Physician Specialty. N Engl J Med. 2011 Aug. doi: 10.1056/NEJMsa1012370.
2. Chong RIH, et al. Scoping review of the second victim syndrome among surgeons: Understanding the impact, responses, and support systems. Am J Surg 2024 Mar. doi: 10.1016/j.amjsurg.2023.09.045.
3. Seabury S, et al. On Average, Physicians Spend Nearly 11 Percent Of Their 40-Year Careers With An Open, Unresolved Malpractice Claim. Health Aff Proj Hope. 2013 Jan. doi: 10.1377/hlthaff.2012.0967.
4. CRICO Strategies. Medical Malpractice in America: A 10-Year Asessment with Insights. 2018. Accessed Apr 28, 2025.
5. Studdert DM, Hall MA. Medical Malpractice Law — Doctrine and Dynamics. N Engl J Med 2022 Oct. doi: 10.1056/NEJMp2201675.
6. Schaffer AC, et al. Rates and Characteristics of Paid Malpractice Claims Among US Physicians by Specialty, 1992-2014. JAMA Intern Med. 2017 May. doi: 10.1001/jamainternmed.2017.0311.
7. Gandhi TK, et al. Missed and Delayed Diagnoses in the Ambulatory Setting: A Study of Closed Malpractice Claims. Ann Intern Med. 2006 Oct. doi: 10.7326/0003-4819-145-7-200610030-00006.
8. Studdert DM, et al. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N Engl J Med. 2016 Jan. doi: 10.1056/NEJMsa1506137.
9. Studdert DM, et al. Changes in Practice among Physicians with Malpractice Claims. N Engl J Med. 2019 Mar. doi: 10.1056/NEJMsa1809981.
10. Ries NM, Jansen J. Physicians’ views and experiences of defensive medicine: An international review of empirical research. Health Policy. 2021 May. doi: 10.1016/j.healthpol.2021.02.005.
11. Hiyama T, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006 Dec. doi: 10.3748/wjg.v12.i47.7671.
12. Elli L, et al. Defensive medicine practices among gastroenterologists in Lombardy: Between lawsuits and the economic crisis. Dig Liver Dis. 2013 Jun. doi: 10.1016/j.dld.2013.01.004.
13. Jena AB, et al. Physician spending and subsequent risk of malpractice claims: observational study. BMJ. 2015 Nov. doi: 10.1136/bmj.h5516.
14. Scott SD, et al. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. BMJ Qual Saf. 2009 Oct. doi: 10.1136/qshc.2009.032870.
15. Gómez-Durán EL, et al. Physicians as second victims after a malpractice claim: An important issue in need of attention. J Healthc Qual Res. 2018 Oct. doi: 10.1016/j.jhqr.2018.06.002.
While nearly 75% of physicians in low-risk specialties and 99% of physicians in high-risk specialties may face a malpractice claim in their careers,1 malpractice is rarely discussed openly in medical school, residency, fellowship, or even amongst colleagues. Indeed, one study suggested that more than 10% of practicing gastroenterologists may face a malpractice claim,2 with gastroenterologists expected to spend 10-15% of their careers with an outstanding malpractice claim3 as cases may take 27-29 months to resolve on average.4
Believing that if a physician is sued, one must have done something “wrong” or that speaking about one’s experience may implicate a colleague, creates an intense stigma and isolation that only serves to aggravate the “second victim syndrome” (SVS) that is well documented in the surgical literature.2 Herein,
What is Malpractice? Why Do Physicians Get Sued?
Malpractice is defined as negligence on the part of a physician which causes physical or emotional damage to the patient. This requires a variety of legal issues to be evaluated (e.g. breach of duty between the physicians and patient, breach of standard of care), that often center around the question: would a “reasonable, careful, and prudent” doctor behave in the same manner in the same circumstance?
While some fields of medicine lend themselves better to algorithmic applications of highly evidence-based guidelines, many aspects of GI care and endoscopic practice are highly physician/patient-specific, dependent on local expertise, and based on low-quality evidence. As a result, an assessment of negligence may be quite subjective, depending on the expert retained by a plaintiff. Conflicting expert testimony on what professional custom is and whether practice deviates may hinge on technical details that may or may not be appreciated by a lay jury.
Plaintiffs must prove both that they have sustained an injury and that the injury (emotional or physical) was due to the physician’s negligence. While this may be straightforward in a “slip-and-fall” tort claim, medical malpractice claims usually involve sick patients with multiple comorbidities, where assigning causality to a single intervention/misinterpretation/missed opportunity is difficult to weigh against competing causes of adverse outcomes. Assessing a specific liability requires that the plaintiff prove this to a “more likely than not” standard which may be part of the reason why only 30% of cases are closed with indemnity payments, a figure that has not changed significantly in the past decade.4
While the perception amongst physicians is that tort legislation is ever increasing, data from the National Practitioner Data Bank (NPDB) demonstrates that the number of paid claims against physicians has decreased by 75% in the last 20 years.5 This may reflect a progressive improvement in the quality of care delivered or success of “tort reform” on the state level to limit damages and “nuisance” lawsuits. However, another more problematic possibility is that with the corporatization of medicine, an untold number of physicians may be removed from litigation as a named party, with their institution shielding them from reporting. While the number of cases may or may not be declining, the average indemnity payment appears to be rising to $330,000 on average,4 with one study suggesting a significant growth in paid claims in gastroenterology.6
Historically, studies of closed malpractice claims have demonstrated that 59% involved diagnostic errors involving a cancer diagnosis,7 though why this actually happens may be for a wide variety of reasons including errors in the development of a differential diagnosis, ordering of an appropriate diagnostic test, interpretation of the diagnostic test, or follow-up of an abnormality identified.
What are the Intended/Unintended Consequences of Litigation?
The objective of our tort system is to compensate patients for economic damages (medical costs and lost wages) and non-economic damages (pain and suffering), and to ideally deter negligent behavior of providers. Interestingly, data from the NPDB have suggested that approximately 1% of all physicians account for 32% of all paid claims, with the same study showing that among physicians with paid claims, 4% had at least 3.8
While certain fields are obviously more prone to litigation, the risk of additional claims on a physician with 3 prior claims was more than 3 times that of physicians with 1 lifetime claim. One would assume that the system was built to drive out a small proportion of “bad actors.” Indeed, similar data from the NPDB has demonstrated that the number of claims against physicians was associated both with their leaving the practice of medicine and relocating to smaller practice settings.9
Another frequent question is whether the threat of litigation drives “defensive medicine” (i.e. medical care that is not beneficial) or avoidance medicine (i.e. excluding high risk patients and procedures from ones’ practice). These behaviors have been well documented in physicians around the world,10 as well as several surveys of gastroenterologists specifically suggesting regular ordering of unnecessary imaging/endoscopy and referrals of patients to specialists that may not be necessary.11,12
However, does defensive medicine work: does spending more prevent you from being the target of a lawsuit? In an observational study in Florida from 2000-2009, researchers demonstrated that across specialties, greater average spending by physicians was associated with a reduced risk of incurring a malpractice claim. Indeed, the likelihood of a top quintile spending internist having a malpractice incident vs a bottom quintile spending internist was 0.3% vs 1.5%.13
Approximately 10.4-43.3% of physicians may experience SVS, experiencing trauma after an adverse patient event/medical error, manifesting with psychological trauma (shame, guilt, anxiety) and cognitive limitations (burnout, stress).2 Significant emotional consequences are common on the part of the physician and have well-documented stages to recovery,14 which if ignored may lead to long-term detrimental mental/emotional health of the physician and their future patients.
Specifically, in one study, 80.8% of physicians who had a closed malpractice claim reported significant emotional distress (regardless of the legal outcome), with frequent reports of mood symptoms that affected professional conduct.15 Recognizing these effects and implementing peer counseling and institutional support may help to expedite recovery and mitigate future adverse career outcomes.14
Anatomy/Timeline of a Liability Lawsuit
Medical malpractice cases are heard in state courts, in the jurisdictions where the care was provided. From the time an event occurs to when a jury verdict may be rendered may take 4-5 years or more depending on the local statute of limitations, discovery process, backlog of the local case docket, and specific circumstances of the case. The length of time is important to consider given the likelihood that a physician may advance in training or move practice locations during the course of litigation. Several common myths surrounding this process are summarized in the accompanying box, titled “Myths Surrounding Medical Liability Litigation.”
The plaintiff faces a statute of limitations to file a lawsuit that may range from 1-6 years depending on the state. The first indication that legal action may be pending will generally be a plaintiff’s formal request for medical records. After these records are reviewed, the plaintiff’s attorney will consult one or more experts (often credentialed in the same specialty) to assess if the case is viable and to ultimately form the basis of an affidavit of merit from a plaintiff expert.
Once the lawsuit is filed, the physician(s) named will be assigned an attorney by their employer/insurance company. A state medical board malpractice questionnaire will generally follow that will seek to independently evaluate the alleged malpractice with interrogatives to determine if censure is warranted. There is a formal response to the plaintiff’s petition by the defense and then the discovery phase begins where both sides depose the defendants/plaintiffs and retain medical experts that are favorable to their arguments.
In choosing potential “experts,” physicians must ensure that they are willing/able to be present for a potential trial, do not have any personal/professional/academic conflicts with the defendants, and are willing to provide compelling testimony to a jury. A pre-trial conference and trial date is set which may be >12 months away depending on the local docket. While the amount of time a trial may take is variable, it may be up to 5-7 days that the defendants are expected to be in court in addition to days where depositions are being taken.
During the discovery process, dismissal of the physician from the lawsuit is pursued. In addition, settlement negotiations generally proceed in parallel with discovery process and may result in a pre-trial/pre-verdict settlement. Once a verdict is reached, whether for the plaintiff or the defendant, the case may be appealed, and the trial preparation process may be repeated.
Conclusions
Awareness of the medical liability process is critical for trainees and attendings alike, given the high likelihood of litigation in a gastroenterologist’s career. Specific considerations like local tort law and malpractice coverage are important to be familiar. Ongoing health services research help to shape our understanding on the intended and unintended consequences of litigation on medicine, though detailed data on outcomes/settlements are limited by confidentiality agreements, which may hamper efforts to improve patient safety.
Dr. Das is associate professor of medicine in the Division of Gastroenterology at Washington University School of Medicine, St. Louis, Missouri. He has served as a consultant for Olympus, but has no other relevant conflicts.
References
1. Jena AB, et al. Malpractice Risk According to Physician Specialty. N Engl J Med. 2011 Aug. doi: 10.1056/NEJMsa1012370.
2. Chong RIH, et al. Scoping review of the second victim syndrome among surgeons: Understanding the impact, responses, and support systems. Am J Surg 2024 Mar. doi: 10.1016/j.amjsurg.2023.09.045.
3. Seabury S, et al. On Average, Physicians Spend Nearly 11 Percent Of Their 40-Year Careers With An Open, Unresolved Malpractice Claim. Health Aff Proj Hope. 2013 Jan. doi: 10.1377/hlthaff.2012.0967.
4. CRICO Strategies. Medical Malpractice in America: A 10-Year Asessment with Insights. 2018. Accessed Apr 28, 2025.
5. Studdert DM, Hall MA. Medical Malpractice Law — Doctrine and Dynamics. N Engl J Med 2022 Oct. doi: 10.1056/NEJMp2201675.
6. Schaffer AC, et al. Rates and Characteristics of Paid Malpractice Claims Among US Physicians by Specialty, 1992-2014. JAMA Intern Med. 2017 May. doi: 10.1001/jamainternmed.2017.0311.
7. Gandhi TK, et al. Missed and Delayed Diagnoses in the Ambulatory Setting: A Study of Closed Malpractice Claims. Ann Intern Med. 2006 Oct. doi: 10.7326/0003-4819-145-7-200610030-00006.
8. Studdert DM, et al. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N Engl J Med. 2016 Jan. doi: 10.1056/NEJMsa1506137.
9. Studdert DM, et al. Changes in Practice among Physicians with Malpractice Claims. N Engl J Med. 2019 Mar. doi: 10.1056/NEJMsa1809981.
10. Ries NM, Jansen J. Physicians’ views and experiences of defensive medicine: An international review of empirical research. Health Policy. 2021 May. doi: 10.1016/j.healthpol.2021.02.005.
11. Hiyama T, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006 Dec. doi: 10.3748/wjg.v12.i47.7671.
12. Elli L, et al. Defensive medicine practices among gastroenterologists in Lombardy: Between lawsuits and the economic crisis. Dig Liver Dis. 2013 Jun. doi: 10.1016/j.dld.2013.01.004.
13. Jena AB, et al. Physician spending and subsequent risk of malpractice claims: observational study. BMJ. 2015 Nov. doi: 10.1136/bmj.h5516.
14. Scott SD, et al. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. BMJ Qual Saf. 2009 Oct. doi: 10.1136/qshc.2009.032870.
15. Gómez-Durán EL, et al. Physicians as second victims after a malpractice claim: An important issue in need of attention. J Healthc Qual Res. 2018 Oct. doi: 10.1016/j.jhqr.2018.06.002.
While nearly 75% of physicians in low-risk specialties and 99% of physicians in high-risk specialties may face a malpractice claim in their careers,1 malpractice is rarely discussed openly in medical school, residency, fellowship, or even amongst colleagues. Indeed, one study suggested that more than 10% of practicing gastroenterologists may face a malpractice claim,2 with gastroenterologists expected to spend 10-15% of their careers with an outstanding malpractice claim3 as cases may take 27-29 months to resolve on average.4
Believing that if a physician is sued, one must have done something “wrong” or that speaking about one’s experience may implicate a colleague, creates an intense stigma and isolation that only serves to aggravate the “second victim syndrome” (SVS) that is well documented in the surgical literature.2 Herein,
What is Malpractice? Why Do Physicians Get Sued?
Malpractice is defined as negligence on the part of a physician which causes physical or emotional damage to the patient. This requires a variety of legal issues to be evaluated (e.g. breach of duty between the physicians and patient, breach of standard of care), that often center around the question: would a “reasonable, careful, and prudent” doctor behave in the same manner in the same circumstance?
While some fields of medicine lend themselves better to algorithmic applications of highly evidence-based guidelines, many aspects of GI care and endoscopic practice are highly physician/patient-specific, dependent on local expertise, and based on low-quality evidence. As a result, an assessment of negligence may be quite subjective, depending on the expert retained by a plaintiff. Conflicting expert testimony on what professional custom is and whether practice deviates may hinge on technical details that may or may not be appreciated by a lay jury.
Plaintiffs must prove both that they have sustained an injury and that the injury (emotional or physical) was due to the physician’s negligence. While this may be straightforward in a “slip-and-fall” tort claim, medical malpractice claims usually involve sick patients with multiple comorbidities, where assigning causality to a single intervention/misinterpretation/missed opportunity is difficult to weigh against competing causes of adverse outcomes. Assessing a specific liability requires that the plaintiff prove this to a “more likely than not” standard which may be part of the reason why only 30% of cases are closed with indemnity payments, a figure that has not changed significantly in the past decade.4
While the perception amongst physicians is that tort legislation is ever increasing, data from the National Practitioner Data Bank (NPDB) demonstrates that the number of paid claims against physicians has decreased by 75% in the last 20 years.5 This may reflect a progressive improvement in the quality of care delivered or success of “tort reform” on the state level to limit damages and “nuisance” lawsuits. However, another more problematic possibility is that with the corporatization of medicine, an untold number of physicians may be removed from litigation as a named party, with their institution shielding them from reporting. While the number of cases may or may not be declining, the average indemnity payment appears to be rising to $330,000 on average,4 with one study suggesting a significant growth in paid claims in gastroenterology.6
Historically, studies of closed malpractice claims have demonstrated that 59% involved diagnostic errors involving a cancer diagnosis,7 though why this actually happens may be for a wide variety of reasons including errors in the development of a differential diagnosis, ordering of an appropriate diagnostic test, interpretation of the diagnostic test, or follow-up of an abnormality identified.
What are the Intended/Unintended Consequences of Litigation?
The objective of our tort system is to compensate patients for economic damages (medical costs and lost wages) and non-economic damages (pain and suffering), and to ideally deter negligent behavior of providers. Interestingly, data from the NPDB have suggested that approximately 1% of all physicians account for 32% of all paid claims, with the same study showing that among physicians with paid claims, 4% had at least 3.8
While certain fields are obviously more prone to litigation, the risk of additional claims on a physician with 3 prior claims was more than 3 times that of physicians with 1 lifetime claim. One would assume that the system was built to drive out a small proportion of “bad actors.” Indeed, similar data from the NPDB has demonstrated that the number of claims against physicians was associated both with their leaving the practice of medicine and relocating to smaller practice settings.9
Another frequent question is whether the threat of litigation drives “defensive medicine” (i.e. medical care that is not beneficial) or avoidance medicine (i.e. excluding high risk patients and procedures from ones’ practice). These behaviors have been well documented in physicians around the world,10 as well as several surveys of gastroenterologists specifically suggesting regular ordering of unnecessary imaging/endoscopy and referrals of patients to specialists that may not be necessary.11,12
However, does defensive medicine work: does spending more prevent you from being the target of a lawsuit? In an observational study in Florida from 2000-2009, researchers demonstrated that across specialties, greater average spending by physicians was associated with a reduced risk of incurring a malpractice claim. Indeed, the likelihood of a top quintile spending internist having a malpractice incident vs a bottom quintile spending internist was 0.3% vs 1.5%.13
Approximately 10.4-43.3% of physicians may experience SVS, experiencing trauma after an adverse patient event/medical error, manifesting with psychological trauma (shame, guilt, anxiety) and cognitive limitations (burnout, stress).2 Significant emotional consequences are common on the part of the physician and have well-documented stages to recovery,14 which if ignored may lead to long-term detrimental mental/emotional health of the physician and their future patients.
Specifically, in one study, 80.8% of physicians who had a closed malpractice claim reported significant emotional distress (regardless of the legal outcome), with frequent reports of mood symptoms that affected professional conduct.15 Recognizing these effects and implementing peer counseling and institutional support may help to expedite recovery and mitigate future adverse career outcomes.14
Anatomy/Timeline of a Liability Lawsuit
Medical malpractice cases are heard in state courts, in the jurisdictions where the care was provided. From the time an event occurs to when a jury verdict may be rendered may take 4-5 years or more depending on the local statute of limitations, discovery process, backlog of the local case docket, and specific circumstances of the case. The length of time is important to consider given the likelihood that a physician may advance in training or move practice locations during the course of litigation. Several common myths surrounding this process are summarized in the accompanying box, titled “Myths Surrounding Medical Liability Litigation.”
The plaintiff faces a statute of limitations to file a lawsuit that may range from 1-6 years depending on the state. The first indication that legal action may be pending will generally be a plaintiff’s formal request for medical records. After these records are reviewed, the plaintiff’s attorney will consult one or more experts (often credentialed in the same specialty) to assess if the case is viable and to ultimately form the basis of an affidavit of merit from a plaintiff expert.
Once the lawsuit is filed, the physician(s) named will be assigned an attorney by their employer/insurance company. A state medical board malpractice questionnaire will generally follow that will seek to independently evaluate the alleged malpractice with interrogatives to determine if censure is warranted. There is a formal response to the plaintiff’s petition by the defense and then the discovery phase begins where both sides depose the defendants/plaintiffs and retain medical experts that are favorable to their arguments.
In choosing potential “experts,” physicians must ensure that they are willing/able to be present for a potential trial, do not have any personal/professional/academic conflicts with the defendants, and are willing to provide compelling testimony to a jury. A pre-trial conference and trial date is set which may be >12 months away depending on the local docket. While the amount of time a trial may take is variable, it may be up to 5-7 days that the defendants are expected to be in court in addition to days where depositions are being taken.
During the discovery process, dismissal of the physician from the lawsuit is pursued. In addition, settlement negotiations generally proceed in parallel with discovery process and may result in a pre-trial/pre-verdict settlement. Once a verdict is reached, whether for the plaintiff or the defendant, the case may be appealed, and the trial preparation process may be repeated.
Conclusions
Awareness of the medical liability process is critical for trainees and attendings alike, given the high likelihood of litigation in a gastroenterologist’s career. Specific considerations like local tort law and malpractice coverage are important to be familiar. Ongoing health services research help to shape our understanding on the intended and unintended consequences of litigation on medicine, though detailed data on outcomes/settlements are limited by confidentiality agreements, which may hamper efforts to improve patient safety.
Dr. Das is associate professor of medicine in the Division of Gastroenterology at Washington University School of Medicine, St. Louis, Missouri. He has served as a consultant for Olympus, but has no other relevant conflicts.
References
1. Jena AB, et al. Malpractice Risk According to Physician Specialty. N Engl J Med. 2011 Aug. doi: 10.1056/NEJMsa1012370.
2. Chong RIH, et al. Scoping review of the second victim syndrome among surgeons: Understanding the impact, responses, and support systems. Am J Surg 2024 Mar. doi: 10.1016/j.amjsurg.2023.09.045.
3. Seabury S, et al. On Average, Physicians Spend Nearly 11 Percent Of Their 40-Year Careers With An Open, Unresolved Malpractice Claim. Health Aff Proj Hope. 2013 Jan. doi: 10.1377/hlthaff.2012.0967.
4. CRICO Strategies. Medical Malpractice in America: A 10-Year Asessment with Insights. 2018. Accessed Apr 28, 2025.
5. Studdert DM, Hall MA. Medical Malpractice Law — Doctrine and Dynamics. N Engl J Med 2022 Oct. doi: 10.1056/NEJMp2201675.
6. Schaffer AC, et al. Rates and Characteristics of Paid Malpractice Claims Among US Physicians by Specialty, 1992-2014. JAMA Intern Med. 2017 May. doi: 10.1001/jamainternmed.2017.0311.
7. Gandhi TK, et al. Missed and Delayed Diagnoses in the Ambulatory Setting: A Study of Closed Malpractice Claims. Ann Intern Med. 2006 Oct. doi: 10.7326/0003-4819-145-7-200610030-00006.
8. Studdert DM, et al. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N Engl J Med. 2016 Jan. doi: 10.1056/NEJMsa1506137.
9. Studdert DM, et al. Changes in Practice among Physicians with Malpractice Claims. N Engl J Med. 2019 Mar. doi: 10.1056/NEJMsa1809981.
10. Ries NM, Jansen J. Physicians’ views and experiences of defensive medicine: An international review of empirical research. Health Policy. 2021 May. doi: 10.1016/j.healthpol.2021.02.005.
11. Hiyama T, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006 Dec. doi: 10.3748/wjg.v12.i47.7671.
12. Elli L, et al. Defensive medicine practices among gastroenterologists in Lombardy: Between lawsuits and the economic crisis. Dig Liver Dis. 2013 Jun. doi: 10.1016/j.dld.2013.01.004.
13. Jena AB, et al. Physician spending and subsequent risk of malpractice claims: observational study. BMJ. 2015 Nov. doi: 10.1136/bmj.h5516.
14. Scott SD, et al. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. BMJ Qual Saf. 2009 Oct. doi: 10.1136/qshc.2009.032870.
15. Gómez-Durán EL, et al. Physicians as second victims after a malpractice claim: An important issue in need of attention. J Healthc Qual Res. 2018 Oct. doi: 10.1016/j.jhqr.2018.06.002.
Remembering Why We Are In Medicine
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
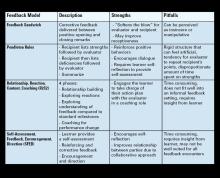
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Positioning Yourself For Success in Private Practice

In this video, Peter Naas, MD, of Gastroenterology Associates in Greenville, South Carolina, shares insights on how young physicians can best position themselves for a successful career in private practice gastroenterology.

In this video, Peter Naas, MD, of Gastroenterology Associates in Greenville, South Carolina, shares insights on how young physicians can best position themselves for a successful career in private practice gastroenterology.

In this video, Peter Naas, MD, of Gastroenterology Associates in Greenville, South Carolina, shares insights on how young physicians can best position themselves for a successful career in private practice gastroenterology.
GLP-1 Receptor Agonist Use in Gastrointestinal Endoscopy: A Review of Current Evidence and Guidelines
The use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has increased over the past several years and has become a cornerstone in both diabetes and weight loss management, particularly because of its unique combination of glucose control, weight reduction potential, and cardiac and metabolic benefits. However, increased use of these agents presents a dilemma in gastrointestinal endoscopy as it pertains to their safety and management during the periprocedural period.
highlighting gaps and future directions.
Pharmacology and Mechanisms of Action
GLP-1 RAs have several mechanisms of action that make them relevant in gastrointestinal endoscopy. These medications modulate glucose control via enhancement of glucose-dependent insulin secretion and reduction of postprandial glucagon, which promotes satiety and delays gastric emptying. This delay in gastric emptying mediated by vagal pathways has been postulated to increase gastric residuals, posing a risk for aspiration during anesthesia.1
It is important to also consider the pharmacokinetics of GLP-1 RAs, as some have shorter half-lives on the order of several hours, like exenatide, while others, like semaglutide, are dosed weekly. Additionally, common side effects of GLP-1 RAs include nausea, vomiting, bloating, and early satiety, which pose challenges for patients undergoing endoscopic procedures.
Current Guidelines
Various societies have published guidelines on the periprocedural use of GLP-1 RAs. The American Society of Anesthesiologist (ASA) in 2023 presented early recommendations to hold GLP-1 RAs either day of procedure or week prior depending on pharmacokinetics, because of the risk of delayed gastric emptying and increased potential for aspiration.2 Soon thereafter, a multi-gastroenterology society guideline was released stating more data is needed to decide if GLP-1 RAs need to be held prior to endoscopic procedures.3
In early 2024, the American Gastroenterological Association (AGA) published a rapid clinical update that advocated for a more individualized approach, particularly in light of limited overall data for GLP-1 RAs and endoscopic procedures.4 In asymptomatic patients who follow typical fasting protocols for procedures, it is generally safe to proceed with endoscopy without holding GLP-1 RAs. In symptomatic patients (nausea, abdominal distension, etc), the AGA advises additional precautions, including performing transabdominal ultrasound if feasible to assess retained gastric contents. The AGA also suggests placing a patient on a clear liquid diet the day prior to the procedure — rather than holding GLP-1 RAs — as another reasonable strategy.
The guidelines continue to evolve with newer multi-society guidelines establishing best practices. While initially in 2023 the ASA did recommend holding these medications prior to endoscopy, the initial guidance was based on expert opinion with limited evidence. Newer multi-society guidance published jointly by the ASA along with various gastroenterology societies, including the AGA in December 2024, takes a more nuanced approach.5
The newer guidelines include two main recommendations:
1. Periprocedural management of GLP-1 RAs should be a joint decision among the procedural, anesthesia, and prescribing team balancing metabolic needs vs patient risks.
- In a low-risk patient, one that is asymptomatic and on standard dosing, among other factors, the guidance states that GLP-1 RAs can be continued.
- In higher-risk patients, the original guidance of holding a day or a week prior to endoscopic procedures should be followed.
2. Periprocedural management of GLP-1 RAs should attempt to minimize the aspiration risks loosely associated with delayed gastric emptying.
- Consider a 24-hour clear liquid diet a day prior to the procedure and transabdominal ultrasound to check gastric contents.
- It is acknowledged that this guidance is based on limited evidence and will be evolving as new medications and data are released.
Recent Clinical Studies
Although there is very little data to guide clinicians, several recent studies have been published that can direct clinical decision-making as guidelines continue to be refined and updated.
A multicenter trial of approximately 800 patients undergoing upper endoscopy found a significant difference in rates of retained gastric contents between those that underwent endoscopy who did and did not follow the ASA guidance on periprocedural management of GLP-1 RAs (12.7% vs 4.4%; P < .0001). However, there were no significant differences in rates of aborted procedures or unplanned intubations.
Furthermore, a multivariable analysis was performed controlling for GLP-1 RA type and other factors, which found the likelihood of gastric retention increased by 36% for every 1% increase in hemoglobin A1c. This study suggests that a more individualized approach to holding GLP-1 RA would be applicable rather than a universal periprocedural hold.6
More recently, a single-center study of nearly 600 patients undergoing upper endoscopy showed that while there were slightly increased rates of retained gastric contents (OR 3.80; P = .003) and aborted procedures (1.3% vs 0%; P = .02), the rates of adverse anesthesia events (hypoxia, etc) were similar between the groups and no cases of pulmonary aspiration were noted.7
One single-center study of 57 patients evaluated the safety of GLP-1 RAs in those undergoing endoscopic sleeve gastrectomy. GLP-1 RAs were continued on all patients, but all adhered to a liquid only diet for at least 24 hours prior to the procedure. There were no instances of retained gastric solids, aspiration, or hypoxia. This study suggests that with a 24-hour clear liquid diet and routine NPO recommendations prior to endoscopy, it would be safe to continue GLP-1 RAs. This study provides rationale for the AGA recommendation for a clear liquid diet 24 hours prior to endoscopic procedures for those on GLP-1 RAs.8
A study looking at those who underwent emergency surgery and endoscopy with claims data of use of GLP-1 RAs found an overall incidence of postoperative respiratory complications of 3.5% for those with GLP-1 RAs fill history vs 4.0% for those without (P = .12). Approximately 800 of the 24,000 patients identified had undergone endoscopic procedures for GI bleeding or food impaction. The study overall showed that preoperative use of GLP-1 RAs in patients undergoing surgery or endoscopy, evaluated as a combined group, was not associated with an increased risk of pulmonary complications.9
Lastly, a systematic review and meta-analysis that included 15 studies that quantified gastric emptying using various methods, including gastric emptying scintigraphy and acetaminophen absorption test, found that there was a quantifiable delay in gastric emptying of about 36 minutes, compared to placebo (P < .01), in patients using GLP-1 RAs. However, compared to standard periprocedural fasting, this delay is clinically insignificant and standard fasting protocols would still be appropriate for patients on GLP-1 RAs.10
These studies taken together suggest that while GLP-1 RAs can mildly increase the likelihood of retained gastric contents, there is no statistically significant increase in the risk of aspiration or other anesthesia complications. Furthermore, while decreased gastric emptying is a known effect of GLP-1 RAs, this effect may not be clinically significant in the context of standard periprocedural fasting protocols particularly when combined with a 24-hour clear liquid diet. These findings support at a minimum a more patient-specific strategy for periprocedural management of GLP-1 RAs.
Clinical Implications
These most recent studies, as well as prior studies and guidelines by various societies lead to a dilemma among endoscopists on proper patient counseling on GLP-1 RAs use before endoscopic procedures. Clinicians must balance the metabolic benefits of GLP-1 RAs with potential endoscopic complications and risks.
Holding therapy theoretically decreases aspiration risk and pulmonary complications, though evidence remains low to support this. Holding medication, however, affects glycemic control leading to potential rebound hyperglycemia which may impact and delay plans for endoscopy. With growing indications for the use of GLP-1 RAs, a more tailored patient-centered treatment plan may be required, especially with consideration of procedure indication and comorbidities.
Currently, practice patterns at different institutions vary widely, making standardization much more difficult. Some centers have opted to follow ASA guidelines of holding these medications up to 1 week prior to procedures, while others have continued therapy with no pre-procedural adjustments. This leaves endoscopists to deal with the downstream effects of inconvenience to patients, care delays, and financial considerations if procedures are postponed related to GLP-1 RAs use.
Future Directions
Future studies are needed to make further evidence-based recommendations. Studies should focus on stratifying risks and recommendations based on procedure type (EGD, colonoscopy, etc). More widespread implementation of gastric ultrasound can assist in real-time decision-making, albeit this would require expertise and dedicated time within the pre-procedural workflow. Randomized controlled trials comparing outcomes of patients who continue GLP-1 RAs vs those who discontinue stratified by baseline risk will be instrumental for making concrete guidelines that provide clarity on periprocedural management of GLP-1 RAs.
Conclusion
The periprocedural management of GLP-1 RAs remains a controversial topic that presents unique challenges in endoscopy. Several guidelines have been released by various stakeholders including anesthesiologists, gastroenterologists, and other prescribing providers. Clinical data remains limited with no robust evidence available to suggest that gastric emptying delays caused by GLP-1 RAs prior to endoscopic procedures significantly increases risk of aspiration, pulmonary complications, or other comorbidities. Evolving multi-society guidelines will be important to establish more consistent practices with reassessment of the data as new studies emerge. A multidisciplinary, individualized patient approach may be the best strategy for managing GLP-1 RAs for patients undergoing endoscopic procedures.
Dr. Sekar and Dr. Asamoah are based in the department of gastroenterology at MedStar Georgetown University Hospital, Washington, D.C. Dr. Sekar reports no conflicts of interest in regard to this article. Dr. Asamoah serves on the Johnson & Johnson advisory board for inflammatory bowel disease–related therapies.
References
1. Halim MA et al. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J Clin Endocrinol Metab. 2018 Feb. doi: 10.1210/jc.2017-02006.
2. American Society of Anesthesiologists. American Society of Anesthesiologists releases consensus-based guidance on preoperative use of GLP-1 receptor agonists. 2023 Jun 20. www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative
3. American Gastroenterological Association. GI multi-society statement regarding GLP-1 agonists and endoscopy. 2023 Jul 25. gastro.org/news/gi-multi-society-statement-regarding-glp-1-agonists-and-endoscopy/.
4. Hashash JG et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr. doi: 10.1016/j.cgh.2023.11.002.
5. Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multi-society Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
6. Phan J et al. Glucagon-Like Peptide Receptor Agonists Use Before Endoscopy Is Associated With Low Retained Gastric Contents: A Multicenter Cross-Sectional Analysis. Am J Gastroenterol. 2025 Mar. doi: 10.14309/ajg.0000000000002969.
7. Panchal S et al. Endoscopy and Anesthesia Outcomes Associated With Glucagon-like Peptide-1 Receptor Agonist use in Patients Undergoing Outpatient Upper Endoscopy. Gastrointest Endosc. 2025 Aug. doi:10.1016/j.gie.2025.01.004.
8. Maselli DB et al. Safe Continuation of glucagon-like Peptide 1 Receptor Agonists at Endoscopy: A Case Series of 57 Adults Undergoing Endoscopic Sleeve Gastroplasty. Obes Surg. 2024 Jul. doi: 10.1007/s11695-024-07278-2.
9. Dixit AA et al. Preoperative GLP-1 Receptor Agonist Use and Risk of Postoperative Respiratory Complications. JAMA. 2024 Apr. doi: 10.1001/jama.2024.5003.
10. Hiramoto B et al. Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A systematic review and meta-analysis with insights for periprocedural management. Am J Gastroenterol. 2024 Jun. doi: 10.14309/ajg.0000000000002820.
The use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has increased over the past several years and has become a cornerstone in both diabetes and weight loss management, particularly because of its unique combination of glucose control, weight reduction potential, and cardiac and metabolic benefits. However, increased use of these agents presents a dilemma in gastrointestinal endoscopy as it pertains to their safety and management during the periprocedural period.
highlighting gaps and future directions.
Pharmacology and Mechanisms of Action
GLP-1 RAs have several mechanisms of action that make them relevant in gastrointestinal endoscopy. These medications modulate glucose control via enhancement of glucose-dependent insulin secretion and reduction of postprandial glucagon, which promotes satiety and delays gastric emptying. This delay in gastric emptying mediated by vagal pathways has been postulated to increase gastric residuals, posing a risk for aspiration during anesthesia.1
It is important to also consider the pharmacokinetics of GLP-1 RAs, as some have shorter half-lives on the order of several hours, like exenatide, while others, like semaglutide, are dosed weekly. Additionally, common side effects of GLP-1 RAs include nausea, vomiting, bloating, and early satiety, which pose challenges for patients undergoing endoscopic procedures.
Current Guidelines
Various societies have published guidelines on the periprocedural use of GLP-1 RAs. The American Society of Anesthesiologist (ASA) in 2023 presented early recommendations to hold GLP-1 RAs either day of procedure or week prior depending on pharmacokinetics, because of the risk of delayed gastric emptying and increased potential for aspiration.2 Soon thereafter, a multi-gastroenterology society guideline was released stating more data is needed to decide if GLP-1 RAs need to be held prior to endoscopic procedures.3
In early 2024, the American Gastroenterological Association (AGA) published a rapid clinical update that advocated for a more individualized approach, particularly in light of limited overall data for GLP-1 RAs and endoscopic procedures.4 In asymptomatic patients who follow typical fasting protocols for procedures, it is generally safe to proceed with endoscopy without holding GLP-1 RAs. In symptomatic patients (nausea, abdominal distension, etc), the AGA advises additional precautions, including performing transabdominal ultrasound if feasible to assess retained gastric contents. The AGA also suggests placing a patient on a clear liquid diet the day prior to the procedure — rather than holding GLP-1 RAs — as another reasonable strategy.
The guidelines continue to evolve with newer multi-society guidelines establishing best practices. While initially in 2023 the ASA did recommend holding these medications prior to endoscopy, the initial guidance was based on expert opinion with limited evidence. Newer multi-society guidance published jointly by the ASA along with various gastroenterology societies, including the AGA in December 2024, takes a more nuanced approach.5
The newer guidelines include two main recommendations:
1. Periprocedural management of GLP-1 RAs should be a joint decision among the procedural, anesthesia, and prescribing team balancing metabolic needs vs patient risks.
- In a low-risk patient, one that is asymptomatic and on standard dosing, among other factors, the guidance states that GLP-1 RAs can be continued.
- In higher-risk patients, the original guidance of holding a day or a week prior to endoscopic procedures should be followed.
2. Periprocedural management of GLP-1 RAs should attempt to minimize the aspiration risks loosely associated with delayed gastric emptying.
- Consider a 24-hour clear liquid diet a day prior to the procedure and transabdominal ultrasound to check gastric contents.
- It is acknowledged that this guidance is based on limited evidence and will be evolving as new medications and data are released.
Recent Clinical Studies
Although there is very little data to guide clinicians, several recent studies have been published that can direct clinical decision-making as guidelines continue to be refined and updated.
A multicenter trial of approximately 800 patients undergoing upper endoscopy found a significant difference in rates of retained gastric contents between those that underwent endoscopy who did and did not follow the ASA guidance on periprocedural management of GLP-1 RAs (12.7% vs 4.4%; P < .0001). However, there were no significant differences in rates of aborted procedures or unplanned intubations.
Furthermore, a multivariable analysis was performed controlling for GLP-1 RA type and other factors, which found the likelihood of gastric retention increased by 36% for every 1% increase in hemoglobin A1c. This study suggests that a more individualized approach to holding GLP-1 RA would be applicable rather than a universal periprocedural hold.6
More recently, a single-center study of nearly 600 patients undergoing upper endoscopy showed that while there were slightly increased rates of retained gastric contents (OR 3.80; P = .003) and aborted procedures (1.3% vs 0%; P = .02), the rates of adverse anesthesia events (hypoxia, etc) were similar between the groups and no cases of pulmonary aspiration were noted.7
One single-center study of 57 patients evaluated the safety of GLP-1 RAs in those undergoing endoscopic sleeve gastrectomy. GLP-1 RAs were continued on all patients, but all adhered to a liquid only diet for at least 24 hours prior to the procedure. There were no instances of retained gastric solids, aspiration, or hypoxia. This study suggests that with a 24-hour clear liquid diet and routine NPO recommendations prior to endoscopy, it would be safe to continue GLP-1 RAs. This study provides rationale for the AGA recommendation for a clear liquid diet 24 hours prior to endoscopic procedures for those on GLP-1 RAs.8
A study looking at those who underwent emergency surgery and endoscopy with claims data of use of GLP-1 RAs found an overall incidence of postoperative respiratory complications of 3.5% for those with GLP-1 RAs fill history vs 4.0% for those without (P = .12). Approximately 800 of the 24,000 patients identified had undergone endoscopic procedures for GI bleeding or food impaction. The study overall showed that preoperative use of GLP-1 RAs in patients undergoing surgery or endoscopy, evaluated as a combined group, was not associated with an increased risk of pulmonary complications.9
Lastly, a systematic review and meta-analysis that included 15 studies that quantified gastric emptying using various methods, including gastric emptying scintigraphy and acetaminophen absorption test, found that there was a quantifiable delay in gastric emptying of about 36 minutes, compared to placebo (P < .01), in patients using GLP-1 RAs. However, compared to standard periprocedural fasting, this delay is clinically insignificant and standard fasting protocols would still be appropriate for patients on GLP-1 RAs.10
These studies taken together suggest that while GLP-1 RAs can mildly increase the likelihood of retained gastric contents, there is no statistically significant increase in the risk of aspiration or other anesthesia complications. Furthermore, while decreased gastric emptying is a known effect of GLP-1 RAs, this effect may not be clinically significant in the context of standard periprocedural fasting protocols particularly when combined with a 24-hour clear liquid diet. These findings support at a minimum a more patient-specific strategy for periprocedural management of GLP-1 RAs.
Clinical Implications
These most recent studies, as well as prior studies and guidelines by various societies lead to a dilemma among endoscopists on proper patient counseling on GLP-1 RAs use before endoscopic procedures. Clinicians must balance the metabolic benefits of GLP-1 RAs with potential endoscopic complications and risks.
Holding therapy theoretically decreases aspiration risk and pulmonary complications, though evidence remains low to support this. Holding medication, however, affects glycemic control leading to potential rebound hyperglycemia which may impact and delay plans for endoscopy. With growing indications for the use of GLP-1 RAs, a more tailored patient-centered treatment plan may be required, especially with consideration of procedure indication and comorbidities.
Currently, practice patterns at different institutions vary widely, making standardization much more difficult. Some centers have opted to follow ASA guidelines of holding these medications up to 1 week prior to procedures, while others have continued therapy with no pre-procedural adjustments. This leaves endoscopists to deal with the downstream effects of inconvenience to patients, care delays, and financial considerations if procedures are postponed related to GLP-1 RAs use.
Future Directions
Future studies are needed to make further evidence-based recommendations. Studies should focus on stratifying risks and recommendations based on procedure type (EGD, colonoscopy, etc). More widespread implementation of gastric ultrasound can assist in real-time decision-making, albeit this would require expertise and dedicated time within the pre-procedural workflow. Randomized controlled trials comparing outcomes of patients who continue GLP-1 RAs vs those who discontinue stratified by baseline risk will be instrumental for making concrete guidelines that provide clarity on periprocedural management of GLP-1 RAs.
Conclusion
The periprocedural management of GLP-1 RAs remains a controversial topic that presents unique challenges in endoscopy. Several guidelines have been released by various stakeholders including anesthesiologists, gastroenterologists, and other prescribing providers. Clinical data remains limited with no robust evidence available to suggest that gastric emptying delays caused by GLP-1 RAs prior to endoscopic procedures significantly increases risk of aspiration, pulmonary complications, or other comorbidities. Evolving multi-society guidelines will be important to establish more consistent practices with reassessment of the data as new studies emerge. A multidisciplinary, individualized patient approach may be the best strategy for managing GLP-1 RAs for patients undergoing endoscopic procedures.
Dr. Sekar and Dr. Asamoah are based in the department of gastroenterology at MedStar Georgetown University Hospital, Washington, D.C. Dr. Sekar reports no conflicts of interest in regard to this article. Dr. Asamoah serves on the Johnson & Johnson advisory board for inflammatory bowel disease–related therapies.
References
1. Halim MA et al. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J Clin Endocrinol Metab. 2018 Feb. doi: 10.1210/jc.2017-02006.
2. American Society of Anesthesiologists. American Society of Anesthesiologists releases consensus-based guidance on preoperative use of GLP-1 receptor agonists. 2023 Jun 20. www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative
3. American Gastroenterological Association. GI multi-society statement regarding GLP-1 agonists and endoscopy. 2023 Jul 25. gastro.org/news/gi-multi-society-statement-regarding-glp-1-agonists-and-endoscopy/.
4. Hashash JG et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr. doi: 10.1016/j.cgh.2023.11.002.
5. Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multi-society Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
6. Phan J et al. Glucagon-Like Peptide Receptor Agonists Use Before Endoscopy Is Associated With Low Retained Gastric Contents: A Multicenter Cross-Sectional Analysis. Am J Gastroenterol. 2025 Mar. doi: 10.14309/ajg.0000000000002969.
7. Panchal S et al. Endoscopy and Anesthesia Outcomes Associated With Glucagon-like Peptide-1 Receptor Agonist use in Patients Undergoing Outpatient Upper Endoscopy. Gastrointest Endosc. 2025 Aug. doi:10.1016/j.gie.2025.01.004.
8. Maselli DB et al. Safe Continuation of glucagon-like Peptide 1 Receptor Agonists at Endoscopy: A Case Series of 57 Adults Undergoing Endoscopic Sleeve Gastroplasty. Obes Surg. 2024 Jul. doi: 10.1007/s11695-024-07278-2.
9. Dixit AA et al. Preoperative GLP-1 Receptor Agonist Use and Risk of Postoperative Respiratory Complications. JAMA. 2024 Apr. doi: 10.1001/jama.2024.5003.
10. Hiramoto B et al. Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A systematic review and meta-analysis with insights for periprocedural management. Am J Gastroenterol. 2024 Jun. doi: 10.14309/ajg.0000000000002820.
The use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has increased over the past several years and has become a cornerstone in both diabetes and weight loss management, particularly because of its unique combination of glucose control, weight reduction potential, and cardiac and metabolic benefits. However, increased use of these agents presents a dilemma in gastrointestinal endoscopy as it pertains to their safety and management during the periprocedural period.
highlighting gaps and future directions.
Pharmacology and Mechanisms of Action
GLP-1 RAs have several mechanisms of action that make them relevant in gastrointestinal endoscopy. These medications modulate glucose control via enhancement of glucose-dependent insulin secretion and reduction of postprandial glucagon, which promotes satiety and delays gastric emptying. This delay in gastric emptying mediated by vagal pathways has been postulated to increase gastric residuals, posing a risk for aspiration during anesthesia.1
It is important to also consider the pharmacokinetics of GLP-1 RAs, as some have shorter half-lives on the order of several hours, like exenatide, while others, like semaglutide, are dosed weekly. Additionally, common side effects of GLP-1 RAs include nausea, vomiting, bloating, and early satiety, which pose challenges for patients undergoing endoscopic procedures.
Current Guidelines
Various societies have published guidelines on the periprocedural use of GLP-1 RAs. The American Society of Anesthesiologist (ASA) in 2023 presented early recommendations to hold GLP-1 RAs either day of procedure or week prior depending on pharmacokinetics, because of the risk of delayed gastric emptying and increased potential for aspiration.2 Soon thereafter, a multi-gastroenterology society guideline was released stating more data is needed to decide if GLP-1 RAs need to be held prior to endoscopic procedures.3
In early 2024, the American Gastroenterological Association (AGA) published a rapid clinical update that advocated for a more individualized approach, particularly in light of limited overall data for GLP-1 RAs and endoscopic procedures.4 In asymptomatic patients who follow typical fasting protocols for procedures, it is generally safe to proceed with endoscopy without holding GLP-1 RAs. In symptomatic patients (nausea, abdominal distension, etc), the AGA advises additional precautions, including performing transabdominal ultrasound if feasible to assess retained gastric contents. The AGA also suggests placing a patient on a clear liquid diet the day prior to the procedure — rather than holding GLP-1 RAs — as another reasonable strategy.
The guidelines continue to evolve with newer multi-society guidelines establishing best practices. While initially in 2023 the ASA did recommend holding these medications prior to endoscopy, the initial guidance was based on expert opinion with limited evidence. Newer multi-society guidance published jointly by the ASA along with various gastroenterology societies, including the AGA in December 2024, takes a more nuanced approach.5
The newer guidelines include two main recommendations:
1. Periprocedural management of GLP-1 RAs should be a joint decision among the procedural, anesthesia, and prescribing team balancing metabolic needs vs patient risks.
- In a low-risk patient, one that is asymptomatic and on standard dosing, among other factors, the guidance states that GLP-1 RAs can be continued.
- In higher-risk patients, the original guidance of holding a day or a week prior to endoscopic procedures should be followed.
2. Periprocedural management of GLP-1 RAs should attempt to minimize the aspiration risks loosely associated with delayed gastric emptying.
- Consider a 24-hour clear liquid diet a day prior to the procedure and transabdominal ultrasound to check gastric contents.
- It is acknowledged that this guidance is based on limited evidence and will be evolving as new medications and data are released.
Recent Clinical Studies
Although there is very little data to guide clinicians, several recent studies have been published that can direct clinical decision-making as guidelines continue to be refined and updated.
A multicenter trial of approximately 800 patients undergoing upper endoscopy found a significant difference in rates of retained gastric contents between those that underwent endoscopy who did and did not follow the ASA guidance on periprocedural management of GLP-1 RAs (12.7% vs 4.4%; P < .0001). However, there were no significant differences in rates of aborted procedures or unplanned intubations.
Furthermore, a multivariable analysis was performed controlling for GLP-1 RA type and other factors, which found the likelihood of gastric retention increased by 36% for every 1% increase in hemoglobin A1c. This study suggests that a more individualized approach to holding GLP-1 RA would be applicable rather than a universal periprocedural hold.6
More recently, a single-center study of nearly 600 patients undergoing upper endoscopy showed that while there were slightly increased rates of retained gastric contents (OR 3.80; P = .003) and aborted procedures (1.3% vs 0%; P = .02), the rates of adverse anesthesia events (hypoxia, etc) were similar between the groups and no cases of pulmonary aspiration were noted.7
One single-center study of 57 patients evaluated the safety of GLP-1 RAs in those undergoing endoscopic sleeve gastrectomy. GLP-1 RAs were continued on all patients, but all adhered to a liquid only diet for at least 24 hours prior to the procedure. There were no instances of retained gastric solids, aspiration, or hypoxia. This study suggests that with a 24-hour clear liquid diet and routine NPO recommendations prior to endoscopy, it would be safe to continue GLP-1 RAs. This study provides rationale for the AGA recommendation for a clear liquid diet 24 hours prior to endoscopic procedures for those on GLP-1 RAs.8
A study looking at those who underwent emergency surgery and endoscopy with claims data of use of GLP-1 RAs found an overall incidence of postoperative respiratory complications of 3.5% for those with GLP-1 RAs fill history vs 4.0% for those without (P = .12). Approximately 800 of the 24,000 patients identified had undergone endoscopic procedures for GI bleeding or food impaction. The study overall showed that preoperative use of GLP-1 RAs in patients undergoing surgery or endoscopy, evaluated as a combined group, was not associated with an increased risk of pulmonary complications.9
Lastly, a systematic review and meta-analysis that included 15 studies that quantified gastric emptying using various methods, including gastric emptying scintigraphy and acetaminophen absorption test, found that there was a quantifiable delay in gastric emptying of about 36 minutes, compared to placebo (P < .01), in patients using GLP-1 RAs. However, compared to standard periprocedural fasting, this delay is clinically insignificant and standard fasting protocols would still be appropriate for patients on GLP-1 RAs.10
These studies taken together suggest that while GLP-1 RAs can mildly increase the likelihood of retained gastric contents, there is no statistically significant increase in the risk of aspiration or other anesthesia complications. Furthermore, while decreased gastric emptying is a known effect of GLP-1 RAs, this effect may not be clinically significant in the context of standard periprocedural fasting protocols particularly when combined with a 24-hour clear liquid diet. These findings support at a minimum a more patient-specific strategy for periprocedural management of GLP-1 RAs.
Clinical Implications
These most recent studies, as well as prior studies and guidelines by various societies lead to a dilemma among endoscopists on proper patient counseling on GLP-1 RAs use before endoscopic procedures. Clinicians must balance the metabolic benefits of GLP-1 RAs with potential endoscopic complications and risks.
Holding therapy theoretically decreases aspiration risk and pulmonary complications, though evidence remains low to support this. Holding medication, however, affects glycemic control leading to potential rebound hyperglycemia which may impact and delay plans for endoscopy. With growing indications for the use of GLP-1 RAs, a more tailored patient-centered treatment plan may be required, especially with consideration of procedure indication and comorbidities.
Currently, practice patterns at different institutions vary widely, making standardization much more difficult. Some centers have opted to follow ASA guidelines of holding these medications up to 1 week prior to procedures, while others have continued therapy with no pre-procedural adjustments. This leaves endoscopists to deal with the downstream effects of inconvenience to patients, care delays, and financial considerations if procedures are postponed related to GLP-1 RAs use.
Future Directions
Future studies are needed to make further evidence-based recommendations. Studies should focus on stratifying risks and recommendations based on procedure type (EGD, colonoscopy, etc). More widespread implementation of gastric ultrasound can assist in real-time decision-making, albeit this would require expertise and dedicated time within the pre-procedural workflow. Randomized controlled trials comparing outcomes of patients who continue GLP-1 RAs vs those who discontinue stratified by baseline risk will be instrumental for making concrete guidelines that provide clarity on periprocedural management of GLP-1 RAs.
Conclusion
The periprocedural management of GLP-1 RAs remains a controversial topic that presents unique challenges in endoscopy. Several guidelines have been released by various stakeholders including anesthesiologists, gastroenterologists, and other prescribing providers. Clinical data remains limited with no robust evidence available to suggest that gastric emptying delays caused by GLP-1 RAs prior to endoscopic procedures significantly increases risk of aspiration, pulmonary complications, or other comorbidities. Evolving multi-society guidelines will be important to establish more consistent practices with reassessment of the data as new studies emerge. A multidisciplinary, individualized patient approach may be the best strategy for managing GLP-1 RAs for patients undergoing endoscopic procedures.
Dr. Sekar and Dr. Asamoah are based in the department of gastroenterology at MedStar Georgetown University Hospital, Washington, D.C. Dr. Sekar reports no conflicts of interest in regard to this article. Dr. Asamoah serves on the Johnson & Johnson advisory board for inflammatory bowel disease–related therapies.
References
1. Halim MA et al. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J Clin Endocrinol Metab. 2018 Feb. doi: 10.1210/jc.2017-02006.
2. American Society of Anesthesiologists. American Society of Anesthesiologists releases consensus-based guidance on preoperative use of GLP-1 receptor agonists. 2023 Jun 20. www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative
3. American Gastroenterological Association. GI multi-society statement regarding GLP-1 agonists and endoscopy. 2023 Jul 25. gastro.org/news/gi-multi-society-statement-regarding-glp-1-agonists-and-endoscopy/.
4. Hashash JG et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr. doi: 10.1016/j.cgh.2023.11.002.
5. Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multi-society Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
6. Phan J et al. Glucagon-Like Peptide Receptor Agonists Use Before Endoscopy Is Associated With Low Retained Gastric Contents: A Multicenter Cross-Sectional Analysis. Am J Gastroenterol. 2025 Mar. doi: 10.14309/ajg.0000000000002969.
7. Panchal S et al. Endoscopy and Anesthesia Outcomes Associated With Glucagon-like Peptide-1 Receptor Agonist use in Patients Undergoing Outpatient Upper Endoscopy. Gastrointest Endosc. 2025 Aug. doi:10.1016/j.gie.2025.01.004.
8. Maselli DB et al. Safe Continuation of glucagon-like Peptide 1 Receptor Agonists at Endoscopy: A Case Series of 57 Adults Undergoing Endoscopic Sleeve Gastroplasty. Obes Surg. 2024 Jul. doi: 10.1007/s11695-024-07278-2.
9. Dixit AA et al. Preoperative GLP-1 Receptor Agonist Use and Risk of Postoperative Respiratory Complications. JAMA. 2024 Apr. doi: 10.1001/jama.2024.5003.
10. Hiramoto B et al. Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A systematic review and meta-analysis with insights for periprocedural management. Am J Gastroenterol. 2024 Jun. doi: 10.14309/ajg.0000000000002820.
Top 5 Tips for Becoming an Effective Gastroenterology Consultant
Gastroenterology (GI) subspecialty training is carefully designed to develop expertise in digestive diseases and gastrointestinal endoscopy, while facilitating the transition from generalist to subspecialty consultant. The concept of effective consultation extends far beyond clinical expertise and has been explored repeatedly, beginning with Goldman’s “Ten Commandments” in 1983.1,2 How should these best practices be specifically applied to GI? More importantly, what kind of experience would you want if you were the referring provider or the patient themselves?
Below are
1. Be Kind
Survey studies of medical/surgical residents and attending hospitalists have demonstrated that willingness to accept consultation requests was the single factor consistently rated as most important in determining the quality of the consultation interaction.3,4 Unfortunately, nearly 65% of respondents reported encountering pushback when requesting subspecialty consultation. It is critical to recognize that when you receive a GI consult request, the requester has already decided that it is needed. Whether that request comports with our individual notion of “necessary” or “important,” this is a colleague’s request for help. There are myriad reasons why a request may be made, but they are unified in this principle.
Effective teamwork in healthcare settings enhances clinical performance and patient safety. Positive relationships with colleagues and healthcare team members also mitigate the emotional basis for physician burnout.5 Be kind and courteous to those who seek your assistance. Move beyond the notion of the “bad” or “soft” consult and seek instead to understand how you can help.
A requesting physician may phrase the consult question vaguely or may know that the patient is having a GI-related issue, but simply lack the specific knowledge to know what is needed. In these instances, it is our role to listen and help guide them to the correct thought process to ensure the best care of the patient. These important interactions establish our reputation, create our referral bases, and directly affect our sense of personal satisfaction.
2. Be Timely
GI presents an appealing breadth of pathology, but this also corresponds to a wide variety of indications for consultation and, therefore, urgency of need. In a busy clinical practice, not all requests can be urgently prioritized. However, it is the consultant’s responsibility to identify patients that require urgent evaluation and intervention to avert a potential adverse outcome.
We are well-trained in the medical triage of consultations. There are explicit guidelines for assessing urgency for GI bleeding, foreign body ingestion, choledocholithiasis, and many other indications. However, there are often special contextual circumstances that will elevate the urgency of a seemingly non-urgent consult request. Does the patient have an upcoming surgery or treatment that will depend on your input? Are they facing an imminent loss of insurance coverage? Is their non-severe GI disease leading to more severe impact on non-GI organ systems? The referring provider knows the patient better than you – seek to understand the context of the consult request.
Timeliness also applies to our communication. Communicate recommendations directly to the consulting service as soon as the patient is seen. When a colleague reaches out with a concern about a patient, make sure to take that request seriously. If you are unable to address the concern immediately, at least provide acknowledgment and an estimated timeline for response. As the maxim states, the effectiveness of a consultant is just as dependent on availability as it is on ability.
3. Be Specific
The same survey studies indicate that the second most critical aspect of successful subspecialty consultation is delivering clear recommendations. Accordingly, I always urge my trainees to challenge me when we leave a consult interaction if they feel that our plan is vague or imprecise.
Specificity in consult recommendations is an essential way to demonstrate your expertise and provide value. Clear and definitive recommendations enhance others’ perception of your skill, reduce the need for additional clarifying communication, and lead to more efficient, higher quality care. Avoid vague language, such as asking the requester to “consider” a test or intervention. When recommending medication, specify the dose, frequency, duration, and expected timeline of effect. Rather than recommending “cross-sectional imaging,” specify what modality and protocol. Instead of recommending “adequate resuscitation,” specify your target endpoints. If you engage in multidisciplinary discussion, ensure you strive for a specific group consensus plan and communicate this to all members of the team.
Specificity also applies to the quality of your documentation. Ensure that your clinical notes outline your rationale for your recommended plan, specific contingencies based on results of recommended testing, and a plan for follow-up care. When referring for open-access endoscopy, specifically outline what to look for and which specimens or endoscopic interventions are needed. Be precise in your procedure documentation – avoid vague terms such as small/medium/large and instead quantify in terms of millimeter/centimeter measurement. If you do not adopt specific classification schemes (e.g. Prague classification, Paris classification, Eosinophilic Esophagitis Endoscopic Reference Score, etc.), ensure you provide enough descriptive language to convey an adequate understanding of the findings.
4. Be Helpful
A consultant’s primary directive is to be of service to the consulting provider and the patient. As an educational leader, I am often asked what attributes separate a high-performing trainee from an average one. My feeling is that the most critical attribute is a sense of ownership over patient care.
As a consultant, when others feel we are exhibiting engagement and ownership in a patient’s care, they perceive that we are working together as an effective healthcare team. Interestingly, survey studies of inpatient care show that primary services do not necessarily value assistance with orders or care coordination – they consider these as core aspects of their daily work. What they did value was ongoing daily progress notes/communication, regardless of patient acuity or consulting specialty. This is a potent signal that our continued engagement (both inpatient and outpatient) is perceived as helpful.
Helpfulness is further aided by ensuring mutual understanding. While survey data indicate that sharing specific literature citations may not always be perceived positively, explaining the consultant’s rationale for their recommendations is highly valued. Take the time to tactfully explain your assessment of the patient and why you arrived at your specific recommendations. If your recommendations differ from what the requester expected (e.g. a procedure was expected but is not offered), ensure you explain why and answer questions they may have. This fosters mutual respect and proactively averts conflict or discontent from misunderstanding.
Multidisciplinary collaboration is another important avenue for aiding our patients and colleagues. Studies across a wide range of disease processes (including GI bleeding, IBD, etc.) and medical settings have demonstrated that multidisciplinary collaboration unequivocally improves patient outcomes.6 The success of these collaborations relies on our willingness to fully engage in these conversations, despite the fact that they may often be logistically challenging.
We all know how difficult it can be to locate and organize multiple medical specialists with complex varying clinical schedules and busy personal lives. Choosing to do so demonstrates a dedication to providing the highest level of care and elevates both patient and physician satisfaction. Having chosen to cultivate several ongoing multidisciplinary conferences/collaborations, I can attest to the notion that the outcome is well worth the effort.
5. Be Honest
While we always strive to provide the answers for our patients and colleagues, we must also acknowledge our limitations. Be honest with yourself when you encounter a scenario that pushes beyond the boundaries of your knowledge and comfort. Be willing to admit when you yourself need to consult others or seek an outside referral to provide the care a patient needs. Aspiring physicians often espouse that a devotion to lifelong learning is a key driver of their desire to pursue a career in medicine. These scenarios provide a key opportunity to expand our knowledge while doing what is right for our patients.
Be equally honest about your comfort with “curbside” consultations. Studies show that subspecialists receive on average of 3-4 such requests per week.7 The perception of these interactions is starkly discrepant between the requester and recipient. While over 80% of surveyed primary nonsurgical services felt that curbside consultations were helpful in patient care, a similar proportion of subspecialists expressed concern that insufficient clinical information was provided, even leading to a fear of litigation. While straightforward, informal conversations on narrow, well-defined questions can be helpful and efficient, the consultant should always feel comfortable seeking an opportunity for formal consultation when the details are unclear or the case/question is complex.
Closing Thoughts
Being an effective GI consultant isn’t just about what you know—it’s about how you apply it, how you communicate it, and how you make others feel in the process.
The attributes outlined above are not ancillary traits—they are essential components of high-quality consultation. When consistently applied, they enhance collaboration, improve patient outcomes, and reinforce trust within the healthcare system. By committing to them, you establish your reputation of excellence and play a role in elevating the field of gastroenterology more broadly.
Dr. Kahn is based in the Division of Gastroenterology and Hepatology at Mayo Clinic, Scottsdale, Arizona. He reports no conflicts of interest in regard to this article.
References
1. Goldman L, et al. Ten commandments for effective consultations. Arch Intern Med. 1983 Sep.
2. Salerno SM, et al. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007 Feb. doi: 10.1001/archinte.167.3.271.
3. Adams TN, et al. Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services. J Hosp Med. 2018 May. doi: 10.12788/jhm.2882.
4. Matsuo T, et al. Essential consultants’ skills and attitudes (Willing CONSULT): a cross-sectional survey. BMC Med Educ. 2021 Jul. doi: 10.1186/s12909-021-02810-9.
5. Welp A, Manser T. Integrating teamwork, clinician occupational well-being and patient safety - development of a conceptual framework based on a systematic review. BMC Health Serv Res. 2016 Jul. doi: 10.1186/s12913-016-1535-y.
6. Webster CS, et al. Interprofessional Learning in Multidisciplinary Healthcare Teams Is Associated With Reduced Patient Mortality: A Quantitative Systematic Review and Meta-analysis. J Patient Saf. 2024 Jan. doi: 10.1097/PTS.0000000000001170.
7. Lin M, et al. Curbside Consultations: The Good, the Bad, and the Ugly. Clin Gastroenterol Hepatol. 2016 Jan. doi: 10.1016/j.cgh.2015.09.026.
Gastroenterology (GI) subspecialty training is carefully designed to develop expertise in digestive diseases and gastrointestinal endoscopy, while facilitating the transition from generalist to subspecialty consultant. The concept of effective consultation extends far beyond clinical expertise and has been explored repeatedly, beginning with Goldman’s “Ten Commandments” in 1983.1,2 How should these best practices be specifically applied to GI? More importantly, what kind of experience would you want if you were the referring provider or the patient themselves?
Below are
1. Be Kind
Survey studies of medical/surgical residents and attending hospitalists have demonstrated that willingness to accept consultation requests was the single factor consistently rated as most important in determining the quality of the consultation interaction.3,4 Unfortunately, nearly 65% of respondents reported encountering pushback when requesting subspecialty consultation. It is critical to recognize that when you receive a GI consult request, the requester has already decided that it is needed. Whether that request comports with our individual notion of “necessary” or “important,” this is a colleague’s request for help. There are myriad reasons why a request may be made, but they are unified in this principle.
Effective teamwork in healthcare settings enhances clinical performance and patient safety. Positive relationships with colleagues and healthcare team members also mitigate the emotional basis for physician burnout.5 Be kind and courteous to those who seek your assistance. Move beyond the notion of the “bad” or “soft” consult and seek instead to understand how you can help.
A requesting physician may phrase the consult question vaguely or may know that the patient is having a GI-related issue, but simply lack the specific knowledge to know what is needed. In these instances, it is our role to listen and help guide them to the correct thought process to ensure the best care of the patient. These important interactions establish our reputation, create our referral bases, and directly affect our sense of personal satisfaction.
2. Be Timely
GI presents an appealing breadth of pathology, but this also corresponds to a wide variety of indications for consultation and, therefore, urgency of need. In a busy clinical practice, not all requests can be urgently prioritized. However, it is the consultant’s responsibility to identify patients that require urgent evaluation and intervention to avert a potential adverse outcome.
We are well-trained in the medical triage of consultations. There are explicit guidelines for assessing urgency for GI bleeding, foreign body ingestion, choledocholithiasis, and many other indications. However, there are often special contextual circumstances that will elevate the urgency of a seemingly non-urgent consult request. Does the patient have an upcoming surgery or treatment that will depend on your input? Are they facing an imminent loss of insurance coverage? Is their non-severe GI disease leading to more severe impact on non-GI organ systems? The referring provider knows the patient better than you – seek to understand the context of the consult request.
Timeliness also applies to our communication. Communicate recommendations directly to the consulting service as soon as the patient is seen. When a colleague reaches out with a concern about a patient, make sure to take that request seriously. If you are unable to address the concern immediately, at least provide acknowledgment and an estimated timeline for response. As the maxim states, the effectiveness of a consultant is just as dependent on availability as it is on ability.
3. Be Specific
The same survey studies indicate that the second most critical aspect of successful subspecialty consultation is delivering clear recommendations. Accordingly, I always urge my trainees to challenge me when we leave a consult interaction if they feel that our plan is vague or imprecise.
Specificity in consult recommendations is an essential way to demonstrate your expertise and provide value. Clear and definitive recommendations enhance others’ perception of your skill, reduce the need for additional clarifying communication, and lead to more efficient, higher quality care. Avoid vague language, such as asking the requester to “consider” a test or intervention. When recommending medication, specify the dose, frequency, duration, and expected timeline of effect. Rather than recommending “cross-sectional imaging,” specify what modality and protocol. Instead of recommending “adequate resuscitation,” specify your target endpoints. If you engage in multidisciplinary discussion, ensure you strive for a specific group consensus plan and communicate this to all members of the team.
Specificity also applies to the quality of your documentation. Ensure that your clinical notes outline your rationale for your recommended plan, specific contingencies based on results of recommended testing, and a plan for follow-up care. When referring for open-access endoscopy, specifically outline what to look for and which specimens or endoscopic interventions are needed. Be precise in your procedure documentation – avoid vague terms such as small/medium/large and instead quantify in terms of millimeter/centimeter measurement. If you do not adopt specific classification schemes (e.g. Prague classification, Paris classification, Eosinophilic Esophagitis Endoscopic Reference Score, etc.), ensure you provide enough descriptive language to convey an adequate understanding of the findings.
4. Be Helpful
A consultant’s primary directive is to be of service to the consulting provider and the patient. As an educational leader, I am often asked what attributes separate a high-performing trainee from an average one. My feeling is that the most critical attribute is a sense of ownership over patient care.
As a consultant, when others feel we are exhibiting engagement and ownership in a patient’s care, they perceive that we are working together as an effective healthcare team. Interestingly, survey studies of inpatient care show that primary services do not necessarily value assistance with orders or care coordination – they consider these as core aspects of their daily work. What they did value was ongoing daily progress notes/communication, regardless of patient acuity or consulting specialty. This is a potent signal that our continued engagement (both inpatient and outpatient) is perceived as helpful.
Helpfulness is further aided by ensuring mutual understanding. While survey data indicate that sharing specific literature citations may not always be perceived positively, explaining the consultant’s rationale for their recommendations is highly valued. Take the time to tactfully explain your assessment of the patient and why you arrived at your specific recommendations. If your recommendations differ from what the requester expected (e.g. a procedure was expected but is not offered), ensure you explain why and answer questions they may have. This fosters mutual respect and proactively averts conflict or discontent from misunderstanding.
Multidisciplinary collaboration is another important avenue for aiding our patients and colleagues. Studies across a wide range of disease processes (including GI bleeding, IBD, etc.) and medical settings have demonstrated that multidisciplinary collaboration unequivocally improves patient outcomes.6 The success of these collaborations relies on our willingness to fully engage in these conversations, despite the fact that they may often be logistically challenging.
We all know how difficult it can be to locate and organize multiple medical specialists with complex varying clinical schedules and busy personal lives. Choosing to do so demonstrates a dedication to providing the highest level of care and elevates both patient and physician satisfaction. Having chosen to cultivate several ongoing multidisciplinary conferences/collaborations, I can attest to the notion that the outcome is well worth the effort.
5. Be Honest
While we always strive to provide the answers for our patients and colleagues, we must also acknowledge our limitations. Be honest with yourself when you encounter a scenario that pushes beyond the boundaries of your knowledge and comfort. Be willing to admit when you yourself need to consult others or seek an outside referral to provide the care a patient needs. Aspiring physicians often espouse that a devotion to lifelong learning is a key driver of their desire to pursue a career in medicine. These scenarios provide a key opportunity to expand our knowledge while doing what is right for our patients.
Be equally honest about your comfort with “curbside” consultations. Studies show that subspecialists receive on average of 3-4 such requests per week.7 The perception of these interactions is starkly discrepant between the requester and recipient. While over 80% of surveyed primary nonsurgical services felt that curbside consultations were helpful in patient care, a similar proportion of subspecialists expressed concern that insufficient clinical information was provided, even leading to a fear of litigation. While straightforward, informal conversations on narrow, well-defined questions can be helpful and efficient, the consultant should always feel comfortable seeking an opportunity for formal consultation when the details are unclear or the case/question is complex.
Closing Thoughts
Being an effective GI consultant isn’t just about what you know—it’s about how you apply it, how you communicate it, and how you make others feel in the process.
The attributes outlined above are not ancillary traits—they are essential components of high-quality consultation. When consistently applied, they enhance collaboration, improve patient outcomes, and reinforce trust within the healthcare system. By committing to them, you establish your reputation of excellence and play a role in elevating the field of gastroenterology more broadly.
Dr. Kahn is based in the Division of Gastroenterology and Hepatology at Mayo Clinic, Scottsdale, Arizona. He reports no conflicts of interest in regard to this article.
References
1. Goldman L, et al. Ten commandments for effective consultations. Arch Intern Med. 1983 Sep.
2. Salerno SM, et al. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007 Feb. doi: 10.1001/archinte.167.3.271.
3. Adams TN, et al. Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services. J Hosp Med. 2018 May. doi: 10.12788/jhm.2882.
4. Matsuo T, et al. Essential consultants’ skills and attitudes (Willing CONSULT): a cross-sectional survey. BMC Med Educ. 2021 Jul. doi: 10.1186/s12909-021-02810-9.
5. Welp A, Manser T. Integrating teamwork, clinician occupational well-being and patient safety - development of a conceptual framework based on a systematic review. BMC Health Serv Res. 2016 Jul. doi: 10.1186/s12913-016-1535-y.
6. Webster CS, et al. Interprofessional Learning in Multidisciplinary Healthcare Teams Is Associated With Reduced Patient Mortality: A Quantitative Systematic Review and Meta-analysis. J Patient Saf. 2024 Jan. doi: 10.1097/PTS.0000000000001170.
7. Lin M, et al. Curbside Consultations: The Good, the Bad, and the Ugly. Clin Gastroenterol Hepatol. 2016 Jan. doi: 10.1016/j.cgh.2015.09.026.
Gastroenterology (GI) subspecialty training is carefully designed to develop expertise in digestive diseases and gastrointestinal endoscopy, while facilitating the transition from generalist to subspecialty consultant. The concept of effective consultation extends far beyond clinical expertise and has been explored repeatedly, beginning with Goldman’s “Ten Commandments” in 1983.1,2 How should these best practices be specifically applied to GI? More importantly, what kind of experience would you want if you were the referring provider or the patient themselves?
Below are
1. Be Kind
Survey studies of medical/surgical residents and attending hospitalists have demonstrated that willingness to accept consultation requests was the single factor consistently rated as most important in determining the quality of the consultation interaction.3,4 Unfortunately, nearly 65% of respondents reported encountering pushback when requesting subspecialty consultation. It is critical to recognize that when you receive a GI consult request, the requester has already decided that it is needed. Whether that request comports with our individual notion of “necessary” or “important,” this is a colleague’s request for help. There are myriad reasons why a request may be made, but they are unified in this principle.
Effective teamwork in healthcare settings enhances clinical performance and patient safety. Positive relationships with colleagues and healthcare team members also mitigate the emotional basis for physician burnout.5 Be kind and courteous to those who seek your assistance. Move beyond the notion of the “bad” or “soft” consult and seek instead to understand how you can help.
A requesting physician may phrase the consult question vaguely or may know that the patient is having a GI-related issue, but simply lack the specific knowledge to know what is needed. In these instances, it is our role to listen and help guide them to the correct thought process to ensure the best care of the patient. These important interactions establish our reputation, create our referral bases, and directly affect our sense of personal satisfaction.
2. Be Timely
GI presents an appealing breadth of pathology, but this also corresponds to a wide variety of indications for consultation and, therefore, urgency of need. In a busy clinical practice, not all requests can be urgently prioritized. However, it is the consultant’s responsibility to identify patients that require urgent evaluation and intervention to avert a potential adverse outcome.
We are well-trained in the medical triage of consultations. There are explicit guidelines for assessing urgency for GI bleeding, foreign body ingestion, choledocholithiasis, and many other indications. However, there are often special contextual circumstances that will elevate the urgency of a seemingly non-urgent consult request. Does the patient have an upcoming surgery or treatment that will depend on your input? Are they facing an imminent loss of insurance coverage? Is their non-severe GI disease leading to more severe impact on non-GI organ systems? The referring provider knows the patient better than you – seek to understand the context of the consult request.
Timeliness also applies to our communication. Communicate recommendations directly to the consulting service as soon as the patient is seen. When a colleague reaches out with a concern about a patient, make sure to take that request seriously. If you are unable to address the concern immediately, at least provide acknowledgment and an estimated timeline for response. As the maxim states, the effectiveness of a consultant is just as dependent on availability as it is on ability.
3. Be Specific
The same survey studies indicate that the second most critical aspect of successful subspecialty consultation is delivering clear recommendations. Accordingly, I always urge my trainees to challenge me when we leave a consult interaction if they feel that our plan is vague or imprecise.
Specificity in consult recommendations is an essential way to demonstrate your expertise and provide value. Clear and definitive recommendations enhance others’ perception of your skill, reduce the need for additional clarifying communication, and lead to more efficient, higher quality care. Avoid vague language, such as asking the requester to “consider” a test or intervention. When recommending medication, specify the dose, frequency, duration, and expected timeline of effect. Rather than recommending “cross-sectional imaging,” specify what modality and protocol. Instead of recommending “adequate resuscitation,” specify your target endpoints. If you engage in multidisciplinary discussion, ensure you strive for a specific group consensus plan and communicate this to all members of the team.
Specificity also applies to the quality of your documentation. Ensure that your clinical notes outline your rationale for your recommended plan, specific contingencies based on results of recommended testing, and a plan for follow-up care. When referring for open-access endoscopy, specifically outline what to look for and which specimens or endoscopic interventions are needed. Be precise in your procedure documentation – avoid vague terms such as small/medium/large and instead quantify in terms of millimeter/centimeter measurement. If you do not adopt specific classification schemes (e.g. Prague classification, Paris classification, Eosinophilic Esophagitis Endoscopic Reference Score, etc.), ensure you provide enough descriptive language to convey an adequate understanding of the findings.
4. Be Helpful
A consultant’s primary directive is to be of service to the consulting provider and the patient. As an educational leader, I am often asked what attributes separate a high-performing trainee from an average one. My feeling is that the most critical attribute is a sense of ownership over patient care.
As a consultant, when others feel we are exhibiting engagement and ownership in a patient’s care, they perceive that we are working together as an effective healthcare team. Interestingly, survey studies of inpatient care show that primary services do not necessarily value assistance with orders or care coordination – they consider these as core aspects of their daily work. What they did value was ongoing daily progress notes/communication, regardless of patient acuity or consulting specialty. This is a potent signal that our continued engagement (both inpatient and outpatient) is perceived as helpful.
Helpfulness is further aided by ensuring mutual understanding. While survey data indicate that sharing specific literature citations may not always be perceived positively, explaining the consultant’s rationale for their recommendations is highly valued. Take the time to tactfully explain your assessment of the patient and why you arrived at your specific recommendations. If your recommendations differ from what the requester expected (e.g. a procedure was expected but is not offered), ensure you explain why and answer questions they may have. This fosters mutual respect and proactively averts conflict or discontent from misunderstanding.
Multidisciplinary collaboration is another important avenue for aiding our patients and colleagues. Studies across a wide range of disease processes (including GI bleeding, IBD, etc.) and medical settings have demonstrated that multidisciplinary collaboration unequivocally improves patient outcomes.6 The success of these collaborations relies on our willingness to fully engage in these conversations, despite the fact that they may often be logistically challenging.
We all know how difficult it can be to locate and organize multiple medical specialists with complex varying clinical schedules and busy personal lives. Choosing to do so demonstrates a dedication to providing the highest level of care and elevates both patient and physician satisfaction. Having chosen to cultivate several ongoing multidisciplinary conferences/collaborations, I can attest to the notion that the outcome is well worth the effort.
5. Be Honest
While we always strive to provide the answers for our patients and colleagues, we must also acknowledge our limitations. Be honest with yourself when you encounter a scenario that pushes beyond the boundaries of your knowledge and comfort. Be willing to admit when you yourself need to consult others or seek an outside referral to provide the care a patient needs. Aspiring physicians often espouse that a devotion to lifelong learning is a key driver of their desire to pursue a career in medicine. These scenarios provide a key opportunity to expand our knowledge while doing what is right for our patients.
Be equally honest about your comfort with “curbside” consultations. Studies show that subspecialists receive on average of 3-4 such requests per week.7 The perception of these interactions is starkly discrepant between the requester and recipient. While over 80% of surveyed primary nonsurgical services felt that curbside consultations were helpful in patient care, a similar proportion of subspecialists expressed concern that insufficient clinical information was provided, even leading to a fear of litigation. While straightforward, informal conversations on narrow, well-defined questions can be helpful and efficient, the consultant should always feel comfortable seeking an opportunity for formal consultation when the details are unclear or the case/question is complex.
Closing Thoughts
Being an effective GI consultant isn’t just about what you know—it’s about how you apply it, how you communicate it, and how you make others feel in the process.
The attributes outlined above are not ancillary traits—they are essential components of high-quality consultation. When consistently applied, they enhance collaboration, improve patient outcomes, and reinforce trust within the healthcare system. By committing to them, you establish your reputation of excellence and play a role in elevating the field of gastroenterology more broadly.
Dr. Kahn is based in the Division of Gastroenterology and Hepatology at Mayo Clinic, Scottsdale, Arizona. He reports no conflicts of interest in regard to this article.
References
1. Goldman L, et al. Ten commandments for effective consultations. Arch Intern Med. 1983 Sep.
2. Salerno SM, et al. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007 Feb. doi: 10.1001/archinte.167.3.271.
3. Adams TN, et al. Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services. J Hosp Med. 2018 May. doi: 10.12788/jhm.2882.
4. Matsuo T, et al. Essential consultants’ skills and attitudes (Willing CONSULT): a cross-sectional survey. BMC Med Educ. 2021 Jul. doi: 10.1186/s12909-021-02810-9.
5. Welp A, Manser T. Integrating teamwork, clinician occupational well-being and patient safety - development of a conceptual framework based on a systematic review. BMC Health Serv Res. 2016 Jul. doi: 10.1186/s12913-016-1535-y.
6. Webster CS, et al. Interprofessional Learning in Multidisciplinary Healthcare Teams Is Associated With Reduced Patient Mortality: A Quantitative Systematic Review and Meta-analysis. J Patient Saf. 2024 Jan. doi: 10.1097/PTS.0000000000001170.
7. Lin M, et al. Curbside Consultations: The Good, the Bad, and the Ugly. Clin Gastroenterol Hepatol. 2016 Jan. doi: 10.1016/j.cgh.2015.09.026.
The Aftermath of Kennedy vs. Braidwood
In our June issue, I highlighted the potentially seismic clinical implications of the U.S. Supreme Court’s then-pending decision in the Kennedy vs. Braidwood Management, Inc., case. That ruling, recently released at the conclusion of the Court’s term, ultimately affirmed the Affordable Care Act’s mandate requiring insurers to cover certain preventive services, including colorectal cancer screening tests, without cost-sharing.
In doing so, however, the court determined that members of the U.S. Preventive Services Task Force (USPSTF), which recommends these services, are “inferior officers” appropriately appointed by the Secretary of Health and Human Services (HHS), rather than needing Senate confirmation. Thus, the decision reinforced the HHS Secretary’s authority to oversee and potentially influence USPSTF recommendations in the future. While the decision represented a victory in upholding a key provision of the ACA, it also signaled a potential threat to the scientific independence of the body charged with making those preventive care recommendations in a scientifically rigorous, unbiased manner.
As anticipated, the HHS Secretary responded to the Supreme Court’s ruling by abruptly canceling the USPSTF’s scheduled July meeting. This decision, coupled with his recent disbanding of the entire 17-member Advisory Committee on Immunization Practices — the group responsible for shaping evidence-based vaccine policy — has raised serious concerns across the healthcare field. On July 9th, AGA joined a coalition of 104 health organizations in submitting a letter to the Chair and Ranking Members of the Senate Committee on Health, Education, Labor and Pensions and the House Committee on Energy and Commerce, urging them to protect the integrity of the USPSTF.
The fight to protect science-based health policy is far from over — effective advocacy necessitates that clinicians use their professional platforms to push back against the politicization of science – not only for the integrity of the medical profession, but for the health and future of the patients we serve.
Megan A. Adams, MD, JD, MSc
Editor in Chief
In our June issue, I highlighted the potentially seismic clinical implications of the U.S. Supreme Court’s then-pending decision in the Kennedy vs. Braidwood Management, Inc., case. That ruling, recently released at the conclusion of the Court’s term, ultimately affirmed the Affordable Care Act’s mandate requiring insurers to cover certain preventive services, including colorectal cancer screening tests, without cost-sharing.
In doing so, however, the court determined that members of the U.S. Preventive Services Task Force (USPSTF), which recommends these services, are “inferior officers” appropriately appointed by the Secretary of Health and Human Services (HHS), rather than needing Senate confirmation. Thus, the decision reinforced the HHS Secretary’s authority to oversee and potentially influence USPSTF recommendations in the future. While the decision represented a victory in upholding a key provision of the ACA, it also signaled a potential threat to the scientific independence of the body charged with making those preventive care recommendations in a scientifically rigorous, unbiased manner.
As anticipated, the HHS Secretary responded to the Supreme Court’s ruling by abruptly canceling the USPSTF’s scheduled July meeting. This decision, coupled with his recent disbanding of the entire 17-member Advisory Committee on Immunization Practices — the group responsible for shaping evidence-based vaccine policy — has raised serious concerns across the healthcare field. On July 9th, AGA joined a coalition of 104 health organizations in submitting a letter to the Chair and Ranking Members of the Senate Committee on Health, Education, Labor and Pensions and the House Committee on Energy and Commerce, urging them to protect the integrity of the USPSTF.
The fight to protect science-based health policy is far from over — effective advocacy necessitates that clinicians use their professional platforms to push back against the politicization of science – not only for the integrity of the medical profession, but for the health and future of the patients we serve.
Megan A. Adams, MD, JD, MSc
Editor in Chief
In our June issue, I highlighted the potentially seismic clinical implications of the U.S. Supreme Court’s then-pending decision in the Kennedy vs. Braidwood Management, Inc., case. That ruling, recently released at the conclusion of the Court’s term, ultimately affirmed the Affordable Care Act’s mandate requiring insurers to cover certain preventive services, including colorectal cancer screening tests, without cost-sharing.
In doing so, however, the court determined that members of the U.S. Preventive Services Task Force (USPSTF), which recommends these services, are “inferior officers” appropriately appointed by the Secretary of Health and Human Services (HHS), rather than needing Senate confirmation. Thus, the decision reinforced the HHS Secretary’s authority to oversee and potentially influence USPSTF recommendations in the future. While the decision represented a victory in upholding a key provision of the ACA, it also signaled a potential threat to the scientific independence of the body charged with making those preventive care recommendations in a scientifically rigorous, unbiased manner.
As anticipated, the HHS Secretary responded to the Supreme Court’s ruling by abruptly canceling the USPSTF’s scheduled July meeting. This decision, coupled with his recent disbanding of the entire 17-member Advisory Committee on Immunization Practices — the group responsible for shaping evidence-based vaccine policy — has raised serious concerns across the healthcare field. On July 9th, AGA joined a coalition of 104 health organizations in submitting a letter to the Chair and Ranking Members of the Senate Committee on Health, Education, Labor and Pensions and the House Committee on Energy and Commerce, urging them to protect the integrity of the USPSTF.
The fight to protect science-based health policy is far from over — effective advocacy necessitates that clinicians use their professional platforms to push back against the politicization of science – not only for the integrity of the medical profession, but for the health and future of the patients we serve.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Experiencing DDW as an Early Career GI
Dear Friends,
Like many readers, I just returned from Digestive Disease Week® (DDW) in San Diego, California. For the first time in my early career, my experience was not just overwhelming and exhausting. Before, I wanted to do everything – lectures, posters, meetings with friends, prospective research collaborators, and more! This year, I acknowledged that instead of spreading myself thin and not fully engaging, I made a focused daily schedule mixed with productivity and social events, selecting only what was most important to me at this time in my career. This time, after DDW, instead of giving in to my inner introvert and holing myself in my house for a week to recover, I am invigorated by what I learned and the people I met. I can’t wait to see what’s to come next year!
In this issue’s “In Focus”, Dr. Evan Dellon describes his diagnostic approach, including a clear history, endoscopic evaluation with biopsy, and ruling out other causes of esophageal eosinophilia. He emphasizes that treatment should target both inflammation and fibrostenosis and reviews the guidelines and evidence behind first-line treatments, surveillance, and long-term maintenance.
In the second of a two-part series in the “Short Clinical Review” section, Dr. Christopher Vélez, Dr. Rosa L. Yu, and Dr. Jennifer Dimino discuss care for patients with disorders of brain-gut interaction from historically marginalized communities. They highlight ways to improve care for these patients in day-to-day clinical practice.
The transition from trainee to a practicing gastroenterologist may bring with it responsibilities of giving feedback to trainees and/or colleagues to improve. In the “Early Career” section, Dr. Michelle Baliss and Dr. Christine Hachem give practical tips on how best to deliver feedback, with a focus on creating time, building rapport, bidirectional communication, and more.
Lastly, in the “Finance/Legal” section, John S. Gardner, a financial advisor, guides trainees and early career gastroenterologists through estate planning – why it’s important, how to do it effectively, and long-term benefits to starting early.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: the first case of eosinophilic esophagitis was only first described in 1978 and became a distinct entity in the early 1990s.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
Like many readers, I just returned from Digestive Disease Week® (DDW) in San Diego, California. For the first time in my early career, my experience was not just overwhelming and exhausting. Before, I wanted to do everything – lectures, posters, meetings with friends, prospective research collaborators, and more! This year, I acknowledged that instead of spreading myself thin and not fully engaging, I made a focused daily schedule mixed with productivity and social events, selecting only what was most important to me at this time in my career. This time, after DDW, instead of giving in to my inner introvert and holing myself in my house for a week to recover, I am invigorated by what I learned and the people I met. I can’t wait to see what’s to come next year!
In this issue’s “In Focus”, Dr. Evan Dellon describes his diagnostic approach, including a clear history, endoscopic evaluation with biopsy, and ruling out other causes of esophageal eosinophilia. He emphasizes that treatment should target both inflammation and fibrostenosis and reviews the guidelines and evidence behind first-line treatments, surveillance, and long-term maintenance.
In the second of a two-part series in the “Short Clinical Review” section, Dr. Christopher Vélez, Dr. Rosa L. Yu, and Dr. Jennifer Dimino discuss care for patients with disorders of brain-gut interaction from historically marginalized communities. They highlight ways to improve care for these patients in day-to-day clinical practice.
The transition from trainee to a practicing gastroenterologist may bring with it responsibilities of giving feedback to trainees and/or colleagues to improve. In the “Early Career” section, Dr. Michelle Baliss and Dr. Christine Hachem give practical tips on how best to deliver feedback, with a focus on creating time, building rapport, bidirectional communication, and more.
Lastly, in the “Finance/Legal” section, John S. Gardner, a financial advisor, guides trainees and early career gastroenterologists through estate planning – why it’s important, how to do it effectively, and long-term benefits to starting early.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: the first case of eosinophilic esophagitis was only first described in 1978 and became a distinct entity in the early 1990s.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
Like many readers, I just returned from Digestive Disease Week® (DDW) in San Diego, California. For the first time in my early career, my experience was not just overwhelming and exhausting. Before, I wanted to do everything – lectures, posters, meetings with friends, prospective research collaborators, and more! This year, I acknowledged that instead of spreading myself thin and not fully engaging, I made a focused daily schedule mixed with productivity and social events, selecting only what was most important to me at this time in my career. This time, after DDW, instead of giving in to my inner introvert and holing myself in my house for a week to recover, I am invigorated by what I learned and the people I met. I can’t wait to see what’s to come next year!
In this issue’s “In Focus”, Dr. Evan Dellon describes his diagnostic approach, including a clear history, endoscopic evaluation with biopsy, and ruling out other causes of esophageal eosinophilia. He emphasizes that treatment should target both inflammation and fibrostenosis and reviews the guidelines and evidence behind first-line treatments, surveillance, and long-term maintenance.
In the second of a two-part series in the “Short Clinical Review” section, Dr. Christopher Vélez, Dr. Rosa L. Yu, and Dr. Jennifer Dimino discuss care for patients with disorders of brain-gut interaction from historically marginalized communities. They highlight ways to improve care for these patients in day-to-day clinical practice.
The transition from trainee to a practicing gastroenterologist may bring with it responsibilities of giving feedback to trainees and/or colleagues to improve. In the “Early Career” section, Dr. Michelle Baliss and Dr. Christine Hachem give practical tips on how best to deliver feedback, with a focus on creating time, building rapport, bidirectional communication, and more.
Lastly, in the “Finance/Legal” section, John S. Gardner, a financial advisor, guides trainees and early career gastroenterologists through estate planning – why it’s important, how to do it effectively, and long-term benefits to starting early.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: the first case of eosinophilic esophagitis was only first described in 1978 and became a distinct entity in the early 1990s.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Practical Tips on Delivering Feedback to Trainees and Colleagues
Feedback is the purposeful practice of offering constructive, goal-directed input rooted in the power of observation and behavioral assessment. Healthcare inherently fosters a broad range of interactions among people with unique insights, and feedback can naturally emerge from this milieu. In medical training, feedback is an indispensable element that personalizes the learning process and drives the professional development of physicians through all career stages.
If delivered effectively, feedback can strengthen the relationship between the evaluator and recipient, promote self-reflection, and enhance motivation. As such, it has the potential to impact us and those we serve for a lifetime. Feedback has been invaluable to our growth as clinicians and has been embedded into our roles as educators. However, Here, we provide some “tried and true” practical tips on delivering feedback to trainees and co-workers and on navigating potential barriers based on lessons learned.
Barriers to Effective Feedback
- Time: Feedback is predicated on observation over time and consideration of repetitive processes rather than isolated events. Perhaps the most challenging factor faced by both parties is that of time constraints, leading to limited ability to engage and build rapport.
- Fear: Hesitancy by evaluators to provide feedback in fear of negative impacts on the recipient’s morale or rapport can lead them to shy away from personalized corrective feedback strategies and choose to rely on written evaluations or generic advice.
- Varying approaches: Feedback strategies have evolved from unidirectional, critique-based, hierarchical practices that emphasize the evaluator’s skills to models that prioritize the recipient’s goals and participation (see Table 1). Traditionally employed feedback models such as the “Feedback Sandwich” or the “Pendleton Rules” are criticized because of a lack of proven benefit on performance, recipient goal prioritization, and open communication.1,2 Studies showing incongruent perceptions of feedback adequacy between trainees and faculty further support the need for recipient-focused strategies.3 Recognition of the foundational role of the reciprocal learner-teacher alliance in feedback integration inspired newer feedback models, such as the “R2C2” and the “Self-Assessment, Feedback, Encouragement, Direction.”4,5
But which way is best? With increasing abundance and complexity of feedback frameworks, selecting an approach can feel overwhelming and impractical. A generic “one-size-fits-all” strategy or avoidance of feedback altogether can be detrimental. Structured feedback models can also lead to rigid, inauthentic interactions. Below, we suggest a more practical approach through our tips that unifies the common themes of various feedback models and embeds them into daily practice habits while leaving room for personalization.
Our Practical Feedback Tips
Tip 1: Set the scene: Create a positive feedback culture
Proactively creating a culture in which feedback is embedded and encouraged is perhaps the most important step. Priming both parties for feedback clarifies intent, increases receptiveness, and paves the way for growth and open communication. It also prevents the misinterpretation of unexpected feedback as an expression of disapproval. To do this, start by regularly stating your intentions at the start of every experience. Explicitly expressing your vision for mutual learning, bidirectional feedback, and growth in your respective roles attaches a positive intention to feedback. Providing a reminder that we are all works in progress and acknowledging this on a regular basis sets the stage for structured growth opportunities.
Scheduling future feedback encounters from the start maintains accountability and prevents feedback from being perceived as the consequence of a particular behavior. The number and timing of feedback sessions can be customized to the duration of the working relationship, generally allowing enough time for a second interaction (at the end of each week, halfway point, etc.).
Tip 2: Build rapport
Increasing clinical workloads and pressure to teach in time-constrained settings often results in insufficient time to engage in conversation and trust building. However, a foundational relationship is an essential precursor to meaningful feedback. Ramani et al. state that “relationships, not recipes, are more likely to promote feedback that has an impact on learner performance and ultimately patient care.”6 Building this rapport can begin by dedicating a few minutes (before/during rounds, between cases) to exchange information about career interests, hobbies, favorite restaurants, etc. This “small talk” is the beginning of a two-way exchange that ultimately develops into more meaningful exchanges.
In our experience, this simple step is impactful and fulfilling to both parties. This is also a good time for shared vulnerability by talking about what you are currently working on or have worked on at their stage to affirm that feedback is a continuous part of professional development and not a reflection of how far they are from competence at a given point in time.
Tip 3: Consider Timing, assess readiness, and preschedule sessions
Lack of attention to timing can hinder feedback acceptance. We suggest adhering to delivering positive feedback publicly and corrective feedback privately (“Praise in public, perfect in private”). This reinforces positive behaviors, increases motivation, and minimizes demoralization. Prolonged delays between the observed behavior and feedback can decrease its relevance. Conversely, delivering feedback too soon after an emotionally charged experience can be perceived as blame. Pre-designated times for feedback can minimize the guesswork and maintain your accountability for giving feedback without inadvertently linking it to one particular behavior. If the recipient does not appear to be in a state to receive feedback at the predesignated time, you can pivot to a “check-in” session to show support and strengthen rapport.
Tip 4: Customize to the learner and set shared goals
Diversity in backgrounds, perspectives, and personalities can impact how people perceive their own performances and experience feedback. Given the profound impact of sociocultural factors on feedback assimilation, maintaining the recipient and their goals at the core of performance evaluations is key to feedback acceptance.
A. Trainees
We suggest starting by introducing the idea of feedback as a partnership and something you feel privileged to do to help them achieve mutual goals. It helps to ask them to use the first day to get oriented with the experience, general expectations, challenges they expect to encounter, and their feedback goals. Tailoring your feedback to their goals creates a sense of shared purpose which increases motivation. Encouraging them to develop their own strategies allows them to play an active role in their growth. Giving them the opportunity to share their perceived strengths and deficiencies provides you with valuable information regarding their insight and ability to self-evaluate. This can help you predict their readiness for your feedback and to tailor your approach when there is a mismatch.
Examples:
- Medical student: Start with “What do you think you are doing well?” and “What do you think you need to work on?” Build on their response with encouragement and empathy. This helps make them more deliberate with what they work on because being a medical student can be overwhelming and can feel as though they have everything to work on.
- Resident/Fellow: By this point, trainees usually have an increased awareness of their strengths and deficiencies. Your questions can then be more specific, giving them autonomy over their learning, such as “What are some of the things you are working on that you want me to give you feedback on this week?” This makes them more aware, intentional, and receptive to your feedback because it is framed as something that they sought out.
B. Colleagues/Staff
Unlike the training environment in which feedback is built-in, giving feedback to co-workers requires you to establish a feedback-conducive environment and to develop a more in-depth understanding of coworkers’ personalities. Similar strategies can be applied, such as proactively setting the scene for open communication, scheduling check-ins, demonstrating receptiveness to feedback, and investing in trust-building.
Longer working relationships allow for strong foundational connections that make feedback less threatening. Personality assessment testing like Myers-Briggs Type Indicator or DiSC Assessment can aid in tailoring feedback to different individuals.7,8 An analytical thinker may appreciate direct, data-driven feedback. Relationship-oriented individuals might respond better to softer, encouragement-based approaches. Always maintain shared goals at the center of your interactions and consider collaborative opportunities such as quality improvement projects. This can improve your working relationship in a constructive way without casting blame.
Tip 5: Work on delivery: Bidirectional communication and body language
Non-verbal cues can have a profound impact on how your feedback is interpreted and on the recipient’s comfort to engage in conversation. Sitting down, making eye contact, nodding, and avoiding closed-off body posture can project support and feel less judgmental. Creating a safe and non-distracted environment with privacy can make them feel valued. Use motivating, respectful language focused on directly observed behaviors rather than personal attributes or second-hand reports.
Remember that focusing on repetitive patterns is likely more helpful than isolated incidents. Validate their hard work and give them a global idea of where they stand before diving into individual behaviors. Encourage their participation and empower them to suggest changes they plan to implement. Conclude by having them summarize their action plan to give them ownership and to verify that your feedback was interpreted as you intended. Thank them for being a part of the process, as it does take a partnership for feedback to be effective.
Tip 6: Be open to feedback
Demonstrating your willingness to accept and act on feedback reinforces a positive culture where feedback is normalized and valued. After an unintended outcome, initiate a two-way conversation and ask their input on anything they wish you would have done differently. This reaffirms your commitment to maintaining culture that does not revolve around one-sided critiques. Frequently soliciting feedback about your feedback skills can also guide you to adapt your approach and to recognize any ineffective feedback practices.
Tip 7: When things don’t go as planned
Receiving feedback, no matter how thoughtfully it is delivered, can be an emotionally-charged experience ending in hurt feelings. This happens because of misinterpretation of feedback as an indicator of inadequacy, heightened awareness of underlying insecurities, sociocultural or personal circumstances, frustration with oneself, needing additional guidance, or being caught off-guard by the assessment.
The evaluator should always acknowledge the recipient’s feelings, show compassion, and allow time for processing. When they are ready to talk, it is important to help reframe the recipients’ mindsets to recognize that feedback is not personal or defining and is not a “one and done” reflection of whether they have “made it.” Instead, it is a continual process that we benefit from through all career stages. Again, shared vulnerability can help to normalize feedback and maintain open dialogue. Setting an opportunity for a future check-in can reinforce support and lead to a more productive conversation after they have had time to process.
Conclusion
Effective feedback delivery is an invaluable skill that can result in meaningful goal-directed changes while strengthening professional relationships. Given the complexity of feedback interactions and the many factors that influence its acceptance, no single approach is suitable for all recipients and frequent adaptation of the approach is essential.
In our experience, adhering to these general overarching feedback principles (see Figure 1) has allowed us to have more successful interactions with trainees and colleagues.
Dr. Baliss is based in the Division of Gastroenterology, Washington University in St. Louis, Missouri. Dr. Hachem is director of the Division of Gastroenterology and Digestive Health at Intermountain Medical, Sandy, Utah. Both authors declare no conflicts of interest.
References
1. Parkes J, et al. Feedback sandwiches affect perceptions but not performance. Adv Health Sci Educ Theory Pract. 2013 Aug. doi:10.1007/s10459-012-9377-9.
2. van de Ridder JMM and Wijnen-Meijer M. Pendleton’s Rules: A Mini Review of a Feedback Method. Am J Biomed Sci & Res. 2023 May. doi: 10.34297/AJBSR.2023.19.002542.
3. Sender Liberman A, et al. Surgery residents and attending surgeons have different perceptions of feedback. Med Teach. 2005 Aug. doi: 10.1080/0142590500129183.
4. Sargeant J, et al. R2C2 in Action: Testing an Evidence-Based Model to Facilitate Feedback and Coaching in Residency. J Grad Med Educ. 2017 Apr. doi: 10.4300/JGME-D-16-00398.1.
5. Liakos W, et al. Frameworks for Effective Feedback in Health Professions Education. Acad Med. 2023 May. doi: 10.1097/ACM.0000000000004884.
6. Ramani S, et al. Feedback Redefined: Principles and Practice. J Gen Intern Med. 2019 May. doi: 10.1007/s11606-019-04874-2.
7. Woods RA and Hill PB. Myers-Briggs Type Indicator. StatPearls. StatPearls Publishing. 2022 Sept. https://www.ncbi.nlm.nih.gov/books/NBK554596/
8. Slowikowski MK. Using the DISC behavioral instrument to guide leadership and communication. AORN J. 2005 Nov. doi: 10.1016/s0001-2092(06)60276-7.
Feedback is the purposeful practice of offering constructive, goal-directed input rooted in the power of observation and behavioral assessment. Healthcare inherently fosters a broad range of interactions among people with unique insights, and feedback can naturally emerge from this milieu. In medical training, feedback is an indispensable element that personalizes the learning process and drives the professional development of physicians through all career stages.
If delivered effectively, feedback can strengthen the relationship between the evaluator and recipient, promote self-reflection, and enhance motivation. As such, it has the potential to impact us and those we serve for a lifetime. Feedback has been invaluable to our growth as clinicians and has been embedded into our roles as educators. However, Here, we provide some “tried and true” practical tips on delivering feedback to trainees and co-workers and on navigating potential barriers based on lessons learned.
Barriers to Effective Feedback
- Time: Feedback is predicated on observation over time and consideration of repetitive processes rather than isolated events. Perhaps the most challenging factor faced by both parties is that of time constraints, leading to limited ability to engage and build rapport.
- Fear: Hesitancy by evaluators to provide feedback in fear of negative impacts on the recipient’s morale or rapport can lead them to shy away from personalized corrective feedback strategies and choose to rely on written evaluations or generic advice.
- Varying approaches: Feedback strategies have evolved from unidirectional, critique-based, hierarchical practices that emphasize the evaluator’s skills to models that prioritize the recipient’s goals and participation (see Table 1). Traditionally employed feedback models such as the “Feedback Sandwich” or the “Pendleton Rules” are criticized because of a lack of proven benefit on performance, recipient goal prioritization, and open communication.1,2 Studies showing incongruent perceptions of feedback adequacy between trainees and faculty further support the need for recipient-focused strategies.3 Recognition of the foundational role of the reciprocal learner-teacher alliance in feedback integration inspired newer feedback models, such as the “R2C2” and the “Self-Assessment, Feedback, Encouragement, Direction.”4,5
But which way is best? With increasing abundance and complexity of feedback frameworks, selecting an approach can feel overwhelming and impractical. A generic “one-size-fits-all” strategy or avoidance of feedback altogether can be detrimental. Structured feedback models can also lead to rigid, inauthentic interactions. Below, we suggest a more practical approach through our tips that unifies the common themes of various feedback models and embeds them into daily practice habits while leaving room for personalization.
Our Practical Feedback Tips
Tip 1: Set the scene: Create a positive feedback culture
Proactively creating a culture in which feedback is embedded and encouraged is perhaps the most important step. Priming both parties for feedback clarifies intent, increases receptiveness, and paves the way for growth and open communication. It also prevents the misinterpretation of unexpected feedback as an expression of disapproval. To do this, start by regularly stating your intentions at the start of every experience. Explicitly expressing your vision for mutual learning, bidirectional feedback, and growth in your respective roles attaches a positive intention to feedback. Providing a reminder that we are all works in progress and acknowledging this on a regular basis sets the stage for structured growth opportunities.
Scheduling future feedback encounters from the start maintains accountability and prevents feedback from being perceived as the consequence of a particular behavior. The number and timing of feedback sessions can be customized to the duration of the working relationship, generally allowing enough time for a second interaction (at the end of each week, halfway point, etc.).
Tip 2: Build rapport
Increasing clinical workloads and pressure to teach in time-constrained settings often results in insufficient time to engage in conversation and trust building. However, a foundational relationship is an essential precursor to meaningful feedback. Ramani et al. state that “relationships, not recipes, are more likely to promote feedback that has an impact on learner performance and ultimately patient care.”6 Building this rapport can begin by dedicating a few minutes (before/during rounds, between cases) to exchange information about career interests, hobbies, favorite restaurants, etc. This “small talk” is the beginning of a two-way exchange that ultimately develops into more meaningful exchanges.
In our experience, this simple step is impactful and fulfilling to both parties. This is also a good time for shared vulnerability by talking about what you are currently working on or have worked on at their stage to affirm that feedback is a continuous part of professional development and not a reflection of how far they are from competence at a given point in time.
Tip 3: Consider Timing, assess readiness, and preschedule sessions
Lack of attention to timing can hinder feedback acceptance. We suggest adhering to delivering positive feedback publicly and corrective feedback privately (“Praise in public, perfect in private”). This reinforces positive behaviors, increases motivation, and minimizes demoralization. Prolonged delays between the observed behavior and feedback can decrease its relevance. Conversely, delivering feedback too soon after an emotionally charged experience can be perceived as blame. Pre-designated times for feedback can minimize the guesswork and maintain your accountability for giving feedback without inadvertently linking it to one particular behavior. If the recipient does not appear to be in a state to receive feedback at the predesignated time, you can pivot to a “check-in” session to show support and strengthen rapport.
Tip 4: Customize to the learner and set shared goals
Diversity in backgrounds, perspectives, and personalities can impact how people perceive their own performances and experience feedback. Given the profound impact of sociocultural factors on feedback assimilation, maintaining the recipient and their goals at the core of performance evaluations is key to feedback acceptance.
A. Trainees
We suggest starting by introducing the idea of feedback as a partnership and something you feel privileged to do to help them achieve mutual goals. It helps to ask them to use the first day to get oriented with the experience, general expectations, challenges they expect to encounter, and their feedback goals. Tailoring your feedback to their goals creates a sense of shared purpose which increases motivation. Encouraging them to develop their own strategies allows them to play an active role in their growth. Giving them the opportunity to share their perceived strengths and deficiencies provides you with valuable information regarding their insight and ability to self-evaluate. This can help you predict their readiness for your feedback and to tailor your approach when there is a mismatch.
Examples:
- Medical student: Start with “What do you think you are doing well?” and “What do you think you need to work on?” Build on their response with encouragement and empathy. This helps make them more deliberate with what they work on because being a medical student can be overwhelming and can feel as though they have everything to work on.
- Resident/Fellow: By this point, trainees usually have an increased awareness of their strengths and deficiencies. Your questions can then be more specific, giving them autonomy over their learning, such as “What are some of the things you are working on that you want me to give you feedback on this week?” This makes them more aware, intentional, and receptive to your feedback because it is framed as something that they sought out.
B. Colleagues/Staff
Unlike the training environment in which feedback is built-in, giving feedback to co-workers requires you to establish a feedback-conducive environment and to develop a more in-depth understanding of coworkers’ personalities. Similar strategies can be applied, such as proactively setting the scene for open communication, scheduling check-ins, demonstrating receptiveness to feedback, and investing in trust-building.
Longer working relationships allow for strong foundational connections that make feedback less threatening. Personality assessment testing like Myers-Briggs Type Indicator or DiSC Assessment can aid in tailoring feedback to different individuals.7,8 An analytical thinker may appreciate direct, data-driven feedback. Relationship-oriented individuals might respond better to softer, encouragement-based approaches. Always maintain shared goals at the center of your interactions and consider collaborative opportunities such as quality improvement projects. This can improve your working relationship in a constructive way without casting blame.
Tip 5: Work on delivery: Bidirectional communication and body language
Non-verbal cues can have a profound impact on how your feedback is interpreted and on the recipient’s comfort to engage in conversation. Sitting down, making eye contact, nodding, and avoiding closed-off body posture can project support and feel less judgmental. Creating a safe and non-distracted environment with privacy can make them feel valued. Use motivating, respectful language focused on directly observed behaviors rather than personal attributes or second-hand reports.
Remember that focusing on repetitive patterns is likely more helpful than isolated incidents. Validate their hard work and give them a global idea of where they stand before diving into individual behaviors. Encourage their participation and empower them to suggest changes they plan to implement. Conclude by having them summarize their action plan to give them ownership and to verify that your feedback was interpreted as you intended. Thank them for being a part of the process, as it does take a partnership for feedback to be effective.
Tip 6: Be open to feedback
Demonstrating your willingness to accept and act on feedback reinforces a positive culture where feedback is normalized and valued. After an unintended outcome, initiate a two-way conversation and ask their input on anything they wish you would have done differently. This reaffirms your commitment to maintaining culture that does not revolve around one-sided critiques. Frequently soliciting feedback about your feedback skills can also guide you to adapt your approach and to recognize any ineffective feedback practices.
Tip 7: When things don’t go as planned
Receiving feedback, no matter how thoughtfully it is delivered, can be an emotionally-charged experience ending in hurt feelings. This happens because of misinterpretation of feedback as an indicator of inadequacy, heightened awareness of underlying insecurities, sociocultural or personal circumstances, frustration with oneself, needing additional guidance, or being caught off-guard by the assessment.
The evaluator should always acknowledge the recipient’s feelings, show compassion, and allow time for processing. When they are ready to talk, it is important to help reframe the recipients’ mindsets to recognize that feedback is not personal or defining and is not a “one and done” reflection of whether they have “made it.” Instead, it is a continual process that we benefit from through all career stages. Again, shared vulnerability can help to normalize feedback and maintain open dialogue. Setting an opportunity for a future check-in can reinforce support and lead to a more productive conversation after they have had time to process.
Conclusion
Effective feedback delivery is an invaluable skill that can result in meaningful goal-directed changes while strengthening professional relationships. Given the complexity of feedback interactions and the many factors that influence its acceptance, no single approach is suitable for all recipients and frequent adaptation of the approach is essential.
In our experience, adhering to these general overarching feedback principles (see Figure 1) has allowed us to have more successful interactions with trainees and colleagues.
Dr. Baliss is based in the Division of Gastroenterology, Washington University in St. Louis, Missouri. Dr. Hachem is director of the Division of Gastroenterology and Digestive Health at Intermountain Medical, Sandy, Utah. Both authors declare no conflicts of interest.
References
1. Parkes J, et al. Feedback sandwiches affect perceptions but not performance. Adv Health Sci Educ Theory Pract. 2013 Aug. doi:10.1007/s10459-012-9377-9.
2. van de Ridder JMM and Wijnen-Meijer M. Pendleton’s Rules: A Mini Review of a Feedback Method. Am J Biomed Sci & Res. 2023 May. doi: 10.34297/AJBSR.2023.19.002542.
3. Sender Liberman A, et al. Surgery residents and attending surgeons have different perceptions of feedback. Med Teach. 2005 Aug. doi: 10.1080/0142590500129183.
4. Sargeant J, et al. R2C2 in Action: Testing an Evidence-Based Model to Facilitate Feedback and Coaching in Residency. J Grad Med Educ. 2017 Apr. doi: 10.4300/JGME-D-16-00398.1.
5. Liakos W, et al. Frameworks for Effective Feedback in Health Professions Education. Acad Med. 2023 May. doi: 10.1097/ACM.0000000000004884.
6. Ramani S, et al. Feedback Redefined: Principles and Practice. J Gen Intern Med. 2019 May. doi: 10.1007/s11606-019-04874-2.
7. Woods RA and Hill PB. Myers-Briggs Type Indicator. StatPearls. StatPearls Publishing. 2022 Sept. https://www.ncbi.nlm.nih.gov/books/NBK554596/
8. Slowikowski MK. Using the DISC behavioral instrument to guide leadership and communication. AORN J. 2005 Nov. doi: 10.1016/s0001-2092(06)60276-7.
Feedback is the purposeful practice of offering constructive, goal-directed input rooted in the power of observation and behavioral assessment. Healthcare inherently fosters a broad range of interactions among people with unique insights, and feedback can naturally emerge from this milieu. In medical training, feedback is an indispensable element that personalizes the learning process and drives the professional development of physicians through all career stages.
If delivered effectively, feedback can strengthen the relationship between the evaluator and recipient, promote self-reflection, and enhance motivation. As such, it has the potential to impact us and those we serve for a lifetime. Feedback has been invaluable to our growth as clinicians and has been embedded into our roles as educators. However, Here, we provide some “tried and true” practical tips on delivering feedback to trainees and co-workers and on navigating potential barriers based on lessons learned.
Barriers to Effective Feedback
- Time: Feedback is predicated on observation over time and consideration of repetitive processes rather than isolated events. Perhaps the most challenging factor faced by both parties is that of time constraints, leading to limited ability to engage and build rapport.
- Fear: Hesitancy by evaluators to provide feedback in fear of negative impacts on the recipient’s morale or rapport can lead them to shy away from personalized corrective feedback strategies and choose to rely on written evaluations or generic advice.
- Varying approaches: Feedback strategies have evolved from unidirectional, critique-based, hierarchical practices that emphasize the evaluator’s skills to models that prioritize the recipient’s goals and participation (see Table 1). Traditionally employed feedback models such as the “Feedback Sandwich” or the “Pendleton Rules” are criticized because of a lack of proven benefit on performance, recipient goal prioritization, and open communication.1,2 Studies showing incongruent perceptions of feedback adequacy between trainees and faculty further support the need for recipient-focused strategies.3 Recognition of the foundational role of the reciprocal learner-teacher alliance in feedback integration inspired newer feedback models, such as the “R2C2” and the “Self-Assessment, Feedback, Encouragement, Direction.”4,5
But which way is best? With increasing abundance and complexity of feedback frameworks, selecting an approach can feel overwhelming and impractical. A generic “one-size-fits-all” strategy or avoidance of feedback altogether can be detrimental. Structured feedback models can also lead to rigid, inauthentic interactions. Below, we suggest a more practical approach through our tips that unifies the common themes of various feedback models and embeds them into daily practice habits while leaving room for personalization.
Our Practical Feedback Tips
Tip 1: Set the scene: Create a positive feedback culture
Proactively creating a culture in which feedback is embedded and encouraged is perhaps the most important step. Priming both parties for feedback clarifies intent, increases receptiveness, and paves the way for growth and open communication. It also prevents the misinterpretation of unexpected feedback as an expression of disapproval. To do this, start by regularly stating your intentions at the start of every experience. Explicitly expressing your vision for mutual learning, bidirectional feedback, and growth in your respective roles attaches a positive intention to feedback. Providing a reminder that we are all works in progress and acknowledging this on a regular basis sets the stage for structured growth opportunities.
Scheduling future feedback encounters from the start maintains accountability and prevents feedback from being perceived as the consequence of a particular behavior. The number and timing of feedback sessions can be customized to the duration of the working relationship, generally allowing enough time for a second interaction (at the end of each week, halfway point, etc.).
Tip 2: Build rapport
Increasing clinical workloads and pressure to teach in time-constrained settings often results in insufficient time to engage in conversation and trust building. However, a foundational relationship is an essential precursor to meaningful feedback. Ramani et al. state that “relationships, not recipes, are more likely to promote feedback that has an impact on learner performance and ultimately patient care.”6 Building this rapport can begin by dedicating a few minutes (before/during rounds, between cases) to exchange information about career interests, hobbies, favorite restaurants, etc. This “small talk” is the beginning of a two-way exchange that ultimately develops into more meaningful exchanges.
In our experience, this simple step is impactful and fulfilling to both parties. This is also a good time for shared vulnerability by talking about what you are currently working on or have worked on at their stage to affirm that feedback is a continuous part of professional development and not a reflection of how far they are from competence at a given point in time.
Tip 3: Consider Timing, assess readiness, and preschedule sessions
Lack of attention to timing can hinder feedback acceptance. We suggest adhering to delivering positive feedback publicly and corrective feedback privately (“Praise in public, perfect in private”). This reinforces positive behaviors, increases motivation, and minimizes demoralization. Prolonged delays between the observed behavior and feedback can decrease its relevance. Conversely, delivering feedback too soon after an emotionally charged experience can be perceived as blame. Pre-designated times for feedback can minimize the guesswork and maintain your accountability for giving feedback without inadvertently linking it to one particular behavior. If the recipient does not appear to be in a state to receive feedback at the predesignated time, you can pivot to a “check-in” session to show support and strengthen rapport.
Tip 4: Customize to the learner and set shared goals
Diversity in backgrounds, perspectives, and personalities can impact how people perceive their own performances and experience feedback. Given the profound impact of sociocultural factors on feedback assimilation, maintaining the recipient and their goals at the core of performance evaluations is key to feedback acceptance.
A. Trainees
We suggest starting by introducing the idea of feedback as a partnership and something you feel privileged to do to help them achieve mutual goals. It helps to ask them to use the first day to get oriented with the experience, general expectations, challenges they expect to encounter, and their feedback goals. Tailoring your feedback to their goals creates a sense of shared purpose which increases motivation. Encouraging them to develop their own strategies allows them to play an active role in their growth. Giving them the opportunity to share their perceived strengths and deficiencies provides you with valuable information regarding their insight and ability to self-evaluate. This can help you predict their readiness for your feedback and to tailor your approach when there is a mismatch.
Examples:
- Medical student: Start with “What do you think you are doing well?” and “What do you think you need to work on?” Build on their response with encouragement and empathy. This helps make them more deliberate with what they work on because being a medical student can be overwhelming and can feel as though they have everything to work on.
- Resident/Fellow: By this point, trainees usually have an increased awareness of their strengths and deficiencies. Your questions can then be more specific, giving them autonomy over their learning, such as “What are some of the things you are working on that you want me to give you feedback on this week?” This makes them more aware, intentional, and receptive to your feedback because it is framed as something that they sought out.
B. Colleagues/Staff
Unlike the training environment in which feedback is built-in, giving feedback to co-workers requires you to establish a feedback-conducive environment and to develop a more in-depth understanding of coworkers’ personalities. Similar strategies can be applied, such as proactively setting the scene for open communication, scheduling check-ins, demonstrating receptiveness to feedback, and investing in trust-building.
Longer working relationships allow for strong foundational connections that make feedback less threatening. Personality assessment testing like Myers-Briggs Type Indicator or DiSC Assessment can aid in tailoring feedback to different individuals.7,8 An analytical thinker may appreciate direct, data-driven feedback. Relationship-oriented individuals might respond better to softer, encouragement-based approaches. Always maintain shared goals at the center of your interactions and consider collaborative opportunities such as quality improvement projects. This can improve your working relationship in a constructive way without casting blame.
Tip 5: Work on delivery: Bidirectional communication and body language
Non-verbal cues can have a profound impact on how your feedback is interpreted and on the recipient’s comfort to engage in conversation. Sitting down, making eye contact, nodding, and avoiding closed-off body posture can project support and feel less judgmental. Creating a safe and non-distracted environment with privacy can make them feel valued. Use motivating, respectful language focused on directly observed behaviors rather than personal attributes or second-hand reports.
Remember that focusing on repetitive patterns is likely more helpful than isolated incidents. Validate their hard work and give them a global idea of where they stand before diving into individual behaviors. Encourage their participation and empower them to suggest changes they plan to implement. Conclude by having them summarize their action plan to give them ownership and to verify that your feedback was interpreted as you intended. Thank them for being a part of the process, as it does take a partnership for feedback to be effective.
Tip 6: Be open to feedback
Demonstrating your willingness to accept and act on feedback reinforces a positive culture where feedback is normalized and valued. After an unintended outcome, initiate a two-way conversation and ask their input on anything they wish you would have done differently. This reaffirms your commitment to maintaining culture that does not revolve around one-sided critiques. Frequently soliciting feedback about your feedback skills can also guide you to adapt your approach and to recognize any ineffective feedback practices.
Tip 7: When things don’t go as planned
Receiving feedback, no matter how thoughtfully it is delivered, can be an emotionally-charged experience ending in hurt feelings. This happens because of misinterpretation of feedback as an indicator of inadequacy, heightened awareness of underlying insecurities, sociocultural or personal circumstances, frustration with oneself, needing additional guidance, or being caught off-guard by the assessment.
The evaluator should always acknowledge the recipient’s feelings, show compassion, and allow time for processing. When they are ready to talk, it is important to help reframe the recipients’ mindsets to recognize that feedback is not personal or defining and is not a “one and done” reflection of whether they have “made it.” Instead, it is a continual process that we benefit from through all career stages. Again, shared vulnerability can help to normalize feedback and maintain open dialogue. Setting an opportunity for a future check-in can reinforce support and lead to a more productive conversation after they have had time to process.
Conclusion
Effective feedback delivery is an invaluable skill that can result in meaningful goal-directed changes while strengthening professional relationships. Given the complexity of feedback interactions and the many factors that influence its acceptance, no single approach is suitable for all recipients and frequent adaptation of the approach is essential.
In our experience, adhering to these general overarching feedback principles (see Figure 1) has allowed us to have more successful interactions with trainees and colleagues.
Dr. Baliss is based in the Division of Gastroenterology, Washington University in St. Louis, Missouri. Dr. Hachem is director of the Division of Gastroenterology and Digestive Health at Intermountain Medical, Sandy, Utah. Both authors declare no conflicts of interest.
References
1. Parkes J, et al. Feedback sandwiches affect perceptions but not performance. Adv Health Sci Educ Theory Pract. 2013 Aug. doi:10.1007/s10459-012-9377-9.
2. van de Ridder JMM and Wijnen-Meijer M. Pendleton’s Rules: A Mini Review of a Feedback Method. Am J Biomed Sci & Res. 2023 May. doi: 10.34297/AJBSR.2023.19.002542.
3. Sender Liberman A, et al. Surgery residents and attending surgeons have different perceptions of feedback. Med Teach. 2005 Aug. doi: 10.1080/0142590500129183.
4. Sargeant J, et al. R2C2 in Action: Testing an Evidence-Based Model to Facilitate Feedback and Coaching in Residency. J Grad Med Educ. 2017 Apr. doi: 10.4300/JGME-D-16-00398.1.
5. Liakos W, et al. Frameworks for Effective Feedback in Health Professions Education. Acad Med. 2023 May. doi: 10.1097/ACM.0000000000004884.
6. Ramani S, et al. Feedback Redefined: Principles and Practice. J Gen Intern Med. 2019 May. doi: 10.1007/s11606-019-04874-2.
7. Woods RA and Hill PB. Myers-Briggs Type Indicator. StatPearls. StatPearls Publishing. 2022 Sept. https://www.ncbi.nlm.nih.gov/books/NBK554596/
8. Slowikowski MK. Using the DISC behavioral instrument to guide leadership and communication. AORN J. 2005 Nov. doi: 10.1016/s0001-2092(06)60276-7.
Vital Partners in GI Care
Demand for specialized GI care has skyrocketed in recent years, eclipsing the supply of gastroenterologists and impairing patient access to high-quality GI care, particularly in rural and other underserved areas. In this environment,
Across specialties, APPs are estimated to constitute roughly a third of the US clinical workforce, and demand is only growing. A June 2024 MGMA Stat poll found that 63% of medical groups planned to add new APP roles in the next year. As the GI APP workforce grows, so too will demand for advanced training tailored to the APP role.
AGA has invested heavily in professional development opportunities for NPs and PAs, in recognition of their vital role in providing high-quality GI care. The newly formed AGA NPPA Task Force, co-chaired by Abigail Meyers (who we featured in GIHN’s April issue) and Kimberly Kearns, works closely with the Education and Training Committee to develop education programs to meet the specific needs of NPs and PAs, and advocate for more APP involvement in AGA programming. One example of this is AGA’s 2025 Principles of GI for the NP and PA course, which will be held in Chicago in early August – I encourage you to spread the word and support your APP colleagues in getting involved in these important initiatives as our vital partners in GI care delivery.
In this month’s issue of GIHN, we present the exciting results of the BOSS trial, showing no survival difference between regular and at need surveillance for Barrett’s esophagus, suggesting that at need endoscopy may be a safe alternative for low-risk patients. Continuing our coverage of potentially practice-changing research from DDW, we highlight another recent RCT challenging the use of papillary sphincterotomy as a treatment for pancreas divisum.
In our July Member Spotlight, Eric Shah, MD, MBA (University of Michigan), a past AGA Research Scholar Award recipient, highlights how this critical research support aided him in his journey to develop a now FDA-approved point-of care screening tool used to evaluate patients with chronic constipation for pelvic floor dysfunction during a routine clinic visit. In our quarterly Perspectives column, Dr. David Wan (a GI hospitalist) and Dr. Zeyed Metwalli (an interventional radiologist) discuss best practices in management of lower GI bleeding. We hope you have a restful summer!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Demand for specialized GI care has skyrocketed in recent years, eclipsing the supply of gastroenterologists and impairing patient access to high-quality GI care, particularly in rural and other underserved areas. In this environment,
Across specialties, APPs are estimated to constitute roughly a third of the US clinical workforce, and demand is only growing. A June 2024 MGMA Stat poll found that 63% of medical groups planned to add new APP roles in the next year. As the GI APP workforce grows, so too will demand for advanced training tailored to the APP role.
AGA has invested heavily in professional development opportunities for NPs and PAs, in recognition of their vital role in providing high-quality GI care. The newly formed AGA NPPA Task Force, co-chaired by Abigail Meyers (who we featured in GIHN’s April issue) and Kimberly Kearns, works closely with the Education and Training Committee to develop education programs to meet the specific needs of NPs and PAs, and advocate for more APP involvement in AGA programming. One example of this is AGA’s 2025 Principles of GI for the NP and PA course, which will be held in Chicago in early August – I encourage you to spread the word and support your APP colleagues in getting involved in these important initiatives as our vital partners in GI care delivery.
In this month’s issue of GIHN, we present the exciting results of the BOSS trial, showing no survival difference between regular and at need surveillance for Barrett’s esophagus, suggesting that at need endoscopy may be a safe alternative for low-risk patients. Continuing our coverage of potentially practice-changing research from DDW, we highlight another recent RCT challenging the use of papillary sphincterotomy as a treatment for pancreas divisum.
In our July Member Spotlight, Eric Shah, MD, MBA (University of Michigan), a past AGA Research Scholar Award recipient, highlights how this critical research support aided him in his journey to develop a now FDA-approved point-of care screening tool used to evaluate patients with chronic constipation for pelvic floor dysfunction during a routine clinic visit. In our quarterly Perspectives column, Dr. David Wan (a GI hospitalist) and Dr. Zeyed Metwalli (an interventional radiologist) discuss best practices in management of lower GI bleeding. We hope you have a restful summer!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Demand for specialized GI care has skyrocketed in recent years, eclipsing the supply of gastroenterologists and impairing patient access to high-quality GI care, particularly in rural and other underserved areas. In this environment,
Across specialties, APPs are estimated to constitute roughly a third of the US clinical workforce, and demand is only growing. A June 2024 MGMA Stat poll found that 63% of medical groups planned to add new APP roles in the next year. As the GI APP workforce grows, so too will demand for advanced training tailored to the APP role.
AGA has invested heavily in professional development opportunities for NPs and PAs, in recognition of their vital role in providing high-quality GI care. The newly formed AGA NPPA Task Force, co-chaired by Abigail Meyers (who we featured in GIHN’s April issue) and Kimberly Kearns, works closely with the Education and Training Committee to develop education programs to meet the specific needs of NPs and PAs, and advocate for more APP involvement in AGA programming. One example of this is AGA’s 2025 Principles of GI for the NP and PA course, which will be held in Chicago in early August – I encourage you to spread the word and support your APP colleagues in getting involved in these important initiatives as our vital partners in GI care delivery.
In this month’s issue of GIHN, we present the exciting results of the BOSS trial, showing no survival difference between regular and at need surveillance for Barrett’s esophagus, suggesting that at need endoscopy may be a safe alternative for low-risk patients. Continuing our coverage of potentially practice-changing research from DDW, we highlight another recent RCT challenging the use of papillary sphincterotomy as a treatment for pancreas divisum.
In our July Member Spotlight, Eric Shah, MD, MBA (University of Michigan), a past AGA Research Scholar Award recipient, highlights how this critical research support aided him in his journey to develop a now FDA-approved point-of care screening tool used to evaluate patients with chronic constipation for pelvic floor dysfunction during a routine clinic visit. In our quarterly Perspectives column, Dr. David Wan (a GI hospitalist) and Dr. Zeyed Metwalli (an interventional radiologist) discuss best practices in management of lower GI bleeding. We hope you have a restful summer!
Megan A. Adams, MD, JD, MSc
Editor in Chief
The Essential Guide to Estate Planning for Physicians: Securing Your Legacy and Protecting Your Wealth
As a physician, you’ve spent years building a career that not only provides financial security for your family but also allows you to make a meaningful impact in your community. However, without a comprehensive estate plan in place, much of what you’ve worked so hard to build may not be preserved according to your wishes.
Many physicians delay estate planning, assuming it’s something to consider later in life. However, the most successful estate plans are those that are established early and evolve over time. Proper planning ensures that your assets are protected, your loved ones are provided for, and your legacy is preserved in the most tax-efficient and legally-sound manner possible.1
This article explores why estate planning is particularly crucial for physicians, the key elements of a strong estate plan, and how beginning early can create long-term financial advantages.
Why Estate Planning Matters for Physicians
Physicians are in a unique financial position compared to many other professionals. With high earning potential, specialized assets, and significant liability exposure, their estate planning needs differ from those of the average individual. A well-structured estate plan not only facilitates the smooth transfer of wealth but also protects assets from excessive taxation, legal complications, and potential risks such as malpractice claims.
1. High Net-Worth Considerations
Physicians often accumulate substantial wealth over time. Without a clear estate plan, your estate could face excessive taxation, with a large portion of your assets potentially going to the government rather than your heirs. Estate taxes, probate costs, and legal fees can significantly erode your legacy if not properly planned for.
2. Asset Protection from Liability Risks
Unlike most professionals, physicians are at a higher risk of litigation. A comprehensive estate plan can incorporate asset protection strategies, such as irrevocable trusts, family limited partnerships, or liability insurance, to shield your wealth from lawsuits or creditor claims.
3. Family and Generational Wealth Planning
Many physicians prioritize ensuring their family’s financial stability. Whether you want to provide for your spouse, children, or even charitable causes, estate planning allows you to dictate how your wealth is distributed. Establishing trusts for your children or grandchildren can help manage how and when they receive their inheritance, preventing mismanagement and ensuring financial responsibility.
4. Business and Practice Continuity
If you own a medical practice, succession planning should be part of your estate plan. Without clear directives, the future of your practice may be uncertain in the event of your passing or incapacitation. A well-drafted estate plan provides a roadmap for ownership transition, ensuring continuity for patients, employees, and business partners.
Key Elements of an Effective Estate Plan
Every estate plan should be customized based on your financial situation, goals, and family dynamics. However, certain fundamental components apply to nearly all high-net-worth individuals, including physicians.
1. Revocable Living Trusts
A revocable living trust allows you to manage your assets during your lifetime while providing a clear path for distribution after your passing. Unlike a will, a trust helps your estate avoid probate, ensuring a smoother and more private transition of wealth. You maintain control over your assets while also establishing clear rules for distribution, particularly useful if you have minor children or complex family structures.2
2. Irrevocable Trusts for Asset Protection
For physicians concerned about lawsuits or estate tax exposure, irrevocable trusts can offer robust asset protection. Since assets placed in these trusts are no longer legally owned by you, they are shielded from creditors and legal claims while also reducing your taxable estate.2
3. Powers of Attorney and Healthcare Directives
Estate planning isn’t just about what happens after your passing—it’s also about protecting you and your family if you become incapacitated. A durable power of attorney allows a trusted individual to manage your financial affairs, while a healthcare directive ensures your medical decisions align with your wishes.3
4. Life Insurance Planning
Life insurance is an essential estate planning tool for physicians, providing liquidity to cover estate taxes, debts, or income replacement for your family. A properly structured life insurance trust can help ensure that policy proceeds remain outside of your taxable estate while being efficiently distributed according to your wishes.4
5. Business Succession Planning
If you own a medical practice, a well-designed succession plan can ensure that your business continues to operate smoothly in your absence. This may involve buy-sell agreements, key-person insurance, or identifying a successor to take over your role.5
The Long-Term Benefits of Early Estate Planning
Estate planning is not a one-time event—it’s a process that should evolve with your career, financial growth, and family dynamics. The earlier you begin, the more control you have over your financial future. Here’s why starting early is a strategic advantage:
1. Maximizing Tax Efficiency
Many estate planning strategies, such as gifting assets or establishing irrevocable trusts, are most effective when implemented over time. By spreading out wealth transfers and taking advantage of annual gift exclusions, you can significantly reduce estate tax liability while maintaining financial security.
2. Adjusting for Life Changes
Your financial situation and family needs will change over the years. Marriages, births, career advancements, and new investments all impact your estate planning needs. By starting early, you can make gradual adjustments rather than facing an overwhelming restructuring later in life.1
3. Ensuring Asset Protection Strategies Are in Place
Many asset protection strategies require time to be effective. For instance, certain types of trusts must be in place for a number of years before they fully shield assets from legal claims. Delaying planning could leave your wealth unnecessarily exposed.
4. Creating a Legacy Beyond Wealth
Estate planning is not just about finances—it’s about legacy. Whether you want to support a charitable cause, endow a scholarship, or establish a foundation, early planning gives you the ability to shape your long-term impact.
5. Adapt to Ever Changing Legislation
Estate planning needs to be adaptable. The federal government can change the estate tax exemption at any time; this was even a topic of the last election cycle. Early planning allows you to implement necessary changes throughout your life to minimize estate taxes. At present, unless new policy is enacted, the exemption per individual will reduce by half in 2026 (see Figure 1).
Final Thoughts: Taking Action Today
The complexity of physician finances—ranging from high income and significant assets to legal risks—makes individualized estate planning an absolute necessity.
By taking proactive steps today, you can maximize tax efficiency, safeguard your assets, and ensure your wishes are carried out without unnecessary delays or legal battles. Working with a financial advisor and estate planning attorney who understands the unique needs of physicians can help you craft a plan that aligns with your goals and evolves as your career progresses.
Mr. Gardner is a financial advisor at Lifetime Financial Growth, LLC, in Columbus, Ohio, one of the largest privately held wealth management firms in the country. John has had a passion for finance since his early years in college when his tennis coach introduced him. He also has a passion for helping physicians, as his wife is a gastroenterologist at Ohio State University. He reports no relevant disclosures relevant to this article. If you have additional questions, please contact John at 740-403-4891 or john_s_gardner@glic.com.
References
1. The Law Offices of Diron Rutty, LLC. https://www.dironruttyllc.com/reasons-to-start-estate-planning-early/.
2. Physician Side Gigs. https://www.physiciansidegigs.com/estateplanning.
3. Afshar, A & MacBeth, S. https://www.schwabe.com/publication/estate-planning-for-physicians-why-its-important-and-how-to-get-started/. December 2024.
4. Skeeles, JC. https://ohioline.osu.edu/factsheet/ep-1. July 2012.
5. Rosenfeld, J. Physician estate planning guide. Medical Economics. 2022 Nov. https://www.medicaleconomics.com/view/physician-estate-planning-guide.
As a physician, you’ve spent years building a career that not only provides financial security for your family but also allows you to make a meaningful impact in your community. However, without a comprehensive estate plan in place, much of what you’ve worked so hard to build may not be preserved according to your wishes.
Many physicians delay estate planning, assuming it’s something to consider later in life. However, the most successful estate plans are those that are established early and evolve over time. Proper planning ensures that your assets are protected, your loved ones are provided for, and your legacy is preserved in the most tax-efficient and legally-sound manner possible.1
This article explores why estate planning is particularly crucial for physicians, the key elements of a strong estate plan, and how beginning early can create long-term financial advantages.
Why Estate Planning Matters for Physicians
Physicians are in a unique financial position compared to many other professionals. With high earning potential, specialized assets, and significant liability exposure, their estate planning needs differ from those of the average individual. A well-structured estate plan not only facilitates the smooth transfer of wealth but also protects assets from excessive taxation, legal complications, and potential risks such as malpractice claims.
1. High Net-Worth Considerations
Physicians often accumulate substantial wealth over time. Without a clear estate plan, your estate could face excessive taxation, with a large portion of your assets potentially going to the government rather than your heirs. Estate taxes, probate costs, and legal fees can significantly erode your legacy if not properly planned for.
2. Asset Protection from Liability Risks
Unlike most professionals, physicians are at a higher risk of litigation. A comprehensive estate plan can incorporate asset protection strategies, such as irrevocable trusts, family limited partnerships, or liability insurance, to shield your wealth from lawsuits or creditor claims.
3. Family and Generational Wealth Planning
Many physicians prioritize ensuring their family’s financial stability. Whether you want to provide for your spouse, children, or even charitable causes, estate planning allows you to dictate how your wealth is distributed. Establishing trusts for your children or grandchildren can help manage how and when they receive their inheritance, preventing mismanagement and ensuring financial responsibility.
4. Business and Practice Continuity
If you own a medical practice, succession planning should be part of your estate plan. Without clear directives, the future of your practice may be uncertain in the event of your passing or incapacitation. A well-drafted estate plan provides a roadmap for ownership transition, ensuring continuity for patients, employees, and business partners.
Key Elements of an Effective Estate Plan
Every estate plan should be customized based on your financial situation, goals, and family dynamics. However, certain fundamental components apply to nearly all high-net-worth individuals, including physicians.
1. Revocable Living Trusts
A revocable living trust allows you to manage your assets during your lifetime while providing a clear path for distribution after your passing. Unlike a will, a trust helps your estate avoid probate, ensuring a smoother and more private transition of wealth. You maintain control over your assets while also establishing clear rules for distribution, particularly useful if you have minor children or complex family structures.2
2. Irrevocable Trusts for Asset Protection
For physicians concerned about lawsuits or estate tax exposure, irrevocable trusts can offer robust asset protection. Since assets placed in these trusts are no longer legally owned by you, they are shielded from creditors and legal claims while also reducing your taxable estate.2
3. Powers of Attorney and Healthcare Directives
Estate planning isn’t just about what happens after your passing—it’s also about protecting you and your family if you become incapacitated. A durable power of attorney allows a trusted individual to manage your financial affairs, while a healthcare directive ensures your medical decisions align with your wishes.3
4. Life Insurance Planning
Life insurance is an essential estate planning tool for physicians, providing liquidity to cover estate taxes, debts, or income replacement for your family. A properly structured life insurance trust can help ensure that policy proceeds remain outside of your taxable estate while being efficiently distributed according to your wishes.4
5. Business Succession Planning
If you own a medical practice, a well-designed succession plan can ensure that your business continues to operate smoothly in your absence. This may involve buy-sell agreements, key-person insurance, or identifying a successor to take over your role.5
The Long-Term Benefits of Early Estate Planning
Estate planning is not a one-time event—it’s a process that should evolve with your career, financial growth, and family dynamics. The earlier you begin, the more control you have over your financial future. Here’s why starting early is a strategic advantage:
1. Maximizing Tax Efficiency
Many estate planning strategies, such as gifting assets or establishing irrevocable trusts, are most effective when implemented over time. By spreading out wealth transfers and taking advantage of annual gift exclusions, you can significantly reduce estate tax liability while maintaining financial security.
2. Adjusting for Life Changes
Your financial situation and family needs will change over the years. Marriages, births, career advancements, and new investments all impact your estate planning needs. By starting early, you can make gradual adjustments rather than facing an overwhelming restructuring later in life.1
3. Ensuring Asset Protection Strategies Are in Place
Many asset protection strategies require time to be effective. For instance, certain types of trusts must be in place for a number of years before they fully shield assets from legal claims. Delaying planning could leave your wealth unnecessarily exposed.
4. Creating a Legacy Beyond Wealth
Estate planning is not just about finances—it’s about legacy. Whether you want to support a charitable cause, endow a scholarship, or establish a foundation, early planning gives you the ability to shape your long-term impact.
5. Adapt to Ever Changing Legislation
Estate planning needs to be adaptable. The federal government can change the estate tax exemption at any time; this was even a topic of the last election cycle. Early planning allows you to implement necessary changes throughout your life to minimize estate taxes. At present, unless new policy is enacted, the exemption per individual will reduce by half in 2026 (see Figure 1).
Final Thoughts: Taking Action Today
The complexity of physician finances—ranging from high income and significant assets to legal risks—makes individualized estate planning an absolute necessity.
By taking proactive steps today, you can maximize tax efficiency, safeguard your assets, and ensure your wishes are carried out without unnecessary delays or legal battles. Working with a financial advisor and estate planning attorney who understands the unique needs of physicians can help you craft a plan that aligns with your goals and evolves as your career progresses.
Mr. Gardner is a financial advisor at Lifetime Financial Growth, LLC, in Columbus, Ohio, one of the largest privately held wealth management firms in the country. John has had a passion for finance since his early years in college when his tennis coach introduced him. He also has a passion for helping physicians, as his wife is a gastroenterologist at Ohio State University. He reports no relevant disclosures relevant to this article. If you have additional questions, please contact John at 740-403-4891 or john_s_gardner@glic.com.
References
1. The Law Offices of Diron Rutty, LLC. https://www.dironruttyllc.com/reasons-to-start-estate-planning-early/.
2. Physician Side Gigs. https://www.physiciansidegigs.com/estateplanning.
3. Afshar, A & MacBeth, S. https://www.schwabe.com/publication/estate-planning-for-physicians-why-its-important-and-how-to-get-started/. December 2024.
4. Skeeles, JC. https://ohioline.osu.edu/factsheet/ep-1. July 2012.
5. Rosenfeld, J. Physician estate planning guide. Medical Economics. 2022 Nov. https://www.medicaleconomics.com/view/physician-estate-planning-guide.
As a physician, you’ve spent years building a career that not only provides financial security for your family but also allows you to make a meaningful impact in your community. However, without a comprehensive estate plan in place, much of what you’ve worked so hard to build may not be preserved according to your wishes.
Many physicians delay estate planning, assuming it’s something to consider later in life. However, the most successful estate plans are those that are established early and evolve over time. Proper planning ensures that your assets are protected, your loved ones are provided for, and your legacy is preserved in the most tax-efficient and legally-sound manner possible.1
This article explores why estate planning is particularly crucial for physicians, the key elements of a strong estate plan, and how beginning early can create long-term financial advantages.
Why Estate Planning Matters for Physicians
Physicians are in a unique financial position compared to many other professionals. With high earning potential, specialized assets, and significant liability exposure, their estate planning needs differ from those of the average individual. A well-structured estate plan not only facilitates the smooth transfer of wealth but also protects assets from excessive taxation, legal complications, and potential risks such as malpractice claims.
1. High Net-Worth Considerations
Physicians often accumulate substantial wealth over time. Without a clear estate plan, your estate could face excessive taxation, with a large portion of your assets potentially going to the government rather than your heirs. Estate taxes, probate costs, and legal fees can significantly erode your legacy if not properly planned for.
2. Asset Protection from Liability Risks
Unlike most professionals, physicians are at a higher risk of litigation. A comprehensive estate plan can incorporate asset protection strategies, such as irrevocable trusts, family limited partnerships, or liability insurance, to shield your wealth from lawsuits or creditor claims.
3. Family and Generational Wealth Planning
Many physicians prioritize ensuring their family’s financial stability. Whether you want to provide for your spouse, children, or even charitable causes, estate planning allows you to dictate how your wealth is distributed. Establishing trusts for your children or grandchildren can help manage how and when they receive their inheritance, preventing mismanagement and ensuring financial responsibility.
4. Business and Practice Continuity
If you own a medical practice, succession planning should be part of your estate plan. Without clear directives, the future of your practice may be uncertain in the event of your passing or incapacitation. A well-drafted estate plan provides a roadmap for ownership transition, ensuring continuity for patients, employees, and business partners.
Key Elements of an Effective Estate Plan
Every estate plan should be customized based on your financial situation, goals, and family dynamics. However, certain fundamental components apply to nearly all high-net-worth individuals, including physicians.
1. Revocable Living Trusts
A revocable living trust allows you to manage your assets during your lifetime while providing a clear path for distribution after your passing. Unlike a will, a trust helps your estate avoid probate, ensuring a smoother and more private transition of wealth. You maintain control over your assets while also establishing clear rules for distribution, particularly useful if you have minor children or complex family structures.2
2. Irrevocable Trusts for Asset Protection
For physicians concerned about lawsuits or estate tax exposure, irrevocable trusts can offer robust asset protection. Since assets placed in these trusts are no longer legally owned by you, they are shielded from creditors and legal claims while also reducing your taxable estate.2
3. Powers of Attorney and Healthcare Directives
Estate planning isn’t just about what happens after your passing—it’s also about protecting you and your family if you become incapacitated. A durable power of attorney allows a trusted individual to manage your financial affairs, while a healthcare directive ensures your medical decisions align with your wishes.3
4. Life Insurance Planning
Life insurance is an essential estate planning tool for physicians, providing liquidity to cover estate taxes, debts, or income replacement for your family. A properly structured life insurance trust can help ensure that policy proceeds remain outside of your taxable estate while being efficiently distributed according to your wishes.4
5. Business Succession Planning
If you own a medical practice, a well-designed succession plan can ensure that your business continues to operate smoothly in your absence. This may involve buy-sell agreements, key-person insurance, or identifying a successor to take over your role.5
The Long-Term Benefits of Early Estate Planning
Estate planning is not a one-time event—it’s a process that should evolve with your career, financial growth, and family dynamics. The earlier you begin, the more control you have over your financial future. Here’s why starting early is a strategic advantage:
1. Maximizing Tax Efficiency
Many estate planning strategies, such as gifting assets or establishing irrevocable trusts, are most effective when implemented over time. By spreading out wealth transfers and taking advantage of annual gift exclusions, you can significantly reduce estate tax liability while maintaining financial security.
2. Adjusting for Life Changes
Your financial situation and family needs will change over the years. Marriages, births, career advancements, and new investments all impact your estate planning needs. By starting early, you can make gradual adjustments rather than facing an overwhelming restructuring later in life.1
3. Ensuring Asset Protection Strategies Are in Place
Many asset protection strategies require time to be effective. For instance, certain types of trusts must be in place for a number of years before they fully shield assets from legal claims. Delaying planning could leave your wealth unnecessarily exposed.
4. Creating a Legacy Beyond Wealth
Estate planning is not just about finances—it’s about legacy. Whether you want to support a charitable cause, endow a scholarship, or establish a foundation, early planning gives you the ability to shape your long-term impact.
5. Adapt to Ever Changing Legislation
Estate planning needs to be adaptable. The federal government can change the estate tax exemption at any time; this was even a topic of the last election cycle. Early planning allows you to implement necessary changes throughout your life to minimize estate taxes. At present, unless new policy is enacted, the exemption per individual will reduce by half in 2026 (see Figure 1).
Final Thoughts: Taking Action Today
The complexity of physician finances—ranging from high income and significant assets to legal risks—makes individualized estate planning an absolute necessity.
By taking proactive steps today, you can maximize tax efficiency, safeguard your assets, and ensure your wishes are carried out without unnecessary delays or legal battles. Working with a financial advisor and estate planning attorney who understands the unique needs of physicians can help you craft a plan that aligns with your goals and evolves as your career progresses.
Mr. Gardner is a financial advisor at Lifetime Financial Growth, LLC, in Columbus, Ohio, one of the largest privately held wealth management firms in the country. John has had a passion for finance since his early years in college when his tennis coach introduced him. He also has a passion for helping physicians, as his wife is a gastroenterologist at Ohio State University. He reports no relevant disclosures relevant to this article. If you have additional questions, please contact John at 740-403-4891 or john_s_gardner@glic.com.
References
1. The Law Offices of Diron Rutty, LLC. https://www.dironruttyllc.com/reasons-to-start-estate-planning-early/.
2. Physician Side Gigs. https://www.physiciansidegigs.com/estateplanning.
3. Afshar, A & MacBeth, S. https://www.schwabe.com/publication/estate-planning-for-physicians-why-its-important-and-how-to-get-started/. December 2024.
4. Skeeles, JC. https://ohioline.osu.edu/factsheet/ep-1. July 2012.
5. Rosenfeld, J. Physician estate planning guide. Medical Economics. 2022 Nov. https://www.medicaleconomics.com/view/physician-estate-planning-guide.