User login
Lump in breast
Given clinical and imaging outcomes, as well as results on IHC assay, this patient is diagnosed with triple-negative breast cancer and is referred for further consultation with a multidisciplinary care team.
Triple-negative breast cancer accounts for 15% of all female breast cancer cases. The term "triple-negative" refers to the absence of estrogen receptor (ER) and progesterone receptor (PR) targets and the HER2 target for treatment. Otherwise, triple-negative breast cancer is highly heterogeneous and includes multiple molecular subtypes. Typically, triple-negative breast cancer occurs in younger patients, has a higher grade, a greater tumor size, a higher clinical stage at diagnosis, and a poorer prognosis than non–triple-negative breast cancers.
Biopsy samples from patients with a new primary or newly metastatic breast cancer diagnosis should undergo HR testing, both ER and PR, as well as HER2 receptor testing. Results of ER and PR testing are negative if a validated IHC assay shows 0% to < 1% of nuclei stain, according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HR testing guideline. HER2 results are negative if a validated IHC assay shows weak to moderate complete membrane staining in < 10% of tumor cells, according to ASCO/CAP guidelines. If HER2 results of a primary tumor are negative and specific clinical criteria suggest further testing, the oncologist may choose to order another IHC assay or a validated dual probe in situ hybridization (ISH) assay to aid in clinical decision-making.
Pathologic classification of triple-negative breast cancers includes multiple subgroups determined by therapeutic possibilities: low-risk histologic types (salivary gland–like carcinomas, fibromatosis-like carcinoma, low-grade adenosquamous breast carcinoma), immune activation (tumor-infiltrating lymphocytes, CD8, programmed death–ligand 1, tumor mutational burden), HER2-low status (IHC score 1+/2+ nonamplified), associated germline BRCA mutations, and other targets (trophoblast cell-surface antigen 2, HER3).
Family history is the most common risk factor for developing breast cancer. If a person's mother or sister had breast cancer, the lifetime risk for that individual is four times greater than that of the general population. Risk for breast cancer grows even more if two or more first-degree relatives are diagnosed, or if one first-degree relative was diagnosed with breast cancer at ≤ 50 years of age. About 25% of triple-negative breast cancer cases have accompanying germline BRCA mutations. A recent study by Ahearn and colleagues found that 85 variants were linked to one or more tumor feature of ER-negative disease, and 32 of those variants were associated with triple-negative disease.
For a breast cancer diagnosis, the National Comprehensive Cancer Network recommends multidisciplinary care as well as the development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Because triple-negative breast cancer lacks HR and HER2 therapeutic targets, it can be difficult to treat. Standard therapy for high-risk triple-negative tumors includes chemotherapy with taxanes (paclitaxel or docetaxel) plus anthracycline-based treatment (cyclophosphamide plus doxorubicin). Neoadjuvant chemotherapy is recommended for patients with stage 2 and 3 tumors to reduce locoregional surgical extension and to allow for personalized treatment based on pathologic response. In 2018, two poly ADP ribose polymerase inhibitors, olaparib and talazoparib, were approved for use in select patients with triple-negative breast cancer, significantly changing the treatment paradigm for this disease. Other promising treatments have emerged recently in clinical trials for triple-negative disease, including immune checkpoint inhibitors, PI3K/AKT pathway inhibitors, and cytotoxin-conjugated antibodies.
Daniel S. Schwartz, MD, is Medical Director of Thoracic Oncology, St. Catherine of Siena Medical Center, Catholic Health Services, Smithtown, New York.
Dr. Schwartz serve(d) as a member of the following medical societies:
American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, Western Thoracic Surgical Association.
Disclosure: Daniel S. Schwartz, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given clinical and imaging outcomes, as well as results on IHC assay, this patient is diagnosed with triple-negative breast cancer and is referred for further consultation with a multidisciplinary care team.
Triple-negative breast cancer accounts for 15% of all female breast cancer cases. The term "triple-negative" refers to the absence of estrogen receptor (ER) and progesterone receptor (PR) targets and the HER2 target for treatment. Otherwise, triple-negative breast cancer is highly heterogeneous and includes multiple molecular subtypes. Typically, triple-negative breast cancer occurs in younger patients, has a higher grade, a greater tumor size, a higher clinical stage at diagnosis, and a poorer prognosis than non–triple-negative breast cancers.
Biopsy samples from patients with a new primary or newly metastatic breast cancer diagnosis should undergo HR testing, both ER and PR, as well as HER2 receptor testing. Results of ER and PR testing are negative if a validated IHC assay shows 0% to < 1% of nuclei stain, according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HR testing guideline. HER2 results are negative if a validated IHC assay shows weak to moderate complete membrane staining in < 10% of tumor cells, according to ASCO/CAP guidelines. If HER2 results of a primary tumor are negative and specific clinical criteria suggest further testing, the oncologist may choose to order another IHC assay or a validated dual probe in situ hybridization (ISH) assay to aid in clinical decision-making.
Pathologic classification of triple-negative breast cancers includes multiple subgroups determined by therapeutic possibilities: low-risk histologic types (salivary gland–like carcinomas, fibromatosis-like carcinoma, low-grade adenosquamous breast carcinoma), immune activation (tumor-infiltrating lymphocytes, CD8, programmed death–ligand 1, tumor mutational burden), HER2-low status (IHC score 1+/2+ nonamplified), associated germline BRCA mutations, and other targets (trophoblast cell-surface antigen 2, HER3).
Family history is the most common risk factor for developing breast cancer. If a person's mother or sister had breast cancer, the lifetime risk for that individual is four times greater than that of the general population. Risk for breast cancer grows even more if two or more first-degree relatives are diagnosed, or if one first-degree relative was diagnosed with breast cancer at ≤ 50 years of age. About 25% of triple-negative breast cancer cases have accompanying germline BRCA mutations. A recent study by Ahearn and colleagues found that 85 variants were linked to one or more tumor feature of ER-negative disease, and 32 of those variants were associated with triple-negative disease.
For a breast cancer diagnosis, the National Comprehensive Cancer Network recommends multidisciplinary care as well as the development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Because triple-negative breast cancer lacks HR and HER2 therapeutic targets, it can be difficult to treat. Standard therapy for high-risk triple-negative tumors includes chemotherapy with taxanes (paclitaxel or docetaxel) plus anthracycline-based treatment (cyclophosphamide plus doxorubicin). Neoadjuvant chemotherapy is recommended for patients with stage 2 and 3 tumors to reduce locoregional surgical extension and to allow for personalized treatment based on pathologic response. In 2018, two poly ADP ribose polymerase inhibitors, olaparib and talazoparib, were approved for use in select patients with triple-negative breast cancer, significantly changing the treatment paradigm for this disease. Other promising treatments have emerged recently in clinical trials for triple-negative disease, including immune checkpoint inhibitors, PI3K/AKT pathway inhibitors, and cytotoxin-conjugated antibodies.
Daniel S. Schwartz, MD, is Medical Director of Thoracic Oncology, St. Catherine of Siena Medical Center, Catholic Health Services, Smithtown, New York.
Dr. Schwartz serve(d) as a member of the following medical societies:
American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, Western Thoracic Surgical Association.
Disclosure: Daniel S. Schwartz, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given clinical and imaging outcomes, as well as results on IHC assay, this patient is diagnosed with triple-negative breast cancer and is referred for further consultation with a multidisciplinary care team.
Triple-negative breast cancer accounts for 15% of all female breast cancer cases. The term "triple-negative" refers to the absence of estrogen receptor (ER) and progesterone receptor (PR) targets and the HER2 target for treatment. Otherwise, triple-negative breast cancer is highly heterogeneous and includes multiple molecular subtypes. Typically, triple-negative breast cancer occurs in younger patients, has a higher grade, a greater tumor size, a higher clinical stage at diagnosis, and a poorer prognosis than non–triple-negative breast cancers.
Biopsy samples from patients with a new primary or newly metastatic breast cancer diagnosis should undergo HR testing, both ER and PR, as well as HER2 receptor testing. Results of ER and PR testing are negative if a validated IHC assay shows 0% to < 1% of nuclei stain, according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HR testing guideline. HER2 results are negative if a validated IHC assay shows weak to moderate complete membrane staining in < 10% of tumor cells, according to ASCO/CAP guidelines. If HER2 results of a primary tumor are negative and specific clinical criteria suggest further testing, the oncologist may choose to order another IHC assay or a validated dual probe in situ hybridization (ISH) assay to aid in clinical decision-making.
Pathologic classification of triple-negative breast cancers includes multiple subgroups determined by therapeutic possibilities: low-risk histologic types (salivary gland–like carcinomas, fibromatosis-like carcinoma, low-grade adenosquamous breast carcinoma), immune activation (tumor-infiltrating lymphocytes, CD8, programmed death–ligand 1, tumor mutational burden), HER2-low status (IHC score 1+/2+ nonamplified), associated germline BRCA mutations, and other targets (trophoblast cell-surface antigen 2, HER3).
Family history is the most common risk factor for developing breast cancer. If a person's mother or sister had breast cancer, the lifetime risk for that individual is four times greater than that of the general population. Risk for breast cancer grows even more if two or more first-degree relatives are diagnosed, or if one first-degree relative was diagnosed with breast cancer at ≤ 50 years of age. About 25% of triple-negative breast cancer cases have accompanying germline BRCA mutations. A recent study by Ahearn and colleagues found that 85 variants were linked to one or more tumor feature of ER-negative disease, and 32 of those variants were associated with triple-negative disease.
For a breast cancer diagnosis, the National Comprehensive Cancer Network recommends multidisciplinary care as well as the development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Because triple-negative breast cancer lacks HR and HER2 therapeutic targets, it can be difficult to treat. Standard therapy for high-risk triple-negative tumors includes chemotherapy with taxanes (paclitaxel or docetaxel) plus anthracycline-based treatment (cyclophosphamide plus doxorubicin). Neoadjuvant chemotherapy is recommended for patients with stage 2 and 3 tumors to reduce locoregional surgical extension and to allow for personalized treatment based on pathologic response. In 2018, two poly ADP ribose polymerase inhibitors, olaparib and talazoparib, were approved for use in select patients with triple-negative breast cancer, significantly changing the treatment paradigm for this disease. Other promising treatments have emerged recently in clinical trials for triple-negative disease, including immune checkpoint inhibitors, PI3K/AKT pathway inhibitors, and cytotoxin-conjugated antibodies.
Daniel S. Schwartz, MD, is Medical Director of Thoracic Oncology, St. Catherine of Siena Medical Center, Catholic Health Services, Smithtown, New York.
Dr. Schwartz serve(d) as a member of the following medical societies:
American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, Western Thoracic Surgical Association.
Disclosure: Daniel S. Schwartz, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
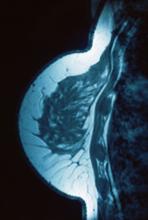
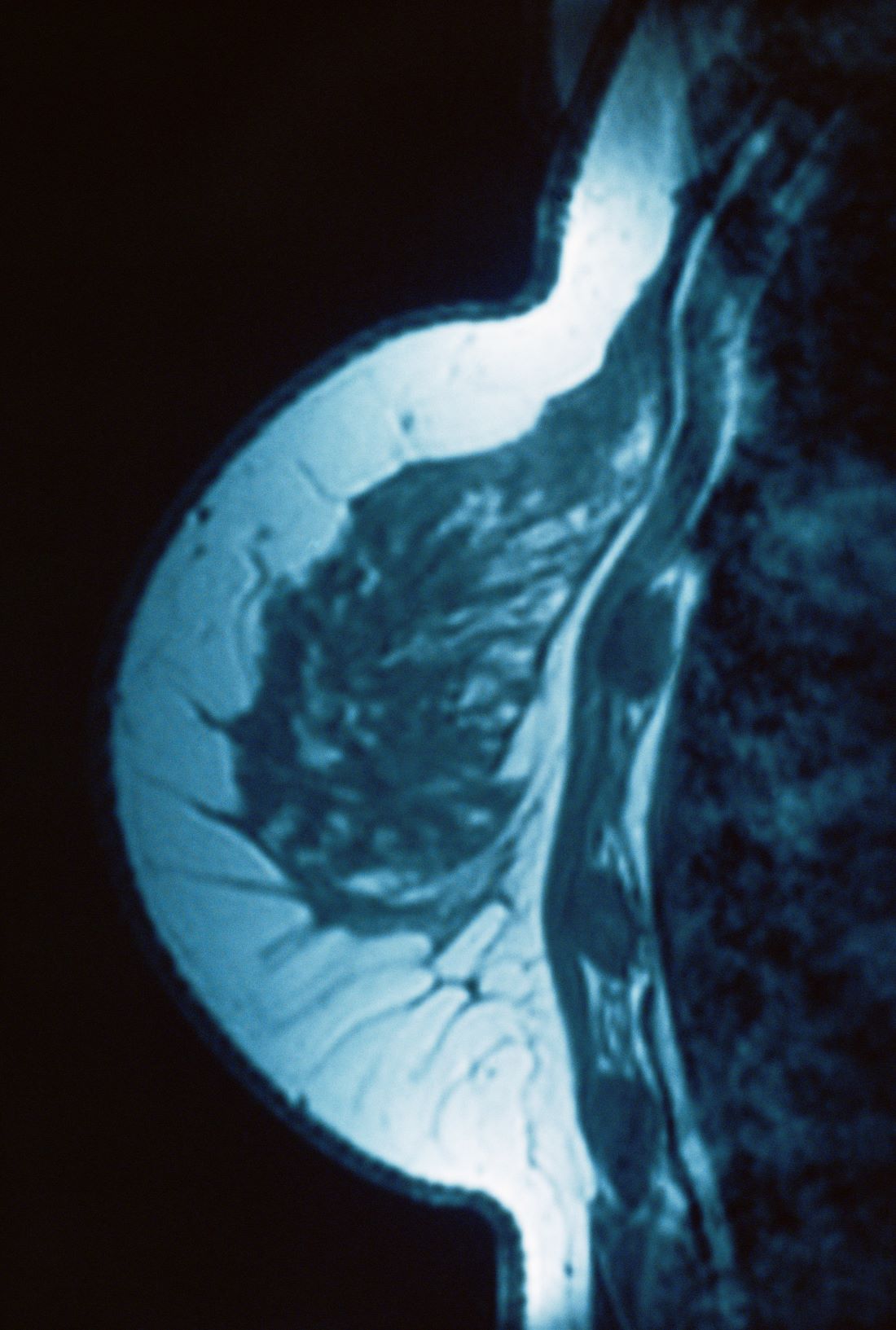
A 34-year-old woman visits her primary care physician after detecting a lump in her right breast. The physician conducts a physical exam and finds a hard, poorly mobile mass in the outer upper quadrant. Ultrasonography reveals an irregular hypoechoic mass of 22.1 x 15.1 mm with several abnormal axillary lymph nodes. MRI of the right breast shows an irregularly shaped and lobulated mass in the outer upper quadrant with enlarged axillary lymph nodes. On further radiologic examination, there is no detectable metastatic spread to the brain, chest, or upper abdomen. The patient undergoes a core needle biopsy of the tumor, which confirms ductal carcinoma and right axillary lymph node metastasis. Immunohistochemistry (IHC) assay of the sample is negative for hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2).
Optimizing Care in Metastatic Breast Cancer
Discomfort in right breast
Breast cancer is the most common tumor type and second only to lung cancer as a cause of cancer death in women. Nearly 300,000 women (and 2800 men) will receive a new diagnosis of breast cancer in the United States in 2023. Despite improvements in treatment options, breast cancer still will lead to 43,700 deaths among women this year.
Breast tumors generally are either ductal or lobular in origin. Ductal carcinomas arise in the lining of the milk ducts and are the most commonly found tumor type in breast cancer. Almost 3 in 4 diagnosed breast cancers histologically are invasive ductal carcinomas, which have spread from the source into surrounding structures. It has no specific histologic indicators and is differentiated from ductal carcinoma in situ by its having spread outside the duct. The presence of lymph node involvement in this patient also confirms invasive ductal carcinoma without metastatic spread. Invasive lobular carcinomas occur much less frequently and have a different histologic appearance of smaller cells that appear to be arranged in linear groups.
Mammography involves low-dose radiation and is used in diagnosis and is the most widely used screening technique for breast cancer. As a screening tool, mammography may detect lesions 1-2 years before they become palpable on breast self-examination. While the incidence of breast cancer in women has slowly increased over the past 20 years, mortality has decreased largely as a result of improved awareness and uptake of screening mammography. Current American Cancer Society guidelines recommend continued mammography screenings at least every other year after age 55 and continued for as long as a woman has a life expectancy > 10 years. Screening or diagnosis using MRI is usually reserved for individuals at high risk for breast cancer or a history of breast cancer before age 50.
For all newly diagnosed primary invasive breast cancers, biomarker testing for estrogen and progesterone receptor expression and HER2 expression are part of the standard workup recommended by the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) to help guide treatment decisions. Biomarker testing showed that her tumor was ER+ and HER2-negative, the most common finding in breast cancer. The patient's history did not suggest a risk for BRCA or other familial cancer mutations, but molecular testing was done and was negative for BRCA1 and BRCA2. In postmenopausal women, ASCO and NCCN also recommend use of risk assessment tools, such as Oncotype DX, to determine whether chemotherapy will add benefit to systemic endocrine therapy. Postmenopausal patients with ER+/HER2-negative breast cancer, one to three positive nodes, and a score < 26 on the 21-gene Oncotype DX can safely forego cytotoxic chemotherapy and derive maximum survival benefit from hormonal therapy alone.
This patient was diagnosed with a primary tumor of approximately 25 mm, metastasis to two ipsilateral nodes, and no distant metastasis, or stage IIIA. Her risk recurrence score was 20, indicating that she could safely forego the rigors of cytotoxic chemotherapy. She underwent localized breast-conserving surgery and began adjuvant tamoxifen therapy (x 2 years) to be followed with an aromatase inhibitor (x 3 years).
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Breast cancer is the most common tumor type and second only to lung cancer as a cause of cancer death in women. Nearly 300,000 women (and 2800 men) will receive a new diagnosis of breast cancer in the United States in 2023. Despite improvements in treatment options, breast cancer still will lead to 43,700 deaths among women this year.
Breast tumors generally are either ductal or lobular in origin. Ductal carcinomas arise in the lining of the milk ducts and are the most commonly found tumor type in breast cancer. Almost 3 in 4 diagnosed breast cancers histologically are invasive ductal carcinomas, which have spread from the source into surrounding structures. It has no specific histologic indicators and is differentiated from ductal carcinoma in situ by its having spread outside the duct. The presence of lymph node involvement in this patient also confirms invasive ductal carcinoma without metastatic spread. Invasive lobular carcinomas occur much less frequently and have a different histologic appearance of smaller cells that appear to be arranged in linear groups.
Mammography involves low-dose radiation and is used in diagnosis and is the most widely used screening technique for breast cancer. As a screening tool, mammography may detect lesions 1-2 years before they become palpable on breast self-examination. While the incidence of breast cancer in women has slowly increased over the past 20 years, mortality has decreased largely as a result of improved awareness and uptake of screening mammography. Current American Cancer Society guidelines recommend continued mammography screenings at least every other year after age 55 and continued for as long as a woman has a life expectancy > 10 years. Screening or diagnosis using MRI is usually reserved for individuals at high risk for breast cancer or a history of breast cancer before age 50.
For all newly diagnosed primary invasive breast cancers, biomarker testing for estrogen and progesterone receptor expression and HER2 expression are part of the standard workup recommended by the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) to help guide treatment decisions. Biomarker testing showed that her tumor was ER+ and HER2-negative, the most common finding in breast cancer. The patient's history did not suggest a risk for BRCA or other familial cancer mutations, but molecular testing was done and was negative for BRCA1 and BRCA2. In postmenopausal women, ASCO and NCCN also recommend use of risk assessment tools, such as Oncotype DX, to determine whether chemotherapy will add benefit to systemic endocrine therapy. Postmenopausal patients with ER+/HER2-negative breast cancer, one to three positive nodes, and a score < 26 on the 21-gene Oncotype DX can safely forego cytotoxic chemotherapy and derive maximum survival benefit from hormonal therapy alone.
This patient was diagnosed with a primary tumor of approximately 25 mm, metastasis to two ipsilateral nodes, and no distant metastasis, or stage IIIA. Her risk recurrence score was 20, indicating that she could safely forego the rigors of cytotoxic chemotherapy. She underwent localized breast-conserving surgery and began adjuvant tamoxifen therapy (x 2 years) to be followed with an aromatase inhibitor (x 3 years).
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Breast cancer is the most common tumor type and second only to lung cancer as a cause of cancer death in women. Nearly 300,000 women (and 2800 men) will receive a new diagnosis of breast cancer in the United States in 2023. Despite improvements in treatment options, breast cancer still will lead to 43,700 deaths among women this year.
Breast tumors generally are either ductal or lobular in origin. Ductal carcinomas arise in the lining of the milk ducts and are the most commonly found tumor type in breast cancer. Almost 3 in 4 diagnosed breast cancers histologically are invasive ductal carcinomas, which have spread from the source into surrounding structures. It has no specific histologic indicators and is differentiated from ductal carcinoma in situ by its having spread outside the duct. The presence of lymph node involvement in this patient also confirms invasive ductal carcinoma without metastatic spread. Invasive lobular carcinomas occur much less frequently and have a different histologic appearance of smaller cells that appear to be arranged in linear groups.
Mammography involves low-dose radiation and is used in diagnosis and is the most widely used screening technique for breast cancer. As a screening tool, mammography may detect lesions 1-2 years before they become palpable on breast self-examination. While the incidence of breast cancer in women has slowly increased over the past 20 years, mortality has decreased largely as a result of improved awareness and uptake of screening mammography. Current American Cancer Society guidelines recommend continued mammography screenings at least every other year after age 55 and continued for as long as a woman has a life expectancy > 10 years. Screening or diagnosis using MRI is usually reserved for individuals at high risk for breast cancer or a history of breast cancer before age 50.
For all newly diagnosed primary invasive breast cancers, biomarker testing for estrogen and progesterone receptor expression and HER2 expression are part of the standard workup recommended by the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) to help guide treatment decisions. Biomarker testing showed that her tumor was ER+ and HER2-negative, the most common finding in breast cancer. The patient's history did not suggest a risk for BRCA or other familial cancer mutations, but molecular testing was done and was negative for BRCA1 and BRCA2. In postmenopausal women, ASCO and NCCN also recommend use of risk assessment tools, such as Oncotype DX, to determine whether chemotherapy will add benefit to systemic endocrine therapy. Postmenopausal patients with ER+/HER2-negative breast cancer, one to three positive nodes, and a score < 26 on the 21-gene Oncotype DX can safely forego cytotoxic chemotherapy and derive maximum survival benefit from hormonal therapy alone.
This patient was diagnosed with a primary tumor of approximately 25 mm, metastasis to two ipsilateral nodes, and no distant metastasis, or stage IIIA. Her risk recurrence score was 20, indicating that she could safely forego the rigors of cytotoxic chemotherapy. She underwent localized breast-conserving surgery and began adjuvant tamoxifen therapy (x 2 years) to be followed with an aromatase inhibitor (x 3 years).
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
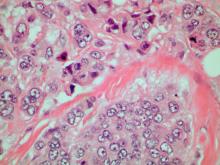
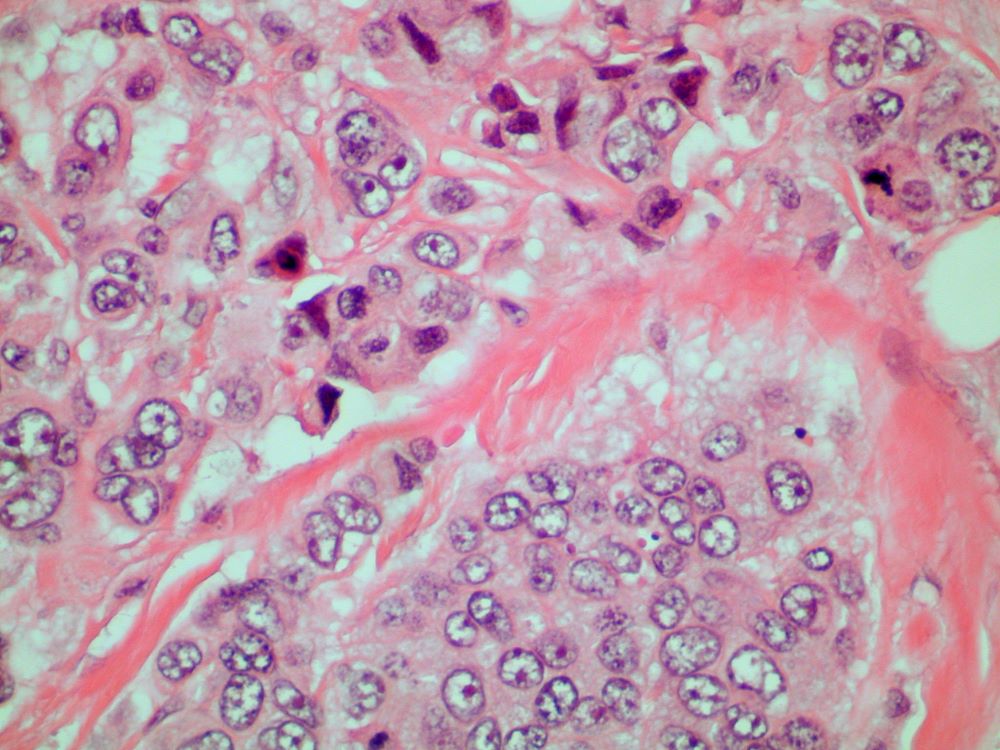
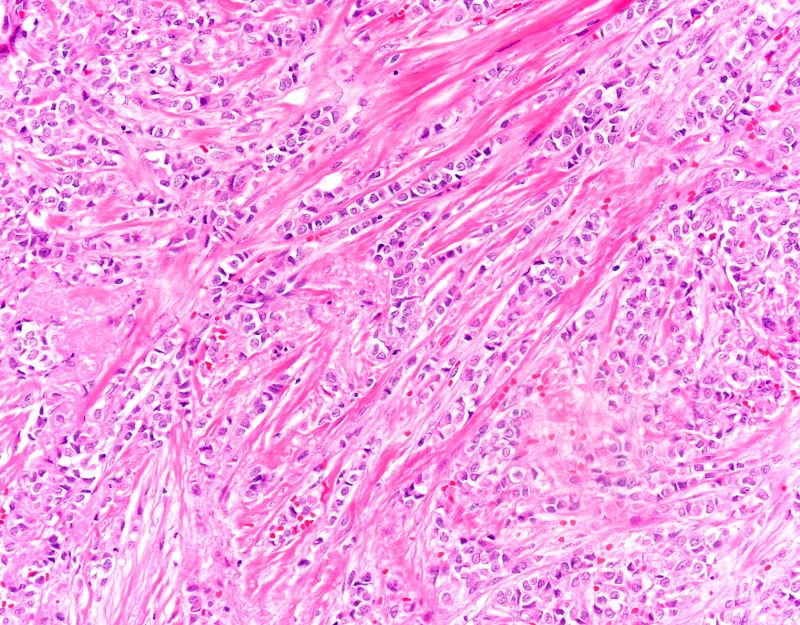
A 70-year-old woman presents for an annual exam and reports development of a firm lump in her right breast. She remarks that she has had discomfort in the same area for "at least a year," but the lump has become noticeable with even a cursory self-exam over the past 2 months. She has no previous history of breast cancer, hypertension, type 2 diabetes, or other cardiometabolic disease. She had no previous abnormal findings on mammograms through age 65 but has not had one since. Her family history includes a grandmother who died of breast cancer at age 64 and her father who lived with prostate cancer for 20 years after diagnosis at age 60. The physical exam reveals a firm, palpable lump in the upper right quadrant of her right breast. The exam is otherwise normal for the patient's age, with minor osteoarthritis that she remarks has worsened over the past year. Mammography, image-guided biopsy, and biomarker and molecular testing are ordered. Lymph node testing reveals two involved nodes, and histology reveals the following (see image).
Management of Metastatic Triple-Negative Breast Cancer
Palpable mass on exam
Given the age of the patient and the results of imaging, histology, and immunohistochemistry, the diagnosis is mucinous (colloid) carcinoma. The patient and oncologist discuss prognosis and discuss treatment options, such as breast-conserving surgery, local radiation, and possible adjuvant endocrine therapy.
Mucinous (colloid) carcinoma is a rare histologic subtype of invasive breast cancer that occurs in < 5% of patients and generally develops in those who are ≥ 60 years old. Patients with mucinous (colloid) carcinoma generally present with a palpable mass or, on imaging, a poorly defined tumor with rare calcifications. The histologic hallmark of mucinous (colloid) carcinoma is mucin production. There are two subtypes of mucinous breast carcinoma: pure and mixed. A pure mucinous tumor is defined as a carcinoma consisting of ≥ 90% intracellular or extracellular mucin. This pure subtype occurs more frequently than mixed mucinous breast carcinoma and is also less likely to metastasize to the lymph nodes.
Differential diagnosis can be challenging because mucinous (colloid) carcinoma can mimic a benign tumor on imaging, which is why it is important to include multiple factors when diagnosing in daily practice. According to the National Comprehensive Cancer Network (NCCN), diagnosing nonmetastatic invasive breast cancer like mucinous (colloid) carcinoma involves patient history and physical exam, diagnostic bilateral mammography (ultrasound and breast MRI, as needed), pathology review, tumor estrogen/progesterone receptor status, HER2 status, and genetic counseling for those with a family history. In most cases of mucinous (colloid) carcinoma, tumors are ER- and PR-positive and HER2-negative.
A pure mucinous histologic subtype is generally associated with a favorable prognosis; 10-year survival rates of mucinous (colloid) carcinoma are > 80%. The tumor is generally not high grade and is most often classified on surgical excision. Two main types of lesions exist — A and B — as does a combination of AB. Type A has larger quantities of extracellular mucin and is considered the classic form of mucinous carcinoma. Type B is a distinct variant with endocrine differentiation. In addition, glycoproteins MUC2 and MUC6 are predominantly expressed in mucinous (colloid) carcinoma; ductal carcinoma in situ is not often found in this setting.
NCCN recommends multidisciplinary care and development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Breast-conserving surgery and local radiation therapy are often the two modalities used to treat mucinous (colloid) carcinoma, especially because prognosis is so favorable. NCCN recommends the consideration of adjuvant endocrine treatment for patients with pure mucinous tumors that are HER2-negative and ER-positive and/or PR-positive; staged at pT1, pT2, or pT3, and pN0 or pN1mi; and ≤ 2.9 cm. Adjuvant endocrine therapy is recommended for patients with the same disease characteristics whose tumor is ≥ 3 cm.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the age of the patient and the results of imaging, histology, and immunohistochemistry, the diagnosis is mucinous (colloid) carcinoma. The patient and oncologist discuss prognosis and discuss treatment options, such as breast-conserving surgery, local radiation, and possible adjuvant endocrine therapy.
Mucinous (colloid) carcinoma is a rare histologic subtype of invasive breast cancer that occurs in < 5% of patients and generally develops in those who are ≥ 60 years old. Patients with mucinous (colloid) carcinoma generally present with a palpable mass or, on imaging, a poorly defined tumor with rare calcifications. The histologic hallmark of mucinous (colloid) carcinoma is mucin production. There are two subtypes of mucinous breast carcinoma: pure and mixed. A pure mucinous tumor is defined as a carcinoma consisting of ≥ 90% intracellular or extracellular mucin. This pure subtype occurs more frequently than mixed mucinous breast carcinoma and is also less likely to metastasize to the lymph nodes.
Differential diagnosis can be challenging because mucinous (colloid) carcinoma can mimic a benign tumor on imaging, which is why it is important to include multiple factors when diagnosing in daily practice. According to the National Comprehensive Cancer Network (NCCN), diagnosing nonmetastatic invasive breast cancer like mucinous (colloid) carcinoma involves patient history and physical exam, diagnostic bilateral mammography (ultrasound and breast MRI, as needed), pathology review, tumor estrogen/progesterone receptor status, HER2 status, and genetic counseling for those with a family history. In most cases of mucinous (colloid) carcinoma, tumors are ER- and PR-positive and HER2-negative.
A pure mucinous histologic subtype is generally associated with a favorable prognosis; 10-year survival rates of mucinous (colloid) carcinoma are > 80%. The tumor is generally not high grade and is most often classified on surgical excision. Two main types of lesions exist — A and B — as does a combination of AB. Type A has larger quantities of extracellular mucin and is considered the classic form of mucinous carcinoma. Type B is a distinct variant with endocrine differentiation. In addition, glycoproteins MUC2 and MUC6 are predominantly expressed in mucinous (colloid) carcinoma; ductal carcinoma in situ is not often found in this setting.
NCCN recommends multidisciplinary care and development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Breast-conserving surgery and local radiation therapy are often the two modalities used to treat mucinous (colloid) carcinoma, especially because prognosis is so favorable. NCCN recommends the consideration of adjuvant endocrine treatment for patients with pure mucinous tumors that are HER2-negative and ER-positive and/or PR-positive; staged at pT1, pT2, or pT3, and pN0 or pN1mi; and ≤ 2.9 cm. Adjuvant endocrine therapy is recommended for patients with the same disease characteristics whose tumor is ≥ 3 cm.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the age of the patient and the results of imaging, histology, and immunohistochemistry, the diagnosis is mucinous (colloid) carcinoma. The patient and oncologist discuss prognosis and discuss treatment options, such as breast-conserving surgery, local radiation, and possible adjuvant endocrine therapy.
Mucinous (colloid) carcinoma is a rare histologic subtype of invasive breast cancer that occurs in < 5% of patients and generally develops in those who are ≥ 60 years old. Patients with mucinous (colloid) carcinoma generally present with a palpable mass or, on imaging, a poorly defined tumor with rare calcifications. The histologic hallmark of mucinous (colloid) carcinoma is mucin production. There are two subtypes of mucinous breast carcinoma: pure and mixed. A pure mucinous tumor is defined as a carcinoma consisting of ≥ 90% intracellular or extracellular mucin. This pure subtype occurs more frequently than mixed mucinous breast carcinoma and is also less likely to metastasize to the lymph nodes.
Differential diagnosis can be challenging because mucinous (colloid) carcinoma can mimic a benign tumor on imaging, which is why it is important to include multiple factors when diagnosing in daily practice. According to the National Comprehensive Cancer Network (NCCN), diagnosing nonmetastatic invasive breast cancer like mucinous (colloid) carcinoma involves patient history and physical exam, diagnostic bilateral mammography (ultrasound and breast MRI, as needed), pathology review, tumor estrogen/progesterone receptor status, HER2 status, and genetic counseling for those with a family history. In most cases of mucinous (colloid) carcinoma, tumors are ER- and PR-positive and HER2-negative.
A pure mucinous histologic subtype is generally associated with a favorable prognosis; 10-year survival rates of mucinous (colloid) carcinoma are > 80%. The tumor is generally not high grade and is most often classified on surgical excision. Two main types of lesions exist — A and B — as does a combination of AB. Type A has larger quantities of extracellular mucin and is considered the classic form of mucinous carcinoma. Type B is a distinct variant with endocrine differentiation. In addition, glycoproteins MUC2 and MUC6 are predominantly expressed in mucinous (colloid) carcinoma; ductal carcinoma in situ is not often found in this setting.
NCCN recommends multidisciplinary care and development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Breast-conserving surgery and local radiation therapy are often the two modalities used to treat mucinous (colloid) carcinoma, especially because prognosis is so favorable. NCCN recommends the consideration of adjuvant endocrine treatment for patients with pure mucinous tumors that are HER2-negative and ER-positive and/or PR-positive; staged at pT1, pT2, or pT3, and pN0 or pN1mi; and ≤ 2.9 cm. Adjuvant endocrine therapy is recommended for patients with the same disease characteristics whose tumor is ≥ 3 cm.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
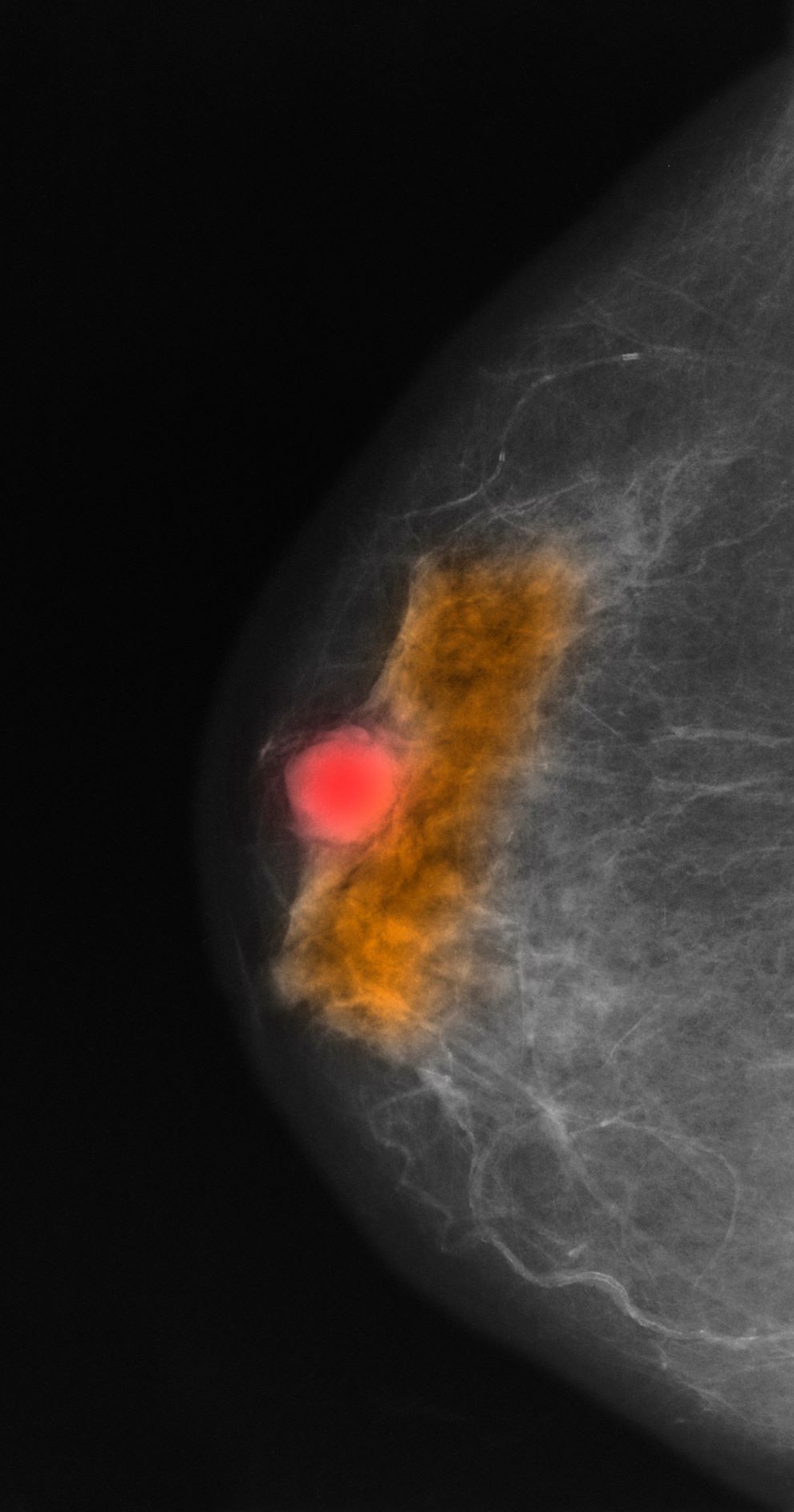
A 64-year-old woman with no prior history of cancer presents to an oncologist after referral from her primary care physician (PCP). The referral came after the patient reported feeling a lump in her left breast during self-examination. She made an appointment with her PCP, who confirmed a palpable mass on physical examination and ordered mammography. Bilateral mammography revealed a poorly defined tumor with rare calcifications in the left breast. Size of the tumor was 1.8 cm. Now, the oncologist orders a percutaneous vacuum-assisted large-gauge core-needle biopsy with image guidance. Results show the tumor is pure mucinous, ER-positive and PR-positive, and HER2-negative; staging is pT2/pN0. Immunohistochemistry reveals that the predominantly expressed glycoproteins are MUC2 and MUC6.
Advanced Breast Cancer Practice Essentials
Postmenopausal screening mammogram
The findings in this case are suggestive of invasive lobular carcinoma (ILC).
Globally, breast cancer remains the most common life-threatening cancer diagnosed and the second leading cause of cancer-related deaths in women. In the United States, approximately 287,850 new cases of invasive breast cancer were diagnosed in 2022 and 43,250 deaths were attributed to breast cancer in the same year. Worldwide, approximately 2.3 million new diagnoses and 685,000 breast cancer-related deaths were reported in 2020.
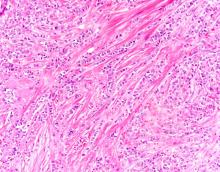
ILC is one of the leading histologic types of invasive carcinoma, second in incidence only to invasive carcinoma of no special type. ILC accounts for 5%-15% of all invasive breast cancers, and its incidence has been steadily increasing — particularly among postmenopausal women — over the past two decades. ILC has distinct molecular and histopathologic features, including the loss of cell-cell adhesion molecule E-cadherin, resulting in small, discohesive cells proliferating in single-file strands; positivity for both the estrogen and progesterone receptor; and human epidermal growth factor receptor 2 negativity.
The diagnosis of ILC can be challenging, as it is difficult to detect both on physical examination and with standard imaging techniques. Patients are often diagnosed with late-stage disease, characterized by large tumors and lymph node involvement. The signs of ILC are often vague, such as skin thickening or dimpling. In addition, measuring the extent of ILC can be challenging, as traditional screening methods (eg, mammography and ultrasonography) have a low sensitivity for detecting ILC compared with other invasive breast tumors. This difficulty is usually ascribed to the diffuse infiltrative growth pattern of ILC. MRI has a greater sensitivity for detecting ILC.
Risk factors for the development of ILC have been identified and include:
• Alcohol consumption
• Use of combined hormone replacement therapy
• Early menarche (menarche before the age of 12 years)
• Late-onset menopause (menopause after the age of 55 years)
• Nulliparity/low parity (defined by World Health Organization as fewer than five pregnancies with gestation periods of ≥ 20 weeks)
• Late age at birth (> 30 years)
• Family history (eg, hereditary diffuse gastric cancer syndrome)
• Genetics (eg, CDH1 mutations)
Treatment protocols for ILC align with those used in other breast cancer subtypes and typically involve a multidisciplinary approach comprising surgery, radiotherapy, and systemic therapies. Cancers that are deemed resectable will typically be managed surgically upfront, although some patients may require neoadjuvant therapy to reduce tumor burden and facilitate surgical intervention. Breast-conserving surgery using a wide local excision can frequently be performed; however, in up to 65% of cases, a second surgery will be required (re-excision or mastectomy). Axillary lymph node status is a crucial factor in the prognosis of all breast cancers and affects surgical planning. Sentinel node biopsy is the standard method of assessing the axilla.
Systemic therapy is an integral part of the multidisciplinary approach to treating breast cancer and usually involves the use of chemotherapy. However, because of the unique molecular biology of ILC, treatment response to chemotherapy is often poor, resulting in lower rates of complete pathologic response and higher rates of mastectomy. Conversely, ILC has been shown to respond well to endocrine therapy, making it the optimal treatment choice. Novel therapeutic approaches are under investigation.
Detailed guidance on the treatment of ILC is available from the National Comprehensive Cancer Network.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The findings in this case are suggestive of invasive lobular carcinoma (ILC).
Globally, breast cancer remains the most common life-threatening cancer diagnosed and the second leading cause of cancer-related deaths in women. In the United States, approximately 287,850 new cases of invasive breast cancer were diagnosed in 2022 and 43,250 deaths were attributed to breast cancer in the same year. Worldwide, approximately 2.3 million new diagnoses and 685,000 breast cancer-related deaths were reported in 2020.
ILC is one of the leading histologic types of invasive carcinoma, second in incidence only to invasive carcinoma of no special type. ILC accounts for 5%-15% of all invasive breast cancers, and its incidence has been steadily increasing — particularly among postmenopausal women — over the past two decades. ILC has distinct molecular and histopathologic features, including the loss of cell-cell adhesion molecule E-cadherin, resulting in small, discohesive cells proliferating in single-file strands; positivity for both the estrogen and progesterone receptor; and human epidermal growth factor receptor 2 negativity.
The diagnosis of ILC can be challenging, as it is difficult to detect both on physical examination and with standard imaging techniques. Patients are often diagnosed with late-stage disease, characterized by large tumors and lymph node involvement. The signs of ILC are often vague, such as skin thickening or dimpling. In addition, measuring the extent of ILC can be challenging, as traditional screening methods (eg, mammography and ultrasonography) have a low sensitivity for detecting ILC compared with other invasive breast tumors. This difficulty is usually ascribed to the diffuse infiltrative growth pattern of ILC. MRI has a greater sensitivity for detecting ILC.
Risk factors for the development of ILC have been identified and include:
• Alcohol consumption
• Use of combined hormone replacement therapy
• Early menarche (menarche before the age of 12 years)
• Late-onset menopause (menopause after the age of 55 years)
• Nulliparity/low parity (defined by World Health Organization as fewer than five pregnancies with gestation periods of ≥ 20 weeks)
• Late age at birth (> 30 years)
• Family history (eg, hereditary diffuse gastric cancer syndrome)
• Genetics (eg, CDH1 mutations)
Treatment protocols for ILC align with those used in other breast cancer subtypes and typically involve a multidisciplinary approach comprising surgery, radiotherapy, and systemic therapies. Cancers that are deemed resectable will typically be managed surgically upfront, although some patients may require neoadjuvant therapy to reduce tumor burden and facilitate surgical intervention. Breast-conserving surgery using a wide local excision can frequently be performed; however, in up to 65% of cases, a second surgery will be required (re-excision or mastectomy). Axillary lymph node status is a crucial factor in the prognosis of all breast cancers and affects surgical planning. Sentinel node biopsy is the standard method of assessing the axilla.
Systemic therapy is an integral part of the multidisciplinary approach to treating breast cancer and usually involves the use of chemotherapy. However, because of the unique molecular biology of ILC, treatment response to chemotherapy is often poor, resulting in lower rates of complete pathologic response and higher rates of mastectomy. Conversely, ILC has been shown to respond well to endocrine therapy, making it the optimal treatment choice. Novel therapeutic approaches are under investigation.
Detailed guidance on the treatment of ILC is available from the National Comprehensive Cancer Network.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The findings in this case are suggestive of invasive lobular carcinoma (ILC).
Globally, breast cancer remains the most common life-threatening cancer diagnosed and the second leading cause of cancer-related deaths in women. In the United States, approximately 287,850 new cases of invasive breast cancer were diagnosed in 2022 and 43,250 deaths were attributed to breast cancer in the same year. Worldwide, approximately 2.3 million new diagnoses and 685,000 breast cancer-related deaths were reported in 2020.
ILC is one of the leading histologic types of invasive carcinoma, second in incidence only to invasive carcinoma of no special type. ILC accounts for 5%-15% of all invasive breast cancers, and its incidence has been steadily increasing — particularly among postmenopausal women — over the past two decades. ILC has distinct molecular and histopathologic features, including the loss of cell-cell adhesion molecule E-cadherin, resulting in small, discohesive cells proliferating in single-file strands; positivity for both the estrogen and progesterone receptor; and human epidermal growth factor receptor 2 negativity.
The diagnosis of ILC can be challenging, as it is difficult to detect both on physical examination and with standard imaging techniques. Patients are often diagnosed with late-stage disease, characterized by large tumors and lymph node involvement. The signs of ILC are often vague, such as skin thickening or dimpling. In addition, measuring the extent of ILC can be challenging, as traditional screening methods (eg, mammography and ultrasonography) have a low sensitivity for detecting ILC compared with other invasive breast tumors. This difficulty is usually ascribed to the diffuse infiltrative growth pattern of ILC. MRI has a greater sensitivity for detecting ILC.
Risk factors for the development of ILC have been identified and include:
• Alcohol consumption
• Use of combined hormone replacement therapy
• Early menarche (menarche before the age of 12 years)
• Late-onset menopause (menopause after the age of 55 years)
• Nulliparity/low parity (defined by World Health Organization as fewer than five pregnancies with gestation periods of ≥ 20 weeks)
• Late age at birth (> 30 years)
• Family history (eg, hereditary diffuse gastric cancer syndrome)
• Genetics (eg, CDH1 mutations)
Treatment protocols for ILC align with those used in other breast cancer subtypes and typically involve a multidisciplinary approach comprising surgery, radiotherapy, and systemic therapies. Cancers that are deemed resectable will typically be managed surgically upfront, although some patients may require neoadjuvant therapy to reduce tumor burden and facilitate surgical intervention. Breast-conserving surgery using a wide local excision can frequently be performed; however, in up to 65% of cases, a second surgery will be required (re-excision or mastectomy). Axillary lymph node status is a crucial factor in the prognosis of all breast cancers and affects surgical planning. Sentinel node biopsy is the standard method of assessing the axilla.
Systemic therapy is an integral part of the multidisciplinary approach to treating breast cancer and usually involves the use of chemotherapy. However, because of the unique molecular biology of ILC, treatment response to chemotherapy is often poor, resulting in lower rates of complete pathologic response and higher rates of mastectomy. Conversely, ILC has been shown to respond well to endocrine therapy, making it the optimal treatment choice. Novel therapeutic approaches are under investigation.
Detailed guidance on the treatment of ILC is available from the National Comprehensive Cancer Network.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 58-year-old postmenopausal woman presents for screening mammography. The patient's last mammogram was 18 months ago and showed dense breast tissue with no abnormalities. The patient states that she has no breast symptoms. She is 5 ft 3 in and weighs 196 lb (BMI 34.7). Her previous medical history is unremarkable. There is a family history of breast cancer (two maternal cousins) and colon cancer (paternal grandmother). Bilateral mammography reveals an irregular mass that is approximately 2.4 cm and calcifications in the upper outer quadrant of the right breast. Physical examination reveals no palpable abnormalities. The patient undergoes a stereotactic breast biopsy. Pathology findings include malignant monomorphic cells that form loosely dispersed linear columns encircling the mammary ducts and infiltrating breast tissue and fat.